8
Action Agendas for Oversight, Regulation, and Payment
CHAPTER SUMMARY
Legislation, regulation, accreditation, payment mechanisms, and the media shape the way health care is delivered. This chapter proposes ways for these functions to motivate the adoption of practices and technologies that can reduce medication errors, and to ensure that professionals have the competencies required to deliver medications safely.
Earlier chapters of this report have presented the committee’s recommended action agendas for patients (Chapter 4) and health care providers (Chapters 4 and 5). Those two chapters are concerned with the first three of the four levels of the chain of effect framework (Berwick, 2002). This framework characterizes the American health care system as comprising the following four levels: the experience of the patient (level A); the functioning of small units of care delivery (“microsystems”) (level B); the functioning of the organizations that house or otherwise support the microsystems (level C); and the environment of policy, payment, regulation, accreditation, and professional education (level D) that shapes the behavior, interests, and opportunities of the organizations at level C. Players at the environmental level include legislators, regulators, accreditors, payers, patient safety organizations,1 and educators. The following recommendation addresses this environmental layer.
Recommendation 7: Oversight and regulatory organizations and payers should use legislation, regulation, accreditation, and payment mechanisms and the media to motivate the adoption of practices and technologies that can reduce medication errors, as well as to ensure that professionals have the competencies required to deliver medications safely.
-
Payers and purchasers should continue to motivate improvement in the medication-use process through explicit financial incentives.
-
CMS should evaluate a variety of strategies for delivering medication therapy management.
-
Regulators, accreditors, and legislators should set minimum functionality standards for error prevention technologies.
-
States should enact legislation consistent with and complementary to the Medicare Modernization Act’s electronic prescribing provisions and remove existing barriers to such prescribing.
-
All state boards of pharmacy should undertake quality improvement initiatives related to community pharmacy practice.
-
Medication error reporting should be promoted more aggressively by all stakeholders (with a single national taxonomy used for data storage and analysis).
-
Accreditation bodies responsible for the oversight of professional education should require more training in improving medication management practices and clinical pharmacology.
The remainder of this chapter provides a detailed discussion of this recommendation.
GUIDING PRINCIPLES
In developing the above recommendation, the committee took the view that environmental-level stakeholders—legislators, regulators, accreditors, payers, patient safety organizations, and educational accreditors—should:
-
Encourage recognition that the use of drugs should take place in a learning environment in which there will always be more to learn about the balance of the effectiveness and safety of drugs in terms of both their intrinsic properties and the ways in which they can be used.
-
Use laws, accreditation practices, payment mechanisms, and the media to foster the safety and quality of medication use.
MOTIVATION FOR PROCESS IMPROVEMENT
Process improvement is primarily the responsibility of providers who must redesign processes at the microsystem level (see Chapter 5). There are, however, key roles in process improvement for legislators, regulators, accreditors, payers, and patient safety organizations.
There are two separate but linked pathways to quality improvement using the measurement of health care performance (see Figure 8-1) (Berwick et al., 2003). Pathway 1 uses performance measurement for accountability purposes—allowing patients, accreditors, and regulators to know how well a particular unit is performing—and for selection purposes—helping patients, referring clinicians, and purchasers decide which providers to use for the services they wish to purchase. Pathway 2 uses performance measurement to design and implement new processes for delivering higher-quality care. The two pathways are linked through the motivation for process
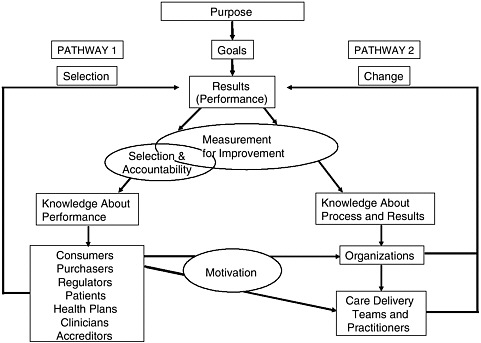
FIGURE 8-1 Two pathways to quality improvement.
SOURCE: Berwick et al., 2003.
improvement (Pathway 2) provided by accountability/selection (Pathway 1). Motivation for process improvement may be influenced by good publicity, higher payments, or access to larger markets.
A number of Pathway 1 motivations relating to medication safety have already been implemented on a trial basis:
-
Public recognition. Since 1999, the Centers for Medicare and Medicaid Services (CMS) has produced comparative performance reports for Medicare providers. These reports are available online through the CMS website. In Hospital Compare (DHHS, 2005), many of the measures used are medication-related. For heart attack patients, for example, the measures include percent of patients given angiotensin-converting enzyme (ACE) inhibitors, percent given aspirin at arrival, percent given aspirin at discharge, percent given beta-blockers at arrival, percent given beta-blockers at discharge, and percent given thrombolytic medication.
-
Preferred provider status. The Leapfrog Group (Leapfrog, 2005) is a consortium of buyers of health care. Members have agreed to base their purchase of health care on principles that encourage quality improvement on the part of providers. The Leapfrog Group introduced three safety practices, one of which—the use of computerized provider order entry (CPOE)— directly relates to medication safety; the other two are evidence-based hospital referral and staffing of intensive care units with doctors who have specialized clinical care training. A fourth leap has also been added, consisting of the National Quality Forum’s (NQF) 30 safe practices (NQF, 2003), many of which are medication-related.
-
Rewarding investment in information technology. Bridges to Excellence is an employer-led group aimed at improving the quality of care by recognizing and rewarding health care providers for implementing high-quality care delivery processes (BTE, 2003). For instance, through the Physician Office Link (POL), Bridges to Excellence rewards practices (in specific geographic areas) according to the number of modules implemented from a schedule monitored by the National Committee for Quality Assurance (NCQA, 2004). These include (1) clinical information systems/ evidence-based medicine (electronic capabilities for prescriptions and texts, use of electronic systems for prescribing and checking for safety and efficiency, contents of patient information in electronic health records (EHRs), and use of EHRs for decision support), (2) patient education and support, and (3) care management.
-
Pay for performance. Good performance by hospitals participating in the first year of a joint Premier Inc.–CMS demonstration project (Premier, 2005b) has made hospitals eligible for increased payments of $8.85 million (Premier, 2005a). The initiative covers five conditions (acute myocardial infarction, coronary artery bypass graft, heart failure, community
-
acquired pneumonia, hip and knee replacement) and 34 quality measures, many of which are medication-related (e.g., ACE inhibitors for heart failure, beta-blockers after myocardial infarction).
-
Innovative approaches to improving quality. Rewarding Results is a joint initiative of The Robert Wood Johnson Foundation and the California HealthCare Foundation, with support from the Commonwealth Fund, administered by the Leapfrog Group (Rewarding Results, 2002). There are seven grantees, including Blue Cross Blue Shield of Michigan, which received a grant to evaluate its hospital incentive program; this program has several elements related to medication safety (BCBS of Michigan, 2002).
Interest in pay for performance is growing. According to a 2004 survey, nearly 100 pay-for-performance initiatives are under way (Baker and Carter, 2005). Many such initiatives, such as Rewarding Results, are currently being evaluated (RWJF, 2005). As yet there is a limited evidence base validating these initiatives. In a 2004 review of the literature sponsored by the Agency for Healthcare Research and Quality (AHRQ), the authors found only nine randomized controlled trials of pay-for-performance initiatives. They concluded there is some evidence supporting the effectiveness of both payment and reputation incentives, but that there is little unequivocal evidence on which to establish quality-based purchasing strategies (Dudley et al., 2004). There are also complex methodological problems to address in evaluating pay-for-performance initiatives. A lengthy editorial in the Journal of the American Medical Association has set forth some guidelines for carrying out pay-for-performance research (Dudley, 2005).
Against this backdrop of uncertainties, the committee recommends that payers and purchasers continue to experiment with pay for performance and value-based purchasing to motivate improvement in the medication-use process The Institute of Medicine’s (IOM) Quality Chasm report drew attention to the disincentives to quality improvement imbedded within current payment approaches (IOM, 2001). The committee believes incentives should be crafted so that the profitability of hospitals, clinics, pharmacies, insurance companies, and manufacturers is aligned with patient safety goals; that is, the incentives should strengthen the business case for quality and safety.
The committee notes that a majority of the pay-for-performance and value-based purchasing initiatives undertaken to date have been for institutional care (for example, hospitals and nursing homes). The committee recommends that such initiatives also be used to foster improvements in the medication-use process in ambulatory care. Such initiatives might incorporate measures from the National Voluntary Consensus Standards for Ambulatory Care, endorsed in late 2005 by the NQF (NQF, 2005b).
The committee recognizes that the successful application of pay-for-
performance and value-based purchasing initiatives requires valid and comprehensive patient data. The use of robust EHRs with interoperable data exchange will greatly assist in the implementation of such initiatives.
MEDICATION THERAPY MANAGEMENT
Medication therapy management is a relatively ill-defined set of services aimed at optimizing the outcomes of drug therapy for individual patients. These services can be provided by appropriately qualified health care providers independently or in conjunction with the provision of medications by pharmacists. While the concept of medication therapy management is promising, there is as yet no clear view as to what services should be provided or will be cost-effective.
Experiments with medication therapy management have demonstrated benefits at several levels for diabetes patients. For example, two large self-insured employers in North Carolina compensated pharmacists on a fee-for-service basis for providing advisory services to employees with diabetes mellitus (the Asheville Project). As a result, hemoglobin A1c levels were better controlled, and employers’ total mean medical costs decreased by $1,622 per patient to $3,356 per patient per year (Cranor et al., 2003). Both employers have permanently added the benefit to their health plans. Another study examining the impact of pharmacy care services for patients with diabetes also produced good results (Garrett and Bluml, 2005). Over the initial year of the program, patients participating in the study showed significant improvement in clinical indicators and higher rates of self-management. Mean total health costs (including the costs of the medication therapy) were $918 per patient per year less than employers’ expected total costs.
Under the new Medicare Part D prescription drug benefit, Medicare beneficiaries who use multiple medications, have multiple chronic conditions, and generate high expenses will be eligible for medication therapy management at no cost. Congress provided the framework for a prescription drug benefit, and CMS is working out the details (Zagaria, 2005). Among other services, medication therapy management may include formulating a medication treatment plan; selecting, initiating, modifying, or administering medication therapy; monitoring and evaluating the patient’s response to therapy, including safety and effectiveness; performing comprehensive medication reviews to identify, resolve, and prevent medication-related problems, including adverse drug events (ADEs); providing verbal education and training designed to enhance patient understanding and appropriate use of medications; and coordinating and integrating medication therapy management services within the broader health care–management services being provided to patients (NACDS Foundation, 2005).
The implementation of the Medicare Part D drug benefit offers an
opportunity to investigate medication therapy management. The committee recommends that CMS carry out studies on the use of medication therapy management addressing the following issues:
-
The specific services that should be provided as part of medication therapy management
-
The target populations that would benefit most from these services
-
The types of health care personnel that would provide the lowest-cost, highest-value outcomes through these services
-
Whether and how medication therapy management should be reimbursed
-
How potential savings might be shared between insurers and providers
MINIMUM FUNCTIONALITY STANDARDS FOR INFORMATION TECHNOLOGY
Recent IOM reports have strongly recommended greater use of information technology in the delivery of health care (IOM, 2000, 2001, 2004). A national health information infrastructure—a foundation of systems, technologies, applications, standards, and policies—is required (IOM, 2004). The IOM’s report on patient safety (IOM, 2004) called upon the federal government to facilitate the deployment of this infrastructure through the provision of targeted financial support and the ongoing promulgation and maintenance of standards for data needed to improve patient safety. That report also called on health care providers to invest in EHR systems that would enable the provision of safe and effective care and the continuous redesign of care processes to improve patient safety (IOM, 2004).
Less than 1 year after the IOM report To Err Is Human: Building a Safer Health System (IOM, 2000) was released, the California legislature enacted Senate Bill 1875, requiring all California hospitals to submit a plan to the Department of Health that would substantially eliminate medication-related errors (SB 1875, 2000). A 2003 analysis of 344 hospital plans revealed that California hospitals were planning on average to implement 2.8 error-reducing technology applications by 2005 (Spurlock et al., 2003). The most frequently cited technology was CPOE (46 percent of hospitals), followed by pharmacy information systems (44 percent), automated dispensing units (38 percent), and electronic medication administration records (31 percent).
In September 2005, an expert panel published estimates of the likely investment by health care providers in EHRs and CPOE systems, based on current trends. The experts projected that in 5 years, 25–38 percent of office practices, 29–41 percent of hospitals, 14 percent of skilled nursing
facilities, and 21 percent of home health agencies would have implemented EHRs, and that 21–32 percent of office practices, 26–54 percent of hospitals, and 14 percent of skilled nursing facilities would have implemented CPOE (Kaushal et al., 2005). The committee believes this projected rate of adoption is too slow and that efforts should be made to speed it up.
The committee believes the California legislation discussed above is an important step toward the implementation of technologies for reducing medication errors. The committee believes further that this initiative should be expanded. Accordingly, the committee recommends that regulators, accreditors, and legislators set minimum functionality standards for information technology as conditions of participation, accreditation requirements, and licensing requirements, drawing on existing functionality models for electronic prescribing (to meet the 2010 deadline recommended in Chapter 5), CPOE, and EHRs. Several models exist on which to base these minimum functionality standards:
-
The Veterans Health Administration operates one of the largest integrated health information systems in the United States (IOM, 2002). The Veterans Health Information Systems and Technology Architecture (VistA), now known as HealtheVet-VistA, is an EHR system that incorporates CPOE, a clinical ordering and decision-support system providing drug– drug and drug–disease interactions. This system is available as free, public-domain software obtainable under the Freedom of Information Act through e-FOIA at ftp://ftp.va.gov/VistA.2
-
The Medicare Modernization Act of 2003 mandated that the National Committee on Vital and Health Statistics develop recommendations for uniform standards to enable electronic prescribing in ambulatory care. In a September 2004 letter to the secretary of the Department of Health and Human Services (DHHS), the committee addressed message format standards (NCVHS, 2004); in a March 2005 letter, the committee addressed electronic signatures and other issues (NCVHS, 2005).
-
An eHealthInitiative report (eHI, 2004) and several journal articles have outlined functionality standards for electronic prescribing/clinical decision support (Bates et al., 2003; Bell et al., 2004; Teich et al., 2005). The Leapfrog Group, with support from the California Health Care Foundation and The Robert Wood Johnson Foundation, is also active in promoting standards for CPOE (Metzger and Turisco, 2001; Forester et al., 2003), including electronic prescribing in ambulatory care (Classen, 2005).
-
The IOM’s report on patient safety (IOM, 2004: Appendix E) proposed a set of functionality standards for EHRs in hospitals, nursing homes, and ambulatory care and for the personal health record. These standards were used as input to Health Level 7’s Electronic Health Record Functional Model (HL7, 2005).
STATE ELECTRONIC PRESCRIBING LAWS
In November 2001, the California Healthcare Foundation published a report on electronic prescribing that described a patchwork of laws governing the practice; for example, 11 states prohibited electronic prescribing by both in-state and out-of-state providers, and only 4 states allowed it with the exception of certain drug types (e.g., controlled drugs) (Kilbridge and Gladysheva, 2001). Laws in many more states are now favorable to electronic prescribing. By 2004, 43 states allowed prescriber-to-pharmacy electronic medication orders (NABP, 2004). Allowing electronic connectivity is not enough, however; some states require dispense-as-written requirements that cannot be met using electronic technologies.
In November 2005, CMS issued the final rule for electronic prescribing of drugs covered under Medicare Part D. This rule contains a preemption covering state laws that prohibit electronic prescribing; that prohibit the transmission of electronic prescriptions through intermediaries; that require certain language to be used, such as “dispense as written,” to indicate whether generic drugs may or may not be substituted; and that require handwritten signatures or other handwriting on prescriptions (FR, 2005). As the rule is currently drafted, the scope of preemption includes electronic prescribing for Part D–eligible individuals (whether or not they are enrolled in a Part D plan) for drugs that may be covered by Part D in at least some circumstances (FR, 2005). Thus the preemption does not cover electronic prescribing for those under 65 and for controlled substances.
The Medicare preemption would create different rules for Medicare and other payers—which would be costly for prescribers, pharmacies, and plans to address and administer—and limit the uptake of electronic prescribing. Hence, states should enact legislation consistent with and complementary to the Medicare Modernization Act’s electronic prescribing provisions and remove existing barriers to the practice. The DHHS and the Drug Enforcement Administration are working on ways to enable electronic prescribing to encompass controlled substances, an effort the committee believes to be important.
STATE PHARMACY BOARDS
With a few exceptions, there is currently little or no oversight of community pharmacies related to medication safety. State boards do send sur-
veyors out, but they may or may not be pharmacists. What they look for are issues related to state practice acts; there is no focus on the types of issues that parallel the requirements hospital pharmacies must meet under the National Patient Safety Goals of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) (JCAHO, 2005) or accreditation requirements for medication management systems (Rich, 2004).
In the community pharmacy setting, there is little understanding of error rates; for example, the committee could find only one study (Flynn et al., 2003) on error rates when medications are refilled. In addition, much greater focus is needed on error prevention strategies. The committee believes state boards should assume a larger role in learning from errors and sharing lessons learned with all pharmacies while avoiding punitive measures in response to reported errors.
A small number of states have developed medication safety initiatives (NABP, 2004). In 2001, in response to medication error rates and medication distribution issues, the Massachusetts Board of Registration in Pharmacy issued a set of best-practice recommendations as standards of professional practice to be considered for implementation as appropriate by all pharmacies (MBRP, 2005b), For example, the first recommendation calls for incident reports to be completed and submitted to the United States Pharmacopeia (USP)–Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program (MERP). These recommendations were followed up in January 2005 with regulations that require all pharmacies to establish continuous quality improvement programs by the end of 2005 (MBRP, 2005a). To aid in this process, a quality improvement specialist is available to advise individual pharmacies. Based on errors reported by consumers to the board, this specialist can give advice proactively to individual pharmacies and in certain situations visit pharmacies to review progress.
Another important initiative is requiring continuing education on the topic of medication errors. The state board of New York has implemented such a requirement, and Pennsylvania will do so soon. New Mexico is one of a few states that require dispensing errors associated with ADEs to be reported to the board. The New Mexico Board of Pharmacy is also active in providing information about preventing medication errors through its website (NMBP, 2005).
The committee recommends that all state boards of pharmacy implement quality improvement programs. In particular, the committee recommends that each state convene a voluntary panel of pharmacists (including a state board member and representatives of hospitals, the community, and consumers) to review major quality and safety issues associated with medication dispensing. These issues could be derived from reviews of error reports. Information about error prevention measures provided by patient safety organizations could be shared, as well as any reports from state
reporting programs, recognizing that much can be learned from efforts in other states and through national programs. The panel should focus on errors that are most serious and most likely to occur, and review all available information on the chosen topics. After consulting with experts and patient safety organizations, as appropriate, the panel should publish its findings in the state newsletter and ask for voluntary compliance with new procedures (although oversight by means of surveying would be better), and perhaps make recommendations to the state board for regulatory changes.
Quality improvement programs might also include approaches similar to those adopted in Massachusetts: requiring all pharmacists and pharmacy technicians to take a few continuing education credits specifically directed at medication error issues, distributing information about good practice and recent examples of hazardous situations through a regular newsletter, and informing patients that complaints regarding medication errors can be directed to state boards.
The funding of a quality improvement program may be difficult for many state pharmacy boards. The committee believes Congress should fund a study on the development and funding of a national medication error prevention effort in community pharmacies, coordinated by state pharmacy boards.
REPORTING PROGRAMS FOR MEDICATION ERRORS/ADVERSE DRUG EVENTS
In Chapter 2, external and internal error reporting programs were discussed briefly, while the committee’s recommendations for internal monitoring programs were presented in Chapter 5. This section addresses the committee’s recommendations for external reporting programs.
Ways to Encourage Reporting
Generally, rates of reporting of errors and hazardous situations to external programs have been low (Leape, 2002). Although it may take only a few reports to raise awareness about a hazardous condition that requires immediate attention, errors need to be reported and analyzed if improvements in care are to be effected. Accordingly, the committee recommends that medication error and ADE reporting both internally and to external programs be promoted by care providers, accreditation agencies, state professional boards, and the relevant state and federal agencies.
Reporting programs are the primary means of providing early warnings of new types of errors, errors at the interfaces between care providers, and errors in care settings without EHRs (the majority of care settings today) or
settings that may never have EHRs (for example, the home). Voluntary reporting to an external program is often the only way providers can effect change outside their organization. Computerized analysis of patient records using a database trigger system (see Chapter 5) will be an important way of identifying many medication errors, but will not eliminate the need for reporting programs.
Legal impediments likely represent one key barrier to external reporting. The signing into law of the Patient Safety and Quality Improvement Act of 2005 (P.L. 109-41), which contains legal liability protections related to reporting, should encourage more reporting. The legislation calls for the establishment of patient safety organizations that will receive confidential patient safety data, including error reports; analyze the data; and disseminate recommendations for ways to reduce the risk of errors. The information provided to patient safety organizations will not be usable as evidence in the event of civil or administrative legal proceedings. Work is currently under way on defining the certification process for patient safety organizations.
Given the sometimes negative attitudes toward reporting of errors, multiple channels of reporting should be encouraged. Some systems will accept the simplest form of reporting, such as a narrative of the event delivered orally or in written form, while others will require a narrative plus structured data items using a computer system. For the latter, a single national taxonomy should be agreed upon and used.
A National Taxonomy: Better Coordination of Reporting Programs
In an institutional setting, reports are often funneled through a patient safety office that provides multiple outputs to local institutions (e.g., hospital systems), state reporting systems, federal systems (e.g., MedWatch), and proprietary programs (e.g., USP MedMarx, University Hospital Consortium Patient Safety Net) as appropriate. A national taxonomy with sufficient granularity would facilitate reporting to multiple programs.
The committee believes better coordination of all reporting systems is needed. Regulation of drugs is done at the federal level through the Food and Drug Administration (FDA), yet many of the reporting systems are implemented at the state level, with great variability in the incidents that must be reported and the data collected for each incident. There is a need for greater uniformity among state-based and other systems so the data can be aggregated to aid in shaping health policy. The use of a national taxonomy by all reporting systems would greatly facilitate such coordination. Using a single taxonomy would also enable databases to merge for datamining purposes. The difficulty of developing a single taxonomy is illustrated by the experience of a nursing faculty member at MD Anderson, who
studied 50 different incident reporting systems and found 856 different data fields (Personal communication, Deborah Simmons, October 6, 2005). Further, the development of a consistent taxonomy for patient safety is a critical bottleneck affecting the rapidity with which automated safety surveillance systems can be deployed.
The past few years have seen significant progress toward the establishment of a national taxonomy for patient safety. As noted in Chapter 7, JCAHO has taken a leadership role in the development of such a taxonomy (Chang et al., 2005). The goals of this effort are to promote a national reporting system for adverse events through the use of a standardized patient safety taxonomy and ontology. The Patient Safety Event Taxonomy (PSET) developed by JCAHO (and approved by NQF in August 2005) combines and classifies data from disparate reporting systems to facilitate comparisons across hospitals (NQF, 2005a). The second application developed under the study is the Hospital Incident Reporting Ontology (HIRO), which examines the relationships among the variables collected and classified by the PSET to facilitate data mining and sharing of patient safety data among hospitals. Lessons learned during the study will be disseminated to the health care community (http://wwwcf.nlm.nih.gov/hsr_project/view_ hsrproj_record.cfm?PROGRAM_CAME=search_fields.cfm&NLMUNI QUE_ ID= 20051166&SEARCH_FOR=reporting).
The IOM report on patient safety (IOM, 2004) described the need for a common patient safety reporting format, as well as the minimum data that should be collected in a standard report. The domain areas described include the following:
-
The discovery
-
The event itself
-
A narrative of the event, including contributing factors
-
Ancillary information
-
Detailed causal analysis
-
Lessons learned
Under the Patient Safety and Quality Improvement Act of 2005 (P.L. 109-41), the Secretary of the DHHS may determine common formats for reporting to and among a network of patient safety databases. Consideration should also be given to using the World Health Organization’s Draft Guidelines for Adverse Event Reporting and Learning Systems, which are designed to promote an international reporting system (WHO, 2005).
Reporting of Practice-Related Errors
Practice-related reports make up a minority of the reports in MedWatch, an FDA system focused on ADEs. These practice-related error re-
ports are an important resource but are difficult to retrieve for those outside the FDA. A Freedom of Information Act request may be filed, but without knowing the nature of the reports, it is difficult to know what needs to be retrieved. Moreover, the retrieval process may take several months to complete. The committee believes it might be more conducive to learning if practice-related medication errors were reported initially to USP or ISMP-MERP, which would automatically pass all such error reports on to the FDA MedWatch program.
Administrative Databases
Until the widespread implementation of EHRs is realized, the committee believes claims databases should continue to be used for pay-for-performance, accountability reporting, and policy development purposes. The addition of diagnostic test results to administrative data will expand the range of possible quality measurement. For example, it will be possible to use Health Plan Employer Data and Information Set (HEDIS) measures for glycemic control and for achievement of goals for reducing LDL (low-density lipoprotein) cholesterol.
In testimony to the Senate Finance Committee in July 2005 (MedPAC, 2005), MedPAC stated that claims data are an important source for assessing the performance of providers of Medicare services. MedPAC recommended that the information on claims forms be expanded. Measurement of the rate of adverse events in hospitals would require information on the conditions present in the patient on arrival at the hospital. In the ambulatory setting, claims data would be an even better source for quality measures if they could be linked to prescription data from the Medicare Part D program (when available) and laboratory data.
Data from the Part D program also have the potential to be a useful resource for understanding and preventing medication errors, especially for medication use by the elderly and the chronically ill (Platt and Ommaya, 2005). These data will be more valuable still if Medicare drug claims can be linked with diagnosis and procedure claims, as has been proposed by CMS (CMS, 2005). Such linked databases would help provide evidence on the occurrence of ADEs and the costs of such events.
Reporting Back
Reporting programs should provide feedback locally to reporters to the extent possible. Similarly, state- and federally based databases that aggregate and analyze these data should regularly provide feedback to health care practitioners, health care organizations, industry, and policy makers. Providing feedback is often a challenge as there may be only a small number of reported events, the programs may lack the resources to carry out the
analyses, and user-friendly reporting formats are difficult to craft. In this regard, in 2005 the National Academy for State Health Policy produced some important guidance on how state adverse event data can be used to improve patient safety (Rosenthal and Booth, 2005).
The ideal reporting system would facilitate widespread access to the databases to speed improvement in the accuracy of medication prescribing, dispensing, and administration processes. An individual health care professional should eventually be able to review errors and effective preventive actions by searching the Internet.
WORKFORCE DEVELOPMENT AND RETOOLING
The training of health care professionals addresses medication safety insufficiently, despite frequent recommendations to increase the emphasis on medication safety in training programs (HRSA, 2000; IOM, 2000, 2003; AAMC, 2003). In an interview coinciding with the fifth anniversary of the release of the IOM’s To Err Is Human report (IOM, 2000), Timothy Flaherty, MD, chairman of the board of the National Patient Safety Foundation, commented that medical education is an area in which patient safety has seen no dramatic improvements (NPSF, 2004).
A 2000 survey of U.S. internal medicine clerkships and internal medicine residency programs for third-year medical students found that little or none of the curriculum had been dedicated to clinical pharmacology during medical school, and only modest amounts during internal medicine resident training (Rosebraugh et al., 2002). The committee is very concerned about this low level of training in clinical pharmacology given the amount of medication prescribing in clinical practice. A 2001 survey of schools of pharmacy in the United States found that the quality and quantity of instruction in medication errors varied significantly, and that key domains of knowledge were lacking in some programs (Johnson et al., 2002). A small survey of the state members of the National Council of State Boards of Nursing revealed that nursing education programs are required by most states to include generic content on medication administration safety, but only one state (Florida) mandates continuing nursing education specifically focused on medication errors (Personal communication, Kathleen Stevens, EdD, RN, December 6, 2005).
Within individual institutions, considerable variability can be found across the various professional schools. Prior to the introduction of an interprofessional patient safety course at Creighton University (Galt et al., in press), for example, the patient safety materials already included in the curriculum for each of the health professions (nursing, medicine, pharmacy, physical therapy, and dentistry) were narrowly focused and integrated into other courses. Further, despite the recognition that interprofessional col-
laboration is a major element in the delivery of quality care (IOM, 2001), there is limited interprofessional education related to patient safety (Mitchell et al., 2005).
A number of institutions are beginning to offer courses in medication/ patient safety:
-
The Faculty Leadership in Interprofessional Education to Promote Patient Safety project created a patient safety–oriented curriculum for the training of health profession faculty leaders (Mitchell et al., 2005).
-
Creighton University has an interprofessional patient safety course available for students in business, law, social work, medicine, pharmacy, physical therapy, occupational therapy, nursing, and dentistry (Creighton, 2005).
-
The British Pharmacological Society’s Clinical Section Committee has developed a core curriculum for the teaching of safe and effective prescribing in U.K. medical schools (Maxwell and Walley, 2003).
-
With the help of a grant from AHRQ, a continuing education curriculum in ambulatory care aimed at advancing patient safety and incorporating a medication errors module was developed (Mottur-Pilson, 2005).
-
The University of Wisconsin-Madison Center, again with funding from AHRQ, has developed a graduate certificate in patient safety (BT Karsh). The certificate requires five courses (including a mandatory course on medication-use safety), a patient safety practicum, and a series of seminars by guest lecturers.
-
Through a grant from AHRQ, the National Patient Safety Foundation partnered with the Medical College of Wisconsin to develop web-based educational patient safety materials for physicians, nurses, and patients (Hendee et al., 2005; NPSF, 2005).
Other sources of educational material are the Centers for Education and Research on Therapeutics, a research program administered by AHRQ in consultation with the FDA and agencies within DHHS. The mission of the Centers for Education and Research on Therapeutics is to conduct research and provide education that will advance the optimal use of drugs, medical devices, and biological products. In early 2006, four more centers, including one devoted to consumer use of medication, were added to the network (CERTS, 2006).
Finally, regarding the use of information technology systems to improve medication safety, a joint American Health Information Management Association/American Medical Informatics Association report has pointed out that no systematic plan exists for training the current health care workforce to use information technology tools to do their jobs (AHIMA/AMIA, 2006). This report called on the health care industry to educate its employees at all levels that information technology is an integral
part of health care work. To address this challenge, the American Medical Informatics Association announced its 10-by-10 program, which aims to realize a goal of training 10,000 health care professionals, especially in applied clinical informatics, by the year 2010 (AMIA, 2005).
The committee recommends that the relevant accreditation organizations—the Liaison Committee on Medical Education, Accreditation Council for Graduate Medical Education, Accreditation Council for Continuing Medical Education, Accreditation Council for Pharmacy Education, American Society of Health-System Pharmacists, National League for Nursing Accrediting Commission, and Commission on Collegiate Nursing Education—ensure that the curricula of undergraduate and graduate pharmacy, nursing, and medical schools and continuing education include:
-
Appropriate medication safety modules to cover an overview of the system for drug development, regulation, distribution, and use; an understanding of where medication errors can take place; the need to monitor continuously for medication errors; how to recognize medication errors and the tools for identifying such errors; what to do once a medication error has been found; reporting and analysis of medication errors; and ways of improving the safety of the medication-use process.
-
Appropriate clinical pharmacology training commensurate with the amount of medication prescribing in clinical practice.
-
Training in the delivery of patient-centered care and the use of information technology tools to enable implementation of the recommendations presented in Chapters 4 and 5.
REFERENCES
AAMC (Association of American Medical Colleges). 2003. Patient Safety and Graduate Medical Education. Washington, DC: AAMC.
AHIMA/AMIA (American Health Information Management Association/American Medical Informatics Association). 2006. Building the Work Force for Health Information Transformation. Chicago, IL: AHIMA and Bethesda, MD: AMIA.
AMIA (American Medical Informatics Association). 2005. Training Health Care Professionals to Serve as Local Informatics Leaders and Champions, 2005. [Online]. Available: http://www.amia.org/10x10 [accessed May 7, 2006].
Baker G, Carter B. 2005. Provider Pay-for-Performance Incentive Programs: 2004 National Study Results. San Francisco, CA: Med-Vantage.
Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B. 2003. Ten commandments for effective clinical decision support: Making the practice of evidence-based medicine a reality. Journal of the American Medical Informatics Association 10(6):523–530.
BCBS of Michigan. 2002. Rewarding Results Grantees: Blue Cross Blue Shield of Michigan. [Online]. Available: http://www.leapfroggroup.org/RewardingResults/bcbsmi.htm [accessed October 30, 2005].
Bell DS, Cretin S, Marken BS, Landman AB. 2004. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. Journal of the American Medical Informatics Association 11(1):60–70.
Berwick DM. 2002. A user’s manual for the IOM’s “Quality Chasm” report. Health Affairs (Millwood) 21(3):80–90.
Berwick DM, James B, Coye MJ. 2003. Connections between quality measurement and improvement. Medical Care 41(Suppl. 1):130–138.
BTE (Bridges to Excellence). 2003. Bridges to Excellence: Rewarding Quality Across the Healthcare System. [Online]. Available: http://www.bridgestoexcellence.org/bte [accessed October 30, 2005].
CERTS (Centers for Education and Research on Therapeutics). 2006. AHRQ Expands Therapeutics Education and Research Network to Focus on Critical Issues Facing the Health Care System. [Online]. Available: http://www.certs.hhs.gov/whats_new/archive/2006/ 20060425_01.html [accessed May 7, 2006].
Chang A, Schyve PM, Croteau DJ, O’Leary DS, Loeb JM. 2005. The JCAHO patient safety event taxonomy: A standardized terminology and classification schema for near misses and adverse events. International Journal for Quality in Health Care 17(2):95–105.
Classen D. 2005. A national standard for medication use. In: Building a Better Delivery System: A New Engineering/Health Care Partnership, NAE/IOM. Washington, DC: The National Academies Press.
CMS (Centers for Medicare and Medicaid Services). 2005. Medicare Prescription Drug Data Strategy: Improving Evidence for Patient Care Through the Medicare Prescription Drug Benefit. Washington, DC: Department of Health and Human Services.
Cranor CW, Bunting BA, Christensen DB. 2003. The Asheville Project: Long-term clinical and economic outcomes of a community pharmacy diabetes care program. Journal of the American Pharmaceutical Association 43(2):173–184.
Creighton. 2005. Interprofessional IPE 410 Foundations in Patient Safety. [Online]. Available: http://www.creighton.edu/ipe/ptsafetyspring05.htm [accessed November 2, 2005].
DHHS (U.S. Department of Health and Human Services). 2005. Hospital Compare. [Online]. Available: http://www.hospitalcompare.hhs.gov [accessed October 17, 2005].
Dudley RA. 2005. Pay-for-performance research: How to learn what clinicians and policy makers need to know. Journal of the American Medical Association 294(14):1821–1823.
Dudley RA, Frolich A, Robinowitz DL, Talavera JA, Broadhead P, Luft HS. 2004. Strategies to Support Quality-Based Purchasing: A Review of the Evidence. Rockville, MD: Agency for Healthcare Research and Quality.
eHI (eHealth Initiative). 2004. Electronic Prescribing: Toward Maximum Value and Rapid Adoption. Washington, DC: eHI.
Flynn EA, Barker KN, Carnahan BJ. 2003. National observational study of prescription dispensing accuracy and safety in 50 pharmacies. Journal of the American Pharmaceutical Association 43(2):191–200.
Forester AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. 2003. The incidence and severity of adverse events affecting patients after discharge from the hospital. Annals of Internal Medicine 138(3):161–167.
FR (Federal Register). 2005. Department of Health and Human Services: Centers for Medicare and Medicaid Services: 42 CFR Part 423: Medicare Program; E-Prescribing and Prescription Drug Program; Final Rule. Washington, DC: National Archives and Records Administration.
Galt KA, O’Brien R, Paschal K, Clark B, Bramble JD, Gleason J, McQuillan R, Graves J, Harris B, Hoidal P, Mahern C, Mu K, Rule A, Scheirton L, Gerardi D, Sonnino R, Bradberry JC. In press. Description and evaluation of an interprofessional patient safety course for health professions and related sciences students. Journal of Patient Safety.
Garrett DG, Bluml BM. 2005. Patient self-management program for diabetes: First-year clinical, humanistic, and economic outcomes. Journal of the American Pharmaceutical Association 45(2):130–137.
Hendee WR, Keating-Christensen C, Loh YH. 2005. Development of a patient safety web-based education curriculum for physicians, nurses, and patients. Journal of Patient Safety 1(2):90–99.
HL7 (Health Level 7). 2005. HL7 Electronic Health Record (EHR) Technical Committee’s Home Page. [Online]. Available: http://www.hl7.org/ehr [accessed October 16, 2005].
HRSA (Health Resources and Services Administration). 2000. Collaborative Education to Ensure Patient Safety: Council on Graduate Medical Education and National Advisory Council on Nurse Education and Practice. Washington, DC: DHHS.
IOM (Institute of Medicine). 2000. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press.
IOM. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press.
IOM. 2002. Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: The National Academies Press.
IOM. 2003. Health Professions Education: A Bridge to Quality. Washington, DC: The National Academies Press.
IOM. 2004. Patient Safety: Achieving a New Standard for Care. Washington, DC: The National Academies Press.
JCAHO (Joint Commission on Accreditation of Healthcare Organizations). 2005. 2006 Critical Access Hospital and Hospital National Patient Safety Goals. [Online]. Available: http://www.jcipatientsafety.org/show.asp?durki=10293&site=164&return=10289 [accessed August 22, 2005].
Johnson MS, Latif DA, Gordon B. 2002. Medication error instruction in schools of pharmacy curricula: A descriptive study. American Journal of Pharmaceutical Education 66:364–371.
Kaushal R, Bates DW, Poon EG, Jha AK, Blumenthal D. 2005. Functional gaps in attaining a national health information network. Health Affairs (Millwood) 24(5):1281–1289.
Kilbridge P, Gladysheva K. 2001. E-Prescribing. Oakland, CA: California HealthCare Foundation.
Leape LL. 2002. Reporting of adverse events. New England Journal of Medicine 347(20): 1633–1638.
Leapfrog. 2005. The Leapfrog Group Fact Sheet. [Online]. Available: http://www.leapfrog group.org/about_us/leapfrog-factsheet [accessed October 17, 2005].
Maxwell S, Walley T. 2003. Teaching safe and effective prescribing in UK medical schools: A core curriculum for tomorrow’s doctors. British Journal of Clinical Pharmacology 55(6): 496–503.
MBRP (Massachusetts Board of Registration in Pharmacy). 2005a. April Newsletter: Item 5. The Board Adopts New Regulations to Improve Patient Outcomes. Boston, MA: MBRP.
MBRP. 2005b. Massachusetts Board of Registration in Pharmacy. [Online]. Available: http: //www. mass.gov/dpl/boards/ph/cmr/24175.htm [accessed January 10, 2006].
MedPAC (Medicare Payment Advisory Commission). 2005. Testimony: Pay for Performance in Medicare (July 27, 2005). U.S. Senate, Committee on Finance. [Online]. Available: http://www.medpac.gov/publications/generic_report_display.cfm?report_type_id= 2&sid=2&subid=0 [accessed October 17, 2005].
Metzger J, Turisco F. 2001. Computerized Physician Order Entry: A Look at the Vendor Marketplace and Getting Started. Washington, DC: The Leapfrog Group.
Mitchell PH, Robins LS, Schaad D. 2005. Creating a curriculum for training health profession faculty leaders. In: Henrikson K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality.
Mottur-Pilson C. 2005. An ambulatory care curriculum for advancing patient safety. In: Henrikson K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality.
NABP (National Association of Boards of Pharmacy). 2004. Survey of Pharmacy Law. Mount Pleasant, IL: NABP.
NACDS Foundation (National Association of Chain Drug Stores Foundation). 2005. National Association of Chain Drug Stores Foundation: Medication Therapy Management in Community Pharmacy Practice. [Online]. Available: http://www.nacdsfoundation.org/user-assets/Documents/PDF/MTM%20Model%20final.pdf [accessed November 13, 2005].
NCQA (National Committee for Quality Assurance). 2004. Bridges to Excellence: Physician Office Link. [Online]. Available: http://www.ncqa.org/pol [accessed October 17, 2005].
NCVHS (National Committee on Vital and Health Statistics). 2004. Letter to Secretary Thompson at DHHS, September 2, 2004. [Online]. Available: http://www.ncvhs.hhs.gov/ 040902lt2.htm [accessed October 30, 2005].
NCVHS. 2005. Letter to Secretary Leavitt at DHHS, March 4, 2005. [Online]. Available: http://www.ncvhs.hhs.gov [accessed October 30, 2005].
NMBP (New Mexico Board of Pharmacy). 2005. New Mexico Board of Pharmacy: Adverse Drug Events and Medication Errors. [Online]. Available: http://www.state.nm.us/ pharmacy [accessed January 10, 2006].
NPSF (National Patient Safety Foundation). 2004. Focus on Patient Safety Newsletter. Vol. 7, No. 3. Five Years After To Err Is Human: A Look at the Patient Safety Landscape. [Online]. Available: http://www.npsf.org/html/Focus.html [accessed October 13, 2005].
NPSF. 2005. National Patient Safety Foundation: Patient Safety Programs and Opportunities. [Online]. Available: http://www.npsf.org/html/programs.html [accessed November 2, 2005].
NQF (National Quality Forum). 2003. Safe Practices for Better Healthcare: A Consensus Report. Washington, DC: NQF.
NQF. 2005a. National Quality Forum Endorses Voluntary Consensus Standard for Standardizing a Patient Safety Taxonomy. August 3, 2005. [Online]. Available: http://www. qualityforum.org/news/home.htm [accessed November 26, 2005].
NQF. 2005b. NQF Endorses Additional Voluntary Consensus Standards for Standardizing Measures of Physician-Focused Ambulatory Care. October 11, 2005. [Online]. Available: http://www.qualityforum.org/news/home.htm [accessed November 26, 2005].
Platt R, Ommaya A. 2005. A beneficial side effect of the Medicare drug benefit. New England Journal of Medicine 353(26):2742–2743.
Premier. 2005a. CMS/Premier Pay-for-Performance Model Produces Remarkable Quality Improvements Among Nation’s Hospitals. [Online]. Available: http://www.premierinc. com/all/newsroom/press-releases/05-nov/cms-pay-for-performance-year-one-results.jsp [accessed November 26, 2005].
Premier. 2005b. HQI Demonstration Overview. [Online]. Available: http://www.premierinc. com/all/quality/hqi/index.jsp [accessed October 17, 2005].
Rewarding Results. 2002. Rewarding Results: About the Program. [Online]. Available: http:// www.leapfroggroup.org/RewardingResults/about.htm [accessed October 30, 2005].
Rich DS. 2004. New JCAHO medication management standards for 2004. American Journal of Health-System Pharmacy 61(13):1349–1358.
Rosebraugh CJ, Honig PK, Yasuda SU, Pezzullo JC, Woosley RL. 2002. Centers for education and research on therapeutics report: Survey of medication errors education during undergraduate and graduate medical education in the United States. Clinical Pharmacology and Therapeutics 71(1):4–10.
Rosenthal J, Booth M. 2005. Maximizing the Use of State Adverse Event Data to Improve Patient Safety. Portland, ME: National Academy for State Health Policy.
RWJF (Robert Wood Johnson Foundation). 2005. Robert Wood Johnson Foundation: Evaluation of the Rewarding Results Program. [Online]. Available: http://www.rwjf.org/research/researchdetail.jsp?id=2154&ia=142 [accessed October 30, 2005].
SB 1875 (Senate Bill 1975). 2000. California Senate Bill 1875. [Online]. Available: http:// info.sen.ca.gov/pub/99-00/bill/sen/sb_1851-1900/sb_1875_bill_20000928_chaptered.html [accessed October 30, 2005].
Spurlock B, Nelson M, Paterno J, Tandel S. 2003. Legislating Medication Safety: The California Experience. Oakland, CA: California Healthcare Foundation.
Teich JM, Osheroff JA, Pifer EA, Sittig DF, Jenders RA, The CDS Expert Review Panel. 2005. Clinical decision support in electronic prescribing: Recommendations and an action plan: Report of the joint clinical decision support workgroup. Journal of the American Medical Informatics Association 12(4):365–376.
WHO (World Health Organization). 2005. World Alliance for Patient Safety: WHO Draft Guidelines for Adverse Event Reporting and Learning Systems. Geneva, Switzerland: WHO.
Zagaria ME. 2005. Senior care: Medication therapy management services. U.S. Pharmacist 4:35–42.





















