5
Addressing Complexity in Microbial and Host Communities
OVERVIEW
In keeping with the goal of placing host-microbe relationships within an ecological context, the two contributions that make up this chapter discuss important sources of complexity in host-microbiota ecosystems: the biodiversity of endogenous microbial communities and the networks of host-microbe relationships among pathogens that typically infect multiple hosts (which are in turn infected by multiple pathogens).
In his workshop presentation, microbial ecologist David Stahl predicted that a better understanding of the natural history of microbes—their diversity, as well as their phylogenetic and ecological relationships to each other and to other living things—will improve researchers’ ability to develop questions and hypotheses that probe host-microbe relationships at the molecular level (see Summary and Assessment). His paper, which describes the use of DNA microarrays to characterize endogenous oral microbes in humans, speaks to the promise of pursing this research strategy.
Stahl and coworkers demonstrate how links between human health status and oral microbiota composition can be explored with microarray-based tools that detect oral microorganisms and monitor their responses to environmental changes. In the near future, microarray-based diagnostics may allow dentists and clinicians to detect microbial sentinels of disease in the oral cavity, permitting earlier and more definitive diagnosis, as well as improved treatment. Such diagnostics could play a key role in elucidating the apparent relationships between
oral microbes and such chronic conditions as cardiovascular disease, diabetes, and arthritis, and potentially in the detection and treatment of such ailments.
The second paper, contributed by veterinary biologist Mark Woolhouse, expands the ecological perspective to address the relationships among all hosts colonized by the same microbial species, and in particular, the web of relationships surrounding the transmission and survival of zoonotic pathogens in novel hosts. Woolhouse and coworker Sonya Gowtage-Sequeira present quantitative data on the diversity of human pathogens, investigate the association between emerging infectious diseases and host range, and examine the implications of having multiple hosts for pathogen evolution. Their findings reveal the importance of taking a broad, multidisciplinary, and ecological approach to the study of infectious disease rather than focusing on the interactions between individual host and microbe species.
DNA MICROARRAYS AS SALIVARY DIAGNOSTIC TOOLS FOR CHARACTERIZING THE ORAL CAVITY’S MICROBIAL COMMUNITY1
Laura M. Smoot, James C. Smoot, Hauke Smidt, Peter A. Noble, Martin Könneke, Z. A. McMurry, David A. Stahl2
The interest in using saliva as a diagnostic medium has increased during the last decade, and recent technological developments are responsible for the advancement of its use as a diagnostic fluid (Streckfus and Bigler, 2002). There are several advantages to using saliva as a diagnostic fluid. Saliva is easy to collect, store, and ship, and, compared with the collection of blood, saliva collection is inexpensive and noninvasive, which is much safer for health-care workers (Slavkin, 1998). In the near future, salivary diagnostic devices based on highly parallel data collection methods (e.g., DNA microarrays) will be very useful tools for health-care professionals. DNA microarrays are now used as tools for developing a comprehensive characterization of oral diseases. For example, Li et al. (2004) used high-density oligonucleotide microarrays to profile transcripts found in saliva from head and neck cancer patients, and found that thousands of human mRNAs are present in cell-free saliva. In conjunction with collaborators, our laboratory is using DNA microarrays to detect microorganisms from the human oral cavity and, ultimately, to develop a microarray-based device for clinical applications.
|
1 |
Reprinted with permission from Advances in Dental Research. 2005;18:6–11. |
|
2 |
LM Smoot, JC Smoot, PA Noble, M Könneke, ZA McMurry, and DA Stahl are from the Civil and Environmental Engineering, 302 More Hall, Box 352700, University of Washington, Seattle, WA 98195. H Smidt is from Wageningen University, Wageningen, Netherlands. DA Stahl is also the corresponding author, dastahl@u.washington.edu. |
The Oral Cavity’s Microbiota and Human Health
The oral microbiota play critical roles in human health and are directly linked to diseases such as dental caries and periodontitis. Although it is clear that microorganisms are intimately involved in disease, studies are revealing that the composition of the complex microbial assemblages resident in the human oral cavity is strongly associated with pathology, resistance, and predisposition to dental caries and periodontal diseases, which remain the most common chronic illnesses in humans. For example, Actinobacillus actinomycetemcomitans is strongly associated with juvenile periodontitis, and Streptococcus mutans is the primary etiologic agent of dental caries. In addition, the structure and activity of oral microbial populations may serve as sentinels of human systemic diseases. Evidence is accumulating that periodontal microbiota are involved in the development of various systemic diseases (Greenstein and Lamster, 2000; Kinane and Marshall, 2001; Scannapieco, 1998; Teng et al., 2002), including cardiovascular disease (Beck et al., 1998; Glurich et al., 2002; Kinane and Lowe, 2000), pneumonia (Scannapieco et al., 1998; Terpenning et al., 2001), arthritis (Mercado et al., 2001), diabetes (Grossi and Genco, 1998; Miller et al., 1992), and preterm low-weight birth (Madianos et al., 2001; Offenbacher et al., 1998, 2001).
More than 600 microbial species are known to inhabit the human oral cavity (Kolenbrander, 2000; Moore and Moore, 1994; Paster et al., 2001). The oral microbiota are broadly distributed among many taxonomically distinct groups, and all domains of life have representatives in the oral cavity (Figure 5-1). Only about half of the oral microorganisms have been successfully cultured (Paster et al., 2001), and the identification of uncultured and novel microbial phylotypes from oral biofilms with small subunit ribosomal DNA (rDNA) clone libraries highlights the need for culture-independent methods for the accurate description of oral microbial communities (Kroes et al., 1999; Paster et al., 2001; Relman, 1999; Sakamoto et al., 2000).
Most of the oral microbiota are organized in complex multispecies biofilms attached to hard and soft surfaces of teeth and oral tissues. Characterization of microbial population structure within oral biofilms has been studied on a spatial, temporal, and disturbance basis with a variety of strategies, including chemostat studies of oral mixed cultures (Bradshaw and Marsh, 1998), fluorescent in situ visualization of microorganisms in native and artificial biofilms with confocal laser scanning microscopy (Guggenheim et al., 2001; Kolenbrander et al., 1999; Wecke et al., 2000), and checkerboard DNA-DNA hybridization (Haffajee and Socransky, 2001; Haffajee et al., 2001; Socransky et al., 1998). Oral biofilm structure and microbial virulence are influenced by various host-associated factors, such as genetic predisposition, activity of the immune system, diabetes, and estrogen deficiency (Greenstein and Lamster, 2000). Although human health status and oral microbiota appear to be linked, the ecology of the oral microbiota (i.e., interactions of microbes with biotic and abiotic factors in the mouth) is not yet
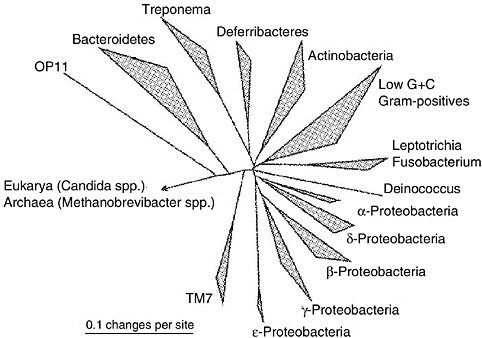
FIGURE 5-1 Phylogenetic tree generated from small subunit rRNA sequences of oral microorganisms. Sequences not yet present in the latest available ARB database [http://www.arbhome.de/] were added from public databases. Alignment and phylogenetic analysis were performed with the ARB software (Strunk and Ludwig, 1995), and the tree was constructed with the use of the “neighbor joining” method (E. coli position 40-1406, Olsen correction) (Saitou and Nei, 1987). The reference bar indicates 10 nucleotide exchanges per 100 nucleotides. Eukaryotic Archaeal (Methanobrevibacter sp.) branches were removed.
SOURCE: Smoot et al. (2005).
fully understood. Thus, it is essential that tools be developed for the detection of microorganisms and the monitoring of their responses to factors important in the relationship with their host, such as physical and chemical changes (e.g., pH and anaerobiosis) or perturbations (e.g., brushing and smoking) in the local environment. Ultimately, associating these responses with disease development and progression is paramount to human oral cavity research.
Whole saliva provides a convenient and reliable means to sample the oral cavity microbiota. However, salivary flow rates vary based on an individual’s circadian rhythms and factors such as stress and exercise (Lawrence, 2002). Many bacteria survive and grow in saliva (de Jong et al., 1984; Palmer et al., 2001; Rudney, 2000), despite the important antimicrobial functions of saliva (Rudney, 2000; Tenovuo, 1998). Microorganisms attached to the surfaces of the mouth are continuously shed into the salivary fluid, and bacteria residing in the periodontal
pockets are constantly washed into saliva by the gingival crevicular fluids (Umeda et al., 1998). The presence of these micro-organisms can be indicative of health status (e.g., salivary levels of periodontal pathogens reflect the periodontal status of the patient) (Sakamoto et al., 2000; Umeda et al., 1998; von Troil-Linden et al., 1995). Thus, the development of salivary diagnostic tools to monitor and detect microbes in the human oral cavity will provide significant benefits to the field of clinical dentistry.
Highly sensitive instruments and highly parallel methods of analysis are needed to identify the microbial sentinels of disease and to listen to their messages. Conventional microbiological approaches that rely on cultivation for the detection of microorganisms in the oral cavity are not sufficient for such comprehensive and intensive monitoring. These techniques are time consuming, require many specialized and complex growth media, capture only a minor fraction of the oral microbiota, and do not provide in vivo data of gene expression during infection and subsequent disease. Molecular techniques such as clone libraries, quantitative polymerase chain reaction (PCR), and fluorescent in situ hybridization analyses, although informative, are labor intensive and impractical for routine patient monitoring. Thus, the field needs to develop tools that provide high-fidelity data in a high-throughput format to characterize the complex microbial communities of the human oral cavity.
Application of DNA Microarray Technology to Dentistry and Oral Diagnostics
The genetic information of all living organisms is present in the nucleic acid polymers DNA and RNA. This information identifies an organism, its genotype, and its potential phenotype. DNA microarrays, ordered displays of genetic material deposited on a surface or matrix, provide a highly parallel means for the analysis of genetic information. For instance, from hundreds to hundreds of thousands of ordered DNA oligonucleotide probes may be present on a single microarray. Several types of DNA microarrays have been developed, and many reviews of the technology and its application exist (e.g., there are 34 citations for reviews of microarray technology in the PubMed database for the first half of 2004). DNA oligonucleotide microarrays are assemblages of short (from 8 to 70 bases long) nucleic acid sequences (probes) linked to a matrix. Sources of target nucleic acids (RNA or DNA molecules with sequences complementary to the probe) include reference or model organisms in pure culture, clinical specimens, and environmental samples. Target nucleic acids are isolated from a sample, labeled, and hybridized to the microarray. The nucleic acid target may be an amplified product of a gene of interest that is either labeled directly or indirectly during the PCR, or it may be directly isolated from a sample and labeled. Hybridization occurs when target nucleic acids bind to their complementary oligonucleotide probes.
DNA microarray analysis is an emerging technology that is being used in a diverse set of molecular applications (Cummings and Relman, 2000; Stears et al., 2003; Zhou, 2003). DNA microarrays were first used for the simultaneous measurement of differential gene expression of 45 Arabidopsis genes (Schena et al., 1995). Since the seminal work of Schena et al., multitudes of studies incorporating microarray analysis have been done (e.g., when the PubMed database was queried on microarray and expression, there were 1055 citations for the first half of 2004). These types of experiments provide information about what genes are up-regulated and down-regulated under certain environmental conditions, under regulator control, or in specific tissue samples. Additional applications of microarrays include the examination of pathogen genetic diversity (Cummings et al., 2004; Fitzgerald et al., 2001; Smoot et al., 2002) and the detection of single nucleotide polymorphisms (SNPs). Microarray SNP analysis provides a simultaneous analysis of thousands of genetic loci and provides insight on chromosomal regions associated with particular diseases (Kuo et al., 2003). For example, high-density DNA microarrays can be used as molecular screens for certain cancers and tumor subtypes. Specific examples include the evaluation of genes involved in head and neck squamous cell carcinoma of the oral cavity (Kuo et al., 2002). In addition to the study of oral cancers, microarrays can be used to study infectious diseases of the oral cavity. In fact, the National Institute of Dental and Craniofacial Research recently funded the Institute of Genomic Research to produce oligonucleotide microarrays (70mers) for S. mutans and Porphyromonas gingivalis. Studies with these arrays will undoubtedly lead to the discovery of novel disease-causing attributes, identification of targets for novel therapeutics, and characterization of the genetic network that allows for biofilm formation on the hard and soft surfaces in the oral cavity.
Another example of DNA microarrays is those composed of DNA oligonucleotide probes complementary to different regions of the rRNA molecules. Typically, these types of microarrays contain oligonucleotide probes designed to regions that vary in conservation, providing a phylogenetic hierarchy to probe specificity (e.g., species, genus, division, domain). Two strategies have been used to detect specific rRNA gene sequences with DNA oligonucleotide microarrays. In both cases, target rRNA hybridizes to multiple hierarchically nested probes, thereby providing a high level of information redundancy, which is an essential design feature required for confident data interpretation (Amann et al., 1995; Stahl, 1995). Using a rational probe design approach, Guschin et al. (1997) used a microarray composed of oligonucleotide probes complementary to a region of the rRNA molecule spanning bases 156–1390. In contrast, Wilson et al. (2002) used a high-density hierarchical microarray composed of over 60,000 oligonucleotide probes complementary to bases 1409–1491 of the rRNA molecule. A significant feature of these types of microarrays is that they provide the phylogenetic signature of an organism. Hence, they can be used in applications that simulta-
neously detect specific pathogens and characterize entire microbial populations, such as flora resident in the human oral cavity.
Our laboratory is currently using rRNA phylogenetic microarrays in the MAGIChip (MicroArray of Gel-immobilized Compounds) format (Figure 5-2B). In this format, oligonucleotide probes are covalently immobilized in three-dimensional polyacrylamide gel pads (Yershov et al., 1996). This format provides specific advantages over conventional glass microarrays with respect to microbial detection: (1) higher probe density, and thus larger dynamic range of target sequence capture; (2) a local environment suitable for performing real-time measurements of probe-target duplex stability; and (3) a reusable format that may reduce experimental variation and cost (Guschin et al., 1997). In the current format, target RNA is fragmented with a hydroxyl radical-based reaction and is simultaneously end-labeled with a fluorescent dye (Figure 5-2A; Bavykin et al., 2001). Following hybridization and washing at room temperature, the fluorescent signal from each gel element is quantified with the use of a custom-designed epifluorescence microscope equipped with a charge-coupled device camera. Nonequilibrium dissociation curves are determined with the use of image analysis software to capture intensity readings for each array element during controlled heating on a temperature-controlled stage (Figure 5-2C; Fotin et al., 1998; Yershov et al., 1996).
Although microarray technology is currently used primarily by the biomedical research community to identify disease-related genes and to characterize gene targets for clinical intervention and novel therapeutic discovery, its use in an applied setting such as clinical dentistry is imminent. Microarrays hold great promise for the analysis of oral cavity diseases, and with the continued evolution and improvement of the technology, dentists will be able to use these tools to better manage patients’ health care. There are several advantages to the use of DNA microarrays in the dental setting. The ability to screen simultaneously for infectious disease agents, cancer markers, and other common oral disorders, as well as monitor general oral health over time, is clearly an advantage. The technique is sensitive, and the assay is relatively quick. Both of these issues are very important in the detection and/or monitoring of oral cavity microorganisms, especially those that are uncultivable or difficult to grow in the laboratory. Furthermore, microarray technology will allow the dentist to fingerprint a patient’s oral cavity and monitor changes. This focus on preventive and personalized medicine should result in healthier patients and, in the long run, reduced health care costs.
Despite the many advantages and capabilities of microarrays, the dental community has several challenges to face before microarray-based assays are used routinely in the dental office. Once the device is developed, individuals will need training on the new technique and instruction on data interpretation and analysis. Other challenges include the high costs associated with start-up of the technology and the management and analysis of data generated by the assays. Technical promise does exist for overcoming these obstacles. For example, costs for per-
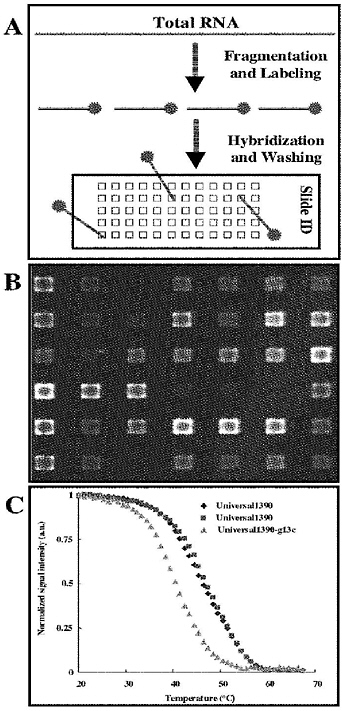
FIGURE 5-2 Description of the MAGIChip DNA oligonucleotide microarray technology used in the Stahl laboratory. (A) Simplified schematic overview of the protocol used to fragment and end-label total RNA directly from saliva. The RNA pool is complex, and targets can range in size from 100 bases to several kilobases. Fragmentation is a chemical nuclease reaction, where the average desired size of molecules ranges from 80–50 bases. (B) Image from a MAGIChip. DNA oligonucleotide microarray following hybridization with total RNA isolated from a human saliva sample. Total RNA was isolated from 300 µL of a human saliva sample. RNA was fragmented, labeled, and hybridized to the microarray. A portion of the microarray image is shown. Individual gel pads are 100 × 100 × 20 µm. Gel pads are arrayed in a 26 × 26 matrix and have 300 µm center-to-center spacing. (C) Example of nonequilibrium thermal dissociation curves measured from 3 gel pads (replicate perfectly matched probes designed to detect all life, and a probe with a SNP that is used as a reference mismatch hybridization).
SOURCE: Smoot et al. (2005).
forming microarray analyses have dropped significantly since their inception, and the competition among, and continuous establishment of, new manufacturers of microarray equipment and reagents keep costs on the decline. Scientists are also now developing and using integrated databases to store the massive amounts of raw microarray data from multiple laboratories. These databases, which are typically Internet-accessible, are invaluable resources, as they allow for the storage, retrieval, and cross-comparison of data from experiments that were conducted in different laboratories.
Technology Integration Required for Point-of-Care Instruments
Recent reviews of microfluidic technology used in point-of-care and molecular diagnostic devices highlight the advances made in microelectromechanical systems (MEMS) (Cunningham, 2001; Gardeniers and van den Berg, 2004; Huang et al., 2002). To produce microarray-based in vitro diagnostic devices for clinical use, scientists must couple DNA microarray technology with microfluidics. Microfluidics enables small sample sizes to be used, avoids reagent and waste costs, and allows for new types of assays that are impossible at macroscopic scales. Practical use of the technology in the clinical setting will require cost-effective integration of several microfluidic processes. This integrated circuitry of fluidic processes will overcome several limitations in microarray technology and move microarrays from basic science tools to devices suitable for application in the clinic. In fact, several different strategies have been used to improved hybridization kinetics and microarray fabrication with microfluidics (e.g., Cheek et al., 2001; Dill et al., 2004; Santacroce et al., 2000). However, few have attempted to manufacture fully integrated systems (Anderson et al., 2000; Baum et al., 2003; Liu et al., 2004). The merger of these technologies produces new challenges. Use of microarrays and their corresponding microfluidic support systems as diagnostic devices requires robust design and validation strategies, traditional quality assurance process control, and quality control testing. Devel-
opment of point-of-care devices that interrogate oral microbiota nucleic acid targets will incorporate the economy of scale provided by microfluidics, a high-fidelity DNA microarray readout, and a highly integrated system consisting of components to miniaturize and automate cell lysis, target isolation, and target detection.
The initial steps in sample preparation are critical to the success of microfluidic microarray devices, and the variability in saliva viscosity, particle load, and microbial biomass makes these processes quite challenging. Preparation of saliva samples for DNA microarray analysis requires concentration of cells and disruption of microbe cell envelopes to make the RNA or DNA target molecules available for processing. An overview of different lysis and purification strategies is presented in a recent molecular diagnostics MEMS review (Huang et al., 2002). However, to date, no single universal cell lysis protocol exists for the quantitative extraction and purification of cellular nucleic acids from microorganisms. Integration of efficient lysis and extraction processes within a microfluidic component is essential for the development of a point-of-care microarray-based device for use in dental applications.
The method of detection and desired assay specifications dictate what processes are performed within the microfluidic component of the device. For example, if the goal is to detect a specific gene, which is present in low copy in the chromosome or is expressed at low levels, amplification strategies such as PCR will likely need to be incorporated. The current reported limit of detection with microarray technology is approximately 107 microorganisms, with direct detection of rRNA (El Fantroussi et al., 2003; Small et al., 2001) and with PCR-amplification of functional genes (Taroncher-Oldenburg et al., 2003). Based on recent quantitative PCR amplification studies with subgingival samples (Lepp et al., 2004), this level of detection may not detect subtle changes in the microbial community in response to changes in health status. Currently, in our laboratory, we can detect on the order of 106 microorganisms with direct detection of rRNA using improved labeling techniques. Further advancements in detection are enabled by microfluidic technology via increased target movement and hybridization buffer-mixing (Adey et al., 2002; Asbury et al., 2002; Liu et al., 2003).
Microarrays provide great promise for advancements in oral cavity biology for dentists in the 21st century. They should be especially useful for the diagnosis of microbes in the oral cavity because they have a high probe density that allows for the simultaneous detection of multiple microbes. As the technology matures, pivotal issues in the sample collection and processing component of point-of-care microfluidic devices include: (1) cell lysis efficiency, (2) target-labeling reactions in MEMS, (3) material compatibility with solvents and reagents, and (4) integration of multiple microfluidic processes. Key areas of future development for microarrays and detection instrumentation include: (1) improved methods of discrimination between perfectly matched hybridizations and cross-hybridization
events, (2) heightened sensitivity and dynamic range of microarrays and detectors, and (3) miniaturization of detectors. The use of microarray-based devices in the dental field will allow dentists and clinicians to detect microbial sentinels in the oral cavity and provide improvements in diagnoses, prevention, and monitoring methods, which will lead to better management of patients’ dental care.
Acknowledgments
The research of DA Stahl and PA Noble is supported by NIH grant U01 DE 14955-02. We thank our collaborators, J. Jackman, D. Relman, R. Lamont, and P. Milgrom. We are particularly indebted to M. Donlon, DARPA, and the Biosensor group at ANL, led by D. Chandler, for supporting our MAGIChip studies.
POPULATION BIOLOGY OF MULTIPLE HOSTS AND MULTIPLE PATHOGENS
Mark E. J. Woolhouse and Sonya Gowtage-Sequeira3
Introduction
Humans can be infected by many different pathogen species, and the majority can also infect other host species (Taylor et al., 2001). The same is true for those pathogens associated with emerging or reemerging human diseases (Morse, 1995). Moreover, the majority of human pathogens have probably been acquired by jumping into humans from a nonhuman reservoir (Diamond, 2002; Woolhouse et al., 2005). Hence if, as the title of this workshop demands, we are to successfully unravel the host-microbe relationship it seems obvious that we need to understand how multiple pathogens interact with multiple hosts, from pathological to evolutionary time scales and from the molecular level to the population level.
As a step towards this goal, we present some quantitative data on numbers of human pathogens, investigate the association between emerging infectious diseases and host range, and briefly review the implications of having multiple hosts for pathogen evolution. The message to take will be that it is often insufficient to consider the interaction between a single host and a single pathogen in isolation. Consequently, a broad, multidisciplinary approach that cuts across the traditional divides between human and veterinary medicine and between virology, bacteriology, and parasitology will be needed to tackle many infectious disease problems.
Numbers of Pathogens
We define a human pathogen as “a species infectious to and capable of causing disease in humans under natural transmission conditions.” We confine atten-
tion to the major pathogen groups: viruses and prions, bacteria and rickettsia, fungi, and protozoa. Helminths we do not consider ectoparasites. We include pathogens that have only been reported as causing human disease in a single case, and those which only cause disease in immunocompromised people. We also include instances of accidental laboratory infection, but exclude deliberate laboratory infections (of which a disturbingly large number have been reported in the scientific literature).
We obtain counts of pathogen species using an updated version of a previously published database (Taylor et al., 2001). Novel pathogens listed online by the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO) and ProMED were added to the database. Taxonomic changes were sourced from the online Index virum (International Committee on Taxonomy of Viruses) and its published updates (Mayo, 2002), the online National Centre for Biotechnology Information (NCBI) taxonomy browser, the online CABI bioscience database of fungal names, and standard texts (Collier et al., 1998; Schmidt and Roberts, 2000).
Total number of pathogen species in the major groups are given in Table 5-1. The grand total is 1406, which differs slightly from Taylor et al. (2001) due to new species recognition (e.g., metapneumovirus, SARS coronavirus) and of classification changes. The most diverse group is the bacteria with over 500 species known to be pathogenic to humans. We note that the definition of a species is problematic for many microorganisms, and so these numbers represent fairly crude measures of pathogen diversity: much of the diversity relevant to pathogenicity exists within species rather than between species.
Only a subset of these pathogens are associated with emerging or reemerging disease problems. There are several published definitions of emerging or reemerging infectious diseases. The CDC suggest: “diseases of infectious origin whose incidence in humans has increased within the past two decades or threatens to
TABLE 5-1 Counts of All, Emerging or Reemerging and Zoonotic Species of Human Pathogens and Comparison of the Relative Risks of Emergence for Zoonotic and Nonzoonotic Species for Each of the Major Pathogen Groups
|
|
No. Species (%) |
No. (Re-)Emerging Species (%) |
No. Zoonotic Species (%) |
No. (Re-)Emerging Zoonotic Species (%) |
Relative Risk* |
||||
|
Viruses |
208 |
(15) |
77 |
(44) |
143 |
(18) |
56 |
(43) |
1.2 |
|
Bacteria |
538 |
(38) |
53 |
(30) |
253 |
(31) |
42 |
(32) |
4.3 |
|
Fungi |
317 |
(23) |
22 |
(13) |
113 |
(14) |
14 |
(11) |
3.2 |
|
Protozoa |
56 |
(4) |
13 |
(7) |
42 |
(5) |
10 |
(8) |
1.1 |
|
Helminths |
287 |
(20) |
10 |
(6) |
266 |
(33) |
8 |
(6) |
0.3 |
|
Total |
1406 |
(100) |
175 |
(100) |
817 |
(100) |
130 |
(100) |
2.1 |
|
*Risk of emergence for zoonotic species relative to risk of emergence for nonzoonotic species. SOURCE: Woolhouse (2005). |
|||||||||
increase in the near future.” Woolhouse and Dye (2001) suggest: “an infectious disease whose incidence is increasing following its first introduction into a new host population or whose incidence is increasing in an existing population as a result of long-term changes in its underlying epidemiology.” In practice, pathogens are usually regarded as emerging or reemerging based on the more subjective judgments of individual investigators, and this must be kept in mind when interpreting any apparent patterns in the data on these organisms.
We obtain counts of emerging and reemerging pathogen species using the updated version of the Taylor et al. (2001) database, as described above.
Total numbers of (re-)emerging pathogen species in the major groups are given in Table 5-1. The grand total is 175, which again differs slightly from the previously published value. The largest group is the viruses and prions, and these, together with the protozoa, are overrepresented in the list of emerging species. An obvious question is whether this reflects genuine differences in the biology and epidemiology of these groups or whether it merely reflects definition bias within the scientific community. But, at the very least, this is a potentially interesting observation that merits further investigation (Woolhouse, 2002).
Zoonotic Pathogens
Zoonoses are defined by the WHO as: “diseases or infections which are naturally transmitted between vertebrate animals and humans.” It has often been noted (e.g., Morse, 1995) that most emerging and reemerging pathogens are zoonotic, but the hypothesis that being zoonotic is in some way associated with emergence cannot be tested formally without also knowing what fraction of nonemerging species are zoonotic.
We can test this hypothesis using the updated version of the Taylor et al. (2001) database. Some care is required in the use of the WHO definition of zoonoses: for example, we do not consider pathogens with complex life cycles, with vertebrate intermediate hosts, and with humans as the only known definitive host as zoonotic. Nor do we consider pathogens—with recent zoonotic origins, but with animal-human transmission no longer being important—as zoonotic, such as the human immunodeficiency viruses (HIV). However, we do include as zoonotic the so-called anthroponoses where the main reservoir is in humans, but other vertebrates can play a minor role in their epidemiology.
Total numbers of zoonotic pathogen species and of zoonotic (re-)emerging pathogen species in the major groups are given in Table 5-1. There are several key observations. Firstly, the number of zoonotic pathogen species is large, comprising 58 percent of the total, over 800 in all. In other words, most human pathogens have a zoonotic component to their epidemiology. Secondly, the zoonotic fraction varies between the major pathogen groups; it is highest for the helminths, over 90 percent of which also infect other vertebrates. Thirdly, it is true that zoonotic species are overrepresented in the list of emerging and reemerging patho-
gens: 74 percent of these are zoonotic, corresponding to a relative risk of 2.1. However, the association is most marked in certain groups, notably the bacteria and the fungi; zoonotic helminths are, in fact, less likely to emerge (Table 5-1).
Cleaveland et al. (2001) looked at the nature of the nonhuman hosts of zoonotic pathogens and for (re-)emerging zoonotic pathogens (noting that many zoonotic pathogens have multiple nonhuman hosts). Perhaps the most striking observation is the breadth of possible nonhuman hosts. Across all zoonotic species the most common nonhuman hosts are carnivores and ungulates (both associated with over 300 species of human pathogen), followed by rodents, primates and nonmammals (all over 100), and then marine mammals and bats (both less than 50). The rank order is very similar for the (re-)emerging species, although these tend to have broader host ranges. Cleaveland et al. also noted that human pathogens were as likely to be associated with wildlife as they were with domestic animals, perhaps reflecting that emerging infectious diseases are often associated with human incursions into previously undeveloped regions.
Species Jumps
So far we have not distinguished between genuinely novel pathogens (emerging) and established pathogens undergoing a resurgence (reemerging). Novel pathogens can be acquired from a variety of sources (e.g., the environmental origins of Legionella pneumophila), but by far the most common source appears to be by jumping into humans from a nonhuman host. Recent examples of species jumps (reviewed in Woolhouse et al., 2005) include: monkeypox virus from prairie dogs (first reported in 2003); SARS coronavirus from (possibly) palm civets (2003); Nipah virus from bats (1999); H5N1 influenza A virus from chickens (1997); Australian bat lyssavirus from bats (1996); the variant Creutzfeldt-Jakob disease agent from cows (1996); Hendra virus from bats (1994); HIV-1 and HIV-2 from primates (1983 and 1986 respectively); Borrelia burgdorferi from (possibly) rodents or birds (1982); and Escherichia coli O157 from cattle (1982).
We can make several observations about this list. First, species jumps appear to be remarkably frequent. If the data are taken at face value then the human species has acquired at least 11 new pathogens from nonhuman reservoirs in the last 25 years. It may be that some of these pathogens are not truly novel and/or that they will quickly disappear from the human population. Even so, it seems highly unlikely that species jumps could have been occurring at this rate over evolutionary time scales. It is, however, more consistent with the idea that most human infectious diseases have been acquired relatively recently (Diamond, 2002). Second, humans can acquire novel pathogens from a wide range of vertebrate hosts—the taxonomic relatedness of host species does not appear to be a major factor constraining jumps. Third, the pathogens in this list are disproportionately single-stranded RNA viruses. We discuss a possible explanation for this below.
A species jump requires several steps to be successfully completed. These have been described as exposure, infection, spread, and adaptation (Antia et al., 2003; Woolhouse et al., 2005). Exposure refers to potentially infectious contacts between the new and existing host populations. This will reflect their geographic distributions, ecologies, and behaviors, as well as the modes of transmission of the pathogen (e.g., vector-borne pathogens do not require close contact between the two hosts).
Infection refers to the ability of the pathogen to invade, survive, and reproduce within the new host—sometimes termed and expression compatibility. Compatibility in turn reflects the pathogen’s host range and the magnitude of any species barriers that inhibit transmission from one host species to another. The determinants of host range are, in general, quite poorly understood. However, for viruses one important determinant is the nature of the receptor that the virus uses to gain access to host cells. Receptors are known for approaching 100 virus species covering most families. Robertson (2002) defined conserved receptors as those with at least 85 percent amino acid sequence homology between humans and mice (as obtained from GenBank) and found a strong association between use of conserved receptors (e.g., vitronectin, coxsackie-adenovirus, and epidermal growth factor receptors) and breadth of host range. This is consistent with the hypothesis that use of conserved receptors predisposes a virus to jump between host species.
Spread refers to the transmission potential of the pathogen in the new host and is usually expressed in terms of the basic reproduction number, R0, defined as the average number of secondary cases produced by a primary case of infection introduced into a large population of previously unexposed hosts. Knowledge of R0 provides a basis for assessing the probability that a pathogen is capable of invading the new host population and causing a major epidemic (May et al., 2001). If R0 is greater than one then each primary case is more than capable of replacing itself and can cause a major epidemic (and will do so provided it does not go extinct through chance events while the number of cases is still low). However, if R0 is less than one then each primary case will, on average, fail to replace itself and the pathogen will die out, although short chains of transmission corresponding to minor outbreaks may still occur. In practice, only a minority of pathogens with zoonotic origins (such as HIV and SARS coronavirus) appear capable of causing major epidemics in humans; the majority (e.g., rabies and, so far, H5N1 influenza A) are not sufficiently transmissible, and most human cases are acquired from the animal reservoir rather than from other humans.
Finally, adaptation refers to genetic changes in the pathogen that occur soon after it enters the new host population and increase its transmission potential and/or its pathogenicity. Adaptive genetic change can occur via a variety of mechanisms: nucleotide substitutions (e.g., canine parvovirus); gene capture (e.g., Salmonella enterica); recombination or reassortment (e.g., influenza virus); or hybridization (e.g., the plant fungal pathogen Phytophthora alni). Adaptations which
increase R0 are of special interest when R0 in the new host population is initially below one. A theoretical analysis by Antia et al. (2003) shows that the probability that the pathogen will successfully invade now becomes a race between how fast the pathogen goes extinct and how rapidly it can adapt to its new host. Pathogens with high rates of genetic change are expected to be far more likely to establish themselves in the new host. This may explain or partly explain why single-stranded RNA viruses appear to be overrepresented among pathogens jumping into the human population: their mutation rate is orders of magnitude greater than that of most DNA-based pathogens.
Pathogen Evolution
The expected course of pathogen evolution, once it has successfully invaded a new host population, is a much discussed topic. A particularly problematic issue is the evolution of virulence (which we take here to be broadly equivalent to pathogenicity, meaning the degree of harm that a pathogen does to its host). The naive expectation that a pathogen should evolve to be less virulent to its host is no longer accepted as a valid generalization; evolutionary biologists prefer to think in terms of an optimum level of virulence that maximizes the chances of successful transmission to other hosts (e.g., Ewald, 1994; Stearns,1999). Even so, in a one-pathogen-one-host system reduced virulence may indeed be favored in many circumstances. However, that is less likely to be the case when dealing with more complex systems (Woolhouse et al., 2001). For a pathogen which coexists in two or more host species, the optimum evolutionary strategy may well be to become more virulent in one of them. If one host is a dead end from which no further transmission occurs, then there are no evolutionary constraints on virulence in that host at all. Emerging pathogens that have jumped from a reservoir host will not have been subject to any evolutionary constraints in the novel host either, and therefore may well, at least initially, be unusually virulent.
In the longer term, pathogen evolution does not happen in isolation; the pathogen and host will coevolve. There are a number of conceptual models of coevolution. These include “arms races” in which genetic changes accumulate in both host and pathogen, and “Red Queen dynamics” in which genetic changes take the form of cycles (see Woolhouse et al., 2002). There are numerous examples of pathogen-host coevolution in the literature, but the majority of these involve invertebrate hosts. The most commonly cited example involving a vertebrate host is the myxoma virus-European rabbit interaction in Australia, in which evolution of the virus from extremely high to more moderate virulence was followed by increased host tolerance of infection and an associated partial recovery of pathogen virulence (Fenner and Fantini, 1999). There are no definitive examples of coevolution involving humans and their pathogens, but evidence consistent with coevolution has been reported for both Plasmodium falciparum and
HIV infections in humans. Even so, the ingredients for coevolution have frequently been reported: there are numerous examples of polymorphisms in genes involved on both sides of human-pathogen interactions and some examples of positive selection in those genes. It seems probable that many features of the human genome (including some genetic polymorphisms that predispose to noninfectious diseases) have been influenced by coevolution with pathogens.
Although pathogens do not evolve independently of their hosts, neither do they do so independently of other pathogens. Pathogens interact in various ways (Woolhouse et al., 2002). Within the host, viruses may share receptors (e.g., sialic acid is used by influenza viruses, reoviruses, and others). Some infections may predispose hosts to other infections; for example, the immunosuppressive effects of HIV or P. falciparum can result in other, opportunistic infections. Conversely, infections may inhibit one another; for example, GB virus C may inhibit the progression of HIV to AIDS. Pathogen interactions can also be indirect, mediated by cross-immunity, such as the smallpox and monkeypox viruses. The interactions can even be at the population level; for example, depletion of the population of susceptible children in the prevaccination era may have led to asynchronous epidemics of measles and whooping cough (Rohani et al., 2003).
Conclusions
We live in a multiple pathogen-multiple host world. A single host species—humans—has over 1,400 recognized pathogen species, over 12 percent of which are regarded as novel and/or causing increasing disease problems. Over half of all human pathogens, and three-quarters of emerging and reemerging pathogens, are shared with at least one other vertebrate host species. Moreover, pathogens not previously known to affect humans apparently make the jump from a nonhuman reservoir every few years.
All of this influences the way we need to think about host-pathogen interactions. Having multiple host species can have a major impact on pathogen epidemiology, as can sharing a single host species with other pathogens. What is more, pathogens evolve, sometimes very rapidly, but they do not evolve in isolation either from their hosts or other pathogens of those hosts. In such circumstances, we cannot view a single host-pathogen interaction in isolation, and any changes that occur, whether unplanned or as a result of human interventions, may have complex and perhaps unexpected effects.
Finally, taking a multihost-multipathogen perspective underlines the importance of a multidisciplinary approach to infectious disease problems. This in turn requires better communication, whether between medical, veterinary, and biological scientists, between virologists, bacteriologists, mycologists, and parasitologists, or between molecular geneticists, immunologists, pathologists, epidemiologists, and evolutionary biologists.
HOST RANGE AND EMERGING AND REEMERGING PATHOGENS4
Mark E.J. Woolhouse5and Sonya Gowtage-Sequeria6
An updated literature survey identified 1,407 recognized species of human pathogen, 58% of which are zoonotic. Of the total, 177 are regarded as emerging or reemerging. Zoonotic pathogens are twice as likely to be in this category as are nonzoonotic pathogens. Emerging and reemerging pathogens are not strongly associated with particular types of nonhuman hosts, but they are most likely to have the broadest host ranges. Emerging and reemerging zoonoses are associated with a wide range of drivers, but changes in land use and agriculture and demographic and societal changes are most commonly cited. However, although zoonotic pathogens do represent the most likely source of emerging and reemerging infectious disease, only a small minority have proved capable of causing major epidemics in the human population.
A recent, comprehensive literature survey of human pathogens listed >1,400 different species (Taylor et al., 2001), more than half known to be zoonotic, i.e., able to infect other host species (Taylor et al., 2001; Woolhouse et al., 2001). The survey data showed that those pathogens regarded as emerging and reemerging were more likely to be zoonotic than those that are not (Cleaveland et al., 2001; Taylor et al., 2001), confirming an association between these characteristics which had long been suspected (IOM, 2003; Morse, 1995), but which could not be formally demonstrated without denominator data as well as numerator data.
Here, we revisit these calculations, using updated information on the biology and epidemiology of recognized human pathogens. We pay close attention to possible differences between the major pathogen groups—viruses, bacteria, fungi, protozoa, and helminths. We also examine in detail the relationship between host range and pathogen emergence or reemergence, considering both the type and diversity of nonhuman hosts. We catalog the kinds of proximate factors or drivers that have been linked with pathogen emergence and reemergence and ask whether these differ between the major pathogen groups or between zoonotic and nonzoonotic pathogens.
We focus mainly on pathogen diversity (as numbers of species) rather than on the effects of disease that they impose, noting that many diseases, e.g., infant
|
4 |
Reprinted from Woolhouse, MEJ and S. Gowtage-Sequeria. 2005. Host Range and Emerging and Reemerging Pathogens. Emerging Infectious Diseases 11(12):1842–1847. Available online at http://www.cdc.gov/ncidod/EID/vol11no12/pdfs/05-0997.pdf |
|
5 |
Centre for Infectious Diseases, University of Edinburgh, Edinburgh, United Kingdom. Address for correspondence: M.E.J. Woolhouse, Centre for Infectious Diseases, University of Edinburgh, Ashworth Laboratories, Kings Buildings, West Mains Rd. Edinburgh EH9 3JT, UK; fax: 44-131-650-6564; email: mark.woolhouse@ed.ac.uk |
|
6 |
Centre for Infectious Diseases, University of Edinburgh, Edinburgh, United Kingdom. |
diarrhea, can be caused by more than one species of pathogen. However, we comment on the transmissibility of pathogens once they have been introduced into the human population because transmissibility is an important determinant of the potential public health problem.
Methods
We obtained counts of pathogen species from an updated version of the previously published database (Taylor et al., 2001). As before, we defined a human pathogen as “a species infectious to and capable of causing disease in humans under natural transmission conditions.” We included pathogens that have only been reported as causing a single case of human disease and those that only cause disease in immunocompromised persons. We also included instances of accidental laboratory infection but excluded infections resulting from deliberate exposure in the laboratory. We added recently recognized pathogens listed online by the Centers for Disease Control and Prevention, ProMED, and elsewhere (CDC, 2003; Ecker et al., 2005; ProMed, 2001; WHO, 2006). We obtained taxonomic classifications online from the International Committee on Taxonomy of Viruses, the National Centre for Biotechnology Information, the CAB International Bioscience database of fungal names, and from standard texts (CAB International Bioscience, 2004; Center for Biotechnology Information, 2000; Collier et al., 1998; International Committee on the Taxonomy of Viruses, 2005; Schmidt and Roberts, 2000).
Pathogen species were categorized as emerging or reemerging based on previously published reviews of the literature (Cleaveland et al., 2001; Taylor et al., 2001), again updated from online sources (CDC, 2003; ProMed, 2001; WHO, 2006). A species was regarded as emerging or reemerging if any recognized variant fell into this category (e.g., Escherichia coli O157, H5N1 influenza A).
We considered the following pathogen groups: viruses (including prions), bacteria (including rickettsia), fungi (including microsporidia), protozoa, and helminths. We did not consider ectoparasites (ticks and lice). Each group was further divided into subgroups (families) to test whether biases existed in numbers of emerging and reemerging species at this level. The viruses were also divided according to genome type (e.g., negative single stranded RNA viruses).
We examined three aspects of host range, both for all pathogens combined and separately for each of the viruses, bacteria, fungi, protozoa, and helminths. First, we distinguished pathogen species according to whether they were known to be zoonotic, using the WHO definition “diseases or infections which are naturally transmitted between vertebrate animals and humans” (WHO, 1959). Note that this definition includes pathogens for which humans are the main host and other vertebrates are only occasional hosts, as well as the opposite, but excludes purely human pathogens that recently evolved from nonhuman pathogens, e.g., HIV. We then compared the fraction of emerging or reemerging species that were
or were not zoonotic across the major pathogen groups and within each group by family.
Second, for all zoonotic species we identified the types of nonhuman vertebrate host they are known to infect, using the following broad categories: bats, carnivores, primates, rodents, ungulates, and other mammals and nonmammals (including birds, reptiles, amphibians, and fish). We excluded vertebrate intermediate hosts of parasites with complex life cycles. Host types were ranked by the number of zoonotic pathogen species associated with them, and rankings were compared by using Spearman rank correlation coefficient.
Third, we obtained a crude index of the breadth of host range by counting the number of the host types that each pathogen species is known to infect: 0 (i.e., not zoonotic), 1, 2, and 3 or more. We compared the fraction of emerging and reemerging species across these four classes.
For the emerging and reemerging pathogen species, we identified the main factors believed to drive their increased incidence, geographic range, or both, by conducting a systematic review of the emerging diseases literature. We allocated these drivers to 1 or more broad categories (Table 5-2). Note that although we chose categories that we considered to be useful and informative for our immediate purposes, and which were similar to those listed elsewhere (IOM, 2003), this is inevitably a subjective procedure and alternative categorizations may be equally valid. We then ranked the drivers (by number of emerging and reemerging pathogen species associated with each) and compared the ranking of drivers for the major pathogen groups and for zoonotic versus nonzoonotic pathogens.
For the zoonotic species, we distinguished those known to be transmissible between humans, allowing that this might be through an indirect route (e.g., a vector or an intermediate host), from those for which humans can only acquire infection (directly or indirectly) from a nonhuman source. For the transmissible
TABLE 5-2 Main Categories of Drivers Associated with Emergence and Reemergence of Human Pathogens
|
Rank* |
Driver |
|
1 |
Changes in land use or agricultural practices |
|
2 |
Changes in human demographics and society |
|
3 |
Poor population health (e.g., HIV, malnutrition) |
|
4 |
Hospitals and medical procedures |
|
5 |
Pathogen evolution (e.g., antimicrobial drug resistance, increased virulence) |
|
6 |
Contamination of food sources or water supplies |
|
7 |
International travel |
|
8 |
Failure of public health programs |
|
9 |
International trade |
|
10 |
Climate change |
|
*Ranked by the number of pathogen species associated with them (most to least). |
|
zoonotic species, we further distinguished those that are sufficiently transmissible to cause major epidemics in human populations from those that cause only relatively minor outbreaks. This classification was intended to distinguish between pathogens with R0>1 in humans from those with R0<1, where R0 is the basic reproduction number, i.e., the average number of secondary infections produced by a single primary infection introduced into a large population of previously unexposed hosts. Direct estimates of R0 are unavailable for most zoonotic pathogens.
Throughout the study, we quantified associations as the relative risk (RR) and tested for statistical significance using a standard χ2 test (with correction for small expected values). Although these statistical analyses are susceptible to bias introduced by related species (e.g., several species of hantavirus exist, most of which are zoonotic and many of which are regarded as emerging or reemerging), the analysis at the family level is an indication of the extent of any such bias.
Results
The survey of human pathogens produced a count of 1,407 human pathogen species, with 177 (13%) species regarded as emerging or reemerging (online Appendix, available at www.cdc.gov/ncidod/EID/vol11no12/05-0997_app.htm). Of all pathogen species, 208 are viruses or prions, including 77 (37%) regarded as emerging or reemerging. For bacteria, the counts were 538 and 54 (10%), respectively; for fungi, 317 and 22 (7%), respectively; for protozoa, 57 and 14 (25%), respectively; and for helminths, 287 and 10 (3%), respectively. These numbers differ slightly from those previously published (Cleaveland et al., 2001; Taylor et al., 2001) as a result of adjustments to taxonomies and the discovery of previously unknown pathogen species. Clear differences were found between the pathogen groups (χ24 = 154.3, p<<0.001), with viruses greatly overrepresented among emerging and reemerging pathogens and helminths underrepresented.
Pathogen Taximony
More than 20 virus families contain human pathogens, with just 4, the Bunyaviridae, Flaviviridae, Togaviridae, and Reoviridae, accounting for more than half of the species affecting humans and, likewise, more than half of the emerging and reemerging species. Overall, no significant difference was found between the nine largest families (pooling the remainder) in the fraction of species regarded as emerging or reemerging (χ29 = 14.9, p = 0.09). Nor were any significant differences found according to genome type, e.g., between RNA and DNA viruses (χ21 = 0.77, p = 0.38) or between positive and negative single-stranded RNA viruses (χ21 = 3.1, p = 0.08).
More than 60 bacteria families contain human pathogens; the enterobacteria and the mycobacteria account for the most species and for the most emerging and
reemerging species. Overall, no significant difference was found between the six largest families (pooling the remainder) in the fraction of species regarded as emerging or reemerging (χ26 = 13.6, p = 0.14). Numbers of species of emerging and reemerging fungi, protozoa, and helminthes were too small for meaningful comparisons between families, but no indication was found that emerging and reemerging species are concentrated in any particular taxa.
Host Range
Of the 1,407 human pathogen species, 816 (58%) are known to be zoonotic. In comparison, of the 177 emerging or reemerging pathogens, 130 (73%) are known to be zoonotic. This corresponds to an RR of 2.0 and confirms the expectation that zoonotic pathogens are disproportionately likely to be associated with emerging and reemerging infectious diseases. This pattern varies somewhat across the different pathogen groups: for bacteria and fungi the association is strongest with RRs of 4.0 and 3.2, respectively; for viruses and protozoa, no obvious association was found, with RRs of 1.2 and 0.9, respectively; and for helminths (which are almost all zoonotic but very rarely emerging or reemerging), RR is 0.3. However, the numbers involved are small (particularly for protozoa and helminths), and these differences were not statistically significant (χ24 = 4.03, p = 0.40).
All the defined host types are potential sources of zoonotic infections, but differences occurred in their importance (ranked by number of pathogen species supported) across viruses, bacteria, fungi, protozoa, and helminths and no 1 type consistently dominates (Figure 5-3A), although ungulates are the most important overall, supporting over 250 species of human pathogen. Emerging and reemerging pathogens show similar trends (Figure 5-3B), with ungulates again the most important overall, supporting over 50 species. In general, ranking of host types in terms of numbers of species correlates well both overall (rs = 0.79, n = 7, p<0.05) and individually for each pathogen group. The general impression is that the emerging and reemerging zoonotic pathogens are not unusual in the types of nonhuman hosts they infect.
However, when the fraction of emerging and reemerging species is compared with the breadth of host range (as the number of host types other than humans), a pattern becomes apparent (Figure 5-4). Overall, the fraction tends to increase with host range: >40% of pathogens with the broadest host ranges (3 or more types of nonhuman host) are emerging or reemerging (exact p = 0.042). However, this trend does not hold for the protozoa and helminthes (although the numbers for these groups are small).
Drivers of Emergence
We identified 10 main categories of drivers of emergence and reemergence and ranked these by the total number of pathogen species associated with them
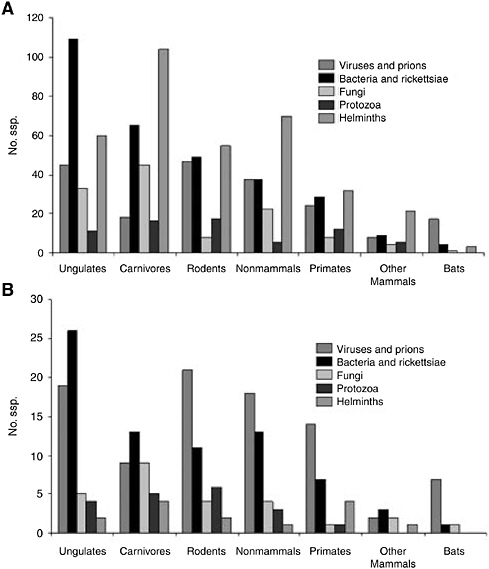
FIGURE 5-3 Numbers of species of zoonotic pathogens associated with different types of nonhuman host. Note that some pathogens are associated with >1 host. A) All zoonotic species. B) Emerging and reemerging zoonotic species only.
(Table 5-2). The ranking of drivers across different categories of pathogen showed poor concordance (e.g., Spearman rank correlation for bacteria vs. viruses, rs = 0.41, n = 10, p = 0.24). The most striking discrepancies were as follows: (1) the marked association of emerging or reemerging fungi with hospitalization, poor population health, or both; (2) the greater importance of pathogen evolution and
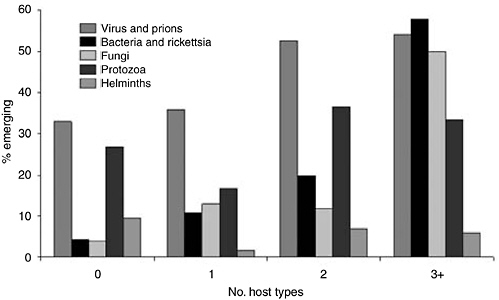
FIGURE 5-4 Relationship between breadth of host range (as number of nonhuman host types, as listed in Figure 5-3) and the fraction of pathogen species regarded as emerging or reemerging. A total of 122 zoonotic species (10 of them emerging or reemerging) for which the host range is unknown are omitted.
contaminated food and water and the lesser importance of international travel and changes in land use and agriculture for bacteria in comparison with viruses; and (3) the greater importance of changing land use and agriculture for zoonoses than for nonzoonoses.
Transmissibility
Overall, most zoonotic pathogens are either not transmissible (directly or indirectly) between humans at all (i.e., humans are a dead-end host) or are only minimally transmissible. Examples include rabies virus, Rift Valley fever virus, and Borrelia burgdorferi (the agent of Lyme disease). A small minority (≈10%) of pathogen species that are technically zoonotic are, in fact, spread almost exclusively from person to person (e.g., Mycobacterium tuberculosis or measles virus) or can do so once successfully introduced from a nonhuman source (e.g., some strains of influenza A, Yersinia pestis, or severe acute respiratory syndrome (SARS) coronavirus). However, a substantial minority of zoonotic pathogens (about 25%, i.e., 200 species) are capable of some person-to-person transmission but do not persist without repeated reintroductions from a nonhuman reservoir (e.g., E. coli O157, Trypanosoma brucei rhodesiense, or Ebola virus). This pattern is fairly consistent across the major pathogen groups.
Discussion
Humans are affected by an impressive diversity of pathogens; 1,407 pathogenic species of viruses, bacteria, fungi, protozoa, and helminths are currently recognized. Of this total, 177 (13%) pathogen species are considered emerging or reemerging. This number must be viewed with some caution, given that these terms are still used somewhat subjectively. More rigorous definitions of emerging and reemerging have been proposed (IOM, 2003; OIE, 2006; Woolhouse and Dye, 2001), but these are difficult to apply universally because they require long-term data on distributions and incidences which are available for only a small subset of infectious diseases (e.g., malaria [Hay et al., 2004] and tuberculosis [Corbett et al., 2003]). Moreover, the counts of emerging and reemerging pathogen species reported here are subject to ascertainment bias. Despite these caveats, our results suggest that pathogens associated with emerging and reemerging diseases share some common features.
First, emerging and reemerging pathogens are disproportionately viruses, although they are not disproportionately different kinds of viruses. Numerically, RNA viruses dominate, comprising 37% of all emerging and reemerging pathogens. RNA viruses are also prominent among the subset of emerging pathogens that have apparently entered the human population only in the past few decades, such as HIV or the SARS coronavirus (Burke, 1998; Woolhouse et al., 2005). A possible explanation for this observation is that much higher nucleotide substitution rates for RNA viruses permit more rapid adaptation, greatly increasing the chances of successfully invading a new host population (Burke, 1998; Woolhouse et al., 2005).
Second, emerging and reemerging pathogens are not strongly associated with particular nonhuman host types, although emerging and reemerging pathogens more often are those with broad host ranges that often encompass several mammalian orders and even nonmammals. This pattern is consistent across the major pathogen groups. The determinants of host range in general remain poorly understood, but among viruses for which the cell receptor is known, an association exists between host range and whether the receptor is phylogenetically conserved (as measured by the homology of the human and mouse amino acid sequences) (Woolhouse, 2002).
Emerging and reemerging pathogens have been likened to weeds (Dobson and Foufopoulos, 2001), and that the associations reported above are likely reflecting underlying “weediness,” that is, a degree of biologic flexibility that makes certain pathogens adept at taking advantage of new epidemiologic opportunities. This characteristic seems to be reflected in the broad range of drivers of the emergence or reemergence of pathogens, ranging from changes in land use and agriculture, through hospitalization to international travel. Although some drivers are numerically more important than others, the overall impression is that pathogens are exploiting almost any change in human ecology that provides new opportuni-
ties for transmission, either between humans or to humans from a nonhuman source.
Even if a pathogen is capable of infecting and causing disease in humans, most zoonotic pathogens are not highly transmissible within human populations and do not cause major epidemics. The possible magnitude of an infectious disease outbreak is related to the basic reproduction number, R0 (Figure 5-5). For pathogens that are minimally transmissible within human populations (R0 close to 0), outbreak size is determined largely by the number of introductions from the reservoir. For pathogens that are highly transmissible within human populations (R0>>1), outbreak size is determined largely by the size of the susceptible population. For pathogens that are moderately transmissible within human populations (corresponding to R0 ≈1), notable outbreaks are possible (especially if multiple introductions occur), but the scale of these outbreaks is very sensitive to small changes in R0. In other words, small changes in the nature of the host-pathogen interaction can lead to large increases (or decreases) in the scale of the public health problem (Figure 5-5). Such pathogens may be likely sources of emerging infectious disease problems in the future. However, we currently have no way of predicting whether a novel human pathogen will behave like rabies (frequently introduced into the human population, but not capable of causing major epidemics) or HIV (probably rarely introduced, but capable of causing a global pandemic).
In conclusion, this study suggests that biologic and epidemiologic correlates of pathogen emergence or reemergence may be identified. However, the most striking feature of emerging and reemerging pathogens is their diversity (online Appendix). For this reason, surveillance and monitoring of infectious disease trends may have to be broadly targeted to be most effective. Given that three-fourths of emerging and reemerging pathogens are zoonotic, in many cases this targeting might usefully be extended beyond at-risk human populations to include populations of potential animal reservoirs.
Acknowledgments
We thank Louise Taylor and Sophie Latham for their work on the original database and Ben Evans for his contribution to the updated database.
Dr. Woolhouse is professor of infectious disease epidemiology in the Centre for Infectious Diseases at the University of Edinburgh. His research interests include foot-and-mouth disease, E. coli O157, scrapie, and sleeping sickness. He is an advisor to the UK government on issues relating to infectious disease epidemiology.
Dr. Gowtage-Sequeira is a postdoctoral research assistant in the Division of Animal Health and Welfare at the University of Edinburgh. Her doctoral research, for the Institute of Zoology in London, was on the epidemiology of viral infections of canids in Namibia. She is currently studying the ecology of wild dogs in eastern Kenya.
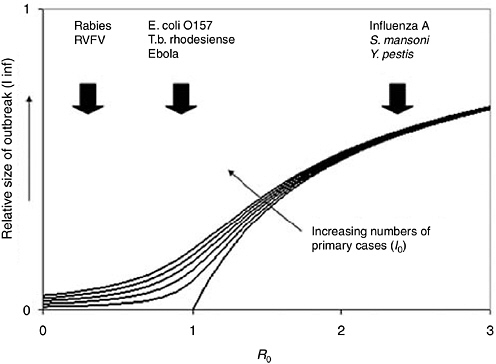
FIGURE 5-5 Expected relationship between outbreak size (as fraction of the population affected) and two key epidemiologic parameters: I0 is the number of primary cases of infection introduced into the human population from an external source such as a zoonotic reservoir (increasing in the direction indicated); R0 is the basic reproduction number, a measure of the transmissibility of the infection with the human population (see text). The curves are obtained from a modified version of the Kermack-McKendrick equation and show that expected outbreak size is particularly sensitive to small changes in I0 or R0 when R0 is close to 1. Examples of zoonotic pathogens with R0>1, R0<1 and R0 close to 1 are shown. RIVF, Rift Valley fever virus. (Reprinted with permission from [Woolhouse, 2002]).
REFERENCES
Adey NB, Lei M, Howard MT, Jensen JD, Mayo DA, Butel DL, Coffin SC, Moyer TC, Amann RI, Ludwig W, Schleifer KH. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiology Review 59(1):143–169.
Anderson RC, Su X, Bogdan GJ, Fenton J. 2000. A miniature integrated device for automated multi-step genetic assays. Nucleic Acids Research 28(12):E60.
Antia R, Regoes RR, Koella JC, Bergstrom CT. 2003. The role of evolution in the emergence of infectious diseases. Nature 426(6967):658–661.
Asbury CL, Diercks AH, van den Engh G. 2002. Trapping of DNA by dielectrophoresis. Electrophoresis 23(16):2658–2666.
Baum M, Bielau S, Rittner N, Schmid K, Eggelbusch K, Dahms M, Schlauersbach A, Tahedl H, Beier M, Guimil R, Scheffler M, Hermann C, Funk JM, Wixmerten A, Rebscher H, Honig M, Andreae C, Buchner D, Moschel E, Glathe A, Jager E, Thom M, Greil A, Bestvater F, Obermeier F, Burgmaier J, Thome K, Weichert S, Hein S, Binnewies T, Foitzik V, Muller M, Stahler CF, Stahler PF. 2003. Validation of a novel, fully integrated and flexible microarray benchtop facility for gene expression profiling. Nucleic Acids Research 31(23):E151.
Bavykin SG, Akowski JP, Zakhariev VM, Barsky VE, Perov AN, Mirzabekov AD. 2001. Portable system for microbial sample preparation and oligonucleotide microarray analysis. Applied Environmental Microbiology 67(2):922–928.
Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. 1998. Periodontitis: A risk factor for coronary heart disease? Annals of Periodontology 3(1):127–141.
Bradshaw DJ, Marsh PD. 1998. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Research 32(6):456–462.
Burke DS. 1998. Evolvability of emerging viruses. In: Pathology of emerging infections 2, Nelson AM and Horsburgh CR, ed. Washington, D.C: American Society for Microbiology. Pp. 1–12.
CAB International Bioscience. 2004. Index fungorum. [Online]. Available at: http://194.131.255.4/Names/Names.asp [accessed May 2, 2006].
CDC (Centers for Disease Control and Prevention). 2003. Emerging Infectious Diseases. [Online]. Available at: www.cdc.gov/ncidod/diseases/eid/index.htm [accessed May 2, 2006].
Cheek BJ, Steel AB, Torres MP, Yu YY, Yang H. 2001. Chemiluminescence detection for hybridization assays on the flow-thru chip, a three-dimensional microchannel biochip. Analytical Chemistry 73(24):5777–5783.
Cleaveland S, Laurenson MK, Taylor LH. 2001. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(1411):991–999.
Collier L, Balows A, Sussman M. eds. 1998. Topley and Wilson’s Microbiology and Microbial Infection, Volume 4: Medical Mycology. London, UK: Arnold.
Cummings CA, Relman DA. 2000. Using DNA microarrays to study host-microbe interactions. Emerging Infectious Diseases 6(5):513–525.
Cummings CA, Brinig MM, Lepp PW, van de Pas S, Relman DA. 2004. Bordetella species are distinguished by patterns of substantial gene loss and host adaptation. Journal of Bacteriology 186(5):1484–1492.
Cunningham DD. 2001. Fluidics and sample handling in clinical chemical analysis. Analytica Chimica Acta 429:1–18.
de Jong MH, van der Hoeven JS, van Os JH. 1984. Growth of oral Streptococcus species and Actinomyces viscosus in human saliva. Applied Environmental Microbiology 47(5):901–904.
Diamond J. 2002. Evolution, consequences and future of plant and animal domestication. Nature 418(6898):700–707.
Dill K, Montgomery DD, Ghindilis AL, Schwarzkopf KR. 2004. Immunoassays and sequence-specific DNA detection on a microchip using enzyme amplified electrochemical detection. Journal of Biochemical and Biophysical Methods 59(2):181–187.
Dobson A, Foufopoulos J. 2001. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(1411):1001–1012.
Ecker DJ, Sampath R, Willett P, Wyatt JR, Samant V, Massire C, Hall TA, Hari K, McNeil JA, Büchen-Osmond C, Budowle B. 2005. The Microbial Rosetta Stone database: a compilation of global and emerging infectious microorganisms and bioterrorist threat agents. BMC Microbiology 5(1):19.
El Fantroussi S, Urakawa H, Bernhard AE, Kelly JJ, Noble PA, Smidt H, Yershov GM, Stahl DA. 2003. Direct profiling of environmental microbial populations by thermal dissociation analysis of native rRNAs hybridized to oligonucleotide microarrays. Applied Environmental Microbiology 69(4):2377–2382.
Ewald PW. 1994. The Evolution of Infectious Diseases. New York: Oxford University Press.
Fenner F, Fantini B. 1999. Biological Control of Vertebrate Pests: The History of Myxomatosis—an Experiment in Evolution. Wallingford, CT: CABI Publishing.
Fitzgerald JR, Sturdevant DE, Mackie SM, Gill SR, Musser JM. 2001. Evolutionary genomics of Staphylococcus aureus: Insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic [online]. Proceedings of the National Academy of Sciences USA 98(15):8821–8826.
Fotin AV, Drobyshev AL, Proudnikov DY, Perov AN, Mirzabekov AD. 1998. Parallel thermodynamic analysis of duplexes on oligodeoxyribonucleotide microchips. Nucleic Acids Research 26(6):1515–1521.
Gardeniers JG, van den Berg A. 2004. Lab-on-a-chip systems for biomedical and environmental monitoring. Analytical and Bioanalytical Chemistry 378(7):1700–1703.
Glurich I, Grossi S, Albini B, Ho A, Shah R, Zeid M, Baumann H, Genco RJ, De Nardin E. 2002. Systemic inflammation in cardiovascular and periodontal disease: Comparative study. Clinical and Diagnostic Laboratory Immunology 9(2):425–432.
Greenstein G, Lamster I. 2000. Changing periodontal paradigms: Therapeutic implications. International Journal of Periodontics and Restorative Dentistry 20(4):336–357.
Grossi SG, Genco RJ. 1998. Periodontal disease and diabetes mellitus: A two-way relationship. Annals of Periodontology 3(1):51–61.
Guggenheim M, Shapiro S, Gmür R, Guggenheim B. 2001. Spatial arrangements and associative behavior of species in an in vitro oral biofilm model. Applied Environmental Microbiology 67(3):1343–1350.
Guschin DY, Mobarry BK, Proudnikov D, Stahl DA, Rittmann BE, Mirzabekov AD 1997. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Applied Environmental Microbiology 63(6):2397–2402.
Haffajee AD, Socransky SS. 2001. Relationship of cigarette smoking to the subgingival microbiota. Journal of Clinical Periodontology 28(5):377–388.
Haffajee AD, Smith C, Torresyap G, Thompson M, Guerrero D, Socransky SS. 2001. Efficacy of manual and powered toothbrushes (II). Effect on microbiological parameters. Journal of Clinical Periodontology 28(10):947–954.
Huang Y, Mather EL, Bell JL, Madou M. 2002. MEMS-based sample preparation for molecular diagnostics. Analytical and Bioanalytical Chemistry 372(1):49–65.
International Committee on the Taxonomy of Viruses. 2005. Index virum. [Online]. Available at: http://life.anu.edu.au/viruses/Ictv/index.html [accessed May 10, 2005].
IOM (Institute of Medicine). 2003. Microbial Threats to Health: Emergence, Detection, and Response. Washington, D.C: The National Academies Press.
Kinane DF, Lowe GD. 2000. How periodontal disease may contribute to cardiovascular disease. Periodontology 23:121–126.
Kinane DF, Marshall GJ. 2001. Periodontal manifestations of systemic disease. Australian Dental Journal 46(1):2–12.
Kolenbrander PE. 2000. Oral microbial communities: Biofilms, interactions, and genetic systems. Annual Reviews in Microbiology 54:413–437.
Kolenbrander PE, Andersen RN, Kazmerzak K, Wu R, Palmer RJ Jr. 1999. Spatial organization of oral bacteria in biofilms. Methods in Enzymology 310:322–332.
Kroes I, Lepp PW, Relman DA. 1999. Bacterial diversity within the human subgingival crevice. Proceedings of the National Academy of Sciences USA 96(25):14547–14552.
Kuo WP, Whipple ME, Sonis ST, Ohno-Machado L, Jenssen TK. 2002. Gene expression profiling by DNA microarrays and its application to dental research. Oral Oncology 38(7):650–656.
Kuo WP, Whipple ME, Jenssen TK, Todd R, Epstein JB, Ohno-Machado L, Sonis ST, Park PJ. 2003. Microarrays and clinical dentistry. Journal of the American Dental Association 134(4):456–462; comment 134(4):1046.
Lawrence HP. 2002. Salivary markers of systemic disease: Noninvasive diagnosis of disease and monitoring of general health. Journal of the Canadian Dental Association 68(3):170–174.
Lepp PW, Brinig MM, Ouverney CC, Palm K, Armitage GC, Relman DA. 2004. Methanogenic Archaea and human periodontal disease. Proceedings of the National Academy of Sciences USA 101(16):6176–6181.
Li Y, Zhou X, St. John MA, Wong DT. 2004. RNA profiling of cell-free saliva using microarray technology. Journal of Dental Research 83(3):199–203.
Liu RH, Lenigk R, Druyor-Sanchez RL, Yang J, Grodzinski P. 2003. Hybridization enhancement using cavitation microstreaming. Analytical Chemistry 75(8):1911–1917.
Liu RH, Yang J, Lenigk R, Bonanno J, Grodzinski P. 2004. Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection. Analytical Chemistry 76(7):1824–1831.
Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL Jr, Beck JD, Offenbacher S. 2001. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Annals of Periodontology 6(1):175–182.
May RM, Gupta S, McLean AR. 2001. Infectious disease dynamics: What characterizes a successful invader? Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(1410):901–910.
Mayo MA. 2002. A summary of taxonomic changes recently approved by ICTV. Archives of Virology 147(8):1655–1663.
Mercado FB, Marshall RI, Klestov AC, Bartold PM. 2001. Relationship between rheumatoid arthritis and periodontitis. Journal of Periodontology 72(6):779–787.
Miller LS, Manwell MA, Newbold D, Reding ME, Rasheed A, Blodgett J, Kornman KS. 1992. The relationship between reduction in periodontal inflammation and diabetes control: A report of 9 cases. Journal of Periodontology 63(10):843–848.
Moore WE, Moore LV. 1994. The bacteria of periodontal diseases. Periodontology 2000 5:66–77.
Morse SS. 1995. Factors in the emergence of infectious diseases. Emerging Infectious Diseases 1(1): 7–15.
National Center for Biotechnology Information. 2000. Taxonomy browser. [Online]. Available at: www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/ [accessed May 2, 2006].
Offenbacher S, Jared HL, O’Reilly PG, Wells SR, Salvi GE, Lawrence HP, Socransky SS, Beck JD. 1998. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Annals of Periodontology 3(1):233–250.
Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, McKaig RG, Jared HL, Mauriello SM, Auten RL Jr, Herbert WN, Beck JD. 2001. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Annals of Periodontology 6(1):164–174.
OIE (World Organization for Animal Health). 2006. Terrestrial animal health code. General definitions. [Online]. Available at: www.oie.int [accessed May 2, 2006].
Palmer RJ Jr, Kazmerzak K, Hansen MC, Kolenbrander PE. 2001. Mutualism versus independence: Strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infection and Immunity 69(9):5794–5804.
Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A and Dewhirst FE. 2001. Bacterial diversity in human subgingival plaque. Journal of Bacteriology 183(12): 3770–3783.
ProMED. 2001. The ProMED-mail archives. [Online]. Available at: www.promedmail.org [accessed May 2, 2006].
Relman DA. 1999. The search for unrecognized pathogens. Science 284(5418):1308–1310.
Robertson J. 2002. Molecular biology of emerging diseases [BSc thesis]. University of Edinburgh, Scotland.
Rohani P, Green CJ, Mantilla-Beniers NB, Grenfell BT. 2003. Ecological interference between fatal diseases. Nature 422(6934):885–888.
Rudney JD. 2000. Saliva and dental plaque. Advances in Dental Research 14:29–39.
Saitou N, Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406–425.
Sakamoto M, Umeda M, Ishikawa I, Benno Y. 2000. Comparison of the oral bacterial flora in saliva from a healthy subject and two periodontitis patients by sequence analysis of 16S rDNA libraries. Microbiology and Immunology 44(8):643–652.
Santacroce R, Ratti A, Caroli F, Foglieni B, Ferraris A, Cremonesi L, Margaglione M, Seri M, Ravazzolo R, Restagno G, Dallapiccola B, Rappaport E, Pollak ES, Surrey S, Ferrari M, Fortina P. 2000. Analysis of clinically relevant single-nucleotide polymorphisms by use of microelectronic array technology. Clinical Chemistry 48(12):2124–2130.
Scannapieco FA. 1998. Position paper of The American Academy of Periodontology: Periodontal disease as a potential risk factor for systemic diseases. Journal of Periodontology 69(7): 841–850.
Scannapieco FA, Papandonatos GD, Dunford RG. 1998. Associations between oral conditions and respiratory disease in a national sample survey population. Annals of Periodontology 3(1): 251–256.
Schena M, Shalon D, Davis RW, Brown PO. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270(5235):467–470; comment 270(5235): 368–369, 371.
Schmidt GD, Roberts LS. 2000. Foundations of Parasitology. 6th ed. New York: McGraw Hill Higher Education.
Slavkin HC. 1998. Toward molecularly based diagnostics for the oral cavity. Journal of the American Dental Association 129(8):1138–1143.
Small J, Call DR, Brockman FJ, Straub TM, Chandler DP. 2001. Direct detection of 16S rRNA in soil extracts by using oligonucleotide microarrays. Applied Environmental Microbiology 67(10): 4708–4716.
Smoot JC, Barbian KD, Van Gompel JJ, Smoot LM, Chaussee MS, Sylva GL, Sturdevant DE, Ricklefs SM, Porcella SF, Parkins LD, Beres SB, Campbell DS, Smith TM, Zhang Q, Kapur V, Daly JA, Veasy LG, Musser JM. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proceedings of the National Academy of Sciences USA 99(7):4668–4673.
Smoot LM, Smoot JC, Smidt H, Noble PA, Konneke M, McMurry ZA, Stahl DA. 2005. DNA microarrays as salivary diagnostic tools for characterizing the oral cavity’s microbial community. Advances in Dental Research 18(1):6–11.
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. 1998. Microbial complexes in subgingival plaque. Journal of Clinical Periodontology 25(2):134–144.
Stahl DA. 1995. Application of phylogenetically based hybridization probes to microbial ecology. Molecular Ecology 4:535–542.
Stearns SC, ed. 1999. Evolution in Health & Disease. Oxford, UK: Oxford University Press.
Stears RL, Martinsky T, Schena M. 2003. Trends in microarray analysis. Nature Medicine 9(1): 140–145.
Streckfus CF, Bigler LR. 2002. Saliva as a diagnostic fluid. Oral Diseases 8(2):69–76.
Strunk O, Ludwig W. 1995. A Software Environment for Sequence Data. Munich, Germany: Department of Microbiology, Technical University of Munich.
Taroncher-Oldenburg G, Griner EM, Francis CA, Ward BB. 2003. Oligonucleotide microarray for the study of functional gene diversity in the nitrogen cycle in the environment. Applied Environmental Microbiology 69(2):1159–1171.
Taylor LH, Latham SM, Woolhouse MEJ. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(1411):983–989.
Teng YT, Taylor GW, Scannapieco F, Kinane DF, Curtis M, Beck JD, Kogon S. 2002. Periodontal health and systemic disorders. Journal of the Canadian Dental Association 68(3):188–192.
Tenovuo J. 1998. Antimicrobial function of human saliva—how important is it for oral health? Acta Odontologica Scandinavica 56(5):250–256.
Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. 2001. Aspiration pneumonia: Dental and oral risk factors in an older veteran population. Journal of American Geriatrics Society 49(5):557–563.
Umeda M, Contreras A, Chen C, Bakker I, Slots J. 1998. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. Journal of Periodontology 69(7):828–833.
von Troil-Lindén B, Torkko H, Alaluusua S, Jousimies-Somer H, Asikainen S. 1995. Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. Journal of Dental Research 74(11):1789–1795.
Wecke J, Kersten T, Madela K, Moter A, Göbel UB, Friedmann A, Bernimoulin J. 2000. A novel technique for monitoring the development of bacterial biofilms in human periodontal pockets. Federation of European Microbiology Societies (FEMS) Microbiology Letters 191(1):95–101.
Wilson KH, Wilson WJ, Radosevich JL, DeSantis TZ, Viswanathan VS, Kuczmarski TA, Andersen GL. 2002. High-density microarray of small-subunit ribosomal DNA probes. Applied Environmental Microbiology. 68(5):2535–2541.
Woolhouse MEJ. 2002. Population biology of emerging and re-emerging pathogens. Trends in Microbiology 10(10 Suppl):S3–S7.
Woolhouse MEJ. 2005 (March 17). Session II: Ecology of the Host-Microbe Interactions. Presentation at the Forum on Microbial Threats Workshop Ending the War Metaphor: The Changing for Unraveling the Host-Microbe Relationship, Washington, D.C., Institute of Medicine, Forum on Microbial Threats.
Woolhouse MEJ, Dye C. 2001. Population biology of emerging and re-emerging pathogens. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(Preface):981–982.
Woolhouse MEJ, Taylor LH, Haydon DT. 2001. Population biology of multi-host pathogens. Science 292(5519):1109–1112.
Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR. 2002. Biological and biomedical implications of the coevolution of pathogens and their hosts. Nature Genetics 32(4): 569–577.
Woolhouse MEJ, Haydon DT, Antia R. 2005. Emerging pathogens: The epidemiology and evolution of species jumps. Trends in Ecology and Evolution 20:238–244.
WHO (World Health Organization). 1959. Zoonoses: Second report of the joint WHO/FAO expert committee. Geneva: The World Health Organization.
WHO. 2006. Emerging diseases. [Online]. Available: www.who.int/topics/emerging_diseases/en/ [accessed May 2, 2006].
Yershov G, Barsky V, Belgovskiy A, Kirillov E, Kreindlin E, Ivanov I, Parinov S, Guschin D, Drobishev A, Dubiley S, Mirzabekov A. 1996. DNA analysis and diagnostics on oligonucleotide microchips. Proceedings of the National Academy of Sciences USA 93(10):4913–4918.
Zhou J. 2003. Microarrays for bacterial detection and microbial community analysis. Current Opinions in Microbiology 6(3):288–294.
































