4
Health Risks Associated with Seafood Consumption
This chapter reviews the potential risks associated with chronic exposure to particular seafoodborne contaminants and risks associated with certain more acute seafoodborne hazards. The discussion includes consideration of the extent to which seafood consumption might increase consumers’ risk of adverse health impacts due to exposure to toxicants, depending upon the critical dose-response relationships for the contaminant, the distribution of contaminant body burden in the population, and the extent to which the body burden is due to seafood consumption rather than to other sources and pathways of exposure. The chapter concludes with a discussion of the interaction between nutrients and contaminants—in particular, selenium and methylmercury—in seafood, and measures that consumers can take to reduce exposure to contaminants that may be present in seafood.
ENVIRONMENTAL CHEMICALS
Consumers seeking the health benefits associated with the consumption of seafood are concerned about potential health risks associated with the presence of chemical contaminants, both those occurring naturally and those resulting from human activities, in seafood. These contaminants include inorganic compounds such as methylmercury and other metals, as well as persistent organic pollutants (POPs) such as dioxins and polychlorinated biphenyls (PCBs). Of these, methylmercury is the contaminant that has elicited the most concern among consumers.
Methylmercury
Mercury is a heavy metal that is present in the environment as a result of both human activities (referred to as anthropogenic sources) and natural processes. The primary anthropogenic source is the combustion of fossil carbon fuels, particulary from coal-fired utility boilers; other sources include municipal, medical, and hazardous waste incineration (NRC, 2000). The natural sources include volcanic emissions and the weathering of rock containing mercury ore. Mercury can be deposited locally or travel long distances in the atmosphere and contaminate sites far from its point of release. Further, the complex biogeochemistry of mercury fate and transport creates uncertainty in efforts to apportion the relative contributions of these processes to global mercury pollution. The US Environmental Protection Agency (US EPA) estimated that 50 to 75 percent of the total yearly input of mercury into the environment is anthropogenic (US EPA, 1997), while the United Nations Environment Programme (UNEP) suggests that this source accounts for more than half of the inputs (UNEP, 2002).
Mercury exists in the environment in several different forms, including metallic, inorganic, and organic, and interconversion between forms can occur. The form of mercury of greatest concern with regard to seafood consumption is methylmercury (MeHg). Methylmercury results when mercury in other forms is deposited in water bodies and biotransformed through the process of methylation by microorganisms. It bioaccumulates up the aquatic trophic food chain as smaller organisms are consumed by larger organisms. Because methylmercury is persistent, this bioaccumulation process results in large long-lived predatory species, such as certain sharks, swordfish, and tuna, or freshwater species such as bass, walleye, and pickerel having the highest concentrations (Kraepiel et al., 2003). Methylmercury levels can also be high in marine mammals such as whales, and in animals that feed on marine life, such as polar bears and sea birds. Consumption of aquatic life is the major route of human exposure to methylmercury. The seafood choices a consumer makes, and the frequency with which different species are consumed, are thus important determinants of methylmercury intake. Because of the global dispersion of methylmercury and migration of species, the extent of regional variation in body burdens among different aquatic animals is less striking than the regional variations in certain other water contaminants, such as PCBs or dioxin-like compounds (DLCs). This implies that the location in which an aquatic animal was caught might provide relatively little information about its methylmercury content.
Methylmercury is not lipophilic (lipid soluble) and is thus present in the largest concentrations in the muscle tissue of aquatic animals rather than in fat or oils. Approximately 95 percent of ingested methylmercury is absorbed across the gastrointestinal tract into the blood. The half-life
of methylmercury in blood in humans is estimated to be 50 days, and the whole-body half-life to be 70–80 days, although the residence time of mercury in the brain appears to be considerably longer (NRC, 2000). Hair is frequently used as an exposure biomarker for methylmercury. Hair is a route of methylmercury excretion, and approximately 80 to 90 percent of the total mercury found in hair is in the methylated form. Hair mercury is a good biomarker in fish-consuming populations. Autopsy studies suggest that maternal hair mercury level correlates reasonably well with the level of mercury in the fetal brain (Cernichiari et al., 1995).
Mercury Burdens in the US Population
The first nationally representative estimates of blood and hair mercury levels were provided by the National Health and Nutrition Examination Survey (NHANES) of 1999–2000. Among women 16–49 years old, the geometric mean hair mercury level was 0.2 parts per million (ppm), with 75th, 90th, and 95th percentiles of 0.42, 1.11, and 1.73 ppm, respectively (McDowell et al., 2004). The geometric mean blood mercury level was 1.02, with 75th, 90th, and 95th percentiles of 2.07, 4.84, and 7.13 ppm, respectively (Mahaffey et al., 2004). The prevalence of levels in excess of 5.8 µg/L (benchmark dose lower bound [BMDL] adjusted for uncertainty and for population variability) was 5.66 percent. Levels were 50 percent higher among older women (30–49 years) compared to younger women, and levels were highest among women who self-identified as “Other” racial/ethnic category (Asians, Native Americans, Pacific Islanders). Mercury burdens were strongly associated with the amount of self-reported fish consumption (Mahaffey et al., 2004). Among women reporting eating 5–8 fish meals per month, these figures were 2.56, 4.54, 8.80, and 11.60 ppm, respectively. Levels were seven times greater among women who reported eating nine or more fish meals in the previous 30 days, compared to women who reported no consumption. Among these relatively high fish-consumers, the 50th, 75th, 90th, and 95th percentiles for blood mercury were 3.02, 6.68, 12.00, and 13.40 ppm, respectively.
Data on blood and hair mercury levels in adult men in the United States were not collected as part of NHANES until 2003, and no data for this group has been reported. Therefore, estimates must be made based on mercury biomarker data reported as part of large cohort studies. Urine and blood mercury levels of 1127 Vietnam-era pilots were measured for a study of the health effects of exposure to dental amalgam (Kingman et al., 1998). The mean blood mercury level in this group of men was 3.1 ppm, with a range up to 44 ppm, but the contribution of fish consumption to blood mercury levels is unknown because data were not collected on fish intake.
An important limitation of NHANES as a source of data on population exposures to methylmercury is that the sampling plan used to identify the 3637 women who contributed data in the 1999–2002 survey is likely to have missed subgroups of high fish-consumers, including sport fishers and subsistence fishers. Examples of such groups include individuals living in areas that provide ready access to seafood (e.g., island populations) (Ortiz-Roque and Lopez-Rivera, 2004), fishers (Burge and Evans, 1994; Bellanger et al., 2000), groups for whom fish or marine mammals are an especially important component of overall diet, and individuals who consume a high-fish diet for its cardioprotective effects. For example, one report described a case series of 116 patients who consumed large quantities of fish and had their blood tested; almost all (89 percent) had blood mercury levels greater than 5 µg/L, ranging up to 89 µg/L (Hightower and Moore, 2003). Evidence from the Third National Report on Human Exposure to Environmental Chemicals (CDC, 2005b) suggests that population exposures to mercury might have decreased between 1999–2000 and 2001–2002. Among women 16–49 years of age, the geometric mean declined from 1.02 µg/L (95% CI 0.825-1.270) to 0.833 (95% CI 0.738-0.940). An even greater decline was evident at the high end of the distribution, as the level corresponding to the 95th percentile in the earlier survey was 7.10 (95% CI 5.30-11.30) compared to 4.6 (95% CI 3.7-5.9) in the later survey. Because of the short time period covered by these data, however, the possibility that the observed time trend reflects sampling variability cannot be rejected.
Health Effects in Critical Target Organs
Organs of the central nervous and cardiovascular systems are considered to be the critical target organs with regard to methylmercury.
Neurological Toxicity The tragic epidemic of frank neurological disease that was identified in the late 1950s in Minamata, Japan, first brought to the world’s attention the devastating effects of methylmercury on the developing fetal brain. Children exposed in utero to high levels of MeHg presented with cerebral palsy, mental retardation, movement and coordination disorders, dysarthria, and sensory impairments.The neuropathological lesions associated with Congenital Minamata Disease (mercury poisoning) were diffuse, occurring throughout the brain. In individuals exposed only in adulthood, the lesions were highly focal, clustering in regions that matched clinical presentation (e.g., motor disorders = precentral gyrus and cerebellum, constriction of visual fields = calcarine fissure of occipital cortex). The major molecular mechanisms of MeHg neurotoxicity include inhibition of protein and macromolecular synthesis, mitochondrial dysfunction, defective calcium and ion flux, disruption of neurotransmitter homeostasis, initiation
of oxidative stress injury, microtubule disaggregation, and post-translational phosphorylation (Verity, 1997). The diffuse injury associated with prenatal exposure is attributable to the ability of MeHg to arrest mitotic cells in metaphase, disrupting the exquisitely choreographed processes of cell proliferation, differentiation, and migration. The result is a brain in which there are reduced cortical cell densities, islands of heterotopic neurons in cerebral and cerebellar white matter, anomalous cytoarchitecture, disturbance in laminar pattern of cerebral cortex, absence of granule and Purkinje cells in the cerebellum, incomplete myelination in the hypoplastic corpus callosum, glial proliferation (“bizarre astrocytes in the white matter”), and limited gyral differentiation (Choi, 1989).
No cases of Congenital Minamata Disease have been reported in the United States, where the primary concern has been whether chronic exposure to MeHg, as the result of seafood consumption among the general population, is associated with subtle adverse health outcomes. Therefore, several risk assessments have been conducted in the past decade in which the goal was to identify a fetal mercury burden that can be interpreted as being without appreciable risk. The basis for most risk assessments for MeHg exposure has been one or more of the three major epidemiologic studies available: the New Zealand study (Kjellstrom et al., 1986), the Faroe Islands study (Grandjean et al., 2001), and the Seychelles study (Myers et al., 2003) (see Box 4-1).
The New Zealand and Faroe Islands studies, but not the Seychelles study, have generally been regarded as providing evidence of harm from MeHg exposures at which clinical effects are not evident, although it should be noted that benchmark dose analyses of the data from the 9-year evaluation of children in the Seychelles study cohort produced BMDLs in the range of 17–23 ppm (Van Wijngaarden et al., 2006), only slightly higher than the BMDLs based on the New Zealand and Faroe Islands studies data. In view of the perceived discrepancies in the findings of the three studies, the choice of critical study has stimulated considerable controversy. Some risk assessors chose the Faroe Islands study (US EPA, 2001; NRC, 2000), while others chose the Seychelles study (ATSDR, 1999). In an effort to use all of the best available data, the Joint Expert Committee on Food Additives and Contaminants (JECFA), a joint committee of the World Health Organization (WHO) and the Food and Agricultural Organization of the United Nations (FAO), averaged the effect estimates reported for the Faroes and Seychelles studies; including the New Zealand study did not significantly change the results (FAO/WHO JECFA, 2003). In all these assessments, however, the final result was a single number interpreted as a reference level for intake for the most sensitive subgroup, the fetus, as shown in Table 4-1. These reference levels differ largely because of differences in the uncertainty factors applied. These levels were derived on the basis of health effects observed, rather than
|
BOX 4-1 Three Major Epidemiological Studies on Methylmercury These three studies were conducted among geographically disparate island populations with a high availability of seafood (tuna is an important export product of Seychelles, approximately one-third of the Faroese workforce is employed in the fishing industry, and both aquaculture and marine fishing feature in the economy of New Zealand). Cohen (2004) summarized these three cohorts in reviews. Seychelles Child Development Study The Seychelles Child Development Study (SCDS) is an ongoing collaboration between the Ministry of Health of Seychelles, a small archipelago country in the Indian Ocean, and the University of Rochester, New York. “Initially the objectives focused on two primary questions. Firstly, could clinical neuro-development effects be found in children after exposure to methylmercury (MeHg) in utero from a maternal diet high in fish and, secondly, what is the lowest level of foetal [sic] exposure to cause such effects?” (Shamlaye, 2004). Seychelles was determined to be a favorable location for this study for a number of reasons: the Seychellois regularly consume fish (an average of 12 meals per week), and the number of annual births allowed for recruitment of a large cohort of mothers and children in a short period of time (Shamlaye, 2004; Myers et al., 2003). The Seychelles Child Development Study enrolled 779 mother-infant pairs between 1989 and 1990, of which 717 were eligible for analysis. Among the tests administered at 107 months were the Wechsler Intelligence Scale for Children—Third Edition, the Boston Naming Test, the California Verbal Learning Test, the Bruininks-Oseretsky Test of Motor Proficiency, a Continuous Performance Test, the Developmental Test of Visual-Motor Integration, the Grooved Pegboard, and selected subtests of the Woodcock-Johnson Tests of Achievement. The children were evaluated (i.e., cognitive, language, motor, adaptive behavior, and social-emotional development) at 6, 19, 29, 66, and 107 months. Maternal hair samples were also collected at enrollment. The information provided here, along with the results from the study, can be accessed in the Special Issue of the Seychelles Medical and Dental Journal, Volume 7, Issue 1, 2004. [Online]. Available: http://www.seychelles.net/smdj/ [accessed July 7, 2005]. Also, in 2000, Clarkson et al. recruited a new cohort of mother-infant pairs in Seychelles, and this project is due for completion in 2006. Faroe Islands Study The Faroe Islands Study, conducted in this North Atlantic Ocean archipelago located between Scotland, Norway, and Iceland, consisted of a cohort of |
|
1022 consecutive singleton births from 1986–1987. The objective of this study was to investigate possible neurobehavioral effects of prenatal exposure to neurotoxicants, such as methylmercury. The Faroese are high consumers of seafood, including pilot whale, which exposes them to high levels of methylmercury. The study team analyzed maternal hair mercury concentrations and cord blood mercury concentrations at birth and conducted neurobehavioral examinations on 917 of the children just before school entry (about 7 years of age) and at 14 years of age. The detailed examinations, which lasted about 5 hours for each child, took place mostly in the National Hospital in Torshavn, the capital of the Faroes Island. The examination included finger tapping; hand-eye coordination; reaction time on a continuous performance test; Wechsler Intelligence Scale for Children—Revised Digit Span, Similarities, and Block Design; Bender Visual Motor Gestalt Test; Boston Naming Test; and California Verbal Learning Test. The parent accompanying the child (usually the mother) was also asked to fill out a self-administered questionnaire on the child’s past medical history, current health status, and social factors (Grandjean, 1997). New Zealand Study The New Zealand Study involved the screening of 11,000 children born in 1978, over 900 of whose mothers consumed fish more than four times per week during pregnancy. As with the other cohorts, the objective of this study was to investigate the association between prenatal mercury exposure and subsequent development during childhood (Crump, 1998). Maternal hair samples were collected at birth to assess mercury exposure during pregnancy. At 4 years of age, the Denver Developmental Screening Test and a set of neurological screening tests were completed on 74 children, 38 with “high” maternal hair mercury levels (> 6µg/g) and 36 with “low” maternal hair mercury levels, matched on maternal demographic characteristics, age, hospital where the birth took place, and date of birth. Maternal interviews about the ages at which the child achieved developmental milestones were also conducted (Kjellstrom et al., 1986). At 6 years of age, 238 children were evaluated. A child with a high maternal hair mercury was matched with three children with low hair mercury levels, but similar in gender, maternal ethnic group, age, smoking habits, location of residence, and number of years living in New Zealand (Kjellstrom et al., 1989). The tests administered included the Test of Oral Language Development, the Weschlar Intelligence Scale for Children-Revised, and the McCarthy Scales of Children’s Abilities. |
TABLE 4-1 Reference Levels for Fetal Exposure to Methylmercury
|
Source |
Reference Level |
|
JECFA provisional tolerable weekly intake |
1.6 µg/kg body weight/week |
|
US EPA reference dose |
0.1 µg/kg body weight/day |
|
Agency for Toxic Substances and Disease Registry minimal risk level |
0.3 µg/kg body weight/day |
|
SOURCES: FAO/WHO JECFA, 2003; US EPA, 2001; ATSDR, 1999. |
|
the general population, and are risk management guidelines rather than estimates of threshold of effect. While such numbers can be used to estimate the number of individuals at potential risk (i.e., for whom the margin of exposure is less than 10-fold), they convey nothing about the quantitative characteristics of the dose-response relationship, i.e., for the risk associated with each unit increase in mercury burden above the reference level.
A variety of hypotheses have been proposed to explain the apparent discrepancy between the results of the Seychelles and Faroe Islands studies. The National Research Council (NRC) committee did not consider that any of them is clearly supported by the evidence, however. The issues evaluated include differences between populations in the temporal characteristics of exposure (presumed to be stable among the Seychellois, but potentially episodic among the Faroese due to occasional consumption of pilot whale), reliance on different biomarkers of exposure (cord blood mercury vs. maternal hair mercury), population differences in vulnerability to methylmercury, the influence of other aspects of nutrition on methylmercury toxicity, and differences in the neuropsychological tests administered and the ages at which children were assessed. Consideration has also been given to the possibility of residual confounding in one or both studies, particularly with regard to the high exposures of the Faroese to PCBs and other POPs.
Although considerable debate has ensued seeking to identify the reasons for the apparent discrepancies among the three major studies of fetal MeHg neurotoxicity, their magnitude might be less dramatic than commonly supposed. As the analyses of the National Research Council Committee on the Toxicological Effects of Methylmercury showed, the BMDLs calculated for the three major studies vary by much less than the 10-fold (one order of magnitude) uncertainty factor applied to the BMDL to achieve the Reference Dose (RfD) (NRC, 2000). Figure 4-1 shows a qualitative effort to assess the degree of concordance among studies of the “no observed adverse effect levels” (NOAEL) estimated for each study on the basis of benchmark dose analysis. An estimate of 10 to 20 ppm appears to be reasonably accurate. Interestingly, this is the range identified by WHO (1990) based solely on the relatively poor-quality data available from a mass poisoning episode
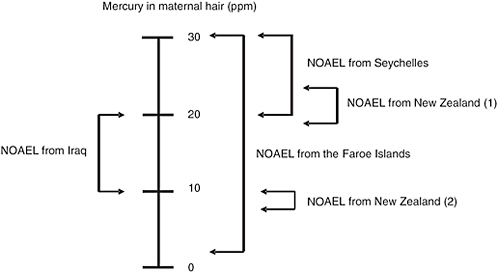
FIGURE 4-1 Integration of data from the New Zealand, Faroe Islands, and Seychelles studies of prenatal methylmercury neurotoxicity. Two ranges are provided for the NOAEL from the New Zealand study. The estimate labeled (1) was derived when the data for a child with a very high maternal hair mercury level (86 ppm) were included in the analyses. The estimate labeled 2 was derived when the data for this child were excluded. This child’s mercury level was more than fourfold higher than the level for any of the 236 other children in this cohort.
NOTE: NOAEL = No observed adverse effect level.
SOURCE: Personal communication, Clarkson et al., University of Rochester, March 2005.
that occurred in Iraq in the 1970s (Personal communication, Clarkson and colleagues, University of Rochester, March 2005).
Ryan (2005) conducted an analysis of data from the three previously described studies using maximum likelihood and Bayesian hierarchical models to derive an estimate of the slope of the dose-response relationship between children’s neurodevelopment and their prenatal methylmercury exposure. This analysis, presented to the Committee on Nutrient Relationships in Seafood (Ryan, 2005), suggested that children’s IQ scores decline by 0.1 to 0.25 points for each ppm increase in maternal hair mercury level. The point estimates were nearly identical in the three studies (results for the New Zealand study differed considerably depending on whether one particular observation was included or excluded) (see Figure 4-2).
The point estimates of the slopes for the other neurodevelopmental endpoints measured in the three studies, some of which were common across studies, were also surprisingly similar (Figure 4-3).
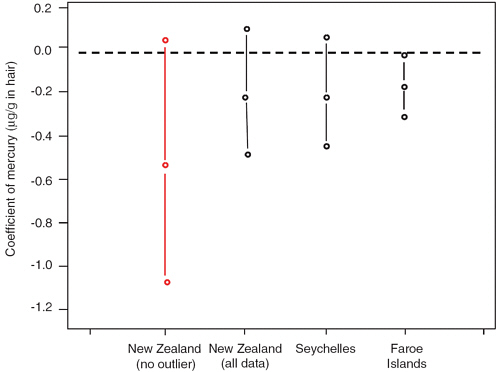
FIGURE 4-2 Point estimates and 95 percent confidence intervals, based on regression analyses, for the changes in full scale IQ (“coefficients”) associated with each ppm increase in maternal hair mercury reported in the three studies. A coefficient with a negative sign indicates that the IQ scores for children within a study cohort decreased with increasing hair mercury level. Two estimates are provided for the New Zealand study, one based on the inclusion of the child with a maternal hair mercury level of 86 ppm and one based on the exclusion of this child.
SOURCE: Ryan, 2005.
These analyses, therefore, suggest that although the findings of the Seychelles study appear discrepant from those of the Faroe Islands and New Zealand studies if one focuses only on the p-values of the reported analyses, at a deeper, quantitative level that focuses on the rates of decline in scores as mercury burden increases, the findings of the three studies are remarkably concordant.
Part of the challenge in characterizing the health risks associated with increased MeHg exposure in seafood is related to the fact that this source also provides nutrients that might have health effects which mitigate those of MeHg. Thus, studies tend not to provide a “pure” estimate of MeHg toxicity but an estimate that represents the balance between the putative harm caused by the contaminant and the putative benefits provided by the
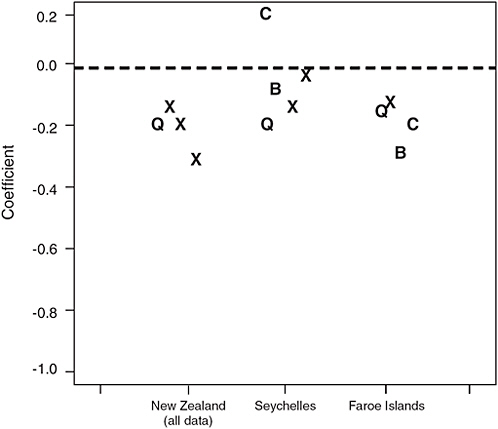
FIGURE 4-3 Coefficients for achievement and cognition-related endpoints from the three studies. The symbols Q, C, and B denote the three endpoints that are common to two or more studies, namely IQ (Q), California Verbal Learning Test (C), and Boston Naming Test (B), respectively. X’s indicate endpoints that were unique to one of the studies. Coefficients reflect the change in test score for each ppm increase in maternal hair mercury. A coefficient with a negative sign indicates that a test score decreased as maternal hair mercury level increased. New Zealand estimates are based on including the child with a maternal hair mercury level of 86 ppm. The Faroe Islands median hair:cord blood ratio of 200 (Budtz-Jorgensen, 2004b) was used to convert the Faroe Islands results to units of hair mercury.
SOURCE: Ryan, 2005.
nutrients in seafood. This issue is critical, however, because the goal in giving advice regarding seafood consumption should be to enable people to obtain the greatest benefit for the least risk.
An illustration of the delicacy of this balance is provided by a study of 135 mother-infant pairs in Boston (Oken et al., 2005). Mothers reported consuming an average of 1.2 fish servings per week during the second trimester of pregnancy (range 0–5.5 servings/week), and had a mean hair
mercury level at delivery of 0.55 ppm (range 0.02–2.38; 10 percent had levels >1.2 ppm). At 6 months of age, infants’ scores on a visual recognition memory task were positively associated with maternal fish intake during the second trimester (4 points for each additional weekly serving), but inversely associated with maternal hair mercury level (7.5 points per ppm). Performance was best among infants whose mother consumed more than two servings of fish per week but whose hair mercury level was less than 1.2 ppm. This study was designed as a study of nutrition rather than of methylmercury intake, however, so women were asked about their fish intake using categories (canned tuna, shellfish, “dark meat” fish, other fish) that relate more directly to omega-3 fatty acid levels than to MeHg levels (see Box 3-1).
Data germane to the balance between the benefits and risks associated with consumption of fish and development in children were also reported from the Avon Longitudinal Study of Parents and Children (ALSPAC), a large ongoing birth cohort study in the UK (Daniels et al., 2004). In a subsample of 1054 of 10,092 eligible children, associations were evaluated between maternal fish consumption during week 32 of gestation, reported on a food frequency questionnaire, and maternal reports of children’s language development at 15 months and general development at 18 months. The categories used in collecting data on the types of fish consumed were “white fish” (cod, haddock, plaice, fish sticks, etc.) and “oily fish” (pilchards, sardines, mackerel, tuna, herring, kippers, trout, salmon, etc.). Most women (88 percent) reported eating fish during pregnancy. Of these, 65 percent reported eating fish from both categories. Unfortunately, this way of classifying fish results in groupings that differ from those that would result if classification were based on mercury levels. Overall, children’s developmental abilities, as reported by mothers, increased modestly with increased maternal fish intake during pregnancy. Most of the benefit appeared to be associated with any fish consumption, compared to none, as maternal consumption of fish more than one to three times per week did not seem to confer additional benefits, at least with regard to the child development outcomes assessed. Higher mercury concentration in umbilical tissue, for which the median was 0.01 µg/g wet weight, was not associated with adverse developmental outcomes in children, although cord tissue mercury is not a well-established biomarker of exposure. Cord mercury level did increase across strata of maternal fish intake, although the greatest increase was between the “none” and “1 per 2 weeks” strata, with little increase evident in the two strata representing greater fish intake (“1–3 per week” and “4+ per week”) (see Box 3-1).
Jensen et al. (2005) reported that the usual substantial neuropsychological benefits associated with breastfeeding were not evident among the children in the Faroe Islands cohort. The authors speculated that contaminants pres-
ent in the breast milk of the Faroese women mitigated the benefits to their children.
Increasing attention is being paid to the neurotoxicities observed in adults exposed to MeHg, although the findings are mixed and do not support firm conclusions about the dose-response/dose-effect relationships. In a small case series report, patients who were clinically referred for paresthesias, in 50 percent of whom mixed peripheral neuropathy with axonal loss was confirmed by electrodiagnostic studies, blood mercury levels ranged from 27 to 96 µg/L (Saint-Phard et al., 2004). Most of the patients reported consuming fish at least twice weekly. These blood mercury levels are considerably higher than those of the general US population. As noted earlier, the geometric mean among US women of child-bearing age is 0.833 µg/L (95% CI 0.738-0.94). In a study involving 129 residents of Brazilian fishing communities, in whom the mean hair mercury level was 4.2 µg/g (range 0.56–13.6), dose-dependent reductions in performance on tests of fine motor speed, dexterity, and concentration were found (Yokoo et al., 2003). In reanalyses of data from a 1977 study of 366 Québec Cree (First Nation) adults, Auger et al. (2005) reported that a 6 ppm increase in hair mercury level was associated with an odds ratio of 2.2 (95% CI 1.15-4.26) for tremor in a proportional odds ordinal regression model. Scalp hair mercury levels ranged from 0.5 to 46.1 ppm. Blood mercury level (mean 37.7 µg/L, range 1–150) was not associated with an increased risk of tremor, however. In a cross-sectional study of 106 elderly (≥ 75 years) Swedes with mercury levels of 2–80 nmol/L (mean 17, standard deviation 11; values for 101 subjects were ≤28 nmol/L), blood mercury level was not associated with scores on the Mini-Mental Status Examination (Johansson et al., 2002). In the only large study conducted on US adults, among 474 adults aged 50 to 70 years, blood mercury level (median 2.1 µg/L; range 0–16) was not consistently associated with performance on a battery of 12 neuropsychological tests (Weil et al., 2005).
Cardiovascular Toxicity The hypothesis that elevated exposures to methylmercury might impair cardiovascular health was suggested by a series of observational studies conducted by Finnish investigators. Men with the highest level of hair mercury (>2 µg/g) had a twofold increase in risk (95% CI 1.2-3.1) (adjusted for age, examination year, ischemic exercise electrocardiogram (ECG) and maximal oxygen uptake) of an acute (fatal or nonfatal) myocardial infarction (MI) and had a 2.3-fold increased risk (95% CI 0.9-5.8) (adjusted for age, examination year, ischemic exercise ECG and maximal oxygen uptake) of death from coronary heart disease (CHD) (Salonen et al., 1995). In addition, self-reported fish consumption of 30 g per day or more was associated with a doubling of risk of an acute MI. Mercury burden was more strongly related to the amounts of nonfatty
freshwater fish (turbot, vendace, northern pike, whitefish) consumed rather than fatty fish (salmon, herring, domestic rainbow trout, tuna) (Salonen et al., 1995). Follow-up examinations of this cohort conducted 4 years later indicated that high hair mercury level at baseline was a significant predictor of the increase in the common carotid intima-media thickness (IMT), suggesting accelerated carotid atherosclerosis (Salonen et al., 2000). Among men in the highest quintile of hair mercury level (>2.81 ppm), the IMT increase was 32 percent greater than among men in the rest of the cohort. The increased cardiovascular risk associated with higher fish consumption reported by Salonen et al. (1995, 2000) and Virtanen et al. (2005) might, for example, be associated with food preparation techniques (see Chapter 5) rather than methylmercury levels in the fish consumed by Finnish men, although this variable was not addressed in these reports.
In a case-control study conducted in nine countries involving 684 men less than 70 years of age with a first diagnosis of MI (Guallar et al., 2002), the adjusted (including docosahexaenoic acid [DHA]) odds ratio for men in the highest, compared to the lowest, quintile of toenail mercury level was 2.16 (95% CI 1.09-4.29). Adjusting for toenail mercury level, the risk of MI was inversely related to adipose tissue DHA level (OR=0.59, 95% CI 0.30-1.19, for highest vs. lowest quintile).
In contrast to the findings of the Finnish studies and the Guallar et al. (2002) study, essentially null findings were reported in a nested case-control study of toenail mercury levels (an alternative biomarker) and coronary heart disease (coronary artery surgery, nonfatal MI, fatal coronary heart disease) in 33,737 male health professionals (Yoshizawa et al., 2002). In the highest, compared to the lowest, quintile of mercury level, the relative risk of coronary heart disease was 0.97 (95% CI 0.63-1.50). Adjustment for omega-3 fatty acid intake did not alter this. A major uncertainty about the interpretation of these two studies is the status of toenail mercury level as a biomarker of mercury burden attributable to fish consumption. In the Yoshizawa et al. study, more than half of the study cohort consisted of dentists, and the mean toenail mercury level in dentists was more than twice the mean among the nondentist health professionals. Although toenail mercury level was modestly correlated with reported fish consumption (correlation of 0.42), toenail mercury level apparently also reflects exposures to mercury from nonfish sources, such as elemental mercury from dental amalgams and dental amalgam preparation. In this regard, it is noteworthy that when the dentists were excluded from analyses in the Yoshizawa et al. (2002) study, increased toenail mercury was associated with increased risk of coronary heart disease. The increase in risk was not statistically significant, however, at least in part because of the reduced sample size.
As noted, because the primary vehicle in which methylmercury is delivered is a food that also contains nutrients that might have health effects
that are antagonistic to those of methylmercury, it is difficult to obtain “pure” estimates of methylmercury toxicities. For example, a follow-up study of the Finnish men reported on by Salonen et al. (1995) showed that men in the highest quintile of docosapentaenoic acid and docosahexaenoic acid intake, compared to men in the lowest quintile, had a 44 percent lower risk of CHD over a 4-year period (Rissanen et al., 2000). Analyses stratified by hair mercury level suggested, however, that the reduction was greater (52 percent) for men with hair mercury (Hg) levels <2 ppm than among men with hair Hg levels >2 ppm (only 24 percent). A similar shift in the balance of the risks of methylmercury and the benefits of omega-3 fatty acids was found in a study of blood mercury level and blood pressure among US women (NHANES 1999–2000; Vupputuri et al., 2005). In the entire cohort of 1240 women aged 16–49 years, blood mercury level was not significantly associated with either systolic or diastolic blood pressure. When analyses were stratified by reported fish intake (759 consumers, 481 nonconsumers), systolic blood pressure increased significantly with blood mercury level among nonconsumers, corresponding to an approximately 5 mmHg difference between the lowest quintile (0.1–0.4 µg/L) and the highest quintile (2.1–21.4). Among the fish-consumers, systolic blood pressure declined (nonsignificantly) with increasing blood mercury level. The findings were similar for changes in diastolic blood pressure with increasing blood mercury level. Overall, this pattern suggests that increased exposure to mercury, obtained from sources other than fish consumption, is associated with higher blood pressure. When mercury exposure occurs in conjunction with fish consumption, however, the effects on blood pressure are blunted and, at the levels in most US women, may be counteracted by protective factors in fish. This interpretation is consistent with the null findings of a study of hair mercury levels and blood pressure in fish-consuming Indian tribes of the Amazon rain forest (Dorea et al., 2005).
Methylmercury Reference Dose
A report from the National Research Council of the National Academies reviewed the US EPA’s process in deriving the RfD (see Box 4-2). It concluded that the existing RfD of 0.1 µg/kg per day was a “scientifically justifiable level for the protection of public health,” although it recommended that it be derived on the basis of the findings of the newer epidemiological studies rather than of the Iraqi study (NRC, 2000). Such a calculation is subject to numerous uncertainties, however. Among these are the choice of the functional form of the statistical model used to identify the methylmercury dose at which a doubling of the target response occurs (e.g., linear vs. supralinear vs. sublinear models), the choice of the adverse health effect, the choice of the point estimate for the excess prevalence to be prevented,
|
BOX 4-2 Reference Dose for Methylmercury The US Environmental Protection Agency (US EPA, 2001) established a Reference Dose (RfD) for methylmercury (MeHg) that it defines as “…an estimate of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime.” To derive the RfD for MeHg, the US EPA applied benchmark dose modeling. In this approach, the benchmark dose (BMD) is identified at which the prevalence of a defined health abnormality exceeds the background prevalence of the abnormality by a specified amount. The abnormality can be defined distributionally (e.g., scores more than 2 standard deviations below the mean) or clinically (e.g., the presence of a particular abnormal finding on neurologic examination). Once the critical dose is identified, the dose corresponding to the lower bound of its 95 percent confidence interval (the Benchmark Dose lower bound [BMDL]) is taken as the “point of departure” for calculating the Hg intake that would result in that dose. In other words, the BMDL is the lowest hair mercury (Hg) level that is statistically consistent with the observed increased in the prevalence of the target outcome. Although the US EPA used the Boston Naming Test results from the Faroe Islands study to illustrate the process by which it derived the RfD for Hg, it considered all of the data from the Faroe Islands and New Zealand studies and an integrative analysis that included the Seychelles study. A test score at the 5th percentile or below was selected as the critical health effect, and a doubling of the prevalence of such scores to be prevented (Rice, 2004). The US EPA selected 12 ppm in maternal hair as the critical BMDL (or 58 µg/L in cord blood). A one-compartment pharmacokinetic model, involving assumptions about factors such as the elimination constant, blood volume, MeHg absorption, fraction of absorbed dose in the blood, and the ratio of cord blood mercury to maternal blood mercury, was used to determine that an MeHg intake of 0.1 µg/kg/day over a lifetime would not result in a hair Hg level exceeding 1.2 ppm. |
the choices of the point estimates for the assumptions made in fitting the one-compartment model, and the size of the aggregate uncertainty factor that should be applied to take account of all these unknowns (Rice, 2004). Reference Dose calculations are sensitive to the assumptions made about factors such as the ratio of cord blood Hg:maternal blood Hg. Although the RfD is intended to address a pregnant woman’s MeHg intake, the fetal risk estimates for the Faroe Islands study, the critical study, were expressed as
cord blood Hg levels. The US EPA assumed a ratio of 1:1, but recent Monte Carlo analyses suggest that the Hg level in cord blood might be as much as 70 percent higher than the Hg level in maternal blood (Stern and Smith, 2003). The results of these analyses suggest that reducing the RfD so that maternal blood Hg levels do not exceed 3.4 µg/L would prevent cord blood Hg levels from exceeding 5.8 µg/L.
The NRC (2000) study identified several other important data gaps that contribute to uncertainty, e.g., the possibility of interindividual variation in susceptibility to MeHg. Factors that might affect susceptibility include age, sex, genetics, health status, nutritional status, and toxicokinetic and toxicodynamic processes. The role of nutritional factors as potential confounders or effect modifiers of MeHg neurotoxicity is particularly important (Chapman and Chan, 2000). The many differences between the diets of the Faroese and Seychellois have been suggested as a possible explanation for apparent differences between findings. The specific dietary components suggested as possibly important are DHA, iodine, choline, and iron (Clarkson and Strain, 2003). One study found that greater consumption of tropical fruit is associated with lower hair Hg levels, although it could not be determined whether this reflected altered absorption, distribution, or excretion (Passos et al., 2003). Other data gaps pertain to the lack of information about possible late-emerging neurodevelopmental effects as children mature and the lack of dose-response analyses for other potential adverse health effects of MeHg, such as cardiovascular disease. A third class of data gaps pertains to the characterization of exposure. Factors that contribute to this are a lack of dietary intake data, the extrapolation from a biomarker such as maternal hair Hg to maternal MeHg intake, confounding by coexposures to other neurotoxic contaminants (e.g., PCBs), and the impracticality of characterizing short-term temporal variations in exposure using currently available biomarkers, particularly during potentially critical windows of brain vulnerability. Using bootstrap analyses, Budtz-Jorgensen et al. (2004b) showed that the BMDL is overestimated by 25 percent if it is not adjusted for error in measuring cord blood Hg and by 40 percent if it is not adjusted for error in measuring hair Hg. The authors argued that a failure to take these sources of error into account would result in a reference dose that is too high, and thus insufficiently protective.
Summary of Evidence
Interpretations of data from the three major epidemiologic methylmercury studies are not entirely concordant. The Faroe Islands and New Zealand studies are regarded as providing evidence that children prenatally exposed to methylmercury as the result of maternal seafood consumption during pregnancy are at increased risk of manifesting subtle neurodevelopmental
deficits. The Seychelles study is regarded as not providing such evidence. A new statistical approach revealed similarities between the three studies not previously evident in published analyses. Results of this approach reduced the degree of discordance, which might have been overestimated due to a focus on p-values. This yielded greater consistency between findings of the three studies, indicating a decline of 0.1 to 0.25 points, on a scale of IQ-like measurement, for each part-per-million increase in maternal hair mercury level during pregnancy.
Observational studies in adult men from the general population have produced mixed results regarding the associations between fish consumption, mercury level, and cardiovascular health. Overall, the data considered suggests an increased risk of myocardial infarction among men with higher hair Hg levels. For both child neurodevelopment and adult cardiovascular health, emerging evidence suggests that the health benefits of seafood consumption are greater among individuals whose body burden of methylmercury is lower.
Other Metals
Metal contaminants other than mercury, including lead, manganese, chromium, cadmium, and arsenic may be present in seafood, although on a population basis, seafood consumption does not appear to be a major route of exposure to these metals. In analyses of farmed Atlantic and wild salmon, Foran et al. (2004) found that for none of nine metals measured did the levels exceed federal standards. For three of the metals measured (cobalt, copper, and cadmium), levels were significantly higher in wild than farmed salmon. Burger and Gochfeld (2005) measured the levels of seven metals (arsenic, cadmium, chromium, lead, manganese, mercury, selenium) in fish obtained from New Jersey markets. Although these levels sometimes exceeded health-based standards, the intercorrelations among the different metals were low, leading the authors to conclude that consuming a variety of fish species will reduce a consumer’s risk. The source of fish is an important consideration, however. Kong et al. (2005) found levels of lead and chromium in farmed tilapia from China that exceeded local guidelines.
Persistent Organic Pollutants
Persistent organic pollutants are defined as organic chemicals that remain intact in the environment for long periods, become widely distributed geographically, bioaccumulate up the food chain by amassing in fatty tissues of animals, and are toxic to humans, wildlife, and the environment (Bidleman and Harner, 2000; IOM, 2003; UNEP Global Environmental Facility, 2003; Robson and Hamilton, 2005). Many POPs are chlorinated
compounds, but brominated and fluorinated compounds also exist (e.g., brominated flame retardants and Freon) and may have a detrimental impact on the environment.
Evidence for long-range transport (to regions distant from the original source) and the threats posed to the environment (Fries, 1995a,b; UNEP Global Environmental Facility, 2003) has prompted regulatory action to reduce emissions (CFSAN, 2001; also reviewed in IOM, 2003). As a result of concerns about global circulation through the atmosphere, oceans, and other pathways, the US signed an agreement on POPs at a diplomatic conference in Stockholm, Sweden (UNEP Global Environmental Facility, 2003). Under this Convention, signatory countries were committed to reduce and/or eliminate the production, use, and/or release of the 12 POPs of greatest concern to the global community and to establish a mechanism by which additional chemicals may be added to the treaty in the future. The POPs initially targeted by the agreement, informally called the “dirty dozen” (Table 4-2), include:
-
Certain insecticides, such as DDT and chlordane, once commonly used to control pests in agriculture and building materials;
-
Polychlorinated biphenyls, used in electrical, heat transfer, and hydraulic equipment and as plasticizers in paints, plastics, and rubber products;
-
Certain chemical byproducts, such as dioxins and furans, which are produced unintentionally from most forms of combustion, including municipal and medical waste incinerators, open barrel burning, and industrial processing.
The POPs to which seafood consumers are most likely exposed are the dioxins, dioxin-like compounds (DLCs), and PCBs.
Dioxins and Dioxin-like Compounds
Dioxins and DLCs are unintentional by-products of combustion of organic material. Sources of dioxins include herbicides (2,4,5-T), wood preservatives, diesel and gasoline fuel combustion, and industrial combustion and backyard barrel burning. Currently, new dioxin releases into the environment are mostly from backyard and agricultural burning (IOM, 2003). Because of the long half-life of dioxins, they will persist in the environment. Furthermore, even if all anthropogenic sources could be eliminated, low levels of naturally occurring dioxins will continue to be produced (US EPA, 2003).
Since 1987, the US EPA has been taking action to effectively reduce environmental release of dioxins and furans to land, air, and water from
TABLE 4-2 The “Dirty Dozen” Identified in United Nations Environment Programme
|
The “Dirty Dozen” |
|
Aldrina |
|
Chlordanea |
|
DDTa |
|
Dieldrina |
|
Endrina |
|
Heptachlora |
|
Mirexa |
|
Toxaphenea |
|
Polychlorinated dibenzo-p-dioxins (Dioxins)c |
|
Polychlorinated dibenzo-p-furans (Furans)c |
|
NOTES: The United States has taken strong domestic action to reduce emissions of POPs. Currently, none of the pesticide POPs are registered for sale and distribution in the United States. In 1978, the US Congress prohibited the manufacture of any new PCBs and severely restricted the use of remaining stocks. aPesticides. bIndustrial Chemical. cBy-products. SOURCES: UNEP Global Environmental Facility, 2003; IISD, 1998. |
sources within the continental United States. Regulatory action has resulted in a 77 percent decline in total dioxin and furan releases between 1987 and 1995 (US EPA, 2005) (for more information see also US EPA 1987, 1991, 1994, 1995). Overall, levels of dioxins and DLCs in the environment have been declining for the past three decades. However, since dioxins are persistent compounds, they can be expected to remain in the environment and the food supply for many years to come (IOM, 2003).
Toxic Equivalency Factors (TEFs) are a convenient method for assessing the toxicity of mixtures containing dioxins and DLCs but there are uncertainties associated with calculating TEF values for individual congeners because of variability in their half-lives and differences in toxicity to humans. The reference compound for the TEF is the dioxin compound 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). WHO recommends a tolerable daily intake of DLCs and PCBs of 1–4 pg/TEQ/kg/day (IOM, 2003). The US EPA has estimated 0.001 pg/kg/day of TCDD as the level associated with a 1 in 1 million excess risk for human health effects from exposure to DLCs and PCBs (IOM, 2003). The NRC committee on EPA’s Exposure and
Human Health Reassessment of TCDD and Related Compounds (NRC, 2006) noted that the classification of DLCs as “carcinogenic to humans” vs. “likely to be carcinogenic to humans” is dependent on “the definition and interpretation of the specific criteria used for classification, with the explicit recognition that the true weight of evidence lies on a continuum with no bright line that easily distinguishes between these two categories.”
Bioaccumulation of Dioxins in Seafood Exposure to dioxins and DLCs occurs when fish consume aquatic invertebrates that come in direct contact with dioxin particles that settle in sediment; through direct absorption through the gills; or by eating contaminated sediment, insects, and smaller fish (Evans, 1991). Because of their lipophilic character, dioxins and DLCs are distributed to fatty tissues in fish, including the liver and gonads. Muscle tissue is less contaminated, depending on the fat content of the muscle, which is likely to be greater in the older, larger, and oily fish.
Adverse Health Effects TCDD is used as the reference congener as a measure of toxicity for all dioxin-like compounds. Adverse health effects associated with exposure to dioxins have been identified in populations exposed through unintended industrial releases. One of the largest population exposures to TCDD occurred from an unintended industrial release in Seveso, Italy. Those who were exposed to the highest doses, primarily children, exhibited chloracne (Mocarelli et al., 1999), a severe skin disease with acne-like lesions that occur mainly on the face and upper body. Other adverse health outcomes included an increased risk for cancer. When compared to the nonexposed general population, the exposed population did not show an increased overall cancer mortality, but did have a significant excess mortality risk for esophageal cancer in males and bone cancer in females among those who were exposed to the lowest doses (Bertazzi et al., 1997). The US EPA (2000a) concluded that the cancer data on the Seveso population was difficult to interpret because of the small number of cases, exposure classification problems, and limited follow-up.
In 1997, the International Agency for Research on Cancer (IARC) placed TCDD in a Group I (agents with sufficient evidence of carcinogenicity for humans) designation, but weaknesses and inconsistencies among the positive studies published have made this designation controversial (Cole et al., 2003). The US EPA (2000a) considers TCDD to be a human carcinogen and other DLCs likely carcinogens, based on epidemiological and animal studies. Although epidemiological evidence alone does not support a causal relationship between dioxin exposure and cancer, US EPA (2000a) describes TCDD as a non-genotoxic carcinogen and a potent tumor promoter.
Polychlorinated Biphenyls
Polychlorinated biphenyls are also long-lived chlorinated aromatic compounds. They include over 200 chemical compounds in the form of oily fluids to heavier grease or waxy substances. Production of PCBs began in 1929, and the compounds were used as coolants and lubricants in transformers and other electrical equipment. Because of their noncombustible insulating characteristics, PCBs were used to reduce the flammability of materials used in schools, hospitals, factories, and office buildings. A variety of commercial products, including paints, plastics, newsprint, fluorescent light ballasts, and caulking materials contained PCBs until production was banned in the 1970s.
Local sources of PCBs may be more important than local sources of dioxins and DLCs for contamination of aquatic organisms. PCBs were legally widely discharged into rivers, streams, and open landfills between 1940 and the early 1970s. In 1976, the Toxic Substance Control Act (TSCA) was passed, calling for a ban on the manufacture, processing, distribution, and use of PCBs in all products in which the PCBs were not totally enclosed. The TSCA was based on three concerns: first, PCBs persist in the environment and resist biodegradation; second, a population-wide incident of human poisoning in Japan in 1968 was attributed to introduction of PCB-contaminated oil into a community; and third, in 1975 the CDC reported that, in rat experiments, oral gavage with Aroclor 1260 (a mixture of PCBs) caused liver cancer (Kimbrough et al., 1975). As a result of the TSCA, transformers and electrical capacitors that contained PCB compartments were sealed. Such transformers remain in place unless the seals leaked or were damaged, and by 1990, any PCB transformer within 30 meters of a commercial or public access building should have been replaced, registered, or provided with protection (US EPA, 1994).
Bioaccumulation of PCBs A significant correlation has been observed between blood PCB levels and the quantity of fish consumed by humans (Fein et al., 1984; Humpfrey, 1988; Jacobson et al., 1990; Smith and Gangolli, 2002). Bioaccumulation of dioxins and PCBs in the fatty tissues of food animals contributes to human body burdens through ingestion of animal fats in foods such as meat and full-fat dairy products. These foods are the largest contributors of dioxins and DLCs from the US food supply. The levels of dioxins, DLCs, and PCBs in seafood are generally greater than those in meat; however, actual exposure levels are far lower because of the lower consumption of fish among the general population (IOM, 2003). Fish oils that are used for supplements tend to have lower levels of dioxins, DLCs, and PCBs than fatty or oily fish as a result of processing methods
that remove these compounds from the final product (Source: http://www.ocean-nutrition.com/inside.asp?cmPageID=158).
Adverse Health Effects An extensive experimental literature on rodent and nonhuman primate models demonstrates that prenatal exposure to PCBs can interfere with neurodevelopment (Rice, 2000; Faroon et al., 2001; Bowers et al., 2004; Nguon et al., 2005). This literature is complemented by numerous prospective epidemiological studies of children conducted in Michigan; North Carolina; Oswego, NY; Germany; Faroe Islands; and the Netherlands (Schantz et al., 2003). The cohorts were often chosen to include children born to women who consumed fish from waters known to be contaminated with PCBs. The results of these epidemiological studies are generally congruent with those using animal models, although, as in most areas of observational research in humans, results are not always consistent across studies or consistent over time in a particular study.
Higher prenatal exposures have been associated with deficits in various functional domains including intelligence, attention, response inhibition, activity, and play behaviors (Jacobson and Jacobson, 1996; Patandin et al., 1999; Walkowiak et al., 2001; Vreugdenhil et al., 2002a,b; 2004; Jacobson and Jacobson, 2003; Stewart et al., 2003). However, there are some uncertainties about many key issues. One issue is the shape of the dose-effect relationship curve and, specifically, whether a threshold exists. A second is whether PCB exposure leading to adverse effects occurs prenatally or postnatally. Although most of the focus has been on prenatal exposures, some recent studies suggest that early postnatal exposures are also associated with neurotoxicities (Huisman et al., 1995; Walkowiak et al., 2001; Winneke et al., 2002). A third issue is the relative potency of the different congeners. For some neurodevelopmental outcomes, it is exposure to the dioxin-like congeners that is most strongly associated with deficits. A fourth issue is the impact of synergism between PCBs and other toxicants. Some studies suggest that adverse effects arise only when PCB exposure occurs in the presence of methylmercury or in environments in which individuals may be exposed to increased levels or multiple exposures (Grandjean et al., 2001; Roegge et al., 2004; Roegge and Schantz, 2006).
The PCB exposures identified in these study samples were considerably greater than those of the general US population. The median concentration of PCB 153 in the 10 studies, the only basis for direct comparison, ranged from 30 to 450 ng/g serum lipid, and the median of the 10 medians was 110 ng/g. The exposure levels in the two recent US studies were about one-third of those in the four earlier US studies or recent Dutch, German, and northern Québec studies (Longneker et al., 2003), consistent with exposure surveys indicating that PCB levels in human tissues in the United States have declined in recent decades (Sjodin et al., 2004a). In the most recent
Centers for Disease Control and Prevention (CDC) National Report of Human Exposure to Environmental Chemicals, the 95th percentile of the distribution of PCB 153 levels in the US population was 126 ng/g serum lipid (CDC, 2005d).
Animal studies carried out by CDC suggest that it is likely cancer risks were overstated and animal-specific. PCBs have been associated with health effects in laboratory animals, but typically at very high doses, possibly not relevant to noncatastrophic exposure for humans. Similar conclusions have been derived from looking at animal studies of exposure to high levels of PCBs resulting in tumor formation. Although there is evidence to substantiate PCB-associated health problems, several epidemiological studies of occupational workers exposed to PCBs found no evidence of ill health associated with their exposure. Even the PCB-chloracne association may be due to co-exposure to DLCs, and there is concern that multiple confounding factors make it difficult to interpret epidemiological studies in the workplace. Some studies of PCB workers found increases in rare liver cancers and malignant melanoma (US EPA, 2006). Thus, the US EPA found that the epidemiological studies are inconclusive; based on animal and recent human studies, PCBs are probable human carcinogens.
The earliest reported incidents of adverse effects from PCB poisoning occurred in Japan and Taiwan following widespread consumption of contaminated rice oil. The high-level exposure to PCBs resulted in skin lesions (acneform dermatitis) and peripheral nerve damage among adults, and similar effects among their offspring. Children born to exposed mothers also showed inhibition of growth and tissue maintenance (Kimbrough, 1987; Erickson, 1997). NRC (1999) also identified low birth weight and shorter gestation, and both neurological and neuromuscular deficits as adverse outcomes associated with prenatal PCB exposure.
Reports from occupational exposure to PCBs have identified several subclinical adverse health effects. The US EPA reviewed and identified many potentially serious noncancer adverse health effects associated with PCB exposure. These adverse effects included impairment of immune, reproductive, and neurological systems. The long-term impact of low-level exposure to PCBs is unclear, particularly on the endocrine system (US EPA, 2006) and will require further research to understand.
As PCB exposure levels continue to decline subsequent to federal laws banning PCB production, it may be difficult to characterize adverse health effects from low-level exposure (WHO Consultation on Risk Assessment of Non-Dioxin-Like PCBs, 2001; Ross, 2004) and to determine the significance of these exposure levels to health outcomes among the general population. Advances in analytic techniques may enhance data gathering and analysis efforts and improve our understanding of risks associated with low-level
TABLE 4-3 TEF Values from WHO (1998)
|
Compound |
TEF valuea |
|
2,3,7,8-TCDD |
1 |
|
Octachlorodibenzo-p-dioxins |
0.0001 |
|
1,2,3,4,6,7,8,9-octachlorodibenzofuran |
0.0001 |
|
3,3’,4,4’-tetrachlorobiphenyl (PCB 77) |
0.0001 |
|
aTEF = Toxicity Equivalency Factor, a numerical index that is used to compare the toxicity of different congeners and substances. SOURCE: Van den Berg et al., 1998. |
|
exposure as well as the role of specific PCB congeners or classes of congeners in health outcomes (Schantz et al., 2003; Ulbrick and Stahlmann, 2004).
Toxicity and Recommended Intake Limits for Dioxins, DLCs, and PCBs
Toxicity and Estimates of Risk The biological activity of dioxins, DLCs, and PCBs varies due to differences in toxicity and half-life of the various congeners. Variations in toxicity among congeners are related to a number of factors, including binding interaction at the cellular level with the arylhydrocarbon receptor (AhR) and variability in pharmacokinetics in vivo. Not all factors apply to all congeners; for example, many PCBs that do not have dioxin-like characteristics do not bind to the AhR. Van den Berg et al. (1998) describes factors used to determine the TEF values for dioxins, DLCs, and PCBs that include (but are not universal to all congeners):
-
Structural relationships between congeners;
-
Binding to the AhR;
-
Toxic responses mediated through AhR activation; and
-
Persistence and bioaccumulation.
The TEF value expresses the activity or toxicity of a specific congener relative to the toxicity of reference congeners, 2,3,7,8-TCDD; it is assigned a TEF of 1 and the toxicity of other congeners is expressed relative to TCDD (Van den Berg et al., 1998; IOM, 2003; SACN, 2004). Examples of some TEF values established by WHO are shown in Table 4-3. Toxicity can be additive in a mixture of congeners and so the Toxicity Equivalency (TEQ) of a mixture of DLCs is calculated by multiplying the concentration of each congener by its TEF, and summing across all DLCs in the mixture.
The Toxic Equivalency system is difficult to use, but it does permit extrapolation from 2,3,7,8-TCDD, a congener for which much is known.
WHO has recommended a Tolerable Daily Intake (TDI) of 1–4 pg/kg body weight per day for TCDD, and the TDI is applied to mixtures of dioxins and PCBs (IOM, 2003). Based on its estimate of cancer potency for DLCs, the US EPA concludes that intakes should not exceed 1–4 pg TEQ/kg/day in the general population (IOM, 2003).
DLC Exposure Limits in Foods With the exception of Canada and the United States, most countries utilize the TDI for assessing adverse health effects from exposure to DLCs and for setting acceptable limits in foods. The TDI represents an index for a contaminant similar to the adequate dietary intake (ADI) used for food additives. These limits are based on the assumption of an experimental threshold dose level below which no toxic effect is found in animal models, and include an additional uncertainty factor for extrapolation to humans.
The FDA and US EPA utilize probabilistic models to derive a Risk Specific Dose (RsD) for a contaminant. This model assumes the lowest dose that could result in a specific risk to humans, i.e., the dose with a lifetime cancer risk of 1 in 1 million. The use of the RfD, as previously described for methylmercury, was not applied to DLCs by the US EPA in its Draft Reassessment; the margins of exposure in the range of 100–1000 are generally considered inadequate to rule out the likelihood of significant effects occurring in humans, based on sensitive animal responses within the TEQ (US EPA, 1994; Foran et al., 2005a). Guidance on the development of risk-based meal consumption limits for 25 high-priority contaminants and analytes has been described by the US EPA (US EPA, 2000b). As described by the US EPA, a cancer slope factor (CSF) for carcinogenic risk can be calculated for DLC exposure of 1 × 10−3/pg TEQ/kg/day (US EPA, 2000c). These risks are described later for analyzing benefits and risks associated with consuming farmed salmon (Foran et al., 2005b).
Exposure to DLCs from Seafood In 2002, the IOM Committee on the Implications of Dioxin in the Food Supply commissioned an exposure estimate for DLCs using intake estimates from the Continuing Survey of Food Intake by Individuals (CSFII) imputed to data from the FDA’s Total Diet Study (Source: http://www.cfsan.fda.gov/~lrd/dioxdata.html). This analysis estimated that for all males and females in the general population, 1 year of age and older, the percentage contribution of fish and fish mixtures to the total DLC exposure from all foods was approximately 8 percent (IOM, 2003). When the data was analyzed for specific subgroups within the general population, the estimated contribution from fish and fish mixtures for pregnant and lactating women and for children (both males and females) aged 1 to 5 years was approximately 4 percent. By comparison, the estimated
contribution of meat and meat mixtures to the total DLC exposure for these groups was approximately 37 and 35 percent, respectively, for pregnant and lactating women compared to children aged 1–5 years (IOM, 2003).
Polybrominated Diphenyl Ethers
Polybrominated diphenyl ethers (PBDEs) are synthetic compounds that are added to a variety of materials to increase their fire resistance. PBDEs are structurally similar to PCBs, and can exist, theoretically, as 209 distinct isomers. PBDEs are released into the environment as emissions from facilities manufacturing them and as a result of degradation, recycling, or disposal of products that contain them. The patterns of use of PBDEs are changing rapidly.
Bioaccumulation of PBDEs As with other persistent organic pollutants, PBDEs are cycled globally (de Wit et al., 2004). PBDE levels in aquatic wildlife have increased rapidly in recent decades (Ikonomou et al., 2002; Law et al., 2003), with doubling times of between 1.6 years and 6.0 years (Lunder and Sharp, 2003; Rayne et al., 2003; Hites et al., 2004a). PBDE tissue (blood, milk, and adipose) levels in humans have followed a time course similar to that in wildlife. The concentrations in human milk samples in Sweden, British Columbia, and the United States have increased manyfold over recent decades (Darneud et al., 2001; Ryan et al., 2002; Hites, 2004; Sjodin et al., 2004a; Schecter et al., 2005), with doubling times of 10 years or less (Meironyte et al., 1999; Ryan et al., 2002). For reasons that are not known, the concentrations of PBDEs in biological tissues collected in North America are at least 10 times greater than those collected in Europe or Japan (Peele, 2004). Although ingestion is considered to be an important route of exposure to PBDEs, the importance of other routes, such as indoor air and dust, are poorly characterized and could be important in certain settings (Sjodin et al., 2004b).
Although the concentrations of PBDEs have been found to vary widely across countries, market basket surveys, total diet studies, duplicate diet studies, and commodity-specific surveys have repeatedly shown that, within a region, fish and shellfish tend to have PBDE concentrations that are greater than those found in dairy products, eggs, fats, and oils, and other meat products are important sources of exposure to PBDEs. This has been found in Canada, Finland, Germany, Japan, the Netherlands, Sweden, and the United States. In terms of total intake of PBDEs, fish and shellfish are the major contributors in Europe and Japan, while meats and poultry are the major contributors in the United States and Canada (FAO/WHO JECFA, 2005). The PBDE concentration tends to be greater in fish at higher trophic levels, i.e., predatory fish (Rice, 2005). In a market basket survey
conducted in Dallas, Texas (Schecter et al., 2004), the highest levels of total PBDEs were found in samples of salmon, catfish, and shark. It is notable that the congener pattern was highly variable across samples, even within types (e.g., catfish), perhaps reflecting site specificity in the magnitude and nature of the problem of PBDE contamination. Total PBDE levels were also greater in meats with relatively high fat content, such as pork sausage, hot dogs, and duck; and in dairy products with higher fat content, such as cheese and butter (Schecter et al., 2004). Similar findings were reported in a market basket survey of foods conducted in California (Luksemburg et al., 2004), in which the highest PBDE levels were found in swordfish, Alaskan halibut, and Atlantic salmon. PBDE levels were 15 times greater in Pacific farm-raised salmon than in Pacific wild salmon (Easton et al., 2002). PBDE levels are higher in salmon farmed in the United States and Europe than in Chile (Hites et al., 2004a). Limited data are available, however, on the association between seafood consumption and PBDE levels in human tissues. In a small study of 94 urban anglers in the New York–New Jersey area, greater consumption of locally caught fish was not significantly related to blood PBDE levels, suggesting that, at least at this time and in this study population, consumption of local fish is not a major route of exposure to PBDEs (Moreland et al., 2005).
Adverse Health Effects The data available on the toxicity of PBDEs are extremely limited. Experimental animal studies indicate that PBDEs affect the nervous (Viberg et al., 2003), endocrine (Stocker et al., 2004), and immune systems (Fowles et al., 1994), and that the potency of PBDEs might be comparable to that of PCBs, although considerable uncertainty remains (Kodavanti and Ward, 2005). No population-based epidemiological studies have evaluated the human health effects of environmental exposure to PBDEs. It is not known whether all PBDEs share a common mechanism of action, complicating any effort to characterize toxicity using a toxic equivalence factor approach. In light of the fact that in vitro studies with purified PBDE congeners do not show AhR activation, it is possible that the presence of trace amounts of DLCs have confounded these assessments of PBDE toxicity (FAO/WHO JECFA, 2006). The FAO/WHO Joint Expert Committee on Food Additives and Contaminants concluded that the toxicological data available on PBDEs were insufficient to establish a Provisional Tolerable Weekly Intake (FAO/WHO JECFA, 2005). The data, however, are not sufficient to identify “no observed adverse effect levels” (NOAELs) for congeners of greatest interest, and thus to draw inferences about the prevalence of exposures of concern in the US population.
Levels of POPs in Seafood
Because of their lipophilic character, persistent organic pollutants are absorbed and transported to fatty tissues in fish and marine mammals. Uptake of POPs can occur through exposure from sediments in water or via consumption of smaller fish by predatory species (Geyer et al., 2000).
Farmed fish are exposed to these contaminants to the extent that they are present in feed (Hites et al., 2004a). Recently, Hites et al. (2004a) found that, perhaps because of their higher fat levels, some farmed salmon contain significantly higher concentrations of certain organochlorine contaminants, including PCBs, than wild-caught salmon. In addition, PCB concentrations in samples of commercial salmon feed purchased in Europe were higher than those in samples purchased in North and South America, suggesting that regional differences in the composition of feed contribute to regional differences in the PCB concentrations in farmed salmon. The mean wet weight concentration of PCBs in farmed salmon was 50 ng/g or below (Hites et al., 2004a), regardless of source, and thus below the Food and Drug Administration (FDA) action level of 2 ppm for PCBs in food. Using the US EPA risk assessment for PCB and cancer risk, Hites et al. (2004a) concluded that, given the PCB levels in the fish samples, a consumer’s risk will not be increased if consumption is limited to no more than 1 meal per month of farmed salmon. Given the substantial regional differences found in PCB levels, however, these analyses demonstrated the importance for the consumer of knowing whether a fish was farmed or wild-caught and also its region of origin.
In a subsequent paper, the same group of investigators reported a quantitative analysis of competing risks and benefits associated with consuming farmed Atlantic and wild-caught Pacific salmon, for both cancer and noncancer end points (Foran et al., 2005b). Sixteen organic contaminants were considered. A benefit/cancer risk ratio was calculated for cancer using cancer slope factors developed by the US EPA (assuming that a 1×10−5 risk is acceptable) and a benefit/noncancer risk ratio using reference doses established by the US EPA. Foran et al. (2005b) concluded that neither farmed nor wild-caught salmon can be consumed in quantities that would provide 1 g/day of EPA/DHA while still maintaining an acceptable level of carcinogenic risk (1×10−5). In contrast, they determined that based on the benefit/noncarcinogenic risk ratio, wild-caught salmon could be consumed in amounts consistent with EPA/DHA intake levels recommended by the American Heart Association (see Chapter 2).
As expected, however, the results differed for farmed and wild-caught salmon. Consuming farmed salmon in amounts that provides 1 g/day of EPA/DHA would produce a cumulative cancer risk that is 24 times the acceptable cancer risk level. For wild-caught salmon, the cumulative cancer risk would be eight times the acceptable level. Both farmed and wild-caught
salmon could be consumed in amounts that provide at least 1 g/day of EPA/DHA per unit of noncarcinogenic risk (Foran et al., 2005b).
These analyses were conducted assuming salmon intake needed to provide 1 g/day of EPA/DHA. The authors interpreted the WHO intake recommendation for omega-3 fatty acids as corresponding to 2–3 g/day; this includes alpha-linolenic acid (ALA) intake, which is derived primarily from plant sources such as soy, flaxseed, and walnut oils (see Chapter 1). The analysis of Foran et al. (2005b) was based on the assumption that the 2–3 g/day of omega-3 fatty acids applied only to EPA/DHA and did not take ALA into consideration. The WHO (2003) recommendation for fish consumption is 1–2 servings per week; it assumes that this level of consumption would provide 200–500 mg of EPA/DHA, considerably less than the intake of 1 g/day EPA/DHA from fish that Foran et al. assumed. These analyses represent a “worst case” scenario in that it is assumed that consumption of salmon would be the sole source of omega-3 fatty acids. Further, it assumed that salmon would provide all omega-3 fatty acids (DHA, EPA, and ALA) and salmon is not a source of ALA. Their analysis was based on data obtained prior to the implementation of industry safety measures for the prevention of POP contamination of aquaculture products (Santerre, 2004). It is worth emphasizing that because the food supply is dynamic, benefit-risk analyses are not static (Willett, 2006).
Body Burdens of POPs
Body burden can be defined as the total amount of a chemical in the human body or in human tissue from exposure to contaminants found in the environment (DeCaprio, 1997; Mendelsohn et al., 1998; IOM, 2003). CDC monitors over 200 contaminants with the aim of identifying baseline concentrations of specific substances and determining trends in body burdens among the general population (http://www.cdc.gov/biomonitoring/overview.htm; Kamrin, 2004). CDC reports (CDC, 2004; 2005b) include data on human exposure to approximately 150 compounds, including potential seafood contaminants such as lead, mercury, and many POPs. Technological advancements now afford the ability to detect minute levels of contaminants in human tissue, although detection of such contaminants does not indicate that a hazard or risk is present. For example, individuals regularly consuming fish from the Great Lakes were reported to have higher serum dichlorodiphenyl dichloroethene (DDE) concentrations (median 10 µg/L) compared to those who did not eat fish (1 µg/L); however, they did not show impaired motor function, impaired visuospatial function, or reduced memory and learning (Schantz et al., 1999; 2001; Rogan and Chen, 2005).
Body burdens for PCBs have been reviewed in studies of fish-consuming populations by the US EPA (US EPA, 2000a,c). The review did not show any
cases among the general population of PCB exposure through fish consumption that exceeded the upper limit of background exposure, although it did find that consumers who had higher consumption levels of fish with typical PCDD/PCDF profiles than the general population may receive up to five times the mean intake exposure level of the general population (Armstrong, 2002). To illustrate, sport fishers living near an industrial release site in the United States had blood PCB levels (both dioxin-like and nondioxin-like congeners) three times higher than control groups eating fish from areas that were not highly contaminated (US EPA, 2000c).
Toxicological and epidemiological data suggests that the population does not necessarily incur adverse health effects from the majority of chemicals currently detected in biomonitoring programs (US EPA, 2005). Thus, biomonitoring measures the level of the contaminant in a biological sample, which is not used to correlate such data to toxicology studies in animals; rather, biomonitoring gives a picture of a person’s body burden at one particular point in time, and it can be difficult to determine when the exposure might have occurred (Paustenbach and Galbraith, 2005). Biomonitoring measurements are relevant exposure assessment tools because they indicate body burden levels from all environmental sources (e.g., air, soil, water, dust, food) combined. The purpose of the CDC national biomonitoring programs is to determine which environmental chemicals are absorbed, measure exposure levels, assess health impacts of exposure on population groups (e.g., pregnant women and children), determine exposure risks among population groups, and monitor trends over time (Source: http://www.cdc.gov/biomonitoring/overview.htm). Research investigations may be utilized to identify specific sources of the elevated exposure and action to deal with the sources (Paustenbach and Galbraith, 2005).
Summary of Evidence
Evidence for specific adverse health effects associated with exposure to POPs is inconsistent. Among confounding factors related to this class of contaminant is the uncertainty that accompanies association of specific disease outcomes with low-level exposures. An issue of particular concern is the inability to determine a threshold for an adverse effect. Thus, the determination of toxicity related to exposure to POPs is challenging and requires further research.
INTERACTIONS BETWEEN NUTRIENTS AND CONTAMINANTS IN SEAFOOD
Selenium and Seafood Contaminants
There are several distinct ways that selenium may influence the impact of toxicants, e.g., through hepatic and extrahepatic detoxification mechanisms, effects on oxidative stress, modulating the immune response, and some novel sequestering mechanism rendering toxicants, e.g., heavy metals, inactive.
As noted previously, mercury, i.e., elemental mercury (Hg), ionic mercury (Hg+), and organic mercury (MeHg), may exist in three different states, and each state likely governs how selenium may interact with this element. MeHg has been implicated as a neurotoxicant, a mutagen, and a teratogen in various organisms. Epidemiological studies have been conducted on the exposure of humans to mercury through consumption of fish and marine mammals in different geographical areas including Seychelles, the Canadian North, the Amazon, Faroe Islands, Papua New Guinea, and Sweden. There are inconsistencies among these studies in the toxic dose, which may be due to differences in dietary patterns between the populations studied, e.g., more whale meat is consumed in Faroe Islands and more fish in Seychelles (Chapman and Chan, 2000) (see Box 4-1). Additionally, toxicity assessments were not conducted in all of the study locations and where they were the results may not be comparable in terms of populations examined and outcomes assessed.
Coexposure to selenium may diminish the toxic effects of some forms of mercury and other heavy metals, including cadmium and silver (Whanger, 1985). The mechanisms for these interactions are only partially understood but their occurrence certainly influences the determination of safe and toxic levels of such metals for persons in the general population. Selenium was first reported to counteract acute mercuric chloride toxicity by Parizek and Ostadalova (1967). Later, Ganther et al. (1972) showed the mitigating effect of sodium selenite on the toxicity of methylmercury. When selenite and mercuric chloride are co-adminstered, these elements react in the bloodstream forming complexes at an equimolar ratio. This reaction may explain the consistent equimolar ratio of selenium and mercury in tissues of seals and other marine mammals (Koeman et al., 1973; 1975) and mercury mine workers (Kosta et al., 1975). In nearly all marine fish sampled, the stoichiometric mercury-to-selenium ratio was less than 1. In contrast, freshwater fish accumulate mercury in such a way that the stoichiometric ratio was greater than 1 (Luten et al., 1980; Whanger, 1985; Cuvin-Aralar and Furness, 1991; Ikemoto et al., 2004). Despite extremely high values for mercury and selenium, sea fish are protected against toxicity of either element. It is interesting to note that adding selenium to lakes contami-
nated with mercury has been shown to be an effective remediation process (Paulsson and Lundbergh, 1989).
Selenide has one of the highest known mercury binding constants and will avidly partner with mercury (Whanger, 1985). Therefore, if dietary or tissue selenium is limited, selenide will be bound by mercury resulting in a lack of selenocysteine for incorporation into vital selenoproteins. The sequestered complex of mercury-selenium is highly insoluble and can be localized in lysosomes. Such sequestered material seems to be an inconsequential accumulation, with no toxicity to the animal.
It should be noted that ocean fish, though not lake fish, are a rich source of selenium. The US Department of Agriculture (USDA) ranked fish sources as 16 of the top 25 (out of a total of 1100) selenium-containing foods (USDA, Release 18. It has been suggested that the differences in mercury toxicity found in the Faroe Islands study compared to the Seychelles study may be due to the fact that mothers in the Faroe Islands ate whale meat, which is low in selenium, while mothers in the Seychelles studies ate seafood, which is rich in selenium (Ralston, 2005). This hypothesis is likely too simplistic, however, given that the Faroese also consume large amounts of seafood other than pilot whale.
Overall, selenium in the form of selenide is pivotal with regards to forming key selenoproteins and interacting with various heavy metals, such as mercury, to form a sequestered inert complex of mercury and seleno-compounds, i.e., bis(methylmercuric)selenide (BMS) (Yoneda and Suzuki, 1997; Watanabe, 2002). MeHg might be acting as a methyl donor, thereby sparing the amount of S-adenosylmethionine (SAM) required for methylation. The net effect would be demethylation of MeHg by selenide, which then could lead to other interactions between selenide molecules and newly formed ionic Hg (Gregus et al., 2001; Watanabe 2002). Evidence suggests that the two elements interact with protein through basic amino acids in the molecule and also that the protein may be one of the heparin-binding proteins (Yoneda and Suzuki, 1997).
RISKS ASSOCIATED WITH MORE ACUTE SEAFOODBORNE HAZARDS
Microbiological Hazards
The best measures of seafood safety in the United States are based on illness reports compiled by the CDC (Source: http://cdc.gov/foodnet/) and the respective epidemiology programs in each state. Complementary lists of seafoodborne illness were also compiled by the Center for Science in the Public Interest (CSPI) (DeWaal and Barlow, 2004). Although these data are compromised by limited reporting and a large portion of unidentified etio-
logical agents or nonspecific food vehicles, they remain the best measures of causes and trends in seafoodborne illnesses.
CDC estimates during the years prior to 1980 with less informative reporting suggested approximately 11 percent of all foodborne outbreaks (an outbreak involves two or more cases from a common source) implicated fish, mollusks, or crustaceans as the food vehicle (Bryan, 1980). Compilations of CDC data from 1978–1987 indicated fish and shellfish constituted only 10.5 percent of foodborne outbreaks and 3.6 percent of total cases (IOM, 1991). Estimates from the same data indicated “both [the percentage of] people made ill from beef (4) and turkey (3.7) exceeded total illnesses from seafood (3.5), whereas pork (2.7) and chicken (2.6) were each slightly lower. When shellfish (2.3 percent) and fish (1.2 percent) were considered separately, the number of reported cases from each was lower than for any other animal meat category” (IOM, 1991). These comparisons did not adjust for per capita consumption. FAO’s compilation of CDC’s data indicated that the number of cases remained higher for shellfish, but that outbreaks for fish, of which 90 percent could be linked to the cause, are more common (Huss et al., 2004). The association between exposure and illness is considered higher for seafood than for other foods due to the early onset of symptoms and the particular symptoms per types of seafood. This situation reinforces reporting of seafoodborne illnesses. Table 4-4 summarizes commonly encountered hazards and risks associated with classes of seafood consumed in the United States, and systems in place to control or minimize potential exposure risks.
Estimating Frequency of Seafoodborne Illnesses
CDC estimates for foodborne illnesses (76 million/year) and related deaths (5000/year) (Mead et al., 1999) indicated that the number of cases were reduced by 6.2 and 44.4 percent, respectively, from previous estimates from Archer and Kvenberg (1985), which were based on foodborne diarrheal diseases alone. These reports did not include any specific references to seafood other than data involving various food-related Vibrio infections. Although Vibrio-related illnesses are not a CDC-reportable disease and therefore may be underreported, Mead et al. (1999) included cases and outbreaks involving Vibrios, which frequently implicate seafood, particularly raw molluscan shellfish, as a likely vehicle. This study accounted for underreporting of foodborne illnesses through the use of multipliers (see Table 4-5). The less serious an illness experienced by an individual, the less likely they are to seek medical attention and thus minor illnesses are less likely to be reported. A low multiplier is needed to accurately depict the actual number of cases of a serious illness, such as those associated with Vibrio vulnificus.
Huss et al. (2004) compiled data from CSPI indicating consumption of molluscan shellfish caused the highest percentage of seafoodborne cases (individual illnesses) during 1990–1998, but the primary causative agent was reported in the collective category for noroviruses (see Table 4-6). The general norovirus category accounted for the large majority of seafoodborne outbreaks reported by CSPI (DeWaal and Barlow, 2004) from their survey of reported illness during 1990–2003. The most recent CDC report (2005a) suggested the incidence of infections involving Vibrio bacteria has been increasing (Figure 4-4), using the estimated number of total incidences based on laboratory-confirmed infections divided by the population estimates. The extent of the estimated affected population was equated to 15.2 percent of the US population. This report did not distinguish the Vibrio species or food vehicles involved, but it did suggest some of the Vibrio infections may have resulted from nonfoodborne sources, e.g., previous wounds.
With the exception of concerns for Vibrio and Norovirus infections, in general, the CDC report (2005a) does not reflect an increase in seafood-borne illnesses, particularly of microbial origin (most commonly associated with consumption of raw molluscan shellfish). Figures reported by CDC (2005a) are imprecise due to inclusion of other nonfoodborne causes and changes in state reporting. Since 1988, CDC has maintained only a voluntary Vibrio surveillance system for culture-confirmed infections in the states contiguous to the Gulf of Mexico. In 1999, the Foodborne and Diarrheal Disease Branch of CDC encouraged all state epidemiology programs to improve Vibrio reporting and the Council for State and Territorial Epidemiologists has drafted a resolution calling for more mandatory reporting of Vibrio-related illnesses (Personal communication, K. Moore, Interstate Shellfish Sanitation Conference, November, 2005).
Reducing Risk of Seafoodborne Illness
Vibrio-Associated Illness The Vibrio family of bacteria are indigenous to most coastal environments; the particular types and amounts present are influenced by salinity and water temperature (IOM, 1991). Filter-feeding animals, e.g., oysters, will take up chemical and microbial flora, including Vibrios, in their immediate environment. The two species of concern are Vibrio vulnificus (Vv) and Vibrio parahaemolyticus (Vp). Like the related Vibrio cholera species, Vv and Vp can live in warm seawater and have been isolated from oysters, clams, crabs, and finfish. In contrast, Vv can cause serious illness (wound infections, gastroenteritis, or a syndrome known as “primary septicemia”) and death in persons with pre-existing liver disease or compromised immune systems while Vp typically causes less severe illness. Vp can infect healthy consumers yet severe disease is rare.
TABLE 4-4 Current Seafood Safety Hazards, Controls, and Risks
|
Hazardous Seafooda |
Hazardb |
Severity to Consumers |
Occurrence and Trend |
|
Raw bivalve mollusks (oysters, clams, mussels) |
(1) Viruses, enteric bacteria (2) Vibrio vulnificus (3) Vibrio parahaemolyticus |
(1&3) Mostly mild gastroenteritis; certain types and serotypes can be more harmful for very few consumers (2) Severe for “at-risk” consumersc |
(1) Random by location and time; improper water classification; recreational harvests (2) Rare yet persistent; primarily warm-water products involving at-risk consumers (3) Sporadic; may be increasing |
|
Natural toxins Finfish (1&2) Mollusk (3) |
(1) Ciguatera (2) Scombroid poisoning (3) PSP, NSP, DSP, ASP |
(1) Moderate to severe and reported prolonged symptoms in some cases (2) Mild and short duration (3) Mild to severe relative to toxin type |
(1) Limited to certain fish species from certain areas; could increase with more imports (2) Limited but persistent for certain species subject to thermal abuse; could increase with more imports (3) Rare but can be very serious; could increase with global warming and related environmental changes |
|
Processed seafood |
(1) Salmonella (2) Listeria monocytogenes (3) C. perfringens (4) C. botulinum (5) Shigella (6) Staphylococcus aureus (7) HAV and NLV |
Usually mild; can be severe to very severe for (2) and (4), respectively, yet occurrence very rare |
Very limited and decreasing; more prevalent in ready-to-eat items; could increase with more and certain imports |
|
Allergies |
Host specific |
Host specific; can be mild to severe |
Seafood ranked in top four food allergies; could increase for preformulated, value-added products |
|
NOTE: PSP = paralytic shellfish poisoning; NSP = neurotoxic poisoning; DSP = diarrhetic shellfish poisoning; ASP = amnesic shellfish poisoning; HAV = hepatitis A virus; NLV = Norwalk-like viruses (Noroviruses) spp. aFish or shellfish, the consumption of which can lead to disease. Ranked in order of concern per occurrence and risk. |
|||
TABLE 4-5 Multipliers Used by CDC to Estimate Total Cases for Different Foodborne Bacterial Illnesses Based on Actual Reported Cases
|
Agent Causing Illness |
Multiplier for Estimated Cases |
|
Campylobacteria |
38 |
|
Clostridium perfringens |
|
|
Salmonella, nontyphoidal |
|
|
Staphylococcus aureus |
|
|
E. coli O157:H7 |
20 |
|
Shigella |
|
|
Vibrio, other spp. |
|
|
Bacillus cereus |
10 |
|
Clostridium botulinum |
2 |
|
Salmonella typhi |
|
|
Vibrio cholerae, toxigenic |
|
|
Vibrio vulnificus |
|
|
SOURCES: Derived from Mead et al., 1999; 2006. |
|
TABLE 4-6 Seafoodborne Diseases Traced to “Molluscan Shellfish” in the United States from 1990 to 1998, and Outbreaks and Cases for Which the Etiological Agent Has Been Identified
|
Agent |
Outbreaks |
Cases |
||
|
Total |
Percent |
Total |
Percent |
|
|
V. parahaemolytics |
18 |
27 |
733 |
22 |
|
Noro-/Norwalk-like virusa |
15 |
23 |
2175 |
66 |
|
PSP/toxin |
14 |
20 |
92 |
3 |
|
Salmonella |
6 |
9 |
183 |
6 |
|
Scombroid |
2 |
3 |
4 |
— |
|
Ciguatera |
3 |
5 |
5 |
— |
|
Shigella |
2 |
3 |
17 |
0.5 |
|
Campylobacter |
2 |
3 |
6 |
— |
|
V. vulnificus |
1 |
— |
2 |
— |
|
V. alginolyticus |
1 |
— |
4 |
— |
|
C. perfringens |
1 |
— |
57 |
2 |
|
Giardia |
1 |
— |
3 |
— |
|
Total |
66 |
93 |
3281 |
100 |
|
aNorovirus was recently approved as the official genus name for the group of viruses provisionally described as “Norwalk-like viruses” (NLV) (http://www.cdc.gov/ncidod/dvrd/revb/gastro/norovirus.htm). |
||||
FIGURE 4-4 Relative rates (compared with 1996) of laboratory-confirmed cases of Yersinia, Escherichia coli O157, Campylobacter, and Salmonella, by year; from Foodborne Diseases Active Surveillance Network, United States, 1996–2004.
SOURCE: CDC, 2005a.
More detailed assessment of the Vibrio illnesses from seafood can be found in state reports for Vibrio vulnificus (see Table 4-7). These reports provide more specific data for infections resulting from consumption of raw molluscan shellfish of commercial origin. With the exception of California, the majority of reported illnesses involve the oyster-producing regions about the Gulf of Mexico, due to the relative prevalence of Vv in warmer coastal waters. The number of Vv illnesses involving commercial shellfish harvests per year across all states reporting (32) during 1995–2004 averages less than one case per year (0.98 cases/year/state). The justification for the persistent concern about Vv stems from the potentially severe consequences for consumers in a higher risk category for infection (e.g., immunocompromised) for whom there may be as much as a 50 percent mortality rate (IOM, 1991; Hlady and Klontz, 1996).
California, Florida, Louisiana, and Texas represent “core states” designated in a Vv management plan designed by FDA through cooperation with the Interstate Shellfish Sanitation Conference (ISSC, 2002) to reduce Vv illnesses from raw oyster consumption (see Table 4-8). This plan was
TABLE 4-7 Individual Reported Vibrio vulnificus Cases of Illness Involving Commercial Oyster Products
complemented with a formal risk assessment conducted for Vv (FAO/WHO, 2001). The plan includes specified industry performance goals for illness reduction rates and consequences if the goals are not attained.
Commercial operations have responded with post-harvest processing procedures such as high-pressure, low-temperature pasteurization and
TABLE 4-8 Abbreviated Table of Compliance for Core States as Specified in the ISSC’s Vibrio vulnificus Management Plan
|
Deadline |
Post-Harvest Treatmentsa |
Illness Reductionsb |
Florida Examplec |
|
December 2004 |
25% capacity |
|
9/year—baseline |
|
2005–2006 |
|
40% (average) |
5/year—goal |
|
December 2006 |
50% capacity |
|
|
|
2007–2008 |
|
60% (average) |
4/year—goal |
|
|
If the 60 percent illness reduction rate is not collectively achieved by 2008, additional controls can be imposed, including harvest restrictions or closures relative to water temperatures and special labels designating product to be shucked by a certified oyster dealer. |
||
|
NOTE: Core states are California, Florida, Louisiana, and Texas. aPost-harvest treatment “capacity will be based on all oysters intended for raw, half-shelled market during the months of May through September harvested from source states, to include the capacity of all operational plants and the capacity of plants under construction.” bIllness reductions will be based on the average illness rate for years 1995–1999 of 0.036/ million persons, using data from California, Florida, Louisiana, and Texas. Adjustments in methodology can be adopted based on further review. cThe Florida example indicates the performance goals (total reported illnesses per year) that must be attained for compliance relative to the initial established baseline. SOURCES: ISSC, 2001, 2002, 2003b. |
|||
“frosting” methods to kill Vv in the oysters as part of the illness reduction efforts. FDA (2005a) has recently approved the use of irradiation as another post-harvest processing option.
This risk management plan also includes recommendations for a public education component including programs targeting consumers of raw oysters, both those with and those without health conditions that increase their risk of Vv infection. In the states that were required to develop risk-management plans, Flattery and Bashin (2003) conducted a survey to elicit new information about media exposure, attitudes, and consumption behavior from consumers of raw oysters. They found that (1) generally, consumer awareness of who should avoid consuming raw oysters is limited; (2) many at-risk consumers are already taking some actions, albeit ineffective, to avoid illness; (3) one in three consumers are eating raw oysters less frequently. International Conference on Emerging Infections and Diseases (2006) also reported a decline in “risky food consumption.” This survey response showed a decline of one-third in the number of individuals who reported consuming foods, including raw oysters, associated with a higher risk for foodborne disease.
Flattery and Bashin (2003) determined that to increase the effectiveness of the educational component of the plan, key messages should identify at-
risk consumers; communicate effective action to prevent illness (i.e., refrain from eating raw oysters); and address popular myths about preventing illness.
It may be difficult to determine precisely the reduction in illnesses achieved because of the large standard deviation about the annual mean (the average illness rate for 1995–1999 of 0.036/million persons, based on data from California, Florida, Louisiana, and Texas) reported by ISSC (2001, 2002). At present, the number of Vibrio illnesses reported annually is small. For example, in Florida the 40 and 60 percent illness reduction goals represent a drop in reported illnesses from nine/year to five/year by 2006, and to four/year by 2008 (see Table 4-8 and Table 4-9). The recent increase in reporting from states that had not previously monitored Vibrio illnesses shows that national trends for reported illnesses are similar to those for the core states.
Management plans are also being considered to address illnesses due to Vibrio parahaemolyticus (Vp) from raw shellfish. Illnesses resulting from this source are less severe than for Vv, but occurrence is not confined to at-risk consumers. Further, international occurrence of certain Vp strains suggests the possibility of a pandemic infection because these strains have been identified as deriving from a known pathogenic strain of Vp (Chowdjury et al., 2000). Vp is a leading cause of seafoodborne illnesses in Japan and eastern Asian countries noted for higher consumption of raw seafood.
Infections in the United States are more sporadic and have involved crabs, shrimp, and crayfish, with cross-contamination of raw and previously cooked product as a contributing factor, although raw oysters remain the primary vehicle for Vp infections. Vp elicited little response from the National Shellfish Sanitation Program (NSSP) prior to two major outbreaks in the Gulf Coast region in 1997 and 1998, some cases of which involved a previously unreported and more virulent serotype (03:K6) from Asia (FDA, 1999). Recent outbreaks of Vp have also occurred in the more temperate waters of the northwest United States and Canada (Fyfe et al., 1998; CDC, 2006a,b). Infection prevention is complicated by the ability of Vp to grow in the oysters after harvest, particularly in the absence of adequate temperature control. Preventive measures for Vp are similar to those for Vv and include cooking, post-harvest temperature controls, and consumer and processor education and processing innovations. In the absence of regulatory mandates the FDA offers voluntary guidance indicating that no more than 10,000 bacteria per gram of raw shellfish (FDA, 2001b) should be present.
Other Bacterial Hazards
In addition to Vibrios, a variety of potentially pathogenic bacteria have been associated with seafood safety risks, though actual occurrence is very
TABLE 4-9 Human Pathogens Associated with Seafood
|
Pathogens |
Isolated from Seafoods |
Proven Pathogen in Seafood |
Pathogen Sourcea |
|
Organisms That Can Cause Disease in Normal, Healthy Adults |
|||
|
Bacteria |
|||
|
Vibrio cholerae O1 |
Yes |
Yes |
1, 2 |
|
Vibrio chloerae non-O1b |
Yes |
Yes |
1 |
|
Vibrio parahaemolyticus |
Yes |
Yes |
1 |
|
Vibrio mimicus |
Yes |
Yes |
1 |
|
Vibrio fluvialis |
Yes |
Yes |
1 |
|
Vibrio furnissii |
Yes |
Yes |
1 |
|
Vibrio hollisae |
Yes |
Yes |
1 |
|
Salmonella typhic |
Yes |
Yes |
2, 3 |
|
Salmonella (nontyphoidal) |
Yes |
Yes |
2, 3 |
|
Campylobacter jejuni |
Yes |
Yes |
2, 3 |
|
Escherichia coli |
Yes |
No |
2, 3 |
|
Yersinia enterocolitica |
Yes |
No |
2, 3 |
|
Clostridium botulinum |
Yes |
Yes |
2, 3 |
|
Shigella |
Yes |
Yes |
2, 3 |
|
Staphylococcus aureus |
Yes |
Yes |
3 |
|
Hekminths |
|||
|
Anisakis simplex |
Yes |
Yes |
1 |
|
Other helminths |
Yes |
Yes |
1 |
|
Viruses |
|||
|
Poliovirus |
Yes |
No |
2 |
|
Other picornaviruses |
Yes |
No |
2 |
|
Norwalk/Snow Mountain/small round viruses (SRVs) |
No |
Yes |
2 |
|
Enteral non-A, non-B, hepatitis |
No |
Yes |
2 |
|
Hepatitis A |
Yes |
Yes |
2, 3 |
|
Organisms That Cause Disease Most Often in Special Population Groups |
|||
|
Vibrio vulnificusd |
Yes |
Yes |
1 |
|
Rotaviruse |
Yes |
No |
2 |
|
Listeria |
Yes |
No |
1, 3 |
|
Organisms with Uncertain Roles as Foodborne Pathogens |
|||
|
Aeromonas hydrophilaf |
Yes |
Yes |
1 |
|
Plesiomonas shigelloides |
Yes |
Yes |
1 |
|
Edwardsiella tarda |
Yes |
No |
1 |
|
a(1) Harvest water/associated with naturally occurring aquatic bacteria; (2) harvest water/associated with fecal pollution; (3) associated with processing and preparation (cross-contamination or time/temperature abuse, infected food handlers). bCauses gastroenteritis in normal, healthy hosts; can cause septicemia in persons in high-risk groups. cPrimarily of historical association in the United States, but remains a problem in some foreign countries and could affect imports. dIllness usually confined to high-risk groups. eIllness generally occurs in children under the age of 2; older persons are usually immune. fAeromonas can cause serious wound infections and septicemia; however, conclusive data on its role as a cause of gastroenteritis are lacking. Studies suggesting that it is a gastrointestinal pathogen have not implicated seafood as a risk factor for illness. SOURCE: IOM, 1991. |
|||
rare or not reported due to lack of severity of symptoms (see Table 4-9) (IOM, 1991). The two principle pathogens of concern are Salmonella spp. and Listeria monocytogenes.
Salmonella is a bacterium of widespread occurrence in animals, especially in poultry and swine. Environmental sources of the organism include water, soil, insects, factory and kitchen surfaces, animal feces, raw meats and poultry, and raw seafoods. S. typhi and the paratyphoid bacteria normally cause septicemia and produce typhoid or typhoid-like fever in humans. Other forms of salmonellosis generally produce milder symptoms (Source: http://www.cfsan.fda.gov/~mow/chap1.html).
Listeria monocyotogenes is a bacterium that can cause a serious infection in humans called listeriosis. Foodborne illness caused by L. monocytogenes in pregnant women can result in miscarriage, fetal death, and severe illness or death of a newborn infant. Others at risk for severe illness or death are older adults and those with weakened immune systems. L. monocytogenes can grow at refrigerator temperatures and is found in ready-to-eat foods (Source: http://www.cfsan.fda.gov/~dms/adlister.html 2003).
Federal regulation prohibits the sale of any raw or cooked seafood products contaminated with any Salmonella, or cooked, ready-to-eat seafood products contaminated with any L. monocytogenes (CFSAN, 2001) (see Appendix Table B-4). The zero tolerance policy for Salmonella on any seafood product is historically based on concerns for unsanitary practices that contaminated a food after harvest. The presence of any Salmonella on seafood from freshwater or saltwater harvests is considered an adulterant. Inland aquacultural production can expose farmed seafood to Salmonella from other animal sources including neighboring wildlife. Koonse et al. (2005) evaluated both product and environments (source water and grow-out pond water) from shrimp aquaculture across six countries, and found a significant association between the concentrations of Salmonella and both fecal coliforms and E. coli. Nevertheless, the occurrence of seafoodborne salmonellosis is rare. Reported cases are usually the result of cross-contamination or unsanitary handling practices (Koonse et al., 2005). Proper sanitation that includes adherence to Hazard Analysis and Critical Control Point (HACCP) regulatory requirements for daily sanitary monitoring and records plus cooking of seafood appear to be adequately controlling Salmonella in seafood.
Likewise, proper sanitary practices and cooking temperature remain the primary control points to prevent potential illnesses due to contamination from particular types and amounts of L. monocytogenes (Lm) that have been found on certain seafood products (Gombas et al., 2003). The most likely vehicle of transmission is previously cooked and ready-to-eat (RTE) seafood products with prolonged refrigerated storage that could al-
low further growth of these more cold-tolerant pathogens. The incidence of reported illnesses for L. monocytogenes for all foods significantly declined by 32 percent from 1996 to 2005 (CDC, 2006a), and there are few reports involving seafood products confirmed by CDC.
Regulatory control of C. botulinum includes processing for canned products, reduced oxygen packaging, smoking, fermenting, and pickling (FDA, 2001a). The C. botulinum is destroyed by heat processing. Botulinum poisoning is rare and usually involves previously cooked and ready-to-eat (RTE) products that have not been properly heat processed. Through 2004, there have been no documented cases of botulism from any fresh seafood product regardless of packaging (Austin and Smith, 2006) with one exception. This involved a whole fresh fish including uncooked viscera, prepared and consumed in Hawaii (CDC, 1991). None of the 19 incidences of botulism cited by Bryan (1980) involved fresh fish. In addition, the CDC (2005a) reported no incidences of C. botulinum for foodborne illnesses cited.
The remaining bacteria with proven pathogenicity in seafood have not posed any significant risk beyond occurrences and causes recognized with other foods. The controls for these hazards are similar in terms of sanitation, proper cooking, and proper refrigeration. They currently pose no unique trend in occurrences that suggest increased exposure risk through seafood consumption.
Viruses
There are a large number of seafoodborne illnesses of unknown etiology generally classified as norovirusal. Evidence is lacking due to limitations in the methodology for culturing and enumerating viruses; further identification is difficult. Current controls rely on monitoring of harvest waters used in production of molluscan shellfish intended for raw consumption (ISSC, 2003a). Water classification or approval protocols rely on indicators associated with the presence of viruses rather than actual measures for a particular virus. Contamination of water with human fecal matter on or near oyster beds has resulted in shellfishborne “Norwalk-like” viruses (NLV) and hepatitis A (HAV) infections in consumers of raw oysters harvested from the contaminated waters (Kohn et al., 1995).
Monitoring of water for indicators associated with the presence of viruses remains the primary control for products to be consumed raw because most new post-harvesting processing methods, including irradiation, used to reduce Vv in raw oysters have not been proven effective for reduction of viruses. Improvements in water classification programs may include advancing methodologies using PCR-based identification and monitoring for viruses in water and seafood products.
Parasites
Consumption of raw or undercooked seafood products that had not been previously frozen has been implicated in certain human parasitic infections. Table 4-10 lists the parasites and seafood choices that have been involved in previous documented illnesses. Incidence of parasitic infection is far more common in regions of the world where raw consumption is more frequent (Table 4-11). Seafoodborne infections are more prevalent in these regions than in the United States due to agricultural practices and reliance on freshwater sources that support the life cycle of certain hazardous parasites (Rodrick and Cheng, 1989; Sakanari et al., 1995). Since their adoption, HACCP programs, which include specific controls to prevent parasite infections, suggested incidence levels were underreported and expected to increase as consumer trends favored more consumption of raw selections (Jackson, 1975; Olson, 1986; McKerrow et al., 1988). The American Gastroenterological Association (AGA) surveyed approximately 30 percent (996 members) of the active AGA practitioners located in coastal states along the Pacific, Atlantic, and Gulf of Mexico, areas prone to parasite exposure (Personal communication, G. Hoskins, FDA Office of Seafood, December 2005). Survey respondents (over 58 percent) estimated
TABLE 4-10 Parasites and Products Involved in Documented Incidences of Parasitic Infection
|
Fishborne parasites involved in human infections resulting from consumptiona |
Some raw and undercooked seafood dishes involved in parasitic infections for products and recipes that are not previously frozenb |
Diphyllobothrium latum Diphyllobothrium pacificum |
|
Clonorchis sinensis Opisthorchis viverrini Heterophyes heterophyes Metagonimus yokogawai |
|
Gnathostoma spinigerum Capillaria philippinensis Anisakis simplex Phocanema spp. |
|
|
SOURCES: aHigashi, 1985; FDA, 2001a; bSakanari et al., 1995; FDA, 2001a. |
|
TABLE 4-11 Estimated Annual Occurrence of Parasitic Infections Due to Consumption of Seafood, Based on Original Compilation by FDA
|
Parasite |
Worldwide |
USA |
Source |
|
Tapeworm |
9,000,000 |
100,000 |
Bylund, 1982 |
|
Fluke |
20,000,000 |
Relatively low |
Rim, 1982 |
|
Roundworm |
2000+ |
50 |
Higashi, 1985 |
|
SOURCE: As referenced in FDA, 1987. |
|||
the number of cases over 24 months during 1998–2000 at 38 parasitic infections, of which 17 were anisikiasis, 16 were diphyllobothriasis, and 5 were pseudoterranoviasis. In the final report, the AGA estimated the actual number of infections would likely be 270 cases. This survey is considered one of the most current estimates for seafoodborne parasite infections in the United States but as a single survey, it is also considered underreporting. Nevertheless, seafoodborne parasitic infections are not common in the United States.
The guidelines for seafood processing and handling that accompanied the FDA mandate for HACCP regulations introduced additional specific controls to further prevent seafoodborne parasitic infections (FDA, 2001a). The FDA identified seafood species of concern (Table 4-12) and controls for HACCP program compliance (Table 4-13). Cooking and freezing had previously been reported as effective methods to kill parasites in order to
TABLE 4-12 Seafood Identified by the FDA that Could Involve Potential Parasite Hazards If Consumed Raw and Not Previously Frozen
|
Bass, Sea Caplin Cobia Cod Corvina Eelpout Flounders,a Sole, Dab, and Fluke Grouper Halibut Herrings Hogfish Jacks Kahawai Mackerels Monkfish Mullet Perch, Ocean Plaice Pollock Rockfish Sablefish Salmona Scad Sea trout Snapper Sprat Thorny head Tomcod Tongue sole Trevally Trout Tunab Turbot Wolffish Octopus Squid Snails |
|
NOTES: The general market names can include numerous species from various locations. The original sources should be referenced for actual species identified. aIncludes wild and aquacultured sources if fresh fish or plankton used as feed. bOnly applies to small tuna species; excludes large tuna species such as the yellowfin, bigeye, bluefin, and albacore. SOURCE: FDA, 2001a. |
TABLE 4-13 FDA Recommended Controls to Reduce or Eliminate Potential Parasite Hazards from Seafood
|
Procedure |
FDA Recommendation |
Comment |
|
Parasite Removal |
Trimming away of suspect and identified portions and/or portions identified with candling |
Not recommended as sole preventive method |
|
Cooking |
Heating of raw fish sufficient to kill bacterial pathogens |
FDA Food Code (2005e) definition for cooked seafood is an internal product temperature of 145°F for 15 seconds |
|
Freezing |
Freezing and storing at −4°F or below for 7 days or freezing at −31°F or below until solid and storing at −31°F or below for 15 hours, or freezing at −31°F or below until solid and storing at −4°F or below for 24 hours |
FDA’s Food Code recommends these freezing conditions to retailers who provide fish intended for raw consumption |
|
SOURCE: FDA, 2001a, International Food Safety Council (http://www.foodsafety.gov/~dms/sept/99-week1.html). |
||
prevent infections (Bier, 1976; Deardorff and Throm, 1988; FDA, 2001a). The current HACCP program requires freezing for certain species intended for commercial use as sushi and related raw seafood products (FDA, 2001a). Given the widespread adoption of HACCP and infrequent incidence of reported infections, concern about parasitic infection may not be deterring consumers from raw seafood consumption. Consumers may still choose to consume raw seafood products that have not been frozen previously.
Naturally Occurring Toxins
Ciguatera and Scombroid
Ciguatera and scombrotoxin are the two most persistent seafoodborne toxicants (IOM, 1991). Ciguatoxins, acquired through the local environmental food chain prior to harvest, may involve a variety of toxins from certain dinoflagellates. Ciguatera arises in certain fish harvested from specific tropical to subtropical regions about South Florida, the Caribbean region, and Hawaii. Reports in Florida suggest there is no evidence for increasing incidence (Personal communication, R. Hammond, Florida Department of Health, Tallahassee, FL, December 2005) (see Table 4-14). These data do not distinguish harvest source, but they do identify the more probable species of concern. Occurrence involves recreational as well as commercial harvests. Risk of ciguatera may increase with illegal recreational sales (not subject to HACCP controls) and with increasing imports of certain fish from affected areas.
TABLE 4-14 Reported Incidences for Ciguatera and Scombroid in the United States and by Selected States
|
Years and Locations |
Ciguatera |
Scombroid Poisoning |
||
|
Outbreaks |
Cases |
Outbreaks |
Cases |
|
|
1993–1997a |
60 total |
205 total |
69 total |
297 total |
|
USA |
Avg-12/year |
Avg-41/year |
Avg-14/year |
Avg-59/year |
|
1990–1998b |
|
|
|
|
|
Hawaii |
73 total |
260 total |
46 total |
287 total |
|
|
Avg-8/year |
Avg-29/year |
Avg-5/year |
Avg-32/year |
|
Florida |
16 total |
82 total |
10 total |
55 total |
|
|
Avg-2/year |
Avg-9/year |
Avg-1/year |
Avg-6/year |
|
Floridac |
|
|
|
|
|
1994 |
3 |
13 |
5 |
14 |
|
1995 |
2 |
4 |
6 |
55 |
|
1996 |
8 |
30 |
5 |
9 |
|
1997 |
9 |
30 |
4 |
11 |
|
1998 |
5 |
37 |
5 |
14 |
|
1999 |
5 |
21 |
5 |
19 |
|
2000 |
9 |
31 |
3 |
10 |
|
2001 |
5 |
27 |
8 |
20 |
|
2002 |
5 |
10 |
3 |
4 |
|
2003 |
3 |
5 |
6 |
35 |
|
|
Avg-5.4/year |
Avg-21/year |
Avg-5.0/year |
Avg-19/year |
|
SOURCES: aOlson et al., 2000; bHuss et al., 2004; cPersonal communication, R. Hammond, Florida Department of Health, Tallahassee, FL, December 2005. |
||||
Subsequent handling, storage, or cooking cannot substantially reduce the risk. The toxic dose and consumer susceptibility remain in question while regulatory controls simply call for avoidance of certain fish from suspect areas. Absence of testable material, errant recall, and consumer misnaming can confuse species identification in reported illnesses. Despite product claims for utility, there are no reliable test kits to screen for ciguatera due to limited specificity for the toxins (Hungerford, 2005). Species avoidance may be the best control to reduce the potential hazard (Lange et al., 1992; Lehane, 1999). Barracuda is a common culprit that should be avoided (Morton and Burklew, 1970). Federal regulations for controls of ciguatoxins advise against consumption of certain species, and avoidance of fish from harvest locations with prior evidence of occurrence (FDA, 2001a). This advice is compromised by lack of area designations, unpredictable changes in the local food chains, and fish migration. Future controls could involve species restrictions from designated areas.
Scombroid poisoning, also known as histamine poisoning, involves thermal abuse of certain fish resulting in elevated levels of histamine con-
centrations that can invoke allergic-type reactions in susceptible consumers of raw or cooked fish. Cooking does not diminish these toxins. The primary fish involved include tunas and mackerels from the Scombridae family of fish—thus the name—and related species mahi-mahi (Coryphaena hippurus), escolar (Lepidocybium flavobrunneum), and others. The common feature distinguishing these fish is a higher proportion of free amino acids, i.e., histidine, lysine, and ornithine, naturally occurring in the muscle tissue, which can be decarboxylated to histamine, cadaverine, and putrascine. This conversion is driven by temperatures that allow growth of certain bacteria to generate the decarboxylating enzymes. Although regulatory action levels for histamine content (<50 ppm) have been established to prevent illnesses, cadaverine and putrascine have the potential to cause illness even in the absence of histamine (FDA, 2001a). Inadequate cooling at the point of harvest is considered the primary problem, and subsequent abuse can increase the potential hazard. Temperature control from harvest until consumption is recommended by the FDA (2001a). In the United States, HACCP mandates thermal controls from harvest through processing; most illnesses which continue to appear involve recreational harvests and imports. The incidences of illness could increase as more supply of affected species is imported and the illegal sale of recreational fish is not addressed with pertinent enforcement.
Shellfish Toxins
Naturally occurring toxins that have been associated with illnesses resulting from the consumption of certain molluscan shellfish such as oysters, clams, and mussels harvested from locations with specific environmental conditions include:
-
Paralytic Shellfish Poisoning (PSP)
-
Neurotoxic Shellfish Poisoning (NSP)
-
Diarrhetic Shellfish Poisoning (DSP)
-
Amnesic Shellfish Poisoning (ASP)
The filter-feeding mollusks accumulate the toxins in their viscera from the waters harboring naturally occurring marine algae (phytoplankton) that produce the toxins. Occurrence has involved both domestic and imported marine mollusks from tropical and temperate waters, depending on the particular species of phytoplankton and water conditions. Recent international reports include a comprehensive assessment of the potential occurrences that warrant closer scrutiny of particular algal species in various locations (FAO/IOC/WHO, 2004).
Related illnesses are rare but poisoning from shellfish toxins can be severe and deadly. Cooking is not considered sufficient to control potential
toxic levels in seafood (FDA, 2001a). Regulatory monitoring programs have been effective; but new toxins and plankton blooms are emerging worldwide, particularly in areas less subject to surveillance. Incidences of toxicity could increase without controls, although the likelihood for an outbreak is low. Appendix Table B-4 identifies tolerances and action levels set by federal agencies for potentially problematic products.
Chemotherapeutants
Most aquaculture operations depend on the use of various chemotherapeutants to control infectious diseases (FAO/NACA/WHO Study Group, 1999; FDA, 2001a). Aquaculture initially relied upon the same antimicrobials employed for production of beef and poultry and other land-based farming. The resultant food safety concerns, as for land-based agriculture, include possible toxic residue in the edible portions, contributions to potential antibiotic-resistant diseases (for both animals and consumers), and concomitant issues involving environmental contamination. Although the volume of chemotherapeutants used in aquaculture is far less than for other medical practices and agricultural production, international aquacultural use with less scrutiny may increase. Product seizures due to the presence of chemotherapeutants in some imported farm-raised seafood have occurred. (Allshouse, 2003; http://www.fda.gov/ora/oasis/3/ora_oasis_i_16.html; http://www.fda.gov/ora/oasis/1/ora_oasis_i_16.html).
Compounds of concern have included chloramphenicol, nitrofurans, fluoroquinolone, malachite green, and others (Table 4-15). All of these antimicrobial/antifungal agents have been used at some time for aquacultural production in the United States, prior to the implemention of restrictions by federal agencies (FDA, 2005f). The established level of controls is zero toler-
TABLE 4-15 Antimicrobial/Antifungal Agents Used at Some Time for Aquaculture Production in the United States
ance, based on the most current limits for analytical detection (Hanekamp, 2003), which currently range in parts per billion (ppb) for residuals. Similar limits are in place in other developed nations that depend on seafood imports (Kulkarni, 2005).
The actual food safety risk resulting from the use of chemotherapeutants in aquaculture has been difficult to assess for lack of surveillance for the types and extent of use, and uncertainty about the hazards (FAO/NACA/ WHO Study Group, 1999; Caprioli, 2000; Hanekamp, 2003). Toxic effects from the very low, ppb levels encountered in some aquacultured foods have been questioned (Hanekamp, 2003). Some studies have suggested probable transmission of antimicrobial-resistant bacteria in aquacultured food (Ervik et al., 1994; Weinstein et al., 1997; Angulo, 1999; Duran and Marshall, 2005). Yet recent reviews compiled by the Institute of Food Technologists (ITF, 2006) indicate the use of “chemical and biological antimicrobials and physical preservation systems has been remarkably successful in providing safe foods and has not been compromised by the occurrence of resistent microorganisms.” The list of chemotherapeutants approved for use in aquaculture is limited and there is strict monitoring of finished products.
Analytical procedures for detecting chemotherapeutants in the ppb range are expensive and time-consuming which may deter routine sampling of aquacultured products. Prevention of illegal use of chemotherapeutants may be achieved through education and development of “best aquaculture practices” (Florida Department of Agriculture and Consumer Services, 2005). Programs are emerging to address this need in both domestic (Otwell et al., 2001; ACC, 2004) and international settings (http://www.gaalliance.org/resp.html; http://www.aquaculturecertification.org/index.html; Otwell et al., 2001). Agencies in the United States have developed programs to advance approval and use of additional chemotherapeutants, as exemplified by the recent recognition for use of florfenicol in catfish farms (FDA, 2005b).
Seafood Allergens
According to the recommended definitions for adverse food reactions (Anderson, 1986; O’Neil and Lehrer, 1995; Adverse Reactions to Food Committee, 2003), a seafood allergy involves an immunologic reaction following exposure to a seafood. The true prevalence of seafood allergies in the United States is unknown and difficult to estimate (Bush, 1995), although they remain among the most common food-induced allergies (Taylor and Bush, 1988; O’Neil et al., 1993; Hefle, 1996) (see Table 4-16). They are more commonly associated with adults (Taylor and Bush, 1988). In general, the prevalence of food allergies is overestimated (Sampson, 1992; O’Neil and Lehrer, 1995) for lack of proper diagnosis or confusion with other food sensitivities, but actual occurrence of seafood allergies is estimated
TABLE 4-16 Most Commonly Implicated Foods in Food Allergy Listed by Most Common Age Group Involved According to the Original Source
|
Adults |
Children |
|
Peanuts |
Cow milk |
|
Tree nuts |
Eggs |
|
Soybeans |
Soybeans |
|
Fish |
Peanuts |
|
Crustaceans |
Wheat |
|
|
Tree nuts |
|
SOURCE: Hefle, 1996. |
|
to affect less than 2 percent of the US population (Hefle, 1996). Of this group, 280,000 to 500,000 consumers may be at risk for developing allergic reactions to seafood (Lehrer, 1993; O’Neil and Lehrer, 1995). Since exposure is the mediating factor, occurrence tends to be more prevalent near coastal regions and will likely increase as per capita seafood consumption increases (Lehrer, 1993; O’Neil et al., 1993; O’Neil and Lehrer, 1995).
Exposure can involve ingestion, inhalation (of vapors), or product handling for consumption or occupation. Likewise, potential exposure can be hidden as the presence of the particular seafood item may not be obvious or expected due to an unidentified ingredient or misidentified ingredients (i.e., fish-based surimi used in a “crab” salad). It can also result from cross-contamination of nonallergenic foods from handling either with the same improperly cleaned utensils or through subsequent cooking in the same containers or cooking media (frying oil or boiling water) as seafood (O’Neil and Lehrer, 1995; Hefle, 1996).
Similar food intolerances that are misidentified as a seafood allergy can involve an abnormal physiological or sensitive response to components accompanying the seafood (Taylor and Nordlee, 1993). Exposure to sulfiting agents is a common suspect. Sulfites are among the most widely used food additives in the food industry (IOM, 1991; Otwell et al., 2001). They are approved for use in preventing discoloration caused by indigenous enzyme activity on shrimp, lobsters, and other crustaceans (FDA, 2001a). If the sulfite residual on certain foods is excessive and not bound to the food matrix, exposure for certain asthmatic consumers could result in serious reactions. The prevalence of such reactions has been estimated at approximately 3.9 percent of asthmatic patients (Bush et al., 1986). Adverse reactions to sulfite residuals on properly treated seafood are rare, since the sulfiting agents are usually bound to the food protein matrix and are not readily released in the throat or nasal areas during consumption. Regulatory HACCP mandates
also specify requirements for distinct labeling of any seafood exposed to sulfiting agents.
Consumer awareness and labeling remain the most effective measures to prevent exposure to seafood that could elicit a food sensitivity response. Commercial practices for dual processing or preparation of other foods in facilities or with utensils used for seafood must avoid potential cross-contamination that could result in unanticipated exposures. Requirements to identify seafood or any use of seafood ingredients, as well as certain food additives, have been emphasized by the HACCP mandate (FDA, 2001a) requiring appropriate hazard analysis to identify any potential food sensitivity risks controlled through proper cleaning, product segregation, or product identification in order to prevent a potential hazard.
Adverse Effects Associated with Omega-3 Supplementation
While there is extensive research suggesting health benefits from the consumption of EPA/DHA found in fish oils, there are also data that indicate that overconsumption of fish oils could have adverse consequences. Evidence suggests that EPA and DHA may increase bleeding time, specifically by reducing platelet aggregability, and prolonged bleeding times in humans whose diets were supplemented with fish oil have been observed (e.g., Jensen et al., 1988; Rodgers and Levin, 1990; Harris et al., 1991). After reviewing this literature, FDA concluded that prolonged bleeding is not a significant risk at levels of consumption of up to 3 grams per day of EPA and DHA (Source: http://www.cfsan.fda.gov/~dms/ds-ltr11.html). This conclusion was the basis for FDA’s recommendation, which remains in force, that consumption of EPA and DHA combined should be limited to 3 grams per day, of which 2 might come from supplements.
Other potentially adverse effects of excessive consumption of fish oils include reduced glycemic control among diabetics, increased levels of lowdensity lipoprotein (LDL) cholesterol among diabetics and hyperglycemics, and immunosuppressive effects. FDA determined that limiting consumption of EPA and DHA to 3 grams per day would protect against these effects also.
Since some contaminants that may be found in seafood are lipophilic, including PCBs, DDT and its metabolites, DLCs, and polyaromatic hydrocarbons (PAHs), they may tend to concentrate in the fish oil. It is important to recognize that dietary supplements, including fish oils sold as supplements, are subject to the same regulations regarding adulteration as are conventional foods. A food is considered adulterated if it “bears or contains any poisonous or deleterious substance which may render it injurious to health” or if it “has been prepared, packed, or held under insanitary condi-
tions whereby it may have become contaminated with filth, or whereby it may have been rendered injurious to health” (21 USC 342(a)(1) & (4)).
Fish-oil supplements are regulated by FDA under the provisions of the Dietary Supplement Health and Education Act (DSHEA). This law provides that no FDA safety notification is needed for dietary supplement ingredients that were already on the US market prior to October 15, 2004. Fish oils are “grandfathered” under this provision, and thus there are no standards of identity for commercial fish-oil dietary supplements. Further, there is no provision under any law or regulation that requires a firm to disclose to FDA or consumers the information they have about the safety or purported benefits of their fish-oil products.
FDA is, however, empowered to remove from the market any fish-oil supplement that is not of adequate purity to ensure consumer safety under normal conditions of use. Since FDA has limited resources to analyze the composition of food products, including dietary supplements, it focuses these resources first on public health emergencies and products that may have caused injury or illness. Enforcement priorities then go to products thought to be unsafe or fraudulent or in violation of the law.
Fish-oil products, as opposed to fish themselves, can be processed to remove undesirable constituents. An industry trade association, the Council for Responsible Nutrition, established voluntary standards for its members in October of 2002. These standards limit concentrations of contaminants in fish-oil products as follows:
-
DLCs: ≤2 pg TEQ/g
-
PCBs: ≤0.09 µg/g
-
Heavy metals (lead, cadmium, mercury, arsenic): all <1 µg/g
Data submitted to FDA by the National Fish Meal and Oil Association on pesticide and PCB analysis in fish oil, conducted under Title 21, Code of Federal Regulations, Parts 109 and 509 “Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed,” indicated that multiple samples of menhaden and fish oil (refined and crude) did not contain detectable levels of a panel of pesticides, PCBs, and dioxins (Source: http://www.fda.gov/ohrms/dockets/dailys/02/Jul02/070202/99p-5332_sup0003_01_vol1.pdf).
Summary of Evidence
In summary, while certain hazards associated with specific species (e.g., scombroid poisoning) and lack of compliance with food safety guidelines (e.g., eating raw molluscan shellfish) persist, reviews of reported seafoodborne illnesses indicate that more acute seafood safety hazards are not
increasing. This trend seems to be due in part to the food safety control measures mandated since 1997 (e.g., HACCP and monitoring of sanitation control procedures); the new labeling requirements providing educational support; and specific management plans implemented by regulatory and industry partnerships to address the more serious illnesses associated with consumption of raw molluscan shellfish. However, the potential for misuse of chemotherapeutants in domestic and imported aquaculture products is a source for concern about the presence of toxins and increased antimicrobial resistance in seafood, particularly in light of increasing dependence on aquacultured products.
CHAPTER CONCLUSION
The committee’s review of evidence on risks associated with consumption of seafood drew on current research reports and reviews, published reviews from stakeholder groups, invited presentations made to the committee, and correspondance with experts in areas relevant to the statement of work. One component of the committee’s charge was to identify and prioritize the potential for adverse health effects from both naturally occurring and introduced toxicants in seafood. The conclusion from the committee’s review of evidence is that, among chemical contaminants, methylmercury presents as a greater concern for adverse health effects, whereas the risk associated with dioxins and PCBs in seafood remains uncertain due to both the availability of evidence and the strength of the findings, and that microbial hazards, particularly those associated with handling and cooking practices, pose a more controllable yet persistent seafood-related risk from the standpoint of public health concerns.
FINDINGS
-
Levels of contaminants in seafood depend on several factors, including species, size, harvest location, age, and composition of feed. Methylmercury is the seafoodborne contaminant for which the most exposure and toxicity data are available; levels of methylmercury in seafood have not changed substantially in recent decades. Exposure to dioxins and PCBs varies by location and vulnerable subgroups (e.g., some American Indian/Alaskan Native groups living near contaminated waters) may be at increased risk. Microbial illness from seafood is acute, persistent, and a potentially serious risk, although incidence of illness has not increased in recent decades.
-
Methylmercury is the seafoodborne contaminant for which the most comprehensive exposure and toxicity data are available for the purpose of deriving quantitative estimates of the risks.
-
The evidence pertaining to the health risks associated with consumption of seafoodborne contaminants derives from observational studies, primarily cross-sectional and prospective cohort in design. The use of a randomized clinical trial to evaluate contaminant risks would be unethical.
-
The metrics used to characterize the risks associated with consumption of seafoodborne contaminants, such as the reference dose, are useful in identifying a contaminant intake level that is considered, based on available data, to be “without an appreciable risk of deleterious effects during a life-time.” Such metrics are not useful, however, in characterizing the increase in risk that is associated with intake levels that are above the reference dose. The dose-response modeling used to identify the BMD and BMDL could be used to characterize the risks although it is important to recognize that the estimates will be influenced by the assumptions made regarding, for example, the appropriate dose-response function.
-
Reference levels for the intake of contaminants, such as the RfDs for methylmercury and dioxin-like compounds, can be misinterpreted as “bright lines,” i.e., that intakes above the level are “harmful” and intakes below the level are “safe.”
-
With regard to trends in population exposures to chemical contaminants,
-
On the basis of nationally representative data on the US population, the median blood mercury level was unchanged over the period 1999–2002.
-
Exposures to PCBs and dioxin-like compounds are decreasing on a population basis, but exposures can vary greatly according to geographic region and consumption patterns so that particular subgroups of the population could be at increased risk.
-
-
Increased methylmercury exposure might be a risk factor for adult cardiovascular toxicity, although the data available are not extensive and uncertainties remain.
-
Considerable uncertainties are associated with estimates of the health risks to the general population from exposures to MeHg and POPs at levels present in commercially obtained seafood. The available evidence to assess risks to the US population is incomplete and useful to only a limited extent.
-
Estimates for trends in chemical contaminants in the seafood supply depend on harvest location and products of concern.
-
Concerns regarding levels of PCBs and DLCs in certain aquacultured products can be addressed by means of further scrutiny of feed content and uses.
-
The levels of methylmercury in marine seafood do not appear to have changed systematically in recent decades.
-
-
New potential chemical-associated risks continue to be identified
-
in seafood and other foods (e.g., polybrominated diphenyl ethers and other persistent organic pollutants), although inadequate data on exposure, toxicities, or both make it difficult to define the dimensions of the potential risks.
-
Consumers are exposed to a complex mixture of dietary and non-dietary contaminants whereas most studies of the risks associated with seafood focus on a single contaminant. The extent to which such co-exposures might affect the toxicity of seafoodborne contaminants is largely unknown. Similarly, few data are available on the extent to which beneficial components of seafood, such as selenium and omega-3 fatty acids, might mitigate the risks associated with seafoodborne contaminants.
-
Reported seafoodborne illnesses indicate acute hazards are not increasing, but certain hazards associated with specific species and consumer preference (e.g., eating raw molluscan shellfish) persist.
-
Increased dependence on aquacultured and imported products is raising concerns for certain potential hazards.
-
Use of illegal chemotherapeutants in certain aquaculture operations.
-
Various microbial and chemical contaminants in products subject to limited regulatory surveillance.
-
RECOMMENDATIONS
Recommendation 1: Appropriate federal agencies (the National Oceanic and Atmospheric Administration [NOAA], the US Environmental Protection Agency [US EPA], and the Food and Drug Administration [FDA] of the US Department of Health and Human Services) should increase monitoring of methylmercury and persistent organic pollutants in seafood and make the resulting information readily available to the general public. Along with this information, these agencies should develop better recommendations to the public about levels of pollutants that may present a risk to specific population subgroups.
Recommendation 2: Changes in the seafood supply (sources and types of seafood) must be accounted for—there is inconsistency in sampling and analysis methodology used for nutrient and contaminants data that are published by state and federal agencies. Analytical data is not consistently revised, with separate data values presented for wild-caught, domestic, and imported products.
Research Recommendations
Recommendation 12: More complete data are needed on the distribution of contaminant levels among types of fish. This information should be
made available in order to reduce uncertainties associated with the estimation of health risks for with specific seafoodborne contaminant exposures.
Recommendation 13: More quantitative characterization is needed of the dose-response relationships between chemical contaminants and adverse health effects in the ranges of exposure represented in the general US population. Such information will reduce uncertainties associated with recommendations for acceptable ranges of intake.
Recommendation 14: In addition, the committee recommends more research on useful biomarkers of contaminant exposures and more precise quantitative characterization of the dose-response relationships between chemical contaminants and adverse health effects in the ranges of exposure represented in the general US population, in order to reduce uncertainties associated with recommendations for acceptable ranges of intake.
REFERENCES
ACC (Aquaculture Certification Council). 2002–2006. Aquaculture Certification. [Online]. Available: http://www.aquaculturecertification.org [accessed March 6, 2006].
ACC. 2004. Aquaculture Facility Certification, Guidelines for BAP Standards (Shrimp Hatcher-ies). [Online]. Available: http://www.aquaculturecertification.org/ACC-PDFS/hgud1204.pdf [accessed April 3, 2006].
Adverse Reactions to Food Committee. 2003. Academy Practice Paper: Current Approach to the Diagnosis and Management of Adverse Reactions to Foods. Milwaukee, WI: American Academy of Allergy, Asthma, and Immunology. [Online]. Available: http://www.aaaai.org/media/resources/academy_statements/practice_papers/adverse_reactions_to_foods.asp [accessed April 3, 2006].
Allshouse J, Buzby JC, Harvey D, Zorn D. 2003. International trade and seafood safety. In: Buzby JC, ed. International Trade and Food Safety: Economic Theory and Case Studies. Agricultural Economic Report Number 828. Washington, DC: Economic Research Service. [Online]. Available: http://www.ers.usda.gov/publications/aer828/aer828i.pdf [accessed May 1, 2006].
Anderson JA. 1986. The establishment of common language concerning adverse reactions to food and food additives. Journal of Allergy and Clinical Immunology 78 (1 Part 2):140–143.
Angulo F. 1999. Antibiotic Use in Aquaculture: Center for Disease Control Memo to the Record. [Online]. Available: http://www.nationalaquaculture.org/pdf/CDC%20Memo%20to%20the%20Record.pdf#search=%22angulo%20and%20transmission%20of%20antimicrobial-resistant%20bacteria%20in%20aquacultured%20food%201999%22 [accessed August 26, 2006].
Archer DL, Kvenberg JE. 1985. Incidence and cost of foodborne diarrheal disease in the United States. Journal of Food Protection 48(10):887–894.
Armstrong SR. 2002. Synopsis of Dietary Exposure to Dioxin-Like Compounds. Paper prepared for the Committee on the Implications of Dioxin in the Food Supply. Washington, DC: Institute of Medicine.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Mercury. Atlanta, GA: US Department of Health and Human Services, Public Health Service. [Online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp46.html [accessed December 2, 2005].
Auger N, Kofman O, Kosatsky T, Armstron B. 2005. Low-level methylmercury exposure as a risk factor for neurologic abnormalities in adults. Neurotoxicology 26(2):149–157.
Austin JW, Smith JP. 2006. Botulism from fishery products: History and control. In: Otwell WS, Kristinsoon HG, Balaban MO, eds. Modified Atmospheric Processing and Packaging of Fish. Ames, IA: Blackwell Publishing. Pp. 193–216.
Bellanger TM, Caesar EM, Trachtman L. 2000. Blood mercury levels and fish consumption in Louisiana. Journal of Louisiana Medical Society 152(2):64–73.
Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, Pesatori AC. 1997. Dioxin exposure and cancer risk. A 15-year mortality study after the “Seveso accident.” Epidemiology 8(6):646–652.
Bidleman TF, Harner T. 2000. Chapter 10: Sorption to aerosols. In: Boethling RS, Mackay D, eds. 2000. Handbook of Property Estimation Methods for Chemicals: Environmental and Health Sciences. Boca Raton, FL: Lewis Publishers. Pp. 233–260.
Bier JW. 1976. Experimental anisakis: Cultivation and temperature tolerance determination. Journal of Milk and Food Technology 39(2):132–137.
Bowers WJ, Nakai JS, Chu I, Wade MG, Moir D, Yagminas A, Gill S, Pulido O, Meuller R. 2004. Early developmental neurotoxicity of a PCB/organochlorine mixture in rodents after gestational and lactational exposure. Toxicological Sciences 77(1):51–62.
Bryan FL. 1980. Epidemiology of foodborne diseases transmitted by fish, shellfish and marine crustaceans in the United States, 1970–1978. Journal of Food Protection 43:859–876.
Budtz-Jorgensen E, Keiding N, Grandjean P. 2004a. Effects of exposure imprecision on estimation of the benchmark dose. Risk Analysis 24(6):1689–1696.
Budtz-Jorgensen E, Grandjean P, Jorgensen P, Weihe P, Keiding N. 2004b. Association between mercury concentrations in blood and hair methylmercury-exposed subjects at different ages. Environmental Research 95(3):385–393.
Burge P, Evans S. 1994. Mercury contamination in Arkansas gamefish: A public health perspective. Journal of the Arkansas Medical Society 90(11):542–544.
Burger J, Gochfeld M. 2005. Heavy metals in commercial fish in New Jersey. Environmental Research 99(3):403–412.
Bush RK. 1995. Seafood allergy: Implications for industry and consumers. Food Technology 49(10):20.
Bush RK, Taylor SL, Holden K, Nordlee JA, Busse WW. 1986. Prevalence of sensitivity to sulfiting agents in asthmatic patients. American Journal of Medicine 81(5):816–820.
Bylund BG. 1982. Diphyllobothriasis. In: Jacbos L, Arambulo P. CRC Handbook Series in Zoonoese. Section C: Parasitic Zoonoses, Volume 1. Boca Raton, FL: CRC Press, Inc. Pp. 217–225.
Caprioli A, Busani L, Martel JL, Helmuth R. 2000. Monitoring of antibiotic resistance in bacteria of animal origin: Epidemiological and microbiological methodologies. International Journal of Antimicrobial Agents 14(4):295–301.
CDC (Centers for Disease Control and Prevention). 1991. Epidemiologic notes and reports fish botulism—Hawaii, 1990. Morbidity and Mortality Weekly Report 40(24):412–414. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00014498.htm [accessed April 5, 2006].
CDC. 2004. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—selected sites, United States, 2003. Morbidity and Mortality Weekly Report 53(16):338–343. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5316a2.htm [accessed January 12, 2006].
CDC. 2005a. FoodNet—Foodborne Diseases Active Surveillance Network. [Online]. Available: http://www.cdc.gov/foodnet/ [accessed October 6, 2005].
CDC. 2005b. National Biomonitoring Program. Overview. [Online]. Available: http://www.cdc.gov/biomonitoring/overview.htm [accessed May 11, 2006].
CDC. 2005c. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 Sites, United States, 2004. Morbidity and Mortality Weekly Report 54(14):352–356. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5414a2.htm [accessed January 10, 2006].
CDC. 2005d. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention. [Online]. Available: http://www.cdc.gov/exposurereport/3rd/pdf/thirdreport.pdf [accessed March 16, 2006].
CDC. 2006a. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. Morbidity and Mortality Weekly Review 55(14):392–395.
CDC. 2006b. Vibrio parahaemolyticus infections associated with consumption of raw shellfish—three states, 2006. Morbidity and Mortality Weekly Review 55(31):854–856. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5531a5.htm [accessed September 7, 2006].
Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, Shamlaye CF, Berlin M, Davidson PW, Clarkson TW. 1995. Monitoring methylmercury during pregnancy: Maternal hair predicts fetal brain exposure. Neurotoxicology 16(4):705–710.
CFSAN (Center for Food Safety and Applied Nutrition). 2000. Letter Regarding Dietary Supplement Health Claim for Omega-3 Fatty Acids and Coronary Heart Disease. [Online]. Available: http://www.cfsan.fda.gov/~dms/ds-ltr11.html [accessed August 28, 2006].
CFSAN (Center for Food Safety and Applied Nutrition). 2001. Fish and Fisheries Products Hazards and Controls Guidance: Third Edition. Appendix 5: FDA and EPA Safety Levels in Regulations and Guidance. Washington, DC: Food and Drug Administration. [Online]. Available: http://www.cfsan.fda.gov/~comm/haccp4x5.html [accessed March 2, 2006].
CFSAN. 2003. How to Safely Handle Refigerated Ready-To-Eat Foods and Avoid Listeriosis. [Online]. Available: http://www.cfsan.fda.gov/~dms/adlister.html [accessed May 11, 2006].
CFSAN. 2005. Salmonella spp. [Online]. Available: http://www.cfsan.fda.gov/~mow/chap1.html [accessed May 11, 2006].
CFSAN. 2006. USDA Total Diet Study: Dioxin Analysis Results/Exposure Estimates. [Online]. Available: http://www.cfsan.fda.gov/~lrd/dioxdata.html [accessed August 27, 2006].
Chapman L, Chan HM. 2000. The influence of nutrition on methyl mercury intoxication. Environmental Health Perspectives 108(Supplement 1):29–56.
Choi BH. 1989. The effects of methylmercury on the developing brain. Progress in Neurobiology 32(6):447–470.
Chowdhury NR, Chakraborty S, Ramamurthy T, Nishibuchi M, Yamasaki S, Takeda Y, Nair GB. 2000. Molecular evidence of clonal Vibrio parahaemolyticus pandemic strains. Emerging Infectious Diseases 6(6):631–636.
Clarkson TW, Strain JJ. 2003. Nutritional factors may modify the toxic action of methyl mercury in fish-eating populations. Journal of Nutrition 133(5 Supplement 1):1539S–1543S.
Cohen JT. 2004. A Summary of the Major Studies of Prenatal Mercury Exposure and Cognitive Function. Boston, MA: Harvard Center for Risk Analysis. [Online]. Available: http://www.hcra.harvard.edu/Supplement_Fish_3IQ.pdf [accessed March 1, 2006].
Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS. 2003. Dioxin and cancer: A critical review. Regulatory Toxicology and Pharmacology 38(3):378–388.
Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. 1998. Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a New Zealand cohort. Risk Analysis 18(6):701–713.
Cuvin-Aralar ML, Furness RW. 1991. Mercury and selenium interaction: A review. Ecotoxicology and Environmental Safety 21(3):348–364.
Daniels JL, Longnecker MP, Rowland AS, Golding J, the ALSPAC Study Team. 2004. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology 15(4):394–402.
Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. 2001. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environmental Health Perspectives 109(Supplement 1):49–68.
de Wit C, Alaee M, Muir D. 2004. Brominated flame retardants in the Arctic—An overview of spatial and temporal trends. Organohalogen Compounds 66:3811–3816.
Deardorff TL, Throm R. 1988. Commercial blast-freezing of third-stage Anisakis simplex larvae encapsulated in salmon and rockfish. Journal of Parasitology 74(4):600–603.
DeCaprio AP. 1997. Biomarkers: Coming of age for environmental health and risk assessment. Environmental Science and Technology 31(7):1837–1848.
DeWaal CS, Barlow K. 2004. Outbreak Alert! Closing the Gaps in Our Federal Food-Safety Net. Washington, DC: Center for Science in the Public Interest. [Online]. Available: http://www.cspinet.org/new/pdf/outbreakalert2004.pdf [accessed October 6, 2005].
Dorea JG, de Souza JR, Rodrigues P, Ferrari I, Barbosa AC. 2005. Hair mercury (signature of fish consumption) and cardiovascular risk in Munuruku and Kayabi Indians of Amazonia. Environmental Research 97(2):209–219.
Duran GM, Marshall DL. 2005. Ready-to-eat shrimp as an international vehicle of antibiotic-resistant bacteria. Journal of Food Protection 68(11):2395–2401.
Easton MD, Luszniak D, Von der GE. 2002. Preliminary examination of contaminant loadings in farmed salmon, wild salmon and commercial salmon feed. Chemosphere 46(7):1053–1074.
Erickson MD. 1997. Analytical Chemistry of PCBs, Second Edition. Boca Raton: CRC/Lewis. P. 667.
Ervik A, Thorsen B, Eriksen V, Lunestad BT, Samuelsen OB. 1994. Impact of administering antibacterial agents on wild fish and blue mussels Mytilus edulis in the vicinity of fish farms. Diseases of Aquatic Organisms 18(1):45–51.
Evans MS, Noguchi GE, Rice CP. 1991. The biomagnification of polychlorinated biphenyls, toxaphene, and DDT compounds in a Lake Michigan offshore food web. Archives of Environmental Contamination and Toxicology 20(1):87–93.
FAO/IOC/WHO (Food and Agriculture Organization/Intergovernmental Oceanographic Commission of UNESCO/World Health Organization). 2004. Report of the Joint FAO/IOC/WHO Ad Hoc Expert Consultation on Biotoxins in Bivalve Molluscs. Oslo, Norway, September 26–30, 2004. [Online]. Available: ftp://ftp.fao.org/es/esn/food/biotoxin_report_en.pdf [accessed May 1, 2006].
FAO/NACA/WHO Study Group. 1999. Food Safety Issues Associated with Products from Aquaculture. Technical Report Series, No. 883. Geneva, Switzerland: World Health Organization. [Online]. Available: http://www.who.int/foodsafety/publications/fs_management/en/aquaculture.pdf [accessed April 3, 2006].
FAO/WHO JECFA (Food and Agriculture Organization/World Health Organization Joint Expert Committee on Food Additives). 2003. Summary and Conclusions, 61st Meeting, Rome, Italy, 10–19 June, 2003. [Online]. Available: http://www.who.int/ipcs/food/jecfa/summaries/en/summary_61.pdf [accessed March 9, 2006].
FAO/WHO JECFA. 2005. Summary and Conclusions, 64th Meeting, Rome, Italy, 8–17 February 2005. [Online]. Available: http://www.who.int/ipcs/food/jecfa/summaries/summary_report_64_final.pdf [accessed March 22, 2006].
FAO/WHO JECFA. 2006. Evaluation of Certain Food Contaminants (Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series, No. 930. Geneva, Switzerland: World Health Organization. [Online]. Available: http://whqlibdoc.who.int/trs/WHO_TRS_930_eng.pdf [accessed August 26, 2006].
FAO/WHO. 2001. Hazard Identification, Exposure Assessment and Hazard Characterization of Campylobacter spp. in Broiler Chickens and Vibrio spp. in Seafood. Geneva, Switzerland: World Health Organization. [Online]. Available: http://www.who.int/foodsafety/publications/micro/en/july2001_en.pdf [accessed March 2, 2006].
Faroon O, Jones D, de Rosa C. 2001. Effects of polychlorinated biphenyls on the nervous system. Toxicology and Industrial Health 16(7–8):305–333.
FDA (Food and Drug Administration). 1987. Food Preparation—Raw, Marinated or Partially Cooked Fishery Products. Retail Food Protection Program Information Manual. Part 6—Inspection. Washington, DC: Center for Food Safety and Applied Nutrition.
FDA. 2001a. Fish and Fishery Products Hazards Controls Guidance: Third Edition. Washington, DC: Center for Food Safety and Applied Nutrition. [Online]. Available: http://www.cfsan.fda.gov/~comm/haccp4.html [accessed January 11, 2006].
FDA. 2001b. Draft Risk Assessment on the Public Health Impact of Vibrio parahaemolyticus in Raw Molluscan Shellfish. Washington, DC: Center for Food Safety and Applied Nutrition. [Online]. Available: http://www.cfsan.fda.gov/~dms/vprisk.html [accessed April 5, 2006].
FDA. 2005a. 21 Code of Federal Regulations, Part 179. Irradiation in the Production, Processing, and Handling of Food. Federal Register 70(157):48057–48073. [Online]. Available: http://frwebgate.access.gpo.gov/cgi-bin/getpage.cgi [accessed March 6, 2006].
FDA. 2005b. 21 Code of Federal Regulations, Parts 556 and 558. New Animal Drugs: Florfenicol. Federal Register 70(223):70046–70047. [Online]. Available: http://a257.g.akamaitech.net/7/257/2422/01jan20051800/edocket.access.gpo.gov/2005/05-22935.htm [accessed April 3, 2006].
FDA. 2005c. 21 Code of Federal Regulations, Part 510—New Animal Drugs, Volume 6. [Online]. Available: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=510&showFR=1 [accessed April 3, 2006].
FDA. Refusal Actions by FDA as Recorded in OASIS. [Online]. Available: http://www.fda.gov/ora/oasis/3/ora_oasis_i_16.html and http://www.fda.gov/ora/oasis/1/ora_oasis_i_16.html [accessed May 11, 2006].
Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK. 1984. Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestational age. Journal of Pediatrics 105(2):315–320.
Flattery J, Bashin M. 2003. A Baseline Survey of Raw Oyster Consumers in Four States. Columbia, SC: Interstates Shellfish Sanitation Conference. [Online]. Available: http://www.epa.gov/gmpo/pubinfo/pdf/accomp-app-d.pdf [accessed April 11, 2006].
Florida Department of Agriculture and Consumer Services. 2005. Aquaculture Best Management Practices Rule. Tallahassee, FL: Florida Department of Agriculture and Consumer Services. [Online]. Available: http://www.floridaaquaculture.com/publications/BMP%20Rule-Manual112805.pdf [accessed April 10, 2006].
Foran JA, Hites RA, Carpenter DO, Hamilton MC, Mathews-Amos A, Schwager SJ. 2004. A survey of metals in tissues of farmed Atlantic and wild Pacific salmon. Environmental Toxicology and Chemistry 23(9):2108–2110.
Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. 2005a. Risk-based consumption advice for farmed Atlantic and wild Pacific salmon contaminated with dioxins and dioxin-like compounds. Environmental Health Perspectives 113(5):552–556.
Foran JA, Good DH, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. 2005b. Quantitative analysis of the benefits and risks of consuming farmed and wild salmon. Journal of Nutrition 135(11):2639–2643.
Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. 1994. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 86(1–2):49–61.
Fries GF. 1995a. A review of the significance of animal food products as potential pathways of human exposures to dioxins. Journal of Animal Science 73(6):1639–1650.
Fries GF. 1995b. Transport of organic environmental contaminants to animal products. Reviews of Environmental Contamination and Toxicology 141:71–109.
Fyfe M, Kelly MT, Yeung ST, Daly P, Schallie K, Buchanan S, Waller P, Kobayashi J, Therien N, Guichard M, Lankford S, Stehr-Green P, Harsch R, DeBess E, Cassidy M, McGivern T, Mauvais S, Fleming D, Lippmann M, Pong L, McKay RW, Cannon DE, Werner SB, Abbott S, Hernandez M, Wojee C, Waddell J, Waterman S, Middaugh J, Sasaki D, Effler P, Groves C, Curtis N, Dwyer D, Dowdle G, Nichols C. 1998. Outbreak of vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. Morbidity and Mortality Weekly Report 47(22):457–462. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00053377.htm [accessed April 11, 2006].
Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P, Oh S-H, Hoekstra WG. 1972. Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science 175(26):1122–1124.
Geyer HJ, Rimkus GG, Scheunert I, Kaune A, Schramm K-W, Kettrup A, Zeeman M, Muir DCG, Hansen LG, Mackay D. 2000. Bioaccumulation and occurrence of endocrine-disrupting chemicals (EDC), persistent organic pollutants (POPs), and other organic compounds in fish and other organisms including humans. In: Hutzinger O, Beek B, eds. Bioaccumulation, New Aspects and Developments. The Handbook of Environmental Chemistry, Vol. 2, Part J. Berlin, Germany: Springer Verlag. Pp. 1–166.
Global Aquaculture Alliance. 2004. Responsible Aquaculture Program. [Online]. Available: http://www.gaalliance.org/resp.html [accessed April 3, 2006].
Gombas DE, Chen Y, Clavero RS, Scott VN. 2003. Survey of Listeria monocytogenes in Ready-to-Eat Foods. Journal of Food Protection 66(4):559–569.
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. 1997. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology 19(6):417–428.
Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H, Ellefsen P, Budtz-Jorgensen E, Keiding N, White RF. 2001. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicology and Teratology 23(4):305–317.
Gregus Z, Gyurasics A, Csanaky I, Pinter Z. 2001. Effects of methylmercury and organic acid mercurials on the disposition of exogenous selenium in rats. Toxicology and Applied Pharmacology 174(2):177–187.
Guallar E, Sanz-Gallardo I, Veer PV, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ. 2002. Mercury, fish oils, and the risk of myocardial infarction. New England Journal of Medicine 347(22):1747–1754.
Hanekamp JC, Frapporti G, Olieman K. 2003. Chloramphenicol, food safety and precautionary thinking in Europe. Environmental Liability 11:209–221. [Online]. Available: http://www.richel.org/theodoc/pdf/Chloramphenicol.pdf [accessed May 1, 2006].
Harris WS, Windson SL, Dujovne CA. 1991. Effects of four doses of n-3 fatty acids given to hyperlipidemic patients for six months. Journal of the American College of Nutrition 10(3):220–227.
Hefle SL. 1996. The chemistry and biology of food allergens. Food Technology 50(3):86–92.
Higashi GJ. 1985. Foodborne parasites transmitted to man from and other aquatic foods. Food Technology 39(3):69–74.
Hightower JM, Moore D. 2003. Mercury levels in high-end consumers of fish. Environmental Health Perspectives 111(4):604–608.
Hites RA. 2004. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environmental Science and Technology 38(4):945–956.
Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. 2004. Global assessment of organic contaminants in farmed salmon. Science 303(5655):226–229.
Hlady W, Klontz K. 1996. The epidemiology of Vibrio infections in Florida, 1981–1993. Journal of Infectious Diseases 173(5):1176–1183.
Huisman M, Koopman-Esseboom C, Lanting CI, van der Paauw CG, Tuinstra LG, Fidler V, Weisglas-Kuperus N, Sauer PJ, Boersma ER, Touwen BC. 1995. Neurological condition in 18-month-old children perinatally exposed to polychlorinated biphenyls and dioxins. Early Human Development 43(2):165–176.
Humphrey H. 1988. Human exposure to persistent aquatic contaminants. In: Schmidtke NW, ed. Toxic Contamination in Large Lakes. Chelsea, MI: Lewis Publishers. Pp. 227–238.
Hungerford JM. 2005. Committee on Natural Toxins and Food Allergens: Marine and freshwater toxins. Journal of the Association of Official Analytical Chemists International 88(1):299–324.
Huss HH, Ababouch L, Gram L. 2004. Assessment and Management of Seafood Safety and Quality. Technical Paper No. 444. Rome, Italy: Food and Agricultural Organization. [Online]. Available: http://www.fao.org/DOCREP/006/Y4743E/y4743e06.htm [accessed March 30, 2006].
ICEID (International Conference on Emerging Infectious Diseases). 2006. Consumption of Risky Foods Declines. Atlanta, GA: International Conference on Emerging Infectious Diseases. [Online]. Available: http://www.asm.org/ASM/files/LeftMarginHeaderList/DOWNLOADFILENAME/000000002017/risky%20foods.pdf [accessed April 11, 2006].
IFT (Institute of Food Technologists). 2006. Antimicrobial Resistance: Implications for the Food System, Summary. IFT Expert Report. [Online]. Available: http://members.ift.org/NR/rdonlyres/17A8181C-143C-4D94-8885-9093C4AFC533/0/OnePagerSummary.pdf [accessed August 26, 2006].
IISD (International Institute for Sustainable Development). 1998. Report of the first session of the INC for an international legally binding instrument for implementing international action on center persistent organic pollutants (POPS): 29 June–3 July 1998. Earth Negotiations Bulletin 15(10):1. [Online]. Available: http://www.iisd.ca/download/pdf/enb1510e.pdf [accessed March 13, 2006].
Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S. 2004. Detoxification mechanism of heavy metals in marine mammals and seabirds: Interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Archives of Environmental Contamination and Toxicology 47(3):402–413.
Ikonomou MG, Rayne S, Addison RF. 2002. Exponential increases of the brominated flame retardants, polybrominated diphenyl ethers, in the Canadian Arctic from 1981 to 2000. Environmental Science and Technology 36(9):1886–1892.
IOM (Institute of Medicine). 1991. Seafood Safety. Washington, DC: National Academy Press.
IOM. 2003. Dioxins and Dioxin-Like Compounds in the Food Supply: Strategies to Decrease Exposure. Washington, DC: The National Academies Press.
ISSC (Interstate Shellfish Sanitation Conference). 2001. Vibrio Management Committee Meeting, Biloxi, MS, November 8–9, 2001. [Online]. Available: http://issc.org/Archives/old-newsletters/Nov-2002/VMCNOV.htm [accessed April 28, 2006].
ISSC. 2002. Vibrio vulnificus Risk Management Plan for Oysters. [Online]. Available: http://www.issc.org/Vibrio_vulnificus_Education/1/II%20A%20Vibrio%20vulnificus%20Risk%20Management%20Plan.doc [accessed March 2, 2006].
ISSC. 2003a. Guide for the Control of Molluscan Shellfish 2003. National Shellfish Sanitation Program, Interstate Shellfish Sanitation Conference. [Online]. Available: http://www.cfsan.fda.gov/~ear/nss2-toc.html [accessed April 20, 2006].
ISSC. 2003b. Florida Vibrio vulnificus Risk Reduction Plan for Oysters. [Online]. Available: http://www.issc.org/Vibrio_vulnificus_Education/1/II%20B%20a%20Florida%20Vibrio%20Vulnificus%20Risk%20Reduction%20Plan%20for%20Oy.rtf [accessed April 28, 2006].
Jackson GJ. 1975. The new disease status of human anisakiasis and North American cases: A review. Journal of Milk and Food Technology 38(12):769–773.
Jacobson JL, Jacobson SW. 1996. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): The Michigan and North Carolina cohort studies. Toxicology and Industrial Health 12(3–4):435–445.
Jacobson JL, Jacobson SW. 2003. Prenatal exposure to polychlorinated biphenyls and attention at school age. Journal of Pediatrics 143(6):780–788.
Jacobson JL, Jacobson SW, Humphrey HE. 1990. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. Journal of Pediatrics 116(1):38–45.
Jensen CD, Spiler GA, Wookey VJ, Wong LG, Whitman JH, Scala J. 1988. Plasma lipids on three levels of fish oil intake in healthy human subjects. Nutrition Reports International 38:165–171.
Jensen TK, Grandjean P, Jorgensen EB, White RF, Debes F, Weihe P. 2005. Effects of breast feeding on neuropsychological development in a community with methylmercury exposure from seafood. Journal of Exposure Analysis and Environmental Epidemiology 15(5):423–430.
Johansson N, Basun H, Winblad B, Nordberg M. 2002. Relationship between mercury concentration in blood, cognitive performance, and blood pressure, in an elderly urban population. BioMetals 15(2):189–195.
Kamrin MA. 2004. Bisphenol A: A scientific evaluation. Medscape General Medicine 6(3):7.
Kimbrough RD. 1987. Human health effects of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annual Review of Pharmacology and Toxicology 27: 87–111.
Kimbrough RD, Squire RA, Linder RE, Strandberg JD, Montalli RJ, Burse VW. 1975. Induction of liver tumor in Sherman strain female rats by polychlorinated biphenyl aroclor 1260. Journal of the National Cancer Institute 55(6):1453–1459.
Kingman A, Albertini T, Brown LJ. 1998. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. Journal of Dental Research 77(3):461–471.
Kjellstrom T, Kennedy S, Wallis S, Mantell C. 1986. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage I: Preliminary Tests at Age 4. Solna, Sweden: National Swedish Environmental Protection Board.
Kjellstrom T, Kennedy S, Wallis S, Stewart A, Friberg L, Lind B, Wutherspoon T, Mantell C. 1989. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage II: Interviews and Psychological Tests at Age 6. Solna, Sweden: National Swedish Environmental Protection Board.
Kodavanti PR, Ward TR. 2005. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicological Sciences 85(2):952–962.
Koeman JH, Peeters WH, Koudstaal-Hol CH, Tjioe PS, de Goeij JJ. 1973. Mercury-selenium correlations in marine mammals. Nature 245(5425):385–386.
Koeman JH, van de Ven WS, de Goeij JJ, Tjioe PS, van Haaften JL. 1975. Mercury and selenium in marine mammals and birds. Science of the Total Environment 3(3):279–287.
Kohn MA, Farley TA, Ando T, Curtis M, Wilson SA, Jin Q, Monroe SS, Baron RC, McFarland LM, Glass RI. 1995. An outbreak of Norwalk virus gastroenteritis associated with eating raw oysters. Implications for maintaining safe oyster beds. Journal of the American Medical Association 273(6):466–471.
Kong KY, Cheung KC, Wong CK, Wong MH. 2005. Residues of DDTs, PAHs and some heavy metals in fish (Tilapia) collected from Hong Kong and mainland China. Journal of Environmental Science and Health. Part A: Toxic/Hazardous Substances and Environmental Engineering 40(11):2105–2115.
Koonse B, Burkhardt W, Chirtel S, Hoskins GP. 2005. Salmonella and the sanitary quality of aquacultured shrimp. Journal of Food Protection 68(12):2527–2532.
Kosta L, Byrne AR, Zelenko V. 1975. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 254(5497):238–239.
Kraepiel AM, Keller K, Chin HB, Malcolm EG, Morel FM. 2003. Sources and variations of mercury in tuna. Environmental Science and Technology 37(24):5551–5558.
Kulkarni P. 2005. The Marine Seafood Export Supply Chain in India: Current State and Influence of Import Requirements. Winnipeg, Manitoba, Canada: International Institute for Sustainable Development. [Online]. Available: http://www.tradeknowledgenetwork.net/pdf/tkn_marine_export_india.pdf [accessed May 2, 2006].
Lange WR, Snyder FR, Fudala PJ. 1992. Travel and ciguatera fish poisoning. Archives of Internal Medicine 152(10):2049–2053.
Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, Stern GA. 2003. Levels and trends of polybrominated diphenylethers and other brominated flame retardants in wildlife. Environment International 29(6):757–779.
Lehane L. 1999. Ciguatera Fish Poisoning: A Review in a Risk-Assessment Framework. Canberra, Australia: National Office Animal and Plant Health, Agriculture, Fisheries and Forestry. [Online]. Available: http://www.affa.gov.au/corporate_docs/publications/pdf/animalplanthealth/chief_vet/ciguatera.pdf [accessed April 3, 2006].
Lehrer SB. 1993. Seafood allergy. Introduction. Clinical Reviews in Allergy 11(2):155–157.
Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, Korrick SA, Rogan WJ, Weisglas-Kuperus N, Hertz-Picciotto I, Ayotte P, Stewart P, Winneke G, Charles MJ, Jacobson SW, Dewailly E, Boersma ER, Altshul LM, Heinzow B, Pagano JJ, Jensen AA. 2003. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environmental Health Perspectives 111(1):65–70.
Luksemburg W, Wenning R, Maier M, Patterson A, Braithwaite S. 2004. Polybrominated diphenyl ethers (PBDE) and polychlorinated dibenzo-p-dioxins (PCDD/F) and biphenyls (PCB) in fish, beef, and fowl purchased in food markets in northern California U.S.A. Organohalogen Compounds 66:3982–3987.
Lunder S, Sharp R. 2003. Tainted Catch: Toxic Fire Retardants Are Building Up Rapidly in San Francisco Bay Fish—And People. Washington, DC: Environmental Working Group. [Online]. Available: http://www.ewg.org/reports_content/taintedcatch/pdf/PBDEs_final.pdf [accessed September 12, 2005].
Luten JB, Ruiter A, Ritskes TM, Rauchbaar AB, Riekwel-Booy G. 1980. Mercury and selenium in marine- and freshwater fish. Journal of Food Science 45:416–419.
Mahaffey KR, Clickner RP, Bodurow CC. 2004. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environmental Health Perspective 112(5):562–570.
McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, Mahaffey KR. 2004. Hair mercury levels in US children and women of child-bearing age: Reference range data from NHANES 1999–2000. Environmental Health Perspectives 112(11):1165–1171.
McKerrow JH, Sakanari J, Deardorff TL. 1988. Anisakiasis: Revenge of the sushi parasite. New England Journal of Medicine 319(18):1228–1229.
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffine PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerging Infectious Diseases 5(5):607–625. [Online]. Available: http://www.cdc.gov/ncidod/eid/vol5no5/mead.htm [accessed January 12, 2006].
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffine PM, Tauxe RV. 2006. Food-related illness and death in the United States. Emerging Infectious Diseases 5(5):607–625.
Meironyté D, Norén K, Bergman A. 1999. Analysis of polybrominated diphenyl ethers in Swedish human milk: A time-related trend study, 1972–1997. Journal of Toxicology and Environmental Health, Part A 58(6):329–341.
Mendelsohn ML, Mohr LC, Peeters JP. 1998. Biomarkers: Medical and Workplace Applications. Washington, DC: Joseph Henry Press.
Mocarelli P, Gerthoux PM, Brambilla P, Marocchi A, Beretta C, Bertona M, Cazzaniga M, Colombo L, Crespi C, Ferrari E, Limonta G, Sarto C, Signorini, Tramacere PL. 1999. Dioxin health effects on humans twenty years after Seveso: A summary. In: Ballarin-Denti A, Bertazzi PA, Facchetti S, Mocarelli P, eds. Chemistry, Man, and Environment: The Seveso Accident 20 Years On: Monitoring, Epidemiology and Remediation. Pp. 41–52.
Moreland KB, Landrigan PJ, Sjödin A, Gobeille AK, Jones RS, McGahee EE, Needham LL, Patterson DG. 2005. Body burdens of polybrominated diphenyl ethers among urban anglers. Environmental Health Perspectives 113(12):1689–1692.
Morton RA, Burklew MA. 1970. Incidence of ciguatera in barracuda from the west coast of Florida. Toxicon 8(4):317–318.
Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. 2003. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet 361 (9370):1686–1692.
National Fish Meal and Oil Association. 2002. Certificate of Analysis and Pesticide Scans. Provided to the FDA by Thionville Laboratories, Inc. [Online]. Available: http://www.fda.gov/ohrms/dockets/dailys/02/Jul02/070202/99p-5332_sup0003_01_vol1.pdf [accessed August 28, 2006].
Nguon K, Baxter MG, Sajdel-Sulkowska EM. 2005. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female rat neonates. Cerebellum 4(2):112–122.
NRC (National Research Council). 1999. Hormonally Active Agents in the Environment. Washington, DC: National Academy Press.
NRC. 2000. Toxicological Effects of Methylmercury. Washington, DC: National Academy Press.
NRC. 2006. Health Risks from Dioxin and Related Compounds: Evaluation of the EPA Reassessment. Washington, DC: The National Academies Press.
Ocean Nutrition Canada. 2004. Manufacturing: MEG-3. [Online]. Available: http://www.ocean-nutrition.com/inside.asp?cmPageID=158/ [accessed May 11, 2006].
O’Neil CE, Lehrer SB. 1995. Seafood allergy and allergens: A review. Food Technology 49(10):103–116.
O’Neil C, Helbling AA, Lehrer SB. 1993. Allergic reactions to fish. Clinical Reviews in Allergy 11(2):183–200.
Oken E, Wright RO, Kleinman KP, Bellinger D, Hu H, Rich-Edwards JW, Gillman MW. 2005. Maternal fish consumption, hair mercury, and infant cognition in a US cohort. Environmental Health Perspectives 113(10):1376–1380.
Olsen SJ, MacKinon LC, Goulding JS, Bean NH, Slutsker L. 2000. Surveillance for foodborne disease outbreaks—United States, 1993–1997. Morbidity and Mortality Weekly Report 49(Surveillance Summary 1):1–51. [Online]. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss4901a1.htm [accessed March 31, 2006].
Olson RE. 1986. Marine fish parasites of public health importance. In: Kramer DE, Liston J, eds. Seafood Quality Determination. Amsterdam, the Netherlands: Elsevier Science Publishers. Pp. 339–355.
Ortiz-Roque C, Lopez-Rivera Y. 2004. Mercury contamination in reproductive age women in a Caribbean island: Vieques. Journal of Epidemiology and Community Health 58(9):756–757.
Otwell S, Garrido L, Garrido V, Benner R. 2001. Farm-raised Shrimp: Good Aquacultural Practices for Product Quality and Safety. Florida Sea Grant Publication SGEB-53. Gainesville, FL: Florida Sea Grant University. [Online]. Available: http://www.uhh.hawaii.edu/~pacrc/Mexico/files/manual/08_shrimp_farming_methods.pdf [accessed May 3, 2006].
Parizek J, Ostadalova I. 1967. The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23(2):142–143.
Passos CJ, Mergler D, Gaspar E, Morais S, Lucotte M, Larribe F, Davidson R, de Grosbois S. 2003. Eating tropical fruit reduces mercury exposure from fish consumption in the Brazilian Amazon. Environmental Research 93(2):123–130.
Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. 1999. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. Journal of Pediatrics 134(1):33–41.
Paulsson K, Lundbergh K. 1989. The selenium method for treatment of lakes for elevated levels of mercury in fish. Science of the Total Environment 87–88:495–507.
Paustenbach D, Galbraith D. 2005. Biomonitoring: Measuring Levels of Chemicals in People—and What the Results Mean. New York, NY: American Council on Science and Health. [Online]. Available: http://www.acsh.org/docLib/20050721_biomonitoring.pdf [accessed March 28, 2006].
Peele C. 2004. Washington State Polybrominated Diphenyl Ether (PBDE) Chemical Action Plan: Interim Plan. Olympia, WA: Washington State Department of Ecology, Department of Health. [Online]. Available: http://www.ecy.wa.gov/pubs/0403056.pdf [accessed March 22, 2006].
Ralston NVC. 2005 (April 11). Selenium Modulation of Toxicants in Seafood. Presented to the Committee on Nutrient Relationships in Seafood: Selections to Balance the Benefits and Risks, Washington, DC, Institute of Medicine.
Rayne S, Ikonomou MG, Antcliffe B. 2003. Rapidly increasing polybrominated diphenyl ether concentrations in the Columbia River system from 1992 to 2000. Environmental Science and Toxicology 37(13):2847–2854.
Rice DC. 2000. Identification of functional domains affected by developmental exposure to methylmercury: Faroe Islands and related studies. Neurotoxicology 21(6):1039–1044.
Rice DC. 2004. The US EPA reference dose for methylmercury: Sources of uncertainty. Environmental Research 95(3):406–413.
Rice, DC. 2005 (February). Brominated Flame Retardants: A Report to the Joint Standing Committee on Natural Resources, 122nd Maine Legislature. Prepared by the Bureau of Health and Department of Environmental Protection. [Online]. Available: http://mainegov-images.informe.org/dep/rwm/publications/legislativereports/pdf/bromfeb2005.pdf#search=%22rice%20brominated%20flame%20retardants%3A%20a%20report%20to%20the%20joint%20standing%20committee%20on%20natural%20resources%22 [accessed August 26, 2006].
Rim H. 1982. Clonorchiasis. In: Hillyer G, Hopla C, eds. CRC Handbook Series in Zoonoses, Volume II. Boca Raton, FL: CRC Press Inc. Pp. 17–32.
Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. 2000. Fish oil-derived fatty acids, docosahexenoic acid and docosapentaenoic acid, and the risk of acute coronary events—The Kuopio Ischaemic Heart Disease Risk Factor Study. Circulation 102(22):2677–2679.
Robson MG, Hamilton GC. 2005. Pest control and pesticides. In: Frumkin H, ed. Environmental Health: From Global to Local. San Fransisco: John Wiley and Sons, Inc. Pp. 544–580.
Rodgers RPC, Levin J. 1990. A critical reappraisal of the bleeding time. Seminars in Thrombosis and Hemostasis 16(1):1–20.
Rodrick GE, Cheng TG. 1989. Parasites: Occurrence and significance in marine animals. Food Technology 43(11):98–102.
Roegge CS, Schantz SL. 2006. Motor function following developmental exposure to PCBS and/or MEHG. Neurotoxicology and Teratology 28(2):260–277.
Roegge CS, Wang VC, Powers BE, Klintsova AY, Villareal S, Greenough WT, Schantz SL. 2004. Motor impairment in rats exposed to PCBs and methylmercury during early development. Toxicological Sciences 77(2):315–324.
Rogan WJ, Chen A. 2005. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 366(9487):763–773.
Ross G. 2004. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicology and Environmental Safety 59:275–291.
Ryan LM. 2005. Effects of Prenatal Mercury Exposure on Childhood IQ: A Synthesis of Three Studies. Report to the US Environmental Protection Agency.
Ryan JJ, Patry B, Mills P, Beaudoin NG. 2002. Recent trends in levels of brominated diphenyl ethers (BDEs) in human milks from Canada. Organohalogen Compounds 58:173–176.
SACN (Scientific Advisory Committee on Nutrition). 2004. Advice on Fish Consumption: Benefits and Risks. London, England: The Stationery Office.
Saint-Phard D, Gonzalez P, Sherman P. 2004. Unsuspected mercury toxicity linked to neurologic symptoms: A case series. Archives of Physical and Medical Rehabilitation 85:E25 (abstract).
Sakanari JA, Moser M, Deardorff TL. 1995. Fish Parasites and Human Health: Epidemiology of Human Helminthic Infections. California Sea Grant College, University of California.
Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. 1995. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 91(3):645–655.
Salonen JT, Seppanen K, Lakka TA, Salonen R, Kaplan GA. 2000. Mercury accumulation and accelerated progression of carotid atherosclerosis: A population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 148(2):265–273.
Sampson HA. 1992. Food hypersensitivity: Manifestations, diagnosis, and natural history. Food Technology 46:141–156.
Santerre CR. 2004. Farmed salmon: Caught in a numbers game. Journal of Food Technology 58(2):108. [Online]. Available: http://seafood.ucdavis.edu/organize/santerresalmon.pdf [accessed April 27, 2006].
Schantz SL, Gardiner JC, Gasior DM, Sweeney AM, Humphrey HE, McCaffrey RJ. 1999. Motor function in aging Great Lakes fisheaters. Environmental Research 80(2 Part 2): S46–S56.
Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HE, Gardiner JC. 2001. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environmental Health Perspectives 109(6):605–611.
Schantz SL, Widholm JJ, Rice DC. 2003. Effects of PCB exposure on neuropsychological function in children. Environmental Health Perspectives 111(3):1–27.
Schecter A, Päpke O, Tung K-C, Staskal D, Birnbaum L. 2004. Polybrominated diphenyl ethers contamination of United States food. Environmental Science and Technology 38(20):5306–5311.
Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. 2005. Polybrominated diphenyl ether flame retardants in the U.S. population: Current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. Journal of Occupational and Environmental Medicine 47(3):199–211.
Seychelles Medical and Dental Journal. 2004. Special Issue: Volume 7, Number 1. [Online]. Available: http://www.seychelles.net/smdj/ [accessed January 12, 2006].
Shamlaye C, Davidson PW, Myers GJ. 2004. The Seychelles Child Development Study: Two decades of collaboration. Seychelles Medical and Dental Journal, Special Issue 7(1):92–99. [Online]. Available: http://www.seychelles.net/smdj/SECIVB.pdf [accessed March 9, 2006].
Sjödin A, Jones RS, Focan J-F, Lapeza C, Wang RY, McGahee EE III, Zhang Y, Turner WE, Slazyk B, Needham L, Patterson DG Jr. 2004a. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environmental Health Perspectives 112(6):654–658.
Sjödin A, Päpke O, McGahee E III, Jones R, Focant J-F, Pless-Mulloli T, Toms L-M, Wang R, Zhang Y, Needham L, Herrmann T, Patterson D Jr. 2004b. Concentration of polybrominated diphenyl ethers (PBDEs) in house hold dust from various countries—inhalation a potential route of human exposure. Organohalogen Compounds 66:3817–3822.
Smith AG, Gangolli SD. 2002. Organochlorine and chemicals in seafood: Occurrence and health concerns. Food and Chemical Toxicology 40:767–779.
Stern AH, Smith AE. 2003. An assessment of the cord:maternal blood methylmercury ratio: Implications for risk assessment. Environmental Health Perspectives 111(12):1465–1470.
Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicology and Teratology 25(1):11–22.
Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. 2004. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicological Sciences 78(1):144–155.
Taylor SL, Bush RK. 1988. Allergy by ingestion of seafood. In: Tu AT, ed. Handbook of Natural Toxins, Volume 3, Marine Toxins and Venoms. New York: Marcel Dekker. Pp. 149–183.
Taylor SL, Nordlee JA. 1993. Chemical additives in seafood products. Clinical Reviews in Allergy 11(2):261–291.
Ulbrich B, Stahlmann R. 2004. Developmental toxicity of polychlorinated biphenyls (PCBs): A systematic review of experimental data. Archives in Toxicology 78(5):252–268.
UNEP (United Nations Environment Programme). 2002. Global Mercury Assessment. Geneva, Switzerland: UNEP Chemicals. [Online]. Available: http://www.chem.unep.ch/mercury/Report/GMA-report-TOC.htm [accessed March 1, 2006].
UNEP Global Environmental Facility. 2003. Regionally Based Assessment of Persistent Toxic Substances. Global Report 2003. Geneva, Switzerland: United Nations. [Online]. Available: http://www.chem.unep.ch/pts/gr/Global_Report.pdf [accessed April 26, 2006].
US EPA (United States Environmental Protection Agency). 1987. National Dioxin Study. Tiers 3, 5, 6, and 7. EPA-440/4-87-003. Washington, DC: Office of Water Regulations and Standards.
US EPA. 1991. Guidelines for developmental toxicity risk assessment. Federal Register 56: 63798-63826. [Online]. Available: http://www.epa.gov/ncea/raf/pdfs/devtox.pdf [accessed April 26, 2006].
US EPA. 1994. Exposure and Human Health Reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin ((TCDD) and Related Compounds. National Academy of Sciences (NAS) Review Draft. Washington, DC: National Center for Environmental Assessment.
US EPA. 1995. The Use of the Benchmark Dose Approach in Health Risk Assessment. EPA/630/R-94-007, Washington, DC: Office of Research and Development.
US EPA. 1997. Mercury Report for Congress. Volume 1. Executive Summary. EPA-452/R-97-003. Washington, DC: Office of Air Quality Planning and Standards and Office of Research and Development. [Online]. Available: http://www.epa.gov/ttn/oarpg/t3/reports/volume1.pdf [accessed January 12, 2006].
US EPA. 2000a. Exposure and Human Health Reassessment of 2,3,7,8-tetrachlorodibenso-p-dioxin (TCDD) and Related Compounds. Draft Final Report. Washington, DC: US EPA. [Online]. Available: http://cfpub.epa.gov/ncea/cfm/part1and2.cfm?ActType=default [accessed March 2, 2006].
US EPA. 2000b. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. Volume II: Risk Assessments and Consumption Limits. Third Edition. Washington, DC: Environmental Protection Agency. [Online]. Available: http://www.epa.gov/ost/fish-advice/volume2/index.html [accessed March 2, 2006].
US EPA. 2000c. Exposure and Human Health Reassessment of 2,3,7,8-tetrachlorodibenso-p-dioxin (TCDD) and Related Compounds. Part III: Dioxin: Draft Integrated Summary and Risk Characterization for 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds. [Online]. Availabe: http://cfpub.epa.gov/ncea/cfm/part1and2.cfm?ActType=default [accessed August 26, 2006].
US EPA. 2001. Water Quality Criterion for the Protection of Human Health: Methylmercury. Chapter 4: Risk Assessment for Methylmercury. Washington, DC: Office of Sciences and Technology, Office of Water, EPA. [Online]. Available: http://www.epa.gov/waterscience/criteria/methylmercury/merc45.pdf [accessed September 12, 2005].
US EPA. 2003. 2001 Toxics Release Inventory Data Release Questions and Answers. [Online]. Available: http://www.epa.gov/tri/tridata/tri01/external_qanda_for_revision.pdf [accessed March 13, 2006].
US EPA. 2005. The Inventory of Sources and Environmental Releases of Dioxin-Like Compounds in the United States: The Year 2000 Update. [Online]. Available: http://www.epa.gov/ncea/pdfs/dioxin/2k-update/ [accessed March 13, 2006].
US EPA. 2006. Health Effects of PCBs. [Online]. Available: http://www.epa.gov/pcb/pubs/effects.html [accessed March 2, 2006].
USDA (United States Department of Agriculture). 2005. National Nutrient Database for Standard Release 18. [Online]. Available: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/sr18.html) [accessed May 11, 2006].
Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environmental Health Perspectives 106(12):775–792.
Van Wijngaarden E, Beck C, Shamlaye CF, Cernichiari E, Davidson PW, Myers GJ, Clarkson TW. 2006. Benchmark concentrations for methyl mercury obtained from the 9-year follow-up of the Seychelles Child Development Study. Neurotoxicology 27(5):702–709.
Verity MA. 1997. Pathogenesis of methyl mercury neurotoxicity. In: Yasui M, Strong MJ, Ota K, Verity MA, eds. Mineral and Metal Neurotoxicology. Boca Raton, FL: CRC Press. Pp. 159–167.
Viberg H, Fredriksson A, Eriksson P. 2003. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory and decreases hippocampal cholinergic receptors in adult mice. Toxicology and Applied Pharmacology 192(2):95–106.
Virtanen, JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppanen K, Laukkanen JA, Salonen JT. 2005. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, Thrombosis and Vascular Biology 25(1):228–233.
Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N. 2002a. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. Journal of Pediatrics 40(1):48–56.
Vreugdenhil HJ, Slijper FM, Mulder PG, Weisglas-Kuperus N. 2002b. Effects of perinatal exposure to PCBs and dioxins on play behavior in Dutch children at school age. Environmental Health Perspectives 110(10):A593–A598.
Vreugdenhil HJ, Mulder PG, Emmen HH, Weisglas-Kuperus N. 2004. Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology 18(1):185–193.
Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. 2005. Blood mercury level and blood pressure among US women: Results from the National Health and Nutrition Examination Survey, 1999–2000. Environmental Research 97(2):195–200.
Walkowiak J, Wiener JA, Fastabend A, Heinzow B, Kramer U, Schmidt E, Steingruber HJ, Wundram S, Winneke G. 2001. Environmental exposure to polychlorinated biphenyls and quality of the home environment: Effects on psychodevelopment in early childhood. Lancet 358(9293):1602–1607.
Watanabe C. 2002. Modification of mercury toxicity by selenium: Practical importance? Tohoku Journal of Experimental Medicine 196(2):71–77.
Weil M, Bressler J, Parsons P, Bolla K, Glass T, Schwartz B. 2005. Blood mercury levels and neurobehavioral function. Journal of the American Medical Association 293(15):1875–1882.
Weinstein MR, Litt M, Kertesz DA, Wyper P, Ross D, Coulter M, McGreer A, Facklam R, Ostach C, Willey BM Borczyk A, Low DE, and the Investigative Team. 1997. Invasive infection due to a fish pathogen: Streptococcus iniae. New England Journal of Medicine 337(9):589–594.
Whanger PD. 1985. Metabolic interactions of selenium with cadmium, mercury, and silver. Advances in Nutritional Research 7:221–250.
WHO (World Health Organization). 1990. Environmental Health Criteria 101: Methylmercury. Geneva, Switzerland: WHO. [Online]. Available http://www.inchem.org/documents/ehc/ehc/ehc101.htm [accessed March 9, 2006].
WHO. 2001. Consultation on Risk Assessment of Non-Dioxin-Like PCBs. 2001 (September 3–4). Presented at the Federal Institute for Health Protection of Consumers and Veterinary Medicine (BgVV), Berlin, Germany. [Online]. Available: http://www.who.int//pcs/docs/consultation_%20pcb.htm [accessed March 17, 2006].
WHO. 2003. Diet, Nutrition, and the Prevention of Chronic Diseases. Geneva, Switzerland: WHO. [Online]. Available: http://www.who.int/hpr/NPH/docs/who_fao_expert_report.pdf [accessed March 2, 2006].
Willett, WC. 2006. Fish: Balancing health risks and benefits. American Journal of Preventive Medicine 29(4):320–321.
Winneke G, Walkowiak J, Lilienthal H. 2002. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology 181–182:161–165.
Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. 2003. Low level methylmercury exposure affects neuropsychological function in adults. Environmental Health 2(1):8.
Yoneda S, Suzuki KT. 1997. Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasma protein. Toxicology and Applied Pharmacology 143(2):274–280.
Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh C-C, Spiegelman D, Stampfer MJ, Willett WC. 2002. Mercury and the risk of coronary heart disease in men. New England Journal of Medicine 347(22):1755–1760.










































































