3
Dispersant-Oil Interactions and Effectiveness Testing
Dispersants are mixtures of solvents, surfactants, and other additives that are applied to oil slicks to reduce the oil-water interfacial tension (NRC, 1989; Clayton et al., 1993). Interfacial tension is the free energy change that is associated with a change in the contact area at the interface between two immiscible phases (e.g., solid-liquid, liquid-liquid, liquid-gas). The term surface tension is also used to describe this phenomenon. Although these two terms are often used interchangeably, interfacial tension is considered to be the more general term, which can be applied to describe the free energy at the interface between any two phases, whereas surface tension applies specifically to those cases in which one of the phases is a gas (Lyklema, 2000). Reduction of the interfacial tension between oil and water by addition of a dispersant promotes the formation of a larger number of small oil droplets when surface waves entrain oil into the water column. These small submerged oil droplets are then subject to transport by subsurface currents and other natural removal processes, such as dissolution, volatilization from the water surface, biodegradation, and sedimentation resulting from interactions with suspended particulate material (SPM).
For the purpose of this and subsequent discussions, it is important to define two terms that are used interchangeably in the dispersant literature: entrainment and dispersion. In this report, entrainment is specifically the transport of oil from a surface slick into the water column by wind and waves, while dispersion includes both entrainment and subsurface transport (mixing and advection) by turbulent forces. It should also be mentioned that in the hydrodynamics literature the term dispersion
(sometimes shear dispersion) refers to a specific mixing process resulting from the combination of shear in the mean velocity coupled with turbulent mixing (or other transport mechanism) in the direction of the shear. This process will be discussed in Chapter 4 and will be denoted as hydrodynamic dispersion to avoid confusion.
The following sections address dispersant chemistry, the physical and chemical interactions of dispersants with oil slicks and droplets, oil chemistry and weathering behavior and how they affect the window of opportunity for effective dispersant applications, and the importance of turbulence for introducing the energy necessary to entrain oil droplets into the water column as well as their subsequent transport by dispersive and advective processes. Next is a discussion of effectiveness testing and related issues, including laboratory systems, wave-tank tests, field studies, and studies involving spills of opportunity. Several of these topics are only considered briefly because there are a number of excellent reviews that consider the mechanisms of dispersant action and laboratory and field testing of dispersant performance (e.g., Meeks, 1981; Rewick et al., 1981; Mackay et al., 1984; Nichols and Parker, 1985; NRC, 1989; Clayton et al., 1993; Trudel, 1998; Etkin, 1999). Topics for which there are still major uncertainties or where data gaps exist are considered in greater detail, along with explicit findings and recommendations for areas requiring additional research.
COMMERCIAL DISPERSANT PRODUCTS AVAILABLE FOR USE IN U.S. WATERS
A typical commercial dispersant is a mixture of three types of chemicals: solvents, additives, and most importantly, surface-active agents (i.e., surfactants). Solvents are added primarily to promote the dissolution of surfactants and additives into a homogeneous dispersant mixture. In addition to keeping the surfactants in solution, these solvents reduce the product’s viscosity and affect the dispersant’s solubility in oil. Also, solvents determine to what extent the dispersant may be premixed with water for some spraying applications. Because aqueous-based solvent systems freeze in spray nozzles at ambient temperatures below 0° C (roughly 32° F) their usefulness is often limited in arctic or subarctic environments. Additives may be present for a number of purposes, such as improving the dissolution of the surfactants into an oil slick and increasing the long-term stability of the dispersant formulation.
Surfactants are compounds containing both oil-compatible (i.e., lipophilic or hydrophobic) and water-compatible (i.e., hydrophilic) groups. Because of this amphiphatic nature (i.e., opposing solubility tendencies), the surfactant molecules will reside at the oil-water interface as shown in
Figure 3-1. The surfactant reduces the oil-water interfacial tension by orienting with the hydrophilic groups interacting with the water phase and the hydrophobic groups interacting with the oil. Reduction of the oil-water interfacial tension facilitates the formation of a large number of small oil droplets that can be entrained into the water column.
Commercial formulations of modern chemical dispersants are usually comprised of two or more surfactant molecules that have differing solubilities in both water and oil. One parameter that has been used to characterize these different solubilities is the hydrophile-lipophile balance (HLB). The HLB ranges from 0 (no hydrophilic group) to 20 (no hydrophobic group), and the specific value characterizes the tendency of the surfactant to preferentially dissolve in either the oil phase (low HLB) or the aqueous phase (high HLB). The dominant group of the surfactant molecule will tend to orient in the outer phase to form a droplet of either oil or water (Porter, 1991). Therefore, a predominantly lipophilic surfactant (with a HLB below 7) will favor water-in-oil emulsions (mousse) where oil forms the continuous phase with discrete water droplets entrained within it (Porter, 1991). Natural components that promote the for-
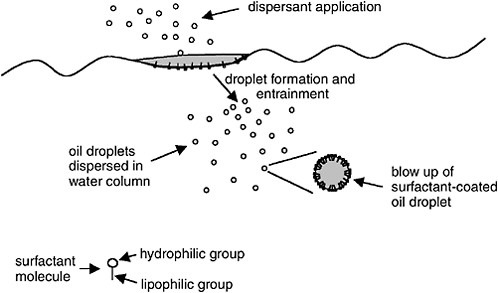
FIGURE 3-1 Mechanism of chemical dispersion: surfactant accumulates at oil-water interface, facilitating formation of small oil droplets that become entrained in the water column. Blow-up of oil droplet shows orientation of surfactant at the droplet surface with the hydrophilic group projecting into the water phase and the lipophilic group projecting into the oil phase.
mation of mousse (e.g., the resin and asphaltene fractions of crude oil) are generally lipophilic. In contrast, a predominantly hydrophilic surfactant (with an HLB greater than 7) will favor oil-in-water dispersions (i.e., entrained oil droplets in a water body) (Porter, 1991). The blend of surfactants in commercial dispersant formulations tend to be hydrophilic and the current formulations usually consist of surfactant mixtures with an overall HLB in the range of 9 to 11 (Clayton et al., 1993).
An example of the orientation of surfactant molecules at the oil-water interface is presented in Figure 3-2. Compound A is sorbitan monooleate (HLB = 4.3; predominantly lipophilic). Compound B is similar to A but has been ethoxylated with molecules of ethylene oxide to make it more hydrophilic (HLB = 15). The dispersant formulation shown in Figure 3-2 contains more compound B than A. Such a balance will promote formation of stable oil-in-water dispersions (entrained oil droplets in the water column) because the dominant hydrophilic group of the surfactant mixture favors the formation of oil droplets in water. The use of two or more surfactants with differing HLB values, but an overall average HLB in the range of 9-11, allows for closer physical interactions (i.e., packing) of the surfactant molecules at the oil-water interface compared to a single surfactant with an HLB value in this range (Porter, 1991). This produces a stronger interfacial surfactant film. Although ionic surfactants can inhibit coalescence of small droplets into larger droplets that would resurface more quickly by providing an electrostatic repulsion barrier (Porter, 1991), recent measurements suggest that this barrier is too small to significantly affect the collision efficiency (i.e., the fraction of collisions that result in coalescence), at least for dispersants (e.g., Corexit 9500) that consist mainly of nonionic surfactants, even when the dispersant-to-oil ratio (1:10) is relatively high (Sterling et al., 2004c).
Exact compositions for commercial dispersant formulations are proprietary, but their generic chemical characteristics are broadly known (e.g., Wells et al., 1985; Brochu, et al., 1986; NRC, 1989; Fingas et al., 1990; Singer et al., 1991, 1996; George-Ares and Clark, 2000). In general, a limited number of surfactant agents are currently used. Current dispersant formulations consist of mixtures of one or more surfactants, which may be either nonionic or anionic. Cationic (positively charged) surfactants are not used in current formulations (Clayton et al., 1993) because they are usually quaternary ammonium salts that are inherently toxic to many organisms.
The Corexit products are by far the most prevalent of all dispersants held in industry stockpiles within the United States, making up as much as 95 percent is some instances (J. Clark, ExxonMobil Research and Engineering Company, Fairfax, Virginia, written communication, 2005). Corexit 9527 was developed in the 1980s; it was supplemented in the 1990s
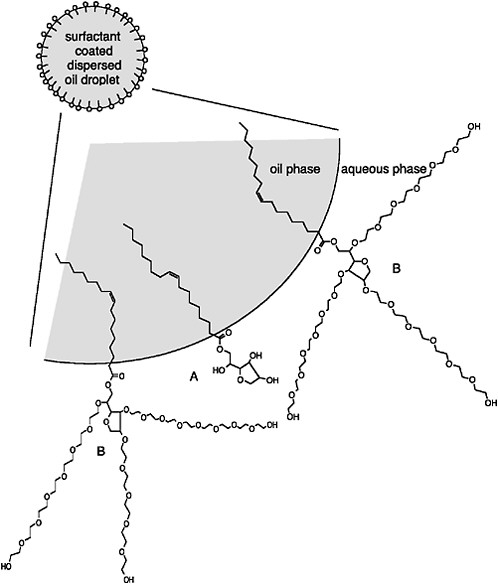
FIGURE 3-2 Orientation of surfactants at oil-water interface in dispersed oil droplets. Surfactant A is sorbitan monooleate (a.k.a., Span 80; HLB ≈ 4.3); surfactant B is ethoxylated (E20) sorbitan monooleate (a.k.a., Tween 80; HLB ≈ 15).
by the introduction of Corexit 9500, which includes the same surfactants incorporated into a different solvent (George-Ares and Clark, 2000). Both products contain a mixture of nonionic (48 percent) and anionic (35 percent) surfactants. The major nonionic surfactants include ethoxylated sorbitan mono- and trioleates and sorbitan monooleate; the major ionic sur-
factant is sodium dioctyl sulfosuccinate (Singer et al., 1991). Neither Corexit product contains polyethoxylated alkyl phenols (J. Clark, ExxonMobil Research and Engineering Company, Fairfax, Virginia, written communication, 2004). A different solvent was used in Corexit 9500 for two reasons. First, prolonged exposure to Corexit 9527 caused adverse health effects in some responders. These effects were attributed to its glycol ether solvent (2-butoxyethanol). Therefore, the solvent was replaced by a mixture of food-grade aliphatic hydrocarbons (Norpar 13; n-alkanes ranging from nonane to hexadecane) in Corexit 9500 (Varadaraj et al., 1995). The second reason for changing the solvent in the reformulated dispersant was to extend the window of opportunity for dispersant use. This window of opportunity is limited by the effects of weathering on the chemical and physical properties of the spilled oil, especially the increase in oil viscosity. Corexit 9500 has been shown to be slightly more effective with high-viscosity oils than Corexit 9527.
THE PHYSICAL CHEMISTRY OF DISPERSANT-OIL INTERACTIONS AND THE ENERGY REQUIREMENTS FOR EFFECTIVE OIL-DROPLET ENTRAINMENT AND DISPERSION
The objective of an oil-spill dispersant application is to lower the oil/water interfacial tension to enhance entrainment of small oil droplets into the water column at lower energy inputs. Entrainment of small oil droplets into the water column (by either physical or chemical means) increases the oil-water interfacial area, which as shown in Eq. (3-1), requires energy:
(3-1)
where WK is the mixing energy (ergs or g-cm2-s−2; 1 erg equals 10−7 joule (kg-m2-s−2)), γo/w is the oil-water interfacial tension (dynes-cm−1, where 1 dyne equals 1 g-cm-s−2; equivalent to ergs-cm−2), and Ao/w is the oil-water interfacial area (cm2). Therefore, reduction of the oil-water interfacial tension allows creation of a larger amount of interfacial area for the same level of energy input. Note that Eq. (3-1) provides an estimate of the minimum energy input that is required to disperse oil as droplets in the water column. Additional energy, which is proportional to viscosity, will be required to form droplets by stretching a continuous oil layer to the point at which it breaks.
The seven requirements for a chemical dispersant to enhance the formation of oil droplets (NRC, 1989) are:
-
The dispersant must hit the target oil at the desired dosage.
-
The surfactant molecules in the dispersant must have time to penetrate and mix into the oil.
-
The surfactant molecules must orient at the oil-water interface with the hydrophilic groups in the water phase and the lipophilic groups in the oil phase.
-
The oil-water interfacial tension must decrease due to the presence of the surfactant molecules at the oil-water interface, thereby weakening the cohesive strength of the oil film.
-
Sufficient mixing energy must be applied at the oil-water interface (by wind and/or wave action) to allow generation of smaller oil droplets (with a concomitant increase in interfacial surface area).
-
The droplets must be dispersed throughout the water column by a combination of diffusive and advective processes to minimize droplet-droplet collisions and coalescence to form larger droplets (which can resurface in the absence of continued turbulence).
-
After entrainment, the droplets must be diluted to nontoxic concentrations and remain suspended in the water column long enough for the majority of the oil to be biodegraded.
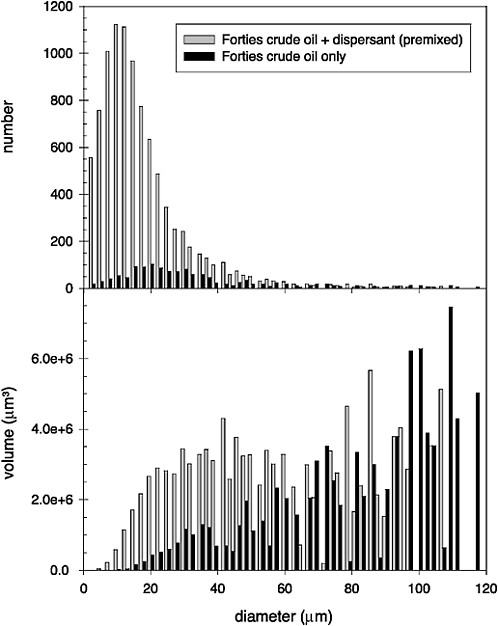
Turbulent energy is the environmental parameter most responsible for generating and transporting dispersed oil droplets in the ocean. Delvigne and Sweeney (1988) studied natural dispersion and argue that the smallest scales of turbulence, with the greatest shear, are responsible for initial droplet formation, while the larger eddy scales are responsible for the subsequent vertical transport (described in more detail in Chapter 4—Transport and Fate). Conversely, Li and Garrett (1998) argue that natural dispersion is generated mainly by dynamic pressures associated with larger eddy scales, resulting in the creation of relatively large droplets (i.e., order of 100 µm diameter) that resurface relatively quickly. They suggest that reduction of the oil-water interfacial tension by chemical dispersants allows the mechanism of turbulent shear to govern droplet formation, which leads to smaller droplets (i.e., order of 10 µm diameter), which is more consistent with the diameters observed for “permanently dispersed” droplets. Unfortunately, the droplet-size distributions of chemically dispersed oil have only rarely been compared directly to those produced when untreated oil was dispersed under identical conditions (see Box 3-1). In the few cases where direct comparisons were made, however, the volume mean diameter was reduced by 30–40 percent by dispersants (Jasper et al., 1978; Lunel, 1995b). Figure 3-3, which was reconstructed from data presented by Lunel (1995b), shows the effect of a chemical dispersant (premixed Dasic Slickgone NS) on the droplet-size distribution produced when Forties crude oil was dispersed at sea: the number of small droplets (<50 mm) increased by about 5- to 30-fold,
|
BOX 3-1 When oil is entrained in the water column due to input of turbulent energy, droplets of various sizes are produced, regardless of whether the process is enhanced by addition of dispersants. Droplet-size distributions describe the relative abundance of droplets of various sizes, which may range from <1 µm to >100 µm in diameter. These distributions can be based on either droplet number or volume, although the volume distribution may be most informative, because the relationship between droplet volume and oil mass is constant regardless of droplet size (i.e., the proportionality constant is the density), whereas the relationship between droplet number and oil mass is not. The most common metrics for characterizing the central tendency of droplet-size distributions are the mean and median diameter, which will be approximately the same if the droplet sizes are normally distributed. The number mean diameter (NMD) is a simple average of droplet diameters, whereas the volume mean diameter (VMD) is the diameter of a droplet with the average volume (i.e., the mean of the volume distribution): 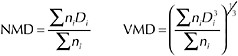
(3-2) where ni is the number of droplets with diameter Di. The VMD is larger than the NMD. Number and volume median diameters (also commonly referred to as NMD and VMD) are those droplet diameters that divide the number and volume distributions in half (i.e., 50 percent of the oil volume is present as droplets smaller than the volume median diameter). |
whereas the number of large droplets (>50 mm) produced from dispersant-treated and untreated oil were similar. Note that although there were relatively few very large droplets produced from either treatment, these represented a significant fraction of the oil mass in both treatments, because the volume of oil in each droplet is proportional to the diameter cubed. Therefore, the volume distribution is extremely sensitive to uncertainty in the number of large droplets. This uncertainty can be seen in the reconstructed volume distribution shown in Figure 3-3. It is not clear whether the differences in characteristic droplet size are statistically significant, but if real, they would result in a 50–65 percent decrease in droplet rise velocity. Therefore, this phenomenon is potentially important and should be investigated further.
|
Droplet-size distributions result from the interaction of two processes: (1) droplet formation due to turbulent shear and (2) size fractionation due to differential rise velocities (Lunel, 1995b). Although the mechanism of droplet formation has not been proven, the initial size distribution of chemically dispersed oil droplets is thought to be related to the scale of the smallest eddies (i.e., microscale turbulence; Delvigne and Sweeney, 1988; Lunel, 1995b; Li and Garrett, 1998), but the distribution will be shifted toward smaller droplets following a period of quiescence due to resurfacing of larger droplets (Daling et al., 1990; Lunel, 1995b). Lunel (1995b) has suggested that dispersant effectiveness tests should be conducted in laboratory-scale systems and wave tanks that generate microscale turbulence similar to that which prevails in surface seawater, because such similarity suggests that the droplet-formation mechanisms will also be similar. Therefore, effectiveness testing should include measurement of droplet-size distributions, preferably in the presence of turbulent mixing energy, so that the observed size distribution will not be affected by size fractionation. Although droplet-size distributions have been measured in some lab-scale effectiveness-testing systems (Byford et al., 1984; Daling et al., 1990a; Lunel, 1995b; Fingas et al., 1995d), the effects of energy dissipation rate, oil and dispersant characteristics, and dispersant treatment should be more thoroughly investigated, because the existing database is not sufficient to support general conclusions regarding how (or whether) these factors affect the droplet-formation mechanisms and kinetics. Even fewer data are available regarding droplet-size distributions formed during dispersant effectiveness tests in wave tanks (Lunel, 1995b). Since one argument for increased use of these systems is their presumed ability to simulate sea surface conditions, it would be prudent to test this hypothesis by measuring droplet-size distributions and comparing them to those measured at sea. |
More effort has been focused on studying the relationship between droplet size and dispersant effectiveness, but conflicting results have been obtained. For example, one study demonstrated an inverse relationship between dispersant effectiveness and the volume median droplet diameter (Byford et al., 1984), whereas others observed no correlation between effectiveness and characteristic droplet size (Daling et al., 1990a; Fingas et al., 1995d; Lunel, 1995b). Although the relationship between effectiveness and droplet-size distribution is uncertain, the droplet-size distributions clearly vary among different experimental systems: volume mean diameters of about 3 mm were observed in a system that was mixed by a six-blade vaned-disk turbine (Jasper et al., 1978), whereas significantly larger diameters (volume median diameters of 20 to 45 µm) were observed in
experimental apparatuses that are more commonly used in dispersant testing (e.g., the Warren Springs Laboratory, Mackay-Nadeau-Steelman, and swirling flask tests) (Daling et al., 1990a; Fingas et al., 1995d). The strong dependence of droplet-size distributions on the characteristics of the experimental system are consistent with the hypothesis that they reflect microscale turbulence (Delvigne and Sweeney, 1988; Lunel, 1995b; Li and Garrett, 1998), and Lunel (1995b) suggested that laboratory-scale or wave-tank effectiveness tests should be evaluated based on their ability to produce size distributions similar to those observed at sea.
In the ocean, turbulent energy is provided mainly by the wind, either by its direct action in shearing the water surface, or through the generation of surface waves. Above a critical wind speed, waves break, creating local areas of intense mixing. Internal waves, bottom shear stress caused by tidal or wind-driven currents interacting with a fixed bottom, and river inflows may also provide turbulent energy. Because of the variety of energy sources and mechanisms for oil droplet generation, it is unlikely that any single parameter can completely characterize the mixing energy responsible for oil dispersion. This is particularly true when including consideration of bench-scale lab tests (see below) in which mixing is produced by other mechanical means such as stirring, swirling, or tumbling. Nonetheless, the parameter that is most likely to be correlated with effective entrainment and dispersion is energy dissipation rate.
Turbulent energy enters a water body at large length scales and is transferred to smaller scales by the process of vortex stretching until it is dissipated by viscosity into thermal energy at the smallest scales. At equilibrium, the rate of energy input equals the rate of energy transferred at each scale, and hence the rate of energy dissipation (Tennekes and Lumley, 1972). Energy dissipation rates can be expressed in units of energy loss per volume per time, e (J-m−3-s−1) where J is joules (kg-m2-s−2). So, the volumetric energy dissipation rate, e, can also be expressed as kg-m−1-s−3. The energy dissipation rate can also be expressed as energy loss per unit mass per time, denoted by ε (J-kg−1-s−1 or m2-s−3). The latter is numerically smaller than e by a factor of the water density (about 103 kg-m−3). Table 3-1, adapted from Delvigne and Sweeney (1988), gives approximate ranges of e and ε for a variety of water bodies.
In-situ values of the dissipation rate can be determined from highly resolved velocity measurements. Doron et al. (2001) describe several methods involving either evaluation of fine-scale velocity gradients or finding a fit to the spectrum of turbulent kinetic energy
(2)
where E(k) is the turbulent kinetic energy density as a function of wave
TABLE 3-1 Energy Dissipation Rates for Different Water Bodies
|
Water Body |
e (J-m−3-s−1) |
e (m2-s−3) |
|
Deep sea |
10−4 to 10−2 |
10−7 to 10−5 |
|
Estuary |
10−1 to 1 |
10−4 to 10−3 |
|
Surface layer |
1 to 10 |
10−3 to 10−2 |
|
Breaking waves |
103 to 104 |
1 to 10 |
|
SOURCE: Modified from Delvigne and Sweeney, 1988. |
||
number, k. The turbulent kinetic energy (i.e., the integral of E(k) over k), expressed per unit mass (units of J-kg−1 or m2-s−2), equals
where ui are the turbulent velocity fluctuations in up to i = 3 coordinate directions (Tennekes and Lumley, 1972).
Turbulent velocities themselves have been measured using a variety of techniques, some more appropriate to the lab and others more appropriate to the field. Point measurements can be made using airfoils, acoustic time-of-travel current meters, drag-sphere devices based on the instantaneous acceleration of a small sphere, hot-wire anemometers, and acoustic and laser Doppler velocimeters (Osborn, 1974; Agrawal et al., 1992; Terray et al., 1996; Doron et al., 2001). A one-dimensional velocity field can be determined using an acoustic Doppler current profiler (Veron and Melville, 2001), or by attaching probes to a vertical profiler, glider, or moving vessel. A two-dimensional velocity field can be obtained simultaneously using particle image velocimetry where a laser is used to illuminate a plane, and velocities are determined by correlating the displacement of natural particles observed in successive images captured with a charge-coupled device camera (Doron et al., 2001; Bertuccioli et al., 1999). In a laboratory flask, column, or tank, the rate of energy dissipation can also be determined indirectly by the rate of energy input by assuming that all input energy turns into turbulence. For example, in their “grid column,” Delvigne and Sweeney (1988) determined ε by measuring the hydraulic resistance of their oscillating grid, while in their wave flumes, they determined ε by measuring the decline in wave energy as a function of distance along their tank. To the extent that ε uniquely determines oil dispersion, designing a laboratory experiment with values of ε equal to
those expected in the field allows one to directly apply observations of dispersion effectiveness in the laboratory to predict dispersion effectiveness in the field. Unfortunately, this approach has not been typically utilized in laboratory and flume studies to date.
FACTORS THAT AFFECT THE OIL/DISPERSANT INTERACTION—THE WINDOW OF OPPORTUNITY AS CONTROLLED BY OIL CHEMISTRY AND WEATHERING STATE
When crude oil or refined petroleum products are released at sea, they are immediately subject to a wide variety of weathering processes that affect the resulting oil’s chemical composition and physical (rheological) properties. These properties, including the chemical components responsible for stabilizing water-in-oil emulsions, are described more fully in Chapter 4. With regard to interactions with dispersants, the two most important weathering factors include evaporation and the formation of stable water-in-oil emulsions, because they both affect the spilled oil’s in-situ viscosity on the water surface. Not surprisingly both of these processes are influenced by temperature (evaporation occurs more rapidly at higher temperatures, while emulsification can occur more rapidly at lower temperatures). Figure 3-4 summarizes the changes in bulk physical properties and water content in weathered Prudhoe Bay crude oil measured in experiments conducted in three 2,800-liter outdoor flow-through wave tanks over a 13 month period at Kasitsna Bay (lower Cook Inlet), Alaska (Payne et al., 1984, 1991a). The residence time of water flowing through the tanks was 4 hours, and the water temperature ranged from about 2° C (roughly 35° F) in the winter to 14° C (roughly 57° F) in the summer. Note the rapid change in properties after as little as 1–2 days of weathering under subarctic conditions. Although the initial oil-water ratio in these experiments was relatively high (1:175) and surface spreading of the oil was limited by the walls of the tank, the changes in oil chemistry and rheological properties that occurred in this oil over time were remarkably similar to those that were observed in the Alaska North Slope crude oil released from the T/V Exxon Valdez oil spill in Prince William Sound, Alaska (Payne et al., 1991a).
Viscosity is typically reported in dynamic units of centipoise (cP; 0.01 dyne-s-cm−2 or 0.01 g-cm−1-s−1). It may also be reported in kinematic units of centistokes (cSt; 0.01 cm2 s−1) by dividing the dynamic viscosity by the fluid density. Because the density of oil is usually between 0.8 and 1.0 g-cm−3, viscosities reported as cP and cSt are numerically similar, but the kinematic viscosity may be up to 25 percent larger than the dynamic viscosity. To provide perspective on the viscosity of weathered oil, Table 3-2 presents data for the water-in-oil emulsions from the wave-tank
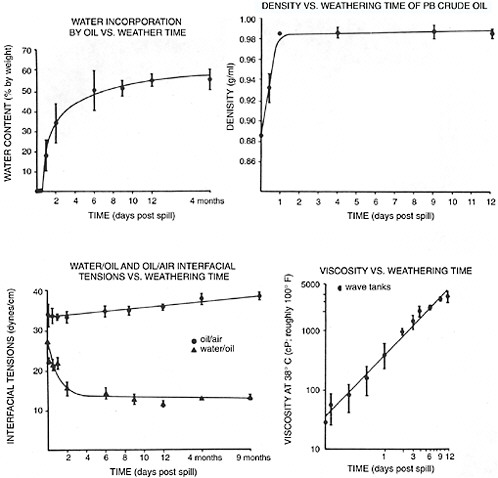
FIGURE 3-4 Changes in various physical properties of Prudhoe Bay crude oil as a function of weathering time. The values given are means from the three replicate summer wave tank experiments ± one standard deviation.
SOURCE: Payne et al., 1991a.
studies along with examples of the viscosities for several common food and household items.
During most of the 1980s, oils or emulsions with viscosities greater than 2,000 cP were considered to be difficult or impossible to chemically disperse (NRC, 1989). More recent studies (Fiocco et al., 1999; Guyomarch et al., 1999a) have shown that a number of intermediate fuel oils and weathered water-in-oil emulsions with viscosities approaching 20,000 cP can at least be partially dispersed in laboratory and field trials with multiple applications of newer hydrocarbon-solvent-based dispersants and demulsifiers (e.g., Corexit 9500, Inipol IP 90, Slickgone NS, Alcopol,
TABLE 3-2 Example Viscosities of Foods and Other Liquids.
|
Product |
Temperature (°C) |
Viscosity (cP) |
|
Water |
20 |
1.0 |
|
Ethyl alcohol |
20 |
1.2 |
|
Olive oil |
40 |
36 |
|
Fresh Prudhoe Bay crude oil (PBCO) |
14 |
68 |
|
Olive oil |
20 |
84 |
|
Olive oil |
10 |
138 |
|
Castor oil |
20 |
986 |
|
48-hr weathered PBCO water-in-oil emulsion |
14 |
1,080 |
|
72-hr weathered PBCO water-in-oil emulsion |
14 |
2,350 |
|
Pancake syrup |
20 |
2,500 |
|
144-hr weathered PBCO water-in-oil emulsion |
14 |
5,400 |
|
Honey |
20 |
10,000 |
|
Chocolate syrup |
20 |
25,000 |
|
Ketchup |
20 |
50,000 |
|
Peanut butter |
20 |
250,000 |
|
SOURCE: Data from CRC (1967), Transtronics (2000), and outdoor subarctic wave-tank experiments described by Payne et al. (1984, 1991a). |
||
Demoussifier, Gamabreak, and Demulsip). As a result, these researchers have concluded that there is no hard and fast rule for the upper viscosity limit for dispersibility of water-in-oil emulsions.
If the pour point of the oil or refined product is above the ambient temperature encountered during a spill, the oil will not flow (it behaves as a semi-solid plastic-like material) and cannot be chemically dispersed. It has also been noted that certain highly paraffinic (waxy) crude oils can form a surface film due to evaporation of light ends (Berger and Mackay, 1994) and that photooxidation can lead to the formation of tar and gum residues (Payne and Phillips, 1985a,b; NRC, 1985, 2003), and it has been suggested that such surface layers may inhibit dispersant penetration into those oils.
Extensive research has been undertaken on the numerous factors responsible for the formation of stable water-in-oil emulsions with different oils (Bridie et al., 1980a,b; Zagorski and Mackay, 1982; Payne and Phillips, 1985b; Mackay, 1987; Bobra, 1990, 1991; Fingas and Fieldhouse, 1994, 2003, 2004a,b; Fingas et al., 1995a,b, 1996b, 1998, 1999, 2000a,b; Walker et al., 1993a,b, 1995), and the major findings from much of this research are briefly summarized in Chapter 4. The current consensus among researchers is that the type and stability of the emulsions is controlled by the properties of the starting oil, especially the asphaltene and resin content and
initial oil viscosity (Fingas and Fieldhouse, 2003). Notwithstanding these advances, most of the existing knowledge on whether or not a particular oil will emulsify under given environmental conditions is empirical, and Fingas and Fieldhouse (2003) compiled a comprehensive data set that was used to develop a model of emulsification rate and stability (Fingas and Fieldhouse, 2004a, b). Although the predictions of this model are reasonably accurate, it is not always possible to predict whether a particular oil will emulsify under specified environmental conditions in the field and what the final water content will be. Often at the time of a spill, the critical compositional data (percent saturates, asphaltenes, resins, etc.) for the oil are not immediately available, and as a rough approximation the >343° C (roughly >650° F) boiling point fraction has been used as a surrogate in predicting whether mousse formation is likely (NRC, 1989). If that fraction is greater than 40 percent, the oil may emulsify and be difficult to disperse.
Empirical models of oil dispersibility with Corexit 9500—as measured in the swirling-flask laboratory test—were also recently developed (Fingas et al., 2003b). These models, which range in complexity from two (viscosity and density) to fourteen parameters, were developed by determining the effects of twenty-nine physical and chemical properties on oil dispersibility. Viscosity was found to be the most important physical property in determining dispersibility, but various aspects of chemical composition (e.g., the concentrations of n-dodecane, n-hexacosane, and naphthalenes) were more highly correlated. The most effective models were used to predict the dispersibility of 295 oils in the Environment Canada oil properties catalog (Environment Canada, 2005). Although these correlations may be useful for predicting and ranking the dispersibility of a large number of oils, the authors caution that the laboratory tests (upon which the correlations are based) may not provide a direct representation of what can be obtained in the field where different salinity and energy regimes are likely to be encountered.
Based on the above considerations and from practical experience, it is evident that response actions using dispersants should be initiated as soon as possible, and every effort should be made to apply the dispersants before significant oil weathering has occurred (usually within 24–72 hours in temperate conditions and possibly within 12–24 hours during the winter and under arctic conditions) to improve the probability of success. It should be noted that increased viscosity and water content in an emulsion also affect the ability to treat spilled oil by other response methods. For example, increased viscosity makes oil harder to pump, and increased water content increases the volume of material that must be handled and stored. For heavier oils, water contents above 20–30 percent make in-situ combustion essentially impossible (Twardus, 1980; Fingas and Punt, 2000).
Weather Considerations and the Window of Opportunity
Another important factor to be considered in evaluating the window of opportunity for effective dispersant applications is the energy regime at the time of dispersant application. As discussed in the previous section, a certain minimum energy (i.e., wind speed of 5 m/s; Allen, 1988; Fingas and Ka’aihue, 2004a), is required to break up the oil slick into small droplets, but applications under higher energy conditions can be plagued by other factors, such as:
-
dispersant drift in the wind (missing the target as discussed in Chapter 2),
-
possibly washing the dispersant off the slick before it penetrates into the oil phase, and
-
the fact that the benefits of dispersant application begin to diminish compared to natural dispersion at wind speeds of 12–14 m/s (Allen, 1988; Fingas and Ka’aihue, 2004a).
Likewise, when dispersants are applied under low-energy conditions (little or no wind and/or reduced sea states), there may be a time lag between dispersant application and a subsequent increase in sea state (energy regime) to enhance dispersion. This delay also can lead to the potential for leaching of the dispersant from the oil phase before there is sufficient energy to promote droplet dispersion. It is believed that this problem might be avoided with some of the newer hydrocarbon-solvent-based dispersant formulations (or by additional adjustments to the HLB), but no studies on leaching of surfactants from the oil phase have been conducted at realistic oil-to-water ratios and under different energy regimes to test this hypothesis. In particular, the effects of surfactant leaching on the effectiveness of initial oil dispersion and the potential for dispersed oil droplet coalescence should be understood better. Recent laboratory studies have shown that surfactants do not appear to inhibit droplet coalescence, but the behavior of dispersed droplets and the concomitant leaching of surfactant under conditions of high dilution have not been studied. This is important because it will eventually affect dispersed oil behavior and the potential for re-surfacing in the field.
HISTORY OF DISPERSANT USE IN THE UNITED STATES
At the time of this writing, dispersant use in the United States had been limited to spills in Alaska (i.e., the T/V Exxon Valdez spill in 1989) and a series of smaller spills in the Gulf of Mexico (spanning 1999 to 2004). Understanding the circumstances and results of these actions provides
some insight into the consequences of dispersant use, and thus is summarized below.
T/V Exxon Valdez Oil Spill (EVOS), Prince William Sound, Alaska (1989)
Spilled Oil Type/Volume/Conditions
An estimated 38,000 tonnes (roughly 250,000 bbls) of Alaska North Slope crude oil were released from T/V Exxon Valdez when it grounded on Bligh Reef in northeast Prince William Sound, Alaska, on March 23, 1989. Alaska North Slope crude has an API gravity of 29.8, a relatively high asphaltene content, and tends to form stable emulsions. Weather conditions were calm and clear.
Physical and Biological Setting
Prince William Sound includes many narrow fiords with deep, cold (<5° C [roughly 41° F]) seawater of low salinity and modest circulation. Rocky outcroppings and gravel beaches are common. There are an extensive local fisheries for both finfish and shellfish, as well as robust sport-fishing and tourist industries throughout the Sound.
Dispersant Application
Two weeks prior to the spill, the Alaska Regional Response Team had adopted the first pre-approval zones for dispersant use in the United States. The spill occurred in Zone 1, where the state and federal coordinators could approve dispersant use on their own authority. Stockpiles of Corexit 9527 were available locally in Valdez, Anchorage, and Kenai. Both helicopters and large military C-130s were available within the state. However, there were no large capacity application packages (e.g., ADDS pack) in Alaska, and only a single helicopter bucket spray system was stored in Kenai (Alaska Oil Spill Commission, 1990)
Twelve hours into the spill, the helicopter bucket system arrived in Valdez and was immediately loaded with Corexit 9527 and used on the evening of March 24, and again on the morning of March 25. A third attempt on the morning of March 26 failed due to applicator malfunction. A fourth and final helicopter application occurred late in the afternoon of March 26. The first large-scale dispersant application occurred on the morning of March 27, 80 hours into the spill. In total, 5,500 gallons (roughly 20,800 liters) of Corexit 9527 were applied by C-130 (Alaska Department of Environmental Conservation [ADEC], 1993).
Monitoring Results
The U.S. Coast Guard and State of Alaska agreed that, on the first two days of helicopter applications, calm conditions did not supply sufficient mixing energy to achieve any noticeable effects. On the evening of the third day, visibility was poor and visual monitoring of the final helicopter application was inconclusive. Nevertheless, with the weather picking up, the decision was made to allow full-scale application in Zone 1 with a one-mile exclusion zone around the grounded tanker. Unfortunately, both T/V Exxon Valdez and the lightering tanker Baton Rouge were heavily sprayed during the next application, forcing a suspension of this extremely vital and difficult operation in order to decontaminate both personnel and equipment. No other effects of this dispersant application were observed. The State of Alaska, citing Exxon’s inability to “accurately and effectively target the dispersant,” declined to allow further dispersant application outside of Zone 1 (Alaska Department of Environmental Conservation, 1989). In any event, a large storm arrived with 40–70 knot (roughly 74–129 kilometers per hour) winds. The window for dispersant use was closed.
In its final report on the T/V Exxon Valdez oil spill, ADEC felt it necessary to state, “There was never a case in which loaded dispersant planes were held on the ground because the government couldn’t or wouldn’t make a decision” (Alaska Department of Environmental Conservation, 1993, p. 58).
Gulf of Mexico (1999 to 2004)
Between 1999 and 2004, dispersants were used seven times to combat oil spills in the Gulf of Mexico. In six of these cases, dispersants were used under the existing pre-approval plan for oil spills greater than 3 nautical miles offshore and in waters of greater than 10 m depth. Four of these dispersant cases are summarized below.
High Island Pipeline Spill (January 1998)
Approximately 360 tonnes (roughly 2,500 bbls) of South Louisiana crude (API gravity 38.2) were treated with Corexit 9527 using DC3 and DC4 aircraft. The application was very successful, based on aerial observations, SLAR measurements that showed decreased slick size, and SMART monitoring using field fluorometers that showed increased dispersed oil concentrations under the treated slick (Gugg et al., 1999).
BP-Chevron Pipeline Spill (October 1998)
Between 530 and 1,070 tonnes (roughly 3,700–7,500 bbls) of South Louisiana crude (API gravity 28.6) were released during a routine pipeline transfer operation at an offshore oil platform. Approximately 12,000 L of Corexit 9500 and 6,650 L of Corexit 9527 were applied to two of the three oil slicks over a period of two days using DC3 and DC4 aircraft. Visual observations suggested that the dispersant application was successful, but no confirmatory water-column data were obtained due to malfunction of the in-situ fluorometer that was deployed with the on-water monitoring team. Chemical analysis of water samples collected from the area of one of the treated slicks on the second day of dispersant operations showed only low concentrations of dispersed oil in the water column. British Petroleum estimated that approximately 160 tonnes (15 to 31 percent) of oil were chemically dispersed based on an assumed 80 percent effectiveness on the first day and 60 percent effectiveness on the second day, but these values were not independently confirmed by NOAA or the U.S. Coast Guard. ADIOS modeling predicted that about 33 percent of the oil was removed by evaporation. Only about 3 bbls of oil were recovered by mechanical response. This dispersant operation was considered to be successful due in part to the quick and aggressive chemical-treatment response and the good dispersibility of the oil (C. Henry, National Oceanic and Atmospheric Administration, New Orleans, Louisiana, written communication, 2004).
M/V Blue Master Spill (August 1999)
Approximately 17 tonnes (roughly 100 bbls) of IFO 180 (specific gravity of 0.988) were released from the M/V Blue Master following a collision with a fishing vessel 55 km south of Galveston, Texas. With light winds and calm seas, the oil was concentrated in a current-generated convergence zone. Within 12 hours after the spill and just before dark, 2,660 L of Corexit 9500 were applied (ratio of 1:6). Next-day observers reported a marked reduction in heavy concentrations of oil. It was considered a “cautious success” because only 0.25 tonne (roughly 1.8 bbls) of tarballs stranded onshore two weeks later (Kaser et al., 2001). Water-column oil concentrations were not measured to confirm that dispersion occurred.
Poseidon Pipeline Spill (January 2000)
Approximately 290 tonnes (roughly 2,000 bbls) of S. Louisiana crude (API gravity 31.5) were released from a 24 inch (roughly 60 cm) pipeline 65 miles (roughly 110 km) south of Houma, Louisiana that was caught
and dragged by a large anchor. Due to 1–2 m seas, mechanical recovery was determined to be ineffective. Within 7 hours after the release, 11,400 L of Corexit 9527 were applied by DC3 and DC4 aircraft, resulting in an estimated 75 percent effectiveness, based on visual observations and fluorometry measurements. The next day, another 3,800 L were applied to the remaining patches of dispersible oil. There was no visual observation of a dispersed oil plume, but fluorometry did detect increased oil concentrations in the water under the treated oil. By the end of the second day of the release, it was determined that the remaining oil slicks were not dispersible. The applications were considered to be highly successful (Stoermer et al., 2001).
In summary, dispersants have been used successfully on oil spills in the Gulf of Mexico on several occasions in the past seven years. Because of the close proximity of dispersant application resources, responders were able to mobilize dispersant operations relatively quickly, which may have contributed to the overall success. Effectiveness, however, was evaluated primarily by visual observation, and not all operations included confirmation by measurement of dispersed oil in the water column. Therefore, the reliability of effectiveness estimates is unknown.
EFFECTIVENESS TESTING AND EFFECTIVENESS ISSUES
The overall effectiveness of oil dispersion has three components: (1) operational effectiveness, which describes the encounter probability of the dispersant application and the ability of the dispersant to become incorporated into the floating oil, (2) chemical effectiveness, which is measured by the fraction of treated surface oil that becomes stably entrained as small droplets in the water column, and (3) hydrodynamic effectiveness, which describes the transport of the chemically dispersed oil plume and its dilution by turbulent diffusion through horizontal and vertical mixing processes. The main focus of this section is a review of the experimental methods that have been used to investigate the chemical effectiveness of oil dispersants, but because the effectiveness that would be realized during spill-response operations at sea is determined by the interaction of all three components, those aspects of operational and hydrodynamic effectiveness that can be studied in effectiveness tests are identified and discussed where appropriate.
Operational effectiveness is determined by site-specific parameters, such as the patchy distribution of oil on the water surface, the ability to accurately target and hit the thicker parts of oil slicks with the dispersant spray, and the size distribution and impact velocity of dispersant droplets that hit the floating oil (as discussed in Chapter 2). It is difficult to simulate important characteristics of dispersant application in laboratory-scale
experimental systems due to their relatively small size. Some large wave tanks can investigate many, but not all aspects of operational effectiveness. Operational effectiveness can be tested best in studies conducted at sea, provided the scale of the experiment is sufficient. Monitoring of operational effectiveness is the primary objective during real spill applications.
Hydrodynamic effectiveness is discussed primarily in Chapter 4 because it is governed by the transport of the dispersed oil plume. Hydrodynamic effectiveness cannot be tested in laboratory-scale systems or wave tanks, because significant dilution can only occur due to externally imposed flow through the system, not due to eddies of varying scales (e.g., turbulent diffusion and hydrodynamic dispersion). In principle, full-scale field studies can test hydrodynamic effectiveness, but appropriate measurements can be difficult and this is not always done.
Chemical effectiveness has been investigated in the laboratory, in wave tanks, and at sea. In many of these studies, effectiveness was defined based on chemical effectiveness, which was quantified as the mass fraction of oil that was measured in samples collected from the water column or the mass fraction that was not recovered from the water surface as floating oil. This definition has resulted in some confusion when attempting to compare studies conducted using different experimental systems, because these effectiveness metrics are operationally defined and measure different things in different systems. For example, some experimental designs include oil droplets that are large enough to resurface relatively quickly in the dispersed-oil concentration (e.g., those that measure water-column oil concentrations during periods of intense mixing), whereas others do not (e.g., those that include a settling period before measurement of dispersed-oil concentrations). Similarly, oil that is not recovered on the water surface may have been transferred to any of several compartments, of which the water column is only one. The droplet-size distribution of dispersed oil is a particularly important factor for chemical and hydrodynamic effectiveness, because it will determine whether the entrained oil will remain in the water column or float back to the surface under low energy conditions, which are unlikely to be the same during spill-response operations and effectiveness tests, regardless of the scale of the test. Future studies should include measurement of droplet-size distribution or some related metric to facilitate comparison among treatments. Lunel (1995b) has suggested that effectiveness tests should produce droplet-size distributions similar to those observed at sea, because this indicates similarity in the droplet-formation mechanisms.
Objectives of Effectiveness Testing
Dispersant effectiveness testing is performed using experimental systems that encompass a wide range of physical scales, from small (hundreds of milliliters) bench-scale systems to large (thousands of cubic meters) wave tanks, to open-ocean testing. All experimental systems used to evaluate dispersant effectiveness suffer from significant limitations; thus, it is important to clearly identify the objectives of the investigation before selecting an experimental system and designing an effectiveness study. Investigations of dispersant effectiveness are conducted for several common reasons: product screening; comparison of commercially available products for specific applications; fundamental investigations into the mechanisms that control dispersion of floating oil; and prediction of dispersant effectiveness under spill-response conditions. These objectives are quite different, and the experimental designs should reflect the differing requirements for data quality and application.
Effectiveness tests can be grouped into four broad categories: bench-scale tests; wave-tank tests; planned field studies; and spills of opportunity. Bench-scale tests often involve relatively common equipment, such as flasks and separatory funnels that are adapted or modified for the specific purpose of testing dispersants. They are also called laboratory-scale tests. Most wave tanks or hydraulic flumes are relatively small, but at least one, the Oil and Hazardous Materials Simulated Environmental Test Tank (OHMSETT), is very large. Although both are considered in the same category, the advantages and disadvantages of large vs. small wave tanks for dispersant effectiveness tests can be significant. Planned field studies and spills of opportunity also have many similarities, but the advantages and disadvantages are sufficiently different that they are considered separately. In general, as the physical scale of an effectiveness test increases, the cost and realism (i.e., the degree to which the test includes all three components of effectiveness) increase, but the degree to which the factors that affect dispersion effectiveness can be controlled and the ability to quantitatively measure effectiveness decrease. As a result of these competing trends, especially between realism and control, effectiveness tests at different scales are appropriate for achieving different objectives, and experimenters should be careful to match the objectives with the appropriate experimental scale.
Screening of dispersant products is often conducted for regulatory purposes. In the United States, dispersant products must be on the National Contingency Plan (NCP) Product Schedule to be considered for use as a response alternative for oil spills in U.S. marine and coastal waters (EPA, 2003). Inclusion on the NCP Product Schedule is contingent on demonstration that the candidate dispersant is capable of dispersing at least
45 percent of South Louisiana crude oil and Prudhoe Bay crude oil in the laboratory-scale swirling flask test. Although the specific method used in this procedure is likely to change in the near future, the objective of this test remains the simple demonstration of a prescribed degree of chemical effectiveness as measured by the concentration of oil in water samples collected from the bottom of the flask after a specified settling period to allow larger droplets to return to the water surface. The outcome is a pass-fail decision: if the product achieves the prescribed degree of dispersion, it may be included on the NCP Product Schedule (assuming it meets all other required criteria, such as successful toxicity testing); if it does not, the dispersant will not be included on the NCP Product Schedule, and it cannot be used in the United States as an oil spill countermeasure.
A related objective of effectiveness testing is comparison of available dispersants for specific applications, such as their ability to disperse specific crude oils or refined products under the environmental conditions that are known to prevail in certain regions (Blondina et al., 1997; Moles et al., 2002; White et al., 2002; Stevens and Roberts, 2003). These tests often attempt to compare the performance of specific oil-dispersant combinations under defined or standardized testing conditions. The results of these studies are intended to provide guidance for spill responders and regulators regarding selection of appropriate response actions or products. Due to the very large number of potential oil-dispersant combinations, the wide range of environmental conditions that may need to be considered, and the difficulty of extrapolating performance data beyond specifically tested conditions, these tests should be relatively simple. As a result, these comparisons are often conducted in bench-scale systems, but more limited testing has also been conducted in wave tanks and at sea.
Effectiveness tests may also be used in fundamental investigations of the mechanisms that control natural or chemically enhanced dispersion of oil into water (Belk et al., 1989; Fingas et al., 1991; Blondina et al., 1999; Canevari et al., 2001; Chandrasekar et al., 2003). Factors that have been investigated include dispersant-to-oil ratio (DOR), salinity, dispersant characteristics (e.g., hydrophilic-lipophilic balance, surfactant chemical structure, solvent characteristics), mixing energy, and the physical-chemical characteristics of the oil. Again, due to the wide range of conditions that may be of interest, the requirement for appropriate control treatments, and the need to rigorously control experimental conditions to facilitate testing of specific mechanisms, bench-scale systems are often used for these studies.
Ultimately, the objective of most effectiveness tests is to provide insight into the potential effectiveness of dispersants under actual spill-response conditions. Although most spill responders agree that quantitative prediction of dispersant performance is extremely difficult—if not
impossible—based on current understanding of the factors that control it, the decision-making process during oil spill response involves implicit assumptions regarding expected effectiveness. For example, most oil transport and fate models that include an option for simulating dispersant application (e.g., French-McCay and Payne, 2001; Lehr et al., 2002; Simecek-Beatty et al., 2002; French-McCay, 2004) use effectiveness estimates as model inputs. These estimates are often based on experience and professional judgment rather than extrapolation from effectiveness tests and, as such, are not predictive. A major goal of chemical dispersant research should be development of quantitative tools for predicting dispersant performance (i.e., mathematical models) that can systematically incorporate many different types of information and the best current scientific understanding regarding droplet-formation and transport mechanisms. Ideally, dispersant effectiveness would be an output of a mathematical model, and the inputs would be factors such as oil characteristics, weather conditions, and other operational factors (e.g., dispersant type, effective DOR). Although multiple-regression models that relate oil dispersibility in a lab-scale effectiveness test to chemical composition have been proposed (Fingas et al., 2003b), these are completely empirical and cannot predict performance in the field—due at least in part to the inability to scale performance predictions from laboratory conditions to the field—and are not, therefore, useful for this purpose. Regardless of whether the predictions are quantitative (i.e., based on a mathematical model) or qualitative (i.e., based on the judgment of experienced professionals), effectiveness tests may provide the needed input parameters. In order to be useful, however, the effectiveness tests should be properly designed and the results should be interpreted with appreciation of their strengths and limitations.
Design of Effectiveness Tests
Effectiveness tests, regardless of the specific objectives or configuration of the experimental system, should explicitly consider how the experimental design will affect the results. Factors that are known to affect the extent of oil dispersion should be carefully controlled or characterized to the extent that is possible given the configuration of the experimental system. Examples of such factors include but may not be limited to the following: physical and chemical characteristics of the oil; physical characteristics of the surface slick; oil-water and dispersant-oil ratios; salinity and temperature; physical and chemical characteristics of the dispersant; method used to apply the dispersant to the oil; energy provided to disperse the oil; and the method used to measure effectiveness. In addition, the experimental design should include a clear description of the data
analysis procedures that will be used, especially those used to estimate the random error term in the response variables, which is required in order to compare treatments. When possible, experimental designs should include independent replication of treatments and appropriate controls. Positive as well as negative controls (as discussed below) should be included whenever possible. Although these principles can be applied at all scales at which dispersant effectiveness can be tested, time and financial constraints will limit the degree to which they can be implemented as the scale of the test system increases. Such practical limitations, however, make clear definition of objectives and careful experimental design more—not less—important with increasing scale.
Among the factors that affect dispersion efficiency, the physical characteristics (e.g., pour point, viscosity, density) and chemical composition (especially aliphatic, aromatic, and asphaltic hydrocarbon concentrations) of the oil have received considerable attention. These characteristics are important because they can vary greatly among oils from different sources and change relatively quickly as oil weathers following a spill. Viscosity, which is roughly correlated with API gravity and density (Speight, 1991), has long been recognized to be an important parameter controlling the efficiency of oil dispersion (Daling, 1988), but viscosity alone is an insufficient predictor of dispersion efficiency (Fingas et al., 1991; Canevari et al., 2001). As a result, the chemical composition of the oil has also been considered, with various investigators identifying either positive or negative correlations between chemical effectiveness and the aliphatic, aromatic, polar, and asphaltene fractions of oil (Fingas et al., 1991, 2003b; Blondina et al., 1999; Canevari et al., 2001). Unfortunately, the nature of the relationships between composition and dispersion effectiveness is not well understood, and many of the results are contradictory. So, additional well-planned investigations are needed. It seems likely that some of the confusion may be due to unrecognized or unquantified differences among the experimental systems, such as the energy input or the characteristics of the oil droplets that are measured as dispersed. Therefore, future experiments should measure energy dissipation rates and the droplet-size distributions of dispersed oil. The dynamic changes that can occur in physical properties and chemical composition of oil during weathering make empirical investigation of these relationships particularly complex.
An important interaction likely exists between the physical characteristics of the oil and the method of dispersant addition. The dispersant must penetrate into the oil phase to effect dispersion, and certain physical characteristics (e.g., high viscosity) of the oil can prevent this from occurring efficiently (Canevari, 1984). Some investigators have suggested that evaporative weathering of waxy crude oils can lead to the formation of a viscous “skin” (Berger and Mackay, 1994) that may provide additional
resistance to dispersant penetration. Few dispersant effectiveness tests use realistic weathering or dispersant application methodologies, and pre-mixing the dispersant with oil is not uncommon. Many bench-scale tests add dispersant to floating oil, but the drop size is typically much larger (>1,600 µm diameter) than would be expected from a typical spray system (350–500 µm; NRC, 1989). For example, several studies involved addition of dispersant to floating oil in volumes ranging from 2 to 10 µL (Blondina et al., 1997, 1999; Venosa et al., 2001; Sorial et al., 2004a), which correspond to droplet diameters ranging from about 1,600 to 2,700 µm. Droplet velocity at impact with the oil is another important aspect of dispersant application that is not adequately simulated in existing bench-scale effectiveness tests. In general, this aspect of dispersant effectiveness, which would be considered operational, is not adequately characterized or controlled in most existing effectiveness tests at any scale. Wave tanks provide the most appropriate system for investigating the relationship between dispersant penetration and oil characteristics, because these systems are large enough to use realistic dispersant application systems (e.g., spray booms with typical nozzles) and can be controlled well enough to characterize the fraction of dispersant droplets that come into contact with floating oil. Therefore, the effects of oil characteristics (e.g., chemical composition, rheological properties, extent and mechanism of weathering) on the ability of dispersants to interact effectively with the oil should be investigated in future wave-tank studies and should be considered when interpreting the results of field-scale effectiveness tests.
The DOR and oil-to-water ratio (OWR), both typically measured on a volume-to-volume basis, are critical factors affecting dispersion effectiveness. Several investigators have shown a direct relationship between DOR and dispersion efficiency (Fingas et al., 1991; White et al., 2002); a DOR of 1:25 is commonly used, but this value can vary by a factor of two or more in either direction in some studies. The OWR of experimental systems for testing the chemical effectiveness of different dispersants can vary over a much larger range, with the values of lab-scale systems reportedly ranging from 1:1 to 1:120,000 (Fingas et al., 1989). The OWR affects the efficiency of oil dispersion in a variety of ways, some of which can have opposing effects. For example, anionic and nonionic surfactants with a high HLB will tend to partition into the aqueous phase where they cannot effectively promote formation of small oil droplets. The extent of partitioning will be determined in part by the OWR: when the OWR is high, more of the surfactant will be associated with the oil phase where it can facilitate droplet formation. Alternatively, high OWR could reduce the observed dispersion efficiency by increasing the rate of droplet coalescence, which is proportional to the number concentration of oil droplets (NRC, 1989). Droplet coalescence will produce larger oil droplets that can resur-
face more quickly and reduce the mass of oil entrained in the aqueous phase.
One of the most important factors in dispersant effectiveness testing is energy dissipation rate (e.g., mixing energy). Energy is required to create new oil-water interfacial area, which occurs when an oil slick breaks up into dispersed oil droplets. Successful oil dispersion will increase the oil-water interfacial area by a factor of ten or more, and sufficient energy should be provided to form the new oil-water interfacial area. Increased mixing energy, therefore, should result in the formation of smaller droplets (i.e., larger oil-water interfacial area). Because smaller droplets will have less tendency to resurface, higher mixing energy should result in more efficient and more stable dispersion. Energy dissipation rate is a parameter that varies widely among experimental systems, and differences among the results obtained with various systems are often attributed to differences in this parameter. Despite its importance, the energy dissipation rate is not measured in most dispersant effectiveness tests, and the relationship between mixing energy and effectiveness is only rarely investigated (Kaku et al., 2002; Fingas, 2004b). When it is, however, dispersion effectiveness is found to be directly proportional to mixing energy, but the proportionality varies among oil-dispersant combinations (Fingas et al., 1996a; Sorial et al., 2001; Chandrasekar et al., 2003). Predicting dispersant effectiveness for spill response based on bench-scale or wave-tank studies is hampered by our lack of understanding of the effect of mixing energy on oil dispersion for specific oil-dispersant combinations and the relationship between energy dissipation rates that prevail in common experimental systems and typical values at sea (1 to 10 J-m−3-s−1 in open-ocean surface) (Delvigne and Sweeney, 1988).
An important aspect of any experimental design is identification and measurement of the endpoint. For dispersant effectiveness testing, the endpoint is often defined to be the percent of added oil that is dispersed into the water column. For larger-scale systems, such as wave tanks and field studies, the water-column sample collection protocols can affect the observed effectiveness because the distribution of dispersed oil droplets is likely to be heterogeneous (Brown et al., 1987; Brown and Goodman, 1988; Lewis and Aurand, 1997). The concentration that is measured will depend on the location at which the sample is collected, and multiple samples will be required to characterize the distribution and estimate the total mass of dispersed oil. The mass of floating (non-dispersed) oil remaining on the surface is sometimes measured in wave tanks (Brown et al., 1987; Brown and Goodman, 1988; Louchouarn et al., 2000; Belore, 2003; Bonner et al., 2003) and field studies (Lewis et al., 1995a,b, 1998a), but many errors can be reflected in these measurements, including incomplete recovery of floating oil, unquantified losses due to evaporation, dis-
solution, or sorption to surfaces in the experimental system, and uncertainty in the distribution of floating oil (e.g., the size of the slick and variations in slick thickness with position; Fingas and Ka’aihue, 2004c).
In laboratory-scale tests, chemical effectiveness measured as percentage of oil dispersed into the water column is very sensitive to the settling time that precedes collection of samples, regardless of which method is used to measure the dispersed oil concentration (Fingas et al., 1989; Daling et al., 1990b; Venosa et al., 1999). This sensitivity is largely due to resurfacing of large oil droplets. Experimental methods that measure the dispersed oil concentration while mixing is occurring (e.g., the Institute Francais du Petrole and Mackay-Nadeau-Steelman tests) tend to result in greater “effectiveness” than those that involve a discrete settling period (e.g., the Labofina, swirling flask, and baffled flask tests). Coalescence of oil droplets, which is promoted by high oil-to-water ratios, can further decrease the measured effectiveness for tests that involve a settling period. Like mixing energy, settling periods vary among effectiveness tests, ranging from zero to about ten minutes. Because mixing energy affects the droplet-size distribution, which will affect the fraction of dispersed oil that resurfaces during the settling period, interpretation of dispersion effectiveness is difficult when the only endpoint is percentage of oil dispersed into the water column. As a result, more generally useful information would be obtained if effectiveness tests measured droplet-size distribution in addition to the mass fraction of oil dispersed into the water column or remaining on the water surface.
An objective of dispersant effectiveness testing at all levels is to determine whether addition of a chemical dispersant to a floating oil slick will increase the amount of oil that is transferred into the water column as small droplets relative to the amount that would be transferred from an untreated oil slick or from a slick treated with a different dispersant. This implies that a comparison should be performed to achieve the objectives of the experiment. For example, if one wishes to determine whether a particular dispersant is effective on a particular oil, the extent of dispersion that occurs for the oil-dispersant combination under specified conditions of temperature, salinity, and mixing energy should be compared to the extent of dispersion that occurs when the oil is exposed to the same conditions in the absence of dispersant. Such a comparison should involve estimation of the uncertainty in the amounts of dispersed oil measured in the presence and absence of the dispersant. The statistical significance of the effect of the dispersant is determined by estimating the probability that the difference in the amount of dispersed oil observed in the presence and absence of dispersant could be due to chance (i.e., the probability that a similar difference would be observed if the experiment were conducted without application of dispersant to either treatment).
The most reliable method for estimating the uncertainty in a measurement is to repeat it several times under identical but independent conditions. Independence of replicate measurements requires, at a minimum, that they be performed in separate experimental units (Hurlbert, 1984; Ruxton and Colegrave, 2003). In addition, some experimental designs, especially those involving large physical scales (e.g., field studies, large wave tanks), may require replication over time (see Box 3-2). Because the experimental conditions (e.g., weather) may vary from day to day, the replicates for different treatments should be interspersed in time to preclude the possibility that factors other than the treatment(s) under investigation will result in endpoint differences that are correlated with the treatment.
When an experimental design requires tests to be conducted over a prolonged period of time or by different analysts, precautions should be taken to ensure that results are comparable. That is, a mechanism should
|
BOX 3-2 Dispersant effectiveness is often quantified by measuring the amount of oil that is transferred to the water column or remains on the surface (or both) following application of a dispersant and mixing energy. All measurements are subject to some error, thus, the measured effectiveness is an estimate of the true effectiveness. The quality of this estimate is determined by its accuracy and precision. Accuracy is a measure of the agreement between the estimate and the true value, whereas precision provides an estimate of the reproducibility of replicate measurements. Since the true effectiveness is unknown, the accuracy cannot be independently evaluated, but the precision is used to identify a range that is likely to contain the true value. This range of values, sometimes called a confidence interval, is often used to compare one estimate of dispersant effectiveness to another (e.g., the extent of dispersion observed for dispersant-treated oil might be compared to the extent observed for an untreated control or to a threshold value specified by a regulatory agency). Statistical analysis is used to determine the probability that the two values that are being compared both estimate the same true effectiveness and appear to be different only due to the effects of random errors. Two types of errors can cause measured estimates of dispersant effectiveness to be different from the true values: systematic errors and random errors. Systematic errors affect all measurements in the same direction, and therefore, bias the estimate. Evaporation of volatile compounds and incom- |
exist to identify errors caused by differences in procedures or reagents that are not related to the treatment that is under investigation. One such mechanism is the use of controls. Controls are treatments (i.e., tests) that are performed periodically throughout a study for the purpose of quality control. Positive controls usually involve treatment with a reagent or procedure that produces a well-known and predictable result. A negative control usually involves measurement of the background response variable in experimental systems that are either untreated or treated with a mixture containing the inert ingredients (e.g., solvent) but lacking the active ingredients (e.g., surfactants). For oil dispersant effectiveness tests, a positive control might involve treatment of a standard easily dispersible oil with a standard dispersant under standard conditions. A negative control might involve subjection of the same standard oil to the physical conditions that would be applied in the dispersant test but without application of a chemical dispersant. Positive and negative controls are often
|
plete recovery of floating oil are two examples of systematic errors that can introduce a positive bias in estimates of dispersant effectiveness when the mass of oil remaining on the surface after treatment is used as the measure of effectiveness (i.e., the measured effectiveness will be greater than the true effectiveness because processes other than dispersion can reduce the mass of recovered oil). Random errors, which can be introduced by uncontrolled (or uncontrollable) variations in experimental conditions or measurement technique, will reduce the likelihood that two independent measurements of dispersant effectiveness will produce the same result even when they are made under nominally identical conditions. For example, small variations in the energy input or dispersant-to-oil ratio may cause the measured extent of dispersion to be different in replicate effectiveness tests. If a sufficiently large number of independent replicate measurements are made, however, positive errors will be offset by negative errors, and the mean (or another appropriate measure of the central tendency of the distribution) will be approximately equal to the true effectiveness. The more replicate measurements that are made, the closer the mean of those replicates is likely to be to the true effectiveness. Statistics can be used to quantify and correct for the effects of random errors, but systematic errors can only be mitigated by proper experimental design (including using appropriate experimental systems, sample collection procedures, and measurement techniques) and careful experimental technique. Proper experimental design should include provisions that eliminate systematic errors, minimize the size of random errors by controlling known sources of variation, and quantify the magnitude of unknown or uncontrollable random errors. |
compared to expected values, which may be determined from experience with the experimental system, and if the results are outside of a predetermined range, the tests should be repeated. Use of a standard oil in controls during dispersant effectiveness testing requires that the characteristics of the oil remain constant over time. As such, the oil should be stored under conditions that prevent evaporation, photooxidation, and other changes in the physical and chemical properties that can affect its dispersibility.
In the following sections, all of the four categories of tests for dispersant effectiveness are discussed, in terms of their roles and objectives, the types of systems and methods used, and advantages and disadvantages.
Bench-Scale Tests
Role of Bench-Scale Testing in Evaluating Dispersant Performance
Due to their relative simplicity, bench-scale tests are widely used to evaluate the performance of dispersants and the physical and chemical mechanisms of oil dispersion. Bench-scale testing has been used to screen dispersants for inclusion on both state and federal product lists (Blondina et al., 1997; Venosa et al., 1999; Sorial et al., 2001; Venosa et al., 2002), compare the relative effectiveness of specific dispersant-oil combinations (Fingas et al., 1991; Moles et al., 2002; Venosa et al., 2002; Stevens and Roberts, 2003), and investigate the effects of environmental conditions or oil composition on dispersion effectiveness (Belk et al., 1989; Fingas et al., 1991, 1996a; Blondina et al., 1999; Canevari et al., 2001; Moles et al., 2002; White et al., 2002; Chandrasekar et al., 2003). A critical review and comparison of bench-scale dispersant effectiveness tests was presented by Clayton and others (1993). In many cases, the ultimate goal of these studies was to provide guidance to spill responders regarding which dispersants are likely to work on which types of oil under what range of conditions. Although it is generally recognized that these results cannot be used to quantitatively predict dispersant effectiveness in the field, their use for the objectives described above implies a belief that they provide reliable relative rankings. Although the results of several bench-scale effectiveness tests may be weakly correlated, this assumed relationship has not been thoroughly investigated or subjected to rigorous peer review (Fingas et al., 1994; Fiocco et al., 1999).
Descriptions of Common Bench-Scale Testing Systems
Although bench-scale effectiveness testing is used to achieve a set of common objectives, several different types of experimental systems have
been used. The most common of these include the Warren Springs rotating flask or Labofina test, the Exxon dispersant effectiveness test (EXDET), the Mackay-Nadeau-Steelman (MNS) apparatus, the swirling flask test, and the baffled flask test.
The MNS apparatus (Figure 3-5), which is the largest of these four test systems, contains about 6 L of seawater in a 29 cm (ID) by 29 cm (depth) glass vessel (Mackay et al., 1978). Mixing is provided by tangential airflow (velocity usually between 6 and 20 m/s) over the water surface, which creates a circular flow pattern and surface waves between 2 and 4 cm in height. Oil is added to the water surface in the center of the vessel inside a 9-cm diameter aluminum containment ring. Dispersant is added to the oil inside the ring and allowed to soak for one minute before starting the airflow. The containment ring is removed as soon as airflow is started, and the oil spreads over the water surface while dispersion occurs. After mixing for ten minutes, but without stopping the airflow, a 500-mL water sample is collected from a sample port located 3 cm from the bottom of the vessel and 2 cm from the wall. Dispersed oil is extracted from the water sample into methylene chloride and the concentration is determined by measuring the absorbance at 580 nm. The circular motion of the water tends to minimize losses of oil to the vessel walls, but Fingas et al. (1994) found that, in the absence of dispersant, 13 to 19 percent of a light Bunker C adhered to the walls. Another effect of the circular motion of the water is that the radial distribution of dispersed oil is not uniform (Mackay et al., 1978). Because the sample is collected from a discrete location, the measured concentration may not be representative of the volume-averaged dispersed oil concentration. Although the MNS apparatus provides mixing in a way that is similar to what would be expected at the ocean surface (i.e., wind- and wave-driven shear at the air-water interface), the observed dispersion efficiency is very sensitive to small differences in the air-flow rate and angle of entry (Mackay et al., 1978; NRC, 1989), and the reproducibility, as measured by the performance of two apparatuses operating side-by-side in the same lab, was reported to be unacceptable (Fingas et al., 1989; Fingas et al., 1994). Note that the basis for this conclusion was that the standard error of dispersant effectiveness in replicate MNS apparatuses was 9 percent versus 3 percent in replicate swirling flask tests (described below) (Fingas et al., 1989) with maximum errors of 40 percent and 20 percent, respectively. Others, however, have raised similar objections to the swirling flask test, finding an average coefficient of variance (CV) of 22 percent with a maximum CV of about 160 percent (Sorial et al., 2004a,b). So, the reproducibility of many of these tests may be highly operator dependent.
The Warren Springs Laboratory (WSL; Labofina) test involves mixing of 5 mL of oil with 250 mL of synthetic seawater in a conical separatory
funnel (Figure 3-6). Dispersant is added to the oil surface dropwise, and the funnel is rotated end-over-end at 34 ± 2 rpm for five minutes. After mixing stops, the oil-water dispersion is allowed to stand for one minute to allow large oil droplets to rise, and a 50-mL sample is removed through the stopcock at the bottom. The oil in the sample is extracted into methylene chloride and the oil concentration is determined by measuring the absorbance at 580 nm. The WSL test is simple and reproducible, but observed performance was sensitive to the geometry of the separatory funnel. In addition, the standard test involves a very high oil-water ratio (1:50), which, as described above, tends to favor droplet coalescence. Alternatively the high oil-water ratio could result in unrealistically high aqueous-phase dispersant concentrations, which could increase the efficiency of dispersion by increasing the equilibrium concentration of the dispersant in the oil phase (NRC, 1989). Of these competing effects, droplet coalescence probably dominates in most cases, because Fingas et al. (1989) found that dispersion efficiency generally decreased with increasing oil-water ratio in the swirling flask test when the oil-water ratio was greater than 1:1000.
The Exxon dispersant effectiveness test (EXDET) is similar to the WSL test in that it is conducted in 250-mL separatory funnels, which are available in many laboratories. In the EXDET procedure, however, mixing energy is provided by a wrist-action shaker, which is also available in many
FIGURE 3-6 Picture of Warren Springs Laboratory (WSL; a.k.a., Labofina) dispersant effectiveness testing apparatus.
SOURCE: M. Fingas, Environment Canada.
laboratories (Becker et al., 1993; Clayton et al., 1993). So, EXDET has an advantage over many other dispersant effectiveness tests in that it does not require specialized equipment. Another advantage of the EXDET method is that it implicitly incorporates a mass balance. The procedure involves addition of a known volume of oil (e.g., 1 mL of oil premixed with dispersant at the desired DOR) to 250 mL of water in the separatory funnel, followed by shaking for 15 minutes on the wrist-action shaker. At the end of this mixing interval, but while shaking continues, a small absorbent pad is added to each funnel to collect the undispersed oil, and shaking continues for 5 minutes longer. Finally, the water is drained from each funnel and the dispersed oil is extracted with an appropriate solvent (e.g., methylene chloride, chloroform). The oil remaining in each 250-mL funnel, including that collected by the sorbent pad, is also extracted, and the concentrations of oil in both fractions (dispersed and undispersed oil) is measured by colorimetry (Becker et al., 1993; Clayton et al., 1993). The fraction dispersed is calculated by taking the ratio of the oil recovered in the aqueous phase to the sum of the oil recovered in both fractions. As long as the absorbances of the dispersed and undispersed oil fractions are within the linear range of the instrument, there is no need to calculate the exact mass of oil in each fraction, so calibrations curves are unnecessary. Of course, this procedure assumes that the oil collected by the absorbent pad is recovered completely and that no undispersed oil is transferred with the aqueous fraction, and any deviations from these assumptions will tend to cause the measured effectiveness to be higher than the actual effectiveness.
The swirling flask test (Figure 3-7) was developed to provide a simple method for screening dispersants (ASTM, 2000) and was adopted by the Environmental Protection Agency (EPA) for testing products for inclusion on the NCP Product Schedule (EPA, 2003; Sorial et al., 2004a,b). This test involves addition of 0.1 mL of oil to 120 mL of synthetic seawater in a modified 125-mL Erlenmeyer flask. Dispersant may be either premixed with oil (Sorial et al., 2001) or added to oil floating on the water surface (Blondina et al., 1997; Venosa et al., 1999). The flasks are mixed by swirling at 150 rpm on a gyratory shaker table, then allowed to settle for 10 minutes before a sample of the aqueous phase is collected by pouring through a glass spout that extends from the bottom of the flask upward to the neck. A recent variant of the swirling flask test, that involves collection of samples by draining water through a stopcock installed in the bottom of the Erlenmeyer flask, was developed to avoid reintroduction of oil droplets into the water phase when the flasks are tilted to pour from the spout (Blondina et al., 1997; Sorial et al., 2004a,b). This modification significantly improved the reproducibility of dispersion effectiveness measurements and reduced the extent of dispersion that was observed for five
FIGURE 3-7 Schematic representation of the glassware used in the swirling flask test. Water samples containing dispersed oil are collected by pouring through the spout attached at the bottom of the flask.
SOURCE: A. Venosa, Environmental Protection Agency.
of six oil-dispersant combinations. The most common method for quantifying dispersion effectiveness in the swirling flask test is measurement of the absorbance of long-wave ultraviolet light (e.g., averaging the absorbance at 340 nm, 370 nm, and 400 nm) by methylene chloride extracts of the aqueous samples collected after the settling period. Some investigators, however, have concluded that gas chromatographic analysis of these extracts is preferable, because this measurement is less sensitive to interference by dispersants and some oils have very low absorbance at the wavelengths of interest (Fingas et al., 1995c; Blondina et al., 1997). Others contend that the increased time required for analysis by gas chromatography coupled with its much lower precision make this alternative less attractive (Sorial et al., 2004a). Measurement of dispersed oil concentration by absorbance requires the use of appropriate calibration procedures, but with the exception of oils with extremely low absorbance at the target wavelengths, agreement between the two methods is very good (Fingas et al., 1995c; Fingas and Ka’aihue, 2004b). This difference in the method used to quantify the concentration of dispersed oil is only one of several ways in which the swirling flask test used by EPA to evaluate products for inclusion on the NCP Product Schedule (EPA, 2003) differs from the ASTM standard method (ASTM, 2000). Other important differences between these two versions of the swirling flask test include the use of synthetic seawater in the EPA version of the test versus sodium chloride in the ASTM test, use of a higher DOR in the EPA test (1:10 vs. 1:25 for the ASTM standard), and specification of a 0.75-inch orbital diameter for the
gyratory shaker used in the EPA test versus a 1-inch orbital diameter in the ASTM standard.
Another modification of the swirling flask test was introduced to overcome perceived limitations due to low energy input. This modification is known as the baffled flask test (Venosa et al., 2002; Sorial et al., 2004a,b). The baffled flask test uses a modified 150-mL trypsinizing flask that contains a stopcock near the bottom of the flask (Figure 3-8). These flasks have four baffles (i.e., indentations in the glass) at the bottom of the flask that increase turbulence during mixing by preventing development of a vortex due to the swirling motion of the gyratory shaker. The baffled flask
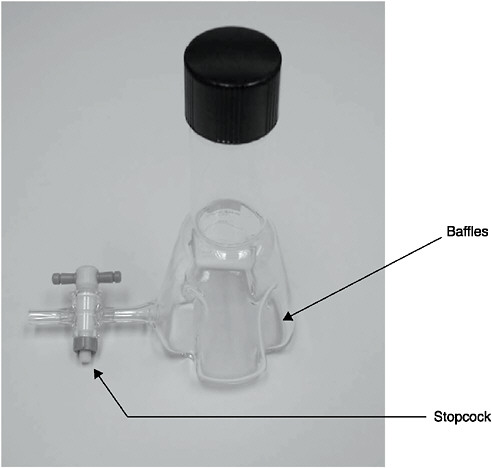
FIGURE 3-8 Photograph of the glassware used in the baffled flask test. This flask has four glass baffles at its base and a stopcock that allows collection of water samples containing dispersed oil by draining rather than pouring.
SOURCE: A. Venosa, Environmental Protection Agency.
test resulted in an average of four to five times more dispersed oil than the swirling flask test when eighteen dispersants were tested with two crude oils (South Louisiana and Prudhoe Bay). More importantly, the baffled flask test was much more precise than the swirling flask test (Sorial et al., 2004a). Dispersion effectiveness can be measured using the same methods as have been used for the swirling flask test, but measurement of the absorbance of methylene chloride extracts between 340 and 400 nm is preferred. Recently, the energy dissipation rates in the swirling flask and baffled flask tests were compared using a Hot Wire Anemometer to characterize the turbulence characteristics (e.g., the velocity gradient, G, and the energy dissipation rate per unit mass) of both systems (Kaku et al., 2002). Flask average energy dissipation rates in the swirling flask were about two orders of magnitude smaller than those in the baffled flask, and it was concluded that the turbulence in the baffled flask more closely resembled the turbulence occurring at sea during breaking waves.
Advantages and Disadvantages of Bench-Scale Testing
Bench-scale tests can be very useful for determination of the chemical effectiveness of oil dispersants. Because they are rapid and relatively inexpensive, bench-scale tests can evaluate a wide variety of experimental conditions in a relatively short period of time. As such, they are ideally suited for studies that are fundamentally empirical in nature (e.g., determination of the effectiveness of various dispersant-oil combinations, salinity or temperature effects on chemical effectiveness, relationships between oil composition or weathering and dispersant effectiveness). The relative ease with which treatments can be replicated independently in bench-scale studies is conducive to determining the statistical significance of any observed treatment effects, and statistically significant interactions among treatment factors can be identified using properly designed experiments. In addition, the use of small closed experimental units makes it relatively easy to perform mass balances (although this is often not done) for quality control purposes.
A major disadvantage of bench-scale testing is that it is difficult to scale the results to predict performance in the field because the test conditions do not simulate field conditions, especially energy regimes and dilution due to horizontal and vertical advection and turbulent diffusion. Scaling is difficult because the sensitivity of the response (e.g., dispersion efficiency) to variations in the test conditions is not well understood. In addition, although there has been some recent work in this area (Fingas, 2004a; Kaku et al., 2002), the energy input is rarely measured in common effectiveness testing systems. The generally poor correlation among performance estimates that are provided by different bench-scale systems
may be due, at least in part, to the poor characterization of treatment conditions in these systems.
To be most useful, future bench-scale effectiveness testing should incorporate the following modifications. First, energy dissipation rates should be determined for each system over a range of operating conditions. This will be accomplished more easily in some systems than in others, but this parameter has a very large effect on chemical effectiveness and should be characterized for proper interpretation of the results, especially when an objective is comparison with other experimental systems. Second, the chemical effectiveness should be determined over a range of energy dissipation rates. The strong, and possibly nonlinear, dependence of effectiveness on energy dissipation rate implies that measurement at a single condition will be less useful than determining the relationship between these variables. The wide range of energy dissipation rates that can be experienced at sea reinforces the importance of understanding the relationships between energy input and chemical effectiveness. Finally, the definition of chemical effectiveness should include measurement of the dispersed-oil droplet-size distribution in addition to its concentration. Careful determination of the relationships between energy input and droplet-size distributions for a variety of oils that differ in physical and/or chemical characteristics will provide the information that is necessary to determine whether a general predictive model of chemical effectiveness can be developed.
Wave Tanks
Role and Objectives of Wave-Tank Testing
Wave-tank tests are expensive and messy, but when carefully done, they can bring greater realism to the study of dispersants compared to bench-scale tests. As described above, laboratory studies measure only chemical effectiveness; effectiveness tests conducted in wave tanks have the potential to also include some level of operational effectiveness. In particular, dispersant application equipment that produces dispersant droplets with size distributions and impact velocities that are similar to those encountered in spill-response operations can be used in tank tests. The physical characteristics of most wave tanks, however, imply that the encounter probability of the dispersant with the oil slick will be higher than can be achieved during a real spill response. So, wave-tank tests provide upper limits on operational effectiveness. In addition to the added realism provided by the ability to include some aspect of operational effectiveness in the study design, the mechanism by which energy is provided to the dispersant-treated oil slick in wave-tank studies (i.e., waves)
is more similar to the mechanism that operates in the sea surface than can be accomplished in any bench-scale effectiveness test. As discussed earlier, there appear to be at least two mechanisms by which dispersed oil droplets can be generated: the dynamic pressure force of turbulent flows dominates at high Reynolds numbers and results in formation of relatively large droplets, whereas viscous shear due to small turbulent eddies dominates at low Reynolds numbers causing small droplets to form (Li and Garrett, 1998). Hence, mechanistic similarity might be important (i.e., energy dissipation rate alone might not be an adequate scaling factor for dispersant effectiveness). Of course, all tanks have walls. Therefore, no tank test will ever be completely realistic, because they cannot adequately incorporate the hydrodynamic effectiveness component. Nevertheless, given the costs and parameter-control difficulties associated with field tests, and the subjective nature of much of the data that can be collected, wave-tank tests are an important tool that can be used to tie the artificialities of laboratory studies to the operational realities of dispersant use in spill response. As such, wave-tank tests should be judged primarily on the basis of the additional realism—over laboratory studies—that is incorporated into their test design while remaining sufficiently controlled to allow replication and collection of quantitative data.
Before dispersants can be accepted by an informed public as a potential primary tool in oil spill response, several important issues need to be thoroughly investigated. In each of these areas, well-designed tank tests should be capable of furthering knowledge considerably.
(1) Structural Effects. Weathering is not uniform throughout oil slicks. Photooxidation and evaporation, especially for certain high wax-content oils, can result in formation of a highly viscous “skin” that may provide significant resistance to penetration of chemical dispersants (Berger and MacKay, 1994; Payne and Phillips, 1985a,b). Effectiveness tests that use oil that has been artificially weathered by evaporation of volatile components from a well-mixed bulk phase may overestimate the operational effectiveness (i.e., dispersant penetration of the floating oil) by underestimating the resistance provided by the viscous film that could be encountered by a dispersant droplet that contacts oil that weathered as a floating slick under natural sunlight. Thus, wave-tank tests that can simulate oil weathering as it would occur at sea (i.e., as floating slicks) should be conducted. These studies should also investigate the evolution of the physical-chemical characteristics and the operational dispersibility, as oil weathers in a slick.
In this regard, the formation of water-in-oil emulsions is particularly important in inhibiting dispersant effectiveness. To date, large wave tanks have not been used to examine the performance of dispersants on water-
in-oil emulsions generated from weathering of oil on the sea surface. Ideally, these emulsions would be generated in adjacent wave tanks or other systems that can provide continuous mixing of oil and water for hours to days. The effectiveness of dispersants on these blended emulsions could then be tested under more realistic field conditions. The rheological and chemical properties of the test emulsions should be characterized and compared to data from emulsified oil samples collected during actual oil spills. The dispersibility of the artificially generated emulsions should be tested over a range of temperatures, including cold, subarctic conditions. If this approach is successful, it could be expanded to investigate dispersant effectiveness on water-in-oil emulsions in the presence of ice.
(2) Dispersant Application. Dispersant application efficiency is affected by dispersant droplet size and velocity. If this aspect of operational effectiveness is to be investigated in wave tanks, the dispersant application system should simulate the droplet-size distributions and impact velocities that are characteristic of specific application methods (e.g., aircraft, helicopter, vessel). These parameters should be measured to verify that the desired characteristics have been achieved. The dispersant distribution over the target area also should be characterized at some point during these tests. In at least one instance, plastic sheet walls surrounding the tank were used to capture drifting spray, and trays were set up within the target area to measure the dosage that was applied to the oil (S.L. Ross, 2002). Although it is unlikely that the characteristics of real dispersant application systems can be accurately reproduced in a wave tank (even a very large one), measurement of effectiveness as a function of dispersant droplet-size distributions and impact velocity may provide information that can be used as input to dispersant effectiveness models.
(3) Mixing Energy. As described previously, mixing energy is one of the most important factors determining dispersant efficiency. Many oils will physically disperse even in the absence of chemical dispersants if sufficient mixing energy is provided. As with effectiveness tests at laboratory scales, mixing energy should be measured as a routine part of system characterization, and effectiveness should be measured over a range of mixing energies that span the range that can be realistically expected in the environment of interest. The wave energies used in the experimental system should be scalable to actual sea states.
(4) Coalescence and Resurfacing of Dispersed Oil Droplets. In the past decade there have been several studies that looked at the effects of dispersant stripping, droplet coalescence, and resurfacing of dispersed oil (Fingas et al., 2002a; Bonner et al., 2003; Sterling et al., 2004c). The extent
to which this occurs will depend to a large extent on the hydrodynamic effectiveness of dispersion (i.e., the relative rates of coalescence and dilution of dispersed oil droplets by turbulent diffusion) and will exert a strong influence on the ultimate fate of the dispersed oil. The coalescence rate depends on the number concentration of dispersed oil droplets (Sterling et al., 2004c), which will decrease as the dispersed oil plume spreads and mixes with surrounding seawater. As described previously, however, hydrodynamic effectiveness cannot, in general, be investigated in the laboratory or in wave tanks, because these are closed systems with little or no dilution potential, and coalescence will be promoted by providing mixing energy over a prolonged period of time (i.e., by increasing the frequency of droplet-droplet collisions). The relative role of coalescence may be significantly reduced in very large wave tanks where dilution more closely approximates natural conditions. The extent to which coalescence and resurfacing will occur in the field, however, can only be fully investigated in field studies or by incorporating coalescence into a comprehensive dispersed oil fate and transport model. Coalescence kinetic parameters can be estimated in the laboratory (Sterling et al., 2004c). The effects of temperature and ice on dispersed oil droplet size, coalescence, and resurfacing also should be investigated to evaluate the range of conditions under which dispersants are likely to be effective, and these investigations would probably be most realistic in very large wave tanks where dilution more closely approximates natural conditions.
Description of Wave Tanks Available for Mesoscale Dispersant Testing
This section provides brief descriptions of some facilities that are available for testing of dispersant effectiveness in wave tanks. These descriptions focus primarily on the physical facilities and the tools available for measuring experimental conditions and results. Large tanks or facilities created to allow complex inter-comparative studies are discussed first, while smaller and simpler tanks are included for completeness.
Oil and Hazardous Materials Simulated Environmental Test Tank (OHMSETT) The largest test tank available in the world for dispersant testing is the Oil and Hazardous Materials Simulated Environmental Test Tank (OHMSETT), operated by the Minerals Management Service and located on the grounds of the Naval Weapons Station Earle in Leonardo, New Jersey (Figure 3-9). This facility was originally designed for testing mechanical oil recovery equipment, such as booms and skimmers. In recent years, it has been modified to accommodate dispersant testing, including the ability to chill the seawater in the tank to arctic temperatures and modifying the seawater filtration system to improve removal of dis-

FIGURE 3-9 Aerial view of the OHMSETT test tank facility.
SOURCE: J. Mullin, Minerals Management Service, http://www.ohmsett.com/.
persed oil and dissolved dispersant (J. Lane, U.S. Minerals Management Service, Herndon, Virginia, written communication, 2005).
The OHMSETT facility includes an aboveground, concrete tank that is 203 m long, 20 m wide, and 3.4 m deep. Six viewing windows are located at intervals along one side to allow for underwater observations. Brackish water is pumped from a nearby bay, filtered, and the salinity adjusted by the addition of salt with major ion composition similar to sea salt. The tank is usually filled to a depth of 2.4 m, giving a working volume of approximately 9,700 m3. The tank is spanned by three movable bridges, which can move along the tank at speeds up to 3.3 m/s. In addition to the administrative building alongside the tank, there is a multistory control complex at one end of the tank affording a complete view of the facility. At the south end of the tank is a paddle-type wave generator, capable of producing either smooth or cresting regular waves. At the north end there is an artificial “beach” that can be raised or lowered to either absorb or reflect wave energy, which allows users to produce waves with specific characteristics (e.g., long, even swells or harbor chop). Oil for dispersant tests has typically been added to the water surface in an approxi-
mately 10,000 ft2 area within containment booms. This represents approximately 23 percent of the available surface area of the tank. Note, however, that the entire volume of the tank is potentially available for dilution of the dispersed oil plume.
In dispersant tests that have been conducted at OHMSETT—beginning in March 2002—the test oil has been applied to the water surface through a manifold mounted to the leading edge of the main bridge (Figure 3-10). Oil has been applied while the main bridge advanced at a speed of 0.5 m/s, and the dispersant has been sprayed on the resulting oil slick from a nozzle array hanging below the trailing edge of the same bridge (Figure 3-11). For these conditions, the time interval between application of the oil and the dispersant is approximately 10 seconds. The short time period between application of oil and dispersant is the basis of some criticism of cold-water tests that were conducted at this facility, because the oil was heated to allow it to be pumped through the oil distribution manifold, and some suggest that 10 seconds is not enough time for the floating oil to cool to the temperature of seawater (PWSRCAC, 2004). To address this potential problem, MMS has funded research to investigate the cool-

FIGURE 3-10 OHMSETT oil distribution system.
SOURCE: S.L. Ross, 2002.

FIGURE 3-11 OHMSETT dispersant spray bar in operation.
SOURCE: S.L. Ross, 2002.
ing rate of heated oil in contact with cold seawater and has redesigned the oil-distribution manifold to allow application of cold, highly viscous oil in future cold-water tests (J. Mullin, U.S. Minerals Management Service, Herndon, Virginia, written communication, 2005). Pending peer review of this research, or a repeat of the cold-water tests using an improved oil-distribution system that does not require heating the oil, OHMSETT test results should be used with caution to gauge the effectiveness of chemical dispersants in cold water.
The large size of the OHMSETT tank offers advantages to experimenters wishing to investigate certain aspects of operational effectiveness (e.g., the dispersant application equipment can produce dispersant droplets with realistic size distributions) and hydrodynamic effectiveness (e.g., the facility allows dispersed oil to be transported in a relatively large volume of water). It also permits studies of effectiveness under specialized conditions (e.g., in broken ice). However, the large size of the tank also presents several problems. Primary among these is the high cost of operating a facility of this size (e.g., the cost of chilling 9,700 m3 of seawater is considerable). This financial constraint often leads to experimental designs that
lack sufficient replication to support statistical analysis of the results. Another size-related limitation is the inability to shield the water surface from wind, which can cause the oil slick to drift to one side of the tank over relatively short periods of time and, therefore, requires experimenters to apply the dispersant immediately after application of the oil. As described above, this practice has led to questions regarding the validity of several high viscosity oil tests. In addition, the tank is too large to allow the water to be replaced after each test, and even with improved filtering, some observers are concerned that residual oil and dispersant can affect subsequent tests. The facility operators have determined the maximum dispersant concentration (400 ppm) that can be present in the water without affecting the validity of subsequent effectiveness tests (S.L. Ross, 2000), and to date, this concentration has not been exceeded in sequential tests. The presence of dispersed oil from previous tests, however, affects the water clarity (limiting the visibility of dispersed oil plumes) and precludes determination of the size distribution of dispersed oil droplets in the water column during subsequent tests when the water is not adequately filtered between successive runs. Finally, the size of the OHMSETT tank and its associated equipment is likely to increase the difficultly of closing mass balances through collection of non-dispersed surface oil, measurement of the concentration of dispersed oil droplets in the water column, and quantification of the oil that escaped the boomed test enclosure or adhered to the boom itself. The addition of a secondary containment boom outside the north end of the 10,000 ft2 experimental area has significantly improved collection of surface oil that splashes out of the test enclosure (it is then included with the other non-dispersed oil collected from the water surface within the test area), but quantifying the oil that adheres to the boom itself remains difficult. To date, dispersion efficiencies have been calculated by comparing the volume of surface oil recovered from dispersant-treated slicks to that recovered from control slicks that are not treated with dispersants, but complete mass balances have not been performed at this facility.
EPA/Department of Fisheries and Oceans Wave Tank at the Bedford Institute of Oceanography A new wave tank for investigation of dispersant effectiveness was recently built at the Bedford Institute of Oceanography (BIO) in Halifax, Nova Scotia, with joint funding from the EPA and the Department of Fisheries and Oceans (DFO) Canada (Figure 3-12). Although this facility was designed specifically for testing dispersant effectiveness and evaluating their effects, experiments involving dispersants and oil have not yet been conducted at the time of this writing.
The BIO/EPA wave tank is 16 m long, 0.6 m wide, and 1.2 m deep (total volume of 8.2 m3 when filled to the typical level). The volume of

FIGURE 3-12 Photo of the EPA/DFO wave tank at BIO, Halifax, NS, Canada.
SOURCE: K. Lee, Fisheries and Oceans Canada, Centre for Offshore Oil and Gas Environmental Research.
seawater in this tank is small enough that it can be replaced relatively quickly between tests, reducing concerns about the build-up of dispersant or dispersed oil concentrations between runs. A disadvantage of the small tank volume is that it precludes investigation of hydrodynamic effectiveness (e.g., dilution of the dispersed oil plume by turbulent mixing). The facility has a flow-through capability that will enable it to simulate some aspects of the dilution that can occur in open water, but this capability was included primarily to allow chronic toxicity studies to be conducted under more realistic exposure conditions. A weakness of simulating dilution due to advection and turbulent diffusion under a slick at sea by inducing flow of clean seawater through the tank is that, as described in Chapter 4, the at-sea rate is very scale dependent. Loss of oil to the walls of the tank will be minimized by a bubble curtain, which is created by forcing compressed air through holes in a copper tube that is submerged about 5 to 7.5 cm below the water surface adjacent to the walls of the tank. The effectiveness of this approach, especially at higher wave energies, has not yet been determined. In addition, the effect of turbulence created by
the bubble curtain on dispersion effectiveness at low wave energies should be carefully evaluated before this system is used extensively. The design of this tank enables it to produce a wide range of waves, including breaking and nonbreaking waves, at energy levels that are typical of sea surface conditions. The wave generator is capable of producing regular waves of varying period and repeatable breaking waves. Another objective of this facility was to allow measurements that will facilitate mass balance calculations; protocols are being developed for this purpose.
Shoreline Environmental Research Facility The Shoreline Environmental Research Facility (SERF), located near Corpus Christi, Texas, contains nine wave tanks, each of which is 33.5 m long by 2.1 m wide by 2.4 m deep (Figure 3-13; Page et al., 2000a). Each tank is equipped with a computer-controlled wave generator that can produce variable wave patterns and seawater inlets and outlets that allow the user to vary the water level in the tank to simulate tides. The SERF wave tanks have the ability to simulate nearshore environments by constructing sand beaches, including a flat back-beach area just above the high-tide line. The tanks can be operated with a high-tide water depth of 2.0 m and a tidal range of about 0.6 m.
FIGURE 3-13 Aerial view of SERF wave tanks.
SOURCE: J. Bonner, Texas A&M University-Corpus Christi.
The SERF is unique among wave-tank facilities in two ways. First, and most obviously, it is specifically designed for simulating nearshore environments, which may contain high concentrations of suspended solids due to resuspension of the shoreline sediment. Therefore, it can be particularly useful for evaluating the ability of dispersants to prevent oil contamination of shorelines. Second, and more importantly, it includes multiple identical wave tanks so independent replication of treatments is much simpler than in facilities that contain a single tank. Although not unique, the capability for continuous flow of clean seawater through the tank allows dilution of the dispersed oil plume to be considered in the experimental design. Finally, the SERF testing protocols have been developed over a period of several years with the objective of closing oil mass balances. To this end, investigators at the SERF measure oil concentrations in several compartments, including the water surface, the water column, the shoreline sediments, and the tank walls (Bonner et al., 2003). Although it may not be possible to account for 100 percent of the added oil, the measurements required to perform mass balances provide a much more detailed picture of the dispersed oil fate than do measurements of only one compartment.
S.L. Ross Wave Tank A small, indoor wave tank is available at the S.L. Ross facility in Ottawa, Ontario, Canada (Figure 3-14). This tank is 10 m long, 1.2 m wide, and 1.2 m deep; it is usually operated filled with 0.85 m of 32 percent salt water (total volume = 10.2 m3). A wave-generating paddle is located at one end, and a wave-dissipating beach is at the other. A submerged air diffuser creates a bubble curtain that contains oil within a rectangular region in the tank even in the presence of waves. Dispersant is applied through flat-fan nozzles—similar to those used in full-scale, boat-based dispersant application systems—from an overhead spray boom that is mounted above the center of the tank. The amount of dispersant that is applied is measured by collecting the spray in a tray positioned above the water surface at one edge of the oil containment zone (S.L. Ross, 1997).
SINTEF Flume The SINTEF facility in Norway has an elliptical flume that has been used for oil weathering and dispersion studies. The flume has a circumference of 9 m, with a 4-m long major axis (Figure 3-15). The tank is 0.5 m wide and is operated at a water depth of 0.4 m (total volume of 1.75 m3). The flume is equipped with a wave generator, submerged pumps that circulate the water around the elliptical track, fans that can simulate surface wind, and a UV lamp for photooxidation studies. This facility has been used primarily for oil weathering studies (Daling et al.,
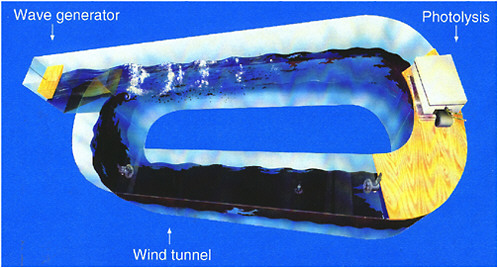
FIGURE 3-15 Schematic diagram of SINTEF hydraulic flume. The flume has a circumference of 9 m, with a 4-meter long major axis.
SOURCE: P. Daling, SINTEF.
1998), but it has been used to study the dispersibility of heavy bunker fuel oil (Fiocco et al., 1999).
The Cedre Polludrome The configuration of the Cedre Polludrome in France is similar to the hydraulic flume at SINTEF, but it is larger (Figure 3-16) (Guyomarch et al., 1999c). The Polludrome flume is 0.6 m wide and is operated with a 1 m water depth (total volume is 10.5 m3). Like the SINTEF flume, the Polludrome is equipped with an adjustable frequency wave generator, a fan to produce wind across the water surface, pumps for generation of currents, and UV lamps that allow experimenters to simulate photooxidation processes. In addition, the Polludrome is connected to a large storage tank that can be used to pump water into and out of the flume to simulate tides. Finally, the Polludrome has a long straight section that extends beyond the elliptical flume—in line with the wave generator—in which a shoreline can be constructed. The Polludrome has been used for a number of dispersant studies, particularly with higher viscosity oils where multiple dispersant applications can be evaluated (Guyomarch et al., 1999c).
Design of Effectiveness Tests in Wave Tanks
The primary advantage of wave-tank studies over laboratory-scale tests is the ability to investigate some components of operational effectiveness and introduce the energy that drives formation of small oil drop-
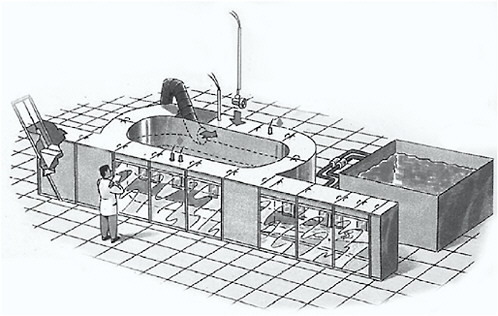
FIGURE 3-16 Schematic diagram of the Cedre Polludrome.
SOURCE: Guyomarch, et al., 1999c; courtesy of J. Guyomarch, Cedre.
lets through a mechanism that is similar to that which occurs at sea (i.e., waves). Whenever possible, the design of mesoscale dispersant effectiveness tests, including hydraulic flumes and wave tanks, should incorporate these factors.
One of the major factors affecting operational effectiveness during spill response operations is the patchy distribution of oil slicks that results from Langmuir circulation and related near-surface transport phenomena, but this is difficult to simulate in wave tanks. A second factor is the interaction of dispersant with floating oil. This requires that the experimental design include dispersant-application equipment that generates realistic droplet-size distributions and impact velocities and that the physical characteristics of the floating oil match those that are expected to exist in-situ as closely as possible. Thus, wave-tank tests should use oil that is weathered in a realistic manner, preferably on the water surface in the presence of waves and at a temperature that is representative of the environment of interest. Penetration of the oil by the dispersant may be affected by the viscosity of the oil, especially in the thin film in contact with the oil-air interface, which will depend on the extent to which the oil has evaporated and formed a water-in-oil emulsion. Similarly, the dispersant properties at the time of application to the floating slick should be representative of those that would be expected to prevail during a spill
response operation (e.g., if cold-water dispersion is under investigation, the dispersant should be applied at a temperature that is similar to the expected ambient temperature of the application vehicle, because the viscosity of the dispersant will affect the size of the droplets that are formed during spraying; Byford et al., 1983).
Many factors that affect chemical effectiveness have been investigated in laboratory-scale experimental systems, including the effects of water characteristics (e.g., salinity, temperature, and suspended sediment concentrations) and oil composition on dispersion effectiveness. These also can be investigated in wave-tank tests if one has reason to believe that there may be an interaction between these factors and the mechanism through which energy is provided to produce droplets, but at a minimum, they should be controlled or measured. The ability to reproduce the mechanism of droplet formation is one of the main advantages of wave-tank tests over those conducted in the laboratory. Therefore, wave-tank tests should measure and correlate the turbulent energy dissipation rate used to those that occur in the real world. Because waves produced by local wind are expected to be the main source of turbulent energy to disperse the oil in open coastal waters, wave-tank tests should generate waves that are controlled, well characterized, and reproducible (Bonner et al., 2003). Because wave energy in the sea surface varies over a wide range in short time periods (Delvigne and Sweeney, 1988; Agrawal et al., 1992), it is a parameter that should be investigated. In addition, the spatial variation in turbulent shear should be characterized, especially in larger wave tanks, when hydrodynamic effectiveness is under investigation.
If one is interested in investigating oil dispersion in relatively narrow estuaries or rivers, the current and bottom friction will be additional major sources of turbulent energy generation and dissipation. In this case, the wave tank tests should reproduce the expected estuarine and riverine flow fields. The concept of hydraulic radius, instead of water depth, should be used in scaling the flow field of the wave tank to that in estuaries and rivers (Chow, 1988).
In addition to quantifying the energy dissipation rate, the fraction of added oil that becomes entrained in the water column should be measured in wave-tank studies. This is accomplished by either measuring the amount of oil remaining on the water surface after mixing in the presence of dispersant (Brown et al., 1987; Brown and Goodman, 1988; Louchouarn et al., 2000; Belore, 2003; Bonner et al., 2003) and/or by measuring the oil concentration in the water column (Brown et al., 1987; Brown and Goodman, 1988; Bonner et al., 2003). Both of these techniques suffer from limitations. Measurement of surface oil estimates dispersion effectiveness by difference and, therefore, measurement errors that lead to incomplete recovery (including transport to compartments that are not explicitly con-
sidered, such as the atmosphere and the walls of the tank) are considered to represent dispersion (Fingas and Ka’aihue, 2004c). These losses are not expected to be the same in dispersed and undispersed oil slicks; so, they cannot be estimated using control treatments. Measurement of oil concentrations in the water column is complicated by the heterogeneous distribution of oil in a chemically dispersed plume, which necessitates collection of a large number of samples with high resolution in space and time. Analysis of oil concentrations with appropriate spatial and temporal resolution requires a method that can provide results in real time, such as in-situ fluorometry, but this method should be carefully calibrated and has been criticized as being subject to large systematic errors (Lambert et al., 2001a). Some attempts have been made to close mass balances during dispersant effectiveness tests in wave tanks (Brown et al., 1987; Brown and Goodman, 1988; Bonner et al., 2003), and although none have been completely successful (Fingas and Ka’aihue [2004c] report that oil recovered after wave-tank studies has ranged from about 10 to 100 percent of that added, with recent studies being in the range of 50 to 75 percent), this exercise provides useful information regarding the fate of the oil and the uncertainty in the estimates of dispersion effectiveness. Therefore, mass balances should be attempted in all wave-tank studies of dispersant effectiveness.
In addition to measuring the concentration of dispersed oil, the droplet-size distribution should also be measured. The size and density of the dispersed oil droplets will determine their rise velocity and, therefore, whether they will be stably entrained in the water column under ambient mixing conditions or will eventually float to the surface and reform a floating slick. Efficient chemical dispersion of oil should result in a high concentration of oil droplets with a volume median diameter less than about 50 µm (Byford et al., 1984; Daling et al., 1990a; Lunel, 1995b). Droplets that are larger than this are likely to resurface if the mixing energy is removed or significantly reduced.
Well-designed experiments using wave tanks have an important role in the study and quantification of factors controlling dispersant effectiveness. With more realistic mechanisms for energy input and rigorous measurements, it is hoped that such tests can be used to develop better predictive models of dispersant effectiveness.
Field Studies
Objectives of Field Studies
Historically, one of the major motivations for conducting full-scale sea trials was skepticism of the validity of laboratory and mesoscale tank
tests. These smaller-scale tests are frequently criticized for inaccurately simulating the processes that contribute to dispersion of oil slicks at sea. In particular, uncertain or improper scaling of the laboratory systems (e.g., oil-water ratios, mixing energy) relative to conditions at sea and the effects of system boundaries (i.e., wall effects) on the observed effectiveness are commonly identified as detracting from the realism of laboratory systems and wave tanks. A perceived advantage of full-scale field trials is that they are the best representation of reality that can be achieved while maintaining some degree of control over the design of the experiment. Although this control is desirable because it allows the experimenters to limit or, at least, to identify and measure the uncontrolled variables, it also introduces artificiality into the test.
From a more fundamental perspective, the motivation for studying dispersant effectiveness in field studies derives from the opportunity to study phenomena that cannot be addressed at the smaller scale of laboratory and wave-tank systems. These include, for example, greater opportunity to investigate operational effectiveness issues such as the use of real application equipment (e.g., aircraft) to apply dispersants to oil slicks under real conditions (e.g., patchy oil distribution caused by Langmuir circulation and eddies of various sizes) resulting in realistic encounter rates. Similarly, field studies may present the only opportunity to investigate the hydrodynamic effectiveness of chemical dispersion (i.e., dilution of the dispersed oil plume due to horizontal and vertical diffusion resulting from realistic currents and eddies). Both of these processes, however, require relatively large experimental oil spills to be sufficiently realistic. For a variety of practical reasons, most planned field studies involve small quantities of oil (e.g., 20 to 50 tonnes [roughly 5,000 to 13,000 gallons]) relative to what is released during real oil spills (e.g., see the case studies presented in text boxes and the subsection on the Gulf of Mexico dispersant applications in this chapter; only the M/V Blue Master spill [16 tonnes] was comparable in size to most field studies; other spills ranged in size from about 320 tonnes [the Poseidon pipeline spill] to 87,000 tonnes [the Sea Empress]). Small spills, even when studied under field conditions, will result in operational and hydrodynamic effectiveness that is better than could be achieved under more complex response conditions. That is, the dispersant encounter rates would be too high and dilution of the dispersed plume would be too fast to extrapolate directly for prediction of performance or effects of real oil spills (see discussion of surface transport in Chapter 4 for more details). In addition, although field studies allow dispersant effectiveness to be investigated under realistic conditions, only a few realizations of all possible conditions can be specifically tested due to financial and logistical constraints, and these may not be the conditions of most interest. Instead, the conditions that can be investigated are those
that prevail at the time the study is conducted, and investigators have only limited control over what those conditions will be.
Additional justifications for conducting at-sea trials of dispersant effectiveness include that they can provide opportunities to develop and test instrumentation for monitoring dispersion effectiveness (e.g., surface oil thickness and aerial extent, water column concentrations of dispersed oil), they can be used to train spill response personnel, and they can be used to verify dispersed oil fate and transport models. Although all computer models are simplifications of the real world and, therefore, should not be expected to exactly simulate the complex behavior of oil in the environment, the underlying conceptual models should incorporate the major oil transport processes and the mechanisms that govern its fate. Carefully executed field studies can inform these conceptual models by testing the suspected cause-and-effect relationships that control dispersant effectiveness. In addition, field studies can be used to calibrate model parameters by providing measured dispersed-oil concentration distributions for specific well-characterized initial and boundary conditions that can be compared to model output. Furthermore, field studies can be used to validate model output to evaluate the reliability of the model predictions.
Conversely, design of chemical dispersion field studies should be guided by modeling, especially the expected transport of the surface and dispersed oil plumes. For example, models can be used to identify sampling locations and determine the required sampling frequencies. Furthermore, oil concentration predictions will assist in specifying sampling and analytical methods that will be used (e.g., sample size affecting detection limits and the dynamic range of expected concentrations and phase—dissolved vs. oil droplet—of the oil in the collected samples).
Design of Field Studies
A full-scale field trial can be very costly (e.g., potentially in excess of U.S. $500,000). The major costs include permitting during the planning phase (Payne and Allen, in press), mobilizing the vessels, aircraft, and personnel that are required to carry out the study during the field exercise itself, and analytical chemistry costs associated with measuring oil concentrations and fate (dissolved vs. particulate) in field-collected samples. The experiments are usually carried out far from populated areas, which increases the travel time to the site, further increasing time and costs. Once committed, the experiment is at the mercy of the prevailing weather, which will dictate whether any work can take place at all. For example, in a recent field trial conducted in the United Kingdom, experiments could not be performed on the first scheduled day due to excessively high wind
speeds, which made the small boat operations that were required for monitoring unsafe, whereas on the second day, the experiment was delayed due to insufficient wind speed, such that poor dispersion of the target heavy fuel oils (IFO 180 and IFO 380) was expected (Lewis, 2004). Weather contingencies of this sort can cause huge costs overruns.
In addition to the high costs of field studies, permitting and legal issues can be major impediments to conducting field studies, and they are a major reason that no field studies have been conducted in U.S. waters since 1979. Although there are published guidelines for obtaining an EPA permit for planned spill experiments (EPA, 2001), those requirements can be quite onerous and include a requirement that all parties assume financial and legal responsibility for any unintended consequences of the study. In addition to the EPA, approval may be required from several other federal, state, and county agencies (Payne and Allen, 2004; in press). In some cases, it can be difficult to identify relevant permitting requirements and obtain approval from these agencies.
Although they are often considered to be the best representation of “reality,” field trials are also subject to limitations. A major limitation is that a very limited data set can be obtained from any one trial. As such, the objectives should be clearly defined and reliable procedures should be established to ensure that the required results are achieved. Poor experimental design will produce results that are difficult to interpret unambiguously.
Design of field studies should involve principles similar to those used in the design of any experiment. In particular, a primary objective should be to obtain an unbiased estimate of the variation that exists between two experimental units (i.e., oil slicks) that are treated identically to allow evaluation of the statistical significance of any differences that are observed between experimental units that are subjected to different treatments (e.g., dispersant-treated slicks versus untreated control slicks). This objective requires treatments to be independently replicated and randomly distributed or interspersed throughout the experimental domain (Box et al., 1978; Hurlbert, 1984; Montgomery, 1997; Ruxton and Colegrave, 2003). Independent replication of treatments requires that they be conducted in independent experimental units. It is not sufficient to collect multiple samples from the same experimental unit; this “pseudoreplication” only serves to characterize the spatial and/or temporal heterogeneity of the experimental unit, it cannot characterize the degree of variation that exists between experimental units independently of treatment (Hurlbert, 1984; Ruxton and Colegrave, 2003). Although alternative designs (e.g., BACI—before-after-control-impact designs) may be appropriate when replication is not possible, such as studies involving spills of opportunity, these are not appropriate for planned field studies. Even
when BACI-type experimental designs are required, modifications that include independent replication of controls are recommended (Underwood, 1994). When field studies are conducted over several days, the experimental domain should be interpreted to include both spatial and temporal dimensions. Therefore, independent replicates for specific treatments should be performed on different days, which will presumably sample a range of weather conditions.
In reality, financial and technical constraints limit the degree to which the design of field studies can comply with these principles of sound experimental design. Financial constraints limit the scope and duration of field studies and, consequently, the ability to independently replicate treatments and intersperse the replicates over space and time. This is further complicated by the vagaries of weather, which may increase the within-treatment variance of properly interspersed replicates and obscure the ability to detect statistically significant between-treatment differences. Paired experimental designs may be useful in reducing the effects of weather, but the apparently nonlinear response of dispersion effectiveness to mixing energy (Fingas et al., 1994; Fingas et al., 1996a) may reduce the benefit of this approach, because the difference between the dispersant treatment and the control might vary with energy level.
In addition to the inability to control weather and, therefore, to set mixing energy as an independent variable, field studies are subject to an additional important technical limitation: the inability to quantitatively measure effectiveness for use as an endpoint in statistical comparisons of treatments. Dispersant effectiveness in sea trials has been monitored by measuring surface oil and dispersed-oil concentrations in the water column, but neither method produces satisfactory results. Surface oil is commonly monitored remotely using aircraft-mounted side-looking airborne radar (SLAR) and ultraviolet (UV) and infrared (IR) line scanners. SLAR and UV scanners are not sensitive to oil thickness, and although IR scanners can distinguish between thick and thin oil slicks, they cannot measure the oil thickness (Goodman and Fingas, 1988; Lewis et al., 1995a,b; Lewis and Aurand, 1997). As a result of these limitations, the volume of surface oil cannot be measured, and therefore, the effectiveness of dispersion cannot be quantified using these methods. In fact, treatment of floating oil with a chemical dispersant may cause the thick part of slick, which can be detected by IR scanning, to increase in area due to decreased oil-water interfacial tension (Goodman and MacNeill, 1984; Goodman and Fingas, 1988). More advanced remote-sensing technologies, such as microwave radiometry (Schroh, 1995) and laser-ultrasonic detection (Choquet et al., 1993; Brown et al., 2000), can be used to estimate the volume of floating oil, but interpretation of these data is sometimes difficult (Lewis and Aurand, 1997). For example, successful use of the laser-ultrasonic
technique, known as Laser Ultrasonic Remote Sensing of Oil Thickness (LURSOT), from an aircraft has not yet been reported (Brown et al., 2000).
Measurement of dispersed oil in the water column can be more difficult. Early studies attempted to measure dispersed oil concentrations by collecting grab samples at various locations and times following application of a chemical dispersant, but the heterogeneity of dispersed oil distribution is too great to obtain meaningful results with the limited number of grab samples that can be collected and analyzed (Brown et al., 1987). Real-time measurement of dispersed oil concentrations using continuous fluorometry allows collection of a more dense data set that improves the ability to characterize the concentration distribution of dispersed oil along a transect through the slick, but these measurements are still limited by the inability to collect samples simultaneously at multiple positions within the dispersed oil plume (Lunel, 1995a; Lewis, 2004). Furthermore, careful calibration of fluorometers is necessary to obtain quantitatively useful results (Lambert et al., 2001a). Although calibration methods vary greatly among investigators, the most reliable method appears to be collection of water samples directly from the fluorometer effluent. Even when calibrated appropriately, however, in-situ fluorometry is subject to interferences that can affect its quantitative reliability. Because the nature of the dispersion and dilution processes results in a dispersed oil plume that is heterogeneous in space and time, unambiguous quantitative interpretation of dispersed oil concentrations for the purpose of estimating mass balances is difficult. In this respect, continuous release of oil from a fixed point into a current may be a more effective experimental design (Lunel, 1994b, 1995a), but this design lacks many of the elements of realism that are sought by field studies. For example, operational effectiveness will be unrealistically high due to application of dispersant from a boom mounted close to the oil discharge position, and the short time period between oil discharge and dispersant application allows for no weathering and limited spreading of the slick. Also, the hydrodynamic effectiveness will be artificially high, because this experimental design lays down a very narrow (initially 1-m wide) oil slick. Discharge of the oil into a current is also likely to increase the hydrodynamic effectiveness by increasing the horizontal turbulent diffusion coefficients. So, although this experimental design is useful for many purposes, it will almost certainly overestimate the effectiveness of dispersion relative to a real spill response operation.
The inability to make measurements that are adequately quantitative in sea trials has led some investigators to rely solely on visual observation (Lewis, 2004), which is purely qualitative and very sensitive to viewing conditions (e.g., position of sun relative to viewer, cloud cover, viewing angle). The reliability of data collected by visual observation would be improved by using “blind” observation techniques, in which the observ-
ers are not informed of the treatment that is applied to experimental slicks. This would require treatment of control slicks with formulations that contain the dispersant solvents but lack the surfactants. In addition, the observers must be extremely careful to avoid interacting with other observers when making observations to reduce the potential for nonindependence of the observations. Visual observation is also a central component of the Specialized Monitoring of Advanced Response Technologies (SMART) protocols for monitoring the performance of dispersants in oilspill response operations. Although qualitative data may provide anecdotal evidence for dispersant effectiveness, which may be suitable for some purposes, it cannot be used to validate fate and transport models.
Review of Past Field Studies
A number of controlled field trials of dispersant effectiveness have been conducted in Canada and Europe since the 1989 NRC review (McDonagh and Colcomb-Heiliger, 1992; Lunel and Lewis 1993a,b; Brandvik et al., 1995, 1996; Walker and Lunel 1995; Lunel 1993, 1994a,b, 1995a,b; Lewis et al., 1995a,b, 1998a,b; Lunel et al., 1995a,b,c; Strom-Kristiansen et al., 1995; Walker and Lunel, 1995; Lunel and Davies, 1996; Fiocco et al., 1999). Many of these studies have been reviewed and summarized (S.L. Ross, 1997; Fingas and Ka’aihue, 2004b), and the proceedings of a two-day symposium on oil-spill dispersant applications in Alaska are also available (Trudel, 1998).
No attempt will be made to duplicate or even briefly cover the findings presented in these documents. Instead, several of the most significant lessons learned—specifically with regard to applications and dispersant-treated oil behavior—will be briefly highlighted in the following paragraphs.
It is now known that oil spills are composed of thick slicks (usually thicker than 1 mm) that contain most of the oil volume (the rule-of-thumb is that 90 percent of the oil volume is contained in 10 percent of the area), and that these patches are surrounded by thinner sheens (about 1 to 10 µm or 0.001 to 0.01 mm) (S.L. Ross, 1997). This combined thick and thin slick spreading is of great importance with regard to dispersant effectiveness. From field trials and actual dispersant treatment of accidental oil spills, it is now generally accepted that the one pass concept for dispersant application is not appropriate for dealing with the thicker part of spills, and that the multi-pass approach (as has always been used in United Kingdom) is the only way to completely dose the thicker portions of marine spills (Lunel et al., 1997b).
Daling and Lichtenthaler (1987) compared the results of laboratory effectiveness tests with the results from several small field trials. They
showed that the correlation between effectiveness measured using the three different laboratory test systems and between field and laboratory tests was poor. There was, however, fairly good correlation between the mean results for the different dispersants from the three laboratory tests and field tests. That is, dispersants that performed poorly in the laboratory also performed poorly in the field, but the lab tests were not able to predict the dispersibility of a specific oil by a specific dispersant under defined conditions at sea with any satisfactory level of accuracy. The results of more recent comparisons of laboratory effectiveness data and field trials are shown in Table 3-3, which demonstrate that the field effectiveness was generally lower than values obtained in the laboratory (Fingas and Ka’aihue, 2004b). The higher effectiveness in laboratory studies may indicate that the energy levels were higher in the laboratory tests than in the field studies, which is contrary to what was thought in previous years (Lunel, 1994a).
Based on the monitoring results from field studies and actual spills, it can be concluded that it is difficult to estimate average concentrations under treated slicks because of the significant heterogeneity both horizontally and with depth into the water column (Brandvik et al., 1995; Lewis et al., 1998b). Figure 3-17 shows the horizontal and vertical distribution of total petroleum hydrocarbons from small test spills as determined by UV/fluorescence before and after dispersant treatment. Before treatment, the maximum concentration in the surface waters (<0.5 m) was less than 1 ppm, but during treatment, this increased to nearly 6 ppm
TABLE 3-3 Comparison of Laboratory and Field Dispersant Effectiveness Results (from Fingas and Ka’aihue, 2004b)
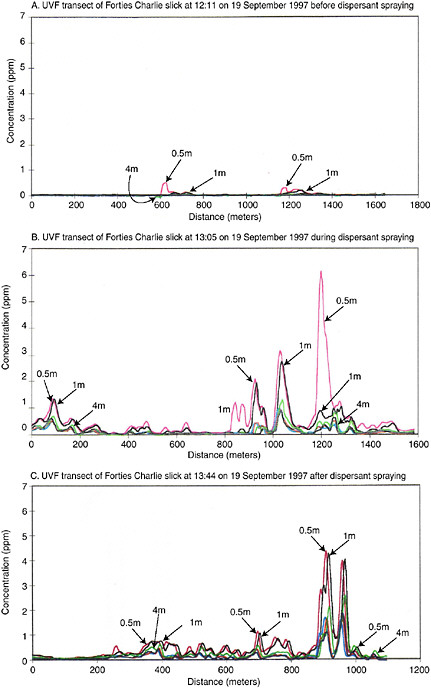
FIGURE 3-17 Dispersed oil concentrations under an approximately 27 m3 surface slick of Forties crude oil (a) before, (b) during, and (c) after spraying with 2,250 liters of Corexit 9500 during the 1997 North Sea field trials. Sampling depths for the major peaks in dispersed oil concentrations are labeled, and in many, but not all cases, the 0.5 and 1.0 m depths were very similar.
SOURCE: Modified from A. Lewis, et al., 1998b; courtesy of AEA Technology.
with lesser concentrations at depth. After approximately 45 minutes, concentrations at depth also increased, but generally to only 1–2 ppm.
The heterogeneity of the dispersed oil plume makes it difficult to obtain reliable estimates of the mass of dispersed oil beneath a treated slick in field studies, which is essential to achieving closure of a mass balance. Fingas and Ka’aihue (2004b) consider estimation of a mass balance to be among the most important factors in obtaining reliable effectiveness estimates from field studies, but they conclude that no field trials have achieved mass balance closure, which is difficult even in more controlled tank tests where up to 70 percent of the oil may be missing from the final mass balance. Other factors considered by these authors to be critical for obtaining reliable results from field studies include the use of proper con-
|
BOX 3-3 Spilled Oil Type/Volume/Conditions: A series of experimental spills in the North Sea were conducted in 1990, 1993, 1994, 1995, and 1997 for various dispersant applications. Test oils included Forties Blend crude oil, Troll crude oil, Alaska North Slope crude oil, IFO-180, and a 50:50 blend of medium fuel oil and gas oil (MFO+GO) with an API gravity of 22. Spill volume was typically 15–50 m3. Physical and Biological Setting: Open-water setting in water depths greater than 90 m. Tests usually conducted in the summer, with water temperatures of 15° C (roughly 59° F), winds 5–10 m/s. Dispersant Application: 1990 Test—Objective was to have a steady-state oil discharge so that replicate measurements could be made of the dispersed oil concentrations under the treated slicks to better quantify dispersant effectiveness. Four continuous releases of 50 liters per minute of MFO+GO, with dispersant application 12–15 minutes later at a dispersant:oil ratio of 1:20, using OSR-5, Slickgone NS, and 1100X, and no dispersant as a control. Winds were up to 14 m/s. 1993 (May) Test—Objective was to determine dispersant effectiveness on a medium fuel oil with low concentrations of light ends, so evaporation would not be significant. Winds were 11–22 miles per hour (roughly 17–35 kilometers per hour). Single releases of 20 m3 of MFO+GO, with application of 2 tonnes (roughly 588 gallons) of Dasic Slickgone NS 1.5 hours after release at a dispersant:oil ratio of 1:10 during 10 spray runs. A second application of 2 tonnes was conducted in the afternoon on the remaining oil. There was a similar untreated oil release as a control. |
trol slicks (i.e., not treated with dispersant), the need for remote sensing, and the use of proper analytical procedures in the field (e.g., fluorometer calibration) and during laboratory analysis of samples collected in the field. In their survey of field trials completed before 1990, the effectiveness estimates averaged around 33 percent, but inclusion of more recent trials decreases the average effectiveness to only 16 percent (Fingas and Ka’aihue, 2004b). This is somewhat surprising, because better experimental designs and methods were used in the more recent tests, but the effectiveness of recent tests may have been reduced by the use of heavier oils.
A large number of at-sea trials were conducted between 1993 and 1994 (see Box 3-3 on the North Sea trials), and Table 3-4 presents a summary of the dispersant efficiency data for different oils tested and the different
|
1995 (August) Test—Objective was to test effectiveness of two application methods on an crude oil: helicopter bucket versus boat-mounted spray arm; and to calibrate aerial remote sensing sensors with ground truth data on the surface oil slicks. Single releases of 15 m3 of topped Troll crude oil with two applications of Corexit 9500 at a final dispersant:oil ratio of 1:20 on the thick oil slicks 2 hours post release. Winds were roughly 17–25 kilometers per hour. 1997 (September) Test—Objectives were test the degree to which multiple applications of dispersants (Corexit 9500 and Dasic Slickgone NS) can disperse high viscosity emulsions formed after 1–2 days at sea and the dispersibility of heavy fuel oils. Monitoring Results: Effectiveness: In all tests, remote sensing aircraft equipped with SLAR, video, ultraviolet, and infrared cameras were used to track the behavior of surface slicks. Oil concentrations in the water column were monitored using field fluorometers towed through the slicks at multiple transects at different depths and distances downcurrent. The field fluorometers were calibrated using discrete samples. 1990 Results—For slicks of a medium fuel oil treated with OSR-5 and Slickgone NS (Type III dispersants), the slick length was reduced compared to the control slick. Using the multiple transect data, it was estimated that, 12–15 minutes after dispersant application, OSR-5 dispersed 21–42 percent of the oil, Slickgone NS dispersed 11–27 percent, 1100x dispersed 6–17 percent, and there was 0–3 percent natural dispersion. 1993 Results—For slicks of a medium fuel oil treated with Dasic Slickgone NS, the surface area of the slick initially increased in area due to spreading by 5 hours after treatment, then by 9 hours after treatment reduced in surface area, compared to the control slick. The first dispersant application |
|
had a temporary effect of lowering the water content and viscosity of the surface oil, however, within 4 hours, the water content and viscosity of the treated oil was the same as the control slick. The oil concentrations under the slick prior to dispersant application were about 1 ppm and only extended down to 1 m. After dispersant application, oil concentrations were typically 1–10 ppm down to 5 m, with a maximum concentration of 25 ppm. There was a 16-fold increase in the volume of dispersed oil under the treated slick compared to the control. 1995 Results—For a slick of stabilized Troll crude oil treated with Corexit 9500 2 hours after release, no thick oil patches were observed 15 minutes (for helicopter application) and 30 minutes (for boat application) after dispersant application. The oil increased from 10 percent water content immediately after release to 20–70 percent water content at the time of dispersant application two hours later. The oil viscosity increased from 20 cSt to 100–1,000 cSt after 2 hours, and 3,000 cSt (control slick) at 6 hours after release. After about 1.5 days, the control slick reached 7,000 cSt, the vis- |
TABLE 3-4 Summary of Dispersant Efficacy Data from 1993–1994 Sea Trials
|
Energy Regime |
Wind Speed (m/s) |
Date |
Oil Dispersant |
Percent Dispersed (mean) |
Standard Deviation |
|
Low |
3 |
7/9/93 |
MFO |
0.8 |
0.7 |
|
Low |
5 |
8/19/94 |
MFO-Slickgone NS |
8 |
4 |
|
High |
10 |
7/9/93 |
MFO |
2 |
0.7 |
|
High |
7 |
8/22/94 |
MFO |
4 |
2 |
|
High |
7 |
8/25/94 |
Forties |
5 |
3 |
|
High |
10 |
7/9/93 |
MFO-1100X |
10 |
4 |
|
High |
10 |
7/9/93 |
MFO-Slickgone NS |
17 |
6 |
|
High |
6 |
8/23/94 |
MFO-Slickgone NS |
16 |
7 |
|
High |
6 |
8/25/94 |
Forties-Slickgone NS |
16 |
6 |
|
High |
7 |
8/22/94 |
MFO-Corexit 9527 |
26 |
10 |
|
High |
10 |
7/9/93 |
MFO-OSR5 |
30 |
7 |
|
Energy Regime |
Percent Dispersed |
Ratio of Chemical Dispersion to Natural Dispersion |
|||
|
MFO |
MFO-Slickgone NS |
||||
|
Low |
0.8 |
8 |
10 |
|
|
|
High |
3 |
17 |
6 |
|
|
|
SOURCE: Data from Lunel et al., 1995b and S.L. Ross, 1997. |
|||||
|
cosity limit for dispersibility of Troll crude oil. Oil concentrations in the water under the control slick were 0.05–0.3 ppm, versus those under the helicopter-treated slick which were 10–20 ppm after 0.5 hours, and 1–3 ppm after 1.5 hours. Under the treated slicks, the oil concentrations were uniform to 8 m depth. 1997 Results—Emulsified crude was rapidly dispersed after two days at sea, even though the Forties crude had a viscosity of 4,000–4,500 cP and the Alaska North Slope crude had a viscosity of 15,000–20,000 cP. Corexit 9500 partially dispersed the IFO-180 after 4 hours at sea, though there was little effect on the heavy fuel oil when the viscosity exceeded 20,000 cP. These field experiments demonstrated that emulsified oils with viscosities up to 20,000 cP can be effectively dispersed, extending the window of opportunity for dispersant use. SOURCE: Summarized from Brandvik et al. (1996), Lunel (1995a), Lunel et al. (1997b), and Lewis et al. (1998a). Effects: Not assessed. |
energy regimes encountered. There is a clear ranking in percentage of oil that the different formulations successfully dispersed into the water column in the field as the encountered energy regime increased; however, it should be noted that the overall percent dispersed values were relatively low. Although this ranking had been well documented for laboratory tests, these data were the first set from field trials where the ranking could be quantified. The tested dispersants increased the rate of dispersion by 6 to 10-fold compared with natural dispersion in the case of a medium fuel oil and 3-fold in the case of Forties crude oil. Comparison of the dispersion data for the low-energy regimes (0–5 m/s wind speed [roughly 0–10 knots]) with the higher-energy regimes (6–10 m/s wind speed [roughly 12–20 knots; 22–37 kilometers per hour; 13–22 miles per hour]) shows that natural entrainment is enhanced through the use of dispersants by about the same factor in low-energy regimes (10-fold) and in higher-energy regimes (6 to 10-fold).
Dispersion effectiveness measured in the 1997 AEA North Sea field trials (Box 3-3), which used Forties blend and Alaska North Slope crude oil weathered on the water surface for 45 and 55 hours, respectively, was much greater than the effectiveness that was observed in the 1993–1994 studies that were described above. In these studies, the naturally formed water-in-oil emulsions were completely dispersed (Lewis et al., 1998a,b; Fiocco et al. 1999). The naturally emulsified Forties oil had a viscosity of 4,000–4,500 cP with a water content of 50 percent by volume, whereas the emulsified ANS oil had a viscosity in the range of 15,000–20,000 cP and a water content of 20–30 percent by volume. The emulsified Forties oil was
rapidly and totally dispersed after multiple aerial applications of either Corexit 9500 or Dasic Slickgone NS. The rate of dispersion of the emulsified ANS resulting from four aerial applications of Corexit 9500 to the thicker oil patches appeared to be slightly slower than that observed for the weathered Forties oil, but it was also totally dispersed. More variable and less effective dispersion was observed after aerial application of Corexit 9500 to 4- to 23-hour weathered and partially emulsified IFO-180 fuel oil with viscosities ranging from 5,000 to 12,000 cP and water content ranging from 20 to 30 percent. Emulsified IFO-180 fuel oil with a viscosity above 20,000–30,000 cP was not dispersible to any degree.
One of the direct charges to the committee was to address “how laboratory and mesoscale experiments could inform potential controlled field trials and what experimental methods are most appropriate for such tests.” The body of work completed to date has provided important, but still limited understanding of many aspects of the efficacy of dispersants in the field and behavior and toxicity of dispersed oil. Developing a robust understanding of these key processes and mechanisms to support decisionmaking in nearshore environments will require taking dispersant research to the next level. Many factors will need to be systematically varied in settings where accurate measurements can be taken. It is difficult to envision the proper role of field testing in a research area that has yet to reach consensus on standard protocols for mesocosm testing. The greater complexities (and costs) of carrying out meaningful field experiments suggest that more effort be placed, at least initially, on designing and implementing a thorough and well-coordinated bench-scale and mesocosm research program. Such work should lead to more robust information about many aspects of dispersed oil behavior and effects. When coupled with information gleaned through more vigorous monitoring of actual spills (regardless of whether dispersants are used effectively in response), this experimental work should provide far greater understanding than is currently available. Upon completion of the work discussed below, the value of further field-scale experiments may become obvious. In any case, such field-scale work would certainly be better and more effectively designed and executed than is currently possible. Future field-scale work, if deemed necessary, should be based on the systematic and coordinated bench-scale and wave-tank testing recommended in this report.
Effectiveness Testing Using Spills of Opportunity
In the arena of public opinion, no test can hope to have the positive impact of an actual success in using dispersants during a real spill. There are several areas around the country where the volume of crude oil traffic is so large that small spills are somewhat common. In these areas, it might
be possible to develop a plan for using dispersants as a first-strike tool to respond to a small spill that would ordinarily be cleaned up mechanically. Resistance from the responsible party due to concerns about additional liability that could result from curtailment of mechanical response activities in the area of the dispersant field study may make it difficult to obtain authorization for this type of research.
The principles of field study design as discussed above should be considered with regard to the additional limitations imposed by spills of opportunity, and many of these have been incorporated into draft Spill of Opportunity Contingency Study Plans that have been or are being prepared for several RRTs in different parts of the country. The most formalized of these documents is the Texas General Land Office “Spill of Opportunity” Dispersant Demonstration Project Description (Aurand et al., 2004). The primary objectives are to evaluate the operational efficiency of dispersant application and monitoring under realistic spill-response conditions, assess the fate of the dispersed oil plume, and evaluate the interaction of the dispersed oil plume with sediments in shallow estuarine waters. Should it be approved and incorporated into a spill response plan (including identification and pre-placement of sampling equipment, and stand-by contracts for personnel), it will provide considerable advantage in marshaling all the components necessary to adequately sample and monitor the results from dispersant applications when and if they occur in the designated areas. Following the lead in Texas, industry and federal agencies should develop and implement detailed plans (including preposition of sufficient equipment and human resources) for rapid deployment of a well-designed monitoring plan for actual dispersant applications throughout the United States.
There were two instances in U.S. waters over the last 17 years where ad hoc spill-of-opportunity studies were conducted during real spill events. During the September 1987 Pac Baroness oil spill off Point Conception, California, the effectiveness of treatment of a 100 meter by 700 meter portion of the slick with 41 gallons of Corexit 9527 by helicopter was documented (Payne et al., 1991c). Continuous subsurface UV fluorescence measurements and grab samples of water from beneath the slick were also obtained from a support vessel before and after dispersant application. Unfortunately, the results of the tests were equivocal because the slick was very thin in the treated area and only a small portion of the slick was treated. In addition, 15- to 20-knot (roughly 27 to 37 kilometers per hour) crosswinds caused significant breakup and dispersion of the surface slick in both treated and untreated control areas. SLAR data did not show definitively that any changes occurred because the resolution of the technique from the observation altitude 5,000 ft (roughly 1,500 m) was not sufficient to observe changes in the small treated area. The aerial UV scans
suggested that changes occurred in the treated slick, but the in-situ UV fluorescence measurements and subsequent chemical analyses did not indicate that significant dispersant-enhanced entrainment occurred.
The lessons learned from this study led to development of detailed plans for investigating dispersant effectiveness at future spills of opportunity. The recommendations included:
-
Detailed plans for different coastal regions should be prepared in advance.
-
During execution of the plan, target areas for dispersant application should be identified by smoke bombs and surface buoys or drogues.
-
The dispersant should be applied into the wind to minimize drift away from the target area, and two surface vessels should be used in addition to a helicopter observation platform for documentation of dispersant application effectiveness. If possible, the vessels should be perpendicular to and along the dispersant flight line to document dispersant drift away from the target area.
-
Both videotape and 35 millimeter (or digital) photography should be used to document the experiment.
-
Water-column oil concentrations should be measured using in-situ UV fluorescence and chemical analyses should be completed on grab samples collected from the output of the fluorometer as well as more traditional water sampling equipment at different depths.
-
For large areas, remotely monitor the slick using SLAR at 5,000 to 7,000 ft (roughly 1,500 to 2,100 m) (which is useful under all weather conditions) and IR/UV at 400 ft (roughly 120 m) (effective only in clear weather). Other remote sensing techniques may also be more appropriate.
Utilizing the lessons learned from the Pac Baroness study, additional spill-of-opportunity dispersant trials were undertaken at the Mega Borg fire and oil spill off Galveston, Texas, in 1990 (Kennicutt et al., 1991; Payne et al., 1993). The ship’s cargo was a light Angola Planca crude oil (API gravity = 38; viscosity = 4.58 cSt at 30° C [roughly 86° F]). Dispersant effectiveness was monitored by concurrent observations from the command control aircraft and dispersed oil concentrations were monitored using UV fluorescence continuously at a depth of 4 m along transects through the slick and a discrete water sampling program.
The distribution of dispersed oil droplets was very heterogeneous and reflected the patchy distribution of oil on the water surface before dispersant application. Maximum concentrations of dispersed hydrocarbons in the center of the treated zone were 22 mg/L for total aliphatics (primarily dispersed droplets) and 5.4 µg/L for total aromatics 60 to 90 minutes after dispersant application. Elevated levels were generally limited to the
upper 1–3 m of the water column. Concentrations in the upper 1–3 m of the untreated control zones were significantly lower (1.2–3.9 mg/L and 0.8–1.7 µg/L for total aliphatic and aromatic hydrocarbons, respectively). The dispersed aliphatic hydrocarbon concentrations at a depth of 9 m in the treated and control areas were similar (2.5–2.7 mg/L), suggesting that they represented a background, steady-state concentration of very fine, physically dispersed oil droplets that were formed by natural dispersion of the slick during the six days before the dispersant tests began. The ratio of the concentrations of aliphatic to aromatic hydrocarbons showed no evidence of significantly enhanced dissolution of lower- and intermediate-molecular-weight aromatics as a result of chemical dispersion. If such dissolution had occurred, however, it is possible that the dissolved-phase polynuclear aromatic hydrocarbons (PAH) were lost to evaporation directly from the 29° C (roughly 102° F) seawater in the upper mixed layer before the water samples were collected.
One of the major disadvantages identified in both of these ad hoc spill-of-opportunity studies was that many of the resources (boats, aircraft, response personnel, etc.) necessary to assist with the execution of the program were tied up with response activities. Also, radio communications among all the operating platforms (observation aircraft, directional aircraft, dispersant application aircraft, sampling and observation boats, and Unified Command personnel) were difficult at best, and often nonexistent during the field operations. Finally, both spill-of-opportunity studies were relatively far from land (16–25 miles [roughly 25–40 kilometers]) and refueling of the observation/command control aircraft coordinating the dispersant trials was problematic.
As with the planned trials discussed earlier, the measured subsurface oil concentrations were extremely patchy, and there was no way to integrate or average the concentrations over time and space to even begin to approach a percent dispersed oil calculation. Finally, during spill-of-opportunity studies, the oils may not be amenable to chemical dispersion, or in the case of the Mega Borg, the oil may be so light, that it disperses naturally, making comparisons of treated vs. untreated areas tenuous at best.
From the early API tests in 1975 and 1979 to the most recent field trials and measurements completed in 1997, only one well-documented spill in which modern dispersants have been used has been studied in an efficient and controlled manner (Lunel et al., 1997a; Lunel, 1998). That was the Sea Empress oil spill in Milford Haven, UK, in 1996 (see case study in Box 3-4).
In future spill-of-opportunity tests, it is recommended that both dissolved-phase and particulate oil droplets be sampled (Payne et al., 1999; Payne and Driskell, 2003) so that measured concentration data can be used to validate computer-model predictions of these phases and so that the
|
BOX 3-4 Spilled Oil Type/Volume/Conditions: T/V Sea Empress grounded outside of Milford Haven on 15 February 1996, releasing a total of 72,000 tons (roughly 23 million gallons) of Forties Blend crude oil over a period of six days. Forties Blend is a relatively light crude oil, with an API gravity of 40°, viscosity of 3.88 cSt at 20°C, and pour point of −3° C. It readily forms emulsions of up to 60–70 percent water. Physical and Biological Setting: The outer coast consists of exposed steep cliffs with pocket beaches of sand and gravel. Water depths are greater than 20 m at 1 km offshore. Milford Haven is a sheltered bay with extensive tidal flats and marshes as well as beaches and rocky shores. There were sustained winds of 15–40 knots (roughly 27–74 kilometers per hour) throughout the spill. Offshore islands are important seabird sanctuaries with internationally important populations of puffins, guillemots, gannets, and Manx shearwaters. The area includes one of three marine nature reserves in the UK, two nature preserves, and 35 sites of Special Scientific Interest. It has popular tourist beaches and a local fishing industry. Dispersant Application: A total of 445 tonnes of seven different dispersant products were applied over seven days, mostly by DC3 spray aircraft. Spotter planes were used to direct the spraying to the thickest parts of the fresh oil releases each day. The first test application conducted 18 hours after the initial release of 2,000 tonnes was not effective, based on visual observations. The reason for the delay in application was that the initial oil release was carried by the ingoing tide into the Milford Haven which was an area where dispersant use was not approved. Oil moving back out of Milford Haven on the subsequent outgoing tide had weathered and was not amenable to dispersant by the time it was targeted. An oil-weathering model was used to predict that, after 12–18 hours, 40 percent of the oil would have evaporated and a 70 percent water-in-oil emulsion would have formed. A second test using both dispersant and a demulsifier 36 hours after the initial release was partially effective. On day 4, there was another release of 2,000 tonnes, where dispersant application on the fresh oil was determined to be highly effective, thus full-scale dispersant application was approved. There were continued daily releases of 5,000–20,000 tonnes of oil during a three-hour period of the falling tide. These slicks formed very discrete targets with limited spread of the oil. Each day, the thickest parts of the fresh oil slicks were repeatedly sprayed until they had been dispersed, then larger patches of more weathered oil offshore were sprayed. The last oil release occurred on day 7, and dispersant applications were terminated on day 8 when it was determined that they were no longer effective on the emulsified oil. Dispersant applications were generally restricted to beyond |
|
1 km of the shoreline, to meet the requirement of a minimum of 20 m water depth, though there were exceptions, particularly on one occasion when a patch of oil that had migrated north of the entrance to Milford Haven returned and there was a threat of it being carried in by the tidal movement, The decision to spray the target was made as the consequence of not doing so was not an acceptable trade-off. Monitoring Results: Effectiveness: There was an extensive monitoring program to document the effectiveness of each dispersant application consisting of visual observations from spotter aircraft, SLAR imagery, visual observations from boats, and measurements of oil concentrations in the water column using field fluorometry. The dispersants were most effective on the fresh oil releases, as indicated by plumes of dispersed oil in the water and large reductions in surface slicks. It was not possible to determine the relative effectiveness of the different dispersant products. Fluorometry measurements through the water column showed some natural dispersion, with oil concentrations of 3 ppm at 1 m, but less than 0.5 ppm at 4–5 m, indicating the formation of relatively large droplets during natural dispersion that remained in the upper water column (Lunel et al., 1997a). Oil concentrations under treated slicks were typically also 3 ppm but uniformly mixed down to 5 m, indicating the formation of smaller dispersed droplets that were vertically mixed under the strong wind conditions. Fluorometry and visual observations from boats were used to document that dispersant application on emulsified oil did increase the oil concentrations and depth of oil mixing into the water column. The first dispersant application appeared to break the emulsion, whereas subsequent applications increased the concentrations of dispersed oil into the water (Lunel et al., 1997a). Even with extensive monitoring, it was difficult to determine dispersant effectiveness. A mass-balance approach was used, as follows (Lunel et al., 1997a): (1) 40 percent was estimated to be lost by evaporation, based on a calibrated oil weathering model; (2) 3 percent was recovered at sea; (3) 7 percent stranded on the shoreline; and (4) the remaining 50 percent was assumed to have been dispersed. Experience and modeling was used to estimate that 10 percent of the oil would have naturally dispersed under the spill conditions (Lunel et al., 1997a). Thus, it was estimated that 40 percent of the oil (about 29,000 tonnes) was chemically dispersed. The dispersant-to-oil ratio was calculated to be 1:65, based on use of 445 tonnes of dispersants and the chemical entrainment of 29,000 tonnes of oil. Effects: The spill impacted 6,900 birds (mostly migrating scoters), and an estimated 5,000 tonnes of oil stranded onshore, resulting in shoreline oiling of 98 km as heavy, 34 km as moderate, and 66 km as light (Harris, 1997; Law et al., 1997). Oil concentrations in the water column below |
|
treated slicks were generally 1–10 ppm and uniformly mixed down to 5 m (the maximum depth of measurement) (Lunel et al., 1997a). Within 5 km downcurrent of the grounding site, oil concentrations in the water column were 0.5–0.6 ppm throughout 4 days after termination of dispersant applications; by 12 days after termination, oil concentration were 0.2 ppm or lower. At distances of >10 km downcurrent, oil concentrations were 0.2–1.0 ppm throughout 6 days after termination of dispersant application; by 12 days after termination, they were 0.2 ppm or lower. There were reported mortalities of shallow sub-tidal and intertidal organisms, with bivalves and urchins washing up by the hundreds in some areas. Wild salmon, other finfish, crab, lobster, and whelk were found to have low levels of PAH but no taint. Intertidal mussels remained contaminated in one bay with heavy shoreline oiling for 19 months after the spill. SOURCE: Summarized from Harris (1997), Law et al. (1997), and Lunel et al. (1997a). |
data can be compared to values typically used in water accommodated fractions (WAF) generated for dispersed oil toxicity evaluations (see Chapter 5).
Monitoring Dispersant Use During Actual Spills
Monitoring of dispersant use means different things to different people. The mental model one has of concepts or definitions is generally associated with their background and stakeholder role. Dispersant-use monitoring can be separated into two basic categories: (1) information collected to help make timely operational decisions; and (2) data gathered for future analyses of fate and effect (Pond et al., 1997). Operational monitoring should provide information on the application platform’s spraying parameters and on whether or not oil is being entrained into the water column. This information should be conveyed immediately to those making the decision on whether or not to continue the operation. The second type of monitoring involves collecting data that can be later used to address the fate and effects of the dispersed oil and may also be used to ground truth some of the operational monitoring information (Hillman et al., 1997). In every dispersant application, operational monitoring is done to some degree. Depending on the circumstances, ground-truth information on fate and effect may or may not be required.
Dispersant effectiveness is a phrase that has been interchangeably used to describe how well the product performs both in the laboratory
and in field applications. As discussed previously, there are three components that will determine dispersant effectiveness during spill response: operational effectiveness, chemical effectiveness, and hydrodynamic effectiveness. The common usage of “dispersant effectiveness” to describe performance in the laboratory and the field is unfortunate because laboratory-derived effectiveness usually does not equate to effectiveness in field applications (e.g., see Table 3-3). This dual usage has fostered misconceptions and misunderstanding throughout the response community and the public. As described previously in this chapter, laboratory tests generally measure chemical effectiveness, whereas effectiveness in the field is also dependent on operational and hydrodynamic factors. Therefore, a laboratory effectiveness of 60 percent does not mean that 60 percent effectiveness will be obtained in field applications. Depending on many factors, the field effectiveness for a product may range from 0 percent to 100 percent.
Effectiveness of a dispersant application in the field has been defined as “the amount of the oil that the dispersant puts into the water column compared to the amount of oil that remains on the surface” considering the total amount of the oil that was treated (Fingas, 2002a,b; 2003; Lewis, 2004). U.S. Coast Guard, et al. (2001) define effectiveness based upon the amount of oil that the dispersant puts in the water compared to the amount of oil that was in the area treated. NRC (1989) concluded that a mass balance approach has given good effectiveness estimates in a few elaborate field tests, but “it is complicated, requires set-up time, and is not practical in real spills.” In field experiments, the release volume is known, the area of the slick can be measured, and the average thickness for this finite area can be calculated. In addition, dispersants are generally applied to the entire test slick; thus mass balance effectiveness estimates may be applicable. In accidental spills, however, only a portion of the total amount of spilled oil is normally treated, the oil thickness of the treated area is unknown and highly spatially variable, and thus the volume of oil in the treatment area is seldom known to any great accuracy in a timely manner. Presently, there is no valid and reliable method of determining slick thickness in the field, and any estimated value may easily be in error by an order of magnitude (Fingas, 2002a,b).
Prior to development and implementation of a monitoring plan, it is imperative that the stakeholders agree on attainable goals and objectives for the monitoring (U.S. Coast Guard et al., 2001). Among these goals and objectives should be a working definition of field dispersant effectiveness and a set of Standard Operating Procedures (SOPs) with data quality objectives. The definition of field effectiveness could parallel the definitions of mechanical recovery (i.e., percent recovery of the entire spill) or in-situ burning (i.e., percent of oil burned from a contained area).
The degree and extent of monitoring should be in proportion to the
sensitivity of the environment. In general, the more sensitive the environment, the more emphasis should be placed on monitoring. Sensitivity can be assigned based upon environmental and political parameters. Basically the resource trustees and stakeholders want to know how well the response works and the extent of the effects. There is a heightened concern as the sensitivity increases. Factors that have a direct relationship to sensitivity include, among others, nearness to shore, special habitats such as marine sanctuaries and parks, biological and migratory seasonality, size of incident, and nature of the spilled product. Nearness to shore generally involves environments with shallower water, lower dilution rates, higher productivity, fish and shellfish nursery grounds, higher concentrations of wildlife, greater commercial and recreational use, and shorter response times.
As discussed in Chapter 2, it is very advantageous for the resource trustees and stakeholders to pre-identify sensitive areas, determine where and when dispersant use should be discussed, and outline monitoring objectives. Unless otherwise stated, pre-approval agreements generally are based on the assumption that use of dispersants, under specified conditions, will protect sensitive shoreline and water-surface resources without causing significant impacts to water-column and benthic resources, even assuming 100 percent dispersion of the slick.
Operational Monitoring
The primary reason to monitor operational aspects of dispersant use is to determine if the dispersant application is operationally effective (e.g., that the dispersant is being applied to the surface oil targets). The secondary purpose is to estimate the relative effectiveness of the operation (Fingas, 2003). Additional data also are needed to provide documentation on what dispersant was used, how much was used, when and where it was used, and the environmental conditions at the dispersal sites. Because there is no truly quantitative method to determine dispersant effectiveness in the field, the best that can be done is to qualitatively estimate if the dispersants are working (Henry, 2004).
Effective/Ineffective Dispersant Applications
It is assumed that some portion of the dispersant spray will miss the target due to wind drift of the spray or turning pumps on too soon or off too late (see earlier discussion of dispersant use in response to the T/V Exxon Valdez spill). Missing the target excessively should be documented in the monitoring report, and controls should be enacted to minimize this to within acceptable limits. An experienced trained observer is the best
TABLE 3-5 Guidelines to Assist in Determination of Effective/Ineffective Application
|
Possible Dispersant Action |
Possible False Positives |
Possible False Negatives |
|
Difference in appearance between treated and untreated slick.a,b |
Suspended solids or algal blooms may resemble dispersed oil.a,b |
Dispersion may not be instantaneous, may take several minutes to a few hours to show dispersed plume.a,b,c,d |
|
Appearance of plume can range from brown to pale yellow.a,b,c,d |
Boat wakes through oil may appear as dispersed paths.a |
Visible cloud or plume not observed, water may be naturally murky.a,b,c |
|
Changes in area and thickness of the oil.c |
Dispersants may have a herding effect on thin oil. May also be seen as lacing.b,d |
|
|
Higher fluorometer readings of dispersed oil in application area vs. background or non-treated slick area.a,b,c,d |
Rapidly dissipating whitish plume may be caused by dispersant alone (missed target).d |
|
|
|
After initial visual assessment, some dispersed oil may resurface.d |
|
|
aUSCG et al., 2001. bNOAA, 1999. cExxonMobil, 2000. dFingas, 2003. |
||
way to assess if the dispersant operation is effective or not (Lewis and Aurand, 1997; U.S. Coast Guard et al., 2001; Fingas, 2003; Goodman, 2003; Henry, 2004). Even though there are difficulties with the interpretation of fluorometer data (Fingas, 2003; Goodman, 2003), the addition of confirmation fluorometer readings will help substantiate visual observations that there has been an increase in the amount of oil entrained into the water column under treated slicks. Table 3-5 contains guidelines to assist in determination of effective/ineffective application.
Special Monitoring of Applied Response Technologies
The protocol used by most if not all U.S. regions for obtaining operational monitoring information for dispersant use and in-situ burning is Special Monitoring of Applied Response Technologies (SMART) (U.S.
Coast Guard et al., 2001). The purpose of the dispersant section of SMART is to outline a protocol that rapidly can collect information to assist in real-time decisionmaking during dispersant applications (Barnea and Laferriere, 1999). SMART only outlines how to determine if the dispersant application is working, but provides no guidance on how to determine a percent dispersant effectiveness. It relies heavily on personnel being trained using job-aids developed to support SMART (Levine, 1999). For much of coastal and offshore waters of the United States, the resource trustees and stakeholders have designated selected areas as pre-approved for dispersant use. All pre-approved areas have a stipulation that requires use of the SMART protocols for operational monitoring, if operationally feasible. In an effort to better document effectiveness, field portable equipment has now been prepared and staged within various RRTs for immediate deployment in the event of a spill (Gugg et al., 1999; Barnea and Laferriere, 1999; Henry et al., 1999; Henry and Roberts, 2001). Some pre-approvals indicate that SMART will be used for fate and effects monitoring; however, SMART specifically states it “does not monitor the fate, effects, or impacts of dispersed oil” (U.S. Coast Guard et al., 2001). The SMART protocol contains three tiers of monitoring:
Tier I is visual monitoring by a trained observer, preferably using an aircraft separate from the “spotter” aircraft directing the dispersant application (U.S. Coast Guard et al., 2001). The protocol recommends documentation via forms, photography, and videotape. Tier I monitoring may be enhanced through the use of remote sensing instruments, such as infrared thermal imaging, if data are available in real-time. The purpose of Tier I is to visually assess if the operation is working and rapidly report the findings to the decisionmakers. Typical observations include: (1) that the dispersant spray hit the slick; (2) a reduction in the amount of oil on the water surface after dispersant treatment; (3) a change in the appearance of the treated slick; and (4) the presence of a milky or cloudy plume in the water column.
Tier II includes Tier I monitoring and adds an on-water component. From a vessel, water samples are analyzed via continuous flow fluorometer collecting water at a 1 m sampling depth. The protocol recommends comparing fluorometer measurements from three general areas: (1) background water outside the spill area, (2) below the surface oil slick before dispersant application, and (3) an area where the oil slick has been treated with dispersants. The purpose of Tier II is to confirm whether or not oil is being entrained into the water column (Barnea and Laferriere, 1999). A few water samples are collected for later laboratory analysis to validate and possibly quantify the fluorometer measurements.
Tier III is presented as “Additional Monitoring” to collect information on transport and dispersion of the oil into the water column. It fol-
lows Tier II procedures but adds multiple depth fluorometer sampling of selected transects and provides for collection of additional environmental parameters, such as water temperature, conductivity, dissolved oxygen, pH, and turbidity.
The SMART protocol includes collection of water samples to validate and quantify the fluorometer readings. Calibration methods and techniques are discussed in Lambert et al. (2001a,b) and Fingas (2002a,b). The validation method can estimate the quantity of “oil” in the water column, but the data cannot be used to differentiate between that part that is dissolved and that part that is in droplets. Fingas (2003, 2004a) discussed the precautions and proper use of fluorometry in the field. His comments on field techniques include awareness of possible contamination using Tygon tubing and maintaining the sampling probe in waters undisturbed by the vessel (in front of or outside the bow wave).
The Alyeska Ship Escort Response Vessel System (SERVS) has developed dispersion monitoring guidelines that are similar to the SMART protocol, but the primary goal of the Alyeska/SERVS protocol is to provide real-time assessment of the environmental effects of dispersion (Hillman et al., 1997). The Alyseka/SERVS protocol relies on aerial monitoring as the primary tool for monitoring dispersant effectiveness and effects with additional support provided by collection of water samples and in-situ fluorometry. This protocol is not intended to provide quantitative estimates of dispersant effectiveness, real-time estimates of water-column dispersed oil concentrations, or estimates of oil mass balance. This protocol attempts to monitor the dispersed oil plume by locating the water-column sampling stations and the in-situ fluorometry transect relative to drogues that drift with subsurface currents (usually at 2-m depth). Whereas SMART and the Alyeska/SERVS protocols rely on conventional filter fluorometers with a single filter for excitation and another for emission for in-situ measurement of dispersed oil concentrations, multiple-wavelength fluorometers and in-situ instruments capable of measuring particle-size distributions have been investigated for research use (Fuller et al., 2003). Unfortunately, the performance of these instruments for monitoring oil dispersion at sea has not yet been evaluated (Ojo et al., 2003).
Additional Operational Monitoring
To better document the operation and to possibly provide clues to future questions, several delivery platform and environmental parameters should be recorded. Pre-application documentation should include the name, lot number, and quantity of dispersant loaded on the aircraft or vessel. A sample should be taken, with proper chain-of-custody, from each dispersant lot (to allow for later analysis if verification of product effec-
tiveness is needed). After each sortie, the amount of dispersant remaining onboard should be documented. Also, other data are needed on the platform performance during the application and on the environmental conditions in the application area. Table 3-6 provides guidance on the additional monitoring data or samples to be obtained. Most of the performance data should be automatically recorded on the platform.
Environmental Monitoring
As discussed in Chapter 2, there is a reasonable degree of confidence in the current ability to assess trade-offs, relative to use of dispersants, in offshore waters. In general, offshore waters are considered to be less sensitive to dispersed oil impacts, because of rapid dilution of dispersed oil, than shallower or nearshore environments. But as shallower or nearer to shore waters are evaluated for dispersant use, the sensitivity of the environment and the degree of uncertainty make the assessment more difficult. The database of oil component acute toxicity is much better than the knowledge of the bioavailability of dispersed oil components in the water column. Unfortunately, most of the measurements on concentrations of
TABLE 3-6 Guidance on the Additional Monitoring Data or Samples
|
Pre-application |
Application |
Post-application |
|
Name of dispersant Dispersant lot number Sample of each dispersant lot number Volume of dispersant onboard Platform (aircraft or vessel) Description of spray system Dispersant pump calibration documentation Spray nozzle test documentation |
Spray time Pump rate Speed during application Spray height during application Sample of weathered and neat oil Dispersant applied neat or diluted Wind speed and direction Current speed and direction Air and surface water temperature Cloud cover Surface salinity Wildlife in area (birds, mammals, turtles) Approximate spray width Approximate spray path length Number of passes over same area to achieve adequate dispersion. Sea state |
Volume of dispersant remaining onboard |
dispersed oil in open water are from fluorometer readings or from the total extraction of unfiltered water samples. Thus at best, the total concentration of oil components in the water column is known, but not whether the component concentrations reside in the water or in oil droplets is not known. Questions the risk assessors need answers to concerning the dispersed oil include: (1) How are the components of dispersed oil distributed in the water column? and (2) What fractions are in the dissolved phase and what fractions are in droplets or adhered to particulates in the water column? These data cannot be obtained through fluorometry, and Page et al. (2000b) have shown that estimations of oil-component partitioning based upon solubility coefficients alone are not reliable for oil-in-water mixtures. The data can be obtained via discrete large-volume water samples that are collected and filtered immediately to differentiate between components that are truly dissolved and those that are present as dispersed oil droplets (Payne et al. 1999; Payne and Driskell, 2001, 2003). These samples, at a minimum, should be analyzed for dispersed oil droplet and dissolved-phase PAH and total petroleum hydrocarbon (TPH) concentrations in the filtered and unfiltered water. Sample collection should be from several depths and repeated over time. Real time in-situ fluorometry data should be used to locate where to take samples and to verify that the discrete samples were taken in the dispersed oil plume. In additional to finite grab examples collected with traditional water-sampling equipment, aliquots of effluent from the fluorometers should also be collected for chemical analysis. Whenever possible, separate fractions for dissolved and particulate/oil-phase components should also be analyzed (Payne et al., 1999; Payne and Driskell, 2001, 2003). Monitoring data, coupled with local transport mechanisms, can be used to validate computer-model predictions, and thus reduce the uncertainty of the fate of dispersed oil components. Ultimately, this will provide decisionmakers with a better tool to assess use of dispersants in sensitive environments.
The trustees of the local resources at risk will determine if other types of monitoring are needed to assess the effects. The extent of monitoring should be based on the sensitivity of the environment and the predicted amount of dispersed oil reaching the resources of concern. The collection and analysis of samples, whether they are sediment, nekton, or benthos, should be conducted so there can be a direct comparison with water-column analytes.
DEVELOPING ADEQUATE UNDERSTANDING OF DISPERSANT EFFECTIVENESS TO SUPPORT DECISIONMAKING
As discussed in Chapter 2 and shown in Figure 2-4, the potential effectiveness of dispersants is a key consideration at several steps in the
decision-making process. Significant work has been done to test dispersant products on a range of oil types or refined products under different test conditions (temperature, salinity, etc.). The test protocols were designed to establish a high degree of reproducibility, but were never intended to replicate actual environmental conditions that may be encountered during a spill. However, these kinds of tests are useful to provide guidance on whether or not a test oil is likely to be dispersible under ideal conditions.
The fourth question in Figure 2-4—“Are conditions conducive?”—addresses the range of factors that affect the overall field effectiveness of dispersant application once the oil starts to spread and weather. Currently, predicted dispersant effectiveness for a specific spill event is based on simple models and past experience. In current fate and transport models, dispersant effectiveness is an input value. In the future, it would be desirable to possess the ability to predict dispersant effectiveness over time through the use of a physical-chemical efficiency model. However, additional research is needed to develop the model. Relevant state and federal agencies and industry should develop and implement a focused series of studies that will enable the technical support staff advising decisionmakers to better predict the effectiveness of dispersant application for different oil types and environmental conditions over time.
Bench-scale effectiveness tests can provide a valuable tool for investigating the factors and interactions that affect the chemical effectiveness of oil dispersion. A particular strength is their ability to inexpensively and quickly test a large number of conditions. Currently, most bench-scale effectiveness tests incompletely characterize the test conditions and do not systematically vary factors, such as mixing energy, that are known to have a strong influence on the process of oil dispersion. In addition, important response variables, such as oil droplet-size distributions, are not routinely measured. As a result, bench-scale effectiveness tests cannot, in general, provide the type of input that is needed for fate and transport models. Experimental systems used for bench-scale effectiveness tests should be characterized to determine the energy dissipation rates that prevail over a wide range of operating conditions. Future effectiveness tests should measure chemical effectiveness over a range of energy dissipation rates to characterize the functional relationship between these variables. Finally, evaluation of chemical effectiveness should always include measurement of the droplet-size distribution of the dispersed oil.
Wave-tank-scale effectiveness tests are particularly useful for investigating factors that cannot be studied in laboratory-scale tests. In addition, the more realistic mechanism of energy input in experiments conducted in wave tanks reduces the sensitivity of results to uncertainties
regarding the mechanism of oil-droplet formation and, therefore, scaling of laboratory- or wave-tank-derived effectiveness estimates to sea-surface conditions.
The design of wave-tank dispersant-effectiveness studies should specifically test hypotheses regarding factors that can affect operational effectiveness. These factors include oil properties that are representative of those expected to prevail under spill-response conditions, such as water-in-oil emulsification and the potential for heterogeneity in the rheological properties of the floating oil (e.g., formation of a “skin” that resists dispersant penetration). Dispersant droplet-size distributions and impact velocities should be similar to those that would be expected to be generated by dispersant application methods commonly used in oil-spill response.
Tank tests that determine the ability of mechanical recovery methods to recover oil that has been treated with dispersant but not effectively dispersed, or re-floated oil, should be carried out. A more complete understanding of what limitations the unsuccessful use of dispersants may have on subsequent mechanical recovery methods could greatly reduce concern over relying on operational testing of the dispersant effectiveness in the early phases of spill response.
Energy-dissipation rates should be determined for wave tanks over the range of operating conditions that will be used in dispersant effectiveness tests. The wave conditions used in dispersant effectiveness tests should represent a specific environment of interest. It may be necessary to conduct experiments over a range of energy dissipation rates to adequately represent the environment of interest.
More robust understanding of dispersant effectiveness can be derived from test tanks, if more rigorous protocols are implemented that better quantify the eventual fate of the test oil. The concentration of oil should be measured in all identifiable compartments to which it could be transferred when dispersant effectiveness is investigated in wave tanks. This includes, but may not be limited to, the water surface, the water column, the atmosphere, and wave-tank surfaces. Oil mass balances should be reported in an effort to better understand the accuracy of effectiveness quantification. In addition, the droplet-size distribution of the dispersed oil should be measured and reported.
Little is known of the potential leaching of surfactant from floating oil and dispersed oil droplets at realistic oil-to-water ratios and under turbulence conditions that might be encountered in the field. In particular, the effects of surfactant leaching on the effectiveness of oil dispersion and the potential for droplet coalescence should be understood better. Coalescence and resurfacing of dispersed oil droplets as a function of mixing time should be studied in flumes or wave tanks with high water-to-oil
ratios (to promote leaching of surfactant into the water column). Periods of wave-induced turbulence should be followed by periods of relative calm to allow droplets to resurface. The surfactant concentration remaining in the resurfaced oil should be measured, and its dispersibility should be measured (by introducing more wave turbulence) to evaluate the ultimate fate of resurfaced oil. Alternatively, oil dispersion should be measured after dispersant is applied and incubated with floating oil under calm conditions to determine the effect of surfactant leaching from a surface oil film on dispersant effectiveness.
Although careful and controlled research in the laboratory or test tank will be important to developing tools to support decisionmaking, the results of dispersant application during real spills will be the most important indicator of whether or not the dispersant application was effective. Field data are essential to a better understanding of the spill-specific conditions that affected the dispersant operation, and they should be used to validate model predictions. To improve the quality of field data collected during dispersant applications, more robust monitoring capabilities should be implemented. Specific attention should be given to:
-
Developing an environmental monitoring guidance manual for dispersant application monitoring with suggested sampling and analytical techniques, sampling methods, and QA/QC to ensure cost effectiveness and maximum utilization of the data
-
Developing a detailed standard operating procedure (including instrument calibrations and data quality objectives) for each sampling and analytical module (SMART is guidance only)
-
Developing a definition of field effectiveness
-
Measuring dispersed oil droplet and dissolved-phase TPH and PAH concentrations with grab samples of filtered and unfiltered water (these data can then be compared to model predictions and toxicity data for both dissolved and particulate/oil-phase components) as a function of location and time.