1
Introduction
Data from biomonitoring studies are becoming widely available and are increasingly used to understand the presence of chemicals in the human body and their effects on human health. At the same time, scientists, public-health officials, and the public have questions about the quality and scope of the available data, what the data tell us about potential risks to human health, and how future research can address these questions. Responding to a congressional request, the National Research Council established the Committee on Human Biomonitoring for Environmental Toxicants to review current practices in and recommend ways to improve the interpretation and uses of human biomonitoring for environmental toxicants.
This report defines biomonitoring as one method for assessing human exposure to chemicals by measuring the chemicals1 or their metabolites2 in human specimens, such as blood or urine (CDC 2005). A biologic indicator of exposure, or “biomarker” is a chemical, its metabolites, or the product of an interaction between the chemical and a target molecule or cell that is measured in an organism (WHO 2001; Metcalf and Orloff 2004).
BACKGROUND
Although biomonitoring has been used in the occupational-health arena since the 1890s to monitor exposure to lead (Sexton et al. 2004), it has recently become more widely used for many applications. Biomonitoring has tremendous utility, providing an efficient and cost-effective means of measuring exposure. Biomonitoring data—when used in conjunction with available epidemiology, toxicology, or pharmacokinetic modeling3 data— can estimate a dose to assess how much has been absorbed into the body and can provide a measure of health risk. When gathered for the U.S. population, biomonitoring data can help to identify new chemicals that are found in the environment and in human tissues, monitor changes in exposures, and establish the distribution of exposures among the general population. The data can also be used to identify populations, such as infants and children, that might have higher exposures than the general population. State and local officials can use biomonitoring data to help to assess environmental risks in specific sites or populations (GAO 2000). In occupational and clinical medicine, biomonitoring can be used as a surveillance tool to help to interpret a clinical problem or to monitor an exposure trend.
Several salient examples highlight the contribution of biomonitoring data to robust public-health decisions and regulations. A case example is the measurement of blood lead, which has been extensively studied. Advances in biomonitoring have allowed scientists to measure blood lead at low concentrations and to correlate these concentrations with adverse health effects. That has resulted in the lowering by the Centers for Disease Control and Prevention (CDC) of blood lead concentrations of concern to 10 µg/dL, although no threshold for effects has been identified (CDC 2005). Blood lead concentrations collected in the CDC National Health and Nutrition Examination Survey (NHANES) from 1976 to 1980 provided impetus for Environmental Protection Agency (EPA) regulations that reduced lead in gasoline, in part on the basis of declining blood lead that paralleled declining gasoline lead (GAO 2000; Jackson et al. 2002).
CURRENT BIOMONITORING ACTIVITIES
The widespread use of biomonitoring, as evidenced by reports citing chemical concentrations in human blood samples (CBRC 2005; IC Wales 2005; WWF 2002, 2003) or in initiatives for developing biomonitoring programs in such states as California and Minnesota (OMB Watch 2005; Risk Policy Report 2005), stems from improvement in analytic methods and laboratory techniques. It is possible to measure smaller concentrations of chemicals in the body and to do so with smaller quantities of biologic samples (such as blood and urine).
In 2001, 2003, and 2005, CDC published the First, Second, and Third Reports on Human Exposure to Environmental Chemicals. Those landmark publications reported the concentrations of chemicals and metabolites in blood and urine of a representative sample of the U.S. civilian population from NHANES, with the first report detailing 27 chemicals and the second and third 116 and 148 chemicals, respectively.
Other federal agencies have participated in biomonitoring efforts, including EPA, which sponsored the National Human Exposure Assessment Survey (NHEXAS) in the 1990s. NHEXAS stemmed from a recommendation in the 1991 National Research Council report Monitoring Human Tissues for Toxic Substances (NRC 1991) that the United States adopt a new program to monitor chemical residues in human tissues. NHEXAS, although not solely focused on biomonitoring, studied the exposure of hundreds of people in three areas of the United States to metals, pesticides, volatile organic compounds, and other chemicals. The survey was designed to evaluate the distribution of human exposure to multiple chemicals by multiple routes and from multiple sources and their association with environmental concentrations and personal activities. In addition, the National Children’s Study—sponsored by the National Institutes of Health, CDC, and EPA—is a national longitudinal study that, if funded, would have the potential to examine environmental influences on the health and development of more than 100,000 children across the United States, following them from before birth to the age of 21 years. The study would analyze a variety of environmental exposures, biomonitoring measures, and health effects (Needham et al. 2005).
CHALLENGES
Despite the powerful nature of biomonitoring (Wilhelm et al. 2004), the utility and interpretation of the data are controversial. The controversy stems in part from the fact that the pace at which biomonitoring data are being generated has eclipsed the development of basic epidemiology, toxicology, and exposure-assessment techniques that are needed to evaluate
whether a chemical measured in an individual or a population may cause a health risk and to determine its sources. Our technical ability to generate new biomonitoring data has essentially exceeded our ability to interpret them. Thus, it has become easier to measure chemicals or their metabolites in the body than to interpret or communicate the findings. As CDC states in its national-exposure reports, the findings of a chemical in people’s blood or urine does not necessarily mean that it causes a health risk or disease (CDC 2005). The challenge for public-health officials is to understand the health implications of the biomonitoring data and to craft appropriate public-health policy responses.
Biomonitoring data are more challenging to interpret than other exposure measures, such as personal air sampling or exposure diaries, in that they provide information on internal doses that are integrated across environmental pathways and routes of exposure and directly reflect the amount of chemicals that are absorbed into the blood and are distributed, stored, metabolized, and excreted. Therefore, not only must the complexities of the biologic system be considered, but also the properties of the chemicals or their metabolites.
Because biomonitoring studies typically measure a concentration of a chemical in a biologic medium (such as blood or urine) without knowledge of when exposure to it occurred, the properties of the chemical or its metabolite that affect how long it remains in the body are critical in trying to understand the biomonitoring results. For persistent chemicals (chemicals that remain in the body for months or years, such as lead in bone or lipophilic organic chemicals in adipose tissue), biomonitoring data provide information on what chemical and how much enters the body and accumulates; in most cases, biomonitoring data do not provide information on the timing, sources, or routes of exposure. For chemicals that remain in the body for shorter periods, biomonitoring data may be much more difficult to interpret; timing and duration of exposure become more critical to the interpretation (Needham et al. 2005).
The most important question for biomonitoring efforts to address is whether exposure to a chemical causes health effects. Few data are available on most of the chemicals measured in population studies, such as NHANES, to address that question (Metcalf and Orloff 2004). For example, the Government Accountability Office (GAO 2005) reports that EPA has limited data on the health and environmental risks posed by chemicals now used in commerce. A survey of risk-assessment practitioners on the extent to which biomarkers are used in risk assessment concluded that the absence of chemical-specific data (for example, toxicologic and epidemiologic data) was the primary limitation in using exposure biomarkers in risk assessment (Maier et al. 2004).
At a minimum, one would like to determine the exposure distribution in a population to establish a “reference range.”4 A reference range can be used to compare individual or subgroup results. With additional analyses, one can determine whether some members of the population have higher exposures than others. However, a biomonitoring value above the upper bound of the reference range does not necessarily mean that there is a health risk, just as a value below the lower bound of the reference range does not mean that there is no health risk.
Putting biomonitoring information into a health-based context is important. It can be done by comparing biomonitoring values with health-based values on the basis of a combination of physiologically based pharmacokinetic modeling and toxicologic and epidemiologic data (described in Chapter 5). As indicated above, very few health-based values have been established for biomonitored chemicals, and there are inherent uncertainties in estimating health risks (discussed further in Chapter 5).
As discussed in Chapter 6, communicating health-based values and other conclusions based on biomonitoring data requires a full discussion of the inherent strengths and limitations of the original data and the related risk conclusions.
Table 1-1 shows that of the 148 chemicals measured by CDC in its third exposure report (CDC 2005), only 25 have established EPA reference values—reference concentrations (RfCs) or reference doses (RfDs)—and/or cancer potency factors. With respect to the occupational arena, only a few have Threshold Limit Value–time weighted averages (TLV-TWAs) and biologic exposure indices (BEIs). Because of the challenges of putting results of biomonitoring studies into context and because human subjects are involved, it is critical to factor communication and ethical considerations into the design, identification, and recruitment of subjects, the handling and use of data, and interpretation of results, including any risk-analysis and risk-management decisions that stem from biomonitoring efforts. The personal nature of biomonitoring raises the bar on ethical and communication challenges because the mere fact that biomonitoring data are results of measurements in human specimens gives them the appearance of being more accurate than traditional sources of exposure information, such as questionnaires and environmental monitoring (Schulte and Sweeney 1995). The challenges include addressing the biomonitoring results on an individual vs group level, the variability within and among individuals, the issue of multiple chemical exposures, and the varied exposures among a population.
TABLE 1-1 Numbers of Chemicals in Third National Report on Human Exposures to Environmental Chemicals for Which Health-Based Values Are Available
|
148a |
Number of chemicals sampled by CDC in third national report |
|
25 |
Number of chemicals for which EPA reference values (i.e., RfCs or RfDs) and/or cancer slope factors are establishedb |
|
23 |
Number of chemicals for which TLV-TWAs are established |
|
5 |
Number of chemicals for which BEIs are established |
|
3 |
Number of chemicals for which RfDs/RfCs, TLVs, and BEIs are established |
|
aThe CDC measures 148 total analytes; however many are similar compounds that are members of a broader class of chemicals, such as polychlorinated biphenyls, dioxins and furans, organophosphorus pesticides, and heavy metals. bMany of the chemicals do not have specific health-based values, but because many are in similar classes of compounds, alternative approaches to evaluate toxicity, such as toxic equivalency factors, are available. Source: CDC 2005. RfC (reference concentration) is an estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. RfD (reference dose) is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. Cancer slope factors are upper bounds, approximating a 95% confidence limit, on the increased cancer risk posed by a lifetime exposure to a carcinogen (EPA 2006). TLV-TWA is the Threshold Limit Value–time weighted average concentration for a conventional 8-hour workday and a 40-hour workweek, to which it is believed that nearly all workers may be repeatedly exposed, day after day, without adverse effect (ACGIH 2001). In general, TLV-TWA values are used to derive BEIs (Biological Exposure Indices). BEIs are the concentrations of chemicals that are most likely to be observed in specimens (blood or urine) collected from healthy workers who have been exposed to chemicals to the same extent as workers with inhalation exposure at the TLV. The exceptions are the BEIs for chemicals for which the TLVs are based on protection against nonsystemic effects (such as irritation or respiratory impairment) where biomonitoring is desirable because of the potential for substantial absorption via an additional route of entry (usually the skin). The BEI generally indicates a concentration below which nearly all workers should not experience adverse health effects (ACGIH 2001). |
|
As Schulte and Sweeney (1995) state, “scientists like to think of gathering and interpreting data as being independent from the social and political context….” But where controversies surround the issue of health risks, as they do in the case of biomonitoring data, the communication and ethical aspects cannot be divorced from the use of the data.
The key challenges in interpreting and using biomonitoring data are summarized in Table 1-2.
TABLE 1-2 Challenges to Interpreting and Using Biomonitoring Data
|
Challenge |
Comment |
|
Data requirements |
Requires knowing properties of chemical or metabolite and complexities of biologic system |
|
Lack of toxicologic, epidemiologic, and toxicokinetic data |
Few data available to assist in determining whether presence of some concentration of a chemical in body may have health effect |
|
Lack of health-based values for comparison |
Few health-based values (such as RfDs, RfCs, cancer slope factors, or BEIs) are available to put biomonitoring results into context |
|
Interpreting data for exposure assessment |
Lack of data on sources of chemicals and how exposures occur makes efforts to identify and control exposures difficult |
|
Interpreting data for health risk |
Because of lack of data and absence of health-based values, it is possible to estimate health risks posed by only a very small fraction of chemicals that can be biomonitored |
|
Communication and ethical challenges |
Personal and complex biomonitoring data require careful planning, informed consent, information sharing, and evaluation to continue to move science of biomonitoring forward |
|
Evaluating and informing the policy process |
Once results from biomonitoring studies are understood, how can data be best used to evaluate and inform public-health decisions? |
BIOMARKERS AND BIOMONITORING
Biologic indicators or biomarkers generally include biochemical, molecular, genetic, immunologic, or physiologic signals of events in biologic systems. The events are depicted as a continuum between an external exposure to a chemical and resulting clinical effect (Schulte and Perera 1993). Biomarkers have traditionally been classified as markers of exposure, effect, or susceptibility (see Figure 1-1).
With respect to environmental chemicals, biomarkers of exposure, effect, and susceptibility are defined as follows (WHO 2001):
Biomarker of exposure. The chemical or its metabolite or the product of an interaction between a chemical and some target molecule or cell that is measured in a compartment in an organism.
Biomarker of effect. A measurable biochemical, physiologic, behavioral, or other alteration in an organism that, depending on the magnitude,
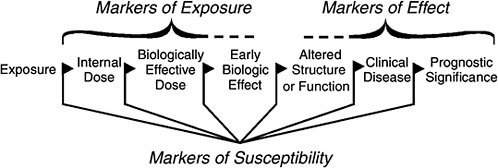
FIGURE 1-1 Simplified flow chart of classes of biomarkers. Source: NRC 1987.
can be recognized as associated with an established or possible health impairment or disease.
Biomarker of susceptibility. An indicator of an inherent or acquired ability of an organism to respond to the challenge of exposure to a specific chemical substance.
Biomonitoring in the context of this report focuses on biomarkers of exposure, that is, the early stages in the process: internal dose (ID), biologically effective dose (BED), and early biologic effect (EBE). ID is the amount of a chemical or its metabolite found in a biologic medium (NRC 1987). BED is the amount of a chemical or its metabolite that interacts with critical subcellular, cellular, and tissue targets. BED represents the integration of external exposure, pharmacokinetics, and interaction of an active chemical form with a target site, such as DNA or a key enzyme. EBE represents an event correlated with, and possibly predictive of, a health effect (for example, lymphocyte chromosomal aberrations, as this effect occurs in a cell type that can be readily screened for the response occurring in the target tissue) (NRC 1987; Schulte and Perera 1993).
Because biomarkers lie on a continuum, it may be difficult to delineate between biomarkers of exposure and effect (Morgan 1997). However, in considering biomarkers in relation to the information that they provide, it is critical to think about what is known about them in terms of exposure vs health effect. (This is discussed in further detail in Chapter 3.) In addition, as scientific knowledge increases, our understanding of where biomarkers lie on the continuum may change (NRC 1987).
This report focuses primarily on biomonitoring as used in population-based studies, such as CDC’s national reports on human exposure or EPA’s NHEXAS, because they raise the most far-reaching and challenging questions regarding the interpretation of biomonitoring data. The population-
based studies sample a large number of chemicals; however, few data are available on most of them to determine the health risks posed by an elevated or even background concentration in a blood or urine sample. Because biomarkers of ID raise the greatest interpretive challenges in the large population-based studies, they are the focus of this report; biomarkers of EBE and BED are equally important, but they are not addressed in detail here.
The committee acknowledges that there has been substantial research developing biomarkers further along the exposure-effect continuum, including prominent work by Gan et al. (2004); Hecht (2003); Joseph et al. (2005); Kensler et al. (2005); Qian et al. (1994); Rappaport et al. (2005); and Yu et al. (1995). The ultimate objective of the biomonitoring research is to link biomarkers of exposure to biomarkers of effect and susceptibility to understand the public-health implications of exposure to environmental chemicals.
Some consideration will also be given to other applications of biomonitoring, beyond population-based studies, because it is being used for myriad purposes, and such uses cannot be ignored. Those applications include the broad categories of risk assessment and management—scoping, evaluating status and trends, conducting exposure and health research, and risk assessment—and are expanded on in Chapter 3.
THE NATIONAL RESEARCH COUNCIL COMMITTEE
To address some of the challenges raised by biomonitoring data, Congress5 asked the National Academies to perform an independent study to identify key uncertainties in estimating exposure, health effects, and human risks potentially associated with biomonitoring and to develop a research agenda for interpreting human biomonitoring data.
In response, the National Research Council established the Committee on Human Biomonitoring for Environmental Toxicants, which prepared this report. Committee members were selected for their expertise in biomonitoring, analytic chemistry, public and environmental health, biostatistics, epidemiology, toxicology, dose-response modeling, toxicokinetics, exposure assessment, human health risk assessment, risk communication, and regulatory decision-making. Members come from universities and other organizations and serve pro bono. Committee members were asked to serve as individual experts, not as representatives of any organization.
The committee was charged with reviewing current practices and recommending ways to improve the interpretation and uses of human bio-
monitoring data on environmental chemicals. The report identifies key principles and uncertainties in estimating and interpreting chemical exposures and health risks from biomonitoring data. The committee was also tasked with developing an overall research agenda for addressing uncertainties to improve evaluations and characterizations of health risks and to improve tracking of changes potentially relevant to public health.
To address its task, the committee held four public sessions in which it heard presentations from officials of EPA’s Office of Research and Development; CDC’s National Center for Environmental Health, National Center for Health Statistics, and National Institute for Occupational Safety and Health; the Washington State Department of Health; the International Life Sciences biomonitoring committee; the American Chemistry Council; Crop-Life America; the Association of Public Health Laboratories; Environmental Defense; and academe.
In addressing its charge, the committee was mindful of several facts. The impetus for the study was the abundance of biomonitoring data that indicated that large numbers of the population have very low concentrations of chemicals in their bodies and that the data and methods needed to interpret these concentrations were not available. Addressing the questions posed by biomonitoring results will require the interdisciplinary collaboration of scientists, public-health officials, and experts in communication and ethics. Therefore, this report is aimed at a diverse audience, including the public-health and medical communities, policy-makers, the federal agencies that sponsor biomonitoring research, and the public.
ORGANIZATION OF THE REPORT
The body of this report is organized into six chapters. Chapter 2 presents an overview of biomonitoring efforts in the United States and internationally. Chapter 3 lays out a systematic framework to characterize the properties of biomarkers and their significance when they are used in biomonitoring studies. Chapter 4 discusses considerations necessary in the design of biomonitoring studies, including communications and ethics. Chapter 5 lays out approaches to interpreting biomonitoring data that depend on the information available. Chapter 6 highlights the challenges that public-health officials face in communicating the results of biomonitoring efforts. Chapter 7 presents a research agenda for improving the interpretation and utility of biomonitoring data.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2001. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 7th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
CBRC (Commonweal Biomonitoring Resources Center). 2005. Taking it all in: Documenting Chemical Pollution in Californians Through Biomonitoring. Commonweal Biomonitoring Resources Center, Bolinas, CA. August 30, 2005. 50pp [online]. Available: http://www.commonweal.org/programs/brc/Taking_It_All_In.html [accessed Nov. 16, 2005].
CDC (Centers for Disease Control and Prevention). 2003. Second National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.jhsph.edu/ephtcenter/Second%20Report.pdf [accessed Nov. 16, 2005].
CDC (Centers for Disease Control and Prevention). 2005. Third National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA [online]. Available: http://www.cdc.gov/exposurereport/3rd/ [accessed Nov. 16, 2005].
EPA (U.S. Environmental Protection Agency). 2006. Glossary of IRIS Terms. Integrated Risk Information System, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/iris/gloss8.htm [accessed May 12, 2006].
Gan, J., P.L. Skipper, M. Gago-Dominguez, K. Arakawa, R.K. Ross, M.C. Yu, and S.R. Tannenbaum. 2004. Alkylaniline-hemoglobin adducts and risk of non-smoking-related bladder cancer. J. Natl. Cancer Inst. 96(19):1425-1431.
GAO (U.S. General Accounting Office). 2000. Toxic Chemicals: Long-term Coordinated Strategy Needed to Measure Exposures in Humans. GAO/HEHS-00-80. U.S. General Accounting Office, Washington, DC. May 2000 [online]. Available: http://www.gao.gov/new.items/he00080.pdf. [accessed Nov. 16, 2005].
GAO (U.S. General Accounting Office). 2005. Chemical Regulation: Options Exist to Improve EPA’s Ability to Assess Health Risks and Manage its Chemical Review Program. GAO-05-458. U.S. General Accounting Office, Washington, DC. June 2005 [online]. Available: http://www.gao.gov/new.items/d05458.pdf [accessed Nov. 16, 2005].
Hecht, S.S. 2003. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 3(10):733-744.
IC Wales. 2005. Chemicals found in stars’ blood. IC Wales, IC Network, Trinity Mirror plc. May 16, 2005 [online]. Available: http://icwales.icnetwork.co.uk/0100news/0600uk/tm_objectid=15520784&method=full&siteid=50082&headline=chemicals-found-in-stars—blood-name_page.html [accessed Nov. 16, 2005].
Jackson, R., P. Locke, J. Pirkle, F.E. Thompson, and D. Sussman. 2002. Will biomonitoring change how we regulate toxic chemicals? J. Law Med. Ethics 30(3):177-183.
Joseph, A.M., S.S. Hecht, S.E. Murphy, S.G. Carmella, C.T. Le, Y. Zhang, S. Han, and D.K. Hatsukami. 2005. Relationship between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol. Biomarkers Prev. 14(12):2963-2968.
Kensler, T.W., J.G. Chen, P.A. Egner, J.W. Fahey, L.P. Jacobson, K.K. Stephenson, L. Ye, J.L. Coady, J.B. Wang, Y. Wu, Y. Sun, Q.N. Zhang, B.C. Zhang, Y.R. Zhu, G.S. Qian, S.G. Carmella, S.S. Hecht, L. Benning, S.J. Gange, J.D. Groopman, and P. Talalay. 2005. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 14(11 Pt 1):2605-2613.
Maier, A., R.E. Savage, Jr., and L.T. Haber. 2004. Assessing biomarker use in risk assessment: A survey of practitioners. J. Toxicol. Environ. Health A 67(8-10):687-695.
McNaught, A.D., and A. Wilkinson. 1997. Compendium of Chemical Terminology: IUPAC Recommendations, 2nd Ed. Malden, MA: Blackwell Science.
Metcalf, S.W., and K.G. Orloff. 2004. Biomarkers of exposure in community settings. J. Toxicol. Environ. Health A 67(8-10):715-726.
Morgan, M.S. 1997. The biological exposure indices: A key component in protecting workers from toxic chemicals. Environ. Health Perspect. 105(Suppl. 1):105-115.
Needham, L.L., H. Ozkaynak, R.M. Whyatt, D.B. Barr, R.Y. Wang, L. Naeher, G. Akland, T. Bahadori, A. Bradman, R. Fortmann, L.J. Liu, M. Morandi, M.K. O’Rourke, K. Thomas, J. Quackenboss, P.B. Ryan, and V. Zartarian. 2005. Exposure assessment in the National Children’s Study: Introduction. Environ. Health Perspect. 113(8):1076-1082.
NRC (National Research Council). 1987. Biologic markers in environmental health research. Environ Health Perspect. 74:3-9.
NRC (National Research Council). 1991. Monitoring Human Tissues for Toxic Substances. Washington, DC: National Academy Press.
OMB Watch. 2005. Minnesota considers “Biomonitoring” to protect public health. OMB Watcher 6(17) [online]. Available: http://www.ombwatch.org/article/articleview/3065/1/97?TopicID=1 [accessed Nov. 18, 2005].
Pirkle, J.L., L.L. Needham, and K. Sexton. 1995. Improving exposure assessments by monitoring human tissues for toxic chemicals. J. Expo. Anal. Environ. Epidemiol. 5(3): 405-424.
Qian, G.S., R.K. Ross, M.C. Yu, J.M. Yuan, Y.T. Gao, B.E. Henderson, G.N. Wogan, and J.D. Groopman. 1994. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 3:3-10.
Rappaport, S.M., S. Waidyanatha, K. Yeowell-O’Connell, N. Rothman, M.T. Smith, L. Zhang, Q. Qu, R. Shore, G. Li, and S. Yin. 2005. Protein adducts as biomarkers of human metabolism. Chem Biol Interact. 153-154:103-109.
Reddy, M.B., R.H. Yang, H.J. Clewell, and M.E. Andersen. 2005. Physiologically Based Pharmacokinetic Modeling: Science and Applications. Hoboken, NJ: John Wiley & Sons.
Risk Policy Report. 2005. California Biomonitoring Bill Clears Hurdle, but Faces Heavy Opposition. Inside EPA’s Risk Policy Report 12(13):9-10. April 5, 2005.
Schulte, P.A., and F.P. Perera, eds. 1993. Molecular Epidemiology: Principles and Practices. San Diego, CA: Academic Press.
Schulte, P.A., and M.H. Sweeney. 1995. Ethical considerations, confidentiality issues, rights of human subjects, and uses of monitoring data in research and regulation. Environ. Health Perspect. 103(Suppl. 3):69-74.
Sexton, K., L.L. Needham, and J.L. Pirkle. 2004. Human biomonitoring of environmental chemicals: Measuring chemicals in human tissue is the “gold standard” for assessing the people’s exposure to pollution. Am. Sci. 92(1):38-45.
WHO (World Health Organization). 2001. Biomarkers in Risk Assessment: Validity and Validation. Environmental Health Criteria 222. Geneva: World Health Organization. 238pp [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc222.htm [accessed Nov. 21, 2005].
Wilhelm, M., U. Ewers, and C. Schulz. 2004. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int. J. Hyg. Environ. Health 207(1):69-73.
WWF (World Wildlife Fund). 2002. Bad Blood? Survey of Chemicals in the Blood of European Ministers. Brussels: World Wildlife Fund [online]. Available: http://worldwildlife.org/toxics/pubs/badblood.pdf [accessed Nov. 18, 2005].
WWF (World Wildlife Fund). 2003. Contamination: The Results of WWF’s Biomonitoring Survey. Brussels: World Wildlife Fund [online]. Available: http://www.wwf.org.uk/filelibrary/pdf/biomonitoringresults.pdf [accessed Nov. 18, 2005].
Yu, M.C., R.K. Ross, K.K. Chan, B.E. Henderson, P.L. Skipper, S.R. Tannenbaum, and G.A. Coetzee. 1995. Glutathione S-transferase M1 genotype affects aminobiphenyl-hemoglobin adduct levels in white, black and Asian smokers and nonsmokers. Cancer Epidemiol. Biomarkers Prev. 4(8):861-864.












