5
Recommendations
Preceding chapters have provided background regarding the laws, regulations, and regulatory agencies responsible for animal transportation (Chapter 2); principles that underlie good practices of animal handling, management, and care essential to maintaining animal comfort, health, and well-being during and immediately after transportation (Chapter 3); and concepts and principles related to preventing exposure of the general public, transported animals, animal handlers, and animal colonies to infectious organisms (Chapter 4). These factors provided the foundation for the following four recommendations.
Recommendation 1: The National Institutes of Health (NIH), through the National Center for Research Resources, should update and reimplement the National Primate Plan to ensure a stable means for transporting nonhuman primates into and within the United States. In addition, research institutions that use nonhuman primates should encourage the development of reliable ground transport for nonhuman primates to protect against the possibility that domestic transportation of nonhuman primates on commercial airlines may one day become unavailable.
For US research institutions, there are several sources for obtaining nonhuman primates for use in biomedical research. Sources include overseas breeding colonies, National Primate Research Center (NPRC) breeding colonies, breeding colonies at academic institutions, and US commercial breeding colonies, with overseas breeding colonies supplying the majority
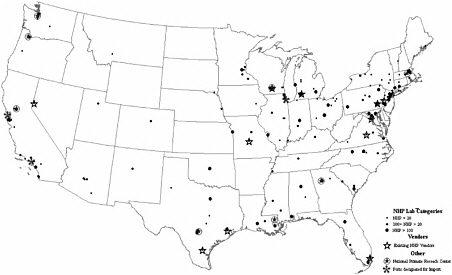
of nonhuman primates (Robinson and Beattie, 2003). Therefore, the majority of nonhuman primate resources in the United States must be transported by air, both into the United States and generally to their final destination (see Figure 5-1 for the locations of research facilities, importation sites, and vendors of nonhuman primates). Currently, few foreign airlines will consistently transport nonhuman primates into the United States, and no US domestic airlines will transport nonhuman primates into the United States. For the purpose of transporting nonhuman primates once they are already in the United States, research institutions may use the one US domestic airline that currently transports nonhuman primates nationally (Kemnitz, J. personal communication, August 4, 2005).
The paucity of carriers that transport live animals is due, in part, to the often unprofitable nature of live-animal transportation in the air-transportation industry. For typical passenger/cargo-configured network carriers, such as the major airlines present in the US market, cargo contributes only 2 to 5% of total operating revenues. The contribution of live-animal transportation is even less significant and involves costs for personnel training and environmental controls to comply with federal regulation. In addition to the economic disincentives discussed above, several other reasons have been identified for the declining number of commercial airlines that will transport nonhuman primates, including the following:
-
concern regarding zoonoses between nonhuman primates and humans;
-
the high cost of training personnel and acquiring protective equipment;
-
potentially higher insurance rates due to liability issues;
-
required disinfection of cargo areas after holding nonhuman primates;
-
pressure from animal rights activists; and
-
the potential danger associated with escapes (DePoyster, 2003).
There is no clear solution to the foreign commercial airline situation. Some organizations have started chartering private jets to import shipment of nonhuman primates to the United States (DeMarcus, 2003). This option increases the cost of transportation (DeMarcus, 2003), and private chartering companies are not immune to the pressures that led the commercial airlines to refuse to transport nonhuman primates. Though corporate research institutions have some ability to cover the cost of private charters, academic researchers utilizing nonhuman primates have no mechanism for absorbing the increased cost of transportation by private charters. In the committee’s judgment, the practical approach is to pre-
pare for the eventuality that all nonhuman primates may have to be transported into the US by private charter. To prevent the financial impact of private charters from restricting research involving nonhuman primates, it is imperative for agencies that fund research using nonhuman primates to ensure that allowances are made for the increased costs associated with private chartering.
In some ways, the domestic air transportation system is more likely to reach a crisis point because currently only one domestic airline will transport nonhuman primates within the United States. A similar situation arose several years ago, when US airlines refused to carry day-old chicks and adult avian species via airmail transport. As a result, Congress enacted Public Law 107-67, the Treasury and General Government Appropriations Act of 2002. Section 651 of this law amended Section 5402(c) of Title 39 so that “the Postal Service may require any air carrier to accept as mail shipments of day-old poultry and such other live animals as postal regulations allow to be transmitted as mail matter.” While legislative action by Congress would virtually eliminate the domestic air transportation problem, that solution is perhaps the least likely to occur, and the committee felt it imperative to provide other pathways to improve the transportation of nonhuman primates.
Some individuals in the academic research community believe that the NPRC breeding colonies will eventually be able to meet the domestic need for nonhuman primates, eliminating the need to transport non-human primates into the United States in large numbers. The source of this assumption is the National Primate Plan (DHEW, 1978). The National Primate Plan was published in 1978 by the Interagency Primate Steering Committee, which included representatives from the National Science Foundation, Department of Defense, Environmental Protection Agency, Veterans Administration, and Department of Health, Education, and Welfare. The National Primate Plan was developed to ensure adequate supplies of primates to meet research needs by coordinating the various federal program activities. One of the recommendations of the National Primate Plan was that domestic primate production be sufficiently expanded to ensure a continuous, stable, and long-term supply of primates and that domestic production must provide for all of the nation’s need for commonly used species, specifically rhesus and cynomolgus macaques. At the time, the Plan’s authors estimated that domestic production was fulfilling about 50% of the domestic need. Since 1980, imported nonhuman primates have comprised, on average, 26% of the nonhuman primates used in research. However, the percentage of nonhuman primates used in research that are imported has steadily increased over the last seven years and, in 2004, imported nonhuman primates comprised 35% of nonhuman primates used in
research.1 This is a cause for concern, as the NPRC’s facilities are limited and being utilized to the maximum (Robinson and Beattie, 2003).
Considering that reliance on imported nonhuman primates is increasing and that the NPRCs are functioning at maximum capacity, it is unlikely that the US research community’s demand for imported nonhuman primates will decrease. Moreover, the mission of the NPRCs is to provide researchers with access to nonhuman primates, not to supply nonhuman primates directly to researchers. Any research using NPRC animals must be conducted at an NPRC, and NIH-funded research projects take precedence over activities funded by other resources (NIH, 2002). The NPRC breeding colonies are not intended to supply nonhuman primates to other academic or corporate research institutions and there is no indication that their mission will be revised. In the committee’s judgment, the most promising solution for permanently addressing the declining availability of transport services is to update and reimplement the National Primate Plan. An updated National Primate Plan could ensure allowances for increased costs associated with domestic private chartering where necessary and relaunch the nation’s efforts to develop domestic production of the most commonly used nonhuman primates to meet national needs.
Recommendation 2: NPRCs and research institutions utilizing non-human primates should work jointly, perhaps through professional societies, to encourage the development of reliable ground transportation for nonhuman primates.
Most producers of small research animals have reacted to the declining availability of economical domestic airline services by developing systems for ground transport of their research animals either by utilizing their own personnel and vehicles or by contracting with independent ground transport companies. Ground transport of nonhuman primates does seem to occur, but is not a widespread occurrence, possibly due to economic constraints and because most ground transport companies are geared toward the transportation of rodents and other small mammals. It would be prudent for the NPRCs and individual research institutions to work jointly, perhaps through professional societies, to encourage the development of reliable ground transport for nonhuman primates to protect against the possibility that domestic transportation of nonhuman primates on commercial airlines may one day become unavailable.
Recommendation 3: Federal agencies that fund nonhuman-primate research and the commercial shipping community should coordinate an initiative to develop a self-contained overshipper to ship non-human primates and other animals that pose a significant risk of zoonotic exposure.
Recommendation 3 is directed to the possibility of cost-effectively shipping nonhuman primates and other infectious animals in standard primary enclosures that would be loaded into closed devices (overshippers). Overshippers could be built in such a fashion as to incorporate HEPA filters, temperature and humidity controls, viewing windows, and outside access. An overshipper with these characteristics would minimize or eliminate exposure to or contact between humans and animals, as well as between animals in the same conveyance, while providing an escape-proof and safe environment. Government agencies, shippers, inspectors, handlers, and carriers alike might prefer the limited human intervention, reduced zoonotic exposure, and convenience this method would provide. Because it is unlikely that the airline industry itself would be in a position to pay for the development of such units, the cost must be borne by the shipping and animal research communities at large. The resulting improvements in terms of safety, security, and convenience may encourage more airlines to transport nonhuman primates and other species that pose similar problems.
Recommendation 4: An interagency working group should be established to coordinate all federal inspection and permitting activities related to transportation of animals and animal products under one entity. In addition, the individual agencies should move to clarify the language of federal regulations or issue guidance documents to increase the readability and understanding of those regulations.
As discussed in Chapter 2 (and diagrammed in Table 2-1), five different federal agencies oversee various aspects of the transportation of research animals and their products. The USDA provides inspection oversight to ensure the welfare of animals during transportation, the Fish and Wildlife Service issues permits and inspects shipments to control the movement of wildlife and conserved species, the CDC registers and inspects shipments to control the introduction of zoonotic disease through imports, the Food and Drug Administration (FDA) performs similar functions to control the intrastate and interstate spread of zoonotic diseases, and the Department of Transportation issues permits to control hazards presented by the transportation of materials such as infectious live animals or radioactive animal products. Due to overlapping regulatory authority, it is possible for one shipment of animals to be inspected by two different
agencies and be required to register or obtain permits from three or more agencies. This overlap presents a significant regulatory burden to individual investigators trying to navigate this complex network of regulatory authority. Establishing one federal entity that can be consulted would greatly reduce regulatory burden for shippers, whether they are individual investigators or large commercial operations. Such a working group also has the potential to centralize and reduce the number of redundant inspections and permits that must be issued for each shipment.
There is also substantial room for improving the comprehensibility of the various federal regulations. The committee was particularly concerned with areas of inconsistency and obscurity in the Animal Welfare Act (AWA) regulations, which are the federal regulations that establish standards for animal welfare that apply to most species of research animals during transportation. The AWA regulations are organized by groups of common species: 9 CFR 3 subpart A details standards for the transportation of dogs and cats, subpart B covers guinea pigs and hamsters, subpart C covers rabbits, subpart D covers nonhuman primates, etc. In this way, species with similar physiology and behavioral characteristics are regulated by the same standards. While differences between species account for the majority of differences in standards between species groupings, there are several cases of inconsistencies that have no apparent underlying scientific reason. For example, the regulations pertaining to dogs and cats state that the cargo area must be pressurized when flying above 8,000 ft (9 CFR 3.15(d)). There is no similar requirement for nonhuman primates, rabbits, guinea pigs, or hamsters. Another example involves acclimation of guinea pigs. For all species besides guinea pigs, there is a provision in the AWA regulations for transporting animals in temperature conditions that fall below the minimum allowed temperature if the animal has been acclimated to lower temperatures as certified by a USDA-accredited veterinarian (e.g., 9 CFR 3.13(e)). However, no such provision exists for guinea pigs, though there is literature suggesting that guinea pigs can be successfully cold-acclimated (Sobel et al., 1960, 1965; Vapaatalo et al., 1984).
There are also a number of inconsistencies in standards that apply to the same animal. For example, for nonhuman primates, the temperature in a terminal facility cannot fall below 45°F for more than four consecutive hours (9 CFR 3.91 (d)), but the temperature during handling (movement into or out of a terminal facility or conveyance) cannot fall below 45°F for more than 45 minutes (9 CFR 3.92(a)(3)). During air transport, the ambient temperature inside the plane must be maintained at a level that ensures the welfare of the nonhuman primate (9 CFR 3.88(d)); however, during surface transportation, the temperature must be maintained between 45°F and 85°F (9 CFR 3.88(e)).
There are also instances in the Animal Welfare Regulations where there are inconsistencies in reporting and documentation. For example, the certification that must accompany dogs, cats, and nonhuman primates must include the name, address, and telephone number of the consignee (9 CFR 3.13(b) and 9 CFR 3.86(b)); however, this information does not have to be included in the certification that must accompany rabbits, guinea pigs, or hamsters. While inconsistencies in reporting and documentation are unlikely to affect the welfare of the animal directly, they make compliance with AWA regulations onerous, thus diminishing their effectiveness.
Though the committee had greater concerns about the AWA regulations because they directly impact the welfare of the animals, the committee found that all of the federal regulatory language lacked clarity. This makes it difficult to determine which federal agencies would oversee a particular transportation situation. In particular, it is exceedingly difficult to determine which federal regulations apply to the transportation of animals or animal products that contain a known or suspected zoonotic/ infectious agent, whether naturally occurring or experimentally induced (see Table 2-1). Federal agencies should work to clarify these issues, either through the development of an interagency working group or the issuance of guidance documents by the individual agencies.
Recommendation 5: Shipments of research animals between institutions should be coordinated between responsible individuals at both the sending and receiving institutions.
In the committee’s judgment, instances of mortality, morbidity, and adverse effects on animal health occur most frequently when individual investigators unfamiliar with the vagaries of the research animal transportation system ship animals to investigators at other institutions. Such problems can be avoided if each institution designates a single individual responsible for ensuring the safe shipment and receipt of research animals. This designated individual would ensure that communication between institutions is effective at all stages of the transport process; that all documentation including health certifications required by the receiving institution and all federal, state, and local permits are in order; and that animals are placed in shipping containers according to the appropriate standard. This designated individual would further ensure that USDA-certified carriers are utilized and that, upon receipt, the consignee is notified and places the animals in the appropriate level of biocontainment/quarantine before introduction into the laboratory colony. Though institutional animal care and use committees are not required to directly review each instance of animal transport, transport activities should be reviewed during the semiannual program evaluation to ensure that appropriate procedures are being followed (Silverman et al., 2000).