5
LEVELS OF ASSOCIATION BETWEEN SELECT DISEASES AND LONG-TERM ADVERSE HEALTH OUTCOMES
Only 10% of the roughly 90 infectious diseases endemic in southwest and south-central Asia are likely to pose a long-term health risk to exposed US military personnel. As noted in Chapter 3, the long-term adverse health outcomes of most diseases endemic in the region would usually become apparent during or immediately after the acute illness, and many of the health outcomes are rare. However, nine of the infectious diseases meet the inclusion criteria outlined in Box 5.1 and discussed in Chapter 2. Those nine diseases and their associated long-term adverse health outcomes are the subject of this chapter (Table 5.1).
Following the paradigm of past Institute of Medicine Committees on Gulf War and Health, the committee determined the strength of association between each infection1 and specific long-term adverse health outcomes in humans. For every health outcome discussed in this chapter, there is limited or suggestive evidence of an association, sufficient evidence of an association, or sufficient evidence of a causal relationship with the infectious disease. Several delayed long-term adverse health outcomes of the nine diseases are listed in Chapter 3 but not reviewed here; the committee determined that there is inadequate or insufficient evidence of an association between these health outcomes and the infectious diseases. To reach its conclusions, the committee assessed the available evidence published in the biomedical literature about the long-term adverse outcomes of the diseases on human health.
|
BOX 5.1 Inclusion Criteria The committee used these questions to evaluate the dozens of infectious diseases endemic in southwest and south-central Asia or commonly found among troops in wartime (Table 2.1). If the answer to every question was yes, a disease met the criteria for in-depth evaluation in this chapter.
|
TABLE 5.1 The Nine Infectious Diseases Studied for Strength of Association with Specific Long-Term Adverse Health Outcomes
|
Infectious Disease |
Long-Term Adverse Health Outcomes Evaluated for Strength of Association |
|
Brucellosis |
Arthritis Cardiovascular system infections Ophthalmologic manifestations Genito-urinary tract manifestations Hepatic abnormalities Neurologic manifestations Respiratory system infections Other symptoms (fatigue, inattention, amnesia, depression) |
|
Campylobacter infection |
Guillain-Barré syndrome Reactive arthritis Uveitis |
|
Leishmaniasis |
Delayed presentation of visceral leishmaniasis (VL)a Reactivation of VL in the context of future immunosuppression Post-kala-azar dermal leishmaniasis |
|
Malaria |
Clinical relapse Late presentation or recrudescence of disease Hematologic manifestations Ophthalmologic manifestations Nephrologic disease Neurologic and neuropsychiatric disease |
|
Coxiella burnetii infection (Q fever) |
Chronic hepatitis Endocarditis Osteomyelitis Post-Q fever fatigue syndrome Vascular infection |
|
Salmonella (nontyphoid) infection |
Reactive arthritis |
|
Shigella infection |
Hemolytic uremic syndrome Reactive arthritis |
|
Tuberculosisb |
Activation of latent tuberculosis infection Late manifestations of pulmonary and extrapulmonary tuberculosis |
|
West Nile virus infectionc |
Persistent deficits in cognition, movement, and daily functioning |
|
a Viscerotropic leishmaniasis is considered a subset of VL for the purposes of this discussion. b Tuberculosis (TB) does not meet inclusion criterion 1 (Box 5.1), because there have been no published reports of military personnel who developed active TB while deployed to the Gulf War, Operation Enduring Freedom (OEF), or Operation Iraqi Freedom (OIF). However, in a presentation to the committee, Kilpatrick (2005) indicated that 2.5% of military personnel deployed to OEF and OIF and given predeployment and postdeployment skin tests for TB seroconverted during their deployment; that is, they acquired new TB infections. Immunocompetent people who are infected with TB have a 10% lifetime risk of developing active TB; this risk increases dramatically in people who become immunosuppressed. Therefore, the committee decided to evaluate TB in depth. c West Nile virus infection does not meet inclusion criterion 4 (Box 5.1), because its health outcomes usually are manifested at the time of the acute illness. However, dramatic changes in the epidemiology of West Nile virus infection since the middle 1990s led the committee to decide to review it in depth. |
|
This chapter contains nine sections, with similar formats: one for each disease. Each begins with an introduction to the disease and its etiologic agent, which is followed by a brief description of the acute illness. Then, a summary of diagnostic criteria and methods and of treatment protocols is presented. Each section ends with an evidence-based discussion of the infection’s known long-term adverse health outcomes and their pathogenesis; this discussion is
the basis of the committee’s conclusions about the strength of association between the primary infection and each long-term adverse health outcome.
DIARRHEAL DISEASES: CAMPYLOBACTER, NON-TYPHOID SALMONELLA, AND SHIGELLA INFECTIONS
Among the many pathogens known to have caused diarrheal disease among US troops deployed to the Gulf War, Operation Enduring Freedom (OEF), or Operation Iraqi Freedom (OIF), three merit an examination of their potential long-term, adverse outcomes to veterans’ health: Campylobacter, Shigella, and Salmonella.
Campylobacter Infection
Campylobacter infections are common causes of acute diarrheal illnesses in humans globally (Blaser 2005). The committee examined three potential long-term adverse health outcomes of Campylobacter infection: Guillain-Barré syndrome, reactive arthritis, and uveitis.
The most common pathogenic Campylobacter species is C. jejuni, but disease may also be caused by other species, especially C. coli, C. upsaliensis, C. lari, and C. fetus. The typical illness is acute diarrheal disease lasting 2-5 days accompanied by abdominal pain and fever. The illness responds well to antibiotic treatment but often is self-limited. Campylobacter occasionally causes an acute systemic infection.
Transmission of Campylobacter
Campylobacter species (spp.) infect humans most often through contaminated food or water. Drinking untreated water is a major risk factor for both sporadic and epidemic campylobacteriosis (Allos 2001; Blaser 2005). Foodborne infections occur chiefly after the consumption of improperly heated foods of animal origin; common vehicles include unpasteurized milk and undercooked chicken. Among wild and domesticated animals, Campylobacter spp. may be normal flora or pathogens (Blaser 2005). Rarely, the bacteria are transmitted by person-to-person contact; this occurs chiefly from the handling of feces of incontinent people, such as infants, who are infected.
People suffering from an enteric illness may be infected with two or more bacterial, viral, protozoan, or helminthic pathogens. Some laboratory analyses of stool specimens from deployed troops who had a diagnosis of diarrheal illness found dual infections in a subset of patients, as described in Chapter 4.
Endemicity in Southwest and South-Central Asia
Campylobacter is a common cause of acute diarrhea in southwest and south-central Asia (Wilson 1991). In the United States, the bacteria frequently instigate both sporadic diarrhea and outbreaks (Wilson 1991).
Acute Illness
Patients with Campylobacter infections often present with a short prodrome of symptoms consisting chiefly of headache, myalgias, back pain, and fever. Within 24 hours, the illness centers on the gastrointestinal tract, producing abdominal pain and diarrhea (either may come
first). Common characteristics of the abdominal pain are unlocalized cramping that may be so severe as to mimic acute appendicitis; however, diarrhea predominates over abdominal pain in most patients.
On the first day of diarrheal illness, the patient usually has four to 20 loose stools, and 25% of them may contain visible blood. Laboratory examination of stool specimens usually reveals gross or microscopic blood in all and leukocytes in 70%. Fever continues from the prodrome and persists for 24-48 hours.
Symptoms usually begin to recede after 48 hours and resolve during the next few days. In rare cases, the illness may last longer. In the absence of antibiotic treatment, relapse occurs in about 20% of cases; relapses are usually milder than the initial episodes.
Some people with Campylobacter infections are bacteremic (Mandell et al. 2005); this condition represents either a primary bacteremia or, rarely, the seeding of a distant organ (Blaser et al. 1986).
Diagnosis During and After Acute Illness
Diagnosis of the acute illness is based on culture of feces and, rarely, of blood. Culture-based tests even in the acute phase can have false-negative results, especially in infection by non-jejuni species, because Campylobacter spp. are difficult to grow in culture. Alternatively, the bacteria can be detected with polymerase-chain-reaction (PCR) assay of genetic material from stool specimens. Antibody testing, which is not commercially available, is less reliable because of the diversity of Campylobacter strains, the time required for a response to occur, and differences in magnitudes of responses among hosts.
Infected people shed Campylobacter in stool for a mean of 2-3 weeks after the onset of symptoms; virtually no immunocompetent hosts are still shedding the organism after 8 weeks (Karmali and Fleming 1979; Svedhem and Kaijser 1980; Taylor et al. 1988). Thus, a culture or PCR test conducted more than 2 months after an acute episode of Campylobacter enteric disease would rarely be positive. After 2 months have elapsed, there is no reliable diagnostic test for exposure to Campylobacter in people who manifest diseases that could be late adverse health outcomes of a Campylobacter infection.
Treatment of Acute Illness
Fluid and electrolyte replacement is the treatment of choice for diarrheal illnesses. In patients who are still symptomatic at the time of diagnosis, antimicrobial treatment is recommended, particularly with fluoroquinolones and macrolides. Clinicians should be cognizant of Campylobacter’s growing resistance to those antimicrobials; the degree of resistance will reflect the use of antimicrobials in animal farming and in the local human population.
Long-Term Adverse Health Outcomes of Campylobacter Infection
On occasion, infection by Campylobacter spp. leads to long-term adverse health outcomes. The most serious health outcome associated with campylobacteriosis is Guillain-Barré syndrome (GBS). Reactive arthritis appears to occur after campylobacteriosis at a frequency greater than the background frequency. There is some evidence that uveitis is associated with Campylobacter infection.
Guillain-Barré Syndrome
The first report of an association between Campylobacter jejuni infection and GBS was published in 1982 (Rhodes and Tattersfield 1982). Numerous scientists have since investigated
the relationship between the two diseases and have published more than 200 reports in peer-reviewed journals. By the year 2000, those investigations had established that infection by C. jejuni causes about 30% of all cases of GBS (Allos 1997; Dingle et al. 2001; McCarthy and Giesecke 2001; Nachamkin 2002; Nachamkin et al. 1998; Nachamkin et al. 2001; Sinha et al. 2004; Tam et al. 2003). A number of other infectious diseases are also associated with GBS.
GBS is a severe acute neurologic disease characterized by ascending paralysis with involvement of motor neurons and sometimes sensory neurons (Rhodes and Tattersfield 1982). Developing over a period of days, the symptoms of GBS may lead to paralysis of the respiratory muscles and death; however, with rapid supportive care, the fatality rate has been reduced from more than 10% to less than 5%. Between 10 and 20% of affected persons have permanent neurologic deficits, such as persistent muscle weakness and contractures. Most patients with GBS require hospitalization, and more than 20% require ventilatory support at some time during their illness. Recommended treatment should be started immediately and may include plasmapheresis and intravenous administration of immunoglobulins.
Approximately 0.01-0.03% of US patients who suffer acute gastrointestinal disease due to C. jejuni will develop GBS (Allos 1997; Tauxe and Blake 1992). The risk of developing GBS during the 2 months after a symptomatic episode of C. jejuni infection is about 100 times greater than the risk in the general population (McCarthy and Giesecke 2001). The symptoms of GBS usually are manifested 7-28 days after the onset of gastrointestinal symptoms (Allos 1997; McCarthy and Giesecke 2001). There is no association between the severity of C. jejuni-induced gastrointestinal illness and the risk of developing GBS (Allos 2001).
Rigorous serologic and culture studies have found and validated evidence of recent infection by C. jejuni in high percentages of patients with GBS. Several studies, including at least two case-control studies, showed that GBS patients were more likely than controls to have increased titers of antibodies to C. jejuni (Liu et al. 2003; Mishu et al. 1993). They demonstrated important trends and associations in populations but are neither standardized nor sufficiently accurate to be used for conclusive diagnosis in an individual patient. In another line of inquiry, seven independent studies found that 8-50% (mean, 30%) of stool specimens obtained from patients with GBS at the onset of symptoms were culture-positive for C. jejuni (Enders et al. 1993; Gruenewald et al. 1991; Hariharan et al. 1996; Kuroki et al. 1993; Rees et al. 1995; Ropper 1988; Speed et al. 1984). A positive culture is sufficient for diagnosis of Campylobacter-induced GBS but may be falsely negative, depending on the accuracy of the cultural procedures used, timing after symptom onset, clinical status, and antibiotic use.
There are several types of GBS, including acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and Miller-Fisher syndrome (MFS). Antecedent Campylobacter infections have been linked with AMAN and MFS (Dingle et al. 2001; Kuwabara et al. 2004; Nachamkin et al. 1998); their association with AIDP is controversial (Kuwabara et al. 2004; Nachamkin et al. 1998).
Molecular mimicry is believed to play a role in the nerve damage that occurs in Campylobacter-associated GBS (Nachamkin et al. 1998). Although the mechanism is unknown, some molecular structures on the surface of particular strains of Campylobacter appear to mimic either the glycolipids of peripheral nerves or specific proteins found in myelin (Allos 2001).
The committee concludes that there is sufficient evidence of an association between Campylobacter jejuni infection and GBS, if the GBS is manifested within 2 months of the infection.
Reactive Arthritis
Reactive arthritis (ReA), an acute nonpurulent form of arthritis, is a complication of many infectious diseases that affect parts of the body distinct from those involved in the acute illness (Yu and Kuipers 2003). The disease chiefly follows urogenital or diarrheal infections by multiple etiologic agents, including Campylobacter. ReA that occurs after an episode of campylobacteriosis usually is manifested within several weeks of the acute gastrointestinal illness (Blaser 2000).
The clinical manifestations ReA range from isolated transient monoarthritis to severe multisystem disease. Although it can be highly inflammatory and severe, ReA usually is moderate in intensity. Patients often manifest such constitutional symptoms as fatigue, malaise, fever, and weight loss. The arthritis typically is asymmetric and additive, with new joints becoming involved over days or weeks. Joints of the lower extremities suffer most. Tendinitis is common, as are urogenital, ocular, and mucocutaneous lesions. Rarely, ReA is associated with aortic insufficiency and cardiac conduction abnormalities. Reiter’s syndrome—the triad of arthritis, urethritis, and conjunctivitis—makes up just one portion of the ReA spectrum and is more closely associated with Shigella and Chlamydia trachomatis infections than with Campylobacter.
ReA following infections by various agents occurs most often, although not exclusively, in people who have the gene that encodes a histocompatibility antigen called HLA-B27. Between 30% and 85% of ReA patients have the HLA-B27 gene. However, only 8% of healthy people have the HLA-B27 gene, and only about 20% of them will develop ReA if they contract the triggering infections (NIH 2002). People who are 18-40 years old are at greatest risk for ReA. Men and women are equally likely to contract ReA from enterically-acquired infections; in contrast, ReA from sexually-acquired infections predominantly affects men.
Long-term followup studies of patients who have ReA suggest that some joint symptoms persist for months in 10-60% of cases and that acute symptoms commonly recur (Hannu et al. 2004a; Hannu et al. 2002; Rees et al. 2004). Up to 25% of affected people must change or curtail their work because of joint symptoms. The symptoms of ReA usually last 1-21 weeks and occasionally up to a year (Skirrow and Blaser 2002). Symptoms that persist beyond a year tend to be mild and nondeforming.
ReA is a clinical diagnosis, but the finding of HLA-B27 positivity is helpful. Treatment is symptomatic and uses primarily anti-inflammatory agents, including nonsteriodal anti-inflammatory agents, especially indomethacin.
Population-based studies have provided the most convincing evidence of an association between Campylobacter infection and ReA. Two such studies found that 7% and 1.8% of patients with laboratory-confirmed Campylobacter infection later developed ReA (Hannu et al. 2002; Rees et al. 2004). They validated the results of three independently conducted rheumatologic surveys administered after distinct outbreaks of Campylobacter infection (Bremell et al. 1991; Eastmond et al. 1983; Hannu et al. 2004a). The surveys found that 0.7-2.6% of adults infected with Campylobacter later developed ReA. The scientific literature also contains reports of at least 40 sporadic cases of ReA associated with Campylobacter infection (Hannu et al. 2002). The disparate geographic locations of the studies—including Finland and California—indicate that the association of Campylobacter with ReA is a general, not local, phenomenon.
The pathogenesis of bacteria-induced ReA is poorly understood. Campylobacter organisms invade such host cells as monocytes and dendritic cells, which transport the bacteria
through the bloodstream to multiple locations, including joints (Yu and Kuipers 2003). How Campylobacter and other ReA-causing bacteria survive persistently in joint cells remains unknown, as does the viability of Campylobacter organisms in those cells. Yu and Kuipers (2003) present a plausible hypothesis for the mechanism by which Campylobacter organisms induce joint-specific inflammation: that macrophages present antigenic peptides to CD8+ T lymphocytes through histocompatibility antigen HLA-B27. The T-cell receptor of CD8+ T lymphocytes is specific for both foreign and self peptides carried by HLA-B27. The process may activate CD8+ T lymphocytes and produce the initial inflammatory response. The mechanism of sustained inflammatory response is unknown.
Despite the ambiguous pathogenesis of postinfection ReA, the weight of epidemiologic evidence convincingly illustrates that a small percentage of people infected by Campylobacter spp. later develop ReA.
The committee concludes that there is sufficient evidence of an association between Campylobacter infection and reactive arthritis (ReA), if the ReA is manifested within 3 months of the infection. Most cases of ReA are manifested within a month of the infection.
Uveitis
Uveitis is an inflammation inside the eye that affects the uvea. Known causes of uveitis include autoimmune disorders, infection, and exposure to toxins (MedlinePlus Medical Encyclopedia 2006). In many cases, the cause is unknown.
Three case reports describe uveitis after C. jejuni infection (Hannu et al. 2004b; Howard et al. 1987; Lever et al. 1984). The first report involves one of 350 patients who contracted C. jejuni infection in an outbreak in Finland in August 2000 (Hannu et al. 2004b). The subject of the report, a 72-year-old woman who had gastritis, developed pain and mucopurulent exudation in her left eye without marked redness after the C. jejuni outbreak. Although C. jejuni infection was not confirmed with a stool culture, it was “epidemiologically highly probable” that her prior gastrointestinal symptoms were caused by C. jejuni (Hannu et al. 2004b). About 3 weeks after the acute illness, the woman sought medical attention for the eye symptoms, and mild acute anterior uveitis was diagnosed. An HLA-B27 antigen test was negative. She was treated with local corticosteroid drops and corticosteroid-antibiotic ointment. The condition resolved about 2 months after the acute illness. In a second case report, a previously healthy 39-year-old woman with a culture-confirmed C. jejuni infection developed redness and pain in her eyes about 4 weeks after the gastritis resolved (Howard et al. 1987). The eye condition was diagnosed as nonspecific anterior uveitis. The eye inflammation was treated and resolved over a period of 2 weeks. An HLA-B27 antigen test was negative. In the third case report, acute anterior uveitis was reported in a 34-year-old woman who had a culture-confirmed C. jejuni infection (Lever et al. 1984). She also had hypogammaglobulinemia and chronic diarrhea. No information was given on how the uveitis was treated, how long after onset of the infection the uveitis developed, or how long it took the condition to resolve.
The committee concludes that there is limited or suggestive evidence of an association between C. jejuni infection and uveitis, if the uveitis is manifested within a month of the infection.
Nontyphoidal Salmonella Infection
The genus Salmonella comprises commensal and pathogenic bacteria found in humans, mammals, reptiles, birds, and insects worldwide. These gram-negative, largely motile bacilli are highly adaptable facultative anaerobes 2-3 µm long that reside mainly in the intestines of their hosts. Salmonellae are classified in two species, S. enterica and S. bongori; the former is divided into six subspecies and more than 2,500 serotypes (or serovars) according to their somatic, surface, and flagellar antigens and their habitats (Box 5.1) (Center for Infectious Disease Research and Policy 2006; Pegues et al. 2005).
|
BOX 5.1 Classification of Salmonella Salmonella enterica subspecies enterica (I) subspecies salmae (II) subspecies arizonae (IIIa) subspecies diarizonae (IIIb) subspecies houtenae (IV) subspecies indica (VI) Salmonella bongori SOURCE: Pegues et al. 2005. |
Salmonella enterica serotypes Typhi and Paratyphi cause life-threatening typhoid fever and paratyphoid fever (typhoidal salmonellosis), respectively. Those diseases’ severity, short incubation period, and other salient characteristics would lead to rapid detection, diagnosis, and treatment in deployed US military personnel (CDC 2005b; Olsen et al. 2003). In contrast, uncomplicated infection with nontyphoidal salmonellae causes an array of generally milder illnesses that appear similar to other diarrheal diseases and usually resolve without medical attention. Therefore, the committee devotes attention exclusively to infection with nontyphoidal salmonellae in this chapter.
Transmission of Nontyphoidal Salmonellae
Nontyphoidal salmonellae are most commonly transmitted by the ingestion of contaminated food, especially food of animal origin. Food derived from infected animals that is uncooked, inadequately cooked, unpasteurized, or inadequately pasteurized may transmit the bacteria to humans. Alternatively, such products may cross-contaminate other food that then becomes a vehicle for transmission. Outbreaks of salmonellosis also have arisen from the consumption of fresh produce contaminated with human or animal feces containing salmonellae (Pegues et al. 2005).
Drinking contaminated water infrequently leads to transmission of nontyphoidal salmonellae to humans (Pegues et al. 2005). Exposure to salmonella-infected pets, especially reptiles, can lead to transmission to humans. Rarely, transmission occurs through the transfusion of tainted blood products (Wilson 1991).
Endemicity in Southwest and South-Central Asia
Salmonella spp. is present in all countries (Wilson 1991). The centralized production and wide distribution of manufactured foods in developed nations periodically facilitates large outbreaks of salmonellosis (Pegues et al. 2005).
Acute Illness
Salmonella Gastroenteritis
Gastroenteritis is the most common syndrome of infection with nontyphoidal Salmonella. Some 60-80% of cases occur sporadically. After an incubation period of 6-72 hours, patients experience sudden onset of diarrhea, nausea, and sometimes vomiting. Those symptoms are frequently accompanied by fever, headache, abdominal pain, and chills. Myalgia is sometimes reported. Rarely, patients manifest pseudoappendicitis or mimicry of the intestinal changes of inflammatory bowel disease (Heymann 2004; Pegues et al. 2005).
Microscopic examination of stool specimens during the acute phase reveals neutrophils and sometimes red blood cells.
Salmonella gastroenteritis is usually self-limited. Fever commonly resolves within 48-72 hours after onset. Diarrhea usually resolves within 3-7 days, after 10 days at most; however, patients continue to shed the agent in stool for 4-5 weeks, depending on the serotype of Salmonella. Patients who receive antimicrobial therapy may shed for longer periods (Pegues et al. 2005).
Severe Salmonella gastroenteritis leads to dehydration and hospitalization in 2.2 cases per million in the US population. The disease causes about 580 deaths per year in the United States, primarily in elderly or immunocompromised people (Pegues et al. 2005).
Salmonella Bacteremia
Bacteremia occurs in 1-4% of immunocompetent patients who have Salmonella gastroenteritis. Any serotype of the agent may be responsible. Among adults, the risk of bacteremia is greater for Salmonella-infected people who are immunocompromised (Pegues et al. 2005).
Diagnosis of Acute Illness
Salmonella infection may be microbiologically confirmed by plating freshly passed stool samples onto a primary culture medium. Selenate-based enrichment broths can facilitate the recovery of low numbers of organisms. Rapid immunoglobulin M (IgM) antibody-based serologic tests may supplement stool culture (Pegues et al. 2005).
Treatment of Acute Illness
Uncomplicated gastroenteritis may be treated simply with ingestion of oral rehydration solution to replace water and electrolytes. Antibiotics are indicated in adults who are debilitated; who have HIV infection, continued fever, or high fever; or who manifest extraintestinal infection. Ciprofloxacin, ampicillin, or amoxicillin may be administered to adults. Trimethoprim-sulfamethoxazole and chloramphenicol may be effective for treating people who have microbial-resistant strains (Heymann 2004).
Coinfection with Nontyphoidal Salmonellae and Human Immunodeficiency Virus
Salmonellosis is sometimes the first manifestation of HIV infection. People with HIV are at much higher risk than the general population for salmonellosis, and the risk of Salmonella
bacteremia is 20-100 times greater. Salmonella bacteremia often recurs in HIV-infected people; indeed, such recurrence is a criterion for the classification of AIDS by the Centers for Disease Control and Prevention (CDC) (CDC 1992; Heymann 2004; Kim et al. 2004; Pegues et al. 2005).
Long-Term Adverse Health Outcome of Nontyphoidal Salmonella Infection
As discussed above, ReA is an acute nonpurulent form of arthritis that complicates infections at other sites of the body. The most commonly affected joints are the knees and ankles (Locht et al. 2002). If ReA follows an acute episode of nontyphoidal Salmonella infection, it is manifested 1-2 weeks after the gastrointestinal illness. The reported incidence of ReA among cases of acute nontyphoidal Salmonella infection ranges from only 1% to as high as 29% (Buxton et al. 2002; Dworkin et al. 2001; Hannu and Leirisalo-Repo 1988; Lee et al. 2005; Leirisalo-Repo et al. 1997; Locht et al. 1993; Locht et al. 2002; Maki-Ikola and Granfors 1992; Maki-Ikola et al. 1991; Maki-Ikola et al. 1992; Mattila et al. 1994; Mattila et al. 1998; Nikkari et al. 1999; Sinha et al. 2003; Thomas and Hedayati 1986; Thomson et al. 1994; Thomson et al. 1992; Thomson et al. 1995). Factors that influence the incidence include older age, longer duration of diarrhea, and the presence of HLA-B27.
The duration of symptoms is variable, ranging from months to years (Lee et al. 2005; Leirisalo-Repo et al. 1997; Mattila et al. 1994; Thomson et al. 1995). Antibiotic treatment for the diarrheal illness does not affect the severity of ReA or its duration (Locht et al. 1993; Mattila et al. 1998). Ankylosing spondylitis occasionally follows ReA.
ReA is a clinical diagnosis, but the presence of HLA-B27 is helpful. Symptom-based treatment involves primarily the administration of anti-inflammatory agents.
The committee concludes that there is sufficient evidence of an association between nontyphoidal Salmonella infection and reactive arthritis (ReA) if the ReA is manifested within 3 months of the infection.
Shigella Infection
Like Campylobacter and nontyphoidal Salmonella infections, Shigella infections are common causes of acute diarrheal illnesses in humans globally (Halpern et al. 1989; Shears 1996; Taylor et al. 1991) and have been diagnosed in US troops during the Gulf War, OEF, and OIF. Occasionally, Shigella infections lead to long-term adverse health outcomes, notably ReA and hemolytic uremic syndrome. Each adverse health outcome appears to occur after an episode of shigellosis at frequencies greater than background rates.
Transmission of Shigella Infection
Humans are the reservoir for the four known species of Shigella: S. dysenteriae, S. flexneri, S. boydii, and S. sonnei. They are transmitted by the fecal-oral route and through fecal contamination of unpurified water, or uncooked or undercooked food. Person-to-person transmission is common and is facilitated by lack of hand-washing facilities and inadequate supply of potable water. In military camps, where sewerage is not regular, shigellosis may become epidemic. Although Shigella spp. occasionally infects other primates, such infections have little impact on transmission among humans.
Endemicity in Southwest and South-Central Asia
Shigella is endemic, hyperendemic, or epidemic in locales with minimal sanitation. Shigellosis is well recognized in southwest and south-central Asia. S. flexneri and S. dysenteriae are more common in southwest and south-central Asia than in the United States, where S. sonnei dominates.
Acute Illness
Shigella infection causes an acute diarrheal illness. Symptoms are constitutional; they frequently include malaise and fever, and they immediately involve abdominal bloating, cramping, and diarrhea.
During shigellosis, diarrhea may be nonbloody and watery or bloody; the latter condition is generally termed dysentery. Laboratory examination of stool specimens usually reveals numerous leukocytes. The number of loose stools can range from several per day to more than 20 on the worst day of the illness. Fever and constitutional symptoms typically peak during the period of most severe diarrheal symptoms. The diarrhea may be accompanied by tenesmus, or painful straining while defecating. The illness usually is self-limiting, and patients recover within a week. In the absence of antibiotic treatment, however, shigellosis can be severe or even, rarely, fatal (Bennish 1991).
Diagnosis of Acute Illness
Diagnosis is based on culture of fecal specimens and very rarely blood. When PCR methods are available, they can be equally valuable. People with acute shigellosis remain culture-positive for up to 4 weeks. Beyond that timeframe, culture is inadequate to confirm or refute any relationship of symptoms with Shigella.
Treatment of Acute Illness
Treatment of all acute gastrointestinal infections must be based first on fluid replacement. The use of antibiotics is recommended because it shortens the duration of shigellosis and the likelihood of transmission to other hosts (Bhattacharya and Sur 2003). Resistance to sulfonamides, chloramphenicol, and tetracyclines is nearly universal, and resistance to ampicillin and trimethoprim-sulfamethoxazole is frequent. Treatment with fluoroquinolines or azithromycin is successful, even in short courses (1-3 days). The use of antimotility agents may induce more severe disease and is contraindicated.
Long-Term Adverse Health Outcomes of Shigella Infection
Reactive Arthritis
As discussed above, ReA is an acute nonpurulent form of arthritis that complicates infections at other sites of the body. If ReA follows an acute episode of shigellosis, it is usually manifested 2-3 weeks after the gastrointestinal illness (Calin and Fries 1976; Chen et al. 2002; Finch et al. 1986; Good 1979; Noer 1966; Sieper et al. 1993; Simon et al. 1981). It is most common after S. flexneri infection; it also follows infection by S. dysenteriae (Good 1979) but rarely S. sonnei (Good 1979; Kaslow RA 1979; Lewis 1982; Simon et al. 1981). Ankylosing spondylitis occasionally follows ReA and may be considered as a consequence of Shigella-induced ReA. The symptoms of ReA cause up to 25% of affected people to change or curtail their work. Followup studies suggest that some joint symptoms persist in 30-60% of patients for up to a year, but most patients recover within a few months (Calin and Fries 1976; Rongnoparat
and Panpanit 1987). ReA after shigellosis is rare: in studies of Israeli soldiers with acute diarrheal illnesses, 336 cases of shigellosis were documented from 1993 to 1997 in the field units under surveillance, and none of the subjects developed ReA or ankylosing spondylitis (Bloom et al. 1994).
The most conclusive evidence regarding the incidence of ReA due to Shigella infection comes from the recent population-based study of Hannu et al. (2005). Of 278 patients with Shigella-positive stool cultures, 7% had ReA, and an additional 2% developed other reactive musculoskeletal symptoms; one of the 597 controls had ReA. In the Shigella-positive patients, the odds ratio for developing ReA was 16.2 (95% CI, 2.1-123.9). Some 36% of the ReA patients were HLA-B27-positive. Several additional studies and case reports support the findings of Hannu et al. (Chen et al. 2002; Davies et al. 1969; Finch et al. 1986; Lauhio et al. 1988; Neithercut et al. 1984; Noer 1966; Sieper et al. 1993; Simon et al. 1981).
The committee concludes that there is sufficient evidence of an association between Shigella infection and reactive arthritis (ReA), if the ReA is manifested within 3 months of the infection. Most cases of ReA will be manifested within 1 month of the infection.
Hemolytic Uremic Syndrome
Acute shigellosis may lead to hemolytic uremic syndrome (HUS), a life-threatening disease that afflicts primarily young children and the elderly (Ilnyckyj et al. 2003; Okhuysen et al. 2004). HUS is defined as a clinical triad of hemolysis, thrombocytopenia, and renal dysfunction. It is usually manifested within 6-10 days of the onset of shigellosis; more rarely, people with shigellosis-associated HUS can come to clinical attention as late as 30 days after the onset of enteritis (Nathoo et al. 1995; Parsonnet and Griffin 1993).
Shiga toxins produced by some Shigella strains (particularly S. dysenteriae) cause HUS by damaging endothelial cells, especially in the kidneys. The damage leads to microangiopathy, which results in microangiopathic hemolytic anemia, renal failure, and systemic illness.
There have been many published cases of HUS that occurred after shigellosis. For example, HUS occurred after Shigella infection in two of 42 US tourists to Mexico in 1988 (Parsonnet et al. 1989), 40 of 320 (12%) patients in Bangladesh admitted to a hospital (Rahaman et al. 1975), nine of 241 (4%) patients in Bangladesh (Koster et al. 1978), and seven of 36 (19%) patients in South Africa (Bloom et al. 1994).
The committee concludes that there is sufficient evidence of an association between Shigella infection and hemolytic uremic syndrome (HUS), if HUS is manifested within 1 month of the infection. Most cases of HUS will be manifested within 10 days of the infection.
BRUCELLOSIS
Human brucellosis is a chronic intracellular infectious process that involves Brucella spp. and the human reticuloendothelial system. The process may harm any organ in the human body. Up to 10% of people infected with brucellae may develop chronic disease, which is often due to relapses after partial therapy or to disease progression after undiagnosed and untreated acute disease. Although brucellosis occurs sporadically in many countries, it is endemic in areas of southwest and south-central Asia. The committee discusses below the clinical spectrum of
chronic brucellosis and determines criteria for linking long-term adverse health outcomes to infection during military service in the Gulf War, OEF, or OIF.
Brucellae are small, gram-negative coccobacilli that are facultative intracellular pathogens with the ability to survive and multiply in mononuclear phagocytic cells of infected hosts. Eight Brucella species have been identified, but only a subgroup is associated with human disease (Table 5.2). At present, all Brucella species are considered biovars of B. melitensis.
B. melitensis contains two circular replicons of 1.1 and 1.2 Mb. Its genome contains 3,197 open reading frames. B. melitensis, B. abortus biotypes 1 and 4, and B. suis biotype 1 are very similar. In contrast, B. suis biotypes 2 and 4 contain two replicons of 1.35 and 1.85 Mb, and B. suis biotype 3 contains a single circular replicon of 3.3 Mb (Pappas et al. 2005).
TABLE 5.2 Nomenclature and Characteristics of Brucella spp.
|
Species |
Biotype |
Animal Hosts |
Human Virulencea |
|
B. melitensis |
1-3 |
Goats, sheep, camels |
++++ |
|
B. abortus |
1-6, 9 |
Cows, camels, yaks, buffalo |
++ to +++ |
|
B. suis |
1-5 |
Pigs (biotypes 1-3), wild hares (biotype 2), caribou (biotype 4), reindeer (biotype 4), wild rodents (biotype 5) |
+ |
|
B. canis |
|
Canines |
+ |
|
B. ovis |
|
Sheep |
- |
|
B. neotomae |
|
Rodents |
- |
|
B. pinnipediae and B. cetaceae |
|
Minke whales, dolphins, porpoises, seals |
+ |
|
a Virulence is graded on a scale from no virulence (-) to the highest degree of virulence (++++). SOURCE: Adapted with permission from Pappas et al. 2005. |
|||
Transmission and Endemicity of Brucellosis
Human brucellosis is a zoonosis; almost all infections are derived directly or indirectly from exposure to animals. Humans may be infected through direct contact of abraded skin or cuts with infected animals, their tissues or fluids, inhalation, inoculation of mucosal or conjunctival membranes, or ingestion of infective animal products (most often unpasteurized dairy products) (Lulu et al. 1988).
Human brucellosis occurs sporadically in many developed or industrialized countries, including the United States, but most cases occur in three distinct endemic zones: the Near East and Middle East, including Iran, Iraq, Kuwait, Saudi Arabia, Israel, Jordan, and Turkey; the Mediterranean region, including Spain, Portugal, Italy, and Greece; and Latin American countries, including Peru, Argentina, and Mexico (Abo-Shehada et al. 1996; Bodur et al. 2003; Geyik et al. 2002; Gottesman et al. 1996; Gungor et al. 2002; Gur et al. 2003; Hasanjani Roushan et al. 2004; Khateeb et al. 1990; Lubani et al. 1989b; Lulu et al. 1988; McLean et al. 1992; Memish and Venkatesh 2001; Mousa et al. 1987; Norton 1984; Tasova et al. 1999; Trujillo et al. 1994; Zaks et al. 1995). Endemic disease in those regions is usually associated with B. melitensis infection.
In the endemic zones, infections are acquired typically through consumption of dairy products, especially unpasteurized goat cheese and untreated milk. Human-to-human transmission of brucellosis species is rare but has been associated with transplantation of infected bone marrow, blood transfusion, and possibly sexual transmission of the organism in semen
(Goossens et al. 1983; Ruben et al. 1991). Brucellosis probably is endemic in Afghanistan, but data on its occurrence there are sparse.
Between 100 and 200 US cases of human brucellosis were reported annually to CDC during the 1990s. Brucellosis cases in the United States have begun to shift from people who are occupationally exposed to animals and animal products (such as butchers, abattoir workers, veterinarians, and farmers) to people who ingest unpasteurized goat-milk products imported from Latin America (Chomel et al. 1994; Taylor and Perdue 1989). The disease is 8 times more prevalent at the US-Mexico border than elsewhere in the United States (Doyle and Bryan 2000; Fosgate et al. 2002). In the United States, cattle-associated B. abortus has been the etiologic agent of human brucellosis acquired directly from animals, and B. melitensis the agent of human brucellosis acquired from dairy products (CDC 1986; Spink 1954).
Acute Brucellosis
The acute form of human brucellosis is usually manifested 2-4 weeks after infection as a nonspecific febrile illness accompanied by profuse sweating, headache, malaise, arthralgia, arthritis, myalgia, back pain, or a combination of these. Hematologic abnormalities may include anemia, leukopenia, thrombocytopenia, and clotting disorders that are usually mild and resolve with therapy (Crosby et al. 1984; Pappas et al. 2004). Severe thrombocytopenia is rare (Young et al. 2000). Brucellosis in animals (especially that caused by B. abortus) is associated with spontaneous abortion. Although brucellosis may result in human abortion, it may be no more common than abortion that occurs during any infectious process (Khan et al. 2001; Makhseed et al. 1998).
Diagnosis of Acute Brucellosis
The diagnosis of brucellosis should be considered in the appropriate clinical setting with appropriate demographic risk factors. Laboratory analysis may disclose mild leukopenia, thrombocytopenia, and anemia with minimally to moderately abnormal liver-function tests. Definitive diagnosis involves recovering organisms, usually from blood or bone marrow. Culturing of bone marrow is the most sensitive method of diagnosis (Gotuzzo et al. 1986). In rare cases, brucellae may also be recovered from synovial fluid, cerebral spinal fluid, urine, or biopsy samples (Gotuzzo et al. 1986). Rapid automated bacterial identification systems may occasionally misidentify brucellae, for instance, as Moraxella phenylpyruvica (Roiz et al. 1998). PCR and other molecular techniques may be used, but they are not yet used widely in clinical settings (Colmenero et al. 2002; Fox et al. 1998; Morata et al. 2001; Queipo-Ortuno et al. 1997). If microbiologic cultures are negative, diagnosis of human brucellosis usually involves serologic analysis.
A number of serologic tests for diagnosing brucellosis exist (Al Dahouk et al. 2003; Young 1991). The most widely used is a serum agglutination test (SAT), which measures IgM and immunoglobulin G (IgG) brucella antibody titers. SAT titers above 1:160 are diagnostic for brucellosis in the appropriate clinical setting (Young 1991). A 2-merceptoethanol assay can increase the specificity of the SAT by distinguishing IgG from IgM responses (Baldi et al. 1996). Drawbacks of the SAT include cross-reactivity and inability to diagnose B. canis infection. In some people with brucellosis, an SAT response will not occur. Blocking antibodies may be present, or a Coombs test may be positive (Pascual et al. 1988). An enzyme-linked immunosorbent assay (ELISA) specific for brucella has higher sensitivity and specificity than the
SAT (Almuneef and Memish 2003; Ariza et al. 1992; Khateeb et al. 1990; Lulu et al. 1988) and may be positive when other tests are negative. In evaluating people for neurobrucellosis when the SAT is negative, an ELISA should be performed (Araj et al. 1988). If neurobrucellosis is considered and serum antibody tests and microbiologic cultures are negative, cerebral spinal fluid can be evaluated for the presence of antibrucella antibodies (Kochar et al. 2000a; McLean et al. 1992).
Treatments for Brucellosis and Related Long-Term Toxicity
Treatment of people for brucellosis usually involves administration of tetracyclines (usually doxycycline) with rifampin for 6 weeks (WHO 1986). However, a regimen of oral doxycycline for 6 weeks and streptomycin for 2-3 weeks is more effective (Solera et al. 1995). Streptomycin may be replaced with gentamicin. Administration of aminoglycoside antibiotics is associated with renal and cranial VIIIth nerve toxicity, although if aminoglycosides are appropriately administered during short-course therapy, such complications are rare and often transient.
Coinfection
Although Brucella spp. are intracellular pathogens, there has been no apparent increase in morbidity and mortality during coinfection with brucellae and other intracellular pathogens or infections that disrupt the cellular immune system, such as HIV infection.
Long-Term Adverse Health Outcomes of Brucellosis
Acute brucellosis may be a nonspecific flu-like illness, so a specific diagnosis might not be made. People with untreated brucellosis are at risk for the relapsing and chronic health outcomes described below. In addition, antimicrobial therapy is not 100% effective, and even treated people are at risk for relapse and chronic disease. Clinical manifestations due to relapsing or chronic brucellosis usually are evident within 2-6 months of acute illness and if untreated can persist for years or decades (Spink 1951). Manifestations may be protean and nonspecific. Focal infections have also been reported up to 30 years after probable acute disease (Ariza et al. 2001; Colmenero et al. 2002; Martin et al. 1961; Mousa et al. 1986; Norton 1984; Williams and Crossley 1982; Zinneman et al. 1961).
Diagnosis of Chronic Brucellosis
Diagnosis during chronic brucellosis is similar to that during acute disease. During chronic brucellosis, bacteriologic confirmation may include detecting the organism in a bone marrow sample or in a focal infectious process or abscess. Serologic evaluation is usually positive. Isolated involvement of the central nervous system is rare and is usually diagnosed with serologic analysis or antibody analysis of cerebral spinal fluid.
Major Manifestations of Chronic Brucellosis
The major manifestations of relapsing or chronic brucellosis include the following conditions and organ systems.
Arthritis
Bone and joint complications are the most common manifestation of chronic and relapsing brucellosis, occurring in 10-80% of cases in various studies (Mousa et al. 1987; Tasova et al. 1999; Zaks et al. 1995). Arthritis is usually peripheral and monoarticular and often involves the knee or hip; however, some patients develop polyarthritis (Geyik et al. 2002; Gotuzzo et al. 1982; Gotuzzo et al. 1987; Hasanjani Roushan et al. 2004). Peripheral arthritis may be infectious (in which case it is usually monoarticular, and the organism may be recovered from the joint) or reactive (in which case involvement is often polyarticular or pauciarticular, and the organism will not be recovered from the joint) (Bravo et al. 2003). Sacroiliitis is the second-most frequent articular lesion (Alarcon et al. 1981; Ariza et al. 1993; Khateeb et al. 1990); it is usually unilateral. Spondylitis may affect 5-10% of patients with Brucella arthritis (Ariza et al. 1985; Gotuzzo et al. 1982; Namiduru et al. 2004; Solera et al. 1999). Radiographic features may include the presence of lytic and blastic lesions, erosion of the anterior superior part of the vertebral body (a “parrot peak” sign) (Ibero et al. 1997), and spondylodiscitis. Postinfection spondyloarthritis, bursitis, tenosynovitis, and infection of joint prostheses have also been reported (Weil et al. 2003). Although any joint might be involved during brucellosis, arthritis of the hips and knees is most common during acute disease and is usually manifested within 12 months of infection; involvement of the axial skeletal system and spondylitis are most common during chronic disease; and sacroiliitis might occur during either acute disease or chronic disease (Akritidis and Pappas 2001; Ariza et al. 1985; Colmenero et al. 1996; Doganay et al. 1993; Gotuzzo et al. 1987; Mousa et al. 1987; Namiduru et al. 2004; Norton 1984).
The committee concludes that there is sufficient evidence of an association between brucellosis and arthritis and spondylitis. Arthritis is usually manifested within 12 months of the acute illness; spondylitis might be manifested later.
Hepatic Involvement
Human brucellosis is often associated with changes in liver function and has been associated with granulomatous hepatitis (Harrington et al. 1982; Lulu et al. 1988; Williams and Crossley 1982). Hepatomegaly may be present (Lulu et al. 1988), but cirrhosis has not been reported. Chronic abscesses of the liver and spleen may occur (Ariza et al. 2001; Colmenero et al. 2002; Vallejo et al. 1996).
The committee concludes that there is sufficient evidence of an association between brucellosis and hepatic abnormalities, including granulomatous hepatitis.
Neurologic Involvement
Neurobrucellosis has been reported in 1-5% of adults who have Brucella infections (al Deeb et al. 1989; Bashir et al. 1985; Bouza et al. 1987; Young 1983). It usually involves meningitis or meningoencephalitis that is often chronic (al Deeb et al. 1989; Bashir et al. 1985; Bodur et al. 2003; Bouza et al. 1987; McLean et al. 1992; Mousa et al. 1986; Pascual et al. 1988). Fever, headache, nuchal rigidity, and altered consciousness may occur (Bodur et al. 2003; Gokul et al. 2000). Evaluation of cerebrospinal fluid usually reveals lymphocytic pleocytosis, increased protein concentration, and normal or moderately decreased glucose (Pascual et al. 1988). Microbiologic cultures of cerebrospinal fluid are positive for brucellae in 10-20% of cases. Rare brain or epidural abscesses, myelitis-radiculoneuritis, demyelinating meningovascular syndromes, deafness, sensorineural hearing loss, and GBS have been reported (Dalrymple-Champneys 1950; Kochar et al. 2000a; Lubani et al. 1989a; McLean et al. 1992;
Mousa et al. 1986; Oliveri et al. 1996; Riestra-Castaneda et al. 1996; Thomas et al. 1993). The diagnosis of neurobrucellosis may be made even in the setting of negative microbiologic cultures of cerebrospinal fluid and negative serologic assays if specific antibodies are found in the cerebrospinal fluid (Kochar et al. 2000a; Sanchez-Sousa et al. 1990).
The committee concludes that there is sufficient evidence of an association between brucellosis and chronic meningitis and meningoencephalitis and between brucellosis and infection of the nervous system.
The committee concludes that there is limited or suggestive evidence of an association between brucellosis and myelitis-radiculoneuritis, demyelinating meningovascular syndromes, deafness, sensorineural hearing loss, and Guillain-Barré syndrome
Ophthalmologic Involvement
Anterior-posterior uveitis is the most common ocular manifestation of brucellosis (al-Kaff 1995; Gungor et al. 2002; Rolando et al. 1985a; Rolando et al. 1985b; Rolando et al. 1987; Tabbara 1990). Papilledema, optic neuritis, episcleritis, nummular keratitis, and multifocal choroiditis have also been reported (Gungor et al. 2002; Lyall 1973; McLean et al. 1992; Rabinowitz et al. 2005; Rolando et al. 1985b; Rolando et al. 1987; Walker et al. 1992). Without proper treatment, secondary glaucoma, cataracts, and retinal detachment may occur (Rabinowitz et al. 2005; Rolando et al. 1985a).
The committee concludes that there is sufficient evidence of an association between brucellosis and uveitis.
The committee concludes that there is limited or suggestive evidence of an association between brucellosis and papilledema, optic neuritis, episcleritis, nummular keratitis, and multifocal choroiditis.
Genitourinary Tract Manifestations
Orchioepididymitis may occur in up to 20% of men with brucellosis (Ibrahim et al. 1988; Memish and Venkatesh 2001; Navarro-Martinez et al. 2001; Papatsoris et al. 2002). It is most often unilateral and accompanied by normal urine sediment (Navarro-Martinez et al. 2001). Pyelonephritis and chronic renal abscesses have been reported in association with brucellosis (Zinneman et al. 1961).
The committee concludes that there is sufficient evidence of an association between brucellosis and orchioepididymitis and between brucellosis and local infections of the genitourinary system (for example, pyelonephritis or renal abscesses).
Cardiovascular System Infections
Endocarditis causes the majority of Brucella-related deaths even though it occurs in less than 2% of chronic cases (al-Harthi 1989). Involvement of the aortic valve is most common, and pericarditis and mycotic aneurysms of blood vessels may occur (McLean et al. 1992).
The committee concludes that there is sufficient evidence of an association between brucellosis and cardiovascular system infections.
Respiratory System Infections
Respiratory tract involvement with brucellosis may include pneumonia, pleural effusion, lung nodules or abscesses, miliary lesions, and thoracic lymphadenopathy (Pappas et al. 2003; Wortmann 2004).
The committee concludes that there is sufficient evidence of an association between brucellosis and respiratory system infections.
Other Symptoms
People who have chronic brucellosis often report fatigue, inattention, amnesia, and depression (Gokul et al. 2000; Imboden et al. 1959; Khateeb et al. 1990; Martin et al. 1961; Sacks and Van Rensburg 1976; Spink 1951).
The committee concludes that there is limited or suggestive evidence of an association between brucellosis and fatigue, inattention, amnesia, and depression.
LEISHMANIASIS
Leishmaniasis is an intracellular infection caused by a diverse group of protozoa in the genus Leishmania. It affects an estimated 12 million people worldwide; there are 1-1.5 million new infections each year.
Leishmaniasis presents as one of three major clinical syndromes: visceral leishmaniasis (VL, also known as kala-azar), cutaneous leishmaniasis (CL) and (infrequently) mucocutaneous leishmaniasis (MCL). About 90% of VL cases occur in India, Bangladesh, Sudan, and Brazil; 90% of CL cases in Afghanistan, Brazil, Iran, Peru, Saudi Arabia, and Syria; and 90% of MCL cases, in Bolivia, Brazil, and Peru (Desjeux 2004; Murray et al. 2005). The three syndromes have been divided into a complex taxonomic and etiologic scheme that is explained briefly here (Table 5.3).
CL is divided into Old World CL (referring to occurrences in southern Europe, the Middle East, and parts of southwest Asia and Africa) and New World CL (southern United States and Latin America). L. tropica, L. major and L. aethiopica occasionally disseminate to cause diffuse cutaneous leishmaniasis (DCL). L. braziliensis can cause mucosal leishmaniasis.
TABLE 5.3 Clinical Syndromes Caused by Leishmania Species and Their Geographic Distribution
|
Clinical Syndromes |
Leishmania species |
Location |
|
Visceral leishmaniasis: |
|
|
|
Kala-azar; generalized involvement of reticuloendothelial system (spleen, bone marrow, liver) |
L. donovani |
Indian subcontinent, northern and eastern China, Pakistan, Nepal |
|
L. infantum |
Middle East, Mediterranean littoral, Balkans, central and southwestern Asia, northern and northwestern China, northern and sub-Saharan Africa |
|
|
L. donovani (archiba) |
Sudan, Kenya, Ethiopia |
|
|
L. chagasi |
Latin America |
|
|
L. amazonensis |
Brazil (Bahia state) |
|
|
L. tropica |
Israel, India; viscerotropic form of disease in Saudi Arabia (US troops) |
Parasites in the L. donovani complex cause VL cases globally. Historically, L. tropica was rarely reported to cause VL; a few cases were reported in east Africa (Kenya) and southwest Asia. However, a handful of US soldiers deployed to the Gulf War developed a mild visceral form of leishmaniasis caused by L. tropica (termed viscerotropic disease). Those cases are described in Chapter 4.
Transmission of Leishmaniasis
The Leishmania organisms have two forms: the promastigote (which is flagellated) and the amastigote. The sand fly is the vector and carries the promastigote form. Sand flies inject the
promastigote form of the parasite into humans. Infection is then established with the amastigote form, which is harbored in human macrophages.
Two transmission cycles have been described. In the zoonotic cycle, dogs are the primary animal reservoir, and humans are an occasional host when they are infected by the bite of the sand fly. In south-central Asia (Afghanistan), great gerbils (Rhombomys opimus) are the vertebrate hosts of L. major and thus determine the clinical distribution of associated CL. In the anthroponotic cycle, humans are the sole reservoir, and sand flies remain the critical vector. Phlebotomus papatasi is the sand fly species that transmits L. major throughout most of the Middle East and is present in south-central Asia. Phlebotomus sergenti was recently identified as the species responsible for transmission of L. tropica in Afghanistan (Wallace et al. 2002).
Sand fly bites are exceedingly common in the Middle East. In August 1943, sand fly fever (caused by a phlebovirus) occurred at a peak rate of 235 per 1,000 military personnel deployed to the Persian Gulf (Hertig and Sabin 1964). Because sand flies are most active during warm months, however, there is seasonal variation in the risk of infection. Only 31 cases of leishmaniasis were diagnosed among 697,000 troops deployed during the Gulf War, and deployment to the open desert during cooler weather was thought to be a partial reason for the low incidence of the disease (Cope et al. 1996). Even in areas that are important foci of Leishmania infection, the prevalence of sand fly-caused infection with Leishmania spp. is unpredictable (Fryauff et al. 1993).
Finally, humans have acquired leishmaniasis through parenteral exposure (because of contaminated injection equipment and blood products) and through sexual contact, but those cases are rare.
Endemicity in Southwest and South-Central Asia
Southwest Asia and south-central Asia are home to Old World CL and VL (Oldfield et al. 1991). The potential for anthroponotic acquisition of CL is especially high in Kabul, Afghanistan, where 270,000 persons (in a population of 2 million) were estimated to be infected in 1996 (World Health Organization as cited in Hewitt et al. 1998). Some 4,700 cases of CL were reported in northern Syria in 1999, an increase from the 3,900 cases reported in 1998 (WHO 2002); most CL in the Middle East is caused by L. major.
Acute Leishmaniasis
Old World CL has an incubation period of 2 weeks to 2 months. The most common etiologic agent is L. major, which causes papular lesions that can ulcerate (Wallace et al. 2002). Most (90-95%) CL lesions heal spontaneously, and they rarely cause persistent disfiguration. L. recidivans can cause a chronic cutaneous (“ring”) lesion.
VL has an incubation period of 2-4 months, although it has been reported to be as long as 2 years. Most infected persons remain asymptomatic during the acute phase. When VL evolves to the clinically evident form, classic symptoms include fever, weight loss, weakness, diarrhea, dysentery, and abdominal swelling. The typical triad of diagnostic findings consists of anemia, fever, and hepatosplenomegaly. Complications of the acute infection arise typically from superimposed bacterial infection, sometimes exacerbated by the neutropenia that can result from bone marrow infiltration. Cytokine disruption is probably critical in determining the clinical presentation and in mediating the outcome of infection, even with treatment (Murray et al. 2005). The predominant cell-mediated immune response is characterized by activity of Th1-type CD4+
cells and associated interferon-γ-induced macrophage activation. That response sets the stage for control, but probably not uniform eradication, of the parasite. Macrophage defenses required to kill Leishmania have been extensively studied, as have the pathogen’s antiphagocytic defenses (Cunningham 2002; Teixeira et al. 2006).
Of the twelve people who had viscerotropic leishmaniasis caused by L. tropica in the Gulf War, one was asymptomatic, and the remainder had a mixed picture involving many of the classic features of VL (Hyams et al. 1995; Magill et al. 1994). Those presentations were distinguished from typical VL in that anemia was typically the sole hematologic sign, and most patients had modest increases in liver enzymes. Three of the patients had an underlying disease of relevance: HIV, acute infection with Epstein-Barr virus, and renal-cell cancer (Hyams et al. 1995).
Diagnosis of Leishmaniasis
Several methods have been used to diagnose the various forms of leishmaniasis. Most CL is diagnosed on the basis of its classic clinical appearance, although if the lesion is atypical, prolonged, or not responsive to therapy, biopsy may be performed at the margin of the lesion. PCR is increasingly used in this setting, especially because misdiagnosis may occur (many lesions clinically diagnosed as CL are bacterial in origin). PCR was the mainstay of diagnosis in a recent description of 237 cases of CL acquired in OIF (Willard et al. 2005). Skin testing based on antigens of L. major demonstrates prior infection with Leishmania spp. and is usually positive in active CL caused by L. major.
VL is often diagnosed on the basis of histopathologic detection of amastigotes in biopsy or aspirate of bone marrow, spleen, or lymph nodes. Indirect immunofluorescent monoclonal antibody can also be applied to those tissues. Biopsy samples can be directly cultured, and isoenzyme analysis used for further speciation. Serum antibody testing, often used in assessment of persons with suspected VL, is most commonly performed with the direct agglutination test. However, the performance of this test is highly variable; in fact, serology was negative in a number of the viscerotropic cases identified in Gulf War soldiers. Available serologic tests are based on L. major antigens, so the relevance to viscerotropic leishmaniasis (caused by L. tropica) is unclear. Finally, some investigators have reported that urine-based assays that detect either Leishmania antigen (Sundar et al. 2005) or Leishmania-specific IgG (Islam et al. 2002) were valuable in diagnosing VL.
Treatments for Leishmaniasis and Related Long-Term Toxicity
Most cases of CL will resolve without specific medical therapy. Oral azoles (fluconazole and ketoconazole), cryotherapy, or paromomycin ointment may hasten resolution. Under study is a device called ThermoMed that delivers radiofrequency-generated heat directly to a lesion through a set of prongs placed on the lesion; the device has Food and Drug Administration 510K clearance as of this writing.
Systemic treatment is always indicated for VL. The mainstay of therapy has been pentavalent antimonials, including sodium stibogluconate and meglumine antimonite (Aronson et al. 1998; Murray 2000; Murray 2004). Liposomal amphotericin B was traditionally reserved for antimony-treatment failures, but it is increasingly used as first-line therapy and has been the regimen of choice for soldiers who acquired VL in OEF. Antimonials are not well tolerated in the acute treatment period. Gastrointestinal intolerance, bone marrow suppression, and
hepatotoxicity occur in up to 50% of patients (and are usually reversible). Pancreatitis and abnormalities of cardiac repolarization also occur; the latter is generally unassociated with arrhythmia and resolves within two months after completion of treatment. At least one case of laryngeal edema has been reported to be associated with antimony therapy. Oral miltefosine has also been used for treatment for VL and CL. None of these drugs appears to be associated with long-term toxicity.
Coinfection by Leishmania Parasite and Human Immunodeficiency Virus
VL is estimated to be the third-most common opportunistic infection in HIV-infected persons in southern Europe (Choi and Lerner 2002). The association emphasizes immune control of the organism and reactivation of quiescent infection in the setting of reduced cell-mediated immune response. Indeed, Leishmania infection might reactivate in patients with CD4 counts below 200/µL (Choi and Lerner 2002). The World Health Organization (WHO) estimates that 25-70% of adult VL cases in southern Europe now occur in HIV-infected patients and that AIDS increases the risk of VL by a factor of 100-1,000 (Choi and Lerner 2002). Clinically, leishmaniasis in HIV-infected persons is characterized by atypical presentations (including pulmonary disease, lingual and esophageal ulcerations, and fever of unknown origin), reduced rates of treatment response, progression from cutaneous to visceral disease, higher rates of death, and reduced sensitivity of serologic tests.
Long-Term Adverse Health Outcomes of Leishmaniasis
Cutaneous Leishmaniasis
Infections with L. major have not led to viscerotropic infection, parenteral or vertical transmission, or presentation as an opportunistic infection associated with HIV. Old World CL as a rule resolves spontaneously and rarely causes chronic scarring. All of the numerous cases of CL that have occurred in soldiers involved in OIF (CDC 2003b; CDC 2004b) have reportedly responded to relatively short courses of sodium stibogluconate (Weina et al. 2004; Willard et al. 2005). However, some cases have been associated with large lesions and long duration. Given the difficulty in diagnosis, unrecognized CL has the potential to cause substantial cosmetic problems.
DCL is not as responsive to therapy as CL and can cause progressive disfiguration and destruction of skin and soft tissue.
Visceral Leishmaniasis
The organisms responsible for VL also infect monocytes and macrophages; however, in contrast with L. major, they may establish latency in these cells. This phenomenon results in a demonstrable risk of recurrence in the setting of immunosuppression induced by chemotherapy, transplantation-related processes, or HIV infection (Basset et al. 2005). As discussed above, immune control of VL involves primarily CD4+ T-cell activity (Th1-type response). Conversely, VL promotes formation of Th2-type cytokines, which can inhibit control of the disease. VL is itself an immunosuppressive disease, partly because of infiltration of reticuloendothelium of liver, spleen, and bone marrow and because it has been associated with polyclonal B-cell activation and increased production of numerous autoantibodies. One case report of GBS that predated the clinical appearance of VL by about a month has been reported;
the authors postulated that the parasite could mediate autoimmune damage to peripheral nerve myelin (Fasanaro et al. 1991).
Because L. infantum has been responsible for most cases of HIV-related VL (Russo et al. 2003), it might be particularly likely to persist in macrophages and monocytes. This organism was identified in one of the two cases of VL acquired in Afghanistan (CDC 2004a) but has not been identified in veterans of other conflicts. Of those two cases, one was diagnosed 14 months after deployment ended in Afghanistan, and the patient had symptoms of clinical recurrence. In addition, VL is estimated to be the third-most common opportunistic infection in HIV-infected persons in southern Europe, as detailed above (Russo et al. 2003).
Because the period of latent infection with VL organisms can be long (10 years is commonly cited), immune suppression can allow reactivation of a latent infection. In the description of the viscerotropic cases that occurred in the Gulf War, the authors stated that “if L. tropica is also capable of surviving in a latent state, visceral leishmaniasis will need to be included in the differential diagnoses of illness in veterans of Operation Desert Storm for years to come” (Magill et al. 1993). Although chronic infection is clearly plausible, no systematic studies have investigated the possibility prospectively, in part because there is no accurate and noninvasive screening test for the infection (Ohl et al. 1993). However, intensive evaluation among 150 Gulf War veterans with complaints was unable to identify prior or current infection with Leishmania spp. (Hyams et al. 1995).
Post-kala-azar dermal leishmaniasis (PKDL) is a well-documented long-term adverse health outcome of VL that occurs on the Indian subcontinent and in east Africa (Zijlstra et al. 2003). On the basis of the Indian experience, this health outcome may develop in 5-10% of patients several years after apparently successful treatment for VL (Zijlstra et al. 2003). PKDL has been mistaken for leprosy, and patients with this presentation remain infectious (Zijlstra et al. 2003). Nerve involvement (as is seen in leprosy) has been reported rarely with PKDL (El Hassan et al. 1992; Khandpur et al. 2004).
The committee concludes that
-
There is sufficient evidence of an association between infection with an etiologic agent of visceral leishmaniasis (VL) and delayed presentation of the acute clinical syndrome.
-
There is sufficient evidence of an association between infection with an etiologic agent of VL and the reactivation of VL in the context of future immunosuppression.
-
There is sufficient evidence of an association between VL and development of post-kala-azar dermal leishmaniasis (PKDL) if PKDL occurs generally within 2 years of the initial infection.
MALARIA
Human malaria is caused by infection with one or more of four species in the genus Plasmodium: P. falciparum, P. vivax, P. ovale, and P. malariae. Although estimates vary, there are probably 350-500 million clinical episodes of malaria each year and 0.7-2.7 million deaths (Breman 2001; WHO 2003). Malaria occurs worldwide in tropical and subtropical regions, typically affecting poor and developing areas most severely. P. falciparum predominates in tropical areas; P. vivax, in temperate regions. The two other species are less frequently
encountered: P. malariae is found worldwide, and the geographic range of P. ovale is limited mostly to tropical Africa, the Middle East, southeast Asia, and the western Pacific.
Transmission of Malaria
Malaria infection occurs when a Plasmodium-infected Anopheles mosquito feeds on a susceptible human host, delivering sporozoites that initially invade hepatocytes and mature into merozoites that then invade erythrocytes. The cycle is completed when a competent female Anopheles mosquito feeds on a parasitemic human, obtaining gametocytes that then initiate infection in the mosquito. Many Anopheles species are potential vectors of malaria in different parts of the world, so mosquito species-specific behaviors, including host feeding preference and daily activity patterns, tend to result in varied regional transmission patterns. Often, several mosquito species will combine to constitute an overall vector profile for a region. In tropical areas, transmission intensity is often linked to rainy seasons—typically one major and another less severe. In temperate or seasonally arid regions, a single transmission period is evident (Guerrant et al. 1999).
Endemicity in Southwest and South-Central Asia
The best recent estimates of overall malaria morbidity and mortality in southwest and south-central Asia are about 6 million cases and 59,000 deaths per year (RBM 2005a). Afghanistan and Yemen alone account for an estimated 5.5 million of all cases, on the basis of 2004 data (RBM 2005b). In the malaria-endemic countries of Tajikistan, Azerbaijan, Armenia, Georgia, Kyrgyzstan, and Uzbekistan, malaria occurred at a rate of 0.11 case per 1,000 population in 1990-2003 (RBM 2005h). In contrast, the case rate was about three per 1,000 during the same period in southwest Asia, Afghanistan, and Pakistan combined (RBM 2005h).
About 70% of all infections are caused by P. vivax, but this varies regionally. P. malariae is not reported to be endemic in most parts of southwest or south-central Asia and is rare in areas where it has been reported. Diagnosis and reporting in some areas, such as Iraq and Afghanistan, have been hindered in recent years because of war-related interruptions to the public-health infrastructure. Transmission is highly seasonal and peaks in late July to September.
In Iraq, malaria is endemic in Duhok, Erbil, Ninawa, Sulaimaniya, Tamim, and Basrah provinces. Some 362 cases were recorded in Iraq in 2003. The disease is due exclusively to P. vivax; peak transmission takes place in May-November. The main vectors are A. sacharovi, A. superpictus, A. maculipennis, A. stephensi, and A. pulcherrimus. Most of the cases occur in the northern governorates, mainly in the Zakho district in Dohuk, where four of the five vector species reside (RBM 2005e).
Malaria is endemic in Afghanistan in all areas below 2,000 m in altitude. Afghanistan reported about 600,000 cases in 2003, 93% of which were caused by P. vivax and 7% by P. falciparum (Kolaczinski et al. 2005). Estimates of the rates of feeding of infective vectors on humans in eastern Afghanistan indicated that A. stephensi would contribute 76% of infective bites and A. fluviatilis and A. culicifacies 7% and 3%, respectively. Because of chloroquine resistance, numbers of P. falciparum infections in eastern Afghanistan have increased from 1% of all infections in 1970 to 20% in 2002 (Kolaczinski et al. 2005; RBM 2005c).
Saudi Arabia tends to have equal percentages of infection with P. vivax and P. falciparum but low case totals (1,700 cases in 2003). The primary vector in Saudi Arabia is A. arabiensis (RBM 2005g).
Pakistan reported more than 125,000 laboratory-confirmed cases in 2003, 4 million probable cases, and 14 deaths. Of the laboratory-confirmed cases, almost 70% were caused by P. vivax. The primary mosquito vectors of malaria in Pakistan are A. culicifacies and A. stephensi (RBM 2005f).
In Iran, three provinces in the southeastern corner account for most of the 23,000 cases reported in 2003, 21% of which were caused by P. falciparum. Primary mosquito vectors include A. fluviatilis, A. stephensi, and A. culicifacies (RBM 2005d).
Acute Malaria
All four Plasmodium species can cause cyclic fevers, particularly in naïve populations. Known as malarial paroxysms, the cycles are characterized by rapid onset of high fever with chills followed by rapid resolution, often with intense diaphoresis. The cycles are associated with erythrocyte lysis that occurs at the end of the erythrocytic cycle of infection. The classical (but infrequently observed) periodic attacks occur every second day with the "tertian" parasites (P. falciparum, P. vivax, and P. ovale) and every third day with the "quartan" parasite (P. malariae).
Among populations in endemic areas, the development of partial immunity leads to milder illness and even asymptomatic infections. However, the immune response does not block repeated infections or infections with multiple strains or species. In temperate climates, the long latent phase with P. vivax and P. ovale appears to provide the opportunity for the resumption of transmission when the mosquito season returns in the next year (Guerrant et al. 1999).
Malaria is diagnosed with microscopic examination of blood smears stained with Giemsa or Wright’s stain. An experienced technician can diagnose most cases with examination of routine blood smears (thin smears), but examination of thick smears is more sensitive in detecting those with less severe parasitemia. The key to diagnosis is recognizing the potential for malaria in a potentially exposed person who has fever, anemia, and thrombocytopenia. Deaths from malaria in travelers returning to the United States, most notably with P. falciparum, continue to occur, often in association with delays in diagnosis and in effective therapy (Newman et al. 2004). Other diagnostic techniques have been developed, including fluorescence microscopy, immunologic diagnosis of falciparum malaria with antibodies to the protein HRP2, DNA probes specifically for P. falciparum, and PCR methods (Amino et al. 2005; Berry et al. 2005; Wilson et al. 2005).
Treatments for Malaria and Related Long-Term Toxicity
Resistance to chloroquine and multiple-drug resistance are major problems with P. falciparum in most of Africa, Asia, and South America. Drug-resistant P. falciparum has also been found in the Middle East and southwest Asia, including Iraq (Guerrant et al. 1999). Resistance to chloroquine is an emergent problem with P. vivax in some parts of Asia, Oceania, and South America (Kurcer et al. 2006).
Antimalarial drugs have well-documented acute adverse effects on the skin, gastrointestinal tract, central nervous system, and other organ systems, but evidence of long-term adverse health outcomes is sparse (Taylor and White 2004). Moderate to severe neuropsychiatric complications have been reported in association with mefloquine, doxycycline, combined chloroquine and proguanil, and combined atovaquone and proguanil (Schlagenhauf et al. 2003; Taylor and White 2004). Although retinopathy associated with high-dose long-term chloroquine use has been described, it has rarely been associated with modern prophylaxis. Additional
discussion on the health effects of antimalarial drugs can be found in the section on ophthalmologic complications below.
Coinfection with Plasmodium Spp. and Human Immunodeficiency Virus
There is clear evidence that HIV infection, particularly with lower CD4+ cell counts, is associated with an increased risk of malaria, higher levels of parasitemia, and higher mortality (Butcher 2005). Nevertheless, malaria and HIV coinfection in a soldier deployed to southwest or south-central Asia would be highly improbable, given the low rates of HIV in southwest and south-central Asia, segregation of US military troops from civilian populations, periodic HIV screening of troops and removal of soldiers testing HIV-positive from overseas service, and low malaria transmission rates.
Long-Term Adverse Health Outcomes of Infection with Plasmodium Spp.
Infection by Plasmodium potentially has long-term repercussions for human health. Long-term adverse health outcomes of infection can be manifested in neurologic, neuropsychiatric, ophthalmologic, hematologic, or renal disease. In addition, malaria itself can present after a latency of months to years or may break out anew because of undertreatment.
The epidemiology of malaria in Afghanistan and Iraq suggests that P. vivax is the principal threat to US troops deployed to OEF and OIF. P. falciparum was not known to circulate in Iraq in 1991-2005, and, although reported in Afghanistan, it is far less prevalent than P. vivax. Although P. falciparum predominates in Saudi Arabia, only seven cases of malaria were reported in US troops during the Persian Gulf War, as described in Chapter 4. P. ovale and P. malariae are rare in southwest and south-central Asia.
Hematologic Complications
Splenic rupture is a well-described complication of malaria, particularly that caused by P. vivax. It can occur weeks to months after the acute infection. Hyperreactive malaria syndrome or tropical splenomegaly can be noted months or years after malarial infection (Metha et al. 1996).
Anemia is a principal complication of malaria. It is expected as an acute event, but it may be detected months or even years after the infection. Only in cases of fulminant falciparum malaria is anemia so severe as to be debilitating or life-threatening. Repeated hemolysis, presumably due to subcurative treatments, is reported as a chronic complication (Metha et al. 1996).
The committee concludes that there is sufficient evidence of a causal relationship between malaria infection and hematologic manifestations weeks or months later, particularly splenic rupture after vivax malaria and anemia after falciparum malaria.
Ophthalmologic Complications
A number of case reports describe the complication of retinal hemorrhage associated with severe cerebral malaria and following vivax malaria. Permanent visual loss may result from this complication (Choi et al. 2004). The capillary permeability associated most notably with falciparum malaria has been associated with retinal hemorrhage and edema, with occasional serious visual impairment, in adults and children (Beare et al. 2003; Hidayat et al. 1993; Kochar
et al. 2000b; Lewallen 1998; Tripathi et al. 1995). Retinal manifestations may be noted months or years after the acute malaria infection (Biswas et al. 1996).
Malaria-associated chronic ophthalmologic disorders include side effects of the chronic prophylactic use of antimalarial drugs, such as hydroxychloroquine and chloroquine (Balo et al. 1996; Easterbrook 1999; Lozier and Friedlaender 1989; Niemeyer and Fruh 1989; Portnoy and Callen 1983; Ruiz and Saatci 1991; Tzekov 2005; Wei et al. 2001). The associations have been recognized for decades (Begue 1964; Bernstein 1967; Giles and Henderson 1965; Rubin 1968; Sugiyama et al. 1967). Chloroquine-based prophylaxis is not being used for US troops deployed to OEF and OIF. Oxidative stress has been cited as a possible contributing etiology (Toler 2004). Rynes and Bernstein (1993) highlight the relative rarity of the retinal complications and the need for long-term administration of the drugs.
The committee concludes that there is sufficient evidence of a causal relationship between malaria infection and ophthalmologic manifestations, particularly retinal hemorrhage and scarring, recognized for the first time months or years after the infection.
Neurologic and Neuropsychiatric Complications
Neurologic complications, particularly cerebral malaria due to P. falciparum, are characterized by confusion, clouding of consciousness progressing to coma, and seizures. Cerebral malaria is due largely to sequestration of infected red blood cells in the cerebral circulation (Renia et al. 2006), but coma can also be caused by such other malaria complications as hypoglycemia, uremia, or hypoxia due to pulmonary edema (Idro et al. 2005). Cerebral malaria is fatal in 15-20% of cases, and residual neurologic deficits have been reported in 1-3% of adults and 10% of children (Bajiya and Kochar 1996). It is notable that over 97% of afflicted adults who survive the cerebral episode of falciparum malaria are left without detectable chronic sequelae.
A postmalaria neurologic syndrome has been described in people who were treated for malaria due to P. falciparum (Falchook et al. 2003). The manifestations include confusion, psychosis, seizures, and a fine tremor (Malviya et al. 2005; Meier et al. 2004). There may be an associated magnetic resonance imaging finding of enhancement of nonspecific white-matter lesions (Dey et al. 2001).
Two other postmalaria neurologic complications have been described in case reports and case series. Acute inflammatory demyelinating polyneuropathy and Guillain-Barré syndrome have been reported after falciparum malaria and less frequently after vivax malaria (Chakravarty et al. 2004; Shubhakaran and Sharma 2003). Onset of neurologic symptoms can occur during the acute stage of the illness or days to weeks after the end of the acute illness (Shubhakaran and Sharma 2003; Kanjalkar et al. 1999). Cerebellar ataxia, often with tremors, has also been described after falciparum malaria, possibly resulting from demyelinating lesions in the cerebellum (Metha et al. 1996; Senanayake and de Silva 1994). All reports of patients who experienced acute inflammatory demyelinating polyneuropathy and cerebellar ataxia described complete recovery within months of onset (Chakravarty et al. 2004; Kanjalkar et al. 1999), although the natural history of these disorders after other conditions has been associated with slow recovery and persistent neurologic deficits in some of those affected (Kanjalkar et al. 1999).
One report dealt with neurologic deficits among veterans who had experienced cerebral malaria from P. falciparum during the Vietnam War (Varney et al. 1997). Veterans with a self-reported history of cerebral malaria were found to have a greater frequency of neuropsychiatric
symptoms than veterans who suffered combat wounds. The report raises questions about the potential for cerebral malaria to produce subtle, persistent neurologic deficits that may not have been apparent in examinations conducted during routine medical treatment and followup (Shamo 2001).
The committee concludes that there is limited or suggestive evidence of an association between Plasmodium vivax and Plasmodium falciparum infections and demyelinating polyneuropathy and Guillain-Barré syndrome.
The committee concludes that there is limited or suggestive evidence of an association between Plasmodium falciparum infection and neurologic disease, neuropsychiatric disease, or both, months to years after the acute infection.
Renal Complications
Chronic untreated P. malariae infection can be manifested with chronic glomerulonephritis even years after the onset of infection (Eiam-Ong 2003; Kibukamusoke 1986). In contrast, the nephrotic syndrome and acute glomerulonephritis are far more common near the onset of infection (days to weeks later), may be associated with any malaria infection, and would be manifested after months or years only very rarely.
The committee concludes that there is sufficient evidence of an association between Plasmodium malariae infection and the manifestation of immune-complex glomerulonephritis years to decades later.
The committee concludes that there is sufficient evidence of a causal relationship between malaria infection and renal disease, especially the nephrotic syndrome that may occur weeks to months after acute infection.
Relapse and Recrudescence of Malaria
Some P. vivax and P. ovale parasites remain dormant as hypnozoites in the liver for months after primary infection. The latent period is generally 6-11 months (Mandell et al. 2005), although one report found the latent period to be less than 4 months (Oh et al. 2001).
At the end of their dormancy, P. vivax or P. ovale hypnozoites initiate the same process that occurs during acute malaria, generating tissue schizonts that rupture and release merozoites into the bloodstream. When the merozoites invade and lyse red blood cells, the patient experiences a relapse with acute symptoms resembling de novo infection (Mandell et al. 2005). Such relapses have been described as occurring periodically but irregularly almost always within 2 years after primary infection (Eliades et al. 2005; Shute et al. 1977).
Thus, relapse of malaria may occur after either symptomatic or asymptomatic infection by P. vivax and P. ovale, particularly in people who are taking such prophylactic antimalarials as chloroquine that neither prevent Plasmodium spp. from infecting hepatocytes nor eliminate Plasmodium hypnozoites (Guerrant et al. 1999). In contrast, treatment with primaquine mitigates hepatic infection, reducing the risk of relapse after primary infection by P. vivax or P. ovale (Baird 2005; Shanks and Edstein 2005; Taylor and White 2004). The diagnosis of persistent hepatic infection by P. vivax or P. ovale can be made only if relapse occurs and blood smears or other suitable diagnostics are confirmatory.
The phenomenon of persistent latent hepatic infection does not occur with P. falciparum or P. malariae. However, there are other mechanisms whereby both these species can lead to hepatic disease months or years after the acute infection. Delayed recurrence or delay in onset
several months after exposure may occur with P. falciparum if drug-resistant parasites are inadequately treated (Guerrant et al. 1999). That would be expected to occur weeks to months later, rather than years later. P. malariae may lead to chronic, low-level parasitemia that may be difficult to detect and may persist for many decades. The unusual cases of truly chronic malaria due to P. malariae may require immunodiagnostic techniques or repeated smears to detect the parasite because of low levels of parasitemia.
The committee concludes that there is sufficient evidence of a causal relationship between malaria infection and relapse of disease (Plasmodium vivax or Plasmodium ovale) or late presentation of disease (Plasmodium malariae) months to years after acute infection.
The committee concludes that there is sufficient evidence of an association between infection by Plasmodium falciparum and recrudescence weeks to months after the primary infection, but only in the case of inadequate therapy.
Q FEVER (INFECTION BY COXIELLA BURNETII)
Coxiella burnetii is the etiologic agent of the zoonosis Q fever, which was first described in abattoir workers in Australia in 1935 (Derrick 1937). The organism has since been demonstrated to have a worldwide distribution and has been isolated in a wide variety of animal and arthropod species. Remarkable for its heterogeneity, it is a highly pleomorphic gram-negative coccobacillus that uses multiple routes of transmission.
In vertebrate hosts, C. burnetii targets the host macrophage, where it survives as an obligate intracellular pathogen in the harsh acidic environment of the phagolysosome. The bacteria exhibits phase variation on passage through cell culture; from the phase I virulent stage observed in natural and animal infections, it can shift to an avirulent phase II stage after repeated passage through cell culture. Under adverse conditions, C. burnetii undergoes sporulation, yielding an atypical spore-like form that can survive extreme environmental conditions. It is highly infectious, producing disease after infection with a single organism.
C. burnetii infection causes a wide array of acute and chronic presentations in humans, as described below. Nonetheless, only about 40% of people infected with it report clinical symptoms. About 7% of the general US population is seropositive for C. burnetii (McQuiston and Childs 2002).
Transmission of Coxiella burnetii
Most human cases of Q fever result from the inhalation of aerosols contaminated with C. burnetii of animal origin (Raoult et al. 2005). Infected aerosols may be generated by domesticated farm animals—especially cattle, sheep, and goats—but can also arise from cats, dogs, and birds. Although the organism is not known to cause overt disease in animals, it is shed in milk, urine, feces, and especially amniotic fluid and products of conception. The placenta of an infected ewe may contain up to a billion infectious doses of C. burnetii per gram of tissue; thus, the parturition of livestock can generate highly infectious aerosols.
Most humans who become infected with C. burnetii are exposed through direct contact with farm animals, domesticated animals, or animals in abattoirs. However, several outbreaks of Q fever appear to have been caused by C. burnetii aerosols transported by wind (Tissot-Dupont
et al. 2004) or through fomites, such as contaminated straw used in industrial packaging (van Woerden et al. 2004). For example, the largest outbreak of Q fever ever reported in the UK occurred in 1989 in large, metropolitan Birmingham (West Midlands) probably as a consequence of the windborne spread of C. burnetii spores from farms outside the city (Hawker et al. 1998; Smith et al. 1993).
Less common routes of C. burnetii transmission include the ingestion of infectious raw milk, direct inoculation with contaminated material, and tick bites. Even rarer are reports of transmission within households, through sexual contact, and through blood transfusion (Milazzo et al. 2001).
Endemicity in Southwest and South-Central Asia
Most countries have reported C. burnetii infections (Wilson 1991). Q fever is widespread in Iran, Afghanistan, and Pakistan and is common in the Arabian Peninsula and Syria (Wilson 1991). Studies conducted in Turkey and Oman have demonstrated that 8-12% of the adult populations of those countries have been exposed to the organism, and rates are higher among those who work with animals (Cetinkaya et al. 2000; Scrimgeour et al. 2003). Several clinical reports document the frequency of Q fever among Israelis; one study found that almost 6% of 346 patients who has a diagnosis of community-acquired pneumonia had laboratory evidence consistent with C. burnetii infection (Oren et al. 2005; Siegman-Igra et al. 1997).
Acute Q Fever
Acute Q fever occurs within 10-17 days after exposure to contaminated aerosols. Patients most frequently present with pneumonia, hepatitis, or a self-limited, influenza-like febrile illness. The clinical presentation of Q fever appears to vary geographically; for instance, C. burnetii-induced pneumonia is more common in eastern Canada, and C. burnetii-induced hepatitis predominates in Spain. The acute phase usually lasts 1-3 weeks and resolves without specific therapy or adverse health outcomes.
Coxiella burnetii Pneumonia
The symptoms of Q fever pneumonia include prominent headache, cough, pleuritic chest pain, and fever (Tissot-Dupont et al. 1992). Radiographic findings can vary widely, although nonsegmental and segmental pleural-based opacities are a common feature. Chest films of patients who have been exposed to parturient cats often show multiple rounded opacities (Gordon et al. 1984). Although some patients with Q fever pneumonia develop acute respiratory distress syndrome, the vast majority of patients’ symptoms resolve without adverse health outcomes.
Coxiella burnetii Hepatitis
Q fever hepatitis is characterized by mildly increased transaminases, thrombocytopenia, and frequent autoantibodies. Liver biopsy often reveals a highly specific histology known as a doughnut granuloma (Travis et al. 1986).
Atypical Presentations of Acute Q Fever
Unusual presentations of acute Q fever include aseptic meningitis, meningoencephalitis, peripheral neuropathy, GBS, myocarditis, pericarditis, thyroiditis, bone marrow necrosis, erythema nodosum, glomerulonephritis, and orchitis. Q fever in pregnancy can lead to miscarriage and neonatal death (Raoult et al. 2002).
Treatment of Acute Q Fever and Related Long-Term Toxicity
Although acute Q fever usually resolves spontaneously, antibiotic treatment can reduce the duration of symptoms and may diminish the risk of complications. The treatment of choice is tetracycline or doxycycline given for 7-14 days. Alternative antibiotic regimens include chloramphenicol, quinolones, rifampin, and trimethoprim. In vitro efficacy of erythromycin is poor, but there is some evidence of clinical efficacy in vivo (Raoult 2003).
Possible long-term toxicity of tetracycline use includes nervous and sensory system effects. Benign intracranial hypertension has been described in children and adults on tetracycline and doxycycline (Digre 2003; Gardner et al. 1995; Lochhead and Elston 2003); this complication has resulted in visual-field loss (Digre and Corbett 2001; Gardner et al. 1995; Lochhead and Elston 2003).
Diagnosing Q Fever
The diagnosis of Q fever should be considered in patients who have an appropriate clinical presentation and substantial animal exposure. Nonspecific laboratory findings include increased erythrocyte sedimentation rate, low platelet counts, increased liver enzymes, and multiple transient autoantibodies.
Specific diagnosis of Q fever is complicated. Growth of C. burnetii in culture is not only difficult, but also fraught with biosafety hazards because of its high infectivity and tendency to aerosolize. Most cases of C. burnetii infection are diagnosed serologically. Acute infection is accompanied by a rise in IgM antibody to phase II antigens followed by an IgG response to phase II antigen. In contrast, chronic infection is characterized by high titers of IgA and IgM to phase I and II antigens. IgM antibodies can remain increased for long periods and are not indicative of recent infection (Fournier et al. 1998).
Current methods of antibody detection include indirect immunofluorescence assay (IFA), ELISA, and the less sensitive and less specific complement-fixation assay. Indirect immunofluorescence is now considered to be the reference for serologic diagnosis. Acute infection can be diagnosed on the basis of a 4-fold rise in titer in paired serum samples. Single IFA titers of 1:50 IgM and 1:200 IgG to phase II antigen are considered diagnostic of acute infection, and a titer of 1:800 IgG to phase I antigen is considered diagnostic of chronic infection. Probes that use DNA amplification with PCR are now available to identify C. burnetii in blood, urine, and tissue samples (Parker et al. 2006).
Coinfection with Coxiella burnetii and Human Immunodeficiency Virus
Relatively little is known about C. burnetii infection in HIV patients. In principle, as an intracellular pathogen with long-term persistence in human hosts, C. burnetii might be expected to cause more frequent and more severe infections in the immunocompromised state. Indeed, Raoult et al. (1993) noted a 10-fold increase in the incidence of Q fever among HIV-seropositive
patients in France. Later studies have yielded conflicting results (Madariaga et al. 2004; Montes et al. 1995; Raoult et al. 1993).
Long-Term Adverse Health Outcomes of Q Fever
C. burnetii persists in circulating monoctyes and bone marrow of healthy people who had a diagnosis of Q fever and recovered from the acute illness.
Complications of Acute Q Fever
About 2% of patients with acute Q fever manifest neurologic involvement. Long-term neurologic deficits have been described in that population: motor weakness, blurred vision, residual paresthesia, sensory loss, peripheral neuropathy, and behavioral changes (Bernit et al. 2002; Drancourt et al. 1991; Ferrante and Dolan 1993; Raoult et al. 2005). There are case reports of other rare neurologic deficits. Although the neurologic deficits can be long-term, onset occurs during the acute syndrome. Thus, the association between acute Q fever with neurologic involvement and long-term neurologic deficits is self-evident.
Chronic Sequelae of Coxiella burnetii Infection
The scientific literature contains evidence of five chronic syndromes associated with C. burnetii infection: post-Q fever chronic fatigue syndrome, culture-negative endocarditis, vascular infection, chronic hepatitis, and osteomyelitis. In general, older age and immunosuppression appear to be risk factors for the development of chronic Q fever (Fenollar et al. 2001). There also appear to be risk factors specific to particular syndromes. Although infection with C. burnetii may be chronic, chronic Q fever itself is rarely reported and usually occurs among those with pre-existing abnormalities of cardiac valves or endovascular grafts.
The largest case series to date reviewed 74,202 suspect cases referred to the French National Reference Center for Rickettsial Diseases during a 14-year period (1985-1998) (Raoult et al. 2000). Serum samples were initially screened with the IFA assay for reactive IgM and IgA antibodies to C. burnetii. Samples that tested positive underwent a second IFA assay to determine antibody titers; a phase II IgG titer of at least 200:1 and a phase II IgM titer of at least 50:1 indicated recent Q fever. With that method, investigators identified 7,543 probable cases. To confirm them, the reference center collected, tested, and cultured additional serum, blood, or tissue samples from the patients. C. burnetii was detected with the IFA assay in the samples of 1,383 cases whose serum had IgG titers of at least 800:1. Clinical data on the confirmed cases indicated that 1,070 of the patients suffered acute Q fever and 313 chronic Q fever. Raoult and colleagues reported the clinical and epidemiologic characteristics of these cases (Raoult et al. 2000). The committee drew on their findings (Table 5.4) and others to reach conclusions about the strength of association between C. burnetii infection and the five long-term adverse health outcomes noted above.
TABLE 5.4 Prevalence of Various Forms of Chronic Q Fever Among 295 Cases from France
|
|
Identified Cases |
|
|
Condition |
No. |
% |
|
Endocarditis |
229 |
73 |
|
Vascular infection |
25 |
8 |
|
Abnormal Pregnancy (outcome) |
13 |
6 |
|
Chronic hepatitis |
8 |
3 |
|
Osteoarticular infection |
7 |
2 |
|
Chronic pericarditis |
3 |
1 |
|
Adenopathy |
1 |
<1 |
|
Splenic pseudotumor |
1 |
<1 |
|
Lung pseudotumor |
1 |
<1 |
|
Chronic neurofoci |
1 |
<1 |
|
No identified foci |
6 |
2 |
|
SOURCE: Reprinted with permission from Raoult et al. 2000. |
||
Endocarditis
The most common and well-studied form of chronic Q fever is endocarditis (Brouqui et al. 1993; Raoult et al. 2000; Saah 2000; Stein and Raoult 1995). Most patients have abnormal or prosthetic cardiac valves; however, any part of the vascular tree may become infected (Raoult et al. 1986; Saah 2000). Fenollar et al. (2001) found that 30-50% of patients who had a diagnosis of acute Q fever and underlying cardiac valvular lesions would develop endocarditis. The delay between infection and the onset of endocarditis remains undefined.
Acute Q fever is not a prerequisite of Q fever endocarditis. In the French study noted above, only one-third of the endocarditis patients reported a previous febrile syndrome of unknown etiology within the year preceding the onset of chronic symptoms (Raoult et al. 2000).
Q fever endocarditis differs from typical endocarditis caused by pyogenic bacteria in that fever is often absent and vegetation can be difficult to detect with echocardiography (Fenollar et al. 2001; Fenollar et al. 2006; Gami et al. 2004). Vegetation is distinct on microscopy, which reveals a chronic inflammatory infiltrate and large, foamy macrophages (Marrie 1990; Marrie 2000).
Untreated endocarditis usually leads to death. Even with treatment, mortality is high (23.5%) (Brouqui et al. 1993). Treatment for chronic Q fever endocarditis usually involves combination antibiotic therapy. Regimens may include doxycycline with quinolone alone or with rifampin. Hydroxycholoroquine has also been used in combination therapeutic regimens (Raoult et al. 1999). The optimal duration of treatment is unclear; some experts treat for 18-24 months, and others recommend lifelong therapy given the high rates of relapse after cessation of antibiotics (Maurin and Raoult 1999).
The committee concludes that there is sufficient evidence of an association between infection by Coxiella burnetii and endocarditis years after primary infection.
Vascular Infection
Vascular infections can occur in aneurysm and vascular grafts and are often accompanied by a nonspecific illness characterized by weight loss and fever. The authors of a recent case series suggest that the incidence of C. burnetii vascular infection is underestimated and recommend that C. burnetii serologic tests be routinely carried out in cases of unexplained illness in patients with a history of underlying vascular disease (Fournier et al. 1998).
The committee concludes that there is sufficient evidence of an association between infection by Coxiella burnetii and vascular infection years after primary infection.
Chronic Hepatitis
Several investigators have documented isolated chronic hepatitis as an infrequent manifestation of chronic Q fever (Raoult et al. 2000; Saah 2000; Turck et al. 1976; Yebra et al. 1988). It presents with mildly increased liver enzymes; granulomatous hepatitis is histologically typical when liver biopsy is performed. Patients whose acute Q fever is manifested as hepatitis or who have underlying alcoholic cirrhosis may be more likely to develop this health outcome (Raoult et al. 2000). The time between acute infection and diagnosis of chronic hepatitis may be as long as 2 years (Yebra et al. 1988). This health outcome appears to account for less than 5% of all chronic manifestations of C. burnetii infection.
The committee concludes that there is sufficient evidence of an association between infection by Coxiella burnetii and chronic hepatitis years after primary infection.
Osteomyelitis
Osteomyelitis is another rare manifestation of chronic Q fever. Of the 313 people with confirmed chronic Q fever as identified by Raoult et al., seven had osteomyelitis. Only one of those had an earlier documented acute infection with C. burnettii, although several reported a febrile illness within the previous year (Raoult et al. 2000). Nourse et al. identified three additional cases and described 11 previously reported cases; almost half the cases were in children, and nearly all the patients had contact with farm animals (Nourse et al. 2004). In summary, chronic Q fever sometimes is manifested as osteomyelitis, which may occur with a previously diagnosed Q fever illness or in the context of a known history of acute Q fever.
The committee concludes that there is sufficient evidence of a causal relationship between exposure to Coxiella burnetii and osteomyelitis.
Post-Q Fever Fatigue Syndrome
A post-Q fever chronic fatigue syndrome has been described in several populations of exposed patients (Ayres et al. 1998; Hatchette et al. 2003; Marmion et al. 1996; 2005; Wildman et al. 2002). Five years after the previously mentioned outbreak in the West Midlands, UK, 42.4% of those with diagnosed Q fever reported symptoms of chronic fatigue compared with only 26% of a control group (Ayres et al. 1998). A second study documented high levels of fatigue in exposed subjects 10 years after exposure (Wildman et al. 2002). Twenty-seven months after an outbreak of Q fever in Newfoundland, 52% of patients had persistent symptoms that hampered their activities to the same extent as type 2 diabetes mellitus and coronary arterial disease affected cohorts of Americans (Hatchette et al. 2003).
Those studies have limitations that prevent the scientific community—including the investigators themselves—from definitively confirming the existence of a post-Q fever fatigue syndrome. For instance, the outbreak of Q fever in Newfoundland led farms to close, leaving
many people unemployed; this socioeconomic factor may have confounded the study results (Hatchette et al. 2003). Data on comorbidity were unavailable, followup serologic data were incomplete, and the study also may have been limited by participation bias: subjects who continued to have symptoms may have been more likely to participate in both questionnaire surveys.
Later studies have reported differences in immune-response genes among those who report post-Q fever fatigue compared with those who are unaffected (Helbig et al. 2005; Helbig et al. 2003). One hypothesis is that after C. burnetii infection, Coxiella may persist universally in the bone marrow and be regulated by the host’s immune response. A subset of patients with subtle differences in their immune response may later develop post-Q fever fatigue. That and other hypotheses are under active investigation in Australia and the UK.
The committee concludes that there is limited or suggestive evidence of an association between infection by Coxiella burnetii and post-Q fever chronic fatigue syndrome years after primary infection.
TUBERCULOSIS
The unique properties and history of tuberculosis (TB) led the committee to approach this section differently from the rest of the chapter in two ways. First, initial infection with Mycobacterium tuberculosis (TB infection) is usually asymptomatic, the onset of the disease (TB) is almost always delayed, and relapse of TB may occur years after successful treatment. Thus, TB infection has the potential for delayed long-term adverse health outcomes both because of the onset of clinically evident TB months to decades after initial infection and because of the long-term consequences of acute disease.
Second, TB has a long history of occurrence and transmission in military settings and remains a cause of potential delayed adverse health outcomes in US troops and veterans of the Gulf War, OEF, and OIF—especially those who are or were deployed to regions where TB is highly endemic. The committee discusses TB in the military at the end of this section.
TB is a chronic necrotizing granulomatous infection caused primarily by the acid-fast bacillus Mycobacterium tuberculosis. An obligate aerobe, M. tuberculosis grows best in such tissues with high oxygen tension as the apices of the lung and the renal cortex; this explains why most infections are manifested as pulmonary disease. M. bovis, a related organism, causes a substantial number of TB cases in regions where milk is not routinely pasteurized and where M. bovis-infected cattle are not identified and destroyed.
Transmission of Tuberculosis
TB is transmitted primarily through exposure to airborne M. tuberculosis. When an infected person coughs, sneezes, yells, or sings, microscopic droplets containing M. tuberculosis are expelled into the air. Heavier particles quickly settle out of the air, and lighter ones remain suspended, often for several hours. Inhaled droplets of 1-5 µm in diameter are small enough to reach the alveoli, where the mycobacteria colonize and infect the lung tissue of their new hosts (IOM 2000).
Detection of Tuberculosis Transmission
The human immune system usually does not recognize M. tuberculosis as a foreign body until 2-6 weeks after inoculation. During that lag time, the organisms proliferate, spreading from the lungs to the lymphatics and disseminating in the bloodstream. Once the human immune system mounts its primary response to M. tuberculosis, further growth and proliferation of the pathogen are usually suppressed; most people maintain a latent TB infection (LTBI) that is believed to persist as a benign condition for life unless progression to active TB develops. The initial infection with M. tuberculosis in adults is usually asymptomatic and results in long-lasting cell-mediated immunity to purified protein derivative (PPD) of M. tuberculosis. Only 1-2% of recently infected people will be found to have active TB (CDC 2000a), so the detection of transmission is based largely on the diagnosis of LTBI.
Diagnosing Latent Tuberculosis Infection
Recently acquired LTBI is detected by conversion of a tuberculin skin test (TST) from negative to positive 2-10 weeks after exposure (CDC 2000a). In development are gamma-interferon release assays that measure cell-mediated immunity to M. tuberculosis protein products more specifically than the TST. The US Food and Drug Administration in 2004 approved the QuantiFERON®-TB GOLD in vitro assay by Cellestis Inc. for diagnosing LTBI (FDA 2005). While noting the need for further research, CDC has recommended that the QuantiFERON-TB GOLD be used in place of the TST (CDC 2005d). The new assay is more specific than TST because it uses antigens that are absent from bacillus Calmette-Guérin (BCG) vaccines and nontuberculous mycobacteria (such antigens can cross-react with the TST to produce false-positive results). In addition, the QuantiFERON-TB GOLD requires only a single draw of blood. Its main limitation is expense. The QuantiFERON-TB GOLD could be used as a confirmatory assay, particularly in TST-positive, BCG-vaccinated people.
Risk Factors for Transmission
TB is not a highly infectious disease, so most transmission occurs in such places where people have close and frequent contact such as households and closed community settings. Occasionally, however, infection follows brief, casual contact in airplanes, buses, hospitals, or prisons. Outbreaks of tuberculosis in closed populations where there is crowding, poor air exchange, or both, may lead to substantial transmission. Exposure to corpses who had active TB has also been identified as posing a high risk for the transmission of the disease.
For US military personnel, the risk of becoming infected with M. tuberculosis depends on occupation, living quarters, exposure to TB-endemic populations, chance exposure in an epidemic setting, and other factors. Cases of TB among active-duty military personnel have the potential to cause extensive TB infection (defined by a positive TST) and outbreaks of active TB among deployed troops, especially shipboard personnel.
The closed shipboard environment and extended periods at sea increase the risk of TB transmission to a level at or above that for most household contacts (Kelley 2005). Notable outbreaks of TB occurred aboard Navy ships in 1966, 1987, and 1998 (Kelley 2005). The most recent of those outbreaks occurred after a US marine with acid-fast bacilli (AFB) smear-positive cavitary pulmonary disease was deployed to a US Navy amphibious ship. More than 18% of the crew and 25% of embarked marines—696 people—converted to TST-positive (Kelley 2005).
Endemicity in Southwest and South-Central Asia
TB is a global disease. An estimated 33% of the world’s population is infected with M. tuberculosis, although the incidence of infection has wide geographic variation (WHO 2006b; Wilson 1991).
In southwest and south-central Asia, TB is highly endemic. WHO estimated the regional incidence in 2004 to be 206 cases per 100,000 population (WHO 2006b). In that year, the estimated incidence in Iraq was 200 cases per 100,000, and in Afghanistan, 661 cases per 100,000 (WHO. 2006a). The burden of TB is particularly severe in Afghanistan, which in 2004 had the 12th highest per capita rate of TB cases in the world (Table 5.5) (WHO 2006a). The United States, in contrast, had only 3.6 cases per 100,000 in 2004 (WHO 2006a).
Thus, for US military personnel, the risk of exposure to TB is much greater in south-central and southwest Asia than domestically. Shipboard personnel and people who have extensive close contact with local populations—in prisons or hospitals, for instance—would be at higher risk than other troops for acquisition of TB during military service.
TABLE 5.5 The 12 Countries with the Highest Rate of TB, 2004
|
Country |
Incidence of TB (All Forms), No. Cases per 100,000 Population |
Rank |
|
Djibouti |
1,137 |
1 |
|
Swaziland |
1,120 |
2 |
|
Kenya |
888 |
3 |
|
Sierra Leone |
847 |
4 |
|
Togo |
718 |
5 |
|
Cambodia |
709 |
6 |
|
Zambia |
707 |
7 |
|
Timor-Leste |
692 |
8 |
|
Somalia |
673 |
9 |
|
Zimbabwe |
673 |
10 |
|
South Africa |
670 |
11 |
|
Afghanistan |
661 |
12 |
|
SOURCE: Adapted with permission from WHO 2006a. |
||
Risk of Progression from Latent Tuberculosis Infection to Active Tuberculosis
Persons with LTBI face a 5-10% lifetime risk of developing active TB. The risk is greatest during the first 2 years after infection (Figure 5.1). In general, the likelihood that TB infection will produce active disease varies with the intensity and duration of exposure, size of induration, and age (Figure 5.2) (Comstock et al. 1974a; Mandell et al. 2005; Vynnycky and Fine 1997). Infants, 15- to 25-year-olds, and the elderly are at greatest risk for progression from LTBI to active TB (Comstock et al. 1974b; Stead and Dutt 1991; Stead and Lofgren 1983; Stead and To 1987).
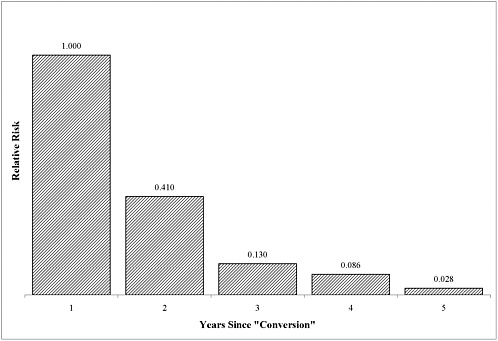
FIGURE 5.1 Relationship between rate at which people experience their first episode of active TB in each year following infection or reinfection and rate at which people experience their first episode of active TB during first year after infection or reinfection. Estimated from distribution of interval between TST conversion and onset of active TB in those who were tuberculin-negative at start of UK Medical Research Council’s trial of BCG vaccine in 1950-1952 (Hart and Sutherland 1977). Relative risk for given year after TST conversion is ratio of (a) proportion of total disease incidence among initially tuberculin-negative people that occurred in that year after conversion to (b) corresponding proportion that occurred during first year after conversion.
SOURCE: Adapted with permission from Vynnycky and Fine 1997.
FIGURE 5.2 Incidence of tuberculosis among initial reactors to tuberculin, by age when tuberculosis was first diagnosed. From 1949 to 1951, investigators administered TSTs to nearly 200,000 Puerto Rican children ages 1-19; 82,269 children tested positive. The investigators followed this cohort through June 1969. During that 18- to 20-year period, 1,400 tuberculin-reactors progressed to active TB. Age was the most important risk factor for progression to active disease, the researchers found. This figure illustrates the age distribution of the 1,400 tuberculin-reactors who progressed to active TB. Incidence rates were highest among children less than 4 years old and second-highest among individuals around 20 years old.
SOURCE: Reprinted with permission from Comstock et al. 1974b.
Another risk factor for progression to active TB is the degree of tuberculin sensitivity, as measured by the size of induration. A 4-year study of 1.2 million recruits to the US Navy found the risk for progression from LTBI to active TB was greater for sailors whose induration at enlistment measured ≥10 mm than for those whose induration was <10 mm (Comstock et al. 1974a). The investigators found that a history of household exposure to TB further increased the risk for progression to active disease. Tuberculin reactors whose induration measured ≥5 mm at enlistment and who had a history of household exposure to TB were more likely to progress to active TB than sailors who lacked such a history.
HIV infection dramatically increases the risk of both primary TB and reactivation TB (Davies 2005). People with LTBI who become infected with HIV face a 5-10% annual risk of developing reactivation TB (Glynn 1998). HIV-infected people exposed to M. tuberculosis have an approximate 40% risk of acquiring the infection and progressing to the active disease within 3 months. Some other conditions also increase the risk of progression to active TB: disorders associated with defects in cell-mediated immunity, such as hematologic malignancies and lymphatic malignancies; diabetes mellitus; renal dialysis; weight loss; intestinal bypass; and gastrectomy. Medical conditions that increase the risk of TB are silicosis, diabetes mellitus, chronic renal failure, leukemias and lymphomas, carcinoma of the head or neck and lung, loss of
at least 10% of ideal body weight, gastrectomy and jejunoileal bypass, radiation therapy, treatment with tumor-necrosis-factor inhibitors, immunosuppression associated with organ transplantation, and corticosteroid therapy. In many cases the stimulating factor is unknown.
Treatment for Latent Tuberculosis Infection to Prevent Active Tuberculosis
To mitigate the risk of LTBI’s becoming active TB, such infections are treated with isoniazid for 9 months. Completing the treatment regimen reduces the risk of active TB by 70-90% (CDC 2000b), but asymptomatic people frequently fail to comply with the regimen.
Active Tuberculosis
Primary Tuberculosis vs Reactivation Tuberculosis
If a chest x-ray picture is taken during initial TB infection, it often shows features of a condition called primary TB: patchy alveolar opacities in the middle- and lower-lung fields, common with unilateral hilar adenopathy. Occasionally, patients with primary TB have fever, nonproductive cough, dyspnea, and—rarely—erythema nodosum. Compression by enlarged lymph nodes may lead to upper- or middlelung collapse. Primary TB generally resolves without treatment. In some patients, however, the immune system cannot contain the infection, and active disease develops, as discussed below. Patients who recover from primary TB (including pleural disease)—particularly those with prior pleuritis—remain at risk for recurrence of active TB.
Historically, a distinction has been made between primary TB occurring at the time of initial TB infection and the more typical adult manifestation of disease, called reactivation TB, developing later. Yet the overlapping temporal and clinical features of the two forms often blur the distinctions between them. One reason for the apparent overlap is uncertainty as to when the primary infection occurred. Therefore, to be consistent with US diagnostic standards, the committee’s discussion of active TB below pertains to both primary and reactivation TB (CDC 2000a).
Diagnosing Active Tuberculosis
The standard approach to diagnosing active TB is through an AFB smear of expectorated sputum. The presence of such mycobacteria as M. tuberculosis in a bodily secretion or tissue specimen can be visually confirmed with the so-called acid-fast test, which exploits the unique properties of the mycobacterial cell envelope. Cells in a specimen are first stained with red carbol fuchsin, then washed with an acidic alcohol solution. The wash decolorizes almost all organisms except mycobacteria because mycobacterial cell envelopes contain mycolic acid, high-molecular-weight lipids, and waxes that prevent the wash from penetrating the cell.
About half of patients with newly diagnosed pulmonary TB have AFB-positive smears. In addition to establishing the likely diagnosis, AFB-positive smears signal highly infectious cases that must be managed through strict isolation. Smears are more likely to be negative in patients with minimal TB or noncavitary TB.
Cultures are performed on such specialized media as Lowenstein-Jensen (an egg-based media), Middlebrook 7H10 (an agar-based media), and Middlebrook 7H102 (a liquid-based media) (CDC 2000a). Using a combination of solid and liquid media will yield positive results
in 85-90% of cultures that contain M. tuberculosis. Culture- and smear-negative cases of suspect TB are treated empirically on the basis of clinical suspicion and lack of an alternative diagnosis.
PCR-based diagnostics provide the diagnosis of TB and, to a lesser extent, extrapulmonary TB, rapidly and with greater sensitivity and specificity compared with sputum smears. Such diagnostics are expensive, however, and offer fewer advantages in cases of paucibacillary (that is, having few bacilli) TB.
Clinical Manifestations of Active Tuberculosis
Pulmonary Tuberculosis
TB presents as pulmonary disease in 80% of reported cases in the United States (CDC 2005c). Similarly, pulmonary TB accounted for 70.7% of cases among hospitalized active-duty US Army personnel from 1980 to 1996 (Table 5.6). The difference in age distribution between the civilian population and the military population probably accounts for much of the 9% difference in the proportion of pulmonary TB between the two groups.
The most common symptoms of pulmonary TB are cough that produces purulent sputum for at least 2 weeks, night sweats, weight loss, and anorexia. Hemoptysis and pleurisy also may occur. Half of patients with pulmonary TB are afebrile, and one-fifth lack pulmonary symptoms altogether.
TABLE 5.6 First Hospitalization Discharge Diagnoses for Tuberculosis Among Active-Duty US Army Personnel, by ICD-9-CM Code, 1980-1996
Signs of consolidation may be present on physical examination. Chest radiographs most frequently show opacities localized to apical and posterior segments of the upper lobes and the superior (dorsal) segment of the lower lobes. Early cavities may be present; these typically are thin-walled and surrounded by opacities, and 10% have air-fluid levels. TB may present atypically in some patients, particularly diabetics, immunocompromised people, and people with HIV infection. In such cases, chest radiographic findings are variable, ranging from dense lobar or segmental consolidation to atelectasis, large-mass lesions, or cavities.
Extrapulmonary Tuberculosis
About 20% of reported cases of active TB occur outside the lungs in such regions as the lymph nodes (9%), pleura (4%), bones and joints (2%), meninges (1%), genitourinary tract (1%),
and peritoneal cavity (1%) (CDC 2005c). Disseminated extrapulmonary TB, known as miliary TB, consists of 1-3 mm nodules throughout the lungs and other tissues.
Tuberculosis Pleurisy
Primary TB is sometimes manifested as an infection of the pleural space, and TB pleurisy may develop later as a progression of LTBI to pleural TB with or without pulmonary TB. The typical presentation is acute onset of fever, cough, and pleuritic chest pains, although there may be a chronic course characterized by fever, general malaise, and loss of up to 10% of body weight. The pleural effusions usually are small to moderate. Concurrent parenchymal disease occurs in one-third to one-half of cases. Diagnosing TB pleurisy usually requires a pleural biopsy, which has a diagnostic yield of 85-95%. The initial TST is negative one-third of the time. Although pleural fluid is exudative, it usually tests negative with an AFB smear and, in 75% of cases, in cultures.
The committee concludes that there is sufficient evidence of a causal relationship between infection with Mycobacterium tuberculosis and occurrence of active tuberculosis months to decades after infection.
Coinfection with Tuberculosis and Human Immunodeficiency Virus
HIV alters the clinical manifestations of TB. For example, pulmonary TB may occur in a lower lobe in a noncavitary fashion. There may be hilar or mediastinal adenopathy, pleural disease, or a normal chest x-ray picture. Extrapulmonary TB is more common among patients coinfected with HIV than among other patients, and it may occur with pulmonary disease or alone. The interactions between chemotherapeutic drugs for TB with antiretroviral drugs for HIV challenge the clinician to treat TB-HIV coinfections effectively.
Treatment for Active Tuberculosis
Treatment for drug-sensitive active TB consists of isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months followed by isoniazid and rifampin for 4 months. That regimen is 95% effective, although about 5% of patients will experience relapses of active TB, most of them in the first 1-2 years after treatment completion (CDC 2003a).
Multiple-drug-resistant TB is more difficult to cure. Regimens should be tailored to known drug susceptibility of the isolates. If an isolate is resistant to isoniazid and rifampin but susceptible to other drugs, treatment regimens would consist of ethambutol, pyrazinamide, and levofloxacin for 12-18 months and an injectible aminoglycoside for the first 2-3 months.
Late Manifestations of Active Tuberculosis
Even after highly effective treatment for acute TB, affected tissues and organs may be functionally impaired or destroyed. Functional impairment may range from imperceptible to severe. The paucity of data on long-term health outcomes of acute TB and the ability of M. tuberculosis to infect and damage virtually any organ of the body challenged the committee to address the late manifestations of this disease comprehensively. This discussion addresses the notable late manifestations of acute TB and the committee’s conclusions about the strength of the association between acute and long-term adverse health outcomes.
Late Manifestations of Pulmonary Tuberculosis
It is well accepted in the biomedical community that disability occurs after pulmonary TB. The most common adverse health outcomes are chronic disabling scarring of the lungs, long-term pulmonary dysfunction, secondary infection of residual cavities, empyema, bronchiectasis, bronchopleural fistulas, and aspergilloma. Rarely, scar carcinoma occurs. Despite the centuries-old history of TB, however, the published data on long-term manifestations are inadequate for the committee to comment on their duration, range, or severity of adverse health outcomes.
A number of studies have compared pulmonary function tests (PFTs) at diagnosis of and after treatment for active TB. For instance, a study of 25 TB patients in Canada found abnormal PFTs (reduced 1-second forced expiratory volume and forced vital capacity) in individuals with cavitary TB but not in those with noncavitary TB (Long et al. 1998). Another study, of 74 TB patients in South Africa, showed a restrictive lung function pattern in 57% of cases and an obstructive lung function pattern in 11%. After treatment for TB, 53% of patients still had abnormal PFTs (Plit et al. 1998). Although neither study commented on the potential disability associated with the findings, none of the patients had residual impairment of oxygenation at rest.
Multiple case reports have been published of patients who have developed “scar cancer”, lung cancer associated with lung scars from TB or other causes (Ardies 2003). The risk of cancer after TB has not been quantified, nor has the percentage of TB patients left with pulmonary scarring. One report from Japan indicated a 2% prevalence of lymphoma of the pleural space among patients with chronic pyothorax, which is associated with tuberculosis (Iuchi et al. 1987). Because pyothorax is a rare complication of TB, the authors could not quantify the overall risk of cancer posed by TB. In summary, the available data are insufficient for the committee to determine whether an association exists between cancer and active TB. If such an association does exist, cancer is undoubtedly a very rare consequence of TB.
Late Manifestations of Extrapulmonary Tuberculosis
The long-term adverse health outcomes of extrapulmonary TB occur with varied frequency depending on the site of active disease. The more common forms of extrapulmonary TB, pleural and lymphatic disease, rarely have long-term adverse health outcomes. Two of the less common forms, TB meningitis and skeletal TB, are more likely to result in irreversible tissue damage. In general, estimates of the long-term prognoses for uncommon forms of TB are based on relatively small series of patients.
Tuberculosis Meningitis and Long-Term Neurologic Disability
It is well accepted in the biomedical community that TB meningitis is associated with long-term neurologic outcomes. The extent of disability depends on the duration and severity of acute symptoms, the age of the patient, and the neurologic deficits (Dube et al. 1992; Kennedy and Fallon 1979). Of TB meningitis patients with stupor or dense paraplegia or hemiplegia, about half either die or recover with severe residual neurologic deficits (Kennedy and Fallon 1979). Until the 1990s, most reports of outcomes for adult patients with TB meningitis focused on mortality. As the recovery rate increased, more studies about the long-term prognoses for these patients emerged. Table 5.7 summarizes the findings of seven studies about long-term neurologic deficits of TB meningitis.
TABLE 5.7 Proportion of Patients Diagnosed with TB Meningitis Who Have Long-Term Neurologic Deficits
|
Study |
No. Patients |
All Adults? |
No. Survivors |
Time of Followup |
No. (%) Survivors with Neurologic Deficits |
|
Prospective Analyses |
|
|
|
|
|
|
Lau et al. 2005 |
156a |
nob |
130 |
3 years |
20 (15) |
|
Kalita and Misra 1999 |
56 |
no |
44 |
1 year |
4 (9) |
|
Retrospective Analyses |
|
|
|
|
|
|
Sutlas et al. 2003 |
|
|
|
24 months-6 years |
|
|
|
61 |
yes |
44 |
(mean, 3 years) |
19 (31) |
|
Hosoglu et al. 1998 |
96a |
yes |
52 |
9 monthsc |
21 (40) |
|
Yechoor et al. 1996 |
30 |
yes |
17 |
9 months |
5 (29) |
|
Verdon et al. 1996 |
48 |
yes |
17 |
1 year |
4 (24) |
|
Bergin et al. 1989 |
28a |
no |
24 |
unspecified |
7 (29) |
|
a Patients not followed for the entire period are excluded from this table. b Only seven patients were >15 years old during the acute phase of illness. c A minimum followup period of 9 months is not directly stated in the article, but is inferred from the minimum duration of treatment reported. The committee was unable to obtain confirmation from the authors of the study. |
|||||
In a recent study of the influence of HIV infection on the outcome of TB meningitis, the authors reported severe neurologic deficits in 5.9% (2/34) of HIV-positive survivors and 17% (53/310) of HIV-negative survivors (Thwaites et al. 2005). HIV status did not alter the neurologic presentation of TB meningitis but significantly reduced the survival rate.
Spinal Tuberculosis and Long-Term Neurologic Disability
It is well accepted in the biomedical community that spinal TB is associated with spinal deformity and neurologic outcomes. A review of 694 patients in Turkey, most of whom were treated both surgically and medically, reported that only 41% had improved after treatment (Turgut 2001). A series of 70 patients in India, of whom only one underwent surgery, reported that 74% had excellent to good results (Nene and Bhojraj 2005).
The committee concludes that there is sufficient evidence of an association between severe forms of pulmonary and extrapulmonary tuberculosis and long-term adverse health outcomes due to irreversible tissue damage.
Relapse of Active Tuberculosis
Even with current therapy under direct observation by health-care providers, relapse can occur in about 5% of treated patients and create a potential for additional late adverse health outcomes (CDC 2003a).
Potential Relationships Between Tuberculosis and Military Service
TB is potentially connected to military service in two ways. First, people who are TST-positive before deployment have LTBI and are at risk for developing active TB during deployment; troops with active TB in the field place other troops at risk for infection and disease. Second, troops who are TST-negative before deployment may become infected with TB during deployment. Such people occasionally manifest active TB shortly after infection but more frequently have LTBI.
Promotion of Tuberculin Skin Testing
The most effective way to mitigate TB transmission and activation is to identify and treat LTBI. That is a compelling argument for testing all military personnel for TB before and after every deployment. Table 5.8 summarizes the policies of each branch of the military regarding TSTs and treatment for LTBI.
Available data suggest that prior M. tuberculosis infection and exposure in the theater of operations contribute about equally to the prevalence of LTBI and the risk of disease among US military personnel. The Department of Defense (DOD) estimates that 2.5% of military personnel deployed to southwest and south-central Asia during OEF and OIF acquired new M. tuberculosis infections during deployments (Kilpatrick 2005). In comparison, the prevalence of TST reactivity among young adults entering the Navy in 1997 and 1998 was 3.5% (Smith et al. 2002); among military police who participated in refugee and humanitarian operations in Guantanamo Bay in 1995, it was 3.7% (Kortepeter and Krauss 2001). No case of active TB has been recognized in troops deployed to the Persian Gulf during Operation Desert Shield or Operation Desert Storm (Hyams et al. 1995).
TABLE 5.8 US Military Requirements Regarding Who Must Receive TSTs and When Tests Must Be Administered
|
Branch of Military |
Name of Policy |
Requirements (Abbreviated) |
Effective Date |
|
Army |
Army LTBI Surveillance and Control Program |
For personnel not previously known to have a positive TST, skin tests will be administered to
For personnel known to have a positive TST previously, per CDC guidelines based on risk, no further TSTs will be applied. Exceptions include clinically valid doubt about previously recorded result, borderline result characterized as positive at prior test time and cases in which a 10-mm increase in reaction size or other factors might warrant treatment. |
May 27, 2003 |
|
Navy and Marines |
Tuberculosis Control Program (Bureau of Medicine and Surgery Instruction 6224.8) |
Each commander, commanding officer, or officer-in-charge is responsible for the maintenance of an effective TB-control program in his or her command. The TST with PPD administered with the Mantoux method is the most sensitive and specific test available for identifying persons infected with M. tuberculosis. Infected persons must be evaluated periodically and kept informed about the symptoms of TB disease. TB screening on entry into Naval service: all persons first entering duty in the regular Navy, Naval Reserve, Marine Corps, or the Marine Corps Reserve for more than 30 days must have the results of TSTs documented in their medical treatment records. Annual TB screening: TSTs must be administered annually to personnel in operational units and in units with a high risk of TB exposure or outbreaks, and the test results must be recorded. The level of risk for a geographic region is based on numerous sources, including reports by, WHO, and PAHO. This policy applies to
|
February 8, 1993 |
|
Branch of Military |
Name of Policy |
Requirements (Abbreviated) |
Effective Date |
|
|
|
Annual TB screening also is required when recommended by cognizant Navy Environmental Preventive Medicine Units (for example, for personnel at some high-risk overseas duty stations). Predeployment and postdeployment screening: All service members must undergo TSTs and have the results recorded within 12 months before deploying and again within 90 days of returning. Ona case-by-case basis, some personnel are required to undergo TSTs just before deploying. Triennial screening: Required for all personnel who are not required to undergo annual testing. Screening before separation from Naval service: All personnel must have TSTs (or annual clinical evaluations in the case of previously known reactors) documented within the 1-year period before separation from the naval service. |
|
|
Air Force |
Air Force Surveillance, Prevention, and Control of Diseases and Conditions of Public Health or Military Significance (Air Force Instruction 48-105) |
The Air Force uses a targeted LTBI screening program. Except for an initial test on accession, personnel are tested only when they have high risk exposures, high risk occupations, or clinical indications for testing. (Air Force Instruction [AFI] 48-105, March 1,2005). The Mantoux TST is the current standard test for identifying LTBI (AFI48-105). The Air Force uses WHO data to determine the prevalence of TB in each country. A country is considered to have a high prevalence if WHO has found the incidence to be at least 30 cases per 100,000 of population (personal communication). The Air Force routinely issues a TB country risk assessment for commanders of bases outside the continental United States. A TST is administered and the results are recorded
|
March 1, 2005 |
Given those data, the only way to determine whether military personnel and reservists have become infected with M. tuberculosis during their service is to test all personnel for TB shortly before and after deployment. Such testing would make it possible to trace cases of active TB to periods of military service if that is when infection occurred.
Anticipating Multiple Drug-Resistant Mycobacterium tuberculosis Infection
Some M. tuberculosis strains are resistant to one or more drugs commonly used to treat LTBI and active TB (WHO 2004). The military’s medical corps should obtain the results of available drug-susceptibility tests for M. tuberculosis in regions where troops are. WHO periodically publishes a report of such data, Resistance in the World: Anti-TB Drug Prevalence and Trends. Those reports could help the military to estimate the likelihood that a person who acquires a TB infection harbors a drug-resistant strain.
WEST NILE VIRUS INFECTION
First isolated in 1937 from a febrile woman in the West Nile Province of Uganda, West Nile virus (WNV) belongs to the Japanese encephalitis virus antigenic complex in the family Flaviviridae (genus Flavivirus) and is closely related to St. Louis encephalitis virus. WNV is a 50-nm-diameter single-stranded RNA virus with a nucleocapsid core surrounded by a host-derived lipid membrane (Campbell et al. 2002).
The first human epidemics of West Nile fever were reported in Israel and occurred in 1951-1954; 2 decades later, an outbreak was reported in South Africa. By 1991, the disease had occurred throughout Africa, south Asia, and Europe. WNV has also occurred in Australia and New Zealand, but cases there were poorly documented (Wilson 1991). Later outbreaks were reported in Tunisia (1997), the Czech Republic (1997), Italy (1998), Romania (1996, 1999), the United States (1999), France (2000), and Israel (1997-2000) (Petersen and Roehrig 2001).
The US outbreak of WNV in 1999 marked the virus’s debut in the Western Hemisphere (CDC 2005e). WNV spread rapidly from its epicenter in New York City; by 2004, 48 states and the District of Columbia had reported human cases (Table 5.9) (CDC 2005a; Nash et al. 2001). It has been found in Canada and Mexico as well (Gould and Fikrig 2004).
TABLE 5.9 Statistics on US Cases of West Nile Neurologic Disease,a 2005
West Nile virus was considered relatively benign to humans before the 1990s (Solomon and Cardosa 2000). WNV usually causes a self-limited illness, West Nile fever, which is
manifested as fever with a variety of other conditions, including rash, arthralgia, myalgia, headaches, and gastrointestinal symptoms.
Since the 1990s, however, there have been reports of increased incidence and severity of WNV illness (Solomon and Cardosa 2000). New neurologic and ophthalmologic manifestations of West Nile encephalitis have been recognized each year since the virus first reached North America in 1999 (Cunha 2004). A small but significant proportion of cases of West Nile neurologic disease (WNND) have led to death, particularly among the elderly. These recent, marked changes in the epidemiology of WNV illness led the committee to include it in this chapter even though the long-term adverse health outcomes of WNV usually are manifest during the acute illness.
Alarm triggered by the sudden change in the incidence and severity of WNV illness must be tempered by the understanding that severe WNV disease remains rare. Only 0.7% of people who become infected with West Nile virus in the United States develop severe neurologic disease, and more than one-third of these recover fully within a year (Klee et al. 2004; Mostashari et al. 2001). About 20% of infected people develop traditional, self-limited West Nile fever, and about 80% are asymptomatic, whereas only 1 in 150 develops neurologic manifestations.
Transmission of West Nile Virus Infection
Although WNV is found in several species of mosquitoes, the vast majority of infections are transmitted by Culex spp. (Campbell et al. 2002). These highly ornithophilic vectors transmit the virus among its natural reservoir: birds. Detected in more than 275 species of birds, WNV is particularly virulent for the family Corvidae, which includes crows and jays. The virus amplifies itself in birds’ bloodstream to a trillion or more virions per milliliter. Mammals are end-stage hosts and may develop disease but do not develop high enough viremia to contribute significantly to the virus’s epidemic spread.
WNV is transmissible from human to human through blood transfusions, transplanted organs, the placenta, and breastfeeding (CDC 2002). Between June and December 2003, WNV nucleic acid amplification testing (NAT) was performed on about 6 million units of blood, which resulted in the removal of at least 818 viremic blood donations. However, even with NAT testing, there were 6 cases of transfusion-associated WNV infection due to low levels of virus not detected by the testing method (minipools from 6-16 donations were used rather than individual testing) (CDC 2004c).
Endemicity in Southwest and South-Central Asia
WNV has been reported in Afghanistan, Pakistan, Iran, and other countries in southwest and south-central Asia (Arsen'eva 1982; Hubalek and Halouzka 1999; Naficy and Saidi 1970; Sugamata et al. 1988; Wilson 1991). In Afghanistan, antibodies to WNV were found in Kunduz, Heart, Bamyan, and Helmand provinces (Arsen'eva 1982). In neighboring Pakistan, 50-65% of the population of Karachi reportedly had antibodies to WNV in 1983 and 1985; new infections were identified in 13% of the population during those years (Sugamata et al. 1988). Similarly, a serum survey conducted in northeastern Iran in the late 1960s found that 30% of surveyed subjects had antibodies to WNV (Naficy and Saidi 1970).
Acute West Nile Fever
Most persons infected with WNV are asymptomatic. A seroepidemiologic study of 677 people who lived in New York City during the 1999 outbreak found that 80% of seropositive subjects never developed symptoms (Mostashari et al. 2001). After an incubation period of 2-14 days, 20% of infected subjects developed a nonspecific febrile illness that lasted 3-6 days. Nausea, vomiting, myalgia, and headache are typical symptoms. A generalized maculopapular rash may occur in up to 20% of patients.
About 1 in 150 symptomatic patients in New York City developed WNND (Mostashari et al. 2001), which is often manifested as meningitis (WNM) or encephalitis (WNE) and sometimes as acute flaccid paralysis (AFP). Patients with WNND frequently have movement disorders with tremor, myoclonus, or Parkinsonism (Sejvar et al. 2003). Muscle weakness is also common. Investigators have reported paresis in about 50% of WNND cases and complete flaccid paralysis in 10%; the latter cases lack deep tendon reflexes and mimick GBS. Seizures and focal neurologic findings have been uncommon.
The development of WNND has been directly correlated with age. Of those over 65 years old, 1 in 50 developed WNM or WNE vs 1 in 300 of those under 65 (Mostashari et al. 2001). In fact, in those over 80 years old, the risk of symptomatic neurologic disease was 43 times higher than in those under 19.
West Nile fever without meningitis is more likely in younger patients. Among those with neurologic involvement, meningitis is more common in younger patients (mean age, 35 years), and encephalitis is more common in older patients (mean age, 70 years) (Sejvar et al. 2003).
Patients with West Nile fever who do not have neurologic manifestations might have residual fatigue, muscle weakness, and headache that can persist for months after resolution of the acute febrile illness (Watson et al. 2004). Of 98 patients with laboratory-confirmed West Nile infection but no clinical evidence of WNM, WNE, or AFP, 96% had fatigue for a median of 36 days, 61% had muscle weakness for a median of 28 days, and 71% had headache for a median of 10 days. The median time for recovery to a point that the patients considered “back to normal” was 60 days.
Diagnosis of West Nile Fever
For patients with acute symptomatic WNV infection, relative lymphocytopenia (less then 20%) is common. The cerebrospinal fluid (CSF) reveals a mild lymphocytic pleocytosis with a mean of 38 white cells/mm3 (range, 0-525) (Nash et al. 2001). Up to one-third may have more then 50% neutrophils on initial evaluation of the CSF. Increased protein with a mean of 104 mg/dL (range, 38-899) can be found. CSF glucose is usually normal.
Imaging studies of the brain usually are normal on computed tomography without evidence of inflammation. Even magnetic resonance imaging scan reveals enhancement of the meninges or periventricular areas in only about 30% of people (Nash et al. 2001). Electromyography reveals a motor axonal polyneuropathy with sparing of the sensory fibers very similar to the findings in poliomyelitis. WNV has a propensity to involve the anterior horn cells of the spinal cord in a manner very similar to poliomyelitis.
Acute infection is diagnosed by demonstration of WNV IgM in serum, which has been found in close to 100% of patients (Tardei et al. 2000). In one study, CSF samples from 94% of patients were WNV-IgM positive (Nash et al. 2001). No cases had virus isolation from CSF, and
only 57% of CSF samples and 14% of serum samples were positive with PCR. Patients with WNV may have persistent IgM antibodies for WNV. In a study by Roehrig et al. (2003), seven of 12 patients with serial samples had IgM persistently positive for WNV for 500 or more days.
Prior infection can be detected with measurement of WNV IgG. However, in a survey of 865 deployed front-line troops, 30 had both predeployment and postdeployment IgG antibodies against WNV. There was no evidence of acquisition of infection during deployment: there were no fourfold rises between predeployment and postdeployment samples, and no IgM antibodies were detected. Infection with dengue virus and prior yellow fever virus vaccine may result in detection of cross-reactive antibodies and make interpretation of serologic tests difficult. The above 30 persons’ serum samples were also reactive to St. Louis encephalitis, dengue, and yellow fever viruses. Because of the high cross-reactivity with St. Louis encephalitis virus, dengue and yellow fever viruses, confirmation of a positive WNV IgG requires testing with the plaque-reduction neutralization test, which requires a biosafety level 3 facility (Gea-Banacloche et al. 2004). Prior WNV can be confidently diagnosed if the WNV neutralizing-antibody titers are 4 times higher than all the other flavivirus titers.
Treatment of West Nile Virus Infection
There is no known effective treatment for WNV infection. Ribavirin, a guanosine analogue with broad-spectrum antiviral activity, has been shown to have activity against WNV in vitro (Jordan et al. 2000). Ribavirin also has concentrations in CSF that are 70% of those in serum. Ribavirin has been used successfully to treat related viruses including LaCrosse encephalitis, Hantaan, Lassa fever, and hepatitis C viruses (Jordan et al. 2000). However, in an outbreak in Israel, patients treated with ribavirin had a higher mortality than those who were not treated (Petersen and Roehrig 2001). The poor outcomes could have been due to patient selection, with sicker patients being treated with ribavirin, inasmuch as this was a nonrandomized study. If it is effective, the predicted required dose would be high, around 4 g intravenously every day, similar to that for treatment of Lassa fever. Interferon has also been noted to have in vitro activity against WNV and has been used in individual cases (Kalil et al. 2005).
A chimeric WNV vaccine with a type 4 dengue virus backbone (an attenuated deletion mutant) with an attached WNV envelop glycoprotein has been tested in Rhesus monkeys with development of high levels of neutralizing antibodies which protected them from infection (Platonov 2001). Clinical trials in humans are in progress.
Long-Term Adverse Health Outcomes of Infection with West Nile Virus
The scientific community is just beginning to unveil the long-term adverse health outcomes of WNV infection. Two teams of investigators have conducted long-term followup studies of the self-reported health outcomes of people who suffered acute episodes of W est Nile fever, WNM, WNE, or West Nile meningoencephalitis (Gottfried et al. 2005; Klee et al. 2004). All cases were diagnosed clinically and confirmed with laboratory analysis. A rigorous study by Klee and colleagues reports the health status at 6, 12, and 18 months of 42 New York City residents whose acute illnesses were manifested in 1999 and required hospitalization in all but two cases. A less rigorous study by Gottfried and colleagues reports the health status at 12 months of 24 Tennessee residents whose acute illnesses were diagnosed and reported to the state’s Department of Health in 2002; all but two of those cases had been hospitalized. About
63% of the New York City patients (22 of 35 patients who participated in a follow-up interview) and 37.5% of the Tennessee patients (nine of 24) suffered persistent cognitive, physical, or functional impairment 12 months after the onset of severe WNV infection (Box 5.2).
|
BOX 5.2 Persistent Signs and Symptoms of WNV Illness 1 year After Onset New York cohort (p ≤ 0.002 relative to baseline function):
Tennessee cohort (five most commonly reported symptoms):
|
Klee and colleagues followed the New York cohort for 18 months. At that time, 30% of the patients continued to report persistent memory loss, confusion, depression, irritability, and the need for assistance with activities of daily living (mostly those requiring increased strength). Many patients continued to report difficulty in walking, muscle weakness, fatigue, and insomnia; more than 40% reported some combination of these symptoms.
The most important risk factor for long-term morbidity in both cohorts was advanced age, defined as over 50 years by Gottfried et al and at least 65 years by Klee et al. Neither the clinical manifestation of acute WN illness nor the prior presence of underlying disease was predictive of physical or cognitive recovery, Klee et al. found, even after adjusting for age. In the Gottfried et al. study, two of the five patients whose acute illness lacked neurologic involvement reported a full recovery during the 1-year followup interview; the degree of recovery of the other three patients, who moved to nursing facilities after their WNV infection, was not ascertained.
Unlike the retrospective case-series studies based on self-reported symptoms, a prospective, clinical case series of long-term morbidity associated with WNND was conducted with 39 suspect cases of acute WNV infection in Louisiana (Sejvar et al. 2003). The patients all resided in the same parish and presented from August 1 to September 2, 2002.
Hospitalized for their acute conditions, the 39 patients were examined by a neurologist and underwent neuroimaging and electrophysiologic and serologic tests. A second neurologist verified the findings in seven patients. Sixteen subjects tested positive for WNV: five had a diagnosis of WNM, eight WNE, AFP, and one classified AFP and WNE. One subject with WNE died after 2.5 months of hospitalization in a comatose state. Five patients—three with AFP and two with WNE—were discharged to long-term rehabilitation facilities. The other 10 subjects who tested positive went home on discharge.
Eight months later, a neurologist re-examined the 15 surviving patients with WNND, who also answered a standardized questionnaire about their symptoms and functional status. The most commonly reported adverse health outcomes were fatigue, tremor, and mild parkinsonism. Eleven subjects were home and functioning independently; three were home but dependent, and
one was still undergoing rehabilitation. The five patients with WNM functioned at normal or nearly normal levels, according to the results of Barthel and modified Rankin scoring systems. Five patients with severe WNE also had recovered premorbid levels of functioning without residual disability; two WNE patients relied on walkers.
The three patients with AFP were faring poorly 8 months after onset. All continued to experience profound muscle weakness; they required wheelchairs and had difficulty in accomplishing such daily activities as grooming and housekeeping. Clinical findings and electrodiagnostic data on two of them suggested a poliomyelitis-like syndrome with involvement of anterior horn cells of the spinal cord. Electromyographic data suggested chronic denervation and permanent loss of motor axons in affected limbs.
Long-Term Prognosis of West Nile Virus-Positive Patients with Focal Neurologic Deficits
A number of studies have been conducted to elucidate the outcomes of patients infected with West Nile virus who develop focal neurologic deficits, especially AFP. Saad and colleagues (2005) reviewed all cases of AFP related to WNV reported in the English-language literature from January 1999 to March 2004 whose clinical characteristics were described in sufficient detail (53 subjects, including the three described above); they added three cases of their own. Forty of the 56 subjects survived the acute phase of disease had a known long-term health outcome. All 40 suffered some degree of persistent neurologic impairment or weakness at the time of long-term followup. As a case in point, the authors noted a survivor who remained quadriplegic and ventilator-dependent after 20 months of followup.
In cases of WNV-induced focal neurologic deficits, the rate and degree of recovery of muscle strength appears to vary by limb and patient; the initial severity of paralysis may not predict the final outcome (Cao et al. 2005). Cao and colleagues reached those conclusions by measuring the muscle strength and overall motor function of 11 subjects for 6-21 months after the onset of AFP. A 36-year-old woman paralyzed in one leg recovered minimal strength during the 21-month period. In contrast, a 44-year-old man with severe four-limb paralysis who was hospitalized for respiratory distress started to walk within 1 month and recovered full strength in all limbs after 9 months (with decreased endurance). Between those extremes, a third patient became paralyzed to various degrees in four limbs and was partially recovered at 21 months. A small case-control study suggested a correlation between the estimated numbers of surviving motor units in a muscle and the degree of improvement of muscle strength (Cao et al. 2005).
Pathologic Plausibility of Long-Term Neurologic Deficits in Patients with West Nile Neurologic Disease
Neurophysiologic, radiologic, and pathologic studies in humans and animals indicate that the underlying mechanism of WNV AFP is damage to the anterior horn cells of the spinal cord akin to the damage caused by poliomyelitis virus (Saad et al. 2005). That suggests that most patients with WNV AFP will not recover completely.
As Klee et al. (2004) noted, WNV infection is clinically similar to St. Louis encephalitis. Patients with the latter disease have reported disability up to 5 years after the acute illness. Persistent symptoms of St. Louis encephalitis have included fatigue, headache, nervousness, inability to concentrate, depression, and problems with gait and balance throughout the convalescent period of 6 months to 3 years.
The committee concludes that there is sufficient evidence of an association between acute West Nile virus infection and variable levels of physical, functional, or cognitive disability that may persist for months, years, or permanently.
Recommendation
It has been just 10 years since the potential long-term adverse health outcomes of WNV infection became an international public-health concern. The body of evidence on which this committee can base its conclusions is small. Future investigators will be able to conduct more robust studies on the long-term adverse health outcomes of patients who suffer acute infection by WNV, and create a broader foundation for conclusions about the acute illness and its long-term adverse health outcomes. The committee recommends that the Department of Veterans Affairs (VA) periodically review the literature on long-term adverse health outcomes of WNV infection to supplement the conclusions of this committee.
REFERENCES
Abo-Shehada MN, Odeh JS, Abu-Essud M, Abuharfeil N. 1996. Seroprevalence of brucellosis among high risk people in northern Jordan. International Journal of Epidemiology 25(2):450-454.
Air Force Office of the Surgeon General. 2003. AFMS Deployment Health Surveillance Implementation Instructions.
Akritidis N, Pappas G. 2001. Ascites caused by brucellosis: A report of two cases. Scandinavian Journal of Gastroenterology 36(1):110-112.
Al Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D. 2003. Laboratory-based diagnosis of brucellosis—a review of the literature. Part II: Serological tests for brucellosis. Clinica y Laboratorio 49(11-12):577-589.
al Deeb SM, Yaqub BA, Sharif HS, Phadke JG. 1989. Neurobrucellosis: Clinical characteristics, diagnosis, and outcome. Neurology 39(4):498-501.
al-Harthi SS. 1989. The morbidity and mortality pattern of Brucella endocarditis. International Journal of Cardiology 25(3):321-324.
al-Kaff AS. 1995. Ocular brucellosis. International Ophthalmology Clinics 35(3):139-145.
Alarcon GS, Bocanegra TS, Gotuzzo E, Hinostroza S, Carrillo C, Vasey FB, Germain BF, Espinoza LR. 1981. Reactive arthritis associated with brucellosis: HAL studies. Journal of Rheumatology 8(4):621-625.
Allos BM. 1997. Association between Campylobacter infection and Guillain-Barre syndrome. Journal of Infectious Diseases 176 (2 Suppl):S125-S128.
Allos BM. 2001. Campylobacter jejuni infections: Update on emerging issues and trends. Clinical Infectious Diseases 32(8):1201-1206.
Almuneef M, Memish ZA. 2003. Prevalence of Brucella antibodies after acute brucellosis. Journal of Chemotherapy 15(2):148-151.
Amino R, Menard R, Frischknecht F. 2005. In vivo imaging of malaria parasites—Recent advances and future directions. Current Opinion in Microbiology 8(4):407-414.
Araj GF, Lulu AR, Khateeb MI, Saadah MA, Shakir RA. 1988. ELISA versus routine tests in the diagnosis of patients with systemic and neurobrucellosis. APMIS: Acta Pathologica, Microbiologica et Immunologica Scandinavica 96(2):171-176.
Ardies CM. 2003. Inflammation as cause for scar cancers of the lung. Integrative Cancer Therapies 2(3):238-246.
Ariza J, Gudiol F, Valverde J, Pallares R, Fernandez-Viladrich P, Rufi G, Espadaler L, Fernandez-Nogues F. 1985. Brucellar spondylitis: A detailed analysis based on current findings. Reviews of Infectious Diseases 7(5):656-664.
Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. 1992. Specific antibody profile in human brucellosis. Clinical Infectious Diseases 14(1):131-140.
Ariza J, Pujol M, Valverde J, Nolla JM, Rufi G, Viladrich PF, Corredoira JM, Gudiol F. 1993. Brucellar sacroiliitis: Findings in 63 episodes and current relevance. Clinical Infectious Diseases 16(6):761-765.
Ariza J, Pigrau C, Canas C, Marron A, Martinez F, Almirante B, Corredoira JM, Casanova A, Fabregat J, Pahissa A. 2001. Current understanding and management of chronic hepatosplenic suppurative brucellosis. Clinical Infectious Diseases 32(7):1024-1033.
Aronson NE, Wortmann GW, Johnson SC, Jackson JE, Gasser RA Jr, Magill AJ, Endy TP, Coyne PE, Grogl M, Benson PM, Beard JS, Tally JD, Gambel JM, Kreutzer RD, Oster CN. 1998. Safety and efficacy of intravenous sodium stibogluconate in the treatment of leishmaniasis: Recent US military experience. Clinical Infectious Diseases 27(6):1457-1464.
Arsen'eva LP. 1982. Natural-focus zoonoses in Afghanistan: A review of the literature. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 51(3):54-59.
Ayres JG, Flint N, Smith EG, Tunnicliffe WS, Fletcher TJ, Hammond K, Ward D, Marmion BP. 1998. Post-infection fatigue syndrome following Q fever. QJM 91(2):105-123.
Baird JK. 2005. Effectiveness of antimalarial drugs. New England Journal of Medicine 352(15):1565-1577.
Bajiya HN, Kochar DK. 1996. Incidence and outcome of neurological sequelae in survivors of cerebral malaria. Journal of the Association of Physicians of India 44(10):679-681.
Baldi PC, Miguel SE, Fossati CA, Wallach JC. 1996. Serological follow-up of human brucellosis by measuring IgG antibodies to lipopolysaccharide and cytoplasmic proteins of Brucella species. Clinical Infectious Diseases 22(3):446-455.
Balo KP, Mensah A, Mihluedo H. 1996. Chloroquine maculopathy and prevention of malaria. Journal Francais d Ophtalmologie 19(12):770-776.
Bashir R, Al-Kawi MZ, Harder EJ, Jinkins J. 1985. Nervous system brucellosis: Diagnosis and treatment. Neurology 35(11):1576-1581.
Basset D, Faraut F, Marty P, Dereure J, Rosenthal E, Mary C, Pratlong F, Lachaud L, Bastien P, Dedet JP. 2005. Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes and Infection 7(13):1370-1375.
Beare NA, Lewis DK, Kublin JG, Harding SP, Zijlstra EE, Molyneux ME. 2003. Retinal changes in adults with cerebral malaria. Annals of Tropical Medicine and Parasitology 97(3):313-315.
Begue JJ. 1964. Ocular complications observed during synthetic antimalarial treatment. Clinique 59:287-291.
Bennish ML. 1991. Potentially lethal complications of shigellosis. Reviews of Infectious Diseases 13 (4 Suppl):S319-S324.
Bergin PS, Haas LF, Miller DH. 1989. Tuberculous meningitis at Wellington Hospital 1962-88. New Zealand Medical Journal 102(878):554-556.
Bernit E, Pouget J, Janbon F, Dutronc H, Martinez P, Brouqui P, Raoult D. 2002. Neurological involvement in acute Q fever: A report of 29 cases and review of the literature. Archives of Internal Medicine 162(6):693-700.
Bernstein HN. 1967. Chloroquine ocular toxicity. Survey of Ophthalmology 12(5):415-447.
Berry A, Fabre R, Benoit-Vical F, Cassaing S, Magnaval JF. 2005. Contribution of PCR-based methods to diagnosis and management of imported malaria. Medecine Tropicale 65(2): 176-183.
Bhattacharya SK, Sur D. 2003. An evaluation of current shigellosis treatment. Expert Opinion on Pharmacotherapy 4(8):1315-1320.
Biswas J, Fogla R, Srinivasan P, Narayan S, Haranath K, Badrinath V. 1996. Ocular malaria. A clinical and histopathologic study. Ophthalmology 103(9):1471-1475.
Blaser MJ. 2000. Campylobacter jejuni and related species. In: Mandell GL, Bennett JE, Dolin R, Editors. Principles and Practice of Infectious Diseases. 5th ed. New York: Churchill Livingstone. Pp. 2276-2285.
Blaser MJ. 2005. Infections due to Campylobacter and related species. In: Kasper DL, Braunwald E, Fauci A, Hauser SL, Longo DL, Jameson JL, Editors. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill. Pp. 907-909.
Blaser MJ, Perez GP, Smith PF, Patton C, Tenover FC, Lastovica AJ, Wang WI. 1986. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: Host factors and strain characteristics. Journal of Infectious Diseases 153(3):552-559.
Bloom PD, MacPhail AP, Klugman K, Louw M, Raubenheimer C, Fischer C. 1994. Haemolyticuraemic syndrome in adults with resistant Shigella dysenteriae type I. Lancet 344(8916):206.
Bodur H, Erbay A, Akinci E, Colpan A, Cevik MA, Balaban N. 2003. Neurobrucellosis in an endemic area of brucellosis. Scandinavian Journal of Infectious Diseases 35(2):94-97.
Bouza E, Garcia de la Torre M, Parras F, Guerrero A, Rodriguez-Creixems M, Gobernado J. 1987. Brucellar meningitis. Reviews of Infectious Diseases 9(4):810-822.
Bravo MJ, Colmenero Jde D, Alonso A, Caballero A. 2003. HLA-B*39 allele confers susceptibility to osteoarticular complications in human brucellosis. Journal of Rheumatology 30(5):1051-1053.
Breman JG. 2001. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. American Journal of Tropical Medicine and Hygiene 64(1-2 Suppl):1-11.
Bremell T, Bjelle A, Svedhem A. 1991. Rheumatic symptoms following an outbreak of campylobacter enteritis: A five year follow up. Annals of the Rheumatic Diseases 50(12):934-938.
Brouqui P, Dupont HT, Drancourt M, Berland Y, Etienne J, Leport C, Goldstein F, Massip P, Micoud M, Bertrand A, et al. 1993. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Archives of Internal Medicine 153(5):642-648.
Butcher GA. 2005. T-cell depletion and immunity to malaria in HIV-infections. Parasitology 130(Pt 2):141-150.
Buxton JA, Fyfe M, Berger S, Cox MB, Northcott KA. 2002. Reactive arthritis and other sequelae following sporadic Salmonella typhimurium infection in British Columbia, Canada: A case control study. Journal of Rheumatology 29(10):2154-2158.
Calin A, Fries JF. 1976. An “experimental” epidemic of Reiter's syndrome revisited. Follow-up evidence on genetic and environmental factors. Annals of Internal Medicine 84(5):564-566.
Camarca MM, Krauss MR. 2001. Active tuberculosis among US Army personnel, 1980 to 1996. Military Medicine: International Journal of AMSUS 166(5):452-456.
Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. 2002. West Nile virus. The Lancet Infectious Diseases 2(9):519-529.
Cao NJ, Ranganathan C, Kupsky WJ, Li J. 2005. Recovery and prognosticators of paralysis in West Nile virus infection. Journal of the Neurological Sciences 236(1-2):73-80.
CDC (Centers for Disease Control and Prevention). 1986. Annual summary 1984. Reported morbidity and mortality in the United States. Morbidity and Mortality Weekly Report 33(54):1-135.
CDC. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Report 41(RR-17):1-19.
CDC. 2000a. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. American Journal of Respiratory and Critical Care Medicine 161(4 Pt 1):1376-1395.
CDC. 2000b. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. Morbidity and Mortality Weekly Report: Recommendations and Reports 49(RR-6):1-51.
CDC. 2002. Update: Investigations of West Nile virus infections in recipients of organ transplantation and blood transfusion. Morbidity and Mortality Weekly Report 51(37):833-836.
CDC. 2003a. Treatment of tuberculosis. Morbidity and Mortality Weekly Report: Recommendations and Reports 52(RR-11):1-77.
CDC. 2003b. Cutaneous leishmaniasis in US military personnel—Southwest/Central Asia, 2002-2003. Morbidity and Mortality Weekly Report 52(42):1009-1012.
CDC. 2004a. Two cases of visceral leishmaniasis in US military personnel—Afghanistan, 2002-2004. Morbidity and Mortality Weekly Report 53 (12):265-268.
CDC. 2004b. Update: Cutaneous leishmaniasis in US military personnel—Southwest/Central Asia, 2002-2004. Morbidity and Mortality Weekly Report 53(12):264-265.
CDC. 2004c. Update: West Nile virus screening of blood donations and transfusion-associated transmission—United States, 2003. Morbidity and Mortality Weekly Report 53(13):281-284.
CDC. 2005a. Statistics, Surveillance, and Control, 2005 West Nile Virus Activity in the United States. [Online]. Available: http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount05_detailed.htm [accessed April 26, 2006].
CDC. 2005b. Typhoid Fever: Fequently Asked Questions. [Online]. Available: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/typhoid_fever_FAQ.pdf [accessed April 26, 2006].
CDC. 2005c. Reported Tuberculosis in the United States, 2004. US Department of Health and Human Services.
CDC. 2005d. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. Morbidity and Mortality Weekly Report: Recommendations and Reports 54(RR-15):1-47.
CDC. 2005e. West Nile Virus Activity—United States, January 1—December 1, 2005. Morbidity and Mortality Weekly Report 54(49):1253-1256.
Center for Infectious Disease Research and Policy, University of Minnesota. 2006. Salmonellosis. [Online]. Available: http://www.cidrap.umn/edu/cidrap/content/fs/food-disease/causes/salmoview.html [accessed April 26, 2006].
Cetinkaya B, Kalender H, Ertas HB, Muz A, Arslan N, Ongor H, Gurcay M. 2000. Seroprevalence of coxiellosis in cattle, sheep and people in the east of Turkey. Veterinary Record 146(5):131-136.
Chakravarty A, Ghosh B, Bhattacharyya R, Sengupta S, Mukherjee S. 2004. Acute inflammatory demyelinating polyneuropathy following plasmodium vivax malaria. Neurology India 52(1):130-131.
Chen M, Delpech V, O'Sullivan B, Donovan B. 2002. Shigella sonnei: Another cause of sexually acquired reactive arthritis. International Journal of STD and AIDS 13(2):135-136.
Choi CM, Lerner EA. 2002. Leishmaniasis: Recognition and management with a focus on the immunocompromised patient. American Journal of Clinical Dermatology 3(2):91-105.
Choi HJ, Lee SY, Yang H, Bang JK. 2004. Retinal haemorrhage in vivax malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 98(6):387-389.
Chomel BB, DeBess EE, Mangiamele DM, Reilly KF, Farver TB, Sun RK, Barrett LR. 1994. Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: A shift toward foodborne transmission. Journal of Infectious Diseases 170(5):1216-1223.
Colmenero JD, Reguera JM, Martos F, Sanchez-De-Mora D, Delgado M, Causse M, Martin-Farfan A, Juarez C. 1996. Complications associated with Brucella melitensis infection: A study of 530 cases. Medicine 75(4):195-211.
Colmenero JD, Queipo-Ortuno MI, Maria Reguera J, Angel Suarez-Munoz M, Martin-Carballino S, Morata P. 2002. Chronic hepatosplenic abscesses in Brucellosis. Clinico-therapeutic features and molecular diagnostic approach. Diagnostic Microbiology and Infectious Disease 42(3):159-167.
Comstock GW, Edwards LB, Livesay VT. 1974a. Tuberculosis morbidity in the US Navy: Its distribution and decline. American Review of Respiratory Disease 110(5):572-580.
Comstock GW, Livesay VT, Woolpert SF. 1974b. The prognosis of a positive tuberculin reaction in childhood and adolescence. American Journal of Epidemiology 99(2):131-138.
Cope SE, Schultz GW, Richards AL, Savage HM, Smith GC, Mitchell CJ, Fryauff DJ, Conlon JM, Corneil JA, Hyams KC. 1996. Assessment of arthropod vectors of infectious diseases in areas of US troop deployment in the Persian Gulf. American Journal of Tropical Medicine and Hygiene 54(1):49-53.
Crosby E, Llosa L, Miro Quesada M, Carrillo C, Gotuzzo E. 1984. Hematologic changes in brucellosis. Journal of Infectious Diseases 150(3):419-424.
Cunha BA. 2004. Differential diagnosis of West Nile encephalitis. Current Opinion in Infectious Diseases 17(5):413-420.
Cunningham AC. 2002. Parasitic adaptive mechanisms in infection by leishmania. Experimental and Molecular Pathology 72(2):132-141.
Dalrymple-Champneys W. 1950. Undulant fever, a neglected problem. Lancet 1:429-777.
Davies NE, Haverty JR, Boatwright M. 1969. Reiter's disease associated with shigellosis. Southern Medical Journal 62(8):1011-1014.
Davies PD. 2005. Risk Factors for Tuberculosis. Monaldi Archives of Chest Disease 63(1):37-45.
Department of the Army. 2003. Army Latent Tuberculosis Infection (LTBI) Surveillance and Control Program. Office of the Surgeon General.
Department of the Army. 2005. Army Regulation 40-5 Medical Services: Preventive Medicine. [Online]. Available: http://www.usapa.army.mil/pdffiles/r40_5.pdf [accessed May 8, 2006].
Department of the Army. 2006. Personnel Policy Guidance (PPG) of Operations Iraqi Freedom (OIF), Enduring Freedom (OEF) and Noble Eagle (ONE). [Online]. Available: http://www.armyg1.army.mil/MilitaryPersonnel/ppg.asp [accessed May 8, 2006].
Department of the Navy. 1993. Bumed Instruction 6224.8 Change Transmittal: Tuberculosis Control Program. [Online]. Available: http://www.nehc.med.navy.mil/nepmu2/pmttoolbox/IMMUNIZATIONS1_files/Instructions%20&%20Notices/BUMEDINST%206224.8%20CH-1.pdf [accessed May 8, 2006].
Department of the Navy. 2001. BUMED, Tuberculosis Control Program - Clarification. [Online]. Available: http://www.nehc.med.navy.mil/nepmu2/pmttoolbox/IMMUNIZATIONS1_files/Immunization%20Messages/TB%20clar%204-01%20241350Z.txt [accessed May 8, 2006].
Derrick E. 1937. Q Fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Medical Journal of Australia 2:281-299.
Desjeux P. 2004. Leishmaniasis: Current situation and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases 27(5):305-318.
Dey AB, Trikha I, Banerjee M, Jain R, Nagarkar KM . 2001. Acute disseminated encephalomyelitis—another cause of post malaria cerebellar ataxia. Journal of the Association of Physicians of India 49:756-758.
Digre KB. 2003. Not so benign intracranial hypertension. British Medical Journal (Clinical Research Ed.) 326(7390):613-614.
Digre KB, Corbett JJ. 2001. Idiopathic intracranial hypertension (pseudotumor cerebri): A reappraisal. The Neurologist 7:2-68.
Dingle KE, Van Den Braak N, Colles FM, Price LJ, Woodward DL, Rodgers FG, Endtz HP, Van Belkum A, Maiden MC. 2001. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barre and Miller-Fisher syndromes are of diverse genetic lineage, serotype, and flagella type. Journal of Clinical Microbiology 39(9):3346-3349.
Doganay M, Aygen B, Inan M, Ozbakir O. 1993. Brucella peritonitis in a cirrhotic patient with ascites. European Journal of Medicine 2(7):441-442.
Doyle TJ, Bryan RT. 2000. Infectious disease morbidity in the US region bordering Mexico, 1990-1998. Journal of Infectious Diseases 182(5):1503-1510.
Drancourt M, Raoult D, Xeridat B, Milandre L, Nesri M, Dano P. 1991. Q fever meningoencephalitis in five patients. European Journal of Epidemiology 7(2):134-138.
Dube MP, Holtom PD, Larsen RA. 1992. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. American Journal of Medicine 93(5):520-524.
Dworkin MS, Shoemaker PC, Goldoft MJ, Kobayashi JM. 2001. Reactive arthritis and Reiter's syndrome following an outbreak of gastroenteritis caused by Salmonella enteritidis. Clinical Infectious Diseases 33(7):1010-1014.
Easterbrook M. 1999. Detection and prevention of maculopathy associated with antimalarial agents. International Ophthalmology Clinics 39(2):49-57.
Eastmond CJ, Rennie JA, Reid TM. 1983. An outbreak of Campylobacter enteritis—A rheumatological followup survey. Journal of Rheumatology 10(1):107-108.
Eiam-Ong S. 2003. Malarial nephropathy. Seminars in Nephrology 23(1):21-33.
El Hassan AM, Ali MS, Zijlstra E, Eltoum IA, Ghalib HW, Ahmed HM. 1992. Post-kala-azar dermal leishmaniasis in the Sudan: Peripheral neural involvement. International Journal of Dermatology 31(6):400-403.
Eliades MJ, Shah S, Nguyen-Dinh P, Newman RD, Barber AM, Nguyen-Dinh P, Roberts JM, Mali S, Parise ME, Barber AM, Steketee R. 2005. Malaria surveillance—United States, 2003. Morbidity and Mortality Weekly Report: Surveillance Summaries 54(2):25-40.
Enders U, Karch H, Toyka KV, Michels M, Zielasek J, Pette M, Heesemann J, Hartung HP. 1993. The spectrum of immune responses to Campylobacter jejuni and glycoconjugates in Guillain-Barre syndrome and in other neuroimmunological disorders. Annals of Neurology 34(2):136-144.
Falchook GS, Malone CM, Upton S, Shandera WX. 2003. Postmalaria neurological syndrome after treatment of Plasmodium falciparum malaria in the United States. Clinical Infectious Diseases 37(2):e22-e24.
Fasanaro AM, Scoleri G, Pizza V, Gaeta GB, Fasanaro A. 1991. Guillain-Barre syndrome as presenting manifestation of visceral leishmaniasis. Lancet 338(8775):1142.
FDA (Food and Drug Administration). 2005. PMA Final Decisions Rendered for December 2004. [Online]. Available: http://fda.gov/cdrh/pma/pmadec04.html [accessed May 8, 2006].
Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. 2001. Risks factors and prevention of Q fever endocarditis. Clinical Infectious Diseases 33(3):312-316.
Fenollar F, Thuny F, Xeridat B, Lepidi H, Raoult D. 2006. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clinical Infectious Diseases 42(6): 818-821.
Ferrante MA, Dolan MJ. 1993. Q fever meningoencephalitis in a soldier returning from the Persian Gulf War. Clinical Infectious Diseases 16(4):489-496.
Finch M, Rodey G, Lawrence D, Blake P. 1986. Epidemic Reiter's syndrome following an outbreak of shigellosis. European Journal of Epidemiology 2(1):26-30.
Fosgate GT, Carpenter TE, Chomel BB, Case JT, DeBess EE, Reilly KF. 2002. Time-space clustering of human brucellosis, California, 1973-1992. Emerging Infectious Diseases 8(7):672-678.
Fournier PE, Casalta JP, Piquet P, Tournigand P, Branchereau A, Raoult D. 1998. Coxiella burnetii infection of aneurysms or vascular grafts: Report of seven cases and review. Clinical Infectious Diseases 26(1):116-121.
Fox KF, Fox A, Nagpal M, Steinberg P, Heroux K. 1998. Identification of Brucella by ribosomal-spacer-region PCR and differentiation of Brucella canis from other Brucella spp. pathogenic for humans by carbohydrate profiles. Journal of Clinical Microbiology 36(11):3217-3222.
Fryauff DJ, Modi GB, Mansour NS, Kreutzer RD, Soliman S, Youssef FG. 1993. Epidemiology of cutaneous leishmaniasis at a focus monitored by the multinational force and observers in the northeastern Sinai Desert of Egypt. American Journal of Tropical Medicine and Hygiene 49(5):598-607.
Gami AS, Antonios VS, Thompson RL, Chaliki HP, Ammash NM. 2004. Q fever endocarditis in the United States. Mayo Clinic Proceedings 79(2):253-257.
Gardner K, Cox T, Digre KB. 1995. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: Case reports and review. Neurology 45(1):6-10.
Gea-Banacloche J, Johnson RT, Bagic A, Butman JA, Murray PR, Agrawal AG. 2004. West Nile virus: Pathogenesis and therapeutic options. Annals of Internal Medicine 140(7):545-553.
Geyik MF, Gur A, Nas K, Cevik R, Sarac J, Dikici B, Ayaz C. 2002. Musculoskeletal involvement of brucellosis in different age groups: A study of 195 cases. Swiss Medical Weekly 132(7-8):98-105.
Giles CL, Henderson JW. 1965. The ocular toxicity of chloroquine therapy. American Journal of the Medical Sciences 249:230-235.
Glynn, JR. Resurgence of tuberculosis and the impact of HIV infection. British Medical Bulletin 54(3): 579-593.
Gokul BN, Paul A, Hussein I. 2000. Neurobrucellosis. Saudi Medical Journal 21(6):577-580.
Good AE. 1979. Shigellae and Reiter's syndrome. Annals of the Rheumatic Diseases 38 (1 Suppl):S119-S122.
Goossens H, Marcelis L, Dekeyser P, Butzler JP. 1983. Brucella melitensis: Person-to-person transmission? Lancet 1(8327):773.
Gordon JD, MacKeen AD, Marrie TJ, Fraser DB. 1984. The radiographic features of epidemic and sporadic Q fever pneumonia. Journal of the Canadian Association of Radiologists 35(3):293-296.
Gottesman G, Vanunu D, Maayan MC, Lang R, Uziel Y, Sagi H, Wolach B. 1996. Childhood brucellosis in Israel. Pediatric Infectious Disease Journal 15(7):610-615.
Gottfried K, Quinn R, Jones T. 2005. Clinical description and follow-up investigation of human West Nile virus cases. Southern Medical Journal 98(6):603-606.
Gotuzzo E, Alarcon GS, Bocanegra TS, Carrillo C, Guerra JC, Rolando I, Espinoza LR. 1982. Articular involvement in human brucellosis: A retrospective analysis of 304 cases. Seminars in Arthritis and Rheumatism 12(2):245-255.
Gotuzzo E, Carrillo C, Guerra J, Llosa L. 1986. An evaluation of diagnostic methods for brucellosis—the value of bone marrow culture. Journal of Infectious Diseases 153(1): 122-125.
Gotuzzo E, Seas C, Guerra JG, Carrillo C, Bocanegra TS, Calvo A, Castaneda O, Alarcon GS. 1987. Brucellar arthritis: A study of 39 Peruvian families. Annals of the Rheumatic Diseases 46(7):506-509.
Gould LH, Fikrig E. 2004. West Nile virus: A growing concern? Journal of Clinical Investigation 113(8):1102-1107.
Gruenewald R, Ropper AH, Lior H, Chan J, Lee R, Molinaro VS. 1991. Serologic evidence of Campylobacter jejuni/coli enteritis in patients with Guillain-Barre syndrome. Archives of Neurology 48(10):1080-1082.
Guerrant RL, Walker DH, Weller PF. 1999. Tropical Infectious Diseases: Principles, Pathogens, and Practice. 1st ed. Philadelphia, PA: Churchill Livingstone.
Gungor K, Bekir NA, Namiduru M. 2002. Ocular complications associated with brucellosis in an endemic area. European Journal of Ophthalmology 12(3):232-237.
Gur A, Geyik MF, Dikici B, Nas K, Cevik R, Sarac J, Hosoglu S. 2003. Complications of brucellosis in different age groups: A study of 283 cases in southeastern Anatolia of Turkey. Yonsei Medical Journal 44(1):33-44.
Halpern Z, Dan M, Giladi M, Schwartz I, Sela O, Levo Y. 1989. Shigellosis in adults: Epidemiologic, clinical, and laboratory features. Medicine 68(4):210-217.
Hannu T, Leirisalo-Repo M. 1988. Clinical picture of reactive salmonella arthritis. Journal of Rheumatology 15(11):1668-1671.
Hannu T, Mattila L, Rautelin H, Pelkonen P, Lahdenne P, Siitonen A, Leirisalo-Repo M. 2002. Campylobacter-triggered reactive arthritis: A population-based study. Rheumatology 41(3):312-318.
Hannu T, Kauppi M, Tuomala M, Laaksonen I, Klemets P, Kuusi M. 2004a. Reactive arthritis following an outbreak of Campylobacter jejuni infection. Journal of Rheumatology 31(3):528-30.
Hannu T, Sihto-Kauppi K, Kotaniemi K, Kauppi M. 2004b. Acute anterior uveitis in association with an outbreak of Campylobacter jejuni infection. Scandinavian Journal of Rheumatology 33(1):55-57.
Hannu T, Mattila L, Siitonen A, Leirisalo-Repo M. 2005. Reactive arthritis attributable to Shigella infection: A clinical and epidemiological nationwide study. Annals of the Rheumatic Diseases 64(4):594-598.
Hariharan H, Naseema K, Kumaran C, Shanmugam J, Nair MD, Radhakrishnan K. 1996. Detection of Campylobacter jejuni/C.coli infection in patients with Guillain-Barre syndrome by serology and culture. New Microbiologica 19(3):267-271.
Harrington PT, Gutierrez JJ, Ramirez-Ronda CH, Quinones-Soto R, Bermudez RH, Chaffey J. 1982. Granulomatous hepatitis. Reviews of Infectious Diseases 4(3):638-655.
Hart PD, Sutherland I. 1977. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. British Medical Journal 2(6082):293-295.
Hasanjani Roushan MR, Mohrez M, Smailnejad Gangi SM, Soleimani Amiri MJ, Hajiahmadi M. 2004. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiology and Infection 132(6):1109-1114.
Hatchette TF, Hayes M, Merry H, Schlech WF, Marrie TJ. 2003. The effect of C. burnetii infection on the quality of life of patients following an outbreak of Q fever. Epidemiology and Infection 130(3):491-495.
Hawker JI, Ayres JG, Blair I, Evans MR, Smith DL, Smith EG, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Farrell ID, Saunders PJ, Wood MJ. 1998. A large outbreak of Q fever in the West Midlands: Windborne spread into a metropolitan area? Communicable Disease and Public Health 1(3):180-187.
Helbig KJ, Heatley SL, Harris RJ, Mullighan CG, Bardy PG, Marmion BP. 2003. Variation in immune response genes and chronic Q fever. Concepts: Preliminary test with post-Q fever fatigue syndrome. Genes and Immunity 4(1):82-85.
Helbig K, Harris R, Ayres J, Dunckley H, Lloyd A , Robson J, Marmion BP. 2005. Immune response genes in the post-Q-fever fatigue syndrome, Q fever endocarditis and uncomplicated acute primary Q fever. QJM 98(8):565-574.
Hertig M, Sabin AB. 1964. Sandfly fever (pappataci, Phlebotomus, three-day fever). In: Hoff EC, Editor. Preventive Medicine in World War II: Communicable Diseases. Volume 7. Office of the Surgeon General, US Department of the Army. Pp. 109-174.
Hewitt S, Reyburn H, Ashford R, Rowland M. 1998. Anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: Vertical distribution of cases in apartment blocks. Transactions of the Royal Society of Tropical Medicine and Hygiene 92(3):273-274.
Heymann DL. 2004. Control of Communicable Diseases Manual. Washington, DC: American Public Health Association.
Hidayat AA, Nalbandian RM, Sammons DW, Fleischman JA, Johnson TE. 1993. The diagnostic histopathologic features of ocular malaria. Ophthalmology 100(8):1183-1186.
Hosoglu S, Ayaz C, Geyik MF, Kokoglu OF, Ceviz A. 1998. Tuberculous meningitis in adults: An eleven-year review. International Journal of Tuberculosis and Lung Disease 2(7):553-557.
Howard RS, Sarkies NJ, Sanders MD. 1987. Anterior uveitis associated with Campylobacter jejuni infection. Journal of Infection 14(2):186-187.
Hubalek Z, Halouzka J. 1999. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerging Infectious Diseases 5(5):643-650.
Hyams KC, Hanson K, Wignall FS, Escamilla J, Oldfield EC, 3rd. 1995. The impact of infectious diseases on the health of US troops deployed to the Persian Gulf during operations Desert Shield and Desert Storm. Clinical Infectious Diseases 20(6):1497-1504.
Ibero I, Vela P, Pascual E. 1997. Arthritis of shoulder and spinal cord compression due to Brucella disc infection. British Journal of Rheumatology 36(3):377-381.
Ibrahim AI, Awad R, Shetty SD, Saad M, Bilal NE. 1988. Genito-urinary complications of brucellosis. British Journal of Urology 61(4):294-298.
Idro R, Jenkins NE, Newton CR. 2005. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurology 4(12):827-840.
Ilnyckyj A, Balachandra B, Elliott L, Choudhri S, Duerksen DR. 2003. Post-traveler's diarrhea irritable bowel syndrome: A prospective study. American Journal of Gastroenterology 98(3):596-599.
Imboden JB, Canter A, Cluff LE, Trever RW. 1959. Brucellosis. III. Psychologic aspects of delayed convalescence. AMA Archives of Internal Medicine 103(3):406-414.
IOM (Institute of Medicine). 2000. Ending Neglect: The Elimination of Tuberculosis in the United States. Washington, DC: National Academy Press.
Islam MZ, Itoh M, Shamsuzzaman SM, Mirza R, Matin F, Ahmed I, Shamsuzzaman Choudhury AK, Hossain MA, Qiu XG, Begam N, Furuya M, Leafasia JL, Hashiguchi Y, Kimura E. 2002. Diagnosis of visceral leishmaniasis by enzyme-linked immunosorbent assay using urine samples. Clinical and Diagnostic Laboratory Immunology 9(4):789-794.
Iuchi K, Ichimiya A, Akashi A, Mizuta T, Lee YE, Tada H, Mori T, Sawamura K, Lee YS, Furuse K, et al. 1987. Non-Hodgkin’s lymphoma of the pleural cavity developing from long-standing pyothorax. Cancer 60(8):1771-1775.
Jordan I, Briese T, Fischer N, Lau JY, Lipkin WI. 2000. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. Journal of Infectious Diseases 182(4):1214-1217.
Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, Fayad P, Freifeld AG. 2005. Use of interferon-alpha in patients with West Nile encephalitis: Report of 2 cases. Clinical Infectious Diseases 40(5):764-766.
Kalita J, Misra UK. 1999. Outcome of tuberculous meningitis at 6 and 12 months: A multiple regression analysis. International Journal of Tuberculosis and Lung Disease 3(3):261-265.
Kanjalkar M, Karnad DR, Narayana RV, Shah PU. 1999. Guillain-Barre syndrome following malaria. Journal of Infection 38(1):48-50.
Karmali MA, Fleming PC. 1979. Campylobacter enteritis in children. Journal of Pediatrics 94(4):527-533.
Kaslow RA RRCA. 1979. Search for Reiter’s syndrome after an outbreak of Shigella sonnei dysentery. Journal of Rheumatology 6:562-566.
Kelley PW. 2005. Military Preventive Medicine: Mobilization and Deployment. Volume 2. Washington, DC: Walter Reed Army Medical Center.
Kennedy DH, Fallon RJ. 1979. Tuberculous meningitis. Journal of the American Medical Association 241(3):264-268.
Khan MY, Mah MW, Memish ZA. 2001. Brucellosis in pregnant women. Clinical Infectious Diseases 32(8):1172-1177.
Khandpur S, Ramam M, Sharma VK, Salotra P, Singh MK, Malhotra A. 2004. Nerve involvement in Indian post kala-azar dermal leishmaniasis. Acta Dermato-Venereologica 84:245-246.
Khateeb MI, Araj GF, Majeed SA, Lulu AR. 1990. Brucella arthritis: A study of 96 cases in Kuwait. Annals of the Rheumatic Diseases 49(12):994-998.
Kibukamusoke JW. 1986. The hazard of malarial nephropathy. Parasitology Today 2(4): 119-121.
Kilpatrick ME. 2005. Presentation to IOM Committee on Gulf War and Health: Infectious Diseases. Washington, DC.
Kim AY, Goldberg MB, Rubin RH. 2004. Salmonella infections. In: Gorbach SL, Bartlett JG, Blacklow NR, Editors. Infectious Diseases. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins. Pp. 618-633.
Klee AL, Maidin B, Edwin B, Poshni I, Mostashari F, Fine A, Layton M, Nash D. 2004. Long-term prognosis for clinical West Nile virus infection. Emerging Infectious Diseases 10(8):1405-1411.
Kochar DK, Agarwal N, Jain N, Sharma BV, Rastogi A, Meena CB. 2000a. Clinical profile of neurobrucellosis—a report on 12 cases from Bikaner (north-west India). Journal of the Association of Physicians of India 48(4):376-380.
Kochar DK, Shubhakaran, Kumawat BL, Vyas SP. 2000b. Prognostic significance of eye changes in cerebral malaria. Journal of the Association of Physicians of India 48(5):473-477.
Kolaczinski J, Graham K, Fahim A, Brooker S, Rowland M. 2005. Malaria control in Afghanistan: Progress and challenges. Lancet 365(9469):1506-1512.
Kortepeter MG, Krauss MR. 2001. Tuberculosis infection after humanitarian assistance, Guantanamo Bay, 1995. Military Medicine 166(2):116-120.
Koster F, Levin J, Walker L, Tung KS, Gilman RH, Rahaman MM, Majid MA, Islam S, Williams RC Jr. 1978. Hemolytic uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. New England Journal of Medicine 298(17):927-933.
Kurcer MA, Simsek Z, Kurcer Z. 2006. The decreasing efficacy of chloroquine in the treatment of Plasmodium vivax malaria, in Sanliurfa, south-eastern Turkey. Annals of Tropical Medicine and Parasitology 100(2):109-113.
Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H. 1993. Campylobacter jejuni strains from patients with Guillain-Barre syndrome belong mostly to Penner serogroup 19 and contain beta-N-acetylglucosamine residues. Annals of Neurology 33(3):243-247.
Kuwabara S, Ogawara K, Misawa S, Koga M, Mori M, Hiraga A, Kanesaka T, Hattori T, Yuki N. 2004. Does Campylobacter jejuni infection elicit “demyelinating” Guillain-Barre syndrome? Neurology 63(3):529-533.
Lamar J. 2006. Navy Environmental Health Center. Personal communication.
Lau KK, Yu IT, Chan AC, Wong LK, Tam CM, Sheng B, Li HL. 2005. A registry of tuberculous meningitis in Hong Kong. International Journal of Tuberculosis and Lung Disease 9(12):1391-1397.
Lauhio A, Lahdevirta J, Janes R, Kontiainen S, Repo H. 1988. Reactive arthritis associated with Shigella sonnei infection. Arthritis and Rheumatism 31(9):1190-1193.
Lee AT, Hall RG, Pile KD. 2005. Reactive joint symptoms following an outbreak of Salmonella typhimurium phage type 135a. Journal of Rheumatology 32(3):524-527.
Leirisalo-Repo M, Helenius P, Hannu T, Lehtinen A, Kreula J, Taavitsainen M, Koskimies S. 1997. Long-term prognosis of reactive salmonella arthritis. Annals of the Rheumatic Diseases 56(9):516-520.
Lever AM, Dolby JM, Webster AD, Price AB. 1984. Chronic campylobacter colitis and uveitis in patient with hypogammaglobulinaemia. British Medical Journal (Clinical Research Ed.) 288(6416):531.
Lewallen S. 1998. The fundus in severe malaria. Archives of Ophthalmology 116(4):542-543.
Lewis RB. 1982. The absence of reactive arthritis after Shigella sonnei infection. Arthritis and Rheumatism 25(10):1267.
Liu GF, Wu ZL, Wu HS, Wang QY, Zhao-Ri GT, Wang CY, Liang ZX, Cui SL, Zheng JD. 2003. A case-control study on children with Guillain-Barre syndrome in North China. Biomedical and Environmental Sciences 16(2):105-111.
Lochhead J, Elston JS. 2003. Doxycycline induced intracranial hypertension. British Medical Journal (Clinical Research Ed.) 326(7390):641-642.
Locht H, Kihlstrom E, Lindstrom FD. 1993. Reactive arthritis after Salmonella among medical doctors—Study of an outbreak. Journal of Rheumatology 20(5):845-848.
Locht H, Molbak K, Krogfelt KA. 2002. High frequency of reactive joint symptoms after an outbreak of Salmonella enteritidis. Journal of Rheumatology 29(4):767-771.
Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. 1998. Pulmonary tuberculosis treated with directly observed therapy: Serial changes in lung structure and function. Chest 113(4):933-943.
Lozier JR, Friedlaender MH. 1989. Complications of antimalarial therapy. International Ophthalmology Clinics 29(3):172-178.
Lubani MM, Dudin KI, Araj GF, Manandhar DS, Rashid FY. 1989a. Neurobrucellosis in children. Pediatric Infectious Disease Journal 8(2):79-82.
Lubani MM, Dudin KI, Sharda DC, Ndhar DS, Araj GF, Hafez HA, al-Saleh QA, Helin I, Salhi MM. 1989b. A multicenter therapeutic study of 1100 children with brucellosis. Pediatric Infectious Disease Journal 8(2):75-78.
Luke T. 2006. Department of the Navy Bureau of Medicine and Surgery. Personal communication.
Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. 1988. Human brucellosis in Kuwait: A prospective study of 400 cases. Quarterly Journal of Medicine 66(249):39-54.
Lyall M. 1973. Ocular brucellosis. Transactions of the Ophthalmological Societies of the United Kingdom 93(0):689-697.
Madariaga MG, Pulvirenti J, Sekosan M, Paddock CD, Zaki SR. 2004. Q fever endocarditis in HIV-infected patient. Emerging Infectious Diseases 10(3):501-504.
Magill AJ, Grogl M, Gasser RA Jr, Sun W, Oster CN. 1993. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. New England Journal of Medicine 328(19):1383-1387.
Magill AJ, Grogl M, Johnson SC, Gasser RA Jr. 1994. Visceral infection due to Leishmania tropica in a veteran of Operation Desert Storm who presented 2 years after leaving Saudi Arabia. Clinical Infectious Diseases 19(4):805-806.
Makhseed M, Harouny A, Araj G, Moussa MA, Sharma P. 1998. Obstetric and gynecologic implication of brucellosis in Kuwait. Journal of Perinatology 18(3):196-199.
Maki-Ikola O, Granfors K. 1992. Salmonella-triggered reactive arthritis. Scandinavian Journal of Rheumatology 21(6):265-270.
Maki-Ikola O, Leirisalo-Repo M, Kantele A, Toivanen P, Granfors K. 1991. Salmonella-specific antibodies in reactive arthritis. Journal of Infectious Diseases 164(6):1141-1148.
Maki-Ikola O, Yli-Kerttula U, Saario R, Toivanen P, Granfors K. 1992. Salmonella specific antibodies in serum and synovial fluid in patients with reactive arthritis. British Journal of Rheumatology 31(1):25-29.
Malviya G, Sinha MK, Bhattacharya SK. 2005. Post-malarial neuropsychiatric syndrome. Journal of the Association of Physicians of India 53:227.
Mandell GL, Bennett JE, Dolin R. 2005. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone
Marmion BP, Shannon M, Maddocks I, Storm P, Penttila I. 1996. Protracted debility and fatigue after acute Q fever. Lancet 347(9006):977-978.
Marmion BP, Storm PA, Ayres JG, Semendric L, Mathews L, Winslow W, Turra M, Harris RJ. 2005. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 98(1): 7-20.
Marrie TJ. 1990. Q Fever: The Disease. Volume 1. Boca Raton, FL: CRC Press.
Marrie TJ. 2000. Coxiella burnetti (Q Fever). In: Mandell GL, Bennett JE, Dolin R, Editors. Principles and Practice of Infectious Diseases. 5th ed. New York: Churchill Livingstone. Pp. 2043-2049.
Martin WJ, Nichols DR, Beahrs OH. 1961. Chronic localized brucellosis. Archives of Internal Medicine 107:43.
Mattila L, Leirisalo-Repo M, Koskimies S, Granfors K, Siitonen A. 1994. Reactive arthritis following an outbreak of Salmonella infection in Finland. British Journal of Rheumatology 33(12):1136-1141.
Mattila L, Leirisalo-Repo M, Pelkonen P, Koskimies S, Granfors K, Siitonen A. 1998. Reactive arthritis following an outbreak of Salmonella Bovismorbificans infection. Journal of Infection 36(3):289-295.
Maurin M, Raoult D. 1999. Q fever. Clinical Microbiology Reviews 12(4):518-553.
McCarthy N, Giesecke J. 2001. Incidence of Guillain-Barre syndrome following infection with Campylobacter jejuni. American Journal of Epidemiology 153(6):610-614.
McLean DR, Russell N, Khan MY. 1992. Neurobrucellosis: Clinical and therapeutic features. Clinical Infectious Diseases 15(4):582-590.
McQuiston JH, Childs JE. 2002. Q fever in humans and animals in the United States. Vector Borne Zoonotic Disease 2(3):179-191.
MedlinePlus Medical Encyclopedia. 2006. MedlinePlus Medical Encyclopedia: Uveitis. [Online]. Available: http://www.nlm.nih.gov/medlineplus/print/ency/article/001005.htm [accessed May 1, 2006].
Meier CR, Wilcock K, Jick SS. 2004. The risk of severe depression, psychosis or panic attacks with prophylactic antimalarials. Drug Safety 27(3):203-213.
Memish ZA, Venkatesh S. 2001. Brucellar epididymo-orchitis in Saudi Arabia: A retrospective study of 26 cases and review of the literature. BJU International 88(1):72-76.
Metha SR, Joshi V, Lazar AI. 1996. Unusual acute and chronic complications of malaria. Journal of the Association of Physicians of India 44(7):451-453.
Milazzo A, Hall R, Storm PA, Harris RJ, Winslow W, Marmion BP. 2001. Sexually transmitted Q fever. Clinical Infectious Diseases 33(3):399-402.
Mishu B, Ilyas AA, Koski CL, Vriesendorp F, Cook SD, Mithen FA, Blaser MJ. 1993. Serologic evidence of previous Campylobacter jejuni infection in patients with the Guillain-Barre syndrome. Annals of Internal Medicine 118(12):947-953.
Montes M, Cilla G, Marimon JM, Diaz de Tuesta JL, Perez-Trallero E. 1995. Coxiella burnetii infection in subjects with HIV infection and HIV infection in patients with Q fever. Scandinavian Journal of Infectious Diseases 27(4):344-346.
Morata P, Queipo-Ortuno MI, Reguera JM, Miralles F, Lopez-Gonzalez JJ, Colmenero JD. 2001. Diagnostic yield of a PCR assay in focal complications of brucellosis. Journal of Clinical Microbiology 39(10):3743-3746.
Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. 2001. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 358(9278):261-264.
Mousa AR, Koshy TS, Araj GF, Marafie AA, Muhtaseb SA, Al-Mudallal DS, Busharetulla MS. 1986. Brucella meningitis: Presentation, diagnosis and treatment—a prospective study of ten cases. Quarterly Journal of Medicine 60(233):873-885.
Mousa AR, Muhtaseb SA, Almudallal DS, Khodeir SM, Marafie AA. 1987. Osteoarticular complications of brucellosis: A study of 169 cases. Reviews of Infectious Diseases 9(3):531-543.
Murray HW. 2000. Treatment of visceral leishmaniasis (kala-azar): A decade of progress and future approaches. International Journal of Infectious Diseases 4(3):158-177.
Murray HW. 2004. Treatment of visceral leishmaniasis in 2004. American Journal of Tropical Medicine and Hygiene 71(6):787-794.
Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366(9496):1561-1577.
Nachamkin I. 2002. Chronic effects of Campylobacter infection. Microbes and Infection 4(4):399-403.
Nachamkin I, Allos BM, Ho T. 1998. Campylobacter species and Guillain-Barre syndrome. Clinical Microbiology Reviews 11(3):555-567.
Nachamkin I, Engberg J, Gutacker M, Meinersman RJ, Li CY, Arzate P, Teeple E, Fussing V, Ho TW, Asbury AK, Griffin JW, McKhann GM, Piffaretti JC. 2001. Molecular population genetic analysis of Campylobacter jejuni HS: 19 associated with Guillain-Barre syndrome and gastroenteritis. Journal of Infectious Diseases 184(2):221-226.
Naficy K, Saidi S. 1970. Serological survey on viral antibodies in Iran. Tropical and Geographical Medicine 22(2):183-188.
Namiduru M, Karaoglan I, Gursoy S, Bayazit N, Sirikci A. 2004. Brucellosis of the spine: Evaluation of the clinical, laboratory, and radiological findings of 14 patients. Rheumatology International 24(3):125-129.
Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. New England Journal of Medicine 344(24):1807-1814.
Nathoo KJ, Sanders JA, Siziya S, Mucheche C. 1995. Haemolytic uraemic syndrome following Shigella dysenteriae type 1 outbreak in Zimbabwe: A clinical experience. Central African Journal of Medicine 41(9):267-274.
Navarro-Martinez A, Solera J, Corredoira J, Beato JL, Martinez-Alfaro E, Atienzar M, Ariza J. 2001. Epididymoorchitis due to Brucella mellitensis: A retrospective study of 59 patients. Clinical Infectious Diseases 33(12):2017-2022.
Navy Environmental Health Center. 2006. Preventive Medicine - Tuberculosis. [Online]. Available: http://www-nehc.med.navy.mil/prevmed/PM/PM_Tuberculosis.htm [accessed May 8, 2006].
Neithercut WD, Hudson MA, Smith CC. 1984. Can erythema nodosum and reactive arthritis be a sequel to Shigella flexneri gastroenteritis? Scottish Medical Journal 29(3):197-199.
Nene A, Bhojraj S. 2005. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. The Spine Journal 5(1):79-84.
Newman RD, Parise ME, Barber AM, Steketee RW. 2004. Malaria-related deaths among US travelers, 1963-2001. Annals of Internal Medicine 141(7):547-555.
Niemeyer G, Fruh B. 1989. Examination strategies in the diagnosis of drug-induced retinal damage. Klinische Monatsblatter Fur Augenheilkunde 194(5):355-358.
NIH (National Institutes of Health). 2002. Questions and Answers About Reactive Arthritis. National Institute of Arthritis and Musckuloskeletal Diseases (NIAMS), Public Health Service, US Department of Health and Human Services.
Nikkari S, Rantakokko K, Ekman P, Mottonen T, Leirisalo-Repo M, Virtala M, Lehtonen L, Jalava J, Kotilainen P, Granfors K, Toivanen P. 1999. Salmonella-triggered reactive arthritis: Use of polymerase chain reaction, immunocytochemical staining, and gas chromatographymass spectrometry in the detection of bacterial components from synovial fluid. Arthritis and Rheumatism 42(1):84-89.
Noer HR. 1966. An “experimental” epidemic of Reiter’s syndrome. Journal of the American Medical Association 198(7):693-698.
Norton WL. 1984. Brucellosis and rheumatic syndromes in Saudi Arabia. Annals of the Rheumatic Diseases 43(6):810-815.
Nourse C, Allworth A, Jones A, Horvath R, McCormack J, Bartlett J, Hayes D, Robson JM. 2004. Three cases of Q fever osteomyelitis in children and a review of the literature. Clinical Infectious Diseases 39(7):e61-e66.
Oh MD, Shin H, Shin D, Kim U, Lee S, Kim N, Choi MH, Chai JY, Choe K. 2001. Clinical features of vivax malaria. American Journal of Tropical Medicine and Hygiene 65(2):143-146.
Ohl CA, Hyams KC, Malone JD, Oldfield EC 3rd. 1993. Leishmaniasis among Desert Storm veterans: A diagnostic and therapeutic dilemma. Military Medicine 158(11):726-729.
Okhuysen PC, Jiang ZD, Carlin L, Forbes C, DuPont HL. 2004. Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in North American travelers to Mexico. American Journal of Gastroenterology 99(9):1774-1778.
Oldfield EC 3rd, Wallace MR, Hyams KC, Yousif AA, Lewis DE, Bourgeois AL. 1991. Endemic infectious diseases of the Middle East. Reviews of Infectious Diseases 13(3 Suppl):S199-S217.
Oliveri R, Matera G, Foca A, Zappia M, Aguglia U, Quattrone A. 1996. Polyradiculoneuropathy with cerebrospinal fluid albuminocytological dissociation due to neurobrucellosis. Clinical Infectious Diseases 23(4):833-834.
Olsen SJ, Bleasdale SC, Magnano AR, Landrigan C, Holland BH, Tauxe RV, Mintz ED, Luby S. 2003. Outbreaks of typhoid fever in the United States, 1960-99. Epidemiology and Infection 130(1):13-21.
Oren I, Kraoz Z, Hadani Y, Kassis I, Zaltzman-Bershadsky N, Finkelstein R. 2005. An outbreak of Q fever in an urban area in Israel. European Journal of Clinical Microbiology and Infectious Diseases 24(5):338-341.
Papatsoris AG, Mpadra FA, Karamouzis MV, Frangides CY. 2002. Endemic brucellar epididymo-orchitis: A 10-year experience. International Journal of Infectious Diseases 6(4):309-313.
Pappas G, Bosilkovski M, Akritidis N, Mastora M, Krteva L, Tsianos E. 2003. Brucellosis and the respiratory system. Clinical Infectious Diseases 37(7):e95-e99.
Pappas G, Kitsanou M, Christou L, Tsianos E. 2004. Immune thrombocytopenia attributed to brucellosis and other mechanisms of Brucella-induced thrombocytopenia. American Journal of Hematology 75(3):139-141.
Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. New England Journal of Medicine 352(22):2325-2336.
Parker NR, Barralet JH, Bell AM. 2006. Q fever. Lancet 367(9511):679-688.
Parsonnet J, Griffin PM. 1993. Hemolytic uremic syndrome: Clinical picture and bacterial connection. Current Clinical Topics in Infectious Diseases 13:172-187.
Parsonnet J, Greene KD, Gerber AR, Tauxe RV, Vallejo Aguilar OJ, Blake PA. 1989. Shigella dysenteriae type 1 infections in US travellers to Mexico, 1988. Lancet 2(8662):543-545.
Pascual J, Combarros O, Polo JM, Berciano J. 1988. Localized CNS brucellosis: Report of 7 cases. Acta Neurologica Scandinavica 78(4):282-289.
Pegues DA, Ohl ME, Miler SI. 2005. Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R, Editors. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier. Pp. 2636-2654.
Petersen LR, Roehrig JT. 2001. West Nile virus: A reemerging global pathogen. Emerging Infectious Diseases 7(4):611-614.
Platonov AE. 2001. West Nile encephalitis in Russia 1999-2001: Were we ready? Are we ready? Annals of the New York Academy of Sciences 951:102-116.
Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, Feldman C. 1998. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. European Respiratory Journal 12(2):351-356.
Portnoy JZ, Callen JP. 1983. Ophthalmologic aspects of chloroquine and hydroxychloroquine therapy. International Journal of Dermatology 22(5):273-278.
Queipo-Ortuno MI, Morata P, Ocon P, Manchado P, Colmenero JD. 1997. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay. Journal of Clinical Microbiology 35 (11):2927-2930.
Rabinowitz R, Schneck M, Levy J, Lifshitz T. 2005. Bilateral multifocal choroiditis with serous retinal detachment in a patient with Brucella infection: Case report and review of the literature. Archives of Ophthalmology 123(1):116-118.
Rahaman MM, JamiulAlam AK, Islam MR, Greenough WB 3rd. 1975. Shiga bacillus dysentery associated with marked leukocytosis and erythrocyte fragmentation. Johns Hopkins Medical Journal 136(2):65-70.
Raoult D. 2003. Use of macrolides for Q fever. Antimicrobial Agents and Chemotherapy 47(1):446.
Raoult D, Piquet P, Gallais H, de Micco C, Drancourt M, Casanova P. 1986. Coxiella burnetii infection of a vascular prosthesis. New England Journal of Medicine 315(21):1358-1359.
Raoult D, Levy PY, Dupont HT, Chicheportiche C, Tamalet C, Gastaut JA, Salducci J. 1993. Q fever and HIV infection. AIDS 7(1):81-86.
Raoult D, Houpikian P, Tissot-Dupont H, Riss JM, Arditi-Djiane J, Brouqui P. 1999. Treatment of Q fever endocarditis: Comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Archives of Internal Medicine 159(2):167-173.
Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, Stein A, Nesri M, Harle JR, Weiller PJ. 2000. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine 79(2):109-123.
Raoult D, Fenollar F, Stein A. 2002. Q fever during pregnancy: Diagnosis, treatment, and follow-up. Archives of Internal Medicine 162(6):701-704.
Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. The Lancet Infectious Diseases 5(4):219-226.
RBM (Roll Back Malaria). 2005a. Roll Back Malaria - Epidemiology. [Online]. Available: http://www.emro.who.int/rbm/Epidemiology-current.htm [accessed May 4, 2006].
RBM. 2005b. Roll Back Malaria - Regional Office for the Eastern Mediterranean: Epidemiological Situation. [Online]. Available: http://www.emro.who.int/rbm/epidemiology-2004.htm [accessed July 17, 2006].
RBM. 2005c. Roll Back Malaria - World Malaria Report: Afghanistan Country Profile. [Online]. Available: http://www.rbm.who.int/wmr2005/profiles/afghanistan.pdf [accessed May 4, 2006].
RBM. 2005d. Roll Back Malaria - World Malaria Report: Iran Country Profile. [Online]. Available at: http://www.rbm.who.int/wmr2005/profiles/iran.pdf [accessed May 5, 2006].
RBM. 2005e. Roll Back Malaria - World Malaria Report: Iraq Country Profile. [Online]. Available: http://www.rbm.who.int/wmr2005/profiles/iraq.pdf [accessed May 4, 2006].
RBM. 2005f. Roll Back Malaria - World Malaria Report: Pakistan Country Profile. [Online]. Available: http://www.rbm.who.int/wmr2005/profiles/pakistan.pdf [accessed May 4, 2006].
RBM. 2005g. Roll Back Malaria - World Malaria Report: Saudi Arabia Country Profile. [Online]. Available: http://www.rbm.who.int/wmr2005/profiles/saudiarabia.pdf [accessed May 4, 2006].
RBM. 2005h. Roll Back Malaria - World Malaria Report 2005 - Malaria Control, By Region. [Online]. Available: http://rbm.who.int/wmr2005/html/2-2.htm [accessed June 27, 2006].
Rees JH, Soudain SE, Gregson NA, Hughes RA. 1995. Campylobacter jejuni infection and Guillain-Barre syndrome. New England Journal of Medicine 333(21):1374-1379.
Rees JR, Pannier MA, McNees A, Shallow S, Angulo FJ, Vugia DJ. 2004. Persistent diarrhea, arthritis, and other complications of enteric infections: A pilot survey based on California FoodNet surveillance, 1998-1999. Clinical Infectious Diseases 38(3 Suppl):S311-S17.
Renia L, Potter SM, Mauduit M, Rosa DS, Kayibanda M, Deschemin JC, Snounou G, Gruner AC. 2006. Pathogenic T cells in cerebral malaria. International Journal for Parasitology 36(5):547-554.
Rhodes KM, Tattersfield AE. 1982. Guillain-Barre syndrome associated with Campylobacter infection. British Medical Journal (Clinical Research Ed.) 285(6336):173-174.
Riestra-Castaneda R, Gonzalez-Garrido A, Gonzalez-Cornejo S. 1996. Brucellosis and polyneuroradiculomyeloencephalitis. A case report. Archives of Medical Research 27(3):331-333.
Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. 2003. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerging Infectious Diseases 9(3):376-379.
Roiz MP, Peralta FG, Valle R, Arjona R. 1998. Microbiological diagnosis of brucellosis. Journal of Clinical Microbiology 36(6):1819.
Rolando I, Carbone A, Gotuzzo E, Carillo CP. 1985a. Circulating immune complexes in the pathogenesis of human brucellar uveitis. Chibret International Journal of Ophthalmology 3:30-38.
Rolando I, Carbone A, Haro D, Gotuzzo E, Carrillo C. 1985b. Retinal detachment in chronic brucellosis. American Journal of Ophthalmology 99(6):733-734.
Rolando I, Tobaru L, Hinostruza S. 1987. Clinical manifestations of 25 Brucella uveitis. Ophthalmic Practice 5:12-17.
Rongnoparat C, Panpanit R. 1987. Hemolytic uremic syndrome associated with shigellosis: Report of two cases. Southeast Asian Journal of Tropical Medicine and Public Health 18(2):226-228.
Ropper AH. 1988. Campylobacter diarrhea and Guillain-Barre syndrome. Archives of Neurology 45(6):655-656.
Ruben B, Band JD, Wong P, Colville J. 1991. Person-to-person transmission of Brucella melitensis. Lancet 337(8732):14-15.
Rubin M. 1968. Prolonged pharmacotherapy and the eye. A symposium. The antimalarials and the tranquilizers. Diseases of the Nervous System 29(3):S67-S76.
Ruiz RS, Saatci OA. 1991. Chloroquine and hydroxychloroquine retinopathy: How to follow affected patients. Annals of Ophthalmology 23(8):290-291.
Russo R, Laguna F, Lopez-Velez R, Medrano FJ, Rosenthal E, Cacopardo B, Nigro L. 2003. Visceral leishmaniasis in those infected with HIV: Clinical aspects and other opportunistic infections. Annals of Tropical Medicine and Parasitology 97(1 Suppl):99-105.
Rynes RI, Bernstein HN. 1993. Ophthalmologic safety profile of antimalarial drugs. Lupus 2 (1 Suppl):S17-S19.
Saad M, Youssef S, Kirschke D, Shubair M, Haddadin D, Myers J, Moorman J. 2005. Acute flaccid paralysis: The spectrum of a newly recognized complication of West Nile virus infection. Journal of Infection 51(2):120-127.
Saah AJ. 2000. Introduction to rickettsioses and ehrlichioses. In: Mandell GL, Bennett JE, Dolin R, Editors. Principles and Practice of Infectious Diseases. 5th ed. New York: Churchill Livingstone. Pp. 2033-2035.
Sacks N, Van Rensburg AJ. 1976. Clinical aspects of chronic brucellosis. South African Medical Journal 50(19):725-728.
Sanchez-Sousa A, Torres C, Campello MG, Garcia C, Parras F, Cercenado E, Baquero F. 1990. Serological diagnosis of neurobrucellosis. Journal of Clinical Pathology 43(1):79-81.
Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. 2003. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: Multicentre, randomised, double blind, four arm study. British Medical Journal (Clinical Research Ed.) 327(7423):1078.
Scrimgeour EM, Al-Ismaily SI, Rolain JM, Al-Dhahry SH, El-Khatim HS, Raoult D. 2003. Q Fever in human and livestock populations in Oman. Annals of the New York Academy of Sciences 990:221-225.
Secretary of the Air Force. 2005. Air Force Instruction 48-105: Surveillance, Prevention, and Control of Diseases and Conditions of Public Health or Military Significance.
Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. 2003. Neurologic manifestations and outcome of West Nile virus infection. Journal of the American Medical Association 290(4):511-515.
Senanayake N, de Silva HJ. 1994. Delayed cerebellar ataxia complicating falciparum malaria: A clinical study of 74 patients. Journal of Neurology 241(7):456-459.
Shamo FJ. 2001. Malaria in Iraq. Meditsinskaia Parazitologiia i Parazitarnye Bolezni (1):46-47.
Shanks GD, Edstein MD. 2005. Modern malaria chemoprophylaxis. Drugs 65(15):2091-2110.
Shears P. 1996. Shigella infections. Annals of Tropical Medicine and Parasitology 90(2):105-114.
Shibukawa-Kent R. 2006. Air Force Institute for Operational Health. Personal communication.
Shubhakaran, Sharma CM. 2003. Acute inflammatory demyelinating polyneuropathy with P. falciparum malaria. Journal of the Association of Physicians of India 51:223-224.
Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, Draper CC, Killick-Kendrick R, Garnham PC. 1977. A strain of Plasmodium vivax characterized by prolonged incubation: The effect of numbers of sporozoites on the length of the prepatent period. Transactions of the Royal Society of Tropical Medicine and Hygiene 70(5-6):474-481.
Siegman-Igra Y, Kaufman O, Keysary A, Rzotkiewicz S, Shalit I. 1997. Q fever endocarditis in Israel and a worldwide review. Scandinavian Journal of Infectious Diseases 29(1):41-49.
Sieper J, Braun J, Wu P, Hauer R, Laitko S. 1993. The possible role of Shigella in sporadic enteric reactive arthritis. British Journal of Rheumatology 32(7):582-585.
Simon DG, Kaslow RA, Rosenbaum J, Kaye RL, Calin A. 1981. Reiter’s syndrome following epidemic shigellosis. Journal of Rheumatology 8(6):969-973.
Sinha R, Aggarwal A, Prasad K, Misra R. 2003. Sporadic enteric reactive arthritis and undifferentiated spondyloarthropathy: Evidence for involvement of Salmonella typhimurium. Journal of Rheumatology 30(1):105-113.
Sinha S, Prasad KN, Pradhan S, Jain D, Jha S. 2004. Detection of preceding Campylobacter jejuni infection by polymerase chain reaction in patients with Guillain-Barre syndrome. Transactions of the Royal Society of Tropical Medicine and Hygiene 98(6):342-346.
Skirrow MB, Blaser MJ. 2002. Campylobacter jejuni. In: Blaser MJ, Smith PD, Raudin JI, Greenberg HB, Guerrant RL, Editors. Infections of the Gastrointestinal Tract. 2nd ed. Philadelphia, PA: Lippincott. Pp. 719-739.
Smith B, Ryan MA, Gray GC, Polonsky JM, Trump DH. 2002. Tuberculosis infection among young adults enlisting in the United States Navy. International Journal of Epidemiology 31(5):934-939.
Smith DL, Ayres JG, Blair I, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Evans M, Farrell ID, et al. 1993. A large Q fever outbreak in the West Midlands: Clinical aspects. Respiratory Medicine 87(7):509-516.
Solera J, Rodriguez-Zapata M, Geijo P, Largo J, Paulino J, Saez L, Martinez-Alfaro E, Sanchez L, Sepulveda MA, Ruiz-Ribo MD. 1995. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrobial Agents and Chemotherapy 39(9):2061-2067.
Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. 1999. Brucellar spondylitis: Review of 35 cases and literature survey. Clinical Infectious Diseases 29(6):1440-1449.
Solomon T, Cardosa MJ. 2000. Emerging arboviral encephalitis. Newsworthy in the West but much more common in the East. British Medical Journal (Clinical Research Ed.) 321(7275):1484-1485.
Speed B, Kaldor J, Cavanagh P. 1984. Guillain-Barre syndrome associated with Campylobacter jejuni enteritis. Journal of Infection 8(1):85-86.
Spink WW. 1951. What is chronic brucellosis? Annals of Internal Medicine 35(2):358-374.
Spink WW. 1954. Family studies on brucellosis. American Journal of the Medical Sciences 227(2):128-133.
Stead WW, Dutt AK. 1991. Tuberculosis in elderly persons. Annual Review of Medicine 42: 267-276.
Stead WW, Lofgren JP. 1983. Does the risk of tuberculosis increase in old age? Journal of Infectious Diseases 147(5):951-955.
Stead WW, To T. 1987. The significance of the tuberculin skin test in elderly persons. Annals of Internal Medicine 107(6):837-842.
Stein A, Raoult D. 1995. Q fever endocarditis. European Heart Journal 16(B Suppl):19-23.
Sugamata M, Ahmed A, Miura T, Takasu T, Kono R, Ogata T, Kimura-Kuroda J, Yasui K. 1988. Seroepidemiological study of infection with West Nile virus in Karachi, Pakistan, in 1983 and 1985. Journal of Medical Virology 26(3):243-247.
Sugiyama T, Okazaki T, Yabe Y, Suda H, Saito T. 1967. Basic and clinical study on chloroquine therapy of rheumatic diseases. Eye disorders due to chloroquine. Saishin Igaku. Recent Medicine 22(10):2331-2335.
Sundar S, Agrawal S, Pai K, Chance M, Hommel M. 2005. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. American Journal of Tropical Medicine and Hygiene 73(2):269-271.
Sutlas PN, Unal A, Forta H, Senol S, Kirbas D. 2003. Tuberculous meningitis in adults: Review of 61 cases. Infection 31(6):387-391.
Svedhem A, Kaijser B. 1980. Campylobacter fetus subspecies jejuni: A common cause of diarrhea in Sweden. Journal of Infectious Diseases 142(3):353-359.
Tabbara KF. 1990. Brucellosis and nonsyphilitic treponemal uveitis. International Ophthalmology Clinics 30(4):294-296.
Tam CC, Rodrigues LC, O'Brien SJ. 2003. Guillain-Barre syndrome associated with Campylobacter jejuni infection in England, 2000-2001. Clinical Infectious Diseases 37(2):307-310.
Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile Virus infection. Journal of Clinical Microbiology 38(6):2232-2239.
Tasova Y, Saltoglu N, Sahin G, Aksu HS. 1999. Osteoarthricular involvement of brucellosis in Turkey. Clinical Rheumatology 18(3):214-219.
Tauxe RV, Blake PA. 1992. Epidemic cholera in Latin America. Journal of the American Medical Association 267(10):1388-1390.
Taylor JP, Perdue JN. 1989. The changing epidemiology of human brucellosis in Texas, 1977-1986. American Journal of Epidemiology 130(1):160-165.
Taylor WR, White NJ. 2004. Antimalarial drug toxicity: A review. Drug Safety 27(1):25-61.
Taylor DN, Echeverria P, Pitarangsi C, Seriwatana J, Bodhidatta L, Blaser MJ. 1988. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. Journal of Clinical Microbiology 26(5):863-868.
Taylor DN, Bodhidatta L, Echeverria P. 1991. Epidemiologic aspects of shigellosis and other causes of dysentery in Thailand. Reviews of Infectious Diseases 13(4):S226-S230.
Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. 2006. Chemokines in host-parasite interactions in leishmaniasis. Trends in Parasitology 22(1):32-40.
Thomas MF, Hedayati H. 1986. Reactive arthropathy (Reiter's syndrome) after salmonellosis: Report of two cases and review of the literature. Journal of the American Osteopathic Association 86(8):504-507.
Thomas R, Kameswaran M, Murugan V, Okafor BC. 1993. Sensorineural hearing loss in neurobrucellosis. Journal of Laryngology and Otology 107(11):1034-1036.
Thomson GT, Chiu B, De Rubeis D, Falk J, Inman RD. 1992. Immunoepidemiology of post-Salmonella reactive arthritis in a cohort of women. Clinical Immunology and Immunopathology 64(3):227-232.
Thomson GT, Alfa M, Orr K, Thomson BR, Olson N. 1994. Secretory immune response and clinical sequelae of Salmonella infection in a point source cohort. Journal of Rheumatology 21(1):132-137.
Thomson GT, DeRubeis DA, Hodge MA, Rajanayagam C, Inman RD. 1995. Post-Salmonella reactive arthritis: Late clinical sequelae in a point source cohort. American Journal of Medicine 98(1):13-21.
Thwaites GE, Duc Bang N, Huy Dung N, Thi Quy H, Thi Tuong Oanh D, Thi Cam Thoa N, Quang Hien N, Tri Thuc N, Ngoc Hai N, Thi Ngoc Lan N, Ngoc Lan N, Hong Duc N, Ngoc Tuan V, Huu Hiep C, Thi Hong Chau T, Phuong Mai P, Thi Dung N, Stepniewska K, Simmons CP, White NJ, Tinh Hien T, Farrar JJ. 2005. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with Tuberculous meningitis. Journal of Infectious Diseases 192(12):2134-2141.
Tissot-Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, Chicheportiche C, Nezri M, Poirier R. 1992. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. American Journal of Medicine 93(4):427-434.
Tissot-Dupont H, Amadei MA, Nezri M, Raoult D. 2004. Wind in November, Q fever in December. Emerging Infectious Diseases 10(7):1264-1269.
Toler SM. 2004. Oxidative stress plays an important role in the pathogenesis of drug-induced retinopathy. Experimental Biology and Medicine (Maywood, NJ) 229(7):607-615.
Travis LB, Travis WD, Li CY, Pierre RV. 1986. Q fever. A clinicopathologic study of five cases. Archives of Pathology and Laboratory Medicine 110(11):1017-1020.
Tripathi BM, Dube S, Biseria S. 1995. Ophthalmic involvement in severe malaria. Journal of the Association of Physicians of India 43(6):441-442.
Trujillo IZ, Zavala AN, Caceres JG, Miranda CQ. 1994. Brucellosis. Infectious Disease Clinics of North America 8(1):225-241.
Turck WP, Howitt G, Turnberg LA, Fox H, Longson M, Matthews MB, Das Gupta R. 1976. Chronic Q fever. Quarterly Journal of Medicine 45(178):193-217.
Turgut M. 2001. Spinal tuberculosis (Pott's disease): Its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurgical Review 24(1): 8-13.
Tzekov R. 2005. Ocular toxicity due to chloroquine and hydroxychloroquine: Electrophysiological and visual function correlates. Documenta Ophthalmologica 110(1):111-120.
Vallejo JG, Stevens AM, Dutton RV, Kaplan SL. 1996. Hepatosplenic abscesses due to Brucella melitensis: Report of a case involving a child and review of the literature. Clinical Infectious Diseases 22(3):485-489.
van Woerden HC, Mason BW, Nehaul LK, Smith R, Salmon RL, Healy B, Valappil M, Westmoreland D, de Martin S, Evans MR, Lloyd G, Hamilton-Kirkwood M, Williams NS. 2004. Q fever outbreak in industrial setting. Emerging Infectious Diseases 10(7):1282-9.
Varney NR, Roberts RJ, Springer JA, Connell SK, Wood PS. 1997. Neuropsychiatric sequelae of cerebral malaria in Vietnam veterans. Journal of Nervous and Mental Disease 185(11):695-703.
Verdon R, Chevret S, Laissy JP, Wolff M. 1996. Tuberculous meningitis in adults: Review of 48 cases. Clinical Infectious Diseases 22(6):982-988.
Vynnycky E, Fine PE. 1997. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiology and Infection 119(2):183-201.
Walker J, Sharma OP, Rao NA. 1992. Brucellosis and uveitis. American Journal of Ophthalmology 114(3):374-375.
Wallace MR, Hale BR, Utz GC, Olson PE, Earhart KC, Thornton SA, Hyams KC. 2002. Endemic infectious diseases of Afghanistan. Clinical Infectious Diseases 34(Suppl 5):S171-S207.
Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, Gerber SI. 2004. Clinical characteristics and functional outcomes of West Nile Fever. Annals of Internal Medicine 141(5):360-365.
Wei LC, Chen SN, Ho CL, Kuo YH, Ho JD. 2001. Progression of hydroxychloroquine retinopathy after discontinuation of therapy: Case report. Chang Gung Medical Journal 24(5):329-334.
Weil Y, Mattan Y, Liebergall M, Rahav G. 2003. Brucella prosthetic joint infection: A report of 3 cases and a review of the literature. Clinical Infectious Diseases 36(7):e81-e86.
Weina PJ, Neafie RC, Wortmann G, Polhemus M, Aronson NE. 2004. Old world leishmaniasis: An emerging infection among deployed US military and civilian workers. Clinical Infectious Diseases 39(11):1674-1680.
WHO (World Health Organization). 1986. Joint FAO/WHO expert committee on brucellosis. World Health Organization Technical Report Series 740:1-132.
WHO. 2002. Strategic Direction for Research: Leishmaniasis. Geneva: World Health Organization.
WHO. 2003. Africa Malaria Report 2003. [Online]. Available: http://www.rbm.who.int/amd2003/amr2003/amr_toc.htm [accessed May 2, 2005].
WHO. 2004. Anti-Tuberculosis Drug Resistance in the World Report No. 3. Geneva: World Health Organization.
WHO. 2006a. WHO Report 2006: Global Tuberculosis Control Surveillance, Planning, Financing. Geneva: World Health Organization.
WHO. 2006b. Tuberculosis Fact Sheet No 104. [Online]. Available: http://www.who.int/mediacentre/factsheets/fs104/en/print.html [accessed May 1, 2006].
Wildman MJ, Smith EG, Groves J, Beattie JM, Caul EO, Ayres JG. 2002. Chronic fatigue following infection by Coxiella burnetii (Q fever): Ten-year follow-up of the 1989 UK outbreak cohort. QJM 95(8):527-538.
Willard RJ, Jeffcoat AM, Benson PM, Walsh DS. 2005. Cutaneous leishmaniasis in soldiers from Fort Campbell, Kentucky returning from Operation Iraqi Freedom highlights diagnostic and therapeutic options. Journal of the American Academy of Dermatology 52(6):977-987.
Williams RK, Crossley K. 1982. Acute and chronic hepatic involvement of brucellosis. Gastroenterology 83(2):455-458.
Wilson ME. 1991. A World Guide to Infections: Diseases, Distribution, Diagnosis. New York: Oxford University Press.
Wilson PE, Alker AP, Meshnick SR. 2005. Real-time PCR methods for monitoring antimalarial drug resistance. Trends in Parasitology 21(6):278-283.
Wortmann G. 2004. Pulmonary manifestations of other agents: Brucella, Q fever, tularemia and smallpox. Respiratory Care Clinics of North America 10(1):99-109.
Yebra M, Marazuela M, Albarran F, Moreno A. 1988. Chronic Q fever hepatitis. Reviews of Infectious Diseases 10(6):1229-1230.
Yechoor VK, Shandera WX, Rodriguez P, Cate TR. 1996. Tuberculous meningitis among adults with and without HIV infection. Experience in an urban public hospital. Archives of Internal Medicine 156(15):1710-1716.
Young EJ. 1983. Human brucellosis. Reviews of Infectious Diseases 5(5):821-842.
Young EJ. 1991. Serologic diagnosis of human brucellosis: Analysis of 214 cases by agglutination tests and review of the literature. Reviews of Infectious Diseases 13(3):359-372.
Young EJ, Tarry A, Genta RM, Ayden N, Gotuzzo E. 2000. Thrombocytopenic purpura associated with brucellosis: Report of 2 cases and literature review. Clinical Infectious Diseases 31(4):904-909.
Yu D, Kuipers JG. 2003. Role of bacteria and HLA-B27 in the pathogenesis of reactive arthritis. Rheumatic Diseases Clinics of North America 29(1):21-36, v-vi.
Zaks N, Sukenik S, Alkan M, Flusser D, Neumann L, Buskila D. 1995. Musculoskeletal manifestations of brucellosis: A study of 90 cases in Israel. Seminars in Arthritis and Rheumatism 25(2):97-102.
Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. 2003. Post-kala-azar dermal leishmaniasis. The Lancet Infectious Dieases 3(2):87-98.
Zinneman HH, Glenchur H, Hall WH. 1961. Chronic renal brucellosis. New England Journal of Medicine 265:872-875.
















































































