4
Considerations in the Design of Biomonitoring Studies
In Chapter 3, the committee delineated a diverse array of uses to which biomonitoring studies are or will foreseeably be put to use (Box 3-1). Detailed discussion of the scientific approaches for each of them is beyond the scope of this report, but there are some issues in the design, conduct, and analysis of all biomonitoring studies for which the committee deemed review of scientific practice crucial to the further development of the field. The committee addresses these issues in this chapter.
Although the need for attention to scientific rigor attends every emerging technology in biomedical and environmental health science, and is thus not peculiar to biomonitoring, some aspects of biomonitoring demand special attention:
-
In developing biomarkers, there are no “gold standards” against which a result or finding can be readily evaluated; in most cases, biomonitoring will afford the first opportunity for scientists to assess even qualitatively the extent to which humans are exposed to, absorb, and might be harmed by innumerable contaminants of the human-made and natural environment.
-
Measured concentrations of biomarkers are often extremely low (in the 1 part per billion range or below) and subject to highly uncertain causes of variation, such as individual genetic differences, age, diet, habits, weather conditions, time of day, recent activity, medication and illness, and other exposures.
-
Compared with measures of contaminants in air, water, or food, biomonitoring results are intrinsically associated with a person and thereby have far greater potential to generate concern and action, for good or ill.
-
The social and political climate in which the new technology of biomonitoring has emerged is itself volatile; contentious and potentially fractious policy debates and litigation surround the field and render it likely that studies will be conducted or interpreted to meet the agendas of specific parties unless great care is taken to establish uniformly agreed on scientific standards against which any study can be transparently judged.
Figure 4-1 presents a schematic diagram of the various considerations in the design of a biomonitoring study addressed in the chapter as well as Chapters 5 and 6 and their relationship to one another. The four stages of any biomonitoring study are study design, study conduct, data analysis, and communication and implementation of results. Each stage incorporates several steps, which follow in chronologic order and are linked in Figure 4-1 by thick arrows. Several disciplines and processes, linked to the main steps by thin arrows, can be engaged in concurrently and are used to inform decisions made for the main steps. For example, biomarker selection and validation usually follow from study hypothesis and population selection, precede participant enrollment and consent, and are informed by statistical considerations, toxicokinetics, ethics, and communication. Study-population selection must take place before study inception. The main steps from population selection through statistical analysis are described in this chapter. Chapter 5 takes up interpretation of results, and Chapter 6 deals with communication of results.
In sum, the purpose of this chapter is to lay out—for the scientific, medical, legal, and policy communities—broad guidelines aimed at guaranteeing that biomonitoring studies will lead efficiently to identification of environmental contaminants that are causing risk or harm while elucidating sufficient information regarding pathways of exposure and health effects to guide their future control and will avoid the creation of widespread anxiety or apathy about contaminants whose potential for personal or societal risk appears not to warrant that reaction.
The discussion will proceed by reviewing the major issues in selection of biomarkers for study, developing the sampling strategy to answer the study questions, and assessing the communication and ethical considerations that must be addressed before the study is conducted. Next, the chapter will review the major considerations regarding the execution of the study, selection of the appropriate matrix (such as, blood or urine), collection of samples, transportation of samples to the laboratory, analysis of the samples, and banking of the specimens, when relevant, for future additional analyses. Finally, we review key considerations in the statistical analysis of the laboratory results.
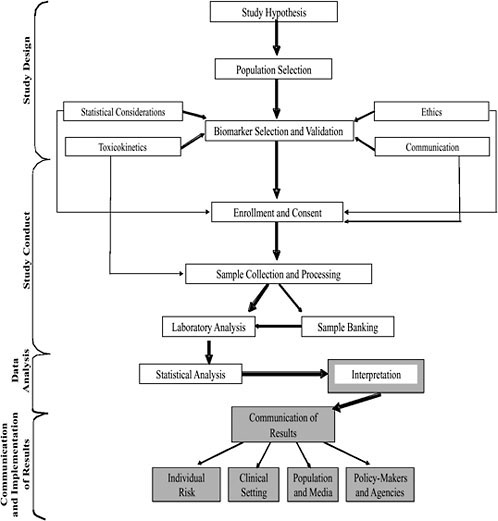
FIGURE 4-1 Stages of a biomonitoring study.
The committee deliberately incorporated discussion of communication and ethical considerations into this chapter not only because these issues present some of the most significant challenges with respect to interpretation and use of the biomonitoring data (key considerations in the committee’s charge), but because it was the committee’s intent to prompt readers to consider these issues as intrinsic in the design of biomonitoring studies.
STUDY DESIGN
Design of a biomonitoring study incorporates several key components, including consideration of the study hypothesis, the properties of the bio-
markers to be used, the selection of the population to be sampled, and ethical and communication issues. Each of those components will depend on the intended uses of the biomonitoring data.
Relevant Considerations in the Selection of Biomarkers
Several criteria must be considered in selecting a biomarker. The criteria—which include sensitivity, specificity, biologic relevance, and practicality—should be met regardless of the intended use of the biomarker (Metcalf and Orloff 2004; NRC 1991). However, rarely does a biomarker satisfy all the criteria (Metcalf and Orloff 2004). The relative strengths of the criteria for a particular biomarker should guide its applications, as discussed in Chapter 3. In addition to the criteria listed above, information on the kinetics of a biomarker is critical to its use (Bernard 1995).
A description of the criteria for biomarkers follows with illustrations of how they may influence a biomarker’s use.
Sensitivity
A biomarker should be capable of measuring a chemical or its metabolites after exposure. It should vary consistently and quantitatively with the extent of exposure (especially at low doses) (Bernard 1995; NRC 1987). However, exposures in community settings are typically lower than exposures in the occupational setting. So, for instance, in measuring chemicals in the workplace, the required limit of detection may be much higher than that needed for assessing environmental exposures in the general population.
Figure 4-2 illustrates the contribution of environmental and occupational exposure to biomarker concentrations and the effect of a method’s limit of detection, LOD (or limit of quantification), on potential uses of the biomarker. The example assumes that the biomarker’s concentration in a given biologic medium results from endogenous processes and from external exposure to a chemical. If the environmental (or community) exposure is sufficiently large compared with the endogenous contribution, then, given the variability of the latter, the environmental exposure might be reliably assessed, provided that the analytic method has sufficient sensitivity. In the graph, a method with a limit of detection LOD1 would be adequate, but not a method with a sensitivity of LOD2.
If workplace exposure to the chemical is large compared with both endogenous and community exposures, the biomarker could be very useful as a tool for preventing adverse health effects, even with the less sensitive analytic LOD2 method. There might also be cases where the extent of community exposure may overlap with occupational exposure and still
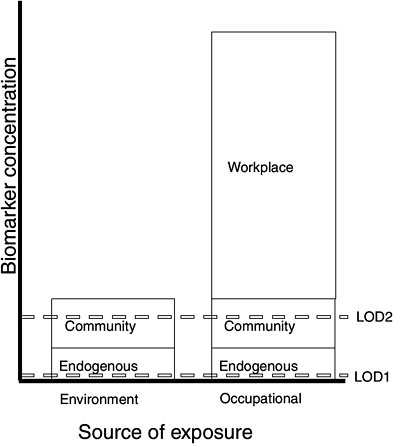
FIGURE 4-2 Contribution of exposures to biomarker concentrations and effect of limit of detection (LOD) on its potential uses.
others where the endogenous contribution to a biomarker concentration exceeds that of community exposure to the parent compound.
Specificity
The biomarker should be specific for the chemical or metabolites of interest; that is, it needs to be an unambiguous marker of exposure. Measurement of the unchanged parent chemical may have greater specificity than that of the metabolite, which may be common to several substances (Bernard and Lauwerys 1986). For example, if the metabolite of the parent chemical is being measured, the result may be equivocal if the same metabolite is produced endogenously or formed after exposure to other compounds. Occupational exposure to high air concentrations of benzene was formerly monitored by testing for its metabolite, phenol, in urine. However, the use of phenol to measure small environmental exposures to benzene is problematic in that many foods contain phenol, and the normal
catabolism of proteins in the body also gives rise to phenol excretion (Metcalf and Orloff 2004).
An example of a nonoccupational exposure is methanol, which is formed endogenously, probably as the result of the activities of intestinal flora or enzymatic processes. It is present in a number of consumer products. Methanol may be present in low concentrations in some foods, juices, and alcoholic beverages. Methanol can also be derived from the intestinal enzymatic hydrolysis of the artificial sweetener aspartame, which results in methanol absorption from the intestine (Butchko et al. 2002). It is estimated that a 355-mL serving of aspartame-sweetened beverages and of various fruit and tomato juices may contribute about 20-100 mg of dietary methanol (Butchko et al. 2002). For comparison purposes, exposure at the current Threshold Limit Value time-weighted average of methanol (262 mg/m3) would result in a daily dose of about 1,500 mg, assuming an 8-hour inhaled volume of 10 m3 of air and absorption of 57%.
Biologic Relevance
The biomarker should be relevant to the exposure-disease continuum. However, as discussed in Chapter 3, depending on the information that a particular biomarker provides, it is critical to consider what is known about it with respect to exposure vs health effect. As our scientific knowledge increases, our understanding of where biomarkers lie on the continuum may change (NRC 1987). That is made clear by Schulte and Talaska (1995), who define biologic relevance of markers as the extent to which they represent the underlying biologic event. The authors state that “without demonstration of a direct relationship to exposure and outcome, each biomarker study is actually a test of the biological relevance of the marker and adds to the web of association.”
However, biomarkers are being used even when there is little information on exposure or health effects. The classification of biomarkers described in Chapter 3 provides a useful framework for thinking about the characteristics of biomarkers that make them relevant and useful for various applications and provides an important assessment of potential research gaps.
Practicality
Several practical considerations are important in the collection and analysis of biologic samples. A sample should be readily obtainable, storable for a certain period, and capable of being analyzed. (More information on the choice of matrix and logistics of sample collection and processing will be presented later, under “Sample Collection and Processing.”) The
cost of analyses is a key consideration in that it often limits the number of participants in community investigations. For instance, analytic costs can be $15-20 for a simple blood analysis or $1,000-2,000 for an analysis of dioxin congeners (Metcalf and Orloff 2004).
In large population-surveillance studies, such as those used by the Centers for Disease Control and Prevention (CDC), biomonitoring is usually conducted on urine and blood samples. However, in research investigations, other matrices—such as breast milk, cord blood, and breath samples—may be used.
The specific biomarker used will also depend on its intended application and the population that is being sampled. For reasons of practicality and study participant convenience, most investigators collect first-morning voids or spot urine samples (Barr et al. 2005a). One recent study in children concluded that measurements of organophosphate metabolites in the first morning void more accurately represented total daily exposure than measurements in spot urine samples collected at other times during the day (Kissel et al. 2005). However, a first morning void specimen might seriously underestimate daily workplace exposure to a rapidly metabolized chemical, whereas one collected at the end of the workday would overestimate 24-hour exposure. Exposures that are episodic over a period of days might be missed entirely with either sampling regimen, but a spot urine sample could be representative of situations involving chronic exposures and intermittent exposures occurring on time scales less than the compound’s metabolic half-life (Barr et al. 2005a).
With regard to blood samples, for some analyses, such as dioxins, large samples of blood (70 mL or more) are required, and collecting this volume may eliminate some susceptible subpopulations, such as children and pregnant women (Metcalf and Orloff 2004). CDC’s National Center for Environmental Health does not collect blood samples on children less than 6 years old except to analyze lead and cadmium (and in the future mercury), because it is difficult to collect the necessary blood volume (J. Osterloh, CDC, personal commun., July 27, 2005).
Pharmacokinetics
A key consideration regarding the practical aspects of biomarkers is the pharmacokinetics of the chemical. The measure usually referred to is the half-life, which reflects both the affinity of the chemical for the biologic matrix and the efficiency of metabolic or elimination processes. Knowledge of half-life is important for several reasons, including its use in determining sampling time (Bernard 1995). For instance, chemicals with short half-lives (a few days or even a few hours)—including cotinine, phthalates, volatile
organic compounds, and current-generation pesticides—are rapidly eliminated from the body.
For chemicals with short half-lives, the biomonitoring result will reflect only very recent exposures, within the past several hours. As Figure 4-3 shows, the shorter the half-life, the more recent the exposure has to be for it to be detected in a biomonitoring sample. This makes the relative timing of the exposure vs the taking of the biomonitoring sample, a critical determinant of the biomonitoring result. Since the biomarker concentration decreases with time, knowledge of the time lapsed between exposure and sampling is needed to calculate the dose correctly. In practice, it is usually difficult, if not impossible, to tell with any accuracy when exposure occurred. Thus, variability in sampling time (in relation to exposure) introduces huge variability in dose estimates. Additional indexes of past exposures (such as, job classification and information from exposure questionnaires) may need to be collected (Bernard 1995). However, a biomarker with a short half-life can still provide a reliable internal dosimeter if exposures are relatively constant. Cotinine, for example, has a half-life of 15-40 hours in serum, but a single determination provides a good dosimeter of steady-state concentrations in people who have stable smoking habits (Kemmeren et al. 1994).
Biological half-lives of most phthalates are also short, on the order of hours, so that urinary metabolites likely reflect exposures over only the preceding day (Hauser et al. 2004). Nonetheless, monoester metabolites of four phthalates have been detected in greater than 75% of urine samples collected in the 1999-2000 NHANES (Silva et al. 2004) indicating that exposures in the United States are commonplace and frequent. In a study of five phthalate metabolites that were measured in repeat spot urine samples collected over three months from ten men (n = 90 samples), Hauser et al. (2004) concluded that the measurements of metabolite levels in a single spot urine sample were moderately predictive of exposures, with sensitivities and specificities ranging from 0.56 to 0.90 for the various phthalate metabolites for a single urine sample to predict the highest 3-month average. The measurement of the phthalate metabolites in two spot urine samples 1-3 months apart was sufficient to capture within person variability, considering both month-to-month and day-to-day variance (Hauser et al. 2004).
For chemicals with half-lives of months or years—such as dioxins, polychlorinated biphenyls (PCBs), polybrominated biphenyl ethers, and first-generation halogenated insecticides—biomarkers can detect exposures months or even years after they have occurred. Such lipophilic chemicals are usually measured in blood, and the principal exposure source is usually diet. After ingestion, they are readily absorbed into the blood supply; blood concentration then decreases rapidly as the blood supply equilibrates with
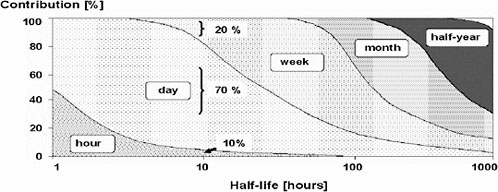
FIGURE 4-3 Effect of half-life on contributions of exposures during the last pre-sampling hour, day, week, month, and half-year to biologic levels of determinants. Half-lives were calculated by a one-compartmental model. For example, if the determinant is eliminated with a half-life of ten hours, the biological level mainly reflects the exposure on the day prior to sampling (contribution of 70%); to a relatively small extent, it reflects the exposure during the previous hour and week (contributions of 10% and 20%, respectively). Source: ACGIH 1995. Reprinted with permission; copyright 1995; American Conference of Governmental Industrial Hygienists.
lipid-rich tissues (Flesch-Janys et al. 1996). After the initial rapid equilibration, the concentration measured in a blood sample is related to body burden and so should not change substantially in the short term.
The importance of the biologic half-life is illustrated with a hypothetical example in Figure 4-4. A biomarker with a short half-life (such as 1 day, in the upper right graph) yields information only about the most recent exposures and does so only if the time of sampling in relation to exposure is known. For a biomarker with a long half-life (such as 1 year, in bottom right graph), the concentration continues to build up over time, so the total exposure duration (and age) of the person is a key factor. In this case, it may be difficult to follow exposure trends in the same people. In geographic population surveys, it may be advantageous to have intermediate half-lives (such as 1 month, in the bottom left graph), in which case a pseudo-steady-state is reached so that the biomarker concentrations reflects continuing average exposure with little influence from sporadic exposure peaks, age, or migration of people to the area under study.
The example given here is representative of many environmental pollutants that are ingested in food. The reasoning can be the same for biomonitoring of ambient air pollutants in the workplace, but the time scales would usually be minutes, hours, or days rather than days, months, or years. In conclusion, the half-life of the chemical needs to be considered in
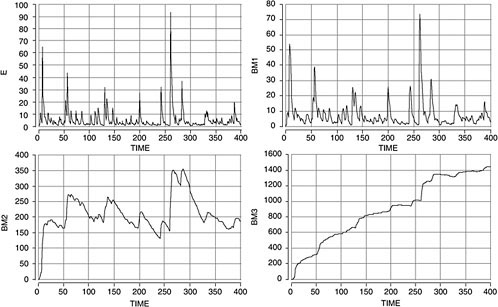
FIGURE 4-4 Influence of biologic half-life relationship between exposure level (E, upper left) and biomarker level (BM). Daily exposure levels were created by Monte Carlo sampling from auto-correlated lognormal distribution. Observation time is 400 days. Biomarker levels were calculated for three half-lives—1 day (upper right), 1 month (lower left), and 1 year (lower right)—with one-compartment pharmacokinetic model.
relation to the time spans of exposure duration, temporal variations in exposure, and sampling.
For the sake of simplicity, simple monophasic pharmacokinetics (one compartment and one half-life) was assumed in the above example and in many other examples in this report. In real life, most chemicals express biphasic or polyphasic pharmacokinetics (several compartments and several half-lives). Squeezing a polyphasic pharmacokinetic behavior into a one-compartment model by assuming a single half-life may lead to negligible errors for some chemicals and serious misinterpretation of biomarker concentrations for others. The same can be said about nonlinear processes, such as metabolic induction, inhibition, and saturation. A good way to check the accuracy of a simple pharmacokinetic model is to verify its performance by comparing with a physiologically based pharmacokinetic (PBPK) model that may encompass the mentioned factors.
In conclusion, great effort should be made to develop a human pharma-cokinetic, preferably PBPK, model early in the study design. The likely influence of, for example, model simplification (such as assuming a single half-life), metabolic saturation (see, e.g., Liira et al. 1990), and sampling time can then be addressed before investment of vast resources in sampling and analyses. By using statistical tools (Monte Carlo simulation and population models) in the model, one can examine additional features, including variability in exposure pattern (e.g., Nihlén and Johanson 1999) and intra-individual and interindividual variability in pharmacokinetic determinants, such as workload (e.g. Droz and Fernandez 1977), body build, and metabolic genotype (e.g., Jonsson and Johanson 2001).
Sampling of Populations
In exploratory investigations, studies of occupational groups, and clinical applications, the choice of subjects on whom biomonitoring should be done is generally straightforward. However, for most other applications (see Box 3-1), values of exogenous chemicals in blood or urine cannot practically be obtained from every member of the group of interest. Instead, researchers or public-health authorities have no choice but to obtain specimens on a sample of the population, from which statistical inferences will later be drawn regarding the (generally much larger) group as a whole. To be successful, the strategy, similar in concept to taking a poll before an election, must be performed in a scientifically valid and efficient way. For one thing, if results are to be extended across all age groups and both sexes, adequate numbers of males and females of all ages must be included in the sample. If there is reason to suspect the importance of some special factor as a determinant of levels, such as proximity to a source or dietary preference, people with various levels of such “risk factors” should be included.
But whether the purpose of a study is exposure surveillance or exploring cause-effect relations between an environmental chemical and ill health, “inclusiveness” alone is not sufficient to ensure that results can be usefully extrapolated to the larger group. The people chosen must also be generally representative of others in their age, sex, risk class, or any other characteristic considered a priori to be important. Polling organizations go to considerable lengths to achieve just that; the three waves of biomonitoring performed by CDC in the National Health and Nutrition Examination Survey (NHANES) are a model effort from this perspective. For various reasons, some scientifically justifiable and some not, some researchers choose samples of convenience. Convenience sampling tends to be the norm rather than the exception. For example, it is far more convenient to obtain the requested numbers of people in a small geographic area or to take specimens from people who appear at a clinic or hospital for unrelated reasons. Such groups may have the right mix of age, sex, and other characteristics specified, but they may be just convenient, and not representative of any larger group. Likewise, there have been numerous reports of groups assembled because they responded to solicitations to participate in studies of environmental effects. Although it may be possible to draw some insights from such groups of self-selected volunteers, they cannot be presumed to be representative of a population of interest, nor can any valid comparisons with unsampled members of the population be made.
Selection bias is the Achilles heel of such samples. Therefore when researchers use convenience samples for assessing population characteristics such as prevalence, incidence, or causal relationships, they must justify the validity of the sample.
At a minimum, when such convenience samples are reported, the strategy used for recruitment and selection must be made completely transparent and explicit so that scientists can assess the distortions or biases that may result from analyzing measurements in such groups as though they were true population samples. The committee recommends that if convenience samples are chosen, then funders, reviewers, and editors of peerreviewed journals must insist on complete characterization of how each sample was chosen so that misinterpretation—intentional or not—is less likely.
Even if those principles are rigorously adhered to, there remains in every situation an important degree of uncertainty because of random variation—who was sampled and who was not—so all results will ultimately need be expressed with respect to that uncertainty (see Statistical Analysis below). In general, the smaller the group sampled and the larger the variation in values because of interindividual differences and laboratory variation, the more uncertain the results will be. Two strategies should be used by researchers in assessing precision. If a single population is of interest (intrinsic inference),
means or other statistics, such as medians or interquartile ranges, can be estimated with a required degree of precision which determines sampling effort. If the comparison of two populations is of interest (comparative inference), the power of the proposed sampling strategy should be assessed in order to detect meaningful differences between the two populations. This will allow the study subjects, ethical review panels, and funding agencies to assess the likelihood that the study will answer the intended questions. The questions surrounding biomarkers will involve comparisons of populations; therefore the emphasis should be on statistical power.
The committee recommends that biomarker researchers, public or private, should adhere to appropriate statistical principles when sampling populations for biomonitoring. Editors of peer-reviewed journals as well as agency administrators and reviewers should insist on explicit attention to such information to minimize the possibility of incorrect inferences—even more while the biomonitoring field remains exploratory and public understanding remains incomplete.
Covariation–Socioeconomic Status, Race, and Ethnicity
As mentioned previously, one of the central dilemmas in the development of biomonitoring strategies is the lack of information about the factors that may determine which people have higher or lower concentrations of widely disseminated chemicals in their bodies. Little is known about how some people might be exposed to many of them, let alone how they are absorbed, metabolized, or excreted or what other genetic or environmental factors may modulate exposure. It is essential, therefore, that in all biomonitoring studies as much information as possible be obtained about each study subject, including not only the obvious factors—such as age, sex, and geographic location—but other relevant characteristics such as personal habits, lifestyle, and living circumstances whenever feasible. When biomonitoring results are presented in relation to each of those characteristics, valuable insights may be gleaned regarding sources of exposure and factors that modify exposure.
Covariates of particular importance are factors related to socioeconomic status (SES) and related demographic factors, including race and ethnicity. There are several reasons for collecting that information rigorously and displaying results of biomonitoring studies in a way that readily elucidates the relationship between biomarker concentrations and SES characteristics. Because income (or wealth), occupation (or social standing), and educational attainment may be closely associated with exposure, “stratification” of the data could provide clues about particular aspects of life that are most closely associated with magnitude of exposure. Such information may be important for understanding of the social distribution of whatever
potential health risk that exposure to the measured chemical may confer. There is widespread belief that environmental risks are not evenly shared by the population—that poorer people and ethnic minorities are subject to a disproportionate share of the potential harm (IOM 1999). Whether environmental injustice is typical of many chemicals or is limited to a few serious toxic chemicals, such as, lead, remains to be established. If biomonitoring data were consistently presented in association with SES data, the extent of such “maldistribution” might be clarified. Because of the sampling strategy, the first three waves of the CDC NHANES had, by design, sufficient power to provide all results in relation to race but not in relation to SES factors more generally.
For all those social and lifestyle-related factors, there is an even more important reason for rigorous attention to covariates during study design. The goal of biomonitoring is ultimately to identify the health effects of environmental factors so that risk can be controlled. Therefore, it is crucial that the relationship between health and environment not be “confounded” by other factors associated with both high biomarker concentrations and adverse health characteristics. Any factor associated with both a higher biomarker concentration and a disease of interest is a potential confounder. It is well established that the risk of many chronic diseases, including most heart and lung diseases and cancers, correlates inversely with education, income, and social position (Marmot et al. 1991); and it is likely that at least some biomarkers will, like lead, correlate directly with such factors as low standards of housing, job type, and diet. In tandem, those two relationships render it likely that cause-effect relationships may be erroneously inferred between some biomarkers and adverse health effects and that the wrong control measures will be chosen. Alternatively, it may turn out that one or more of the measured chemicals may actually explain an SES-health link or part of a link. But this possibility cannot be meaningfully evaluated without detailed knowledge of the relevant characteristics for each subject in biomonitoring or epidemiologic studies that use biomarkers.
The committee recommends that investigators, including CDC, that conduct surveys of biomarkers in the population should routinely collect detailed information about SES, lifestyle, and other cofactors on each subject and routinely present results organized so as to address the question of whether biomarker concentrations vary as a function of each. Epidemiologic analyses of biomarkers in relation to health should routinely include appropriate adjustments for such covariates.
Intra-individual and Interindividual Variability
In addition to SES, race, and ethnicity, biologic variation between and within people must be considered in sampling populations because at a
given exposure dose or dose rate, the true concentration (assuming no sampling or measurement error) may vary considerably between people owing to differences in pharmacokinetics (absorption, distribution, biotransformation, and excretion of a chemical). Most pharmacokinetic factors may also change within a person, causing variation within people. For example, the absorbed amount of inhaled pollutants varies with pulmonary ventilation, which in turn depends on body size and level of physical exercise. Fat-soluble chemicals tend to be redistributed to and accumulated in adipose tissues. The distribution of such substances depends on the amount of and blood flow to adipose tissue in the body. Body build and obesity depend on genetic factors, dietary habits, and exercise, all of which may vary between ethnic and socioeconomic groups and geographic regions. Accumulation in adipose tissue (or any other tissue) tends to lower a chemical’s concentration in blood and urine during exposure and increase the concentration after exposure, causing additional variation.
The rate of biotransformation of a chemical depends on the amount and efficiency of the pertinent biotransformation enzymes. Enzyme activity is partly genetically determined but may also vary between and within people because of enzyme induction caused by previous exposure to the same or related chemicals. Variation in enzyme activity may also be caused by enzyme inhibition due to concurrent exposures.
There is increasing concern for infants and children as susceptible sub-populations (Daston et al. 2004), in that differences in physiology and behavior can lead to higher exposures in young children than in adults. Young children eat, drink, and breathe more air per unit body weight than adults (Daston et al. 2004, NRC 1993), but children also differ from adults with respect to ratios of fat, muscle, and water; higher metabolic rates per unit of body weight; and immaturities in enzymatic systems (Ginsberg et al. 2004). Those differences can increase or decrease risk, depending on the mechanisms of action (Ginsberg et al. 2004).
An important aspect to consider in biomarker population variability is polymorphisms in the biotransformation enzymes. A polymorphism is typically defined as a genetic variant that appears in at least 1% of a population (Nebert 2000). Those genetic variants may in some cases produce enzymes that have higher or lower activity. Most of the more than five dozen human pharmacogenetic differences described represent alterations in the drug-metabolizing enzyme genes. Pharmacogenetic differences in metabolism can be striking, often 10- to more than 40-fold (Nebert 2000). One or both alleles1 may also be lacking. For example, in the case of glutathione transferase T1 (GST T1), people with two normal alleles for GST T1 will have
high enzyme activity, those with one normal and one lacking allele will have intermediate activity, and those with two absent alleles will have zero activity of GST T1 (Lof et al. 2000; Warholm et al. 1994). The frequencies of polymorphisms vary between ethnic groups and between different parts of the world (see for example, Kaneko et al. 1999; Mizutani 2003). As a consequence, the relationship between magnitude of exposure and biomarker concentration will vary. At present, there are limited data available on the relations between genotype and population variability in biotransformation rate of environmental toxicants. However, with the ongoing development of high-throughput techniques and pharmacogenomics, the knowledge in this field is likely to grow rapidly.
The influence of pharmacokinetic factors—such as physical exercise, body build, diet, and polymorphisms—on interindividual and intra-individual variability in biomarker concentration depends on the physico-chemical characteristics of the chemicals. The relationships are often difficult to describe with simple, general rules. It may be advantageous to use PBPK models to understand their influence. (These are described in Chapter 5 and Appendix B.)
Ethical Aspects of Biomonitoring
Despite the extensive experience with biomedical ethics, the infrastructure of institutional review boards (IRBs), and the equivalent for protection of human subjects (Schulte et al. 1997; but see Soskolne 1997), it is essential to address questions of ethics that may be particular to the design of biomonitoring studies. In this section, the committee considers some practical and research issues in biomonitoring ethics but makes no pretense that the list is exhaustive. Ethical issues can stop specific studies, and the field in general, dead in their tracks. Therefore, it is incumbent on investigators, policy-makers, and others to consider these issues carefully.
Practical Issues
First, IRBs’ scope—and thus potential protection for human subjects in biomonitoring studies—is not universal. Fairchild and Bayer (2004) noted with regard to the United States the “necessity of ethical review of public health surveillance activities at both state and federal levels, whether such activities fall neatly under the classification of research or practice or exist in a gray borderland.” Similar gray or black areas exist for many agencies that lack any IRB infrastructure or even awareness that it might be valuable, perhaps for some corporate and activist-group operations, and for for-profit biomonitoring firms soliciting business from individuals. The committee would encourage all entities that sponsor or conduct biomoni-
toring studies to either establish an in-house IRB or equivalent, or arrange with an external organization to provide that service, so that any gaps in formal ethical assessment and informed consent are filled.
Second, IRBs can become overzealous in insisting that biomonitoring data be anonymous rendering biomarker results untraceable to an individual and his or her personal characteristics. The only value in biomonitoring efforts in the long run is the ability to assess human health effects, and this makes it necessary to link sample results back to human health and risk-factor information. Unless a sample is taken solely to establish ranges in a population, no biomonitoring researcher should render samples useless by severing links between them and current or future health data. The European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC 2005) endorsed the notion that anonymous data are “problematic” if they concern a treatable or preventable health impairment, but the committee considers that the problem is larger and could present a major barrier to progress in biomonitoring. IRBs and researchers have enough experience with methods for keeping personal data confidential—for example, the pharmaceutical industry’s use of double coding, recommended by ECETOC (2005)—for this allowance for biomonitoring data to be neither unethical nor impractical even without the support offered by legislation in some countries (such as the Health Insurance Portability and Accountability Act in the United States, HIPAA).
Third, a generic warning about potential consequences of group results and the significance of personal measurements should be a default provision in informed-consent forms for biomonitoring studies in general. For example, if a biomonitoring study reveals that the internal dose of a population living near a polluting plant has reached intolerable levels, a policy decision might be made to close the plant or move neighboring residents. It is crucial that health, economic, regulatory, and other ramifications for all potential constituencies be considered by both study designers and study subjects before the study begins. In practice, that may be difficult, particularly when results of a “research” study never intended to provide “policy” advice are used by policy-makers—with or without the concurrence of researchers—to decide such major issues. However, difficulty is not a proper reason for limiting ethical obligations. A similar situation arises when an individual learns of an elevated concentration of the chemical in the body, but the relationship to health effects is not known. The committee considers that it is better for the individual to possess this information than not, as sampling in the individual provides very valuable information for population-based exposure studies that can advance public-health knowledge and ultimately benefit the individual.
Fourth, despite debates over what to tell study subjects (e.g., Schulte et al. 1997; Veach et al. 2001; Grill and Hansson 2005; Kass 2005), the
committee considers that subjects should be told (or offered the chance to be told) whatever researchers know (or do not know—see uncertainty discussion in Chapter 6) about the topic. If the meaning of results for potential health effects is clear, but potential options are not obvious (for example, because biomarkers are from groups V-VII, for which data on external exposure pathways or sources may not be available—see Chapter 3), the disclosure method used for some kinds of genetic testing should be considered. For Huntington’s disease, for example, genetic testing can with high confidence confirm whether an individual will get this fatal disease, but there is no known treatment or cure. As a result, people with a family history of the disease vary widely in whether they choose to get the test (for instance, “I want to know so I can be prepared” vs “What good does it do to know if nothing can be done?”). Giving biomonitoring research subjects the same option is desirable. In other situations (such as for group III or IV substances), the health implications of biomarker concentrations for an individual may be unclear, but possible actions are obvious, such as when chemical exposure can be easily reduced by individuals’ actions. In this case, the relative contribution of different sources to exposure or of different actions to exposure reduction should be explained. If there is some uncertainty about the information, it could be accompanied by a warning that action may be wasteful or counterproductive from the perspective of the individual subject. Therefore, understanding the subjects’ mental models about biomonitoring (see Chapter 6) is an important aspect to obtaining true informed consent. Empirical research on this topic will be necessary to improve the conduct of biomonitoring studies from the ethical perspective.
Fifth, studies proposing to sample human tissues must carefully lay out tradeoffs—without exaggerating either benefits or risks implied by the sampling, the body tissue, or sources of biomarker chemicals—in both consent forms and reports of final results. The challenge of how to search for and convey potential dangers without ignoring or downplaying potential benefits of risk-bearing activities is exemplified by efforts to use breast milk for biomonitoring. Concerns over how to communicate risks vs benefits of breastfeeding in 2005 helped to block recent California legislation on biomonitoring. Breastfeeding offers great benefits of nutrition and immunity to infants but also might transfer a relatively high concentration of chemicals to those infants at a vulnerable point in their development. Given the undeniable benefits of breast-feeding and the fact that a substantial portion of babies in the United States do not receive them now, poorly designed and publicized breast-milk biomonitoring studies could yield more harm than good. Yet breast-milk studies should be conducted, because little can be done to keep chemicals out of breast milk without such information (Risk Policy Report 2001), and a single measurement provides the chemical-exposure profile for the mother and the exposure dose for the fetus. This
issue is more challenging than warning people about mercury or PCB exposures from eating specific fish species without discouraging their obtaining the benefits of fish oils. There are benefit-equivalent but lower-risk alternatives for fish that are not available for breast milk. However, the mercury analogy underscores the importance of identifying and conveying guidance to these mothers on potential sources of chemicals in their breast milk so that (as with fish consumers) choices can be made about how to reduce exposures.
Sixth, committee deliberations raised two ethical issues whose occurrence, seriousness, and resolution were uncertain but that deserve tracking and possible future practical or research action. Only open discussion between scientists and ethicists can promote consensus on the issues, and the committee would be remiss if it did not encourage that discussion by mentioning them here. One question is how to report and respond to those with higher exposure levels when health implications are unknown. Secondly, is it ever appropriate to “require” biomonitoring of higher risk populations such as those occupationally exposed?
Research Needs
There are three high-priority research needs for biomonitoring ethics. The first is for research that develops, evaluates, and disseminates methods for ethically and practically informing subjects (during both recruitment and debriefing) in studies that use high-output, high-throughput technologies. For studies that develop a biomarker, researchers should provide as much information as possible on the biomarker and its parent chemical. Currently, participants in a public-health study measuring hundreds of biomarkers might give “informed consent” only with respect to the general objective of the study (although some biomarkers might not be fully validated). Detailed discussion of each biomarker is prohibitive. However, failing to provide more detailed information about all biomarkers to be measured, no matter how many chemicals are involved in the study, raises ethical questions that merit consideration.
The second research recommendation is for the development of new methods for obtaining blanket consent for future uses of biomonitoring data that are ethical and practical. Concern that blanket consent (for instance, for future testing of tissue samples with genomic or metabonomic assays that are not available at the time of study recruitment) would be open to abuse has increased the difficulty of obtaining IRB approval. The committee is sympathetic to such concerns but is aware of the ethical (and practical) problems posed by the undue replication of tissue sampling that this implicit ban forces as each new sample application is imagined. In pursuing this research, it would be prudent to assess “mental models” of
various constituencies (see Chapter 6) about blanket consent in the context of biomonitoring. In addition to understanding the beliefs and concerns of IRBs about blanket consent and how they might overlap with those about anonymizing data (see previous section), this step could inform development of blanket-consent approaches that are as widely understood and acceptable as possible.
The third research recommendation is to conduct empirical research on the mental models of the general population about biomonitoring, as also recommended in Chapter 6 for the purpose of communication results, interpretations, and uses. One ethical reason for this research would be to understand the population’s concerns about participating in biomonitoring studies, since participation rates in some countries have been depressed to the extent that the apparent need for incentives could compromise ethics if incentives are too high. Research on biomonitoring concerns can address these issues, as well as help ensure that better informed consent will be obtained from biomonitoring study participants.
Two issues have been identified in addition to those three high-priority research needs. Better understanding is required on how the framing of research, through the language used, can affect subject recruitment, policy implications of study results, and other applications (Schulte and Sweeney 1995). For example, inhalation exposure to a solvent in an occupational setting can often be assessed by roughly equivalent measures for the purpose of controlling exposure: measuring its atmospheric concentration or a urinary metabolite. But the fact that a urinary metabolite is a “biologic” substance sometimes creates far different legal and ethical obligations from its equivalent “chemical” counterpart. A biomarker concentration considerably below guideline levels might have to be declared to public-health authorities, whereas there is no such obligation regarding air concentrations even just below legal limits. In another example of language’s power to bias responses, some people may refuse to participate in a study because of the methods and terms used—for example, urine connotes testing for illegal drugs, “genetic” connotes a revelation of health problems to insurers—thus potentially affecting a study’s outcome or even whether it is feasible. Research that directly examines the extant framings (intended and unintended) and alternative framings of biomonitoring by researchers, policy-makers, stakeholders, and the mass media will help to advance the field and encourage all parties to consider possibilities that might otherwise not be apparent in biomonitoring ethics and communications.
Finally, it is important that biomonitoring projects document (in widely available reports or in the communications database discussed in Chapter 6) the ethical challenges and solutions (and evaluations of those solutions) that they face. Biomonitoring sponsors could aid in this task by requiring that project proposals address how they will document the issues. The data can
then become grist for research that identifies potential new ethical challenges and procedures. Just as all future uses of biomonitoring tissue samples cannot be envisaged, neither can all ethical issues related to biomonitoring. Ethics documentation provides a resource that may be productively mined for further illumination of this important but all-too-neglected field.
Communication in Biomonitoring Design
Communication is usually seen as a late stage in environmental management: a biomonitoring study, risk assessment, or project or policy decision has been produced, and results must be conveyed to decision-makers and observers. (This is covered in Chapter 6.) Before then, scientific research and public-health investigations focus on “doing the science right” (Stern and Fineberg 1996). Yet early incorporation of communication issues into study design can greatly enhance biomonitoring’s value for every biomarker group (Table 3-1). While study purpose and design can certainly influence the content and means of communicating study results, anticipation of communication issues can—and often should—influence study purpose and design. It is for this reason that communication themes discussed below, including constituency assessment, consideration of partnerships, and planning for evaluation, are crucial to the design of a biomonitoring study, as they cannot be easily or effectively incorporated at the end of the study. Therefore the committee recommends that every biomonitoring project explicitly include communication issues in project design.
Two issues critical for designing biomonitoring studies are early assessment of constituency2 views and planning for evaluation of communication effectiveness.
Early Constituency Assessment
Careful consideration of project goals early in the design can help to identify the nature and scope of likely communication goals and problems and thus begin to identify solutions (Pflugh et al. 1994; see Schulte 2005, on biomonitoring specifically). Project goals and communication goals are not identical (Pflugh et al. 1994), and early thinking through of how project and communication goals might support or conflict with each other can improve the eventual success of both. Project and communication goals are
|
2 |
Some people might prefer the term audience(s) for those considered in biomonitoring-project design, and those targeted for messages about biomonitoring results (Chapter 6). But audience implies passive receipt of information, which is rare and often undesirable in communication. “Constituents” vary widely in the type and quantity of their activity, including being passive audiences, but this term more accurately conveys the appropriate tone. |
intimately entwined with the constituencies that will be served by or interested in study results; constituencies and goals mutually determine each other, rather than having a simple one-way link. The constituencies include, at a minimum, study subjects, scientific peers, sponsors, stakeholders, policy-makers (European Commission 2004), the supporting community and larger publics, and the mass media, although these distinctions are somewhat artificial. The intended constituencies (those which project managers tend to plan for) need not include all desirable or actual recipients of project communication, so potential “unintended” constituencies should be planned for as well (with the aim of turning them into intended ones).
Assessment of constituencies entails, at a minimum, identifying their beliefs, attitudes, relevant values, behaviors and behavioral intentions, concerns and questions. Biomonitoring designers can use this information to determine whether and how project design could answer potential constituencies’ questions and concerns, and revise the design to improve its ability to meet those needs. That will make their studies more robust in the face of criticisms of poor design or irrelevance, focus disputes on interpretation of data rather than on missing data, and aid the development of appropriate informed-consent procedures and content (Kass 2005). If detailed enough, it can yield the foundation for specific, effective messages about study results (see the “mental models” discussion in Chapter 6). Guides for understanding audience concerns and the communication context (also see Chapter 6) include both overviews of communication planning (Pflugh et al. 1994) and specific assessment methods, such as methods that help to grasp social and cultural aspects of community-based environmental protection (EPA 2002). A given biomonitoring project need not address every possible question of every possible audience, nor will all biomonitoring projects evoke identical interest or questions. However, most biomonitoring research will have eventual applications; researchers who think they will not need to communicate and thus ignore constituency assessment are likely to be unpleasantly surprised.
Practical Issues
First, biomonitoring funders should require communication planning in any application for funding. It is hoped that investigators would spontaneously recognize the value of communication planning and constituency assessment, but sponsor requirements for proposal and study content would clearly signal their importance.
Second, the biomonitoring communication database (see Chapter 6) should include results of studies’ experiences: biomonitoring reveals the questions, concerns, beliefs, and attitudes of various constituencies for biomonitoring data, and each practitioner need not reinvent this knowledge
anew. There is likely to be enough overlap in these issues—and how to address them in project design—across projects to make such a database invaluable for later funders and researchers. Relevant data would include the constituency questions considered, the sources of the questions (such as, direct interviews with constituency members on researcher assumptions), and their effects (if any) on project design.
Third, the often tense political and social context in which biomonitoring studies are conducted (see Chapter 4 introduction) suggests that some such studies could benefit from incorporating joint decision-making. This partnership with study subjects or stakeholders in project design is one example of analytic-deliberative processes for environmental decision-making (Stern and Fineberg 1996). The Community-Based Environmental Protection program of the Environmental Protection Agency sponsors one kind of such project-design partnerships; “the community” (or a community association) is not the only or best candidate for all biomonitoring studies, and the “partnership” can vary widely in how much control the nonresearcher partners have. These processes recognize that effective identification of values and application of science pertinent to the values are both essential to good decisions and bring scientists, officials, stakeholders, and citizens together to pursue these goals. Partnerships in policy decision-making are more common, but partnerships in defining research agendas and designs can heighten the breadth and quality of research, build confidence in the research process, and make the results more credible and more likely to be used (e.g., Stern and Fineberg 1996; Stinson and Ehrmann 1998; Ehrmann and Stinson 1999; Lasker and Weiss 2003; Adler 2002; Andrews 2002; Beecher et al. 2005). Among the largest challenges for either implementation or success of analytic-deliberative processes are erroneous beliefs “that analysis = science and deliberation = participation” and “that we analyze only facts but deliberate only about values” (Webler 1998; emphasis in original). Other issues that deserve early consideration include large power and expertise imbalances among the potential partners (for example, are study sponsors and partners willing and able to set up a process that minimizes the imbalances?) and whether some benefits of “joint fact-finding” might be produced largely by ensuring that constituency questions are answered by study design (see above). Some constituencies might be represented indirectly by other partners (for example, unions might stand in for workers in occupational studies) but only when study constituencies have interests that can be adequately represented.
Research Recommendations
First, research should be conducted on the best ways to link constituency assessment, project design, and constituency satisfaction and other
evaluation criteria (see next section). Whether it consists of independent empirical studies or is based on analysis of biomonitoring communication database contents, this research would allow a step back from the ad hoc response of individual biomonitoring projects to examine the larger picture. For example, some quick, low-resource assessment methods may be quite useful, whereas others could be misleading about constituency views compared with more intensive methods; some project designs could reliably produce successful outcomes, whereas others might work not at all or only in unique situations. It would be too much to assume that individual investigators could garner that information themselves.
Second, although there is some work on design of analytic-deliberative processes, it is recognized that much more research is needed to understand how to improve their functioning, particularly as the scientific proportion of issue analysis—as in biomonitoring studies—increases (Stern and Fineberg 1996). As noted above, joint fact-finding and other efforts at partnerships in designing scientific studies are less understood than policy-focused analytic-deliberative processes. The committee considers that structuring research projects around such processes applied specifically to biomonitoring issues would be valuable. Research projects around nonbiomonitoring partnerships are likely to be few, with unknown generalizability to biomonitoring, so it would be prudent for biomonitoring sponsors and investigators to pursue this research themselves rather than wait for enlightenment from other fields.
Designing for Communication Evaluation
A second main reason to consider communication in project design is that this is the time to plan for evaluation of communication. There is inevitable interaction between communication goals and evaluations. One needs to know the goals to decide how to measure success or failure in achieving them; if achievement of the goals cannot be validly or reliably measured, goals need to be reframed or revised, because this problem implies that goals have not been fully thought out. Thus, planning for evaluation during or after project implementation is likely to be too late to be useful. An early start also enhances the likelihood that midcourse evaluations can feed into regular reassessments of goals (and possible changes to them), just as goal revision during a project feeds into changes to communication practice and evaluation (Pflugh et al. 1994).
Ultimately, success will be defined by a particular study’s goals and thus vary from one study to another. Table 4-1 outlines two approaches for determining and measuring criteria of success adaptable to specific projects: a goal-based approach proposed by Weinstein and Sandman (1993; also see Rohrmann 1992) and a process-based approach focusing more on whether
TABLE 4-1 Goal-Based and Process-Based Criteria for Evaluating Communication
|
Goal-Baseda |
Process-Basedb |
|
Comprehension: Message content is understood Agreement: Interpretation or recommendation in message gains assent Dose-response consistency: Greater risk perception or readiness for action among those who face a higher dose of a hazard Hazard-response consistency: Greater risk perception or readiness for action for a higher-risk hazard than for a lower-risk hazard Uniformity: Similar responses among those facing the same level of risk Audience evaluation: Message’s perceived utility, clarity, credibility, helpfulness, accuracy, and so on Types of communication failures: Whether types that occur are more acceptable, given communication goals |
Exposure: Message received by audience Attention: Message read or heard Comprehension: Message content is understood Confirmation (optional): Complementary information sought from other sources Acceptance: Interpretation or recommendation in message gains assent Retention: Message content memorized so that it can be retrieved when needed Realization: Audience action complies with the message |
|
aAdapted from Weinstein and Sandman 1993. bAdapted from Rohrmann 2000, Figure 2. |
|
intermediary steps to comprehension, agreement, and so on are being met (Rohrmann 2000; also see Rohrmann 1992), although these methods often also include goal-based criteria. Further details on these approaches can be obtained from the cited references; Balch and Sutton (1995) also offer suggestions for providing government agencies useful communication evaluations.
Practical Issues
Quality assurance and quality control of biomarker data get far more attention than whether biomonitoring communication goals are achieved and why (Balch and Sutton 1995), despite the importance of both issues. The committee suggests practical ways to improve biomonitoring evaluation below.
First, the benefits of incorporating evaluation planning into project design are more likely if it is explicitly funded as a separate task. Funders should require discussion of communication evaluation in proposals for funding; researchers should explicitly remind sponsors that these are important tasks by including them in proposals even when this is unsolicited. If neither group explicitly includes evaluation tasks in the funding application process, the inadequate status quo will endure (Balch and Sutton 1995).
Second, project designers should consider defining communication success more broadly than by criteria of the study’s manager or sponsor or solely with goal-based and process-based “theoretical” criteria. Santos and Chess (2003) showed in evaluating citizen advisory boards that such participants as citizens, officials of different agencies, and activists had criteria for success differing from each other and from those derived from theory. The authors concluded that “using both theoretical and participant-based criteria for evaluation can provide more comprehensive feedback than either method alone” although they noted a need for evidence that mixing these criteria “enhances the legitimacy of the effort and increases use of the evaluation.”
Third, the committee recommends that biomonitoring sponsors and researchers consider development of a standard set of criteria and measures to be applied as a default (in addition to study-specific criteria and measures) so as to compare the relative effectiveness of different communication approaches (Santos and Chess 2003).
Fourth, the biomonitoring communication database (Chapter 6) should include study-specific communication methods, evaluation planning, and evaluation results. Little health or risk communication research is currently relevant to biomonitoring (Chapter 6), and this condition is likely to endure if it is shaped solely by investigator-driven communication research. The large and useful literature on evaluation methods and issues (e.g., Comfort 1985; Fisher et al. 1991; Rohrmann 1992; Tinker 1994) does not identify problems and solutions peculiar to biomonitoring. Knowing messages and methods of communication that work, and why, would be invaluable to projects with similar communication goals or biomonitoring data (e.g., see Schulte et al. 1997 on evaluating notifications of individual results).
Research Recommendation
The biomonitoring communication database (Chapter 6) should be used to identify “good” evaluation criteria and feed into development of the “standard” default criteria and measures recommended above. For example, research could contrast goal- vs process-based evaluation criteria, or theory-, investigator-, and constituent-derived criteria, in terms of their ease of elicitation or use or utility in reliably predicting successful commu-
nication. In addition, evaluation research might identify kinds of study goals or designs that are less successful than others, motivating further research on how they might be improved or whether these goals or designs should be modified or abandoned.
Incorporating communication into project planning and design is not difficult, futile, or irrelevant to biomonitoring but is an essential element. Careful attention to effective communication at the beginning of a biomonitoring study will pay off with much easier communication at the end and may even make the technical side of the project more effective.
STUDY CONDUCT
Execution of the study consists of collecting the biologic samples (including considerations regarding sampling time and quality assurance, depending on the matrix—such as blood or urine—used), transporting them from the field to the laboratory, and processing them in the laboratory. If samples are to be saved for future analyses, biobanking may be a consideration.
Sample Collection and Processing
Choice of Specimen
Historically, adipose tissue has been considered the most appropriate matrix for biomonitoring focused on persistent chemicals that bioaccumulate in fatty tissues (see Chapter 2 for more details). In the last 15-20 years, blood and urine have been used more commonly.
The 1991 National Research Council report Monitoring Human Tissues for Toxic Substances recommended that any new program to assay chemical concentrations in tissues of the U.S. population be based primarily on analysis of blood. The use of blood permits sampling of a wider sector of the population, better comparison of exposed populations with national averages, repeat sampling of persons who have high tissue concentrations, and opportunities to follow chemical clearance with time. The 1991 report also advised analysis of adipose tissue (especially for persistent pesticides) that would provide continuity with previous studies and confirmation that a survey based on blood also detects important tissue residues of persistent chemicals (NRC 1991).
It is not feasible to study a wide array of tissues in a general population sample, so it is important to identify tissues that most accurately account for the body burden of most of the chemicals of concern. Figure 4-5 shows the main routes of exposure and the matrices available for analysis of biomarkers based on the metabolism and pattern of bioaccumulation and
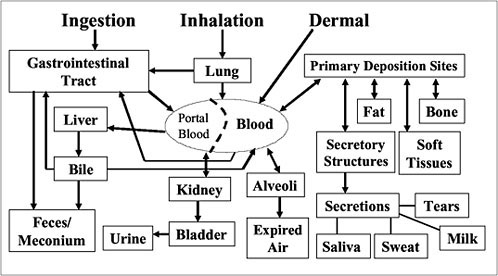
FIGURE 4-5 Pharmacokinetics of environmental chemicals in body and what matrices are available for analyses. Source: Needham et al. 2005.
excretion (that is, feces or meconium, urine, expired air, saliva, sweat, and milk). The choice of matrix for specific biomonitoring depends on the chemical of interest, its persistence, and its deposition.
Not all matrices are readily available for large population studies and for all age groups (Table 4-2). For example, breast milk can be collected only for several days or months and only from lactating women. Cord blood and meconium, which both reflect intrauterine fetal exposure, can be collected immediately after or shortly after delivery.
Matrix choice is also informed by the invasiveness of collection and whether it is appropriate at a given life stage. Urine is one of the least invasive matrices to collect, but its amount varies widely, from several milliliters in very young infants (collected with specially designed diapers) to several dozen milliliters in 2- to 4-year-old children who are already toilet-trained and can use disposable collection units. Finally, in older children and adults, the amount of urine is no longer a limiting factor, and the main focus in consideration of study design shifts to the timing of collection. For example, a morning void may be needed to acquire a more concentrated sample for low-level chronic exposures, and the end of the work shift can be more appropriate if the goal of the study is to monitor occupational exposure. Urine serves as a route and medium of elimination for many chemicals, especially nonpersistent chemicals (chemicals with short biologic half-lives); the persistent chemicals are eliminated primarily in feces. The nonpersistent chemicals are generally found in the urine not only
TABLE 4-2 Matrices for Biomonitoring Studies
|
Collection |
Stages of Life |
||||||
|
Matrices |
Procedure |
Invasive Yes/No |
Fetal Period |
Delivery |
Children 0-5 years old |
Children 5-18 years old |
Adults |
|
Blood |
Venipuncture or prick |
Y |
– |
– |
+ |
+ |
+ |
|
Cord blood |
Drained into sterile container from cord after delivery |
N |
– |
+ |
– |
– |
– |
|
Urine |
Collection cups or diapers |
N |
– |
– |
+ |
+ |
+ |
|
Saliva |
Sterile plastic pipette or specially prepared cotton swab |
N |
– |
– |
+ |
+ |
+ |
|
Expired air |
Spirometer attachment |
N |
– |
– |
+ |
+ |
+ |
|
Hair |
In container after cut or falling out |
N |
– |
– |
+ |
+ |
+ |
|
Fingernails |
Clippings in sterile container |
N |
– |
– |
+ |
+ |
+ |
|
Teeth |
Collected in sterile container after loss or extraction |
N |
– |
– |
+ |
+ |
+ |
|
Meconium |
Collected from diapers |
N |
– |
+ |
– |
– |
– |
|
Amniotic fluid |
Amniocentesis (mother) |
Y |
+ |
– |
– |
– |
– |
|
Adipose tissue |
Biopsy or postmortem collection |
Y |
– |
– |
+ |
+ |
+ |
|
Bone marrow |
Spinal tap |
Y |
– |
– |
+ |
+ |
+ |
|
Breast milk |
Breast pump |
N |
– |
– |
– |
– |
+ |
|
Semen |
Cup |
N |
– |
– |
– |
– |
+ |
|
Feces |
Container |
N |
– |
– |
– |
– |
+ |
as their original “parent” structure but more frequently as metabolites. Urine is an unregulated body fluid, and it varies from void to void in volume and in the concentration of endogenous and exogenous chemicals (Barr et al. 2005a; Wessels et al. 2003). That may be less true of very young children (less than12 months old), because they feed and urinate frequently; but variability in urinary dilution in this age group has not been evaluated. Creatinine adjustment of urinary metabolites has been the standard method for accounting for urine dilution. However, urinary creatinine concentrations vary with age, sex, race and ethnicity, and body-mass index (Barr et al. 2005b). Adjustments of urinary pesticide concentrations with creatinine therefore may not be appropriate in pregnant women and children. A recent study suggests that for multiple regression analyses in epidemiology studies, the analyte concentration unadjusted for creatinine should be included in the model, and urinary creatinine added as a separate independent variable (Barr et al. 2005b).
Blood has inherent advantages for biomonitoring. Regardless of the route of exposure, a chemical must be absorbed into the bloodstream and circulate to the tissues to have an effect (exceptions include direct inhalation effects on the lung and blistering agents on skin) (Needham et al. 2005). Blood is also a “regulated” matrix; therefore, there is a constant amount of blood for a given body weight, so measurements can be “normalized” to this amount. Blood can be collected with vacutainers and other suitable containers without anticoagulant or with heparin, EDTA, or citrate, depending on the types of assays expected to be conducted (Holland et al. 2003). Serum and plasma are most commonly used for measurements of chemicals in blood. Red cells and white cells can be isolated with centrifugation or by using special separation substances (such as Lymphoprep). It is important to decide on the type of vacutainers to be used and the number and volumes of resulting aliquots that will be used for planned analyses without additional freeze-thaw cycles, which may affect sample integrity.
For persistent organic chemicals, a blood sample can be taken years after exposure has occurred. Exposure can still be accurately identified, but the investigator will have no information about when the exposure occurred. Sample collection for nonpersistent-chemical measurements should reflect the residence time of the chemical in each individual matrix. The half-lives of nonpersistent chemicals are typically much shorter in blood than in urine; thus, blood samples may need to be collected within minutes or hours of exposure, whereas urine samples may be collected several hours or even days after exposure (Barr et al. 2005b).
Many other matrices can be used for biomonitoring studies, including saliva, breast milk, hair, fingernails, meconium, bone marrow, and feces (Table 4-2). They differ in level of invasiveness and amounts required for
analysis, which also strongly depend on the stages of life and the types of biomarkers measured. For example, breast milk can contain chemicals from exposures that occurred earlier in a woman’s life and thus can reflect cumulative exposure, but measuring these chemicals in her breast milk can be used as a measure of her infant’s intake. Investigators have to account for the timing of collection because early milk, called colostrum, significantly differs substantially in content from mature milk. Furthermore, collecting breast-milk samples soon after delivery, although most convenient for the research team, was challenging for mothers in one study (Eskenazi et al. 2005). For most, the milk supply had not yet fully developed, and some new mothers (particularly primiparas) found it difficult to provide samples with a breast pump. In addition, some mothers feared that milk was being taken away from their babies. Later collection of breast milk avoided some of those problems, but timing problems arose for other sample types as well.
Saliva samples typically mimic blood, whereas meconium samples may provide a longer window of time for capturing an exposure.
It is desirable to collect as many different matrices from each study participant as is feasible and to process them with consideration of both immediately planned analyses of biomarkers and future uses. For example, several Children’s Environmental Health Centers obtained urine, peripheral blood, cord blood, breast milk, meconium, saliva, hair, placental tissue, infant formula, indoor and outdoor air, and house dust from longitudinal birth cohort studies (Eskenazi et al. 2005). The centers have analyzed concentrations of numerous compounds in those biologic and environmental samples, such as pesticides, phthalates, mercury, lead, cotinine, polycyclic aromatic hydrocarbone (PAHs), PAH-DNA adducts, allergens, endotoxin, antioxidant micronutrients, cholinesterase, and thyroid hormones. Most centers also banked samples for future analyses.
Quality Assurance for Sample Collection
To ensure the quality of specimens for current and future use, most researchers develop protocols for collecting, shipping, processing, and banking samples—standard operating procedures (SOPs) (Heinrich-Ramm et al. 1996; Gunter 1997; Landi and Caporoso 1997; Holland et al. 2003). Pilot studies are conducted to determine the collection and storage conditions necessary for the stability of particular chemicals and their range of concentrations in the cohort. Separating specimens into several aliquots to eliminate the need for repeated thawing and freezing helps to avoid potential contamination and degradation. Field blanks, spikes, and duplicates are included in the analytic batch of samples that are bar coded for making
sample handling less error-prone. Labels include the participant’s unique, coded identifier, the sample type, and the aliquot.
A paper trail for each sample is necessary to ensure the integrity of the sample. A complete paper trail includes collection details (date, sample number, type, and volume), shipping information (receipts and tracking numbers), and chain-of-custody forms. In accordance with HIPAA, personal information on the participant must be encrypted. Electronic databases are quickly replacing hard copies, and bar codes allow for quick and accurate encoding and processing of samples.
From Field to Laboratory
Depending on the type of monitoring study, sample collection can take place in the clinic, in the field office, or even in the home of a participant. Each scenario imposes specific requirements and limitations on the type, volume, and potential use of a sample.
Researchers find it helpful to consult with community physicians to determine the amount of blood or other matrix collected that is both clinically and culturally acceptable to the target population (Eskenazi et al. 2005).
Studies conducted in rural areas face additional barriers to successful collection and processing of samples, including laboratory facilities that are inadequately equipped to process samples and a lack of skilled phlebotomists, especially for blood collection from pediatric populations (Eskenazi et al. 2003).
Using noninvasive collection of samples, such as urine, saliva, or exhaled breath tends to increase participation in a study because more participants are willing to subject themselves to a buccal swipe than to a blood draw. When children in one study could not provide urine samples during scheduled visits, investigators gave parents the supplies and instructions to collect the samples at home and arranged to pick them up on the following day (Eskenazi et al. 2003). Noninvasive sample collection also eliminates the need for highly trained personnel to draw blood or perform a biopsy. Some specimens—such as saliva, hair, and nail clippings—can even be obtained by participants at home and mailed to the researcher, making studies in the remote areas more feasible. However, hair and nails are susceptible to contamination in some settings and require specimen consideration in handling.
Sample Stability
Some biomarkers are unstable, so timing of processing, temperature, addition of stabilizing chemicals and buffers, and sterility are important
considerations. Time between collection and processing of a sample is a vital concern. Some processes can be done 24-48 hours after collection; others must be started immediately after collection (Landi and Caporaso 1997). Samples that must be shipped to a processing laboratory cannot be used for the more unstable biomarkers, or processing (including preparing of aliquots, serum and clot separation, and freezing at –80°C) should begin in the field to ensure stability during transportation (Holland et al. 2005).
That different components of blood must be stored at different temperatures should be considered in the study design. Most samples retain their integrity for years if stored in nitrogen tanks (–196°C ) or deep freezers (–80°C) (Gunter 1997; Landi and Caporaso 1997). In addition to the preservation of biologic components in the collected samples, it is important to ensure that measurement of environmental pollutants in the samples is not confounded by contaminants in the containers or tools used for sample collection. Prescreening of the materials may be necessary, depending on the toxicant of interest.
Shipping
Sample-shipping requirements depend on the time, distance, climate, season, method of transportation, applicable regulations, type of specimen, and markers to be assessed. Usually, polyurethane boxes containing dry ice are used to ship and transport samples that require low temperatures. The quantity of dry ice should be carefully calculated on the basis of the estimated time of the trip (dry ice evaporates with time, depending on external temperature), the number of samples to be transported in boxes, and an ample safety factor that takes likely delays into account. If the boxes have to be shipped by air, it is suggested that they be placed in the hold of the plane, where the temperature during the trip is very low. Each box should be accompanied by a typed chart describing in detail the contents of the box and the location of each tube in the box. For samples that require very low temperatures, shipping in a liquid-nitrogen container can be optimal.
Communication between field personnel is especially important when samples need to be shipped or transported from the field to the laboratory (Eskenazi et al. 2003). A system of e-mail or telephone messages and confirmations should be established with backup contacts to avoid missed shipments or improper handling on arrival.
Biologic Banks
The main goals of a biologic bank, or biorepository, are to make future analysis of currently unknown biomarkers possible and to minimize research costs of future studies by using previously collected specimens. A
biorepository is “a system which will store one or many types of biologic specimens for later analysis from single or multiple studies under conditions which permit efficient retrieval and optimum stability of the sample” (Winn et al. 1990). Recently, the International Society for Biological and Environmental Repositories published a document that reflects the collective experience of its members with collection and banking of human samples (ISBER 2005); it contains comprehensive information on the handling of blood, urine, nail clippings, saliva, breast milk, and other tissues.
Chapter 2 describes several repositories from completed or current projects in Europe and the United States. The human-matrix repositories vary widely and include commercial banks, national or international project banks, and much smaller biorepositories associated with scientific laboratories or universities. The latter may have 100,000 samples or fewer; larger biorepositories, such as CDC or the National Cancer Institute, have many millions of samples (Gunter 1997; Goodman et al. 2006). Each sample needs to have a secure chain of custody, processing, location, and temperature-stability records compiled in an accessible location. Nanobarcoding and partial to complete automation of a biorepository help to address these issues (Holland et al 2005).
Laboratory Analysis
Laboratory analysis of human specimens is an integral part of any biomonitoring study. Various characteristics of a laboratory method should be evaluated before it is chosen for use in one of the diverse biomonitoring applications discussed in Chapter 3. Accuracy reflects the agreement between the measured value and the “true” value. Sources of bias or systematic error are numerous. For example, contamination introduced during specimen collection or analysis may bias results upward, whereas incomplete recovery of the analyte from the specimen may bias results toward lower values. Three related terms describe the random variation of data produced from identical specimens. Precision is a measure of the degree of agreement among individual results obtained from the same or identical specimens with the same method and by the same analyst and laboratory. Numerous sources of scatter or imprecision exist in laboratory procedures, including random variations in introduced contamination, analyte recovery, and instrument response. Ruggedness describes a method’s reproducibility under the influence of variation in analyst, instrumentation, day of testing, and laboratory. Robustness is a measure of intralaboratory day-to-day variation induced by small changes in procedure. The characteristic of greatest relevance to a given study depends on whether samples are tested in the same or different laboratories, on different days, and so on. The ability to identify and quantify the target analyte in the presence of chemically
similar interfering compounds reflects the specificity of the method. Specificity may vary widely between methods on the basis of, for example, the rigor of sample cleanup and the method of instrumental analysis; for example, mass spectrometric detection will typically be more specific than detection by electron capture. A method’s limit of detection (the lowest concentration that can be measured) also may affect variation. Typically, precision is poorest near the limit of detection. Somewhat arbitrary procedures must be used to “assign” concentrations to specimens whose actual concentrations are below the limit of detection. The results of some statistical calculations, such as the mean concentration in a population, will be influenced by the method used to handle results below the limit of detection, and comparisons between datasets that hold different limits of detection compound the problem (Özkaynak et al. 2005).
In the absence of cost and sample-throughput considerations, users of biomonitoring laboratory data might prefer an analysis that has a limit of detection adequate to measure background exposures, and that can measure the concentration of the target chemical or metabolite with absolute accuracy and precision. Laboratory methods inevitably introduce unwanted error into the dataset, so the last two goals can never be attained. The practical effect of those errors depends substantially on the reason for generating the data. For example, an occupational physician seeking to compare concentrations of a chemical in blood samples drawn from a few employees with national reference values will be concerned about interlaboratory variability but will not necessarily require a very low limit of detection. Alternatively, an epidemiologist conducting a study of an “unexposed” population may require a low limit of detection to classify individual exposure status but, if all samples are analyzed in a single laboratory, may be less concerned about interlaboratory variability introduced by methodologic differences. Study design is important in establishing the optimal balance among variation-related factors.
If an analytic method lacks sufficient specificity, chemical interferences will result in an erroneously high reported concentration. If a measured chemical is introduced as an artifactual contaminant during sample collection or analysis, reported concentrations will also be overestimated. For example, credibly estimating human exposure to phthalates was hindered by the difficulties involved in avoiding specimen contamination with these ubiquitous chemicals; the problem was resolved by focus on the much less prevalent metabolic product, the phthalate half-ester (Silva et al. 2004). Alternatively, a chemical measured as a marker of exogenous exposure may be identical with a chemical formed by an unrelated endogenous metabolic pathway. In each of those cases, a rigorous laboratory-method validation should detect the problem before data are reported. More subtly, the measured biomarker of exposure may be chemically identical with a dietary
chemical not directly relevant to the exposure of interest. For example, organophosphorus-insecticide exposure can be assessed by measuring the metabolic hydrolysis product, the corresponding dialkylphosphate in urine. However, the dialkylphosphate can itself be a dietary component formed by degradation of the parent pesticide in the environment or in a food product (Lu et al. 2005). Under those circumstances, the urinary metabolite may lead to overestimation of exposure to the intact insecticide.
One important characteristic of laboratory-introduced variation is that the expected variation can be statistically defined before an analytic method is first used. A method-validation sequence should be completed before an analytic method is placed into service. Specimens of known concentration are obtained, usually by spiking an appropriate matrix with known concentrations of the target chemical or by using externally provided materials with well-defined and known concentrations. Those specimens are then treated as unknowns and analyzed under conditions closely approximating those to be used in future applications. Thus, depending on study design, the validation specimens might be analyzed by multiple analysts using different instruments on different days. After completion of the validation sequence (which often requires analysis of hundreds of specimens), the resulting data should allow quantitative statements about the expected accuracy, precision, and robustness of the method at multiple concentrations and should establish the method’s limit of detection. That information should be provided to the end user of the data because it can influence overall study design (Needham and Wang 2002; Needham et al. 2005).
When the method has been validated and is in regular use, laboratories must maintain rigorous quality-assurance procedures to verify that analysis is being properly performed. Typically, each batch of 10-20 study-related specimens will be analyzed in association with one or two samples of known concentration (either purchased standard reference materials or materials independently prepared by the laboratory): a method blank (a specimen containing an undetectable or very low concentration of the target analyte) and a duplicate. If the results with the quality-control specimens are inconsistent with measurements determined in the validation sequence, the laboratory should investigate the cause of discrepancies, make appropriate corrections, and reanalyze the batch of specimens in question. Those safeguards should ensure that the data quality predicted by the validation sequence is maintained during the analysis of unknown samples.
Participation in interlaboratory comparisons and proficiency-testing programs provides additional information especially pertinent to controlling interlaboratory variation. Aliquots of homogeneous samples containing the analytes of interest are drawn and distributed to each participating laboratory. The participants’ results are used to calculate overall and method-specific statistics, such as means, medians, and standard devia-
tions. Such programs allow participants to assess the performance of their laboratory vs those of other participants and encourage laboratories to investigate reasons for any discrepancies observed. Voluntary participation in such programs usually indicates that a laboratory’s management is committed to producing high-quality data. In the United States, the federal Clinical Laboratory Improvement Act of 1988 (CLIA-88) requires that any facility that performs diagnostic or treatment-related laboratory testing of human specimens have CLIA approval (Rivers et al. 2005). The CLIA program includes evaluation of staff qualifications, quality-control and quality-assurance (QC-QA) procedures, SOPs, onsite laboratory inspections, and mandatory proficiency testing. However, the current CLIA regulations are not focused on tests relevant to biomonitoring. For example, CLIA recognizes three subspecialties in chemistry (routine chemistry, endocrinology, and toxicology); environmental or occupational chemistry is not a listed subspecialty (42 CFR 493.839 [2005]). Furthermore, CLIA regulations require enrollment in a proficiency-testing program approved by the Centers for Medicare and Medicaid Services (CMS) only for “regulated” analytes. However, the only CLIA-regulated analyte in the chemistry specialty clearly relevant to biomonitoring is blood lead (42 CFR 493.929 [2005]). CLIA requires that laboratories that test analytes not specifically regulated participate in non-CMS-approved proficiency testing, split sampling, or internally generated proficiency tests at least twice a year to demonstrate satisfactory performance (42 CFR 493.1236c [2005]). However, the scarcity of well-validated proficiency tests and interlaboratory comparison studies for unusual or cutting-edge biomonitoring analytes complicates comparison of data generated in different laboratories. The problem may become more acute as the list of chemicals with well-established population-based reference ranges continues to be expanded by the NHANES program, facilitating the interpretation of patient values.
Selecting a laboratory and an analytic method that can provide data of sufficient quality for their intended purpose can be difficult. As a first screen, users of biomonitoring laboratory data should focus on laboratories that systematically work to characterize and minimize laboratory-induced variation. For routinely performed analyses, a laboratory should have completed method-validation sequences and be able to provide statistically based estimates of expected accuracy, precision, limit of detection, and so on. For new methods, the laboratory must conduct an appropriate method validation before initiating sample analysis. The laboratory should also maintain a rigorous QA-QC program to verify that these goals are attained during routine analysis. Particularly when interlaboratory variation can affect data interpretation, the laboratory should participate in available interlaboratory comparisons and proficiency-testing programs. CLIA certification, a legal requirement in the United States for all diagnostic or treatment-related
testing, provides some assurance that test methods are documented and that laboratory staff are appropriately qualified to perform them.
STATISTICAL ANALYSIS
The first principle of analysis is that it follows design. That is particularly true of biomonitoring data. At the beginning of this chapter, we emphasized the importance of describing the selection of the sampled population. Ideally, specific analyses (based on specific hypotheses) have been outlined at the beginning of the study. The statistical jargon phrase for characterizing the sampled population is the target of inference. A dataset may have different targets of inference—with resulting differences in the statistical analysis. For example, an occupational physician faced with a specific biomarker concentration in a specific worker is faced with the inferential question of whether the worker belongs to the group of workers “exposed” or “not exposed.” The inference will be drawn by bringing in other factors, such as a careful work history. If the same physician is interested in the worker’s result for a study of occupational disease, the target of inference is different, and different statistical analyses will be required. Statisticians would couch the first question in terms of fixed-effect analyses and the second in terms of random-effects analyses. Closely tied to the target of inference is the measurement process and its precision. As mentioned previously, if only a single population of inference is of interest, the precision of the measurement is the key driver and sample size determinations should reflect the required precision. If comparisons between two populations are desired, then rather than the precision of the measurements, the magnitude of the difference between the two populations becomes the key driver in the sampling effort.
Before complicated statistical models are constructed and run—increasingly easy with more and more powerful statistical computing packages—it is absolutely necessary to describe the basic characteristics of each variable—number of observations, mean, standard deviation, minimum, and maximum. That will reveal which data are below the limits of detection, are missing, are miscoded, and are outliers. If the study involves three or four key variables, associations among the variables should also be examined. Histograms and scatterplots will reveal data structures unanticipated from the numerical summaries.
If the study is exploratory, it is not uncommon to have a multitude of creative ideas about the nature of the biomarker response and its relationship to the exposure. Ideally, some comparisons of particular interest are specified in advance. The analyses can then be divided into confirmatory and exploratory phases. Two key considerations in such exploratory studies are selection bias and confounding; both were discussed in the section on the sam-
pling of populations, and they should be addressed specifically at the analytic stage. Often, they appear as a throwaway paragraph in the “Study Limitations” section of a paper or a thesis. They deserve a more prominent place.
Longitudinal data are increasingly common. Some of the larger national surveys are considering incorporating longitudinal features. The primary reason is that such approaches can detect shifts more precisely. The Achilles heel of longitudinal studies is missing data. Causes of missing data include death, moving from the area, and refusal to continue with the study. Statisticians have developed a useful terminology for patterns of missingness. Longitudinal data are usually analyzed with fairly complicated statistical models, such as mixed-effect regression models, generalized estimating equations, and logistic-regression models. The validity of a particular model depends heavily on the missing-data pattern. Given that an appropriate class of models has been identified, secondary concerns enter, such as precision of the estimates. The models are not for the statistically naïve, and a professional statistician or someone well versed in the field needs to be consulted. A specific example of longitudinal data involves the monitoring of pesticide handlers in the eastern part of Washington state. The pesticides of concern are cholinesterase inhibitors. A baseline acetylcholinesterase (AChE) concentration is obtained from each handler before the spraying season begins, and handlers are monitored after every 30 hours of handling activity. The end point is AChE depression from baseline expressed as a percentage. If the depression is greater than 20%, specific regulatory actions are begun. The data collected are relevant for each handler but also for the agricultural industry. Issues of false-positive and false-negative depressions also have to be dealt with. In this example, the nature of the missing data becomes very important. Suppose that workers become too sick from the pesticide exposure and simply quit work. This would introduce selection bias. The growers tend to focus on false positives, the workers on false negatives. Resolution of these conflicting aims requires a careful understanding of the within-subject variability and measurement error.
The analyst needs to understand that statistical models vary in their inferential utility. Linear models of some type or other are the most common and the most easily analyzed with statistical computing packages, but they may be only rough approximations of the real world. An oft-quoted aphorism of G.E.P. Box is that “all models are wrong, some are useful.” That is no doubt true, but it misses another level of detail of such models as follows. At the simplest level, a model fits the data. At the next level, a model predicts the data. At its most useful level, a model shows unanticipated features of the data and the research, and this is the ideal especially for biomarker research. The most exquisite characterization of association
does not necessarily constitute evidence of causation. As new biomarkers are investigated, the causal link to exposure is a first requirement.
It is usual whenever biologic samples are obtained in medical practice or in any health encounter for results to be expressed in relation to “normal.” In fact, a relationship to “normal” is the question almost every subject asks first when presented with such information. For biomonitoring results, it is a generally meaningless question—“normal” for human-made chemicals or chemicals that did not enter the environment except for human activity, is actually zero. Despite that, many laboratories report results, such as blood lead concentrations, in just this way—a practice likely to result in confusion. For example, it is not rare to see reports of blood concentrations that contain the phrase “normal for industrial workers.” In truth, such concentrations may be common in industrial workers, or even typical, but hardly normal in the usual meaning of that word in relation to health. Such comparisons are especially problematic for biomarkers whose relationship to health and environmental sources is less well studied. The discussion below touches briefly on the scientific issues raised by such implicit or explicit comparisons of results with reference ranges.
Although the notion of “normal” is not appropriate to biomonitoring, there are two axes of potential reference around which comparisons may be pertinent and indeed important. The first is in relation to comparable subjects. For example, where adequate information exists, it would be entirely appropriate to express values in relation to one or more groups of subjects. For example, it would be reasonable to report an individual or group of blood lead concentrations in children as “comparable with those measured (extensively) in populations of children not known to have a specific exposure source” or “suggestive of an identifiable source of exposure.” In adults, it might be reasonable to express lead concentrations as “comparable with concentrations seen in the population not occupationally exposed to lead” “within the range of concentrations seen in workers exposed to lead under well-controlled conditions,” or the like. Those are not assessments of normality or health effects, merely comparisons with other potentially relevant populations that could lead to research inferences or clinical or public-health interventions.
A second axis for interpretation of results, relevant only in the small number of situations where information is available to link a biomarker concentration to risk or clinical effects, is comparison with such risk or health-effect concentrations, for example, at or below the concentrations previously associated with developmental delays in children. The point is that the health comparison must be explicit and referenced to the source of the inference, such as an epidemiologic study or reference publication.
SUMMARY AND CONCLUSIONS
Biomonitoring challenges laboratory methods, ethical considerations, and communications strategies. It also challenges the technical limits of epidemiology and biostatistics. This chapter has provided guidelines for the conduct of biomonitoring studies, including study design, study conduct, and data analysis. Because not all biomonitoring studies are conducted with the same rigor, it is important that the guidelines presented be followed to ensure, to the extent possible, that biomonitoring studies will lead to the identification of chemicals that are causing risks or health effects, will provide information on exposure pathways and health effects to guide future control efforts, and will avoid anxiety or apathy about chemicals where personal or societal risk appears not to warrant that reaction.
Wherever blood or other matrices are being collected from a sample of any population—whether for surveillance, etiologic study, or clinical evaluation—the highest standards of sampling theory should be adhered to, and the approach should be explicitly described in publications and reports so that biases may be recognized. If it is feasible, results should be expressed in terms not only of age groups, sex, and race, but also in relation to quantifiable lifestyle factors, such as occupation, income, and education.
A great deal of effort and time is involved in getting studies approved. Hence, it is absolutely essential to address questions of ethics at the design stage. Informed consent and IRB approval became especially important when more than one research site or jurisdiction is involved, as this can introduce a problematic cycle of approval at one level and modification at another. Because the value of biomonitoring in the long run is the ability to link the sample result back to human health and risk factor information, the links between the biomonitoring and health data should not be severed, while at the same time ensuring confidentiality of the data.
A topic linked to ethics but with its own issues and strategies is communication. The committee is convinced that communication starts with the study proposal and continues through study design. Each of the many constituencies associated with a proposed study requires careful consideration in planning communication strategy and content. Most biomonitoring studies will eventually have applications, and researchers need to anticipate potentially affected communities and plan for communication with them.
A carefully, comprehensively designed study facilitates study conduct. Two key elements of study conduct are sample collection and laboratory analysis. The choice of matrix depends on both theoretical and practical considerations. Those include the tissue most likely to represent the biomarker route of exposure and the ease of obtaining samples. Careful attention to collection and shipping from field to laboratory is a prerequisite for valid laboratory analysis. The choice of analytic laboratory methods is
determined by cost, sensitivity, and specificity. Quality control surrounds both the collection and the laboratory analysis of samples.
Analysis of biomonitoring data, following the strategies initiated in study design, begins with description of the data in ways that are transparent. Sources of a priori importance and those introduced by random variation must be explicitly addressed in any statistical model, as must the context for tests of statistical significance. In every case, diligent attention to noncausal relationships within the data—associations due to bias and confounding— demand the greatest professional attention because such spurious associations are likely when so many chemicals are studied, and hypotheses remain so open-ended. Data analysts should approach results mindful that for the vast majority of biomarker measurements, results can neither be claimed to be normal in the usual sense—zero is probably the original human condition for most—nor be assigned value judgments, such as “high.” Any such designations should emerge from the interpretation of the data in context, not from the results of a single individual study or survey.
RECOMMENDATIONS
-
A biomonitoring-study report should contain a detailed description of the origin of the sample of subjects selected for study. The vast majority of biomonitoring studies are not based on a CDC-like probabilistic sample of the population. Investigators should state explicitly the population that their results apply to. Editors of journals should insist that this information be included in any submission.
-
Any analysis of biomonitoring data should include an assessment of the importance of sources of variation. Basic sources to be considered are laboratory, intra-individual, interindividual, and variability attributable to groups.
-
Great effort should be made to develop a human pharmacokinetic, preferably PBPK, model early in the study-design process. The likely influence of, for example, model simplification (for example, assuming a single half-life), metabolic saturation, and influence of sampling time may then be addressed before spending vast resources on sampling and analyses. By adding statistical tools (Monte Carlo simulation, population models) to the model, additional features may be examined including variability in exposure pattern and intra- and interindividual variability in pharmacokinetic determinants.
-
Investigators, including CDC, that conduct surveys of biomarkers in the population should routinely collect detailed information about SES, lifestyle, and other cofactors on each subject and should routinely present results organized in a way that addresses the issue of whether biomarker concentrations vary as a function of each. Epidemiologic analyses of bio-
-
markers in relation to health should routinely include appropriate adjustments for such covariates.
-
Incorporating communication in the design of a biomonitoring study is essential. The type of communication needed will depend on the goals of the biomonitoring study. Careful attention to planning effective communication at the beginning of a biomonitoring study will make communication at the end of the study easier and may make the technical aspects of the study proceed more smoothly. Biomonitoring funders should require communication planning in any application for support.
-
Research is needed to develop and disseminate effective methods for evaluating communication of study results.
-
With respect to providing information to study participants, the committee considers that failing to provide detailed information about all biomarkers to be measured, no matter how many chemicals are involved in the study, raises ethical questions.
-
Blanket consent for use of biomonitoring samples at some future time has the potential to result in abuse. However, there are practical difficulties in repeated tissue sampling that the absence of blanket consent reinforces. Research is needed to develop new approaches for obtaining consent for future uses of biomonitoring data.
-
Biologic and environmental specimens should be carefully collected with rigorous QA-QC provisions and should be processed and banked in multiple aliquots with comprehensive characterization of their origin and history.
-
Laboratory analysis of human samples for trace contaminants or their metabolites inevitably produces results that deviate quantitatively from the actual concentrations. Such deviations can, for example, complicate exposure classifications in epidemiologic studies, detection of time trends in exposure, and comparison of studies that use data produced with different analytic methods. Individual laboratories can use standard QA-QC methods to minimize and define the magnitude of the variations. However, federal agencies and statutes, such as CDC, the National Institute of Standards and Technology, and statutes such as CLIA, could play important roles in improving the overall quality of biomonitoring laboratory data and their utility in health-related applications.
-
NHANES and other studies are rapidly expanding the list of chemicals for which population-based reference values are available. Health practitioners will increasingly seek to compare data from potentially occupationally or environmentally exposed patients with those reference values. That application requires consistency between data generated from different laboratories. CMS, through the CLIA program, regulates clinical-laboratory testing for nonresearch-related purposes. However, the program does not emphasize testing for chemicals associated with environmental or occupational
-
exposure. The quality of such data would be improved if CMS created a new chemistry subspecialty related to environmental and occupational medicine and expanded the array of regulated analytes supplied by approved proficiency-test providers.
-
It is essential to develop and use noninvasive and ultrasensitive specimen-collection techniques for the biomonitoring of children and other groups that can provide only small samples.
-
Researchers should anticipate maintenance of samples in biorepositories (banks) for future generations of researchers. Such banking requires attention, at the planning and design stages, to ethical considerations, communication strategies, location and storage of samples, and—last but not least—the costs involved.
-
As soon as practicable, researchers should standardize and harmonize biomonitoring measurements to make scientific communication feasible. That may involve the development of reference materials that can be shared among laboratories. Standardization and harmonization are particularly important for international collaboration.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1995. Topics in Biological Monitoring: A Compendium of Essays. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
Adler, P.S. 2002. Science, politics, and problem solving: Principles and practices for the resolution of environmental disputes in the midst of advancing technology, uncertain or changing science, and volatile public perceptions. Penn. St. Environ. Law Rev. 10(2):323-343.
Andrews, C.J. 2002. Humble Analysis: The Practice of Joint Fact-Finding. Westport, CT: Praeger.
Balch, G.I., and S.M. Sutton. 1995. Putting the first audience first: Conducting useful evaluation for a risk-related government agency. Risk Anal. 15(2):163-168.
Barr, D.B., R.Y. Wang, and L.L. Needham. 2005a. Biologic monitoring of exposure to environmental chemicals throughout the life stages: Requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 113(8):1083-1091.
Barr, D.B., L.C. Wilder, S.P. Caudill, A.J. Gonzalez, L.L. Needham, and J.L. Pirkle. 2005b. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 113(2):192-200.
Beecher, N., E. Harrison, N. Goldstein, M. McDaniel, P. Field, and L. Susskind. 2005. Risk perception, risk communication, and stakeholder involvement for biosolids management and research. J. Environ. Qual. 34(1):122-128.
Bernard, A.M. 1995. Biokinetics and stability aspects of biomarkers: Recommendations for application in population studies. Toxicology 101(1-2):65-71.
Bernard, A.B. and R. Lauwerys. 1986. Present status and trends in biological monitoring of exposure to industrial chemicals. J. Occup. Med. 28(8):558-562.
Bernard, A., and R. Lauwerys. 1987. General principles for biological monitoring of exposure to chemicals. Pp. 1-16 in Biological Monitoring of Exposure to Chemicals: Organic Compounds, M.H. Ho, and H.K. Dillon, eds. New York: Wiley.
Butchko, H.H., W.W. Stargel, C.P. Comer, D.A. Mayhew, C. Benninger, G.L. Blackburn, L.M. de Sonneville, R.S. Geha, Z. Hertelendy, A. Koestner, A.S. Leon, G.U. Liepa, K.E. McMartin, C.L. Mendenhall, I.C. Munro, E.J. Novotny, A.G. Renwick, S.S. Schiffman, D.L. Schomer, B.A. Shaywitz, P.A. Spiers, T.R. Tephly, J.A. Thomas, and F.K. Trefz. 2002. Aspartame: Review of safety [review]. Regul. Toxicol. Pharmacol. 35(2 Pt 2):S1-S93.
Comfort, L.K. 1985. Action research: A model for organizational learning. J. Pol. Anal. Manage. 5:100-118.
Daston, G., E. Faustman, G. Ginsberg, P. Fenner-Crisp, S. Olin, B. Sonawane, J. Bruckner, W. Breslin, and T.J. McLaughlin. 2004. A framework for assessing risks to children from exposure to environmental agents. Environ. Health Perspect. 112(2):238-256.
Droz, P.O., and J.G. Fernandez. 1977. Effect of physical workload on retention and metabolism of inhaled organic solvents. A comparative theoretical approach and its applications with regards to exposure monitoring. Int. Arch. Occup. Environ. Health. 38(4):231-246.
European Commission. 2004. Baseline Report on “Biomonitoring for Children” in the Framework of the European Environment and Health Strategy (COM(2003)338 final). Prepared by the Technical Working Group on Integrated Monitoring. January 9, 2004 [online]. Available: http://www.brussels-conference.org/Download/baseline_report/BR_Biomonitoring_final.pdf [accessed June 3, 2005].
ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals). 2005. Guidance for the Interpretation of Biomonitoring Data. Document No. 44. Brussels, Belgium: ECOTOC.
Ehrmann, J.R., and B.L. Stinson. 1999. Joint fact-finding and the use of technical experts. Pp. 375-400 in The Consensus Building Handbook: A Comprehensive Guide to Reaching Agreement, L. Susskind, S. McKearnan, and J. Thomas-Larmer, eds. Thousand Oaks, CA: Sage.
EPA (U.S. Environmental Protection Agency). 2002. Community, Culture and the Environment: A Guide to Understanding a Sense of Place. EPA 842-B-01-003. Office of Water, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ecocommunity/pdf/ccecomplete.pdf [accessed Dec. 1, 2005].
Eskenazi, B., A. Bradman, E.A. Gladstone, S. Jaramillo, K. Birch, and N.T. Holland. 2003. CHAMACOS, A longitudinal birth cohort study: Lessons from the fields. J. Child. Health 1(1):3-27.
Eskenazi, B., E. Gladstone, G. Berkowitz, C. Drew, E. Faustman, N.T. Holland, S. Lanphear, S. Meilsel, F. Perera, V. Rauh, A. Sweeney, R. Whyatt, and K. Yolton. 2005. Methodologic and logistic issues in conducting longitudinal birth cohort studies: Lessons learned from the Center of Children’s Environmental Health and Disease Prevention Research. Environ. Health Perspect. 113(10):1419-1429.
Fairchild, A.L., and R. Bayer. 2004. Ethics and the conduct of public health surveillance. Science 303(5658):631-632.
Fisher, A., M.T. Pavlova, and V.T. Covello, eds. 1991. Evaluation and Effective Risk Communications: Workshop proceedings. EPA/600/9-90/054. Interagency Task Force on Environmental Cancer and Heart and Lung Disease, Center for Environmental Research Information. U.S. Environmental Protection Agency, Washington, DC.
Flesch-Janys, D., H. Becher, P. Gurn, D. Jung, J. Konietzko, A. Manz, and O. Papke. 1996. Elimination of polychlorinated dibenzodioxins and dibenzofurans in occupationally exposed persons. J. Toxicol. Environ. Health Part A 47(4):363-378.
Ginsberg, G., D. Hattis, and B. Sonawane. 2004. Incorporating pharmacokinetic differences between children and adults in assessing children’s risks to environmental toxicants. Toxicol. Appl. Pharmacol. 198(2):164-183.
Goodman, G.E., M.D. Thornquist, C. Edelstein, and G.S. Omenn. 2006. Biorepositories: Let’s not lose what we have so carefully gathered! Cancer Epidemiol. Biomarkers Prev. 15(4):599-601.
Grill, K., and S.O. Hansson. 2005. Epistemic paternalism in public health. J. Med. Ethics 31(11):648-653.
Gunter, E.W. 1997. Biological and environmental specimen banking at the Centers for Disease and Control and Prevention. Chemosphere 34(9/10):1945-1953.
Hauser, R., J.D. Meeker, S. Park, M.J. Silva, and A.M. Calafat. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Persp. 112(17):1734-1740.
Heinrich-Rumm, R., G. Lehnert, and J. Angerer. 1996. The German external quality assessment scheme in occupational and environmental medicine. Ann. Ist. Super Sanita. 32(2):2 47-251.
Holland, N.T., M.T. Smith, B. Eskenazi, and M. Bastaki. 2003. Biological sample collection and processing for molecular epidemiological studies. Mutat. Res. 543 (3):217-234.
Holland, N.T., L. Pfleger, E. Berger, A. Ho, and M. Bastaki. 2005. Molecular epidemiology biomarkers: Sample collection and processing considerations. Toxicol. Appl. Pharmacol. 206(2):261-268.
IOM (Institute of Medicine). 1999. Toward Environmental Justice: Research, Education, and Health Policy Needs. Washington, DC: National Academy Press.
ISBER (International Society for Biological and Environmental Repositories). 2005. Best practices for repositories I: Collection, storage, and retrieval of human biological materials for research. CPT 3(1):5-48.
Jonsson F., and G. Johanson. 2001. A Bayesian analysis of the influence of GSTT1 polymorphism on the cancer risk estimate for dichloromethane. Toxicol. Appl. Pharmacol. 174(2):99-112.
Kaneko, A., J.K. Lum, L. Yaviong, N. Takahashi, T. Ishizaki, L. Bertilsson, T. Kobayakawa, and A. Bjorkman. 1999. High and variable frequencies of CYP2C19 mutations: Medical consequences of poor drug metabolism in Vanuatu and other Pacific islands. Pharmacogenetics 9(5):581-590.
Kass, N.E. 2005. Research Ethics and Human Biomonitoring Research. Presentation at the Second Meeting on Human Biomonitoring of Environmental Toxicants, April 28, 2005, Washington, DC.
Kemmeren, J.M., G. van Poppel, P. Verhoef, and M.J. Jarvis. 1994. Plasma cotinine: Stability in smokers and validation of self-reported smoke exposure in nonsmokers. Environ. Res. 66(2):235-243.
Kissel, J.C., C.L. Curl, G. Kedan, C. Lu, W. Griffith, D.B. Barr, L.L. Needham, and R.A. Fenske. 2005. Comparison of organophosphorous pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State. J. Expo. Anal. Environ. Epidemiol. 15(2):164-171.
Landi, M.T., and N. Caporaso. 1997. Sample collection, processing and storage. Pp. 223-236 in Application of Biomarkers in Cancer Epidemiology, P. Toniolo, P. Boffetta, and D.E.G. Shuker, eds. IARC Scientific Publications 142. Lyon: IARC.
Lasker, R.D., and E.S. Weiss. 2003. Broadening participation in community problem-solving: A multidisciplinary model to support collaborative practice and research. J. Urban Health 80(1):14-60.
Liira, J., G. Johanson, and V. Riihimäki. 1990. Dose-dependent kinetics of inhaled methyl ethyl ketone in man. Toxicol. Lett. 50(2-3):195-201.
Lof, A., A. Johanson, M. Rannug, and M. Warholm. 2000. Glutathione transferase T1 phenotype affects the toxicokinetics of inhaled methyl chloride in human volunteers. Pharmacogenetics 10(7):645-653.
Lu, C., R. Bravo, L.M. Caltabiano, R.M. Irish, G. Weersekera, and D.B. Barr. 2005. The presence of dialkylphosphates in fresh fruit juices: Implications for organophosphorus pesticide exposure and risk assessments. J. Toxicol. Environ. Health A 68(3):209-227.
Marmot, M.G., G.D. Smith, S. Stansfeld, C. Patel, F. North, J. Head, I. White, E. Brunner, and A. Feeney. 1991. Health inequalities among British civil servants: The Whitehall II study. Lancet 337(8754):1378-1393.
Metcalf, S.W., and K.G. Orloff. 2004. Biomarkers of exposure in community settings. J. Toxicol. Environ. Health Part A 67(8-9):715-726.
Mizutani, T. 2003. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab. Rev. 35(2-3):99-106.
Nebert, D.W. 2000. Suggestions for the nomenclature of human alleles: Relevance to ecogenetics, pharmacogenetics and molecular epidemiology. Pharmacogenetics 10(4): 279-290.
Needham, L.L., and R.Y. Wang. 2002. Analytic considerations for measuring environmental chemicals in breast milk. Environ Health Perspect. 110(6):A317-A324.
Needham, L.L., H. Ozkaynak, R.M. Whyatt, D.B. Barr, R.Y. Wang, L. Naeher, G. Akland, T. Bahadori, A. Bradman, R. Fortmann, L.J. Liu, M. Morandi, M.K. O’Rourke, K. Thomas, J. Quackenboss, P.B. Ryan, and V. Zartarian. 2005. Exposure assessment in the national Children’s Study: Introduction. Environ. Health Perspect. 113(8):1076-1082.
Nihlén, A., and G. Johanson. 1999. Physiologically based toxicokinetic modeling of inhaled ethyl tertiary-butyl ether in humans. Toxicol. Sci. 51(2):184-194.
NRC (National Research Council). 1987. Biologic markers in environmental health research. Environ Health Perspect. 74:3-9.
NRC (National Research Council). 1991. Monitoring Human Tissues for Toxic Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. Pesticides in the Diets of Infants and Children. Washington, DC: National Academies Press.
Özkaynak, H., R.M. Whyatt, L.L. Needham, G. Akland, and J. Quackenboss. 2005. Exposure assessment implications for the design and implementation of the National Children’s Study. Environ. Health Perspect. 113(8):1108-1115.
Pflugh, K.K., J.A. Shaw, and B.B. Johnson. 1994. Establishing Dialogue: Planning for Successful Environmental Management; A Guide to Effective Communication Planning. Trenton, NJ: New Jersey Department of Environmental Protection and Energy.
Risk Policy Report. 2001. Chemicals in breast milk could act as indicator, shape regulatory policy. Inside EPA’s-Risk Policy Report 8(2):24-26.
Rivers, P.A., A. Dobalian, and F.A. Germinario. 2005. A review and analysis of the clinical laboratory improvement amendment of 1988: Compliance plans and enforcement policies. Health Care Manage Rev. 30(2):93-102.
Rohrmann, B. 1992. The evaluation of risk communication effectiveness. Acta Psychol. 81(2):169-192.
Rohrmann, B. 2000. A socio-psychological model for analyzing risk communication processes. Australasian J. Disaster Trauma Studies [online]. Available: http://www.massey.ac.nz/~trauma/issues/2000-2/rohrmann.htm [accessed April 21, 2006].
Santos, S.L., and C. Chess. 2003. Evaluating citizen advisory boards: The importance of theory and participant-based criteria and practical implications. Risk Anal. 23(2):269-280.
Schulte, P.A. 2005. Application and Design Issues in Human Biomonitoring. Presentation at the Second Meeting on Human Biomonitoring of Environmental Toxicants, April 28, 2005, Washington, DC.
Schulte, P.A., and M.H. Sweeney. 1995. Ethical considerations, confidentiality issues, rights of human subjects, and uses of monitoring data in research and regulation. Environ. Health Perspect. 103(suppl. 3):69-74.
Schulte, P.A., and G. Talaska. 1995. Validity criteria for the use of biological markers of exposure to chemical agents in environmental epidemiology. Toxicology 101(1-2): 73-88.
Schulte, P.A., D. Hunter, and N. Rothman. 1997. Ethical and social issues in the use of biomarkers in epidemiological research. IARC Sci Publ. (142):313-318.
Silva, M.J., D.B. Barr, J.A. Reidy, N.A. Malek, C.C. Hodge, S.P. Caudill, J.W. Brock, L.L. Needham, and A.M. Calafat. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Study (NHANES) 1999-2000. Environ. Health Perspect. 112(3): 331-338.
Soskolne, C.L. 1997. Ethical, social, and legal issues surrounding studies of susceptible populations and individuals. Environ. Health Perspect. 105(suppl. 4):837-841.
Stern, P.C., and H.V. Fineberg, eds. 1996. Understanding Risk: Informing Decisions in a Democratic Society. Washington, DC: National Academy Press.
Stinson, B.L., and J.R. Ehrmann. 1998. Joint fact-finding in consensus-building processes. Inside EPA’s-Risk Policy Report 5(11):36-38.
Thompson, K.M., and D.L. Bloom. 2000. Communication of risk assessment information to risk managers. J. Risk Res. 3(4):333-352.
Tinker, T. 1994. Case Studies of Applied Evaluation for Health Risk Communication: Workshop Proceedings, June 9, Annapolis, MD. U.S. Department of Health and Human Services, Washington, DC.
Veach, P.M., D.M. Bartels, and B.S. LeRoy. 2001. Ethical and professional challenges posed by patients with genetic concerns: A report of focus group discussions with genetic counselors, physicians, and nurses. J. Genet. Couns. 10(2):97-119.
Warholm, M., A.K. Alexandrie, J. Hogberg, K. Sigvardsson, A. Rannug. 1994. Polymorphic distribution or glutathione transferase activity with methyl chloride in human blood. Pharmacogenetics 4(6):307-311.
Webler, T. 1998. Beyond science: Deliberation and analysis in public decision making. Human Ecol. Rev. 5(1):61-64.
Weinstein, N.D., and P.M. Sandman. 1993. Some criteria for evaluating risk messages. Risk Anal. 13(1):103-114.
Wessels, D., D.B. Barr, and P. Mendola. 2003. Use of biomarkers to indicate exposure of children to organophosphate pesticides: Implications for a longitudinal study of children’s environmental health. Environ. Health Perspect. 111(16):1939-1946.
Winn, D.M., M.E. Reichman, and E. Gunter. 1990. Epidemiologic issues in the design and use of biologic specimen banks. Epidemiol. Rev. 12:56-70.
















































