5
Afternoon Breakout Sessions with Invited Speakers
DEVELOPING AND TESTING MODELS OF SURVIVORSHIP CARE
Moderator: Patricia Ganz, University of California, Los Angeles
We have four individuals with us who will be discussing and representing different types of models for delivering survivorship care. There may certainly be other models that are available. Our first speaker is Steve Woolf, a member of our committee who is a primary care physician in Virginia.
Steven Woolf, Virginia Commonwealth University
I am a family physician in the Department of Family Medicine at Virginia Commonwealth University. Let me begin by introducing the three models of survivorship care that were discussed in the report: shared care, nurse-led care, and survivorship follow-up clinics. I am going to focus mainly on the first one, but I want to set the stage for the discussions that will follow. The following figure from data reported in the IOM report shows the number of cancer-related physician office visits, by specialty, estimated from the National Ambulatory Medical Care Survey (NAMCS) (Figure 5-1). One of the points that it makes is of the 36.6 million physician office visits made for cancer care, nearly one-third (32 percent; 11.7 million visits) are made to primary care providers. That is the perspective I am trying to represent.
From the perspective of the primary care physician, however, these 11.7 million cancer-related visits made by adults are a very small fraction of
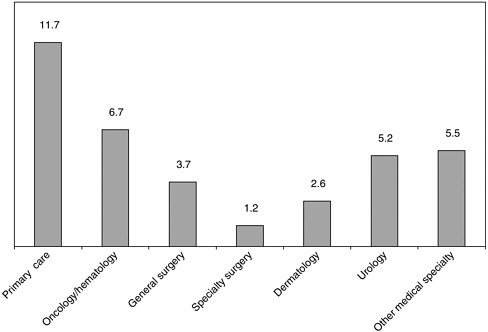
FIGURE 5-1 Cancer-related physician office visits made by adults (ages 25 and older), by specialty, United States, 2001-2002 (average annual, in millions).
their practices. There were 558.4 million visits made to primary care physician offices in 2002 (all ages), so again, the relative proportion of visits that are designated in NAMCS as cancer-related visits is small. The point of showing this contrast is to say that there is a larger holistic perspective that occurs in primary care. This notion of shared care is very prevalent across the spectrum of health conditions that primary care providers care for, not just cancer. Primary care providers report that other physicians share care for the patient’s problem in nearly one-in-five visits (18 percent) (Woodwell and Cherry, 2004).
We talk about shared care in the report and at this meeting as a new, evolving idea. How exactly would it work? Are the delivery systems available to do it? Whose role would it be to handle which aspects of care? Is the primary care provider capable of dealing with it? These issues are well traveled territory in the primary care world, because it is already done for a wide variety of conditions. There is a regular relationship of shared care between primary care providers and specialists that often works very well and has been in place for many years. In the management of coronary artery disease, primary care physicians and cardiologists work together regularly in well-coordinated systems. There are lots of exceptions, and I
am not saying that we never have problems in those areas, but this is not uncharted territory. The management of diabetes is very complicated and involves multiple different care providers and different areas of expertise. Epilepsy, neurologic disorders, Alzheimer’s disease, bipolar disorder, and end stage renal insufficiency—these are all conditions that primary care providers regularly care for in shared care relationships with specialists. These conditions obviously differ in important ways from cancer care and from survivorship care, but nonetheless involve very serious diseases that are chronic, recurring, potentially life threatening, and complicated.
What is the ideal arrangement under shared care that we aspire to, whether it is for cancer care or for these other conditions? Looking at it from the perspective of primary care, the first goal is to address all physical and emotional needs. The primary care provider’s responsibility is to deal not just with the specific condition, but the totality of conditions, both physical and emotional that the patient is facing. In the context of cancer survivorship it is not just taking care of their cancer needs, and the late effects, and other consequences of their cancer treatment. It is taking care of their renal insufficiency, chronic obstructive pulmonary disease, depression, family violence issues, and all the other things that occur in primary care.
Primary care providers also assume responsibility for chronic care needs that are feasible. Primary care physicians can only do so much, both in terms of their knowledge base and in terms of what is possible in their busy office visits. The role of the primary care physician is to do what is possible and then coordinate with other providers to handle the aspects that are not. Primary care physicians should also be referring patients to specialists for periodic evaluations and to address issues that require focused expertise. They also consult with specialists to get advice on how to deal with particular problems that are outside their knowledge.
Ideally, that is the way it ought to work on the primary care side. The way it ought to work on the side of the cancer team is to provide guidance. Based on what is going on with the patient, they should see the patient and provide specialized treatment as needed. They need to keep the primary care clinician informed of the treatment plan. That is where the whole notion behind the survivorship care plan comes in. The cancer team also needs to return the patient to primary care for implementation of the plan and for care of other health needs. This is something that people disagree with as being part of the ideal, but my bias is that it is ideal to return patients to primary care.
The challenges to achieving this ideal are front and center in our IOM report. One is that both the primary care folks and the cancer team have to have a common understanding of the expected components of care. Roles need to be clear, and everyone needs to be on the same page about who is responsible for what. There needs to be a common play book. The absence
of clear, consistent guidelines that was discussed this morning is problematic in this regard. Without them, the primary care clinician and the specialist may not necessarily agree on what ought to be done.
There needs to be clear communication between the cancer specialist and the primary care clinician. I hear oncologists say that they regularly communicate with the primary care clinician and vice versa. There are cases where that actually happens, but unfortunately not often enough, and not where I practice. There needs to be confidence in what the primary care clinician can do. In some settings, there is undue skepticism about the capability of primary care clinicians to handle certain things, when in fact they regularly deal with very complicated diseases on a daily basis.
There also has to be clarity about what primary care clinicians cannot do. What are the limits of their knowledge? What are the limits of their capabilities? The primary care clinician needs to know what is realistic, what is feasible, and what one’s limits are. There also must be an understanding among specialists of what they can count on the primary care provider to do. Finally, there has to be a supportive infrastructure within the healthcare system that facilitates the transfer of information. This is where we need electronic health records and other changes in our system of care to overcome some of the gaps.
Dr. Linda Jacobs, University of Pennsylvania: I just wanted to ask one question. How is this actually implemented at your institution, or are you speaking more generically? How successful is it?
Dr. Woolf: First of all, this idea is not for academic medical centers or similar institutions. The world that I am representing is the community practice physician, not somebody working in a large infrastructure like an academic medical center. The way it is implemented varies, and the integrity with which it is implemented varies from setting to setting. For lots of other conditions, although perhaps not as much for cancer care as there should be, there is a clear understanding of what the roles are. It is understood what the cardiologist does, and what the endocrinologist does, and what the primary care clinician does in the management of these conditions. The details of how primary care physicians partner with specialists to manage other conditions are probably too elaborate to go into in just a few minutes. Infrastructure and good models do exist for other conditions, though.
Dr. Archie Bleyer, American Society of Clinical Oncology: I am waiting for this to happen, and the sooner, the better. I would like cancer to be at the lead, and have all of those other diseases, such as diabetes and renal failure, learning from us. You mentioned six challenges. Another challenge I would
raise is reimbursement. I have been in the private sector now for half a year and watched the practice of oncology. If reimbursement rates for those sharing the responsibility of care are unequal, the person who is reimbursed more will get the burden. Having seen that firsthand on a daily basis, I wonder how that affects implementing this model.
Dr. Woolf: That point was also made this morning. The misalignment of reimbursement and the priorities of health care is a systemic problem that is not limited to cancer. It is something we addressed in the report. It is a larger issue than cancer care. The things that will do the most good to improve the health of the population are not reimbursed accordingly. I will be paid hundreds of dollars to take off a sebaceous cyst, which has absolutely no benefit to a patient. However, I am paid a paltry amount for smoking cessation and cancer prevention. The reimbursement system in the country is askew.
Dr. Noreen Aziz, National Cancer Institute, Office of Cancer Survivorship: Thank you for an excellent presentation. When you talk about the partnership between the cancer specialist and the primary care clinician, I think we do need to acknowledge that an integral part of the partnership is the patient or the survivor. Clear communication to the survivor about who is going to do what is going to be really critical as well. I know we all acknowledge that, but it needs to be said.
Dr. Woolf: Patient-centered care is something we all espouse and emphasize, but it has to be operationalized.
Dr. Aziz: That is a big challenge.
Dr. Woolf: Yes, it is, I agree.
Dr. Ganz: You say that specialists and primary care doctors both know what is going on in the care of patients with diabetes or heart disease. Is that because there are written documents, or is it just that over time enough family practitioners have taken care of patients with diabetes that they know when it is out of their league and they should refer to the endocrinologist?
Dr. Woolf: It is both. Those conditions differ in some respects. There are much clearer guidelines about what needs to get done: How often should diabetic retinopathy screening occur? Therefore, how often do I need to send my diabetic patients to an ophthalmologist? There are clear guidelines, and it is very well known, chapter and verse, among all family physicians. Also, roles are pretty clear. When faced with a patient with acute myocar-
dial infarction (MI), or an exacerbation of their congestive heart failure (CHF), or exertional chest pain from their angina, most primary care physicians are pretty clear when it is time to engage the cardiologist, and when it is time to pick up the phone and make a call.
In the case of cancer survivorship, there is a lack of guidelines. There is a lack of an evidence base to support clear guidelines, as we noted in the report. Roles are still unclear. Specialists may be a bit uncomfortable with the primary care provider playing an active role in the management of the condition. The primary care provider is not exactly sure of the limits of the specialist’s role. Those need to be more clearly defined and articulated.
Dr. Ganz: One last question. Do you think there is a role for collaboration between professional societies? I am thinking about the fact that hormone replacement therapy (HRT) is no longer being given to the general population of women. Primary care physicians have had to learn how to use alternative approaches to HRT. Treating breast cancer survivors for menopause and osteoporosis should be very similar since they generally should not receive HRT. I can imagine that there are probably a lot of areas where the domains of survivorship care are probably already guidelined in a way in primary care. Collaborations could share that between primary care and specialties.
Dr. Woolf: John Ayanian highlighted the need to get the cancer organizations and the primary care organizations working together at the end of the last session. We have a moment of opportunity here. The release of this report is a leverage point where momentum has been created that can be carried forward at the organizational level to try to expedite that.
Dr. Ganz: Thanks very much, and thanks for your contribution. Linda Jacobs from the University of Pennsylvania is going to talk to us about the model at her institution.
Linda Jacobs, University of Pennsylvania
I am going to present a concrete example of what we are doing at the Abramson Cancer Center at the University of Pennsylvania with our Lance Armstrong Foundation Living Well After Cancer Program. I just have a few slides, so that I can then open it up to some discussion.
Our initial and ongoing funding is from the Lance Armstrong Foundation. The funding was initiated in 2001 to set up an infrastructure to develop an adult survivorship program. In designing this program, we hoped it could be a model for the development of other programs across the country. Since we began the program we have acquired some additional
funding from the NCI and the Department of Defense for a few specific research studies looking at particular issues in survivorship. This program bridges the Children’s Hospital of Philadelphia (CHOP) Survivorship Program, which has been in existence for over 20 years, with the Abramson Cancer Center. My co-director is Anna Meadows, who is sitting here in the audience. The CHOP program is staffed by a multidisciplinary team.
One of the strong aspects of our program is consistent team leadership. It helps to have one person who is 100 percent involved in the program as the director. I am a nurse practitioner in oncology, as well as primary care, and I am also a researcher and an educator. We also have very strong institutional support from the University of Pennsylvania, both financially and philosophically. We are a patient-focused program that integrates clinical care, research, and education. Believe me, it has not been easy to get this program up and running. It has really been a trial and error experience. We piloted lots of different approaches until we came upon something that actually worked for us.
What we are trying to do in our program is build upon established surveillance guidelines. We have heard a lot about the lack of evidence-based guidelines, and it is true that there are very few. There are no existing tools or guidelines for the care of adult cancer survivors, other than a few focused on treatment-related issues. However, there are some data-based and consensus guidelines for the care of children. We actually reference the Children’s Oncology Group guidelines quite a bit when we are making decisions such as whether or not someone should have a particular test based upon the treatment that they received.
Our program has several other goals. We hope to establish a standard evaluation approach for our patients. We are developing a database that includes information from a number of research protocols. We aim to disseminate our findings by collaborating with our Penn network of hospitals, which consists of hospitals within a couple hundred mile radius. We also disseminate our work through presentations and publications. In addition, we hope to collaborate with other survivorship centers down the road. Finally, we plan to serve as a model for other survivorship programs in the country.
Our program is an adult cancer survivorship program, with a focus on clinical care, research, and education. However, a relatively new component of our program includes young adult survivors of childhood cancer. We have recently developed a transition program with CHOP to refer the care of young adult survivors to us. We have patients anywhere from 21 years of age to 40 who are still being seen there. They will be seen one last time at CHOP, and then it is recommended that they move their care to us. When they come to us, they are accompanied by a summary of their care, and what treatment they received. It is usually two pages, and it is very
comprehensive. It guides us as we determine what we need to do for the patient. We, in turn, write the same type of report to send to primary care providers and the patient if they want it. We try to involve families as well as providers in the care. In many cases families do come with these patients.
Our team is multidisciplinary. We have a number of medical oncologists and advance practice nurses, as well as psychiatrists, cardiologists, and rehabilitation medicine specialists. Our advance practice nurses are all nurse practitioners, so they can bill. We also have a number of primary care providers who have an interest in survivorship working with us.
Our research is also multidisciplinary. For example, some of the primary care providers are doing research in complementary and alternative medicine. Our rehabilitation specialist does research in lymphedema. We also have an exercise physiologist working with us who is very interested in lymphedema. We have a medical geneticist working with our team looking at the genetics of testicular cancer. We also have an entire service of nutrition and psychosocial counselors. It is actually funded by a grant from another source. We refer almost all of our patients to counseling. Whether or not they go is up to them, but we recommend it. It can include families. We also recommend nutrition counseling for a number of people who have issues related to weight gain, weight loss, lipid profiles, and other problems.
What we have discovered over the last five years is that one model of care does not necessarily work. There are lots of reasons why one model will not work. I think it is institution-dependent, regionally-dependent, and patient population-dependent. We found that we have two different models. We have what we call the practice model, and then what we call the consultative model. I will just briefly go over these.
The practice model is one where we actually see the patients. We tell them that the focus of their care is disease surveillance, health promotion, and disease prevention. A number of protocols and ongoing studies are made available for patient enrollment on an optional basis. If they choose not to enroll, they are still cared for in our program.
Our focus in the area of health promotion and disease prevention is on developing an individualized risk profile largely based on the treatment that they received. It is more treatment-focused than disease-focused—mantle radiation, certain drugs, et cetera. Family history is, of course, a big component of this risk assessment. We recommend screening according to this risk profile. We are using this model with testicular cancer survivors and survivors of childhood cancers.
With such a large population of cancer survivors at our institution, we discovered that we cannot see everyone in the clinic. For example, we have a very large breast service at Penn with 10 different breast oncologists. We could not manage to see all those survivors. It was also a territorial issue, with oncologists not wanting to give up patients, and patients not wanting
to leave their oncologist. For these reasons we have developed the consultative model for our breast cancer patients, which is our largest and growing endeavor. We have a breast cancer survivor protocol which incorporates a questionnaire that elicits information about symptoms and quality of life. We have pilot tested the questionnaire with the patients and after about six months we stepped back to revise and revamp it. We are about to re-submit the protocol revision to move forward. Because of issues related to IRB approval and HIPAA, we cannot put patient information into a database at Penn, even if it is protected and coded, unless the patient has given us permission to do so. Everything that we do is consent-driven. Our research program is evolving.
Our ultimate goal is to adopt this model with each of the disease types. We hope to have a lymphoma survivor protocol, a lung cancer survivor protocol, et cetera. Personnel-wise this can be very expensive, and we do not have designated oncologists who are going to practice exclusively with cancer survivors. Instead we have collaborative practices at Penn that are oncology and nurse practitioner partnerships. We have solid tumor, breast, and bone marrow transplant teams. Each team has a group of physicians and nurses that coordinates their schedules. If we are going to open, for example, a lung cancer survivor protocol, we meet with that group of oncologists and advance practice nurses, and talk to them about our program. The infrastructure will be there, meaning the database for entering the data. We will develop the tools. Then we will say to them, “Who would like to take the lead on this?” It does not really matter whether it is a nurse practitioner or an oncologist who takes the lead as being the PI (principal investigator) on that particular protocol. Their responsibilities will be to make sure that the questionnaires and the tools are distributed or mailed to the patients in their practice. We will have research coordinators who track their return and ensure that the data get entered into the database. This very large database includes different patient populations, but allows analyses of similar variables among the groups. Different populations of cancer survivors can be compared for research purposes. Even though we call this aspect of our program a research protocol, the activity is not driven by hypotheses or specific questions. It is really an effort to gather descriptive data to provide a baseline of information on our survivor population. We re-mail the questionnaires annually, so that we can see if there are changes in the symptoms that people are reporting. Many of the late effects we might expect will not appear for four or five years. Just because they are not having a particular symptom today, does not mean they are not going to be having it a year from now.
I close with a little note of special thanks to the Lance Armstrong Foundation, because we could not be doing this and could not have started doing it without their help. Thank you.
Dr. Ganz: Is there a specific question for Linda?
Susan Leigh: Linda, thank you so much for describing a clinic that many adult survivors have wanted for years. We have always looked at pediatrics and said, “Why can’t we have something like that for adults?” There have been any number of excuses, but now it is happening. Now that we have a number of models of adult clinics around the country, it will be interesting to see the difference in the needs for the adult clinic versus the pediatric clinic. When you were the only one around, there were a number of survivors to whom we have said, “Go to the University of Pennsylvania and see if you can get in and have a consultative session yourself.” Can you give me an idea of how much it would cost for somebody coming from around the country? Is there any kind of a ballpark figure for the cost associated with coming to your clinic for your general consultation?
Dr. Jacobs: We went through trying to develop a billing number when we first started the program, but were not successful. We see survivors in our program as part of their routine follow-up. For example, we will tell people if you are going to come to our program, this replaces your yearly follow-up with your oncologist. The patient has to make the decision whether or not they choose to do that. We do follow-up surveillance, and this is considered a routine medical visit. We have had patients come to us, for example some testicular cancer survivors, who say that their oncologists discharged them from care. They have not seen an oncologist in two years. They want to know what they should be doing now. They can come to us and it is billed as a routine visit, or as a new patient visit if it is their first time coming to us. We do not have an issue with that because patients generally come as part of routine care. If they are having an acute medical issue, the visit can be usually justified through an ICD-9 code.
Dr. Ganz: Linda, can you say your fee for a new patient consultation is such and such or your fee for a follow-up visit is such and such? Do you have a number on that?
Dr. Jacobs: We do, but I generally refer them to the billing office. I do not deal with the numbers.
Dr. Mary Vargo, Case Western Reserve University: I work within a physical medicine and rehabilitation program, and I am interested in the subspecialist component of your program. Specifically, from a rehabilitation perspective, it can be relatively easy to figure out who has lymphedema and needs a referral to our service. However, there are other issues, such as debility, musculoskeletal pain issues, and fatigue. Do you have specific
screening tools that you find useful for capturing patients that have those sorts of needs, or is it a more global kind of assessment?
Dr. Jacobs: As part of the screening we have a list of simple, patient-focused questions. The patient checks off whether or not they are having any of those symptoms. We then get computer-generated feedback for the patient and the patient will get a letter listing the symptoms that they complained about, the issues that they had, and the recommendations that we make for follow-up. Generally, if we have patients who present with those types of symptoms and complain of fatigue and certain musculoskeletal things, we will refer them to our rehabilitation collaborator, Andrea Cheville. They make an appointment with her for an evaluation. Andrea does use the Disabilities of the Arm, Shoulder, and Hand (DASH) measure and a few other assessment tools. We also have physical therapy right there.
We do not incorporate specific tools other than very simple things into our protocols because everything has to get approved by the IRB. If you are going to send a screening instrument to one patient, you would have to send it to the entire group. We would be collecting more and more data. Our general line is that we are collecting this as baseline data. You can query the database, see if there is an issue that you would like to study, and then write a research protocol for it. Then you can collect the data yourself in a research protocol, for example, to test one particular tool. The database is available for people to access patients for further study.
Mary McCabe, Memorial Sloan-Kettering: I would like to thank Linda and her group for being very generous to us when we first started our survivorship efforts. They were enormously helpful in preventing us from making some initial big mistakes. I have a question about your consultative model and its potential for expansion. In the future, how do you see the communication with the oncologists who are continuing to see these patients, but also with the primary care physicians? How might that work?
Dr. Jacobs: As part of our program, a summary letter is dictated for every patient. The initial evaluation is summarized to include treatment information, risk information, side effects the patient has experienced, family history, and medical problems experienced since treatment. Everything is in that initial letter. We also compose subsequent letters for follow-up appointments saying what has or has not been done. Recommendations are also listed under health promotion and disease prevention, such as a recommendation for a baseline echocardiogram. The recommendations are made, and they are sent to the primary care providers or whoever the patient tells us to send them to. The patient has to provide us with that list. The patient also receives a copy of the letter.
The letter summarizing symptoms that is generated from the data that patients give us on the tools, which I mentioned before, goes to patients. We recommend that if they want to follow-up, they need to take it to their primary care provider, their oncologist, or their gynecologist. We have empowered the patient to take responsibility for follow-up. We found when we piloted this and sent letters to the oncologist, it just was not working. The oncologists were too busy. The letter would get lost in their pile in their office, or they would say we are already following up on these things. We found that it was most helpful to provide the patient with this information. They can then say, for example, “My gynecologist is the right person for me to go to about sexual function, or the hot flashes I am having.” That is how we are handling that right now.
Dr. Ganz: If someone has hot flashes, you are not managing that?
Dr. Jacobs: We are managing them, because in many cases we are the only ones the patient is seeing. They do not all have primary care providers. That is definitely a huge issue. We strongly recommend that the people have one. We also refer them to primary care providers that are part of our team who are interested in following survivors. We would choose to have someone else follow them, but if the patient does not want to do that, we certainly treat those things.
Dr. Ganz: Thank you for the clarification.
Lisa Diller, Dana-Farber Cancer Institute
I think it is fitting that my talk follows that of Linda Jacobs because I represent another Lance Armstrong Foundation Center recently established at the Dana-Farber Cancer Institute. We are grateful to the group from Penn for their help in starting our program. I am speaking in the place of Craig Earle, the medical director of the Lance Armstrong Foundation Clinic for Adult Cancer Survivors, who could not make it today. In the spirit of full disclosure I am a pediatric oncologist and run our pediatric survivor program, but I will try to do justice to the adult survivor program.
The Dana-Farber Cancer Institute, as most of you are aware, is a part of the Dana-Farber/Harvard Cancer Center (DF/HCC), a federally designated comprehensive cancer center and affiliated with the Harvard Medical School and its teaching hospitals. The institute is highly focused on research and has a busy disease-center-based outpatient oncology service for adult cancer patients. There is really no primary care, and very limited medical subspecialty care within the building. All the medical subspecialty care is provided at the Brigham and Women’s Hospital.
|
BOX 5-1 Clinical Care
Research
Programs
|
Our center, and the Lance Armstrong Foundation Clinic in particular, has a three-pronged approach of clinical care, research, and support programs (Box 5-1).
One of the things I want to focus on that we have not talked about much today is the way in which we can educate and empower survivors apart from one-on-one traditional medical communication. We have found that as pediatric survivors become adults, group sessions of teaching about survivorship become very important and very empowering. We are also using this delivery model in the adult survivorship program.
We saw our first patient in February 2004, so we are really still in our first year. Our care delivery model relies on advanced nurse practitioners. We have two nurse practitioners who provide a written treatment summary to patients, a comprehensive overview of their expectations, and risk-based recommendations. We have conceptualized survivor care as unique and decided to provide our clinical care separate from acute care. We also wanted it to be separate in the mind of the oncologist who is seeing the survivor, so we have established the clinic in a different physical location and schedule from where acute care is provided.
We also wanted to provide subspecialty care with a focus on cancer survivorship. Even subspecialists in fields like cardiology and endocrinology feel that cancer survivorship is a sub-subspecialty. As Dr. Woolf mentioned earlier, oncologists may think that primary care providers do not really know how to take care of cancer survivors. I have also found that endocrinologists and cardiologists who specialize in cancer do not think that general community-based endocrinologists and cardiologists know how to care for survivors. I do not know whether this is true or not. I know that we have developed sub-subspecialty expertise in what I think is appropriate in a quaternary care setting. We felt that drawing those researchers and clinicians into Dana-Farber would add value to the care of our survivors.
Patients treated for cancer at Dana-Farber are treated within disease-specific centers and tend to stay with their disease center for their follow-up care. Follow-up care is available from our cancer survivor specialist nurse practitioner and an oncologist on a weekly or monthly basis. We provide care flexibly depending upon the preferences of the disease centers or providers. Some of the providers agree to do quick 15 minute or 20 minute visits around disease recurrence. That allows the nurse practitioner to have the rest of the 40 minute hour to have a more educationally-focused visit, and time to talk about some of the anxiety or other psychosocial issues around being a survivor. Other providers take responsibility for every other visit, alternating with the nurse practitioner. We are finding our way with the different oncologists to figure out how they want to work with us and participate in survivors’ care.
We also have a fairly robust survivor center practice which is independent of the disease center visits I just described. These visits are mostly from patients who have been lost to follow-up. If you call Dana-Farber today as a long-term survivor of X disease, you will likely end up in our survivor center practice, although it is not guaranteed. You could also be sent to the “new patient” coordinator of the relevant disease center. In either case, you will probably have an independent visit in the survivor center practice. As I mentioned, we have the ability to do subspecialty consultation with a committed cardiologist, endocrinologist, and genetic counselor.
We have been successful so far because we have committed, experienced nurse practitioners who are really, really good. We also have oncologists who have bought into this model. It is a very academic group of both pediatric and adult survivorship professionals, with a high research focus, so we have cross-fertilization around research ideas. Also, we are fairly unconstrained financially because we do not have to depend on what we bill for our pay. We have a lot of philanthropic support, institutional support, and some grant support to allow us to build a program without waiting for payment from the insurers.
In terms of the future, I am worried about expansion. I am worried that we will soon be overwhelmed in this model by having too many survivors wanting this very time-intensive method of care. I would like to find the right balance between a flexible system that works in different ways for different patient groups, and at the same time provides a consistent standard of care. I think the important goal for the next step is to collaborate with our community partners, including community-based oncology practices and primary care practices, to figure out how we can export certain pieces of this care outside of a tertiary care center. Thank you.
Dr. Vargo: Congratulations. The programs all look really wonderful. I have two questions. First, is there an a priori articulated program goal that you can measure? What is the goal of your program?
Dr. Diller: We are trying to respond to the needs articulated before we started the program. Patients expressed that their needs were not being met by the kind of care they got at a tertiary care center once they became survivors. They were seen in the same clinics where they received treatment, and they felt guilty asking questions because the person next to them was sick from their chemotherapy. They were not sure that their questions were being answered by their primary care provider in the community, nor by their busy oncologist. We were responding to a specific articulated need in that group of patients.
How do we measure progress toward that goal? We are doing a mailed survey after every patient is seen that asks about some patient satisfaction issues. We are early in the process, so we do not have a particular outcome today that we will be measuring a year from now. We are developing that from our first set of surveys.
We have a very strong research goal. Our goal in developing research protocols in survivorship is to inform current care of patients. Because we are a center where new clinical trials are often being developed, I think it is important to understand the late effects of those clinical trials, and using what we understand about late effects to develop new clinical trials. That is where we see ourselves.
Dr. Vargo: I mention that because of the importance of process evaluation. It is good to sometimes do process evaluations rather than evaluations of outcome only, and you are early enough in your development that you could do that.
Dr. Diller: We are looking at what we are doing in the first year. We saw 75 patients last month. We saw 4 patients the first month, so that is a pretty
steep trajectory. At the end of the first year we will sit down and ask ourselves exactly the question that you just asked.
Kym Martin, National Coalition for Cancer Survivorship: I am a 22-year Hodgkin’s survivor. Dana-Farber was one of my stops along the way. I have a question about the development of the program. I hear a lot about clinicians’ perspectives. I am just curious, do you have anybody working within the center that is actually a survivor as well as a clinician?
Dr. Diller: One of our nurse practitioners is a survivor. He is a bone marrow transplant survivor, having had leukemia, and is very involved in patient advocacy, as well as professionally involved in patient care. At Dana-Farber we have a patient family advisory council with a high representation of survivors. We report to them about our developments. We have not brought a specific survivor in as a patient advisor, but we have used those two mechanisms.
Kym Martin: I was curious because you are at an early stage in development, as is the program at the University of Pennsylvania. It seems like a good opportunity to really engage the survivors who are active and find out what they would like to see. Surveying the community is one avenue, and a good one. I would be concerned that you are not going to get as much rich information as you would as if you had someone who was involved in program development.
Dr. Diller: We did a needs assessment early on, but it was done using a convenience cohort of people who happened to come to a couple of survivor events and filled out the forms. It was very informative, nonetheless, about which needs were being met.
Kym Martin: At the NCCS, obviously our focus is survivorship and we are led by a survivor. Developing a survivor program seems like a good window of opportunity for you to have survivors leading the way, in addition to the clinicians.
Dr. Diller: I agree.
Mary McCabe: Lisa, I was interested in your mention of the need to export services to community partners. The reason it comes to mind is that we have clinics at Memorial Sloan-Kettering where 40 percent of the patients who come in are long-term follow-up patients. It presents a conundrum because it makes it harder to see new patients. Are you thinking about how you might partner with primary care, or to hand patients off?
Dr. Diller: Yes, that is exactly what we are thinking about. We are thinking about both the primary care providers and community-based oncologists, and the idea of exporting the care of survivors, or exporting this whole programmatic look at survivorship care. This care does not actually have to be provided at a tertiary care cancer center. A community-based oncology practice can handle pieces of what we do, and a primary care provider other pieces. Our program right now does not really fit into that shared care model that has been talked about. We are very rarified, and we do not represent anywhere near the numbers of cancer survivors out there.
Iris Portney: I am a Y-ME volunteer advocate, and also a seven-year survivor of a stem cell transplant. I have been very involved in follow-up studies on survivorship, although not with your program. I would like to follow-up on the question about whether or not you had any survivors at the center. This is really a process suggestion reinforcing this idea. I heard you say that you are doing surveys. As a person who has answered many surveys that were directed to survivors of my treatments, I hope you have survivors review the questions before you send them out. Many times I have answered questions and found maybe 60 percent of the questions to be sensible. When the survey is over, I feel like I know what it was trying to get at, but it lacked the right questions. Then the researcher or interviewers says, “I am sorry, I can only take answers of the questions I have been told to ask.”
Dr. Ganz: Thanks very much, Lisa. Our last speaker is Eva Grunfeld, who is from Dalhousie in Nova Scotia. She is a primary care physician who has done some very interesting work in the area of collaborative care.
Eva Grunfeld, CancerCare Nova Scotia
Good afternoon. I am going to present to you today a program of research that I have conducted over the past 15 or so years that attempts to test the basic hypothesis that the family physician can provide routine follow-up care to breast cancer patients, which is equivalent to specialist follow-up care. It is a pretty provocative hypothesis. We have done a sequence of studies, and I am going to describe their methodology and some of the key findings.
When we started out, we needed to get some descriptive information. The first study was a series of patient focus groups that we conducted in two district general hospitals in England in the early 1990s. Patients were prevalent cases who were healthy at follow-up. We wanted to know from these patients what was most important to them in their follow-up program. Three really important themes emerged. One was continuity of care. They felt that it was very important to have some continuity, for the clini-
cian to know them specifically. The second was the quality of the consultation, which included information to be provided but also the quality of the physical examination that they received at their follow-up visit. Third was access to specialists when they needed it. Specifically, they were concerned about access to specialist tests. This was a unique aspect of care in England at that time, where certain tests could only be ordered by specialists. You had to see a specialist in order to get specialized tests. These were the three themes that emerged from patients.
We also interviewed family physicians and we conducted a postal questionnaire survey of all breast cancer specialists in the U.K. We included medical oncologists, radiation oncologists, and surgeons who are major players in the follow-up care of breast cancer patients. We asked them mirror image questions about what they considered to be important about follow-up, and the results of those two surveys were very revealing. The first finding was that very few of the oncologists or the specialists followed their patients because they thought it was important for clinical reasons. They did not think that the follow-up was important to improve clinical outcomes for their patients. Rather, they thought it was important for audit and general medical education. They thought it was important for psychosocial support for the patient and for research, but they did not identify clinical outcomes for the patient as important.
When we asked general practitioners, we found that they felt that they had the skills to provide follow-up care to their patients, and felt that they should play a larger role in the follow-up of their patients. We asked both groups what model of follow-up care they most preferred. This, I think, reveals the mismatch between the specialists and the primary care physicians, and the mismatch that I think we are identifying in this session today. Lo and behold, the most preferred method of follow-up according to the specialist was follow-up with the specialist. The most preferred method of follow-up according to the primary care physician was follow-up with the primary care physician. The only agreement we found was that “no follow-up” was not a model either group preferred.
We then went on to conduct a randomized controlled trial in England in 1993. This was an 18-month study that involved prevalent cases of women on well follow-up at these two district general hospitals. We defined delay in diagnosing recurrence as our primary outcome. We had a very rigorous definition of delay. It was from the first presentation of symptoms that were related to recurrence to the time that the patient was seen again by the specialist. Our idea there was that it was not sufficient for the primary care physician to be plugging into the fact that there might be recurrence, but the patient had to be seen by the specialist in order to initiate treatment. We found a median delay of 22 days in the general
TABLE 5-1 Primary Care vs. Specialist Follow-up
|
|
Primary Care Provider (n=148) |
Specialist (n=141) |
Difference (95% Confidence Interval) |
|
Time to diagnosis of recurrence (days) |
22 |
21 |
1.5 (−13 to 22) |
|
Total time with the patient (minutes) |
35.6 |
20.7 |
14.9* (11.3 to 18.4) |
|
Cost per patient (£s) |
65 |
195 |
−130* (−149 to 112) |
|
Time cost to the patient (minutes) |
53 |
82 |
−29* (−37 to 23) |
|
*significance at p<0.001. |
|||
practice group, and a median delay of 21 days for the specialist group (Table 5-1). So, there was no difference in our primary outcome.
We also looked at a series of cost factors, both for the healthcare system and for the patient (Table 5-1). Patients who were seen by the family physician had significantly more time in their follow-up visits than patients seen by the specialist. The costs per one visit for primary care follow-up were less, as you would expect, than costs for follow-up in the specialist clinic. It took significantly less patient time, including travel time and waiting time, to see the primary care physician than to see the specialist. Not only did it take less time, but they also spent more face time with the physician.
Our secondary outcomes were health-related quality of life, and there were no differences in health-related quality of life on any of the standardized subscale measures that we used. Also, there was no difference in anxiety and depression. We hypothesized that anxiety was a particular domain of importance. Patients in the general practice group were more satisfied on a whole range of questions about patient satisfaction.
We then went on to conduct a phase 3 randomized controlled trial in Canada in 1997. Patients were enrolled in the study one year after diagnosis, so that all of their primary treatment was completed. They were followed for a median of 3.5 years, meaning they were 4.5 years from the time of their diagnosis at the end of the study. That is important, because in that interval the majority of recurrences will be diagnosed. We had almost 1,000 patients in the study, and they were randomized to receive follow-up with a primary care physician or a specialist.
TABLE 5-2 Number and Rate of Serious Clinical Events (3.5 median years of follow-up)
|
|
Primary Care Provider (n=483) |
Specialist (n=485) |
Difference (95% CI) |
|
Number (rate) of serious clinical events |
17 (3.5%) |
18 (3.7%) |
0.19% (2.26 to 2.65) |
There are two points that I want to highlight about this randomized trial and the previous randomized trial. In both cases our goal was an effectiveness study that would be as generalizable as possible. That meant that the family physician was the patient’s own family physician. It was not a special cadre of family physicians that were trained to do the work. We provided the family physician with a one-page guideline on follow-up, but that was the extent of the educational intervention.
We defined two primary outcomes for the Canadian trial. For patients who developed recurrence, we determined that the most important outcome is a clinical event related to recurrence that is potentially preventable and would be a catastrophe for the patient if it were missed. Therefore, we defined as primary outcome any one of a series of serious clinical events, which were pathological fracture, spinal cord compressions, hypercalcemia, and uncontrolled local recurrence. For patients who did not develop recurrence, we identified the fact that quality of life was the most important outcome.
For patients who developed recurrence, there were a small number of events in both groups (Table 5-2). I think that is a very important finding of this study, because we have documented prospectively how rare these events are. Over the five years of the study and almost 1,000 patients, 4 percent of cases had a serious clinical event. Clinical events occurred in both groups regardless of who was primarily responsible for follow-up care. In our primary outcome there was no significant difference between the two groups.
In terms of health-related quality of life, we used a range of standardized measures, and described both median differences in quality of life, as well as change scores from baseline. We found no differences in the two groups on health-related quality of life. Now, we have also looked at patient costs and patient satisfaction, but I cannot give you those results now because those data are in the process of being analyzed.
In terms of our original question about the acceptability of primary care follow-up, we found that in this study 55 percent of patients agreed to participate in this study, and in a previous study 67 percent agreed to
participate. That is identifying all prospectively eligible patients. In terms of primary care physicians, 83 percent of family physicians agreed to provide follow-up care to their patients with breast cancer.
Our conclusion from these studies is, first of all, that primary care follow-up of breast cancer patients is a safe and acceptable alternative. We have shown this now in two randomized controlled trials looking at it in two different ways. We think it is a proof of principle for the other of the major prevalent adult cancers, such as prostate cancer and colorectal cancer.
In terms of implementation tools, we used a very simple one page guideline. We were focusing on medical follow-up. Needless to say, there are a range of issues, such as psychosocial issues and late sequela of treatment, that one would want to include in any new guideline. We have published guidelines that include those issues. I like to stick to the paper versions of things until we have confidence in electronic ways of doing things. When we get there, electronic guidelines will be fabulous, but in the meanwhile, paper versions are very useful.
A discharge letter was used in our randomized trials to facilitate communication. Needless to say, Web-based medical records is the brave new world that we are all waiting for. In the interim, a well-structured discharge letter would be very useful.
One of the tacit things that has been discussed, and a couple of people brought it up overtly, is the importance of the patient in all of this. There is evidence to show that when you want to implement guidelines, providing the patient with a version of the guideline and actually having the patient as an active player in the whole process does improve outcomes. When we talk about a care plan, what we are really saying is that we are including the patient in the process of improving their care and improving follow-up care. Thank you very much.
Dr. Ganz: This is just to clarify, you did in fact have a survivorship care plan or a discharge summary that went to the primary care provider?
Dr. Grunfeld: We had a one-page guideline that went to the family physician.
Dr. Ganz: You said there was a discharge summary?
Dr. Grunfeld: There was just a letter that was dictated saying, “You are now taking on responsibility for follow-up care.” Many aspects of it were like the care guideline.
Dr. Ganz: And you did in fact give the same guideline to the patient that you gave to the primary care physician in your study?
Dr. Grunfeld: We did not, not in our study.
Dr. Ganz: So, giving a guideline to the patient has not been tested?
Dr. Grunfeld: That has not been tested, although we have just written a protocol in order to test that component of it.
DEVELOPING GUIDELINES, INSTITUTING QUALITY IMPROVEMENT, AND STRENGTHENING PROFESSIONAL EDUCATION PROGRAMS
Moderator: John Ayanian, Harvard Medical School
I am a general internist at Brigham and Women’s Hospital in Boston, and a health services researcher in healthcare policy at the Harvard Medical School. It is my pleasure to moderate this session on developing guidelines, instituting quality improvement, and strengthening professional education. If we can solve these three issues in the next hour, I think we will have tackled the cause of cancer survivorship. We have four interesting presentations by our panelists today. First, Charlie Shapiro, will be joining us by speaker phone to discuss the ASCO Survivorship Task Force’s guideline effort. Melissa Hudson will then describe the childhood cancer survivorship guidelines that have been developed for young adults. Next, Rodger Winn from the National Quality Forum will discuss quality indicators and their relationship to guidelines and quality improvement. We will wrap up with LuAnn Wilkerson from UCLA, who will describe a new model for educating medical students about cancer survivorship. We will hope to have time for a discussion of how health professionals could be introduced to cancer survivorship and remain well-educated and well-informed about it. We will start with Dr. Shapiro, who will be presenting by speaker phone.
Charles Shapiro, Arthur James Cancer Hospital, Ohio State University
I am a medical oncologist specializing in breast cancer at Ohio State University Medical Center and Comprehensive Cancer Center. I have a long-standing interest in survivorship issues, particularly on the implications of chemotherapy-induced early menopause on osteoporosis for breast cancer survivors. I co-chair the ASCO task force on adult survivor guidelines. Our mission in this effort is to provide healthcare professionals with a blueprint to obtain the necessary knowledge and expertise to decrease morbidity and improve quality of life for adult cancer survivors. We define survivorship as the period following the diagnosis and treatment phases, and the population includes adults who have survived childhood cancers.
This is a very important population, because as you will see from Melissa Hudson’s presentation, the transition period from pediatric oncologists and healthcare professionals to other providers is difficult in that nobody is clear what to do and who is going to take responsibility. We have taken it on ourselves to include adults who have survived childhood cancers. There will be overlap in this population between the guidelines that the Children’s Oncology Group has created and the ones we are in the process of creating.
Why is this effort important at this time? Well, the existing survivorship guidelines are very limited. In the review of the guidelines that is included in the IOM report you can see how limited they actually are. I think it is an opportunity for us to take stock and highlight what we know in terms of care of survivors, and perhaps more importantly, what we do not know. This will suggest areas for future survivorship research and education of healthcare professionals. I think that this effort is timely for several reasons, and I am happy to be a co-chair in this important effort.
We face substantial challenges in creating these guidelines. As I noted before, there is a really limited database of relevant evidence. Furthermore, the science of late effects represents a moving target. The most mature data on late effects come from clinical trials conducted 20 to 25 years ago. The techniques and treatments in these trials differ greatly from treatments we use today. This is most apparent in the radiotherapy literature, and specifically radiotherapy effects on the heart. Twenty to 30 years ago radiation was delivered in a different manner than it is today. They relied on low-energy sources, used larger fraction sizes and a field arrangement that exposed the maximum amount of normal tissue to radiation. Nowadays with modern radiotherapy techniques we use higher energy sources, limited fraction sizes, and techniques that really minimize normal tissue exposure.
In studies conducted 20 to 30 years ago, heart disease induced by radiation exposure was prevalent in the second and subsequent decades following treatment. More recent studies using modern techniques, show no increase in radiation-associated heart disease, or at least no detectable increase. The most mature data on late effects may have limited generalizability because of the changing nature of treatments.
Another difficulty in this area is potential biases associated with data sources. Much of the research on late effects is conducted using information from cancer registries where there can be under- or over-reporting of treatment exposures. Another methodological challenge is confounding due to the prevalence of co-morbid conditions of aging such as heart disease. When a pediatric patient is treated with anthracycline at age 4 and develops congestive heart failure at age 18 or 19, the association between treatment and late effect is pretty clear. However, when a 60-year-old woman receives anthracycline for breast cancer, and at 70 she develops heart problems, it is not as easy to sort out the effects of aging versus the treatment exposure on
the heart. Similar problems arise in studies of treatment-induced osteoporosis. Osteoporosis is a disease of aging. We are again challenged in trying to figure out what is due to the exposure and what is due to simple aging.
It is obvious, but worth stating that the decision to adopt a new therapy is based on studies that assess short-term improvements in efficacy. For example, many will be familiar with the herceptin story that was recently published in the New England Journal of Medicine. A dramatic reduction in recurrence rates were observed among women with early breast cancer treated with a combination of herceptin and chemotherapy as compared to chemotherapy alone (Romond et al., 2005). The combined treatment was associated with a 3 or 4 percent incidence of cardiac toxicity when evaluated in the short-term, however, the ejection fractions improved indicating that the cardiac toxicity appears to be reversible. However, the long-term effects of herceptin are not known, so longer-term studies are needed. Other new treatments might have other long-term consequences.
The ASCO guidelines effort is focused on four areas: cardiovascular late effects; neurocognitive and psychosocial late effects; endocrine disorders; and second cancers. The task force felt that these four areas cover most of the problems of cancer survivors, irrespective of cancer site. For example, we relate exposures like anthracycline and radiation and herceptin to the incidence of long-term cardiac effects, and identify when screening for cardiac effects should be conducted. We also identify lifestyle changes that might be able to mitigate the cardiotoxicity for cancer survivors who are exposed to potential cardiotoxins. In terms of neurocognitive and psychosocial effects, we look at whether there is a role for screening survivors to identify problems in these areas. We also identify certain treatments that, in pilot studies, begin to address the issue of neurocognitive function and psychosocial interventions. Endocrine is a big area which includes addressing sexual dysfunction and osteoporosis. Identifying exposures and risks of second cancers and then specifying screening strategies for second cancers will be an important area for the task force. Lifestyle changes, the obvious one being smoking reduction in cancer patients, are important and these preventive measures will be considered in the guidelines. Again, these four areas will be those that we initially focus on in the ASCO guideline effort.
Finally, I think the future of survivorship, and cancer treatment in general, is dependent on improved drugs that are more selective and improved methods of selecting patients for therapy. In the next 5 to 10 years we can look forward to better methods of selecting patients who really need therapy, and sparing patients who do not. Sorting out who really needs treatment from who should be spared treatment will do a lot for survivorship in that it will eliminate the exposure for a population of patients that we are ordinarily treating at this time.
I think that the other side of the equation is host factors, or individual factors that might influence quality of life and influence the likelihood of developing treatment-related effects. Those are pharmacogenomic considerations. A recently described study related pharmacogenomic incidences in enzyme metabolism to quality-of-life considerations (Sloan, 2004). This is only the first study, but I think that it makes sense that quality of life could be affected by differences in metabolizing enzymes.
I think it is time now for adult survivorship guidelines to summarize the state-of-the-art of what we know and what we do not know in 2005. That will set the stage for future directions in survivorship research. I thank you very much for the opportunity to present, and I appreciate your indulgence in letting me present by speaker phone.
Dr. Ayanian: Any questions for Dr. Shapiro about his comments regarding the ASCO survivorship guidelines?
Dr. James Talcott, Massachusetts General Hospital: I want to thank Charlie and his group for organizing this body of information. However, I noticed, for example, that in describing the four topic areas for the ASCO guidelines, sexual function ended up in the endocrine category, and there was a psychosocial category in which it could have been included. There is a tendency to take a somewhat reductionist focus in medicine to deal with specific medical events that we define, count up, and hopefully try to pare down later. I wonder to what extent your group thought about the broader existential psychosocial issues of survivors?
Dr. Shapiro: These are supposed to be evidence-based guidelines that follow a careful review of the literature. We will be trying to come up with guidelines based on evidence. I think that Jim’s point is well taken in that this effort will have to go beyond the data to really truly get at the heart of survivorship. There are a whole host of issues beyond the medical considerations, including psychosocial issues such as employment. The IOM report is a comprehensive report and it deals with these issues and medical issues on an equal footing.
ASCO, in this effort, is taking a first step. Our goal is to first review the available evidence on these four topics, appreciating that these four topics are not inclusive of everything we need to address. There is so much to consider, and we did pare it down. On your specific point, I agree with you that there is overlap between sexual dysfunction and psychosocial issues and a focused look at mechanical and functional issues is incomplete. Your point is well taken, but the ASCO initiative is really an initial crack at a comprehensive attempt at survivorship guidelines.
Dr. Ayanian: I am curious how the ASCO process integrates with the NCCN process for guideline development.
Joan McClure, National Comprehensive Cancer Network: We went a short way down the road of trying to develop survivorship guidelines and found that there were very disease-specific issues that were coming up in our discussions. I was wondering to what extent the ASCO guidelines are going to be global guidelines for all survivors versus disease-specific guidelines for particular problems, long-term toxicities, and second primaries that follow-up on individual diseases?
Dr. Shapiro: Whether the guidelines are disease-specific versus global in application remains to be sorted out. I think that there are certainly instances of cancer-specific exposures that merit awareness, such as chemo-therapy-induced menopause, androgen deprivation in prostate cancer survivors, and second cancers specific to exposure late effects. I would not be surprised if we come up with some overarching general principles applicable to all cancer survivors, and then specifically focus on the exposures or specific cancers that are related to the late effects. I think it is a work in progress, and I am not sure that I fully understand how the ASCO effort will integrate with NCCN guidelines.
Dr. Ayanian: Our next speaker will be Dr. Melissa Hudson, who is speaking on behalf of the Children’s Oncology Group about the guidelines they have developed for survivors of childhood, adolescent, and young adult cancers.
Melissa Hudson, St. Jude Children’s Research Hospital
I am a pediatric oncologist, and I supervise the After Completion of Therapy Clinic, which monitors long-term survivors treated at St. Jude Children’s Research Hospital. We have almost 5,000 five-or-more-year survivors that we are monitoring. I also have the privilege of co-chairing the initiative for the Children’s Oncology Group (COG) developing long-term follow-up screening guidelines for children, adolescents, and young adults who have survived cancer, and I want to share a little bit about that experience with you now.
I would like to start by setting the context of our work. Children come to pediatric cancer centers seeking curative therapy with their cancer diagnosis, and we provide these primary interventions and administer therapy based on core prognostic factors, tumor responsiveness, and their specific risk for treatment complications. We may even modify their treatment for cancer in light of known late effects. As these children achieve long-term
survival, which we typically designate as five or more years from diagnosis, we begin to lose them as they transition into the adult healthcare system from the pediatric healthcare systems. This transition occurs just when it is important for us to intervene with secondary interventions such as health education, cancer screening, and risk-reducing interventions that are going to encourage health and resilience. Providers in the adult healthcare system are generally unfamiliar with our children’s specific issues.
There are challenges in providing this type of care across this continuum, this age spectrum. First, pediatric cancers and treatments are heterogeneous. They are very diverse, and it is impractical and improbable that any one provider will be familiar with the host of pediatric cancer treatments. There is a relatively low incidence of pediatric cancers, so there is really no incentive for providers to have a great deal of knowledge of these cancers and treatments. Our therapies even now continue to evolve, as do the late effects of treatment. There is a very long latency with some of these effects. We discovered early on we had to have children complete their growth to really understand musculoskeletal effects and neurocognitive effects. Now we are in the second phase of that understanding, and we are seeing what is happening as our survivors are aging.
Multiple factors contribute to cancer-related morbidity. Cancer treatment is just one of them, but there are many others including genetics and behavioral factors that can be discussed with the survivor. We have some unknown effects associated with aging, but we do have a fair amount of experience with the general population. Survivors’ cancer-related risk may be enhanced through the aging process or specific treatments.
Overall, there is a lack of consensus regarding screening guidelines and risk reduction methods. While we have a huge body of literature about treatment complications, we do not have a good evidence base to support recommendations for screening and risk-reducing interventions. In fact, in contrast to what Dr. Winn said of the medical oncology literature, we have textbooks on late treatment complications after pediatric cancers, and we have many medical manuscripts that have been published. It is important for us to move forward in that direction.
The main problem we have in providing survivorship care is the lack of familiarity and lack of comfort by providers, particularly community providers, in accepting these patients who move into their busy practices. Among pediatric oncology providers, the buzz words, when seeing cancer patients for long-term follow-up, is that they deserve “risk-based” care. I want to tell you what “risk-based” care implies. This is the screening and prevention planning that integrates the cancer experience with their healthcare needs. We have to consider risk associated with a variety of issues: the host; the age at diagnosis; race; their age at follow-up; the cancer location; their specific treatment modalities, and if there were treatment complica-
tions; genetic and familial predispositions, some of which we know, many of which we do not understand; and certainly include areas for which we need to pursue research. Finally, there are lifestyle issues that can certainly contribute to an increased risk of morbidity. It is important to work with the survivor on these issues, since this is the one area of risk reduction where they can personally be involved. Of course, many patients and survivors bring into their cancer experience co-morbid conditions or later on develop co-morbid health conditions that should be considered during the follow-up.
After the childhood cancer survivorship report that came from the Institute of Medicine (IOM, 2003) a few years ago, the COG undertook a huge initiative to organize survivorship guidelines. These are clinical practice guidelines for survivors, specifically of childhood, adolescent, and young adult cancers. They are exposure related. We did this because of the heterogeneity I discussed earlier. We wanted to have a broad compendium of information to guide providers. They are risk-based, accounting for all factors that I just discussed—host, genetics, and lifestyle issues.
The recommendations for screening and management are drawn from the literature in that we have an evidence base linking a late effect with a treatment exposure. However, the specific screening recommendations that we made are based on the consensus of late effects experts, because we do not have the evidence base to guide those recommendations. In developing and working through this process of organizing the guidelines, we have been able to identify priority areas of research. Further research will help us gain the evidence base to make more appropriate recommendations.
We have patient education materials called health links. There are now 35 health links that accompany the guidelines. They are meant to broaden the application of the guidelines, and deal with topics such as heart disease, kidney risk, psychosocial issues such as insurance access, and neurocognitive issues, in a format that can be downloaded, printed, and used within clinics.
The goals of the guidelines are to educate providers and patients about late effects, and to standardize and enhance follow-up care over the age spectrum. We also want to facilitate early identification of late effects. Our aims are to promote a healthy lifestyle in long-term survivors, to provide ongoing monitoring of health status, and ultimately to provide timely interventions for late effects.
I will leave you with the URL: www.survivorshipguidelines.org. This web site has Version 1.2 in the PDF format. We have 18 task forces that are system-based, cardiovascular, endocrine, et cetera, that have reviewed the literature since we distributed or made available Version 1.2 of the guidelines. The guidelines have been completely updated, and Version 2 will be posted probably sometime this spring.
We are working with Baylor College of Medicine researchers on a Passport for Care application that will ultimately make these guidelines interactive and more user friendly. We have not started our health services research initiatives to judge how the guidelines are being used, but we are tracking their use on the COG web site. We would love to have a more user-friendly application of the guidelines, because currently it is a huge document. You can go and see the guidelines and how we have done it: exposure-related, whether a specific therapeutic agent, specific radiation volume, or specific surgery. I think this will be of use to the guideline development for adult cohorts. Even though they are focusing specifically on some target areas, some of the information that we have can be readily applicable to the adult populations.
Ms. Shawn Kennedy, American Journal of Nursing: With your guidelines, do you have a standardized care plan type of thing that you use to communicate this information?
Dr. Hudson: Yes, we do. In order to use the guidelines, you have to have a treatment summary to know the specific exposures. A treatment summary template was developed by the nursing late effects group through the Children’s Oncology Group. Their plan is ultimately for everyone who is exiting or completing COG trials to have that information. Then from that treatment summary, the guideline provides information such as potential late effects, recommended screening, health counseling, and the frequency of the recommended screenings and interventions. Does that answer your question?
Ms. Kennedy: Yes, I was curious as to when the care plan comes into play, because especially at St. Jude’s you have people coming from different parts of the country. At what points in treatment follow-up does all this get done?
Dr. Hudson: The timing varies. The COG guidelines pertain to care provided at least two years after completion of therapy. They are for the asymptomatic survivor who has had these specific exposures. How individual institutions are using the guidelines to develop a care plan may vary. The St. Jude After Completion of Therapy Clinic does not accept our patients until they begin long-term follow-up, when they are at least five years from diagnosis, and we develop a care plan. It is given once a year. We outline a treatment summary, and we update it. It has not only their specific late effects, it has a problem list that is system-based, and then it has the recommendations for screening, along with our special concerns, whether it is increased risk of heart disease, infertility, et cetera. However,
I think the process really varies across the centers regarding how they are using guidelines and care planning in providing follow-up care.
Participant: How much does it vary within St. Jude’s?
Dr. Hudson: With the After Completion of Therapy unit, we have a fairly large initiative. I work with two other oncologists. We are fortunate in that our institution funds this initiative. So, we have three days of long-term follow-up clinics. One is for brain tumor survivors, one is for solid tumor survivors, and one is for survivors of hematologic malignancies. The oncologist works with the staff physician who is a generalist, specifically, an internist. In this case she has medical oncology training as well. At St. Jude, I am the only one who does long-term follow-up within the context of the treatment sections. I would expect if you go to any other institutions, their application or adherence would not be very consistent. It is going to vary according to individuals.
Dr. Ayanian: Next, I would like to introduce Dr. Rodger Winn from the National Quality Forum.
Rodger Winn, National Quality Forum
I would like to start by examining where we stand vis-à-vis a measure set that could realistically assess the quality of survivorship care. If we look at the three interventions that are the topic of this breakout session, education, guidelines, and measures, I would say on a scale from 1 to 10, that education is probably at step seven. Mechanisms are in place to improve educational opportunities, and it is now just a question of tooling up the content. I think the guidelines still need some work in conceptualization and methodologically, so I put them at about a four. I think measures are very solidly on square one. What I want to suggest, though, is that square one is real, and that there are enough general precepts of what measures should look like that we can go ahead.
Just to let you know, the National Quality Forum, which endorses measures, recently looked at a set of symptom and end-of-life care measures and found virtually none that were ready for long-term use. The field of cancer is really lagging behind other fields in terms of measures.
Very briefly, what are the purposes of measures? Are we where we can really talk about accountability and public reporting yet? Obviously, that takes very rigorously derived measures. The problem is there is a tsunami coming at us where people in fact are demanding that we have accountability and public reporting measures, not the least of which is the Centers for Medicare & Medicaid Services. It is also driven very heavily by our con-
|
BOX 5-2 Care that is:
SOURCE: (IOM, 2001). |
sumer and purchaser constituencies who are saying, “We want to make decisions, and we cannot make decisions unless we are given information.” The drive is very real.
Most of what we as professional organizations look at are quality improvement measures. We use them internally to monitor trends and compare to benchmarks. We use the results, not to “blame” providers for their performance, but instead to develop approaches to improve the performance of a system of care.
Finally, there is a surveillance role for measures. How do you use these measures to look at our national priorities? Measures can be used to monitor at the community, regional, or national level to establish policy and to allocate resources.
The IOM actually came out with a list of the aims of high-quality care (Box 5-2). Most of what we use are effectiveness measures that look at how well an intervention leads to a clinical outcome. In reality, there are these five other domains. If the goal is, in fact, a comprehensive measure set, we are going to have to look at all of them.
Safety—what does that mean in survivorship? A question addressed to a patient, “How confident are you in knowing when to call your doctor about an emergency?” might be a realistic measure of safety for survivorship.
Timeliness—are follow-up studies done on time?
Patient centeredness—does the patient participate in shared decision-making? Does the patient get adequate information?
Efficiency—a lot of what we look at in this area are measures of overutilization. An example of this might be, “Does your stage I breast cancer patient still get a bone scan and a liver scan?” Such measures of overutilization could be put in place, but they are really not great efficiency measures. What you really want to look at is given the amount of resources, what kind of outcome did you get?
Equitable—these measures could assess the extent to which patients have access to specific services.
The criteria used to evaluate measures include, first, that they be evidence-based. This appears to be straightforward, but sometimes it takes on some complexity. We measure structure and processes of care that we know are linked to outcomes. Unfortunately, there is a paucity of data that allow us to make those linkages in the area of survivorship. What would be a good structural measure for survivorship? Did you have clinical practice guidelines in place? A process measure might be, “Were the guidelines implemented in your system?” In order for these to be good measures, we have to be able to link their presence to a favorable outcome. In other words, if the patient achieves this status, which is an outcome, was it due to the fact that something happened to their healthcare delivery, or was it something totally unrelated?
Once you have an evidence-based indicator, then you can move on to the measure. There has to be some parsimony in developing the measure set. Unfortunately, it would be nice if every one of us had a Cray computer in our kitchens to measure 800 measures, but it is not going to work that way. We are really going to have to try to pick the 6, 7, or 8 measures, whatever they are.
Having said that, then the next question is, “How important is the measure?” The measure has to make a difference to the patient. There are many scales that have been developed to gauge relevance to patients. Dana-Farber, for example, has a model to assess how to balance patient preferences. Another important attribute of a measure is the extent to which it varies. There is no use looking at something if 98 percent of clinicians are already doing it. Having a measure for a healthcare attribute that can, in fact, be improved is also very important. If you measure and find a deficit, can the healthcare delivery system, in fact, work on improving it?
Once all of these criteria are met, then the measure must prove to be scientifically acceptable. This is a formidable task. This is high tech. This is rocket science. This is not back of the envelope and it would be impossible to invent a measure today and go out tomorrow and put it into play. Measures must be evaluated for several critical characteristics, for example, their reliability and potential for risk adjustment that relate to their usability. To have value, a measure needs to be understood in the context of quality, and it has to be feasible to implement. For example, data sources must be necessary for measurement.
Once we have a measure set that meets these criteria, there must be agreement or standardization of the measurement. One of the worst things would be to have nine different measures out there of what is good quality care, especially if some of them were contradictory, which in fact can happen. In a plug for the home team, this is what the National Quality
Forum does. We try to collect measures and then put them through an endorsement process. We come out with an agreed upon measurement set to use going forward. Standardization of data is also an issue. Once again a very technical area, but it is important to assess whether valid information is being extracted as part of a quality-of-care program.
There was a mention this morning of attribution, the issue of designating responsibility for meeting a particular quality standard. In the context of the primary care and oncology shared care models, we need to decide who would be responsible for follow-up care and how to make these decisions. Finally, we will have to risk adjust some of the measures to make sure that when you report these you are comparing providers taking into account the risk profiles of their patients, in other words comparing apples to apples.
My overall assessment is that the first step has to be the gathering of evidence, and here, the best way to move forward is through the process of developing clinical practice guidelines. Once those are developed, we can then extract some of the measures that meet these criteria and apply them in areas where we feel we can make a difference.
Dr. Ayanian: Let me just invite brief comments from Phyllis Torda and Beth Kosiak. They represent, in Phyllis’s case, the National Committee for Quality Assurance, and Beth, the Agency for Healthcare Research and Quality (AHRQ). They are two of the lead organizations thinking about quality indicators.
Ms. Phyllis Torda: Thank you. First of all, I would like to underscore Rodger’s point that measures should be evidence-based, and that good evidence is a prerequisite of quality measurement. I am a little concerned that people might come away overly daunted by his talk. I would just inject that there are actually some tricks of the trade that can be used to make measurement more feasible in the short-term.
Clinical performance measures that are used to compare one entity to another are the gold standard of measures. The broader the number of entities that you want to measure, the more complex the issues become, and rigorous methodology is needed. If your goal is to single out excellence in care and focus on centers, individuals, or certain types of practices rather than others, it can become a little easier. If you were using a series of measures to assess an individual’s performance, that also can make it easier. Another trick of the trade is to use a cutpoint. Instead of comparing entities directly on their performance score, for example, one entity scoring 95 percent and another scoring 85 percent, you can look at whether entities scored over 80 percent. These are examples of what I mean by tricks of the trade which can simplify implementation and facilitate quality measurement.
Dr. Beth Kosiak: I want to very briefly mention one aspect of this measurement agenda. The AHRQ is responsible, as some of you may know, for the production on an annual basis of the congressionally mandated National Healthcare Quality Report and National Healthcare Disparities Report. One of the issues that comes up is what measures should go into those reports. That is where this issue of consensus and standardization and support in the community is critical, because we are going to establish some measures at baseline, and then track them over time. There has to be support for those measures, not only in the community, but also evidence to show that those are the right measures. One of the issues that has come up as Rodger and others have noted is the limited number of available measures. To some extent that means that the national agenda on quality, at least on one level, is missing the degree of comprehensiveness that we would want for cancer care. This represents one of the activities that the AHRQ is involved in that I wanted to bring to your attention.
Dr. Ayanian: A quick comment?
Participant: The one thing that has not come out is that the standards of care in oncology are changing fairly rapidly with the evolution of treatments. The quality measures are also going to have to evolve right alongside of these developments.
Ms. Torda: I think there has been a resurgence of interest in process measures rather than outcome measures. Ten years ago everybody talked about outcome measures. I think the changing evidence base has informed the shift a little bit back to broad processes of care.
Dr. Ayanian: Our concluding speaker is LuAnn Wilkerson from the University of California, Los Angeles (UCLA). She is going to address the challenge of, “If we knew what the evidence base, quality indicators, and right care were, how do we get health professionals to provide that care?”
LuAnn Wilkerson, David Geffen School of Medicine, University of California, Los Angeles
If there is a gap anywhere, I imagine there is a gap here. How do we get guidelines adopted and measures used? I am going to just give you one example of what we have been doing in a consortium that involves UCLA, the University of California, San Francisco and the Drew University of Medicine and Science. My colleague, Margi Stuber, is here with me. We are funded by the National Cancer Institute on an R25 grant to begin to develop educational tools for cancer survivorship. We invite you to help us
think about adapting these tools for different kinds of health professionals and different levels of trainees.
We began by asking the question, “What do we want our medical students to know about survivorship?” We decided to develop a guideline to help us in this domain. We used 17 experts, drawing heavily on oncology, of course, but also the generalists who cared for those patients following the acute treatment period. We generated at our three institutions a very long list of knowledge, skills, and attitudes, everything in the world that a medical student might need to know. We used a two-round Delphi process to narrow that list down to 19 objectives. Those objectives have served over the last two years as a guide for developing a series of modules.
The objectives are published in the IOM report and shown in Box 5-3. They come down on the side of being general, rather than specific principles and broadly applicable to the training of medical students, residents, and nursing students. We have a cohort study underway to look at the quality and outcomes of educational materials developed from these objectives, both the processes and outcomes of this kind of educational program.
To date, we have developed 20 instructional modules, and they are all patient-based and interactive. They have a flexible format, so that you can choose to use different components of a module, and they are highly adaptable for different healthcare fields and levels of trainee. These are open source materials that you can change, pick, choose, adjust as you wish. They are available free upon request at www.medsch.ucla.edu/public/cancer/, but they do not actually appear on this web site; instead there is a template that describes each tool or module. This web site will also serve as a dissemination point for the video on cancer survivorship that was produced by the IOM committee. It is of wonderful quality, and has raised a lot of interest in the last few days at the Association of American Medical Colleges’ (AAMC’s) annual meeting that Margi and I are here in town to attend.
Let me just give you a couple of examples of some of the elements of our educational program. In learning to take a cancer survivor history, our objectives are that the students will understand the long-term physiologic and psychological impact of the cancer diagnosis and treatment, appreciate follow-up preventive care, and demonstrate effective communication in counseling a cancer survivor. The format for this particular module is a standardized patient. The standardized patient meets with a group of students, is interviewed with a time-out freeze frame interaction so that the group, which might well include a cancer survivor, can talk about best practices and approaches. The module includes a template for a patient record which we based on the care plan, so that the students can begin to learn what a survivor care plan is like.
The second example I wanted to share with you uses a very different format, a CD-ROM video case. It can be used on the Web for asynchronous
|
BOX 5-3 Attitudes
Knowledge
Skills
SOURCE: UCLA (2005). |
discussion, or it can be used in a face-to-face group discussion. It features a lung cancer survivor and is focused more on discussing the factors that affect quality of life after diagnosis and after treatment. This product was funded additionally by a grant from the U.S. Department of Education.
We have been developing tools for outcome and process assessment, and these represent other products of our work. In medicine and medical education, including resident education, we provide things called OSCEs, Objective Structured Clinical Exams. In OSCEs, the trainee sees a standardized patient and is scored by either an expert observer or the patient himself/herself using a checklist. We have developed four cases that come complete with a standardized patient script, instructions on how to use that script, and a checklist for scoring. The checklist scores at a more general level, but for higher levels of training it could easily be adjusted to include the more content-specific issues that you might be interested in testing. We have standardized patient scripts on four topics: (1) screening for colon cancer risks; (2) counseling for smoking cessation; (3) assessing cognitive effects of chemotherapy; and (4) planning for advanced directives
We have also developed a knowledge test. This is targeted at the survivorship objectives, and includes two to four questions for each objective. We have only pilot tested it so far, so I cannot tell you anything except that senior medical students know very little about this domain. The last tool that we have developed and then pilot tested is an experience survey. We ask about the students’ direct instruction in a number of domains relevant to the objectives, the amount of practice that they have had, and also the number of times they might have seen this particular skill demonstrated by their clinical faculty. We do not have any results yet. The biggest challenge that we face is not so much in educating the younger trainees, but in educating the physicians, particularly in primary care that will need to implement the survivorship modules.
At the recent AAMC meeting, we had two days of exhibiting some of these materials, and cancer as a chronic disease is a very good concept for medical educators to begin to think about. We have a long way to go.
Dr. Ayanian: Thank you. Questions?
Dr. Talcott: This is for Melissa Hudson. There is a rationale for expert opinion-based guidelines, and in particular, in pediatrics where you have a smaller number of patients, so it is harder to do randomized studies. I am just wondering what you have done to try and constrain the recommendations? My experience is that physicians do not pay much attention to punchy, well-supported, evidence-based guidelines when the guidelines consist of a bunch of people getting together and recommending things that look an awful lot like their practice, with no constraints on what they are
recommending. How do you make those things user friendly and really constrain them to the available evidence?
Dr. Hudson: In the context of developing guidelines, it is interesting to think about constraining individuals. Probably 70 percent or more of the guidelines pertain to taking the history, doing the proper examination, ordering a very limited amount of lab work, and only rarely recommending imaging. Some of the health information or the additional information that you would consider for a patient is listed in a separate category specifically because there is no evidence to provide greater levels of testing, but we wanted it on the physician’s radar screen. You are absolutely right that somebody could look at that and say, “Well, I will just go ahead and order the X, Y, Z, rather than do the physical examination or sit there and take the history to see if there is a clinical indication to provide this intervention or to do this test.”
Throughout the guidelines we have clinician information links. We envision that when this tool is interactive it will pop up when the physician is in a certain section. They discuss more broadly the limitations of the literature. For example, for the guideline about breast cancer surveillance in young women who have had chest radiation therapy, our guideline is comparable to the guidelines for other high-risk populations, such as the BRCA-positive populations. We recommend mammography, with a caveat that you should start within a specified time period after radiation is completed. We also have a very broad clinician information link that discusses the problems with imaging in a pre-menopausal breast, the ongoing, evolving literature about MRI, and the need for future studies. We include all these areas that are very contentious.
Osteopenia and osteoporosis is an additional one. We do not have a broad population database of normative data for making those assessments. We explain what the issues are in some of the measurement tools for some of these outcomes, and how it may not be appropriate within that population. We really have the goal of them understanding that it should be on the radar screen.
I cannot respond more specifically on how to constrain individuals, other than trying to give them information that we hope they will review appropriately, but they may not. They may choose to do the test, which is sometimes easier to do than sitting down with the patient and taking the history and reading through the literature.
Dr. Ayanian: I would like to thank our presenters, including Dr. Shapiro joining us by phone.
MAKING BETTER USE OF PSYCHOSOCIAL AND COMMUNITY SUPPORT SERVICES; ADDRESSING EMPLOYMENT AND INSURANCE ISSUES
Moderator: Ellen Stovall, National Coalition for Cancer Survivorship
Good afternoon. This is the last session of the day, and we are getting down to the nitty-gritty in terms of how to make the best use of one of the most important recommendations of the report, the use of psychosocial support services for people with cancer and their families, and access to insurance for cancer survivors. We have four presenters today. Our first presenter will be Diane Blum from CancerCare.
Diane Blum, CancerCare
I am really pleased to be part of this. I will be talking about the survivorship services in the community. What I related to most in the IOM report was Chapter 4, where it talks about delivering survivorship care. There is a whole section in that chapter about survivorship services in the community, where in fact most people with cancer are. I just heard about wonderful programs at the University of Pennsylvania, Dana-Farber, and Memorial Sloan-Kettering in another session. However, most people are in the community, and they are not necessarily going to access programs that are at comprehensive cancer centers.
I am going to be giving a snapshot of CancerCare, of which I am the executive director, and how we provide survivorship services in the community. There are many other organizations besides us who are also providing services who are represented here: The Wellness Community, the NCCS, the Research Advocacy Network, the Lung Cancer Alliance. I do not want to leave anybody out, as there are many organizations that do this. I want to acknowledge that this really is a group process, with services that are focused on people living in the community.
I will just take a moment to introduce myself. I am Diane Blum, the executive director of CancerCare. I am also editor-in-chief of People Living with Cancer, the ASCO patient web site referred to this morning (ASCO, 2005). I am also on the ASCO Survivorship Task Force.
CancerCare was founded in 1944, and its services were confined to the person with advanced cancer. Most of the work was done with the families of the person with advanced cancer, because that person was dying, and usually died within weeks or perhaps a month or so that CancerCare offered services. That was the total focus of the organization, providing counseling and financial assistance to these families for the care of someone who was dying. However, today, 61 years later, we provide free professional
support to over 90,000 people a year. That support is now provided to people at all stages of cancer, and all diagnoses. The change was made in the early 1980s, when the board of CancerCare decided that providing services to people with advanced cancer no longer reflected the nature of cancer. More and more people were being treated with adjuvant chemotherapy, they were dealing with the issues of being treated aggressively, but they did not have advanced stage cancer. For that reason, the mission was extended to include people at all stages of cancer.
In 1990, CancerCare, influenced by the NCCS, which I became involved with at that time, started to focus on the issues of the work site and employment, and concerns that reflected survivorship. As an organization, CancerCare has evolved along with the needs of people with cancer. Most of our patients live for a long time now. They use our services for a long time, and we see them through all stages of their treatment and into a survivorship period. We offer services to survivors in three broad categories: emotional support, education, and financial assistance.
The first category is providing emotional support to survivors. We provide individual, family, and group counseling to people who are in the post-treatment stage. I am defining survivors here as people who have finished their active treatment. The services are provided face-to-face at 10 offices in the eastern part of the United States, they are provided over the telephone all over the country, and since 1996, online through our web site, which was launched in that year.
The issues that people who are in the post-treatment stage confront were described this morning. They are somewhat different than the issues people that are in active treatment face, as they focus more on uncertainty and fear of recurrence. We hear a lot from people about dealing with old problems. When you are actively being treated for cancer, many of your ongoing problems are pushed aside and you do not have to worry about them for the moment. Then you are told you are going to be okay, and all of these old problems come back and need attention.
People often describe to us their unpleasant sense of uniqueness, particularly those who are in developmental stages of their life where cancer is not common. They will feel very uncomfortable, and different from their peer group because of the fact that they are dealing with cancer.
CancerCare offers a program called the SurvivorCare Program. This is funded by the Lance Armstrong Foundation. The LAF has gotten a lot of thanks today, and we extend our thanks to them as well. CancerCare employs 50 social workers who are trained specifically to work with people in the post-treatment stage. We provide education, which mirrors our education program for those who are in active treatment. Our education is geared to help the person in the post-treatment stage understand, participate, and anticipate the challenges of post-treatment. Although we have
heard today about the survivorship plan, most of the people we deal with do not have any kind of written document like that. We try to help them understand what they should be looking for, the kinds of questions they should be asking, and how they should be participating.
Education may be done on a one-to-one basis with a social worker and a survivor. It is done most significantly through our teleconference programs, reaching thousands each year. The cornerstones of our educational programs on survivorship are the Annual Cancer Survivorship Series, which is done in collaboration with the National Cancer Institute, the Office of Cancer Survivorship, the NCCS, and the LAF. This past spring we offered three of these programs. More than 6,000 people participated in the live programs, and thousands more have accessed the archives on the web site. They are hour-long programs delivered in a supportive way, available to anyone who has a telephone. These telephone education workshops focus on long-term side effects such as fatigue and cognitive difficulties; insurance, including legal protection and trying to get through the insurance process; workplace issues; and health-promoting behaviors.
CancerCare also provides financial assistance to survivors. One of the gaps that was identified in the IOM report was this terrible problem of financial assistance. CancerCare has been providing financial assistance since 1944, and this last fiscal year we provided over $3 million in financial assistance. Just in the past year we have been able to extend the financial assistance to people with specific survivor needs—transportation for follow-up appointments, unreimbursed medication needs after $500, medical co-payments up to $500, and neuro-psychological assessments up to $750. These are small amounts of money, and our clients have enormous needs. Many people come to us who are uninsured or underinsured. However, small grants do make a difference to people, particularly for people who have little money. The financial assistance is also an entry point into our services. We can then try and work with the survivors to help them apply for entitlements and utilize other community programs. The financial assistance again mirrors what we have been doing since 1944, now extended to survivors.
We have organizational limitations in helping survivors, and I think these are the limitations that we have been talking about today. We are committed to extending our services because we see more and more of the people who we have helped come back post-treatment and say, “We still need help.” However, we are one privately funded organization, and many of the post-treatment problems we observe are societal problems. On the issue of insurance, we are just filling a gap. We are helping pay for a ride to treatment, but we are not overcoming a lack of insurance. We are one privately funded organization, joined by a whole group of other privately funded organizations, but I would argue that the problems require solutions beyond the private sector.
CancerCare’s mission for 61 years has been to provide free professional support services to anyone with cancer and anyone who cares about them. We are serving survivors now, but we have not made a direct mission change. If we were to go out and say our mission is now to serve anyone who has had cancer, we would have another potential 10 million people who could be making use of our services, and we would have a difficult time meeting the need. We have discussed this with our board, and although we are responding to this need and meeting the services, we have not done a full-fledged mission change to actually reflect this need.
The third limitation is highlighted in the report: the issue of reaching people in need. I personally think we have great resources, and many of you in other organizations also have excellent resources. However, it is highlighted in the report that people do not know about existing resources. One aspect of the survivorship care plan is people should be given a list of resources when they complete treatment. Those lists exist. All of our organizations have these lists, and they are readily available. The challenge is to try not only to develop this comprehensive list, which most of us are well on the way to doing, but also to figure out a distribution mechanism, so that patients actually are able to have access to help when they complete treatment. Many of the challenges that we are discussing today are going to be harder to meet, but this is a test that is realistic to complete. Thank you.
Ms. Stovall: Any specific questions for Diane, before we move on to Bonnie?
Dr. Mitch Golant, The Wellness Community: I am Vice President of Research and Development for The Wellness Community International, and on the board of directors for the American Psychosocial Oncology Society. Diane, thanks for your presentation. As a long-time practitioner in the world of cancer survivorship, it is really a pleasure to hear you talk about CancerCare. I want to make one pitch in this conversation and get the reactions from some of the others on the panel as well.
I was looking at the final presentations, and truthfully I was torn between attending this one and the other one. So, I was imaging this idea of lost in transition, and the challenge of survivorship is really about an evidence-based and community-based level of care. I imagine a workshop someday in which we would be making better use of psychosocial and community support services by investing in survivorship research through community-initiated research collaborations. We as a community have been serving so many cancer survivors, and the challenge has been measuring the effects that we have had historically.
Archie Bleyer gives a talk called “Minding the Gap Between the Needs of Children and the Needs of Adults with Cancer.” There is a gap, and I think we in the community have been serving that gap for a long time. I
think there is a need to invest in this kind of collaborative research endeavor. I know many of us in this room are passionate about what we do, and this report, which is like a bible to us, provides direction in some places. So, I would be interested in others’ thoughts about those challenges.
Dr. Teschendorf: Actually, I am going to address that in my presentation as well. I think it is critically important that we begin to look at the service delivery in a different way. We need to think very hard about the effects and the impact of our service delivery, and build in a quality improvement process. Then we can come back in another year and say what we know works in the community and what we know we have to modify or change over time.
Diane Blum: I guess the question I have is how do you actually make it happen? Do you use the structure that is already here and proceed?
Dr. Golant: At The Wellness Community we have done randomized clinical trials through the California Breast Cancer Research Program in order to look at the effectiveness of our programs. We work with Stanford University in particular, because that was an academic model, and we wanted to compare that academic model to a community model and services delivered in the community where there are such limited resources. We agree that The Wellness Community could not create an infrastructure like Stanford or Dana-Farber, but we could plug into that mainframe. We could collaborate with the American Cancer Society or others. It is the idea that the community has a plethora of options and services available that could be looked at in order to find out what is best in terms of the needs of cancer patients. So, that is how I would think about it, Diane.
Bonnie Teschendorf, American Cancer Society
I am very pleased to be here today, and as an IOM committee member I was also involved in the work that went on before getting here. It is very exciting for me to see the response of all the people who have come as well. When I started thinking about what we were going to discuss in this particular session, I decided to narrow it down. I am going to respond to four of the recommendations, and try and weave this together in the context of what the American Cancer Society is doing now and what is on our plate at the moment. Next week, we are going to convene a meeting to look at how we can weave these recommendations from this report into our future work. Some issues I want to discuss we are already doing.
We synthesize the science and find ways to translate evidence into practice. When I talk about practice, I am not talking about medical prac-
tice. I am talking about community-based practice, things that we would do for patients and families. We also want to be able to anticipate the needs of survivors and families. I will tell you a little bit about how we do that. It is a very important function, to be able to synthesize this body of information. Linking people to existing community services is also important. Diane addressed this issue. I will tell you a little bit about how the ACS does it. Then I will discuss some designs for targeted applications and service delivery, which I think is what Mitch was raising as an issue.
In terms of synthesizing science, we actually have a couple of departments in our organization at the national home office. The department I am seated in is the Cancer Control Science Department. Our mission is fairly complete, because there are several of us who are involved in taking science from several domains and then weaving together how we might translate that into work that would be done in the Health Promotion Department, the applied portion of our organization. They are using science-based initiatives as we move forward. We are also beginning to think about how we can analyze the portions of our organization that I call the legacy programs, the programs that we have traditionally offered.
Anticipating the needs of survivors and families is really a very important function for us. I think that we have learned that a call center is like a canary in the mine. When we listen to a number of people who are telling us the same story, we consider how we can meet those needs by developing program initiatives or developing new materials to distribute, either at the web site or through actual publications. In addition, the literature reviews that are produced out of our department often lead into some sort of research potential for other departments.
We have a number of relevant publications through our Health Promotions Department. They are not necessarily generated on a schedule or in response to certain time frames as would be the case with our guideline development, but these are focused on what we are hearing from cancer survivors as important issues. We recently published a book on palliative care and also have issued books on lymphedema and pain. Our web site is always in a state of development, if you will, in that we are trying to find new delivery methods and new ideas for engaging people, both families as well as the survivors.
We introduced the clinical trial mapping service about two years ago. It has been very successful, and people have appreciated it. We have most recently had an opportunity to interact more with physicians in clinical practice. One way which we are doing this is that we have started a detailing project, where we have hired staff to go out and talk to physicians in their practices.
I recently had the most interesting experience of being sent to the American Academy of Family Physicians to be an exhibitor. I am not usually an exhibitor, but I went because they needed someone who knew the science.
It was a revelation to me to see how interested family practice physicians were in what we were doing, and the kinds of issues that they raised. I came back with lots of ideas about applications that would meet the needs of this group, which I have now begun to transmit to other departments. We should always be at the AAFP conference. This was a new opportunity for us to learn from physicians and to find out what they want to know about cancer and need in order to work with their patients. They talked about patients who come back to them after they finish their treatment, and how there is a sense that they are not quite sure what they are supposed to be doing, but are working their way through it.
Linking people to community services has been a hallmark for the American Cancer Society for some time. We think that we are really good at it, but I am sure there is room for us to learn. We have a community resource database that has been developed through each of our division offices. In this database we can enter all of the services that are accessible in a particular geographic area. It might be support groups, or it might be some sort of family interaction. It might be information. There are a number of ways in which that information might be delivered, and we are looking for additional ways to respond to people who have requested information about service delivery in their geographic area. How can we link them through more than our call center? Can we do it in another way, maybe through our web site as well?
We will also use our web site to disseminate disease and treatment information. That has been in a steady state of development for some time. In the last two years, we moved from simply focusing on early diagnosis and early treatment, into looking at the whole disease trajectory. And that is a big change for our organization.
We also provide options for patients and survivors to communicate. We have had the Cancer Survivors Network online for some time. People tell their stories there and exchange ideas with one another, and it has been a nice source of emotional support for many of them. We have a call center that is open 24/7. The middle of the night is often the busiest time, or a very busy time, when people really want some answers. They wake up and are unable to sleep, because a particular problem is bothering them. More recently we have developed some navigation models that are being tested. This goes back to Mitch’s comment about testing in the community, doing some kind of pilot projects, and then going forward with development of a more detailed product.
Designing targeted applications is going to be part of the future for our organization. We need to help promote the idea of follow-up clinics. We need to move forward with the idea of detailing, and see what our results are, and we will have an analysis of that data. We really want to promote the idea of self-advocacy to get people involved.
Finally, I want to talk about exploring and testing new service delivery models. It has only been in the last several months that we have begun to put together people from several departments to begin to look at how we can use science to drive delivery of new services, and how to evaluate them. The program evaluation process is being formalized and built in as a combination of work from several different departments. Our Behavioral Research Center is in the process of hiring people who are used to doing program evaluation, our Health Promotions Department is coming up with ideas of ways that they would like to have more targeted interventions, and our Cancer Control Science Department is hoping to supply a non-stop service of information about the science. That is a little snapshot of what is going at the ACS.
Ms. Stovall: Bonnie, thank you very much. That is very helpful. Questions for Bonnie?
Ms. Iris Portny: I am a volunteer with Y-ME, and a seven-year survivor of inflammatory breast cancer. I have a couple of questions. First, when you talk about detailing to physicians’ offices, can you define detailing? Can you give us a sense of what that means? When you are contacting physicians to ask them if there might be information they want, what exactly are you focusing on there? Are you looking for feedback? Are you trying to find out what they think they need? Are you also giving them suggestions of information that they might need?
Dr. Teschendorf: Detailing is a model that is used in the pharmaceutical industry. There are pharmaceutical representatives that go out to physicians’ offices to provide samples and information about new drugs. We have adopted that model, and we are testing it to see if providing information in that way makes a difference in terms of physicians’ response. Detailing is very active and interactive.
Ms. Portny: So, it is looking for ways that you can better deliver the information that you already have, as opposed to also looking for new information you might want to be developing and collecting?
Dr. Teschendorf: I think it might be a little bit of both. I am sure that we will learn from this what they need, just as I said I did at the AAFP conference. At that meeting, we learned a lot about what physicians are looking for.
Ms. Stovall: Thank you.
Loria Pollack, Centers for Disease Control and Prevention
Thank you. I am glad to be participating in this breakout session. I am Loria Pollack and I work for the Centers for Disease Control and Prevention. I plan to give a brief overview of comprehensive cancer control, and how it may be useful in addressing psychosocial survivorship issues including those related to employment and insurance.
Comprehensive cancer control is a collaborative process through which communities and partners pool resources to promote cancer prevention, improve early detection, increase access to health and social services, enhance survivorship, and reduce the burden of cancer. Participants in comprehensive cancer control are diverse by design, and include many public and private organizations that come together to address these goals. The CDC’s role is to provide support for building an infrastructure for states, tribes and territories to form coalitions, to give guidance in comprehensive cancer control planning, and to assist coalitions in sharing approaches and strategies.
Comprehensive cancer control plans are one outcome of these coalitions. The process of developing these plans facilitates organizations to come together, plan and articulate goals and strategies unique to their state, tribe, and territory. The Colorado plan, for example, has an entire chapter that articulates the survivorship burden, objectives, and strategies to meet those objectives (Colorado Cancer Coalition, 2005). Currently, over 40 states, 2 tribes, and 2 territories have developed a plan, and others are in the process of either updating or completing a new one (Figure 5-2).
Most comprehensive cancer plans recognize survivorship issues, but the plans vary in how they address them. In some, survivorship is an overarching theme. Other plans mention survivorship in terms of a general goal or recommendation. For example, they may aim to ensure access to adequate supportive services, but lack specific strategies to achieve this aim. Other plans have a specific chapter with clear survivorship goals and strategies outlined. Few plans address survivorship-related financial issues, access to care, and legislation and policy as outlined so well in Chapter 6 of Lost in Transition.
I propose that comprehensive cancer control coalitions could be a vehicle to educate and advocate on the employment, economic, and other important psychosocial concerns of survivors. The established coalitions could be used to address these issues at a state, tribe, and territory level to help reach target populations and disseminate already established programs.
In the upcoming year, cancer leaders will meet at a Comprehensive Cancer Control Leadership Institute. Survivorship was chosen as one of six specific topic areas for the meeting. These leadership institutes can be a forum to recognize, include, and expand upon the various employment
FIGURE 5-2 Status of state comprehensive cancer control plans.
protection policies and legislation, financial aid programs, and other wonderful survivorship programs that are described in this report.
In response to Mitch’s question about CDC’s role in community-based health, we have established community-based resource networks in which there are community practices that we will ask to answer specific programmatic research questions. In the upcoming year I would like to see existing survivorship educational materials evaluated through these community-based programs so we reach beyond just those people who log on to peoplelivingwithcancer.org, or call CancerCare, or these other wonderful organizations. There are a good number of people who are not connecting with the programs that we are working so hard to develop and improve. CDC’s role in survivorship has been catalyzed with the development of the National Action Plan, which is now two years old (CDC and LAF, 2004). We are doing a lot to build upon that plan.
Cancer control planning is a process, not a product, because plans are always being developed and revised. Having the cancer control plans, being involved in the coalitions that are drafting and redrafting these plans, and connecting with the state, tribal, or territorial cancer coalition is a good way to get out to providers and survivors. More information about the CDC’s Comprehensive Cancer Control Programs is available at our web site and through recent publications (Pollack et al., 2005; CDC, 2005a; 2005a,b).
Ms. Stovall: Thank you, Loria. Any questions for Loria about CDC’s programs?
Dr. Golant: Please elaborate on CDC’s role in the planning process. That planning process is so robust, and I am thinking about all the different roles that are so meaningful in the process. Gathering information, planning, and disseminating information among all the community organizations is just so valuable. That is also what makes this Lost in Transition report such an important resource. I wanted to hear more about it.
Dr. Pollack: I think our role at the CDC was to help establish these coalitions in the states, but in no way to control or direct what they should be doing. It should be a very bottom-up process. What we can do, since we are a national organization and have a bird’s eye view of what different organizations are doing, is to facilitate the sharing of information. If one state has a fully developed chapter on survivorship and talks about incorporating survivorship and addressing insurance programs through the development of a high-risk insurance pool, we can take that to another state that is really struggling to address these issues, and they can use the developed plan as a guide and a model for their state. CDC’s role in survivorship with these programs is always to support any research programs that could establish ways to better outcomes and to improve care. However, as we heard today, we cannot always wait for the results of these research efforts, and have to support programs as they are.
Ms. Stovall: Thank you very much. Last, we have Pam Short, another member of the IOM Committee on Survivorship.
Pamela Farley Short, Pennsylvania State University
I am a Professor of Health Policy and Administration at Pennsylvania State University. I am a principle investigator on a grant from the National Cancer Institute that is following a cohort of about 1,800 cancer survivors, looking particularly at the economic consequences of cancer survival. In our cohort, which is from a relatively high socioeconomic status, our focus has been not so much on insurance as it has been on employment. I also have to confess that I am one of the people who, for my entire career, has been working on trying to solve the problem of the uninsured, which may take more than my career. We may have to interest some more young people in thinking about it. I served as one of the thousands of people on the Clinton task force, and had a chance to work in the White House when we were trying to see if we could do something about health security.
As I was sitting at the National Press Club yesterday, listening to reporters ask questions of some of my fellow committee members, I could
not help but think that this report that we have written asks a lot of its audience, because it is actually full of mixed messages. There is good news and bad news. Maybe that comes out of the fundamental ambiguity of being a cancer survivor. The good news is you are going to live, but the bad news is you are going to live with the risks and the unpleasant consequences of your cancer.
As we were reviewing what is known about employment and cancer survivorship, we wanted to bring out both the good news and the bad news. We wanted to emphasize that most cancer survivors continue working and remain productive after their treatment. For them, it is not really an issue of needing a lot of extra services or rehabilitation. For many, the big issue is discrimination of one sort or another, if there is an issue. But at the same time, the bad news is that there is a significant minority of cancer survivors, about 1 out of 5 according to the results from the Penn State cancer survivor study, who have ongoing disabilities that affect them at work (Short et al., 2005). While emphasizing the good news, we did not want the bad news or those problems to be lost.
I have to confess that I am pretty comfortable with these ambiguities in our report because I am an economist. Maybe you know that economists are very well known for using both hands when they give you the answer to any problem. Both of my hands start to twitch when I try to decide how hard we ought to be pushing to get more survivors to work more. The research shows that cancer survivors do work less than otherwise similar people. This is a good news/bad news ambiguous kind of situation, because on the one hand, working is a good thing. As the one survivor said in the film we saw this morning, work is about a lot more than a paycheck. However, on the other hand, working is not always a good thing.
My friend and fellow researcher Cathy Bradley is concerned about the statistics showing that now something like 60 percent of cancer survivors work throughout their treatment. Is that necessarily good? Are they working, wanting to be able to take that time for themselves, but continuing to work because they do not have the sick leave or they are not able to take the time away from work? Are they worried that if they somehow let down the team at work, they will not have the job to come back to?
My response to Fitzhugh Mullan’s issue that he raised about 11 percent of cancer survivors who are uninsured is that if you are a cancer survivor and you have any way to keep your health insurance, or if you have any way to get health insurance, you are going to do that. It does not surprise me that insurance rates are higher and that the percentage of cancer survivors who are uninsured is lower than it is in the general population. We even have some public programs like Medicare and Medicaid programs for people with disabilities that are designed to fill in those gaps. However, is it
a good thing that people are working for health insurance, when their health and their long-term quality of life would be better if they were not working through treatment?
It is also not necessarily a bad thing that some cancer survivors stop working and retire. If it is because they faced down a deadly disease, reassessed their life’s priorities, and found that their priorities do not necessarily involve going to work 50 hours a week, I am not prepared to say that that is a bad thing.
Now, when we move on to the subject of health insurance, I think there are fewer ambiguities. It is really much more clear-cut in my mind. First of all, clearly the changes in clinical care of cancer survivors that the committee has recommended are going to have to be paid for. That may require an agreement to expand the services covered by most policies, to provide reimbursement for services that are not otherwise covered, or to provide more generous reimbursement to get providers to do the things that we want them to do. I think it is unambiguous that these services are not going to reach cancer survivors who have little or no health insurance. It is not going to happen without the health insurance, and the committee wanted to point that out. That is one one-handed statement.
I will give you another one-handed statement: all cancer survivors live with more economic risks than the rest of us. Although cancer survivors are a very heterogeneous group, I think this is true of all cancer survivors, even the ones who have essentially no long-term effects. If they tell the truth on those applications, they are going to find that they have difficulty getting life insurance. They face risks involving what Karen Pollitz taught us to think of as the three As: access, adequacy, and affordability of health insurance. They may find that the legal protections that experts seem to agree have improved the situation do not necessarily eliminate the more subtle forms of discrimination in the workplace.
I propose one more one-handed statement—I want you to testify that you heard an economist willing to make a few one-handed statements—to say that these economic risks are everyone’s risks. We tried to point this out in our report. In this sense, while cancer survivors need healthcare financing reform and universal coverage or universal access to health insurance, I think perhaps that universal coverage and universal access may benefit from the political smarts and the political power of cancer survivors too. We are all at risk for cancer. The statistics are 1 out of 3 during a lifetime. That means we all have a stake in reducing these economic risks associated with cancer.
When I talk to people about insuring the uninsured or healthcare reform, what I try to emphasize—in fact what the health security idea tried to emphasize—is this is not about them. It is about our security. Knowing that the American public is as afraid as it is of cancer, and that this is an
issue that touches almost all of us, this may be a way that we can make a little progress towards reducing some of these economic risks.
There are things that we can all do. There has been a lot of talk about what oncologists can do, and what primary care physicians can do, and other sorts of specialists, but these are issues for all citizens, for all of us. There are things we can do in our own workplaces, in how we treat our co-workers, in how we make accommodations for our employees, and through our own willingness to pay for public and private insurance that is going to share these risks. These risks are shared through life insurance, they are shared through health insurance, and they are shared through disability insurance.
Ms. Stovall: Thank you, Pam. I love ending on that last message. It was such a privilege to work with both Pam and Bonnie on this committee. I look forward to continuing to work with all of you, every one of you on this panel. Are there any questions for Pam or for anybody else on our panel as we are attempting to end on time?
Mr. Robert Weiss, National Lymphedema Network: Just a comment. I am very heavily involved in legislative issues. I know that many of these organizations here today are either government organizations or 501(c)3 charities, and are not allowed to do much legislative work.
When you realize that eventually the cancer survivor is going to reach the age of 65 and be subject to Medicare, and that Medicare does not cover the treatment of lymphedema, you see that there is a terrible situation. There are something like 1.6 million cancer survivors who are at risk for lymphedema, and when you consider that it is not being covered, that is a terrible situation.
I urge you to consider setting up non-profit or lobbying organizations to do some grassroots work in getting legislation in the various states for insurance, and in the government for Medicare. There are a handful, I think six states now, that cover lymphedema care. Thanks to the ACS of Northern Virginia, which was the first organization that was able to get a state law for the treatment of lymphedema in Virginia. In January 2004 it went into effect. New York, Massachusetts, Connecticut, Georgia, and California have bills in the assembly or the senate right for the treatment of lymphedema. These are all grassroots efforts. There are teams in the various states.
We have had very few words on legislation in this conference. I urge you to get involved with that aspect of providing for the cancer survivor. It is so important. I will help to set up a team in your state. I have all kinds of materials. You just have to provide the people who are going to go into
your legislators’ offices with materials and have them urge their representatives to pass this.
Ms. Stovall: Thank you so much for your comment. I think that Bonnie and I were just thinking the same thing. The reason that the rocky road of survivorship has been so long is that we have a lot of reports over the years that have recommended precisely the kinds of interventions and support and reimbursement you are talking about, but they just sat on the shelf. Getting this report done by the Institute of Medicine is going to take organizations like the American Cancer Society and my organization and many others in this room a much longer way toward getting there than anything that has been put out there before, just because of the respect that these reports command with our lawmakers. You are in the right place at the right time.
Mr. Weiss: Thank you for the report. You can be guaranteed it will be used as early as tomorrow when I testify in Georgia to a health committee in legislation in Georgia.
Ms. Stovall: Wonderful. Congratulations. That is a great way to end our workshop. Thank you all very much.
INVESTING IN SURVIVORSHIP RESEARCH
Moderator: Patricia Ganz, University of California, Los Angeles
I would like to welcome our first presenter, Lois Travis, who is from the NCI Epidemiology Program. Lois has a lot of experience with second malignancies, and she is going to be talking to us about that.
Lois Travis, National Cancer Institute
Good afternoon. Chapter 7 of the IOM report was entitled “Research,” and in the last few pages the overall findings and recommendations were summarized. The section began, “Research is especially needed to improve understanding of mechanisms of late effects experienced by cancer survivors, how to identify and intervene to alleviate symptoms and improve function, and the prevalence and risk of late effects.” Today I am going to focus on the first and third items, which relate to mechanisms and risks. As noted in the report several times, transdisciplinary teams will be needed to further the research agenda.
To understand the mechanisms of the long-term complications of cancer and its treatment, we must first identify the relevant etiologic factors. It
FIGURE 5-3 Long-term complications of cancer and its treatment: Etiologic factors.
SOURCE: Travis, 2002.
is well established that radiation and chemotherapy are associated with late effects, however, numerous other factors can contribute to the development of late effects (Figure 5-3). These include lifestyle choices, such as tobacco, alcohol and diet; environmental determinants; and host factors, such as genetic susceptibility and co-morbidities. In addition, interactions between various factors occur.
A promising area for future research is that of gene-environment interactions. These include the effects of individual variability in carcinogen processing and detoxification. For chemotherapeutic agents, these relate to differences in drug absorption, metabolism, distribution, and excretion. The roles of pharmacogenetics, DNA repair, and other host factors should also be studied.
Characterization of interactions between exposures also will be important. A first step is the identification of the individual components, for example, treatment, tobacco, alcohol, or other risk factors. Second, what is the impact of the combined exposures on late effects? Is it additive, multiplicative, intermediate? With this information, high-risk groups of patients can be identified, with implications for screening and prevention.
It is important to be able to estimate the risk or magnitude of late effects. One type of epidemiologic study design that is mentioned in the report is the cohort approach. Well-defined cohort studies with complete follow-up can yield a number of risk measures including the relative risk and the absolute excess risk. The latter measure is frequently expressed as the excess number of events per 10,000 patients per year, and serves as a useful measure of disease burden. The cumulative absolute risk is the per-
centage of patients diagnosed with the event of interest in a specified time interval. For example, Mitch Gail and I, along with other investigators, recently published estimates of the risk of breast cancer after Hodgkin’s lymphoma by age, follow-up time, and type of treatment (Travis et al., 2005b). For women treated at age 25 years with a mantle dose of at least 40 gray without alkylating agents, the estimated cumulative absolute risk of breast cancer by age 35, 45, and 55 years was 1.4 percent, 11.1 percent, and 29.0 percent, respectively. This measure seems particularly useful for healthcare providers and patients.
It is always important to keep in mind that the risk of late effects varies not only by treatment, but also by age at exposure, gender, length of follow-up, co-morbidities, and other variables (Travis et al., 2005a). In addition, it should be kept in mind that these estimates may reflect the effects of therapies administered decades ago, which are no longer used.
As a foundation for future research in one area of cancer survivorship, the National Cancer Institute held a workshop last November on genetic susceptibility and second primary cancers. Over the last few decades, the number of second cancers has steadily increased. In 2002, over 120,000 new invasive cancers were reported to the Surveillance Epidemiology and End Results (SEER) program. Of these, second or higher order neoplasms comprised 16.3 percent, or 1 in 6. Second cancers are also important in terms of the significant impact that they can have on morbidity and mortality, often in patients who had considered themselves cured of cancer.
During the workshop, we brought together a trans-disciplinary group of investigators in epidemiology, molecular genetics, statistics, and many other specialties. This group proposed several recommendations for future research which are applicable to cancer survivorship in general, and which are reported in the Journal of the National Cancer Institute (Travis et al., 2006). The core recommendations from the workshop include the development of a national research infrastructure for studies of cancer survivorship; the creation of a coordinated system for biospecimen collection; the development of new technology, bioinformatics, and biomarkers; the design of new epidemiologic methods; and the development of evidence-based clinical practice guidelines. Thank you for your attention.
Dr. Ganz: I think in the interest of time we will hear our other presenters, and have questions at the end.
Sandra Horning, Stanford University
I have been interested in late effects of treatment since my initial publication as an institutional and cooperative group investigator. Today I thought I would focus on the American Society of Clinical Oncology activi-
ties and initiatives in cancer survivorship research as a means of demonstrating what a medical society might bring to this area. I mentioned our Survivorship Task Force earlier today, which Patti Ganz and I co-lead. This has been another link in a chain of activities at ASCO.
ASCO has activities and initiatives in: research methods; policy and advocacy; the promotion of scientific exchange; awards for survivorship research; the preparation of guidelines, which can then be used as the basis of survivorship research; and in communications, which we know are important to the many stakeholders in this area.
First, some of the research methods initiatives that are underway deal with barriers to participation. For some period of time ASCO, led by Lowell Schnipper, has been involved in efforts to promote central Institutional Review Boards. We have partnered with many others in this effort. We are co-sponsoring a workshop with the Association of American Medical Colleges and the Office of Human Research Protections (OHRP) to try to further understand the sticking points with central IRBs, and to discuss what the next steps may be.
We are working with the NCI on implementation of the Clinical Trials Working Group (CTWG) recommendations. Jim Doroshow, who was part of our cancer research committee, is helping us to understand how ASCO can best work with others to implement recommendations that will overcome research barriers. This, again, is an effort undertaken in partnership with many others groups.
We also have a number of education and training efforts related to research. One is the Vail Methods in Clinical Trials course, conducted in conjunction with the American Association for Cancer Research (AACR). Survivorship research methods are part of the course, which Patti Ganz participated in last year. We also have a workshop on clinical trials for the community oncology team, which is in its second year. It is important that the community oncologist be involved, because a lot of survivorship research will need to be done in the community. We are discussing efforts to bring this to a larger proportion of community oncologists, and also to integrate this kind of methodology into the training of oncologists who are going to go into community practice. In addition, we have a half-day symposium planned for our annual meeting in 2006, which will be a distillation of the Vail methods course, in order to bring this content to a larger group of individuals.
In the area of advocacy and policy, we have our Government Relations Council and Cancer Research Committee. We have partnerships with the Food and Drug Administration (FDA) and the NCI. We have provided commentary on drug safety issues, and on National Institutes of Health reauthorization and funding. We work with the FDA on critical path and clinical trial endpoints issues. We also work with the Translational Research
Working Group of the NCI; a subgroup of our Cancer Research Committee is currently considering ways to disseminate clinical trial results to study patients.
A major effort in the past several months has been to integrate survivorship into our scientific and educational programs. In our science program, we have a new track which is a home for survivorship research and presentation. Of note, this appeared in the plenary session 2005. We have given special awards for survivorship research. Survivorship has a place in the Journal of Clinical Oncology.
Our communications efforts have included our 2004 Meet the Experts media event focused on survivorship. The print media coverage of that event reached more than 110 million people, and there was additional national broadcast media coverage as well. We also have the People Living with Cancer web site (peoplelivingwithcancer.org) featuring articles on survivorship, where we partner with the Lance Armstrong Foundation on survivorship stories and content.
In the area of awards, in 2004, we gave four young investigator and career development awards. In 2005, we are identifying additional award needs and potential partnerships.
In the area of health services research, we are developing guidelines for long-term medical care of adult survivors, which cover cardiovascular effects, hormone replacement therapy, bone health, sexual function, neurocognitive and psychosocial concerns, and second cancers.
We have task forces that are looking at imaging and biomarkers and the integration of translational research. I think this is extremely important as we think about both the therapy that we are delivering now, and how therapy may be changing by early assessment with biomarkers, imaging or both. Changes in therapies may translate into changes in late effects. As we move closer to understanding mechanisms such as DNA repair, biomarkers become even more relevant. Bringing investigators working in these areas into the fold as part of the transdisciplinary research team is an extremely important effort.
In summary, our initiatives and activities fall into these important areas: research methods; advocacy and policy; scientific exchange; awards; guidelines; communications; and strategic partnerships. Thank you for your attention.
Julia Rowland, National Cancer Institute
I would like to take this opportunity to add my deep appreciation to the Institute of Medicine, and in particular the 17 superb members of the committee who were midwives, if you like, of the birth of this report that our office has been very eagerly awaiting. We are very excited that it has
now appeared and is on the street. I also want to publicly acknowledge my gratitude to the Institute of Medicine, ASCO, and the NCCS for convening this symposium, and for putting in place other meetings down the road to really pick up on the momentum generated and not let this report be shelved.
As director of the National Cancer Institute’s Office of Cancer Survivorship, I am going to make a public commitment that I will do everything in my power to help us realize and implement the recommendations of this report, as long as I am in this position. In particular, I note those recommendations that have identified the National Cancer Institute as needing to take a leadership role. The most significant of these being to grow, support, and shepherd the survivorship research arena.
I want to also thank my predecessor and founder of the position as the director of the Office of Cancer Survivorship, Anna Meadows, for saying that despite being included as the last chapter in the report, it is all about the research. We cannot have guidelines or develop care if we do not know what the issues are, where they are appearing, who does and does not get them, and what interventions are needed. Anna, my response to your comment about the place of research in the report is that it is the backbone of the book. I am a psychologist, and for those of us who do cognitive therapy and cognitive studies, we know that it is the last thing on the list that you remember. We are all going to walk off knowing that research is the most important thing in there, because it is the last chapter. It is the last piece of information you heard that you will walk away with.
John Ayanian already delivered my talk during the plenary. I told him that and he said, “Do not worry about it. That is the music. You come back and tell them where the crescendos are.” My job is to tell you the high points in here. I want to talk about three things: the challenges to doing this kind of research; the opportunities that we have right now; and where I think we need to go in the future.
I think one of the critical points that we forget about or take for granted is that the long-term or late effects of surviving cancer are literally and figuratively a moving target. We heard Doug Ulman speak about the fact that our survivors are a mobile population. It is hard to keep track of them, regardless of whether they are pediatric or adult survivors. We lose them pretty much about two years after the completion of their treatment. Many of them disappear. Furthermore, as they continue to age, the issues are changing over time. If we want to know the long-term and late effects, we must be following survivors long-term. We also know their health is a moving target. If you consider the fact that 60 percent of those who are survivors today are 65 and older, it is quite likely that they had one or more co-morbid illnesses when they were originally diagnosed. Trying to understand and tease out what is cancer-related and what was pre-existent, but may be exacerbated or affected by the diagnosis and treatment of cancer, is
a real challenge for us. When we get five years beyond diagnosis, which is the least-studied time period, we know even pitifully less.
Much of the research that has been published historically has been atheoretical. We cannot go forward in this fashion. We must be thoughtful about why we think these kinds of problems are going to develop, generate a hypothesis, and consider the models that we are testing, because that is the only way we are going to answer the question of what the interventions should be. We have to have appropriate tools. We have outstanding measures now for active treatment, including quality-of-life measures and quality-of-care indicators. Post-treatment, though, there are very few tools that are available to us. There is also the issue of how we are going to look at co-morbidities, and how we measure those and compare these conditions in survivors with those reported in the non-cancer or general population.
We have already heard many speakers talk about reaching this population. The Health Insurance Portability and Accountability Act is not going to make it easier. Institutional Review Boards are a hurdle for many. Informed consent for patients is also going to be an issue. We clearly have to do extra work to include our minority and underserved, harder-to-reach populations. This will require a bigger investment in efforts to understand how to access these individuals, recruit and retain them in our studies and research, and to make these data available.
Then there is the issue of securing funding. Here we need to realize that it is not enough to study the problems of survivorship without developing interventions. NIH-wide, about 40 percent of the studies that were funded in survivorship in fiscal year 2004, 217 if I remember the number correctly, have an intervention component. That is about the right portfolio mix, as we would like to see 40-50 percent at any given time. We know new treatments are going to have new late effects. We have to find out what those are, and then develop interventions. That is going to be very important.
However, we know that intervention research tends to be very expensive if you are not just giving a pill. Behavioral intervention and education, which is the backbone of almost everything we do in the intervention arena, tend to be expensive. They are personnel intensive. We need to monitor for fidelity of delivery of these interventions. They often involve behavior and lifestyle changes and again, we need to be mindful of the costs and difficulties associated with these areas. It is very hard to get this research funded as we are competing with basic science and treatment-focused research. Further, we, specifically behavioral scientists, sometimes defeat ourselves at the review table. If we are going to fund this type of research, we have to take ourselves very seriously and say that we need to be supporting this research, including at the review table.
Finally, the transdisciplinary aspect of this research is an issue (Box 5-4). This really is a very special kind of science. It is not just different disciplines
|
BOX 5-4
|
in the same room thinking in their own way. It is not even just an intersection, such as psycho-oncology where mental health professionals are addressing cancer issues. It is truly broad spectrum. You want pediatric perspectives and geriatric perspectives. You want the physiologists, clinicians, and mental health professionals involved. It really is a complicated science that has to be done in a transdisciplinary fashion. Again, that requires attention to recruiting diverse expertise and promoting cross-training.
We also need to sustain the necessary infrastructures that we have already built, and ensure the continuity and support of key resources and repositories. Importantly, it does not matter what resources we have if we do not have the personnel both to support and utilize these resources. That means we need training programs. It is wonderful to hear that ASCO is supporting these kinds of initiatives. Certainly, at the NIH, we need to do better in adding more money to training and retaining dedicated researchers in this field. People get scared when money gets tight. If junior investigators cannot envision a secure future in this field, I am worried we will not be able to recruit them. That is an important concern.
I think we also cannot overestimate the need to change the mindset, as we have heard again and again today, about survivors’ care. It has to be conceptualized as spanning the continuum starting at diagnosis and extending long-term. Interestingly, we are seeing some of our cancer survivor population demanding and getting what we do not even provide for many individuals who do not have cancer, and that is preventive health, health promotion, and disease prevention. We do not have that as a standard model of care. We haven’t designed a healthcare delivery system for adults that encourages anybody to deliver preventive medicine, ask for it, or to engage in it. It is a real problem. Maybe cancer will be the model or driver for helping us solve this issue. It is something we have to be thinking about all the time.
The final challenge to investment is the competition for funds. I have said earlier that we compete for much of the dollars and resources coming to the NCI, monies that are rarely distributed evenly across the cancer control continuum. We always have to be cognizant of that, and reflect on what the best mix of those dollars is, and what is the most equitable.
Despite these challenges to survivorship research, I am very excited, and feel it is wonderful to be here at this point in time. I think as Patti said we are at a critical juncture. We have tremendous momentum that is building. We have cancer survivors in leadership positions (e.g., NCI, ASCO), which is incredibly empowering. We have this new report on the street that we can build on with an evidence base. And, we have now invested, particularly at the Cancer Institute, in a variety of platforms to pursue survivorship research. These data resources include our clinical trials groups to our cohort and epidemiologic studies that are looking at big populations to see who gets cancer. Within these latter studies, we now have growing numbers of cancer cases that can be used to ask questions such as “What were they like before they developed cancer?” “What can we tell about them on the other side in terms of their survivorship?” “Is there information buried in those studies that we can leverage?”
Various administrative, linked data sources are all well described in the IOM report: SEER-Medicare linkages; the Cancer Research Network; the CanCORs database; and the practice-based research networks we heard about. There are also descriptive population-based data sets from the National Center for Health Statistics, including the National Health Interview Survey and the Behavioral Risk Factor Surveillance System, which you heard John Ayanian talk about earlier. Again, all of these resources allow us to compare our cancer survivors with non-cancer populations, and examine the relative burden of having a diagnosis of cancer. We can use this information to, over time, monitor these burdens, and determine whether we are making progress in reducing cancers’ cost.
The ongoing samples of survivors that the American Cancer Society is supporting and various registries abroad that we have heard about represent additional outside sources of information. In summary, we have a rich and broad array of data resources that we are poised to extend and operationalize to address survivorship issues, and to me that represents an incredible opportunity.
I want to end with what I think are several key targets for investment, and I speak on behalf of our office. We talk regularly about these issues, and this list reflects our collective experience (Box 5-5). I want to emphasize two issues in the area of essentially fundamental research.
Understanding the role of, and impact of, survivorship on caregivers is a fundamental research area in need of pursuit. For the most part, these individuals are family members. As more care is being pushed out into the
|
BOX 5-5
|
community, and as more people experience cancer as a chronic illness, caregivers and family members are going to be a key element in sustaining, supporting, providing interventions to, and altering outcomes for survivors. As a consequence they need to be a population that is targeted for research.
Surprisingly, when we look at our nationwide topical portfolio analysis, what is fundamentally lacking in survivorship-focused research are basic science studies. We do not see gene-environment studies that address survivorship questions. We do not see mechanistic studies of what it is about specific drugs and treatment exposures that, whether due to their biological, biochemical, or molecular effects cause toxicities long-term. We do not have an established base of bench science in survivorship research. It is simply not someplace that our scientific community has gone. I think hidden in this neglected arena may be some provocative and rewarding frontiers to explore.
Clearly, nobody is going to achieve the many mandates outlined alone. Fortunately, the IOM and ASCO have brought all the partners together at this meeting. This effort has to be all about partnerships. By way of illustration, we at the NCI need to find a way to integrate what we do not just across the NCI, but also across the other institutes: Aging, Mental Health, Nursing, Neuromuscular Diseases, and Heart, Lung, and Blood. We need to draw upon that collective scientific expertise and bring it into the cancer arena. We also need to interface with our advocacy partners, our voice out there, and our champions if you like, in promoting what we do. We need to
partner with diverse entities so that we can coordinate our efforts, not be duplicative, and use precious resources in the best way possible.
A third target for future investment is exploring delivery systems with the potential to bolster the larger research agenda. Can we use the Cancer Information Service, personal digital assistants, the Internet, and the other new technologies that are available to deliver interventions? Can we use the Community Clinical Oncology Programs to get interventions out there? Are cancer centers using them as delivery platforms for promoting cancer survivorship research or care? Does the research say how survivorship care should be delivered? What is the best way to do it? What are the costs and what are the associated benefits? What are the various models that are going to be most effective, efficient, and equitable that we can use to deliver care that will improve survivors’ outcomes?
A fourth area for investment is in communication. It will do us no good at all if we do not communicate what we know or have learned. Fundamentally, what the survivorship care plan is all about is communicating what science has taught us. We have to be able to talk to one another, not just the researchers, but also the clinician, patient, consumer, and payer communities. Communication is going to be very key, and we live in a big communication world. There is more and more information being pushed out there. Helping people access it and understand it is increasingly important. It will be vital to know what they need, when they need it, and how they need it.
Finally, as mentioned earlier, while we are at this very momentous point, we need to think about what our benchmarks for success are going to be. Is it to have 15 million survivors? I do not think so, because knowing that we have 15 million survivors is really not very helpful. We need to know what it is that we are trying to improve. We need to know about the quality of life of those survivors and the quality of their care. We need to know whether we are reducing the cancer burden, or whether this cost is simply escalating. We need to know if we can prevent some of the long-term effects. Importantly, we need benchmarks now before we go too far down the road. I know that even if we set them up, we will later think, “Gee, why didn’t I ask that?” as all of us in research have done. However, we have to be thoughtful now, because we have an obligation to mark our progress, and not just programmatically. When you go back down to Congress, or to the cancer institute director for that matter, you need to say where you expect to go and what you expect to achieve with the public’s investment. We need to be able to say that we are going in the right direction, and that we are doing what it is that we set out to do. I think that is going to require us to have a thoughtful dialogue, and to look at the many levels of metrics that we may need in place to be able to do that. Thank you.
Dr. Ganz: Next we will hear from Frank Johnson, a surgeon who was a member of our IOM panel from St. Louis University.
Frank Johnson, St. Louis University
Thank you. Our committee has endorsed a previous recommendation of the Institute of Medicine dealing with health insurance. A rational national universal-access medical system that is affordable, accessible, and acceptable to meet society’s needs would benefit cancer survivors probably more than anything else that has been discussed today, but I do not want to dwell on this. Quite a bit can be accomplished with the current system that we have, and also could be accomplished within a rational national healthcare system that I just mentioned. I am a surgical oncologist, and cancer patient follow-up has been one of my academic interests over the last several years. I am particularly interested in the follow-up of patients that have been treated with primary curative intent surgery, plus or minus chemotherapy and radiation therapy.
Research from this work has shown that existing guidelines, whether published in books or journals, from prestigious societies and institutions, are largely based on the opinions of experts. They vary widely. My colleagues and I have estimated that the cost difference among the recommendations of highly acclaimed institutions and organizations varies, usually by a factor of five, sometimes up to 100 fold. We have also discovered wide variability among experienced, highly credentialed clinicians who are caring for patients with particular cancers in terms of the intensity of follow-up that they provide. Such patient groups include survivors of colon cancer, lung cancer, prostate cancer, melanoma, sarcoma, and rectal cancer. In all of these instances we have found a great deal of variation, which we think is probably unwarranted. This conclusion relates to recommendation 10 of the report: “Answers to the following basic questions about survivorship care are needed: How frequently should patients be evaluated following their primary cancer therapy? What tests should be included in the follow-up regimen?”
How do we get rid of the apparently unwarranted variability in the clinical practice guidelines that have been put forward in the literature? How do we settle on guidelines? How can we get evidence-based guidelines? Low-quality evidence such as expert opinion, retrospective data, and evidence from registries has not proved very persuasive, because we do have variability in recommendations from the leaders of this discipline. We know that randomized clinical trials are feasible. They do change practice, and as evidence for this I cite the two Italian trials of breast cancer patient follow-up that were published over a decade ago (GIVIO, 1994). Over 1 million randomized clinical trials have been published in the literature since the
first one in the late 1940s. Nonetheless, the Institute of Medicine has estimated that only about 4 percent of the medical decisions we make as clinicians are based on high-quality evidence (IOM, 1992). Therefore, we have to select the targets for randomized clinical trials very carefully. We know that prevalent clinical problems can be effectively studied with randomized clinical trials. Things like treatment of hypertension, whether patients with coronary artery disease should be treated with surgery or angioplasty, and tight or routine glycemic control of diabetics have been addressed in clinical trials. I think we all would agree that proper cancer patient follow-up should be subject to randomized clinical trials of the same sort that has proved very effective in defining the standards of care in our country and, for that matter, around the world.
Using reasonable assumptions, I estimate that about 1 million people enter cancer patient follow-up each year in the United States after primary curative intent treatment. Almost all of the trials that have been conducted so far dealing with cancer patient follow-up after initial treatment have been underpowered. Many trials have been based on very few patients and purport to define a standard of care, but they are not persuasive. Large randomized trials, however, are difficult to carry out, as they take a lot of cooperation and institutional support, several years to accrue enough patients to meet the target goals, and several more years to get results. Other speakers have addressed the potential drawbacks of these trials, namely the moving target, different strategies, and improvement in treatment.
The goals of follow-up include, first, detection of the recurrence of the index cancer. This is what patients come to your office or my office and ask about. Has my cancer come back? I think this is job number one. Detection of second cancers is, in my opinion, a very important goal as well, because often these are relatively easy to treat and they tend not to be as advanced as the earlier primary. Quality-of-life issues have been discussed at some length today. Detection of long-term effects of therapy is another very laudable goal. These can be, and have been, inserted into randomized controlled trials.
As I said, there have been a few adequately powered randomized clinical trials of cancer patient follow-up. The two breast cancer trials were done by Italian researchers. The Italians have also almost reached the target for completion of a colorectal cancer patient follow-up trial, comparing intensive versus minimalist follow-up strategies. There is a British trial in the works on sarcoma patient follow-up, comparing minimalist versus aggressive follow-up. There is another British trial of colorectal cancer patient follow-up, which amazingly includes a no follow-up arm. But where are the American trials? There are no American trials of adequate power that I am aware of in any of the solid organ tumors dealing with the best way to follow such patients. The committee feels that the United States should
explicitly set out to design such trials and carry them out. I am glad to know that some of the decision-makers that can authorize such trials are present in the audience.
As Floyd Bloom said in his presidential address to the American Association for the Advancement of Science, there are a huge number of potential variables: a large number of described medical conditions, a large number of drugs and dosages, many guidelines, and millions of rules. We already know that there are hundreds of different kinds of cancers, and many hundreds of potential follow-up tests to be used. We can only focus on the most important problems to deal with in randomized clinical trials. We know that the anecdotal and other low-quality evidence has not been persuasive. We know that the actual practice of expert clinicians varies widely. The variation in actual practice does not seem to be influenced greatly by the age of the doctor, the initial stage of the tumor, the geographic location of the doctor, or by the managed care organization penetration rate in the area where he or she practices. These are the conclusions of some of the research that we have done, and I do not have time to show you the data on which those conclusions are based. We have concluded that the major reason for the variability in actual practice and promulgated in guidelines is the lack of high-quality evidence on which doctors, patients, and insurance companies can base their decisions. This is not news to this audience. This variability, however, results undoubtedly in overuse, underuse, and misuse of medical care resources, and probably costs some patients their lives.
A research finding from Phelps and Parente (1990) indicated that there is some potential that the investment of money in trials to determine the best form of follow-up for patients after potentially curative treatment may actually save money in the long run. It could be by one or two orders of magnitude. Such research has been felt to be beneficial.
What I hope to do today is to help the decision-makers allow us to do what I think everybody in this room would like to do. That is to base the care of patients that have had curative treatment for various sorts of cancers on high-quality evidence. Making these investments in research is working towards a highly attainable and very obvious goal. It involves billions of dollars, because cancer patient follow-up testing, counseling, and administration of corrective actions is very expensive. The benefits of figuring out how best to carry out post-treatment follow-up include better outcomes for our patients, and possibly some cost savings. Thank you very much for your attention.
Dr. Ganz: I think we have about five or ten minutes for questions.
Dr. Eva Grunfeld, CancerCare Nova Scotia: I wanted to thank everybody for their presentations. Julia, you were so exciting, you made me want to
rush out and write that protocol right away. I am on the Research Advisory Committee for the Canadian Breast Cancer Research Alliance, and we have just launched our first ever RFA on cancer survivorship. It has been an up-hill battle keeping it on the table as we vied for funds with the basic scientists.
Frank, I would like to comment on your issue about the payback on clinical trials, on the cost of doing the clinical trials, and whether we actually see a cost savings from the results of those clinical trials. We did a modeling study in which we modeled the cost of doing a randomized controlled trial on intensive versus minimalist follow-up for colorectal cancer, and assessed how long it would be before we would get the payback on that clinical trial. We estimated it would be about four years if the results are implemented.
As a slight outsider, I do want to raise one point, because I was a little perplexed about the report, and I wonder if you can give me some insight on it. The report I think is excellent. I was struck, however, that in the models of follow-up care which you identify worthy of further investigation you identified the shared care model, a nurse-led model, and the survivorship clinic models. I am struck by the fact that the primary care model, which has the strongest, largest evidentiary base to it, was not one that was recommended for further testing. I think there is a slight disconnect between the evidence you are presenting in the report and the conclusions that you are coming to. I would be interested in your views on that.
Dr. Ganz: I am not sure if others want to respond to this, but I think your work was in fact cited in the report. One of our concerns is that in the United States many people do not have primary care or they do not turn to their primary care physician as the first source of care in the way they do in the U.K. or in Canada. The shared care model seemed to be more appropriate in our setting.
Dr. Grunfeld: I appreciate that there is a different system. What strikes me is that you are suggesting three models for testing. You are not suggesting three models for implementation. Given that this report recommends a research agenda, it would be consistent with the evidence to include researching a primary care model.
One point, indeed, the two countries in which I tested both of those models have the same response. It is inappropriate. The family care physician cannot do it. They came to it equally cynical. That is an outsider’s view on the report that you might be interested in hearing.
Dr. Ganz: I think part of the situation is we have people in this country who never go to see their primary care physician. I have patients who are in
health maintenance organizations who have a primary care physician that they have never seen. They are assigned only in name to that physician. That is often the norm here, rather than a relationship that has been established. We would not even have a primary care physician (PCP) to test the model with. You at least had PCPs that people had identified as caregivers.
Dr. Rowland: Hopefully, we are going to have some data from the SEER registry soon looking at where people are getting their follow-up care for the major cancers in this country. At present, we do not even know where the bulk of our older survivors actually are getting their care. We suspect it is largely with primary care providers. While survivors may come in for specialty care when diagnosed, they go back out into the community after treatment ends. You raise an interesting empirical question for which I hope we can get answers. Also, I cannot wait to see your research. The hook is to get the basic sciences interested in survivorship, and then we will get the money.
Dr. Jerome Yates, American Cancer Society: Julia, I would take the opposite tack. Unless you get funding with RFAs in very targeted, well-defined subsets of populations, I think it will be extremely difficult to get this kind of research funded and to compete with the basic sciences. All you have to do is watch what is going on at the NCI now. At the ACS, 70 to 80 percent of the money goes to basic bench laboratory research. I am hoping to twist that around a little bit in the future, but this is a problem.
One of the biggest problems with research in this area is you are dealing with low event rates. We see only the serious complications in relatively early follow-up. We also have large populations that are really heterogeneous. We need to think about being able to shift some of the administrative data sets from CMS and some of the large insurers to collect the information that will tell us about exposures. Maybe old people who have had a fair amount of platinum lose enough nephrons that they get into renal trouble when they are 75, when they would not have if they had not been exposed to these drugs. We do not have that kind of information, and I think that that is a real problem. It is something that we could try to address and see if we could use these large administrative data sets to help us sort out some of these problems.
Lastly, I think we do know some of the things that cause problems. Clearly adriamycin affects heart disease in combination with atherosclerosis and some of the neurotoxic agent’s effects in older people may be worse than they are in younger people. We ought to take some of those things that we actually know can cause problems, subset those populations, and study them in terms of the follow-up, rather than studying the whole general
group of breast cancer patients, or colon cancer patients. We need to break it down if we want to get some payoff with a relatively modest investment.
Dr. Horning: In preparing for this session, and speaking with Lois Travis, I was reminded that earlier this year I got a note from our cooperative group saying that they wanted to discontinue follow-up in as many clinical trials as possible because our budgets are flat and expenses are going up. I was advised to check off the trials for which we could discontinue follow-up in five years. I do think we are missing opportunities to think about economies of scale in coordinating survivorship research with ongoing clinical trials intervention. I agree fully with that last statement that we should be selective about the clinical trials that we choose for follow-up. We should choose trials that are representative of the different questions that we want to ask, such as questions about underserved populations and questions about differences in age and exposure. We have clinical trials now that have very different interventions, and those would certainly be at the top of the list to highlight.
I think that we are missing opportunities to work together in thinking across the broad clinical trials efforts, and selecting the menu that can cross horizontally and vertically to catch most of these areas that we would like to study further.
Dr. Sheldon Greenfield, University of California Irvine: If we followed these notions of imaginatively focusing on a limited number of questions, it could lead to quality measures. We could pick out a half a dozen.
I wonder if an NCI goal for the next two or three years might be to find data, not necessarily from trials, and come up with a half a dozen measures given the pressure that is put upon us by everybody. No institute would ordinarily take up that goal, but the pressure is mounting. If we wait for trials, we may not get there in time. Julia, maybe you could respond to that.
Dr. Rowland: Thanks, Shelly, a very thoughtful comment. The large shift that has occurred at the NCI is the growing commitment to dissemination. We are looking at what the quality indicators should be, and how we push the science out. I think the answer to it used to be that we were content just to be the generator of the evidence base, and then we let everybody else worry about how it is applied. I do not think that is true any more.
There are a number of ongoing projects, including collaborations with other entities, to look at some of the benchmarks. Molla Donaldson at NCI could generate a list of projects better than I can.
Maybe one of the reasons that I put that point at the end of my slides is in part because I am perennially frustrated when people ask me about the
numbers of survivors. Well, 10 million. So what? It just tells me who is alive after a diagnosis. It tells me nothing about the health and well being of that population, or where they are in the trajectory. Just as a simple marker I would love to be able to tell you more about who is in active treatment, who is really in this post-treatment survivorship period, who has progressive disease, what their health status is, and how they are different from peers who do not have a cancer history. I think those are very real questions, and a very appropriate task to ask NCI to take a lead on, but certainly not as the only stakeholder.
Dr. Winn: We really need a parsimonious set of measures moving ahead. The difficulty right now is what they should be. One of the next steps could be to think in terms of a framework of quality measures which would get at the parameters, the scope, the priorities, and the best practices out there. Eventually you can come to say, “Let’s look at these 20, and then maybe we can get down to the 5 or 6 that are the ones to go forward.”
Dr. Travis: I want to thank Julia for the nice explanation of transdisciplinary science versus interdisciplinary and multidisciplinary science. It is possible to include basic scientists in our research, because we have ongoing a large international study of survivors of Hodgkin’s, breast cancer, or testicular cancer. We are looking at second cancer risk in these patients, and have the basic scientists helping us in this new field of molecular epidemiology. They are trying to figure out the markers of DNA repair and other markers that might determine who is at the highest risk of second cancers.
For people who are studying gene-environment interactions, this is a wonderful forum to do this type of research because it is one of the few situations where you have humans deliberately exposed to carefully measured amounts of carcinogens such as radiation and chemotherapy. You can look at the measured doses, compare them to the outcome, look at differences in various DNA repair genes and other markers, and then determine who is at the highest risk and how you predict that. It is possible to incorporate them, and I have several bench scientists involved in our new study.
Dr. Johnson: The American College of Surgeons Oncology Group represents a large group of surgeons. Surgeons, I will remind the audience, are responsible for most of the cures of cancer in this country, with or without chemotherapy and radiation therapy, to give those other disciplines their due. This group has the advantage over other groups of being able to easily obtain a piece of tumor tissue, as well as a sample of normal body tissue or fluid. Important information can be gotten from these carefully stored bits of tissue.
The American College of Surgeons Oncology Group has proposed trials of long-term follow-up in patients treated surgically with or without chemotherapy and radiation therapy, using the repository containing the original tumor tissue and a sample of non-tumor tissue. Those trials have been rejected by the Cancer Therapy Evaluation Program (CTEP) of NCI. I speak for the American College of Surgeons Oncology Group when I say this should change. There should be an acceptance of this group’s strengths, and a willingness to fund follow-up trials using the available tissue to do the correlative studies that everybody agrees are so valuable.
Dr. Ganz: I am going to let Dr. Horning have the last word.
Dr. Horning: I wanted to support what Lois just said. My strong feeling is that the integration of the basic science is absolutely imperative to move this field forward. Among the biggest news in science in the last couple of weeks are the results of HapMap project.1 People are debating about what this is going to mean in terms of the prediction of disease and possibly implications for prevention. There is a feeling that this is going to impact our understanding of how individuals handle different drugs. Cancer chemotherapeutic agents are going to be lead candidates for study.
The other point I want to make is that I do not think it is an either/or situation. It is not, and should not be, that we are competing for the same funding. This is a perfect time for team science. It is a perfect time for multiple principle investigators. I think we have to think about how we can work together and collaborate.
What we really talked about a lot today is the fact that we wish we had more evidence-based guidelines and measures for today’s patients. We also know that there is a built in latency period for many of these side effects to play out over time. We critically need the biomarkers and surrogate markers to predict them as early as possible, so that intervention strategies can be employed.
Dr. Ganz: So, I think we are about ready to have the rest of the group join us. Thank you all.
|
1 |
The International HapMap Project is a partnership of scientists and funding agencies from Canada, China, Japan, Nigeria, the U.K., and the United States to develop a public resource that will help researchers find genes associated with human disease and response to pharmaceuticals (www.hapmap.org, accessed December 28, 2005). |







































































