4
Trends in the Patenting and Licensing of Genomic and Protein Inventions and Their Impact on Biomedical Research
This chapter reports the committee’s findings with respect to its charge to determine current trends in the patenting of genomic- and protein-related inventions, licensing practices, and the impact on biomedical research and innovation. To address these issues to the greatest extent possible within the limits of the available time and resources, the committee consulted the existing research literature and received testimony from scholars in various fields, government officials, and stakeholders. In addition, the committee engaged in three original research efforts:
-
a search for issued patents and published patent applications in selected biotechnology categories;
-
a small survey of university licensing of selected categories of patents. This and the first effort supplemented information being gathered systematically by other investigators in larger-scale research studies; and
-
a survey of biomedical research scientists to ascertain their experience with intellectual property and its effects on research.
The first and second tasks were performed by committee staff. The third, more ambitious project was a survey of approximately 2,000 randomly selected researchers in universities, industry, and government laboratories. It was conducted by John Walsh and Charlene Cho, University of Illinois at Chicago, and Wesley Cohen, Duke University, and it was supported by funding from the com-
mittee.1 It builds on a more limited interview-based survey by Walsh, Cohen, and Ashish Arora—work carried out for the National Academies’ predecessor Committee on Intellectual Property Rights in the Knowledge-Based Economy (NRC, 2003). The new survey represents the first systematic effort to shed light on the concerns expressed by members of the academic community that patents on upstream discoveries may impede follow-on research and development if access to the foundational intellectual property is restricted or is too difficult, time consuming, or costly to obtain. The new survey goes further, however, to try to determine the extent of biomedical researchers’ involvement with intellectual property, its role—positive as well as negative—in decisions to initiate, redirect, or suspend research, and investigators’ experience with sharing of research data and materials, whether or not protected by intellectual property. The survey achieved a modest response rate and is subject to the limitations of an inquiry relying on memory and self-reporting, but its results are largely consistent with the findings of the earlier nonrandom interviews. The results of these inquiries and the committee’s interpretation of those results and of closely related studies are presented in this chapter.
TRENDS IN PATENTING GENOMIC AND PROTEIN INVENTIONS
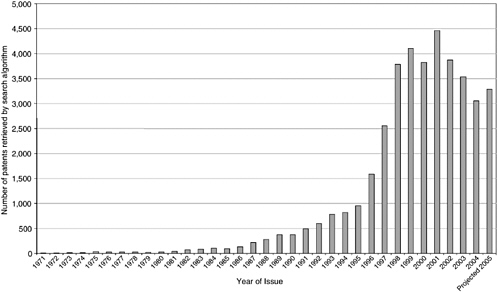
Although not the only source of data on genomic and proteomic patents,2 the most extensive database of U.S. “gene” patents was initiated by the congressional Office of Technology Assessment in the early 1990s with assistance from the United States Patent and Trademark Office (USPTO) and Georgetown University scholars and was transferred to Georgetown University, where it is maintained and continually updated with the support of the National Institutes of Health (NIH) and the Department of Energy. Using a proprietary patent database, Delphion, the investigators have compiled a comprehensive set of patents from several broad biology-related patent classes. These are patents that refer to nucleic acids and closely related terms assembled into an algorithm to search in their claims. From 1971 until 2006, approximately 33,000 issued nucleic acid patents have been identified. The annual rate of patenting did not exceed 500, however,
|
1 |
The full report, J. Walsh, C. Cho, and W. Cohen, Patents, Material Transfers, and Access to Research Inputs in Biomedical Research, June 2005, is available at http://www.uic.edu/~jwalsh/NASreport.html. |
|
2 |
See also A.M. Michelsohn, Biotechnology Innovation Report 2004: Benchmarks and Biotechnology Innovation Report. Washington, DC: Finnegan, Henderson, Farabow, Garett & Dunner, LLP. These sources have reported numbers and ownership of patents in several biotechnology categories, identified by key word searches. The results are not incompatible with those described below, but the use of carefully delineated search algorithms yields more discriminating results than do keyword searches. |
until 1991 when it began accelerating, peaking at 4,500 in 2001. The number of issued patents declined sharply in 2002 and again in 2003 and 2004 (Figure 4-1).
A more refined analysis has been done using bioinformatics methods to compare nucleotide sequences claimed in U.S. patents to the human genome (Jensen and Murray, 2005). This analysis shows that approximately 20 percent of human genes (4,382 of the 23,685 genes currently in the public databank) are explicitly claimed, not merely disclosed, in issued U.S. patents owned by 1,156 different assignees. A number of genes, including BRCA1, are subjects of multiple patents asserting rights to various gene uses and manifestations. In a few cases, single patents claim multiple genes, usually as probes on a DNA microarray. None of these circumstances is by itself indicative of a “thicket” or “blocking” problem absent information on patent claims, licensing, and corporate relationships.
Large numbers of applications for patents with such claims are still pending, some of them since the early 1990s. Many of these can be retrieved from the database, because USPTO began publication of most 18-month-old applications in March 2001; but there are two reasons why the precise number for each year cannot be ascertained. First, under the American Inventors Protection Act of 1999, an application can be withheld from publication if the filer agrees not to seek a patent on her or his invention outside the United States. In biotechnology and organic chemistry the “withholding” rate was about 6 percent through 2002 (NRC, 2004). Second and more important, USPTO has not been systematic about publishing applications after 18 months of filing. For example, applications filed in 2001 and 2002 continue to appear for the first time in the database in 2005.3
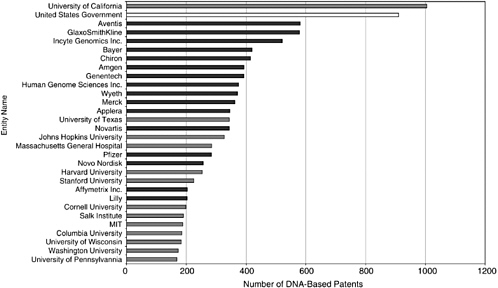
Approximately 5,000, or 15 percent, of the issued patents in the Georgetown database are managed by universities, led by the University of California, the largest patent holder in the field overall. The dominance of the University of California is somewhat misleading, however, because the number is a compilation of the patenting activity of 10 major research institutions, including the University of California at San Francisco, the University of California at Berkeley, and the University of California at San Diego. More than 800 are assigned to the U.S. government. The government also has an “interest” in as many as 60 percent of the patents held by the leading academic patenting institutions, meaning that they derived from federally funded research.4 The majority of patents are held by U.S.-based biotechnology and pharmaceutical companies. Figure 4-2 shows the 30 largest holders of DNA-based U.S. patents.
In his charge to the committee at its first meeting, Francis Collins, Director, National Human Genome Research Institute, requested data on what patents have been issued or applied for, by whom, and in which countries for nine more specific categories of genomic- and protein-related patents: gene regulatory sequences, single nucleotide polymorphisms (SNPs) and haplotypes, protein structures, protein-protein interactions, gene expression profiling, genetically modified organisms, and related software, algorithms, and databases.
Further discussions among the committee resulted in the selection of three additional patent categories, each representing a distinct disease-related molecular pathway: Cytotoxic T-Lymphocyte Associated Protein-4 (CTLA4), Epidermal Growth Factor (EGF), and Nuclear factor-Kappa B (NF-kB).5 CTLA4, EGF, and NF-kB were chosen from a longer list of known pathways on the basis of four criteria:
-
they are seen as involved in or correlated with more than one category of disease, spanning cancer and autoimmune or inflammatory diseases;
-
there is significant scientific research interest, as indicated by frequent citation in the scientific literature;
-
they exhibit some variance in the number of related patents; and
-
there is some but varying industry involvement, represented by pharmaceutical or biotechnology firm patenting activity, licensing of university patents, or clinical testing or even marketing of therapeutic products.
In short, it is reasonable to hypothesize that to the extent they arise at all, intellectual property complications will be greater in research involving at least some of these pathways than in genomic and proteomic research in general.
Methods
In consultation with USPTO supervising examiners in technology center “1600” (biotechnology), committee staff developed search algorithms for each of the categories of patents (see Appendix C). These searches were run on the patent claims field to obtain the number of U.S. patents and assignees, assignee countries, inventor countries, application years, and ultimate assignees over the period from January 1, 1995, to February 1, 2005. An independent search using the same algorithms for the same period was made subsequently by staff of the Georgetown University project. The numbers of patents found in the two sets of searches corresponded very closely but not exactly. In addition to U.S.-assigned patents, the searches included published U.S. patent applications and, for comparison, patents and applications issued by the European Patent Office (EPO). The “software,” “database,” and “algorithm” categories were limited to patent classification 435 (chemistry: molecular biology and microbiology). Table 4-1 summarizes the results. Especially for the “software” and “algorithm” categories, the class restriction may limit the results, because biologically related patents may have been placed in other patent classifications. It was not possible with the re-
TABLE 4-1 Issued U.S. and European Patents and Patent Applications in Selected Categories of Biotechnology Inventions, 1995-2005
|
Category |
U.S. Granted |
U.S. Application |
EPO Granted |
EPO Application |
|
Genes and gene regulation |
6,145 |
7,105 |
1,327 |
1,153 |
|
Haplotype/SNPs |
1,482 |
2,292 |
266 |
293 |
|
Gene expression profiling |
7,428 |
16,983 |
2,635 |
3,043 |
|
Protein structure |
39 |
230 |
28 |
31 |
|
Protein-protein interactions |
6,964 |
12,845 |
3,590 |
2,066 |
|
Modified animals |
652 |
2,767 |
177 |
334 |
|
Software |
60 |
209 |
11 |
28 |
|
Algorithms |
91 |
325 |
64 |
113 |
|
Databases |
1,466 |
3,765 |
A |
A |
|
EGF pathway |
765 |
1,045 |
212 |
166 |
|
CTLA4 pathway |
63 |
149 |
19 |
19 |
|
NF-kB pathway |
94 |
206 |
42 |
81 |
|
NOTE: A = No biological class restriction is available for this category. |
||||
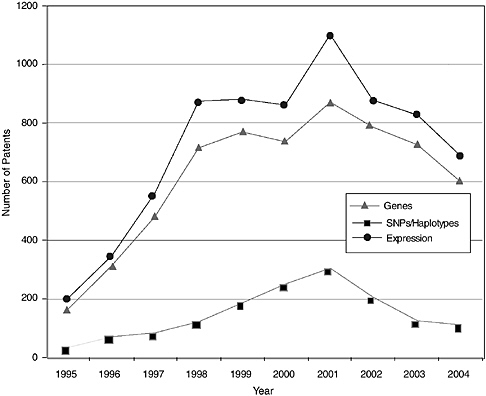
FIGURE 4-3 DNA patent trends, 1995-2004.
sources available to restrict the searches to human material, excluding plants, animals, microorganisms, and synthetic molecules.6
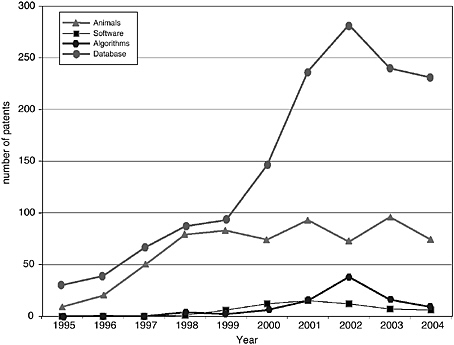
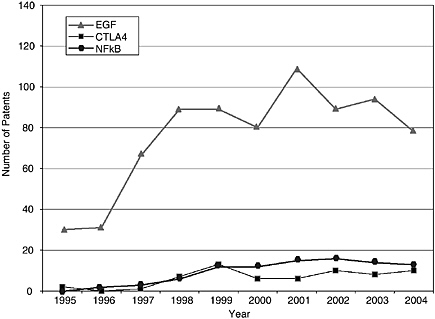
The patenting trends and published applications by year from 1995 to 2004 are shown in Figures 4-3 through 4-7, with the nine categories grouped as follows: DNA patents (including genes and gene regulation, haplotypes and SNPs, and gene expression profiling) and tools (modified animals, software, algorithms, and databases). Protein structures and protein-protein interactions are shown separately because of the vast difference in patenting activity, which is characteristic of other categories. Genes and gene regulation, gene expression profiling, and protein-protein interactions are by far the most active categories, followed by haplotypes and SNPs and databases. There are few protein structure patents and
|
6 |
This will be apparent in Table 4-2, in which some agricultural biotechnology firms appear as leading patent holders in some categories, especially genes and gene regulatory sequences and SNPs and haplotypes. |
pending applications, as well as few biologically related software and algorithm patents. There are also sharp differences among the pathways. Indeed, that was a criterion of selection. The area of EGF shows considerably more activity than those of CTLA4 or NF-kB. The EPO data show lower levels of patenting in every category but similar variations among categories. Compared to the United States, the low European levels of patenting of haplotypes and SNPs and genetically modified animals are particularly striking and perhaps attributable to greater conservatism on the part of EPO in approving patents in those domains.
Similar to the Georgetown University DNA patent data, patenting declined in most categories beginning in 2000-2001. The only case in which this is not readily apparent is protein structures, where the numbers are very low to begin with. Does this signify a general decline in biotechnology patenting that can be expected to continue? It is of course too early to tell. However, several possible explanations can be ruled out or considered unlikely: (1) public research funding was not declining during this period; in fact, the decline begins at a time when the NIH budget was being doubled; (2) research productivity was not declining; if anything it was increasing, with the automation of sequencing and improvements in other techniques; and (3) the economic environment could not have played a role, at least initially, because the patents that issued after 2000 derived from applications filed two or more years earlier, at the height of the boom.
Greater conservatism on the part of USPTO is almost certainly a factor in the decline, perhaps especially in categories such as haplotypes and SNPs. Partly in response to criticisms of the standards being applied to genomic patent applications, the office conducted a broad review of its examination standards and practices and in January 2001 released new guidelines clarifying the written description and utility requirements. The guidelines are written to be generic to all technologies, but they had a significant effect on claims involving DNA and proteins, and most of the training examples given to examiners are in biotechnology. The written description guidelines were intended to bring USPTO practice into line with the Federal Circuit’s decision in Regents of the University of California v. Eli Lilly and Co.,7 in which the court ruled that simply describing a method for isolating a gene or other sequence of DNA is insufficient to show possession and that the complete sequence or other identifying features must be disclosed. The guidelines declared that the claimed utility of the invention must be “specific, substantial, and credible” and extend beyond a mere description of its biological activity. The guidelines were widely interpreted as raising the bar to patents on genomic inventions (see Chapter 3).
But the question of what practical effect the measures had on examiners’ behavior and USPTO output is difficult to answer. It is complicated by the lag between application filings and patent grants and other nearly simultaneous developments, such as the deposit of large amounts of human DNA sequence data in the public domain, where they became prior art. Other factors to be weighed in interpreting patent grants over time are the finite nature of the human genome and USPTO’s “restriction” practice of forcing patent applicants to separate DNA sequences into different applications.
Markets may have had another kind of influence. The rising cost of patenting may have discouraged some from vigorously pursuing all of the patenting opportunities presented by the flourishing of biomedical research.8 Moreover, there is evidence that technology licensing offices become more sophisticated and selective as they accumulate experience about what technologies are licensable (Mowery et al., 2004).
Another change can be documented and may be the principal effect of the new examination policies—the lengthening of patent application pendency. By the end of 2002, applications in the principal category affected by the guidelines (526/23.1, DNA/RNA fragments, including ESTs) were taking more than 3 years to yield patents, several months longer than the 24-month average in 2002 for all applications (Figure 4-8).
The data indicate that there are at least as many applications pending in USPTO as there are patents already issued in each of the patent categories, and in some cases—for example, gene expression profiling, protein-protein interactions, and modified animals—two to four times as many.9 In EPO, the numbers of patents and applications are less divergent, but in most categories more applications are pending than patents have been issued.
No one can predict how many of the pending applications eventually will issue, let alone how many will be filed in the future. The grant rate in USPTO (the proportion of applications that result in issued patents) is substantial—two-thirds to 90 percent or more by various calculations, and probably higher than the approval rates in the European and Japanese patent offices.10 The committee concluded that the patent landscape, which already is crowded in areas such as gene expression and protein-protein interactions, could become considerably more complex over time.
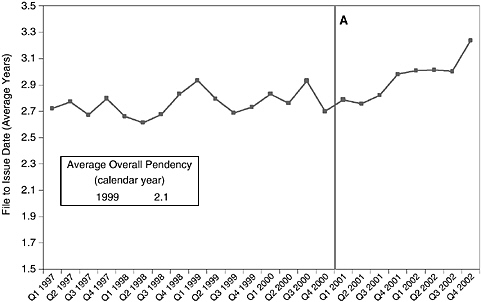
FIGURE 4-8 Patent Class 536/23.1 (DNA/RNA fragments) application pendency by quarter, 1997-2002. NOTE: Pendency is calculated based on original file date and issue date for all issued patents in Class 536/23.1. Overall pendency is calculated by USPTO and also includes an estimate of the time from filing date to abandonment of the application. A = Utility and written description guidelines implemented.
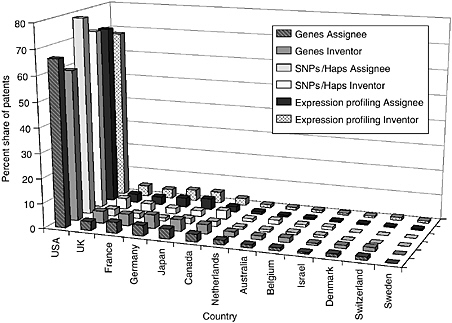
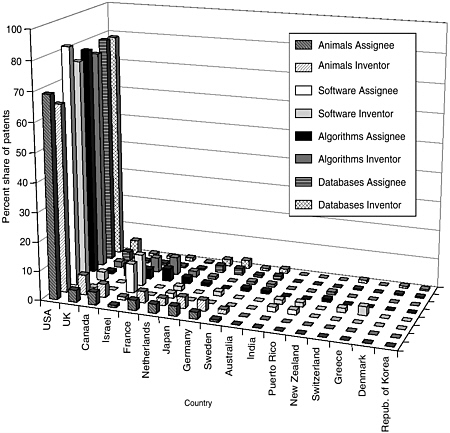
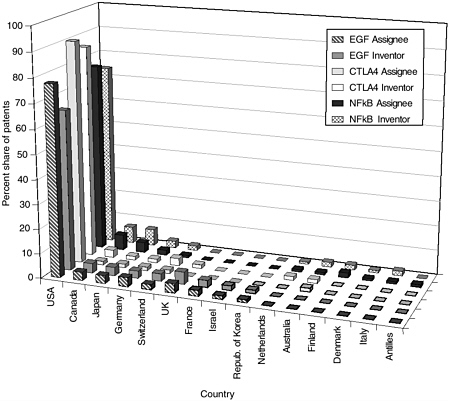
The committee staff analysis also looked at the inventor and assignee country for the patents in each of the categories. The United States leads the world in both inventors and assignees, holding 65 to 80 percent of the worldwide patent share, followed by the United Kingdom, France, Germany, Japan, and Canada. Other active countries are Israel and the Netherlands. For the U.S.-granted patents and pending U.S. applications, the top assignees were all in the United States—sometimes by a factor of 15 or more. Additionally, the United States is the top inventor country, but by a smaller margin. Figures 4-9 through 4-12 show these data for the DNA-related categories, the protein-related categories, tools, and the three pathways.
In the United States, the institutions constituting the University of California have been active patentees across all categories, with a combined patenting frequency at the level of a commercial entity. Categories that developed more recently (software, databases, and gene expression profiling) typically are dominated by biotechnology firms. The protein structure category has been led by pharmaceutical companies because of its proximity to drug discovery, while universities have been dominant in the modified animals category. NIH (through the
Department of Health and Human Services) also has been active in several categories, especially gene-expression profiling and protein-protein interactions (Table 4-2). Table 4-2 shows the leading assignees of patents in the 12 categories described above and identified using the search algorithms set out in Appendix C.
TABLE 4-2 Principal Assignees of Patents by Category
|
|
Total Patents |
Top Assignees |
|
Genes and Gene Regulatory Sequences |
6,145 |
U. California (188) Pioneer Inc. (150) Ludwig Inst. (72) Monsanto (72) Chiron Corp. (71) General Hosp. (71) |
|
SNPs and Haplotypes |
1,482 |
Pioneer (183) Dekalb Genetics (107) Stine Seed Farm (48) U. California (39) John Hopkins (25) |
|
Gene Expression Profiling |
7,428 |
U. California (215) Incyte (170) Affymetrix (117) Gen-Probe (100) DHHS (96) |
|
Protein Structure |
39 |
Abbott Labs (3) Connaught Labs (3) U. California (3) U. Alberta (3) |
|
Protein-Protein Interactions |
6,964 |
Genentech (181) U. California (178) DHHS (84) Chiron (82) Immunex (78) |
|
Modified Animals |
652 |
U. California (26) General Hosp. (11) Pharming BV (10) Abgenix Inc. (9) |
|
Software |
60 |
Millennium (8) Rosetta (4) Pioneer Hi-Bred (3) |
|
|
Total Patents |
Top Assignees |
|
Algorithms |
91 |
Cytokinetics (42) All others (2 or 1) |
|
Databases |
1,466 |
Affymetrix (108) U. California (45) Agilent Tech. (34) Nanogen (22) Sequenom (18) |
|
EGF |
765 |
Sugen (23) Genentech (16) U. California (12) DHHS (12) Yale (11) |
|
CTLA4 |
63 |
Bristol-Myers Squibb (20) Dana Farber (6) Repligen (4) Genetics Inst. Inc. (3) Pfizer (3) |
|
NF-kB |
94 |
U. California (7) Bristol-Myers Squibb (6) Tularik (5) Ariad (3) Dalhousie Univ. (3) |
|
NOTE: The assignee is the company or organization assigned ownership on the original patent. Through consolidations, mergers and acquisitions, and other transactions, ownership may change. Private organizations, foundations, and hospitals are distinguished from commercial entities by italics. Government entities are indicated by bold typeface. |
||
TRENDS IN UNIVERSITY LICENSING OF GENOMIC AND PROTEOMIC INVENTIONS
Licensing is the principal means of accessing the use of patented technology, and it occurs under terms that are infinitely varied and complex and whose effects are not straightforward. Thus, whether a patented upstream invention is available for licensing—and under what conditions—is possibly the principal determinant of whether exclusive rights can impede or alternatively, facilitate the conduct of follow-on research. Unfortunately, data on licensing are very limited for two reasons. First, unlike patents, government administrative processes generate information on only some licenses whose representativeness is unknown and for which detailed information may be lacking and not publicly available. Licenses to or by
publicly traded companies are required to be reported to the U.S. Securities and Exchange Commission, but only if they are “material” to the financial performance of the firms. Research grantees are required to report invention disclosures, patents, and licenses to technologies developed with federal support to a repository—the Edison database, which is maintained by NIH—but these data were not accessible by the committee. Generally speaking, not only firms but also universities consider licenses and licensing terms proprietary information that they voluntarily disclose very selectively and only when it is to their advantage.
Despite these sensitivities, some progress has been made in identifying the parameters of licenses of university-owned biotechnology patents.11 In particular, the Georgetown University group surveyed 30 U.S. academic institutions owning 75 or more of the DNA-based patents in their database (which have been described previously) at the beginning of 2003 (Pressman et al., 2005). Nineteen institutions provided data on licensing frequency for about 2,700 patents. Detailed data were obtained on 200 licensing agreements involving 500 patents, supplemented by qualitative responses to open-ended policy questions and phone interviews.
The principal findings were as follows:
-
Approximately 70 percent of the patents managed by survey respondents were licensed at least once, in a large majority of cases before the patents were issued; approximately 2 percent were licensed more than 9 times.
-
For patents licensed once, 56 percent were licensed exclusively for all fields, 36 percent were licensed exclusively by field of use, and only 8 percent were nonexclusive. For patents licensed 2 to 9 times, 36 percent were nonexclusive, 46 percent were exclusive by field, 13 percent were exclusive for all fields (often sequentially), and 5 percent were licensed on other terms. The types of licenses used vary a great deal by company type, with all-field exclusive licensing dominant in the case of start-up enterprises but less frequent with established companies regardless of size (Figure 4-13).
-
Nevertheless, the results underscore the fact that exclusivity is not a reliable indicator of the extent to which patented inventions are available for others to use.12 Some patents are licensed exclusively but to multiple entities for many different fields of use. Exclusive licenses terminate for a variety of reasons, and patents are subsequently relicensed. In some cases, licenses are renegotiated and their exclusivity modified, and some exclusive licenses permit sublicensing with or without the agreement of the patent holder.
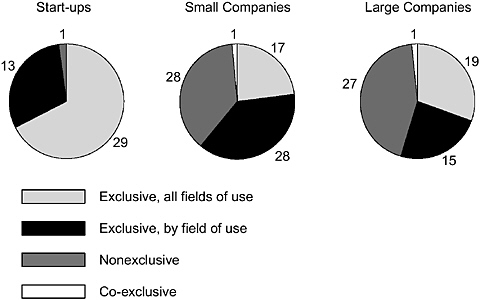
FIGURE 4-13 Patterns of university licensing of DNA inventions by company type.
SOURCE: Pressman et al., 2005.
-
Qualitative responses from the universities suggest that the utility and development potential associated with a technology have an important influence on both patenting and licensing behavior. When known utility and the presumed potential for commercial development are low, universities are less inclined to patent, and when they do, licensing tends to be nonexclusive. On the other hand, when utility and presumed commercial potential are both high, universities are inclined not only to patent but also to license exclusively.
-
Most exclusive licenses contain nonfinancial due-diligence requirements, as do about 45 percent of nonexclusive licenses. These are requirements to report progress in further development of the technology and steps in commercialization. Among the 62 responding universities, 78 percent said that they had terminated research because of due-diligence problems.
-
Most institutions report reserving rights to use a patented technology for their own investigators even though it is licensed exclusively to a commercial entity. An increasingly common university practice in recent years is to reserve such rights for investigators at other nonprofit institutions, but this is often subject to the patent holder’s case-by-case approval.
In short, interview respondents reported practices broadly consistent with the NIH Research Tool guidelines issued in 1998 and with the Guidelines for Licensing of Genomic Inventions, which were in draft form and published for comment
at the time the survey was conducted and the results analyzed. Further, university technology transfer offices reported considering the NIH guidelines de facto regulations binding on grantee institutions.
This committee also obtained the cooperation of the five university assignees with the most patents (in all but one case) holding patents on inventions related to the three molecular pathways—CTLA4, EGF, and NF-kB—to supply data on the licensing of these inventions. The results were similar to those obtained by the Georgetown team for DNA-based patents held by an overlapping set of research institutions. Eleven institutions reported on a total of 122 patents—86 EGF patents, 17 CTLA4 patents, and 19 NF-kB patents. Approximately two-thirds of the patents have been licensed at one time or another—75 once and 7 two or more times. The remaining patents have been abandoned or their licensing histories are unknown. Of 90 licenses reported, two-thirds (60) are exclusive for all fields, 21 percent are exclusive by field of use, and 12 percent (11) are nonexclusive. Most of the agreements (90 percent) allowed sublicensing. Approximately 60 percent were licensed to start-up firms (overwhelmingly on exclusive terms), about 21 percent to firms identified as biotechnology companies, and 20 percent to pharmaceutical firms. Diligence requirements of some sort are included in nearly all license agreements involving these pathway patents.
EFFECTS OF INTELLECTUAL PROPERTY PRACTICES ON RESEARCH13
The implications of patenting and licensing practices are likely to vary from one stage of research and development to another—for example, basic, curiosity-driven research; drug discovery and development; clinical and diagnostic testing—and depend on a variety of circumstances, including the resources of the respective parties and their awareness of the existence and use of intellectual property. A patent on an upstream discovery may encourage downstream development if it gives a developer necessary protection from free riding by others. A patent on an upstream discovery may be an impediment to downstream research if it results in lack of access by downstream researchers not in need of exclusivity or to a foundational discovery or indispensable research tool (a “blocking” problem), or if it renders access to multiple patented technologies excessively difficult or costly (the “thicket” or “anti-commons” problem). Efficient licensing practices can help lower transactions costs and reduce these problems of access to key upstream technologies.
To collect more extensive and systematic but still preliminary information on these relationships, the committee arranged with Walsh and colleagues to de-
sign and conduct a post-mail survey of biomedical researchers in academia, industry, government, and other nonprofit institutions. The committee also provided comments on a draft survey instrument and the proposed sampling methodology, as did other experts consulted. The sample was drawn from the membership lists of relevant professional societies.14 Excluded were researchers in academic institutions not among the top 70 recipients of NIH research awards. Investigators identified as working in government or industry automatically were included in the sampling frame because of their under-representation in the lists relative to university investigators. In fact, industry researchers were over-sampled to ensure that they constituted about one-third of the total. To ensure that the survey respondents contained sufficient numbers of individuals who work in fields of biomedical sciences of high commercial interest (because of their association with normal and diseased cellular processes), a specially selected sample of approximately 100 researchers working on each of the three molecular pathways described earlier (EGF, CTLA4, and Nf-kB) also was included.
The total sample of 1,125 included investigators in universities, government laboratories, and other nonprofit institutions; 563 industry scientists; and 299 researchers working on one of the signaling proteins. In all, 655 responses were received (a 33 percent unadjusted response rate15), 414 from “academia,” including government and nonprofit sectors,16 and 144 from industry. The pathway samples yielded about 30 respondents each.17
In keeping with the committee’s interests, the Walsh et al. survey asked re-
spondents to identify their fields, their research objectives, and the size of their research groups in order to distinguish between the fields of genomics (mapping and sequencing of genes and researching gene functions and associations with diseases) and proteomics (determining protein structures, interactions, and cell signaling), and across the various stages of research and development and projects of different scale (e.g., large projects might be expected to use larger numbers of patented research elements). About 40 percent of the academic respondents reported doing genomics research, and just under 40 percent were doing proteomics research. Ten percent indicated that they were doing drug development, clinical research, or developing diagnostic tests, and almost 80 percent said they were performing basic research, with the remainder developing research tools or doing other work. About 70 percent are associated with research groups of 3 to 10 people; 20 percent were 1- or 2-investigator projects; and just fewer than 10 percent were groups of more than 10 people.
Before trying to ascertain the effects of patents on research, the 159-item questionnaire inquired about researchers’ involvement in commercial activity and in the generation of intellectual property and about their awareness of other intellectual property bearing on their work. Twenty-seven percent of academic respondents have some research tie with small or medium-sized enterprises, and 16 percent have ties with large firms. Nineteen percent of academic respondents receive funding from industry (an average of 4 percent of their research budgets), a slight decline from 5 years ago when 23 percent reported receiving industry funding.18 The average academic respondent spends about 3 percent of her or his time on commercial activity, defined as paid consulting, negotiation of intellectual property rights, or working with a start-up based on the researcher’s own invention.
Forty-three percent of academic respondents have applied for a patent at some point in their research careers, with about 22 percent having done so in the last two years. They averaged 0.37 percent patents each in the last two years. Thirty percent of academics have been involved in negotiations over the rights to their inventions; 11 percent had begun developing a business plan or laying other groundwork for starting a firm; 8 percent had a start-up based on their invention; 13 percent had a product or process on the market; and 18 percent had some licensing income, with 5 percent of this group receiving more than $50,000 in total. Not surprisingly, for those academics conducting drug discovery, clinical testing, or diagnostics, industry funding and patenting rates are higher. In general, there is much more business activity and there is more licensing income in particular than for those engaged in basic research. For academic investigators work-
ing on one of the three molecular pathways, commercial activity was somewhat higher than the average for academics, especially for those involved with NF-kB and EGF, but less so for those working on CTLA4.
The rather high level of commercial involvement, including patenting, contrasts with the rather low awareness of the existence of relevant, already-existing intellectual property bearing on investigators’ work, despite the proliferation of patents on elements of upstream research. Only 8 percent of academic respondents (32) indicated that their research over the previous 2 years involved information or knowledge covered by someone else’s patent. Nineteen percent reported not knowing, and the other 73 percent expressed confidence that they did not need access to other intellectual property. But do academic biomedical scientists attempt to find out if there are patents impinging on their research? Only 5 percent of respondents do so on a regular basis. The percentage is about twice as high for investigators engaged in drug discovery and research involving NF-kB, but not for those working on other pathways.
In the aftermath of the Madey v. Duke decision, both institutional concerns and patent asserters are raising awareness somewhat. Approximately 22 percent of academic respondents have been notified by their institutions to be careful with respect to patents on research inputs, up from 15 percent five years ago. Five percent have been notified at one time or another that their own research may be infringing upon another’s intellectual property. Those external influences are having only a very modest effect on behavior, however. In the two years since the Madey v. Duke decision, only 2 percent of academic bench scientists have begun to check regularly for patents that might impinge on their research. Cautionary notifications from institutions are seemingly ineffectual: 5.9 percent of those who report receiving such notices regularly check for patents, compared with 4.5 of those who recall no such advice to consider the intellectual property rights of others.
Does patenting provide a positive incentive for academic investigators to conduct certain kinds of research, apart from the reputational rewards, competitive influences, and norms that govern the behavior of the scientific community?
Although motivations are exceedingly difficult to disentangle, it appears that the patentability of results is not a negligible factor in academic research choices—only 7 percent consider it more than moderately important—but it pales in comparison to scientific importance (97 percent), personal interest (95 percent), feasibility (88 percent), and access to funding (80 percent) as reasons to do the work. Of course, patentability and commercial potential rank much higher (19 percent and 22 percent, respectively) for those conducting research on drugs and other therapies than for the average academic scientist engaged in basic research (4 percent and 6 percent, respectively). Furthermore, intellectual property prospects may have a bearing on the availability of research funding, especially from industry.
To what extent do patents negatively impinge upon research by leading aca-
demic investigators to abandon lines of work they otherwise might pursue or to modify research protocols or by raising costs or causing delays?
To probe the adverse impact of patents on research, the survey questionnaire asked respondents to “… think about the most recent case where you seriously considered initiating a major research project and decided not to pursue it at that time” and to rank responses on a 1 (“not at all important”) to 5 (“very important”) scale. Table 4-3 shows the percentage of academic respondents in each research category and in the random sample as a whole scoring a given reason more than a “3,” or more than moderately important.19
The reasons for project abandonment were, in order of frequency, lack of funding, conflict with other priorities, a judgment that the project was not feasible, not scientifically important, or not that interesting, and the perception that the field was too crowded with competing investigators. Technology access issues—“unreasonable” terms for obtaining research inputs (10 percent) or too many patents covering needed research inputs (3 percent)—are less frequently cited as important factors. Terms of access weigh more heavily on investigators involved in work on drugs and therapies than on basic researchers (21 percent versus 9 percent), on researchers working on NF-kB than on those involved with other pathways (19 percent versus 7 to 9 percent), on those involved in genomics than on those in proteomics, and on those involved in industry-funded research or other commercial activity than on those who are not.
It is important to follow the experience of the academic respondents, although few in number (32), who concluded that they needed a research input covered by someone else’s patents. Twenty-four contacted the patent owner to obtain permission to using the patented input; five proceeded without contacting the owner; and one modified a project to avoid the input. None abandoned the work as a consequence of either delay or inability to receive permission. Of those who sought permission, seven reported not receiving it within one month. A higher proportion of those intending to use the patented technology as a drug experienced delays or difficulty than those intending to use it as a research tool. Of those seeking permission, only one encountered a demand for licensing fees, in the range of $1 to $100.
Overall, the number of projects abandoned or delayed as a result of technology access difficulties is extremely small, as is the number of occasions in which investigators revise their protocols to avoid intellectual property issues or pay high costs to obtain one. Thus, it appears that for the time being, access to patents or information inputs into biomedical research rarely imposes a significant burden for academic biomedical researchers.
|
19 |
Without an index allowing comparison across an individual’s answers to all questions, the percentages in Table 4-3 do not reflect relative importance. That is, the data do not correct for the fact that some individuals may answer that everything is a 3 or higher. |
TABLE 4-3 Reasons for Not Pursuing Projects, by Research Goal and Pathway
|
|
Random Sample |
Research Goal |
Pathways |
||||
|
Drug Discovery |
Basic Research |
Other |
CTLA4 |
EGF |
NF-kB |
||
|
No Funding |
62 |
86 |
60 |
58 |
63 |
54 |
82 |
|
Too Busy |
60 |
55 |
60 |
59 |
53 |
58 |
48 |
|
Not Feasible |
46 |
41 |
46 |
47 |
33 |
55 |
53 |
|
Not Scientifically Important |
40 |
24 |
41 |
45 |
40 |
36 |
50 |
|
Not Interesting |
35 |
24 |
36 |
33 |
20 |
30 |
29 |
|
Too Much Competition |
29 |
21 |
32 |
21 |
27 |
29 |
29 |
|
Little Social Benefit |
15 |
21 |
14 |
15 |
13 |
5 |
22 |
|
Unreasonable Terms |
10 |
21 |
9 |
6 |
7 |
9 |
19 |
|
Not Help w/Promotion/Job |
10 |
21 |
7 |
15 |
0 |
13 |
5 |
|
Too Many Patents |
3 |
3 |
2 |
3 |
0 |
4 |
0 |
|
New Firm Unlikely |
3 |
3 |
2 |
3 |
0 |
4 |
0 |
|
Little Commercial Potential |
2 |
3 |
2 |
3 |
0 |
4 |
0 |
|
Little Income Potential |
1 |
3 |
1 |
3 |
0 |
4 |
0 |
|
Not Patentable |
1 |
3 |
1 |
3 |
0 |
4 |
0 |
|
Respondents |
274 |
28 |
213 |
33 |
16 |
24 |
22 |
|
SOURCE: Walsh et al., 2005. |
|||||||
There are, however, reasons to be concerned about the future in addition to the earlier observation that the patent landscape is becoming more complex in many domains of research. First, the lack of substantial evidence for a patent thicket or a patent-blocking problem is associated with the general lack of awareness or concern among investigators about existing intellectual property.20 That could change dramatically and possibly even abruptly in two circumstances. Institutions, aware that they currently enjoy no legal protection, may become more concerned about their potential patent infringement liability and take more active steps to raise researchers’ awareness or even to try to regulate their behavior. The latter could be both burdensome on research and largely ineffective because of researchers’ autonomy and their ignorance, or at best uncertainty, about what intellectual property applies in what circumstances. It is much easier for corporations to exercise due diligence in the context of research that is centralized and directed than it is for universities, where research is highly decentralized and decisionmaking is fragmented.
On the other hand, patent holders, equally aware that universities are not shielded from liability by a research exception, could take more active steps to assert their patents. The latter may not extend to more patent suits against universities—indeed, established companies may be reluctant to pursue litigation against research universities—but it could involve more demands for licensing fees, grant-back rights, and other terms that raise transaction costs that are burdensome to research. More assertions would, in all likelihood, prompt more defensive behavior on the part of institutions that traditionally are risk averse. Whether proactively in planned research or defensively in response to claims of infringement, established companies typically go to great lengths and considerable expense to determine what constitutes a “valid” patent. If necessary, the in-house legal department will consult outside counsel to verify its views. The resources necessary to conduct patent literature searches and arrive at validity judgments on a frequent or routine basis probably are beyond the capacity of most nonprofit research institutions and a wasteful diversion, in any case. Nevertheless, failure to perform due diligence could limit research institutions’ ability to approach demands for licenses by distinguishing between patents that probably are valid and patents that likely would be held invalid in litigation.
According to information collected from 66 research universities by the American Association for the Advancement of Science,21 there was an increase in patent infringement notifications received in the aftermath of the Madey deci-
sion. Most of them involved biomedical research, although it is not known how many instances pertained to genomic and proteomic patents. The number reported increased from 16 in the January-June 2003 6-month period to 36 in the July-December period. In about half of the cases, the notice was in the form of a request to take a license. These led to background investigations in only four cases and licensing agreements in only about one-fifth of the cases. At the same time, only 9 of the 66 institutions reported having a written policy encouraging faculty to consider whether they might be infringing on intellectual property rights in planning and conducting research.22 Although so far little disruption of research has occurred, few precautions have been taken to limit the infringement liability exposure of universities. A few cases of successful patent assertions could have a powerful demonstration effect and upset this equilibrium.
The second source of concern is that biomedical research is becoming more complex and increasingly requires larger-scale efforts. The pattern of a single investigator working on a single gene or gene sequence is giving way to more multi-investigator projects entailing work on many genes or proteins simultaneously, more and more of them patented. The Walsh et al. survey, the sample for which included research teams of significant size, did not indicate that intellectual property-related complications are greater in proportion to the number of investigators involved in the effort, but it is a reasonable presumption that such would be the case with more research inputs. Of course, the resources to address intellectual property complexities also are likely to be greater the more substantial the project. Even if the status quo continues—with many investigators and research institutions not taking precautions to avoid infringement and not subject to frequent patent assertions—the absence of any shield from infringement liability raises a further concern. Institutions may encounter difficulties in licensing the inventions of their researchers in the future.
Are the effects of intellectual property on research different for work on the three molecular pathways than for academic biomedical research in general?
Research on EGF and NF-kB exhibits high levels of commercial activity, including patenting, while CTLA4 research is much closer to the norm for biomedical research. This is probably partly attributable to the more recent discovery of the functions of CTLA4 but not entirely; CTLA4, for whatever reasons, is not yet a target of much commercial interest.
For all three fields, respondents choose their project primarily on the basis of scientific importance, interest, feasibility, and funding. However, EGF investigators are more likely to cite personal income (11 percent versus 2 percent for the random academic sample) and the opportunity to start a new firm (7 percent versus 1 percent) as additional reasons to choose projects. Those working on NF-kB were above average in citing unreasonable terms for research inputs as a rea-
son to avoid pursuing a project (19 percent versus 10 percent for the norm). In none of the fields do “too many patents” appear to deter research.
The adverse effects of patents nevertheless occur more frequently for those who work on the pathways than for the random sample of academic biomedical researchers. Investigators working on the three pathways were two to three times more likely to indicate a need for access to a third-party patent than researchers in the random sample and were more likely to report adverse consequences. In CTLA4 research, there were no delays or modified projects, but one person abandoned a project. In EGF research, two researchers abandoned projects, three experienced delays, and one changed a research protocol. There were three reported NF-kB cases of delay and three of project redirection. Still the number of adverse incidents is small, representing less than 15 percent of the sample; and the number who had to abandon some project represents just 3 percent of those working on these pathways.
What are the effects of intellectual property on biomedical research and development in industry?
Presuming that patenting and commercialization are strong incentives to industrial research and development, especially in the biomedical arena, and that investigators would not report neglecting third-party intellectual property, the survey did not explicitly ask industry respondents about their reactions to upstream biomedical patents. However, a small number of industry respondents (17) answered related questions in which 60 percent said that they regularly check for third-party intellectual property and 35 percent acknowledged needing access to a third-party patent. Two out of the 17 said they had aborted a project for lack of such property, and 4 reported other adverse effects. It is unclear how many of these incidents were the result of being in direct market competition with the patent owner, but for this or other reasons it appears that the incidence of intellectual property-related problems is somewhat greater in industry than in academe.
Patents, Publications, and Citations
Fiona Murray and Scott Stern (2004) and Bhaven Sampat (forthcoming) have taken an entirely different approach in studying the effects of patents on scientific research and the anti-commons hypothesis regarding biomedical research in particular. Using slightly different methodologies, they examined what happens to the citations to a scientific article before and after a patent is issued on its subject matter. They found that articles associated with patents are more highly cited than articles not associated with patents, but that the citations are about 9 to 16 percent fewer than expected after the patent is awarded, suggesting some avoidance of the research direction and possibly some modest decline in “knowledge accumulation.”
The finding is intriguing, especially in light of its corroboration by investigators using two different approaches. Nevertheless, for a host of methodologi-
cal reasons, it should be interpreted with caution. Both papers’ authors refrain from causal inferences or speculation about what lies behind their observations. Do investigators in fact know that a patent has issued? At least for academic researchers, this seems unlikely in view of the survey evidence that they neither search for patents nor respond to notices to pay attention to potential infringement. If they become aware of patents, do they cease working in an area or continue working but cite other research? In industry, where there is little premium on publication, the legal department often reviews external publications and may withhold them to avoid provoking patentees. In either case the effect, if real, may be more on publication and citation behavior than on research conduct. The effect, if real, ultimately may be more on citation behavior than on research conduct.
SHARING RESEARCH MATERIALS
In the meantime, the Walsh et al. survey turned up evidence of a more immediate and potentially remediable burden on research, private as well as public, stemming from difficulties in accessing proprietary research materials, patented or unpatented. Conflicts arising from scientific as well as commercial competition have to be addressed in addition to simply the burden and cost of providing such materials. Concern over the flow of research materials, which may be critical inputs for the success of a research project, is not new. Nor has it gone unaddressed; the NIH research tool guidelines address the process of materials exchanges, and NIH has developed a model Material Transfer Agreement (MTA).
The survey found that impediments to the exchange of biomedical research materials remain prevalent and may be increasing. For the period 1997 to 1999, Campbell and colleagues (2002) reported on the basis of a previous survey that academic genomics researchers denied 10 percent of material transfer requests. In the Walsh et al. study, the comparable number for 2003-2004 is 18 percent (95 percent confidence interval: +/− 3.7 percent). Other pertinent findings were as follows:
-
Requests for material transfers between and within the industrial and academic sectors are widespread, although not of high frequency. About 60 percent of industry respondents and 75 percent of academic respondents initiated at least one request in the last two years. Approximately 40 percent of industry respondents and 69 percent of academic scientists had received such a request in the same period. Rates of initiation and receipt of requests are about the same for those doing drug discovery and those doing basic research.
-
Between 7 percent (suppliers’ estimate) and 18 percent (consumers’ estimate) of university to university requests are denied. Typically, approximately half of respondents have had at least one request denied over a two-year period.
TABLE 4-4 Sharing of Research Materials, by Consumer Sector and Supplier Sector
|
Sectors |
Average Percent Non-Compliance |
||
|
Consumer |
Supplier |
Consumer Estimate (%) |
Supplier Estimate (%) |
|
University |
University |
18 |
7 |
|
University |
Industry |
32 |
27 |
|
Industry |
University |
25 |
38 |
|
Industry |
Industry |
22 |
26 |
|
SOURCE: Walsh et al., 2005. |
|||
-
Rates of refusal or noncompliance are highest for university to industry, followed by industry to university and industry to industry requests (Table 4-4)
-
The consequences of being denied a tangible research input can be more severe than the inability to license another’s intellectual property, because in the latter case work may proceed, albeit at some liability risk. The survey asked about four possible adverse impacts—abandonment, delay, change in research approach, or the need to develop the research input in the requester’s own laboratory. The results are shown in Table 4-5.
What stands out is the higher incidence of adverse effects for drug discovery and pathway researchers, especially those working on NF-kB.
-
Fewer than half of material requests entail an MTA, and the presence or absence of a formal agreement does not appear to be central to whether ultimately the materials are shared. But the process of negotiating an MTA frequently entails costs in terms of restricted freedom of action, delays in proceeding, and financial costs to institutions. Reach-through claims are common as are publication restrictions, more so than royalty payments. Negotiations over MTA terms frequently occasion delays (with 11 percent of the requests leading to negotiations taking more than one month to conclude), especially when the suppliers are industrial firms. Industry suppliers also are much more likely to require MTA than academic suppliers.
-
Although agreements for transfer of patented technologies are more likely to contain restrictive terms and have protracted negotiation histories than are agreements involving unpatented technologies, one cannot infer that patenting per se was the cause of the difficulties. Both patenting and complex drawn out negotiations derive from the commercial potential of the technology and the desire of the supplier and conceivably the consumer to capture a greater share of the rents from that potential.
-
For academics, the most common reason given for denying or ignoring a request was simply the effort involved and the need to protect publication. For
TABLE 4-5 Average Number of Adverse Effects from Not Receiving Research Inputs, Academic Respondents by Research Goal and for Pathways and Industry Respondents
|
|
Random Sample |
Research Goal |
Pathways |
Industry |
||||
|
Drug Discovery |
Basic Research |
Other |
CTLA4 |
EGF |
NF-kB |
|||
|
Academic Supplier |
||||||||
|
Delay >1 month |
0.68 |
0.98 |
0.69 |
0.33 |
0.83 |
1.2 |
2.85 |
0.78 |
|
Change Research Approach |
0.56 |
0.89 |
0.54 |
0.3 |
0.45 |
0.7 |
2.24 |
0.68 |
|
Abandon |
0.22 |
0.07 |
0.24 |
0.21 |
0.27 |
0.2 |
0.62 |
0.39 |
|
Make In-house |
0.67 |
0.88 |
0.65 |
0.59 |
0.93 |
1.2 |
2.29 |
1.01 |
|
Industry Supplier |
||||||||
|
Delay >1 month |
0.4 |
0.75 |
0.39 |
0.18 |
1.02 |
1.1 |
0.87 |
0.35 |
|
Change Research Approach |
0.46 |
0.66 |
0.42 |
0.56 |
0.68 |
0.7 |
1.66 |
0.49 |
|
Abandon |
0.27 |
0.08 |
0.3 |
0.26 |
0.58 |
0.9 |
0.28 |
0.32 |
|
Make In-house |
0.31 |
0.44 |
0.28 |
0.47 |
0.69 |
0.8 |
0.71 |
0.33 |
|
Respondents |
242 |
24 |
195 |
23 |
21 |
24 |
26 |
62 |
|
SOURCE: Walsh et al., 2005. |
||||||||
-
industry respondents, the key reported reasons were the need to protect commercial value and the consumer’s unwillingness to accept restrictive terms.
Gene-Based Diagnostic Test Patents
An area where patents seem to be having an inhibitory effect on research and related clinical practice involves gene-based diagnostic tests (see Chapter 2 for a discussion of breast cancer diagnostics). This was not a focus of the committee’s survey, in part because Mildred Cho and colleagues (2003) have conducted telephone surveys of U.S. clinical laboratory directors who were members of the Association for Molecular Pathology. The first concern is that a patent owner’s refusal to make a single patented gene available for licensing on reasonable terms will inhibit follow-on research on the incidence of mutations in the gene as well as limit patient access to testing at a reasonable cost and the possibility of obtaining a second opinion on the result. Exclusive licenses also limit the opportunity for the development of improvements in the test and verification of the result. An anti-commons effect also can be anticipated in the future as clinicians begin to develop tests for multigenic traits.
Cho and colleagues’ sampling frame of 211 laboratory directors combined listings in the most recent Association of Molecular Pathology directory and on the genetests.org website maintained by the University of Washington with federal funding. The result was a sample of corporate, university, private hospital, federal government, and other nonprofit laboratories. They analyzed the responses of 122 individuals, a large majority of whom had licenses to perform genetic tests for a wide variety of conditions, including hereditary breast and ovarian cancer (BRCA1/2), Canavan Disease, Hereditary Hemochromatosis, and Fragile X syndrome, among others.
The results suggest that holders of gene-based diagnostic patents are active in asserting their intellectual property rights. Sixty-five percent of respondents reported having been contacted by a patent or license holder regarding their potential infringement in performing a test. Twenty laboratories had received notification for 1 test; 51 had received notifications for up to 3 tests, and 26 laboratories for 4 or more tests. These enforcement efforts focused on 12 tests that, as a result, 1 or more laboratories had ceased to perform. In all, 30 laboratories responded that they had ceased administering at least 1 test. This number included almost all of the corporate laboratories and about one-quarter of university laboratories. Asked to evaluate their experience, respondents indicated that patents had had a negative effect on all aspects of clinical testing and reported a decline in information sharing between laboratories. Inclination to undertake test development, too, was adversely affected, according to respondents. Thus, patents do appear to be blocking the clinical use of tests insofar as such clinical use is closely related to follow-on research. Because clinical research often is more efficiently
done with an entire battery of tests, both blocking and an anti-commons might be in effect.
CONCLUSION
After reviewing the existing research literature and conducting research on (1) issued patents and published patent applications in a subset of biotechnology categories; (2) a small set of university licensing practices of selected categories of patents; and (3) biomedical research scientists’ experiences with intellectual property and its effects on research, the committee identified four areas of concern.
First, the apparent lack of substantial evidence for a patent thicket or a patent-blocking problem is associated with a general lack of awareness or concern among investigators about existing intellectual property. This situation could change dramatically as institutions increasingly realize that they enjoy no legal protection and concerns are raised about possible patent infringement liability; this may lead them to take more steps to raise awareness and regulate their behavior.
Second, although the survey did not reveal significant differences in experience between investigators working independently and those working in multimember teams, the growing complexity of biomedical research may make intellectual property more problematic as work on a single gene or gene sequence gives way to research entailing far more extensive inputs, more and more of them patented.
Third, the licensing of some gene-based diagnostic tests does appear to be having an inhibiting effect on research and related clinical practice.
Finally, impediments to the exchange of research materials among laboratories exist, although these impediments appear to be largely independent of intellectual property. Instead, they are associated with scientific competition and the lack of rewards for the time and effort entailed in meeting requests for research inputs and academic respondents’ commercial interests.