4
Systems to Support Organ Donation
Organ transplantation involves a complex, collaborative set of interactions among patients, family members, healthcare professionals, organ procurement and transplant coordinators, the hospital where the donation occurs, the organ procurement organization (OPO) that facilitates the acquisition and distribution of organs, and the transplant center. Although any hospital may have a patient who is a potential organ donor, specialized hospitals that treat many patients with traumatic injuries and other serious conditions (particularly those that can result in neurologic determination of death) are the ones that, to date, have been most likely to have potential organ donors in their patient populations.
Current debate and discussions about changing the organ donation process to increase the supply of transplantable organs occur within the context of ongoing efforts to improve the healthcare system as a whole. One major challenge facing the United States is improving the overall quality of health care. The increasing complexity of new technologies, the inadequate organization of the healthcare delivery system, the increased proportion of health care devoted to chronic conditions, and current constraints on taking full advantage of changes in information technology have all contributed to a healthcare system that is overburdened, inefficient, and often inequitable. In view of these problems, the Institute of Medicine (IOM) reports on healthcare quality have identified six improvement aims for the healthcare system (IOM, 2001, 2004a):
-
Safe—avoiding injuries to patients from the care that is intended to help them
-
Effective—providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit (avoiding underuse and avoiding overuse, respectively)
-
Patient centered—providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions
-
Timely—reducing waits and sometimes harmful delays for both those who receive and those who give care
-
Efficient—avoiding waste, including waste of equipment, supplies, ideas, and energy
-
Equitable—providing care that does not vary in quality because of personal characteristics such as gender, ethnicity, geographic location, and socioeconomic status
These goals are highly relevant to the challenges facing the organ donation and transplantation system. These challenges include ensuring a patient- and family-centered system, providing equitable access to transplantation through the elimination of economic and ethnic or cultural barriers, increasing trust and confidence in the system, and providing the care needed to optimize the value of the transplant to the recipient.
As previous IOM studies on improving the quality and safety of U.S. health care have concluded, improving care requires a focus on systems, not on the individuals within those systems (IOM, 2000a). “[E]very system is perfectly designed to achieve the results it achieves” (Berwick, 1996, p. 619). Hence, if the results are unacceptable, the system’s design must be changed.
This chapter focuses on the clinical systems issues that are specific to the organ donation process. It is important to note, however, that an equitable allocation system and ongoing attention to the recipient are vital to maintaining the value of the donated organ and sustaining the integrity of the entire organ transplantation system (Box 4-1). Although these allocation issues are beyond the scope of this report, addressing them is indispensable to achieving a trustworthy organ donation system and thereby increasing the rates of donation. Additionally, several aspects of tissue donation and the market for tissue-related products need attention in order to ensure the credibility of the entire donation system.
The chapter begins with a brief overview of the issues facing the U.S. organ donation system, provides a snapshot of ongoing efforts, and concludes with recommendations for next steps to improve the system. As discussed throughout this chapter, there are many opportunities to build on existing practices and to leverage ongoing quality improvement efforts to expand knowledge and implement best practices. Clinical care at the end of life receives the main attention in this chapter, while emergency care and resuscitative care are examined in Chapter 5.
|
BOX 4-1 Issues in Allocation and Distribution of Organs Equitable Access to Transplantation: Medical, technical, ethical, and other issues arise in the referral for transplantation, admission to the waiting list, and selection to receive a donated organ. The criteria for each of these actions continue to need close attention by both professionals and the public to ensure equity. Particularly important is the role of the so-called green screen (which screens for insurance coverage and the ability to pay for the transplant) for nonrenal transplants and, often relatedly, the problems of access faced by minority populations. Access to Immunosuppressive Medications Following Transplantation: Immunosuppressive medications, estimated to cost more than $10,000 per patient annually, are needed for the rest of the patient’s life after transplantation; their discontinuation can result in organ rejection and the need for retransplantation (Chisholm and Garrett, 2001). It has been estimated that medication noncompliance results in the loss of 13 to 35 percent of transplanted kidneys (Yen et al., 2004), but it is difficult to determine the extent to which noncompliance is tied to the high cost of medication. Medicare currently provides initial coverage for immunosuppressive medications after most solid-organ transplants that occur in Medicare-approved facilities. However, for many patients this coverage is limited to 3 years posttransplantation. An analysis of the effects of extending immunosuppression coverage from one to three years found improved graft survival (Woodward et al., 2001) suggesting the importance of uninterrupted access to immunosuppressive medications for optimal outcomes from donation. Research models that explored extending to lifetime coverage found improved transplant and economic outcomes (Yen et al., 2004). For individuals who are eligible for Medicare because of age or disability, the Benefits Improvement and Protection Act (incorporated into Public Law 106-554) extended the benefits to lifetime coverage of immunosuppressive medications; however, this full coverage applies to only a fraction of total transplant recipients. Private insurers offer some patient assistance programs to provide medications to patients who lack Medicare coverage or the ability to pay, but these programs are highly individualized to specific insurance companies. Immunosuppressive medications offer the dual protection of maintaining the health of the recipient and protecting a scarce resource, a transplanted organ. A 2000 Institute of Medicine analysis of the effectiveness and cost savings of extending the Medicare coverage benefit found strong evidence for eliminating the time limits for coverage of immunosuppressive drugs for all solid-organ transplant recipients (IOM, 2000b). |
CONTEXT OF THE CURRENT U.S. ORGAN DONATION SYSTEM
The U.S. organ donation system has evolved over the past half century, having been shaped by a series of federal and state laws and regulations, private-sector oversight, and individual hospital policies. The system has focused primarily on deceased donors with neurologic determination of death. More recently, living donation, which is rapidly increasing, has
largely been regulated through hospital policies (Chapter 9). Internationally, some countries have developed extensive systems for organ donation and recovery, particularly in countries with national systems of health care. In addition, individuals whose deaths are determined by circulatory criteria represent a largely untapped group of potential organ donors (Chapter 5).
In the United States, people become organ donors either after the determination of death by established criteria (together with their prior consent or the consent of their family) or after an autonomous decision by a living person to donate all or part of an organ. Each circumstance presents distinct clinical, ethical, legal, emotional, spiritual, and cultural issues. This chapter focuses on the donation of organs by deceased donors (deceased organ donation). Organ donation by living donors is discussed in Chapter 9.
Under the existing legal and ethical framework of deceased organ donation in the United States, organ donation must not cause or hasten death (the dead donor rule) and the individual’s wishes regarding donation must be honored. Some donors have indicated their preferences to donate before their death (e.g., on their driver’s license, in an advance directive, or by signing a donor registry); others have not and the decision to donate organs is left to the family or surrogate at the time of the individual’s death. Regardless of the cause of death or the decision-making pathway, one person’s death creates the opportunity for others to benefit from the donation of one or more organs.
With some exceptions (for instance, where there is valid evidence of the deceased person’s wish to donate, perhaps backed by state law and OPO policy), the current practice of obtaining consent for deceased organ donation generally involves hospital or OPO staff talking with the patient’s family or surrogate about what is known about the potential donor’s wishes and providing information on the opportunity for organ donation. This practice is consistent with other decisions made by surrogates at the end of life and the requirements for informed consent (Beauchamp and Childress, 2001; National Consensus Project Steering Committee, 2004). The family’s knowledge of the wishes of the deceased individual is often a key factor in the decision about donation (Sque and Payne, 1996; Siminoff and Lawrence, 2002; Jacob Arriola et al., 2005; Sque et al., 2005; Rodrigue et al., 2006). There is wide variation from case to case and among hospitals and OPOs in who talks with the family and when and how those discussions occur (HRSA, 2003).
Systems of Care: An Organizational Perspective
Organ transplantation generally involves interactions among three organizations: the donor hospital, the OPO, and the transplant center(s).
Each organization has unique features but must meet conditions of participation established and regulated by the Centers for Medicare & Medicaid Services (CMS). To be accredited each organization must meet the accreditation criteria of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and those of other organizations (Box 4-2). The challenges to the system—as well as the opportunities for improvement—are found in the variations among organizations.
Of the 58 OPOs (recently designated collectively as “donate life organizations”) in the United States, 50 are independent and 8 are hospital or university based. Each OPO serves a unique geographic area (a donation service area [DSA])1 with specific donor hospitals and transplant centers; in 2003, the populations served by individual OPOs ranged from 1 million to 17 million people and the OPO coverage areas ranged from approximately 3,600 to more than 850,000 square miles (AOPO, 2004). Similarly, the range in the number of acute-care hospitals in a DSA is also wide, with from 12 to 220 hospitals in a single DSA. Some OPOs recover organs from many different hospitals, whereas others work with only a few hospitals. In 2003, from 6 to 78 hospitals within a single DSA reported one or more organ donors (AOPO, 2004). Also in 2003, the number of organ donor referrals to an OPO varied from a low of 52 to a high of 2,627 and the number of organs locally recovered by OPOs ranged from 86 to 1,181 (AOPO, 2004).
As a result of the demographics of a region, the demand for services in a particular region, and the administration and management of OPOs, the structures, staffing, and financing of OPOs vary widely. Most OPOs (72 percent in the 2003 Association of Organ Procurement Organizations [AOPO] survey) recover tissue as well as solid organs, and a few OPOs are involved in tissue processing; additionally, several OPOs operate an eye bank or a histocompatibility testing laboratory. The OPOs in St. Louis and Philadelphia have built, or are in the process of building, facilities for organ recovery.
The financing of organ transplantation largely occurs through the health insurance costs paid by Medicare or private insurers. In 2004, private insurance was the primary payment source for 47.9 percent of transplant recipients, with 39.3 percent having Medicare as the primary source, and 9.0 percent using Medicaid (HRSA and SRTR, 2006).
OPOs develop standardized fees (standard acquisition charges) representing the average cost for each type of organ. The fees include direct costs, organ acquisition overhead, and general and administrative costs.
Direct costs consist of donor-related medical costs after declaration of death (including operating room costs, surgeon fees, tissue typing, transportation of the organ and surgical teams). Organ acquisition overhead includes donor approach and public awareness costs. General and administrative costs include finance, human resources, information technology, and occupancy costs. The standard acquisition charge is billed to the transplant center receiving the organ. The center develops its own organ acquisition fees in addition to the transplantation fees and bills them to the recipient’s insurance company or other payer. The OPO does not conduct any financial transaction with the recipient or the recipient’s insurance company (third party payer). In 2003, the median local standard acquisition charge for kidneys was $20,500 (AOPO, 2004).
Medicare Part A, specifically the End-Stage Renal Disease program, is authorized to pay acquisition fees for organ acquisition and organ transplantation. OPOs are subject to annual federal audits after each fiscal year to reconcile their costs and the acquisition fees billed to transplant centers. In 2003, total Medicare expenditures totaled $14.8 billion for dialysis and $0.8 billion for organ acquisition and transplantation (USRDS, 2005). CMS provides oversight to the transplantation system through the Medicare/Medicaid conditions of participation. Only transplant centers that meet the conditions of participation are eligible for Medicare or Medicaid reimbursement. OPOs must meet separate certification criteria. CMS is currently revising the conditions of participation including the process and outcome measures and the certification cycles (Box 4-2).
OPOs also vary in their approaches to staffing and hospital interaction. In 2003, 28 OPOs responding to a survey reported that they had 31 or more employees, with only one OPO reporting fewer than 10 employees (AOPO, 2004). OPO staff members include organ and tissue procurement staff (e.g., laboratory managers and technicians, coordinators), executive staff, donation request and family care staff, public education personnel, and call center staff.
Some OPOs have established in-house coordinator programs in which full-time OPO staff work at Level I trauma centers and are fully integrated into hospital operations (HRSA, 2003). Other OPOs have staff who are based in multiple hospitals, whereas others, particularly those in rural settings, have staff based at the OPO office who travel to various hospitals in the region as needed.
The heterogeneity of OPOs is evident in the range of donation rates. The Scientific Registry of Transplant Recipients calculates observed donation rates (calculated as the number of actual donors per the number of individuals eligible for donation) and expected donation rates (the expected donation rate accounts for the variations in age, sex, race, and cause of death among notifiable deaths). Among OPOs the observed donation rates
|
BOX 4-2 Regulation and Accreditation Centers for Medicare & Medicaid Services (CMS): CMS is responsible for certifying OPOs and transplant center hospitals for Medicare coverage. The conditions of participation for OPOs and transplant centers are in the process of being revised to address requirements of the Organ Procurement Organization Certification Act of 2000. The proposed rules increase the recertification cycle from 2 to 4 years and establish multiple outcome and process performance measures, including changes in measurements of donor potential by replacing the current use of population data with data based on hospital referral calls to OPOs. Data submission and outcome requirements for transplant centers are also proposed to be modified to consider the care being provided rather than relying on underlying policies and procedures. The proposed rule expands outcome measures to include graft survival rates (CMS, 2005). Organ Procurement and Transplantation Network (OPTN): OPTN develops policies and procedures for organ recovery, allocation, and transplantation and conducts reviews and evaluations of each member OPO and transplant center for compliance with OPTN policies. Joint Commission on Accreditation of Healthcare Organizations (JCAHO): Since January 1988, JCAHO has required its member hospitals, as a prerequisite for accreditation, to develop policies and procedures on the identification and referral of potential donors. JCAHO requires hospitals that recover organs to have an agreement with an appropriate OPO and at least one tissue bank and one eye bank. JCAHO describes procedures for notifying the family of the option to donate and for maintaining the records of potential donors. A standard which became effective in July 2005 requires hospitals to measure the effectiveness of their organ procurement efforts. According to this standard, the conversion rate data must be collected and analyzed, and steps must be taken to improve the rate whenever possible (JCAHO, 2005). Association of Organ Procurement Organizations (AOPO): AOPO sets organizational and ethical standards for OPOs and offers a voluntary accreditation program to its members. The peer review accreditation process helps ensure compliance with federal regulations as well as AOPO standards. The period of accreditation is 3 years, after which the OPO must apply for reaccreditation to maintain its status. Recent standards include an emphasis on continuous quality improvement practices. American Board for Transplant Certification (ABTC): ABTC develops certification standards and programs for certification testing for transplantation clinicians. Specifically, clinicians may receive certification as a certified procurement transplant coordinator, a certified clinical transplant coordinator, or a certified clinical transplant nurse. |
in 2004 varied from 34.3 percent (with an expected rate of 54.9 percent) to 77.9 percent (with an expected rate of 51.3 percent) (SRTR, 2005) (Table 4-1). These statistics refer only to deaths determined by neurologic criteria. Wide variations in the number of cases of donation after circulatory determination of death (DCDD) also occur (Chapter 5). In 2004, 21 of the 58 OPOs had completed five or more DCDD cases, whereas 18 OPOs had not had a DCDD case in that year. Variations among OPOs and among transplant centers are being addressed through quality improvement processes and practices through the Organ Donation and Transplantation Breakthrough Collaboratives, which are described below.
According to the best estimates, approximately 200 of the nation’s hospitals host half of the nation’s eligible donors (HRSA, 2005a). A 2003 report by the Office of the Inspector General of the U.S. Department of Health and Human Services found that the 190 transplant centers obtained consent from a mean of 51 percent of potential donors, with 30 centers (16 percent) having consent rates of 70 percent or higher and 18 centers having consent rates of less than 30 percent (DHHS, 2003). Hospitals with the largest potential for donation may also be the most stressed because their intensive care units (ICUs) typically run at peak capacity, despite shortages of nurses and other key personnel (JCAHO, 2004). Because of ongoing concerns about the serious shortages of nurses (IOM, 2004b) and other personnel, the extent to which the current system will be able to accommodate large increases in the numbers of donors and transplant recipients is unclear. Currently, specialized operating room and critical care professionals must be available for donation surgery and the
TABLE 4-1 OPO Donation Rates, 2004
|
Number of OPOsa |
Donation Rateb (%) Range |
|
7 |
34–45 |
|
8 |
45–50 |
|
14 |
50–55 |
|
7 |
55–60 |
|
8 |
60–65 |
|
9 |
65–69 |
|
6 |
70–78 |
|
aIn 2004, there were 59 OPOs; a merger occurred in 2005, and currently there are 58 OPOs. bThe donation (or conversion) rate is calculated as the number of actual donors per the number of eligible donors expressed as a percentage. Eligible donors are defined as heart-beating individuals who meet or who will imminently meet criteria for neurologic determination of death, who are aged 70 years or under, and who have not been diagnosed with exclusionary medical conditions. SOURCE: SRTR (2005). |
|
subsequent transplantation procedures. In an already stressed system, shortages of nurses or anesthesiologists, for example, could limit the options for timely donation and the quality of care of either the donor or the recipient. Given these system challenges, strategic planning for such increases would need to be undertaken to ensure that these scarce resources will be able to be used the most effectively and that the necessary human resources are available.
Required Request and Required Referral
Several systemwide initiatives have focused on increasing the donation rate by ensuring that all potential donor families are asked about donation (required request) and that OPOs are notified of all imminent deaths (required referral). Beginning in 1986, the Health Care Financing Administration (HCFA; now CMS) stipulated that Medicare reimbursement to hospitals was contingent on the hospital having a required request policy to ensure that the families of patients who are eligible to be donors are given the opportunity to donate (Nathan et al., 2003). In 1998, HCFA published the stipulations, known as the Final Rule, that imposed several additional requirements, including the requirement for hospitals to have an agreement with an OPO and to put in place mechanisms to contact the OPO in a timely manner about individuals who die or whose death is imminent (required referral) (DHHS, 1998). The regulations also require hospitals working with the local OPO to inform the family of every potential donor of the option to donate organs or tissues. JCAHO has incorporated required request and referral policies into requirements for hospital accreditation (JCAHO, 2005).
Increases in the numbers of referrals to OPOs have occurred; from 2002 to 2003 the number of referrals increased by nearly 10 percent (from 1,022,280 to 1,121,392) (Delmonico et al., 2005). However, the extent to which increases in donation rates can be attributed to required referral practices alone is unclear. One potential gap in the current system is that the referrals most often involve patients whose deaths are imminent as determined by neurologic criteria and do not often include deaths determined by circulatory criteria (Bernat et al., 2006) (Chapter 5).
ORGAN DONATION AND TRANSPLANTATION BREAKTHROUGH COLLABORATIVES
In recent years, many initiatives to increase the rates of organ donation have been undertaken. Several of these efforts are early in their implementation phases and have not yet been fully evaluated. Although organ donation rates have risen in the past 5 years, it is difficult, if not impossible, to
determine exactly how much each initiative or regulatory change has contributed to that rise. Taken together, the data suggest that a multipronged approach is necessary to realize the potential of organ donation in the United States.
A major new initiative is the series of Organ Donation Breakthrough Collaboratives. Directed by the Health Resources and Services Administration (HRSA), the collaboratives attempt to increase rates of organ donation by encouraging hospitals and OPOs to use methods of continuous quality improvement to enhance the process of deceased organ donation.
The process of identifying and referring potential donors, requesting donation, recovering the organs, and ensuring that they are successfully transplanted is complex. It takes place under difficult circumstances, with time pressure and emotional stress, and in environments that vary widely across the country. In these circumstances, mobilization of the personnel involved in the local settings to identify ways to improve the process, use quality improvement methods to explore the process, and share the results
|
BOX 4-3 Quality Improvement in Health Care Quality improvement can be defined as systematic activities that are guided by empirical evidence and that are designed to bring about immediate positive change. A wide variety of methods are used to achieve quality improvement, all of which involve deliberate actions to improve processes involving the provision of health care in local settings. These actions are guided by data on the effects of the methods that have been introduced. For example, introducing quality improvement methods often means encouraging people in the local setting to use their daily experience to identify promising ways to improve the quality of the care that they provide, implement changes on a small scale, collect data, and assess the results. The goal is to find interventions that work well, implement those interventions more broadly, and thereby improve local practice. Alternatively, a quality improvement activity might begin with a review of aggregate data at the patient, provider, clinical unit, or organizational level to identify a clinical care or management change that can be expected to be an improvement over the existing procedures. The change is made, the effects are monitored, and conclusions are drawn about whether the change should be made permanent. Quality improvement can also be brought about by collecting data from multiple organizations, analyzing those data to understand what drives positive change, and using the results to design and implement a strategy to achieve a specific improvement across organizations (Berwick, 1989; Kilo, 1998; McLaughlin and Kaluzny, 1999). |
with others has significant potential to increase the supply of organs available for donation (Box 4-3).
The collaboratives began with HRSA partnering with the Institute for Healthcare Improvement (IHI), a nonprofit organization that is a leader in promoting the application of quality improvement methods to healthcare systems. IHI developed the Breakthrough Collaborative model, which brings healthcare organizations together to identify, learn, adapt, replicate, and celebrate breakthrough practices that can help them achieve specific improvement goals (IHI, 2003).
The collaborative’s initial analysis of data on organ donation found wide variations in donation rates (also termed conversion rates) among hospitals and OPOs. (It is important to note that conversion rates are based on data from donation after neurologic determination of death and do not reflect the larger potential population of donors after circulatory determination of death [Chapter 5].) The data also showed that large urban hospitals with busy trauma centers hold the greatest potential for increasing donor conversion rates because approximately half of the eligible donors are concentrated in slightly more than 200 of the largest hospitals nationwide (HRSA, 2005a).
On the basis of site visits, extensive interviews, and a review of the best practices at six OPOs and 16 OPO-affiliated hospitals with high donor conversion rates (HRSA, 2003), the collaborative leaders developed a change package that comprised a set of shared strategies to boost organ donation rates. Broadly, those strategies
-
advocate organ donation as the mission,
-
involve senior leadership to achieve results,
-
deploy a self-organizing OPO/hospital team,
-
practice early referral and rapid response,
-
learn effective requesting, and
-
implement DCDD.
The first two collaboratives focused on organ donation. In 2003 and 2004, the first collaborative began with 95 hospitals and 43 OPOs as participants (personal communication, D. Wagner, HRSA). To share best practices among participating OPOs and hospitals, the collaboratives conduct regular learning sessions, in which participants describe their experiences, discuss problem-solving techniques, and celebrate their successes. Through these interactions, a community with a shared vision and a commitment to improving the donation process has developed. Data on several process and outcome measures are voluntarily submitted: the organ transplant conversion rate, the referral rate, the number of medical examiner denials, the use of timely notification, the use of the appropriate requester, and donor before nondonor statistics. These measures serve as benchmarks
for individual hospitals and collective members of the collaborative to measure the impacts of their specific initiatives.
Although Collaborative 1, the first phase, ended in September 2004, member hospitals continued reporting data at regular intervals through October 2005. The second collaborative, launched in September 2004, included 131 hospitals of the nation’s 500 largest hospitals, 50 OPOs, and a large number of satellite teams. Again, members regularly reported process and outcome measures on a shared website, offering a venue for comparison and learning.
An analysis of the impact of the first collaborative compared trends in donation rates between the 95 hospitals that participated in the first Breakthrough Collaborative and 99 control hospitals that had at least five eligible donors2 in the year before the first Breakthrough Collaborative and affiliated with OPOs that did not participate in the first collaborative (Howard et al., under review). Collaborative and control hospitals were similar in size (535 versus 505 beds), but collaborative hospitals are more likely to be members of the Council of Teaching Hospitals (64 percent versus 20 percent). OPOs report the number of eligible donors by hospital and by month to the United Network for Organ Sharing. Because not all eligible donors are identified as such and the reliability of the classification of potential donors as “eligible” and “ineligible” is unknown, the level of the conversion rates should be interpreted cautiously. However, differential trends in conversion rates across collaborative and control hospitals provide evidence of the impact of the Breakthrough Collaboratives on donation rates (Figure 4-1).
In the year prior to the first of the Breakthrough Collaboratives, conversion rates were the same among Collaborative 1 hospitals and control hospitals (both 52 percent). By the final 6 months of the first collaborative, conversion rates among Collaborative 1 and control hospitals differed substantially (60.3 and 52.2 percent, respectively), suggesting that the Breakthrough Collaborative was successful in increasing organ donation rates. During the second of the Breakthrough Collaboratives, when best practices were disseminated more widely, conversion rates increased among control hospitals and continued to increase among Collaborative 1 hospitals as well.
The impact of the Breakthrough Collaboratives also shows up in aggregate nationwide donation trends. Figure 4-2 displays the annual increase in the number of organ donors nationwide from the prior year for the period
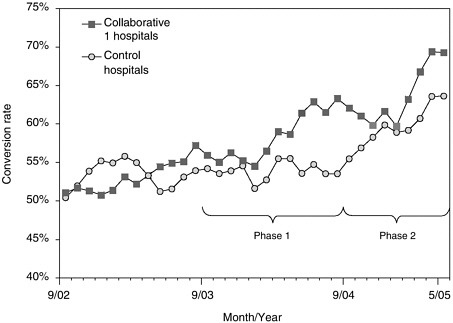
FIGURE 4-1 Conversion rates among member hospitals of the first breakthrough collaborative and non-collaborative-member hospitals.
NOTE: Phase 1 refers to the time period of the first collaborative and Phase 2 refers to the time period of the second collaborative.
SOURCE: Howard et al. (under review).
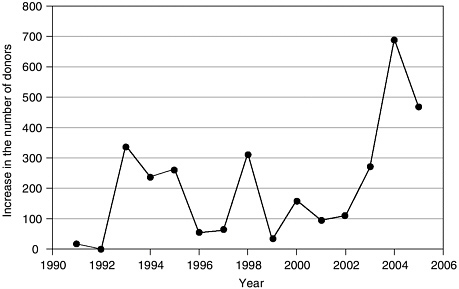
FIGURE 4-2 Annual increase in the number of deceased organ donors.
SOURCE: Howard et al. (under review).
from 1991 to 2005. The average increase from 1991 to 2003 was 150 donors, but reached 691 donors in 2004 and 445 donors in 2005 (projected on the basis of data through September 2005) (Howard et al., under review). The increase in the number of deceased donors nationwide in 2004 over the prior year was 11 percent (HRSA and SRTR, 2006).
The Organ Transplantation Breakthrough Collaborative (the third, and current, phase) is focused on increasing organ utilization. In particular, this initiative targets transplant centers, surgeons, and OPO and hospital administrators to evaluate how their institutions can increase the number of organs recovered and transplanted, with a goal of 3.75 organs per donor.
Sustaining the quality improvement efforts is critically important. The Breakthrough Collaboratives have been instrumental in enhancing the coordination, processes, and practices within hospitals and OPOs as well as among all of the relevant organizations and individuals. To maintain this level of coordination and communication between organizations and to continue to improve conversion rates will require efforts focused on instilling quality improvement into the evaluation, accreditation, and reimbursement processes and infrastructure. As recommended at the end of the chapter, resources should be invested in sustaining these quality improvement initiatives.
ONGOING EVOLUTION OF THE REQUEST PROCESS
The process of talking with a family about the opportunity for organ donation3 has evolved considerably over the past 30 years. Various approaches regarding who does the requesting, what is discussed, how the topic is broached, and when the discussion occurs have been explored (Bires, 1999; Nathan et al., 2003); however, only a limited amount of research has been done to ascertain the most effective models.
A growing body of research shows that families’ perceptions of their experience during the patient’s critical illness are a major factor in their decision to donate organs. One study found that nondonor families were often less satisfied with the quality of care received in the hospital and less likely to believe that they had sufficient time and privacy to consider organ donation (DeJong et al., 1998). By contrast, donor families report greater satisfaction with the request for organ donation (Burroughs et al., 1998). In one study, 94 percent of donor families indicated that they were satisfied
with their decision (DeJong et al., 1998). These studies suggest that communication and the relational aspects of the donation process are instrumental in creating a positive environment for considering and consenting to donation.
Formulation of the Request
The basic approach to the request process has evolved and is still changing. Although the process has been known as a process of requesting organ donation and asking for familial consent, the approach is changing in the direction of providing an opportunity for donation. As a result of these shifts, specialized skills, processes, and behaviors are needed.
One new model for the request process takes a more positive approach, which uses open-ended questions to guide the discussion with the family, presents organ donation as the expected path for families (Zink, 2004), and focuses the family’s attention on the benefit to the potential recipients. This approach has been described as the “presumptive approach” (Zink, 2004). To avoid confusion with presumed-consent terminology, the committee prefers the phrase “expected donation,” as suggested by Siegal and Bonnie (2006).
While this approach frames the opportunity for donation in the affirmative, clear boundaries must be maintained between offering an opportunity to consider donation, on the one hand, and using undue pressure and coercion on the other hand. This is particularly true when families are reapproached after they initially declined the opportunity to donate. In addition, further research is needed on family responses to this approach, its impact on donation rates, and its consistency with key ethical principles, such as the ones presented in this report.
Timing of Requests
The decoupled model of care that arose in response to concerns about conflicts of interest among physicians has received mixed reviews since its inception (Garrison et al., 1991; Cutler et al., 1993). This approach requires requests for organ donation to occur in a separate interaction (by separate staff, often OPO staff) after the family has been informed that the patient has died. This approach was developed largely in response to patient and familial mistrust of the healthcare system and the family’s concerns that dying individuals who were identified as potential donors would not receive the full extent of life-saving measures. One early retrospective review of 155 consecutive donor referrals reported that decoupling resulted in higher consent rates (Garrison et al., 1991). Another retrospective review demonstrated a nonsignificant trend toward more probable consent if the
donation request was made subsequent to the notification of death (Cutler et al., 1993); a similar study indicated that the timing of the request made no difference in the consent rate (Morris et al., 1989). Niles and Mattice (1996) found that asking families to donate at the time of death was associated with a lower consent rate, with no difference in consent rates when requests were made before and after death. Finally, a large prospective study failed to find support for decoupling. Instead, that study found that asking families to donate before they were notified of the patient’s death was associated with higher consent rates compared with the consent rates achieved when the family was asked to donate after the pronouncement of death (63.0 percent versus 56.6 percent) (Siminoff et al., 2002). This result suggests that the content of the communication and the relationships with healthcare professionals and OPO staff may be more important than the timing of the request.
Requesters
The early and consistent involvement of staff members with the family increases organ donation rates when it is coupled with effective communication. In a small study of a single hospital in Texas, the use of early notification and an in-house coordinator was associated with an increase in donation rates from 45 to 74 percent (Shafer et al., 1997). Other studies of in-house coordinators by the same group have replicated these results (Shafer et al., 2004). A recent descriptive study of the donor request process by Siminoff and colleagues (2001b) reported that the most important extrinsic factor to obtaining consent to organ donation was the time that the family decision makers spent with the requesters and the amount of time that they spent discussing specific donation-related issues.
The benefit of early and consistent involvement by OPO staff may be explained in part by the focused attention that is given to the donor family and the resultant benefit of a relationship with a knowledgeable, caring person. In an era of shortages of key healthcare personnel and hospital units stretched beyond capacity, the addition of personnel who can focus on this essential aspect of the donation process is clearly essential. Other members of the healthcare team could also be effective providers of these services, with the necessary training and the time to devote to talking with the family.
Best practices identified through the Breakthrough Collaboratives emphasize flexibility in determining who is the appropriate person to talk with the family, when to have the discussion, and how to approach the topic. This point is evident in the trend in requesting that has gone from designated requester to effective requester to effective requesting. A recent break-
through collaborative learning session (HRSA, 2005b) identified several characteristics of effective requesting. These include:
-
Focus on the family and provide compassionate care. Acknowledge the uniqueness of each family situation, and do not rely on a set of scripted statements.
-
Determine the most appropriate requester and the timing on a case-by-case basis. Families of patients who have been in the ICU for extended lengths of time may have developed a bond with a specific nurse or physician or may be more accepting of an impending death and willing to discuss organ donation at an earlier point than families in a more acute crisis.
-
Question assumptions regarding ethnic and cultural variations among those who are likely to donate or not donate.
-
Discuss donation as an opportunity, and consider the use of language that emphasizes the benefits to the transplant recipient and the healing that this can bring to the donor family.
-
Continue to provide excellent-quality care to the family, regardless of the donation decision.
These requesting practices are consistent with other end-of-life practices surrounding other decisions during the dying process, such as discussions on the withdrawal of life-sustaining therapies or the use of cardiopulmonary resuscitation. The integration of these requesting and inquiring practices increases the likelihood that the healthcare professionals caring for critically ill and dying persons will see organ donation not as a totally separate activity but as one integral to the end-of-life care that they are already providing.
Hospitals and OPOs use a variety of structures to optimize the staffing that will work for their geographic area and population. For example, Life Gift in Houston, Texas, has OPO staff working as in-house coordinators in the core hospitals with maximum donor potential. An OPO covering a wide geographic area works closely with the hospital staff who often talk with the families about organ donation. Given the time-intensive efforts required to meet the needs of the family during end-of-life care (Truog et al., 2001), many OPOs have hired family advocates who work on staff in addition to transplantation coordinators. The California Transplant Donor Network uses family resource teams who provide quality care to the family, irrespective of the family’s decision to donate.
South Carolina’s OPO, Life Point, restructured the procurement coordinator position into five separate positions (clinical services liaison, family support counselor, donor clinician, recovery coordinator, and aftercare coordinator), each of which has specific functions and goals (Sade et al., 2002). Such a structure allows the formation of an interdisciplinary team in which each member can use his or her expertise to support the family (for
example, family support counselors provide bereavement counseling and social work expertise). Such models are consistent with interdisciplinary palliative care models. In institutions with palliative care programs, natural collaborations may develop among palliative care providers, critical care professionals, and OPO staff that provide the families of potential organ donors with the needed services.
Some data suggest that the rates of donation by members of minority groups can be increased through the use of ethnically sensitive in-house coordinators or like-to-like requesters (Kappel et al., 1993; Gentry et al., 1997; Shafer et al., 1997). Although these results appear to be convincing, not all studies support the use of race- or ethnicity-specific requesters. With improved training in cultural competence, it is possible that ethnically sensitive requesters could be just as effective as requesters of the same ethnicity as the family.
As seen in the progress made in a short time by the Organ Donation Breakthrough Collaboratives, the methods used to request organ donation will continue to evolve. Research to explore the optimal models for organ donation requests, identify the key components of those models, and provide validation of the models being disseminated through training and other educational initiatives would be beneficial.
Several curricula and methods are used throughout the country to train healthcare professionals (including nurses, physicians, OPO staff, bereavement staff, and family advocates) on effective requesting. Although a standard curriculum is not necessary, the committee concludes that research is needed to explore the key components of training modules and to validate the effectiveness of those models.
A FRAMEWORK OF TRUST
One of the key elements in the success of organ donation and transplantation is the level of trust in healthcare professionals and the healthcare system. A recent IOM report (2000c) recommended aligning organ donation with quality care at the end of life. It asserted that “trust depends on the knowledge that the best care will be provided to all patients regardless of decisions about organ and tissue donation” (IOM, 2000c, p. 17). Indeed, opinion polls often indicate that distrust or mistrust of the healthcare system is a reason for the reluctance of individuals to become donors or of families to donate a deceased relative’s organs (Chapter 2).
The provision of health care is a human activity based on trust between patients and healthcare professionals. Everyone wants, needs, and deserves trust in healthcare relationships. Yet, trust is a complex concept with a variety of meanings (Reina and Reina, 2006). At a minimum, trust is confidence in and reliance upon others, whether individuals, healthcare profes-
sionals, or organizations, to act in accord with accepted social, ethical, and legal norms. In organ donation, a key donation-related factor for individuals and families is whether it is reasonable for them to place their trust in the healthcare professionals and the organizations seeking to obtain organs from their deceased loved ones. For healthcare professionals and organizations, a key task is to ensure the reasonableness of the trust placed in them. Unless healthcare professionals and organizations can count on widespread trust, many of the proposals examined in this report will not be effective.
Consider, for instance, using an “expected donation” approach with the family of a deceased person whose donation wishes are not known but who, if alive, the family believes would have wanted to donate his or her organs. Approaching such a family in this way will be ineffective and even counterproductive if the family lacks trust in the professionals and organizations involved. At best, trust is fragile; but it is particularly fragile when the families, whose trust is needed to consent to organ donation, are confronting a major tragedy in their lives: a loved one’s death.
If trust is confidence in and reliance upon others to act in accord with social, ethical, and legal norms, the basis of that confidence then becomes the question. The most immediate basis is the trustworthiness of the requester, whether an individual healthcare professional or an organization. Yet, trustworthiness has at least two dimensions. One is demonstrated competence, that is, the capacity or ability to perform certain tasks; the other is a demonstrated commitment to the social, ethical, and legal norms. The first dimension is especially but not only technical; the second dimension is especially but not only a matter of dispositional character (or, in organizational terms, the makeup and direction of the system). The former has been termed competence trustworthiness (Reina and Reina, 2006), and the latter has been termed normative trustworthiness (i.e., the enacted commitment to social, ethical, and legal norms). Both are essential in a system that seeks to improve the rates of deceased organ donation.
Several tasks are central to competence trustworthiness in the context of seeking higher rates of organ donation: quality end-of-life care, accurate determination of death, and effective organ recovery. These competencies, which have been established by professional societies and regulatory agencies, create clear expectations about professional responsibilities, and involve a mechanism for monitoring and accountability. Moreover, these competencies align with ethical and legal standards of care. Embedded in these competencies are technical skills (i.e., determination of death); interpersonal, relational, and communication skills; and decision-making and organizational skills. Although the provision of quality end-of-life care is improving across the country, concurrent improvements in organ donation processes and end-of-life practices and processes are needed to create the trustworthiness necessary to improve donation rates.
The development of valid and reliable programs for certification of the professionals with various roles in the process of organ recovery can further enhance competence trustworthiness. The certification process aims to define and validate the knowledge base and competencies that are necessary to perform a particular role. Certification offers a method for establishing benchmarks for professional competence. To discharge well their responsibilities to the whole range of stakeholders—patients, families, and the public—healthcare professionals and OPO staff members must have access to effective ways to gain competence in end-of-life care, determination of death, and organ recovery. Evidence-based, validated curricula are necessary to support the achievement of certification and reliability in donation processes. This will be particularly important as innovative practices develop, for example, in DCDD.
Healthcare organizations demonstrate competence trustworthiness by putting into place integrated systems with clinical, educational, and administrative infrastructures that enable healthcare professionals to practice in accord with these competencies and achieve the outcomes that the process was designed to reach. Interdisciplinary teams of healthcare professionals and OPO staff who work collaboratively must be able to design systems to promote organ recovery that are flexible; that are aligned with the systems, structures, and cultures of their institutions; and that are effective. The methods used in the Breakthrough Collaboratives to engage interdisciplinary teams within a variety of healthcare organizations illustrate the flexibility needed to both respect and work effectively with the diverse organizational structures and climates where organ donation occurs. Through self-organizing teams, members of the Breakthrough Collaboratives designed unique approaches that have produced significant increases in the rates of organ donation.
Normative trustworthiness is also indispensable. One essential condition for normative trustworthiness is transparency. Patients and families need to be able to understand and appreciate the professionals’ and organizations’ commitments to social, ethical, and legal norms. As outlined in Chapter 3, these norms include the dead donor rule; acceptance of the decedent’s previously stated preferences about donation as prima facie binding; honest disclosure of information relevant to end-of-life care and other decisions, including organ donation; respect for the family’s preferences when the decedent’s preferences are unknown; avoidance of coercion, exploitation, and unfairness; and protection of confidentiality. Several of these are broad norms applicable to most areas of health care, not just to end-of-life care and organ recovery.
Within the context of efforts to obtain transplantable organs, it is important that the processes used be perceived to be impartial. Hence, consistency and reliability are essential. For example, the consistent and
reliable application of the criteria used to determine death, the use of reliable processes for determining donation preferences and acting upon them, and the use of consistent criteria for organ allocation are necessary to assure the public that the organ donation system is indeed trustworthy. Another crucial factor for trust appears in collaborative arrangements: a division of roles and responsibilities is essential to avoid the reality and the perception that a conflict of interest exists. Of particular importance is the clear separation of processes and personnel who are simultaneously caring for both donors and organ recipients.
Several of these norms identify what should be done or what should not be done, but other important questions arise about how the tasks should be discharged. For instance, the honest disclosure of information about end-of-life care decisions, including organ donation, should be sensitive, particularly in addressing cultural or religious issues. Intentional recognition of the ways that trust can be built and inadvertently broken during the donation and organ recovery process is essential in designing a system that can fulfill its mission.
NEXT STEPS
In building on the existing system and the many changes that are under way, it is important to sustain the momentum in part by ensuring that healthcare and transplantation professionals have the training and support that they need to carry out their tasks. It is the committee’s belief that the next steps to improving the organ donation system are to sustain continuous quality improvement, integrate organ donation requests with end-of-life care, enhance training in end-of-life communication and decision making, improve training on the criteria used in both neurologic and circulatory determinations of death, and continue to refine the process for donation requests.
Sustain Continuous Quality Improvement
As described above, the Breakthrough Collaboratives focus specifically on improving the current system for obtaining and transplanting organs. However, the goal of a collaborative is not merely to achieve the specific breakthrough improvement around which it is organized. Its long-term goal is to lay the foundation for ongoing quality improvement through the use of quality improvement methods, in the particular area of the original collaborative, and in the organization as a whole. Ideally, a breakthrough collaborative teaches quality improvement methods, enables participants to experience success in achieving a significant improvement, and begins a transformation of the organization’s culture that will enable it to continue
to improve. In fact, quality improvement is also referred to as continuous quality improvement, which reflects the fact that quality improvement is properly understood as an ongoing part of the organization’s normal healthcare operations and mindset rather than a set of discrete projects. To the extent that this happens as a result of the collaboratives, organizations will sustain the improvements achieved and may, in fact, find new ways to improve outcomes in the future.
To facilitate continuous quality improvement, HRSA, CMS, and private insurers (where relevant) should ensure that quality improvement efforts are recognized as part of normal organizational healthcare operations and should reimburse accordingly. It would also be desirable to provide some ongoing funding for technical assistance and the ongoing interaction of organizations around the use of quality improvement methods to improve the organ donation process. An emphasis should be placed on rigorous data reporting and analysis to ensure that the data collected are complete and of the highest quality.
Quality improvement methods are well suited for exploring the implications of the integration of organ donation and end-of-life care, as described below.
Integrate Organ Donation and End-of-Life Care
One of the overarching principles set forth by the Organ Donation Breakthrough Collaboratives is to “integrate organ donation fully into routine roles and responsibilities” while recognizing the diverse characteristics of hospitals involved in organ donation and transplantation (HRSA, 2003, p. v). Given this call for integration, the committee believes that the process of organ donation rightly belongs within the context of end-of-life care. To date, efforts to increase donation have not explicitly acknowledged this potential (Williams et al., 2003), even though policies and clinical practices in some hospitals and OPOs may include them (DHHS, 2000; HRSA, 2003).
The framework of end-of-life care, a component of palliative care,4 is grounded in respect for individuals and families, respectful communication and decision making, and compassionate care, all of which are essential to the organ donation process. Over the past 10 years, palliative and end-of-life care have been recognized as an integral part of the care provided to critically ill and injured patients and their families (Lo et al., 1999;
Shannon, 2001; Truog et al., 2001; IOM, 2003; Curtis, 2004; Tulsky, 2005). Building on the recommendations of previous IOM committees (1997, 2003) and others (Hastings Center, 1987; AACN, 1998), clinical practice guidelines are now available for quality palliative and end-of-life care (National Consensus Project Steering Committee, 2004). Furthermore, progress has been made in integrating end-of-life care into the guidelines, protocols, and standards for critical care professionals (Lo et al., 1999; Shannon, 2001; Truog et al., 2001; Gilmer, 2002; Rushton et al., 2002; IOM, 2003; Curtis, 2004; Tulsky, 2005). Although a gap remains between the guidelines and the systematic integration of those guidelines into clinical practice across the country, progress in integration is being made.
An integrated model would emphasize patient- and family-centered care, interdisciplinary teams, and institutional alignment. Although unintended, one result of the current supply-demand approach is the perception that the goal of the donation request process is to get consent rather than to offer dying patients or their families the opportunity to consider donation as a natural part of dying and death (Australian and New Zealand Intensive Care Society, 1998; Streat and Silvester, 2001; Rocker, 2002; Streat, 2003, 2004; Williams et al., 2003; JCAHO, 2004).
Use of an integrated approach would expand the measures of success of the organ donation process to include whether appropriate donors were identified, whether the family was offered the option of donation (if the deceased person’s wishes were unknown), and whether quality end-of-life care was provided. Other measures would include the quality of the communication about the patient’s dying process and death, the availability of resources for emotional and spiritual support, the management of pain and other symptoms, the offering of end-of-life closure and rituals, and the provision of bereavement services and resources. The assumption, which has not yet been established empirically, is that the provision of excellent end-of-life care would translate into higher rates of organ donation (Williams et al., 2001).
Quality end-of-life care is still a goal rather than a reality for many institutions and organizations. Quality improvement processes and practices need to be implemented in tandem with efforts to enhance organ donation rates. Further, there is still much to be learned about how to develop and follow through on advance care directives. Nationwide efforts have focused more on the designation of healthcare agents who are able to effectively advocate for incapacitated persons. It will be incumbent on OPOs and the transplant community, as they work with hospitals and other healthcare facilities, to improve support for individuals and their families regarding end-of-life care decisions particularly through communications with healthcare professionals, patient and family education about end-of-life decisions and decision-making, and provision of opportunities for or-
gan donation. The confidence and trust in quality health care needed to promote and encourage organ donation are the same goals as those needed to ensure optimal end-of-life care. To isolate organ donation from the broader context of end-of-life care has the potential to ultimately undermine the effectiveness of efforts to increase donation rates.
Emphasis on Patient and Family Relationships
An end-of-life care framework acknowledges that care is for the patient and his or her family and continues through the patient’s death and the family’s bereavement. Respect for individual choices is central to a framework of end-of-life care. Likewise, respect for a person’s competent choice about organ donation will guide decisions at the end of life.
Ideally, patients will have formally designated a proxy decision maker by executing a durable power of attorney for health care. In some cases, however, patients may not know or may not have discussed their preferences with family members or healthcare professionals. In a study focusing on surrogate decision making in end-of-life care, researchers found that the surrogate decision makers experienced less stress when they knew their loved one’s preferences and when those preferences were documented in an advance directive (Tilden et al., 2001). By themselves, however, advance directives cannot eliminate the burdens of decision making at the end of life, in part because they are rarely clear enough or specific enough to dissolve all uncertainty and ambiguity (Tulsky, 2005).
In situations in which the deceased person’s wishes have not been documented, an end-of-life care framework suggests that conversations with families be framed in terms of what most people would want if they were alive to state their desires and what most aggrieved families would want if they were able to step outside their grief. Attitude surveys consistently show that most people would want to donate and that most families are touched by the message that organ donation is an opportunity to bring some good out of their tragedy.
Because families are the most common surrogate decision makers at the end of life, it is essential that a framework for end-of-life care and organ donation be family centered. Shannon (2001) asserts that the families of critically ill patients play three important roles: they contextualize the patient’s life, they serve as proxy decision makers, and they are themselves the recipients of care. Families are often the people who know the critically ill patient best and are able to help healthcare professionals understand the patient’s values; preferences; and interpretations of the meaning of treatment, dying, and death. They also commonly serve as proxy decision makers when patients are no longer able to speak for themselves, for instance, because of a severe brain injury or some other catastrophic disease process.
Although concerns are frequently raised about families overriding patient’s preferences, there is little or no empirical evidence that this practice is widespread (Chapter 2). In an end-of-life care framework, the presumption would be that decisions about organ donation would be consistent with the patient’s recorded wishes in the absence of current and compelling evidence that the patient changed his or her mind. This presumption is consistent with clinical, ethical, and legal practices in decisions about other forms of end-of-life care. In view of the importance of the potential donor’s prior wishes, it is appropriate to elicit and document the individual’s views about organ donation at different times in the context of discussing a range of preferences about end-of-life care.
Use of Interdisciplinary Healthcare Teams
Increasingly, hospitals are developing interdisciplinary palliative care teams, inpatient hospice units, and bereavement programs (Manfredi et al., 2000). Consistent with an end-of-life care model, interdisciplinary teamwork is essential (National Consensus Project Steering Committee, 2004). Recently, models of training in the organ donation process have highlighted the importance of an interdisciplinary team that includes OPO staff and that closely collaborates with OPO staff. Although various roles within the process can be handled by individuals from different disciplines, there is a consensus that an interdisciplinary team approach is the most effective for achieving positive donation outcomes (DHHS, 2000).
An expansion of this approach would include the integration of organ donation and recovery practices into end-of-life protocols. The Nebraska Health System, for example, created the Acute Bereavement Service to ensure a consistent and caring approach to potential donor families (DHHS, 2000). Its interdisciplinary team provides comprehensive services to potential donor families and reports a nearly 100 percent rate of referral of hospital deaths to the OPO. New partnerships among critical care professionals, palliative care and bereavement specialists, and OPO staff would help to achieve full integration.
Enhance Training in End-of-Life Communication and Decision Making
The healthcare professional’s level of comfort in discussing organ donation is associated with an increased likelihood that families will donate their loved one’s organs (Siminoff et al., 1996, 2002). Increasingly, critical care professionals have access to education and resources aimed at enhancing their competencies in palliative and end-of-life care, particularly communication skills (Tulsky, 2005). These skills, which are transferable to the process of caring for a dying person who is also a donor candidate, include
the provision of humane and compassionate care, whatever the decision about donation; the relief of suffering; maintenance of respect and dignity; and nonabandonment of the patient or the patient’s family (Heyland et al, 2002, 2003). Also important is the responsibility of all members of the team to foster an environment with an adequate infrastructure and sufficient resources in which patients and their families are given the opportunity to consider the option of organ and tissue donation (HRSA, 2003). Building on the successes of the Organ Donation Breakthrough Collaboratives and HRSA’s demonstration projects, national initiatives for interdisciplinary training and models of collaboration should continue to be supported and expanded.
Effective communication about organ donation and end-of-life care is difficult in a culture of critical care that focuses on rescuing patients from death rather than integrating the goals of critical care with palliative and end-of-life care (Danis et al., 1999; Lo et al., 1999; Rushton et al., 2002; Curtis and Rubenfeld, 2005). Families of ICU patients often express concerns about the adequacy, reliability, and timeliness of communication about healthcare issues (SUPPORT Principal Investigators, 1995; Pierce, 1999; Azoulay et al., 2000; Heyland et al., 2002, 2003); and research on communication at the end of life continues to document deficiencies (Pierce, 1999; Azoulay et al., 2000; Kirchhoff et al., 2000; Heyland et al., 2002). Dissatisfaction with care at the end of life focuses on communication and decision making (SUPPORT Principal Investigators, 1995) and often arises from the perception of a lack of complete and consistent information and of inadequate respect and compassion for the patient and family (Heyland et al., 2002, 2003). These problems likely affect the process of organ donation in critical care settings.
Although there are curricula for end-of-life care, there are “few, if any, validated training models in EOL [end of life] care developed specifically for organ donation” (Williams et al., 2003, p. 1571). Few physicians and nurses have been trained in the skills of communication and problem resolution that may be important when clinicians broach end-of-life issues and options with patients and their families (Murphy et al., 2001). Clinicians often have misconceptions about basic ethical principles and beliefs that are widely shared in the bioethics literature and promoted by national professional organizations (May et al., 2000). Such misconceptions can make end-of-life decision making more difficult by erecting unwarranted barriers. Added to this mix are the comfort levels of healthcare team members discussing organ donation, along with misconceptions and misperceptions about neurologic determination of death, organ donation, and procurement (Siminoff et al., 2001a,b). Recent preliminary work with an experiential learning model suggests that interdisciplinary training can significantly increase consent rates (Tartaglia and Linyear, 2000; Williams et al., 2001).
A comprehensive meta-analysis of the effectiveness of such models is warranted, and the best ones should be replicated for interdisciplinary training.
SUMMARY AND RECOMMENDATIONS
This report is being written at a time when significant efforts are under way to improve the organ donation system, particularly those focused on improving the consent rates for donation after neurologic determination of death. The Organ Donation Breakthrough Collaboratives are working to galvanize the efforts of hospitals and OPOs to develop and implement continuous quality improvement methods and thereby create changes in policies, practices, and structures. Future support for continuous quality improvement efforts is critical to the progress of organ donation efforts.
The opportunity to decide whether to be an organ donor is an important dimension of end-of-life decision making. Patients and their families should be offered this opportunity as standard end-of-life care, and information on organ donation processes should be an integral part of the many other decisions that are faced at this time. For the organ donation process to be fully integrated into end-of-life care, a wide range of healthcare professionals need enhanced awareness of and training regarding the donation process. Moreover, it will be necessary for healthcare institutions to develop or improve their end-of-life care processes and competencies.
Recommendation 4.1 Sustain Continuous Quality Improvement Initiatives.
HRSA should be sufficiently funded to provide technical assistance to hospitals and OPOs for continuous quality improvement efforts, including the identification and dissemination of best practices. An infrastructure that can support the collaboration, the dissemination of findings, and evaluations of the Breakthrough Collaboratives should be funded. Furthermore,
-
Individual OPOs and hospitals should develop, implement, and evaluate continuous quality improvement processes.
-
Accrediting and monitoring organizations, such as the Association of Organ Procurement Organizations, Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and National Committee for Quality Assurance (NCQA) should require and monitor measures of continuous quality improvement, including process measures as well as conversion rates.
-
HRSA, the Centers for Medicare & Medicaid Services, and private insurers (where appropriate) should ensure that organizational quality improvement efforts are recognized as part of normal healthcare operations and should be reimbursed accordingly.
Recommendation 4.2 Increase Research on Innovative System Changes.
HRSA, the National Institutes of Health, and the National Center on Minority Health and Health Disparities should be allocated funds sufficient to increase research efforts to identify further innovative and effective system changes for improving the organ donation process and increasing the rates of organ donation and to evaluate the impacts of such changes on the healthcare system. Research efforts should be evidence based, interdisciplinary, and culturally relevant.
Recommendation 4.3 Strengthen and Integrate Organ Donation and Quality End-of-Life Care Practices.
Hospitals, OPOs, and other healthcare entities should consider how best to integrate the organ donation process with quality end-of-life care practices. Interdisciplinary teams should align end-of-life protocols, practices, and guidelines with organ donation protocols.
Recommendation 4.4 Enhance Training for Healthcare Professionals.
HRSA, in collaboration with palliative care and other professional associations representing diverse disciplines and specialties (including, but not limited to, critical care professionals, transplantation professionals, social workers, and clergy), should strengthen training in end-of-life practices and organ donation, including processes of communication and decision making, with the goal of establishing a knowledgeable and positive environment that supports organ donation.
REFERENCES
AACN (American Association of Colleges of Nursing). 1998. Peaceful Death: Recommended Competencies and Curricular Guidelines for End-of-Life Nursing Care. [Online]. Available: http://www.aacn.nche.edu/Publications/deathfin.htm [accessed March 20, 2006].
AOPO (Association of Organ Procurement Organizations). 2004. 2003 AOPO Annual Report. McLean, VA: Association of Organ Procurement Organizations.
Australian and New Zealand Intensive Care Society. 1998. Recommendations on Brain Death and Organ Donation. [Online]. Available: http://www.anzics.com.au/uploads/200005braindeathorgandonation.pdf [accessed March 20, 2006].
Azoulay E, Chevret S, Leleu G, Pochard F, Barboteu M, Adrie C, Canoui P, Le Gall JR, Schlemmer B. 2000. Half the families of intensive care unit patients experience inadequate communication with physicians. Critical Care Medicine 28(8):3044–3049.
Beauchamp TL, Childress J. 2001. Principles of Biomedical Ethics, 5th ed. New York: Oxford University Press.
Bernat JL, D’Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, Rosenbaum SH, DeVita MA, Gaston RS, Merion RM, Barr ML, Marks WH, Nathan H, O’Connor K, Rudow DL, Leichtman AB, Schwab P, Ascher NL, Metzger RA, McBride V, Graham W, Wagner D, Warren J, Delmonico FL. 2006. Report of a national conference on donation after cardiac death. American Journal of Transplantation 6(2):281–291.
Berwick DM. 1989. Continuous improvement as an ideal in health care. New England Journal of Medicine 320(1):53–56.
Berwick DM. 1996. A primer on leading the improvement of systems. British Medical Journal 312(7031):619–622.
Bires MH. 1999. Comparison of consent rates between hospital-based designated requestors and organ procurement coordinators. Journal of Transplant Coordination 9(3): 177–180.
Burroughs TE, Hong BA, Kappel DF, Freedman BK. 1998. The stability of family decisions to consent or refuse organ donation: Would you do it again? Psychosomatic Medicine 60(2):156–162.
Chisholm MA, Garrett CJ. 2001. Increasing transplant patients’ access to medications: Medicare and beyond. American Journal of Health-System Pharmacy 58(21):2081–2084.
CMS (Centers for Medicare & Medicaid Services). 2005. Medicare News, January 28, 2005. Medicare Proposes Conditions of Participation for Transplant Centers and Organ Procurement Organizations. [Online]. Available: http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=1335 [accessed March 15, 2006].
Curtis JR. 2004. Communicating about end-of-life care with patients and families in the intensive care unit. Critical Care Clinics 20(3):363–380.
Curtis JR, Rubenfeld GD. 2005. Improving palliative care for patients in the intensive care unit. Journal of Palliative Medicine 8(4):840–854.
Cutler JA, David SD, Kress CJ, Stocks LM, Lewino DM, Fellows GL, Messer SS, Zavala EY, Halasz NA. 1993. Increasing the availability of cadaveric organs for transplantation: Maximizing the consent rate. Transplantation 56(1):225–228.
Danis M, Federman D, Fins JJ, Fox E, Kastenbaum B, Lanken PN, Long K, Lowenstein E, Lynn J, Rouse F, Tulsky J. 1999. Incorporating palliative care into critical care education: Principles, challenges, and opportunities. Critical Care Medicine 27(9):2005–2013.
DeJong W, Franz HG, Wolfe SM, Nathan H, Payne D, Reitsma W, Beasley C. 1998. Requesting organ donation: An interview study of donor and nondonor families. American Journal of Critical Care 7(1):13–23.
Delmonico FL, Sheehy E, Marks WH, Baliga P, McGowan JJ, Magee JC. 2005. Organ donation and utilization in the United States, 2004. American Journal of Transplantation 5(4 Pt 2):862–873.
DHHS (U.S. Department of Health and Human Services), Health Care Financing Administration. 1998. Medicare and Medicaid programs: Hospital conditions of participation: Identification of potential organ, tissue, and eye donors and transplant hospitals’ provisions of transplant-related data. Final Rule. Federal Register 119:33856–33875 (Codified at 42 CFR §482.45).
DHHS, Health Resources and Services Administration and Health Care Financing Administration. 2000. Roles and Training in the Donation Process: A Resource Guide. Prepared by the Lewin Group, Inc. [Online]. Available: http://www.organdonor.gov/finalresourceguide1.pdf [accessed April 18, 2006].
DHHS, Office of the Inspector General. 2003. Variation in Organ Donation Among Transplant Centers. [Online]. Available: http://oig.hhs.gov/oei/reports/oei-01-02-00210.pdf [accessed March 15, 2006].
Garrison RN, Bentley FR, Raque GH, Polk HC Jr, Sladek LC, Evanisko MJ, Lucas BA. 1991. There is an answer to the shortage of organ donors. Surgery, Gynecology & Obstetrics 173(5):391–396.
Gentry D, Brown-Holbert J, Andrews C. 1997. Racial impact: Increasing minority consent rate by altering the racial mix of an organ procurement organization. Transplantation Proceedings 29(8):3758–3759.
Gilmer MJ. 2002. Pediatric palliative care: A family-centered model for critical care. Critical Care Nursing Clinics of North America 14(2):207–214.
Hastings Center. 1987. Guidelines on the Termination of Life-Sustaining Treatment and the Care of the Dying. Briarcliff Manor, NY: The Hastings Center.
Heyland DK, Rocker GM, Dodek PM, Kutsogiannis DJ, Konopad E, Cook DJ, Peters S, Tranmer JE, O’Callaghan CJ. 2002. Family satisfaction with care in the intensive care unit: Results of a multiple center study. Critical Care Medicine 30(7):1413–1418.
Heyland DK, Rocker GM, O’Callaghan CJ, Dodek PM, Cook DJ. 2003. Dying in the ICU: Perspectives of family members. Chest 124(1):392–397.
Howard DH, Siminoff LA, McBride V, Lin M. Under review. Evaluation of a nationwide quality improvement initiative to increase organ donation rates.
HRSA (Health Resources and Services Administration), Office of Special Programs, Division of Transplantation. 2003. The Organ Donation Breakthrough Collaborative: Best Practices Final Report. Prepared by the Lewin Group, Inc. [Online]. Available: http://www.organdonor.gov/bestpractice.htm [accessed March 20, 2006].
HRSA Organ Donation Breakthrough Collaborative. 2005a. Collaborative Charter. [Online]. Available: http://www.organdonationnow.org [accessed March 21, 2006].
HRSA Organ Donation Breakthrough Collaborative. 2005b (January 18–20). Presentations at the 2005 Learning Session 2. Birmingham, AL.
HRSA and SRTR (Scientific Registry of Transplant Recipients). 2006. 2005 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1995–2004. [Online]. Available: http://www.optn.org/AR2005/default.htm [accessed March 3, 2006].
IHI (Institute for Healthcare Improvement). 2003. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. [Online]. Available: http://www.ihi.org/NR/rdonlyres/3F1925B7-6C47-48ED-AA83-C85DBABB664D/0/TheBreakthroughSeriespaper.pdf [accessed March 22, 2006].
IOM (Institute of Medicine). 1997. Approaching Death: Improving Care at the End of Life. Washington, DC: National Academy Press.
IOM. 2000a. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press.
IOM. 2000b. Extending Medicare Coverage for Preventive and Other Services. Washington, DC: National Academy Press.
IOM. 2000c. Non-Heart-Beating Organ Transplantation: Practice and Protocols. Washington, DC: National Academy Press.
IOM. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press.
IOM. 2003. When Children Die: Improving Palliative and End-of-Life Care for Children and Their Families. Washington, DC: The National Academies Press.
IOM. 2004a. The 1st Annual Crossing the Quality Chasm Summit: A Focus on Communities. Washington, DC: The National Academies Press.
IOM. 2004b. Keeping Patients Safe: Transforming the Work Environment of Nurses. Washington, DC: The National Academies Press.
Jacob Arriola KR, Perryman JP, Doldren M. 2005. Moving beyond attitudinal barriers: Understanding African Americans’ support for organ and tissue donation. Journal of the National Medical Association 97(3):339–350.
JCAHO (Joint Commission on Accreditation of Healthcare Organizations). 2004. Health Care at the Crossroads: Strategies for Narrowing the Organ Donation Gap and Protecting Patients. [Online]. Available: http://www.jointcommission.org/NR/rdonlyres/E4E7DD3F-3FDF-4ACC-B69E-AEF3A1743AB0/0/organ_donation_white_paper.pdf [accessed March 20, 2006].
JCAHO. 2005. Comprehensive Accreditation Manual for Hospitals. Oakbrook Terrace, IL: Joint Commission Resources.
Kappel DF, Whitlock ME, Parks-Thomas TD, Hong BA, Freedman BK. 1993. Increasing African American organ donation: The St. Louis experience. Transplantation Proceedings 25(4):2489–2490.
Kilo CM. 1998. A framework for collaborative improvement: Lessons from the Institute for Healthcare Improvement’s Breakthrough Series. Quality Management in Health Care 6(4):1–13.
Kirchhoff KT, Spuhler V, Walker L, Hutton A, Cole BV, Clemmer T. 2000. Intensive care nurses’ experiences with end-of-life care. American Journal of Critical Care 9(1):36–42.
Lo B, Quill T, Tulsky J. 1999. Discussing palliative care with patients. Annals of Internal Medicine 130(9):744–749.
Manfredi PL, Morrison RS, Morris J, Goldhirsch SL, Carter JM, Meier DE. 2000. Palliative care consultations: How do they impact the care of hospitalized patients? Journal of Pain and Symptom Management 20(3):166–173.
May T, Aulisio MP, DeVita MA. 2000. Patients, families, and organ donation: Who should decide? Milbank Quarterly 78(2):323–336.
McLaughlin CP, Kaluzny AD, eds. 1999. Continuous Quality Improvement in Health Care: Theory, Implementation, and Applications, 2nd ed. Gaithersburg, MD: Aspen Publishers.
Morris JA, Slaton J, Gibbs D. 1989. Vascular organ procurement in the trauma population. Journal of Trauma 29(6):782–788.
Murphy PA, Price DM, Stevens M, Lynn J, Kathryn E. 2001. Under the radar: Contributions of the SUPPORT nurses. Nursing Outlook 49(5):238–242.
Nathan HM, Conrad SL, Held PJ, McCullough KP, Pietroski RE, Siminoff LA, Ojo AO. 2003. Organ donation in the United States. American Journal of Transplantation 3(Suppl 4):29–40.
National Consensus Project Steering Committee. 2004. Clinical Practice Guidelines for Quality Palliative Care. [Online]. Available: http://www.nationalconsensusproject.org/Guideline.pdf [accessed March 20, 2006].
Niles PA, Mattice BJ. 1996. The timing factor in the consent process. Journal of Transplant Coordination 6(2):84–87.
Pierce SF. 1999. Improving end-of-life care: Gathering suggestions from family members. Nursing Forum 34(2):5–14.
Reina DS, Reina ML. 2006. Trust & Betrayal in the Workplace: Building Effective Relationships in Your Organization, 2nd ed. San Francisco, CA: Berrett-Koehler Publishers.
Rocker GM. 2002. Organ and tissue donation in the intensive care unit. Canadian Medical Association Journal 167(11):1248–1249.
Rodrigue JR, Cornell DL, Howard RJ. 2006. Organ donation decision: Comparison of donor and nondonor families. American Journal of Transplantation 6(1):190–198.
Rushton CH, Williams MA, Sabatier KH. 2002. The integration of palliative care and critical care: One vision, one voice. Critical Care Nursing Clinics of North America 14(2): 133–140.
Sade RM, Kay N, Pitzer S, Drake P, Baliga P, Haines S. 2002. Increasing organ donation: A successful new concept. Transplantation 74(8):1142–1146.
Shafer T, Wood RP, Van Buren CT, Guerriero G, Davis K, Reyes DA, Sullivan H, Levert-Cole T. 1997. A success story in minority donation: The LifeGift/Ben Taub General Hospital in-house coordinator program. Transplantation Proceedings 29(8):3753–3755.
Shafer TJ, Ehrle RN, Davis KD, Durand RE, Holtzman SM, Van Buren CT, Crafts NJ, Decker PJ. 2004. Increasing organ recovery from level 1 trauma centers: The in-house coordinator intervention. Progress in Transplantation 14(3):250–263.
Shannon SE. 2001. Helping families prepare for and cope with a death in the ICU. In: Curtis JR, Rubenfeld GD, eds. Managing Death in the ICU: The Transition from Cure to Comfort. New York: Oxford University Press.
Siegal G, Bonnie R. 2006. Closing the organ donation gap: A reciprocity-based social contract approach. Journal of Law, Medicine & Ethics 34(2):415–423.
Siminoff LA, Lawrence RH. 2002. Knowing patients’ preferences about organ donation: Does it make a difference? Journal of Trauma 53(4):754–760.
Siminoff LA, Arnold RM, Caplan AL. 1996. Asking for altruism when death occurs: Who asks for organ donation and why? Transplantation Proceedings 28(6):3632–3638.
Siminoff LA, Arnold RM, Hewlett J. 2001a. The process of organ donation and its effect on consent. Clinical Transplantation 15(1):39–47.
Siminoff LA, Gordon N, Hewlett J, Arnold RM. 2001b. Factors influencing families’ consent for donation of solid organs for transplantation. Journal of the American Medical Association 286(1):71–77.
Siminoff LA, Lawrence RH, Zhang A. 2002. Decoupling: What is it and does it really help increase consent to organ donation? Progress in Transplantation 12(1):52–60.
Sque M, Payne SA. 1996. Dissonant loss: The experiences of donor relatives. Social Science & Medicine 43(9):1359–1370.
Sque M, Long T, Payne S. 2005. Organ donation: Key factors influencing families’ decision-making. Transplantation Proceedings 37(2):543–546.
SRTR (Scientific Registry of Transplant Recipients). 2005. National Reports: National Summary by OPO by Table, Table 3 (Measures of Donation Rates). [Online]. Available: http://www.ustransplant.org/csr/current/nats.aspx [accessed December 19, 2005].
Streat S. 2003. Organ donation. In: Bersten AD, Soni N, Oh TE, eds. Oh’s Intensive Care Manual, 5th ed. Edinburgh, UK: Butterworth-Heinemann.
Streat S. 2004. Clinical review: Moral assumptions and the process of organ donation in the intensive care unit. Critical Care 8(5):382–388.
Streat S, Silvester W. 2001. Organ donation in Australia and New Zealand—ICU perspectives. Critical Care and Resuscitation 3(1):48–51.
SUPPORT Principal Investigators. 1995. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). Journal of the American Medical Association 274(20):1591–1598.
Tartaglia A, Linyear AS. 2000. Organ donation: A pastoral care model. Journal of Pastoral Care 54(3):277–286.
Tilden VP, Tolle SW, Nelson CA, Fields J. 2001. Family decision-making to withdraw life-sustaining treatments from hospitalized patients. Nursing Research 50(2):105–115.
Truog RD, Cist AF, Brackett SE, Burns JP, Curley MA, Danis M, DeVita MA, Rosenbaum SH, Rothenberg DM, Sprung CL, Webb SA, Wlody GS, Hurford WE. 2001. Recommendations for end-of-life care in the intensive care unit: The Ethics Committee of the Society of Critical Care Medicine. Critical Care Medicine 29(12):2332–2348.
Tulsky JA. 2005. Beyond advance directives: Importance of communication skills at the end of life. Journal of the American Medical Association 294(3):359–365.
USRDS (U.S. Renal Data System). 2005. USRDS 2005 Annual Data Report: Costs of CKD & ESRD. [Online]. Available: http://www.usrds.org/2005/pdf/11_econ_05.pdf [accessed March 20, 2006].
Williams MA, Lipsett PA, Shatzer JH, Rushton CH, Berkowitz ID, Mann SL, Lane K, Knapp JC, Humphreys SL, Zaeske R, Haywood C. 2001. Experiential, interdisciplinary training in end-of-life care and organ donation. Critical Care Medicine 28(Suppl):A204.
Williams MA, Lipsett PA, Rushton CH, Grochowski EC, Berkowitz ID, Mann SL, Shatzer JH, Short P, Genel M. 2003. The physician’s role in discussing organ donation with families. Critical Care Medicine 31(5):1568–1573.
Woodward RS, Schnitzler MA, Lowell JA, Spitznagel EL, Brennan DC. 2001. Effect of extended coverage of immunosuppressive medications by Medicare on the survival of cadaveric renal transplants. American Journal of Transplantation 1(1):69–73.
Yen EF, Hardinger K, Brennan DC, Woodward RS, Desai NM, Crippin JS, Gage BF, Schnitzler MA. 2004. Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. American Journal of Transplantation 4(10):1703–1708.
Zink S. 2004. The Presumptive Approach to Consent for Organ Donation. [Online]. Available: http://www.presumptivity.com/files/9.%20Introduction.ppt [accessed March 20, 2006].


































