1
Microbial Communities of the Gut
OVERVIEW
The gastrointestinal tract represents an important and challenging system for exploring how microbial communities become established within their hosts, how their members maintain stable ecological niches, and how these dynamics relate to host health and disease. The complex, dynamic, and spatially diversified microbial community of the human gut is believed to be composed of at least 1013 microorganisms, including more than 800 species of bacteria (most of which have not yet been successfully cultured in the laboratory), numerous viral species including bacteriophages, archaea (e.g., methanogens), and eukaryotes (e.g., helminths and protozoa). The collective genome of the microbiota in the human gut is approximately one hundred-fold larger than that of its host. Therefore, as Bäckhed et al. (2005) state in their contribution to this chapter, “It seems appropriate to view ourselves as a composite of many species and our genetic landscape as an amalgam of genes embedded in our Homo sapiens genome and in the genomes of our affiliated microbial partners.”
The first paper in this chapter, contributed by workshop presenter Karen Guillemin, focuses on the establishment of the gut microbiota (in humans, during the early days of infancy), its influence on host development (e.g., immunity), and the mechanisms by which hosts perceive and respond to the presence of colonizing microbes. Guillemin and coworkers pursue these fundamental questions in germ-free (GF) zebrafish, an experimental system that simplifies analyses of microbial influence on host development, while closely approximating gastrointestinal tract and immune system maturation, as well as gut microbiota
diversity, in mammals. This approach has demonstrated the pervasive influence of the microbiota over a variety of events in the maturation of the gastrointestinal tract, but it raises further questions regarding the potential for individual developmental variation arising from differences in microbiota from one member of a species to another. In humans, such variation could accrue among contemporaries who live in different environments or have different diets, as well as over the course of history.
Once established, the gut microbiota acts as an exquisitely tuned metabolic “organ” within the host, according to presenter Jeffrey Gordon, senior author of the second paper in this chapter. He and coworkers review current knowledge of the structure and function of the human gut microbiota, as well as recent research that reveals coevolution between humans and gut microbes to their mutual benefit. Over the course of evolution, symbiotic gut bacteria have become, in Gordon’s words, “master physiological chemists,” employing a broad range of strategies to manipulate host genomes.
Details of these microbial strategies are revealed in the final contribution to this chapter, in which presenter Abigail Salyers surveys the microbial activities in the human colon that are influenced by diet and that in turn affect human health. These include genetic exchanges among microbes that occur through transformation, phage transduction, and conjugation—interactions that are known to contribute to antibiotic resistance and which may also influence the evolution and virulence of pathogens. Salyers notes that several basic and longstanding questions regarding the composition, function, and evolution of the human intestinal microflora can now be investigated with the advent of molecular technology.
THE ROLE OF THE INDIGENOUS MICROBIOTA IN ZEBRAFISH GASTROINTESTINAL TRACT DEVELOPMENT
Karen Guillemin, Ph.D.
University of Oregon
Although the anatomy of the human gastrointestinal (GI) tract has been explored since at least the time of Leonardo da Vinci, who secretly produced detailed drawings of human organs at a time when such studies were considered heretical, our understanding of this organ is still largely incomplete. That is because we know so little about its cellular composition, which is dominated by microbes. The bacterial community of the GI tract contains an enormous wealth of unsequenced genomic information, and it raises important questions as to its function in the normal physiology and development of this organ.
My coworkers and I are interested in the role commensal bacteria play in animal development, a phenomenon that has gone largely unexplored by developmental biologists. We are using a model vertebrate, the zebrafish, to study GI tract development in the presence and absence of the microbiota. Here I will
describe how we have used this system to explore the establishment and role of the intestinal microbiota in early development and the means by which the host perceives and responds to its microbiota.
The Zebrafish Model
Zebrafish offer a number of advantages as a model for microbiota development and establishment. The embryos develop ex utero, which facilitates our ability to create GF animals. We harvest embryos and surface-sterilize them with a bleaching procedure that has been shown not to cause developmental defects; the embryos are then grown in sterile tissue culture flasks containing sterile media. The larvae are transparent, allowing us to follow the development of internal organs and monitor the dynamics of bacteria in live animals. GI tract development and physiology in zebrafish closely resemble those of mammals. Zebrafish also possess both adaptive and innate immune systems similar to those in mammals. Finally, zebrafish are readily amenable to genetic analysis.
The zebrafish gut is simpler than the human equivalent, but it exhibits regional specialization similar to the human organ: the proximal tract is specialized for lipid absorption, while most protein absorption occurs in the distal tract. This area near the anal vent is thought to be involved in osmoregulation. Figure 1-1 shows some of the key events in gut maturation and immune system development; our focus is on zero through eight day postfertilization (dpf). The embryos hatch out of their eggshells between 2 and 3 dpf, prior to the completion of gut maturation.
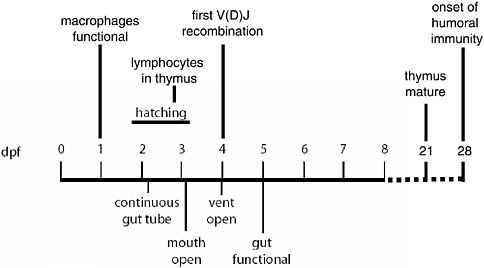
FIGURE 1-1 Important events in zebrafish development.
SOURCE: Guillemin (2005).
One of the first questions we addressed was how the gut microbiota is established. We have used scanning electron microscopy to visualize the rich microbial community on the surface of the embryonic chorion. We have also performed fluorescent in situ hybridization to look at the distribution of bacteria during development. Using a combination of three eubacterial probes that recognize sequences of 16S ribosomal RNA genes, we found that there is a dense community of microbes on the surface of the early embryo, but the interior of the embryo appears sterile. Microbes do not begin to accumulate within the embryo’s internal organs, as viewed in transverse sections of the GI tract, until after the embryo hatches. In adult zebrafish, microbes are distributed throughout the lumen of the gut; the fish also appear to have immune cells that sample the microbial contents of the gut, possibly like mammalian dendritic cells. We quantified the bacterial load over the course of zebrafish development using quantitative polymerase chain reaction (PCR), producing results similar to those obtained through in situ hybridization and suggesting that the microbial load of the zebrafish GI tract, relative to its weight, is roughly comparable to that in humans.
Enumeration studies of the zebrafish microbiota reveal a preponderance of Aeromonas and Pseudomonas species, so we wanted to examine the distribution of these genera during developmental time. We hybridized the proximal and distal intestine of zebrafish at both 4 and 8 dpf with the eubacterial probes, as well as with specific probes for Aeromonas or Pseudomonas. At 4 dpf, we found a preponderance of Pseudomonas species in the distal intestine. By 8 dpf, these were largely replaced with Aeromonas species, but a large number of Pseudomonas species remained in the proximal intestine of these animals. Acquisition of this microbiota happens concurrently with the later events of gut maturation; a similar process of postnatal gut maturation occurs in mammals as they acquire their microflora.
Role of the Microbiota in Gut Development and Function
To examine the functional significance of the temporal relationship between microbiota establishment and gut maturation, we turned to gnotobiology, the use of GF animals (see subsequent paper by Bäckhed et al.). We raised GF zebrafish under sterile conditions, as previously described. For both GF and control fish, the egg yolk was the sole source of nutrients during these experiments. We verified the sterility of the GF zebrafish by plating fish homogenates on tryptic soy agar, by PCR with 16S panbacterial primers, and by conventional and scanning electron microscopy. To determine whether adding bacteria to GF zebrafish at a later stage of development would restore them to the same status as control animals, we raised GF animals until 5 dpf, when the conventionally-reared animals’ guts become functional, then exposed the GF fish to a mixture of bacteria found in the water of their conventionally reared clutch mates.
We examined a variety of traits to compare the phenotypes of control and
experimental animals, including the expression of an enzyme on the brush border of the intestinal epithelium, alkaline phosphatase. This enzyme is known to be induced during the developmental period; by staining for its activity in transverse sections of the gut, we found that by 8 dpf, alkaline phosphatase activity was considerably higher in the control zebrafish than in the GF animals. Alkaline phosphatase activity was restored to control levels in GF animals that were exposed, 5 dpf, to bacteria from their siblings.
Another marker of maturation we examined was glycan expression. The glycan landscape of the GI tract is known to be very sensitive to the presence of microbes, so we used a number of different lectins to sensitively detect the expression of a variety of sugar moieties. Using image analysis software to quantify sugar expression, we found that galactoseα1,3galactosyl (Galα1,3Gal) is down-regulated in conventionally reared animals, but persists at high levels in GF zebrafish; exposure to bacteria reduces the expression of this glycan in formerly GF fish. Experiments in GF rats have found that the number of mucus-secreting goblet cells in the GI tract is reduced in the GF animals (Ishikawa et al., 1986; Sharma and Schumacher, 1995). We found that in GF zebrafish the number of goblet cells at 8 dpf is less than in conventionally reared 8 dpf animals and similar to the number found in 5 dpf conventionally reared animals. Exposure to the microbiota again reverses this GF trait to that of a conventional animal of the same age.
We are also examining the relationship between microbiota establishment and GI tract function. For example, we have compared the ability of GF and control zebrafish to absorb proteins in their distal intestines. This trait can be evaluated by feeding the fish a high concentration of protein in the form of the enzyme horseradish peroxidase, then assaying the enzyme’s activity after it is absorbed into cells. In these experiments, the GF animals—which we know to be equally proficient at swallowing as the conventionally raised controls—were found to be dramatically defective in protein absorption. This deficiency was reversed in GF animals following exposure to the microbiota. We also visualized GI motility in GF and control animals, which is possible in the transparent zebrafish. The GF animals had markedly shorter and more regular peristaltic waves along their GI tract, a trait that was once again reversed by exposure to the microbiota.
Host Perception of and Response to the Microbiota
Having accumulated evidence that the microbiota plays important roles in maturation and function of the zebrafish GI tract, we next explored how the host perceives the presence of the microbes, and attempted to identify the types of signals sent by the microbiota to its host that promote gut development. To do this, we created monocolonized animals by inoculating GF zebrafish, at 5 dpf, with pure cultures of either of two major bacterial constituents of the zebrafish GI
tract: Aeromonas sobria and Pseudomonas fluorescens. We found that monoassociation with either bacterial strain was sufficient to reverse the previously discussed traits of low alkaline phosphatase activity and high Galα1,3Gal levels in GF zebrafish.
We next tested whether live bacteria were required to reverse these phenotypes or whether a heat-killed preparation of the microbiota was sufficient to signal to the host to promote gut maturation. We found that heat-killed microbiota was sufficient to induce alkaline phosphatase activity in GF animals; preliminary studies also indicate that bacterial lipopolysaccharide produces the same effect. By contrast, heat-killed bacteria failed to suppress the expression of Galα1,3Gal in GF animals. Thus, we have found evidence for two different modes of host perception for the presence of microbes: one that uses a generic signal of microbial-associated molecular patterns and another that requires active signaling from constituents of the microbiota.
Conclusion
Our findings on the role of the microbiota in the development of the GI tract in zebrafish show this to be a promising model for investigating the role of microbial communities in the developmental biology of the host. We have been able to show that the microbiota is required for gut maturation—as indicated by patterns of expression of glycans, by alkaline phosphatase activity, and by goblet cell census—and that the microbiota is important for such functions as protein absorption and GI motility.
The possibility that alkaline phosphatase enzymatic activity can be up-regulated by a heat-killed bacterial preparation immediately brings to mind the toll-like receptors (TLRs) of the innate immune system, and their ability to recognize generic microbial-associated molecular patterns. Much attention has been focused on the role of TLRs in protection against infection, but it is also important to consider these molecules in the context of gut development and homeostasis. A recent publication by Rakoff-Nahoum et al. (2004) examines susceptibility to intestinal injury in animals that are deficient for TLR signaling. In this study, wild-type mice survived an intestinal injury, while animals deficient in MyD88, an adaptor molecule essential for TLR-mediated induction of inflammatory cytokines, manifested severe morbidity and mortality in response to the same insult. A similar phenotype was observed in animals in which the ligand for TLRs was depleted by an antibiotic reduction of the microbiota. This trait was reversed, and the animals’ viability restored, by exposing them to lipopolysaccharide and lipoteichoic acid, two conserved molecular products of microorganisms recognized by TLRs. These results indicate that the constituents of the microbiota continually shape GI tract homeostasis.
Given such findings, it will now be important to determine the extent to which the microbiota is stereotyped during development. Most of us would agree
that the human microbiota has probably changed very much since the time of Leonardo da Vinci, due to the onset of antimicrobial therapies and modern sanitation. In that regard, it may be somewhat comforting to imagine that certain aspects of the microbial-directed maturation of the GI tract require generic signals that many different possible constituents could supply. However, it is also clear that certain complex and specific signaling events influence GI tract maturation and function. The gnotobiotic zebrafish model will help elucidate the various signaling mechanisms between animals and their resident microbes.
HOST-BACTERIAL MUTUALISM IN THE HUMAN INTESTINE
Fredrik Bäckhed, Ruth E. Ley, Justin L. Sonnenburg, Daniel A. Peterson, Jeffrey I. Gordon1
Reprinted with permission from Science (Bäckhed et al. 2005).
Copyright 2005 AAAS.
The distal human intestine represents an anaerobic bioreactor programmed with an enormous population of bacteria, dominated by relatively few divisions that are highly diverse at the strain/subspecies level. This microbiota and its collective genomes (microbiome) provide us with genetic and metabolic attributes we have not been required to evolve on our own, including the ability to harvest otherwise inaccessible nutrients. New studies are revealing how the gut microbiota has coevolved with us and how it manipulates and complements our biology in ways that are mutually beneficial. We are also starting to understand how certain keystone members of the microbiota operate to maintain the stability and functional adaptability of this microbial organ.
The adult human intestine is home to an almost inconceivable number of microorganisms. The size of the population—up to 100 trillion—far exceeds that of all other microbial communities associated with the body’s surfaces and is ~10 times greater than the total number of our somatic and germ cells (Savage, 1977). Thus, it seems appropriate to view ourselves as a composite of many species and our genetic landscape as an amalgam of genes embedded in our Homo sapiens genome and in the genomes of our affiliated microbial partners (the microbiome).
Our gut microbiota can be pictured as a microbial organ placed within a host organ: It is composed of different cell lineages with a capacity to communicate with one another and the host; it consumes, stores, and redistributes energy; it mediates physiologically important chemical transformations; and it can maintain and repair itself through self replication. The gut microbiome, which may
|
1 |
Materials and methods are available as supporting material on Science Online. We thank L. Angenent for many helpful discussions. Work cited from the authors’ lab is supported by the NIH and NSF. F.B. and J.L.S. are supported by postdoctoral fellowships from the Wenner-Gren and W. M. Keck Foundations, respectively. Supporting online material: www.sciencemag.org/cgi/content/full/307/5717/1915/DC1MaterialsandMethodsTablesS1toS3References.10.1126/science.1104816. |
contain > 100 times the number of genes in our genome, endows us with functional features that we have not had to evolve ourselves.
Our relationship with components of this microbiota is often described as commensal (one partner benefits and the other is apparently unaffected) as opposed to mutualistic (both partners experience increased fitness). However, use of the term commensal generally reflects our lack of knowledge, or at least an agnostic (noncommittal) attitude about the contributions of most citizens of this microbial society to our own fitness or the fitness of other community members.
The guts of ruminants and termites are well-studied examples of bioreactors “programmed” with anaerobic bacteria charged with the task of breaking down ingested polysaccharides, the most abundant biological polymer on our planet, and fermenting the resulting monosaccharide soup to short-chain fatty acids. In these mutualistic relationships, the hosts gain carbon and energy, and their microbes are provided with a rich buffet of glycans and a protected anoxic environment (Brune and Friedrich, 2000). Our distal intestine is also an anaerobic bioreactor that harbors the majority of our gut microorganisms; they degrade a varied menu of otherwise indigestible polysaccharides, including plant-derived pectin, cellulose, hemicellulose, and resistant starches.
Microbiologists from Louis Pasteur and Ilya Mechnikov to present-day scientists have emphasized the importance of understanding the contributions of this microbiota to human health (and disease). Experimental and computational tools are now in hand to comprehensively characterize the nature of microbial diversity in the gut, the genomic features of its keystone members, the operating principles that underlie the nutrient foraging and sharing behaviors of these organisms, the mechanisms that ensure the adaptability and robustness of this system, and the physiological benefits we accrue from this mutualistic relationship. This review aims to illustrate these points and highlight some future challenges for the field.
Microbial Diversity in the Human Gut Bioreactor
The adult human gastrointestinal tract contains all three domains of life—bacteria, archaea, and eukarya. Bacteria living in the human gut achieve the highest cell densities recorded for any ecosystem (Whitman et al., 1998). Nonetheless, diversity at the division level (superkingdom or deep evolutionary lineage) is among the lowest (Hugenholtz et al., 1998); only 8 of the 55 known bacterial divisions have been identified to date (Figure 1-2A), and of these, 5 are rare. The divisions that dominate—the Cytophaga-Flavobacterium-Bacteroides (CFB) (e.g., the genus Bacteroides) and the Firmicutes (e.g., the genera Clostridium and Eubacterium)—each comprise ~30 percent of bacteria in feces and the mucus overlying the intestinal epithelium. Proteobacteria are common but usually not dominant (Seksik et al., 2003). In comparison, soil (the terrestrial biosphere’s GI tract, where degradation of organic matter occurs) can contain 20 or more bacterial divisions (Dunbar et al., 2002).
Our knowledge of the composition of the adult gut microbiota stems from culture-based studies (Moore and Holdeman, 1974), and more recently from culture-independent molecular phylogenetic approaches based on sequencing bacterial ribosomal RNA (16S rRNA) genes. Of the > 200,000 rRNA gene sequences currently in GenBank, only 1,822 are annotated as being derived from the human gut; 1,689 represent uncultured bacteria. rRNA sequences can be clustered into relatedness groups based on their percent sequence identity. Cutoffs of 95 and 98 percent identity are used commonly to delimit genera and species, respectively. Although these values are somewhat arbitrary and the terms “genus” and “species” are not precisely defined for microbes, we use them here to frame a view of human gut microbial ecology. When the sequences (n = 495 greater than 900 base pairs) are clustered into species, and a diversity estimate model is applied, a value of ~800 species is obtained (Figure 1-3). If the analysis is adjusted to estimate strain number (unique sequence types), a value > 7,000 is obtained (Figure 1-3). Thus, the gut microbiota, which appears to be tremendously diverse at the strain and subspecies level, can be visualized as a grove of eight palm trees (divisions) with deeply divergent lineages represented by the fan(s) of closely related bacteria at the very top of each tree trunk.
Diversity present in the GI tract appears to be the result of strong host selection and coevolution. For example, members of the CFB division that are predominantly associated with mammals appear to be the most derived (i.e., farthest away from the common ancestor of the division), indicating that they underwent accelerated evolution once they adopted a mutualistic lifestyle. Moreover, a survey of GenBank reveals that several subgroups in CFB are distributed among different mammalian species (Figure 1-2B), suggesting that the CFB-mammal symbiosis is ancient and that distinct subgroups coevolved with their hosts.
The structure and composition of the gut microbiota reflect natural selection at two levels: at the microbial level, where lifestyle strategies (e.g., growth rate and substrate utilization patterns) affect the fitness of individual bacteria in a competitive ensemble, and at the host level, where suboptimal functionality of the microbial ensemble can reduce host fitness. Microbial consortia whose integrated activities result in a cost to the host will result in fewer hosts, thereby causing loss of their own habitat. Conversely, microbial consortia that promote host fitness will create more habitats. Thus, the diversity found within the human GI tract, namely, a few divisions represented by very tight clusters of related bacteria, may reflect strong host selection for specific bacteria whose emergent collective behavior is beneficial to the host. This hypothesis has two important implications: (1) A mechanism exists to promote cooperation, and (2) the structure promotes functional stability of the gut ecosystem.
To benefit the host, bacteria must be organized in a trophic structure (food web) that aids in breaking down nutrients and provides the host with energetic substrates. Cooperative behavior that imposes a cost to the individual while benefiting the community can emerge within groups of bacteria (Rainey and Rainey,
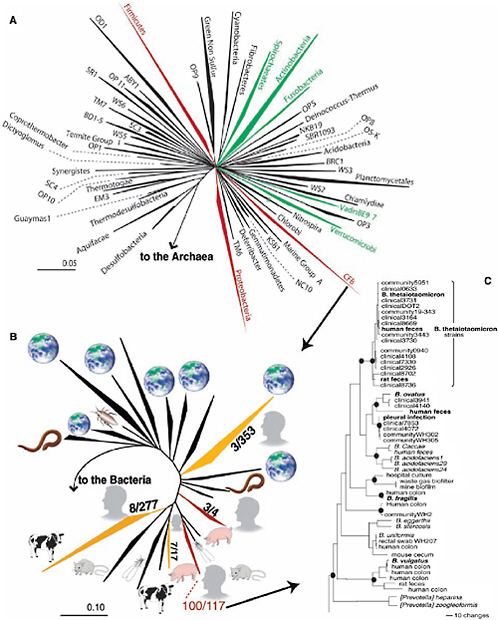
FIGURE 1-2 Representation of the diversity of bacteria in the human intestine. (A) Phylogenetic tree of the domain bacteria based on 8,903 representative 16S rRNA gene sequences. Wedges represent divisions (superkingdoms): Those numerically abundant in the human gut are red, rare divisions are green, and undetected are black (for colors please refer to the original article). Wedge length is a measure of evolutionary distance from the common ancestor. (B) Phylogenetic tree of the CFB division based on 1561 sequences from GenBank (> 900 nucleotides) and their ecological context. Wedges are major subgroups of CFB; symbols are sources of the sequences [Earth, environmental; cow, ruminants; rodent, rat and/or mouse; person, human GI tract; others are termite, cockroach, worm (including hydrothermal), and pig]. Ratios are the number of sequences represented in the human gut relative to the total number in the subgroup; red, yellow, and black indicate majority, minority, and absence of sequences represented in human GI tract, respectively. (C) Phylogenetic (parsimony) tree of Bacteroides. Strains classified as B. thetaiotaomicron based on phenotype are in red; 16S rRNA analysis did not confirm this classification for all strains. Bacteroides spp. with sequenced genomes are in bold. Black circles indicate nodes with high (> 70 percent) bootstrap support. Scale bars indicate the degree of diversity (evolutionary distance) within a division or subgroup ([A] and [B]), respectively] in terms of the fraction of the 16S rRNA nucleotides that differ between member sequences; in (C), the evolutionary distance between organisms is read along branch lengths, where scale indicates number of changes in 16S rRNA nucleotide composition.
SOURCE: Bäckhed et al. (2005).
2003) and can be maintained by group selection as long as consortia are isolated and new consortia form periodically (e.g., new GI tracts). Furthermore, selection must act simultaneously at multiple levels of biological organization (Travisano and Velicer, 2004). These criteria are met in the human GI tract where new acts of colonization occur at birth, with a small founding population of noncheaters from the mother, and selection occurs both at the microbial and host level.
Diversity is generally thought to be desirable for ecosystem stability (McCann, 2000). One important way diversity can confer resilience is through a wide repertoire of responses to stress (referred to as the insurance hypothesis [Yachi and Loreau, 1999]). In man-made anaerobic bioreactors used to treat wastewater (a system analogous to the gut but where no host selection occurs), rates of substrate degradation can remain constant, whereas bacterial populations fluctuate chaotically as a result of blooms of subpopulations (Fernandez et al., 2000). Functional redundancy in the microbial community ensures that key processes are unaffected by such changes in diversity (Goebel and Stackebrandt, 1994). By contrast, in the human gut, populations are remarkably stable within individuals (Zoetendal et al., 1998), implying that mechanisms exist to suppress blooms of subpopulations and/or to promote the abundance of desirable bacteria. A study of adult monozygotic twins living apart and their marital partners has emphasized the potential dominance of host genotype over diet in determining microbial composition of the gut bioreactor (Zoetendal et al., 2001). The role of the immune system in defining diversity and suppressing subpopulation blooms remains to be defined. One likely mediator of bacterial selection is secretory immunoglobulin A (Suzuki et al., 2004).
The human gut is faced with a paradox: How can functional redundancy be maintained in a system with low diversity (few divisions of bacteria), and how
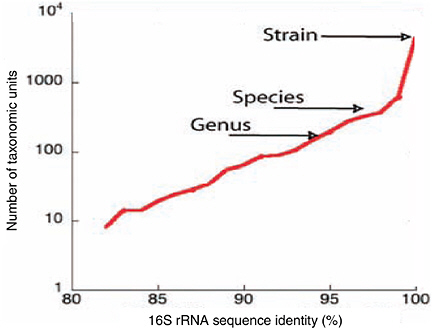
FIGURE 1-3 Taxon richness estimates for bacteria in the human GI tract. Taxon richness estimates for varying levels of 16S rRNA sequence identity, ranging from below the “genus” level (95 percent identity), to the “species” level (98 percent identity), to the strain level (unique sequences). Estimates are based on sequences available in GenBank, annotated as derived from the human GI tract, after alignment and clustering into taxonomic units ranging from 80 to 100 percent identity.
SOURCE: Bäckhed et al. (2005).
can such a system withstand selective sweeps in the form of, for example, phage attacks? The estimated 1,200 viral genotypes in human feces (Breitbart et al., 2003) suggest that a phage attack is a powerful shaper of the gut’s microbial genetic landscape (Fuhrman, 1999; Winter et al., 2004). Enough diversity of genome and transcriptome must be represented at the subspecies level to lend resilience to the gut ecosystem. Ecological studies of macroecosystems have shown that less diversity is required to maintain stability if individual species themselves have a wide repertoire of responses (Yachi and Loreau, 1999). In the following section we discuss recent genome-based studies exploring how a presumed keystone bacterial species in our gut is able to adapt to (1) changing dietary conditions in ways that should stabilize the microbiota’s food web, and (2) changing immune and phage selective pressures in ways that should stabilize the ecosystem.
Bacteroides thetaiotaomicron—A Highly Adaptive Glycophile
Bacteroides thetaiotaomicron is a prominent mutualist in the distal intestinal habitat of adult humans. It is a very successful glycophile whose prodigious capacity for digesting otherwise indigestible dietary polysaccharides is reflected in the fully sequenced 6.3-Mb genome of the type strain (ATCC 29148; originally isolated from the feces of a healthy adult human) (Xu et al., 2003). Its “glycobiome” contains the largest ensemble of genes involved in acquiring and metabolizing carbohydrates yet reported for a sequenced bacterium, including 163 paralogs of two outer membrane proteins (SusC and SusD) that bind and import starch (Shipman et al., 2000), 226 predicted glycoside hydrolases, and 15 polysaccharide lyases (CAZY, 2005). By contrast, our 2.85-Gb genome only contains 98 known or putative glycoside hydrolases and is deficient in the enzyme activities required for degradation of xylan-, pectin-, and arabinose-containing polysaccharides that are common components of dietary fiber (we have one predicted enzyme versus 64 in B. thetaiotaomicron; see Annex 1-1, Table 1-1).
The carbohydrate foraging behavior of B. thetaiotaomicron has been characterized during its residency in the distal intestines (ceca) of gnotobiotic mice colonized exclusively with this anaerobe (Sonnenburg et al., 2005). Scanning electron microscopy studies of the intestines of mice maintained on a standard high-polysaccharide chow diet, containing xylose, galactose, arabinose, and glucose as its principal monosaccharide components, revealed communities of bacteria assembled on small undigested or partially digested food particles, shed elements of the mucus gel layer, and exfoliated epithelial lining cells (Sonnenburg et al., 2005). Whole-genome transcriptional profiling of B. thetaiotaomicron (Sonnenburg et al., 2005) disclosed that this diet was associated with selective up-regulation of a subset of SusC and SusD paralogs, a subset of glycoside hydrolases (e.g., xylanases, arabinosidases, and pectate lyase), as well as genes encoding enzymes involved in delivering the products of mannose, galactose, and glucose to the glycolytic pathway and arabinose and xylose to the pentose phosphate pathway. In contrast, a simple sugar (glucose and sucrose) diet devoid of polysaccharides led to increased expression of a different subset of SusC and SusD paralogs, glycoside hydrolases involved in retrieving carbohydrates from mucus glycans, as well as enzymes that remove modifications that make these host glycans otherwise resistant to degradation (O-acetylation of sialic acids and sulfation of glycosoaminoglycans) (Sonnenburg et al., 2005).
These findings provide insights about how functional diversity and adaptability are achieved by a prominent member of the human colonic microbiota (Figure 1-4). Dining occurs on particulate nutrient scaffolds (food particles, shed mucus, and/or exfoliated epithelial cells). For a bacterium such as B. thetaiotaomicron, which lacks adhesive organelles, seating at the “dining table” is determined in part by the repertoire of glycan-specific outer membrane-binding proteins it produces, and this repertoire is itself shaped by the menu of available
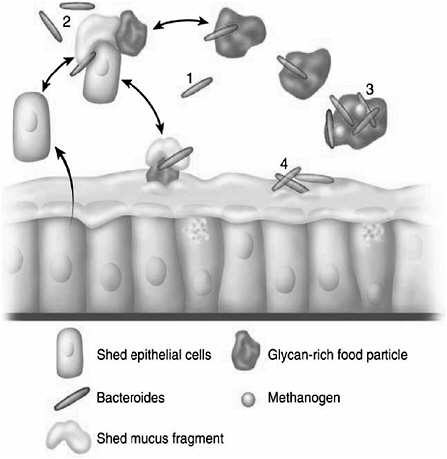
FIGURE 1-4 Lessons about adaptive foraging for glycans obtained from B. thetaiotaomicron. (1) B. thetaiotaomicron does not have adhesive organelles. Without outer membrane polysaccharide-binding protein-mediated attachment to glycan-rich nutrient platforms, it is at risk for being washed out from the intestinal bioreactor. Substrate access is limited under these conditions. (2) Small nutrient platforms are composed of undigested or partially digested food particles (e.g., dietary fiber), shed host epithelial cells, and/or mucus fragments. These platform elements may be in dynamic equilibrium with one another and with the mucus layer overlying the intestinal epithelium. Microbial fermentation of otherwise indigestible polysaccharides in these platforms is made possible by induced expression of substrate-appropriate sets of bacterial polysaccharide-binding proteins and glycoside hydrolases. (3) Mesophilic methanogens drive carbohydrate utilization by removing products of fermentation (H2 and CO2 are converted to methane), thereby improving the overall efficiency of energy extraction from polysaccharides. (4) When dietary polysaccharides are scarce, B. thetaiotaomicron turns to host mucus by deploying a different set of polysaccharide binding proteins and glycoside hydrolases. This adaptive foraging reflects the coevolved functional versatility of B. thetaiotaomicron’s glycobiome and the structural diversity of the host’s mucus glycans.
SOURCE: Bäckhed et al. (2005).
glycans (Sonnenburg et al., 2005). Attachment to nutrient platforms helps avoid washout from the intestinal bioreactor, in much the same way as dense, well-settling, granular biofilms help oppose elimination from engineered (man-made) anaerobic upflow bioreactors (Sonnenburg et al., 2004). Attachment also presumably increases the efficiency of oligo- and monosaccharide harvest by adaptively expressed bacterial glycoside hydrolases and their subsequent distribution to other members of the microbiota whose niche overlaps that of B. thetaiotaomicron. In this conceptualization, microbial nutrient metabolism along the length of the intestine is a summation of myriad selfish and syntrophic relationships expressed by inhabitants of these nutrient platforms. Diversity in these microhabitats and mutualistic cooperation among their component species (including the degree to which sanctions must be applied against cheats) are reflections of a dynamic interplay between the available nutrient foundation and the degree of flexible foraging (niche breadth) expressed by microhabitat residents. Bacteroides spp., such as B. thetaiotaomicron, impart stability to the gut ecosystem by having the capacity to turn to host polysaccharides when dietary polysaccharides become scarce. The highly variable outer chain structures of mucus and epithelial cell surface glycans are influenced by host genotype and by microbial regulation of host glycosyltransferase gene expression. Coevolution of host glycan diversity and a large collection of microbial glycoside hydrolases that are regulated by nutrient availability provides insurance that the “system” (microbiota and host) can rapidly and efficiently respond to changes in the diet, and maximize energy harvest, without having to undergo substantial changes in species composition. Rather than minimizing genome size, a keystone species such as B. thetaiotaomicron has evolved an elaborate and sizable genome that can mobilize functionally diverse adaptive responses.
Diet-associated changes in the glycan-foraging behavior of B. thetaiotaomicron are also accompanied by changes in expression of its capsular polysaccharide synthesis loci, indicating that B. thetaiotaomicron is able to change its carbohydrate surface depending upon the nutrient (glycan) environment. This could be part of a strategy for evading an adaptive immune response. Whole-genome genotyping studies of B. thetaiotaomicron isolates, with the use of GeneChips designed from the sequenced genome of the type strain, disclose that their CPS loci differ, whereas their housekeeping genes are conserved (Ley and Gordon, Unpublished data). Because selective sweeps are most likely to come from the immune system and phages, both of which respond to surface structures, the associated genes are likely to be the most diverse in the genome. Accordingly, B. thetaiotaomicron has a remarkable apparatus for altering its genome content. The sequenced type strain contains a plasmid, 63 transposases, 43 integrases, and 4 homologs of a conjugative transposon (Xu et al., 2003). Gene transfer and mutation mechanisms endow strains of bacterial species with the (genetic) versatility necessary to withstand selective sweeps that would eradicate more clonal populations (Taddei et al., 1997).
The Gut Microbiota as a “Host” Factor That Influences Energy Storage
Comparisons of mice raised without exposure to any microorganisms, (Germ-Free), with those that have acquired a microbiota since birth, or conventionally raised (CONV-R), have led to the identification of numerous effects of indigenous microbes on host biology (see Annex 1-1, Table 1-2), including energy balance. Young adult CONV-R animals have 40 percent more total body fat than their GF counterparts fed the same polysaccharide-rich diet, even though CONV-R animals consume less chow per day (Bäckhed et al., 2004). This observation might seem paradoxical at first but can be explained by the fact that the gut microbiota allows energy to be salvaged from otherwise indigestible dietary polysaccharides (Yamanaka et al., 1977). “Conventionalization” of adult GF mice with cecal contents harvested from CONV-R donors increases body fat content to levels equivalent to those of CONV-R animals (Bäckhed et al., 2004). The increase reflects adipocyte hypertrophy rather than hyperplasia and is notable for its rapidity and sustainability (Bäckhed et al., 2004).
The mutualistic nature of the host-bacterial relationship is underscored by mechanisms that underlie this fat-storage phenotype. Colonization increases glucose uptake in the host intestine and produces substantial elevations in serum glucose and insulin (Bäckhed et al., 2004), both of which stimulate hepatic lipogenesis through their effects on two basic helix-loop-helix/leucine zipper transcription factors—ChREBP and SREBP-1c (Bäckhed et al., 2004; Towle, 2001). Short-chain fatty acids, generated by microbial fermentation, also induce lipogenesis (Rolandelli et al., 1989). Triglycerides exported by the liver into the circulation are taken up by adipocytes through a lipoprotein lipase (LPL)-mediated process. The microbiota suppresses intestinal epithelial expression of a circulating LPL inhibitor, fasting-induced adipose factor (FIAF, also known as angiopoietin-like protein-4) (Bäckhed et al., 2004). Comparisons of GF and conventionalized wild-type and FIAF –/– mice established FIAF as a physiologically important regulator of LPL activity in vivo and a key modulator of the microbiota-induced increase in fat storage (Bäckhed et al., 2004).
The caloric density of food items is portrayed as a fixed value on package labels. However, it seems reasonable to postulate that caloric value varies between individual “consumers” according to the composition and operation (e.g., transit time) of their intestinal bioreactors, and that the microbiota influences their energy balance. Relatively high-efficiency bioreactors would promote energy storage (obesity), whereas lower efficiency reactors would promote leanness (efficiency is defined in this case as the energy-harvesting and storage-promoting potential of an individual’s microbiota relative to the ingested diet).
The idea that individual variations in bioreactor efficiencies may be a significant variable in the energy balance equation is supported by several observations. First, individual variations in the composition of the microbiota occur and are influenced by host genotype (Zoetendal et al., 2001). Second, small but chronic differences between energy intake and expenditure can, in principle, produce
major changes in body composition [e.g., if energy balance is +12 kcal/day, 90.45 kg of fat could be gained per year if there are no compensatory responses by the host; this is the average weight increase experienced by Americans from age 25 to 55 (Flegal and Troiano, 2000)]. Third, the microbiota is a substantial consumer of energy. One group estimated that individuals on a “British Diet” must ferment 50 to 65 g of hexose sugars daily to obtain the energy required to replace the 15 to 20 g (dry weight) of bacteria they excrete per day (McNeil, 1984).
These considerations emphasize the need to assess the representation of species with large capacities for processing dietary polysaccharides, such as Bacteroides, in lean versus morbidly obese individuals, and in cohorts of obese individuals before, during, and after weight reduction achieved by high-polysaccharide/low-fat versus high-fat/low-polysaccharide diets, or by bariatric (gastric bypass) surgery. The results, coupled with coincident assessments of energy extraction from the diet, should provide a proof-of-concept test of whether differences in the composition of the microbiota are associated with differences in gut bioreactor efficiency (and predisposition to obesity).
Lessons that have been learned by environmental engineers who study how to optimize the efficiency of man-made anaerobic bioreactors (see Annex 1-1, Table 1-3) suggest that these enumeration studies should also include members of archaea. Thermodynamics dictates that the energy obtained from substrate conversions will be higher if low concentrations of products are maintained (Stams, 1994; Thauer et al., 1977). In the human gut, methanogenic archaea provide the last microbial link in the metabolic chain of polysaccharide processing. Bacteria degrade polysaccharides to short-chain fatty acids, carbon dioxide, and hydrogen gas. Methanogens lower the partial pressure of hydrogen by generating methane, and thereby may increase microbial fermentation rates. Defining the representation of mesophilic methanogens in the colonic microbiota of individuals, sequencing their genomes [as we are currently doing with Methanobrevibacter smithii, a prevalent isolate from the human colon (Miller and Wolin, 1982)], and characterizing archaeal-bacterial syntrophy in simplified gnotobiotic mouse models consuming different diets should provide a starting point for defining the role of archaea in shaping the functional diversity, stability, and beneficial contributions of our distal gut microbiota. Devising ways for manipulating archaeal populations may provide a novel way for intentionally altering our energy balance.
Looking to the Future
A comprehensive 16S rRNA sequence-based (bacterial and archaeal) enumeration of the microbiotas of selected humans, representing different ethnic groups, living in similar or distinct milieus, would provide an invaluable database for studying normal and diseased populations (Chacon et al., 2004). The concept of using the microbiota as a biomarker of impending or fully manifest diseases within or outside of the GI tract and for monitoring responses to therapeutic interventions needs to be explored.
Several groups are embarking on metagenome sequencing projects to define gene content in the human gut microbiome. If we view ourselves as being a composite of many species, this represents a logical continuation of the Human Genome Project. A complementary approach to metagenomic analysis is to determine genome-level diversity among bacterial populations belonging to a specific genus or species residing within a defined gut habitat of a single individual or a few individuals. Members of Bacteroides provide a natural experiment for examining the impact of habitat on genome content since they have yet to be encountered in any environment other than animal GI tracts. Figure 1-2C illustrates how a collection of just 29 isolates phenotyped as B. thetaiotaomicron provided a broad range of 16S rRNA sequences, including several new species. We are close to producing finished genome sequences for two prominent members of the colonic microbiota, B. vulgatus and B. distasonis (GSC, 2005). B. fragilis, a less prominent member, has recently been sequenced (Cerdeño-Tárraga et al., 2005; Kuwahara et al., 2004). The results will allow us to ask how evolutionary history relates to genome content and what constitutes a minimal Bacteroides genome.
We also need to obtain a direct view of how the metabolites originating from the microbiome influence host physiology. This will be a formidable task, requiring new techniques for measuring metabolites generated by single and defined collections of symbionts during growth under defined nutrient conditions in single-vessel chemostats, in more elaborate mechanical models of the human gut, and in vivo after colonization of specified habitats of the intestines of gnotobiotic mice. The results should help formulate and direct hypothesis-based investigations of the microbiota’s “metabolome” in humans.
Databases that connect molecular data with ecosystem parameters are still rare (Galperin, 2004). A human intestinal microbiome database is needed to organize genomic, transcriptomic, and metabolomic data obtained from this complex natural microbial community, and would provide a substrate for generating testable hypotheses.
Finally, just as microbiotas have coevolved with their animal hosts, this field must coevolve with its academic hosts and their ability to devise innovative ways of assembling interactive interdisciplinary research groups necessary to advance our understanding.
ACTIVITIES OF HUMAN COLONIC MICROBES
Abigail A. Salyers
Department of Microbiology
University of Illinois
Overview of the Interaction
The microbes that inhabit our colons have a complex relationship with us
(Salyers, 1986; Salyers and Shipman, 2002). We are a food source for them, but they also contribute to our nutrition. Through their activities, they influence many aspects of human physiology. In fact, one could view the human intestine as an organ that was largely shaped by them during evolution. For example, the small intestine is designed to promote a fast flow of contents, a feature that has the effect of discouraging microbial growth by washing microbes through before they can establish themselves. The flow of contents through the colon, by contrast, is so slow that microbes can easily establish themselves and reach concentrations that are high enough to make up at least 30 percent of human colonic contents (Figure 1-5). In the colon, bacteria perform a service for us by digesting substances in the diet that the human stomach and small intestine cannot. The intestinal microbes also take an energy toll from us; they stimulate the turnover of intestinal mucosal cells. This constant sloughing of intestinal mucosal cells is a very effective defense that prevents bacteria that have attached to the mucosal cells from staying in the site long enough to invade.
In the colon, resident microbes also interact with each other genetically. Bacteria exchange genes in order to acquire new traits. One type of gene that is exchanged is antibiotic resistance genes, but many other genes are also exchanged. Diet influences this genetic interaction because it can include antibiotics or even antibiotic-resistant bacteria that can interact with the normal inhabitants of the site.
Finally, colonic bacteria modify substances such as bile acids, cholesterol, and xenobiotic compounds. The effects of these modifications on human health are still uncertain, but they surely occur.
In this chapter, we survey in detail the different types of microbial activities that are affected by the human diet and, in turn, affect human health. We also identify some basic questions, most of which were posed years ago, but are only now beginning to be addressed because modern molecular technology has made such investigations much more feasible than were previously possible.
Composition of the Intestinal Microflora
The microbial population of the human colon consists primarily of bacteria. It has been estimated that there are at least several hundred to a thousand distinct species of bacteria, although a smaller number, about 20 species, account for the majority of isolates (Bäckhed et al., 2005; Salyers, 1986). A smaller group of microbes in the colon are the methanogenic archaea. These methane-producing microbes are sufficiently active, and the methane they produce can be detected, not only in intestinal contents, but also in the breath. Most of the numerically predominant bacteria are carbohydrate fermenters. These methanogens live on byproducts of carbohydrate fermentation such as carbon dioxide and acetate.
The colonic microflora is unusual in that in contrast to most microbial populations about 50 percent of the microbes can be cultivated. More recently, molecular techniques such as 16S rRNA PCR amplification and sequencing have
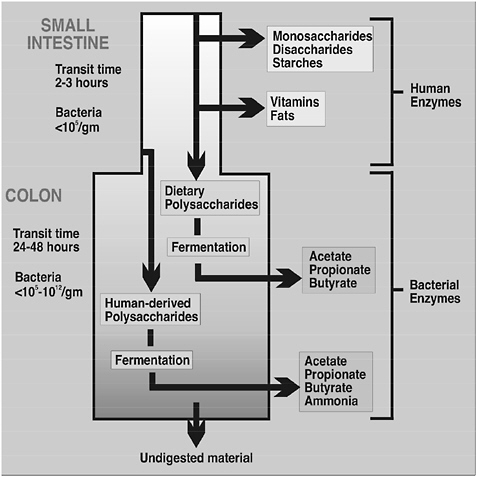
FIGURE 1-5 The flow of contents through the small intestine and colon.
SOURCE: Salyers (2005).
been applied to take a molecular census of the bacterial population (Wilson and Blitchington, 1996). Although molecular techniques have revealed some new species, there is general agreement between cultivation-based and molecular characterizations of the population. A striking feature of the colonic microflora is that the majority of colonic bacteria are gram-positive bacteria, virtually all of which are obligate anaerobes (Bäckhed et al., 2005; Salyers, 1986; Salyers and Shipman, 2002; Wilson and Blitchington, 1996). Gram-positive anaerobes account for at least 65 percent of the isolates. Gram-negative anaerobes, such as Bacteroides species, account for another 20 to 30 percent. Numerically minor populations, such as the enterics and the enterococci, account for less than 2 percent (Bäckhed et al., 2005).
It is important to note that numerically minor populations may still play an important role despite their relatively low numbers. Because the numerically major populations of bacteria are packed in the colon lumen, separated from the colonic mucosa by a mucin layer, any numerically minor population that succeeds in reaching the mucosa and adhering to mucosal cells could have a disproportionate effect on the colonic mucosa. Little is known about the bacteria that occupy the mucin layer, but colonic bacteria that degrade mucin have been isolated (McGarr et al., 2005).
A question that has not been answered conclusively is whether there are different populations of bacteria on the mucosal surface compared to the population in the lumen of the colon or whether there is population variation in the varying parts of the colon. It has not been easy to answer this question because mucosal samples from unmedicated healthy volunteers cannot be ethically obtained. Samples that are available tend to come from people with underlying conditions that require surgery, and these patients have invariably been treated extensively with antibiotics.
There is one older study in which samples were obtained from the intestinal tracts of sudden death victims. This was made possible by the fact that in some U.S. states, autopsy of such persons is mandated by law (Moore et al., 1978). At the time, such a study was possible only because some pathologists would provide post-autopsy samples. Such a study could probably not be done today. The results of this study supported the hypothesis that there were no distinct microbial subpopulations in different colonic locations, a conclusion that is difficult to believe in view of the fact that most environments contain niches that support distinct bacterial populations. The mouth and skin are human examples of such niche differentiation. A major limitation of this study, however, was that at least four hours elapsed between death and the availability of the specimens. During this time, extensive sloughing of the colonic mucosa would have occurred and peristalsis would have ceased. This might have eliminated, to some extent, any unique populations that exist in living humans. Because the human colon is not as accessible to sampling as other areas of the human body, mice—or some other animal such as the pig—will have to be accepted as models for colonic populations if progress in understanding niches in the colon is to be made.
As has already been mentioned, gram-positive bacteria account for at least 65 percent of colonic isolates. This is also true of other areas of the body such as the mouth, skin, and vaginal tract. The numerical predominance of gram-positive bacteria is a curious feature of the microflora of the human body because gram-positive bacteria represent only a minority of the types of bacteria found in the world at large. Gram-negative bacteria are much more diverse and much more numerous. To our knowledge, no one has yet proposed an explanation of why gram-positive bacteria should be such prominent members of the human microflora. Part of the problem with speculating about the reasons for this predominance is that virtually nothing is known about the gram-positive majority in the human colon.
Dore and coworkers (De La Cochetiere et al., 2005) were among the first to identify colonic bacteria using molecular methods. Surprisingly, most of them proved to be members of the genus Clostridium. Earlier cultivation-based studies had classified them in such genera as Eubacterium, Peptostreptococcus, and Bifidobacterium. This apparent difference between the results of molecular analyses and cultivation-based analyses may not be due so much to the presence of uncultivated bacteria as to the fact that much of the cultivated gram-positive bacteria may have been misclassified using phenotypic traits. It has certainly been the case that the relatively easily cultivable gram-negative colonic anaerobes have undergone many reclassifications in recent years based on 16S rRNA phylogenetic analysis. Two groups of the numerically predominant clostridia emerged, the Clostridium leptum group and the Clostridium coccoides group. Subsequent molecular studies have confirmed this identification. If you have never heard of these species, you are in good company. Virtually no studies of their metabolic characteristics have been done, and so far no genome sequences are available. These bacteria may receive more attention in the future due to interest in the possibility that the colonic microflora play a role in inflammatory bowel disease and colon cancer.
The preponderance of these previously unknown gram-positive bacteria in the normal human colon raises an interesting practical question. Should probiotics, preparations of bacteria ingested intentionally with a view of maintaining or restoring a healthy colonic microflora, include these species of gram-positive anaerobes? At present, probiotics that are available on the market consist of species of Lactobacillus or Bifidobacterium that are either not present in the human colon or are present in very low concentrations.
Given that C. leptum, C. coccoides, and their close relatives are normally predominant components of the colonic microflora, they are the logical species to use in probiotic products. Selling C. leptum or C. coccoides to the probiotics industry and to the regulatory agencies, however, is going to be a tough job. The genus Clostridium has a widely known reputation for pathogenicity, with Clostridium botulinum (botulism), Clostridium perfringens (gas gangrene), Clostridium tetani (tetanus), and Clostridium difficile (pseudomembraneous colitis) being its best known representatives. Of course, most genera that contain serious pathogens also contain a much greater number of benign species. But, at present, Clostridium is definitely considered a “scarce genus.”
The issue of probiotic formulation is not just the concern of those who patronize health food stores and natural healers. Restoration of the colonic microflora in people who have taken antibiotics that diminish the normally predominant anaerobes or have had cancer chemotherapy, which for some reason affects the microflora in some people, is a serious medical problem that has long worried gastroenterologists.
Post-antibiotic or post-chemotherapy diarrhea is a nuisance, but the consequences of microflora changes can be much more serious. The best documented
example is pseudomembraneous colitis, a condition in which depletion of the normally predominant population allows the pathogenic Clostridium difficile—usually a very minor component of the microflora and one that is found in only about five percent of the human population—to overgrow and produce two potent toxins (Brierley, 2005). The resulting damage to the colonic mucosa can be rapidly lethal within a few days.
The Forgotten Eukaryotes
A group of microbes in the colonic microflora that has been routinely ignored in most studies is the eukaryotic microbes (Moreels and Pelckmans, 2005). In developing countries, there is a eukaryotic component to the microflora that consists of protozoans, flatworms, and helminths. This eukaryotic component of the microflora has been a fact of human life for millions of years. Only during the last couple of centuries, and only in certain parts of the world, has the eukaryotic component of the microflora been virtually eliminated due to clean water and a high-quality food supply.
We tend to think of protozoa and helminths as pathogens, but is this picture entirely correct? In areas of the world where such eukaryotes are endemic, a majority of the population maintains them without any adverse effects. Is it possible that the abrupt (in evolutionary terms) loss of the eukaryotic component of the microflora by people who live in developed countries has had some adverse effects?
One recent, but still very speculative, answer to this question arises from the fact that helminthes—and possibly protozoa as well—stimulate the arm of the immune system that consists of eosinophils, mast cells, and other cell types that are also associated, on the negative side, with allergies and inflammatory bowel disease (Moreels and Pelckmans, 2005). The hypothesis that arises from this observation is that early stimulation of the GI immune system by eukaryotes allows this part of the immune response to develop normally. Conversely, failure to experience this type of stimulation may, in some people, predispose them to autoimmune disease such as allergies and inflammatory bowel disease.
Is there a eukaryotic component of the microflora in people from developed countries today? Although the eukaryotic intestinal pathogens are rarely seen, normal eukaryotic microflora may still exist. This population deserves investigation.
Nutritional Interactions
The colon has been called a second organ of digestion because dietary material that is not digested in the small intestine by human enzymes is fermented by the colonic microflora to produce short-chain fatty acids such as acetate, propionate, and butyrate (Bäckhed et al., 2005; Salyers, 1986; Salyers and Shipman,
2002). See Figure 1-5. These short-chain fatty acids are absorbed through the colonic mucosa and can be detected in the bloodstream. They are used by mammalian cells as sources of carbon and energy. In humans, the digestion of such materials as plant polysaccharides (dietary fiber) has been estimated to account for as much as 8 percent of the nutrition of the average person. In hard times, when only low quality, non-nutritious foods low in dietary fiber are available, the contribution to human nutrition may be higher.
There is also evidence that colonic bacteria produce vitamins (Salyers, 1986; Salyers and Shipman, 2002). The extent to which vitamin production is important to us is unknown, but it is interesting that scientists who breed and maintain populations of mice that lack any bacterial microflora (gnotobiotic or GF mice) have found it necessary to provide them with vitamin-enriched chow.
An extreme example of the importance of bacteria as sources of mammalian nutrition is the rumen of a cow. A cow relies absolutely on the bacterial population of its rumen for survival. Fermentation of plant polysaccharides by the ruminal bacteria provide the cow with almost 100 percent of the carbon and energy it needs. A cow unfortunate enough to experience an imbalance in its rumen microflora suffers from a painful and deadly condition called bloat.
Given that the residuum of the human diet that reaches the human colon is an important part of the diet of colonic microbes, a question that naturally arises is whether changes in the human diet cause changes in the microflora. Although numerous attempts have been made in the past, using cultivation-based techniques, to answer this question, the results are ambivalent (Salyers, 1986). Most studies report diet-associated changes in the microflora, but these changes are usually small and differ with the studies. No consensus emerges. It makes sense that the colonic microflora is in some sense “buffered” from changes in the human diet by the ability of colonic bacteria to utilize an amazing variety of substances and to change their activities very quickly (Salyers, 1986; Sonnenburg et al., 2005). The newly emerging molecular techniques should make such studies easier and more reliable and may perhaps reveal changes that were missed previously.
Possible Detoxifying Activities of the Human Colonic Microflora
Another interesting example of a beneficial interaction between cows and bacteria comes from scientists who were trying to solve the problem of animals in Australia and many tropical and subtropical countries that cope with toxic plants. The main toxin is mimosine, a plant compound that can kill a ruminant. Scientists observed that some small tropical ruminants ate plants that produced this compound with impunity. From the rumens of these animals, they isolated bacteria capable of detoxifying mimosine. These bacteria are now being introduced into cattle in Hawaii and Australia and they seem to confer some protection from mimosine-producing weeds (Jones and Megarrity, 1986).
The possible toxin-inactivating activities of the human colonic microflora have attracted some attention. There are a number of studies that demonstrate the ability of some colonic microbes to modify xenobiotics such as food dyes and other potential carcinogens. So far, the actual contribution of this type of activity to human health has not been demonstrated, and it is not clear that the compounds studied are the ones that may actually cause human health problems. It is an area that deserves attention because such materials in the diet spend a much longer time in the colon than in the rest of the GI tract. For this reason, toxic substances in the diet are likely to have a disproportionate effect on colonic mucosal cells or on cells they encounter if they are absorbed.
Looming behind interest in this topic is the question of whether colon cancer risk might be affected, positively or negatively, by activities of the colonic microflora. It is important to note that, although the assumption is generally made that xenobiotic-modifying activities of the colonic microbes is beneficial, scientists who are interested in bioremediation of toxic waste dumps have found many examples in which bacterial transformations of toxic waste components have produced substances that are even more toxic. More careful studies are needed of xenobiotic transformation that take a careful account of what compounds are actually likely to be found regularly in the human colon and of possible negative effects of bacterial activities.
Yet another activity of the colonic microflora that has not received much attention is recycling of human-produced substances, such as mucin and sloughed mucosal cells. These substances, mostly polysaccharides, contain—in addition to fermentable sugars—a substantial amount of nitrogen in the form of amino-sugars. Fermentation of these sugars releases not only short-chain fatty acids, but also ammonia. If the ammonia is absorbed from the colon by mucosal cells, it may contribute to the host’s nitrogen balance.
Genetic Interactions Among Colonic Bacteria
Recent studies in our laboratory and other laboratories have produced evidence for an old idea called the reservoir hypothesis. This hypothesis, which so far has focused on antibiotic resistance genes, is illustrated in Figure 1-6. The ability of bacteria to transfer DNA to each other by a direct cell-to-cell transfer process called conjugation has been well documented in laboratory experiments. This process is of interest because it is the type of genetic exchange between bacteria that can cross species and genus barriers. Thus, one could imagine a scenario in which bacteria in the colon are not only constantly exchanging genes with each other, but also with bacteria that have been swallowed and are only transiently present in the colon (Salyers et al., 2004).
Why would such a scenario be of any interest to us, especially since most of the bacteria coexist with us without causing any problems? The reason is that a subset of these bacteria can cause infections. For example, swallowed bacteria
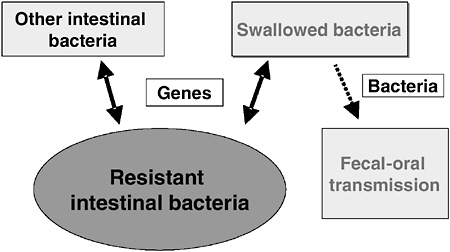
FIGURE 1-6 Flow of antibiotic resistance genes (hypothetical).
SOURCE: Salyers (2005).
include Streptococcus pneumoniae, the main cause of bacterial pneumonia and a major cause of infectious disease deaths in developed, as well as developing, countries. Moreover, normal colonic inhabitants such as Bacteroides species, E. coli, and Enterococcus species are notorious causes of potentially lethal post-surgical infections. Increasingly, these bacteria are becoming resistant to many antibiotics.
In recent years, molecular methods have been used to show how resistance gene transfers have been occurring at a surprising level in the human colon. The amount of transfer detected in the colon was surprising because in the laboratory, under supposedly optimum conditions, frequencies of transfer are relatively low. Only one in 10,000 or 100,000 possible recipients acquire transferred DNA. Yet, in the colon, some antibiotic resistance genes have obviously transferred widely within a period of a few decades (Salyers et al., 2004).
This extensive transfer has loomed large in the debate over possible adverse consequences of the use of antibiotics on the farm. The concern is that antibiotic-resistant bacteria that arise due to selection from the use of antibiotics as prophylactic treatments or as feed additives are moving through the food supply and into the human intestinal tract, where the resistance genes could be transferred to bacteria that permanently or temporarily reside in the human colon. How important this process is for the development of resistance in bacteria—that are serious causes of human infections—has not been conclusively established, but it remains a concern.
The subject of genetic interactions could be extended from concern about bacterium-to-bacterium transfer to a larger scale. Conjugation can transfer DNA, at least in the laboratory, not only from prokaryote to prokaryote but also from
prokaryote to yeast. Do the bacteria that come into contact with our mucosal cells ever transfer DNA to them, and if they do, is this DNA ever fixed in the eukaryotic recipients? The latter question is important because it is unlikely that there will be much sequence homology between the bacteria and eukaryotic cell, nor are bacterial genes that encode the integrases that mediate homology-independent integration events likely to be expressed. Are there forms of illegitimate recombination that mediate integration of bacterial DNA into the genome of a eukaryotic host? Such events have been well documented in the case of the transfer of DNA from Agrobacterium tumifaciens to plants, but this phenomenon has not been reported for other eukaryotes. The lack of such reports may be more a reflection of lack of studies than lack of actual phenomena. Moreover, one could argue that even if such events occur in the colon, most mucosal cells are short lived and are sloughed into the lumen of the colon. There are, however, colonic stem cells whose germline is much more permanent. If the stem cells receive bacterial DNA and if it becomes fixed in their genome, such an event could have long-term effects. This is yet another area that has so far received no attention, but might be worth considering in the future.
At the very least, it is certain that transfer among bacteria in the colon involves many genes other than antibiotic resistance genes. Genes that enhance the ability of bacteria to cause disease or to survive in new niches such as the roots of plants are known to be transferred as well. The trafficking in genes among intestinal bacteria could have more global effects than just the transfer of antibiotic resistance genes.
What Needs to Be Done?
This discussion has surveyed briefly some of the issues that have been raised in connection with the normal microflora of the human colon and its possible role, if it becomes unbalanced, in human disease. A theme that has run through this article is the surprising lack of information about some very basic aspects of the human microflora. We know remarkably little about the “silent majority” of gram-positive anaerobes that are the basis of the microflora. There is little reliable information about how much person-to-person variation there is, whether nonhuman animals are good models for the human microflora or the way in which changes in diet or medical therapies affect the microflora.
One excuse given in the past for this dismal record is that cultivation-based analyses were not only time consuming and expensive, but also inherently unreliable given the variety of bacteria involved. The new molecular methods remove this technical barrier and make it possible to survey large numbers of samples cheaply and reliably. Surprisingly, there is still no microarray that represents the predominant species of colonic bacteria, although rumors have been circulating during the past several years that one laboratory or another is hard at work on developing such a microarray. At present, no such microarray is commercially available.
Moreover, having a microarray is only the first step to having a valuable screening tool. Given the large number of bacteria species in the colon and the possible presence of interfering substances in human intestinal contents or feces, it will be necessary to do a substantial amount of work on optimizing protocols for use of such a microarray in the real world. Microarrays that could detect expression of bacterial genes in the colon are also needed, but pose even more daunting challenges. This remark is not meant to make it sound as if such challenges pose a barrier that is unscalable at the present time. Microbiologists working with even more challenging environments such as soil have managed to make impressive progress in recent years. Enlisting their help makes a lot of sense.
An even bigger challenge facing us, however, is how to switch from a mind set that focuses on one microbe at a time, with disease as the end point, to a mind set that can comprehend population changes. Once again, the environmental microbiologists have been blazing this intellectual trail for the last decade and would be valuable allies in making the transition from viewing colonic bacteria one microbe at a time to viewing it as a population. Some promising work of this type is beginning to appear.
Perhaps the greatest challenge of all is educating the funding agencies. Understandably, research on human health issues has focused on disease. “Normal” is not an easily fundable research objective. This perspective needs to be changed. The irony is that the funding agencies that have supported the revolution in bacterial population studies, such as the Department of Energy and the National Science Foundation, have been discouraged from making any forays into issues traditionally associated with human or animal health. This means that the microfloras of mammalian bodies have fallen into a funding vacuum unless they can be tied to disease.
No one questions the importance of studies on inflammatory bowel disease or colon cancer, but without a normal baseline or criterion for how external factors such as diet might affect this baseline, modern studies of these diseases are likely to fall to the same fate that was previously met by the cultivation-based studies: studies whose results contradict each other because they lack any consensus as to what is normal and what amount of variation can be counted as significant. The one group that seems to have become immune to this problem is the dental microbiologists. Their example would be a good one for scientists and funding agencies interested in emulating the colonic microflora and its multifaceted interactions with the human body.
Philosophers and theologians are fond of telling us that humans are the crown of creation, but in reality we are microbial planets, the proverbial free lunch for microbes. They were here billions of years before we appeared. They made the Earth habitable for us, but it is well to remember that we were born into their world and we can only know ourselves if we understand our complex interactions with them. Disease is only a tiny part of this picture. Perhaps the time has come to remove the jeweler’s eyepiece we have been using to examine a detail in the painting and take a good look at the whole picture.
ANNEX 1-1
TABLE 1-1 Comparison of the Glycoside Hydrolase and Polysaccharide Lyases in the Human, Mouse, and B. thetaiotaomicron Genomes
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
GH 1 |
beta-glucosidase (EC 3.2.1.21) beta-galactosidase (EC 3.2.1.23) beta-mannosidase (EC 3.2.1.25) beta-glucuronidase (EC 3.2.1.31) beta-D-fucosidase (EC 3.2.1.38) Phlorizin hydrolase (EC 3.2.1.62) 6-phospho-beta-galactosidase (EC 3.2.1.85) 6-phospho-beta-glucosidase (EC 3.2.1.86) strictosidine beta-glucosidase (EC 3.2.1.105) lactase (EC 3.2.1.108) prunasin beta-glucosidase (EC 3.2.1.118) oligoxyloglucan beta-glycosidase (EC 3.2.1.120) raucaffricine beta-glucosidase (EC 3.2.1.125) thioglucosidase (EC 3.2.1.147) beta-primeverosidase (EC 3.2.1.149) hydroxyisourate hydrolase (EC 3.-.-.-) |
6 |
5 |
0 |
|
GH 2 |
beta-galactosidase (EC 3.2.1.23) beta-mannosidase (EC 3.2.1.25) beta-glucuronidase (EC 3.2.1.31) |
3 |
2 |
33 |
|
GH 3 |
beta-glucosidase (EC 3.2.1.21) xylan 1,4-beta-xylosidase (EC 3.2.1.37) beta-N-acetylhexosaminidase (EC 3.2.1.52) glucan 1,3-beta-glucosidase (EC 3.2.1.58) glucan 1,4-beta-glucosidase (EC 3.2.1.74) exo-1,3-1,4-glucanase (EC 3.2.1.-) alpha-L-arabinofuranosidase (EC 3.2.1.55) |
1 |
0 |
10 |
|
GH 5 |
chitosanase (EC 3.2.1.132) beta-mannosidase (EC 3.2.1.25) cellulase (EC 3.2.1.4) glucan 1,3-beta-glucosidase (EC 3.2.1.58) licheninase (EC 3.2.1.73) glucan endo-1,6-beta-glucosidase (EC 3.2.1.75) |
0 |
0 |
1 |
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
|
mannan endo-1,4-beta-mannosidase (EC 3.2.1.78) endo-1,4-beta-xylanase (EC 3.2.1.8) cellulose 1,4-beta-cellobiosidase (EC 3.2.1.91) endo-1,6-beta-galactanase (EC 3.2.1.-) beta-1,3-mannanase (EC 3.2.1.-) |
|
|
|
|
GH 9 |
endoglucanase (EC 3.2.1.4) cellobiohydrolase (EC 3.2.1.91) beta-glucosidase (EC 3.2.1.21) |
1 |
0 |
0 |
|
GH 13 |
alpha-amylase (EC 3.2.1.1) pullulanase (EC 3.2.1.41) cyclomaltodextrin glucanotransferase (EC 2.4.1.19) cyclomaltodextrinase (EC 3.2.1.54) trehalose-6-phosphate hydrolase (EC 3.2.1.93) oligo-alpha-glucosidase (EC 3.2.1.10) maltogenic amylase (EC 3.2.1.133) neopullulanase (EC 3.2.1.135) alpha-glucosidase (EC 3.2.1.20) maltotetraose-forming alpha-amylase (EC 3.2.1.60) isoamylase (EC 3.2.1.68) glucodextranase (EC 3.2.1.70) maltohexaose-forming alpha-amylase (EC 3.2.1.98) branching enzyme (EC 2.4.1.18) trehalose synthase (EC 5.4.99.16) 4-alpha-glucanotransferase (EC 2.4.1.25) maltopentaose-forming alpha-amylase (EC 3.2.1.-) amylosucrase (EC 2.4.1.4) sucrose phosphorylase (EC 2.4.1.7) malto-oligosyltrehalose trehalohydrolase (EC 3.2.1.141) isomaltulose synthase (EC 5.4.99.11) |
7 |
5 |
10 |
|
GH 16 |
xyloglucan:xyloglucosyl transferase (EC 2.4.1.207) keratan-sulfate endo-1,4-beta-galactosidase (EC 3.2.1.103) |
0 |
0 |
3 |
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
|
glucan endo-1,3-beta-D-glucosidase (EC 3.2.1.39) endo-1,3(4)-beta-glucanase (EC 3.2.1.6) licheninase (EC 3.2.1.73) agarase (EC 3.2.1.81) kappa-carrageenase (EC 3.2.1.83) |
|
|
|
|
GH 18 |
chitinase (EC 3.2.1.14) endo-beta-N-acetylglucosaminidase (EC 3.2.1.96) non-catalytic proteins: xylanase inhibitors concanavalin B narbonin |
8 |
11 |
7 |
|
GH 20 |
beta-hexosaminidase (EC 3.2.1.52) lacto-N-biosidase (EC 3.2.1.140) |
2 |
2 |
15 |
|
GH 22 |
lysozyme type C (EC 3.2.1.17) and alpha-lactalbumins |
6 |
7 |
0 |
|
GH 23 |
lysozyme type G (EC 3.2.1.17) |
4 |
1 |
3 |
|
GH 24 |
lysozyme (EC 3.2.1.17) |
1 |
0 |
0 |
|
GH 25 |
lysozyme (EC 3.2.1.17) |
0 |
0 |
1 |
|
GH 27 |
alpha-galactosidase (EC 3.2.1.22) alpha-N-acetylgalactosaminidase (EC 3.2.1.49) isomalto-dextranase (EC 3.2.1.94) |
2 |
2 |
5 |
|
GH 28 |
polygalacturonase (EC 3.2.1.15) exo-polygalacturonase (EC 3.2.1.67) exo-polygalacturonase (EC 3.2.1.82) rhamnogalacturonase (EC not defined) |
0 |
0 |
9 |
|
GH 29 |
alpha-L-fucosidase (EC 3.2.1.51) |
2 |
2 |
9 |
|
GH 30 |
glucosylceramidase (EC 3.2.1.45) beta-1,6-glucanase (EC 3.2.1.75) |
2 |
1 |
2 |
|
GH 31 |
alpha-glucosidase (EC 3.2.1.20) glucoamylase (EC 3.2.1.3) sucrase-isomaltase (EC 3.2.1.48) (EC 3.2.1.10) |
8 |
7 |
6 |
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
|
alpha-xylosidase (EC 3.2.1.-) alpha-glucan lyase (EC 4.2.2.13) isomaltosyltransferase (EC 2.4.1.-) |
|
|
|
|
GH 32 |
invertase (EC 3.2.1.26) inulinase (EC 3.2.1.7) levanase (EC 3.2.1.65) exo-inulinase (EC 3.2.1.80) sucrose:sucrose 1-fructosyl transferase (EC 2.4.1.99) fructan fructan 1-fructosyltransferase (EC 2.4.1.100) |
0 |
0 |
4 |
|
GH 33 |
sialidase or neuraminidase (EC 3.2.1.18) trans-sialidase (EC 2.4.1.-) |
4 |
4 |
1 |
|
GH 35 |
beta-galactosidase (EC 3.2.1.23) |
5 |
4 |
3 |
|
GH 36 |
alpha-galactosidase (EC 3.2.1.22) alpha-N-acetylgalactosaminidase (EC 3.2.1.49) stachyose synthase (EC 2.4.1.67) raffinose synthase (EC 2.4.1.82) |
0 |
0 |
3 |
|
GH 37 |
trehalase (EC 3.2.1.28) |
1 |
1 |
0 |
|
GH 38 |
alpha-mannosidase (EC 3.2.1.24) (EC 3.2.1.114) |
5 |
5 |
2 |
|
GH 39 |
alpha-L-iduronidase (EC 3.2.1.76) beta-xylosidase (EC 3.2.1.37) |
1 |
1 |
0 |
|
GH 42 |
beta-galactosidase (EC 3.2.1.23) |
0 |
0 |
1 |
|
GH 43 |
beta-xylosidase (EC 3.2.1.37) alpha-L-arabinofuranosidase (EC 3.2.1.55) arabinanase (EC 3.2.1.99) xylanase (EC 3.2.1.8) |
0 |
0 |
31 |
|
GH 47 |
alpha-mannosidase (EC 3.2.1.113) |
8 |
8 |
0 |
|
GH 51 |
alpha-L-arabinofuranosidase (EC 3.2.1.55) endoglucanase (EC 3.2.1.4) |
0 |
0 |
4 |
|
GH 53 |
endo-1,4-beta-galactanase (EC 3.2.1.89) |
0 |
0 |
1 |
|
GH 56 |
hyaluronidase (EC 3.2.1.35) |
6 |
7 |
0 |
|
GH 57 |
alpha-amylase (EC 3.2.1.1) 4-alpha-glucanotransferase (EC 2.4.1.-) |
0 |
0 |
1 |
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
|
alpha-galactosidase (EC 3.2.1.22) amylopullulanase (EC 3.2.1.41) |
|
|
|
|
GH 59 |
galactocerebrosidase (EC 3.2.1.46) |
1 |
1 |
0 |
|
GH 63 |
processing alpha-glucosidase (EC 3.2.1.106) |
1 |
1 |
0 |
|
GH 65 |
trehalase (EC 3.2.1.28) maltose phosphorylase (EC 2.4.1.8) trehalose phosphorylase (EC 2.4.1.64) kojibiose phosphorylase (EC 2.4.1.-) |
1 |
1 |
0 |
|
GH 66 |
cycloisomaltooligosaccharide glucanotransferase (EC 2.4.1.-) dextranase (EC 3.2.1.11) |
0 |
0 |
1 |
|
GH 67 |
alpha-glucuronidase (EC 3.2.1.139) |
0 |
0 |
1 |
|
GH 73 |
endo-beta-N-acetylglucosaminidase (EC 3.2.1.96) beta-1,4-N-acetylmuramoylhydrolase (EC 3.2.1.17) |
0 |
0 |
1 |
|
GH 76 |
alpha-1,6-mannanase (EC 3.2.1.101) |
0 |
0 |
10 |
|
GH 77 |
amylomaltase or 4-alpha-glucanotransferase (EC 2.4.1.25) |
0 |
0 |
1 |
|
GH 78 |
alpha-L-rhamnosidase (EC 3.2.1.40) |
0 |
0 |
6 |
|
GH 79 |
endo-beta-glucuronidase / heparanase (EC 3.2.1.-) |
2 |
1 |
0 |
|
GH 84 |
N-acetyl beta-glucosaminidase (EC 3.2.1.52) hyaluronidase (EC 3.2.1.35) |
1 |
1 |
1 |
|
GH 85 |
endo-beta-N-acetylglucosaminidase (EC 3.2.1.96) |
1 |
1 |
0 |
|
GH 88 |
unsaturated glucuronyl hydrolases (EC 3.2.1.-) |
0 |
0 |
4 |
|
GH 89 |
alpha-N-acetylglucosaminidase (EC 3.2.1.50) |
1 |
1 |
3 |
|
GH 92 |
alpha-1,2-mannosidase (EC 3.2.1.-) |
0 |
0 |
23 |
|
GH 93 |
exo-arabinanase (EC 3.2.1.55) |
0 |
0 |
1 |
|
GH 95 |
alpha-L-fucosidase (EC 3.2.1.51) |
0 |
0 |
5 |
|
GH 97 |
alpha-glucosidase (EC 3.2.1.20) |
0 |
0 |
10 |
|
Glycoside Hydrolase (GH) or Polysaccharide Lyase (PL) Family |
Known Activities |
Homo sapiens |
Mus musculus |
B. theta |
|
PL 1 |
pectate lyase (EC 4.2.2.2) pectin lyase (EC 4.2.2.10) |
0 |
0 |
5 |
|
PL 8 |
hyaluronate lyase (EC 4.2.2.1) chondroitin ABC lyase (EC 4.2.2.4) chondroitin AC lyase (EC 4.2.2.5) xanthan lyase (EC 4.2.2.12) |
0 |
0 |
4 |
|
PL 9 |
pectate lyase (EC 4.2.2.2) exopolygalacturonate lyase (EC 4.2.2.9) |
0 |
0 |
2 |
|
PL 10 |
pectate lyase (EC 4.2.2.2) |
0 |
0 |
1 |
|
PL 11 |
rhamnogalacturonan lyase (EC 4.2.2.-) |
0 |
0 |
1 |
|
PL 12 |
heparin-sulfate lyase (EC 4.2.2.8) |
0 |
0 |
2 |
|
PL 13 |
heparin lyase (EC 4.2.2.7) |
0 |
0 |
1 |
|
NOTE: Based on information available at http://afmb.cnrs-mrs.fr/CAZY/. SOURCE: Bäckhed et al. (2005). |
||||
TABLE 1-2 Effects of the Microbiota on Host Biology, Defined by Comparing Germ-Free (GF) and Conventionally-Raised (CONV-R) Rodents
|
Phenotype |
Comments |
References |
|
Gut structure and function |
||
|
GF mice and rats have enlarged ceca |
Likely a consequence of accumulation of undegraded host and dietary polysaccharides that bind water |
(Gordon et al., 1966a; Wostmann and Bruckner-Kardoss, 1959) |
|
GF mice villi are thinner |
Mesenchymal core is less cellular; reduced immune population (see below) |
(Banasaz et al., 2002) |
|
GF mice have slower epithelial renewal rates |
Evolutionary conserved response, also seen in germ-free zebrafish; mechanisms to be defined (e.g., what is contribution of undeveloped mucosal immune system). Epithelial regeneration markedly reduced in GF mice with dextran sodium sulfate-induced colitis: effect requires Myd88-dependent signaling through mesenchymal cells and recruitment of pericryptal macrophages. |
(Banasaz et al., 2002; Pull et al., 2005; Rawls et al., 2004; Savage et al., 1981) |
|
GF mice have slower gut motility |
Migrating motor complexes move more slowly with more restricted spatial distribution; detailed comparisons of the enteric nervous system in GF versus CONV-R animals are needed; potential implications concerning the pathogenesis of irritable bowel syndrome in humans |
(Abrams and Bishop, 1967) |
|
GF rats have reduced bile acid deconjugation |
Impaired excretion of bile acids |
(Gustafsson et al., 1966) |
|
GF rats produce more bile acids |
This effect, together with deconjugation defect, results in increased bile acid pools; impact on cholesterol homeostasis |
(Wostmann, 1973) |
|
GF rats have fewer enteroendocrine cells |
There are multiple subpopulations on enteroendocrine cells, defined by their peptide hormone products. The effects of microbiota on these subpopulations has not been well-defined. |
(Uribe et al., 1994) |
|
GF rats have mesenteric microvaculture that is hyporesponsive to norepinephrine and pitressin |
Kallikrein-like molecules produced by GF cecum may contribute to phenotype |
(Baez and Gordon, 1971; Gordon, 1964) |
|
Phenotype |
Comments |
References |
|
GF mice have reduced capillary network complexity in their villus mesenchymal cores |
Potential influence on nutrient absorption and mucosal barrier functions |
(Stappenbeck et al., 2002) |
|
Cardiac function |
||
|
GF mice and rats have lower cardiac weight |
Detailed morphometric studies lacking, mechanism not described |
(Bruckner-Kardoss and Wostmann, 1974; Gordon et al., 1966a, 1966b; Wostmann et al., 1982) |
|
GF rats have lower cardiac output |
Physiological mechanisms, myocardial energetics not defined |
(Gordon et al., 1963; Wostmann et al., 1968) |
|
Endocrine system |
||
|
GF mice are more insulin sensitive than CONV-R animals |
Could reflect, at least in part, reduced fat stores; other similarities to mice that are subjected to chronic caloric restriction need to be delineated |
(Bäckhed et al., 2004) |
|
GF mice have lower circulating levels of leptin and adiponectin |
Associated with reduced fat stores |
(Bäckhed et al., 2004; Bäckhed and Gordon, unpublished observation) |
|
Nutrition/metabolism |
||
|
GF mice are vulnerable to vitamin deficiencies |
The gut microbiota produces vitamin K, B6, B12, biotin, folic acid, and pantothenate |
(Gustafsson et al., 1962; Sumi et al., 1977; Wostmann et al., 1963) |
|
GF rats do not extract as much energy from their diet |
See text for details |
(Wostmann et al., 1983; Yamanaka et al., 1977) |
|
Phenotype |
Comments |
References |
|
GF mice and rats have lower metabolism (VO2) |
Hexose monophosphate shunt and TCA cycle activity reduced. Mechanisms remain ill-defined; possible role for leptin |
(Bruckner-Kardoss and Wostmann, 1978; Levenson et al., 1969; Wostmann et al., 1982, 1968) |
|
GF mice absorb more cholesterol |
GF mice have large bile acid pools |
(Gustaffsson et al., 1975) |
|
Immune system development |
||
|
B-cells and immunoglobulin secretion |
GF mice have 40–1000 fold less serum IgM/IgG and intestinal IgA |
(Horsfall et al., 1978) |
|
Natural immunoglobulin |
GF mice have normal levels of natural Ig and B1 B-cells. However the majority of IgA that reacts with components of the microbiota originates from B2 B-cells. While class switching can occur in a T-cell independent pathway, most reactive antibodies are generated in a classical T-cell dependent manner. |
(Bos et al., 2001; Macpherson and Uhr, 2004) Ochsenbein et al., 1999; Thurnheer et al., 2003) |
|
Anti-‘commnesal’ IgA |
The segmented filamentous bacteria (SBF; Clostridia) are stronger inducers of intestinal IgA than other bacteria (including Bacteroidetes). Interestingly, SBF found to be enriched in the intestines of mice lacking secretory IgA. |
(Fagarasan et al., 2002; Suzuki et al., 2004) |
|
Intraepithelial lymphocytes |
Both γδ and αβ T-cells are significantly decreased in the intraepithelial lymphocyte population of GF mice: γδ>αβ |
(Bandeira et al., 1990) |
|
Cytotoxic T lymphocytes |
Size and composition of CD8 repertoire to non-gut related antigen is unaffected by presence of absence of the microbiota. |
(Bousso et al., 2000) |
|
T-cells (αβ) |
General T-cell function and number is not reduced in GF rats |
(Nielsen, 1972) |
|
T-cells (γδ) |
Decreased number of mesenteric lymph node γδ T-cells in GF mice |
(Yoshikai et al., 1988) |
|
Mucosal-associated invariant T-cells |
Invariant Vα19-Jα33 TCR+ cells located in lamina propria, fail to develop in GF mice |
(Treiner et al., 2003) |
|
CD4+CD25+ T-cells |
Levels and function are the same in GF and CONV-R mice |
(Gad et al., 2004) |
|
Phenotype |
Comments |
References |
|
Modulation of Immunity and Disease |
||
|
GF mice are resistant to developing inflammatory bowel disease |
GF mice with aberrations in T-cell development and function (IL2–/–, IL10–/–, TCRα–/–) do not develop spontaneous colitis, unlike their CONV-R counterparts |
(Dianda et al., 1997; (Mizoguchi et al., 2000; Sadlack et al., 1993; Sellon et al., 1998) |
|
GF rodents have reduced susceptibility to arthritis |
CONV-R β2-microglobulin-HLA-B27 transgenic rats develop spontaneous colitis and arthritis; B10.BR mice develop enthesopathy; GF counterparts do not |
(Rath et al., 1996; Rehakova et al., 2000) |
|
GF NOD mice have higher rate of autoimmune diabetes |
No primary papers; phenotype mentioned in reviews |
(Bach, 1994a,1994b; Wicker et al., 1987) |
|
Xenobiotic metabolism |
||
|
The gut microbiota metabolizes dietary oxalates |
50 percent of GF rats have kidney stones versus 0 percent for CONV-R. Oxalate-degrading Oxalobacter formigenes reduces kidney stone formation in a rat model; currently in clinical trials. |
(Allison et al., 1985; (Gustaffson and Norman, 1962; Sidhu et al., 2001) |
|
The gut microbiota is required for nitroreduction of xenobiotics |
See http://umbbd.ahc.umn.edu/ for a database of microbial biocatalytic/biodegradation reactions involving xenobiotics and various chemical compound classes. Consider microbiota when defining factors that influence bioavailability of orally administered drugs. |
(Larsen et al., 1998) |
|
General health and disease |
||
|
GF rats live longer than their CONV-R counterparts |
Mechanism to be determined; possible similarities to insulin-sensitive, calorie-restricted animals |
(Gordon et al., 1966b; Pollard and Wostmann, 1985) |
|
Removing the microbiota decreases incidence of intestinal neoplasia in some mouse models |
GF IL-10–/– mice and Tgfβ-1–/–, Rag2–/–; Tcrb–/–, p53–/–; and Gpx1–/–,Gpx2–/– compound homozygous knockout mice have reduced inflammation and tumor formation. Microbial communities associated with pre-neoplastic and neoplastic lesions in mouse models and humans have yet to be enumerated. |
(Balish and Warner, 2002; Chu et al., 2004; Engle et al., 2002; Kado et al., 2001) |
|
GF mice are more radioresistant |
Mechanism to be defined; potential implications for ameliorating GI syndrome in patients receiving radiotherapy for abdominal/pelvic malignancies |
(Matsuzawa, 1965) |
|
SOURCE: Bäckhed et al. (2005). |
||
TABLE 1-3 Six Ways Environmental Engineers Improve Bioreactor Efficiency
|
Approach |
Method |
Reference |
|
Engineering |
Altering reactor configuration: e.g., changing to a plug-flow reactor with higher substrate levels at the entrance to the reactor increases conversion rates according to Monod kinetics and promotes staging of the reactor by compartmentalization. This results in differing local environmental conditions that are optimal for sequential functional subpopulations. |
(Angenent et al., 2002; Rittmann and McCarty, 2001) |
|
Engineering |
Removal of product. Even if the product is not inhibiting, thermodynamics dictate that the energy for the conversion will be higher if the concentration of products are maintained at a very low level. |
(Stams, 1994; Thauer et al., 1977) |
|
Operational |
Elongation of the sludge retention time (mean residence time) by adding an attachment matrix to the reactor. |
(Zaiat et al., 2001) |
|
Microbial Ecology |
Selection of a different community: e.g., sulfate reducers (SRBs) are not wanted in anaerobic methanogenic bioreactors because they produce a toxic product—H2S. Preventing SRBs improves reactor efficiency. |
(Elferink et al., 1994) |
|
Microbial Ecology |
Bioaugmentation: addition of a functional microbe that can remove a specific substrate at higher rates. |
(Abeysinghe et al., 2002; Patureau et al., 2001) |
|
Microbial Ecology |
Bacteriophage addition: bacteriophage modulate bacterial population dynamics in bioreactors and, therefore, can be used to optimize reactor efficiency (e.g., as a biocontrol agent to prevent foam formation by microbes). |
(Hantula et al., 1991; Lu et al., 2003; Thomas et al., 2002) |
|
SOURCE: Bäckhed et al. (2005). |
||
REFERENCES
Abeysinghe DH, De Silva DG, Stahl DA, Rittmann BE. 2002. The effectiveness of bioaugmentation in nitrifying systems stressed by a washout condition and cold temperature. Water and Environmental Research 74(2):187–199.
Abrams GD, Bishop JE. 1967. Effect of the normal microbial flora on gastrointestinal motility. Proceedings for the Society of Experimental Biology and Medicine 26(1):301–304.
Allison MJ, Dawson KA, Mayberry WR, Foss JG. 1985. Oxalobacter formigenes gen. nov., sp. nov.: Oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Archives of Microbiology 141(1):1–7.
Angenent LT, Zheng D, Sung S, Raskin L. 2002. Microbial community structure and activity in a compartmentalized, anaerobic bioreactor. Water and Environmental Research 74(5):450–461.
Bach JF. 1994a. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocrine Reviews 15(4):516–542.
Bach JF. 1994b. Predictive medicine in autoimmune diseases: From the identification of genetic predisposition and environmental influence to precocious immunotherapy. Clinical Immunology and Immunopathology 72(2):156–161.
Bäckhed F, Gordon JI. Unpublished observation as cited in Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920.
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Science USA 101(44):15718–15723.
Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920.
Baez S, Gordon HA. 1971. Tone and reactivity of vascular smooth muscle in germfree rat mesentery. Journal of Experimental Medicine 134(4):846–856.
Balish E, Warner T. 2002. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. American Journal of Pathology 160(6):2253–2257.
Banasaz M, Norin E, Holma R, Midtvedt T. 2002. Increased enterocyte production in gnotobiotic rats mono-associated with Lactobacillus rhamnosus GG. Applied Environmental Microbiology 68(6):3031–3034.
Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, Coutinho A. 1990. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. Journal of Experimental Medicine 172(1):239–244.
Bos NA, Jiang HQ, Cebra JJ. 2001. T cell control of the gut IgA response against commensal bacteria. Gut 48(6):762–764.
Bousso P, Lemaitre F, Laouini D, Kanellopoulos J, Kourilsky P. 2000. The peripheral CD8 T cell repertoire is largely independent of the presence of intestinal flora. International Immunology 12(4):425–430.
Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. 2003. Metagenomic analyses of an uncultured viral community from human feces. Journal of Bacteriology 185(20): 6220–6223.
Brierley R. 2005. Clostridium difficile—a new threat to public health? Lancet Infectious Diseases 5(9):535.
Bruckner-Kardoss E, Wostmann BS. 1974. Blood volume of adult germfree and conventional rats. Laboratory Animal Science 24(4):633–635.
Bruckner-Kardoss E, Wostmann BS. 1978. Oxygen consumption of germfree and conventional mice. Laboratory Animal Science 28(3):282–286.
Brune A, Friedrich M. 2000. Microecology of the termite gut: structure and function on a microscale. Current Opinion in Microbiology 3(3):263–269.
CAZY (Carbohydrate-Active Enzymes). 2005. Homepage. [Online]. Available: http://afmb.cnrs-mrs.fr/CAZY [accessed December 20, 2005].
Cerdeoño-Tárraga AM, Patrick S, Crossman LC, Blakely G, Abratt V, Lennard N, Poxton I, Duerden B, Harris B, Quail MA, Barron A, Clark L, Corton C, Doggett J, Holden MTG, Larke N, Line A, Lord A, Norbertczak H, Ormond D. 2005. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 307(5714):1463–1465.
Chacon O, Bermudez LE, Barletta RG. 2004. Johne’s disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annual Review of Microbiology 58:329–363.
Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. 2004. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Research 64(3):962–968.
De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. 2005. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. Journal of Clinical Microbiology 43(11):5588–5592.
Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. 1997. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. American Journal of Pathology 150(1):91–97.
Dunbar J, Barns SM, Ticknor LO, Kuske CR. 2002. Empirical and theoretical bacterial diversity in four Arizona soils. Applied Environmental Microbiology 68(6):3035–3045.
Elferink O, Visser A, Stams A. 1994. Sulfate reduction in methanogenic bioreactors. FEMS Microbiology Review 15(2–3):119.
Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. 2002. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Research 162(22):6362–6366.
Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. 2002. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298(5597):1424–1427.
Fernandez AS, Hashsham SA, Dollhopf SL, Raskin L, Glagoleva O, Dazzo FB, Hickey RF, Criddle CS, Tiedje JM. 2000. Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Applied Environmental Microbiology 66(9):4058–4067.
Flegal KM, Troiano RP. 2000. Changes in the distribution of body mass index of adults and children in the US population. International Journal of Obesity-Related Metabolic Disorders 24(7): 807–818.
Fuhrman JA. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399(6736): 541–548.
Gad M, Pedersen AE, Kristensen NN, Claesson MH. 2004. Demonstration of strong enterobacterial reactivity of CD4+CD25− T cells from conventional and germ-free mice which is counter-regulated by CD4+CD25+ T cells. European Journal of Immunology 34(3):695–704.
Galperin MY. 2004. The Molecular Biology Database Collection: 2004 update. Nucleic Acids Research 32(Database issue):D3–D22.
Genome Sequencing Center (GSC). 2005. Comparative microbial genome analysis of the human-Bacteroides symbiosis. [Online]. Available: http://genomeold.wustl.edu/projects/bacterial/cmpr_microbial/index.php?cmpr_microbial01 [accessed: December 21, 2005].
Goebel BM, Stackebrandt E. 1994. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Applied Environmental Microbiology 60(5):1614–1621.
Gordon HA. 1965. A bioactive substance in the caecum of germ-free animals: Demonstration of a bioactive substance in caecal contents of germ-free animals. Nature 205(4971):571.
Gordon HA, Wostmann BS, Bruckner-Kardoss E. 1963. Effects of microbial flora on cardiac output and other elements of blood circulation. Proceedings of the Society of Experimental Biology and Medicine 114:301–304.
Gordon HA, Bruckner-Kardoss E, Wostmann BS. 1966a. Acta Anatomica 64:367.
Gordon HA, Bruckner-Kardoss E, Wostmann BS. 1966b. Aging in germ-free mice: Life tables and lesions observed at natural death. Journal of Gerontology 21(3):380–387.
Guillemin K. 2005 (March 17). Session I: Host-Pathogen Interactions: Defining the Concepts of Pathogenicity, Virulence, Colonization, Commensalism, and Symbiosis. C. Presentation at the Forum on Microbial Threats Workshop Ending the War Metaphor: The Changing Agenda for Unraveling the Host-Microbe Relationship. Washington, D.C., Institute of Medicine, Forum on Microbial Threats.
Gustafsson BE, Norman A. 1962. Urinary calculi in germfree rats. Journal of Experimental Medicine 116:273–284.
Gustafsson BE, Daft FS, McDaniel DE, Smith JC, Fitzgerald RJ. 1962. Effects of vitamin K-active compounds and intestinal microorganisms in vitamin K-deficient germfree rats. Journal of Nutrition 78:461–468.
Gustafsson BE, Midtvedt T, Norman A. 1966. Isolated fecal microorganisms capable of 7-alpha-dehydroxylating bile acids. Journal of Experimental Medicine 123(2):413–432.
Gustafsson BE, Einarsson K, Gustafsson J. 1975. Influence of cholesterol feeding on liver microsomal metabolism of steroids and bile acids in conventional and germ-free rats. Journal of Biological Chemistry 250(21):8496–8502.
Hantula J, Kurki A, Vuoriranta P, Bamford DH. 1991. Ecology of bacteriophages infecting activated sludge bacteria. Applied Environmental Microbiology 57(8):2147–2151.
Horsfall DJ, Cooper JM, Rowley D. 1978. Changes in the immunoglobulin levels of the mouse gut and serum during conventionalisation and following administration of Salmonella typhimurium. Austrian Journal of Experimental Biology and Medical Science 56(6):727–735.
Hugenholtz P, Goebel BM, Pace NR. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. Journal of Bacteriology 180(18):4765–4774.
Ishikawa K, Satoh Y, Tanaka H, Ono K. 1986. Influence of conventionalization on small-intestinal mucosa of germ-free Wistar rats: Quantitative light microscopic observations. Acta Anaomicat (Basel) 127:296–302.
Jones RJ, Megarrity RG. 1986. Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome the toxicity of Leucaena. Australian Veterinary Journal 63(8):259–262.
Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Morotomi M. 2001. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Research 61(6):2395–2398.
Kuwahara T, Yamashita A, Hirakawa H, Nakayama H, Toh H, Okada N, Kuhara S, Hattori M, Hayashi T, Ohnishi Y. 2004. Genomic analysis of Bacteroides fragilis reveals extensive DNA inversions regulating cell surface adaptation. Proceedings of the National Academy of Science USA 101(41):14919–14924.
Larsen GL, Huwe JK, Bakke JE. 1998. Intermediary metabolism of pentachloronitrobenzene in the control and germ-free rat and rat with cannulated bile ducts. Xenobiotica 28(10):973–984.
Levenson SM, Doft F, Lev M, Kan D. 1969. Influence of microorganisms on oxygen consumption, carbon dioxide production and colonic temperature of rats. Journal of Nutrition 97(4):542–552.
Ley RE, Gordon JI. Unpublished data as cited in Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920.
Lu Z, Breidt F, Plengvidhya V, Fleming HP. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Applied Environmental Microbiology 69(6):3192–3202.
Macpherson AJ, Uhr T. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303(5664):1662–1665.
Matsuzawa T. 1965. Survival time in germfree mice after lethal whole body x-irradiation. Tohoku Journal of Experimental Medicine 85:257–263.
McCann KS. 2000. The diversity-stability debate. Nature 405(6783):228–233.
McGarr SE, Ridlon JM, Hylemon PB. 2005. Diet, anaerobic bacterial metabolism and colon cancer: A review of the literature. Journal of Clinical Gastroenterology 39(2):98–109.
McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. American Journal of Clinical Nutrition 39(2):338–342.
Miller TL, Wolin MJ. 1982. Enumeration of Methanobrevibacter smithii in human feces. Archives of Microbiology 131(1):14–18.
Mizoguchi A, Mizoguchi E, Saubermann LJ, Higaki K, Blumberg RS, Bhan AK. 2000. Limited CD4 T-cell diversity associated with colitis in T-cell receptor alpha mutant mice requires a T helper 2 environment. Gastroenterology 119(4):983–995.
Moore WE, Holdeman LV. 1974. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Applied Microbiology 27(5):961–979.
Moore WE, Cato EP, Holdeman LV. 1978. Some current concepts in intestinal bacteriology. American Journal of Clinical Nutrition 31(10 Suppl):S33–S42.
Moreels TG, Pelckmans PA. 2005. Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflammatory Bowel Disease 11(2):178–184.
Nielsen HE. 1972. Reactivity of lymphocytes from germfree rats in mixed leukocyte culture and in graft-versus-host reaction. Journal of Experimental Medicine 136(3):417–425.
Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286(5447):2156–2159.
Patureau D, Helloin E, Rustrian E, Bouchez T, Delgenes JP, Moletta R. 2001. Combined phosphate and nitrogen removal in a sequencing batch reactor using the aerobic denitrifier, Microvirgula aerodenitrificans. Water Research 35(1):189–197.
Pollard M, Wostmann BS. 1985. Increased life span among germfree rats. Progress in Clinical Biology Research 181:75–76.
Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proceedings of the National Academy of Science USA 102(1):99–104.
Rainey PB, Rainey K. 2003. Evolution of cooperation and conflict in experimental bacterial populations. Nature 425(6953):72–74.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118(2): 229–241.
Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. Journal of Clinical Investigation 98(4):945–53.
Rawls JF, Samuel BS, Gordon JI. 2004. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proceedings of the National Academy of Science USA 101(13): 4596–4601.
Rehakova Z, Capkova J, Stepankova R, Sinkora J, Louzecka A, Ivanyi P, Weinreich S. 2000. Germ-free mice do not develop ankylosing enthesopathy, a spontaneous joint disease. Human Immunology 61(6):555–558.
Rittmann BE, McCarty PL. 2001. Environmental Biotechnology: Principles and Applications. Boston: McGraw Hill International.
Rolandelli RH, Koruda MJ, Settle RG, Leskiw MJ, Stein TP, Rombeau JL. 1989. The effect of pectin on hepatic lipogenesis in the enterally-fed rat. Journal of Nutrition 119(1):89–93.
Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75(2):253–261.
Salyers AA. 1986. Diet and the colonic environment: Measuring the response of human colonic bacteria to changes in the host’s diet. In: Vahouny G, Kritchevsky D, eds. Dietary Fiber: Basic and Clinical Aspects. New York: Plenum Press. Pp. 119–130.
Salyers AA. 2005 (March 16). Session I: Host-Pathogen Interactions: Defining the Concepts of Pathogenicity, Virulence, Colonization, Commensalism, and Symbiosis. Presentation at the Forum on Microbial Threats Workshop Ending the War Metaphor: The Changing Agenda for Unraveling the Host-Microbe Relationship, Washington, D.C., Institute of Medicine, Forum on Microbial Threats.
Salyers AA, Shipman JA. 2002. Getting in touch with your prokaryotic self: Mammal-microbe interactions. In: Staley JT, Reysenbach AL, eds. Biodiversity of Microbial Life: Foundation of Earth’s Biosphere. New York: Wiley-Liss, Inc. Pp. 315–341.
Salyers AA, Shoemaker NB, Bonheyo GT. 2002. The ecology of antibiotic resistance genes. In: Bacterial Resistance to Antimicrobials. New York: Marcel Dekker. Pp. 1–17.
Salyers AA, Gupta A, Wang Y. 2004. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends in Microbiology 12(9):412–416.
Savage DC. 1977. Microbial ecology of the gastrointestinal tract. Annual Review of Microbiology 31:107–133.
Savage DC, Siegel JE, Snellen JE, Whitt DD. 1981. Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Applied Environmental Microbiology 42(6):996–1001.
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52(2):237–242.
Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and Immunity 66(11): 5224–5231.
Sharma R., Schumacher U. 1995. Morphometric analysis of intestinal mucins under different dietary conditions and gut flora in rats. Digestive Diseases and Sciences 40:2532–2539.
Shipman JA, Berleman JE, Salyers AA. 2000. Characterization of four outer membrane proteins involved in binding starch to the cell surface of Bacteroides thetaiotaomicron. Journal of Bacteriology 182(19):5365–5372.
Sidhu H, Allison MJ, Chow JM, Clark A, Peck AB. 2001. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. Journal of Urology 166(4): 1487–1491.
Sonnenburg JL, Angenent LT, Gordon JI. 2004. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nature of Immunology 5(6):569–573.
Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307(5717):1955–1959.
Stams AJ. 1994. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Van Leeuwenhoek 66(1–3):271–294.
Stappenbeck TS, Hooper LV, Gordon JI. 2002. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proceedings of the National Academy of Science USA 99(24):15451–15455.
Sumi Y, Miyakawa M, Kanzaki M, Kotake Y. 1977. Vitamin B-6 deficiency in germfree rats. Journal of Nutrition 107(9):1707–1714.
Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. 2004. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proceedings of the National Academy of Science USA 101(7):1981–1986.
Taddei F, Matic I, Godelle B, Radman M. 1997. To be a mutator, or how pathogenic and commensal bacteria can evolve rapidly. Trends in Microbiology 5(11):427–428.
Thauer RK, Jungermann K, Decker K. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriological Reviews 41(1):100–180.
Thomas JA, Soddell JA, Kurtboke DI. 2002. Fighting foam with phages? Water Science and Technology 46(1–2):511–518.
Thurnheer MC, Zuercher AW, Cebra JJ, Bos NA. 2003. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. Journal of Immunology 170(9):4564–4571.
Towle HC. 2001. Glucose and cAMP: adversaries in the regulation of hepatic gene expression. Proceedings of the National Academy of Science USA 98(24):13476–13478.
Travisano M, Velicer GJ. 2004. Strategies of microbial cheater control. Trends in Microbiology 12(2):72–78.
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. 2003. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422(6928):164–169.
Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. 1994. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 107(5):1259–1269.
Whitman WB, Coleman DC, Wiebe WJ. 1998. Prokaryotes: The unseen majority. Proceedings of the National Academy of Science USA 95(12):6578–6583.
Wicker LS, Miller BJ, Coker LZ, McNally SE, Scott S, Mullen Y, Appel MC. 1987. Genetic control of diabetes and insulitis in the nonobese diabetic (NOD) mouse. Journal of Experimental Medicine 165(6):1639–1654.
Wilson K, Blitchington R. 1996. Human colonic biota studied by ribosomal DNA sequence analysis. Applied Environmental Microbiology 62(7):2273–2278.
Winter C, Smit A, Herndl GJ, Weinbauer MG. 2004. Impact of virioplankton on archaeal and bacterial community richness as assessed in seawater batch cultures. Applied Environmental Microbiology 70(2):804–813.
Wostmann B. 1973. Intestinal bile acids and cholesterol absorption in the germfree rat. Journal of Nutrition 103(7):982–990.
Wostmann B, Bruckner-Kardoss E. 1959. Development of cecal distention in germ-free baby rats. American Journal of Physiology 197:1345–1346.
Wostmann BS, Knight PL, Keeley LL, Kan DF. 1963. Metabolism and function of thiamine and naphthoquinones in germfree and conventional rats. Federation Proceedings 22:120–124.
Wostmann BS, Bruckner-Kardoss E, Knight PL. 1968. Cecal enlargement, cardiac output, and O2 consumption in germfree rats. Proceedings of the Society of Experimental Biology and Medicine 128(1):137–141.
Wostmann BS, Bruckner-Kardoss E, Pleasants JR. 1982. Oxygen consumption and thyroid hormones in germfree mice fed glucose-amino acid liquid diet. Journal of Nutrition 112(3):552–559.
Wostmann BS, Larkin C, Moriarty A, Bruckner-Kardoss E. 1983. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Laboratory Animal Science 33(1):46–50.
Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-bacteroides thetaiotaomicron symbiosis. Science 299(5615):2074.
Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proceeding of the National Academy of Science USA 96(4):1463–1468.
Yamanaka M, Nomura T, Kametaka M. 1977. Influence of intestinal microbes on heat production in germ-free, gnotobiotic and conventional mice. Journal of Nutritional Science and Vitaminology (Tokyo) 23(3):221–226.
Yoshikai Y, Matsuzaki G, Kishihara K, Nomoto K, Yokokura T, Nomoto K. 1988. Age-associated increase in the expression of T-cell antigen receptor gamma-chain gene in conventional and germfree mice. Infection and Immunity 56(8):2069–2074.
Zaiat M, Rodrigues JA, Ratusznei SM, de Camargo EF, Borzani W. 2001. Anaerobic sequencing batch reactors for wastewater treatment: A developing technology. Applied Microbiology and Biotechnology 55(1):29–35. Review.
Zoetendal EG, Akkermans AD, De Vos WM. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Applied Environmental Microbiology 64(10):3854–3859.
Zoetendal EG, Akkermans AD, Vliet WMA, Arjan J, de Visser GM, de Vos WM. 2001. The host genotype affects the bacterial community in the human gastro-intestinal tract. Microbial Ecology in Health and Disease 13(3):129–134.













































