5
The Ethical Framework for Research Involving Prisoners
In 1976, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (NCPHSBBR) addressed the ethics of research with prisoners in a document entitled Report and Recommendations: Research Involving Prisoners. The commission focused on respect for persons and justice as the two key ethical considerations guiding their recommendations.
The intervening decades have offered few reasons to quarrel with the commission’s identification of these two factors as fundamental ethical principles guiding the conduct and regulation of research with prisoners. The goal of this chapter is to demonstrate that the principles of justice and respect for persons, although having evolved in meaning over the past three decades, should still be the basis for determining whether to conduct research with prisoners.
As part of this ethical evolution, the committee suggests that collaborative responsibility should be added to an updated ethical framework as a derivative of the principle of justice. The national commission thought of justice as primarily a matter of the distribution of the benefits and burdens of research, and that is certainly a legitimate understanding. Recent scholarship has offered reason to elevate some other concerns under the heading of justice. In particular, attention to distributive justice should be complemented by attention to the needs and responsibilities of all parties who will be involved with or affected by a research endeavor.
This focus on collaboration will more effectively facilitate the implementation of ethical research. For research to be truly ethical, it must be tailored to the individual setting; a one-size-fits-all approach is inadequate. Every research setting and population presents unique challenges and concerns. Ethically appropriate subject protections in one institution may be grossly inadequate in another. Only through close cooperation and communication with all relevant parties, in every implicated setting, can researchers ensure that they are creating ethical conditions that are favorable for respect and unfavorable for exploitation in any research context.
THE 1976 COMMISSION’S ETHICAL FRAMEWORK
Historical Context
The commission’s deliberations took place against a background that included the Nazi experiments with concentration camp prisoners followed by the adoption of a stringent standard of voluntary consent in the Nuremburg Code. Many interpreted the code’s statement that potential subjects “be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, overreaching, or other ulterior form of constraint or coercion” (U.S. GPO, 1953) as precluding participation by prisoners.
This thinking was reinforced by publicity about the realities of research in prisons. The late 1960s and early 1970s saw a series of exposés documenting abuses in connection with nontherapeutic research in U.S. prisons (Mitford, 1973a,b; Rugaber, 1969). Many of those who were most vocal about the plight of prisoners (i.e., journalists and prisoner advocacy and civil liberties groups) saw research with prisoners largely under the twin headings of coercion and exploitation (Mitford, 1973a,b; Rugaber, 1969). For the most part, these groups pushed for restriction rather than reform of the prison research enterprise.
Although the commission did not recommend a ban on all research with prisoners, their work and the subsequent scholarship and regulation have been described as tending toward that result: “The result of these regulations has been, as was their goal, the virtual elimination of biomedical research activity in prisons and jails” (Dubler and Sidel, 1989). According to one informed estimate, in the late 1990s only about 15 percent of institutions that engaged in clinical research in the United States included prisoners in their research protocols (Hoffman, 2000). In 1997, New York State had the largest estimated number of HIV-infected prisoners of any prison system (9,456), but only 8 (less than .001 percent) were enrolled in clinical trials (Lazzarini and Altice, 2000).
Justice and Respect for Persons
The commission’s emphasis on limiting research involving prisoners was guided by its choice of ethical framework. Congress’s charge to the commission concerning research with prisoners identified informed consent as the primary locus of ethical concern. In particular, Congress directed the commission to attend to three components of informed consent: (1) nature of the consent; (2) adequacy of the information given; and (3) competence and freedom of the prisoners or their legal representatives to make a choice.1
In carrying out this charge, the commission used elements of a principle-based ethical framework that would be more fully fleshed out in the Belmont Report. The commission identified two basic ethical dilemmas arising in connection with the use of prisoners as research subjects and linked these dilemmas to two basic ethical principles (NCPHSBBR, 1976). The first issue was whether prisoners bear a fair share of the burdens of research and receive a fair share of the benefits. The commission linked this dilemma to the principle of justice, specified as the fair treatment of persons and groups. The second issue was whether prisoners could give truly voluntary consent. The commission linked this dilemma to the principle of respect for persons, specified as involving the protection and promotion of personal autonomy. The principles of beneficence and nonmaleficence (providing benefit and doing no harm) were not discussed by the commission in its report on prison research; however, they were prominently featured in the Belmont Report, and both maintain an underlying importance in the ethics of human subjects research. These concepts have been well developed in other works (Beauchamp and Childress, 2001).
The commission then reviewed two possible perspectives on prisoner participation in research involving two very different interpretations of respect for persons and justice. According to the first perspective, the principles of respect for persons and justice require that prisoners not be deprived of the opportunity to volunteer for research. Here the emphasis is on the freedom of prisoners to decide questions of research participation for themselves and on the possible benefits of research participation.
According to the second perspective, the principles of respect for persons and justice require (1) that prisoners be protected from exploitation, safeguards be introduced to reduce elements of constraint, and, when that proves impossible, participation be prohibited; and (2) that prisoners as a group not bear a disproportionate share of research burdens without a commensurate share of benefits. Here the emphasis is on structural conditions that create special vulnerabilities in prison populations and the pos-
sible burdens of research participation. The commission adopted the second perspective.
The commission explicitly stated that its stance was influenced by its understanding of the realities of prison life, including conditions of social and economic deprivation and the possibility or even likelihood of manipulation or corruption on the part of prison authorities and prisoners in positions of privilege. Recognizing that they were inclining toward protectionism, the commissioners commented that “should coercion be lessened and more equitable systems for the sharing of burdens and benefits be devised, respect for persons and concern for justice would suggest that prisoners not be deprived of the opportunity to participate in research” (NCPHSBBR, 1976).
Flowing from this protectionist perspective, the commission’s ethical framework and current federal regulations permit therapeutic research2 with prisoners as long as multiple safeguards are in place, but they do not encourage or provide incentives for therapeutic research with prisoners. The perception that all forms of research involving prisoners are equally ethically problematic, or subject to blanket prohibition or to conditions so onerous that the research is not worth doing, may be responsible for the dwindling of prisoner research participation to the point that justice concerns have been expressed about the exclusion of prisoners from clinical trials.
AN UPDATED ETHICAL FRAMEWORK
The committee developed a new ethical framework that utilizes the ethical principles applied by the national commission in the 1976 report, with several new, important components. The framework builds on the principles of respect for persons and justice by shifting from a categorical approach to review to a risk-benefit analysis approach, and by adding a derivative of justice, called collaborative responsibility to research proposal development.
Ideas about justice and respect for persons have evolved over the past three decades. To construct a comprehensive ethical framework for thinking about research in prisons, this chapter explores recent research ethics scholarship.3 Changes in the way these principles have been conceptualized have influenced the shape of our recommendations. However, before beginning to address how this new ethical framework is different than that of the
original commission, it is important to emphasize that it does not deviate from their core ethical principles.
Respect for persons requires that research subjects be treated as autonomous individuals. As discussed in Chapter 1, it is clear that prisoners are still an extremely vulnerable population, with severely restricted autonomy; thus, this issue requires special attention. Prisoners still need to be protected from the risk of coercion, undue inducement, and exploitation. The historical pattern of research abuses in prisons underscores the need to have an ethical framework that, first and foremost, is concerned with the welfare of prisoners. Similarly, justice still requires a careful consideration of the fair distribution of burdens and benefits. Prisoners, as a vulnerable population, are in jeopardy of receiving a disproportionate share of the risk associated with human subjects research. As stated by the National Bioethics Advisory Commission (NBAC), “In research involving human subjects, risk is a central organizing principle, a filter through which protocols must pass (NBAC, 1998). Like the original commission, our recommendations start with a baseline ethical concern for the protection of prisoners.
Respect for Persons
In this section, the committee expresses its support for a broadened view of the principle of respect for persons, to consider more than a narrow focus on informed consent issues, which are still vital but not the whole picture. It also suggests a shift from a categorical approach to research review to a risk-benefit approach.
An Expanded View of Respect for Persons
In accord with its emphasis on the principle of respect for persons, the original commission’s report focused on informed consent. Although informed consent is still an ethically important means of ensuring respect for the right of persons to engage in autonomous decision making, recent scholarship has questioned the myopia caused by such a narrow focus.
Kahn, Mastroianni, and Sugarman (1998) are the editors of a volume that captures a major research ethics reform agenda in its title: Beyond Consent: Seeking Justice in Research. One question the editors raise is whether research ethics has been too concerned with informed consent to the neglect of other matters. There seems to be agreement from a variety of perspectives that informed consent forms have consumed too much time and energy. Critics of the preoccupation with forms are not necessarily interested in shifting attention away from informed consent. Rather, they may emphasize that documentation should be but a part of an informed consent process that involves opportunities for questions and answers and
allows time for reflection before a decision is made, and that more attention should be paid to ameliorating basic power and knowledge differentials, which may undermine information sharing, understanding, and voluntariness. One proposal for reform advises simply raising the consciousness of investigators and ancillary personnel. Another suggests the use of external measures such as third-party monitoring to guard against deficiencies. This could be accomplished by the integration of third-party research subject advocates in the informed consent process, especially for studies that are considered unusually sensitive or risky or that involve subjects with impaired autonomy (see prison research subject advocate [PRSA] discussion in Chapter 6).
A more fundamental question is whether too much weight has been placed on informed consent in the framework of research ethics and research regulation. As noted previously, the National Research Act charge to the commission focused on informed consent issues, so the centrality of consent issues in the report is neither surprising nor necessarily indicative of a judgment on the part of the commissioners that the most compelling issues in research with prisoners are those of consent.
An alternate perspective, discussed by Emanuel et al. (2000), focuses on directing attention to risks and to risk-benefit analysis. According to this view, only health-related benefits derived from the research can be counted as benefits to individual subjects, meaning that extraneous benefits, such as payments or medical services unrelated to the research, are excluded in this analysis. Further, although the process of weighing risks against benefits is inherently subjective, the analysis should be based on data permitting identification of the types of potential harms and benefits, their probability of occurrence, and their long-term consequences. For example, a placebo-controlled trial of new antiemetic therapy for patients undergoing chemotherapy could be rejected because the investigators failed to give adequate weight to the discomfort associated with nausea and vomiting and failed to take steps to minimize this potential harm by using available antiemetic agents in the control group (Emanuel et al., 2000).
These questions about an undue focus on informed consent influence our recommendations. More attention needs to be paid to risks and risk-benefit analysis rather than the formalities of an informed consent document. The ethical risks associated with research involving prisoners cannot be solved by focusing only on the informed consent document.
The Role of Protectionism
A risk-benefit paradigm is necessarily more flexible than the current categorical approach. Although some might view this flexibility as opening
the door for potential abuses, this new approach should actually increase the protection of prisoners involved in research.
This committee, like the original commission, is focused on the protection of prisoners as our core ethical concern. However, there are many approaches one can take to accomplish this goal, involving different levels of protective oversight mechanisms. One scholar outlines three types of protectionism:
Weak protectionism is the view that this problem is best resolved through the judgment of virtuous scientists. Moderate protectionism accepts the importance of personal virtue but does not find it sufficient. Strong protectionism is disinclined to rely, to any substantial degree, on the virtue of scientific investigators for purposes of subject protection (Moreno, 2001).
The movement over time has been from weaker to stronger forms of protectionism as a means of addressing a fundamental problem, specifically, the tension between protecting the interests of subjects and promoting scientific progress. Strong protectionism sharply limits investigator discretion and demands external assurances through measures such as third-party monitors of consent, conflict-of-interest committees, and other procedures. These external assurances can be associated with costs, thus leading to an ethical critique of strong protectionism. For example, an emphasis on external assurances may weaken the sense of personal moral responsibility on the part of investigators. Similarly, rigid external assurances, like those seen in the current regulations, can direct attention away from an analysis of risks and benefits, where the key ethical issues can be found.
Simultaneously, there has been a countervailing force in the march toward strong protectionism, exemplified in the push by acquired immune deficiency syndrome (AIDS) activists for greater access to clinical trials and by progressives for the inclusion of women and children in research studies. More recently, there has been a similar movement to ensure that racial and ethnic minority groups are included in research. These tendencies form one basis for a somewhat different reading of the history. This reading indicates a trend away from viewing certain types of research participation (especially clinical trials) as mostly risky or burdensome toward viewing them as mostly beneficial.
This represents a change in thinking about distributive justice. The commission focused on the equitable distribution of risks and worried that prisoners would bear more than their fair share. However, an equally valid case can be made for attention to the distribution of benefits. For example, Mastroianni and Kahn (2001) wrote that, in the 1970s, federal “policies emphasized the protection of human subjects from the risks of harm in
research, and justice was seen as part of this protection,” but since the early 1990s “justice as applied in research ethics has emphasized the need to ensure access to the potential benefits that research has to offer” (Mastroianni and Kahn, 2001).
|
During the committee’s October 2005 meeting, the prisoner liaison panel spent a great deal of time debating the appropriateness of including prisoners in research, with special concerns for biomedical research. “We have 275 million people in this country. We have 2 million in prisons. What is the allure to this population, if it is not the fact that it is a controlled population?” asked Daniel Murphy, Ph.D., a former prisoner in the Federal Bureau of Prisons and now professor in the Department of Political Science and Justice Studies at Appalachian State University. In other words, why conduct studies with prisoners when there are many more people outside of prison who are potential participants? |
Some fundamental changes in the nature of the research conducted with human subjects provide support for this account of the recent history of research practices. For example, although the paradigmatic studies with prisoners in the period leading up to the report were studies in which investigators induced disease to learn more about it, biomedical research is more likely now to be discussed in terms of clinical trials comparing alternative beneficial treatments. The last several years have also seen the publication of studies comparing the outcome between patients who participate in clinical trials and those who receive standard care outside such trials; the results have tended to favor the former (Agrawal and Emanuel, 2003).
The two accounts can be reconciled in several ways. Increased protectionism is quite visible over the past century, whereas movements demanding greater access to clinical trials are far more recent. Further, protectionism as distrust of individual investigators can coexist with a view that participation in research subject to external oversight can often offer benefits to individuals and groups. One can simultaneously believe that the piling on of more rules and oversight bodies at some point becomes counterproductive and that human subjects are presently inadequately protected. Indeed, many modern ethicists seem to hope for a reawakening of scientific conscience rather than additional fortifications to the citadel of regulations.
|
“It is so much easier for indiscretions or bad intentions to take place behind those prison walls and razor wire. I have seen it in so many cases, where doctors who were sworn to save lives and do good have become so consumed by that intellectual scientific quest that they forget about the test subject. It is just so easy to abuse the situation,” stated Allen Hornblum, author of Acres of Skin (Hornblum, 1998) and former member of the Philadelphia Prison System Board of Trustees. |
This committee concurs. The critique of strong protectionism, combined with a new understanding of research as a potential benefit, requires a reexamination of the current regulations. Advances in ethical thinking about protectionism suggest a new regulatory model. In particular, the committee rejects strong protectionism because it discounts the notion that researchers can be trusted to act virtuously in the protection of subjects. Researchers have responsibility for protecting subjects in their studies, especially those who are most vulnerable. However, given the troubling history of research abuses in prisons, weak protectionism is not an option. The recommendations in this chapter, and throughout this report, reflect a moderate protectionist stance, acknowledging that robust protections are needed but that they need not be rigid or absolute.
This position should not be perceived as a call for the relaxation of prison research ethics. Justice and respect for persons are as vital today as they were three decades ago; research still must be constrained by these ethical principles. The prison continues to be a setting in which it may be difficult to avoid contamination through contact with what will often be a culture of, at best, deprivation and dysfunction and, at worst, corruption, brutality, and degradation (Hornblum, 1997, 1998; Murphy, 2005; Rhodes, 2005).
Perhaps some unease is appropriate about removing what prisoners themselves, given full information and understanding, might regard as acceptable or even desirable options in light of their circumstances, circumstances that are unlikely to be changed for the better by research bans. A prisoner’s ability to participate in research need not be completely precluded.
The original commissioners talked to actual prisoner-subjects during a fact-finding visit to Jackson State Prison on November 14, 1975. The prison, in southern Michigan, was at the time home to one of the largest nontherapeutic biomedical research programs in the country. The report notes that commission members spoke with a representative sample of
research participants and nonparticipants selected by commission staff from a master list of all prisoners and found that, overall, participants valued the opportunity to participate in research and felt they were sufficiently informed and free to enroll or withdraw at will, and nonparticipants did not object to this opportunity being available to others (NCPHSBBR, 1976).
|
“My experience has really been that prisoners want access to innovative intervention programs. They want to change. They want to have access to the things that are going to help them, and that is one reason why people become involved, at least in working with us,” said Olga Grinstead, Ph.D., adjunct associate professor at the University of California, San Francisco’s Center for AIDS Prevention Studies, when she spoke to the committee in July 2005. She continued, “From the issue of equity or the issue of justice, there are advantages to being involved in research. We need to be aware that prisoners are motivated to be involved in research. They are motivated to give back, and that should be taken into account too.” |
This message continues to be articulated today. This committee visited one prison and one prison medical facility to discuss experimentation with current prisoners and peer educators (see Appendix A). The prisoners actively expressed the desire to have access to research. They stated they would feel they had a choice as to whether to participate and that they know their rights when it comes to study participation. The prisoners and peer educators at those site visits also echoed the sentiment that prisoners possess sufficient autonomy to make informed decisions about whether to participate in a given study.4
This, combined with the myopic emphasis on informed consent, is why the current categorical regulatory approach should be abandoned in favor of a risk-benefit paradigm. The following recommendations strive to acknowledge that, in limited circumstances, the potential benefit of a research protocol can justify research involving prisoners. These limited circumstances cannot be captured by a rigid categorical approach but need to be
|
Doris James, of the Bureau of Justice Statistics, added that some studies are very specific to the experiences and actions of prisoners. “Offenders are the only source of some of this data, data that are needed to provide programs, to produce policies to help meet their needs.” |
rooted in a risk-benefit analysis that grapples with the balance between a need for protection and access to potentially beneficial research protocols.
Recommendation 5.1 Apply a risk-benefit framework to research review. The U.S. Department of Health and Human Services should revise regulations regarding research with prisoners from a model based on categories to a system based on weighing of risks and benefits for the individual human subject, similar to the approach currently used in Subpart D.5
The risks and benefits of human subjects research are the ethically relevant issues, not the category of the research. The current categorical approach is dependent on stipulated research categories that are subject to various interpretations. This approach does not provide sufficient or reliable protection for the human subject. In addition, the present structure does not address the actual conditions of confinement or the restrictions on liberty that attach to any prisoner (whether incarcerated or subject to restraints on liberty in connection with community-based alternatives to incarceration) who may consider becoming a research subject and for whom the regulations are intended to provide protection. A risk-based approach is preferable because it requires institutional review boards (IRBs) and the Office for Human Research Protections (OHRP) to (1) focus on the potential benefits and harms of each suggested research protocol, and (2) identify the particular ethical issues that each protocol raises in the specific context of the correctional setting.
The general principle holds for all research: Ethically permissible research must offer benefits to prisoners that outweigh the risks. On the risk side of the equation, it will be important to analyze all potential risks, even something as seemingly innocuous as an interview. Certain questions can trigger harmful emotional or psychological responses; these questions cannot be allowed among prisoners unless there is an associated benefit.
On the benefit side, there may be research protocols, most likely epidemiological or social/behavioral, that carry very low risks for the prisoner-
subjects but no personal benefit for the subjects. Instead, the potential benefits may be for prisoners as a class (e.g., studies to identify factors that predict recidivism). Application of a risk-benefit analysis may determine that, because the risks are very low and important knowledge or benefits may accrue for prisoners as a class, the research may be considered ethically acceptable. The same may hold true for epidemiological studies (as distinct from biomedical research) that require analysis of biomedical samples, such as tissue, blood, or urine, but are not designed to assess outcomes of an intervention.
The idea of benefit can be flexible enough to include minimal risk protocols where the benefit to prisoners is indirect and/or temporally distant. It will be up to IRBs to determine whether there is a convincing affirmative reason for conducting research in a prison setting. When reviewing minimal risk research that does not present a direct benefit to subjects or prisoners as a class, IRBs should consider whether the research has the potential to yield important scientific information and the extent to which that information can only be obtained in a prison setting. For example, it would be appropriate to allow a prisoners’ continued participation in a minimal risk longitudinal study (i.e., epidemiological study) that they began before being incarcerated. Such studies may not benefit either the individual prisoner or prisoners as a class, but may generate important information about the community to which the participant belonged before incarceration. This would be permissible because the subject was selected for reasons other than incarceration, and the subject’s decision to participate is unlikely to have been influenced by the pressures of prison life. However, it should be noted that the flexible notion of benefit has distinct limits. In the absence of benefit, either to the prisoner-subject or to prisoners as a class, the research should be conducted in other settings
This balancing framework represents a departure from the way that decisions are currently made for approving research protocols. The present system utilizes the idea of “minimal risk” to evaluate the dangers associated with a protocol; studies are often characterized as presenting either minimal risk or more than minimal risk. The committee believes that this categorical approach is problematic and needs to be balanced with a consideration of benefit. Under a new risk-benefit framework, studies should be evaluated through a dynamic process of balancing risks and benefits, thus removing the need to rely on static definitions and categories. Nonetheless, as discussed in Chapter 6, the idea of minimal risk can be a useful tool for evaluating the risk side of a risk-benefit analysis. IRBs are accustomed to this starting point in their analyses, but should also move beyond strict reliance on this specific term in favor of a consideration of the balance between risks and benefits. Moreover, given the particularities of being a prisoner, the committee believes that the definition of “minimal risk” pres-
ently in Subpart C should be replaced by a slightly modified version of the definition, as follows:
The probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental, or psychological examination of healthy persons living outside the correctional setting.
This definition reflects the fact that prisoners are faced with a high baseline level of daily risk, thus making prison life an inappropriate reference point for determining whether a research protocol presents more than minimal risk.
Guidance on biomedical research The following guidance suggests how the risk-benefit framework should be applied to biomedical research. Specific direction is being supplied for this area because of the history and controversy surrounding medical and pharmacological studies in prisons. For other types of research (i.e., epidemiological, behavioral, and so on), these very specific limitations are not relevant.
There are two narrow circumstances in which biomedical research might be ethically acceptable:
-
In normal circumstances, a biomedical research study may be ethically acceptable if:
-
for research on new therapies or preventive measures, there is already some evidence of safety and efficacy, as in phase 3 testing for new drugs, as defined by the Food and Drug Administration (FDA); and
-
the ratio of prisoner to nonprisoner-subjects does not exceed 50 percent.
-
In exceptional circumstances, a biomedical research study may be ethically acceptable even if the benefit of an intervention has not been completely established, or if the research population is disproportionately composed of prisoners. This requires a federal-level review, for example, if the research addresses a condition that is solely or almost solely found in incarcerated populations. For studies of this nature to proceed, the protocol must be submitted to a national, specially convened panel of experts, who, in a public process, consider the ethical acceptability of the protocol (as is the process for Subpart D [45 C.F.R. § 46.407]), and make recommendations to the responsible government authority (the OHRP) regarding the special circumstances that do or do not provide a basis for research and the safeguards that must apply.
Rationale This approach starts with the presumption that biomedical research should be severely restricted and is allowable only in limited circumstances. Biomedical research involving prisoners as subjects is only permitted when the potential benefit to the prisoner-participants outweighs the risk to which the subjects are exposed. Under this framework, studies that offer no benefit to potential subjects would be precluded (e.g., testing of cosmetic products).The goal of the risk-benefit analysis is to prevent prisoners from being burdened by more than their fair share of risk, while allowing access to research that has potential benefits. This is especially relevant in circumstances in which effective treatments have not been developed to address a life-threatening or life-altering condition.
The guidelines articulated above illustrate how these principles would be applied in practice. The first allowable situation involves a treatment that appears to be safe and effective based on small-scale trials. The potential benefit of an experimental intervention must be established before engaging in a risk-benefit analysis. As such, phase 1 and phase 2 studies, as defined by the FDA to determine safety and toxicity levels, would not be allowable. Since these trials are principally designed to study a drug’s safety and efficacy, potential benefits are not yet clear. In these cases, risks to the prisoner might well overshadow the uncertainty of unproven benefits. Only phase 3 studies would be allowed, since basic efficacy would already have been demonstrated.
This approach reflects the growing view that research presents not only burdens but can also present benefits that should be fairly distributed to prisoners. However, the distribution of burdens must still be considered— thus the requirement that the ratio of prisoner to nonprisoner-subjects does not exceed 50 percent. Biomedical research should involve prisoners only to provide a benefit to individual prisoner participants, not because they are a convenient source of subjects. This 50 percent limit represents the committee’s strongly held view that prisoners should not compose the majority of a biomedical study’s enrollment when nonincarcerated subjects are available. The just distribution of risks and the potential for abuse require that researchers not be permitted to unnecessarily rely on prisoners as subjects.
It should be noted that the 50 percent limit is a ceiling that should only be approached when extensive use of prisoners as subjects can be justified by potential benefit. If a disease is less common in prisons, ethical guidelines would suggest a lower proportion of prisoner-subjects. Inmates should only be part of the subject pool to the extent that the disease affects the prisoner population. A study that extensively enrolls prisoners when nonprisoner-subjects are available should be examined closely to ensure that benefit to the prisoner population, and not convenience, is the true justification.
Under guideline 2, the 50 percent ceiling can be exceeded in exceptional circumstances, such as for conditions that solely or almost exclusively affect prisoners (for example, repetitive sexual assault; see Example 7, Chapter 6, page 167). Due to the inherent risks associated with research involving prisoners, increased oversight is needed when a biomedical study enrolls a high proportion of prisoners or when the potential benefits are expected but not yet established. Thus, the second exception requires more stringent safeguards. In this instance, the protocol would need to be submitted to an expert panel of medical and ethical scholars, whose opinions would be collected by the supervising agency and published on the agency’s Web site. The agency would then need to publicly post an opinion regarding its acceptance or rejection of the expert testimony and the reasons for either. This process is analogous to the process used under Subpart D § 407 (IOM, 204).
The preceding discussion should not be construed as an abandonment of the commission’s “primarily protective framework.” The goal of a risk-benefit framework is to maximize the safety and well-being of prisoners. As the commission emphasized, respect for persons requires that the risk of coercive practices and research abuses be negated (or at least minimized) by the use of protective measures. The commission’s approach emphasized the prevention of deleterious research protocols, but it did not properly account for potential that research can offer positive benefits to prisoner-subjects. A risk-benefit framework is still primarily concerned with preventing harm, but does so in a manner that allows for participation in research when the potential for benefit to prisoners greatly outweighs potential risks.
Risk-benefit analyses of the type illustrated here provide the bases for the kinds of specific safeguards discussed and described in greater detail in Chapter 6.
Justice
In this section, the committee lays out its expansion of the principle of justice in two ways: To include: (1) collaborative responsibility for research proposals and setting a research agenda, and (2) enhancing the welfare of the prisoner population.
Collaborative Responsibility
The conceptualization of justice has expanded since the original commission’s work. They primarily thought of justice in terms of the distribution of risks and benefits. Although this is still a legitimate concern, some recent scholarship suggests elevating collaborative responsibility under the rubric of justice.
|
“We need to emphasize and reemphasize the voluntary nature of research in our collaborations with community-based agencies, with the Department of Corrections, and with the prisoners themselves. We need to be sure we are getting input from the incarcerated community,” said Olga Grinstead, Ph.D., University of California, San Francisco Center for AIDS Prevention Studies, at its July 2005 meeting. Dr. Grinstead has been developing and evaluating human immunodeficiency virus, (HIV), sexually transmitted diseases (STD), and hepatitis prevention programs for incarcerated men and their female partners since 1993. |
Specifically, Eckenwiler (2001) develops a proposal for incorporating particularity into the research review process that reflects the interest in subjectivities and participatory justice in contemporary feminist scholarship. She notes that analyses of potential harms and benefits, and trade-offs between them, can vary considerably depending on personal characteristics and on the social, economic, and institutional contexts. A determination to be impartial or to put oneself in the shoes of a particular kind of research subject will be inadequate in many circumstances: “When differences between the social positions of the deliberators and the targeted beneficiaries involve relations of privilege and oppression attempts to ignore one’s own situation or imagine the perspectives of others are especially unlikely to be successful. Furthermore, as most IRB members are health-care providers and scientists, they are less disposed to question features of research that collude in perpetuating inequalities … [and may also be] acutely aware of the financial environment in which they operate and of the importance of clinical research in their institutions’ economic viability” (Eckenwiler, 2001). Eckenwiler recommends that, among other things, efforts be undertaken to enhance the participation of affected or interested groups. This involves acknowledging that groups are not monolithic and are themselves subject to a range of problems that should be addressed in the consultation process. This recommendation has two aspects: (1) including more laypeople who match the local population and common subject groups in key respects; and (2) shaping IRBs so they are hospitable places for lay members.
This committee agrees with this perspective. Thus, a new risk-benefit approach needs to be accompanied by an emphasis on collaboration. The ethical problems associated with research involving prisoners will manifest themselves differently in each correctional setting. The one-size-fits-all approach characterized by a focus on informed consent cannot adequately address the unique concerns presented by each setting. Thus, all relevant parties should be involved (prisoners, correctional officers, medi-
|
“I would like to know more about how we could have a liaison between the inmates and the researchers. I really think that that is an important issue,” stated Debra Breuklander at the committee’s October 2005 meeting. Ms. Breuklander is a former prisoner who is now a nurse consultant at MECCA, a residential inpatient substance abuse treatment program in Des Moines, Iowa, and a member of the committee’s prisoner liaison panel. |
cal staff, administrators) when creating and implementing a research protocol. This effort, combined with a more specific focus on risks and benefits, can lead to research practices that better incorporate justice and respect for persons.
Recommendation 5.2 Use a collaborative research approach. Under an ethic of collaborative responsibility, investigators should find ways to obtain input from prisoners and other stakeholders on the design and conduct of any research protocol involving prisoners.
To satisfy the spirit of the Belmont Report principles in modern correctional settings requires recognition of an additional ethical imperative. Collaborative responsibility is a necessary ethical underpinning for research in correctional settings. Collaborative responsibility is a phrase intended to convey the idea that, to the extent feasible, all aspects of research (design, planning, and implementation) should include the active participation of relevant institutional stakeholders (prisoners, correctional officers, medical staff, administrators). Efforts should be made to consult with major stakeholders within the local institution, particularly prisoners as well as former prisoners and prison staff, to particularize the protocol to local conditions. A focus on collaboration would help cope with the reality that each institution has its own unique conditions and also may facilitate openness of the research environment. With collaborative input, research design and implementation could be tailored to the issues, needs, and capacities of a given setting. Prisoners have an interest in being consulted as part of the collaborative process. The responsibility for collaboration lies with investigators, who need to make the effort to engage prison administration and prisoners themselves for their input, and with the human research participant protection program, which must determine that the effort was made.
A valuable model can be found in Responsible Research: A Systems Approach to Protecting Research Participants (IOM, 2003), which sets forth the phases of human research (see Figure 5-1). Most of these phases
provide an opportunity for collaboration that can facilitate the conduct of research in accordance with the principles of justice and respect for persons.
At the outset, a researcher must construct a research question. Collaboration with prisoners and prison staff at this stage can be a productive means of addressing topics that will have the most benefit for the prison population. Representatives from within the prison system can encourage study of the most pressing problems or discourage protocols that address insignificant issues. It is at this first stage that prisoners in particular can voice their thoughts on whether a given research question provides benefits that outweigh the risks.
Collaboration during protocol development is important because each correctional setting presents unique strengths and weaknesses. This is an opportunity to assess a specific prison’s infrastructure (e.g., to determine what is feasible). In particular, collaboration during protocol development can reveal setting-specific characteristics that will make it significantly more difficult to conduct ethical research (i.e., presence of gangs, mistrust of outsiders, inadequate medical infrastructure). When such barriers exist, the researcher, in collaboration with prisoners, correctional officers, medical staff, and administrators, must consider whether it is even appropriate to perform human subject research in that setting, and if so, to design safeguards that protect subjects and overcome impediments.
Recruitment and enrollment make up a third area of potential collaboration. Involving prisoners in the recruitment process can go a long way toward minimizing potential coercion or undue inducement. If prisoners have a voice in how subjects are enrolled, they can help protect themselves from inappropriate recruitment practices that infringe on their autonomy (respect for persons) or unfairly distribute risks and benefits within the prison population (justice).
This model and discussion are not meant to provide a comprehensive list but merely to illustrate that collaboration can and should occur at every level of the research process. There is no objective way to say collaboration occurred. However, the human research participants protection program (HRPPP) can ask if all relevant people were consulted and determine that the process was transparent and fair. As long as all parties are consulted fully and fairly, given an opportunity to be heard, the goal is met.
The committee acknowledges that this will generally be a new model to many researchers and within most correctional settings, and, thus will require a significant commitment to implement.
Welfare of the Prisoner Population
Recent decades have seen an explosion in writing on justice, specifically in the context of research. As discussed previously, this has involved a
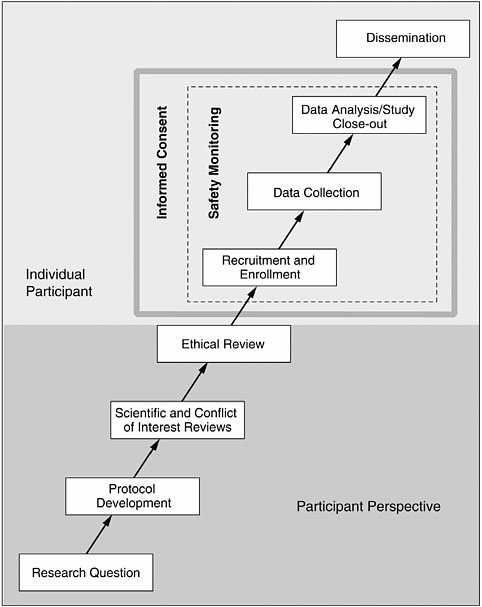
FIGURE 5-1 The phases of human research.
SOURCE: IOM (2003).
change in the way we view distributive justice. The commission thought of distributive justice as requiring the fair distribution of research risks and burdens. However, much of the recent philosophical work argues that although justice requires the protection of vulnerable subjects from exploitative research, sometimes it also mandates that research be done to improve
the welfare of these populations. This perspective focuses on the idea that justice requires the fair distribution not only of risks but also of benefits.
In this area, the volume Beyond Consent: Seeking Justice in Research has synthesized and extended prior work on justice. In the chapter “Race, Justice, and Research,” King (1998) argues that justice should take us beyond purely distributive concerns to the evaluation and modification of “institutional arrangements” and structures of “decision making and other procedural aspects of research.” Another chapter, “Convenient and Captive Populations,” provides a history of the regulation of research with prisoners, institutionalized persons, military personnel, and students in the United States. It concludes by endorsing a protectionist stance toward these populations, based on histories of abuse, while noting that “there are circumstances in which justice may permit, or even require, access to research” for these populations, such as “the prevalence of a disease that poses a particular threat” to its members and “cannot be studied as effectively with other subjects” (Moreno, 1998).
Similarly, two other philosophers have also thought about the idea that justice requires more than the protection of subjects from exploitation; to be truly ethical, research must actively consider what is best for a population. London, in his work on international research, argues that the permissibility of clinical research should rest in part on its contribution to “filling the gaps between the most important health needs in a community and the capacity of its social structures to meet them” (London, 2005). Even if a particular research project is not, strictly speaking, exploitative, it may still be ethically problematic if it is not the project that has the greatest potential to address the health problems and concerns of the community. Powers (1998) argues that “freedom of choice is important, but the availability of choice-worthy options also is important.” He calls for a complex, comprehensive concept of justice in research that not only synthesizes elements of the various norms (e.g., adopting a dual focus on individuals and groups, benefits and burdens, and upstream and downstream) but also considers the connections between research and other realms of health (or, for that matter, social) policy.
The committee believes that this expanded concept of justice is an important ethical development. Justice requires more than the protection of prisoners from harm caused by the research itself. Ethical research carries with it a responsibility to grapple with the fact that potential harm is ubiquitous in everyday prison life, creating an environment for research in which the choice to participate in a study can be inherently coercive and potentially dangerous. Thus, in order for research to be ethical, justice requires that it must be done in a setting in which there is an adequate standard of health care in place.
How to assess the adequacy of a correctional health-care system? The committee acknowledges that the vast majority of researchers and IRBs do
not have the expertise to directly measure health-care quality in correctional settings. Certain indirect measures, however, may help with this determination. For example, has the specific correctional system’s health-care services been found to be unconstitutional by a court? Is it under a consent decree, settlement agreement, or a similar process relevant to the adequacy of the health-care services? Such situations create a presumption that the system was inadequate at that time. Research in a correctional facility that involves subject matter addressed under a court order/settlement/consent decree should be presumptively disapproved. (The concern is primarily biomedical research, but behavioral research could be implicated under the standards proposed here if the underlying mental health support is seriously lacking in the institution and if the proposed research project has mental health as an integral component of the behavioral research.)
To allow biomedical research under such circumstances, the IRB must apply the risk-benefit analysis the committee proposes in a heightened form and find that the research proposed is permissible only after reviewing the specific components of the research and its interaction with the specific components of the system presumed to be deficient. This risk-benefit application should address the theoretical aspects of the proposed research, as well as its administration and monitoring, including the informed consent. The IRB must assure itself that no prisoner is choosing to be a research subject in order to bypass the presumptively deficient system.
Other factors, which would be useful though not definitive, include:
-
Is the system accredited by a third party, such as National Commission on Correctional Health Care (NCCHC), American Correctional Association (ACA), or Joint Commission on Accreditation of Healthcare Organizations (JCAHO)?
-
Have any recent internal or external assessments been made of the health-care system?
-
Do relevant quality improvement studies exist?
-
Do other relevant assessments exist?
Recommendation 5.3 Ensure adequate standards of care. Human research participant protection programs,6 together with the prison administration and prison health-care professionals, are responsible for
ensuring that research with prisoners occurs in an environment that is appropriate to the health and well-being of prisoners, including access to existing medical and mental health care that is adequate, protection from inmate attempts to coerce or manipulate participation or non-participation in research, and prompt access to decent health-care services in case the research causes physical or mental harm.
Ethical research requires an environment that is humane and provides reasonable access to supportive care, particularly when human subjects are exposed to physical or psychological risks. Without adequate medical or psychological care, subjects may be vulnerable to undue inducements to participate in research such that they would consent in order to gain access to medical care or other benefits they would not normally have. Finally, researchers have an ethical obligation, if they expose subjects to risk, to rapidly and professionally remedy any harms caused by the research.
HRPPPs can meet their obligations under Recommendation 5.3 by engaging in due diligence and going through a careful process to discover whether adequate heath care exists within the correctional setting, including analysis of the factors described above and any others that might reflect on the quality of the correctional setting. Obtaining answers to these questions would likely require visiting the setting, speaking to health-care staff, and reviewing relevant court cases.
Lastly, if research is to be done in prisons, there is an ethical responsibility to devote much of this research effort to determine how best to achieve all of the legitimate purposes of the criminal justice system.
Recommendation 5.4 Support critical areas of correctional research. Government agencies should fund and researchers should conduct research to identify needed supports to facilitate prisoners’ successful reentry into society, reduce recidivism, and inform policy makers about the most humane and effective strategies for the operation of correctional systems.
Society creates a correctional system for clear purposes, such as deterrence to future crime and rehabilitation of those who are convicted of committing offenses. It is of utmost social importance to better understand how best to achieve the purposes of incarceration, including reduction of recidivism and successful introduction back into the community. Perhaps unavoidably, the criminal justice system inflicts some harm on those it punishes. As ethical people, we constantly strive to develop and use corrective measures that are effective and humane, without causing unnecessary physical or mental harm to prisoners. However, prisoners are a vulnerable population subject to abuse and exploitation. Indeed, several subclasses of prisoners make up some of society’s most vulnerable populations, such as
young people, persons with mental disabilities, racial minorities, women, and people with diseases (addiction, hepatitis, HIV, hypertension, diabetes) that may or may not be treated during imprisonment. It is, therefore, especially important to better understand how to protect and promote the welfare and well-being of this large and growing segment of our society. Scientific knowledge and information about “best practices” gained from high-quality research is critically important to understanding how best to achieve all of the legitimate purposes of the criminal justice system.
REFERENCES
Agrawal M, Emanuel E. 2003. Ethics of phase 1 oncology studies: Reexamining the arguments and data. Journal of the American Medical Association 290:1075–1082.
Beauchamp TL, Childress JF. 2001. Principles of Biomedical Research Ethics. 4th ed. New York: Oxford University Press.
Dubler NN, Sidel VW. 1989. On research on HIV infection and AIDS in correctional institutions. Milbank Quarterly 67(2):171–207.
Eckenwiler L. 2001. Moral reasoning and the review of research involving human subjects. Kennedy Institute of Ethics Journal 11(1):37–69.
Emanuel EJ, Wendler D, Grady C. 2000. What makes clinical research ethical? Journal of the American Medical Association 283:2701–2711.
Hoffman S. 2000. Beneficial and unusual punishment: An argument in support of prisoner participation in clinical trials. Indiana Law Review 33:475.
Hornblum AM. 1997. They were cheap and available: Prisoners as research subjects in twentieth century America. British Medical Journal 315:1437–1441.
Hornblum AM. 1998. Acres of Skin: Human Experiments at Holmesburg Prison. New York: Routledge.
IOM (Institute of Medicine). 2003. Responsible Research: A Systems Approach to Protecting Research Participants. Washington, DC: The National Academies Press.
IOM. 2004. The Ethical Conduct of Clinical Research Involving Children. Washington, DC: The National Academies Press.
Kahn JP, Mastroianni AC, Sugarman J, eds. 1998. Beyond Consent: Seeking Justice in Research. New York: Oxford University Press.
King PA. 1998. Race, justice, and research. In: Kahn JP, Mastroianni AC, Sugarman J, eds. Beyond Consent: Seeking Justice in Research. New York: Oxford University Press. Pp. 88–110.
Lazzarini Z, Altice FL. 2000. A review of the legal and ethical issues for the conduct of HIV-related research in prisons. AIDS & Public Policy Journal 15(3/4):105–135.
London AJ. 2005. Justice and human development approach to international research. Hastings Center Report 35(1):24–37.
Mastroianni A, Kahn J. 2001. Swinging on the pendulum. Hastings Center Report 31(3):21–28.
Mitford J. 1973a. Experiments behind bars: Doctors, drug companies, and prisoners. Atlantic Monthly 76:64–73.
Mitford J. 1973b. Kind and Usual Punishment: The Prison Business. New York: Alfred A. Knopf.
Moreno JD. 1998. Convenient and captive populations. In: Kahn JP, Mastroianni AC, Sugarman J, eds. Beyond Consent: Seeking Justice in Research. New York: Oxford University Press. Pp. 111–130.
Moreno JD. 2001. Goodbye to all that: The end of moderate protectionism in human subjects research. Hastings Center Report 31(3):9–17.
Murphy D. 2005. Health care in the federal bureau of prisons: Fact or fiction. California Journal of Health Promotion 3(2):23–37.
NBAC (National Bioethics Commission). 1998. Research Involving Persons with Mental Disorders That May Affect Decision–Making Capacity, Volume I: Report and Recommendations of the National Bioethics Advisory Commission. U.S. Department of Commerce, National Technical Information Service, Sterling, VA. PB99143919. P. 89.
NCPHSBBR (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research). 1976. Report and Recommendations: Research Involving Prisoners. Washington, DC: NCPHSBBR.
Powers M. 1998. Theories of justice in the context of research. In: Kahn JP, Mastroianni AC, Sugarman J, eds. Beyond Consent: Seeking Justice in Research. New York: Oxford University Press. Pp. 147–165.
Rhodes, LA. 2005 Pathological effects of the supermaximum prison. American Journal of Public Health 95(10):1692–1695.
Rugaber W. 1969, July 9. Prison drug and plasma projects leave fatal trail. New York Times, p. 20.
























