Modern Paints
Tom Learner
Senior Conservation Scientist
Tate
London
ABSTRACT
Few would argue that oil paint has been the most important type of paint over the last 500 years. The use of oil as the film-forming component of paint—the binding medium—was well established by the start of the fifteenth century, and for many artists oil paints still remain the preferred choice today. However, throughout the twentieth century a wide and varied range of synthetic polymers have been developed, many of which have been used as binding media in modern paints. The introduction of these synthetic binders, most notably acrylic, alkyd, and polyvinyl acetate, has undoubtedly enabled great advances to be made in paint technology, in terms of reduced yellowing, greater flexibility, faster drying times, and in the case of emulsion formulations, the elimination of organic solvents as thinners and diluents. Many artists have utilized these modern paint types, including those that were never intended specifically for artists’ use, and have explored and exploited their distinct handling and optical properties.
Establishing the constituents of paint is frequently necessary prior to any kind of conservation treatment and for developing long-term preventive conservation strategies, as well as for technical art historical studies and issues surrounding authenticity. The identification of binding media is particularly important, as this component appears to have the largest influence on many of the properties of the resulting dried paint film. Although noninvasive/nondestructive techniques would clearly be favorable, at present the most useful analysis is obtained from high-sensitivity techniques that
require the removal of submilligram paint samples. Two analytical techniques—pyrolysis-gas chromatography-mass spectrometry (PyGCMS) and Fourier transform infrared spectroscopy (FTIR)—are now routinely used at Tate to identify and characterize modern paints from works of art. This paper will summarize the three principal classes of synthetic binder and how PyGCMS and FTIR have been utilized to analyze them.
INTRODUCTION
Despite the great variety of modern paint formulations (see Figure 1), there are three principal classes of synthetic binder that have been widely used by artists: acrylic, alkyd, and polyvinyl acetate (PVA) (Crook and Learner, 2000; Learner, 2000). The main binder used in the artists’ paint market has been acrylic, although there are two quite distinct forms: acrylic solution, where the acrylic

FIGURE 1 Selection of modern paints.
polymer is dissolved in a mineral spirit or turpentine, and acrylic dispersion (i.e., emulsion), where the acrylic polymer is dispersed in water (with the aid of a surfactant and other additives). The solution form consists of a poly (n-butyl methacrylate) homopolymer, which was developed in the late 1940s, whereas the emulsion form consists of an acrylic copolymer, typically between methyl methacrylate (MMA) and either ethyl acrylate (EA) or n-butyl acrylate (nBA), and only became available in the late 1950s. The two types have quite distinct mechanical properties and exhibit very different sensitivities to organic solvents and water. It is important therefore to be able to distinguish between them analytically.
Acrylic binders are also used in the house paint market, but two other important types of synthetic binder—alkyd and PVA—are also widely utilized. Alkyd paints are oil-modified polyester paints, introduced in the late 1930s, although they did not make a significant impact on the paint industry until the late 1950s in Europe and slightly earlier in the United States. Since then, the vast majority of oil-based house paints have incorporated an alkyd resin as the principal binder. Perhaps somewhat surprisingly, they have received only limited use by artists’ colormen. Alkyd resins are produced from three main components: a polyhydric alcohol, a polybasic carboxylic acid, and a source of monobasic fatty acid, which is often added in the form of a drying oil. The polyhydric alcohol (also called “polyol”) and polybasic acid constituents in the vast majority of alkyd house paints are actually limited to just three compounds: glycerol and/or pentaerythritol as the polyol and phthalic anhydride—the dehydrated version of ortho-phthalic acid (1,2-benzenedicarboxylic acid)—as the polybasic acid.
PVA has also been used in waterborne polymer emulsions, although it requires some slight modification to lower its glass transition temperature, either by the addition of a plasticizer (common in early formulations) or by copolymerization with a softer monomer (the preferred option since the 1960s). A number of different plasticizing methods have been used for this since the introduction of PVA paints, and PyGCMS is able to differentiate between them. In early emulsion formulations an external plasticizer, such as dibutyl phthalate (DBP), was added and often in appreciable quantities (up to 20 percent by weight) (Martens, 1981, p. 81). The problems caused by these plasticizers migrating out of the paint film were overcome during the 1960s by the copolymerization of PVA with softer monomers, often called internal plasticization. This has been achieved with a variety of other vinyl monomers, including some of the softer acrylates but also commonly achieved with vinyl versatates or VeoVa monomers, which are commercial mixtures of highly branched C9 and C10 vinyl esters manufactured by Shell (Slinckx and Scholten, 1994).
The choice of which binder is used in a household emulsion formulation appears to be dependent on such factors as cost (acrylic is more expensive), durability (acrylic is considered more durable and therefore often used for exterior paints), surface finish (acrylic has a superior binding power and is therefore
sometimes used for matt paints where less binder is present), and age (PVA emulsions were developed in the 1940s, earlier that the acrylics).
The ability to identify the binding medium in paints is often essential for conservation reasons. Since different paint types will respond differently to cleaning solvents and reagents, paint characterization is often needed prior to treatment. It is also necessary when examining the aging properties of paints. Reactions such as oxidation, cross-linking or chain-scission all affect the physical and chemical properties of a paint; so understanding the likely reactions is an important consideration. It is, after all, better to prevent deterioration than try to reverse it. Much effort is currently being put into the general understanding of artists’ materials and techniques, in other words, what did an artist use and how? Analysis can also play an important role in authentication issues.
Many of the techniques used for traditional medium analysis, such as gas and liquid chromatography, are not totally suited to all these modern paint binders, largely because of their high molecular weights (i.e., they are nonvolatile and frequently insoluble in solvents) and the inability to extract diagnostic components from the polymer matrix. Nevertheless, these polymeric materials can be effectively broken down into volatile fragments through pyrolysis (i.e., heat in the absence of oxygen), and these fragments can consequently be separated and identified by gas chromatography (GC). This technique—pyrolysis-gas chromatography (Py-GC)—has been used since the 1960s by forensic scientists for the identification of synthetic binders in house paints, car paints, and various industrial coatings (Jain et al., 1965; Challinor, 1983; Wheals, 1985) but was not properly assessed by the conservation profession for its capability to identify the synthetic binders used in artists’ painting materials until the 1990s (Sonoda and Rioux, 1990; Stringari and Pratt, 1991; Sonoda, 1998). More recently a wider range of paint binders has been investigated with the many advantages of using a mass spectrometer as a detector (i.e., with PyGCMS) (Learner, 1995a, 2001).
Another technique frequently adopted for the analysis of traditional binding media is Fourier transform infrared spectroscopy (FTIR). FTIR is normally used as a comparative technique with the spectra of each unknown material being matched either visually with a library of known standards or through a computer search. Although this technique requires no instrumental modifications to enable the analysis of synthetic polymers, an entire set of new reference standards has to be generated. The technique is also semiquantitative; so it is normally possible to assess the relative proportions of two components in a mixture if the spectra of each are available and adequately different.
At Tate, FTIR has been widely used as a nondestructive analytical method (see FTIR section below for details) to be carried out prior to PyGCMS (Learner, 1996). More recently it has also been used in attenuated total reflectance (ATR) mode to examine the migration of surfactants to the surface of acrylic emulsion films as part of an ongoing study into the effects of surface cleaning (Learner et al.,
2002a,b). The use of PyGCMS and FTIR in the analysis of modern paints will now be outlined.
PYROLYSIS-GAS CHROMATOGRAPHY-MASS SPECTROMETRY
Acrylic Solutions
Artists’ acrylic solution paints, bound with a poly n-butyl methacrylate (pnBMA) homopolymer resin, produce extremely simple pyrograms consisting of a single peak of nBMA monomer. The acrylic binder undergoes complete depolymerization (a mechanism common to all polymethacrylates [Irwin, 1979]) on pyrolysis.
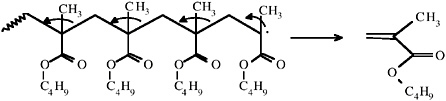
Figure 2 shows a pyrogram of Paraloid F-10 (Rohm and Haas)—the acrylic binder used in acrylic solution paints—with the mass spectrum of the single peak identified as nBMA. The two most intense ions in the mass spectrum are those of m/z = 69 (loss of the n-butoxy side group) and m/z = 41 (loss of a further C=O),
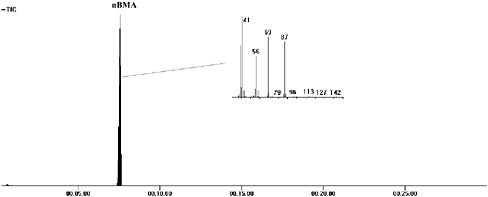
FIGURE 2 Pyrogram of Paraloid F-10 (pnBMA acrylic resin) with mass spectrum of nBMA.
and these are both seen with all methacrylate monomers. Strong peaks at m/z = 87 (from the protonation of methacrylic acid) and m/z = 56 (from butene) are also seen. The molecular ion of nBMA (m/z = 142) is extremely weak and often not observed.
Acrylic Emulsions
Figure 3 shows the overall pyrogram from Plextol B-500 (Röhm), a p(EA/ MMA) emulsion that has been used in artists’ acrylic paint formulations, and from Spectrum polymer medium (Spectrum), a p(nBA/MMA) acrylic copolymer artists’ product. Also shown in the pyrogram of Plextol B-500 is the mass spectrum from the most intense peak, MMA. The mass spectrum of MMA shows a similar fragmentation pattern to nBMA seen with acrylic solution paints, with
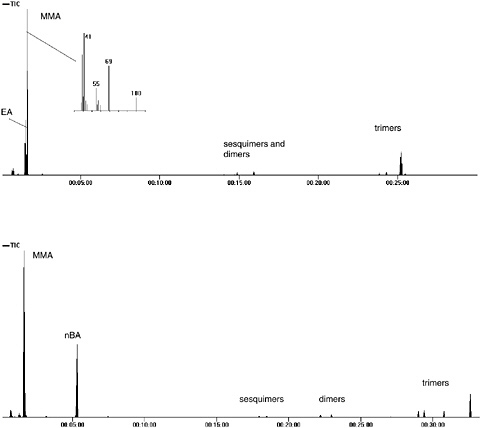
FIGURE 3 Pyrogram of Plextol B-500, a p(EA/MMA) emulsion with mass spectrum of MMA (top), and Spectrum polymer medium, a p(nBA/MMA) emulsion (bottom).
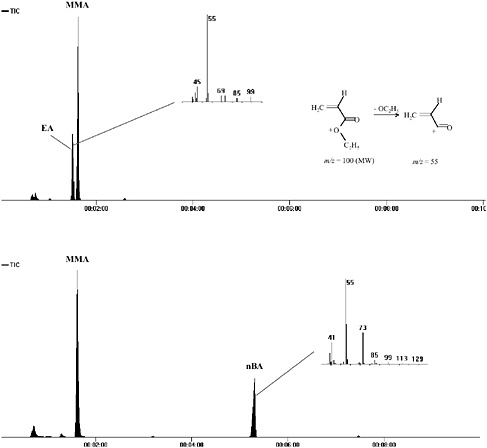
FIGURE 4 Details of early sections of pyrograms of Plextol B-500 (top) and Spectrum polymer medium (bottom) with mass spectra of acrylate components (EA and nBA, respectively).
prominent ions at m/z = 69 and 41, but here the molecular ion (m/z = 100) is clearly visible.
Figure 4 shows a detail from the early part of both of these pyrograms with the mass spectrum of the EA and nBA monomers shown, respectively. The ability to separate the EA and MMA monomers, despite their similar retention times, is clearly seen in this detail. The mass spectra of both acrylate monomers are dominated by a peak of m/z = 55, corresponding to the loss of the alkoxy side group to produce a CH2CH.CO+ fragment ion (as shown for EA).
The overall pyrograms (see Figure 3) of both copolymers contain a number of later additional peaks, which are the result of incomplete depolymerization when an acrylate component is present in the polymer. These have been identi-
fied as a series of sesquimers, dimers, and trimers of acrylate or acrylate-MMA combinations. Sesquimer is a term meaning 1.5 monomer units (i.e., a molecule consisting of a three-carbon atom backbone with an acrylate/methacrylate group at either end).
Some materials labeled acrylic emulsions can actually be copolymers with other monomers, such as styrene (to create styrene-acrylics) or vinyl acetate (to create vinyl-acrylics). Although not shown here, both are readily distinguished by pyrolysis-gas chromatography-mass spectrometry (PyGCMS), by the detection of styrene monomer and acetic acid (see below), respectively.
Polyvinyl Acetate (PVA) Emulsions
On pyrolysis, PVA emulsion paints produce principally ethanoic (acetic) acid and benzene by a side group elimination mechanism. Figure 5 shows the overall pyrogram observed from Emultex VV536 (Harco), with the mass spectrum from the intense peak at the start of the pyrogram. This spectrum is mainly that of ethanoic acid (with a molecular ion of m/z = 60, and strong fragment ions at m/z = 43, 45), although the peak of m/z = 78 corresponds to the molecular ion of benzene.
It is usually possible to confirm the presence of an emulsion form of PVA (as opposed to a solution form) by the detection of a plasticizer, since pure PVA is slightly too hard to form a continuous film from an emulsion.
This particular emulsion, Emultex VV536 (Harco), actually contains both a vinyl versatate resin and a phthalate plasticizer (in the majority of PVA emulsions only one kind is used). The sharp peak at the far right of the pyrogram is identified as DBP, whose mass spectrum (also shown) has a very intense fragment ion of m/z = 149, which is the characteristic fragment ion of all dialkyl phthalates. In the center of the pyrogram is the band of rather broad peaks produced by the VeoVa plasticizer. Although these peaks are not fully resolved, the overall peak pattern does conform to a very distinctive profile.
Alkyds
For alkyd paints based on ortho-phthalic acid, phthalic anhydride is the principal peak detected on pyrolysis and therefore used as the diagnostic peak. Figure 5 shows the pyrogram of 75045 alkyd resin (Croda), a typical ortho-phthalic alkyd resin, with the mass spectrum of the very dominant phthalic anhydride peak on the left. The molecular ion (m/z = 148) is clearly seen, with the most intense peak at m/z = 104 produced from the loss of CO2. The mass spectrum on the right is from palmitic acid (with a molecular ion clearly visible at m/z = 256), normally the most intense fatty acid observed from a dried oil component. The suspected mechanism of phthalic anhydride liberation from the alkyd’s polyester structure is as follows:
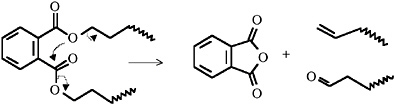
More recently work has been carried out to assess the advantages of carrying out an in situ methylation step at the time of pyrolysis, which appears to give a quantitative method of analysis (Cappitelli et al., 2002). This is being investigated to see whether oil type can be obtained reliably.
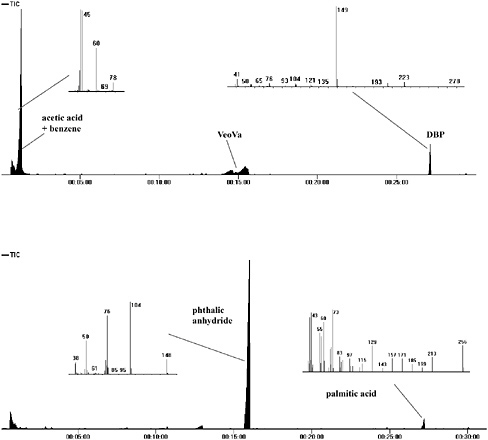
FIGURE 5 Top: Pyrogram of Emultex VV536, a PVA emulsion with mass spectrum of ethanoic acid/benzene (left) and dibutylphthalate plasticizer (right). Bottom: Pyrogram of Croda 75045 alkyd resin with mass spectrum of phthalic anhydride (left) and palmitic acid (right).
FOURIER TRANSFORM INFRARED SPECTROSCOPY
There are many ways of introducing a sample to a Fourier transform infrared spectroscopy (FTIR) instrument. The main technique currently employed at Tate is to compress the sample in a diamond cell and make the measurement through an infrared microscope, although a beam condenser seems to give equally good results (Learner, 1995b).
Although spectra obtained from a diamond cell are arguably inferior to those from a KBr disc, the use of the diamond cell has three major advantages. First, the technique is nondestructive, which permits the sample to be retrieved and then reanalyzed by a complementary technique. Second, there is no sample preparation necessary. The diamond cell simply compresses the sample to a sufficiently reduced thickness for reliable transmission spectra to be obtained. Use of a diamond cell can be problematic with hard and brittle materials, but fortunately the majority of twentieth-century paints are fairly soft materials, which permit easy compression in the cell. Third, this soft nature of most synthetic polymers used in paints (in particular, the acrylics) makes grinding them into KBr powder very difficult. The main disadvantage of the diamond cell is the possibility of pressure effects on the spectrum, although the actual pressures used in the diamond cell are not thought to be particularly high.
FTIR is an excellent way of obtaining information quickly about the basic chemical class of a binding material. For homogeneous samples, such as certain synthetic varnishes, this is relatively straightforward. Figure 6 shows the FTIR
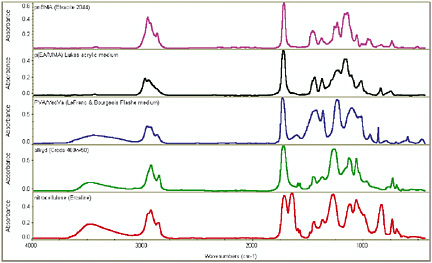
FIGURE 6 FTIR spectra of unpigmented media. From top to bottom: acrylic solution (pink line), pEA/MMA-type acrylic emulsion (black line), PVA emulsion (blue line), alkyd resin (green line), and nitro-cellulose (red line).
spectra of five different synthetic binders, all obtained from films of unpigmented media. These are (shown from top to bottom) an acrylic solution, an acrylic emulsion, a PVA emulsion, an alkyd resin, and a nitrocellulose resin (one of the other less common types of modern paint binder).
The FTIR spectra of paints are much more complicated as each additional component of the paint formulation, in particular the pigment(s) and extender(s), will exhibit their own individual vibrations and absorb the infrared radiation at those characteristic frequencies. Sometimes this can be a distinct disadvantage, especially if the spectrum from a particular pigment completely dominates the spectrum, thereby in effect masking out the absorptions from the binding media. However, in some instances the absorptions of the various components are so characteristic that it may be possible to sort out the individual bands by visual methods. Here the presence of overlapping bands can be turned into an advantage, as information can be gathered from all the individual components from a paint sample from a single analysis.
An example of how FTIR can successfully identify each of the three main components in a paint is given in Figure 7, which shows four overlaid spectra. The pink curve is the overall spectrum, obtained from an acrylic emulsion paint:
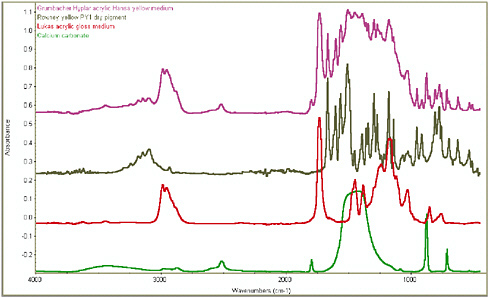
FIGURE 7 Overall FTIR spectrum of Hyplar Hansa yellow medium acrylic emulsion paint (Grumbacher 1994 range) (pink line), with spectra of individual principal components: PY1 azo yellow pigment (black line), pEA/MMA binder (red line), chalk extender (green line).
Hansa yellow medium artists’ acrylic color (Grumbacher). The other three curves are reference curves taken from each individual constituents.
-
Red curve: The frequencies of the C-H stretching bands at 2986 cm−1 and 2955 cm−1, the overall profile of the C-H stretching region, C=O stretching at 1732 cm−1, and skeletal vibrations at 1179 cm−1 are all indicative of a p(EA/MMA) acrylic emulsion.
-
Grey curve: The sharp peaks seen at 1667 cm−1, 1602 cm−1, 1562 cm−1, 1508 cm−1, 1296 cm−1, 1140 cm−1, 953 cm−1, and 774 cm−1 are all present as strong absorptions in the spectrum of the pigment PY1, one of the common organic monoazo yellow pigments. The profile of absorptions in the region between 3000 cm−1 and 3300 cm−1, with peaks at 3098 cm−1, 3145 cm−1, 3181 cm−1, and 3243 cm−1 is also highly diagnostic of pigment PY1.
-
Green curve: the two absorptions at 2520 cm−1 and 1799 cm−1 are immediately indicative of the presence of chalk (calcium carbonate). Although relatively weak absorptions, these two wave numbers are normally found to be completely separated from the absorptions from all the binding media, pigments, and other extenders. The two very sharp peaks at 877 cm−1 and 713 cm−1 confirm the presence of chalk as extender and the strong and very broad absorption between 1400 cm−1 and 1500 cm−1 is also clearly visible.
Recently, attenuated total reflectance (ATR), a reflective mode of FTIR, has proved useful at identifying the differences at the surface of a paint film, compared with its bulk properties (as measured in transmission mode). This mode is showing great potential for following chemical surface changes on a paint film with age and after certain conservation treatments, such as cleaning. Figure 8 shows three stacked spectra measured with ATR. The top spectrum is from the upper surface of an unpigmented acrylic medium (Golden) that has been cast on a glass slide and been left for approximately five years. The middle spectrum is from the lower surface of the same sample, after removal from the slide. There are clear differences between the two. The peak assignments on each of these spectra that are placed to the left of the relevant peak are characteristic of a p(EA/MMA) acrylic emulsion. All peak labels that are placed to the right of their peaks are indicative of polyethylene glycol, a common class of surfactant. In this example the PEG has gathered at the upper surface of the paint film, a phenomenon that could have significant ramifications for a painting’s appearance and its change with age and/or cleaning.
CONCLUSIONS AND LOOKING AHEAD
It is possible to identify, characterize, and differentiate the principal classes of synthetic binders used in modern paints with a combination of pyrolysis-gas chromatography-mass spectrometry (PyGCMS) and Fourier transform infrared
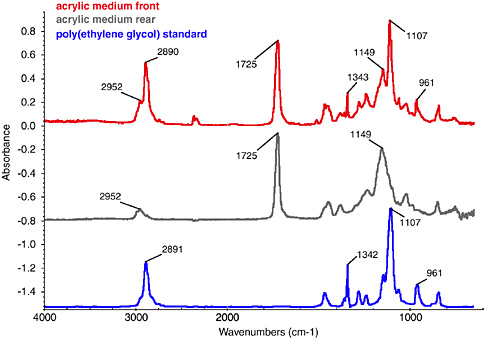
FIGURE 8 FTIR-ATR spectrum of unpigmented pEA/MMA acrylic medium (Golden 1993 range): upper surface (red line), lower surface (after removal from glass slide support) (black line), and polyethylene glycol (PEG) reference spectrum (blue line).
spectroscopy (FTIR). However, there still remain a great many analytical needs for modern paints, including
-
improved quantitative methods of medium analysis;
-
analytical techniques for organic pigments and the additives added to paint formulations;
-
surface analysis methods for chemical, physical, and optical changes on aging and conservation treatments; and
-
high spatial resolution techniques to analyze individual layers from layered paint structures.
REFERENCES
Cappitelli, F., T. Learner, and O. Chiantore. 2002. An initial assessment of thermally assisted hydrolysis and methylation—gas chromatography/mass spectrometry for the identification of oils from dried paint films. Journal of Analytical and Applied Pyrolysis 63:339-348.
Challinor, J. 1983. Forensic applications of pyrolysis gas chromatography. Forensic Science International 21:269-285.
Crook, J., and T. Learner. 2000. The Impact of Modern Paints. London: Tate Gallery Publishing.
Irwin, W. 1979. Analytical pyrolysis—an overview. Journal of Analytical and Applied Pyrolysis 1:3-25.
Jain, N., C. Fontain, and P. Kirk. 1965. Identification of paints by pyrolysis gas chromatography. Journal of the Forensic Science Society 5:102-109.
Learner, T. 1995a. The analysis of synthetic resins found in twentieth century paint media. In Resins Ancient and Modern, eds. M. Wright and J. Townsend, pp. 76-84. Edinburgh: Scottish Society for Conservation and Restoration.
Learner, T. 1995b. The use of a diamond cell for the FTIR characterisation of paints and varnishes available to twentieth century artists. Postprints: IRUG2 Meeting, pp. 7-20, available at http://www.irug.org/documents/1Learner.pdf.
Learner, T. 1996. The use of FTIR in the conservation of twentieth century paintings. Spectroscopy Europe 8(4):14-19.
Learner, T. 2000. A review of synthetic binding media in twentieth century paints. The Conservator 24:96-103.
Learner, T. 2001. The analysis of synthetic paints by pyrolysis-gas chromatography-mass spectrometry (PyGCMS). Studies in Conservation 46:225-241.
Learner, T., O. Chiantore, and D. Scalarone. 2002a. Ageing studies of acrylic emulsion paints. Preprints of the 13th Triennial meeting of the ICOM Committee for Conservation, Rio de Janeiro, pp. 911-919 . London: James and James.
Learner, T., M. Schilling, and R. de la Rie. 2002b. Modern paints: A new collaborative research project. Conservation. The Getty Conservation Institute Newsletter 17(3):18-20, available at http://www.getty.edu/conservation/resources/newsletter/17_3/news_in_cons1.html.
Martens, C. 1981. Waterborne Coatings. New York: Van Nostrand Reinhold.
Slinckx, M., and H. Scholten. 1994. Veova9/(meth)acrylates, a new class of emulsion copolymers. Journal of the Oil and Colour Chemists’ Association 77:107-112.
Sonoda, N. 1998. Application des méthodes chromatographiques a la caractérisation des peintures alkydes pour artistes. Techne 8:33-43.
Sonoda, N., and J.-P. Rioux. 1990. Identification des matériaux synthétiques dans les peintures modernes. 1. Vernis et liants polymères. Studies in Conservation 35:189-204.
Stringari, C., and E. Pratt. 1991. The identification and characterization of acrylic emulsion paint media. In Saving the 20th Century: The Conservation of Modern Materials, ed. D. Grattan, pp. 411-439. Ottawa: Canadian Conservation Institute.
Wheals, B. 1985. The practical application of pyrolytic methods in forensic science during the last decade. Journal of Analytical and Applied Pyrolysis 8:503-514.
APPENDIX
EXPERIMENTAL CONDITIONS
Pyrolysis-Gas Chromatography-Mass Spectrometry
FOM-4LX Curie point pyrolysis unit mounted directly onto the injection port of a Hewlett-Packard 5890 gas chromatograph and interfaced to a Finnigan MAT Incos 50 quadrupole mass spectrometer. Pyrolysis conditions: 610°C for eight seconds. Pyrolysis chamber kept at 200°C. GC injection port kept at 180°C. BPX-5 (SGE) nonpolar column: 25 meters long, 0.32 µm internal diameter and 0.1 µm film thickness. Temperature program: 40°C held for two minutes, then ramped at 10°C/min−1 to 350°C and held for two minutes. The transfer line was kept at 250°C. Incos 50 utilized EI ionization at 70 eV, and scanned from mass 35-500 every second.
Fourier Transform Infrared Spectroscopy
Transmission work carried out on a Nicolet Avatar 360 instrument with SpectraTech IR Plan microscope. Sample held in a diamond cell and 128 scans were averaged at 4 cm−1 resolution. ATR work carried out on a Nicolet Magna IR 560 instrument and Nicolet Nic Plan IR microscope with a Spectra-Tech ATR objective with zinc selenide crystal and purged with dry air. Two hundred scans were averaged at 4 cm−1 resolution.
ACKNOWLEDGEMENTS
This work was made possible by the support of the Tate Gallery and the Leverhulme Trust, and the generosity of the FOM Institute (which loaned the PyGCMS instrument). The FTIR was purchased with a grant from the Clothworkers’ Foundation in London. Herant Khanjian at the Getty Conservation Institute carried out the ATR measurements during the author’s guest scholarship there in 2001. The author is extremely grateful to all those involved.















