Paint Media Analysis
Michael R. Schilling
Senior Scientist
The Getty Conservation Institute
Pigments and organic binding media are the two principle components of paint. Whereas pigments impart color to paint, it is the role of the organic binding media to bind together the grains of pigment and adhere them to the work of art. Synthetic polymers are the binding media of choice for most of today’s commercial paints. Nevertheless, the continued use by contemporary artists of such natural products as egg, milk, animal hides, vegetable oils, plant gums, waxes, and natural resins (which were the only binding media available from antiquity through the end of the nineteenth century [Kühn, 1986]) attests to their durability, versatility, and working properties that artists value.
Conservation scientists are often called upon to analyze organic binding media and pigments in painted works of art. Knowledge obtained from the study of artists’ materials and techniques enriches our understanding of the history of art, informs the decisions of conservators who must develop appropriate conservation treatments, and reveals compositional changes in artists’ materials brought about by age, weathering, and environmental factors. Although many instrumental analysis techniques now exist for identifying organic substances, several key factors limit the actual number of techniques that are suitable for identifying organic binding media. To begin, typical samples removed from paintings weigh in the range of 1 to 50 micrograms; in many instances the medium simply may be present below instrumental detection limits. Mixtures of organic binding media may present problems of overlapping signals. Physical aging and pigment interferences may complicate data interpretation by changing the original composition. Moreover, it is extremely difficult, if not impossible, to resolubilize some organic binding media (such as egg tempera) once they have become dried into
paint films. For those instrumental techniques that are capable of detecting organic binding media, application of simple qualitative analysis limits the extent to which the analytical test results may be interpreted.
Quantitative gas chromatography-mass spectrometry (GC-MS) is one of the few analytical techniques capable of overcoming the myriad of problems associated with identification of natural organic binding media in painted works of art. In research at the Getty Conservation Institute, quantitative GC-MS procedures were developed for identifying organic binding media based on proteins, oils, and plant gums. The procedures were validated on test paints that were subjected to two types of artificial aging (six weeks at 80°C and 500 hours in a Weather-O-Meter light exposure chamber at 50 percent RH and 50°C). The present study illustrates the utility of quantitative GC-MS in the study of paintings by two prominent American artists.
A TECHNICAL STUDY OF PAINTINGS BY JACOB LAWRENCE
Jacob Lawrence was known for his simplified, brilliant graphic forms that depict African American history and experience (Steele, 2000). Throughout his long career he favored working in various water-based organic binding media commonly referred to as tempera: casein, egg, plant gums, and animal glue (Mayer, 1940). It is known that Lawrence mixed some of his own tempera paints from artists’ recipe books, whereas in many of his later works he used commercially available tube colors. Manufacturers often add materials to tube colors, in addition to the binding media, to modify the working properties of the paints and stabilize the mixtures. These additives include glycerol, seed oils (such as linseed, poppy, and walnut), natural resins (dammar, rosin), phthalate plasticizers, and sugar. From these lists it is quite clear that Lawrence’s paint media may be complicated mixtures of many substances (Steele and Halpine, 1993). It should also be noted that it is nearly impossible to differentiate these tempera media based solely on the appearance of the painted surface, yet because this is sometimes the only means available to museum registrars when cataloguing their collections, these records are sometimes erroneous.
Recently a technical study of samples from a number of Lawrence’s paintings (see Table 1) was undertaken to learn more about his painting technique, check the accuracy of museum archival records, and contribute to a catalogue raisonné of Lawrence’s paintings (Schilling et al., 2000). Pigments were identified in the paint samples using polarized-light microscopy. Some samples were tested using Fourier transform infrared microspectrometry (FTIR) to identity the paint components.
To test for proteinaceous media in paint samples, amino acids were liberated by acid hydrolysis and analyzed by quantitative GC-MS in the form of (tert-butyl-dimethylsilyl) derivatives (see Appendix A for experimental details) (Simek et al., 1994; Columbini et al., 1998; Schilling and Khanjian, 1996a). The quantitative
TABLE 1 Jacob Lawrence Paintings Analyzed in This Study
|
The Metropolitan Museum of Art, New York Blind Beggars, 1938 |
|
National Museum of American Art, Washington, D.C. Painting the Bilges, 1944 New Jersey, 1946 Men Exist for the Sake of One Another, 1958 Library, 1960 |
|
Hirshhorn Museum and Sculpture Garden, Washington, D.C. African Gold Miners, 1946 Vaudeville, 1951 The Cue and the Ball, 1956 Magic Man, 1958 Playing Card (Joker) or (King), 1962 Harriet and the Promised Land No.10, 1967 In a Free Government, 1976 |
|
Worcester Art Museum The Checker Players, 1947 |
|
The Museum of Modern Art, New York Sedation, 1950 |
|
Private Collection Struggle Series No.11: Informers Coded Message, 1955 Ordeal of Alice, 1963 |
|
National Gallery of Art, Washington, D.C. Street to Mbari, 1964 Daybreak-A Time to Rest, 1967 |
|
Merril C. Berman Collection Students with Books, 1966 |
|
Jacob and Gwen Knight Lawrence Collection Other Rooms, 1975 |
results for the alkyl- and imino-substituted amino acids (the so-called “stable” amino acids) were normalized to 100 mole percent. The quantitative yields for the other amino acids are often unreliable due to pigment interferences in the hydrolysis and/or derivatization procedures, or due to aging (Halpine, 1992; Ronca, 1994; Schilling and Khanjian, 1996b); these amino acids were excluded from the final dataset.
Samples tested for plant gum media were hydrolyzed in trifluoroacetic acid, and the monosaccharides were analyzed as O-methyloxime acetate derivatives (see Appendix B for details) (Murphy and Pennock, 1972; Neeser and Schweizer, 1983). For comparative purposes the monosaccharide dataset, excluding glucose and fructose, were normalized to 100 weight percent.
Test Results for Jacob Lawrence Paintings
Table 2 lists the quantitative stable amino acid test results for the Lawrence paint samples with those of several common proteinaceous and plant-gum-binding media included for reference (the reference data originated from inhouse tests and from published sources [Schilling et al., 1996]). Carbohydrate compositions for selected Lawrence samples and various reference materials are listed in Table 3.
Using the method of correlation coefficients, the quantitative stable amino acid composition for each sample was compared to those of the common binding media in order to find the closest match, as listed in Table 4 (Anderson, 1987); the same method was employed for identification of plant gums (see Table 3). Most samples correlated very closely either to glue, egg, casein, or gum arabic. In two paint samples, however, there were indications in the test data that two proteinaceous media were present. This situation may arise either because the artist intentionally mixed two binding media together in the paint, or because the paint sample was contaminated with medium from a second paint layer (this last situation occurs frequently in samples from egg tempera paintings that have ground layers mixed with glue). And so, for the two Lawrence samples, simple algebraic equations were used to find the most likely pair of proteinaceous media that gave the closest correlations to those in the paint samples (Schilling and Khanjian, 1996c). Thus, the red from Blind Beggars had a 0.99 correlation to a mixture (1:2) of casein and glue, whereas the green from Sedation had a 0.95 correlation to a mixture (1:3) of casein and glue.
In general, good agreement was evident between the analytical findings and the medium attributions in the museum archives. One notable exception was the detection of glue as the medium of Playing Card, which had been previously misidentified in the archives as plant gum. Another exception was Street to Mbari, which had been assessed visually as having a gouache medium.
GC-MS was useful for detecting components in the paints that were unrelated to the protein or plant gum media. Some samples revealed the presence of commercial paint additives, such as Struggle Series Number 11. The brown paint contained egg medium plus high amounts of glycerol, gallic acid, and rosin; this formulation is consistent with an artists’ tube color (Steele, 2000; Steele and Halpine, 1993; Schilling et al., 2000). Moreover, a few samples showed evidence of biodeterioration of the paint medium. For instance, oxalic acid (a common byproduct of microbial activity [Matteini, 1998]) was detected in the dark paint from The Checker Players. It may be that the relatively poor quality of the correlation for the medium in this paint to the reference materials (0.91 to casein, 0.74 to egg) may be due in part to the effects of biodeterioration.
TABLE 2 Stable Amino Acid Compositions of Jacob Lawrence Paint Samples
|
Painting |
Sample |
Concentration (mole percent) |
|||||||
|
ALA |
VAL |
ILE |
LEU |
GLY |
PRO |
HYP |
% Protein |
||
|
Blind Beggars |
Red |
13.5 |
10.8 |
7.8 |
13.7 |
28.8 |
20.9 |
4.6 |
4 |
|
Checker Players |
Paint & ground |
16.1 |
14.9 |
11.3 |
22.1 |
13.1 |
22.3 |
0.2 |
7 |
|
Daybreak |
Yellow |
22.2 |
16.3 |
12.3 |
21.9 |
17.2 |
10.2 |
0.0 |
4 |
|
Library |
Ochre |
20.6 |
18.3 |
16.4 |
25.7 |
16.3 |
2.7 |
0.0 |
2 |
|
|
Brown |
22.4 |
20.8 |
15.7 |
25.8 |
9.5 |
5.3 |
0.5 |
6 |
|
Magic Man |
Light black |
24.3 |
19.7 |
14.2 |
23.3 |
15.9 |
2.3 |
0.3 |
9 |
|
Men Exist |
Blue & ground |
21.5 |
16.3 |
13.5 |
24.0 |
16.4 |
8.3 |
0.0 |
3 |
|
Ordeal of Alice |
Brown |
27.2 |
19.5 |
14.1 |
21.7 |
13.3 |
3.7 |
0.4 |
2 |
|
Parade |
Blue |
20.5 |
16.1 |
12.9 |
22.7 |
21.3 |
6.4 |
0.1 |
9 |
|
Playing Card (Joker) |
Blue & paper |
14.9 |
3.5 |
2.1 |
4.3 |
51.6 |
13.1 |
10.4 |
12 |
|
Playing Card (King) |
Red & fibers |
14.2 |
2.7 |
1.6 |
3.2 |
55.8 |
12.3 |
10.2 |
18 |
|
|
Black & paper |
17.1 |
5.5 |
3.6 |
6.9 |
52.6 |
10.8 |
3.5 |
11 |
|
Sedation |
Green & paper |
14.0 |
13.1 |
9.0 |
16.8 |
25.6 |
21.0 |
0.5 |
5 |
|
Street to M’Bari |
Yellow & blue |
18.7 |
3.7 |
2.0 |
3.5 |
52.4 |
12.6 |
7.2 |
14 |
|
|
Blue |
17.8 |
3.1 |
1.7 |
3.2 |
51.2 |
13.2 |
9.7 |
13 |
|
Struggle Series #11 |
Brown |
22.2 |
16.2 |
12.4 |
24.1 |
14.4 |
10.8 |
0.0 |
12 |
|
Students and Books |
Yellow |
25.0 |
17.8 |
13.1 |
23.5 |
10.4 |
8.7 |
1.5 |
16 |
|
|
Brown & ground |
23.1 |
18.3 |
13.2 |
22.4 |
15.4 |
6.7 |
0.9 |
6 |
|
|
Yellow |
22.0 |
15.1 |
12.2 |
22.3 |
20.6 |
7.8 |
0.0 |
8 |
|
Vaudeville |
Blue |
24.0 |
17.0 |
12.9 |
26.1 |
17.9 |
2.1 |
0.0 |
6 |
|
|
Ground |
12.4 |
16.8 |
11.1 |
21.8 |
8.8 |
28.9 |
0.2 |
3 |
A STUDY OF WILLEM DE KOONING’S PAINTINGS FROM THE 1960S AND 1970S
Willem de Kooning was born in the Netherlands in 1904, and immigrated to the United States in 1926. He trained in guild and craft traditions of wood graining, gilding, marbleizing, lettering, and sign painting; he also spent time as a house painter and a commercial artist. These experiences gave him a thorough mastery of materials and a craftsman’s skills (Lake et al., 1999).
De Kooning routinely exploited unconventional materials for his pictures. Historical and anecdotal records report that he mixed house paint, safflower cooking oil, water, egg, and even mayonnaise into his artists’ paints to achieve the desired appearance and texture. During the course of creating a painting, he scraped the painted canvas at day’s end, and repeatedly reworked it; for this process to be successful, soft, slow-drying paints were required. Such paints were abundant in the 1940s and 1950s, when oil was the typical medium in house paints. Unfortunately for de Kooning, alkyd paint formulations became popular in the retail trade industry in the 1960s and 1970s; their fast-drying properties were incompatible with his chosen technique. In paintings from this period, sources document his use of Bellini Bocour artists’ tube colors, which contained heat-bodied linseed oil, to which he occasionally added safflower oil and water (Lake et al., 1999).
Although his methods and materials have been well documented, it was not clear what his actual practices were at specific times in his career. Moreover, there was concern that his unusual paint formulations could negatively affect the long-term stability of his paintings. The paintings executed during the 1960s and 1970s, in particular, are problematic for conservators, with passages that remain soft and sticky. Such paint surfaces are easily deformed when touched and they readily pick up surface dust. To learn more about de Kooning’s materials and techniques, a study was undertaken to analyze the binding media and pigments of a selection of his paintings from the period of 1960-1977 (Lake et al., 1999). Table 5 provides a complete list of the paintings that were sampled.
Chemistry of Oil Paints
The chemistry of oil paint is very complex, and even with modern analytical equipment, it is difficult to understand the precise details of the interactions between the polymerized oil media and pigments. Nonetheless, a substantial body of knowledge has been developed that sheds some light on the drying and subsequent aging of oil paints (van den Berg, 2002). Essentially, seed oils differ in terms of their fatty acid distribution on the triglyceride molecules. The so-called drying oils that are favored by artists (e.g., linseed, walnut, poppy seed) have a high proportion of multiple unsaturated fatty acids, whereas the semidrying and non-drying oils (such as castor, safflower, sunflower) do not. All of the aforemen-
TABLE 3 Gum Sugar Compositions of Jacob Lawrence Paint Samples and Plant Gum Standards, with Correlation Coefficient Data
|
Sample |
Weight % Gum Sugars |
Normalized Weight % of Gum Sugars |
||
|
Rhamnose |
Fucose |
Arabinose |
||
|
African Gold Miners: blue paint |
10.4 |
13.0 |
0.1 |
36.3 |
|
African Gold Miners: black paint |
6.3 |
10.2 |
0.1 |
29.1 |
|
Blind Beggars: blue paint |
3.8 |
17.0 |
0.0 |
35.5 |
|
New Jersey: red paint |
11.8 |
12.9 |
0.1 |
32.8 |
|
Painting the Bilges: blue paint |
1.9 |
15.4 |
0.0 |
37.7 |
|
Gum Arabic standard |
57.0 |
16.0 |
0.0 |
36.0 |
|
Cherry gum standard |
95.7 |
0.6 |
0.0 |
46.6 |
|
Gum tragacanth standard |
46.4 |
3.3 |
8.8 |
48.4 |
tioned seed oils contain approximately 10 percent by weight of glycerol, plus small amounts of saturated fatty acids (the two most important being hexadecanoic acid and octadecanoic acid, more commonly known as palmitic acid and stearic acid, respectively) (Mills, 1966).
As oil paints dry, unsaturated fatty acids react with oxygen to form a polymerized oil matrix; triglycerides and diglycerides provide additional cross-links to the polymerized oil matrix via their glycerol backbones. The saturated fatty acids, being less reactive, neither oxidize nor cross-link, and so remain as marker compounds in oil paint. During the drying and aging processes, chain scission products such as dicarboxylic fatty acids are formed in oil paint. The most important dicarboxylic fatty acid marker compound is nonanedioic acid (azelaic acid), but other straight-chain dicarboxylic fatty acids (that contain from two to ten carbon atoms) also form (Mills, 1966).
Other reactions also occur during aging that further alter the fatty acid distribution of oil paints. For example, free fatty acids are produced by hydrolysis of glycerides (e.g., glycerol esters of fatty acids). As hydrolysis progresses there is a reduction in residual triglycerides and diglycerides, and formation of free fatty acids. Complete hydrolysis of an oil paint would eventually yield three moles of free fatty acids per every mole of glycerol (van den Berg, 2002).
Reaction of the carboxylate groups of free fatty acids with pigments that contain coordinating metal cations (e.g., lead, copper, cobalt) produces metal soaps. Evidence has shown that aged oil paints are essentially ionomeric polymers of fatty acids coordinated with pigments (van den Berg et al., 1999).
Figure 1 illustrates the results from a Monte Carlo simulation of the hydrolysis of a model triglyceride, in which the extent of hydrolysis is estimated by the
|
|
Correlation Coefficient to Plant Gum Standards |
||||
|
Xylose |
Mannose |
Galactose |
Gum Arabic |
Cherry Gum |
Gum Tragacanth |
|
0.7 |
0.4 |
49.4 |
1.00 |
0.90 |
0.39 |
|
0.7 |
0.5 |
59.3 |
0.97 |
0.84 |
0.24 |
|
0.5 |
0.5 |
46.5 |
1.00 |
0.88 |
0.37 |
|
0.5 |
0.2 |
53.6 |
0.99 |
0.87 |
0.31 |
|
0.6 |
0.0 |
46.3 |
1.00 |
0.90 |
0.42 |
|
0.0 |
0.0 |
48.0 |
|
||
|
10.4 |
2.2 |
40.2 |
|||
|
27.5 |
0.0 |
12.1 |
|||
percent of free fatty acids. This figure shows that nontrivial amounts of triglycerides and diglycerides remain even after significant hydrolysis of the model compound. Undoubtedly this model is extremely simple, and does not account for differing rates of hydrolysis of the middle ester group on a glyceride compared to the end positions. Nonetheless, it does suggest that residual triglycerides and diglycerides may remain even in extremely hydrolyzed oil paints, which can additionally stabilize oil paint ionomeric polymers.
Additional alteration of the fatty acid composition of oil paints occurs by evaporation of free fatty acids. So-called ”ghost images” that develop on the glass next to framed and glazed oil paintings provide clear evidence of fatty acid evaporation (Williams, 1989). Thermogravimetric analysis indicated that palmitic acid evaporated approximately twice as rapidly as stearic acid or azelaic acid (Schilling et al., 1999). Oil paints made with lead white pigment produced no visible ghost image, which is consistent with the fact that lead pigments readily coordinate free fatty acids.
Analysis of Oil Paints
Two GC-MS procedures were employed to analyze the de Kooning paint samples. A quantitative procedure for fatty acid and glycerol analysis of food oils (Mason et al., 1964) was modified to work on oil paint samples (Schilling and Khanjian, 1996d). In this procedure FAMEs and isopropylidene glycerol (IPG, a volatile glycerol derivative) were produced quantitatively by overnight treatment with sodium methoxide in 2,2-dimethoxypropane, followed by addition of methanolic hydrochloric acid. The FAMEs and IPG were separated in a single
TABLE 4 Correlation Between Jacob Lawrence Paint Sample Data (Table 2) and Reference Material Data (Table 8)
|
Painting |
Paint Sample |
Correlation Coefficient |
|||
|
Collagen |
Egg Yolk |
Casein |
Other |
||
|
Blind Beggars |
Red |
0.82 |
0.29 |
0.33 |
0.99 (1 part glue, 2 parts casein) |
|
Checker Players |
Paint & ground |
−0.06 |
0.79 |
0.91 |
|
|
Daybreak |
Yellow |
0.06 |
0.98 |
0.39 |
|
|
Library |
Ochre |
0.13 |
0.90 |
0.19 |
0.99 (yolk & ultramarine, light aged) |
|
|
Brown |
−0.40 |
0.91 |
0.32 |
0.96 (yolk, aged at 80 deg C) |
|
Magic Man |
Light black |
−0.10 |
0.90 |
0.12 |
1.00 (Rowney cadmium red) |
|
Men Exist |
Blue & ground |
−0.03 |
0.98 |
0.36 |
|
|
Ordeal of Alice |
Brown |
−0.17 |
0.89 |
0.14 |
0.96 (yolk, light aged) |
|
Parade |
Blue |
0.19 |
0.90 |
0.21 |
0.96 (yolk & lead white, light aged) |
|
Playing Card (Joker) |
Blue & paper |
0.99 |
−0.06 |
−0.27 |
|
|
Playing Card (King) |
Red & fibers |
0.99 |
−0.07 |
−0.28 |
|
|
|
Black & paper |
0.96 |
0.13 |
−0.19 |
|
|
Sedation |
Green & paper |
0.61 |
0.55 |
0.56 |
0.95 (1 part glue, 3 parts casein) |
|
Street to M’Bari |
Yellow & blue |
0.98 |
0.02 |
−0.25 |
|
|
|
Blue |
0.99 |
−0.03 |
−0.28 |
|
|
Struggle Series #11 |
Brown |
−0.09 |
1.00 |
0.47 |
|
|
Students and Books |
Yellow |
−0.28 |
0.95 |
0.36 |
|
|
|
Brown & ground |
−0.08 |
0.95 |
0.26 |
|
|
|
Yellow |
0.20 |
0.92 |
0.25 |
0.96 (yolk, light aged) |
|
Vaudeville |
Blue |
−0.01 |
0.90 |
0.00 |
0.99 (Rowney cadmium red) |
|
|
Ground |
−0.21 |
0.55 |
1.00 |
|
TABLE 5 Willem de Kooning Paintings Analyzed in This Study
|
Door to the River, 1960, Whitney Museum of American Art, New York |
|
Spike’s Folly II, 1960, Robert and Jane Meyerhoff, Phoenix, MD |
|
Rosy-Fingered Dawn at Louse Point, 1963, Stedelijk Museum, Amsterdam |
|
Pastorale, 1963, private collection, New Orleans |
|
Woman, Sag Harbor, 1964, Hirshhorn Museum and Sculpture Garden, Washington, D.C. |
|
Woman, 1965, Hirshhorn Museum and Sculpture Garden, Washington, D.C. |
|
The Visit, 1966-67, Tate Gallery, London |
|
Two Figures in a Landscape, 1967, Stedelijk Museum, Amsterdam |
|
Amityville, 1971, private collection |
|
… Whose Name Was Writ in Water, 1975, Solomon R. Guggenheim Museum, New York |
|
Untitled I, 1977, Adriana and Robert Mnuchin |
|
Untitled V, 1977, Albright-Knox Art Gallery, Buffalo, New York |
chromatogram using an HP-INNOWAX capillary column. This procedure derivatizes all three forms of fatty acids in oil paints (free fatty acids, pigment soaps, and glycerides). The second quantitative procedure used hexamethylene disilazane with trichloromethyl silane catalyst (HMDS/TMCS) in pyridine to form trimethyl silyl esters of the free fatty acids and soaps, and separation of the derivatives on a DB-5MS column (Pierce, 1968).
In evaluating the quantitative test data several key parameters are calculated for identification and diagnostic purposes. For instance, the minimum content of oil in the paint sample can be approximated from the glycerol content, although the accuracy of this estimation is limited by loss of glycerol due to aging and solvent extraction. Second, the molar ratio of palmitic acid to stearic acid (P/S) is useful for identifying the type of oil present (Mills, 1966). Third, the extent of hydrolysis may be estimated by comparing the content of free azelaic acid and its soap to the total azelaic acid content. To probe the extent of alteration of the dried oil matrix, the two most diagnostic ratios are palmitic to glycerol (P/G), and the ratio of dicarboxylic fatty acids to glycerol (D/G, where D is the sum of all dicarboxylic fatty acids from C3 to C8 plus C10). Reduction in P/G from its original value in the fresh oil is caused by loss of palmitic acid due to evaporation or migration into the canvas and ground layer. Photo-oxidative reactions are responsible for increases in D/G (Schilling and Khanjian, 1996d; Schilling et al., 1997).
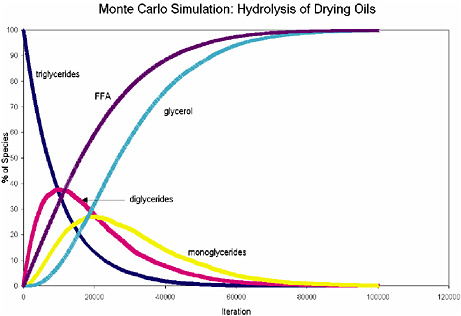
FIGURE 1 Monte Carlo simulation of the hydrolysis of a model triglyceride.
NOTE: 10,000 triglyceride molecules and 100,000 iterations were used in this simulation, which was run using an Excel macro program.
Test Results for de Kooning Paintings
Table 6 lists the analytical results for the de Kooning paint samples. From an examination of the test results a few generalizations can be made. The medium in the paints with the highest P/S ratios was identified either as poppy oil, or poppy oil mixed with linseed or castor oil. These paints tended to have the lowest values for oil content, extent of hydrolysis, and content of dicarboxylic fatty acid degradation products (as measured by D/G).
In contrast, the medium in the paints with the lowest P/S ratios was identified either as linseed oil, or linseed oil mixed with castor oil; it is likely that de Kooning used unmixed tube colors in these paints. They had the highest values for oil content, extent of hydrolysis, and D/G ratios. Paints with intermediate P/S ratios contained linseed oil and safflower oil mixtures, and differed little from the high P/S paints in terms of hydrolysis and D/G.
Void spaces inside paint samples indicate that de Kooning mixed water into them, and the test results show that this procedure had no deleterious effect on the extent of hydrolysis. Another finding was that the sticky paints tended to contain cadmium pigments or synthetic organic dyestuffs, whereas no clear relationship to the drying rate of the oil medium was apparent. The sticky paints exhibited the highest degree of hydrolysis, as measured by the free fatty acid
TABLE 6 Test Results for Willem de Kooning Paint Samples
|
Painting, Date |
Sample Description |
P/S |
P/G |
D/G |
% Hydrolyzed |
% Oil |
Oil(s) |
|
Door…, 1960 |
Pink, voids, matte |
3.5 |
0.26 |
0.32 |
32 |
25 |
Poppy/linseed |
|
|
White, voids, matte, brittle |
4.1 |
0.26 |
0.36 |
19 |
29 |
Poppy |
|
Spike’s…, 1960 |
Yellow, soft, matte |
0.9 |
0.04 |
0.66 |
40 |
52 |
Castor |
|
|
Blue, brittle, matte |
3.7 |
0.24 |
0.34 |
12 |
23 |
Poppy/castor |
|
Pastorale, 1963 |
Light blue, voids, brittle |
4.8 |
0.2 |
0.36 |
32 |
48 |
Poppy |
|
Rosy…, 1963 |
Bright yellow, soft |
1.9 |
0.07 |
0.57 |
48 |
50 |
Castor/linseed |
|
Woman…, 1964 |
Pink, soft |
3.4 |
0.1 |
0.23 |
29 |
18 |
Poppy/linseed |
|
Woman, 1965 |
Amber drip |
2.6 |
0.14 |
0.65 |
17 |
52 |
Linseed/safflower |
|
Visit, 1966 |
Orange, soft |
2.6 |
0.12 |
0.5 |
33 |
24 |
Linseed/safflower |
|
|
Wrinkled gray-green, soft |
2.6 |
0.15 |
0.23 |
34 |
31 |
Linseed/safflower |
|
Two…, 1967 |
Orange, soft, soft |
2.1 |
0.17 |
0.32 |
29 |
31 |
Linseed/safflower |
|
|
Pink, wrinkled, soft |
2.7 |
0.16 |
0.3 |
32 |
33 |
Safflower |
|
|
Amber gel, wrinkled flesh |
2.8 |
0.09 |
0.35 |
22 |
59 |
Safflower |
|
Amityville, 1971 |
Yellow, wrinkled, soft |
3.9 |
0.21 |
0.28 |
28 |
23 |
Poppy/linseed |
|
|
White, soft |
3.7 |
0.24 |
0.25 |
15 |
38 |
Poppy/linseed |
|
Whose…, 1975 |
Amber drip, sticky |
2.6 |
0.09 |
0.28 |
25 |
29 |
Linseed/safflower |
|
|
Red, soft, sticky |
2.7 |
0.14 |
0.26 |
26 |
67 |
Safflower |
|
|
Maroon, soft, stringy |
3.8 |
0.13 |
0.34 |
26 |
24 |
Poppy/castor |
|
Untitled I, 1977 |
White, wrinkled |
1.3 |
0.05 |
0.36 |
23 |
59 |
Linseed |
|
|
Pink-white, wrinkled |
1.4 |
0.04 |
0.34 |
37 |
67 |
Linseed |
|
Untitled V, 1977 |
Gray, wrinkled, voids |
1.8 |
0.07 |
0.31 |
17 |
40 |
Linseed |
|
|
Black, wrinkled |
0.91 |
0.03 |
0.72 |
66 |
48 |
Castor |
|
|
Dark blue, wrinkled, soft |
2.0 |
0.07 |
0.48 |
37 |
59 |
Linseed |
content. In conclusion, the results supported the anecdotal evidence that de Kooning did occasionally add semidrying oils to his paints, which would have retarded their drying rate.
CONCLUSIONS
Quantitative GC-MS is an important analytical technique for characterizing natural products that have been used by artists as organic binding media. In the study of modern paintings the technique provides valuable information that enhances our understanding of artists’ materials and techniques, permits changes in material composition to be monitored, and contributes to the development of appropriate conservation strategies.
ACKNOWLEDGEMENTS
The following colleagues from the Getty Conservation Institute made invaluable contributions to the analytical research presented in this paper: Herant Khanjian, Joy Keeney, David Carson, Narayan Khandekar, Andrew Parker, and Luiz Souza. Jim Druzik was the creative influence behind the Monte Carlo simulation study. I am especially grateful to Dusan Stulik, who developed the organic binding medium research project and who was its director for many years. Our principal collaborator in the Jacob Lawrence study was Elizabeth Steele, paintings conservator at the Phillips Collection, whose extensive knowledge of Lawrence’s technique and materials greatly enhanced the interpretation of our research. I am also thankful for the enthusiastic support of Peter Nesbett, director of the Jacob Lawrence Catalogue Raisonné project, and Michelle DuBois, associate director. In the Willem de Kooning study we collaborated with Susan Lake, chief conservator at the Hirshhorn Museum and Sculpture Garden, who contributed in countless ways to the success of the research. Suzanne Quillen-Lomax, organic chemist at the National Gallery of Art, Washington, D.C., studied a number of de Kooning paintings and was an important partner in the collaboration.
REFERENCES
Anderson, R. L. 1987. In Practical Statistics for Analytical Chemists. New York: Van Nostrand Reinhold.
Columbini, M. P., R. Fuoco, A. Giacomelli, and B. Muscatello. 1998. Characterization of proteinaceous binders in wall painting samples by microwave-assisted acid hydrolysis and GC-MS determination of amino acids. Studies in Conservation 43:33-41.
Halpine, S. 1992. Amino acid analysis of proteinaceous media from Cosimo Tura’s The Annunciation with Saint Francis and Saint Louis of Toulouse. Studies in Conservation 37:22-38.
Kühn, H. 1986. In Conservation and Restoration of Works of Art and Antiquities, vol. 1, pp. 157-167. London: Butterworths.
Lake, S., S. Lomax, and M. Schilling. 1999. A technical investigation of Willem de Kooning’s paintings from the 1960s and 1970s. In ICOM Committee for Conservation Preprints, 12th Triennial Meeting, Lyon, France 29 August-3 September 1999, ed. J. Bridgland, pp. 381-385. London: James and James.
Mason, M. E., M. E. Eager, and G. R. Waller. 1964. A procedure for the simultaneous quantitative determination of glycerol and fatty acid contents of fats and oils. Analytical Chemistry 36(3):597-590.
Matteini, M. 1998. Different integrated analytical methods for the study of the pictorial techniques in the Vasari and Zuccari wall paintings of Florence Cathedral: Comparison and discussion. Science and Technology for Cultural Heritage 7(1):83-94.
Mayer, R. 1940. In The Artists Handbook of Materials and Techniques, p. 223. New York: Viking Press.
Mills, J. S. 1966. The gas chromatographic examination of paint media. Part I. Fatty acid composition and identification of dried oil films. Studies in Conservation 11:92-107.
Murphy, D., and C. A. Pennock. 1972. Gas chromatographic measurement of blood and urine glucose and other monosaccharides. Clinica Chimica Acta 42:67-75.
Neeser, J. R., and T. F. Schweizer. 1983. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugar (as O-methyloxime acetates). Analytical Biochemistry 142:58-67.
Pierce, A. E. 1968. In Silylation of Organic Compounds, pp. 160-162. Rockford, Ill.: Pierce Chemical Co.
Ronca, F. 1994. Protein determination in polychromed stone sculptures, stuccoes and gesso grounds. Studies in Conservation 39:107-120.
Schilling, M., and H. Khanjian. 1996a. Gas chromatographic investigations of organic materials in art objects: New insights from a traditional technique. In Innovation et Technologie au Service du Patrimoine de l’Humanite, pp. 137-143. Paris: UNESCO/Admitech.
Schilling, M., and H. Khanjian. 1996b. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. II. Effects of pigments and accelerated aging on the identification of proteinaceous binding media. Journal of the American Institute of Conservation 35:123-144.
Schilling, M., and H. Khanjian. 1996c. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. III. Identification of proteinaceous binding media by interpretation of amino acid composition data. In ICOM Committee for Conservation Preprints, 11th Triennial Meeting, Edinburgh, Scotland 1-6 September 1996, ed. J. Bridgland, pp. 220-227. London: James and James.
Schilling, M., and H. Khanjian. 1996d. Gas chromatographic determination of the fatty acid and glycerol content of lipids. I. The effects of pigments and aging on the composition of oil paints. In ICOM Committee for Conservation Preprints, 11th Triennial Meeting, Edinburgh, Scotland 1-6 September 1996, ed. J. Bridgland, pp. 220-227. London: James and James.
Schilling, M., D. Carson, and H. Khanjian. 1999. Gas chromatographic determination of the fatty acid and glycerol content of lipids. IV. Evaporation of fatty acids and the formation of ghost images by framed oil paintings. In ICOM Committee for Conservation Preprints, 12th Triennial Meeting, Lyon, France 29 August-3 September 1999, ed. J. Bridgland, pp. 242-247. London: James and James.
Schilling, M., H. Khanjian, and D. Carson. 1997. Fatty acid and glycerol content of lipids; effects of ageing and solvent extraction on the composition of oil paints. Techné 5:71-78.
Schilling, M., H. Khanjian, and L. Souza. 1996. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. I. Composition of proteins associated with objects of art and monuments. Journal of the American Institute of Conservation 35:45-59.
Schilling, M., N. Khandekar, J. Keeney, and H. Khanjian. 2000. Identification of binding media and pigments in the paintings of Jacob Lawrence . In Over the Line: The Art and Life of Jacob Lawrence, eds. P. T. Nesbett and M. DuBois, pp. 266-269. Seattle: University of Washington Press.
Simek, P., A. Heydová, and A. Jegorov. 1994. High resolution capillary gas chromatography and gas chromatography-mass spectrometry of protein and non-protein amino acids, amino alcohols, and hydroxycarboxylic acids as their tert-butyldimethylsilyl derivatives. Journal of High Resolution Chromatography 17:145-152.
Steele, E. 2000. The materials and techniques of Jacob Lawrence. In Over the Line: The Art and Life of Jacob Lawrence, eds. P. T. Nesbett and M. DuBois, pp. 247-265. Seattle: University of Washington Press.
Steele, E., and S. M. Halpine. 1993. Precision and spontaneity: Jacob Lawrence’s materials and techniques. In Jacob Lawrence: The Migration Series. Washington, D.C.: Rappahannock Press in association with the Phillips Collection.
Van den Berg, J. D. J. 2002. In Analytical Chemical Studies on Traditional Linseed Oil Paints. Ph.D. thesis, pp. 45-52. University of Amsterdam.
Van den Berg, J. D. J., K. J. van den Berg, and J. Boon. 1999. Chemical change in curing and aging oil paints. In ICOM Committee for Conservation Preprints, 12th Triennial Meeting, Lyon, France 29 August-3 September 1999, ed. J. Bridgland, pp. 248-253. London: James and James.
Williams, S. R. 1989. Blooms, blushes, transferred images and mouldy surfaces: What are these distracting accretions on art works? In Proceedings of the Fourteenth Annual IIC-CG Conference, pp. 65-84. Ottawa: IIC-Canadian Group.
APPENDIX A
PROCEDURE FOR QUANTITATIVE GC-MS ANALYSIS OF AMINO ACIDS, FATTY ACIDS, AND GLYCEROL AS (TERT -BUTYL-DIMETHYLSILYL) DERIVATIVES
All analytical standards were obtained from Aldrich Chemical Company. Weigh paint sample on an ultra-microbalance and then transfer to a 0.1 ml conical vial. Add norleucine internal standard to give a final concentration of 20 ppm in the final injection volume. Add 100 µl of 6.0N hydrochloric acid (Pierce, sequanal-grade) to the sample vial and close the vial with a screw-top lid and PTFE septum. Heat the vial at 105°C for 24 hours in an oven. Remove vial from oven, let stand until cool, and centrifuge.
Evaporate the contents to dryness under a stream of nitrogen gas while warming the vial to 60°C. Add 40 µl of HPLC-grade water (VWR Scientific), replace lid, stir on a vortex mixer, centrifuge, and evaporate the contents to dryness. Add 40 µl of absolute ethanol (Spectrum Chemical), replace lid, stir with a vortex mixer, centrifuge, and evaporate the contents to dryness.
Prepare a solution of 40 mg of pyridine hydrochloride (Aldrich) to 1 ml of silylation-grade pyridine (Pierce Chemical). The silylating reagent consists of 300 µl of 99 percent MTBSTFA/1 percent TBDMCS mixture (Pierce Chemical) in 700 µl of pyridine hydrochloride solution.
Add silylating reagent to the vial and replace lid (Note: Use 1 µl of reagent per 2 µg of sample for typical paint samples; use 300 µl per 50 to 100 µg of pure
proteinaceous reference materials. Use a minimum of 20 µl of reagent if an oven is used for heating and 50 µl if a heating block is used).
Stir with a vortex mixer, warm the vial at 60°C for 30 minutes on a hotplate, and heat in an oven at 105°C for five hours. Remove vial from heat, let stand until cool, centrifuge, and transfer solution to an injection vial.
GC-MS Conditions for 30 M × 0.25 mm × 1 µm DB-5MS Column: Helium carrier set to a linear velocity of 45 cm/sec; splitless injector at 300°C with a 60 sec purge off time; MS transfer line set to 300°C. GC oven temperature program: 80°C for one minute; 75°C/m to 180°C; 10°C/m to 320°C; isothermal for three minutes; solvent delay of seven minutes. MSD source temperature is approximately 200°C. Figure 2 shows the GC-MS result for a standard mixture of amino acids, fatty acids, and glycerol.
Calibration parameters: See Table 7 for the list of quantitation ions for the TBDMS derivatives. Using a quadratic curve fit forcing through the origin gives correlation coefficients of 0.995 or better for most analytes over the calibration range of 2 to 50 ppm. Stable amino acid compositions for various reference materials are listed in Table 8.
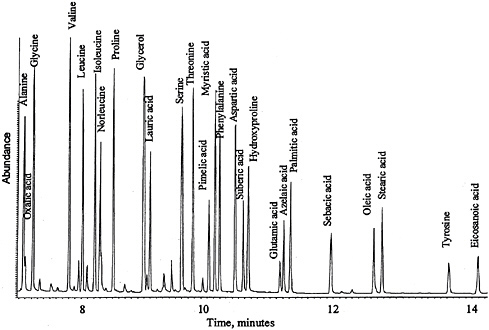
FIGURE 2 GC-MS analysis of (tert-butyl dimethylsilyl) derivatives of amino acid, fatty acid, and glycerol standards.
TABLE 7 Quantitation Ions for TBDMS Derivatives
|
Analyte |
m/z |
Analyte |
m/z |
|
Norleucine |
200.1 |
Glycerol |
189.1 |
|
Alanine |
260.1 |
Decanoic acid |
229.1 |
|
Glycine |
246.1 |
Lauric acid |
257.1 |
|
Valine |
186.1 |
Myristic acid |
285.1 |
|
Leucine |
200.1 |
Pentadecanoic acid |
299.2 |
|
Isoleucine |
200.1 |
Palmitic acid |
313.2 |
|
Proline |
184.1 |
Heptadecanoic acid |
327.2 |
|
Methionine |
218.1 |
Oleic acid |
339.2 |
|
Serine |
390.2 |
Stearic acid |
341.2 |
|
Threonine |
303.2 |
Nonadecanoic acid |
355.2 |
|
Phenylalanine |
234.1 |
Eicosanoic acid |
369.2 |
|
Aspartic acid |
302.2 |
Oxalic acid |
261.1 |
|
Hydroxyproline |
416.2 |
Malonic acid |
115.1 |
|
Glutamic acid |
432.2 |
Succinic acid |
289.1 |
|
Lysine |
488.2 |
Glutaric acid |
303.2 |
|
Arginine |
460.2 |
Adipic acid |
317.2 |
|
Histidine |
196.1 |
Pimelic acid |
331.2 |
|
Tyrosine |
302.2 |
Subaric acid |
345.2 |
|
|
Azelaic acid |
359.2 |
|
|
Sebacic acid |
373.2 |
||
APPENDIX B
PROCEDURE FOR QUANTITATIVE GC-MS ANALYSIS OF SUGARS IN PLANT GUMS AS O-METHYLOXIME ACETATE DERIVATIVES
All analytical standards were obtained from Aldrich Chemical Company. Weigh sample on an ultra-microbalance, transfer to a conical reaction vial, and add allose internal standard to give a final concentration of 20 ppm in the injection volume. Add 100 µl of 1.2N trifluoroacetic acid (Pierce Chemical), purge vial with nitrogen for 30 seconds, and cap. Heat the vial for one hour at 125°C, remove from heat, let stand until cool, and centrifuge.
Transfer contents to a 2 ml autosampler vial, rinse conical vial with 40 µl of water, and combine in the 2 ml vial. Evaporate the contents to dryness using a nitrogen stream while warming the vial to 50°C. Rinse with 40 µl of absolute ethanol (Spectrum Chemical), and evaporate to dryness.
Add 80 µl of a 1 percent solution of methoxyamine hydrochloride (Sigma) in pyridine (Pierce Chemical), replace cap, and heat for 10 minutes at 70°C. Remove from heat, let stand until cool, and add 40 µl of acetic anhydride (Supelco). Replace the cap, and heat the vial for 10 minutes at 70°C. Remove vial from heat, let stand until cool, and centrifuge.
TABLE 8 Stable Amino Acid Compositions of Various Reference Materials
Evaporate the contents using a nitrogen stream while warming the vial to 50°C. Reconstitute the contents in 100 µl chloroform (Spectrum Chemical), and transfer into a clean 2 ml vial. Rinse the first vial with 100 µl chloroform, and combine. Evaporate chloroform to about 50 µl under a nitrogen stream while warming the vial to 50°C. Transfer the chloroform to a conical glass insert. Evaporate to dryness and reconstitute to desired final volume. Reconstitute the contents in chloroform (use an amount equivalent to 1 µg of gum per 5 µl), and inject into GC-MS.
GC-MS Conditions for a 15 M × 0.25 mm × 0.25 µm DB-WAX Capillary Column: Helium carrier set to a linear velocity of 60 cm/sec; splitless injector at 240°C with a 60 sec purge off time; MS transfer line set to 240°C. GC oven temperature program: 105°C for one minute; 30°C/m to 180°C; 5°C/m to 240°C; isothermal for two minutes; solvent delay of seven minutes. MSD source temperature is approximately 200°C. Figure 3 shows the GC-MS result for a standard mixture of carbohydrates.
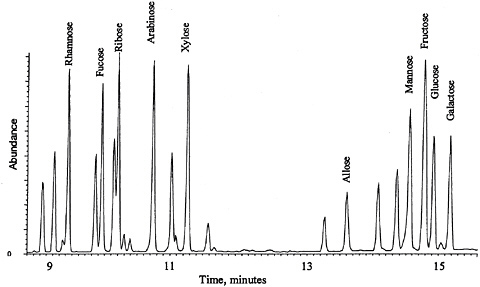
FIGURE 3 GC-MS analysis of O-methyloxime acetate derivatives of carbohydrate standards.
Calibration parameters: See Table 9 for the list of quantitation ions for the MOA derivatives. Using a linear curve fit forcing through the origin gives correlation coefficients of 0.995 or better for most analytes over the calibration range of 2 to 50 ppm.
TABLE 9 Quantitation Ions for MOA Derivatives
|
Analyte |
m/z |
Analyte |
m/z |
|
Allose |
115.1 |
Xylose |
115.1 |
|
Rhamnose |
129.2 |
Mannose |
131.2 |
|
Fucose |
129.2 |
Fructose |
203.2 |
|
Ribose |
115.1 |
Glucose |
89.2 |
|
Arabinose |
115.1 |
Galactose |
131.2 |




















