4
Environments on Mars Relative to Life
Currently, we do not know whether there is, or has ever been, life on Mars, nor do we know if there are environments on Mars today that can sustain life—terrestrial or martian. However, as knowledge of Mars grows and understanding of the planet matures, it is increasingly evident that there may currently be diverse environments on Mars that may be or might have been hospitable to life, either now or in the recent past. This chapter presents a snapshot of current understanding of the possibilities for potentially habitable martian environments. Analyses of new spacecraft data are revolutionizing many aspects of scientific understanding of Mars, including the possibilities for habitable environments. These possibilities could expand further or contract considerably as additional new data from Mars missions are acquired and analyzed. However, the general trend is now toward increasing prospects for habitability, a trend that should be factored into future policies regarding forward contamination.
Based on terrestrial experience, life has three minimum prerequisites: biogenic elements (such as carbon, nitrogen, and other elements critical for biological molecules), a utilizable source of energy, and liquid water (to provide the medium for biochemistry). The presence of life as we know it implies the presence of organic molecules, and many theories of the origin of life require certain prebiotic organics to be present in the environment prior to life’s origin.1
An ideal habitable environment would have all three prerequisites continuously available. However, on Earth, life is able to flourish in environments where all three prerequisites are not continuously coincident in space and time (see Chapter 5). Therefore, consideration of environments on Mars that are relevant to life necessarily requires factoring in temporal variability, which may occur on daily, seasonal, interannual, and climatic (104 to 107 year) timescales. The next three sections outline current understanding of the three minimum prerequisites for martian habitability. The rest of the chapter discusses the implications for the definition of special regions2 and the need for additional observations and studies.
|
1 |
Organic molecules are molecules based on carbon bonded to hydrogen or nitrogen and perhaps other atoms: organic molecules can be produced by both biological and nonbiological processes. For a review of current thinking in orgins-of-life and astrobiology research, see Chyba and Hand (2005). |
|
2 |
For the COSPAR definition of special region, see Box 1.1. |
BIOGENIC MATERIALS
Mars is known to be well endowed with most of the elemental building blocks of life, although the martian inventory of nitrogen, in particular, remains poorly understood (Mancinelli, 1996). Mars’s inventory of these elements is being continuously supplemented through the influx of cometary and meteoritic material.3 A significant fraction (1 to 10 percent) by weight of infalling material is in the form of organic material synthesized by abiotic processes. The annual delivery of reduced organic compounds to the martian surface, most of it due to interplanetary dust particles and micrometeorites, is estimated to be 2.4 × 108 g/yr (Flynn and McKay, 1990; Benner et al., 2000).
Present data on martian organics come from two sources. The first are the Viking pyrolysis gas chromatographmass spectrometer (GCMS) analyses of martian soils, which detected the solvents used to clean the instrument on Earth but found no traces of martian organics to the limits of the instrument’s sensitivity (Biemann et al., 1977). The second source of data is that fraction of the organics contained in martian meteorites that is not due to terrestrial contamination (Jull et al., 1998; Becker et al., 1999). The organics identified in martian meteorites include polycyclic aromatic hydrocarbons (PAHs) and kerogens, some of which exhibit 13C/12C ratios similar to those observed in organics in primitive meteorites, suggesting that Mars may contain a record of extraterrestrial organic carbon compounds delivered by meteorites (e.g., Jull et al., 2000). The failure of the Viking GCMS to detect martian organics has been explained by (1) the absence of organics in the strongly oxidizing near-surface soil environment (Biemann et al., 1977), (2) insufficient instrument sensitivity (Glavin et al., 2001), (3) inability to achieve the high oven temperatures required to volatilize complex kerogen-like components (Becker, 2002), and (4) inability to detect the presence of the nonvolatile products of oxidative degradation such as the salts of organic acids (Benner et al., 2000).
Oxidants (such as hydrogen peroxide, H2O2) derive from photochemical processes in the martian atmosphere and from the interaction of solar ultraviolet radiation with surface minerals to form superoxides (Yen et al., 2000). However, Mancinelli (1989) has shown that certain Earth soil bacteria can survive much higher concentrations of H2O2 than are implied by the Viking measurements. Moreover, geologic processes have managed to preserve organics in terrestrial sedimentary rocks for billions of years (e.g., Foriel et al., 2004), despite oxidizing surface conditions. Therefore, it is unlikely that the presence of oxidants in the martian atmospheric and near-surface environments in itself represents an insurmountable challenge for martian habitability.
UTILIZABLE ENERGY
Earth’s biota derive useful energy either from light or from chemical reactions. Sunlight is abundant at the surface of Mars, but in the absence of evidence for the absorption of sunlight by martian biota, most considerations of biologically useful energy on Mars have focused on chemical sources. Utilizable chemical energy becomes available when disequilibrium redox conditions are created in the environment by processes such as volcanism, chemical weathering, and atmospheric photochemistry. There is evidence that all three processes either currently occur, or have occurred recently, on Mars. Crater counts in the calderas of martian volcanoes suggest that that eruptions have occurred as recently as 2 million years ago (Neukum et al., 2004). The weathering of iron minerals has been considered as a potential energy source for an early martian biosphere (Jakosky and Shock, 1998), and like the crust of the Earth, martian meteorites show evidence of incomplete chemical weathering (Treiman et al., 1993). Photochemical processes in the martian atmosphere result in the ongoing production of reactive species, such as H2, O2, and CO (Nair et al., 1994) that could be an energy source for martian biota (Weiss et al., 2000; Summers et al., 2002). The relatively good agreement between the observed concentrations of these species and chemical models that do not include surface sinks (biotic or abiotic) has been used to place upper limits on the overall metabolic activity of hypothetical biota. Also, it is concluded that Mars’s present-day biotic carbon flux
can be at most 4 × 10–5 times that of Earth’s (Weiss et al., 2000). These results suggest that distribution of hypothetical martian biota may not be limited by the availability of energy, but rather by the availability of liquid water.
The metabolic activity of a potential martian biosphere is one of several possible explanations for recent claims of the detection of roughly 10 ppb of methane in the martian atmosphere (Mumma et al., 2004; Krasnapolsky et al., 2004; Formisano et al., 2004). If this detection is correct, the approximately 300-year photochemical lifetime of methane in the martian atmosphere implies the presence of a significant and recent methane source. Candidate abiogenic methane sources include recent volcanism, hydrothermal sources, cometary and meteorite impacts, and the serpentization of basalt (Wallendahl and Treiman, 1999; Krasnopolsky et al., 2004; Formisano et al., 2004). Candidate biogenic sources include fossil biogenic methane diffusing out of the martian crust and ongoing methane production from a hypothetical martian biosphere (Farmer, 1996; Fisk and Giovannoni, 1999; Max and Clifford, 2000; Krasnopolsky et al., 2004; Formisano et al., 2004). The global atmospheric source strength of methane on Mars is less than one-millionth that of methane on Earth. This suggests that, if the amount of methane that appears to be present in the atmosphere is in equilibrium with its production rate (i.e., there is no net accumulation of methane in the subsurface), then the metabolic rate of any hypothetical martian biosophere is quite small. Neither the validity of this assumption nor the accuracy of the identification of atmospheric methane can currently be confirmed. If subsequent investigations were to verify the presence of methane and also confirm that it is of biological origin, the motivation and requirements for preventing the forward contamination of Mars would have to be thoroughly reassessed.
LIQUID WATER
On Mars today, the limiting requirement for habitability appears to be the presence of liquid water. The potential for forward contamination of Mars by Earth microorganisms is therefore closely tied to the existence, state, and distribution of water reservoirs across the planet. Thus, water is the key indicator for special regions (see Box 1.1) on Mars. Knowledge of the distribution and behavior of water in all its forms on Mars is at present incomplete, particularly with respect to liquid water.
The subsections below summarize current understanding of the total martian reservoir and discuss the prospects for liquid water in the deep and near subsurface.
The Total Martian Water Reservoir
Mars exhibits widespread evidence of extensive modification by the effects of impacts, volcanism, liquid water, ice and wind—processes that appear to have been more active in the planet’s past. Evidence for a water-rich Mars is provided by the geomorphic interpretation of a long list of landforms (e.g., Carr and Schaber, 1977; Rossbacher and Judson, 1981; Carr, 1986, 1996; Squyres et al., 1992; Malin and Edgett, 2000, 2003) and by geochemical and sedimentary evidence, recently acquired by the Mars Exploration Rover (MER), of episodic inundation by shallow surface water at Meridiani Planum (Squyres et al., 2004a,b).4
The most persuasive geomorphic evidence for large amounts of water on Mars are the outflow channels—broad scoured depressions hundreds of kilometers long that exhibit braided and streamlined forms within their beds. The channels generally emerge abruptly from large areas of collapsed and disrupted terrain, the apparent result of a massive release of groundwater. The distribution, size, and range of ages of these features suggest that a significant body of groundwater was present on Mars throughout much of its geologic history and may still persist today (Baker, 1982; Tanaka, 1986; Tanaka and Scott, 1987; Carr, 1986, 1996; Baker et al., 1991).
Using a conservative estimate of the volume of water required to erode the outflow channels, and the likely extent of their subsurface source regions, Carr (1986) estimates that Mars may possess a planetary inventory of
water equivalent to a global ocean 0.5 to 1 km deep if uniformly distributed over the planet’s surface. Because the peak in outflow channel activity appears to have occurred ~2 to 3 Gya (billion years ago) (Tanaka, 1986), it significantly post-dates the period when the most efficient mechanisms of planetary water loss (impact erosion and hydrodynamic escape) are thought to have been active (>4 Gya). Thus, it is expected that the vast bulk of this water still resides on Mars. Studies of recent (less than a few hundred million years old) martian meteorites further support this conclusion; there is evidence that the parent magma from which several of these meteorites were derived outgassed significant amounts of water (Dann et al., 2001).
Little of the 0.5 to 1 km estimated inventory of existing water is in the martian atmosphere or apparent at the martian surface. Only about 0.000001 percent of the inventory is found in the atmosphere (Farmer and Doms, 1979), while ~5 to 10 percent is thought to be stored as ice in the perennial polar ice caps and layered deposits (Clifford et al., 2000). That leaves ~90 to 95 percent of the total H2O unaccounted for, almost all of which is thought to be stored as ground ice and groundwater within the planet’s crust (Carr, 1996). To understand the distribution and state of water on Mars, one must understand the distribution and state of its subsurface water.
Deep Subsurface Water
A comprehensive geophysical survey for deep subsurface water on Mars has yet to be conducted. In its absence, knowledge of the distribution and state of deep subsurface water depends strongly on theoretical models and analogies to Earth. This distribution is likely to be influenced by an equivalent level of geologic complexity and the spatial variability of such crustal characteristics as lithology, structure, stratigraphy, porosity, permeability, ice content, mechanical strength, and thermal properties.5
The distribution of the two principal reservoirs of deep subsurface water on Mars—ground ice and groundwater—is thought to be determined by the thermal structure of the crust. Current mean annual surface temperatures on Mars range from ~154 kelvin (K) at the poles to ~218 K at the equator, with the geothermal heating, produced by the decay of natural radioactive materials in the crust, expected to result in increasingly warmer temperatures at depth. Consideration of the current best estimates of the planet’s mean geothermal heat flux, about 15 to 45 milliwatts per square meter (mW/m2) and the plausible range of freezing temperature (~252 to 273 K) of any saline groundwater that may be present at depth, suggests that the thickness of frozen ground (cryosphere) on Mars should vary from ~2.5 to 5 km at the equator to ~6.5 to 13 km at the poles (Fanale, 1976; Rossbacher and Judson, 1981; Kuzmin, 1983; Clifford, 1993). (See Figure 4.1.) However, natural variations in crustal heat flow, thermal conductivity, and the presence of potent freezing-point-depressing salts are likely to result in significant local differences in the thickness of frozen ground, when compared with estimates based on the assumption of latitudinally averaged values (Clifford and Parker, 2001; Travis et al., 2003).
At equatorial and midlatitudes on Mars, the low relative humidity of the atmosphere will tend to cause near-surface ground ice to sublime at a rate dependent on the local surface temperature and diffusive properties of the crust (Smoluchowski, 1968; Clifford and Hillel, 1983; Fanale et al., 1986; Mellon and Jakosky, 1993). Depending on the nature of these properties, their variation with depth, and the potential for replenishment from any deeper reservoir of subpermafrost groundwater, the result may be the progressive loss of ice by sublimation from the local regolith to depths that range from centimeters to as much as a kilometer (Smoluchowski, 1968; Clifford, 1993, 1998; Mellon and Jakosky, 1995).
If the martian inventory of water exceeds what can be stored as ice within the pore volume of the cryosphere, then the bulk of the excess will be present as a liquid, saturating the lowermost porous regions of the crust—which, as illustrated in Figure 4.1, may reside at depths that range from about 3 to as much as 13 km below the martian surface, depending on the local surface temperature and heat flow (Clifford, 1993; Carr, 1996). Given a large-scale crustal permeability comparable to that of Earth, and the lack of any recent rainfall, the influence of gravity should
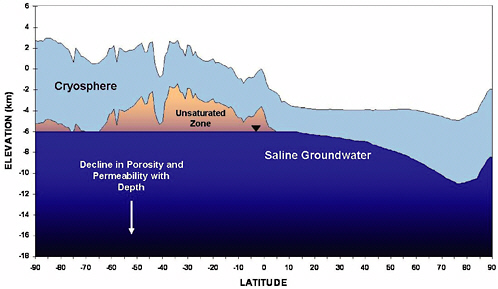
FIGURE 4.1 Hypothetical pole-to-pole cross section of the present day martian crust along 157° longitude, illustrating the potential relationship between surface topography, ground ice, and groundwater. The depicted thickness of frozen ground (the cryosphere) assumes the present latitudinal range of mean annual surface temperatures (~154 to 218 K), a column-average thermal conductivity of 2 W m–1 K–1, and a global mean geothermal heat flux of 30 mW m–2 (modified from Clifford and Parker, 2001).
result in a present-day groundwater system that is in effective equilibrium (i.e., characterized by a relatively flat water table)—except where it may be locally perturbed by tectonic, seismic, or thermal processes. Because of the low porosity expected at depth,6 comparatively little water is required to produce a groundwater system of substantial extent. Therefore, if a subpermafrost groundwater system is present on Mars, it may underlie much of the planet’s surface—although the extent to which it may be interconnected is unknown (Clifford, 1993; Carr, 1996).
The distribution of ground ice is expected to follow the thermal structure of the crust, whereas the distribution of groundwater, under the influence of gravity, will drain and saturate the lowermost porous regions present at depth. For this reason, the vertical distance separating these subsurface reservoirs may vary considerably, such that the intervening unsaturated zone is maximized in regions of high elevation and minimized, or absent, at lower elevations (see Figure 4.1). Within the unsaturated zone, water vapor will tend to diffuse from the higher-temperature (higher-vapor-pressure) depths to the colder (lower-vapor-pressure) region just below the base of the cryosphere. As this moisture-laden air rises and cools, some of the vapor will condense, creating a low-temperature hydrothermal convection system of rising vapor and descending liquid condensate. Such a system may have resulted in complicated variations in saturation state between the base of the cryosphere and the regional ground-
water table and may also have contributed to the development of the underlying groundwater into a highly mineralized brine (Clifford, 1993; Carr, 1996).
Although the outflow channels provide persuasive evidence that Mars once possessed a sizable inventory of subpermafrost groundwater, it is possible that today such a reservoir no longer survives—a potential consequence of the cold-trapping of a once-large inventory into the pore volume of the thickening cryosphere, as the planet’s internal heat flow declined with time (Clifford and Parker, 2001). Thus, the present state of deep subsurface water on Mars is bracketed by two extremes: one in which a small planetary inventory, combined with the progressive cooling of the crust, has eliminated any persistent reservoir of groundwater, and another in which the planetary inventory is sufficiently large that a sizable reservoir of liquid may still survive at depth over much of the planet.
Near-Surface Water
Near-surface water on Mars is defined here as any water—whether present as ice, liquid (including brine), absorbed on mineral surfaces, or as vapor—that exists within the several hundred meters of the planet’s surface, whether above, below, or on. Scientists further define two specific zones within the shallow subsurface where water may potentially be exchanged with the atmosphere in response to variations in atmospheric water vapor concentration and surface temperature: (1) the diurnally active layer, which extends down to a depth of ~20 cm, and (2) the seasonally active layer, which includes the top ~2 to 3 m of the regolith. These zones represent the approximate depths at which the amplitudes of the diurnal and seasonal temperature waves decay to zero. It is difficult for atmospheric water vapor to diffuse below the depth of the seasonally active layer because it would be acting against the geothermal temperature gradient, which causes water to diffuse from the warmer (higher-vaporpressure) depths to the colder (lower-vapor-pressure), shallower regions of the crust.
As defined above, near-surface water may be found in the atmosphere, on the surface, and below the surface. The distribution and state of water in the near-surface region of Mars today are determined by a range of properties and processes that to a large extent are analogous to those that operate in cold dry regions on Earth. Cold soils that are in good diffusive contact with the atmosphere7 act as local cold traps for water vapor and can accumulate surface and subsurface ice. As this ice warms, it acts as a local source of water vapor, and it can also melt to form transient liquid water if local atmospheric and temperature conditions permit. The overall behavior of the martian global water cycle is determined by the combined, time-varying influences of local sources and sinks, and the circulation and transport of water by the martian atmosphere (Jakosky et al., 1997; Richardson and Wilson, 2002).
As on Earth, the distribution and behavior of near-surface water on Mars are likely to exhibit substantial spatial and temporal variability. This is especially true in the subsurface, where local differences in the thermal, diffusive, and physical properties of the soil can exert a substantial influence on the exchange and retention of H2O (Clifford and Hillel, 1983; Fanale et al., 1986; Mellon and Jakosky, 1993; Jakosky et al., 1995). Indeed, given geologically reasonable variations in the actual values and spatial distribution of soil properties, it is possible for near-surface liquid water and ice to become diffusively isolated from the atmosphere, even at depths as shallow as several centimeters. Examples of low-permeability materials capable of producing this effect include sulfate-cemented regolith, a layer of very fine clay, or rock. In such instances, liquid water and ice could survive in disequilibrium with the water vapor content of the atmosphere for billions of years (e.g., see Smoluchoski, 1968). Unfortunately, for the case of subsurface water on Mars, researchers are lacking many of the key observables necessary to understand and quantify fully its distribution and behavior.
The next four subsections outline current understanding of some of the key known near-surface water reservoirs. Possibilities for liquid water in the near-surface environment are then discussed.
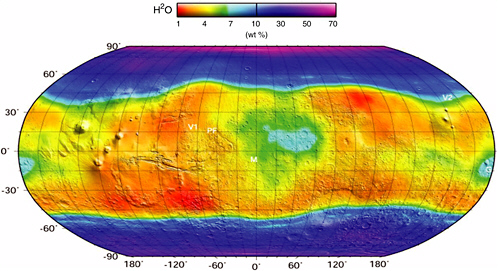
FIGURE 4.2 Global distribution and abundance of near-surface (top meter) hydrogen on Mars as inferred from the Mars Odyssey gamma ray spectrometer (GRS) data. Also shown on the map are the Viking 1 and 2 (V1 and V2), Pathfinder (PF), and Mars Exploration Rover Meridiani (M) and Gusev (G) landing sites. SOURCE: Courtesy of the GRS instrument team, Lunar and Planetary Institute, University of Arizona.
Near-Surface Ground Ice
Near-surface ground ice represents the most significant and most widely distributed near-surface martian water reservoir. Because thermal conduction from the planet’s interior results in generally increasing temperatures with depth, the near-surface regions of Mars are actually the coldest places on the planet; therefore, they should be effective long-term cold traps for martian water (Clifford, 1991). The expected distribution of near-surface ground ice has been predicted by various models over the past 40 years (e.g., Leighton and Murray, 1966; Fanale, 1976; Fanale et al., 1986; Mellon and Jakosky, 1993). Those models show generally good agreement with global near-surface hydrogen abundance measurements made by the Mars Odyssey gamma ray spectrometer (GRS) instrument (see Figure 4.2) (Boynton et al., 2002).
The GRS high-latitude data are usually interpreted in the context of a simple two-layer model, where an ice-rich (>60 percent by volume) layer is assumed to underlie a desiccated layer of variable thickness. If taken literally, this model implies that the depth to ground ice varies from ~13 cm near the poles to ~50 cm at 40 to 50° latitude (Boynton et al., 2002). However, with a surface resolution of ~1 × 105 km2, the hydrogen abundances determined from the GRS data are averaged over areas that are many orders of magnitude larger than the scale of variability observed in the physical and thermal properties of the planet’s surface (Malin and Edgett, 2001; Christensen et al., 2003)—properties that may have a significant influence on the local distribution of near-surface ground ice. Thus, what appears as a uniform distribution of ice at a resolution of 105 km2 may exhibit considerable variability at a scale of 1 km2, such that regions possessing very high concentrations of near-surface ice may be interspersed with smaller regions that are ice-poor.
In the equatorial region below 40° latitude, the GRS data indicate that Mars is not completely dry. In this latitude range, GRS measures bulk soil water contents ranging from 2 to 8 percent. Mean equatorial annual surface
temperatures are high enough, and the concentration of atmospheric water vapor low enough, that near-surface ground ice is unstable if it is in diffusive equilibrium with the atmosphere. However, here too, small-scale variations in physical and thermal properties may contribute to substantial differences in the extent and magnitude of local desiccation. Thus, the lower abundance of hydrogen observed at mid- to equatorial latitudes by the GRS instrument can be interpreted in several ways: (1) it may reflect a region that is uniformly devoid of near-surface ice (at least, within the top meter) but that exhibits adsorbed water and hydrated minerals, as observed at the Mars Exploration Rover (MER) landing sites (Squyres et al., 2004a,b), by the Mars Express Orbiter OMEGA instrument (Bibring et al., 2005), and by the Viking Lander 1 GCMS instrument (Biemann et al., 1977)—the abundance of which could vary over large scales; (2) it may consist of broad areas where the top meter of the regolith is ice-free, but where the diffusion-limiting properties of the local soil have permitted shallow ice to survive over smaller regions (and, perhaps, more widely at depths >1 meter); or (3) it may be attributable to a combination of both explanations.
The Martian North and South Residual Polar Caps
The martian north and south residual polar caps also represent significant near-surface water reservoirs. Together, they have an exposed surface area roughly the size of the Greenland Ice cap and have been estimated to be ~3 to 5 km thick (Smith et al., 1999; Johnson et al., 2000). They contain exposed scarps that display continuous fine-scale layering that may be evidence for astronomically forced climate variability (see Figure 4.3) (Laskar and Robutel, 2004), as well as a variety of other geomorphic features suggesting recent glacial and/or aeolian activity (Thomas et al., 2000; Fishbaugh and Head, 2001).
Seasonal Surface Water Deposits
Seasonal surface water deposits occur when water ice is condensed directly on the surface in the form of frost, or deposited onto the surface in the form of snow. There is evidence for the existence of seasonal water deposits (Kahn, 1990), particularly at high latitudes where water is cold-trapped onto the seasonal solid carbon-dioxide deposits that constitute the martian seasonal polar caps (Bibring et al., 2005). The depths of seasonal water ice deposits on Mars under present climatic conditions are estimated to be on the order of tens of microns (Mischna et al., 2003). Figure 4.4 depicts a winter panorama at the Viking 2 landing site showing the accumulation of surface condensation.
Atmospheric Water
Atmospheric water is a minor constituent of the atmospheres of Mars and Earth, but it plays important roles in the climate systems of both planets. The water-vapor-holding capacity of the martian atmosphere is limited by the saturation vapor pressure, which at low martian temperatures is less than 1,000 times lower than typical values on Earth. Water on Mars achieves mobility in the near-surface region through atmospheric transport in the vapor phase and by the precipitation of condensed phases. Figure 4.5 shows measurements of seasonal variations in atmospheric water vapor and water ice clouds from the Mars Global Surveyor (MGS) orbiter thermal emission spectrometer (TES) instrument (Smith et al., 2001). Atmospheric water shows strong seasonal variations associated with the holding capacity of the martian atmosphere and the condensation and sublimation of water from near-surface reservoirs such as ground ice, adsorbed water, and surface ice.
Liquid Water Stability
The properties and processes that determine the stability of liquid water in the martian near-surface environment are analogous to those on Earth. Figure 4.6 shows a phase diagram for pure water. At total pressures above the triple point of 6.1 mbar, pure liquid water can exist on the surface of Mars at temperatures above the freezing temperature of ~273 K and below the boiling temperature of ~283 K, where its saturated vapor pressure exceeds
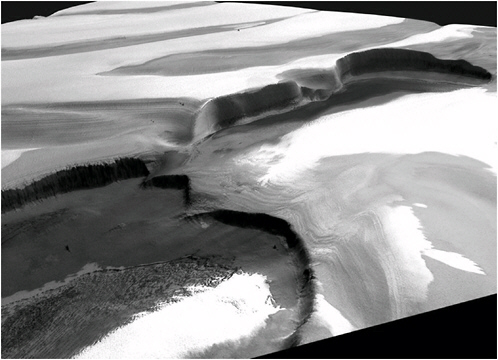
FIGURE 4.3 Mars Express high-resolution stereo camera three-dimensional image of the martian north polar residual cap showing surface ice, layered deposits, scarps, and sand dunes. SOURCE: Courtesy of European Space Agency/Deutsches Zentrum für Luft- und Raumfahrt e.V./Freie Universität Berlin-G. Neukum.
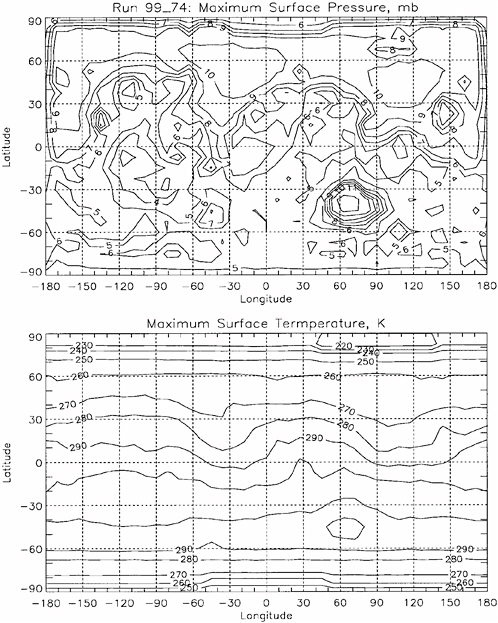
the total atmospheric pressure. Because of the low partial pressure of water vapor in the martian atmosphere, liquid water in diffusive contact with the atmosphere will evaporate rapidly, and so the stability of liquid water at the surface today is at best transient. The relatively narrow temperature range at which pure water is transiently stable at the martian surface is controlled by the atmospheric surface pressure, which exhibits both geographic and seasonal variations (Haberle et al., 2001). Figure 4.7 shows general circulation model (GCM)8 calculations of current martian annual maximum surface pressures and annual maximum surface pressures. Figure 4.8 shows GCM calculations of the locations and length of time in a Mars year in which martian surface temperatures are estimated to be above the triple point and below the boiling point (Haberle et al., 2001).
The results shown in Figures 4.6, 4.7, and 4.8 assess the potential stability of liquid water to freezing and boiling, but they do not address the issue of evaporation and stability in detail. In general, water is stable to evaporation only when the local partial pressure of atmospheric water vapor equals the vapor pressure of the liquid. That situation rarely occurs on the surface of Earth for extended periods, so consistent with common

FIGURE 4.4 Winter scene at the Viking 2 landing site at Utopia Planitia, +47.96°N latitude. SOURCE: Courtesy of NASA.
experience, liquid water tends to evaporate unless it is actively replenished. Similarly, transient liquid water is also possible on the surface of Mars, although as is discussed, there is no direct evidence for its existence, much less its distribution.
While pure liquid water freezes at temperatures below 273 K, there are two mechanisms that could depress both the freezing temperature and the corresponding minimum pressure required for liquid water stability. The first is the well-documented presence of thin films of liquid water on soil and ice grain boundaries that can persist down to temperatures as low as ~20 K below the freezing point (Anderson and Tice, 1973; see also Figure 4.9). It has been suggested that such water films could extend the geographic range of survivability for martian biota (Jakosky et al., 2003).
A second mechanism for depressing the freezing point is the presence of dissolved solutes (Brass, 1980; Clark and Van Hart, 1981; Knauth and Burt, 2002). Observations at the MER landing sites (Squyres et al., 2004a,b) and by the Mars Express Orbiter OMEGA spectrometer (Bibring et al., 2005) suggest the widespread distribution of sulfates and other evaporite minerals on the martian surface. The sulfate content of rocks and soils at the MER Meridiani landing sites ranges from 5 to 25 percent by weight (Rieder et al., 2004). Models predict eutectic9
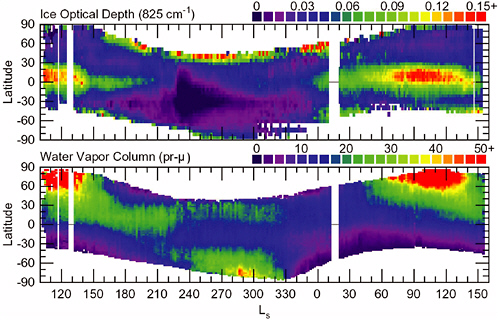
FIGURE 4.5 Mars Global Surveyor thermal emission spectrometer observations of atmospheric water ice cloud optical depth (top) and column water vapor (bottom) as a function of martian season (Ls). SOURCE: Smith et al. (2001). Copyright 2001 American Geophysical Union. Reproduced by permission of American Geophysical Union.
freezing point temperatures for plausible martian brines of 225 K or lower (see Figure 4.10) (Brass, 1980; Knauth and Burt, 2002; Madden and Bodnar, 2002). If brines with high salt concentrations are present in the near-surface environment, then there is the potential for near-surface liquid water almost everywhere on the planet.
In general, the available information paints a highly uncertain picture regarding the present existence of liquid water on Mars. From a thermodynamic standpoint, pure liquid water can exist only in restricted geographic locations during restricted time periods. However, such water would evaporate rapidly into the dry martian atmosphere, and it would have to be replenished in order to be present over many diurnal or annual cycles. Liquid water in the near-subsurface faces similar challenges, but the requirements for resupply could be significantly reduced by the presence of diffusive barriers such as fine-grained soil, duricrust, ice, or rocks. The recent verification of the presence of high concentrations of water-soluble minerals on Mars greatly increases the prospects for liquid water environments enabled by freezing-point depression. Researchers also know that, at least to the spatial resolution of the GRS instrument, water molecules are globally distributed throughout the martian near-surface environment, at spatially averaged concentrations that exceed 1 percent. Taken together, these facts do not add up to a convincing case for or against the presence of liquid water. Instead, they underscore the need for additional measurements and analysis. Very cold yet liquid water environments could potentially currently exist at many locations in the martian near-surface.
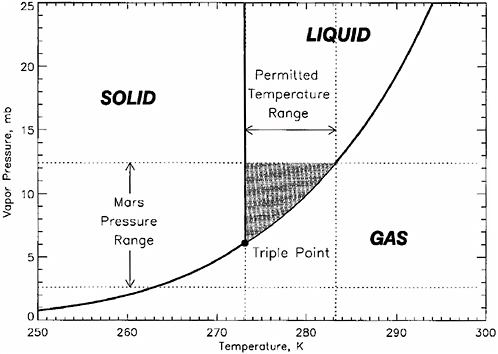
FIGURE 4.6 Phase diagram for pure water. The shaded region shows the conditions under which pure liquid water on the surface of Mars today is transiently stable. SOURCE: Haberle et al. (2001). Copyright 2001 American Geophysical Union. Reproduced by permission of American Geophysical Union.
Potential Influence of Obliquity-Induced Climate Change
The preceding discussion assumes global environmental conditions characteristic of the present martian climate. However, the presence of both large- and fine-scale layering in the martian polar stratigraphy suggests that the climate has undergone quasiperiodic change in response to a variety of astronomical variables operating on many different time scales. Of these, the martian obliquity exerts the greatest influence. The obliquity oscillates about its current mean value (i = ~25°) with a period of 1.2 × 105 years. The amplitude of this oscillation also varies and is modulated with a period of 1.3 × 106 years (Ward, 1992). At low obliquity, both seasonal temperature fluctuations and mean annual polar temperatures are at a minimum. This situation is reversed at times of high obliquity, when summers of continuous illumination alternate with dark winters to produce both extreme seasonal variations and higher mean annual temperatures at the poles.
Recent studies have demonstrated that the evolution of the martian obliquity is chaotic, varying from ~0o to 60° (Touma and Wisdom, 1993; Laskar and Robutel, 1993; Laskar et al., 2004). This behavior places an upper limit of several million years on how far back (or forward) the present obliquity can be reliably extrapolated. At obliquities above ~54° the mean annual insolation at the poles actually exceeds that at the equator (Ward, 1992). However, even at lower obliquities, maximum daytime temperatures at the poles can still exceed 273 K throughout much of the spring and summer (Toon et al., 1980; Pathare and Paige, 1998). Under these conditions, high-latitude
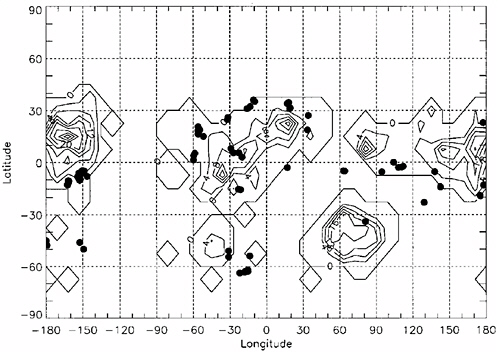
FIGURE 4.8 General circulation model calculations of the locations and length of time (in sols or martian days) during a Mars year at which martian surface temperatures are estimated to be above the triple point and below the boiling point (Haberle et al., 2001). Also shown (black dots) are the locations of possible Amazonian paleolakes from Cabrol and Grin (2001). SOURCE: Modified from Haberle et al. (2001). Copyright 2001 American Geophysical Union. Reproduced by permission of American Geophysical Union.
sublimation rates for exposed ice may reach ~0.1 m/yr averaged over a single obliquity cycle (Jakosky et al., 1995). There could also be widespread melting of the polar ice, as well as high-latitude snow packs and near-surface ground ice (Pathare and Paige, 1998; Costard et al., 2002; Christensen, 2003), creating episodic liquid water environments, lasting for days or many months, that would be repeated on an annual basis for as long as the high-obliquity phase of the cycle persisted (up to ~104 years). Theoretical studies suggest that such conditions might recur on 106- to 107-year timescales.
A CATALOG OF POTENTIALLY SPECIAL REGIONS
As mentioned above, COSPAR currently defines a special region on Mars as “a region within which terrestrial organisms are likely to propagate, or a region which is interpreted to have a high potential for the existence of extant martian life forms. Given current understanding, this applies to regions where liquid water is present or may occur.” (See Table 2.1.) The following is a catalog of potentially accessible special regions on Mars:
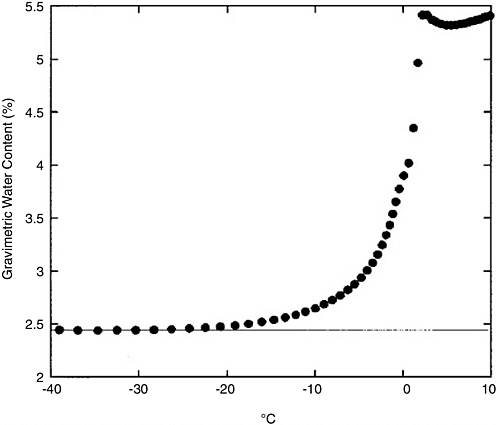
FIGURE 4.9 Liquid water content measurements in loam soil as a function of temperature. The minimum water content represents unfrozen adsorbed water (Jakosky et al., 2003). NOTE: “Loam soil” is a porous soil composed of sand, silt, clay, and organic matter.
-
Near-surface liquid water. During the summer seasons at noon, temperature and pressure conditions at the surface in low-altitude equatorial and southern latitudes may permit the transient occurrence of pure liquid water at the surface (see Figure 4.8). Over much of the rest of the planet, surface water films and liquid brines may potentially exist during the warmer parts of the day. Because the vapor pressure of condensed phase water increases with temperature, there will be a strong tendency for warm liquid water to evaporate and ultimately recondense as ice in colder areas. Therefore, the repeatable formation of ephemeral liquid water on Mars will generally require a source of water ice that can be replenished on diurnal, seasonal, and climatic timescales (Hecht, 2002). Environments that are isolated from diffusive contact with the atmosphere, such as the interiors of rocks or regolith overlain by duricrust, can form liquid water whenever melting temperatures are reached. Even with liquid water periodically present, on a mean annual basis, these will be cold environments.
-
Geothermal hot spots. Although regions of current active volcanism or enhanced heat flow have not yet been identified, if they exist such regions and their immediate surroundings may well be special. By analogy to Earth, martian hot spots could provide abundant utilizable energy and liquid water.
-
Segregated ground ice. Geologic evidence for extensive and repeated flooding by outflow channel activity (Baker, 1982; Carr, 1996) suggests that massive ice deposits, preserved at comparatively shallow depth beneath a protective cover of lava flows and aeolian sediment, may now reside beneath the northern plains (Carr, 1990; Clifford and Parker, 2001; Murray et al., 2005). Although the case for such deposits remains controversial, it is conceivable that the volatile stratigraphy of the northern plains is quite complex, having potentially been built up through multiple episodes of flooding, freezing, sublimation, and burial. The presence of embedded solutes or
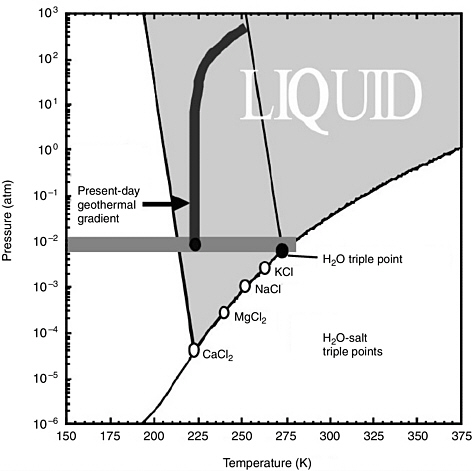
FIGURE 4.10 Calculated eutectic triple points for four molal water-salt compositions (Madden and Bodnar, 2002). SOURCE: Courtesy of R.J. Bodnar.
-
pockets of brine, the purposeful or accidental melting of massive ice as a consequence of exploration activities, or (on 105-year timescales) high obliquities could result in the production of substantially more liquid water where segregated ground ice occurs than would be produced for dispersed ice in soil under equivalent environmental conditions.
-
Ice-rich frozen ground. The GRS data shows that near-surface soil in high-latitude regions is nearly saturated with water ice. If some of this ice has melted in the recent past, then it likely contains high concentrations of solutes derived from adjacent martian rocks and soil, which could significantly depress melting points. Current observations cannot rule out the diurnal or seasonal melting of frozen brines in the near-surface environment.
-
Polar caps and surface ice. Dark regions on the north and south residual polar caps achieve seasonal maximum temperatures that could permit the presence of ephemeral water in the form of surface films and brines. Recent Mars Express OMEGA observations have revealed the presence of sulfate minerals in the dark polar sand sea adjacent to the north residual cap that indicate the past presence of at least ephemeral liquid water (Langevin et al., 2005). Theoretical models have shown that surface ice deposits containing small quantities of dust may support melting under certain circumstances (Clow, 1987). The melting of recent surface ice at midlatitudes is one of several explanations put forward to explain the origin of gullies (Christensen, 2003).
-
Subpermafrost groundwater. If the martian inventory of water exceeds what can be stored as ice within the pore volume of the cryosphere, then the bulk of the excess will be present as a liquid, saturating the lowermost
-
porous regions of the crust. The zonally averaged depth to the bottom of the cryosphere is estimated to be ~3 to 13 km, varying from its lowest to highest values with increasing latitude. These estimated depths are not well constrained by observations and are likely to exhibit significant variations due to localized differences in crustal thermal conductivity and heat flow. In areas of exceptionally high geothermal heat flow, or where potent freezing-point-depressing salts are abundant in the crust, near-surface environments may exist with possible hydrologic connections to a deeper and more widespread reservoir of subpermafrost groundwater.
-
Gullies. Mars exhibits widespread evidence for gully landforms on crater walls and other steep slopes that have been interpreted as geomorphic evidence for recent seepage and runoff of liquid water (Malin and Edgett, 2000). The timescales over which martian gully features have been hydrogically active is currently not well constrained, but it has been suggested that large-scale obliquity variations may have resulted in sufficient solar heating to cause localized melting of ground ice on million-year timescales (Costard et al., 2002). Although the mechanisms for gully formation on Mars are controversial (see Appendix F), gullies are likely to be regions where liquid water has a higher probability of occurring. Therefore, gullies and other landforms that suggest the presence of recent liquid water hold special significance for preventing forward contamination.
Taken together, the foregoing catalog of potentially special regions on Mars covers the entire surface of the planet. No specific region or range of latitudes, including the equatorial region, can currently be excluded, given available observations and current understanding. While certain regions on Mars may have a lower probability of being special than others, sufficient uncertainty remains, especially at smaller spatial scales, to warrant caution. A greater understanding of the distribution and state of subsurface (especially near-subsurface) water on Mars is needed. How this dilemma could be mitigated by upcoming exploration is discussed below.
TECHNIQUES FOR ASSESSING THE DISTRIBUTION AND STATE OF SUBSURFACE WATER ON MARS
Substantial uncertainties remain regarding the amount and distribution of subsurface water on Mars, yet these factors are of great importance for evaluating the potential for forward contamination of the martian environment by microbes carried on spacecraft. This section considers techniques currently available or soon to be available for providing an improved assessment of these factors.
Theoretical Modeling
The earliest efforts to model the stability and distribution of ground ice on Mars made many simplifying assumptions in order to focus on large-scale average effects.10 More recent investigations (Paige, 1992; Mellon and Jakosky, 1993, 1995) have examined how local variations in the radiative and thermophysical properties of the surface could lead to significant geographic differences in ground ice stability. These investigations suggest that in some regions, under favorable conditions, fossil ground ice might survive at shallow depth even at the equator.
The acquisition of high-spatial-resolution thermal data alone is not sufficient to allow the prediction of the present local distribution of ground ice, because the thermal properties of the regolith cannot be extrapolated with any confidence beyond the depth that experiences diurnal temperature variations (essentially the top 10 to 25 cm). In fact, knowledge of many fundamental characteristics of the martian near-surface is poor, especially with respect to how the thermal and diffusive properties of the crust vary both geographically and at depth. Such variations play a determining role in the evolution and vertical distribution of ground ice.
Drill cores on Earth, lunar soil profiles, and high-resolution images of the walls of the canyon Valles Marineris on Mars reveal visible stratgraphic variations that demonstrate that the structure, lithology, and thermophysical properties of a planetary crust can vary dramatically on size scales that range from millimeters to kilometers. Given the sheer number of properties that may vary, the potential amplitude of these variations, and the unknown number and thickness of possible layers, the transport properties of the crust can potentially change by many orders of magnitude over a depth interval as small as a few millimeters. Thus, plausible permutations of crustal diffusive and thermal properties can result in intricate combinations of low- and high-permeability strata that yield substantial differences in the local distribution of ground ice.
Understanding of the subsurface distribution of ice is further complicated by the lack of knowledge regarding the nature and duration of processes involved in the geologic evolution of the local crust, among the most important of which are geographic and temporal variations in crustal heat flow. Measurements of continental heat flow on Earth (Sclater et al., 1980; Pollack et al., 1993; Stein, 1995) suggest that regional-scale (i.e., ~107 km2) differences of ±50 percent can occur about the crustal mean. However, over smaller areas, the potential range of local thermal conditions on Mars is likely to be far greater. Given the scale of features and geologic diversity observed on the planet (Carr, 1996), as well as the measured heat flow of similar environments on Earth (e.g., Morgan et al., 1977; Sclater et al., 1980; Pollack et al., 1993; Stein, 1995; Dragoni et al., 2002), the thermal structure of the martian crust may vary from nearly isothermal conditions in the thickest regions of ancient highland crust to gradients of as much as 1 to 10 K/m in the vicinity of recent magmatic, volcanic, or hydrothermal activity.
This review suggests that although predictions of subsurface volatile distribution based on theoretical modeling and surface thermal observations can be of value in understanding potential global and regional-scale behavior, their use at the local scale is likely to provide little insight much below the depth that experiences daily temperature variations (~0.2 to 0.5 m), regardless of the precision and resolution to which the present thermal and radiative properties of the surface may be known. This conclusion applies not only to understanding of the distribution of ground ice, but also to virtually every other aspect of the local volatile and physical characteristics of the crust.
Geomorphic Analysis
Another major approach that has been used to infer the distribution of subsurface H2O is geomorphic analysis, in which the size, density, and geographic distribution of landforms, attributed to the presence of subsurface liquid water or ice, have been used to infer local crustal properties. However, as a potential indicator of the present three-dimensional distribution and state of subsurface water, geomorphic analysis has two principal shortcomings: (1) its interpretations are generally not unique, and (2) the information it conveys about the potential nature and distribution of subsurface H2O may be millions to billions of years old. These deficiencies seriously limit the utility of geomorphic indicators as reliable and quantitative guides to the present location of liquid water or ground ice, although they may provide a useful guide for assessing where more definitive investigations should be directed. Appendix F illustrates such difficulties in the context of geomorphic interpretations of martian gullies.
Geophysical Approaches
It is clear that theoretical and geomorphic approaches to assessing the distribution and state of subsurface water on Mars face many challenges. Terrestrial experience suggests that geophysical techniques may be better suited to the task. A number of such techniques are considered here.
Mars Odyssey Gamma-Ray Spectrometer
The first application of a geophysical technique to the search for subsurface water on Mars has been the GRS experiment onboard the Mars Odyssey spacecraft. The data (see Figure 4.2) indicate that, at mid- to high latitudes, the top meter of the soil is hydrogen-rich, suggesting a volumetric water ice content of as much as ~50 to 75 percent (Boynton et al., 2002; Mitrofanov et al., 2003; Feldman et al., 2003). At these latitudes, mean annual
surface temperatures fall below the ~196 K frost point temperature of atmospheric H2O (Farmer and Doms, 1979). As a result, the level of confidence in the association of abundant hydrogen with near-surface ground ice is high.
However, for the lower abundances of hydrogen detected at equatorial and midlatitudes (where mean annual temperatures exceed the frost point), the association with ground ice is less clear. For example, at the Viking Lander 1 site (22.5°N, 48°W), which lies within the equatorial zone of reduced hydrogen abundance, data obtained by the spacecraft’s GCMS instrument suggest that the soil contains ~0.1 to 1 percent by weight chemically bound H2O (Biemann et al., 1977), most likely in the form of adsorbed water and hydrated minerals. Thermodynamically, ice at these latitudes is unstable if it is in diffusive equilibrium with the atmosphere, a condition that is likely to characterize much of the equatorial regolith, at least down to the half-meter depth sensed by the GRS instrument. However, the presence of near-surface ground ice at these latitudes cannot be ruled out. Geographic and stratigraphic variations in regolith thermal and diffusive properties, as well as the potential for subsurface replenishment, can allow ice to survive in disequilibrium with the atmosphere, at shallow depth, for billions of years—even at the warmest locations on the planet (Smoluchowski, 1968; Clifford, 1998).
Although the data acquired by the GRS suite of instruments indicate a marked contrast in the abundance of near-surface hydrogen between low and high latitudes, the magnitude and uniformity of this appearance may be deceiving. With a best integrated instrument surface spatial resolution of the order of ~ 3 × 105 km2, the indicated abundances of hydrogen in Figure 4.2 are averaged over areas that are many orders of magnitude larger than the scale of heterogeneity in physical, thermal, and compositional properties exhibited by the planet’s surface. Thus, at high latitudes, there may be many localized areas where the concentration of near-surface ground ice is actually quite small (i.e., <10 percent), interspersed with larger regions where the volumetric ice content approaches 90 to 100 percent. The same may be true of ground ice at lower latitudes, although there the relative abundance of ice-rich to ice-poor regions could be reversed. The GRS data show low-latitude regions with bulk water abundances that approach maximum values of ~10 percent, but the forms of that water, be it chemically bound, adsorbed, or ice, or spatially varying combinations of all three, have not yet been determined.
For mission planners attempting to locate or avoid ice-rich regions, the dilemma is that, given the currently available data, one cannot assess the characteristic scale and magnitude of local heterogeneity with respect to the near-surface distribution of ground ice. A second problem is that, although the GRS investigations provide a wealth of data on the large-scale distribution of hydrogen within the top meter of the regolith, they provide no insights regarding the presence of water at greater depths. Both higher spatial resolution and probing of greater depths are needed. One approach to obtain these data is through active radar sounding.
Radar Sounding of the Martian Subsurface
On Earth, radar sounding from surface, aerial, and orbital platforms has been used successfully to investigate a variety of terrains and subsurface environments, including hot arid deserts (McCauley et al., 1982, 1986; Daniels et al., 2003), Arctic permafrost (Annan and Davis, 1976; Arcone et al., 1998), glaciers (Arcone et al., 2000; Degenhardt and Giardino, 2003), and polar ice sheets (Robin et al., 1969, 1977; Siegert, 2000). Radar sounding was also successfully conducted from lunar orbit by Apollo 17 (Phillips et al., 1973), although in comparison with terrestrial studies, the interpretation of those data have proved far more challenging, due in large part to the lack of available ground truth (Peeples et al., 1978; Sharpton and Head, 1982).
Terrestrial experience has demonstrated that ground-based geophysical exploration, using a combination of different techniques, is well suited for the search for subsurface water on Mars (Stoker, 1998; Beaty et al., 2000; Clifford et al., 2001). But a strategy to search for subsurface water on Mars by proceeding directly to the use of ground-based instruments has the drawback that such instruments provide no global context. For this reason, the European Space Agency selected the Mars Advanced Radar for Subsurface and Ionospheric Sounding (MARSIS) among the instruments to fly on the Mars Express spacecraft, which successfully entered Mars orbit on December 25, 2003.11 One of the primary goals of the MARSIS investigation is to conduct a preliminary global reconnaissance
|
11 |
For further background on MARSIS and Mars Express, see <www.esa.int/SPECIALS/Mars_Express/>. |
for subsurface water. The results will help target more capable and higher-resolution surface investigations in the future. A technical discussion of the mode of operation of MARSIS is provided in Box 4.1.
The depth achieved by a radar sounder is maximized at low frequencies. For this reason, MARSIS was designed with a low operational frequency range of 0.5 to 5 MHz, with the lower limit imposed by the potential for interference from the ionosphere. At these frequencies, MARSIS will have a vertical subsurface resolution of approximately 100 m and a maximum projected penetration depth (under ideal sounding conditions) of as much as several kilometers. However, propagation losses due to the electrical and magnetic properties of the crust are likely to have a significant impact on the sounding performance of MARSIS (Beaty et al., 2001a; Heggy et al., 2001, 2003; Grimm, 2003). Specifically, the transmission, reflection, and loss experienced by a radar pulse depend on the electromagnetic properties and volumetric contribution of the regolith’s various components, as modified by its porosity and the degree of pore saturation by air, ice, and liquid water or brine (Olhoeft, 1998; Leuschen et al., 2003; Heggy et al., 2003; Grimm, 2003). Transmission is maximized in high-porosity, low-iron-content lithic materials whose pores are saturated with nonconducting air or ice, while the strength of a reflection from the
|
BOX 4.1 MARSIS is a multifrequency, coherent-pulse, synthetic aperture radar sounder whose operation resembles that of a more traditional ground-penetrating radar (GPR). MARSIS operates by emitting a short electromagnetic pulse whose transmitted power is reduced at the planet’s surface by 1/R2, where R is the sounder’s altitude above the ground. When this pulse reaches the boundary between two materials of differing dielectric properties (such as the atmosphere and the ground), a portion of the incident energy is reflected back to the sounder (once again, undergoing a 1/R2 loss)—while the remainder continues to propagate into the subsurface, where it may suffer additional losses due to scatter by embedded objects and absorption by the host material. As successive dielectric interfaces are encountered, the signal experiences additional reflections and losses by absorption (affecting both the transmitted and the reflected portions of the signal). As the reflected signals are received by the orbital sounder, the depth of the corresponding interface can be determined by measuring the time delay between transmission and reception, as long as the returned signal is still detectable above the background noise. Given the high dielectric contrast between liquid water and ice or rock, it has been predicted that MARSIS will be able to detect a liquid water interface over a depth range of 0.3 to 5 km with a signal >3 dB above the noise, assuming a sharp transitional boundary, a saturated porosity >10 percent, and favorable conditions of surface roughness (rms slopes <0.5°, which characterize ~20 percent of the planet). Under the most favorable conditions of surface roughness, target geometry, and rock composition, MARSIS may also be capable of detecting the much smaller dielectric contrast between massive lenses of segregated ice (where the saturated porosity is near 100 percent) and ice-free or ice-saturated frozen ground (Beaty et al., 2001a). The unambiguous identification of crustal H2O may prove difficult or impossible using orbital sounding alone (Beaty et al., 2001a,b). But the geometry and contrast associated with the volatile targets of greatest scientific interest, such as liquid water and massive ground ice (MEPAG, 2004), are expected to be sufficient so that, under favorable observing conditions (and with the additional interpretive context provided by the analysis of other remote sensing data), probable occurrences should be identifiable. In this way, it is hoped that MARSIS will provide a first look at the lithology, structure, and volatile stratigraphy of the martian subsurface.1 |
interface between two materials is aided by a high dielectric contrast and sharp boundary transition. In contrast, the presence of abundant iron (particularly as magnetic phases), liquid water, and especially electrically conductive brines can reduce a radar sounder’s performance by as much as several orders of magnitude, even at low frequencies. Given the range of composition, physical and electromagnetic properties, and local environmental conditions found on Earth (many of which may also be present on Mars), the actual sounding depth achieved by MARSIS is likely to vary enormously from essentially no penetration in some areas to as much as several kilometers in others.
The combination of the low frequencies used by MARSIS and interference arising from off-nadir reflections creates a potential blind zone that may prohibit MARSIS from resolving variations in dielectric contrast at depths shallower than several hundred meters. In 2005, NASA plans to launch the Mars Reconnaissance Orbiter (MRO), which includes the shallow radar (SHARAD) instrument (Seu et al., 2004). As with MARSIS, the primary mission of SHARAD is to search for dielectric evidence of subsurface water and ice, but at shallower depth and higher resolution—a capability derived from SHARAD’s higher (20 MHz) operating frequency and 10-MHz bandwidth. Additional radar instruments either are planned or have been proposed, as part of both surface- and orbiter-based missions to Mars in 2009 and beyond.
MEASUREMENTS NEEDED TO IDENTIFY SPECIAL REGIONS
For mission planners and scientists who wish to identify special regions in order to either avoid or explore them, there are three principal concerns: (1) What types of measurements are necessary to identify special regions? And to what resolution? (2) What instruments are capable of making these measurements? And on what platforms are they most effectively employed? (3) What are the ambiguities associated with this identification? And how can they be reduced?
Identifying the distribution of special regions on Mars requires understanding the present three-dimensional distribution and state of water in the subsurface. As noted above, orbital and surface geophysical investigations appear to offer the best approach for acquiring this knowledge—yet, no single technique, or even combination of techniques, is capable of providing a complete understanding. To make the greatest progress in assessing the distribution and state of subsurface water it is necessary to identify those investigations that maximize knowledge of the location of the most sensitive environments.
The chief immediate concern in locating special regions is to identify where liquid water and massive ground ice are present within the top ~25 m of the regolith, the maximum depth to which currently envisioned investigations by robotic spacecraft will be capable of reaching over the next decade. In addition, because variations in regolith thermal and diffusive properties—capable of preserving liquid water or ice at shallow depth—can occur at small scales, an understanding of the distribution and state of subsurface H2O at as high a resolution as practically possible (ideally, at a scale equivalent to that defined by the operational activities and landing accuracy of the investigating robotic spacecraft) would provide the greatest level of assurance in researchers’ ability to access or avoid special regions.
Terrestrial experience has demonstrated that the accurate identification of subsurface groundwater and ground ice is likely to require the application of multiple geophysical techniques (Stoker, 1988)—investigations that are most effectively conducted on (or in close proximity to) the planet’s surface. But the relative fraction of the martian surface that can be investigated by a single lander, rover, or even aerial platform is small, requiring a prohibitively large number of spacecraft to conduct a global survey. Therefore, local investigations are most effectively employed following the completion of an initial global reconnaissance to identify regions of interest, a survey that is most easily accomplished from orbit.
Orbital Measurements
The electrical and magnetic properties of the crust are particularly diagnostic of the presence of conducting liquid water and brine. However, the much smaller contrast between the dielectric properties of rock and ice makes the unambiguous identification of ice much more difficult, except where it may be present in the form of massive
segregated deposits. Thus, geophysical investigations can provide valuable insights regarding the occurrence of two of the most sensitive special-region environments, those where liquid water or massive ground ice are present, but may be unable to exclude the potential presence of ice-rich frozen ground without the assistance of invasive surface investigations using penetrators or shallow drills.
MARSIS and SHARAD were not optimized to collect data on the near-surface (~1 to 100 m) range of greatest immediate concern in the identification of special regions (see Box 4.1). Because of its low operating frequencies (1 to 5 MHz), MARSIS may have an effective blind zone that extends from the surface down to a depth of ~300 m, a consequence of the instrument’s inability to distinguish between off-nadir reflections due to local surface clutter and reflections from the shallow subsurface. The higher operational frequencies of SHARAD (15 to 25 MHz) could reduce this blind zone to ~50 m (Beaty et al., 2001b), but that would still be a depth exceeding that of greatest concern for the identification of special regions. The actual capabilities of these instruments in the martian environment will soon be known, allowing such expectations to be reassessed in the context of actual data.
The depth of the blind zone should shrink dramatically at higher frequencies, which also significantly improve the horizontal and vertical resolution of the sounder. The principal disadvantage of higher frequencies is a reduction in maximum penetration depth. This penalty can be offset by operating the sounder over a broad range of frequencies that provide high spatial resolution near the surface and coarser resolution at depth. For depths of 0 to 25 m, a frequency range of 100 MHz to 1 GHz appears ideal, particularly if such a sounder were complemented with a synthetic aperture imaging radar. Such a combination would be expected to detect any liquid water or brine present in the top 25 m of the subsurface, provided its volumetric fraction was more than a few percent, and would also offer a significant enhancement in the ability to identify deposits of massive ice in the near-subsurface (such as a fossil snowpack or the ponded frozen discharge of the gullies, outflow channels, or a relic of a former sea or ocean).
As described earlier, gamma ray and neutron spectrometry from orbit can provide strong quantitative constraints on the distribution of hydrogen in the near-surface environment. The chief limitation of this technique as it has been applied to date, is its low spatial resolution. This limitation could be improved in future experiments by using higher-resolution collimated sensors as with the Lunar Exploration Neutron Detector (LEND) experiment to fly on the 2008 Lunar Reconnaissance Orbiter, or by measurements from lower-altitude airborne platforms.
Surface Measurements
Global orbiter measurements intended to identify special regions with good spatial resolution will require ground truth measurements of the electromagnetic, physical, and compositional properties of the top meter of the regolith at multiple locations around the planet. The need to obtain ground truth at multiple sites is driven by both the natural heterogeneity of the surface and the various factors that may contribute to the origin of subsurface reflections and signal loss. The acquisition of such data would significantly reduce the level of ambiguity associated with the interpretation of orbital radar data—especially if combined with ground-based geophysical investigations at these same locations. Possible candidates include the addition of magnetotelluric and permitivity instruments, active and passive seismometers, and ground-penetrating radar (GPR). Such an approach would greatly improve the ability to discriminate between lithologic and volatile units.
For platforms such as landers, rovers, and penetrators that can directly access the near-surface region, a variety of measurements can be deployed that could shed light on the existence of and/or potential for special environments. Examples include measurements of physical parameters such as temperature, pressure, soil thermal and adsorptive properties, electromagnetic properties, and gas and liquid permeability; measurements of gas composition, with particular emphasis on the partial pressure of atmospheric water vapor; measurements of the soil and rock chemistry, including the abundances of potential solutes in brines, and parameters such as pH and eH; and direct time-dependent measurements of the abundance of water in its various forms. It should be noted that most of the above measurements have yet to be successfully acquired at any location on Mars.
SPACECRAFT ACCESS AND SPECIAL REGIONS
In the wake of a best-effort orbital reconnaissance for special regions, there will always be some uncertainty regarding the designation of any region as nonspecial—either because of the presence of dispersed ice or because of the concentration of liquid water or massive ice at spatial scales that are much smaller than can be surveyed from orbit. The characterization of special regions should evolve with improved technological capabilities for exploring the near-surface and subsurface of Mars. Further, the degree to which robotic spacecraft could accidentally or intentionally access the subsurface (e.g., as a consequence of mission failure that results in a spacecraft’s high-velocity impact into the surface) or by purposeful intent (e.g., by trenching with a robotic arm, drilling into the subsurface, or by the emplacement of a penetrator, or, in the case of ice, by melting down with a cryobot) affects the depth and resolution of understanding about the subsurface regolith that may be required to prevent contamination and could eventually necessitate an understanding of depths greater than 25 m. For example, if deep-drilling investigations of the crust were anticipated, then a knowledge of the distribution of liquid water and massive ground ice at even greater depth (e.g., ~100 to 1000 m) could be desirable. In Chapter 8, the committee presents its conclusions regarding planetary protection requirements that should be followed by spacecraft exploring regions that cannot confidently be defined as nonspecial.
Between 2005 and 2020, it appears unlikely that such activities will access depths much greater than ~25 m. However, efforts are already under way to identify and develop techniques capable of accessing depths ranging from many tens to thousands of meters beneath the surface (Blacic et al., 2000) to conduct astrobiological research.
SUMMARY
By the end of the Viking mission, Mars was broadly viewed as a very inhospitable world (e.g., Horowitz, 1986). The data available then suggested that the planet had little surface water, and the low atmospheric pressure, surface temperature, and high UV flux appeared extremely hostile to even the hardiest terrestrial organisms known. Knowledge of Mars has since progressed enormously, as has knowledge of the limits of terrestrial life (see Chapter 5). Although the surface of Mars continues to appear inhospitable to Earth life (e.g., Schuerger et al., 2003), the inventory of martian water in all its forms seems substantial.
The martian surface environment is salt-rich, and plausible combinations of salts could result in the transient presence of cold liquid water environments in the near-surface regolith on a diurnal and seasonal basis over much of the planet. Indeed, at low latitudes, it is possible that brines may persist continuously at all depths within the crust. Even in the absence of salts, summertime temperatures at low latitudes can still exceed the freezing point for up to several hours a day within the top 1 to 2 cm. More persistent occurrences of liquid water may be found in association with localized volcanic and magmatic activity and at depths of several kilometers or more, where geothermal heating may elevate crustal temperatures above the freezing point. It is not known whether geothermal hot spots may exist in the near-subsurface at some locations. Water in the form of ice is more widely distributed, appearing to be the dominant constituent of the polar-layered deposits and the near-surface regolith at mid- to high latitudes. Near-surface ice may also be present in smaller amounts at equatorial latitudes and is likely present globally at depth.
Currently, there are no data to distinguish between areas that are water-rich versus water-poor at spatial resolution better than ~3 × 105 km2. Yet, recent observations show that Mars exhibits significant horizontal and spatial diversity on spatial scales of kilometers to centimeters. This fact makes it difficult currently to designate with confidence any specific region of the planet as special or nonspecial. Coming to grips with the many issues relating to Mars’s current habitability will require a continued broad and sustained program of interdisciplinary scientific exploration of Mars, and also measurements specifically targeted to locating special regions, especially to identify where liquid water and massive ground ice are present within the near-surface. In addition, because variation in regolith thermal and diffusive properties—capable of preserving liquid water or ice at shallow depth—can occur at small scales, an understanding of the distribution and state of subsurface H2O at a resolution and at a scale equivalent to that defined by spacecraft operational activities and landing accuracy would improve confidence in the ability to access or avoid special regions.
REFERENCES
Anderson, D.M., and A.R. Tice. 1973. The unfrozen interfacial phase in frozen soil water systems. Pp. 107-124 in Ecological Studies. Analysis and Synthesis, Vol. 4, A. Hadas, D. Swartzendruber, P.E. Rijtema, M. Fuchs, and B. Yaron, eds. Springer-Verlag, New York.
Annan, A.P., and J.L. Davis. 1976. Impulse radar sounding into permafrost. Radio Sci. 11: 383-394.
Arcone, S.A., D.E. Lawson, A.J. Delaney, J.C. Strasser, and J.D. Strasser. 1998. Ground-penetrating radar reflection profiling of groundwater and bedrock in an area of discontinuous permafrost. Geophys. 63: 1573-1584.
Arcone, S.A., D.E. Lawson, M. Moran, and A.J. Delaney. 2000. 12-100-MHz profiles of ice depth and stratigraphy of three temperate glaciers. P. 377 in GPR 2000 Conference Proceedings, Eighth International Conference on Ground-Penetrating Radar, Gold Coast,
Australia, May 23-26. Available from GPR 2000 Conference Secretariat, Department of Computer Science and Electrical Engineering, University of Queensland, Australia.
Baker, V.R. 1982. The Channels of Mars. University of Texas Press, Austin, Tex.
Baker, V.R., R.G. Strom, V.C. Gulick, J.S. Kargel, G. Komatsu, and V.S. Kale. 1991. Ancient oceans, ice sheets and the hydrologic cycle on Mars. Nature 352: 589-594.
Beaty, D.W., G. Briggs, and S.M. Clifford. 2000. Strategic planning for exploration of the martian subsurface. Workshop on Concepts and Approaches for Mars Exploration. Abstract No. 6233. Available at <www.lpi.usra.edu/meetings/robomars/pdf/6233.pdf>.
Beaty, D.W., A. Coradini, S. Clifford, J. Grant, P. Gogineni, J. Plaut, K. Raney, and A. Safaeinili. 2001a. Analysis of the potential of a Mars orbital ground-penetrating radar instrument in 2005. Mars Program Office White Paper. Available at <www.lpi.usra.edu/meetings/geomars2001/radar.pdf>.
Beaty, D.W., S.M. Clifford, P. Gogineni, R. Grimm, C. Leuschen, G.R. Olhoeft, K. Raney, and A. Safaeinili. 2001b. Report of the virtual instrument science definition team on: Facility Orbital Radar Sounder Experiment for MRO 2005 (FORSE). Mars Program Office White Paper. Available at <www.lpi.usra.edu/meetings/geomars2001/virtual.pdf>.
Becker, L. 2002. Organic detection. Pp. 164-173 in Signs of Life: A Report Based on the April 2000 Workshop on Life Detection Techniques. The National Academies Press, Washington, D.C.
Becker, L., B. Popp, T. Rust, and J.L. Bada. 1999. The origin of organic matter in the martian meteorite ALH84001. Earth Planet. Sci. Lett. 167: 71-79.
Benner, S.A., K.G. Devine, L.M. Matveeva, and D.H. Powell. 2000. The missing orbanic molecules on Mars. Proceedings of the National Academy of Sciences 97: 2425-2430.
Bibring, J.P., Y. Langevin, A. Gendrin, B. Gondet, F. Poulet, M. Berthé, A. Soufflot, R. Arvidson, N. Mangold, J. Mustard, P. Drossart, S. Erard, O. Forni, A. Gendrin, M. Combes, T. Encrenaz, T. Fouchet, R. Merchiorri, G. Belluci, F. Altieri, V. Formisano, G. Bonello, F. Capaccioni, P. Cerroni, A. Coradini, S. Fonti, V. Kottsov, N. Ignatiev, V. Moroz, D. Titov, L. Zasova, N. Mangold, P. Pinet, S. Douté, B. Schmitt, C. Sotin, E. Hauber, H. Hoffmann, R. Jaumann, U. Keller, T. Duxbury, and F. Forget. 2005. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 307: 1576.
Biemann, K., J. Oro, P. Toulmin III, L.E. Orgel, A.O. Nier, D.M. Anderson, D. Flory, A.V. Diaz, D.R. Rushneck, and P.G. Simmonds. 1977. The search for organic substances and inorganic volatile compounds in the surface of Mars. J. Geophys. Res. 82: 4641-4658.
Blacic, J., D. Dreesen, T. Mockler, and G. Briggs. 2000. How to access and sample the deep subsurface of Mars. Workshop on Concepts and Approaches for Mars Exploration. Abstract No. 6065. Available at <www.lpi.usra.edu/meetings/robomars/pdf/6065.pdf>.
Boynton, W.V., W.C. Feldman, S.W. Squyres, T.H. Prettyman, J. Brückner, L.G. Evans, R.C. Reedy, R. Starr, J.R. Arnold, D.M. Drake, P.A.J. Englert, A.E. Metzger, I. Mitrofanov, J.I. Trombka, C. d’Uston, H. Wänke, O. Gasnault, D.K. Hamara, D.M. Janes, R.L. Marcialis, S. Maurice, I. Mikheeva, G.J. Taylor, R. Tokar, and C. Shinohara. 2002. Distribution of hydrogen in the near surface of Mars: Evidence for subsurface ice. Science 297: 81-85.
Brass, G.W. 1980. Stability of brines on Mars. Icarus 42: 20-28.
Cabrol, N.A., and E.A. Grin. 2001. The evolution of lacustrine environments on Mars: Is Mars only hydrologically dormant? Icarus 149: 291-318.
Carr, M.H. 1986. Mars: A water-rich planet? Icarus 68: 187-216.
Carr, M.H. 1990. D/H on Mars: The effect of floods, volcanism, impacts and polar processes. Icarus 87: 210-227.
Carr, M.H. 1996. Water on Mars. Oxford University Press, New York.
Carr, M.H., and G.G. Schaber. 1977. Martian permafrost features. J. Geophys. Res. 82: 4039-4055.
Christensen, P.R. 2003. Formation of recent martian gullies through melting of extensive water-rich snow deposits. Nature 422: 45-48.
Christensen, P.R., J.L. Bandfield, J.F. Bell III, N. Gorelick, V.E. Hamilton, A. Ivanov, B.M. Jakosky, H.H. Kieffer, M.D. Lane, M.C. Malin, T. McConnochie, A.S. McEwen, H.Y. McSween, Jr., G.L. Mehall, J.E. Moersch, K.H. Nealson, J.W. Rice, Jr., M.I. Richardson, S.W. Ruff, M.D. Smith, T.N. Titus, and M.B. Wyatt. 2003. Morphology and composition of the surface of Mars: Mars Odyssey THEMIS results. Science 300: 2056-2061, doi: 10.1126/science.1080885.
Chyba, C.F. 1990. Impact delivery and erosion of planetary oceans in the early inner solar system. Nature 343: 129-133.
Chyba, C.F., and K.P. Hand. 2005. Astrobiology: The study of the living universe. Annu. Rev. Astron. Astrophys. 43: 2.1-2.44, doi: 10.1146/ annurev.astro.43.051804.102202.
Clark, B.C., and D.C. Van Hart. 1981. The salts of Mars. Icarus 45: 370-378.
Clifford, S.M. 1991. The role of thermal vapor diffusion in the subsurface hydrologic evolution of Mars. Geophys. Res. Lett. 18: 2055-2058.
Clifford, S.M. 1993. A model for the hydrologic and climatic behavior of water on Mars. J. Geophys. Res. 98: 10973-11016.
Clifford, S.M. 1998. Mars: The effect of stratigraphic variations in regolith diffusive properties on the evolution and vertical distribution of equatorial ground ice. Lunar and Planetary Science Conference XXIX. Abstract No. 1922. Available at <www.lpi.usra.edu/meetings/LPSC98/pdf/1922.pdf>.
Clifford, S.M., and D. Hillel. 1983. The stability of ground ice in the equatorial region of Mars. J. Geophys. Res. 88: 2456-2474.
Clifford, S.M., and T.J. Parker. 2001. The evolution of the Martian hydrosphere: Implications for the fate of a primordial ocean and the current state of the northern plains. Icarus 154: 40-79.
Clifford, S.M., D. Crisp, D.A. Fisher, K.E. Herkenhoff, S.E. Smrekar, P.C. Thomas, D.D. Wynn-Williams, R.W. Zurek, J.R. Barnes, B.G. Bills, E.W. Blake, W.M. Calvin, J.M. Cameron, M.H. Carr, P.R. Christensen, B.C. Clark, G.D. Clow, J.A. Cutts, D. Dahl-Jensen, W.B. Durham, F.P. Fanale, J.D. Farmer, F. Forget, K. Gotto-Azuma, R. Grard, R.M. Haberle, W. Harrison, R. Harvey, A.D. Howard, A.P. Ingersoll, P.B. James, J.S. Kargel, H.H. Kieffer, J. Larsen, K. Lepper, M.C. Malin, D.J. McCleese, B. Murray, J.F. Nye, D.A. Paige, S.R. Platt, J.J. Plaut, N. Reeh, J.W. Rice, Jr., D.E. Smith, C.R. Stoker, K.L. Tanaka, E. Mosley-Thompson, T. Thorsteinsson, S.E. Wood, A. Zent, M.T. Zuber, and H.J. Zwally. 2000. The state and future of Mars polar science and exploration. Icarus 144: 210-242.
Clifford, S.M., R. Bianchi, M.C. De Sanctis, M. Duke, S. Kim, R. Mancinelli, D. Ming, Q. Passey, S. Smrekar, and D. Beaty. 2001. Science rationale and priorities for subsurface drilling in ’07. Mars Program Office White Paper. Available at <www.lpi.usra.edu/meetings/geomars2001/drilling.pdf>.
Clow, G.D. 1987. Generation of liquid water on Mars through the melting of a dusty snowpack. Icarus 72: 95-127.
Costard, F., F. Forget, N. Mangold, and J.P. Peulvast. 2002. Formation of recent martian debris flows by melting of near-surface ground ice at high obliquity. Science 295: 110-113.
Daniels, D.J., D.G. Blumberg, L.D. Wilson, A.L. Kotlyar, V. Freiliker, G. Ronen, and J. Ben-Asher. 2003. Microwave remote sensing of physically buried objects in the Negev desert: Implications for subsurface martian exploration. J. Geophys. Res. 108(E4): 8033.
Dann, J.C., A.H. Holzheid, T.L. Grove, and H.Y. McSween, Jr. 2001. Phase equilibria of the Shergotty meteorite: Constraints on pre-eruptive water contents of martian magmas and fractional crystallization under hydrous conditions. Meteoritics and Planetary Science 36: 793-806.
Degenhardt, J.J., and J.R. Giardino. 2003. Subsurface investigation of a rock glacier using ground penetrating radar: Implications for locating stored water on Mars. J. Geophys. Res. 108(E4): 8036, doi: 10.1029/2002JE001888.
Dragoni, M., F. D’Onza, and A. Tallarico. 2002. Temperature distribution inside and around a lava tube. J. Volcanol. Geotherm. Res. 115: 43-51.
Fanale, F.P. 1976. Martian volatiles: Their degassing history and geochemical fate. Icarus 28: 170-202.
Fanale, F.P., J.R. Salvail, A.P. Zent, and S.E. Postawko. 1986. Global distribution and migration of subsurface ice on Mars. Icarus 67: 1-18.
Farmer, C.B., and P.E. Doms. 1979. Global and seasonal variation of water vapor on Mars and the implications for permafrost. J. Geophys. Res. 84: 2881-2888.
Farmer, J.D. 1996. Hydrothermal processes on Mars: An assessment of present evidence. Pp. 273-299 in Evolution of Hydrothermal Eco-systems on Earth (and Mars?), G.R. Bock and J.A. Goode, eds. John Wiley and Sons, New York.
Feldman, W.C., T.H. Prettyman, W.V. Boynton, J.R. Murphy, S. Squyres, S. Karunatillake, S. Maurice, R.L. Tokar, G.W. McKinney, D.K. Hamara, N. Kelly, and K. Kerry. 2003. CO2 frost cap thickness on Mars during northern winter and spring. J. Geophys. Res. 108(E9): 5103.
Fishbaugh, K.E., and J.W. Head III. 2001. Comparison of the north and south polar caps of Mars: New observations from MOLA data and discussion of some outstanding questions. Icarus 154: 145-161.
Fisk, M.R., and S.J. Giovannoni. 1999. Sources of nutrients and energy for a deep biosphere on Mars. J. Geophys. Res. 104: 11805-11815.
Flynn, G.J., and D.S. McKay. 1990. An assessment of the meteoritic contribution to the Martian soil. J. Geophys. Res. 95(B9): 14497.
Foriel, J., P. Philippot, J. Susini, P. Dumas, A. Somogyi, M. Salomé, H. Khodja, B. Ménez, Y. Fouquet, D. Moreira, and P. López-García. 2004. High-resolution imaging of sulfur oxidation states, trace elements, and organic molecules distribution in individual microfossils and contemporary microbial filaments. Geochim. Cosmochim. Acta 68: 1561-1569.
Formisano, V., S. Atreya, T. Encrenaz, N. Ignatiev, and M. Giuranna. 2004. Detection of methane in the atmosphere of Mars. Science 306: 1758-1762.
Glavin, D.P., M. Schubert, O. Botta, G. Kminek, and J.L. Bada. 2001. Detecting pyrolysis products from bacteria on Mars. Earth Planet. Sci. Lett. 185: 1-5.
Grimm, R.E. 2003. A comparison of time domain electromagnetic and surface nuclear magnetic resonance sounding for subsurface water on Mars. J. Geophys. Res. 108(E4): 8037.
Haberle, R.M., C.P. McKay, J. Schaeffer, N.A. Cabrol, E.A. Grin, A.P. Zent, and R. Quinn. 2001. On the possibility of liquid water on present-day Mars. J. Geophys. Res. 106: 23317-23326.
Hecht, M.H. 2002. Metastability of liquid water on Mars. Icarus 156(2): 373-386.
Heggy E., P. Paillou, G. Ruffie, J.M. Malezieux, F. Costard, and G. Grandjean. 2001. On water detection in the martian subsurface using sounding radar. Icarus 154(2): 244-257.
Heggy E., P. Paillou, F. Costard, N. Mangold, G. Ruffie, F. Demontoux, G. Grandjean, and J.M. Malezieux. 2003. Local geoelectrical models of the martian subsurface for shallow groundwater detection using sounding radars. J. Geophys. Res. 108(E4): 8030.
Horowitz, N.H. 1986. To Utopia and Back: The Search for Life in the Solar System. W.H. Freeman and Co., New York.
Jakosky, B.M., and E. Shock. 1998. The biologic potential of Mars, the early Earth, and Europa. J. Geophys. Res. 103: 19359-19364.
Jakosky, B.M., B.G. Henderson, and M.T. Melon. 1995. Chaotic obliquity and the nature of the martian climate. J. Geophys. Res. 100: 1579-1584.
Jakosky, B.M., A.P. Zent, and R.W. Zurek. 1997. The Mars water cycle: Determining the role of exchange with the atmosphere. Icarus 130: 87-95.
Jakosky, B.M., K.H. Nealson, C. Bakermans, R.E. Ley, and M.T. Mellon. 2003. Subfreezing activity of microorganisms and the potential habitability of Mars’ polar regions. Astrobiol. 3(2): 343-350.
Johnson, C.L., S.C. Solomon, J.W. Head, R.J. Philips, D.E. Smith, and M.T. Zuber. 2000. Lithospheric loading by the north polar cap of Mars. Icarus 144: 313-328.
Jull, A.J., C. Courtney, D.A. Jeffrey, and J.W. Beck. 1998. Isotopic evidence for a terrestrial source of organic compounds found in martian meteorites Allan Hills 84001 and Elephant Moraine 79001. Science 279: 366.
Jull, A.J.T., J.W. Beck, and G.S. Burr. 2000. Isotopic evidence for extraterrestrial organic material in the martian meteorite, Nakhla. Geochim. Cosmochim. Acta 64: 3763.
Kahn, R. 1990. Ice haze, snow, and the Mars water cycle. J. Geophys. Res. 95: 14677-14693.
Knauth, L.P., and D.M. Burt. 2002. Eutectic brines on Mars: Origin and possible relation to young seepage features. Icarus 158: 267-271.
Krasnopolsky, V.A., J.P. Maillard, and T.C. Owen. 2004. Detection of methane in the martian atmosphere: Evidence for life? Icarus 272: 537-547.
Kuzmin, R.O. 1983. Cryolithosphere of Mars. Nauka Press, Moscow.
Langevin, Y., F. Poulet, J.-P. Bibring, and B. Gondet. 2005. Sulfates in the north polar region of Mars detected by OMEGA/Mars Express. Science 307: 1584.
Laskar, J., and P. Robutel. 1993. The chaotic obliquity of the planets. Nature 361: 608-612.
Laskar, J., A.C.M. Correia, M. Gastineau, F. Joutel, B. Levrard, and P. Robutel. 2004. Long-term evolution and chaotic diffusion of the insolation quantities of Mars. Icarus 170: 343-364.
Leighton, R.B., and B.C. Murray. 1966. Behavior of CO2 and other volatiles on Mars. Science 153: 136-144.
Leuschen, C., S. Clifford, and P. Gogineni. 2003. Simulation of a surface-penetrating radar for Mars exploration. J. Geophys. Res. 108(E4): 8035.
Madden, M.D.E., and R.J. Bodnar. 2002. Geochemical modeling of basalt-brine interactions as an analog for Mars near-surface processes. Lunar and Planetary Science Conference XXXIII. Abstract No. 1211. Available at <www.lpi.usra.edu/meetings/lpsc2002/pdf/1211.pdf>.
Malin, M.C., and K.S. Edgett. 2000. Evidence for recent groundwater seepage and surface runoff on Mars. Science 288: 2330-2335.
Malin, M.C., and K.S. Edgett. 2001. Mars Global Surveyor Mars orbiter camera: Interplanetary cruise through primary mission. J. Geophys. Res. 106: 23429-23570.
Malin, M.C., and K.S. Edgett. 2003. Evidence for persistent flow and aqueous sedimentation on early Mars. Science 302: 1931-1934.
Mancinelli, R.L. 1989. Peroxides and the survivability of microorganisms on the surface of Mars. Adv. Space Res. 9(6): 191-195.
Mancinelli, R.L. 1996. The search for nitrogen compounds on the surface of Mars. Adv. Space Res. 18(12): 241-248.
Max, M.D., and S.M. Clifford. 2000. The state, potential distribution, and biological implications of methane in the Martian crust. J. Geophys. Res. 105: 4165-4171.
McCauley, J.F., G.G. Schaber, C.S. Breed, M.J. Grolier, C.V. Haynes, B. Issawi, E. Elachi, and R. Blom. 1982. Subsurface valleys and geomarcheolology of Egypt and Sudan revealed by radar. Science 218: 1004-1020.
McCauley, J.F., C.S. Breed, G.G. Schaber, W.P. McHugh, B. Issawi, C.V. Haynes, M.J. Grolier, and A.E. Kilani. 1986. Paleodrainages of the Eastern Sahara—The radar rivers revisted (SIR-A/B implications for a mid-tertiary trans-African drainage system). IEEE Trans. Geosci. Remote Sensing GE-24: 624-648.
Mellon, M.T., and B.M. Jakosky. 1993. Geographic variations in the thermal and diffusive stability of ground ice on Mars. J. Geophys. Res. 98: 3345-3364.
Mellon, M.T., and B.M. Jakosky. 1995. The distribution and behavior of Martian ground ice during past and present epochs. J. Geophys. Res. 100: 11781-11799.
Melosh, H.J., and A.M. Vickery. 1989. Impact erosion of the primordial atmosphere of Mars. Nature 338: 487-489. MEPAG (Mars Exploration Program Analysis Group). 2004. Scientific Goals, Objectives, Investigations, and Priorities: 2003, unpublished document. Available at <mepag.jpl.nasa.gov/reports/index.html>.
Mischna, M.A., M.I. Richardson, R.J. Wilson, and D.J. McCleese. 2003. On the orbital forcing of martian water and CO2 cycles: A general circulation model study with simplified volatile schemes. J. Geophys. Res. 108(E6): 5062.
Mitrofanov, I.G., M.T. Zuber, M.L. Litvak, W.V. Boynton, D.E. Smith, D. Drake, D. Hamara, A.S. Kozyrev, A.B. Sanin, C. Shinohara, R.S. Saunders, and V. Tretyakov. 2003. CO2 snow depth and subsurface water-ice abundance in the northern hemisphere of Mars. Science 300: 2081-2084.
Morgan, P., D.D. Blackwell, R.E. Spafford, and R.B. Smith. 1977. Heat flow measurements in Yellowstone Lake and the thermal structure of the Yellowstone caldera. J. Geophys. Res. 82: 3719-3732.
Mumma, M.J., R.E. Novak, M.A. DiSanti, B. Bonev, and N. Dello Russo. 2004. Detection and mapping of methane and water on Mars: Evidence for local enhancements in methane. Bulletin of the American Astronomical Society 36(4): Abstract No. 26.02.
Murray, J.B., J.-P. Muller, G. Neukum, S.C. Werner, S. Van Gasselt, E. Hauber, W.J. Markiewicz, J.W. Head III, B.H. Foing, D. Paige, K.L. Mitchell, G. Portyankina, and the HRSC Co-Investigator Team. 2005. Evidence from the Mars Express High Resolution Stereo Camera for a frozen sea close to Mars’ equator. Nature 434: 352-356.
Nair, H., M. Allen, A.D. Anbar, Y.L. Young, and R.T. Clancy. 1994. A photochemical model of the martian atmosphere. Icarus 111: 124-150.
Neukum, G., R. Jaumann, H. Hoffmann, E. Hauber, J.W. Head, A.T. Basilevsky, B.A. Ivanov, S.C. Werner, S. van Gasselt, J.B. Murray, T. McCord, and the HSRC Team. 2004. Recent and episodic volcanic and glacial activity on Mars revealed by the High Resolution Stereo Camera. Nature 432: 23-30.
Olhoeft, G.R. 1998. Electrical, magnetic and geometric properties that determine ground penetrating radar performance. Pp.177-182 in Proceedings of GPR’98, Seventh International Conference on Ground Penetrating Radar, May 27-30, University of Kansas, Lawrence.
Paige, D.A. 1992. The thermal stability of near-surface ground ice on Mars. Nature 356: 43-45.
Pathare, A.V., and D.A. Paige. 1998. Recent liquid water in the polar regions of Mars. P. 31 in First International Conference on Mars Polar Science and Exploration, LPI Contribution No. 953, Lunar and Planetary Institute, Houston, Tex.
Peeples, J., W. Sill, T. May, S. Ward, R. Phillips, R. Jordan, E. Abbott, and T. Killpack. 1978. Orbital radar evidence for lunar subsurface layering in Maria Serenitatis and Crisium. J. Geophys. Res. 83: 3459-3468.
Phillips, R.J., G.F. Adams, W.E. Brown, Jr., R.E. Eggleton, P. Jackson, R. Jordan, W.J. Peeples, L.J. Porcello, J. Ryu, G.G. Schaber, W.R. Sill, T.W. Thompson, S.H. Ward, and J.S. Zelenka. 1973. The Apollo 17 lunar sounder. Pp. 2821-2831 in Proceedings of the Fourth Lunar Science Conference, R. Brett, W.C. Phinney, and D.W. Strangway, eds. Pergamon Press, Inc., New York.
Pollack, H.N., S.J. Hurter, and J.R. Johnson. 1993. Heat flow from the earth’s interior: Analysis of the global data set. Rev. Geophys. 31: 267-280.
Richardson, M.I., and R.J. Wilson. 2002. Investigation of the nature and stability of the martian seasonal water cycle with a general circulation model. J. Geophys. Res. 107(E5): 5031.
Rieder, R., R. Gellert, R.C. Anderson, J. Brückner, B.C. Clark, G. Dreibus, T. Economou, G. Klingelhöfer, G.W. Lugmair, D.W. Ming, S.W. Squyres, C. d’Uston, H. Wänke, A. Yen, and J. Zipfel. 2004. Chemistry of rocks and soils at Meridiani Planum from the alpha particle x-ray spectrometer. Science 306: 1746-1749.
Robin, G. de Q., S. Evans, and J.T. Bailey. 1969. Interpretation of radio echo sounding in polar ice sheets. Philos. Trans. R. Soc. London Ser. A 265: 437-505.
Robin, G. de Q., D.J. Drewry, and D.T. Meldrum. 1977. International studies of ice sheets and bedrock. Philos. Trans. R. Soc. London Ser. B 279: 185-196.
Rossbacher, L.A., and S. Judson. 1981. Ground ice on Mars: Inventory, distribution, and resulting landforms. Icarus 45: 39-59.
Schuerger, A.C., R.L. Mancinelli, R.G. Kern, L.J. Rothschild, and C.P. McKay. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: Implications for the forward contamination of Mars. Icarus 165: 253-276.
Sclater, J.G., C. Jaupart, and D. Galson. 1980. The heat flow through oceanic and continental crust and the heat loss of the Earth. Rev. Geophys. 18: 269-311.
Seu, R., D. Biccari, R. Orosei, L.V. Lorenzoni, R.J. Phillips, L. Marinangeli, G. Picardi, A. Masdea, and E. Zampolini. 2004. SHARAD: The MRO 2005 shallow radar. Planetary and Space Science 52: 157-166.
Sharpton, V.L., and J.W. Head III. 1982. Stratigraphy and structural evolution of the southern Mare Serenitatis: A reinterpretation based on Apollo Lunar Sounder Experiment data. J. Geophys. Res. 87: 10983-10998.
Siegert, M.J. 2000. Antarctic subglacial lakes. Earth Science Reviews 50: 29-50.
Smith, D.E., M.T. Zuber, S.C. Solomon, R.J. Phillips, J.W. Head, J.B. Garvin, W.B. Banerdt, D.O. Muhleman, G.H. Pettengill, G.A. Neumann, F.G. Lemoine, J.B. Abshire, O. Aharonson, C.D. Brown, S.A. Hauck, A.B. Ivanov, P.J. McGovern, H.J. Zwally, and T.C. Duxbury. 1999. The global topography of Mars and implications for surface evolution. Science 284: 1495-1503.
Smith, M.D., J.C. Perl, B.J. Conrath, and P.R. Christenson. 2001. One martian year of atmospheric observations by the thermal emission spectrometer. Geophys. Res. Lett. 29(22): 4263-4266.
Smoluchowski, R. 1968. Mars: Retention of ice. Science 159: 1348-1350.
Squyres, S.W., S.M. Clifford, R.O. Kuzmin, J.R. Zimbelman, and F.M. Costard. 1992. Ice in the martian regolith. Pp. 523-554 in Mars, H.H. Kieffer, B.M. Jakosky, C.W. Snyder, and M.S. Matthews, eds. University of Arizona Press, Tucson, Ariz.
Squyres, S.W., R.E. Arvidson, J.F. Bell III, J. Brückner, N.A. Cabrol, W. Calvin, M.H. Carr, P.R. Christensen, B.C. Clark, L. Crumpler, D.J. Des Marais, C. d’Uston, T. Economou, J. Farmer, W. Farrand, W. Folkner, M. Golombek, S. Gorevan, J.A. Grant, R. Greeley, J. Grotzinger, L. Haskin, K.E. Herkenhoff, S. Hviid, J. Johnson, G. Klingelhöfer, A.H. Knoll, G. Landis, M. Lemmon, R. Li, M.B. Madsen, M.C. Malin, S.M. McLennan, H.Y. McSween, D.W. Ming, J. Moersch, R.V. Morris, T. Parker, J.W. Rice, Jr., L. Richter, R. Rieder, M. Sims, M. Smith, P. Smith, L.A. Soderblom, R. Sullivan, H. Wänke, T. Wdowiak, M. Wolff, and A. Yen. 2004a. The Opportunity Rover’s Athena science investigation at Meridiani Planum, Mars. Science 306: 1698-1703.
Squyres, S.W., J.P. Grotzinger, R.E. Arvidson, J.F. Bell III, W. Calvin, P.R. Christensen, B.C. Clark, J.A. Crisp, W.H. Farrand, K.E. Herkenhoff, J.R. Johnson, G. Klingelhöfer, A.H. Knoll, S.M. McLennan, H.Y. McSween, Jr., R.V. Morris, J.W. Rice, Jr., R. Rieder, and L.A. Soderblom. 2004b. In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306: 1709-1722.
Stein, C.A. 1995. Heat flow of the Earth. Pp. 144-157 in A Handbook of Physical Constants. American Geophysical Union, Washington, D.C.
Stoker, C. 1998. Summary of the NASA Ames Mars Deep Water Sounding Workshop. Available at <astrobiology.arc.nasa.gov/workshops/1998/marswater/index.html>.
Summers, M.E., B.J. Lieb, E. Chapman, and Y.L. Yung. 2002. Atmospheric biomarkers of subsurface life on Mars. Geophys. Res. Lett. 29(24): 2171.
Tanaka, K.L. 1986. The stratigraphy of Mars. J. Geophys. Res. 91: 139-158.
Tanaka, K.L., and D.H. Scott. 1987. The youngest channel system on Mars. Pp. 44-45 in Martian Geomorphology and Its Relation to Subsurface Volatiles, S.M. Clifford, L.A. Rossbacher, and J.R. Zimbelman, eds. LPI Technical Report 87-02. Lunar and Planetary Institute, Houston, Tex.
Thomas, P.C., M.C. Malin, K.S. Edgett, M.H. Carr, W.K. Hartmann, A.P. Ingersoll, P.B. James, L.A. Soderblom, J. Veverka, and R. Sullivan. 2000. North-south geological differences between the residual polar caps on Mars. Nature 404: 161-164.
Toon, O.B., J.B. Pollack, W. Ward, J.A. Burns, and K. Bilski. 1980. The astronomical theory of climatic change on Mars. Icarus 44: 552-607.
Touma, J., and J. Wisdom. 1993. The chaotic obliquity of Mars. Science 259: 1294-1296.
Travis, B.J., N.D. Rosenberg, and J.N. Cuzzi. 2003. On the role of widespread subsurface convection in bringing liquid water close to Mars’ surface. J. Geophys. Res. 108(E4): 8040.
Treiman, A.H., R.A. Barrett, and J.L. Gooding. 1993. Preterrestrial aqueous alteration of the Lafayette (SNC) meteorite. Meteoritics 28: 86-97.
Wallendahl, A., and A.H. Treiman. 1999. Geochemical models of low-temperature alteration of martian rocks. 30th Lunar and Planetary Science Conference. Abstract No. 1268. Lunar and Planetary Institute, Houston, Tex.
Ward, W.R. 1992. Long-term orbital and spin dynamics of Mars. Pp. 298-320 in Mars, H.H. Kieffer, B.M. Jakosky, C.W. Snyder, and M.S. Matthews, eds. University of Arizona Press, Tucson, Ariz.
Weiss, B.P., Y.L. Yung, and K.H. Nealson. 2000. Atmospheric energy for subsurface life on Mars? Proc. Natl. Acad. Sci. U.S.A. 97: 1395-1399.
Yen, A.S., S.S. Kim, M.H. Hecht, M.S. Frant, and B. Murray. 2000. Evidence that the reactivity of the martian soil is due to superoxide ions. Science 289: 1909-1912.