4
The U.S. Military Malaria Vaccine Research and Development Program—Scientific Aspects
MALARIA THREAT
Malaria presents a serious medical threat to U.S. military capability for operations in any environment where malaria is endemic—specifically, the developing tropical and subtropical regions of the world. This includes essentially all of Africa, most of Southeast Asia, much of India, Pakistan and Bangladesh, parts of Central Asia and the Middle East, parts of South America, and most of Central America. Many of these regions are characteristically politically and economically unstable with brittle infrastructures and often social unrest. Current malaria countermeasures include drug prophylaxis and treatment, vector control and personal protection efforts (topical repellents, clothing, and bed nets)—but no vaccine.
Prophylactic malaria drugs are currently the major preventive measure for military personnel. The Department of Defense (DoD) continues to produce new such drugs, including, most recently, tafenoquine. However, there are serious issues around the effective use of antimalarial drugs that include increasing multidrug resistance to P. falciparum (Asia and Africa) and P. vivax (Asia), problems with compliance both in terms of personal discipline and concerns over possible toxic side effects, and potential logistical failures. Consequently, a malaria vaccine that protects military personnel against infection and severe disease, although requiring a long and expensive research and development commitment to bring the product to Food and Drug Administration (FDA) approval, affords
the most cost-effective, safest, and least encumbering solution to the malaria threat.1
Department of Defense Mandate for a Malaria Vaccine
The U.S. Army was designated by Congress (1982) as the lead agent for infectious disease research. Renewed emphasis on the importance of vaccines and other countermeasures was given in Executive Order 13139 (1999) directing that “It is the policy of the United States government to provide our military personnel with safe and effective vaccines, antidotes, and treatments that will negate or minimize the effects of these health threats.” These health threats include diseases endemic to an area of operations, such as malaria.
Under the direction of the U.S. Army Medical Research and Materiel Command (USAMRMC), the tri-service Military Infectious Diseases Research Program (MIDRP) coordinates malaria vaccine research and development at the Walter Reed Army Institute of Research (WRAIR), the Naval Medical Research Center (NMRC), and at DoD laboratories overseas in Kenya, Thailand, Indonesia, Peru, and Egypt.
MALARIA VACCINE REQUIREMENTS
The current requirements for a malaria vaccine (Appendix B) are formulated in a U.S. Army operational requirements document dated March 13, 1997, which has now expired and is due to be replaced. Although the current requirements were prepared under the auspices of the Army, the requirements are no different for the Navy and Marines. The requirements are used to guide the MIDRP Malaria Vaccine Program through the vaccine research and development process, including aiding in the decision process of when to move a product to advanced development.
The current requirements are expressed in terms of “development threshold” and “development objective,” but the consequences of a product reaching either of these benchmarks was not clear. In addition, the requirement for efficacy was subject to different interpretations since it did not distinguish between prevention of infection, clinical attacks, or severe malaria.
Rather than attempting to interpret and revise the military requirements using the same terminology as in the operational requirements
document, the committee opted to express its views about the desirable targets for a malaria vaccine in terms of two types of vaccine, a “first-generation vaccine” and an “ideal” vaccine (Table 4-1). The former would be a vaccine worth using by the military in addition to chemoprophylaxis, while the latter represents the most desirable vaccine in all characteristics and could be used to replace the routine use of chemoprophylaxis.
The desired levels of protection specified in Table 4-1 (60 percent for a first-generation vaccine and at least 95 percent for a second-generation vaccine) represent the consensus Delphian judgment of the committee members based on their expert opinion and by analogy with other diseases for which vaccines are being developed. For the first-generation vaccine, there is no objective criterion justifying the level of 60 percent—it represents what the committee felt would likely be useful in addition to chemoprophylaxis.
Vaccine development often follows a “generational” process whereby the first vaccine licensed and marketed is not fully effective, but is later replaced with a second-generation vaccine with greater efficacy, a different schedule, a different target age-group, and/or better safety. This has occurred for example with the Haemophilus influenzae type b (Hib), pertussis, pneumococcal, and hepatitis B vaccines. This adjunctive strategy is very
TABLE 4-1 Important Characteristics of a First-Generation Malaria Vaccine and a Later-Generation Ideal Vaccine
|
Characteristic |
First-Generation Vaccine |
Ideal Vaccine |
|
Species |
P. falciparum |
All malaria species |
|
Efficacy end point |
Clinical disease |
Blood-stage infection |
|
Level of vaccine efficacya |
60% |
≥95% |
|
Duration of protection |
6 months |
3 years |
|
Immunization schedule |
Reasonably rapid, convenient, and compatible with concurrent other vaccines |
Rapid, convenient, and compatible with concurrent other vaccines |
|
Chemoprophylaxis still required |
Yes |
No |
|
aThe proposed levels are intended as point estimates. The lower limit for the first-generation vaccine might be of the order of 30–40 percent, by analogy with what is regarded as potentially useful for seasonal influenza and HIV. |
||
likely to be the scenario for malaria vaccines given the difficulty of producing an initial vaccine with high efficacy. Achievement of the first-generation vaccine should not lead to the cessation of further vaccine development, given the importance of malaria to the military and the continuing need for chemoprophylaxis with the first-generation vaccine.
P. falciparum is a more important target than other malaria species. Because it seems unlikely that the same vaccine would protect against multiple species of Plasmodium, more than one vaccine may be needed. Although it would not be the most desirable outcome, one could envisage a situation in which there is a vaccine that is more than 95 percent effective against P. falciparum, but prophylaxis is still needed because there is not yet such a vaccine against P. vivax.
The generational approach is similar to that recommended for a public health-oriented vaccine as proposed by the Malaria Vaccine Technology Roadmap Working Group (Roadmap, 2006). The consensus of the roadmap working group was that the goal for a first-generation public health-oriented vaccine is 50 percent efficacy, lasting for a year or more, against severe disease and death in children under 5 years (by 2015). The goal for a second-generation vaccine is 80 percent efficacy, that lasts longer than 4 years, against clinical disease and death.
Recommendation 4.1: For a first-generation vaccine, a level of 60 percent efficacy (with a lower limit of 30 percent for the 95 percent confidence interval around the 60 percent point estimate of efficacy) against the clinical effects of P. falciparum would be a useful adjunct to chemoprophylaxis for military use. Nevertheless, research to develop a more effective second-generation vaccine that can be used in the absence of chemoprophylaxis and that would confer a much higher level of efficacy against infection should continue.
CLINICAL TRIALS TO TEST EFFICACY OF A FIRST-GENERATION MALARIA VACCINE
The MIDRP Malaria Vaccine Program has the goal of licensing a “U.S. military/travelers” vaccine that might not have relevance for preventing disease in indigenous pediatric populations in malarious areas. A series of carefully designed clinical trials executed in a step-by-step fashion move a vaccine candidate incrementally towards licensure. Phase 1 trials preliminarily examine the vaccine candidate’s safety and immunogenicity in small numbers of healthy adults. These early phase 1 trials detect adverse reactions that occur at high frequency and provide an initial glimpse of whether the candidate elicits relevant immune responses.
Subsequent phase 2 trials that assess the vaccine in increasingly larger numbers of subjects are typically placebo controlled to better measure the rate of adverse events versus background complaints. For vaccines that will ultimately target infants or young children, phase 1 and 2 trials must be undertaken in progressively younger subjects until the target age is reached. If a vaccine candidate combines several distinct antigens, each of which may independently contribute to protection, phase 2 studies must document that immune responses are elicited to all the component antigens.
For some vaccines such as candidate malaria vaccines, it is possible to obtain preliminary assessments of the efficacy of the vaccine by performing experimental challenge studies at late phase 1 as well as early phase 2. In performing experimental malaria challenges, vaccinated and control subjects are each exposed to the bites of five insectary-reared mosquitoes infected with P. falciparum. These challenge trials are critical to selection of effective preerythrocytic vaccine candidates.
Large-scale randomized controlled phase 3 efficacy trials remain the “gold standard” for demonstrating the efficacy of a vaccine to prevent the disease under natural conditions of exposure. In general, prelicensure phase 3 trials are expensive, logistically demanding, require multiple years to complete, and are subject to the vagaries of year-to-year variation in disease incidence. Phase 3 trials represent the ability of a vaccine to protect under “idealized conditions” where there is a pristine cold chain, analyzed subjects have received full dosage of vaccine, and case detection is intensive.
Once the clinical acceptability, safety, and immunogenicity of the leading vaccine candidate (see following sections on various candidates) have been successfully demonstrated in phase 2 trials and its efficacy documented in several experimental challenge studies in U.S. subjects exposed to insectary-reared Anopheles, it is appropriate to transition the vaccine to phase 3 efficacy field studies. These phase 3 field efficacy studies, if they can be accomplished, are key to licensure.
Given the crucial importance of providing a clear path to licensure for a potential vaccine, the committee concluded that it was appropriate to discuss their view of the feasibility of undertaking such daunting key studies within the DoD system. In contrast, the various other clinical trials, including phase 1 and 2 safety and immunogenicity trials, Phase 2 experimental challenge model studies, and assessment of the large-scale safety and immunogenicity testing of three production lots of the vaccine are generic and well within the capability of the MIDRP Malaria Vaccine Program testing infrastructure.
The MIDRP Malaria Vaccine Program investigators have long held the view that it may be possible to perform phase 3 trials in which cohorts of malaria-naïve adult subjects from the U.S. military or other immuno-
logically naïve adult populations might be immunized with the experimental malaria vaccine or a control vaccine prior to being sent to endemic areas of very high seasonal P. falciparum transmission (Brown et al., 1994; Sherwood et al., 1996). The initial type of study to be carried out in U.S. adults brought to such a malaria “hot spot” for several months would be a rigorous test of the vaccine’s ability to confer 60 percent efficacy compared to a control vaccine. Some examples of a control vaccine that would confer an independent benefit upon the control subjects include a quadrivalent meningococcal conjugate or rabies vaccine; this benefit for the control subjects would be important from the bioethical perspective. During the high transmission period (usually 3–5 months in duration) the subjects would be kept under intensive clinical surveillance using active case detection to identify cases of P. falciparum malaria. For ethical reasons, physicians, nurses, corpsmen, and other health care providers would accompany the subjects to the field area to assure prompt and vigorous therapy.
For many years, DoD researchers have maintained a field site in Western Kenya where the dynamics of transmission during high season are such that 89 percent of semi-immune adults developed clinical P. falciparum malaria during the 5-month peak period of transmission (January through May) (Sherwood et al., 1996). In Western Kenya, approximately 100 percent of immunologically naïve older infants and toddlers develop confirmed clinical malaria during high season. It is expected that in the absence of compulsive rigorous chemoprophylaxis, approximately 100 percent of immunologically naïve U.S. adults would develop clinical malaria if they spent several months at this site during peak transmission season. The extremely high attack rates of malaria that occur in susceptibles in such an area suggest that phase 3 efficacy trials could be conducted with numbers of subjects that would be logistically feasible while maintaining close clinical supervision.
Because the point estimate of efficacy expected of the vaccine is relatively low (i.e., only 60 percent), it would be important to design the trial to include a rigorous lower limit of efficacy. A lower limit of 30 percent for the 95 percent confidence interval (CI) around the 60 percent point estimate of efficacy is recommended.
The committee considered two types of trials in nonimmune U.S. adults living temporarily in a malaria endemic area—without and with prophylaxis. Details of such proposed studies are given in Appendix C. The MIDRP Malaria Vaccine Program investigators working at AFRIMS in conjunction with Royal Thai Army rangers established the feasibility of carrying out an efficacy trial of a malaria vaccine in (semi-immune) volunteers who were not given chemoprophylaxis when sent to a field area of high malaria transmission (Brown et al., 1994). The precedent for this
type of study was also established with volunteers from the Colombian Army who participated in an efficacy evaluation of SPf66 vaccine (Amador et al., 1992).
Preliminary calculations were performed in order to assess the sample sizes necessary for such trials. The assumptions were that the malaria attack rate would be 70 percent (without chemoprophylaxis) and that the vaccine efficacy is 60 percent (with a lower limit of 30 percent for the 95 percent CI around the 60 percent point estimate of efficacy). The estimates in Appendix C show that the sample sizes required are reasonable: approximately 400 persons (200 per group) would be needed to give 90 percent power of detecting such an effect for initial trials without chemoprophylaxis. In trials with chemoprophylaxis, it was assumed that 10 percent would fail to take it; thus 4,000 persons would be needed for these trials in order to have 400 persons for the analysis.
Because of the investment made heretofore in maintaining field sites and conducting trials in Africa, such as in Western Kenya and in Ghana, a first-generation malaria vaccine that conferred 60 percent efficacy for 6 months would constitute a product that could be successfully evaluated for efficacy in prelicensure phase 3 trials both without and with chemoprophylaxis. These suggestions are not intended to minimize in any way the daunting challenge that would be faced by the MIDRP Malaria Vaccine Program in having to enroll the large number of U.S. subjects necessary to carry out the large phase 3 efficacy trial in Africa under recommended chemoprophylaxis. On the other hand, this major logistical challenge can be overcome by allocation of sufficient resources and by making such a trial a high priority.
Recommendation 4.2: Small, carefully designed and executed clinical efficacy trials involving U.S. military personnel (or other groups of immunologically naïve, nonmilitary personnel) off chemoprophylaxis (initial proof of principle studies) or on chemoprophylaxis (later study) should be carried out to assess the efficacy of the leading MIDRP Malaria Vaccine Program candidate in field sites in endemic areas. In this regard, field sites currently maintained by the DoD in Africa are a critical resource.
CURRENT AND PLANNED SCIENTIFIC PROGRAM
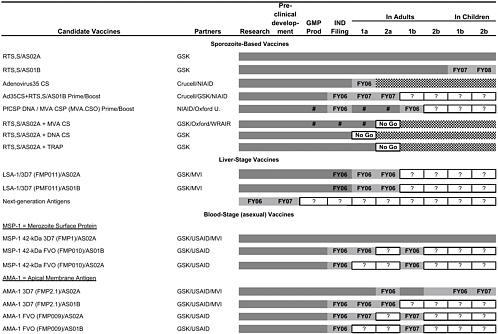
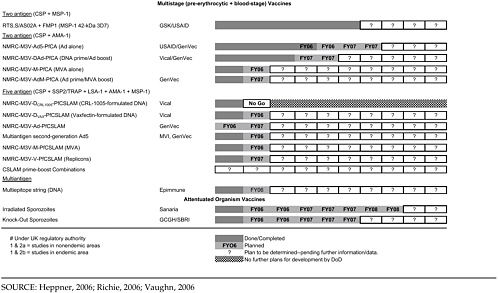
Table 4-2 shows the very large program of different constructs currently being tested by the MIDRP Malaria Vaccine Program, as well as the partners involved and projected time lines. The particular categories of antigens or constructs will be briefly discussed.
RTS,S
The WRAIR group has a strongly focused research and development strategy with an emphasis on the RTS,S recombinant protein preerythrocytic vaccine with GlaxoSmithKline (GSK) adjuvants. This continues a line of research that began 20 years ago after the sequencing of the circumsporozoite protein (CSP) and the preparation of the first recombinant proteins at WRAIR. Current efforts to improve immunogenicity and protective efficacy of RTS,S focus on new adjuvant formulations, comparing oil in water MPL/QS21 (AS02A) with a MPL/QS21 liposome formulation (AS01B), heterologous prime-boosts using naked DNA or viral vectors expressing CSP to boost RTS,S responses, and multistage antigen combinations (Ballou, 2005; Heppner et al., 2005). Direct comparisons of AS01B and AS02A are required and in progress with certain contructs, but constraints imposed by commercial partners limit some comparisons of the RTS,S/AS02A or AS01B constructs with and without particular viral vectors.
Recombinant Proteins
Other current WRAIR efforts focus on expressing recombinant blood-and liver-stage proteins, including MSP-1, AMA-1, and LSA-1 in E. coli with the correct conformation to elicit inhibitory antibodies. This is a particular challenge as both inhibitory and blocking antibody have been shown to develop following immunization and infection (Nwuba et al., 2002). To address these concerns both WRAIR and NMRC are developing growth inhibition assays to measure functional antibodies. Clinical trials are planned or in progress for all three antigens, and the intention is to assess the immunogenicity and efficacy of each separately before deciding whether to include them in a multicomponent vaccine.
For MSP-1, early studies of MSP-119 conjugated to tetanus toxoid showed poor immunogenicity in immunized volunteers (Keitel et al., 1999), and therefore efforts have focused on MSP-142, which contains additional T-cell epitopes. Combinations of two alleles (FVO/3D7) are planned for both MSP-1 and AMA-1 in order to overcome potential problems with polymorphism and strain specificity of vaccine-induced immunity. Both blood-stage antigens will be tested with AS02A adjuvant and AS01B formulations in fiscal year 2006–2007. These approaches are similar to those that are planned by the National Institutes of Health (NIH) with their Pichia pastoris-expressed MSP-142 3D7/FVO and AMA-1 C1 (FVO/3D7) in phase 1 trials (Malkin et al., 2005). However, the MIDRP Malaria Vaccine Program is more advanced in the phase 2 trial process for the 3D7 forms of MSP-1.
Gene-Based Vaccines and Prime-Boost Approaches
The NMRC group is focused on gene-based vaccines with a wide-ranging and diverse program. They are pursuing several specific strategies of DNA-based constructs. These revolve around the group of sequences known as CSLAM (CSP, SSP-2/TRAP, LSA-1, AMA-1, MSP-1) that were down-selected from a much larger group of DNA sequences previously included in the earlier MuStDO5 vaccine, which failed to show immunogenicity in human trials in the U.S. Antigen interference is suspected as part of the reason for that failure, and therefore noninterfering antigens were selected based on data from animal models.
The following are the specific subunit approaches:
-
Plasmid DNA (five plasmids each encoding one of the CSLAM antigens) in collaboration with Vical, using novel adjuvants such as Vaxfectin, either alone or as prime for subsequent boost for viral constructs (below)
-
Adenovirus 5 vectors in collaboration with GenVec, with two to five antigen sequences (CSLAM or some of its constituent antigens) used in DNA priming and vector boosting
-
Poxvirus vectors used in a similar way to adenovirus 5 vectors and containing the same antigens
-
Virus replicon particles in collaboration with Alphavax
-
Viral priming followed by recombinant protein boosting, in collaboration with the NIH Malaria Vaccine Development Unit and WRAIR
-
Multiepitope vaccines (T- and B-cell epitopes in viral vectors that bind to multiple HLA types, removed from surrounding unnecessary sequences) in collaboration with EpiImmune
The NMRC has immediate plans for a phase 1 clinical trial of the adenovirus 5/CSP + AMA-1 construct in U.S. volunteers in the near future, followed by trials of different prime-boost combinations of DNA and adenovirus 5 constructs for CSP/AMA-1.
The WRAIR scientists have also been investigating heterologous prime-boost regimens, in which different immunogens are used for prime versus boost, to further optimize immune responses. WRAIR is collaborating with Crucell and GSK in exploring use of Ad35 virus expressing CSP to boost RTS,S primed responses (although these studies are contingent on resolving intellectual property issues of both companies). Vaccinia-based prime-boost strategies are also being considered but are not proving to be successful.
The above description summarizes the aspects of research, particularly those funded directly by MIDRP Malaria Vaccine Program, that were
described to the committee. The program has been very effective in generating additional funds for research that may have different emphases.
Antigen Discovery Using Genomics and Proteomics
In addition to focusing on empirically defined antigens, both WRAIR and NMRC have initiated antigen discovery programs using genomics and/or proteomics. NMRC has extensively explored algorithms and new methods to identify and express potential candidates for preclinical studies. At least one new preerythrocytic antigen (AgX) recognized by cells from sporozoite-challenged volunteers has been identified by these methods. Exploration of the parasite transcriptome has led to the identification of critical genes required for hepatic-stage development, and deletion of these genes has provided genetically attenuated parasites for vaccine development.
Attenuated Sporozoites
The use of genetically or radiation-attenuated sporozoites as a military vaccine is being pursued (Mueller et al., 2005). WRAIR scientists are collaborating on a genetically attenuated sporozoite approach under a Gates Foundation Grand Challenges in Global Health grant with the Seattle Biomedical Research Institute. The radiation-attenuated sporozoite strategy is being pursued by a private company, Sanaria, founded by a former NMRC director, with National Institute of Allergy and Infectious Diseases (NIAID) support and strong links to the NMRC (Hoffman et al., 2002). Attenuated sporozoite vaccines would provide a multistage vaccine without the need to identify relevant antigens, and although the logistical and regulatory challenges of this approach are significant, the high vaccine efficacy of attenuated sporozoite vaccines merits efforts to test these vaccines in phase 1 and 2 trials.
Planned Clinical Trials
To summarize the status of current vaccine candidates, ongoing and pending clinical trials are listed in Table 4-3.
OVERALL ASSESSMENT OF SCIENTIFIC PROGRAM
The committee noted the impressive scientific program and achievements and the unparalleled opportunities provided by the availability of the human sporozoite challenge model. The work has resulted in hundreds of publications in the peer-reviewed scientific literature. From 2001
TABLE 4-3 Current and Pending MIDRP Malaria Vaccine Program Clinical Trials
|
Lead Agency |
Construct |
Objective |
Site |
IRB Approved |
|
WRAIR |
MSP-1/AS02A |
Safety, immunogenicity in immune 1–5-year-oldsa |
Kenya |
Yes |
|
WRAIR |
MSP-1/AS02A |
Efficacy in immune 1–5 year-oldsa |
Kenya |
Yes |
|
WRAIR |
AMA-1/AS02A |
Safety, immunogenicity in immune adultsb |
Mali |
Yes |
|
WRAIR |
RTS,S |
Efficacy AS02A vs. AS01B in naïve adults |
United States |
Yes |
|
WRAIR |
RTS,S |
Safety, immunogenicity AS02A vs. AS01B in immune adults |
Kenya |
Yes |
|
WRAIR |
LSA-1/AS02A |
Safety, immunogenicity, efficacy in naïve adults |
United States |
Pending |
|
WRAIR |
LSA-1/AS01B |
Safety, immunogenicity, efficacy in naïve adults |
United States |
Pending |
|
NMRC |
Ad Pf CA |
Safety, immunogenicity, efficacy in naïve adults |
United States |
Pending |
|
aThese trials funded by the Malaria Vaccine Initiative for a pediatric vaccine goal. bFunded by NIAID. |
||||
to 2005 alone, WRAIR scientists contributed to 149 papers and NMRC scientists to 147 papers on malaria vaccine-related studies, the largest number for any MIDRP program area. Since 1990, the NMRC malaria vaccine program has had six patent applications approved and WRAIR has had eight (see Appendix D). One additional WRAIR patent is expected to be assigned a patent number in the next few months, giving a total of nine from WRAIR. The MIDRP Malaria Vaccine Program has also been successful in leveraging additional funds and has had very successful collaborations.
The research funded by the MIDRP Malaria Vaccine Program has been enormously beneficial to the overall global vaccine effort. Highlights include the success in clinical trials with RTS,S, the expansion of activities beyond preerythrocytic vaccines to include erythrocytic antigens in multistage vaccines (both protein and gene based), the advanced production of these antigens to good manufacturing practices (GMP) standards, and the advances in gene-based prime-boost technologies with combination vaccines in animal models. Progress on the genetically and irradiated-attenuated sporozoite vaccine strategy is also very encouraging.
However there is concern over the large number of vaccine candidate constructs currently under evaluation, with numerous external partners involved. Lack of financial independence sometimes puts the partners in control. The pathway from preclinical research to clinical trials for the numerous gene-based products is sometimes not clear. In some cases the partners appear to be excessively influencing the research agenda while simultaneously imposing restrictions on what can be accomplished. For example, the use of two different adenovirus vectors needs to be reconciled, as do the two adjuvants being used by GSK. Head-to-head efficacy comparisons of constructs and vectors from different commercial partners are unlikely to occur and may not be necessary in any case. Other decision methods such as standardized comparative immunogenicity tests could be used to narrow the scope.
Increased focus on a smaller number of potential constructs is required, but the program lacks a prospective process or objective criteria for down-selection of candidates. Performance should be evaluated by established humoral and cellular immune response assays in a reference laboratory if possible, and additionally for blood-stage candidate vaccines by a functional immune response assay such as the merozoite growth inhibition assay. Development of these mutually agreed upon criteria should be the responsibility of Joint Task Force for Malaria Vaccine (JTF-MV) discussed below. Down-selection criteria should utilize all available information from the rapidly progressing HIV vaccine field to assess vaccine delivery platform leads.
The committee noted that increased focus on fewer candidate antigens and constructs does not imply decreased funding as development of even one of the current constructs will require a greatly increased budget. Maintaining a larger number of constructs is likely to lead to failure to complete development or to delay substantially the development of candidates.
From the information presented, the committee felt that there is no vaccine candidate yet available that is likely to meet the military requirements (even the suggested revised requirements) in the next 5 to 10 years. The likelihood of eventual success appears to be high, but a more realistic target date for availability of a licensed P. falciparum vaccine (even with more resources) is 2015–2020.
Recommendation 4.3: Research on all three main malaria vaccine development strategies—gene-based (e.g., DNA, plasmid, or viral vector vaccines) protein-based, and attenuated sporozoite approaches—should be continued. However, as research progresses, the number of candidate products must be limited by dropping those that perform less well. The MIDRP Malaria Vaccine Program
should aggressively move into clinical trials to test specific vaccine products, and select two to three leads at phase 1 and one lead at phase 2 for each strategy. For protein-based and gene-based strategies, the focus should be on specific vaccine products that combine the lead antigens (CSP, SSP-2/TRAP, LSA-1, AMA-1, and MSP-1) including their use in heterologous prime-boost combinations.
Recommendation 4.4: Finding correlates for protection in humans relevant to each of the above vaccine strategies should be a research priority.
Recommendation 4.5: The MIDRP Malaria Vaccine Program should continue research on human immune processes and responses to malaria. The current incomplete understanding of the mechanisms of protective immunity to malaria in humans constitutes a barrier that impedes malaria vaccine development.
After thorough review of the MIDRP Malaria Vaccine Program in order to understand the current situation and the program’s quality, the committee concluded that it is crucial to narrow the focus to a smaller number of candidate antigens. Given the limited time available for this review, the committee did not wish to give more detailed specific advice other than that given above and in Recommendation 4.3. Despite having extensive expertise in all scientific aspects of the program, the committee concluded that instead of offering one-time advice on specific antigens or approaches, it would be more productive to recommend a structure and process for ongoing review and decision making about the scientific direction of the work.