5
Cancer
This chapter reviews the U.S. Environmental Protection Agency (EPA) assessment of the carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), commonly referred to as dioxin, other dioxins, and dioxin-like compounds (DLCs), including EPA’s qualitative characterization of their carcinogenicity, the assumption that the dose-response relationship is linear, and the use of animal bioassay and epidemiological data to quantify the dose response. The final section summarizes the committee’s conclusions.1
QUALITATIVE EVALUATION OF CARCINOGENICITY
EPA concludes that dioxin is “carcinogenic to humans” based on the following evidence (Reassessment, Part III, pp. 6-7 to 6-8): evidence from the occupational cohort studies that dioxin exposure increases mortality from cancer aggregated over all sites and from lung cancer “and, perhaps, other sites”; evidence from bioassays of cancer in both sexes of multiple species at multiple sites; and evidence regarding dioxin’s mode of action, including mechanistic evidence that dioxin acts as a tumor promoter via receptor-mediated pathway(s) and the finding that the receptor-mediated pathways that may give rise to cancer in laboratory animals appear to be present and functional in human tissues.
In this chapter, the committee reviews the epidemiological, bioassay, and mode of action evidence and then presents conclusions regarding both qualitative and quantitative measures of carcinogenicity of TCDD, other dioxins, and DLCs.
Epidemiological Evidence
The epidemiological evidence that provided the basis for EPA’s assessment consists primarily of studies following four cohorts. Of these, the Reassessment reviewed in detail those related to the three cohorts that provided quantitative dose-response estimates linking serum dioxin to cancer mortality (Ott and Zober 1996; Becher et al. 1998; Steenland et al. 2001). The cohorts were quite variable in size and exposure ranges. Ott and Zober (1996) studied a relatively small number of men exposed to an accidental release of dioxin in 1953 (N = 243, 13 cancer deaths). Becher et al. (1998) examined a cohort of 1,189 men employed in pesticide and herbicide production, from which 124 cancer deaths were identified. The third cohort represents a large occupational population originally studied by Fingerhut et al. (1990, 1991), who examined 5,172 male employees in 12 manufacturing facilities. An update on this cohort was provided by Steenland et al. (1999), who applied “job-exposure matrix”2 estimates to 5,132 workers in the original cohort who were followed for 6 more years. The total number of cancer deaths in this cohort was 377. In 2001, Steenland et al. updated this study again on a subcohort of 3,538 workers (with 256 cancer deaths) and used data from 170 members of this cohort for which estimated external exposures and known serum dioxin levels were available to establish a quantitative dose-response assessment.
Each study identified a cohort of workers who had been employed in industrial settings in which dioxin was a by-product. These settings included pesticide production (Ott and Zober 1996; Becher et al. 1998) or chemical plants more broadly (Steenland et al. 2001). In each instance, current serum dioxin measurements were available for a subset of workers. Development of exposure estimates for the entire cohort required two extrapolations: from current serum dioxin measurements to historical exposure levels using estimates of serum dioxin half-life, and from workers with current serum dioxin measurements to those without by linking available serum dioxin measurements to job characteristics based on knowledge of the industrial processes. Although these extrapolations decrease the accu-
racy of the assessment, they were necessary to provide historical exposure estimates so that there would be a sufficient number of cohort members who could be included in the analysis.
In addition to these three cohorts, Part II, section 7.5.4 of the Reassessment describes studies that reported on an occupational cohort of 2,310 workers in two plants that prepared and manufactured phenoxy herbicides in the Netherlands (see Reassessment, Part II, Table 7-21 for a summary of all four studies). Bueno de Mesquita et al. (1993) found no statistically significant increases in cancer mortality among all workers (31 deaths, standardized mortality ratio [SMR] = 107, 95% confidence interval [CI] = 73 to 152) and among a subset of 139 workers involved in a 1963 industrial accident (10 deaths, SMR = 137, 95% CI = 66 to 252). Comparing exposed workers (N = 963) to unexposed workers (N = 1,111), both total cancer mortality (rate ratio, [RR] = 1.7, 95% CI = 0.9 to 3.4) and respiratory cancer mortality (RR = 1.7, 95% CI = 0.5 to 6.3) were nonsignificantly increased.
A follow-up study by Hooiveld et al. (1996) reported a statistically significant increase in cancer mortality among workers in one of the two plants (SMR = 146, 95% CI = 109 to 192). No such increase was observed in the other factory. Follow-up analysis by Hooiveld et al. (1998) reported a statistically increased incidence of malignant neoplasms among 140 workers involved in the 1963 industrial accident (SMR = 1.7, 95% CI = 1.1 to 2.7). The incidence of malignant neoplasms was also increased in a larger group of 549 workers (SMR = 1.5, 95% CI = 1.1 to 1.9). A comparison of this group of 549 exposed workers to 482 unexposed workers, also from this cohort, yielded an increased total cancer mortality risk (RR = 4.1, 95% CI = 1.8 to 9.0) and an increased respiratory cancer mortality risk (SMR = 7.5, 95% CI = 1.0 to 56.1).
There are three major issues to consider regarding EPA’s review of the epidemiological studies investigating the relationship between dioxin exposure and cancer. First, although EPA identified the cohort studies capable of generating quantitative dose-response information for the dose-response modeling and considered the broader epidemiological literature in the background documents, Part III of the Reassessment did not provide a thorough and systematic analysis of the body of epidemiological evidence from which these three studies were chosen. In particular, although Part II described the complete array of studies, including those by Kogevinas et al. (1997) and Bertazzi et al. (1998), the Reassessment did not analyze site-specific tumors consistently across all studies but rather emphasized the positive findings in each paper without a full discussion of consistency, or lack thereof, across studies.
A second issue is EPA’s decision to focus on total cancers instead of specific types of cancer. EPA argues that because dioxin is not genotoxic
and is instead presumed to act primarily as a promoter rather than an initiator of cancer, the lack of specificity in tumor type is to be expected. If dioxin promotes cancer through the Ah receptor mechanism, however, then an increased tumor incidence would require expression of the receptor in that tissue. The Ah receptor is expressed in most tissues but to varying degrees. It is uncertain whether the level of expression is an important determinant of tumor promotion. There are also many downstream events from ligand-receptor interaction that are tissue specific and essential for tumor promotion to occur via a receptor-mediated response, and these downstream events differ from tissue to tissue (see also discussion on mode of action later in this chapter). In any case, EPA reasons that, in the face of limited power, increased risk of total cancers (which would reflect the increased incidence across the multiple sites affected by dioxin) is easier to detect than an increased risk of individual cancer types (see Part III, pp. 2-9 to 2-10). This rationale would be valid for a given relative risk (e.g., a doubling of the incidence or mortality). However, a given absolute incremental risk (e.g., an additional 10 cancers due to exposure) would be more readily identified for a specific cancer site than for cancers in the aggregate.
The more compelling argument for aggregating across cancer types is the practical one that the results for specific cancers are extremely imprecise in these cohorts of modest size. If, in fact, multiple cancer types all showed a small increment in risk of equal magnitude, there would be greater precision and statistical power for the aggregation. For example, in the case of ionizing radiation, the aggregation across a series of radiosensitive cancers, each with small increases in risk, yields a more statistically precise indication of an increase in cancers related to radiation exposure than do any of the individual cancers. In the case of dioxin, it is not clear that a specific set of cancers is affected that can then be aggregated to enhance statistical power.
To evaluate the patterns across cancer sites, the committee examined selected papers from the three cohorts (Ott and Zober 1996; Flesch-Janys et al. 1998; Steenland et al. 1999). This evaluation revealed that only limited information is available regarding numbers of cases at specific sites, hence limiting the opportunity to examine consistency across studies. As noted by others, there is some consistency across studies for respiratory cancers, but there is a general lack of concordance for the other cancer sites reported in more than one study. The degree of replication or lack thereof should not be overstated given the small number of studies and imprecise information on specific cancer sites from all but the Steenland et al. (1999) report.
Overall, the committee concurs with the value of conducting analyses of total cancers, given the potential for dioxin to affect multiple types of cancer and the limited precision of risk estimates for individual cancer types. Nonetheless, the potential for effects limited to specific types of
cancer, as has been found for other causes, also warrants an analysis of major cancer types (e.g., respiratory cancers), the imprecision notwithstanding.
Another concern is the potential role of confounding by lifestyle factors such as smoking and by occupational exposures that co-occur with dioxin. Although smoking is a powerful lung carcinogen, quite capable of generating spurious relative risks on the order of those reported in the epidemiological studies for dioxin of around 1.5, the design of those studies makes its potential role as a confounder unlikely in this case. The key comparisons were not between industrial workers and the general population, which is quite susceptible to confounding by lifestyle factors, but among subsets of workers with different levels of estimated dioxin exposure. It is not likely that smoking histories would differ markedly among men located at different jobs within the industrial plant or in relation to duration of employment. In contrast, there is greater potential for confounding by other workplace agents given that the industrial cohorts had exposure to pesticides and potentially carcinogenic chemicals in addition to dioxin. Although these accompanying workplace hazards likely differed for the three cohorts that contributed to the quantitative risk assessment, confounding could have occurred in each to yield a similar falsely elevated measure of association. The difficulties in isolating the health effects of single agents from the complex mixtures encountered in chemical manufacturing must be recognized.
Epidemiological evidence for an association between cancer and exposure to DLCs has been characterized as “inadequate but suggestive” (EPA 1987) and “limited” (IARC 1997). ATSDR (2000) concluded that the epidemiological evidence “taken in totality, indicates a potential cancer causing effect for PCBs.”
On the whole, it was the committee’s impression that EPA’s narrative in discussing epidemiological studies in Part III of the Reassessment tended to focus on positive findings without fully considering the strengths and limitations of both positive and negative findings. Part III of the Reassessment would be strengthened if EPA clearly identified specific inclusion criteria for those studies for which quantitative risk estimates were determined.
Bioassay Data
Several large and well-conducted dioxin-related cancer bioassays (Kociba et al. 1978; NTP 1982a,b; NTP 2004) have reported induction of several types of cancer in both rats and mice. The study in hamsters was confounded by use of dioxane, which is a potential carcinogen, as the delivery vehicle. Table 5-1 summarizes these studies. In all studies in which dioxin elicited an increase in tumors, the increase was site specific. With
oral administration, the organ most frequently affected was the liver, reflecting the mode of action of carcinogenicity, as discussed below.
Of the 21 DLCs of concern, EPA (Part II, p. 6-30) reported that carcinogenicity bioassays have been conducted on only two pure polychlorinated dibenzo-p-dioxin (PCDD) and 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PeCDD), and a mixture of two congeners (1,2,3,6,7,8- and 1,2,3,7,8,9-hexachlorodibenzo-p-dioxin [HxCDD]). Carcinogenicity bioassays have also been conducted on one polychlorinated dibenzofuran (PCDF) (2,3,4,7,8-pentachlorodibenzofuran [PeCDF]) and one PCB (126; 3,3′,4,4′, 5-pentachlorobiphenyl (PeCB) (Table 5-2).
However, the ability of a variety of dioxins other than TCDD and DLCs to enhance the carcinogenicity of known carcinogens (promoter assays) has also been reported for PeCDD, HpCDD, 2,3,7,8-tetrachlorodibenzofuran (TeCDF), PeCDF, and 1,2,3,4,7,8-hexachlorodibenzofuran (HxCDF) (summarized by IARC 1997). Bioassays have also been conducted on mixtures of PCBs, and although they provide some information on the carcinogenicity of components, they do not identify the specific responsible chemical(s).
Mode of Action
Dioxin does not have structural features that would lead to a reactive electrophile, and it is clearly not DNA reactive, as no DNA binding or adducts were found in rodent tissues (Poland and Glover 1979; Randerath et al. 1988; Turteltaub et al. 1990). Absence of DNA reactivity is supported by negative findings in genetic toxicological assays (IARC 1997).
Nevertheless, EPA notes (Part II, p. 6-1) the hypothesis that dioxin might be indirectly genotoxic, either through induction of oxidative stress or by altering the DNA damaging potential of some endogenous compounds, including estrogens. No evidence is available for estrogen-mediated DNA damage resulting from dioxin exposure, but oxidative DNA damage has been documented after 30 weeks administration of dioxin (Tritscher et al. 1996; Wyde et al. 2001). Indirect genotoxicity has been postulated to initiate carcinogenicity, but there is insufficient evidence that dioxin has initiating activity.
Dioxin was reported to have weak initiating activity in one study (DiGiovanni et al. 1977) in which it was applied to mouse skin prior to a promoting agent. This finding has not been corroborated, and in contrast to what would be expected from an initiating agent, application of dioxin to mouse skin at a dosage greater than that required for a promoting effect did not induce skin tumors (Poland et al. 1982).
Moreover, dioxin has not been specifically tested as an initiator in standard models in rat or mouse liver in which chemicals can be evaluated
TABLE 5-1 Dioxin Cancer Bioassays
|
Species/Strain |
Route and Dose |
Sex |
Sites of Tumor Increases |
Reference |
|
Rat/Sprague Dawley |
Oral in feed 1, 10, 100 µg/kg/day |
Male |
Oral cavity |
Kociba et al. 1978 |
|
Female |
Lung, oral cavity, liver |
NTP 1982a |
||
|
Rat/Osborne Mendel |
Gastric instillation 10, 50, 500 µg/kg/week for 104 weeks |
Male |
Thyroid |
|
|
Female |
Liver |
|
||
|
Rat/Sprague-Dawley |
Gastric instillation 3, 10, 22, 46, or 100 mg/kg, 5 days/week for 104 weeks |
Female |
Liver, lung, oral cavity, uterus |
NTP 2005 |
|
Mouse/B6C3F1 |
Gastric instillation 0.01, 0.05, 0.5 mg/kg/wk for 104 weeks (males) |
Male |
Liver |
NTP 1982a |
|
|
0.04, 0.2, 2.0 mg/kg/wk for 104 weeks (females) |
Female |
Liver, thyroid |
|
|
Mouse/Swiss Webster |
Topical application 0.005 µg 3 days/week for 104 weeks |
Female |
Skin |
NTP 1982b |
|
Mouse/B6C3 and B6C |
Intraperitoneal injection 1, 30, 60 µg/kg/week for 5 weeks |
Male |
Thymus (both), liver (B6C3 only) |
DellaPorta et al. 1987 |
|
Female |
Thymus (both), liver (B6C3) |
|||
|
Mouse/B6C3 |
Gastric instillation 2.5, 5.9 µg/kg/week for 52 weeks |
Male |
Liver |
DellaPorta et al. 1987 |
|
Female |
Liver |
|||
|
Mouse/Swiss |
Gastric instillation 0.007, 0.7, 7.0 µg/kg/week for 52 weeks |
Male |
Liver |
Toth et al. 1979 |
|
Mouse/TG. AC |
Topical application for 24 weeks |
Male |
Skin papillomas |
Eastin et al. 1998 |
|
Female |
Skin papillomas |
|||
|
Mouse/TP53+/− |
Gastric instillation 250 µg/kg, 1,000 µg/kg twice weekly for 24 weeks |
Male |
None |
Eastin et al. 1998 |
|
Female |
None |
|||
|
Hamster/Syrian Golden |
Intraperitoneal or subcutaneous injection 50 or 100 µg/kg every 4 weeks |
Male |
Skin |
Rao et al. 1988 |
TABLE 5-2 TCDD, Other Dioxins, and DLC Cancer Bioassays
|
Congener |
Bioassay |
|
Dioxins |
|
|
2,3,7,8-TCDD |
Rat (M,F)/mouse (M,F) |
|
1,2,3,7,8-PeCDD |
Rat (M,F)/mouse (M,F)/promoter rat (F) |
|
1,2,3,4,7,8-HxCDD |
No bioassay conducted |
|
1,2,3,6,7,8-HxCDD |
Combination study |
|
1,2,3,7,8,9-HxCDD |
Combination study |
|
1,2,3,4,6,7,8-HpCDD |
Promoter rat (F) |
|
1,2,3,6,7,8- and 1,2,3,7,8,9-HxCDD mix |
Rat (M,F)/mouse (M,F) |
|
OCDD |
No bioassay conducted |
|
Furans |
|
|
2,3,7,8-TCDF |
Promoter mouse (F) |
|
1,2,3,7,8-PeCDF |
No bioassay conducted |
|
2,3,4,7,8-PeCDF |
Rat (F)/promoter mouse (F) and rat (M) |
|
1,2,3,4,7,8-HxCDF |
Promoter mouse (F)/rat (M) |
|
1,2,3,6,7,8-HxCDF |
No bioassay conducted |
|
1,2,3,7,8,9-HxCDF |
No bioassay conducted |
|
2,3,4,6,7,8-HxCDF |
No bioassay conducted |
|
1,2,3,4,6,7,8-HpCDF |
No bioassay conducted |
|
1,2,3,4,7,8,9-HpCDF |
No bioassay conducted |
|
OCDF |
No bioassay conducted |
|
Non-ortho PCBs |
|
|
3,3′,4,4′-TCB (77)a |
No bioassay conducted |
|
3,4,4′,5-TCB (81) |
No bioassay conducted |
|
3,3′,4,4′,5-PeCB (126) |
Rat (F) |
|
3,3′,4,4′,5,5′-HxCB (169) No |
Bioassay Conducted |
|
Mono-ortho PCBs |
|
|
2,3,3′,4,4′-PeCB (105) |
No Bioassay Conducted |
|
2,3,4,4′,5-PeCB (114) |
No Bioassay Conducted |
|
2,3′,4,4′,5-PeCB (118) |
No Bioassay Conducted |
|
2′,3,4,4′,5-PeCB (123) |
No Bioassay Conducted |
|
2,3,3′,4,4′,5-HxCB (156) |
No Bioassay Conducted |
|
2,3,3′,4,4′,5′-HxCB (157) |
No Bioassay Conducted |
|
2,3′,4,4′,5,5′-HxCB (167) |
No Bioassay Conducted |
|
2,3,3′,4,4′,5,5′-HpCB (189) |
No Bioassay Conducted |
|
aInternational Union of Pure and Applied Chemistry numbers in parentheses. Abbreviations: OCDD, octachlorodibenzo-p-dioxin; OCDF, octachlorodibenzofuran; TCB, -tetrachlorobiphenyl. |
|
as initiators followed by administration of a promoting substance (Enzmann et al. 1998). Also, in several chronic bioassay studies in which dioxin was administered to female Sprague-Dawley rats for 30 weeks at dosages associated with an increased incidence of liver tumors in carcinogenicity studies, no increase in hepatic preneoplastic lesions indicative of initiation was
found (Lucier et al. 1991; Maronpot et al. 1993). Thus, at present, there is no direct experimental evidence that dioxin acts as an initiator in rat liver.
A lack of initiating activity would be consistent with an absence of direct genotoxicity (Williams 1992). Nevertheless, some dose-response modeling of data that show a promoting effect of dioxin on rat liver preneoplastic lesions suggested that dioxin also had “a weak” (Moolgavkar and Luebeck, 1995) or “a slight” (Portier et al. 1996) initiating effect. In contrast, analysis of a two-cell clonal growth model reproduced such data without presuming an effect on mutation rates (that is, initiation) (Conolly and Andersen 1997).
Resolution of the question of initiating activity of dioxin awaits experimental evidence. Also, the postulated linkage between potential initiating activity and oxidative DNA damage is not established. In an investigation of the mode of action of hepatocarcinogenicity of pentachlorophenol, oxidative DNA damage was not found to produce liver initiation (Umemura et al. 1999).
The committee agrees with EPA that TCDD, other dioxins, and DLCs appear to enhance tumor development in female rat liver via tumor promotion. The promoting activity and liver tumor-enhancing activity of dioxin seem to be mediated through activation of the Ah receptor (aromatic hydrocarbon receptor [AHR]), which in turn leads to a variety of changes in gene expression, including notably induction of cytochromes P450 (CYPs) (Whitlock 1989) and genes related to cell proliferation (Puga et al. 1992) (see Figure 5-1). Whether those gene changes mediate the reported oxidative stress is not known. Nevertheless, both CYP induction and oxidative stress could be involved in liver cytotoxicity, which was found in studies that examined this parameter (Maronpot et al. 1993; Viluksela et al. 2000). Cytotoxicity, in turn, elicits regenerative cell proliferation (Williams and Iatropoulos 2002), as reported in several dioxin studies (Lucier et al. 1991). Dioxin-induced changes in gene expression, however, can occur without enhancement of hepatocelluar proliferation (Fox et al. 1993). In fact, increases in cell proliferation have been documented only after 30 weeks of dioxin administration (Lucier et al. 1991). The enhanced cell proliferation arising from either altered gene expression or cytotoxicity or both could be the principal factor leading to promotion of hepatocellular tumors (Busser and Lutz 1987; Whysner and Williams 1996). The sensitivity of female rat liver to dioxin, which apparently does not extend to the mouse, clearly depends on ovarian hormones (Lucier et al. 1991; Wyde et al. 2001). This sensitivity has been ascribed to induction of estradiol metabolizing enzymes (Graham et al. 1988) and is hypothesized to lead either to generation of reactive metabolites of endogenous estrogen or to active oxygen species of estrogens. Oxidative DNA damage has been implicated in liver tumor promotion (Umemura et al. 1999). In contrast to the extensive work on hepa-
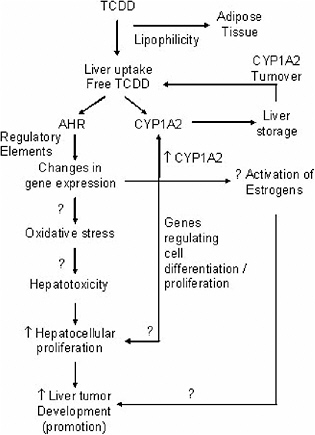
FIGURE 5-1 Possible mechanism for TCDD hepatocarcinogenicity.
tocellular neoplasia, little is known about the pathogenesis of the bile-duct tumors (Table 5-3).
Mechanistic issues are discussed in greater detail below in the context of evaluating whether the dose-response relationship is likely to be linear. In any case, the committee agrees with EPA’s general conclusion that there is sufficient evidence from epidemiological studies, animal bioassays, and mode of action studies to support the qualitative conclusion that TCDD, other dioxins, and DLCs are likely to cause cancer in humans with adequate conditions of dose and duration of exposure.
Committee’s Perspective on Whether the Scientific Evidence Supports Classification of Dioxin As a Known Human Carcinogen
After extensive discussion of EPA’s revised definition of “carcinogenic to humans” and “likely to be carcinogenic to humans” provided in EPA’s
TABLE 5-3 Dioxin Rat Bioassays
|
Study End Point |
Kociba et al. (1978) (1, 10, 100 ng/kg/day) |
NTP (1982a) (3, 10, 100 ng/kg/wk) |
NTP (2005) (10, 22, 46, 100 ng/kg) |
|
Survival |
Decreased at 100 ng/kg |
No effect |
|
|
NOEL for tumors |
1 ng/kg |
|
10.22 ng/kg |
|
Liver |
Adenoma/carcinoma (58%); bile-duct adenoma, some in low dose |
Cholangiocarcinoma, adenoma (30%), cholangioma, hepatocholangioma |
Cholangiocarcinoma (46, 100 ng/kg), adenoma (100 ng/kg) |
|
Lung |
Keratinizing squamous cell carcinoma (100 ng/kb) |
Cystic keratinizing epithelioma (100 ng/kg) |
Cystic keratinizing epithelioma (100 ng/kg) |
|
Oral cavity |
Squamous cell carcinoma, hard palate |
Squamous cell carcinoma, gingival |
Squamous cell hyperplasia (all) |
|
Abbreviation: NOEL, no-observed-effect level. |
|||
2005 Guidelines for Carcinogen Risk Assessment (EPA 2005a, see also Appendix B) and consideration of the points above, the committee agrees that there is strong and convincing evidence that dioxin is likely to be a human carcinogen. Although the committee does not reject outright the somewhat higher classification of dioxin as “carcinogenic to humans,” there was not unanimous agreement that the available scientific information on human dioxin carcinogenicity met condition (a) of EPA’s cancer guidelines, which states “there is strong evidence of an association between human exposure and either cancer or the key precursor events of the agent’s mode of action but not enough for a causal association” (EPA 2005a, p. 2-54).
The committee was in general agreement that the epidemiological evidence, although not “strong,” was generally consistent with a positive association between occupational dioxin exposure and mortality from all cancers, but the magnitude of the effect was modest, and the limited evidence for any specific tumor type being significantly associated was of some concern. This conclusion is in fact quite similar to EPA’s assessment of the relative strength of the epidemiological evidence (Reassessment, Part III, p. 2-21). In its discussion, the committee remained uncertain about the intent of the language in the 2005 Guidelines for Carcinogen Risk Assessment stating that condition (a) could be satisfied if there is “strong evidence of an association between human exposure and either cancer or the key precursor events of the agent’s mode of action but not enough for a causal association” (EPA 2005a, p. 2-54). The committee agreed that there is convincing evidence supporting the interaction of dioxin with the human Ah receptor and that the interaction with the receptor was necessary, but not sufficient, to cause cancer in animals. However, the committee was not in complete agreement about whether these conditions met the stated criterion of a “key precursor event of the agent’s mode of action” (EPA 2005a, p. 2-54). For example, it was noted that, even though TCDD binds to the human Ah receptor, several endogenous and exogeous substances, including bilirubin, biliverdin, and β-naphthoflavone, also bind to the Ah receptor but are not carcinogenic in rodent models (Seidel et al. 2000); hence, some other key precursor event(s) may need to be identified to meet that criterion. However, it was also recognized that persistence of the Ah receptor activation may be a key determinant for carcinogenicity because genetic modification of the AhR gene, causing activation in the absence of any ligand, results in a tumorigenic response in mice (Andersson et al. 2002).
Furthermore, there is evidence that prolonged stimulation of AHR by the nonpersistent ligand indole-3-carbinol (or its acid condensation products; derived from broccoli and other cruciferous vegetables) can promote a variety of tumor types after initiation with different genotoxic compounds (Pence et al. 1986; Bailey et al. 1987; Dashwood et al. 1991; Kim et al.
1997; Dashwood 1998; Yoshida et al. 2004). The committee recommends that EPA use the 2005 Guidelines for Carcinogen Risk Assessment (EPA 2005a, see also Appendix B) specifically in its final assessment and carefully delineate its interpretation of what constitutes “strong” evidence and a “key precursor event” under condition (a) of the definition of “carcinogenic to humans.”
The committee noted that classification artificially places an apparent bright line distinguishing a substance as “carcinogenic to humans” and “likely to be carcinogenic to humans,” whereas the actual scientific evidence lies on a continuum. In the context of a weight-of-evidence continuum, the committee found that the scientific evidence favored the high end of the “likely to be a human carcinogen” classification or the lower end of the “carcinogenic to humans” classification and emphasized that dioxin remains unique with respect to the International Agency for Research on Cancer (IARC) Group 1 designation based to a large extent on total cancers instead of a specific cancer type.
The committee recognizes that the 2003 Reassessment used a different definition of “carcinogenic to humans” based on EPA’s 2003 draft carcinogen risk assessment guidelines. The committee found that the argument provided by EPA in the 2003 Reassessment to support its position that the epidemiological data met the criterion of “strong evidence of an association” between dioxin exposure and cancer risk was unconvincing. However, the committee questioned whether it is worth EPA’s investment of significant efforts to further qualitatively classify the carcinogenicity of dioxin. The committee considers that quantitative risk estimates for dioxin should not depend on which side of the artificial bright line between “likely to be a human carcinogen” and “carcinogenic to humans” EPA ultimately places dioxin. The committee urges EPA to focus on improved quantitative characterization of risks and to reduce the emphasis on qualitative characterization of hazard in this case.
QUANTITATIVE CONSIDERATIONS IN ASSESSING TCDD, OTHER DIOXINS, AND DLC CARCINOGENICITY
EPA’s Assumption That the Dose-Response Relationship Is Linear
To estimate a cancer slope factor (CSF) for dioxin using either animal bioassay data or epidemiological data, EPA estimated a point of departure (POD) dose as the dose yielding an excess cancer risk of 1% and then extrapolated back to zero incremental dose using a straight line. The dose (mg/kg-day) corresponding to a 1% excess cancer risk is referred to as the ED01 (effective dose). Dividing the ED01 dose into 0.01 yields the CSF, expressed in units of (mg/kg-day)−1. A more conservative, but widely used,
estimate of the CSF can be calculated by estimating the lower confidence limit on the ED01 (designated the LED01 [lower confidence bound on the effective dose]) and then dividing that value into 0.01.
EPA describes its approach as follows (Part III, p. 5-15):
Extrapolation from the POD to lower doses is conducted using a straight line drawn from the POD to the origin—zero incremental dose, zero incremental response—to give a probability of extra risk. The linear default is selected on the basis of the agent’s mode of action when the linear model cannot be rejected and there is insufficient evidence to support an assumption of non-linearity.
Because EPA’s assumption of linearity at doses below the 1% excess risk level for carcinogenic effects of TCDD, other dioxins, and DLCs is central to the ultimate determination of regulatory values, it is important to critically address the available scientific evidence on the most plausible shape of the dose-response relationship at doses below the POD (LED01). On the basis of a review of the literature, including the detailed review prepared by EPA and presented in Part II of EPA’s Dioxin Risk Assessment and new literature available since the last EPA review, the committee concludes that, although it is not possible to scientifically prove the absence of linearity at low doses, the scientific evidence, based largely on mode of action, is adequate to favor the use of a nonlinear model that would include a threshold response over the use of the default linear assumption. The committee concludes that four major considerations of the scientific evidence support the use of a nonlinear model for low-dose extrapolation.
TCDD, Other Dioxins, and DLCs Are Not Directly Genotoxic
As noted earlier, available evidence suggests that TCDD, other dioxins, and DLCs are not directly genotoxic. There is general consensus in the scientific community that nongenotoxic carcinogens that act as tumor promoters exhibit nonlinear dose-response relationships, and that thresholds (doses below which the expected response would be zero) are likely to be present. In addition, even among compounds that covalently react with DNA, the dose response may be nonlinear (Williams et al. 2005). For example, the ED01 study (Staffa and Mehlman 1979) used more than 24,000 mice to evaluate the shape of the dose-response relationship over a 5-fold range of administered dose (30 to 150 parts per million) of the potent carcinogen 2-acetylaminofluorene, which is metabolized to a highly genotoxic metabolite that forms DNA adducts. The results of the lifetime feeding study showed a dose-related increase in bladder and liver tumors. The dose-response relationship for the liver tumors appeared to be linear, whereas the bladder tumor dose-response was markedly sublinear at the lower end of the curve.
Receptor-Mediated Agents Have Sublinear Dose-Response Relationships
Some studies suggest that TCDD (and, presumably, other dioxins and DLCs) may cause DNA damage indirectly via generation of reactive oxygen species that may result in oxidative DNA damage and intrachromosomal recombination, although, as noted above, initiating activity has not been demonstrated. However, these effects are secondary to a series of downstream events that are secondary to Ah receptor activation, a phenomenon that would be likely to cause the dose-response relationship to be sublinear at low doses. It is recognized that a roughly linear increase in response with increasing dose will occur at doses above a minimal response level (e.g., 1% or 5% excess risk), as would be expected for any receptor-mediated response. These comments are focused on extrapolation of the dose-response relationship to doses well below those associated with a minimum response level (POD).
The observation that adverse effects caused by TCDD, other dioxins, and DLCs depend on AHR activation underlies mechanistic considerations for these compounds. Part III, p. 3-1 (lines 5 to 14), of the Reassessment states that
much evidence indicates that TCDD acts via an intracellular protein (the AhR) which functions as a ligand-dependent transcription factor in partnership with a second protein (ARNT [AHR nuclear translocator protein]). Therefore, from a mechanistic standpoint, TCDD’s adverse effects appear likely to reflect alterations in gene expression that occur at an inappropriate time and/or for an inappropriate long time. Mechanistic studies also indicate that several other proteins contribute to TCDD’s gene regulatory effects and that the response to TCDD probably involves a relatively complex interplay between multiple genetic and environmental factors. If TCDD operates through such a mechanism, as all evidence indicates, then there are certain constraints on the possible models than can plausibly account for TCDD’s biological effects, and, therefore, on the assumptions used during the risk assessment process.
EPA cites further mechanistic studies describing interactions between the AHR and other critical regulatory proteins and transcription factors (Rb, SIM, HIF1-α, REL-A, among others) as evidence of the complex interplay between dioxin and other genetic and environmental factors. Table 3-1 in the Reassessment appropriately describes early molecular events.
There is widespread agreement in the scientific community that all or nearly all the adverse effects of TCDD, other dioxins, and DLCs depend on a receptor-mediated mechanism. Both IARC and EPA (see above) conclude that these compounds act through a mechanism involving the AHR. As noted in the Reassessment (Part III, p. 2-19, lines 28 to 33):
Despite this lack of a defined mechanism at the molecular level, there is a consensus that 2,3,7,8-TCDD and related compounds are receptor-mediated carcinogens in that (1) interaction with the AhR is a necessary early event, (2) 2,3,7,8-TCDD modifies a number of receptor and hormone systems involved in cell growth and differentiation, such as the EGFR [epidermal growth factor receptor] and estrogen receptor, (3) sex hormones exert a profound influence on the carcinogenic action of 2,3,7,8-TCDD.
Mechanistic considerations for DLCs and dioxins other than TCDD are less well established, although it is widely held that most of the toxic and carcinogenic effects of other dioxins and DLCs are mediated via the same receptor signaling pathways as those for TCDD. The Reassessment cites “comparative binding studies and other data” (Part III, p. 2-3, lines 25 and 26) to suggest that DLCs and dioxins other than TCDD exhibit TCDD-like responses in proportion to their receptor binding affinity (generally reflected in their toxic equivalency factors [TEFs]). Although this association may hold for most toxic and biochemical responses, there are few, if any, biochemical or mechanistic studies describing interactions when DLCs and dioxins other than TCDD are the ligands/inducers. It is not clear if those interactions play a role in the adverse health effects of TCDD, other dioxins, and DLCs, nor have such interactions been characterized.
There is a large body of scientific data on receptor-mediated responses. However, while the relationship of receptor binding and effects on tumor development in rodents remains uncertain from a mechanistic point of view (Whysner and Williams 1996), the recent National Toxicology Program (NTP) bioassay results using the TEF/TEQ (toxic equivalent quotient) approach (which is dictated by AHR binding) strongly supports the role of AHR in hepatocarcinogenicity of DLCs. Receptor binding appears necessary, but insufficient, because many tissues with receptors are not sites of TCDD-induced (or, by inference, induced by other TCDD, other dioxins, and DLCs) preneoplastic changes or tumors.
A fundamental concept in pharmacology is that receptor-mediated responses show sigmoidicity in the shape of the log dose-response relationship, although ligand-receptor interactions and subsequent “down stream” events that ultimately produce drug efficacy or toxicity are complex (Ross and Kenalkin 2001). Response is a function of the number of occupied and activated receptors, which typically exhibit steep dose-response relationships. For example, Kohn and Melnick (2002) modeled the shape of the dose-response relationship for receptor-mediated responses, using the estrogen receptor and various xenoestrogens as a model receptor and ligands, respectively. The model included a variety of assumptions with regard to receptor number, ligand binding affinity, and partial agonist activities, yet in every instance clear sublinear responses were observed at low doses. In
all instances modeled, a predicted response indistinguishable from the background response was seen at doses less than one order of magnitude lower than the dose providing the lowest detectable response (conceptually similar to a POD). The model parameters were based on ligands with relatively short half-lives and reversible binding to the receptor and thus may not be directly applicable to TCDD, other dioxins, and DLC binding to AHR.
Carcinogenicity of DLCs is not solely and quantitatively related to receptor binding. There are numerous synthetic and naturally occurring AHR ligands, to which humans are exposed through diet and the environment, that bind to and activate the receptor (and induce a transcriptional response as measured by cytochrome P4501A protein [CYP1A] mRNA and enzyme activity) and yet do not seem to act as tumor promoters or directly produce AHR-dependent toxic responses commonly seen with TCDD, other dioxins, and DLCs. Thus, although binding to and activation of AHR appears to be required for tumor promotion, it is not sufficient. On the other hand, others (Carney et al. 2004) have shown that morpholinos to AHR block cardiovascular toxicity in zebrafish, but morpholinos to CYP1A do not. This conclusion strongly suggests that additional downstream events are critical to the promotional effects of these chemicals. Because multiple additional steps are necessary, each probably with homeostatic mechanisms functional at low doses but perhaps overwhelmed at high doses, sublinearity with a response approaching zero at low doses would be expected.
EPA determined in previous evaluations of receptor-mediated carcinogens that a nonlinear, low-dose model, that may accommodate a threshold is appropriate. For example, numerous pesticides found to cause thyroid cancer secondary to modulation of thyroid hormone levels have been evaluated as threshold-type carcinogens (EPA 1998). In the recent NTP studies with dioxin, the observed thyroid tumors are undoubtedly due to perturbation of thyroid homeostasis (NTP 2005). Similarly, the induction of liver tumors from peroxisome proliferators was also deemed to occur via a threshold-type response but was further deemed largely irrelevant to humans because of species differences in peroxisome proliferator activated receptor (PPAR) function (EPA 2003d).
The final cancer guidelines (EPA 2005a, see also Appendix B) provide the following guidance on choosing between linear and nonlinear risk extrapolation approaches: “A nonlinear approach should be selected when there are sufficient data to ascertain the mode of action and conclude that it is not linear at low doses and the agent does not demonstrate mutagenic or other activity consistent with linearity at low doses” (p. 3-22). This is an important decision, as it will influence the methodology adopted in subsequent risk assessments. The final EPA cancer guidelines also make the following statement about risk assessment for carcinogens with a nonlinear mode of action (EPA 2005a, p. 3-20).
TABLE 5-4 Hepatic Toxicity in TCDD Rat Bioassays
|
Kociba et al. (1978) |
NTP (1982a) |
NTP (2005) |
|
0 ng/kg, severity = 0.6 (57%) |
0 ng/kg, 0 incidence |
0 ng/kg |
|
1 ng/kg, severity = 1.2 (88%) |
|
|
|
|
3 ng/kg, severity = 1.0 (4%) |
|
|
10 ng/kg, severity = 2.1 (95%) |
10 ng/kg, severity = 1.3 (15%) |
10 ng/kg severity + |
|
|
|
22 ng/kg severity |
|
|
|
2+46 ng/kg severity |
|
|
|
3+ |
|
100 ng/kg, severity = 3.6 |
100 ng/kg, severity = 3.5 |
100 ng/kg severity 4+ |
|
(100%) |
(100%) |
|
At this time, safety assessment is the default approach for tumors that arise through a nonlinear mode of action; however, EPA continues to explore methods for quantifying dose-response relationships over a range of environmental exposure levels for tumors that arise through a nonlinear mode of action. (EPA 2002)
Evidence That Liver Tumors Are Secondary to Hepatotoxicity
In the Reassessment, EPA used the female rat liver tumor data from the Kociba et al. (1978) study to develop a dose-response relationship. In that study, the liver was the main site of carcinogenic activity (see Table 5-3).
Dioxin is retained preferentially in the liver in rats (Fries and Marrow 1975; Kociba et al. 1978), in addition to adipose tissue, which may underlie the liver susceptibility. In the rat liver, hepatic toxicity was accompanied by increases in liver tumors (Table 5-4), and numerous studies have shown that hepatotoxicity results in increased cell proliferation (Williams and Iatropoulos 2002). In the most recent dioxin bioassay (NTP 2004), toxic hepatopathy was found at 31 weeks at 100 mg/kg and at 53 weeks at 46 mg/kg and 100 mg/kg, but not at low dosages. Hepatocellular labeling indices were consistently elevated at these dosages at 31 and 53 weeks. In other studies, hepatotoxicity was less pronounced in male rats, for which no increase in tumors was seen. The hepatocarcinogenicity in female rats is related to estrogens and may be due to elevation of estrogen catechol levels resulting from AHR-dependent induction of cytochromes P450 in the CYP1 family responsible for generating catechols from estradiol. Accordingly, toxicity and cell proliferation may have been key events for hepatocarcinogenicity in these studies, as has been delineated for a variety of other rodent hepatocarcinogens (Williams 1997).
The cancer guidelines (EPA 2005a, see also Appendix B) caution against
using tumor data for quantitative, low-dose extrapolation when clear evidence of cytotoxicity is present:
Studies that show tumor effects only at excessive doses may be compromised and may or may not carry weight, depending on the interpretation in the context of other study results and other lines of evidence. Results of such studies, however, are generally not considered suitable for dose-response extrapolation if it is determined that the mode(s) of action underlying the tumorigenic responses at high doses is not operative at lower doses…. Studies that show tumors at lower doses, even though the high dose is excessive and may be discounted, should be evaluated on their own merits. (EPA 2005a, p. 2-18)
Earlier in the document, EPA states,
In addition, overt toxicity or altered toxicokinetics due to excessively high doses may result in tumor effects that are secondary to the toxicity rather than directly attributable to the agent. (EPA 2005a, p. 2-17)
Thus, based on these criteria, evidence of substantial hepatoxicity in tumor-bearing animals would raise questions about the use of hepatic tumors in female rats for quantitative, low-dose extrapolation.
Although there is evidence that the liver tumors observed may be due to hepatotoxicity and have a sublinear dose-response relationship, the committee notes that two other types of epithelial tumors (keratinizing epithelioma of the lung and squamous cell tumors of the oral mucosal epithelium) were increased in a dose-dependent manner with no apparent indication of cytotoxicity in these tissues. However, the shape of the dose-response relationship for these tumors suggests that they may be nonlinear, as described below.
Bioassay Evidence of Nonlinearity
The recent NTP bioassay data (NTP 2004; Walker et al. 2005) show a consistent sigmoidicity to the tumor dose response. Walker et al. (2005) reported a Hill coefficient3 of 2.81 (standard error [SE] = 0.68) for cholangiocarcinoma, 3.74 (SE = 1.5) for hepatocellular adenoma, 23.4 (SE insufficiently stable to report) for keratinizing epithelioma of the lung, and 2.14 (SE insufficiently stable to report) for squamous cell tumors of the oral mucosal epithelium. The central estimates for these coefficients all exceed
2, hence suggesting nonlinearity, although the 95% CIs do not exclude a Hill coefficient of 1, which corresponds approximately to a linear dose response at low doses. Nonetheless, although the data alone do not rule out a linear tumor response at doses below a 5% response level (because of small sample size and limited statistical power), the observed data are more consistent with a sublinear response that approaches zero at low doses rather than a linear dose response. On the other hand, the tumor data would also probably fit a linear, low-dose model because of the small number of data points in the low-dose region.
EPA Evaluation of Bioassay Data to Estimate the CSF
For the purpose of estimating a CSF for dioxin based on animal data, EPA considered the assays conducted by Kociba et al. (1978) and NTP (1982a). In each case, EPA restricted attention to those tumor types for which incidence increased with dioxin exposure (five types in the Kociba et al. study and eight types in the NTP study). Based on an analysis by Portier et al. (1984) using a simple multistage model (order up to 3), the ED01 body burdens for these 13 dose-response relationships ranged from 14 to 1,190 ng/kg. The corresponding LED01 values ranged from 10 to 224 ng/kg.
EPA also considered two alternative ED01 estimates developed using the Kociba et al. (1978) female rat liver tumor data. First, EPA described an ED01 estimate calculated from a model developed by Portier and Kohn (1996). That model combined a pharmacokinetic model characterizing gene expression induced by dioxin with a two-stage carcinogenesis model to analyze the female rat liver tumors from the Kociba et al. study. Using that model, EPA calculated ED01 = 2.7 ng/kg. Second, EPA reported an estimate for ED01 equal to 31.9 ng/kg (LED01 = 22.2 ng/kg). EPA developed this estimate using its benchmark dose software and a reevaluation of the Kociba et al. (1978) pathology results by Goodman and Sauer (1992). The revised estimate also reflected other changes in the procedure for fitting a function to the data.
In 2003, when EPA’s Reassessment was issued, the most recent NTP bioassay results (NTP 2004) were not yet published. Because this study represents an extensive data set developed using state-of-the-art methodology, EPA should integrate this information into its analysis.
In addition, EPA should specify criteria used to identify those data sets to be included in its analysis. The EPA Reassessment does not explain why EPA chose to rely on a single site (liver) from one sex (female) in one species (rat), as measured in a single study (Kociba et al. 1978). Consideration of other data sets that were available to EPA would have yielded a substantially wider range of potency estimates. Whereas the LED01 for liver tumors in female rats from the Kociba et al. study is 22.2 ng/kg, the ED01 values
calculated using all the animal bioassay data sets considered by EPA (data sets from the NTP and Kociba et al. studies that suggested tumor incidence increases with dose) range as high as 1,190 ng/kg. In addition, use of the mechanistic model developed by Portier and Kohn (1996) to analyze the Kociba et al. female rat liver tumor data yields a substantially lower ED01 value (2.7 ng/kg). The range of values would be even broader if EPA had also estimated upper ED01 (UED01 [upper confidence bound on the effective dose]) values. Like the LED01 values, these values are indicative of the range of estimates that are consistent with the data and hence are indicative of inherent uncertainty. Calculations of a slope factor that considers the effects of dioxin on ALL tumor sites, as was done for the human epidemiological data, would probably further broaden the range of plausible ED01 values. Because dioxin is presumed to promote tumor growth at a wide range of sites, EPA should explain why it chose not to evaluate the dose-response relationship for “all tumors combined” in the animal studies if it considers this approach to be appropriate for use in human epidemiological studies.
The committee notes that extrapolation of results across species is highly uncertain, even when dose is scaled to account for body burden. Although data from animal and human cells and tissues suggest a qualitative similarity across species in the response to DLCs (Reassessment, Part III, p. 2-3, lines 28 and 29, and p. 3-10, lines 30 to 33), they do not support the hypothesis that the responses across species are quantitatively similar. For example, there is no explanation for the observation that the LD50 (50% lethal dose) for dioxin in guinea pigs and hamsters differs by more than a factor of 8,000 (Part II, p. 3-1) even though their respective receptors do not differ substantially in terms of dioxin binding and other responses (e.g., CYP1A1 induction differs by only a factor of 4). Similarly, whereas the LD50 for dioxin in two strains of rats differs by a factor of 300 to 1,000 (Part II, pp. 3-1 to 3-3), AHR ligand binding in these two strains has similar affinities, and CYP1A1 inducibility does not differ. These observations complicate interspecies comparisons. Recent studies comparing the response of human hepatocytes to dioxin with that of rat and mouse hepatocytes further illustrate that quantitative extrapolation of rodent data to humans is highly uncertain (Silkworth et al. 2005).
TCDD, other dioxins, and DLCs act as potent inducers of CYP, a property that can affect both the hepatic sequestration of these compounds and their half-lives. Hepatic sequestration of dioxin may influence the quantitative extrapolation of the rodent liver tumor results because the body-burden distribution pattern in highly dosed rats would differ from the corresponding distribution in humans subject to background levels of exposure. EPA should consider the possible quantitative influence of dose-dependent toxicokinetics on the interpretation of animal toxicological data.
EPA’s Characterization of Uncertainty for CSF Estimates
As part of their quantification of risk, it is important for risk assessors to provide a full characterization of the uncertainty inherent in their estimates. Although risk managers may choose to focus on conservative estimates of risk, the risk assessment must be kept distinct (NRC, 1983) and should describe the lower and upper ranges of plausibility for risk estimates. A complete characterization of a risk’s uncertainty facilitates (1) comparison of that risk estimate with other risk estimates that may have the same point value but a different degree of uncertainty, (2) comparison of risks with the costs and countervailing risks associated with interventions to address the primary risk, and (3) evaluation of research needs. A number of reports have addressed the need for a comprehensive treatment of uncertainty in risk assessment, including a National Research Council (NRC 1994) report.
Part III, Chapter 5, of the Reassessment describes EPA’s development of a CSF for TCDD, other dioxins, and DLCs. EPA identifies 1 × 10−3 pg TEQ/kg of body weight per day (pg/kg-day)−1 “as an estimator of upper bound cancer risk for both background intakes and intakes above background” (Part III, pp. 5-28 to 5-29). While EPA qualitatively notes many of the factors contributing to this estimate’s uncertainty, the Reassessment does not adequately discuss how these factors contribute quantitatively to the underlying uncertainty. By omitting the quantitative implications of these factors, the Reassessment understates the uncertainty inherent in these estimates and overstates the consistency of the data and risk estimates across all studies.
EPA should have addressed quantitatively the following sources of uncertainty:
-
Basis for risk quantification: (1) bioassay data, (2) occupational cohort data.
-
Epidemiology data to use: (1) risk estimate developed with data aggregated from all suitable studies, (2) risk estimate or estimates developed using each study individually.
-
Factors affecting extrapolation from occupational to general population cohorts, including differences in baseline health status, age distribution, the healthy worker survivor effect, and background exposures.
-
Bioassay data to use: (1) risk estimate developed with the single data set implying the greatest risk (that is, single study, tumor site, gender), (2) risk estimate developed with multiple data sets satisfying an a priori set of selection criteria.
-
Dose-response model: (1) linear dose response, (2) nonlinear dose.
-
Dose metric: (1) average daily intake, (2) area under the blood concentration–time curve, (3) lifetime average body burden, (4) peak body burden, (5) other.
-
Dose metric—biological measure: (1) free dioxin, (2) bound dioxin.
-
POD: (1) ED10, (2) ED05, (3) ED01.
-
Value from ED distribution to use: (1) ED, (2) lower confidence bound value for the ED (LED), (3) upper confidence bound for the ED (UED).
Where alternative assumptions or methodologies could not be ruled out as implausible or unreasonable, EPA could have estimated the corresponding risks and reported the impact of these alternatives on the risk assessment results. The potential impacts of four sources of uncertainty are discussed below.
-
The full range of plausible parameter values for the dose-response functions used to characterize the dose-response relationship for the three occupational cohort studies selected by EPA (Ott and Zober 1996; Becher et al. 1998; Steenland et al. 2001).
-
Use of other points of departure, not just the ED01 (or LED01), to develop a CSF.
-
Alternative dose-response functional forms as well as goodness of fit of all models, especially at low doses.
-
Uncertainty introduced by estimation of historical occupational exposures.
If these factors are considered, the range of plausible CSF values becomes much larger, with more extreme upper and lower bound estimates, as shown below.
EPA’s development of a CSF value emphasizes the analysis of three occupational cohort studies (Ott and Zober 1996; Becher et al. 1998; Steenland et al. 2001). In all cases, the studies estimated SMRs or rate ratios (RRs) as a function of cumulative dioxin lipid burden (CLB, nanogram of dioxin per kilogram of lipid weight × years) (see Reassessment, Part III, Table 5-2). Using these dose-response relationships, EPA summarizes its ED01 and CSF calculations (Part III, Table 5-4). The ED01 values are reported as lifetime average body burdens for dioxin (LABB, ng/kg). Although the relationship EPA used to convert from CLB to LABB was not transparent in the Reassessment, the committee assumes it can be described as CLB = 4 × 75 × LABB. Here, the factor of 4 accounts for conversion from nanogram per kilogram of lipid to nanogram per kilogram of body weight (see Reassessment note (a) of Table 5-
4 in Part III for this assumption), and 75 corresponds to the “average” lifetime in years.
We note that EPA identified the dose corresponding to 1% excess risk (the ED01) from the relationship.
EPA estimated the risk function in the above equation from the occupational cohort studies by converting the hazard function to a probability of death by age 75 years. This risk estimate satisfies the requirement that risk (infinity) = 1—that is, as dose increases, the risk approaches 100%. Critical findings are reproduced in Table 5-5.
The committee also notes that EPA’s analyses of the Hamburg and BASF cohorts considered all cancer deaths with no latency, but the analysis of the National Institute of Occupational Safety and Health (NIOSH) cohort considered a 15-year lag. The committee is aware of the problem with cancer mortality studies in which the subjects are without cancer at baseline (first exposure) so that the cancer mortality at the start of follow-up (in the few years after first exposure) will be artificially low. There is thus good reason to consider deaths only after some fixed time, and in dose-response calculations, one has to estimate cumulative dose appropriate to the date of occurrence of the cancer. These considerations were not part of the basis for determining the latency used in the NIOSH analysis, which was based on the assumption that effects should not be seen for many years after first exposure and the dose calculations ignored all doses for the immediately preceding 15 years. The committee is unconvinced of the validity of such assumptions in the context of dioxin as a promoter and furthermore sees no justification for considering the NIOSH results any differently than the other two cohorts.
Full Range of Plausible Parameter Values
In Part III of the Reassessment, EPA makes use of only the ED01 and LED01 for the purpose of estimating a CSF. Of course, a more complete range of plausible CSF values can be developed by considering parameter estimates corresponding to dose-response relationships that are less than the central estimate relationship (used to identify the ED01). EPA’s recently released cancer guidelines (EPA 2005a) recommends use of both lower- and upper-bound values. In section 3.2.4 of the document, which is entitled Point of Departure (POD), EPA states, “risk assessors should calculate, to the extent practicable, and present the central estimate and the correspond-
TABLE 5-5 EPA Inputs to CSF Estimates Using Epidemiological Dataa
|
Study |
Function RR(x)b |
Central Estimate for bb |
P Value for bb |
ED01c |
LED01c |
|
Becher et al. 1998 |
Power: (1 + kx)b |
b = 0.326 for |
0.026 |
6 |
NAd |
|
|
k = 1.7 × 10−4 |
|
|
|
|
|
|
Additive: 1 + bx |
b = 1.6 × 10−5 |
0.031 |
18.2 |
NAd |
|
|
Multiplicative: ebx |
b = 8.69 × 10−6 |
0.043 |
32.2 |
NAd |
|
Steenland et al. 2001 |
Power: (x/background)b |
b = 0.097 |
0.003 |
1.38e |
0.71e |
|
|
Piecewise linear: ebx |
b = 1.5 × 10−5 |
NAf |
18.6 |
11.5 |
|
Ott and Zober 1996 |
ebx |
b = 5.03 × 10−6 |
0.05 |
50.9 |
25 |
|
aED01 values here represent estimates only for males (as is the case in EPA’s Part III, Table 5-4). Female ED01 values are modestly larger because the background cancer rate for females is less than it is for males. For example, the Ott and Zober (1996) ED01 value for females is 62 ng/kg and the corresponding LED01 is 30.5 ng/kg. bSee Part III, Table 5-2. Note that x is exposure expressed in terms of cumulative lipid burden (CLB), ng of dioxin/kg of fat × years. cSee Part III, Table 5-4. The ED01 values are expressed in terms of lifetime average body burden (LABB), ng of dioxin/kg of body weight. dNot available. EPA did not estimate LED01 values (or the corresponding upper bound for ED01), although these values can be calculated, as described below. eEPA reported these values in Part III, Table 5-3. Note that EPA omitted further consideration of the power function for this dataset, stating that “this formula predicts unreasonably high attributable risks at background dioxin levels in the community due to the steep slope of the power curve formula at very low levels” (Part III, p. 5-37). fNot available. Steenland et al. (2001) did not report the P value for this parameter, although EPA reported a value for LED01. |
|||||
ing upper and lower statistical bounds (such as confidence limits) to inform decision makers” (EPA 2005a, p. 3-17).
To illustrate the quantitative impact on the range of uncertainty from this one assumption, the committee considered the upper ED01 (UED01) values that correspond to the lower 95% confidence interval on the dose-response relationship. EPA provides the UED01 values for the Steenland et al. (2001) and Ott and Zober (1996) studies (see Table 5-3 in Part III of the Reassessment). As explained below, the committee has calculated the UED01 values for the Becher et al. (1998) study. Together with the ED01 and LED01 values, the UED01 values help to describe the range of plausible ED01 values and hence the uncertainty that attends the CSF estimates due to finite sampling.
To estimate the range of plausible ED01 values, the committee assumes that the set of plausible values for the dose-response relationship parameter
(b—see column 3 of Table 5-5 in this report) is normally distributed with a mean equal to the parameter’s central estimate (b—see column 3 of Table 5-5) and a standard deviation equal to the estimate’s standard error (bSE).4 When possible, the committee estimated the value of bSE using the P value for the dose-response relationship parameter, as reported in the Reassessment, Part III, Tables 5-2 and 5-3 (shown in column 4 of Table 5-5 of this report). In particular, bm, bSE, and P satisfy the relationship
where N−1 is the inverse cumulative normal function. For example, if bm = 0.1 and P = 0.05, then bSE = 0.051. Designating bUED = bm − 1.96bSE, the value of b yielding the UED01, and bLED = bm + 1.96bSE, the value of b yielding the LED01, the UED01 satisfies the relationship RR(bUED, UED01) = RR(bLED, LED01), where the function RR is the dose-response relationship (rate ratio) taking two arguments (the parameter b and a dose).
When the P value is not available but both LED01 and ED01 are specified, the committee assumed that bLED − bm = bm − bUED. If necessary, the value of bLED was estimated from the relationship RR(bLED, LED01) = RR(bm, ED01) and UED01 from the relationship RR(bUED, UED01) = RR(bLED, LED01). In the case of the Becher et al. (1998) study, inserting bm into any of the RR formulas along with the ED01 value for that dose-response function yields an RR of approximately 1.09. It was assumed that the UED01 is the dose that yields an RR of 1.09 when inserted into the dose-response function along with bUED. For example, the Becher et al. power function yields an RR of 1.09 if a LABB of 6 ng/kg (CLB = 1,800 ng/kg-year) is used along with the exponent parameter bm = 0.326. In particular, (1 + 0.00017 × 1,800)0.326 = 1.09. The value of bUED is 0.039 and (1 + 0.00017 × 45,300)0.039 = 1.09. That is, CLB = 45,300 ng/kg-year produces the same RR when used with b = bUED. Dividing CLB by 4 × 75 = 300 yields a LABB of 151 ng/kg. The committee assumed that because the LABB of 151 ng/kg also yields an RR of 1.09, this dose is the UED01.
Table 5-6 summarizes the ED01, LED01, and UED01 values for the dose-response relationships listed in Table 5-2 of Part III of the Reassessment. The results in Table 5-6 indicate that the set of plausible ED01 values spans at least one or two orders of magnitude for the Becher et al. (1998) study and the Ott and Zober (1996) study.
TABLE 5-6 ED01, LED01, and UED01 Values
|
|
|
LABB (ng/kg) |
||
|
Study |
Function RR = |
ED01 |
LED01 |
UED01 |
|
Becher et al. 1998 |
Power: (1 + kx) |
6 |
3 |
150 |
|
|
Additive: 1 + bx |
18.2 |
9.5 |
200 |
|
|
Multiplicative: ebx |
32.2 |
16 |
1,000 |
|
Steenland et al. 2001 |
Power: (x/background) |
1.38 |
0.71 |
8.95 |
|
|
Piecewise linear: ebx |
18.6 |
11.5 |
49 |
|
Ott and Zober 1996 |
ebx |
50.9 |
25 |
Infinite |
|
NOTES: Bolded values represent the committee’s estimates of UED01 for the Becher et al. (1998) study. These values were estimated by assuming a normal error distribution for b (see column 3 in Table 5-5 of this report). All other values were as reported by EPA in Table 5-3 of Part III of the Reassessment. |
||||
Consideration of Alternative Points of Departure
EPA explains that while a 10% level is generally used as a POD (that is, an ED10 is generally used to estimate the CSF), “where more sensitive data are available, a lower point for linear extrapolation can be used to improve the assessment (e.g., 1% response for dioxin, ED01)” (Part III, p. 5-15). EPA’s cancer guidelines (EPA 2005a, see also Appendix B) state, “Conventional cancer bioassays, with approximately 50 animals per group, generally can support modeling down to an increased incidence of 1-10%; epidemiologic studies, with larger sample sizes, below 1%” (p. 3-17).
However, these generalities do not imply that extrapolation down to low levels is justified in all circumstances. EPA’s carcinogen risk assessment guidelines document explains, “Various models commonly used for carcinogens yield similar estimates of the POD at response levels as low as 1%…. Consequently, response levels at or below 10% can often be used as the POD” (EPA 2005a, p. 3-17). The key point here is that a lower response level is justified only if the estimated dose corresponding to this response is insensitive to the functional form (provided the other functional forms fit the data to a comparable degree). The dose-response functions for the epidemiological data identified by EPA suggest this criterion is not satisfied. For example, as detailed in Table 5-3 of Part III of the Reassessment, the ED01 for males in the Steenland et al. (2001) study is 1.38 ng/kg of body burden if the power function is used, more than an order of magnitude less than the ED01 of 18.6 ng/kg calculated using the piecewise linear function. In the Becher et al. (1998) study, the ED01 spans a factor of five, depending on which dose-response function is used.
Although EPA states that a 1% response above background (corresponding to RR ≈ 1.09) is within the range of observed response for the three occupational cohort studies considered, it is clearly at the low end of the observed range. For example, among the five exposure groups defined in the Becher et al. (1998) study (excluding the comparison group, for which SMR is fixed at 100), the lowest RR is 1.12. Of the six exposure groups in the Steenland et al. (2001) study (excluding the comparison group), one has an RR value below 1.09 (RR = 1.02 for the second lowest exposure group). RR values for the other five groups were 1.26 or greater. For the Ott and Zober (1996) study, RR values for the three comparison groups were 1.2, 1.4, and 2.0.
The use of alternative points of departure for the power dose-response relationships would greatly increase the range of plausible CSF values. Table 8-2 (Reassessment, Part II) demonstrates this point. For the Steenland et al. power function, the 95% confidence interval for ED01 spans approximately one order of magnitude. As a result, the CSF calculated with this set of ED01 estimates likewise spans approximately an order of magnitude. In contrast, the 95% confidence interval for ED05 spans approximately three orders of magnitude. Similarly, the ED01 confidence interval derived from the Becher et al. power function spans a factor of approximately 50. The corresponding range for ED05 spans nearly four orders of magnitude.
Thus, it is evident that the choice of POD can have a substantial impact on the uncertainty of the final risk estimate, especially if both upper and lower confidence limits are provided. The importance of this assumption is not readily evident in the Reassessment. The transparency of the uncertainty of CSF calculations, and thus risk estimates, would be substantially improved if the document presented CSF ranges and risk estimates calculated from both ED01 and ED05 values to illustrate the importance of this assumption.
Consideration of Alternative Dose-Response Functional Forms
Because there are so many functional forms from which to choose for the purpose of modeling dose response, EPA should establish criteria for selecting acceptable solutions. For example, there are formal goodness-of-fit tests that can help to identify the best candidates. Note that a higher statistical significance for a positive dose response does not necessarily imply that, using standard statistical criteria, the model adequately fits the data. Evaluating the goodness of fit for the occupational cohort analyses was complicated by EPA’s lack of ready access to the original data. Despite these complications, it is important that EPA provide a cogent set of criteria for determining which functional forms were used. This section identifies four instances in which EPA eliminated from consideration alternative dose-
response functional forms without providing adequate justification. EPA should describe the range of ED01 and ED05 values implied by dose-response functions that are statistically consistent with the occupational cohort data and the inclusion criteria established for this assessment.
First, EPA eliminated from consideration the power function dose-response relationship calculated from the Steenland et al. (2001) study, explaining only that this relationship “predicts an unrealistic risk for the background exposure” (Reassessment, Part II, p. 8-67) and that it “leads to unreasonably high risks at low exposure levels, based on calculations of the attributable risk that this model would predict from background dioxin levels in the general population” (Reassessment, Part III, p. 5-13). EPA provided no criteria by which it judged the reasonableness of the Steenland et al. power function, nor does EPA provide any further explanation on this point. The Reassessment should provide further scientific rationale for excluding the Steenland et al. power function, or it should be considered as valid as any of the other dose-response relationships.
Second, EPA considered only dose-response relationships based on the assumption of no background incremental risk (that is, SMR = 100 at background exposure levels). This assumption is inconsistent with the findings of two analyses identified by EPA (Starr 2001, 2003; Crump et al. 2003) (Part III, p. 5-14) that rejected the assumption on statistical grounds that SMR = 100 at baseline exposure levels. If relaxing this assumption yields an estimate of SMR > 100 at background exposures, the resulting dose-response relationship would tend to be shallower, yielding smaller CSF values. For example, EPA (Part III, p. 5-15) noted that in a pooled analysis of Ott and Zober (1996), Flesch-Janys et al. (1998), and Steenland et al. (2001), fixing SMR = 100 at background exposure levels yielded ED01 = 51 ng/kg-day, whereas dropping this assumption resulted in ED01 = 91 ng/kg. EPA should provide an explanation for assuming SMR = 100 at background exposure levels. Short of doing so, EPA should consider the impact of relaxing this assumption on the estimated value of the ED01.
Third, for the piecewise linear dose-response function developed for the Steenland et al. data set, EPA considered only one cut point (40,000 ng/kg × years) (the cut point, or changing point, is the dose at which the slope of the piecewise linear dose-response relationship changes). Although this is the best-fit cut-point estimate and the only relationship of this form reported by the authors, other cut-point values are plausible. (Other cut points would yield dose-response relationships that could not be statistically rejected.)
Finally, EPA considered only a subset of the plausible dose-response relationships that could be fit to the data in the Becher et al. (1998) study. Becher et al. considered a family of dose-response relationships of the form RR = (kx + 1)β, where the value of k is chosen arbitrarily. The best-fit value
of β depends on the value of k selected. The relative plausibility of different values of k can be determined by comparing likelihood function values. Finally, holding β = 1 yields a linear function where the value of k is uncertain. Becher et al. reported that k = 0.00017 maximizes the likelihood function and that, for this value of k, the corresponding value of β = 0.326. Holding β = 1 yields k = 0.000016. However, Figure 1 of Becher et al. indicates that the likelihood function is relatively insensitive to the value of k selected. Hence, other dose-response relationships are plausible. Estimating the ED01 values corresponding to these alternative dose-response relationships would require further primary analysis of the data.
Uncertainty Associated with Estimation of Historical Exposures
The assumed half-life for dioxin in humans plays a major role in the back-extrapolation of dioxin lipid concentrations to the estimation of peak body burdens in occupational cohorts. The Reassessment states, “Using published first-order back-calculation procedures, the relatively small difference (<10-100-fold) in body burden between exposed and controls in the dioxin epidemiology studies makes exposure characterization in the studies a particularly serious issue” (Part III, p. 5-7). The high exposures in the occupational cohorts suggest a high likelihood of enzyme induction during the period of occupational exposure that may have led to a reduction of the half-life to less than the assumed value of 7.1 years. Aylward et al. (2005) discussed the issue of half-lives and the impact of this parameter on risk estimates.
EPA’s Reassessment compared the impact of using either a half-life of 4 years or the default of 7.1 years on the back-extrapolation estimate. EPA reported that using a 4-year half-life increases the peak body burden and the area under the curve (AUC) by 4.6-fold and 3.8-fold, respectively. This difference would have increased the estimated ED01 values by the same amount and hence decreased the CSF estimates, resulting in a lower risk estimate. Given the potential importance of this issue, the committee finds the following statement by EPA surprising: “This bounding exercise suggests that impacts on back-calculated peak and AUC values may become significant if the models predict prolonged periods with half-lives of less than 4 years” (Part III, p. 5-8).
Because the impact of the half-life used for back-extrapolation depends on the back-extrapolation duration required in any particular study, EPA should have estimated the impact of using the 4-year alternative value for each of the main epidemiological studies separately. EPA should also consider the issues raised by Aylward et al. (2005). Overall, the Reassessment does not provide sufficient quantification of the impacts of these choices, and the committee believes these decisions influence the estimated dose-response relationships.
Overall Uncertainty
Table 5-4 (Reassessment, Part III) summarizes EPA’s all-site cancer ED01 values. For the three occupational cohort studies, these values span less than an order of magnitude (6 to 50.9 ng/kg). The corresponding CSF values range from 5.7 × 10−4 to 5.1 × 10−3 (pg/kg-day)−1. On the basis of this range, EPA concludes that “A slope factor estimate of approximately 1 × 10−3 per pg of dioxin per kg of body weight per day represents EPA’s most current upper bound slope factor for estimating human cancer risk based on human data” (Part III, p. 5-28). The animal-based ED01 values listed in EPA’s Table 5-4 range from 22 to 30.9 ng/kg, leading to the conclusion that “A slope factor of 1.4 × 10−3 per pg dioxin/kg body weight/ day represents EPA’s most current upper bound slope factor for estimating human cancer risk based on animal data” (Part III, p. 5-28).
Whereas a CSF of approximately 1 × 10−3 per pg/kg-day (equivalently, an ED01 of approximately 30 ng/kg LABB) lies within the range of plausible values, this discussion has focused on the relative magnitude of the range of plausible values. Consideration of the set of all plausible parameter values (that is parameter values within the 95% confidence interval) for the dose-response functions considered by EPA considerably widened the range of values estimated from the Becher et al. and Ott and Zober studies (see Table 5-6). The CSF values (risk per pg/kg-day) can be calculated by first converting the ED01 expressed as ng/kg LABB to an ED01 expressed as a daily intake (ng/kg-day) using EPA equation 5-1 (Part III, p. 5-18) and then dividing this intake into a risk of 0.01. For the Becher et al. (1998) study, the resulting CSF values range from 3.0 × 10−5 to 1.0 × 10−3 per pg/kg-day, more than two orders of magnitude. The 95% confidence interval for the CSF calculated from the Ott and Zober study (1996) has a lower bound of zero and an upper bound of 1.2 × 10−3. Only the Steenland et al. (2001) study retains an ED01 range (CSF range) with a span confined to less than two orders of magnitude (CSF 6.1 × 10−4 to 3.0 × 10−2). Figure 5-2 compares the range of plausible CSF values identified by EPA with the range of plausible values consistent with the dose-response parameter 95% confidence intervals.
Consideration of alternative points of departure can greatly inflate the confidence interval for the power function dose-response relationships. Using an ED05 (rather than an ED01) broadens the confidence interval for the function to more than three orders of magnitude. Consideration of alternative dose-response relationship forms could further broaden the range of plausible CSF values, although primary analysis of the data would be required to quantify the impact. The Reassessment could develop a distribution for the CSF by assigning some probability to different options for each of the assumptions discussed here (McKone and Bogen 1992; Evans et al.
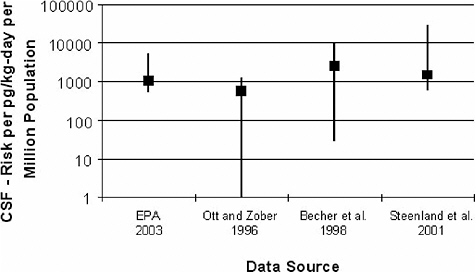
FIGURE 5-2 Range of plausible CSF values: consideration of parameter confidence intervals only. Solid blocks are central estimate values. For EPA, this value (1,000) corresponds to EPA’s stated central estimate of dioxin’s CSF (1 × 10−3 per pg/kg-day). For the Becher et al. (1998) study, the central estimate in the figure corresponds to the average of the three ED01 values that EPA reports in Part III, Table 5-4. For the Ott and Zober (1996) and Steenland et al. (2001) studies, the central estimate corresponds to the individual ED01 values listed in EPA, Part III, Table 5-4.
1994a,b). In any case, a more thorough consideration of plausible alternative values for key assumptions is needed to portray accurately to risk managers the magnitude of uncertainty that underlies the quantitative risk estimates derived from epidemiological studies.
CONCLUSIONS AND RECOMMENDATIONS
Qualitative Weight-of-Evidence Carcinogen Classification
-
The committee concluded that the classification of dioxin as “carcinogenic to humans” versus “likely to be carcinogenic to humans” depends greatly on the definition and interpretation of the specific criteria used for classification, with the explicit recognition that the true weight of evidence lies on a continuum with no bright line that easily distinguishes between these two categories. The committee agreed that, although the weight of epidemiological evidence that dioxin is a human carcinogen is not strong, the human data available from occupational cohorts are consistent with a
-
modest positive association between relatively high body burdens of dioxin and increased mortality from all cancers. Positive animal studies and mechanistic data provide additional support for classification of dioxin as a human carcinogen. However, the committee was split on whether the weight of evidence met all the necessary criteria described in the cancer guidelines (EPA 2005a, see also Appendix B) for classification of dioxin as “carcinogenic to humans.” EPA should summarize its rationale for concluding that dioxin satisfies the criteria set out in the most recent cancer guidelines (EPA 2005a, see also Appendix B) for designation as either “carcinogenic to humans” or “likely to be carcinogenic to humans.”
-
The committee agreed that other DLCs are most appropriately classified as “likely to be carcinogenic to humans.”
-
Should EPA continue to classify dioxin as “carcinogenic to humans,” more justification will be required to rationalize why a mixture containing dioxin would not also meet the classification of “carcinogenic to humans.”
-
If EPA continues to designate dioxin as “carcinogenic to humans,” it should explain whether this conclusion reflects a finding that there is a strong association between dioxin exposure and human cancer or between dioxin exposure and a key precursor event of dioxin’s mode of action (presumably AHR binding). If EPA’s finding reflects the latter association, EPA should explain why that end point (e.g., AHR binding) represents a “key precursor event.”
-
The committee considers the distinction between these two categories to be based more on semantics than on science and recommends that EPA spend its energies and resources more carefully delineating the assumptions used in quantitative risk estimates for TCDD, other dioxins, and DLCs derived from human and animal studies.
Quantitative Risk Estimation of Cancer Potency
-
The committee concludes that there is an adequate scientific basis to support the hypothesis that the shape of the relationship between dioxin dose and cancer risk is sublinear at low doses, perhaps reflecting responses indistinguishable from background risk at doses below which dose-response data are available, including evidence that (1) TCDD, other dioxins, and DLCs are not genotoxic; (2) dioxin acts through receptor mediation, and receptor-mediated carcinogens tend to exhibit sublinear dose-response relationships; (3) dioxin-induced liver tumors are secondary to hepatotoxicity and enhanced rates of cell proliferation; (4) bioassay results suggest sublinearity (Hill coefficient central estimates substantially greater than 1); and (5) epidemiological results do not help to distinguish between zero and nonzero responses at the low-dose end of the dose-response curve. Accordingly, a risk assessment can be conducted without resorting to default assumptions.
-
To the extent that EPA favors using default assumptions for regulating dioxin as though it were a linear carcinogen, such a conclusion should be supported with scientific evidence. (For example, EPA could explore whether background exposures raise the population to the linear portion of the dose-response relationship.) Alternatively, the decision to use the linear dose-response relationship could be made as a part of risk management, although the risk assessment should provide the scientific strengths and weaknesses for both linear and nonlinear approaches. EPA should adhere to the division between risk assessment, which is a scientific activity, and risk management, which takes into account other considerations, as described by the National Academy of Sciences more than two decades ago (NRC 1983).
-
EPA has not adequately justified use of the 1% excess risk level as the POD for the analysis of either the epidemiological or animal bioassay data. Although demonstrating that the POD is within the range of the data is necessary, it is not sufficient to justify its use. Other conditions, such as demonstrating that the POD is relatively insensitive to functional form (as noted in EPA’s cancer guidelines), must also be satisfied. EPA should acknowledge the larger extrapolation from justifiable POD values down to environmentally relevant doses that would be necessitated by use of a higher-response-level POD.
-
Regarding EPA’s review of the animal bioassay data, the committee recommends that EPA establish clear criteria for including different data sets. The reliance on one site from one gender of one species, as reported by a single study, does not adequately represent the full range of data available. The committee recommends that EPA consider the full range of data, including the new NTP animal bioassay study on TCDD for quantitative dose-response assessment.
Characterization of Uncertainty Surrounding Cancer Risk Estimates
-
EPA should characterize more completely the uncertainty associated with risk estimates inferred from the epidemiological data by (1) taking into account the full range of EDxx values statistically consistent with the data (not just the central and lower estimates), (2) considering alternative PODs, (3) considering alternative dose-response functional forms consistent with the data, and (4) considering uncertainty associated with the half-life estimates of dioxin in humans for the purpose of back-extrapolating exposures in occupational cohort studies.
-
The committee recognizes that explicit characterization of uncertainty could result in an especially wide range of risk estimates. Narrowing consideration to a subset of those estimates could be made as part of the risk management task. For example, a “health protective” (conservative)
-
estimate could be used to support an imperative to protect public health. Alternatively, if the goal is to compare that risk with other risk management priorities, countervailing risks, or the economic costs of risk mitigation, a central or arithmetic mean value could be used. Finally, to address uncertainty associated with specification of the dose-response relationship functional form below the POD (that is, linear vs. nonlinear), EPA could choose to use a margin of exposure approach in place of estimating population risk. These options are the purview of risk management rather than risk assessment.
-
On the whole, it was the committee’s impression that EPA’s narrative in discussing epidemiological studies in Part III of the Reassessment tended to focus on positive findings without fully considering the strengths and limitations of both positive and negative findings. Part III of the Reassessment would be strengthened if EPA clearly identified specific inclusion criteria for those studies for which quantitative risk estimates were determined.




































