2
HEMATOPOIETIC STEM CELL TRANSPLANTATION
BIOLOGICAL CHARACTERISTICS OF UMBILICAL CORD BLOOD
Blood cell differentiation begins with multipotent hematopoietic progenitor cells (HPCs), which are located in the marrow spaces of the bone. These primitive cells undergo division and differentiation to form the various peripheral blood cells. As the cells reproduce, they commit to a particular task or cell line and become known as committed progenitor cells. These committed progenitor cells are difficult to discern from the original multipotent cells but can be cultured to form colonies of specific types of blood cells (Guyton and Hall, 2000). These cultured cells, or colony-forming units (CFUs), are coded according to the type of cells that they will ultimately produce (e.g., CFU-M cells will produce megakaryocyte cells) (Figure 2-1). Umbilical cord blood is a rich source of these committed progenitor cells and, presumably, multipotent HPCs (Knudtzon, 1974). In fact, cord blood has a significantly higher concentration per volume of primitive HPCs than does bone marrow (Nakahata and Ogawa, 1982; Smith and Broxmeyer, 1986), thereby making it a potential source of cells for transplantation (Bodger, 1987). In laboratory analyses, these cells were found to have higher proliferative responses (indicating a higher engraftment potential) than similar doses of marrow (per 105 nucleated cells per kilogram [kg] of graft) (Mayani and Lansdorp, 1998; Barker and Wagner, 2003b). In the last decade, the number of transplantations of HPCs derived from cord blood has increased, particularly for children.
Numerous literature reports document the feasibility and efficacy of the transplantation of cord blood from a related donor for the treatment of
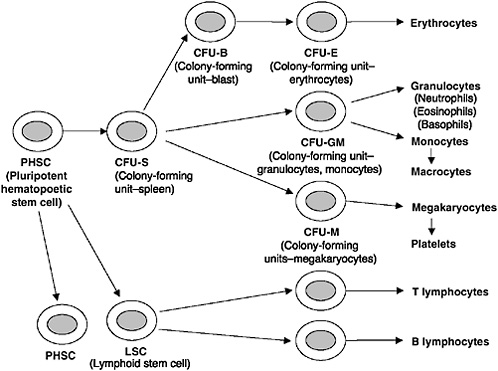
FIGURE 2-1 Formation of the multiple peripheral blood cells from multipotent hematopoietic stem cells.
SOURCE: Guyton and Hall, 2000.
a broad range of disorders for which transplantion of HPCs from an adult donor is also successful, including hematological malignancies, solid tumors, constitutional and acquired bone marrow failure syndromes, hemoglobinopathies, congenital immune deficiencies, and inherited disorders of metabolism (Gluckman et al., 1997; Locatelli et al., 1999; Rocha et al., 2000; Locatelli et al., 2003). After the early success of transplantation of cord blood from related donors, cord blood banks were established to provide rapidly accessible, human leukocyte antigen (HLA)-typed units predominantly for transplantation of HPCs from unrelated donors. Since then cord blood banking programs throughout the world have expanded rapidly (Broxmeyer, 1998), with the estimated number of units stored to date exceeding 155,000 (BMDW, 2004).
The establishment of at least three independent, international registries of outcome data—the International Cord Blood Transplant Registry (ICBTR) in 1992 (which was transferred to the International Bone Marrow Transplant Registry [IBMTR] in 1996, and to the Center for International
Blood and Marrow Transplant Research [CIBMTR] in 2004), the European Research Project on Cord Blood Transplantation (Eurocord) in 1993, and the Japanese Cord Blood Banking Network in 1996—expedited the clinical evaluation of the efficacy and safety of transplantation of cord blood from unrelated donors. With more than 6,000 transplants of cord blood from related and unrelated donors performed thus far, cord blood has emerged as an acceptable, alternative source of HPCs that has some advantages over adult sources of HPCs and the availability of which represents an important development in the field.
One advantage of cord blood over adult sources of HPCs is the fact that cord blood banking does not have the same donor attrition issues because the units are collected and stored before they are needed. In addition, the time from unit identification to transplantation can be a matter of days to weeks rather than the weeks to months required for adult HPC donation. Another major benefit of cord blood is the reduced capacity of cord blood cells to produce an alloreactive response (i.e., an immune response against the recipient). This results in less frequent and less severe graft versus host disease (GVHD) and the ability to perform transplants with greater degrees of donor-patient HLA disparity compared with the disparity associated with adult HPC transplants, which must be minimized as much as possible (see Box 2-1). This is thought to be due to both quantitative and qualitative differences in the lymphoid cell content of cord blood (Risdon et al., 1995; Roncarolo et al., 1996; Leung et al., 1999; Gluckman and Locatelli, 2000).
Compared with adult HPCs, cord blood cells have immune naiveté because of their minimal previous exposure to antigens (Sirchia and Rebulla, 1999). However, immune suppression is still required, as is the case for adult HPC transplantation, to prevent both rejection and GVHD. In addition, although the recipient can tolerate some degree of HLA mismatch, the clinical results are generally better with closer HLA matches. Furthermore, cord blood-derived HPCs possess higher expansion and proliferation potentials than HPCs derived from bone marrow. Similar differences may also account for the replicative capacities of cells (Allsopp et al., 1992; Vaziri et al., 1994; Lansdorp, 1995a, 1995b).
However, cord blood has some important limitations. Even though cord blood units have higher concentrations of HPCs, they have relatively small volumes (~100 milliliters) and, therefore, fewer total cells than bone marrow grafts. This can result in very low cell doses (the number of cells in the graft per kilogram patient weight) for larger children and adults (HRSA, 2000). In addition, the use of very low overall cell doses (<1.5 × 107 nucleated cells in the graft per kilogram of patient weight) results in a higher risk of nonengraftment. Immune reconstitution with cord blood can also be slower, and, on account of this delayed immune reconstitution, the
|
BOX 2-1 Proteins that control tissue compatibility were first detected on the surface of white blood cells and were named human leukocyte antigens (HLAs). Located in a cluster on chromosome 6, the genes encoding these cell surface molecules are named the major histocompatibility complex (MHC) (Dausset, 1981; Benacerraf, 1992; Snell, 1992; Janeway et al., 2005). These molecules are then further divided into class I, which is made up of HLA-A-, HLA-B, and HLA-C, and class II, which is made up of HLA-DR, HLA-DQ, and HLA-DP. Although, these two classes are composed of different polypeptide chains, they assume a very similar structure on the cell surface (Bjorkman and Parham, 1990). Figure 2-2 provides a diagram of the HLA protein, its location on chromosome 6, and its function. An individual’s HLA genotype reflects two haplotypes (the genes inherited on a single chromosome), one inherited from mother and one inherited from the father. Within a family, siblings have a one-in-four chance of inheriting the same two haplotypes and, thus, of being HLA identical. Outside the family, the situation is very different. HLA antigens are highly polymorphic, with hundreds of different HLA antigens found in the human population (the HLA-A, HLA-B, and HLA-DR antigens alone have roughly 750,000 possible combinations). However, many of these potential combinations have not yet been encountered because HLA alleles are in linkage disequilibrium. Only a subset of all possible HLA haplotypes have been encountered in the entire population. At present, the donated HPC cells (including cord blood) are routinely typed for HLA-A, HLA-B, and HLA-DR loci. Each individual has two antigens at each of these sites. Thus, when a transplant physician speaks of a 4/6, 5/6, or 6/6 match, he is talking about the number of antigens a particular donor has in common with the patient. Many transplant centers also type the units for HLA-C and HLA-DQB1 because some data suggest that disparities at these loci can also affect the outcome of a transplant (Hurley et al., 2003). There is ongoing research about which, if any, loci are better able to tolerate a mismatch. |
rate of infection can sometimes be higher. Some data indicate that this can be overcome by combining cells from multiple units or by combining cord blood with highly purified HPCs from mismatched related donors. Several investigators have also explored the ex vivo expansion of cord blood cells, but no data supporting faster engraftment by this approach are available and few comparative studies of the clinical outcomes obtained by transplantation of HPCs from adult donors and cord blood have been conducted.
The fact that cord blood transplants can be successful, even though they involve degrees of HLA disparity typically associated with an increased risk of GVHD in the adult donor setting, allows transplant physicians some leeway in determining which units can be selected for transplantation.
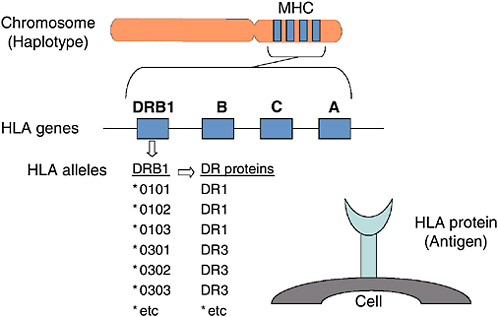
FIGURE 2-2 The human histocompatibility genes.
SOURCE: Hurley (2005). See also Appendix F.
Transplant physicians can select units with higher cell counts instead of a closer HLA match to increase the chances of a successful engraftment. However, some degree of HLA compatibility is necessary, although the minimum acceptable level is somewhat controversial (see also Appendix G). Most cord blood transplants are done with units mismatched for a maximum of two HLA antigens (HLA-A and HLA-B) using low-resolution typing techniques and for DRB1 antigens by using high-resolution typing techniques.
The detection of a suitable cord blood unit for a given patient is difficult. First, significant differences in HLA types exist among various ethnic populations. For example, HLA-Bw53 is found in 5.4 percent of the haplotypes in African Americans but only 0.05 percent of the haplotypes in Caucasians (Margolis and Casper, 2000). Because of the number of possible HLA combinations, which are enhanced by the differences inherent in the various ethnic populations, the likelihood of finding an exact match is extremely small.
HLA-types have traditionally been identified with serologic typing (also called “low/intermediate resolution” typing) methods. Sequencing (one of the many methods referred to as “high resolution typing”) of HLA genes revealed that each of the serologically defined HLA antigens represent many different protein structures. Thus, a unit typed at the low resolution level
may appear to be a match but may be mismatched when typed at high resolution. More information about HLA and matching can be found in Appendix F.
CORD BLOOD TRANSPLANTATION
Allogeneic Transplantation
Most cord blood banking involves the collection, storage, and distribution of cells for transplantation to unrelated individuals. These units are typed and stored anonymously so that any patient who might have a medical need may use them. This transplantation involving the transfer of cells from one individual to another genetically different individual is referred to as allogeneic transplantion, and the individual receiving the transplant may or may not be related to the donor.
HPC transplantation can also be done using autologous cells, in which an individual’s own cells are collected and stored and then reinfused, generally after high-dose anticancer therapy. Allogeneic and autologous transplantation each have benefits and disadvantages. The benefits of allogeneic transplantation include the ability to treat a wide range of conditions, both inherited and acquired, with HPCs from a healthy, unaffected donor. Allogeneic cells may also have immune effects that may aid in eradicating the recipient’s malignancy. Autologous transplantation carries the risk of the reinfusion of abnormal cells if the cells are collected after the onset of the disease and it lacks any immune-mediated anticancer effects. The major disadvantage of allogeneic transplantation is the use of non-native tissue for the graft, which necessitates the use of immunosuppressive therapy to avoid rejection and GVHD. Table 2-1 describes the details of conditions that may be treated by allogeneic or autologous HPC transplantation.
Outcomes of Transplantation of HPCs from an Unrelated Donor
Engraftment of an HPC transplant is generally considered successful when neutrophil recovery is found. Neutrophils are the white blood cells that form the first line of defense against infection. Neutrophil recovery is an indication that the patient has begun to generate new blood cells and hematopoiesis1 is being restored. Neutrophil recovery, however, is not synonymous with the restoration of full immune function, which requires the production and maturation of various other types of white blood cells and
TABLE 2-1 Indications for Allogeneic and Autologous Stem Cell Support
|
Disease |
Allogeneic Transplantation |
Autologous Transplantation |
|
Leukemia (acute lymphoblastic, acute myelogenous, chronic myelogenous) |
|
|
|
Lymphomas (Hodgkin’s disease, non-Hodgkin’s lymphoma) |
|
|
|
Myelodysplastic Syndromes |
|
|
|
Neuroblastoma (stage IV) |
|
|
|
Bone and soft tissue sarcomas, Wilms’ tumor, brain tumors |
|
|
|
Aplastic anemia and other cytopenias (not environmentally caused) |
|
|
|
Immune deficiency (e.g., severe combined immunodeficiency disease) |
|
|
|
Hemoglobinopathies, thalassemia, sickle cell anemia |
|
|
|
Metabolic storage disorders, Hurler’s syndrome, metachromatic leukodystrophy |
|
|
|
Disease |
Allogeneic Transplantation |
Autologous Transplantation |
|
Renal Cell Cancer/Melanoma |
|
|
|
Autoimmune disease |
|
|
|
SOURCE: AAP (1999). |
||
which can take many months. It is, however, an important milestone, indicating a high likelihood of normal hematopoiesis.
The incidence of neutrophil recovery2 after cord blood transplantation, as reported in a larger series of studies, ranges from 65 to 92 percent (Laughlin et al., 2001) and is generally lower than that after adult bone marrow or peripheral blood transplantation. More rapid neutrophil and platelet recovery occurs in patients receiving higher cord blood cell doses (Wagner et al., 1996; Barker et al., 2001; Rubinstein et al., 2001; Grewal et al., 2003). The nucleated cell dose in a graft required for consistent engraftment, as reported by Gluckman et al. (1997), is greater than 3.7 × 107 cells in the graft per kilogram of patient weight; this was associated with a shorter time to neutrophil recovery (25 days with lower doses versus 35 days with higher doses). Rubinstein et al. (1998) suggested the use of a threshold cryopreserved nucleated cell dose greater than or equal to 2.5 × 107/kg of graft, whereas Wagner et al. (2002) reported success with a threshold dose of infused CD34+ cells of greater than or equal to 1.7 × 105/kg of graft.
It has also been demonstrated that a stepwise increase in the nucleated cell dose in the graft is associated with a progressively shortened time to neutrophil recovery (Rubinstein et al., 1998; Rubinstein and Stevens, 2000) and that the CD34+-cell dose predicts the speed of recovery (Wagner et al., 2002).
Several but not all studies show a relationship between HLA match and neutrophil recovery (Rubinstein et al., 1998; Gluckman et al., 2004). Using data provided by the National Marrow Donor Program, the New York Blood Center, and the National Heart, Lung, and Blood Institute (NHLBI) Cord Blood Transtplantation Study (COBLT), the committee conducted its
own analysis of the significance of the degree of HLA match and cell dose on outcome. This report can be found in its entirety in Appendix G.
The probability of survival after cord blood transplantation, as reported in a large series of studies, has ranged from 18 to 78 percent (Gluckman et al., 1997; Rubinstein et al., 1998). The variation is explained, in large part, by marked differences in patient characteristics (Wagner et al., 2002). In terms of graft parameters, however, nearly all studies demonstrate a significant relationship between cell dose and survival after cord blood transplantation.
The association between the level of HLA match and survival is controversial, in part because of the limited numbers of patients who have been studied and a lack of data for all age groups. For example, Locatelli et al. (1999) reported on the outcomes for pediatric patients with acute leukemia reported in Eurocord3 registry and found that the number of HLA mismatches did not influence survival. Similarly, in a study of 68 adult recipients ofcord blood with zero to three HLA mismatches, Laughlin et al. (2001) also found no association between the degree of HLA mismatch and the overall rate of survival. In contrast, Rubinstein et al. (1998), Rubinstein and Stevens (2000), and Wagner et al. (2002) observed a significant negative association between HLA mismatch and survival. Other studies have attempted to link the association between HLA mismatch and the occurrence of GVHD but have so far been unsuccessful in formulating reliable estimates of the risk of GVHD from transplants with HLA mismatches (Laughlin et al., 1998), although a positive association between the two has been reported (Amos and Gordon, 1995; Gluckman et al., 2004).
Outcomes of HPC Transplantation from Unrelated Adult Donors
Transplantation of HPCs from unrelated donors for the treatment of acute leukemia in adults is associated with a high risk of GVHD and treatment failure (Ash et al., 1991; Laughlin et al., 2004). Cord blood transplantation may have an advantage in adult patients because of a potentially decreased risk of GVHD. However, as the cell dose is an important determinant of the outcome and adults require higher absolute numbers of cells to achieve adequate doses, the pool of cord blood units suitable for adult patients is considerably smaller than that suitable for children.
Laughlin et al. (2001) reported on the first major series of cord blood transplants done in adults by pooling data from five U.S. centers. The patients (68 subjects) weighed a median of 69.2 kg (weight range, 40.9 to
115.5 kg); 54 patients had a hematological cancer, and 50 of these patients were considered at intermediate or high risk of rejection. In 97 percent of the grafts, HLAs between the donor and the patient were mismatched at one of three antigens, and the median nucleated cell dose infused was 2.1 × 107/kg of graft (range, 1 × 107 to 6.3 × 107/kg of graft). The rate of engraftment was similar to that in studies with pediatric populations, with an estimated probability of myeloid recovery of 90 percent by day 42 (median time to engraftment was 28 days).4 The probabilities of grade II-IV and grade III-IV GVHD (see below) were 60 and 20 percent respectively. Nineteen of 68 recipients survived 22 months after the engraftment. Faster rates of myeloid recovery as well as longer lengths of disease-free survival were reported for the patients who received higher nucleated cell and CD34+-cell doses. No significant association between the extent of HLA mismatching and the kinetics of myeloid recovery, graft failure, and acute GVHD was reported and the risk of severe acute and chronic GVHD was lower than that typically reported after the transplantation of bone marrow from unrelated donors. (Hansen et al., 1990, 1998; Kernan et al., 1993; McGlave et al., 1993; Schiller et al., 1994; Szydlo et al., 1997; Cornetta et al., 2005).
Sanz et al. (2001) reported on the results of a study in which 20 of 22 adult patients (weight range, 41 to 85 kg) who received an unrelated cord blood transplant (total nucleated cell dose ranging from 1.01 × 107 to 4.96 × 107/kg of graft) survived more than 30 days and showed myeloid engraftment at a median of 22 days. In a separate Eurocord report of 42 adults receiving cord blood from unrelated donors, the median time to neutrophil recovery was 35 days, and no patient who received less than 1.0 × 107 nucleated cells per kilogram of graft survived (Locatelli et al., 1999). Both of these studies underscore the importance of the graft cell dose to achieving the optimal results after cord blood transplantation, suggesting that the minimum effective nucleated cell dose is at least 1.5 × 107 nucleated cells/kg of graft.
Two recent comparative studies of bone marrow and cord blood transplantation in adults retrospectively examined the results of transplants performed over several years (Laughlin et al., 2004; Rocha et al., 2004). Both studies concluded that cord blood grafts in adults were acceptable alternatives to bone marrow grafts if no suitably matched bone marrow donors were available, even though the cord blood units transplanted contained lower numbers of nucleated cells. The two studies differed in that Rocha
and colleagues reported that the results obtained with HLA-matched bone marrow were similar to those obtained with HLA-matched and mismatched cord blood, whereas Laughlin et al. found somewhat better results with HLA-matched bone marrow than with HLA-mismatched cord blood. Both found lower rates of acute GVHD among individuals who received cord blood, although Laughlin and colleagues (2004) found that this was true only when they compared the outcomes for HLA-mismatched bone marrow transplants receipients with those for HLA-mismatched cord blood transplant recipients. The rates of recurrence of leukemia and the incidence of chronic GVHD did not differ significantly by graft type. The outcomes of transplants of cord blood HLA mismatched at one and two antigens did not differ significantly (Laughlin et al., 2004).
Cornetta et al. (2005) recently published a report describing the results of cord blood transplantation in high-risk adults in connection with the COBLT study. Of the 30 subjects infused with cord blood as a part of that study, 19 achieved engraftment in an estimated median time of 31 days. The survival probability of these 30 subjects was 47 percent to day 180, although only 17 percent were alive at 1 year. Analysis on the basis of HLA match showed no significant difference in survival. Significant causes of death included relapse and acute GVHD.
With the paucity of cord blood units available in general and the even smaller number of units with an acceptable cell dose, adults are more frequently offered a unit that is mismatched at two HLAs and that also contains a suboptimal cell dose, a scenario that is especially true for non-white adult patients.
Autologous Transplantation
Autologous transplantation is the process by which blood or bone marrow samples from a patient are saved from a patient before the patient receives extensive chemo- or radiation therapy as treatment for a disease. The reintroduction of one’s own progenitor cells generally results in the rapid reconstitution of the immune and blood systems and avoids the need for immunosuppressive therapy.
Private cord blood banks perform this service for people who wish to be prepared in the event their child becomes ill. This type of treatment is useful only for a limited number of diseases or disorders, however, as preexisting genetic conditions are not treatable because the saved cord blood contains the same dysfunctional genetic code that results in the expression of the condition in the first place. For this reason, saving cord blood for autologous use on a large scale may be impractical because of the limitations to its use and the infrequency of the conditions that can be treated with the unit (Catlin et al., 2000).
Eurocord has taken the public position that that there is little scientific justification for preservation unless: (1) the disorder may be corrected by reinfusion of the preserved cord blood cells after correction of the genetic defect, and (2) it would not be considered advantageous to attempt such a correction with cells from any other source (Fernandez, 1998). However, the committee is aware of at least 14 autologous cord blood transplant procedures. With only one autologous transplant reported in the literature thus far (in a child with aplastic anemia), the technique’s usefulness remains speculative (Fruchtman et al., 2004).
Outcomes of Transplantation of HPCs from Related Donors
Information reported in IBMTR, ICBTR, and the Eurocord registry has demonstrated that the transplantation of cord blood from a related donor will reliably reconstitute hematopoiesis after myeloablative therapy. In these studies the probability of neutrophil recovery by day 60 after engraftment was 84 percent in one such study, with a median time to neutrophil recovery of 17 days. Platelet recovery5 was observed at a median of 56 days with a probability of platelet engraftment of 85 percent by day 180 after engraftment (Locatelli et al., 1999). In their report of the results a comparative study, Rocha et al. (2000) reported that cord blood transplantation results in a delayed and lower cumulative incidence of hematopoietic recovery compared with that achieved with bone marrow transplantation. The factors significantly associated with superior engraftment included younger age (Gluckman et al., 1997; Rocha et al., 2000), lower weight (Gluckman et al., 1997), the use of GVHD prophylaxis regimens that did not contain methotrexate (Atkinson, 1990; Rocha et al., 2000; Locatelli et al., 2003), the use of a higher cell dose (Gluckman et al., 1997; Locatelli et al., 1999) and the transplantation of units with closer HLA matches (Gluckman et al., 1997).
In patients with bone marrow failure syndromes, inborn errors of metabolism, and hemoglobinopathies, there appears to be a trend toward a higher risk of primary graft failure, regardless of graft source (Gluckman et al., 1997).
The assessment of the risk of a relapse after the transplantation of cord blood from a related donor is limited by the short duration of monitoring in published studies (median 24 to 34 months) and the inclusion of patients with a range of malignancies at various stages of disease (Gluckman et al., 1997; Locatelli et al., 1999; Rocha et al., 2000). The risk of a relapse of the malignancy in the studies in the Eurocord registry was 26 percent with a
median follow-up time of 29 to 34 months (Nash et al., 1992; Gluckman et al., 1997; Locatelli et al., 1999) and was significantly higher in those who received the transplant during an advanced stage of disease and those with low body weights (<20 kg) (Locatelli et al., 1999). The probability of event-free survival in the studies reported in IBMTR was 41 percent at 2 years. Notably, no increase in the rate of disease-related mortality in cord blood recipients compared with that in those receiving bone marrow was observed (48 and 49 percent, respectively, after a median follow-up time of 27 months) in IBMTR and the Eurocord registry.
IBMTR and the Eurocord registry have reported overall survival rates after the transplantation of cord blood from a related donor of 0.61 (95 percent confidence intervals [CI] 0.49 to 0.83) at 2 years (Barker and Wagner, 2003b) and 0.63 (95 percent CI, 0.57 to 0.69) at 1 year, respectively (Gluckman et al., 1997). In the HLA-identical sibling donor setting, there was no significant difference in the 3-year survival rates between cord blood transplants (survival rate, 0.64; 95 percent CI, 0.53 to 0.74) and bone marrow transplants (survival rate, 0.66; 95 percent CI, 0.64 to 0.68).
These studies demonstrate that the transplantation of cord blood from a related donor results in reliable engraftment, a reduction in the incidence of acute and chronic GVHD, and an overall survival rate equivalent to that achieved with bone marrow transplants.
Graft Versus Host Disease
GVHD is one of the primary complications of transplantation of HPCs from any source. Donor T cells in the transplanted graft attack the tissues of the recipient, a process that occurs even after the transplantation of HPCs from an HLA-identical sibling. The T cells can be depleted from the graft to reduce the possibility of GVHD; however, T cells also have some beneficial effect. Donor T cells can destroy residual recipient lymphocytes, thereby reducing the likelihood that the recipient cells will reject the graft. Evidence also indicates that T cells from the donor actually attack residual cancer cells, thereby increasing the likelihood of a cure.
GVHD is classified into two forms: acute, which generally occurs within the first 100 days post-transplantatation, and chronic, which generally occurs later. Different immune cells and cytokines appeared to be involved in each form of the disease, and different subsets of organs are most affected in each form. Acute GVHD is generally graded from I (mild) to IV (severe); chronic GVHD is generally graded as limited or extensive. There is, however, extensive variability on the part of transplant centers in the diagnosis of acute GVHD. For this reason, any discussion of the relative instances of GVHD should be viewed with careful scrutiny.
The transplantation of cord blood from a related donor is associated
with incidences of grade II to IV (3 to 18 percent) and grade III and IV (0 to 5 percent) acute GVHD and chronic GVHD (4 to 14 percent) lower than incidents that would be expected with bone marrow or peripheral blood transplants (Gluckman et al., 1997; Rocha et al., 2000; Locatelli et al., 2003).
The incidence of grade II to IV acute GVHD (33 to 44 percent), grades III and IV acute GVHD (11 to 22 percent), and chronic GVHD (0 to 25 percent), as reported in studies with large numbers of tranplantations of cord blood from an unrelated donor, varies widely (Gluckman et al., 1997; Rubinstein et al., 1998; Wagner et al., 2002; Grewal et al., 2003). However, all of these incidences are lower than those that would be expected with bone marrow transplants, because most donor-patient pairs were HLA mismatched at one or two antigens. However, most recipients of cord blood have been young (children generally have lower rates of GVHD) and far fewer data are available for adult patients. Rubinstein et al. (1998) reported a low rate of acute GVHD in recipients of HLA-matched cord blood grafts and no increase with increasing HLA disparity (one, two, or three antigen mismatches).
Current data indicate that an HLA mismatch may be better tolerated with cord blood grafts. An IBMTR and Eurocord study that compared the outcomes from children who received a cord blood transplant from an HLA-identical sibling with those from children who received bone marrow from an HLA-identical sibling observed significantly lower incidences of acute and chronic GVHD in the cord blood transplant group (Rocha et al., 2000). That study perhaps provides the clearest indication of a difference in biological properties between the two progenitor cell sources, as the interpretation of GVHD after transplantation of unrelated cord blood and after transplantation of bone marrow is often complicated by different levels of HLA histocompatibility and other patient heterogeneities.
Two other studies have compared the frequencies of GVHD in the receipients of bone marrow and cord blood from unrelated donors (Barker et al., 2001; Rocha et al., 2001). The findings of a matched-pair analysis from a single institution found that the risk of acute GVHD and that of chronic GVHD were similar when the outcomes for recipients of unmanipulated bone marrow matched for HLA-A, HLA-B, and HLA-DRB1 were compared with those for the recipients of cord blood from an unrelated donor mostly mismatched by one or two antigens (Barker et al., 2003). In another study, transplants involving cord blood at zero to three antigen mismatches were associated with a significantly lower risk of acute and chronic GVHD compared with the risk of GVHD from transplants involving unmanipulated, mostly HLA-matched bone marrow transplants (Barker et al., 2001). Study data indicate that despite the greater degrees of HLA disparity that are accepted in cord blood transplantation, the risk of devel-
oping acute and chronic GVHD after the transplantation of cord blood from an unrelated donor with one to two HLA mismatches is similar to or even less than that reported after the transplantation of HLA-matched bone marrow (Barker et al., 2001; Rocha et al., 2001).
The reason for this lower risk of GVHD after cord blood transplantation is not clear. Researchers speculate however that the functional and phenotypic immaturity of cord blood lymphocytes or the reduced T-cell dose infused with cord blood grafts may contribute to the reduced alloreactivity of cord blood (Barker and Wagner, 2003a).
Relapse and Graft-Versus-Leukemia Effect
Current experience comparing the risk of relapse after cord blood transplantation with that after bone marrow transplantation is limited. In an analysis of transplants between HLA-matched siblings, the 3-year survival rates among patients with a diagnosis of a malignancy were comparable after cord blood and bone marrow transplantation (p = 0.69) (Laughlin et al., 2004). In another study of HPC transplantation as treatment for acute leukemia in children, Rocha et al. (2001) compared the outcomes among children receiving cord blood and those receiving unmanipulated bone marrow or bone marrow depleted of T cells. The proportion of individuals with advanced-stage leukemia was larger among the individuals receiving unmanipulated bone marrow and cord blood than among the individuals receiving T-cell-depleted bone marrow (9 percent). Interestingly, although the recipients of both T-cell-depleted bone marrow and cord blood had lower incidences of acute and chronic GVHD than the recipients of unmanipulated bone marrow, only the group that received T-cell-depleted bone marrow and not the group receiving cord blood had an increased risk of relapse (p = 0.02).
Overall, no evidence available thus far suggests that the risk of a leukemia relapse is higher after cord blood transplantation.
A study in mice and humans showed that the infusion of donor-derived alloreactive natural killer (NK)6 cells not only provides a graft-versus-leukemia (GVL)7 effect, but may also protect against GVHD by targeting patient antigen presenting cells (Harris, 1995). Cord blood contains levels of NK cells and inducible NK-like cytotoxic activity similar to those in adult peripheral blood (Harris et al., 1994), which might explain the preserved GVL effect.
NHLBI Cord Blood Transplantation Study
NHLBI began the Cord Blood Banking and Transplantation Study (COBLT), a prospective, multicenter study, in 1996 to examine the nascent field of cord blood transplantation. The intent of the study was to collect data from studies on the banking and transplantation of cord blood to create a coherent set of guidelines and standards for the collection, preservation, and transplantation of cord blood (COBLT, 2000b). The primary end point of the COBLT study was the 180-day survival rate among patients who had received a cord blood transplant for the treatment of hematopoietic or immune system disorders. Patients were considered eligible to receive a cord blood unit if they were either unable to find an appropriate bone marrow match or unable to wait for the transplant because of the severity of their illness. An appropriately HLA-matched cord blood unit (a high-resolution match at HLA-DRB1 and a low-resolution match at HLA-A and HLA-B) for the patients also had to be identified, and the patients were required to provide informed consent for the transplantation of cord blood (COBLT, 2000a).
Although the original goal of the study was to bank more than 15,000 cord blood units from a racially and ethnically diverse population, University of California, Los Angeles (UCLA) and the Carolinas Cord Blood Bank of Duke University banked only 8,000 units from 1998 to 2000. Between 1999 and 2003, 326 patients were enrolled to receive a cord blood transplant at 1 of the 28 transplant centers around the country (Carter, 2004). After the patients were enrolled in the study, their HLA types were determined by high-resolution DNA typing methods and were categorized into the 10 strata shown in Box 2-2.
In addition to the 180-day survival end point, the study also collected data on long-term patient survival; incidences of neutrophil engraftment, primary and secondary graft failure, platelet engraftment, red blood cell engraftment, complications, relapses, and appearance of other malignancies; and immune reconstitution. A medical coordination center managed the coordination and statistical analysis of the data from the banks and transplant centers. The center created and maintained a World Wide Web-based data management system to track the multiple variables and provided periodic statistical analysis of the results.
Eligibility
Mothers who gave birth at hospitals affiliated with the UCLA or Duke University banks between 1998 and 2000 were considered possible cord blood donors for the study. Donors were recruited through brochures placed in obstetrical office waiting rooms and presentations to community groups
|
BOX 2-2
SOURCE: COBLT (2003). |
in the areas of the collecting hospitals. Informed consent was obtained before admission to the hospital for labor. However, in the event that it was not possible for the mother to provide informed consent, verbal consent or preliminary informed consent was obtained immediately before or during labor and was reaffirmed after delivery. Of the 35,799 available donors, 20,710 consented to the collection of their cord blood. After the exclusion of units with low cell counts, microbial contamination, and positive serological test results for an infectious disease, and of the units that could not be collected after consent was obtained because of a lack of collection staff, the study was able to cryopreserve 8,731 cord blood units (Cairo et al., 2004).
In 2000, NHLBI convened an ad hoc committe when it realized that the study was not going to meet its goals for collection and transplantation on schedule. The study staff was having difficulty encouraging potential transplant recipients to use what was essentially an experimental product (i.e., cord blood transplantation), and it was competing with other banks outside the study that often had more suitably matched units for the transplant patients. Participation in the COBLT study was increased to include an additional 21 U.S. transplant centers, as well as access to cord blood units banked at the New York Blood Center, NMDP-approved banks, or U.S.
banks meeting FACT/Netcord standards. All transplant centers followed COBLT protocols and reported transplant outcomes according to COBLT protocols. The increase in the number of transplant centers and access to additional units facilitated the completion of the COBLT study. In addition, cord blood units were made available to patients who did not meet specific transplant criteria for the COBLT study strata through a separate protocol called the Expanded Access Protocol. Transplant centers using COBLT units in the Expanded Access Protocol were required to report the transplant outcome data to the COBLT medical coordinating center. The study allowed the use of these external transplant data, as long a COBLT laboratory performed the HLA typing and the transplant center complete the forms required for participation in the COBLT protocol. These requirements were developed to ensure that all outcomes data for the patients with cord blood transplants analyzed in the COBLT study were consistent with those from the centers already participating in the study.
The study made a concerted effort to increase the ethnic and racial diversity of their cord blood inventory, which by extension would increase the diversity of the HLA types with different ethnic and racial populations. The collection centers were specifically tasked to retrieve specified proportions of cord blood specimens from targeted minority populations to ensure that potential minority recipients had a similar chance of locating a unit to any other group. The target distribution of the sources of the cord blood specimens in the bank was 43 percent Caucasian, 30 percent African American, 17 percent Hispanic, and 10 percent Asian American (COBLT, 2000b). As of 2003, the distribution of the blood specimens in the COBLT cord blood banks by ethnicity and race were 42 percent Caucasian, 15 percent African American, 22 percent Hispanic, 9 percent Asian, 11 percent mixed, and 1 percent other (Baxter-Lowe et al., 2003). The study team came to the conclusion that it was possible to provide at least one unit matched at four of six HLAs (minimum cell dose, 1 × 107 total nucleated cells) to 94 percent of patients who were searching for a cord blood unit for transplantation (Baxter-Lowe et al., 2003).
Although the study results are still being evaluated, a definite relationship between the volume of cord blood collected and the nucleated cell counts and CD34+ levels was observed. The study has reported that cord blood samples from African-American women generally have lower nucleated cell and CD34+-cell counts per milliliter of cord blood (Kurtzberg et al., 2004) than samples from Caucasian women. The study also reported that birth weight, gender, gestational age, and type of delivery (vaginal versus cesarean) affect the size and the quality of the cord blood units. It has found that these factors are significant in the selection of units and are important in the development of plans to recruit donors (Cairo et al., 2004).
SUMMARY
This chapter summarizes what is known to date about the relative effectiveness of cord blood transplantation, either allogeneic or autologous in terms of engraftment and GVHD compared to HPC transplantation from other sources (bone marrow or peripheral blood). It also describes the federally funded COBLT, which aims to collect data from the banking and transplantation of cord blood to create a coherent set of guidelines and standards for the collection, preservation, and transplantation of cord blood.
REFERENCES
AAP (American Academy of Pediatrics). 1999. Cord blood banking for potential future transplantation: Subject review. Work Group on Cord Blood Banking. Pediatrics 104 (1 Pt 1):116–118.
Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proceedings of the National Academy of Sciences (U. S. A.) 89(21):10114–10118.
Amos TA, Gordon MY. 1995. Sources of human hematopoietic stem cells for transplantation—A review. Cell Transplantation 4(6):547–569.
Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC, Henslee PJ, Kolb HJ, Lowenberg B, Masaoka T. 1991. Bone marrow transplantation from related donors other than HLA-identical siblings: Effect of T cell depletion. Bone Marrow Transplantation 7(6):443–452.
Atkinson K. 1990. Reconstruction of the haemopoietic and immune systems after marrow transplantation. Bone Marrow Transplantation 5(4):209–226.
Barker JN, Wagner JE. 2003a. Umbilical-cord blood transplantation for the treatment of cancer. Nature Review Cancer 3(7):526–532.
Barker JN, Wagner JE. 2003b. Umbilical cord blood transplantation: Current practice and future innovations. Critical Reviews in Oncology-Hematology 48(1):35–43.
Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. 2001. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: Results of a matched-pair analysis. Blood 97(10):2957–2961.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. 2003. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 102(5):1915–1919.
Baxter-Lowe LA, Kim Y, Carter S, Fernandez-Vina M, Wagner E, Jensen L, Fraser J, Kernan N, Kurtzberg J. 2003. Ability of minority patients to find donors from an ethnically diverse cord blood bank. Presentation at the 29th Annual Meeting of the American Society for Histocompatibility and Immunogenetics, October 28-November 1, 2003, Miami, FL.
Benacerraf B. 1992. The role of MHC gene products in immune regulation and its relevance to alloreactivity. In: Lindsten J, ed. Nobel Lectures, Physiology or Medicine, 1971–1980. Singapore: World Scientific Publishing Co. [Online] Available: http://nobelprize.org/medicine/laureates/1980/benacerraf-lecture.html [accessed January 2005].
Bjorkman PJ, Parham P. 1990. Structure, function, and diversity of class I major histocompatibility complex molecules. Annual Review Biochemistry 59:253–288.
BMDW (Bone Marrow Donors Worldwide). 2004. Bone Marrow Donors Worldwide Annual Report 2003. Leiden, The Netherlands: Europdonor Foundation.
Bodger MP. 1987. Isolation of hemopoietic progenitor cells from human umbilical cord blood. Experimental Hematology 15(8):869–876.
Broxmeyer HE. 1998. The past, present, and future of cord blood transplantation. In: Broxmeyer HE, ed. Cellular Charactristics of Cord Blood and Cord Blood Transplantation. Bethesda, MD: AABB Press. Pp. 1–9.
Caro MS, Cohen G, Wagner EL, Fraser J, Jensen L, Carter S, Kernan N, Kurtzberg, J. 2004. Cord blood (CB) hematopoietic progenitor cell (HPC) characterization and correlation with ethnicity: A report from the COBLT study. Presentation at the 33rd Annual Scientific Meeting of the International Society for Experimental Hematology, July 17-20, 2004, New Orleans, LA.
Carter S. 2004. Cord Blood Transplantation Study (COBLT). Presentation to the Institute of Medicine Committee on Establishing a National Cord Blood Stem Cell Bank Program, September 29, 2004, Woods Hole, MA.
Catlin AJ, Gonzalez-Ryan L, Van Syckle K, Coyne KD, Glover N. 2000. Umbilical cord blood banking: Procedural and ethical concerns for this new birth option. Pediatric Nursing 26(1):105–111.
COBLT (Cord Blood Transplantation Study). 2000a. Cord Blood Transplantation Study Expanded Access Protocol. The National Heart, Lung and Blood Institute, National Institutes of Health. Potomac, MD: The EMMES Corporation.
COBLT. 2000b. Cord Blood Transplantation Study Protocol. The National Heart, Lung and Blood Institute, National Institutes of Health. Potomac, MD: The EMMES Corporation.
COBLT. 2003. Cord Blood Transplantation Study Protocol, The National Heart, Lung and Blood Institute, National Institutes of Health. Potomac, MD: The EMMES Corporation.
Cornetta K, Laughlin M, Carter S, Wall D, Weinthal J, Delaney C, Wagner J, Sweetman R, McCarthy P, Chao N. 2005. Umbilical cord blood transplantation in adults: Results of the prospective cord blood transplantation (COBLT). Biology of Blood and Marrow Transplantation 11(2):149–160.
Dausset J. 1981. The major histocompatibility complex in man. Science 213(4515):1469–1474.
Fernandez MN. 1998. Eurocord position on ethical and legal issues involved in cord blood transplantation. Bone Marrow Transplantation 22(Suppl. 1):S84–S85.
Fruchtman SM, Hurlet A, Dracker R, Isola L, Goldman B, Schneider BL, Emre S. 2004. The successful treatment of severe aplastic anemia with autologous cord blood transplantation. Biology of Blood and Marrow Transplantation 10(11):741–742.
Gluckman E, Locatelli F. 2000. Umbilical cord blood transplants. Current Opinion in Hematology 7(6):353–357.
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C. 1997. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. New England Journal of Medicine 337(6):373–381.
Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, Takahashi TA, Ortega J, Filipovich A, Locatelli F, Asano S, Fagioli F, Vowels M, Sirvent A, Laporte JP, Tiedemann K, Amadori S, Abecassis M, Bordigoni P, Diez B, Shaw PJ, Vora A, Caniglia M, Garnier F, Ionescu I, Garcia J, Koegler G, Rebulla P, Chevret S, Eurocord Group. 2004. Factors associated with outcomes of unrelated cord blood transplant: Guidelines for donor choice. Experimental Hematology 32(4):397–407.
Grewal SS, Barker JN, Davies SM, Wagner JE. 2003. Unrelated donor hematopoietic cell transplantation: Marrow or umbilical cord blood? Blood 101(11):4233–4244.
Guyton A, Hall J, eds. 2000. Red blood cells, anemia, and polycythemia. In: Textbook of Medical Physiology. 10th ed. Philadelphia, PA: W. B. Saunders and Co. Pp. 382–391.
Hansen JA, Anasetti C, Beatty PG, Martin PJ, Sanders JE, Storb R, Thomas ED. 1990. Treatment of leukemia by marrow transplantation from HLA incompatible donors. Effect of HLA-disparity on GVHD, relapse and survival. Bone Marrow Transplantation 6(Suppl. 1):108–111.
Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, Petersdorf EW, Radich J, Sanders JE, Storb RF, Sullivan KM, Anasetti C. 1998. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. New England Journal of Medicine 338(14):962–968.
Harris DT. 1995. In vitro and in vivo assessment of the graft-versus-leukemia activity of cord blood. Bone Marrow Transplantation 15(1):17–23.
Harris DT, LoCascio J, Besencon FJ. 1994. Analysis of the alloreactive capacity of human umbilical cord blood: Implications for graft-versus-host disease. Bone Marrow Transplantation 14(4):545–553.
HRSA (Health Resources and Services Administration). 2000. Report to Congress on the Status of Umbilical Cord Blood Transplantation.
Hurley CK. 2005. HLA Overview: An analysis prepared for the Committee on Establishing a National Cord Blood Stem Cell Bank, Institute of Medicine, Washington, DC.
Hurley CK, Hegland J, Fernandez-Vina M, Maiers M, Lazaro A, Hartzman RJ, Cao K, Ng J, Janzen M, Setterholm M. 2003. Designing a typing system for a hematopoietic stem cell registry. In: Hansen JA, Dupont B, eds. HLA 2002: Immunobiology of the Human MHC. Seattle, WA: IHWG Press.
Janeway C, Travers P, Walport M, Shlomchik M, 2005. Immunobiology. 6th ed. New York: Garland Publishing.
Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, Gajewski J, Hansen JA, Henslee-Downy J, McCullough J, McGlave P, Perkins H, Phillips G, Sanders J, Strocek D, Thomas ED, Blume KG. 1993. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. New England Journal of Medicine 328(9):593–602.
Knudtzon S. 1974. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood 43(3):357–361.
Kurtzberg J, Wagner EL, Fraser JK, Cairo M, Jensen L, Cohen G, Carter S, Kernan N. 2003. Results of the Cord Blood Transplantation Study (COBLT) unrelated donor banking program from donor screening to characterization of banked units. Presentation at the 2004 Tandem BMT Meeting, February 13-17, 2004, Orlando, FL.
Lansdorp PM. 1995a. Telomere length and proliferation potential of hematopoietic stem cells. Journal Cell Science 108(Pt 1):1–6.
Lansdorp PM. 1995b. Developmental changes in the function of hematopoietic stem cells. Experimental Hematology 23(3):187–191.
Laughlin MJ, Rizzieri DA, Smith CA, Moore JO, Lilly S, McGaughey D, Martin P, Carrier C, Stevens CE, Rubinstein P, Buckley R, Kurtzberg J. 1998. Hematologic engraftment and reconstitution of immune function post unrelated placental cord blood transplant in an adult with acute lymphocytic leukemia. Leukemia Research 22(3):215–219.
Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, Gerson SL, Lazarus HM, Cairo M, Stevens CE, Rubinstein P, Kurtzberg J. 2001. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. New England Journal of Medicine 344(24):1815–1822.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM. 2004. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. New England Journal of Medicine 351(22):2265–2275.
Leung W, Ramirez M, Mukherjee G, Perlman EJ, Civin CI. 1999. Comparisons of alloreactive potential of clinical hematopoietic grafts. Transplantation 68(5):628–635.
Locatelli F, Rocha V, Chastang C, Arcese W, Michel G, Abecasis M, Messina C, Ortega J, Badell-Serra I, Plouvier E, Souillet G, Jouet JP, Pasquini R, Ferreira E, Garnier F, Gluckman E. 1999. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Eurocord-Cord Blood Transplant Group. Blood 93(11): 3662–3671.
Locatelli F, Rocha V, Reed W, Bernaudin F, Ertem M, Grafakos S, Brichard B, Li X, Nagler A, Giorgiani G, Haut PR, Brochstein JA, Nugent DJ, Blatt J, Woodard P, Kurtzberg J, Rubin CM, Miniero R, Lutz P, Raja T, Roberts I, Will AM, Yaniv I, Vermylen C, Tannoia N, Garnier F, Ionescu I, Walters MC, Lubin BH, Gluckman E. 2003. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood 101(6):2137–2143.
Margolis DA, Casper JT. 2000. Alternative-donor hematopoietic stem-cell transplantation for severe aplastic anemia. Seminars in Hematology 37(1):43–55.
Mayani H, Lansdorp PM. 1998. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells 16(3):153–165.
McGlave P, Bartsch G, Anasetti C, Ash R, Beatty P, Gajewski J, Kernan NA. 1993. Unrelated donor marrow transplantation therapy for chronic myelogenous leukemia: Initial experience of the National Marrow Donor Program. Blood 81(2):543–550.
Nakahata T, Ogawa M. 1982. Hemopoietic colony-forming cells in umbilical cord blood with extensive capability to generate mono- and multipotential hemopoietic progenitors. Journal of Clinical Investigation 70(6):1324–1328.
Nash RA, Pepe MS, Storb R, Longton G, Pettinger M, Anasetti C, Appelbaum FR, Bowden RA, Deeg HJ, Doney K, Martin PJ, Sullivan KM, Sanders J, Witherspoon RP. 1992. Acute graft-versus-host disease: Analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood 80(7):1838–1845.
Risdon G, Gaddy J, Horie M, Broxmeyer HE. 1995. Alloantigen priming induces a state of unresponsiveness in human umbilical cord blood T cells. Proceedings of the National Academy of Sciences (U. S. A.) 92(6):2413–2417.
Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. 2000. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. New England Journal of Medicine 342(25):1846–1854.
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, Tiedemann K, Busca A, Chan KW, Kato S, Ortega J, Vowels M, Zander A, Souillet G, Oakill A, Woolfrey A, Pay AL, Green A, Garnier F, Ionescu I, Wernet P, Sirchia G, Rubinstein P, Chevret S, Gluckman E. 2001. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 97(10):2962–2971.
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, Frassoni F, Gluckman E. 2004. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. New England Journal of Medicine 351(22):2276–2285.
Roncarolo MG, Bigler M, Martino S, Ciuti E, Tovo PA, Wagner J. 1996. Immune functions of cord blood cells before and after transplantation. Journal of Hematotherapy 5(2): 157–160.
Rubinstein P, Stevens CE. 2000. Placental blood for bone marrow replacement: The New York Blood Center’s program and clinical results. Bailliere’s Best Practice in Clinical Haematology 13(4):565–584.
Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE. 1998. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. New England Journal of Medicine 339(22):1565–1567.
Rubinstein P, Kurtzberg J, Loberiza FR, et al. 2001. Comparison of unrelated cord blood and unrelated bone marrow transplants in leukemia in children: A collaborative study of the New York Blood Center and the International Bone Marrow Transplant Registry. Blood 98:814a.
Sanz GF, Saavedra S, Planelles D, Senent L, Cervera J, Barragan E, Jimenez C, Larrea L, Martin G, Martinez J, Jarque I, Moscardo F, Plume G, Andreu R, Regadera AI, Garcia I, Molla S, Solves P, De la Rubia J, Bolufer P, Benlloch L, Soler MA, Marty ML, Sanz MA. 2001. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood 98(8):2332–2338.
Schiller G, Feig SA, Territo M, Wolin M, Lill M, Belin T, Hunt L, Nimer S, Champlin R, Gajewski J. 1994. Treatment of advanced acute leukaemia with allogeneic bone marrow transplantation from unrelated donors. British Journal of Haematology 88(1):72–78.
Sirchia G, Rebulla P. 1999. Placental/umbilical cord blood transplantation. Haematologica 84(8):738–747.
Smith S, Broxmeyer HE. 1986. The influence of oxygen tension on the long-term growth in vitro of haematopoietic progenitor cells from human cord blood. British Journal Haematology 63(1):29–34.
Snell GD. 1992. The Nobel Lectures in Immunology. Lecture for the Nobel Prize for Physiology or Medicine, 1980: Studies in histocompatibility. Scandinavian Journal Immunology 36(4):513–526.
Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, Bradley BA, Casper JT, Flomenberg N, Gajewski JL, Gluckman E, Henslee-Downey PJ, Hows JM, Jacobsen N, Kolb HJ, Lowenberg B, Masaoka T, Rowlings PA, Sondel PM, van Bekkum DW, van Rood JJ, Vowels MR, Zhang MJ, Horowitz MM. 1997. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. Journal of Clinical Oncology 15(5):1767–1777.
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. 1994. Evidence for a mitotic clock in human hematopoietic stem cells: Loss of telomeric DNA with age. Proceedings of the National Academy of Sciences (U. S. A.) 91(21):9857–9860.
Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, Sender L, Cairo MS. 1996. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: Analysis of engraftment and acute graft-versus-host disease. Blood 88(3):795–802.
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NKC, Davies SM. 2002. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: Influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100(5):1611–1618.























