1
Introduction
Trichloroethylene is a chlorinated solvent widely used as a degreasing agent in industrial and manufacturing settings. It is also used as a chemical intermediate in making other chemicals and is a component of products such as typewriter correction fluid, paint removers, adhesives, and spot removers (ATSDR 1997a). Trichloroethylene is released into the environment as the result of its use and disposal practices, primarily from vapor degreasing operations. It is also found in soils and surface waters as a result of direct discharges and in groundwater due to leaching from disposal operations.
Trichloroethylene is a common environmental contaminant at Superfund sites, Department of Defense facilities, and certain manufacturing operations (e.g., aircraft, spacecraft). It has been found at approximately 852 of the 1,416 sites proposed for inclusion on the U.S. Environmental Protection Agency (EPA) National Priorities List. On the basis of data reported to the EPA Toxic Release Inventory, it was estimated that approximately 42 million pounds of trichloroethylene were released into the environment in 1994 (Scott and Cogliano 2000).
In 2001, EPA issued a draft health risk assessment and proposed exposure standards for trichloroethylene. EPA’s Scientific Advisory Board (SAB) reviewed the draft and it was issued for public comment. A number of scientific issues were raised during the course of these reviews by SAB, federal agencies, the scientific community, environmental organizations, citizen groups, and other interested parties. To help address these issues, EPA, the Department of Defense, the Department of Energy, and the National Aeronautics and Space Administration asked the National Research Council (NRC) to review and provide advice on the key scientific issues raised. In
response to this request, NRC convened the Committee on Human Health Risks of Trichloroethylene, which prepared this report.
STATEMENT OF TASK
The committee was asked to identify and assess the key scientific issues relevant to analyzing the human health risks of trichloroethylene. In performing its task, the committee was asked to consider pertinent toxicologic, epidemiologic, population susceptibility, and other available information, including relevant published scientific literature, EPA’s 2001 draft health risk assessment of trichloroethylene, scientific and technical comments received by EPA from public and private sources, and additional relevant information to be provided by the sponsoring agencies. The committee was tasked with holding one or more information-gathering sessions open to the public to gain additional insights into the issues from federal agencies, concerned parties, and other scientists.
The committee was asked to highlight issues critical to the development of an objective, realistic, and scientifically balanced trichloroethylene health risk assessment. The focus was to be on hazard characterization and mode of action for trichloroethylene toxicity, possible approaches to synthesize epidemiologic data in informing the hazard characterization of trichloroethylene, differential susceptibility in different subpopulations or life stages, the evidence for effects from trichloroethylene exposures alone compared with that for effects from mixtures of chemicals that include trichloroethylene, physiologically based pharmacokinetic modeling, dose-response assessment, and quantitative assessment of cancer and noncancer risks. The availability of appropriate data and methods to implement the committee’s advice as well as the distinction between data analysis and data generation were to receive special attention. The committee was asked to distinguish between issues that can be addressed through short-term analyses and issues that are more appropriately addressed through medium- or long-term research projects.
The committee was not asked to develop its own risk assessment or to address any risk management issues.
COMMITTEE’S APPROACH
The committee held five meetings between March and November 2005. The first three meetings involved data-gathering sessions, where the committee heard from sponsors, invited speakers, representatives of citizen groups, and members of the public. The committee reviewed a large body of written material on trichloroethylene, including research articles, literature reviews,
position papers, and unpublished data submitted by various sources, including the public. It focused its review on new data generated since EPA’s 2001 draft risk assessment, pertinent older information, and their implications for conducting a scientifically balanced risk assessment.
The committee is aware that some readers expect this report to evaluate EPA’s 2001 draft health risk assessment or to provide a comprehensive evaluation of the literature on trichloroethylene. However, the scope of this project did not encompass either of these tasks. The statement of task directed the committee to evaluate specific issues related to performing a risk assessment on trichloroethylene. EPA’s draft assessment was to be factored into the committee’s evaluation, but it was not to be the main focus of the review. Thus, although the report comments on certain aspects of the draft assessment, the scientific validity of the proposed standards for trichloroethylene is not assessed.
As directed in the statement of task, the committee’s review of the different cancer and noncancer end points focused primarily on mode-of-action information and how it contributes to the hazard characterization of trichloroethylene. With regard to cancer, the committee’s evaluation focused on the assessment of kidney cancer. Guidance is provided on how to evaluate the epidemiologic evidence and to consider this information in conjunction with animal evidence and mechanistic data. The assessment provided for kidney cancer is intended to be a model for how other types of cancer should be evaluated. For the other cancer and noncancer end points, a qualitative review of some epidemiologic evidence is provided to give a sense of whether the animal evidence is supported by observations in human populations. However, these data sets were not rigorously reviewed.
OVERVIEW OF PHARMACOKINETICS
Understanding the absorption, distribution, metabolism, and elimination of trichloroethylene is critical to the qualitative and quantitative assessment of human health risks from environmental exposures. Qualitatively, pharmacokinetics is helpful in identifying the chemical species that might be causally associated with observed toxic responses. This is particularly important for trichloroethylene because many of its toxic effects are thought to be due to metabolites rather than to trichloroethylene. The delineation of interspecies and intraspecies pharmacokinetic differences can provide insights into how laboratory animal and epidemiologic data might reveal overall human health risks and the basis for individual differences in susceptibility. Furthermore, physiologically based pharmacokinetic models can quantify the relationship between external measures of exposure and internal measures of a toxicologically relevant dose. Selecting the appropriate
dose metric for use in risk assessment depends on the understanding of the target tissue, active chemical, and mode of action for a particular toxic effect as well as the reliability of physiologically based pharmacokinetic models.
Trichloroethylene is rapidly and extensively absorbed by all routes of environmental exposures, including ingestion, inhalation, and dermal contact. Once absorbed, trichloroethylene distributes throughout the body via the circulatory system. Most trichloroethylene taken into the body is metabolized; direct exhalation of the parent compound is the other major route of elimination (Lash et al. 2000a).
Figure 1-1 presents a postulated scheme for the pathways of trichloroethylene metabolism, adapted from the work of Clewell et al. (2000), Lash et al. (2000a), and recent studies described below. Trichloroethylene metabolism occurs through two main, irreversible pathways—oxidation via the microsomal mixed-function oxidase system (cytochrome P-450s [CYPs]) primarily to chloral [C2HCl30]or chloral hydrate [CCl3CH(OH)2] and trichloroethylene oxide), and conjugation with glutathione by glutathione S-transferases to S-1,2-dichlorovinyl-L-glutathione. For trichloroethylene oxidation, CYP2E1 is thought to be most important in vivo. Subsequent important metabolic branch points include the production of trichloroethanol, regeneration of chloral and chloral hydrate from trichloroethanol, and further metabolism of S-1,2-dichlorovinyl-L-cysteine.
A number of important issues relate to understanding trichloroethylene pharmacokinetics: (1) enterophepatic recirculation of trichloroethylene and trichloroethanol; (2) diffusion-limited tissue distribution in fat and liver; (3) plasma binding of trichloroacetic acid and dichloroacetic acid; (4) dichloroacetic acid formation, pharmacokinetics, and the role of trichloroethylene oxide in its formation; and (5) pathways of gluthathione conjugation and subsequent metabolism. These and other pharmacokinetic considerations are presented in Appendix C.
EXPOSURE CONSIDERATIONS
Trichloroethylene
People can be exposed to trichloroethylene from contaminated air (outdoor and indoor), water, and soil. Data from 2004 on ambient air concentrations of trichloroethylene indicate an average of 0.37 µg/m3 (range, 0-6.32 µg/m3), a concentration that has remained fairly consistent since 1996 (EPA, unpublished material, June 2005). Mean concentrations at various land-use sites include 1.84 µg/m3 in commercial areas, 1.54 µg/m3 in industrial areas, 1.08 µg/m3 in agricultural areas, and 0.89 µg/m3 in residential areas. Trichloroethylene concentrations measured in ambient air at various U.S. locations are provided in Table 1-1.
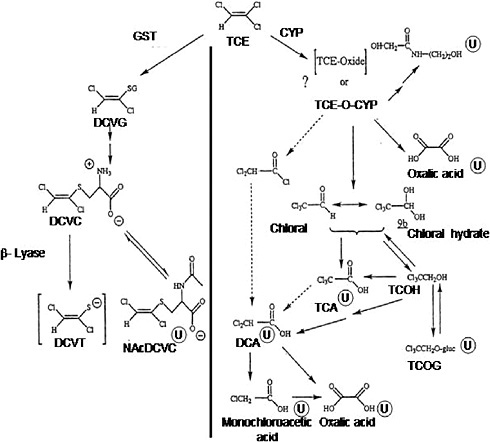
FIGURE 1-1 Metabolism of trichloroethylene. Metabolites marked with ![]() are known urinary metabolites. Arrows with broken lines indicate other possible steps in forming dichloroacetic acid. Abbreviations: CYP, cytochrome P-450; DCA, dichloroacetic acid; DCVC, S-(1,2-dichlorovinyl)-L-cysteine; DCVG, S-(1,2-dichlorovinyl)glutathione; DCVT, S-(1,2-dichlorovinyl)thiol; GST, glutathione S-transferase; NAcDCVC, N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine; TCA, trichloroacetic acid; TCE, trichloroethylene; TCE-O-CYP, trichloroethylene-oxide-cytochrome P-450 complex; TCOH, trichloroethanol; TCOG, trichloroethanol glucuronide.
are known urinary metabolites. Arrows with broken lines indicate other possible steps in forming dichloroacetic acid. Abbreviations: CYP, cytochrome P-450; DCA, dichloroacetic acid; DCVC, S-(1,2-dichlorovinyl)-L-cysteine; DCVG, S-(1,2-dichlorovinyl)glutathione; DCVT, S-(1,2-dichlorovinyl)thiol; GST, glutathione S-transferase; NAcDCVC, N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine; TCA, trichloroacetic acid; TCE, trichloroethylene; TCE-O-CYP, trichloroethylene-oxide-cytochrome P-450 complex; TCOH, trichloroethanol; TCOG, trichloroethanol glucuronide.
SOURCE: Adapted from Lash et al. 2000a.
Indoor air can become contaminated by certain consumer products (e.g., adhesives, tapes) and by volatilization from contaminated water supplies. Vapor intrusion through walls and floors can also be a source of indoor exposure when buildings are near contaminated groundwater.
TABLE 1-1 Concentrations of Trichloroethylene in Ambient Air
|
Area |
Year |
Concentration, µg/m3 |
Reference |
|
|
Mean |
Range |
|||
|
Rural |
|
|
|
|
|
Whiteface Mountain, NY |
1974 |
0.5 |
<0.3-1.9 |
IARC 1995a |
|
Badger Pass, CA |
1977 |
0.06 |
0.005-0.09 |
IARC 1995a |
|
Reese River, NV |
1977 |
0.06 |
0.005-0.09 |
IARC 1995a |
|
Jetmar, KS |
1978 |
0.07 |
0.04-0.11 |
IARC 1995a |
|
Urban and Suburban |
|
|
|
|
|
New Jersey |
1973-1979 |
9.1 |
ND-97 |
IARC 1995a |
|
New York City, NY |
1974 |
3.8 |
0.6-5.9 |
IARC 1995a |
|
Los Angeles, CA |
1976 |
1.7 |
0.14-9.5 |
IARC 1995a |
|
Lake Charles, LA |
1976-1978 |
8.6 |
0.4-11.3 |
IARC 1995a |
|
Phoenix, AZ |
1979 |
2.6 |
0.06-16.7 |
IARC 1995a |
|
Denver, CO |
1980 |
1.07 |
0.15-2.2 |
IARC 1995a |
|
St. Louis, MO |
1980 |
0.6 |
0.1-1.3 |
IARC 1995a |
|
Portland, OR |
1984 |
1.5 |
0.6-3.9 |
IARC 1995a |
|
Philadelphia, P A |
1983-1984 |
1.9 |
1.6-2.1 |
IARC 1995a |
|
Southeast Chicago, IL |
1986-1990 |
1.0 |
— |
Sweet and Vermette 1992 |
|
East St. Louis, IL |
1986-1990 |
2.1 |
— |
Sweet and Vermette 1992 |
|
District of Columbia |
1990-1991 |
1.94 |
1-16.65 |
Hendler and Crow 1992 |
|
Urban Chicago, IL |
Pre-1993 |
0.82-1.16 |
— |
Scheff and Wadden 1993 |
|
Suburban Chicago, IL |
Pre-1993 |
0.52 |
— |
Scheff and Wadden 1993 |
|
300 cities in 42 states |
Pre-1986 |
2.65 |
— |
Shah and Singh 1988 |
|
Several Canadian cities |
1990 |
0.28 |
— |
Bunce and Schneider 1994 |
|
Several U.S. cities |
1990 |
6.0 |
— |
Bunce and Schneider 1994 |
|
Phoenix, AZ |
1994-1996 |
0.29 |
0-1.53 |
Zielinska et al. 1998 |
|
Tucson, AZ |
1994-1996 |
0.23 |
0-1.47 |
Zielinska et al. 1998 |
|
SOURCE: EPA, unpublished material, June 2005. |
||||
Information on measured concentrations of trichloroethylene in indoor air is presented in Table 1-2. Some of the studies used outdoor concentrations and personal samples for comparison.
Trichloroethylene is the most frequently reported organic contaminant in groundwater. The Agency for Toxic Substances and Disease Registry (ATSDR 1997a) estimates that between 9% and 34% of drinking water supply sources tested in the United States contain some trichloroethylene. Example concentrations of trichloroethylene found in effluents, surface water, rainwater, groundwater, and drinking water are presented in Table 1-3. Groundwater concentrations of trichloroethylene have been extensively sampled in California. A statewide survey conducted in 1984-1985 found
TABLE 1-2 Example Concentrations of Trichloroethylene in Indoor Air
|
Setting |
Mean Trichloroethylene Concentration, µg/m3 |
Reference |
||
|
Indoor |
Outdoor |
Personal |
||
|
Baltimore Harbor Tunnel toll boothsa |
3.11 |
0.08 |
|
Sapkota et al. 2005 |
|
Residential, Ottawa, Canada |
0.06 |
0.08 |
|
Zhu et al. 2005 |
|
Residential, Minnesota (Children’s Pesticide Exposure Study) |
0.6 |
0.6 |
0.8 |
Adgate et al. 2004 |
|
Residential, Minneapolis/St. Paul, Minnesota |
0.5 |
0.2 |
1.0 |
Sexton et al. 2004 |
|
U.S., Canada, Europe |
<1-165 |
|
|
Hers et al. 2001 |
|
Residences and workplaces in the United States |
7.2 |
|
|
Shah and Singh 1988 |
|
Bathrooms in two homes using trichloroethylene-contaminated well water |
500-40,000 |
|
|
Andelman et al. 1986 |
|
aDry-cleaning residues on uniforms is thought to be the source of exposure. |
||||
TABLE 1-3 Concentrations of Trichloroethylene in Water
|
Water Type |
Location (No. of Samples) |
Year |
Trichloroethylene Concentrations, µg/L |
Reference |
||
|
Mean |
Median |
Range |
||||
|
Industrial effluent |
U.S. |
1983 |
|
0.5 |
|
IARC 1995a |
|
Surface water |
U.S. |
1983 |
|
0.1 |
|
IARC 1995a |
|
Rainwater |
Portland, OR |
1984 |
0.006 |
|
0.002-0.02 |
Ligocki et al. 1985 |
|
Groundwater |
MN |
1983 |
|
|
0.2-144 |
Sabel and Clark 1984 |
|
|
NJ |
1976 |
|
|
≤1,530 |
Burmaster 1982 |
|
|
NY |
1980 |
|
|
≤3,800 |
Burmaster 1982 |
|
|
PA |
1980 |
|
|
≤27,300 |
Burmaster 1982 |
|
|
MA |
1976 |
|
|
≤900 |
Burmaster 1982 |
|
|
AZ |
— |
|
|
8.9-29 |
IARC 1995a |
|
Drinking water |
U.S. |
1976 |
|
|
0.2-49 |
IARC 1995a |
|
|
U.S. |
1977 |
|
|
0-53 |
IARC 1995a |
|
|
U.S. |
1978 |
|
|
0.5-210 |
IARC 1995a |
|
|
MA |
1984 |
|
|
Max. 267 |
IARC 1995a |
|
|
NJ (1,130) |
1984 |
23.4 |
|
Max. 67 |
Cohn et al. 1994 |
|
|
CA (486) |
1985 |
|
|
8-12 |
EPA 1987 |
|
|
CA (486) |
1984 |
66 |
|
|
EPA 1987 |
|
|
NC (48) |
1984 |
5 |
|
|
EPA 1987 |
|
|
ND (48) |
1984 |
5 |
|
|
EPA 1987 |
|
SOURCE: EPA 2001a. |
||||||
trichloroethylene in 187 of 2,947 wells at concentrations up to 440 µg/L (DHS 1986). The most contaminated wells were typically found in more urbanized areas.
Metabolites
Trichloroethylene toxicity comes primarily from its metabolites, but people may be exposed to the metabolites from sources other than trichloroethylene. For example, chlorination of drinking water produces the by-products chloral, chloral hydrate, monochloroacetic acid, dichloroacetic acid, and trichloroacetic acid. Chloral is used in the production of polyurethanes and as a chemical intermediate for the herbicide trichloroacetic acid. Chloral hydrate is a pharmaceutical used as a hypnotic and sedative. The metabolite monochloroacetic acid is used in pharmaceuticals, as an herbicide, and as a chemical intermediate in the production of indigoid dyes. Trichloroacetic acid is also used as a chemical intermediate and in the production of herbicides (EPA 2001a).
Other chemical compounds have some of the same metabolites as trichloroethylene, including tetrachloroethylene, 1,1,1-trichloroethane, 1,2-dichloroethylene (cis-, trans-, and mixed isomers), 1,1,1,2-tetrachloroethane, and 1,1-dichloroethane. Tetrachloroethylene is used in textile dry cleaning, as part of the processing and finishing in cleaning and degreasing metals, and as a chemical intermediate in the synthesis of some fluorocarbons. 1,1,1-Trichloroethane is used as a solvent and in pesticides, textile processing, cutting oil formulations, and printing inks. 1,2-Dichloroethylene, 1,1,1,2-tetrachloroethane, and 1,1-dichloroethane are used primarily as solvents in cleaning, degreasing, and extracting processes (EPA 2001a). EPA’s preliminary set of dose estimates for these compounds and the metabolites they share with trichloroethylene are presented in Table 1-4.
ORGANIZATION OF THE REPORT
Guidance for hazard characterization of trichloroethylene is presented in Chapters 2 through 10. Chapter 2 provides guidance for evaluating large sets of epidemiologic data. In Chapter 3, the committee applies this guidance as an example in its evaluation of the epidemiologic data on trichloroethylene and kidney cancer, and this example should help guide evaluations of other cancer risks. Chapter 3 also assesses new information on the kidney toxicity of trichloroethylene and its metabolites and potential modes of action. Chapters 4, 5, 6, 7, and 8 evaluate the key issues regarding liver toxicity and cancer, reproductive and developmental toxicity, neurotoxicity, respiratory tract toxicity and cancer, and immunotoxicity, respectively. While these chapters are divided by target organ site, it is important to
TABLE 1-4 Preliminary Intake Estimates of Trichloroethylene and Related Chemicals
|
Chemical |
Population |
Media |
Range of Estimated Adult Exposures, µg/day |
Range of Adult Doses, mg/kg/day |
Data Sources |
|
Trichloroethylene |
General |
Air |
11-33 |
1.57E-04-4.71E-04 |
ATSDR 1997a |
|
|
General |
Water |
2-20 |
2.86E-05-2.86E-04 |
ATSDR 1997a |
|
|
Occupational |
Air |
2,232-9,489 |
3.19E-02-1.36E-01 |
ATSDR 1997a |
|
Tetrachloroethylene |
General |
Air |
80-200 |
1.14E-03-2.86E-03 |
ATSDR 1997b |
|
|
General |
Water |
0.1-0.2 |
1.43E-06-2.86E-06 |
ATSDR 1997b |
|
|
Occupational |
Air |
5,897-219,985 |
8.43E-02-3.14 |
ATSDR 1997b |
|
1,1,1-Trichloroethane |
General |
Air |
10.8-108 |
1.54E-04-1.54E-03 |
ATSDR 1995 |
|
|
General |
Water |
0.38-4.2 |
5.5E-06-6.00E-05 |
ATSDR 1995 |
|
1,2-Dichloroethylene |
General |
Air |
1-6 |
1.43E-05-8.57E-05 |
ATSDR 1996 |
|
|
General |
Water |
2.2 |
3.14E-05 |
ATSDR 1996 |
|
Cis-1,2-Dichloroethylene |
General |
Air |
5.4 |
7.71E-05 |
HSDB 1996a |
|
|
General |
Water |
0.5-5.4 |
7.14E-06-7.71E-05 |
HSDB 1996a |
|
1,1,1,2-Tetrachloroethane |
General |
Air |
142 |
2.03E-03 |
HSDB 2002b |
|
1,1-Dichloroethane |
General |
Air |
4 |
5.71E-05 |
HSDB 2002b |
|
|
General |
Water |
2.47-469.38 |
3.53E-05-6.71E-03 |
ATSDR 1990 |
|
Chloral |
General |
Water |
0.02-36.4 |
2.86E-07-5.20E-04 |
HSDB 1996a |
|
Monochloroacetic acid |
General |
Water |
2-2.4 |
2-86E-05-3.43E-05 |
EPA 1994a |
|
Dichloroacetic acid |
General |
Water |
10-266 |
1.43E-04-3.80E-03 |
IARC 1995a |
|
Trichloroacetic acid |
General |
Water |
8.56-322 |
1.22E-03-4.60E-03 |
IARC 1995a |
|
aData from Hazardous Substances Data Bank 1996. bData from Hazardous Substances Data Bank 2002 (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB). SOURCE: EPA 2001a. |
|||||
note that concordance of target organ effects in animals and humans was not a requirement in evaluating the implications of animal data for human risk. Site concordance of tumors is usually judged as providing strong evidence of an association, and the committee did consider this relationship in evaluating the data. However, the committee’s review focused on mode-of-action information to understand how trichloroethylene might affect certain processes differently in different species. Chapter 9 discusses susceptibility to trichloroethylene and its metabolites, and Chapter 10 describes important factors in considering trichloroethylene in mixtures. Physiologically based pharmacokinetic models are evaluated in Chapter 11, and guidance is provided on future directions for model development. Finally, Chapter 12 considers issues related to dose-response assessment and quantitative assessment of risk.










