3
Today’s CDC Quarantine Stations at U.S. Ports of Entry
This chapter presents the committee’s findings about the activities and composition of the present CDC quarantine stations at U.S. ports of entry and the nature of their relationships with other organizations. Most of the available information is qualitative in nature, deriving from direct observation and interviews. The chapter begins with a description of the broad statutory and regulatory foundation of the CDC quarantine stations’ activities. A conceptual framework for understanding the stations’ organizational environment follows. Then the committee discusses its findings about the processes and outcomes of four fundamental activities:
-
Identification of ill passengers and crew.
-
Responding to reports of ill passengers.
-
Assuring immigrant and refugee health.
-
Inspection of animals, animal products, etiologic agents, hosts, and vectors.
The conclusions and recommendations in Chapter 4 are based upon these findings.
STATUTORY AND REGULATORY FOUNDATION OF CDC QUARANTINE STATION ACTIVITIES
The secretary of the Department of Health and Human Services (DHHS) has statutory responsibility for preventing the introduction, transmission,
and spread of communicable diseases from foreign countries into the United States and its possessions (42 U.S.C. §264). To implement this statute, the secretary develops and enforces regulations through the Centers for Disease Control and Prevention (CDC) (8 U.S.C., 42 U.S.C. §70 and §71). CDC has authorized its Division of Global Migration and Quarantine (DGMQ) to carry out many of these regulations through a variety of activities, including the operation of quarantine stations at select ports of entry and the administration of regulations that govern the movement of people, animals, cargo, and conveyances into the United States. For example, DGMQ can detain, medically examine, or conditionally release individuals at U.S. ports of entry who are reasonably believed to be carrying a communicable disease of public health significance (42 CFR §70–71). Also, DGMQ and CDC can set policies to prevent certain animals that pose a public health threat from entering the country (42 CFR §71.32).
QUARANTINE CORE, SYSTEM, AND NETWORK
The committee found the CDC quarantine stations to be one component of a large, complex network of organizations whose collective actions provide limited protection to residents of and travelers to the United States from microbial threats of foreign origin. It became apparent that understanding the role of the CDC quarantine stations in this network would be essential to developing realistic conclusions and recommendations. Consequently, the committee developed a conceptual diagram and vocabulary to visualize and articulate the interrelationships among the stations, the network, and other key actors. The committee used this diagram and vocabulary both during its deliberations and in its report.
The committee found that some members of the network interact primarily or exclusively with the headquarters staff of DGMQ, rather than with individual stations. For instance, the Director of DGMQ has direct contact with the World Health Organization and the Air Transport Association of America.
Other members of the network interact with CDC’s quarantine operations at multiple levels. For example, the issuance of a joint FDA–CDC ban on the importation and interstate trade of African rodents in the wake of the monkeypox outbreak was accomplished through communication among DGMQ leadership, relevant officials at CDC headquarters, and their counterparts at FDA’s main offices in the Washington, DC, area. By contrast, FDA’s field inspectors at U.S. ports of entry interact primarily with the CDC quarantine station in their region.
In addition, the committee found, a subgroup of organizations in the network interacts with the quarantine stations more closely and frequently than the rest of the network. Within this subgroup, some organizations and
individuals have weekly or even daily contact with the quarantine station staff. The FDA field inspectors are a part of this subgroup.
Figure 3.1 presents the visual schematic created by the committee to illustrate these findings. The following text describes this diagram in detail, as well as the corresponding set of terms coined by the committee for use throughout the report.
The Quarantine Core
At the center of the diagram is what the committee has dubbed the “Quarantine Core,” which consists of the CDC quarantine stations, DGMQ headquarters, and the organizational and scientific capacity of CDC (Figure 1.1 illustrates the relationship among these entities). The quarantine stations lie at the center of this diagram because they are the only members of the network whose primary purpose is the mitigation of imported microbial threats at U.S. ports of entry.
The Quarantine System
In the ring around the Core lies the subgroup of organizations that have (or should have) especially close ties to the Core. Together, this subgroup and the Quarantine Core form what the committee calls the “Quarantine System.” The organizations within the System are responsible for performing the critical quarantine functions of planning, surveillance, assessment and response, and communication to mitigate the risks posed by microbial threats of foreign origin to residents of and travelers to the United States.
In addition to the entities within the Core, the Quarantine System includes local emergency responders and hospitals, local health care providers, local and state health departments, state public health laboratories, port authorities, port staff, airlines, cruise lines, shipping companies, shipping agents, the International Organization for Migration, overseas panel physicians, the U.S. Coast Guard, the Federal Bureau of Investigation, and federal inspectors from U.S. Customs and Border Protection (CBP),1 the U.S. Fish and Wildlife Service, and the Food and Drug Administration. As will be seen in Chapter 4, the consistency and quality of relationships within the System are the subject of several conclusions and recommendations.
The Quarantine Network
In the outermost ring lie the organizations and people that interact with DGMQ leadership and the organizational and scientific capacity of CDC,
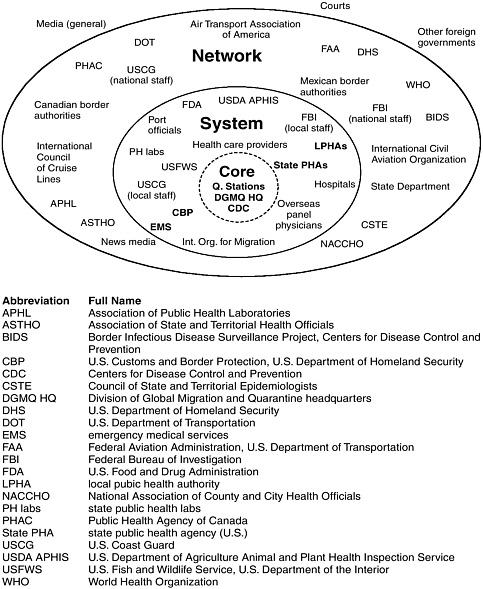
FIGURE 3.1 The relationships among the Quarantine Core, System, and Network for U.S. ports of entry. The circle around the Core is a dotted line to reflect the interdependence of the quarantine stations and their partners in the System. CBP, EMS, LPHAs, and State PHAs are bolded to reflect the especially close collaboration of those entities with the stations on virtually a daily basis. Some organizations interact with the quarantine stations at the System level as well as with CDC or DGMQ at the Network level; these organizations appear in both places in the diagram.
not with individual stations. Together, these entities plus the Quarantine System and Core form a multijurisdictional, multisectoral, multinational “Quarantine Network” that protects both travelers entering the United States and the population within U.S. borders from microbial threats of public health significance2 that originate abroad. In so doing, this Network helps protect the health of the global community.
The members of the Network that lie outside the System include the national-level staff of federal agencies active at the System level, the U.S. Department of State and its embassies, the Department of Homeland Security, national and international transportation industry associations, Mexican and Canadian officials responsible for border activities and disease control, the World Health Organization, and the news media. Although these organizations do not interact with the CDC quarantine stations on a daily basis, they are essential partners whose actions and decisions affect the functioning of the CDC quarantine stations at U.S. ports of entry.
IDENTIFICATION OF AND RESPONSE TO ILL PASSENGERS AND CREW
While the United States has 474 ports of entry3 (CRS, 2004), CDC quarantine stations were established at only 8 of them as of May 2005 (Table 1.1, Table 3.1).The absence of Quarantine Core personnel at most U.S. ports of entry impedes the establishment of critical relationships, plans, and protocols with members of the System to lay the groundwork for identifying and responding to cases of illness there (Committee, 2005). As a
|
2 |
Definition: A microbial threat of public health significance causes serious or lethal human disease and is transmissible from person to person, from animal to person, or potentially both; it also may be transmissible from food or water to people. Because of their potential for wide dispersal, concern is greatest for microbes that spread readily from person to person. A microbial threat may be introduced intentionally—as in bioterrorism—or unintentionally. Additional threats of public health significance of concern to the quarantine stations include the release of chemical or radiological substances and of biological substances other than microbes (e.g., microbial toxins). |
|
3 |
This report uses the term “port of entry” to mean any air, land, or sea port through which people, cargo and conveyances may legally enter the United States from abroad. It should be noted that “port of entry” has a slightly different meaning when used by the Department of Homeland Security’s U.S. Customs and Border Protection (CBP). In CBP’s case, a Port of Entry is an administrative center whose jurisdiction may include more than one entry facility in a certain geographic area. For instance, the Philadelphia Port of Entry services Philadelphia International Airport, Philadelphia’s seaport, Trenton Mercer Airport, Atlantic City International Airport, and ports in Lehigh Valley, PA (http://www.cbp.gov/xp/cgov/toolbox/contacts/ports/pa/1101.xml). Thus, the United States has fewer CBP Ports of Entry (331) than literal ports of entry (474). |
TABLE 3.1 Number of Ports of Entry to the United States Compared with Number of Official Ports of Entry (POEs) Defined by U.S. Customs and Border Protection, 2005
|
|
Air |
Sea |
Land |
TOTAL |
|
No. of ports of entry |
216 |
143 |
115 |
474 |
|
No. of official POEs |
— |
— |
— |
331a |
|
NOTE: Official U.S. Ports of Entry (POEs) are determined by U.S. Customs and Border Protection (CBP). According to CBP, “a ‘Port of Entry’ is an officially designated location (seaports, airports, and or land border locations) where CBP officers or employees are assigned to accept entries of merchandise, clear passengers, collect duties, and enforce the various provisions of CBP and related laws.” Some POEs are not staffed. Staffed POEs may be responsible for overseeing inspections at multiple entry facilities (airports, seaports, and land crossings) within a single geographic area. Thus, the total number of entry facilities is greater than the total number of ports of entry. aFourteen of these are CBP preclearance stations in Canada and the Caribbean. SOURCES: CBP, 2005d; CBP, 2005e; CRS, 2004. |
||||
result, identification and responses to signs of microbial threats in persons, animals, cargo and conveyances generally are unsystematic.
For example, in one recent experience, an inbound international ship reported to the U.S. Coast Guard that a crew member had a fever and rash, potentially signs of a quarantinable disease (Personal communication, P. Edelson, DGMQ, March 24, 2005). The potential microbial threat is typically isolated in such cases by having the ship anchor offshore and transporting a clinician there to examine the patient and decide whether the sailor’s illness is likely a communicable disease of public health concern. Because of lack of preparation for such an event, however, the local health department could not identify a clinician in the county to examine the sailor. The quarantine station with jurisdiction over the case believes that clinicians with relevant expertise were present in the county at the time, but the local health department lacked the resources and planning to identify an appropriate clinician on the spot. Consequently, the ship docked at port before the patient underwent clinical evaluation. Had the sailor been infected with a microbial threat of public health significance, the community might have suffered an outbreak that cost lives. The larger the port and community, the greater the likely impact of an ineffective Quarantine System.
Visual Screening of Passengers and Crew for Signs of Illness
With tremendous growth in the volume of international air traffic, significant cuts in the Quarantine Core’s resources, and limited federal investment to fill the gap, the CDC quarantine stations inspect just a small
fraction of arriving international air travelers and crew for clinical signs of a communicable disease of public health significance. To make the most of its limited resources at airports where there is a quarantine station, the CDC staff sets priorities for which international flights it will meet to screen disembarking passengers and crew. The highest-priority flights are those arriving from points of origin where an infectious disease outbreak is known or believed to have occurred and flights whose pilot has reported a suspect or probable case of disease or injury on board. High-priority flights at the present time include those arriving from Africa and from the Southeast Asian countries where avian influenza is endemic (Committee, 2005).
In addition to inspecting travelers who self-identify or are identified prior to arrival by crew members, quarantine inspectors screen arriving travelers for physical signs suggestive of quarantinable communicable disease: rash, unusually flushed or pale complexion, jaundice, shivering, profuse sweating, diarrhea, and inability to walk without assistance (DQ, 2000). So as not to impede the movement of travelers through airports and customs stations, the brief visual inspections are conducted from a distance of several feet (Committee, 2005). The inspectors stand at various locations in the terminal, depending on the airport. In many locations, the travelers are walking past the staff member while the inspection takes place. Such locations include
-
The gate where arriving passengers enter the terminal.
-
Hallways where passengers from multiple arriving flights walk toward customs (or through which international travelers pass en route to connecting flights).
-
The side of passport control booths located inside U.S. territory.
Partners in the Identification of Ill Passengers
Aircraft Commanders and Ship Masters
The quarantine stations rely not only on CBP but also on airline crews and ship masters to identify ill passengers. The commanders of aircraft destined for the United States have a regulatory responsibility for notifying the quarantine station nearest their first U.S. destination of “the occurrence, on board, of any death or ill person among passengers or crew” (42 CFR §71.21b). Similarly, the masters of ships sailing to the United States have a regulatory responsibility for immediately notifying the quarantine station nearest the port of arrival of “the occurrence, on board, of any death or any ill person among passengers or crew (including those who have disembarked or been removed) during the 15-day period preceding the date of expected arrival” (42 CFR §71.21a). Ideally, when a quarantine
station receives prearrival notification of illness or death, a quarantine inspector, a local emergency responder, a clinician, or a combination of the three boards the conveyance to evaluate the ill passenger before anyone disembarks, thus containing the potential microbial threat (Committee, 2005).
Anecdotal evidence suggests that pilots and ship masters fail to comply with these prearrival reporting regulations out of ignorance, neglect, or other factors (Committee, 2005; personal communication, P. Edelson, DGMQ, March 24, 2005). The penalty for sidestepping this regulation—$5,000 or less per vessel—is inconsequential (42 U.S.C. §271b). Officers of CBP and the U.S. Coast Guard (USCG) have statutory responsibility “to aid in the enforcement of quarantine rules and regulations” (42 U.S.C. §268b); it is unclear how they do so in relation to the aforementioned regulations.
A 2001 report by CDC found that many pilots were unfamiliar with the requirement to report arriving ill passengers aboard a flight (CDC, 2001). More recently, flight attendants aboard a plane en route from London to the United States failed to identify a man with signs and symptoms of chills, fever, diarrhea, and severe sore throat. The man was hospitalized within hours of his arrival and diagnosed with Lassa fever, a quarantinable communicable disease, after having exposed 19 passengers in flight (CDC, 2004c; personal communication, M. Cetron, DGMQ, October 21, 2004). Three of the five quarantine stations visited by the committee, as well as DGMQ HQ officials, agreed that many pilots do not follow the regulatory notification procedures and instead radio intermediaries, such as MedLink, a service available to most commercial airlines that directly links pilots to emergency care physicians4 (Committee, 2005; MedAire, 2005). When radioed, MedLink’s physicians give the pilots medical instructions, contact emergency response teams to meet the plane when it lands, and contact hospitals to receive the patient or patients. The company that owns MedLink, MedAire Inc. of Tempe, Arizona, offers a similar service called MAS to cargo vessels.
The executive vice president of the Air Transport Association of America Inc. (ATA) recently testified that CDC and his staff “have made sure that our members fully understand the reporting requirements for passengers with suspected communicable diseases” (Meenan, 2005, p.3). To further encourage airline pilots to comply with prearrival notification procedures, CDC quarantine station personnel make an effort to meet any plane reporting an ill passenger, even if (as is frequently the case) the reported signs and symptoms clearly indicate that the passenger does not have a quarantinable communicable disease (Committee, 2005).
ATA’s members are reportedly trying to train their crews better to recognize and report passengers who display signs of communicable disease of public health concern (Personal communication, K. Andrus, ATA, October 21, 2004). The 2001 CDC report mentioned above recommended that commercial in-flight manuals be updated to include procedures for managing an ill passenger and detailed information on how to contact the closest quarantine station (CDC, 2001); whether such action has been taken is unclear.
The quarantine stations receive such infrequent prearrival notifications from ship masters that some CDC staff believe only the veteran ship masters are aware of the regulation (Appendix D). As noted above, USCG is responsible for assisting in the enforcement of prearrival notification rules among ships, and federal regulation requires vessels to immediately notify the nearest USCG group office of hazardous conditions. Such conditions include injury or illness to persons aboard the vessel (33 CFR 160.1–160.215). For a thorough description of the relationship between the CDC quarantine stations and U.S. seaports, see Appendix D.
The failure to notify a quarantine station of on-board illness could, in certain cases, represent a missed opportunity for the Quarantine Core to contain a potential outbreak of a serious communicable disease, compromising public health and national security.
U.S. Customs and Border Protection (CBP)
More than 460 U.S. ports of entry and preclearance ports lack personnel from the Quarantine Core to screen arriving travelers for signs of communicable disease of public health significance. For every full-time-equivalent quarantine station staff member, there are approximately 300 CBP field officers.5 Consequently, the Core relies on CBP inspectors to watch for ill passengers at most ports and to carry out other regulatory responsibilities on their behalf. In addition, because the CDC quarantine stations are not open 24 hours a day, 7 days a week, on-site CBP personnel sometimes act as surrogates for quarantine inspectors during their off-hours. The Core has created a field manual for these surrogates (DQ, 2000).
Port-based CBP officials are officially responsible for performing this surrogate function for CDC among their “duties as assigned” (Personal communication, P. Gaddini, CBP, June 3, 2005). The officers receive
|
5 |
Calculation based on DGMQ forecast of 66 field staff in October 2005 (see Chapter 1) and assumption that the number of CBP inspection staff at ports of entry in fiscal 2004—19,230—remained approximately the same in fiscal 2005 (see Appendix E, Table E.4) (CRS, 2004). |
|
BOX 3.1 Ports of entry are responsible for daily port specific operations. There are 317 official ports of entry in the United States and 14 preclearance stations in Canada and the Caribbean, a total of 331 official manned and unmanned ports. Port personnel are the face at the border for most cargo and visitors entering the United States. Here CBP enforces the import and export laws and regulations of the U.S. federal government and conducts immigration policy and programs. Ports also perform agriculture inspections to protect the USA from potential carriers of animal and plant pests or diseases that could cause serious damage to America’s crops, livestock, pets, and the environment. SOURCE: CBP, 2005d. |
on-the-job training for this function when they are newly hired; they receive additional training from CDC quarantine station staff when new diseases of public health significance are detected. Job performance in the area of “public health measures” is discussed with these officers during their mid-year and year-end performance reviews.
The primary duties of port-based CBP officials are summarized in Box 3.1. The protection of humans from infectious disease of public health concern—whether introduced intentionally or unintentionally—is absent from this statement, as well as from the public mission statements of CBP and its Immigration Inspection Program (Boxes 3.2 and 3.3). This function also was absent from the CBP Commissioner’s 2005 testimony outlining his department’s duties and functions to the U.S. House of Representatives Committee on Appropriations (Bonner, 2005). By contrast, the protection of U.S. agriculture—and, by extension, the U.S. economy—from “potential carriers of animal and plant pests or diseases” appears explicitly in the mission statement of CBP Ports of Entry (CBP, 2005d).
Data were unavailable for the committee to quantitatively measure the extent and quality of disease surveillance conducted at U.S. ports of entry. On the basis of the committee’s observations, CBP does conduct disease inspection to varying degrees at some ports (Committee, 2005). CBP inspectors collocated at airports with a CDC quarantine station tend to be trained by station staff to conduct public health screening. The DGMQ 2003 Program Review lists 157 public health training sessions for 2,034 federal inspection service inspectors and other agencies (DGMQ, 2004). These CBP inspectors appear to take the responsibility seriously as much for their own safety as for the health of the public. By contrast, anecdotal
|
BOX 3.2 We are the guardians of our Nation’s Border. We are America’s frontline. We safeguard the American homeland at and beyond our borders. We protect the American public against terrorists and the instruments of terror. We steadfastly enforce the laws of the United States while fostering our Nation’s economic security through lawful international trade and travel. We serve the American public with vigilance, integrity, and professionalism. SOURCE: CBP, 2005b. |
evidence indicates that quarantine station staff can provide only minimal training to CBP inspectors at subports—the majority of U.S. air, sea, and land ports of entry—because of limited travel budgets, frequent turnover among CBP personnel, and unclear lines of authority due to reorganization within the U.S. Department of Homeland Security (DHS) (Committee, 2005). The Core has for more than a decade lacked sufficient funds for quarantine station staff to visit subports6 with reasonable frequency (Personal communication, M. Becker, DGMQ, March 24, 2005).
The extent to which CBP staff inspect passengers, animals, and cargo for disease appears to be influenced in part by the goodwill that quarantine station staff have fostered through relationships with their CBP counterparts; these relationships are strongest at ports that contain quarantine stations or that quarantine station staff have recently visited to conduct on-site training sessions. Concern among CBP inspectors of contracting a serious communicable disease that originates abroad also seems to motivate them to perform disease inspections on the Quarantine Core’s behalf (Committee, 2005; personal communication, A. Lombardi and P. Gaddini, CBP, January 20, 2005).
On the basis of the information presented above, the committee concluded that the CBP function as a surrogate to the CDC quarantine stations
|
6 |
Subport: DGMQ refers to ports of entry without a CDC quarantine station located on site as subports. The eight established CDC quarantine stations are responsible for the subports located within their jurisdictions (Table 1.1). |
|
BOX 3.3 Mission The mission of the inspections program is to control and guard the boundaries and borders of the United States against the illegal entry of aliens. In a way that:
Role Individuals seeking entry into the United States are inspected at Ports-of-Entry (POEs) by CBP officers who determine their admissibility. The inspection process includes all work performed in connection with the entry of aliens and United States citizens into the United States, including preinspection performed by the Immigration Inspectors outside the United States. “An officer is responsible for determining the nationality and identity of each applicant for admission and for preventing the entry of ineligible aliens, including criminals, terrorists, and drug traffickers, among others. U.S. citizens are automatically admitted upon verification of citizenship; aliens are questioned and their documents are examined to determine admissibility based on the requirements of the U.S. immigration law.” SOURCE: CBP, 2005c. |
is an inherited role historically performed as well as possible given the limitations of training, time, and resources. But the back-up that CBP provides to the CDC quarantine stations clearly falls outside the domain for which CBP officials are hired and for which they are best trained. The port-
based staff of CBP and other federal agencies have multiple responsibilities that, from their perspective, take precedence over the duties of the Core. The committee addresses this shortcoming in its recommendations in Chapter 4.
Overview of Response
The Quarantine System lacks a nationally standard protocol for responding to suspect and probable cases of quarantinable communicable disease that have been reported to or identified by a quarantine station. Although states and localities bear primary responsibility for the management and monitoring of these cases, local public health laws governing the response to and treatment of infectious disease in general vary widely across the United States (IOM, 2003, pp. 101-111). The following paragraphs provide an overview of the System’s response to ill passengers, then focus on the robust role played by local public health and health care entities.
If an ill passenger (reported during travel) arrives at a U.S. port of entry that has a quarantine station, CDC staff evaluate the individual for signs, symptoms, and travel history consistent with a quarantinable communicable disease (Box 1.3). If the passenger arrives at a port of entry lacking a CDC quarantine station and a station is notified of the passenger, the station with jurisdiction7 over the port (see Table 1.1) consults the physician on call at DGMQ headquarters or alerts the local health department to evaluate the individual for signs and symptoms of a quarantinable disease. If the index of suspicion is high, the individual is sent to a health care facility for diagnosis (Personal communications: J. Barrow and M. Remis, DGMQ, December 28, 2004; S. Maloney, DGMQ, January 18, 2005).
Recently identified diseases of public health concern among air travelers include measles, meningitis, viral hemorrhagic fevers, and tuberculosis (CDC, 2004c; Lasher et al., 2004; LoBue and Moser, 2004). Many of the illnesses identified by pilots or quarantine inspectors, however, are found to be either noncommunicable diseases or communicable diseases of insignificant public health concern. Table 3.2 presents the number of cases of illness reported to or found by the quarantine stations in 2003 and the types of medical control measures taken in response.
Although the Quarantine Core does not have jurisdiction over departing flights, the quarantine stations provide advice upon request to CBP, port authorities, or airlines regarding suspected cases of illness among passengers on departing flights (Committee, 2005). CBP, which has jurisdic-
|
7 |
Each quarantine station is responsible for many ports of entry without a quarantine station located within a specific geographic area. For example, Hartsfield International Airport in Atlanta has jurisdiction over all ports in Georgia, Alabama, Arkansas, Louisiana, Oklahoma, Mississippi, North Carolina, South Carolina, and Tennessee (Table 1.1). |
TABLE 3.2 All Cases of Illness Reported to or Found by DGMQ in 2003 and Medical Control Measures Taken
tion over international departures, can contact the Quarantine Core for medical advice if an ill passenger is identified on an outbound flight. Local public health authorities also will occasionally contact a quarantine station to alert the staff that a departing passenger may be infectious. In such cases, the quarantine station notifies the airline, which almost always follows the Quarantine Core’s advice regarding the health risk posed by the passenger (Committee, 2005).
Partners in Response: Local Public Health and Health Care Entities
Certain state and local entities appear to be involved most of the time in the response to suspect or probable cases of quarantinable communicable disease that come to the attention of the Quarantine Core. Below is a description of these entities and their roles, in rough chronological order (CDC, 2004a; Checko and Libbey, 2005; Committee, 2005; personal communication, P. Edelson, DGMQ, March 24, 2005):
-
The local public health authority (LPHA) is notified.
-
At airports, local emergency medical services (EMS) personnel provide urgent medical care, if necessary, and conduct a preliminary clinical evaluation.
-
At seaports, a local physician is dispatched to the ship at anchor to provide urgent medical care, if necessary, and to conduct a preliminary clinical evaluation.
-
The LPHA and the CDC quarantine station staff (in consultation with a physician at DGMQ headquarters) share their conclusions and decide what steps should be taken next.
-
Most of the time, the Quarantine Core and the LPHA agree upon next steps. Infrequently, the Quarantine Core believes a patient should be hospitalized for evaluation, but the LPHA either disagrees or lacks the resources and authority to mandate such evaluation. Under these circumstances, the Core has the federal authority to order hospital-based evaluation and monitoring of the patient. To prepare for such eventualities, the Core has entered into memoranda of agreement (MOAs) with more than 130 hospitals near ports of entry around the country (DGMQ, 2004; personal communications: P. Edelson, DGMQ, March 24, 2005, and M. Remis, DGMQ, August 29, 2005). Participating hospitals must accept suspect or probable cases of quarantinable communicable diseases or “other conditions of urgent public health significance” for diagnosis and management (CDC, 2004a). An abbreviated MOA appears in Appendix G.
-
If the case calls for hospitalization, a local ambulance transports the patient to the hospital, where local physicians examine the patient and collect tissue samples for laboratory tests by a hospital or state public health laboratory.8
-
The LPHA, the CDC quarantine station, or both sometimes receive follow-up calls from the laboratory and hospital with the patient’s diagnosis. At other times, the CDC quarantine station must call the hospital to learn the diagnosis; however, some operators at hospitals do not recognize the station’s authority to obtain health information and refuse to provide it due to Health Insurance Portability and Accountability Act (HIPAA) regulations.
Suspect and probable cases of quarantinable communicable disease remain under federal jurisdiction for a very short period of time. In general, the transfer of the patient to EMS personnel represents the transfer of legal authority from the Quarantine Core—the federal government—to the locality or state. From that point forward, the locality or state bears ultimate responsibility for medical care administered by EMS, the choice of hospital to which the patient is delivered, the medical care provided by the hospital, laboratory tests conducted by public health laboratories, and medical follow-up conducted by local or state public health authorities.
The CDC quarantine station staff do not provide medical treatment, even since the decision in 2004 to begin hiring physicians (generally one per
station) for the first time in decades (Committee, 2005). Before then, no one on the quarantine station staff had medical training, although some of the inspectors had been trained as nurses or in provision of EMS in an earlier career. The staff could always consult by phone with a physician at DGMQ HQ. The relevance of clinically trained staff for the stations is discussed in Chapter 4 and Appendix A.
Both reported and anecdotal evidence suggests that small numbers of commercial travelers routinely enter the United States during the symptomatic stage of quarantinable communicable diseases without coming to the attention of air commanders, ship masters, or CDC quarantine station staff at U.S. ports of entry. Such cases have been identified through retrospective studies (CDC, 2001; CDC, 2004c).
Obtaining Passengers’ Contact Information During an Outbreak Investigation
At present, the process of obtaining passengers’ contact information for contact tracing is a labor-intensive, time-consuming process. When this information is obtained from the airlines, it arrives by mail or fax in a lengthy paper form called a passenger manifest. The handover of this information to the Quarantine Core appears to be delayed by logistical obstacles and legal concerns. Customs declarations, which contain travelers’ local contact addresses, also are challenging to obtain (Committee, 2005; DGMQ, 2004; Meenan, 2005; personal communications: K. Andrus, ATA, October 21, 2004, and P. Edelson, DGMQ, March 18, 2005).
The difficulty in obtaining passengers’ contact information to alert them to a microbial threat of public health significance has several detrimental consequences: first, the quarantine stations cannot identify or locate a significant percentage of potentially exposed passengers; second, by the time many passengers are contacted, it may be too late to implement effective preventive measures; and third, affected passengers may spread the disease among their close contacts, as happened during the outbreak of SARS (Personal communication, P. Edelson, DGMQ, March 18, 2005).
The airline industry, as represented by the ATA, and the Federal Aviation Administration (FAA) have been collaborating with DGMQ and CDC on both intermediate and long-term solutions to expedite the transfer of passenger contact information to the quarantine stations (Meenan, 2005; Jordan, 2005).
Intermediate Solution: Passenger Locator Card
The intermediate solution has been to develop a card on which passengers’ contact information is collected in a machine-readable format
(DGMQ, 2004; Meenan, 2005). When needed, these so-called passenger locator cards would be quickly scanned and the data transmitted electronically to a quarantine station or to DGMQ headquarters. One advantage of these cards over passenger manifests is that the traveler records his or her actual seat, which sometimes differs from the assigned seat listed in the manifest (Personal communication, K. Andrus, ATA, October 21, 2004). The intended use of passenger locator cards appears to be confined to two particular circumstances (Meenan, 2005):
-
During an outbreak of communicable disease of public health significance that affects international travelers, CDC would identify affected countries and, in conjunction with airlines, would identify specific flights on which cards should be distributed.
-
When a pilot has reported an ill passenger or crew member while the flight is in the air, cards could be distributed.
Passenger locator cards had not been field-tested as of April 2005 (Personal communication, M. Remis, DGMQ, April 7, 2005). The International Air Transport Association and the International Civil Aviation Organization are reportedly reviewing and modifying the cards for international use (DGMQ, 2004). The member airlines of ATA are prepared to use passenger locator cards when and if directed by CDC to do so (Meenan, 2005).
Long-Term Solution 1: eManifest
In the long run, both the airline industry and CDC would prefer that passenger contact information already collected by airlines be transmitted electronically to public health officials for contact tracing to contain a microbial threat. This information is stored in so-called electronic passenger manifests (eManifest). DGMQ and Delta Airlines have been working for the past few years on a model system for the transmittal of eManifests from Delta to the Quarantine Core (DGMQ, 2004). In general, legal, technical and political challenges must be overcome to make eManifests from any airline accessible to the Quarantine Core (Meenan, 2005).
One challenge is the issue of privacy in the electronic age. As the Core and its airline partners pursue the idea of utilizing eManifests for contact tracing, they face important concerns over who may obtain the manifest’s content, for what purposes, and for how long. The privacy policies of airlines based in the United States require that they maintain the confidentiality of passengers’ personal information unless compelled to disclose it by law. The American airline industry would reportedly welcome a regulatory change that compels airlines to give the quarantine stations access to elec-
tronic passenger manifests during an outbreak investigation (Personal communication, K. Andrus, ATA, October 21, 2004). The privacy issue is more challenging in the European Union, however, and a solution to the issue there remains unclear (DGMQ, 2004).
Other challenges to the use of eManifests for contact tracing include incompatible computer systems in use by airlines and the Quarantine Core and the question of reciprocity with other countries (Meenan, 2005).
Collaboration among the Quarantine Core, CBP, the Transportation Security Administration, and other federal entities that seek passenger information—and face similar obstacles to obtaining it—may expedite the development of solutions to these challenges (Meenan, 2005).
Passenger manifests alone—whether delivered electronically or on paper—do not always provide sufficient or accurate information for contact tracing, the committee found. A study conducted by the CDC quarantine station in Hawaii and the Hawaii Department of Health concluded that the information from passenger manifests should be supplemented by other information sources to conduct rapid contact tracing of airline passengers after they have disembarked (Lasher et al., 2004). In another case, the director of DGMQ reported that a cell-phone number, not usually contained in passenger manifests, was one of the most useful tools for tracing 20 contacts of a man who was ill with Lassa fever while traveling from London to Newark, NJ (Personal communication, M. Cetron, DGMQ, October 21 2004). Also, as noted above, passenger manifests list travelers’ assigned seats, which may differ from where they actually sat during the flight.
Long-Term Solution 2: National Architecture for Collecting Passenger Information
In parallel with its work on eManifests, the Core reportedly has begun to develop a national information architecture that may be an alternative source of passengers’ contact information in the future (DGMQ, 2004). This information would be extracted from the Advanced Passenger Information System,9 the Global Distribution System, the computerized passenger profile system, and other sources. The Core is collaborating on this project with the John A. Volpe National Transportation Systems Center, a research and development organization within the U.S. Department of Transportation, and MITRE Corporation, a not-for-profit, federally char-

FIGURE 3.2 CDC Health Alert Notice. The staff of CDC quarantine stations distribute Health Alert Notices to passengers arriving at a U.S. port of entry from an area experiencing an outbreak of communicable disease of public health significance. These paper notices are one of the Quarantine Core’s principal tools for educating international travelers who have potentially been exposed to a microbial threat.
SOURCE: DGMQ, 2002.
tered organization whose expertise includes systems engineering, information technology, and operational concepts.
Response Protocols During Public Health Emergencies
During the outbreak of severe acute respiratory syndrome (SARS), the Quarantine Core and a multitude of volunteers spent hundreds of hours educating international travelers about the new disease and appropriate forms of health care and distributing health alert cards (Figure 3.2), which provided educational and contact information (DGMQ, 2004; Meenan, 2005). For years, the Core has monitored electronic sentinel surveillance systems for reports of infectious disease outbreaks around the world. Since U.S. residents generally lack immunity to diseases that are not endemic in this country, outbreaks of communicable diseases that are common in other parts of the world can pose a serious risk to the health of Americans (CDC, 2004c; Ndao et al., 2005; Shu, 2005). In addition, outbreaks of infectious
disease of public health concern to individuals of any nationality come to the Core’s attention; one example is meningitis (CDC, 2001). To the extent possible, given the quarantine stations’ limited human resources and geographic reach, the staff meet such flights upon arrival and inform travelers of how to identify and report signs and symptoms of the disease. The CDC quarantine station staff are expected to participate in emergency responses that affect their entire airport, and a station’s role differs from one airport to the next according to the nature of the emergency plan. John F. Kennedy International Airport in New York City (JFK), for instance, establishes an operations center in the event of a large-scale emergency. The staff of all federal inspection agencies located at JFK must coordinate their response activities through the operations center, which manages media relations so as to communicate consistent messages to the public with one voice (Committee, 2005).
IMMIGRANTS AND REFUGEES: ROLE OF THE QUARANTINE CORE
The scope of this study precluded a thorough examination of the many complex issues surrounding the health of migrants to the United States in relation to the Quarantine Core. Thus, the following discussion is limited, and the topic is ripe for further study.
Some quarantine stations spend more time on immigrant and refugee health than any other issue (Committee, 2005). Table 3.3 presents the total number of immigrants, refugees, and asylees admitted into the United States in 2003. These populations, particularly refugees, generally carry a greater burden of disease than the average traveler because they tend to come from developing nations where access to preventive and curative care is limited and where a relatively high number of communicable diseases of public health concern are endemic. As a rule, refugees enter the United States through a port with a CDC quarantine station (8 U.S.C. §1522).
To protect the health of U.S. communities, immigrants and refugees
TABLE 3.3 Numbers of Immigrants, Refugees, and Asylees Accepted into the United States, 2003
|
Category of Arrivals |
Number Admitted in 2003 |
|
Immigrants |
705,827 |
|
Refugees |
28,306 |
|
Persons granted asylum |
15,470 |
|
SOURCE: OIS, 2004. |
|
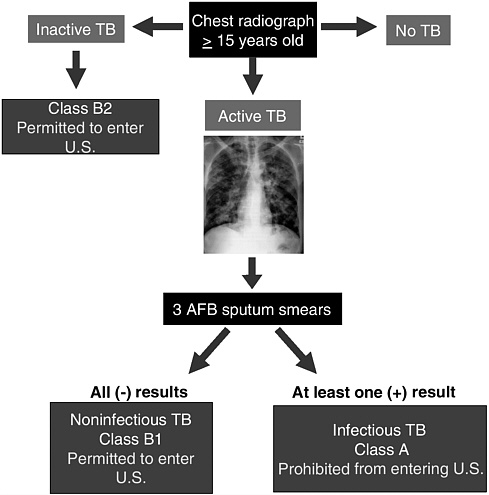
FIGURE 3.3 Algorithm for screening prospective immigrants, refugees, and asylees to the United States for tuberculosis (TB). First, individuals who are 15 years old or older must have a chest x ray. An overseas physician selected by the U.S. Department of State examines the x ray to determine whether the person has tuberculosis (TB), and if so, whether the Mycobacterium tuberculosis complex is active or inactive. Individuals who are diagnosed with inactive TB receive Class B2 status and may enter the United States but are required to undergo medical follow-up after arrival. Individuals who are diagnosed with active TB then undergo a laboratory test to determine whether the infectious agent is communicable. If sputum smears are negative for acid-fast bacilli (AFB) on 3 consecutive days, the individual has a noninfectious form of TB and may enter the United States with Class B1 status. These individuals may begin a course of treatment before leaving their home country and must receive medical follow-up in the United States. If one or more sputum samples test positive, the individual has infectious TB—a Class A communicable disease—and may not enter the United States without a special waiver (DQ, 1991; LoBue and Moser, 2004; Royce, 2005).
SOURCE: Maloney, 2001.
TABLE 3.4 Communicable Diseases of Public Health Concern Diagnosed in Immigrants and Refugees to the United States by Panel Physicians, 1999–2003
|
Health Condition |
Immigrants |
Refugees |
Total |
|
Infectious (AFB+) active TB: class A |
29 |
7 |
36 |
|
Noninfectious (AFB-) active TB: class B1 |
19,206 |
2,140 |
21,346 |
|
Inactive TB: class B2 |
17,026 |
8,025 |
25,051 |
|
HIV |
102 |
735 |
837 |
|
Syphilis |
209 |
62 |
271 |
|
Hansen’s disease (leprosy) |
13 |
4 |
17 |
|
TOTAL |
36,585 |
10,973 |
47,558 |
|
SOURCE: Adapted from personal communication, S. Maloney, DGMQ, August 29, 2005. |
|||
applying for a visa to the United States are required to undergo medical screening in their country of origin. The purpose of screening is to identify foreign-born individuals who have a communicable disease of public health concern classified as a Class A or B disease (Box 1.2). The screening process includes a physical exam and an x ray of the lungs to identify signs of tuberculosis (Figure 3.3). U.S. embassy staff select the clinicians, known as panel physicians, who perform these medical evaluations (DQ, 1991; LoBue and Moser, 2004) (42 CFR §34.1–34.8).
Panel physicians identified more than 46,000 suspect cases of noninfectious active tuberculosis (TB) and inactive TB among the 2 million immigrants and 280,000 refugees who came to the United States between 1999 and 2003 (Table 3.4).
The effectiveness of overseas medical screening depends on accurate diagnoses by the clinicians overseas as well as further medical evaluation and follow-up by LPHAs of foreign-born individuals after their arrival in the United States. The CDC quarantine stations at U.S. ports of entry facilitate the follow-up of immigrants and refugees by notifying the LPHAs and forwarding medical paperwork of all refugees and of immigrants who have admissible, medically notifiable conditions. The stations are responsible for identifying such immigrants who enter the United States through a port that contains a quarantine station, while CBP personnel are responsible for identifying those individuals who enter through all other ports and forwarding their paperwork to one of the stations (Cetron, 2004b; Committee, 2005; Royce, 2005; personal communication, S. Maloney, DGMQ, January 18, 2005).
A small-scale, unpublished study conducted by DGMQ in 1999, as well as anecdotal evidence, suggests that both the CDC quarantine stations at ports of entry and CBP miss a significant percentage of immigrants who have medically notifiable conditions and are admissible into the United
States (Royce, 2005; personal communication, P. Edelson, DGMQ, March 24, 2005). Because CBP personnel are under pressure to move through immigrants’ paperwork quickly, the quarantine station staff must reportedly “scramble” to obtain the Class B forms from subports (Personal communication, P. Edelson, DGMQ, March 24, 2005).
Flights carrying refugees and parolees generally enter the United States at an airport with a quarantine station (8 U.S.C. §1522). In theory, CDC quarantine station staff meet every arriving refugee flight, visually screen the passengers for signs and symptoms of illness, and notify local health departments of their arrival. Because of the number of demands on a small workforce, however, the quarantine inspectors at JFK meet only the refugee flights known to carry passengers with medical histories of concern. All other refugee flights are met by the International Organization for Migration, which acts on DGMQ’s behalf (Committee, 2005; personal communication, M. Becker, DGMQ, April 19, 2005).
In addition to visual screening, the quarantine station staff review the results of refugees’ and parolees’ overseas medical examinations to identify individuals with Class A or Class B conditions. If such cases are identified, local public health authorities receive notification so they may monitor the patients’ health and health care for the safety of the individual and his or her community (Personal communication, S. Maloney, DGMQ, January 18, 2005). The hard copies of refugees’ and parolees’ medical exams are mailed in batches to the appropriate public health authority (Committee, 2005).
To reduce the volume of refugees arriving at JFK, they have been permitted for several years to enter the country through Newark’s international airport. There, personnel from the International Organization for Migration review the medical paperwork and send it in batches to the quarantine station at JFK (Personal communication, M. Remis, DGMQ, January 18, 2005).
Immigrants may enter the United States through any port, and they are not visually screened upon arrival; only their medical paperwork is reviewed by federal authorities. The CDC quarantine stations are ultimately responsible for identifying immigrants with Class A or B diseases. Anecdotal evidence suggests that a significant number of immigrants who have Class A or B diseases are missed because of human error in the scanning of paperwork (Committee, 2005; personal communication, P. Edelson, DGMQ, March 24, 2005). This appears to be particularly true at subports.
If the immigrant enters through a subport, CBP personnel in the Immigration Inspection Program collect and scan the overseas medical exam reports for conditions of public health concern; the paperwork of individuals found to have Class A or B diseases is sent to the CDC quarantine station with jurisdiction over the subport (Table 1.1). The station then sends the paperwork to the local health department where the immigrant will reside. If
the immigrant arrives at a port with a CDC quarantine station, a quarantine inspector reviews the paperwork. Should the immigrant’s overseas medical exam identify a disease of public health significance, the quarantine station mails information to the relevant state and local health departments about the immigrant’s final destination, the suspect disease, and the results of his or her overseas medical exams. The station also notifies the immigrant, advising him or her to report to the local health department (Committee, 2005; personal communications: J. Barrow and M. Remis, DGMQ, December 28, 2004, and S. Maloney, DGMQ, January 18, 2005).
One of DGMQ’s three branches (Figure 1.1) is dedicated to immigrant and refugee health. Many of this branch’s accomplishments in 2003 illustrate overlap and potential synergy with the branch containing the quarantine stations. These accomplishments include (DGMQ, 2004):
-
Screening and treatment for malaria, varicella, and measles of Liberians in Cote d’Ivoire who were awaiting transport to the United States.
-
Provision of full antimalarial and anti-intestinal parasitosis treatment for 1,030 (out of 1,468) refugees from sub-Saharan Africa prior to departure for the United States during fiscal year 2002.
-
Strengthening the training of overseas panel physicians and improving standardization of U.S. visa applicant medical screening by providing guidelines for interpreting chest radiographs suggestive of active TB.
-
Finalizing of new and more complete technical instructions for medical examination of aliens in the United States (performed by licensed physicians known as civil surgeons) for tuberculosis.
This information demonstrates how another branch of DGMQ also has responsibilities that help protect the U.S. population and travelers to this country from microbial threats of public health significance that originate abroad.
INSPECTION OF ANIMALS AND ANIMAL PRODUCTS, ETIOLOGIC AGENTS, HOSTS, AND VECTORS10
Jurisdiction of the Quarantine Core
A small number of imported animal species are regulated by the Quarantine Core: domestic dogs, domestic cats, nonhuman primates (NHPs), turtles, tortoises, and terrapins (Foreign Quarantine—Importations, 42 CFR
|
10 |
Much of the information in this section comes from interviews that the authors of a commissioned paper conducted with DGMQ and staff at partner agencies. The paper, Microbial Threats of Public Health Significance Originating in Animals or Animal Products at U.S. Ports of Entry appears in Appendix E and includes a list of interviewees. |
§71.51–71.53, §71.56). The Core can add other animals or animal groups to its authority on the basis of a specific threat or through the federal rule-making process (42 CFR §71.32). Two contemporary examples of restrictions on the import of specific animals perceived to pose a public health threat are the bans on the importation of civet cats, an important potential source of the SARS coronavirus (Zhong, 2004), and African rodents, the probable source of human monkeypox virus that emerged in the United States in 2003 (Di Giulio and Eckburg, 2004).
The CDC quarantine stations are technically responsible for inspecting all imports of animals under their authority to ensure that the animals do not display signs of communicable disease. In practice, however, this responsibility usually is carried out by CBP veterinary and animal health inspectors on behalf of the Quarantine Core (Appendix E). The principal exception is the inspection of shipments of NHPs, which must be imported according to a strict protocol intended to protect the people involved from contracting a zoonotic disease, as discussed below (Cetron, 2004a; Committee, 2005). Legal and illegal imports of animal products, etiologic agents, hosts, and vectors that may pose a public health threat also lie within CDC jurisdiction (42 CFR §71.54).
Jurisdictions of CBP and the U.S. Fish and Wildlife Service
Agricultural Imports
In March 2003, following the establishment of the Department of Homeland Security (DHS), the Agriculture Quarantine and Inspection unit (AQI) of the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS), was shifted to CBP (APHIS, 2003). AQI’s veterinary and animal health inspectors continue to screen agricultural imports to protect the United States from potential carriers of animal and plant pests or diseases that could cause serious damage to America’s crops, livestock, pets, and the environment (CBP, 2005d). Such pests and diseases may be naturally occurring or intentionally introduced.
CBP inspectors generally notify a CDC quarantine station when they have identified importations of animals, animal products, etiologic agents, and other items under DGMQ’s jurisdiction. These inspectors sometimes notify a CDC quarantine station when they believe they have identified other animals or animal products of possible public health significance, although the high volume of imports frequently impedes CBP in doing so (Appendix E).
The transfer of AQI from USDA to DHS has created additional layers of communication that have impeded the rapid delivery of critical information to port inspectors (Appendix E). The Government Accountability Of-
fice (GAO) found that CBP’s agricultural inspectors do not always receive timely information about high-risk cargo that should be held for inspection (GAO, 2005). For instance, CBP inspectors at a seaport in a major agricultural state did not receive an alert in 2004 about an outbreak of a highly pathogenic, zoonotic strain of avian influenza until a week after the warning was issued (GAO, 2005). In addition, farm groups and some members of Congress have questioned whether CBP officers will receive sufficient training to properly inspect agricultural imports (FASS, 2003). Despite an overall increase in the number of agricultural imports to the United States during the past 2 years, GAO reported a decrease in the number of these imports that have been inspected since CBP assumed primary responsibility for the inspection of farm animals and agricultural products at U.S. ports of entry (GAO, 2005).
Inspection of Wildlife
The Department of the Interior’s U.S. Fish and Wildlife Service (USFWS) is yet another agency involved in the regulation and inspection of animal imports. USFWS enforces U.S. and international laws regarding the trade and transport of wildlife (Division of Law Enforcement, 2002). The service has statutory authorities over the importation of nonfarm animals, including birds, fish, reptiles, and amphibians, and such animal byproducts as pelts, skins, coats, and game trophies. These authorities derive from the Endangered Species Act (16 U.S.C. §1531–1543), the Lacey Act (18 U.S.C. §703–712), and the Migratory Bird Treaty Act (16 U.S.C. §668–668C).
Passengers and conveyances arriving in the United States from a foreign point of origin are required by law to declare all wildlife importations to USFWS. Port-based USFWS officers inspect international cargo, baggage, and passengers for endangered and protected species of wildlife (Division of Law Enforcement, 2002). If an officer of USFWS is unavailable, CBP employees inspect and process the imported wildlife instead (PPQ, 2005). When a specific animal or animal product for importation to the United States poses a potential threat to public health, USFWS coordinates the inspection and response to the item with DGMQ, as discussed below (Appendix E).
Jurisdictional Overlap and Zoonotic Disease
When cases of jurisdictional overlap arise, the agencies involved must decide how to apportion operational responsibilities or knit complementary responsibilities together (Personal communication, P. Edelson, DGMQ, March 18, 2005). For example, DGMQ and USFWS both have an interest
in responding to importations of goatskin because it may carry spores of Bacillus anthracis, the causative agent of anthrax.
No federal agency has a mandate and mission that cover all imported animals and zoonoses (Personal communication, P. Arguin, DGMQ, April 8, 2005). In general, a strict protocol defining which agency has jurisdiction over a perceived zoonotic threat does not exist; the decision is made by the local officers in charge from the three agencies on the basis of the nature of the particular situation (Appendix E). The exceptions to this practice occur when such high-profile microbial threats as avian influenza or monkeypox arise. In these cases, the Quarantine Core and the relevant agency collaborate at the federal level to formulate a national response and resolve questions of jurisdictional overlap (Personal communication, R. Koppaka, DGMQ, March 18, 2005). For instance, USDA APHIS and CDC issued complementary orders banning the import of birds from Southeast Asia to guard against avian influenza (CDC, 2004b). In the wake of the monkeypox outbreak, collaboration between CDC and FDA led the agencies to issue a joint order banning the interstate shipment of African rodents (FDA, 2003). In the same order, CDC banned the importation of these rodents into the United States (CDC and FDA, 2003).
A liaison from Veterinary Services frequently visits the CDC quarantine stations and speaks with them about zoonotic diseases. This person then will pass the information on to the other federal groups in making his rounds (Appendix E). It appears that this individual is one of the main means of communication about zoonotic disease among the inspectors from the three agencies.
Jurisdictional Overlap and the Seizure of Bush Meat
Bush meat is a term broadly applied to game meat from wild animals that are hunted for consumption, typically in the bush of Africa but also elsewhere in the world. The illegal importation and trade in bush meat has burgeoned in recent years, along with an increased demand for farmed game meats (Klein, 2005). Bush meat has the potential to carry microbial threats of public health concern, as described below; consequently, CDC quarantine inspectors have found or have been alerted with growing frequency to the presence of bush meat in passengers’ baggage (Committee, 2005). CDC is only one of four federal agencies that have regulatory authority over domestic and imported game meats, however. The following paragraphs explain how CDC, USFWS, USDA APHIS, and CBP manage their overlapping responsibilities for bush meat (Klein, 2005; Appendix E).
Bush meat comes from a wide variety of animals, including NHPs, hoofed animals, reptiles, birds, and rodents, many of which are protected by international wildlife and trade laws. The consumption of bush meat
may pose a public health risk because the animals’ health and origin are often unknown and because any pathogens that lie in bush meat from NHPs have the potential to cross the species barrier into humans with relative ease. Communicable diseases of public health concern that may originate in bush meat include Ebola, HIV/simian immunodeficiency virus (SIV), monkeypox, herpes B, Rift Valley fever, tuberculosis, salmonellosis, and brucellosis. Animal diseases of concern in bush meat may include transmissible spongiform encephalopathies, such as mad cow disease and scrapie (Klein, 2005).
The commercial harvest and importation of bush meat into the United States is often illegal and a violation of international laws. The total amount of bush meat entering the United States is unknown, but USFWS, USDA, and CBP estimate that only a small fraction is intercepted. The United Kingdom’s Department of Food and Rural Affairs estimates that about 12,000 tons of smuggled bush meat enters that country annually (Klein, 2005; Appendix E).
USDA APHIS, USFWS, FDA, and CDC all have jurisdiction over bush meat based on the following laws and regulations (Klein, 2005):11
-
USDA APHIS has jurisdiction under the Animal Health Protection Act to inspect, detain, quarantine, seize, and destroy animals, meat, and meat products in interstate commerce or those being imported into the United States that pose a risk of introducing a pest or foreign animal disease, such as foot-and-mouth disease or avian influenza.
-
USFWS has authority under the Endangered Species Act, the Lacey Act, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and the Wild Bird Conservation Act to prohibit the importation of any wild animals or animal products that may threaten native wildlife or violate state, federal, or local wildlife laws.
-
CDC has jurisdiction under the Public Health Service Act to prohibit the importation of animals and animal products and to regulate foreign quarantine to prevent introduction of communicable diseases that threaten public health. CDC bans include importation of all NHPs, African rodents (42 CFR §71.56), civets, and Asian birds. These bans are specifically designed to protect the U.S. population from Ebola, SIV, monkeypox, SARS, and avian influenza.
-
FDA has jurisdiction under the Federal Food, Drug, and Cosmetic Act, which says that all foods not covered by standard meat and poultry inspections must meet the same safety standards applied to all domestic
|
11 |
See Table E.1 in Appendix E for further details. |
-
foods. In addition, under the Public Health Safety Act, the FDA can prohibit the interstate commerce of animal products to prevent the transmission of communicable disease harmful to humans.
When multiple federal agencies have jurisdictions over a single product (such as bush meat), determining responsibility is based primarily on the particular situation at hand. The local heads of each agency will contact one another and determine whose jurisdiction involves the most stringent regulation. For example, if endangered monkey meat is discovered at a port of entry, the risk posed by pathogens that could be in the meat leads CDC to have primary responsibility even though endangered species are the responsibility of USFWS.
Since CDC has very few local inspectors and no disposal facilities, it will often rely on inspectors from other groups (usually APHIS) to notify it of any confiscated bush meat. Then, CDC can either seize the product or instruct APHIS to seize and dispose of the product on its behalf, since APHIS would have access to the proper disposal facilities (Appendix E).
The CDC Animal Inspection Process
Paper shipping manifests—lists of cargo coming into a port—are the principal tool employed by the CDC quarantine stations at ports of entry to identify the animals, animal products, etiologic agents, and so on that warrant inspection. The staff study these lists each day and often ask CBP field staff to conduct the physical inspections of specific shipments on the Core’s behalf. CDC is infrequently called by other agencies to conduct a physical inspection of these imports (Appendix E).
In addition to shipping manifests, the CDC quarantine staff review the vaccination certificates of imported dogs and cats, answer telephone queries, and follow up on calls from officials of other federal inspection agencies—particularly CBP’s veterinary inspectors and USFWS inspectors—who have identified items that appear to fall within the Quarantine Core’s jurisdiction. CBP inspectors do not actively seek animal products and related items of public health concern, but as a courtesy, they generally notify CDC quarantine station staff if they come across such an item in the course of their work. At times, local law enforcement officials, individuals from airlines and cargo carriers, local veterinarians, and local health groups also inform the Quarantine Core when they perceive a possible public health threat in imported animals or animal products (Appendix E).
When quarantine station staff inspect an animal in person, they conduct a visual inspection for outward signs of illness. If such signs are visible, the animal is confined until a veterinarian from CBP or the private sector conducts a clinical examination at the importer’s cost (Appendix E).
The Core’s Reliance on Federal Partners
Since the Quarantine Core lacks sufficient staff to conduct all the necessary physical inspections of cargo and items carried by passengers, it often delegates this responsibility to other parties, most often CBP. Some imports are cleared by telephone and others by fax (Personal communication, J. Barrow and M. Remis, DGMQ, November 9, 2004). The CDC quarantine stations allow CBP inspectors to sign through their materials and goods when the stations are closed (in general, CDC quarantine stations are open only during regular business hours). The station staff occasionally train CBP inspectors to recognize outward signs of disease of public health concern in animals, but the stations’ small workforce and travel budget limit the frequency of such training. CDC quarantine stations that have jurisdiction over an especially large volume of cargo shipments frequently request assistance from state and local partners to enforce quarantine regulations over imported animals and animal products perceived to be a potential public health threat. The Quarantine Core also requests assistance at times from private individuals, such as local veterinarians, or from local law enforcement officers. The need for assistance is particularly acute at subports. In such cases, a product will be held until a CDC quarantine inspector either arrives or communicates directions on how to proceed (Appendix E).
Some at-risk cargo enters the United States without being inspected or cleared (Personal communication, J. Barrow and M. Remis, DGMQ, November 9, 2004). In addition to the factors noted above, this is a result of the tremendous volume of imports, the stations’ broad geographical jurisdictions, the inefficiency of the paper-based process, and the inability to identify intentionally or unintentionally mislabeled cargo. For instance, if the samples of the 1957 pandemic strain of influenza virus accidentally shipped worldwide by a private U.S. company in early 2005 had been imported to the United States, those shipments would not have captured the attention of the Quarantine Core because they were not labeled as pandemic strains (Stein and Vedantam, 2005).
Electronic Cargo Manifest Systems
The Trade Act of 2002, slowly being implemented at the ports of entry, is converting to paperless shipping manifests. DGMQ is pursuing the possibility of accessing the International Trade Data System with the goal of reviewing incoming cargo at all ports and identifying those items that might need further scrutiny (Personal communication, J. Barrow and M. Remis, DGMQ, November 9, 2004). At present CBP has access to the Automated Manifest System (AMS), another electronic manifest tool whose utility de-
pends in part on the accuracy of the importer’s labeling. According to staff at every quarantine station the committee visited, having access to AMS would significantly enhance their ability to identify cargo that poses a public health threat (Committee, 2005). The major barrier to access is financial. Security clearance is required for anyone who accesses this system and DGMQ has not allocated resources for this yet, although the committee understands it is under active consideration. In addition, stations might require updated communications links.
Inspection of Nonhuman Primates
CDC quarantine station staff always observe the importation of NHPs to determine whether regulations designed to protect people are followed. These animals are so genetically similar to humans that an infectious agent in an NHP could cross the species barrier to humans relatively easily. NHP importers are reportedly diligent about notifying USFWS of a pending shipment, and procedures are in place for both USFWS and CBP to notify CDC of such shipments. When the animals arrive, inspectors from the Quarantine Core
-
Ensure that people remain 10 feet or more from the NHPs.
-
Check that the aircraft door separating the crew from the NHPs is securely closed to prevent air exchange, which could potentially transport respiratory droplets containing microbial threats.
-
Check that no animal excretions remain in the aircraft once the animals are offloaded.
Clearing a shipment of NHPs takes 3 to 4 hours. One shipment consists on average of 120 NHPs. Los Angeles International Airport receives approximately 10,000 NHPs per year, more than any other CDC quarantine station (Cetron, 2004a; Committee, 2005; personal communication, M. Marty, DGMQ, May 16, 2005; Appendix E).
CONCLUSION
This chapter has illustrated the complexity of the Network within which the CDC quarantine stations operate. Clearly, the actions of the Core alone do not assure the effective protection of travelers to and people within the United States from microbial threats of public health significance that originate abroad. In the next chapter, the committee presents a vision that encompasses the entire Quarantine Network. The subsequent recommendations are designed to help the Core achieve its part of that vision and influence its partners in the Network to do the same.
REFERENCES
APHIS (Animal and Plant Health Inspection Service, United States Department of Agriculture). 2003. APHIS Fact Sheet. The Animal and Plant Health Inspection Service and Department of Homeland Security: Working Together to Protect Agriculture. [Online] Available: http://www.aphis.usda.gov/lpa/pubs/fsheet_faq_notice/fs_aphis_homeland.pdf [accessed April 4, 2005].
Bonner RC, Commissioner, U.S. Customs and Border Protection. 2005. Fiscal 2006 Appropriations: Homeland Security: Statement of Robert C. Bonner, Commissioner, U.S. Customs and Border Protection. Statement at the March 15, 2005 hearing of the Subcommittee on Homeland Security, Committee on House Appropriations, U.S. House of Representatives.
CBP (U.S. Customs and Border Protection, Department of Homeland Security). 2005a. Advance Passenger Information System (APIS) Fact Sheet. [Online] Available: http://www.customs.gov/xp/cgov/travel/inspections_carriers_facilities/apis/apis_factsheet.xml [accessed Jun 14, 2005].
CBP. 2005b. CBP Mission Statement and Core Values. [Online] Available: http://www.cbp.gov/xp/cgov/toolbox/about/mission/guardians.xml [accessed April 19, 2005].
CBP. 2005c. Immigration Inspection Program. [Online] Available: http://www.cbp.gov/xp/cgov/border_security/port_activities/overview.xml [accessed April 19, 2005].
CBP. 2005d. Ports of Entry. [Online] Available: http://www.cbp.gov/xp/cgov/toolbox/ports/ [accessed April 19, 2005].
CBP. 2005e. Ports of Entry and User Fee Airports. [Online] Available: http://www.cbp.gov/xp/cgov/import/communications_to_industry/ports.xml [accessed July 11, 2005].
CDC (Centers for Disease Control and Prevention). 2001. Exposure to Patients with Meningococcal Disease on Aircrafts—United States, 1999-2001. MMWR Weekly 50(23): 485-489.
CDC. 2004a. Memorandum of Agreement to Prevent the Introduction, Transmission, and Spread of Communicable Diseases in the United States (Version 4). Memorandum of Agreement, 2004.
CDC. 2004b. Order of the Centers for Disease Control and Prevention, Department of Health and Human Services. [Online] Available: http://www.cdc.gov/flu/avian/pdf/embargo.pdf [accessed June 14, 2005].
CDC. 2004c. Imported Lassa fever—New Jersey, 2004. MMWR Morb Mortal Wkly Rep 53(38): 894–897.
CDC, FDA. 2003. Control of communicable diseases; restrictions on African rodents, prairie dogs, and certain other animals. Federal Register 68(213): 62353–62369.
Cetron M. 2004a. Animal Importations. Presentation at the October 21, 2004, Meeting of the IOM Committee on Measures to Enhance the Effectiveness of the CDC Quarantine Station Expansion Plan for U.S. Ports of Entry, Washington, DC.
Cetron M. 2004b. Immigrant Refugee and Migrant Health Branch, DGMQ. Presentation at the October 21, 2004, Meeting of the IOM Committee on Measures to Enhance the Effectiveness of the CDC Quarantine Station Expansion Plan for U.S. Ports of Entry, Washington, DC.
Checko P, Libbey P. 2005. Measures to Enhance the Effectiveness of CDC Quarantine Station Expansion Plan for U.S. Ports of Entry. Presentation at the January 20, 2005, Meeting of the IOM Committee on Measures to Enhance the Effectiveness of the CDC Quarantine Station Expansion Plan for U.S. Ports of Entry, Baltimore, MD.
Committee (IOM Committee on Measures to Enhance the Effectiveness of the CDC Quarantine Station Expansion Plan for U.S. Ports of Entry). 2005. Unpublished. Notes on Site Visits to DGMQ Quarantine Stations.
CRS (Congressional Research Service, The Library of Congress). 2004. Border Security: Inspection Practices, Policies, and Issues. [Online] Available: http://fpc.state.gov/documents/organization/33856.pdf [accessed April 7, 2005].
DGMQ (Division of Global Migration and Quarantine, National Center for Infectious Diseases, Centers for Disease Control and Prevention). 2002. Health Alert Notice. Informational card for public distribution.
DGMQ. 2004. Program Review Fiscal Year 2003. Program review, May 7, 2004.
Di Giulio DB, Eckburg PB. 2004. Human monkeypox: an emerging zoonosis. The Lancet Infectious Diseases 4(1): 15–25.
Division of Law Enforcement, U.S. Fish & Wildlife Service. 2002. Annual Report FY 2001. [Online] Available: http://library.fws.gov/Pubs9/LEannual01.pdf [accessed August 16, 2005].
DQ (Division of Quarantine, National Center for Infectious Diseases, Centers for Disease Control and Prevention). 1991. Technical Instructions for Medical Examination of Aliens. [Online] Available: http://www.cdc.gov/ncidod/dq/pdf/ti-alien.pdf [accessed May 5, 2005].
DQ. 2000. Public Health Screening at U.S. Ports of Entry: A Guide for Federal Inspectors. [Online] Available: http://www.cdc.gov/ncidod/dq/pdf/hguide.pdf [accessed January 25, 2005].
FASS (Federation of Animal Science Societies). 2003. No Retraining for Agriculture Inspectors in Border Agency Plan. [Online] Available: http://www.fass.org/fasstrack/news_item.asp?news_id=1646 [accessed August 16, 2005].
FDA (U.S. Food and Drug Administration, Department of Health and Human Services). 2003. Joint Order of the Centers for Disease Control and Prevention and the Food and Drug Administration, Department of Health and Human Services. [Online] Available: http://www.fda.gov/oc/opacom/hottopics/monkeypox/monkeypox.html [accessed June 15, 2005].
GAO (United States Government Accountability Office). 2005. Homeland Security: Much Is Being Done to Protect Agriculture From a Terrorist Attack, but Important Challenges Remain. GAO–05–214. Washington, DC: GAO.
IOM (Institute of Medicine). 2003. The Future of the Public’s Health in the 21st Century. Washington, DC: The National Academies Press.
Jordan JL, Federal Air Surgeon, Federal Aviation Administration. 2005. Efforts to Prevent Pandemics by Air Travel. Statement at the Apr. 6, 2005 hearing of the Subcommittee on Aviation, Committee on Transportation and Infrastructure, U.S. House of Representatives.
Klein PN. 2005. Regulatory report: game meat: a complex food safety and animal health issue. Food Safety Magazine 10(96).
Lasher LE, Ayers TL, Amornkul PN, Nakatab MN, Effler PV. 2004. Contacting passengers after exposure to measles on an international flight: implications for responding to new disease threats and bioterrorism. Public Health Reports 119(5): 458–463.
LoBue PA, Moser KS. 2004. Screening of immigrants and refugees for pulmonary tuberculosis in San Diego County, California. Chest 126(6): 1777–1782.
Maloney, SA. 2001. Overseas Screening and Stateside Evaluation of Immigrants and Refugees with Tuberculosis. Presentation at the July 2001 Annual Meeting of the Tuberculosis and Respiratory Diseases Institute, American Lung Association of North Carolina, Black Mountain, NC.
MedAire. 2005. MedAire: A Health and Security Company. [Online] Available: www.medaire.com [accessed April 13, 2005].
Meenan JM, Executive Vice President and Chief Operating Officer, Air Transport Association of America Inc. 2005. Statement of John M. Meenan. Statement at the Apr. 6, 2005 hearing of the Subcommittee on Aviation, Committee on Transportation and Infrastructure, U.S. House of Representatives.
Ndao M, Bandyayera E, Kokoskin E, Diemart D. 2005. Malaria “epidemic” in Quebec: diagnosis and response to imported malaria. Canadian Medical Association Journal 172(1): 46–50.
OIS (Office of Immigration Statistics, U.S. Department of Homeland Security). 2004. 2003 Yearbook of Immigration Statistics. [Online] Available: http://uscis.gov/graphics/shared/statistics/yearbook/2003/2003Yearbook.pdf [accessed April 6, 2005].
PPQ (Plant Protection and Quarantine, Animal and Plant Health Insepction Service, United States Department of Agriculture). 2005. Animal Product Manual. [Online] Available: http://www.aphis.usda.gov/ppq/manuals/pdf_files/APM.pdf [accessed August 16, 2005].
Royce S. 2005. Quarantine Stations and the Control of M. tuberculosis. Presentation at the January 20, 2005, Meeting of the IOM Committee on Measures to Enhance the Effectiveness of the CDC Quarantine Station Expansion Plan for U.S. Ports of Entry, Baltimore, MD.
Shu P. 2005. Fever screening at airports and imported dengue. Emerging Infectious Diseases 11(3): 460–462.
Stein R, Vedantam S. 2005, Apr. 13. Deadly flu strain shipped worldwide: officials race to destroy samples. The Washington Post. A1.
Zhong N. 2004. Management and prevention of SARS in China. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 359(1447): 1115–1116.


































