3
The Medical and Psychological Concerns of Cancer Survivors After Treatment
The medical and psychological effects of cancer and its treatment have been recognized for many years, but it is only recently that survivorship is coming to be recognized as a distinct phase of the cancer trajectory. Findings from research studies that have tracked the health and well-being of individuals long after cancer treatment has ended have identified risks that both the survivors and their health care providers should recognize. Advances in knowledge of how to manage conditions that arise in the post-treatment period have led to the development of some guidelines for health care providers to follow. The survivorship period provides many opportunities to improve the health and quality of life of cancer survivors. This chapter begins with a general overview of the potential medical and psychological consequences of cancer and its treatment. Brief descriptions are then provided on the late effects associated with four cancer types (breast, prostate, colorectal, and Hodgkin’s disease) as well as information on the need for services to ameliorate them. Lifestyle issues of interest to cancer survivors are reviewed—smoking cessation, physical activity, nutrition and diet, healthy weight, and the use of complementary and alternative medicine. The chapter concludes with a review of the committee’s findings and recommendations.
OVERVIEW
The meaning of health and life itself can be altered following a diagnosis of cancer (Herold and Roetzheim, 1992; Muzzin et al., 1994; Vachon, 2001). Cancer survivors report ongoing struggles to achieve a balance in
their lives and a sense of wholeness and life purpose after a life-altering experience (Ferrell, 2004). Individuals may reappraise their lives following a diagnosis of cancer and search for a sense of control and meaning. Survivors of cancer, although free of the cancer for which they were treated, may be immobilized by fears of recurrence and have difficulties making life decisions, for example, proceeding with vocational plans or marriage. Existential and spiritual issues may also arise related to concerns about death and dying, having a new orientation to time and future, and changed values and goals. The survivorship experience is dynamic, changing over time, with particular moments of stress being transitions, such as the transition from treatment to long-term follow-up. Cancer survivors face these psychosocial concerns and worries about the physical effects of their treatment across the continuum of cancer care (Ganz, 2000).
Cancer’s effects are not isolated to an individual. Instead, it has an impact on the entire family, and the needs of children, spouses, partners, and other loved ones all need to be considered. Family members routinely provide personal care and emotional support for the duration of the cancer experience. Financial concerns may also arise because family income, insurance status, and employment can all be profoundly affected by cancer (see Chapter 6). Caregivers and family members often require, but do not receive, the respite, health care, psychosocial, and financial assistance they need in meeting the many needs of cancer survivors in their lives.
Quality of life (QOL) is a term used widely to describe an individual’s assessment of his or her own general well-being. There is no one agreed-on conceptual model or definition for health-related QOL, and investigators continue to work on developing ways to measure outcomes that matter to patients (Ganz, 2002a; Zebrack et al., 2003). Central to the concept of QOL, however, is the importance of capturing the perspective of the patient across multiple “domains” or areas of well-being. Standardized, self-administered questionnaires are generally used to assess symptoms and functioning in physical, psychological, social, and spiritual domains (Mandelblatt and Eisenberg, 1995; Cella, 1995; Dow et al., 1996; Montazeri et al., 1996; Ferrell et al., 1997a,b, 1998; Ferrans, 2005).1 An example of a conceptual model of QOL is shown in Figure 3-1.
This chapter reviews what is known about these various dimensions of quality of life for cancer survivors. The recognition of these health effects of
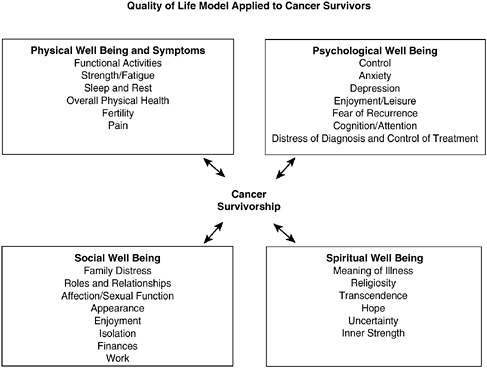
FIGURE 3-1 Quality of life: conceptual model.
SOURCE: City of Hope Beckman Research Institute (2004). Reprinted with permission from Betty R. Ferrell, PhD, FAAN; and Marcia Grant, DNSc, FAAN, City of Hope National Medical Center.
cancer and its treatment, sometimes referred to as “the price of survival,” follows investments in cancer survivorship research directed to better understand the long-term consequences of cancer (Ganz, 2002b). Because most of the research conducted to assess QOL of cancer issues among survivors involves individuals with certain types of cancer (or certain treatments), descriptions of the cancer survivorship experience are provided by selected cancer site. What follows are brief reviews of the quality of life literature for individuals with a history of cancer of the breast, prostate, and colon and rectum, and Hodgkin’s disease. The terms “late effects” and “long-term effects” can be used to distinguish health effects according to their onset (Box 3-1). However, in this report, the general term “late effects” is used to describe the consequences of cancer and its treatment, regardless of their date of onset.
There is limited information on the prevalence of late effects, but there is a general recognition that they have become more common, largely as a result of the more frequent use of complex cancer interventions,
|
BOX 3-1 Late effects refer specifically to unrecognized toxicities that are absent or subclinical at the end of therapy and become manifest later with the unmasking of hitherto unseen injury because of any of the following factors: developmental processes, the failure of compensatory mechanisms with the passage of time, or organ senescence. Long-term effects refer to any side effects or complications of treatment for which a cancer patient must compensate; also know as persistent effects, they begin during treatment and continue beyond the end of treatment. Late effects, in contrast, appear months to years after the completion of treatment. SOURCE: Aziz and Rowland (2003). |
often combinations of surgery, chemotherapy, radiation, and hormone treatments.
Of particular concern for cancer survivors are psychological effects. There may be cancer-specific concerns, such as fear of recurrence, to more generalized symptoms of worry, fear of the future, fear of death, trouble sleeping, fatigue, and trouble concentrating (Box 3-2). The pervasive uncertainty associated with cancer survival has been labeled the “Damocles syndrome” (Smith and Lesko, 1988; Quigley, 1989; Herold and Roetzheim, 1992). In Greek mythology, Damocles was invited to the king’s banquet for dinner. Once there, he found himself seated beneath a sword suspended over his head by a single horsehair. Damocles was happy to be at the king’s feast, but any movement he made while reaching for food or drink might knock the sword loose and spell a quick death. For cancer survivors, fears of recurrence can result in persistent anxiety and difficulties in planning for the future (Lee-Jones et al., 1997).
Individuals with cancer may also experience a mental disorder as a result of cancer or treatment, or they may experience an exacerbation of a prior psychiatric disorder (e.g., recurrent depression). Major depression and depressive symptoms occur frequently in cancer patients (Massie, 2004). According to a recent review of the literature, prevalence rates varied from 10 to 25 percent for major depressive disorders, a rate at least four times higher than in the general population (AHRQ, 2002). The timing and method of the assessment, concurrent treatment, medical morbidity, pain, gender, and age of subjects contributed to the wide range of estimates. The higher rates are usually seen in patients with more advanced illness and uncontrolled pain or other physical symptoms.
The term “psychosocial distress” has been coined to reflect a broader
|
BOX 3-2 Negative
Positive
SOURCE: Ganz (2002c). |
set of concerns (NCCN, 1999). As conceived, distress is a “multi-factorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment. Distress extends along a continuum, ranging from common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis” (NCCN, 1999). Distress may be experienced as a reaction to the disease and its treatment and also as a result of the consequences of the disease on employment, health insurance, and social functioning, including family relationships (McEvoy and McCorkle, 1990; Kornblith, 1998) (see Chapter 6 for a discussion of employment and insurance issues).
Brief screening tools can be used to identify individuals with symptoms of distress so that clinical assessment by the primary oncology team and referral to psychosocial providers can take place (Trask, 2004). The Distress Thermometer, for example, is a visual analogue scale that the National Comprehensive Cancer Network (NCCN) guidelines suggest for the screening of psychosocial distress (NCCN, 1999).
Many survivors function at high levels and do not report excess depres-
sive symptoms. Importantly, not all of the psychological effects are negative. Cancer survivors are often grateful to be alive and have an enhanced appreciation of life. Their self-esteem and sense of mastery may also be enhanced. Social late effects may be negative (alienation and isolation) or positive (affinity and altruism). Socioeconomic concerns may arise following treatment, particularly financial concerns related to costs of care, access to health insurance, and the ability to return to work or school (see Chapter 6). Recent evidence suggests that there are income-related disparities in the QOL of cancer survivors that cannot be explained by the effect of health on earnings. High-income patients are not only more likely to survive cancer, but they enjoy better QOL as survivors (Short and Mallonee, in press).
Aside from psychosocial distress, there are two main categories of late effects. First, cancer survivors are at increased risk for cancer, either a recurrence of the cancer for which they were initially treated, or the independent development of a second cancer (either of the same type or a different type from the original cancer).2 The increased risk of developing a second cancer may be due to cancer treatment (e.g., chemotherapy-induced leukemia and bladder cancer), genetic or other susceptibility, or some interaction between treatment and an inherent susceptibility. In addition to concerns about the risk of cancer following treatment, cancer survivors are at increased risk for a wide range of treatment-related problems notable for their variability and unpredictability. Their variability can be traced, in part, to the complexity of cancer itself (e.g., the type of tumor and stage of disease), the wide array of therapies that can be employed, the intensity of treatment (e.g., doses of chemotherapy or radiation, the extent of surgery needed), and the age and underlying health status of the individual at the time of treatment.
A number of tissues and body systems can potentially be impaired as a consequence of cancer and its treatment, as illustrated in Tables 3-1 and 3-2. Some of the late effects associated with certain chemotherapeutic agents, for example, can result in significant changes in physical functioning, leading to effects such as post-treatment fatigue or sexual or urinary problems. Clinicians, in designing initial treatment plans, consider the potential for late effects and attempt to be as conservative as guidelines warrant to maximize treatment effectiveness while minimizing late effects. Late effects will likely be reduced in the future with the advent of therapies that are tailored to the characteristics of an individual and their cancer. In addition, advances in methods to assess individuals risk for late effects (e.g., their DNA repair mechanisms related to radiation-induced DNA damage) and to personalize treatments will improve the outlook for cancer survivors.
TABLE 3-1 Examples of Possible Late Effects of Radiation Therapy, Chemotherapy, and Hormonal Therapy Among Survivors of Adult Cancers
|
Organ System/Tissue |
Radiation Therapy Late Effects |
Chemotherapy/Hormonal Therapy |
|
|
Late Effects |
Agent Responsible |
||
|
All tissues |
Second cancers |
Second cancers |
Steroids, alkylating agents, nitrosureas, topoisomerase inhibitors, anthracyclines |
|
Bone and soft tissue |
Atrophy, deformity, fibrosis, bone death |
Bone death and destruction, risk of fractures |
Steroids |
|
Cardiovascular |
Scarring or inflammation of the heart, coronary artery disease; scarring of heart sac (pericardium) |
Inflammation of the heart, congestive heart failure |
Anthracylines, high-dose cyclophosphamide, cisplatin, herceptin, taxanes |
|
Dental/oral health |
Dental caries, dry mouth |
— |
— |
|
Endocrine-pituitary |
Various hormone deficiencies |
Diabetes |
Steroids |
|
Endocrine-thyroid |
Low thyroid function, thyroid nodules |
— |
— |
|
Endocrine-gonadal |
Men: Sterility, testosterone deficiency Women: Sterility, premature menopause |
Men: Sterility, testosterone deficiency Women: Sterility, premature nitrosureas |
Alkylating agents, Procarbazine hydrochloride, menopause |
|
Gastrointestinal |
Malabsorption, intestinal stricture |
Motility disorders |
Vinca drugs |
|
Genitourinary |
Bladder scarring, small bladder capacity |
Hemorrhagic cystitis (symptoms include urinary frequency, urgency, bleeding, and pain) |
Cyclophosphamide, ifosfamide, transplant therapy |
|
Hematologic |
Low blood counts, myelodysplastic syndrome and acute leukemia |
Myelodysplastic syndrome and acute leukemia |
Alkylating agents, nitrosureas, topoisomerase inhibitors, purine analogs, any high-dose therapy with autologous transplantation |
|
Hepatic |
Abnormal liver function, liver failure |
Abnormal liver function, cirrhosis, liver failure |
Methotrexate, carmustine (BCNU) |
|
Immune system |
Impaired immune function, immune suppression |
Impaired immune function, immune suppression |
Steroids, anti-thymocyte globulin (ATG), methotrexate, rituximab, alemtuzumab, purine analogs, and any high-dose therapy with autologous transplantation |
|
Lymphatic |
Lymphedema |
— |
— |
|
Nervous system |
Problems with thinking, learning, memory; structural changes in the brain; bleeding into the brain |
Problems with thinking, learning, memory; structural changes in the brain; paralysis; seizure |
Methotrexate, multiagent chemotherapy, bortezomib |
|
|
|
Numbness and tingling, hearing loss |
Cisplatin |
|
|
|
Numbness and tingling |
Vinca alkaloids, taxanes. oxaliplatin |
|
Ophthalmologic |
Cataracts, dry eyes, visual impairment |
Cataracts |
Steroids |
|
Pulmonary |
Lung scarring, decreased lung function |
Lung scarring, inflammation |
Bleomycin sulfate, carmustine (BCNU), methotrexate |
|
|
|
Potentiation of radiation therapy effects (gemcitabine) |
Actinomycin D/doxorubicin (Adriamycin) |
|
Renal |
Hypertension, impaired kidney function |
Impaired kidney function, delayed-onset renal failure |
Cisplatin, methotrexate, nitrosoureas |
TABLE 3-2 Examples of Possible Late Effects of Surgery Among Survivors of Adult Cancers
|
Procedure |
Late Effect |
|
Any procedure |
Pain, cosmetic, psychosocial, impaired wound healing |
|
Surgery involving neurologic structures (brain, spinal cord) |
Impairment of cognitive function, motor sensory function, vision, swallowing, language, bowel and bladder control |
|
Head and neck surgery |
Difficulties with communication, swallowing, and breathing; cosmetic; damage to muscles affecting movement |
|
Removal of lymph nodes |
Lymphedema, retrograde ejaculation in testicular cancer |
|
Abdominal surgery |
Risk of intestinal obstruction, hernia, altered bowel function |
|
Pelvic surgery |
Sexual dysfunction, incontinence, hernia, risk of intestinal obstruction |
|
Removal of spleen |
Impaired immune function, increased risk of sepsis, hernia |
|
Amputation; limb-sparing procedures |
Functional changes; cosmetic deformity; psychosocial impact; accelerated arthritis in other joints; post-surgical, phantom, and/or neuropathic pain |
|
Lung resection |
Difficulty breathing, fatigue, generalized weakness |
|
Prostatectomy |
Urinary incontinence, sexual dysfunction, poor body image |
|
Oophorectomy |
Premature menopause and infertility |
|
Orchiectomy |
Infertility, testosterone deficiency |
|
Ostomy |
Bowel obstruction, constipation, nausea, vomiting, loss of appetite, fatigue, poor body image |
Second cancers are perhaps the most frequent life-threatening late effect, but other disabling conditions may occur. Some of these are identified early in follow-up and resolve without consequence (e.g., treatment-related fatigue). Other late effects may persist, become chronic problems, and influence the progression of other diseases associated with aging (e.g., radiation-induced changes in the lung called “radiation pneumonitis,” renal failure). Some late effects may only become evident years after treatment (e.g., congestive heart failure, graft versus host disease, neurological syndromes).
Certain late effects are easy to identify because they are visible or have direct effects on function. Examples include major paralysis from brain or spine neoplasms, communication and swallowing problems from head and neck cancers, and limb loss or deformity due to osteosarcoma or another
type of sarcoma. Many affected individuals, in addition to their medical surveillance needs, require expensive equipment, such as wheelchairs or prostheses, to maintain functional independence and quality of life. Such equipment requires maintenance and often replacement over the lifespan.
Other effects, however, can be subtle and apparent only to the trained observer (e.g., change in posture secondary to osteoporosis) or are not directly observable and identified only through diagnostic tests (e.g., for hypothyroidism, infertility). It is sometimes difficult to distinguish among cancer-related changes, age-related changes, and those caused by comorbid conditions (see Chapter 2 for a description of the survivor population by age and comorbidity). Cancer can be considered a chronic disease, in part because of the serious consequences and persistent nature of some of cancer’s late effects.
The limited empirical evidence on the late effects of adult cancer treatment is primarily confined to small case series that are not population-based. There are relatively few longitudinal cohort studies available to understand the link between specific treatment regimens and late physical and psychological effects, making it difficult to describe the natural history of late effects for patients and their health care providers. Unfortunately, absent data from longitudinal studies, the degree of risk of late effects to individual patients cannot be predicted.
To illustrate the range of late effects and the diversity of the cancer survivor population, one could consider the individual who had an early-stage melanoma successfully removed, leaving an inconspicuous scar, to have had cancer with minimum late effects and impact on life. Such a person would have concerns regarding subsequent risk of cancer, but likely would not suffer serious long-term health effects of treatment. At the other extreme might be an individual with a hematological cancer undergoing intensive chemotherapy followed by a bone marrow transplant. Such a person would face substantial long-term health problems associated with treatment. This variation in survivorship experience is more fully described in the next section, where late effects and interventions to ameliorate them are more fully described for four cancer types: cancer of the breast, prostate, and colon and rectum, and Hodgkin’s disease. These sites were selected because more than half of all cancer survivors have had these types of cancer. In addition, they were selected because investigators have focused research on these cancers and there is an extensive survivorship literature available. Other cancer sites, while not covered at length in this review, also have potential for major, varied, and often lifelong disabling effects. For example, individuals with brain or spine tumors may develop severe neurologic deficits (Mukand et al., 2001); survivors of head and neck cancer may have impaired eating, communication, and musculoskeletal functions of the neck and shoulder (Hammerlid and Taft, 2001); and individuals with bone
cancers may require amputations or limb-sparing procedures that interfere with mobility (Hoffman et al., 2002).
SITE-SPECIFIC REVIEW
The following brief site-specific summaries of late effects of cancer and its treatment are based on selected reviews and literature to which the reader is referred for more detailed information. Information on interventions that are available to ameliorate these health effects are also described, as are available clinical practice guidelines (CPGs) for the management of late effects. CPGs are “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances” (IOM, 1990).
Female Breast Cancer3
The experience of survivors of breast cancer has been the most extensively researched. Women with a history of breast cancer are the largest group of cancer survivors, representing 22 percent of the survivorship population (see Chapter 2 for a description of breast cancer survivors). The evolving nature of breast cancer treatment has generated a heterogeneous group of breast cancer survivors (Box 3-3). Elderly survivors treated 20 to 30 years ago, for example, had fewer treatment options and likely experienced mastectomy. The issues of concern to those women were often linked to late effects of surgery such as lymphedema and body image. Younger cohorts of women, in contrast, have benefited from a wider range of options, but may be concerned about a broader set of late effects related to their treatment.
Quality of Life
At the conclusion of primary treatment for breast cancer, women generally report good emotional functioning, but decreased physical function-
ing, especially those women who have had a mastectomy or receive chemotherapy (Ganz et al., 2004a). Persistent symptoms one year following either lumpectomy or mastectomy to treat early-stage breast cancer can include numbness in the chest wall or axilla, tightness, pulling or stretching in the arm or axilla, less energy or fatigue, difficulty in sleeping, and hot flashes (Shimozuma et al., 1999). Despite these symptoms most women report high levels of functioning and quality of life, with no relationship between the type of surgery and quality of life. By 2 to 3 years following surgery, breast cancer survivors in one study rated their quality of life more favorably than outpatients with other common medical conditions, and they identified many positive aspects from the cancer experience (Ganz et al., 1996). However, some aspects of quality of life (e.g., sexual function and interest, body image) and rehabilitation problems (e.g., physical functioning) worsened after that time. Among the factors that have been associated with poorer ratings of quality of life among breast cancer survivors are impaired physical functioning, poor body image, a lack of social support, coping strategies, and aspects of care such as poor communication with physicians (Mandelblatt et al., 2003; Ganz et al., 2003b, Avis et al., 2005).
Several studies of the long-term consequences of breast cancer and its treatment have been conducted. The largest of these assessed the quality of life of disease-free survivors of Stage I or II breast cancer at 1 to 5 years (baseline) and then at 5 to 10 years following their diagnosis (Ganz et al., 1998b, 2002).4 At baseline, breast cancer survivors were found to function at a high level, similar to healthy women without cancer. However, compared to survivors with no adjuvant therapy, those who received chemotherapy had significantly more sexual problems, and those treated with tamoxifen experienced more vasomotor symptoms such as hot flashes and night sweats (Ganz et al., 1998b). At the 5- to 10-year follow-up, physical well-being and emotional well-being were excellent. The minimal changes between the baseline and follow-up assessments reflected expected age-related changes. Complaints at baseline of hot flashes, night sweats, vaginal discharge, and breast sensitivity were reported less frequently at follow-up. However, symptoms of vaginal dryness and urinary incontinence were increased. In this study, survivors with no past systemic adjuvant therapy had a better quality of life than those who had received systemic adjuvant therapy (chemotherapy, tamoxifen, or both) (Ganz et al., 2002). The asso-
|
BOX 3-3 Research that demonstrated that breast-conserving therapy followed by radiation is an efficacious alternative to mastectomy in most women has contributed to less disfigurement and reduced morbidity among women (Fisher et al., 2002). In research conducted over the past three decades, clinical trials have demonstrated that chemotherapy given to women shortly after their primary surgery and/or radiation treatment (called adjuvant therapy) reduces the risk of recurrence by 20 to 40 percent and reduces mortality by 10 to 30 percent at 10 years following treatment (NIH, 2000; Shapiro and Recht, 2001; Early Breast Cancer Trialists’ Collaborative Group, 2004a). For women whose tumors are hormone receptor positive (with either estrogen or progesterone receptor expression), which includes about 70 percent of breast cancer patients, endocrine therapies (e.g., aromatase inhibitors, tamoxifen, surgical removal of the ovaries) have been found to reduce recurrence rates by nearly 50 percent and death rates by more than 25 percent (Early Breast Cancer Trialists’ Collaborative Group, 2004b; Mrozek and Shapiro, 2005). Adjuvant chemotherapy, endocrine therapy, or both are widely recommended for women with invasive breast tumors greater than 1 cm in diameter, irrespective of whether axillary lymph nodes are involved (NIH, 2000; NCCN, 2004b). Although these interventions are beneficial, they can lead to late effects, and decision making about the approach to adjuvant therapy can be complex (Langer, 2001; Ganz, 2001a). During the 1990s, many women with metastatic breast cancer underwent high-dose chemotherapy and bone marrow transplantation, which was later shown not to be more effective than standard-dose chemotherapy alone for advanced disease. Women who survived this treatment experienced not only the late effects, but also the financial costs of this expensive procedure. Most women alive today |
ciation of lower quality of life among women treated with systemic chemotherapy as compared to local therapy has been observed in more recent studies (Ahles et al., 2005).
Information on the long-term consequences of breast cancer are also available from the longitudinal Nurses’ Health Study, a study that began in 1976 and has prospectively followed 121,700 female nurses ages 30 to 55 (Michael et al., 2000). The unique contribution of this study is that information on functional health status is available about women both before and after their diagnosis of cancer. In addition, the study was able to control for age-related changes in functional status by comparing women with a history of breast cancer to the large cohort of women in the Nurses’ Health Study without breast cancer. In this study, there were greater than expected declines in physical function and role function due to physical and emotional problems, vitality, social function, and increased bodily pain among the breast cancer survivors relative to the control population. Risk
|
after transplantation recei ved it for extensive nodal disease without distant metastases. Contemporary treatment for breast cancer usually involves various combinations of surgery, radiation therapy, cytotoxic chemotherapy, and hormone therapy. Selection of therapy is influenced by the age and menopausal status of the patient, stage of the disease, and certain characteristics of the tumor (e.g., its histologic and nuclear grade,a presence of estrogen and progesterone receptors, measures of proliferative capacity, and genetic characteristics such as overexpression of some growth factor receptors such as human epidermal growth factor receptor 2, or HER2/neu) (NCI, 2004a). The effectiveness of adjuvant chemotherapy can be improved by administering a higher dose of drug per unit time (called dose density). In a recent study, for example, women with node-positive breast cancer were more likely to survive when a given dose of adjuvant chemotherapy was administered over a period of 22 weeks instead of 33 weeks (Citron et al., 2003; Stearns and Davidson, 2004). This intensification in dose increases the drugs’ toxicity, but data are not yet available to determine if the risk of late effects is increased. Genetic profiling methods are becoming available that can help predict which women will benefit most from chemotherapy and adjuvant therapies. As such methods become part of the standard initial evaluation of patients, treatment of late effects may decline as therapies are tailored to individual risk (Paik et al., 2004; Mrozek and Shapiro, 2005). |
of decline was attenuated with increasing time since diagnosis, but remained significant for some domains of function up to 4 years after diagnosis. Prediagnosis level of social integration is an important factor in future health-related QOL among breast cancer survivors, pointing to the need for adequate social support (Michael et al., 2002). In a subsequent study of breast cancer survivors participating in the Nurses’ Health Study (NHS I and II), investigators found significant functional declines among breast cancer survivors who had been diagnosed at age 40 or younger (Kroenke et al., 2004). Relative to their peers, these women experienced declines in physical roles, bodily pain, social functioning, and mental health. Declines observed among breast cancer survivors aged 65 and older were those expected with age.
Younger breast cancer survivors (under age 50) have reported good quality of life and high levels of functioning when assessed 5 to 10 years after their diagnosis (Bloom et al., 2004; Casso et al., 2004). Mild impair-
ment, however, has been observed in the area of sexual functioning.5 Recent evidence suggests that among women of reproductive age, concerns about reproduction lower ratings of quality of life (Schover, 2005; Wenzel et al., 2005).
There is limited information on racial or ethnic differences in quality of life among women diagnosed with breast cancer. One study that compared outcomes of African-American and white breast cancer survivors found that differences in reported quality of life were attributable to socioeconomic and life-burden factors and not to race/ethnicity (Ashing-Giwa et al., 1999). African-American women demonstrated better quality of life outcomes as compared to white women in a study of younger breast cancer survivors (aged 50 years or younger) who were also disease-free survivors for 2 to 10 years (Ganz et al., 2003a). African-American women found more meaning in life as a result of having had breast cancer, while Hispanic women reported more physical symptoms, according to a study of breast cancer survivors followed up within 5 years of their diagnosis (Giedzinska et al., 2004).
Table 3-3 summarizes specific late effects found among breast cancer survivors. These late effects are described more fully below.
Cancer Recurrence
Women with recurrent disease in the breast or regional lymph nodes can be treated and potentially cured. Disease that has metastasized to distant organs, however, is not curable, but some women live years or even decades after such metastases are discovered. Most recurrences in the breast are detected within 5 years of diagnosis with a peak rate of recurrence during the second year following diagnosis (Burstein and Winer, 2000; Emens and Davidson, 2003). There is not a defined time at which breast cancer survivors can be considered definitively cured of their disease because recurrences can occur more than 20 years after primary therapy. More than three-quarters of recurrences are identified through symptoms (e.g., shortness of breath, bone pain) or by physical examination (e.g., feeling a mass). Recommendations for follow-up include routine history, physical examination, and annual mammogram.
Second Primary Cancer
Women with a history of breast cancer, in addition to being at risk for a recurrence of their original cancer, are at risk of developing another cancer, independent of the first occurrence. The risk of developing these so-called “second primary cancers” depends not only on an individual’s inherent predisposition, but also on the treatments used for the initial cancer. The underlying risk of developing a second primary cancer in the contralateral breast is estimated to be 0.5 to 1 percent per year and is greater in women whose first cancer was diagnosed at a younger age and women with heritable or familial breast cancer (Burstein and Winer, 2000). Radiation therapy contributes to a higher risk of cancer in exposed areas (e.g., soft-tissue sarcomas of the thorax, shoulder, and pelvis; lung cancer) (Matesich and Shapiro, 2003; Levi et al., 2003). Adjuvant chemotherapy, including alkylating agents and topoisomerase II inhibitors (e.g., anthracyclines), can increase the risk for acute myelogenous leukemia (Mrozek and Shapiro, 2005). Little is known about long-term side effects of a class of drugs called taxanes (i.e., paclitaxel, docetaxel)6 due to their relatively recent introduction into standard practice in the adjuvant setting (Mrozek and Shapiro, 2005).
Tamoxifen is usually administered for 5 years to women with estrogen receptor- (ER-) positive tumors.7 While providing survival benefits, serious medical risks associated with tamoxifen include endometrial cancer, strokes, and blood clots. Women taking tamoxifen have a two- to threefold increase in the risk of developing endometrial cancer (about 80 excess cases per 10,000 treated women at 10 years) (Matesich and Shapiro, 2003). This increase occurs primarily in women over the age of 50. Most of the endometrial cancers that develop are early-stage and low-grade tumors that can be successfully treated (Burstein and Winer, 2000). Women taking tamoxifen are advised to undergo an annual pelvic examination while taking tamoxifen, and to see a gynecologist if they have irregular bleeding (Shapiro and Recht, 2001).8
Two small groups of breast cancer survivors face relatively high risks of
TABLE 3-3 Possible Late Effects Among Breast Cancer Survivors
|
Late Effect |
Population at Risk |
Risk |
Interventions |
|
Cancer recurrence |
All women with a history of breast cancer |
Varies by stage and tumor characteristics |
Mammography, physical examination |
|
Second primary cancer |
All women with a history of breast cancer |
Varies by treatment, age, and genetic predisposition (women with BRCAa mutations are at higher risk) |
Mammography, pelvic examination, general physical examination, patient education |
|
Psychosocial distress |
All women with a history of breast cancer |
Approximately 30 percent experience distress at some point; distress declines over time |
Assessment for distress Some psychosocial interventions are effective in reducing distress |
|
Arm lymphedema |
Women who had axillary dissection and/or radiation therapy |
Across treatments and time since treatment, approximately 12 to 25 percent of women develop lymphedema |
Massage and exercise (manual lymphatic drainage), use of elastic compression garments, complex decongestive therapy |
|
Premature menopause and related infertility and osteoporosis |
Women who received adjuvant chemotherapy (e.g., alkylating agents such as cyclophosphamide) Women with BRCA mutations who elect oopherectomy |
Risk depends on the chemotherapy regimen, the cumulative dose, and patient age (see details below) |
New reproductive technologies for infertility Diagnostic and preventive strategies for osteoporosis Assessment of sexual function |
|
Symptoms of estrogen deprivation (e.g., hot flashes, sweats, vaginal discharge) |
Women taking endocrine therapy |
More than half report symptoms, although mild in most cases |
Promising nonhormone treatments include antidepressants, dietary changes. and exercise |
second cancers. First, women with BRCA mutations (5 to 10 percent of women with breast cancer) are at increased risk of ovarian cancer, non-colonic gastrointestinal cancers, and second primary breast cancer. Women with BRCA1 and BRCA2 mutations who do not undergo prophylactic surgery have a risk of breast cancer of 45 to 84 percent by age 70 (Ford et al., 1998; Antoniou et al., 2003; King et al., 2003; Easton et al., 2004). Such women may benefit from genetic counseling, breast cancer early detection tools (i.e., breast self-examination, clinical breast examinations, annual mammograms, magnetic resonance imaging (MRI) examinations) (Warner et al., 2004), and ovarian cancer detection tools (e.g., transvaginal ultrasound, annual pelvic examination) (Isaacs et al., 2004). Counseling can be provided regarding prophylactic measures such as mastectomy and tamoxifen use to reduce the risk of breast cancer, and oophorectomy to minimize the risk of ovarian cancer. A second small group of women at significantly higher risk of second cancer are those treated with intensive-dose chemotherapy (Fisher et al., 1999). These women are at higher risk of myelodysplasia and acute myelogenous leukemia, and if symptomatic can be evaluated with blood counts.
Psychosocial Distress9
Most of the literature on the psychosocial aspects of breast cancer suggests that the vast majority of women adjust well to the diagnosis of breast cancer, and manage the complex and sometimes aggressive treatments associated with primary treatment and recurrent disease (Maunsell et al., 1992; Schag et al., 1993; Ganz et al., 1996; Dorval et al., 1998; Ganz et al., 1998a; Hanson Frost et al., 2000; Ganz et al., 2002). When cancer-related distress occurs, it generally dissipates with time for the majority of individuals diagnosed with breast cancer.
The frequency and patterns of psychosocial distress that occur among women with breast cancer depend greatly on which concerns are included in the operational definition of distress and how it is measured. The highest distress levels appear to occur at transition points in treatment: at the time of diagnosis, awaiting treatment, during and on completion of treatment, at follow-up visits, at time of recurrence, and at time of treatment failure (Box 3-4). Taken overall, around 30 percent of women show significant distress at some point during the illness. At higher risk for psychosocial distress are
|
BOX 3-4 “After my very last radiation treatment for breast cancer, I lay on a cold steel table hairless, half-dressed, and astonished by the tears streaming down my face. I thought I would feel happy about finally reaching the end of treatment, but instead, I was sobbing. At the time, I wasn’t sure what emotions I was feeling. Looking back, I think I cried because this body had so bravely made it through 18 months of surgery, chemotherapy, and radiation. Ironically, I also cried because I would not be coming back to that familiar table where I had been comforted and encouraged. Instead of joyous, I felt lonely, abandoned, and terrified. This was the rocky beginning of cancer survivorship for me.” SOURCE: McKinley (2000). |
women who are relatively young, have a history of preexisting depression or psychological distress, have other serious comorbid conditions, and have inadequate social support (Maunsell et al., 1992; Ganz et al., 1992, 1993; Schag et al., 1993; Mor et al., 1994; Schover, 1994; Maunsell et al., 1995; Wenzel et al., 1999; Leedham and Ganz, 1999; Shimozuma et al., 1999). The specific type of breast cancer surgery or taking tamoxifen does not influence the level of distress (Maunsell et al., 1989; Ganz et al., 1992, 1993, 1998a,b; Omne-Ponten et al., 1994; Schover et al., 1995; Day et al., 1999, 2001; Rowland et al., 2000; Fallowfield et al., 2001).
Functional status, sense of well-being, and self-perceived health reported by disease-free breast cancer survivors were found to be similar or more positive than those from healthy women of comparable ages in a large cross-sectional study (Figure 3-2) (Ganz et al., 1998a). This and other studies have shown that marital relationships are generally maintained and are often reported to have strengthened following breast cancer treatment (Kornblith and Ligibel, 2003; Schover, 2004; Dorval et al., 2005). Assessing the factors that contribute to resilience, effective coping with cancer, and positive psychological outcomes associated with the cancer experience is of increasing interest to researchers (Petrie et al., 1999; Justice, 1999; Cordova et al., 2001; Brennan, 2001; Tomich and Helgeson, 2002).
For a minority of women, however, a diagnosis of breast cancer contributes to significant psychosocial distress that can interfere with functioning and well-being (Massie and Holland, 1991). In a review of the literature on depression in patients with cancer, Massie found breast cancer to be among the sites that had especially high prevalence, ranging from 2 to 46 percent, in the studies reviewed (Massie, 2004). This range of estimates is in part due to variation in assessment procedures (Trask, 2004). In terms of
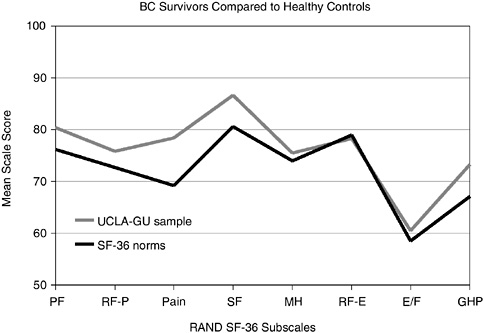
FIGURE 3-2 Breast cancer survivors compared to healthy controls. BC = breast cancer; PF = physical functioning; RF-P = role limitations attributed to physical problems; SF = social functioning; MH = mental health; RF-E = role limitations attributed to emotional problems; E/F = energy and fatigue; GHP = general health perception; UCLA-GU sample = University of California-Los Angeles and Georgetown University sample of breast cancer survivors; SF-36 norms = healthy controls.
SOURCE: Reprinted with permission from the American Society of Clinical Oncology. Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. 1998a. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 16(2):501–514.
extreme psychiatric morbidity, some evidence points to breast cancer as potentially leading to the development of post-traumatic stress disorder (PTSD).10 For example, in one study that assessed breast cancer survivors 20 years after treatment, relatively few women (5 percent) had clinical
levels of distress, but 15 percent reported two or more symptoms of PTSD that were moderately to extremely bothersome (Kornblith et al., 2003).
Beneficial effects of a range of psychosocial interventions have been found in randomized trials in women with breast cancer (IOM, 2004). Notably, there is evidence for the benefit of individual interventions and relaxation/hypnosis/imagery for women with early-stage breast cancer. Group interventions are effective for women with both early and metastatic breast cancer. According to a recent clinical trial, relatively simple interventions (e.g., a videotape on issues related to reentry transitions, sessions with a cancer educator) helped to reduce common symptoms experienced by women during the transition from active treatment to survivorship (Stanton et al., 2004). Another recent clinical trial suggests that psychological interventions have immunological benefits in addition to relieving distress and improving health behaviors (Andersen et al., 2004). Although it needs strengthening, this body of evidence supports the conclusion that psychosocial interventions can be expected to reduce psychiatric symptoms and improve quality of life in routine clinical care of breast cancer (IOM, 2004). (See Chapter 4, Appendix 4D for a description of the delivery of psychosocial services for women with breast cancer.)
Lymphedema11
Lymphedema is a relatively common late effect of surgery and radiation therapy for breast cancer. Surgery to remove lymph nodes for biopsy and radiation treatment both contribute to an interruption of the flow of fluid within the axillary lymphatic system. When impeded, fluid accumulates in subcutaneous tissue in the arm. Lymphedema and related long-term chronic inflammatory changes can be painful, limit function, increase the risk of infection, and diminish quality of life. In addition to the discomfort associated with lymphedema, women may suffer arm pain and numbness following their treatment.
No large population-based studies of the incidence of lymphedema have been carried out using standardized procedures for diagnosis, measurement, and follow-up time. Consequently, there are no precise estimates of its risk (Erickson et al., 2001; Sparaco and Fentiman, 2002). Available evidence suggests that across treatments and time since treatment, approximately 12 to 25 percent of women develop arm edema after treatment for breast cancer. The onset of lymphedema following breast
cancer treatment varies. For most women it develops within 1 year of treatment, but for others it can occur up to 4 years or more following treatment (Mortimer et al., 1996). The risk appears to vary by extent of treatment with surgery and radiotherapy; however, the relative contributions of these interventions to the development of lymphedema is not clearly understood. Prospective studies of lymphedema are needed that use consistent definitions and measures.12
Lymphedema frequently occurs among women who have lymph nodes removed to determine the extent of cancer spread. Until the late 1990s, most women with early breast cancer had a procedure called axillary dissection, where some or all of the lymph nodes in the armpit area near the affected breast were removed. In 1994, a procedure called sentinel lymph node biopsy was tested on women with breast cancer in an effort to reduce the morbidity associated with axillary dissection while preserving the diagnostic utility of examining lymph nodes for evidence of cancer (Posther et al., 2004).13 Evidence of the effectiveness of sentinel lymph node biopsy will be available toward the end of the decade at the conclusion of clinical trials now underway (National Surgical Adjuvant Breast and Bowel Project, 2004; White and Wilke, 2004; Krag et al., 2004; Posther et al., 2004).14 In the meantime, sentinel lymph node biopsy is widely used in the United States, especially at major cancer centers. Estimates are that sentinel lymph node biopsy, if proven effective, could save 70 percent of women with negative findings at physical examination and negative pathology results following sentinel lymph node biopsy from the morbidity of immediate, complete axillary dissection (Schwartz, 2004). Some descriptive studies suggest that sentinel lymph node biopsy significantly reduces the occurrence of arm lymphedema among women with breast cancer (Blanchard et al., 2003b).
|
BOX 3-5 Shop owner Catherine Pascucci had three lymph nodes removed and a lumpectomy and radiation treatment for breast cancer 3 years ago. After her surgery, she returned to her fragrance shop, lifting boxes and ringing sales, never knowing that she was at risk for lymphedema. About 3 months after cancer surgery, she noticed her bracelet was tight, but her breast surgeon attributed her swollen arm to a reaction to a bug bite. Months later, another doctor told her about lymphedema, and she sought treatment. She now undergoes regular physical therapy treatments and wears compression bandages to control the swelling. SOURCE: Adapted from Parker-Pope (2004). |
There have been relatively few well-designed, randomized trails to test the range of therapies that are available to treat lymphedema (Badger et al., 2004a,b,c). Nonpharmacologic treatments, such as massage and exercise (manual lymphatic drainage), use of elastic compression garments, and a technique called complex physical therapy or complex decongestive therapy, appear to be effective therapies for lymphedema (Kligman et al., 2004). These complex therapies involve skin care, manual lymphatic drainage, and low-stretch compression bandaging followed by a fitted compression garment when the edema has plateaued (Sparaco and Fentiman, 2002). Pharmacologic interventions (e.g., anticoagulants, diuretics) have not been shown to be effective in treating lymphedema itself (Loprinzi et al., 1999; Sparaco and Fentiman, 2002; Kligman et al., 2004), but certain medications may help alleviate discomfort, infection, or other side effects associated with lymphedema (Erickson et al., 2001). Avoidance of activities and factors known to trigger lymphedema (e.g., having blood pressure checked or blood drawn) can reduce its development (NCCN, 2004a) (Box 3-5). The role of exercise and prevention (e.g., use of low-pressure sleeve at specified times of arm use) in reducing the occurrence of lymphedema among women with breast cancer is being examined (Paskett, 2003). Obesity is a risk factor for lymphedema, and maintenance of a healthy weight is recommended (Johansson et al., 2002). Areas in need of further research include assessments of the value of prevention, early diagnosis, surveillance strategies, and treatment (Erickson et al., 2001). (See Chapter 4, Appendix 4D for a description of the delivery of rehabilitation services, including lymphedema services.)
Reproductive/Sexual Function15
Adjuvant chemotherapy improves the survival of women with breast cancer, but is associated with late effects of the reproductive system and in turn sexual function. Menopause can be precipitated among premenopausal women who were treated with certain types of chemotherapy that are directly toxic to the ovaries. Issues related to fertility and lactation are of particular concern to younger breast cancer survivors who may have delayed childbearing and not completed their families.
Premature menopause The risk of amenorrhea (either temporary or permanent) after common adjuvant treatments for breast cancer varies by the agent used, its dose, and the patient’s age (Figure 3-3) (Goodwin et al., 1999b; Burstein and Winer, 2000). Most women over age 40 who receive chemotherapy can expect permanent or prolonged menstrual dysfunction. For example, more than 70 percent of women over age 40, and 40 percent of younger women treated with the chemotherapy regimen CMF, will develop permanent ovarian failure (Mrozek and Shapiro, 2005).16 Younger women are likely to have a transient period of amenorrhea and then resume menses.
Roughly one-third (35 percent) of women newly diagnosed with breast cancer are under age 55. Given that the average age of menopause in North American women is 51 years, many of these women will be subject to immediate menopause, and those who continue to menstruate after chemotherapy are at risk for premature menopause. More than half of all women taking tamoxifen experience hot flashes, sweats, and vaginal discharge; however, in most cases, symptoms are mild and subside over time (Fallowfield et al., 2001; Ganz, 2001a).
Premenopausal women who elect to have their ovaries removed (oophorectomy) as a part of their breast cancer treatment, such as women with BRCA mutations, will also experience premature menopause. Women with ER-positive tumors may have an oophorectomy or have the function of their ovaries temporarily suppressed through treatments with hormones (e.g., luteinizing hormone-releasing hormone analogues such as goserelin).
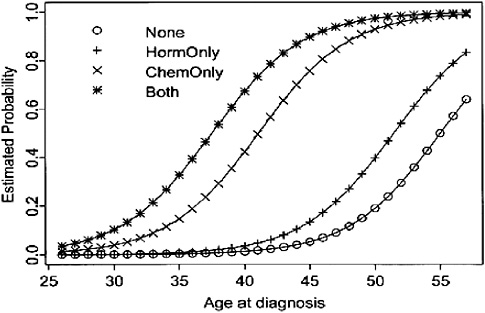
FIGURE 3-3 Estimated probability of amenorrhea among breast cancer survivors, by age at diagnosis and treatment modality.
SOURCE: Reprinted with permission from the American Society of Clinical Oncology. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. 1999b. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370.
The short-term effects of diminished circulating levels of estrogen that occur with menopause include:
-
Hot flashes, sweats, and palpitations (referred to as “vasomotor symptoms”)
-
Vaginal dryness and sexual changes, including pain with sexual intercourse
-
Urinary incontinence
-
Musculoskeletal complaints such as joint pains and skin changes
-
Sleep disturbance
-
Mood changes
Because chemotherapy causes an abrupt change in menopausal status, symptoms can be more severe than those associated with the usual transition that with normal aging lasts from 5 to 10 years (Burstein and Winer, 2000; Ganz, 2001b; Crandall et al., 2004).
Menopausal symptoms are very prevalent among breast cancer survivors, according to the Cancer and Menopause Study, a study designed to
evaluate the quality of life and health outcomes of younger survivors of breast cancer (aged 50 or younger at diagnosis and disease-free for 2 to 10 years) (Ganz et al., 2003a; Crandall et al., 2004). Hot flashes, for example, occurred in 17 percent, 51 percent, and 71 percent of pre-, peri-,17 and post-menopausal breast cancer survivors, respectively. Nearly three-fourths of women had received some form of adjuvant therapy, and amenorrhea frequently resulted. Some have noted the unique menopausal experience of breast cancer survivors and have called for longer term monitoring of the severity and duration of their menopausal symptoms (Fiorica, 2004).18
How to best manage menopausal symptoms among breast cancer survivors is uncertain. Results of the Women’s Health Initiative trial reaf-firmed the small but significant increased risk of breast cancer associated with hormone replacement therapy (HRT). Long-term estrogen use is contraindicated among women with a history of breast cancer, but other nonhormonal strategies are available (Chlebowski et al., 2003; Hoda et al., 2003). For example, treatment of menopausal symptoms with antidepressants (selective serotonin reuptake inhibitors or SSRIs), vitamin E, dietary changes, and exercise appears to be promising (Friedlander and Thewes, 2003). The antidepressant fluoxetine modestly improved hot flashes among women with breast cancer when tested as part of a randomized clinical trial (Loprinzi et al., 2002).
A comprehensive menopausal assessment intervention program delivered by a nurse practitioner succeeded in reducing symptoms and improving sexual functioning among post-menopausal breast cancer survivors with at least one severe menopausal symptom (Ganz et al., 2000; Zibecchi et al., 2003). The program, evaluated through a randomized controlled trial, involved symptom assessment, education, counseling, and, as appropriate, specific pharmacologic and behavioral interventions.
There is much interest in alternative or natural therapies to treat the symptoms of menopause among all women, including those with a history of breast cancer (DiGianni et al., 2002; Canales and Geller, 2003; Sparreboom et al., 2004; Navo et al., 2004). Products on the market range from soy protein in powder form, to evening primrose oil and yam creams
(a source of natural progesterone). A few of these substances have been tested among breast cancer survivors in randomized controlled trials, but have not been found to be effective (Jacobson et al., 2001; Van Patten et al., 2002; Amato et al., 2002; Tice et al., 2003).
Sexual function Understanding sexual functioning following treatment of breast cancer is difficult because there is a general decline in libido and an increase in vaginal dryness with normal aging. These problems are, however, often exacerbated as a result of breast cancer treatment (Ganz, 2001b). Many women who are treated with adjuvant chemotherapy report loss of libido, body image concerns, decreased breast sensitivity, and a decline in sexual activity. However, sexual functioning among a large cohort of breast cancer survivors when assessed on average 3 years after their breast cancer diagnosis was found to be very similar to that of healthy women (Ganz et al., 1998a; Meyerowitz et al., 1999). Predictors of sexual dysfunction in breast cancer survivors include being younger at diagnosis, a history of chemotherapy, and having treatment-induced amenorrhea (Ganz et al., 1998a, 1999). There is little evidence of a link between type of surgical treatment (e.g., lumpectomy versus mastectomy) and sexual functioning, but women who have had a mastectomy report poorer body image (Rowland et al., 2000; Thors et al., 2001). Tamoxifen does not appear to adversely affect sexual functioning among breast cancer survivors (Fallowfield et al., 2001; Ganz, 2001a). Few differences in sexual function between African-American and white breast cancer survivors have been reported; however, studies generally have been limited to women who are well educated, high income, and highly functional (Wyatt et al., 1998). The American Cancer Society’s (ACS’s) website has information on sexuality for women and their partners (ACS, 2004b). Cognitive and behavioral sexual rehabilitation interventions are available to assist persons with cancer in understanding and adjusting to the physical changes caused by cancer treatment (Gallo-Silver, 2000).
Pregnancy and lactation Reproductive-age women making treatment decisions need to be apprised of the benefits and adverse effects of treatment on reproductive function to aid in their decision making (Friedlander and Thewes, 2003). Patients are often advised to wait 2 years after diagnosis before becoming pregnant because of the higher rate of recurrence of breast cancer in this period. Women under age 35 may have a higher likelihood of relapse than older patients, which may affect reproductive decision making. For older women, a decision to delay pregnancy may diminish their chances of becoming pregnant. Evidence on the consequences of breast cancer for the estimated 3 to 7 percent of survivors who become pregnant is limited, but reassuring. To date, most studies have not shown increases in cancer
recurrence among women who bear children and no increase in birth defects among offspring has been observed.
While on tamoxifen, menstrual function may be disrupted and continuous tamoxifen use is believed to suppress ovulation in most women. Women can, however, become pregnant while taking tamoxifen, but its effect on fetal development is not known. It is therefore recommended that women who wish to become pregnant discontinue tamoxifen therapy several months before conceiving (Burstein and Winer, 2000). Because tamoxifen is recommended for 5 years, women with ER-positive tumors wanting to have children must consider delaying childbearing for more than 5 years.
Assisted reproductive techniques are an option to overcome fertility problems (Oktay, 2001; Oktay et al., 2003; Oktay and Sonmezer, 2004; Oktay et al., 2005; Partridge and Winer, 2005). The reproductive strategies typically require exposure to high levels of exogenous steroidal hormones, raising a concern regarding increased risk of recurrence or second cancer, especially for women with ER-positive tumors.19 Some promising approaches to preserve ovarian function have been suggested, but more research is needed (Friedlander and Thewes, 2003). The recent report of a live birth after the transplantation of cryopreserved ovarian tissue from a woman with Hodgkin’s lymphoma holds promise for younger women diagnosed with cancer (Donnez et al., 2004).
The extent and nature of breast-conserving surgery affect the likelihood of successful lactation in the affected breast. An estimated 25 to 30 percent of women are able to lactate after breast-conserving surgery and irradiation, but the majority of women continue to report difficult and inadequate lactation in the affected breast (Burstein and Winer, 2000).
Weight Gain20
At least half of women receiving adjuvant chemotherapy report gaining weight, with mean gains of 2.5 to 5 kg (5.5 to 11 pounds). More significant weight gain, as much as 10 to 20 kg (22 to 44 pounds), has been reported in as many as 20 percent of women. The exact cause of weight gain is uncertain, but it may be explained in part by decreased levels of physical
activity during therapy and changes in metabolic rate that are associated with the menopause transition. Use of adjuvant therapy and onset of menopause are the strongest clinical predictors of weight gain when assessed 1 year from treatment (Goodwin et al., 1999a). Recent evidence suggests that obesity prior to diagnosis and decreased current physical activity, but not adjuvant treatment, were associated with obesity among breast cancer survivors when assessed approximately 6 years from the time of diagnosis (Herman et al., in press). Obesity can have serious health consequences and also impair psychosocial adaptation. Of great concern is the suggestion by some studies that weight gain may increase a woman’s risk of disease recurrence and death (Chlebowski et al., 2002a; Carmichael and Bates, 2004; Dignam and Mamounas, 2004; Kroenke et al., 2005). Exercise and dietary interventions may help alleviate weight gain among women receiving adjuvant breast cancer chemotherapy (Rock and Demark-Wahnefried, 2002; Demark-Wahnefried and Rock, 2003).
Osteoporosis
Estrogen is known to contribute to the risk of breast and endometrial cancer, but to be protective against osteoporosis. Women with breast cancer, who are more likely to have had relatively high exposure to estrogens, have a significantly lower risk of osteoporosis, according to both epidemiologic and clinical research (Lamont and Lauderdale, 2003; Lamont et al., 2003). Premenopausal women who experience ovarian failure following chemotherapy are, however, at much higher risk for accelerated bone density loss.
Osteoporosis is characterized by a reduction in bone density and strength, which predisposes individuals to an increased risk of fractures (Box 3-6). Post-menopausal women average a decline in bone mineral density of about 1 to 2 percent per year, but in one study of 35 premenopausal
|
BOX 3-6 A 53-year-old woman with a 13-year history of breast cancer was seen for multiple fractures that were not related to any trauma she had sustained. The fractures were determined to be due to a marked reduction in bone mineral density following premature menopause, which was secondary to her adjuvant chemotherapy. SOURCE: Ganz (2004). |
breast cancer patients who experienced ovarian failure following chemotherapy, there was an 8 percent loss in bone density after 12 months of treatment (Shapiro et al., 2001). Recent evidence suggests that post-menopausal women are also at increased risk for fractures relative to their peers (Chen et al., 2005). Tamoxifen preserves bone mineral density in post-menopausal women, but may increase bone loss in premenopausal women (Ramaswamy and Shapiro, 2003). Available evidence indicates that women treated with anastrazole (e.g., post-menopausal women with early-stage, ER-positive breast cancer) are at increased risk for fractures relative to those treated with tamoxifen (Ramaswamy and Shapiro, 2003). Aromatase inhibitors may also increase osteoporosis and lead to more bone fractures (NCCN, 2004i; Mackey and Joy, 2005).
A guideline for patient management to help maintain bone health has been published by the American Society of Clinical Oncology (ASCO). Recommended are regular monitoring of bone density, adequate dietary intake of calcium and vitamin D, exercise, and smoking cessation (Hillner et al., 2003; Friedlander and Thewes, 2003; Chlebowski, 2005b). Clinical trials are underway to prospectively monitor bone mineral density and test interventions to reduce or ameliorate the impact of treatment-related bone loss (Hillner et al., 2003).
Musculoskeletal Complaints
There is an emerging role for aromatase inhibitors (e.g., anastrozole, letrozole, exemestane) in post-menopausal women either as primary therapy or after several years of tamoxifen (Winer et al., 2005). This class of drugs completely blocks the production of estradiol in post-menopausal women, and as a result these drugs may lead to an increased risk of fractures, as well as some musculoskeletal complaints and vaginal dryness (Campos, 2004). The late effects of this class of drugs may not be life threatening, but can be very troubling (Box 3-7).
Cardiovascular Disease
One of the most serious and life-threatening late complications of chemotherapy is congestive heart failure, which develops in 0.5 to 1 percent of women treated with standard anthracycline-based chemotherapy regimens (e.g., doxorubicin) (Box 3-8) (Burstein and Winer, 2000). The cardiac dysfunction associated with anthracycline is potentially irreversible, long term, and capable of appearing years or decades following therapy (Ewer and Lippman, 2005).
Although congestive heart failure is the most extreme manifestation of anthracycline cardiotoxicity, a range of problems may arise, from mild
|
BOX 3-7 E-mail from a patient, 3 months after starting aromatase inhibitor therapy after 5 years of tamoxifen: “It has been several months since I started taking Femara. Although I do want to continue taking it and not take any chances with a cancer recurrence, I have encountered some problems. I am experiencing constant pain in my muscles, joints, etc., as if my body was continuously sore from strenuous exercise. The hardest times are in the morning and in the late afternoon, and I am usually very tired in the afternoon as well. I feel much better after exercise, but often I do not have enough energy or willpower after work to go to the gym. Instead I go to my bedroom and sleep. Altogether, this is not me and I want to do something to change it.” SOURCE: Ganz (2004). |
|
BOX 3-8 Nearly 10 years ago, Mrs. O’Donnell found a lump in her breast. At first, she wasn’t worried. A routine mammogram a month earlier showed no signs of a tumor. The lump grew so quickly during a 2-week vacation that Mrs. O’Donnell went to see her doctor days after returning home. The doctor ordered an immediate biopsy. The 42-year-old mother of three boys was diagnosed with advanced breast cancer and told she had only a 5 percent chance of surviving the next year. She proved the doctors wrong. In 1995 Mrs. O’Donnell began chemotherapy treatments, underwent two surgeries, including a mastectomy, and is now considered cancer free. Her survival came at a price. Mrs. O’Donnell, now 51, has chronic health problems arising from her cancer treatment. Just 6 weeks after her last chemotherapy session, her heart failed—a side effect of the chemotherapy. She underwent a heart transplant in 1996. That, in turn, caused other problems (e.g., medication-caused spinal deterioration, kidney disease, blood clots), which have resulted in hospitalizations and physical limitations. SOURCE: Marcus (2004). |
blood pressure changes to thrombosis and myocardial infarction (Theodoulou and Seidman, 2003). Of some concern is the observation that women treated with an anthracycline have subclinical signs of heart trouble (e.g., systolic dysfunction) that may portend later heart disease or cardiac compromise with subsequent cardiac stressors, such as hypertension
(Partridge et al., 2001). Risk factors for cardiac toxicity following anthracycline exposure include older age, preexisting heart disease, higher dose of anthra-cycline, and radiation treatment that includes the heart. Symptoms of heart disease usually develop within several months after chemotherapy, but may develop years after completion of therapy (Theodoulou and Seidman, 2003).
Other chemotherapies can cause long-term heart problems: alkylating agents (e.g., cisplatin) can cause ischemia, hypertension, and congestive heart failure; trastuzumab (Herceptin) can cause myocardial depression;21 and paclitaxel (Taxol) is associated with arrhythmias (Yeh et al., 2004). Tamoxifen has been associated with an increased risk of stroke, but the absolute risk is small, according to a recent meta-analysis (Bushnell and Goldstein, 2004). Some research suggests that tamoxifen may protect against the development of heart disease (Bradbury et al., 2005).
The early onset of menopause precipitated by cancer treatment can also place women at increased risk of atherosclerotic cardiovascular disease. This increased risk has not been well quantified, but is related to the declining levels of estrogen and subsequent increases in cholesterol levels and changes to the circulatory system (Ganz, 2001b). Reassuring data on cardiovascular risk factors among breast cancer survivors come from a cohort study in which women were followed approximately 6 years after the time of diagnosis. The cardiovascular lipid levels and blood pressure among this cohort of breast cancer survivors were within the normal range for women of comparable age and other sociodemographic characteristics (Herman et al., in press).
When radiation therapy is administered even in the absence of anthracyclines, clinically important heart damage can occur, particularly if the dose of radiation therapy is high and administered to the left breast. In their review of the evidence regarding the cardiac effects of radiation therapy, Theodoulou and Seidman note that post-operative radiation therapy increases the risk of cardiac mortality, but this mortality is offset by a reduced number of deaths from breast cancer. With new techniques, machines, and planning, these authors conclude that radiation therapy is safer today than in the past (Theodoulou and Seidman, 2003). Some evidence of this lowering of risk comes from a recent study that found differences in heart disease mortality between women diagnosed with left-sided and right-sided breast cancer in the period 1973 to 1979, but not during the period 1980 to 1984 (Giordano et al., 2005; Cuzick, 2005).
Given the increased risk of cardiotoxicity from various treatments, women with breast cancer need to be carefully monitored for risk factors such as hypertension and hypercholesterolemia (Theodoulou and Seidman, 2003). Routine screening of cardiac function is not recommended, although patients with symptoms suggestive of heart disease should be evaluated with electrocardiography and echocardiography (Burstein and Winer, 2000).
Fatigue
Fatigue is a common symptom of cancer and its treatment, and as many as one-third of breast cancer survivors report fatigue by 1 to 5 years after diagnosis. However, this level of fatigue is comparable to age-matched controls in the general community (Bower et al., 2000). A subgroup of survivors appear to have more severe and persistent fatigue. Co-occurring depression and pain are the strongest predictors of fatigue. Other factors potentially contributing to fatigue include menopausal symptoms, changes in weight, difficulties in coping, and a lack of social support (de Jong et al., 2002). Cancer-related fatigue can be a consequence of other treatment-related effects and so is difficult to diagnose (Box 3-9).
Identifying and treating underlying causes of fatigue is the first step in fatigue management. Depression, anemia, pain, and hypothyroidism can all contribute to fatigue and can be treated. Therapies for fatigue include pharmacologic interventions (e.g., psychostimulant and antidepressant medications) as well as nonpharmacologic interventions (e.g., stress management training and energy conservation and restoration) (Sadler and Jacobsen, 2001; Rao and Cohen, 2004). Controlled clinical trials of many of these interventions are underway. Some evidence suggests that exercise is a useful strategy to overcome post-treatment fatigue (Pinto and Maruyama,
|
BOX 3-9 A 38-year-old survivor of breast cancer treated with high-dose chemotherapy and radiation for Stage III breast cancer suffered from chronic anxiety and depression for the first 4 to 5 years following her treatment, but her mental health symptoms improved with medications. Six years following her treatment, she went to the doctor with a new complaint of debilitating fatigue. Following a careful examination, it was determined that she had radiation-induced hypothyroidism. SOURCE: Ganz (2004). |

FIGURE 3-4 NCCN practice guideline on cancer-related fatigue.
NOTE: These Guidelines are a work in progress that will be refined as often as new significant data becomes available.
The NCCN Guidelines are a statement of consensus of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN guideline is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
These guidelines are copyrighted by the National Comprehensive Cancer Network. All rights reserved. These Guidelines and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN.
SOURCE: NCCN (2005). Reprinted with permission from the NCCN 2.2005 Cancer-Related Fatigue Clinical Practice Guideline in Oncology. Available at: http://nccn.org. Accessed July 22, 2005. To view the most recent and complete version of the guideline, go online to www.nccn.org.
1999). The NCCN (2005) has published guidelines on cancer-related fatigue in clinical practice (Figure 3-4).
Cognitive Effects
Cognitive dysfunction has been observed among breast cancer survivors treated with adjuvant chemotherapy (Ganz, 1998; Meyers, 2000; Brezden et al., 2000; Ahles and Saykin, 2002; Rugo and Ahles, 2003; Saykin et al., 2003; Phillips and Bernhard, 2003; Tannock et al., 2004; Wefel et al., 2004a,b). The cognitive dysfunction, sometimes called “chemobrain,” includes deficits in memory, concentration, and executive functioning.22 Such dysfunction can impede attainment of work, education, and general quality of life goals. Underlying mechanisms are unknown, but recent evidence indicates that some degree of cognitive impairment exists prior to chemotherapy, suggesting that the disease itself rather than the treatment may be responsible (Wefel et al., 2004a). In their review of baseline measurements taken as part of three clinical trials, Wefel and colleagues found that 35 percent of women exhibit cognitive impairment before the start of systemic therapy for breast cancer. According to this review, distress was found to be significantly related to cognitive impairment. Other preliminary studies suggest there may be a genetic predisposition to susceptibility to chemotherapy-associated cognitive decline (Ahles et al., 2003). In order to understand its onset and underlying mechanisms, longitudinal studies of cognitive function are needed as well as studies of interventions designed to alleviate such dysfunction.
Risk to Family Members
Approximately 5 to 10 percent of breast cancer is hereditary and accounted for by mutations in the BRCA1 and BRCA2 genes. The likelihood that a woman with breast cancer has a BRCA mutation is estimated at 1 in 50 in women who are not Ashkenazi Jewish, and 1 in 10 in Ashkenazi Jewish women (NCI, 2004b). Only women with family histories or a personal history of breast cancer at a young age are candidates for BRCA testing (NCCN, 2004e). ASCO guidelines recommend that genetic testing only be offered to selected patients with personal or family histories suggestive of a hereditary syndrome, in the context of pre- and post-test counseling to discuss the risks and benefits of genetic testing and cancer early detection and prevention methods, and only when the test results can be
adequately interpreted and will aid in diagnosis or care management (ASCO, 2003). (See Chapter 4, Appendix 4D for a description of the delivery of cancer-related genetic counseling services.)
Breast Cancer Clinical Practice Guidelines
Table 3-4 lists 24 breast cancer clinical practice guidelines that were identified in the committee’s review of survivorship-related CPGs.23,24 These CPGs were evaluated in terms of their coverage of the following domains:
-
Surveillance for recurrent disease
-
Monitoring/prevention of second primary cancer
-
Management of late sequelae of disease
-
Management of late complications of treatment
-
Management of psychological, social, and spiritual issues
-
Management of genetic issues
-
Management of sexuality and fertility issues
-
Locus of care
Twelve of the guidelines address follow-up and include schedules and recommendations regarding testing. The four most comprehensive guidelines, those covering five or more of the eight domains assessed, were promulgated by government-sponsored guidelines groups in Australia, Canada, and Scotland. Eleven of the guidelines were very focused, addressing only one of the specific domains. A few of the guidelines addressed the appropriate use of a particular modality, such as radiotherapy or surgery, but these treatment-related CPGs included some recommendations or discussion that could apply to survivors.
The depth of coverage on survivorship issues varies markedly among guidelines, with some CPGs including both guidance on follow-up and extensive coverage of specific issues such as lymphedema and hormone replacement therapy (e.g., National Breast Cancer Center of Australia, British Columbia Cancer Agency, Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer of Canada). Others cover only one or two topics, with little detail. Some guidelines describe potential late effects of treatment, but have little information on how to manage symptoms.
Only one guideline, from the National Breast Cancer Center of Australia, touches on all of the topics reviewed, although it does not cover each of them with equal depth. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer covers nearly all of the topics; however, the lymphedema and hormone replacement therapy guidelines are published separately from the general breast cancer follow-up guideline. The clinician seeking comprehensive recommendations would be able to find them if multiple sources were searched, however, some of the guidelines are not easily identified. Of note, some major guidelines such as the Australian National Breast Cancer Center guidelines and those of the National Comprehensive Cancer Network were not included in the National Guideline Clearinghouse (NGC) that can be searched at the website of the U.S. Department of Health and Human Services (AHRQ, 2004b).25
All guidelines that address the issue of testing for recurrence advise against routine imaging, and blood and marker testing. The contraindication for such testing comes from randomized trials demonstrating no benefit from these procedures (Rosselli Del Turco et al., 1994; GIVIO, 1994; Liberati, 1995; Palli et al., 1999; Rojas et al., 2005). In terms of frequency of follow-up visits, all guidelines advise that visits occur on more than an annual basis, although one randomized trial assessing visit frequency showed no difference in outcomes or satisfaction for women seen on an annual or more frequent basis (Gulliford et al., 1997). The frequencies of visits in the CPGs reviewed varied within narrow limits from every 3 to 4 months to every 6 months in the first 2 years, and every 6 or 12 months in subsequent years.
Most of the guidelines offer similar schedules for follow-up visits, but recommendations for the content of follow-up visits varies. All reviewed guidelines that address surveillance recommend follow-up mammography. The strength of the mammography recommendations vary markedly, as shown in Table 3-5. Thus, depending on the guideline used, the clinician
TABLE 3-4 Breast Cancer Clinical Practice Guidelines
|
Clinical Practice Guideline |
Follow-up Schedule and Testing |
Monitoring for Second Primary Tumors; Chemoprevention for Second Primary Tumors |
|
|
1. |
National Breast Cancer Center (NBCC) (Australia). Clinical Practice Guidelines for the Management of Early Breast Cancer. Follow-up, Radiotherapy, Surgery (NBCC, 2001). |
• |
• |
|
2. |
Scottish Intercollegiate Guidelines Network (SIGN). Breast Cancer in Women: A National Clinical Guideline. Follow-up, Psychosocial Aspects, Rehabilitation, Menopausal Symptoms, and Complications of Local Treatment (1998) (SIGN, 1998). |
• |
• |
|
3. |
British Columbia Cancer Agency. Breast Cancer. Follow-up, Lymphedema, Hormone Replacement, Pregnancy, Contraception (British Columbia Cancer Agency, 2004a). |
• |
• |
|
4. |
Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer (Canadian). 9. Follow-up After Treatment for Breast Cancer (2005 update). (Grunfeld et al., 2005). |
• |
• |
|
5. |
American College of Radiology (ACR), American College of Surgeons (ACoS), College of American Pathology (CAP), Society of Surgical Oncology (SSO). Standard for Breast Conservation Therapy in the Management of Invasive Breast Carcinoma (Morrow et al., 2002a). |
• |
• |
|
6. |
ACR, ACoS, CAP, SSO. Standard for the Management of Ductal Carcinoma in Situ of the Breast (DCIS) (Morrow et al., 2002b). |
• |
• |
|
Clinical Practice Guideline |
Follow-up Schedule and Testing |
Monitoring for Second Primary Tumors; Chemoprevention for Second Primary Tumors |
|
|
7. |
American Society of Clinical Oncology (ASCO). Post-mastectomy Radiotherapy (Recht et al., 2001). |
|
|
|
8. |
National Comprehensive Cancer Network (NCCN). 2004 Breast Cancer Treatment Guidelines (NCCN, 2004b). |
• |
• |
|
9. |
ASCO. 1998 Update of Recommended Breast Cancer Surveillance Guidelinesa (Smith et al., 1999). |
• |
• |
|
10. |
Canadian Task Force on Preventive Health Care (CTFPHC). Preventive Health Care, 1999 Update: 3. Follow-up after breast cancer (Temple et al., 1999). |
• |
• |
|
11. |
Institute for Clinical Systems Improvement (ICSI). Breast Cancer Treatment (ICSI, 2003). |
• |
• |
|
12. |
American Association of Clinical Endocrinology (AACE). AACE Medical Guidelines for Clinical Practice for Management of Menopause (AACE, 1999). |
|
|
|
13. |
ASCO. 2003 Update on the Role of Bisphosphonates and Bone Health Issues in Women with Breast Cancer (Hillner et al., 2003). |
|
|
|
14. |
ASCO. Technology Assessment of Pharmacologic Interventions for Breast Cancer Risk Reduction Including Tamoxifen, Raloxifene, and Aromatase Inhibition (Chlebowski et al., 2002b). |
|
• |
|
Clinical Practice Guideline |
Follow-up Schedule and Testing |
Monitoring for Second Primary Tumors; Chemoprevention for Second Primary Tumors |
|
|
15. |
ASCO. 2000 Update of Recommendations for the Use of Tumor Markers in Breast and Colorectal Cancer (Bast et al., 2001). |
• |
|
|
16. |
European Society for Medical Oncology (ESMO). Minimum Clinical Recommendations for Diagnosis, Adjuvant Treatment, and Follow-up of Primary Breast Cancer (ESMO, 2003). |
• |
|
|
17. |
European Society of Mastology (EUSOMA). Guidelines on Endocrine Therapy of Breast Cancer (Blamey, 2002). |
|
|
|
18. |
NBCC, National Cancer Control Initiative (Australia). Clinical Practice Guidelines for the Psychosocial Care of Adults with Cancer (NBCC and NCCI, 2004). |
||
|
19. |
NCCN. Genetic/Familial High Risk Assessment: Breast and Ovarian (NCCN, 2004e). |
||
|
20. |
NCCN. Distress Management (NCCN, 2004d). |
||
|
21. |
Society of Obstetricians and Gynaecologists of Canada (SOGC). Breast Cancer, Pregnancy, and Breast Feeding (Helewa et al., 2002). |
||
|
22. |
SOGC. Use of Hormonal Replacement Therapy after Treatment of Breast Cancer (Lea et al., 2004). |
||
may interpret the need for post-operative mammography differently. Other interventions are recommended by only a few guidelines. For example, the Institute for Clinical Systems Improvement Breast Cancer Treatment guideline pointedly addresses the increased risk of cataracts in women taking tamoxifen, and recommends that patients on tamoxifen should have annual eye exams. Few of the other guidelines mention the increased risk of cataracts, much less recommend annual eye exams. Recent evidence from a case control study suggests that tamoxifen does not increase the risk for cataracts (Bradbury et al., 2004).
In terms of the management of menopausal symptoms and the use of HRT to treat them, the recommendations vary (Table 3-6). These guidelines all agree that there is some leeway in the use of HRT, but provide different rationales for the recommendation’s flexibility. There has been considerable controversy regarding the use of HRT since the publication of results of the Women’s Health Initiative study in 2003 (Wassertheil-Smoller et al., 2003). This illustrates the importance of systems to keep guidelines
|
Late Effects of Disease/Treatment |
|||||||
|
Treatment Complications |
Reconstruction/Post Surgery |
Lymphedema |
Sexuality/Fertility |
Menopause/Hormone Replacement |
Genetics |
Psychosocial Issues |
Locus of Care |
|
|
• |
|
|||||
|
|
• |
|
|||||
up to date. In general, it is recommended that CPGs be updated at least every 3 years (Shekelle et al., 2001). Some of the survivorship-related CPGs reviewed here have been updated since their original publication (e.g., American College of Radiology guidelines), but others were published 7 or more years ago and have not been updated (e.g., ASCO).
Prostate Cancer26
Men with a history of prostate cancer make up the second largest group of cancer survivors, representing 17 percent of the survivorship population (see Chapter 2 for a description of prostate cancer survivors). The advent of early detection with prostate-specific antigen (PSA) screening in the early
TABLE 3-5 Examples of Breast Cancer CPG Recommendations on Follow-up Mammography
|
Clinical Practice Guideline |
Recommendation |
|
British Columbia Cancer Agency |
Baseline, post-treatment bilateral mammograms should be performed approximately 6 months after all treatment has been completed and repeated annually thereafter. |
|
ASCO 1998 Update of Recommended Breast Cancer Surveillance Guidelines |
It is prudent to recommend that all women with a prior diagnosis of breast cancer have yearly mammographic evaluation. |
|
Canadian Task Force on Preventive Health Care 1999 Update: 3. Follow-Up After Breast Cancer |
There is no evidence to suggest that mammography decreases mortality by detecting ipsilateral disease in the conservatively treated breast; however there is indirect evidence that it may be beneficial (grade C recommendation).a There is no direct evidence to suggest that physical examination or mammography, or both, should be used to detect contralateral breast cancer, however there is indirect evidence that it may be beneficial (grade C recommendation).a |
|
aGrade C recommendation: Insufficient evidence regarding inclusion or exclusion of the condition or manuvere in a periodic health exam, but recommendations may be made on other grounds. SOURCE: Adapted from Winn (2002). |
|
1990s has contributed to an increase in the number of men diagnosed with localized prostate cancer at younger ages. Nearly all of these men will survive at least 5 years past diagnosis (Ries et al., 2004). With this high level of survival, the late effects of treatment on quality of life become of central importance to this group of cancer survivors. This section of the report will focus on the treatment and late effects associated with localized prostate cancer, but because some men with recurrent disease can live many years with cancer, the late effects of recurrent disease are also discussed. Varying approaches to prostate cancer treatment have resulted in a heterogeneous group of prostate cancer survivors (Box 3-10).
Quality of life is the primary outcome of interest for many men selecting among the available options for the treatment of localized prostate cancer. All of the treatments for localized prostate cancer have side effects that can profoundly affect patients’ sexual, urinary, and bowel function
TABLE 3-6 Examples of Breast Cancer CPG Recommendations on Menopausal Symptom Management
|
Clinical Practice Guideline |
Recommendation |
|
British Columbia Cancer Agency Breast Cancer. Follow-up; Lymphedema; Hormone Replacement; Pregnancy; Contraception |
Does not recommend HRT. If there are symptoms that interfere significantly with a woman’s quality of life and there are no other therapeutic options, HRT should be considered. |
|
American Association of Clinical Endocrinology (AACE) Medical Guidelines for Clinical Practice for Management of Menopause |
A history of breast cancer or uterine cancer is still the main contraindication to HRT, except in special circumstances (e.g., investigational studies). The conventional prohibition against HRT in survivors of breast cancer and endometrial cancer is currently being reexamined. |
|
Scottish Intercollegiate Guidelines Network (SIGN). Breast Cancer in Women: A National Clinical Guideline: Follow-up; Psychosocial Aspects; Rehabilitation, Menopausal Symptoms, and Complications of Local Treatment |
Although HRT is widely advocated for the treatment of menopausal symptoms, its use in the treatment of women with a personal or family history of breast cancer remains controversial and alternative methods of coping with menopause have not been fully explored. |
|
SOURCE: Adapted from Winn (2002). |
|
and, in turn, their quality of life (Penson and Litwin, 2003a). Men who receive combination therapy for early-stage prostate cancer generally experience additional decrements in health-related quality of life (Litwin, 2003). Although most late effects associated with prostate cancer relate to aggressive treatment, studies of men who choose watchful waiting have shown that prostate cancer itself can contribute to late effects such as urinary incontinence (Penson and Litwin, 2003b). Table 3-7 summarizes certain late effects found among prostate cancer survivors. These late effects are described more fully below.
Cancer Recurrence
There is limited information on cancer recurrence among men with prostate cancer. In one study, 15 percent of men with localized disease who were treated with prostatectomy developed elevated PSA levels indicative of recurrence by 15 years of follow-up (Pound et al., 1999). Among these men, 34 percent developed metastatic disease within the 15-year study period.
|
BOX 3-10 The most common treatments for localized prostate cancer are surgical removal of the prostate (prostatectomy), external beam radiation therapy, or brachytherapy (implanting radioactive “seeds”). These modalities may be used singly or in combination in the case of men considered to be at higher risk. Because prostate cancer is usually a slow-growing cancer, providing none of these therapies and instead monitoring the course of the disease for signs of progression (called “watchful waiting”) is another option, especially for men who are elderly or suffer from other major health problems. Evidence from clinical trials on the relative effectiveness of these approaches is not yet available, and it is recommended that clinicians provide men with information about alternative treatments and their side effects and be supportive as decisions about treatment are made. In one study of national practice patterns, about half of men with low-risk prostate cancer had elected prostatectomy (Cooperberg et al., 2004). Many men (approximately 20 percent over 5 years) treated for localized prostate cancer require follow-up cancer treatments such as radiation therapy, cryosurgery (freezing malignant areas of the prostate with cooled metal probes), prostatectomy (after the cancer has not responded to other treatments), or androgen deprivation therapy (Lu-Yao et al., 1996; Grossfeld et al., 1998). Additional treatment may be given prophylactically to men at high risk for disease recurrence (e.g., those with positive surgical margins, high-grade tumors, or positive lymph nodes) or therapeutically following biochemical (i.e., based on rising or elevated PSA levels) or clinical disease recurrence. For locally advanced disease or recurrent prostate cancer that is localized, prolonged disease control is often possible with radiation and/or hormonal therapy. For disseminated recurrent disease, hormone therapy may be used along with palliative radiation therapy. |
Practice guidelines are available for surveillance for prostate cancer recurrence. The National Comprehensive Cancer Network, for example, recommends that clinicians measure PSA every 6 months for 5 years after initial definitive therapy and then every year (NCCN, 2004g). An annual digital rectal examination (DRE) is also recommended. These guidelines are not supported by high-quality evidence from randomized clinical trials.
Second Primary Cancer
Rates of bladder cancer appear to be higher than expected among men with prostate cancer (Chun, 1997). According to a large Swedish study, rates of second primary cancers among men with prostate cancer were increased in the first 6 months of follow-up, most likely due to increased surveillance (Thellenberg et al., 2003). An increased risk of endocrine-
related second primary cancers such as male breast cancer was observed in this study. A recent study indicates that prostate irradiation increases the risk of rectal cancer (Grady and Russell, 2005; Baxter et al., 2005). The authors recommend that endoscopic evaluation for rectal cancer begin 5 years after prostate cancer radiotherapy.
Psychosocial Distress
Relatively little is known about the psychologic effects of prostate cancer on men and their family members. Concerns about having cancer, fears of recurrence, and the effects of post-treatment symptoms on quality of life may all contribute to psychosocial distress (Bacon et al., 2002). Excess levels of anxiety and depression have been found among prostate cancer survivors and their wives (Manne, 2002). Spouses and partners play an integral role in the adjustment to prostate cancer and some research has shown that having a partner positively effects quality of life (Gore et al., 2005; Soloway et al., 2005).
Younger men appear to have more trouble with psychological adjustment following treatment for prostate cancer. This could be explained if older men have accommodated to preexisting urinary and sexual problems or if they are more inclined to expect that physical health problems would occur with treatment (Eton et al., 2001). The implications of prostate cancer among men according to their age, race and ethnicity, socioeconomic status, and sexual orientation are not well understood (Visser and van Andel, 2003; Pierce et al., 2003; Blank, 2005).
Undergoing treatment for prostate cancer can decrease fears that the cancer will recur but, according to one study, significant levels of fear remained after treatment, and the fear persisted even 2 years after treatment (Mehta et al., 2003). Some men express regret about their treatment decisions. In one study, 16 percent of men treated for early-stage prostate cancer regretted their treatment decisions. Men most likely to feel regret were those with less than a college education and those who had lower quality of life ratings (Hu et al., 2003).
Groups that provide education and support—such as “Man-to-Man” and “Us TOO!”—are available to men with prostate cancer, but few such groups have been evaluated (Manne, 2002). An exception is a university-based group education and support intervention for men recently diagnosed with prostate cancer. It was evaluated through a randomized controlled trial. Group education and support were found to be successful in enhancing quality of life, especially for men with less formal education (Lepore et al., 2003). Increased knowledge about prostate cancer, adoption of healthy behaviors, improvements in general physical functioning, greater employment stability, and improved QOL related to sexual dysfunction
TABLE 3-7 Possible Late Effects Among Prostate Cancer Survivors
|
Late Effect |
Population at Risk |
Risk |
Interventions |
|
Cancer recurrence |
All men |
Varies by stage and tumor characteristics |
PSA testing every 6 months, annual digital rectal exam; bone scans only indicated if PSA level rises |
|
Second primary cancer |
All men |
Possible increase in risk of bladder, rectal, and male breast cancer |
Surveillance |
|
Psychosocial distress |
All men |
Increased anxiety and depression, but prevalence is not well documented |
Assessment for distress Support groups can be helpful |
|
Sexual dysfunction |
All men |
Rates of erectile dysfunction vary by patient age, cancer characteristics, and treatment |
Assessment for sexual function Oral agents: sildenafil, tadalafil, and vardenafil |
|
|
|
Men treated with prostatectomy at highest risk |
External mechanical devices |
|
|
|
Men treated with testosterone-suppressing hormones may have reduced libido |
Penile injection therapy Penile prostheses |
|
Bladder dysfunction |
All men |
Varies by treatment |
Assessment for urinary function |
|
|
|
Stress incontinence more common among men who had a prostatectomy |
Medication for urge incontinence (e.g., oxybutynin, tolterodine) Diet and fluid intake (e.g., reduction in fluid intake; avoidance of substances that irritate the bladder such as coffee, tea, acid juices; treatment of constipation) |
|
|
|
Irritative voiding symptoms and urge incontinence are more common among men who had radiation therapy |
|
|
|
Both symptoms tend to improve with time |
Pelvic floor rehabilitation (e.g., pelvic muscle exercise) |
|
|
|
|
Supportive interventions (e.g., good skin care, use of absorbent pads) |
|
|
|
|
Surgical interventions (e.g., prosthetic urethral sphincters) are available for men with persistent or severe post-prostatectomy incontinence |
|
Bowel dysfunction |
All men |
Varies by treatment |
Assessment for bowel function |
|
|
|
Rates of fecal incontinence low after prostatectomy |
Prescription antispasmotics (e.g., Levsin) or over-the-counter Anusol suppositories |
|
|
|
Risk among brachytherapy patients lower than for those with external beam radiotherapy |
Surgery for rectal necrosis (includes colostomy) |
|
|
|
Most symptoms decline over the course of 1 to 2 years |
|
|
Osteoporosis, fatigue, muscle wasting |
Men treated with testosterone-suppressing hormones |
Degree of symptoms related to dose and duration of treatment |
Preventive measures (e.g., calcium, vitamin D, weight-bearing exercise) |
|
|
|
|
Assessment of mineral bone density, consider treatment of osteoporosis with bisphosphonates |
|
Cognitive function |
Men treated with testosterone-suppressing hormones |
When therapy is used less than 1 year, the effects are mostly reversible; mental deficits may become persistent with treatment over 2 years |
Interventions to ameliorate the effects have not been evaluated |
were among the benefits of the intervention. The results of a randomized controlled trial conducted to assess the impact of a psychoeducational intervention aimed at wives of men with prostate cancer suggest that group interventions targeting spouses may benefit both members of the couple (Manne, 2002).
Sexual Dysfunction
Significant sexual dysfunction can occur after all three therapies commonly used to treat localized prostate cancer. Reported rates of erectile dysfunction at 1 year after therapy are 66 percent for nerve-sparing prostatectomy, 75 percent for non-nerve-sparing prostatectomy, 24 percent for brachytherapy, 40 percent for those who received brachytherapy plus external beam radiotherapy, and 40 percent for those receiving external beam radiotherapy alone (Robinson et al., 2002). Improvement in sexual function usually occurs during the first year after treatment, but further improvement into the second year appears to be more likely for men treated with radical prostatectomy as compared to external beam radiotherapy. In a recent study of long-term outcomes among localized prostate cancer survivors, sexual function and urinary and bowel symptoms were similar when evaluated at a median of 2.6 years and then 6.2 years following radical prostatectomy (Miller et al., 2005). Some symptoms improved while others worsened (e.g., urinary incontinence) for men who had undergone external radiation and brachytherapy.
The effect of erectile dysfunction on patients’ quality of life is variable and highly idiosyncractic—some men with severe dysfunction are troubled very little while others with modest levels of dysfunction view it as a significant problem (Stanford et al., 2000). Clinicians need to assess both sexual function and how men feel their sexual function has affected their quality of life. When asked about their perceptions, a significant portion reported dissatisfaction with their sexual function following treatment. In one study, 42 percent of men reported that their sexual function was a moderate to big problem at 2 years following radical prostatectomy (Stanford et al., 2000). The use of nerve-sparing techniques has modestly improved sexual function following this procedure. In a study of men undergoing external beam radiotherapy for localized prostate cancer, half reported that their overall quality of life had decreased much, or very much, as a direct result of decreased erectile function. Aggressive treatment for early prostate cancer may confer confidence in cancer control, yet be countered by sexual dysfunction, which can diminish intimate relationships and feelings of masculinity (Clark et al., 2003).
Interventions to improve sexual function following prostate cancer in-
clude use of a vacuum erection device, oral medications (e.g., sildenafil), penile injection therapy, and penile prostheses. There are no clinical practice guidelines specific to the management of sexual dysfunction among men with prostate cancer,27 but a review article is available that describes treatment options (Teloken, 2001). Some investigators have tested a progressive local treatment protocol, trying interventions sequentially and moving on to the next intervention only if they failed the previous one (Baniel et al., 2001). In this study, nearly all of the men (94 percent) were treated sucessfully and continued to respond well after one year of follow-up. Not all men who are bothered by sexual dysfunction seek medical help, and one large survey of men with erectile dysfunction after prostate cancer suggests that interventions to overcome men’s negative beliefs about seeking help for sexual dysfunction could potentially increase help-seeking behavior (Schover et al., 2002, 2004).
Bladder Dysfunction
Urinary dysfunction is seen in nearly all men with prostate cancer in the immediate post-therapy period, but function improves for most men during the first 2 years after therapy. Men who have radical prostatectomies are more likely to report urinary leakage when they cough or strain, whereas men undergoing either external beam radiotherapy or brachytherapy often experience significant pain, frequency, or urgency with urination. Although the type of urinary dysfunction differs among treatments, the impact on quality of life is considerable with both surgery and radiotherapy and represents a significant burden of disease for patients. In a study of men who had undergone external beam radiotherapy for prostate cancer, 54 percent (as compared to 31 percent of controls) reported urinary problems 8 years after treatment (Fransson and Widmark, 1999). A study of men treated with brachytherapy found that at 6 months after treatment, 40 percent reported urinary frequency and 17 percent reported pain upon urination (Arterbery et al., 1997). Even without aggressive treatment, men with localized prostate cancer can have lower urinary tract symptoms. Like sexual dysfunction, the significance of urinary dysfunction is highly individualized: not all men are bothered by it. In one study of men following radical prostatectomy, 2 percent had no urinary control, 7 percent reported frequent leakage, 40 percent reported occasional leakage, and 32 percent reported total urinary control 2 years after surgery. When questioned as to
how big a problem their incontinence was, 38 percent said it was no problem, 34 percent said it was a small problem, and 9 percent said it was a moderate to big problem.
A number of interventions are available to treat the urinary problems associated with prostate cancer treatment:
-
Medication for urge incontinence (e.g., oxybutynin, tolterodine)
-
Diet and fluid intake (e.g., reduction in fluid intake; avoidance of substances that irritate the bladder such as coffee, tea, and acid juices; treatment of constipation)
-
Pelvic floor rehabilitation (e.g., pelvic muscle exercise)
-
Supportive interventions (e.g., good skin care, use of absorbent pads, condom catheters)
-
Surgical interventions (e.g., urethral sphincters) are available for men with persistent or severe post-prostatectomy incontinence
There are no clinical practice guidelines specific to the management of urinary dysfunction for men with a history of prostate cancer, but a review article is available that describes these treatment options (Grise and Thurman, 2001).
Bowel Dysfunction
Radiotherapy, either external beam or brachytherapy, can lead to significant bowel dysfunction, including bowel necrosis and symptoms such as rectal urgency or diarrhea (Penson and Litwin, 2003b). While many gastrointestinal problems were viewed as minor following treatment with external beam radiotherapy, a small proportion of men (10 percent or less) have reported severe bowel symptoms, including fecal soiling. For men treated with brachytherapy, bowel necrosis can occur, and it is estimated that problematic diarrhea may occur for 12 percent of men at 9 months following surgery (Krupski et al., 2000). Bowel dysfunction is fairly uncommon after prostatectomy. Interventions for bowel dysfunction include medication for cramping and diarrhea. Surgery, including colostomy, may be required for severe problems such as bowel necrosis.
Osteoporosis
Osteoporosis is a potentially serious complication of androgen deprivation therapy for prostate cancer (Smith, 2003). Such therapy may be used for men with advanced disease or recurrent prostate cancer. Androgen deprivation therapy either by bilateral orchiectomies (i.e., surgical removal of the testicles) or by treatment with a gonadotropin-releasing hormone
agonist/antagonist decreases bone mineral density and increases the risk of fracture (Krupski et al., 2004). Lifestyle modification should be encouraged, including smoking cessation, moderation of alcohol consumption, and regular weight-bearing exercise. Recommended also are preventive measures such as taking supplemental calcium and vitamin D. Treatment with bisphosphonates may be warranted for men with osteoporosis, fractures, or high rates of bone loss during androgen deprivation therapy.
Cognitive Dysfunction
Androgen deprivation therapy for prostate cancer may be associated with impaired memory, attention, and executive functions (i.e., the brain’s supervisory or regulatory functions) (Green et al., 2002a; Koupparis et al., 2004). In a recent study of men with prostate cancer treated with androgen deprivation therapy, cognitive effects were mostly reversible when therapy was used less than 1 year. However, mental deficits persisted with treatment that lasted more than 2 years (Salminen et al., 2005). Interventions to improve cognitive function by administering estrogen replacement therapy have not been shown to be effective (Taxel et al., 2004).
Clinical Practice Guidelines
Available prostate cancer CPGs focus on surveillance for recurrence and do not provide information on management of late effects (Finnish Medical Society Duodecim, 2002; Villers et al., 2003; British Columbia Cancer Agency, 2004b; NCCN, 2004g). All guidelines recommend routine surveillance with digital rectal examination and PSA testing, but the frequency of recommended follow-up vary somewhat. For example, the NCCN CPG recommends an annual DRE and PSA testing every 6 months for 5 years, and then every year thereafter. The British Columbia CPG recommends DRE and PSA testing at regularly scheduled intervals (e.g., every 3 months in the first year, increasing to every 6 months thereafter). The CPGs for follow-up of patients with prostate cancer are not based on clinical trials. Such trials are needed to test the effectiveness of the various follow-up measures and strategies.
In summary, prostate cancer treatment can result in high rates of urinary, sexual, and bowel dysfunction that can adversely affect quality of life. Treating physicians should actively inquire about these adverse effects and provide early treatment to maximize quality of life (Penson and Sokoloff, 2004). Validated questionnaires are available to assist clinicians in the ascertainment and documentation of complications such as urinary and fecal incontinence, erectile dysfunction, and intestinal inflammation, and effective treatments are available (Yao and Dipaola, 2003; Litwin et al.,
2004). Evidence-based clinical practice guidelines are needed to assist clinicians in the management of late effects of prostate cancer treatment.
Colorectal Cancer
Individuals with a history of colorectal cancer make up the third largest group of cancer survivors, representing 11 percent of the survivorship population. As a group, survivors of colorectal cancer are elderly, with 76 percent aged 65 and older (see Chapter 2 for a description of colorecal cancer survivors). Box 3-11 summarizes the most common treatments for colon and rectal cancers. Fortunately, 80 percent of people with colorectal cancer have local or locally advanced cancer and curative-intent surgery is performed (Meyerhardt and Mayer, 2003). However, up to 40 percent of these patients will subsequently develop recurrent disease.
Most long-term survivors of colorectal cancer report very good quality of life following their treatment, but certain deficits are still observed in some patients (Ramsey et al., 2002; Trentham-Dietz et al., 2003). According to one study, individuals who had survived colorectal cancer for at least 5 years reported a relatively uniform and high quality of life, irrespective of stage at diagnosis and time from diagnosis (Ramsey et al., 2002). Compared to age-matched individuals, however, cancer survivors reported higher
|
BOX 3-11 Colon cancer: Surgical removal of the cancer and nearby lymph nodes is the standard treatment for patients with colon cancer. Sometimes a temporary colostomy is needed. Some very early-stage cancers may be removed endoscopically, with good results. 5-fluorouracil-based adjuvant chemotherapy is considered standard for patients with Stage III colon cancer, and an option for some with Stage II disease. Newer regimens incorporating oxaliplatin may be used. Adjuvant radiation therapy is sometimes given for patients with locally advanced colon cancer, but its use is controversial. Rectal cancer: Surgery alone is often sufficient for individuals with low-stage rectal cancer. When the tumor is in the low rectum, the rectum and anus are removed and a permanent colostomy is necessary. In men, such surgery can damage genital nerves and impair bladder and sexual function. Sphincter-preserving surgery is feasible for patients with a tumor located in the upper or middle part of the rectum. Some of these procedures, however, may also damage the pelvic nerves involved in sexual function. For those with Stage II or III rectal cancer, radiation and chemotherapy are recommended. Radiation is increasingly being given preoperatively to increase the local control rate. |
rates of depression and nearly half reported frequent bowel movements or chronic diarrhea. Long-term, disease-free survivors of rectal cancer have reported good quality of life, but residual pain and constipation sometimes negatively affected quality of life (Rauch et al., 2004). Table 3-8 summarizes some of the late effects associated with colorectal cancer and its treatment. Details regarding these late effects and their management are described below.
Cancer Recurrence and Second Primary Cancer
Up to 40 percent of individuals treated for local or locally advanced colorectal cancer will have their disease recur. Following treatment, periodic evaluations can lead to the earlier identification and management of recurrent disease, but the impact of such monitoring on overall mortality is limited by the relatively small proportion of patients in whom localized, potentially curable metastases or local recurrences are found. Survivors of colorectal cancer are also at risk of developing a second primary colorectal cancer. In a follow-up study of individuals with localized colon cancer, the incidence of a second primary colorectal cancer remained high (1.5 percent at 5 years) (Green et al., 2002b). The risk of other cancers developing is also higher among survivors of colorectal cancer, including cancers of the small intestine, cervix, uterus, and ovary (Evans et al., 2002).
The optimal regimen and frequency of follow-up examinations to detect cancer recurrence and second primary cancers are not well defined. No large-scale randomized trials have been completed to document the efficacy of any overall post-operative monitoring program (i.e., involving carcinoembryonic antigen (CEA) testing, imaging studies, office visits). Two such trials are now in progress, but the results will not be available for several years (Johnson et al., 2004; FACS, 2005). Guidelines concerning colonoscopy in high-risk groups such as those who have completed treatment are fairly consistent and supported by high-quality data (Table 3-9). However, there is variation in recommendations on other follow-up tests. In the area of routine CEA28 testing for the early detection of recurrence, for example, several guidelines29 recommend that patients who would be candidates for resection of metastases receive regular CEA testing. Other evidence suggest it is of no value (Moertel et al., 1993; Northover, 2003).
|
28 |
Carcino-embryonic antigen is a serum glycoprotein that can be detected in the blood of individuals with colon cancer. |
|
29 |
Guidelines making this recommendation include those of The Finnish Medical Society Duodecim, British Columbia Cancer Agency, ASCO, American Society of Colon and Rectal Surgeons and NCCN (see Table 3-10). |
TABLE 3-8 Possible Late Effects Among Colorectal Cancer Survivors
|
Late Effect |
Population at Risk |
Risk |
Interventions |
|
Cancer recurrence |
All survivors |
40 percent among those treated with local or locally advanced cancer |
Follow-up imaging recommended. Periodic testing for carcino-embryonic antigen (CEA) may be indicated for some survivors in the first few years after diagnosis. |
|
Second primary cancer |
All survivors |
Increased risk of cancers of the colon, rectum, small intestine, cervix, uterus, and ovary |
Follow-up colonoscopy recommended |
|
Psychosocial distress |
All survivors |
Higher rates of depression have been reported |
Assessment for distress |
|
|
|
|
Evidence on the effectiveness of psychosocial interventions among survivors of colorectal cancer is limited |
|
Bowel dysfunction: diarrhea and fecal leakage/incontinence, constipation, bowel obstruction, pain |
Variable |
Hemicolectomy can lead to loose stools that usually improve over time. Surgery can also lead to adhesions. Rectal cancer patients are at higher risk of fecal incontinence. Radiation may lead to small bowel scarring and bowel obstruction. |
Dietary counseling, use of over-the-counter medications (e.g., fiber laxative, stool softeners, antidiarrheals), and anal sphincter biofeedback training |
|
Colostomy |
Rectal cancer survivors who had tumors located in the lower part of the rectum |
Approximately 15 to 25 percent of survivors will have permanent colostomies |
Enterostomal nurses provide education, training, and counseling |
|
Sexual function |
Rectal cancer survivors |
Erectile dysfunction and ejaculatory difficulties in men. Painful coitus in women. Infertility, especially among women. Abnormal bowel function can affect sexual functioning. |
Assessment for sexual function: For men, drugs for erectile dysfunction (e.g., sildenafil citrate); for women, vaginal dilatation, over-the-counter lubricants. For infertility, men can bank sperm. Effective options for fertility preservation in women are limited. Ovarian pexy, pinning the ovaries up out of the radiation field at the time of surgery, may preserve ovarian function, but the uterus will be damaged. |
|
Peripheral neuropathy |
Survivors who received oxaliplatin |
Numbness or painful sensations |
Prescription medications (i.e., vitamin B6, amitriptyline, gabapentin) |
|
Risk to family members |
All survivors |
Most colorectal cancer is sporadic and relatives are not at higher risk. Family history and clinical characteristics of the cancer may suggest a genetic etiology. |
Genetic counseling; in addition, those at high risk are counseled to begin colonoscopy 10 years before the earliest colorectal cancer in the family (or age 50, whichever comes first). Genetic tests are commercially available for some genetic disorders (e.g., hereditary nonpolyposis colorectal cancer [HNPCC], familial adenomatous polyposis [FAP]). |
TABLE 3-9 Examples of Colorectal Cancer CPG Recommendations on Follow-up Colonoscopy
|
Clinical Practice Guideline |
Recommendation |
|
The American Society of Colon and Rectal Surgeons (ACSRS), Practice Parameters for the Detection of Colorectal Neoplasms |
Pre-operative colonoscopy; repeat colonoscopy in 1-3 years, then 3 years, and then every 5 years if free of disease. If no pre-operative exam, colonoscopy 3 to 6 months post-surgery |
|
American Society of Clinical Oncology (ASCO), 2000 Update of American Society of Clinical Oncology Colorectal Cancer Surveillance Guidelines |
If polyp free, colonoscopy every 3 to 5 years. |
|
British Columbia Council on CPGs, Protocol for Follow-Up of Patients After Curative Resection of Colorectal Cancer |
Pre-operative colonoscopy; repeat once every 3 years; if free of disease, repeat every 5 years |
|
SOURCE: Adapted from Winn (2002). |
|
Several other guidelines30 say that evidence is insufficient to make any recommendation regarding regular CEA testing. None of the guidelines recommend regular computed tomography (CT) scanning, although one guideline (i.e., Management of Colorectal Cancer, Scottish Intercollegiate Guidelines Network) says that regular scanning may be beneficial. Surveillance methods including CEA immunoscintigraphy and positron-emission tomography (PET) scan are under evaluation (NCI, 2005b).
Psychosocial Distress
There have been relatively few studies of the psychosocial impact of colorectal cancer, however, in one study, depression was more prevalent among survivors of colorectal cancer than expected in the general population (14 percent versus 10 percent) (Ramsey et al., 2002). Higher levels of psychosocial distress have been reported among individuals with perma-
|
30 |
These guidelines include Scottish Intercollegiate Guidelines Network, Cancer Care Ontario, and British Society of Gastroenterology/Association of Coloproctology for Great Britain and Ireland (see Table 3-10). |
nent colostomies (Sprangers et al., 1995). Among female survivors of colorectal cancer, contacts with relatives and friends and other measures of the extent of social networks appeared to improve mental health (Sapp et al., 2003). Another study of female survivors of colorectal cancer found health-related quality of life comparable with that of similarly aged women in the general population (mean follow-up was 9 years) (Trentham-Dietz et al., 2003).
Bowel Dysfunction
Some individuals with colon cancer experience bowel symptoms. Among rectal cancer patients, permanent colostomies represent a major life adjustment. For most colon cancer patients, there are often frequent bowel movements, but few disabling problems. Whether or not quality of life differed between those survivors who had had a permanent colostomy and those who hadn’t was the subject of a review of the literature (Sprangers et al., 1995). According to this review, many patients are troubled by frequent or irregular bowel movements and diarrhea. Some patients, however, are not troubled (e.g., those who had constipation prior to surgery), and many individuals with colostomies are able to adapt very successfully. Patients with very early-stage cancer treated with polypectomy may have no change in bowel function.
Sexual Function
Survivors of colorectal cancer can have poor sexual functioning, in part as a consequence of the irregular bowel function that may occur. Most of what is known about sexual function in this group of survivors relates to rectal cancer. For women surviving rectal cancer, age at surgery and characteristics of the surgery are predictive of sexual functioning. For men, dry ejaculate and erectile dysfunction may occur among 25 to 45 percent of men following rectal surgery. Sexual function was consistently more impaired among survivors who had lost sphincter control following their surgery for rectal cancer than among patients with intact sphincters, according to a review of quality of life among colorectal survivors (Sprangers et al., 1995). For men with erectile dysfunction, prescription medications (e.g., sildenafil citrate) and devices (e.g., prostheses) are available. For women, vaginal dilatation31 is an option as are over-the-counter vaginal lubricants.
Risk to Family Members32
About 70 percent of cases of colorectal cancer occur sporadically, with no evidence of familial or inherited predisposition (Calvert and Frucht, 2002). An inherited polyposis syndrome33 accounts for fewer than 10 percent of individuals with colorectal cancer. For up to 25 percent of cases, the cancer is considered familial, meaning there is a family history of colorectal cancer, but it cannot be accounted for by the known inherited syndromes. Genetic testing is becoming more available, but its clinical indications are still limited. The testing should be limited to persons whose family history suggests an inherited syndrome or who exhibit specific features of an inherited cancer syndrome (e.g., colon cancer before age 50). If the genetic test results of the individual with colorectal cancer identify a specific mutation, phenotypically unaffected first-degree relatives can then be tested. However, if the results of a particular test are negative, unaffected first-degree relatives should not be tested for that genetic disorder because the test will be uninformative. When genetic testing is indicated, it should be preceded by a pretest counseling session detailing the limitations of the test and the potential psychological, ethical, legal, and societal implications for the individual with cancer and his or her family members.
Clinical Practice Guidelines
The committee identified and reviewed 15 CPGs that include recommendations on the follow-up care of colorectal cancer survivors (Table 3-10).34 Despite the wide range of late effects associated with colorectal cancer, most of these CPGs address only two domains of survivorship: (1) surveillance testing, especially colonoscopy, and (2) the screening issues related to monitoring the genetic variants of colorectal carcinoma, which account for a small fraction of all disease. Notably absent is guidance regarding the functional sequelae that may follow surgical interventions (e.g., colostomy, bowel dysfunction, sexual dysfunction). Only one guide-
line mentions enterostomal therapy (i.e., care for colostomy) and it provides little detail. Of the 15 colorectal cancer guidelines, 7 are specifically targeted to follow-up, 5 are oriented to screening or genetics, and 3 are part of general treatment guidelines.
The guidelines currently available are not uniform, and the possible reasons for variability among guidelines are numerous. The most important is probably the absence of adequately powered, well-controlled trials of high-intensity versus low-intensity follow-up after potentially curative initial therapy. As mentioned earlier, two such trials are now in progress, but the results will not be available for several years (Johnson et al., 2004; FACS, 2005). Funding agencies such as the U.K. Medical Research Council support the Follow-up After Colorectal Surgery (FACS) trial.
Hodgkin’s Disease35
Survivors of Hodgkin’s disease (HD) make up a small fraction (about 1 percent) of the population of cancer survivors (see Chapter 2 for a description of HD survivors). However, most individuals diagnosed with HD are relatively young and will be long-term survivors of their disease. Late effects of HD treatment have been recognized for many years due to the high survival rates, and are among the first to be well documented. Long-term follow-up studies have shown higher than expected death rates among HD survivors. Second cancers and cardiovascular disease attributable to HD treatment account for much of this excess mortality (Ng and Mauch, 2004). Modification of HD therapies have been made to reduce the serious late effects of treatment (Donaldson et al., 1999). Changes in therapy that have maintained good survival while minimizing late effects have included: elimination of the use of surgical staging with splenectomy; minimizing radiation doses and large volumes of the body irradiated; shifting to chemotherapy drugs that are less toxic and delivered over shorter periods of time; and therapy adapted to the patient’s risk of recurrence. Box 3-12 outlines the main strategies for initial treatment for HD.
Quality of Life
Reductions in the toxicity of treatment for HD have improved survivors’ quality of life. A recent prospective study assessed the quality of life of
TABLE 3-10 Colorectal Cancer Clinical Practice Guidelines
|
Clinical Practice Guideline |
Follow-up Schedule and Testing |
|||
|
Colonoscopy |
Imaging |
CEA |
||
|
1. |
Scottish Intercollegiate Guidelines Network Management of Colorectal Cancer (SIGN, 2003) |
• |
• |
• |
|
2. |
The Finnish Medical Society Duodecim Evidence-based Medicine Guidelines: Postoperative Follow-up of Colorectal Cancer (Finnish Medical Society Duodecim, 2001) |
• |
• |
• |
|
3. |
British Columbia Cancer Agency Colon: Follow-up (British Columbia Cancer Agency, 2002a) |
• |
• |
• |
|
4. |
American Society of Clinical Oncology (ASCO) 2000 Update of American Society of Clinical Oncology Colorectal Cancer Surveillance Guidelines (Benson et al., 2000) |
• |
• |
• |
|
5. |
Cancer Care Ontario Program in Evidence-Based Care Follow-up of Patients with Curatively Resected Colorectal Cancer: Practice Guideline (Figueredo et al., 2003) |
• |
• |
• |
|
6. |
National Comprehensive Cancer Network (NCCN) Colon Cancer Version 2.2004 (NCCN, 2004h) |
• |
• |
• |
|
7. |
American Society of Colon and Rectal Surgeons (ASCRS) Practice Parameters for the Surveillance and Follow-Up of Patients with Colon and Rectal Cancer (Anthony et al., 2004) |
• |
• |
• |
|
8. |
ASCRS Practice Parameters for the Detection of Colorectal Neoplasms (Simmang et al., 1999) |
• |
|
|
|
9. |
British Columbia Guidelines and Protocols Advisory Committee Follow-up of Patients After Curative Resection of Colorectal Cancer (British Columbia Guidelines and Protocols Advisory Committee, 2004) |
• |
|
• |
|
Clinical Practice Guideline |
Follow-up Schedule and Testing |
|||
|
Colonoscopy |
Imaging |
CEA |
||
|
10. |
British Society of Gastroenterology (BSG), Association of Coloproctology for Great Britain and Ireland (ACPGBI) Guidelines for Follow-up After Resection of Colorectal Cancer (Scholefield and Steele, 2002) |
• |
• |
|
|
11. |
U.S. Multisociety Task Force on Colorectal Cancer Colorectal Cancer Screening and Surveillance: Clinical Guidelines and Rationale—Update Based on New Evidence (Winawer et al., 2003) |
• |
|
|
|
12. |
ASCO 2000 Update of Recommendations for the Use of Tumor Markers in Breast and Colorectal Cancer (Bast et al., 2001) |
|
|
• |
|
13. |
ASCRS Practice Parameters for the Treatment of Patients with Dominantly Inherited Colorectal Cancer (Church and Simmang, 2003) |
|
||
|
14. |
BSG, ACPGBI Guidance on Gastrointestinal Surveillance for Hereditary Non-Polyposis Colorectal Cancer, Familial Adenomatous Polyposis, Juvenile Polyposis, and Peutz-Jeghers Syndrome (Dunlop, 2002) |
|||
|
15. |
NCCN Colorectal Screening Version 1.2004 (NCCN, 2004c) |
|||
247 survivors of early-stage HD treated as part of a clinical trial (Ganz et al., 2003c). Short-term decrements in quality of life were observed, but the scores at 1 year were similar to baseline scores before treatment, without further improvement at the 2-year assessment. HD survivors perceived that their health had declined following treatment. The adverse consequences of treatment are greater for those with more advanced disease. In one study of survivors of Stage III or IV HD assessed an average of 5 years after their treatment, nearly one-quarter (23 percent) of survivors had problems directly related to HD therapy (e.g., hypothyroidism, peripheral neuropathy)
|
Late Effects of Disease/Treatment |
||||||
|
Bowels/Stoma |
Sexuality/Fertility |
Post – Radiotherapy |
Menopause/Hormone Replacement |
Genetics |
Psychosocial Issues |
Locus of Care |
|
|
||||||
|
|
• |
|
|
|||
|
|
||||||
|
|
• |
|
||||
|
• |
||||||
|
• |
||||||
(Kornblith et al., 1998). Table 3-11 provides information on some of the late effects experienced by HD survivors. These are described more fully below.
Cancer Recurrence
A minority of long-term survivors of HD will have their cancer recur. The risk is related to the effectiveness of the initial therapy. In one study 22
|
BOX 3-12 The majority of patients are treated with risk-adapted therapy. For those with early stage disease, the standard is combined modality therapy with radiation directed to initially involved sites and a brief course of chemotherapy selected to reduce late effects. Recent studies suggest that chemotherapy alone may be an alternative to combined modality therapy for select early-stage HD but the mature results of randomized trials are needed to compare late effects with these approaches. High dose therapy and autologous transplantation represents a potentially curative option for HD patients with recurrence after initial therapy. |
percent of patients had experienced a relapse at a median of 1.9 years (Torrey et al., 1997). Only 15 percent of relapses occurred after 5 years. Treatment of recurrent HD is often successful. Given the time course of recurrence, it is recommended that post-treatment surveillance for recurrence be concentrated in the first few years after primary treatment.
Second Cancers
At 15 years of follow-up, the risk of second solid tumors (cancers of the lung, breast, thyroid, bone/soft tissue, stomach, esophagus, uterine cervix, and head and neck) is approximately 13 percent, and at 25 years, approximately 22 percent (Dores et al., 2002). The risk of lung cancer is increased among HD survivors, especially among those who smoke and were treated at an older age (Travis et al., 2002). The risk of breast cancer is high among women treated with chest radiation before age 30, and the incidence increases substantially after 15 years of follow-up (Hancock et al., 1993). Women with therapy-related premature menopause have a lower risk of subsequent breast cancer (Travis et al., 2003). The risk of skin cancer is also increased and routine skin examinations are recommended. Counseling regarding healthy lifestyle, including smoking cessation, is recommended along with follow-up physical examination and selected laboratory and imaging studies (e.g., mammograms for females).
Psychosocial Distress
Survivors of HD often report post-treatment fatigue that can affect work and leisure activities and in turn contribute to psychological distress
(Fobair et al., 1986; Loge et al., 2000). One French study that compared psychosocial outcomes of HD survivors to those among healthy controls (matched for sex, age, and residency) found HD survivors to have more physical, role, and cognitive functioning impairments than their peers, but to report good overall health and psychologic status (Joly et al., 1996). Survivors of aggressively treated HD have been found to be at increased risk for psychological distress (Cella and Tross, 1986), and in one study, 22 percent of advanced HD survivors met the criterion suggested for a psychiatric diagnosis (Kornblith et al., 1992, 1998).
Infertility and Gonadal Dysfunction
Infertility can be a problem for HD survivors as a result of treatment with either chemotherapy or radiation therapy. Some survivors retain or regain fertility after treatment. In one study of 391 adult patients of reproductive age, female patients who attempted conception had pregnancy rates similar to those observed in the general population (81 percent versus 85 percent). The female partners of male patients, however, had a much lower frequency of pregnancy (49 percent) (Aisner et al., 1993). In this study, there was no apparent increase in complications of pregnancy, spontaneous abortions, or congenital abnormalities after treatment compared with pregnancies in this patient group before treatment or with pregnancies in the general population. Counseling regarding reproduction is advised.
Hypothyroidism
According to one study of 177 survivors of HD, more than one-quarter (27 percent) had developed hypothyroidism when examined after an average follow-up of 6 years (Bethge et al., 2000). Only those treated with radiotherapy were at risk. Patients who received radiation to the region of the thyroid gland should be evaluated by physical examination and have periodic thyroid function tests.
Cardiovascular Disease
HD survivors treated with radiation or cardiotoxic chemotherapy may experience cardiovascular effects, and aggressive risk reduction is warranted. Cardiovascular conditions that have been observed among HD survivors include pericarditis, coronary artery disease, heart valve damage, cardiomyopathy, pancarditis, and conduction abnormalities. The use of modern radiation techniques and low radiation doses can reduce the risk of cardiovascular late effects. Recommended risk reduction strategies for HD survivors at risk of cardiovascular disease include: smoking cessation; avoid-
TABLE 3-11 Possible Late Effects Among Survivors of Hodgkin’s Disease
|
Late Effect |
Population at Risk |
Risk |
Interventions |
|
Cancer recurrence |
All HD survivors |
Risk highest within first few years of primary therapy |
Post-treatment surveillance (physical examination, including skin examination) Prevention strategies (e.g., smoking cessation) For women: routine breast self-examination, mammography |
|
Second primary cancer |
Individuals receiving certain chemotherapies Individuals receiving radiation therapy |
Increased risk of leukemia and lymphoma Increased risk of cancers of the lung, breast, thyroid, bone/soft tissue, gastrointestinal tract, and skin |
|
|
Psychosocial distress |
All survivors |
Not well understood |
Not well investigated |
|
Infertility and gonadal dysfunction |
Individuals receiving pelvic irradiation and those receiving high cumulative doses of alkylating agent chemotherapy drugs |
High cumulative alkylating agent exposure sterilizes nearly all males, and females over age 25 undergo menopause. Sexual dysfunction may occur with sex hormone deficiency |
Pretreatment reproductive counseling, semen and embryo cryopreservation, and referral to specialists Hormone replacement therapy counseling based upon treatment received |
|
Hypothyroidism |
Individuals receiving high-dose neck radiation |
Risk varies by radiation dose and area exposed |
Routine physical examination of the thyroid; periodic thyroid function testing |
|
Cardiovascular disease |
Individuals receiving radiation to the heart or cardiotoxic chemotherapy |
Patients treated with a lower radiation dose are at lower risk |
Aggressive risk reduction (e.g., management of lipids, glucose intolerance, and hypertension; |
|
|
Individuals receiving radiation to the head and neck (vascular arterial changes) |
Risk related to cumulative dose of anthracycline |
smoking cessation) |
|
Impaired pulmonary function |
Individuals receiving high doses of radiation; bleomycin increases the risk |
Worsening lung function depends on dose of radiation therapy and bleomycin |
Smoking cessation, avoidance of other pulmonary toxins Annual influenza vaccine Steroid and antibiotic therapy |
|
Increased risk of infection |
Individuals who have undergone splenectomy or splenic radiation; patients exposed to intensive chemotherapy |
Infection risk (e.g., sepsis) increases in asplenic patients, and with higher degree of immunosuppression |
Pretreatment and periodic immunization; prophylactic antibiotics |
|
Fatigue |
All survivors |
Some HD survivors have fatigue; it may be related to aspects of the disease rather than to treatment |
Exercise may be beneficial |
|
Nerve damage |
Individuals receiving neurotoxins: vinca alkaloids and platinum compounds |
Peripheral neuropathy and autonomic neuropathy |
Avoid other neurotoxins: heavy metals, radiation, drugs |
|
Osteoporosis; avascular necrosis |
Individuals receiving corticosteroid therapy |
Risk reduced through radiation shielding of the femoral head, minimizing prednisone exposure |
Assessments and preventive strategies for osteoporosis (e.g., bone density examinations, calcium, and vitamin D) Orthopedic surgery |
|
Musculoskeletal atrophy |
Individuals receiving radiation to the head and neck |
Risk related to dose of radiation and age at therapy |
Rehabilitation if severe loss of muscle mass |
|
Dental caries |
Individuals receiving salivary gland radiation |
Risk reduced through radiation shielding of the salivary glands |
Dental prophylaxis (e.g., fluoride) |
ance of obesity; and management of lipids, glucose intolerance, and hypertension. Unusual symptoms (e.g., chest or arm pressure, unexpected, profound exertional fatigue) should prompt careful cardiologic assessment (Hancock, 1999).
Impaired Pulmonary Function
HD survivors may experience damage to the lung if they are treated with radiation therapy that is of high dose and involves large volumes of the chest area or if they receive certain chemotherapy agents, such as bleomycin. Smoking cessation programs are very important. Severe pneumonitis may require steroid therapy.
Increased Risk of Infection
Individuals with HD are at increased risk of infection if they had a splenectomy, splenic radiation, or were treated with high-dose therapy and autologous transplantation. Such individuals should be immunized with Haemophilus influenza type B conjugate, meningococcal, and pneumococcal vaccines before treatment. Reimmunization with all three vaccines 2 years after completion of treatment and with pneumococcal vaccine every 6 years thereafter has also been recommended. Patients exposed to aggressive immunosuppressive treatment programs may benefit from antibiotic use. Patient education is important to alert HD survivors to the importance of medical attention during episodes of fever.
Fatigue
Fatigue has been observed among HD survivors in several studies (Bloom et al., 1993; Loge et al., 1999; Knobel et al., 2001; Flechtner and Bottomley, 2003), but according to a recent prospective study conducted by Ganz and colleagues, increased fatigue was evident prior to treatment. This suggests that an underlying disease process may be responsible (Ganz et al., 2003c). Further analyses of this study cohort found pretreatment fatigue not to be associated with medical factors related to disease or to hematologic status (Ganz et al., 2004b). Instead, fatigue was significantly associated with patient-reported symptoms and physical and psychosocial well-being. Post-treatment fatigue was related to depressed pretreatment vitality. Exercise may help cancer survivors who experience fatigue (Holtzman et al., 2004).
Nerve Damage
Those with preexisting neuropathies and those who receive neurotoxic drugs or radiation are at risk for nerve damage. The risk depends on dosages of radiation and certain chemotherapies. Survivors with nerve damage should avoid further exposure to neurotoxins.
Bone Damage
Chemotherapy programs using prolonged and high doses of steroids predispose to osteopenia and osteoporosis. Measures to reduce the risk of osteoporosis include using prescription medications (e.g., alendronate), performing bone density examinations, recommending the use of calcium and vitamin D supplements, and counseling survivors about the benefits of regular exercise, weight-bearing exercise, and a healthy weight. High radiation doses to bone, especially the mandible and femoral heads, has been associated with bone necrosis. However, these late effects are rarely seen with modern treatment programs.
Dental Caries
Radiation to large areas including the salivary glands can decrease the amount of saliva and change its quality so that it is less effective in cleansing normal oral bacteria from the mouth. This sets the stage for possible dental caries. People who get radiotherapy to the neck and mouth areas should have dental care pretreatment. After therapy, survivors need to take good care of their teeth. Regular dental visits, use of fluoride mouth washes, drinking fluorinated water, and use of dental floss are recommended. Antibiotic therapy may be needed before a tooth extraction. Pulling teeth after radiation therapy increases the risk of necrosis of the mandible and maxilla, and some patients elect to get all of their teeth pulled prior to radiation to avoid this. There are dentists available who specialize in the care of the mouth following radiation.
Clinical Practice Guidelines
The committee identified two clinical practice guidelines that describe management strategies for HD survivors.36 The NCCN Hodgkin’s disease CPG provides a visit schedule, vaccination recommendations, and sugges-
|
BOX 3-13 Interim health visit and physical examination:
Laboratory studies:
Chest imaging:
Abdominal/pelvic CT:
Annual mammographic screening:
Counseling:
Recommend written follow-up instructions for the patient
NOTE: The frequency and types of tests may vary depending on clinical circumstances; age and stage at diagnosis, social habits, treatment modality, etc. SOURCE: NCCN (2004f). |
tions for laboratory studies, including those for thyroid function, imaging, mammograms for women, and counseling (Box 3-13) (NCCN, 2004f). Somewhat more comprehensive coverage of survivorship issues can be found in the HD CPG of the British Columbia Cancer Agency (British Columbia Cancer Agency, 2002b). Included in this CPG is a follow-up schedule for visits, tests, and immunizations, as well as information on cancer relapse, second cancers, dental caries, hypothyroidism, and infertility. None of the CPGs for the follow-up of patients with HD are based on clinical trials. Such trials are needed to test the worth of high-intensity
versus low-intensity strategies, assess quality of life prospectively, and measure the effectiveness of various follow-up measures.
Summary
There are many late effects associated with the treatment of breast cancer, prostate cancer, colorectal cancer, and Hodgkin’s disease. CPGs exist for all of these sites, but they are incomplete and do not cover most of the essential elements of survivorship care. There have been relatively few population-based, longitudinal studies to accurately assess the prevalence of late effects among cancer survivors. Little is known regarding appropriate follow-up because few large clinical trials of specific strategies have been conducted, even for common cancers.
LIFESTYLE FOLLOWING CANCER TREATMENT
Cancer survivors are at increased risk for developing a second cancer and, depending on their treatment, may be at increased risk for cardiovascular disease, osteoporosis, and other chronic illnesses. If lifestyle behaviors that may have contributed to the onset of cancer, such as smoking and unhealthy diet, persist, they can continue to threaten survival and quality of life. Given this heightened level of risk, cancer survivors represent a large and important target population for health promotion interventions (Demark-Wahnefried et al., 2000; Blanchard et al., 2003a; Demark-Wahnefried et al., 2005; Ganz, 2005). After a diagnosis of cancer, individuals are often motivated to change their diet, exercise habits, and other lifestyles (Satia et al., 2004). Many are also interested in learning more about dietary supplements and nutritional complementary therapies to manage persistent symptoms of disease or treatment. This section of the chapter reviews evidence on some common issues of interest to cancer survivors regarding smoking cessation, physical activity, diet and nutrition, and the use of complementary and alternative medicine.
Smoking Cessation
Nearly one-third of cancers are caused by smoking. Declines in smoking prevalence in the United States have reduced deaths from lung and other respiratory cancers. Many cancer patients and survivors, however, continue to smoke after their diagnosis and providers may not encourage smoking cessation because they believe it is “too late,” “it doesn’t matter,” or “it is too difficult” for their patients to quit (Dresler, 2003). However, smoking cessation has benefits even after cancer has developed. Effective behavioral therapy and pharmacotherapy are available to help smokers quit (Cox et
TABLE 3-12 Prevalence of Smoking by Self-Reported History of Cancer, by Age, United States, 1999–2000
|
Self-Reported History of Cancer and Age |
Smoking Status |
||
|
Current |
Former |
Never |
|
|
History of Cancer |
|||
|
All ages |
20 |
38 |
42 |
|
18-44 |
41 |
17 |
43 |
|
45-64 |
24 |
38 |
38 |
|
65+ |
9 |
46 |
45 |
|
No History of Cancer |
|||
|
All ages |
24 |
22 |
54 |
|
18-44 |
27 |
13 |
60 |
|
45-64 |
24 |
30 |
46 |
|
65+ |
11 |
39 |
50 |
|
SOURCE: Hewitt et al. (2003). |
|||
al., 2003; McBride and Ostroff, 2003). Guidelines of the U.S. Preventive Services Task Force recommend that clinicians screen all adults for tobacco use and provide tobacco cessation interventions for those who use tobacco products (USPSTF, 2003). The committee believes that oncology providers’ encounters with cancer patients represent “teachable moments,” and a failure to routinely assess smoking status and provide smoking cessation counseling is a lost opportunity. According to two large surveys of cancer survivors, roughly 65 to 70 percent of individuals who reported that they smoked said their physician recommended they quit smoking (Demark-Wahnefried et al., 2000; Blanchard et al., 2003a). Evidence that physician smoking cessation advice is not provided routinely at each visit comes from national surveys of the content of ambulatory care (i.e., non-hospitalized) visits. Physicians are providing smoking cessation counseling for fewer than one in five cancer-related ambulatory care visits made by patients who use tobacco, according to national surveys of ambulatory care providers (see Chapter 4, Table 4-1).
The problem of smoking among cancer survivors appears to be substantial. As many as 20 percent of cancer survivors report that they currently smoke, a rate only slightly lower than the rate among individuals without a history of cancer (Table 3-12). Smoking rates are alarmingly high among young cancer survivors (ages 18 to 44), substantially higher than among their counterparts without a cancer history (41 versus 27 percent)
|
BOX 3-14 Brief tobacco cessation counseling interventions, including screening, brief counseling (3 minutes or less), and/or pharmacotherapy, have been proven to increase tobacco abstinence rates, although there is a dose-response relationship between quit rates and the intensity of counseling. Effective interventions may be delivered by a variety of primary care clinicians. The “5-A” behavioral counseling framework provides a useful strategy for engaging patients in smoking cessation discussions:
Helpful aspects of counseling include providing problem-solving guidance for smokers to develop a plan to quit and to overcome common barriers to quitting, and providing social support within and outside of treatment. Common practices that complement this framework include motivational interviewing, the “5 R’s” used to treat tobacco use (relevance, risks, rewards, roadblocks, repetition), assessing readiness to change, and more intensive counseling and/or referrals for quitters needing extra help. Telephone “quit lines” have also been found to be an effective adjunct to counseling or medical therapy. SOURCE: Agency for Healthcare Policy and Research (USPSTF, 2003). |
(Table 3-12). Many (38 percent) cancer survivors are former smokers and so are at considerable risk for relapse of their smoking habit.
Persistent smoking following diagnosis contributes to poor long-term outcomes (Dresler, 2003). Cessation of cigarette smoking has been associated with a reduction in treatment complications, improved survival, and a decrease in risk for second cancers (Dresler, 2003; Cox et al., 2003; Garces and Hays, 2003; McBride and Ostroff, 2003). Benefits of smoking cessation following a diagnosis of cancer also include reductions in the risk for cardiovascular and pulmonary disease.
Guidance on how to provide smoking cessation counseling is available and has been shown to be effective, in combination with pharmacotherapy, to help smokers quit (Box 3-14) (Carter et al., 2001). Smoking cessation interventions that have been evaluated in cancer patient populations have generally been associated with relatively high rates of cessation in the short term. However, relapse rates are high, suggesting that sustained and/or
repeated cessation efforts are needed (Pinto et al., 2000, 2002; Cox et al., 2003; McBride and Ostroff, 2003). The studies to date have generally been limited to hospitalized cancer patients and have been of insufficient size to detect significant effects of interventions. The results of a recent clinical trial to test physician-initiated smoking cessation interventions in oncology settings are discouraging (Schnoll et al., 2003). According to this trial, training physicians to provide smoking cessation treatment to cancer patients enhanced physician adherence to clinical practice guidelines, but the physician interventions failed to yield significant gains in long-term quit rates among cancer patients.
Barriers to smoking cessation among cancer patients can include a strong nicotine dependence because of a long history of heavy tobacco use, fatalistic beliefs, psychological distress, and social influences (McBride and Ostroff, 2003). Building smoking cessation counseling into important cancer transitions has been suggested as a way to promote smoking cessation. Teachable moments for smoking cessation counseling and relapse prevention include the time of diagnosis, time of active treatment, and time of transition from inpatient to outpatient care and follow-up visits. In each of these clinical settings, involvement of family members is important given the likelihood that smoking is common among the family members of cancer patients (McBride and Ostroff, 2003).
The provision and acceptance of smoking prevention services are enabled when they are covered by insurance. However, smoking cessation counseling and pharmacotherapies are not consistently covered as paid services by Medicaid, health insurance plans, and managed care organizations (IOM, 2003). Medicare has recently added coverage of smoking and other tobacco use cessation services for certain beneficiaries (CMS, 2005). Coverage of cessation services is limited to beneficiaries who have an illness caused or complicated by tobacco use and to those who take any of the many medications whose effectiveness is complicated by tobacco use (e.g., agents to treat hypertension, thrombosis, and depression, as well as insulin to treat diabetes).
Research is needed to identify specific strategies for smoking cessation that are tailored to the specific needs of cancer survivors. How smoking cessation effects risks of recurrence and quality of life and the effectiveness of family-oriented interventions are issues that have not been extensively explored, but are worthy of future research (Cox et al., 2003).
Physical Activity
Many cancer patients reduce their levels of activity during treatment and do not resume activity at their prediagnosis levels (Irwin et al., 2003;
Blanchard et al., 2003a). The effectiveness of behavioral interventions to modify physical activity behaviors among cancer survivors was the subject of a 2004 Agency for Healthcare Research and Quality (AHRQ) evidence report (Holtzman et al., 2004).37 According to this review, controlled trials of behavioral interventions to increase physical activity among cancer survivors show positive and consistent effects of physical activity on the following outcomes:
-
Vigor and vitality
-
Cardiorespiratory fitness
-
Quality of life
-
Depression
-
Anxiety
-
Fatigue/tiredness
Similar findings come from a recent systematic review of randomized controlled clinical trials (Knols et al., 2005; Pinto et al., 2005). The exercise prescription associated with these positive outcomes in cancer survivors was generally moderate- to vigorous-intensity aerobic activity on 3 or more days per week, for 10 to 60 minutes per session. The findings for many of these outcomes parallel the results in generally healthy populations. The effect of physical activity on cancer recurrence or survival is unknown, but physical activity might improve prognosis through beneficial effects on cardiovascular disease (McTiernan, 2004) or through hormonal mechanisms (Holmes et al., 2005). Resistance training has beneficial effects on muscle and bone and may counteract some of the side effects of cancer treatment (e.g., bone and muscle loss) and help improve survivors’ physical function and quality of life (Galvao and Newton, 2005).
For physical activity to be recommended for cancer survivors, it must be safe and not associated with adverse outcomes. The results of the studies reviewed by AHRQ generally indicate that it is safe for cancer survivors to be physically active. Questions about the safety of physical activity remain, however. For example, one concern is that exercise by breast cancer survivors could induce or exacerbate lymphedema. Most studies have reported no adverse effects of upper body exercise on breast cancer survivors at risk for lymphedema. However, current clinical guidelines from multiple sources
(NCI, ACS, National Lymphedema Network, Susan G. Komen Foundation) include recommendations to breast cancer survivors to avoid ever lifting anything heavier than 5 to 15 pounds. This recommendation has negative health promotion and quality of life implications. According to the AHRQ review, “There is too little research on this topic thus far to appropriately and safely prescribe physical activity for breast cancer survivors at risk for (or with a diagnosis of) lymphedema.” Further research on this topic is needed to guide the more than 2 million American breast cancer survivors.
There is an additional concern that too-vigorous physical activity could depress the immune system and promote the spread of cancer. In generally healthy adults, moderate-intensity physical activity is associated with overall improvement in immune parameters, while high-intensity, high-volume physical activity is associated with a temporary worsening of immune function. According to the AHRQ review, additional studies are needed to clarify the effects on certain immune parameters, with specificity as to timing across the cancer experience as well as physical activity mode, frequency, intensity, and duration (Holtzman et al., 2004).
There is limited evidence regarding the extent to which physicians are providing guidance regarding exercise to their patients who are cancer survivors. According to two relatively large surveys, 20 to 35 percent of cancer survivors reported that their physician recommended changes in their exercise behavior. One study included a sample of cancer survivors with several types of cancer (Blanchard et al., 2003a) while the other study was limited to survivors of breast and prostate cancer (Demark-Wahnefried et al., 2000). An oncologist’s recommendation to exercise may increase exercise behavior, according to a randomized trial that involved breast cancer survivors (Jones et al., 2004). One study suggests that cancer survivors prefer that their oncologist initiate a discussion about exercise (Jones and Courneya, 2002).
A framework for examining physical activity across the cancer experience (Framework PEACE) has been proposed based on the cancer control perspective (Courneya and Friedenreich, 2001). The framework includes six possible cancer control outcomes after the point of cancer diagnosis, including buffering prior to treatment (i.e., building up physical condition before treatment), coping during treatment, rehabilitation immediately after treatment, health promotion and survival for those with positive treatment outcomes, and palliation for those without positive treatment outcomes. The AHRQ review concludes that additional research is needed on the effects of physical activity on pretreatment outcomes, health promotion, survival, and palliation.
Nutrition and Diet
A limited but growing body of evidence shows that nutritional interventions for cancer survivors reduce the risk of recurrence (Chlebowski et al., 2005). It is therefore reasonable to recommend that cancer survivors follow dietary guidelines established for primary prevention of cancer as well as other diseases (e.g., cardiovascular disease, osteoporosis, and diabetes). Cancer survivors can obtain information and guidance on nutrition and diet from the ACS and the American Institute for Cancer Research (AICR) (Brown et al., 2003; AICR, 2004; ACS, 2004a). In general, these guidelines for cancer survivors are similar to general recommendations for the primary prevention of cancer. The rationale for this guidance for cancer survivors is that the same factors that increase cancer incidence might also be important in promoting cancer recurrence after treatment. Data are most compelling for breast cancer, where the risk of recurrence might be increased by obesity and perhaps by diets high in fat and low in fruits and vegetables (Holmes and Kroenke, 2004; Chlebowski et al., 2005).38 Prostate cancer recurrence might also be increased by a high saturated fat intake, with increased intakes of meat and dairy products associated with more aggressive cancers (Brown et al., 2003). Adherence to these dietary guidelines may also be the most effective method for preventing the growth of second primary cancers and to improve overall health. AICR’s dietary recommendations for cancer survivors are shown in Box 3-15.
Most cancer survivors make at least some dietary changes following their diagnosis. In one survey of a general survivorship population, 51 percent of survivors said they had reduced their fat intake, 44 percent increased their fiber intake, and 43 percent reduced their red meat intake. More than one-quarter (28 percent) indicated their physician recommended that they reduce their fat intake, and 15 percent reported that their physician suggested they increase their fiber intake (Blanchard et al., 2003a). Findings from a survey of breast and prostate cancer survivors were similar, with 29 percent reporting that their doctor recommended that they reduce fat intake and 16 percent reporting a recommendation to increase their fruit and vegetable intake (Demark-Wahnefried et al., 2000).
Healthy Weight
There is convincing evidence that obesity is associated with an increased risk of several cancers, including cancers of the colon, breast, and
|
BOX 3-15
SOURCE: AICR (2004). |
endometrium (IOM, 2003). In some cases, being overweight has been shown to reduce survival. Overweight and obese women with breast cancer, for example, have poorer survival compared with thinner women (Kroenke et al., 2005; Chlebowski, 2005a). Diminished survival among obese women with breast cancer may be caused by higher concentrations of tumor-promoting hormones found in association with higher degrees of adiposity (McTiernan et al., 2003). Obesity also has been found to be a poor prognostic factor for prostate cancer (Freedland et al., 2004; Amling et al., 2004). To date, relatively little research on interventions to help cancer survivors lose weight has been conducted, and much of it has been confined to survivors of breast cancer (Djuric et al., 2002; Jenkins et al., 2003; Jen et al., 2004). Interventions to improve self-confidence may be needed because some research suggests that low self-esteem among overweight and obese breast cancer survivors interferes with their ability to adopt healthy lifestyles (Pinto et al., 2002). As in healthy populations, exercise also has been found to play a major role in weight management of cancer survivors (Goodwin et al., 1998).
Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) is a group of diverse medical and health care systems, therapies, and products that are not cur-
rently considered to be part of conventional medicine (NCCAM, 2002). The use of CAM is very common among the general population. In 2002 an estimated 62 percent of adults used some form of CAM therapy during the past year when the definition of CAM therapy included prayer specifically for health reasons (Barnes et al., 2004). When prayer was excluded from the definition, 36 percent of adults used some form of CAM therapy during the past year.
Individuals with cancer frequently use CAM products with the belief that their use will arrest their disease, alleviate symptoms, promote well-being, and increase their sense of control over their health (Burstein, 2000; Richardson et al., 2000; Antman et al., 2001). Common categories of CAM therapies used by cancer survivors include dietary modification and supplementation, herbal products and other biological agents, acupuncture, massage, exercise, and psychological and mind-body therapies (Weiger et al., 2002). In their review of the effectiveness and safety of such products, Weiger and colleagues found several CAM therapies that offer potential benefits for patients with cancer. For CAM therapies intended for palliation of symptoms associated with cancer or side effects of conventional treatment, the authors advised physicians to consider recommending and monitoring massage for anxiety or pain, moderate exercise, and psychological and mind-body therapies (e.g., support groups, relaxation training, imagery). Other CAM therapies, however, may be ineffective, and many present risks to cancer survivors (e.g., phytoestrogens for breast cancer survivors taking tamoxifen). The authors recommend that physicians communicate openly with patients about CAM use. Recent studies suggest, however, that many cancer patients do not discuss their use of CAM with their physicians (Lee et al., 2000; Navo et al., 2004). Such discussions are especially important given the association in some studies between the use of CAM and greater psychosocial distress (Burstein et al., 1999; DiGianni et al., 2002).
Cancer survivors can obtain comprehensive information about CAM from the American Cancer Society’s Guide to Complementary and Alternative Cancer Methods (ACS, 2000) and from the NCI’s Office of Cancer Complementary and Alternative Medicine (NCI and NCCAM, 2004).
Summary
Clinical encounters with cancer survivors provide “teachable moments” for health prevention and promotion (Demark-Wahnefried et al., 2005; Ganz, 2005). The adoption of healthy lifestyle behaviors provides an opportunity for cancer survivors to assume control of some aspects of their health and improve outcomes from cancer and other chronic illnesses. There are opportunities to intervene to help cancer survivors quit smoking, exer-
cise, and adopt healthy diets. As many as 20 percent of cancer survivors smoke, and evidence suggests that not all are receiving assistance with smoking cessation during routine clinical visits. Moderate exercise has many benefits for cancer survivors, including improved vigor and vitality, cardiorespiratory fitness, quality of life, and mental health. Questions remain regarding the safety of exercise for some cancer survivors, for example, breast cancer survivors with, or at risk for, lymphedema, but for most cancer survivors, moderate exercise is beneficial. A healthy diet low in saturated fat and rich in fruits and vegetables is recommended for the general public to prevent cancer, but also for cancer survivors to reduce their risk for subsequent cancer. Data are limited, but physicians do not appear to be routinely counseling cancer survivors regarding diet and nutrition. Obesity is a risk factor for several cancers, and researchers are beginning to test interventions to help overweight and obese cancer survivors lose weight.
CAM interventions are used frequently by cancer survivors and, when tested, some CAM interventions have been shown to be beneficial. Among CAM therapies that can be recommended for cancer survivors are massage for anxiety or pain, moderate exercise, and psychological and mind-body therapies (e.g., support groups, relaxation training, imagery). Cancer survivors are sometimes reluctant to discuss CAM therapies with their providers. It is recommended that physicians openly discuss these therapies because some have been shown to be harmful, to interfere with cancer treatment, or to be ineffective.
FINDINGS AND RECOMMENDATIONS
Cancer survivorship, as defined in this report, is a distinct phase of the cancer trajectory, but has been relatively neglected in advocacy, education, clinical practice, and research. Raising awareness of the medical and psychosocial needs that may follow cancer treatment will help both survivors and their health care providers to ensure that appropriate assessments are completed and available interventions employed. The constellation of cancer’s long-term and late effects varies by cancer type, treatment modality, and individual characteristics, but there are common patterns of symptoms and conditions that must be recognized so that health and well-being can be improved.
Recommendation 1: Health care providers, patient advocates, and other stakeholders should work to raise awareness of the needs of cancer survivors, establish cancer survivorship as a distinct phase of cancer care, and act to ensure the delivery of appropriate survivorship care.
Cancer patients and their advocates can call attention to their survivor-
ship experiences and the need for change. The leadership of organizations representing physicians, nurses, and psychosocial care providers can collaborate to improve care. Third-party payors of health care and health plans can improve access to needed services through reimbursement policies and improvements in systems of care. Employers can ensure fair workplace policies and accommodations. Sponsors of research can improve the opportunities to increase what we know about survivorship and appropriate care. Congress and state legislatures can enact policies and ensure the support needed to improve survivorship care and quality of life.
Providing a Care Plan for Survivorship
The recognition of cancer survivorship as a distinct phase of the cancer trajectory is not enough. A strategy is needed for the ongoing clinical care of cancer survivors. There are many opportunities for improving care—psychosocial distress can be assessed and support provided; cancer recurrences and second cancers may be caught early and treated; bothersome symptoms can be effectively managed; preventable conditions such as osteoporosis may be avoided; and potentially lethal late effects such as heart failure averted.
Recommendation 2: Patients completing primary treatment should be provided with a comprehensive care summary and follow-up plan that is clearly and effectively explained. This “Survivorship Care Plan” should be written by the principal provider(s) that coordinated oncology treatment. This service should be reimbursed by third-party payors of health care.
Such a care plan would summarize critical information needed for the survivor’s long-term care:
-
Cancer type, treatments received, and their potential consequences;
-
Specific information about the timing and content of recommended follow-up;
-
Recommendations regarding preventive practices and how to maintain health and well-being;
-
Information on legal protections regarding employment and access to health insurance; and
-
The availability of psychosocial services in the community.
These content areas, adapted from those recommended by the President’s Cancer Panel (President’s Cancer Panel, 2004), are elaborated upon in Box 3-16.
The content of the survivorship care plan could be reviewed with a
|
BOX 3-16 Upon discharge from cancer treatment, including treatment of recurrences, every patient should be given a record of all care received and important disease characteristics. This should include, at a minimum:
Upon discharge from cancer treatment, every patient and his/her primary health care provider should receive a written follow-up care plan incorporating available evidence-based standards of care. This should include, at a minimum:
|
patient during a formal discharge consultation. Clinicians would likely have discussed some aspects of the survivorship care plan before or during treatment, for example, short- and long-term treatment effects and their implications for work and quality of life.39 However, during acute treatment, much time is spent dealing with the acute toxicities of treatment that little emphasis is given to the post-treatment care plan. A substantial amount of information needs to be communicated during this consultation and then documented in an end-of-treatment consultation note. Examples of such consultation notes are provided in Appendix 3A of this chapter. Appropri-
SOURCE: Adapted from the President’s Cancer Panel (2004). |
ate reimbursement should be provided for such a visit, given the complexity and importance of the consultation.
The member of the oncology treating team who would be responsible for this visit could vary depending on the exact course of treatment. The responsibility could be assigned either to the oncology specialist coordinating care or to the provider responsible for the last component of treatment. Oncology nurses could play a key role. The survivorship care plan may need revision as new knowledge concerning late effects and interventions to ameliorate them, genetic disorders, and surveillance methods is identified. Cancer survivors can help to ensure that the plan is followed. The consultation at the conclusion of primary treatment could serve as a teaching event for survivors and their family members and provide opportunities to discuss with clinicians their prognosis, concerns, lifestyle issues, and follow-up schedules. The plan could be used by survivors subsequently to raise questions with doctors and prompt appropriate care during follow-up visits.
Agencies that accredit health plans and other providers could build compliance with the recommended consultation into their evaluation criteria (see discussion of quality measures in chapter 4). With 61 percent of cancer survivors aged 65 and older, the Medicare program could play a key role in ensuring that the survivorship care plan is written, communicated, and reimbursed. A formal assessment of survivorship care planning should be undertaken to assess its value.
Survivorship care plans have been recommended by the President’s Cancer Panel and by the IOM committee, however, the implementation of such plans has not yet been formally evaluated. Despite the lack of evidence to support the use of survivorship care plans, the committee concluded that some elements of care simply make sense—that is, they have strong face validity and can reasonably be assumed to improve care unless and until evidence accumulates to the contrary. Having an agreed-upon care plan that outlines goals of care falls into this “common sense” area. Health services research should be undertaken to assess the impact and costs associated with survivorship care plans, and to evaluate their acceptance by both cancer survivors and health care providers.
Developing Clinical Practice Guidelines for Survivorship Care
The “Survivorship Care Plan” would inform clinicians involved in the subsequent care of cancer survivors about treatment exposures, signs and symptoms of late effects, and, in some cases, would provide concrete steps to be taken. To carry out this plan, an organized set of clinical practice guidelines based on the best available evidence is needed to help ensure appropriate follow-up care. Guidelines should be derived by a formal process and, depending on the predominant methodology used to develop them, CPGs may be characterized as evidence based or consensus derived (Woolf, 1992). Because the goal is to assist in clinical decision making, the guideline should reflect the major clinical decisions that must be made as the disease entity is managed (Winn and Botnick, 1997). Furthermore, the interventions recommended in a CPG must be appropriate, that is, the expected benefits must outweigh the expected risks and harms by a sufficient amount to make the intervention worthwhile (Park et al., 1986).
Unfortunately, the status of cancer-related guidelines falls far short of these ideals. Deficiencies exist both in the availability and content of the guidelines. Relatively few cancer-related CPGs are available to clinicians, and of those that are available, most focus on the most common cancers (Smith and Hillner, 2001). Many of the tumor-specific guidelines are limited to one phase of the care trajectory (e.g., screening, primary treatment, therapy with limited chance for cure), or are modality oriented and address
issues related to a particular oncologic intervention (e.g., surgery, radiotherapy, adjuvant therapy).
Evidence-based guidelines would provide specific information on how to manage the complex issues facing survivors of adult cancers. Assessment tools and screening instruments for common late effects are also needed to help identify cancer survivors who have, or who are at high risk for, late effects and who may need extra surveillance or interventions.
Recommendation 3: Health care providers should use systematically developed evidence-based clinical practice guidelines, assessment tools, and screening instruments to help identify and manage late effects of cancer and its treatment. Existing guidelines should be refined and new evidence-based guidelines should be developed through public- and private-sector efforts.
Cancer survivors represent a very large at-risk population, and without evidence-based clinical practice guidelines, health care providers will vary widely in their practices, leading to inefficiencies in care delivery. Evidence suggests that some tests are being overused in the context of routine surveillance care after cancer treatment (Elston Lafata et al., 2005). The critical need for more rational, consistent, and efficient cancer follow-up practices has been widely recognized (Johnson and Virgo, 1997; Schwartz et al., 2000). As a nation, we have not invested in the research on cancer survivors on which such clinical practice guidelines would be based. Without high-quality evidence on the benefits, harms, and relative cost-effectiveness of follow-up strategies, cancer survivors face the health and financial hazards of overuse, underuse, and misuse of resources. The adoption of evidence-based guidelines has the potential to reduce this variation, improve patient outcomes, and reduce health care costs. Health services research is needed to evaluate the impact of such guidelines in the context of survivorship care.
The most comprehensive CPGs included in the committee’s review were created under the auspices of regional or national health policy organizations (e.g., Australia; British Columbia, Canada; Scotland). Similar support from appropriate bodies in the United States would facilitate guideline development. Public and private support of studies to generate evidence for guideline development is needed. The Centers for Medicare and Medicaid Services is the primary payor of care for cancer survivors and therefore have a stake in developing clinical practice guidelines. The Agency for Healthcare Research and Quality maintains a National Guideline Clearinghouse and supports Evidence-Based Practice Centers that review relevant scientific literature on clinical, behavioral, organizational, and financial topics to produce evidence reports and technology assessments (AHRQ, 2004a,b). Such reviews can form the foundation of evidence-based guidelines. Profes-
sional organizations (e.g., those representing oncology, primary care, nursing) also have a role to play in developing interdisciplinary guidelines. Achieving consensus on CPGs across medical specialties and provider groups is essential in promoting conformance to CPGs. The guideline development process is a costly one, and public and private support is needed to improve and expedite the development process. The development of guidelines is currently impeded by the lack of good evidence to support most surveillance strategies.
A model for guideline development can be found in the efforts of the Children’s Oncology Group (COG). COG has developed systematic guidelines for long-term follow-up of survivors of childhood, adolescent, and young adult cancers and has made them widely available through the internet (Children’s Oncology Group, 2005; Landier et al., 2004). A complementary set of patient educational materials has also been developed to broaden the application of the guidelines.
Rigorously developed evidence-based guidelines can minimize the potential harms of surveillance (e.g., morbidity and mortality associated with the follow-up of false-positive screening tests) (Woolf et al., 1999; Woolf, 2000). They are, however, only one option for improving the quality of care. On a practical level, it is difficult for providers to obtain reimbursement from insurance companies for needed surveillance (e.g., cardiac and pulmonary function testing) without evidence-based CPGs. Balancing this is the likelihood that some testing strategies will be found to be excessively intensive; savings are likely to result from discontinuing ineffective tests and procedures.
Cancer treatments are constantly evolving and consequently, what is known about today’s cohort of cancer survivors may not be relevant to those benefiting from new therapies. Newer therapies hold the promise of limiting the late effects of cancer, but mechanisms to monitor long-term effects need to put in place. The science on which clinical decisions must be based is far from perfect. Compared to the number of studies on the effectiveness of cancer therapies, relatively few have addressed late effects and the value of cancer follow-up policies. A greater investment in research is needed to learn more about late effects and their management. Mechanisms are also needed to communicate new research findings of relevance to cancer survivors and their providers.
APPENDIX 3A
EXAMPLES OF END-OF-TREATMENT CONSULTATION NOTES
Example of an End-of-Treatment Consultation Note: Breast Cancer
Date of note: April 12, 2005
Name: Jane Doe Age: 39
Date of tissue diagnosis of cancer: August 4, 2004
Diagnosis: Breast cancer
Stage of cancer: T1N1M0 Stage II
Pathologic findings: 1.5 cm. infiltrating ductal cancer in the left breast, moderately differentiated, ER positive, PR negative, Her2Neu negative; 3 of 10 nodes positive for metastatic cancer
Initial treatment plan:
-
Surgery: Lumpectomy and axillary dissection
-
Radiation therapy: 6 weeks of radiation therapy to the left breast
-
Chemotherapy: 4 cycles of AC followed by Taxol; dose-dense regimen
Treatment received (specify dates, location, and providers):
Surgery performed as planned by Dr. David Smith at Happy Valley Hospital on 8/23/04.
Chemotherapy administered by Dr. Mary Scott at Westside Oncology Center from 9/15/04 to 2/1/05. Patient received full dose as specified in published protocol Citron et al., JCO, 2003, CALGB 9751 trial, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 q 2 weeks × 4 cycles followed by paclitaxel 175 mg/m2 q 2 weeks × 4 cycles. Total dose of doxorubicin was 240 mg/m2.
Radiation therapy was given to the left breast by Dr. Mark Schwartz at Happy Valley Hospital from 2/15/05 to 4/6/05.
Dr. Scott initiated therapy with tamoxifen on 4/12/05.
Unusual or unexpected toxicities during treatment:
There were some treatment delays due to neutropenia and patient required blood transfusions on two occasions.
Expected short- and long-term effects of treatment:
Patient has some fatigue and alopecia at this time, but these are likely to recover over the next 3–6 months. The patient became amenorrheic after the first two cycles of chemotherapy and has severe hot flashes at this time
that may worsen on tamoxifen. She may well have resumption of menses and should use some form of barrier contraception at this time as she may still ovulate. If hot flashes persist, then she may want to consider one of several non-estrogen therapies, as described in the March 21, 2005, NIH State of the Science conference on management of menopausal symptoms. This patient also requires a baseline bone density with follow-up every 2 years to assess for premature osteoporosis.
Late toxicity monitoring needed:
The dose of radiation received is unlikely to cause much risk for hypothyroidism, but periodic evaluation should be considered. Patient needs to be reminded of lymphedema precautions re: trauma and infection.
She will need regular pelvic examinations to monitor for tamoxifen effects and second malignancies.
Surveillance needed for potential recurrence of cancer:
Needs annual mammograms and breast examinations every 6 months forever. No recommendations for radiological studies or blood tests except to monitor for potential tamoxifen toxicity with annual CBC and chemistry panel.
Surveillance needed for second malignancies:
This patient has a strong family history of breast and ovarian cancer. Given her young age, she may benefit from consideration of genetic testing for BRCA1/2, as well as preventive oophorectomy.
Physicians responsible for monitoring of toxicity, recurrence, second malignancies:
Dr. Scott will see patient every 3–4 months for the next 2 years, and then every 6 months to monitor for tamoxifen therapy and local recurrence of breast cancer.
Dr. Ian Chen, the patient’s family physician, will monitor patient with pelvic examination and bone density as well as routine health maintenance issues (contraception, hot flashes); he will arrange for referral for genetic counseling.
Identified psychosocial issues or concerns:
The patient is very concerned about her potential loss of fertility and possible risk for permanent menopause. She is attending the support group at Happy Valley Hospital, but may need individual counseling, depending on whether or not her menses resume. Her husband is very supportive, but he is also concerned about this lost opportunity in their lives.
Recommended preventive behaviors, interventions, or genetic testing:
This patient already has a very health lifestyle and habits, but she is encouraged to avoid weight gain and to remain physically active. Genetic testing has been advised as noted earlier. Patient given NCI booklet, Life After Cancer Treatment, and the NCCS “Cancer Survival Toolbox: An Audio Resource Program” that address medical and psychosocial issues, including those related to health insurance and employment.
NOTE: All individual and hospital names are fictitious.
SOURCE: Patricia Ganz, committee member, 2005.
Example of an End-of-Treatment Consultation Note: Prostate Cancer
Date of note: April 20, 2005
Name: John Doe Age: 65
Date of tissue diagnosis of cancer: October 21, 2001
Diagnosis: Prostate cancer
Stage of cancer: Clinical T1c
Pathologic findings: pT2cN0M0, Gleason 4+4, 2.3 cm
Initial treatment plan:
-
Surgery: Yes
-
Radiation therapy: None
-
Chemotherapy: None
Treatment received (specify dates, location, and providers):
Radical prostatectomy with nerve sparing on December 1, 2001, Eastside Medical Center, Dr. Roger Smith
Unusual or unexpected toxicities during treatment:
None
Expected short- and long-term effects of treatment:
Mild urinary leakage for 3 weeks, now dry
Sexual dysfunction for 3 months, now potent with occasional sildenafil
Late toxicity monitoring needed:
None
Surveillance needed for potential recurrence of cancer:
Semi-annual PSA until 5 years post-op, then annual PSA; annual digital rectal exam
Surveillance needed for second malignancies:
None
Physicians responsible for monitoring of toxicity, recurrence, second malignancies:
Dr. Smith will be following Mr. Doe for recurrence and will also assess treatment side effects.
Identified psychosocial issues or concerns:
Short-term depression following surgery, resolved with counseling and support group. Assess psychosocial distress during follow-up visits.
Recommended preventive behaviors, interventions, or genetic testing:
Patient counseled regarding diet/exercise (avoidance of obesity). At follow-up visits assess sexual function and depression. Patient given NCI booklet, Life After Cancer Treatment, and the NCCS “Cancer Survival Toolbox: An Audio Resource Program” that address medical and psychosocial issues, including those related to health insurance and employment.
NOTE: All individual and hospital names are fictitious.
SOURCE: Mark Litwin, committee member, 2005.
Example of an End-of-Treatment Consultation Note: Colorectal Cancer
Date of note: April 18, 2005
Name: John Smith Age: 70
Date of tissue diagnosis of cancer: September 15, 2004
Diagnosis: Colon cancer
Stage of cancer: T3N2M0 (IIIB)
Pathologic findings: Moderately differentiated adenocarcinoma penetrating through the muscularis propria. No lymphovascular or perineural invasion. 5/13 regional lymph nodes positive for cancer.
Initial treatment plan:
-
Surgery: Left hemicolectomy 9/28/04
-
Radiation therapy: None
-
Chemotherapy: FOLFOX (5-FU 400 mg/m2 bolus followed by
1,200 mg/m2/d for 2 days, leucovorin 400 mg/m2, oxaliplatin 85 mg/m2) × 12 cycles
Treatment received (specify dates, location, and providers):
Received FOLFOX from 11/10/04 to 04/13/05 at Northside Cancer Institute under the supervision of Dr. Jane Marks.
Unusual or unexpected toxicities during treatment:
None
Expected short- and long-term effects of treatment:
Sixth cycle held 1 week for thrombocytopenia, requiring a dose reduction in oxaliplatin to 65 mg/m2. Experienced cold-induced paresthesias in the hands and feet, but no residual neuropathy.
Late toxicity monitoring needed:
None
Surveillance needed for potential recurrence of cancer:
Clinical assessments and bloodwork including CEA every 3 months for 2 years, every 4 months for 1 year, then every 6 months for 2 years. After 5 years, either follow up on an as-needed basis or every 1–2 years, depending on patient choice.
Surveillance needed for second malignancies:
Colonoscopy 1 year after hemicolectomy. Subsequent schedule to depend on the findings. If not polyps or other disease, repeat every 3 to 5 years.
Physicians responsible for monitoring of toxicity, recurrence, second malignancies:
Dr. Jane Marks
Identified psychosocial issues or concerns:
Normal anxiety. Has contact with social worker, David Jones, as needed.
Recommended preventive behaviors, interventions, or genetic testing:
None specific for this cancer. Routine medical care recommended. Patient counseled regarding diet/nutrition. Patient given NCI booklet, Life After Cancer Treatment, and the NCCS “Cancer Survival Toolbox: An Audio Resource Program” that address medical and psychosocial issues, including those related to health insurance and employment.
NOTE: All individual and hospital names are fictitious.
SOURCE: Craig Earle, committee member, 2005.
Example of an End-of-Treatment Consultation Note: Hodgkin’s Disease
Date of note: April 15, 2005
Name: Jane Smith Age: 28
Date of tissue diagnosis of cancer: November 15, 2004
Diagnosis: Hodgkin’s disease
Stage of cancer: Clinical stage IIA
Pathologic findings: Classical Hodgkin’s disease
Initial treatment plan:
-
Surgery: Biopsy, left supraclavicular lymph note
-
Radiation therapy: 30 Gy radiation, to modified mantle field (i.e., bilateral supraclavicular and mediastinal), as consolidation after chemotherapy
-
Chemotherapy: Stanford V chemotherapy for 12 weeks
Treatment received (specify dates, location, and providers):
Stanford V chemotherapy 12/1/04–3/2/05; full doses, on schedule; Dr. Kay, Eastern University Medical Center
Radiation therapy 3/15/05–4/15/05; Dr. Smith, Eastern University Medical Center
Unusual or unexpected toxicities during treatment:
None
Expected short- and long-term effects of treatment:
Short term—partial alopecia, hospitalization for fever with neutropenia, 2/15/05 to 2/17/05—given granulocyte colony-stimulating factor and red blood cell transfusion.
Late toxicity monitoring needed:
Thyroid function tests, annually—thyroid-stimulating hormone (TSH) and free T4.
Pulmonary function tests and echocardiograms are not customary or recommended to perform routinely. In particular, it is established that pulmonary function tests within 12 months of thoracic radiation may show mild abnormalities which improve over time.
Careful auscultation of the heart is recommended during follow-up, particularly for patients receiving chest irradiation and anthracycline chemotherapy.
Assessment of fertility—birth control pills for at least 2 years. Monitoring of menstrual functioning. Referral to GYN if requested.
Surveillance needed for potential recurrence of cancer:
History and physical examination every 3 months × 1 year; every 4 months × 1 year; every 6 months × 1 year;: annually thereafter. Appropriate laboratory and imaging studies if symptomatic.
Surveillance needed for second malignancies:
Annual mammograms, beginning 2010; breast self-exam, monthly; breast exam by all follow-up physicians (primary care provider, medical oncologist, radiation oncologist).
Skin assessment annually.
Thyroid exam annually.
Laboratory and imaging studies according to National Comprehensive Cancer Network guidelines for the follow-up of Hodgkin’s disease.
Physicians responsible for monitoring of toxicity, recurrence, second malignancies:
Primary care provider: Assess for general medical issues, weight, exercise, diet, annual influenza vaccination.
Medical oncologist: Assess for fertility, infections, cardiopulmonary function, surveillance imaging exams. Visits alternated with radiation oncologist. Radiation oncologist: Assess for thyroid function, second malignancy, surveillance imaging exams. Visits alternated with medical oncologist.
Identified psychosocial issues or concerns:
None identified, but needs evaluation and consultation as appropriate if specific issues arise. Patient has no children and desires them in the future. Although her therapy is not known to cause fertility problems, counseling was provided on fertility and reproduction.
Recommended preventive behaviors, interventions, or genetic testing:
Patient was counseled regarding diet and exercise for cardiovascular health, and avoidance of sun exposure to minimize risk of skin cancer. Annual flu vaccination is recommended. Recommend psychosocial assessment at follow-up. Patient given NCI booklet, Life After Cancer Treatment, and the NCCS “Cancer Survival Toolbox: An Audio Resource Program” that address medical and psychosocial issues, including those related to health insurance and employment.
NOTE: All individual and hospital names are fictitious.
SOURCE: Sarah Donaldson, committee member, 2005.
REFERENCES
AACE (American Association of Clinical Endocrinologists). 1999. AACE medical guidelines for clinical practice for management of menopause. Endocrine Practice 5(6):354–366.
ACS (American Cancer Society). 2000. Guide to Complementary and Alternative Cancer Methods. Atlanta, GA: ACS.
ACS. 2004a. Eating Well, Staying Well During and After Cancer. Atlanta, GA: ACS.
ACS. 2004b. Sexuality for Women and Their Partners. [Online]. Available: http://www.cancer.org/docroot/MIT/MIT_7_1x_SexualityforWomenandTheirPartners.asp [accessed September 30, 2004].
Ahles TA, Saykin AJ. 2002. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer 3(Suppl 3):S84–S90.
Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. 2003. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12(6):612–619.
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Titus-Ernstoff L, Skalla K, Bakitas M, Silberfarb PM. 2005. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol 23(19):4399-4405.
AHRQ (Agency for Healthcare Research and Quality). 1998. Invitation to Submit Guidelines to the National Guideline Clearinghouse. [Online]. Available: http://www.ahrq.gov/fund/ngcguidl.htm [accessed April 19, 2005].
AHRQ. 2002. Management of Cancer Symptoms: Pain, Depression, and Fatigue. Summary, Evidence Report/Technology Assessment: Number 61. AHRQ Publication No. 02-E031. [Online]. Available: http://www.ahrq.gov/clinic/epcsums/csympsum.htm [accessed December 21, 2004].
AHRQ. 2004a. Evidence-based Practice Centers. [Online]. Available: http://www.ahrq.gov/clinic/epc/ [accessed October 18, 2004].
AHRQ. 2004b. National Guideline Clearinghouse. [Online]. Available: http://www.guideline.gov [accessed December 6, 2004].
AICR (American Institute for Cancer Research). 2004. Nutrition Guidelines for Cancer Survivors After Treatment. [Online]. Available: http://www.aicr.org/information/survivor/guidelines.lasso [accessed December 8, 2004].
Aisner J, Wiernik PH, Pearl P. 1993. Pregnancy outcome in patients treated for Hodgkin’s disease. J Clin Oncol 11(3):507–512.
Amato P, Christophe S, Mellon PL. 2002. Estrogenic activity of herbs commonly used as remedies for menopausal symptoms. Menopause 9(2):145–150.
American Psychiatric Association. 1994. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association.
Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG. 2004. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 22(3):439–445.
Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE III. 2004. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol 22(17):3570–3580.
Anthony T, Simmang C, Hyman N, Buie D, Kim D, Cataldo P, Orsay C, Church J, Otchy D, Cohen J, Perry WB, Dunn G, Rafferty J, Ellis CN, Rakinic J, Fleshner P, Stahl T, Gregorcyk S, Ternent C, Kilkenny JW III, Whiteford M. 2004. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum 47(6):807–817.
Antman K, Benson MC, Chabot J, Cobrinik D, Grann VR, Jacobson JS, Joe AK, Katz AE, Kelly K, Neugut AI, Russo D, Tiersten A, Weinstein IB. 2001. Complementary and alternative medicine: The role of the cancer center. J Clin Oncol 19(18 Suppl):55S–60S.
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. 2003. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130.
Arterbery VE, Frazier A, Dalmia P, Siefer J, Lutz M, Porter A. 1997. Quality of life after permanent prostate implant. Semin Surg Oncol 13(6):461–464.
ASCO (American Society of Clinical Oncology). 2003. American Society of Clinical Oncology policy statement update: Genetic testing for cancer susceptibility. J Clin Oncol 21(12):2397–2406.
Ashing-Giwa K, Ganz PA, Petersen L. 1999. Quality of life of African-American and white long term breast carcinoma survivors. Cancer 85(2):418–426.
Avis N, Crawford S, Manuel J. 2005. Quality of life among younger women with breast cancer. J Clin Oncol 23(15):3322-3330.
Aziz NM, Rowland JH. 2003. Trends and advances in cancer survivorship research: Challenge and opportunity. Semin Radiat Oncol 13(3):248–266.
Bacon CG, Giovannucci E, Testa M, Glass TA, Kawachi I. 2002. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer 94(3):862–871.
Badger C, Preston N, Seers K, Mortimer P. 2004a. Benzo-pyrones for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev (2):CD003140.
Badger C, Preston N, Seers K, Mortimer P. 2004b. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev (4):CD003141.
Badger C, Seers K, Preston N, Mortimer P. 2004c. Antibiotics/anti-inflammatories for reducing acute inflammatory episodes in lymphoedema of the limbs. Cochrane Database Syst Rev (2):CD003143.
Baniel J, Israilov S, Segenreich E, Livne PM. 2001. Comparative evaluation of treatments for erectile dysfunction in patients with prostate cancer after radical retropubic prostatectomy. BJU Int 88(1):58–62.
Barnes PM, Powell-Griner E, McFann K, Nahin RL. 2004. Complementary and alternative medicine use among adults: United States, 2002. Adv Data (343):1–19.
Bast RC Jr, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG, Somerfield MR. 2001. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 19(6):1865–1878.
Baxter NN, Tepper JE, Durham SB, Rothenberger DA, Virnig BA. 2005. Increased risk of rectal cancer after prostate radiation: A population-based study. Gastroenterology 128(4):819–824.
Benson AB III, Desch CE, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Smith TJ, Somerfield MR. 2000. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol 18(20):3586–3588.
Bethge W, Guggenberger D, Bamberg M, Kanz L, Bokemeyer C. 2000. Thyroid toxicity of treatment for Hodgkin’s disease. Ann Hematol 79(3):114–118.
Blamey RW. 2002. Guidelines on endocrine therapy of breast cancer EUSOMA. Eur J Cancer 38(5):615–634.
Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, Gesme DH, Reding D, Flynn T, Kennedy JS. 2003a. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav 27(3):246–256.
Blanchard DK, Donohue JH, Reynolds C, Grant CS. 2003b. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 138(5):482–487; discussion 487–488.
Blank TO. 2005. Gay men and prostate cancer: Invisible diversity. J Clin Oncol 23(12):2593–2596.
Bloom JR, Fobair P, Gritz E, Wellisch D, Spiegel D, Varghese A, Hoppe R. 1993. Psychosocial outcomes of cancer: A comparative analysis of Hodgkin’s disease and testicular cancer. J Clin Oncol 11(5):979–988.
Bloom JR, Stewart SL, Chang S, Banks PJ. 2004. Then and now: Quality of life of young breast cancer survivors. Psychooncology 13(3):147–160.
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. 2000. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol 18(4):743–753.
Bradbury BD, Lash TL, Kaye JA, Jick SS. 2004. Tamoxifen and cataracts: A null association. Breast Cancer Res Treat 87(2):189–196.
Bradbury BD, Lash TL, Kaye JA, Jick SS. 2005. Tamoxifen-treated breast carcinoma patients and the risk of acute myocardial infarction and newly-diagnosed angina. Cancer 103(6):1114–1121.
Brennan J. 2001. Adjustment to cancer—coping or personal transition? Psychooncology 10(1):1–18.
Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. 2000. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18(14):2695–2701.
British Columbia Cancer Agency. 2002a. Gastrointestinal—05 Colon. [Online]. Available: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Gastrointestinal/05.Colon/default.htm [accessed March 16, 2004].
British Columbia Cancer Agency. 2002b. Hodgkin’s Lymphoma. [Online]. Available: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Lymphoma/ HodgkinsDisease.htm [accessed March 16, 2004].
British Columbia Cancer Agency. 2004a. Breast. [Online]. Available: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Breast/Followup/default.htm [accessed March 16, 2004].
British Columbia Cancer Agency. 2004b. Genitourinary—Prostate. [Online]. Available: http://www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Genitourinary/Prostate/default.htm [accessed March 16, 2004].
British Columbia Guidelines and Protocols Advisory Committee. 2004. Follow-up of Patients after Curative Resection of Colorectal Cancer. [Online]. Available: http://www.healthservices.gov.bc.ca/msp/protoguides/gps/colorectal.pdf [accessed December 6, 2004].
Brown JK, Byers T, Doyle C, Courneya KS, Demark-Wahnefried W, Kushi LH, McTiernan A, Rock CL, Aziz N, Bloch AS, Eldridge B, Hamilton K, Katzin C, Koonce A, Main J, Mobley C, Morra ME, Pierce MS, Sawyer KA. 2003. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin 53(5):268–291.
Burstein HJ. 2000. Discussing complementary therapies with cancer patients: What should we be talking about? J Clin Oncol 18(13):2501–2504.
Burstein HJ, Winer EP. 2000. Primary care for survivors of breast cancer. N Engl J Med 343(15):1086–1094.
Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. 1999. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med 340(22):1733–1739.
Bushnell CD, Goldstein LB. 2004. Risk of ischemic stroke with tamoxifen treatment for breast cancer: A meta-analysis. Neurology 63(7):1230–1233.
Calvert PM, Frucht H. 2002. The genetics of colorectal cancer. Ann Intern Med 137(7):603–612.
Campos SM. 2004. Aromatase inhibitors for breast cancer in postmenopausal women. Oncologist 9(2):126–136.
Canales MK, Geller BM. 2003. Surviving breast cancer: The role of complementary therapies. Fam Community Health 26(1):11–24.
Cancer Care Ontario. 2005. Supportive Care Practice Guidelines. [Online]. Available: http://www.cancercare.on.ca/index_supportiveCareguidelines.htm [accessed May 9, 2005].
Carmichael AR, Bates T. 2004. Obesity and breast cancer: A review of the literature. Breast 13(2):85–92.
Carter CL, Key J, Marsh L, Graves K. 2001. Contemporary perspectives in tobacco cessation: What oncologists need to know. Oncologist 6(6):496–505.
Casso D, Buist DS, Taplin S. 2004. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes 2(1):25.
Cella DF. 1995. Methods and problems in measuring quality of life. Support Care Cancer 3(1):11–22.
Cella DF, Tross S. 1986. Psychological adjustment to survival from Hodgkin’s disease. J Consult Clin Psychol 54(5):616–622.
Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, Gass M, Leboff MS. 2005. Fracture risk among breast cancer survivors: Results from the Women’s Health Initiative Observational Study. Arch Intern Med 165(5):552–558.
Children’s Oncology Group. 2005. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. [Online] Available: http://www.survivorshipguidelines.org/ [accessed July 15, 2005).
Chlebowski RT. 2005a. Obesity and early-stage breast cancer. J Clin Oncol 23(7):1345–1347.
Chlebowski RT. 2005b. Bone health in women with early-stage breast cancer. Clin Breast Cancer 5(Suppl 2):S35–S40.
Chlebowski RT, Aiello E, McTiernan A. 2002a. Weight loss in breast cancer patient management. J Clin Oncol 20(4):1128–1143.
Chlebowski RT, Blackburn GL, Elashoff RE, Thomson C, Goodman MT, Shapiro A, Giuliano AE, Karanja N, Hoy MK, Nixon DW. 2005 (May 13–17). Dietary fat reduction in postmenopausal women with primary breast cancer: Phase III Women’s Intervention Nutrition Study (WINS). Presentation at the ASCO Annual Meeting, Orlando, FL. [Online]. Available: http://www.asco.org/ac/1,1003,_12-002643-00_18-0034-00_19-0031414,00.asp [accessed May 20, 2005].
Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG III, Burstein HJ, Eisen A, Lipkus I, Pfister DG. 2002b. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol 20(15):3328–3343.
Chlebowski RT, Kim JA, Col NF. 2003. Estrogen deficiency symptom management in breast cancer survivors in the changing context of menopausal hormone therapy. Semin Oncol 30(6):776–788.
Chun TY. 1997. Coincidence of bladder and prostate cancer. J Urol 157(1):65–67.
Church J, Simmang C. 2003. Practice parameters for the treatment of patients with dominantly inherited colorectal cancer (familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer). Dis Colon Rectum 46(8):1001–1012.
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. 2003. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21(8):1431–1439.
City of Hope Beckman Research Institute. 2004. Quality of Life. [Online]. Available: http://www.cityofhope.org/prc/pdf/cancer_survivor_QOL.pdf [accessed September 7, 2004].
Clark JA, Inui TS, Silliman RA, Bokhour BG, Krasnow SH, Robinson RA, Spaulding M, Talcott JA. 2003. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 21(20):3777–3784.
CMS (Centers for Medicare and Medicaid Services). 2005. Medicare Adds Coverage of Smoking and Other Tobacco Use Cessation Services. [Online]. Available: http://www.cms.hhs.gov/media/press/release.asp?Counter=1395 [accessed March 29, 2005].
Cooperberg MR, Broering JM, Litwin MS, Lubeck DP, Mehta SS, Henning JM, Carroll PR. 2004. The contemporary management of prostate cancer in the United States: Lessons from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), a national disease registry. J Urol 171(4):1393–1401.
Cordova MJ, Cunningham LL, Carlson CR, Andrykowski MA. 2001. Post-traumatic growth following breast cancer: A controlled comparison study. Health Psychol 20(3):176–185.
Courneya KS, Friedenreich CM. 2001. Framework PEACE: An organizational model for examining physical exercise across the cancer experience. Ann Behav Med 23(4):263–272.
Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. 2003a. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 12(4):347–357.
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. 2003b. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol 21(9):1660–1668.
Cox LS, Africano NL, Tercyak KP, Taylor KL. 2003. Nicotine dependence treatment for patients with cancer. Cancer 98(3):632–644.
Crandall C, Petersen L, Ganz PA, Greendale GA. 2004. Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11(5):519–530.
Cuzick J. 2005. Radiotherapy for breast cancer. J Natl Cancer Inst 97(6):406–407.
Day R, Ganz PA, Costantino JP. 2001. Tamoxifen and depression: More evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst 93(21):1615–1623.
Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. 1999. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol 17(9):2659–2669.
de Jong, N, Courtens AM, Abu-Saad HH, and Schouten HC. 2002. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nurs. 25(4):283–297; quiz 298–299.
Demark-Wahnefried W, Rock CL. 2003. Nutrition-related issues for the breast cancer survivor. Semin Oncol 30(6):789–798.
Demark-Wahnefried W, Aziz NM, Rowland JH, and Pinto BM. 2005. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol 23(24):1–17.
Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. 2000. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 88(3):674–684.
DiGianni LM, Garber JE, Winer EP. 2002. Complementary and alternative medicine use among women with breast cancer. J Clin Oncol 20(18 Suppl):34S–38S.
Dignam JJ, Mamounas EP. 2004. Obesity and breast cancer prognosis: An expanding body of evidence. Ann Oncol 15(6):850–851.
Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CK, Mood D, Bradley E, Hryniuk WM. 2002. Combining weight-loss counseling with the Weight Watchers plan for obese breast cancer survivors. Obes Res 10(7):657–665.
Donaldson SS, Hancock SL, Hoppe RT. 1999. The Janeway Lecture. Hodgkin’s disease—finding the balance between cure and late effects. Cancer J Sci Am 5(6):325–333.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A. 2004. Live birth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 364(9443):1405–1410.
Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, van Leeuwen FE, Holowaty EJ, Andersson M, Wiklund T, Joensuu T, van’t Veer MB, Stovall M, Gospodarowicz M, Travis LB. 2002. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J Clin Oncol 20(16):3484–3494.
Dorval M, Guay S, Mondor M, Mâsse B, Falardeau M, Robidoux A, Deschênes L, Maunsell E. 2005. Couples who get closer after breast cancer: Frequency and predictors in a prospective investigation. J Clin Oncol 23(15):3588-3596.
Dorval M, Maunsell E, Deschenes L, Brisson J. 1998. Type of mastectomy and quality of life for long-term breast carcinoma survivors. Cancer 83(10):2130–2138.
Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. 1996. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat 39(3):261–273.
Dresler CM. 2003. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer 39(2):119–124.
Dunlop MG. 2002. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 51(Suppl 5):V21–V27.
Early Breast Cancer Trialists’ Collaborative Group. 2004a. Multi-agent chemotherapy for early breast cancer (Cochrane Review). In: The Cochrane Library. Issue 3. Chichester, UK: John Wiley & Sons, Ltd.
Early Breast Cancer Trialists’ Collaborative Group. 2004b. Tamoxifen for early breast cancer (Cochrane Review). In: The Cochrane Library. Issue 3. Chichester, UK: John Wiley & Sons, Ltd.
Easton DF, Hopper JL, Thomas DC, Antoniou A, Pharoah PD, Whittemore AS, Haile RW. 2004. Breast cancer risks for BRCA1/2 carriers. Science 306(5705):2187–2191; author reply 2187–2191.
Elston Lafata J, Simpkins J, Schultz L, Chase GA, Johnson CC, Yood MU, Lamerato L, Nathanson D, Cooper G. 2005. Routine surveillance care after cancer treatment with curative intent. Med Care 43(6):592-599.
Emens LA, Davidson NE. 2003. The follow-up of breast cancer. Semin Oncol 30(3):338–348.
Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. 2001. Arm edema in breast cancer patients. J Natl Cancer Inst 93(2):96–111.
ESMO (European Society of Medical Oncology). 2003. Minimum Clinical Recommendations for Diagnosis, Adjuvant Treatment and Follow-up of Primary Breast Cancer. [Online]. Available: http://www.esmo.org/reference/referenceGuidelines/pdf/new_pdf/ESMO_01_primary_breast.pdf [accessed December 13, 2004].
Eton DT, Lepore SJ. 2002. Prostate cancer and health-related quality of life: A review of the literature. Psychooncology 11(4):307–326.
Eton DT, Lepore SJ, Helgeson VS. 2001. Early quality of life in patients with localized prostate carcinoma: An examination of treatment-related, demographic, and psychosocial factors. Cancer 92(6):1451–1459.
Evans HS, Moller H, Robinson D, Lewis CM, Bell CM, Hodgson SV. 2002. The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut 50(5):647–652.
Ewer MS, Lippman SM. 2005. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol 23(13):2900–2902.
FACS. 2005. FACS Trial. [Online]. Available: http://www.facs.soton.ac.uk/ [accessed April 19, 2005].
Fallowfield L, Fleissig A, Edwards R, West A, Powles TJ, Howell A, Cuzick J. 2001. Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized controlled trials. J Clin Oncol 19(7):1885–1892.
Ferrans CE. 2005. Definitions and conceptual models of quality of life. In: Outcomes Assessment in Cancer: Measures, Methods, and Applications. Cambridge, UK: Cambridge University Press. Pp. 14–30.
Ferrell BR. 2004 (October 27–29). Quality of Life Issues: Cancer Patients’ Perspectives. Presentation at the meeting of the IOM Committee on Cancer Survivorship Meeting, Irvine, CA.
Ferrell BR, Grant M, Funk B, Otis-Green S, Garcia N. 1997a. Quality of life in breast cancer. Part I: Physical and social well-being. Cancer Nurs 20(6):398–408.
Ferrell BR, Grant MM, Funk B, Otis-Green S, Garcia N. 1997b. Quality of life in breast cancer survivors as identified by focus groups. Psychooncology 6(1):13–23.
Ferrell BR, Grant M, Funk B, Otis-Green S, Garcia N. 1998. Quality of life in breast cancer. Part II: Psychological and spiritual well-being. Cancer Nurs 21(1):1–9.
Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C. 2003. Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer 3(1):26.
Finnish Medical Society Duodecim. 2001. Postoperative Follow-up of Colorectal Cancer. [Online]. Available: http://195.236.0.10/ltk-root/eng/htm/ebm/ebm00199.htm [accessed June 2, 2004].
Finnish Medical Society Duodecim. 2002. Carcinoma of the Prostate. [Online]. Available: http://195.236.0.10/ltk-root/eng/htm/ebm/ebm00247.htm [accessed June 2, 2004].
Fiorica J. 2004. Association of breast cancer and its therapy with menopause-related symptoms. Menopause 11(5):502–504.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. 2002. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233–1241.
Fisher B, Anderson S, DeCillis A, Dimitrov N, Atkins JN, Fehrenbacher L, Henry PH, Romond EH, Lanier KS, Davila E, Kardinal CG, Laufman L, Pierce HI, Abramson N, Keller AM, Hamm JT, Wickerham DL, Begovic M, Tan-Chiu E, Tian W, Wolmark N. 1999. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol 17(11):3374–3388.
Flechtner H, Bottomley A. 2003. Fatigue and quality of life: Lessons from the real world. Oncologist 8(Suppl 1):5–9.
Fobair P, Hoppe RT, Bloom J, Cox R, Varghese A, Spiegel D. 1986. Psychosocial problems among survivors of Hodgkin’s disease. J Clin Oncol 4(5):805–814.
Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M, et al. 1998. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 62(3):676–689.
Fransson P, Widmark A. 1999. Late side effects unchanged 4–8 years after radiotherapy for prostate carcinoma: A comparison with age-matched controls. Cancer 85(3):678–688.
Freedland SJ, Aronson WJ, Kane CJ, Presti JC Jr, Amling CL, Elashoff D, Terris MK. 2004. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol 22(3):446–453.
Friedlander M, Thewes B. 2003. Counting the costs of treatment: The reproductive and gynaecological consequences of adjuvant therapy in young women with breast cancer. Intern Med J 33(8):372–379.
Gallo-Silver L. 2000. The sexual rehabilitation of persons with cancer. Cancer Pract 8(1): 10–15.
Galvao DA, Newton RU. 2005. Review of exercise intervention studies in cancer patients. J Clin Oncol 23(4):899–909.
Ganz PA. 1998. Cognitive dysfunction following adjuvant treatment of breast cancer: A new dose-limiting toxic effect? J Natl Cancer Inst 90(3):182–183.
Ganz PA. 2000. Quality of life across the continuum of breast cancer care. Breast J 6(5):324–330.
Ganz PA. 2001a. Impact of tamoxifen adjuvant therapy on symptoms, functioning, and quality of life. J Natl Cancer Inst Monogr 30:130–134.
Ganz PA. 2001b. Menopause and breast cancer: Symptoms, late effects, and their management. Semin Oncol 28(3):274–283.
Ganz PA. 2002a. What outcomes matter to patients: A physician-researcher point of view. Med Care 40(6 Suppl):III11–19.
Ganz PA. 2002b. The price of anticancer intervention. Treatment-induced malignancy. Lancet Oncol 3(9):575–576.
Ganz PA. 2002c. Adult Cancer Survivors: Understanding the Late Effects of Cancer and Its Treatment. Paper commissioned for the National Cancer Policy Board. Unpublished.
Ganz PA. 2004 (July 27). Understanding the Late Effects of Cancer Treatment: Making the Case for Systematic Follow-up. Presentation at the meeting of the IOM Committee on Cancer Survivorship, Woods Hole, MA.
Ganz PA. 2005. A teachable moment for oncologists: Cancer survivors, 10 million strong and growing! J Clin Oncol 23(24):1–3.
Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. 1996. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat 38(2):183–199.
Ganz PA, Desmond KA, Belin TR, Meyerowitz BE, Rowland JH. 1999. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol 17(8):2371–2380.
Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. 2002. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst 94(1):39–49.
Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. 2003a. Breast cancer in younger women: Reproductive and late health effects of treatment. J Clin Oncol 21(22):4184–4193.
Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. 2000. Managing menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Natl Cancer Inst 92(13):1054–1064.
Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. 2003b. Breast cancer in older women: Quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol 21(21):4027–4033.
Ganz PA, Hirji K, Sim MS, Schag CA, Fred C, Polinsky ML. 1993. Predicting psychosocial risk in patients with breast cancer. Med Care 31(5):419–431.
Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. 2004a. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst 96(5):376–387.
Ganz PA, Lee JJ, Sim MS, Polinsky ML, Schag CA. 1992. Exploring the influence of multiple variables on the relationship of age to quality of life in women with breast cancer. J Clin Epidemiol 45(5):473–485.
Ganz PA, Moinpour CM, McCoy S, Pauler DK, Press OW, Fisher RI. 2004b. Predictors of vitality (energy/fatigue) in early stage Hodgkin’s disease (HD): Results from Southwest Oncology Group (SWOG) Study 9133. J Clin Oncol 22(14S):569S.
Ganz PA, Moinpour CM, Pauler DK, Kornblith AB, Gaynor ER, Balcerzak SP, Gatti GS, Erba HP, McCoy S, Press OW, Fisher RI. 2003c. Health status and quality of life in patients with early-stage Hodgkin’s disease treated on Southwest Oncology Group Study 9133. J Clin Oncol 21(18):3512–3519.
Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. 1998a. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol 16(2):501–514.
Ganz PA, Rowland JH, Meyerowitz BE, Desmond KA. 1998b. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res 152:396–411.
Garces YI, Hays JT. 2003. Tobacco dependence: Why should an oncologist care? J Clin Oncol 21(9):1884–1886.
Giedzinska AS, Meyerowitz BE, Ganz PA, Rowland JH. 2004. Health-related quality of life in a multiethnic sample of breast cancer survivors. Ann Behav Med 28(1):39–51.
Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. 2005. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 97(6):419–424.
GIVIO. 1994. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA 271(20):1587–1592.
Goodwin P, Esplen MJ, Butler K, Winocur J, Pritchard K, Brazel S, Gao J, Miller A. 1998. Multidisciplinary weight management in locoregional breast cancer: Results of a Phase II study. Breast Cancer Res Treat 48(1):53–64.
Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, Trudeau M, Hood N, Redwood S. 1999a. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol 17(1):120–129.
Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. 1999b. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370.
Gore JL, Krupski T, Kwan L, Maliski S, Litwin MS. 2005. Partnership status influences quality of life in low-income, uninsured men with prostate cancer. Cancer 104 (1):191–198.
Grady WM, Russell K. 2005. Ionizing radiation and rectal cancer: Victims of our own success. Gastroenterology 128(4):1114–1117.
Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, Mactaggart PN, Swanson C, Watson RB, Gardiner RA. 2002a. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: A randomized controlled trial. BJU Int 90(4):427–432.
Green RJ, Metlay JP, Propert K, Catalano PJ, Macdonald JS, Mayer RJ, Haller DG. 2002b. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: An analysis of Intergroup 0089. Ann Intern Med 136(4):261–269.
Grise P, Thurman S. 2001. Urinary incontinence following treatment of localized prostate cancer. Cancer Control 8(6):532–539.
Grossfeld GD, Stier DM, Flanders SC, Henning JM, Schonfeld W, Warolin K, Carroll PR. 1998. Use of second treatment following definitive local therapy for prostate cancer: Data from the CaPSURE database. J Urol 160(4):1398–1404.
Grunfeld E, Dhesy-Thind S, Levine M. 2005. Clinical practice guidelines for the care and treatment of breast cancer: 9. Follow-up after treatment for breast cancer (2005 update). [Online]. Available: http://www.cmaj.ca/cgi/data/172/10/1319/DC1/2 [accessed July 14, 2005].
Guay AT, Spark RF, Bansal S, Cunningham GR, Goodman NF, Nankin HR, Petak SM, Perez JB, Law B Jr, Garber JR, Levy P, Jovanovic LG, Hamilton CR Jr, Rodbard HW, Palumbo PJ, Service FJ, Stoffer SS, Rettinger HI, Shankar TP, and Mechanick JI. 2003. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: A couple’s problem—2003 update. Endocr Pract 9(1):77–95.
Gulliford T, Opomu M, Wilson E, Hanham I, Epstein R. 1997. Popularity of less frequent follow up for breast cancer in randomised study: Initial findings from the hotline study. BMJ 314(7075):174–177.
Hammerlid E, Taft C. 2001. Health-related quality of life in long-term head and neck cancer survivors: A comparison with general population norms. Br J Cancer 84(2):149–156.
Hancock SL. 1999. Cardiovascular Late Effects After Treatment of Hodgkin’s Disease. New York, NY : Lippincott Williams & Wilkins.
Hancock SL, Tucker MA, Hoppe RT. 1993. Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst 85(1):25–31.
Hanson Frost M, Suman VJ, Rummans TA, Dose AM, Taylor M, Novotny P, Johnson R, Evans RE. 2000. Physical, psychological and social well-being of women with breast cancer: The influence of disease phase. Psychooncology 9(3):221–231.
Harris JR, Lippman ME, Morrow M, Osborne CK. 2004. Diseases of the Breast. 3rd ed. New York, NY: Lippincott Williams & Wilkins.
Harris SR, Hugi MR, Olivotto IA, Levine M. 2001. Clinical practice guidelines for the care and treatment of breast cancer: Lymphedema. CMAJ 164(2):191–199.
Helewa M, Levesque P, Provencher D, Lea RH, Rosolowich V, Shapiro HM. 2002. Breast cancer, pregnancy, and breastfeeding. J Obstet Gynaecol Can 24(2):164–180; quiz 181–184.
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E III, Schilsky RL, Wood WC, Muss HB, Norton L. 2003. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983.
Herman DR, Ganz PA, Petersen L, Greendale GA. In press. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast Cancer Res Treat.
Herold AH, Roetzheim RG. 1992. Cancer survivors. Prim Care 19(4):779–791.
Hewitt M, Rowland JH, and Yancik R. 2003. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci 58(1):82–91.
Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S. 2003. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21(21):4042–4057.
Hoda D, Perez DG, Loprinzi CL. 2003. Hot flashes in breast cancer survivors. Breast J 9(5):431–438.
Hoffman RD, Saltzman CL, Buckwalter JA. 2002. Outcome of lower extremity malignancy survivors treated with transfemoral amputation. Arch Phys Med Rehabil 83(2):177–182.
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. 2005. Physical activity and survival after breast cancer diagnosis. JAMA 293(20): 479–2486.
Holmes MD, Kroenke CH. 2004. Beyond treatment: Lifestyle choices after breast cancer to enhance quality of life and survival. Women’s Health Issues 14(1):11–13.
Holtzman J, Schmitz K, Babes G, Kane RL, Duval S, Wilt TJ, MacDonald RM, Rutks I (Minnesota Evidence-based Practice Center, under Contract No. 290-02-009). 2004. Effectiveness of Behavioral Interventions to Modify Physical Activity Behaviors in General Populations and Cancer Patients and Survivors. Evidence Report/Technology Assessment No. 102. Rockville, MD: Agency for Healthcare Research and Quality.
Hu JC, Kwan L, Saigal CS, Litwin MS. 2003. Regret in men treated for localized prostate cancer. J Urol 169(6):2279–2283.
Hurria A, Hudis C. 2003. Follow-up care of breast cancer survivors. Crit Rev Oncol Hematol 48(1):89–99.
ICSI (Institute for Clinical Systems Improvement). 2003. Health Care Guideline: Breast Cancer Treatment. Bloomington, MN: ICSI.
IOM (Institute of Medicine). 1990. Clinical Practice Guidelines: Directions for a New Program. Field MJ, Lohr KN, eds. Washington, DC: National Academy Press.
IOM. 2003. Fulfilling the Potential of Cancer Prevention and Early Detection. Curry SJ, Byers T, Hewitt M, eds. Washington, DC: The National Academies Press.
IOM. 2004. Meeting the Psychosocial Needs of Women with Breast Cancer. Hewitt M, Herdman R, Holland J, eds. Washington, DC: The National Academies Press.
Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. 2003. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 97(7):1746–1757.
Isaacs C, Peshkin BN, Schwartz M. 2004. Evaluation and management of women with a strong family history. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Diseases of the Breast. 3rd ed. New York, NY: Lippincott Williams & Wilkins. Pp. 315–345.
Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, Lo KM, Moore A, Rosenman PJ, Kaufman EL, Neugut AI, Grann VR. 2001. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 19(10):2739–2745.
Jen KL, Djuric Z, DiLaura NM, Buison A, Redd JN, Maranci V, Hryniuk WM. 2004. Improvement of metabolism among obese breast cancer survivors in differing weight loss regimens. Obes Res 12(2):306–312.
Jenkins I, Djuric Z, Darga L, DiLaura NM, Magnan M, Hryniuk WM. 2003. Relationship of psychiatric diagnosis and weight loss maintenance in obese breast cancer survivors. Obes Res 11(11):1369–1375.
Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. 2002. Factors associated with the development of arm lymphedema following breast cancer treatment: A match pair case-control study. Lymphology 35(2):59–71.
Johnson FE, Virgo KS. 1997. Cancer Patient Follow-Up. St. Louis, MO: Mosby.
Johnson FE, Virgo KS, Fossati R. 2004. Follow-up for patients with colorectal cancer after curative-intent primary treatment. J Clin Oncol 22(8):1363–1365.
Joly F, Henry-Amar M, Arveux P, Reman O, Tanguy A, Peny AM, Lebailly P, Mace-Lesec’h J, Vie B, Genot JY, Busson A, Troussard X, Leporrier M. 1996. Late psychosocial sequelae in Hodgkin’s disease survivors: A French population-based case-control study. J Clin Oncol 14(9):2444–2453.
Jones LW, Courneya KS. 2002. Exercise discussions during cancer treatment consultations. Cancer Pract 10(2):66–74.
Jones LW, Courneya KS, Fairey AS, Mackey JR. 2004. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: A single-blind, randomized controlled trial. Ann Behav Med 28(2):105–113.
Justice B. 1999. Why do women treated for breast cancer report good health despite disease or disability? A pilot study. Psychol Rep 84(2):392–394.
Kattlove H, Winn RJ. 2003. Ongoing care of patients after primary treatment for their cancer. CA Cancer J Clin 53(3):172–196.
King MC, Marks JH, Mandell JB. 2003. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302(5645):643–646.
Kligman L, Wong RK, Johnston M, Laetsch NS. 2004. The treatment of lymphedema related to breast cancer: A systematic review and evidence summary. Support Care Cancer 12(6):421–431.
Knobel H, Havard Loge J, Brit Lund M, Forfang K, Nome O, Kaasa S. 2001. Late medical complications and fatigue in Hodgkin’s disease survivors. J Clin Oncol 19(13):3226–3233.
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. 2005. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 23(16):3830–3842.
Kornblith AB. 1998. Psychosocial adaptation of cancer survivors. In: Holland JC, ed. Psycho-Oncology. New York: Oxford University Press.
Kornblith AB, Ligibel J. 2003. Psychosocial and sexual functioning of survivors of breast cancer. Semin Oncol 30(6):799–813.
Kornblith AB, Anderson J, Cella DF, Tross S, Zuckerman E, Cherin E, Henderson E, Weiss RB, Cooper MR, Silver RT, et al. 1992. Hodgkin’s disease survivors at increased risk for problems in psychosocial adaptation. The Cancer and Leukemia Group B. Cancer 70(8):2214–2224.
Kornblith AB, Herndon JE II, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, Mertz M, Payne D, Massie MJ, Holland JF, Wingate P, Norton L, Holland JC. 2003. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer 98(4):679–689.
Kornblith AB, Herndon JE II, Zuckerman E, Cella DF, Cherin E, Wolchok S, Weiss RB, Diehl LF, Henderson E, Cooper MR, Schiffer C, Canellos GP, Mayer RJ, Silver RT, Schilling A, Peterson BA, Greenberg D, Holland JC. 1998. Comparison of psychosocial adaptation of advanced stage Hodgkin’s disease and acute leukemia survivors. Cancer and Leukemia Group B. Ann Oncol 9(3):297–306.
Koupparis A, Ramsden A, Persad R. 2004. Cognitive effects of hormonal treatment for prostate cancer. BJU Int 93(7):915–916.
Krag DN, Julian TB, Harlow SP, Weaver DL, Ashikaga T, Bryant J, Single RM, Wolmark N. 2004. NSABP-32: Phase III, randomized trial comparing axillary resection with sentinal lymph node dissection: A description of the trial. Ann Surg Oncol 11(3 Suppl):208S–210S.
Kroenke CH, Chen WY, Rosner B, Holmes MD. 2005. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23(7):1370–1380.
Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. 2004. Functional impact of breast cancer by age at diagnosis. J Clin Oncol 22(10):1849–1856.
Krupski T, Petroni GR, Bissonette EA, Theodorescu D. 2000. Quality-of-life comparison of radical prostatectomy and interstitial brachytherapy in the treatment of clinically localized prostate cancer. Urology 55(5):736–742.
Krupski TL, Smith MR, Lee WC, Pashos CL, Brandman J, Wang Q, Botteman M, Litwin MS. 2004. Natural history of bone complications in men with prostate carcinoma initiating androgen deprivation therapy. Cancer 101(3):541–549.
Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeny T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. 2004. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22(24):4979–4990.
Lamont EB, Christakis NA, Lauderdale DS. 2003. Favorable cardiac risk among elderly breast carcinoma survivors. Cancer 98(1):2–10.
Lamont EB, Lauderdale DS. 2003. Low risk of hip fracture among elderly breast cancer survivors. Ann Epidemiol 13(10):698–703.
Langer AS. 2001. Side effects, quality-of-life issues, and trade-offs: The patient perspective. J Natl Cancer Inst Monogr 30:125–129.
Lea R, Bannister E, Case A, Levesque P, Miller D, Provencher D, Rosolovich V. 2004. Use of hormonal replacement therapy after treatment of breast cancer. J Obstet Gynaecol Can 26(1):49–60; quiz 62–64.
Lee MM, Lin SS, Wrensch MR, Adler SR, Eisenberg D. 2000. Alternative therapies used by women with breast cancer in four ethnic populations. J Natl Cancer Inst 92(1):42–47.
Lee-Jones C, Humphris G, Dixon R, Hatcher MB. 1997. Fear of cancer recurrence—a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology 6(2):95–105.
Leedham B, Ganz PA. 1999. Psychosocial concerns and quality of life in breast cancer survivors. Cancer Invest 17(5):342–348.
Lepore SJ, Helgeson VS, Eton DT, Schulz R. 2003. Improving quality of life in men with prostate cancer: A randomized controlled trial of group education interventions. Health Psychol 22(5):443–452.
Levi F, Te VC, Randimbison L, La Vecchia C. 2003. Cancer risk in women with previous breast cancer. Ann Oncol 14(1):71–73.
Liberati A. 1995. The GIVIO trial on the impact of follow-up care on survival and quality of life in breast cancer patients. Interdisciplinary Group for Cancer Care Evaluation. Ann Oncol 6(Suppl 2):41–46.
Litwin MS. 2003. Quality of life following definitive therapy for localized prostate cancer: Potential impact of multiple therapies. Curr Opin Urol 13(2):153–156.
Litwin MS, Ron DH, Fink A, Ganz PA, Leake B, Brook RH. 2004. UCLA Prostate Cancer Index, including the RAND 36-Item Health Survey v2 (SF-36 v2). [Online]. Available: http://www.proqolid.org/public/UCLA-PCI.html [accessed February 15, 2005].
Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. 1999. Hodgkin’s disease survivors more fatigued than the general population. J Clin Oncol 17(1):253–261.
Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S. 2000. Fatigue and psychiatric morbidity among Hodgkin’s disease survivors. J Pain Symptom Manage 19(2):91–99.
Loprinzi CL, Kugler JW, Sloan JA, Rooke TW, Quella SK, Novotny P, Mowat RB, Michalak JC, Stella PJ, Levitt R, Tschetter LK, Windschitl H. 1999. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med 340(5):346–350.
Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA. 2002. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 20(6):1578–1583.
Lu-Yao GL, Potosky AL, Albertsen PC, Wasson JH, Barry MJ, Wennberg JE. 1996. Follow-up prostate cancer treatments after radical prostatectomy: A population-based study. J Natl Cancer Inst 88(3–4):166–173.
Mackey JR, Joy AA. 2005. Skeletal health in postmenopausal survivors of early breast cancer. Int J Cancer 114(6):1010–1015.
Mandelblatt JS, Eisenberg JM. 1995. Historical and methodological perspectives on cancer outcomes research. Oncology (Huntingt) 9(11 Suppl):23–32.
Mandelblatt J, Figueiredo M, Cullen J. 2003. Outcomes and quality of life following breast cancer treatment in older women: When, why, how much, and what do women want? Health Qual Life Outcomes 1(1):45.
Manne SL. 2002. Prostate cancer support and advocacy groups: Their role for patients and family members. Semin Urol Oncol 20(1):45–54.
Marcus AD. 2004, September 8. A wife’s struggle with cancer takes an unexpected toll. The Wall Street Journal. P. A1.
Massie MJ. 2004. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 32:57–71.
Massie MJ, Holland JC. 1991. Psychological reactions to breast cancer in the pre- and post-surgical treatment period. Semin Surg Oncol 7(5):320–325.
Matesich SM, Shapiro CL. 2003. Second cancers after breast cancer treatment. Semin Oncol 30(6):740–748.
Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM, eds. 1999. Hodgkin’s Disease. New York, NY: Lippincott, Williams & Wilkins.
Maunsell E, Brisson J, Deschenes L. 1989. Psychological distress after initial treatment for breast cancer: A comparison of partial and total mastectomy. J Clin Epidemiol 42(8):765–71.
Maunsell E, Brisson J, Deschenes L. 1992. Psychological distress after initial treatment of breast cancer. Assessment of potential risk factors. Cancer 70(1):120–125.
Maunsell E, Brisson J, Deschenes L. 1995. Social support and survival among women with breast cancer. Cancer 76(4):631–637.
McBride CM, Ostroff JS. 2003. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control 10(4):325–333.
McEvoy MD, McCorkle R. 1990. Quality of life issues in patients with disseminated breast cancer. Cancer 66(6 Suppl):1416–1421.
McKinley ED. 2000. Under Toad days: Surviving the uncertainty of cancer recurrence. Ann Intern Med 133(6):479–480.
McTiernan A. 2004. Physical activity after cancer: Physiologic outcomes. Cancer Invest 22(1):68–81.
McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, Gilliland F, Stanczyk FZ, Yasui Y, Ballard-Barbash R. 2003. Adiposity and sex hormones in post-menopausal breast cancer survivors. J Clin Oncol 21(10):1961–1966.
Mehta SS, Lubeck DP, Pasta DJ, Litwin MS. 2003. Fear of cancer recurrence in patients undergoing definitive treatment for prostate cancer: Results from CaPSURE. J Urol 170(5):1931–1933.
Meyerhardt JA, Mayer RJ. 2003. Follow-up strategies after curative resection of colorectal cancer. Semin Oncol 30(3):349–360.
Meyerowitz BE, Desmond KA, Rowland JH, Wyatt GE, Ganz PA. 1999. Sexuality following breast cancer. J Sex Marital Ther 25(3):237–250.
Meyers CA. 2000. Neurocognitive dysfunction in cancer patients. Oncology (Huntingt) 14(1):75–79; discussion 79, 81–82, 85.
Michael YL, Berkman LF, Colditz GA, Holmes MD, Kawachi I. 2002. Social networks and health-related quality of life in breast cancer survivors: A prospective study. J Psychosom Res 52(5):285–293.
Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. 2000. The persistent impact of breast carcinoma on functional health status: Prospective evidence from the Nurses’ Health Study. Cancer 89(11):2176–2186.
Miller DC, Sanda MG, Dunn RL, Montie JE, Pimentel H, Sandler HM, McLaughlin WP, Wei JT. 2005. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol 23(12):2772–2780.
Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. 1993. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 270(8):943–947.
Montazeri A, Gillis CR, McEwen J. 1996. Measuring quality of life in oncology: Is it worthwhile? Meaning, purposes and controversies. Eur J Cancer Care (Engl) 5(3):159–167.
Mor V, Malin M, Allen S. 1994. Age differences in the psychosocial problems encountered by breast cancer patients. J Natl Cancer Inst Monogr (16):191–197.
Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, Harris JR, O’Malley F, Schnitt SJ, Singletary SE, Winchester DP. 2002a. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin 52(5):277–300.
Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Harris JR, O’Malley F, Schnitt SJ, Singletary SE, Winchester DP. 2002b. Standard for the management of ductal carcinoma in situ of the breast (DCIS). CA Cancer J Clin 52(5):256–276.
Mortimer P, Bates D, Brassington H, Stanton A, Strachan D, Levick J. 1996. The prevalence of arm oedema following treatment for breast cancer. QJM 89(5):337–380.
Mrozek E, Shapiro CL. 2005. Survivorship and complications of treatment in breast cancer. Clinical Advances in Hematology & Oncology 3(3):211–222.
Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. 2001. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil 80(5):346–350.
Muzzin LJ, Anderson NJ, Figueredo AT, Gudelis SO. 1994. The experience of cancer. Soc Sci Med 38(9):1201–1208.
National Surgical Adjuvant Breast and Bowel Project. 2004. NSABP Clinical Trials Overview: Protocol B-32. [Online]. Available: http://www.nsabp.pitt.edu/b-32.htm [accessed August 30, 2004].
Navo MA, Phan J, Vaughan C, Palmer JL, Michaud L, Jones KL, Bodurka DC, Basen-Engquist K, Hortobagyi GN, Kavanagh JJ, Smith JA. 2004. An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies. J Clin Oncol 22(4):671–677.
NBCC (National Breast Cancer Centre). 2001. Clinical Practice Guidelines for the Management of Early Breast Cancer. Canberra, Australia: National Health and Medical Research Council.
NBCC and NCCI (National Breast Cancer Center and National Cancer Control Initiative). 2004. Clinical Practice Guidelines for the Management and Support of Younger Women With Breast Cancer. Camperdown, NSW, Australia: National Breast Cancer Center.
NCCAM (National Center for Complementary and Alternative Medicine). 2002. What Is Complementary and Alternative Medicine (CAM)? [Online]. Available: http://nccam.nih.gov/health/whatiscam/ [accessed February 26, 2005].
NCCN (National Comprehensive Cancer Network). 1999. NCCN practice guidelines for the management of psychosocial distress. Oncology (Huntingt) 13(5A):113–147.
NCCN. 2004a. Breast Cancer Treatment Guidelines for Patients, Version VI. Breast Cancer Treatment. [Online]. Available: http://www.nccn.org/patients/patient_gls/_english/_breast/5_treatment.asp#consider [accessed May 9, 2005].
NCCN. 2004b. Clinical Practice Guidelines in Oncology-v.1.2004. Breast Cancer. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf [accessed December 6, 2004].
NCCN. 2004c. Clinical Practice Guidelines in Oncology-v.1.2004. Colorectal Screening. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/colorectal_screening.pdf [accessed December 6, 2004].
NCCN. 2004d. Clinical Practice Guidelines in Oncology-v.1.2004. Distress Management. [Online]. Available: http://www.nccn.org/physician_gls/f_guidelines.html [accessed December 6, 2004].
NCCN. 2004e. Clinical Practice Guidelines in Oncology-v.1.2004. Genetic/Familial High-Risk Assessment: Breast and Ovarian. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf [accessed December 6, 2004].
NCCN. 2004f. Clinical Practice Guidelines in Oncology-v.1.2004. Hodgkin’s Disease. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/hodgkins.pdf [accessed December 6, 2004].
NCCN. 2004g. Clinical Practice Guidelines in Oncology-v.1.2004. Prostate Cancer. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf [accessed December 6, 2004].
NCCN. 2004h. Clinical Practice Guidelines in Oncology-v.2.2004. Colon Cancer. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf [accessed December 6, 2004].
NCCN. 2004i. NCCN Treatment Guidelines for Patients. [Online]. Available: http://www.nccn.org/patients/patient_gls.asp [accessed September 11, 2004].
NCCN. 2005. Clinical Practice Guidelines in Oncology-v.2.2005. Cancer-Related Fatigue. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf [accessed July 22, 2005].
NCI (National Cancer Institute). 2004a. Breast Cancer (PDQ): Treatment. [Online]. Available: http://www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional/allpages [accessed September 2004].
NCI. 2004b. Genetics of Breast and Ovarian Cancer (PDQ) Health Professional Version. [Online]. Available: http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian/healthprofessional [accessed October 6, 2004].
NCI. 2005a. Adult Hodgkin’s Lymphoma (PDQ®): Treatment. [Online]. Available: http://www.nci.nih.gov/cancertopics/pdq/treatment/adulthodgkins/Patient/page4 [accessed February 10, 2005].
NCI. 2005b. Colon Cancer PDQ: Treatment. [Online]. Available: http://www.cancer.gov/cancertopics/pdq/treatment/colon/healthprofessional [accessed February 7, 2005].
NCI and NCCAM (National Cancer Institute and National Center for Complementary and Alternative Medicine). 2004. Cancer Facts: Complementary and Alternative Medicine. [Online]. Available: http://cis.nci.nih.gov/fact/9_14.htm [accessed December 8, 2004].
Ng AK, Mauch PM. 2004. Late complications of therapy of Hodgkin’s disease: Prevention and management. Curr Hematol Rep 3(1):27–33.
NIH (National Institutes of Health). 2000. Adjuvant therapy for breast cancer. NIH Consensus Statement 17(4):1–35.
Northover J. 2003. Follow-up after curative surgery for colorectal cancer. Scand J Surg 92(1):84–89.
Oktay K. 2001. Ovarian tissue cryopreservation and transplantation: Preliminary findings and implications for cancer patients. Hum Reprod Update 7(6):526–534.
Oktay K, Sonmezer M. 2004. Ovarian tissue banking for cancer patients: Fertility preservation, not just ovarian cryopreservation. Hum Reprod 19(3):477–480.
Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. 2003. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 18(1):90–95.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. 2005. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 23(19):4347–4353.
Omne-Ponten M, Holmberg L, Sjoden PO. 1994. Psychosocial adjustment among women with breast cancer Stages I and II: Six-year follow-up of consecutive patients. J Clin Oncol 12(9):1778–1782.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. 2004. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826.
Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, Pacini P. 1999. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 281(17):1586.
Park RE, Fink A, Brook RH, Chassin MR, Kahn KL, Merrick NJ, Kosecoff J, Solomon DH. 1986. Physician ratings of appropriate indications for six medical and surgical procedures. Am J Public Health 76(7):766–772.
Parker-Pope T. 2004 (June 1). Efforts mount to combat lymphedema, a devastating side effect of cancer care. The Wall Street Journal. P. D1.
Partridge AH, Winer EP. 2005. Fertility after breast cancer: Questions abound. J Clin Oncol 23(19):4259-4261.
Partridge AH, Burstein HJ, Winer EP. 2001. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 30:135–142.
Partridge AH, Winer EP, Burstein HJ. 2003. Follow-up care of breast cancer survivors. Semin Oncol 30(6):817–825.
Paskett ED. 2003. Empowering women with breast cancer: One survivor’s story. Semin Oncol 30(6):814–816.
Penson DF, Litwin MS. 2003a. The physical burden of prostate cancer. Urol Clin North Am 30(2):305–313.
Penson DF, Litwin MS. 2003b. Quality of life after treatment for prostate cancer. Curr Urol Rep 4(3):185–195.
Penson DF, Sokoloff MH. 2004. Management of side effects of prostate cancer therapy. In: Carroll P, Nelson WG, eds. Report to the Nation on Prostate Cancer. Santa Monica, CA: Prostate Cancer Foundation.
Petrie KJ, Buick DL, Weinman J, Booth RJ. 1999. Positive effects of illness reported by myocardial infarction and breast cancer patients. J Psychosom Res 47(6):537–543.
Phillips KA, Bernhard J. 2003. Adjuvant breast cancer treatment and cognitive function: Current knowledge and research directions. J Natl Cancer Inst 95(3):190–197.
Pierce R, Chadiha LA, Vargas A, Mosley M. 2003. Prostate cancer and psychosocial concerns in African American men: Literature synthesis and recommendations. Health Soc Work 28(4):302–311.
Pinto BM, Maruyama NC. 1999. Exercise in the rehabilitation of breast cancer survivors. Psychooncology 8(3):191–206.
Pinto BM, Eakin E, Maruyama NC. 2000. Health behavior changes after a cancer diagnosis: What do we know and where do we go from here? Ann Behav Med 22(1):38–52.
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. 2005. Home-based physical activity intervention for breast cancer patients. J Clin Oncol 23(15):3577–3587.
Pinto BM, Maruyama NC, Clark MM, Cruess DG, Park E, Roberts M. 2002. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc 77(2):122–129.
Posther KE, Wilke LG, Giuliano AE. 2004. Sentinel lymph node dissection and the current status of American trials on breast lymphatic mapping. Semin Oncol 31(3):426–436.
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. 1999. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281(17):1591–1597.
President’s Cancer Panel. 2004. Living Beyond Cancer: Finding a New Balance. Bethesda, MD: National Cancer Institute.
Pritchard KI, Khan H, Levine M. 2002. Clinical practice guidelines for the care and treatment of breast cancer: The role of hormone replacement therapy in women with a previous diagnosis of breast cancer. CMAJ 166(8):1017–1022.
Quigley KM. 1989. The adult cancer survivor: Psychosocial consequences of cure. Semin Oncol Nurs 5(1):63–69.
Ramaswamy B, Shapiro CL. 2003. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol 30(6):763–775.
Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR. 2002. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol 97(5):1228–1234.
Rao A, Cohen HJ. 2004. Symptom management in the elderly cancer patient: Fatigue, pain, and depression. J Natl Cancer Inst Monogr 32:150–157.
Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. 2004. Quality of life among disease-free survivors of rectal cancer. J Clin Oncol 22(2):354–360.
Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, Fleming GF, Formenti S, Hudis C, Kirshner JJ, Krause DA, Kuske RR, Langer AS, Sledge GW Jr, Whelan TJ, Pfister DG. 2001. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 19(5):1539–1569.
Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. 2000. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 18(13):2505–2514.
Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, eds. 2004. SEER Cancer Statistics Review, 1975–2001. Bethesda, MD: National Cancer Institute.
Robinson JW, Moritz S, Fung T. 2002. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 54(4):1063–1068.
Rock CL, Demark-Wahnefried W. 2002. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J Clin Oncol 20(15):3302–3316.
Rojas M, Telaro E, Russo A, Moschetti I, Coe L, Fossati R, Palli D, Roselli TM, Liberati A. 2005. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev (1):CD001768.
Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. 1994. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA 271(20):1593–1597.
Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. 2000. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. J Natl Cancer Inst 92(17):1422–1429.
Rugo HS, Ahles T. 2003. The impact of adjuvant therapy for breast cancer on cognitive function: Current evidence and directions for research. Semin Oncol 30(6):749–762.
Sadler IJ, Jacobsen PB. 2001. Progress in understanding fatigue associated with breast cancer treatment. Cancer Invest 19(7):723–731.
Salminen EK, Portin RI, Koskinen AI, Helenius HY, Nurmi MJ. 2005. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer 103(7):1381–1387.
Sapp AL, Trentham-Dietz A, Newcomb PA, Hampton JM, Moinpour CM, Remington PL (University of Wisconsin Comprehensive Cancer Center). 2003. Social networks and quality of life among female long-term colorectal cancer survivors. Cancer 98(8):1749–1758.
Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. 2004. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev 13(6):1022–1031.
Saykin AJ, Ahles TA, McDonald BC. 2003. Mechanisms of chemotherapy-induced cognitive disorders: Neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry 8(4):201–216.
Schag CA, Ganz PA, Polinsky ML, Fred C, Hirji K, Petersen L. 1993. Characteristics of women at risk for psychosocial distress in the year after breast cancer. J Clin Oncol 11(4):783–793.
Schnoll RA, Zhang B, Rue M, Krook JE, Spears WT, Marcus AC, Engstrom PF. 2003. Brief physician-initiated quit-smoking strategies for clinical oncology settings: A trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 21(2):355–365.
Scholefield JH, Steele RJ. 2002. Guidelines for follow up after resection of colorectal cancer. Gut 51(Suppl 5):V3–V5.
Schover LR. 1994. Sexuality and body image in younger women with breast cancer. J Natl Cancer Inst Monogr 16:177–182.
Schover LR. 2004. Myth-busters: Telling the true story of breast cancer survivorship. J Natl Cancer Inst 96(24):1800–1801.
Schover LR. 2005. Motivation for parenthood after cancer: A review. J Natl Cancer Inst Monogr 34:2–5.
Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, Kupelian PA. 2002. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer 95(11):2397–2407.
Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, Kupelian PA. 2004. Seeking help for erectile dysfunction after treatment for prostate cancer. Arch Sex Behav 33(5):443–454.
Schover LR, Yetman RJ, Tuason LJ, Meisler E, Esselstyn CB, Hermann RE, Grundfest-Broniatowski S, Dowden RV. 1995. Partial mastectomy and breast reconstruction. A comparison of their effects on psychosocial adjustment, body image, and sexuality. Cancer 75(1):54–64.
Schwartz D, Billingsley K, Wallner K. 2000. Follow-up care for cancer: Making the benefits equal the cost. Oncology (Huntingt) 14(10):1493–1498, 1501; discussion 1502–1505.
Schwartz GF. 2004. Clinical practice guidelines for the use of axillary sentinel lymph node biopsy in carcinoma of the breast: Current update. Breast J 10(2):85–88.
Shapiro CL, Recht A. 2001. Side effects of adjuvant treatment of breast cancer. N Engl J Med 344(26):1997–2008.
Shapiro CL, Manola J, Leboff M. 2001. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 19(14):3306–3311.
Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, Grimshaw JM, Woolf SH. 2001. Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA 286(12):1461–1467.
Shimozuma K, Ganz PA, Petersen L, Hirji K. 1999. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Res Treat 56(1):45–57.
Short PF, Mallonee EL. In press. Income disparities in the quality of life of cancer survivors. Med Care.
SIGN (Scottish Intercollegiate Guidelines Network). 1998. Breast Cancer in Women: A National Clinical Guideline. Edinburgh, Scotland: SIGN.
SIGN. 2003. Management of Colorectal Cancer. Edinburgh, Scotland: SIGN.
Simmang CL, Senatore P, Lowry A, Hicks T, Burnstein M, Dentsman F, Fazio V, Glennon E, Hyman N, Kerner B, Kilkenny J, Moore R, Peters W, Ross T, Savoca P, Vernava A, Wong WD. 1999. Practice parameters for detection of colorectal neoplasms. The Standards Committee, The American Society of Colon and Rectal Surgeons. Dis Colon Rectum 42(9):1123–1129.
Singh GK, Miller BA, Hankey BF, Edwards BK. 2003. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. NCI Cancer Surveillance Monograph Series, No. 4. Bethesda, MD: National Cancer Institute.
Smith K, Lesko LM. 1988. Psychosocial problems in cancer survivors. Oncology (Huntingt) 2(1):33–44.
Smith MR. 2003. Bisphosphonates to prevent osteoporosis in men receiving androgen deprivation therapy for prostate cancer. Drugs Aging 20(3):175–183.
Smith TJ, Hillner BE. 2001. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J Clin Oncol 19(11):2886–2897.
Smith TJ, Davidson NE, Schapira DV, Grunfeld E, Muss HB, Vogel VG III, Somerfield MR. 1999. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol 17(3):1080–1082.
Soloway CT, Soloway MS, Kim SS, Kava BR. 2005. Sexual, psychological and dyadic qualities of the prostate cancer “couple”. BJU International 95:780-785.
Sparaco A, Fentiman IS. 2002. Arm lymphoedema following breast cancer treatment. Int J Clin Pract 56(2):107–110.
Sparreboom A, Cox MC, Acharya MR, Figg WD. 2004. Herbal remedies in the United States: Potential adverse interactions with anticancer agents. J Clin Oncol 22(12):2489–2503.
Sprangers MA, Taal BG, Aaronson NK, te Velde A. 1995. Quality of life in colorectal cancer. Stoma vs. nonstoma patients. Dis Colon Rectum 38(4):361–369.
Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, Albertsen PC, Harlan LC, Potosky AL. 2000. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: The Prostate Cancer Outcomes Study. JAMA 283(3):354–360.
Stanton AL, Ganz PA, Bower JE, Kwan L, Meyerowitz BE, Rowland JH, Krupnick JL. 2004. The Moving Beyond Cancer Trial: A psycho-educational intervention for women with breast cancer. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 22(14S):8011.
Stearns V, Davidson NE. 2004. Adjuvant chemotherapy and chemoendocrine therapy. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Diseases of the Breast. 3rd ed. New York: Lippincott Williams & Wilkins. Pp. 893–919.
Tannock IF, Ahles TA, Ganz PA, Van Dam FS. 2004. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol 22(11):2233–2239.
Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. 2004. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J Am Geriatr Soc 52(2):269–273.
Teloken C. 2001. Management of erectile dysfunction secondary to treatment for localized prostate cancer. Cancer Control 8(6):540–545.
Temple LK, Wang EE, McLeod RS. 1999. Preventive health care, 1999 update: Follow-up after breast cancer. Canadian Task Force on Preventive Health Care. CMAJ 161(8):1001–1008.
Thellenberg C, Malmer B, Tavelin B, Gronberg H. 2003. Second primary cancers in men with prostate cancer: An increased risk of male breast cancer. J Urol 169(4):1345–1348.
Theodoulou M, Seidman AD. 2003. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol 30(6):730–739.
Thors CL, Broeckel JA, Jacobsen PB. 2001. Sexual functioning in breast cancer survivors. Cancer Control 8(5):442–448.
Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. 2005. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol 23(10):2378–2388.
Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. 2003. Phytoestrogen supplements for the treatment of hot flashes: The Isoflavone Clover Extract (ICE) Study: A randomized controlled trial. JAMA 290(2):207–214.
Tomich PL, Helgeson VS. 2002. Five years later: A cross-sectional comparison of breast cancer survivors with healthy women. Psychooncology 11(2):154–169.
Torrey MJ, Poen JC, Hoppe RT. 1997. Detection of relapse in early-stage Hodgkin’s disease: Role of routine follow-up studies. J Clin Oncol 15(3):1123–1130.
Trask PC. 2004. Assessment of depression in cancer patients. J Natl Cancer Inst Monogr 32:80–92.
Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E, Storm H, Glimelius I, Pukkala E, Stovall M, Fraumeni JF Jr, Boice JD Jr, Gilbert E. 2002. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst 94(3):182–192.
Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Lynch CF, Van’t Veer MB, Glimelius I, Storm H, Pukkala E, Stovall M, Curtis R, Boice JD Jr, Gilbert E. 2003. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin’s disease. JAMA 290(4):465–475.
Trentham-Dietz A, Remington PL, Moinpour CM, Hampton JM, Sapp AL, Newcomb PA (University of Wisconsin Comprehensive Cancer Center). 2003. Health-related quality of life in female long-term colorectal cancer survivors. Oncologist 8(4):342–349.
USPSTF (U.S. Preventive Services Task Force). 2003. Counseling to Prevent Tobacco Use and Tobacco-Related Diseases: Recommendation Statement. [Online]. Available: http://www.ahrq.gov/clinic/3rduspstf/tobacccoun/tobcounrs.htm [accessed December 8, 2004].
Vachon ML. 2001. The meaning of illness to a long-term survivor. Semin Oncol Nurs 17(4):279–283.
Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, Wattie A, Prior JC. 2002. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: A randomized, controlled clinical trial. J Clin Oncol 20(6):1449–1455.
Villers A, Pommier P, Bataillard A, Fervers B, Bachaud JM, Berger N, Bertrand AF, Bouvier R, Brune D, Daver A, Fontaine E, Haillot O, Lagrange JL, Molinie V, Muratet JP, Pabot du Chatelard P, Peneau M, Prapotnich D, Ravery V, Richaud P, Rossi D, Soulie M. 2003. Summary of the Standards, Options and Recommendations for the management of patients with nonmetastatic prostate cancer (2001). Br J Cancer 89(Suppl 1):S50–S58.
Visser A, van Andel G. 2003. Psychosocial and educational aspects in prostate cancer patients. Patient Educ Couns 49(3):203–206.
Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, Cutrara MR, DeBoer G, Yaffe MJ, Messner SJ, Meschino WS, Piron CA, Narod SA. 2004. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 292(11):1317–1325.
Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. 2003. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 289(20):2673–2684.
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. 2004a. ‘Chemobrain’ in breast carcinoma?: A prologue. Cancer 101(3):466–475.
Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. 2004b. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100(11):2292–2299.
Weiger WA, Smith M, Boon H, Richardson MA, Kaptchuk TJ, Eisenberg DM. 2002. Advising patients who seek complementary and alternative medical therapies for cancer. Ann Intern Med 137(11):889–903.
Wenzel L, Dogan-Ates A, Habbal R, Berkowitz R, Goldstein DP, Bernstein M, Kluhsman BC, Osann K, Newlands E, Seckl MJ, Hancock B, Cella D. 2005. Defining and measuring reproductive concerns of female cancer survivors. J Natl Cancer Inst Monogr 34:94–98.
Wenzel LB, Fairclough DL, Brady MJ, Cella D, Garrett KM, Kluhsman BC, Crane LA, Marcus AC. 1999. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer 86(9):1768–1774.
White RL Jr, Wilke LG. 2004. Update on the NSABP and ACOSOG breast cancer sentinel node trials. Am Surg 70(5):420–424.
Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C. 2003. Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology 124(2):544–560.
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR. 2005. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report 2004. J Clin Oncol 23(3):619–629.
Winn RJ. 2002. Clinical Practice Guidelines and Survivorship. Paper commissioned for the National Cancer Policy Board. Unpublished.
Winn RJ, Botnick WZ. 1997. The NCCN Guideline Program: A conceptual framework. Oncology (Huntingt) 11(11A):25–32.
Wooldridge JE, Link BK. 2003. Post-treatment surveillance of patients with lymphoma treated with curative intent. Semin Oncol 30(3):375–381.
Woolf SH. 1992. Practice guidelines, a new reality in medicine. Methods of developing guidelines. Arch Intern Med 152(5):946–952.
Woolf SH. 2000. Evidence-based medicine and practice guidelines: An overview. Cancer Control 7(4):362–367.
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. 1999. Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 318(7182):527–530.
Wyatt GE, Desmond KA, Ganz PA, Rowland JH, Ashing-Giwa K, Meyerowitz BE. 1998. Sexual functioning and intimacy in African American and white breast cancer survivors: A descriptive study. Women’s Health 4(4):385–405.
Yao SL, Dipaola RS. 2003. An evidence-based approach to prostate cancer follow-up. Semin Oncol 30(3):390–400.
Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. 2004. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 109(25):3122–3131.
Zebrack BJ, Ganz PA, Bernaards CA, Peterson L, Abraham L. 2003. Impact of cancer instrument: A new assessment tool for long-term cancer survivors. Proceedings of ASCO 22:531.
Zibecchi L, Greendale GA, Ganz PA. 2003. Continuing education: Comprehensive menopausal assessment: An approach to managing vasomotor and urogenital symptoms in breast cancer survivors. Oncol Nurs Forum 30(3):393–407.

























































































































