3
Causes of Pollinator Declines and Potential Threats
It is difficult to determine whether North American pollinator species are declining, and no less challenging is determining the causes of putative declines or local extirpations. Many explanations have been invoked to account for declines in pollinator populations in North America, including, among others, exposure to pathogens, parasites, and pesticides; habitat fragmentation and loss; climate change; market forces; intra- and inter-specific competition with native and invasive species; and genetic alterations. Careful evaluation of the literature allows some causes to be assigned, but explanations are ambiguous or elusive for other species losses.
DECLINE IN ACTIVELY MANAGED POLLINATORS
Honey Bees (Apis mellifera L.)
The best evidence of specific pollinator decline is seen in the western honey bee, Apis mellifera L., the primary commercial pollinator of agricultural crops in North America and the most widely used, actively managed pollinator in the world (Delaplane and Mayer, 2000; Kearnes et al., 1998; McGregor, 1976). The population losses among honey bees are elucidated in a large body of literature on honey bee pests, parasites, and pathogens (Morse and Flottum, 1997), most notably on the parasitic mites Varroa destructor (varroa mite) and Acarapis woodi Rennie (tracheal mite), the pathogen Paenibacillus larvae (American foulbrood, [AFB]), and the invasive Africanized honey bee.
Parasitic Mites
Varroa Mite (Varroa destructor)
The supply of healthy and affordable honey bee colonies for crop pollination clearly has been threatened by the arrival of parasitic mites Varroa destructor and Acarapis woodi. Since 1981—just before the arrival of A. woodi—stocks of honey bee colonies in the United States have declined by 39 percent (Figure 2-1; USDA-NASS, 1995, 1999, 2005, 2006). Parasitism by mites of honey bees is a relatively recent problem in North America. A 1980–1982 survey of samples from 4,400 apiaries in the United States and Canada revealed no evidence of mite infestation (Shimanuki et al., 1983). The varroa mite was first reported in the United States in 1987 (Anonymous, 1987) and within a decade it had become established throughout the United States.
Varroa destructor (Anderson and Trueman, 2000) has caused dramatic declines in honey bee abundance in North America and throughout the world (DeJong, 1990; DeJong et al., 1982a; Sammataro et al., 2000). The varroa mite is an obligate external parasite of A. mellifera and Apis cerana (eastern honey bee) that was first described as V. jacobsoni (Oudemans, 1904) in Java. It exists there in a stable and sustainable association with A. cerana, its native host (Rath, 1999). In eastern honey bee colonies, female varroa mites reproduce almost exclusively on male (drone) larvae or pupae (Koeniger et al., 1983), so they do not affect the population size of the female worker force. The biology of A. cerana, including its relationship with the varroa mite, is discussed by Kevan et al. (1996) and by Oldroyd and Wongsiri (2006).
The association of V. destructor with the western honey bee, A. mellifera, reportedly began in the 1950s (Matheson, 1995) when the mites moved into honey bee colonies brought into the home range of A. cerana. Subsequently, the varroa mite has established a nearly cosmopolitan distribution with respect to its new host, and Australia is now the only mite-free continent (Matheson, 1995). It is not known how this parasite entered the United States.
In A. mellifera, female varroa mites reproduce on both worker and male larvae. Infestation of honey bee colonies of European origin (the source of most A. mellifera introduced to North America) is fatal if untreated, and colony mortality usually occurs 6 months to 2 years after the initial infestation (DeJong, 1990).
Newly emerged adult worker bees parasitized as pupae exhibit a range of symptoms: substantial loss of adult weight (DeJong et al., 1982a,b; Engels and Schatton, 1986), reduced concentrations of serum proteins (Engels and Schatton, 1986), impaired development of (brood food-producing) hypopharyngeal glands (Schneider and Drescher, 1987), severe deformations of
the wings (Akratanakul and Burgett, 1975), and reduced longevity (DeJong and DeJong, 1983).
Varroa parasitism of A. mellifera drones also can affect the ability of the queen to obtain adequate supplies of healthy sperm during mating. Parasitism has been associated with reduced sperm quality (Collins and Pettis, 2001) and with decreases in adult weight, size of seminal vesicles, and mucus. Effects of parasitism on male behavior include a decline in the frequency of flight (Schneider, 1986) and decreased flight performance, sperm production, and mating efficiency (Bubalo et al., 2005; Duay et al., 2002).
Varroa parasitism of honey bees is associated with viral pathogens, and some damage attributed to varroa mites is actually viral in origin (Allen and Ball, 1996). Although some viral diseases of honey bees are associated with varroa infestations (Kevan et al., 2006; Oldroyd and Wongsiri, 2006), which negative effects are exclusively attributable to direct actions of the mites or to their associated pathogens is unknown (Chen et al., 2005). “Parasitic mite syndrome” is used to describe colonies that exhibit a constellation of symptoms, including the presence of diseased adult and immature bees, adults with deformed wings, and crawling bees at hive entrances (Shimanuki et al., 1994). Once this syndrome is apparent, the colony begins a rapid decline in adult worker population and viable replacement brood. It dies, typically within 3–6 weeks of the onset of symptoms.
The rate at which the varroa mite population increases in a honey bee colony depends in part on the rate at which individual mites reproduce (Fries et al., 1994). Some stocks of honey bees, such as neotropical Africanized honey bees (see section on Invasive Species in this chapter), are less susceptible to varroa mites than are other stocks, apparently because they have slightly faster developmental times, thus depriving the mites of the time necessary for successful reproduction (Camazine, 1986).
Twenty years after its introduction to the United States, V. destructor continues to devastate honey bee populations. High losses have been reported locally (Burgett, 1994; Loper, 1995) and nationally. During the winter of 1995–1996, northern U.S. beekeepers experienced their largest losses in history; in some states, 30 to 80 percent of colonies were lost (Finley et al., 1996). Similar losses were observed in the winters of 2000–2001 and 2004–2005 (Caron and Hubner, 2001; Lumkin, 2005). Data on colony losses are derived from informal surveys of beekeepers, and the exact causes of colony deaths have not been established. However, except for the large loss of honey bee colonies in the 1940s from the bacterial disease, AFB, losses on this scale were never reported before the detection of parasitic mites (Finley et al., 1996). These honey bee losses have occurred despite the industry’s heavy reliance on pesticides to control mite populations. Pesticide resistance has become widespread (Elzen et al., 1998, 1999d) and many beekeepers are no longer able to use the few registered pesticides for varroa control.
New miticides formulated from natural products (Calderone, 2000; Calderone and Nasr, 1999; Calderone and Spivak, 1995; Calderone et al., 1997) and fungal pathogens Hirsutella thompsonii and Metarhizium anisopliae have shown promise, but problems with temperature sensitivity and treatment methods remain unresolved (Kanga et al., 2003a,b).
Operating costs for beekeepers have increased because of varroa mite infestations (Kemp, 2000); expenses include those for pesticide treatment (material and labor) and to replace colonies killed by the mites. Replacing colonies also requires additional labor and, because the new colonies are smaller, they produce less honey the first year than healthy colonies that have successfully wintered (Morse, 1994). The bee industry badly needs improved methods for managing varroa mites, including methods of breeding for resistance in hosts (Chapter 6).
Tracheal Mite (Acarapis woodi)
The tracheal mite Acarapis woodi is an internal parasite of A. mellifera. Initially identified in the United Kingdom in 1921 (Imms, 1921; Rennie, 1921), tracheal mites were first detected in the United States in 1984 in Texas, where they most likely entered into the country on swarms of bees from Mexico (Eischen et al., 1990; Hall and Eischen, 1991; Pettis et al., 1987). At first, tracheal mites caused serious damage to colonies in the United States (Eischen, 1987; Eischen et al., 1989; Frazier et al., 1994; Otis, 1990; Sammataro et al., 2000; Scott-Dupree and Otis, 1991), but attention to tracheal mites has diminished as beekeepers struggle to manage the more problematic varroa mite. Perhaps this is also related to findings of heritable variation in honey bees for resistance to tracheal mites (Gary et al., 1990; Nasr et al., 2001; Page and Gary, 1990). Several chemical treatments have been identified to control tracheal mites (Calderone and Shimanuki, 1995; Clark, 1990; Delaplane, 1992; Wilson and Collins, 1993; Wilson et al., 1989, 1990). The current status of the tracheal mite and its impact on honey bees are unknown.
Pathogens
Paenibacillus larvae (formerly Bacillus larvae: White, 1920) is the most serious honey bee pathogen. It causes AFB, a disease of larval honey bees. AFB is highly virulent and easily spread among colonies as a result of beekeeper activity and bee behavior, and it is generally fatal if untreated (Shimanuki, 1997). During the first half of the 20th century, AFB was the most serious threat to beekeeping, and it caused tremendous loss of colonies, amounting to hundreds of thousands in the 1940s (Barrett, 1955). The incidence of AFB was reduced dramatically by the introduction of antibiotics
and by state apiary inspection programs that required the burning of infected hives (Barrett, 1955).
Sulfathiozole (Hasemans and Childers, 1944) was the first effective chemotherapeutic agent used to control AFB, but its use was discontinued in the United States because of concerns with residues in honey (Lodesani and Costa, 2005). Gochnauer (1951) reported good control with oxytetracycline (Terramycin®) and was a mainstay in AFB prevention until the 1990s (Wilson, 1970; Wilson et al., 1973). However, AFB is troublesome because its spores are refractory to antibiotics (Shimanuki, 1997) and can persist on contaminated equipment for more than 80 years. Treatment of colonies with active cases of AFB eliminates disease symptoms, but withdrawal of antibiotics is generally followed by disease recurrence (Allipi et al., 1999).
Even when infected colonies are treated with antibiotics, there is still a major threat to nearby healthy colonies because the infected colonies can serve as reservoirs of infective spores. Consequently, the use of oxytetracycline is recommended as a preventive rather than as a treatment for active cases. Most states still require that colonies with active cases of AFB be destroyed and the equipment be burned or buried (Ratnieks, 1992).
Resistance to the antibiotics used against AFB was not observed in the United States until about 1994 (Shimanuki and Knox, 1994), but it has become widespread, and AFB is now a resurgent threat to the industry (Cox et al., 2005; Evans, 2003). Tylosin tartrate (Tylan®) is an effective control agent (Alippi et al., 1999; Elzen et al., 2002; Hitchcock et al., 1970) that recently received Food and Drug Administration approval (FDA-CVM Update, October 20, 2005). However, a single chemical treatment is only a short-term solution, as has demonstrably been the case with treatment for varroa mites.
Pesticides
The application of pesticides, especially insecticides used to control crop pests, kills or weakens thousands of honey bee colonies in the United States each year (Johansen and Mayer, 1990). Local bee kills have occurred sporadically for decades and likely have not contributed significantly to the recent national decline in colony populations (Chapter 2). Most pesticidecaused bee kills are the result of accidents, careless application, or failure to adhere to label recommendations and warnings (Johansen and Mayer, 1990).
A few examples illustrate the nature of the problem: mosquito control programs have resulted in major losses of honey bees in Canada and the United States (Dixon and Fingler, 1982, 1984). In Manitoba, efforts to combat serious outbreaks of western equine encephalitis by controlling its mosquito vectors resulted in colony losses that amounted to $850,000 in
1983 (Dixon and Fingler, 1984). In California, between 1966 and 1979, before the emergence of the varroa mite, insecticides caused the death of more than 1 million colonies—accounting for 47 percent of bee colony deaths in that period—causing a 10 percent decrease in population (NRCC, 1981, Table 6, p. 83).
Recent trends in North America and many other parts of the world are toward reducing the use of pesticides in agriculture and forestry, to mitigate problems associated with pesticide applications, and adopting such practices as restricting spraying to times when pollinators are not foraging (Adey et al., 1986; Johansen and Mayer, 1990). In a lawsuit against the State of Minnesota and the International Paper Company (the landowners), beekeepers alleged that the landowners sprayed carbaryl insecticide (Sevin XLR Plus) to control cottonwood leaf beetles (Chrysomela scripta) despite the knowledge that the tree plantations were within the foraging range of beekeepers’ apiaries. Although the case was disposed by the Minnesota District Court, the Supreme Court reversed the District Court’s decision (http://www.beyondpesticides.org/news/daily_news_archive/2005/03_10_05.htm). The State of Minnesota settled out of court with a $335,000 payment to beekeepers (Anonymous, 2005; Schell, 2005).
Sublethal effects of pesticides on bee foraging behavior have been reported (Pham-Delegue et al., 2002). For example, there have been reports in Europe that exposure to Gaucho® (imidacloprid) impairs the navigational and foraging abilities of honey bees. These results have not been obtained in all studies (Pham-Delegue et al., 2002), and the effect of imidacloprid on honey bees is controversial. However, other pesticides have been shown to impair bee behavior, so the threat of sublethal effects of pesticides on bee foraging behavior is real.
The negative impact of pesticides on managed honey bee colonies suggests that feral bee populations could be similarly affected by pesticides, but there are no studies on the latter subject to the committee’s knowledge. Feral honey bees have not been studied intensively (see Chapter 2). Pesticides can potentially harm many bee species and even eliminate some pollinator populations in ecosystems. However, bee populations seem to recover once pesticide application ceases (for example, Kevan et al., 1997) unless the populations are eliminated over a very large area.
Transgenic Crops
Transgenic crops were developed in part to reduce the unintended effects of pesticides. However, the deployment of crop plants genetically engineered to express insecticidal proteins in pollen raised questions about direct effects on nontarget species, including some pollinators (Losey et al., 1999). For honey bees, the concerns involved the potential lethality of insecticidal
transgenic proteins, the sublethal effects of these proteins on insect behavior, physiology, and reproduction and the economic effects of transgenic pollen as a contaminant of honey. Malone and Pham-Delègue (2001) reviewed the small literature on this topic and concluded that, in some cases, there are negative but sublethal effects attributable to consumption of transgenic pollens. These effects varied with the identity of the transgene and the amount of its expression but in no case have any effects of transgenic crops on honey bee populations been documented.
Invasive Species
Africanized Honey Bees
The Africanized honey bee is a hybrid of the African race, A. mellifera scutellata, intentionally introduced to Brazil in the early 1950s (Winston, 1992), and European races of A. mellifera introduced with European colonists in the 1600s (Sheppard, 1989a,b). The Africanized honey bee gained some measure of notoriety because it is more defensive than most European races of honey bee; when disturbed, colonies of Africanized honey bees respond more aggressively and with more rapid and prolonged stinging behavior (Winston, 1992). The spread of the Africanized honey bee from South to North America is one of history’s most spectacular examples of biological invasion (Roubik, 1989; Schneider et al., 2004). Several traits have facilitated the establishment of Africanized honey bees: their colonies grow faster than do those of the European honey bees, and there are genetic incompatibilities in hybrids that favor loss of European traits; African drones exhibit mating advantages; Africanized bees have a greater ability to establish nests in a broader variety of locations; and they exhibit more nest usurpation behavior than do European bees (Schneider et al., 2004). The influx of Africanized bees into the United States began several years after resident honey bee populations had experienced sharp declines (Chapter 2); Africanization of U.S. bees was not a cause of those declines.
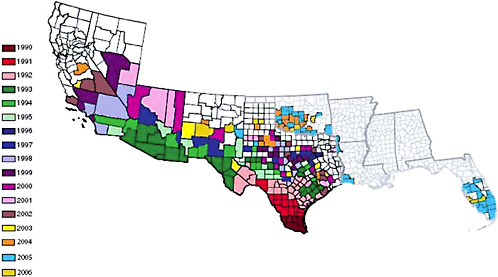
Africanized honey bees were first detected in the United States in Hidalgo, Texas, in 1990 (Hunter et al., 1993). They have spread throughout the Southwest, parts of California, and most recently (National Plant Board, 2005), parts of the Southeast including Florida (Figure 3-1). Africanized honey bees have not spread into Canada because the border between the two countries has been closed to transport of honey bees since tracheal mites were detected in the United States (see Chapter 2). If the border were to reopen to allow imports of packaged bees and queens from the United States, there would be potential for Africanized bees to be imported to Canada.
Although the Brazilian (DeJong, 1996; Goncalves et al., 1991) and Mexican (Guzman-Novoa and Page, 1994a, 1999) bee industries eventually
have adjusted well to Africanized honey bees, beekeeping in Mexico and Brazil differs in many fundamental ways from that in the United States and Canada, and experiences with Africanized bees in those countries might not serve as useful models for the rest of the hemisphere.
The presence of Africanized bees throughout the southern and southeastern United States could exacerbate losses of European honey bee colonies documented in Chapter 2 (Winston, 1992). Africanized bees reproduce (“swarm”) more often than do European bees (Danka and Rinderer, 1986; Winston, 1979; Winston et al., 1981), which has two important consequences. First, colonies are weakened as a result, and weaker colonies do not pollinate as efficiently as stronger colonies (Winston, 1987). Second, swarms of honey bees often settle in places near humans, posing an increased health hazard.
Colonization by Africanized honey bees might make it more difficult to obtain replacement queens and packages from desirable stocks. As Africanized honey bees move northward, farther into California, and eastward into the southeastern United States, they will enter the principal queen- and package-producing regions (northern California, Georgia, Alabama, Louisiana, Mississippi, Florida) and wintering areas for migratory beekeepers (Florida, Louisiana, South Carolina, Texas), who move their colonies as crops come into bloom. Each year, the bee packaging industry supplies at least a million queens and packages of bees (Schiff and Sheppard, 1995, 1996) for replacement of colonies that succumb to winter stress, mites, or the rigors of migratory beekeeping. About half of the queens are produced in the western United States and half in the southeastern United States.
Africanized honey bees will also encroach on prime agricultural regions such as the almond orchards in California. Africanized honey bees are less desirable than European honey bees as commercial pollinators because they forage over relatively short distances (Danka et al., 1993) so they are not appropriate for some crops. They also are more likely to abandon their colonies altogether when food is scarce (Danka et al., 1987; Winston et al., 1979, 1983), and shortages of nectar can occur when honey bee colonies are used at high densities to pollinate orchards or fields. Most important, Africanized honey bees’ aggressive behavior contributes to increased liability costs and regulations banning the movement of bees into or through certain areas (Danka et al., 1987). Beekeepers are almost entirely dependent on the goodwill of rural property owners to find locations for their apiaries.
Predictions about the eventual distribution of Africanized honey bees in the United States vary. Some researchers suggest Africanized bees will become established across all the southern states (Dietz and Vergara, 1995; Rinderer, 1986; Taylor, 1977). Africanized bees are not likely to survive in the interior portions of the United States, but partially Africanized colonies (European or Africanized queens mated to one or more Africanized drones)
could (Dietz and Vergara, 1995). The seasonal movement of packages, queens, and beekeepers servicing crops both within and outside areas with Africanized honey bees could contribute to an increase in range, even if temporary in some locales.
Small Hive Beetle (Aethina tumida)
Native to South Africa (Lundie, 1940), the small hive beetle (Aethina tumida) was detected in the United States in 1998 (Thomas, 1998), but how it arrived is not known. Adults and larvae eat pollen, honey, and brood, in the process damaging colonies and beekeeping equipment (Thomas, 1998). They especially damage combs of unextracted honey stored in honey houses (Hood, 2004a); the larvae burrow through the combs and defecate in them, causing the honey to ferment and leak from the combs (Headings, 2000).
The small hive beetle is found throughout the eastern United States, although it is considered an economically important pest only along the southeastern coast (Hood, 2004b). In northern states, it is a manageable pest of honey houses that is not associated with colony losses (Neumann and Elzen, 2004). Whether it becomes a more noxious pest could depend on its capacity to expand its range; its ultimate distribution in the United States could be affected partly by soil conditions (Ellis et al., 2004a). Whether it will remain manageable is an open question as well. Chemical controls are available (Elzen et al., 1999b), and biological control agents show promise (Ellis et al., 2004b; Richards et al., 2005). Racial variation in the response of honey bees to A. tumida infestation (Elzen et al., 2001) suggests that selection for resistance to this pest, like selection for resistance to AFB and varroa mites, is possible.
Bumble Bees (Bombus spp.)
Starting in the early 1990s, companies in Europe (Banda and Paxton 1991), Israel, and Canada (Kevan et al., 1991) developed commercial insectary techniques for mass-rearing bumble bees year-round to pollinate tomatoes, sweet peppers, and several other greenhouse crops (Banda and Paxton, 1991; Kevan et al., 1991). In the United States and Canada, Bombus impatiens and B. occidentalis have been the main species used commercially, although B. occidentalis has not been reared by Koppert since 1998 (M. Tacolla, Koppert Biological Systems, Inc., personal communication, March 2006), and the other company is closing its rearing program soon (R. Ward, Biobest Canada Ltd., personal communication, June 2006). Because bumble bees are reared and deployed for pollination at high densities—as many as 23,000 individuals per greenhouse (Morandin et al., 2001)—they are particularly vulnerable to pathogens and parasites.
Parasites
Bumble bees suffer from infestation by several parasites, notably the protozoans Nosema bombi and Crithidia bombi and the tracheal mite Locustacris buchneri (Imhoof and Schmid-Hempel, 1999; Shykoff and Schmid-Hempel, 1991). The two protozoan parasites often occur together in European bumble bees. Although C. bombi is suggested not to be native to North America (its natural host is the European B. terrestris), it has been found in B. impatiens and B. occidentalis in North America, likely because the three species were reared together in the same insectary facilities in Europe (Colla et. al, 2006; Thorp, 2003; Winter et al., 2006) during the years when queens collected in North America were sent to Europe to establish colonies and then returned to North America. Presumably, cross-infected bees returned to North America for pollination of greenhouse crops also foraged outside the greenhouses and infected wild bees (Colla et al., 2006).
A new threat to bumble bees is deformed wing virus, originally a disease of honey bees, that has been found in B. terrestris in European commercial breeding operations and in a feral colony of B. pascuorum in Germany (Genersch et al., 2006). The frequency of this disease in honey bees is increasing because of oral transmission and transmission by varroa mites; in bumble bees, transmission appears to be exclusively oral. Discovery of this virus in bumble bees raises questions about transmission and cross-infectivity among bumble bees and between bumble bees and honey bees, as well as the potential risks of commercial trafficking in bumble bees (R. Thorp, University of California, Davis, personal communication, April 2006).
There is growing evidence of the proliferation of exotic pathogens and parasites in populations of commercially reared bumble bees in the United States and Canada (Colla et al., 2006). Bumble bees used in greenhouse pollination frequently harbor high levels of different pathogens (Colla et al., 2006), and infected colonies exhibit reduced survival and reproduction and diminished foraging efficiency (Brown et al., 2003; Fisher and Pomeroy, 1989; Gegear et al., 2005; Husband and Sinha, 1970; Macfarlane et al., 1995; Otterstatter et al., 2005). Nosema bombi is globally associated with bumble bees, and its ubiquity in managed colonies presents a palpable risk to wild bumble bee populations in North America (Flanders et al., 2003; Thorp et al., 2003).
Pesticides
The damage to honey bees inflicted by insecticides suggests that similar problems occur for other managed and unmanaged bee species (Helson et al., 1994; Johansen and Mayer, 1990; Torchio, 1973). Nontarget effects, however, particularly in unmanaged populations, tend to be poorly documented,
so the scope of the problem is unclear. The few comparative studies give evidence that pesticide toxicities are not necessarily predictive of the hazards to other bee species (Johansen and Mayer, 1990; Kevan and Plowright, 1995; NRCC, 1981). Like honey bees, bumble bees can be exposed to pesticides while foraging (Gels et al., 2002; Tasei et al., 2001). Ground-nesting bumble bees, however, are uniquely susceptible to pesticides applied to turf or lawns for grub control (Gels et al., 2002). Effects can be sublethal (Belzunces et al., 2001; Tasei et al., 1994); for example, imidacloprid (Morandin et al., 2005) and clothianidin (Franklin et al., 2004) can hamper foraging and pollinating. Morandin and Winston (2003), however, reported no effects of the transgenic (insecticidal) proteins Cry1ac or chitinase.
Because almost all bumble bee deaths caused by pesticide exposure are unreported, determining their implications for bumble bee population declines is difficult, if not impossible. Thompson (2001) suggested that nontarget exposures can disproportionately affect bumble bee numbers if they occur early in the season when queens are still foraging and when colonies are very small (Thompson, 2001). During studies on the environmental effects of fenitrothion on pollination and bumble bees in New Brunswick, Canada, Plowright and his colleagues (1978) noted severe bumble bee population reductions that were evidenced in changed foraging behavior of surviving colonies. Foraging trip times declined because there was less competition with congeners for floral resources and the bumble bee populations rebounded quickly (reviewed in Kevan and Plowright, 1995).
Alfalfa Leafcutting Bees (Megachile rotundata F.)
Alfalfa (= lucerne) is a major forage crop grown for free-ranging livestock and as hay for livestock feed. In the United States, about 25 million acres (about 10 million hectares) of alfalfa are planted annually, and the crop has an estimated value of more than $5 billion (Flanders and Radcliff, 2000). The primary pollinator is the introduced alfalfa leafcutting bee Megachile rotundata F. Management techniques for this solitary bee are well developed (Peterson et al., 1992; Stephen, 2003); however, chalkbrood disease nearly destroyed the production of leafcutting bees in the United States.
Chalkbrood is a fungal disease caused by Ascosphaera aggregata Skou (Goettel et al., 1997; Vandenberg and Stephen, 1983). Larvae contract chalkbrood by ingesting pollen contaminated with fungal spores. After germinating in the midgut, the fungus infiltrates the hemocoel and mycelia proliferate, turning the body chalk-white (Vandenberg and Stephen, 1983). The disease was first noted in Nevada in 1973 and has since spread to most areas in western North America (Stephen et al., 1981), where total bee mortality rates can exceed 60 percent.
Because chalkbrood disease is less common in Canada, most leafcutting
bee production in North America is in British Columbia, Saskatchewan, Alberta, and Manitoba. Although some seed growers in the United States can replace bee stocks in a good year, a loss of 50 percent or more is typical. Consequently, large numbers of alfalfa leafcutting bees are imported from Canada each year (R. Bitner’s presentation to the committee, January 14, 2006). Canadian beekeepers produce large numbers of healthy bees, perhaps because of increased resistance to chalkbrood in Canadian stocks (Vandenberg, 1991). But if chalkbrood disease becomes endemic in Canada, leafcutting bee production could be jeopardized (Peterson et al., 1992). Efforts to select for resistant stocks of M. rotundata in the United States have been unsuccessful (Stephen and Fichter, 1990a,b). Although recent records are difficult to find, there have been losses of alfalfa leafcutting bees caused by pesticides in the western United States (Johansen, 1977). In Manitoba, law protects alfalfa leafcutting bees from pesticide applications (Tang et al., 2005).
Alkali Bee (Nomia melanderi Cockerell)
The alkali bee, Nomia melanderi Cockerell, is the world’s only intensively managed ground-nesting bee. In regions of the western United States, particularly in southeastern Washington and formerly in several other areas (among them, Lovelock, Nevada), alfalfa seed growers construct large subirrigated nest sites with salt-crusted surfaces for this bee. Densities of 400 nests per square meter over a hectare or more can be obtained with this gregarious bee (Bohart, 1970, 1972a; Fronk, 1963).
Alkalli bee mortality can be caused by a variety of vertebrate and invertebrate predators, microbial pathogens, inadvertent pesticide exposure (especially aerial applications of pesticide for rangeland grasshoppers), vehicular traffic (which can kill bees crossing roads near their nest sites), and nest site flooding. Economic factors, however, were primarily responsible for declines in North American populations. Low prices for alfalfa leafcutting bees led growers to abandon the maintenance of nesting sites, leading to a decrease from peak populations of more than 400,000 nesting females per site to a few thousand per site in Touchet Valley, Washington. Prices of leafcutting bees have risen recently, as have prices of alfalfa, leading to an increase in the cultivation of alkali bees (Stephen, 2003).
DECLINE IN NATURAL OR WILD POLLINATORS
Pathogen Spillover
Nosema bombi could be the most important factor responsible for the extinction of Bombus franklini (Thorp et al., 2003), perhaps via “patho-
gen spillover” (Colla et al., 2006; Box 3-1), which can occur when heavily infected, domestic hosts interact with closely related wild populations. Commercially produced bumble bees used for greenhouse pollination often have extensive pathogen infections that can spread to wild bees when the commercial bees escape from greenhouses and interact with their wild
|
BOX 3-1 Unintended Consequences of Greenhouse Pollination and Native Pollinators When commercial growers began to grow tomatoes in greenhouses, they realized that good fruit set required pollination (Velthuis, 2002). Tomato flowers have poricidal anthers that are typically pollinated by buzz pollination—the vibration of the wing muscles of large bees that land on flowers to collect pollen. Different techniques were investigated to achieve pollination mechanically: blowers were installed to move pollen around, overhead wires used as trellises were struck or shaken, and hand-held electric vibrators were used to shake individual flowers. Introducing bees into greenhouses proved the most economical strategy. Although some pollination can be accomplished by honey bees, bumble bees are much more efficient. In the 1980s, large-scale bumble bee rearing operations began in Europe for use by growers of greenhouse tomatoes and other crops. Two unintended consequences have resulted from this commercially successful effort. First, because greenhouses are not generally airtight and sometimes are not screened, bumble bees escape and establish in areas of the world where they are not native. The effects have not yet been widely studied, but they are likely to include competition with native bees. Second, the commercial rearing and export of bees has also resulted in transport of bumble bee parasites and diseases, possibly causing the apparent local extinction of B. occidentalis from the west coast of the United States in recent years and the disappearance of B. franklini from its relatively small range in the area along the Oregon and Washington border. Because there are no large-scale monitoring operations for bumble bee distribution and abundance, it is difficult to determine the consequences of introducing bees and their diseases. Internal tracheal mites of European origin have been found in wild bumble bees in Japan, and Nosema bombi, a microsporidian parasite of bumble bees, has been found in colonies imported from Europe (Colla et al., 2006; Thorp et al., 2003). |
counterparts at nearby flowers. In Canada and elsewhere, foraging bumble bees can escape from greenhouses and survive; Whittington and colleagues (2004) reported that as much as 73 percent of the pollen carried by Bombus foragers in greenhouses comes from native plants and weeds growing outside, and the European species B. terrestris is now established in Japan after its introduction for greenhouse pollination (Matsumura et al., 2004). Managed, greenhouse-reared bumble bees are likely to come in contact with wild Bombus species. If extinction of B. franklini was caused by pathogen spillover, this species has the unfortunate distinction of providing the first known example of this phenomenon in wild invertebrates; pathogen spillover has been reported in vertebrates and plants (Power and Mitchell, 2004).
Interspecific Competition in Bees
Pollinators have been introduced from one part of the world to another for at least three centuries, resulting in the establishment of one species (Apis mellifera) and several species of another genus (Bombus) on most continents. Many other introduced species have become established in the United States and Canada (Table 3-1). The major damage caused by introduced species includes competition with native pollinators for floral resources and nest sites (Barthell and Thorp, 1995; Barthell et al., 1998; Thorp et al., 2000; Box 3-2), inadvertent introduction of natural enemies (Butz-Huryn, 1997; Dupont et al., 2004; Kato et al., 1999; Paton et al., 1992, 1996; Roubik, 1978), especially pathogens that can escape into wild populations of native pollinators, enhanced pollination of exotic weeds and furthering their spread by increasing seed set (Barthell et al., 2001; Goulson and Derwent, 2004), and disruption of the pollination of native plants via deposition of heterospecific pollen on the stigma.
The extent to which introduced species disrupt native communities remains equivocal (Goulson, 2003a; Schaffer et al., 1983). Schaffer and colleagues monitored agave blossoms in Arizona before, during, and after the introduction of genetically marked honey bees (Schaffer et al., 1983). They reported that honey bees lowered the available amount of Agave pollen and nectar, and their introduction led to shifts in the numbers of foraging native bumble bees and nectar-feeding ants. Short-term effects of interspecific competition have been documented, including beneficial effects to plants (Dick, 2001), but long-term population effects have not been documented.
Honey bees are highly polylectic (they collect pollen from many unrelated plants) and because even a few colonies can collect hundreds of kilograms of nectar and dozens of kilograms of pollen annually (Buchmann, 1996), they can lower the available amount of nectar and pollen in diverse natural plant communities (Paton, 1990, 1993, 1996). The flower-visiting behavior of native flower visitors—such as bees, hummingbirds, ants, and
TABLE 3-1 Exotic Bee Species Now Established in the United States and Canada (Polylectic Species Collect Pollen from Many Unrelated Plants)
|
Scientific Name |
Introduction |
Dates |
|
ANDRENIDAE |
|
|
|
Andrena wilkella (Kirby) |
Accidental |
1700–1800s |
|
APIDAEa |
|
|
|
Apis mellifera L. |
Introduced |
1620s |
|
Apis mellifera scutellata Lepeletier |
Introduced |
1950s |
|
Anthophora plumipes (Pallas) |
Introduced |
1980s |
|
Ceratina cobaltina Cressonb |
Accidental |
1970s |
|
Ceratina dalltoreana Friese |
Accidental |
1940s |
|
Centris eisenii Foxc |
Accidental |
1990s |
|
Xylocopa tabaniformis parkinsonae Cockerelld |
Accidental |
1980s |
|
COLLETIDAE |
|
|
|
Hylaeus bisinuatus Forster |
Accidental |
1990–1910 |
|
Hylaeus hyalinatus Smith |
Accidental |
1990s |
|
Hylaeus punctatus Brulle |
Accidental |
1980s |
|
MEGACHILIDAE |
|
|
|
Anthidium manicatum L. |
Accidental |
1960s |
|
Anthidium oblongatum (Illiger) |
Accidental |
1990s |
|
Chelostoma campanularum (Kirby) |
Accidental |
1960s |
|
Chelostoma fuliginosum (Panzer) |
Accidental |
1960s |
|
Hoplitis anthocopoides (Schenck) |
Accidental |
1960s |
|
Lithurgus chrysurus Fonscoloombe |
Accidental |
1970s |
|
Megachile apicalis (Spinola) |
Accidental |
1930s |
|
Megachile concinna Smith |
Accidental |
1940s |
|
Megachile lanata (F.) |
Accidental |
1700–1800s |
|
Megachile rotundata (F.) |
Accidental |
1920–1945 |
|
Megachile sculpturalis Smith |
Accidental |
1990s |
|
Osmia coerulescens (L.) |
Accidental |
1800s |
|
Osmia cornifrons (Radoszkowski) |
Introduced |
1960s |
|
Osmia cornuta (Latreille) |
Introduced |
1980s |
|
aCeratina (Pithitis) smaragdula (F.) was deliberately introduced to California and Florida, but failed to establish (Daly et al., 1971). bThe neotropical bee Ceratina cobaltina Cresson has been collected sporadically in Texas since 1978 and may be adventive (J. Neff, Central States Melittological Institute, Austin, Texas, personal communication, October 2005). cThe centridine Centris eisenii has been collected at horticultural plantings of Callaeum macropterum (its floral oil host plant) from Nogales to Tucson, Arizona, since the 1990s, and is likely adventive and established in southern Arizona (S. Buchmann, unpublished data). |
||
|
BOX 3-2 Competition Between Managed and Wild Pollinators If a plant community is close to its carrying capacity for pollinators, introduction of additional pollinators by moving in managed colonies of honey bees or other bees, or the introduction of more native bees, presents the potential for increasing competition for floral resources. This potential for competition has been a concern for areas where honey bees are not native but have been introduced or proposed for introduction, and also for areas (Australia) where bumble bees are not native but have been proposed for introduction. In a literature review in 1997, Butz-Huryn concluded that “the presence of honey bees, however, alters the foraging behavior and abundance of some native fauna on flowers, but no studies have shown detrimental impacts of honey bees on population abundances of any native animals or plants.” More recently, in a combination of observational and experimental studies, Thomson (2006) found that niche overlap between honey and bumble bees reached levels as high as 80 to 90 percent during times of resource scarcity, but only in 1 of 7 months of observation was there a significant negative relationship between them. In an experimental study, however, the mean numbers of bumble bee foragers observed on a given transect increased significantly with greater distance from introduced honey bee colonies. Of the three measures (niche overlap, correlations in abundances, and effects of experimental introductions) that Thomson considered, only the experimental data on forager abundances accurately estimated competitive effects on colony reproductive success. These studies suggest that it may not be easy to detect competition between pollinators, even if it is affecting reproductive success. If feral honey bee colonies increase again in North America, for example if disease-resistant strains are developed, there may be subtle, unintended, but significant effects on native bees that use the same floral resources. If this alters visitation and pollination rates of native plants, there may be consequences for their populations as well. |
wasps—shifted after the experimental introduction of honey bees to a chaparral area in southern Arizona (Schaffer et al., 1979, 1983).
An experimental study in California examined the effects of competition with Apis mellifera on colony foraging behavior and reproductive success of a native eusocial bee, B. occidentalis (Thomson, 2004). Bumble bee colonies in competition with honey bees experienced increased nectar scarcity and had lowered rates of larval production. Thus, A. mellifera can competitively suppress a native bee species that is a known important pollinator. The
competitive effects of introduced pollinators depend on phenology (seasonal timing), abundance, and overlap in resource use. Competition among bees is likely because some bumble bee species have proboscides (“tongues”) that are the same length as the proboscis of A. mellifera (Inouye, 1977); proboscis length determines in part the range of plants that can be used as nectar sources (Harder, 1982, 1983, 1986; Stang et al., 2006). After Africanized bees were experimentally introduced into a community of neotropical stingless bees, native bee numbers declined, as did their use of floral resources (Roubik, 1978, 1980; Roubik and Wolda, 2001).
In addition to competitively suppressing native bees, exotic bees can affect ecosystem function by virtue of their foraging habits. Many exotic species demonstrate a preference for visiting the flowers of weedy plants on disturbed sites (Goulson, 2003a; Roubik, 1983; Thorp, 1996) and accordingly can be less likely to pollinate native plant species. Effects of introduced Africanized honey bees on populations of native stingless bees in Central America have been reported by Roubik (1978, 1980). Those studies indicate that the potential of a nonnative pollinator species to affect native populations must be considered before introduction.
Habitat Losses for Insect Pollinators
Habitat alteration, fragmentation, and loss pose major problems for populations of many organisms, and pollinator populations are no exception (Kearns and Inouye, 1997; Kevan, 2001; Kevan et al., 1990). Bees and other insect pollinators require nesting sites (suitable soil, dead wood, abandoned mouse nests, burrows) and floral resources (nectar and pollen) to persist. These environmental resources are at risk through disruption caused by row-crop agriculture, grazing, and fragmentation of habitat into patches too small to support diverse communities of pollinators (Kearns et al., 1998; Kevan, 1999, 2001; Kevan et al., 1990). Changes in agriculture, caused by large plantings of monocultures, loss of field margins, abandonment of crop rotation involving legumes (which have been replaced by fertilizers), and lower diversity of weeds in fields and pastures (caused by herbicide use) are all detrimental to pollinator populations (Goulson, 2003b; Kevan, 1999; Kevan et al., 1990). The loss of flower-rich grasslands, and in particular the long-tubed flowers in the Fabaceae, seems to underlie the decline of at least three previously common bumble bee species in England (Goulson et al., 2005). Grazing can disrupt ground-nesting bees, affect availability of water (for nest construction) and nectar, and decrease the diversity and abundance of floral resources (Gess and Gess, 1993; Vinson et al., 1993). Fragmentation makes it more difficult for pollinators to maintain metapopulation structures, decreasing the availability of corridors and source populations for recolonization. Bumble bees seem particularly susceptible to such effects,
and more than half of the species in the United Kingdom are either already extinct or could face extinction in the next few decades (Goulson, 2003c). The apparent loss of two species in the United States in the past few years suggests that North American bumble bees are similarly imperiled as a result of the combined effects of numerous anthropogenic factors, including habitat loss, degradation, conversion, pesticide use, pollution, and pathogen spillover from commercial bumble bee cultures (Thorp, 2003, 2005).
The urbanization of many pollinator habitats also can have unintended, detrimental consequences. Pollinator populations can be reduced by exposure to city lights and other artificial light sources, including “bug zappers” (Frick and Tallamy, 1996), and by traffic on roadways (McKenna et al., 2001). Some researchers, however, report positive effects of urban or suburban growth on selected bee species when floral resources and nest sites are available (Cane et al., 2006; Frankie et al., 2005).
A decline in habitat quality can occur even if the overall diversity of vegetation is static or increases; floral composition is key to determining suitability. Rasmont and colleagues (2006) suggested that the loss of predominantly longer-tongued bee taxa in Belgium and France is the result of a loss of floral resources, especially plants with long corollas (Fabaceae, Lamiaceae, Scrophulariaceae, Boraginaceae). They also concluded that anthropogenic disturbances—excessive mowing of embankments, road sides, and public areas—could have led to the loss of floral hosts and their specialized bee pollinators. They further hypothesized that decreases in native, solitary, ground-nesting bees could have been caused by afforestation and the negative effects of poisoning or the sublethal effects of exposure to insecticides, fungicides, and herbicides. Afforestation occurred on chalky dry grasslands (prime bee habitats), especially in the Namur province, where habitats have been converted to pine plantations or housing. Bees and other pollinators can survive in urban or suburban settings if nesting sites are available and if there is appropriate floral diversity to provide nectar and pollen throughout the growing season (Cane et al., 2006; Frankie et al., 2005).
Invasive Plant Species and Bees
The literature on biological control is rife with accounts of accidental or even deliberate introduction of plants that have become noxious weeds. In North America, exotic grasses accidentally introduced or grown as livestock fodder are spreading rapidly (D’Antonio and Vitousek, 1992; Larson et al., 2001; Zavaleta et al., 2001). Exotic grasses, such as red brome (Bromus madritensis) and buffel grass (Cenchrus ciliaris) in the southwestern United States and northern Mexico, are rapidly choking out other plants, decreasing nectar and pollen-producing wildflowers, and providing fuel for intense wildfires. Because buffel grass and other highly invasive grasses cover bare
ground, they provide optimal nesting sites for ground-nesting solitary bees (Buchmann, 1996), but such benefits could be outweighed by elevated risks of wildfires and by reductions in available forage species (Asner et al., 2004; Daehler, 2003).
Bee Genetics and Diploid Males: An Extinction Vortex?
Like the entire order of Hymenoptera, bees exhibit haplodiploidy, in which males develop from unfertilized eggs while females are derived from fertilized eggs. The genetic basis of sex determination in haplodiploid species appears to be diverse (Bull, 1983; Cook, 1993). In some hymenopteran insects, including bees, a complementary sex-determining mechanism is present (Cook, 1993). Females develop when the alleles at the sex determining locus are different (heterozygous). Unfertilized eggs develop into males because they are hemizygous at this locus. And diploid males, which are not viable, arise when the alleles at the sex-determining locus are the same (homozygous). The gene for complementary sex determination (csd) has recently been identified in honey bees (Beye et al., 2003), providing strong molecular support for understanding complementary sex determination.
A recent theoretical analysis suggests that complementary sex determination could be a risk factor for the decline, and even extinction, of bee pollinators (Zayed and Packer, 2005). Zayed and Packer (2005) developed a stochastic mathematical model that predicts that if population sizes decrease (because of other intrinsic or extrinsic factors, such as those discussed above), the frequency of diploid males will increase because of inbreeding and the loss of heterozygosity at the csd locus. According to this model, the increase in diploid males leads to inbreeding depression, reducing the effective breeding size of a population and decreasing the production of females, thus further depressing populations. Thus, under some conditions, single-locus complementary sex determination can create substantial genetic load.
Support for the “extinction vortex” hypothesis currently is limited. Zayed and Packer (2001) estimated the frequency of diploid drones could be as high as 50 percent in small populations of the primitively eusocial bee Halictus poeyi in central and south Florida, much higher than earlier estimates by Kukuk (1989) of 2 to 14 percent. Hedrick et al. (2006) suggested that the deleterious effects of low variation at the csd locus in Hymenoptera might be stronger than for self-incompatibility genes or Major Histocompatability Complex genes; these loci are generally thought to be particularly important in the population dynamics of plants and vertebrates, respectively. The extinction vortex hypothesis is noteworthy because prior to its development, haplodiploid organisms were thought to be relatively less sensitive to genetic factors that can cause population declines, such as the
founder effect (the effect of establishing a new population by a small number of individuals, carrying only a small fraction of the original population’s genetic variation), the Allee effect (the positive effect of population density on population growth rate), genetic drift, and deleterious mutations (Hartl and Clark, 1997) because deleterious alleles have a higher probability of being purged in haploid males. But the extinction vortex hypothesis predicts that haplodiploid species with particularly small populations, and thus fewer alleles at csd, are particularly at risk (Hedrick et al., 2006). Solitary Hymenoptera have lower fecundity and population sizes than do eusocial species. This novel hypothesis in pollination conservation genetics deserves serious examination (Zayed and Packer, 2005).
Transgenic Crops and Butterflies
Concerns about transgenic crops and nontarget species have been studied most extensively for butterflies, with a particular focus on the influence of “Bt corn.” Initial genetic transformations of corn (Zea mays) used Bacillus thuringiensis (Bt) endotoxins, specifically Cry1ab, Cry1ac, or Cry9c, for control of the European corn borer, Ostrinia nubilalis (Minorsky, 2001). Before the 1996 release of Bt corn, most industry testing focused on nontarget predators and the honey bee, all of which are taxa not expected to be affected by the Lepidoptera-specific toxins (Malone and Pham-Delègue, 2001; O’Callaghan et al., 2005). By 1999, more than 20 million acres (9.6 million hectares) of Bt corn had been planted in the United States—more than 20 percent of all corn acreage (NRC, 2000)—and concerns over consequences to nontarget organisms had increased.
In a small-scale laboratory bioassay, Losey and colleagues (1999) demonstrated that larvae of the monarch butterfly (Danaus plexippus) experienced substantial mortality after ingesting Bt corn pollen. Partly because of the iconic nature of the monarch butterfly—its striking appearance and thousand-mile migration to a narrow range of overwintering sites led to its designation as the state insect in Alabama, Idaho, Illinois, Texas, West Virginia, and Minnesota (http://www.adver-net.com/states.html)—the discovery of the potential for damage caused by Bt corn led to widespread public alarm (Berenbaum, 2001). The dramatic increase in acreage of transgenic corn between 1996 and 2000 notwithstanding, no documentation of declines in monarch populations, either in the midwestern United States, where much of the nation’s transgenic corn was planted, or in the overwintering sites was reported (see Chapter 2). The implications of the work of Losey and colleagues (1999) were questioned for natural populations (Shelton and Rousch, 1999).
Concern in the scientific community and among the public at large prompted multiple studies to estimate risks associated with monarch butter-
fly exposure to corn pollen and to quantify the effects of pollen ingestion (Hellmich et al., 2001; Oberhauser et al., 2001; Pleasants et al., 2001; Sears et al., 2001; Stanley-Horn et al., 2001; Zangerl et al., 2001). Collectively, the work showed that the asclepiaceous host plants of D. plexippus are found in cornfields throughout much of eastern North America, so D. plexippus is in fact potentially vulnerable to the consequences of exposure to Bt corn pollen. Despite the proximity of monarchs to transgenic corn pollen, however, the risks of adverse effects are low. A combination of factors provides protection: selection for particular genetic transformations of corn (Hellmich et al., 2001; Zangerl et al., 2001), caterpillar behavior (Anderson et al., 2005), lack of pollen persistence (Pleasants et al., 2001), and phenological displacement (Bartholomew and Yeargan, 2001). Sears and colleagues (2001) conducted a risk assessment on the basis of available laboratory and field data and they concluded that an adoption rate of the demonstrably less harmful Bt corn transformations of 80 percent of the total corn crop would place only 0.05 percent of the monarch population at risk. This estimated risk is substantially lower than the risk presented by pesticides conventionally used for control of European corn borer (Stanley-Horn et al., 2001).
Although the public focus on nontarget effects of genetically modified corn originated with the report on monarchs (Losey et al., 1999), other studies have estimated negligible nontarget effects for a few other Lepidoptera: Papilio polyxenes, the black swallowtail (Wraight et al., 2000), and Euchaetes egle, the milkweed tussock caterpillar (Jesse and Obrycki, 2002). Laboratory and field studies of Bt corn on other continents (Li et al., 2005) also failed to demonstrate damage to a nontarget lepidopteran, Antheraea pernyi, which is used as a natural silk source (Li et al., 2005).
Transgenic crops could pose secondary reasons for concern for pollinators, in the form of genetically modified, herbicide-tolerant (GMHT) crops. Weeds in agricultural monocultures can be important host plants for lepidopteran pollinators (milkweed for monarch butterflies; Oberhauser et al., 2001) and nectar or pollen resources for a variety of pollinator species. They can provide resources for more of the growing season than does the crop, and they attract pollinators that the crop does not (for example, long-tubed corollas for long-tongued pollinators). Evidence for this effect is provided by the British Farmscale Study, a 5-year project that assessed the effects of farm management of GMHT crops on farmland biodiversity relative to conventional agriculture. Heard and colleagues (2003) reported that weed populations were reduced in most (but not all) fields of GMHT crops, and Haughton and co-workers (2003) reported reduced abundances of butterflies in transgenic beet and spring canola fields and smaller numbers of bees in transgenic beet fields compared with non-GMHT crops. In field margins, butterfly numbers were lower by 24 percent adjacent to transgenic
spring canola (Roy et al., 2003). In general, pollinator numbers reflected nectar source abundance (Hawes et al., 2003). Whether the reduced abundances in the field could lead to reduced pollinator populations over time would depend on the proportion of GMHT crops within the foraging ranges of these insects. Moreover, it is not known whether those findings are applicable to agroecosystems outside of Britain.
Habitat Destruction and Bats
Bats face important extinction threats (Chapter 2). Mickleburgh and colleagues (2002) reported that 11 bat species have become extinct in the past 400 years, 65 are either critically endangered or endangered, and 177 more are vulnerable to extinction, according to the criteria of the World Conservation Union (Mickleburgh et al., 2002). The 242 bat species represent about 24 percent of the world’s total number of bat species, a proportion that is consistent with the 25 percent of the mammals of the world considered at risk of extinction (IUCN, 1996).
The loss of bat populations is mostly the result of habitat destruction, especially of roosting sites in caves. About half of Mexico’s 140 bat species (Arita, 1993; Medellín et al., 1997) and half of the United States’ 45 species (Pierson, 1998) roost in caves. Among nectar-feeding bats (12 species in Mexico and the United States), only two do not use caves as roosts (Arita, 1993). The others, including the three migratory species with seasonal ranges in the United States, rely on caves to some extent. Bats spend more than half of their time roosting in caves (Kunz, 1982) and they attain high numbers in cave environments (Tuttle, 2000), so the destruction of caves is a significant threat to bats (Medellín, 2003). Severe declines of cave bat populations have been documented in Mexico and elsewhere (Hutson et al., 2001; Medellín, 2003; Moreno, 1997).
The most common causes for the destruction of cave-dwelling bat populations involve misguided attempts to control the vampire bat (Desmodus rotundus), vandalism such as setting fires in caves, disturbance during critical times such as birth peaks, and persecution of such mythical creatures as the chupacabras1 (Arita and Santos-del-Prado, 1999; Medellín, 2003). These causes are linked to a lack of understanding of the bats’ ecological purposes and economic benefits (Medellín et al., 2004).
Habitat destruction also threatens migratory pollinivorous bats. Species that migrate seasonally (Arita and Santos-del-Prado, 1999; Medellín et al., 2004; Wilkinson and Fleming, 1996) need a nectar trail or corridor along their migratory route (Allen-Wardell et al., 1998; Buchmann and Nabhan,
1996; Fleming et al., 1993; Nabhan et al., 2004) that is continuous and sufficiently conserved to provide the bats with resources. Natural habitats have been destroyed or fragmented along the migratory corridors of western Mexico and other areas (Valiente-Banuet, 2002), but it is not known whether the destruction is damaging bat populations.
Some nectar-feeding bats are habitat specialists that could depend largely on the availability of intact dry tropical forest for survival (Quesada et al., 2003). That group includes nectarivorous bats with restricted distributions, such as the Mexican banana bat (Musonycteris harrisoni) and the Moreno long-tongued bat (Glossophaga morenoi). The bats inhabit the dry tropical forest of western Mexico, a region that has experienced considerable deforestation and fragmentation (Trejo and Dirzo, 2000). Declines in their populations could affect the reproductive biology of their food plants (Quesada et al., 2003).
Habitat Changes and Hummingbirds
Most studies of threats to landbirds have focused on nonhummingbird species (for example, Rappole and McDonald, 1994; Robbins et al., 1989), partly because no hummingbird is included on the U.S. Endangered Species Act list. Migratory bird species (including some pollinating species) show declines that have been linked to deforestation and forest fragmentation in the tropical wintering ranges of those species (Robbins et al., 1989). The Audubon Society’s WatchList (http://www.audubon.org/bird/watch/) has six species of hummingbird identified by Partners in Flight at the national level as moderately high or moderate priority: the Allen (Selasphorus sasin), buff-bellied (Amazilia yucatanensis), calliope (Stellula calliope), Costa (Calypte costae), lucifer (Calothorax lucifer), and rufous (Selasphorus rufus) hummingbirds. Threats listed for the six species on the WatchList include habitat destruction that results from human encroachment (urbanization, agriculture, conversion of grasslands for cattle ranching). Another important identified threat is the replacement of native plants by invasive species that are unproductive for hummingbirds.
Calder (2004) identified the destruction of stopover habitat—considered critical to migration—along the migratory corridors as a cause of population decline. He also identified habitat destruction attributable to the invasion of African exotic buffel grass, which could damage Sonoran Desert vegetation (Burquez and Martínez-Yrízar, 1997). Abnormal weather, primarily cold winters or drought along desert migratory corridors, also could pose an important threat to hummingbirds (Calder, 2004). After one particularly cold winter (1957–1958), Bailey and Niedrach (1965) reported that less than one-fourth of the 1957 population of broad-tailed hummingbirds reap-
peared in 1958 and 1959. After another unusually cold winter in 1995, the population declined by an estimated 57 percent (Calder, 2004).
At least 7 (5 threatened, 2 endangered) of the 23 hummingbird species are shown on the Mexican list of species at risk of extinction (SEMARNAT, 2002). These seven species were Campylopterus excellens, Lophornis brachylopha, Thalurania ridgwayi, Hylocharis xanthusii, Eupherusa cyanophrys, Amazilia viridifrons, and Eupherusa poliocerca (Ornelas, 2000). All face similar threats: they have restricted distributions (all seven are endemic to small areas in the south, west, or northwest of Mexico) and all have experienced severe habitat destruction or fragmentation caused by conversion of grasslands to use for cattle ranching or agriculture. Urban or suburban domestic cats that are allowed outside have been implicated in mortality of ruby-throated hummingbirds, a species of conservation concern in some parts of its range (Lepczyk et al., 2004).
One threat is associated only with the (primarily Mexican) lucifer hummingbird: trade in individuals in past decades (although confirmation is lacking) (http://audubon2.org/webapp/watchlist/viewSpecies.jsp?id=127), which is likely to have affected several other species. It is not known whether the hummingbird trade continues to be a factor of concern.
Climate Change
Global, regional, and local climate changes can alter or disrupt plant-pollinator relationships. Included in the global climate change forecast are shifts in temperature and precipitation, concentrations of carbon dioxide (CO2) and ozone, and ultraviolet light levels. All are important to plant growth and flowering, and those changes could alter plant and pollinator phenology and distribution along altitudinal and latitudinal gradients, generate changes in plant and pollinator mutualisms and community compositions, and cause local extinctions.
There is evidence that the latitudinal and altitudinal ranges of some plants and pollinators have changed in the past 30 years, presumably in response to global warming (Walther, 2004). For example, some butterflies in Britain and North America have expanded ranges north (Crozier, 2003; Hill et al., 1999; Parmesan et al., 1999), and others in Montana (Lesica and McCune, 2004), Spain (Wilson et al., 2005), and Norway (Klanderud and Birks, 2003) have contracted ranges at lower altitudes and latitudes.
An increase in atmospheric CO2 could alter production of nectar (reviewed by Davis, 2003). Typically, elevated CO2 concentrations alter nectar volume and secretion rate, sometimes negatively and sometimes positively, but not sugar concentration or composition (for example, Lake and Hughes, 1999). Increases in CO2 could benefit at least one species of
melon (Cucumis melo). Average nectar volumes per flower were significantly higher, sometimes by as much as 100 percent (Dag and Eisikowitch, 2000), in greenhouses enriched with CO2. No comparable greenhouse or field studies seem to have addressed the potential for CO2 enrichment to affect pollen production.
Elevated intensities of ultraviolet-B radiation (UV-B; wavelengths between 280 and 320 nanometers) result from diminished concentrations of atmospheric ozone and can delay flowering and diminish lifetime flower production in some plants. Sampson and Cane (1999) reported idiosyncratic responses in flowering phenology and flower production in two annual plants, traits that could affect plant competition for pollinator services, and plant and pollinator reproductive success. Stephanou and colleagues (2000) reported that UV-B increased nectary size in another species, which apparently resulted in an observed increase in pollination, but no differences were reported in honey bee foraging behavior on brassicaceous nectar plants exposed to and protected from UV-B (Collins et al., 1997).
In the Washington, D.C. area, Abu-Asab and colleagues (2001) reported that 89 plant species had advanced flowering time by an average of 4.5 days (although 11 species showed later flowering times). Primack and colleagues (2004) used herbarium specimens of the same individual plants in the Arnold Arboretum in Boston, Massachusetts, to compare flowering times from 1885 to 2002. Plants flowered 8 days earlier from 1980 to 2002 than they did from 1900 to 1920. Flowering by agricultural species also is influenced by global warming: a 40-year study of white clover (Trifolium repens) revealed that flowering has advanced by 7.5 days per decade since 1978 (Williams and Abberton, 2004).
Several studies demonstrate that pollinator phenology can be influenced by changing global temperatures. The first appearance of most British butterflies has advanced in the past two decades; peak appearance also occurs earlier, and multibrooded species exhibit longer flight periods (Roy and Sparks, 2000). Forister and Shapiro (2003) documented a similar change in California butterflies. The mean date of first flight trended toward earlier dates for 16 species (70 percent of the fauna studied), and the trend was statistically significant for 4 of them (average shift of 24 days). Seven species showed trends toward later appearance that were not statistically significant. Some Spanish butterflies (8 of 19 species studied from 1988 to 2002) also showed significant advances in mean flight dates (Stefanescu et al., 2003).
If the phenology of flowering and pollinator activities does not change synchronously, there is the potential for disruption of coordinated interactions. Plants might flower before or after the period of seasonal activity of their pollinators and different groups of pollinators might respond differ-
ently to a change in temperature. A record-early spring in Japan resulted in drastic decreases in seed set of two species normally pollinated by bees, but not in two others pollinated by flies (Kudo et al., 2004). A long-term study of life cycles of Mediterranean plants and animals showed that the phenology of plant leafing out, flowering, and fruiting changed at different rates, and all were different from changes recorded for butterfly emergence and the arrival of migratory birds (Peñuelas et al., 2002). The authors suggested that these changes could alter ecosystem structure and function. Migrating pollinators (for example, hummingbirds that overwinter in Mexico and reproduce in the United States) depend on corridors with flowers that bloom at the appropriate times during spring and fall migrations. If the timing of the migration does not coincide with flowering, the plants could suffer a loss of pollinators and the pollinators could face energetically expensive migratory flights with no opportunity to forage and replenish metabolic fuel along the way.
Thus, the evidence indicates that plants and their pollinators could respond differentially to climate change. Depending on the degree of variations in their responses, the consequences of climate change could range from subtle to dramatic. Alterations in nectar abundance or concentration could change the foraging behavior of pollinators, increasing or decreasing pollination of one flower by another of the same plant (geitonogamy); changing the quantities of pollen collected or deposited or the distances that pollen is transported—all can have significant effects on plant mating systems and genetic parameters. Changes in floral abundance could in turn influence the abundance and distribution of pollinators. The loss of synchrony that could result from differential responses in phenology of plants and pollinators could be important and possibly result in the loss of some historical mutualisms or the creation of new ones. It appears that this area of research warrants more attention, in view of the potential for climate change to disrupt plant-pollinator interactions significantly in the future.
The combined effects of climate change and other environmental changes (such as habitat fragmentation) have not been assessed for most pollination systems, but Warren and colleagues (2001) reported that 34 of 46 British butterfly species that might be expected to respond positively to climate warming at their northern climatic range margins in fact declined, as negative consequences of habitat loss outweighed the positive responses to climate warming over the past 30 years. Although half of the habitat generalists that also were mobile species increased their distributions, the other generalists and 89 percent of the habitat specialists declined in distribution, suggesting that the diversity of pollinators could decline substantially in the face of the combined pressures of climate change and habitat loss. The potent combination of environmental changes could cause substantial harm to many plant-pollinator interactions.
CONCLUSIONS
Just as different species of pollinators differ in the degree to which their diversity and populations have declined, the causes that underlie decline vary widely. Some mortality is particularly important in a narrow range of pollinators; in managed pollination systems, there is clear evidence of reductions in pollinator numbers caused by introduced parasites and pathogens. The evidence indicates that these agents of mortality also could operate in wild pollinator declines. Other causes of mortality affect a cross-section of pollinators (albeit to different extents); habitat degradation and habitat loss, in their many manifestations, have contributed to declines in many vertebrate and invertebrate pollinators.