2
Scientific Background of Human Embryonic Stem Cell Research
INTRODUCTION
Human embryonic stem cells (hES cells) are primitive (undifferentiated) cells that can self-renew or differentiate into most or all cell types found in the adult human body (Edwards, 2004; Gardner, 2004). Differentiation is the process whereby an unspecialized cell acquires specialized features, such as those of a heart, liver, or muscle cell.
Fertilization of an oocyte by a sperm results in a one-cell zygote, which begins to divide without any increase in size (Figure 2.1). By 3-4 days after fertilization, cell division results in a compact ball of 16-32 cells known as a morula. By 5-6 days, a blastocyst is formed consisting of a sphere of about 200-250 cells. The sphere is made up of an outer layer of cells (the trophectoderm), a fluid-filled cavity (the blastocoel), and a cluster of cells in the interior (the inner cell mass). Up to this point, there has been no net growth (Figure 2.1). The cells of the inner cell mass will give rise to the embryonic disk and ultimately the fetus, but not the placenta, which arises from the trophectoderm. Neither the trophectoderm nor the inner cell mass alone can give rise to a developing fetus. After the blastocyst implants into the uterus (day 6), the cells of the inner cell mass differentiate to form the embryonic tissue layers of the developing fetus. Embryonic stem cells are usually derived from the primitive (undifferentiated) cells of the inner cell mass, which have the potential to become a wide variety of specialized cell types. Because embryonic stem cells can become all cell types of the body, they are considered to be pluripotent. Study of embryonic stem cells provides information about how an organism develops from a single cell and how healthy cells can potentially replace damaged cells in adult
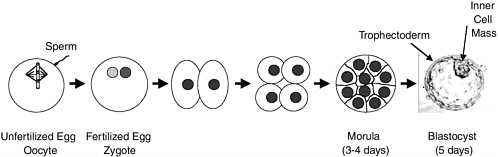
FIGURE 2.1 Preimplantation development. The oocyte (unfertilized egg) combines with sperm to form a zygote (fertilized egg). Each gamete (oocyte or sperm) is haploid (has a single set of chromosomes); the zygote and all later cells are diploid (have two sets of chromosomes). The zygote then divides approximately once a day. Since there is no growth during this period of cell division (cleavage), the cells become progressively smaller. By 3-4 days, a ball of cells (morula) has formed. By 5 days, it has become hollowed out to form a blastocyst, which consists of a sphere 0.1-0.2 mm in diameter comprising two cell types—an outer shell of trophectoderm cells and an inner collection of 30-34 cells called the inner cell mass. By day 6, the blastocyst would normally implant into the uterine wall, the trophectoderm would begin to form the placenta, and the inner cell mass would begin to form the cells and tissues of the fetus. At the blastocyst stage, cells of the inner cell mass are undifferentiated and pluripotent; that is, they have the potential to differentiate into all cells of the fetus except the placenta. If separated from the blastocyst and cultured, the cells of the inner cell mass can be converted into embryonic stem cells that are also pluripotent and can be propagated extensively while maintaining that potential. Blastocyst picture from http://stemcells.nih.gov/info/scireport/chapter3.asp.
organisms. The latter subject raises possibilities of cell-based therapies to treat disease, often referred to as regenerative medicine.
Scientists discovered how to obtain or derive embryonic stem cells from mouse blastocysts in the early 1980s (Evans and Kaufman, 1981; Martin, 1981) by culturing inner cell masses on feeder layers of mouse fibroblasts. It was later discovered that feeder cells could be replaced with culture medium containing the growth factor leukemia inhibitory factor (LIF)(Smith et al., 1988; Williams et al., 1988). Mouse ES cells (mES cells) have been studied in the laboratory, and a great deal has been learned about their essential properties and what makes them different from specialized cell types.
mES cells are shown to be pluripotent using three kinds of tests. The first and most rigorous test is to inject mES cells into the blastocoel cavity of a blastocyst (Stewart, 1993). The blastocyst is then transferred to the uterus of a pseudopregnant female (a female primed to accept implanted blastocysts). If the mES cells are pluripotent, the resulting progeny will be a chimera because it consists of a mixture
of tissues and organs derived from both the donor mES cells and the recipient blastocyst. In some cases, a fetus can be derived entirely from mES cells by providing trophectoderm cells from another source (Nagy et al., 1990, 1993). However, mES cells cannot themselves form a functional placenta and therefore are not equivalent to an intact blastocyst. The ability of mES cells to generate a complete embryo tends to decline with the number of times the cells have divided (or been “passaged”) in culture.
A second approach for testing pluripotency of mES cells is to inject them into the testis or under the skin or kidney capsule of an immunodeficient mouse. If pluripotent, the injected cells form benign tumors known as teratomas. The teratomas contain differentiated tissues from all three germ layers (ectoderm, mesoderm, and endoderm). Such structures as gut, muscle (smooth, skeletal, and cardiac), neural tissue, cartilage, bone, and hair are found, but they are arranged in a disorganized manner (Martin, 1981).
A third approach for testing pluripotency of mES cells is by in vitro differentiation (Wiles, 1993). Spontaneous differentiation can occur if the mES cells are grown in suspension without feeders or LIF. The cells will form fluid-filled clumps called embryoid bodies, which will differentiate along the ectoderm, mesoderm, and endoderm pathways. If the embryoid bodies are allowed to attach to the tissue culture dish, they will differentiate into multiple tissue types much like teratomas.
Developmentally relevant signaling factors can also be used to induce mES cells to differentiate into specific cell types in vitro, including hematopoietic stem cells, beating cardiac muscle cells, neuronal progenitors, endothelial cells, and bone cells. In some cases, those differentiated cell types can be transplanted into animals to form functional tissues (Lanza et al., 2004). Such work engenders excitement about regenerative medicine using hES cells. One of the milestones of mES cell research was the development of methods to modify the cells genetically (Doetschman et al., 1987; Thomas and Capecchi, 1987). The evolution of those methods has revolutionized animal models for biomedical research by allowing one to modify endogenous genes or to tag the cells so that they can be easily visualized in the animal.
Bongso et al. (1994) first described isolation and culture of cells of the inner cell mass of human blastocysts in 1994, and techniques for deriving and culturing stable hES cell lines were first reported in 1998 (Thomson et al., 1998). The trophectoderm was removed from day-5 blastocysts, and the inner cell mass, consisting of only 30-34 cells, was placed into tissue culture. Cell lines similar to mES cells were derived after fairly extensive culture and passaging of the cells. Cells with similar properties were reported at about the same time from culturing cells isolated from fetal genital ridges—so-called human embryonic germ (hEG) cells (Shamblott et al., 1998). It had previously been shown that the germ cells in fetal mouse gonads can give rise to permanent pluripotent stem cell lines in culture, mEG cells (Matsui et al., 1992; Resnick et al., 1992). Under appropriate culture conditions, hES cells were shown to be pluripotent by differentiating into multiple tissue types (Itskovitz-Eldor et al., 2000; Reubinoff et al., 2000). Since 1998, research teams have refined the
techniques for growing hES cells in vitro (Amit et al., 2000; Itskovitz-Eldor et al., 2000; Klimanskaya and McMahon, 2004; Reubinoff et al., 2000). Collectively, the studies indicate that it is now possible to grow karyotypically normal hES cells (that is, with correct chromosome number) for more than a year in serum-free medium on mouse fibroblast feeder layers. Both XX (female) and XY (male) hES cell lines have been established. The cells express markers characteristic of pluripotent and proliferating cells. Work with hEG cells has also shown pluripotency and extended self-renewal, but more extensive work has been done with hES than with hEG cells.
There are differences between mouse and human ES cells (Pera and Trounson, 2004). For example, mES cells grow as rounded colonies with indistinct cell borders, while hES cell colonies are flatter and display more distinct cell borders. The two cell types also demonstrate differences in growth regulation. In general, both mES and hES cells require fibroblast feeder cell support. Current attempts to substitute for that support have required different approaches for the two species. The soluble growth factor, LIF, can substitute for a feeder cell layer in maintaining mES cells, but hES cells require a solid extracellular matrix (Matrigel) in place of the fibroblasts (Xu et al., 2005). Those examples of interspecies differences indicate that if one is to identify signals that cause stem cells to differentiate into specialized cells, work needs to continue with both hES and mES cells.
Embryonic stem cells have three important characteristics that distinguish them from other types of cells. First, hES cells express factors—such as Oct4, Sox2, Tert, Utf1 and Rex—that are associated with pluripotent cells (Carpenter and Bhatia, 2004). Second, they are unspecialized cells that renew themselves through many cell divisions. A starting population of stem cells that proliferates for many months in the laboratory can yield millions of cells. An important research challenge is to understand the signals that cause a stem cell population to remain unspecialized and to continue to proliferate until they are needed for repair of a specific tissue.
A third characteristic of hES cells is that under some physiological or experimental conditions in tissue culture they can be induced to become cells with special functions, such as cardiomyocytes (the beating cells of the heart), liver cells, nerve cell precursors, endothelial cells, hematopoietic cells, and insulin-secreting cells (Assady et al., 2001; Chadwick et al., 2003; Kaufman et al., 2001; Kehat et al., 2001; Levenberg et al., 2002; Mummery et al., 2002; Reubinoff et al., 2001; Reubinoff et al., 2000; Xu et al., 2002; Zhang et al., 2001). However, because hES cells have not yet been used in blastocyst chimera studies, researchers have been able to assess in vivo differentiation only after injection of hES cells into immunodeficient mice. There, the cells create teratomas in which tissues of the three embryonic germ layers are found (Thomson et al., 1998). Examples are bone and cartilage tissue, striated muscle, gut-like structures, neural rosettes, and glomerulus-like structures. More organized structures—such as hair follicles, salivary glands, and tooth buds—also form. hES cells will also create embryoid bodies and differentiate in vitro (Itskovitz-Eldor et al., 2000). However, those types of differentiation assays do not provide conclusive evidence that the resulting cell types are functioning
normally, nor whether hES cells have the capacity to participate in normal development in the context of the three-dimensional embryo in the reproductive tract. Such conclusive evidence requires testing in blastocyst chimeras as is routinely done with mES cells.
Understanding why ES cells are able to proliferate essentially indefinitely and retain the ability to be induced to differentiate and stop proliferating will provide important information about the regulation of normal embryonic development and the uncontrolled cell division that can lead to cancer. It is known that external signals for cell differentiation include chemicals secreted by other cells, physical contact with neighboring cells, and molecules in the microenvironment. Identifying such factors would allow scientists to find methods for controlling stem cell differentiation in the laboratory and thereby allow growth of cells or tissues that can be used for specific purposes, such as cell-based therapies.
Several methods have been shown to be effective for delivering exogenous genes into hES cells, including transfection by chemical reagents, electroporation, and viral infection (Eiges et al., 2001; Gropp et al., 2003; Ma et al., 2003; Pfeifer et al., 2002; Zwaka and Thomson, 2003). Those are all critical methodological objectives that must be met if hES cells are to be used as the basis of therapeutic transplantation.
NUCLEAR TRANSFER TO GENERATE STEM CELLS
Most work on hES cells has taken place with a relatively small number of cell lines obtained from excess blastocysts donated from in vitro fertilization (IVF) programs. The genetic makeup of the cells is not controlled in any way, and genetic variation among lines needs to be considered when results from different lines are compared. Experience from research with mES cells shows that ES cell lines can differ markedly in their differentiation efficiencies. Being able to control the genotype of ES cells would be valuable for various reasons, most notably the desire to generate ES cells with genotypes known to predispose to particular diseases. In the case of single-gene defects, one could achieve that goal by deriving hES cells from discarded morulae or blastocysts that were identified with preimplantation genetic diagnosis (PGD) procedures (Verlinsky et al, 2005) as carrying mutations or by generating the appropriate mutation by gene targeting of established hES cell lines. However, such approaches cannot be used if the genetic predisposition has an unknown basis or arises from multiple gene effects. Availability of hES cell lines from patients with Alzheimer’s disease, type I diabetes, or many other complex diseases would provide a source of cells that could be differentiated into appropriate cell types; and the progression of the disease could then be modeled and potentially modified in culture. Given the complex interplay between genotype and environment that typifies complex chronic diseases, the availability of cell-line models would provide major new tools for diagnosis and therapy. In this context, hES cells are research tools for the study of disease, not therapeutic agents themselves.
Controlling the genotype of ES cells will also be important in the future if they are to be used directly as therapeutic tools in regenerative medicine. Transplantation of hES cells will face issues of tissue rejection common to all forms of organ or tissue transplants. As in organ or bone marrow transplantation, one solution is to develop large banks of genetically diverse hES cells to increase the chances that matches can be found for all patients who need them. That is one strong medical reason for generating additional hES cell lines from a wider spectrum of the population. Other methods to overcome tissue rejection, including genetic modification of hES cells to reduce immunogenicity and use of immunosuppressive drugs may be helpful. However, in the long run, one obvious solution would be autologous transplantation, using hES cells genetically identical with the recipient of the graft.
Generation of ES cells using nuclear transfer (NT) has the potential to produce ES cells of defined genotype to address both genetic diversity and avoidance of rejection. NT is the process by which the DNA-containing nucleus of any specialized cell (except eggs and sperm, which contain only half the DNA present in other cells) is transferred into an oocyte whose own nuclear genome has been removed (Figure 2.2). The egg can then be activated to develop and will divide to form a blastocyst, whose genetic material and genetically determined traits are identical with those of the donor of the specialized cell, not those of the donor of the oocyte. The oocyte does provide a very small amount of genetic information in the mitochondria, the “energy factories” of the cell, but the genes in the nucleus are of overriding importance, nuclear genes being responsible for the vast majority of the traits of the animal. If such a blastocyst were transferred to a uterus, the transferred blastocyst could potentially develop into a live-born offspring—a clone of the nuclear donor. NT was first developed with frog embryos and later successfully used to generate Dolly the sheep, the first mammal cloned from an adult cell (Campbell et al., 1996). Since the birth of Dolly, live cloned offspring of several other mammalian species have been reported, including mice, goats, pigs, rats, cats, and cows. The success rate of live births is very low, however, and a variety of abnormalities have been found in cloned animals (NRC, 2002b), so this is currently an unreliable technology and unsafe for application to humans. Given the safety issues associated with NT for human reproduction, there is a worldwide consensus that such efforts should be not be conducted at this time. Despite some well-publicized but undocumented claims of production of live cloned babies, the scientific community in general and this committee in particular support that moratorium.
Blastocysts derived using NT can be an important source of genetically defined ES cells. If the inner cell mass of the NT-derived blastocyst, comprising a few dozen undifferentiated cells, is removed and grown in culture, ES cells can be derived and their genotype will be identical with that of the nuclear donor. Successful derivation of pluripotent mES cells from cloned NT blastocysts has been demonstrated in mice by several groups (Kawase et al., 2000; Munsie et al., 2000; Wakayama et al., 2001). In addition, the principle of alleviating a genetic disease was demonstrated by transplantation of genetically repaired mouse NT ES cells in an immunodeficient
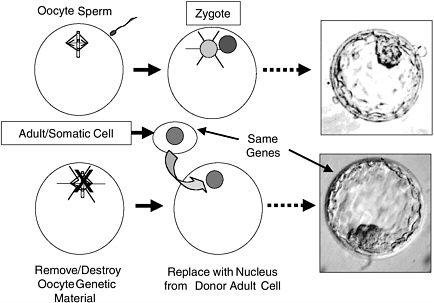
FIGURE 2.2 Comparison of Normal Preimplantation Development with Nuclear Transfer (NT). In NT, the genetic material of the oocyte is removed and replaced with a diploid nucleus from a somatic (body) cell. This divides to yield an NT blastocyst whose genes are identical with those of the donor somatic cell. NT blastocysts, like normal blastocysts, can be used to derive embryonic stem cells from their inner cell masses. The picture shown is of a normal human blastocyst (http://www.fosep.org/images/blastocyst2.gif) because pictures of human NT blastocysts are scarce and normal and NT blastocysts appear indistinguishable.
mouse (Rideout et al., 2002). Although production of normal live offspring from NT blastocysts is not very successful in any species, NT ES cells seem to be able to differentiate normally in mice and have been able to contribute extensively to adult tissues, including the germ line, in chimeras (Wakayama et al., 2001). The rate of successful production of ES cells from NT-derived blastocysts is, however, still quite low (less than 5 percent).
In 2004, the first report of an NT-derived hES cell line was made by Woo Suk Hwang and colleagues in South Korea (Hwang et al., 2004). One line was produced by transfer of a nucleus from donated ovarian cumulus cells to an enucleated host oocyte derived from the same donor. The line appeared to be pluripotent and chromosomally normal. Successful production of hES cells was again inefficient—
over 200 oocytes were used in the course of the experiments that generated a single line. However, the scientists made a number of improvements in the procedure as the experiments progressed, increasing the yield of blastocysts and suggesting that the success rate will be improved in the future. This proof of the principles behind generating NT hES cells has made plausible the derivation of more such lines from specifically defined genetic backgrounds.
It is important to note that stem cells made using NT result from an asexual process that does not involve the generation of a novel combination of genes from two “parents.” In this sense, it may be more acceptable to some than the creation of blastocysts for research purposes by IVF (NIH HERP, 1994). It has also been suggested (Hurlbut, 2004) that transfer of genetically altered nuclei incapable of directing full development might make NT acceptable. However, it has been pointed out (Melton et al., 2004) that this approach faces many technical hurdles and does not avoid the need for oocyte donation. At least three methods for generating hES cells from defective embryos have been suggested. One such method involves the use of viable blastomeres extracted from a morula or blastocyst that has been declared dead due to cleavage arrest (Landry and Zucker, 2004). This proposal is untested and is technically challenging. Even if it were possible to identify unequivocally embryos with no chance of further development, the likelihood of then isolating a viable blastomere and generating an ES line is small. There has been only one published report claiming derivation of mES cell lines from isolated 8-cell blastomeres (Delhaise et al., 1996). One cell line was obtained from 52 fully viable, dissociated 8-cell stage morulae.
Two other methods of generating hES cells from defective embryos have been considered: parthenogenesis and androgenesis. In parthenogenesis, an oocyte can be activated to develop without being fertilized by a sperm. The genomic DNA of the resulting embryo is completely maternally derived, which is not compatible with survival to term. Both mouse and nonhuman primate parthenogenetic ES cell lines have been established (Kaufman et al., 1983; Cibelli et al., 2002). The results are of interest because deriving stem cells from parthenogenetic blastocysts could eliminate the requirement to produce and destroy viable blastocysts. Parthenogenetic ES cells could serve as an alternative source for autologous cell therapy. However, parthenogenetic mES cells show restricted tissue contributions in chimeras and in teratomas formed by grafting the cells under the kidney capsule (Allen et al., 1994); this is related to the lack of expression of key imprinted genes that are normally expressed from the paternal genome. In contrast with parthenogenesis, in androgenesis the entire genome comes from the male parent. Such embryos also do not survive to term. Diploid androgenetic mES cells have been derived (Mann et al., 1990), but many androgenetic ES cell chimeras died at early postnatal stages, and the ones that survived developed skeletal abnormalities. Again, the imprinting status of the cells differed from that of wild-type ES cells (Szabo et al, 1994). Thus, although the results show that androgenetic and parthenogenetic ES cells have broad developmental potential, their imprinted gene expression status is likely to
restrict their therapeutic applications. Moreover, no human parthenogenetic or androgenetic stem cell lines have been established, and more research is needed to determine whether these techniques can be applied to human oocytes for production of stem cell lines.
SOURCES OF OOCYTES FOR NT ES CELLS
At current rates of success of generation of NT blastocysts and ES cells, one major limitation of expansion of this approach will be the availability of oocytes for NT. Current and possible future sources of such oocytes include excess oocytes and unfertilized oocytes from IVF procedures, oocytes matured from ovariectomies or fetal ovaries from pregnancy terminations, oocyte donation, derivation of oocytes from nonreproductive material, and use of nonhuman oocytes.
-
Excess oocytes and unfertilized eggs from IVF procedures. During IVF, hormonal induction is used to generate oocytes for fertilization in vitro. Often, more oocytes are generated than are needed for reproductive purposes, and some oocytes may be available for research donation. In addition, after IVF, not all oocytes are successfully fertilized, and unfertilized oocytes would otherwise be discarded if not donated for research. Experiments to explore use of such oocytes for NT derivation of hES cells have been approved and initiated in the United Kingdom. However, this source of oocytes is limited, and the unfertilized oocytes may be of lower quality for cell line production. It is ethically problematic to consider alteration of the IVF clinical procedure to deliberately induce more oocytes than needed for reproduction, even with the consent of the participants. Thus, this source of oocytes is likely to be limited and unreliable for any major NT ES cell program.
-
Oocytes matured from ovariectomies or fetal ovaries from pregnancy terminations. Adult as well as fetal ovaries contain a large supply of immature oocytes, which in principle could be harvested from adult ovaries donated after removal for clinical reasons or from fetal ovaries that are obtained from legal pregnancy terminations. In the case of other mammals, it is possible to mature such oocytes in culture and achieve fertilization and normal development, although the process is not efficient (O’Brien et al., 2003). In humans, success has been limited and requires an intermediate xenograft (transplantation into an animal) of the ovarian tissue for oocyte maturation. Research on how to expand the supply and how to mature human oocytes in vitro could make this a reasonable source of donated material.
-
Oocyte donation. The most reliable source of oocytes for NT ES cells today seems to be direct donation of oocytes by female donors after hormonal induction and oocyte recovery. Such third-party donation has much in common with organ donation and already occurs in some IVF programs for
-
reproductive purposes. However, this option raises significant issues about the risks to the donors, about a possible profit motive if excessive payment is made for donated oocytes, and about the nature of informed consent in such circumstances. Altruistic donation of oocytes by family members for generation of disease-related NT ES cells might be a good alternative source of material.
-
Derivation of oocytes from nonreproductive material. The problems of the limited pool of oocytes for NT would be alleviated if a renewable source of oocytes can be found. The recent report that cells resembling oocytes could be formed from mouse ES cells in culture (Hubner et al., 2003) is intriguing in this regard. If confirmed and extended to human ES cells, this approach could eventually provide an extensive source of oocytes or something resembling oocytes for NT.
-
Use of nonhuman oocytes. Obtaining large numbers of oocytes from nonhuman mammals is relatively easy, and the use of such oocytes to derive NT blastocysts and stem cells has been considered. If this were successful, the nuclear genome would be entirely human, but there could be some persistence of nonhuman mitochondria in the cells. The relevance of such interspecies mixing for the growth, potential, and safety of such cells would need to be evaluated. There has been one report of putative ES cell lines produced after transfer of human nuclei to rabbit oocytes (Chen et al., 2003), but the finding needs to be confirmed and extended before this approach can be considered feasible.
Given the strong scientific rationale for generating human NT ES cells, there is an urgent need to develop new ethically acceptable sources of cytoplasmic material for reprogramming adult nuclei. Further research into the molecular mechanisms by which the oocyte cytoplasm reprograms the adult nucleus for pluripotency should lead to methods to bypass altogether the need for oocytes to achieve NT reprogramming. In the long run, it may be possible to reprogram adult cells or nuclei directly—not by transfer into oocytes but by other means, such as fusion with pluripotent ES cells or exposure to factors from such pluripotent cells.
INTERSPECIES MIXING
Interspecies mixing happens in nature, and deliberate human-made examples, such as mules, raise no ethical concerns. However, when one of the species involved is human, there is a clear need to consider ethical issues. Hybrids, such as mules, are animals derived from interbreeding between two different species. In the case of a mule, chromosomes from a horse and a donkey are brought together through the fusion of horse and donkey gametes in fertilization to produce an animal whose every cell contains genes from both parental species. Interspecies hybrids are rarely viable and no one proposes to generate interspecies hybrids involving human ga-
metes, even if it were possible. However, there are valid scientific reasons for creating a second sort of interspecies mix in the context of hES cell research—a chimera. Chimeras, unlike genetic hybrids, consist of mixtures of cells (or, in some cases, tissues) from two different kinds of animals. Unlike the situation in hybrids, there is no commingling of genetic material in individual cells of a chimera.
Chimeras are widely used in research and medicine—xenotransplants of, for example, human skin onto mice, of human tumors into mice, and of human bone marrow into mice are already subject to regulation (for example, use of human material is regulated by Institutional Review Boards (IRBs) and animal care issues are regulated by Institutional Animal Care and Use Committees (IACUCs)). Thus, there seem to be no new ethical or regulatory issues regarding chimeras themselves. Nonetheless, because of the pluripotency of hES cells, the extent of their contributions to interspecies chimeras is uncertain, and both the need for and value of chimera experiments involving hES cells and related ethical concerns need to be considered (see Chapter 3). In stem cell research, the possible utility of interspecies mixing arises in several contexts.
Incorporation of hES Cells or Cells Derived from Them into Postnatal Animals of Another Species
Such experiments will be essential to test the potential of hES cells or their derivatives to differentiate into the desired cells and tissues and to ensure that hES cells or their derivatives do not give rise to inappropriate cell types or to tumors or have any other deleterious consequences. Such “preclinical testing” is analogous to the standard testing of drugs, transplants, and medical devices in animals before human clinical trials. It will inevitably be required by the Food and Drug Administration (FDA) en route to any application of hES cells or their derivatives or, indeed, of adult stem cells in therapeutic applications. As mentioned above, many experiments of this type have been done before and are well covered by existing regulations concerning use of human tissues and animals. The use of pig heart valves in humans is an example of routine clinical use of interspecies chimeras. The issues that are particular to hES cells concern the possibility that such cells, because of their pluripotency, could give rise to cells of the germline or the brain. That would be of less or no concern in the case of hES cell derivatives that had differentiated down particular developmental paths, for example, into cells able to make cartilage, bone, skin, or blood. But it needs consideration when pluripotent hES cells or their neural derivatives, such as neural stem cells, are used.
It seems highly unlikely that hES cells could contribute to the germline after implantation into a postnatal animal because the germline is set aside very early in fetal development. Nonetheless, the possibility could readily be addressed by ensuring that animals receiving hES cell transplants do not breed. The possibility of contribution to the brain is harder to evaluate. One purpose of introducing hES cells or human neural progenitor cells is to have them contribute to repair or regenerative
processes and to yield neurons. Production of motor neurons, sensory neurons, or neurons that secrete mediators, such as dopamine, might all contribute to combating spinal-cord injuries and neurodegenerative diseases. However, the idea that human neuronal cells might participate in “higher-order” brain functions in a nonhuman animal, however unlikely that may be, raises concerns that need to be considered. Indeed, if such cells are to be used in human therapeutic interventions, one needs to know whether they could participate in that way in the context of a treatment. Thus, there are good reasons to explore this sort of issue through animal experiments. Studies on the brain are proceeding rapidly, but there is clearly a need for more investigation, and hES cell research in this field should proceed with due care (see Chapter 3).
Incorporation of hES Cells or Cells Derived from Them into Postgastrulation Stages of Another Species
Such experiments would allow a greater opportunity for hES cells to be properly incorporated into appropriately organized tissues and would therefore offer greater opportunities to reveal the potential of such cells. Similar experiments have been invaluable in testing the capacity of neuronal progenitors derived in vitro from mES cells by transplantation into chicken embryos (Wichterle et al., 2002); it seems clear that there will be a need or desire to conduct similar experiments to test the potential of hES cells and their derivatives. Indeed, preliminary experiments showing that hES cells can survive and differentiate after transplantation into chicken embryos have been reported (Goldstein et al., 2002). As noted at the outset, there seems little ethical concern about many such experiments, which resemble research approaches that have been used often in the past. For example, human hematopoietic stem cell transplantation would be equivalent to current human-to-mouse bone marrow transplantation and the same could be said for many other tissues. The sensitivities, again, arise concerning neuronal and germline cells and are perhaps more of a concern than in the case of transplantation into a postnatal animal, because the hES cells might be expected to have greater opportunity to participate. As above, the issue of germline contribution could be addressed by preventing any such chimeras from breeding. The potential for incorporation into brain functions needs research and monitoring as mentioned above.
Incorporation of hES Cells into Nonhuman Blastocysts
This approach is an obvious extension of techniques widely used in research with mES cells—namely, aggregation of morulae from two mice or injection of mES cells into mouse blastocysts. In both cases, the cells can contribute extensively to any mouse that arises from implantation of such a chimeric blastocyst. Clearly, an animal (e.g., mouse) blastocyst into which human cells are transplanted raises other issues because potentially the inner cell mass, the progenitor of the fetus, would
consist of a mixture of human and mouse cells. It is not now possible to predict the extent of human contribution to such chimeras. If the recipient blastocyst were from an animal that is evolutionarily closer to a human, the potential for human contributions would appear to be greater. For these reasons, research that involves the production of such chimeras should be performed first using nonhuman primate ES cells in mouse blastocysts before proceeding to use of hES cells. The need for the use of blastocysts from larger mammals would need to be very clearly justified and nonhuman primate blastocysts should not be used at this time. Any chimeric experiments using hES cells should be subject to careful review by the institutional oversight committees described in Chapter 3. (Also see Chapter 3 for additional discussion of the ethical concerns surrounding chimeras.)
Use of Nonhuman Oocytes as Recipients of Human Somatic Nuclei in NT with the Aim of Generating hES Cell Lines Without the Need for Human Oocytes
The possibility of using nonhuman oocytes as recipients for NT was mentioned above. The procedure is not in wide use, and it is not clear how useful it will be, but it might constitute a solution to the problem of limited supplies of human oocytes. More immediately, interspecies combinations (human nucleus into nonhuman oocytes) are potentially valuable research tools that could be used to learn about reprogramming of somatic nuclei, which could be one long-term solution to the problems of tissue rejection and limited supplies of human oocytes. Such an interspecies construct would be similar to the product of human NT and would be subject to similar guidelines regarding implantation or culture beyond 14 days (the primitive streak stage) while still permitting the recovery of ES cells.
PRIORITIES FOR hES CELL RESEARCH
Although the potential for future therapeutic use of hES cells seems clear, many technical issues remain to be solved before the potential can be realized. More than a decade of research with mES cells has amply demonstrated their potential to differentiate into all cells of the body. Nonetheless, there is only limited understanding of how to direct their differentiation into well-defined paths, as would be necessary if hES cells are to be used to generate cells of specific developmental potential for therapeutic purposes. A clear example of how such research must proceed is offered by a study in which mES cells were coaxed to develop in vitro into precursors of motor neurons (restricted potential neuronal progenitors or neuronal stem cells), which were then transplanted into chicken embryos, where they differentiated into motor neurons (Wichterle et al, 2002). ES-cell-derived hematopoietic cells can also be used to achieve long-term hematopoietic reconstitution (Kyba et al., 2002), and cardiomyocytes from mouse ES cells have achieved reintegration into cardiac muscle (Klug et al., 1996). Much more of this type of differentiation and
transplantation research will need to be done if hES cells are to be used in regenerative medicine, and much research is needed into the various steps of such protocols.
Experimental manipulations that will need to be developed with hES cells to achieve successful applications in human medicine are described below in sequence from hES cell derivation and culture, through preclinical testing and other research uses to illustrate the spectrum of hES cell research that will be necessary in the coming years and to point out the biomedical rationales for the experiments. These are the types of essential experiments for which the guidelines proposed later in this report are designed to provide a framework for ethical and responsible conduct.
-
Additional hES cell lines must be generated because experience from studies of mES cells shows that lines differ in their potential and do not always retain their potential on extended culture. Furthermore, the hES cells now available do not have adequate genetic diversity.
-
hES cells of defined genetic backgrounds need to be generated. In the future, such cells could be used in autologous cellular therapy, which would avoid problems of immune rejection, but that prospect is some years away. In the immediate term, hES cells with genotypes known to predispose to particular diseases would be invaluable for research into the bases of the diseases in question and for developing tests for diagnostic and therapeutic approaches (for example, drug testing). Few such genetically defined hES cells now exist, but several sources are possible. Excess blastocysts will necessarily be produced in the course of IVF and PGD procedures designed to derive blastocysts that lack disease-promoting genotypes. Excess blastocysts that are genotypically unsuitable for reproduction would normally be discarded but instead they can be used to generate hES cells (Verlinsky et al., 2005). Such blastocysts could also be generated with IVF procedures specifically for that purpose; families with genetic predispositions might well be motivated to contribute gametes altruistically. Alternatively, hES cells of the desired genotype could be generated using NT; again, altruistic donation of oocytes and nuclei would be a suitable route.
-
Genetic manipulation of hES cells is another route to the generation of hES cells with defined genetic defects where the diseases are well enough understood for the relevant genes to be known. Research with such procedures would also lay the groundwork for future manipulations, such as gene therapy, to generate autologous cells in which genetic defects have been “fixed.” Such in vitro manipulations could eventually allow gene modifications to be controlled with precision to avoid deleterious side effects. hES cells can be genetically modified by introduction of transgenes with a variety of approaches, and homologous recombination to alter the endogenous genes of the cells is also possible (Zwaka and Thomson, 2003). Further research into genetic modification of hES cells is important.
-
As discussed in the section on NT, a major current limitation of widespread use of NT is the restricted availability of human oocytes, and research into the many different possibilities for alternative sources is needed. The possibilities include the maturation of immature oocytes derived from therapeutic ovariectomies or from fetal ovaries and, perhaps, of unfertilized oocytes from IVF clinics. However, a better long-term solution of the problem would be development of methods for producing renewable sources of oocytes, such as differentiation of hES cells. Studies on the latter possibility would be invaluable.
-
Nonhuman oocytes might also be used for NT, and this needs further research.
-
A means of reprogramming the nuclei of somatic cells, either by culturing cells under different growth conditions or by exposing the nuclei to factors from oocyte or hES cell cytoplasm, is essential. Research on the nature of epigenetic modification and means of modifying it so that somatic cell nuclei could be reprogrammed to a state equivalent to that of ES cells would make oocytes and embryos unnecessary for generating hES cells. Success in this effort would be a major advance and, therefore, while not imminent, seems a high priority for research.
-
Research is needed to understand how to maintain the self-renewing capacity of hES cells over long-term culture and expansion. In the mouse, the LIFJAK-STAT pathway of signaling molecules is necessary and sufficient for self-renewal, but it is not sufficient to maintain hES cells in the stem cell state (Daheron et al., 2004). For therapeutic applications, it will be essential to be able to propagate and expand hES cells.
-
It will also be necessary to develop culture conditions that do not include mouse feeder cells and bovine serum as in most current research. Animal products will introduce complications in any future therapeutic use of hES cells, both with respect to FDA requirements and because nonhuman materials can contribute biochemical precursors to the hES cells that render them immunogenic and therefore unsuitable for transplantation (Martin et al., 2005). Initial success has been reported in replacing mouse feeder layers (Xu et al., 2005) but additional improvements in culture conditions will need to be developed and tested.
-
Detailed investigation will be needed to determine the best means of ensuring stability of genotype, epigenetic status, and phenotypic properties of ES cells grown in long-term cultures for use in human therapies.
-
Research is needed to determine how to direct the development of hES cells down particular pathways to generate cells restricted to specific developmental fates. It will involve exploration of different culture conditions and investigation of growth and differentiation factors that promote specified developmental fates. Such investigations will rely on ongoing research into the developmental biology of other species but will require direct studies of
-
hES cells because there will be differences between ES cells of different species. Studies of nonhuman ES cell models and of hES cells must proceed in parallel.
-
A related challenge will be the development of methods to separate progenitors of restricted developmental potential from hES cells (or methods to ensure complete conversion of hES cells into the desired cellular derivatives). mES cells transplanted to ectopic sites can generate benign tumors and such an outcome clearly would be undesirable in any cellular therapy. One can imagine methods for separating or removing persisting hES cells (such as sorting of undifferentiated cells or inducible suicide of inappropriate cells), but research will be required to ensure that such methods are effective.
-
All the foregoing procedures will necessitate means of testing the potential of the derived cells to contribute usefully when implanted and for adverse side effects; such tests will undoubtedly be required by FDA before any therapeutic use. That requirement will necessitate development of protocols for effective and ethical testing of the potential of hES cells and their derivatives (or adult stem cells). Many tests can be conducted in vitro but in vivo tests will also be mandatory. As discussed above, some such tests present no particular ethical problems, and the technical issues can be addressed with further experimentation. However, some chimera experiments that can be easily envisaged raise issues pertaining to the possibilities of hES cell contributions to the brain or the germline. Research is needed to determine the likelihood of those potential concerns. It has been argued that their potential may be quite limited but a main purpose of developing hES cell-based therapies is to promote some participation of the implanted cells. Research will be necessary to discover the extent to which this is possible both to exploit the therapeutic potential and to avoid undesired contributions.
-
One issue arising in any cell or tissue transplantation is immune rejection due to histocompatibility antigenic differences between people. This problem is confronted every day in organ transplantation and has been addressed with tissue-matching and immune suppression. Nevertheless it remains a problem and will affect any stem cell-based therapies (adult or embryonic) unless means can be found to avoid it. One such means is the use of autologous hES cells derived using a patient’s own nuclei to generate genetically identical hES cells through NT. That approach is feasible and likely to be exploited, but it will face hurdles, such as oocyte availability, if it is to be widely used. The more genetically diverse hES cells there are available, the more likely that a histocompatible matching line can be found. That is a strong argument for development of stem cell banks (see Chapter 5). In parallel, research into ways of avoiding immune rejection should be encouraged both for standard organ transplantation and for future hES cell therapies. With ES cells and their derivatives, it may be possible to devise means
-
of suppressing histocompatibility antigens, which clearly is not feasible with organ transplants.
-
In addition to therapeutic transplantation, hES cells are good candidates for testing of therapeutic drugs. If hES cells can be directed to differentiate into specific cell types, they may be more likely to mimic the in vivo response of cells and tissues to the drug being tested and so offer safer models for drug screening. Similarly, hES cells could be used to screen potential toxins. Toxic agents often have different effects on different animal species and cell types, and this makes it critical to have the best possible in vitro models for evaluating their effects on human cells. However, it remains to be determined which differentiation stages of hES-derived cells are optimal for such practical applications. For example, what differentiation stages of ES-derived cells would be best for screening drugs or toxins or for delivering potentially therapeutic drugs?
CONCLUSION
The list of hES cell research priorities underlines the need for a broadly accepted set of guidelines to assist researchers and regulators in their design of investigations, whether funded by federal, state, philanthropic, or industrial sources. The research has great promise, but much further investigation is needed to realize the potential, and the sensitivities surrounding research with hES cells require continuing attention to the ethical and public policy issues. The next chapter discusses many of the ethical concerns raised by this research and proposes a system of oversight to address ethical and public concerns.


















