4
Research Issues Related to Risk Assessment of Low-Level Exposure to Chemical Warfare Agents
As noted earlier, one charge to the committee was to review and consider the U.S. Department of Defense’s (DOD’s) research plans for evaluating low-level exposures to chemical warfare agents (CWAs), identify data gaps, and make recommendations for further research. The second part of the charge asked the committee for guidance on appropriate risk assessment methods for assessing health risk to military personnel from low-level exposures to CWAs. To address this, the committee took the approach of coming up with a series of questions that must be addressed as part of the risk assessment of low-level exposure to CWAs, as a way to focus research on the most important issues.
QUESTION 1. WHAT IS THE CRITICAL ADVERSE RESPONSE TO EXPOSURE TO LOW LEVELS OF CWAS?
The U.S. Environmental Protection Agency (EPA 2004) defines the critical response as “The first adverse effect, or its known precursor, that occurs to the most sensitive species as the dose rate of an agent increases,” and it must be considered whether the most sensitive species is relevant to humans. Other organizations use somewhat different definitions, but identifying the first effect of concern for the population of interest as dose increases is a key step in the development of exposure
guidelines. For CWAs, an adverse effect is considered an operationally relevant performance decrement or an adverse health effect.
Throughout the DOD report, low-level exposures to CWAs that may cause miosis have been emphasized (pupillary constriction); that effect occurs earlier and more reliably than other adverse effects. Although miosis typically has been considered the critical effect and most frequently used indicator of toxicity, questions about other adverse effects at low-level exposures remain. As noted in Chapter 3, Hartgraves and Murphy (1992) and Wolfe et al. (1992) observed decrements in equilibrium performance in primates at soman (GD) doses that generally do not produce classic signs of cholinergic toxicity, and Chilcott et al. (2003) found that mastication consistently preceded miosis and salivation in minipigs treated with high percutaneous doses of VX. Therefore, DOD should conduct research to determine whether there are more sensitive toxicity end points than miosis from low-level exposures to CWAs.
QUESTION 2. HOW MUCH OF A DECREMENT IN THE CRITICAL RESPONSE IS ADVERSE?
In the military operational risk management (ORM) context, this means identifying the degree of decrement that is operationally relevant, including the potential for the decrement to result in a field unit to become ineffective for combat or other mission-related activities. This information is currently not available. As noted above, if miosis is selected as the critical response, studies must determine what level of pupillary constriction from miosis is operationally relevant. For example, determining that a specific exposure or dose will cause a 10% decrement in pupil size is not sufficient without knowing whether that decrement is operationally relevant and determining what other operational factors must be considered. For example, a degree of pupillary constriction that represents no decrement in performance for daylight operations may represent a significant impairment for evening or night operations. Under daylight conditions with adequate lighting, a moderate degree of miosis may not be operationally relevant. It is normal for human pupil diameters to decrease by 50% when moving from dim to bright light conditions, such as when one moves from a dark room to outside when the sun is shining brightly. This suggests that different CWA exposure limits may be appropriate for use under different lighting conditions. Furthermore, miosis has been reported to improve visual acuity of presbyopic
individuals (people who have lost elasticity of the lens due to advancing age) under well-lit conditions, a finding attributed to the pinhole effect, but miosis would decrease visual acuity in dim light (Sidell 1977). If visual acuity is affected, how much loss of acuity is important and how long does it last? Risk managers generally prefer a more cautious or protective approach when the health effect of interest is permanent or severe. In contrast, implementation of ORM risk-risk comparison requires the development of exposure limits and probabilities of adverse outcomes for exceedances of those limits for each level of severity and operationally relevant exposure duration. The field commander must consider the severity of delayed effects influencing future performance as well as possible immediate effects both from exposure to CWA and from heat stress resulting from wearing protective gear (mission-oriented protective posture [MOPP]).
Although it is desirable that the demarcation between acceptable and adverse be defined by the content specialists (e.g., how much miosis would impact a war fighter’s ability to perform his/her mission successfully), for neurobehavioral effects that are measured on a continuous scale, there often is no clear point of demarcation between an acceptable and adverse level, particularly for studies in laboratory animals. In such cases, the distribution of effects in unexposed control animals may be used to establish an abnormal range—for example, above the 95th and 99th percentile. Then, the probability of animals exhibiting responses in the abnormal (not necessarily adverse) range can be estimated as a function of exposure, using the available concentration-duration-response data. Such procedures have been used for behavioral data (Gaylor and Slikker 1990).
QUESTION 3. ARE THERE BIOMARKERS OF CWA EXPOSURE THAT CAN BE MEASURED AND USED IN RISK COMPARISONS?
Biomarkers of exposure and of effect are essential to understanding the relationships between concentration and duration of exposure and the resulting effect. A biomarker of exposure is a xenobiotic substance or metabolite or the product of an interaction between a xenobiotic and a target in the organism (NRC 1989). A biomarker of effect is a measurable biochemical, physiologic, or other alteration within an organism that, depending on magnitude, can be recognized as an established or
potential health impairment or disease (NRC 1999). A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism’s ability to respond to the challenge of exposure to a specific xenobiotic. It can be an intrinsic genetic or other characteristic or a pre-existing disease that affects the toxicokinetics of the chemical or the target tissue response to the chemical.
Recent research has used protein binding and fluoride-induced regeneration of the agent as a biomarker of exposure to CWAs. Biomarkers of exposure can be used as forensic biomarkers to aid in the reconstruction of exposure levels and the normalization across different exposure levels to a common dose metric. They can also be used to determine post hoc whether someone was exposed under combat conditions and to determine whether any apparent delayed effects are related to exposure. Such use, however, depends on the stability of the biomarker of interest, because it may be weeks or months before exposed personnel are removed from the field and assayed for possible exposure. Another research question is whether sampling weeks or months after exposure can be used to identify whether someone was exposed, and, if so, to quantify that exposure. Although the uses of biomarkers described in this report may have limited relevance to immediate ORM decisions, it is likely they might be useful for research on postexposure sequelae, for refining interspecies extrapolations, and for exposure reconstruction (the DOD Research Plan only mentions biomarkers briefly).
See discussion of Tasks IID1, IIIB1, and IIIB2 for other issues related to this question.
Decreased blood cholinesterase generally is considered a biomarker of exposure, although some scientists consider it to be sufficiently closely tied to adverse end points that they consider it to be a biomarker of effect. However, as noted earlier, it is not always a reliable indicator of toxicity after exposure to organophosphates (OPs). While decreased brain cholinesterase activity is a more appropriate biomarker of effect, it is obviously not a useful end point that can be studied in the field.
Biomarkers of susceptibility might be useful before exposures to CWAs to evaluate the potential increased risk to susceptible individuals. People with polymorphisms (genetically determined variants) that result in very low plasma cholinesterase activity may be unusually sensitive to systemic effects of some anticholinesterase compounds (Young et al. 1999). Similarly, people with genetic polymorphisms leading to lower activity in the enzymes that metabolize CWAs to inactive forms may have enhanced sensitivity, although the implications of such polymor-
phisms for determining tissue dose (and therefore risk) depend on the degree to which enzyme activity limits metabolism (Gentry et al. 2002). This discussion is probably most relevant for systemic toxic effects. It may not be relevant for miosis if this response is primarily the result of direct contact with a CWA.
QUESTION 4. HOW SHOULD EXPOSURES IN “WORK” CONDITIONS BE CONSIDERED WHEN EXTRAPOLATING ANIMAL MODELS TO HUMAN EXPERIENCE?
Military personnel are likely to be exposed to CWAs while physically active. Thus, the use of animal models in which exposures are conducted while the animals are physically restrained or inactive may be problematic. In occupational settings, the higher respiration associated with physical activity will result in higher systemic exposures, while restraint-induced stress may lead to increased adrenergic stimulation. Also, depending on the location of deployment, military personnel often operate under severe environmental conditions, such as extremely high or low ambient temperatures. These environmental conditions could affect respiration and uptake of CWAs and may alter the expected degree of impairment associated with exposure to the agent. Experimental design should consider incorporating such factors (e.g., physical exertion with treadmill exercise, increased temperature) as variables where feasible.
Adjustments are also needed in extrapolating from studies in the general population or worker population to military scenarios. Previously, when human volunteer data were used to develop airborne exposure guidelines for G agents in occupational settings (Mioduszewski et al. 1998), it was necessary to extrapolate from the resting respiratory rate associated with the human volunteer studies to that associated with an occupational setting. The extrapolation assumed a human resting respiratory rate of 10 liters (L) per minute and an occupational respiratory rate of 20.8 L per minute over 8 hours. Different minute-volume adjustments would be necessary for deployed military personnel because, depending on the military missions, their activity levels and exposure durations would be different from a typical 8 hour/day worker. Strenuously working individuals will absorb more agent than people in the general population because of higher pulmonary ventilation and higher cardiac output.
Appropriate consideration of activity levels also may depend on the toxicity end point used to derive exposure limits. For example, systemic
and respiratory effects are closely tied to the minute volume under many conditions, and ocular effects resulting from topical exposure, such as miosis, may be a function primarily of the exposure concentration and duration of exposure.
QUESTION 5: WHAT IS THE RELATIONSHIP BETWEEN EXPOSURE CONCENTRATION-DURATION AND RISK?
The setting of exposure limits based on dose and duration has been considered in a variety of contexts. For example, Jabarek (1995) presented a collection of potential dose metrics along with an accompanying illustration. Although ease of use has made it a common practice to invoke Haber’s law (the assumption that adverse response is related to the product of agent concentration and duration of exposure, or Ct or cumulative exposure metric), Atherley (1985) provided a historical summary and challenge to the routine use of this assumption.
ten Berge et al. (1986) provided a generalization of this relationship that weights concentration more than time (for n > 1), stating that adverse response is related to Cnt. Using this relationship, they found that, for acute lethality, the exponent (n) was greater than 1 for 19 of 20 tested chemicals and the highest exponent was 3.5. Similar broad analyses of the ten Berge et al. (1986) modification have not been conducted for end points other than lethality. Use of the ten Berge relationship for extrapolating to durations well beyond experimental design is problematic because its applicability for those scenarios has not been studied or validated. Mechanistic considerations can also aid in improving on the ten Berge relationship. Empirical curve-fitting methods can also be used if the data span the time period of interest. Physiologically based pharmacokinetic (PBPK) modeling (see Question 8) can also be used to improve the evaluation of concentration-duration-response relationships.
Another tool that has been used to evaluate concentration-duration relationships, particularly for acute inhalation exposures, is categorical regression. This is a method by which a dose-response model may be fit to data where only severity ratings are available (Hertzberg and Miller 1985; Hertzberg 1989; Guth et al. 1997; reviewed by Haber et al. 2001). For example, a study may note that exposure to 15 milligrams per cubic meter (mg/m3) for 10 minutes caused miosis, while clinical signs of toxicity occurred after exposure to the same concentration for 1 hour. Thus, an advantage of categorical regression is that it can be used to analyze
the concentration-time severity of response relationship in the absence of quantitative data.
Often, acute inhalation data from one study are insufficient to describe the full concentration-duration-response spectrum of interest. However, data on the effects of interest may be available from studies conducted in different species and strains of animals and different concentration-time patterns. Categorical regression can be used to combine these data in the development of acute inhalation exposure limits (NRC 1993; Guth et al. 1997). EPA has developed guidance on use of categorical regression (EPA 2000), and their free software can be used to conduct the modeling (EPA 2003).
Several other methods of combining studies are also available, although there is less experience in applying these methods for toxicological risk assessment. For example, meta-analytic methods are frequently used to combine estimates across independent studies (Hedges and Olkin 1985; Gurevitch and Hedges 1993; Piegorsch 1998; Manly 2001; Guth et al. 1997). These estimates are typically weighted by some estimate of variability/uncertainty associated with each of the potency estimates. Alternatively, analyses considering strain and species as random effects and concentration and duration as fixed effects might be conducted (Diggle et al. 1994; Verbeke and Molenberghs 1997). Risk estimation in the context of these mixed effects models may represent an area of interesting risk assessment research.
The methods developed for acute exposure guideline levels (AEGLs) are very pertinent for exposures to CWAs. The generalizations of Haber’s law to effects related to Cnt (ten Berge et al. 1986) is a valuable contribution. The National Research Council (NRC) (2001) recommends the use of this approach for developing AEGLs for CWAs. The NRC (2003) used the ten Berge et al. (1986) approach in developing AEGLs for exposures of 10 minutes to 8 hours. For sarin (GB) (and subsequently tabun [GA], soman [GD], cyclosarin [GF], and VX), they used an exponent of n = 2, based on both miosis and lethality data. For sulfur mustard (HD), a value of n = 1 was used for the lower-severity values (AEGL-1 and AEGL-2). For AEGL-3, which is designed to protect against life-threatening effects or death, the NRC (2003) used the health-protective approach of using an exponent of n = 3 for extrapolating to shorter periods (resulting in a flatter curve in the time-exposure-duration dimension relative to the concentration-dose dimension) and n = 1 for AEGL-3 for extrapolating to longer periods.
For military operations, the U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM) has developed air military exposure guidelines (MEGs) for CWAs (USACHPPM 2002a,b). These MEGs are derived based on existing data and assumptions about critical end points such as miosis and lethality. The findings from research on neurobehavioral and cognitive effects would have great importance on future updates and revisions of the MEGs. As such, coordination and communication of research strategies between DOD risk assessors and USACHPPM is strongly recommended. The exposure durations associated with these guidelines are 10 minutes, 1 hour, 8 hours, and 24 hours. These durations are presumed to reflect the duration of various military missions and the environmental fate of CWAs (in the air pathways). Estimates of the 24-hour exposure guidelines are based on one-third of the 8-hour level (by using Haber’s law). However, for end points that are primarily minimal local effects (e.g., reversible miosis, eye pain), Haber’s rule generally is thought not to apply because it overestimates the importance of the duration component. For these local effects, toxicity is determined primarily by the exposure concentration, with the exposure duration (“time”) making a smaller contribution. The degree, if any, to which toxicity depends on the duration is not known, and the approach for extrapolating across significantly different exposure durations is not well characterized.
Finally, mechanistic considerations would be informative regarding the conditions and dose metrics for which Haber’s law is and is not applicable. A number of mechanistic reasons can be hypothesized as to why the Ct relationships for OP acetylcholinesterase (AChE) inhibitors may not follow Haber’s law. First, the rate of AChE inhibition may be an important determinant in the toxicologic response—that is, rapid AChE inhibition may not be tolerated as well as more protracted inhibition. Adaptive processes—for example, receptor desensitization or receptor internalization/down regulation—may occur more effectively with slower AChE inactivation than with rapid inhibition. As G agents and VX are all rapid, direct-acting AChE inhibitors, this may influence the Ct relationship. Finally, synapses are thought to have “spare” AChE molecules—that is, some degree of AChE inhibition can be tolerated at the synapse before cholinergic neurotransmission is functionally affected. This could influence the concentration-duration-response relationship if the rate of synthesis of spare molecules is slower than the rate of inactivation. Other factors that could contribute to the shape of the concentration-duration-response relationship include the potential for regenerative repair.
QUESTION 6. HOW SHOULD EXPOSURE-DURATION RISK BE COMMUNICATED FOR CWAS?
The risk to human health is a function of the concentration and the exposure duration. The best strategy for communicating this risk has yet to be determined. Field commanders ultimately must be able to use the information generated by the DOD Research Plan. Those commanders will only rarely find that traditional formats for presenting toxicologic data meet their needs. Accordingly, one of the challenges of the DOD research program will be to find ways to express or relate relevant data in adequate and useful ways that consider both the need to adequately protect forces from the harmful effects of low levels of CWAs and the off-setting costs of impairing the capacity of forces to accomplish their battle objectives consequent to the use of protective gear or MOPP. DOD risk assessors should confer with operations personnel to determine the nature and form of information that would be most useful. For example, a chart with exposure duration along one axis and exposure concentration along the other axis would allow a readily understood pictorial presentation of the exposure conditions (duration and concentration) associated with various probabilities of adverse effects (e.g., work impairment due to miosis). The committee recommends consultation with risk communication specialists to further assist with this task. Some general remarks related to this topic are given below. At a simple level, a tabular display may suffice. For example, a hypothetical table of the probabilities of adverse response associated with different exposure durations at different CWA concentrations (arbitrary units) is presented (Table 4-1). A figure analogous to this table might also be constructed (see figures at the end of this chapter for different ways to represent these types of data).
Depending on how short, medium, and long exposure durations are defined, it is necessary to describe the range of exposure durations in
TABLE 4-1 Probability of Adverse Response Associated with Various CWA Concentrations and Durations (Hypothetical)
|
Exposure Duration |
CWA Concentration |
||||
|
0 |
10 |
20 |
30 |
40 |
|
|
Short |
0 |
0.40 |
0.60 |
0.90 |
0.90 |
|
Medium |
0 |
0.60 |
0.80 |
0.95 |
0.95 |
|
Long |
0 |
0.80 |
0.90 |
1.00 |
1.00 |
which CWA concentration limits are valid as well as a method for adjusting estimates of exposure limits when those ranges are exceeded.
QUESTION 7. WHAT ARE APPROPRIATE APPROACHES FOR EVALUATING THE RISK OF EXPOSURE TO A CWA MIXTURE?
In general, DOD considers the most likely CWA exposures to be those that involve single agents. However, multiple CWAs might be simultaneously deployed. In the development of AEGLs for nerve agents, the National Research Council (NRC) (2003) suggested that the effects of concurrent exposures to commercial insecticides and G agents would be dominated by the more potent nerve agents. This conclusion is based on large differences in lethal concentration in 50% of exposed individuals (LC50) values among the G agents, agent VX, and commercial insecticides. The NRC (2003) further pointed out that when administered together in laboratory animals, the toxicity of two G-series agents was “basically additive” (Clement 1994; Luo and Liang 1997), which is expected for compounds sharing anticholinesterase properties. No potentiation has been observed in toxicologic tests of nerve agent mixtures.
Unless detection methods are available to discriminate among agents in the field, it is reasonable to focus on the most potent chemical for setting exposure guidelines for mixtures relevant to battlefield conditions. The committee assumes that if a mixture were present, it would be composed of a relatively small number of CWAs. Existing data characterizing the toxicity of nerve agent mixtures indicate that the toxicity of the mixture would approximate that of the most potent component. Thus, it is reasonable to evaluate the risk for a nerve agent mixture assuming that the entire mixture was composed of its most potent constituent.
When detection methods to discriminate among different agents are available, standard methods for mixture risk assessment can be used. For chemicals that act via similar modes of action, the default approach for assessing the risk from combined exposure is dose addition. For nerve agents, experimental data collected from administration of a G-agent mixture confirms the validity of this approach (reviewed by NRC 2003). This approach adds the scaled doses of the different components of the mixture, accounting for relative toxicity. Dose-additive approaches include the hazard index, toxicity equivalence factor, and relative potency factors.
Existing and well-conducted experimental studies indicate that the toxicity of G-series nerve agents is additive. Relative-potency analyses documented in the NRC (2003) report support this determination for nerve agent VX as well. Consideration of other available data could define the degree of toxicity independence between and among CWAs possessing different mechanisms (e.g., alkylating agents vs. anticholinesterase agents); this area would benefit from further exploration. The results of such exploration would inform the need and direction for any further model considerations. Such an approach would support the application of ORM, which considers the likelihood of adverse effects associated with various exposures.
QUESTION 8. WHAT INFORMATION IS NEEDED TO BEST SUPPORT EXTRAPOLATION FROM ANIMAL STUDIES TO HUMAN RESPONSES? HOW AND WHEN MIGHT A PBPK MODEL BE USEFUL FOR AIDING IN SUCH EXTRAPOLATIONS?
Determining the probability of a toxic response from human data is straightforward if the population for which data exist is comparable to the population of interest and if data are available for the exposure duration of interest. (See Question 5.) Methods for extrapolating threshold levels from animal data are well defined (albeit there are uncertainties inherent in the approach), but extrapolation of response probabilities is more complex. Essentially, it is often assumed that humans have the same probability of response as animals when both receive an equivalent dose, although this approach does not address interspecies differences in sensitivity to a given dose (due to differences in tissue susceptibility, repair, defense, and other factors). The definition of this “equivalent dose” is the challenge of species extrapolation. Hence, establishment of species equivalency in response, or lack thereof, is critical. Biologically based dose-response modeling makes it possible to predict probabilities in humans, but it is very labor intensive. PBPK modeling can aid in extrapolating exposures to humans. These models incorporate information on the physiology and kinetics of metabolism of the chemical of interest in humans and the experimental animal species and so allow for determination of the human exposure that results in the same tissue dose as received by the experimental animals. As an important aside, however, a PBPK model may not be required for extrapolating a toxic response from
a direct contact (e.g., miosis may be an example of this if direct contact of the CWA with the eye causes this response).
A key issue when a PBPK model is used is identification of the appropriate measure of tissue dose, the “dose metric.” The correct dose metric will describe the dose response for the chemical under multiple sets of exposure conditions. Common dose metrics include, but are not limited to, the area under the curve of the chemical or its metabolite in the plasma or target tissue, peak concentrations, and amount metabolized. The use of any animal model in risk assessment would require considering whether the model has been adequately validated in the experimental animal species, whether it has been validated for humans, and whether the mode of toxicity for CWAs in the modeled species is relevant to other mammals, notably humans. Maxwell et al. (1988) developed a physiologically based pharmacodynamic (PBPD) model for GD in the rat, while Gearhart et al. (1990, 1994) developed a similar combined PBPK/PBPD model for the OP diisopropyl fluorophosphate (DFP), a model OP with some resemblance to nerve agents. This model allows one to estimate not only tissue levels of DFP but also the toxic response in terms of AChE levels in the brain or plasma. More recent work is exploring the use of fluoride ion regeneration as a dose metric that is more sensitive and less variable than changes in AChE at low exposure levels. Further work in extending this sort of model to other CWAs will be useful in improving the interspecies extrapolation for CWAs as well as in addressing exposure duration extrapolation issues. For example, Yu et al. (2004) have reported on the preliminary development of a PBPK/PBPD model for GB in miniature swine to aid in interpretation of results from toxicity studies with swine. The model includes an ocular compartment, including ocular absorption of GB and binding to AChE, to aid in addressing miosis. Incorporating data on human variability may make it possible to predict the proportion of exposed humans expected to be adversely affected.
The toxicokinetics of GD is complicated by the high in vivo reactivity and distinct differences in metabolic properties of the toxic C(+/−)P(−)-stereoisomers and the less toxic C(+/−)P(+)-stereoisomers. For VX, the overall stereospecificity for sequestration of (+)- versus (−)-VX is much less pronounced than for the stereoisomers of G agents, based on the observed marked in vivo persistence of VX. The first PBPK model that took into consideration the chirality of GD was by Langenberg et al. (1997; cited by Benschop and De Jong 2001). In this model, binding in blood becomes less dominant in overall toxicokinetics
when the dose of C(+/−)P(−)-GD exceeds the capacity of the binding sites in blood. This highlights the need for further refinement by discriminating among the four stereoisomers of GD.
Benschop and De Jong (2001) and the NRC (2003) reviewed the data on interspecies differences. In view of the large differences in susceptibilities to G agents between rats and guinea pigs and the relative role of plasma carboxylesterases (i.e., these esterases are found in relatively high concentrations in rat but not in guinea pig plasma), the guinea pig is often considered a better model for these toxicants. Carboxylesterases do not play a significant role in detoxification of VX, however, leading the committee to conclude that rats are not a less-appropriate model with this nerve agent. Thus, the most appropriate animal model to use based on these species differences in agent detoxification can vary with the particular agent in question.
Although biologically based dose-response modeling allows one to predict response in humans in response to a specified external exposure, PBPK modeling calculates only tissue dose. Additional extrapolation is needed to consider toxicodynamics (pharmacodynamics) or interspecies differences in tissue response. Several of the existing PBPK models for CWAs include a pharmacodynamic component (Maxwell et al. 1988; Gearhart et al. 1990; Yu et al. 2004), addressing, for example, how AChE levels change with tissue dose. This addresses a major component of the pharmacodynamic differences, although it does not address any interspecies differences in operationally relevant performance decrements for a given AChE level. U.S. and international agencies typically use a factor of 3 or 3.2 (half the default factor of 10 on a log scale) to address interspecies differences in toxicodynamics when data from a PBPK model are used to replace the toxicokinetic portion of the interspecies uncertainty factor (IPCS 2001). Toxicologic judgment could be used to reduce this factor for toxicodynamic differences to a factor of 1 if the critical effect is in a species that is judged to react very similarly to humans, such as many end points in nonhuman primates. Similarly, the use of validated PBPK/PBPD models, when available for the species and end point of interest, would address most, if not all, of the interspecies differences in toxicodynamics, markedly reducing or eliminating the need for uncertainty factors to address this area of uncertainty. NRC (2003) notes that the miotic response of the mammalian eye appears to be quantitatively very similar across species, including humans, indicating that no toxicodynamic uncertainty factor is needed for this end point.
QUESTION 9. WHAT IS THE IMPACT OF EXPERIMENTAL DESIGNS ON RISK ASSESSMENTS AND SUBSEQUENT EFFORTS TO ESTABLISH EXPOSURE GUIDELINES FOR MILITARY PERSONNEL PROTECTION?
The lack of experimental data for longer exposure durations (e.g., 8 hours, 24 hours) and at lower concentrations is an acknowledged limitation in efforts to establish military exposure guidelines for exposure durations greater than 8 hours. As a consequence, the protective assumption of response linearity from 8 to 24 hours was applied by USACHPPM to develop 24-hour military exposure guidelines for nerve and mustard agents from the 8-hour AEGL (e.g., assuming Haber’s law for exposure duration greater than 8 hours; it should be noted that Haber’s law is a special case of the ten Berge expression when n = 1). The 8-hour AEGLs were time-scaled using the techniques described by NRC (2001) from human and laboratory animal experimental exposure durations of less than 8 hours, assuming the ten Berge et al. (1986) expression of dose-response and chemical-specific determination of n. For example, the 8-hour AEGL-1, AEGL-2, and AEGL-3 were extrapolated from 4-hour, 30-minute, and 6-hour experimental data, respectively. The agent GB data set is robust (NRC 2003) and supports the assumptions used. As a general rule, the greater the extrapolation from the original data, the greater the resulting uncertainty. Grotte and Yang (2001) had speculated that inhalation concentration for the G agents probably could be extrapolated from 2 minutes through 60 minutes with reasonable confidence. Without access to the more recent experimental data of Mioduszewski et al. (2000, 2001, 2002a, 2002b) and of van Helden et al. (2001, 2002, 2003), Grotte and Yang (2001) further speculated that the accuracy of extrapolating below 2 minutes and beyond 60 minutes is unknown. This assumption has been superseded by current assessments with more recent nerve agent experimental data (NRC 2003). Model extrapolation beyond the tested concentration-time scenarios can be done with reasonable precautions. Experiments that include durations of exposure and concentrations that are operationally relevant are preferred. Conducting studies at operationally relevant levels helps alleviate extrapolation in the risk characterization of low-level exposures to CWAs.
To operationalize the ORM concept, the risk assessor will require information on response probabilities from the experimental study. For each critical response associated with CWA exposure, the research should study the complete dose-duration-response profile and not simply
some single end point, such as ECt50 (the concentration and time that causes an effect in 50% of subjects) and EC01 (concentration effective in 1% of subjects) for a specific effect for a specified exposure duration. Access to recently generated experimental data sets and contact with investigators should be pursued to develop other response probabilities (e.g., 15%, 30%, 40%) that would be needed in operational risk assessment. Experimental design consideration of adequate size for each exposure-response group would help minimize uncertainties in subsequent extrapolations.
QUESTION 10. HOW DOES ONE ACCOUNT FOR VARIABILITY IN HUMAN RESPONSE TO CWAS, AND DO CWA-SUSCEPTIBLE SUBPOPULATIONS OF HUMANS EXIST?
Factors contributing to human variability depend on the end point and the mechanistic basis for the end point. Miosis that can occur from local exposure in the absence of systemic cholinesterase inhibition is a local effect. For such effects, variability would be determined by the chemistry and physiology of the eye, including metabolic enzymes in the eye that can deactivate nerve agents, and the variability in response in the neurons controlling miosis.
For systemic effects of nerve agents, variability in human response can be due to differences in the kinetics of agent uptake and metabolism or to differences in susceptibility to a given tissue dose. Thus, it can be predicted that variability in response to systemic exposure would be higher than that involving local effects. Both plasma cholinesterase and red blood cell AChE activities generally are lower in women than in men by about 10%. This means that a given percent decrease in cholinesterase generally results in a lower absolute activity for women, so that there is a smaller “buffer” between normal cholinesterase levels and levels that can result in toxicity. It has also been shown that a small subpopulation of men and women have genetically determined variants in their plasma cholinesterase resulting in very low activity levels. Individuals with these genetic variants may be unusually susceptible to some anticholinesterase compounds (Young et al. 1999). As reviewed by NRC (2003), the frequency of the homozygous pheno-type is estimated at 0.025% (Hayes 1982), and plasma cholinesterase activity is less than 25% of normal in these individuals (Bonderman and Bonderman 1971).
Variability in nerve agent kinetics is due to genetic factors, such as polymorphisms in the paraoxonase gene (PON1), as well as variability in expression of the relevant enzymes (Furlong et al. 2002) and variability in breathing rate, which affects the amount of agent taken up into the body. As discussed above, variability in breathing rate may be particularly important for military personnel. Young animals have lower levels of paraoxonase, as well as carboxylesterase, but this age-related difference in susceptibility is not of concern in the context of military operational performance.
Because many of the sources of variability would also apply to military populations, the committee considers this group likely to be as variable as the general population. Further characterization of the implications of human variability in response to CWAs under military deployment conditions would be useful for ORM.
QUESTION 11. WHAT STATISTICAL MODELS ARE APPROPRIATE FOR CONCENTRATION-TIME DATA?
The modeling of dichotomous responses in dose-duration studies is discussed below. The committee’s focus is on the evaluation of data from an experimental study of a single agent. In particular, the structure imposed by Haber’s law and possible generalizations are described. As mentioned above, Haber’s Law states that cumulative exposure determines the toxic response (e.g., an exposure at 100 parts per million [ppm] for 6 hours leads to the same toxic response as 600 ppm for 1 hour). To illustrate the impact of Haber’s law on a statistical model, we focus on a dichotomous response (e.g., the presence of significant impairment). For a dichotomous response, the probability of a material impairment is modeled as a function of dose (or concentration) and duration (or time). Denoting the concentration by C and duration by t, the probability of an adverse response given exposure to concentration C of an agent for t units of time is π(C, t) = function(C, t). A generalized linear model (McCullagh and Nelder 1989) with a logit link or a probit link and a binomial response distribution is commonly used in this situation. In this case, under Haber’s law, the model can be written as follows:
(1)
where logit(x) = log[x/(1 − x)]. A contour plot of this pattern is shown in Figure 4-1. The “X” points in this figure provide one option of a facto-
rial experimental design where combinations of concentrations and durations are tested. Table 4-2 provides an example of a better experimental design that more specifically targets the experimental data points to provide the most information on the dose-response.
As an aside, this model provides a prediction of responses in an animal study. In general, extrapolation to humans may require other adjustments, although, as observed previously, this may not be an issue for miosis caused by direct contact with CWAs.
The contour plot in Figure 4-1 corresponds to a projection of the three-dimensional surface (Figure 4-2). The region in the top right corner of the plot (region in the Ct plan located above the line labeled “0.95”) corresponds to concentration-duration combinations resulting in >95% of adverse response.
Note that the models shown do not include a mathematical threshold, even though such thresholds are assumed biologically for the primary effects of concern for CWAs. The absence of a mathematical threshold would not have a substantial impact on the model estimates in
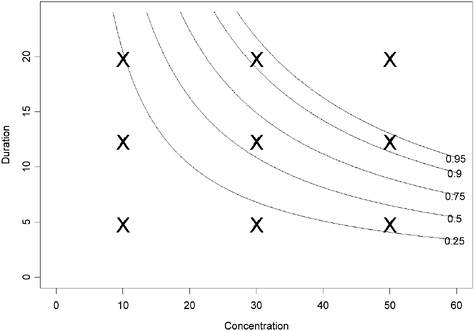
FIGURE 4-1 Contour plot of hypothetical data assuming that Haber’s law applies (using parameter values α = −2.944439, β = 0.009036316 in equation 1). The probability of adverse response is displayed as a function of concentration and duration.

FIGURE 4-2 Perspective plot of hypothetical data assuming Haber’s law applies (using parameter values α = −2.944439, β = 0.009036316 in equation 1).
TABLE 4-2 Example of Experimental Designa That Might Be Considered an Alternative to a Factorial Design
|
Duration (t) |
C = 1 |
C = 3 |
C = 9 |
|
16 |
X |
|
|
|
8 |
X |
X |
|
|
4 |
X |
X |
X |
|
2 |
|
X |
X |
|
1 |
|
X |
|
|
aArbitrary units are for illustration. X indicates a tested duration-concentration combinations, and Ct ranges from 4 to 36. |
|||
the range of the data, but these models should not be used to extrapolate response predictions to conditions well below the range of the data.
Departures from this model often can be incorporated by adding an additional time term to the model. For example, this is illustrated with the following logistic regression model:
(2)
where α, β, and γ are variable parameters used to fit data.
An illustration of a hypothetical contour plot when Haber’s law does not apply (γ ≠ 0) is given below. Note that this model can be expressed as follows:
(3)
and highlights one possible modification of the pattern imposed by Haber’s law. In this case, if γ > 0, then toxicity greater than predicted by Haber’s law is observed. This is illustrated in Figures 4-3 and 4-4. As an aside, this model assumes that the probability of an adverse response increases with time even in unexposed individuals (C = 0). Model 3 implies ![]() . If the probability of adverse response is assumed constant in the absence of dose (C = 0) and Haber’s law does
. If the probability of adverse response is assumed constant in the absence of dose (C = 0) and Haber’s law does
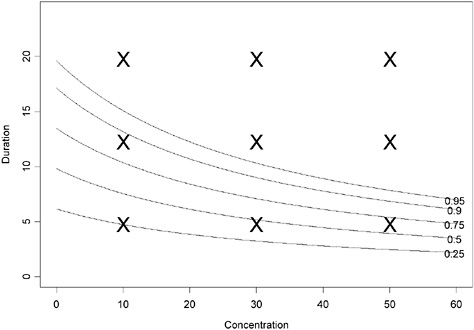
FIGURE 4-3 Contour plot of hypothetical data assuming Haber’s law does not apply (using parameter values α = −2.944439, β = 0.009036316, γ = 0.3 in equation 2). The probability of adverse response is displayed as a function of concentration and duration.
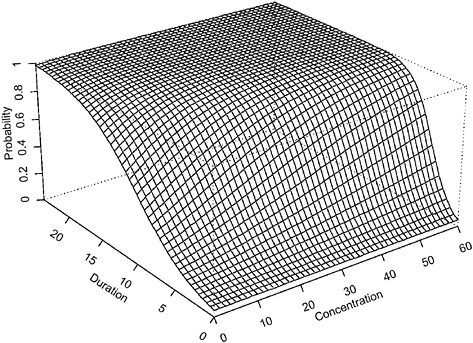
FIGURE 4-4 Perspective plot of hypothetical data assuming Haber’s law does not apply (using parameter values α = −2.944439, β = 0.009036316, γ = 0.3 in equation 2).
not apply, then an alternative model might be written as ![]()
![]() t or with ten Berge’s modification
t or with ten Berge’s modification ![]() .
.
The “X” points in the figure provide the same hypothetical sample design points as shown in Figure 4-1, assuming that 10 ≤ concentrations ≤ 60 and 5 ≤ durations ≤ 20 (units arbitrary) are of interest. However, unlike the results in Figure 4-1, this sampling design would result in most of the data being collected at concentration-duration combinations that result in ≥95% response. In this case, one should conduct range-finding studies to better define the concentration-duration range of interest or test lower concentrations for the exposure durations of interest.
This contour was associated with the three-dimensional surface shown in Figure 4-4. This full model reduces to the model suggested by Haber’s law when γ = 0. What do these figures illustrate about Haber’s law being violated? If one compares the contour plots (Figures 4-1 and 4-3), one sees that for constant values of α and β, much lower exposures for the same duration lead to higher probabilities of adverse response
when Haber’s law does not apply. For example, a concentration = 20 × duration = 10 (units arbitrary) exposure is associated with an estimated probability of adverse response of <0.25 when Haber’s law is assumed; however, this same concentration-duration pattern is associated with >0.75 probability of adverse response when Haber’s law is not operative. The perspective plots (Figures 4-2 and 4-4) confirm the observation that higher probabilities of adverse response are predicted for each concentration-time exposure combination. The implication of this observation is that the naive and incorrect application of Haber’s law to the setting of CWA exposure guidelines may lead to erroneous and, in this example, unprotective risk estimates.
While many investigators use the available experimental data to calculate the coefficients α and β, others would (erroneously) apply Haber’s law directly to extrapolate from concentration-response data at one time point to other time points. The implications for prediction of response at other concentration-duration combinations can be considered by using ten Berge’s generalization of this relationship, which states that Cn × t is a constant. Values of n > 1 tend to flatten the concentration-time contour plot, making the plot more like Figure 4-3 and less like Figure 4-1. Again, focusing on the contour plot, this means that as one moves to shorter durations from the available data, erroneously applying Haber’s law when n > 1 would tend to overestimate the concentration one can be exposed to for a given response contour and thus would not be a health-protective approach. Conversely, erroneously applying Haber’s law in extrapolating to longer exposure conditions when n > 1 would tend to underestimate the concentration one can be exposed to for a given response contour and thus would be overprotective.
REGRESSION METHODS FOR CONTINUOUS OR ORDERED RESPONSES
The analyses presented above assumed that adverse response was dichotomous (e.g., impaired or not impaired). Pupillary dilation could be measured as a continuous parameter and standard regression techniques (Neter et al. 1996) might be used. In other words, if pupillary constriction is a measure on a continuous scale, then the response can be used in a normal-theory regression model. Equation 1 and extensions would be modified accordingly. For a continuous response, say, Y = continuously measured pupillary constriction,
(4)
Note that the assumed structure for the relationship of the response and dose and duration should be evaluated for any response. Finally, if severity scales are used for the response—that is, the response may be categorized into a set of ordered categories—then ordinal response regression methods should be considered (Agresti 1984).
EXPERIMENTAL RECOMMENDATIONS
For detecting deviations from Haber’s law, a minimum of two concentrations multiplied with two durations resulting in four Ct treatments would be required. The committee recommends conducting studies with a minimum of nine Ct treatments (three concentrations multiplied by three durations). In general, the committee believes that if models are to be used to predict risk at a variety of concentrations and durations, then more extensive data are required to capture the nuances of the relationship between response, concentration, and exposure duration. Such relationships are not well established for CWAs.
THE COMMITTEE’S RECOMMENDATIONS
Although miosis typically has been considered the critical effect and most frequently used indicator of toxicity, questions remain about other adverse effects at low-level exposures. DOD should conduct research to identify whether there are more sensitive toxicity end points, other than miosis, from exposures to low levels of CWAs.
If miosis is selected as the critical adverse response, studies must determine what level of pupillary constriction in miosis is operationally relevant. This information is needed for ORM risk-risk comparison, which must consider the range of exposure limits for and probabilities of adverse outcomes from exceedances of those limits for each level of severity and operationally relevant exposure duration.
Although the uses of biomarkers may have limited relevance to immediate ORM decisions, they might be useful for research on postexposure sequelae, refining interspecies extrapolations, and exposure reconstruction. Future research should focus on the practical application of
the biomarker of interest—that is, whether samples taken weeks after exposure can be used to identify and quantify exposure.
Military personnel are likely to be exposed to low levels of CWAs while physically active. The use of animal models in which exposures are conducted while the animals are physically restrained may be problematic unless PBPK/PBPD models are used to address the implications of differences in breathing rates. Even then, care needs to be taken to ensure that measured effects are not due to stress or interaction between the agent and the stress. Appropriate consideration of extreme environmental conditions and high activity levels during deployment needs to be taken in future research.
The setting of exposure limits for CWAs usually has involved extrapolation to other concentrations or durations by invoking Haber’s law (adverse response is related to the product of agent concentration and duration of exposure) or ten Berge calculation, a generalization of Haber’s law that can (1) weight concentration more than time when n > 1, (2) weight concentration less than time when n < 1, or (3) weight concentration and time equally. Naive applications of Haber’s law can lead to erroneous risk estimates. PBPK/PBPD modeling should be used to improve evaluation of concentration-duration-response relationships. Mechanistic considerations would also be informative regarding the conditions and dose metrics for which Haber’s law is and is not applicable. Studies that allow for the testing and validation of duration-exposure models are encouraged.
Ask combat doctrine developers to list tactical decision requirements and then ask researchers to identify which requirements would be addressed by proposed research. This should not discourage basic research but should help keep toxicologists focused on the most critical problems demanding ultimate solutions.
Field commanders ultimately must be able to use the information generated by this research program to make decisions. The DOD toxicology establishment should confer with operations personnel to determine the nature and form of information that would be most useful. The committee recommends consultation with risk communication specialists to further assist with this task.
There is a possibility that multiple CWAs could be used simultaneously. Unless methods are available to discriminate among agents in the field, it is reasonable to focus on the most potent agent for setting exposure guidelines for mixtures relevant to battlefield conditions and assume
that the entire mixture would be less potent than an equal exposure to the most potent component of the mixture.
PBPK modeling can aid in extrapolating exposures from animals to humans. Recent work exploring the use of fluoride ion regeneration as a dose metric that is more sensitive and less variable than changes in AChE may be useful in improving the interspecies extrapolation for CWAs as well as addressing the exposure duration extrapolation issue and should be further explored. It should be noted that a PBPK model may not be required for extrapolating a direct contact toxic response (e.g., miosis may be an example of this if direct-contact of the CWA with the eye leads to this response).
To make the ORM concept operational, the risk assessor will require robust information on response probabilities from the experimental study. For CWAs, for each critical response (e.g., miosis), access to recently generated experimental data sets and contact with investigators should be pursued to develop other response probabilities that would be needed in operational risk assessment beyond single values, such as ECt50 or EC01 (e.g., 15%, 30%, 40%).
The toxicity end point and the mechanistic basis for the end point are factors contributing to human variability. For miosis alone, variability would be determined by the chemistry and physiology of the eye. Ambient light conditions can also contribute to the variability in miosis. For systemic effects of CWAs, variability in human response can be due to differences in the kinetics of CWA uptake and metabolism or differences in sensitivity to a given tissue dose of CWA. Further characterization of the implications of human variability in response to CWAs under military conditions would be useful for ORM.
The methodology developed for deriving AEGL values for CWAs is pertinent. The generalization of Haber’s law to effects related to Cnt (ten Berge method) is a valuable contribution. The committee recommends that DOD utilize information and techniques developed for deriving AEGLs.
























