FRANK ALDEN BOVEY
June 4, 1918–January 19, 2003
BY FREDERIC C. SCHILLING AND ALAN E. TONELLI
THE NAME OF Frank Alden Bovey will always be associated with nuclear magnetic resonance spectroscopy of polymers. Frank published some of the earliest scientific papers demonstrating the use of NMR to reveal detailed structural information of synthetic and natural polymers. Through his textbooks he introduced several generations of scientists to the power of NMR spectroscopy. At the time of his retirement Frank had published nearly 200 original publications and authored or coauthored nine books. Frank was an associate editor of Macromolecules (the preeminent research journal in polymer science) for more than 25 years, beginning with its first publication in 1968. The significance of his research is reflected in the many awards bestowed upon him, including the Polymer Chemistry Award (1969), the Nichols Medal (1978), and the Applied Polymer Science Award (1983), all from the American Chemical Society; the High-Polymer Physics Award (1974) of the American Physical Society; the Carothers Award (1991); and the Silver Medal (1991) of the Japanese Polymer Society. He was elected to the National Academy of Sciences in 1975.
Frank is remembered foremost as a modest gentleman of great intellect. His personal style was rather formal, from his Brooks Brothers clothing to his interactions with col-
leagues. However, he was always open to new ideas, treated everyone with respect, and appreciated a good sense of humor.
EARLY LIFE, EDUCATION, CAREER, FAMILY, AND PERSONAL REFLECTIONS (BY FREDERIC C. SCHILLING)
Frank Alden Bovey was born on June 4, 1918, in Minneapolis. His family traces its roots back to John Alden and Priscilla Mullins, who came to America aboard the Mayflower in 1620. Longfellow made famous this same Priscilla in his poem “The Courtship of Miles Standish.” For seven generations the Alden line came down only through women, until 1856 when Hannah Caroline Brooks married Charles Argalis Bovey (Frank Bovey’s grandfather).
Charles’s father had come to America as a young man from the little town of Bovey Tracey in Devonshire, England. In 1869 Charles Bovey moved his family (six children) to Minneapolis. He played a major role in the commercial and civic development of the city, attempting to bring the cultured lifestyle of New York and Boston to this Midwestern community. In Minneapolis he established one of the first lumber companies in the area. Later he helped develop a small townsite in the Western Mesabi iron range, which had been recently discovered. Against the many protestations of Charles, the inhabitants insisted on naming the town Bovey. Charles, being a teetotaler and a very religious man, objected to the use of the Bovey name for a booming mining town with many saloons.
The family of Charles Bovey and descendants remained in Minneapolis. John Bovey (one of Charles’s sons and Frank’s father) also worked in the lumber business and early in the 1900s married Margaret Jackson. They and their three children (John, Frank, and Eugenia) lived in a beautiful brick
colonial home that remains today and is used as a place to host catered parties. During Frank’s childhood years, the family spent the winter months in Minneapolis. However, when the hot summer months arrived, the Bovey families all moved out to Lake Minnetonka, where his father, uncles, and grandfather commuted to work by streetcar, following the custom of the wealthy and influential families of Boston and New York.1
The family of John Bovey suffered financial misfortunes common to many in the 1920s and 1930s. The lumber firm was lost in bankruptcy. Frank’s father struggled but managed a transition from lumber to investment banker, and Frank’s mother, Margaret, against all feminine precedent, conducted a successful insurance business. As a result the family was able to maintain a semblance of the lifestyle the Bovey families had come to know.
As a young boy Frank showed an early interest in art, in particular painting. As a child he drew hundreds of cartoons. Frank and his older brother, John, loved to read, while their sister, Genie, became the athlete and social butterfly of the family. The children inherited from their parents and grandparents the Bostonian disposition toward literature and reflection. They “went East to college,” the boys going to Harvard and their sister to Smith.
At Harvard, Frank and John shared a Model T Ford, which they drove back and forth between Cambridge and Minneapolis. This experience probably began Frank’s lifelong love affair with cars. Later in life he always had two or three cars: a Cadillac, his car of choice for the family and for long trips, and a small sports car just for fun, from the red 1957 MGA roadster to the red Nissan 300Z.2
Knowing he had to make a living, Frank chose to study chemistry at Harvard, although he would have preferred English literature. While he chose to make his professional
life that of a scientist, his interest and study of the arts continued throughout his life.
While studying at Harvard, Frank met Shirley Elfman, and after graduation in 1940 they married and returned to Minneapolis. In 1942 Frank moved his family to Louisville, Kentucky, and began work at the National Synthetic Rubber Corp., where he helped to develop a formula for synthetic rubber, an important contribution to the war effort. In 1945 the Boveys moved to St. Paul, Minnesota, where Frank entered graduate school at the University of Minnesota. In 1948 he received his Ph.D. in physical chemistry with Professor Izaak M. Kolthoff. Upon graduation Frank joined the research group at the 3M company. During his years at 3M and later at Bell Laboratories, he enjoyed phenomenal success in his research endeavors and was always a leader in the field of polymer science.
In the early 1950s Frank moved his family to White Bear Lake, a suburb of St. Paul, where their 20-acre homesite included an apple orchard, not unlike the summer home on Lake Minnetonka where Frank enjoyed his childhood summers. Whenever possible, Frank and Shirley kept with the family tradition of including European travel as part of a proper education. For example, they took their teenage children to England where Frank was presenting a series of lectures. For six weeks the children studied their lessons and sent their assignments back to the United States, meanwhile absorbing the culture of the Bovey ancestral homeland.
The homes of Frank Bovey always included a book-lined den for his vast book collection, as well as magazines and newspapers from subscriptions to Eastern publications like the New Yorker and the New York Times. This collection and frequent visits to the Minneapolis Symphony helped his children grow up with a strong sense of history and the
arts. As was the Bovey custom, Frank and Shirley sent their children (Margaret, Peter, and Victoria) to college in the East.
Frank had many interests beyond the polymer chemistry that filled his professional life. He had a passion for photography, astronomy, music, art, and architecture. His camera recorded events throughout his life, and his collection of slides provided him much joy in his later years. Frank’s interest in astronomy provided an opportunity to introduce his children to the world of science, whether it was a lesson in using the telescope or pouring over prints from the Palomar 200-inch telescope. He also obtained his pilot’s license and loved the study of navigation (precomputer age). In music his taste was classical, particularly Mozart, Bach, Beethoven, and Brahms. Frank traveled extensively, principally in Europe.
In 1962 Frank moved his family to New Jersey, where he lived and worked at Bell Laboratories until his retirement. Even at age 75 he could be found in his office nearly every day, reading and studying what the younger generations of scientists were discovering. In his final years Frank moved to Amherst, Massachusetts, where he lived with his daughter Margaret. There he continued to spend his time enjoying literature, music, and watching his favorite movies. He passed away on January 19, 2003.
For more than 20 years Frank Bovey was my mentor, teacher, colleague, and friend. He taught me a great deal not only about polymer chemistry and NMR spectroscopy but also, by example, about the patience and determination required of a successful scientist. As a prolific and accomplished author Frank demonstrated that accuracy, brevity, and clarity are the hallmarks of successful publications.
Frank willingly shared his interests in areas outside of science. He loved to discuss architecture, particularly, of
early American homes like Mount Vernon, Monticello, and the Biltmore Estate. Travel was something Frank enjoyed heartily, both in the doing and in the telling. From his descriptions, I think he enjoyed visiting Prague more than any other city. He always amazed me with his depth of knowledge in so many fields, from art and history to automobiles and astronomy. The one exception was sports, where he clearly felt little need to venture. Frank appreciated good humor especially if witty or wry in nature. I was pleasantly surprised when I discovered his love of Monty Python’s antics.
Frank loved to sample the food at fine restaurants, be it in Summit, New Jersey, or Rome, Italy. He had no fear of red meat, and we shared a fondness for dessert. His ability to discuss almost any issue or subject with ease made him an excellent dinner host for groups large or small.
I would like to thank the children of Frank Bovey for their contributions and assistance in the preparation of his memoir.
SCIENTIFIC CAREER (BY ALAN E. TONELLI)
The professional career of Frank Bovey began in 1943 when he joined the National Synthetic Rubber Corp. This was a U.S. government-sponsored consortium of companies charged by the U.S. Synthetic Rubber Program (USSRP) with developing a general purpose synthetic rubber to replace natural rubber from Southeast Asia, which came under Japanese control at the beginning of Word War II. In collaboration with a network of government and academic researchers, the USSRP successfully developed and manufactured in record time enough synthetic rubber to meet the needs of the United States and its allies during World War II.
Early in the USSRP it was decided to produce GR-S rubber, a styrene(25%)-butadiene(75%) copolymer that could be vulcanized, as the most likely synthetic replacement for natural rubber. Professor Izaak Maurits (“I. M.”) Kolthoff of the University of Minnesota, often called the father of modern analytical chemistry, was an academic collaborator in the USSRP. Frank Bovey, while at the National Synthetic Rubber Corp., joined the Kolthoff research group to study various aspects of the emulsion polymerization and copolymerization of styrene,3 which later resulted in his coeditorship of a book on emulsion polymerization.4 This collaboration culminated in 1948, when Frank received his Ph.D. in physical chemistry for his work with Prof. Kolthoff.
After receiving his Ph.D., Frank joined the 3M company, where he remained for 14 very productive years and became head of the 3M Polymer Research Department. Here Frank studied the synthesis and characterization of fluoropolymers; the electron irradiation of polymers, about which he wrote a book5; biopolymers, including the synthesis of the polysaccharide dextran and the denaturation of the protein enzyme pepsin; and the 1H-NMR spectroscopy of polymers. The NMR work was conducted with his able 3M collaborator George Tiers, who also collaborated with future Nobelist Paul C. Lauterbur (NMR imaging/MRI). The seminal studies of polymers by 1H-NMR spectroscopy begun by Frank Bovey at 3M resulted in his becoming one of the earliest pioneers and leading researchers in this field, a role he continued during the remainder of his career of nearly 40 years. At 3M Frank Bovey successfully employed 1H-NMR techniques to characterize the motions, configurations, and polymerizations of synthetic polymers. Most notable were his studies of the stereosequences of vinyl polymers and introduction of the m (meso) and r (racemic) repeat unit diad and i = mm (isotactic), s = rr (syndiotactic), and h = mr or mr
(heterotactic) repeat unit triad terminology to describe the relative attachments of side-chains to polymer backbones,6 and which is still used to describe their stereochemistry. Also in this paper 1H-NMR was used for the first time to unambiguously determine the absolute stereochemistry of stereoregular vinyl polymers. This was particularly noteworthy, because X-ray diffraction often cannot distinguish the stereochemical nature of crystalline polymers.
Of course, the stereochemistry of vinyl polymers is critically important to their physical properties, and very early on Frank recognized this important structure-property relationship. This was later evidenced in a humorous incident on the occasion of his receipt of the High-Polymer Physics Prize of the American Physical Society in Philadelphia at the March 1974 meeting. In his award address Frank was making the point that the stereochemistry of vinyl polymers is key to understanding their properties. He did this in a manner that was very unusual and certainly uncharacteristic of his somewhat formal, intensely private, and shy nature. Frank noted that the regular and irregular attachment of vinyl polymer side-chains in isotactic and atactic vinyl polymers leads to strong crystalline and weak amorphous materials, respectively. He then displayed color slides of two well-known women: Twiggy, the very slender, almost emaciated, boyish British model and pop-culture icon of that period, and Raquel Welch, the voluptuous American model and movie actress. This was followed by “need I say more,” delivered in a manner that let the audience know Frank realized this humorous, slightly irreverent, but very effective analogy was very “out of character” for him.
On a personal note, I was a graduate student at Stanford working in Paul Flory’s research group. It was Paul’s custom to have members of his research group present informal lunchtime seminars. Sometime in late 1966, when my
turn to present such a seminar arrived, I decided to describe the efforts of Bovey and Tiers to use 1H-NMR to elucidate the microstructures of vinyl polymers. This choice was a personal one, because my Ph.D. thesis research dealt with the conformational properties (sizes and shapes) of poly(lactic acids) and vinyl polymers and establishing the sensitivities of their conformations to their stereochemistries. Flory had a general interest in this area, and in fact a few years later published two papers dealing with the configurational sensitivities of the conformations and 1H-NMR spectra of vinyl polymers. Upon completion of my Ph.D. in 1968 I eagerly accepted a position in the Polymer Chemistry Research Department at Bell Laboratories, which was headed by Frank Bovey. Thus began my fortunate 23-year association with Frank Bovey.
Also while at 3M, Frank and Charles Johnson7 and simultaneously Waugh and Fessenden8 developed quantitative estimates of the effects produced by magnetically anisotropic groups, like phenyl rings, on 1H-NMR spectra. The Johnson-Bovey Tables of ring-current NMR shieldings have been widely used to determine the conformations of polymers, both synthetic and biological. For example, the Johnson-Bovey ring-currents, as modified by Giessner-Prettre and Pullman9 for the bases in DNA, were utilized to determine the detailed double-helical conformations of several DNA fragments in aqueous solution before and after binding antibiotics like Actinomycin D, containing aromatic rings. For example, the location and orientation of Actinomycin D bound to these deoxyribonuleotides were derived from a comparison of the 1H-NMR spectra observed before and after binding with those calculated using the Johnson-Bovey ring-current effects. This information is critically important in the design of new and more effective drugs.
In 1962 Frank Bovey moved to AT&T-Bell Laboratories,
where he remained for more than 30 years. During much of this time, he was head of the Polymer Chemistry Research Department, and more importantly he wrote nine books covering various aspects of NMR spectroscopy and its application to polymers. Not only did Frank Bovey pioneer this important field in his research but with his books he also introduced and educated generations of polymer scientists to the great utility of elucidating the microstructures, conformations, organization, and mobilities of polymers, through their observation with NMR, in order to gain fundamental understanding of their physical properties. Although he never held a permanent academic position, Frank Bovey successfully taught countless students, professors, and industrial scientists, principally through his books. The underlying reason for his success lay in Frank’s twofold expertise in NMR spectroscopy and in polymer science, which is apparent when reading his books and which makes them both very relevant and readily understandable to researchers studying synthetic and biological macromolecules.
During his tenure of more than 30 years at Bell Laboratories, Frank Bovey conducted significant research into synthetic and biological polymers employing NMR spectroscopy, but he also conducted important studies using ORD, CD, fluorescence, and electronic spectroscopies. Frank used solution NMR to determine the microstructures of a wealth of different polymers and solid-state NMR to learn about the organization and mobilities of solid polymers. As new NMR techniques were developed Frank Bovey was among the first to adapt and apply them successfully to polymers. In the early 1970s high-magnetic-field superconducting NMR spectrometers were introduced and Frank Bovey made sure that Bell Laboratories obtained one of the first of these instruments. As a consequence of his foresight, many biologically relevant polymer samples were made available by a
host of investigators from around the world for study at high resolution in Frank’s laboratory. Frank amassed hundreds of such samples, and in fact he and his colleagues studied many of them. The late Murray Goodman, then at Brooklyn Poly (now New York Polytechnic), became a significant collaborator in the NMR study of biopolymers. I can remember many Saturday mornings driving from New Jersey to Brooklyn to discuss our NMR observations and their interpretations with Prof. Goodman and his students. Each of these trips was eventful, if not always for the science then surely for the excitement of driving with Frank, an automotive aficionado without the motoring skills that matched his enthusiasm for driving.
A good example of the extensive NMR studies of biopolymers conducted by Frank Bovey can be found in a paper10 dealing with the solution conformation of the 9-amino acid polypeptide hormone lysine-vasopressin. The constraints provided by 1H-NMR data were used to limit possible conformations/shapes for lysine-vasopressin. Subsequent conformational searches were conducted to determine those conformations that were consistent with the constraints provided by the 1H-NMR observations. These NMR studies were clearly the precursors of the currently used approach for determining the solution conformations of native proteins by searching their conformational spaces under the constraints provided by multifaceted NMR observations.
Frank Bovey also pioneered the use of multidimensional NMR observations to determine the conformations of flexible polymers in solution.11 These provide information concerning the dihedral angles of rotation about the bonds in the polymer backbones (polymer conformations, sizes, and shapes) and permit determination of the average local bond conformations for randomly coiling, disordered polymers in solution. The direct measurement of the local conforma-
tional preferences in polymers is vital to understanding their overall global sizes and shapes, which are so intimately tied to their physical behaviors.
At Bell Laboratories Frank also continued the NMR study of vinyl polymer stereochemistry, which he initiated while at 3M. In 1966 he and two colleagues12 presented the mathematical relations describing the probabilities for observing all possible stereosequences of various lengths (2-repeat unit diads, 3-repeat unit triads, 4-repeat unit tetrads, 5-repeat unit pentads, etc.) in vinyl polymers formed in a random manner during polymerization to yield Pm and Pr = 1 − Pm fractional m and r diad populations. This was a major breakthrough, because now NMR could provide a quantitative measure of stereosequence populations in vinyl polymers and could distinguish between polymers produced by polymerization mechanisms that were random (Bernoullian) or not (possibly Markovian).
Beginning in the early 1970s with the advent of commercial NMR spectrometers that employed pulsed field detection and fast Fourier transformation of time-domain resonance signals, high-resolution 13C-NMR observation of polymers became possible, and Frank Bovey was among the first to take advantage of this development. 13C resonance frequencies (δ13C) are much more sensitive to chemical microstructure than δ1Hs, by ~ an order of magnitude. As a consequence, polymer microstructures are generally more easily and sensitively determined by observation of their solutions with 13C-NMR. Frank and his Bell Laboratories colleagues took full advantage of this development to determine in great detail the microstructures and mobilities of many homo- and copolymers.13
An illuminating and important example was the determination of the contents of short- and long-chain branching produced by intrachain and interchain transfer of radi-
cals during the high-pressure, free-radical synthesis of low-density polyethylene (PE).14 Using the Grant-Paul 13C chemical shift (δ13C) rules,15 which describe the additive effects on δ13Cs (resonance frequencies) of carbon substituents located α, β, and γ to the observed carbon, and model copolymers, Frank Bovey and his colleagues were able to determine that low-density PE typically contained 10-12 four-carbon, 4 five-carbon, and 2 two-carbon short branches per 1,000 repeat units and ~ 1-2 long branches (6 carbons or more), resulting from intrachain and interchain radical transfer during free-radical polymerization, respectively. These results were very significant, because aside from molecular weight and its distribution and stereoregularity the types and amounts of branching in vinyl polymers are the most important structural variables influencing their physical properties.
Very early on,16 Frank Bovey realized that the conformational sensitivity of the γ-substituent effect on δ13Cs, first suggested by Grant and Cheney,17 could have important consequences in the determination of the configurations and conformations of polymers by 13C-NMR. This is most clearly illustrated by the 13C-NMR spectra of polypropylenes (PPs), where, for example, nearly all 36 possible heptad stereosequences (seven consecutive repeat units) are observed18 in the methyl carbon region of the spectrum for atactic-PP (a-PP). To assign the methyl resonances observed in the 13C-NMR spectrum of a-PP to its individual constituent stereosequences, model compounds (3,5,7,9,11,13,15-heptamethylheptadecanes) –
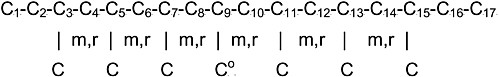
mimicking the heptad stereosequences in a-PP were synthesized, each with 13C enrichment of the 9-methyl carbon, and their 13C-NMR spectra were observed.19 Alternatively, the relative amounts of proximal gauche arrangements between the 9-CH3 and its γ-substituents, the 7- and 11-methine carbons, were evaluated.20 When multiplied by −5 ppm (the
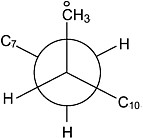
View along the C8–C9 backbone bond when it is trans and CoH3 and C7 are close in space and shielded from the magnetic field.
γ-gauche shielding effect), the calculated 13C chemical shifts faithfully reproduced the methyl carbon region of the 13C-NMR spectra of a-PP and its heptad stereosequence model compounds. This was made possible only after the development of an appropriate description of the conformational characteristics of PPs.21
Though Frank Bovey had previously suspected16 that the conformationally sensitive γ-gauche shielding effect was the
source of the stereosequence dependent δ13Cs observed in PPs and other vinyl polymers, it was not until an appropriate description of the conformational characteristics of PPs was developed that this important relationship was convincingly demonstrated. From that point in time onward the 13C-NMR spectra of vinyl polymers could be effectively assigned to their stereosequences by evaluating their γ-gauche shieldings from knowledge of their conformational characteristics.
In 1976 Schaefer and Stejskal22 first proposed and obtained high-resolution 13C-NMR spectra for polymer solids by combination of several techniques. This made possible the study of the conformations, organizations, and mobility of polymer chains in solid samples, which is their most practical use. Within a few years of this development Frank Bovey and his colleagues at Bell Laboratories were applying the solid-state 13C-NMR technique to a wide variety of solid homopolymers, copolymers, polymer blends, and guest polymers included in noncovalent clathrate compounds formed with small-molecule hosts.23 Frank continued these studies until his retirement in 1993. His high-resolution solid-state NMR studies of polymers were not limited to 13C-NMR but also included observation of the 19F, 29Si, and 31P nuclei, as well.
1,4-trans-Polybutadiene (TPBD) was known to exist in two crystalline structures, Forms I and II, stable below and above ~65°C, respectively. Observations of variable-temperature, high- and low-resolution solid-state 13C-NMR spectra of TPBD and surface-epoxidized TPBD crystals, which immobilizes their fold surface, permitted Bovey and colleagues24 to conclude that TPBD chains are conformationally ordered and rigid in Form I crystals but disordered and mobile in Form II crystals. Epoxidation of the fold surfaces
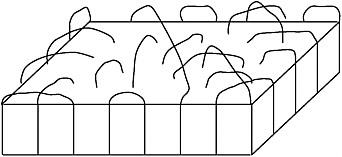
Folded chains on polymer crystal surfaces.
of TPBD crystals did not affect the Form I → Form II transition temperature nor the mobility of TPBD chains in the interior of Form II crystals. This demonstrated that the fold surfaces of TPBD crystals were not involved in the Form I → Form II crystal → crystal transition. Rather it is the TPBD chains in the crystalline interiors alone, which undergo transition from a rigid single conformation to a collection of mobile conformations at ~65°C.
Somewhat related NMR studies of TPBD chains that are extended and isolated in the narrow (~5Å) channels of the crystalline inclusion compound (IC) formed with perhydrotriphenylene (PHTP) host revealed a close correspondence between the conformations and mobility of the included guest TPBD chains and those in bulk Form II TPBD crystals. Even more remarkable were the observations by 2H (deuterium) NMR of deuterated TPBD and polyethylene (PE) when included as guests in their PHTP-ICs. This was surprising, because, while the included TPBD chains were thought to undergo conformational transitions, the motions of included PE chains were believed to be limited to librations (small-amplitude vibrations) around the extended pla-
nar, zigzag conformation. As a consequence, this comparative study of polymer mobility provided the first documented example that the motion of C-2H bonds as revealed by 2H-NMR may not always be unambiguously interpreted in terms of the underlying segmental motions of polymers.
Frank Bovey and collaborators employed both high-resolution 13C and wide-line 13C and 1H-NMR techniques to study the organization and mobilities of the phase-segregated blocks in thermoplastic elastomeric copolymers made with poly(butylene terephthalate) hard blocks (semicrystalline) and poly(tetramethylene oxide) soft blocks (amorphous). They found the soft blocks to be mobile but with an angular range of motion that decreased with increasing content of hard blocks. In addition, the motions of CH2 carbons were observed to be more facile than the OCH2 carbons in the butylene diol portions of the hard blocks. 2H-NMR observation of copolymers made with deuterated hard blocks (-O-CD2-CD2-CD2-CD2-O-) revealed the ability of the butylene diol segments to undergo trans-gauche conformational transitions, while in the amorphous regions of samples with perdeuterated phenyl rings, 2H-MR observations revealed phenyl ring flipping. Solid-state NMR observations of the motions occurring in the phase-segregated regions of these copolymers permitted a much improved, detailed understanding of their mechanical properties.
Finally, Frank Bovey also used NMR to study compatible polymer blends. Mirau and Bovey25 employed concentrated solutions of polymer mixtures and/or bulk, molten polymer mixtures to obtain resolution sufficient to perform 1H-NMR studies. In this manner they were able to determine which protons belonging to poly(vinyl methyl ether) (PVME)
chains interact with (are within ~4Å of) the protons in polystyrene (PS) and poly(ε-caprolactone) (PCL) chains in the
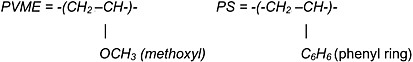
compatible PVME/PS and PVME/PCL blends. They found the phenyl protons of PS to give cross-peaks with the methoxy protons (strong or closer) and methine protons (weak or farther) of PVME. For the PVME/PCL blend the interactions, distances between the methoxy protons of PVME and the C1,C3 protons of PCL were observed to be weaker, farther than those of the C2/C4 and C5 protons of PCL.
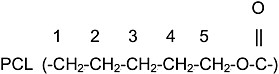
Frank Bovey’s scientific legacy was, and remains, the influence he exerted, and still commands, on generations of polymer scientists through illustration of the methods and utilities of NMR spectroscopy as applied to polymers to understand in detail their microstructures, conformations, mobilities, and organization. He accomplished this by example during his long career in research, and more for-
mally by the many books he authored. For all of these we, and I in particular, remain grateful.
NOTES
SELECTED BIBLIOGRAPHY
1950 With I. M. Kolthoff. Mechanism of emulsion polymerizations. IV. Kinetics of polymerization of styrene in water and detergent solutions and studies of retarders and inhibitors in the emulsion polymerization of styrene. II. Inhibitors. J. Polym. Sci. 5(4):487; 5(5):569.
1959 With G. V. D. Tiers and G. Filipovich. Polymer NSR spectroscopy. I. The motion and configuration of polymer chains in solution. J. Polym. Sci. 38(133):73.
1960 With D. W. McCall. Note on proton resonance in polystyrene solutions. J. Polym. Sci. 45(146):530.
Polymer NSR spectroscopy. III. The rates of the propagation steps in the isotactic and syndiotactic polymerization of methyl methacrylate. J. Polym. Sci. 46(147):59.
With G. V. D. Tiers. Polymer NSR spectroscopy. IV. The stereochemical configuration of polymethacrylic anhydride. J. Polym. Sci. 47(149):479.
1962 Polymer NMR spectroscopy. VI. Methyl methacrylate-styrene and methyl methacrylate-α-methylstyrene copolymers. J. Polym. Sci. 62(173):197.
1963 With S. S. Yanari and R. Lumry. Fluorescence of styrene homopolymers and copolymers. Nature 200(490):242.
1967 NMR Tables for Organic Compounds. New York: Interscience.
With F. P. Hood. Circular dichroism spectrum of poly-L-proline. Biopolymers 5(3):325.
1969 Nuclear Magnetic Resonance Spectroscopy. New York: Academic Press.
Polymer Conformation and Configuration. New York: Academic Press.
1972 High Resolution NMR of Macromolecules. New York: Academic Press.
With A. I. Brewster and A. E. Tonelli. Determination of the solution conformations of cyclic polypeptides. Acc. Chem. Res. 5(6):193.
1979 With F. H. Winslow, eds. Macromolecules. New York: Academic Press.
1980 With A. E Woodward, eds. Polymer Characterization by ESR and NMR. ACS Symposium Series No. 142. Washington, D.C.: American Chemical Society.
1982 Chain Structure and Conformation of Macromolecules. New York: Academic Press.
With L. W. Jelinski, J. J. Dumais, and F. C. Schilling. Solid-state C-13 NMR studies of polyester thermoplastic elastomers. ACS Symposium Series No. 191, p. 345. Washington, D.C.: American Chemical Society.
1984 With F. C. Schilling, A. E. Tonelli, S. Tseng, and A. E. Woodward. A solid-state carbon-13 NMR study of the fold surface of solution-grown 1,4-trans-polybutadiene crystals. Macromolecules 17:728.
With L. A. Belfiore, F. C. Schilling, A. E. Tonelli, and A. J. Lovinger. Magic angle spinning carbon-13 NMR spectroscopy of three crystalline forms of isotactic poly(1-butene). Macromolecules 17:2561.
1988 With P. A. Mirau and L. W. Jelinski. Nuclear Magnetic Resonance Spectroscopy. New York: Academic Press.
1989 With R. A. Quintero-Arcaya, J. M. Fernandez-Santin, and J. A. Subirana. Carbon-13 solid-state NMR of solution prepared polymorphs of poly-.alpha-isobutyl L-aspartate (PAIBLA). Macromolecules 22:2533.
With P. Sozzani, R. W. Behling, F. C. Schilling, S. Bruckner, E. Helfand, and L. W. Jelinski. Traveling defects in 1,4-trans-polybutadiene as an inclusion complex in perhydrotriphenylene canals and a comparison with molecular motions in the crystalline solid state. Macromolecules 22:3318.
1990 With C. A. Walsh, F. C. Schilling, A. J. Lovinger, D. D. Davis, and J. M. Zeigler. Electronic absorption and structural properties of poly(di-n-butylsilylene) precipitated from solution at low temperature. Macromolecules 23(6):1742.
1991 With F. C. Schilling and P. Sozzani. Chain conformation and dynamics of crystalline 1,4-trans-polyisoprene and its inclusion compound with perhydrotriphenylene. Macromolecules 24:4369.
With P. Sozzani and F. C. Schilling. Characterization of isolated polyethylene chains in the solid state. Macromolecules 24:6764.
1993 With F. C. Schilling, A. J. Lovinger, D. D. Davis, and J. M. Zeigler. Solid-state structures, phase transitions, and thermochromism in polysilylene copolymers. Macromolecules 26:2716.

























