5
Use of Data in Human Health Risk Assessment
Data from toxicity testing and epidemiologic studies are used in risk assessments and in environmental decision-making to identify potential hazards, to rank environmental problems, to determine the need for further cleanup at a contaminated site, and ultimately to establish environmental standards and exposure guidelines. A conceptual framework for risk assessment was proposed by the National Research Council (NRC 1983) in Risk Assessment in the Federal Government: Managing the Process. The framework consists of the following four components:
-
Hazard identification. What kind of adverse effects might a substance cause? For example, does developmental toxicity, neurotoxicity, or cancer result from exposure?
-
Dose response. What is the risk of effects at different exposure levels? Is there a level below which no effects are observed?
-
Exposure. How are humans exposed, and at what levels and frequencies do exposures occur?
-
Risk characterization. Given human exposure scenarios, what is the probability of adverse effects? How does risk vary across the population? What are the uncertainties in our understanding of the risk or safety?
For risk assessment, data from animal studies and, less often, from epidemiologic investigations are evaluated to identify the types of adverse effects that may occur. Dose-response data from such studies are also analyzed to predict exposures in humans that should be without risk or pose no more than some predetermined level of risk (Lowrence 1976).
The U.S. Environmental Protection Agency (EPA) and other institutions have issued risk-assessment guidelines1 that outline the array of studies used and how they might be interpreted for risk assessment. The guidelines cover specific end points—such as developmental toxicity (EPA 1991), reproductive toxicity (EPA 1996a), and neurotoxicity (EPA 1995)—and, more broadly, noncancer dose-response assessments (EPA 2004a). Risk-assessment guidelines for carcinogens have also been issued that provide detailed guidance on the procedures for assembling evidence and evaluating modes of action to identify carcinogens and for conducting quantitative dose-response assessments (EPA 1986, 1999, 2005a; IARC 2005a). Noncancer and cancer guidelines are fundamentally different in that noncancer end points are typically evaluated with diverse studies on a wide variety of specific outcomes whereas carcinogenicity is evaluated with a specific set of bioassays that focus on the degree to which a chemical might increase neoplasia in different organs and cell types in the body. EPA also has published a review to explain its approach to risk assessment (EPA 2004b).
This chapter first outlines risk-assessment guideline documents for neurotoxicity, developmental toxicity, and reproductive toxicity. It then discusses generic guidelines for noncancer dose-response assessments, including the requirements resulting from the Food Quality Protection Act (FQPA). Cancer risk-assessment guidelines are discussed next. Throughout, the chapter notes the use and limitations of toxicologic and epidemiologic data typically available for drawing conclusions about hazards, dose-response relationships, and risk based on the guidelines. The chapter focuses on current institutional practices, emphasizing those of EPA to assess environmental agents, and on the types of data generated through regulatory testing strategies, such as those discussed in Chapter 4. The chapter concludes with observations regarding strengths and weaknesses of the current system for generating toxicologic data to assess environmental risks.
NONCANCER RISK-ASSESSMENT GUIDANCE
Neurotoxicity
EPA (1998), the International Programme on Chemical Safety (IPCS) (2001), and the Organisation for Economic Co-operation and Development (OECD) (2004) have provided guidance on the use and interpretation of data generated by neurotoxicity tests. A few of the key principles used to evaluate neurotoxicity data for risk-assessment purposes are highlighted here to show what the regulatory data needs for toxicity testing are and where available data may fall short.
Definition of Neurotoxic Effects
EPA, IPCS, and OECD define neurotoxicity as an adverse change in the structure or function of the central or peripheral nervous system after exposure to a chemical, physical, or biologic agent. Adverse effects include alterations from baseline or normal conditions that diminish an organism’s ability to survive, reproduce, or adapt to the environment.
As discussed in Chapter 2, neurotoxic effects can be functional or structural. Functional effects are neurochemical, neurophysiological, or behavioral and include adverse changes in somatic, autonomic, sensory, motor, and cognitive function (IPCS 2001). Structural neurotoxic effects are adverse neuroanatomic changes at any level of nervous system organization. Central nervous system (CNS) neurons generally cannot be replaced after damage, so toxic damage to the brain or spinal cord that results in neuronal loss is usually permanent. If axons in peripheral nerves are damaged, they can regenerate and reach their original target site if the neuronal cell bodies are not damaged. Axons in the CNS, most notably in the spinal cord, may also regenerate but are less likely to reach their original targets.
Neurotoxic effects may be either direct or indirect. Direct effects result when agents or their metabolites act directly on sites in the nervous system. Indirect effects result if agents or metabolites produce their effects primarily by interacting with sites outside the nervous system—that is, the effects are secondary to other systemic toxicity. To determine whether neurotoxic effects are direct or indirect, gross toxicity, losses in body weight, and alterations in normal metabolism are evaluated for their possible relationship to the observed effects (IPCS 2001). However, dis-
tinguishing between direct and indirect effects may be difficult, particularly when the mechanisms of neurotoxicity are not known. EPA, IPCS, and OECD discuss and provide guidance for study interpretation when neurotoxic effects are found at doses that also cause other systemic toxicity.
EPA Concern Levels
EPA neurotoxicity risk assessment distinguishes among levels of concern on the basis of the magnitude of effect, the duration of exposure, and the reversibility of some neurotoxic effects. In general, there is less concern about effects that are rapidly reversible or transitory—specifically those measured in minutes, hours, or days—and that appear to be associated with the pharmacokinetics of the causative agent and its presence in the body. However, EPA and OECD caution that reversible effects should not be readily dismissed, because reversible changes that occur in the occupational setting or the environment may be of high concern—for example, if a short-acting solvent interferes with operation of heavy equipment in an industrial plant. Also, reversible effects resulting from cell death could require activation of repair capacity that decreases future potential adaptability. That is of special concern for the nervous system because neurons, unlike other cells, do not repair damage to DNA or undergo a continual cycle of programmed cell death and replacement. Clear, demonstrable, irreversible change in either the structure or function of the nervous system causes greater concern. Evidence of progressive, delayed-onset, residual, or latent effects also generates a high level of concern.
Assessment of Neurotoxic End Points
EPA’s assessment of commonly measured neurotoxic end points is discussed in this section to illustrate some of the approaches used to evaluate neurotoxicity data. Behavioral end points are measured with a functional observational battery and motor-activity tests. Those tests are designed to detect and measure major overt behavioral, physiologic, and neurologic signs. EPA, IPCS, and OECD guidelines emphasize the importance of evaluating data in terms of patterns of effects rather than as isolated independent end points. There is a potential for false-positive statistical findings because of the large number of end points typically
evaluated. Thus, the relevance of statistically significant test results should be evaluated according to the number of signs affected; the pattern of effects with respect to functional domains (such as neuromuscular, sensory, and autonomic); the doses at which effects are observed; the nature, severity, and persistence of the effects; and their incidence compared with that in control animals.
If only a few unrelated measures are affected or the effects are unrelated to dose, the results might not indicate a neurotoxic effect (EPA 1998). If several neurologic signs are affected, but only at the high dose and in conjunction with other overt signs of toxicity, EPA does not consider it to be persuasive evidence of a direct neurotoxic effect. For example, body-weight changes can affect measurements of auditory startle, and temperature can affect conduction velocity. If several related measures in a battery of tests are affected and the effects appear to be dose-dependent, the data are considered to be evidence of a direct neurotoxic effect, especially in the absence of other systemic toxicity. However, the observation of some specific end points, such as body tremors and convulsions, even of short duration and even if they are the only observable changes, may be sufficiently important to raise a high level of concern (OECD 2004).
Tests that measure more complex behaviors—such as tests of schedule-controlled operant behaviour, learning, and memory—often require that the test animals have adequate motivation or intact sensory and motor function. Improved performance of a complex task does not necessarily indicate the absence of neurotoxicity; both increases and decreases in neurobehavioral performance may result from deleterious chemical interactions with neurons (IPCS 2001). Thus, EPA does not consider an improvement to be adverse or beneficial until it is so demonstrated by converging evidence.
Some neurotoxicity-testing protocols suggest using a high dose that produces minimal toxicity because behavioral and functional findings can be difficult to interpret when substantial systemic toxicity is observed. In designing studies that include special behavioral, morphologic, neurochemical, or neurophysiologic measures, OECD recommends that careful consideration be given to doses and study conditions that minimize confounding effects of generalized systemic toxicity.
The development of specific and selective biomarkers of neurotoxicity could theoretically improve assessment of the neurotoxic potential of chemicals. IPCS observed that neurotoxicology lags behind other branches of toxicology in the development of such biomarkers. The lack
of progress can be attributed partially to the complexity of the nervous system, the multiplicity of expressions of neurotoxic effects, and the limited understanding of the mechanism of action of many neurotoxic agents (IPCS 2001).
Developmental Neurotoxicity
Although the general principles for evaluating adult neurotoxic end points apply to the developing animal, there are issues of particular importance in the evaluation of animal developmental neurotoxicity studies that affect risk assessment. The development of the mammalian nervous system is a highly complex process with specialized morphologic and biochemical patterns of organogenesis that continue as a carefully timed multistage process guided by chemical messengers (IPCS 2001). A relatively minor disturbance resulting in a perturbation of the developmental interactions between selective cells for a short time may have major effects on the developing CNS. In addition, blood-brain barriers that will eventually protect much of the adult brain, spinal cord, and peripheral nerves are incomplete. As a result, risk assessment of acute and repeated exposures to females of childbearing age (13 years old and older) should include careful consideration of potential exposure of the fetus and its effects on the developing nervous system. Toxicity data can assist in determining whether developmental effects are due primarily to acute or repeated exposures in utero or postnatally.
EPA states that chemical agents that produce developmental neurotoxicity at a dose that is not toxic to the maternal animal are of special concern, whereas EPA generally discounts developmental neurotoxic effects when overt maternal toxicity is moderate or greater. However, EPA cautions that current information is inadequate to assume that developmental effects at doses that cause minimal maternal toxicity result only from maternal toxicity. Another possibility is that both the mother and the developing nervous system are equally sensitive to a given dose. More important, EPA notes that “whether developmental effects are secondary to maternal toxicity or not, the maternal effects may be reversible while the effects on the offspring may be permanent” (EPA 1995).
EPA emphasizes that developmental neurotoxicity should be evaluated in light of other toxicity data, including those on other types of developmental toxicity. Methods of and approaches to toxicity testing that improve the understanding of the mechanisms of neurotoxicity and of the
mechanisms by which maternal toxicity and stress can cause structural and functional effects on the developing fetus could lead to improved risk assessment.
Categories and Overall Evaluation of Neurotoxicity Evidence
EPA (1995) guidelines call for summarizing the evidence from the neurotoxicity database into categories of “sufficient evidence,” “sufficient human evidence,” “sufficient experimental animal evidence/limited human data,” and “insufficient evidence.” The “sufficient evidence” category includes data that collectively provide enough information to judge whether a human neurotoxic hazard could exist. The “sufficient experimental animal evidence/limited human data” category is used when the evidence is judged to support a conclusion of potential or lack of potential neurotoxic hazard. Strong findings from one guideline study are sufficient to establish potential, whereas findings from more than one study and in multiple species are needed to establish that neurotoxic potential does not exist. EPA, IPCS, and OECD emphasize the importance of evaluating overall patterns of effects relative to other neurotoxicologic measures and systemic toxicity end points to determine level of concern and severity of effect and to address possible confounding by systemic toxicity.
OECD (2004) emphasizes an iterative approach to determining whether experimental neurotoxicity data are sufficient for risk assessment. OECD considers initial neurotoxicology testing to be standard acute and repeated-dose toxicity studies in which functional or histologic information is gathered on all major organ systems, including the nervous system. All available data, including human and animal neurotoxicology data on structurally related chemicals, are assessed. The need for additional studies is based on hazard characterization and exposure assessment to determine whether the available data are sufficient to evaluate risk in light of the intended use, foreseeable misuse, and special considerations of exposed populations, such as sex and age.
Reproductive and Developmental Toxicity
EPA has developed guidelines for developmental-toxicity risk assessment (1991) and reproductive-toxicity risk assessment (1996a). As discussed in Chapter 2, reproductive and developmental toxicity testing
includes a broader category of end points than most kinds of toxicity testing because of the multiple life stages of exposure and the variety of effects that can result. Exposure of sexually mature animals before conception can result in infertility and decreased fertility; exposure during pregnancy can result in embryonic death, congenital malformations, fetal growth retardation, and premature or delayed parturition; and exposure of the neonatal, immature, or adolescent organism may result in death, growth retardation or stimulation, endocrine abnormalities, immunologic deficits, neurobehavioral effects, or cancer.
In 1991, EPA published Guidelines for Developmental Toxicity Risk Assessment (1991), which outlines the principles and methods for evaluating exposure data from animal and human studies to characterize risks to human development, growth, survival, and function. The EPA document provides guidance on the relationship between maternal and developmental toxicity, characterization of the health-related database for developmental-toxicity risk assessment, use of the benchmark-dose approach in dose-response assessment, and application of the reference-dose or reference-concentration approach to developmental-toxicity assessment.
In 1996, EPA published Guidelines for Reproductive Toxicity Risk Assessment (1996a). Those guidelines focus on the reproductive-system function as related to sexual behavior, fertility, pregnancy outcomes, lactating ability, and effects on gametogenesis, gamete maturation and function, reproductive organs, and components of the endocrine system that directly support reproductive functions. The guidelines concentrate on the integrity of the male and female reproductive system necessary to ensure successful procreation. They also emphasize the importance of maintaining the integrity of the reproductive system for overall physical and psychological health.
The guidelines used by EPA, the UN, and OECD are fairly comparable regarding definition and evaluation of end points. However, in the European Union (EU), the data are used to classify chemicals into three hazard categories: substances that are known to cause (category 1), should be regarded as causing (category 2), or cause concern about (category 3) impairment of fertility or developmental toxicity in humans (EU 2001). The UN, in its Global Harmonization System of Classification and Labeling of Chemicals (UN 2003), has combined the first two of the EU categories and formed two main hazard categories: “known or presumed” (category 1) and “suspect” (category 2) human reproductive or developmental toxicant (UN 2003). Category 1 is subdivided into categories of known human reproductive or developmental toxicant
(1A), indicating evidence primarily from human studies, and presumed human reproductive or developmental toxicant (1B), indicating clear evidence primarily from animal experiments. A chemical is in category 1B if animal studies provide clear evidence of toxicity that is not found to be a secondary, nonspecific consequence of other toxic effects. When mechanistic data raise substantial doubt about the relevance of the findings to humans or there is some (but not clear) evidence from animal and human studies, a chemical may be placed in category 2. The EU and UN systems generally require direct evidence from animal or human studies for a chemical to be placed in known, presumed, or suspect categories.
Noncancer Dose-Response Assessment
Risk assessments for end points other than cancer are based on the idea that there is a magnitude of exposure—a threshold—at or below which effects do not occur and above which they do. The rationale and methods for characterizing noncancer dose-response relationships are described in guidance documents by EPA (2004a), IPCS (1999, 2001), the National Research Council (NRC 1977, 2001), and other institutions. According to the EPA (2005a) cancer risk-assessment guidelines, noncancer dose-response methods apply when carcinogens are judged to have a threshold mode of action.
In practice, it has not been possible to define objective criteria for establishing thresholds (Daston 1993). Thresholds may be biologically plausible for some end points, but the variation in response of sensitive members of the population makes it difficult to determine an absolute threshold for the entire population. Nonlinear, or threshold, mathematical models are generally not used to describe the relationship between risk and dose at low doses quantitatively. Instead, the dose-response relationship is characterized by values, such as a no-observed-adverse-effect level (NOAEL) or a benchmark dose (BMD). NOAELs and BMDs can then serve as the basis for identifying a reference dose (RfD), a reference concentration (RfC), a tolerable intake, or a guidance value—exposure levels at or below which significant adverse effects are not thought to occur (EPA 2004a; IPCS 1994). Occasionally, a nonthreshold dose-response relationship is judged plausible or likely for a noncancer end point, at least at doses to which humans are commonly exposed. When that occurs, different assessment approaches may be applied.
Guidance values, such as RfDs and RfCs, are used by regulatory agencies to establish levels of daily exposure below which no adverse
effects would be expected to occur even in potentially sensitive individuals, such as children. Examples include maximum contaminant levels for drinking-water contaminants, ambient-air quality concentrations of air pollutants, acceptable daily intakes of pesticides and other food residues, acute-exposure guideline levels for accidental releases, and other advisories.
The Reference-Dose Method
The toxicity database on a compound typically includes studies that assess a multitude of biologic end points and evaluate its effect on various organ systems. The RfD approach, which is analogous to the approach used by IPCS for developing tolerable intakes, begins by identifying studies of suitable quality and then selecting the most sensitive study from among them. The key study provides dose-response data on the critical effect, which is the most sensitive adverse response that occurs at the lowest dose. The dose-response data are examined to derive or select a “point of departure,” which is the starting dose for the calculation of the RfD. The point of departure can be a NOAEL (the highest dose that is not significantly different from the control group) or the lowest-observed-adverse-effect level (LOAEL) (the lowest dose where there is a significant increase compared with the control group). Alternatively, a BMD can be derived. The BMD is the dose that is estimated through model-fitting to produce a specified response rate (for example, 1% or 10%). The BMD is chosen to be in the lower end of the dose-response range for which there are sufficient data. The point of departure is used in conjunction with uncertainty and correction factors (for simplicity, denoted UFs) to derive the RfD (IPCS 1994, 1999; EPA 1991, 1995, 1996a; Dourson 1994).
UFs are used to account for several specific issues, including interspecies extrapolation (UFA), human intraspecies variability (UFH), extrapolation between subchronic and chronic exposure durations (UFS), extrapolation of a LOAEL to a NOAEL if a LOAEL is used (UFL), and concerns about the quality or breadth of the database (UFDB).
IPCS (1999) assumes factors of 10 for both the interspecies and intraspecies UFs and separates them into pharmacokinetic and pharmacodynamic components (Figure 5-1) when there are sufficient data to derive one of the components (Renwick 2000; Renwick and Lazarus 1998). For example, the pharmacokinetics of interspecies differences may be sufficiently well understood to build and assign parameters to a physiologically based pharmacokinetic model. Model predictions of interspe-
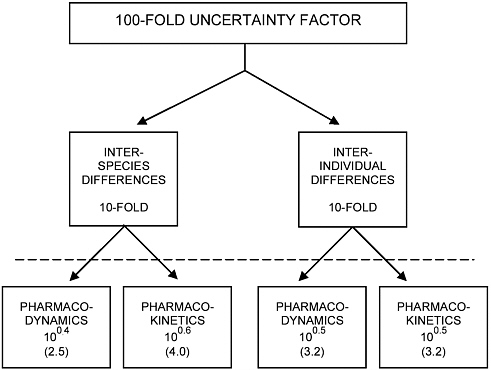
FIGURE 5-1 IPCS subdivision of interspecies and intraspecies uncertainty factors into pharmacokinetic and pharmacodynamic components. Source: IPCS 1999. Reprinted by permission of the International Programme of Chemical Safety, copyright 1999.
cies differences may replace the pharmacokinetic component of the interspecies factor. The remaining uncertainty in interspecies pharmacodynamic differences would be addressed by an interspecies pharmacodynamic factor adopted by IPCS to be 2.5. EPA (2002a) has recognized the IPCS approach and now uses chemical-specific adjustment factors instead of default values when it finds the available data sufficient to derive them.
Individual UFs—usually with values of 10, 3, or 1—are multiplied together to produce an aggregate or composite UF. When the composite UF is large, EPA may judge the data to be insufficient for establishing an RfD and refrains from setting one. The agency has not used a composite UF exceeding 3,000 in over a decade, and an EPA technical panel has recommended that it become policy not to do so (EPA 2002a). EPA (2002a, 2002b) has reviewed the extent to which the composite UF results in adequate protection.
When animal data are used to derive the RfD, the BMD (or the NOAEL or LOAEL) is first adjusted to represent a daily continuous ex-
posure of a human population. For example, the adjustment factor (ADJ) might be 5/7 to adjust a laboratory exposure of 5 days/week to an exposure of 7 days/week. The resulting value is divided by the composite uncertainty, as illustrated in Equation 1.
(1)
The Reference-Concentration Method
Guidelines for determining inhalation RfCs have been refined to base the derivation on dose delivered to epithelial regions of the respiratory tract (EPA 1994). In a procedure similar to that for RfD derivation, the RfC is calculated from a benchmark concentration (BMC) or a no-observed-adverse-effect concentration. It is first adjusted to represent a daily continuous exposure and then corrected with dosimetric adjustment factors (DAFs) to provide a human equivalent benchmark concentration (BMC(HEC)). The DAFs are based on general knowledge of particle and regional gas deposition characteristics in the specific regions of the respiratory tract in the test animals and humans. The BMC(HEC) is estimated from the following relationship:
(2)
The DAF correction is to estimate an equivalent deposition in the human respiratory tract at the animal BMD(Adjusted). After the “above-the-line” corrections in Equation 2, UFs are applied. Because the BMC(HEC) includes an adjustment for delivered dose between species, EPA applies a UFA of 3.15 rather than 10, where 3.15 is about the square root of 10. The UFA is reduced because the correction with the DAF is intended to account for species pharmacokinetic differences but does not account for pharmacodynamic uncertainties in interspecies extrapolation.
Uncertainty Factor for Susceptibility of the Fetus and the Young—Application of the Food Quality Protection Act
The approach of dividing the dose at which responses are observed in animals by some factor or group of factors began in the 1950s when FDA used a factor of 100 to determine allowable human daily intakes of food additives and other compounds on the basis of animal studies
(Lehman and Fitzhugh 1954). In the first application, the adjustments were considered to be safety factors. The major grouping of current UFs discussed above was developed in the 1980s as EPA began a process of standardizing the risk-assessment approach for end points other than cancer (Dourson and Stara 1983; Dourson 1994). In 1993, NRC addressed the question of whether the regulatory approaches for controlling pesticide residues in foods adequately protected infants and children (NRC 1993). After reflecting on the testing and standard-setting system, NRC recommended that a UF of up to 10 be applied when there is evidence of fetal and postnatal developmental toxicity and when data from toxicity testing relative to children are incomplete. Its report stated that “the committee wishes to emphasize that this is not a new, additional uncertainty factor but, rather, an extended application of an uncertainty factor now routinely used by the agencies for a narrower purpose [to address fetal developmental toxicity]. In the absence of data to the contrary, there should be a presumption of greater toxicity to infants and children.” The FQPA, signed into law in August 1996, directed EPA to use an additional 10-fold margin of safety in setting pesticide tolerances for infants and children to take into account the potential for prenatal and postnatal toxicity and the completeness of the toxicology and exposure databases. The law provides for departure from the 10-fold margin when reliable evidence shows that a different margin is protective of infants and children [Section 408(b)(2)(C)].
In implementing the FQPA, the EPA Office of Pesticide Programs considers the FQPA factor as part of the tolerance evaluation. The toxicology and exposure data are evaluated, and then it is determined whether there are important residual uncertainties regarding potential risks to the young. EPA uses a weight-of-evidence approach to judge the degree of concern for potential prenatal and postnatal toxicity to determine whether the 10-fold margin, referred to by EPA as the 10X factor, should be used or a different value should be assigned. The approach considers several factors, including available human and animal data on prenatal and postnatal toxicity, the nature of the dose-response relationship, and information on the human relevance of data from animal experiments, such as pharmacokinetics, mechanism of action, and similarity of the biologic response in different species. Table 5-1 illustrates how EPA may weigh those factors in evaluating the necessary FQPA margin.
In practice, EPA’s Office of Pesticide Programs usually has found that application of the traditional factors provides adequate protection.
TABLE 5-1 EPA’s Weight-of-Evidence Approach for Evaluating Degree of Concern for Prenatal and Postnatal Toxicity on the Basis of Human and Animal Data
|
Factor |
Degree of Concern |
|
|
Increasing Weight—Higher Degree of Concern |
Decreasing Weight—Lower Degree of Concern |
|
|
Pre- and postnatal toxicity |
Effects found in humans related to exposure Same types of effects seen in more then one species Effects of a different type with greater potential consequences in young than in adults Persistence or relatively longer recovery of effects in young than in adults |
No adverse human or animal effects associated with exposure Similar response in young with relatively shorter recovery than in adults |
|
Dose-response relationship |
Effects observed at a lower dose in young than in adults NOAEL not identified Poor data on dose-response relationship |
Effects at higher dose in young than in adults or only at high dose in presence of severe generalized toxicity Good data on dose-response relationship that allows confident identification of NOAEL or BMD |
|
Pharmacokinetics |
Metabolic profile indicates higher internal dose of active moiety in young than in adults or in humans than in animals |
Metabolic profile indicates lower internal dose of active moiety in young than in adults or in humans than in animals |
|
Mode of action |
Mode of action supports relevance to humans and concern for animal findings Mode of action may lead to several adverse consequences in offspring |
Evidence indicates that mode of action is species-specific and thus not relevant to humans Evidence indicates that humans are less sensitive than animal model |
|
Source: Adapted from EPA 2002a. |
||
Pesticide Fact Sheets summarizing many pesticide evaluations conducted since the passage of the FQPA in 1996 are posted on EPA’s web site. Of the 59 chemicals posted, EPA found it unnecessary to apply an FQPA factor—that is, it uses a factor of 1—for all but 11 chemicals. For five pesticides, the agency applied the full FQPA factor of 10 for at least one exposure group and exposure circumstance, such as acute dietary exposure of women of childbearing age. For six pesticides, EPA applied an FQPA factor of 3. In the five cases where the factor of 10 was applied, severe developmental toxicity end points, such as multiple malformations and fetal death, were observed in laboratory animals. In two cases in which an FQPA factor of 1 was applied, a database UF of 10 was used. Both cases were driven by findings from existing studies suggesting effects at lower doses and the need for specific studies to resolve the uncertainty (for example, a developmental-immunotoxicology test).
EPA (2002b) notes that there should be consistency among agency programs, including the pesticide program, in deriving RfDs and RfCs for the same chemical. EPA (2002b) found that broad use of the database UF under the FQPA is characteristic of other agency practices and provided the following guidance on its use in risk assessments:
The [database] “completeness” inquiry should be a broad one that takes into account all data deficiencies. In other words, the risk assessor should consider the need for traditional uncertainty factors not only when there are inadequacies or gaps in currently required studies on pesticides, but also when other important data needed to evaluate potential risks to children are missing or are inadequate.
EPA (2002b) also noted that all agency programs have traditionally considered a group of five studies to be the minimum for deriving a “high-confidence” chronic RfD—two chronic oral studies in different species, two prenatal-development studies in different species, and a multigeneration reproductive-toxicity study in rats. EPA concluded that
the absence of any of these studies suggests that the existing data are not sufficient to address and relieve uncertainties regarding the hazards of the chemical and would typically give rise to the need for a database uncertainty factor to protect the safety of infants and children.
In addition to considering any data gaps involving these five studies, the risk assessor should as is now standard Agency practice evaluate other data gaps, particularly those that pertain to evaluating risk to children and other sensitive subpopulations.
However, EPA (2002b) continued that when determining the need for a database UF, the risk assessor should evaluate how likely the missing or inadequate study will substantially change the outcome of the overall risk assessment. EPA (2002a) found that when the traditional UFs are appropriately applied, they are usually adequate and that an additional factor, such as an FQPA factor, was not needed to address concerns regarding prenatal and postnatal toxicity.
EPA (2002b) is considering new studies and modification of existing guideline studies to provide a more comprehensive coverage of life stages, a more systematic evaluation of pharmacokinetics, and a more focused evaluation of structural and functional toxicity in the young. Such studies might include pharmacokinetics in fetuses or young animals, direct dosing of offspring before weaning, enhanced developmental-neurotoxicity studies, developmental-immunotoxicity studies, and enhanced evaluations related to endocrine disruption.
CANCER RISK-ASSESSMENT GUIDANCE
Principles for assessing cancer hazards and risks have been in use at least since the early 1970s (OTA 1987). In the early 1980s, NRC recommended periodic updating of risk-assessment guidelines to keep pace with scientific advances and to clarify science policy positions (NRC 1983). Several agencies and organizations have developed and implemented guidelines or principles for cancer risk assessment. EPA (1986, 1996b, 1999, 2005a) has developed, revised, and conducted peer reviews of its cancer risk-assessment guidelines over the years as the scientific basis of evaluation has evolved. The current EPA (2005a) carcinogen guidelines emphasize cancer hazard identification and dose-response assessment and provide limited guidance for carcinogen exposure assessment and risk characterization. EPA (2005b) has also published supplemental guidelines to describe possible approaches for assessing risks resulting from early-in-life exposures to carcinogens.
Cancer Hazard Identification
Carcinogen hazard-identification guidelines provide approaches for evaluating data to determine a chemical’s cancer-causing potential. The National Toxicology Program (NTP 2005) has general guidance to identify a chemical “known to cause cancer” or “reasonably anticipated to be a human carcinogen.” The International Agency for Research on Cancer (IARC 1999) has developed more detailed guidance to categorize a chemical as a known, probable, or possible human carcinogen or as a chemical for which inadequate evidence is available or for which evidence suggests lack of carcinogenicity. Similar classifications have been adopted by EPA (2005a) and the Institute of Medicine (IOM 2004).
Classification systems typically involve two steps. First, the different types of evidence on a chemical are evaluated for sufficiency for establishing causal relationships between cancer and exposure to the chemical. Second, there is an overall evaluation of the entire body of evidence.
There are three types of evidence. There is human evidence from cancer epidemiology studies; bias, confounding, and chance are critically evaluated to determine the extent to which they might explain observed relationships. There is evidence from animal cancer bioassays; finding the evidence sufficient to establish causality requires multiple studies showing increases in cancers or tumors that can progress to cancer. And there are other relevant data, such as “data on preneoplastic lesions, tumour pathology, genetic and related effects, structure-activity relationships, metabolism and pharmacokinetics, physicochemical parameters and analogous biological agents” (IARC 1999). Data are “considered to be especially relevant if they show that the agent in question has caused changes in exposed humans that are on the causal pathway to carcinogenesis” (IARC 1999).
Table 5-2 shows how the overall evidence from human, animal, and other relevant studies may be used by various agencies to reach conclusions about potential carcinogenicity. Positive categorizations of carcinogenicity—such as “known,” “sufficient,” “likely,” and “suggestive”—require, at a minimum, direct observations of cancer in humans or laboratory animals. Rarely, structural analogy to an established carcinogen may be used. IOM has the most stringent criteria and requires epidemiologic evidence for drawing any positive conclusions about potential carcinogenicity; animal evidence and other test information are used only to confirm cancer causation once epidemiologic associations have
TABLE 5-2 Level of Evidence in Carcinogen Classification Schemes or Narrative Descriptions
|
Type of Evidence |
IARC |
EPA |
NTP |
IOM |
|
Sufficient human, sufficient animal |
Carcinogenic to humans |
Carcinogenic to humans |
Known to be human carcinogen |
Sufficient evidence of causal relationship |
|
Sufficient human |
Carcinogenic to humans |
Carcinogenic to humans |
Known to be human carcinogen |
Sufficient evidence of association |
|
Limited human, sufficient animal, strong evidence in exposed humans that agent acts through relevant mechanism of carcinogenicity |
Carcinogenic to humans |
Carcinogenic to humans |
Known to be human carcinogen |
Limited/suggestive evidence of association |
|
Limited human, sufficient animal |
Probably carcinogenic to humans |
Likely to be carcinogenic to humans |
Reasonably expected to be human carcinogen |
Limited/suggestive evidence of association |
|
Inadequate human, sufficient animal, strong evidence that carcinogenesis is mediated by a mechanism that also operates in humans |
Probably carcinogenic to humans |
Likely to be carcinogenic to humans |
Reasonably expected to be human carcinogen |
Inadequate/insufficient evidence to determine whether association exists |
|
Inadequate human, limited animal, strong supporting evidence from other relevant data |
Possibly carcinogenic to humans |
Likely to be carcinogenic to humans |
Reasonably expected to be human carcinogen |
Inadequate/insufficient evidence to determine whether association exists |
|
Type of Evidence |
IARC |
EPA |
NTP |
IOM |
|
Inadequate human, sufficient animal |
Possibly carcinogenic to humans |
Likely to be carcinogenic to humans |
Reasonably expected to be human carcinogen |
Inadequate/insufficient evidence to determine whether association exists |
|
Limited human, limited or inadequate animal |
Possibly carcinogenic to humans |
Suggestive evidence of carcinogenicity potential |
Reasonably expected to be human carcinogen |
Limited/suggestive evidence of association |
|
Inadequate human, limited animal |
Not classifiable as to carcinogenicity in humans |
Suggestive evidence of carcinogenicity potential |
(No statement) |
Inadequate/insufficient evidence to determine whether association exists |
|
Inadequate human, inadequate animal, convincing relevant inform ation that the agent acts through mechanisms indicating it would likely cause cancer in humans |
Not classifiable as to carcinogenicity to humans |
Inadequate evidence to assess carcinogenic potential |
Reasonably expected to be human carcinogen |
Inadequate/insufficient evidence to determine whether association exists |
|
Sufficient animal, strong evidence that mechanism of carcinogenicity in animals does not operate in humans |
Not classifiable as to carcinogenicity in humans |
Inadequate evidence to assess carcinogenic potential |
(No statement) |
Inadequate/insufficient evidence to determine whether association exists |
|
Evidence suggesting lack of carcinogenicity in humans and experimental animals or evidence suggesting lack of carcinogenicity in experimental animals consistently and strongly supported by broad range of other relevant data |
Probably not carcinogenic in humans |
Not likely to be carcinogenic in humans |
(No statement) |
Inadequate/insufficient evidence to determine whether association exists |
|
Sources: IARC 1999; IOM 2004; EPA 2005a; NTP 2005. |
||||
been demonstrated. IARC and EPA use direct evidence of cancer from animal bioassays to determine whether a chemical may or is likely to cause cancer. IARC requires more evidence for a conclusion of probable carcinogenicity than EPA needs for a conclusion of likely carcinogenicity. Multiple studies are usually required to establish a positive categorization. IARC (2005b), however, is now considering modifying its rules of evidence so that “possible carcinogenicity can be assessed solely on the basis of strong evidence from mechanistic and other relevant data.” Current NTP guidance indicates that an agent can be classified as reasonably expected to be a human carcinogen when there is “convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans” (NTP 2005). In practice, however, this criterion has not been used in the absence of direct evidence of carcinogenicity of a chemical or a closely related structural analogue.
The hazard-identification guidelines of IARC and EPA discuss in detail how some design features of the bioassay may influence inferences. EPA’s guidelines (2005a) note that study findings can be compromised by dose selection. High doses that cause excessive toxicity complicate the interpretation of tumor observations. Doses that are set too low render a study insensitive. Too few doses or too few animals at each dose limit the dose-response characterization. Guidelines also discuss study quality, reporting, and interpretation with regard to statistical and biologic significance, use of historical and concurrent control animals, and tumor type and progression.
In the IARC, EPA, and NTP guidance, indirect evidence is used to affect the categorization that would otherwise be based on the direct evidence alone. Indirect evidence from genotoxicity assays, comparative human and animal metabolism and receptor profiles, and structure-activity, biomarker, and other studies may increase the confidence that cancer findings in animals are relevant or irrelevant to humans. Such data have been used to classify some chemicals (for example, dioxin and ethylene oxide) as carcinogenic to humans in the absence of definitive epidemiologic data. A potential for carcinogenicity should not be based on indirect evidence in the absence of direct findings of cancer in animal or human studies.
Cancer Dose-Response Assessment
In the EPA (2005a) guidelines, mode-of-action data guide the dose-response assessment. A two-step process is used: the first step involves
the determination of mode of action for each tumor finding, and that dictates the approach for the dose-response analysis, which may be a linear analysis that presumes a linear dose-response relationship, a nonlinear analysis that reflects the assumption of a threshold, or both. When there is strong evidence of genotoxicity from multiple test systems, a linear relationship is assumed. A nonlinear mode of action is determined from data usually at the cellular, tissue, organ, and organism level that together indicate that exposures at some dose would be without cancer effect. The guidelines provide general criteria for the evaluation of data in assessing the mode of action.
In a linear analysis, mathematical models are fitted to dose-response data to estimate a benchmark dose. The BMD is a dose that causes a specified fraction of subjects to develop tumors. The BMD is then used to infer lower risk-specific doses. In a threshold, nonlinear analysis, the standard approach used for setting an RfD for noncancer end points, as discussed above, is used.
The EPA (2005a) guidelines provide for the application of a biologically based model for agents on which quantitative data relate specific key events in the cancer process to neoplasia. A large amount of data is required to support biologically based modeling. Standards and guidance for data generation are not available, nor is specific guidance available for the use of such data in dose-response evaluation.
The EPA guidelines note the importance of considering potentially susceptible populations, such as children and other “subpopulations of individuals who are particularly vulnerable to the effects of an agent because of pharmacokinetic or metabolic differences (genetically or environmentally determined)” (Bois et al. 1995). In practice, few assessments quantitatively characterize human variability in cancer risk. There is a large degree of heterogeneity among humans compared with the relative homogeneity of bioassay animals used as the basis for many cancer dose-response characterizations.
There is no systematic approach for identifying sensitive populations in conducting cancer risk assessment. Human and animal studies indicate that the young can be (but are not always) more sensitive than adults. EPA has developed guidance for assessing the contribution of early life exposures to lifetime cancer risk (EPA 2005b).
The EPA guidelines discuss in many places the issue of cross-species site concordance—that is, whether the specific tumors observed in an animal bioassay should also be assumed to occur in humans. EPA (2005a) states that
site concordance of tumor effects between animals and humans should be considered in each case. Thus far, there is evidence that growth control mechanisms at the level of the cell are homologous among mammals, but there is no evidence that these mechanisms are site concordant. Moreover, agents observed to produce tumors in both humans and animals have produced tumors either at the same (e.g., vinyl chloride) or different sites (e.g., benzene) (NRC, 1994). Hence, site concordance is not assumed between animals and humans. On the other hand, certain processes with consequences for particular tissue sites (e.g., disruption of thyroid function) may lead to an anticipation of site concordance.
Although that is EPA’s stated position, in many dose-response analyses that use pharmacokinetic information, site concordance has been assumed. There is no clear guidance on when it is appropriate to assume it.
COMMITTEE OBSERVATIONS CONCERNING TOXICITY DATA AVAILABLE FOR RISK ASSESSMENT
Guidelines for assessing hazards and dose-response relationships from toxicologic and epidemiologic data have coevolved with scientific developments and laboratory capabilities. In some respects, the human and animal data being generated as described in Chapters 2 and 3 mesh well with the evidence requirements. In other respects, there is a disconnect between the data needed for evaluating risk and the data generated in the laboratory or field. The following discussion presents the committee observations on data availability and needs for risk assessment.
Coverage of End Points
For widely used drugs, food additives, and pesticides, there is a reasonably good basis for risk-based decision-making. That is not the case for industrial chemicals, partly because there is no mandatory testing of industrial chemicals; and the rules of evidence applied in assessing the hazards and risks they pose do not always relate well to the test data being generated. For example, although adequate cancer bioassay and epi-
demiologic data are not available on many chemicals, short-term test data and structural alerts are. Indeed, in some testing strategies, carcinogen bioassays are not performed or infrequently performed, and genotoxicity data are generated to provide presumptive evidence of carcinogenicity. Under EPA and IARC carcinogen guidelines, direct evidence of cancer in animals or humans is required if a chemical is to be identified as having carcinogenic potential. In practice, when such data are not available, the chemical is classified as having, for example, “inadequate information to assess carcinogenic potential”; cancer risk is not estimated; and the chemical is generally treated as posing zero cancer risk. A system for using indirect evidence from emerging test strategies and genotoxicity batteries could be developed to guide the assessment of chemicals that lack adequate cancer bioassay or epidemiologic data. Similarly, systems and guidance could be created for identifying a potential for neurotoxicity, developmental toxicity, and other toxicities on the basis of short-term tests and high-throughput approaches using end points that are more specific to the relevant toxicologic processes that are conserved across species.
Coverage of Life Stages
Most toxicologic tests do not provide sufficient information to assess health risks associated with exposures at different life stages, including pregnancy, infancy, childhood, and old age. To characterize health effects of exposures at different life stages fully, more elaborate study designs would be needed. The extent to which existing risk-assessment procedures for establishing guidance levels may adequately address health protection in relation to exposures at different life stages is an issue of current scientific inquiry and discussion.
Development of Epidemiologic Evidence
High-quality human evidence is given the most weight in hazard identification. Existing regulatory programs and data-generation requirements do not encourage the development of epidemiologic data, but they could. Followup studies long enough to identify carcinogenic hazards are not now required after the introduction of pharmaceuticals, pesticides, or other chemicals.
Use of Standardized Toxicity Tests
Standardization of animal toxicity tests (for example, standardized as to species and strain) provides stability and predictability in testing and regulatory processes but appears in some cases to work against the development of findings of greatest relevance to humans. The finding of lack of relevance of results does not usually prompt explorations with alternative animal models that may be more relevant. When specific animal findings are not relevant to humans, the lack of additional testing in a more appropriate species biases the process toward the creation of false negatives. However, adherence to the standard study-design features and the current testing guidelines generally does produce data that are valuable for hazard identification. The high doses used in the toxicity tests limit their applicability for characterizing risks at the low doses typical of environmental exposures, but accurate characterizations at the low doses would require impracticably large numbers of animals.
The issue of indirect, systemic toxicity resulting from high-dose testing and the challenges it poses for interpreting findings of cancer, reproductive toxicity, developmental toxicity, and neurotoxicity is discussed in risk-assessment guidelines for these end points. For example, neurotoxicity testing currently relies on apical tests that have a strong emphasis on behavioral end points that can be confounded by other systemic toxicity, such as can be seen at or above the maximum tolerated dose or indicated by moderate maternal toxicity. The development of specific and selective biomarkers of toxicity could theoretically improve assessment of such effects when they occur in the presence of systemic toxicity.
Default Dose-Response Assessments
Regardless of the specific noncancer end point, the dose-response relationship is typically characterized with the use of reference doses and reference concentrations. The starting point for analysis is the selection of the point of departure—a NOAEL, LOAEL, or BMD. Use of toxicity data for establishing a point of departure is often not taken into account in designing experiments. Attention to the number of animals, magnitude of dose, number of dose groups, and dose spacing can lead to NOAELs that fall closer to the actual no-effect levels or more reliable estimates of the BMD. For noncancer end points, a reference level is derived from a
BMD, LOAEL, or NOAEL for the most sensitive adverse end point and specified uncertainty and adjustment factors; and, unless scientific information indicates that it is inappropriate, the same process is applied for carcinogens that are judged to act through a nonmutagenic mechanism. The uncertainty and adjustment factors reflect a variety of considerations, including human heterogeneity, interspecies differences, and completeness of the available data. Some time has passed since the currently used values were adopted. Comprehensive testing and data-collection strategies are needed to re-evaluate those factors or alternatives to their use, such as probabilistic risk assessment for noncancer end points; a general framework for evaluating factors used for developmental-toxicity assessment has been described (NRC 2000).
For mutagenic carcinogens or carcinogens of unknown mechanism, a linear no-threshold dose-response model is used by EPA for low-dose extrapolation. Risk-assessment guidelines for assessing the dose-response relationship of mutagenic carcinogens from animal data assume that each individual faces the same risk of cancer at a given dose. Noncancer guidance applies a generic default factor to adjust for variability. Testing strategies do not reflect a systematic approach for developing data to assess the variability of human responses to chemicals quantitatively. Such data would aid in understanding whether the current default procedures for estimating cancer risk are conservative overall or may in some cases understate the risk for some segments of the population.
Data and Framework for Nondefault Assessment
For most environmental agents of concern, the initial default assessment of risk involves extrapolation of findings from studies in very small homogeneous animal populations that are exposed for a portion of their lifespan at doses typically considerably higher than environmental levels to large heterogeneous human populations. The extrapolations have the potential to overestimate or underestimate risk; when the difference between expected human exposure and effect level is relatively small or the costs of indicated exposure reductions are high, additional study may be undertaken either by regulatory authorities or by affected industries.
With the exception of standard genotoxicity testing, the generation of data for mode-of-action evaluations, pharmacokinetic modeling, and other nondefault approaches is typically ad hoc. The data may be sup-
plied by interested parties or otherwise available in the literature but are not required by the agencies. Although the guidelines may provide a loose framework for nondefault approaches, they provide little guidance on data-generation issues, such as hypothesis-testing of modes of action and plausible competing hypotheses. Guidance is also limited or nonexistent for developing other information useful for nondefault analyses, including data for models for assessing human variability, age dependence, site concordance, and high- to low-dose and cross-species differences in pharmacokinetics.
Optimizing further testing to improve the initial characterization of a particular chemical or class of chemicals can be highly context-dependent. Nonetheless, a general framework and further guidance on developing a testing strategy to improve specific risk assessments would be useful. In the process for setting national ambient air quality standards (NAAQS) for criteria air pollutants, data generation, assessment, and standard-setting itself are components of an iterative and cyclic process. As EPA is adopting the new standard, the stage is set for further study and generation of information to improve assessment in the next round of standard-setting. Formal reviews, such as NRC (2004), can be part of the process that leads to coherent and effective testing strategies.
REFERENCES
Bois, F.Y., G. Krowech, and L. Zeise. 1995. Modeling human interindividual variability in metabolism and risk: The example of 4-aminobiphenyl. Risk Anal. 15(2):205-213.
Daston, G.P. 1993. Do thresholds exist for developmental toxicants? A review of the theoretical and experimental evidence. Pp. 169-197 in Issues and Reviews in Teratology, Vol. 6, H. Kalter, ed. New York: Plenum Press.
Dourson, M.L. 1994. Methods for establishing oral reference doses (RfDs). Pp. 51-61 in Risk Assessment of Essential Elements, W. Mertz, C.O. Abernathy, and S.S. Olin, eds. Washington, DC: ILSI Press.
Dourson, M.L., and J.F. Stara. 1983. Regulatory history and experimental support of uncertainty (safety) factors. Regul. Toxicol. Pharmacol. 3(3):224-238.
EPA (U.S. Environmental Protection Agency). 1986. Guidelines for Carcinogen Risk Assessment. EPA/630/R-00/004. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ncea/raf/car2sab/guidelines_1986.pdf [accessed March 30, 2005].
EPA (U.S. Environmental Protection Agency). 1991. Guidelines for Developmental Toxicity Risk Assessment. EPA/600/FR-91/001. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ncea/raf/pdfs/devtox.pdf [accessed March 30, 2005].
EPA (U.S. Environmental Protection Agency). 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8-90/066F. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC. October 1994.
EPA (U.S. Environmental Protection Agency). 1995. Guidelines for Neurotoxicity Risk Assessment. EPA/630/R-95/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: www.epa.gov/ncea/raf/pdfs/neurotox.pdf [accessed March 30, 2005].
EPA (U.S. Environmental Protection Agency). 1996a. Guidelines for Reproductive Toxicity Risk Assessment. EPA/630/R-96/009. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ncea/raf/pdfs/repro51.pdf [accessed March 30, 2005].
EPA (United States Environmental Protection Agency). 1996b. Proposed Guidelines for Carcinogen Risk Assessment. EPA/600/P-92/003C. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://cfpub.epa.gov/ncea/raf/cra_prop.cfm [accessed April 4, 2005].
EPA (U.S. Environmental Protection Agency). 1998. Health Effects Test Guidelines OPPTS 870.6300. Developmental Neurotoxicity Study. EPA 712-C-98-239. Office of Prevention, Pesticides, and Toxic Substances, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/opptsfrs/OPPTS_Harmonized/870_Health_Effects_Test_Guidelines/Series/870-6300.pdf [accessed March 15, 2005].
EPA (U.S. Environmental Protection Agency). 1999. Guidelines for Carcinogen Risk Assessment. Review Draft NCEA-F-0644. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ttn/atw/toxsource/carcinogens.html [accessed March 30, 2005].
EPA (U.S. Environmental Protection Agency). 2002a. A Review of the Reference Dose and Reference Concentration Processes. Final Report. EPA/630/P-02/002F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/iris/RFD_FINAL%5B1%5D.pdf [accessed March 11, 2005].
EPA (U.S. Environmental Protection Agency). 2002b. Determination of the Appropriate FQPA Safety Factor(s) in Tolerance Assessment. Office of Pesticide Programs, U.S. Environmental Protection Agency, Washington,
DC. February 28, 2002 [online]. Available: http://www.epa.gov/oppfead1/trac/science/determ.pdf [accessed March 30, 2005].
EPA (U.S. Environmental Protection Agency). 2004a. Risk Assessment for Non-cancer Effects. Air Toxics Website. U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/ttn/atw/toxsource/noncarcinogens.html [accessed April 1, 2005].
EPA (U.S. Environmental Protection Agency). 2004b. An Examination of EPA Risk Assessment Principles and Practice, Staff Paper Prepared for the U.S. Environmental Protection Agency by Members of the Risk Assessment Task Force. EPA/100/B-04/001. Office of the Science Advisor, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/OSA/ratf-final.pdf [accessed March 22, 2005].
EPA (U.S. Environmental Protection Agency). 2005a. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001B. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/iris/cancer032505.pdf [accessed Nov. 10, 2005].
EPA (U.S. Environmental Protection Agency). 2005b. Supplemental Guidance for Assessing Susceptibility for Early-Life Exposure to Carcinogens. EPA/630/R-03/003F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: http://www.epa.gov/iris/children032505.pdf [accessed Nov. 10, 2005].
EU (European Union). 2001. European Commission Directives: 67/548/EEC, updated 2001/59/EC. Official Journal of European Communities 225:1-333 [online]. Available: http://europa.eu.int/eur-lex/pri/en/oj/dat/2001/l_225/l_22520010821en00010333.pdf [accessed Nov. 10, 2005].
IARC (International Agency for Research on Cancer). 1999. Preamble to the IARC Monographs. 12. Evaluation. IARC Monographs Programme on the Evaluation of Carcinogenic Risk to Humans [online]. Available: http://monographs.iarc.fr/monoeval/eval.html [accessed April 4, 2005].
IARC (International Agency for Research on Cancer). 2005a. IARC Monographs Programme on the Evaluation of Carcinogenic Risk to Humans [online]. Available: http://monographs.iarc.fr/ [accessed March 30, 2005].
IARC (International Agency for Research on Cancer). 2005b. DRAFT Preamble to the IARC Monographs. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer, World Health Organization, Lyon, France [online]. Available: http://monographs.iarc.fr/past&future/PreambleDraft2005.pdf. [accessed Oct. 28, 2005].
IOM (Institute of Medicine). 2004. Gulf War and Health: Updated Literature Review of Sarin. Washington, DC: The National Academies Press.
IPCS (International Programme on Chemical Safety). 1994. Assessing Human Health Risks of Chemicals: Derivation of Guidance Values for Health-Based Exposure Limits. Environmental Health Criteria 170. Geneva:
World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc170.htm [accessed March 31, 2005].
IPCS (International Programme on Chemical Safety). 1999. Principles for the Assessment of Risk to Human Health from Exposure to Chemicals. Environmental Health Criteria 210. Geneva: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc210.htm [accessed March 30, 2005].
IPCS (International Programme on Chemical Safety). 2001. Guidance Document for the Use of Data in Development of Chemical-Specific Adjustment Factors (CSAFs) for Interspecies Differences and Human Variability in Dose/Concentration–Response Assessment. WHO/PCS/01.4. Geneva: World Health Organization. July 2001 [online]. Available: http://www.who.int/ipcs/publications/methods/harmonization/en/csafs_guidance_doc.pdf [accessed March 31, 2005].
Lehman, A.J., and O.G. Fitzhugh. 1954. 100-fold margin of safety. Assoc. Food Drug Off. U.S.Q. Bull. 18(1):33-35.
Lowrence, W. 1976. Of Acceptable Risk: Science and the Determination of Safety. Los Altos, CA: W. Kaufman.
NRC (National Research Council). 1977. Drinking Water and Health. Washington, DC: National Academy of Science.
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Pesticides in the Diets of Infants and Children. Washington, DC: National Academy Press.
NRC (National Research Council). 1994. Science and Judgment in Risk Assessment Washington, DC: National Academy Press.
NRC (National Research Council). 2000. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 1. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Research Priorities for Airborne Particulate Matter. IV. Continuing Research Progress. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 2005. Report on Carcinogens, 11th Ed. National Toxicology Program, Research Triangle Park, NC [online]. Available: http://ntp.niehs.nih.gov/ntpweb/index.cfm?objectid=035E5806-F735-FE81-FF769DFE5509AF0A [ accessed April 4, 2005].
OECD (Organisation for Economic Cooperation and Development). 2004. Guideline Document for Neurotoxicity Testing. ENV/JM/MONO(2004) 25. OECD Series on Testing and Assessment No. 20. Organisation for Economic Cooperation and Development, Paris [online]. Available:
http://appli1.oecd.org/olis/2004doc.nsf/linkto/env-jm-mono(2004)25 [accessed April 1, 2005].
OTA (Office of Technology and Assessment). 1987. Identifying and Regulating Carcinogens. OTA-BP-FI-42-P. Washington, DC: U.S. Government Printing Office.
Renwick, AG. 2000. The use of safety or uncertainty factors in the setting of acute reference doses. Food Addit. Contam. 17(7):627-635.
Renwick, A.G., and N.R. Lazarus. 1998. Human variability and noncancer risk assessment—an analysis of the default uncertainty factor. Regul. Toxicol. Pharmacol. 27(1 Pt. 1):3–20.
UN (United Nations). 2003. Globally Harmonized System of Classification and Labelling of Chemicals. United Nations Economic Commission for Europe [online]. Available: http://www.unece.org/trans/danger/publi/ghs/ghs_rev00/00files_e.html [accessed April 1, 2005].































