7
Respiratory Tract Toxicity and Cancer
This chapter reviews information on the effects of trichloroethylene on the respiratory system, particularly information generated since the U.S. Environmental Protection Agency released its draft health risk assessment (EPA 2001b). In keeping with the committee’s charge, the chapter focuses on hazard characterization and the mode of action for trichloroethylene toxicity, assessing the available information from in vitro, animal, and human studies. Cancers of the respiratory tract are also considered.
RESPIRATORY TRACT TOXICITY
In Vitro Studies
Trichloroethylene, tested in swine trachea in vitro for its effects on smooth muscle contraction and epithelial release of prostanoids, did not alter the basal tone of tracheal smooth muscle but did potentiate the muscle contractile responses to acetylcholine and histamine in a concentration-dependent manner. Trichloroethylene increased epithelial prostaglandin E2 release and decreased acetylcholinesterase activity. Such responses are consistent with the reported effects of trichloroethylene exposure on increasing airway hyperresponsiveness and asthma (Chen et al. 2005).
In Vivo Studies
The time course of trichloroethylene-induced pulmonary injury was followed in CD-1 male mice exposed to [14C]trichloroethylene in a single
oral dose of 2,000 mg/kg (Forkert and Birch 1989). Clara cells of the bronchiolar epithelium showed necrotic changes within 1 hour of dosing and most Clara cells were severely vacuolated by 24 hours. Twenty percent of the lung burden at 4 hours was covalently bound. The study indicates that the highly metabolic Clara cells are targets of trichloroethylene toxicity in the respiratory tract.
Human Studies
There are few reports of noncancer pulmonary toxicity in trichloroethylene-exposed humans. A study of respiratory findings in gun factory workers exposed to multiple solvents indicated significant effects of smoking and exposure to solvents, with smoking having the most important effect on asthma-related symptoms. Trichloroethylene was only one of many solvents to which the workers were exposed (Cakmak et al. 2004).
Toxicokinetics and Mode of Action
Pulmonary toxicity induced by trichloroethylene is associated with cytochrome P-450-dependent bioactivation to reactive metabolites (see Figure 7-1). The predominant pathway of trichloroethylene metabolism is oxidation via the cytochrome P-450 system, mainly by CYP2E1, although other P-450 enzymes including CYP1A1/2, CYP2B1/2, CYP2C11/6, and CYP2F have been implicated (Guengerich et al. 1991; Nakajima et al. 1992a; Forkert et al. 2005). Oxidative metabolism of trichloroethylene yields the primary metabolites chloral, trichloroethylene oxide, and dichloroacetyl chloride. Chloral, a predominant metabolite of trichloroethylene, is rapidly converted to chloral hydrate, which then undergoes oxidation and reduction by aldehyde-dehydrogenase and alcohol-dehydrogenase enzymes to form trichloroacetic acid and trichloroethanol (Green and Prout 1985; Dekant et al. 1986a). Clara cells isolated from the mouse lung are known to efficiently metabolize trichloroethylene to chloral and trichloroacetic acid. Recent studies of recombinant (r) cytochrome P-450s and rodent and human lung microsomes revealed that rat and human rCYP2E1, rCYP2F, and rCYP2B1 were all capable of mediating trichloroethylene metabolism to chloral hydrate (Forkert et al. 2005). Rat rCYP2E1 exhibited greater affinity than rat rCYP2F4 and rCYP2B1 and human rCYP2E1. More recently, the same investigators (Forkert et al. 2006) suggested that CYP2F2 might play a greater role than CYP2E1 in the metabolism of trichloroethylene in the mouse lung. Treatment of CYP2E1-null and wild-type mice with trichloroethylene led to bronchiolar damage that correlated with the formation of dichloroacetyl adducts in the Clara cells. These findings provide evidence
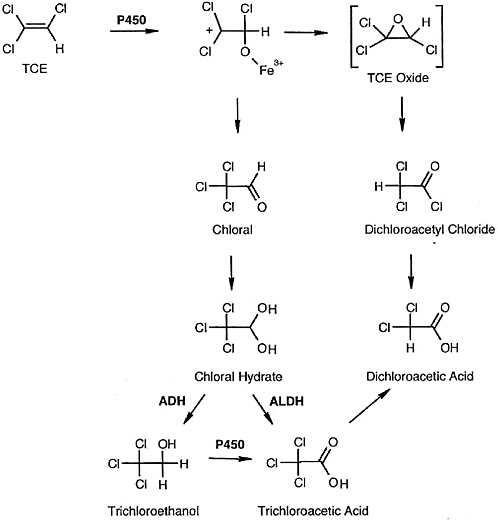
FIGURE 7-1 Proposed scheme of trichloroethylene metabolism. ABBREVIATIONS: TCE, trichloroethylene; ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase. Source: Forkert et al. 2005. Reprinted with permission; copyright 2005, American Society for Pharmacology and Experimental Therapeutics.
for bioactivation of trichloroethylene within the Clara cells, predominantly involving CYP2F2, that correlates with bronchiolar cytotoxicity.
The rates of chloral hydrate production in human lung microsomes were low and were detected in only three of eight subjects (Forkert at al. 2005). Furthermore, the rates of chloral hydrate production were substan-
tially higher in murine than in human lung. Alcohol dehydrogenase, the enzyme responsible for metabolizing chloral to trichloroethanol, is known to be at low concentrations in lung tissue (Sorokin 1970), with chloral being the major metabolite in these cells. Trichloroethanol glucuronide, a major metabolite in the liver, is not formed in the Clara cells due to lack of glucuronyltransferase (Odum et al. 1992). Recent studies of mice treated intraperitoneally with high doses (500-1,000 mg/kg) of trichloroethylene found dichloroacetyl protein adducts in Clara cells (Forkert et al. 2006). The proximate toxicant for the Clara cell, whether chloral, dichloroacetyl chloride, or another metabolite, is still under study.
Exposure to trichloroethylene occurs mainly through inhalation and oral routes and rapid absorption occurs by both routes. Absorption of inhaled trichloroethylene is both rapid and extensive. Regardless of the route of exposure, unmetabolized trichloroethylene is eliminated by exhalation. Consequently, pulmonary airways are exposed to trichloroethylene regardless of the route of exposure. However, the amount of pulmonary exposure to trichloroethylene after an oral exposure is dose dependent and will be high only after an oral dose exceeds the capacity of the liver to metabolize the trichloroethylene. After inhalation exposure, trichloroethylene is rapidly absorbed through the alveolar endothelium due to a high blood-gas partition coefficient. However, the blood-gas partition coefficient in humans is 1.5- to 2.5-fold lower than that in mice and rats, respectively, which suggests that delivery of trichloroethylene to the circulatory system for translocation to target organs may be significantly less efficient in humans. This factor should be taken into account when using animal data in risk assessment analysis for trichloroethylene (Sato et al. 1977; Prout et al. 1985; Clewell et al. 1995).
The Clara cells develop vacuoles (Forkert and Birch 1989; Odum et al. 1992) after exposure to trichloroethylene and proliferate with continued exposure (Green et al. 1997b). Considering the site of induced tumors in mice and the observed toxicity sites, it appears that the Clara cell is the most sensitive site in the respiratory tract with respect to the toxicity of inhaled trichloroethylene. CYP2E1 and CYP2F2 are highly concentrated in mouse Clara cells (Buckpitt et al. 1995; Forkert 1995) and the presence of dichloracetyl protein adducts in these same cells (Forkert et al. 2006) suggests that the bioactivation of trichloroethylene takes place in these cells. The low CYP2E1 concentration in human Clara cells suggests that humans are not as sensitive as mice for the development of lung tumors as a result of trichloroethylene exposures at ambient levels. This hypothesis agrees with the results of most epidemiology studies (discussed later in this chapter), which do not indicate a strong association between trichloroethylene exposure and increased incidence of lung tumors.
Whereas trichloroethylene is both acutely toxic and carcinogenic to the
mouse lung after exposure by inhalation, it is not carcinogenic in the rat lung and is markedly less toxic after acute exposure (Stewart et al. 1979; Fukuda et al. 1983; Maltoni et al. 1986, 1988; Davidson and Beliles 1991). The lack of toxicity or carcinogenicity in the lungs of mice after oral dosing is presumably due to extensive hepatic metabolism reducing the amount of trichloroethylene that reaches the lungs. Adverse effects on human lungs have not been reported. The primary effects of trichloroethylene on mouse lungs in all studies have been morphologic and biochemical changes in the nonciliated Clara cells. The only other toxicologic responses noted in the lung after exposure to trichloroethylene were fibrosis in the mouse (Forkert and Forkert 1994) and a decrease in surfactant phospholipid after exposure of rats and mice to high doses (3,000 mg/kg) of trichloroethylene (Scott et al. 1988). Loss of cytochrome P-450 activity and morphologic recovery of the Clara cells with repeated daily exposure of mice to trichloroethylene suggest that loss of metabolic capacity in these cells is an adaptive mechanism (Lewis et al. 1984; Forkert et al. 1985, 2005; Odum et al. 1992).
RESPIRATORY TRACT CANCER
Animal Studies
Animal carcinogenicity studies are summarized in Table 7-1. Trichloroethylene inhalation exposure caused an increased incidence of pulmonary tumors in mice (Fukuda et al. 1983; Maltoni et al. 1986, 1988) but not in rats and hamsters (Henschler et al. 1980; Fukuda et al. 1983; Maltoni et al. 1986, 1988). Oral administration of trichloroethylene did not result in lung tumors (NCI 1976; Van Duuren et al. 1979; Henschler et al. 1984; Maltoni et al. 1986; NTP 1988, 1990) in any species studied. Details of those studies follow.
Inhalation Exposure
The inhalation studies by Fukuda et al. (1983) included exposing female ICR mice and female Sprague-Dawley rats to trichloroethylene at 0, 50, 150, or 450 ppm for 7 hours/day, 5 days/week for 104 weeks followed by an observation period of 3 weeks. They observed a threefold increase in lung tumors per mouse in those exposed to the two higher concentrations but saw no increase in lung tumors in the rats.
In the studies by Maltoni et al. (1986, 1988), male and female Swiss mice and Sprague-Dawley rats were exposed to trichloroethylene at 0, 100, and 600 ppm for 7 hours/day, 5 days/week for 8 weeks and for 104 weeks (rats only). B6C3F1 and Swiss mice were also exposed for 78 weeks to trichloroethylene at 0, 100, 300, or 600 ppm. Animals were held for obser-
TABLE 7-1 Animal Carcinogenicity Studies of Trichloroethylene
|
Reference |
Animals (Sex) |
Exposure Route |
Stabilizers |
|
Fukuda et al. 1983 |
ICR mice (F) S-D rats (F) |
Inhalation, 7 h/day, 5 days/week, 104 wk, hold 3 wk |
Epichlorohydrin |
|
Maltoni et al. 1986, 1988 |
S-D rats (M, F) |
Inhalation, 7 h/day, 5 days/week, 104 wk; hold until death |
No stabilizer |
|
|
Swiss mice (M, F) B6C3F1 mice |
Inhalation, 7 h/day, 5 days/week, 78 wk; hold until death |
No stabilizer |
|
Henschler et al. 1980 |
Wistar rats (M, F) Syrian hamsters (M, F) NMRI mice |
Inhalation, 6 h/day, 5 days/week, 18 months |
Triethanolamine |
|
Van Duuren et al. 1979 |
Swiss mice (M, F) |
Gavage, 1/wk, 89 wk |
Unknown |
|
NCI 1976 |
Osborne-Mendel rats (M, F) B6C3F1 mice (M, F) |
Gavage, 5/wk, 78 wk, hold 110 wk (rats) or 90 wk (mice) |
Epoxybutane, epichlorohydrin |
|
NTP 1988 |
4 strains of rats |
Gavage, 1/day, 5 days/week, 103 wk |
No stabilizer |
|
NTP 1990 |
F344 rats (M, F) B6C3F1 mice (M, F) |
Gavage, 1/day, 5/wk, 103 wk |
No stabilizer |
|
Maltoni et al. 1986 |
S-D rats (M, F) |
Gavage, 1/day, 4-5 days/week, 56 wk; hold until death |
No stabilizer |
|
ABBREVIATIONS: F, female; M, male; S-D, Sprague-Dawley. |
|||
vation until spontaneous death. Excess tumors were not observed in either species after the 8-week exposures. Excess pulmonary tumors were observed in both strains of mice after 78 weeks of exposure, but no excess pulmonary tumors were observed in rats after the 104-week exposures.
Henschler et al. (1980) exposed NMRI mice, WIST rats, and Syrian hamsters of both sexes to trichloroethylene at 0, 100, or 500 ppm for 6 hours/day, 5 days/week for 18 months. They observed no pulmonary tumors in any of the species. The trichloroethylene used was technical grade and contained traces of epichlorohydrin and 1,2-epoxybutane—two known carcinogenic compounds used as stabilizers.
Gavage Exposure
Van Duuren et al. (1979) tested trichloroethylene among 14 other halogenated compounds for carcinogenicity in Swiss mice. The mice (both sexes)
|
Doses/Exp Conc. |
Exposed |
Results |
|
0, 50, 150, 450 ppm |
50/group |
Threefold increase in lung tumors in mice at two higher concentrations; no increase in any tumors in rats. |
|
0, 100, 300, 600 ppm |
130-145/ group |
Increase in tumors of testis and kidney (high dose only) in males. |
|
0, 100, 300, 600 ppm |
90/group |
Excess lung tumors in both strains; liver tumors in male Swiss mice. |
|
0, 100, 500 ppm |
30/group |
No increase in any tumors, except increase in lymphomas in female mice. |
|
0, 0.5 mg |
30/group |
No excess tumors in lung, liver, or stomach. |
|
0, 500, 1,000 mg/kg (rats) 0, 1,000, 3,000 mg/kg (mice) |
50/group except 20/control |
No excess pulmonary tumors; increase in liver tumors. |
|
0, 500, 1,000 mg/kg |
50/group |
No excess pulmonary tumors; some renal and testicular tumors in two strains. |
|
0, 500, 1,000 mg/kg (rats) 0, 1,000 mg/kg (mice) |
50/group |
No pulmonary tumors; increase in liver tumors. |
|
0, 50, or 250 mg/kg |
30/group |
No excess tumors. |
were administered trichloroethylene at 0.5 mg per intragastric intubation once a week for 622 days (89 weeks). No excess tumors were observed.
The National Cancer Institute (NCI 1976) and the National Toxicology Program (NTP 1988, 1990) reported three carcinogenicity studies of trichloroethylene administered by gavage to rats and mice. The first study (NCI 1976) used both sexes of Osborne-Mendel rats and B6C3F1 mice. Animals were dosed with trichloroethylene at approximately 500 or 1,000 mg/kg (rats) or approximately 1,000 or 2,000 mg/kg (mice), 5 times/week for 78 weeks. Rats were observed for 110 weeks and mice were observed for 90 weeks. No increase in pulmonary tumors was observed in either species, but the study was not considered valid because of early mortality among the rats. The trichloroethylene was technical grade and was stabilized with epichlorohydrin and 1,2-epoxybutane. In a later study (NTP 1988), trichloroethylene stabilized by diisopropylamine was administered orally to four strains of rats at 0, 500, or 1,000 mg/kg per day, 5 days/week, for 103
weeks. No pulmonary tumors were observed, but the study was considered inadequate because of chemical toxicity and early mortality in the rats. The third study (NTP 1990) was conducted in male and female F344 rats and B6C3F1 mice with epichlorohydrin-free trichloroethylene. Doses of 500 or 1,000 mg/kg in rats and 1,000 mg/kg in mice were given by gavage 5 days/week for 103 weeks. No pulmonary tumors were observed. The study was considered adequate for demonstrating no carcinogenicity in female rats, but the male rats did not survive long enough to test adequately for carcinogenicity.
Epidemiology Studies
Because it was not possible to provide a comprehensive evaluation of the epidemiologic evidence on trichloroethylene and different cancers, the committee borrowed a previously compiled summary of the epidemiologic evidence on lung cancer from the Institute of Medicine (IOM 2003) to give some perspective on the evidence for lung cancer (see Table 7-2). The list was updated with one study published since the IOM report. Epidemiology studies do not indicate an increase in pulmonary tumors in association with trichloroethylene exposure, except for the new study in Denmark (Raaschou-Nielsen et al. 2003), which was done on a cohort of more than 40,000 blue-collar workers in 347 Danish companies where trichloroethylene was used. The standardized incidence ratio did not increase with duration of exposure, and was highest among workers with less than 1 year of exposure, particularly among females (SIR = 2.5). The authors suggested that smoking might be a confounding factor for cancers known to be related to tobacco use because of the lower socioeconomic status of the cohort and the higher prevalence of smoking among the least educated groups in Denmark. The authors also estimated that only 41% of the cohort were likely to have been exposed to trichloroethylene on the job, because the only marker of exposure used was employment in a blue-collar job at a trichloroethylene-using company. The large excess of lung cancer among females with the shortest exposures argues strongly against a causal interpretation for these findings.
Mode of Action
When trichloroethylene is inhaled, a large portion is absorbed (a greater percentage of what is inhaled is absorbed at lower concentrations) and is rapidly distributed throughout the body in the bloodstream. The liver is the major site of metabolism, but the fact that inhaled trichloroethylene causes lung tumors in mice, whereas orally administered trichloroethylene does not, suggests that pulmonary metabolism may play a role in lung
TABLE 7-2 Epidemiologic Data on Lung Cancer and Exposure to Trichloroethylene
|
Reference |
Study Population |
Exposed Cases |
Estimated Relative Risk (95% confidence interval) |
|
Cohort Studies—Incidence |
|||
|
Raaschou-Nielsen et al. 2003 |
Workers in Denmark |
|
|
|
|
Males |
|
|
|
|
<1 yr |
181 |
1.6 (1.4-1.9) |
|
|
1-4.9 yr |
193 |
1.3 (1.1-1.5) |
|
|
≥5 yr |
185 |
1.4 (1.2-1.6) |
|
|
Females |
|
|
|
|
<1 yr |
28 |
2.5 (1.6-3.6) |
|
|
1-4.9 yr |
25 |
1.6 (1.1-2.4) |
|
|
≥ 5 yr |
20 |
1.6 (1.0-2.5) |
|
Hansen et al. 2001 |
Biologically monitored workers in Denmark |
|
|
|
|
Males, ever exposed |
16 |
0.8 (0.5-1.3) |
|
|
Females, ever exposed |
1 |
0.7 (0.01-3.8) |
|
Blair et al. 1998 |
Aircraft maintenance workers in Utah |
|
|
|
|
Males |
|
|
|
|
No exposure |
22 |
1.0 (0.5-1.9) |
|
|
<5 unit-yr |
24 |
1.0 (0.6-2.0) |
|
|
5-25 unit-yr |
11 |
0.8 (0.4-1.6) |
|
|
>25 unit-yr |
15 |
0.8 (0.4-1.7) |
|
|
Females |
|
|
|
|
No exposure |
0 |
— |
|
|
<5 unit-yr |
1 |
0.6 (0.1-5.3) |
|
|
5-25 unit-yr |
0 |
— |
|
|
>25 unit-yr |
0 |
— |
|
Anttila et al. 1995 |
Biologically monitored workers in Finland |
|
|
|
|
Entire period since first measurement |
25 |
0.92 (0.59-1.35) |
|
|
0-9 yr |
11 |
1.19 (0.59-2.13) |
|
|
10-19 yr |
9 |
0.67 (0.30-1.26) |
|
|
≥20 yr |
5 |
1.11 (0.36-2.58) |
|
|
Mean personal U-TCA concentration |
|
|
|
|
<100 μmol/L |
16 |
1.02 (0.58-1.66) |
|
|
100+ μmol/L |
7 |
0.83 (0.33-1.71) |
|
Reference |
Study Population |
Exposed Cases |
Estimated Relative Risk (95% confidence interval) |
|
Cohort Studies—Mortality |
|||
|
Boice et al. 1999 |
Aircraft manufacturing workers in California |
|
|
|
|
All exposed factory workers |
78 |
0.76 (0.60-0.95) |
|
|
Duration of potential exposure (routine or intermittent) |
|
|
|
|
<1 yr |
66 |
0.85 (0.65-1.13) |
|
|
1-4 yr |
63 |
0.98 (0.74-1.30) |
|
|
≥5 yr |
44 |
0.64 (0.46-0.89) |
|
Blair et al. 1998 |
Aircraft maintenance workers in Utah |
|
|
|
|
Males |
|
|
|
|
No exposure |
51 |
1.0 (0.7-1.6) |
|
|
<5 unit-yr |
43 |
1.0 (0.6-1.6) |
|
|
5-25 unit-yr |
23 |
0.9 (0.5-1.6) |
|
|
>25 unit-yr |
38 |
1.1 (0.7-1.8) |
|
|
Females |
|
|
|
|
No exposure |
2 |
0.4 (0.1-1.6) |
|
|
<5 unit-yr |
2 |
0.6 (0.1-2.4) |
|
|
5-25 unit-yr |
11 |
0.6 (0.1-4.7) |
|
|
>25 unit-yr |
2 |
0.4 (0.1-1.8) |
|
Morgan et al. 1998 |
Aerospace workers in Arizona |
|
|
|
|
Entire TCE-exposed cohort Cumulative |
97 |
1.10 (0.89-1.34) |
|
|
Low |
45 |
1.49 (1.09-1.99) |
|
|
High |
52 |
0.90 (0.67-1.20) |
|
|
Peak: medium and high versus low and no exposure |
64 |
1.07 (0.82-1.40) |
|
Greenland et al. 1994 |
White male transformer-assembly workers, ever exposed |
NA |
1.01 (0.69-1.47) |
|
Wilcosky et al. 1984 |
White male rubber-industry workers in Ohio, cumulative exposure of more than 1 yr |
11 |
0.64 |
|
ABBREVIATIONS: NA, not available; TCE, trichloroethylene; U-TCA, urinary trichloroacetic acid. SOURCE: Adapted from IOM 2003. |
|||
tumor formation in mice. Trichloroethylene is not mutagenic but some of its metabolites are. Because trichloroethylene is lipid soluble, one would expect the compound to be delayed long enough in crossing the lung tissue into the blood to be partially metabolized by metabolically active epithelial cells (Clara cells and Type II cells) (Gerde et al. 1993). The metabolic activity of such cells toward several lipophilic xenobiotics is known to be higher in mouse cells than in rat or hamster cells, which may be part of the reason for the greater susceptibility of mouse tissue than of tissue in rats and hamsters.
Some investigators have reported that the capacity of the mouse lung to metabolize trichloroethylene to the mutagenic metabolite chloral is an order of magnitude higher than in the rat lung, whereas human lung samples have no detectable activity (Green et al. 1997b). The CYP450 (2E1) enzyme responsible for this oxidation is present in high amounts in the Clara cells of mice and in lesser amounts in Clara cells of rats. The enzyme was not detected in human Clara cells, although mRNA for the enzyme has been detected and variable amounts of CYP2E1-like metabolism has been observed in human lung microsomes (Forkert et al. 2001).
The Clara cell is the site of toxicity induced by inhalation of trichloroethylene in mice. A number of acute toxicity studies have shown that trichloroethylene selectively targets the mouse lung Clara cell, suggesting the role of Clara cells in the development of mouse lung tumors (Buckpitt et al. 1995; Forkert and Forkert 1994). Although there is no direct evidence that mouse lung tumors are derived from Clara cells, the lack of other types of epithelial cells such as Type II cells responding by cell division suggests that Clara cells play a key role in the development of lung tumors in mice exposed to trichloroethylene. Similarly, differences in the metabolic capacity of Clara cells in mice and rats are consistent with species differences in toxicity and carcinogenicity (Green 2000). Clara cells are thought to be the cells of origin of some chemically induced mouse lung tumors (Sorokin 1970; Kauffman et al. 1979; Sato and Kauffman 1980; Kauffman 1981; Thaete and Malkinson 1991; Palmer 1985; Rasmussen et al. 1986; Rehm et al. 1991). However, evidence from antigenic staining for detailed morphologic characterization of the tumors is lacking.
ISSUES
Possible Modes of Action for Cancer and Noncancer Effects
Cytotoxicity and increased cell division form the basis of a plausible mode of action for trichloroethylene-induced lung tumors in mice. Both are known risk factors for mouse lung carcinogenesis because of significant background tumor incidences (Green 2000). In addition, chloral appears to
have genotoxic potential (Salmon et al. 1995), although in the whole lung two studies failed to find evidence of DNA binding in mice exposed to trichloroethylene (Forkert and Birch 1989; Leuschner and Leuschner 1991). In conclusion, a number of known risk factors for the development of tumors, such as cytotoxicity, increased cell proliferation, and possibly aneuploidy, correlate well with the observed species-specific pulmonary carcinogenicity of trichloroethylene.
Relevance of Animal Studies to Humans
The acute responses believed to be causally related to the development of lung tumors in mice exposed to trichloroethylene have been attributed to the high metabolic capacity of the mouse lung Clara cells. Comparisons of the metabolic capacity of mouse, rat, and human lung tissue found that mouse lung microsomes metabolized trichloroethylene to chloral at a rate 3-fold higher than the rat lung microsomes. A metabolic rate could not be detected in the human lung (Green et al. 1997b). With an antibody to CYP2E1, the enzyme responsible for metabolism of trichloroethylene to chloral (Green et al. 1997b; Cruzan et al. 1997), the highest concentrations of the enzyme were found in the mouse lung. Significantly lower amounts were found in the rat lung. This enzyme could not be detected in the human lung in any cell types or in human lung sections by Western blotting (Green et al. 1997b). Other studies (Guengerich et al. 1991; Wheeler et al. 1992; Willey et al. 1996) reported the presence of CYP2E1 in human lung detectable only by reverse transcriptase-polymerase chain reaction. The total cytochrome P-450 content of the human lung is reported to be only 3.7% (27-fold lower than) that in the rat lung (Raunio et al. 1998). This is consistent with the lack of a measurable metabolic rate for trichloroethylene (Green et al. 1997b). Studies with recombinant cytochrome P-450s have revealed that, although rat and human rCYP2E1, rCYP2F, and rCYP2B1 were all capable of mediating trichloroethylene metabolism to chloral hydrate, the rat rCYP2E1 exhibited greater affinity than rat rCYP2F4 and rCYP2B1 and human rCYP2E1 (Forkert et al. 2005). These studies provide evidence supporting the role of CYP2E1, CYP2F, and CYP2B1 in the metabolism of trichloroethylene in the mouse lung. Studies with mouse and human lung microsomes indicated that the rates of chloral hydrate formation in human lung microsomes were low and were detected in only three of eight subjects (Forkert at al. 2005). Overall, the data suggest that the capacity of the human lung to metabolize trichloroethylene is 600-fold lower than the mouse lung (Green 2000).
The number of Clara cells and their morphology differ in human and rodent lungs, and this should also be considered in evaluating human risk from exposure to trichloroethylene. Clara cells differ significantly between
rodents and among rodents and humans in number and structure (ReznikSchuler 1976; Buckpitt et al. 1995). In mice, Clara cells are numerous and are spread throughout the airways, whereas in rats they are significantly fewer, particularly in the terminal bronchiolar region. In human lung, Clara cells are rare, as they are found in small numbers in the distal bronchioles. Mouse lung Clara cells are packed with endoplasmic reticulum, but human Clara cells are devoid of these membranes (Reznik-Schuler 1976; Buckpitt et al. 1995). The membranes of the endoplasmic reticulum in the Clara cell are the site of origin of the trichloroethylene-induced lesion in the mouse, consistent with the location of high concentrations of cytochrome P-450 enzymes that metabolize trichloroethylene in those membranes.
In summary, the metabolic data suggest that humans will be much less sensitive than mice to trichloroethylene-induced Clara cell toxicity and lung tumor development. One would not expect humans to develop lung tumors after exposure to ambient levels of trichloroethylene, which is in agreement with the results of epidemiology studies that show no association between trichloroethylene exposures and an increased in incidence of lung tumors.
FINDINGS AND RECOMMENDATIONS
Trichloroethylene has been shown to induce lung tumors in rodents. It is well documented that the mode of action for this effect is localization of the metabolite chloral in Clara cells of the lungs and that pulmonary metabolism of trichloroethylene to chloral is species dependent. The weight of evidence indicates that rodents and humans differ significantly in their capacity to metabolize trichloroethylene in their lungs, with humans having less capacity to metabolize the compound. This is supported by the results of most epidemiologic studies of occupational exposure to trichloroethylene, which do not show a strong association between trichloroethylene exposure and an increased incidence of lung tumors. Thus, pulmonary cancer is does not appear to be a critical end point in assessing human health risks of trichloroethylene.













