A
Data Sources and Methods
To comprehensively address the committee’s overarching task of reviewing ethical considerations for protection of prisoners involved in research, the committee cast a broad net for the collection and assessment of information. These sources included commissioned papers (Box A-1), open sessions and workshops, telephone interviews and e-mail surveys to the state departments of corrections (DOCs), a survey of recent literature (to assess basic characteristics of research with prisoners), and two site visits to the correctional facilities.
In addition, a liaison panel of former prisoners and prisoner advocates was assembled for the committee to consult with throughout the project (Box A-2). The committee organized two meetings with the liaison panel to receive their expert advice and guidance in framing the issues, identifying important sources of information, and ensuring a comprehensive analysis. A summary description of the committee’s evidence gathering activities and results follows.
OPEN SESSIONS AND WORKSHOPS
Over the course of the study, the committee sought and received input from former prisoners, representatives of the prisoner advocacy community, bioethics researchers, health professionals, prison services researchers, and other organizations involved with research in prisons. To help accomplish this, the committee held three open meetings. The first was part of the first committee meeting on March 16, 2005. Staff from the Office for
|
BOX A-1 Commissioned Papers Ethical Issues Regarding HIV/AIDS Research Among Prisoners Theodore M. Hammett, Ph.D., Abt Associates, Inc. 10 Years of HIV/AIDS Research Behind Bars: Time for Change Jason Farley, Ph.D.(c), MPH, CRNP, The Johns Hopkins University Rethinking the Ethics of Research Involving Prisoners Alex London, Ph.D., Carnegie Mellon University Research with Prisoners: A Reexamination of Ethical Foundations Mary Anderlik Majumder, J.D., Ph.D., Center for Medical Ethics and Health Policy Current Status of the Process of Mental Health Research and Substance Abuse Research with Prisoners: Practical Burdens and Benefits of the Current System Robert Trestman, Ph.D., M.D., University of Connecticut Health Center |
|
BOX A-2 Former Prisoners/Prisoner Advocates Liaison Group Edward Anthony, Philadelphia, PA Jack Beck, Esq., Correctional Association of New York Debra Breuklander, MECCA James J. Dahl, Ph.D., Phoenix House Allen Hornblum, M.A., M.P.A., Temple University Daniel S. Murphy, Ph.D., Appalachian State University Barry Nakell, Esq., North Carolina Prisoner Legal Services, Inc. Osvaldo Rivera, LADC I, Span, Inc. Jeffrey Ian Ross, Ph.D., University of Baltimore Jean Scott, Phoenix House |
Human Research Protections (OHRP) discussed the current federal regulations and their goals for this Institute of Medicine (IOM) project. Perspectives on the current federal regulation and needed changes were also provided by representatives of the prisoner advocacy community, bioethics researchers, prison services researcher, and a representative from the federal Bureau of Prisons (BOP). The second was a workshop in Washington, D.C., on May 4, 2005. This public workshop focused on the ethical, legal, regulatory frameworks that underlie research involving prisoners. The committee also heard from representatives of the corrections industry about the practicalities of conducting research in correctional settings. A panel of
|
BOX A-3 Individuals and Organizations that Addressed the Committee Elizabeth Alexander, J.D., National Prisoner Project of the American Civil Liberties Union (ACLU) Edward Anthony, Philadelphia, PA Larry Bench, Ph.D., Utah Department of Corrections Jessica Berg, J.D., Case Western Reserve University Joseph Bick, M.D., California Medical Facility Debra Breuklander, MECCA Alvin J. Bronstein, J.D., ABA Task Force on Legal Status of Prisoners, ACLU National Prison Project James Childress, Ph.D., University of Virginia Gwendolyn C. Chunn, M.A., American Correctional Association Hazel D. Dean, Sc.D., M.P.H., Centers for Disease Control and Prevention Nancy Dubler, LL.B., Montefiore Medical Center Bernice Elger, Ph.D., Timothy Harding University of Geneva, Switzerland Gerald Gaes, Ph.D., National Institute of Justice Julia Gorey, J.D., Office for Human Research Protections Olga Grinstead, Ph.D., M.P.H., University of California, San Francisco (UCSF) Alison Hardy, J.D., Prison Law Office Edward Harrison, CCHP, National Commission on Correctional Health Care Allen Hornblum, M.A., M.P.H., Temple University Doris J. James, M.A., Bureau of Justice Statistics Denise Johnston, M.D., Center for Children of Incarcerated Parents Patricia King, J.D., Georgetown University Peter Leone, Ph.D., University of Maryland at College Park Phillip Lyons, J.D., Ph.D., Sam Houston State University Philip Magaletta, Ph.D., Federal Bureau of Prisons Monika Markowitz, MSN, RN, M.A., Virginia Commonwealth University Mary Faith Marshall, Ph.D., University of Minnesota Nena Messina Ph.D., University of California, Los Angeles Daniel S. Murphy, Ph.D., Appalachian State University David Paar, M.D., University of Texas Medical Branch, Galveston Darrel A. Regier, M.D., M.P.H., American Psychiatric Association Bernard Schwetz, DVM, Ph.D., Department of Health and Human Services Vera Hassner Sharav, M.L.S., Alliance for Human Research Protection Christopher Slobogin, J.D., LL.M., University of Florida School of Law Susan Sniderman, M.D., IRB Chair, UCSF Irene Stith-Coleman, Office for Human Research Protections T. Howard Stone, J.D., LL.M., University of Louisville David Thomas, M.D., Nova Southeastern University College of Medicine Dan Wikler, Ph.D., Harvard University Gary Zajac, Ph.D., Pennsylvania Department of Corrections |
former prisoners and prisoner advocates talked about needed protections for research involving prisoners. The third workshop was held in San Francisco on July 18, 2005. This workshop focused on topical research areas and methodological issues related to conducting research with correctional populations. Former prisoners and prisoner advocates also presented their views of needed protections. The organizations and individuals that addressed the committee in these open sessions are listed in Box A-3. In
|
BOX A-4 Public Meeting Participants Sue Allison, Federal Bureau of Prisons Susan Bankowski, Johns Hopkins School of Public Health Jessica Baumann, Bureau of National Affairs Francis Beylotte, American Psychological Association Laura Bishop, Kennedy Institute of Ethics Kristina Borror, Office for Human Research Protections Bret Bucklen, Pennsylvania Department of Corrections Scott Camp, Federal Bureau of Prisons Michael Carome, Office for Human Research Protections Erika Check, Nature Michael D. Cohen, New York State Office of Children and Family Services Jennifer Couzin, Science Joyce Cutler, Bureau of National Affairs Pamela Diamond, University of Texas School of Public Health Erik Dietz, Federal Bureau of Prisons Glen Drew, Office for Human Research Protections Jessica Ebert, Nature David Egilman, Brown University Bernice Elger, University of Geneva and National Institutes of Health (NIH) Patricia El-Hinnawy, Office for Human Research Protections Julie Falk, CorrectHELP Christine Fornwalt, Johns Hopkins Bloomberg School of Public Health Gerald Gaes, National Institute of Justice Doreen Geiger, Washington State Department of Corrections Harold Goldstein, American Psychiatric Institute for Research and Education Te Guerra Erica Hall, KPFT Pacifica Radio, Houston News Shirley Hicks, Office for Human Research Protections |
addition, many other individuals attended and participated in the three public meetings (Box A-4).
LITERATURE SURVEY TO ASSESS GENERAL CHARACTERISTICS OF RESEARCH WITH PRISONERS
To help characterize the landscape of published research with prisoners (i.e., who is doing what type of research in what type of prisoner settings), the committee conducted an assessment of prisoner research published in peer-reviewed journals. The preliminary search consisted of English language articles published since 1990, using the following databases: MedLine, PsychLit, Sociological Abstracts, Cumulative Index to Nursing & Allied Health Literature, Criminal Justice Abstracts, Education Resources
|
Terry Hill, Lumetra Sally Hillsman, American Sociological Association Bill Holman, Gilead Sciences, Inc. Craig Hutchinson, UCSF Center for AIDS Prevention Studies Victoria Joseph, Bureau of Prisons Julie Kaneshiro, Office for Human Research Protections Alexa Kasdan, San Francisco AIDS Foundation Steven Krosnick, NIH/Center for Scientific Review Dan Landrigan, Report on Research Compliance Molly Lang, The Blue Sheet, F-D-C Reports Elizabeth Mendelsohn, UCSF Office of Research Leah Mendelsohn, Johns Hopkins Bloomberg School of Public Health Virginia Morrison, Health Care Mediations, Inc. Janet Myers, UCSF Medicine Edward Opton, Jr. Sangeeta Panicker, American Psychological Association Kevin Prohaska, Office for Human Research Protections Mercedes Rubio, American Sociological Association William Ruby, Gilead Sciences, Inc. Sandra Sanford, George Mason University Jeffrey Schomisch, Guide to Good Clinical Practice Angela Sharpe, Consortium of Social Science Associations Barbara Solt, Institute for the Advancement of Social Work Research Anne Spaulding, Medical College of Georgia/Georgia Correctional Health Care, Infectious Disease Mary Sylla, Centerforce Sara Tobin, Stanford University Center for Biomedical Ethics Christie Visher, The Urban Institute Cheryl Crawford Watson, National Institute of Justice Donna Willmott, Legal Services for Prisoners with Children |
Information Center, National Technical Information Service, and Excerpta Medica Database. Search terms used included IRB composition, multisite study/studies, risk-benefit, informed consent, undue influence, vulnerable populations, payment, biomedical research, behavioral research, environment, clinical trials, medication development, FDA, data storage, record keeping, privacy, placebo-control trials, standard of care, follow-up care, follow-up monitoring, data monitoring, HIV/AIDS, tuberculosis, infectious diseases, substance abuse, mental health, women, females, juveniles, adolescents, and mental illness. These search terms were cross-matched with the following subject terms: inmate(s), prisoner(s), incarcerate(d), jail(s). The preliminary search resulted in more than 14,000 articles. The search was then limited to the past 5 years, which resulted in a selection of 1,870 articles.
A random sampling of 20 percent of the 1,870 articles was selected as the final sample. Of these 374 studies, a total of 327 were studies that included human subjects. The remaining 47 included the following types of articles: review articles, commentaries, introductions to special editions, letters to the editor, position pieces, editorials, theory articles, news articles, legal reviews, opinion pieces, discussion pieces, and news type articles.
All of the articles were reviewed and coded using the standard criteria. The results follow.
Results
The results of the survey to assess the general characteristics of published research with prisoners are summarized in the following figures.
Funding Sources
Funding stemmed from a variety of sources, including the federal government, state agencies, universities, and the private sector (Figures A-1, A-2).
Mechanism of Approval
Most studies (66 percent) did not report the mechanism by which they were approved (Figure A-3). Fifteen percent indicated institutional review board approval; other entity review was 19 percent.
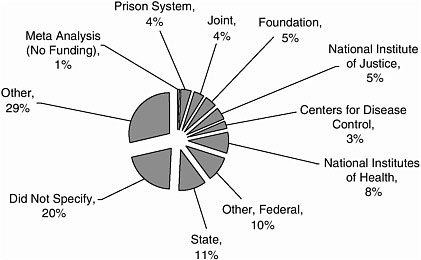
FIGURE A-1 Source of funding.
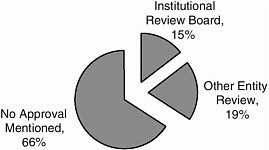
FIGURE A-3 Mechanism of approval.
Study Design
As shown in Figure A-4, epidemiological studies were the most common (39 percent). Other common study designs included correlational studies (27 percent) and those assessing behavioral outcomes (14 percent).
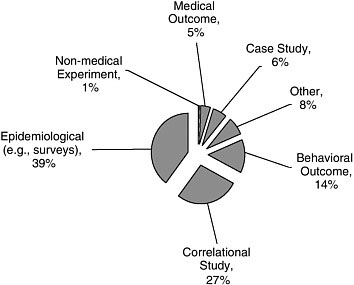
FIGURE A-4 Study design.
Type of Study
Most of the studies (41 percent) in the sample had a sociobehavioral focus, lacked a therapeutic purpose, and had minimal risk to participants (Figure A-5). Program evaluations (26 percent) and record reviews (21 percent) were also common.
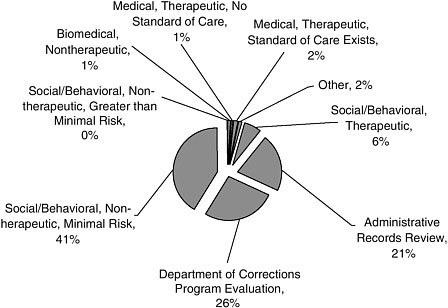
FIGURE A-5 Type of study. (Same as Figure 2-3).
NOTE: Greater than minimal risk included any biomedical (nontherapeutic) study; any medical therapeutic study (regardless of the existence of a standard of care); any social/behavioral therapeutic study; and any nontherapeutic study involving a manipulation that the research assistant judged to involve potentially serious physical or emotional stress (e.g., long sleep deprivation). Not greater than minimal risk included any study based on review of administrative records; any program evaluation study; any nontherapeutic social/behavioral study that either involved no manipulation (e.g., innocuous questionnaires/surveys) or involved a manipulation that the research assistant judged did not involve potentially serious physical or emotional stress (e.g., long sleep deprivation).
Studies were largely focused on health status (43 percent) and personality characteristics (19 percent) (Figure A-6).
Facilities/Locations
More than half of the studies (53 percent) were conducted in prisons or jails (Figure A-7). Another large proportion of the studies (37 percent) were conducted in alternate settings, such as treatment programs or postincarceration settings (Figures A-7, A-8).
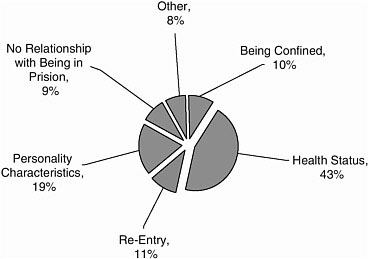
FIGURE A-6 Categories of research.
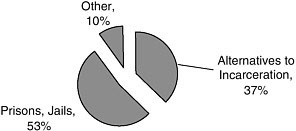
FIGURE A-7 Facilities/location of studies.
Number and Demographics of Research Participants
The number of participants in a published article ranged from 1 to 336,668. Most studies (272) included 1,000 or fewer participants (Figure A-9).
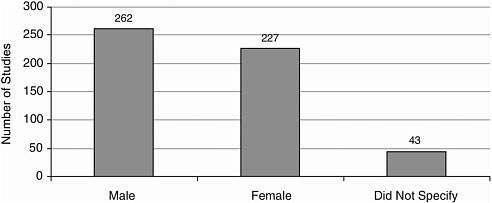
Gender More studies included male participants than female participants (Figure A-10).
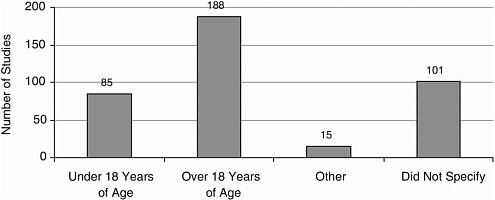
Age Most studies included adult participants; few included participants younger than 18 years (Figure A-11).
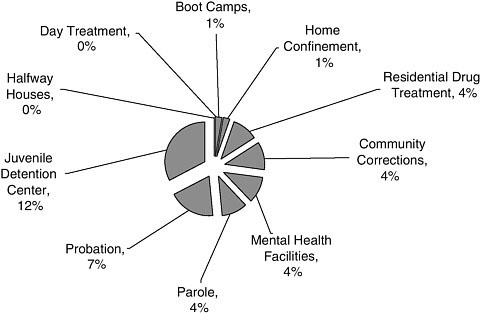
FIGURE A-8 Alternatives to incarceration research settings.
NOTE: This graph corresponds to the “Alternatives to Incarceration” slice in Figure A-7. Juvenile detention centers were included in this analysis because the committee decided to limit its focus to adults after this literature assessment was conducted.
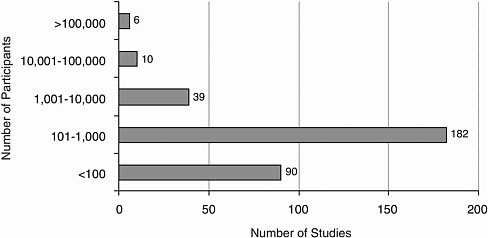
FIGURE A-9 Number of research participants.
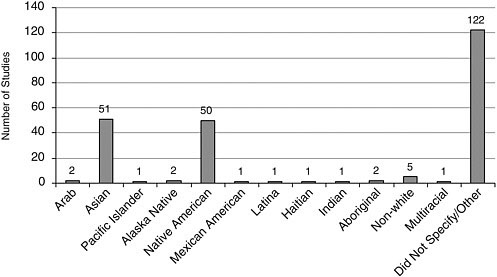
FIGURE A-13 Number of studies with participants of “other” race/ethnicity.
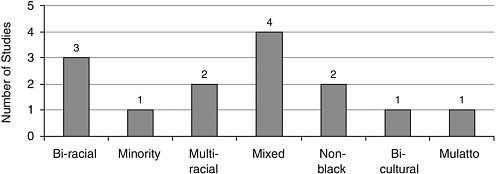
FIGURE A-14 Number of studies with nonwhite participants.Mulatto
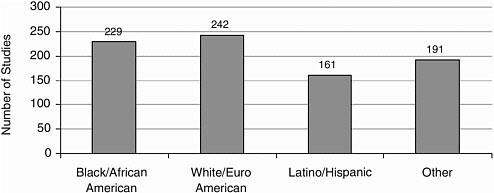
Race/Ethnicity Most studies included Caucasians, closely followed by African Americans, and Latinos/Hispanics (Figure A-12). Other racial and ethnic groups were represented to a lesser extent (Figures A-13, A-14).
SITE VISITS
The committee conducted two site visits to correctional facilities. On July 20, 2005, the committee visited San Quentin Prison in San Francisco and the California Medical Facility (CMF) at Vacaville, California. During their site visits, the committee had guided tours of both facilities and un-
structured discussions with peer educators (i.e., inmates who are trained to be peer educators) about research experiences and needed protections when participating in research.
SURVEY OF STATE DEPARTMENTS OF CORRECTIONS
As part of its data collection activities, the committee collected information from state DOCs regarding research policies and practices. The committee conducted telephone interviews with six states and sent a survey to the remaining states and District of Columbia by e-mail.
Telephone Interviews
The committee collected information from DOCs in six states via telephone interviews: New York, California, Iowa, Texas, Florida, and Utah. The interviews covered the following:
-
types of research that are conducted,
-
number of studies that have been undertaken in recent years,
-
requirements for informed consent,
-
degree of risk to which research subjects are subjected,
-
procedures for processing research proposals,
-
credentials and qualifications of the people charged with the responsibility of approving research,
-
problems or concerns that have arisen in connection with such research, and
-
impact of laws and regulations on proposed or actual research projects.
Chapter 2 includes summary results of those telephone interviews.
E-Mail Survey
In the interest of reaching all 50 states and the District of Columbia, similar information to that which was collected by telephone interviews was requested from the DOCs of the remaining 44 states and Washington, D.C., in an e-mail survey. The purpose of this survey was to poll the states’ DOCs about their research activities and practices. All but three (Delaware, Illinois, Wyoming) DOCs responded, bringing to 48 the total number of DOCs about which the committee had information (6 from telephone interviews, 42 from e-mail survey). Table A-1 presents the survey questions and a summary of DOC responses.
TABLE A-1 Summary of Results from Department of Corrections (DOC) Survey
|
Question |
Yes |
No |
Other |
|
|
For questions 1–8, is this type of research permitted in your DOC? |
40 |
2 |
|
|
|
1. |
Purely DOC records review, typically descriptive studies (e.g., demographics of prison population) or correlational studies (e.g., association of prisoner characteristics with type of index crime, number/type of disciplinary infractions) based on information routinely gathered by the DOC outside the framework of aspecific research protocol |
|
|
|
|
2. |
Evaluation studies of DOC programs that evaluate the process or outcomes of an internal DOC program such as an educational program (e.g., impact of new classroom technique on GED test performance), or health or mental program (e.g., drug/substance abuse education; sex offender treatment) |
40 |
2 |
|
|
3. |
Nontherapeutic social/behavioral studies involving minimal risk such as administration of interviews and/or questionnaires to assess personality features and personal history for development of a risk assessment measure; reaction time studies (e.g., how quickly inmates respond to different visual stimuli presented on a computer screen) |
30 |
10 |
1/case by case 1/yes–noa |
|
4. |
Nontherapeutic social/behaviors studies involving greater than minimal risk (e.g., evaluate the effects of prolonged sleep deprivation) |
4 |
34 |
1/not likely 2/case by case 1/yes–nob |
|
5. |
Evaluation of behavioral clinical interventions developed and administered by outside agencies (e.g., university researchers implement and evaluate a group therapy treatment protocol for PTSD that is not part of DOC standard services). |
20 |
19 |
2/case by case 1/yes–noa |
|
6. |
Medical research–therapeutic studies (e.g., AIDS, hepatitis C, breast/prostate cancer, reproductive medicines/devices) in which study involvement permits inmates to have access to experimental treatments that would not be otherwise available |
13 |
27 |
1/case by case 1/yes–noa |
|
Question |
Yes |
No |
Other |
|
|
7. |
Medical research–therapeutic studies of diseases for which there is an established standard of care (e.g., new asthma medications) |
14 |
26 |
1/case by case 1/yes–noa |
|
8. |
Biomedical studies of a nontherapeutic nature, including studies that involve exposure to a biological or chemical agent to assess the effects on and reactions of humans (e.g., effects of cosmetic or cleaning agents on skin) |
3 |
38 |
1/not likely |
|
9. |
If you answered “no” to any of the questions above, is it the case that some of these types of research are explicitly prohibited by your DOC policy or by legislation? Answer the following questions only if at least one of the types of research described above is permitted in your DOC. To ensure the safety of research subjects, in many research settings any study that involves human beings as research participants must be evaluated and approved by an institutional review board (IRB) before the study can commence. Please answer each of the questions below regarding IRB nvolvement in research at your organization. |
31 |
8 |
3/NA |
|
10. |
Does your DOC require IRB approval before research can commence? |
29 |
10 |
3/NA |
|
11a. |
Does your DOC have its own IRB within the organization? |
13 |
26 |
3/NA |
|
11b. |
If you answered yes to Question 11a, are there prisoner representatives on the DOC’s IRB? |
5 |
13 |
24/NA |
|
12. |
Does your DOC have an adverse events reporting process or procedure? |
18 |
20 |
4/NA |
|
NOTE: DOC, Department of Corrections; IRB, institutional review board; NA, not applicable; GED, General Education Development (tests); PTSD, post-traumatic stress disorder. aVermont stated that the DOC is part of an umbrella Agency of Human Services (AHS). The AHS operates an IRB for review of all research, including DOC-related studies. No research involving minimal or greater risk to participants may proceed without IRB approval. bOnly with IRB approval. |
||||