3
Good Practices in the Transportation of Research Animals
Animals are transported to facilitate research, teaching, and training, and for breeding-colony establishment and maintenance. Stress during transportation is unavoidable and can affect the quality of ensuing research activities. However, when science-based good practices in animal handling and transport are identified and implemented, the transportation experience can be made less stressful.
There are a few publications and articles that discuss common practices for the transportation of research animals, including the AATA Manual for the Transportation of Live Animals (AATA, 2000), the IATA Live Animals Regulations (IATA, 2005), and a Report of the Transport Working Group Established by the Laboratory Animal Science Association (Swallow et al., 2005). In addition, an extensive collection of scientific literature relating to the effects of transportation on agricultural animals has been produced, in part because the livestock industry often requires that animals be shipped to new locations during the production cycle, which involves social and economic pressures for the animals to arrive in optimal condition. Much of this literature is summarized in the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 1999).
Unfortunately, there is sparse scientific literature on the effects of transportation on most common research animals, but good practices for all research animals can be established by drawing some universal concepts from the available scientific literature and by understanding species-specific needs. Although precise engineering standards are often preferred
by human assessors, the scientific literature supports few engineering standards. This report emphasizes science-based performance standards, which define an outcome (such as animal well-being or safety) and provide criteria for assessing that outcome without limiting the methods by which to achieve that outcome (NRC, 1996). The use of performance standards allows researchers and shippers the flexibility to adjust their procedures to optimize animal welfare on the basis of the species being transported, the mode of transportation, and local environmental conditions.
STRESS DURING TRANSPORTATION
Although the word stress generally has adverse connotations, stress is a familiar aspect of life—a stimulant for some, a burden for others. Numerous definitions have been proposed for stress. Each definition focuses on aspects of an internal or external challenge, disturbance, or stimulus; on perception of a stimulus by an organism; or on a physiological response of the organism to the stimulus (Goldstein, 1995; Sapolsky, 1998; Selye, 1975). An integrated definition states that stress is a constellation of events including a stimulus (stressor), a reaction in the brain precipitated by the stimulus (stress perception), and an activation of the body’s physiological fight or flight systems (stress response) (Dhabhar and McEwen, 1997). Transportation stressors can be physical (changes in temperature, humidity, or noise), physiological (limitation of access to food and water), and psychological (exposure to novel individuals or environments).
It is important to recognize that stress does not always have adverse consequences (Dhabhar and McEwen, 2001; Pekow, 2005), and it is often overlooked that a stress response has healthful and adaptive effects (Dhabhar and McEwen, 1996; McEwen, 2002).
Stress can be harmful when it is long-lasting and animals are unable to adapt successfully to it (Dhabhar and McEwen, 1997; Irwin, 1994; Kiecolt-Glaser et al., 2002; McEwen, 2002); therefore, an important distinguishing characteristic of stress is its duration. Acute stress is defined as stress that lasts for minutes, hours, or a few days; and chronic stress as stress that persists for months or years (Dhabhar and McEwen, 1997; McEwen, 2002). Most transportation events last only a few days and are considered acute stress events. Even the transportation of animals from overseas does not take more than a few days, so there is little concern about chronic stress during transportation. However, care must be taken to minimize post-trip stress in order to ensure that animals are not chronically stressed.
Transportation of animals involves three phases or periods: pretrip, intermodal, and post-trip. During the intermodal period, trip time has a large effect on the stressfulness of the experience. Animals experience a
sudden and large stress response at the initiation of transportation. That response declines until a lower plateau is reached. Then, after a longer period, the stress of transportation gradually increases, especially if feed and water are not consumed. For small species in extreme thermal conditions, the length of time that an animal remains at a plateau of stress response before the stress of transportation begins to increase can be rather short (minutes). However, an animal with a large body store of nutrients in extreme thermal conditions may remain at that plateau of stress response for many days.
A main issue of concern during transportation is an animal’s psychological experience. Normally, animals live in a uniform, familiar environment; during transport, almost every aspect of their environment changes. The transportation enclosure, motion, human handling, temperature, light, and perhaps social group mates, odors, sounds, floor surface, food and water availability, vibrations, unusual gravitational forces (such as during acceleration, braking, or turning of vehicles), and other factors all change from moment to moment. That change in multiple sensory experiences will be perceived as stressful, even under the best of conditions, for two major psychological reasons: the transportation experience is not part of the normal routine, and the animal has no control of the situation. Stress during transportation is unavoidable, so the optimal conditions for moving animals from one location to another would be those that minimize the intensity and duration of excessive stress. Reduction in the number of transportation experiences and in novelty are two ways to make transportation more predictable and to minimize stress; however, most animals will travel only once in their lifetime—from the location where they are bred to the research location. In that case, the goal is to make the single transportation experience as predictable as is practically possible, for example, by providing access to familiar bedding during transportation.
Efforts to minimize excessive stress should be implemented from the time animals are removed from their home cages in the shipping location to the time they are delivered to home cages in the receiving location. Minimizing the intensity and duration of stress in animal home cages is also important and is under the purview of animal caretakers at each institution where animals are housed. However, it must be recognized that even mild manipulations such as moving an animal from one room to another in the same animal facility have been shown to increase corti-costerone levels and result in transient but marked changes in endocrine, serological, and hematological measures (Gartner et al., 1980). Repeated transportation from one location to another in the same building was shown to increase numbers of sulfomucin-producing cells in mucosa of the descending colon of Sprague Dawley rats (Rubio and Huang, 1992). A simple rule of thumb for stress minimization during transportation
involves trying to mimic the animal’s accustomed living conditions as closely as possible while recognizing that animals are resilient and can adapt to an array of conditions provided that their optimal living conditions are restored within a reasonable time frame.
Acute stress from successful transportation is not likely to affect the long-term health of an animal adversely, but it can substantially change important psychophysiological measures in ways that could alter the outcome of research if it is performed before these measures normalize. Most studies suggest that animal responses to transportation stress include activation of the brain, changes in behavior, neuroendocrine and peripheral endocrine responses, and activation of homeostatic mechanisms, but these responses can vary with age, species, and strain. They are generally of short duration. Some studies have attempted to define post-transportation recovery times that are required for normalization of specific measures after transport. Physiological changes due to transportation and recovery times are outlined below for the major species of research animals. Generally, physiological changes return to normal within a day or two of transportation. However, it is important to recognize that the sparse literature suggests that some psychophysiological measures may take longer to normalize after transportation and that the time until normalization can be influenced by the duration and intensity of the stress of transportation and the particular stress-responsivity characteristics of the species or strain being transported. In practice, many investigators allow 2 to 3 days to a week or more for animals to recover after transportation and to acclimate to their new environment.
Rodents
The level of plasma corticosterone, one of the prinicipal stress hormones, increases substantially after transportation (Aguila et al., 1988; Drozdowicz et al., 1990; Landi et al., 1982). The increase is accompanied by changes in immune characteristics, such as a decrease in splenic natural killer cell activity (Aguila et al., 1988), total white cell numbers, lymphocyte counts, thymus weight (Drozdowicz et al., 1990), and humoral immunity (Landi et al., 1982). Body weight also decreases, even in rodents that have access to food and water during transportation (Dymsza et al., 1963; Wallace, 1976; Weisbroth et al., 1977). It has been suggested that normalization of most physiological changes (including corticosterone and body weight) occurs in 2 to 4 days (van Ruiven et al., 1996). However, other measures may take several weeks to normalize. For example, in animals that experience a light-dark shift (as can occur during transportation between continents), the corticosterone circadian rhythm can take more than 2 weeks to resynchronize (Weinert et al., 1994).
The strain of animal can also influence the magnitude of physiological changes caused by transportation. Reproduction in some strains of mice is adversely affected by transportation (Hayssen, 1998), and some murine strains (A/J, DBA/1, SWR, and other strains with different haplotypes of the H-2 histocompatibility complex) may show a greater incidence of shipping-associated development of isolated cleft palate when pregnant females are shipped during the 5 days of gestation before embryonic palate closure (Barlow et al., 1975; Brown et al., 1972; Gasser et al., 1981). Studies have also shown that laboratory mice may be more resilient to transportation-associated stressors than wild-caught animals (Wallace, 1976).
Nonhuman Primates
There are only sparse data on the effects of transportation in nonhuman primates (Wolfensohn, 1997). One study involving owl monkeys documented the effect of international transportation on body weight (Malaga et al., 1991). All animals in the study lost weight, but the amount of body weight lost was a function of age and not the length of transportation (3 to 14 days). Younger animals lost more weight than mature animals but regained more weight than adults during the 30 days after transportation, irrespective of the length of transportation.
Pregnancy outcome and reproduction rates after transportation have also been studied in nonhuman primates. Pregnancy outcomes of pig-tailed macaques, long-tailed macaques, and baboons were studied by Sackett (1981). He found that shipment during any trimester of pregnancy had no effect on the production of viable offspring versus unshipped controls. Rates of reproduction in the three species were also tracked over a period of 8 years. In general, numbers of offspring produced were unchanged after air transport and in some cases slightly greater. The only adverse effect, not present in all species, was increased latency in rebreeding after transportation.
Livestock
In cattle, as in other farm species, body temperature rises, heart and respiration rates increase, the hypothalamic pituitary adrenal axis (HPA) activates, and there is an increase in levels of nonesterified fatty acids, blood cortisol, glucocortoid, and glucose after transportation (Marahrens et al., 2003; Nyberg et al., 1988; Warriss et al., 1992). Creatine kinase, albumin, and total plasma protein concentrations also tend to increase with the duration of the journey (Warriss et al., 1995).
In general, physiological changes are largely determined by the age of the animal. For example, transported calves that are less than 4 weeks old
do not appear to exhibit as large an HPA response as do mature cattle (Cole et al., 1988; Mormede et al., 1982). At 8 weeks, the response begins to change. Corticosteroids increase, but glucose is variable, either increasing or remaining unchanged (Crookshank et al., 1979; Kent and Ewbank, 1983, 1986a, 1986b; Simensen et al., 1980). In general, those changes return to baseline immediately after transportation (Knowles et al., 1999; Warriss et al., 1992), although some genotypes may have altered endocrine concentrations for months after transport (Nyberg et al., 1988). Young pigs and calves have also been found to have an unstable metabolic rate after transportation, requiring 6 to 9 days to stabilize (del Barrio et al., 1993; Heetkamp et al., 2002; Schrama et al., 1992).
The limited physiological responses observed in adult animals can be much more pronounced after extended periods of food and water deprivation. Livestock are often transported without access to food and water for safety reasons, and the longer the period of deprivation, the longer the time necessary for normalization. Extended periods (more than a day) without food and water may result in 5 days or more before normalization of physiological measures (Warriss et al., 1995).
ALLOMETRIC SCALING AND IMPLICATION FOR TRANSPORTATION PRACTICES
Transported research animals vary greatly in size, from small rodents to very large sea mammals, and within each species animals can vary in size from neonates to adults. As the size of animals varies, so do the biological processes that affect transportation practices. However, variations in processes such as heat production, metabolic rate, and space requirement are not linear functions of animal size (Lindstedt and Schaeffer, 2002). In other words, an animal that is twice the size of another animal does not have twice its metabolic rate. Rather, the relationship is exponential. The term allometric scaling is used to describe methods of quantifying the dependence of biological processes on body mass (West et al., 1997). Implicit in allometric scaling is the principle that small animals occupy more space per unit of body weight than larger animals. Small animals also produce more heat per unit of body weight than larger animals.
The relationships between surface area, metabolic rate, and space required by mammals are defined by the following allometric equations:
Surface area (m2) = 0.1 × weight2/3
(Curtis, 1983)
Basal metabolic rate (kcal/hr) = 3.0 × weight3/4
(Curtis, 1983)
Floor area (lateral recumbency, m2) = 0.1 × weight1/3
(Baxter, 1984)
The larger surface area and basal metabolic rates per unit of body weight of smaller animals means that they evaporate more water and lose more heat per unit of body weight than larger animals. The practical implication is that smaller animals are more susceptible to changes in temperature (cold or hot), wind speed, and humidity. The core temperatures of smaller animals can decrease in cold environments more quickly than those of larger animals.
Smaller animals also become dehydrated more quickly than larger animals and cannot live without water as long. That is because of the larger evaporative skin area and/or respiration rate of small animals. Some species have special adaptations to conserve water, but even among these species, the general relationships between young (smaller) and older (larger) animals apply.
Smaller animals generally have higher metabolic rates per unit of body weight than larger animals. That means that smaller animals can go without food for less time than larger animals, which, because they are larger, have relatively greater nutrient reserves.
THERMAL ENVIRONMENT
Provision of a proper thermal environment is the most important element of safe and humane animal transportation. Temperature has been implicated as the major factor in leading to animal mortality during transportation in many species (Abbott et al., 1995; e.g., Bayliss and Hinton, 1990; Slanetz et al., 1957). The principles of a safe thermal environment during transportation are not different from those in normal housing. The goal is to identify the range of ambient temperatures over which an animal is able to maintain a physiologically normal core body temperature. In this section, the basic principles of thermoregulation in warm-blooded animals are discussed to provide the scientific basis of the committee’s recommendations and to inform the professional judgment of researchers, staff, and institutional animal care and use committees in meeting performance standards.
Principles of Thermoregulation
Warm-blooded animals are known as homeotherms because they maintain a constant body temperature through a high metabolic rate. That process keeps body temperature constant, independent of the ambient temperature. The average body temperatures of the most common research animal species are listed in Table 3-1.
The thermoneutral zone (TNZ) is the range of ambient temperatures within which an animal’s metabolic rate is at a minimum and body tem-
TABLE 3-1 Thermoregulation Data on Common Research Animal Species
|
Species |
Average Rectal or Intraperitoneal Temperature (°C) |
Reference |
Thermo-neutral Zone (°C)a |
Reference |
|
Mouse |
36.5 ± 1.3 |
Herrington, 1939 |
26 to 34 |
Gordon, 1985; Herrington, 1940; Oufara et al., 1987 |
|
Rat |
36.7 ± .9 |
Herrington, 1940 |
26 to 33 |
Gordon, 1990; Gwosdow and Besch, 1985; Swift and Forbes, 1939; Szymusiak and Satinoff, 1981 |
|
Guinea pig |
39.2 ± .7 |
Herrington, 1940 |
28 to 30 |
Fewell, Kang, and Eliason, 1997 |
|
Rabbit |
39.5 (38.6 to 40.1) |
Robertshaw, 2004 |
15 to 20 |
Brody, 1945 |
|
Hamster |
36.8 ± .2 |
Jones et al., 1976 |
28 to 32 |
Jones et al., 1976 |
|
Rhesus macaque |
39.1 (37.9 to 40.0) |
Johnson and Elizondo, 1979 |
24.7 to 30.6 |
Johnson and Elizondo, 1979 |
|
Dog |
38.9 (37.9 to 39.9) |
Robertshaw, 2004 |
20 to 26 |
Brody, 1945 |
|
Pig |
39.2 (38.7 to 39.8) |
Robertshaw, 2004 |
16 to 23 |
Huynh et al., 2005 |
|
Cat |
38.6 (38.1 to 39.2) |
Robertshaw, 2004 |
35 to 38 |
Adams et al., 1970 |
|
Sheep |
39.1 (38.3 to 39.9) |
Robertshaw, 2004 |
21 to 25 |
Brody, 1945 |
|
Beef cow |
38.3 (36.7 to 39.1) |
Robertshaw, 2004 |
−18 to 23 |
Hahn, 1999 |
|
Dairy cow |
38.6 (38.0 to 39.3) |
Robertshaw, 2004 |
−15 to 26 |
Hahn, 1999 |
|
Stallion |
37.6 (37.2 to 38.1) |
Robertshaw, 2004 |
5 to 25 |
Morgan, 1998 |
|
Mare |
37.8 (37.3 to 38.2) |
Robertshaw, 2004 |
5 to 25 |
Morgan, 1998 |
|
Goat |
39.1 (38.5 to 39.7) |
Robertshaw, 2004 |
13 to 21 |
Brody, 1945 |
|
aThermoneutral zones can vary by strain, age, and reproductive or health status. The measurement of an animal’s thermoneutral zone may also be influenced by the room temperature and caging condition of the animal’s regular housing. bThat results in no substantial change in core temperature over the time period indicated in parentheses. In some cases, lowest and highest tolerated ambient temperatures were determined in acclimated animals. |
||||
|
Lowest Tolerated Ambient Temperatureb (°C) |
Reference |
Highest Tolerated Ambient Temperatureb (°C) |
Reference |
|
−5 (3 hr) |
Oufara et al., 1987 |
34 (2 to 3hr) |
Oufara et al., 1987 |
|
−15 (3 hr) |
Depocas et al., 1957 |
34 (100 min) |
Gordon, 1987 |
|
−20 (1.5 hr) |
Huttunen, 1982 |
36 (30 min) |
Fewell et al., 1997 |
|
−10 (2 hr) |
Harada and Kanno, 1975 |
32.2 (2 hr) |
Besch and Brigmon, 1991 |
|
−30 (1hr) |
Pohl, 1965 |
32 (60 to 80 min) |
Jones et al., 1976 |
|
15 (1 hr) |
Johnson and Elizondo, 1979 |
40.0 (1 hr) |
Johnson and Elizondo, 1979 |
|
−35 (30 min) |
Good and Sellers, 1957 |
35.0 (2 hr) |
Besch et al., 1984 |
|
−20 (indefinitely) |
FASS, 1999 |
35 (indefinitely) |
FASS, 1999 |
|
−5 (1.5 hr) |
Hensel and Banet, 1982 |
35 (1.5 hr) |
Adams et al., 1970 |
|
— |
— |
— |
— |
|
— |
— |
— |
— |
|
— |
— |
— |
— |
|
— |
— |
— |
— |
|
— |
— |
— |
— |
|
−13 (indefinitely) |
Schaeffer et al.., 2001 |
— |
— |
perature is maintained solely through autonomic responses (piloerection and peripheral vasomotor tone) and behavioral responses (adjusting posture to bring limbs close to or away from body) (Bligh and Johnson, 1973). In Figure 3-1, the committee has modeled the relationship between metabolic rate and ambient temperature in homeotherms. The TNZ is represented in Figure 3-1 as the area between C, the lower critical temperature, and D, the upper critical temperature (UCT). This zone is narrow in some species, particularly the smaller research animals (see Table 3-1). The midpoint of the TNZ is usually 7 to 10°C below normal rectal temperature and 5°C below normal skin temperature (Brody, 1945).
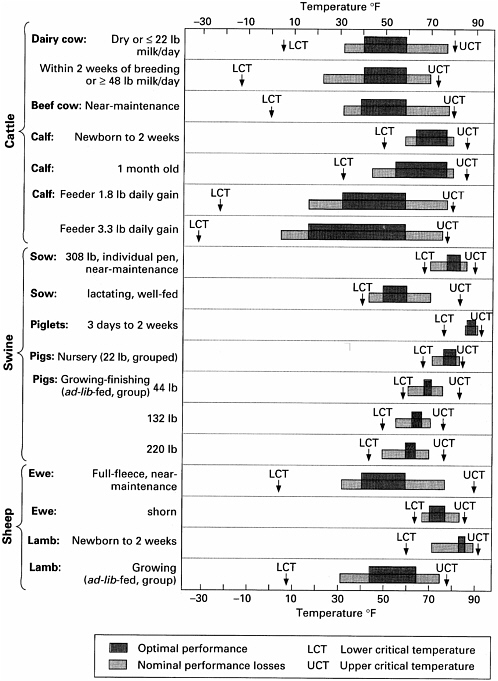
The TNZs for rats and mice (Table 3-1) vary in the scientific literature because TNZ estimates depend on the environments in which the animals are housed and assessed. TNZs can also vary considerably with age and reproductive status, as illustrated for chickens in Figure 3-2 and several other livestock species in Figure 3-3. Comparable data on commonly used laboratory species are not available.
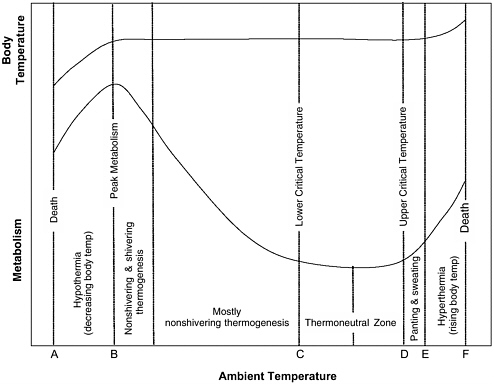
FIGURE 3-1 Graph representing relationship between metabolic rate and ambient temperature in homeotherms.
When the ambient temperature falls below the TNZ, physiological mechanisms collectively referred to as nonshivering thermogenesis (oxidation of fatty acids and brown adipose tissue) are initiated; they increase metabolic rate, balance heat production and loss, and maintain body temperature (Robertshaw, 2004). As ambient temperatures continue to fall, nonshivering thermogenesis is no longer adequate to offset heat loss and maintain body temperature. Shivering thermogenesis (involuntary contractions of skeletal muscles) then occurs and further increases heat production (Robertshaw, 2004). As ambient temperatures continue to decrease, heat production through nonshivering and shivering thermo-genesis reaches the maximum rate that can be sustained over long periods. This point of maximal heat production is known as peak metabolism (B in Figure 3-1) (Robertshaw, 2004). The peak metabolic rate is 3 to 4 times the basal metabolic rate in most species (Brody, 1945). If the ambient
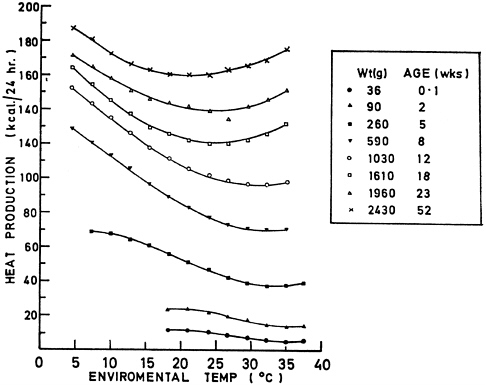
FIGURE 3-2 Changes in thermoneutral zone (range of ambient temperatures at which an animal’s heat production is at a minimum) with age and size in chickens. TNZ of chickens shifts to lower temperatures as chickens grow and age. Reprinted from Fuller, 1969.
temperature decreases further (below B in Figure 3-1), heat production decreases, body temperature decreases (hypothermia), and death eventually occurs. Animals can boost exertions at peak metabolic rates 10 to 15 times their basal metabolic rates during periods of high exertion (Robertshaw, 2004), as occurs in humans during a 100-m race. However, these peak metabolic rates cannot be sustained and are of little value in maintaining body temperature during prolonged cold exposure.
The range of ambient temperatures over which an animal can maintain its core body temperature is depicted in Figure 3-1 between B and E. The lowest ambient temperature at which an animal can maintain its body temperature is generally much lower than its TNZ (see Table 3-1), but the highest ambient temperature at which an animal can maintain its body temperature is generally close to the upper limit of its TNZ. This is because most research animals are nonsweating species (Brody, 1945) and have a limited capacity for dissipating heat. When the ambient temperature rises above the upper limit of the TNZ, heat is dissipated by increasing radiant heat loss (through increased peripheral vascular flow) and evaporative heat loss (through panting, sweating, and saliva spreading). When the ambient temperature is at body temperature, there is no radiant heat loss. Panting can still dissipate heat when ambient temperature reaches body temperature, but it has less capacity to dissipate heat because the energy expended (and heat produced) as panting increases eventually offsets the heat lost through panting (Brody, 1945).
Sweating provides the largest capacity for heat dissipation in homeotherms, so it is important to recognize that most common research animals are nonsweating species and do not have the same capacity to dissipate heat as profusely sweating species such as humans and horses. For example, at an ambient temperature of about 36°C, all of the heat produced by a human can be dissipated through sweating, but only 30 to 40% of the heat produced by nonsweating species—such as rodents, cats, dogs, rabbits, swine, sheep, and cattle—can be so dissipated (Brody, 1945). It should be noted that most nonhuman primates—rhesus macaques are an exception (Johnson and Elizondo, 1979)—have not been observed to sweat over their general body surface and should be considered non-sweating species for the purposes of determining safe ambient temperatures for transportation (Stitt and Hardy, 1971).
Animal handlers must be acutely aware that although they as humans may be able to remain reasonably comfortable because sweating reduces their heat load, the nonsweating animals they care for are not able to remain comfortable through sweating. That must be emphasized during the training of handlers, because temperatures well within human tolerance zones can be deadly to many species of research animals (Ohara et al., 1975; Oufara et al., 1987; Wright, 1976).
Safe Temperature Ranges During Transport
An animal must be transported in an environment in which it can maintain its body temperature, becoming neither hyperthermic nor hypothermic. Due to the many and often interacting physiological and environmental variables that can affect body temperature, it is impossible to specify exact ambient temperature ranges that guarantee that animals will remain homeothermic during transportation. The thermal requirements of animals vary widely depending on age, physiological state, and the environment in which they were previously housed and to which they have adapted. For example, newborns are less tolerant of thermal extremes than adults, and late-pregnancy and lactating animals are less tolerant of heat. In addition, animals previously maintained in cold environments with high feed intakes and high rates of resting heat production (Young, 1975) do not tolerate heat as well as animals previously maintained in a hot environment with low feed intakes and low metabolic rates (NRC, 1981). The effect of the higher rates of heat production associated with pregnancy, lactation, and cold acclimation is an increase in the amount of heat that animals must dissipate as environmental temperatures approach and exceed body temperature, that is, a reduction in heat tolerance.
It could be suggested that homeothermic animals be transported in ambient temperatures within their TNZ. The range of the TNZ for most agricultural animals is large, allowing for safe transportation over a relatively wide range of ambient temperatures (see Table 3-1). The Livestock Weather Safety Index was developed to guide decision making for transporting swine during weather extremes (Livestock Conservation Institute, 1970). More information about the Livestock Weather Index can be found at the National Institute for Animal Agriculture (formerly known as the Livestock Conservation Institute) website:
The TNZ for the more common research animals (rodents, cats and dogs, and nonhuman primates) is narrower than in livestock. However, upper limit of the safe range for transport of those animals is also based on the UCT of an animal’s TNZ. Unlike humans, who can maintain their body temperatures above their TNZ through sweating, most research animals are nonsweating species and have a limited capacity for dissipating heat.
Though the highest ambient temperature at which those animals can maintain their body temperature is close to the upper limit of its TNZ, the lowest ambient temperature at which those animals can maintain their
body temperature is generally much lower than its TNZ. In order to determine the lower limit of the range of ambient temperatures for safe transportation, the committee turned to the acclimation literature. It is well established in the cold-acclimation literature that the more common research animals can be housed in conditions around 6°C for weeks or months with no ill effects (Besch et al., 1984; Depocas et al., 1957; Gordon, 1988; Whiting and Brandt, 2002). Therefore, the lower limit of the temperature range for safe transport was defined as the lowest temperate to which a species had been successfully acclimated (see Table 3-2).
The temperature ranges in Table 3-2 should be considered only as a general guideline. In many cases, air temperature alone is insufficient to determine whether a particular animal or species can be transported safely. Wind chill, sun exposure, and particularly humidity can greatly influence the temperature that an animal effectively experiences (see Table 3-3). For example, air feels colder with even a slight wind. In addition to environmental factors, physiological factors can affect the ambient temperature at which an animal can maintain its core body temperature. These factors include physiological states such as late pregnancy and lactation, previous acclimation, disease status, hydration state, feeding level, exercise, anesthesia, and body fat.
Nevertheless, short-term exposures to thermal extremes during transportation do not generally result in adverse physiological effects except when the short-term temperature exposures are particularly high. As documented in Table 3-1, most common research animal species are able to maintain their core temperature over the course of several hours at extreme temperatures. Even animals with narrow TNZs (e.g., Xin, 1997) can tolerate high temperatures and large fluctuations in temperature over extended periods if provided appropriate food and hydration. The committee recommends that professional judgment be considered the final determinant of whether the ambient temperatures that animals will be exposed to during transportation are safe. Many factors must be considered if professional judgment is utilized, including humidity, stocking density, the characteristics of the transportation cage, plumage and hair coat, previous adaptation, metabolic and behavioral characteristics, physiologic status, food and water consumption, trip length, and potential temperature extremes.
The effects of physiological and environmental factors on the effective temperature that an animal experiences during transportation have been well documented in birds and can be generalized to most other species. Birds with feather loss or poor feather condition lose insulating capability. This loss can be detrimental under cold conditions and beneficial in hot conditions. Likewise, thin birds are less able to maintain their body temperature in cold conditions than birds with more adequate body
TABLE 3-2 Ambient Temperature Range for Safe Transportation of Common Adult Research Animalsa
|
Species |
Ambient Temperature Range for Safe Transportationb (°C) |
References |
|
Mouse |
4c to 34 |
Oufara et al., 1987 |
|
Rat |
6c to 33 |
Depocas et al., 1957 |
|
Guinea pig |
4c to 34 |
Himms-Hagen et al., 1995 |
|
Rabbit |
Cooper et al., 1980; Honda et al., 1962 |
|
|
Hamster |
Jones et al., 1976; Pohl, 1965 |
|
|
Macaca mulatta |
6c to 35 |
Oddershede and Elizondo, 1980, 1982 |
|
Dog |
−10c to 28 |
Nagasaka and Carlson, 1965 |
|
Pig |
−20 to 35d |
FASS, 1999 |
|
Cat |
Adams, 1963; Hensel and Banet, 1982 |
|
|
Sheep |
−12c to 25 |
Horton, 1981 |
|
Beef cow |
−18 to 23 |
|
|
Dairy cow |
−15 to 26 |
|
|
Stallion |
5 to 25 |
|
|
Mare |
5 to 25 |
|
|
Goat |
−13c to 21 |
Schaeffer et al., 2001 |
|
aHumidity, wind chill, sun exposure, hydration state, physiological state, age, acclimation, and so on, can greatly influence these ranges (see Table 3-3). Professional judgment must be used in determining safe transportation of research animals. bThe maximum and minimum temperatures of the range were derived from the upper limit and lower limit, respectively, of that species thermoneutral zone as described in Table 3-1, unless otherwise noted. Most larger mammals can be transported when the temperature is below freezing as long as the temperature inside the transport compartment does not cause frostbite or other signs of extreme cold. Conditions inside transport compartments, especially warm, deep bedding will allow animals to establish a microenvironment that is comfortable. Professional judgment should be used to assess risks to animal welfare when animals are preconditioned or not preconditioned for transport. cTemperature derived from the lowest or highest temperature to which that species has been acclimated. It is possible that animals could be safely transported at more extreme temperatures; however, the literature neither supports nor negates the possibility. dRecommended thermal conditions for swine. |
||
conditions (Schrama et al., 1996). The age of the animal also influences the thermal conditions that are suitable for safe transport. As shown in Figure 3-2, young birds have a narrower TNZ than mature birds. Thus the temperature at which thermogenesis begins is higher in younger birds, making these animals more susceptible to cold.
The phenotypes of some animal strains and transgenic animals must also be considered when the animals have abnormal metabolic characteristics. Some pigs carry a mutation in a gene that causes malignant hyper-
TABLE 3-3 Effects of Various Factors on Effective Environmental Temperature and Relative Risk to Animal Health and Welfare
|
Factor |
Very Cold Outside Temperature |
Risk |
Very Hot Outside Temperatures |
Risk |
|
High relative humidity |
Little effect |
Low |
Much warmer |
Very high |
|
High air velocity |
Much colder |
High |
Feels colder as long as air temperature is below animal core temperature |
Low |
|
Food deprivation |
Much colder |
High |
Little effect |
Low |
|
Water deprivation |
Colder |
Medium |
Much warmer |
High |
|
High stocking density |
Warmer |
Zero or positive effect |
Much warmer |
Very high |
|
Illness (fever) |
Colder |
Medium |
Warmer |
Medium |
|
Fat (high subcutaneous insulation) |
Warmer (protective) |
Low |
Warmer |
High |
|
Stress susceptible genotypesa |
Colder |
High |
Warmer |
Very high |
|
Surface cover (plumage and hair coat) |
Warmer |
Protective |
Warmer |
High |
|
Previous experience or adaptation to cold temperatures |
Warmer |
Zero or positive effect |
Warmer |
High |
|
Previous experience or adaptation to hot temperatures |
Colder |
High |
Colder |
Zero or positive effect |
|
aMay include malignant hyperthermia or transgenic animals that have thermoregulatory or physiological dysfunction. SOURCE: Table adapted from Schrama et al., 1996. |
||||
thermia when stress is experienced (McGlone and Pond, 2003). These animals become warm and red-skinned during stress and develop muscle tremors and an inability to walk. In some instances, animals may die as a result of malignant hyperthermia.
Behavior can affect the thermal experience. The stocking density of birds within the transportation crate may inhibit or encourage thermal regulating behaviors, such as stretching and fanning wings during heat episodes or huddling close to other birds under cold conditions. Posthatch-fasted male chicks held in shipping containers exhibited dispersal behaviors at 35°C (Xin and Harmon, 1996), and birds transported at high densities during hot weather are more prone to heat stress (Schrama et al., 1996). As chicks were subjected to lower temperatures, huddling became more evident, and the most huddling occured at 20°C (Xin and Harmon, 1996). Those behaviors also altered moisture production within the container; there was more moisture loss from animals as they spread out at higher temperatures.
Safe temperature ranges for transportation are more difficult to establish for poikilotherms such as reptiles and amphibians. In poikilotherms, a decrease or increase in body temperature of a few degrees is not a cause for concern. Animal activity and alertness may be better indicators of comfort temperature than body temperature (which varies considerably with air temperature).
Effect of Transportation Caging on Thermal Environment
A major factor that can influence an animal’s effective environmental temperature is the nature of its transportation cage. Transportation containers for small research animals (such as rodents and chicks) are almost always stacked. That feature potentially restricts air flow into or around the containers and can increase the temperature in a container to exceed that of the surrounding environment. For example, when a commonly used commercial chick container was stacked six high in four stacks with 2.5 cm of vertical distance between containers and 5.1 cm between the stacks, the temperature inside the containers was about 5.5 to 10°C above the ambient temperature (Tanaka and Xin, 1997). The IATA Live Animals Regulations (Container Standard #84) and the Animal Welfare Act (9 CFR 3.14(c)(2), 9 CFR 3.36(a)(6), 9 CFR 3.61(a)(5), 9 CFR 3.87(c)(2), 9 CFR 3.137(a)(5)) provide guidance on using spacers or projecting rims in order to prevent obstruction of the ventilation openings and provide space for air circulation.
Transportation containers with biocontainment filters can similarly restrict air flow into a container, increasing the container temperature. In some facilities, rodent transportation caging is autoclaved for reuse. It has
been suggested that such autoclaving may increase the air resistance of the biocontainment filter and restrict air flow (White, 2004). Until it can be established that reautoclaving biocontainment filters does not restrict air flow below acceptable levels, the committee suggests that the prudent course of action is to avoid reautoclaving and reuse of the containers.
Behavioral Monitoring of Thermal Environment
Ideally, all animals should be transported in environmentally controlled vehicles. That method would eliminate concerns about thermal stress. However, such transportation is often unavailable, particularly for nonrodent species, and other precautions and procedures, such as monitoring animal behavior, must be used to ensure the animals’ welfare.
As discussed above, when ambient temperatures change, animals use both physiological and behavioral mechanisms either to increase heat production or to promote heat loss and maintain a homeothermic state. Evidence of those mechanisms does not necessarily indicate that an animal has become hypothermic or hyperthermic. However, prolonged display of the behaviors listed in Table 3-4 is an indication that the animal is stressed and may not remain homeothermic. Although the temperature ranges in Table 3-2 can generally be considered safe for the transportation of research animals, many factors can affect the effective ambient temperature (see Table 3-3). Therefore, when animals cannot be transported in environmentally controlled vehicles, the committee recommends frequent visual inspection of the animals when practical, as signs of thermogenesis or heat loss may indicate that the animal’s thermal environment should be adjusted. Exposure to unaccustomed temperature extremes, particularly high temperature, can be more stressful, harmful, and deadly than suspected by researchers and staff who are accustomed to handling and observing animals in controlled housing conditions.
Thermal Acclimation
When it is anticipated that an animal will encounter extreme temperatures during transportation, as may occur during loading of animals onto airplanes during winter and summer in some parts of the United States, prior acclimation may be appropriate, if practical. During acclimation, prolonged exposure to a single component of the environment—in this case either heat or cold—results in physiological changes that allow an animal to respond more effectively to that component (Robertshaw, 2004). For example, rats acclimatized to 6°C can maintain their body temperature when exposed to −15°C for a period of at least 3 hr (Gordon, 1990).
TABLE 3-4 Behavioral and Physiological Signs of Thermal Status
|
Species |
Signs of Thermogenesis |
Signs of Heat Loss |
|
Rodents |
Piloerection Cutaneous vasoconstriction (paleness of skin of ears or feet) Shivering Drawing limbs close to body (curling up) |
Saliva spreading Cutaneous vasodilation (redness of skin of the ears or feet) Closed-mouth panting (increased respiratory frequency) |
|
Dogs and cats |
Piloerection Cutaneous vasoconstriction (paleness of skin of ears or feet) Shivering Drawing limbs close to body (curling up) |
Open-mouth panting Extending limbs (maximizing surface area) Cutaneous vasodilation (redness of skin of ears or feet) |
|
Agricultural animals (cows, sheep, goats, and horses) |
Piloerection Cutaneous vasoconstriction (paleness of skin of ears or feet) Shivering Drawing limbs close to body (curling up) |
Sweating Closed-mouth panting (increased respiratory frequency) |
|
Birds |
Piloerection Cutaneous vasoconstriction (paleness of skin of ears or feet) Shivering Drawing limbs close to body (curling up) |
Open-mouth panting |
|
SOURCE: Adapted from Robertshaw, 2004. |
||
SPACE ALLOCATION
The need for space allocations during transportation of research animals is the subject of public concern and regulations—but few scientific studies. The space needs of animals during transportation are different from their space needs in resident housing. Space needs also vary with animal temperament, social relationships, thermal environment, and species-specific behavioral requirements.
It is clear that the floor space required during transportation is different from the floor space required during long-term housing. In conventional housing, there are often few adverse effects of providing too much space, but this is not the case during transportation. If transported animals have too much space, they can fall, injure themselves, or even be killed.
Animals should never be in a situation in which they may come into contact with container walls with force or roll around. At the other end of the spectrum, animals should never have so little space that they pile on top of one another; this situation can cause animal injury and may potentially lead to suffocation.
Although smaller animals occupy less space than larger animals, they occupy more space per unit of body weight. Thus, 10 1-kg animals require more space than one 10-kg animal if other considerations are equal. However, the space that animals occupy depends on their posture. All species commonly adopt distinct postures during transportation. For example, horses and adult cattle stand during the entire trip, but rodents, pigs, young calves, dogs, and cats lie down during stable parts of the trip. The order of space occupancy from greatest to least is lateral recumbency, sternal recumbency, sitting, and standing. The space needs of individual animals also depend on whether they are transported alone or in groups and on whether they normally stand or lie down during transportation. The personnel responsible for placing animals in transportation caging must be familiar with the normal behavior of each species to assess the adequacy of the floor space provided.
Determining the appropriate density of animals in a transportation cage or vehicle must take into account weather conditions, the physical characteristics of the species (such as horns, pilage condition), and the preferred posture, if any, adopted during transportation. Simply providing transported animals large amounts of space may not be conducive to their welfare in all instances. For example, low stocking densities can present problems with balance during transportation. Many species, including cattle, rarely adopt a vulnerable posture such as lateral recumbency. If they remain standing, those animals may fall down as the transportation vessel experiences movement in different directions. Even if they eventually adopt a sternal recumbent posture, they can fall or roll in the transportation compartment and be injured. Cattle tend to prefer standing, so it is imperative that they can adjust footing or brace against other cattle to prevent slipping and falling.
Some people have suggested that a high stocking density is preferable for horses and cattle because the animals “hold each other up,” preventing injuries due to falls or rolls (Friend, 2001). However, studies of stocking density indicate that high stocking densities are associated with a higher rate of injury (Friend, 2001). Pushing and mounting behaviors tend to increase with stocking density (Tarrant et al., 1992), which can lead to injury. Also, the ability of animals to rise after a fall is hampered at high stocking densities, leading to more injuries and a greater severity of injury (Collins et al., 2000). In addition, high stocking densities decrease social interactions among animals and may prevent them from assuming a
preferred orientation (parallel or perpendicular to motion) during travel (Eldridge et al., 1988; Kenny and Tarrant, 1987; Lambooy and Hulsegge, 1988; Tarrant et al., 1992). Based on the literature, a moderate stocking density for cattle and horses maximizes animal welfare (Swanson and Morrow-Tesch, 2001; Tarrant and Grandin, 2000).
There is abundant literature on the space requirements or stocking densities necessary to optimize the welfare of agricultural animals during transportation, but none on the most common species of research animals—rats and mice. Rodent vendors have developed space allowances for rodents on the basis of practical experience. When a regression of the space allowances for agricultural animals and rodents was performed (Table 3-5), the trend line (Figure 3-4) had a very high coefficient of determination (r2 = 0.9915). That value suggests that there is a mathematical algorithm that describes the transportation space required to maximize the well-being of group-transported animals, and it provides information on the transportation space required for unusual research animals for which space requirements are unknown. Transportation space requirements for guinea pigs and hamsters mandated in the Animal Welfare Act also follow the trend line; although they are not obviously based on empirical data, those space requirements might be appropriate. The algorithm would be useful to people who are attempting to determine the transportation space needs for an uncommon species of research animal for whose transportation there are neither guidelines nor much practical experience.
FOOD AND WATER
Most animals react to the experience of being transported by becoming anorexic and adipsic. The stressful experiences of a novel environment, movement of the transportation vehicle, and food and water sources that differ from those in the animal’s previous environment for logistical reasons inhibit food and water consumption. However, animals lose weight more rapidly when transported than they would normally during the same period without feed and water. That consequence implies that transportation is stressful for reasons beyond the lack of feed and water.
Provision of feed or water during transportation can be problematic because of food spoilage and water spillage; wetting of the floor by spilled water, which results in chilling, slipping, and injuries; animals’ lack of ability to eat or drink while in motion; motion sickness; and lack of motivation to eat or drink during the trip. Thus, providing food or water may not be of any benefit during short trips because of lack of motivation to consume food and water. Provision of feed and water during very long trips requires special attention, especially if the vehicle stops or has periods of stability during which animals may seek food and water. In
TABLE 3-5 Space Allowances for Group-Transported Animalsa
|
Species |
(lb) |
(kg) |
(ft2) |
(m2) |
Source |
|
Mice |
0.053 |
0.024 |
0.09 |
0.008 |
Harlanb |
|
Mice |
0.055 |
0.025 |
0.04 |
0.004 |
Jackson Laboratoriesb |
|
Mice |
0.075 |
0.034 |
0.10 |
0.009 |
Harlanb |
|
Mice |
0.077 |
0.035 |
0.07 |
0.006 |
Charles Riverb |
|
Gerbils |
0.077 |
0.035 |
0.08 |
0.007 |
Charles Riverb |
|
Gerbils |
0.110 |
0.050 |
0.11 |
0.010 |
Charles Riverb |
|
Gerbils |
0.132 |
0.060 |
0.13 |
0.012 |
Charles Riverb |
|
Gerbils |
0.154 |
0.070 |
0.18 |
0.017 |
Charles Riverb |
|
Rats |
0.110 |
0.050 |
0.13 |
0.012 |
Charles Riverb |
|
Rats |
0.110 |
0.050 |
0.10 |
0.009 |
Taconicb |
|
Rats |
0.163 |
0.074 |
0.16 |
0.015 |
Harlanb |
|
Rats |
0.165 |
0.075 |
0.16 |
0.015 |
Charles Riverb |
|
Rats |
0.165 |
0.075 |
0.11 |
0.010 |
Taconicb |
|
Rats |
0.218 |
0.099 |
0.19 |
0.018 |
Harlanb |
|
Rats |
0.220 |
0.100 |
0.21 |
0.019 |
Charles Riverb |
|
Rats |
0.220 |
0.100 |
0.12 |
0.011 |
Taconicb |
|
Rats |
0.273 |
0.124 |
0.22 |
0.020 |
Harlanb |
|
Rats |
0.276 |
0.125 |
0.27 |
0.025 |
Charles Riverb |
|
Rats |
0.276 |
0.125 |
0.13 |
0.012 |
Taconicb |
|
Rats |
0.328 |
0.149 |
0.25 |
0.023 |
Harlanb |
|
Rats |
0.331 |
0.150 |
0.30 |
0.028 |
Charles Riverb |
|
Rats |
0.331 |
0.150 |
0.15 |
0.014 |
Taconicb |
|
Rats |
0.384 |
0.174 |
0.29 |
0.027 |
Harlanb |
|
Rats |
0.386 |
0.175 |
0.18 |
0.017 |
Taconicb |
|
Rats |
0.441 |
0.200 |
0.33 |
0.031 |
Charles Riverb |
|
Rats |
0.494 |
0.224 |
0.35 |
0.032 |
Harlanb |
|
Rats |
0.505 |
0.229 |
0.43 |
0.040 |
Harlanb |
|
Rats |
0.551 |
0.250 |
0.44 |
0.041 |
Charles Riverb |
|
Rats |
0.551 |
0.250 |
0.24 |
0.023 |
Taconicb |
|
Rats |
0.606 |
0.275 |
0.29 |
0.027 |
Taconicb |
|
Rats |
0.661 |
0.300 |
0.53 |
0.050 |
Charles Riverb |
|
Rats |
0.717 |
0.325 |
0.36 |
0.034 |
Taconicb |
|
Rats |
0.882 |
0.400 |
0.67 |
0.062 |
Charles Riverb |
|
Rats |
0.882 |
0.400 |
0.49 |
0.045 |
Taconicb |
|
Rats |
0.992 |
0.450 |
0.89 |
0.083 |
Charles Riverb |
|
Hamsters |
0.110 |
0.050 |
0.11 |
0.010 |
Charles Riverb |
|
Hamsters |
0.132 |
0.060 |
0.13 |
0.012 |
Harlanb |
|
Hamsters |
0.176 |
0.080 |
0.13 |
0.012 |
Charles Riverb |
|
Hamsters |
0.220 |
0.100 |
0.15 |
0.014 |
Harlanb |
|
Hamsters |
0.287 |
0.130 |
0.17 |
0.015 |
Harlanb |
|
Guinea Pigs |
0.549 |
0.249 |
0.27 |
0.025 |
Harlanb |
|
Guinea Pigs |
0.769 |
0.349 |
0.33 |
0.031 |
Harlanb |
|
Guinea Pigs |
0.772 |
0.350 |
0.27 |
0.025 |
Charles Riverb |
|
Guinea Pigs |
1.210 |
0.549 |
0.44 |
0.041 |
Harlanb |
|
Guinea Pigs |
1.323 |
0.600 |
0.44 |
0.041 |
Charles Riverb |
|
Guinea Pigs |
1.764 |
0.800 |
0.53 |
0.050 |
Charles Riverb |
|
Species |
(lb) |
(kg) |
(ft2) |
(m2) |
Source |
|
Rabbits |
7.915 |
3.59 |
1.44 |
0.134 |
Harlanb |
|
Swine |
10.00 |
4.54 |
0.70 |
0.065 |
Whiting and Brandt, 2002 |
|
Swine |
20.00 |
9.07 |
0.90 |
0.084 |
Whiting and Brandt, 2002 |
|
Swine |
30.00 |
13.60 |
1.00 |
0.093 |
Whiting and Brandt, 2002 |
|
Swine |
50.00 |
22.70 |
1.50 |
0.139 |
Whiting and Brandt, 2002 |
|
Swine |
60.00 |
27.20 |
1.70 |
0.158 |
Whiting and Brandt, 2002 |
|
Swine |
70.00 |
31.20 |
1.80 |
0.167 |
Whiting and Brandt, 2002 |
|
Swine |
80.00 |
36.30 |
1.90 |
0.177 |
Whiting and Brandt, 2002 |
|
Swine |
90.00 |
40.80 |
2.10 |
0.195 |
Whiting and Brandt, 2002 |
|
Swine |
100.00 |
45.40 |
2.20 |
0.204 |
Whiting and Brandt, 2002 |
|
Swine |
110.00 |
49.90 |
2.30 |
0.214 |
Whiting and Brandt, 2002 |
|
Swine |
120.00 |
54.40 |
2.50 |
0.232 |
Whiting and Brandt, 2002 |
|
Swine |
130.00 |
59.00 |
2.60 |
0.242 |
Whiting and Brandt, 2002 |
|
Swine |
140.00 |
63.50 |
2.80 |
0.260 |
Whiting and Brandt, 2002 |
|
Swine |
150.00 |
68.00 |
2.90 |
0.269 |
Whiting and Brandt, 2002 |
|
Sheep (Full Fleece) |
60.00 |
27.00 |
2.20 |
0.210 |
FASS, 1999 |
|
Sheep (Full Fleece) |
80.00 |
36.00 |
2.60 |
0.240 |
FASS, 1999 |
|
Sheep (Full Fleece) |
100.00 |
45.00 |
3.00 |
0.270 |
FASS, 1999 |
|
Sheep (Full Fleece) |
120.00 |
55.00 |
3.40 |
0.310 |
FASS, 1999 |
|
Calves |
200.00 |
91.00 |
3.50 |
0.320 |
FASS, 1999 |
|
Calves |
300.00 |
136.00 |
4.80 |
0.460 |
FASS, 1999 |
|
Calves |
400.00 |
182.00 |
6.40 |
0.570 |
FASS, 1999 |
|
Calves |
600.00 |
273.00 |
8.80 |
0.800 |
FASS, 1999 |
|
Cattle (Horned) |
800.00 |
364.00 |
10.90 |
1.000 |
FASS, 1999 |
|
Cattle (Horned) |
1,000.00 |
455.00 |
12.80 |
1.200 |
FASS, 1999 |
|
Cattle (Horned) |
1,200.00 |
545.00 |
15.30 |
1.400 |
FASS, 1999 |
|
Cattle (Horned) |
1,400.00 |
636.00 |
19.00 |
1.800 |
FASS, 1999 |
|
aMore space may be given during transportation than is listed, but more floor space increases the risk of animal injury. More space per animal is needed in warm weather and during long trips (over 48 hr; FASS, 1999). Space allowances are to be tempered with professional judgment to accommodate strains, species, thermal conditions, special models, and protocol requirements. bSpace allowances calculated from caging density and cage specification data available in corporate literature. |
|||||
cases where an animal may refuse food because it is presented in a novel form or source, animals should be adapted to the travel and post-travel diets and to feed and water dispensers before travel. Exposure to the food forms and water sources that will be used during travel before the trip may help to reduce dehydration and weight losses during transportation.
Small animals (young animals or small animal species of any age) can survive less time without food and water than larger animals. Water is the
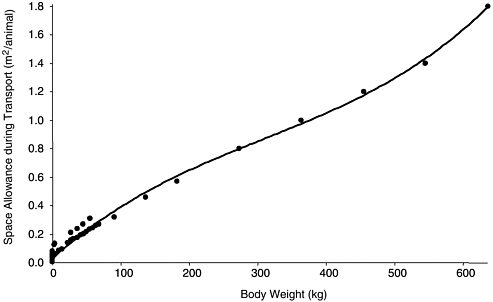
FIGURE 3-4 Space allowances during transportation. Based on transportation space allowances in Table 3-5. Second-order polynomial regression resulted in trend line (y = 8−9x3 − 8−6x2 + 0.0043x + 0.0302) with a coefficient of determination (r2) of 0.9929.
most important consideration for trips of intermediate length for most species. Small animals lose more heat, require more calories per unit of body mass, and become dehydrated more quickly than larger animals. Schlenker and Muller (1997) identified the duration of water and food deprivation as factors in the high mortality of air-shipped chicks. Post-hatching metabolic changes and physical development of chicks exacerbate the development of pathological conditions.
In most cases, small animals (less than 1 kg) will require a source of food and water during transportation that lasts more than a few hours. Several commercially available gel moisture sources have been developed to provide an alternative to the use of water bottles during transportation (Maher and Schub, 2004). These gel moisture sources provide uniform, spill-proof, and contamination-free hydration for rodents; however, they are not nutritionally complete, and a food source should also be utilized during transportation. Xin and Lee (1996) found that the provision of water (or a substitute) and feed were also important for sustaining male day-old chicks during long trips (experiments were conducted under simulated conditions for a duration of 72 hr).
Larger animals can go longer without food or water without ill effects. Studies indicate that only after 24 hr of road transportation does a lack of water and physical fatigue become detrimental to cattle welfare (Knowles et al., 1997; Tarrant and Grandin, 2000). Cattle are typically fasted for 6 to 12 hr before transportation (Lapworth, 2004), and this state must be considered when assessing the physical condition of cattle during transportation. The primary reason for fasting is to limit manure accumulation in the trailer and thus prevent slipping and falling.
Horses can also experience dehydration after 24 hr of transportation (Friend, 2000; Friend et al., 1998; Stull and Rodiek, 2000). Friend et al. (1998) found that horses transported for long distances during hot weather drank less water (20.9 L) than horses penned under similar conditions (38.2 L). In a later study, Friend (2000) found that respiration, heart rate, blood sodium, osmolality, and chloride were significantly higher in nonwatered horses after 30 hours of transportation in hot conditions (indicating dehydration), than in horses that had received water during similar transport. However, offering of water to horses transported under cool conditions appeared to result in no added benefit to their well-being. Stull and Rodiek (2000) assessed the condition of show horses transported in a commercial van during the summer. They, too, concluded that after 24 hr of transportation, horses begin to show changes in physiological markers of hydration. In general, it appears that horses in good physical condition can be safely transported in hot weather for at least 24 hr, when provided with water.
Estimating the amounts of food and water that should be placed in the enclosure during transportation is relatively simple. Initially, the caloric and water requirements of the species must be determined. That information can be found in the Nutrient Requirements of Domestic Animals series, a group of reports from the National Academies that cover farm animals, laboratory species, wildlife, and companion animals. When the minimal requirements have been determined, several other factors must be considered, including (Wallace, 1976):
-
Expected duration of the journey;
-
Initial weight and life stage of the animal (for example, caloric and water requirements vary with age);
-
Special requirements of the species or strain of animal (for example, some transgenic animals may have altered nutritional or caloric requirements); and
-
Expected environmental conditions (for example, animals may consume more water in low-humidity environments).
SOCIAL INTERACTION AND GROUP TRANSPORTATION
Animals can be transported in individual or group enclosures (caging or vehicles). Isolation, such as during transportation, can minimize social stress in solitary animals and species, but isolation can induce stress in social species (Tamashiro et al., 2005). New social groups of nonsocial animals constitute a stressor, and these animals should be transported individually. Socially dominant pigs are less adversely influenced by the stress of transportation than are socially intermediate or submissive pigs (McGlone et al., 1993). Some animals, particularly large ones, are aggressive and are best transported alone or with conspecifics in sensory but not physical contact. Baldock and Sibly (1990) found that spatial isolation (4 to 90 m) alone did not have a substantial effect on heart rate in sheep, but that visual isolation produced a substantial increase in heart rate, vocalization, and activity within the first 5 min of treatment.
For prey species such as sheep, being shipped near a predator species such as a dog is especially stressful and should be avoided. In contrast, having familiar conspecifics in the same compartment reduces the stress of a new experience. Most laboratory and farm animals are social animals, and they are often housed in compatible social groups at the site of trip origin. If social groups are transported, it is recommended that the groups be established before transportation where appropriate so that dominance orders will not need to be established during or after transportation. However, it has been found that rats adapt quickly to unfamiliar social environments (Sharp et al., 2005) and unfamiliar social environments have no negative effect when chickens travel together in the same shipping enclosure (Knowles and Broom, 1990). The performance standard for social interaction is the lack of social aggression and injury resulting from aggressive social interactions.
HANDLING
An animal’s experience can greatly affect its response to the transportation environment. Animals can be preconditioned to transportation by being exposed to the transportation container and the food or water that will be available during transportation. In addition, frequent human handling before the handling associated with transportation will help animals to respond better to the transportation experience. Animals that have been socialized with people and have been handled respond more favorably to the handling associated with transportation than those not similarly exposed. In many cases, preconditioning animals to handling already occurs as part of routine husbandry procedures. For example, rodents are often handled on a daily or weekly basis in breeding and
research facilities; additional handling to precondition the animals to transportation handling is probably not necessary.
Many species of research animals are typically handled and then caged for transportation, and this practice can produce an additive stress effect of both the handling and the novel enclosure. Although an animal’s stress response to human handling associated with transportation may not be completely ameliorated, the method of handling can reduce or exacerbate the stress response. Kannan and Mench (1996) demonstrated substantial physiological response differences between methods of handling of laying hens. Either birds were captured and then held and carried inverted (single carry or multi-bird carry) or single birds were captured and then held upright and carried gently. When compared with unhandled controls, both methods produced an alarm response. However, gentle upright handling yielded a lower response than inverted handling. Kannan and Mench (1996) also found that caging of the birds produced a powerful fear response. Capture, carrying, and caging were found to be less stressful to chickens when conducted under low light (Knowles and Broom, 1990).
The activity of horses during road transportation can contribute to the increased incidence of injury or stress. The physiological responses of horses to head restraint (cross-tied vs. loose) after 24 hr of transportation were measured by Stull and Rodiek (2000). Cross-tied horses had higher blood glucose and cortisol concentrations, neutrophil-to-lymphocyte ratios, and white blood cell counts than horses traveling loose in small compartments. The authors recommended that horses be allowed to travel loose during long periods of transportation.
MONITORING TRANSPORTATION
For facilities or people that transport large numbers of animals, the quality of transportation can be monitored by tracking mortality, morbidity, and injury during transportation and comparing these measures with published data. For instance, Malaga et al. (1991) reported that in-transit mortality for air transport of owl monkeys (Aotus nancymai) was 0.67% and total mortality at the end of a 30-day observation period was 2.44%. When mortality, morbidity, or injury exceed published norms (for instance, exceeding 2 standard deviations from the mean), action should be taken to adjust protocols or provide training. If small numbers of animals are occasionally transported, careful attention should be paid to ensuring that a reputable shipper is used and that the entire trip is adequately planned to transfer the animals smoothly from consignor to carrier, shipper, and consignee.
EMERGENCY PROCEDURES
Emergencies may occur during any phase of the shipping process. During the peritrip period, emergencies encountered have included extended delays before the start of long trips, exposure to extreme temperatures, animal escapes, and mechanical problems with transportation vehicles.
To ensure animal comfort and safety, all plans for animal shipments must include instructions for emergency responses in accordance with the mode of transportation used. For example, Appendix B of the International Air Transport Association Live Animals Regulations contains a section covering emergency responses. The section provides a summary of actions appropriate to emergency situations, including delays, container damage, escapes, illness, and segregation. It is important that when an emergency occurs, those directly involved with the transport of the animals (the shipper and the organization and individual(s) providing transport) need to be able to contact each other and the means of contact be established prior to transport. Planning must also include procedures to follow in the event of an emergency. Both a primary plan and a backup plan should be available for each phase of the trip. For example, if animals are to be transported by plane or truck and a mechanical problem causes a long delay, animal needs must be accommodated to avoid tragedy. Animals should not remain unprotected from extreme weather for more than a few minutes, and comfortable accommodations should be available.
In rare circumstances, a situation may arise in which it must be determined whether euthanasia of an animal is necessary. For example, an animal might become moribund during transportation, or might endanger the safety of the human handlers, as can happen if a horse becomes uncontrollable during a flight and kicks at the aircraft’s doors. A part of the emergency procedure plan should document specifics that identify which persons are trained and qualified to make and carry out decisions (usually a veterinarian) and the methods and equipment to administer anesthesia or perform euthanasia safely in the transportation situation.
PERSONNEL TRAINING
Personnel who handle animals must be properly trained in routine and emergency procedures for the species they handle. Training should include procedures applicable to the mode of transportation and should cover at least
-
Shipper and carrier responsibilities;
-
Inspection of primary enclosures;
-
Documentation;
-
Acceptance, handling, and delivery;
-
Loading and off-loading procedures and precautions;
-
Operator and government regulations; and
-
Emergency procedures.
Personnel must also be trained in species-specific husbandry and environmental requirements of animals. They can be deemed competent when they possess, as appropriate to the species and mode of transportation, the following:
-
Ability to recognize when an animal becomes ill or unfit for transport;
-
Ability to recognize signs of stress and alleviate the cause, if possible;
-
Knowledge of how to contact and interact with local emergency personnel, including veterinarians who have skills in the treatment of injuries; and
-
Knowledge of the administration of veterinary drugs and methods of euthanasia.
Personnel must also be trained to recognize physiological signs that a problem is developing in a particular animal or group of animals. The signs may include
-
Increased respiratory rate (in warm weather);
-
Excessive sweating (in species that sweat during warm weather);
-
Excessive shivering or huddling (in cool weather);
-
Aggressive interactions and injuries associated with fighting;1
-
Excessive weight loss;1 and
-
Dehydration.1
At least one person associated with each segment of the trip should be fully trained. Employers should provide training (initial and recurrent) for employees with respect to transportation of animals so that their employees will be able to ensure the safety of animals and of their own equipment and can explain to shippers the conditions under which animals are transported. Personnel that simply move containers into, out of, or between conveyances also must have at least minimal training to recognize potentially unsafe conditions (for the animal or the handler) and to know whom to contact in case of questions or problems.
An American Veterinary Medical Association animal air-transportation study group evaluated, on a national basis, the adequacy of employee training as related to the protection of dogs and cats in air transportation (AVMA, 2002). The group found that although initial training was adequate for all of the airlines, continuing education and education of contractors were inconsistent. The group recommended the establishment of a formal training program that would incorporate:
-
A time line for recurrent training;
-
A consistent standard and frequency of training for ground handling staff, especially for outside contractors that are used more frequently by smaller airports; and
-
A standard training program to minimize the amount of informal on-the-job training and thereby avoid omission of important considerations for safe animal care and transportation; this would also minimize delays in training during staff turnovers.