3
Toward Absolute Zero
When breakthrough science happens it defines a new frontier. AMO science is camped on one of the most exotic frontiers in science—the push toward ever-lower temperatures. When this report was written, the record low temperature stood at about a billionth of a degree above absolute zero. This is the coldest temperature in the universe (see Figure 3–1). By contrast, intergalactic space is a relatively hot 2.7 K above absolute zero owing to the cosmic microwave background. The frontier of this research is at the intersection of AMO with other fields, particularly condensed matter physics, low-temperature physics, plasma physics, and even theoretical nuclear physics. The interaction of researchers in these different fields is leading to exciting new physics, promising a decade of rapid advancement in these areas of science and blurring the line between research fields. In the last decade, six physicists have won Nobel prizes for their work at the frontier of the ultracold, and we are just beginning.
THE PROMISE OF ULTRACOLD SCIENCE
The first step in almost any ultracold gas experiment consists in cooling a gas of atoms to “degeneracy.” This means that it is so cold that the de Broglie wave of one atom starts to overlap with that of its nearest neighbor (see Box 3–1). When the atoms in question are bosons, the result is a Bose-Einstein condensate (BEC). Below a certain critical temperature, systems of trapped bosonic atoms become Bose-Einstein condensed superfluids, with a macroscopic fraction of the particles occupying the lowest-energy “wave” in the box in which the atoms are kept. In 1995 the first Bose-Einstein condensed gases of bosonic alkali atoms were produced (see Figure 3–2). BECs are by now routinely studied worldwide in systems of millions or more atoms, down to temperatures some billionths of a degree above absolute zero.
The striking characteristic of a superfluid is its ability to flow without even the
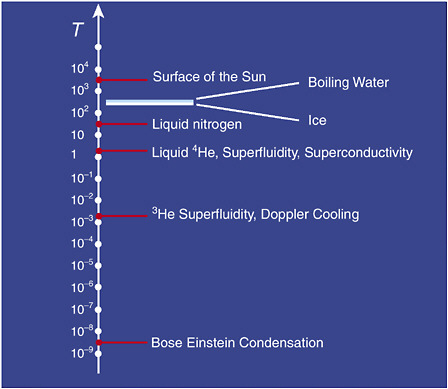
FIGURE 3–1 Temperatures of some familiar objects, on a scale of powers of 10.
|
BOX 3–1 de Broglie Waves It has been known since the work of Max Planck and Albert Einstein that one must sometimes think of light as consisting of (massless) particles, now called photons. This idea complements the more common notion that light is a wave. Likewise, quantum mechanics teaches us that atoms, as well as all particles, also possess wavelike properties. This wave-particle duality of both light and atoms is the cornerstone of quantum mechanics, yet it remains one of its most unsettling aspects. The quantum-mechanical wavelength of massive particles in thermal equilibrium is inversely proportional to the square root of their temperature. It is exceedingly small at normal (room) temperatures, a small fraction of a nanometer or so. As atoms get colder, though, their wavelength becomes longer—for the temperatures we will discuss here, these wavelengths can be hundreds of micrometers. Another way to say this is that at ever-lower temperatures, quantum mechanics becomes progressively more dominant. |
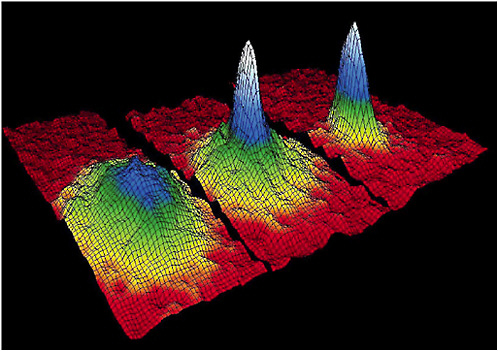
FIGURE 3–2 The original demonstration of Bose-Einstein condensation (BEG) in a dilute gas of rubidium atoms. These false-color images show the velocity distribution of a cloud of cold atoms near and below the BEG critical temperature. Left: Above the transition temperature, the atomic velocities show that this is an ordinary cold gas at an extraordinary temperature of 400 billionths of a degree above absolute zero (400 nK) . Center: Further cooling to 200 nK leads to the onset of BEG, shown as a clumping of atoms near zero velocity. Right: Still more cooling to 50 nK shows nearly all of the remaining gas in the BEG phase. SOURCE: E.Cornell, C.Wieman, JILA.
slightest resistance, to—as if by magic—overcome friction. Superfluidity is very much analogous to superconductivity, the ability of a metal to conduct electricity without any loss. Indeed, in a very real sense superconductivity is superfluidity, with the electrical current being carried along through the superconducting metal as a superfluid of electrons. A superfluid is to a regular fluid what a superconductor is to a regular electrical conductor. Until the mid-1990s the only laboratory superfluids known were liquids of both helium isotopes, 4He and the rarer 3He.
In principle, a superfluid gas flowing around a closed loop can keep flowing (or “persist”) forever. At a conceptual level, persistent superflow around a loop can be understood as analogous to a twisted loop of ribbon. Imagine taking a length of ribbon, putting a twist in it, and then bringing the two ends of the ribbon together and permanently gluing them. The twist in the loop of ribbon represents the flow
|
BOX 3–2 Fermions and Bosons According to the basics of quantum theory, any two electrons are identical. In fact, any two atoms, so long as they are the same kind of atom, are also identical. This simple statement has some extremely far-reaching consequences. If we exchange any two identical particles, the system will act in precisely one of two ways, depending on whether the particles are fermions or bosons. Composite particles composed of fermions may be either fermions or bosons, depending on the number of fermionic constituents. Particles composed of an even number of fermions are themselves bosons; those composed of an odd number of fermions are themselves fermions. Fermions are constrained by the Paul! exclusion principle, which states that only one fermion can occupy a given quantum state at a time. Bosons, on the other hand, are not constrained by the Paul! exclusion principle. At low temperatures, bosons can therefore collect into the same energy state. In fact, the more bosons that are in a state, the more likely it is that still more will join, resulting in Bose-Einstein condensation. |
of the superfluid. The persistence of the flow is just a consequence of the difficulty one would have in removing the twist from the loop without cutting the loop. One can crumple the loop, one can stretch it, but the twist is trapped in the loop by topology. “Cutting the loop” in this analogy means destroying the BEC locally. But at sufficiently low temperatures, the gas wants to remain in a condensed state. So while the unavoidable effects of friction along the flow of the fluid try to stop the flow, until there is a concentrated source of energy to destroy the condensate, it will flow forever.
Atoms come in two kinds: “social” bosons and “loner” fermions (see Box 3–2). The fermions are as important as the bosons that we have talked about so far. Indeed, there would be no chemical elements and no life as we know it if fermions were not the way they are. Aside from fermionic atoms, a fermionic particle of particular importance is the electron. Most properties of chemical elements are determined by electrons, and many properties of solids are determined by them as well. This includes not just their electrical conductivity (or superconductivity) but also the thermal conductivity and magnetic properties of metals. Atomic physicists in the last few years have extended the study of ultracold, degenerate gases to include fermionic atoms.
CONDENSED MATTER PHYSICS IN DILUTE ATOMIC SYSTEMS
In ordinary liquid or solid matter, atoms are tightly packed and pressed against each other, typically separated by a fraction of a nanometer. Interactions between atoms are complicated and often not exactly known. In contrast, in dilute gases
the density is a billion times lower: The atoms are separated by thousands of nanometers, a regime where the interactions are well known and characterized. In addition, instead of interacting with a large number of neighbors, the interactions between atoms tend to be largely pairwise only, considerably simplifying the theoretical description of these systems as well as their control in the laboratory. At such distances, however, the normal short-range effects of quantum mechanics, so critical to the physics of solids, are largely suppressed.
One important application of ultracold atom physics is to develop scale models that can tell us about condensed matter systems. To construct such systems as we scale up the distance between atoms, we have to scale the temperature down, way down, so as to increase their de Broglie wavelength. This renders quantum mechanics once more dominant. Indeed, temperatures a billion times lower than usual are required to mimic, in a gas, the physics of a solid. Fortunately, nano- or even picokelvin temperatures can now be achieved in many laboratories using a combination of cooling techniques.
Tuning the Interactions Between Atoms
A very powerful experimental tool available in cold-atom studies is the abil ity to adjust the strength, and even the nature, of the interaction between nearby atoms. This is accomplished simply by adjusting the ambient magnetic field near a so-called Feshbach resonance (see Box 3–3). As the magnetic field approaches the resonant value from one side, the interactions are attractive and become extremely strong. On the other side of the resonance, the interactions are effectively repulsive. One can literally dial in the interaction one wants—whether strong, weak, or zero, whether attractive or repulsive—and one can even change it as an experiment is in
|
BOX 3–3 Feshbach Resonances During a collision, two atoms can stick together for a brief time and form a short-lived molecule. If the molecular state has a different energy than the colliding atoms, this time is very short. If the magnetic field produced by the electrons in the molecule differs from that of the atoms, one can use an external magnetic field to vary the energy difference between the atomic and molecular states. Near the field where the energies match, the so-called Feshbach resonance, the atoms can stick together for a long time. On one side of the resonance, the effective interaction between the colliding atoms is strongly repulsive; on the other side it is strongly attractive. Right at the resonant field, atoms can form molecules without release of any heat. This has opened up the possibility of ultracold chemistry and coherent conversion of atoms into molecules. Recently, such ultracold association processes have been extended to three and four atoms. |
progress. This extremely powerful tool, without a counterpart in condensed matter physics, has led to remarkable developments such as the discovery of high-temperature superfluidity in a fermionic gas and the creation of molecular condensates. It also offers extraordinary future promise—for instance, in quantum information science, the topic of Chapter 7.
When the effective interaction between two ultracold fermionic atoms is repulsive, then there is a closely lying bound state of a bosonic diatomic molecule. When the effective interaction is weakly attractive, on the other hand, and for low enough temperatures, the fermions favor arranging themselves into weakly bound pairs of atoms of opposite momentums called Cooper pairs. This pairing mechanism was discovered by Bardeen, Cooper, and Schrieffer (BCS) to lie at the origin of superconductivity.
As noted, it is possible simply by varying the ambient magnetic field to tune a system from the “repulsive” side of the Feshbach resonance, where the atoms can combine into stable molecules and form a molecular BEC, to the “attractive” side of the resonance, where the situation is as in superconductivity. The difference is that the formation of Cooper pairs is now between fermionic atoms instead of between electrons. In this way it is possible to transform a molecular condensate into a superfluid of paired fermionic atoms. It had never been possible in the past to study this BEC-BCS crossover in the laboratory, but the availability of ultracold fermionic atomic gases now makes such studies possible.
High-temperature superconductivity, a discovery that made the newspaper headlines in the 1980s, is not yet fully understood and remains a daunting challenge for theoretical condensed matter physics. In ultracold fermionic gases, a new form of high-temperature superfluidity was discovered in a breathtaking series of experiments performed in 2004 and 2005. These observations are now the starting point for an investigation of different mechanisms for pairing fermions and superfluidity, and there is much hope that this work will contribute significantly to understanding high-temperature superconductivity in solids. A goal of such studies is to develop systems that have the more realistic features of high-temperature laboratory superconductors.
Optical Lattices
Many experiments with cold gases are performed with the samples confined in bowllike traps. Electrons and atoms in solids, on the other hand, live not in a bowl but in a corrugated, undulating, egg-carton-like potential that arises from the ions of the solid being arrayed in a crystalline lattice. An increasing number of ultracold atom experiments are being done in periodic potentials as well, with the periodicity being imposed experimentally by means of an optical lattice.
Using optical lattices—formed by counterpropagating lasers producing standing electromagnetic waves—one can control to an unprecedented degree the environment in which the atoms sit. For example, one can produce lattices in one, two, and three dimensions with a wide variety of lattice spacings and structures in which neutral atoms can be trapped (see Figure 3–3). When the depth of the corrugation is relatively shallow, the atoms moving through it pick up the qualities of electrons in a good conductor and can move almost freely within the crystal.
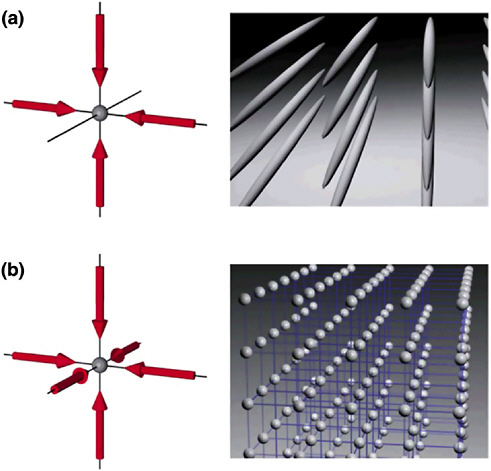
FIGURE 3–3 Optical lattices are periodic potentials formed by the intersection of several laser beams in ultra-high-vacuum chambers. The left-hand panels show laser configurations forming (a) two- and (b) three-dimensional potentials. The right-hand panels are schematics of the corresponding arrays of neutral atoms that can be trapped by these potentials. SOURCE: I.Bloch, Johannes Gutenberg University, Mainz, Germany.
As the lattice wells become deeper, more exotic condensed matter behavior kicks in. For instance, one can convert from a superconducting state, where the atoms are delocalized over the whole lattice much like electrons in a superconductor, to a so-called Mott insulator state. In the latter state, each lattice site is host to a precise number of atoms, and there is no possible transport of atoms from one site to the next (see Figure 3–4).
Optical lattices give us the ability to simulate a vast range of conditions expected in condensed matter systems, such as high-temperature superconductivity in layered oxides and numerous other exotic states of quantum matter. Indeed, in such situations the trapped atomic systems can be thought of as a form of quantum simulator (see discussion in Chapter 7). Using trapped atoms holds great promise; using cold atom systems directly to simulate the behavior of various physical systems is already a possibility with optical lattices. For example, the Hubbard model, a collection of particles localized on a lattice and interacting with their nearest neighbors, is a key system studied in condensed matter physics. By constructing in the laboratory an analog system of fermionic or bosonic atoms trapped in an optical lattice, it is possible to measure the properties of the Hubbard model over wide ranges of dimensionality, lattice filling, interaction strength, temperature, and more. In addition, one can also exploit the atomic spin to achieve an additional class of simulations: In both fermionic and bosonic systems, the atomic spin is an important feature that influences both microscopic, single-atom behavior and collective behavior, like superfluidity. Recent experiments provide evidence for a new
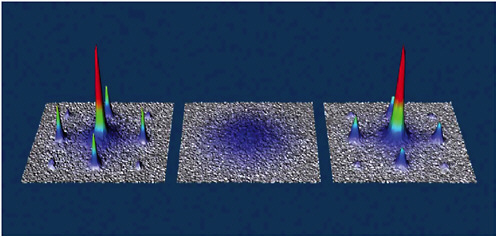
FIGURE 3–4 Momentum distribution of ultracold atoms in an optical lattice, illustrating the transition from a superfluid to a Mott insulator state and back. SOURCE: I.Bloch, Johannes Gutenberg University, Mainz, Germany.
state of matter, a supersolid, in which solid helium exhibits superfluidity at low temperatures. This remarkable coexistence of ordering of atomic states underlying superfluidity and crystalline ordering in the absence of an external periodic potential was generally unexpected. In optical lattices, which have such a potential, the two kinds of order have been observed to coexist. Studying the behavior of bosonic atoms in such lattices as the potential is turned down to zero might give us insight into viable scenarios for superfluidity in solid helium. Finally, the extension of optical lattices to molecular systems increases the size and utility of the ultracold atom “toolbox” even more, a point to which we return shortly.
Vortices
Another tool in the simulated solid toolbox is the vortex. When a superfluid is rotated, the rotation is concentrated into tiny tornado-like quantized units called vortices (see Figure 3–5). At fast rotations, which can be generated simply by “stirring up” the bowl in which the condensate is confined, the superfluid is pierced by hundreds of these tiny twisters, which organize themselves into regular arrays, rows, and columns. A whole new class of exotic states of matter is predicted to occur at high rotational speeds, when the number of vortices approaches the number of atoms in the condensate. This system is mathematically closely related to the quantum Hall effect of electrons in two-dimensional semiconductors, which has led to an unprecedented accuracy in the measurement of electrical resistances. Such exotic states are now close to being produced in Bose systems and are expected in Fermi systems as well.
The overlap between cold-atom physics and condensed matter physics described in the previous paragraphs will create stunning opportunities for advances in experiment and theory over the next decade.
MOLECULES AND CHEMISTRY
While some of the most fascinating science in the past decade has emerged from the study of ultracold atoms, the coming decade may well be the decade of cold molecules. In the last 2 or 3 years, there have been a number of breakthroughs in the efforts to extend the techniques of cold atoms to the nascent field of cold molecules.
A molecule in the simplest case with only two atoms is much like a tiny barbell with one atom at each end. The molecule can vibrate, with the barbell becoming alternately longer and shorter, and it can rotate, whirling end over end like a tiny baton. Chemical reactions take place during collisions, when molecules hit each other randomly, sometimes end to end, sometimes in the middle. A key means of
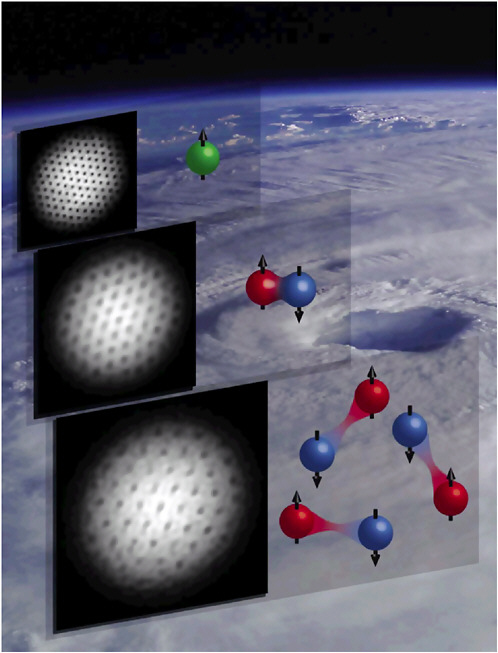
FIGURE 3–5 Vortices in gases. Shown are a vortex pattern in bosonic sodium atoms (green cartoon) in a magnetic trap; vortices in tightly bound lithium molecules (red-blue cartoon); and a vortex lattice in loosely bound fermion pairs on the Bardeen-Cooper-Schrieffer (BCS) side of a Feshbach resonance. The background shows a classical vortex (Hurricane Isabel in summer 2003). SOURCE: W.Ketterle, Massachusetts Institute of Technology.
controlling these collisions, and thereby controlling molecular chemistry, is to cool the molecules to low temperatures.
Why is control of chemistry so dependent on the temperature of the colliding molecules? The point is that the random molecular orientations that are normally present create a lack of control over the collision processes that lead molecules to form new compounds. In many molecules, one end is electrically positive and the other is electrically negative. The electrical charge separation in a molecule means that pairs of molecules interact with each other over relatively large distances. At low temperature, simply applying an electric field can make all the molecules line up. This allows the detailed study of chemical reactions with exquisite control, allowing scientists to tease out the fundamental nature of molecular interactions. This fundamental understanding will likely affect numerous areas of science and technology 20 years from now.
In the last half-decade, there have arisen two rather distinct approaches for creating cold molecules. The first proceeds by first creating molecules using conventional “hot” chemistry, then cooling them down via collisions with cold noble gases. The second approach is to first create ultracold atoms and then induce them to stick together, to react and form molecules, in such a way as to preserve the initial low temperatures. These two basic approaches have their own relative strengths and weaknesses. The first approach is more general: It can work for many different kinds of molecules. The second approach is more spectacular: The ultimate temperature reached by recombination can be nearly as low as the temperature of the coldest atomic BECs and recently led to the formation of a molecular BEC. It is hard to predict which will be the more widely practiced method 10 years hence. Most likely both will still be in play, because their strengths are complementary and the choice will depend on the ultimate application.
The concept of superfluidity is discussed earlier in this report, with the persistence of the flow explained as analogous to the difficulty in removing a twist from a ribbon loop without cutting the ribbon. A fascinating possibility with molecular condensates is that the internal degrees of freedom of the molecules may change the rules that govern taking the twist out of a loop. The metaphorical ribbon that describes the superfluid flow in a molecular gas is embedded in a higher-dimensional space than an ordinary twisted ribbon. Under certain circumstances, one maybe able to bend and stretch this higher dimensional ribbon to remove the twist without tearing the ribbon! Some higher dimensional “ribbon worlds” allow for this untwisting, and some do not. The consequences of this seemingly unworldly but very real distinction might be seen in the presence or absence of friction in a BEC of molecules.
Once the formation of ultracold molecules has become somewhat routine, it will also become possible to start exploring chemistry near absolute zero. For
example, it is possible that in the future Feshbach resonances could be exploited to enhance the rate of specific chemical reactions by orders of magnitude and to achieve an exquisite degree of selectivity between competing product channels.
Chapter 7 contains an extended discussion of quantum information and quantum computing. In the context of a discussion of future research in cold molecules, it is worth noting that the key technical challenge in quantum computing is to develop a system of quantum bits that interact with one another so as to form gates but that do not interact with their environment, so as to avoid decoherence. Trapped neutral atoms present one possible AMO realization of this, as do trapped ions (see Chapter 7). Neutral atoms are well isolated from the environment, and do not easily interact with one another; on the other hand, ions interact easily with one another, but their interactions are too long range, and they are more sensitive to their environment. Ultracold molecules in an optical lattice may offer an optimal compromise here.
ATOM OPTICS
The de Broglie wavelength of atoms is inversely proportional to the square root of their temperature. It is exceedingly small at normal (room) temperatures, about the size of the atom itself. This is why it is normally difficult—although not impossible—to observe the wave nature of atoms. At the extreme low temperatures that can now be achieved by laser cooling and other cooling techniques, however, the wave nature of atoms and molecules completely dominates their behavior. The analogy between atomic and optical waves can then be exploited in the emerging field of atom optics. For example, it is possible to build a matter-wave analog of an optical interferometer, a device that holds great promise for both fundamental science and applications (Box 3–4). Chapter 2 discusses how these interferometers are beginning to find applications in precision measurements. See Figure 3–6 for an example of a matter-wave interferogram.
Before the realization of atomic Bose-Einstein condensation in 1995, the situation in atom interferometry had been similar to the situation in optics before the invention of the laser, and the spatial and temporal coherence of sources required to produce interference fringes was largely obtained by filtering techniques, much as had been the case in traditional optics. This approach, which does not necessitate the use of ultracold atomic or molecular beams, let alone quantum-degenerate ones, is still used in many groundbreaking experiments on matter-wave interferometry.
While it is relatively easy to observe quantum interferences with small objects such as electrons and atoms, observations become harder for larger and larger molecules, which interact more strongly with their environment. Molecular-interference
|
BOX 3–4 Atom Interferometers In a conventional optical interferometer, a light beam is split into two or more paths that are recombined later on a detector. If the beams from the different paths are in phase, their fields add up and the detector measures bright light. If they are out of phase, on the other hand, the fields cancel each other and the signal at the detector indicates darkness. Similarly, in an atom interferometer, as in Figure 3–4–1, one can observe the coherent addition or cancellation of matter waves. By carefully monitoring the variations of “bright” and “dark” periods, one can determine with exquisite precision the physical processes that influenced the dynamics of the atoms in the two paths of the interferometer. For instance, atom interferometers have been used to carry out tests of Einstein’s equivalence principle at the atomic level and are being developed into rotation and inertial sensors with unprecedented sensitivity and accuracy. 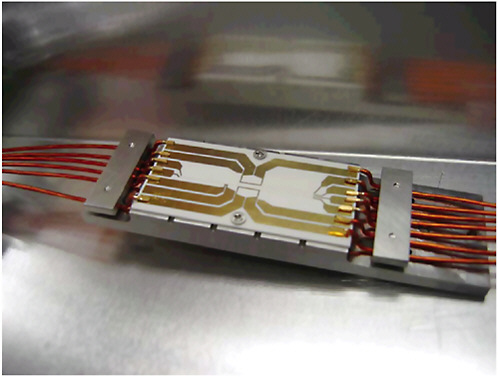 FIGURE 3–4–1 Atom interferometer chip mounted and wired. SOURCE: Quantum Sciences and Technology Group, Jet Propulsion Laboratory, National Aeronautics and Space Administration. |
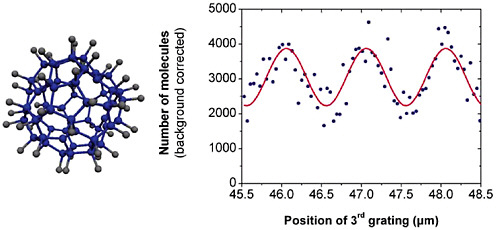
FIGURE 3–6 Interferogram (right) of C60F48(left). This molecule currently holds the record in complexity (108 atoms) and mass (1,632 amu) for matter-wave interferometry. This molecule exists in two isomers of different symmetry. Both were present in the same experiment. SOURCE: M.Arndt, L.Hackermuller, and A.Zeilinger, Faculty of Physics, University of Vienna, Austria.
experiments are far from easy, but recent work shows that large, complex molecules can indeed interfere and reveal their quantum nature. Such experiments have now been carried out with molecules containing 100 or more atoms bound in a single interfering object (see Figure 3–6). Especially in the context of quantum information science, understanding the mechanisms of decoherence is a central issue. In matter-wave interferometry, decoherence can be traced back to the fact that the molecule exchanges information with its environment via thermal photons, the radiation emitted by any object with a temperature that is not absolute zero. Specifically, each photon emitted by the molecule transfers information about the molecule position, and indeed decoherence due to heat radiation is of particular relevance for macroscopic objects.
In a BEC near absolute zero, practically all atoms are in the same quantum state. This situation is similar to that in a laser, where a vast number of photons have precisely the same wavelength, direction, and phase. Extracting an atomic beam from the BEC makes it possible to realize the matter-wave analog of a laser beam (see Figure 3–7).
Nonlinear Atom Optics
Many laser applications rely on the ability to mix photons of different wavelengths and/or directions of propagation using techniques of nonlinear optics. For
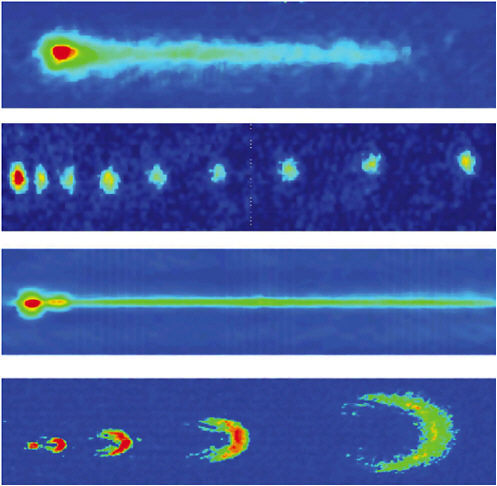
FIGURE 3–7 Picture gallery of coherent atom beams, so-called atom lasers, extracted from a BEG, the red dot at the left of each figure. The beams are shown both pulsed and continuous. SOURCE: W.Ketterle, Massachusetts Institute of Technology; I.Bloch, Johannes Gutenberg University, Mainz, Germany, and T.W.Hänsch, Max-Planck-Institut für Quantenoptik, Garching, Germany; M.Kasevich, Yale University; and W.D.Phillips, National Institute of Standards and Technology.
instance, it is possible to annihilate two photons of one frequency to produce a new photon at double the frequency (second-harmonic generation) or to mix three optical fields to produce a fourth one (four-wave mixing). There is also an atomoptic analog to nonlinear optics. Four-wave mixing in matter waves—the coherent generation of a new matter wave out of three incident waves—and the launching of solitons (waves that can propagate long distances without changing their shape)
soon followed the first experiments in BEC. The mechanisms by which two atoms combine into a diatomic molecule can be thought of as analogous to secondharmonic generation in optics, except that instead of annihilating two photons to create a new one with double the frequency, we now have the annihilation of two atoms and the creation of one molecule (with double the mass). In addition, it has proven possible to mix optical and matter waves.
The Pauli exclusion principle forbids putting two identical fermions in the same quantum state and hence building a fermionic atom laser. Nonetheless, fermionic atoms may offer a viable alternative to bosons for interferometric applications that require the detection of tiny path differences in the arms of the interferometer. This is somewhat analogous to the situation in white-light interferometry, which has proven exceedingly useful in many conventional optics applications. An advantage of quantum-degenerate fermions in this context is that they do not suffer the kind of collisions that bosons are subject to. These collisions can lead to decoherence and the destruction or random shift of an interference pattern. The absence of collisions between ultracold fermions is another direct consequence of the Pauli exclusion principle.
Integrated Atom Optics
In electronics and in conventional optics, the miniaturization of components and their integration into networks leads to powerful tools and devices. Similarly, integrated atom optics could result in powerful devices that combine many atom optical elements into integrated quantum matter-wave circuits. Current research seeks to develop atom chips to implement atom-based devices on a small scale. On a single substrate one can, for example, incorporate conducting wires, magnetic elements, and optical components to produce miniaturized atom optical elements that confine, control, and manipulate matter waves. On that same substrate one can also incorporate atom detection and signal conversion elements. Atom chips are now being developed for a whole new range of applications, from novel sources of quantum-degenerate gases to compact atom interferometers. In combination with techniques of cavity quantum electrodynamics and with microfabricated resonators, they may also lead to the development of novel systems for quantum information technologies.
Quantum Atom Optics
Improvements in detectors will transform atom optics to quantum atom optics, the matter-wave analog of quantum optics. Many future applications of atom optics will be tied to the development of improved neutral atom detection schemes.
As experiments become more and more sophisticated, it will become increasingly important to go from simple digital photographs of the clouds and moving pictures that accurately record the density and spin patterns of the samples, to detection schemes that can address questions related to the precise quantum state of the system under study. They will include single-atom detectors using detectors based on optical resonators developed for cavity quantum electrodynamics, charge-coupled devices, or sophisticated image-processing techniques.
REACHING OUT: PLASMAS, NUCLEAR PHYSICS, AND MORE
New and unforeseen bridges are developing between ultracold physics and areas of research that might at first sight seem far removed, including systems under extreme conditions such as plasma physics, high-energy physics, and astrophysics.
Cold Plasmas
Plasmas are ubiquitous in the universe. Most of observable space is in a plasma state, so that plasma physics is the natural science that we apply to understand the phenomena of space. Clouds of charged particles, electrons, and ions surround Earth. Approximately 99 percent of the interstellar medium is composed of interstellar gas that consists partly of neutral atoms and molecules, as well as charged particles such as ions and electrons. Plasma physics and astrophysics require detailed atomic spectra and structure calculations to diagnose and predict properties of plasmas, as well as to understand the temperatures and velocities of astrophysical objects.
Ultracold atoms add a new frontier to plasma physics: the creation of plasmas colder than any ever created before (Box 3–5). Cold neutral plasmas, with equal numbers of positive and negative charges, are challenging systems for computational physics, in particular for the types of molecular dynamics calculations that are also used to simulate thermonuclear plasmas. Cold neutral plasmas can recombine into neutral atoms—and this is at the heart of the experiments looking at the formation of the antimatter version of hydrogen, antihydrogen. Another fascinating aspect of cold plasmas is that they can become so cold that instead of acting like a gas, they behave more like a liquid or even a crystalline solid. Such strongly coupled plasmas are thought to lie at the core of white dwarf stars and possibly massive Jupiter-sized planets.
Nonneutral plasmas are created from laser-cooled ions held together by strong magnetic fields. These plasmas can form beautiful “crystals,” with symmetries and structures depending on the shape of the confining field (see Figure 3–8). These
|
BOX 3–5 Cold Plasmas At cryogenic temperatures, trapped-ion plasmas become strongly coupled and, at sufficiently low temperatures, form crystalline states. These crystalline states have been observed and studied with laser-cooled atomic ion plasmas stored in Penning and radio-frequency traps. Strongly coupled plasmas are models of dense astrophysical matter, as well as quark-gluon plasmas produced in ultrarelativistic heavy ion collisions. Recently it was theoretically shown that laser-cooled ion plasmas in a Penning trap could be used to measure the exponential enhancement of close collisions in a strongly coupled plasma. This is exactly the same enhancement predicted over 50 years ago for the nuclear fusion rate in dense stellar interiors. Future work can provide the first precision measurements of this enhancement. This is yet another example of how measurements done on model AMO systems can advance our understanding of condensed matter systems (in this case, our understanding of nuclear reactions in dense stellar interiors). Small, one-dimensional crystal strings of three or four ions have been used to demonstrate quantum gates and some of the basic building blocks of a quantum computer. These few ion experiments demonstrate the control that is possible with trapped ions. Long relaxation times of greater than 100 seconds have been measured on ground-state hyperfine coherences in cold plasmas of trapped ions. This combination of control and long coherence times makes cold plasmas of trapped ions a fertile ground for investigating the generation of entangled or squeezed spin states, which can be used in sub-shot-noise spectroscopy. In recent years, a significant effort in the AMO community has been going into cooling, trapping, and controlling molecules in the gas phase. In trapped-ion plasmas, cooling proceeds quite simply by using an atomic ion species that can be laser-cooled to sympathetically cool molecular ions. In this manner crystals of molecular ions have been formed in an ultrahigh vacuum, without perturbation of the internal states for periods longer than many minutes. These translationally cold molecular ions provide a good starting point for precision spectroscopy and for the investigation of techniques to control the molecular populations. |
systems probe the fundamental aspects of self-organization, with only simple electric repulsion leading to highly organized states. Further applications of cold, trapped plasmas range from the generation of intense positron beams for material science studies to the generation of squeezed spin states that can be used to improve atomic clocks.
The Bose condensation of Cooper pairs, discussed earlier in this report in the context of superconductivity in metals, is also familiar in nuclear physics, where BCS pairing explains reduced moments of inertia of heavier nuclei. In astrophysics, condensates of pi mesons and of K mesons have been studied as possible states of neutron star matter, where both the neutron and proton components undergo BCS pairing to become superfluid. Such superfluidity most likely underlies the observed glitches, or sudden rotational speedups, observed in some 30 pulsars to date.
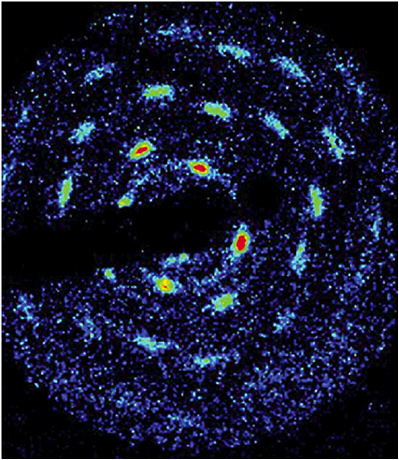
FIGURE 3–8 Time-resolved Bragg scattering is used to obtain information on the spatial correlations of trapped and laser-cooled beryllium. The pattern is consistent with Bragg scattering off a single body-centered cubic (bcc) crystal. Analysis of gated and time-averaged patterns like those shown here indicates that with several hundred thousand trapped ions, bcc crystals are always observed. SOURCE: National Institute of Standards and Technology.
A close connection has emerged between the quark-gluon plasmas formed in heavy ion accelerators such as Brookhaven National Laboratory’s relativistic heavy ion collider (RHIC) facility and systems of ultracold trapped laboratory fermions near the BEC-BCS crossover (Box 3–6). Both systems are extremely strongly coupled forms of matter. As observed both at RHIC and in the AMO laboratory, such matter flows with minimal viscosity. Understanding such connections will be a fascinating challenge to both high-energy nuclear and AMO theories.
|
BOX 3–6 Boson Condensates at the Relativistic Heavy Ion Collider (RHIC) Bosonic condensates formed from the pairing of spontaneously created quarks and antiquarks are fundamental features of the vacuum and the structure of elementary particles such as the nucleon; such condensates underlie the spontaneous breaking of the chiral symmetry (the symmetry between right- and left-handedness) of the strong interactions. One of the important aims of ultrarelativistic heavy ion collisions at the RHIC and, starting in 2007, at the Large Hadron Collider (LHC) is to produce chirally restored matter in the form of quarkgluon plasmas. There, one is asking the opposite of the question asked in condensed matter physics—namely, What are the properties of Bose-Einstein “de-condensed” matter? |
THE SYNERGY BETWEEN EXPERIMENT AND THEORY
AMO science enjoys a very close interaction and synergy between theory and experiment, which benefit each other in numerous ways. Indeed, the hallmark of science is the parallel hand-in-hand progress that theory and experiment make together, each playing a vital role, sometimes with theory leading the way, other times with experiment doing so. Nowhere is this more true than for the areas of research described in this chapter.
While experimental breakthroughs constantly challenge theorists, the reverse is also true, with theorists suggesting new experimental paths and novel ways to reach exciting regimes where new physics can be explored. For example, the possibility of using Feshbach resonances to achieve new regimes of ultracold physics was suggested by theorists. This proposal led to the creation of molecular condensates and opened the way to one of the most exciting recent discoveries in AMO physics: observation of the crossover between Bose condensation and Cooper pairing of fermions. As a result, there is a new link between atomic and condensed matter physics. Likewise, the idea of using ultracold atoms trapped in optical lattices to study the transition between superfluidity and another quantum state called a Mott insulator originated in theoretical studies. The experimental realization of these states opens the way to exciting new potential approaches to quantum information processes and to the realization of quantum simulators to investigate in detail key problems in condensed matter physics. Nonlinear atom optics and the generation of vortex lattices in BECs, along with many other examples of theory leading experiment, illustrate that in AMO science, there is close cooperation between theory and experiment.




















