Panel III—
Partnerships Against Bioterrorism
INTRODUCTION
Larry Kerr
Department of Homeland Security
Dr. Kerr observed that this panel would present “the absolute experts in this area” who had gained from “nearly a year’s worth of hindsight on the events of last fall.” He recalled that “several distinguished panels” had already met over the past year to discuss the strengths and weaknesses of our bioterrorism preparedness. These include the NRC’s Branscomb and Klausner report,13 a blue-ribbon panel of the National Institute of Allergy and Infectious Diseases, the Presidents’ Council of Advisors on Science and Technology (PCAST) letter, the President’s national strategy on homeland security, and the House and Senate draft legislation for the new Department of Homeland Security.
All these documents had a common theme, he said: our strongest weapon against terrorism will be partnership. And in partnering, we have to create a technological and operational advantage over enemies. “As we work to create the Department of Homeland Security,” he said, “the transition planning office has been mandated by the President and by Governor Ridge to create a
groundswell within the nation’s R&D efforts and to establish a new paradigm for preparedness.”
He said that his office had been mandated to find ways to bring disparate partners together into mutually beneficial collaborations: government with academia, government with the private sector; federal with state and local partners; big business with small business; military with civilian.
The office planned to bring scientists, engineers, intelligence, and law enforcement groups together to focus not only on research and development, but also on testing, validation, and evaluation; on procurement and distribution; on concepts of operation; on all vital technologies necessary to defend the homeland. He noted that the office had a unique mandate within the bio-defense arena, and a unique opportunity. “I am constantly reminded that as we seek to guard our people and agriculture against biological attack,” he said, ”we do so by strengthening the resources and technologies that ultimately contribute to our public health infrastructure.” An improved arsenal of diagnostics, therapeutics, and vaccines; better protective equipment for first responders; and a national disease surveillance system—all help improve the public health while offering powerful deterrents to biological attack. “This National Academy of Sciences symposium,” he said, “could not have come at a better time for us.”
PARTNERING FOR VACCINES: THE NIAID PERSPECTIVE
Carole Heilman
National Institute of Allergy and Infectious Diseases
Dr. Heilman began by saying she would offer some perspective on the National Institutes of Health (NIH), especially on their vaccine programs, and then focus more specifically on NIAID’s biodefense activities. She would refer often to partnerships, she said, because “one of our assets at NIH is our history of partnering.”
NIH supports several but not all aspects of the vaccine R&D pipeline, she said, and this necessitates partnerships with other entities that bring needed skills to vaccine development. This development process is long and tedious, she said, both because vaccines are difficult biologics and because regulatory hurdles slow the process. Thus, partnerships make both technical and financial sense. About 90 percent of NIH’s budget goes off-campus to support the activities of academic and business researchers; the balance covers intramural research programs and support functions at NIH.
Vaccine development requires information that is difficult to produce before decisions can be made, she said. Researchers have to understand the pathogen, its components, and how it interacts with the body. Only when those questions are answered, can researchers begin identifying targets and move from those targets toward making the tools that will be needed for actual development of a vaccine.
Once a vaccine is made, laborious preclinical testing begins. This is often a cyclical process—an initial try, adjustments, a retry, tweaking again, retrying, and so on. After that preclinical work, a sample lot must be produced and approved for human trials—a “huge hurdle.” The vaccine must go through a series of clinical tests to assure that it is not only effective but also extremely safe. Because vaccines are generally given to healthy people, the standards required for ensuring safety are very high.
This process requires multiple players, all of whom contribute in different ways to new or improved vaccines. The NIH in general conducts basic research and develops medical interventions. The Centers for Disease Control and Prevention (CDC) is involved in surveillance, training local response teams, and maintaining stockpiles of vaccines and antimicrobials. The Food and Drug Administration (FDA) is responsible for regulating vaccines, therapeutics, and diagnostics. Both large and small companies contribute in different ways toward vaccine development. In addition to the academic community, there are strong partners among nonprofits and global organizations. The Gates Foundation, in particular, provides critical “pull,” guaranteeing purchase of vaccines for developing countries. This provides a needed incentive to many companies.
Partnering for Vaccine Development
The NIH has long maintained robust vaccine development programs, she said, and they have become more robust as a result of post-9/11 activities. Virtually all of the vaccine development of NIH involves partnering in a variety of ways. This requires flexibility on the part of both partners, especially for industrial partnerships. Each partner needs something different, or they need to negotiate in a different way.
She turned to biodefense, and why the Department of Health and Human Services (DHHS, the parent agency of the NIH) is concerned about vaccines as they relate to this topic. Traditionally this has been the domain of the Department of Defense (DoD), but for three reasons the NIH has now moved into this area.
First, biodefense is different from biowarfare. Vaccines developed by the DoD are usually developed with the goal of preparing troops for situations where they may encounter a bioweapon. The licensed anthrax vaccine, which involves six shots over 18 months followed by yearly boosters, can be used for troops, but is not helpful in the event of a terrorist attack. Because it is impossible to guarantee an amount of time to prepare for a bioterrorist attack, the goal is to develop a vaccine that works quickly.
Second, in the military, the population to be defended is fairly homogeneous. The population at large is not homogeneous; it includes infants, people with HIV, and people on chemotherapy, the well elderly, and other special populations with less than robust levels of immunogenicity. This requires different approaches to prevention and treatment.
Third, the threats one may encounter in civilian settings are usually different from those in military settings. A terrorist might decide to lace all muffins in a coffee shop with giardia and succeed in causing considerable confusions and even panic; in a military situation, soldiers with giardia would be expected to tough it out.
She said that the two most important pathogens on the list of category A pathogens are smallpox and anthrax; the third is Ebola virus (see Figure 13). Category B and C priority pathogens contain a larger number of biological agents; little is known about some of these pathogens.
A Six-Fold Increase in Funding for Biodefense
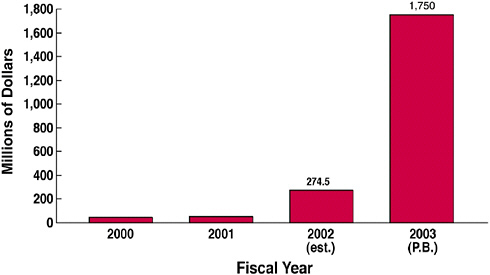
The Department of Homeland Security, in a document about strengthening the nation, recognized the importance of vaccines, antimicrobials, and a strong infrastructure for medical research. As a result of many factors, including that publication, said Dr. Heilman, the biodefense budget of the NIH was increased about six-fold from FY2002 to FY2003 (see Figure 14).
Little is known about some of the potential agents of bioterror, because of the difficulty of studying them in either a natural or laboratory setting. Consequently, some of the additional funds are being used to develop a robust basic research program, including genomics and proteomics. More Biosafety Laboratory (BSL)-3 and BSL-4 facilities are being built to address the safety of individuals working on pathogens and the general public. One of the immediate
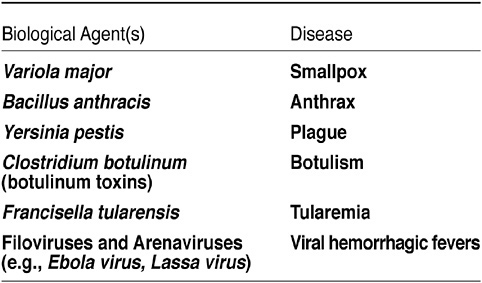
FIGURE 13 Biodefense: Category A agents. SOURCE: CDC.
goals is to distribute research finding to scientists through bioinformatics resource centers with databases that will allow scientists to access a large amount of genomic and related data.
She added that the American people expect products at the end of all this research, so there are specific objectives focused on producing drugs, vaccines, and diagnostics. In addition, the agency plans to expand the clinical research component, which must accompany the development of any particular product.
The NIH has an understanding with Congress that these projects require a long-term commitment of support in order to succeed. Providing homeland security is a continuing need and objective that cannot be reached in a brief burst of activity, no matter how well funded. As the need for research facilities is gradually met, researchers will continue to need flexibility to develop drugs and vaccines, and conduct basic research that makes them possible.
It is an NIH tradition to recruit groups of outside experts to provide objective guidance and help develop appropriate strategies for addressing timely issues. In February 2002, she said, NIAID convened a panel of experts to develop a strategic plan to guide the implementation of basic and translational biodefense research emphasizing specifically the Category A priority pathogens (see Figure 16). At the end of October, the institute repeated this exercise for Category B and
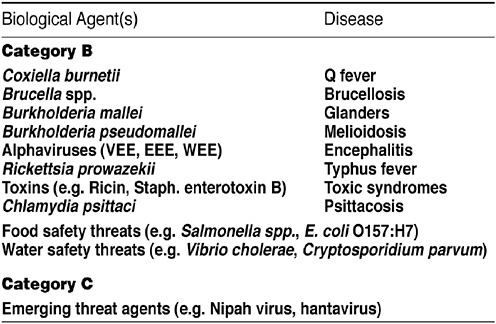
FIGURE 16 Bioterrorism: Category B and C agents. SOURCE: Rotz et al., Emerging Infectious Diseases, February 2002.
C priority pathogens. From those meetings, the institute determined that it should be prepared to cover a wide variety of areas relating to research on the biology of the microbe, host response, and basic and applied research aimed at developing diagnostics, therapeutics, and vaccines against these agents. These areas include genomics, proteomics, antimicrobials, vaccines, and the expansion of research capability. The results of the meetings guided the institute in building its budgetary plan.
Goals for Biodefense at NIAID
The groups set the following goals:
-
Conduct basic research on the biology of the microbe and host response;
-
Conduct basic and applied research aimed at developing diagnostics, therapeutics, and vaccines against these agents;
-
Develop improved vaccines against microbes for which vaccines currently exist but may not be useful for the civilian population;
-
Develop new vaccines for microbes against which no vaccines currently exist;
-
Establish needed research resources and make them available to the scientific community.
NIH’s extramural research grants provide a mechanism to attract not only the academic community but also private firms. The money goes out in several forms: general grants, cooperative agreements, and contract resources. She highlighted the importance of SBIR grants to the small business community, which “really rallied after 9/11.” Within about a month, she said, NIAID had put out a solicitation to the small business community, detailing exactly what was needed. This drew about 300 responses within a month. “It was a phenomenal expression of interest and capability and good application, with extremely thoughtful approaches.”
She described a recent effort to develop new models. One was a challenge grant model, in which the government grant must be matched 50–50 by the grantee, to use in developing products. This had been refined with the realization that a balance of 80–20, or even 90–10 was more realistic for most companies, while maintaining the principle of industry participation. The institute recognized that the way industry does business is different from the way academia does business, and most of the institute’s mechanisms had previously been based on academic models.
The institute also developed a series of contract infrastructures. One, a contract for vaccine products, was unusual for the NIH. It meant that in some ways, the institute was operating like a medium-sized manufacturing company, offering to do some screening, animal model evaluation, preclinical testing, and clinical
trials, and to help the company submit an application for an investigational new drug (IND). The flexibility of this model contributed to its success, because each company was likely to have different needs. Some companies might have the money to achieve all these steps; most would not. Some companies would encounter stumbling blocks; the institute could look for ways to help the firm move past them.
She said that NIAID did recognize that this kind of partnership was an important vehicle for business, especially in biodefense. Companies may not have in their own labs such specialized resources as assays for anthrax or nonhuman primates to do an aerosolized challenge model.
She also described other forms of partnering—with non-governmental organizations (NGOs), for example. She said that many people and organizations had tried to fill some of the post-development gaps, such as guaranteed purchases. Various collaborators had also provided surveillance data or needs assessments for particular products.
She focused then on vaccine production as an “example of the kind of things we can do.” Within each program in the biodefense research pathway, NIAID supported 30 to 40 to which it was encouraging people to apply. Each initiative was targeted toward a certain area, with its own specific approach and needs.
Models for Vaccines
Three specific models were evolving around biodefense vaccines. In the first, DHHS awarded a $428 million contract to produce a smallpox vaccine. This was a guaranteed-purchase model, which was a usual model for the government, and it inspired differences of opinion on its viability.
A second model was a “medium-sized pharma model” designed to build infrastructure. Since NIAID was created in 1962, it has been building and working with Vaccine and Treatment Evaluation Units for developing vaccines. This kind of infrastructure allowed NIAID to respond quickly to biodefense needs. Because the units were under contract to NIAID, the institute could arrange the priorities.
As the institute waited for its post-9/11 contracts to be implemented, it performed an inventory of smallpox defenses. It found available only 15 million doses of smallpox vaccine for a U.S. population of some 280 million. Researchers then asked themselves whether they could dilute the dosage, and found that the answer was yes: They could dilute it at least 1:5, and perhaps 1:10 if necessary. That raised the number of doses to 75 or 150 million. They also analyzed what could be expected in the way of clinical reactions if the entire population had to be vaccinated, which was very important for policy making. They found that in a normal population that is vaccinated with smallpox vaccine, there are many adverse reactions, but most are not serious or life-threatening.
In addition, NIAID was able to find additional stocks of vaccine that had been made by Aventis Pasteur three or four decades ago. They obtained this vac-
cine and found that it was still potent and could also be diluted. So there was now at least enough known smallpox vaccine for the entire U.S. population.
The third model, designed to develop a vaccine against Ebola virus, had been developed over the past four years at an intramural facility called the Vaccine Research Center in Bethesda, Maryland. For Ebola, NIH formed a Cooperative Research and Development Agreement Opportunities (CRADA) partnership with a small company to produce and market the vaccine, and determined what would be the most useful assistance NIH could provide in order to make the partnership work.
As her last point, Dr. Heilman announced a piece of breaking news. Just 4 hours previously, she said, NIAID had announced a new contract for the development of an anthrax vaccine. This would be different approach, using a recombinant protective antigen, in which researchers use a fragment of the anthrax agent instead of the whole organism as the antigen. From preliminary studies performed jointly with DoD for the past 5 years, the institute had concluded that the product had “a good safety profile and seems to have a nice antibody profile.” Now that the decision had been made to proceed with the technique, she said, this particular program would be fast-tracked.
She concluded by reaffirming NIAID’s support for public-private partnerships. “Our job is not to market vaccines,” she said. “It doesn’t make sense for us to develop vaccines if we have no partner at the other end to bring them to market. So for us, a very important component in deciding how heavily to invest in vaccines is the commitment of the private partner.”
PARTNERING FOR COUNTER MEASURES: THE PRIVATE RESEARCH PERSPECTIVE
Gail Cassell
Lilly Research Laboratories, Eli Lilly & Co.
Dr. Cassell said she would talk about three different topics: (1) the current status of countermeasures against biothreat agents—focusing not on vaccines, which Dr. Heilman addressed, but on antibiotics and antivirals; (2) the partnerships that had already been established and seemed to be working; and (3) issues that may need to be resolved to encourage more partnerships for future development of countermeasures.
With the diversity of biological weapons and the ever-increasing possibilities to create new weapons through genetic engineering, she said, there was no simple way to develop countermeasures to biothreat agents. A sobering consideration was that it might take only 3 to 6 months to develop a new biological weapon, but at least 8 to 10 years to develop a new antibiotic, antiviral, or vaccine.
She also pointed out that because of the diversity of biological weapons, including a number of different viruses and bacterial agents, many infectious agents require broad-spectrum therapies.14 The same holds true with respect to development of anti-viral treatments. In the past, research on anti-virals had focused mostly on agents for specific viruses in a one-to-one relationship. Researchers would like to be able to use new technologies to develop broad-spectrum antivirals for use in emergencies. To date, this had not been possible, she said. In our armamentarium today, we had 13 different viruses on the “select” list, but only a single anti-viral was being tested, cidovifir,15 a derivative of which was indicated for treatment of vaccinia and other pox viruses. Even this drug could only be administered intravenously, limiting its usefulness in time of emergency. In addition, it is nephrotoxic, as antivirals often are, which may limit its safe use in children.
In the face of the large number of major viral diseases, health officials had at their disposal only a small number of anti-virals to naturally occurring viruses. The only antivirals that had been approved and marketed for use were those used against HIV, hepatitis B, and herpes.
For antibiotics, the situation was somewhat better, she said, but still worrisome. Over the last 30 to 40 years, only two new classes of antibiotics have been developed and introduced. The more recent was approved several years ago, but unfortunately, resistance to it developed even before FDA approval and launch. Before that—about a decade ago—companies were excited when genetic sequencing was completed for a number of important bacterial pathogens; this heralded two decades of drug development now regarded as the “golden age of antibiotic discovery.” Again, however, results have been disappointing. Even with millions of dollars invested in trying to develop new classes of antibiotics, the products that had reached phase one through three development, and even late preclinical stages, still fell short of completely new classes of antibiotics or new broad-spectrum antibiotics.
At first glance, a tally of the antibiotics that had reached phase one through three development seemed encouraging, she said, with a total of some 20 new drugs. But a closer examination would reveal that these were not truly new classes of antibiotics, but slight modifications of tetracycline and other macrolides and a larger number of quinolones. Quinolones can be effective drugs, she said, but bacteria develop resistance to them quickly. In China, for example, some 50 per-
cent of the strains of E. coli, the common intestinal bacterium, are already resistant to commercial quinolones. A new class of broad-spectrum antibiotics is needed, because no one knows what specific agent might be used in bioterrorism. The best strategy, she said, would be to develop a drug with broad-spectrum activity to hold in reserve for immediate response; that drug could then be replaced by drugs that are more selective when the agent was identified.
To summarize her discussion of antivirals, she emphasized there exist virtually no broad-spectrum anti-virals in phases one through three in the development pipeline. She did say that a few immune modulators hold promise, but no broad-spectrum antivirals.
The Puzzling Failure of High-Throughput Screens
She described a puzzling technical challenge that stands in the way of both broad-spectrum antibiotics and antivirals. There are many companies performing high-throughput screens that contain over a million different chemical entities, and yet they have not found new effective agents. “What we need is a scientific explanation for that,” she said. “It’s not the targets; these are numerous and well validated. The problem is that we are not finding new chemical entities that can actually inhibit the growth of the organism.”
There are also financial hurdles, said Dr. Cassell. The failure rates for drug discovery, in areas from organ identification to launch of a new drug, average about 90 percent. In the area of antibiotic drug discovery, the failure rates had been even higher. This reality had dampened enthusiasm among firms for investing in antibiotic drug discovery. In fact, she said, a recent competitive analysis indicated that most large pharmaceutical companies and many smaller companies had actually reduced their activity in this area. Because of the daunting technical and financial challenges, they had emphasized therapeutic areas with unmet medical needs and larger market opportunities.
Another reason companies have shifted away from the complex challenges of bacteria and viruses is the new pharmaceutical opportunities opened by completion of the human genome. Geneticists have shown that researchers have been focusing on only about 10 percent of the potential targets for drug discovery in fields such as cancer, endocrinology, and neuroscience. Now there were hundreds of new potential drug targets in the form of genetic segments. Drugs with potential activity against specific gene targets are less complex and far easier to develop than those needed to inhibit the growth of bacteria or other natural products.
In short, she said, what we have now in our antibiotic and antiviral armamentarium is quite limited compared to the ideal. The picture is also clouded by concerns about increasing antibiotic resistance. Despite a great deal of public and Congressional attention, it is clear that no public health response to bioterrorism is likely to prove effective without (1) addressing the overall problem of antimi-
crobial resistance and (2) making better progress against both technical and financial challenges of drug discovery, both for bio-agents released intentionally and those occurring naturally.
The need for partnerships is no greater in any area of bioterrorism, she said, than for developing new countermeasures for biological threats, particularly in the areas of antibiotics and antivirals. She regarded these tasks as more challenging scientifically than development of vaccines, where “in some cases you can get away with rather crude vaccines that provide excellent protection. Our easy antibiotics have already been discovered, as well as the antivirals.”
In the face of these challenges, she said, partnerships are crucial. The very best and brightest scientists were spread throughout NIH, the universities, and the pharmaceutical and biotechnology industry. No single institution or sector could address the challenges alone. In addition, partnerships were needed in order to share the high financial risks, particular in the area of antibiotic drug discovery, and to pool dispersed knowledge about health risks.
Testing the Utility of Existing Drugs
She then turned to some partnerships that had been established since October 2001, some initiated by Lilly, others through various pharmaceutical companies. One was a working group with representation from the NIH, DoD, CDC, and FDA, charged with assessing the utility of existing antibiotics and antivirals. The importance of such an exercise, she said, was illustrated by the usefulness of older antibiotics when the anthrax attacks took place. Older antibiotics are likely to have efficacy against the agents discussed by Dr. Heilman, but proof is needed, both in vitro and in vivo, and other questions must be addressed. For example, what kinds of standards are needed to demonstrate in vitro activity against intra-cellular organisms? Can this be shown in routine laboratory facilities, or does one have to do susceptibility tests in the presence of cells? When does efficacy in vitro demonstrate efficacy in vivo? The last question is complicated because it is not ethical to perform clinical trials or even experimental studies on humans. There is little experience with naturally occurring human cases or the use of antibody therapy, particularly for organisms on the select list.
The FDA uses a rule stating that efficacy based on animal data must include at least two different species before it can be accepted as a standard. A problem for some bacterial agents and virals, she said, is that there are no accepted animal models. Researchers had begun the task of determining what standards to use and what body sites were the correct sites to use experimentally. In addition, a vaccine working group was being established, composed of companies that produced vaccines, to decide which viruses to focus on in developing new vaccines and to assist NIH-funded investigators. Industry investigators had also agreed to share information and data in other ways that might point to promising directions or avoid known dead-ends.
She also described an information technology initiative that would take advantage of expertise in the pharmaceutical industry and in IT companies such as IBM. This group would advise the WHO, CDC, NIH, DoD, and other entities in establishing a global surveillance network, with special coverage of certain countries. The DHHS had committed $10 million to this effort, and others were committing resources and expertise. Such an international effort was important, she said, because modern travel and trade systems can transport microbes to and from every region.16 She noted that recent international discussions on weapons verification had emphasized surveillance as one of the most important components of any system to control biological weapons. One participant in these discussions, she said, was a training program for international fellows established by Lilly at the CDC, which had recently been expanded to included fellows working on biothreat issues.
Another partnership established between HHS and CDC, she added, distributed educational materials about the highest select threats. This included hand-delivery of protection materials by pharmaceutical sales forces to practicing physicians. HHS regarded this as important because it placed practical information at the point of delivery.
Important Issues to Address
Some important working groups had been established, she said, while others needed to be formed and nurtured. These must address some complex issues that had not been resolved. For example, the nation still had significant vaccine shortages even for normally occurring childhood diseases. Many other issues remained to be addressed around the issues of antibiotic drug discovery, liability, and indemnification. The question of liability, in particular, is extraordinarily complex in relation to human health and biothreat agents, both because of the animal model rule and the inability to gather sufficient data to show safety and efficacy in humans. If anti-trust issues that preclude company consortia could be resolved, the resulting partnerships could provide working relationships that allow risks and expertise to be shared. Dr. Cassell said she served on the Global Alliance for Tuberculosis, and even that forum is hindered by antitrust issues from determining the manufacturing capacity of existing antibiotics. One of the most thorny issues to be resolved was that products related to bio-agents have a limited and undefined market. Collaboration will be required to develop the ability to project the real costs of producing and safeguarding products that may be needed in the future.
In addressing all these challenges, she said, government incentives and contracts needed to be realistic about the costs required. “The important increase of $1.5 billion to NIAID,” she said, “is really only a drop in the bucket to what’s needed when you consider that it costs almost $800 million to develop a single product.” She pleaded also for realistic estimates of the time and vast amount of research needed to develop countermeasures. Any legislation, she said, should also encourage the broadest participation to ensure the best products and the lowest cost. “Excluding or disadvantaging some sectors of the industry,” she said, “would work against this goal.”
She ended by concluding that the best deterrent against the use of a biological weapon of mass destruction may be a constant stream of new, innovative antibiotics, antivirals, and vaccines. “Knowledge of such commitment and successful development would surely help dissuade our enemies in such an arena,” she said. “But successful innovation and development will require successful and effective partnerships.”
DISCUSSANT
Kathy Behrens
RS Investment Management
Dr. Behrens reminded the participants that there is a “silver lining for this set of storm clouds”: All the work we do on bioterrorism will also contribute to understandings and solutions to natural causes of disease and infection.
She began by saying she would reinforce some of the reasons in favor of government-industry partnerships. For the area of bioterrorism, she said, “the good news is that we have a strong and favorable history of partnerships at many levels”—government-academia, academia-industry, industry-government—where many organizations and enterprises were already working together. She said that she hoped the urgency of the task ahead would help to reduce some barriers that in the past had slowed the development of some drug products. She said that by the evidence presented by the panel so far, “it’s very clear that a lot of agencies, private organizations, and academic enterprises have put pencil to paper in trying to solve these problems, in spite of the fact that you can’t tell from day to day.”
She expressed optimism about partnerships on the basis of what the STEP organization had done through its series of conferences. She said that the group had learned that one key to partnership success was to have well-defined objectives and goals. In the bioterrorism area, she said, whether for protection, vaccine manufacture, or passive immunization, “it’s clear that we have a target set of organisms we need to be working on and some very specific results we’re looking for to provide protection for individuals.”
The first step, she said, had already been identified historically for government-industry partnerships, and was already in place. It began with personal interaction and good engagement between the individuals who represent different entities. She reminded her listeners that all organizations established for the purpose of providing advice should feel reasonably sure that they are there because their viewpoints are respected, and because people want their feedback and advice.
Key Areas: Liability and Regulation
She suggested that the panels look further at some of the areas discussed by Dr. Cassell, especially in determining the kinds of projects government-industry partnerships are equipped to do, discovering whether the dialogs that will be necessary in initiating partnerships have yet begun. Key items, she suggested, were the broad areas of liability and regulation, which had been major contributors to the cost and time of development for many therapeutic agents. She stressed the importance of good communication with the private sector, including assurances that these issues would be addressed thoroughly during the design of any partnership.
Dr. Cassell then joined the discussion, affirming that those issues had indeed been raised and discussed over a number of years—usually in regard to the development of vaccines that would be used in developing countries. The issue of liability had received serious attention in regard to infectious diseases. About 15 years ago, it was noticed that as drugs became more effective at eliminating infectious diseases, the reactigenicity associated with existing vaccines became a contentious issue. These examples, she said, could serve as precedents to build on during discussion of the same issues in the bio-defense arena.
She elaborated on the issue of liability. As vaccine safety became more of an issue, it prompted the development of a vaccine compensation program. That program had allowed for vaccines to be taxed, and for that tax revenue to flow into a compensation program. As adverse reactions were identified, by almost anyone, sufferers were able to go to the compensation program for relief instead of to the legal system. The value of this system was that during the mid-1980s, when law suits became prevalent, fewer vaccine companies were lost than had been feared. Therefore, the model of compensation was being studied as a one that might be used in developing and using bio-defense agents.
In addition, she said, the Department of Defense had had experience with liability issues in developing their vaccines. Most of the test vaccines were used under IND (investigational new drug) status, and the authorization for DoD already contained liability capabilities. She said that she believed this kind of authority already existed within the DHHS, although it may not have been exercised yet.
In the area of regulatory issues, Dr. Cassell pointed to “another silver lining” in government-industry relationships: “We not only knew each other before, but have trusted each other and worked together.” This was true both for DHHS and its regulatory counterpart, the FDA. She described a productive precedent in a model used for the accelerated development of acellular pertussis vaccine. Under that model the FDA, NIH, CDC, the DHHS, and the drug companies came together periodically “and resolved issues then and there.” She said that this model was then being used for development of improved smallpox vaccine by two private firms17 and that a similar model was being implemented to develop rPA vaccines against anthrax.18 The model benefits from the use of simple conventions, such as entering data in a format used by the FDA; this saves the time that might have been spent re-entering data into a different format before it could be evaluated.
She said that the animal model rule would probably be used for the first time on bio-defense drugs, and the FDA had proposed a different paradigm for approaching it. The customary FDA procedure had been to ask industry to first lay out a plan that the agency would then respond to. In the new paradigm, the agency invited companies to sit down together to define what needed be resolved at the outset. The agency would suggest the guidelines, but invite the companies to discuss any problems they might have with those guidelines. “Those kinds of activities are ongoing now,” said Dr. Cassell.
The Difficulty of Using a Procurement Model
Dr. Behrens asked whether a procurement model would be the best vehicle for ensuring adequate return for the industry partners. Dr. Cassell agreed that the procurement model already used for vaccines is an important one to study. She said that a problem in using the model with antibiotics and other therapeutics was that it may not be possible to project the need as accurately as it is for a vaccine, where a fixed dose gives protection. She said that the model would have to be studied further with respect to many variables, such as what problems does the model currently present; what will be the source of funding for procurement; how stable will the drug be; and how variable might the need be over the next decade.
“In the case of therapeutics,” she said, “there is a finite shelf life, so we have to know if it will need to be replenished.”
Dr. Heilman said that these issues had indeed been brought to the attention of the department, and she acknowledged that any model would have to be complex if it is to address the many variables already anticipated. “The good thing,” she said, “is that there’s a dialog and we’re trying to resolve the challenges. The hard thing is that there’s a lot of complexity in finding an answer that can indeed be valuable for everybody.”
Dr. Behrens continued with the topic of finding the best match with industry in planning for research and possibly development activities. The good news, she said, is that there are a small number of players, and the relationships between them and government had always been good. But, she asked, was there a mechanism that will allow us to match up concepts with both small and large entities that are best able to conduct the work, and an efficient way to bring the parties together?
Dr. Heilman said that the agency’s Web site had been very helpful. She assured the panel that NIAID had a 40-year history of vaccine development and it was familiar with every competent participant. She also applauded Dr. Cassell for working tirelessly on behalf of the industry as a whole to optimize the interaction between government and industry. She cited the example of Dr. Cassell’s extensive effort to understand the full scope of industry’s research on adjuvants,19 so as not to repeat work already done or to follow known dead ends during new programs. She noted that the agency benefited from good relations with PhRMA and similar organizations, which allowed government agencies to communicate their priorities directly to responsive industry representatives.20
Dr. Behrens added that efficient communication is essential when there are so many pending actions in different places, all on “parallel tracks. The right people have to be in the room to bring these tracks together.”
Praise for the New “HSARPA”
Dr. Kerr said that the new Department of Homeland Security planned an entity called HSARPA, the Homeland Security Advanced Research Projects Agency, under the Undersecretary for S&T. HSARPA, he said, would be “the systems equivalent of DARPA, but with many of the procurement issues and problems put aside.” HSARPA would be the “major facilitator to couple the research and development testing and evaluation enterprise with the actual entities, whether they be in the private sector or in academia, and the actual end-users.”
This facilitation will occur in the entity that reports directly to the undersecretary.21
A questioner volunteered that the idea of HSARPA was an excellent one, but said that the published budget figure of $200 million seemed far too low, given the agency’s responsibility for both animal and human clinical research. Dr. Kerr replied that the initial budget should be thought of as “an administrative setting-up period in which the actual roadwork and technological and administration will be set in place.”
Dr. Behrens praised the job of Dr. Cassell in identifying the historical issues on the pharma side, especially in the case of anti-infective and similar agents. She then asked Dr. Heilman whether industry should be doing anything now that was not being done. Dr. Heilman answered that she could not think of an example, but that “people seem to be coming together on this issue more so than around any area I’ve seen.”
A Need for More Manufacturing Capacity
A questioner asked about procurement, and how long it might take to create a new manufacturing facility. Dr. Cassell answered that the point was important. For even the existing antibiotics, she said, there is little or no excess manufacturing capacity. If the nation had to gear up to meet a surge in demand, it would probably require months, not days. This is true for biologics, because the manufacturing is so complex and the quality control standards are high. She said that at a meeting at the National Academies in December 2002, the FDA admitted the possibility that it might have to build a new facility for manufacturing biologics. She had concluded that constructing a dedicated facility, which would have to be maintained at high standards even it was not being used, was not cost efficient. At the same time, she said it was difficult to say how a demand surge for anti-infectives could be met without sacrificing some needed product for which there was continuing demand. “Do you take away antibiotics that are needed to treat sepsis over here because we have a biothreat over there?” she asked. “Those are not easily answered questions.”
Suspending Patents Could Have a “Chilling Effect”
Steve Merrill of the National Research Council returned to the issue of liability. He recalled that HHS Secretary Thompson had raised the possibility of abrogating the Bayer patent on the antibiotic Cipro if that was necessary to obtain an
adequate supply of the antibiotic for an emergency.22 At the time, he said, some warned that this comment could have a “chilling effect” on companies’ willingness to develop new antibiotics, vaccines, and anti-virals. He asked if such an effect had occurred, and whether the government had developed a position on protecting such intellectual property rights.
Dr. Heilman said that in order to have success in this area, the government would have to set a policy environment that not only would nurture public-private partnerships, but also would provide strong intellectual property rights. Without them, she said, companies would not support the innovation that is required. This was a lesson that had been learned through the experience of the vaccine industry—that innovation is essential, “especially if we take into account the National Security Council’s warning that infectious diseases in general are the most serious threat for humanity over the next two decades.” The finding of this report, released 3 years ago, had become even more urgent, she said, because it was written before the anthrax attacks.
Dr. Behrens said that in small and mid-sized companies the topic of liability is raised more often than any other. “The secretary’s comment really struck fear in the hearts of organizations that finance the business,” she said. “It was resolved reasonably amicably, but had the potential to cause serious harm.”
Dr. Heilman agreed that questions of rights and liability pertained not only to single-use products developed for select agents, but also to multiple-use products like ciprofloxacin.
A questioner asked Dr. Heilman whether sufficient numbers of trained people, both in government and industry, were available to work on bioterrorism research, given the fifteen or so years required to train a first-class biotechnology researcher. He also asked whether the specific skills needed to combat terrorism were being taught at all, and whether there needed to be incentives for candidates.
The Lack of Trained Biomedical People
Dr. Heilman said that that was in fact her largest concern—not only are there insufficient numbers of people now trained in microbiological and immunological sciences, but there are few incentives to attract them away from other important research. She recalled a recent discussion about workforce issues related to bio-defense. She said that available data about immunologists, especially those well trained in the clinical microbiology needed in surveillance and research in public health, indicated the existence of “maybe three people” in the United States with expertise in plague and anthrax.
She also said that the nation lacked sufficient numbers of in vivo biologists—people trained in whole-body physiology. The need for DVM/PhDs and
veterinarians is a major issue, she said, not only to help establish infectivity models, but also to address animal diseases and agroterrorism. “The manpower issue is tremendous,” she concluded.
The government is making efforts to address this issue. Dr. Heilman said that DHHS does have a targeted initiative for training in the area of bio-defense. A second effort was to identify regional centers of excellence that would partner with public health service systems within the region and with the CDC. Those partnerships could be regarded as additional surge capacity, she said, should the need occur. “It’s better to partner now than during an event,” she said, adding that the partnerships would also form units to train people on site in various skill and techniques.
Marc Stanley, director of the NIST ATP program, referred to the long history of the ATP in sponsoring government partnerships with both industry and academia, and suggested the use of this vehicle for bioterrorism as well. “If the effort of the government is to get advanced technology commercialized quickly,” he said, “it seems to me having a viable program tested over eleven years would be a unique way to utilize those capabilities without having to reinvent the wheel.” He also suggested that the effort make use of existing relationships in the DNA diagnostic field between NIH, NIST, and Sandia Laboratories.
Dr. Heilman responded that a partnership for DNA sequencing had already been developed along the lines the questioner suggested, with the goals of sharing sequencing information, identifying priorities, and finding the best people.
With regard to the question about NIST, she said that NIAID was working with a DoD organization called the Chemical, Biological, and Radiological Technology Alliance (CBRTA). “In some ways they have been able to solve the IP issue,” she said, “and have allowed interaction along the whole spectrum of biodefense. It’s a fascinating concept to see whether we can interact with them as they’re doing their mission for the DoD.”
A questioner referred to the expense of developing drugs by current processes, and asked whether new techniques such as robotics would reduce the cost and time of drug development, and what public-private mechanisms might advance development of these new robots. Dr. Cassell said that the “short answer is that it hasn’t yet reduced the price, and the reason is that the new technologies being used are more expensive. Unfortunately the science is expensive, and biologics, because they are more complex, are more costly.” She said it is also appropriate to focus on the value of the outcomes—how many lives will be saved and how much health care costs decline by applying new discoveries. She did say that the failure rate in the drug discovery process is likely to decline because of new data from microbial genome sequences, the human genome, and pharmacotoxicology. “We’ll be able to better predict potential toxicities before we actually try a new substance in animals and humans. But we’re not quite there yet.”