4
Resources for Completing the Care Plan
INTRODUCTION
Moderator: Ms. Ellen Stovall
This second day of the workshop is devoted to implementation issues. Kevin Oeffinger and Charles Shapiro will provide an overview of the status of survivorship guidelines. Diane Blum will then discuss appropriate use of available psychosocial support services. Recommendations for healthy lifestyle behaviors will be reviewed by Wendy Demark-Wahnefried. Information technology is critical to the success of survivorship care planning, and David Poplack, Mark Horowitz, and Michael Fordis will demonstrate its promise with an introduction to the Passport for Health program for survivors of childhood cancer. Lawrence Shulman will then reflect on the state of information technology as it pertains to survivorship care planning. Finally, Tim Byers will discuss regional approaches to cancer survivorship planning.
SURVIVORSHIP GUIDELINES
Two Perspectives
Presenter: Dr. Kevin Oeffinger
I would like to share some guideline-related lessons learned from two perspectives: (1) from working with the Children’s Oncology Group’s Late
Effects Steering Committee to disseminate and implement follow-up guidelines, and (2) from sitting on the American Academy of Family Physicians’ Commission on Clinical Policy and Research, the body that reviews and collaboratively develops guidelines that are adopted by members of the American Academy of Family Physicians.
Evidence-based guidelines are useful in promoting high-quality care, standardizing and facilitating the care of complex patients, and providing a rubric or a set of accepted measures for process evaluation. While they are valuable, there are some pitfalls associated with the use of guidelines. First, the large number of published guidelines may overwhelm and confuse practicing physicians. Second, some physicians reject guidelines because they may not take into consideration the complexities facing their patients. Guidelines may also be perceived to be dictating clinical decisions.
The Children’s Oncology Group (COG) long-term follow-up guidelines were developed by a late effects committee cochaired by Melissa Hudson and Wendy Landier over the course of several years. COG is a 244-institution clinical trial consortium. The pediatric community has over the past 15 years wrestled with the issues under discussion at this workshop. Much has been learned from the pediatric experience, and it would be unfortunate if this experience were not applied productively to the challenges ahead in the adult survivor arena.
The goal of the COG guideline effort was to standardize and enhance follow-up care throughout the life span of the survivor. The focus has been on screening for late effects rather than screening for relapse or recurrence, as these were already embedded in ongoing protocols. The COG guidelines start to apply at 2 years following the completion of cancer therapy. The intended users of the guidelines are clinicians who provide health care for pediatric cancer survivors regardless of their age and their care setting.
To develop the guidelines, more than a year was spent conducting in-depth literature reviews, synthesizing the literature, achieving multidisciplinary group consensus, and submitting the guidelines to external review. Some refinements have been made following their initial dissemination in September 2003.
A hybrid approach was used in guideline development. The large body of evidence linking therapeutic exposures and late effects was reviewed and scored according to quality. There are very few studies that examine how surveillance affects outcomes, in part because of the relatively small numbers of pediatric cancer survivors. Consequently, expert clinical experience and principles of screening in the general population and other high-risk groups was relied on for the aspects of care considered as part of the guideline development process.
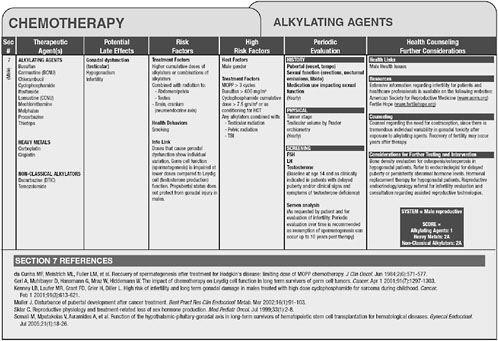
Version 2.0 of the long-term follow-up guidelines is available online at www.survivorshipguidelines.org. The guidelines are based on therapeutic
exposures rather than on cancer type. Shown in Figure 4-1 are some of the late effects of alkylating agents, associated risk factors, recommendations for follow-up and health counseling, and references to the literature.
Throughout the guideline development process, dissemination efforts have been a priority. A methods paper was published in the Journal of Clinical Oncology in 20041 and general review articles have subsequently been published. Presentations have been made at specialty and primary care conferences. Having the guidelines posted online permits wide accessibility. A computer-based Passport for Care has been developed to tailor the guidelines to individual patients. This effort will be described by David Poplack and colleagues later in this session.
Maintenance of the guidelines depends on 18 multidisciplinary task forces that include pediatric oncologists, radiation oncologists, surgeons, cardiologists, organ-specific specialists, primary care physicians, nurse practitioners, social workers, and psychologists. These groups review the literature, develop and recommend revisions to the guidelines, and engage in dissemination activities.
A publications committee was established through the COG late effects committee to review all concept proposals for literature that would reflect on the COG guidelines. Two types of publications are highlighted: (1) detailed and in-depth systematic reviews on focused topics such as chest radiation and its relationship with breast cancer development; and (2) general reviews that are geared more toward the practicing primary physician and the community-based pediatric oncologist.
Relationships with professional societies have been developed and endorsements, or what one might call “seals of approval” of the guidelines, have been sought. The guidelines are included in the National Guideline Clearinghouse™ (http://www.guideline.gov/).
The backbone of dissemination efforts is through long-term follow-up programs. These programs are generally based at children’s hospitals or cancer centers at which a team approach is taken, with physicians working with survivors, nurse practitioners, social workers, and psychologists. There is also a multidisciplinary network of adult and pediatric-based subspecialists. The three core components of the long-term follow-up programs are: (1) a cancer summary and treatment plan that are discussed with the patient; (2) the COG long-term follow-up guidelines; and (3) delivery of risk-based survivorship care.
Long-term follow-up is not uniformly available across the 244 COG institutions. Although more than half of these institutions have a mechanism for long-term follow-up care, only one-quarter of them have a pro-
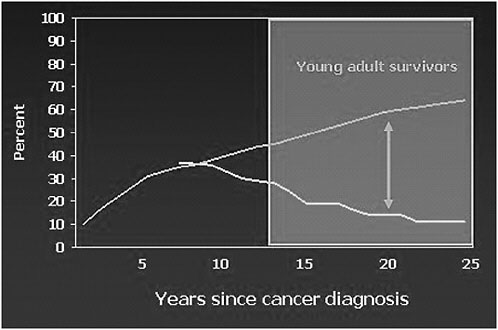
FIGURE 4-2 Cancer center visits and late effects: Results from the Childhood Cancer Survivor Study.
NOTE: Top line represents percent of survivors with incidences of late effects; bottom line represents percent of survivors seen during cancer center visits.
SOURCE: Oeffinger, 2006.
gram that provides comprehensive long-term follow-up care, and only one-tenth of these institutions can follow adult survivors of pediatric cancer.
The proportion of survivors of childhood cancer with a cancer center visit within the past 2 years is about 35 percent at 7 years after cancer therapy or from the cancer diagnosis, according to results from the Childhood Cancer Survivor Study published a few years ago.2 As the bottom line on Figure 4-2 illustrates, the further survivors are from their cancer diagnosis, the less likely they are to have been seen at a cancer center. These results are based on patients who were treated from 1970 to 1986. When the cumulative incidence of late effects is superimposed, as shown in the top line on Figure 4-2, a very significant gap becomes evident as they are 15, 20,
and 25 years from their cancer therapy. By this time, they are experiencing their highest rate of morbidity but are not being seen at a cancer center.
Some barriers to comprehensive survivorship care extend beyond the reach of long-term follow-up programs. Primary care physicians are not familiar with pediatric cancer survivors, which is not unexpected because, on average, a general internist or a family physician has three to five pediatric cancer survivors in their practice. One of these patients might be a leukemia survivor, one a Hodgkin’s disease survivor, and one a brain tumor survivor. Primary care physicians cannot be expected to keep up with this complex and rapidly evolving area of medicine, when it so rarely relates to their practices.
Insurance-related barriers are very significant for survivors of childhood cancer. Many are uninsured, and if insured, their coverage may not adequately cover components of follow-up care, or it may exclude specialized care entirely if the long-term follow-up program is not a designated part of their health care network. There are no survivor-based delivery models in health maintenance organizations or U.S.-based single-payer systems.
Efforts to improve survivor health care have focused on increasing the number and the quality of long-term follow-up programs in COG. Innovative tools, such as the Passport for Care to be demonstrated at the workshop, will facilitate improvements in care not only at cancer centers but also in community-based oncology practices. Work is also under way with insurance companies to develop cost-effective models of survivorship care. Other efforts are aimed at bridging the gap as patients age and transition from pediatric to adult care and transition from specialty to primary care. Engaging primary care physicians in survivorship care is key to improved care. If current practice is to change, efforts must extend beyond the dissemination efforts described.
It is important to note some key differences between pediatric and adult cancer survivors. First, pediatric cancer is rare and, as mentioned, it is not a commonly encountered problem in primary care. In contrast, primary care physicians in the adult arena are engaged in cancer prevention efforts as well as care for the over 10 million survivors of adult cancer. Pediatric cancer survivors, especially as they age, do not maintain strong ties with oncologists, whereas adults survivors, as we learned from the Institute of Medicine (IOM) focus groups, tend to be “followed for life” by their oncologist.
These features of pediatric and adult survivorship suggest different approaches to knowledge transfer. Primary care physicians encountering a pediatric cancer survivor in their practice need a single national site or source of information. In contrast, primary care providers caring for adult cancer survivors usually have a local source of information, the community-based oncologist. In both cases, translating knowledge goes
well beyond simply providing follow-up guidelines. Primary care providers need contact with expertise and avenues for continued communication.
Disseminating clinical practice guidelines through journals, continuing medical education opportunities, and postings on websites is insufficient to change practice behavior. What will change practice behaviors? Some recent research conducted at the University of Toronto has provided some clues to effective knowledge translation and subsequent transfer of that knowledge.3 These investigators have defined knowledge translation as the “exchange, synthesis, and ethically sound application of researcher findings within a complex system of relationships among researchers and knowledge users.” To improve knowledge translation and transfer, mechanisms must be developed to “strengthen relationships among health researchers and users of health knowledge, enhance capacity for knowledge uptake, and accelerate the flow of knowledge into beneficial health applications.”
In the context of survivorship care, components needed for knowledge transfer include:
-
a user-friendly version of survivorship guidelines;
-
a patient version of the treatment summary and care plan;
-
training to prepare practices for guideline adoption (both the office staff and the clinicians);
-
facilitated communication (e.g., between nurse managers, oncologists, and primary care physicians); and
-
innovative technology (e.g., cross-platform electronic health records or tools such as the Passport for Care).
In terms of models of care, Eva Grunfeld’s series of randomized clinical trials illustrates how follow-up care for breast cancer survivors can be accomplished in the primary care community.4 The shared care model can be stratified by risk. Some high-risk survivors will need continued follow-up with their oncologist. Lower-risk patients may not need the resources of
|
3 |
Information about this program is available at http://www.ktp.utoronto.ca/index.htm. |
|
4 |
Grunfeld E. et al., 2006. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 24(6):848-55. Grunfeld E. et al., 1999. Comparison of breast cancer patient satisfaction with follow-up in primary care versus 2specialist care: Results from a randomized controlled trial. British Journal of General Practice 49(446):705-710. Grunfeld E. et al., 1999. Follow-up of breast cancer in primary care vs specialist care: Results of an ecomonic evaluation. British Journal of General Practice 79(7-8):1227-1233. Grunfeld E. et al., 1995. Evaluating primary care follow-up of breast cancer: Methods and preliminary results of three studies. Annals of Oncology 6(Suppl 2):47-52. |
a cancer center or an oncologist and may be best seen by a primary care physician. There is a unique opportunity to develop and test models of care through partnerships of cancer centers, Community Clinical Oncology Practices (CCOPs), and practice-based research networks supported by the federal Agency for Healthcare Research and Quality (AHRQ). An example of such a network is the Federation of Practice-Based Research Networks, which was established in 1997. There are 50 different networks involving over 6,000 primary care clinicians, over 5 million patients, and 24 million patient encounters a year. There are over 100 active research studies through these networks. There are opportunities for cancer centers and CCOPs to collaborate with these practice-based research networks to develop and test survivorship care models and then disseminate successful models for adoption.
In engaging primary care providers in survivorship care, it is important to remember that effective strategies tend to work from the bottom up, not from the top down. Involving primary care physicians in the process at the outset is critical. The American Academy of Family Physicians, the American College of Physicians, and the American Academy of Pediatrics have been integrally involved in developing and disseminating guidelines for many years and have an established process and experience working with other professional societies. Some guideline strategies fail if: (1) there is a “top-down” mentality; (2) guidelines are based on consensus rather than good evidence; and (3) the focus is on who should do it, rather than what should be done. To bring primary care providers and other constituencies into the dialogue, a summit meeting could be held on collaborative approaches to survivorship care.
Survivorship researchers have examined issues related to quality of life, late effects, and health outcomes, but what is needed now are research initiatives to test models of care. A program announcement that established a common set of outcomes, including measures for adherence to guidelines, would allow the research community to test stratified risk models of care and alternative methods to collaborate with primary care providers. A funding mechanism is needed to encourage the development and testing of innovative technology. Support could be used to explore the potential for exciting technologies, like the Passport for Care and electronic health records to further survivorship health care.
Additional research is also needed to better understand the survivorship care paradigm. Cancer survivors are being characterized as having a chronic condition, but cancer is quite distinct from cardiovascular disease and diabetes. Cancer survivors may have a late effect that becomes a chronic health problem, but often it is their risk for developing new problems that presents a different paradigm.
ASCO INITIATIVE
Presenter: Dr. Charles Shapiro
The American Society of Clinical Oncology’s (ASCO) initiative is to develop long-term medical care guidelines for adult cancer survivors. The effort began in 2005, and its purpose is to provide health care professionals with the knowledge and expertise to decrease morbidity and to improve the quality of life for adult cancer survivors. The initial audience for the guidelines is health care providers, but the effort could be expanded to include companion patient-friendly survivorship guidelines. The ASCO guidelines will address issues arising during the posttreatment phase of the cancer trajectory.
In contrast to pediatrics, there are few guidelines for adult survivorship care. A limited evidence base has impeded guideline development. The ASCO initiative will be key to highlighting what is known and not known for this phase of cancer care and suggesting critical areas for future survivorship research.
In contrast to the treatment modality approach taken in pediatrics, the ASCO guidelines will be developed using a symptom or organ site paradigm. For many aspects of survivorship care there is limited clinical trial-based evidence, and generalizations about care from cancer registries are difficult because registries do not capture complete information on treatment and late effects. Furthermore, adult cancer survivors often have comorbid conditions that can confound interpretation of outcomes by researchers as they attempt to establish relationships among treatments, late effects, and health outcomes. It has been difficult, for example, to clearly establish the link between doxorubicin and cardiac problems because heart disease increases naturally as patients age. There is also a dependence on surrogate endpoints, for example, using bone mineral density in research on the effects of various treatments on fractures. The more clinically relevant endpoint, fractures, has not been well studied because follow-up periods have not been long enough.
Another challenge facing clinicians as they attempt to use evidence to guide their practice is that data from long-term follow-up studies may reflect outmoded treatment techniques. For example, 30 years ago, radiation techniques were very different from what they are today. These older techniques were associated with an increased incidence of cardiovascular effects that presented in the second decade. With more modern techniques, the latest data show a markedly reduced incidence of cardiac effects. With treatments advancing rapidly, evaluation of late effects of treatments becomes a moving target. It is axiomatic that new therapies will be adopted into standard practice based on short-term improvements in efficacy. For
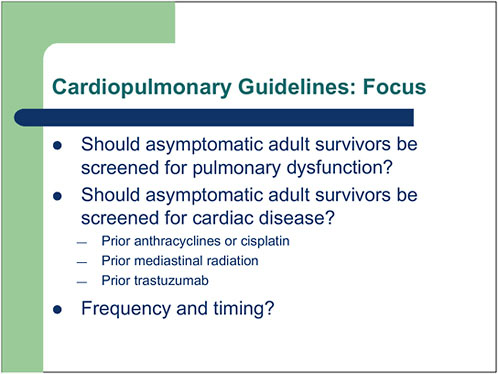
FIGURE 4-3 ASCO’s cardiopulmonary guidelines.
SOURCE: Shapiro, 2006.
example, trastuzumab (Herceptin) is markedly beneficial in the early stage of breast cancer according to clinical trials, but the median follow-up period in these trials is 2 to 3 years. It has become the standard of care for women with breast cancer who overexpress the growth factor protein HER2. What is known of trastuzumab cardiotoxicity is reassuring, but absent are long-term follow-up studies and information on interactions between radiation and trastuzumab or anthracyclines and trastuzumab. This represents another kind of moving target that necessitates a continuous reexamination of guidelines after they are created.
The ASCO guidelines will focus on five areas in the following order: cardiopulmonary late effects; bone health; second cancers; hormone deficiency; and anxiety and depression. The guideline development process is moving forward very quickly, and the first guideline is already being reviewed by the ASCO board. Many people have volunteered to expedite the guidelines process.
The focus of the guidelines is on screening questions, for example, whether asymptomatic adult survivors should be screened for pulmonary
dysfunction or cardiac disease (Figure 4-3), and if so, when should such screening begin, and at what interval should it be repeated?
One of the recommendations included in the ASCO cardiopulmonary guideline was based on consensus and may be considered controversial. The ASCO guideline suggests screening of asymptomatic high-risk survivors every 5 years and treatment for asymptomatic left ventricular (LV) systolic or diastolic dysfunction based on recommendations of the American College of Cardiology, the American Heart Association, and the Heart Failure Society. Survivors are considered to be at high risk if they have one of the following: age less than 18 years at exposure; prior cardiac disease; greater than 300 mg/m2 of doxorubicin; or mediastinal radiation. This consensus-based recommendation seems to be reasonable, at least as a starting point.
In terms of challenges and implementation barriers, the first is the paradigm shift to thinking of cancer as a chronic condition. It will be important to raise awareness that people live a long time with cancer and that there are long-term consequences of treatment. A second issue relates to the major legal and financial implications of guidelines. ASCO’s consensus guideline states that it is reasonable to screen for cardiac dysfunction in women who have had greater than 300 mg/m2 doxorubicin or who had breast radiation that involved the chest wall. Such screening is not now part of standard breast cancer follow-up. Physicians are being asked to consider screening for cardiac dysfunction when they have not routinely done so and when there is only consensus among experts to back up the recommendation.
Another challenge in issuing guidelines is establishing who is to accept responsibility for providing guideline-recommended care. ASCO originally viewed this as a responsibility of the oncology community, but more recently, and certainly at this meeting, primary care providers and survivors themselves should be considered as potential constituents for taking action based on the guidelines.
Publishing the guidelines is a starting point, and it may ultimately have the most value in identifying gaps in knowledge and setting the research agenda. The ASCO guideline effort is also an opportunity to link academic and community centers to collaborate on research interventions, education, and information dissemination. The Lance Armstrong Foundation Centers of Excellence, to be described later today, are a model that needs to be developed to fulfill its potential of driving the agenda for research and improvements in survivorship care. The Quality Oncology Practice Initiative (QOPI) is a potential mechanism to assess compliance with the ASCO guidelines. The ASCO guideline initiative will attempt to learn from the experiences of other clinical practice guideline efforts, such as those of the National Comprehensive Cancer Network (NCCN). We need to under-
stand what worked and what did not work, so as not to duplicate effort but rather to learn from the experience of existing programs that are similar to ASCO’s in scope and purpose.
Engaging survivors in efforts to improve care is also extremely important. One recent example of a successful engagement is a survey on fertility conducted by a coalition of young cancer survivors. The Internet survey of the members of this coalition resulted in a peer-reviewed publication in the Journal of Clinical Oncology. Fertility guidelines issued by ASCO in May 2006 were in part completed as a result of this effort. Directly asking survivors about what they need and want, as well as what is lacking in their care, can inform development of practice guidelines. This experience with the coalition of young survivors represents a good model to emulate. There need to be more opportunities for survivors to be empowered to participate in their own path to wellness and health maintenance. Survivorship care planning, including the development of guidelines, provides such an opportunity.
Discussion
Dr. Sheldon Greenfield asked the speakers whether the timing is right for oncologists to meet with generalists to discuss survivorship care. He suggested that a standing meeting be held, perhaps on a yearly or biennial basis, to discuss advances in clinical practice guidelines and psychosocial issues. Both Dr. Oeffinger and Dr. Shapiro agreed that such a meeting was a very good idea. It would bring together relevant stakeholders and provide an opportunity to collaborate and learn what each other wants and needs. The reality is that the oncology community does not have the resources or capacity to provide care for survivors, and until a partnership is established with primary care providers, quality survivorship care will not be achieved. There are some good precedents for collaboration. The American College of Cardiology has worked with the American Academy of Family Physicians and the American College of Physicians through the Society of General Internal Medicine to develop collaborative guidelines on myocardial infarction posttreatment that have been widely adopted.
Dr. Lawrence Shulman of the Dana-Farber Cancer Institute made the distinction between areas of survivorship care for which there is no or little evidence and areas where there is incomplete evidence. For example, while not all of the evidence is in, it is well established that premature menopause after chemotherapy rapidly affects bone health. In this case, there are interventions to improve bone health. In Dr. Shulman’s opinion, the evidence is weaker for the recommendation to screen asymptomatic survivors for cardiac disease after doxorubicin therapy. Dr. Oeffinger reiterated that guideline development will highlight what is known and unknown and help to
set a research agenda to fill in the identified gaps. Experience with the pediatric guidelines illustrates the dynamic nature of guideline development in terms of identifying gaps in knowledge, trying to fill those gaps with current research, and then updating the guidelines as new findings are made available.
Ms. Martha Gaines of the University of Wisconsin Law School pointed out that there were many opportunities for survivors to be involved in their own care from its very beginning. An analogy can be made between patients and the captain of a ship. The captain is not always at the helm, but the ship neither leaves the shore nor heads for any destination without the captain’s approval. Dr. Oeffinger agreed with this focus on patient empowerment and described how the pediatric survivorship guidelines include over 50 health links that are written on specific topics that can be downloaded, read, and shared by survivors with their physicians. The Passport for Care empowers survivors with information, clinical recommendations, and guidance on improving their own health. Dr. Shapiro suggested that the culture of oncologist practice will have to change to accommodate broader survivor involvement in care.
PSYCHOSOCIAL SUPPORT RESOURCES
Presenter: Ms. Diane Blum
The IOM recommended that the Survivorship Care Plan include information on the availability of community-based psychosocial services. That information and a list of resources be provided to cancer patients at the conclusion of treatment was also called for 3 years ago in the President’s Cancer Panel report.5 It is imperative for an informed cancer survivor in 2006 to be able to use community-based resources. People are increasingly on their own while they are being treated for cancer and in the posttreatment phase of care. They are expected to make decisions, manage treatment, and integrate the cancer experience into their lives as best they can without resources that might have been provided in a hospital. Twenty years ago, being a cancer patient was very much a full-time job. People spent much of their time in a hospital and had access there to education and support. Progress in treatment has shifted care to the outpatient setting. In this environment, patients are seeing nurses less often and are rarely encountering social workers. While there has been a decline in onsite sources
of education, counseling, and support, there has been tremendous growth in Internet-based resources.
The problem for cancer survivors is not that there are too few resources. There are many national and local resources. The American Cancer Society (ACS), for example, has 3,300 offices and thousands of people a day telephone their call center. ACS also has an online searchable database to find local resources by zip code. CancerCare, the National Coalition for Cancer Survivorship (NCCS), the Lance Armstrong Foundation (LAF), the Wellness Community, and numerous disease-specific organizations are among the many other national organizations that provide services. There are also many regional and local resources, particularly for women with breast cancer. Excellent fact sheets and printed guides to resources are also available. The National Cancer Institute (NCI) booklet Facing Forward: A Guide for Cancer Survivors, for example, describes the roles of and access to social workers, nutritionists, and physical therapists. CancerCare will issue the fifth edition of its national guide to resources, A Helping Hand, that includes tips on evaluating them.6 “People Living with Cancer” is ASCO’s patient website that includes a wealth of information.7 NCCS, in collaboration with the Oncology Nursing Society and the Association of Oncology Social Work, has produced the Cancer Survivor Toolbox in CD format and online. The toolbox addresses many survivorship issues, including those related to employment and insurance.8 These sorts of materials should be distributed to every person with cancer in the country, especially those who have finished treatment. The bountiful resources available in print, by telephone, and online are very underutilized.
According to a fairly recent survey of 2,000 oncology professionals, fewer than 60 percent recommended support services or thought such services were helpful. This survey, published in Cancer Practice in 2002, included responses from members of professional organizations representing oncologists, oncology nurses, and social workers.9 Professionals who thought that support services were not helpful were least likely to make referrals and least likely to know about them. These results are alarming, given that the respondents were oncology professionals who belong to their professional organizations. The lack of attention to the psychosocial needs
|
6 |
Information on the guide can be found at http://www.cancercare.org/get_help/assistance/helping_hand.php. |
|
7 |
ASCO’s People Living With Cancer website is at http://www.plwc.org/portal/site/PLWC. |
|
8 |
The toolbox can be found at http://www.cancersurvivaltoolbox.org/default.aspx. |
|
9 |
This study, “Healthcare Professionals’ Awareness of Cancer Support Services,” by B. Alex Matthews, Frank Baker, and Rachel Spillers was published in Cancer Practice, Vol. 10, No. 1, January/February 2002 (pages 36-44). |
of cancer patients was also prominent in the findings from the IOM focus groups (see Chapter 3).
The 2004 IOM report, Meeting Psychosocial Needs of Women with Breast Cancer, found psychosocial interventions to be effective but under-used for many reasons, including stigma, inadequate insurance coverage, and, very importantly, lack of knowledge on the part of health care professionals.10
How can problems related to lack of knowledge and underutilization be addressed? How can one ensure that available materials and services reach the people they are intended to help? The problem lies in the absence of a systematic distribution system and no clear-cut allocation of responsibility. Pharmaceutical companies reach into every place where people with cancer are treated, and they might be enjoined to hand resource materials to a physician or a nurse, but experience suggests that such materials often do not reach the patient. Social workers could assume some responsibilities, but they rarely work in the community settings in which most people with cancer are treated. An additional challenge is keeping resource guides and materials up-to-date. Online guides are somewhat easier to update, but this is time-consuming and has to be assumed as an ongoing responsibility.
Assessing individuals for their psychosocial needs allows providers to refer survivors to a program that is more likely to be individually tailored to their circumstances. Some survivors will require psychological support, while others will need financial or insurance counseling. Making these assessments, however, takes time and should be undertaken only when appropriate resources are available to meet identified needs. Assessments and referrals may be complicated in the case of special populations and the “hard-to-reach.” Making resources available across differences in culture, age, and literacy can be a major challenge. These challenges must be overcome, because it is often the economically disadvantaged and individuals with other limitations who are most in need of resources.
Examples of programs that offer resources and resource information to survivors are CancerCare; www.plwc.org, the patient website of the American Society of Clinical Oncology; and the American Cancer Society. CancerCare is a nonprofit organization that provides free professional support services to 90,000 individuals a year. Clients include people with all cancers and their families and friends throughout the country. The services offered include counseling, education, and financial assistance provided by 120 staff, 75 of whom are either trained social workers or health educators. The social work staff works hard to offer quality service to the nearly 1,000 callers each week. CancerCare provides a number of special survivorship
|
10 |
Information on this report can be found at: http://www.nap.edu/catalog/10909.html. |
programs. A three-part telephone education workshop on survivorship is conducted annually. This program, supported by LAF and NCI, reaches thousands of people with good survivorship educational material. The program is archived on CancerCare’s website.11 CancerCare is part of the LAF LIVESTRONG™ initiative and responds to calls to the foundation from people who need psychosocial services. The potential demand for assistance is very high, and with additional resources, many more individuals could be helped.
ASCO’s website, People Living with Cancer, was developed as a member benefit. ASCO members were going to be able to refer their patients to the website for credible up-to-date information developed by a trusted organization. Three million business cards were printed with the website’s URL for members to distribute to their patients. Four years later, the website is a success. It contains quality content, and hundreds of thousands of people visit the site each year. However, it is not used well by ASCO members. Plans are for the site to be promoted more to the public, who seem to be enthusiastic users of it.
The American Cancer Society Call Center in Austin, Texas, is staffed by 400 well-trained counselors who provide a 24-hour, 365-day service. They receive about 2,500 calls a day that are patient related. The counselors link callers to resources according to zip code, but this approach may be limiting insofar as many resources are now virtual. An online service, for example, may be extremely valuable, but it would not necessarily be linked to a caller by zip code. The ACS has very high name recognition with the public and, given the volume of calls and visits to their survivorship-related web content, provides an important dissemination mechanism for information on survivorship.
The ultimate goal in terms of psychosocial services for cancer survivors is for each person at the completion of treatment to have a psychosocial assessment. The distress guidelines developed by NCCN are reasonable. Assessments are, however, appropriate only if resources are available to address the identified needs. Optimally, up-to-date resource materials would be distributed and tailored to the developmental needs of the patient—for example, young adults, older adults, parents. Survivors would then be given guidance on how to use the resources, when to use them, and how to evaluate them. Follow-up with survivors would ensure that appropriate resources were accessed. This is the optimal scenario. At a bare minimum, people should receive at least a resource guide and some basic materials. If every person who is completing treatment could be handed something, that
|
11 |
Information on these cancer care programs can be found at: http://www.cancercare.org/get_help/tew_calendar.php. |
would put them one step ahead of the game. Further steps could then be taken toward the ideal.
Although admonitions to increase awareness have become a cliché, some awareness campaigns have been very successful, for example, those launched in mid-1980s to raise awareness of breast cancer. An organization like the LAF could probably facilitate this kind of awareness program. Going directly to the survivor is another strategy that has been successful. Women have played a major role in changing the treatment of breast cancer, and they have also changed childbirth practices. Improvements in the management of cancer pain have been made by going directly to the person for whom pain is an issue. Physicians are key to change and also must continue to be targeted through professional organizations to raise awareness of psychosocial resources for their patients. Incentives might considered to aid in implementation efforts.
In conclusion, there are many quality resources, but no systematic method of distributing them and no health care professional identified as being in charge of this particular area of care, especially in community-based practices, in which most people are receiving their care. Lacking also are good evaluations of programs to assess their value.
Discussion
Dr. Lee Newcomer of the UnitedHealth Group started the discussion by asking whether research has shown what steps a new cancer patient takes to find information. Do we know, for example, where patients seek information and their level of reliance on the Internet? Dr. Julia Rowland described some unpublished research on information use that suggests that physicians are key providers, nurses are important, especially during active treatment, and the Internet is actively used by many. Ms. Blum mentioned that the “digital divide” seemed to be decreasing, indicating that Internet services are becoming accessible to more people. When the topic of finding information about cancer was raised in several patient focus groups conducted by Dr. Catherine Harvey, patients often said that they started with a neighbor, a church, a friend, or somebody at work. Information was initially sought through one of these informal networks. In the 10 to 12 communities in which focus groups were held, there was never a mention of a systematic approach to providing cancer-related information. This would seem to be a missed opportunity on the part of both oncology and primary care providers.
Ms. Blum pointed out that many patients do not know how to seek information and do not have the resources even to know where to start. Half of the people who call CancerCare each week have trouble getting transportation to treatment. That is their basic and overwhelming problem.
Dr. Lari Wenzel of the University of California, Irvine questioned
whether use of psychosocial resources would increase if good evidence were available on their effectiveness and, more specifically, who benefits and when they benefit. Ms. Blum responded that there is already a robust body of literature showing that a number of psychosocial interventions are effective. Much of the work has been in the context of women with breast cancer and was reviewed in the IOM report on meeting psychosocial needs. Some good evidence exists on the benefits of psychosocial interventions for other diagnostic areas as well.
Dr. Al Marcus of the AMC Cancer Research Center mentioned that a particular challenge from the perspective of a regional cancer center is identifying resources for patients coming to the center from a broad and diverse geographic area. Resources are needed to help patients get local help as they return to their homes. Listing some of the national resources on the care plan should be routine. Another resource, not yet mentioned, is NCI’s 1-800-4-CANCER telephone resource, which could also be very important in terms of providing referrals.
Dr. Rowland of the NCI Office of Cancer Survivorship suggested that the cancer-related call centers analyze their data to better understand consumers’ and patients’ information needs. The NCI cancer information service will do so to determine who is calling and what kind of information is being sought (e.g., prevention, diagnosis, treatment, survivorship, or end-of-life concerns). The LAF and the ACS are also examining the content of their calls. These data can help determine the adequacy of the available information and referral resources.
RECOMMENDATIONS FOR HEALTHY LIFESTYLE BEHAVIORS
Presenter: Dr. Wendy Demark-Wahnefried
Lifestyle factors, such as diet, exercise, and smoking cessation, should be considered in the care plans that are given to cancer patients as they finish their primary treatment. Cancer survivors are at greater risk for cardiovascular disease, osteoporosis, and diabetes, and changes in health behaviors may reduce these treatment-related conditions, cancer-related symptoms, comorbidity, and functional declines. Accumulating evidence also suggests that the pursuit of healthy lifestyles after treatment also may reduce cancer recurrence and improve both cancer-specific and overall death rates.
Only about 20 percent of oncologists provide any sort of guidance to patients on lifestyle issues. When counseling is offered, it is more often for smoking cessation than for diet and exercise. When asked why they do not provide this information, oncologists say that they lack time and are unsure of the science, the appropriate messages, and how to deliver them. Primary care providers have more familiarity and experience in this area. What
messages regarding health behaviors have sufficient evidence to support their delivery to cancer patients? What follows is a summary of a background paper prepared for the workshop in the areas of weight management, nutrition and diet, exercise, smoking, alcohol consumption, bone health, protection against skin cancer, and complementary and alternative medicines.12
Weight Management: Weight management is a key concern for cancer survivors since there are considerable risks associated with either underweight or overweight status. Anorexia and cachexia are prevalent problems for patients with cancers in advanced stages and certain gastrointestinal, respiratory, and childhood cancers. In these cases, weight gain is recommended to speed recovery, improve well-being, and increase functional status. These patients benefit from information and counseling to improve their nutritional intake. Often this requires additional counseling to increase their physical activity in an effort to stimulate appetite and reduce constipation, and in some cases pharmacologic interventions are required, for example, megestrol acetate.
In contrast, overweight and obesity are risk factors for several cancers, including cancers of the endometrium, esophagus (adenocarcinoma), colon, kidney, and postmenopausal breast cancer. The majority of breast and prostate cancer survivors are overweight or obese, and the high prevalence of overweight among these major subgroups of survivors is a key health concern. Being overweight at diagnosis is a risk factor for subsequent cancers. Weight gain is common during and after cancer treatment and also is linked with progressive disease, second primary cancers, and comorbid conditions (e.g., diabetes, cardiovascular disease) that may play a role in functional decline.
Shown in Figure 4-4 are data on breast cancer survivors enrolled in the Nurses Health Study. Women who increased their body mass index from 0.5 to 2 units were at significantly higher risk for breast cancer recurrence, breast cancer mortality, and overall mortality when compared with women who maintained their weight (represented by the second set of bars from the left). This unit increase in weight is not large and can be anywhere from 3 to 13 lb, depending on a woman’s height.
More definitive work needs to be done in the area of energy balance and survivorship because these findings come from longitudinal observational studies. Intervention studies are needed to see if weight reduction and maintenance of healthy weights are effective in reducing cancer recurrence and mortality, as well as comorbidity and functional decline.
|
12 |
For more information, see the background paper prepared by Dr. Demark-Wahnefried and Dr. Lee Jones in Appendix D.2. |
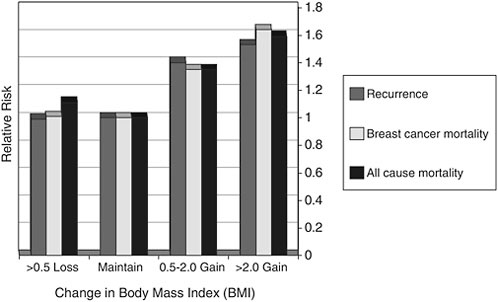
FIGURE 4-4 Outcomes related to weight gain following breast cancer: Results from the Nurses Health Study.
SOURCE: Demark-Wahnefried presentation of information adapted from Kroenke et al., 2005.
Body composition changes that occur during or after treatment also are a concern. Even if cancer survivors are able to maintain their prediagnostic body weight, their body composition often changes, especially during treatment with adjuvant chemotherapy. Figure 4-5 shows changes observed among breast cancer patients who received adjuvant chemotherapy (solid lines) or radiation therapy (dotted lines). The lightly shaded lines show the changes in adipose tissue (in kilograms) from diagnosis to 1 year after diagnosis. The darker lines show changes in lean body mass. Women who received adjuvant chemotherapy had significant increases in fat mass and significant decreases in lean mass, so that even if they were able to maintain weight, they were significantly fatter by the end of the 1-year treatment period. These changes in body composition are equivalent to what would be observed during 10 years of normal aging. This unique form of weight gain, called sarcopenic obesity, has implications for quality of life and metabolically in terms of insulin resistance.
What can the oncologist or primary care provider advise for the patient who come in following treatment with the same prediagnosis body weight, but with complaints that they no longer fit into their clothes and have increased body size? Resistance training is the hallmark treatment for sarcopenic obesity, and recommendation of an exercise program that in-
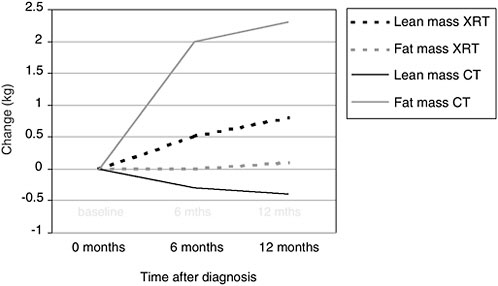
FIGURE 4-5 Body composition changes associated with treatment among women with breast cancer.
NOTE: XRT = x-ray therapy; CT = chemotherapy.
SOURCE: Demark-Wahnefried et al., 2001.
cludes resistance training can be important for regaining lean body mass and resuming previous body habitus.
Figure 4-6 shows the ACS guidelines for weight management. If underweight or at risk for underweight, patients should avoid further weight loss and consider nutritional counseling, physical activity, and the possible use of pharmacologic agents.
Patients of normal weight should try to maintain their weight with exercise and a healthy diet. Overweight or obese patients should be encouraged to lose weight and adhere to guidelines published by the National Heart Lung and Blood Institute (NHLBI). Research is needed to identify weight loss interventions that are effective among cancer survivors.
Nutrition and Diet: ACS guidelines recommend a prudent diet for cancer survivors that includes high proportional intakes of fruits, vegetables, whole grains, and low-fat dairy and lower proportional intakes of meat, refined grains, and high-fat dairy products. This guidance needs to be emphasized, because according to some surveys, fewer than 25 percent of cancer survivors eat at least five servings of fruits and vegetables a day, and fewer than half eat the recommended amounts of saturated fat.
The results of two studies, the Women’s Intervention Nutrition Study (WINS) and the Women’s Healthy Eating and Living (WHEL) study should

FIGURE 4-6 ACS recommendation for weight management.
NOTE: PA = physical activity; NHLBI = National Heart, Lung, and Blood Institute; NAASO = North American Association for the Study of Obesity.
SOURCE: Demark-Wahnefried, 2006.
provide information to inform future guideline development. WINS is a randomized trial comparing women with postmenopausal breast cancer assigned either to a group asked to adhere to a very low-fat diet (15 percent or less of calories from fat) or a control group that received counseling on a well-balanced diet. Preliminary results of this study suggest a 24-percent risk reduction in recurrence among women in the intervention group compared with the control group, with the greatest reduction among estrogen receptor–negative patients (Figure 4-7).
The WHEL study is currently examining the impact of increased fruit and vegetable consumption and a low-fat diet on breast cancer recurrence and mortality, and results are anticipated in 2008. More research is needed on specific dietary components, for example, soy and flax.
The ACS publication Nutrition and Physical Activity During and After Cancer Treatment: A Guide for Informed Choices was first published in 2003, was revised, and was rereleased in fall 2006. In 2004, the AHRQ also published an evidence-based review on physical activity and cancer, Effectiveness of Behavioral Interventions to Modify Physical Activity Behaviors in General Populations and Cancer Patients and Survivors.
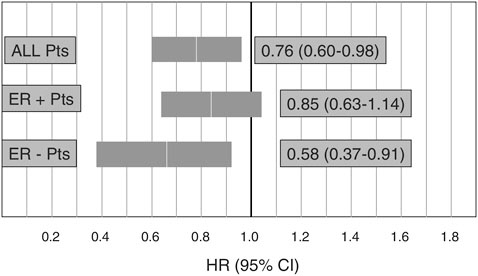
FIGURE 4-7 Preliminary results from the Women’s Intervention Nutrition Study on cancer recurrence in postmenopausal women with primary breast cancer (n=2,437).
NOTE: ER = estrogen receptor.
SOURCE: Demark-Wahnefried presentation of information adapted from Chlebowski et al., 2005.
Exercise: Systematic reviews of the literature and consensus reports suggest that exercise is safe for cancer survivors and has consistent positive effects on common symptom management issues, such as vigor, vitality, cardiorespiratory fitness, quality of life, depression, anxiety, and fatigue. A number of groups, including the ACS and the Centers for Disease Control and Prevention (CDC), recommend at least 30 minutes of exercise a day, 5 days a week. There are some patient groups, however, that may need to be evaluated before engaging in this level of exercise. For example, childhood cancer survivors who received anthracycline-based chemotherapy or chest radiation should undergo cardiac screening before starting an exercise regimen.
Data are accumulating to suggest that exercise has a protective effect in terms of cancer recurrence and survival. While data suggest that cancer survivors may exercise somewhat more (9 percent more, according to some surveys) than the general population, most survivors still fail to achieve recommended levels of exercise.
Shown in Figure 4-8 are data on cancer recurrence, breast cancer mortality, and all-cause mortality among a cohort of breast cancer survivors enrolled in the Nurses Health Study according to their levels of physical activity. The sedentary referent group is represented by the far left-hand set of bars. These sedentary women had significantly higher rates of recur-
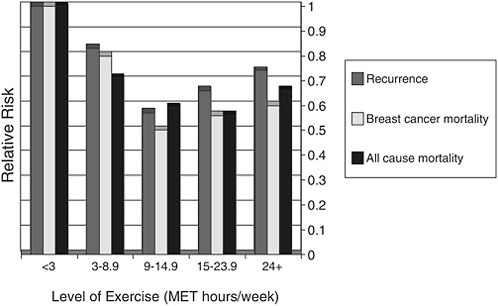
FIGURE 4-8 Exercise and cancer recurrence and mortality: Results from the Nurse’s Health Study (n=2,987).
SOURCE: Demark-Wahnefried presentation of information adapted from Holmes et al., 2005.
rence, breast cancer mortality, and all-cause mortality compared with women who had increased physical activity. The cut point of 9 hours per week is similar to the American College of Sports Medicine guidelines, that is, 30 minutes a session, 5 sessions a week. The level of risk reduction observed with exercise in this study is of the same magnitude (or better) than that observed from chemotherapeutic agents. While encouraging, these results must be interpreted with caution, because the data are observational. Randomized intervention studies to evaluate exercise with respect to these outcomes has yet to be conducted.
These promising results are from only one study in breast cancer; however, the Journal of Clinical Oncology has recently published two similar studies regarding the association of physical activity and colon cancer outcomes.13
Smoking: Many cancers are caused by smoking, and persistent tobacco use is associated with complications of treatment, progressive disease, second primary cancers, and increased comorbidity. Smokers diagnosed with smoking-related cancers have relatively high quit rates; however, these smokers often relapse. Clinicians must discuss smoking cessation during the “teachable moments” surrounding diagnosis and treatment and then consistently remind and support patients in sustaining healthy behaviors. The vigilant, long-term follow-up recommended by the U.S. Preventive Services Task Force needs to be applied to cancer survivors. Smokers represent a group in need of attention, because people who smoke often have unhealthy diet and poor exercise habits.
Alcohol Consumption: Alcohol is linked to cancers of the head and neck, kidney and breast. Head and neck cancer survivors who continue to drink have higher treatment-related complications, comorbidity, second primary cancers, and mortality. These patients need to be warned about the risk associated with alcohol consumption and referred to counseling services if necessary.
The prevalence of “risky drinking” (more than 2 drinks a day for men and more than 1 drink a day for women) among most cancer survivors is really no different than that seen in the general population. Higher rates of risky drinking have been noted among survivors of prostate cancer, head and neck cancers, and lung cancer. Moderate alcohol consumption is protective for cardiovascular disease, and the recommendation from the American Cancer Society is that “if you do drink alcohol, do so in moderation.”
Bone Health: Bone health is an important survivorship concern because various cancer therapies reduce skeletal integrity (e.g., luteinizing hormone-releasing hormone [LHRH] antagonists, glucocorticoids, select chemotherapeutic agents, radiation therapy). ASCO has published guidelines for breast cancer patients that recommend monitoring bone density and intervening as necessary with pharmacologic agents. For preventive measures, patients should ingest adequate amounts of calcium and vitamin D, undertake weight-bearing exercise, quit smoking, and curb excess consumption of alcohol, protein, caffeine, and sodium. The role of calcium and vitamin D needs further examination because, in the case of prostate cancer, evidence suggests that increased calcium intake may be associated with more aggressive disease.
Protection Against Skin Cancer: Cancer survivors who received X-ray therapy are at higher risk for skin cancer, especially childhood cancer survivors. Thus, skin examinations should be performed routinely. In general,
sun protection should be encouraged among cancer survivors. Some controversy has arisen, however, as to the degree of sun protection warranted for survivors of other cancers, given evidence that vitamin D from sun exposure may be protective against some solid tumors and their subsequent progression (e.g., prostate cancer).
Complementary and Alternative Medicine: Complementary and alternative medicine (CAM) includes specific diet and exercise regimens, diet and herbal supplements, acupuncture, massage, and mind-body therapies. No CAM therapies have proven to be beneficial in terms of clinical outcomes; however, some are effective in reducing anxiety (e.g., relaxation therapy). Most cancer survivors use some form of CAM therapy, for example, 60-89 percent of survivors take supplements, and 40-50 percent initiate additional supplements at the time they are diagnosed. Providers need to maintain open communication with their patients regarding CAM, and they should refer patients to reliable sources of information (e.g., the National Center for Complementary and Alternative Medicine). They also should be aware of supplement use with harmful implications (e.g., beta-carotene in smokers and PC-SPES use among men with prostate cancer) so that they can advise their patients accordingly. Additional research is needed to determine the effects of supplements to determine either their potential benefits or adverse events in the survivor population.
In summary, cancer provides a teachable moment for making positive lifestyle changes. Interventions need to be tested to determine how to best capitalize on this opportunity so that healthy behaviors that are initiated are sustained. Oncologists can play a key role in catalyzing behavior change. Primary care providers, nurses, and allied health professionals have key roles to play. Increasingly, Web-based programs, telephone counseling, and mailed interventions may prove to be acceptable and effective in promoting and sustaining lifestyle change. It is very difficult to change lifestyle behaviors, and sustained interventions to ensure long-term adherence are needed.
Cancer survivors are very interested in such lifestyle factors as diet, exercise, and smoking. When asked “How do you like to receive assistance?” survivors report the most interest in interventions that are delivered via the mail, with less interest reported for clinic-based programs, telephone counseling, and computer-based approaches. Recently, however, a telephone-based intervention for smoking cessation in young adults was shown to be highly effective.
A mailed material intervention called Fresh Start has been very successful in changing behaviors of newly diagnosed breast and prostate cancer patients. A new program called RENEW is currently testing a hybrid program of mailed materials (exercise bands, portion-guided tableware and
workbooks) and telephone counseling among long-term survivors of breast, prostate and colorectal cancer. Its goal is to facilitate weight loss and diet and exercise behaviors in an effort to improve functional status.
In summary, cancer survivors are at risk for cancer recurrence, comorbidity, functional decline, and decreased survival. Adherence to behavioral health guidelines that have been developed for survivors may help reduce these risks. More research is needed to determine the optimal content, formats, and delivery channels for interventions in these areas. It is clear, however, that sustained lifestyle modification is crucial to achieving optimal health among cancer survivors. Related assessments, education and counseling, and appropriate referrals need to be incorporated into survivorship care planning.
Discussion
Dr. Patricia Ganz questioned whether the education and counseling needed in this area can be accomplished successfully by oncologists. She advised establishing links with primary care physicians, who are providing these interventions for many of their patients. Although it is very important to sensitize the oncology community about these issues, primary care physicians, in following these patients over the long term, will need to be especially vigilant in addressing them. It is critical for the oncologist to say, “This is important,” because doctor recommendations are extremely powerful motivators, but at the same time, it is important to somehow establish linkages with other resources.
Dr. Demark-Wahnefried agreed, reinforcing the fact that oncologists play an important role in persuading patients to change their behaviors and in catalyzing appropriate action. If suggestions for behavioral change are made and included in the care plan, primary care physicians will be prompted to raise these issues and to be proactive when it comes to making referrals.
As a primary care provider, Dr. Jean Kutner recommended that oncology providers inform primary care providers of the increased risks facing cancer survivors. Behavioral counseling is very central to what general internists do, especially as preventive health is incorporated into pay for performance initiatives. Primary care providers, however, are unlikely to be familiar with the heightened risks facing cancer survivors.
Dr. Kenneth Schellhase, a family physician from Milwaukee, cautioned that there is no evidence that primary care providers who counsel patients to lose weight are effective. He pointed out that there is actually good evidence that such counseling does not work. Cancer survivors, however, are a motivated group, and so they provide a golden opportunity to test whether, in this context, advice might actually be followed.
PHYSICIAN AND SURVIVOR DECISION SUPPORT USING INFORMATION TECHNOLOGY: THE PASSPORT FOR CARE
Presenters: Dr. David Poplack, Dr. Marc Horowitz, and Dr. Michael Fordis
The Passport for Care is an online national resource for survivors of childhood cancer that is under development by Baylor College of Medicine’s Texas Children’s Cancer Center and Baylor’s Center for Collaborative and Interactive Technologies, in collaboration with the COG through a working group and steering committee.14
As discussed throughout this workshop, cancer survivors face the following serious issues:
-
Medical late effects;
-
Lack of consistent long-term medical follow-up;
-
Psychosocial concerns;
-
Employment and insurance problems; and
-
Discrimination.
Complicating their situation, childhood cancer survivors have frequent changes in health care providers, and they often see primary care physicians who are unfamiliar with survivorship issues. For example, a 28-year-old survivor of Wilm’s tumor who received radiation therapy at age 4 and is seeing a primary care provider because he has developed hematuria may not know that this symptom may be associated with his history of cancer treatment. The primary care physician is unlikely to make this connection, especially if the survivor lacks details of his diagnosis and treatment history.
The IOM report identified the need for every survivor to have a Survivorship Care Plan that contains detailed information regarding cancer diagnosis and treatments, recommended follow-up evaluations, preventive practices and health maintenance information, and guidance on available resources related to psychosocial concerns, employment, and health insurance.
The Passport for Care is being developed to provide this care plan information to childhood cancer survivors. It is an Internet-based resource that provides survivors and their physicians or caregivers immediate access to a portable care summary of the survivor’s treatment history, individualized guidelines for care, information and links on prevention and healthy lifestyles, alerts to guideline changes and new information on late effects of therapies that the survivor may have received, general news on survivor-
|
14 |
For additional information on the Passport For Care, see the background paper prepared by Drs. Poplack, Horowitz, and Fordis in Appendix D.3. |
ship, a customized list of national and local resources, provider contact information, and opportunities to participate in research. The passport also provides access to a survivor forum, which allows the survivors to contact others who have had the same disease or who have had similar treatments. There are video stories of individual survivors with the same disease. The Passport for Care is a means of empowering survivors and facilitating their long-term follow-up.
Survivor participation in the Passport for Care will be voluntary and will require consent. One of its most notable features is that the survivor will control the sharing of information, both what is shared and how it is shared. Both the information in the passport and the process of sharing information are secured using encryption technology. The Passport for Care has been designed to be in compliance with requirements of the Health Insurance Portability and Accountability Act (HIPAA).
The Passport for Care will ultimately contain portals for the survivor, the primary care physician, and the pediatric oncology physician following survivors. The portal for pediatric oncology physicians is being developed first in order to meet the needs of providers in the Children’s Oncology Group, which treats over 90 percent of the children with cancer in the United States.
The Passport for Care is based on the Comprehensive Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers that have been developed by COG’s nursing discipline and late effects committee. COG members have enthusiastically endorsed broader dissemination and use of the guidelines in helping childhood cancer survivors and other health professionals who provide care to survivors to recognize and manage health risks related to late effects of treatment. However, because of the length of the guidelines and the detail contained in them, clinical utility of the paper-based version of the guidelines on a day-to-day basis in a busy clinical practice is limited. It is precisely for this reason that the Passport for Care, with its ability to generate individualized follow-up recommendations, is anticipated to be attractive to the practicing clinician.
Use of the guidelines is likely to increase when they are accessible through the Passport for Care. It can serve as a clinical guide to the physician in terms of what questions he or she needs to ask the survivor, what the physician should be looking for on the physical examination, what studies need to be done, and how often. Physicians will have access to detailed information embedded with the guidelines, including access to the latest references that relate to any particular recommendation. They will also be able to access links on the COG website that provide detailed information on pediatric cancer and survivorship issues.
It is important to keep in mind that the Passport for Care is not a
comprehensive medical record, nor is it intended to be a substitute for an ongoing physician follow-up effort. Workshop participants were shown a brief demonstration of the Passport for Care prototype to illustrate how the passport will be used by survivors and their health care providers.
What is the current status of the Passport for Care? The COG guidelines are now housed in the passport’s database and can be easily updated or modified. A consensus has been reached on the elements to be included in the COG care summary, and these elements have been integrated into the passport database. The survivor and primary care portals are being completed, and pilot testing is scheduled for selected COG clinics in early 2007. An evaluation will be conducted to validate the feasibility, utility, and impact of the Passport for Care on the follow-up of long-term survivors.
The passport as envisioned will play an important role in research. It will serve as a way to recruit survivors into studies and will also provide opportunities for educational and outcomes research. In terms of training, continuing medical education credits could be given to those physicians who use it.
What are some of the challenges faced in developing the Passport for Care that are relevant when considering such a tool for survivors of adult cancer? The first and most important prerequisite for such a tool is having evidence-based guidelines and adapting them to online use. A care summary is then needed. A major problem in the development of the Passport for Care has been securing financial support. Most representatives of federal funding agencies, although excited about the passport, do not have funding mechanisms to support the development of these sorts of applied tools. More readily available is funding for research to demonstrate the value of the passport. For its development, support has come from a variety of organizations, including the LAF, Ronald McDonald House Charities, and the Hearst Foundations. What is needed for the long term is a source of support to maintain this type of resource. Possibilities being considered include the development of a survivorship foundation or an endowment that would allow us to maintain and improve this technology over the long term. The Passport for Care represents a paradigm and a model that could potentially be applied to survivors of adult cancer. Adult oncology care differs markedly from pediatric oncology care. Nevertheless, it is likely that the Passport for Care could eventually be adapted for survivors of adult cancers.
Discussion
Dr. Peter Raich of Denver Health Medical Center applauded how the Passport for Care has been designed to meet the needs of survivors while at the same time facilitating entry into clinical trials. Such a system for adults,
even using some of the templates under discussion at the workshop, could potentially increase clinical trial enrollment among adults, which now stands at 3 to 5 percent. Dr. Poplack is optimistic that the passport can be adapted for survivors of adult cancer, but he cautioned that “the devil is in the details.” In the context of adult cancer, he advised starting with a discrete population of patients, for example, breast cancer survivors. For this group, evidence-based guidelines can be developed and integrated into a passport-like tool that can be tested as a “proof of principle.”
Dr. Shapiro congratulated the passport developers for their outstanding work and pointed out that the Passport for Care comprehensively applied principles embodied in the IOM report. He asked how the passport works in practice, for example, “Who inputs data into the system?” Dr. Poplack responded that, in the COG, the care summary information can be entered by oncology physicians, nurse practitioners, or certified research administrators. The physician checks and verifies the information. This aspect of the passport is key, because the underlying algorithms use this information to generate the individualized guidelines. Once the treatment summary information is entered, the rest is automatically generated.
Dr. Poplack reiterated the monumental nature of the guideline development process. While difficult, the process of adapting the guidelines into the standard format required by the passport system has improved the guidelines.
Dr. Ganz asked about interoperability and wondered if oncology providers could download guideline recommendations from the passport system into the physician’s own electronic record. Dr. Poplack described how physicians can read or can access and retrieve information electronically and then can use it in their own systems. The passport will be interoperable, and one of the goals will be to have automatic population of the passport with information that is entered into the treatment summary record. The COG has developed a very comprehensive care summary that, once completed, will be linked with the Passport for Care.
REACTION
Dr. Lawrence N. Shulman
As an invited reactant to the presentation given by Dr. Poplack and his colleagues on the Passport for Care, Dr. Shulman credited the pediatric community with leadership in all aspects of survivorship. Examples include not only the Passport for Care effort, but also early adoption of survivorship clinics and an organized system to develop comprehensive guidelines. In his view, incorporating information technology into adult care systems
presents some unique challenges. Dr. Shulman shared some of the lessons he has learned over the 15 years he has applied information technology solutions to improving adherence to guidelines and ensuring patient safety.
-
Tools to create a Survivorship Care Plan must have a high usability rating by the physicians, nurses, and certified research administrators who are going to be completing it. Without their acceptance, the system will not be adopted.
-
Tools must be easily changeable as new information becomes available. Programmers or analysts must be able to enter new information into the system overnight to reflect the latest scientific evidence.
-
Decision support should be a component to improve the output and benefit of the tool.
-
In relation to completion of forms: knowing the last bit of data should lead to entry of the next, for example, choosing breast cancer should lead to breast cancer chemotherapy and hormonal regimens and breast radiation options.
-
In relation to delineation of risks and recommendations: selecting certain treatments should preselect risks and follow-up recommendations.
-
-
Development of tools should be iterative: if you try to develop and deploy the perfect system, you will never implement any system. Start simple and develop a system that can grow and improve with experience.
-
Tools must be widely available for practicing clinicians in both academic and community settings, for those using and not using electronic medical records. The vast majority of adult patients get their care in community-based programs.
-
Tools should have customized output that may be different for patients, referring physicians, and oncology records.
-
Tools should be able to use disease- and treatment-specific information as decision supports to develop individualized risk assessments and follow-up care recommendations.
-
Tools might incorporate information for which there are different levels of evidence. The level of evidence may be specified.
-
Clinicians completing the form should be able to edit statements regarding risks and recommendations on assessments.
-
As risk factor and patient-specific information changes, the tool needs to be able to incorporate new data, revising risk assessments and recommendations.
-
Tools should supply risk assessments and recommendations around:
-
medical issues specific to individual cancer and treatment history;
-
psychological issues and support; and
-
routine health care, as indicated.
-
Dr. Shulman presented slides showing a pilot tool being developed at the Dana-Farber Cancer Institute. It represents a simple approach and a place to start in adult survivorship care. The first slide shows the entry of the diagnosis of Hodgkin’s lymphoma (Figure 4-9).
Once the diagnosis is entered, the chemotherapy page pulls up the specific chemotherapies that are used for that cancer (Figure 4-10). The provider can check the agents and then enter the total doses.
The radiation page profiles what the Hodgkin’s lymphoma patient is likely to have gotten, and the provider specifies the doses and the dates (Figure 4-11).
The information provided on chemotherapy and radiation therapy is summarized on a treatment summary page (Figure 4-12).
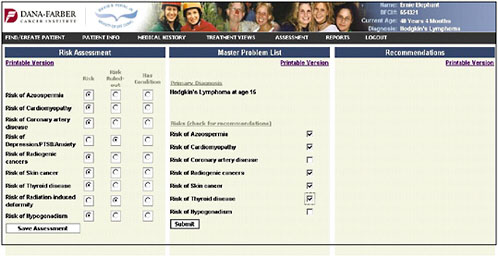
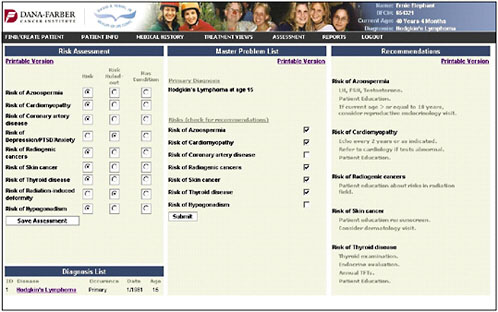
An assessment/problem list is then generated based on the patient’s age, sex, and treatments (Figure 4-13). The provider can then go down the list and check risks—for example, in this case, azoospermia, cardiomyopathy, coronary artery disease, depression and anxiety, and risk of second cancers. The provider can indicate that the patient’s risk has been ruled out or that the patient has the listed problem.
Once the provider has identified the pertinent risk factors, information and recommendations can be generated in a bulleted format (the information shown in the figure is in draft form and is shown only to illustrate how the system might work) (Figure 4-14).
A patient summary can be generated and printed. This draft example is written in patient-friendly language and is called an oncology long-term follow-up summary (Figure 4-15). It instructs the survivor to share the summary the doctors and to keep it in their personal files.
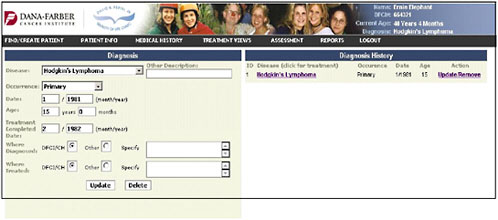
FIGURE 4-9 Diagnosis entry.
SOURCE: Shulman, 2006.
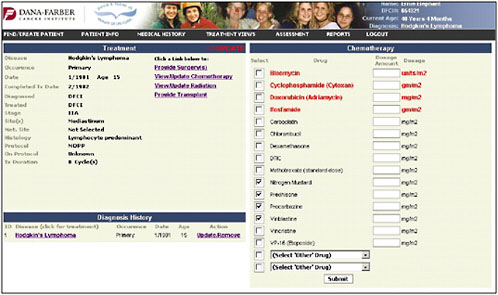
FIGURE 4-10 Chemotherapy entry.
SOURCE: Shulman, 2006.
A summary suitable for distribution to the primary care provider can also be generated (Figure 4-16).
Dr. Shulman raised several questions for the workshop participants to consider regarding implementation:
-
Will physicians and nurses want to use such a tool? Will its potential value make them feel that its use is worthwhile? Will peer pressure motivate its use?
-
Could the treatment summary become the equivalent of a clinic note or admission note and be considered a part of normal practice?
-
Could the treatment summary be required by regulatory agencies such as the Joint Commission on the Accreditation of Healthcare Organizations?
-
Could the use of this tool be tied to pay for performance and reimbursement to improve the quality and rationality of follow-up care? Payers and insurers want well-codified care plans.
-
Could patients help push this as an expectation of their care?
Dr. Shulman described mechanisms to encourage the development of information technology that will facilitate survivorship care. A centralized,
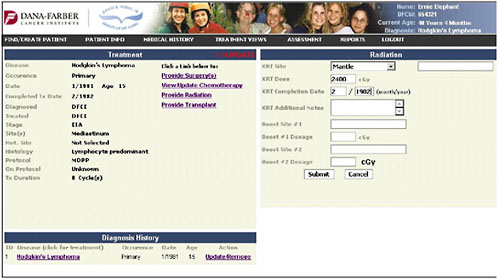
FIGURE 4-11 Radiation entry.
SOURCE: Shulman, 2006.
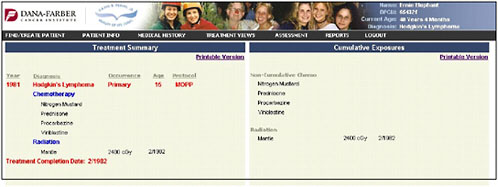
FIGURE 4-12 Treatment summary.
SOURCE: Shulman, 2006.
national effort, similar to the COG guideline development project, is needed in adult oncology. Teams will need to be assembled by cancer type to achieve consensus on the content of the care plan—for example, the chemotherapy and radiation therapy regimens that need to be included, the risks for late effects, and recommended follow-up. This collaborative effort might be similar to the NCCN’s efforts to develop guidelines. There is a need to start simply but on a platform that will facilitate enhancements with time.

FIGURE 4-15 Patient treatment summary.
SOURCE: Shulman presentation, 2006.
A central organization needs to be established to assume this task. It will need an administrative arm, clinical teams, and an information technology group that manages the development of the tool. Funding will have to support this effort—including the development, implementation, maintenance, and evaluation costs.
REGIONAL APPROACHES TO CANCER SURVIVORSHIP PLANNING
Presenter: Dr. Tim Byers
As clinicians become engaged in survivorship care planning and as tools are developed to aid them, it is important to be thinking about the role of organizations and institutional systems at the state, regional, and local

FIGURE 4-16 Primary care provider (PCP) summary.
SOURCE: Shulman, 2006.
levels.15 These could be involved in both responding to and perhaps motivating national opportunities. A recent example of this interplay between local and national opportunities is the decision by the companies making Coca-Cola and Pepsi-Cola to remove high-calorie carbonated beverages from U.S. schools. This national solution was reached because of activities at the local level. It became clear to drink manufacturers that they were going to lose their access to schools city by city, and so a number of bottom-up efforts created a top-down solution. Both kinds of solutions will be necessary to improve U.S. cancer survivorship care. There are going to be national efforts, but also some state, regional, and local ideas and demonstration projects that will emerge.
|
15 |
For additional information on regional approaches to cancer survivorship planning, see the background paper prepared by Dr. Byers in Appendix D.4. |
State-level cancer control efforts are beginning to focus on cancer survivorship. Across the country there are 44 states with comprehensive cancer control plans. The CDC has a strategy to capitalize on their state-based investments in epidemiologic surveillance, tobacco control, and breast and cervical cancer screening. They are providing support to states to help them coordinate these varied efforts into cohesive and comprehensive cancer control programs.
States have received assistance for planning, but they now face the challenge of implementing their plans with limited support. Most have specified that they want to cut cancer death rates, decrease smoking, address the problems of obesity and physical inactivity, and increase screening for cervical and breast cancer. Without the necessary federal support, the 44 states that are implementing their plans are trying to identify new sources of support and partners. In response to a question that Dr. Byers posed to the audience about involvement with state plans, about 10 percent of the workshop participants indicated that they were well engaged with their state’s cancer control program, two-thirds indicated that they were unfamiliar with their state’s plan, and the balance fell somewhere in between.
Dr. Byers introduced Dr. Loria Pollack, a medical officer at CDC, and asked her about its vision for state cancer programs over the next 5 years. Dr. Pollack indicated that CDC sees itself as providing an impetus, guidance, and expertise to states as they design their own plans. CDC does not want to dictate how states create their plans or what goes into them. CDC has provided guidance to address the entire continuum along the cancer trajectory, from prevention and early detection to palliative and end-of-life care. The budget for comprehensive cancer control planning has gone up, but more and more states, tribes, and territories are getting this support.
CDC encourages states to create coalitions of clinicians, public health departments, businesses, and advocacy organizations. The comprehensive cancer control plan is an impetus to bring these divergent groups together. CDC expects the partners to also provide some funding. In Georgia, tobacco settlement money has gone into the planning efforts. In Connecticut, $6 million has been directed to cancer planning from the state budget, the hospital fund, and other sources. CDC takes a background role as these coalitions take ownership of the cancer control plan to serve their own communities.
Dr. Byers noted the existence of a wide gap between public health and clinical care in the United States, resulting in some state coalitions lacking the necessary clinical partners. Most of the systems involved in care for cancer patients are not represented in the cancer coalitions. Creative ways to engage clinicians are needed. The cancer plans, in order to be considered comprehensive, need to diversify to include clinical perspectives. A content
analysis of the 44 state plans shows that tobacco control, epidemiologic surveillance, and cancer screening goals are all well represented. There is very little coverage of clinical issues.
There is an enormous opportunity to use the state cancer planning groups and the state cancer coalitions to move survivorship care planning forward into demonstration projects. Every one of the 44 state cancer plans mentions cancer survivorship as an important issue. Most of these plans mention survivorship only briefly. There are at least two states, Oregon and Minnesota, that have some focus on cancer survivorship planning. All of the states recognize that they need to do more to address the needs of cancer survivors, and they are looking for something specific to do. Including survivorship care issues in the state plans may engage some of the clinical partners who have not yet come to the table, especially if they can participate in a specific demonstration project. Survivorship as a public health issue is something that both NCI and CDC have been talking about, and the LAF is now joining with both of those organizations to try to move this forward.
There are opportunities in the short term to have demonstration projects in partnership with public health agencies and some of the NCI-supported cancer centers. There are 39 comprehensive cancer centers around the United States whose primary mission is cancer research. Each one of them has an unfunded mandate to earn that adjective “comprehensive” in its name and provide community service through education and outreach.
In the short term, NCI-designated comprehensive cancer centers, CCOPs, and other cancer care systems could take a look at their clinical trials and examine the opportunities to answer questions related to cancer survivorship. Dr. Raich, asked to comment on this suggestion, pointed out that few adult cancer patients participate in clinical trials and that relatively few trials have been designed with survivorship issues in mind. Nevertheless, these programs could provide excellent settings for doing pilot studies in cancer survivorship care planning. The big obstacle is funding such demonstration projects. CCOPs can receive “cancer control credits” for conducting this kind of research, and this may represent a small motivator for them to get involved.
Dr. Byers mentioned that many patients leaving clinical trials are not given a Survivorship Care Plan at the conclusion of their treatment, and they probably should have such a plan. This could be a good place to pilot test paper- or Web-based versions that have been discussed. Cancer centers are supposed to be innovative and cutting edge, and the treatment provided as part of any trial is very well defined. This and the fact that quality-of-life outcomes are often measured as part of trials would seem to make this an ideal place to start.
Other opportunities for demonstration programs at the state or regional level may be through the Centers for Medicare and Medicaid Services’ (CMS) quality improvement organizations (QIOs). QIOs may examine claims data to identify problems and work in concert with providers to improve care. Some cancer quality programs have been undertaken, but most of the successful efforts have been in the area of cardiovascular disease and diabetes management. A QIO-run demonstration project would be very natural because they already work closely with providers to monitor and evaluate care. A joint project could be undertaken by two or three of them around the country. If the demonstration project were successful, both operationally and in terms of improved outcomes, then it would be expected to become a standard of care. Once a program was established in Medicare, other payers would be likely to come on board.
Sometimes local health care systems can have an enormous impact. For example, if the Mayo Clinic adopts a new policy, then southern Minnesota is affected as a region. When the Marshfield Clinic moves ahead with a new program, then central Wisconsin is affected as a region. This is also the case with large managed care programs such as Kaiser Permanente.
In summary, in terms of the various organizations that can affect state and regional approaches to survivorship care planning, state public health departments have a key role to play. They are the conveners of the state-level cancer control programs. The state public health agencies will have to learn how to accommodate multisectorial involvement, especially private-sector involvement and leadership in improving the cancer situation in states. State health agencies must be active partners in the process, but they will need to step aside to let others move the process forward. Comprehensive cancer centers have an important role to play, but they will need to avoid ivory tower approaches and design demonstrations projects that have a business model for the private sector so that they can be sustainable. QIOs have statewide reach. They are generally not active partners in state cancer programs, but there is an immediate opportunity for CMS to conduct demonstration projects and affect state-level practice along these lines. Health professional societies tend to be more national than local, but in some areas there are some active local health professional societies that might come to bear on this.
To move the agenda forward, cancer survivorship planning should be a key element in every state comprehensive cancer control plan. Inclusion in the plan does not ensure its implementation. However, if there are actionable items in the plans, and if there is interest in the community, then putting some specific goals and objectives into plans for cancer survivorship care planning could be very helpful.
Should the plan say that, statewide, by the year 2010 everybody is going to have a care plan? That might be a long-term vision, but the most
useful action item for the next 2 to 3 years would be to conduct some demonstration projects, evaluate them, publicize the results, and then move toward standardizing the practice. It may be the case that the imperative for care planning will be established nationally in the next two to three years, but it is also likely that local initiatives will be needed to motivate change at the national level. As demonstrations are proceeding, business models must be developed to sustain survivorship care planning.
Dr. Demark-Wahnefried suggested that for states to take an active role, CDC will have to encourage demonstration projects on survivorship. Dr. Pollack said that while CDC is very interested in this area, they have no dedicated funds for survivorship projects.
Dr. Byers pointed out that CDC does not have the resources for 98 percent of the items that are in cancer plans. This is why diversification is necessary. Dedicated tobacco tax revenues, the private sector, and nongovernmental organizations can be mobilized to support the implementation of these plans.
Dr. William Kraybill identified another potential partner in state or regional cancer control, the American College of Surgeons’ Commission on Cancer (ACS-COC). Most large and small cancer programs around the country are organized in a multidisciplinary fashion and abide by a set of standards promulgated by the ACS-COC. They are partially funded by the American Cancer Society and are likely to be enthusiastic about moving survivorship forward. Dr. Byers agreed and suggested that the combination of the American Cancer Society, the ACS-COC, and the state tumor registries represent a potentially powerful triad that has not been fully taken advantage of.
Dr. James Talcott characterized survivorship as crosscutting and mentioned organizational barriers that make it difficult to cut across disease entities. Academic medicine is often organized by disease, and often, one must duplicate effort for breast cancer, prostate cancer, lymphoma, and every other oncology subspecialty. It is sometimes hard to engage in activities that cut across diagnoses. There are almost no opportunities for researchers to work with other investigators who are doing the same thing “one disease over.”
Finally, Dr. Byers discussed how the traditional public health role of surveillance could be enhanced to further survivorship care planning. Perhaps methods could be devised to routinely monitor outcomes of confusion, anxiety, fatigue, dissatisfaction with care, and poor quality care. Survivorship-related quality-of-care measures could include those related to poor transitions in care or underuse of tamoxifen among women with breast cancer who were prescribed the medication. Cancer registries could be monitoring outcomes after cancer apart from death and (in some) recurrence. Demonstration projects on enhanced cancer registration to capture
some of these data elements could be considered. Extending cancer registration into these areas on a fairly routine basis would help to shine a light on the problem.
In summary, Dr. Byers reiterated the value of working with state cancer programs to build survivorship issues into the comprehensive cancer control plans. This planning effort should be moved out of public health agencies, so that public health is a partner but not a convener of these plans. Extending the public health function of surveillance to include cancer outcomes that are common and important to cancer survivors should be considered and tested. Demonstration projects could be initiated soon to begin to test these ideas and processes and to move them forward.