4
The Host Response to Pathogens
OVERVIEW
A complete understanding of pathogenesis must consider not only how microorganisms inflict damage—the primary focus of research under the war metaphor—but also the mechanisms by which hosts discriminate among microbes and convert that information into an immune response. This expanded field of inquiry is yielding intriguing results, as illustrated in the two contributions that comprised by this chapter: the first characterizes the complex host-microbe interactions that establish mucosal immunity in the gut; the second describes insights gained from analyzing patterns of global host gene expression in response to infection.
Much of the intestinal epithelium features a combination of innate defenses, but these defenses are reduced in cells that lie between lymphoid follicles and the intestinal lumen. This follicle-associated epithelium, which serves as a key interchange in signaling between microbes on one side of the epithelial barrier and host immune and inflammatory cells on the other, is the focus of research in Marian Neutra’s laboratory. She describes the structure and function of these rare and specialized regions of the intestinal epithelium and their vital role in the development of mucosal immunity; particular attention is paid to the role of M cells, which transport pathogens and antigens from the lumen to the lymphoid tissue. Understanding the molecular means by which M cells fulfill their gatekeeping role could inform the design of mucosal vaccines to prevent infection by pathogens such as human immunodeficiency virus (HIV) and provide insights on the prevention and treatment of inflammatory bowel disease (IBD) and associated immune disorders.
Researchers have greatly expanded our understanding of the complexities of
host-microbe interactions through the use of molecular techniques that monitor microbial and host cell gene expression. In his contribution to this chapter, David Relman describes studies conducted in his laboratory that employ DNA microarrays to monitor host transcript abundance in blood cells following exposure to known bacterial and viral pathogens. Their findings illustrate variation in the indigenous microbial flora of healthy humans and the range of host transcriptional response to infection; Relman and colleagues also identify recurring patterns and possible sources of variability in that response. Relman also describes the use of rDNA polymerase chain reaction (PCR) to explore the possible pathogenic activity of archaea, focusing on their potential role in peridontitis.
One factor that has not been found to produce significant differences in host gene expression is the distinction between pathogen- versus commensal-associated stimuli. Detailed comparisons of host transcriptional responses to various pathogens over time are, however, revealing subtle transcriptional signatures upon the host response. Such variability—which is thought to result from pathogen-specific mechanisms that co-opt, subvert, or modify the stereotypical host response to microbial stimuli—offers the possibility of a new approach to disease prevention, diagnosis, and treatment.
THE INTESTINAL EPITHELIUM: AN INTERACTIVE BARRIER BETWEEN HOST AND MICROBE
Marian Neutra
Those of us who study mucosal immunity and mucosal protection are in the habit of using the war metaphor when we talk about the epithelial monolayer that lines the human gut. We have described it as a barrier and the mucus it secretes as our “front line” of defense. However, this impression is being revised as we discover that the intestinal epithelium also acts as a sensor of the contents of the lumen, and as an “intelligent” mediator of immune signaling between ourselves and the microbes we encounter.
By “intelligent,” I mean that the intestinal epithelium appears capable of filtering a tremendous amount of information regarding the antigens borne by the huge and diverse population of microorganisms in the lumen. Specialized epithelial cells survey the contents of the gut and report it to a highly developed mucosal immune recognition system that resides in adjacent lymphoid cells—and, remarkably, this occurs without provoking all-out war in the form of chronic intestinal inflammation. The following essay recounts several recent discoveries that show how these specialized intestinal epithelial cells accomplish this critical balancing act.
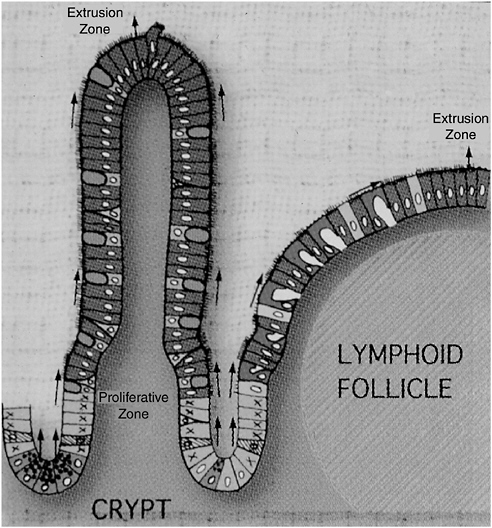
FIGURE 4-1 The follicle-associated epithelium and MALT: a close collaboration.
SOURCE: Neutra (2005).
Structure of the Follicle-Associated Epithelium
The sampling of luminal contents occurs in rare, specialized regions of the intestinal epithelium, as depicted in Figure 4-1. These include mucosa-associated lymphoid tissue (MALT) and associated epithelial cells. Because the epithelial cells in these immune induction sites are associated with underlying collections of lymphoid cells known as lymphoid follicles, they are known as the follicle-associated epithelium (FAE). In contrast to villus-associated epithelium, which covers the vast majority of the intestinal surface, FAE is sparsely distributed along
the length of the gut. Peyer’s patches, located in the ileum, contain the best-known example of lymphoid-associated epithelium in humans; these sites are most numerous in the colon and rectum, but they are also found in the oral cavities, the tonsils and adenoids, and perhaps also on minor salivary glands. The distribution of immune induction sites in the body generally reflects the local abundance of foreign material and microorganism (Neutra et al., 2001).
Villus-associated epithelium, which functions primarily to absorb nutrients, is heavily defended against microbial intrusion. Its cells secrete mucus, digestive enzymes, defensins,1 and lysosyme, as well as IgA (which plays a role in both innate and adaptive immunity, as demonstrated below). Such defenses are significantly reduced in the FAE—in fact, rather than preventing microbial adherence or contact, specialized cells in the FAE, known as M cells, appear to invite microbes to enter. M cells appear to make their luminal surfaces readily accessible to microbes, which are then endocytosed and delivered to dendritic cells that lie just beneath the epithelial surface. The dendritic cells rapidly capture antigens and pathogens from the M cells and migrate to nearby deposits of T or B cells where the antigens are presented. Thus, the inductive system of the adaptive immune response, as well as that of innate immunity, occurs in these MALTs. The relative rarity of immune induction sites limits host exposure to potential pathogens.
Pathogen-M-Cell Interactions
Inevitably, some pathogens have evolved to exploit the FAE. Microbes—including Salmonella, Shigella, and poliovirus—gain entry to their hosts by selectively adhering to the M cell. A mouse reovirus that exclusively infects Peyer’s patches causes a severe infection in newborn mice that spreads to the brain, but weaned mice with mature immune systems can clear such infections with a mucosal immune response. This reovirus provides a good model for examining how M cells select pathogens and how pathogens can select M cells.
To locate its target, the reovirus uses a carbohydrate “fingerprint” that is present on all the epithelial cells but is accessible to viral particles exclusively on the surface of M cells (Helander et al., 2003). We were able to show that one form of this reovirus (reovirus 1) targets a specific sialic acid-containing trisaccharide epitope, while another form of the reovirus (reovirus 3) uses a different
epitope. The reovirus has extended attachment proteins on its surface that look like landing gear; these attachment proteins recognize the specific trisaccharide.
The carbohydrate epitope recognized by reovirus 1 is also bound by lectins2 MAL I and MAL II, so we expected that the lectins would also specifically recognize M cells. However, when we applied MAL I and MAL II to mucosal tissue, they bound all epithelial cell surfaces readily and nonspecifically—until we displayed the lectins on particles. Clearly, the specificity for M-cell binding is a matter of accessibility, as well as carbohydrate recognition. As shown in Figure 4-2, M cells are differentiated by their lack of a thick glycoprotein coat, which
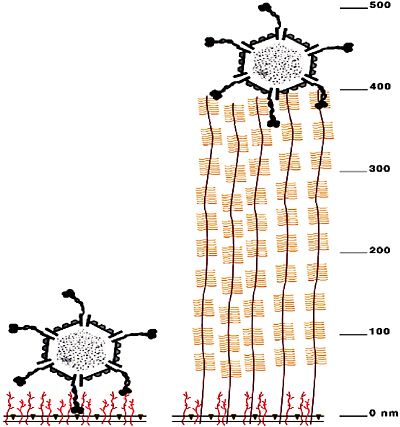
FIGURE 4-2 Reovirus has access to carbohydrate receptors on M cells. Drawn to (nanometer) scale, the virus is about 80 nm in diameter, and has 40-nm extensions (“landing gear”) made of trimers of a protein called sigma-1. With its sigma 1 protein extended, the virus can reach through the M cell’s glycoprotein coat (shown on the left), which is only about 30 nm thick. The absorptive epithelial cells that cover the intestinal mucosa bear a thick glycoprotein coat (as shown on the right) that cannot be penetrated by the extended sigma 1 “landing gear.”
SOURCE: Helander et al. (2003).
renders certain carbohydrates on their surfaces accessible to binding by the reovirus surface protein, sigma-1. Moreover, the reovirus can only bind the M-cell if the viral sigma-1 protein is in an extended conformation. It achieves this structure through the action of gut proteases, which process the folded reoviral surface protein on the native virion into this infectious form.
Because we are interested in mucosal immunity, we wanted to determine an immune response that could protect M cells from attachment of the virus and prevent reinfection of the mucosa. We found that only antibodies against the head of the sigma-1 protein were successful, and that protection depended upon blocking the interaction between sigma-1 and the carbohydrate epitope it recognizes on M cells.
The Dual Role of IgA
Another series of studies on the FAE led us to discover how IgA is both an adaptive and an innate protective mechanism. Using a technique we call the backpack tumor method, we inject hybridoma tumor cells under the skin on the backs of syngeneic (genetically identical) mice. When the resulting tumor reaches about 1 centimeter in diameter, it starts producing large amounts of antibody. These mice produce only one type of monoclonal antibody, which is determined by the hybridoma used; otherwise, they have no means of immune protection against challenge with a specific pathogen. In mice with a tumor expressing anti-sigma-1 IgG, a great deal of IgG is produced by the hybridoma and enters the blood, but only a negligible amount of IgG is secreted into the intestine; thus there is no IgG in the gut lumens of such mice (Hutchings et al., 2004). We also made mice with tumors that expressed dimeric IgA. These animals secrete the monoclonal antibody, and it is also present in their blood. In addition, we examined control animals that expressed anticholera toxin IgG and anticholera toxin dimeric IgA, as well as some without tumors.
When we orally challenged all five of these types of mice (which are not transgenic) with reovirus, we found, as expected, that only the mice secreting the anti-sigma-1 IgA were completely protected against oral reovirus challenge (see Figure 4-3) (Hutchings et al., 2004). However, we also found that IgA knockout mice that have never been exposed to reovirus before had especially high viral levels in their mucosa compared with the normal mouse controls, indicating that IgA might act as a nonspecific defense mechanism. This view is supported by our finding that the irrelevant IgA (against cholera toxin) offered significant (but not complete) protection against reoviral entry. We have seen this with other pathogens as well, so we conclude that it is good to have IgA, whether it is specific or not. This nonspecific protective effect of IgA might be especially important early in life; for example, by permitting an innate immune response to a variety of pathogens or even as an adjuvant following exposure to a single pathogen.
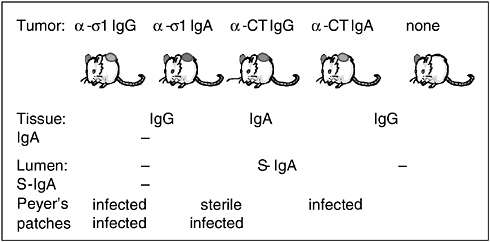
FIGURE 4-3 The “backpack” hybridoma tumor protocol.
SOURCE: Neutra (2005).
One might question what these mice, which secrete such excessive amounts of monoclonal IgA, could really tell us about what is going on in a normal human mucosa. However, when we actually captured IgA secreted by human tissues (with little wicks and sponges) and measured it, we found large amounts—for example, as much as three milligrams per milliliter of IgA, and some IgG as well—on the epithelial surface of the human rectum. After a local immunization in the rectum with a strong antigen, 10 to 15 percent of the secreted antibody may be antigen-specific IgA.
We also were surprised to discover that, when we labeled antigen-specific mouse monoclonal IgAs and introduced them into the lumen of a mouse or rabbit, they would adhere selectively to M cells. This was consistent with a previous report that newborn rabbits have collections of IgA on the surfaces of their M cells and nowhere else. Nick Mantis, a member of my laboratory, then showed that human IgA2 (but not IgA1) binds a unique receptor on M cells that has not yet been identified. This receptor is specific to antibody containing a constant domain 1 and domain 2 of the heavy chain in close proximity; if the domains are separated by a long linker region, as they are in human IgA1, the antibody can no longer be recognized by the M-cell receptor. M cells also did not bind hybrid antibody containing homologous constant domains 1 and 2 of IgG.
What advantages might the display of IgA on the FAE confer upon the host? Our current hypothesis is that IgA interacts specifically or nonspecifically with gut microbes, then brings them back to be sampled or promotes further sampling by the organized mucosal lymphoid tissues.
Toll-Like Receptors in the FAE
Another unique feature of the FAE concerns the display and function of toll-like receptors (TLRs). Villus-associated epithelial cells bear TLRs that can send signals either directly or via subepithelial macrophages, as shown by Jeffrey Gordon and others to dampen inflammation and, at the same time, promote epithelial health and renewal (Bäckhed et al., 2004; Rakoff-Nahoum et al., 2004). Signaling through these TLRs, which occurs in response to the commensal flora, prompts the constant migration and turnover of epithelial cells in the intestine. Given the role of the FAE in mucosal immunity, it would seem paradoxical that it would bear receptors that repress the immune response, yet we found that several types of TLRs are indeed displayed in these sites.
To explore this conundrum, Sophie Chabot, in my laboratory, examined how TLRs in the FAE respond to various ligands. Her studies focused on TLR2, which recognizes peptidoglycans and several other bacterial molecules. When she labeled the FAE with a fluorescent lectin to identify M cells and also with a fluorescent antibody against TLR2 this is what she saw: the FAE cells bearing TLR2 appear largely distinct from the M cells, although a few M cells displayed TLR2. Interestingly, we also found that TLR2 is displayed on both the apical and basolateral surfaces of the FAE, whereas it is only present on the apical surfaces of villus and crypt cells. The purpose of the basolateral receptors has not yet been determined.
Little is known about the non-M cells of the FAE, which are called FAE enterocytes, but it is known that these cells produce chemokines that attract dendritic cells. When we injected peptidoglycan, a TLR2 ligand, to the intestinal lumen, the apical TLR2s disappeared from the FAE enterocytes within 90 minutes, but not from the M cells. We therefore suspect that the binding of the ligand caused the enterocyte TLR2s to be endocytosed and degraded, and we hypothesize that these events resulted in release of signaling molecules by the FAE. By staining the dendritic cells just below the FAE with a specific fluorescent marker, we could also visualize what happened to them: after the FAE was exposed to the TLR2 ligand, some dendritic cells rushed into the epithelial layer. Because we know that dendritic cells can enter M-cell pockets, and we know that dendritic cells are involved in antigen capture, these observations suggest to us that TLR2 binding sets off a signaling cascade that alerts the underlying dendritic cells to prepare for subsequent antigen delivery by M cells.
Antigen Transfer in the FAE
Another effect we observed following exposure of the FAE to peptidoglycan was a dramatic increase in the rate of transcytosis of particles across the epithelium. We noted similar reactions (both the increased rate of transcytosis and the migration of dendritic cells into the epithelium) after exposing the FAE to cholera toxin; this suggests that a number of different compounds may signal the FAE to
increase transport of antigens to the dendritic cells. We have also seen that over longer periods of time adjuvants, or bacterial products similar to toxins, can influence the further movement of the dendritic cells.
We monitored these phenomena using virus-sized polystyrene fluorescent particles, which, if fed to mice, are taken up exclusively by their M cells. A postdoctoral student in my laboratory, Vijay Shreedhar, found that when he did this, the particles accumulated, as expected, in the dendritic cells. However, contrary to our expectations, those dendritic cells did not proceed to deliver their cargo to the nearby T-cell areas. Instead, the particles remained in the FAE indefinitely (we stopped checking after two weeks). Suspecting that another signal was needed to complete antigen transfer to the T cells, he fed the mice cholera toxin; the dendritic cells then moved to the T-cell areas. The same thing happened when the mice were fed Salmonella instead of cholera toxin.
Summary and Conclusion
As demonstrated by this gallery of recent snapshots of the MALT and the associated FAE in action, these sites are the focus of intense communication between the outside world and the mucosal immune system, between microbe and host. M cells, which act as gatekeepers at this boundary, have reduced antimicrobial defenses and instead promote contact with antigens and potential pathogens in the lumen. Many details of the mechanisms of signaling in the FAE remain to be determined, but it appears that the FAE is especially sensitive to TLR ligands (e.g., peptidoglycan). This localization may allow the host to respond to pathogens without creating havoc throughout the intestinal epithelium.
Understanding the differences between the FAE and the rest of the intestinal epithelium, between antigen sampling sites and nutrient absorption sites, is likely to be critical to the design of mucosal vaccines. The gut will generally not mount an immune response to a nutrient peptide or a noninfectious particle alone. Mucosal immune responses clearly require extra signals, such as ligands recognized by TLRs, enterotoxins, and probably others yet unknown; such information is being used to boost the effectiveness of mucosal vaccines. However, much more needs to be learned about how these signals work and how they might be manipulated to promote human health.
HOW THE HOST “SEES” AND RESPONDS TO PATHOGENS
David A. Relman3
Traditional perspectives of human responses to infection suggest that these responses are dominated by a limited number of effector molecules, such as
cytokines and a limited number of signaling pathways such as those associated with TLRs. Genomic tools now allow a more comprehensive assessment of human responses to infectious agents. DNA microarrays, in particular, permit one to examine human genomewide RNA transcript abundance patterns during the course of an infectious disease. Although this effort is in its early days, the resulting data reveal previously unappreciated sterotyped patterns, suggesting choreography and great complexity. The following set of observations on these kinds of data comes with several disclaimers. First, complexity and subtlety will be described at a superficial level. Second, these observations are based on transcript abundance patterns, which provide only one prism for viewing host-microbe interactions. Third, the data discussed in this essay were derived from blood, and are, therefore, limited to one anatomic compartment, albeit highly distributed, within the highly compartmentalized human host. Nevertheless, some early and important lessons have been learned concerning the variability of the host response to different kinds of microbial stimuli and the possible sources of that variability.
Conserved Response Structures and Pathways
Given that hosts have prominent mechanisms for recognizing conserved patterns among microbes (see previous contribution from Marian Neutra), one might expect to find evidence of shared responses to pathogens and commensals. To obtain a genomic perspective on the question of whether hosts can distinguish pathogen from commensal, we and other groups have conducted a broad variety of experiments in which human cells are exposed to various kinds of microbial stimuli in vitro, and their RNA transcripts are then monitored with DNA microarrays. Many of these experiments have employed human blood cells and subsets thereof, such as peripheral blood mononuclear cells (PBMCs). Although there have been many such studies with a variety of methodological and biological nuances, they seem to convey a common message: much of the response by human cells to microbial components, and to microorganisms themselves, is in fact shared. The most dominant responses are similar across time and fairly similar across different kinds of agents.
Some of the mechanisms that govern these common patterns result from well-known response systems in host cells, such as the NF-kappa-B regulon, and are probably mediated by conserved microbial pattern recognition systems. As demonstrated by Rakoff-Nahoum et al. (2004), the TLR system, an important pattern-recognition system, also recognizes commensal organisms. In fact, it is the constant stimulation by commensals that imparts or stimulates a protective, health-associated response at the mucosal barrier. The same system that recognizes pathogen-associated patterns also recognizes these same patterns in nonpathogenic microbes. The NOD-LRR family of conserved pattern-recognition
receptors also recognizes both commensals and pathogens (Eckmann and Karin, 2005; Kobayashi et al., 2005). However, we (and others) have also noted that there are differences in the response of human cells in vitro to different intact or live pathogens that cannot be explained by simple corrections for equivalent dose or time (Boldrick et al., 2002). These differences have supported the concept that the human host is capable of discrimination among pathogens. But, are the responses observed in vitro necessarily similar to those that occur in vivo?
How Conserved and Shared Are the Responses Seen in a Primate Host?
These observations lead to the question of disease mechanism from the perspective of host response systems. There are lessons to be learned from examining changes in transcript abundance patterns triggered by conserved signaling systems such as the NF-kappa-B regulon. Stress, infection, cytokines, and other factors trigger transcriptional responses in a large set of common genes with some variation based on the nature of the stimulus and set of cognate receptors. Yet, we know that a diverse set of pathogens have evolved the means of perturbing or subverting the NF-kappa-B response system. We have worked with several groups of collaborators in order to explore established in vivo models of disease in nonhuman primates caused by five diverse, overwhelming systemic infectious agents, all of which cause similar, fulminant disease in humans: Marburg and Ebola viruses, anthrax, smallpox, and monkeypox. Macaques were deliberately infected with these microbes, so that we could establish time points for infection and standardize sampling procedures, and their PBMCs were sampled over many time points, until death, which was the most common outcome in all of these infections. The overall transcriptional patterns, over time, suggested some degree of conservation among the responses to diverse stimulating agents. Yet there was also evidence of variability. At least one source of variability appears to be the infectious agent (see below). Changes in the relative abundance of blood cell subsets can also affect the apparent abundance of cell type-specific gene transcripts. However, the source of variability is often difficult to resolve due to confounding, biologically important parameters such as time and dose.
Based on the “30,000-foot view” of host transcriptional response that we obtained from these primate model hosts, we now suspect that, although the set of host responses to all microbes is largely conserved, disease—a specific host response to pathogens—acquires its agent-specific features when conserved responses are triggered at a time point, or at a location within the host, or to a magnitude, that is not usually seen when the same responses are triggered by commensals. Thus, pathogens may set off host response programs when the host recognizes that microbes are present at an inappropriate location or at concentrations higher than normally found with commensals.
Can Archaea Act as Pathogens?
Archaea are the most abundant of the three domains of life on earth, and yet it is curious that they have not been frequently implicated in human disease. These organisms do not possess the microbial patterns—such as lipopolysaccharides and peptidoglycans—that are recognized by pattern-recognition receptors such as the TLRs and NOD-LRRs. Perhaps humans (and other higher organisms) do not interface with or confront archaea with the necessary cell types or at the proper sites, or perhaps the archaea do not present the necessary patterned molecules or virulence factors to allow them to cause disease. On the other hand, it is also possible that the archaea do cause disease. However, because they are difficult to detect, we are not aware of them as the causal factors in these cases. To pursue some of these possibilities, we have focused on a common disease, chronic (adult) periodontitis (Lepp et al., 2004). In this disease, deep pockets form between the gums and teeth. The bacterial communities in these pockets may include 500 or more species and strains, a number that has been associated with the severity of disease. We wanted to determine whether archaea are present in these pockets, as some have previously suggested, and if so, identify them and examine whether they are associated in any meaningful way with the disease state. Using broad range archaeal rDNA PCR, we detected archaeal sequences only at sites with moderate or severe disease. The relative abundance of the archaeal sequences was significantly higher in severe disease than in lesser degrees of disease, and decreased in association with favorable responses to treatment (scaling and root planing) at diseased sites.
The archaeal sequences we obtained were restricted to a discrete number of clades of methanogenic archaea, suggesting that there is limited archaeal diversity in such communities. We, therefore, suspected that these particular methanogens play a role in some (but not all) cases of periodontitis. As noted by Jeffrey Gordon (see paper by Bäckhed et al. in Chapter 1), methanogenic archaea represent the “final microbial link” in the polysaccharide processing chain. In the human mouth, archaea may participate in syntropic relationships with secondary bacterial fermentors, resulting in benefits for all participants. At sites of severe peridontitis without methanogens, other syntropic partners, such as the treponemes, may substitute for the methanogens; for example, we found an inverse relationship between treponeme ribosomal DNA abundance and methanogen ribosomal DNA abundance at sites of moderate and severe disease. This suggests that treponemes—and possibly other organisms—can take the place of methanogens in the community enterprise that is periodontitis. Thus, this pathologic host response appears to be generated by a disturbed community structure, one component of which may be archaeal. This example illustrates the complexity and variability of the microbial stimuli that are capable of eliciting the same gross pathology, and emphasizes how much we still need to learn about the microbial ecology and synergy of molecular mechanisms responsible for some common forms of pathology.
Sources of Variation in Host Response to Pathogens
From a simple, conceptual framework, there are three factors that might help to determine the nature of the host response to a pathogen: host genetics, environmental factors that condition the host response, and pathogens. A recent study, which compared patterns of transcript abundance in the PBMCs of 77 healthy people, found relatively little variation among them (Whitney et al., 2003). Sources of variability among these subjects included gender, age of host, and time of the day (see Figure 4-4). Some of the most “host-intrinsic” genes may turn out to be previously unrecognized determinants of human individuality. In comparison, among individuals with hematologic malignancies, there was a high degree of variability in these patterns.
Individuals with fever and systemic infection caused by different known microbial agents displayed an intermediate degree of variability in their blood-associated transcript abundance patterns (between that associated with health and cancer). Although our analysis of patients from one study of fever and infection is so far only preliminary, the data suggest that microbiological diagnosis is an important source of this intermediate variability (Personal Communication, Popper SJ, Brown PO, Relman DA, Department of Microbiology and Immunology,
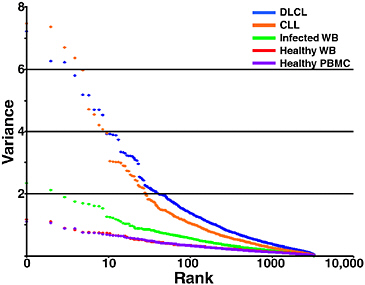
FIGURE 4-4 Variation in gene expression in health and disease. Global gene expression was measured in 45 samples from each of five studies. See text for details on each sample set. Of the well-measured microarray elements, 3,826 were randomly selected from each group and variance was calculated for each. Variance was plotted in rank order (highest to lowest) for the genes in each of the specimen groups. DLCL, diffuse large B-cell lymphomas; CLL, chronic lymphocytic leukemia; WB, whole blood.
SOURCE: Whitney et al. (2003).
Stanford University School of Medicine, March 17, 2005). Similarly, detailed analysis of the in vivo responses of nonhuman primates to the five systemic infectious agents described earlier reveals significant numbers of genes with transcript abundance patterns that distinguish otherwise similar responses of macaques infected with smallpox from those infected with monkeypox, and a similar general finding when any two of the infections are compared to each other. One of these distinguishing features in smallpox appears to be down-regulation of the NF-kappa-B regulon (Rubins et al., 2004). In contrast, Ebola produces a very strong NF-kappa-B response in the same host, as do many kinds of fulminant infectious disease agents. Thus, it appears that one of the sources of variability in host transcriptional response to pathogens results from pathogen-specific mechanisms that co-opt, subvert, or modify the stereotypical host response to microbial stimuli. These subtle signatures could potentially be exploited for diagnostic purposes, even during the early, asymptomatic stages of overwhelming diseases such as Ebola.
Among the other environmental factors that may determine the nature of variability in host responses to noxious stimuli (and influence the set-point of any given individual, prior to infection, for example) is the composition and structure of the host indigenous microbial communities.
Variation in the Indigenous Microbial Flora Among Healthy Humans
In my laboratory, we recently undertook a large-scale survey of the surface-adherent colonic and fecal microbial communities in a small number of healthy individuals (Eckburg et al., 2005). Microbes at six different colonic mucosal sites, ranging from the cecum to the rectum, were sampled in each of three healthy people, as were feces. Using broad-range rDNA PCR, we analyzed more than 11,000 bacterial and 1,500 archaeal sequences from these samples. Every archaeal sequence was identical, and corresponded to the known rDNA sequence from Methanobrevibacter smithii. Among the bacteria, we found great diversity, but concentrated within seven phyla, with a preponderance of members from the phyla Bacteroidetes and Firmicutes. Among the latter, the enormously diverse Clostridia class and the Molicutes and Bacilli classes were highly abundant. About 60 percent of the sequences we discovered were novel, and about 80 percent corresponded to bacteria that have not apparently yet been cultivated in the laboratory, or at least have not been recognized as having been cultivated. Among the surprises were some Cyanobacteria-like sequences, as well as sequences from Verrucomicrobia.
We looked at a number of analytical approaches for comparing microbial diversity within and between these different specimens and subjects. When we analyzed the species-level abundance of the various bacterial taxa for each individual by sample location, we found that the greatest degree of variability existed between individuals, rather than between the various sampling sites. We employed
double principle coordinate analysis to assess the relative magnitude and sources of differences in community diversity between and among subjects. Once again, we found distinct clustering by individual. Although the community composition of the mucosal samples within any given individual resembled each other more than they did the fecal community from that same individual, the latter specimens were collected one month after the mucosal specimens. Thus, the distinct microbial communities that are in constant contact with the human host at sites such as the intestines and the mouth may prove to be another important source of human individuality, and may precondition the host for the subsequent responses that occur when confronted with a pathogen.
Future Inquiries
A prerequisite to answering some of the questions that I have raised in the above discussion is the generation of more complete time- and anatomic site-dependent profiles of genomewide transcript abundance in humans with natural infectious disease of various kinds, as well as during various states of health. To be most useful, such data sets should include analyses from patients infected by closely related microbial pathogens (by taxonomy or by virulence mechanism), and patients with the same clinical syndrome—but caused by different microbial agents, or patients with defined genetic differences who have been infected with the same agent. It will also be important to develop relevant animal models of disease in order to control such variables.
Concurrent analyses of host mucosal transcript abundance patterns and the composition of the adjacent microbial community might reveal the degree to which host and community determine the responses and physiological state of the other, as well as the mechanisms behind these responses. An understanding of these issues might lead to novel approaches for preserving human health, predicting disease, and treating the latter with much more meaningful end points.
REFERENCES
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. Proceedings of the National Academy of Sciences USA 101(44):15718–15723.
Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. 2002. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proceedings of the National Academy of Sciences USA 99(2): 972–977.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308(5728): 1635–1638.
Eckmann L, Karin M. 2005. NOD2 and Crohn’s disease: Loss or gain of function? Cell 118(2): 229–241.
Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, Nibert ML, Neutra MR. 2003. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. Journal of Virology 77(14): 7964–7977.
Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, Neutra MR. 2004. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer’s patches. Journal of Virology 78(2):947–957.
Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. 2005. Nod2 dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307(5710): 731–734.
Lepp PW, Brinig MM, Ouverney CC, Palm K, Armitage GC, Relman DA. 2004. Methanogenic Archaea and human periodontal disease. Proceedings of the National Academy of Sciences USA 101(16):6176–6181.
Neutra MR. 2005 (March 17). Session II: Ecology of Host-Microbe Interactions. Presentation at the Forum on Microbial Threats Workshop Ending the War Metaphor: The Changing Agenda for Unraveling the Host-Microbe Relationship, Washington, D.C., Institute of Medicine, Forum on Microbial Threats.
Neutra MR, Mantis NJ, Kraehenbuhl JP. 2001. Collaboration of epithelial cells with organized mucosal immune lymphoid tissues. Nature Immunity 2(11):1004–1009.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118(2): 22–41.
Rubins KH Hensley LE, Jahrling PB, Whitney AR, Geisbert TW, Huggins JW, Owen A, Leduc JW, Brown PO, Relman DA. 2004. The host response to smallpox: Analysis of the gene expression program in peripheral blood cells in a nonhuman primate model. Proceedings of the National Academy of Sciences USA 101(42):15190–15195.
Whitney A, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. 2003. Individuality and variation in gene expression patterns in human blood. Proceedings of the National Academy of Sciences USA 100(4):1896–1901.
















