2
Noise-Induced Hearing Loss
The purpose of this chapter is to provide background material on noise-induced hearing loss to facilitate understanding of the evidence on noise-induced hearing loss in military personnel presented in Chapter 3. The chapter begins with a general discussion of the structure and function of the auditory system, with particular emphasis on the periphery, and the impact of noise on the peripheral auditory system. The effects of noise on hearing thresholds are reviewed next, followed by a review of the time course for the development of hearing loss from noise exposure. Next, exogenous and endogenous risk factors that may alter an individual’s susceptibility to noise-induced hearing loss are reviewed. This is followed by a discussion of national and international standards that have been developed to estimate the amount of noise-induced hearing loss to be expected from a given noise exposure and to separate the effects of noise from age-related changes in hearing.
MECHANISMS AND MODELS OF NOISE-INDUCED HEARING LOSS
Structure and Function of the Hearing Apparatus
In humans and other mammals, the auditory system consists of the external, middle, and inner ears (Figure 2-1), as well as the central auditory pathways in the brain. Sound waves enter the external ear through the pinna, travel through the external ear canal, and strike the eardrum. The external ear boosts high-frequency (2000–5000 Hz in humans) sound en-
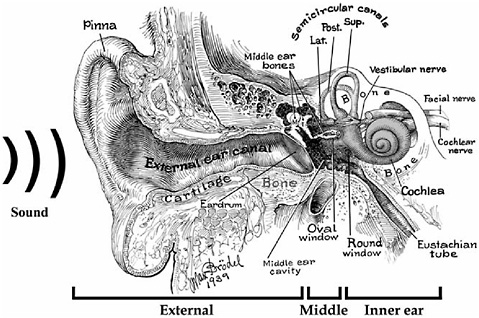
FIGURE 2-1 Semi-schematic drawing of the human ear. Sound waves enter the pinna, travel through the external ear canal, and strike the eardrum, setting it in motion. Motion of the eardrum sets the middle ear bones (malleus [M], incus [I], and stapes [S]) in motion and ultimately generates pressure waves in the fluids of the inner ear. Sensory cells in the hearing portion of the inner ear (i.e., cochlea) are then stimulated. When the fibers of the cochlear nerve are stimulated by the sensory cells, auditory information is transmitted to the brain.
SOURCE: Modified from Brödel (1946).
ergy by about 20 dB before it strikes the eardrum (Shaw, 1974). The eardrum vibrates when sound waves strike it, setting the middle-ear bones (malleus, incus, stapes) (Figure 2-1) in the air-filled middle-ear cavity in motion. The base of the stapes is fitted into the oval window of the hearing portion of the fluid-filled inner ear, the cochlea. Movement of the stapes sets up pressure waves in the fluids inside the cochlea, which contains the organ of Corti, the sensory organ for hearing, spiraling from base to apex. The primary sensory receptors for hearing, the inner hair cells, are found within the organ of Corti as are the outer hair cells, which primarily facilitate the sensory response of the inner hair cells. The pressure waves within the cochlea vibrate the basilar membrane and the attached organ of Corti (Figure 2-2). Specific sound frequencies vibrate specific places along the length of the cochlea, with high-frequency sound causing maximum vibration in the base of the cochlea and low-frequency sound causing maximum vibration in the apex. In addition, as the intensity of sound increases, the
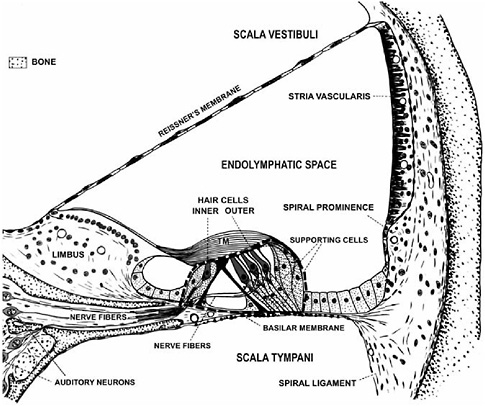
FIGURE 2-2 Cross-section of one turn of the spiral-shaped cochlea. The organ of Corti (outlined by the black dashed line) is attached to the flexible basilar membrane and is surrounded by large fluid spaces (i.e., scala vestibuli, endolymphatic space, scala tympani). The organ of Corti contains sensory cells (i.e., inner and outer hair cells) that respond to pressure waves in the fluid spaces by releasing neurotransmitter from their bases. The nerve fibers that terminate on the hair cell bases are extensions of the auditory neurons. The nerve fibers conduct auditory information to the brain when the hair cells release neurotransmitter. TM = tectorial membrane.
SOURCE: Modified from Davis and Associates (1953).
amplitude of basilar membrane vibration also increases, although in a nonlinear, compressive manner over much of its operating range.
The mechanical activity of the basilar membrane leads to mechanical stimulation of the inner and outer hair cells. From the surface of each hair cell, thin hair-like processes (stereocilia) project into the overlying gelatinous tectorial membrane (Figure 2-2). Movement of the basilar membrane and organ of Corti relative to the tectorial membrane deflects the stereocilia and opens ion channels in the hair cells. Channel opening depolarizes the hair cells so they release a neurotransmitter from their bases. This conver-
sion of mechanical energy from basilar membrane vibration to neuro-electrical energy by the sensory cells in the organ of Corti is a process involving high levels of metabolic activity. The nerve fibers connected to the hair cells, primarily the inner hair cells, are excited by the neurotransmitter and transfer the auditory information to the brain.
Effects of Noise on Hearing
The magnitude of hearing loss that results from excessive exposure to noise depends on factors associated with the exposure (e.g., sound pressure level [SPL], duration, type of noise, and frequency), as well as the characteristics of the individual being exposed (e.g., susceptibility to noise damage, age, prior history of hearing/ear damage). In the next section, we examine the influence of the type of noise in greater detail.
Impulse/Impact Noise
High-level, short-duration noise can arbitrarily be categorized as impulse noise, which is the product of explosive devices (e.g., gunfire), or impact noise, which is generated by the forceful meeting of two hard surfaces (e.g., a hammer to a nail, impact wrenches). The typical measures of impulse noise are the initial peak level and the duration of the first over-pressure. This is the A-duration and is less than 1 millisecond (msec) for handguns and several msec for large cannons. For impact noise, the two principal descriptors are the highest peak in a series of successive peaks (reverberations) and the so-called B-duration, the duration from the highest peak level to a point in time when the reverberations have decayed either 10 or 20 dB. B-durations range from 50 to 300+ msec. The distinction between impulse and impact noise becomes blurred in many real-life situations because impulse noise can reflect off the ground, or other surfaces, and the reflections add to the initial impulse noise, creating a large, more complicated waveform that is best described using the B-duration (Hamernik and Hsueh, 1991).
Impulse noise creates several special hazards to the auditory system. First, the high peak levels associated with gunfire (140–190 dB pe SPL)1 may damage the cochlea by causing rapid mechanical failure and injury (Henderson and Hamernik, 1986). A series of rapidly occurring impulses
|
1 |
As noted in Chapter 1, various metrics have been used in the literature to quantify the sound levels associated with impulse and impact noise, including dBP and dB pe SPL. Sound levels for steady-state noise, on the other hand, are more commonly expressed as dBA. Since simple conversions among these various metrics are not possible, the committee chose to report sound levels using the specific metric employed in the studies reviewed. |
can be partially attenuated by the acoustic reflex, a reflexive contraction of the middle-ear muscles, while isolated impulses reach the cochlea before the activation of the acoustic reflex. Thus, intense explosions may result in large cochlear lesions and significant hearing losses. This damage is termed “acoustic trauma,” and hearing at most frequencies may be affected (e.g., Ward and Glorig, 1961). Additional symptoms include a sense of fullness in the ears, speech sounding muffled, and a ringing in the ears (i.e., tinnitus) (Kraus, 1959; Ward and Glorig, 1961). Although some recovery of hearing takes place after an acoustic trauma episode, the individual is often left with a severe, permanent hearing loss (Ward and Glorig, 1961; Van Campen et al., 1999). Exposure to impulse noise can result in acoustic trauma from a limited number of exposures, including a single exposure, but can also result in conventional noise-induced hearing loss from extended periods of exposure to impulse noise over many weeks, months, or years.
The relationship between noise-induced hearing loss and the peak amplitude of an impulse or impact noise is complicated. Systematic research with the chinchilla has shown that at the lower range of exposure to impulse noise (< 140 dB pe SPL) or impact noise (< 115 dB pe SPL), the chinchilla develops a hearing loss that is proportional to the total energy of the exposure (peak level × number of impulses). However, above these peak sound pressure levels, the auditory system is damaged primarily by the large displacements caused by high peak levels. The dividing line between the “energy” and “peak-level” behavior is referred to as the “critical level.” It should be noted that the critical levels of about 140 dB SPL for impulse noise and 115 dB SPL for impact noise are general approximations for the chinchilla. The actual critical level is dependent on the specific waveform of the impulse and impact noise (Henderson and Hamernik, 1986). Based on across-species comparisons from chinchillas to humans, the critical levels for humans are likely to be approximately 10 dB higher than those observed in chinchillas. However, because of the high risk of hearing loss from high-level impulses and the variability in subsequent noise-induced hearing loss, a more conservative criterion of 140 dB SPL has been adopted for humans.
Below the critical level, hearing loss grows by the rate of approximately 1–3 dB of hearing loss for each dB of increase in peak level. However, above the critical level, hearing loss grows 3–7 dB for each dB increase in the level of the impulse or impact noise. This accelerated growth of hearing loss with increase in peak sound pressure level above the critical level is one of the factors that make high-level impulse and impact noise particularly dangerous (Henderson and Hamernik, 1986).
Impulse and impact noise also present a heightened risk when either occurs with other steady-state background noise (approximately 85–95
dBA). Experimental studies with laboratory animals have shown that exposure to combinations of relatively benign impact and steady-state noise can lead to multiplicative interactions with hearing loss and cochlear damage, with the effects of the combined exposure being greater than the simple additive effects of impulse or continuous noise (Hamernik et al., 1981). Lei et al. (1994) have developed a metric, based on the distribution of noise levels during exposure, that captures the extra hazard to hearing associated with such combined exposures in laboratory animals.
Intermittent and Continuous Exposures to Steady-State Noise
Exposure to less intense noise (i.e., < 90 dBA) for short durations (i.e., ≤ 24 hrs) may result in a mild (< 30 dB) temporary hearing loss (Mills et al., 1970; Melnick, 1976). A noise-induced temporary hearing loss, or temporary threshold shift (TTS), is characterized by an increase in the hearing thresholds at some frequencies immediately following exposure, depending on the frequencies comprising the noise and its intensity. The threshold shift generally disappears within 24–48 hours after the exposure terminates (Mills et al., 1970; Melnick, 1976). Typically, exposure to more intense noise (> 90 dBA) or moderate noise for longer durations (> 24 hours) results in a larger amount of TTS (i.e., > 40 dB). In these cases, postexposure improvement of thresholds may continue for 30 days or longer, but in general, thresholds will not return to preexposure values. The individual likely will be left with a residual permanent threshold shift (PTS) (Taylor et al., 1965; Mills and Talo, 1972; Mills, 1973; Henderson et al., 1974a; Henderson and Hamernik, 1982).
Hearing loss that results from exposure to sound with energy spread across a wide range of frequencies, such as many broad-band noises and impulses common to most industrial and military settings, is typically characterized by a gradual increase in threshold as frequency increases. Typically, the hearing loss abruptly reaches a maximum between 3000 and 6000 Hz, followed by a return toward normal hearing at still higher frequencies. This particular pattern of hearing loss, as illustrated in Figure 2-3, is typically referred to as the “noise-notch” audiogram. It is a clinical hallmark often used to distinguish noise-related high-frequency hearing loss from that associated with other etiologies, such as ototoxic medications or aging. Several mechanisms have been offered to explain the extra vulnerability of the higher frequencies to the damaging effects of a broad-band noise, including better transmission of the higher frequencies through the outer and middle ears to the inner ear (e.g., Saunders and Tilney, 1982; Rodriguez and Gerhardt, 1991) and specific vascular (e.g., Axelsson and Vertes, 1982) or metabolic (e.g., Thalmann, 1976) vulnerabilities of this region of the cochlea. However, none of these mechanisms can fully explain
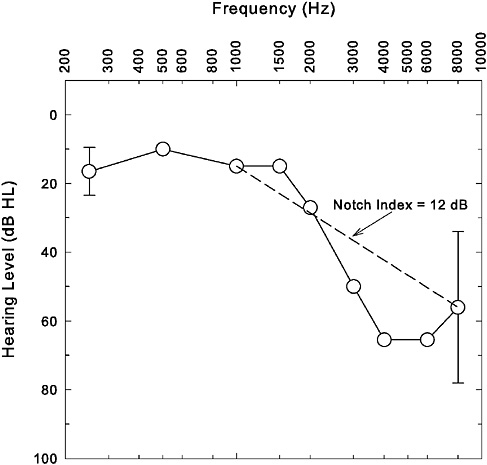
FIGURE 2-3 Illustration of a typical noise-notch audiogram. Average audiogram (n = 450 ears) from Cooper and Owen (1976) shown here. Error bars at 250 and 8000 Hz represent ±1 standard deviation and were the only standard deviations reported by the authors of this study for the average pure-tone thresholds at individual frequencies. The dashed line connecting thresholds at 1000 and 8000 Hz provides a visual representation of the Notch Index (NI) metric.
all of the features of the increased vulnerability of the 3000–6000 Hz region of the cochlea to noise damage.
Although the group data from Cooper and Owen (1976) in Figure 2-3 reveal a clear decrease in hearing from 1000 Hz to 4000 Hz followed by a return toward better hearing at still higher frequencies (8000 Hz), a pattern that typifies a noise notch, this is not always readily apparent for individual data. Discerning a noise notch in the pattern of hearing loss may be especially challenging in older adults for whom age-related hearing loss is super-imposed on a preexisting noise notch (see pp. 62–63). As a result, there
have been attempts to define the presence or absence of a noise notch more objectively than by simply relying on visual inspection of the pattern of hearing loss in the high frequencies, the latter approach not being particularly reliable (e.g., McBride and Williams, 2001a,b). One such approach to objectively define the presence or absence of a noise notch was advocated initially by Coles et al. (2000) and further refined by Dobie and Rabinowitz (2002). A graphic demonstration is provided by drawing a line to connect the hearing thresholds at 1000 and 8000 Hz, as illustrated by the dashed line in Figure 2-3. Having thresholds between 1000 and 8000 Hz (especially those at 2000, 3000, and 4000 Hz) that fall at or below the dashed line is thought to indicate the presence of a high-frequency notch in the hearing loss. Dobie and Rabinowitz (2002) describe a corresponding metric, referred to as the notch index (NI), that is simply the mean of the hearing thresholds at 1000 and 8000 Hz subtracted from the mean of the hearing thresholds at 2000, 3000, and 4000 Hz. Values of NI greater than 0 dB are thought to indicate the presence of a notch, whereas those less than 0 dB do not. For the hearing thresholds displayed in Figure 2-3, the notch index is 12 dB and is consistent with poorer hearing thresholds at 2000–4000 Hz than at 1000 and 8000 Hz. Other approaches to objective determination of the presence or absence of a noise notch have been described previously (e.g., Gates et al., 2000). The simplicity of the notch index and similar metrics is appealing, although additional research is needed to establish its reliability, as well as sensitivity and specificity in the identification of noise-induced hearing loss in the general population.
In summary, there are four key acoustic parameters of a given noise exposure that determine the type and amount of the resulting hearing loss. These are the sound pressure level of the noise, the duration and temporal pattern of the exposure (hours/day, impulses/day, number of years), the type of noise (steady-state, impulse/impact, blast), and the spectral content of the noise. Knowledge of values for each of these four parameters is necessary, but not sufficient, to fully assess the hazard of a given exposure to hearing. Although there can be some variation in the audiometric pattern of hearing loss for pure-tone thresholds following exposure to noise, the hallmark of noise-induced hearing loss is a characteristic noise notch in the audiogram that typically occurs between 3000 and 6000 Hz.
Effects of Noise on the Structure of the Hearing Apparatus
Acoustic trauma can occur following exposure to very intense noise, typically blasts > 150 dBA. Humans experiencing blasts at very high sound levels (~ 180 dB SPL) may suffer damage to the middle ear, including hemorrhage in or perforation of the eardrum and fracture of the malleus (Davis et al., 1949; Hirsch, 1968; Ward, 1973; Henderson et al., 1974b;
Roberto et al., 1989). If the eardrum does not rupture during such an intense exposure, the organ of Corti is likely to rupture off the basilar membrane (Ward, 1973; Henderson et al., 1974a,b; Roberto et al., 1989). When a portion of the organ of Corti ruptures, it does not reattach to the basilar membrane. Rather, it eventually degenerates. As noted, the hearing loss associated with acoustic trauma often is severe and spans a wide range of frequencies, much broader than that represented by the high-frequency, noise-notch pattern of hearing loss associated with other types of noise exposures.
Because sound levels in areas free from reflective surfaces, known as free fields, decay 6 dB per doubling of distance from the sound source, a key factor in such exposures may be the proximity of the individual to the blast. For example, consider two individuals, A and B, such that A is located 1 meter from a blast and the sound level recorded at that location was 160 dBA. Individual B, on the other hand, is located 32 meters from this same blast. Assuming that the blast can be modeled as a point source in a free field, the sound level at the location occupied by B will be 130 dBA. Whereas A may experience acoustic trauma from such a blast, including the development of a severe hearing loss affecting a wide range of frequencies, this will be much less likely for B, who might, through repeated such exposures over time, develop the more common noise-notch pattern of hearing loss. Importantly, individuals A and B may each subsequently report having heard a loud blast in a case history, but the hazard to hearing will be much greater for individual A, who is closer to the blast. Observations from Operation Iraqi Freedom suggest that even when personnel are close enough to suffer a blast injury that results in medical evacuation, four out of ten such individuals escape without permanent hearing loss, although many do experience acoustic trauma resulting in a severe or profound hearing loss (Chandler, 2005).
Individuals with mild or moderate permanent noise-induced hearing losses typically have some structural damage in their cochleas. The damage may initially involve scattered loss of sensory cells, primarily outer hair cells, in the organ of Corti (undamaged sensory cells shown in Figures 2-2 and 2-4, part A). Permanent noise-induced hearing loss may also result in damage to or destruction of other important structures in the cochlea, including fibrocytes in the spiral ligament and limbus and cells of the stria vascularis (Liberman and Mulroy, 1982; Hirose and Liberman, 2003) (Figure 2-2). In humans and other mammals, outer hair cells are usually the first type of sensory cell to be damaged or destroyed by excessive noise (Bredberg, 1968; McGill and Schuknecht, 1976) (Figure 2-4, part B). With larger permanent hearing losses, the degeneration involves both outer and inner hair cells (Bredberg, 1968; Liberman and Mulroy, 1982; Bohne and Harding, 2000) (Figure 2-4, part C). With severe permanent hearing losses,
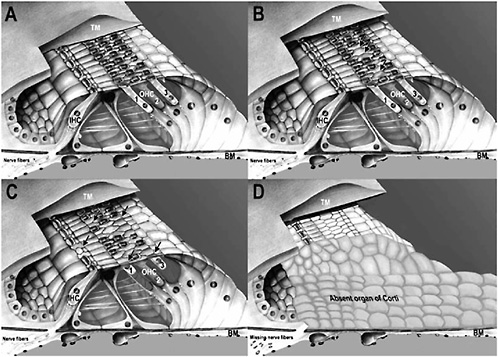
FIGURE 2-4 Drawings of the organ of Corti showing: (A) undamaged organ of Corti where all inner hair cells (IHC) and outer hair cells (OHC 1, 2, 3) are present; (B) beginning noise damage where 3 outer hair cells are missing (arrows); (C) moderate noise damage where 11 outer hair cells and 1 inner hair cell are missing (extent of loss indicated by arrows); and (D) severe noise damage where an entire portion of the organ of Corti is absent and is replaced on the basilar membrane (BM) by an undifferentiated, squamous epithelium. Nerve fibers to the area are also missing. TM = tectorial membrane.
SOURCE: Modified from an original painting by David Bellucci.
a variable amount of the organ of Corti (i.e., both sensory and supporting cells) is missing. In these cases, an undifferentiated layer of squamous epithelium covers the basilar membrane where the organ of Corti degenerated, and the nerve fibers that originally innervated the missing sensory cells also disappear (Johnsson and Hawkins, 1976; McGill and Schuknecht, 1976; Bohne and Harding, 2000) (Figure 2-4, part D).
The time course of cell degeneration and scar formation in the cochlea following a damaging noise exposure can be determined from animal studies only. A number of studies have shown that outer hair cells often begin to degenerate during an exposure. Additional outer hair cells, as well as inner hair cells and various supporting cells, may degenerate for days to a few weeks following termination of the exposure. While the various cells are degenerating, scars are forming in the organ of Corti. Phalangeal scars, formed from supporting-cell processes, replace missing hair cells, and squamous epithelial scars, formed from supporting cells on the basilar membrane, replace degenerated portions of the organ of Corti. Nearly all scar formation is completed by 1 month postexposure (e.g., Stockwell et al., 1969; Bohne, 1976; Fredelius, 1988; Wang et al., 2002).
Although there are some exceptions, especially for high-intensity, low-frequency sounds (e.g., Jerger et al., 1966; Burdick et al., 1978; Mills et al., 1983), good consistency has been observed in human and animal studies between the frequency content of the exposure stimulus and the location in the cochlea experiencing the greatest damage or injury (e.g., Johnsson and Hawkins, 1976; Moody et al., 1976). For narrow-band stimuli, the maximum cochlear insult is often one-half to one octave higher in frequency than the exposure stimulus (Ward, 1973). For broad-band noises and impulses, much more commonly encountered in military and industrial settings, the damage is greatest in the high-frequency (i.e., basal) portion of the cochlea (e.g., Gravendeel and Plomp, 1959; Ward, 1973; Ylikoski and Ylikoski, 1994). Furthermore, these differences in location of the greatest cochlear damage are accurately reflected in the pattern of hearing loss. For example, the noise-notch pattern of hearing loss (Figure 2-3) is associated with underlying damage to the sensory cells in the basal portion of the cochlea; that is, the portion of the cochlea tuned to those frequencies. In addition, as suggested by the sequence of illustrations shown in Figure 2-4, there is also a positive correlation between the amount of damage at a particular location in the cochlea and the severity of the hearing loss measured for a frequency associated with that location, although this correlation is believed to be weaker for low-frequency sounds (e.g., Bredberg, 1968). The pattern of hearing loss measured following noise exposure provides valuable information about the extent and severity of the underlying damage, especially in the middle and high frequencies following exposures to broad-band sounds.
In summary, although the specific site of lesion varies with the type of noise to which one is exposed (steady-state, impulse/impact, blast), the sensory receptors within the cochlea are the most common site of permanent destruction from noise exposure. Behavioral pure-tone thresholds, although not perfect indicators of the underlying cochlear pathology, represent the best available and most widely used measure of the underlying damage in humans.
TIME RELATION BETWEEN EXPOSURE TO NOISE AND THE DEVELOPMENT OF HEARING LOSS AND COCHLEAR DAMAGE
Consider exposure to an intense sound for a specified duration, with the measurement of hearing thresholds performed at periodic intervals during the exposure and at several times following the exposure. Thresholds measured in brief intervals during the exposure represent the growth or development of hearing loss, whereas those measured after the exposure is terminated represent the recovery at specific postexposure time intervals. For humans, the research conducted on the growth and recovery of hearing loss associated with noise exposure has primarily used changes in behavioral measures of hearing threshold, either TTS or PTS. Thus, conclusions about cochlear damage resulting from noise exposure in humans are limited by the imperfect association between behavioral measures of hearing threshold and underlying cochlear damage noted previously (e.g., Bredberg, 1968).
This less than perfect correlation has also been noted in the specific context of the study of recovery from noise exposure. For example, Davis et al. (1950) repeatedly exposed humans to a series of intense sounds with the exposures spaced so that there was complete recovery of TTS from one exposure before beginning the next exposure. In only 3 of more than over 60 intensity-duration exposure combinations, the individuals’ hearing thresholds failed to fully recover to baseline levels established before the series of exposures; that is, some PTS occurred in only 3 of 60 cases (5 percent). On this basis, it was assumed that if an individual completely recovered from a TTS, the inner ear had not sustained permanent structural damage. However, because the inner ears of these individuals were not examined microscopically, the occurrence of permanent structural damage could not be ruled out. Subsequently, laboratory animal studies in the 1960s and 1970s using cats and chinchillas, two common animal models of human hearing, showed that animals who had completely recovered from TTS of at least 50 dB always sustained some permanent cochlear damage (e.g., Miller et al., 1963; Carder and Miller, 1972). Although it is likely that the same phenomenon is true for humans, there is insufficient evidence to support this generalization.
With regard to the effects of the duration of the exposure, when the
duration is defined in terms of hours of continuous exposure to intense noise, hearing thresholds in humans begin to deteriorate after about 1–4 hours of exposure to moderately intense noise (i.e., ~85 dBA) and reach a maximum threshold shift of 10–18 dB after 8–12 hours (Mills et al., 1970; Melnick, 1976; Mills et al., 1979). With exposure to more intense noise (i.e., 92–120 dBA), hearing thresholds begin to deteriorate less than 30 minutes after the start of the exposure in both humans (Davis et al., 1950; Mills et al., 1970; Mills et al., 1979) and other mammals (Miller et al., 1963; Carder and Miller, 1972; Mills, 1973). With regard to recovery following such continuous exposures to noise, generally, threshold shifts that are less than 20 dB fully recover by 48 hours after termination of the exposure (Mills et al., 1970; Carder and Miller, 1972; Melnick, 1976). Threshold shifts of about 30 dB may require 3–6 days for the temporary component of the threshold shift to disappear. With larger maximum thresholds shifts (e.g., > 40 dB), recovery may be complete or incomplete. In animals, threshold shifts that require a week or more for recovery have been shown to be associated with permanent cochlear damage, primarily the loss of outer hair cells (Eldredge et al., 1973a; Eldredge et al., 1973b).
We speculate that delayed recovery of thresholds in humans also signifies the occurrence of cochlear damage. Mills (1976) exposed four chinchillas to a 4-kHz octave-band noise at 80 dB SPL for 90 days and measured thresholds and threshold shifts behaviorally before, during, and for 150 days after the exposure. Threshold shifts grew to an asymptote of 50 dB by 24 hours of exposure. After termination of the exposure, thresholds recovered to a 30-dB shift over 8 days, reached a plateau, and then recovered another 10 dB between 60 and 150 days.
It is important to note, however, that with the exception of a very short-term “bounce” in the recovery process that occurs typically within the first 2 minutes following cessation of the sound exposure (Hirsh and Ward, 1954), thresholds measured following these exposures are all consistent with a process of recovery. That is, thresholds always improve or remain stable as postexposure time increases.
Limited data on PTS in humans appear to be consistent with these data from laboratory animals on recovery following noise exposure. Segal et al. (1988), for example, using a classification scheme based on the severity of hearing loss (mild, moderate, or severe), reported that approximately 91 percent of the 1,514 hearing losses observed following acoustic trauma in military personnel remained in the same severity classification for follow-up periods of 4 months to 4 years, 7 percent improved, and 2 percent worsened by one category. Similar findings (4 percent classified as having worse hearing at follow-up) were reported by Melinek et al. (1976) for a smaller sample of 626 ears from military personnel with acoustic trauma. In both studies, additional noise exposure between the initial and final
measurement of hearing thresholds was possible because these individuals remained in the military for the period between these measurements. Given the reliability of clinical threshold measurements and the use of a severity classification scheme based on those measurements, these data provide evidence in humans of the stability of the postexposure hearing thresholds over an extended period of time.
Laboratory studies of TTS and PTS in humans and laboratory animals that have focused on recovery of hearing thresholds have typically been terminated soon after the thresholds have fully recovered (TTS) or appear to have stabilized for a period of days or weeks (PTS). Although at least some studies have followed participants of various ages with hearing loss for up to 15 years (e.g., Macrae, 1991), no life-span studies of humans have followed the same subjects longitudinally for their remaining lifetime after a noise exposure that produced a significant TTS or PTS early in life. In laboratory animals, Mills et al. (1997) exposed one ear of six 18-month-old (“middle-aged”) gerbils to high-intensity sound that produced PTS of 10–15 dB at 6 weeks postexposure. The animals were no longer exposed to noise and thresholds were assessed again at age 36 months, which is near the end of the gerbil’s life. During the period from 18 to 36 months of age, hearing losses of 0–50 dB can occur in gerbils that are non-noise exposed at a young age and reared entirely in a quiet environment. At age 36 months, hearing thresholds in the “noise-exposed and aged” ears had increased further by about 3 dB (or, a total shift of 13–18 dB compared with the preexposure baseline). Thresholds in the “non-noise exposed and aged” ears (of the same animals), on the other hand, had increased by about 10–20 dB over this same time period. In other words, at an age of 36 months, hearing thresholds in the ears subject to both noise exposure and aging were nearly equivalent to the hearing levels in the control ears subject only to aging (and substantially lower than the thresholds predicted using ISO/ANSI rules for the combined effects of noise and aging; see below). Clearly, the noise exposed animals in this study did not have unusual changes in hearing levels that were manifested long after the noise exposure was terminated. Other than this study, however, there are no other data from studies of laboratory animals in which hearing thresholds were measured throughout the animals’ life spans after recovery appeared to be complete. Thus, few data are available from laboratory animals or humans with which one can address the issue of “delayed effects” in later life of a noise exposure experienced much earlier in life.
It is noteworthy, however, that the modeling of noise-induced hearing loss and age-related hearing loss in ISO-1999 suggests that noise-induced hearing loss is almost asymptotic beginning at about 10–20 years of exposure and continuing through 40 years of exposure, especially for the milder amounts of hearing loss (ISO, 1990). (Also see Figure 2-5 in the subsequent
discussion of estimating noise-induced hearing loss.) Thus, if continued exposure to noise for an additional two decades (from 20 to 40 years of exposure) results in little additional noise-related hearing loss following an initial 10–20 years of exposure, it hard to imagine that removal from the noise after 10–20 years of exposure would result in further declines in hearing.
Finally, for the study of PTS in humans, “duration” may also be defined by years of daily exposure (typically, 5 days/week) to intense sounds of various types. Here, cross-sectional data from humans indicate that PTS increases with years of exposure, typically growing most rapidly during the first 10–15 years of exposure at 4000 Hz and the first 10–20 years at 2000 Hz (e.g., Nixon and Glorig, 1961; Gallo and Glorig, 1964; Taylor et al., 1965).
FINDING: The evidence from laboratory studies in humans and animals is sufficient to conclude that the most pronounced effects of a given noise exposure on pure-tone thresholds are measurable immediately following the exposure, with the length of recovery, whether partial or complete, related to the level, duration, and type of noise exposure. Most recovery to stable hearing thresholds occurs within 30 days.
FINDING: There is not sufficient evidence from longitudinal studies in laboratory animals or humans to determine whether permanent noise-induced hearing loss can develop much later in one’s lifetime, long after the cessation of that noise exposure. Although the definitive studies to address this issue have not been performed, based on the anatomical and physiological data available on the recovery process following noise exposure, it is unlikely that such delayed effects occur.
RISK FACTORS FOR NOISE-INDUCED HEARING LOSS: INDIVIDUAL DIFFERENCES AND NONACOUSTIC FACTORS
Thus far, the focus in the preceding pages has been placed on the effects of noise on the hearing of the “typical” or “average” person. For at least 175 years, however, it has been known that one of the hallmarks of noise-induced hearing loss is the wide range of individual differences in hearing loss that can result from seemingly identical noise exposures (Temkin, 1933; Ward, 1965, 1968, 1995). Various factors, some inherent to the individual (endogenous) and some external to the individual (exogenous), have been examined by researchers to assess their role in susceptibility to noise-induced hearing loss (e.g., Humes, 1984; Boettcher et al., 1987; Boettcher et al., 1992; Henderson et al. 1993; Ward, 1995). In some cases, such as exposures to solvents or use of aminoglycoside antibiotics, the exogenous factors not only interact with noise exposure but also produce hearing loss themselves. Here these factors are examined with regard to whether they
interact with noise exposure to increase the hearing loss beyond that resulting from exposure to either agent alone. The details of earlier reviews will not be repeated here. Rather, the emphasis in this section is placed on their conclusions and recent findings (since 1990) that serve to supplement these earlier reviews.
Although an effort was made to focus on recent human studies, for some factors, the only studies have been in laboratory animals. In many of the animal studies, the exposures were exploratory and often not relevant to the occupational setting. In addition, in the human studies, details of the exposures to noise and other agents were not always available, especially for an individual subject. Dose-response information was rarely available. Finally, the studies reviewed included a wide range of methods to assess hearing loss. With these caveats in mind, we proceed to a review of evidence on the effects of certain exogenous and endogenous factors on the amount of noise-induced hearing loss measured following noise exposure.
Exogenous Factors
As noted in reviews by Humes (1984), Boettcher et al. (1987), and Boettcher et al. (1992), interactions of the effects of noise exposure and various drugs and chemical agents have received considerable attention. When noise, diuretics, or aminoglycosides (common antibiotics, such as gentamicin) are used in combination, for example, synergistic interactions occur such that the hearing loss from the combination of agents is greater than the hearing loss from either agent alone. This result was supported by Aran et al. (1992), who found, using guinea pigs, that exposure to sound at moderate to high levels increased the ototoxic effects of the drugs. Gratton et al. (1990) studied the interaction of a drug commonly used in chemotherapy, cisplatin, with concurrent noise exposure. Using chinchillas, administration of cisplatin during noise exposure resulted in greater hearing loss and hair cell loss than occurred for either agent alone, but the interaction was dependent on the noise level. For sodium salicylate, a compound related to aspirin, conclusions regarding the interaction with effects of noise exposure have been somewhat equivocal, with most studies suggesting that the addition of salicylates does not make the ear more susceptible to the damaging effects of noise (e.g., Spongr et al., 1992, in chinchillas).
Although hearing loss in the industrial or military environment is most often attributed to noise exposure, there is increasing attention to whether co-exposure to chemical agents present in these environments may potentiate noise-induced hearing loss. Carbon disulfide, for example, is a chemical common in the clothing industry and is often neurotoxic; an interaction between noise exposure and exposure to carbon disulfide has been observed in some human studies (Franks and Morata, 1996).
Vapors from organic solvents, such as toluene, are often found in occupational environments involved in printing and painting, as well as in shipyards where paint spraying is common. Effects of toluene have been shown in laboratory animal studies to combine in an additive or synergistic manner with noise (e.g., Cary et al., 1997; Fechter, 2004; see also Table D-1 in Appendix D). Styrene, which is widely used in manufacturing and in the chemical and petroleum industries, is another organic solvent whose effects have been shown in animal studies to combine synergistically with noise (Morata and Campo, 2001). Overall, results from animal studies generally suggest that although exposure to noise and solvents individually may be at safe levels, simultaneous or successive exposures to both may increase susceptibility to noise-induced hearing loss. However, inconsistencies in results of animal studies have been observed, and the extent of the interaction is highly species-specific. Some of the inconsistencies may relate to differences in the sites of damage of the ototoxins and their mode of transmission. For example, the damaging effects of noise affect primarily the inner ear and occur via acoustic transmission, whereas chemical exposures may reach the inner ear and the central nervous system by being inhaled or absorbed through the skin and circulated through the bloodstream.
Results of laboratory studies with animals showing increased risk for noise-induced hearing loss with exposure to solvents led to efforts to confirm these results in humans, given the widespread occupational exposures to these chemicals in noisy environments. Many observational and epidemiological studies of noise-induced hearing loss have been conducted in humans exposed to solvents (e.g., Morata et al., 1993; reviews in Morata et al., 1994; Morata and LeMasters 1995; Morata, 1998, 2003). Some studies show an increased prevalence of hearing loss in workers exposed to noise and solvents relative to workers exposed to either agent alone (e.g., Morata et al., 1993). An increased risk of hearing loss was also observed for aircraft maintenance personnel exposed to jet fuel and noise on an Air Force base (Kaufman et al., 2005). However, taken together, results of human studies are equivocal, and the design of these occupational studies is often confounded by a lack of control groups and poor quantification of the exposures. Typically, the hearing of a group of unexposed workers is compared to the hearing of groups of workers exposed to noise only, to solvent only, and to both noise and solvent. However, the noise or solvent exposure within the “noise and solvent” group may not be equivalent to the exposures within the noise-only or solvent-only groups. Moreover, noise and chemical exposure records for individual study participants often are not available. Therefore, exposures must be estimated from area surveys rather than from dosimetry or biological monitoring. Nonoccupational noise exposures are also difficult to estimate and control for in analyses. In addition, workers of different ages or with different durations of employment
may be subject to uncontrolled cohort differences in their histories of noise or solvent exposure and in other factors that could affect hearing. Given these design limitations, there is insufficient evidence in humans to reach conclusions regarding the interactive effects of solvents and noise on noise-induced hearing loss.
Exposure to chemical asphyxiants, such as hydrogen cyanide and carbon monoxide, is common in some workplaces, and the interaction of these asphyxiants with noise exposure has also received attention recently. Cyanides are used in electroplating and metal leaching. Carbon monoxide is among the most common workplace air pollutants, especially for individuals working around gas-combustion engines, such as mechanics and other engine workers. Recent reviews of research in animal models indicate that low to moderate levels of carbon monoxide and hydrogen cyanide potentiate noise-induced hearing loss, especially at high noise levels (Cary et al., 1997; Fechter et al., 2000; also see Table D-2 in Appendix D). This potentiating effect, moreover, has been observed whether the exposures to each agent were simultaneous or successive. A recent review by Fechter (2004) notes that solvents (toluene, ethyl benzene, styrene) are likely to result in an additive effect to noise whereas asphyxiants (carbon monoxide, hydrogen cyanide) appear to result in synergistic effects to noise. There are no known human studies on the interaction of the effects of chemical asphyxiants and noise on noise-induced hearing loss.
Cigarette smoking may have a negative effect on hearing (e.g., Cruickshanks et al., 1998), but it is unclear whether it interacts with noise exposure to increase noise-induced hearing loss (see Table D-3 in Appendix D). Recent studies have yielded mixed results, with some finding no synergistic effects between smoking and noise exposure (Starck et al., 1999; Toppila et al., 2000), one observing a small interaction (Palmer et al., 2004), and one showing a significant increase in the prevalence rate for noise-induced hearing loss among factory workers who smoked (Mizoue et al., 2003). The design limitations described previously for human studies of the interactive effects of noise and solvents are also evident in these cross-sectional studies of the effects of smoking on noise-induced hearing loss.
Finally, whole-body vibration increases temporary hearing loss when noise is present (see review by Humes, 1984), but only when body temperature is elevated (Manninen, 1988). An increase in body temperature is known to increase the effects of noise on hearing (e.g., Drescher, 1976). Exercise during noise exposure and cardiovascular fitness have been shown to decrease (Manson et al., 1994), increase, or have no effect on temporary hearing loss (Wilson and Herbstein, 2003). The effect of exercise on noise-induced hearing loss may also relate to increases in body temperature (Pekkarinen, 1995). Many of these studies included relatively small (n < 20) convenience samples, with no randomized designs or control groups. Re-
sults from studies of the interactive effects of noise and electromagnetic fields in magnetic resonance imaging devices have been equivocal, and only limited results are available (Pekkarinen, 1995).
FINDING: Nonacoustic factors may interact with the effects of noise to increase the measured noise-induced hearing loss. For many exogenous factors, evidence in animal models reveals that the effects of drugs or chemical agents may combine in an additive or synergistic manner with the effects of noise to increase noise-induced hearing loss. In particular, aminoglycosides, cisplatin, and solvents (toluene and styrene) interact in laboratory animals with noise presented simultaneously or sequentially to increase the amount of noise-induced hearing loss. However, there is not sufficient evidence to confirm this finding in humans. In particular, the evidence is not conclusive in humans with regard to additive or synergistic effects of noise and the following exogenous factors on hearing: aminoglycosides, cisplatin, diuretics, salicylates, solvents, carbon disulfide, carbon monoxide, cigarette smoking, whole-body vibration, body temperature, exercise, and electromagnetic fields.
Endogenous Factors
Previous reviews of endogenous factors affecting noise-induced hearing loss have been performed (Humes, 1984; Henderson et al., 1993; Ward, 1995). Factors covered in these review papers include age (both young and old), gender, race, eye color, and prior hearing loss. With regard to age, the focus here, given the committee’s charge, will be only on the older end of the age continuum. In each of the reviews, the basic thesis is that there have been inconsistent trends reported in the literature for each of these endogenous factors, but in general, the research points to a lack of effect of each of these factors on noise-induced hearing loss. It is certainly not the case that one could adjust a general prediction of noise-induced hearing loss for the “average person” to be more appropriate for a given individual with knowledge of any of these endogenous factors.
With regard to gender differences in susceptibility to noise-induced hearing loss, audiometric surveys of employees in noisy industries have shown that women, on average, have better hearing than men, even when gender-specific age corrections have been applied (e.g., Berger et al., 1978). It is not clear, however, that the source of these differences can be attributed to gender alone (Ward et al., 2000).
Most of the more recent publications that have appeared since the latest of these general reviews by Ward (1995) have been concerned with the effects of old age on susceptibility to noise-induced hearing loss. These recent publications are reviewed first, followed by several citations with
regard to the effects of race and prior hearing loss on noise-induced hearing loss.
The general conclusion from several recent studies (Sun et al., 1994; Erway et al., 1996; Mills et al., 1997; McFadden and Campo, 1998; Ohlemiller et al., 2000; Boettcher, 2002; Fraenkel et al., 2003), all of which were conducted with laboratory animals, is that aging per se does not enhance susceptibility to noise-induced hearing loss or cochlear damage. However, there is a suggestion that genetic factors that lead to hearing loss, including a predisposition to age-related hearing loss, may render an individual more susceptible to noise-induced hearing loss (Erway et al., 1996; Ohlemiller et al., 2000). This is also consistent with recent work by Holme and Steel (2004), who have identified a gene in mice, Cdh23, that is linked to increased susceptibility to noise-induced hearing loss. Other studies in mice indicate that a genetic basis for resistance to noise-induced hearing loss may exist as well (Yoshida et al., 2000).
The basic approach pursued in most of the foregoing studies on aging and susceptibility was to examine the effects of specific noise exposures on two or more groups of subjects differing in age. As noted, in general, older adult animals were neither more nor less susceptible than younger adults to the effects of noise on hearing. A related, but separate, question regarding interactions of noise and aging is whether prior noise-induced hearing loss impacts the subsequent progression of age-related hearing loss. Two recent studies with older humans (Gates et al., 2000; Rosenhall, 2003) suggest that evidence of prior noise exposure results in a more rapid deterioration of hearing with aging, at least for some frequencies (e.g., 2000 Hz). On the other hand, two other recent studies (Cruickshanks et al., 2003; Lee et al., 2005), also with humans, failed to find evidence supporting differences in the amount of age-related hearing loss or the progression of age-related hearing loss based on a history of prior noise exposure. (Table D-4 in Appendix D summarizes the key features of these four studies.) The reasons for these differences in findings are unclear, and this is an area that deserves further attention and research.
Race is another endogenous factor that has received some attention recently by researchers studying noise-induced hearing loss. Henselman et al. (1995), for example, conducted a retrospective study among soldiers in the U.S. Army and noted that, for similar noise exposures and length of service exceeding 10 years, black soldiers demonstrated less age-corrected noise-induced hearing loss than white soldiers. It is unclear, however, whether this is a valid indication of racial differences in noise susceptibility. The study used the same (primarily white) unscreened database to generate the age corrections to the audiograms. African Americans have been observed to have less age-related hearing loss than whites (Royster et al., 1978). Thus, the reduced noise-induced hearing loss reported for African Americans in this
study could be due to the subtraction of an inappropriately high age-related threshold derived from data for white subjects to generate the age-corrected noise-induced hearing loss for African Americans.
Ishii and Talbott (1998) conducted another retrospective study of noise-induced hearing loss in industry and found that white workers in a metal fabricating plant had significantly higher high-frequency (3000–8000 Hz) hearing levels than nonwhites (86 percent African American, 14 percent Hispanic) working in the same plant. The differences in hearing levels in the high frequencies, however, are similar to the race differences in age-related hearing loss noted previously. This suggests that appropriate race-specific age correction of these hearing levels, rather than the use of the same age correction for all racial/ethnic groups, would have resulted in little difference between racial/ethnic groups in the actual noise-induced hearing loss.
Although the effects of prior hearing loss, including previous noise-induced hearing loss, on the susceptibility to subsequent noise-induced hearing loss have not received much attention in recent research, several earlier studies in laboratory animals and humans have addressed this topic (e.g., Glorig et al., 1961; Resnick et al., 1962; Ward, 1968, 1973; Mills, 1973; Man et al., 1975; Howell, 1978; Botte and Variot, 1979; Humes, 1984; Humes and Jesteadt, 1991). The subjects in two of these earlier studies were military personnel (Resnick et al., 1962; Man et al., 1975). In most of the studies cited, the existing hearing loss resulted from prior noise exposure. When expressed in terms of relative threshold shift (TTS or PTS), the observed amount of postexposure threshold shift always decreased as the amount of prior hearing loss increased, giving the impression that individuals with prior hearing loss are less susceptible to noise-induced hearing loss. When examined in terms of the hearing threshold to which the individuals have been shifted (so-called “shifted threshold” in dB HL or dB SPL), however, it is apparent that individuals with and without prior hearing loss are equally susceptible to noise-induced hearing loss (Mills, 1973; Humes, 1980; Humes and Koval, 1981; Humes and Jesteadt, 1991).
Consider a noise exposure that is known to produce, on average, a noise-induced hearing loss of 20 dB HL at 4000 Hz. An individual who enters this noise exposure with a hearing threshold of 0 dB HL at 4000 Hz will demonstrate a threshold shift of 20 dB, whereas another individual who enters this noise exposure with a hearing threshold of 40 dB HL at 4000 Hz will show a threshold shift of 0 dB. On the other hand, if a given noise exposure is known to produce a noise-induced hearing loss of 40 dB HL at 4000 Hz, individuals with preexposure thresholds of 0 and 20 dB HL at 4000 Hz will demonstrate, on average, threshold shifts of 40 and 20 dB, respectively. Thus, the relative threshold shift in individuals with preexisting noise-induced hearing loss is always less than that observed in individuals with normal hearing prior to exposure, but the shifted thresholds (in dB
HL or dB SPL) are frequently similar, depending on the particular combination of thresholds and exposure conditions involved. Basically, the nonlinear additivity rules used to combine hearing thresholds from age-related hearing loss and noise-induced hearing loss, reviewed in detail below, also apply to the addition of previous and current noise-induced hearing loss. The basic conclusion from review of the research in this area is that individuals with previous noise-induced hearing loss are neither more nor less susceptible to subsequent noise-induced hearing loss than individuals without such pre-existing hearing loss.
FINDING: Several endogenous factors have been examined, including (old) age, gender, race, eye color, and prior hearing loss, but there is not sufficient evidence in humans to conclude that any of these factors predicts susceptibility to noise-induced hearing loss.
ESTIMATING NOISE-INDUCED HEARING LOSS
Large-scale, systematic investigations of the temporary and permanent effects of noise on hearing, in both human and laboratory animal subjects, began in the 1940s and early 1950s at several research laboratories, largely because of the extreme sound levels to which military personnel were exposed during World War II. Soon, a need developed for the scientific community to coordinate, evaluate, and guide the research in this area, and the National Academy of Sciences, with support of various branches of the military, created the Committee on Hearing and Bioacoustics (CHABA; subsequently appending “and Biomechanics” to its name while leaving the acronym, CHABA, intact). Over the ensuing decades, CHABA and subsidiary working groups generated several critical reviews and summaries of the state of our knowledge with regard to noise-induced hearing loss (e.g., Kryter et al., 1966; CHABA, 1968; NRC, 1992, 1993). These reports frequently led to additional research that, in turn, led to updated reports. Through this iterative process, much came to be known about the effects of noise on hearing.
There was particular interest in being able to estimate the average permanent hearing loss, as well as its distribution, expected from years of exposure to various noise types and levels. The greatest progress was made in the understanding of noise-induced hearing loss resulting from years of continuous or intermittent daily exposures to steady-state noise. The work in this area resulted in the adoption of an international standard (ISO-1999 [ISO, 1990]) and an American standard (ANSI S3.44 [ANSI, 1996]) designed to estimate the median noise-induced hearing loss for a given exposure, along with some measures of the statistical distribution of the resulting noise-induced hearing loss (e.g., 10th and 90th percentiles). Although
both standards state that they can be applied to a wide variety of noise exposure conditions, including intermittent and time-varying exposures to steady-state noise and exposures to impulse noise, the formulas included in each standard were derived primarily from data on noise-induced hearing loss among industrial workers with 10–40 years of exposure to broad-band, steady-state noise for 8 hours per day (5 days/week). In addition, both standards restrict their application to equivalent continuous sound levels of 75–100 dBA for a normal 8-hour working day. As a result, the ISO/ANSI predictions are considered to be most valid for similar exposure conditions. The standards, therefore, are less likely to provide valid estimates of permanent hearing loss in humans for other types of noise (e.g., impulse), noise exposures (e.g., daily exposures that differ significantly from 8 hours), or exposure durations (less than 10 or more than 40 years). These limitations of the standards should be kept in mind when they are applied to estimate noise-induced hearing loss, including their use to estimate noise-induced hearing loss in the military.
These national and international standards represent the synthesis of the best available data on industrial noise-induced hearing loss. The available data, however, are not without limitations. For example, sample sizes were often small, the studies were cross-sectional rather than longitudinal and subject to cohort effects, and specification of the noise exposure was by group or area of the industry, rather than for each individual.
Figure 2-5 provides an illustration of the age-corrected “noise-induced permanent threshold shift,” NIPTS, estimated with ISO-1999 (1990).2 The top panel demonstrates the development of NIPTS at one frequency, 4000 Hz, with increasing years of exposure to 8-hour equivalent continuous noise levels of 85, 90, 95, or 100 dBA. These estimates reveal two key features of NIPTS: (1) that NIPTS increases with noise level and, for an 8-hour equivalent continuous noise level of 85 dBA (or less), is negligible at 4000 Hz, the frequency showing the greatest amount of NIPTS; and (2) that NIPTS grows most rapidly during the first 10–15 years, with only slight increases beyond that. It is important to emphasize here, however, that this is the hearing loss associated with noise exposure only (NIPTS). Also, the values displayed are the predicted median values from ISO-1999. The same standard provides for the generation of other percentiles or quartiles. In general, the interquartile ranges (the difference between
|
2 |
Although the relative NIPTS in dB is calculated in ISO-1999, the committee chose to plot this both as NIPTS in dB and noise-only hearing loss in dB HL in Figures 2-5 and 2-6 for consistency with the other figures in this report. Since it is assumed in both figures that the age-related component is 0 dB HL throughout, X dB NIPTS is also X dB HL of “pure” noise-induced hearing loss. Such “pure” noise-induced hearing loss, in the absence of concomitant age-related hearing loss, can only occur hypothetically and within such a model framework. |
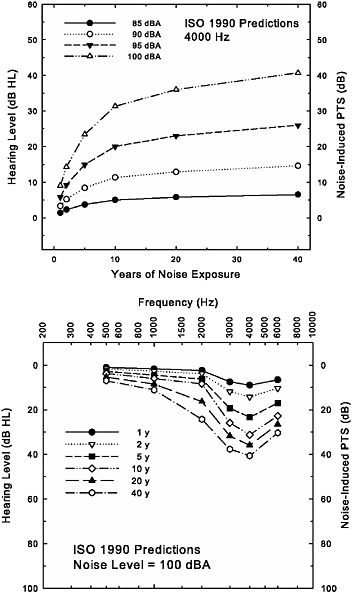
FIGURE 2-5 Depiction of the development of the noise-induced permanent threshold shift (NIPTS) in dB, or the noise-only noise-induced hearing loss in dB HL, at 4000 Hz (top) and at several frequencies (bottom) with increasing years of noise exposure as estimated by ISO-1999 (1990). The 8-hour equivalent continuous noise level is the parameter in the top panel, whereas the noise exposure is fixed at the highest level (100 dBA) included in the standards in the bottom panel, with length of exposure as the parameter. In both panels, age-related hearing loss is fixed at 0 dB HL throughout to illustrate that portion of the hearing loss associated with noise exposure only. This enables the hypothetical NIPTS in dB (left ordinate) to be plotted as hearing loss in dB HL (right ordinate).
thresholds at the 25th to 75th percentiles) around the median values in Figure 2-5 are typically 10–20 dB. Of course, while the individual is being exposed to such noise for 40 years, the individual also ages, and aging by itself can result in elevated hearing thresholds. This issue is addressed in more detail below. Here it is important to note that because aging effects are not included, it is unlikely that these would be the actual thresholds measured, especially for more than 10–20 years of noise exposure (assuming that the exposure begins in early adulthood).
The bottom panel of Figure 2-5 shows ISO-1999 (1990) predictions for NIPTS for an 8-hour equivalent continuous noise level of 100 dBA, and for progressively longer durations of exposure up to the maximum of 40 years. Here, however, NIPTS in dB (left ordinate), as well as the equivalent hearing thresholds in dB HL (right ordinate), for the noise-only portion of the hearing loss have been illustrated in conventional clinical format for frequencies from 500 through 6000 Hz to demonstrate the typical pattern of hearing loss at specific ages. Note the emergence of a noise-notch pattern over time, with the maximum hearing loss occurring at 4000 Hz. It is noteworthy that, consistent with the data depicted in the top panel, hearing loss at 4000 Hz increases most with durations of exposure up to 10 years, but that this is not the case at 2000 Hz, where increases in hearing loss continue after the first 10 years of exposure. Nonetheless, the noise-notch pattern of hearing loss is maintained and the average hearing loss associated with noise exposure only is about 40 dB HL in the high frequencies after 40 years of exposure to the highest noise level included in the ISO/ANSI standards.
One of the key issues covered in the ISO/ANSI standards is the way each addresses the combined effects of noise-induced and age-related hearing loss. Consider, for example, a man who either begins his job in industry or enlists in the military at 20 years of age. Thirty years later, this same individual retires. Estimates of the resulting noise-induced hearing loss using either standard would take into consideration this individual’s 30 years of noise exposure. However, while this individual was exposed to noise, he also aged and was 50 years old when the daily noise exposure ended. It is well known that hearing loss also increases with age and that the average 50-year-old man will have worse hearing, especially in the high frequencies, than the average 20-year-old man. Thus, both the 30 years of noise exposure and advancing age can have an effect on high-frequency hearing, and it is the combined effects of both factors (as well as others) that will contribute to the hearing loss measured in this 50-year-old man.
How should the separate effects of noise and aging on hearing be disentangled? Continuing with this hypothetical example, let’s assume that the noise exposure alone is expected to produce a permanent hearing loss corresponding to a hearing level of 25 dB HL at 4000 Hz after 10 years of
exposure, with no further changes for another 20 years until retirement at age 50. Let us also assume that the median hearing level for a 50-year-old man in the United States is 20 dB HL at 4000 Hz. What hearing level would one expect to measure in a 50-year-old man who has worked in this noise for 30 years? One model that has been considered is simple decibel additivity of the hearing levels associated with aging and noise exposure, or 25 dB HL (NIPTS) + 20 dB HL (aging) = 45 dB HL. Another model suggests simple additivity of the corresponding sound powers, expressed as 10 log10 [10(25/10) + 10(20/10) ] or 26 dB HL. Based on empirical fits to the existing data, the ISO and ANSI standards both adopted the same compressive nonlinear model that yields values between these two extremes. The model used in the standards also is similar to a more general nonlinear compressive model that has proven successful when adding or combining elevated thresholds of various types (Humes et al., 1987; Humes and Jesteadt, 1989), including those arising from noise exposures (Humes and Jesteadt, 1991).
The specific form of the model used in the ISO and ANSI standards is as follows:
where HTLAN is the hearing threshold level from the combined effects of age (A) and noise (N), HTLA is the hearing threshold level associated with age alone, and HTLN is the hearing threshold level associated with the noise exposure alone. In the hypothetical example considered previously, according to this model, the 50-year-old man would be expected to have a hearing threshold at 4000 Hz of about 41 dB HL after 30 years of noise exposure [i.e., 20 + 25 = 45; (20 × 25) / 120 = 4.2; 45 − 4.2 = 40.8 dB HL]. In this case, the result is much closer to the prediction made according to simple decibel additivity (45 dB HL) than with power additivity (26 dB HL), but this is less true as the amount of hearing loss expected from each factor increases. For example, assume that both noise and age result in expected hearing threshold levels of 50 dB HL at 4000 Hz, then the ISO/ANSI additivity model predicts a threshold of about 79 dB HL, which is 21 dB less than simple decibel additivity (100 dB HL). In many situations, the ISO/ANSI additivity model is applied in the reverse direction. That is, the thresholds for a group or individual following X years of continuous exposure to noise at an 8-hour equivalent continuous level of Y dBA, as well as the age and gender of the group or individual are known, and the task is to determine how much of the hearing loss is associated with the noise exposure and how much is associated with age-related changes.
Regardless of the way such models are used, many assumptions are involved. One key assumption has to do with the time course of the development of hearing loss associated with each factor. For example, consider
two men, A and B, both 20 years old when they began work in the same noisy industrial or military environment. Individual A happens to be susceptible to the damaging effects of noise and develops a 40-dB hearing loss within the first few years of employment in this noisy environment, then experiences no additional decline in hearing over the next several decades (not susceptible to age effects). Individual B, on the other hand, is not susceptible to the damaging effects of noise and shows typical or average age-related hearing loss throughout adulthood. Let us assume further that, at age 70, the median hearing loss at this same frequency for males is 40 dB. So, at age 70, both A and B have a 40-dB hearing loss. Given the foregoing, the ISO/ANSI additivity model would attribute all of the hearing loss for each 70-year-old male to aging, an accurate estimate for individual B. Yet, for individual A, who had noise-induced hearing loss most of his adult life, attributing the hearing loss entirely to age would be inappropriate.
The foregoing examples demonstrate a key point regarding models designed to estimate noise-induced hearing loss and allocate the hearing loss to either noise-related or age-related components: These models were built from and are designed for group data, not individual data. Given the same 40-dB HL hearing loss of individuals A and B at age 70 in the foregoing example, it is virtually impossible without other data to determine whether the scenario represented by A or that represented by B is the actual underlying scenario for a given individual. This serves to underscore the critical importance of periodic measurement of hearing thresholds for those exposed to high levels of noise. Given the wide range of individual differences in susceptibility, the possible effects of exogenous factors, and our inability to predict these effects from other measures, regular measurement of hearing thresholds is the only way to determine if a change in hearing has occurred in an individual during the period of a particular noise exposure. In the absence of such information, estimates of noise-induced hearing loss must be confined to statistical descriptions of population values (i.e., medians, percentiles) expected from the specific noise exposure, but there is no way to determine where among the population a particular individual would be found (e.g., “average,” “most susceptible 10 percent,” etc.).
ISO-1999 and ANSI S3.44 both allocate a portion of the observed hearing loss to age-related hearing loss and a portion to noise-induced hearing loss. We have noted several concerns with regard to the noise-induced component in the preceding paragraphs. Concerns have also been expressed, however, regarding the validity of the component representing age-related hearing loss. Specifically, the validity of the screened database (database A) on age-related hearing loss has been called into question, due, in part, to its derivation from data that are now several decades old and may be subject to cohort bias (Wiley et al., 2001). This serves to underscore the critical importance of annual measurement of hearing thresholds for
individuals not exposed to noise, who can serve as a control group for the noise-exposed workers to document the effects of aging.
Thus far in our discussion of the estimation of noise-induced hearing loss from models such as ISO-1999 and ANSI S3.44, hearing thresholds at only one particular frequency have been considered. Although both exposures to industrial or military noise and advancing age result in high-frequency hearing loss, the pattern of hearing loss across frequencies differs for these two etiologies. As noted previously, it is the noise-notch pattern of hearing loss, together with detailed case-history information, that is used clinically to distinguish noise-induced hearing loss from other forms of high-frequency hearing loss, such as age-related hearing loss. This difference in pattern of hearing loss for noise and age is illustrated in Figure 2-6. The top panel in this figure shows the average hearing loss as a function of age in males when data from several cross-sectional studies of age-related hearing loss were synthesized (Robinson and Sutton, 1979). These data form the basis of one of the databases (database A) of age-related hearing loss included in the ISO-1999 and ANSI S3.44 standards. They represent composite values from studies that took some care in screening out subjects with prior exposures to noise or previous hearing loss attributable to other etiologies (based on self-report)—so-called “highly screened” samples. From the functions depicted in the top panel of Figure 2-6, it is apparent that hearing loss is greatest in the highest frequencies initially, and at older ages, the hearing loss is still worse at the highest frequencies, but lower frequencies are also affected. The ISO and ANSI standards both make provision for the use of other databases representing age-related hearing loss. Database B in both standards represents the hearing thresholds for a large unscreened sample from a U.S. Public Health Service survey completed in 1962 (Glorig and Roberts, 1965). Although the specific values for hearing thresholds at each frequency differ between databases A and B, with better hearing thresholds found in database A, greater high-frequency hearing loss at older ages is common to both databases.
In contrast, the patterns of NIPTS, or noise-only hearing loss in dB HL, in the lower panel of Figure 2-6 illustrate hypothetical data on the progression of noise-induced hearing loss patterned after actual data (Robertson et al., 1978). Of course, the actual amount of noise-induced hearing loss will depend on a variety of factors, as noted previously, such as the type of noise, the level of the noise, the noise spectrum, and the duration and pattern of exposure. The lower panel of Figure 2-6 illustrates the increase in hearing loss with longer durations (in years) of continuous exposure to broad-band, steady-state noise at a specific noise level, similar to that depicted in Figure 2-5. Unlike the patterns of hearing loss associated with age in the top panel of Figure 2-6, the hearing loss in the bottom panel of this figure reaches a maximum at 6000 Hz and then returns toward milder
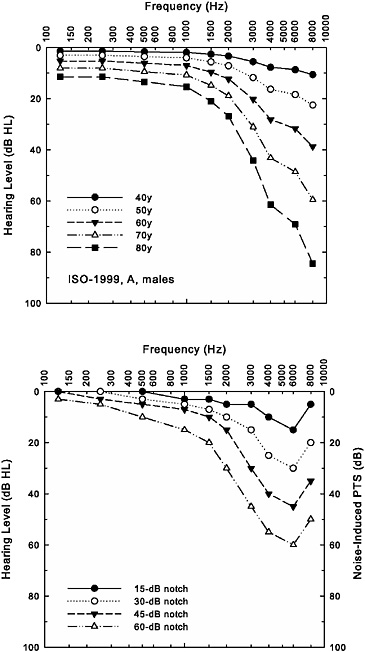
FIGURE 2-6 Age-related hearing loss for men (ISO-1999, database A, top panel) and hypothetical progression of noise-induced hearing loss with increased length of exposure in years (bottom panel). The bottom panel displays hypothetical NIPTS in dB, as well as the noise-only hearing loss data plotted in dB HL.
hearing loss at 8000 Hz. This demonstrates the characteristic noise-notch pattern of hearing loss in which the notch is located at 6000 Hz. As noted previously, the notch location varies with the noise exposure and across individuals experiencing the same noise exposure, but it is generally located at 3000, 4000, or 6000 Hz. A noise notch located at 6000 Hz was chosen for illustration purposes in the bottom panel of Figure 2-6 because of the frequent appearance of notches at this frequency in the data on military noise-induced hearing loss reviewed subsequently in Chapter 3.
Perhaps, therefore, the pattern of hearing loss across frequency can assist in determining how much, if any, of an older adult’s hearing loss can be attributed to prior noise exposure. That is, rather than just considering the hearing threshold at one frequency for the two 70-year-old individuals, A and B, in our previous example, one of whom had no prior noise-induced hearing loss and the other who had sizable noise-induced hearing loss, perhaps the presence or absence of a noise notch will assist in sorting this out. To examine the impact of aging on the pattern of hearing loss across frequency, the additivity model of ISO-1999 and ANSI S3.44 was applied to the two sets of data in the top and bottom panels of Figure 2-6. The top panel was assumed to represent a “pure” age-related hearing loss for each age decade, and the bottom panel was interpreted as four different degrees of noise notch developed in four young men during the first few years of noise exposure (rather than the progression of noise-induced hearing loss over time, as originally indicated). The case represented by the “45-dB notch” has a threshold at 4000 Hz that is about 40 dB HL and is representative of individual A in our previous examples. Individual B, on the other hand, was assumed to have no noise-induced hearing loss, and thresholds for this individual would be best represented by the age-only curves (“no notch”) depicted in the top panel of Figure 2-6.
Figure 2-7 illustrates the combined effects of noise (noise notches of various depths) and age (50-, 60-, 70-, and 80-year-olds) that result from using the ISO/ANSI additivity model to combine the sets of hearing thresholds from the two panels of Figure 2-6. When examining the predictions for each age, clear notching is visible in patterns of hearing loss for those individuals with initial noise-notch patterns at ages 50 and 60 years, but appears to be absent at ages 70 and 80 years. The other clear trend with age is the convergence of all the hearing losses by the age of 80 years. Hearing losses that differed by about 50 dB in the high frequencies for 50-year-olds differ by about half that much for 80-year-olds. Thus, there is less difference in pattern of hearing loss by the time these individuals reach their 70s and 80s, and the severity of the loss no longer differs as much among these individuals. As a result, two individuals who have similar hearing thresholds when measured at 70 or 80 years of age may have had entirely different patterns of hearing loss as young adults and throughout much of their
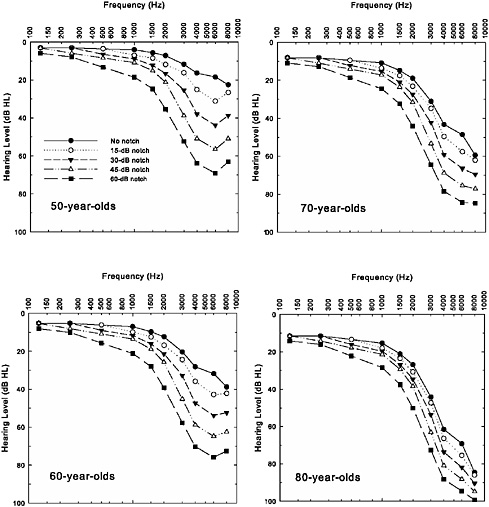
FIGURE 2-7 Illustrations of the combined effects of aging (top panel from Figure 2-6) and noise exposure (bottom panel from Figure 2-6) using the ISO-1999/ANSI S3.44 model for additivity. Each panel depicts the combined hearing loss for a separate decade (50-, 60-, 70-, or 80-year-old men).
adult lives. Once again, this underscores the critical importance of measuring hearing thresholds periodically (preferably annually) for individuals exposed to noise and, ideally, before and after employment or military service. With only the hearing thresholds from a much later stage in life, it is virtually impossible to discern how much, if any, of an individual’s hearing loss can be attributed to noise exposure or for how long this hearing loss might have been present.
With regard to the estimation of noise-induced hearing loss, the following represents a summary of the main points of this section of the chapter:
-
Without measurement of pure-tone thresholds prior to and following a given exposure to noise, it is impossible to document the effects of that exposure on hearing or to know what portion of the hearing loss in an older individual is due to earlier noise exposure.
-
ISO-1999 and ANSI S3.44 provide estimates of median values and range of variation in noise-induced hearing loss for a given noise exposure. Predictions are best for the noise-induced hearing loss that results from continuous or intermittent exposures to steady-state noise at levels between 75 and 100 dBA for 8 hours per day (for an assumed 5-day work week) for periods of 10 to 40 years.
-
Age-related hearing loss occurs at several of the same frequencies for which noise-related hearing loss occurs, and the measured thresholds are presumed to be a combination of these two forms of hearing loss.
-
Combined effects of noise and age on hearing thresholds range from energy summation to decibel summation. The combined effects of noise and age included in ISO-1999 and ANSI S3.44 lie somewhere between these two extremes.
FINDING: The evidence from cross-sectional studies of noise-induced hearing loss in humans is sufficient to conclude that daily time-weighted average noise exposures greater than approximately 85 dBA for 8 hours for periods of many years pose a hazard to human hearing and that the hazard increases as the time-weighted average exposure exceeds this value.
FINDING: The evidence is not sufficient to determine the probability of acquiring a noise-induced hearing loss, or to estimate the magnitude of the noise-induced hearing loss, that a specific individual is likely to experience from a given noise exposure.
REFERENCES
ANSI (American National Standards Institute). 1996. ANSI S3.44 Determination of Occupational Noise Exposure and Estimation of Noise-Induced Hearing Impairment. New York: Acoustical Society of America.
Aran JM, Hiel H, Hayashida T, Erre JP, Aurousseau C, Gulhaume A, Dulon D. 1992. Noise, aminoglycosides, and diuretics. In: Dancer AL, Henderson D, Salvi RJ, Hamernik RP, eds. Noise-Induced Hearing Loss. St. Louis, MO: Mosby Year Book. Pp. 188–195.
Axelsson A, Vertes D. 1982. Histological findings in cochlear vessels after noise. In: Hamernik RP, Henderson D, Salvi R, eds. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press. Pp. 49–68.
Berger EH, Royster LH, Thomas WG. 1978. Presumed noise-induced permanent threshold shift resulting from exposure to an A-weighted Leq of 89 dB. Journal of the Acoustical Society of America 64:192–197.
Boettcher FA. 2002. Susceptibility to acoustic trauma in young and aged gerbils. Journal of the Acoustical Society of America 112(6):2948–2955.
Boettcher FA, Henderson D, Gratton MA, Danielson RW, Byrne CD. 1987. Synergistic interactions of noise and other ototraumatic agents. Ear and Hearing 8(4):192–212.
Boettcher FA, Gratton MA, Bancroft BR, Spongr V. 1992. Interaction of noise and other agents: Recent advances. In: Dancer AL, Henderson D, Salvi RJ, Hamernik RP, eds. Noise-Induced Hearing Loss. St. Louis, MO: Mosby Year Book. Pp. 175–187.
Bohne BA. 1976. Healing of the noise-damaged inner ear. In: Hirsh SK, Eldredge DH, Hirsh IJ, Silverman SR, eds. Hearing and Davis: Essays Honoring Hallowell Davis. St. Louis, MO: Washington University Press. Pp. 85–96.
Bohne BA, Harding GW. 2000. Degeneration in the cochlea after noise damage: Primary versus secondary events. American Journal of Otology 21(4):505–509.
Botte MC, Variot MH. 1979. [Auditory fatigue in individuals having sustained an acoustic trauma (author’s transl)]. Annales d’Oto-Laryngologie et de Chirurgie Cervico-Faciale 96(12):827–833.
Bredberg G. 1968. Cellular pattern and nerve supply of the human organ of Corti. Acta OtoLaryngologica Supplement 236:1–135.
Brödel M. 1946. Three Unpublished Drawings of the Anatomy of the Human Ear. Assisted by Malone PD, Guild SR, Crowe SJ. Philadelphia, PA: W.B. Saunders Co.
Burdick CK, Patterson JH, Mozo BT, Camp RT. 1978. Threshold shifts in chinchillas exposed to octave bands of noise centered at 63 and 1000 Hz for three days(a). Journal of the Acoustical Society of America 64(2):458–466.
Carder HM, Miller JD. 1972. Temporary threshold shifts from prolonged exposure to noise. Speech and Hearing Research 15(3):603–623.
Cary R, Clarke S, Delic J. 1997. Effects of combined exposure to noise and toxic substances—critical review of the literature. Annals of Occupational Hygiene 41(4):455–465.
CHABA (National Academy of Sciences–National Research Council Committee on Hearing, Bioacoustics, and Biomechanics). 1968. Proposed Damage–Risk Criterion for Impulse Noise (Gunfire). Report of Working Group 57 (Ward WD, ed.). Washington, DC: National Academy of Sciences.
Chandler D. 2005. Current Scope of Problem. Washington, DC: Walter Reed Army Medical Center.
Coles RR, Lutman ME, Buffin JT. 2000. Guidelines on the diagnosis of noise-induced hearing loss for medicolegal purposes. Clinical Otolaryngology 25(4):264–273.
Cooper JC, Owen JH. 1976. Audiologic profile of noise-induced hearing loss. Archives of Otolaryngology 102(3):148–150.
Cruickshanks KJ, Klein R, Klein BEK, Wiley TL, Nondahl DM, Tweed TS. 1998. Cigarette smoking and hearing loss: The Epidemiology of Hearing Loss Study. Journal of the American Medical Association 279(21):1715–1719.
Cruickshanks KJ, Tweed TS, Wiley TL, Klein BEK, Klein R, Chappell R, Nondahl DM, Dalton DS. 2003. The 5-year incidence and progression of hearing loss: The Epidemiology of Hearing Loss Study. Archives of Otolaryngology and Head and Neck Surgery 129:1041–1046.
Davis H and Associates. 1953. Acoustic trauma in the guinea pig. Journal of the Acoustical Society of America 25(6):1180–1189.
Davis H, Parrack H, Eldredge D. 1949. Hazards of intense sound and ultrasound. Annals of Otology, Rhinology and Laryngology 58(3):732–738.
Davis H, Morgan CT, Hawkins JE, Galambos R, Smith FW. 1950. Temporary deafness following exposure to loud tones and noise. Acta Oto-Laryngologica Supplement 88: 1–56.
Dobie RA, Rabinowitz PM. 2002. Change in audiometric configuration helps to determine whether a standard threshold shift is work-related. Spectrum 19(Suppl 1):17.
Drescher DG. 1976. Effect of temperature on cochlear responses during and after exposure to noise. Journal of the Acoustical Society of America 59(2):401–407.
Eldredge DH, Mills JH, Bohne BA. 1973a. Anatomical, behavioral, and electrophysiological observations on chinchillas after long exposures to noise. Advances in Oto-Rhino-Laryngology 20:64–81.
Eldredge DH, Miller JD, Mills JH, Bohne BA. 1973b. Behavioral, physiological and anatomical studies of threshold shifts in animals. In: Proceedings of the International Congress on Noise as a Public Health Problem. Washington, DC: U.S. Environmental Protection Agency.
Erway LC, Shiau YW, Davis RR, Krieg EF. 1996. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hearing Research 93(1-2):181–187.
Fechter LD. 2004. Promotion of noise-induced hearing loss by chemical contaminants. Journal of Toxicology and Environmental Health 67(8-10):727–740.
Fechter LD, Chen GD, Rao D, Larabee J. 2000. Predicting exposure conditions that facilitate the potentiation of noise-induced hearing loss by carbon monoxide. Toxicological Sciences 58(2):315–323.
Fraenkel R, Freeman S, Sohmer H. 2003. Susceptibility of young adult and old rats to noise-induced hearing loss. Audiology Neurootology 8(3):129–139.
Franks JR, Morata TC. 1996. Ototoxic effects of chemicals alone or in concert with noise: A review of human studies. In: Axelsson A, Borchgrevink HM, Hamernik RP, Hellstrom PA, Henderson D, Salvi RJ, eds. Scientific Basis of Noise-Induced Hearing Loss. New York: Thieme. Pp. 437–446.
Fredelius L. 1988. Degeneration Pattern in the Guinea Pig Organ of Corti After Pure Tone Acoustic Overstimulation. A Morphological and Electrophysiological Investigation. Ph.D. dissertation. Stockholm, Sweden.
Gallo R, Glorig A. 1964. Permanent threshold shift changes produced by noise exposure and aging. American Industrial Hygiene Association Journal 25:237–245.
Gates GA, Schmid P, Kujawa SG, Nam B, D’Agostino R. 2000. Longitudinal threshold changes in older men with audiometric notches. Hearing Research 141(1-2):220–228.
Glorig A, Roberts J. 1965. Hearing levels of adults by age and sex, United States, 1960–1962. Vital and Health Statistics. Series 11, No. 11. Washington, DC: Department of Health, Education, and Welfare.
Glorig A, Ward WD, Nixon J. 1961. Damage risk criteria and noise-induced hearing loss. Archives of Oto-Rhino-Laryngology 74:413–423.
Gratton MA, Salvi RJ, Kamen BA, Saunders SS. 1990. Interaction of cisplatin and noise on the peripheral auditory system. Hearing Research 50(1-2):211–223.
Gravendeel DW, Plomp R. 1959. The relation between permanent and temporary noise dips. American Medical Association Archives of Otolaryngology 69(6):714–719.
Hamernik RP, Hsueh KD. 1991. Impulse noise: Some definitions, physical acoustics and other considerations. Journal of the Acoustical Society of America 90(1):189–196.
Hamernik RP, Henderson D, Coling D, Salvi R. 1981. Influence of vibration on asymptotic threshold shift produced by impulse noise. Audiology 20(3):259–269.
Henderson D, Hamernik RP. 1982. Asymptotic threshold shift from impulse noise. In: Hamernik RP, Henderson D, Salvi R, eds. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press. Pp. 265–281.
Henderson D, Hamernik RP. 1986. Impulse noise: Critical review. Journal of the Acoustical Society of America 80(2):569–584.
Henderson D, Hamernik RP, Sitler R. 1974a. Audiometric and anatomical correlates of impulse noise exposure. Archives of Otolaryngology 99:62–66.
Henderson D, Hamernik RP, Sitler RW. 1974b. Audiometric and histological correlates of exposure to 1-msec noise impulses in the chinchilla. Journal of the Acoustical Society of America 56(4):1210–1221.
Henderson D, Subramaniam M, Boettcher FA. 1993. Individual susceptibility to noise-induced hearing loss: An old topic revisited. Ear and Hearing 14(3):152–168.
Henselman LW, Henderson D, Shadoan J, Subramaniam M, Saunders S, Ohlin D. 1995. Effects of noise exposure, race, and years of service on hearing in U.S. Army soldiers. Ear and Hearing 16(4):382–391.
Hirose K, Liberman MC. 2003. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. Journal of the Association for Research in Otolaryngology 4(3):339–352.
Hirsch FG. 1968. Effects of overpressure on the ear—a review. Annals of the New York Academy of Science 152(1):147–162.
Hirsh IJ, Ward WD. 1954. Recovery of the auditory threshold after strong acoustic stimulation. Journal of the Acoustical Society of America 24:131–141.
Holme RH, Steel KP. 2004. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. Journal of the Association for Research in Otolaryngology 5(1):66–79.
Howell RW. 1978. A seven-year review of measured hearing levels in male manual steelworkers with high initial thresholds. British Journal of Industrial Medicine 35(1):27–31.
Humes LE. 1980. Temporary threshold shifts for masked pure tones. Audiology 19:335–345.
Humes LE. 1984. Noise-induced hearing loss as influenced by other agents and by some physical characteristics of the individual. Journal of the Acoustical Society of America 76(5):1318–1329.
Humes LE, Jesteadt W. 1989. Models of the additivity of masking. Journal of the Acoustical Society of America 85(3):1285–1294.
Humes LE, Jesteadt W. 1991. Modeling the interactions between noise exposure and other variables. Journal of the Acoustical Society of America 90(1):182–188.
Humes LE, Koval CB. 1981. Temporary threshold shifts for masked pure tones. II. Broad-band masker. Audiology 20(4):325–335.
Humes LE, Dirks DD, Bell TS, Kincaid GE. 1987. Recognition of nonsense syllables by hearing-impaired listeners and by noise-masked normal hearers. Journal of the Acoustical Society of America 81(3):765–773.
Ishii EK, Talbott E. 1998. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. Journal of Occupational and Environmental Medicine 40(8):661–666.
ISO (International Organization for Standardization). 1990. ISO 1999: Acoustics—Determination of Occupational Noise Exposure and Estimation of Noise-Induced Hearing Impairment. Geneva, Switzerland: ISO.
Jerger J, Alford B, Coats A. 1966. Effects of very low frequency tones on auditory thresholds. Journal of Speech and Hearing Research 9(1):150–160.
Johnsson LG, Hawkins JE. 1976. Degeneration patterns in human ears exposed to noise. Annals of Otology, Rhinology and Laryngology 85(6 Pt 1):725–739.
Kaufman LR, LeMasters GK, Olsen DM, Succop P. 2005. Effects of concurrent noise and jet fuel exposure on hearing loss. Journal of Occupational and Environmental Medicine 47(3):212–218.
Kraus RN. 1959. An evaluation of patients suspected of having noise induced hearing loss. Laryngoscope 69(11):1429–1442.
Kryter KD, Ward WD, Miller JD, Eldredge DH. 1966. Hazardous exposure to intermittent and steady-state noise. Journal of the Acoustical Society of America 39(3):451–464.
Lee FS, Matthews LJ, Dubno JR, Mills JH. 2005. Longitudinal study of pure-tone thresholds in older persons. Ear and Hearing 26:1–11.
Lei SF, Ahroon WA, Hamernik RP. 1994. The application of frequency and time domain kurtosis to the assessment of hazardous noise exposures. Journal of the Acoustical Society of America 96(3):1435–1444.
Liberman CM, Mulroy MJ. 1982. Acute and chronic effects of acoustic trauma: Cochlear pathology and auditory nerve pathophysiology. In: Hamernik RP, Henderson D, Salvi R, eds. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press. Pp. 106–135.
Macrae JH. 1991. Presbycusis and noise-induced permanent threshold shift. Journal of the Acoustical Society of America 90(5):2513–2516.
Man AL, Naggan L, Swet RT. 1975. Bilateral hearing loss as a sequel to unilateral acoustic trauma. Israel Journal of Medical Sciences 11:5–9.
Manninen O. 1988. Changes in hearing, cardiovascular functions, haemodynamics, upright body sway, urinary catecholamines and their correlates after prolonged successive exposures to complex environmental conditions. International Archives of Occupational and Environmental Health 60(4):249–272.
Manson J, Alessio HM, Cristell M, Hutchinson KM. 1994. Does cardiovascular health mediate hearing ability? Medicine and Science in Sports and Exercise 26(7):866–871.
McBride DI, Williams S. 2001a. Audiometric notch as a sign of noise induced hearing loss. Occupational and Environmental Medicine 58(1):46–51.
McBride DI, Williams S. 2001b. Characteristics of the audiometric notch as a clinical sign of noise exposure. Scandinavian Audiology 30(2):106–111.
McFadden SL, Campo P. 1998. Cubic distortion product otoacoustic emissions in young and aged chinchillas exposed to low-frequency noise. Journal of the Acoustical Society of America 104(4):2290–2297.
McGill TJI, Schuknecht HF. 1976. Human cochlear changes in noise induced hearing loss. Laryngoscope 86(9):1293–1302.
Melinek M, Naggan L, Altman M. 1976. Acute acoustic trauma—a clinical investigation and prognosis in 433 symptomatic soldiers. Israel Journal of Medical Sciences 12(6):560–569.
Melnick W. 1976. Human asymptotic threshold shift. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, eds. Effects of Noise on Hearing. New York: Raven Press. Pp. 277–289.
Miller JD, Watson CS, Covell WP. 1963. Deafening effects of noise on the cat. Acta OtoLaryngologica Supplement 176:1–91.
Mills JH. 1973. Temporary and permanent threshold shifts produced by nine-day exposures to noise. Speech and Hearing Research 16(3):426–438.
Mills JH. 1976. Threshold shifts produced by 90-day exposure to noise. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, eds. Effects of Noise on Hearing. New York: Raven Press. Pp. 265–275.
Mills JH, Talo SA. 1972. Temporary threshold shifts produced by exposure to high-frequency noise. Speech and Hearing Research 15(3):624–631.
Mills JH, Gengel RW, Watson CS, Miller JD. 1970. Temporary changes of the auditory system due to exposure to noise for one or two days. Journal of the Acoustical Society of America 48(2, Pt 2):524–530.
Mills JH, Gilbert RM, Adkins WY. 1979. Temporary threshold shifts in humans exposed to octave bands of noise for 16 to 24 hours. Journal of the Acoustical Society of America 65(5):1238–1248.
Mills JH, Osguthorpe JD, Burdick CK, Patterson JH, Mozo B. 1983. Temporary threshold shifts produced by exposure to low-frequency noises. Journal of the Acoustical Society of America 73(3):918–923.
Mills JH, Boettcher FA, Dubno JR. 1997. Interaction of noise-induced permanent threshold shift and age-related threshold shift. Journal of the Acoustical Society of America 101(3): 1681–1686.
Mizoue T, Miyamoto T, Shimizu T. 2003. Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. Occupational and Environmental Medicine 60:56–59.
Moody DB, Stebbins WC, Johnsson LG, Hawkins JE. 1976. Noise-induced hearing loss in the monkey. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, eds. Effects of Noise on Hearing. New York: Raven Press. Pp. 309–322.
Morata TC. 1998. Assessing occupational hearing loss: Beyond noise exposures. Scandinavian Audiology Supplement 48:111–116.
Morata TC. 2003. Chemical exposure as a risk factor for hearing loss. Journal of Occupational Environmental Medicine 45(7):676–682.
Morata TC, Campo P. 2001. Auditory function after single of combined exposure to styrene: A review. In: Henderson D, Prasher D, Kopke R, Salvi R, Hamernik R, eds. Noise Induced Hearing Loss: Basic Mechanisms, Prevention and Control. London: Noise Research Network Publications. Pp. 293–304.
Morata TC, Lemasters GK. 1995. Epidemiologic considerations in the evaluation of occupational hearing loss. Occupational Medicine 10(3):641–656.
Morata TC, Dunn DE, Kretschmer LW, Lemasters GK, Keith RW. 1993. Effects of occupational exposure to organic solvents and noise on hearing. Scandinavian Journal of Work, Environment & Health 19(4):245–254.
Morata TC, Dunn DE, Sieber WK. 1994. Occupational exposure to noise and ototoxic organic solvents. Archives of Environmental Health 49(5):359–365.
Nixon JC, Glorig A. 1961. Noise-induced permanent threshold shift at 2000 cps and 4000 cps. Journal of the Acoustical Society of America 33:904–908.
NRC (National Research Council). 1992. Hazardous Exposure to Impulse Noise. Committee on Hearing, Bioacoustics, and Biomechanics. Washington, DC: National Academy Press.
NRC. 1993. Hazardous Exposure to Steady-State and Intermittent Noise. Committee on Hearing, Bioacoustics, and Biomechanics. Washington, DC: National Academy Press.
Ohlemiller KK, Wright JS, Heidbreder AF. 2000. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: A dose-response approach in CBA, C57BL, and BALB inbred strains. Hearing Research 149(1-2):239–247.
Palmer KT, Griffin MJ, Syddall HE, Coggon D. 2004. Cigarette smoking, occupational exposure to noise, and self reported hearing difficulties. Occupational and Environmental Medicine 61:340–344.
Pekkarinen J. 1995. Noise, impulse noise, and other physical factors: Combined effects on hearing. Occupational Medicine 10(3):545–559.
Resnick DM, Albrite JP, Shutts RE. 1962. Noise-induced hearing loss among army helicopter trainees. Laryngoscope 72:262–273.
Roberto M, Hamernik RP, Turrentine GA. 1989. Damage of the auditory system associated with acute blast trauma. Annals of Otology, Rhinology and Laryngology (Suppl 140):23–34.
Robertson RM, Page JC, Williams CE. 1978. The Prevalence of Hearing Loss Among Selected Navy Enlisted Personnel. NAMRL-1251. Pensacola, FL: Naval Aerospace Medical Research Laboratory.
Robinson DW, Sutton GJ. 1979. Age effect in hearing—a comparative analysis of published threshold data. Audiology 18(4):320–334.
Rodriguez GP, Gerhardt KJ. 1991. Influence of outer ear resonant frequency on patterns of temporary threshold shift. Ear and Hearing 12(2):110–114.
Rosenhall U. 2003. The influence of ageing on noise-induced hearing loss. Noise Health 5(20):47–53.
Royster LH, Thomas WG, Royster JD, Lilley D. 1978. Potential hearing compensation cost by race and sex. Journal of Occupational Medicine 20(12):801–806.
Saunders JC, Tilney LG. 1982. Species difference in susceptibility to noise exposure. In: Hamernik RP, Henderson D, Salvi R, eds. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press. Pp. 229–248.
Segal S, Harell M, Shahar A, Englender M. 1988. Acute acoustic trauma: Dynamics of hearing loss following cessation of exposure. American Journal of Otology 9(4):293–298.
Shaw EA. 1974. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. Journal of the Acoustical Society of America 56(6):1848–1861.
Spongr VP, Boettcher FA, Saunders SS, Salvi RJ. 1992. Effects of noise and salicylate on hair cell loss in the chinchilla cochlea. Archives of Otolaryngology–Head and Neck Surgery 118(2):157–164.
Starck J, Toppila E, Pyykko I. 1999. Smoking as a risk factor in sensory neural hearing loss among workers exposed to occupational noise. Acta Oto-Laryngologica 119(3):302–305.
Stockwell CW, Ades HW, Engström H. 1969. Patterns of hair cell damage after intense auditory stimulation. Annals of Otology, Rhinology and Laryngology 78:1144–1168.
Sun JC, Bohne BA, Harding GW. 1994. Is the older ear more susceptible to noise damage? Laryngoscope 104(10): 1251–1258.
Taylor W, Pearson J, Mair A, Burns W. 1965. Study of noise and hearing in jute weaving. Journal of the Acoustical Society of America 38:113–120.
Temkin J. 1933. Die Schädigung des Ohres durch Lärm und Erschütterung. Monatsschrift für Ohrenheilkunde und Laryngo-Rhinologie 67:257–299.
Thalmann RR. 1976. Quantitative biochemical techniques for studying normal and noise-damaged ears. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, eds. Effects of Noise on Hearing. New York: Raven Press. Pp. 129–154.
Toppila E, Pyykko II, Starck J, Kaksonen R, Ishizaki H. 2000. Individual risk factors in the development of noise-induced hearing loss. Noise Health 2(8):59–70.
Van Campen LE, Dennis JM, Hanlin RC, King SB, Velderman AM. 1999. One-year audiologic monitoring of individuals exposed to the 1995 Oklahoma City bombing. Journal of the American Academy of Audiology 10(5):231–247.
Wang Y, Hirose K, Liberman MC. 2002. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. Journal of the Association for Research in Otolaryngology 3:248–268.
Ward WD. 1965. The concept of susceptibility to hearing loss. Journal of Occupational Medicine 7(12):595–607.
Ward WD. 1968. Susceptibility to auditory fatigue. In: Neff WD, ed. Contributions to Sensory Physiology. Vol. 3. New York: Academic Press. Pp. 191–226.
Ward WD. 1973. Noise-induced hearing damage. Otolaryngology 2:377–390.
Ward WD. 1995. Endogenous factors related to susceptibility to damage from noise. Occupational Medicine 10(3):561–575.
Ward WD, Glorig A. 1961. A case of firecracker-induced hearing loss. Laryngoscope 71:1590–1596.
Ward WD, Royster JD, Royster LH. 2000. Auditory and nonauditory effects of noise. In: Berger EH, Royster LH, Royster JD, Driscoll DP, Layne M, eds. The Noise Manual. 5th ed. Fairfax, VA: American Industrial Hygiene Association. Pp. 101–147.
Wiley TL, Torre P, Cruickshanks KJ, Nondahl DM, Tweed TS. 2001. Hearing sensitivity in adults screened for selected risk factors. Journal of American Academy of Audiology 12(7):337–347.
Wilson WJ, Herbstein N. 2003. The role of music intensity in aerobics: Implications for hearing conservation. Journal of the American Academy of Audiology 14(1):29–38.
Ylikoski ME, Ylikoski JS. 1994. Hearing loss and handicap of professional soldiers exposed to gunfire noise. Scandinavian Journal of Work, Environment & Health 20(2):93–100.
Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC. 2000. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hearing Research 141(1-2):97–106.







































