1
Introduction
The U.S. Environmental Protection Agency (EPA) and other organizations, such as the World Health Organization (WHO), began assessing the potential risks to human health from exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, commonly referred to as dioxin) decades ago. Early studies suggested very high toxicity at very low doses in test animals and potential carcinogenicity. The history of dioxin risk assessment is complicated and contentious (Thompson and Graham 1997). In 1985, EPA produced an initial assessment of the human health risks from environmental exposure to dioxin. Three years later, EPA, other federal agencies, and the scientific community began developing a broad research program to identify the biological response mechanisms and to explore other key scientific issues related to dioxin. In light of significant advances in the scientific understanding of the mechanisms of dioxin toxicity, new studies of dioxin’s carcinogenic potential in humans, and increased evidence of other adverse health effects primarily after the 1985 assessment, EPA announced in 1991 that it would conduct a scientific reassessment of the health risks of human exposure to TCDD and related compounds, that is, dioxins, other than TCDD, and dioxin-like compounds (DLCs). The reassessment would respond to emerging scientific knowledge of the biological, human health, and environmental effects of TCDD, other dioxins, and DLCs.
EPA conducted the reassessment process as an open and participatory exercise, involving chapter authorship by scientists outside the agency, a series of public meetings and peer-review workshops, and reviews by EPA’s Science Advisory Board (SAB). EPA’s National Center for Environmental
Assessment (NCEA) headed the reassessment efforts with participation of scientific experts in EPA, the National Institutes of Health’s (NIH) National Institute of Environmental Health Sciences (NIEHS), and other federal agencies and scientific experts in the private sector and academia. EPA sponsored open meetings in 1991 and 1992 to inform the public about the assessment, receive public comments on plans and activities of the reassessment process, and obtain additional relevant scientific information. Peer-review workshops were convened in 1992 and 1993 to review initial drafts of all background chapters. The workshops were followed by extensive revision and additional review of some chapters. In 1994, EPA released for public review all the chapters plus the first draft of a summary risk characterization chapter, received public comments on the drafts, and submitted the documents to the SAB for review.
In 1995, the SAB, commenting on the 1994 draft assessment, proposed several substantive and contingent recommendations, including revision of the chapter on dose-response modeling for TCDD, development of a chapter on dioxin toxic equivalency factors (TEFs), and an external peer review of redrafted or new chapters, including the chapter on risk characterization. The SAB also recommended that EPA involve outside scientists from the public and private sectors to help determine approaches for revising what was then called Chapter 9: “Risk Characterization of TCDD and Related Compounds.”
In 1996, EPA initiated interaction with a group of 40 stakeholders from the public and private sectors to gather input on approaches for conducting the risk characterization revision. EPA met regularly with the group to ensure ongoing input as recommended by the SAB and shared with them the initial post-SAB revision of the draft risk characterization.
EPA, with NIEHS, revised Chapter 8, developed a new Chapter 9 on TEFs, and revised the former Chapter 9 and renamed it as a free-standing report, “Part III—Integrated Summary and Risk Characterization for 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds.” The dioxin report consisted of two other parts: “Part I—Estimating Exposure to Dioxin-Like Compounds” and “Part II—Health Assessment for 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds.” All three parts are collectively referred to as the Reassessment.
On February 24, 1997, the Federal Register announced the public external peer review and 60-day comment period of the revised Chapter 8, “Dose-Response Modeling for 2,3,7,8-TCDD.” On June 12, 2000, the Federal Register announced a similar peer review and public comment period on the revised Part III—Integrated Summary and Risk Characterization and the revised TEFs Chapter 9 in Part II.
Recognizing the broad policy implications of the dioxin reassessment, the National Science and Technology Council established an interagency
working group on dioxin (IWG) in the summer of 2000 to ensure a coordinated federal approach to dioxin-related health, food, and environmental issues. Specifically, the IWG was charged with fostering information sharing, developing a common language for dioxin science and science policy across governmental agencies and programs, identifying gaps and needs in the dioxin risk assessment, and facilitating coordination of risk management strategies. The IWG includes representatives from the following federal agencies: U.S. Department of Health and Human Services, U.S. Department of Agriculture, U.S. Department of State, U.S. Department of Veterans Affairs, U.S. Department of Defense, the Executive Office of the President, and EPA.
In the winter of 2000, the SAB held a 3-day public review of Part III of the Reassessment and additional information on the toxic equivalence of dioxins, other than TCDD, and DLCs. In the spring of 2001, the SAB recommended that EPA proceed expeditiously to complete and release its report, taking appropriate note of the SAB’s findings and recommendations and public comments. In response, EPA revised its draft Reassessment and submitted it to the IWG in late 2003, requesting input about the need and benefit of further review. EPA appropriations language for fiscal year 2003 also called for an IWG evaluation of the need for further review and provided specific issues to consider. The IWG recommended that the National Academies’ National Research Council (NRC) review the draft Reassessment. The scope of work for the NRC review and interagency agreements for funding were developed through the IWG in the spring of 2004. Ultimately, the NRC review would seek to inform and assure the risk characterization of TCDD, other dioxins, and DLCs and to benefit EPA in finalizing its Reassessment.
TCDD, OTHER DIOXINS, AND DLCS
The Reassessment addresses a limited number of chemical compounds within three subclasses of the halogenated aromatic hydrocarbons (HAHs): the polychlorinated dibenzo-p-dioxins (PCDDs), the polychlorinated dibenzofurans (PCDFs), and the polychlorinated biphenyls (PCBs). These compounds contain the basic aromatic structure of a benzene ring, a hexagonal carbon structure with conjugated double bonds connecting the carbons (Figure 1-1). PCDDs and PCDFs have tricyclic (triple-ring) structures consisting of two benzene rings, with varying numbers of chlorines, connected by an oxygenated ring, with the oxygenated ring of PCDDs having two oxygen atoms (a dioxin, Figure 1-2a) and the oxygenated ring of PCDFs having a single oxygen atom (a furan, Figure 1-2b). PCBs have a variable number of chlorines attached to a biphenyl group (two benzene rings with a carbon-to-carbon bond between carbon 1 on the first ring and carbon 1'
FIGURE 1-1 Benzene ring (a) with conjugated bonds and (b) with inner ring depicting conjugated bonds.
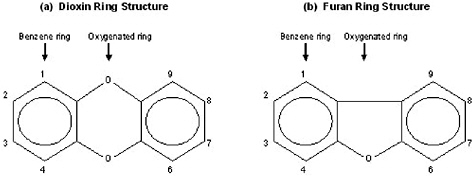
FIGURE 1-2 Double benzene ring structures of (a) dioxins and (b) furans.
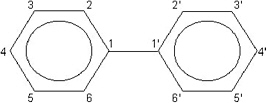
FIGURE 1-3 Biphenyl ring structure of PCBs.
on the second ring) (Figure 1-3). Examples of some PCDDs, PCDFs, and PCBs of interest are shown in Figure 1-4. Each chemical compound from any of these subclasses is referred to as a congener. Brominated or mixed halogenated congeners within these classes of compounds or within other chemical classes, such as the polyhalogenated naphthalenes, benzenes, azobenzenes, and azoxybenzenes, have not been evaluated as extensively and are not addressed in the Reassessment. TCDD, the most studied and one of the most toxic members of these classes of compounds, is the designated reference chemical for the Reassessment and for other related litera-
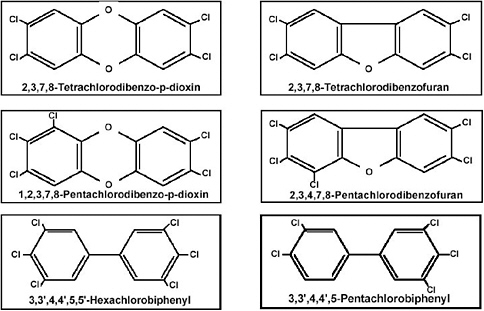
FIGURE 1-4 Examples of toxic PCDDs, PCDFs, and PCBs of interest in the Reassessment.
ture. PCDDs and PCDFs are tricyclic aromatic compounds with similar physical and chemical properties—properties shared by specifically configured, or coplanar (a flat configuration), dioxin-like PCBs. The Reassessment uses the terms “dioxins” and “dioxin-like compounds” in reference to any individual or any mixture of the addressed chemicals. These are general terms that describe chemicals that share defined similarities, including chemical structure and biological and toxicological character. However, of the several hundred HAH congeners, only 29 are considered to have significant toxicity and to induce a common battery of toxic responses through similar biological modes of action. The evaluation of dioxin-like congeners within the Reassessment focuses on dioxins and DLCs with TCDD-like toxicity and those generally considered the most associated with environmental and human health risks. These chemicals include PCDDs and PCDFs that retain chlorine substitutions at positions 2, 3, 7, and 8 on the benzene rings (see Figure 1-2). The remaining evaluated TCDD-like congeners include the PCBs with four or more chlorines in the lateral positions (3, 3', 4, 4', 5, or 5'), with established TCDD-like environmental and biological behaviors, and particularly the mono- and non-ortho PCBs—that is, PCBs with one or no, respectively, chlorine substitution in the ortho position (2, 2', 6, or 6') on the benzene rings (see Figure 1-3). Studies show that the TCDD-like toxicity of the PCB congener increases with a larger number of
chlorines in the lateral positions and one or no chlorines in the ortho position. Also, when a PCB has only one or no substitution in the ortho position, the atoms of the PCB congener can line up in a coplanar or flat configuration, making these the most toxic of the TCDD-like PCBs. Evaluation of the chemical congeners addressed in the Reassessment is considered sufficient to characterize environmental chlorinated dioxins (Reassessment, Part I, p. 1-5, lines 4 to 6).
Experimental evidence indicates that TCDD acts by way of binding to an intracellular protein, the aromatic hydrocarbon receptor (AHR), a ligand-dependent transcription factor that functions in partnership with a second protein, the AHR nuclear translocator protein (ARNT) to stimulate alterations in gene expression that result in toxic and biological effects. AHR is present throughout the animal kingdom, including invertebrates like the fruit fly and the clam. In addition to AHR binding, several other molecular events are necessary for AHR-dependent biological and toxic effects to occur, and there are significant species differences in those events, so quantitative cross-species comparisons based only on AHR binding may not provide accurate or dependable information about their AHR responsiveness or the possible AHR-dependent responses. The invertebrate AHR does not bind xenobiotic ligands (that is, TCDD, other dioxins, and DLCs), and it is not associated with toxic end points, suggesting that the role of AHR as a mediator of toxic or adaptive responses might be a function acquired during vertebrate evolution and superimposed on an endogenous physiological role.
TOXIC EQUIVALENCY FACTORS
TCDD, other dioxins, and DLCs are generally present as complex mixtures in environmental, food, and biological matrices, including humans and other animals. To address the complexity of risk assessment for TCDD-related compounds, the concept of TEFs is used in the Reassessment. The TEF concept originated as a method for evaluating health risks associated with closely related chemicals with similar mechanisms of action but different potencies. The criteria for including a chemical in the TEF concept are described in detail in the Reassessment, Part II, Chapter 9. The TEF of each chemical congener is determined by evaluating available congener-specific data (primarily in vivo data), and the congener is then assigned an “order of magnitude” estimate of relative toxicity compared with the prototypical and most potent HAH, 2,3,7,8-TCDD. By using those factors, the toxicity of a mixture is expressed in terms of its total toxic equivalent quotient (TEQ), which is the amount of TCDD that it would take to equal the combined toxic effect of all contributing congeners within the mixture. The TEF value of each congener within a mixture is multiplied
by its concentration, and the products (TEQs) are summed to yield the total TEQ of the mixture, which is the estimate of the total toxicity of the mixture.
In 1997, a team of experts convened by the WHO European Center for Environment and Health and the International Program on Chemical Safety (IPCS) evaluated a large database of experimental data of the relative potencies for PCDDs, PCDFs, and dioxin-like PCBs to establish consensus TEF values for these compounds in mammals, birds, and fish. Human and mammalian TEFs were constructed by an approach that gave more weight to in vivo toxicity data than to in vitro data. Moreover, among the different in vivo studies available for establishing TEFs, the basis for selecting the most relevant in vivo toxicity study was the length of exposure, with chronic exposures ranking highest and acute exposures lowest in relevance. The team concluded that an additive TEF model served as the most feasible risk assessment method for complex mixtures of TCDD-like PCDDs, PCDFs, and PCBs. Although several TEF/TEQ schemes exist for TCDD-related compounds, the Reassessment recommends using the international WHO TEF scheme of values, proposed and published by WHO (IPCS 1998a), to assign toxic equivalency for the Reassessment. Table 1-1 presents WHO TEFs established for humans and mammals for 7 PCDDs, 10 PCDFs, and 12 dioxin-like PCBs. TEF assignments continue to evolve in accordance with emerging science and iteration, and WHO recommended revisiting TEF values every 5 years, with review in 2005. For additional information on the TEF/TEQ approach, see Chapter 3 of the Reassessment, Part II.
To facilitate evaluation of human health risks and regulatory control of exposure to mixtures of TCDD, other dioxins, and DLCs, EPA, using all available data, has incorporated the TEF concept and method into the risk assessment process since 1987. The Reassessment considers the application, limitations, and uncertainties when using TEFs. Part II, Chapter 9, of the Reassessment describes the application of the TEF method for TCDD, other dioxins, and DLCs and addresses the uncertainties in detail. Overall, the use of the TEF method is currently the most reliable and best evaluated approach for assessing the potential toxic potency of complex mixtures of DLCs. TEFs/TEQs are addressed in further detail in Chapter 3.
EXPOSURE CHARACTERIZATION
EPA classifies sources of TCDD, other dioxins, and DLCs into five categories—(1) combustion; (2) metal smelting, refining, and processing; (3) chemical manufacturing and processing; (4) biological and photochemical processes; and (5) reservoir sources. Combustion sources include incineration of various types of waste (municipal solid, sewage sludge, medical, and hazardous), burning of fuels (coal, wood, and petroleum products),
TABLE 1-1 TEFs for Humans and Nonhuman Mammals
|
PCDD Congeners |
|
WHO TEF |
|
2,3,7,8-TCDD |
|
1 |
|
1,2,3,7,8-PeCDD |
|
1 |
|
1,2,3,4,7,8-HxCDD |
|
0.1 |
|
1,2,3,7,8,9-HxCDD |
|
0.1 |
|
1,2,3,6,7,8-HxCDD |
|
0.1 |
|
1,2,3,4,6,7,8-HpCDD |
|
0.01 |
|
1,2,3,4,6,7,8,9-OCDD |
|
0.0001 |
|
PCDF Congeners |
|
WHO TEF |
|
2,3,7,8-TCDF |
|
0.1 |
|
1,2,3,7,8-PeCDF |
|
0.05 |
|
2,3,4,7,8-PeCDF |
|
0.5 |
|
1,2,3,4,7,8-HxCDF |
|
0.1 |
|
1,2,3,7,8,9-HxCDF |
|
0.1 |
|
1,2,3,6,7,8-HxCDF |
|
0.1 |
|
2,3,4,6,7,8-HxCDF |
|
0.1 |
|
1,2,3,4,6,7,8-HpCDF |
|
0.01 |
|
1,2,3,4,7,8,9-HpCDF |
|
0.01 |
|
1,2,3,4,6,7,8,9-OCDF |
|
0.0001 |
|
PCB Congeners |
|
WHO TEF |
|
IUPAC Number |
Structure |
|
|
077 |
3,3',4,4'-TCB |
0.0001 |
|
081 |
3,4,4',5-TCB |
0.0001 |
|
105 |
2,3,3',4,4'-PeCB |
0.0001 |
|
114 |
2,3,4,4',5-PeCB |
0.0005 |
|
118 |
2,3’,4,4',5-PeCB |
0.0001 |
|
123 |
2',3,4,4',5-PeCB |
0.0001 |
|
126 |
3,3',4,4',5-PeCB |
0.1 |
|
156 |
2,3,3',4,4',5-HxCB |
0.0005 |
|
157 |
2,3,3',4,4',5'-HxCB |
0.0005 |
|
167 |
2,3',4,4',5,5'-HxCB |
0.00001 |
|
169 |
3,3',4,4',5,5'-HxCB |
0.01 |
|
189 |
2,3,3',4,4',5,5'-HpCB |
0.0001 |
|
Abbreviations: PeCDD, pentachlorodibenzo-p-dioxin; HxCDD, hexachlorodibenzo-p-dioxin; HpCDD, heptachlorodibenzo-p-dioxin; OCDD, octachlorodibenzo-p-dioxin; TCDF, tetrachlorodibenzofuran; PeCDF, pentachlorodibenzofuran; HxCDF, hexachlorodibenzofuran; HpCDF, heptachlorodibenzofuran; OCDF, octachlorodibenzofuran; TCB, tetrachlorobiphenyl; PeCB, pentachlorobiphenyl; HxCB, hexachlorobiphenyal; HpCB, heptachlorobiphenyl. SOURCE: IPCS 1998a. Reprinted with permission; copyright 1998, World Health Organization. |
||
forest fires and open burning of waste materials, and high-temperature processes (e.g., cement kiln operations). Combustion sources produce PCDDs, PCDFs, and limited amounts of PCBs (commercially manufactured in large quantities from about 1930 until 1977). Metallurgical operations (e.g., iron ore sintering, steel production, and scrap metal recovery) can produce PCDDs and PCDFs, which are also formed as by-products of chemical processing (e.g., manufacture of chlorine-bleached wood pulp and phenoxy herbicides). PCDDs and PCDFs can also be formed under such environmental conditions as composting via microorganism action on chlorinated phenolic compounds. Studies have also reported that these chemicals form during photolysis of highly chlorinated phenols, such as pentachlorophenol, although it has been demonstrated only under laboratory conditions. Four of the source categories (combustion, metallurgical processing, chemical manufacturing and processing, and biological and photochemical processes) are collectively referred to as contemporary formation sources. In contrast, reservoir sources are not considered in the quantitative inventory of contemporary formation sources because they involve the recirculation of previously formed compounds that have already partitioned into air, water, soil, sediment, and biota. However, the Reassessment recognizes that the contribution of reservoir sources to human exposure may be significant, perhaps contributing half or more of total background TEQ exposure. For any given time period, releases from both contemporary formation sources and from reservoir sources determine the overall amount of TCDD, other dioxins, and DLCs released to the accessible environment.
The Reassessment gives an inventory of environmental releases of PCDDs, PCDFs, and TCDD-like PCBs for the United States based on two reference years, 1987 and 1995. An updated inventory for reference year 2000 was published in 2005 and was included in the committee’s review. EPA’s best estimate of releases of these compounds to air, water, and land from reasonably quantifiable sources in 2000 was approximately 1,500 g TEQDF-WHO ([DF] dioxins and furans), representing an 89% decrease from a 1987 best estimate of 14,000 g TEQDF-WHO. U.S. environmental releases of PCDDs and PCDFs occur from an expansive variety of sources but are dominated by releases to the air from combustion sources. The decrease in estimated releases of PCDDs and PCDFs from 1987 to 2000 is largely attributed to reductions in air emissions from municipal and medical waste incinerators; further reductions are anticipated. Three types of combustion sources contributed approximately 70% of all quantifiable environmental releases in 1995: municipal waste incinerators, backyard burning of refuse in barrels, and medical waste incinerators, representing 38%, 19%, and 14% of total environmental releases, respectively. A number of investigators have proposed that the U.S. inventory underestimates releases from contemporary formation sources partly because of the lack of
sufficient data from sources that can emit TCDD and related compounds, such as land fires; unquantifiable or poorly quantifiable sources, such as agricultural burning; and the possibility of unknown sources. Additional observations in the Reassessment regarding sources are concerns about insufficient data or estimates from nonpoint sources (e.g., urban stormwater runoff and rural soil erosion) and the likelihood that total nonpoint-source releases are substantially larger than point-source releases. Evidence also indicates that current emissions of TCDD, other dioxins, and DLCs to the U.S. environment result principally from anthropogenic activities, as supported by correlations in the rise in environmental levels of these compounds and a period of rapid increase in industrial activities, lack of significant natural sources, and observations of higher body burdens in industrialized versus less industrialized countries. PCDDs, PCDFs, and PCBs share similar properties, including lipophilicity, hydrophobicity, and resistance to degradation. Consequently, these intrinsically stable compounds are found throughout the world in practically all environmental media, including air, water, soil, sediment, food, and food products. The amount of time required for a chemical to lose one-half of its original concentration, known as its half-life, varies by substance. The chemical half-lives of mixtures change with time, as the shorter-lived substances disappear and the proportion with longer half-lives increases. (For further discussion on chemical half-lives, see commentary (Part II, Volume 2, Chapter 2).
The Reassessment defines background exposure to TCDD, other dioxins, and DLCs as exposure that would occur in an area without known point sources of the contaminants. Background exposure includes exposure via the commercial food supply, air, or soil but not any significant occupational exposure. Background exposure estimates are based on the monitoring of data from environmental sites and other media void of known contaminant sources and on pharmacokinetic models using body burden data from nonoccupationally exposed populations. High concentrations, measured in parts per trillion (ppt) and higher, are found in soil, sediments, and biota because of their recalcitrant nature and their physical-chemical properties. Low concentrations, measured in parts per quadrillion (ppq) and picograms per cubic meter (pg/m3), are found in water and air, respectively.
Estimates for background concentrations of DLCs in environmental media and in food are based on studies conducted at various locations in North America. The number of locations examined for environmental media estimates in those studies was small, and it is not known whether the estimates adequately reflect the full range of variation across the United States. Food estimates were derived from statistically based national surveys, nationwide sampling networks, food fat concentration measurements, samples collected from retail stores, and samples obtained from biohabitat.
PCDD, PCDF, and PCB TEQ-WHO concentrations in environmental
media and food are presented in Table 1-2. Measurable quantities of these in environmental media and food in the United States were found to be similar to quantities measured in Europe. Evidence from Europe suggests a decline in dioxin and furan concentrations in food products during the 1990s. Although no systematic study of temporal trends in dioxin concentrations in food has been conducted in the United States, at least one study determined that meat now contains lower concentrations of TCDD, other dioxins, and DLCs than samples from the 1950s through the 1970s contained. The U.S. Department of Agriculture is conducting a nationwide survey of dioxin concentrations in beef, pork, and poultry that should allow for a time-trend analysis.
The average PCDD, PCDF, and PCB tissue concentration for the general adult U.S. population in the late 1990s, based on EPA’s estimate, was 25 ppt TEQDFP-WHO ([DFP] dioxins, furans, and PCBs), lipid basis (Reassessment, Part III, p. 4-15). This estimate suggests average tissue concentrations have declined from the estimated 55 ppt in the late 1980s and early 1990s. Because new emissions of TCDD, other dioxins, and DLCs have been declining since the 1970s, it is reasonable to expect that concentrations in food, human diet, and, ultimately, human tissue have also declined during this time.
The Reassessment acknowledges that characterization of national background concentrations of TCDD, other dioxins, and DLCs in tissue is uncertain because current data are not statistically representative of general populations. Also, tissue concentrations are a function of age and year of birth.
HEALTH EFFECTS
On a global scale, exposure to TCDD, other dioxins, and DLCs resulting from accidental, occupational, or incidental exposure through dermal contact, inhalation, or ingestion has been associated with adverse effects on human health. In the early 1900s, workers involved in distilling, processing, or producing chlorine-based chemicals presented with symptoms characteristic of those currently associated with TCDD poisoning, including severe cases of chloracne and various degrees of fatigue. Soil contaminated with TCDD at 300 ppb caused the 1983 evacuation of the town of Times Beach, Missouri, and allegedly was responsible for the deaths of local animals and for a variety of human and animal illnesses. In a January 2003 press release, the Institute of Medicine announced that reexamination of six studies of herbicide-exposed veterans revealed sufficient evidence of an association between herbicide defoliants, or their contaminants, which included TCDD, sprayed by U.S. forces in Vietnam and the risk of developing chloracne, chronic lymphocytic leukemia, and soft tissue sarcoma.
Some studies suggest that exposure of chemical workers to very high concentrations of TCDD, other dioxins, and/or PCBs (body burdens of 100-1,000 times background) are associated with an increased incidence of cancer (Flesch- Janys et al. 1995; Hooiveld et al. 1998; Steenland et al. 1999). Other studies of highly exposed populations suggest that TCDD, other dioxins, and PCBs can have reproductive and developmental effects (Eskenazi et al. 2000; Kogevinas 2001; Revich 2002; Vreugdenhill et al. 2002a; Pesatori et al. 2003). The long-term effects of low-level exposure to TCDD, other dioxins, or DLCs normally experienced by the general population are not known, nor is the clinical significance of biochemical biomarkers, such as enzyme induction at or near background-level exposures. Focal points of research include organ and organ-system effects and elucidation of the cellular mechanisms through which these effects occur.
COMMITTEE CHARGE AND RESPONSE
In May 2004, EPA asked the NRC to review the revised draft reassessment titled Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Related Compounds (2003 version, publicly released in October 2004) and to assess whether EPA’s risk estimates are scientifically robust and whether there is a clear delineation of all substantial uncertainties and variability (see Box 1-1 for the complete statement of task). In response, the NRC formed the Committee on EPA’s Exposure and Human Health Reassessment of TCDD and Related Compounds, a panel of 18 members that included experts in exposure assessment; food exposure pathways; pharmacokinetics; physiologically based pharmacokinetic modeling; benchmark dose modeling; dose-response modeling; molecular and cellular aspects of receptor-mediated responses; toxicology with specialties in cancer, reproduction, development, and immunology; epidemiology; reproductive physiology and medicine; pediatric biology and medicine; statistics; risk assessment (both qualitative and quantitative); and uncertainty analysis (see Appendix A for details).
The committee held three public meetings in Washington, DC, to collect information, meet with researchers and decision makers, and accept testimony from the public. The committee met two additional times, in executive session, to complete its report. Although the committee reviewed all three parts of the Reassessment, it focused primarily on Part III— Dioxin: Integrated Summary and Risk Characterization for 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds, as directed by the study charge. The committee also considered new peer-reviewed studies published since Part III of the Reassessment was last revised and before the committee held its final meeting in July 2005. However, because the committee was charged to review EPA’s Reassessment, conducting a compre-
TABLE 1-2 Summary of North American PCDD, PCDF, and PCB TEQ WHO Concentrations in Environmental Media and Food (Whole Weight Basis)
|
Media |
PCDDs, PCDFsa |
Referencesb |
PCBsa |
Referencesb |
Mean Total PCDD, PCDF, PCBs |
|
Urban soil, ppt |
n = 270 9.3 ± 10.2c Range = 2 to 21 |
EPA 1985, 1996a, 2000a; Nestrick et al. 1986; Birmingham 1990; Pearson et al. 1990; NIH 1995; Rogowski et al. 1999 |
n = 99 2.3 |
EPA 2000a |
11.6 |
|
Rural soil, ppt |
n = 354 2.7c Range = 0.11 to 5.7 |
EPA 1985, 1996a, 2000a; Birmingham 1990; Pearson et al. 1990; Reed et al. 1990; MRI 1992; van Oostdam and Ward 1995; Tewhey Assoc. 1997; Rogowski et al. 1999; Rogowski and Yake 1999 |
n = 62 0.59 |
EPA 2000a |
3.3 |
|
Sediment, ppt |
n = 11 5.3 ± 5.8c Range = <1 to 20 |
Cleverly et al. 1996 |
n = 11 0.53 ± 0.69c |
Cleverly et al. 1996 |
5.8 |
|
Urban air, pg/m3 |
n = 106 0.12 ± 0.094c Range = 0.03 to 0.2 |
CDEP 1988, 1995; Hunt et al. 1990; Hunt and Maisel 1990; Maisel and Hunt 1990; OHEPA 1995; Smith et al. 1989, 1990 |
n = 53 0.0009d |
Hoff et al. 1992 |
0.12 |
|
Rural air, pg/m3 |
n = 60 0.013c Range = 0.004 to 0.02 |
CDEP 1995; OHEPA 1995; Cleverly et al. 2000 |
n = 53 0.00071 |
Cleverly et al. 2000 |
0.014 |
|
Freshwater fish and shellfish, ppt |
n = 222 1.0c |
EPA 1992; Fiedler et al. 1997; Jensen et al. 2000; Jensen and Bolger 2001 |
Mes and Weber 1989; Mes et al. 1991, Schecter et al. 1997 |
2.2 |
|
|
Marine fish and shellfish, ppt |
n = 158 0.26c |
Fiedler et al. 1997; Jensen et al. 2000 |
Mes et al. 1991; Schecter et al. 1997 |
0.57 |
|
|
Water, ppq |
n = 236 0.00056 ± 0.00079 |
Meyer et al. 1989; Jobb et al. 1990 |
—g |
— |
0.00056 |
|
Milk, ppt |
n = 8 composites 0.018f |
Lorber et al. 1998 |
n = 8 composites 0.0088 |
Lorber et al. 1998 |
0.027 |
|
Dairy, ppt |
n = 8 composites 0.12f |
Based on data from Lorber et al. 1998 |
n = 8 composites 0.058 |
Based on data from Lorber et al. 1998 |
0.18 |
|
Eggs, ppt |
n = 15 composites 0.081f |
Hayward and Bolger 2000 |
Mes and Weber 1989; Mes et al. 1991; Schecter et. 1997 |
0.13 |
|
|
Beef, ppt |
n = 63 0.18 ± 0.11 Range = 0.11 to 0.95 |
Winters et al. 1996a |
n = 63 0.084 |
Winters et al. 1996b |
0.26 |
|
Media |
PCDDs, PCDFsa |
Referencesb |
PCBsa |
Referencesb |
Mean Total PCDD, PCDF, PCBs |
|
Pork, ppt |
n = 78 0.28 ± 0.28 Range = 0.15 to 1.8 |
Lorber et al. 1997 |
n = 78 0.012 |
Lorber et al. 1997 |
0.29 |
|
Poultry, ppt |
n = 78 0.068 ± 0.070 Range 0.03 to 0.43 |
Ferrario et al. 1997 |
n = 78 0.026 |
Ferrario et al. 1997 |
0.094 |
|
Vegetable fats, ppt |
n = 30 0.056 ± 0.24h |
Versar 1996 |
n = 5 composites 0.037f |
Mes et al. 1991 |
0.093 |
|
aValues are the arithmetic mean TEQs, in ppt, and standard deviations. Nondetects were set to one-half the limit of detection, e xcept for soil, PCDDs, and PCDFs in vegetable fats for which nondetects were set to zero. bA full list of references is found in Part I, Vol. 2, Chapter 3 of the Reassessment NAS review draft (December 2003). cThe values for environmental media are means of the data but lack the spatial representativeness to be considered true national means. dBased on data from Canadian air, as reported by Hoff et al. (1992). Not used in U.S. background exposure estimates in Part I, V ol. 2, Chapter 4 of the Reassessment NAS review draft (December 2003). eThe values for fish lack the statistical significance to be considered true means; the values of the other food groups were derived from statistically based surveys and can be considered true national means. The PCCD and PCDF concentrations are species-specific ingestion-weighted average values. fStandard deviations could not be calculated because of limitations of the data (composite analyses). gCongener-specific PCB data are sparse. hTEQ calculated from Versar (1996) by setting nondetects to zero. SOURCE: EPA 2003a. |
|||||
|
BOX 1-1 Statement of Task The National Academies’ National Research Council will convene an expert committee that will review EPA’s 2003 draft reassessment of the risks of dioxins and dioxin-like compounds to assess whether EPA’s risk estimates are scientifically robust and whether there is a clear delineation of all substantial uncertainties and variability. To the extent possible, the review will focus on EPA’s modeling assumptions, including those associated with the dose-response curve and points of departure; dose ranges and associated likelihood estimates for identified human health outcomes; EPA’s quantitative uncertainty analysis; EPA’s selection of studies as a basis for its assessments; and gaps in scientific knowledge. The study will also address the following aspects of the EPA reassessment: (1) the scientific evidence for classifying dioxin as a human carcinogen; and (2) the validity of the non-threshold linear dose-response model and the cancer slope factor calculated by EPA through the use of this model. The committee will also provide scientific judgment regarding the usefulness of toxicity equivalence factors (TEFs) in the risk assessment of complex mixtures of dioxins and the uncertainties associated with the use of TEFs. The committee will also review the uncertainty associated with the reassessment’s approach regarding the analysis of food sampling and human dietary intake data, and, therefore, human exposures, taking into consideration the Institute of Medicine’s report Dioxin and Dioxin-Like Compounds in the Food Supply: Strategies to Decrease Exposure. The committee will focus particularly on the risk characterization section of EPA’s reassessment report and will endeavor to make the uncertainties in such risk assessments more fully understood by decision makers. The committee will review the breadth of the uncertainty and variability associated with risk assessment decisions and numerical choices, for example, modeling assumptions, including those associated with the dose-response curve and points of departure. The committee will also review quantitative uncertainty analyses, as feasible and appropriate. The committee will identify gaps in scientific knowledge that are critical to understanding dioxin reassessment. |
hensive and thorough review of all TCDD-related materials published since 2003, reassessing TEF values, and re-creating the risk assessment were outside of the scope of the statement of task.
The present report is the product of the efforts of the entire NRC committee and underwent extensive, independent, external review overseen by the NRC’s Report Review Committee. It specifically addresses and is limited to the statement of task as agreed upon by the NRC and EPA.
The remaining chapters of this report comprise the findings of the Committee on EPA’s Exposure and Human Health Reassessment of TCDD and Related Compounds. Chapter 2 provides conceptual text on how to address variability and uncertainty in risk assessment. Chapter 3 evaluates the usefulness and uncertainties of TEFs in the risk assessment of complex mixtures of TCDD, other dioxins, and DLCs and discusses various ap-
proaches to dose metrics. Chapter 4 addresses exposure characterization in terms of sources, environmental fate, environmental media concentrations, food concentrations, background exposures, and potentially highly exposed populations. Chapter 5 reviews EPA’s assessment of the carcinogenicity of TCDD other TCDD, other dioxins, and DLCs, including the qualitative characterization of their carcinogenicity, the validity of the nonthreshold linear dose-response model, and the use of the animal bioassay and epidemiological data to quantify the dose response. Chapter 6 reviews EPA’s assessment of noncancer end points, including immune function, reproduction, and development. Chapter 7 focuses on risk characterization. Chapter 8 summarizes the committee’s conclusions and recommendations and succinctly addresses each component of the statement of task.

















