4
Delivering Cancer Survivorship Care
Cancer survivorship is a distinct phase of the cancer trajectory, with many opportunities to intervene to improve care. The current system for delivering care to the growing number of cancer survivors is inadequate. This chapter begins with a description of the attributes of an ideal follow-up system that would meet the needs of individuals surviving their cancer. Next, the gap between this ideal system and the current health care delivery system is illustrated in terms of problems faced by survivors in obtaining care and by providers in delivering care. Barriers that patients face in receiving appropriate care include a fragmented and poorly coordinated health care system, an absence of a locus of responsibility for follow-up care, and a lack of guidance on how cancer survivors can maximize their own health outcomes. Barriers that health care providers face in delivering care include not having necessary tools to provide consistent quality care, such as evidence-based clinical practice guidelines. Providers also lack delivery system supports such as information technology that would allow them to overcome some of the obstacles posed by the fragmented nature of cancer care in the United States. The chapter next reviews alternative models for delivering survivorship care. Survivorship clinics are being developed at a few cancer centers to meet the long-term needs of cancer survivors, but other promising models for delivering survivorship care are emerging and are examined. A description of the U.S. cancer care infrastructure is then described, highlighting existing programs to meet the needs of cancer survivors. Finally, the chapter puts forward steps that could be taken to implement the envisioned cancer survivorship system of care. Issues related to provider education and training are covered in Chapter 5. Overriding prob-
lems in accessing care due to a lack of health insurance coverage and inadequate insurance coverage are described in Chapter 6.
OPTIMAL CANCER SURVIVORSHIP CARE
For years cancer survivors have voiced concerns about access to appropriate services following their primary treatment. A decade ago, the National Coalition for Cancer Survivorship promulgated 12 principles that it believed were imperatives for quality cancer care (NCCS, 1996). Two of the principles relate to the delivery of care to cancer survivors:
-
“People with histories of cancer have the right to continued medical follow-up with basic standards of care that include the specific needs of long-term survivors.” (Principle 6)
-
“Long-term survivors should have access to specialized follow-up clinics that focus on health promotion, disease prevention, rehabilitation, and identification of physiologic and psychological problems. Communication with the primary care physician must be maintained.” (Principle 7)
The committee agreed with the underlying premise of these principles—that an organized system of care is needed to ensure the provision of survivorship care. In its deliberations, the committee sought a clear definition of the essential components of survivorship care and examples of delivery models that could be adopted throughout the nation in communities with varying characteristics and needs. The committee, following its review of the post-treatment clinical and psychosocial needs of cancer survivors, concluded that survivorship care represents a distinct phase of the cancer care trajectory. In its effort to better define this phase of care, the committee addressed key questions concerning the content of survivorship care, its recipients, and attributes of a system of care for this population.
What Are the Essential Components of Survivorship Care?
Survivorship care includes four components: (1) prevention and detection of new cancers and recurrent cancer; (2) surveillance for cancer spread, recurrence, or second cancers; (3) intervention for consequences of cancer and its treatment (e.g., medical problems such as lymphedema and sexual dysfunction; symptoms, including pain and fatigue; psychological distress experienced by cancer survivors and their caregivers; and concerns related to employment and insurance); and (4) coordination between specialists and primary care providers to ensure that all of the survivor’s health needs are met (e.g., health promotion, immunizations, screening for both cancer and noncancerous conditions, and the care of concurrent conditions).
Essential to survivorship care is a patient-centered approach, including responsiveness to patients’ needs, effective communication and information sharing, encouragement of the adoption of healthy lifestyles, and assistance in accessing community support services. Survivorship care has a focus on prevention—identifying treatable cancer recurrences, second cancers, and late effects; ensuring access to effective interventions; and helping patients to improve their quality of life.
Who Should Receive Survivorship Care?
Every individual should receive survivorship care following their treatment. The need for specific services will vary from survivor to survivor because of the heterogeneity of cancer and late effects. Survivors of early-stage cancer whose treatment was limited to surgery may require minimal follow-up care. In contrast, survivors with more advanced disease treated with combinations of surgery, chemotherapy, radiation, and hormone therapies may need long-term rehabilitative and supportive care. Some individuals treated for a predisposition to cancer (e.g., those who have genetic mutations, such as BRCA mutations) may also benefit from survivorship care.
When Does Survivorship Care Start and End?
An organized plan for survivorship care should be developed by the time primary treatment ends.1 Discussions of long-term effects of cancer and its treatment often begin at the time when treatment decisions are made. Later in the course of care, discussion of a survivorship care plan can provide hope and practical guidance. The transition from primary treatment into survivorship care is not always clear cut because some individuals require ongoing treatment such as adjuvant therapy. The committee viewed this period of adjuvant therapy as within the spectrum of survivorship care. Survivorship care lasts until recurrence, a second cancer, or death. Individuals who experience a recurrence or second cancer may reenter the acute phase of care for a time and then resume survivorship care. Individuals with chronic or intermittent disease may receive ongoing treatment for their disease, but benefit from survivorship care as they live with their disease (Figure 4-1). These individuals are generally under the long-term care of an oncology provider who can help ensure that survivorship needs are met. Some individuals who cease treatment prematurely may not benefit from a care plan if they are not formally discharged from care.
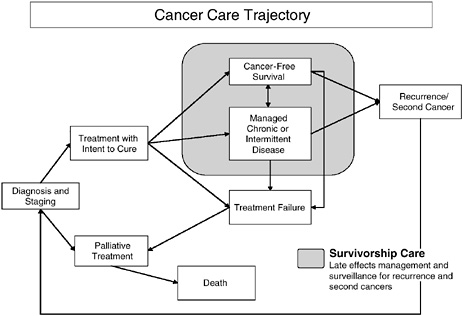
FIGURE 4-1 Cancer care trajectory.
NOTE: Palliative care is provided throughout the cancer care trajectory.
Who Should Provide Survivorship Care?
Survivorship care can be provided by either specialists or primary care providers. These providers can come from various care backgrounds—physicians, nurses, psychologists, and social workers—but optimally a designated individual is responsible for coordinating survivorship care, and care is viewed as a shared responsibility. Cancer survivors should be informed care partners, but providers within the health care system must take primary responsibility for coordinating care.
How Should Survivorship Care Be Provided?
Different models can be used to deliver optimal cancer survivorship care (see discussion below). Information technology, electronic medical records, and other health care delivery support systems can facilitate the delivery of integrated, coordinated, and multidisciplinary survivorship care. Survivorship care should embody rules set forth by the Institute of Medicine’s (IOM’s) Committee on Health Care Quality in America in its report Crossing the Quality Chasm (Box 4-1).
Receipt of optimal survivorship care depends on a patient-centered
|
BOX 4-1 Recommendation: Private and public purchasers, health care organizations, clinicians, and patients should work together to redesign health care processes in accordance with the following rules:
SOURCE: IOM (2001). |
approach in which care is structured around the needs and preferences of patients themselves (Berry et al., 2003). A call for such an approach has been made by physician-researchers William Tierney and Elizabeth McKinley in their description of their cancer experience from the patient’s perspective (Tierney and McKinley, 2002):
Providers must try to understand the impact of cancer on their patients’ lives and the lives of their patients’ caregivers. They should focus on both the negative and positive effects of cancer and its treatment, and be as energetic and considerate in treating the cancer patient (and hopefully, survivor) as they are in treating the cancer itself.
BARRIERS TO OPTIMAL CANCER SURVIVORSHIP CARE
Cancer survivors now generally receive some kind of follow-up, either from their cancer care specialist or primary care physician, but the focus of care has usually been on surveillance for recurrence and second cancers, not on the other key elements of care identified above. What barriers impede the delivery of optimal survivorship care? As this phase of care has only recently gained wide public attention, there is relatively little experience and research on how to deliver comprehensive and multidisciplinary survivorship care. This section of the chapter reviews significant barriers that both cancer survivors and their caregivers face in achieving satisfactory survivorship care.
Barriers Facing Cancer Survivors
Fragmented Delivery System
Individuals with chronic conditions face many obstacles in obtaining medical care that meets their needs for effective clinical management, psychological support, and information (Wagner et al., 2001). Cancer survivors, like other individuals with chronic conditions, face a common set of challenges—dealing with symptoms, disability, emotional upheaval, complex medication regimens, difficult lifestyle adjustments, and the need to obtain helpful medical care. While in treatment, cancer patients often see multiple specialists—surgeons, medical oncologists, and radiation oncologists—in addition to their primary care provider. Assuring coordinated, multidisciplinary care for primary treatment can be difficult and may affect access to subsequent survivorship care. It is generally the primary treatment specialist who informs survivors of their need for long-term follow-up, but continuity of that care is not always assured. A focus on continuity of care is central to quality of care throughout the cancer care
trajectory, including survivorship. The concept of continuity of care in oncology has been defined as:
The systematic assurance of uninterrupted, integrated medical and psychosocial care of the patient, in accord with the patient’s wishes, from assessment of symptoms in the prediagnostic period, throughout the phase of active treatment, and for the duration of posttreatment monitoring and/or palliative care. (Lauria, 1991)
When the systems responsible for coordinating individuals’ cancer care have been evaluated, they have often come up short. A qualitative study of mechanisms present within several New York hospitals to coordinate care for women with early breast cancer found that no site had the ability to systematically track care provided by multiple specialists (Bickell and Young, 2001). Mechanisms that hospitals relied on included tracking of referrals, patient support such as education and navigator programs, regularly scheduled multidisciplinary meetings, feedback of performance data, use of protocols, computerized systems, and a single physical location for care.
One consequence of poorly coordinated care is poor-quality care. Cancer survivors may not receive necessary noncancer care if their cancer diagnosis shifts attention away from care that is routine but necessary. Colorectal cancer survivors in one study were less likely than controls to receive appropriate follow-up for heart failure, necessary diabetic care, and recommended preventive services (Earle and Neville, 2004). Having both primary care physicians and oncologists involved in follow-up appeared to ameliorate this effect significantly, suggesting that a collaborative approach to follow-up is needed. This study focused on the care experience of Medicare beneficiaries who had survived 5 years past their diagnosis of colorectal cancer. In contrast to the findings in this study, breast cancer survivors received more preventive services (i.e., influenza vaccination, blood lipid testing, cervical and colon cancer screening, bone densitometry for osteoporosis) than controls in a similar study of Medicare beneficiaries (Earle et al., 2003). Breast cancer survivors who were followed by oncology specialists were more likely to receive mammograms; those who were followed by primary care physicians were more likely to receive all other noncancer-related preventive services; and those who saw both types of practitioners received more of both types of services. Both studies point to the importance of care that is coordinated and involves both primary and specialty providers.
Evidence from studies of surveillance practices in the United States suggests that follow-up care is not being provided as guidelines recommend (Johnson and Virgo, 1997) (see Appendix 4A for a summary of relevant studies). Rates of follow-up are not uniformly high for patients with a
history of breast cancer with annual mammography (Hillner et al., 1997; Andersen and Urban, 1998; Schapira et al., 2000; Lash and Silliman, 2001; Geller et al., 2003), for patients with a history of colorectal cancer with colorectal examinations (Cooper et al., 1999, 2000; Elston Lafata et al., 2001, 2005; Knopf et al., 2001; Ellison et al., 2003; Rulyak et al., 2004), and for patients with a history of bladder cancer with cystoscopy (Schrag et al., 2003). When examined, racial/ethnic and income differences usually account for significant variation in surveillance practices. The use of testing for metastatic disease that is not recommneded in guidelines has been found to be commonplace among cancer survivors (Elston Lafata et al., 2005). Adherence to adjuvant tamoxifen therapy among women with breast cancer is not uniformly high, with some studies finding nearly one-fourth of patients at risk for inadequate clinical response because of poor adherence (Demissie et al., 2001; Partridge et al., 2003; Fink et al., 2004). Evidence also suggests that the psychosocial needs of cancer patients are not being addressed. For example, oncologists often underdiagnose depression and fail to refer patients to mental health services (Passik et al., 1998; Fallowfield et al., 2001; Eakin and Strycker, 2001; Ell et al., 2005). Reports of unmet mental health needs because of cost have been reported to be significantly higher among cancer survivors relative to those without such a history (Hewitt and Rowland, 2002).
Optimal survivorship care is characterized by an organized plan for follow-up that is shared with patients so they can take responsibility for their care. There has been little research in the United States on the extent to which such plans are developed or communicated to patients. One Canadian study found that more than a third of cancer survivors surveyed after completion of treatment were not sure which physician was in charge of their cancer follow-up care (Miedema et al., 2003). This study relied on an unrepresentative sample of cancer survivors and so may not be generalizable to the broader population.
Relatively little is known of cancer survivors’ desires and perspectives regarding follow-up. Interviews conducted in England with breast cancer survivors on their views of routine follow-up indicated that women wanted, but were not receiving, continuity of care and an unrushed consultation (Adewuyi-Dalton et al., 1998).
A management model has emerged to guide the redesign of delivery systems and to improve care for individuals with chronic conditions. Six elements of the model are relevant to cancer survivorship care (Improving Chronic Illness Care, 2004):
-
Mobilize community resources to meet needs of patients.
-
Create a culture, organization, and mechanisms that promote safe, high-quality care.
-
Empower and prepare patients to manage their health and health care.
-
Assure the delivery of effective, efficient clinical care and self-management support.
-
Promote clinical care that is consistent with scientific evidence and patient preferences.
-
Organize patient and population data to facilitate efficient and effective care.
The chronic disease model has been implemented in primary care practices to improve care for individuals with diabetes, asthma, and congestive heart failure and has had some success in terms of improved outcome measures and reduced health care costs (Bodenheimer et al., 2002a,b). The Centers for Medicare and Medicaid Services (CMS) has supported several demonstration programs to improve care coordination and disease management in Medicare (MedPAC, 2004b). The Medicare Coordinated Care Demonstration, for example, is testing models of coordinated care to improve quality of services and manage Medicare expenditures at 15 sites, with 1 site focused on cancer care (CMS, 2004). The cancer care coordination project provides Medicare beneficiaries in South Florida with an oncology nurse advocate to help them understand their disease and better manage the side effects and symptoms of cancer and its treatment (Quality Oncology Inc., 2003).
Improvements in cancer care coordination could also come from initiatives aimed at improving care for the chronically ill. For example, the Academic Chronic Care Collaborative, an initiative of the American Association of Medical Colleges Institute for Improving Clinical Care, has been launched in partnership with the Robert Wood Johnson Foundation’s national chronic illness care program. The collaborative involves 22 academic medical centers that will undergo extensive redesign of their chronic care strategies (AAMC, 2005).
The complexities of the health care system can be particularly daunting for those whose language is not English, who are uninsured, who reside in a rural area, or who have other difficulties in accessing care. One mechanism that is being evaluated to reduce cancer health disparities is “Patient Navigation” (Freeman and Clanton, 2004). A patient navigator is a trained patient advocate and guide who helps individuals and their families navigate their way through the maze of doctors’ offices, clinics, hospitals, outpatient centers, insurance and payment systems, patient support organizations, and other components of the health care system (NCI, 2004). Navigation services include: facilitating communication and information exchange for patients; coordinating care among medical service providers; and arranging for financial support, transportation, or child care services.
Lack of Awareness of the Late Effects of Cancer and Its Treatment
A prerequisite to obtaining appropriate cancer follow-up care is an awareness of one’s increased risk and knowledge of what should be done to reduce risk or ameliorate adverse outcomes. Late effects that are known to be associated with cancer treatments may be discussed in the context of making treatment decisions and obtaining informed consent. Given the stressful nature of this phase of care, cancer patients may have difficulty retaining the information. Patients do not routinely receive a summary of their treatments or possible late effects. Cancer survivors are beginning to be informed about what to expect after treatment through the efforts of patient advocacy organizations. For example, the American Cancer Society (ACS) has provided information on “What Happens After Treatment” for most cancer types (ACS, 2005a) and the Lance Armstrong Foundation has provided a guide to help survivors summarize their medical treatment and plan for follow-up care (LAF, 2004a). A few studies have assessed adult cancer survivors’ awareness of their increased risk and need for follow-up:
-
Female adult survivors of Hodgkin’s disease treated at a young age with mantle irradiation are at high risk for subsequent cancer, but only 47 percent reported having had a mammogram in the past 2 years (Diller et al., 2002). As many as 40 percent of women were unaware of their increased risk.
-
Breast cancer survivors report knowing little about lymphedema before developing it, and physicians report not routinely counseling women or providing written information on lymphedema prevention to their patients with breast cancer (Paskett and Stark, 2000).
-
Only about half of men and women with cancer who are of childbearing age receive timely information from their health care providers about their risk of infertility and options to preserve or restore fertility (Canada and Schover, 2005).
-
Breast cancer survivors often do not recall discussing the reproductive health impact of their treatment, and many report that their concerns are not adequately addressed (Partridge et al., 2004; Duffy et al., 2005).
-
Relatively few (22 percent) survivors of colorectal cancer could identify risk indicators for recurrence, but most (64 percent) agreed that they would like to be told what to look for (Papagrigoriadis and Heyman, 2003).
More is known about the awareness of late effects among survivors of childhood cancer. As part of the Childhood Cancer Survivorship Study (CCSS), members of a large cohort of 5-year childhood cancer survivors have been surveyed to learn more about their health status, health care
behavior, attitudes, and perceptions. When 635 members of this cohort were asked if past therapies could cause a serious health problem with the passage of time, 35 percent responded affirmatively, 46 percent responded negatively, and 19 percent did not know (Kadan-Lottick et al., 2002). Only 15 percent reported that they had ever received a written statement of their diagnoses and treatments to keep as a reference in the future. To learn more about the experiences of survivors of adult cancer, a large cohort study, similar in design to the CCSS, could be initiated (see details of its design in Chapter 7).
Barriers to Communication
Some research suggests there is a disjuncture between patients’ expectations and physicians’ perceptions of cancer follow-up. Most women being followed after breast cancer treatment want to be asked about nutrition, pain, and emotional/family problems, but relatively few want to be asked about sexual problems, according to an American study of patients’ expectations of follow-up visits and perceptions of the value of follow-up tests and examinations. Women in this study overestimated the value of laboratory and imaging studies and underestimated the value of the medical history and physical examination (Muss et al., 1991). Studies conducted among European cancer survivors indicate that information on long-term effects of treatment and prognosis, prevention of cancer, and hereditary factors was desired, as was access to cancer expertise, diagnostic tests, and specialist facilities (Adewuyi-Dalton et al., 1998; de Bock et al., 2004).
Anticipation of a follow-up visit can engender anxiety, and providers must balance providing realistic information with remaining hopeful and reassuring. In one British study of asymptomatic and disease-free survivors’ views on follow-up of colorectal cancer, anticipation of the follow-up appointment caused anxiety (35 percent), sleep problems (27 percent), and decreased appetite (8 percent) (Papagrigoriadis and Heyman, 2003). Most patients (78 percent), however, felt reassured and optimistic for the future after receiving results of tests performed at their visit. This finding that follow-up clinic visits are generally perceived as reassuring has been found in other research (Kiebert et al., 1993; GIVIO, 1994; Stiggelbout et al., 1997).
When cancer survivors seek follow-up care, all components of survivorship care may not be addressed. In a study of follow-up care for women with breast cancer in England, for example, visits were focused on detection of recurrent disease by clinical examination, but little attention was paid to patient education and psychosocial needs (Beaver and Luker, 2004). There is anecdotal evidence for this same pattern of care in the United States. One recent unscientific poll of cancer survivors found that nearly half (49 per-
cent) felt their psychosocial needs were not being met by the health care system (LAF, 2004b). Cancer survivors expressed dissatisfaction with their oncologist’s provision of support in dealing with the secondary aspects of cancer, such as depression, fear of recurrence, chronic pain, ongoing health challenges, infertility, sexual dysfunction, difficulty with relationships, and financial or job insecurity.
As part of a major effort to gauge cancer patients’ experience with cancer within the British National Health System, a nationwide survey was conducted in 2000 (Airey et al., 2002).2 Dissatisfaction with some aspects of post-treatment care mirror those identified in the United States. Nearly one in five (19 percent) survivors reported that doctors and nurses did not spend enough time, or spent no time at all, telling them what would happen when they left the hospital after their first treatment; 26 percent reported not being given written or printed information about what they should or shouldn’t do following their discharge; and 36 percent reported not being told about a support or self-help group. Results of surveys were made available to each group of cancer care providers so they could compare their results with other providers and make efforts to improve care.
Relatively little is known about the content of follow-up care provided in the United States. According to national surveys of ambulatory care, relatively little counseling takes place during cancer-related visits.3 Among cancer-related visits made by individuals who use tobacco, for example, physicians report smoking cessation counseling or referral during only 18 percent of visits (Table 4-1). Guidelines for smoking cessation recommend routinely counseling individuals who smoke (USPSTF, 2003). These estimates are for all cancer-related visits and would include visits for both primary treatment and survivorship care.4
Barriers to communication are compounded for the 90 million American adults who lack the needed literacy skills to effectively use the U.S. health system (IOM, 2004a). The problem of limited health literacy is often greater among older adults, people with limited education, and those with limited English proficiency. For individuals whose native language is not
|
2 |
Nearly three-quarters (74 percent) of patients identified through hospital records responded to the survey. See Appendix 4C for more information on the survey and how it has been used to redesign cancer care systems. |
|
3 |
See Appendix 4B for details of the ambulatory care surveys and their analysis. |
|
4 |
A limitation of these estimates is the underreporting by physicians on the delivery of health behavior counseling. There was only fair to moderate agreement among physician reports on the provision of counseling on smoking, exercise, and diet and directly observed physician behaviors during clinical visits. Reporting by physicians of procedures and tests is more accurate (Gilchrist et al., 2004). |
TABLE 4-1 The Provision of Counseling During Adult Cancer-Related Ambulatory Care Visits, United States, 2001–2002a
|
Characteristic |
Total |
|
Annual number of visits (in 1,000s) |
20,574 |
|
Services ordered or provided (% yes) |
|
|
Mental health or psychotherapy |
4 |
|
Diet counseling/education |
11 |
|
Exercise counseling/education |
6 |
|
Smoking cessation |
2 |
|
Smoking cessation for visits made by patients who used tobacco |
18 |
|
(9% of visits made by tobacco users) |
|
|
aAdults were categorized as being aged 25 and older. Visits for non-melanoma skin cancer were excluded. Radiologists were excluded from the sample of office-based physicians. Clinics providing chemotherapy, radiotherapy, physical medicine, and rehabilitation were excluded from the sample of hospital out-patient departments. SOURCE: Committee staff analyses of the 2001 and 2002 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. See Appendix 4B for details of analyses. |
|
English, issues of health literacy are compounded by issues of basic communication and the specialized vocabulary used to convey health information. In addition, communication barriers may arise that relate to sociocultural differences between survivors and their health care providers (IOM, 2002). These differences may relate to commonly held attitudes, norms, beliefs, and practices for those with certain life experiences (e.g., poverty, membership in a racial or ethnic minority group) or environments (e.g., communities with poor access to health care services).
In summary, there is a limited amount of research regarding cancer survivors’ expectations and experience with their care following primary treatment. Available evidence points to systemic problems in health care delivery that in some cases lead to poor-quality care, such as underuse of post-treatment screening for cancer. When evaluated, problems in survivorship care appear to stem from a lack of coordination between primary care providers and cancer care providers. There is anecdotal evidence of general dissatisfaction with post-treatment care, with cancer survivors reporting too little attention being paid to their many psychosocial concerns, such as depression, fear of recurrence, sexual dysfunction, and financial issues. Why the expectations of cancer survivors are not being met is not clear, but factors that could be at play include a lack of recognition of the value of these aspects of care, the presence of communication barriers, and a lack of delineation of responsibility on the part of providers to address these con-
cerns. There is little information regarding the difficulties survivors may have in communication that are related to low literacy levels and to sociocultural factors.
Barriers Facing Providers
Health care providers face many of the same problems as cancer survivors in dealing with a fragmented system of care. Providers are also hampered in their provision of survivorship care by a lack of training on survivorship and an absence of agreed-upon standards of survivorship care. Such standards are essential to ensuring the delivery of the full complement of services that cancer survivors may need (see discussion of guidelines in Chapter 3). Furthermore, without agreed-upon practice guidelines for care, reimbursement for care can be problematic. Communication issues are also a major challenge to those providing and coordinating survivorship care because individuals with cancer often have multiple providers at different sites of care. Compounding these problems are concerns about the capacity of the primary care and oncology care systems to accommodate the follow-up needs of the large and growing population of cancer survivors.
Fragmented Delivery System Hampers Delivery of Coordinated Care
The fragmented nature of the U.S. health care system hampers the delivery of coordinated survivorship care. Providing such care is a challenge because cancer care is delivered by multiple providers over extended periods of time and through multiple phases of illness. These providers often wish to provide coordinated care, but usually do not work within systems of care that facilitate its delivery. The goal of care coordination is to establish and support a continuous healing relationship, enabled by an integrated clinical environment and characterized by the proactive delivery of evidence-based care and follow-up (IOM, 2001). Clinical integration is further defined as the extent to which patient care services are coordinated across people, functions, activities, and sites over time so as to maximize the value of services delivered to patients. Coordination encompasses a set of practitioner behaviors and information systems intended to bring together health services, patient needs, and streams of information to facilitate the delivery of quality care. Such coordination can be facilitated by procedures for engaging community resources, including social and public health services.
Key strategies that enhance care coordination are often lacking. These include: providing educational supports; instituting patient-centered health records supported by modern information technology; ensuring accountability and defining roles for providers of care; and aligning financial incen-
tives to ensure the delivery of coordinated care (IOM, 2001). The extent to which these strategies operate for providers of survivorship care are described in the following sections.
Lack of Education and Training
Often physicians, nurses, and other providers of cancer survivorship care have not had optimal relevant formal education, training, and continuing medical education. The status of education and training for physicians, nurses, social workers, and other providers of survivorship care is detailed in Chapter 5. The recognition of cancer survivorship as a phase of care associated with an extensive set of management issues is relatively new. Educational and training opportunities are likely to increase as a consensus is reached regarding the content of such care and its delivery, but for now the notion of cancer survivorship as a distinct clinical entity is not prevalent in the provider community. In addition to their lack to education and training regarding cancer survivorship, health care providers report being ill-equipped and -trained to manage the care of patients with chronic conditions. According to one survey conducted in 2000 and 2001, practicing physicians reported that their training did not adequately prepare them to coordinate in-home and community services (66 percent), educate patients with chronic conditions (66 percent), or manage the psychological and social aspects of chronic care (64 percent) (Partnership for Solutions, 2002).
Lack of Survivorship Standards of Care
Health care providers, before being held accountable for providing quality care, need to have clear evidence-based guidance. As described in Chapter 3, such guidance for survivorship care exists for some aspects of care, but it is not readily available to clinicians.5 There are practice guidelines for the follow-up of breast and colorectal cancer, but the focus of the guidelines is generally limited to detecting recurrences and second cancers. There are also guidelines for the management of certain late effects (e.g., lymphedema, osteoporosis, depression), but these have not been widely disseminated to the primary care clinicians most likely to encounter patients presenting with these symptoms. For many aspects of survivorship care—for example, health promotion (exercise and healthy diet)—clear
|
5 |
As described in Chapter 3, a comprehensive set of guidelines has been developed by the Children’s Oncology Group for survivors of childhood, adolescent, and young adult cancers (Landier et al., 2004). |
guidance is not available. For most types of cancer, the research needed to support such guidelines has not been conducted, but assembling that information is critical to informing clinicians and patients on appropriate post-treatment care. Clinical guidelines can also help avoid unnecessary and expensive care, and without them physicians may be under considerable pressure from patients to provide follow-up tests (Loprinzi et al., 2000). Without established guidelines, follow-up practices and expenditures have been shown to vary widely (Virgo et al., 1995; Johnson and Virgo, 1997).
Although guidelines for most aspects of survivorship care are lacking, providers are not following the guidelines that are available. This general phenomenon in medicine (Mendelson and Carino, 2005; Timmermans and Mauck, 2005) is apparent in survivorship care as well. For example, adherence to post-treatment surveillance guidelines is not uniformly high; depression is not routinely assessed; patients complete their primary treatment without knowing about their risks of late effects such as lymphedema; and individuals are not apprised of the implications of their cancer history to employment and health insurance (see Appendix 4A for a summary of studies of U.S. surveillance practice patterns).
Ultimately, health care quality measures will be developed to monitor quality problems in survivorship care. There are three types of quality problems in health care: too little care; too much care; and the wrong care (IOM, 1998). Too little care (underuse) is when patients do not receive evidence-based care. Too much care (overuse) is when patients receive unnecessary health care services that may cause side effects or pose other health risks. The wrong care (misuse) is when diagnoses are missed or delayed, ineffective treatments are used, effective procedures are done poorly, or errors are made. A framework has been created for identifying measures of quality for cancer care (McGlynn, 2002; McGlynn and Malin, 2002). Some quality measures have already been developed (Schachter et al., 2004; AHRQ, 2004a; Greenberg et al., 2005; IOM, 2005) or are under review (NQF, 2005). Few of the measures identified thus far are directly related to survivorship care. Some potential quality of care measures relevant to cancer survivorship are shown in Box 4-2. Such measures, if found to be clinically important, evidence based, practical to measure, and meaningful to providers and patients, could facilitate improvements in care.
The use of quality of care measures has a dual purpose: evaluating progress and motivating change (IOM, 2005). Monitoring systems may help to assess progress according to a particular set of indicators, but may also motivate change though a new focus on processes of care and outcomes. Quality of care measures for other chronic conditions such as diabetes have been developed through public/private partnerships and adopted by health systems to improve care (National Diabetes Quality Improvement Alliance, 2005).
|
BOX 4-2 Processes of care
Screening guidelines
Survivorship interventions
Survivor assessments of care
|
When quality measures for survivorship care are developed and then adopted by health systems, office supports such as computerized reminder systems, the involvement of nonphysician providers in care, and standing orders for screening tests that have been shown to be effective in promoting preventive health services will likely also prove useful in prompting the delivery of appropriate survivorship care (IOM, 2003b).
Difficulties in Communication
Communication issues are a major challenge to those providing and coordinating survivorship care because individuals with cancer often have multiple providers at different sites of care. Discharge plans that are clear to
the oncologist may not be clear to the primary care provider. The migration of patients across health plans and the geographic movement that characterizes contemporary mobile society create a turnover in health providers. Large separations of time are characteristic of survivorship care, which can extend over a period of decades. Clinicians unfamiliar with the patient’s medical history may have difficulty in determining the names of the original doctors or health care institutions or in obtaining documentation of the cancer diagnosis and treatment regimen.
Relatively few health care providers have access to information systems and electronic medical records that would facilitate communication regarding survivorship care (Burt and Hing, 2005; Berner et al., 2005; Ash and Bates, 2005). According to a survey of U.S. physicians in 2003, only 7 percent said they routinely use e-mail to communicate with other doctors, and only 27 percent used electronic medical records (Audet et al., 2004). Perceived barriers to the adoption of information technology included the costs of system start-up and maintenance, lack of standards, and lack of time to consider acquiring, implementing, and using a new system. The investigators concluded that widespread adoption of information technology in health care would require federal leadership, potentially in the form of federal grants, increased physician reimbursement, and loans. A 2004 health information technology initiative, if fully implemented, would further the adoption of these communication tools (Thompson and Brailer, 2004). As part of this effort, a strategic framework for action has been developed to inform clinical practice, interconnect clinicians, personalize care, and improve population health. Although these developments are encouraging, improvements in information technology and adoption of electronic medical records must be viewed as enabling technologies. Improvements in the quality and coordination of care will require investments in medical practice support systems, financial rewards for quality improvement, and improved information technology infrastructure (Miller and Sim, 2004).
There are a few examples of technological innovation to improve communication between primary care providers and cancer specialists. An interactive Internet resource, Passport for Care, is being developed for survivors of childhood cancer. Elements of the website include: a guidelines generator that dynamically assembles recommendations for care individualized to each survivor according to his or her treatment history;6 an end-of-treatment summary, completed by the treating institution and available
to the survivor, that can be securely shared with providers at the direction of the survivors; individualized survivor education resources that are customized to the needs of each patient based on his/her disease and its treatment (and accessible to the survivor’s health care provider); an online survivor forum; and a section for survivor news and stories (Personal communication, M. Horowitz, Baylor College of Medicine, February 23, 2005). Once completed and evaluated, it is planned to encompass adult cancer survivors as well. Another initiative is the Cancer Survivor Virtual Information Center, a website with information for cancer survivors and their physicians. It is undergoing a pilot feasibility study targeting survivors of childhood Hodgkin’s lymphoma (Personal communication, K. Oeffinger, University of Texas–Southwestern, June 24, 2004). The website contains information about survivorship, but does not provide specific information about individual patients. As part of a Patient Gateway initiative at Partners HealthCare, a web-based information system is being piloted in oncology to enhance care coordination across multiple practices, including medical oncology, radiation oncology, and primary care (Personal communication, J. Wald, Partners HealthCare, March 22, 2005).
The Improving Cancer Care in Massachusetts (CAMA) project, sponsored by the Dana-Farber/Harvard Cancer Center, aims to improve cancer treatment in Massachusetts through the use of more efficient and timely data on cancer care quality (Ayanian, 2004). The CAMA investigators plan to assess the feasibility of a personal health record that integrates care information from multiple care sites. The plan is to give patients web-based access to relevant information from their medical records, and enable them to share information with their clinicians. The CAMA system would give clinicians more complete and timely medical information on their patients, including information from other care sites.
In Europe, a few systems are already in place. One hospital in Italy has, with cancer patients’ consent, made information about cancer care available to each patient’s primary care physician through a protected website (Personal communication, F. Testore, Head of Oncology Division, Ospedale Civile di Asti, Asti, Italy, November 22, 2004). Community-based physicians can also send e-mail requests to specialists through this system to get information about their patients. In a similar fashion, a secure ONCONET system has been established in the German federal state of Saxony-Anhalt to facilitate the shared care of cancer patients (Blobel, 2000). The system aids communication among 57 clinics and more than 160 general practitioners involved in oncology. The system includes an electronic health care record, scheduling functions, and the creation of doctor’s reports. It also supports research activities and quality assurance efforts for cancer care. Many other systems are being developed to improve communication between patients and physicians, such as Internet-based tools. Such systems
can be used to send e-mail, view the medical chart, provide health information, and send personalized reminders about care.
Until information technology advances and standard systems are in place to facilitate communications, cancer care providers need to rely on mechanisms at hand, such as a letter from a specialist to a primary care provider detailing the nature of a patient’s cancer, a summary of primary treatment, risks of late effects, and a survivorship care plan (see Chapter 3 for more information on individual survivorship care plans). Such a summary and cancer survivorship care plan should also be provided to survivors so they can be alerted to possible late effects, engage in recommended health promotion activities, and actively seek necessary care.
The Capacity for Delivering Survivorship Care
Both oncologists and primary care providers want to provide follow-up care to cancer patients after their treatment (Bope, 1987; Williams, 1994). However, when survivorship care is delivered, there is often no clear plan or designated responsibility. Some models of care that foster shared care with designated responsibilities are emerging, but these have not been extensively tested (see discussion of alternative models below). The creation of alternative models of delivery is needed to accommodate the growing numbers of cancer survivors.
Primary care clinicians, who manage general health and survivorship needs, must have systems in place to coordinate ongoing care with the work of oncologists and other specialists to provide streamlined attention to cancer-related issues. The primary care system is under tremendous stress, and only innovative models of coordination will serve to accommodate the expanded workload that will come from a growing survivor population.
Handling the cancer-related issues of the survivor population may also become more difficult for oncologists. While surveillance for recurrence, cancer spread, and second cancers is usually the responsibility of oncologists, many of the late effects of cancer are most appropriately managed by other providers such as physiatrists, cardiologists, fertility specialists, and psychologists. Oncologists will need the help of other clinicians to steer patients to the most appropriate specialists and to coordinate the delivery of care. The increasing volume of cancer survivors may also hamper their ability to see new patients. Gauging resource use according to the National Comprehensive Cancer Network (NCCN) guidelines provides one indicator of the magnitude of the problem. If NCCN guidelines are adhered to for breast cancer, a breast cancer survivor would make 10 to 15 visits over the course of 5 years. For colorectal cancer, the recommended number of visits is 14. The NCCN site-specific guidelines only cover issues related to the detection of recurrence and second cancer, not the full complement
of survivorship care that the committee recommends. The workload generated for specialists by cancer survivors can be significant. In one study, 210 patients who had achieved a complete or partial remission following treatment for Hodgkin’s disease between 1984 and 1990 generated 2,512 outpatient follow-up visits during the follow-up period (Radford et al., 1997). In another study of resource use, 535 women with breast cancer (all stages) made 8,206 follow-up visits during the first 5 years of follow-up (Kaija et al., 1996). With demographic trends predicting a surge in new cancer patients in need of follow-up care, there is an imperative to assess alternative models that will deliver needed services to cancer survivors.
In summary, physicians share some of the same frustrations as cancer survivors in terms of fragmentation of care, poor mechanisms for communication, and a lack of agreement on what constitutes survivorship care and how it should be provided. Of note is the apparent universality of fragmented chronic care, irrespective of delivery system. Such fragmentation in survivorship care is evident in studies carried out in European countries with national health plans (see Appendix 4C). Overcoming fragmentation rests on building an integrated systems approach—getting primary care providers, oncologists, and other care providers to work together as a team, to agree on how to communicate with each other, and to work out streamlined transitions in care.
Facilitating such an integrated system of care are improvements in communication technology. Efforts underway to improve the health care information technology infrastructure will likely help in overcoming problems of fragmentation and enhance chronic care delivery. Innovative applications of the Internet to promote shared care for cancer patients have been implemented in Europe and hold promise in furthering coordination of care. Until such innovations are more widely available, however, the burden of overcoming problems related to fragmentation largely rests with combined efforts of primary care and oncology providers. Office supports such as reminder systems, standing orders for certain screening tests, and standardized letters to primary care providers are among the tools available now. Providers of survivorship care should welcome consideration of new models for delivering this post-treatment care in light of the enormous resource use posed by the expansion of the survivorship population and a more comprehensive definition of what constitutes good survivorship care.
MODELS FOR DELIVERING SURVIVORSHIP CARE
How different follow-up delivery strategies affect health outcomes and costs, perceptions of quality of life, and satisfaction with care is relatively unexplored. Most research in the area has been conducted in the context of breast cancer care. A recent systematic review on the effectiveness of fol-
low-up services concluded that there is insufficient primary empirical evidence on which to draw broad conclusions regarding best practice for breast cancer follow-up in terms of patient involvement in care, reductions in morbidity, and cost-effectiveness of service provision (Collins et al., 2004).
Some promising models of follow-up care have emerged, including a shared-care model that integrates oncology with primary care follow-up, a nurse-led model of care, and specialized multidisciplinary survivorship follow-up clinics. Relatively little is known regarding cancer survivors’ preferences for care, but there is a growing recognition of the need for flexible options for survivors who may have different needs and circumstances (Koinberg et al., 2002).
Shared-Care Model of Follow-up Care
Shared care has been defined as “care which applies when the responsibility for the health care of the patient is shared between individuals or teams who are part of separate organizations, or where substantial organizational boundaries exist” (Pritchard and Hughes, 1995). Such a model implies personal communication and organized transfer of knowledge from specialists to primary care practitioners as well as patient involvement (Nielsen et al., 2003). Cancer patients may face several care transitions, for example, from their active treatment phase, to survivorship care, to care for a recurrence, and finally to palliative and end-of-life care. With such transitions, the focus of care can shift toward specialty care or toward primary care. When the shift is toward primary care, a smooth transition is more likely when the primary care physician receives relevant and timely information from cancer specialists (Braun et al., 2003).
Primary care physicians are actively providing cancer-related care according to ambulatory care surveys of U.S. office-based and hospital-based physicians. Of all the cancer-related visits that were made to physicians’ offices in 2001 and 2002, nearly one-third (32 percent) were made to primary care physicians (Table 4-2). Relatively fewer such visits were made to oncologists (18 percent). Cancer-related primary care visits were somewhat more common when they were for prostate cancer and lung cancer, which may indicate primary care providers’ active role in symptom management, palliative care, and end-of-life care.
The role of the primary care clinician in the shared-care model is to ensure that all of the physical and emotional health needs of the patient are addressed, to assume responsibility for aspects of care of the chronic disease that are feasible in the primary care setting, to refer the patient to specialists for periodic reevaluations and to address issues that require focused expertise, and to consult with specialists on areas of uncertainty. The role of the
TABLE 4-2 Distribution of Adult Ambulatory Cancer Care Visits, by Site of Visit, Physician Specialty, and Clinic Type, United States, 2001–2002a
|
Visit Characteristic |
Number/Percentage |
|
Annual number of visits (in 1,000s) |
20,574 |
|
Site of visits (%) |
|
|
Physician’s office |
89 |
|
Hospital outpatient department |
11 |
|
Physician office visitsb (%) |
|
|
Oncology |
18 |
|
Primary care |
32 |
|
General surgery |
10 |
|
Specialty surgery |
3 |
|
Dermatology |
7 |
|
Urology |
14 |
|
Other medical specialty |
15 |
|
Hospital outpatient departmentc (%) |
|
|
General medicine |
78 |
|
Surgery |
14 |
|
Other |
8 |
|
aAdults were categorized as being aged 25 and older. Visits for non-melanoma skin cancer were excluded. bRadiologists were excluded from the sample of office-based physicians. cClinics providing chemotherapy, radiotherapy, physical medicine, and rehabilitation were excluded from the sample of hospital outpatient departments. SOURCE: Committee staff analyses of the 2001 and 2002 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. See Appendix 4B for details on analyses. |
|
specialist who participates in shared care is to provide guidance and treatment in the area of expertise, to keep the primary care clinician informed of the treatment plan, and to return the patient to the primary care provider for implementation of the treatment plan and for care of other health needs. This model is applicable for many conditions, including when primary care providers share care for the management of chronic heart failure (working with cardiologists), multiple sclerosis (working with neurologists), bipolar disorder (working with psychiatrists), and chronic renal failure (working with nephrologists).
The shared care model depends on the specialist and generalist having a common understanding of expected components of care and respective roles, and works best when providers communicate clearly with each other. Shared care may not be fully understood or practiced. Specialists may
TABLE 4-3 Proportion of Adult Cancer-Related Ambulatory Care Visits for Which Care Was Shared by Other Physicians, by Site of Care, United States, 2001–2002a
|
Characteristic |
Total |
Physician Office-Based Visits |
Hospital Outpatient Department Visits |
|
Annual number of visits (in 1,000s) |
20,574 |
18,311 |
2,263 |
|
Other physicians share care for problem (%) |
|
||
|
Yes |
47 |
46 |
55 |
|
No |
41 |
43 |
24 |
|
Unknown |
12 |
11 |
20 |
|
aAdults were categorized as being aged 25 and older. Visits for non-melanoma skin cancer were excluded. Radiologists were excluded from the sample of office-based physicians. Clinics providing chemotherapy, radiotherapy, physical medicine, and rehabilitation were excluded from the sample of hospital outpatient departments. SOURCE: Committee staff analyses of the 2001 and 2002 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. See Appendix 4B for details of analyses. |
|||
believe that it is their obligation to follow up on their patients and that patients prefer to see them for their cancer-related care, even when that care could be provided by a primary care physician. They may also question the ability of primary care physicians to handle all components of follow-up care (e.g., detection of recurrence) (Steinberg and Rose, 1996). For their part, primary care physicians may not have been informed by care specialists of the important role they have to play in the ongoing care of cancer survivors. A balance between primary care and specialty care is clearly needed, as evidenced by the research of Earle and colleagues cited above (Earle et al., 2003; Earle and Neville, 2004).
Studies of shared cancer follow-up care in the United States are limited. According to national surveys, U.S. physicians report that care is shared by other physicians for nearly half (47 percent) of cancer-related visits (Table 4-3).7 Shared care is reported more often by physicians in hospital outpa-
tient departments than by physicians in office-based practices (for 55 versus 46 percent of visits, respectively).
In Europe, Canada, and Australia, several research initiatives have been undertaken to promote shared care (see Appendix 4C). Findings from this research suggest that cancer-related follow-up care can be provided by primary care providers and at lower cost without sacrificing patient satisfaction, but that a minority of patients wish to continue to see specialists for their follow-up care (Grunfeld et al., 1996, 1999a,b). The timely transfer of information from one care sector to another is critical to the concept of shared care. Addressing patient anxiety is also key to a successful transfer from specialty to primary care, and simple strategies, such as discussing plans for follow-up with patients and designing a standardized discharge letter, can ease the transition (Glynne-Jones et al., 1997; Braun et al., 2003). Successful shared-care models depend on professional training; general practitioners viewing their role in cancer care as enhancing patient care and improving their job satisfaction; and appropriate remuneration (Nielsen et al., 2003; Maher and Millar, 2003).
In summary, the shared-care delivery model appears to be especially relevant for the transition from active cancer treatment to survivorship care. U.S. primary care physicians are playing a significant role in cancer care, and nearly half of cancer-related ambulatory visits are characterized as shared care, but with available information it is not clear what the relative roles of specialists and primary care providers are in these settings. Research points to the importance of setting expectations and planning for follow-up early in the care process. Demonstrations and evaluations of shared survivorship care are needed, as are assessments of the shared-care model’s effects on resource use and costs.
Nurse-Led Model of Cancer Follow-up Care
Nurses have successfully led comprehensive, long-term follow-up clinics for survivors of childhood cancer throughout the United States (Hobbie and Hollen, 1993; Hollen and Hobbie, 1995; IOM, 2003a). Clinical nurse specialists have also delivered post-treatment oncology care in rural areas (White et al., 1996; Desch et al., 1999), successfully managed cancer symptoms (Given et al., 2002), promoted continuity of care (Smith et al., 1998), played a key role in cancer disease management programs (Lee, 2004), provided survivorship care in research settings (Ganz et al., 2000), and conducted survivorship research (Ferrell et al., 1992, 1995, 1997, 1998, 2003a,b; Dow et al., 1999; Ritz et al., 2000). Nurse-led follow-up services are acceptable, appropriate, and effective, according to a comprehensive review of the literature evaluating the impact of nurse-led follow-up in cancer care. Although the evidence base for the review was far from com-
plete, the review concluded that nurse follow-up can be an efficient means of maintaining contact with a large client group, providing vital support to vulnerable patients during their move into aftercare and beyond (Cox and Wilson, 2003). A nurse-led case management program also appears promising in improving cancer care for individuals with low incomes (Maliski et al., 2004).
Nurses would appear to be very well suited to providing survivorship care, given the emphasis in nursing education and training on patient assessment, symptom management, psychosocial care, and care planning. Nurses have assumed important roles in survivorship care in Europe and Australia. Research related to their integration into care systems is described in Appendix 4C. Most of these studies have found that cancer survivors are satisfied with follow-up care from nurses, but some cancer survivors prefer to remain with a specialist physician for their post-treatment long-term care (Earnshaw and Stephenson, 1997; Pennery and Mallet, 2000; Renton et al., 2002; Brown et al., 2002; Papagrigoriadis and Heyman, 2003; Koinberg et al., 2004). Creative strategies for harnessing the talents of American nurses in cancer survivorship have been proposed (Leigh, 1998; Pelusi, 2001). Despite the obvious appeal of a nurse-led model of cancer follow-up care, such a model has not been widely implemented or evaluated in the United States.
A factor limiting the feasibility of having nurses provide survivorship care is the short supply of nurses (see Chapter 5). In addition, nurses are more likely to work in hospitals than in outpatient or community-based settings, where cancer follow-up care is most likely to be delivered (see Chapter 5).
According to national surveys of ambulatory care, registered nurses or physician assistants are involved in 27 percent of cancer-related ambulatory care visits. Nurses are much more likely to be involved in care during visits to hospital outpatient departments than physician office-based practices (67 versus 22 percent, respectively) (Table 4-4). The focus of the surveys represented in Table 4-4 is on ambulatory care settings where individual encounters with physicians take place. Nurses are often involved in the administration of chemotherapy and in the provision of supportive care, but the estimates provided in Table 4-4 excluded patient visits to freestanding ambulatory care centers and hospital outpatient chemotherapy, radiotherapy, physical medicine, and rehabilitation clinics.
In summary, although a nurse-led model of cancer follow-up appears to be promising, there is relatively little research available to judge its effectiveness and acceptance in the United States. Nurses are central to any interdisciplinary effort in survivorship care and in some instances, nurses may be the best survivorship care providers. Barriers to adopting nurse-led models of survivorship care include a shortage of trained oncology nurses,
TABLE 4-4 Percentage of Adult Cancer-Related Ambulatory Care Visits During Which Patients Saw an RN, PA, or NP, by Site of Care, United States, 2001–2002a
|
Characteristic |
Total |
Physician Office-Based Visits |
Hospital Outpatient Department Visits |
|
Annual number of visits (in 1,000s) |
20,574 |
18,311 |
2,263 |
|
Saw RN, PA, NP during visit (%) |
|||
|
Yes |
27 |
22 |
67 |
|
No |
73 |
78 |
33 |
|
aAdults were categorized as being aged 25 and older. Visits for non-melanoma skin cancer were excluded. Radiologists were excluded from the sample of office-based physicians. Clinics providing chemotherapy, radiotherapy, physical medicine, and rehabilitation were excluded from the sample of hospital outpatient departments. NOTE: RN = registered nurse; PA = physician assistant; NP = nurse practitioner. SOURCE: Committee staff analyses of the 2001 and 2002 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. See Appendix 4B for details of analyses. |
|||
especially in outpatient settings (see Chapter 5), and the potential preference on the part of some cancer patients to receiving follow-up care from physicians.
Survivorship Follow-up Clinics
A few academic centers have developed cancer survivorship clinics that concentrate needed expertise to provide follow-up care in one location. Such programs can facilitate the application of a holistic and coordinated approach to medical and psychosocial problems. One potential disadvantage of such clinics is the separation of survivorship care from other routine care and the attendant difficulties of communication and coordination. Selected attributes of the few clinics for survivors of adult cancers are described in Table 4-5.
According to representatives of these clinics, they are labor intensive and the respective roles of physicians and other personnel are not well established. Many of the services available in the clinics are provided by expert oncology nurses and nurse practitioners. A barrier to the dissemination of such clinics is the uncertainty regarding adequate reimbursement for
TABLE 4-5 Adult Cancer Survivorship Clinics
|
Clinic Name and Location |
Clinic Characteristics |
|
University of Texas M.D. Anderson Cancer Center: Life After Cancer Care |
|
|
No external support |
|
|
University of Michigan: Breast Cancer Survivor Clinic |
|
|
No external support |
|
|
University of Pennsylvania: Living Well After Cancer Program |
|
|
Supported by the Lance Armstrong Foundation |
|
|
Clinic Name and Location |
Clinic Characteristics |
|
|
|
Dana-Farber Cancer Institute: Lance Armstrong Foundation Adult Survivorship Clinic |
|
|
Supported by the Lance Armstrong Foundation |
|
the range of services provided, especially because nonphysician personnel deliver much of the care. In addition, referrals to the clinics are limited because many cancer survivors and oncologists are not aware of the clinics, probably because they were established only in the past few years. Also, some patients prefer to continue seeing their oncologists, and some oncologists would rather follow patients themselves.
Although relatively few survivors of adult cancer are cared for in specialized survivorship clinics, specialized follow-up clinics for survivors of childhood cancer have emerged as an acceptable model in the past decade. There are 35 comprehensive follow-up programs for survivors of
pediatric cancer, according to the website of the Association of Cancer Online Resources (Pediatric Oncology Resource Center, 2003).8 According to a 2000 survey, 44 percent of childhood cancer survivors reported that they had attended a clinic expressly for follow-up of their cancer (Kadan-Lottick et al., 2002). Information collected on health behaviors during an earlier period indicated that 42 percent of young adult survivors reported having had a cancer-related visit and 19 percent a visit at a cancer center (Oeffinger et al., 2004). The clinics diagnose and manage treatment-related sequelae; provide education and counseling; develop surveillance recommendations; address issues related to insurance, education, and employment; and conduct research on late effects (IOM, 2003a).
Pediatric nurse practitioners trained in oncology generally manage the clinics in collaboration with one or more pediatric oncologists. Additional personnel involved, usually on a referral basis, include social workers, psychologists, and other specialists (e.g., cardiologists, fertility specialists, genetic counselors). Well-established programs typically assess 300 to 400 survivors annually, while newer programs or those serving smaller patient populations report seeing only 50 or 60 patients each year. Most programs picked up patients after they had completed their care from their treating oncologist, generally when they were 2 years removed from the completion of therapy and/or 3 to 5 years from diagnosis, and disease free.
Although these comprehensive follow-up programs are addressing the concerns of cancer survivors and their families, there have been no evaluations of their effectiveness or value. As a consequence, a referral to a long-term follow-up program is often initially met by denial from health insurers who contend that such care is not medically necessary. Efforts to overturn these denials usually succeed in securing authorization for follow-up care, but insurers often stipulate that all laboratory and diagnostic tests be performed within network. This can present logistical problems to patients who must travel extended distances to access follow-up care. In addition, reimbursement for services provided in long-term follow-up clinics typically falls far short of compensation for the time and effort required to evaluate and manage these patients. In fact, many services garner no reimbursement for surveillance programs, including those provided by social workers, education specialists, genetic counselors, nutritionists, or dentists. Consequently, hospitals often rely on grant support or philanthropic dona-
tions to partially subsidize the costs of providing long-term follow-up care. Similar issues will likely arise for clinics serving survivors of adult cancer.
Although some cancer centers have focused on survivorship care by creating specialized survivor clinics, Memorial-Sloan Kettering Cancer Center is integrating survivorship care into disease-site-specific clinics. After completing primary therapy, survivors continue to be seen in the same medical clinic where they received treatment, but receive follow-up care from a provider with expertise in survivorship, usually a nurse practitioner. This model is currently being pilot-tested in lung, head and neck, prostate cancer, and lymphoma clinics (Personal communication, M. McCabe, Memorial Sloan-Kettering Cancer Center, March 23, 2005). Core evaluation criteria for survivors are being developed and will be used institutionwide, with specialized items added for each disease site. In addition to medical evaluation, Memorial Sloan-Kettering is also piloting a psychosocial screening effort. A screening questionnaire to evaluate emotional functioning and facilitate immediate referrals is currently being pilot-tested and, if successful, will be put into general use (Personal communication, J. Ford, Memorial Sloan-Kettering Cancer Center, March 23, 2005). A sexual health clinic, smoking cessation services, and fertility preservation services are being established or expanded to supplement the survivorship services that are provided in each site-specific clinic. These efforts expand on Memorial Sloan-Kettering’s existing survivorship infrastructure, which includes the Post-Treatment Resource Program, an education and support center that has served more than 50,000 people since it was established in the 1980s. This program is open to all survivors, regardless of where they received primary treatment, and provides seminars and workshops on late effects and other survivorship issues, consultation on insurance and employment issues, and professionally led educational support groups (MSKCC, 2004).
At cancer centers that do not have a dedicated survivorship clinic, support services and educational programs are still often available to survivors. For example, a program similar to the Sloan-Kettering Post-Treatment Resource Program is being developed at the Nevada Cancer Institute with the support of the Lance Armstrong Foundation (LAF, 2005a; NVCI, 2005). The Lance Armstrong Foundation Cancer Survivorship Center, which will be located in the patient library at the Nevada Cancer Institute, will provide educational programs, translation and interpretation services, navigation services, and general support to survivors and their families. The program, which is currently in development, will be tailored to the needs of survivors in Nevada as identified by a survey being conducted of cancer survivors in the state.
In addition to the general cancer survivorship clinics described in Table 4-5, there are a number of more narrowly focused referral clinics that provide some aspects of survivorship care. For example, The University of
Texas M.D. Anderson Cancer Center has a clinic for the diagnosis and management of fatigue (M.D. Anderson Cancer Center, 2004); Beth Israel Hospital in New York has a sexuality clinic to address post-treatment sexual late effects (Continuum Health Partners, 2005); and the Fox Chase Cancer Center has a family risk assessment program to provide screening, genetic counseling and testing, and follow-up services (Fox Chase Cancer Center, 2004). Psychosocial interventions, lymphedema care, and menopausal symptom management are other types of care that could be handled by such specialized referral clinics.
In summary, a handful of dedicated clinics have been established to meet the needs of survivors of adult cancer, but they see relatively few patients and have not been formally evaluated. Other clinics are available to manage particular late effects, for example, fatigue, sexual dysfunction, genetic risk, and symptoms of menopause. These specialized clinics are available to individuals with and without cancer and so may be more economically viable. There is virtually no information on the cost-effectiveness and acceptability to patients and providers of either generalized cancer survivorship clinics or the more specialized cancer-related ancillary clinics. There is more experience with clinics that have been established to meet the needs of childhood cancer survivors, but here too, there has been no research to evaluate their cost-effectiveness. Difficulties in obtaining reimbursement for services through these clinics will persist until evidence of their effectiveness has been demonstrated.
THE INFRASTRUCTURE FOR DELIVERING SURVIVORSHIP CARE
Much of the research on the organization of cancer survivorship care has been conducted in Europe and Canada (findings from international studies are summarized in Appendix 4C). While much can be learned from the experience in other countries, the U.S. health care system is somewhat unique. First, adequacy of insurance coverage varies greatly, from no coverage at all for an estimated 11 percent of individuals ages 25 to 64 with a history of cancer, to somewhat generous coverage for elderly cancer survivors enrolled in Medicare who, in addition, have private supplemental coverage (see Chapter 6 for a review of insurance issues). In addition to the confusing array of health insurance products that are available, health care consumers in the United States face a heterogeneous delivery system that includes, at one extreme, managed care, with its focus on controlling costs and coordinating care, and, at the other extreme, fee-for-service care that optimizes choice of health care providers, but leaves care coordination to the patient and doctor. Although the United States lacks a comprehensive national system of care, there is an organized federally supported infrastructure for cancer-related clinical research and care.
This section of the chapter reviews the limited information on where cancer-related care is delivered and what survivorship services are available within the U.S. cancer care system. Although most cancer care is provided in outpatient settings, little information is available on survivorship care or service availability in these settings. More information is available on survivorship-related services that are provided in hospitals with well-developed cancer programs. Examples of the delivery of certain cancer survivorship services are described in Appendix 4D (genetic counseling, rehabilitation, and psychosocial services).
Cancer-Related Hospital and Ambulatory Care
Until the early 1980s most cancer care was delivered in hospitals. The dramatic shift from hospital to ambulatory care began in 1983 when Medicare’s inpatient Diagnostic Related Group payment system went into effect. With the added cost-constraining influence of managed care, cancer care has shifted largely to outpatient settings. Mastectomy and other breast surgical procedures, for example, have been increasingly performed in outpatient day-hospital settings (Case et al., 2001). The implication of this shift in site of care is that people cared for in outpatient settings may no longer have access to the many supportive care personnel that are hospital based, such as social workers, nurse educators, psychologists, and clergy. According to national health care surveys, there were an estimated 1.2 million cancer-related hospitalizations and 20.6 million ambulatory care visits in 2002 (Tables 4-6 and 4-2).
When a person with cancer is hospitalized, the availability of ancillary services that might be needed long term (oncology social workers, rehabilitation specialists) often vary by care setting. A rich array of hospital-based specialists and services may, for example, be available in larger hospitals, but absent in smaller hospitals. Fifteen percent of cancer-related hospital discharges are from hospitals with fewer than 100 beds where ancillary and supportive care resources may not be widely available. An equal share of cancer-related hospital discharges are from bigger hospitals (500 or more beds) that likely have more supportive care resources.
In terms of ambulatory care among adults, most cancer-related visits are to physicians’ offices, with only 11 percent of visits made to hospital-based outpatient departments where supportive care specialists tend to be available. For example, a registered nurse, nurse practitioner, or physician’s assistant is three times more likely (67 versus 22 percent) to be involved in a cancer-related visit to a hospital outpatient department than a physician’s office (Table 4-4).
Efforts to improve services to members of minority racial/ethnic groups or to the uninsured could focus on hospital outpatient departments because
TABLE 4-6 Characteristics of Cancer-Related Hospital Discharges, United States, 2002a
|
Characteristic |
Cancer-Related Hospital Discharges (in 1,000s) |
Percent Distribution |
|
Type of cancerb |
||
|
All types |
1,175.1 |
100.0 |
|
Lung, other respiratory |
146.5 |
12.5 |
|
Female breast |
85.1 |
7.2 |
|
Prostate |
92.0 |
7.8 |
|
Colon, rectum |
155.4 |
13.2 |
|
Lymphatic and hematopoietic |
128.6 |
10.9 |
|
Gynecologic |
73.3 |
6.2 |
|
Bladder |
32.6 |
2.8 |
|
All others |
461.5 |
39.3 |
|
Hospital size (no. of beds) |
1,175.1 |
100.0 |
|
6-99 |
171.4 |
14.6 |
|
100-199 |
240.6 |
20.5 |
|
200-299 |
258.1 |
22.0 |
|
300-499 |
324.7 |
27.6 |
|
≥500 |
180.3 |
15.3 |
|
aBased on a sample of 11,033 hospital discharges from nonfederal, short-stay hospitals with a primary diagnosis of cancer. Numbers and percentages are adjusted using sampling weights to produce national estimates. Numbers and percentages may not add to total because of rounding. bAccording to the Ninth Revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM): Lung, other respiratory = 161, 162; female breast = 174; prostate = 185; colon, rectum = 153, 154; lymphatic and hematopoietic = 200-208; gynecologic = 179-184; bladder = 188; other = all other malignancies (ICD-9-CM 140 to 208 and V10). SOURCE: Committee Staff analyses of the 2002 National Hospital Discharge Survey (NCHS, 2004). |
||
disproportionately more cancer-related care is provided to these groups at these sites of ambulatory care than in physician offices (Table 4-7).
Changes in the organization of cancer care can facilitate the shared-care model. Comprehensive breast care programs have been developed over the past decade to put under one roof the many providers and services that an individual with breast cancer might need to make breast care simpler and to provide “one-stop” care. Typically, these programs employ physician specialists, clinical nurse specialists, social workers, psychologists, and other providers to meet the range of breast cancer needs throughout diagnosis, treatment, and follow-up. While care in such settings is likely more
TABLE 4-7 Patient’s Race/Ethnicity and Payment Source for Adult Cancer-Related Ambulatory Care Visits, by Site of Care, United States, 2001–2002a
|
Characteristic |
Total |
Physician Office-Based Visits |
Hospital Outpatient Department Visits |
|
Annual number of visits (in 1,000s) |
20,574 |
18,311 |
2,263 |
|
Race/ethnicity (%) |
|||
|
White, non-Hispanic |
85 |
87 |
73 |
|
White, Hispanic |
3 |
2 |
9 |
|
Black |
9 |
9 |
15 |
|
Other |
2 |
2 |
3 |
|
Main payment source (%) |
|||
|
Private insurance |
41 |
42 |
37 |
|
Medicare |
46 |
47 |
37 |
|
Medicaid |
4 |
3 |
12 |
|
Uninsured (self-pay/no charge) |
2 |
2 |
4 |
|
Other, unknown source |
6 |
6 |
10 |
|
NOTE: Percentages may not add to 100 because of rounding. aAdults were categorized as being aged 25 and older. Visits for non-melanoma skin cancer were excluded. Radiologists were excluded from the sample of office-based physicians. Clinics providing chemotherapy, radiotherapy, physical medicine, and rehabilitation were excluded from the sample of hospital outpatient departments. SOURCE: Committee staff analyses of the 2001 and 2002 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. See Appendix 4B for details of analyses. |
|||
integrated, some evidence suggests that surveillance tests for breast cancer are overused in breast cancer centers (Lash and Silliman, 2001). There are no data on how many individuals with breast cancer are seen in such programs, but it is likely to be a small fraction of the total number of breast cancer patients (Frost et al., 1999; Rabinowitz, 2002). Analyses of care provided to Medicare beneficiaries during 1994–1995 suggest that surgical treatment for older women with breast cancer is decentralized and being provided primarily by surgeons in low-volume settings (Neuner et al., 2004). Most (55 percent) surgeons providing breast cancer surgery worked in solo or two-physician practices. Only 12 percent of surgeons worked in hospital-based practices.
Cancer survivorship is a distinct phase of care, but it is difficult to know where the 10 million cancer survivors are along the cancer care
trajectory. One study of Medicare beneficiaries diagnosed with colorectal cancer between 1975 and 1996 assessed the type of care survivors were receiving in 1996. Investigators classified care into four categories:
-
Initial diagnosis treated with curative intent
-
Post-diagnostic monitoring
-
Treatment for recurrent/metastatic disease or second primaries
-
Terminal care
More than one-third (38 percent) of beneficiaries were determined to have received post-diagnostic monitoring care (Mariotto et al., 2003). The prevalence of type of care in 1996 is shown in Figure 4-2 by years since diagnosis. Those diagnosed within the past 2 years were usually in the initial phase of care. After 2 years, however, the monitoring phase of care predominates. This first study of “care prevalence” relied on analysis of the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database,9 which allows investigators to examine the health care claims of individuals following their diagnosis of cancer.
Survivorship Services Within Cancer Centers
With the limited number of dedicated cancer survivorship programs and large and growing population of cancer survivors, the committee attempted to assess the availability and scope of survivor-oriented services within cancer centers. Information was sought for the following sites of care:
-
National Cancer Institute-designated Comprehensive Cancer Centers
-
Cancer programs approved by the American College of Surgeons’ Commission on Cancer
-
Community cancer centers that are members of the Association of Community Cancer Centers
NCI-Designated Comprehensive Cancer Centers
The National Cancer Institute (NCI) supports 60 major academic and research institutions throughout the United States to sustain interdisciplinary programs in cancer research (NCI, 2005a). A relatively small proportion of individuals with cancer are cared for within these institutions, but
|
9 |
The SEER-Medicare database is described in Chapter 7. |
FIGURE 4-2 Average number of months of phase of care in 1996 among patients diagnosed with colorectal cancer from 1975 to 1996, by years since diagnosis.
SOURCE: Mariotto et al. (2003).
they provide important opportunities for participation in clinical trials and other research. Cancer Center Support Grants fund the scientific infrastructure of the cancer centers, and recipients of these grants are recognized as NCI-designated cancer centers. Two types of cancer centers are designated based on the degree of specialization of their research activities (Figure 4-3): (1) comprehensive cancer centers integrate research activities across laboratory, clinical, and population-based research (there were 38 such centers in 2005); and (2) cancer centers not designated as “comprehensive” have a scientific agenda that is primarily focused on basic sciences, population sciences, or clinical research, or any two of the three components (there were 22 such cancer centers in 2005).
Although NCI grants are used solely to support the research infrastructure at cancer centers, all designated cancer centers also provide clinical care and service for cancer patients. In addition, comprehensive cancer centers have extensive ancillary cancer-related activities such as outreach, education, and information dissemination. The NCI-designated cancer centers are viewed as playing “an important role in their communities and regions and serve to influence standards of cancer prevention and treatment” (NCI, 2005a).
A telephone survey conducted in 2001 of representatives of all NCI-designated comprehensive cancer centers (there were 37 such centers at the time) found varying degrees of availability of medical and psychosocial
FIGURE 4-3 NCI-designated cancer centers.
SOURCE: NCI (2005a).
services for cancer survivors (Table 4-8) (Tesauro et al., 2002). Although most (70 percent) centers had a lymphedema management program in place, fewer than half had professionally led support groups (49 percent) and long-term medical care programs (38 percent). Relatively few (14 percent) had programs to provide counseling regarding nutrition, fertility, and sexual concerns. Surveys of NCI clinical and comprehensive cancer centers conducted in the early 1990s indicate that virtually all of the responding cancer
TABLE 4-8 Survivorship Services in NCI-Designated Comprehensive Cancer Centers
|
Service |
Availability (percentage) |
|
Lymphedema management |
70 |
|
Professionally led support groups |
49 |
|
Long-term medical care |
38 |
|
Nutrition counseling |
14 |
|
Fertility and sexual counseling |
14 |
|
SOURCE: Tesauro et al. (2002). |
|
centers offered group support programs (Presberg and Levenson, 1993; Coluzzi et al., 1995; Gruman and Convissor, 1995). A recent survey on genetics services at NCI clinical and comprehensive cancer centers indicates that that most centers provide such services for evaluation of familial cancer (82 percent) (Epplein et al., 2005).
Cancer Programs Approved by the American College of Surgeons’ Commission on Cancer
Most people with cancer are treated in community hospitals close to their homes. In an effort to assure the quality of cancer care throughout the nation, the American College of Surgeons in 1922 established a Commission on Cancer (CoC) that sets standards for quality multidisciplinary cancer care, surveys hospitals to assess compliance with those standards, collects data from approved hospitals to measure treatment patterns and outcomes, and uses the data to improve cancer care outcomes at the national and local levels (Personal communication, K. Phair, Cancer Liaison Program Administrator, CoC, November 9, 2004).10 As of 2003, there were more than 1,400 CoC-approved cancer programs in the United States and Puerto Rico, representing nearly 25 percent of all hospitals (CoC, 2003). More than 70 percent of all newly diagnosed cancer patients are treated in CoC-approved cancer programs, either as an inpatient or when visiting an outpatient hospital-based practice or clinic. Some of the CoC standards pertain to services of potential benefit to cancer survivors (Box 4-3).
CoC staff provided information to the committee on the supportive care services offered at CoC-approved facilities in the previous year. The information provided is self-reported by the institutions on a web-based application, which is updated twice a year (Personal communication, K. Phair, Cancer Liaison Program Administrator, CoC, November 9, 2004). Cancer centers submit detailed information on their programs in advance of their onsite survey. A service was considered to be present if it was provided at the facility, in a staff physician’s office, or by referral. At least some level of supportive care was available through the reporting sites, for example, psychology and mental health providers were available in 88 percent of programs, a pain management service was available in 92 percent of programs, and lymphedema rehabilitation services were available in 77 percent of programs (Table 4-9).
|
BOX 4-3 Rehabilitation services are provided onsite or by referral (Standard 4.7). Rehabilitation services include, but are not limited to:
Supportive services are provided onsite or coordinated with local agencies and facilities (Standard 6.1). Supportive services include, but are not limited to:
The cancer committee monitors the community outreach activities on an annual basis. The findings are documented (Standard 6.3). Supportive services, prevention, and early detection programs are monitored to ensure that appropriate services are provided to patients and the community. SOURCE: Commission on Cancer (2003). |
Although the level of survivorship services appears relatively high in CoC-approved cancer programs, it is difficult to know precisely how accessible services are to cancer survivors. Many of the positive responses may represent services provided hospitalwide. If there were only one psychologist available within an institution, for example, the availability of psychological services may be stretched thin. Unclear also is the level of training of staff regarding issues related to cancer survivorship. Some services may be limited to patients on active treatment, leaving a void for long-term survivors. Because referral sources were included as positive responses, the logistics of accessing care at a distance may have a major impact on the true availability of services. Although many important areas are included in the CoC survey, information on significant areas of survivorship care such as genetic counseling or sexuality counseling is not available.11 Although these data have some shortcomings, they provide important information on the
|
11 |
Information on the availability of genetic counseling services will be available in subsequent years. See Appendix 4D for a description of the delivery of cancer-related genetic counseling services. |
TABLE 4-9 Number (and Percentage) of Programs Approved by the American College of Surgeons’ Commission on Cancer That Provide Support Services, 2004
|
Servicea |
Programs Offering Serviceb |
|
|
Number |
Percent |
|
|
Support services: |
||
|
Home care |
1,224 |
89 |
|
Hospice |
1,263 |
92 |
|
Nutrition |
1,008 |
73 |
|
Pain management |
1,272 |
92 |
|
Lymphedema rehabilitation |
1,068 |
77 |
|
Family services |
1,143 |
83 |
|
Reference library |
1,272 |
92 |
|
Providers: |
||
|
Oncology Nursing Society-certified nurse |
1,329 |
96 |
|
Physical therapist |
1,290 |
94 |
|
Pastoral care |
1,290 |
94 |
|
Psychiatric |
1,243 |
90 |
|
Psychology/mental health |
1,220 |
88 |
|
Social services |
940 |
68 |
|
Enterostomal care |
1,044 |
76 |
|
Speech therapy |
1,267 |
92 |
|
Support activities: |
||
|
Breast cancer specific: |
||
|
Reach to Recoveryc |
1,198 |
87 |
|
Prostate cancer specific: |
||
|
Man to Mand |
844 |
61 |
|
Us TOOe |
573 |
42 |
|
Any cancer type: |
||
|
CanSurmountf |
490 |
36 |
|
I Can Copeg |
935 |
68 |
|
Other support groups |
951 |
69 |
|
aServices may be available directly from the institution or by referral to appropriate resource. bPediatric hospitals were excluded from these estimates. cA support group sponsored by the American Cancer Society for individuals with breast cancer. dA support group sponsored by the American Cancer Society for men who are prostate cancer survivors. eUs TOO provides information, local support groups, counseling, and educational meetings to assist men with prostate cancer as they make decisions about their treatment and continued quality of life. fA program that puts a patient in touch with a person who has experienced the same kind of cancer. gA 7-week educational series for cancer patients and their families sponsored by the American Cancer Society. |
||
availability of supportive services, at some level, within the CoC-approved cancer program. Unknown entirely is how available support services are for the approximately 30 percent of patients treated in non-CoC-approved hospitals.
Association of Community Cancer Centers
The Association of Community Cancer Centers (ACCC) is a membership organization that includes 650 medical centers, hospitals, oncology practices, and cancer programs that are generally recognized to have structured cancer programs. The organization estimates that its members provide services to 40 percent of all new cancer patients in the United States (ACCC, 2004a). ACCC has issued voluntary guidelines for cancer programs to encourage the development of comprehensive and interdisciplinary programs. Several guidelines relate to care for cancer survivors. Shown in Box 4-4 are guidelines for rehabilitation and patient advocacy and cancer survivorship. Other guidelines are available for pain management, nutritional support services, and genetic risk assessment, counseling, and testing (ACCC, 2004b).
To learn more about survivorship services available within ACCC cancer centers, an informal survey was conducted in 2002 of cancer center program coordinators.12 Program coordinators were asked about the availability of several survivorship-related services.13 The service did not have to be housed in the center: referrals to external organizations or facilities at other centers, including local academic institutions, were considered to serve the center’s needs. As stated above, many of the clinics/organizations served both survivors and patients in active treatment, and it was not possible to identify services that were dedicated only to survivors. Table 4-10 shows the availability of these services across responding centers. The availability of lymphedema services and support groups appears to be somewhat lower in these ACCC-member cancer centers than in the NCI-designated comprehensive cancer centers (Table 4-8).14
With the information at hand, it is difficult to gauge the availability of cancer survivorship clinical and supportive services in cancer centers. In
|
12 |
A convenience sample of 83 centers was selected from the roster of institutions who are ACCC members. Two members were selected from each state with three or more members, one member was selected if there were only one or two members. Community centers were selected from different cities in a state, and larger city or larger institutional centers were preferentially selected. Telephone calls were placed to the designated program coordinator of each of the 83 centers. No follow-up calls were made. Responses were obtained from 56 institutions (67 percent). |
|
13 |
The questions about survivorship services were asked in an open-ended format. Respondents were not asked whether they were provided a given set of survivorship services. |
|
14 |
See Appendix 4D for a description of the delivery of cancer rehabilitation services. |
part, the difficulty in ascertainment may be due to the lack of clear definitions relating to survivorship care. The availability of certain specialized services among cancer centers appears to vary substantially. Lymphedema services were, for example, reported in 77 percent of CoC-approved cancer programs according to 2004 CoC survey data, 70 percent of comprehensive cancer centers according to a 2001 NCI-sponsored telephone survey, and 38 percent of ACCC-member cancer centers according to a 2002 informal survey. Likewise, the availability of general or disease-specific support groups seems to vary widely. Sexuality counseling and dedicated fatigue management appear to be rarely available.
To better understand the adequacy of cancer survivorship clinical and support services, an in-depth survey from the perspectives of both survivors and providers of oncology and primary care is needed. This survey would allow conclusions to be drawn about such issues as the spectrum of services that are needed, levels of unmet need, the respective roles of specialty and primary care providers, and the role of specialized clinics versus integration of survivorship care into routine oncology and primary care practice. Such a survey would also assist in the development of standardized survivor instruments to facilitate needs assessments and remedial interventions. Ultimately, a set of quality indicators and benchmarks is needed so that survivorship care can be evaluated, regardless of the setting in which it is provided or the particular type of practitioner involved in care.
Community-Based Support Services
An important element of the chronic disease model is mobilizing community resources to meet needs of patients. There is a wealth of cancer-related community support services available through voluntary organizations, many of them at no cost. Many supportive services are offered through call centers, web-based information and discussion boards, and direct service delivery. Table 4-11 describes some of the programs that are available nationally. Among the services these programs offer are information, peer support, individual support by telephone, information on nutrition and exercise, and assistance with appearance, for example, wigs and breast prostheses.
Cancer patients make frequent inquiries about supportive services. Health providers are often asked about support groups, counseling, nutrition, financial aid, health insurance, and employment, according to a survey of physicians, nurses, and social workers providing cancer care (Matthews et al., 2004). Although community-based support services are of great interest, some evidence suggests that health care providers are not providing their patients with information or referral to support groups and other resources in their communities (Guidry et al., 1997; Matthews et al., 2002). Furthermore, a recent review of the role of community-based and
|
BOX 4-4 Rehabilitation Guidelines: Comprehensive rehabilitation services are available to cancer patients and their families. Cancer is a chronic disease that may require adjustment in the physical, social, financial, and emotional aspects of life in order to maximize independence and quality of life. Professionals experienced in rehabilitation are best suited to meet the needs of cancer patients. The rehabilitation team includes, but is not limited to:
Each health care discipline is available on staff or by consult to facilitate continuity of care for rehabilitation services. All outsourced services should be provided by properly credentialed individuals whose performance is reviewed annually. Rehabilitation services are a part of the organizational structure of the program and follow proper policies and procedures. |
philanthropic organizations in meeting the needs of cancer patients and caregivers found gaps in service provision for assistance with practical needs such as transportation, home care, child care, financial assistance, and psychosocial support (Shelby et al., 2002). The authors note that with increasing use of outpatient care for cancer patients, a greater demand for practical assistance can be expected in the future.
|
Ongoing educational opportunities are available to members of rehabilitation services. A mechanism is in place to inform patients and family members of the services available. Patient Advocacy and Survivorship Guidelines: Information and programs specific to patient advocacy and survivorship issues are available to cancer patients and their families. Programs and educational resources for survivors and their families should include but are not limited to the following:
Resources are allocated to provide a robust advocacy and survivorship program. National standards for advocacy and survivorship will be incorporated into program planning, implementation, and evaluation. SOURCE: ACCC (2004c,d). |
This section of the chapter highlights selected programs that provide services nationally, those offered by the American Cancer Society and the Wellness Community, selected programs that focus on the needs of Hispanic and African-American cancer survivors, and support available by telephone or online. Appendix 4D includes a description of the delivery of psychosocial services for women with breast cancer to illustrate some of the barriers to access to care.
TABLE 4-10 Survivorship Services in Selected ACCC Cancer Centers
|
Survivorship Service |
Number (n = 56) |
Percentage |
|
At least one survivorship service |
47 |
84 |
|
No survivorship services |
9 |
16 |
|
Lymphedema services |
21 |
38 |
|
Genetic counseling |
19 |
34 |
|
Support groups, professionally run |
||
|
All cancer |
19 |
34 |
|
Breast |
13 |
23 |
|
Prostate |
8 |
14 |
|
Gynecologic |
4 |
7 |
|
Brain |
2 |
4 |
|
Lymphoma/leukemia |
2 |
4 |
|
Support groups, peer led |
13 |
23 |
|
Sexuality counseling |
4 |
7 |
|
Yoga/relaxation |
2 |
4 |
|
Symptom management |
2 |
4 |
|
Prevention |
2 |
4 |
|
Prosthesis lending |
1 |
2 |
|
SOURCE: Winn (2002). |
||
American Cancer Society Programs
The American Cancer Society provides extensive information on cancer patient support and special topics in survivorship issues and sponsors several programs for cancer survivors (see Table 4-11; Box 4-5). Detailed guides on what to expect after treatment for the most common cancers, including surveillance practices, are available at the ACS website. Also available is information on adopting healthy lifestyles after cancer treatment. The ACS’s Cancer Survivors Network is a web-based program supporting online interaction among cancer survivors. Participants can create a personal website; post pictures, poems, and other expressions; listen to or read prerecorded stories of other survivors; and engage in online discussions in English, Spanish, and Chinese.
The I Can Cope program started as a series of classes for individuals with cancer, but has been adapted for use as multiple standalone modules on a variety of topics ranging from financial management to coping with side effects. A health care navigator program is under development by ACS that will direct individuals to resources within their community.
The Reach to Recovery program, offered since 1960, provides peer support to women with breast cancer. Initially designed for women in the hospital after mastectomy, the program is now offered largely in the com-
munity. Attempts are made to match volunteer mentors with women with breast cancer by age, type of procedure, and cancer stage. Trained volunteers made nearly 65,000 visits in 2003 (Teschendorf, 2005). Road to Recovery is a community-based program that provides rides to individuals with cancer who need transportation to treatment. Although the program is already offered in various forms in many states, it continues to expand nationwide. An estimated 66,000 people were served nationally by this program in 2003 (Teschendorf, 2005).
Since 1989, the Look Good…Feel Better (LGFB) program has provided assistance with makeup, skin care, and other aspects of appearance (e.g., information on wigs, turbans). The program is co-sponsored by the Cosmetic, Toiletry, and Fragrance Association Foundation, the National Cosmetology Association, and ACS. In 2003 the LGFB program reached about 31,000 women (Teschendorf, 2005). A magazine and catalogue called tlc, or Tender Loving Care, combines articles, information, and products for women coping with cancer treatment (e.g., wigs, mastectomy forms and products, hats and head coverings, bathing suits, and lingerie) (ACS, 2004).
The ACS’s Man to Man program helps men cope with prostate cancer by providing community-based education and support to patients and their family members. A major part of the program is the self-help and/or support group. Volunteers organize free monthly meetings where speakers and participants learn about and discuss information about prostate cancer, treatment, side effects, and how to cope with the disease and its treatment. Approximately 27,000 men participated in Man to Man in 2003 (Teschendorf, 2005).
The Wellness Community
The Wellness Community, founded in 1982, is an international nonprofit organization that provides free education and support services to individuals with cancer and their families (see Table 4-11) (The Wellness Community, 2004b). The founding principle of The Wellness Community is the “Patient Active Concept,” which states that patients who participate in recovery improve the quality of their lives and may enhance the possibility of recovery. In 2004, The Wellness Community reached more than 150,000 people affected by cancer. The free programs are led by licensed health care professionals, including social workers, psychotherapists, nurses, and psychologists, and all programs and training curricula are uniform throughout the country. Its online resources include webcasts, relaxation exercises, cancer-specific educational materials, continuing education for oncology nurses, and online support groups hosted through “The Virtual Wellness Community” (The Wellness Community, 2004a). Many of the online programs are also available in Spanish.
TABLE 4-11 Selected National Community-Based Psychosocial Resources
|
Program Name, Sponsor |
Services and Availability |
|
Generic Cancer Programs |
|
|
Cancer Care |
Available nationwide by telephone and via the Internet |
Available nationwide |
|
|
The Wellness Community |
Available in 22 locations throughout the United States, and nationwide via the Internet |
|
Cancer Hope Network |
Available nationwide by telephone, or in person at some locations |
|
ACOR (Association of Cancer Online Resources) |
Available nationwide via the Internet |
|
Gilda’s Club |
Available in 18 centers nationwide, with 7 centers in development |
|
Cancer Survivors Project |
Available nationwide via the Internet |
|
Content of Services |
Eligibility |
|
Survivors, family, and loved ones |
|
Survivors and family |
|
Survivors and family |
|
Survivors |
|
Survivors and family |
|
Survivors, family, and friends |
|
Survivors, family, and friends |
|
Program Name, Sponsor |
Services and Availability |
|
LIVESTRONG, Lance Armstrong Foundation and Centers for Disease Control and Prevention |
Available nationwide via the Internet and by telephone |
|
Breast Cancer |
|
Available nationwide |
|
|
Look Good…Feel Better, ACS; Cosmetic, Toiletry, and Fragrance Association Foundation; and the National Cosmetology Association |
Available nationwide |
|
Other groups (Y-Me, Bosom Buddies, Sisters Network, YWCA, Circle of Life, TOUCH) |
Available in regions nationwide |
|
Prostate Cancer |
|
Services and activities vary depending on location |
|
|
Us TOO |
Support groups available nationwide, with more than 330 chapters worldwide |
|
Colon Cancer |
|
|
Colon Cancer Alliance |
Available nationwide through the Internet |
|
Content of Services |
Eligibility |
|
Survivors, family, and friends |
|
Breast cancer survivors |
|
Breast cancer survivors |
|
Breast cancer survivors and family |
|
Prostate cancer survivors |
|
Prostate cancer survivors and partners |
|
Colorectal cancer survivors and family |
|
Program Name, Sponsor |
Services and Availability |
|
Blood Cancers |
|
|
The Leukemia and Lymphoma Society |
59 local chapters, and information available nationwide by telephone |
|
Thyroid Cancer |
|
|
ThyCa: Thyroid Cancer Survivors Association, Inc. |
Available nationwide during the internet, and in person in some locations |
Community-based educational programs are offered at all The Wellness Community locations and at some independent cancer clinics. A variety of topics are covered, and programs are designed to help people with cancer and their caregivers feel a greater sense of hope, control over their situation, and community. National education programs are focused on coping with cancer as a chronic condition, with strategies for managing the disease, its side effects, and a host of lifestyle and emotional concerns that arise over time. The Frankly Speaking about New Discoveries in Cancer program, developed in 2004, covers information about the most current medical and psychosocial advances, and addresses topics such as complementary and alternative medicine and psychological concerns (The Wellness Community, 2005).
Evaluations of The Wellness Community programs are underway and include a comparison of face-to-face and online support services, an examination of provider best practices, and analyses of outcomes associated with interventions (Lieberman et al., 2003). Research partners include Stanford University, University of California–San Francisco, M.D. Anderson Cancer Center, Catholic University, and the National Coalition for Cancer Survivorship.
|
Content of Services |
Eligibility |
|
Leukemia and lymphoma survivors, family, and caregivers |
|
Thyroid cancer survivors and caregivers |
Community-Based Support Targeted to Racial and Ethnic Minority Groups
A number of psychosocial support programs are targeted to members of racial and ethnic minority groups. Nueva Vida, a program to meet the needs of Hispanic women in the District of Columbia, was established in 1996 and offers support groups, crisis intervention, and peer support to breast cancer survivors (Nueva Vida, 2005a). Because many women served are poor, uninsured, and speak Spanish, Nueva Vida provides “patient navigation” services to help women make appointments and get appropriate follow-up care, assistance with health insurance applications and claim translation, and social supports (assistance with transportation, babysitting). Support groups focus on stress reduction, education (e.g., nutrition), and the implications of cancer for families. Nueva Vida maintains a national psychosocial support resource directory for Hispanic women with breast cancer; the directory includes programs in 20 states (Nueva Vida, 2005b). The most pressing needs of Hispanic women identified by programs surveyed thus far are related to poor access to health care, information, and psychosocial support, and difficulties navigating the health care system.
Sisters Network, Inc., was founded in 1993 to address the needs of African-American women with breast cancer (Sisters Network, Inc., 2005). The only national African-American breast cancer survivors’ organization
|
BOX 4-5
SOURCE: ACS (2004). |
in the United States, Sisters Network has 39 affiliate chapters across the country. More than 3,000 members are involved in providing breast health training, attending conferences, and serving on various national boards and review committees. Chapters offer individual and group support, community education, advocacy, and research-related activities (e.g., promoting access to clinical trials). The 2005 national Sisters Network Conference “The New Spirit of Survivorship” focused on disparities, risk factors, and survivorship (CDC, 2005a).
The Prostate Health Education Network (PHEN) was founded by a prostate cancer survivor to raise awareness of prostate cancer among those at high risk, especially African-American men (PHEN, 2005). PHEN is establishing “brotherhoods” of prostate cancer survivors across the country that will focus on educating men about prostate cancer and mentoring and counseling those newly diagnosed with the disease, but will also provide support to each other as survivors. The Dana-Farber Cancer Institute and PHEN recently partnered to establish the first prostate cancer support group for African-American men in the Boston area (Dana-Farber Cancer Institute, 2005).
Support Available by Telephone and Online
Many psychosocial support services are available by phone to residents of rural areas and those living far from cancer centers, and increasingly
online through the World Wide Web. This section of the report describes three such programs: Cancer Care, the Association of Cancer Online Resources (ACOR), and CHESS (Comprehensive Health Enhancement Support System). In addition, a referral resource is available online to help locate psychosocial and mental health services through the American Psychosocial Oncology Society (APOS, 2004). There are other organized online lay information and support groups, including Fertile Hope (FertileHope.org), Living Beyond Breast Cancer (lbbc.org), and Cancer and Careers: Living and Working with Cancer (cancerandcareers.org), and many Internet chat rooms. There are also radio resources, for example, the Group Room weekly cancer talk radio show (and Internet simulcast) provided by Vital Options® International, which can reach rural areas. Chapter 6 reviews some online resources related to employment and insurance programs. The potential for these resources to help survivors, provide psychosocial care, and engage in research is not clear but merits investigation.
CancerCare Since 1944, CancerCare has provided emotional support, information, and practical help to people with cancer and their families. With a staff of more than 40 professional oncology social workers, this nonprofit social service agency provides individual and family counseling, group counseling (in-person, online, or by telephone), referrals to other resources, direct financial assistance, and teleconference programs that allow people to listen via telephone to experts in oncology or related fields (CancerCare, 2005a). Previous teleconference programs have covered coping strategies for side effects, communicating with one’s health care team, and how best to maintain quality of life while living with cancer. CancerCare has recently teamed with the Lance Armstrong Foundation to create a counseling program specifically for cancer survivors that includes an online forum for survivors and individualized counseling (CancerCare, 2005b). In addition, CancerCare also hosts an annual teleconference series on survivorship; the 2005 workshop covered care after treatment, fatigue and memory long-term effects, and health-promoting behaviors. In 2004, CancerCare provided services to more than 90,000 people, and all services are provided free of charge. According to an analysis of requests for assistance made to CancerCare from 1983 to 1997, the most commonly reported problems relate to personal adjustment to illness and financial, home care, and transportation needs (Shelby et al., 2002).
CancerCare is also involved in professional education and training, offering seminars, workshops, and teleconferences in all fields of oncology care. Distance learning programs are conducted entirely on the website. (Chapter 6 includes a description of services related to employment and insurance issues.)
Association of Cancer Online Resources ACOR provides opportunities for individuals with cancer to interact with other cancer patients, therapists, or doctors through chat rooms, e-mail, or listservs. Psychosocial support is available through listservs dedicated to breast cancer, cancer-related depression, and caregivers. Another feature of ACOR is the provision of information about cancer from sources deemed to be credible (ACOR, 2004a).
CHESS (Comprehensive Health Enhancement Support System) CHESS provides information, social support, and decision-making assistance via personal computers placed in a patient’s home. Women of all ages and varied socioeconomic backgrounds have successfully used the program to become active participants in their care following a diagnosis of breast cancer. CHESS allows participants to talk anonymously with peers, question experts, learn where to obtain help and how to use it, read stories about people who have survived similar crises, read relevant articles, monitor their health status, consider decision options, and plan how to implement decisions (Gustafson et al., 1993; Shaw et al., 2000). Support group use is the most popular aspect of CHESS. CHESS support groups are monitored by a facilitator. CHESS is undergoing further development as part of NCI’s Centers of Excellence in Cancer Communications Research Initiative (NCI, 2005c).
In addition to these support services, many sources of information are available on survivorship issues (Box 4-6).
Statewide Comprehensive Cancer Control
Opportunities in the United States to develop regional approaches to care for cancer survivors could be facilitated by the Centers for Disease Control and Prevention’s (CDC’s) efforts to build the capacities of states—and, in turn, their local partners—to both develop and implement comprehensive cancer control plans (Brady, 2004). As part of CDC’s National Comprehensive Cancer Control Program, such plans have been defined as those with an integrated and coordinated approach to reducing the incidence and rates of morbidity and mortality from cancer through prevention, early detection, treatment, rehabilitation, and palliation (CDC, 2004a).
CDC has identified a useful framework for the establishment of a state cancer control program and has provided various models for comprehensive planning and evaluation. Essential elements of a comprehensive plan include (Abed et al., 2000a,b) the following:
-
Strategies and mechanisms for developing and maintaining partnerships,
|
BOX 4-6 Cancer advocacy
Web
Radio
Telephone
|
-
Assessments and surveillance,
-
Infrastructure development,
-
Public education,
-
Professional education,
-
Policy and legislative activities, and
-
Evaluation and monitoring.
An online resource called Cancer Control Planet helps states to assess program priorities, identify potential partners, determine the effectiveness of different intervention approaches, find research-tested programs and products, and plan and evaluate their program (Cancer Control Planet, 2004). A locus at the site on survivorship issues is under development (Rowland, 2005). All but 1 state (Idaho) receive support from CDC for their comprehensive cancer control program; 28 states are implementing their plans; and 21 states and the District of Columbia are developing their plans (Figure 4-4) (CDC, 2004b).
With an understanding that cancer survivors could benefit tremendously from a coordinated public health effort, CDC in collaboration with the Lance Armstrong Foundation developed A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies (CDC and LAF, 2004). The plan includes four goals:
-
Preventing secondary cancers and recurrence of cancer whenever possible.
-
Promoting appropriate disease management following diagnosis and treatment to ensure the maximum number of years of healthy life for cancer survivors.
-
Minimizing preventable pain, disability, and psychosocial distress for those living with, through, and beyond cancer.
-
Assisting cancer survivors in accessing family, peer, and community support and other resources they need for coping with their disease.
This action plan and the strategies outlined within the plan shown in Box 4-7 will serve as a guide to states as they expand their comprehensive cancer control plans to include survivorship. As part of a pilot project, CDC is analyzing four organizations’ survivorship-related activities that are national in scope (i.e., those of the American Cancer Society, CancerCare, the Lance Armstrong Foundation, and the National Coalition for Cancer Survivorship). The results of this pilot project will help CDC determine how it can most effectively address gaps in cancer survivorship within the realm of public health (Personal communication, P. Thompson, CDC, April 26, 2005).
FIGURE 4-4 Status of CDC State Comprehensive Cancer Control Plans.
SOURCE: CDC (2004a).
The CDC-funded comprehensive cancer control activities vary by state depending on local needs and preferences. Comprehensive cancer control plans have been oriented to primary and secondary prevention activities such as tobacco control and cancer screening; however, states have recently been adding some survivorship elements to their plans (True, 2004; Texas Cancer Council, 2005). In the area of data collection, some plans describe expanding current cancer registry activities to obtain information on survival, rehabilitation, and palliative care. In the area of consumer education, some plans address the need for better information regarding side effects of treatment and quality of life issues. Ensuring appropriate and continuous psychosocial support is addressed in some plans. There is little consistency across plans on how survivorship is defined and addressed, but when implemented, these plans provide an opportunity to approach survivorship with a public health focus. The Maryland Comprehensive Control Plan is a good example of how survivorship can be integrated into a cancer control plan.
|
BOX 4-7 The National Action Plan identifies and prioritizes cancer survivorship needs and proposes strategies for addressing those needs within four core public health components:
|
However, the state has limited resources with which to implement its plan (Box 4-8) (Family Health Administration, 2005). The comprehensive cancer control plans have not yet been evaluated and evidence is needed of their effects on statewide access to cancer-related services, the quality and comprehensiveness of those services, and the extent to which the needs of cancer patients and survivors are being met. The National Conference of State Legislators (NCSL) is working with the Lance Armstrong Foundation to develop state-level policy indicators for survivorship (Personal communication, S. Wasserman, NCSL, March 21, 2005). This effort will include a survey of state best practices. An opportunity for state and tribal representatives to meet and discuss issues related to their comprehensive cancer control efforts is provided by the Comprehensive Cancer Control Leader-
SOURCE: CDC and LAF (2004). |
ship Institutes.15 These 2- to 3-day regional meetings bring together up to 15 people from each state to participate in lectures, discussions, and exercises to assist them in their cancer control efforts (Personal communication, P. Thompson, CDC, May 2, 2005). Survivorship will be among the topics addressed at the Institutes in 2006 (Personal communication, P. Thompson, CDC, April 26, 2005).
|
BOX 4-8 The Maryland Comprehensive Cancer Control plan, released in 2004, includes a chapter entitled “Patient Issues and Cancer Survivorship” that recognizes many of the problems faced by cancer survivors, and presents public health strategies for overcoming them, with the overall goal of enhancing quality of life for all cancer survivors in Maryland. The Maryland plan identifies four objectives relating to survivorship:
Selected strategies to reach these goals include (see state plan for a list of all strategies):
SOURCES: Family Health Administration (2005); Maryland Comprehensive Cancer Control Plan; Personal communication, R. Villanueva, Maryland State Council on Cancer Control, February 16, 2005. |
FINDINGS AND RECOMMENDATIONS
Survivorship care represents a unique phase of the cancer care trajectory following primary treatment and lasting until recurrence, a second cancer, or death. An ideal system of survivorship care would provide all cancer survivors with preventive services, surveillance, necessary interventions, and coordination with primary care to ensure that all of the survivor’s care needs are met. Many cancer survivors do not receive comprehensive survivorship care. They are, in effect, lost to follow-up. Some survivors may receive aspects of post-treatment care from their cancer care or primary care providers, but such care is rarely comprehensive or coordinated. Many survivors are not aware of their increased risk for late effects and do not seek the care they need. Primary care physicians are often willing to assume follow-up responsibilities, but do not receive explicit guidance from oncology specialists on what they should do. Improvements in information systems and electronic health records hold promise to improve communications between providers involved in cancer care, but such systems are not yet widely available. Education and training opportunities on survivorship care are limited and comprehensive evidence-based clinical practice guidelines have not been developed. Promising models of delivering survivorship care have been tested in Europe, but have not been formally evaluated in the United States. The chronic disease model of care is emerging in the United States, but has not been applied in the context of cancer survivorship.
Defining Quality Health Care for Cancer Survivors
The National Cancer Policy Board, in its 1999 report, Ensuring Quality Cancer Care, recommended that systems of care “measure and monitor the quality of care using a core set of quality measures” and specified some of the attributes and applications of such measures (Box 4-9) (IOM, 1999). Since the IOM report was published, the American Society of Clinical Oncology, the Susan G. Komen Foundation, the National Cancer Insitute, and others have supported research and activities to further the development of quality measures for cancer care.
For certain types of cancer, some evidence-based measures of quality survivorship care exist. Survivors of breast cancer, for example, need to receive annual mammograms, survivors of prostate cancer need periodic testing with the prostate-specific antigen (PSA) test, and survivors of colon cancer require periodic colon examinations. Other measures could likely be developed with available evidence, for example, the need to monitor some individuals treated with certain chemotherapeutic agents for heart conditions and to monitor certain individuals treated by radiotherapy for thyroid
|
BOX 4-9 Cancer care is optimally delivered in systems of care that “measure and monitor the quality of care using a core set of quality measures.” Cancer care quality measures should:
SOURCE: IOM (1999). |
conditions. In contrast to these disease-specific or treatment-specific measures, some evidence-based measures of quality apply broadly across all types of cancer. For example, routinely assessing cancer survivors for psychosocial distress is warranted because it often exists and effective treatments are available. Given the frequency of other common and treatable symptoms such as fatigue and sexual dysfunction, other measures of survivorship care quality could likely be formulated with available evidence that would be broadly applicable to cancer survivors.
Recommendation 4: Quality of survivorship care measures should be developed through public/private partnerships and quality assurance programs implemented by health systems to monitor and improve the care that all survivors receive.
Overcoming Delivery System Challenges
The problems that cancer survivors face in getting comprehensive and coordinated care are common to those faced by others with chronic health conditions. Because cancer is a complex disease and its management involves the expertise of many specialists, often practicing in different settings, cancer illustrates well the “quality chasm” that exists within the U.S.
health care system and the need for health insurance reforms and innovations in health care delivery.
The Survivorship Committee recognizes that underlying flaws in the organization of health care delivery hamper appropriate survivorship care. The committee endorses the conclusions and recommendations in the IOM report, Crossing the Quality Chasm (IOM, 2001). That report provided the rationale and a strategic direction for redesigning the health care delivery system. It concluded that fundamental reform of health care is needed to ensure that all Americans receive care that is safe, effective, patient centered, timely, efficient, and equitable. To that end, health care organizations need to design and implement more effective organizational support processes to make change in the delivery of care possible. Purchasers, regulators, health professionals, educational institutions, and the Department of Health and Human Services need to create an environment that fosters and rewards improvement by (1) creating an infrastructure to support evidence-based practice, (2) facilitating the use of information technology, (3) aligning payment incentives, and (4) preparing the workforce to better serve patients in a world of expanding knowledge and rapid change.16
Barriers facing cancer survivors and their providers in achieving quality survivorship care include: a fragmented and poorly coordinated cancer care system; the absence of a locus of responsibility for follow-up care; poor mechanisms for communication; a lack of guidance on the specific tests, examinations, and advice that make up survivorship care; inadequate reimbursement from insurers for some aspects of care; and limited experience on how best to deliver care.
Recommendation 5: The Centers for Medicare and Medicaid Services (CMS), National Cancer Institute (NCI), Agency for Healthcare Research and Quality (AHRQ), Department of Veterans Affairs (VA), and other qualified organizations should support demonstration programs to test models of coordinated, interdisciplinary survivorship care in diverse communities and across systems of care.
Several promising models for delivering survivorship care are emerging, including:
-
A shared-care model in which specialists work collaboratively with primary care providers,
-
A nurse-led model in which nurses take responsibility for cancer-related follow-up care with oversight from physicians, and
-
Specialized survivorship clinics in which multidisciplinary care is offered at one site.
There is limited evidence on which of these, or other, delivery strategies is feasible, cost-effective, or acceptable to survivors and clinicians. It is likely that different care models will be preferred and appropriate for different survivor groups and communities. Models for delivering survivorship care should address the fact that oncology specialists, facing an expanding population of cancer survivors, will become overburdened with follow-up care at the expense of being able to evaluate and treat new patients. The proposed demonstration program could include the development of an infrastructure for integrated care to enable primary care clinicians to coordinate with specialists as a team to ensure that cancer survivors receive timely and complete care for cancer-related and other health needs. Such an infrastructure could include advanced information systems, such as electronic health records, virtual consultations, smart cards, and web-based approaches. Less technology-dependent interventions could also yield results. Having a community’s cancer specialists and primary care providers agree to a plan on how to coordinate care for survivors—including clarifying roles, specifying referral procedures, agreeing on consultation letters, and providing feedback to primary care—could all enhance coordinated care. More than 60 percent of cancer survivors are aged 65 and older, so the Medicare program should have a strong interest in identifying cost-effective models of care.
Survivorship as a Public Health Concern
CDC and the Lance Armstrong Foundation have developed a public health approach to survivorship care that may assist communities in identifying and addressing the survivorship needs of individuals, their families, and their health care providers (CDC and LAF, 2004; CDC, 2004a). Among the public health capacities that could be addressed include:
-
Population-based surveillance systems for survivorship care and quality of life;
-
Areawide community-based resource guides for survivors and health care providers;
-
Service needs assessments;
-
A clearinghouse for health care provider education and training opportunities;
-
Provision of primary and secondary prevention services (e.g., smoking cessation, cancer screening);
-
Program evaluation and identification of best practices.
Health departments have had a long tradition of managing cancer registries, offering health education, and providing community-based health promotion and disease prevention activities. Interventions for common chronic public health problems such as heart disease and diabetes could well be germane to cancer survivors and their families. These public health approaches are early in their development and resources are needed to evaluate the effectiveness of community-based services and comprehensive cancer control plans in improving the care and quality of life of cancer survivors.
Recommendation 6: Congress should support the Centers for Disease Control and Prevention (CDC), other collaborating institutions, and the states in developing comprehensive cancer control plans that include consideration of survivorship care, and promoting the implementation, evaluation, and refinement of existing state cancer control plans.
APPENDIX 4A SUMMARY OF ARTICLES DESCRIBING U.S. SURVEILLANCE PRACTICE PATTERNS, BY CANCER SITE (LIMITED TO ARTICLES PUBLISHED FROM 1994 TO 2005)
Breast Cancer
|
Reference/Study Question |
Methods |
|
Mammography surveillance following breast cancer (Geller et al., 2003) _________ |
Method: Linkage of data from seven mammography registries (NCI’s Breast Cancer Surveillance Consortium (BCSC)) to population-based cancer and pathology registries. |
|
When do women diagnosed with breast cancer return for their first mammogram? What factors predict women’s return for surveillance? |
Sample: 2,503 women diagnosed with breast cancer (DCIS or invasive) within 1 year of a 1996 BCSC mammogram were followed to see if they returned for surveillance mammography. |
|
Statistical methods: Kaplan-Meier curves were used to depict time from diagnosis to first surveillance mammogram during the defined follow-up period. |
|
|
Explanatory variables: Age, race, education, stage, detection by screening, family history, treatment. |
|
|
Quality of non-breast cancer health maintenance among elderly breast cancer survivors (Earle et al., 2003) _________ What is the quality of preventive health care for breast cancer survivors? What patient and provider characteristics are associated with high-quality care? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 5,965 elderly women who were diagnosed with nonmetastatic breast cancer in 1991 or 1992 while living in a SEER tumor registry area and who survived to the end of 1998 without evidence of cancer recurrence. |
|
|
Statistical methods: Multivariate analyses were used to develop explanatory models. |
|
|
Explanatory variables: Preventive service use among cancer survivors was compared to controls matched for age, race, and geographic location. |
|
|
Medical surveillance after breast cancer diagnosis (Lash and Silliman, 2001) |
Method: Study of surveillance practices in five Boston hospitals. |
|
Results |
|
78% of women with breast cancer returned for mammography between 7 and 30 months following the diagnosis. Women most likely to undergo surveillance mammography were 60–69 (OR 1.9 relative to women 40–49) with Stage 0, I, or II breast cancer (relative to Stage III) and had received radiation therapy in addition to surgery. |
|
74% of women who returned had two or more mammograms. ASCO guidelines do not recommend biannual surveillance mammography, but many providers are following women after treatment with breast-conserving surgery with mammography every 6 months for the first 2–3 years. |
|
Breast cancer survivors received more preventive services (influenza vaccination, lipid testing, cervical and colon screening, and bone densitometry) in 1997–1998 than matched controls. Survivors who continued to see oncology specialists were more likely to receive appropriate follow-up mammography for their cancer, but those who were monitored by primary care physicians were more likely to receive all other noncancer-related preventive services. Those who saw both types of practitioners received more of both types of services. Among both groups, those who were younger, non-African American, of higher socioeconomic status, living in urban areas, and receiving care in a teaching center were most likely to receive high-quality health maintenance. Mammography use in 1997–1998 was 74% among survivors. |
|
92% of women had some surveillance testing. |
|
47% of women received less than guideline surveillance. |
|
Reference/Study Question |
Methods |
|
_________ What tests are ordered for surveillance of breast cancer recurrence in the 4 years after breast cancer diagnosis by surgeons, medical oncologists, and radiation oncologists? |
Sample: 303 Stage I or II breast cancer patients aged 55 years or older and diagnosed at one of five Boston hospitals and followed for at least 4 years were interviewed and their medical records abstracted. Year of completion of primary therapy ranged from 1992 to 1994. 78% of women eligible to participate did so. Nonparticipants were on average 3 years older than participants. |
|
To what extent are guidelines for follow-up adhered to? |
Medical records were examined for the following nine surveillance tests:
Tests ordered because of suspected recurrence were excluded. Surveillance information from primary care physicians was not examined. |
|
Adherence measured according to ASCO guideline of annual physical examination and mammography. |
|
|
|
Statistical methods: Stepwise proportional hazards regression used to determine predictive model of failure to receive 4 consecutive years of surveillance. |
|
|
Explanatory variables: Physician specialty, patient age, education, marital status, household composition, employment status, cancer stage, treatment, comorbidity. |
|
Underutilization of mammography in older breast cancer survivors (Schapira et al., 2000) _________ |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 3,885 breast cancer survivors aged 65 and older diagnosed with early-stage breast cancer in 1991. |
|
|
How frequently do older breast cancer survivors obtain annual mammography? What determines mammography use? |
Examined mammography use in 2-year period following initial breast cancer treatment. |
|
Statistical methods: Multivariate analyses (logistic regression). |
|
Results |
|
Women ages 75 to 90 years were at higher risk for failure to complete 4 consecutive years of surveillance and for receipt of less than recommended guideline surveillance. |
|
Women treated at a breast center and women treated with radiation therapy were more likely to get mammograms. |
|
In the 4 years of follow-up, a mean of 22 tests were ordered for 279 women getting any type of surveillance. Most tests were ordered by medical oncologists. The most common test was a mammogram (mean = 3.9), which was most often ordered by a surgeon. |
|
Liver function studies were ordered on average 2.5 times over the 4-year follow-up period for 279 women, CEA tests 2 times, and chest X rays 0.4 times. |
|
62% of women underwent annual mammography, an additional 23% underwent mammography in 1 of 2 years, and 15% had no mammography claim in the 2 years evaluated. |
|
Women treated with breast-conserving surgery without radiotherapy, those with higher stage of disease or greater comorbidity were less likely to have had annual mammography. |
|
When use of mammography in the 2-year period was examined, black women were less likely than white women to have any mammography (OR 0.54). |
|
Reference/Study Question |
Methods |
|
|
Explanatory variables: Age, race, cancer stage, type of treatment, comorbidity, census tract income, education, population density. |
|
The use of mammography by survivors of breast cancer (Andersen and Urban, 1998) _________ To what extent are rural breast cancer survivors getting annual mammograms? |
Method: Reinterview of women identified with breast cancer by survey (cancer diagnoses were not confirmed with medical records or registry). |
|
Sample: 248 women who had reported a history of breast cancer in a 1994 survey of women ages 50 to 80 living in 40 communities in predominantly rural areas of Washington state were reinterviewed in 1996 regarding mammography use. 83% (351/423) of eligible women recontacted in 1996 for the survey agreed to participate. Of these 71% (248/351) were included in the study. Excluded women were being treated for cancer, diagnosed recently, or had had a double mastectomy. |
|
|
|
Statistical analysis: Multiple logistic regression analysis. |
|
|
Explanatory variables: Age, insurance status, years since diagnosis, detection of cancer by mammogram, treatment, recent recommendation of mammography. |
|
Measuring standards of care for early breast cancer in an insured population (Hillner et al., 1997) _________ |
Method: Private insurance claims linked to state cancer registry data. Procedural and hospital claims from Blue Cross/Blue Shield of Virginia were linked with clinical stage data from the Virginia Cancer Registry from 1989 to 1991. |
|
Can available information be used to develop measurements regarding standards of quality and efficiency of oncologic care? What surveillance occurs following breast cancer treatment for privately insured women under age 64? |
Sample: 918 women <64 with local or regional breast cancer. |
|
Statistical method: Univariate descriptive statistics used to develop a quality-of-care “report card.” |
|
|
Explanatory variables: Age, node status. |
|
Results |
|
70% of breast cancer survivors reported having received a mammogram within the past year. 72% reported having had a mammogram in the past 2 years. |
|
Women who had a recent physician recommendation for mammography and those whose breast cancer had been originally detected by mammography were more likely to have had a recent mammogram. |
|
Mammography 81% of women had at least one mammogram within 3 years of diagnosis. 76% had a mammogram by 18 months and 66% between 18 and 36 months. |
|
Diagnostic imaging Within 36 months, 34% of women had a bone scan and 21% had a CT scan. For each of the 18-month periods, approximately 24% of women had one or more bone scans and 14% had a CT scan of some type. |
Prostate Cancer
|
Reference/Study Question |
Methods |
|
Breast cancer evaluation and follow-up: A survey of the Ohio Chapter of the American College of Surgeons (Stark and Crowe, 1996) _________ What are the practice philosophies of Ohio general surgeon fellows regarding evaluation and follow-up? |
Method: 1994 survey of 764 general surgeons, fellows in the Ohio chapter of the American College of Surgeons. |
|
Sample: 261 responses to the survey were evaluable (RR = 34%). |
|
|
Statistical method: Descriptive statistics. |
|
|
Explanatory variables: None. |
|
|
Geographic variation in patient surveillance after radical prostatectomy (Powell et al., 2000) |
Method: 1997 physician survey. Examined three frequently used modalities:
|
|
How tumor stage affects American urologists’ surveillance strategies after prostate cancer surgery (Johnson et al., 2000) |
And eight infrequently used modalities:
Attitudes toward follow-up were also assessed. |
|
Current follow-up strategies after radical prostatectomy: A survey of American Urological Association urologists (Oh et al., 1999) |
|
|
The age of the urologist affects the postoperative care of prostate carcinoma patients (Tsai et al., 1999) _________ How does tumor stage and urologist geographic location and age influence variability of surveillance after radical prostatectomy (Stage TNM I-II, III)? |
Sample: 4,467 members of the American Urological Association (randomly selected from total of 12,500 members) mailed survey in 1997. RR = 32% (1,416/4,467); 1,050/1,416 responses were usable; 28% of usable responses (290/1,050) were from urologists practicing outside of United States. |
|
Statistical methods: Repeated measures analysis of variance. |
|
|
Explanatory variables: Location in 24 MSAs, U.S. census region, HMO penetration. |
|
Results |
|
Essentially all surgeons indicated that physical exam and mammograms are important for post-treatment follow-up. Complete blood cell count, liver function studies, and chest X rays are used by more than half of the surgeons. 39% used bone scans. 44% had had difficulty with third-party payors covering the costs of surveillance studies. 88% of surgeons wanted the state chapter of the ACS to establish clinical guidelines or practice parameters. |
|
Geographic variation |
|
Surveillance practices were not affected by MSA, census region, or area HMO penetration rate. |
|
Urologists reported an average of 4.02 office visits, 3.43 PSA tests, and 2.99 urinalyses by patients in the postoperative year after radical prostatectomy (TNM stages I–II). |
|
With the exception of CBC and blood chemistry, other modalities were used very infrequently (0.20 or fewer tests). |
|
Very large standard deviations for the total population estimates indicate wide variation in practices. |
|
72% indicated that the literature does not document significant survival benefits of follow-up testing. Nevertheless, 59% of urologists thought that failure to perform follow-up testing at least once yearly constitutes malpractice. |
|
Stage |
|
The analysis of the effect of stage on follow-up practice by Johnson et al. was limited to 760 U.S. urologists. |
|
Intensity of follow-up differed only slightly by TNM stage (data on Gleason score was not collected). |
|
Urologist age |
|
There was a small but significant influence of urologist age on surveillance practice. Older urologists ordered more PSA levels and CT scans, whereas younger urologists ordered more bone scans. Optimism and belief in the survival benefits of routine surveillance increase with age, but this did not translate into substantially more frequent usage of follow-up modalities. |
Colorectal Cancer
|
Reference/Study Question |
Methods |
|
Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer (Rulyak et al., 2004) |
Method: Analysis of HMO automated clinical records for evidence of colonoscopy or flexible sigmoidoscopy, together with barium contrast radiography. |
|
|
Sample: 1,002 patients diagnosed with local or regional colon or rectal cancer between January 1993 and December 1999. |
|
|
Statistical methods: Survival analysis. |
|
|
Explanatory variables: Cancer site, age, SES, marital status, race/ethnicity. |
|
Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery (Ellison et al., 2003) _________ How does race/ethnicity affect patient surveillance following colorectal surgery? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 44,768 non-Hispanic white, 2,921 black, and 4,416 patients from other racial/ethnic groups aged 65 and older at diagnosis, with a diagnosis of local or regional colorectal cancer between 1986 and 1996, and followed through December 31, 1998. |
|
|
Surveillance procedures examined included colonoscopy, sigmoidoscopy, endoscopy, and barium enema. |
|
|
|
Statistical methods: Cox proportional hazards models. |
|
|
Explanatory variables: Race, gender, age at diagnosis, geographic region, census tract education, comorbidity, hospital ownership, size, teaching status. |
|
Results |
|
61% of patients had colon examinations within 18 months of diagnosis and 80% of patients within 5 years of diagnosis. |
|
Less likely to undergo surveillance were patients over 80 years of age, with rectal cancer, and African Americans. |
|
More likely to undergo surveillance were patients with higher SES, married, and those of “other” race/ethnicity. |
|
The chance of surveillance within 18 months of surgery was 57 percent, 48 percent, and 45 percent for non-Hispanic whites, blacks, and other, respectively. |
|
After adjusting for sociodemographic, hospital, and clinical characteristics, blacks were 25% less likely than whites to receive surveillance if diagnosed between 1991 and 1996 (RR = 0.75). This result was not explained by measured racial differences in sociodemographic, hospital, and clinical characteristics. |
|
More than 70% of the bowel surveillance procedures received were colonoscopy. Blacks were nearly 40% more likely than non-Hispanic whites to receive post-treatment bowel surveillance with barium enema. |
|
Reference/Study Question |
Methods |
|
Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries (Knopf et al., 2001) _________ To what extent are recommendations for postoperative colon surveillance followed for patients with local/regional colorectal cancer? Have practices improved over time? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 52,283 patients aged ≥65 with colorectal cancer treated between 1986 and 1996. Surveillance patterns through 1998 were examined. Proportion of surviving patients’ use of procedures at four time periods after treatment were assessed: 2 to 14 months, 15 to 50 months, 51 to 86 months, and more than 97 months. |
|
|
Surveillance procedures examined included colonoscopy, sigmoidoscopy, endoscopy, and barium enema. |
|
|
|
Statistical methods: Kaplan-Meier method survival analysis. |
|
|
Explanatory variables: Age, stage at diagnosis, year of diagnosis. |
|
Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent (Elston Lafata et al., 2001) _________ Are there differences in surveillance care within an HMO population that are explained by race and/or income? |
Method: Retrospective cohort study based on HMO claims. |
|
Sample: 251 patients aged ≥40 with colorectal cancer diagnosis from 1990 to 1995 and treated with curative intent. |
|
|
Statistical methods: Cumulative incidences of service receipt were estimated using Kaplan-Meier survival analyses. Cox proportional hazard models were used to evaluate the relationship between patient sociodemographic and clinical characteristics and service receipt. Patients were followed through the first of either recurrence, death, health plan disenrollment, or December 1997. Average 8-year medical care expenditures were calculated, adjusted for inflation. |
|
|
|
Explanatory variables: Age, sex, race, census tract income, comorbidity. |
|
Results |
|
Low use of post-diagnosis colon surveillance was observed. No surveillance occurred for 17% of the cohort. The proportions of the cohort that underwent no surveillance during each of the four time periods were 54%, 52%, 60%, and 69%. The proportions of the cohort that underwent surveillance at a greater-than-annual frequency for each of the four periods were 10%, 8%, 5%, and 4%. |
|
Median times to first through fifth surveillance events were 20, 14, 15, 15, and 15 months, respectively. |
|
Surveillance improved with time (1994–1998 versus 1986–1993). Younger patients were more likely to undergo surveillance. Surveillance was not related to stage. |
|
Within 18 months of treatment, 55% of the cohort received a colon examination (colonoscopy, barium enema, sigmoidoscopy), 71% received CEA testing, and 59% received metastatic disease testing (CT scans, ultrasounds, MRI, chest X ray, serum liver transaminases testing). Whites were more likely than minorities to receive CEA testing (RR = 1.47). As the median household income of a patient’s zip code of residence increased, so too did the likelihood of colon examination and metastatic disease testing receipt (RR = 1.09, RR = 1.12, respectively). Average 8-year medical care expenditures among the cohort were $30,247. |
|
Reference/Study Question |
Methods |
|
Patterns of endoscopic follow-up after surgery for nonmetastatic colorectal cancer (Cooper et al., 2000) _________ How frequently are colorectal patients followed up? How do surveillance practices vary by patient characteristics and geography? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 5,716 patients aged ≥65 with local or regional stage colorectal cancer diagnosed in 1991. Medicare claims from 6 months after diagnosis through the end of 1994 were examined to determine use of endoscopic procedures. |
|
|
Statistical methods: Chi-square testing. Study did not account for censoring in the data. |
|
|
|
Explanatory variables: Age, gender, race, SEER site, cancer site, stage. |
|
Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma (Cooper et al., 1999) _________ How frequently are colorectal patients followed up? How do surveillance practices vary by patient characteristics and geography? |
Same sample and study design as above. |
|
Colorectal cancer screening and surveillance practices by primary care physicians: Results of a national survey (Sharma et al., 2000) _________ To what extent are primary care providers providing screening and surveillance tests for colorectal cancer? |
Method: 1997 mailed questionnaire to 2,310 primary care physicians (family practice, internal medicine). Stratified sampling from a national database (TAP Pharmaceutical Inc.) to obtain 40–50 physicians per state. |
|
Sample: Used 417 responses from 2,135 questionnaires that could be delivered (RR 20%). |
|
|
Statistical methods: Descriptive statistics. |
|
|
Explanatory variables: None. |
|
Results |
|
One or more colonoscopies were performed in 51%, with an average of 2.9 procedures performed among those tested; sigmoidoscopy was performed in 17%. The rate of colonoscopy was highest during the initial 18 months. Polypectomy was performed in 21% of all patients, and subsequent primary colorectal tumors were diagnosed in 1.3%. Factors associated with colonoscopy and sigmoidoscopy use included younger age, survival through follow-up, and geographic region; sigmoidoscopy was also more common in relation to rectal cancers. |
|
The most commonly performed tests among the 5,716 patients identified were liver enzymes, chest X rays, colonoscopy, and CT scans. Lower rates of testing generally were observed with older age groups, patients with fewer comorbidities, and patients who did not survive through the follow-up period. Among all procedures studies, there was also significant variation in the rates of testing across the nine SEER areas, varying from 1.5-fold to 3.6-fold. The geographic variation persisted in multivariate models adjusting for potentially confounding factors. |
|
On the question related to follow-up of a patient with an adenomatous colon polyp diagnosed 10 years previously, 83% of physicians recommended colonoscopy, 10% recommended flexible sigmoidoscopy (FS), 4% recommended FS and barium enema, and 3% recommended FOBT. |
|
Reference/Study Question |
Methods |
|
Geographic variation in patient surveillance after colon cancer surgery (Johnson et al., 1996a) |
Method: These five articles describe variation in patient surveillance after colon cancer surgery as reported in two provider surveys. |
|
How practice patterns in colon cancer patient follow-up affected by surgeon age (Johnson et al., 1996c) |
Sample: 1,663 members of the American Society are of Colon and Rectal Surgeons (ASCRS) were asked in 1992 by mailed questionnaire how often they request these nine follow-up evaluations for their patients treated for cure with TNM Stage I, II, or III colon cancer over the first 5 post-treatment years:
46% (757/1,663) of ASCRS members completed the survey and 39% (646/1,663) provided evaluable data. |
|
How tumor stage affects surgeons’ surveillance strategies after colon cancer surgery (Johnson et al., 1995) |
|
|
Surveillance after curative colon cancer resection: Practice patterns of surgical subspecialists (Virgo et al., 1995) |
|
|
Current follow-up strategies after resection of colon cancer: Results of a survey of members of the American Society of Colon and Rectal Surgeons (Vernava et al., 1994) |
|
|
The same questionnaire was administered to 1,070 members of the Society of Surgical Oncology (SSO) in 1993. |
|
|
|
33% (349/1,070) of SSO members provided evaluable responses. |
|
|
Statistical methods: Repeat measures of analysis of variance. |
|
|
Explanatory variables: Tumor stage and year post-surgery, MSA/city and MSA size, surgeon age, training period, and country of practice (United States versus foreign). |
|
Results |
|
According to ASCRS members, routine clinic visits and CEA levels were the most frequently performed items for each of the 5 years. More than three-quarters of surgeons see their patients every 3 to 6 months for years 1 and 2, and then every 6 to 12 months for years 3, 4, and 5. Approximately 80% of respondents obtain CEA levels every 3 to 6 months for years 1, 2, and 3, and every 6 to 12 months for years 4 and 5. Colonoscopy is performed annually by 46% to 70% of respondents, depending on the year. A chest X ray is obtained yearly by 46% to 56%, depending on the year. The majority of ASCRS members do not routinely request CT scans or bone scans. There was great variation in the pattern of use of CBC and liver function tests. |
|
Office visits and CEA tests were performed most frequently. SSO members generally see patients every 3 months in years 1–2, every 6 months in years 3–4, and annually thereafter. There was wide variability in test ordering patterns and moderate variation between SSO and ASCRS members. |
|
With responses from ASCRS and SSO combined, seven of the nine surveillance modalities were performed, significantly more frequently with increasing TNM stage. This effect persisted through 5 years of follow-up. Bone/CT scans were performed too infrequently for analysis. |
|
Surgeon age was not predictive of post-treatment surveillance practice patterns, suggesting that post-graduate physician education homogenizes practitioner behavior. |
|
MSA population size and geographic location generally did not explain variation in surveillance practices. |
Hodgkin’s Disease
|
Reference/Study Question |
Methods |
|
Breast cancer screening in women previously treated for Hodgkin’s disease: A prospective cohort study (Diller et al., 2002) _________ |
Method: Prospective cohort study. |
|
Sample: 90 female long-term survivors of Hodgkin’s disease treated 8 or more years previously with mantle irradiation (ages 24 to 51 years, diagnosed under age 30). 75% of contacted patients were enrolled in the study. |
|
|
How aware are long-term female survivors of Hodgkin’s disease of their risk for breast cancer? What are their breast cancer screening practices? |
Participants completed surveys of their perceptions of risk and screening behaviors and received written recommendations for breast examinations and mammography. Annual follow-up was conducted through medical records, telephone, and/or mailed questionnaires. |
|
|
Statistical methods: Descriptive statistics and multivariate logistic regression modeling. |
|
|
Explanatory variables: Age, age at diagnosis, year of diagnosis, stage, treatment, radiation dose. |
|
Results |
|
At baseline, 40% of women were unaware of their increased risk of breast cancer. Only 47% reported having had a mammogram in the previous 24 months. 10 women developed 12 breast cancers during the study, all evident on mammogram (2 detected at baseline; median time of follow-up 3.1 years). |
|
Six women in the cohort refused mammography because they thought they were “too young” (n = 3), “breasts are too dense according to primary physician” (n = 1), and no reason given (n = 2). |
|
Four patients withdrew from the study because of “no time” (n = 1), “too upsetting to deal with” (n = 1), and no reason (n = 2). |
|
Women who perceived themselves to be at high risk were not more likely to have had a mammogram in the previous 2 years. |
|
Older patients were more likely to have had a mammogram. |
|
Nine patients in the cohort developed other primary tumors during the follow-up period (thyroid, pancreas, stomach, lung, sarcoma, non-Hodgkin’s lymphoma). |
Bladder Cancer
|
Reference/Study Question |
Methods |
|
Adherence to surveillance among patients with superficial bladder cancer (Schrag et al., 2003) _________ How frequently do patients with superficial bladder cancer undergo recommended surveillance procedures? What patient and primary care provider characteristics are associated with nonadherence? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 6,717 patients aged ≥65 years diagnosed with superficial bladder cancer from 1992 through 1996 and who survived for at least 3 years after diagnosis, but did not have a total cystectomy. Surveillance examination of the bladder during each of five contiguous 6-month intervals from month 7 to month 36 were examined. |
|
|
Statistical methods: Logistic regression. |
|
|
Explanatory variables: Patient race, sex, age at diagnosis, year of diagnosis, registry location, census tract income, MSA residence, comorbidity; physician Medicare case volume, characteristics from AMA masterfile (e.g., board certification, year of medical school graduation). |
|
|
|
(AUA and NCCN guidelines recommend that patients with superficial bladder cancer receive cystoscopic surveillance at least every 3–6 months for the first 3 years after diagnosis and at least annually thereafter.) |
|
Results |
|
Only 40% of the entire cohort had an examination during all five intervals; 18% had low-intensity surveillance (bladder exam during fewer than two of the five contiguous 6-month intervals). Patient characteristics that were associated with low-intensity surveillance were being aged 75 years or older (OR = 1.54), being nonwhite (OR = 1.94), having favorable tumor histology (OR = 0.59 for poorly differentiated versus referent well-differentiated tumor grade), and having high comorbidity (OR = 1.72). Residence in an urban area or in a census tract with low median income was also associated with low-intensity surveillance. |
|
Physician characteristics associated with low adherence include solo practice, lower case volume, and year of medical school graduation before 1980. |
Lung Cancer
|
Reference/Study Question |
Methods |
|
Geographic variation in the conduct of patient surveillance after lung cancer surgery (Johnson et al., 1996b) Clinical surveillance testing after lung cancer operations (Naunheim et al., 1995) _________ What are the surveillance practices of thoracic surgeons for their patients with lung cancer? |
Method: Physician mail survey. |
|
Sample: 3,700 active members (U.S. and foreign) of the Society of Thoracic Surgeons (STS) surveyed in 1994. 54% responded (2,009/3,700), and of these, 768 were found to both operate on and provide long-term follow-up for lung cancer patients. |
|
|
Profiles of hypothetical patients suitable for post-operative surveillance and a detailed questionnaire based on the profiles were mailed to STS members. |
|
|
Statistical methods: One-way analysis of variance. |
|
|
|
Explanatory variables: None. |
|
Lung cancer patient follow-up: Motivation of thoracic surgeons (Virgo et al., 1998) _________ Do physician characteristics and beliefs explain follow-up intensity? |
Method: Same as above. |
|
Sample: Same as above. |
|
|
Statistical methods: Logistic regression to examine test use by stage and ordinary least squares regression to examine intensity of use. |
|
|
Explanatory variables: Age, professional society memberships, practice type, percentage of practice that was noncardiac, practice location. |
|
Results |
|
The follow-up methods most frequently used during a 5-year follow-up period include clinic visit, chest X ray, CBC, liver function testing, and chest CT scan. Sputum cytology, head CT scan, bone scanning, chest MRI, and bronchoscopy are used infrequently. Although there is wide variation in the frequency of use of the follow-up methods, there is a decrease in the frequency of testing over time since primary therapy. |
|
Fewer than half of respondents believe surveillance testing would yield a survival benefit for either Stage I (44%) or advanced-stage patients (17%) after lung cancer resection. Only one of four respondents believed that the current literature documents any survival benefit. Other reasons for follow-up include maintenance of rapport with colleagues (42%) or patients (69%) and medicolegal liability concerns (49%). |
|
TNM stage and year post-surgery affect follow-up practice. Geographic setting has rather little effect on surveillance strategies. |
|
Physician characteristics and beliefs predicted a less than expected amount of the variation in self-reported follow-up intensity by TNM stage. |
Melanoma
|
Reference/Study Question |
Methods |
|
Current practice of patient follow-up after potentially curative resection of cutaneous melanoma (Virgo et al., 2000) How surgeon age affects post-treatment surveillance strategies for melanoma patients (Margenthaler et al., 2001) Effect of initial tumor stage on patient follow-up after potentially curative surgery for cutaneous melanoma (Johnson et al., 2001) Geographic variation in post-treatment surveillance intensity for patients with cutaneous melanoma (Margenthaler et al., 2003) |
Method: Physician mail survey. |
|
Sample: 3,032 members of the American Society of Plastic and Reconstructive Surgeons (ASPRS; randomly chosen from the 4,320 members) surveyed in 1998 on use of 14 follow-up modalities during years 1–5 and 10 following primary treatment for patients with cutaneous melanoma.
RR = 38% (1,142/3,032); 35% (395/1,142) were evaluable because practice included follow-up in addition to treatment. |
|
|
|
Statistical methods: Repeated-measures analysis of variance. |
|
|
Explanatory variables: TNM stage, year post-surgery, and physician age. |
|
Results |
|
Plastic surgeons often do not provide post-operative follow-up themselves. When they do, surveillance relies most heavily on office visits, chest X ray, CBC, and liver function tests. All other modalities are used infrequently. Surgeons use the most common modalities similarly by TNM stage. |
|
The intensity of post-treatment surveillance practice patterns of ASPRS members caring for patients with cutaneous melanoma varies markedly. Factors accounting for this variation include geography, MCO penetration rate (chest X ray highest in areas with low MCO penetration rate; 5S-cysteinyl dopa testing highest in areas with high MCO penetration rate). |
|
Surveillance varies only marginally with physician age. |
|
Reference/Study Question |
Methods |
|
Geographic and patient variation in receipt of surveillance procedures after local excision of cutaneous melanoma (Barzilai et al., 2004) What are the surveillance practices for Medicare beneficiaries with invasive melanoma? |
Method: Retrospective cohort study using SEER-Medicare data. |
|
Sample: 3,389 patients aged ≥65 years diagnosed with invasive melanoma from 1992 through 1996 and who survived for at least 2 years after diagnosis. Surveillance examination and tests were examined (CBC, liver enzymes or lactic dehydrogenase, chest X ray, CT scans, and MRI). |
|
|
Statistical methods: Kaplan-Meier analysis and log-rank test. |
|
|
|
Explanatory variables: Gender and age, tumor stage and thickness, geographic area. |
|
|
(The American Academy of Dermatology does not recommend a specific follow-up interval schedule, but recommended follow-up in some situations, including patient education, examination of the skin, and laboratory/radiologic examination between one and four times per year for the first 2 years after diagnosis and one to two times per year thereafter. Routine laboratory tests and imaging tests are not required in asymptomatic patients with melanoma ≤4 mm in depth for initial staging or routine follow-up.) |
|
Results |
|
Surveillance testing was relatively common, ranging from 13% for abdominal ultrasound to 80% for laboratory testing. Follow-up skin examinations were performed in 70% to 90% of patients. The use of most surveillance procedures was associated with younger age, male gender, regional stage tumors, and geographic area. |
Upper Aerodigestive Tract Cancer (UADT)
|
Reference/Study Question |
Methods |
|
How tumor stage affects surgeons’ surveillance strategies after surgery for carcinoma of the upper aerodigestive tract (Johnson et al., 1998) How surgeon age affects post-treatment surveillance strategies for upper aerodigestive tract cancer patients (Clark et al., 1999) Surgical decision making in upper aerodigestive tract cancer patient follow-up (Virgo et al., 2002) _________ Does tumor stage and surgeon age affect surveillance practices? Do clinical beliefs explain follow-up practices? |
Method: Mail survey of head and neck surgeons. |
|
Sample: 824 members of the Society of Head and Neck Surgeons (SHNS) and 522 members of the American Society for Head and Neck Surgery (ASHNS) who were not members of the SHNS were surveyed by mail in 1996 on use of 14 follow-up modalities for patients with resectable UADT cancer during years 1 to 5 after potentially curative primary treatment.
RR = 24% (199/824) for SHNS and 42% (221/522) for ASHNS. |
|
|
|
Statistical methods: Repeated-measures analysis of variance. The relationship between clinical beliefs and test ordering practices was examined using Poisson and negative binomial regression analysis. |
|
|
Explanatory variables: TNM stage, year post-surgery, and surgeon age clinical beliefs. |
|
Results |
|
Ten of the 14 most commonly used surveillance modalities were ordered significantly more frequently with increasing TNM stage. The effect persisted through 5 years of follow-up. |
|
Surveillance practice patterns of surgeons do not vary substantially with practitioner age. |
|
Intensity of follow-up decreases with time post-surgery. |
|
Two clinical beliefs with the greatest impact on surgical decision making are that surveillance: (1) permits palliative treatment and improves quality of life, and (2) provides no survival benefit for patients with TNM Stage I cancer. |
Head and Neck Cancers
|
Reference/Study Question |
Methods |
|
Practice patterns and clinical guidelines for post-treatment follow-up of head and neck cancers (Paniello et al., 1999) _________ How does current clinical practice compare to recommendations in published clinical practice guidelines? |
Method: 1996 mail survey of head and neck surgeons. |
|
Sample: 640 members of the ASHNS and 824 members of the Society of Head and Neck Surgeons (SHNS) (1,322 were members of one society or the other) were asked about the following surveillance tests: Imaging:
Blood tests
Other
RR = 610 were returned (46%); 420 evaluable (32%). |
|
|
|
Statistical methods: Analysis of variance. |
|
|
Explanatory variables: Stage, group membership, post-operative year. |
|
Results |
|
Most surgeons relied on directed history, physical examination, and routine chest radiograph at varying intervals for detection of recurrences and second primary tumors. Other tests were used sporadically. |
|
The proportion of surgeons who followed published guidelines varied from 97% in post-operative year 1 to 62% in post-operative year 5. |
Extremity Soft-Tissue Sarcoma
|
Reference/Study Question |
Methods |
|
Current follow-up strategies after potentially curative resection of extremity soft-tissue sarcomas: Results of a survey of members of the Society of Surgical Oncology (Beitler et al., 2000) How surgeon age affects surveillance strategies for extremity soft-tissue sarcoma patients after potentially curative treatment (Sakata et al., 2002) Extremity soft-tissue sarcoma patient follow-up: Tumor grade and size affect surveillance strategies after potentially curative surgery (Sakata et al., 2003) _________ What surveillance modalities are used? Does tumor stage, grade, and size and surgeon age affect surveillance practices? |
Method: Physician mail survey. |
|
Sample: 1,592 members of the SSO surveyed in 1997 regarding their follow-up practices for extremity sarcoma patients treated for cure. Respondents reported on 12 surveillance modalities performed annually during the first 5 years and the 10th year after surgery.
|
|
|
45% (716/1,592) surgeons completed the survey. Of the 343 respondents who performed sarcoma surgery, 318 (93%) also provided long-term post-operative follow-up. |
|
|
Statistical methods: Repeated measures analysis of variance. |
|
|
|
Explanatory variables: Physician age and years since completion of training, tumor grade and size, and year post-surgery. |
|
Results |
|
Routine office visits and chest X ray were the most frequently performed items for each of the years. The frequency of office visits and chest X ray increased with tumor size and grade and decreased with post-operative year. CBC and liver function tests were the most commonly ordered blood tests, but many respondents did not order any blood tests routinely. Imaging studies of the extremities were performed on the majority of patients with large (> 5 cm) low-grade lesions and on both large and small high-grade lesions during the first post-operative year. |
|
The post-treatment surveillance practice patterns of the members of the SSO vary only marginally with the length of time since completion of training. |
|
Tumor grade and size significantly impacted physician practice patterns in post-treatment follow-up, although the degree of variation attributable to these variables was modest. Office visit, complete blood count, liver function tests, chest X ray, chest CT, extremity CT, and extremity MRI were ordered more frequently with increasing tumor grade and size. |
Multiple Sites
|
Results |
|
All respondents reported an intention to provide some level of follow-up testing of their cancer patients. There was considerable variation in testing practices that was not explained by physician age, year of graduation, practice type, or state of residence. |
|
The most consistently performed screenings reported by nurses were mammogram, professional breast examination, and Pap test and pelvic examination. The least frequently performed screenings were flexible sigmoidoscopy/colonoscopy, and bone mineral density testing. Less than one-third of survivors are offered counseling on strategies to promote bone health. |
|
Most cancer patients received the recommended minimum number of physical examinations after treatment. A sizable number received physical examinations at a frequency in excess of what is currently recommended. Most survivors received recommended testing for local recurrence, however, less than two-thirds of colorectal cancer patients received recommended colon examinations in the initial year after treatment. Evidence of overtesting for local recurrent cancer was found. Testing for metastatic disease was commonplace despite its not being recommended in guidelines. |
APPENDIX 4B
INFORMATION ON AMBULATORY CARE SURVEY DATA
The information on ambulatory care in Tables 4-1 to 4-4, 4-6, and 4-7 comes from two large population-based surveys conducted by the National Centers for Health Statistics, the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Care Survey (NHAMCS). Two years (2001 and 2002) of data from both surveys were combined to yield sufficient sample sizes for cancer site-specific estimates. Information on 3,773 cancer-related ambulatory care visits was available from the 2001 and 2002 NAMCS and NHAMCS. Cancer-related visits are those for which cancer was recorded as the first, second, or third diagnosis associated with the visit according to the International Classification of Diseases (Clinical Modification), Ninth Edition (ICD-9-CM). Cancer-related visits were those where the reason for the visit was coded for history of cancer (V10) and malignant neoplasms (ICD-9 code 140 to 208, but excluding skin, nonmelanoma [173]). All numbers and percentages presented in tables are adjusted using sampling weights to produce national estimates.
NAMCS
NAMCS is a national probability sample survey of visits to office-based physicians. In 2001, information on 24,281 patient visits was received from the 1,230 physicians who participated in NAMCS (64 percent response rate). In 2002, information on 28,738 patient visits was received from 1,474 physicians (70 percent response rate). For each participating physician, a random sample of visits was obtained during a 1-week period. In the tables describing type of physician, “oncology” includes medical oncology and hematology/oncology; “primary care” includes family practice, internal medicine, and general practice; “specialty surgery” includes the following surgical specialties: orthopedic, plastic, vascular, neurological, thoracic, colorectal, and head and neck. “Other medical specialty” includes obstetrics and gynecology, ophthalmology, cardiovascular diseases, psychiatry, gastroenterology, otolaryngology, hematology, pulmonary disease, and others. Estimates of cancer-related ambulatory care are somewhat hampered by the exclusion of radiologists from the sampling frame of office-based providers.
NHAMCS
NHAMCS provides information on ambulatory care provided in hospital outpatient departments. In 2001, information on 33,567 patient visits
was provided by 1,036 clinics in 224 hospitals (overall response rate, 74 percent). In 2002, information on 35,586 patient visits was provided by 1,041 clinics in 224 hospitals (overall response rate, 75 percent). For each participating outpatient department clinic, a random sample of visits was obtained during a 4-week period. Excluded from the sampling frame of the hospital outpatient departments are clinics providing chemotherapy, radiation therapy, infusion therapy, physical medicine, and rehabilitation because, in these settings, much of the care is provided by nonphysician providers (e.g., oncology nurses, radiation technicians, physical therapists). The purpose of the surveys is to capture physician-patient encounters.
APPENDIX 4C WHAT HAS BEEN LEARNED ABOUT MODELS OF SURVIVORSHIP CARE IN OTHER COUNTRIES?
Investigators in Europe, Canada, and Australia have evaluated delivery system issues, often in an effort to improve national health programs. Much of the work of relevance to the delivery of cancer survivorship care comes from the United Kingdom, where cancer care is being reorganized and the results of restructuring efforts are being evaluated through clinical audits (Tattersall and Thomas, 1999). Here, traditional disciplinary divisions of medicine, surgery, obstetrics, and gynecology are being replaced by disease-and organ-based multidisciplinary groupings. The goals of the reorganization are to facilitate improvements in quality, reduce geographic disparities in outcomes of treatment, and provide better coordinated care.
As part of a major effort to gauge cancer patients’ experience with cancer within the British National Health System, a nationwide survey was conducted in 2000 (Airey et al., 2002). The survey covered access to care, diagnosis, first treatment, hospital care, and outpatient care.17 Some aspects of follow-up care were assessed as part of this survey:
-
19 percent of cancer patients reported that doctors and nurses did not spend enough time, or spent no time at all, telling them what would happen when they left the hospital after their first treatment.
-
26 percent of patients were not given written or printed information about what they should or shouldn’t do following their discharge.
-
36 percent of cancer patients were not told about a support or self-help group.
The cancer care survey involved more than 65,000 patients who had been treated in 1999 and 2000. Cancer care in England has recently been organized into 172 networks or “Trusts.” Data from the survey were made available to each Trust so they could compare their results with other care providers. The position of “Primary Care Cancer Lead Clinician” has been created as part of this reorganization to improve communication between primary and specialty care providers, raise awareness of cancer in primary care, and improve palliative care (Leese et al., 2004).
Several clinical trials have been conducted in Europe to assess alternative models of survivorship care. A shared cancer care program implemented and tested as part of a clinical trial had a positive effect on patients’ evaluation of cooperation between the primary care providers and specialists (Nielsen et al., 2003).
The trial involved patients with several types of cancer seen in a Danish university hospital-based practice. The intervention involved knowledge transfer, communication channels, and active patient involvement (Box 4C-1). The shared care program increased contacts with the general practitioner and did not adversely effect quality of life.
A commentary that accompanied the publication of this trial emphasized the importance of the involvement of both patients and providers in formal shared-care arrangements in cancer (Maher and Millar, 2003). In
|
BOX 4C-1 Knowledge transfer
Communication channels
Active patient involvement
SOURCE: Nielsen et al. (2003). |
addition, shared-care models depend on (1) professional training, (2) general practitioners viewing their role in cancer care as enhancing patient care and improving their job satisfaction, and (3) appropriate remuneration.
Long-term cancer survivors who have been followed for many years by specialists are sometimes reluctant to return to their primary care physician for follow-up, even when reassured that they are at low risk of recurrence. One group of British clinicians noted that a feeling often expressed by patients seen for many years in their specialty clinics was “As long as I keep coming here I feel I’ll be alright” (Glynne-Jones et al., 1997). These clinicians concluded that reassurance rather than the detection of recurrence was the most important function of follow-up and so developed a formal system of discharge from their hospital-based oncology clinic to primary care providers for follow-up. As part of the planned discharge, patients were counseled and given a written contract reassuring them of their good prognosis and commitment to continued care from the specialist if necessary. Primary care physicians were informed of the discharge plan. Of the long-term cancer survivors invited to participate, 63 percent agreed to be discharged to primary care. Of the patients who agreed to the contract, 85 percent remained with their primary care provider at 13 months. The planned discharge was viewed as successful, and investigators noted the need to address patients’ expectations regarding follow-up and the duration of specialty follow-up early in the treatment process. Investigators highlighted the need to address anxiety among patients as this is a deterrent to acceptance of a transfer to primary care.
A trial conducted at a breast clinic in London showed that reducing the number of hospital-based follow-up visits was not associated with increased visits to local practitioners or to higher use of a telephone hotline (Gulliford et al., 1997). Women diagnosed within the past 5 years were seen every 3 to 6 months in the conventional arm of the trial, but annually during the visit for mammography in the reduced visit arm (for women treated with lumpectomy). Nearly all (93 percent) women were willing to participate in the trial. Twice as many patients in both groups preferred reducing rather than increasing follow-up visits.18
Evidence that generalists are as effective as specialists in providing follow-up care for women with breast cancer is available from clinical trials. General practitioner follow-up did not increase length of time to diagnosis of a recurrent cancer (as measured at 18 months) or adversely effect quality of life (Grunfeld et al., 1996). Women who had follow-up
care provided by their general practitioner reported greater satisfaction with care than did those followed up in the hospital by specialty providers (Grunfeld et al., 1999a). Costs associated with follow-up were lower when provided by general practitioners (even though they ordered more tests) (Grunfeld et al., 1995, 1996, 1999b).19 This trial was conducted at two district hospitals in England where one-third of women declined to participate in the trial, indicating that this model of care is not acceptable to all patients. As part of the intervention, general practitioners were sent a letter with recommended follow-up protocols that varied according to initial treatment and patient age. Specialists and general practitioners, when surveyed, indicated that their specialty group should provide follow-up care. These findings have prompted some British hospitals to limit specialty follow-up after treatment for breast cancer to 2 years, after which time oncology providers coordinate with local general practitioners and arrange for patients to be seen by their general practitioners with immediate access to specialist review in the breast care unit if needed (Donnelly et al., 2001).
A Canadian trial also examined the question of specialist versus primary care follow-up for women with early breast cancer (Grunfeld et al., 2004). Women randomized to follow-up by cancer center specialists or family physicians had similar rates of death, recurrence, serious clinical events (e.g., poor functional status), and quality of life. Median period of follow-up was 3.5 years. The results of this trial appear to have affected follow-up care in other parts of the country. A survey conducted in Manitoba of cancer patients regarding their care 6 to 12 months after diagnosis found that relatively few (10 percent) were getting mainly specialist care; roughly 40 percent were getting “parallel care” in which cancer specialists looked after everything to do with cancer and the family doctor looked after most other health problems; about 40 percent were getting “shared care” in which the family doctor and cancer specialists both had been involved in taking care of cancer and the family doctor looked after most other health problems; and 10 percent described other health care situations (Sisler et al., 2004). The most commonly cited kinds of help that women reported needing from family practitioners were:
-
Helps with medical problems unrelated to my cancer
-
Gets me an appointment with a surgeon or other cancer specialist fairly quickly
-
Takes extra time with me during a visit
-
Sees me quickly in the office if I think it’s necessary
-
Answers my questions about cancer and cancer treatments
-
Discusses how I am feeling about having cancer
-
Helps with common cancer-related problems, like pain, nausea, depression, and bowel problems
Focus groups held with family physicians throughout Canada to discuss communication between family physicians and oncologists suggest that family physicians would like to have more contact with oncologists, preferably by phone or in person, to negotiate their respective roles, and to discuss the patient’s prognosis and the effectiveness of proposed treatments (Dworkind et al., 1999). According to qualitative interviews with oncologists in a Canadian regional cancer center, collaboration with family physicians in the remission phase was identified as desirable, but inhibited by variable and unpredictable interest, poor communication with family physicians, and patients’ own preferences for follow-up. Oncologists perceived the cancer system structure as a “black box” within which multidisciplinary teams worked well but seldom included family physicians. Oncologists expressed a need to see healthy patients and to have more understanding and support from family physicians, preferably through sharing follow-up care. Developing dialogue and a more collaborative approach were suggested (Wood and McWilliam, 1996; Matthews et al., 2004).
A few studies have been carried out to evaluate how to improve communication among cancer specialists, primary care providers, and cancer patients. To encourage breast cancer patients to seek information about cancer from their general practitioners, one group of investigators in England tested giving women cards with specific information about their treatment to take to their general practitioner (Luker et al., 2000). Results of the small clinical trial suggested that the cards did not encourage women to seek information from their primary care physician. Women’s information-seeking behavior was more determined by their longstanding relationship with their general practitioner, their perception that their general practitioner lacked specialist knowledge, and the perception that information seeking was not a reason to seek primary care.
A standardized discharge letter improved communication from oncologists to family physicians with respect to the relevance, timeliness, format, and amount of information conveyed (Braun et al., 2003). In this Canadian study, the letter was intended to communicate information about the palliative care needs of patients with lung cancer and included information about diagnosis, stage of disease, current problem(s), treatment plan,
potential problems, prognosis, discussion with family, follow-up, and homecare arrangements.
Several European and Australian studies have assessed nurse-led follow-up care. Nurse-led follow-up of patients with lung cancer was found to be acceptable and led to positive outcomes when compared to conventional medical follow-up in an English randomized trial (Moore et al., 2002). Other assessments in the United Kingdom also suggest that breast cancer survivors are accepting of a specialist nurse-led system of follow-up care (Earnshaw and Stephenson, 1997; Pennery and Mallet, 2000; Renton et al., 2002). One British study of patients’ views on follow-up of colorectal cancer, however, indicated that fewer than half (47 percent) would accept follow-up care from a specialist nurse, and even fewer (27 percent) were willing to be followed by their general practitioner (Papagrigoriadis and Heyman, 2003). A Swedish randomized trial compared routine physician follow-up and “on-demand” care by a specialist nurse for women with early-stage breast cancer and found no differences between groups in terms of survivors’ satisfaction, perceptions regarding accessibility of care, anxiety, and depression when measured over a 5-year period (Koinberg et al., 2004). Another smaller clinical trial conducted in the United Kingdom compared standard clinic follow-up with on-demand care through a breast care nurse (Brown et al., 2002). Women in the on-demand care group were given written information on the signs and symptoms of recurrence and were instructed to contact the breast care nurse if they encountered any problems. There were no major differences in quality of life and psychological morbidity between the two groups and no observed adverse effects associated with patient-initiated follow-up. Of note, however, is that half of women approached to participate in the trial refused, indicating that this model for follow-up is not acceptable to many women with early-stage breast cancer. Resistance to this model of follow-up was evident in an earlier study indicating that most women desired specialist, hospital-based follow-up (Brown et al., 2002). In Australia, a comprehensive specialist breast nurse model of care has been developed to improve the delivery of care, especially psychosocial services (Hordern, 2000; Liebert et al., 2001, 2003; Parle et al., 2001).
Some research suggests that nurse-based follow-up can meet the needs of cancer survivors and reduce the medical outpatient workload. A British study of a nurse-led clinic for patients being treated for central nervous system tumors and a nurse “phone clinic” for post-treatment follow-up was acceptable to patients and decreased the medical outpatient workload by 30 percent. This medical audit was conducted in a large neurooncology program in an English hospital (James et al., 1994). At this same hospital, patients were very satisfied with a nurse-led telephone clinic in the follow-up of patients with glioma, a cancer with very poor prognosis. The tele-
phone intervention was believed to be able to replace routine specialist care during the short stable phase of disease following treatment (Sardell et al., 2000).
APPENDIX 4D CHALLENGES IN THE DELIVERY OF SELECTED SURVIVORSHIP SERVICES
This appendix illustrates some of the challenges of delivering three survivorship services:
-
Genetic counseling
-
Cancer rehabilitation
-
Psychosocial services for women with breast cancer
Genetic Counseling
It has long been recognized that cancer can run in families, and that people with close relatives who have had cancer may be at greater risk for a cancer diagnosis. The establishment of concrete links between particular genetic mutations and cancer have opened the possibility of genetic testing for increased cancer risk. The complete sequencing of the human genome will likely lead to additional opportunities for risk assessment. Genetic risk assessment and testing holds great promise in helping survivors and their family members plan appropriate screening regimens, consider preventative measures, and make reproductive decisions (NCCN, 2004a,b). However, the appropriate delivery and management of genetic information creates new challenges for the health care system. Among these challenges is a lack of trained personnel available to provide genetic counseling and testing to cancer survivors and their families. This limitation of capacity, if not addressed, will limit the diffusion of this relatively new and potentially vital technology.
Genetic tests are commercially available and may be of value for certain cancer survivors and their family members. For example, women diagnosed with breast cancer who have a strong family history of breast and/or ovarian cancer are often tested to determine if they are among the estimated 5 to 10 percent of women with breast and ovarian cancer that is caused by mutations in the BRCA1 and BRCA2 genes. Likewise, commercially available genetic tests are available for colon cancer survivors who have a strong family history of the disease. An estimated 5 percent of individuals with colon cancer have either familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer (HNPCC) (Sifri et al., 2004). As the availability of genetic testing becomes more widely known, it will be in-
creasingly important for primary care providers, oncologists, nurses, and other health professionals to be familiar with the process of genetic counseling and testing.
A complete assessment of genetic risk involves much more than genetic testing. American Society of Clinical Oncology (ASCO) guidelines recommend that genetic testing only be offered to selected patients with personal or family histories suggestive of a hereditary syndrome, in the context of pre- and post-test counseling to discuss the risks and benefits of genetic testing and cancer early detection and prevention methods, and only when the test results can be adequately interpreted and will aid in diagnosis or care management (ASCO, 2003).
A recent national survey of physicians indicated that most do not consider themselves qualified to provide genetic counseling to their cancer patients (Table 4D-1) (Freedman et al., 2003). Although nearly a third of U.S. physicians have offered genetic tests or referred patients to be tested, only 8 percent took responsibility for providing pre- and post-test counseling by ordering the tests directly (Wideroff et al., 2003a). Oncologists express more confidence than other physicians in recommending genetic testing (85 percent) and providing counseling (50 percent), however, these estimates suggest that additional education and training in this area is needed for all providers who are likely to encounter cancer survivors in their practices (see Chapter 5).
Individuals with strong family histories of cancer (or who are considered high risk) may be referred to a genetic counselor. Genetic counselors are usually master’s degree-level trained and are certified by the American Board of Genetic Counseling. There are about 1,800 certified genetic counselors in the United States, but only 42 percent provide counseling to cancer patients and their families, and only 16 percent spend more than half their
TABLE 4D-1 Physicians’ Qualifications to Provide Genetic Counseling and Recommend Genetic Testing
|
Specialty |
Percentage Who Feel Qualified to Provide Genetic Counseling |
Percentage Who Feel Qualified to Recommend Genetic Testing |
|
Primary care |
28.8 |
40.8 |
|
Tertiary care (general surgery, gastroenterology, urology) |
30.7 |
58.2 |
|
Oncology |
50.0 |
84.6 |
|
SOURCE: Freedman et al. (2003). |
||
time on cancer genetics (Parrott et al., 2002, 2003). Access to genetic counselors with cancer-related experience may be further limited, as more than half of cancer genetic counselors work in university medical centers, and only 27 percent work in private hospitals or medical facilities (Parrott et al., 2003). Another important resource for genetic counseling is oncology nurses trained in genetics, who may play an increasing role in the delivery of cancer genetic services in the future (Bernhardt et al., 2000; Masny et al., 2003).
The demand for genetic counseling and testing is likely to grow as new tests are developed and marketed, and as cancer survivors and their family members become aware of their potential benefits. Approximately 44 percent of the public is already aware of the availability of genetic tests for cancer susceptibility (Wideroff et al., 2003b). One of the greatest predictors of whether a physician has ordered genetic tests or made a referral for genetic counseling is having patients ask for cancer genetic tests (Wideroff et al., 2003a). Unless education and training programs reach more oncology and primary care providers who care for cancer survivors, the public’s demand for genetic testing and counseling will likely not be met.
Some patients and providers may be concerned about the possibility of genetic information being used as a basis for employment or insurance discrimination. Bills have been introduced in Congress to prevent discrimination based on genetic information, but no federal protection is currently in place. However, 33 states have laws prohibiting genetic discrimination in employment, and most states restrict employer access to genetic information (NCSL, 2005). Some states extend the protections to inherited characteristics, family history, the test results of family members, and information on receipt of genetic services. In addition, an executive order issued in 2000 protects against discrimination based on genetic information in civilian federal employment. The Health Insurance Portability and Accountability Act (HIPAA) provides some protection against genetic discrimination under group insurance plans, but does not provide any protection for those seeking individual insurance, and does not prevent insurers from accessing genetic information (NIH, 2005).
Cancer Rehabilitation
Rehabilitation services can help cancer survivors regain and improve physical, psychosocial, and vocational functioning within the limitations imposed by the disease and its treatment (Ganz, 1990; Watson, 1992). Although cancer rehabilitation has been recognized as valuable, organized rehabilitation programs for cancer survivors are limited and lag behind those organized for patients with other chronic conditions such as heart disease for which rehabilitation is now considered a part of standard care
(Segal et al., 1999). Some have suggested that rehabilitation programs for cancer patients with physical limitations have been slow to develop because of the nature of cancer and its treatment as compared to other causes of disability (Sliwa and Marciniak, 1999). Physical impairments associated with stroke and brain injury are commonly treated through rehabilitation programs and in these cases, the deficits are fixed, acute care treatment has been completed, and the likelihood of survival following the initial injury or episode is good. In such cases rehabilitation occurs after acute medical treatment and addresses static deficits. In contrast, cancer patients experiencing physical limitation may be in treatment when rehabilitation services are needed and the treatment may not be curative. Complicating cancer rehabilitation further is its heterogeneity. It spans lymphedema management, neurologic and orthopedic rehabilitation, and general conditioning. This breadth of cancer rehabilitation may pose the greatest challenge to service delivery.
The recognition of the need for rehabilitation services for cancer survivors is longstanding, and the U.S. Congress has actively encouraged the development of rehabilitation programs for cancer survivors (Box 4D-1). These congressional actions encouraged the development of cancer rehabilitation programs and centers. One of the earliest cancer rehabilitation programs was established in 1969 by Dietz, a physiatrist who coordinated the resources of an acute care hospital and a cancer center (Dietz, 1974; Grabois, 2001). The expansion of the role of the National Cancer Institute into rehabilitation in 1971 led to the development of related training, demonstration, and research projects. Some observers have noted, however, that cancer rehabilitation was hampered in its development because there was no specific implementation plan, a lack of trained personnel, and a failure to educate referring health care professionals (Grabois, 2001).
Despite these early initiatives, there are now relatively few organized cancer rehabilitation programs. Those that have been developed are usually housed within hospital physical medicine and rehabilitation programs or within large cancer centers. With the shift of cancer care from inpatient settings to outpatient care, some are concerned that cancer survivors’ needs are not being met. Oncologists and surgeons report that rehabilitation services are not available, or if available, are not adequately covered by health insurance. While there is anecdotal evidence of problems with access to rehabilitation services for cancer patients, there has been little systematic documentation of such problems among contemporary cancer survivors.
The successful expansion of cancer rehabilitation programs has been hampered by a lack of an evidence base upon which to base decisions regarding: who needs services; what services should be provided; who should deliver services; and where and how services should be delivered. In the absence of evidence, no widely recognized clinical practice guidelines
|
BOX 4D-1 1965—Congress authorized the establishment and maintenance of Regional Medical Programs under the Heart Disease, Cancer and Stroke Amendment (Pub. L. No. 89–239). These programs were to encourage and assist in the establishment of regional cooperative arrangements among medical schools, research institutions, and hospitals for research and training, including continuing education, and for related demonstration of patient care. Fifty-six regions were established across the nation. Rehabilitation units were to be created in association with diagnostic and treatment services. The program was terminated in 1976. 1971—Congress passed the National Cancer Act (Pub. L. No. 92–218) to amend the Public Health Service Act to strengthen the National Cancer Institute. Funds were available for the development of training, demonstration, and research projects in rehabilitation. 1988—Congress passed legislation (Pub. L. No. 100–607) that added rehabilitation research to the mission of the National Cancer Institute as follows: The general purpose of the National Cancer Institute is the conduct and support of research, training, health information dissemination and other programs with respect to cause, diagnosis, prevention, and treatment of cancer, rehabilitation from cancer, and the continuing care of cancer patients and the families of cancer patients (42 U.S.C. 285). 1998—Women’s Health and Cancer Rights Act (Pub. L. No. 105–277) was enacted to require health insurance policies that cover mastectomy to also provide coverage for reconstructive surgery, prostheses, and physical complications of mastectomy, including lymphedema. SOURCES: President’s Commission on Heart Disease (1964); DeLisa (2001); NLM (2005); NCLAC (2005); NCI (2005b). |
have been developed for common cancer-related conditions, and there are few evidence-based mechanisms to ensure appropriate service use. This void has led to the use by Medicare and private insurers of other mechanisms such as caps in benefits or limits on services in order to control rising costs. Such mechanisms can frustrate both providers and patients as they seek care that is viewed as medically necessary.
Evidence Regarding the Risk of Disability and the Need for Services
Relatively few studies adequately document the prevalence of physical and functional limitations among contemporary cancer survivors. Many of
the widely cited studies used to document the prevalence of physical impairments imposed by cancer or its treatment are limited because they were conducted many years ago; did not include representative samples of patients; did not use standard evaluation tools to assess functional limitations; and did not include control groups, sometimes making it difficult to distinguish cancer-related limitations from those due to aging. Many studies have been conducted within inpatient units of individual institutions, and virtually none conducted in outpatient settings where most cancer patients now receive their care. Without such studies it is difficult to gauge how many cancer survivors need rehabilitation services and the extent of any access problem that may exist.
Evidence Regarding What Services Should Be Provided
Relatively few clinical trials have been conducted to assess the effectiveness of cancer rehabilitation services, and those that have been conducted have focused on inpatient rehabilitation (especially for patients with cancers of the brain or spinal cord) (Gerber, et al., 2005). A few clinical trials have been conducted to test the role of exercise in cancer rehabilitation (Segal et al., 1999; AHRQ, 2004b). Exercise has been shown to enhance physical performance, reduce fatigue, and improve psychological well-being (see Chapter 3 section on physical activity). A recommendation to exercise is therefore appropriate for most cancer survivors, however, more research is needed to determine what type of exercise regimen is optimal for survivors of different types of cancer and with various levels of physical limitation. Research could also help distinguish those survivors who could safely engage in exercise on their own from those who need the supervision of rehabilitation personnel during exercise.
Much of the literature documenting gains in functioning following cancer rehabilitation is based on observational studies conducted within a single institution (Sabers et al., 1999; Ganz, 1999). The relative lack of evidence on the effectiveness of rehabilitation treatment modalities has serious implications for patients who are facing cancer-related functional limitations. A recent review of the evidence regarding the treatment of lymphedema related to breast cancer found insufficient high-quality evidence on which to base a clinical practice guideline (Kligman et al., 2004). There is also insufficient evidence upon which to counsel women with breast cancer regarding how to prevent lymphedema (Runowicz, 1998). Well-designed controlled clinical trials are needed to reinvigorate cancer rehabilitation, as well as to evaluate the impact of this important clinical care activity on patient outcomes (Ganz, 1999).
|
BOX 4D-2
SOURCES: Beck (2003); Ragnarsson and Thomas (2000). |
Who Should Deliver Services
Members of the cancer rehabilitation team trained to address the physical, functional, and vocational concerns of cancer survivors are shown in Box 4D-2. Other types of professionals may provide rehabilitative services (e.g., massage therapists, chiropractors), and some referring providers have expressed discomfort over role issues among rehabilitation team members and other medical caregivers (Schmidt, 2001). This discomfort or confusion could lead physicians caring for cancer survivors to fail to refer patients for rehabilitation services. Rehabilitation is underrepresented in medical education and training programs and the role of physical rehabilitation services is not always well understood (Frymark, 1998). This lack of awareness of the specialty may also contribute to underreferral (Schmidt, 2001).
Where and How Services Should Be Delivered
Rehabilitation services are furnished in many different settings, including hospital outpatient departments, outpatient rehabilitation facilities, skilled nursing facilities, home health agencies, and physicians’ offices, and by therapists in private practice. Cancer rehabilitation has generally focused on inpatient services, leaving outpatient services not well developed. The exception to this is the many lymphedema clinics that are available. However, there are no estimates of their number, their organization, or their patient populations.
There are few descriptions of existing models for delivering cancer rehabilitation (Harvey et al., 1982; MacLaren, 2003). One U.S. study pub-
lished in 1982 reviewed 36 cancer rehabilitation programs that were located at cancer centers, university hospitals, or community hospitals. Most programs were organized through oncology departments or departments of rehabilitation medicine (Harvey et al., 1982). Lehmann and colleagues described a model of care that involved systematic assessment and an interdisciplinary team approach to care (Lehmann et al., 1978). A few hospital- or cancer-center-based rehabilitation programs have been described in the literature (Segal et al., 1999; Schmidt, 2001; Grabois, 2001), but there have been no attempts to assess which models of care are more effective or preferred by cancer survivors.
The Consequences of a Lack of Evidence
Without evidence of the effectiveness of services and optimal delivery systems, patients cannot easily make personal health care decisions, health care providers lack the clinical practice guidelines they need to optimize care, and insurers and payors lack the tools they need to ensure that appropriate care is given.
Lacking professionally developed evidence-based guidelines, managed care organizations have attempted to control costs through utilization review mechanisms such as case management programs and authorization for coverage or have set annual limits for coverage. Such programs limit coverage, but without established evidence-based clinical practice guidelines, they cannot distinguish necessary from unnecessary care. Some managed care organizations have attempted to refine their coverage decisions through commercially available therapy guidelines (e.g., InterQual, Milliman & Robertson, Apollo, HealthSouth) and functional assessment and outcome monitoring systems (e.g., LIFEware, Focus on Therapeutic Outcomes or FOTO) (Maxwell and Baseggio, 2000).
Expenditures under Medicare for outpatient therapy services of physical therapists (PTs), occupational therapists (OTs), and speech and language pathologists (SLPs) have increased over time, and Congress has responded by enacting measures to control payment growth (Ciolek and Hwang, 2004). Medicare’s coverage policies are fairly broad (Box 4D-3) and, in general, Medicare beneficiaries (with and without cancer) do not report encountering problems in getting special therapy services (which include PT, OT, and SLP services) (MedPAC, 2004a). No limit currently exists on the amount of medically necessary outpatient therapy (PT, OT, or SLP) a beneficiary may receive under Medicare, but this has not always been the case. Payment caps have been imposed intermittently and were suspended at the end of 2003. Without congressional action, payment caps will resume in January 2006 (Ciolek and Hwang, 2004). Most private payors have adopted some kind of controls to limit rehabilitation service
|
BOX 4D-3 Medicare covers outpatient therapy services as long as the services are furnished by a skilled professional, are appropriate and effective for a patient’s condition, and are reasonable in terms of frequency and duration. Furthermore, a physician must refer the patients; review a written plan of care every 30 days; and, for longer term treatment (extending beyond 60 days), reevaluate the patient. In addition, providers must have a physician on call to support emergency medical care. Beneficiaries are expected to improve significantly in a reasonable period of time. Medicare does not cover physical therapy designed to maintain a level of functioning or serve as a general exercise program. Finally, services are not covered when the expected patient gains from therapy are insignificant in relation to the therapy required to reach them or when it has been decided that a patient will not realize treatment goals. SOURCES: Maxwell and Baseggio (2000); MedPAC, (2004a). |
use (e.g., to a predefined number of days or visits per year, such as 60 calendar days from the beginning of an “event” or 30 visits) (Maxwell and Baseggio, 2000).
Some of Medicare’s reimbursement policies are not always consistent with the latest evidence on effectiveness. For women with breast cancer, for example, available evidence suggests that nonpharmacologic treatments, especially complex decongestive therapy, is effective for lymphedema. This therapy involves skin care, multilayer low-stretch bandaging, exercise, and massage techniques, followed by long-term fitted elastic compression (Sparaco and Fentiman, 2002; Kligman et al., 2004). Medicare and many private health insurers generally cover the most expensive components of complex lymphedema therapy, including physical therapy, but some aspects of the therapy may not be covered. Medicare, for example, does not cover durable medical equipment, including surgical stockings or hose. Expensive pneumatic compression devices are covered if they are determined to be reasonable and necessary for the treatment of lymphedema and if a trial of conservative therapy (including the use of compression bandages or garments) lasting 4 weeks does not result in improvement. Compression garments are covered only if the patient is also prescribed a pneumatic compression pump.
There are some anecdotal reports that pumps are provided to lymphedema patients with little education or follow-up, and that in some cases, these pumps actually worsen the condition irreversibly, especially if excessive pressures are used. Pumps have not been shown to be effective, are expensive, and because they tether users for a few hours daily, they tend to
go unused. Pumps may have a place in patient treatment programs, but it is crucial that other modalities be fully explored first, and to the extent possible, that pumps be combined with other modalities. Although Medicare reimbursement is available for pumps, the scientific evidence supporting their effectiveness is poor compared to that available for most of the other modalities, notably complex decongestive therapy (especially the wrapping component) and compression garments.
Congress has acted to ensure coverage of certain cancer rehabilitation services. The federal Women’s Health and Cancer Rights Act of 1998 requires health insurance policies that cover mastectomy to also provide coverage for reconstructive surgery, prostheses, and physical complications of mastectomy, including lymphedema. Certain states have also mandated coverage. Since 2004, Virginia has required health insurers and plans to provide coverage for lymphedema, including equipment, supplies, complex decongestive therapy, and outpatient self-management training and education for the treatment of lymphedema, if prescribed by a health care professional.
Given the complexities of coverage for lymphedema therapy, help in resolving insurance reimbursement problems is frequently requested from the National Lymphedema Network, an advocacy group representing individuals with lymphedema (NLN, 2005).
In summary, evidence of the effectiveness of cancer rehabilitation services, who should deliver such services, and in what manner is critical to guide the decisions of consumers, providers, educators, and payors. Such evidence is needed before the perceived barriers to access to these services can be overcome. Research is critical to better elucidate the post-treatment rehabilitation needs of cancer survivors.
Psychosocial Services for Women with Breast Cancer
Distress in cancer has been defined as an unpleasant emotional experience that may be psychological, social, or spiritual in nature (see Chapter 3). Distress exists on a continuum beginning with the “normal” and expected feelings of fear, worries, sadness, and vulnerability in coping with cancer and its treatment. However, these normal feelings may extend to become more severe, even disabling, symptoms of anxiety or a formal diagnosis of major depression. Severe distress may relate to the illness or its treatment, a severe social problem, or a family problem, or it also may result from a spiritual or existential crisis created by confronting a threat to life or from the complications of treatment (NCCN, 2004c). Logically, psychosocial issues and distress are likely primarily not cancer site-specific, but they have been studied most extensively among women with breast cancer. In particular, women with breast cancer have been examined for the
impact on psychological function at each stage of disease and during survivorship. The studies show highest distress at transition points in treatment: at the time of diagnosis, awaiting treatment, during and on completion of treatment, at follow-up visits, at time of recurrence, and at time of treatment failure. Taken overall, approximately 30 percent of women show significant distress at some point during the illness, and the number is greater in women with recurrent disease whose family members are also distressed.
Interventions to address psychosocial problems and distress begin with basic information about the disease and treatment options from the breast cancer care clinician (often a medical oncologist). This clinician, regardless of medical specialty, should express support, encourage patients to voice their fears and concerns, encourage coping, and provide medication when needed to control symptoms like insomnia and anxiety. Psychosocial services should be provided by oncology caregivers as a part of total medical care, but referrals to specialists in psychooncology, social workers, pastoral counselors, and other professionals may be necessary when the level of distress is high. The frequency of visits to a psychooncology professional may vary from a single encounter to several, and the timing and duration may also vary from very brief to extending over months or, at times, even years. Today, there are many community-based services available to women with breast cancer at no charge. Evidence from 31 randomized clinical trials, meta-analyses, and nonrandomized studies of the effectiveness of psychosocial interventions among women with breast cancer supports the inclusion of psychosocial interventions in routine clinical care (IOM, 2004b). This body of research documents that several psychosocial interventions reduce psychosocial problems and distress among women with breast cancer. Psychosocial factors and interventions are also related to other aspects of cancer such as pain and other side effects.
Many women with breast cancer rely solely on family, friends, and clergy for social support. Some may find information and support on the Internet, for example, the American Cancer Society’s “Cancer Survivor’s Network” or ASCO’s “People Living with Cancer.” Other women, however, do not have social supports built into their lives. They may also lack access to psychosocial services, either because care providers do not refer them to the available services or because of other barriers (e.g., no health insurance or no reimbursement for services).
Several barriers impede appropriate care. The dramatic shift in the delivery of nearly all cancer care from inpatient hospital to outpatient settings has not included a similar shift in the outpatient psychosocial services to the outpatient clinics and private oncology office practices. Increased complexity of care has limited access even further. Women with breast cancer usually see multiple specialists (e.g., surgeons, radiation
oncologists, medical oncologists), and care is often not well coordinated. Fragmentation of care is an added psychological burden; the patient is not given care by a single, trusted physician. In addition, the outpatient offices and clinics are extremely busy; the length of time doctors can spend with patients is often limited; and the opportunity to bring up psychosocial problems may be lost. Receiving adequate information and the ability to ask questions in a comfortable way are basic needs for addressing psychosocial concerns. Breast cancer care occurs primarily in private office-based practices that routinely do not employ psychosocial professionals.
Another barrier is the lack or inadequacy of health insurance coverage. An estimated 5 percent of women ages 25 to 64 with breast cancer are uninsured, or, if patients are insured, there is coverage of mental health services with lower reimbursement levels or placement of mental health services in behavioral health contracts, separate from medical coverage (see Chapter 6). Still other barriers are the reluctance to discuss psychosocial concerns with the busy oncologist provider; the stigma associated with seeking or using mental health services; physicians’ failure to ask patients about distressing emotional symptoms; and the lack of simple, rapid instruments for screening for psychosocial distress. All are barriers to the symptoms receiving appropriate recognition, diagnosis, and treatment by supportive and psychosocial services. Also, primary oncology teams in outpatient offices are often not familiar with clinical practice guidelines for managing psychosocial distress; they often work in environments that do not provide psychosocial services onsite; and they often are not aware of the psychosocial resources in their local communities. The situation is complicated additionally by the paucity in many communities of identified professionals with skills in managing psychosocial and mental health issues in patients with cancer. As part of an initiative to help locate appropriate professionals, the American Psychosocial Oncology Society (APOS) now provides a directory online (www.apos-society.org) and a toll-free help line for patients and families (1-866-APOS-4-HELP). Overcoming barriers to appropriate use of psychosocial services will require advocacy, monitoring of psychosocial services through quality assurance programs to ensure compliance with standards of care, physician education, training in communication skills, and research relative to identifying and overcoming barriers.
REFERENCES
AAFP (American Academy of Family Physicians). 2002. Cancer: After Treatment. [Online]. Available: http://familydoctor.org/723.xml [accessed April 27, 2005].
AAMC (American Association of Medical Colleges). 2005. AAMC Launches Chronic Care Initiative. [Online]. Available: http://www.aamc.org/newsroom/pressrel/2005/050428.htm [accessed May 2, 2005].
Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. 2000a. Comprehensive cancer control initiative of the Centers for Disease Control and Prevention: An example of participatory innovation diffusion. J Public Health Manag Pract 6(2):79–92.
Abed J, Reilley B, Butler MO, Kean T, Wong F, Hohman K. 2000b. Developing a framework for comprehensive cancer prevention and control in the United States: An initiative of the Centers for Disease Control and Prevention. J Public Health Manag Pract 6(2):67–78.
ACCC (Association of Community Cancer Centers). 2004a. About ACCC. [Online]. Available: http://www.accc-cancer.org/about/ [accessed October 21, 2004].
ACCC. 2004b. Guidelines for Cancer Program. [Online]. Available: http://www.accc-cancer.org/guidelines.asp [accessed October 21, 2004].
ACCC. 2004c. Guidelines for Cancer Programs. Chapter 4: Clinical Management and Supportive Care Services. [Online]. Available: http://www.accc-cancer.org/guidelinesCH4.asp [accessed October 21, 2004].
ACCC. 2004d. Guidelines for Cancer Programs. Chapter 8: Community Services. [Online]. Available: http://www.accc-cancer.org/guidelinesCH8.asp [accessed October 21, 2004].
ACOR (Association of Cancer Online Resources). 2004a. ACOR homepage. [Online]. Available: http://www.acor.org/ [accessed December 3, 2004].
ACOR. 2004b. Mailing Lists: Treatments Late Effects. [Online]. Available: http://www.acor.org/mailing.html?sid=12 [accessed January 31, 2005].
ACS (American Cancer Society). 2004. tlc Catalog. [Online]. Available: http://www.tlccatalog.org/ [accessed December 3, 2004].
ACS. 2005a. All About Cancer (General Information). [Online]. Available: http://www.cancer.org/docroot/CRI/CRI_2x.asp [accessed March 3, 2005].
ACS. 2005b. Cancer Survivors’ Network. [Online]. Available: http://www.acscsn.org/ [accessed April 26, 2005].
Adewuyi-Dalton R, Ziebland S, Grunfeld E, Hall A. 1998. Patients’ views of routine hospital follow-up: A qualitative study of women with breast cancer in remission. Psychooncology 7(5):436–439.
AHRQ (Agency for Healthcare Research and Quality). 2004a. 2004 National Healthcare Quality Report. Rockville, MD: AHRQ.
AHRQ. 2004b. Effectiveness of Behavioral Interventions to Modify Physical Activity Behaviors in General Populations and Cancer Patients and Survivors. Evidence Report/Technology Assessment 102. Rockville, MD: AHRQ.
Airey C, Becher H, Erens B, Fuller E. 2002. National Surveys of NHS Patients—Cancer: National Overview 1999/2000. London, England: Department of Health.
Andersen MR, Urban N. 1998. The use of mammography by survivors of breast cancer. Am J Public Health 88(11):1713–1714.
APOS (American Psychosocial Oncology Society). 2004. Referral Information for Cancer Patients and Caregivers. [Online]. Available: http://www.apos-society.org/patient/default.asp [accessed December 3, 2004].
ASCO (American Society of Clinical Oncology). 2003. American Society of Clinical Oncology policy statement update: Genetic testing for cancer susceptibility. J Clin Oncol 21(12):2397–2406.
ASCO. 2005. People Living with Cancer. [Online]. Available: http://www.plwc.org [accessed April 15, 2005].
Ash JS, Bates DW. 2005. Factors and forces affecting EHR system adoption: Report of a 2004 ACMI discussion. J Am Med Inform Assoc 12(1):8–12.
Audet AM, Doty MM, Peugh J, Shamasdin J, Zapert K, Schoenbaum S. 2004. Information technologies: When will they make it into physician’s black bags? Medscape General Medicine 6(4) [Online]. Available: http://www.medscape.com/viewarticle/493210 [accessed July 15, 2005].
Ayanian J. 2004. Improving Cancer Care in Massachusetts (CAMA). Dana-Farber/Harvard Cancer Center. Unpublished.
Barzilai DA, Cooper KD, Neuhauser D, Rimm AA, Cooper GS. 2004. Geographic and patient variation in receipt of surveillance procedures after local excision of cutaneous melanoma. J Invest Dermatol 122(2):246–255.
Beaver K, Luker KA. 2004. Follow-up in breast cancer clinics: Reassuring for patients rather than detecting recurrence. Psychooncology 14(2):94–101.
Beck LA. 2003. Cancer rehabilitation: Does it make a difference? Rehabil Nurs 28(2):42–47.
Beitler AL, Virgo KS, Johnson FE, Gibbs JF, Kraybill WG. 2000. Current follow-up strategies after potentially curative resection of extremity sarcomas: Results of a survey of the members of the Society of Surgical Oncology. Cancer 88(4):777–785.
Berner ES, Detmer DE, Simborg D. 2005. Will the wave finally break? A brief view of the adoption of electronic medical records in the United States. J Am Med Inform Assoc 12(1):3–7.
Bernhardt BA, Geller G, Doksum T, Metz SA. 2000. Evaluation of nurses and genetic counselors as providers of education about breast cancer susceptibility testing. Oncol Nurs Forum 27(1):33–39.
Berry LL, Seiders K, Wilder SS. 2003. Innovations in access to care: A patient-centered approach. Ann Intern Med 139(7):568–574.
Bickell NA, Young GJ. 2001. Coordination of care for early-stage breast cancer patients. J Gen Intern Med 16(11):737–742.
Blobel B. 2000. Onconet: A secure infrastructure to improve cancer patients’ care. Eur J Med Res 5(8):360–368.
Bodenheimer T, Wagner EH, Grumbach K. 2002a. Improving primary care for patients with chronic illness. JAMA 288(14):1775–1779.
Bodenheimer T, Wagner EH, Grumbach K. 2002b. Improving primary care for patients with chronic illness: The chronic care model, Part 2. JAMA 288(15):1909–1914.
Bope ET. 1987. Follow-up of the cancer patient: Surveillance for metastases. Prim Care 14(2):391–401.
Brady K. 2004 (July 26–27). Survivorship as a Public Health Issue. Presentation at the meeting of the IOM Committee on Cancer Survivorship, Woods Hole, MA.
Braun TC, Hagen NA, Smith C, Summers N. 2003. Oncologists and family physicians. Using a standardized letter to improve communication. Can Fam Physician 49:882–886.
Brown L, Payne S, Royle G. 2002. Patient initiated follow up of breast cancer. Psychooncology 11(4):346–355.
Burt CW, Hing E. 2005. Use of computerized clinical support systems in medical settings: United States, 2001–03. In: Advance Data From Vital and Health Statistics. No. 353. Hyattsville, MD: National Center for Health Statistics.
Canada AL, Schover LR. 2005. Research promoting better patient education on reproductive health after cancer. J Natl Cancer Inst Monogr 34:98–100.
Cancer Control Planet. 2004. Cancer Control Planet. [Online]. Available: http://cancercontrolplanet.cancer.gov/ [accessed November 30, 2004].
CancerCare. 2005a. CancerCare homepage. [Online]. Available: http://www.cancercare.org [accessed May 11, 2005].
CancerCare. 2005b. CancerCare Services for Cancer Survivors. [Online]. Available: http://www.cancercare.org/CancerCareServices/CancerCareServicesList.cfm?c=878 [accessed May 11, 2005].
Case C, Johantgen M, Steiner C. 2001. Outpatient mastectomy: Clinical, payer, and geographic influences. Health Serv Res 36(5):869–884.
CDC (Centers for Disease Control and Prevention). 2004a. National Comprehensive Cancer Control Program. [Online]. Available: http://www.cdc.gov/cancer/ncccp/ [accessed November 29, 2004].
CDC. 2004b. Program Contacts by Funding Status. [Online]. Available: http://www.cdc.gov/cancer/ncccp/cccstatelist.htm [accessed November 29, 2004].
CDC. 2005a. The New Spirit of Survivorship. [Online]. Available: http://www.cdc.gov/cancer/survivorship/newspirit.htm [accessed July 21, 2005].
CDC. 2005b. Working Together for Comprehensive Cancer Control: Institutes for State Leaders. [Online]. Available: http://www.cdc.gov/cancer/ncccp/institutes.htm [accessed April 28, 2005].
CDC and LAF (Centers for Disease Control and Prevention and Lance Armstrong Foundation). 2004. A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies. Atlanta, GA: CDC.
Children’s Oncology Group. 2005. Cure Search. [Online]. Available: http://www.childrensoncologygroup.org/ [accessed February 24, 2005].
Ciolek DE, Hwang W. 2004. Outpatient Rehabilitation Services Payment System Evaluation: Final Project Report. Columbia, MD: AdvanceMed, prepared for the Centers for Medicare and Medicaid Services.
Clark JG, Virgo KS, Clemente MF, Johnson MH, Paniello RC, Johnson FE. 1999. How surgeon age affects posttreatment surveillance strategies for upper aerodigestive tract cancer patients. Am J Otolaryngol 20(4):217–222.
CMS (Centers for Medicare and Medicaid Services). 2004. Medicare Coordinated Care Demonstration. [Online]. Available: http://www.cms.hhs.gov/healthplans/research/coorcare.asp [accessed November 22, 2004].
Collins RF, Bekker HL, Dodwell DJ. 2004. Follow-up care of patients treated for breast cancer: A structured review. Cancer Treat Rev 30(1):19–35.
Coluzzi PH, Grant M, Doroshow JH, Rhiner M, Ferrell B, Rivera L. 1995. Survey of the provision of supportive care services at National Cancer Institute-designated cancer centers. J Clin Oncol 13(3):756–764.
Commission on Cancer. 2003. Cancer Program Standards, 2004. Chicago, IL: American College of Surgeons.
Continuum Health Partners. 2005. Sexual Health and Rehabilitation Program (SHARP). [Online]. Available: http://www.stoppain.org/services_staff/featuredprogsold.html#sexualhealth [accessed February 23, 2005].
Cooper GS, Yuan Z, Chak A, Rimm AA. 1999. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer 85(10):2124–2131.
Cooper GS, Yuan Z, Chak A, Rimm AA. 2000. Patterns of endoscopic follow-up after surgery for nonmetastatic colorectal cancer. Gastrointest Endosc 52(1):33–38.
Cox K, Wilson E. 2003. Follow-up for people with cancer: Nurse-led services and telephone interventions. J Adv Nurs 43(1):51–61.
Dana-Farber Cancer Institute. 2005. New Support Group Launched for African-American Men with Prostate Cancer. [Online]. Available: http://www.dana-farber.org/pat/support/support-2005-03-21.asp [accessed May 11, 2005].
de Bock GH, Bonnema J, Zwaan RE, van de Velde CJ, Kievit J, Stiggelbout AM. 2004. Patient’s needs and preferences in routine follow-up after treatment for breast cancer. Br J Cancer 90(6):1144–1150.
DeLisa JA. 2001. A history of cancer rehabilitation. Cancer 92(4 Suppl):970–974.
Demissie S, Silliman RA, Lash TL. 2001. Adjuvant tamoxifen: Predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19(2):322–328.
Desch CE, Grasso MA, McCue MJ, Buonaiuto D, Grasso K, Johantgen MK, Shaw JE, Smith TJ. 1999. A rural cancer outreach program lowers patient care costs and benefits both the rural hospitals and sponsoring academic medical center. J Rural Health 15(2):157–167.
Dietz JH. 1974. Rehabilitation of the cancer patient: Its role in the scheme of comprehensive care. Clin Bull 4(3):104–107.
Diller L, Medeiros Nancarrow C, Shaffer K, Matulonis U, Mauch P, Neuberg D, Tarbell NJ, Litman H, Garber J. 2002. Breast cancer screening in women previously treated for Hodgkin’s disease: A prospective cohort study. J Clin Oncol 20(8):2085–2091.
Donnelly J, Mack P, Donaldson LA. 2001. Follow-up of breast cancer: Time for a new approach? Int J Clin Pract 55(7):431–433.
Dow KH, Ferrell BR, Haberman MR, Eaton L. 1999. The meaning of quality of life in cancer survivorship. Oncol Nurs Forum 26(3):519–528.
Duffy CM, Allen SM, Clark MA. 2005. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol 23(4):766–773.
Dworkind M, Towers A, Murnaghan D, Guibert R, Iverson D. 1999. Communication between family physicians and oncologists: Qualitative results of an exploratory study. Cancer Prev Control 3(2):137–144.
Eakin EG, Strycker LA. 2001. Awareness and barriers to use of cancer support and information resources by HMO patients with breast, prostate, or colon cancer: Patient and provider perspectives. Psychooncology 10(2):103–113.
Earle CC, Neville BA. 2004. Under use of necessary care among cancer survivors. Cancer 101(8):1712–1719.
Earle CC, Burstein HJ, Winer EP, Weeks JC. 2003. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol 21(8):1447–1451.
Earnshaw JJ, Stephenson Y. 1997. First two years of a follow-up breast clinic led by a nurse practitioner. J R Soc Med 90(5):258–259.
Ell K, Sanchez K, Vourlekis B, Lee PJ, Dwight-Johnson M, Lagomasino I, Muderspach L, Russell C. 2005. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol 23(13):3052–3060.
Ellison GL, Warren JL, Knopf KB, Brown ML. 2003. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res 38(6 Pt 2):1885–1903.
Elston Lafata J, Cole Johnson C, Ben-Menachem T, Morlock RJ. 2001. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care 39(4):361–372.
Elston Lafata J, Simpkins J, Schultz L, Chase GA, Johnson CC, Yood MU, Lamerato L, Nathanson D, Cooper G. 2005. Routine surveillance care after cancer treatment with curative intent. Med Care 43(6):592-599.
Epplein M, Koon KP, Ramsey SD, Potter JD. 2005. Genetic services for familial cancer patients: A follow-up survey of National Cancer Institute cancer centers. J Clin Oncol 23(21):4713–4718.
Fallowfield L, Fleissig A, Edwards R, West A, Powles TJ, Howell A, Cuzick J. 2001. Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized controlled trials. J Clin Oncol 19(7):1885–1892.
Family Health Administration. 2005. The 2004–2008 Maryland Comprehensive Cancer Control Plan: Our Call to Action. [Online]. Available: http://www.marylandcancerplan.org [accessed February 22, 2005].
Ferrell B, Grant M, Schmidt GM, Rhiner M, Whitehead C, Fonbuena P, Forman SJ. 1992. The meaning of quality of life for bone marrow transplant survivors. Part 1. The impact of bone marrow transplant on quality of life. Cancer Nurs 15(3):153–160.
Ferrell B, Smith SL, Cullinane CA, Melancon C. 2003a. Psychological well being and quality of life in ovarian cancer survivors. Cancer 98(5):1061–1071.
Ferrell BR. 1996. The quality of lives: 1,525 voices of cancer. Oncol Nurs Forum 23(6):909–916.
Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. 1995. Quality of life in long-term cancer survivors. Oncol Nurs Forum 22(6):915–922.
Ferrell BR, Grant MM, Funk B, Otis-Green S, Garcia N. 1997. Quality of life in breast cancer survivors as identified by focus groups. Psychooncology 6(1):13–23.
Ferrell BR, Grant MM, Funk BM, Otis-Green SA, Garcia NJ. 1998. Quality of life in breast cancer survivors: Implications for developing support services. Oncol Nurs Forum 25(5):887–895.
Ferrell BR, Smith SL, Ervin KS, Itano J, Melancon C. 2003b. A qualitative analysis of social concerns of women with ovarian cancer. Psychooncology 12(7):647–663.
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. 2004. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol 22(16):3309–3315.
Fox Chase Cancer Center. 2004. The Margaret Dyson Family Risk Assessment Program. [Online]. Available: http://www.fccc.edu/clinicalresearch/familyriskassessment [accessed December 3, 2004].
Freedman AN, Wideroff L, Olson L, Davis W, Klabunde C, Srinath KP, Reeve BB, Croyle RT, Ballard-Barbash R. 2003. US physicians’ attitudes toward genetic testing for cancer susceptibility. Am J Med Genet A 120(1):63–71.
Freeman H, Clanton M. 2004. Patient navigator program reduces cancer health disparities. NCI Cancer Bulletin. 1(33):1–2.
Frost MH, Arvizu RD, Jayakumar S, Schoonover A, Novotny P, Zahasky K. 1999. A multidisciplinary healthcare delivery model for women with breast cancer: Patient satisfaction and physical and psychosocial adjustment. Oncol Nurs Forum 26(10):1673–1680.
Frymark SL. 1998. Taking control. Cancer rehabilitation allows patients to increase the quality of their lives and reclaim independence. Rehab Manag 11(3):80, 84–86.
Ganz PA. 1990. Current issues in cancer rehabilitation. Cancer 65(3 Suppl):742–751.
Ganz PA. 1999. The status of cancer rehabilitation in the late 1990s. Mayo Clin Proc 74(9):939–940.
Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. 2000. Managing menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Natl Cancer Inst 92(13):1054–1064.
Geller BM, Kerlikowske K, Carney PA, Abraham LA, Yankaskas BC, Taplin SH, Ballard-Barbash R, Dignan MB, Rosenberg R, Urban N, Barlow WE. 2003. Mammography surveillance following breast cancer. Breast Cancer Res Treat 81(2):107–115.
Gerber LH, Vargo MM, Smith RG. 2005. Chapter 56, Rehabilitation of the Cancer Patient. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott, Wiliams & Wilkins. Pp. 2719–2746.
Gilchrist VJ, Stange KC, Flocke SA, McCord G, Bourguet CC. 2004. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care 42(3):276–280.
Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, Wojcik C. 2002. Pain and fatigue management: Results of a nursing randomized clinical trial. Oncol Nurs Forum 29(6):949–956.
GIVIO. 1994. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA 271(20):1587–1592.
Glynne-Jones R, Chait I, Thomas SF. 1997. When and how to discharge cancer survivors in long term remission from follow-up: The effectiveness of a contract. Clin Oncol (R Coll Radiol) 9(1):25–29.
Grabois M. 2001. Integrating cancer rehabilitation into medical care at a cancer hospital. Cancer 92(4 Suppl):1055–1057.
Greenberg A, Angus H, Sullivan T, Brown AD. 2005. Development of a set of strategy-based system-level cancer care performance indicators in Ontario, Canada. Int J Qual Health Care 17(2):107–114.
Gruman J, Convissor R. 1995. Psychosocial Services in Cancer Care: A Survey of Comprehensive Cancer Centers. Washington, DC: The Center for the Advancement of Health.
Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi-Dalton R, Stewart J, Cole D, Vessey M. 1999a. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: Results from a randomized controlled trial. Br J Gen Pract 49(446):705–710.
Grunfeld E, Gray A, Mant D, Yudkin P, Adewuyi-Dalton R, Coyle D, Cole D, Stewart J, Fitzpatrick R, Vessey M. 1999b. Follow-up of breast cancer in primary care vs specialist care: Results of an economic evaluation. Br J Cancer 79(7–8):1227–1233.
Grunfeld E, Levine M, Julian J, et al. 2004 (June 5–8). A randomized controlled trial (RCT) of routine follow-up for early stage breast cancer: A comparison of primary care versus specialist care. Paper presented at the Annual Meeting of the American Society for Clinical Oncology, New Orleans, LA. Abstract No. 665.
Grunfeld E, Mant D, Vessey MP, Yudkin P. 1995. Evaluating primary care follow-up of breast cancer: Methods and preliminary results of three studies. Ann Oncol 6(Suppl 2):47–52.
Grunfeld E, Mant D, Yudkin P, Adewuyi-Dalton R, Cole D, Stewart J, Fitzpatrick R, Vessey M. 1996. Routine follow up of breast cancer in primary care: Randomised trial. BMJ 313(7058):665–669.
Guidry JJ, Aday LA, Zhang D, Winn RJ. 1997. The role of informal and formal social support networks for patients with cancer. Cancer Pract 5(4):241–246.
Gulliford T, Opomu M, Wilson E, Hanham I, Epstein R. 1997. Popularity of less frequent follow up for breast cancer in randomised study: Initial findings from the hotline study. BMJ 314(7075):174–177.
Gustafson D, Wise M, McTavish F, Taylor J, Wolberg W, Steward J, Smalley R, Bosworth K. 1993. Development and pilot evaluation of a computer-based support system for women with breast cancer. Journal of Psychosocial Oncology 11(4):69–93.
Harvey RF, Jellinek HM, Habeck RV. 1982. Cancer rehabilitation. An analysis of 36 program approaches. JAMA 247(15):2127–2131.
Hewitt M, Rowland JH. 2002. Mental health service use among adult cancer survivors: Analyses of the National Health Interview Survey. J Clin Oncol 20(23):4581–4590.
Hillner BE, McDonald MK, Penberthy L, Desch CE, Smith TJ, Maddux P, Glasheen WP, Retchin SM. 1997. Measuring standards of care for early breast cancer in an insured population. J Clin Oncol 15(4):1401–1408.
Hobbie WL, Hollen PJ. 1993. Pediatric nurse practitioners specializing with survivors of childhood cancer. J Pediatr Health Care 7(1):24–30.
Hoffman, B. 2004. A Cancer Survivor’s Almanac: Charting Your Journey. 3rd ed. Hoboken, NJ: John Wiley & Sons.
Hollen PJ, Hobbie WL. 1995. Establishing comprehensive specialty follow-up clinics for long-term survivors of cancer. Providing systematic physiological and psychosocial support. Support Care Cancer 3(1):40–44.
Hordern A. 2000. The emerging role of the breast care nurse in Australia. Cancer Nurs 23(2):122–127.
Improving Chronic Illness Care. 2004. The Community—Resources and Policies. [Online]. Available: http://www.improvingchroniccare.org/change/model/community.html [accessed November 16, 2004].
IOM (Institute of Medicine). 1998. Statement on Quality of Care. National Roundtable on Healthcare Quality. Washington, DC: National Academy Press.
IOM. 1999. Ensuring Quality Cancer Care. Hewitt M, Simone JV, eds. Washington, DC: National Academy Press.
IOM. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press.
IOM. 2002. Speaking of Health: Assessing Health Communication Strategies for Diverse Populations. Washington, DC: The National Academies Press.
IOM. 2003a. Childhood Cancer Survivorship: Improving Care and Quality of Life. Hewitt M, Weiner SL, Simone JV, eds. Washington, DC: The National Academies Press.
IOM. 2003b. Fulfilling the Potential of Cancer Prevention and Early Detection. Curry SJ, Byers T, Hewitt M, eds. Washington, DC: The National Academies Press.
IOM. 2004a. Health Literacy: A Prescription to End Confusion. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds. Washington, DC: The National Academies Press.
IOM. 2004b. Meeting Psychosocial Needs of Women with Breast Cancer. Hewitt M, Herdman R, Holland J, eds. Washington, DC: The National Academies Press.
IOM. 2005. Assessing the Quality of Cancer Care: An Approach to Measurement in Georgia. Eden J, Simone JV, eds. Washington, DC: The National Academies Press.
James ND, Guerrero D, Brada M. 1994. Who should follow up cancer patients? Nurse specialist based outpatient care and the introduction of a phone clinic system. Clin Oncol (R Coll Radiol) 6(5):283–287.
Johnson FE, Virgo, KS. 1997. Cancer Patient Follow-Up. St. Louis, MO: Mosby.
Johnson FE, Longo WE, Vernava AM, Wade TP, Coplin MA, Virgo KS. 1995. How tumor stage affects surgeons’ surveillance strategies after colon cancer surgery. Cancer 76(8):1325–1329.
Johnson FE, McKirgan LW, Coplin MA, Vernava AM, Longo WE, Wade TP, Virgo KS. 1996a. Geographic variation in patient surveillance after colon cancer surgery. J Clin Oncol 14(1):183–187.
Johnson FE, Naunheim KS, Coplin MA, Virgo KS. 1996b. Geographic variation in the conduct of patient surveillance after lung cancer surgery. J Clin Oncol 14(11):2940–2949.
Johnson FE, Novell LA, Coplin MA, Longo WE, Vernava AM, Wade TP, Virgo KS. 1996c. How practice patterns in colon cancer patient follow-up are affected by surgeon age. Surg Oncol 5(3):127–131.
Johnson FE, Virgo KS, Clemente MF, Johnson MH, Paniello RC. 1998. How tumor stage affects surgeons’ surveillance strategies after surgery for carcinoma of the upper aerodigestive tract. Cancer 82(10):1932–1937.
Johnson FE, Virgo KS, Johnson DY, Chan D, Goshima K, Handler BS. 2001. Effect of initial tumor stage on patient follow-up after potentially curative surgery for cutaneous melanoma. Int J Oncol 18(5):973–978.
Johnson FE, Virgo KS, Ornstein DK, Johnson ET, Chan D, Colberg JW. 2000. How tumor stage affects American urologists’ surveillance strategies after prostate cancer surgery. Int J Oncol 16(6):1221–1225.
Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R, Hudson M, Greenberg M, Mertens AC. 2002. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 287(14):1832–1839.
Kaija H, Matti H, Tapani H. 1996. Use of hospital services by breast cancer patients by stage of the disease: Implications on the costs of cancer control. Breast Cancer Res Treat 37(3):237–241.
Kiebert GM, Welvaart K, Kievit J. 1993. Psychological effects of routine follow up on cancer patients after surgery. Eur J Surg 159(11–12):601–607.
Kligman L, Wong RK, Johnston M, Laetsch NS. 2004. The treatment of lymphedema related to breast cancer: A systematic review and evidence summary. Support Care Cancer 12(6):421–431.
Knopf KB, Warren JL, Feuer EJ, Brown ML. 2001. Bowel surveillance patterns after a diagnosis of colorectal cancer in Medicare beneficiaries. Gastrointest Endosc 54(5):563–571.
Koinberg IL, Fridlund B, Engholm GB, Holmberg L. 2004. Nurse-led follow-up on demand or by a physician after breast cancer surgery: A randomised study. Eur J Oncol Nurs 8(2):109–117; discussion 118–120.
Koinberg IL, Holmberg L, Fridlund B. 2002. Breast cancer patients’ satisfaction with a spontaneous system of check-up visits to a specialist nurse. Scand J Caring Sci 16(3):209–215.
LAF (Lance Armstrong Foundation). 2004a. Cancer Survivor’s Medical Treatment Summary. [Online]. Available: http://www.livestrong.org/portal/needsAssessment/medical_summary.pdf [accessed February 23, 2005].
LAF. 2004b. LiveStrong Poll Finds Nearly Half of People Living With Cancer Feel Their Non-Medical Needs Are Unmet by the Healthcare System. Austin, TX: LAF.
LAF. 2005a. Cancer Survivorship Centers. [Online]. Available: http://www.laf.org/site/c.beIKLOOrGpF/b.507137/k.8513/Cancer_Survivorship_Centers.htm#5 [accessed May 19, 2005].
LAF. 2005b. Lance Armstrong Foundation LIVESTRONG. [Online]. Available: http://www.livestrong.org [accessed July 26, 2005].
Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeny T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. 2004. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22(24):4979–4990.
Lash TL, Silliman RA. 2001. Medical surveillance after breast cancer diagnosis. Med Care 39(9):945–955.
Lauria MM. 1991. Continuity of cancer care. Cancer 67(6 Suppl):1759–1766.
Lee FC. 2004. Employer-based disease management programs in cancer. Dis Manage Health Outcomes 12(1):9–17.
Leese B, Din I, Darr A, Walker R, Heywood P, Allgar V. 2004. ‘Early Days Yet’: The Primary Care Cancer Lead Clinician (PCCL) Initiative Executive Summary. Leeds, UK: Centre for Research in Primary Care, University of Leeds.
Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. 1978. Cancer rehabilitation: Assessment of need, development, and evaluation of a model of care. Arch Phys Med Rehabil 59(9):410–419.
Leigh SA. 1998. The long-term cancer survivor: A challenge for nurse practitioners. Nurse Pract Forum 9(3):192–196.
Lieberman MA, Golant M, Giese-Davis J, Winzlenberg A, Benjamin H, Humphreys K, Kronenwetter C, Russo S, Spiegel D. 2003. Electronic support groups for breast carcinoma: A clinical trial of effectiveness. Cancer 97(4):920–925.
Liebert B, Parle M, Roberts C, Redman S, Carrick S, Gallagher J, Simpson J, Ng K, Khan MA, White K, Salkeld G, Lewis M, Olver I, Gill G, Marchant M, Coates A, North R, Akers G, Cannon A, Gray C, Liebelt J, Rodger A, Henderson M, Stoney D, Hickey P, Archer S, Metcalf C, Trotter J. 2003. An evidence-based specialist breast nurse role in practice: A multicentre implementation study. Eur J Cancer Care (Engl) 12(1):91–97.
Liebert B, Parle M, White K, Rodger A. 2001. Establishing an evidence base for the specialist breast nurse: A model for Australian breast cancer care. Aust Health Rev 24(1):192–199.
Loprinzi CL, Hayes D, Smith T. 2000. Doc, shouldn’t we be getting some tests? J Clin Oncol 18(11):2345–2348.
Luker K, Beaver K, Austin L, Leinster SJ. 2000. An evaluation of information cards as a means of improving communication between hospital and primary care for women with breast cancer. J Adv Nurs 31(5):1174–1182.
MacLaren JA. 2003. Models of lymphoedema service provision across Europe: Sharing good practice. Int J Palliat Nurs 9(12):538–543.
Maher EJ, Millar D. 2003. Shared care: Step down or step up? Qual Saf Health Care 12(4):242.
Mahon SM, Williams MT, Spies MA. 2000. Screening for second cancers and osteoporosis in long-term survivors. Cancer Pract 8(6):282–290.
Maliski SL, Clerkin B, Litwin MS. 2004. Describing a nurse case manager intervention to empower low-income men with prostate cancer. Oncol Nurs Forum 31(1):57–64.
Margenthaler JA, Meier JD, Virgo KS, Johnson DY, Goshima K, Chan D, Handler BS, Johnson FE. 2003. Geographic variation in post-treatment surveillance intensity for patients with cutaneous melanoma. Am J Surg 186(2):194–200.
Margenthaler JA, Virgo KS, Johnson DY, Sugarbaker EM, Handler BS, Johnson FE. 2001. How surgeon age affects post-treatment surveillance strategies for melanoma patients. Int J Oncol 19(1):175–180.
Mariotto A, Warren JL, Knopf KB, Feuer EJ. 2003. The prevalence of patients with colorectal carcinoma under care in the U.S. Cancer 98(6):1253–1261.
Masny A, Daly M, Ross E, Balshem A, Gillespie D, Weil S. 2003. A training course for oncology nurses in familial cancer risk assessment: Evaluation of knowledge and practice. J Cancer Educ 18(1):20–25.
Matthews BA, Baker F, Spillers RL. 2002. Healthcare professionals’ awareness of cancer support services. Cancer Pract 10(1):36–44.
Matthews BA, Baker F, Spillers RL. 2004. Oncology professionals and patient requests for cancer support services. Support Care Cancer 12(10):731–738.
Maxwell S, Baseggio C. 2000. Outpatient Therapy Services Under Medicare: Background and Policy Issues. Washington, DC: Urban Institute.
McGlynn EA (RAND). 2002. Applying the Strategic Framework Board’s Model to Selecting National Goals and Core Measures for Stimulating Improved Quality for Cancer Care. Background Paper No. 1. Bethesda, MD: National Cancer Institute.
McGlynn EA, Malin J (RAND). 2002. Selecting National Goals and Core Measures of Cancer Care Quality. Background Paper No. 2. Bethesda, MD: National Cancer Institute.
M.D. Anderson Cancer Center. 2004. Fatigue & Cancer. [Online]. Available: http://www.mdanderson.org/topics/fatigue/ [accessed December 3, 2004].
M.D. Anderson Cancer Center. 2005. Life After Cancer Care. [Online]. Available: http://www.mdanderson.org/departments/lacc/ [accessed April 25, 2005].
MedPAC (Medicare Payment Advisory Commission). 2004a. Report to the Congress: Eliminating Physician Referrals to Physical Therapy (December 2004). [Online]. Available: http://www.medpac.gov/publications/congressional_reports/Dec04_PTaccess.pdf [accessed May 17, 2005].
MedPAC. 2004b. Report to the Congress: New Approaches in Medicare. Washington, DC: MEDPAC.
Mendelson D, Carino TV. 2005. Evidence-based medicine in the United States—de rigueur or dream deferred? Health Aff (Millwood) 24(1):133–136.
Miedema B, MacDonald I, Tatemichi S. 2003. Cancer follow-up care. Patients’ perspectives. Can Fam Physician 49:890–895.
Miller RH, Sim I. 2004. Physicians’ use of electronic medical records: Barriers and solutions. Health Aff (Millwood) 23(2):116–126.
Moore S, Corner J, Haviland J, Wells M, Salmon E, Normand C, Brada M, O’Brien M, Smith I. 2002. Nurse led follow up and conventional medical follow up in management of patients with lung cancer: Randomised trial. BMJ 325(7373):1145.
Morris S, Corder AP, Taylor I. 1992. What are the benefits of routine breast cancer follow-up? Postgrad Med J 68(805):904–907.
MSKCC (Memorial Sloan-Kettering Cancer Center). 2004. Post-Treatment Resource Program. [Online]. Available: http://www.mskcc.org/mskcc/html/19409.cfm [accessed April 27, 2005].
Muss HB, Tell GS, Case LD, Robertson P, Atwell BM. 1991. Perceptions of follow-up care in women with breast cancer. Am J Clin Oncol 14(1):55–59.
National Diabetes Quality Improvement Alliance. 2005. National Diabetes Quality Improvement Alliance homepage. [Online]. Available: http://www.nationaldiabetesalliance.org [accessed May 10, 2005].
Naunheim KS, Virgo KS, Coplin MA, Johnson FE. 1995. Clinical surveillance testing after lung cancer operations. Ann Thorac Surg 60(6):1612–1616.
NCCN (National Comprehensive Cancer Network). 2004a. Clinical Practice Guidelines in Oncology-v.1.2004. Breast Cancer Screening and Diagnosis Guidelines. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/breast-screening.pdf [accessed June 3, 2004].
NCCN. 2004b. Clinical Practice Guidelines in Oncology-v.1.2004. Breast Risk Reduction. [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/breast_risk.pdf [accessed June 3, 2004].
NCCN. 2004c. Clinical Practice Guidelines in Oncology-v.1.2004. Distress Management. [Online]. Available: http://www.nccn.org/physician_gls/f_guidelines.html [accessed June 2004].
NCCS (National Coalition for Cancer Survivorship). 1996. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH. Silver Spring, MD: National Coalition for Cancer Survivorship.
NCCS. 2004. Cancer Survival Toolbox. Silver Spring, MD: NCCS.
NCCS. 2005. Cancer: Keys to Survivorship. [Online]. Available: http://www.canceradvocacy.org/programs/keys.aspx [accessed April 26, 2005].
NCHS (National Center for Health Statistics). 2004. National Hospital Discharge Survey, 2002: Public Use Data File Documentation. Hyattsville, MD: NCHS.
NCI (National Cancer Institute). 2002a. Facing Forward: Life After Cancer Treatment. Bethesda, MD: NCI.
NCI. 2002b. Facing Forward Series: Ways You Can Make a Difference in Cancer. Bethesda, MD: NCI.
NCI. 2004. Patient Navigator Research Program Introduction & Overview. [Online]. Available: http://crchd.nci.nih.gov/Navigator/overview.htm [accessed November 8, 2004].
NCI. 2005a. Description of the Cancer Centers Program. [Online]. Available: http://www3.cancer.gov/cancercenters/description.html [accessed May 11, 2005].
NCI. 2005b. National Cancer Act of 1971. [Online]. Available: http://www3.cancer.gov/legis/1971canc.html [accessed May 12, 2005].
NCI. 2005c. Centers of Excellence in Cancer Communications Research. [Online]. Available: http://dccps.nci.nih.gov/ncirb/ceccr [accessed August 29, 2005].
NCLAC (National Cancer Legislation Advisory Committee). 2005. National Cancer Policy Legislative History. [Online]. Available: http://www.cancersource.com/nclac/leghistory.htm [accessed May 10, 2005].
NCSL (National Conference of State Legislatures). 2005. State Genetics Employment Laws. [Online] http://www.ncls.org/programs/health/genetics/ndiscrim.htm [accessed June 15, 2005].
Neuner JM, Gilligan MA, Sparapani R, Laud PW, Haggstrom D, Nattinger AB. 2004. Decentralization of breast cancer surgery in the United States. Cancer 101(6):1323–1329.
Nielsen JD, Palshof T, Mainz J, Jensen AB, Olesen F. 2003. Randomised controlled trial of a shared care programme for newly referred cancer patients: Bridging the gap between general practice and hospital. Qual Saf Health Care 12(4):263–272.
NIH. 2005. Legislative Updates: Genetic Nondiscrimination. [Online]. Available: http://olpa.od.nih.gov/legislation/109/pendinglegislation/geneticnondiscrimination.asp [accessed June 14, 2005].
NLM (National Library of Medicine). 2005. Regional Medical Programs. [Online]. Available: http://rmp.nlm.nih.gov/RM/ [accessed May 12, 2005].
NLN (National Lymphedema Network). 2005. NLN homepage. [Online]. Available: http://lymphnet.org/ [accessed May 9, 2005].
NQF (National Quality Forum). 2005. National Quality Forum Current Activities and Consensus Reports. [Online]. Available: http://www.qualityforum.org/activities/home.htm [accessed April 15, 2005].
Nueva Vida. 2005a. Nueva Vida: A Support Network for Latinas with Cancer. [Online], Available: http://www.nueva-vida.org/index2.htm [accessed May 11, 2005].
Nueva Vida. 2005b. Nueva Vida: Locate a Local Support Program. [Online]. Available. http://www.nueva-vida.org/locate.htm [accessed May 11, 2005].
NVCI (Nevada Cancer Institute). 2005. Survivorship: A Joint Goal of the Nevada Cancer Institute and the Lance Armstrong Foundation. [Online]. Available: http://www.nevadacancerinstitute.org/communityprograms/survivorship.htm [accessed May 18, 2005].
Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, Whitton J, Yeazel M, Yasui Y, Robison LL. 2004. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivors Study. Ann Fam Med 2(1):61–70.
Oh J, Colberg JW, Ornstein DK, Johnson ET, Chan D, Virgo KS, Johnson FE. 1999. Current followup strategies after radical prostatectomy: A survey of American Urological Association urologists. J Urol 161(2):520–523.
ONS (Oncology Nursing Society). 2005. Cancer Symptoms.org. [Online]. Available: http://cancersymptoms.org [accessed April 26, 2005].
Paniello RC, Virgo KS, Johnson MH, Clemente MF, Johnson FE. 1999. Practice patterns and clinical guidelines for posttreatment follow-up of head and neck cancers: A comparison of 2 professional societies. Arch Otolaryngol Head Neck Surg 125(3):309–313.
Papagrigoriadis S, Heyman B. 2003. Patients’ views on follow up of colorectal cancer: Implications for risk communication and decision making. Postgrad Med J 79(933):403–407.
Parle M, Gallagher J, Gray C, Akers G, Liebert B. 2001. From evidence to practice: Factors affecting the specialist breast nurse’s detection of psychological morbidity in women with breast cancer. Psychooncology 10(6):503–510.
Parrott S, Clark C, Mahoney Shannon K. 2002. National Society of Genetic Counselors, Inc. Professional Status Survey 2002. Waltham, MA: Boston Information Solutions.
Parrott S, Clark C, Mahoney Shannon K. 2003. National Society of Genetic Counselors, Inc. Professional Status Survey 2002: Cancer Genetics Analysis. Waltham, MA: Boston Information Solutions.
Partnership for Solutions (Johns Hopkins University). 2002. Chronic Conditions: Making the Case for Ongoing Care. Baltimore, MD: Johns Hopkins University.
Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP. 2004. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 22(20):4174–4183.
Partridge AH, Wang PS, Winer EP, Avorn J. 2003. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606.
Paskett ED, Stark N. 2000. Lymphedema: Knowledge, treatment, and impact among breast cancer survivors. Breast J 6(6):373–378.
Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. 1998. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol 16(4):1594–1600.
Pediatric Oncology Resource Center. 2003. Survivors—Follow-up Clinics. [Online]. Available: http://www.acor.org/ped-onc/treatment/surclinics.html [accessed November 19, 2004].
Pelusi J. 2001. The past sets the stage for the future: Follow-up issues facing long-term cancer survivors. Semin Oncol Nurs 17(4):263–267.
Pennery E, Mallet J. 2000. A preliminary study of patients’ perceptions of routine follow-up after treatment for breast cancer. Eur J Oncol Nurs 4(3):138–145; discussion 146–147.
PHEN (Prostate Health Education Network). 2005. About PHEN. [Online]. Available: http://prostatehealthed.org [accessed May 11, 2005].
PLTC (People Living Through Cancer). 2005. PLTC homepage. [Online]. Available: http://www.pltc.org/ [accessed April 26, 2005].
Powell TM, Thompsen JP, Virgo KS, Johnson ET, Chan D, Colberg JW, Ornstein DK, Johnson FE. 2000. Geographic variation in patient surveillance after radical prostatectomy. Ann Surg Oncol 7(5):339–345.
Presberg BA, Levenson JL. 1993. A survey of cancer support groups provided by National Cancer Institute (NCI) clinical and comprehensive centers. Psychooncology 2:215–217.
President’s Commission on Heart Disease, Cancer, and Stroke. 1964. Report to the President: A National Program to Conquer Heart Disease, Cancer, and Stroke. Washington, DC: U.S. Government Printing Office.
Pritchard P, Hughes J. 1995. Shared Care the Future Imperative? London, England: Royal Society of Medicine Press.
Quality Oncology, Inc. 2003. Quality Oncology, Inc. Announces Progress in Its Federal Pilot Project To Manage Cancer Care for South Florida Medicare Patients. [Online]. Available: http://www.qualityoncology.com/press/medicare_4_18_03.asp [accessed November 22, 2004].
Rabinowitz B. 2002. Psychosocial issues in breast cancer. Obstet Gynecol Clin North Am 29(1):233–247.
Radford JA, Eardley A, Woodman C, Crowther D. 1997. Follow up policy after treatment for Hodgkin’s disease: Too many clinic visits and routine tests? A review of hospital records. BMJ 314(7077):343–346.
Ragnarsson KT, Thomas DC. 2000. Principles of cancer rehabilitation medicine. In: Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, Holland JF, Frei E, Gansler TS, eds. Cancer Medicine. Hamilton, Ontario, Canada: BC Decker. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=cmed.section.17163 [accessed July 18, 2005].
Renton JP, Twelves CJ, Yuille FA. 2002. Follow-up in women with breast cancer: The patients’ perspective. Breast 11(3):257–261.
Richert-Boe KE. 1995. Heterogeneity of cancer surveillance practices among medical oncologists in Washington and Oregon. Cancer 75(10):2605–2612.
Ritz LJ, Nissen MJ, Swenson KK, Farrell JB, Sperduto PW, Sladek ML, Lally RM, Schroeder LM. 2000. Effects of advanced nursing care on quality of life and cost outcomes of women diagnosed with breast cancer. Oncol Nurs Forum 27(6):923–932.
Rowland J. 2005 (March 24–25). Update for the IOM Committee on Cancer Survivorship. Presentation at the meeting of the IOM Committee on Cancer Survivorship, Washington, DC.
Rulyak SJ, Mandelson MT, Brentnall TA, Rutter CM, Wagner EH. 2004. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc 59(2):239–247.
Runowicz CD. 1998. Lymphedema: Patient and provider education: Current status and future trends. Cancer 83(12 Suppl American):2874–2876.
Sabers SR, Kokal JE, Girardi JC, Philpott CL, Basford JR, Therneau TM, Schmidt KD, Gamble GL. 1999. Evaluation of consultation-based rehabilitation for hospitalized cancer patients with functional impairment. Mayo Clin Proc 74(9):855–861.
Sakata K, Beitler AL, Gibbs JF, Kraybill WG, Virgo KS, Johnson FE. 2002. How surgeon age affects surveillance strategies for extremity soft tissue sarcoma patients after potentially curative treatment. J Surg Res 108(2):227–234.
Sakata K, Johnson FE, Beitler AL, Kraybill WG, Virgo KS. 2003. Extremity soft tissue sarcoma patient follow-up: Tumor grade and size affect surveillance strategies after potentially curative surgery. Int J Oncol 22(6):1335–1343.
Sardell S, Sharpe G, Ashley S, Guerrero D, Brada M. 2000. Evaluation of a nurse-led telephone clinic in the follow-up of patients with malignant glioma. Clin Oncol (R Coll Radiol) 12(1):36–41.
Schachter HM, Mamaladze V, Lewin G, Paszat L, Verma S, DeGrasse C, Graham I, Brouwers M, Sampson M, Morrison A, Zhang L, O’Blenis P, Garrity C. 2004. Measuring the Quality of Breast Cancer Care in Women. Evidence Report/Technology Assessment No. 105. (Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02-0021.) AHRQ Publication No. 04-E030-2. Rockville, MD: AHRQ.
Schapira MM, McAuliffe TL, Nattinger AB. 2000. Underutilization of mammography in older breast cancer survivors. Med Care 38(3):281–289.
Schmidt KD. 2001. Cancer rehabilitation services in a tertiary care center. Cancer 92(4 Suppl):1053–1054.
Schrag D, Hsieh LJ, Rabbani F, Bach PB, Herr H, Begg CB. 2003. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst 95(8):588–597.
Segal R, Evans W, Johnson D, Smith J, Colletta SP, Corsini L, Reid R. 1999. Oncology Rehabilitation Program at the Ottawa Regional Cancer Centre: Program description. CMAJ 161(3):282–285.
Sharma VK, Vasudeva R, Howden CW. 2000. Colorectal cancer screening and surveillance practices by primary care physicians: Results of a national survey. Am J Gastroenterol 95(6):1551–1556.
Shaw BR, McTavish F, Hawkins R, Gustafson DH, Pingree S. 2000. Experiences of women with breast cancer: Exchanging social support over the CHESS computer network. J Health Commun 5(2):135–159.
Shelby RA, Taylor KL, Kerner JF, Coleman E, Blum D. 2002. The role of community-based and philanthropic organizations in meeting cancer patient and caregiver needs. CA Cancer J Clin 52(4):229–246.
Sifri R, Gangadharappa S, Acheson LS. 2004. Identifying and testing for hereditary susceptibility to common cancers. CA Cancer J Clin 54(6):309–326.
Sisler JJ, Brown JB, Stewart M. 2004. Family physicians’ roles in cancer care. Survey of patients on a provincial cancer registry. Can Fam Physician 50:889–896.
Sisters Network, Inc. 2005. Sisters Network, Inc. Organization Overview. [Online]. Available: http://www.sistersnetworkinc.org/about-us.asp [accessed May 11, 2005].
Sliwa JA, Marciniak C. 1999. Physical rehabilitation of the cancer patient. Cancer Treat Res 100:75–89.
Smith ED, Walsh-Burke K, Crusan C. 1998. Principles of training social workers in oncology. In: Holland JC, ed. Psycho-Oncology. New York: Oxford University Press.
Sparaco A, Fentiman IS. 2002. Arm lymphoedema following breast cancer treatment. Int J Clin Pract 56(2):107–110.
Stark ME, Crowe JP Jr. 1996. Breast cancer evaluation and follow-up: A survey of The Ohio Chapter of The American College of Surgeons. Am Surg 62(6):458–460.
Steinberg ML, Rose CM. 1996. Posttreatment follow-up of radiation oncology patients in a managed care environment. Int J Radiat Oncol Biol Phys 35(1):113–116.
Stiggelbout AM, de Haes JC, Vree R, van de Velde CJ, Bruijninckx CM, van Groningen K, Kievit J. 1997. Follow-up of colorectal cancer patients: Quality of life and attitudes towards follow-up. Br J Cancer 75(6):914–920.
Tattersall MH, Thomas H. 1999. Recent advances: Oncology. BMJ 318(7181):445–448.
Tesauro GM, Rowland JH, Lustig C. 2002. Survivorship resources for post-treatment cancer survivors. Cancer Pract 10(6):277–283.
Teschendorf B. 2005 (October 27–28). ACS Division Survey 2003 Results. Presentation at the meeting of the IOM Committee on Cancer Survivorship. Irvine, CA.
Texas Cancer Council. 2005. Texas Cancer Plan: A statewide blueprint for cancer prevention and control in Texas. 4th ed. Austin, TX: Texas Cancer Council.
The Wellness Community. 2004a. The Virtual Wellness Community Online Support Groups. [Online]. Available: http://www.thewellnesscommunity.org/virtual_WC/support.htm [accessed December 3, 2004].
The Wellness Community. 2004b. The Wellness Community National. [Online]. Available: http://www.thewellnesscommunity.org/default.asp [accessed October 21, 2004].
The Wellness Community. 2005. Frankly Speaking About New Discoveries in Cancer. [Online]. Available: http://www.thewellnesscommunity.org/programs/frankly/newdiscoveries/newdiscoveries_home.htm [accessed March 2, 2005].
Thompson TG, Brailer DJ. 2004. The Decade of Health Information Technology: Delivering Consumer-Centric and Information-Rich Health Care. Washington, DC: U.S. Department of Health and Human Services.
Tierney WM, McKinley ED. 2002. When the physician-researcher gets cancer: Understanding cancer, its treatment, and quality of life from the patient’s perspective. Med Care 40(6 Suppl):III20–III27.
Timmermans S, Mauck A. 2005. The promises and pitfalls of evidence-based medicine. Health Aff (Millwood) 24(1):18–28.
True S. 2004 (June 17–18). Comprehensive Cancer Control and Survivorship. Presentation at the NCI and ACS Meeting, “Cancer Survivorship: Pathways to Health After Cancer,” Washington, DC.
Tsai DY, Virgo KS, Colberg JW, Ornstein DK, Johnson ET, Chan D, Johnson FE. 1999. The age of the urologist affects the postoperative care of prostate carcinoma patients. Cancer 86(7):1314–1321.
US Oncology. 2005. Life Beyond Cancer. [Online]. Available: http://www.usoncology.com/Resources/LBC.asp [accessed April 25, 2005].
USPSTF (U.S. Preventive Services Task Force). 2003. Counseling to Prevent Tobacco Use and Tobacco-Related Diseases: Recommendation Statement. [Online]. Available: http://www.ahrq.gov/clinic/3rduspstf/tobacccoun/tobcounrs.htm [accessed December 8, 2004].
Vernava AM III, Longo WE, Virgo KS, Coplin MA, Wade TP, Johnson FE. 1994. Current follow-up strategies after resection of colon cancer. Results of a survey of members of the American Society of Colon and Rectal Surgeons. Dis Colon Rectum 37(6):573–583.
Virgo KS, Chan D, Handler BS, Johnson DY, Goshima K, Johnson FE. 2000. Current practice of patient follow-up after potentially curative resection of cutaneous melanoma. Plast Reconstr Surg 106(3):590–597.
Virgo KS, Naunheim KS, Coplin MA, Johnson FE. 1998. Lung cancer patient follow-up: Motivation of thoracic surgeons. Chest 114(6):1519–1534.
Virgo KS, Paniello RC, Johnson MH, Clemente MF, Johnson FE. 2002. Surgical decision making in upper aerodigestive tract cancer patient follow-up. Int J Oncol 21(5):1101–1109.
Virgo KS, Wade TP, Longo WE, Coplin MA, Vernava AM, Johnson FE. 1995. Surveillance after curative colon cancer resection: Practice patterns of surgical subspecialists. Ann Surg Oncol 2(6):472–482.
Vital Options, Inc. 2005. Cancer Survivorship, Program #466 1/9/2005. [Online]. Available: http://www.vitaloptions.org/ [accessed April 27, 2005].
Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. 2001. Improving chronic illness care: Translating evidence into action. Health Aff (Millwood) 20(6):64–78.
Watson PG. 1992. Cancer rehabilitation: An overview. Semin Oncol Nurs 8(3):167–173.
White NJ, Given BA, Devoss DN. 1996. The advanced practice nurse: Meeting the information needs of the rural cancer patient. J Cancer Educ 11(4):203–209.
Wideroff L, Freedman AN, Olson L, Klabunde CN, Davis W, Srinath KP, Croyle RT, Ballard-Barbash R. 2003a. Physician use of genetic testing for cancer susceptibility: Results of a national survey. Cancer Epidemiol Biomarkers Prev 12(4):295–303.
Wideroff L, Vadaparampil ST, Breen N, Croyle RT, Freedman AN. 2003b. Awareness of genetic testing for increased cancer risk in the year 2000 National Health Interview Survey. Community Genet 6(3):147–156.
Williams PT. 1994. The role of family physicians in the management of cancer patients. J Cancer Educ 9(2):67–72.
Winn R. 2002. Clinical Practice Guidelines and Survivorship. Background paper prepared for the National Cancer Policy Board. Unpublished.
Wood ML, McWilliam CL. 1996. Cancer in remission. Challenge in collaboration for family physicians and oncologists. Can Fam Physician 42:899–904, 907–910.







































































































































