4
Exposure and Disposition
EXPOSURE
Introduction
Briefly this section reviews the uses of asbestos, how people may be exposed to it, the magnitude of exposure, and how it is measured. For detailed information, the reader is directed to the more comprehensive reviews conducted by the Agency for Toxic Substances and Disease Registry (ATSDR 2001), the International Agency for Research on Cancer (IARC 1977), and the International Program on Chemical Safety (IPCS 1986).
Asbestos has had a very wide array of uses. It has been used extensively for insulation and in textiles and has been mixed and bonded with cement, plastics, and resins. Production of asbestos products in the United States, Canada, and most other industrialized countries increased rapidly in the 20th century, particularly during World War II, and peaked in the early to mid 1970s. In 1973, the US Environmental Protection Agency (EPA) prohibited spraying of asbestos insulation, and further restrictions were later applied. Many other industrialized countries enacted similar regulations or outright bans, and the use and production of asbestos dropped rapidly.
Although many uses have been discontinued, asbestos is still used in some limited applications. In 2003, asbestos was not produced in the United States, although 6,000 metric tons were consumed—80% for specialized roofing products; 8% for gaskets; 4% for friction products, such as vehicles brake pads, clutches, and transmissions; and 8% for other uses (USGS 2004). Some European countries have banned all uses of asbestos. How-
ever, exposure occurs in all countries when buildings, ships, and other structures insulated with asbestos are demolished, when asbestos is removed, or during maintenance and repair of asbestos-containing materials.
Consideration needs to be given to the different measurement methods used when interpreting and comparing the reported levels of airborne exposure in various settings. Historically, airborne asbestos in workplaces was measured with a midget impinger to collect the fibers, a standard occupational hygiene method, and concentrations were expressed as millions of particles per cubic foot (mppcf). More recently, airborne fibers have been collected on membrane filters, and concentration has been reported in terms of either mass (such as nanograms per cubic meter, ng/m3) or number of fibers (such as fibers per milliliter, f/ml). The latter measure is most commonly used. In water, concentrations may be expressed in terms of fibers per liter. In a given measurement system, fibers may qualify for counting on the basis of criteria such as length (for instance, over 5 μm) or aspect (length:diameter) ratio (for instance, over 3:1), characteristics also relevant to their potential to cause health effects.
Fibers may be counted with either phase-contrast microscopy (PCM) or transmission electron microscopy (TEM). Of the two, TEM is the more sensitive and may measure higher concentrations in the same environment than PCM, because PCM may miss very thin fibers. In addition, PCM may fail to distinguish asbestos from other types of fibers. However, workplace exposures are generally measured with PCM, which is less expensive and considered adequate by regulatory agencies. Conversion between different measures of airborne units is problematic because conversion factors vary with the distribution of fiber thickness and length in the environment of interest. The most valid approach to conversion involves obtaining measurements simultaneously under the same conditions using the different methods for which conversion factors are needed. Using that approach, Dement et al. (1983) found factors for converting PCM to TPM data within the same facilities in the textile industry that ranged from 2.5 to 7.5 f/ml :: 1 mppcf.
Occupational Exposure
Asbestos concentrations observed in occupational settings have been orders of magnitude higher than the highest concentrations observed in residential settings, but some in-home activities, such as shaking out work clothes, can produce levels that may rival those found in the workplace. The highest well-documented exposures have been among workers manufacturing asbestos products or employed in mining and milling operations. Table 4.1 provides selected summary statistics for some asbestos-product manufacturing facilities in the United States based on samples
TABLE 4.1 Concentrations of Fibers in Various US Asbestos-Using Industries
|
Industry |
Plants |
Years |
Range of Means Within Departments (# fibers >5 μm)/ml, PCM |
|
Insulation plants |
5 plants |
1966-1971 |
0.01-74.4 |
|
Textile plants |
8 plants |
1964-1971 |
0.1-29.9 |
|
Friction products plants |
5 plants |
1968-1971 |
0.1-14.4 |
|
Paper, packing, asphalt products |
not reported |
1966-1970 |
0.2-13.6 |
|
Cement pipe |
7 plants |
1969-1970 |
0.2-6.3 |
|
Cement shingle, millboard, gasket |
3 plants |
1966-1970 |
0.1-4.4 |
|
SOURCE: NIOSH (1972). |
|||
collected in 1964-1971 (NIOSH 1972). The table indicates that the highest concentrations were observed in the textile and insulation industries but also that levels of exposure varied considerably between and within industries. Thus, exposure cannot be estimated with any certainty on the basis of descriptions of exposure situations.
The report by Dement et al. (1983) provides a useful example of variability of exposures within an industry. In a large US textile-manufacturing facility, exposures were highest in the 1930s and 1940s and generally, although not uniformly, decreased through the 1970s with the introduction of technologic changes. Concentrations varied among departments by as much as an order of magnitude. In the 1930s, mean concentrations in some areas of fiber preparation were up to 78 f/ml and other areas, such as spinning, had means below 10 f/ml. A similar range of exposures has been reported for the textile industry in Italy (Pira et al. 2005) and in England (Peto et al. 1985).
Concentrations in the mining and milling industries have been similiar to those in manufacturing. For example, in the mid 1960s, mean exposures of 20-100 f/ml were reported at the Wittenoom crocidolite mines and mills in Western Australia; they may have been higher in earlier decades (Armstrong et al. 1988, Reid et al. 2004). Exposures in a similar range have been reported in Quebec (Gibbs and LaChance 1974); Libby, Montana (Amandus et al. 1987); and South Africa (Sluis-Cremer et al. 1992).
Exposure levels of end-users of asbestos are less well documented, at least historically, but appear to be lower when considered as time-weighted averages. Insulation workers constitute a group with potentially high exposures. Although Selikoff et al. (1979) anecdotally reported exposures of 4-12 f/ml, the National Institute of Occupational Safety and Health (NIOSH 1972) reported individual exposures of 0 up to 100 f/ml when shorter-term
exposures were considered. Insulation workers engaged in ship building and repair, in which mean exposures may have been as high as 30 f/ml (NIOSH 1972), are of particular concern. Other end-users may have had lower exposures. For example, Corn et al. (1994) reported means during various maintenance activities of 0.008-0.061 f/ml, and other studies have observed similar, highly variable levels of exposure (ATSDR 2001). Mean levels of exposure during asbestos abatement have been measured at 0.006 to 0.76 f/ml (TEM) depending on the material being removed (ATSDR 2001). However, it is important to note that short-term exposures could be quite high.
Limits considered acceptable for occupational exposure have dropped over time. The American Conference of Governmental Industrial Hygienists first proposed a Threshold Limit Value (TLV) of 5 mppcf in 1946 (ACGIH 1998). However, historical sampling data show that many industries did not adhere to that guideline (for example, see Amandus et al. 1987, Dement et al. 1983, Hughes et al. 1987). With the wider recognition of the hazards of asbestos and the regulatory response, exposures rapidly decreased in the industrialized countries. Reductions in exposure due to improved technology have been well documented. An example from the US textile industry is presented in Table 4.2; Dement et al. (1983) converted older measures in mppcf to f/ml using paired samples collected at the same facilities. NIOSH (1972) documented decreasing exposure in many industries. Others noted further dramatic reductions of workplace concentrations in the early and mid 1970s in North America and Europe. For example, mean exposures in the Quebec mining industry declined from 16 f/ml in 1973 to less than 2 f/ml by the late 1970s (LeBel 1995).
TABLE 4.2 Estimated Mean Concentrations (# fibers over 5 mm per ml, PCM) in a Chrysotile Textile Plant (1930-1975)
|
Operation |
Before Controls |
After Controls |
|
Fiber preparation |
26.2-78.0 |
5.8-17.2 |
|
Carding |
10.8-22.1 |
4.3-9.0 |
|
Twisting |
24.6-36.0 |
5.4-7.9 |
|
Winding |
4.1-20.9 |
4.1-8.4 |
|
Spinning |
4.8-8.2 |
4.8-6.7 |
|
Weaving |
5.3-30.6 |
1.4-8.2 |
|
SOURCE: Dement et al. (1983). |
||
General Population Exposure
Earlier National Academies committees have considered non-occupational exposure to asbestos (NRC 1984, 1993).
Airborne asbestos fibers can be detected and measured in the general, ambient environment even far from industrial sources. The sources include disturbed natural deposits, improper disposal or transportation of asbestos-containing wastes, and uses that result in friable asbestos, such as motor-vehicle brake pads. Although exposure from undisturbed natural sources is possible, it has not been documented. Concentrations measured in outdoor air are highly variable, ranging from below the limit of detection (0.1 ng/ m3, and estimated to be equivalent to about 0.000003 f/ml as measured with PCM) in rural areas to over 100 ng/m3 (about 0.003 f/ml, PCM) near industrial sources. In rural areas, typical concentrations are about 0.00001 f/ml (PCM), while urban measurements are typically higher (up to about 0.0001 f/ml, PCM) (ATSDR 2001).
Higher concentrations have been documented in communities near asbestos-related industries. Table 4.3 presents concentrations measured near asbestos manufacturing facilities in Taiwan (Chang et al. 1999). Although concentration appears to drop off with distance, the decreases are not dra-
TABLE 4.3 Asbestos Concentrations in Ambient Air Around Taiwanese Factories
|
Factory Type |
No. of Factories |
Methoda |
GM (GSD) Asbestos Concentration (f/ml) Distance from Factoryb |
||
|
200 m |
400 m |
600 m |
|||
|
Cement |
5 |
TEM |
0.006 (1.230) |
0.007 (1.487) |
0.006 (1.301) |
|
|
|
PCM |
0.01 (3.49) |
0.01 (2.91) |
<0.01 |
|
Friction |
3 |
TEM |
0.008 (2.441) |
0.008 (1.978) |
0.002 (2.221) |
|
|
|
PCM |
0.01 (3.22) |
0.02 (2.88) |
<0.01 |
|
Textile |
2 |
TEM |
0.012 (2.221) |
0.020 (1.432) |
0.006 (1.765) |
|
|
|
PCM |
0.02 (3.21) |
0.02 (3.33) |
<0.01 |
|
Ground tile |
2 |
TEM |
0.033 (1.412) |
0.021 (1.421) |
0.025 (2.321) |
|
|
|
PCM |
0.4 (3.21) |
<0.01 |
0.01 (2.21) |
|
Insulation |
1 |
TEM |
0.012 (2.321) |
0.020 (2.210) |
0.006 (2.773) |
|
|
|
PCM |
<0.01 |
<0.01 |
<0.01 |
|
Refractory |
1 |
TEM |
<0.0001<0.0001 |
<0.0001 |
|
|
|
|
PCM |
<0.01 |
<0.01 |
<0.01 |
|
aTEM = transmission electron microscopy; PCM = phase contrast microscopy. bGM = geometric mean; GSD = geometric standard deviation. SOURCE: Chang et al. (1999). |
|||||
matic out to at least 600 m, indicating that the fibers remain airborne. Higher concentrations have been measured historically near asbestos-related open-pit mining and milling operations. For example, in towns near open-pit chrysotile mines in the Canadian province of Quebec, concentrations as high as 0.08 f/ml were measured in the early 1970s, although current concentrations are much lower (Case and Sebastien 1987, ICPS 1998). Residential exposure may also occur in communities with asbestos industries from fibers carried home on the clothing or hair of asbestos workers (Anderson et al. 1979, Case and Sebastien 1989).
Asbestos has been measured in the air inside many public and noncommercial buildings (HEI 1991). Sources of fibers released into the indoor air of non-industrial buildings include asbestos insulation, dry wall, ceiling and floor tiles, and materials used primarily for fireproofing. Exposures may occur by disturbance of asbestos-containing materials that are not well encapsulated (HEI 1991). Nicholson (1987) reported concentrations in buildings from about 0.00003 to 0.006 f/ml (PCM). In a survey of 94 public buildings, EPA (1988) reported concentrations ranging from below the limit of detection to about 0.003 f/ml (PCM), with a mean of 0.0004 f/ml (PCM).
Population exposure may also occur through the consumption of asbestos in drinking water. Asbestos may enter drinking water from erosion of natural deposits, mining operations, or asbestos-containing cement pipes (ATSDR 2001). Although most areas have concentrations less than 103 f/ml (PCM), much higher concentrations have been observed, some over 105 f/ml (EPA 1976, Kanarek et al. 1980, Sigurdson et al. 1981).
DOSIMETRY
Introduction
For inhaled contaminants, such as asbestos fibers, concepts of exposure and dose have been developed for the respiratory system. Asbestos fibers are particulate matter that is distinguished from other particles present in air by having a length substantially greater than their width. Aspect ratio is the term used for the ratio of length to width. The Occupational Safety and Health Administration defines a fiber as having a length of at least 5 mm and an aspect ratio of 3:1, whereas EPA defines a fiber as having an aspect ratio of over 5:1 (ATSDR 2003). Airborne particles are generally characterized by their aerodynamic diameter, which is determined in reference to the behavior of a sphere of unit density; the aerodynamic diameter corresponds to the size of a unit-density sphere with the same aerodynamic characteristics as the particle of interest.
Much has been learned about particle size and the handling of particles by the respiratory system from experimental findings and the use of phys-
ical models of the lung. The mechanisms responsible for particle and fiber deposition are impaction, sedimentation, interception, and diffusion (Asgharian and Yu 1988). Aerodynamic diameter is a key determinant of the likelihood of deposition in the respiratory tract and the site of deposition (see Figure 4.1). Particles greater than 10 μm in aerodynamic diameter are generally captured in the upper respiratory tract, the nose and upper airway, whereas smaller particles can penetrate more deeply and reach the airways and alveoli of the lungs. Particles smaller than about 2.5 μm have a greater likelihood of reaching the alveoli and depositing in this region. Ultrafine particles (less than 0.1 μm) also deposit heavily in the nose. These same considerations apply to inhaled fibers, which can have a range of aerodynamic diameters, depending on size and physical characteristics.
In considering the potential risk posed by inhaled pollutants, including fibers, the critical determinant of injury is the amount of material that reaches the target site—a measure generally referred to as the biologically effective dose. As depicted in Figure 4.2, dose, without qualification, gener-
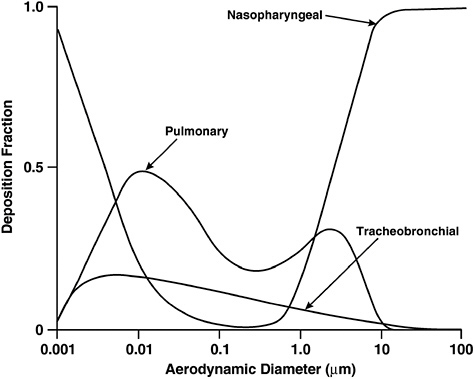
FIGURE 4.1 Effect of aerodynamic diameter on deposition of particles in the respiratory tract.
SOURCE: ICRP (1994).
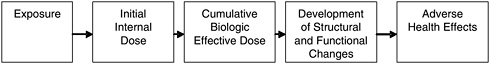
FIGURE 4.2 Exposure and dose-response paradigm in toxicology.
SOURCE: Modified from Lippmann (1992).
ally refers to the amount of material that enters the body; exposure refers to the amount of contact with material, with units expressed as concentration multiplied by time. For the respiratory system, models have been developed that relate dose to exposure for inhaled particles (Jarabek et al. 2005); the models are useful in characterizing the chain that begins with the source of an inhaled pollutant and terminates with injury to target tissues.
Various processes remove particles that are deposited in the lung in ways that depend on their size, physicochemical characteristics, and site of deposition (Table 4.4). Particles that reach the upper airways will generally be removed as mucus is swept toward the nostrils or into the pharynx for passage through the esophagus and the gastrointestinal tract. Particles reaching the bronchi are cleared by the mucociliary apparatus, which moves mucus toward the trachea, where it exits and is swallowed. Particles that reach the smaller airways are gradually scavenged by the lung’s macrophages; their fate depends on their toxicity to the macrophages. Particles may also penetrate the respiratory epithelium and remain in the airways or migrate to bronchopulmonary lymph nodes. Experimental studies show that particles in the ultrafine fraction (less than 0.1 μm in aerodynamic diameter) may be moved across the barriers posed by the respiratory epithelium and the alveolar-capillary membrane and be disseminated systemically (Oberdörster et al. 1983).
TABLE 4.4 Mechanisms of Fiber Clearance from the Lungs
|
Physiological clearance processes of deposited fibers |
|
|
Physicochemical processes reducing fiber burden |
|
|
SOURCE: Modified from Bernstein et al. (2005). |
Fiber dimensions are thought to be important in the pathogenesis of asbestos-related lung diseases (reviewed in Bernstein et al. 2005). Long asbestos fibers are deposited by interception primarily at sites of bifurcations in the conducting airways of the lower respiratory tract (Asgharian and Yu 1988). It is postulated that long asbestos fibers (greater than the diameter of alveolar macrophages about 10-15 μm in rodents and about 14-21 mm in humans) are less likely to be completely phagocytized and are cleared more slowly (reviewed in Bernstein et al. 2005). Fibers that are not removed rapidly by the mucociliary escalator may penetrate into the interstitium of the alveolar walls, be cleared by lymphatic channels, or migrate to the pleura and other extra pulmonary sites. Fibers that are not effectively cleared from the lung may be removed by physicochemical processes, including leaching of ions, dissolution, and breakage (see Chapter 3). Those processes could occur extracellularly in the lung-lining fluid or intracellularly in the phagolysosomal compartment of alveolar and interstitial macrophages. In general, fibers that are long and persistent in the lungs have been shown to be associated with fiber-induced lung disease in animal models (Hesterberg et al. 1996, 1998). The physicochemical properties of asbestos fibers described in Chapter 3 influence the susceptibility of different fiber types to leaching, dissolution, and breakage in the extracellular compartment at neutral pH or in the phagolysosome at acidic pH. In general, amphibole asbestos fibers are more persistent than chrysotile asbestos fibers.
In contrast with studies of fiber deposition in the lower respiratory tract, little is known about fiber deposition and clearance from the upper respiratory tract, particularly the larynx. A recent study by Zhou and Cheng (2005) modeled deposition of carbon fibers with laryngeal casts and predicted that a fraction of inhaled fibers would be deposited in the larynx, especially at the higher ventilation rates associated with moderately heavy work. Gemci et al. (2001) modeled airflow in the larynx by using drug sprays and predicted turbulent flow at the laryngeal constriction. As is seen in the lower airways of cigarette smokers, tobacco-smoking and other causes of chronic laryngeal irritation might impair clearance of fibers from the laryngeal mucosal surfaces.
Considerations for Inhaled Asbestos Fibers
Extension of the general models and definitions to inhaled asbestos fibers provides a framework for considering exposure and the dosimetry of asbestos fibers in the respiratory tract. The exposure measures used in the epidemiologic studies can be considered in the context set by this framework. In the committee’s judgment, the most relevant dose measure for cancer is probably the cumulative number of fibers that reach and persist in the target organ, and the biologically effective dose would be related to
fibers that interact with target cells. Such measurements are not available, but the epidemiologic studies have not incorporated any attempt to estimate dose, for example, by considering the size distribution of the fibers or the activity of the workers. Instead, a variety of indexes of exposure have been used, ranging from crude indicators of potential for contact to more refined, semi-quantitative measures.
Dose refers to the amount of material potentially available for deposition in the respiratory tract—in this instance, the number of fibers in the air inhaled by the exposed person. Only some fraction of that dose is deposited, and much will be exhaled or cleared. However, studies of lung tissues of asbestos-exposed people show that fibers are retained in the lung and that long, thick fibers are coated with iron, protein, and mucopolysaccharides to form asbestos bodies (ABs) visible with light microscopy (Roggli 2004). ABs have also been documented in other tissues, although their presence may reflect contamination occurring during handling and processing of specimens.
For nonrespiratory organs, concepts of dose are not well developed and related experimental and observational data are limited. For the organs of the gastrointestinal tract (esophagus, stomach, and intestines), fibers cleared from the respiratory tract will move through, with the potential for interaction with target cells in the epithelium. For other abdominal organs (including the liver, pancreas, and kidneys), the routes of movement are uncertain, although fibers have been found in those organs on occasion (Borow et al. 1973, Pooley 1974).
Translocation to the Pleura
After inhalation, asbestos fibers that deposit in the alveolar region of the respiratory tract may be cleared or retained (Figure 4.3). Fibers that persist in the alveolar region may be directly toxic to alveolar epithelial cells or incite a chronic inflammatory response that perpetuates tissue injury followed by episodes of epithelial cell proliferation and fibrosis (Oberdörster 1996). It has been proposed that fibers that penetrate the alveolar lining and enter the interstitium may move to lymphatics and regional lymph nodes. Asbestos fibers that accumulate in subpleural lymphatics may produce diffuse visceral pleural fibrosis and pleural effusions, directly and indirectly (Churg 1988). Asbestos-induced pleural effusions are hypothesized to be caused by cytokines (such as interleukin-8) released from mesothelial cells that trigger inflammation and fluid accumulation in the pleural space (Boylan et al. 1992). Inhaled particles could reach the pleural space after direct transpleural penetration, lymphatic translocation, or indirect transport by the blood (Holt 1981, Lee et al. 1981, Oberdörster et al. 1983). Recent studies in hamsters of inhaled refractory ceramic fibers (mean
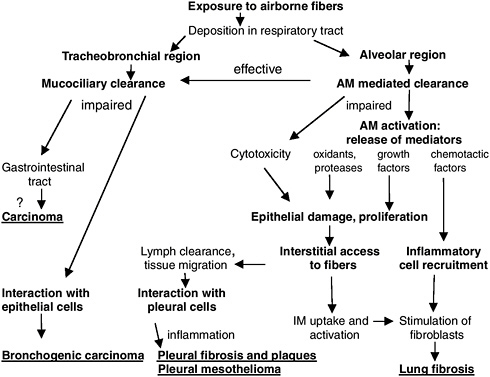
FIGURE 4.3 Translocation of inhaled asbestos fibers and adverse health effects. AM = alveolar macrophage, IM = interstitial macrophage.
SOURCE: Modified from Oberdörster (1996).
length, 20 μm; mean diameter, 1 μm) confirmed rapid translocation of short fibers to the pleural space, followed by inflammation and mesothelial cell proliferation (Gelzleichter et al. 1996a,b).
Few investigators have quantified the mineral-fiber burden in human pleura, but several investigators have found asbestos fibers in the pleural lining of people exposed to asbestos fibers (Dodson et al. 1990, Gibbs et al. 1991, Sebastien et al. 1977, Suzuki and Yuen 2001). Boutin et al. (1996) obtained samples of parietal pleura and lung from workers exposed to asbestos and counted asbestos fibers in dried tissue samples. Anthracotic particles, as well as asbestos fibers, were detected near lymphatic openings on the surface of the parietal pleura. Scanning electron microscopy of these “black spots” revealed focal accumulation of inflammatory cells. The investigators directly visualized black spots with video-assisted fiber-optic thoracoscopy; previous investigators had not been able to identify asbestos fibers in pleural samples, because they sampled tissues at random (Boutin et al. 1996). Identification of particles and fibers at sites of lymphatic drainage on the parietal pleura suggests that local transpleural or lymphatic translo-
cation is likely. Boutin and Rey (1993) have also diagnosed early cases of malignant mesothelioma arising on the parietal pleura; they speculated that focal trapping of long asbestos fibers at sites of lymphatic drainage from the pleura may provoke the development of mesothelioma. The potential relevance of fiber dimensions in induction of cancer at the sites under consideration is unknown.
Extrapulmonary Translocation
It has long been postulated that systemic dissemination of fibers occurs after retrograde transport via lymphatics and the bloodstream (Holt 1981, Lee et al. 1981, Taskinen et al. 1973). In addition, particle movement from the lungs to tracheobronchial lymph nodes by pulmonary alveolar macrophages has been documented in a canine model (Harmsen et al. 1985). Asbestos fibers that are cleared from the lower respiratory tract by the mucociliary escalator can be swallowed from the pharynx and gain access to the upper and lower gastrointestinal tract. It has also been proposed that fibers could penetrate the gastrointestinal mucosa, submucosa, and muscle layers and reach the peritoneal cavity (Selikoff et al. 1979).
Cunningham and Pontefract (1973) analyzed samples of drinking water and various beverages with electron microscopy and detected chrysotile asbestos fibers. To determine whether ingested asbestos fibers could disseminate systemically, they instilled a preparation of short chrysotile asbestos (0.5-2 μm long) in the stomach of rats and sacrificed them after 2-4 days. Using precautions to prevent contamination during necropsy, they sampled blood from the retroorbital plexus, spleen, omentum, heart, brain, and lungs and detected a substantial number of asbestos fibers; the highest concentration was in the omentum (Cunningham and Pontefract 1973). More recent chronic feeding studies conducted by the National Toxicology Program (HHS 1983, 1985, 1988, 1990a, 1990b, 1990c) did not identify any asbestos bodies using light microscopy at the following sites: larynx, lymph nodes, esophagus, stomach, small intestine, colon, cecum, and mesentery (McConnell 2005).
Lee et al. (1981) conducted a subchronic inhalation study with inorganic titanate fibers in hamsters, guinea pigs, and rats and reported fibers and multinucleated giant cells widely disseminated throughout the body, including mediastinal lymph nodes, adipose tissue, spleen, liver, Peyer’s patches in the intestine, and the mucosal, submucosal, and muscular layers of the gastrointestinal tract. Tissue responses were minimal except for mild inflammation and fibrosis in the epicardium.
Cook and Olson’s (1979) demonstration of asbestos in human urine, which abated when drinking water was filtered, provides substantial evidence that fibers enter into systemic circulation.
Case reports based on human autopsies have noted the presence of asbestos fibers or ABs in abdominal organs, including the spleen, abdominal lymph nodes, liver, omentum, and mesentery (Borow et al. 1973, Dodson et al. 2000, Pooley 1974, Suzuki and Yuen 2001). ABs have also been recovered from sites under consideration by this committee: larynx, esophagus, stomach, and small and large intestines (Roggli 2004). Several caveats must be considered, however, in evaluating reports of asbestos fiber translocation to extrapulmonary sites. A taskforce of the European Respiratory Society (De Vuyst et al. 1998) prepared guidelines for mineral-fiber analyses in biologic samples focusing on the tissues involved in asbestosis, lung cancer, and mesothelioma, but the principles are in large part applicable to techniques that would be used to determine fiber presence in the selected sites considered in this review. First, electron microscopy (EM) is necessary to detect most asbestos fibers, with transmission EM being preferable to scanning EM, but light microscopy is adequate for visualizing ABs; limits of detection should be reported. Second, asbestos fibers and ABs can be identified in lung tissue in most people in the general population, even people who have with no occupational history of asbestos exposure and in the absence of clinical or pathologic evidence of lung disease, meaning that comparison with appropriate controls is essential (De Vuyst et al. 1998). Third, cross-contamination of tissue samples with fibers or particles from surgical gloves, specimen containers, fixatives, scalpel blades, and other organs collected in the surgical or autopsy suite is a serious concern (Cook 1983, De Vuyst et al. 1998; Roggli 2004). Those caveats should be considered in evaluation of the published papers.
In two of five cases, Roggli et al. (1980) found ABs in laryngeal tissues gathered at autopsy from asbestos workers with known pulmonary disease. ABs were not recovered, however, from laryngeal tissue from ten autopsies controls.
Auerbach et al. (1980) used light microscopy to detect ABs in paraffin-embedded tissue samples obtained at autopsy from the lungs and kidneys of a series of 37 cases recorded as having asbestosis, mesothelioma, or pleural plaques. ABs were observed in the lung sections of all but one of these cases and in 38% of the kidney samples. Other organs were available from a subset of these cases. Of the organs for which at least 30 samples were available (heart, liver, spleen, adrenal glands, and pancreas), ABs were detected in 32-62% of cases. Of organs with fewer samples available, stomach, duodenum, and colon were among those in which ABs were found; the others with positive findings were brain, prostate, thyroid, mediastinal lymph nodes, bone, omentum, and spinal cord. The prevalence of ABs in other organs reflected the number of asbestos fibers found in a case’s lung sample.
Kobayashi et al. (1987) performed a similar analysis on formalin-fixed
tissues from 26 subjects. Esophagus, stomach, and both large and small intestine were among the 13 extrapulmonary organs from which tissues were examined. ABs were detected in some samples from all these organs, and the pattern corresponded with the degree of a given subject’s pulmonary burden. The number observed was high for the esophagus. Roggli (2004) noted that the reported results would be consistent with contamination by pulmonary ABs via formalin.
Kambic et al. (1989) examined a cohort of 195 asbestos-cement factory workers and controls in Yugoslavia. Chronic laryngitis was found more frequently in workers than in controls; in 10 workers, biopsies showed changes consistent with hyperplastic chronic laryngitis. Chronic laryngitis was diagnosed clinically in asbestos workers who were nonsmokers or former smokers, as well as in current smokers. Four of the biopsies were examined with scanning EM, and asbestos fibers were observed on the epithelial lining in three.
Ehrlich et al. (1991) determined colonic asbestos burdens with light and electron microscopy in 44 asbestos workers with colon cancer. Chrysotile fibers, amosite fibers, or ABs were identified in the colonic wall in 32% of workers. In contrast, no asbestos fibers or ABs were found in 20 patients who had colon cancer but no history of asbestos exposure.
In addition to technical concerns about reported findings of fibers or ABs in extrapulmonary tissues, there are uncertainties that should be borne in mind when interpreting their biologic meaning. The fact that fibers found in tissues are predominantly amphibole, even when the individual’s exposure is documented to have been exclusively to chrysotile with a slight tremolite component, is fully in accord with what is understood about the relative biopersistence of those fiber types (Churg 1994). In the upper respiratory or gastrointestinal tract, are epithelial injury and chronic inflammation (for example, secondary to tobacco-smoking, alcohol use, or persistent bacterial or viral infections) a prerequisite of accumulation of fibers and development of cancer at these sites? Finally, the finding of asbestos fibers or ABs in tissue samples obtained from gastrointestinal tract tumors raises the question of the causal significance of this observation: do the fibers accumulate secondarily at sites of mucosal damage or ulceration associated with a growing tumor?
Few studies have systematically sought evidence of asbestos fibers in the particular extrapulmonary sites of interest in this review, and such investigations are subject to technical difficulties. Nonetheless, there is some documentation that asbestos fibers may disseminate and persist at these selected tissue sites, although not with the regularity that has been established for the lungs, pleura, and lymph nodes.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1998. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th edition. Cincinnati, OH.
Amandus HE, Wheeler R, Jankovic J, Tucker J. 1987. The morbidity and mortality of vermiculite miners and millers exposed to tremolite-actinolite: Part I. Exposure estimates. American Journal of Industrial Medicine 11(1): 1-14.
Anderson HA, Lilis R, Daum SM, Selikoff IJ. 1979. Asbestosis among household contacts of asbestos factory workers. Annals of the New York Academy of Sciences 330: 387-399.
Armstrong BK, de Klerk NH, Musk AW, Hobbs MS. 1988. Mortality in miners and millers of crocidolite in Western Australia. British Journal of Industrial Medicine 45(1): 5-13.
Asgharian B, Yu CP. 1988. Deposition of inhaled fibrous particles in the human lung. Journal of Aerosol Medicine 1: 37-50.
ATSDR (Agency for Toxic Substances and Disease Registry). 2001. Toxicological Profile for Asbestos. Atlanta, GA: US Department of Health and Human Services.
ATSDR. 2003. Case Studies in Environmental Medicine. Atlanta, GA: US Department of Health and Human Services.
Auerbach O, Conston AS, Garfinkel L, Parks VR, Kaslow HD, Hammond EC. 1980. Presence of asbestos bodies in organs other than the lung. Chest 77(2): 133-137.
Bernstein D, Castranova V, Donaldson K, Fubini B, Hadley J, Hesterberg T, Kane A, Lai D, McConnell EE, Muhle H, Oberdörster G, Olin S, Warheit DB. 2005. Testing of fibrous particles: Short-term assays and strategies. Inhalation Toxicology 17(10): 497-537.
Borow M, Conston A, Livornese L, Schalet N. 1973. Mesothelioma following exposure to asbestos: A review of 72 cases. Chest 64(5): 641-646.
Boutin C, Rey F. 1993. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients: Part 1. Diagnosis. Cancer 72(2): 389-393.
Boutin C, Dumortier P, Rey F, Viallat JR, De Vuyst P. 1996. Black spots concentrate oncogenic asbestos fibers in the parietal pleura: Thoracoscopic and mineralogic study. American Journal of Respiratory and Critical Care Medicine 153(1): 444-449.
Boylan AM, Ruegg C, Kim KJ, Hebert CA, Hoeffel JM, Pytela R, Sheppard D, Goldstein IM, Broaddus VC. 1992. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenesis of asbestos-induced pleurisy in rabbits. Journal of Clinical Investigation 89(4): 1257-1267.
Case BW, Sebastien P. 1987. Environmental and occupational exposures to chrysotile asbestos: A comparative microanalytic study. Archives of Environmental Health 42(4): 185-191.
Case BW, Sebastien P. 1989. Fibre levels in lung and correlation with air samples. IARC Scientific Publications 90: 207-218.
Chang HY, Chen CR, Wang JD. 1999. Risk assessment of lung cancer and mesothelioma in people living near asbestos-related factories in Taiwan. Archives of Environmental Health 54(3): 194-201.
Churg A. 1988. Chrysotile, tremolite, and malignant mesothelioma in man. Chest 93(3): 621-628.
Churg A. 1994. Deposition and clearance of chrysotile asbestos. Annals of Occupational Hygiene 38(4): 625-633.
Cook PM. 1983. Review of published studies on gut penetration by ingested asbestos fibers. Environmental Health Perspectives 53: 121-130.
Cook PM, Olson GF. 1979. Ingested mineral fibers: Elimination in human urine. Science 204(4389): 195-198.
Corn M, McArthur B, Dellarco M. 1994. Asbestos exposure of building maintenance personnel. Applied Occupational Environmental Hygiene 9(11): 845-852.
Cunningham HM, Pontefract RD. 1973. Asbestos fibers in beverages, drinking water, and tissues: Their passage through the intestinal wall and movement through the body. Journal—Association of Official Analytical Chemists 56(4): 976-986.
De Vuyst P, Karjalainen A, Dumortier P, Pairon J, Monso E, Brochard P, Teschler H, Tossavainen A, Gibbs A. 1998. Guidelines for mineral fibre analyses in biological samples: Report of the ERS Working Group. European Respiratory Journal 11(6): 1416-1426.
Dement JM, Harris RL Jr, Symons MJ, Shy CM. 1983. Exposures and mortality among chrysotile asbestos workers: Part I. Exposure estimates. American Journal of Industrial Medicine 4(3): 399-419.
Dodson RF, Williams MG Jr, Corn CJ, Brollo A, Bianchi C. 1990. Asbestos content of lung tissue, lymph nodes, and pleural plaques from former shipyard workers. American Review of Respiratory Diseases 142(4): 843-847.
Dodson R, O’Sullivan M, Huang J, Holiday D, Hammar S. 2000. Asbestos in extrapulmonary sites: Omentum and mesentery. Chest 117(2): 486-493.
Ehrlich A, Gordon RE, Dikman SH. 1991. Carcinoma of the colon in asbestos-exposed workers: Analysis of asbestos content in colon tissue. American Journal of Industrial Medicine 19(5): 629-636.
EPA (US Environmental Protection Agency). 1976. Asbestos Fibers in Natural Runoff and Discharges from Sources Manufacturing Asbestos Products: Part II. Non-Point Sources and Point Sources Manufacturing Asbestos Products. Washington, DC: Office of Toxic Substances.
EPA. 1988. EPA Study of Asbestos-Containing Materials in Public Buildings: A Report to Congress. Washington, DC.
Gelzleichter TR, Bermudez E, Mangum JB, Wong BA, Everitt JI, Moss OR. 1996a. Pulmonary and pleural responses in Fischer 344 rats following short-term inhalation of a synthetic vitreous fiber: I. Quantitation of lung and pleural fiber burdens. Fundamental and Applied Toxicology 30(1): 31-38.
Gelzleichter TR, Bermudez E, Mangum JB, Wong BA, Moss OR, Everitt JI. 1996b. Pulmonary and pleural responses in Fischer 344 rats following short-term inhalation of a synthetic vitreous fiber: II. Pathobiologic responses. Fundamental and Applied Toxicology 30(1): 39-46.
Gemci T, Corcoran TE, Yakut K, Shortall B, Chigier N. 2001. Spray dynamics and deposition of inhaled medications in the throat. Institute of Liquid Atomization and Spray Systems Europe: Oral Presentation.
Gibbs AR, Stephens M, Griffiths DM, Blight BJ, Pooley FD. 1991. Fibre distribution in the lungs and pleura of subjects with asbestos related diffuse pleural fibrosis. British Journal of Industrial Medicine 48(11): 762-770.
Gibbs G, LaChance M. 1974. Dust-fiber relationships in the Quebec chrysotile industry. Archives of Environmental Health 28(2): 69-71.
Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. 1985. The role of macrophages in particle translocation from lungs to lymph nodes. Science 230(4731): 1277-1280.
HEI (Health Effects Institute). 1991. Asbestos in Public and Commercial Buildings: A Literature Review and Synthesis of Current Knowledge: Report of the Asbestos Literature Review Panel. Cambridge, MA.
Hesterberg TW, Miiller WC, Musselman RP, Kamstrup O, Hamilton RD, Thevenaz P. 1996. Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Fundamental and Applied Toxicology 29(2): 269-279.
Hesterberg TW, Chase G, Axten C, Miller WC, Musselman RP, Kamstrup O, Hadley J, Morscheidt C, Bernstein DM, Thevenaz P. 1998. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicology and Applied Pharmacology 151(2): 262-275.
HHS (US Department of Health and Human Services). 1983. Lifetime Carcinogenesis Studies of Amosite Asbestos (CAS NO. 12172-73-5) in Syrian Golden Hamsters (Feed Studies). NTP TR 249. Research Triangle Park, NC: National Toxicology Program.
HHS. 1985. Toxicology and Carcinogenesis Studies of Chrysotile Asbestos (CAS No. 12001-29-5) in F344/N Rats (Feed Studies). NTP TR 295. Research Triangle Park, NC: Na-tional Toxicology Program.
HHS. 1988. Toxicology and Carcinogenesis Studies of Crocidolite Asbestos (CAS No. 12001-28-4) in F344/N Rats (Feed Studies). NTP TR 280. Research Triangle Park, NC: National Toxicology Program.
HHS. 1990a. Lifetime Carcinogenesis Studies of Chrysotile Asbestos (CAS No. 12001-29-5) in Syrian Golden Hamsters (Feed Studies). NTP TR 246. Research Triangle Park, North Carolina: National Toxicology Program.
HHS. 1990b. Toxicology and Carcinogenesis Studies of Amosite Asbestos (CAS No. 12172-73-5) in F344/N Rats (Feed Studies). NTP TR 279. Research Triangle Park, NC: National Toxicology Program.
HHS. 1990c. Toxicology and Carcinogenesis Studies of Tremolite (CAS No. 14567-73-8) in F344/N Rats (Feed Studies). NTP TR 277. Research Triangle Park, NC: National Toxicology Program.
Holt PF. 1981. Transport of inhaled dust to extrapulmonary sites. Journal of Pathology 133(2): 123-129.
Hughes JM, Weill H, Hammad YY. 1987. Mortality of workers employed in two asbestos cement manufacturing plants. British Journal of Industrial Medicine 44(3): 161-174.
IARC (International Agency for Research on Cancer). 1977. Asbestos. IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Man 14: 1-106. Lyon, France: World Health Organization.
ICRP (International Commission on Radiological Protection). 1994. Human Respiratory Tract Model for Radiolgical Protection. Oxford: Elsevier.
IPCS (International Programme on Chemical Safety). 1986. Asbestos and Other Natural Mineral Fibers: Environmental Health Criteria 53. Geneva: World Health Organization.
IPCS. 1998. Chrysotile Asbestos: Environmental Health Criteria 203. Geneva: World Health Organization.
Jarabek AM, Asgharian B, Miller FJ. 2005. Dosimetric adjustments for interspecies extrapolation of inhaled poorly soluble particles (PSP). Inhalation Toxicology 17: 317-334.
Kambic V, Radsel Z, Gale N. 1989. Alterations in the laryngeal mucosa after exposure to asbestos. British Journal of Industrial Medicine 46(10): 717-723.
Kanarek MS, Conforti PM, Jackson LA, Cooper RC, Murchio JC. 1980. Asbestos in drinking water and cancer incidence in the San Francisco Bay area. American Journal of Epidemiology 112(1): 54-72.
Kobayashi H, Ming ZW, Watanabe H, Ohnishi Y. 1987. A quantitative study on the distribution of asbestos bodies in extrapulmonary organs. ACTA Pathologica Japonica 37(3): 375-383.
LeBel J. 1995. Review of Fibre Concentrations in Asbestos Mines and Quebec Asbestos Mining Towns. Sherbrooke: Quebec Asbestos Mining Association.
Lee KP, Barras CE, Griffith FD, Waritz RS. 1981. Pulmonary response and transmigration of inorganic fibers by inhalation exposure. American Journal of Pathology 102(3): 314-323.
Lippmann M. 1992. Environmental Toxicants: Human Exposures and Their Health Effects. 1st edition. New York: Wiley.
McConnell EE. 2005 (October 27). Personal Communication to Mary Paxton for the Committee on Asbestos: Selected Health Effects. Available in IOM Public Access Files.
Nicholson WJ. 1987. Airborne levels of mineral fibers in the non-occupational environment. IARC Scientific Publications 90: 239-261.
NIOSH (National Institute for Occupational Safety and Health). 1972. Criteria for a Recommended Standard: Occupational Exposure to Asbestos. Washington, DC.
NRC (National Research Council). 1984. Asbestiform Fibers: Non-Occupational Health Risks. Washington, DC: National Academy Press.
NRC. 1993. Solid-Earth Sciences and Society. Washington, DC: National Academy Press.
Oberdörster G. 1996. Evaluation and use of animal models to assess mechanisms of fibre carcinogenicity. IARC Scientific Publications 140: 107-125.
Oberdörster G, Ferin J, Marcello NL, Meinhold SH. 1983. Effects of intrabronchially instilled amosite on lavagable lung and pleural cells. Environmental Health Perspectives 51: 41-48.
Peto J, Doll R, Hermon C, Binns W, Clayton R, Goffe T. 1985. Relationship of mortality to measures of environmental asbestos pollution in an asbestos textile factory. Annals of Occupational Hygiene 29(3): 305-355.
Pira E, Pelucchi C, Buffoni L, Palmas A, Turbiglio M, Negri E, Piolatto PG, La Vecchia C. 2005. Cancer mortality in a cohort of asbestos textile workers. British Journal of Cancer 92(3): 580-586.
Pooley F. 1974. Locating fibers in the bowel wall. Environmental Health Perspectives 9: 235.
Reid A, Ambrosini G, de Klerk N, Fritschi L, Musk B. 2004. Aerodigestive and gastrointestinal tract cancers and exposure to crocidolite (blue asbestos): Incidence and mortality among former crocidolite workers. International Journal of Cancer 111(5): 757-761.
Roggli V. 2004. Asbestos bodies and nonasbestos ferruginous bodies. In: Roggli V, Oury T, Sporn T, eds. Pathology of Asbestos-Associated Diseases. 2nd edition. New York: Springer. Pp. 34-71.
Roggli VL, Greenberg SD, McLarty JL, Hurst GA, Spivey CG, Heiger LR. 1980. Asbestos body content of the larnyx in asbestos workers: A study of five cases. Archives of Otolaryngology 106(9): 533-535.
Sebastien P, Fondimare A, Bignon J, Monchaux G, Desbordes J, Bonnaudi G. 1977. Topographic distribution of asbestos fibers in human lung in relation to occupational and nonoccupational exposure. In: Walton WH, McGovern B, eds. Inhaled Particles IV. Oxford: Pergamon Press. Pp. 435-444.
Selikoff IJ, Hammond EC, Seidman H. 1979. Mortality experience of insulation workers in the United States and Canada, 1943-1976. Annals of the New York Academy of Sciences 330: 91-116.
Sigurdson EE, Levy BS, Mandel J. 1981. Cancer morbidity investigations: Lessons from the Duluth study of possible effects of asbestos in drinking water. Environmental Research 25(1): 50-61.
Sluis-Cremer GK, Liddell FD, Logan WP, Bezuidenhout BN. 1992. The mortality of amphibole miners in South Africa, 1946-80. British Journal of Industrial Medicine 49(8): 566-575.
Suzuki Y, Yuen SR. 2001. Asbestos tissue burden study on human malignant mesothelioma. Industrial Health 39(2): 150-160.
Taskinen E, Ahlamn K, Wukeri M. 1973. A current hypothesis of the lymphatic transport of inspired dust to the parietal pleura. Chest 64(2): 193-196.
USGS (US Geological Survey). 2004. Mineral Commodity Summaries. Washington, DC: US Government Printing Office. http://minerals.usgs.gov/minerals/pubs/mcs/2004/ mcs2004.pdf.
Zhou Y, Cheng YS. 2005. Particle deposition in a cast of human tracheobronchial airways. Aerosol Science and Technology 39(6): 492-500.


















