2
Enabling Science and Technology That Drives the Application of Sustainable Chemistry
Today, chemists can make virtually any molecule, no matter how structurally complex, using the synthetic methods available to them. On the other hand, only a very small percentage of the chemical products are made following the principles of green chemistry—which is based on the ultimate premise that it is better to prevent waste than to clean it up after it is formed.1,2
Building this capacity to carry out what often needs to be fundamentally new chemical transformations requires a global view as well as a strong tie between academe and industry. Because the design of the chemical synthesis occurs too often without engineering input in the early stages of research and development (R&D) when it can be the most valuable, more collaboration and dialog between chemists and engineers is essential. Overall, approaches are needed to create more cross-talk between all members of the chemical enterprise. Ideally, the science and technology carried out by chemists and chemical engineers will be identical to the practices of green chemistry and engineering3 and the overarching sustainability goals they support.
|
1 |
Anastas, P. T., and J. Warner. 1998. Green Chemistry Theory and Practice. Oxford: Oxford University Press. |
|
2 |
Poliakoff, M., J. M. Fitzpatrick, T. R. Farren, and P. T. Anastas. 2002. Green Chemistry: Science and Politics of Change. Science 297:807–810. |
|
3 |
Green chemistry and engineering is additionally discussed in Chapter 1. Many good web sites also exist at: the EPA (www.epa.gov/greenchemistry/), the Royal Society of Chemistry (www.chemsoc.org/networks/gcn/), and the American Chemical Society’s Green Chemistry Institute (www.chemistry.org). |
|
Chemists need to understand that adding an environmental or sustainability layer over research is not a constraint on creativity but rather is a challenge to creativity … Forward-thinking companies are beginning to realize this point. The economic advantages need to be understood. John B. Carberry, E.I. du Pont de Nemours and Company |
GREEN CHEMISTRY AND ENGINEERING
Important green chemistry and engineering needs and capabilities are provided below.4 In some cases the status of these capabilities may be closer to the ideal state than indicated. For these situations, the most important task is educating chemists and engineers to inform them about the availability and utility of these tools. In order to design the least wasteful process possible, it is essential that the chemist and chemical engineers know the fates and effects of all chemical inputs in the bond forming steps. This will require that they be informed by adequate life cycle analysis and toxicological data, which are discussed later in this chapter.
Efficient Chemical Bonding
Too often, a large excess of the nonlimiting reagent is used to convert the limiting reactant to product in the highest yield possible. The concept that the limiting reagent defines chemical yield encourages waste in chemical processing by focusing more attention on the end product than on how it is produced. One principle of green chemistry is that catalytic processes are preferred to stoichiometric ones. Catalysis is often used in petrochemical and bulk chemical industries where semicontinuous and continuous processing is commonplace, but less so in the fine chemical and pharmaceutical industries where batch processing is the rule. A rapidly growing area of catalysis use for these latter industries is in the area of biotransformations. This is driven, in part, by the need for chiral molecules as building blocks for drugs which have very specific stereochemical (or three-dimensional spatial) relationships with the enzymes and re-
|
4 |
It should also be noted that there are many excellent examples of green chemistry and engineering successes in industry and academe that could not be highlighted in this report. The U.S. EPA Presidential Green Chemistry Challenge web site is an excellent source for such examples: http://www.epa.gov/greenchemistry/presgcc.html. |
ceptors they influence. The emergence of directed enzyme evolution5 to modify natural microorganisms in order to carry out transformations with higher chemo and stereochemical selectivity has helped fuel this growth. Asymetric chiral catalysis is an alternate route.
As pointed out by Glenn Nedwin during the workshop,6 the production of textile derivatives synthesized by means of an enzymatic based process instead of a traditional multi-step chemical synthesis can lead to significant environmental benefits. It was found that utilizing enzymes can (1) reduce aquatic pollution by limiting the utilization of solvents, acids, chlorine derivates, oxidizing agents, sulphides, and other chemicals; (2) save energy by reducing process temperature; and (3) avoid having to resort to useful agricultural raw materials needed for other applications.
There are many other approaches to catalytic bond formation that still need to be explored, such as those being carried out at the Center for Environmentally Beneficial Catalysis (CEBC). CEBC is a multi-university NSF Engineering Research Center, headquartered at the University of Kansas with core partners at the University of Iowa, Washington University in St. Louis, and Prairie View A&M University. The center was created with a mandate to make “sustainable” manufacturing processes available to industry—that is, improved processes that minimize their “environmental footprint” while remaining profitable. CEBC’s approach to doing this is by reducing or eliminating the use of hazardous materials in manufacturing (including the catalysts themselves or hazardous solvents), minimizing the formation of wasteful byproducts, and by improving energy efficiency. In pursuit of its vision and research goals, CEBC is guided by the principles of “Green Engineering” and “Green Chemistry”.
Safer Solvent Selection
Another important green chemistry principle is to use safer solvents and reaction conditions by avoiding use of organic solvents, separation agents, or other auxiliary chemicals. When these chemicals are necessary, innocuous chemicals should then be used to the greatest extent possible.
Manufacturing chemicals can generate significant amounts of waste by-products and pollutants, such as halogenated or toxic organic solvents, volatile toxic or ozone-depleting organic compounds, hazardous air pollutants such as NOx, COx, SOx, and aqueous wastes. Roger Sheldon devel-
|
5 |
For a review of this topic see: Farinas, E. T., T. Butler, and F. H. Arnold. 2001. Directed Enzyme Evolution. Current Opinion in Biotechnology 12:545–551. |
|
6 |
See comments by Glenn Nedwin, Workshop Summary in Appendix D, p. 143. |
TABLE 2.1 Sectors of the Chemical Industry by Quantity of Byproduct per kilogram (kg) of Product Generated.
|
Industry Sector |
Product tonnage |
E-factor (kg byproducts/kg product) |
|
Oil refining |
106-108 ca |
0.1 |
|
Bulk Chemicals |
104-106 |
<1-5 |
|
Fine Chemicals |
102-104 |
5-50 |
|
Pharmaceuticals |
10-103 |
25-100+ |
|
SOURCE: Sheldon, R. A. 2000. Atom Efficiency and Catalysis in Organic Synthesis, Pure Appl. Chem., 72(7):1233–1246. |
||
oped the E-factor as a measure of the efficiency of the chemical industry, and this formula is expressed mathematically as: E = amount of waste (kg)/amount of product (kg) for an overall process (Table 2.1). According to Sheldon,7 “Waste is defined as everything produced in the [chemical] process except the desired product. It consists primarily of inorganic salts (e.g., sodium chloride, sodium sulfate, ammonium sulfate), formed in the reaction or subsequent neutralization steps, or derived from stoichiometric inorganic reagents (e.g., a stoichiometric metal oxidant). The E factor increases dramatically on going downstream from bulk to fine chemicals and specialties such as pharmaceuticals. This is partly owing to the fact that the production of fine chemicals involves multi-step syntheses but is also a reflection of the use of stoichiometric reagents rather than catalytic methodologies.”
Recently scientists at GSK reported8 that in a life cycle study for waste produced from pharmaceutical manufacturing facilities, approximately 80 percent of the waste is solvent-related with the remaining 20 percent being solid-related waste. Therefore, dealing with solvent waste and using green solvents in the process has become very important. Solvents can often be recovered and recycled, but recovery efficiencies typically range from 50-60 percent .9
Process Analytical Technologies
The use of real time, inline, and online analysis for pollution prevention is another principle of green chemistry that relates to solvent selection. Once the desired product has reached its maximum yield in the reaction, further reaction time can lead to yield loss due to degradation to one or more side products. Moreover, traditional analytical methods that require removing a sample for analysis can expose the worker and work environment to chemical hazards. Process Analytical Technologies (PAT) tools are an important emerging technology for cleaner chemical manufacturing. PAT tools include analytical systems for the analysis and control of manufacturing processes based on timely measurements during processing. They also include measurements of critical quality parameters and performance attributes of raw and in-process materials and processes to ensure acceptable end product quality at the completion of the process. The use of inline and online PAT tools to monitor solvent distillation during recovery operations should improve the recovery efficiency. Also, improved technologies for recovering solvents, now typically achieved via distillation, may improve recovery yields. More extensive use of PAT tools in the R&D and manufacturing phases will contribute to lowering the environmental burden of chemical manufacturing.
It is well accepted within chemical processing that the development of green process chemistries to fully utilize green solvents improves product selectivity and conversion as well as reduces aqueous wastes.10 The pharmaceutical industry’s current work with global regulatory agencies to create a harmonized approach to residual solvents may be a useful tool for the rest of the chemical enterprise.
Selection Tools
Synthetic chemists expend much intellectual energy trying to answer the question, “How do I select a green solvent for the process?” This task is difficult because most of them have received little training in green chemistry, either in their academic or industrial experience. Fortunately, there are resources becoming available from a variety of sources. For example, in their guidance document on impurities and residual solvents, the U.S. Food and Drug Administration (FDA) provides information on classes of solvents based on patient safety and environmental considerations (reference: http://www.fda.gov/cder/guidance/Q3Cfinal.htm). In addi-
tion, the EPA Office of Pollution Prevention and Toxics (OPPT) has also produced the Green Chemistry Expert System (GCES)—which is readily available from their web site at http://www.epa.gov/greenchemistry/tools/html. GCES allows users to build a green chemical process, design a green chemical, or survey the field of green chemistry. It includes a green solvents/reaction condition module which compares information on green solvent alternatives to traditional choices based on physiochemical properties. The system is equally useful for new and existing chemicals and their synthetic processes, and includes extensive documentation.
Elsewhere, in a recent publication11 of the American Institute of Chemical Engineers (AIChE), the Center for Waste Reduction Technologies and Center for Chemical Process Safety has reported such a useful tool for selecting solvents—MERITT—which stands for Maximizing EHS (Environmental Health and Safety) Returns by Integrating Tools and Talents. This tool encompasses input regarding pollution prevention, inherent safety, green chemistry, and related topics. MERITT outlines a way to integrate these considerations for a chemist when designing a manufacturing process and includes a solvent selection tool.12 This guide ranks solvent choices according to required waste treatment, impact on health, and safety. The reader is encouraged to study these references for a more comprehensive understanding.
Several other resources exist for selecting green solvents. A spreadsheet tool for solvent selection in chemical processing called CAPEC/CAMD (http://www.capec.kt.dtu.dk) is gaining popularity. There is an excellent tutorial program on solvent selection available at http://www.chemsoc.org/pdf/gcn/solventsystem.ppt. The SAGE alternative solvent guide, which was developed collaboratively by the U.S. EPA Air Pollution Prevention and Control Division (APPCD) and the Research Triangle Institute, is available at http://www.clean.rti.org/index.cfm.
Finally, no matter what scale is employed, chemists should at the very least always consult the material safety data sheet (MSDS) for any solvent they plan to use in a synthesis.
Controlling Thermal Conditions
Photochemistry, microwave chemistry, and ultrasonic chemistry that involve control of thermal reaction conditions offer new or expanded opportunities for greener transformation tools. Long used at laboratory scale, these technologies have not seen a commensurate use at commercial scale
due to the perception of many chemists that these technologies are difficult to use commercially. Consequently, chemists redesign processes, often creating ones that generate more waste, in order to avoid using these tools. Hence, more development is needed to make these thermal control technologies for chemical processes commercially viable at larger scales.
Purification and Recovery
Once a molecule of interest has been created it must be separated and isolated from the reaction at a desired state of purity consistent with product specifications. As reported by Sheldon, and amplified by Constable and coworkers at GSK,12 solvents are a major component of chemical manufacturing waste. Continuing investment in the development of separations tools such as commercial scale chromatography and membranes can play an important role in limiting the formation of waste solvents.
Crystal Engineering
For solid state chemical products, the crystalline form is critical to product performance. Traditional crystallization methods use large amounts of solvent. Once crystals are formed, they must be removed quickly in order to avoid continued growth beyond the specified size. Otherwise, energy intensive and potentially hazardous particle size reduction using high energy grinding must be used to restore the desired size range.
The emergence of crystal engineering as an important tool in the chemist’s toolbox should continue to be encouraged and financially supported. Novel tools such as impinging jet crystallization (IJC) offer a unique opportunity for collaboration between chemists and chemical engineers. There are basically two types of IJC: counter-solvent and reactive crystallization that are used to produce crystals with very narrowly defined particle size distribution. Such size limitations are mandatory for optimum performance of many chemical products. In some parts of the chemical enterprise this tool is well established, while in others it is just emerging.
Equipment Cleaning
The involvement of equipment cleaning in purification and product recovery is another area that would benefit from new technologies, particularly in the batch manufacturing chemical industries represented by the Pharmaceutical Research and Manufacturers of America (PhRMA) and Synthetic Organic Chemical Manufacturers Association (SOCMA).
The traditional way to clean manufacturing equipment is to fill, boil, drain, sample, and assay (usually using an organic solvent). This cycle is repeated until the equipment is clean according to some predetermined specification. Spray balls, which use less solvent, are being used with increasing frequency but for the most part only in non aqueous systems. Ultrasonic cleaning has been studied; however, the common cleaning agents used with aqueous solutions have been found to be abrasive to glass lined equipment. Combing water-based technology (or organics as a last resort) and the use of PAT represents an opportunity for improving the cleaning process.
Formulation
Site-Specific Delivery
In the pharmaceutical industry, research into drug delivery technology offers a great opportunity to reduce its overall environmental impact. This is because for many drugs less than 50 percent is actually used in the body, with the rest being emitted from the body unused. Improved bioavailability by overcoming solubility and permeability limitations can reduce the overall amount of drug a patient consumes. The biggest payoff could come from targeted drug delivery, which is defined as a system to direct the flow of a drug to the target organ, tissue, or synthetic medical structure such as a graft. Site-specific delivery enables a therapeutic concentration of a drug to be administered to the desired target without exposing the entire body to a similar dose. When one considers that a typical daily dose of a pharmaceutical can contain a billion-fold more molecules than are needed to occupy every disease related receptor, the potential benefits of site-specific delivery technology are obvious.
Design for Degradation
Design for degradation is another principle of green chemistry. Chemical products should be designed to break down into innocuous substances after use so that they do not accumulate in the environment. Products need to be stable for their intended use lifetime before being rapidly degraded once they enter the environment. Intended lifetime includes the time to incorporate the drug into the dosage form (tablet, capsule, injectable, etc.) as well as time to produce therapeutic effects within the patient. To address the growing issue of chemicals in the environment, research is needed to study molecular triggers or chemical switches to activate degradation of active pharmaceutical ingredients (API’s) following excretion into the environment after use.
|
In order to change something, you have to be able to measure it, and you have to be able to do it quantitatively, and life cycle analysis gives us one way of doing that. Richard Helling, Dow Chemical Company |
LIFE CYCLE ANALYSIS
As discussed, achieving sustainability requires a broad system view that integrates the multiple factors of social responsibility, environmental stewardship, and economical success. This involves having a keen understanding of the metabolism of chemical products—that is, their industrial ecology13—from the extraction of raw materials and creation of products, to their use and management of any resulting wastes. Life cycle analysis or assessment (LCA) and life cycle inventory (LCI) are tools that provide a means for systematic evaluation of the largest number of issues related to these impacts of the manufacturing of products through their full life cycle.
LCA is sometimes viewed as more comparative than absolute, and is found most useful for internal assessment and process development.14 Some differences exist between the two approaches: LCI is a data intensive method (with quantitative figures, databases, and subsequent analysis), while LCA incorporates damage metrics and consequences through approaches of sometimes questionable subjectivity.
The need for effective LCA is illustrated by the example of chlorofluorocarbons (CFCs), which were developed as safer alternatives to the sulfur dioxide and ammonia refrigerants in the late 1920s and early 1930s. Applying the metrics of what is often considered green—low in toxicity, safe to use (nonflammable, noncorrosive, and nonreactive with other chemical species), and having other superior properties (desirable thermal-conductivity and boiling-point characteristics)—CFCs to a large extent fit the bill. Unfortunately, as pointed out by Brad Allenby during the workshop, what made CFCs desirable on one scale—their stability and safety to humans—made them highly undesirable when they managed to enter the upper atmosphere and destroy ozone. CFCs were found to be present in only trace quantities in the atmosphere and yet, because of the
|
13 |
Frosch, R. A. 1995. The Industrial Ecology of the 21st Century. Scientific American 273(3):178–181. |
|
14 |
See comments by Richard Helling in the Workshop Summary, Appendix D, p. 120. |
dynamics of the system, they turned out to be extremely critical. Today, there continues to be these same gaps in the way that chemistry and its impact on global systems is thought about.
Thus, the challenge going forward is to be able to foresee such unintended consequences by accounting for such properties as stability within a large complex systems analysis. The major areas in which LCI/LCA tools require improvement are:
-
Economics (based on an appropriate standardized matrix such as total cost analysis—TCA)
-
Social issues (for which many options and matrices have been proposed)
-
Management of energy and process-related resources (water, temperature, pressure, etc.)
-
Emissions of pollutants in the different ecosystems (solid waste, aqueous effluents, air, etc.)
-
Availability, accessibility (extraction and related impacts), quality, and supply chain of raw and platform chemicals (including the management of their toxicity—MSDS contents need to be completed and verified for numerous products)
-
Optimization of the chemical processing industry (CPI): solvents, separation chemistry, etc.
-
Replacement of multi-step, wasteful chemistry by more selective and innovative biologically driven procedures
-
Reduction in environmental impacts for workers (occupational regulation, collective and personal protection) and local residents (dispersion and eventual transformation of pollutants)
-
Establishment of routes and yields of formation of waste and by-products, management, and recycling when initiating projects: The end-of-life disposition or recovery of chemical products (especially those generated in large quantities and which present potential long term toxicity) constitutes a strategic element which is now largely used on the commercial side (percentage of recycling materials; set up of a recovery channel; injection of the excess of electric energy into the local network, etc.)
-
Interpretation, utilization, and dissemination of the study results (data sharing with other companies)
Some of these items are priority elements that will be emphasized in the forthcoming European REACH (Registration, Evaluation and Authorisation of Chemicals)15 program and regulations. They are also
|
15 |
European Commission. 2001. White Paper on the Strategy for a Future Chemicals Policy (COM(2001)88); available at: europa.eu.int/comm/environment/chemicals/whitepaper.htm |
incorporated in the principles of green chemistry and green engineering discussed earlier in this chapter.
Despite the fact that there is no systematic, comprehensive method for analyzing opportunities for chemical processing improvement, LCA (now an ISO-standardized methodology of the 14040-14043 series) has expanded the traditional process boundary, considering up and downstream processes in terms of energy and materials use, waste generation, and business value creation.16 Some of these items require further technological developments and other related sustainability research programs in order to provide adequate answers and solutions.
According to Warner and coworkers,17 LCA should function as a strategic link between green chemistry and Environmental Impact Assessment (EIA). As pointed out by Helling during the workshop, many commercial and publicly released software and data that could assist chemists and chemical engineers in this way already exist, such as: Ganzheitliche Bilanzierung, version 4 (GaBi IV, found at www.gabisoftware.de), and Tool for Reduction & Assessment of Chemical & other Environmental Impacts (TRACI) from the U.S. Environmental Protection Agency (EPA). However, use and interpretation of such LCA software largely remains a job for specialists because most chemists and chemical engineers do not have the training to interpret the results they obtain.
Going forward, many scientists believe that more emphasis should be placed on understanding the toxicity of chemicals and on increasing the capacity of chemical sciences and toxicology to provide this necessary basic information. The current unavailability of reliable toxicity data and corresponding uncertainties (discussed in more detail later in this report) constitute major hurdles that hamper the application of efficient LCA studies and hinder progress in sustainability. There is also a need to understand the long-term impacts of chemicals in the environment—such as persistence, bioaccumulation, global warming potential, or ozone depletion—and be able to evaluate within LCA as discussed earlier. However, it is clear that present state-of-the-art LCA methodologies such as GaBi IV and TRACI are very useful for internal comparative assessments of environmental and societal impacts for those who know how to use them. The need for more effective and easier to use tools to guide sustainable chemical process development is essential to the future of the chemical industry.
TOXICOLOGY
Many data related to human and environmental toxicology of chemicals are either questionable or missing, which has significant implications for advancing the application of green chemistry and engineering and overall sustainability goals. For example, ionic liquids,18 which provide both superior properties and environmental benefits, are promising replacements for volatile organic solvents currently used by industry. However, as it has been recently pointed out,19 “Despite the potential for ionic liquids to reduce [volatile organic compound] emissions…. Little is known about the toxicity or mobility of ionic liquids in the environment.”
Thus, there is clearly a role for all involved in the chemical industry to assist in funding and otherwise supporting the collection of critical data related to the most pervasive—and the most potentially useful—chemicals in the industrial environment.
Such a resource is essential for more effective LCA, which requires a large volume of data input in order to produce reliable final figures to support decision making. Current data sources for LCA are derived from MSDS and other technical fact sheets, but numerous others have to come from separate sources that must often be identified, retrieved, and correctly handled by the user on a case-by-case basis. Other information is either inconsistent or not thorough in terms of relevant environmental data.
Human Toxicity Data
In order to increase basic knowledge of the biological impacts of chemicals on human health, and to generate the experimental and/or theoretical values needed for a quantitative toxicological assessment of a larger number of products (all major elements qualified to justify the forthcoming European REACH program), priority should be given to research in the topics discussed in this section.
In Vitro Biological Assays
In vitro biological models (see Figure 2.1) are of primary importance in providing the numerous data needed in LCA studies. This is because management of toxicity pathways plays a key role in LCA. Measurements
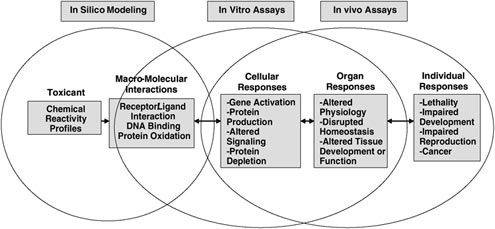
FIGURE 2.1 Example of a toxicity pathway.
SOURCE: A Framework for a Computational Toxicology Research Program U.S. Environmental Protection Agency, 15, http://www.epa.gov/comptox/publications/comptoxframework06_02_04.pdf.
performed on human and animal cells, subcellular fractions, or specific biological receptors or biomolecules have the potential to provide an efficient alternative to tedious, labor intensive, and costly standard toxicity test methods based on in vivo experimentation. Only a small number of chemicals can be processed by animal based toxicological assays. In some industrial countries, green politicians and parties are lobbying to lower the number of animals incorporated into toxicity tests, and regulations in this direction become more stringent each year. At the same time, it is crucial for scientists and toxicologists to generate reliable quantitative information on individual chemicals according to their respective biological effects (from identification of discrete molecular initiating events to adverse outcomes to molecular alterations and linkages across biological levels of organization).
There is pressure to develop sensitive new assay methods for measuring those biological effects that are most likely to cause dramatic human health impacts. These impacts include: reproductive and developmental impacts, neurotoxicity, carcinogenicity and cancer hazard, and endocrine disruption and fertility.
The views presented for in vitro testing above are complementary to those presented by Robert Kavlock during the workshop,20 and several FDA21 and other22 scientific and technical reports. There is agreement that intensive research should be conducted in order to increase the number of toxicological tools and reliable data needed to verify and validate theoretically generated figures (see discussion later in this chapter).
Efforts need to be made to better understand chemical mixtures. New experimental biological assays based on precise study protocols are necessary to approach the impacts of binary and tertiary chemical mixtures. Enzymatic induction, inhibition, or any other mechanisms of interaction with fundamental and critical metabolic pathways related to important endogenous compounds such as thyroidal and steroid hormones should be thoroughly investigated. Identification of synergistic, promoting, inhibitory, and antagonistic effects would be a great benefit to increasing understanding in toxicology. Management of waste, which involves treating complex matrices containing trace amounts of pharmaceuticals or endocrine disrupting chemicals (EDCs), would largely benefit from these
|
20 |
See comments by Robert Kavlock, Workshop Summary in Appendix D, p. 124. |
|
21 |
See the FDA Office of In Vitro Diagnostics (OIVD), www.fda.gov/cdrh/oivd/ |
|
22 |
Sam Brauer (Business Communications Company Inc.), June 2003, The Market for In Vitro Toxicology Testing (B110R report); and ECVAM (European Center for the Validation of Alternative Methods) reports: Workshop Report 45: Novel Advances in Vitro Methods for Long Term Toxicity Testing (2001). |
such advances. Data metrics would also be required to set a priority list of potentially active compounds that could be detrimental to human health.
These experimental data would be integrated into dose-response relationships that are generally used to establish dose of exposure values. This would be accomplished by performing calculations on appropriate models, such as those proposed by implementation of a threshold level to the biologically measured effect. Whenever possible, more sophisticated mathematical models should be promoted, such as the Physiologically Based Pharmacokinetic (PBPK) model, which measures time of exposure to a target dose, and the Biologically Based Dose-Response (BBDR) model, which identifies target organ dose to early biological effects. These models require larger sets of data which could be supplied by experiments. The selectivity and sensitivity of newer assay methods would have a positive impact on the accuracy and overall quality of the data, which in turn would affect the evaluation of uncertainty values that accompany published reference dose or concentration figures. Data issued from complementary toxicity studies (animal in vivo assays, epidemiological studies, computational toxicology, etc.) would complete those databases that provide validated bio-statistical analysis.
Metabolic and Degradation Pathways
The characterization of the various toxicity properties of chemicals requires additional information such as the identification of their metabolic pathways in humans and the (bio)degradation routes of compounds in various environmental ecosystems.
Modern analytical instrumentation based on combined HPLC–mass spectrometry technique offer many opportunities to perform these experiments on biological models, ranging from simplified in vitro tests to in vivo studies on animals. High-throughput, sensitive assays can now be performed at reduced cost. Metabolic and transformation patterns of chemicals would also be useful for generating valuable kinetic information. These patterns could be used to set up priorities in lists of compounds that require additional testing. This type of prioritization would be necessary because such compounds are widely spread into the environment and could enter the food chain as a result of their long life in ecosystems. For example, the part of the food industry that prepares flour-based foodstuff uses nutrients recycled from materials of different origins, and this is a source of great concern in various western European countries. Biotransformation studies will also identify enzymatic reactions related to well known genetic polymorphism. Specific tests are now available to identify individual metabolic deficiencies such as those related to the P-450’s isoenzyme activities or to phase II metabolic pathways like those regulated by
N-acetyltransferase. These data also have positive impacts for the uncertainty linked to heterogeneity in different populations.
Computational Approaches
The gap of missing toxicity data is so large that it will be impossible to perform the complete battery of tests required to fill up databanks. Toxicologists need additional data on numerous chemicals. Agencies such as the EPA, which are charged with protecting human health and the environment from exposure to potentially harmful compounds in water, food, air, and soil, are interested in assessing—by alternative, non invasive methods—possible hazardous effects for tens of thousands of compounds. Such information would allow for prioritization of additional data requirements and accurate risk assessments.
Over the last ten years, advances in theoretical, computational chemistry, and molecular biology have led to generation of data related to potential biological activity and toxicity. The pharmaceutical companies largely rely on these new tools to design drugs. This new area, known as “computational toxicology,” may be able to predict the potential effects of compounds from their chemical (sub)structures if substantial developments, validation steps, and budgets are created to achieve the objectives and validate these computer tools. Understanding how structures are correlated and transformed to chemical functions and biological activities (including deleterious toxic effects), requires additional and sustainable efforts. In 2003, the Office of Research and Development (ORD) of the EPA launched an ambitious research program leading to the installation of a Framework for Computational Toxicology.23 This program already involves the collaboration of several partners: NIEHS, DOE, NERL, NCER, NCEA, NRMRL and NHEERL.
Computational toxicology is designed to address the questions of “when” and “how” to test chemicals because they could be hazardous, improving the prioritization of data requirements, and risk assessment. This multidisciplinary project of the EPA has been peer reviewed and evaluated by the experts in toxicology. Computational toxicology establishes the links with the other resources needed to reach the goals and objectives of sustainability in the chemical industry. The main objectives of the framework are to:
|
23 |
See more details in R. J. Kavlock’s presentation in the Appendix D, p. 124, and on the EPA Web sites: www.epa.gov/comptox/ (a general survey of the project) and www.epa.gov/comptox/comptox_framework.html (the entire text of the proposal). |
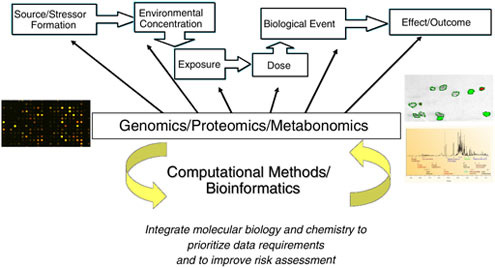
FIGURE 2.2 Source to outcome continuum—Development of Sound Structure-Activity Tools to Predict Health and Environmental Impact of Specific Chemicals.
SOURCE: Robert Kavlock, U.S. EPA.
-
Improve linkage across the source-to-outcome continuum (Figure 2.2)—from adding the chemical into the environment to the eventual biological effects
-
Develop approaches for prioritizing chemicals for subsequent screening, testing, and data generation in order to enhance the predictive understanding of toxicity pathways
-
Produce faster accurate assay methods (e.g. using systems biology to understand cells and organ’s mode of functioning) and provide predictive models for a secure hazard identification and enhancement of reliable quantitative risk assessment. This would permit the classification of chemicals by their potential to influence molecular and biochemical pathways of concern. The program could also integrate developments in cross species extrapolation and in the mixtures issues.
Quantitative Structure Activity Relationship (QSAR) development (see the DSSTox program of the EPA at www.epa.gov/NHEERL/dsstox/About.html) will also be considered by the Europeans in their REACH program as a bearing tool to supply more toxicity data without resorting to complex and costly in vivo experimentation. It is important that there be some collaboration on both sides of the Atlantic Ocean (and elsewhere) to validate harmonized approaches of these computerized tools in order to avoid future controversial discussions and conflicts.
Toxicity studies are ideally focused on the applications of chemicals; a component present in various products at different concentration levels likely has diverse toxicity profiles that should be addressed as well. This is a very challenging issue as is the question of the toxicity of isomers. The scientific literature has reported well-known examples of different dramatic toxic effects observed between isomeric forms. In such cases, experimentation is the only solution to solve this question.
Boundaries of assessment should be delimited in these topics; the main objective is to deliver indicative, pertinent, and relevant sources of information. The proposed solution should not transform scientific consideration into pure speculation that leads to questionable or subjective conclusions. Heuristic tools are also needed to fix boundaries and limitations to the implementation of such computerized solutions in LCA studies.
A strategic approach is recommended in order to provide the most appropriate toxicity data. To set up priorities and risk, computational toxicology should be the first line of investigation. In vitro testing should be carried out for a large number of compounds to provide preliminary dose-response data (and establish temporary Reference Doses (RfDs) or Reference Concentrations (RfCs). Then, in vivo experimentation could take place for a limited number of very hazardous chemicals present in measurable quantities in materials issued from any sources (including environmental ecosystems) and which come in contact with human beings.
|
How do we get into that list of 850 pesticidal inerts and tell the agency, these 63 are the ones you should worry about; and you should worry about 21 of them for birth defects and 32 of them for cancer effects; and be able to put some kind of a priori knowledge in the system? Robert Kavlock, U.S. Environmental Protection Agency |
Ecotoxicity Data
Environmental metrics often expressed as “Eco-Efficiency” do not exist under global standard protocols. Instead, most approaches involve energy intensity and consumption, mass intensities (including fossil resources and water), and pollutant emissions (limited to the major compounds released in the air, water, and soil). However, numerous factors can influence the fate and dispersion of chemicals into the different environmental compartments and ecosystems. Persistence, (bio)degradation, and mobility represent key elements that exert direct threats on surface,
ground water, and soil with consequences for the food supply chain (according to the importance of the enrichment factors associated to each trophic level). The establishment of physico-chemical parameters related to environmental fate, transport, and bioconcentration effects can be regarded as cost-effective surrogates for data generated from more complex laboratory experimentations.
For specific classes of compounds (i.e. endocrine disrupting products and carcinogens), the EPA has launched a series of databases (such as ECOTOX at: www.epa.gov/ecotox/), which contain comprehensive figures on the ecotoxicity of chemicals towards aquatic and terrestrial organisms, including plants. This internationally recognized source of information is also linked to predictive models for ecotoxicity endpoints and physico-chemical properties in the absence of empirical data. Similar trends concern other priority pollutants like pesticides, pesticidal inerts (constituents added to the active ingredients of a registered pesticide), heavy metals, and small particulate matters with high uptake coefficients (e.g. diesel exhaust).
Additional basic research is needed to better screen and identify persistent chemicals that would play an active role in the adverse health effects stressed by eco-toxicologists. Application of emerging technologies into new assay methods should be encouraged to generate complementary robust data sets relative to impacts resulting from the biology of chemicals in various environmental compartments. The development of a new discipline to better understand these issues would be very useful. It should incorporate and integrate basic concepts taken from geology, hydrogeology, agronomy, chemistry, and biology, particularly when it is related to soil and water dispersion.
The feasibility of a separate “Environmental Fact Sheet” assembling the items that should be integrated into LCA studies should be envisioned. Its content should be defined, evaluated, and thoroughly reviewed. There must be guidelines and standardized techniques for supplying such information.
CENTRALIZED DATA COMPILATION AND MANAGEMENT, AND INCREASED COLLABORATION
Many companies are building internal capacity to address sustainability parameters into their daily operations. One excellent example can be found at GlaxoSmithKline (GSK). Part of GSK’s Eco-Design Tool Kit includes a Green Chemistry Guide that offers guidance to GSK scientists and engineers on applying green chemistry concepts. This would allow them to enable more efficient use of resources, reduce environmental, health and safety impacts, and minimize costs. It includes:
-
A ranking and summary of the most used chemistries and ‘best-in-class’ examples from well-developed GSK processes.
-
A ranking and review of issues encountered during process design and development.
-
A ranking and summary of common technology alternatives for chemical processing.
-
Guidance on materials, process alternatives, synthetic route strategies, and metrics for evaluating chemistries, technologies, and processes.
A larger wide-scale effort is needed to compile such information from across industrial and academic R&D institutions, as well as other hightech industries (e.g. defense-related). Such an effort would help identify technologies that have been developed by one member of the enterprise that can be useful, but are unknown, to the other.
The EPA’s Green Chemistry Program is currently compiling and organizing journal articles into specific sub-topics for a literature database on the subject. Topic areas include alternative synthesis methods, catalysis, reaction conditions, and alternative solvents. The goal of this project is to have a database compilation of green chemistry literature which is publicly accessible that will enable researchers across the chemical industry to identify the new approaches to chemical synthesis. In addition to compiling such information, it will be important that greenness parameters such as E-factors24 and atom economy25 be supplied (or even required) for all chemical synthetic routes published in the scientific literature.
There is also a need to expand the availability of sound LCI data and methods. Future LCA studies and risk assessments would largely benefit from additional experimental and computer-generated (eco)toxicological data. In order to facilitate their utilization and incorporation into studies, some complementary practical measures should be taken. Verification and validation operations should be performed by agency senior staff, and centralization on a dedicated site should be made accessible to all (free of charge or at affordable cost) while being duly managed and maintained. This centralized and standardized repository of information, which requires coordinated efforts, must be adequately organized and presented in inventory matrices to provide easy pattern recognition and transfer to LCA platform and templates. Lauren Heine has proposed26 a toxicology
|
24 |
E stands for efficiency, and is the amount of waste (kg) generated per amount of product (kg) for an overall process. This is discussed more later in this chapter under Solvent Selection in the section on Chemistry Tools. |
|
25 |
Atom economy is the ability to avoid loss of atoms in a chemical synthesis—and is one of the 12 principles of green chemistry discussed in the Introduction. |
|
26 |
See Lauren Heine’s comments, Workshop Summary in Appendix D, p. 112. |
and exposure summary table. Improvements and complimentary figures should be incorporated into this model (i.e. the introduction of quantified parameters).
Chemistry is a complex discipline. Providing ways to deal with this complexity and determining where decisions should be made is not an easy task. Therefore, the organization and classification of data should be associated with a quantification process (scoring methods, weighing factors, and heuristics) that will allow for prioritization of hazard and risk and for further multi-criteria based analysis. These metrics should be evaluated extensively in order to reach a balanced agreement, since no validated equation to accurately evaluate “sustainability” has been presented thus far. Communicating the results of a complex analysis into a single figure, or into a simple and readily understood form that does not oversimplify the analysis conclusions, is an additional challenge that needs to be addressed.
CONCLUSIONS AND RECOMMENDATIONS
New scientific developments and more efficient tools to evaluate them are needed to enable the chemical industry to more effectively incorporate sustainability into general practices. Such an effort will require science, technology, and harmonized strategic approaches across disciplines, industries, and geographic boundaries:
Green Chemistry and Engineering. While chemists can currently make virtually any molecule using synthetic methods available to them, much more effort is needed in the development of green chemistry and engineering capabilities. These include the ability to:
-
efficiently form chemical bonds,
-
select solvents,
-
control thermal conditions,
-
purify and recover chemical products,
-
develop analytical methods,
-
formulate products,
-
model chemical reactions, and
-
perform all these tasks in an environmentally benign manner
These are essential for the development of industrial technologies that support sustainability.
Life Cycle Analysis. Life cycle assessment (LCA) is considered to be a powerful tool for comparing the environmental performance of products
generated under different operating conditions. In order to effectively implement LCA, there is a need to be more systematic about the generation and handling of data inputs and models. Improvement in the quantity and quality of data being used is necessary, and agreement on the approach used to evaluate LCA metrics (environmental indicators, impact factors, etc.) is crucial. LCA metrics should be easy to calculate with available data, useful for decision-making, reproducible, scientifically rigorous, usable at multiple scales of analysis, and extendable with improved understanding.27
Toxicology. At the foundation of improved life cycle and chemistry tools is the development of methods to supply toxicity data on chemicals and chemical mixtures. Computational toxicology and QSAR (Quantitative Structure Activity Relationship) analysis will be significantly involved in generating such data, which will also need to be compiled in centralized and accessible databases.
Data Compilation, Management, and Collaboration. There is a need to create a network system of all upstream, lateral, and downstream channels that will enable exchanges between different companies and different countries. Ideally, one end-of-line material will serve as a feedstock supply to another activity to create a chain of supply. Close partnership and collaboration between subcontractors of services, suppliers of materials, clients, and authorities should be identified, encouraged, and demonstrated with concrete actions such as sharing of databases and related scientific resources. It is crucial that closely related aspects of chemical safety and environmental impacts are approached more consistently and managed in all developed countries.






















