F
Cochlear Implants in Children: A Review of Reported Complications, Patterns of Device Failure, and Assessment of Current Approaches to Surveillance
John K. Niparko M.D.,* Janice Leung A.B.,* Debara L. Tucci M.D.,** Martin J. Burton M.D., F.R.C.S.,*** Eric A. Mann M.D., Ph.D.****
INTRODUCTION
Impairment of hair cell function induces profound deafness in approximately 0.3 percent of children younger than 5 years.1,2 In deafness, hair cells of the inner ear fail to trigger auditory nerve fibers in the presence of sound. However, large reserves of auditory nerve fibers exist even in the ear of the profoundly deaf. Furthermore, these nerve fibers retain the ability to respond to electrical activation. Cochlear implants could potentially affect the auditory rehabilitation of an estimated 200,000 United States children with advanced levels of deafness as indicated by a failure to achieve critical milestones in speech and language using conventional hearing aids.
While the impact of hearing loss in an adult varies considerably with the severity of hearing loss and with lifestyle choices, the impact of an advanced level of hearing loss in infancy and early childhood can dramatically affect developmental learning. Because most domains of communication and language learning are subserved by early access to the phonology of speech, deafness effects can extend to the acquisition of visual-language reception (reading) and visual-language production (writing), as well as the constructs of spoken language. A hearing 5-year-old child, for example, has
a vocabulary that ranges between 5,000 and 26,000 words. In comparison, a deaf child of the same age usually has access to a vocabulary of about 200 words and limited ability to structure a spoken sentence.3 Given the substantial impact of deafness on development and the potential benefit of restored hearing, there are risks of underestimating the importance of potential complications associated with a device-oriented approach to deafness. Complications associated with the implantation procedure and device malfunction may arise during critical stages of language acquisition, before a child can be expected to report on their experience with an implanted device.
The cochlear implant is best characterized as a device that provides access to sound. The device enables the hearing pathway to respond to environmental and speech sounds, providing informational cues from the surroundings and from others through direct, electrical activation of auditory nerve fibers tuned to frequencies that span the spectrum of practical hearing.
In October 2004, the U.S. Food and Drug Administration (FDA) developed a website that contains general information on hearing physiology (including animated graphics), information on how a cochlear implant functions, as well as FDA regulatory approvals for these devices. The reader is referred to this website as a comprehensive resource for background information on cochlear implants: http://www.fda.gov/cdrh/cochlear/index.html.
Three different manufacturers of cochlear implant systems provide currently available devices. All three product devices consist of similar component parts
-
An external unit comprised of a microphone, speech processor (Figures F.1 and F.2), and batteries to drive the system.
-
An implanted receiver and electronics package (Figure F.3) with connecting leads that feed an electrode array (Figure F.4).
The design of the electrode array must incorporate biocompatibility, mechanical stability, practical fabrication, and minimize insertion trauma. From a surgical point of view, efforts to reduce insertion trauma must be accomplished at the materials and design levels, as well as through the surgical technique.
For the past two decades, mastoid-implantable internal devices with leads to electrical arrays placed in the basal turn of the cochlea have been applied to deaf children as an increasingly large proportion of all cochlear implants placed (Figures F.5 and F.6). As of 2003, more than half of all newly implanted devices have been placed in children under age 5. It is estimated that approximately 7,500 to 10,000 United States children have received a multichannel cochlear implant prior to the age of 5 years out of

FIGURE F.1 Ear-level processor. (Courtesy of Cochlear Americas Corporation.)

FIGURE F.2 Body-worn processor. (Courtesy of Cochlear Americas Corporation.)
a worldwide population of approximately 90,000 recipients as of February 2005 (synthesis of verbal communication with the three major cochlear implant manufacturers: Advanced Bionics Corporation, Cochlear Corporation, and Med El Corporation, February 2005).
Auditory thresholds of cochlear-implanted children allow access to auditory information beyond that available to deaf children who routinely use conventional amplification (hearing aids). Lowered hearing thresholds offer a substrate for auditory therapy.4 Through developmental learning in the early, formative years, auditory centers of the brain appear capable of processing the additional information from the implant in ways that are not possible at later developmental stages. Speech comprehension and oral lan-

FIGURE F.3 An implanted receiver and electronics package. (Courtesy of Advanced Bionics Corporation.)

FIGURE F.4 An electrode ray. (Courtesy of Advanced Bionics Corporation.)
guage development after implantation occur at a rate that parallels that of normal hearing peers, although gaps due to early deprivation often persist. Phonologic access afforded by of the cochlear implant to children has set the stage for several global perceptions of the intervention:
-
the cochlear implant represents one of many innovative technologies that enable the rapid transfer of processed information from sound to comprehension;
-
implant technology represents an alliance of informational processing strategies that utilize both manufactured and natural neural circuits;
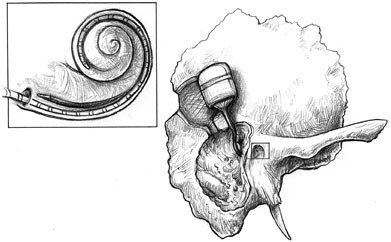
FIGURE F.5 An implanted electronics and receiver package connected to an electrode ray, which is inserted through the cochlea. (Used with permission of Lippincott Williams & Wilkins. Originally appeared in Cochlear Implants: Principles and Practices, 2000. Niparko J, Kirk KI, Mellon NK, eds.)
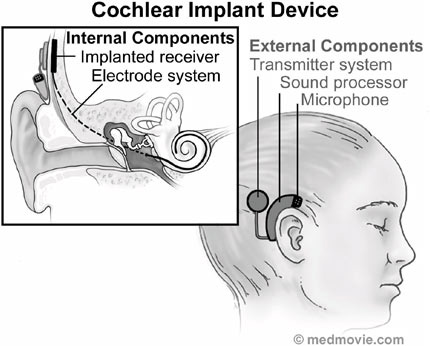
FIGURE F.6 The cochlear implant system comprised of its internal and external components. (Courtesy of Medmovie.com.)
-
to the extent that a cochlear implant can encode the sounds of speech with precision, the device can provide opportunities for developmental and oral language learning in young children with implications for psychosocial development, scholastic achievement, and life chances; and
-
potentially dramatic, life-altering outcomes following early cochlear implantation have fostered high media visibility and enthusiasm about the benefits of early implantation.
Against a two-decade old background of a generally positive global perception of clinical effects of cochlear implants in deaf children, a more open acknowledgement of potential harms has emerged in recent years. This summary provides background, peer-reviewed information regarding current risks posed to implant recipients and suggests approaches to improve surveillance of complications associated with cochlear-implantable devices in children.
We begin with a description of considerations for candidacy selection and then review current understanding of complications and underlying mechanisms. We close with an assessment of the current state of device surveillance and propose several approaches to addressing the considerable challenge of tracking the health of children with cochlear implants.
CANDIDACY EVALUATION: MEDICAL, OTOLOGIC AND RADIOLOGIC ASSESSMENT
General Medical and Otologic Assessment
To date, most cochlear implantations have been performed in children over the age of 2, though recent trends have shifted applications to ever younger patients. The main concerns with implanting infants and toddlers relate to the unreliability of audiologic testing at these ages, the prevalence of otitis media, challenges in the rehabilitation process, surgical obstacles posed by smaller tolerances in surgically approaching the cochlea. Nonetheless, centers have demonstrated promising results with few surgical complications.5,6,7,8,9 Moreover, outcome analyses seem to suggest the value in implanting at earlier ages. Zwolan and colleagues reviewed their series of 295 children implanted between 12 months and 10 years of age and observed that children implanted at younger ages experienced greater gains in speech perception over time than children implanted at a later stage.10 Robbins and colleagues11 noted that children implanted prior to the age of 19 months carry the highest potential for acquiring auditory skills at rates
that are not statistically different from their normal-hearing peers. Likewise, Schauwers and colleagues12 found smaller delays in the onset of babbling with earlier implantation, and Govaerts and colleagues13 found that implantation before the age of 2 was important in achieving optimal results. These case series of children suggest that implant-based percepts promote developmental learning in keeping with constructs of critical-period dictates of early language development.
It does not appear that earlier implantation is associated with higher device-related complications. For example, a review of 34 cochlear implant complications in the Johns Hopkins cohort of 1,030 patients found that 51 percent of implants were placed in children under the age of 4 years. Device failure was found to occur in children under the age of 5 years in 11 of the 34 cases (33 percent).
In addition to age considerations, the child must undergo a series of tests to ensure the proper candidacy for the procedure. Evaluation should include a complete medical history with appropriate laboratory studies and an assessment of the patient’s general health and ability to endure a general anesthetic for the necessary mastoid surgery. For children under the age of 9 to 12 months, general anesthetic may carry increased risk;14 therefore, the benefits of earlier implantation must be carefully assessed against these risks. Although implantation under a local anesthetic has been described, this approach is usually not recommended because it constrains the soft tissue dissection behind the mastoid required for embedding the internal device.
Patients must be physically and psychologically capable of completing the course of recommended programming and therapy. Personality traits and family dynamics that predict program engagement should be assessed. Psychological assessment may be indicated to screen for psychopathology and organic brain disease. However, children with developmental delays and other limitations or disabilities should not be barred from implantation. While such children may not experience the same gains as deaf children without other functional limitations, numerous studies have shown considerable benefit is still possible.15,16,17,18
Both the chronology of deafness and the history of amplification use can help determine the choice of ear to be implanted. Previous otologic operations should be documented, as well as any abnormalities or diseases. In particular, a history of meningitis should prompt a discussion of methods for implanting a cochlea that may be partially or fully ossified as a result of meningitic-related inflammation. While post-meningitic deaf children were previously thought to perform at less impressive levels after implantation due to the neuropathologic consequences of the disease at the level of the inner ear, Francis and colleagues19 found no significant delays in their cognitive or speech perception performance, with the exception of
those children who exhibited evidence of meningitic-related hydrocephalus. Data from this case series of 30 meningitis-deafened children suggest that central factors hold sway in predicting audiologic performance and that ossification of the cochlea does not prevent good outcomes.
Most children who present for cochlear implantation will likely have some history of acute otitis media (AOM). However, this does not constitute a clinical contraindication currently, as long as the condition is under control at surgery. The surgeon, however, should be prepared for an inflamed middle ear mucosa that may complicate and lengthen the surgery.20 Based on clinical experience, ventilation tubes may be removed before implantation, but should be left in if the child has frequent recurrent episodes of AOM. Luntz and colleagues21 suggest the continuous use of ventilation tubes until the child has outgrown susceptibility to AOM based on a study.
In audiologic testing, the cochlear implant candidate should have puretone averages (PTA) greater than 90 dB and speech understanding scores of up to 50 percent on sentence testing, though recent work has suggested that children who retain greater residual hearing may still also significantly benefit from implantation.22 Such guidelines, however, are less compelling as younger candidates are considered. The required testing may prove difficult in young children. For such children, severe to profound levels of hearing loss, severe delays in verbal language acquisition, and substantial elevations in thresholds even with the use of hearing aids may indicate the need for an implant.23
Special consideration should be given to patients for whom magnetic resonance imaging (MRI) may be needed in the future. MRI is conventionally contraindicated in patients with magnetic devices. Recently, devices compatible with low-strength MRI (0.2 Tesla) and devices which can be rendered MRI compatible (by a minor surgical procedure to remove the magnet should the need for an MRI arise) have become available from some of the manufacturers.24,25,26 Even with magnetic devices left in place and intact, though, at least three reports have suggested that MRI can be safely performed.27,28,29 Nonetheless, at least three adverse events are under investigation.
Etiologic Assessment
Determining the cause of a child’s hearing loss can reveal information about the expected histopathology of the inner ear. Although many patient factors are deemed important in predicting success of speech recognition with the cochlear implant, survival of the first-order neurons is thought to be of particular importance. Second, recognition of factors that are associated with cochlear abnormalities (e.g., congenital malformations or ossifi-
cation) is critical for surgical planning and for patient and family counseling before implantation.
Nadol’s studies30 of almost 100 temporal bones from patients with documented profound sensorineural hearing loss reveal patterns of spiral ganglion cells (SGC) survival that are consistent across diagnostic categories. Residual SGC counts were highest in individuals who were deafened by aminoglycoside ototoxicity or sudden idiopathic sensorineural hearing loss and least in those deafened by postnatal viral labyrinthitis or congenital causes. Counts for the two other etiologic categories—temporal bone neoplasms and bacterial labyrinthitis—fall in between. Age at time of death and duration of deafness were less predictive of SGC survival than was the cause of hearing loss.
Labyrinthitis ossificans, or new bone formation in the inner ear, is a common finding in the temporal bones of patients who are deafened by bacterial meningitis. Quantitative assessment of 11 temporal bones of these patients by Nadol revealed a significant negative correlation between SGC survival and the presence of bony occlusion.30 However, even in segments with severe bony occlusion, significant numbers of SGCs remained. In a study of temporal bones from previously implanted patients, Linthicum and colleagues31 found that useful auditory sensations are reported by individuals whose temporal bones were found to have as few as 10 percent of the normal complement of cells. Although the presence of ossification is not considered a contraindication to implantation, the degree of ossification as demonstrated on imaging studies preoperatively should correlate with SGC survival and help guide the implant team in selecting an ear for implantation.
Radiologic Assessment
Radiologic imaging is an essential part of the evaluation of the cochlear implant candidate. High-resolution computerized tomography (HRCT) scans of the temporal bone help to define the surgical anatomy and provide information about cochlear abnormalities that can aid the surgeon in surgical planning and patient counseling. Temporal bone CT scans should be obtained and reviewed for evaluation of temporal bone anatomy with attention to the degree of mastoid pneumatization, position of vascular structures, middle ear anatomy, and position of the facial nerve.32 Scans are also examined for evidence of cochlear malformation, cochlear ossification, enlarged vestibular aqueduct, and other inner ear and skull base anomalies. An absolute contraindication to cochlear implantation detectable by HRCT is the absence of the cochlea in Michel’s aplasia.
Although HRCT is the gold standard for evaluating most aspects of temporal bone anatomy, MRI is ideal in imaging soft tissue structures such as the membranous labyrinth and nerves. MRI can identify the presence or
absence of fluid within the cochlear turns and the size of the cochlear and vestibular nerves within the internal auditory canals. MRI is superior to HRCT in determining cochlear obstruction due to non-ossified scarring. One disadvantage to using MRI in children, though, is the need for sedation.
CRITERIA FOR DEFINING COMPLICATIONS IN COCHLEAR IMPLANTATION
A number of classification schemes have been developed to help define complications in the cochlear implantation process. Cohen and colleagues33 labeled “major” complications as those requiring additional surgery or hospitalization for treatment or correction, while “minor” complications resolve with minimal or no treatment. Exceptions to this distinction include facial nerve palsy or paralysis, considered to be a “major” complication even if no further surgery or inpatient treatment is required. As examples, they cite flap necrosis and improper electrode placement as “major” complications and dehiscence of incisions, infection, facial nerve stimulation, dizziness, and pedestal problems as “minor” complications. The initial reports revealed the rate of “major” and “minor” complications to be 8 percent and 4.3 percent, respectively, but later analyses found the rates to be 5 percent and 7 percent, suggesting a decrease in major complications with experience over time.34
Determining the stage at which complications arise can also be helpful. Luetje and Jackson35 separated “surgical” complications from “nonsurgical” complications. “Surgical” complications were identified as skin flap problems, facial nerve injury and stimulation, and infection. “Non-surgical” complications consisted of device failure, delayed stimulation, educational deficiencies, poor compliance by the family unit, behavioral problems, and socioeconomic disadvantages. In a review of 55 children, no “surgical” complications were found whereas “non-surgical” problems occurred, the most common being device failure in 10.9 percent of the group.
Finally, the timing of events is of interest. Kempf and colleagues36 characterized “early” complications as those that arose within 3 months of surgery and “delayed” complications as those that occurred after this time period. In their retrospective analysis of 366 children, “early” complications such as flap problems, electrode dislocation, facial nerve problems, and incorrect insertion of the electrode, were found in 1–2.5 percent of children. “Delayed” complications, for example, otitis media and facial nerve stimulation, occurred in 14 percent of the children.
Although children were indeed once thought to be at greater risk than adults of major and minor complications from cochlear implantation, stud-
ies have, in fact, suggested no significantly heightened risk in the pediatric population. In a series of 309 children who were implanted with the Nucleus device by 25 surgeons in North America before 1991, the total complication rate (major and minor) was 7 percent, which compared favorably with the adult rate of 12 percent. The incidence of complications was lower in children older than 7 years of age. However, the relatively low rate of operative complications in the pediatric population as reported in this study may have reflected the greater experience of surgeons who initially performed pediatric cochlear implants.44,37
SURGICAL ISSUES RELATED TO COMPLICATIONS
Cochlear Implantation Surgical Procedures
Implantation of the young child requires specific knowledge of the unique anatomy of the temporal bone in this age group and of the impact of skull growth on the implanted device. Although temporal bone growth has been shown to continue through adolescence, anatomy of the facial recess is fully developed at birth.38,39 The most significant developmental changes are in the size and configuration of the mastoid cavity, which has been shown to expand in width, length, and depth from birth until at least the teenage years. Growth of the mastoid during this time parallels the growth patterns of the skull, with two periods of rapid development: one starts at birth and continues through early childhood, and the other occurs at puberty. From 1 year of age to adulthood, the average mastoid can be expected to grow 2.6 cm in length, 1.7 cm in width, and 0.9 cm in depth for males and 2.0 cm in length, 1.7 cm in width, and 0.8 cm in depth for females. Based on these measurements, it has been recommended that 2.5 cm of electrode lead redundancy in the mastoid is necessary to accommodate head growth while avoiding electrode extrusion.40,41
Investigation in the young primate has demonstrated that cochlear implantation had no adverse effects on skull growth.42,43 Moreover, the electrode appears to remain in a stable position with no migration over time.44 These observations strongly suggest that lateral skull base development occurs in a pattern that circumscribes the implanted device, with soft-tissue anchoring of connecting leads.
As for all otologic surgery in children, the surgeon should remember that the lack of development of the mastoid tip, narrow tympanic ring, and lack of subcutaneous tissue in infants and toddlers place the main trunk of the facial nerve just below the skin, where it is easily injured by an incorrectly placed incision. Design of the postauricular skin flap is particularly important. In younger children, who have a thin scalp, elevation of the postauricular tissue in continuity with the skin flap may protect from flap
necrosis secondary to magnet pressure.45 In older children, the lateral skull is usually thick enough to permit the creation of an adequate well for the receiver/stimulator. In younger children, in whom the skull is much thinner, the bone is often drilled to the level of the dura, or a mobile island of thin bone can be created over the dura in the center of the well for protection. Alternatively, many surgeons make attempts to thin the skull to approximate, but not reach, the level of the dura. Retention sutures are often placed between the bone and dura. Electrode insertion and closure are similar to the procedures in the adult.
A new development in the surgical procedure is minimal access surgery, proposed by O’Donoghue and Nikolopoulos.46 With this technique, a short, oblique, and straight postauricular incision of a length no greater than 3 cm is made without shaving any hair, thereby minimizing flap infection and flap necrosis. The authors posit improved aesthetic quality of this procedure that can reduce the psychological trauma of the intervention and can translate into increased acceptance of cochlear implants by children and their families. In general there has been a move to smaller incisions in placing cochlear implants in children as guided by these considerations rather than in response to demonstrated complications.
Implantation of Special Populations
Cochlear Ossification
Labyrinthitis ossificans results from severe inflammation of the inner ear and can be associated with a variety of pathology, including viral or bacterial labyrinthitis, advanced otosclerosis, trauma, autoimmune inner ear disease, occlusion of the labyrinthine artery, and leukemia or other tumors of the temporal bone.47 Labyrinthitis ossification presents one of the greatest challenges to effective, safe cochlear implantation. In children, the most likely cause of cochlear ossification is meningitis. Twenty percent of children acquire profound bilateral sensorineural hearing loss prior to the age of 3 years; 90 percent of these cases are meningitic in origin.48 Green and colleagues demonstrated that ossification due to meningogenic labyrinthitis extended further into the cochlea than ossification due to other causes. The extra bone growth makes the insertion of the electrode a difficult process.47 In addition, the stimulation of surviving neural elements may be compromised by the bony obliteration, and histopathololgic reports have shown an association between the degree of bony occlusion and a decreased number of surviving SGCs,49 particularly in cases of bacterial meningitis. For these reasons, patients with labyrinthitis ossificans were often thought to perform at lower levels than those without ossification.
Nonetheless, certain factors make successful implantation possible. In
most cases, ossification involves only the most basal portion of the cochlea, allowing complete electrode insertion.50 A significant if decreased number of neurons can also remain in ossification.51 El-Kashlan and colleagues52 and Steenerson and Gary53 therefore found that children with postmeningitic hearing loss and cochlear ossification could attain significant benefit from their implants, although children without ossification were likely to perform better. Still, other studies demonstrate that ossified and non-ossified children perform equally well.54 A key factor for success may be the timing of implantation. Ossification may appear as early as 2 months following meningitis, leaving a small time period during which electrode insertion is optimal.55 As mentioned previously, however, central nervous system sequelae of meningitis are likely to hold sway in determining outcome.19 Nonetheless, the implant surgeon should expect that ossification may be present and have an armamentarium of techniques available to deal with potentially unexpected findings.56 Gantz and colleagues57 described a more aggressive approach that optimized electrode insertion by creating a circumodiolar trough for the electrode using an extended transtympanic approach. The ear canal is divided and closed, and the ear canal skin, tympanic membrane, malleus, and incus are removed. The bony canal wall may be retained or taken down, but the prominence of the anterior bony external canal usually must be reduced to allow adequate visualization of the cochlear promontory. A cochleostomy is created, and a bridge of bone at the round window niche is preserved to help secure the electrode. The electrode array is then inserted beneath the bony bridge at the cochleostomy and into the lumen. Fibrous tissue is used to secure the electrode within the lumen. Additional strategies of implantation of the ossified basal turn involve opening the scala tympani.
Cochlear Malformations
Cochlear malformations pose potential risks to the surgical approach and opening of the inner ear. Up to 30 percent of children with congenital hearing loss have bony abnormalities of the labyrinth.58 Cochlear malformation raises concerns about both surgical safety and postimplantation performance. Bony malformations of the cochlea have been associated with absence of the round and oval windows and with an aberrant course of the facial nerve. In addition, a thin cribriform area between the modiolus and a widened internal auditory canal is believed to be the route of cerebrospinal fluid leak when it occurs during surgery or spontaneously, as in the case of microscopic occult leak and recurrent meningitis.59,60 An anomalous internal auditory canal may suggest absence of the auditory nerve, ordinarily a contraindication to implantation.61,62 MRI may be used to delineate the
intracanalicular neural anatomy in detail.63 Histopathologic studies of temporal bones with cochlear malformations reveal substantially diminished and, in one case, bilaterally absent SGC populations.64,65,66,67 Even so, implantation of children with cochlear malformations can be achieved without surgical complications and can result in levels of performance comparable to patients with normal bony cochlear anatomy.60,68,69,70,71,72,73,74 Modifications of conventional surgical implantation techniques are suggested and depend on a knowledge of the different types of malformations. Malformations based on embryogenesis are described by Jackler and colleagues61 (Table F.1) as follows:
-
Cochlear aplasia: no cochlear development; these patients are not usually candidates for implantation, though Govaerts and colleagues75 has suggested that patients with Type IIa aplasia may be implanted with reasonable benefit.
-
Common cavity deformity: combined cochlea and vestibule with no internal structure.
-
Cochlear hypoplasia: small cochlear bud.
-
Incomplete partition: classic Mondini malformation, with loss of interscalar septum between the middle and apical turns; cochlea are often smaller than normal.
Full or near-full electrode insertion can be achieved using routine implantation techniques in patients with incomplete partition (Mondini) deformity. A common cavity malformation is also likely to accommodate a multichannel electrode array, whereas the small size of the hypoplastic cochlea restricts the number of electrodes that can be positioned within the inner ear. Still, the two patients with cochlear hypoplasia in one series were able to use 10 electrodes each (Tucci and colleagues60). Because the electrodes may not be confined by scalar anatomy, electrode migration may occur, and individuals with cochlear malformations may require frequent reprogramming of the electrodes. Electrodes that are not intracochlear or that elicit facial nerve stimulation can be eliminated from the “map” as can electrodes that elicit facial nerve stimulation in implanted normal cochleae.
Abnormalities of the round window and facial nerve anatomy should be expected and the use of facial nerve monitoring is recommended. If the round window is absent, a cochleostomy should be placed according to the measurements described previously. The round window may be found in a position more posterior and superior than usual, consistent with the deformity of the cochlea. Malposition of the facial nerve may necessitate a modification of usual implantation techniques, and implantation through a vestibulotomy has been described. Facial nerve anomalies were found in 17
TABLE F.1 Malformations Based on Embryogenesis as Described by Jackler and Colleagues (Images courtesy of Johns Hopkins Medical Institutions)
|
Name (after Jackler et at.)61 |
Other Designations |
Characteristic |
|
Michel Deformity |
|
Complete absence of vestibule and cochlea. |
|
Cochlear Aplasia |
|
Absent cochlea yet identifiable vestibule or semicircular canals. |
|
Common Cavity |
|
Defective fundus at lateral end of IAC. Risk of CSF gusher. |
|
(No name distinguished by Jackler and colleagues) |
Incomplete partition type 1,10 Empty cochlea,11 Pseudomondini,12 Cystic cochleovestibular malformation |
Cochlea is lacking the entire modiolus resulting in cystic appearance. Vestibule is similarly cystic. |
|
Cochlear Hypoplasia |
Full vestibule-cochlear partition and normal number of turns, but abnormally small cochlea. |
|
|
Incomplete Partition/Mondini |
Incomplete partition type 210 |
Cochlea has 1.5 turns with basal modiolus. The apical turn has a cystic formation. |
|
Anatomic Associations |
Gestational Stage at Developmental Arrest |
Axial CT Anatomy of Cochlea & Labyrinth |
|
Often including absence of IAC. |
Week 3 |
|
|
Absence of promontorium distinguishes this from acquired ossified cochlea. |
Late week 3 |
|
|
IAC may be small, normal or large. Presence of neural tissue in the cavity is variable. |
Week 4 (arrested at otocyst stage) |
|
|
Little more than a common cavity with a rudimentary partition. |
Week 5 |
|
|
|
Approximately week 6 |
|
|
Enlarged vestibular aqueduct. Spiral ganglia and nerve endings present (good implantation outcomes). |
Week 7 |
|
percent of children with malformations by Mylanus and colleagues,76 though rates of up to 32 percent have been reported by Buchman and colleagues.74 Particularly in cases where the nerve crosses the promontory, Hoffman and colleagues warn of facial nerve paralysis and suggest a sacrifice of the posterior canal wall for better visualization in difficult cases.57 While Buchman and colleagues did not encounter either paresis or paralysis, they did note an association with adventitial facial nerve stimulation by the cochlear implant.
CSF leak is also a common hazard when implanting children with inner ear malformations. Rates of up to 50 percent have been reported.70,71,72,73 Once the initial flow of CSF abates, soft tissue packing at the cochleostomy can readily fix the problem. However, in cases of persistent CSF leak, a lumbar spinal drain can be placed at the time of surgery and left in place for 3 to 4 days to allow the fibrous tissue packing in the cochleostomy to seal, preventing further leakage. Control of a CSF leak may also be accomplished by more extensive soft tissue packing of the middle ear space and Eustachian tube, with or without radical mastoidectomy and closure of the ear canal. An important, recently identified concern occurs when a CSF leak follows implantation of a malformed inner ear and results in early or delayed meningitis. Six of 26 cases of pediatric postimplantation meningitis found by Reefhuis and colleagues occurred in such instances.91
Intra-Operative Complications
Electrode Placement Problems
Electrode placement problems occurred in 0.58 percent of children.34 Rather than its proper placement in the scala tympani, the electrode is occasionally misplaced into a hypotympanic air cell. Intraoperative radiography can help to guide the electrode to its precise location, and care should be taken to minimize the trauma to the membranous components of the cochlea during insertion.77 If resistance is encountered, the electrode can be withdrawn slightly, rotated medially (counterclockwise for the right cochlea and clockwise for the left), and carefully advanced.78,79,80 Because buckling of the implant can produce spiral ligament, basilar membrane, and localized neural injury, aggressive insertion attempts should be avoided.
Long-term complications involving the electrode (including migration) were found by Hoffman and Cohen in 1.31 percent of children.121 However, Roland and colleagues have found no substantial migration over time in children.44 Cohen and Hoffman suggest that packing the cochleostomy and facial recess with soft tissue may help to prevent migration.81
Facial Nerve Injury
Though rare, facial nerve injury is a serious potential complication of cochlear implantation. Studies focusing specifically on the incidence of injury in children are still lacking, but Hoffman and Cohen found a rate of facial nerve injury of 0.58 percent in 1,905 children.110 House and Luxford82 described eight cases, three of them pediatric, of facial paralysis or paresis that occurred after implantation. More recently, Fayad and colleagues83 reviewed 705 implant recipients and found 4 to have post-operative facial nerve weakness. Of the 4, one was a 19-month-old child. Surgeons should be aware of aberrant positioning of the facial nerve in cases of cochlear malformation as well as the relatively small tolerances of the smaller corda-facial angle within the pediatric temporal bone.
In House and Luxford’s eight cases, the most frequent mode of injury was the heat of the burr shaft rotating over the facial nerve in the facial recess. Copious irrigation during drilling, maintenance of a thin sheet of bone over the facial nerve in this location, and maintenance of an angle of drilling that keeps the burr shaft lateral and away from the floor of the facial recess are important in preventing injury. Intraoperative facial nerve monitoring may be helpful, but may not prevent injury from occurring. In Fayad and colleagues’ review, the authors proposed neural edema in early onset palsy and acute nerve compromise or reactivation of herpes virus from surgical trauma in delayed onset palsy as possible mechanisms. Steroid treatment has been helpful in some cases of paresis.
INFECTIONS AND OTHER POST-OPERATIVE COMPLICATIONS
Otitis Media
One of the most common conditions affecting children, acute AOM carries small risk for the post-implantation patient. Infection can potentially spread along the electrode through the cochleostomy into the cochlea, particularly in a child who already has a pre-implantation history of AOM. Such a history, however, should not preclude or delay implantation as numerous studies have shown the decreased likelihood of the disease following surgery. House and colleagues84 noted that with (1) the natural decline in cases with age, (2) effective antibiotic, or (3) mastoidectomy treatment, children often develop fewer instances of AOM than prior to implantation. Similarly, Luntz and colleagues85 reported that neither the prevalence nor the severity of AOM increased with implantation, although children with a history of AOM did show a higher risk of developing postimplantation infection. Their data showed a decrease in AOM from 74 percent pre-operatively to 16 percent post-operatively, with all post-
operative cases treated successfully and without any complications. Fayad and colleagues86 also found a significant decrease in cases of otitis media after implantation, both in children with and without a history of bilateral myringotomy and tubes, and Kempf and colleagues87 found only a 5.6 percent post-implantation rate of AOM.
Ideally, AOM should be controlled before surgery. Children with preimplantation histories of AOM should be treated prior to the operation with adenoidectomy or ventilation tubes. If AOM develops during either the peri-operative or post-operative period, Kempf and colleagues recommend prompt treatment with intravenous antibiotics and mastoidectomy if necessary. In cases of severe infection, removal of the device is possible with future reimplantation as an option.87 Papsin and colleagues88 suggest that effusion at the time of implantation does not pose peri-operative difficulties; they recommend peri-implant myringotomy tubes with peri-implant antibiotics to protect against peri-operative infections.
Device Infection
Foreign objects within the inner ear are potential breeding grounds for infection and can lead to life-threatening episodes of meningitis. Nadol and Eddington’s study of 21 temporal bones found that late hematogenous contamination and colonization of the device are likely mechanisms for the pathogenesis of meningitis.89 Such infections of the device can be difficult to treat as biofilms create a barrier between the bacteria and antibiotics. Removal of the implant may be necessary, if incision, drainage, and intravenous antibiotics fail. The possibility of bacterial biofilm development on the surface of the cochlear implant is increasingly recognized in cases of persistent and recurrent abscess or granulation tissue formation.90 This observation is critical as biofilms confer substantial bacterial resistance to host defenses. Such observations carry implications for device design. They suggest, for example, that surface openings to accommodate retention magnets and electronic grounding should be sealed and offer no confines for contaminants to be isolated from immuno-surveillance.
Post-Implantation Meningitis
In 2002, an alarming increase in the number of post-implantation meningitis cases noted anecdotally and through reports to the FDA prompted health care agency investigations in both Europe and North America.91 Initial reports suggested a higher risk of meningitis in patients implanted with a particular device design and particularly when placed with an intracochlear shim (electrode positioner), and the manufacturer ultimately recalled unimplanted devices utilizing the positioner in July 2002. The level
of risk did not suggest the need for positioner removal unless repeated infections of any kind are encountered. Since then, continued studies have revealed a higher risk for the disease in patients with all cochlear implants compared to the general population. Children appear to be particularly affected: of the 52 cases originally reported by the FDA, 33 (63 percent) were under the age of 7.
Reefhuis and colleagues91 conducted a study of 4,264 children implanted between 1997 and 2002 and found 29 cases of bacterial meningitis in 26 of the children. This rate of meningitis (caused by Streptococcus pneumoniae) was 30 times the incidence in the general population. While the use of a positioner increased the likelihood for contracting meningitis, even children who were implanted without a positioner had rates of meningitis 16 times higher than the general population. Case-control analysis found the following risk factors: a history of placement of a ventriculoperitoneal (CSF) shunt, a history of otitis media prior to implantation, the presence of inner-ear malformations with CSF leak and CSF leaks alone, the use of a positioner, incomplete insertion of the electrode, signs of middle-ear inflammation at the time of implantation, and exposure to smoking in the household. While the incidence of meningitis decreased considerably after the peri-operative period, cases still appeared even 2 years after implantation.
The design of the study conducted by Reefhuis and colleagues, as noted by the authors, did not permit the comparison of meningitis risk between deaf children with and without a cochlear implant. That is, there is no comprehensive epidemiologic analysis of the risk of meningitis in deaf children but no cochlear implants. It is biologically plausible that greater risk of meningitis exists in deaf children in general given high levels of associations of deafness with skull base anomalies that predispose them to the disease. It is also possible that any surgical implant placed in or near the skull base of toddlers and young children (e.g., CSF shunts) does indeed carry a prolonged risk of meningitis.
Callanan and Poje92 similarly found an association between meningitis and the use of a positioner and congenital malformations. Another risk factor may be hyperbaric oxygen therapy; in children with cerebral palsy it was associated with middle ear complications. Of the congenital malformations, Mondini dysplasia has generated the most attention for its predisposition to CSF leaks. Page and Eby93 reported a case of CSF otorhinorrhea and meningitis 2 years post-implantation in a child with Mondini dysplasia and suggested careful seals of the cochleostomy to prevent CSF leakage. Suzuki and colleagues94 also reported a case of fatal meningitis with chronic otitis media and bilateral CSF leaks in a 6-year-old boy 2 years following implantation. However, it should be remembered, as O’Donoghue and colleagues95 point out, profoundly deaf patients who are likely to undergo
cochlear implantation may already be at increased risk for meningitis particularly in the context of a CT-evident cochlear anomaly.
Mechanisms for post-implantation meningitis for children with implants have been proposed by Cohen and colleagues.96 Infections may result from the direct spread of otitis media into the scala tympani, either at the time of surgery in immediate cases or some time following surgery in delayed cases. Also, the trauma caused by surgery may activate latent colonies of pneumococci and the drilling of the well for device placement may allow exposure and subsequent translocation of postsurgical, neovascularized dura. Finally, hematogenous spread of infection or factors relating to the surface of the device itself may cause meningitis.
Given the dangers posed by meningitis (mortality rates range from 15 to 60 percent), the Center for Disease Control and Prevention recommends pneumococcal vaccination for all cochlear implant recipients following age-appropriate schedules.97 These vaccinations should be completed 2 or more weeks prior to surgery. Reefhuis and colleagues further suggest careful monitoring for signs and symptoms of meningitis in the post-operative period, timely diagnosis and treatment of acute otitis media to prevent the spread of infection, and awareness of any inner ear malformations or other surgical findings that may predispose the child to meningitis.91 If meningitis develops, however, consideration of removal of the implant is individualized given the risk for surgical complications that might exacerbate the conditions leading to meningitis.
Tinnitus and Vestibular and Balance Function
While no studies have tracked tinnitus in pediatric cochlear implant recipients, many have established an association between cochlear implantation and a reduction in tinnitus.98,99,100 Ruckenstein and colleagues’ review of 38 adult patients with pre-implantation tinnitus found that 92 percent had statistically and clinically significant reductions in their symptoms following implantation.101 Miyamoto and Bichey’s review of recent literature confirmed these results, but noted that the prevalence of tinnitus in pre-operative implant recipients was extremely high at 80 to 90 percent.102 Children are generally believed to have a lower prevalence of tinnitus, yet how they detect and report symptoms remains unclear. Still, Aschendorff and colleagues found a similar reduction in tinnitus following implantation in adolescent patients.103 Further studies are necessary to clarify tinnitus in children receiving implants.
Similarly, research on vertigo in post-implantation children is scarce. In adult populations, vertigo is a common complaint following surgery, with the vast majority of patients noting resolution of active vertigo within 72 hours of surgery. Caloric testing on cochlear implant recipients showed that
while implantation did not abolish vestibular function, temporary disturbances were seen in 20 percent of patients,104 thus yielding the possibility of post-implantation disequilibrium and vertigo. During the first week after surgery, patients often experience some degree of disequilibrium and unsteadiness. True vertigo that persists in the post-operative phase is rare.105 Extended periods of disequilibrium are also rare, but, when present, are treatable with exercise regimens designed to elicit central compensatory mechanisms. Kubo and colleagues106 reported a 49 percent rate of vertigo in 94 adult implant recipients and similar results were found by Ito.107 Fina and colleagues108 found a lower rate of 39 percent while Steenerson and Gary109 reported that up to 75 percent of adults with implants experienced vertigo or imbalance. Most of Fina and colleagues’ 75 patients experienced delayed, episodic vertigo and had a history of pre-operative dizziness. Like tinnitus, vertigo is more likely to affect older populations; the incidence in children is still unknown though clinical observations (e.g., rare instances of obvious nystagmus) suggest that it is uncommon.
Scalp Flap Complications
The most frequently reported major and minor complications are related to the incision and postauricular flap design. Problems range in severity from minor wound dehiscences or infections to major loss of tissue necessitating removal of the device. In a study of 3,064 adults and 1,905 children who received implants, Hoffman and Cohen110 found the overall rate of flap complications to be 4.5 percent. Flap necrosis occurred in 0.37 percent of children while flap infection was found in 0.73 percent of children. Fifty-five percent of all flap complications were considered major and required follow-up surgery.
Many implant surgeons have thus emphasized the importance of good flap design and technical skill to avoid these complications. Ideally, the flap should have adequate blood supply and venous drainage, allow enough exposure of the operative site with sufficient coverage of the device, and be carefully closed in layers without tension. Because it is associated with complications,111 a C-shaped incision is contraindicated when there is a previous post-auricular incision.112 Inverted U- and J-shaped flaps take advantage of the posterior arterial supply. Because these flaps have the disadvantage of crossing the electrode lead as it enters the mastoid cavity, it is necessary to create an anteriorly based musculofacial flap (i.e., Palva flap) under the scalp to ensure electrode coverage.
Other suggestions have included straight, post-auricular incisions. Gibson and colleagues113 developed a 7 cm vertical entry route found to minimize scalp infection and device extrusion in 20 adults and 32 children. O’Donoghue and Nikolopoulos’s minimal access surgical route is a 3 cm
oblique incision.46 Of their 23 pediatric cases, no major complications occurred, and only 3 instances of wound edema were encountered.
As Cohen and Hoffman warn,114 flaps that are too thick will impede the transmission of electrical signals, whereas flaps that are too thin will erode under the magnetic pressure. Careful handling of the flap, with proper moisture and hemostasis, and suture placement away from the surface of the implant are especially important. Signs of flap infection or necrosis should be immediately treated with topical or oral antibiotics to avoid removal of the device. More persistent cases of infection should be treated with intravenous antibiotics and surgery if necessary. Extrusion of the device can result from local flap necrosis, which can be managed by rotation of the device under an extended flap, usually to a more superior location where intact skin covers the device.115,116
Facial Nerve Stimulation
With initial use of the cochlear implant, current flow through the facial nerve may cause undesirable stimulation. Kempf and colleagues found 11 cases out of 66 children with such a problem.87 Facial nerve stimulation is usually easily solved by changing stimulation patterns and eliminating offending electrodes that inject errant electrical signals. In rare cases the stimulation persists, though, and the device may not be able to be used by the patient.
Histopathology of Implanted Temporal Bones
The histologic results of cochlear implantation have been well studied.117,118,119,120 Potential histopathologic findings can be divided into surgical and device-related injuries. Surgical trauma may include fractures of the osseous spiral lamina, perforation of the basilar membrane, and tears of the spiral ligament. Cochlear fibrosis and neoossification are common findings in these studies. In general, traumatic changes appear to be limited to the most basal portions of the cochlea and are unlikely to exert significant negative effects on implant performance. Reactions to extended electrical stimulation (e.g., electrochemical tissue damage, neural degeneration) by current implants appear to be modest. Foreign body reactions and infections that extend along the implant array to involve membranous elements of the cochlea are likely to induce sensorineural degeneration. However, well-documented cases of such occurrences are lacking.
Findings of localized cochlear trauma after cochlear implantation have led to concerns that these traumatic injuries might result in associated SGC degeneration. Although two studies of temporal bone histopathology in individual patients with a unilateral cochlear implant report a decrease in
the normal SGC population ipsilateral to the implant (Zappia and colleagues),119 other studies in chronically implanted animals121,122 and humans123,124,125 showed no differences in SGC populations between the implanted and unimplanted ears. It is likely that many factors influence neuron survival, as evidenced by histopathologic studies, including previous pathology (which may vary between the two ears) and the amount of time between implantation (with possible end organ damage) and the time of death. Because the temporal association between SGC degeneration and end organ damage has not been clearly established for humans, histopathologic findings from implanted temporal bones are not always clearly interpretable. Although the duration of implant stimulation in the Linthicum study ranged from 1 to 14 years, Nadol’s report was limited to the study of one set of temporal bones from a patient implanted 10 weeks before death.120 Animal studies have shown that the reintroduction of electrical activity through a cochlear implant may prevent degenerative changes in the central auditory system.126,127
DEVICE FAILURE
The cochlear implant system may demonstrate failure either as a result of complete loss of electrical connectivity or current shunting/shorting. Either the internal, implanted device or the external processor may be affected.
Device failure as a result of loss of electrical function in the external processor commonly produces a sudden loss of function and, therefore, hearing. Intermittency and rarely popping sensations occur before processor failure. Processor functionality may be lost with direct trauma to the unit, exposure to water, and, most frequently, normal wear and tear of connecting lead-wires linking the processor unit with the magnetically-retained antenna that relays information to the internal device. Manufacturers’ inserts and websites and cochlear implant clinics offer readily available troubleshooting guides to either correct the problem with the external unit or prompt processor replacement.
Although much less prevalent, device failure as a result of loss of electrical function in the internal device is of considerably greater concern. An internal device failure typically presents as either an immediate cessation of function or intermittency that is associated with reduced quality of sound and diminishing periods of function over days to weeks. Reports of painful stimulation have been noted, but are fortunately rare.
As all devices are current-limited at levels well below the threshold for tissue injury, symptoms of painful stimulation probably reflect adventitial stimulation of middle ear sensory nerves as a result of current shunting. However, hermeticity failures may be associated with DC leakage as suggested by failure analysis of explanted devices. The presence of moisture in
the implant case can result in dendrite formation and short circuiting of the safety mechanisms (e.g., capacitors), which are designed to prevent neural overstimulation. To date, there are no clear case reports of DC leakage or shorting of RF energy to the electrode resulting in permanent neurological damage, but such injury may be possible and the likely clinical manifestations would be pain. This would presumably prompt adults and older children to immediately cease use of the device, which provides an additional safety net against permanent neurological damage. Unfortunately, infants and younger children would not necessarily be able to remove the noxious stimuli, and there could be a delay in recognition of the problem by parents and caregivers, leaving this younger population more vulnerable to injury. Such possibilities should be made clear to clinical teams who provide services to families with infants and young children with cochlear implants.
Diagnosis of the mode of failure of an internal device is hampered when the implant lacks telemetry to enable electrical assessment of all contained circuits. When telemetry is available, removing select channels from the “map” of stimulated circuits may allow for continued function. In the absence of telemetry, revision surgery may be the only alternative when the clinical team deems the implant nonfunctional.
Explanted devices are sent to the manufacturer for bench assessment of circuit integrity. Formal data on analyses of explanted devices have yet to be reported. However, for the purposes of this review the senior author interviewed manufacturers’ engineers and found general trends in patterns of failure across manufacturers. Of devices with a recognizable fault, the general pattern of failure mode occurs with a relative incidence of
-
Hermeticity or moisture: 75 percent (0.75 percent total)
-
Electronic / Hybrid failure: 16 percent (0.16 percent total)
-
Electrode / Connecting lead: 9 percent (0.09 percent total)
As a whole, cochlear implants have maintained a historical reliability of 99 percent at 1 year, meaning that 99 percent of devices remain working at the 1 year interval. Reliability data have only recently been disclosed for public review by one of the implant manufacturers. It should be noted, however, that the integrity of the data on reliability is subject to under- or misreporting due to a lack of agreement on what constitutes a “device failure” and the challenges associated with ongoing communications an estimated 1,000 cochlear implant centers internationally.
Notwithstanding these uncertainties, reported trends suggest that device reliability has improved over the past 30 years.128 For example, the failure rate for the third generation Cochlear Corporation device released in
2001 (0.3 percent/year) is approximately one-third of that associated with its first generation device used from 1985 to 1998 (0.8 percent).
The need for revision surgery to replace the internal device has motivated scrutiny of potential sources of manufacturing and material defects. In the past year, FDA has examined manufacturing processes at all three major manufacturers with public disclosure of concerns relating to two of the three (see www.fda.gov/foi/warning_letters/g5089d.htm and www.fda.gov/foi/warning_letters/g5265d.htm).
Although cochlear implant technology has improved significantly over the past few years, children remain at an unspecified level of increased risk for device failure given higher activity levels and predisposition to direct trauma.129 A trend toward device failures with direct device impact, particularly in toddlers and very young children, associated with a particular grade of ceramic casing used in Clarion devices manufactured in the mid-1990s led to a change in the grade of the ceramic used. Subsequently, this manufacturer has adopted a titanium case embedded in soft, solid silastic.
Device failure is the most common indication for revision surgery and cochlear reimplantation. “Upgrades” to more advanced models are rarely indicated as the level of performance enhancement achievable is subject to individual case consideration. Infection and flap breakdown require reimplantation less frequently.130
Luetje and Jackson131 reported a 9 percent rate of device failure in a review of 55 children, which matched results found by Parisier and colleagues.132 The most common failures include fracture of the central pin feed-through for the antenna coil, damaged integrated circuits in the internal receiver from electrostatic discharges (occasionally associated with contact and friction with playground-grade polyethylene), damaged electrodes exiting from the internal receiver, capacitor failure, and electrode-array damage.
Detection of device failure is imperative in the implanted child. Parisier and colleagues’ analysis identified four major risk factors that point to failure: fluctuations in threshold and comfort levels of nine units for more than six electrodes, performance incompatible with age and duration of deafness, complaints of extraneous noises and intermittent shocks, and a high number of external equipment changes.131 Tests on the device showing an absence of recorded output, abnormal pulse configuration, or lack of amplitude increase in response to increased stimulation can verify failure.
Prevention of device failure in children begins with proper securing of the implant, particularly the connecting lead between the receiver/stimulator and the electrode array, which is vulnerable to shearing. The device should be embedded in a well drilled in bone and fixed with permanent suture material. While the evolution of implant design has certainly decreased the
rate of device failure, these precautions may help to shield the device from traumatic events.
Should device failure occur, though, reimplantation is the most viable solution. The most common challenges encountered include fibrosis and ossification of the cochleostomy, as well as skin flap breakdown and CSF leakage.133,134,135,136 Theoretical concerns relate to hair cell and spiral ganglion cell damage from further surgical trauma, though clinical evidence of trauma with reimplantation is lacking. Special attention should be paid to the skin flap given the frequency of atrophy following primary implantation and removal of bone at the cochleostomy may be necessary. Alexiades and colleagues suggest that if significant scar tissue surrounds the lead wire, facial recess, or cochleostomy, the lead wire can be severed away from the electrode array to ease removal of the old device and insertion of a replacement (although this procedure may hamper the subsequent device analysis discussed above).130
Despite technical challenges, insertion of all electrodes is usually possible, as Parisier and colleagues found.136 Balkany and colleagues’134 review of 16 subjects, 11 of them pediatric, found insertion length to be greater than in the primary surgery. Only Miyamoto and colleagues found a small but statistically significant decrease in reimplant insertion and the number of active channels.133
Performance following reimplantation is generally promising. Parisier and colleagues’ review of 25 children found that speech perception abilities had remained the same or had improved overall.136 In particular, these children demonstrated continued improvement in open-set speech recognition. Similar reimplantation results were found in Alexiades and colleagues’ review of 20 children135 and in Haensel and colleagues’ review137 of 11 children. If the technical challenges of reimplantation surgery can be overcome, children may benefit greatly from the revision with little risk of compromised performance.
RISKS FOR POOR COMMUNICATION OUTCOMES
Cochlear implants can have impressive effects on a child’s language abilities, yet outcomes remain variable across the pediatric population. Numerous studies have thus attempted to identify predictors determining post-implantation communication. So far, relevant factors are age at onset of deafness, age at implantation, length of implant use, amount of residual hearing, duration of deafness, educational mode and resources, and psychosocial elements.
A clear factor seems to be the age at implantation: children appear to perform better when implanted at earlier stages.138,139,140,141 On IT-MAIS testing, Robbins and colleagues found that children implanted under the
age of 19 months demonstrated faster progress and higher scores than those implanted between the ages of 2 and 3.140 As Geers142 found, though, this age advantage disappears after 2 years, implying a critical period of development within the first 2 years of life. At older ages, then, other factors begin to affect implant performance. In particular, duration of deafness, pre-operative open-set score, and equivalent language age were found by Dowell and colleagues143 to explain a large percentage of the variance in speech perception scores of children implanted between the ages of 8 and 18. Children with an earlier age at onset of deafness and with greater durations of deafness tend to have poorer outcomes.144
At the same time, educational resources must be attuned to the child with a cochlear implant in order to achieve optimal benefit. Classrooms emphasizing total communication rather than oral communication have been found to limit the speech intelligibility abilities and phonological processing skills of children.145,146 Placement in oral communication settings have allowed a greater number of implanted children to eventually join mainstream classrooms in which speech intelligibility scores are further enhanced through interaction with hearing peers. However, oral communication is not enough to ensure the proper progression of the child through schooling. Cooperation between parents, school administrators, teachers, and speech specialists is also vital to the success of the child. Luetje and Jackson130 noted that insufficient funds for special education programs with trained speech and language professionals and adversarial relationships between all parties involved are detrimental to the child’s progress. These adverse circumstances are made worse in regions of the country where services to deaf children are scarce.
The child’s family unit is another integral part of the rehabilitation process. Nikolopoulos and colleagues147 found family structure and support to be significant predictors of outcome. Without full investment of the family in the child’s learning and development maximum benefit from the implant may never be reached. Unfortunately, many families are under considerable stress due to their children’s condition. Spahn and colleagues148 found that a quarter of parents of cochlear implant recipients suffer from high psychic stress, with 18 percent needing psychosocial support. Awareness and prompt treatment of these issues may prevent further delays in the child’s progress, although the exact effects of parental stress on child development with an implant remain unclear.
Overcoming these obstacles can help the child achieve successful outcomes with a cochlear implant. The gains that can be made through implantation are certainly impressive; not only have implanted children shown remarkable improvements in language ability, educational attainment, and social integration, but they have also proven the procedure to be economically beneficial. Cost–utility analyses by O’Neill and colleagues149 and
Cheng and colleagues150 have demonstrated considerable quality of life gains at reasonable costs, particularly when compared to other health care interventions.
It is clear, however, that early cochlear implantation does not provide a “mainstream” educational and psychosocial developmental opportunity for all childhood recipients. The extent to which device-specific factors may impose constraints in developmental learning will require longitudinal, multivariate analysis. If device-specific factors predicting poor long-term performance were identified, their occurrence would have to be considered in the context of a device-related complication.
TRACKING COMPLICATIONS ASSOCIATED WITH COCHLEAR IMPLANTS IN CHILDREN
The above survey of the peer-reviewed literature presents only the range of reported complications associated with cochlear implantation in children. Substantial uncertainty remains regarding the precise rates of complications, and there is insufficient epidemiologic data with which to better understand potential source(s) of adverse outcomes that may be associated with cochlear implants in children.
The importance of tracking complications associated with cochlear implants in children is underscored by the enthusiasm associated with the application of advancing technologies to a significant disability. For example, microprocessing technology has propelled communication technologies of increasing sophistication for the general population. Advances in information processing, when incorporated into cochlear implants, are commonly perceived as naturally contributing to an improved speech code and an improved outcome for deaf children. Indeed, virtually all introductions of newer models of speech processors for cochlear implants have entailed higher rates of information transfer. Faster processing capabilities result from the integration of literally more millions of transistors into progressively smaller body- and ear-worn speech processors. As ever larger numbers of components are fit onto microchips then, a move to new implant designs that facilitate the delivery of more sophisticated codes has followed in at least seven instances. Such circumstances, however, risk a potentially hazardous scenario wherein technology drives the development of devices for biologic application in the absence of long-term, quality data that track in vivo effects.
The field of pediatric cochlear implantation has generally advanced only after periods of months to years of observation of successful experience with newly designed devices in adults. This cautious approach may still fail to ensure safety for childhood applications. In fact, observations emanating from the identification of the risk posed by cochlear implants for
meningitis in children in 2002 indicated that complications of particular concern to children can arise and that trends can go undetected for years.91 It also appears that complications unique to children can arise after highly variable periods of time following surgical implantation, thus necessitating follow-up for indefinite periods of time. For example, the current database on pediatric cochlear implant users is not sufficiently robust to enable a formal determination of meningitis risk going forward as new implant types are introduced. These observations emphasize the need for mandatory reporting requirements and better quality reporting to facilitate the early detection of health-related complications in cochlear implant users.
Manufacturer-based reports on cochlear implant complications are often supplied in direct correspondences and in newsletters to clinicians. However, such reports are often provided in the context of marketing device durability, suggesting that some datasets may go unreported in manufacturer communications. In addition to reports, independently-tracked data are needed to provide a more useful profile. The above summary of pediatric cochlear implant complications from the peer-reviewed literature represents the best efforts of clinicians to gather information about complications from case series, and manufacturer and FDA datasets.
The cornerstone for FDA’s postmarket surveillance of medical devices is the information provided by both voluntary and mandatory device-related adverse event reports to the Agency. (For additional description, see Chapters 3 and 4 of the Institute of Medicine report in which this appendix appears.151) Voluntary reporting to FDA began in 1973, and the current MedWatch Program, created in 1993, provides a mechanism for consumers and health care professionals to voluntarily report problems with medical devices by phone, mail, or online completion of an adverse event form. More recently, FDA has developed partnerships with major health care organizations in the United States to promote reporting adverse events through this program, particularly those of a serious nature.
In 1984 FDA implemented the MDR (Medical Device Reporting) regulation, which requires manufacturers and importers to report all device-related deaths, serious injuries, and malfunctions to the Agency. Additionally, under the Safe Medical Devices Act of 1990 and other subsequent legislation, user facilities (e.g., hospitals, nursing homes) are also now required to report: (1) device-related deaths to both FDA and the manufacturer, (2) device-related serious injuries to the manufacturer (or to the FDA if the manufacturer is unknown), and (3) an annual summary report of deaths and serious injuries to FDA. Although legal enforcement of these mandatory reporting requirements is possible and FDA does inspect manufacturer facilities for compliance with adverse event record keeping and reporting, FDA has relied on the goodwill and cooperation of manufacturers and user facilities to ensure compliance with the regulation and charac-
terization of the potential sources of the complication. Of note, individual health professionals are not required to report events. This is not an insignificant source of cochlear implant surgeries for children in the United States.
The specific requirements for device-related adverse event reporting are outlined in federal regulation at 21 CFR 803. Briefly, manufacturers are required to report deaths, serious injuries, and malfunctions to FDA within 30 calendar days. When an adverse event requires remedial action to prevent an unreasonable risk to public health (or when another event designated by FDA occurs), manufacturers must report the event to the agency within 5 work days. User facilities must report deaths and serious injuries within 10 work days, and must also file an annual report to FDA of deaths and serious injuries.
In actual practice, reporting from user facilities has been low (less than 3 percent of adverse event reports to the Agency in 1999). Recognizing the value and importance of adverse event reporting experience from user facilities in postmarket surveillance, Congress mandated, under the FDA Modernization Act of 1997, that a focused network of well-trained and highly motivated user facilities be created to enhance detection of emerging device problems and to act as a two-way communication channel between FDA and the clinical community. This network, known as the Medical Product Surveillance Network (MedSun), is currently under development and will consist of at least 250 hospitals and approximately 50 nursing homes. MedSun aims to achieve a reasonably representative sample of user facilities (primarily hospitals) that may allow for national estimates on certain device issues in the future. This network could eventually provide a consistent, reliable source of postmarket clinical experience in children with cochlear implants.
Adverse events, whether from voluntary or mandatory reports, are entered into the FDA’s Manufacturer User Facility and Distributor Experience (MAUDE) database. MAUDE contains reports from user facilities since 1991, voluntary reports to the Agency since June 1993, distributor (e.g., importers) reports since 1993, and manufacturer reports since August 1996. Within FDA’s Center for Devices and Radiological Health (CDRH), the Office of Surveillance and Biometrics routinely monitors and analyzes incoming reports to detect signals of significant postmarket safety and effectiveness concerns. Information from these reports is disclosed to the public and is available on the web in the MAUDE database (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM).
An informal analysis of MAUDE suggests that in its current state, the database lacks sensitivity for complications that can be managed medically or are related to surgical technique; such complications would seem to be unlikely to be reported to FDA.152 The MAUDE dataset is intended for
serious complications that result from errors in the use of a device or device malfunction. Even for complications such as device failure, however, there is often an absence of data, such as device lifespan prior to failure and uniform terminology regarding failure modes (e.g., hermeticity breach, electrode wire breakage). These data would likely prove invaluable in analyzing trends. Information such as patient age, date of implant, surgical approach and relevant surgical anatomy, prior surgeries, and characteristics of the implant center are often not included in the MAUDE reports. To be effective, the MAUDE database will need to be structured to provide detailed information on individual medical devices. This will require a priori form design that offers heightened sensitivity and specificity in characterizing device-related complications. Form 3500A (www.fda.gov/medwatch/safety/3500a.pdf) is quite detailed. One problem, however, is that it often is not filled out completely by the manufacturer or user facility—due either to lack of information or other issues.
For cochlear implants, the MAUDE database might be strengthened with regular, timely provision to FDA of manufacturer and distributor data on the number of devices implanted (i.e., denominator data) and trends with respect to recipient demographics could construct a crucial background of device distributional patterns. For example, when cases of meningitis were reported at an alarming rate in 2002, it was completely unclear as to whether the higher incidence of this complication may have coincided with clinical trends of implantation of younger ages. Epoch analyses are likely to be extremely instructive in determining the source(s) of device-related complications. Such analyses would be afforded with updated, high-quality epidemiologic datasets.
It is apparent, however, that the establishment of a uniform, comprehensive, and up-to-date national database of device-related complications faces monumental obstacles. For example, manufacturers may be reluctant to publicly provide data on devices implanted for defined time periods (i.e., denominator data) because this would reveal potentially sensitive information regarding market share. Another very practical issue that hampers ongoing tracking is that families of children with cochlear implants are young and highly likely to change their address within any 5 to 10 year period. A recent experience is instructive. The Advanced Bionics Corporation made a concerted effort to contact every user of its cochlear implant systems (n = ~12,000) in association with the meningitis reports of 2002 with direct, registered mail. Despite using updated address information based on warranty registration, the corporation was able to contact less than 90 percent of users at a cost of over $300,000. While the inability to contact more than 10 percent of users is less than ideal, it is higher than might be expected. Because children and most adults with cochlear implants require regular follow-up, their regular contact with clinics may
promote high rates of information updating and thus relatively high access when compared with patient groups using other implants.
The above experience underscores that some cochlear implant device users and their families appear to go without close, regular follow-up by a medical facility. Moreover, address changes are not always available from manufacturer and patient records. This experience, however, does suggest that ongoing manufacturer to FDA communications and warranty-related contact information can provide an important means of informing trends in device-related complications. This would seem to be particularly important with respect to childhood recipients. The establishment of a dedicated, pediatric database that draws on multiple sources of information deserves further consideration.
In addition to adverse event reporting requirements, FDA may also mandate that manufacturers perform postmarket studies either as a condition of the original FDA approval or under the FDA Modernization Act (Section 522) authorities. High-risk implantable devices (e.g., cochlear implants) require FDA approval of a premarket approval application (PMA) prior to marketing to ensure their safety and effectiveness. Along with the specific adverse event reporting requirements listed in the PMA Conditions of Approval, the Agency may also include an additional “condition” for the manufacturer to address specific safety and/or effectiveness issues through a postapproval study. For example, cochlear implant manufacturers have often been required to collect additional long-term clinical data on pediatric patients to demonstrate safety and effectiveness of the device in this population. Alternatively, the Agency may impose postmarket study requirements for certain devices under Section 522 of the Federal Food, Drug, and Cosmetic Act. This authority allows FDA, under its discretion and for good reason, to order manufacturers to conduct postmarket surveillance on Class II or III devices that are implanted in the human body for more than 1 year, life-sustaining or life-supporting devices used outside of a device user facility, or devices the failure of which would be reasonably likely to have serious adverse health consequences. These studies are generally reserved for potential device failure situations that would be reasonably likely to have serious adverse health consequences. Under these circumstances, FDA can require prospective surveillance period of up to 36 months.
Reviews of major complications associated with implantable devices suggest that two approaches to monitoring device safety might be expanded: (1) the role of clinicians in reporting potential device-related complications, and (2) the visibility of a centralized data coordination center. Health care providers are the likely contact for virtually all complications related to device use. The responsibility of clinicians to report major device complications is in the interest of the public’s health and should be underscored in licensing requirements. Successful completion of web-based training mod-
ules used recently to inform clinicians of new Health Insurance Portability and Accountability Act and Effort-Reporting regulations could be used to develop new approaches to device-use surveillance and may be held as a requirement for licensure. Such training should also promote complete understanding of mechanisms of reporting to a data coordination center that is singularly recognized as the site for the accrual of data on device complications.
REFERENCES
1Reis R. Prevalence and characteristics of persons with hearing trouble: United States, 1990–1991. National Center for Health Statistics. Vital Health Stat 10 1994:1–134.
2Blanchfield B, Dunbar J, Feldman J, Gardner E. The Severely to Profoundly Hearing Impaired Population in the United States: Prevalence and Demographics. Bethesda, Md: Project HOPE Center for Health Affairs, 1999.
3Berliner K, Eisenberg L. Methods and issues in the cochlear implantation of children: an overview. Ear Hear 1985;6:(Suppl 3) 6S–13S.
4Ling D. Foundations of Spoken Language for Hearing Impaired Children. Washington, DC: Alexander Graham Bell Assoc for the Deaf, 1989.
5Parisier SC, Chute PM, Popp AL, et al. Surgical techniques for cochlear implantation in the very young child. Otolaryngol Head Neck Surg 1997;117:248–254.
6Waltzman SB, Cohen NL. Cochlear implantation in children younger than 2 years old. Am J Otolaryngol 1998;19:158–162.
7Lenarz T. Cochlear implantation in children under the age of two years. Adv Otorhinolaryngol 1997;52:204–210.
8Novak MA, Firszt JB, Rotz LA, et al. Cochlear implants in infants and toddlers. Ann Otol Rhinol Laryngol Suppl 2000;185:46–49.
9Hehar SS, Nikolopoulos TP, Gibbin KP, et al. Surgery and functional outcomes in deaf children receiving cochlear implants before age 2 years. Arch Otolaryngol Head Neck Surg 2002;128:11–14.
10Zwolan TA, Ashbaugh CM, Alarfaj A, et al. Pediatric cochlear implant patient performance as a function of age at implantation. Otol Neurotol 2004;25:112–120.
11Robbins AM, Koch DB, Osberger MJ, et al. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg 2004; 130:570–574.
12Schauwers K, Gillis S, Daemers K, et al. Cochlear implantation between 5 and 20 months of age: the onset of babbling and the audiologic outcome. Otol Neurotol 2004;25:263–270.
13Govaerts PJ, De Beukelaer C, Daemers K, et al. Outcome of cochlear implantation at different ages from 0 to 6 years. Otol Neurotol 2002;23:885–890.
14Young NM. Infant cochlear implantation and anesthetic risk. Ann Otol Rhinol Layngol Suppl 2002;189:49–51.
15Waltzman SB, Scalchunes V, Cohen NL. Performance of multiply handicapped children using cochlear implants. Am J Otol 2000;21:349–335.
16Hamzavi J, Baumgartner WD, Egelierler B, et al. Follow up of cochlear implanted handicapped children. Int J Ped Otorhinolaryngol 2000;56:169–174.
17Fukuda S, Fukushima K, Maeda Y, et al. Language development of a multiply handicapped child after cochlear implantation. Int J Ped Otorhinolaryngol 2003;67:627–633.
18Donaldson AI, Heavner KS, Zwolan TA. Measuring progress in children with autism spectrum disorder who have cochlear implants. Arch Otolaryngol Head Neck Surg 2004;130: 666–671.
19Francis HW, Pulsifer MB, Chinnici J, et al. Effects of central nervous system residua on cochlear implant results in children deafened by meningitis. Arch Otolaryngol Head Neck Surg 2004;130:604–611.
20Luntz M, Teszler CB, Shpak T, et al. Cochlear implantation in healthy and otitis–prone children: a prospective study. Laryngoscope 2001;111:1614–1618.
21Luntz M, Teszler CB, Shpak T. Cochlear implantation in children with otitis media: second stage of a long-term prospective study. Int J Pediatr Otorhinolaryngol 2004;68:273–280.
22Dettman SJ, D’Costa, WA, Dowell RC, et al. Cochlear implants for children with significant residual hearing. Arch Otolaryngol Head Neck Surg 2004;130:612–618.
23Mecklenburg D. Cochlear implants and rehabilitative practices. In: Handbook of Hearing Aid Amplification. Sandlin R, ed. Boston: College Hill Press, 1990, pp. 179–188.
24Weber BP, Neuburger J, Goldring JE, et al. Clinical results of the CLARION magnetless cochlear implant. Ann Otol Rhinol Laryngol Suppl 1999;177:22–26.
25Youssefzadeh S, Baumgartner W, Dorffner R, et al. MR compatibility of Med EL cochlear implants: clinical testing at 1.0 T. J Comput Assist Tomogr 1998;22:346–350.
26Heller JW, Brackmann DE, Tucci DL, et al. Evaluation of MRI compatibility of the modified nucleus multichannel auditory brainstem and cochlear implants. Am J Otol 1996; 17:724–729.
27Baumgartner WD, Youssefzadeh S, Hamzavi J, et al. Clinical application of magnetic resonance imaging in 30 cochlear implant patients. Otol Neurotol 2001;22:818–822.
28Schmerber S, Reyt E, Lavieille JP. Is magnetic resonance imaging still a contraindication in cochlear-implanted patients? Eur Arch Otorhinolaryngol 2003;260:293–294.
29Teissl C, Kremser C, Hochmair ES, et al. Cochlear implants: in vitro investigation of electromagnetic interference at MR imaging—compatibility and safety aspects. Radiology 1998;208:700–708.
30Nadol JB. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg 1997;117:220–228.
31Linthicum FH, Fayad J, Otto SR, et al. Cochlear implant histopathology. Am J Otol 1991;12:245–311.
32Woolley AL, Oser AB, Lusk RP, et al. Pre-operative temporal bone computed tomography scan and its use in evaluating the pediatric cochlear implant candidate. Laryngoscope 1997;108:1100–1106.
33Cohen NL, Hoffman RA, Stroschein M. Medical or surgical complications related to the Nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol Suppl 1988;135:8–13.
34Cohen NL, Hoffman RA. Complications of cochlear implant surgery in adults and children. Ann Otol Rhinol Laryngol 1991;100:708–711.
35Luetje CM, Jackson K. Cochlear implants in children: what constitutes a complication? Otolaryngol Head Neck Surg 1997;117:243–247.
36Kempf HG, Johann K, Weber BP, et al. Complications of cochlear implant surgery in children. Am J Otol 1997;18:S62–S63.
37Gysin C, Papsin BC, Daya H, Nedzelski J. Surgical outcome after paediatric cochlear implantation: diminution of complications with the evolution of new surgical techniques. J Otolaryngol 2000 Oct;29(5):285–289.
38Bielamowiez SA, Coker NJ, Jenkins HA, et al. Surgical dimensions of the facial recess in adults and children. Arch Otolaryngol Head Neck Surg 1988;114:534–537.
39Eby TL. Development of the facial recess: implications for cochlear implantation. Laryngoscope 1996;106(Suppl 80):1–7.
40Eby TL, Nadol JB. Postnatal growth of the human temporal bone: implications for cochlear implants in children. Ann Otol Rhinol Laryngol 1986;95:356–382.
41Dahm MC, Shepherd RK, Clark GM. The postnatal growth of the temporal bone and its implications for cochlear implantation in children. Acta Otolaryngol Suppl 1993;505:1–39.
42Xu J, Shepherd RK, Xu SA, Seldon HL, Clark GM. Pediatric cochlear implantation: radiologic observations of skull growth. Arch Otolaryngol Head Neck Surg 1993;119:525–534.
43Burton MJ, Shepherd RK, Xu SA, et al. Cochlear implantation in young children: histological studies on head growth, leadwire, design, electrode fixation in the monkey model. Laryngology 1994;104:167–175.
44Roland JT Jr, Fishman AJ, Waltzman SB, et al. Stability of the cochlear implant array in children. Laryngoscope 1998;108:1119–1123.
45Wang RC, Parisier SC, Weiss MH, et al. Cochlear implant flap complications. Ann Otol Rhinol Laryngol 1990;99:791–795.
46O’Donoghue GM, Nikolopoulos TP. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol 2002;23:891–894.
47Green JD, Marion MS, Hinojosa R. Labyrinthitis ossificans: histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg 1991;104:320–326.
48Telian SA, Zimmerman-Phillips S, Kileny PR. Successful revision of failed cochlear implants in severe labyrinthitis ossificans. Am J Otol 1996;17:53–60.
49Nadol JB. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg 1997;117:220–228.
50Balkany T, Gantz B, Nadol JB. Multichannel cochlear implants in obstructed and obliterated cochleas. Otolaryngol Head Neck Surg 1988;98:72–81.
51Linthicum FH, Fayad J, Otto SR, et al. Cochlear implant histopathology. Am J Otol 1991;12:245–311.
52El-Kashlan HK, Ashbaugh C, Zwolan T, et al. Cochlear implantation in prelingually deaf children with ossified cochleae. Otol Neurotol 2003;24:596–600.
53Steenerson RL, Gary LB. Multichannel cochlear implantation in children with cochlear ossification. Am J Otol 1999;20:442–444.
54Hodges AV, Balkany TJ, Gomez-Marín O, et al. Speech recognition after implantation of the ossified cochlea. Am J Otol 1999;20:453–456.
55Dodds A, Tyszkiewicz E, Ramsden R. Cochlear implantation after bacterial meningitis: the dangers of dealy. Arch Dis Child 1997;76:139–140.
56Balkany T, Gantz BJ, Steenerson RL, et al. Systematic approach to electrode insertion in the ossified cochlea. Otolaryngol Head Neck Surg 1996;114:4–11.
57Gantz BJ, McCabe BF, Tyler RS. Use of multichannel cochlear implants in obstructed and obliterated cochleas. Otolaryngol Head Neck Surg 1988;98:72–81.
58McClay JE, Tandy R, Grundfast K, et al. Major and minor temporal bone abnormalities in children with and without congenital sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 2002;128:664–671.
59Ohlms LA, Edwards MS, Mason EO, et al. Recurrent meningitis and Mondini dysplasia. Arch Otolaryngol Neck Surg 1990;116:608–612.
60Tucci DL, Telian SA, Zimmerman-Phillips S, et al. Cochlear implantation in patients with cochlear malformations. Arch Otolaryngol Head Neck Surg 1995;21:833–838.
61Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 1987;97(Suppl 40):15–17.
62Shelton C, Luxford WM, Tonokawa LL, et al. The narrow internal auditory canal in children: a contraindication for cochlear implants. Otolaryngol Head Neck Surg 1989;100: 227–231.
63Casselman JW, Offeciers FE, Govaerts PJ, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology 1997; 202:773–781.
64Otte G, Schuknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae: implications for cochlear implantation. Laryngoscope 1978;88:1231–1246.
65Johnsson LG, Hawkins JE, Rouse RC, et al. Four variations of the Mondini inner ear malformations as seen in microdissections. Am J Otol 1984;5:242–257.
66Monsell EM, Jackler RK, Motta G, et al. Congenital malformations of the inner ear. Laryngoscope 1987;97(Suppl 40):18–24.
67Zheng Y, Schachern PA, Cureoglu S, et al. The shortened cochlea: its classification and histopathologic features. Int J Pediatr Otorhinolaryngol 2002;63:29–39.
68Molter DW, Pate BR, McElveen JT. Cochlear implantation in the congenitally malformed ear. Otolaryngol Head Neck Surg 1993;108:174–177.
69Hoffman RA, Downey LL, Waltzman SB, et al. Cochlear implantation in children with cochlear malformations. Am J Otol 1997;18:184–187.
70Weber BP, Dillo W, Dietrich B, et al. Pediatric cochlear implantation in cochlear malformations. Am J Otol 1998;19:747–753.
71Woolley AL, Jenison V, Stroaer BS, et al. Cochlear implantation in children with inner ear malformations. Ann Otol Rhinol Laryngol 1998;107:492–500.
72Eisenman DJ, Ashbaugh C, Zwolan TA, et al. Implantation of the malformed cochlea. Otol Neurotol 2001;22:834–841.
73Incesulu A, Vural M, Erkan U, et al. Cochlear implantation in children with inner ear malformations: report of two cases. Int J Pediatr Otorhinolaryngol 2002;65:171–179.
74Buchman CA, Copeland BJ, Yu KK, et al. Cochlear implantation in children with congenital inner ear malformations. Laryngoscope 2004;114:309–316.
75Govaerts PJ, Casselman J, Daemers K, et al. Cochlear implants in aplasia and hypoplasia of the cochleovestibular nerve. Otol Neurotol 2003;24:887–891.
76Mylanus EAM, Rotteveel LJC, Leeuw RL. Congenital malformation of the inner ear and pediatric cochlear implantation. Otol Neurotol 2004;25:308–317.
77Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg 1997;117:214–216.
78Clark GM. An evaluation of per-scalar cochlear electrode implantation techniques: a histopathological study in cats. J Laryngol Otol 1977;91:185–199.
79Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Acta Otolaryngol (Stockh) Suppl 1983;399:19–31.
80Kennedy DW. Multichannel intracochlear electrodes: mechanism of insertion trauma. Laryngoscope 1987;97:42–49.
81Cohen NL, Hoffman RA. Complications of cochlear implant surgery. In: Complications in Head and Neck Surgery. Krespi YP, Ossoff RH, eds. Baltimore, Md.: Mosby, 1993.
82House JR, Luxford WM. Facial nerve injury in cochlear implantation. Otolaryngol Head Neck Surg 1993;109:1078–1082.
83Fayad JN, Wanna GB, Micheletto JN, et al. Facial nerve paralysis following cochlear implant surgery. Laryngoscope 2003;113:1344–1346.
84House WF, Luxford WM, Courtney B. Otitis media in children following cochlear implant. Ear Hear 1985;6:24S–26S.
85Luntz M, Hodges AV, Balkany T, et al. Otitis media in children with cochlear implants. Laryngoscope 1996;106:1403–1405.
86Fayad JN, Tabaee A, Micheletto JN, et al. Cochlear implantation in children with otitis media. Laryngoscope 2003;113:1224–1227.
87Kempf HG, Stöver T, Lenarz T. Mastoiditis and acute otitis media in children with cochlear implants: recommendations for medical management. Ann Otol Rhinol Laryngol Suppl 2000;185:25–27.
88Papsin BC, Bailey CM, Albert DM, et al. Otitis media with effusion in paediatric cochlear implantees: the role of peri-implant grommet insertion. Int J Pediatr Otorhinolaryngol 1996; 38:13–19.
89Nadol JB, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol 2004;25:257–262.
90Antonelli PJ, Lee JC, Burne RA. Bacterial biofilms may contribute to persistent cochlear implant infection. Otol Neurotol 2004 Nov;25(6):953–957.
91Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med 2003;349:435–445.
92Callanan V, Poje C. Cochlear implantations and meningitis. Int J Pediatr Otorhinolaryngol 2004;68:545–550.
93Page EL, Eby TL. Meningitis after cochlear implantation in Mondini malformation. Otolaryngol Head Neck Surg 1997;116:104–106.
94Suzuki C, Sando I, Fagan JJ, et al. Histopathological features of a cochlear implant and otogenic meningitis in Mondini dysplasia. Arch Otolaryngol Head Neck Surg 1998;124: 462–466.
95O’Donoghue GM, Balkany T, Cohen N, et al. Meningitis and cochlear implantation. Otol Neurotol 2002;23:823–824.
96Cohen NL, Roland JT, Marrinan M. Meningitis in cochlear implant recipients: the North American experience. Otol Neurotol 2004;25:275–281.
97Pneumococcal vaccination for cochlear implant candidates and recipients: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2003;52(31):739–740.
98Ito J, Sakakihara J. Tinnitus suppression by electrical stimulation of the cochlear wall and by cochlear implantation. Laryngoscope 1994;104:752–754.
99Souliere CRJ, Kileny PR, Zwolan TA, et al. Tinnitus suppression following cochlear implantation: a multifactorial investigation. Arch Otolaryngol Head Neck Surg 1992;118: 1291–1297.
100Tyler RS. Tinnitus in the profoundly hearing-impaired and the effects of cochlear implants. Ann Otol Rhinol Laryngol Suppl 1995;165:25.
101Ruckenstein MJ, Hedgepeth C, Rafter KO, et al. Tinnitus suppression in patients with cochlear implants. Otol Neurotol 2001;22:200–204.
102Miyamoto RT, Bichey BG. Cochlear implantation for tinnitus suppression. Otolaryngol Clin North Am 2003;36:345–352.
103Aschendorff A, Pabst G, Klenzner T, et al. Tinnitus in cochlear implant users: the Freiburg experience. Int Tinnitus J 1998;4:162–164.
104Vibert D, Hausler R, Kompis M, et al. Vestibular function in patients with cochlear implantation. Acta Otolaryngol Suppl 2001;545:29–34.
105Dobie R, Jenkins H, Cohen N. Surgical results. Ann Otol Rhinol Laryngol 1995; 104(Suppl 165):6–8.
106Kubo T, Yamamoto K, Iwaki T, et al. Different forms of dizziness occurring after cochlear implant. Eur Arch Otorhinolaryngol 2001;258:9–12.
107Ito J. Influence of the multichannel cochlear implant on vestibular function. Otolaryngol Head Neck Surg 1998;118:900–902.
108Fina M, Skinner M, Goebel JA, et al. Vestibular dysfunction after cochlear implantation. Otol Neurotol 2003;24:234–242.
109Steenerson RL, Cronin GW, Gary LB. Vertigo after cochlear implantation. Otol Neurotol 2001;22:842–843.
110Hoffman RA, Cohen NL. Complications of cochlear implant surgery. Ann Otol Rhinol Laryngol 1995;166:420–422.
111Gibson WPR, Harrison HC. Further experience with a straight, vertical incision for placement of cochlear implants. J Laryngol Otol 1997;111:924–927.
112Harris JP, Cueva RA. Flap design for cochlear implantation: avoidance of a potential complication. Laryngoscope 1987;97:755–757.
113Gibson WPR, Harrison HC, Prowse C. A new incision for placement of cochlear implants. J Laryngol Otol 1995;109:821–825.
114Cohen NL, Hoffman RA. Complications of cochlear implant surgery. In: Complications in Head and Neck Surgery. Baltimore, Md.: Mosby, 1993:723.
115Wang RC, Parisier SC, Weiss MH, et al. Cochlear implant flap complications. Ann Otol Rhinol Laryngol 1990;99:791–795.
116Haberkamp TJ, Schwaber MK. Management of flap necrosis in cochlear implantation. Ann Otol Rhinol Laryngol 1992;101:38–41.
117Kennedy DW. Multichannel intracochlear electrodes: mechanism of insertion trauma. Laryngoscope 1987;97:42–49.
118Fayad J, Linthicum FH, Otto SR, et al. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol 1991;100: 807–811.
119Zappia JJ, Niparko JK, Oviatt DL, et al. Evaluation of the temporal bones of a multichannel cochlear implant patient. Ann Otol Rhinol Laryngol 1991;100:914–921.
120Nadol JB, Ketten DR, Burgess BJ. Otopathology in case of multichannel cochlear implantation. Laryngoscope 1994;104:299–303.
121Zappia JJ, Niparko JK, Oviatt DL, et al. Evaluation of the temporal bones of a multichannel cochlear implant patient. Ann Otol Rhinol Laryngol 1991;100:914–921.
122Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Acta Otolaryngol (Stockh) Suppl 1983;399:19–31.
123Clark FM, Shepherd RK, Franz BK-H. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Acta Otolaryngol (Stockh) Suppl 1988;448:6–65.
124Linthicum, FH, Fayad J, Otto SR, et al. Cochlear implant histopathology. Am J Otol 1991;12:245–311.
125Nadol JB Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol 2001;110:883–891.
126Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol 1999; 412:543–562.
127Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear Res 1999;133:27–39.
128Cochlear Americas Technology Update, Vol.1, Ed. 2, October 2004.
129Weise JB, Muller-Deile J, Brademann G, Meyer JE, Ambrosch P, Maune S. Impact to the head increases cochlear implant reimplantation rate in children. Auris Nasus Larynx 2005 May 26; [Epub ahead of print].
130Alexiades G, Roland JT, Fishman AJ, et al. Cochlear reimplantation: surgical techniques and functional results. Laryngoscope 2001;111:1608–1613.
131Luetje CM, Jackson K. Cochlear implants in children: what constitutes a complication. Otolaryngol Head Neck Surg 1997;117:243–247.
132Parisier SC, Chute PM, Popp AL. Cochlear implant mechanical failures. Am J Otol 1996;17:730–734.
133Miyamoto RT, Svirsky MA, Myres WA, et al. Cochlear implant reimplantation. Am J Otol 1997;18:S60–S61.
134Balkany TJ, Hodges AV, Gómez-Marín O, et al. Cochlear reimplantation. Laryngoscope 1999;109:351–355.
135Alexiades G, Roland JT, Fishman AJ, et al. Cochelar reimplantation: surgical techniques and functional results. Laryngoscope 2001;111:1608–1613.
136Parisier SC, Chute PM, Popp AL, et al. Outcome analysis of cochlear implant reimplantation in children. Laryngoscope 2001;111:26–32.
137Haensel J, Engelke JC, Dujardin H, et al. Cochlear reimplantation—experiences and results. Laryngorhinootologie 2004;83:83–87.
138Brackett D, Zara CV. Communication outcomes related to early implantation. Am J Otol 1998;19:453–460.
139Nikolopoulos TP, O’Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope 1999;109:595–599.
140Hammes DM, Novak MA, Rotz LA, et al. Early identification and cochlear implantation: critical factors for spoken language development. Ann Otol Rhinol Laryngol Suppl 2002;189:74–78.
141Robbins AM, Koch DB, Osberger MJ. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg 2004;130:570–574.
142Geers AE. Speech, language, and reading skills after early cochlear implantation. Arch Otolaryngol Head Neck Surg 2004;130:634–638.
143Dowell RC, Dettman SJ, Hill K, et al. Speech perception outcomes in older children who use multichannel cochlear implants: older is not always poorer. Ann Otol Rhinol Laryngol Suppl 2002;189:97–101.
144Staller SJ, Beiter AL, Brimacombe JA, et al. Pediatric performance with the Nucleus 22-channel cochlear implant system. Am J Otol 1991;12 Suppl:126–136.
145Tobey EA, Rekard D, Buckley K, et al. Mode of communication and classroom placement impact on speech intelligibility. Arch Otolaryngol Head Neck Surg 2004;130:639–643.
146Dillon C, Pisoni DB, Cleary M, et al. Nonword imitation by children with cochlear implants. Arch Otolaryngol Head Neck Surg 2004;130:587–591.
147Nikolopoulos TP, Gibbin KP, Dyar D. Predicting speech perception outcomes following cochlear implantation using Nottingham children’s implant profile (NChIP). Int J Pediatr Otorhinolaryngol 2004;68:137–141.
148Spahn C, Richter B, Zschocke I, et al. The need for psychosocial support in parents with cochlear implanted children. Int J Pediatr Otorhinolaryngol 2001;57:45–53.
149O’Neill C, O’Donoghue GM, Archbold SM, et al. A cost-utility analysis of pediatric cochlear implantation. Laryngoscope 2000;110:156–160.
150Cheng AK, Rubin HR, Powe NR, et al. Cost-utility analysis of the cochlear implant in children. JAMA 2000;284:850–856.
151Institute of Medicine, Committee on Postmarket Surveillance of Pediatric Medical Devices. Safe Medical Devices for Children. Field M, and Tilson H, eds. Washington, DC: The National Academies Press, 2005.
152 Tambyraja EM, Gutman MA, Megerian CA Cochlear implant complications: Utility of federal database in systematic analysis. Archives of Otolaryngology-Head & Neck Surgery, 2005, Mar;131(3):245-250.