5
Organization and Management of the Program
PROGRAM MANAGEMENT
Malaria vaccine research in the Department of Defense (DoD) takes place at the Walter Reed Army Institute of Research (WRAIR), the Naval Medical Research Center (NMRC), and the overseas laboratories. The laboratories in Kenya and Thailand are subordinate units of WRAIR, and laboratories in Indonesia, Peru, and Egypt are subordinate units of NMRC. The DoD-funded research is coordinated within the U.S. Army Medical Research and Materiel Command (USAMRMC) by the Military Infectious Disease Research Program (MIDRP). The relationships between the program elements (USAMRMC, MIDRP, NMRC, WRAIR) are shown in Figure 5-1.
Within MIDRP there are four research areas and 11 program areas, one of which is program area F—malaria vaccine research. This is further subdivided into four task areas:
-
Task F: Malaria vaccine research
-
Task 6A: Protein-based vaccines
-
Task 6B: DNA-based vaccines
-
Task A1: P. vivax vaccines.
The first three task areas are the subject of this program review. In general, projects fall under Task F if they are core activities or relate to either antigen discovery, basic understanding of the malaria parasite (e.g., genetic diversity), or the immune response to it in humans and animals.
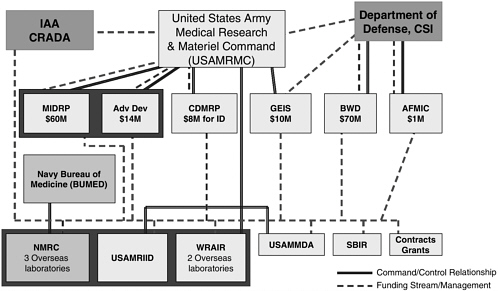
FIGURE 5-1 Military infectious diseases research program working relationships.
NOTE: IAA: interagency agreement; CRADA: cooperative research and development agreement; CSI: congressional special interest; MIDRP: Military Infectious Disease Research Program; Adv Dev: advanced development; CDMRP: congressionally directed medical research programs; GEIS: Global Emerging Infections Surveillance; BWD: Biological Warfare Defense; AFMIC: Armed Forces Medical Intelligence Center; USAMRIID: U.S. Army Medical Research Institute for Infectious Diseases; USAMMDA: U.S. Army Medical Materiel Development Activity; SBIR: Small Business Innovation Research.
SOURCE: Vaughn, 2006.
However, some basic and innovative research including new antigen discovery appears under tasks 6A and 6B as well. Task areas 6A and 6B are relatively new divisions within program area F, and the distinctions are still fluid. Task 6A essentially covers work carried out under the auspices of WRAIR, and 6B covers NMRC-specific projects; Task F is intended to cover joint activities. Within each task area there are three to five subsidiary objectives.
Several specific aspects of program management are discussed in the following sections, noting particularly the programmatic barriers that are impeding progress. Significant reorganization is then suggested in order to overcome these barriers.
Project Management Structure
DoD intramural funds are distributed through MIDRP by a proposal application and funding process with an annual cycle (although some core activities such as sporozoite production and GMP production are automatically renewed and are not subject to review each year). Currently, the objectives and their justifications under each task area are developed by a joint steering committee between WRAIR and NMRC that meets four times per year, mainly to manage the project proposal and approval process. The objectives for each task are described in written research plans distributed on the MIDRP website in order to solicit proposals.
Investigator-initiated proposals are written and submitted by objective, first as preproposals and then, if merited, as full proposals. In MIDRP as a whole, for fiscal year (FY) 2007 a large number (225) of new preproposals and 135 new full proposals were submitted, of which 72 new projects will be funded. The total is 116 funded projects since there are also 13 core projects and 31 multiyear projects. A subset of the total proposals submitted for FY2007 are for malaria vaccines: 42 preproposals and 28 full proposals, of which 14 will be funded, together with 3 core and 5 multiyear projects (total 22 for malaria vaccines). Each year the amounts of funds approved for WRAIR and NMRC projects are approximately equal.
External and onsite review by the American Institute of Biological Sciences (AIBS) occurs in order to review the steering committee prioritization of the proposals; however, MIDRP and the steering committee are not obligated to follow the AIBS recommendations. After prioritization, projects above the funding cutoff are approved. Senior Army and Navy investigators intimated that the quality of the AIBS reviews was erratic, and reviewers’ comments were not always helpful. In contrast, they lauded the broader type of review offered by a U.S.
Agency for International Development (USAID) committee that reviewed work supported by that agency through the DoD. That committee, which includes individuals with considerable product development experience, was considered to provide more helpful practical guidance.
The project management structure imposes several programmatic barriers. Although improved over earlier iterations, the structure seems cumbersome and inflexible and sometimes obstructive to the flow of work. A large number of competing proposals does not allow the program to be managed with a comprehensive investment and down-selection strategy. The application process and and outside review leads to a heavy administrative burden, and the whole process of application, review, and approval takes over a year from start to finish. There is a lack of focus in an extremely diverse and ambitious scope of work, with unrealistic expectations and a constant pressure to move forward even if not directly towards the goals. An inadequate advisory structure is a barrier to effective strategic planning.
IRB Approval Process for Clinical Trials
Separate institutional review boards (IRB) at WRAIR and NMRC must approve clinical trials followed by a second IRB at the level of the Surgeon General (the Human Subjects Research Review Board (HSRRB) review, see Figure 5-2). These two levels are in addition to local IRB approval at the site of external trials (e.g., Mali, Kenya) and any necessary review by external funding agencies or partners (NIH, GSK, etc.).
The requirement for dual ethical and sometimes also scientific review at WRAIR/NMRC as well as HSRRB (in addition to partner and local IRBs) often adds complexity, cost, and time to a necessarily lengthy process. Facilitation of IRB review in a streamlined way with a short time line is needed.
Business and Intellectual Property Issues
The thrust to develop a vaccine that combines multiple antigens and constructs has led to many intellectual property challenges. Commercial interests are often reluctant for their proprietary constructs to be combined or compared with those of competitors. The committee was not shown current agreements between MIDRP Malaria Vaccine Program and commercial partners, and hence is unaware of the restrictions imposed. The procedures for initiating new partnerships seem convoluted, lengthy, and obscure, with separate systems in WRAIR and NMRC. They no doubt discourage new external partnerships from developing.
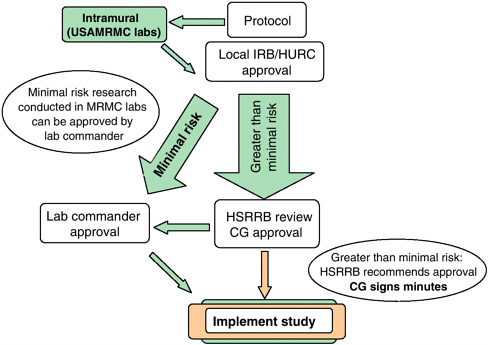
FIGURE 5-2 Process of USAMRMC approval to conduct human subjects research.
NOTE: Excludes additional local and partner IRBs.
SOURCE: Heppner, 2006; Richie, 2006; Vaughn, 2006.
Communication and Resource Sharing
The committee formed the impression that although individual investigators in the two groups (WRAIR and NMRC) communicate informally, there could be much greater interchange and sharing of knowledge, especially at higher management levels. This is despite the fact that there is convergence in the approaches and antigens on which the two groups are now focusing for first-generation vaccines. Both also have discovery efforts in place for second-generation vaccines as well as collaboration with (separate) partners for whole-parasite approaches.
Duplication of core facilities such as flow cytometry, genomics, and immunological assays is inefficient and creates a barrier to standardization of techniques and assays. Although some activities are shared, such as the GMP pilot production facility, opportunities for increased sharing of facilities were noted that should aid standardization and increase pace of progress.
PROGRAM REORGANIZATION
Military malaria vaccine research and development is currently conducted by the U.S. Army (WRAIR) and U.S. Navy (NMRC) in two essentially independent programs nominally coordinated by the MIDRP (USAMRMC) at Ft. Detrick, Maryland. The committee recognized the commitment and energy of the malaria vaccine research groups at WRAIR and NMRC, the strong leadership they have received, and their good intentions to cooperate with each other. Notably, the MIDRP Malaria Vaccine Program has been scientifically productive in spite of a diffuse and cumbersome management structure and inadequate funding that impairs the program’s ability to accomplish the malaria vaccine mission. Moreover it has a limited staff, “one-deep” in some critical areas and subject to assignment for clinical duties.
Although the competition between the separate programs at WRAIR and NMRC creates a healthy tension, the programs appear overextended at present. Supporting two independent vaccine development programs is not sustainable, nor is such an approach consistent with business and industry best practices. In industry, although there may be several candidate products in the pipeline, it is unusual to carry two separate products forward simultaneously beyond the early stages of product development, such as following preclinical studies or phase 1 clinical trials.
The committee recognizes the inherent complexity in the DoD’s centralized oversight for research and development and acquisition of military vaccines, which was extensively spelled out in a previous Institute of Medicine (IOM) report (IOM, 2002), but the committee considers the number of organizational units unnecessarily complex and seemingly incongruous. This situation is made even more difficult by the USAMRMC’s divisions of vaccine research and development (MIDRP), advanced development (U.S. Army Medical Materiel Development Activity [USAMMDA]), and contracting (U.S. Army Medical Research Acquisition Activity [USAMRAA]).
Now that the WRAIR and NMRC are colocated in the same building, there is little justification for the perpetuation of separate malaria vaccine efforts. In making recommendations for reorganization, the committee urges USAMRMC to focus on the goal (a malaria vaccine) and develop structures that will help achieve that goal, rather than a new structure for its own sake. Of course, to do nothing should not be an option.
The committee notes that the concept of reorganization for the malaria vaccine program and MIDRP could be viewed within the theme of U.S. military transformation. Transformation refers to the broad changes the U.S. military must make in its structure, culture, and doctrine to meet the emerging threats challenging our nation in this century. Placed in this con-
text, reorganization of the malaria vaccine program is an opportunity for the MIDRP/USAMRMC to think differently about enabling the program’s capability to address the current and future challenge of the malaria threat. Experimentation has been a tradition of the U.S. military’s approach to an ever-changing strategic environment. At the level of the MIDRP there is an opportunity to experiment with the malaria vaccine program as a pilot project that integrates the WRAIR and NMRC programs in a joint operation. Success in reorganizing the approach to malaria vaccine development could serve as an example for other infectious disease program areas.
Recommendation 5.1: The MIDRP Malaria Vaccine Program, currently composed of two separate entities—WRAIR and NMRC, should be integrated into a unified organizational entity (JTF-MV) that spans the spectrum and life cycle of responsibilities: epidemiological/threat assessment, research and development, advanced product development, clinical trials, licensure, manufacture, technology transfer, procurement, maintenance of manufacturing practice standards, and regulatory compliance.
Recommendation 5.2: The JTF-MV should appoint one scientific director, reporting to the commanding general of the USAMRMC, to provide joint direction and accountability for the program. The scientific director must have operational authority and budgetary as well as scientific control.
This recommendation does not in any way imply criticism of the current outstanding leadership at WRAIR and NMRC. The search committee for this position should be constituted by MIDRP with assistance from a malaria program transition team (see below in Recommendation 5.6). Recruitment for this high-level scientific and managerial position should consider outstanding individuals from both inside and outside the military. The scientific director must be physically located in the WRAIR/ NMRC building (as opposed to the U.S. Army Medical Research Institute for Infectious Diseases at Fort Detrick) in order to provide hands-on management.
Recommendation 5.3: The JTF-MV should organizationally incorporate an industry/business model and be constituted as a single legal entity (able to share proprietary data) that would simplify the external contracting process, including cooperative research and development agreements, interagency agreements, and other con-
tracts. The JTF-MV must include team members with specialized expertise in business and regulatory affairs. Although these individuals would be located in the existing business and regulatory affairs units, adequate staffing for these tasks must be assigned to the JTF-MV in order to avoid or minimize future intellectual property conflicts and other issues.
Recommendation 5.4: The JTF-MV program for vaccine development should have an external senior expert advisory group (scientific advisory board) that conducts yearly face-to-face meetings to provide external review and evaluation of the scientific program, and also gives ongoing advice in a timely manner. The scientific advisory board can assist the program to set clear and appropriate objectives (defined up front), with benchmarks of progress. Draft terms of reference for the scientific advisory board are found in Appendix E.
Recommendation 5.5: The annual proposal cycle should be replaced with a more programmatic and directed approach to project management under the newly reorganized JTF-MV. The MIDRP sets the annual budget and long-range objectives (with input from the scientific advisory board), and implementation is by the JTF-MV with a longer (approximately 3 year) time horizon for projects.
PREVIOUS REPORTS ADDRESSING DOD VACCINE DEVELOPMENT AND ACQUISITION
Three recent expert panels have considered the issue of vaccine development and production in the U.S. military. First, an independent committee of experts chaired by Franklin Top, MD, was convened to make recommendations on improving the DoD acquisition process for Biological Defense Program vaccines that could also include vaccines for the (endemic) Infectious Disease Program. Their report (the Top report) was previously mentioned in Chapter 1 in relation to cost estimates (Top et al., 2000). Subsequently, in 2002 an IOM committee tasked with assessing vaccine policies for naturally occurring infectious diseases produced a report Protecting Our Forces edited by Lemon et al. (IOM, 2002). Although the subsets of diseases emphasized by these two committees were different, both committees pointed out that the vaccines produced for both naturally occurring diseases and biowarfare agents would be used in the same way. The IOM report also noted that vaccines of both types (e.g., adeno-
virus types 4 and 7 and certain biowarfare agents) may have insufficient demand to be marketed to the general public. Therefore, the report recommended that vaccines for both types of diseases be considered jointly in the acquisition process. Although the Protecting Our Forces report was focused on the U.S. Army, their conclusions are highly pertinent to the overall MIDRP Malaria Vaccine Program (IOM, 2002).
The summary recommendations of these two committees are reproduced in Appendixes F and G. It is important to note that both committees recommended rather sweeping reorganization of vaccine research and development and acquisition processes within MIDRP and the DoD, none of which has yet been implemented. For example, the first recommendation of the Protecting Our Forces report was as follows (IOM, 2002):
Combine all DoD acquisition responsibilities under a single DoD authority that includes the entire spectrum of responsibility—from potential threat definition through research and development, advanced product development, clinical trials, licensure, manufacture, procurement, and continued maintenance of manufacturing standards and regulatory compliance.
Similarly, the Top report recommended “changes in DoD policy and organization, legislation, and statutory commitments” as well as to “combine programs from discovery to production” (Top et al., 2000). It was recommended that the DoD acquisition program for all vaccines (biowarfare agents and naturally occurring diseases) be managed as an acquisition category 1 program under a government-owned, contractor-operated vaccine production facility. The Top report generated cost estimates (see Chapter 1) and facility design plans for completing vaccine production for major threats in-house, suggesting that a facility with a 25-year life cycle and producing eight vaccines would require $3.2 billion and a staff of approximately 2,500 people.
A third report, Giving Full Measure to Countermeasures, dealing with biodefense vaccines also supported the Top report (IOM, 2004). The 2004 IOM report recommended giving vaccines and drugs very high visibility as a separate program in the DoD hierarchy at the assistant secretary level in order to compete for funds more effectively.
The Top report said that there should be “accountable, lean DoD management structure.” A similar recommendation was made by the IOM Protecting Our Forces report (IOM, 2002):
Ensure that there is an effective, ongoing senior advisory group—one providing perspectives from both within and outside of DoD—to assess program priorities and accomplishments, to act as a proponent for vaccines and other infectious disease countermeasures, and to maintain active relationships with current science and technology leaders in academic, government, and corporate sectors.
Although these recommendations referred to overall vaccine development, they are very applicable to the MIDRP Malaria Vaccine Program. They reinforce the current committee’s recommendations for major reorganization of the WRAIR/NMRC programs into a JTF-MV, for the formation of a scientific advisory board, and for the streamlining of the prioritization and research proposal approval process into a truly joint programmatic approach. Optimally, the JTF-MV would be a hybrid organization incorporating the best and most relevant features of both military and business organization.
Recommendation 5.6: A malaria program transition team (led by a program manager with a strong business/industry background and reporting to the commanding general of the USAMRMC) should be established to carry out the JTF-MV reorganization and constitution of the scientific advisory board and assist with recruitment of a highly qualified JTF-MV scientific director. This transition team will be disbanded once the reorganization is in place.
HUMAN RESOURCE COMMITMENTS
The numbers of full-time equivalent (FTE) staff dedicated to malaria vaccine research and development in the U.S. is currently 70 at WRAIR and 32 at NMRC. At WRAIR the great majority (60) of these FTEs are in the Department of Immunology. In addition there are 140 FTE staff (mostly support staff) at the United States Army Medical Research Unit in Kenya (USAMRU-K), 8.5 at the Armed Forces Research Institute of Medical Sciences in Thailand (AFRIMS), 8.8 at the Naval Medical Research Unit (NAMRU)-2 in Indonesia, 1 at NAMRU-3 (Egypt), and 3.2 at NMRC-D (Peru).
Figures 5-3 (WRAIR) and 5-4 (NMRC) show the breakdown by staff level (senior scientists, other scientists, technicians, and support staff) in each relevant department or overseas unit. Of the senior scientific staff, 63 percent were based in the United States at NMRC or WRAIR.
Table 5-1 indicates the salary source breakdown for staff in each institution (shown separately for the U.S. labs and overseas labs). Overall in the United States, 17 percent of staff were active-duty military, 13 percent government civilian employees, and 70 percent contract or other.
The above charts and table show that the current programs are dependent on very small numbers of individuals (fewer at NMRC than WRAIR). The committee noted with concern the lack of depth in the program. Experienced staff members are stretched very thin, and programs are affected if personnel are sent to or volunteer for active duty in conflict
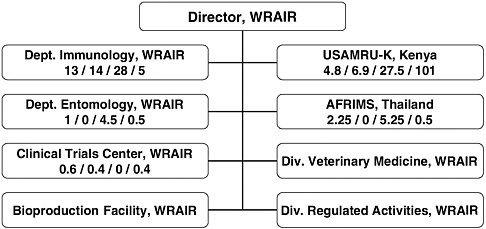
FIGURE 5-3 WRAIR organizational elements involved in the malaria vaccine research and development program and key personnel (expressed in full-time equivalent) dedicated to the malaria vaccine effort.
NOTE: Numbers in each box represent FTEs as follows: senior scientist / other scientist / technician / support staff. If no numbers are given, there are no staff dedicated to the malaria vaccine program.
SOURCE: Vaughn, 2006.
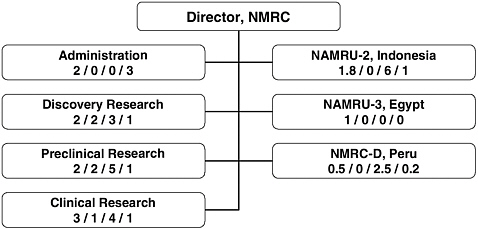
FIGURE 5-4 NMRC organizational elements involved in the malaria vaccine research and development program and key personnel (expressed in full-time equivalent) dedicated to the malaria vaccine effort.
NOTE: Numbers in each box represent FTEs as follows: senior scientist / other scientist / technician / support staff.
SOURCE: Vaughn, 2006.
TABLE 5-1 Numbers of FTE Staff in Different Categories in United States and Overseas Labs
|
|
Government, military |
Government, civilian |
Contract |
Other |
Total |
|
WRAIR labs |
10 |
11 |
45 |
2 |
68 |
|
NMRC labs |
7 |
2 |
23 |
0 |
32 |
|
Total United States labs |
17 |
13 |
68 |
2 |
100 |
|
Army overseas |
5 |
6 |
139 |
1 |
151 |
|
Navy overseas |
2 |
5 |
6 |
0 |
13 |
|
Total overseas |
7 |
11 |
144 |
1 |
164 |
situations. The long-term future of the malaria vaccine research and development program is compromised by the lack of perceived career enhancement opportunities. The ability to attract and retain military and civilian scientists requires appropriate promotion rewards and incentives.
Recommendation 5.7: A workforce plan must be developed and implemented by the JTF-MV. This plan should include training and budgeting for the next generation of scientists in the military program, ways to improve recruitment and retention of civilians and foreign nationals, and succession planning to ensure availability of required staff in 5–10 years time. The DoD should respond to the lack of sufficient depth of human resources to carry through current objectives with increased resources to carry out the workforce plan.
FINANCIAL COMMITMENT
The DoD’s internal funding for malaria vaccine research and development is channeled mainly through MIDRP, which oversees all infectious disease research. The MIDRP’s overall budget for all diseases was about $40 million in 2005, which is a significant decline from a peak of over $50 million in 1998 ($64 million in real [inflation-adjusted] terms). Although a slight rise to over $45 million is projected in the MIDRP’s overall research and development budget over the period 2006 to 2011, this represents a stable or declining trend in real terms.
A limited amount of additional funds are released through additional channels (USAMMDA) when a promising vaccine product enters the advanced development process. However, this represents only 10 percent or less of the MIDRP total.
The MIDRP intramural budget for malaria vaccine research rose from $5.5 million in 1994 to a peak of over $10 million in 2001 (Figure 5-5). This was approximately one-quarter of the total MIDRP budget at that time. After a steep decline in malaria vaccine funding, down to $7 million in 2003, the amount increased slightly in 2004 and 2005 to about $8.5 million. In 2004, the overall amount spent by the DoD on malaria vaccines from both internal and external sources was $22.9 million (Malaria R&D Alliance, 2005). The MIDRP malaria vaccines budget is projected to remain stable at this level through 2011 (as is the overall MIDRP budget), effectively declining in real terms to below the 1994 level (Figure 5-5).
For comparison of malaria vaccine spending with other malaria research and development, MIDRP spent a larger amount (almost $12 million in 2004) on antimalarial drug discovery and development and a smaller amount on vector control research (almost $2 million in 2004). Considering all aspects, malaria research requires about half of the MIDRP’s research budget.
It should be noted that these amounts do not include the salaries of military personnel; however this category of staff constitutes less than 20 percent of the total staff involved (see Table 5-1).
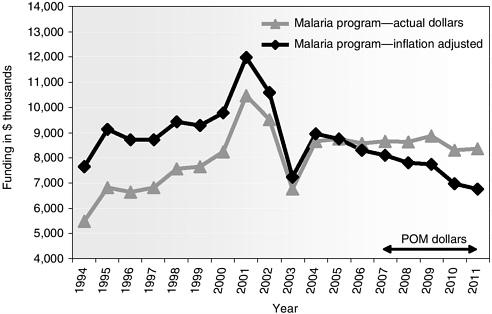
FIGURE 5-5 MIDRP funding for malaria vaccine research, 1994–2011 (projected).
NOTE: Baseline year is 2005, using Biomedical Research and Development Price Index.
SOURCE: Vaughn, 2006.
Because the DoD is a recipient of funds from other organizations, notably USAID, NIAID, and the Gates Foundation, WRAIR and NMRC independently manage funds that at least triple the intramural total.
Worldwide, it was estimated that $323 million was spent on malaria research and development (including drugs, vaccines, vector control, and diagnostics) in 2004 (Malaria R&D Alliance, 2005). About one-quarter of this total (approximately $80 million) was spent on malaria vaccine research and development. Thus with a contribution of over $9 million in 2004, DoD is a significant contributor to the world’s malaria vaccine research and development effort in financial as well as scientific terms (see above).
Notwithstanding the DoD’s major financial contribution, the amount spent by MIDRP on malaria vaccine research and development falls far short of the required amount to bring even one vaccine product to licensure, which was estimated above to cost upwards of $300 million at the very least, and probably much more (see Table 2-3).
It appeared to the committee that lack of funding was dictating suboptimal decision making—for example, the selection by NMRC of only two of the CSLAM antigens rather than the full set of five for the first clinical trials of the DNA-adenovirus prime-boost approach. The committee’s opinion was that limiting the vaccine in this way was not fully capitalizing on previous research and was reducing the chances of success. There are other technical challenges, such as purification and production of large quantities of GMP proteins for clinical trials and licensure, that can also be overcome given sufficient resources and access to facilities.
The Forest Glen GMP pilot production facility is crucial to the program as it overcomes the barrier of producing sufficient material for phase 1 and phase 2 trials of certain antigens. It enables the program to produce in-house many potential antigens and constructs for screening. Currently the facility produces recombinant proteins but could produce certain other potential types of candidate vaccines. The facility time available to the malaria program is currently limited by the need to support the facility by contracting out to other groups.
At present, it is not envisaged that the Forest Glen GMP pilot production facility would produce the material for pivotal phase 3 licensure track trials of the vaccine. Careful planning and costing for the transition from in-house production to a larger-scale facility for phase 3 trials is needed. Ideally, the large-scale manufacture facility that would prepare the phase 3 material would be the site of manufacture of the vaccine postlicensure. External collaboration with industry will almost certainly be required, but such must be carefully managed to enable the DoD to achieve its primary goals.
The overseas laboratories are important sites for planning and carrying out field trials, studying naturally acquired immunity, and training. The funding of the overseas laboratories under the new management structure must be carefully considered during the transition.
It is clear that if the MIDRP Malaria Vaccine Program is to produce a malaria vaccine, a large increase in funding will be necessary. Military needs for a vaccine are unique and specific, and will not be met by others. Although the program has done an impressive amount of work with the relatively small budget available, and has been able to leverage significant outside funding, the overall amount available is not sufficient for advanced development of even one candidate antigen.
Recommendation 5.8: Sufficient funding should be made available to support the infrastructure to produce pilot-lot formulations of MIDRP malaria vaccine candidates in-house at the pilot production plant at Forest Glen (an invaluable part of the MIDRP Malaria Vaccine Program). Although pilot lots of all candidate vaccines cannot be made at Forest Glen, the ability to prepare certain candidates removes a major obstacle that would otherwise impede the program.
Recommendation 5.9: A formal economic analysis would be helpful to clarify current costs of malaria (both P. falciparum and P. vivax) prevention, treatment, and case management. This economic analysis would reveal the direct (monetary) and indirect (lost work time) costs that would be averted by both a first-generation vaccine (to be used in conjunction with chemoprophylaxis) and a second-generation vaccine (to replace chemoprophylaxis).
Recommendation 5.10: Given that malaria remains a major problem for U.S. military personnel deployed to endemic areas and this threat is not diminishing in importance with time, the MIDRP program to develop a malaria vaccine compatible with the needs for protecting U.S. military personnel should be fully supported. To increase the likelihood of achieving the current goals for a first-generation vaccine and to test the limited number of vaccine candidates described above will almost certainly require a several-fold increase in the current malaria vaccine development budget by 2010, with continuation at that level to at least 2015.















