Public Summary
HEALTH RISKS FROM TCDD, OTHER DIOXINS, AND DIOXIN-LIKE COMPOUNDS
Evaluation of the EPA Reassessment
Dioxins and dioxin-like compounds (DLCs) are released into the environment from several sources, including combustion, metal processing, and chemical manufacturing and processing. The most toxic of these compounds is TCDD, often simply called dioxin. Many other types of dioxins, other than TCDD, and DLCs share most, if not all, of the toxic characteristics of TCDD. In the past, occupational exposures to TCDD, other dioxins, and DLCs occurred in a variety of industries, especially those involved in the manufacture of trichlorophenol (used to make certain herbicides) and PCBs. (PCBs contain some forms that are dioxin-like and, when heated to high temperatures, may also be contaminated with dibenzofurans, which are also dioxin-like.) Much of the knowledge about the health effects of TCDD, other dioxins, and DLCs in humans comes from studies of relatively highly exposed workplace populations. Widespread use of certain herbicides containing TCDD, other dioxins, and DLCs, as well as some types of industrial emissions, resulted in local and global contamination of air, soil, and water with trace levels of these compounds. These trace levels built up in the food chain because TCDD, other dioxins, and DLCs do not readily degrade. Instead, they persist in the environment and accumulate in the tissues of animals. The general
public is exposed to TCDD, other dioxins, and DLCs primarily by eating such foods as beef, dairy products, pork, fish, and shellfish.
The health effects of exposures to relatively high levels of dioxin became widely publicized due to the use of the herbicide called Agent Orange in the Vietnam War. Agent Orange contained small amounts of TCDD as a contaminant. Studies suggest that veterans and workers exposed occupationally to TCDD, other dioxins, and DLCs experience an increased risk of developing a potentially disfiguring skin lesion (called chloracne), liver disease, and possibly cancer and diabetes.
Fortunately, background exposures for most people are typically much lower than those seen in either Vietnam veterans or occupationally exposed workers. The potential adverse effects of TCDD, other dioxins, and DLCs from long-term, low-level exposures to the general public are not directly observable and remain controversial. One major controversy is the issue of estimating risks at doses below the range of existing reliable data. Another controversy is the issue of appropriately assessing the toxicity of various mixtures of these compounds in the environment.
In 2004, the U.S. Environmental Protection Agency (EPA), asked the National Research Council (NRC) of the National Academies to review its 2003 draft document titled Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds (the Reassessment). This NRC report describes the Reassessment as very comprehensive in its review and analysis of the extensive scientific literature on TCDD, other dioxins, and DLCs. However, the NRC report finds substantial room for improvement in the quantitative approaches used by EPA to characterize risks. In particular, the committee recommends that EPA more thoroughly justify and communicate its approaches to dose-response modeling for health effects and make its criteria for selection of key data sets more transparent. EPA should also improve how it handles and communicates the substantial uncertainty that surrounds its various estimates of health risks from low-level exposures to TCDD, other dioxins, and DLCs. This NRC report provides a critical review of EPA’s Reassessment, but the report is not a risk assessment and does not recommend exposure levels for TCDD, other dioxins, or DLCs for regulatory consideration. Rather the NRC report provides guidance to EPA on how the agency could improve the scientific robustness and clarity of the Reassessment for its ultimate use in risk management of TCDD, other dioxins, and DLCs in the environment by federal, state, and local regulatory agencies.
Assessing Human Exposure to TCDD, Other Dioxins, and DLCs
People worldwide are exposed to background levels of TCDD, other dioxins, and DLCs. Background exposures include those from the commer-
cial food supply, air, water, and soil. EPA’s 2003 draft Reassessment does not identify many specific direct sources of human exposures to relatively high levels of TCDD, other dioxins, or DLCs. EPA estimated background concentrations based on studies conducted at various locations in North America. Those studies examined a small number of locations and, hence, may not fully characterize national variability. EPA derived its estimates of TCDD, other dioxins, and DLCs in food from statistically based national surveys, nationwide-sampling networks, food fat concentrations, and environmental samples of air, water, soil, and food.
According to recent estimates, background concentrations of TCDD, other dioxins, and DLCs continue to decline. EPA’s estimates of releases of these compounds to air, water, and land from reasonably quantifiable sources in 2000 showed a decrease of 89% from its 1987 estimates. At least one U.S. study determined that meat contains lower levels of TCDD, other dioxins, and DLCs than samples from the 1950s through the 1970s. An ongoing national study by the U.S. Department of Agriculture of the concentrations of TCDD, other dioxins, and DLCs in beef, pork, and poultry should allow for a time-trend analysis of food concentrations.
To assess the total magnitude of emissions of TCDD, other dioxins, and DLCs, EPA used a “bottom-up” approach that attempted to identify all emission-source categories (such as combustion, metal processing, and chemical manufacturing and processing) and then estimated the magnitude of emissions for each category. The committee concludes that a “top-down” approach would also provide useful information and could give rise to significantly different estimates of the historical levels of emissions of TCDD, other dioxins, and DLCs. A top-down approach would account for measured levels in humans and the environment and consider the emission sources required to account for these levels.
The committee also recommends that EPA set up an active database of typical concentrations for TCDD, other dioxins, and DLCs present in food. This database should be based on a collection of all available data and updated on a regular basis with new data as they are published in the peer-reviewed literature.
Cancer Risk and TCDD, Other Dioxins, and DLCs
The EPA Reassessment revisits EPA’s classification of TCDD, other dioxins, and DLCs on their potential to cause cancer in humans. In 1985, EPA classified TCDD as a “probable human carcinogen” based on the data available and EPA’s classification criteria in place at the time. The Reassessment, which revisited this issue given the current evidence and a different draft classification scheme, characterized TCDD as “carcinogenic to humans.” In 2005, after completion of the Reassessment, EPA further revised
its cancer guidelines. In its charge, the NRC committee was specifically asked to address “the scientific evidence for classifying TCDD as a human carcinogen.”1 Referring to the definitions of chemical carcinogens in the EPA’s current cancer guidelines, the NRC committee was split on whether the evidence from available studies met all the criteria necessary for definitive classification of TCDD as “carcinogenic to humans,” although the committee unanimously agreed on a classification for TCDD of at least “likely to be carcinogenic to humans.” The committee believed that the public health implications of the two terms appeared identical and for this reason did not belabor the issue of classification. The committee concluded that because the definition of “carcinogenic to humans” changed somewhat from previous EPA guidelines and after submission of the Reassessment, EPA should reevaluate its 2003 conclusion based on the criteria set out in its 2005 cancer guidelines.
The committee agrees with EPA in classifying other dioxins and DLCs as “likely to be carcinogenic to humans.” However, because mixtures of DLCs and other dioxins may include TCDD, EPA should reconsider its classification of such mixtures as “likely to be carcinogenic to humans” if it continues to classify TCDD as “carcinogenic to humans.”
Estimating Cancer Risks at Very Low Doses
Nearly all relevant cancer-risk data from human epidemiological studies and experimental animal bioassays reflect doses much higher than those typically experienced by humans from exposure to TCDD, other dioxins, and DLCs in the general environment. Consequently, analysts must extrapolate well below the doses observed in the studies to consider typical human exposure levels. This extrapolation involves two critical decisions: (1) selecting a “point of departure” (POD), which corresponds to the lowest dose associated with observable adverse effects within the range of data from a study, and (2) selecting the mathematical model used to extrapolate risk from typical human exposures that are well below the POD.
In general, EPA estimates the POD by setting it equal to the dose producing the smallest positive effect observed in a study. The size of the health effect it produces in the population determines the “effective dose.” For example, the 1% effective dose (referred to as the ED01) elicits an additional 1% response and the ED05 elicits an additional 5% response
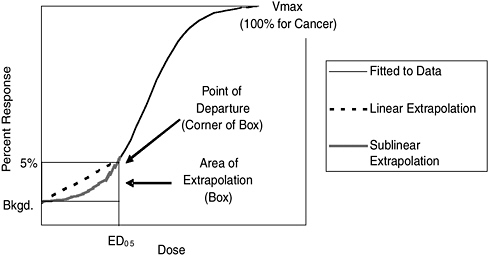
FIGURE S-1 Conceptual illustration of the effect of the selection of the point of departure and the mathematical model used to extrapolate below the point of departure on the risk estimate. Note that the 5% response rate is not drawn to scale. If it were, the area of the extrapolation box would be much smaller. In this illustration, the ED05 has been selected as the point of departure for extrapolation to lower doses.
above the “background” response (the level of response that occurs in the absence of any exposure). The response size depends on the difference between the unexposed population and the largest response possible. For example, consider the case of a 25% lifetime background risk of death from cancer in an unexposed population and a highest possible cancer death rate of 100%. In this case, the ED01 is the dose that increases the cancer death rate by 1% of the difference between 100% and 25%, or by 0.75%. Thus, the ED01 is the dose that increases the risk of dying from cancer from 25% to 25.75%.
Estimating risks below the POD requires making assumptions about how TCDD, other dioxins, and DLCs might cause cancer at lower exposures. For example, in the hypothetical illustration in Figure S-1, a biological mode of action implying that risk is proportional to dose would correspond to use of the dashed line below the POD. A biological mode of action implying a sublinear dose-response relationship would correspond to the shaded line below the POD.
The committee concludes that EPA’s decision to rely solely on a default linear model lacked adequate scientific support. The report recommends that EPA provide risk estimates using both nonlinear and linear methods to extrapolate below PODs. If background exposures to humans result in
doses substantially less than the dose associated with the POD (the most likely case in most instances but perhaps not for occupational exposures), then an estimate of risk for typical human exposures to TCDD, other dioxins, and DLCs would be lower in a sublinear extrapolation model than in the linear model. Given the important regulatory implications of this assumption, the committee recommends that EPA communicate the scientific strengths and weaknesses of both approaches so that the full range of uncertainty generated by modeling of the data is conveyed in the Reassessment.
The committee also concluded that EPA did not adequately quantify the uncertainty associated with responses at the estimated value of the POD. The estimated value of the response at a particular effective dose (like the ED01) is typically uncertain for a variety of reasons related to the challenge of conducting an epidemiological study or an animal study. For example, in epidemiological studies, the number of enrolled subjects is limited, it can be difficult to estimate the actual level of exposure, other factors (such as smoking or exposure to other chemicals) can also cause cancer, and so forth. The committee concludes that, although EPA discussed many of these factors qualitatively, the agency should strive to more comprehensively characterize the impact of these sources of uncertainty quantitatively.
Estimating Noncancer Risk
To characterize the risks of adverse health effects other than cancer, EPA typically identifies a dose, called the reference dose (RfD), below which it anticipates no adverse effects from exposure even among sensitive members of the population. EPA did not estimate an RfD for TCDD, other dioxins, or DLCs in the Reassessment. The committee suggests that estimating an RfD would provide useful guidance to risk managers to help them (1) assess potential health risks in that portion of the population with intakes above the RfD, (2) assess risks to population subgroups, such as those with occupational exposures, and (3) estimate the contributions to risk from the major food sources and other environmental sources of TCDD, other dioxins, and DLCs for those individuals with high intakes.
Given the existing data, the committee concurs with the conclusion in EPA’s Reassessment that TCDD, other dioxins, and DLCs are likely to be human immunotoxicants at “some dose level.” However, the report finds this conclusion inadequate. The committee recommends that EPA add a section or paragraph to its Reassessment on the immunotoxicology of TCDD, other dioxins, and DLCs in the context of the biological mechanisms responsible for health effects relevant to assessing the likelihood of such effects occurring in humans at relatively low levels of exposure. The
risk characterization should provide some insight about the level of risk given actual exposures.
Studies show that TCDD, other dioxins, and DLCs cause embryonic and fetal development and reproduction problems in rodents and some other species. However, the fetal rodent clearly shows more susceptibility to adverse effects of TCDD, other dioxins, and DLCs than the adult rodent. Given the lack of comparable human data, the committee recommends that EPA more thoroughly address how animal pregnancy models might relate to human reproductive and developmental toxicity and risk information.
The committee further recommends that, in areas with substantial amounts of human clinical data and epidemiological data, EPA establish formal, evidence-based approaches, including but not limited to those for assessing the quality of the study and study design for classifying and statistically reviewing all available data.
Communicating Variability and Uncertainty in Risk Estimates
Risk assessors must make many choices as they develop models to characterize risks, including selecting appropriate data sets for low-dose extrapolation, dose-response models, PODs, and so forth. Because risk estimates reflect numerous sources of uncertainty and alternative assumptions, EPA’s Reassessment should include a detailed discussion of variability (the range of risks reflecting true differences among members of the population due to, for example, genetic and age differences) and uncertainty (the range of plausible risk estimates arising because of limitations in knowledge). Although EPA addressed many sources of variability and uncertainty qualitatively, the committee noted that the Reassessment would be substantially improved if its risk characterization included more quantitative approaches. Failure to characterize variability and uncertainty thoroughly can convey a false sense of precision in the conclusions of the risk assessment.
Estimating Toxicity of DLCs and Mixtures in the Environment
Risk managers base their decisions about cleanup and control of chemicals, such as TCDD, other dioxins, and DLCs, in the environment on assessment of the risks. Because of the common mode of action in producing health effects, EPA’s Reassessment assessed the cumulative toxicity of the compounds. The approach taken by EPA and international public health organizations relies on assigning each compound (dioxins, other than TCDD, and DLCs) a “toxic equivalency factor,” which is an estimate of the toxicity of the compound relative to TCDD. For example, a particular DLC
thought to result in one-tenth the risk of TCDD for the same level of exposure would be assigned a toxicity equivalency factor of 0.1.
Because some mixtures may contain little or no measurable TCDD but relatively large amounts of other dioxins and/or DLCs, the toxic equivalency factor plays a critical role in determining the mixture’s overall estimated toxicity (which is called the toxic equivalency quotient). Estimation of TEFs is a critically important part of the risk assessment of environmental mixtures of TCDD, other dioxins, and DLCs, because any environmental sample typically contains a dozen or more similar substances, but often very little TCDD. Also, TCDD, other dioxins, and DLCs break down at different rates in the environment and are eliminated at different rates in humans. Thus, although analysts may reasonably estimate the relative potency value for a given compound based on toxicity tests, the compound’s contribution to total risk in an environmental (or biological) sample may change over a period of many years. This change may occur because the relative concentration in a sample may change with time, even though the potency remains constant, and the estimated risk in a given sample depends on both potency and concentration.
Even with the inherent uncertainties, the committee concludes that the toxic equivalency factor methodology provides a reasonable, scientifically justifiable, and widely accepted method to estimate the relative potency of DLCs. However, the committee noted that the Reassessment should acknowledge the need for better uncertainty analysis of the toxicity values and should provide at least some initial uncertainty analysis of overall toxicity of environmental samples.
CONCLUDING REMARKS
The committee appreciates the dedication and hard work that went into the creation of the Reassessment and commends EPA for its detailed evaluation of an extremely large volume of scientific literature (particularly Parts I and II of the Reassessment). The NRC report focused its review on Part III of the Reassessment and offers its recommendations with the intention of helping to guide EPA in its efforts to make and implement environmental policies that adequately protect human health and the environment from the potential adverse effects of TCDD, other dioxins, and DLCs. The committee recognizes that it will require a substantial amount of effort for EPA to incorporate all the changes recommended in this NRC report. Nevertheless, the committee encourages EPA to finalize the current Reassessment as quickly, efficiently, and concisely as possible after addressing the major recommendations in this report. The committee notes that new advances in the understanding of TCDD, other dioxins, and DLCs could require reevaluation of key assumptions in the EPA risk assessment docu-
ment. The committee recommends that EPA routinely monitor new scientific information related to TCDD, other dioxins, and DLCs, with the understanding that future revisions should provide risk assessment based on the current state-of-the-science. However, the committee also recognizes the importance of stability in regulatory policy to the regulated community and thus suggests that EPA establish criteria for identifying when compelling new information warrants science-based revisions in its risk assessment. The committee finds that the recent dose-response data released by the National Toxicology Program after submission of the Reassessment represent good examples of new and compelling information that warrants consideration in a revised risk assessment.
COMMITTEE’S KEY FINDINGS
The committee identified three areas that require substantial improvement in describing the scientific basis for EPA’s dioxin risk assessment to support a scientifically robust risk characterization:
-
Justification of approaches to dose-response modeling for cancer and noncancer end points.
-
Transparency and clarity in selection of key data sets for analysis.
-
Transparency, thoroughness, and clarity in quantitative uncertainty analysis.
The following points represent Summary recommendations to address the key concerns:
-
EPA should compare cancer risks by using both a linear model and a nonlinear model consistent with a receptor-mediated mechanism of action and by using epidemiological data and the new NTP animal bioassay data. The comparison should include upper and lower bounds, as well as central estimates of risk. EPA should clearly communicate this information as part of its risk characterization.
-
EPA should identify the most important data sets to be used for quantitative risk assessment for each of the four key end points (cancer, immunotoxicity, reproductive effects, and developmental effects). EPA should specify inclusion criteria for the studies (animal and human) used for derivation of the benchmark dose (BMD) for different noncancer effects and potentially for the development of RfD values and discuss the strengths and limitations of those key studies; describe and define (quantitatively to the extent possible) the variability and uncertainty for key assumptions used for each key end-point-specific risk assessment (choices of data set, POD, model, and dose metric); incorporate probabilistic models to the
-
extent possible to represent the range of plausible values; and assess goodness-of-fit of dose-response models for data sets and provide both upper and lower bounds on central estimates for all statistical estimates. When quantitation is not possible, EPA should clearly state it and explain what would be required to achieve quantitation.
-
When selecting a BMD as a POD, EPA should provide justification for selecting a response level (e.g., at the 10%, 5%, or 1% level). The effects of this choice on the final risk assessment values should be illustrated by comparing point estimates and lower bounds derived from selected PODs.
-
EPA should continue to use body burden as the preferred dose metric but should also consider physiologically based pharmacokinetic modeling as a means to adjust for differences in body fat composition and for other differences between rodents and humans.
The committee encourages EPA to calculate RfDs as part of its effort to develop appropriate margins of exposure for different end points and risk scenarios.










