Appendix C
Information on Current Composition of Levenstein Mustard Agent at Blue Grass Army Depot
Tables C-1 through C-5 provide a summary overview of what is known about the current condition of the Levenstein mustard agent (H) in the U.S. chemical stockpile, including that in the projectiles stored at Deseret Chemical Depot.1 This form of mustard agent has degraded from what was originally about 70 percent pure mustard agent (liquid) and 30 percent (liquid) impurities into a mixture that is, on average, 70 percent solids and 30 percent liquid.
TABLE C-1 Composition of Liquid H (Levenstein Mustard), 16-42 wt% (Average = 31 wt%) of Agent Fill in the 10 155-mm H Projectiles Tested During Munitions Washout System Testinga,b
TABLE C-2 Composition of H Heels, 58-84 wt% (Average = 69 wt%) of Agent Fill in the 10 155-mm Projectiles Tested During Munitions Washout System Testinga,b
|
Compound Name |
Structure |
GC/MS–CI Analysis of CH2Cl2 Extract (area%) |
NMR Analysis of Three Extracts (wt%) |
|
HD and degradation compounds |
|
|
CHCl3 Extract |
|
1,4-Dithiane |
|
10 (7.4-15) |
1.3 (0.64-2.9) |
|
1,4-Thioxane |
|
0.23 (0.10-0.50) |
|
|
Bis(2-chloroethyl) sulfide (HD) |
ClCCSCCCl |
58 (52-64) |
28 (10- 37) |
|
1,2-Bis(2-chloroethylthio)ethane (Q) |
ClCCSCCSCCCl |
17 (14-20) |
4.1 (0.99-5.5) |
|
Bis(2-chloroethyl) sulfoxide |
|
0.46 (0.20-0.60) |
|
|
1,4-Dithiane-1-oxide |
|
0.80 (0.30-1.6) |
|
|
2-Chloroethyl vinyl sulfide |
ClCC-S-CH=CH2 |
0.33 (0.020-0.60) |
|
|
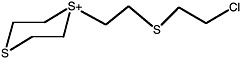
Bis[(2-chloroethylthio)ethyl] sulfide |
ClCCSCC-S-CCSCCCl |
2.6 (1.4-4.3) |
|
|
(2-chloroethylthio)ethyl vinyl sulfide |
ClCCSCCSCH=CH2 |
2.2 (1.5-2.9) |
|
|
C3/C4 HD analogs/impurities |
|
|
|
|
Bis(3-chloropropyl) sulfide |
|
0.13 (0.050-0.20) |
|
|
2-Chloroethyl-4-chlorobutyl sulfide |
|
0.71 (0.60-0.80) |
|
|
2-Chloroethyl-2-chlorobutyl sulfide |
|
0.057 (0.040-0.080) |
|
|
2-Chloropropyl-3-chloropropyl sulfide |
|
0.29 (0.12-0.40) |
|
|
2-Chloroethyl-3-chloropropyl sulfide |
|
0.18 (0.11-0.30) |
|
|
Linear polysulfides |
|
|
|
|
Bis(2-chloroethyl) disulfide, HS2 |
ClCC-S-S-CCCl |
0.29 (0.15-0.50) |
|
|
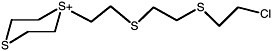
2-(Chloroethylthio) ethyl 2-chloroethyl disulfide |
ClCCSCC-S-S-CCCl |
0.41 (0.30-0.50) |
|
|
Bis(2-chloroethyl) trisulfide, HS3 |
ClCC-S-S-S-CCCl |
0.86 (0.40-1.2) |
|
|
Cyclic sulfides and polysulfides |
|
|
|
|
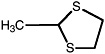
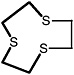
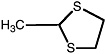
2-Methyl-1,3-dithiolane |
|
0.050 (one sample) |
|
|
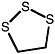
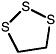
1,2,3-Trithiolane |
|
1.0 (0.40-2.2) |
|
|
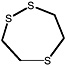
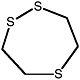
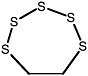
1,2,5-Trithiepane (7-membered ring) |
|
2.1 (1.1-4.4) |
|
|
1,4,7-Trithionane |
|
0.12 (0.080-0.15) |
|
|
1,2,3,4-Tetrathiane |
|
0.18 (0.080-0.40) |
|
|
Compound Name |
Structure |
GC/MS–CI Analysis of CH2Cl2 Extract (area%) |
NMR Analysis of Three Extracts (wt%) |
|
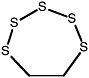
1,2,3,4,5-Pentathiepane (7-membered ring) |
|
0.15 (0.080-0.30) |
|
|
2-Methyl-1,3-dithiolane |
|
0.050 (one sample) |
|
|
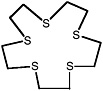
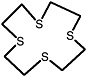
1,4,7,10,13-pentathiacyclopentadecane |
|
0.26 (0.070-0.90) |
|
|
1,4,7,10-tetrathiacyclododecane |
|
0.49 (0.14-1.0) |
|
|
Elemental sulfur |
|
|
|
|
Sulfur S6 |
|
0.10 (0.040-0.15) |
|
|
Sulfur S8 |
|
6.0 (one sample) |
|
|
Unknown MW |
|
118 0.18 (0.11-0.30) |
|
|
Cyclic thioethers |
|
|
|
|
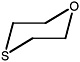
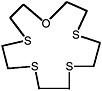
1-oxa-4,7,10,13-tetrathiacyclopentadecane |
0.080 (0.060-0.10) |
|
|
|
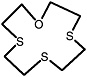
1-oxa-4,7,10-trithiacyclododecane |
|
0.41 (0.30-0.50) |
|
|
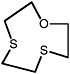
1-oxa-4,7-dithionane |
|
0.30 (0.070-0.70) |
|
|
|
|
|
|
|
Compound Name |
Structure |
GC/MS–CI Analysis of CH2Cl2 Extract (area%) |
NMR Analysis of Three Extracts (wt%) |
|
Cyclic sulfonium ion |
|
|
CH3CN Extract |
|
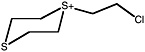
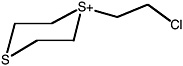
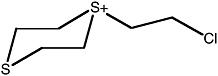
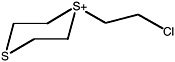
S-(2-chloroethyl)-1,4-dithianium ion |
36 (27-48) |
||
|
Cyclic sulfonium dication |
H2O Extract |
||
|
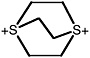
1,4-Dithioniabicyclo[2.2.2]octane dication |
1.4 (0.70-2.7) |
||
|
Total |
|
99.81 |
70.8c |
|
a Results are the average and range for seven samples. bUsing n-hexane extraction and an internal standard, gas chromatography/thermal conductivity detector quantitative measurements gave 13 wt% (5.47-18.4 wt%) HD. cThe main elements in the residual were iron and sulfur. |
|||
TABLE C-3 Liquid Chromatography–Electrospray Ionization–Mass Spectrometry Analysis of 14 Solid H Samplesa
TABLE C-4 Liquid Chromatography–Electrospray Ionization–Mass Spectrometry Analysis of Solids (Filtered from Liquid H Samples)a
TABLE C-5 Estimated Total Iron Contents in Liquid and Solid Phases of H Mustard Agent Fill in 155-mm Projectilesa,b