12
New and Underutilized Research Techniques and the Dietary Reference Intakes1
Looking to the future in terms of Dietary Reference Intakes (DRI) efforts brings a need to consider new techniques, new methodologies, and new research opportunities. This session included three perspectives on new and underutilized research techniques that may be applicable to DRIs. Irwin H. Rosenberg of Tufts University, who served as a member of the Subcommittee on Upper Reference Levels of Nutrients, addressed promising research techniques and a research strategy for setting Tolerable Upper Intake Levels (ULs). Paul B. Pencharz of the University of Toronto, who served on the macronutrient panel, focused on protein and amino acid research. Jose M. Ordovas of Tufts University addressed the “-omics” and systems biology.
NEW AND UNDERUTILIZED RESEARCH TECHNIQUES AND THE DIETARY REFERENCE INTAKES
Presenter: Irwin H. Rosenberg
The presentation covered potential research techniques and also some major research needs that could be addressed with more traditional techniques.
Promising Research Techniques
Genotyping, Epigenetics, and Imprinting
Topics that need consideration with regard to setting nutrient requirements and to considering susceptibility to higher levels of intake of a nutrient include genotyping, epigenetics, and imprinting—including the assessment of effects of single nucleotide polymorphisms (SNPs) on variability in requirements and/or ULs. Earlier in this workshop, Drs. Steven Zeisel and Patrick Stover provided examples, such as the finding that methylation of deoxyribonucleic acid (DNA) at the cytosine position can have a substantial influence on gene expression and that dietary methyl donors can influence the level of methylation to some extent. This is a knowledge area that is important to understand, particularly when it is possible that one can influence not only gene expression but even the total silencing of a gene, resulting in genetic imprinting and some very important phenotypic outcomes. Some interesting modeling challenges can be anticipated.
With regard to the methylene tetrahydrofolate reductase (MTHFR) polymorphisms, the pathway is highly complex; flavin-adenine dinucleotide (FAD) may be able to partially stabilize the heat lability of the variant. Looking at this additional way in which riboflavin interacts with this pathway may serve as another example of how this type of information will enter some of the decision making related to DRIs.
A specific example involves the C677T mutation in the MTHFR gene. Homozygosity for this mutation results in a less active and a more heat-sensitive enzyme protein (Kang et al., 1988). Heat sensitivity results in dissociation of FAD, but the dissociation is prevented by folate substrates (Guenther et al., 1999). The prevalence of the homozygous variant differs among subgroups: the variant exists in about 15 percent of the Caucasian population, is less prevalent in African Americans, and is more prevalent in Hispanics.
Data from Dr. Paul Jacques of Tufts University shows an elevation of homocysteine in the homozygous variant only when folate intake is below the median. This finding raised questions about whether different subgroups would respond differently to intake. The increase in plasma folate by genotype (data provided by Jacques, see Figure 12-1), suggests that the abnormality probably is not a large determinant of the requirement. One possible model of the MTHFR variant related to the folate
requirement is depicted in Figure 12-2. This model assumes that the variant represents a certain part of the distribution of the folate requirement that would shift the curve only slightly to the right. The intent of this model is to illustrate that when new data are obtained, models will be needed to help interpret how findings about requirements of subpopulations should influence requirements set for the overall population.
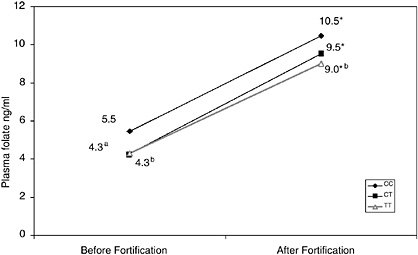
FIGURE 12-1 Increase in plasma folate by genotype.
*p < .05 compared with the same genotype before fortification.
ap < .05 compared to wild type.
bp < .05 compared to wild type. Significance lost after adjustment for multiple comparisons.
SOURCE: Paul Jacques, Tufts University, Boston.
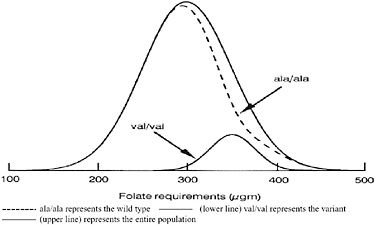
FIGURE 12-2 A possible model of the MTHFR variant related to the folate requirement.
NOTE: Dashed line (ala/ala) represents the wild type; lower line (val/val) represents the variant; upper line represents the entire population.
SOURCE: Irwin Rosenberg, Tufts University, Boston, MA.
Bioavailability Studies
Because nutrition deals with different forms of vitamins and minerals, studies of bioavailability are very important. Dr. Rosenberg views them as absolutely essential in the setting of requirements.
An example is provided by folate, dietary folate equivalents, and folic acid. One of the research recommendations made in the DRI B Vitamins Report (IOM, 1998a) is to obtain more information about monoglutamate versus food folate, which Dr. Rosenberg views as an oversimplification. Instead, he suggested that the research need concerns free folic acid versus folates embedded in foods. The hydrolysis of folate polyglutamates is not the rate-limiting problem in folate absorption. Probably absorption is affected by the release of folate from some of the food matrices. Whether folic acid (the synthetic form of the vitamin) is given with or without food may affect its bioavailability.
Dr. Rosenberg then pointed out that the DRI B Vitamins Report (IOM, 1998a) misapplies dietary folate equivalents to the folate contained in human milk. Doing so is inappropriate because human milk folate is absorbed by a totally different process than is folate provided by other foods. In particular, the infant absorbs the folate from breast milk
in association with a protein binder that passes across a permeable membrane. As a result, the folate in human milk probably is at least as well—if not better—absorbed than is folic acid—the form of the vitamin added to infant formula. Expressing human milk folate in terms of dietary folate equivalents underestimates the bioavailable folate content of human milk and thus the Adequate Intake (AI). This error in the report needs to be corrected.
Stable Isotope Studies
Fruitful areas for research involving stable isotope studies (the subject of other presentations during this workshop) include the conversion of carotenes to vitamin A, studies of amino acid and protein requirements, and doubly labeled water studies.
Research Strategy for Setting ULs
The report of the Upper Levels Subcommittee (IOM, 1998b) did not contain a set of research recommendations, in spite of the fact that the research needs were very great. Of the research recommendations contained in the set of DRI reports, only 2 of 60 in the research methods list refer to the ULs. A few more could be attributed to UL research needs. A look at the complete list of research needs reveals an even lower proportion of needs that apply to ULs.
Dr. Rosenberg called for a commitment to the research base for making the judgments needed to set ULs, including the following:
-
Sharper end points of adverse effects
-
Dose–response analyses with larger numbers—a very important need for ULs and EARs
-
Depletion–repletion studies for adverse effects if feasible and ethical (otherwise, consider ways to obtain better information from cohort studies)
-
Validated surrogate markers of risk
An early finding, such as the presence of esterified vitamin A as evidence of replete stores, might be one example of a surrogate marker of risk. Another possible surrogate marker might be circulating free folic
acid, which indicates that doses of folic acid beyond the ability of dihydrofolate reductase to reduce them have entered the metabolic system. Connecting various observations with other functional markers and functions may help move the process forward.
Dr. Rosenberg concluded that the process for setting ULs is relatively new. Progress in this area will require a commitment to the research that allows making good judgments.
A FOCUS ON PROTEIN AND AMINO ACIDS
Presenter: Paul B. Pencharz
Promising Future Directions for Research
In looking at future directions for research related to the DRIs, this presentation highlighted the following points:
-
Know where we come from—that is, return to first principles and rediscover what people previously knew. For example, in about 1900, Karl Voit reported that 1 gram of protein per kilogram of body weight per day supported good work efficiency.
-
Consider the techniques used to determine the dietary requirements of monogastric farm animals (e.g., pigs), because the animals are easier to study and exhibit less genetic variability.
-
Find surrogate measures that can be used to determine dose– response to define a level to use in studying longer-term functional outcomes.
Dr. Pencharz clarified that his remarks were from the perspective of a researcher who is interested in minimally invasive methods that can be used in children.
Using the Full Range of Intakes to Define the DRI Values
In the DRI process, the Estimated Average Requirement (EAR) is best established by defining the physiological response both below and above the requirement point. This approach also helps define variability and thus the Recommended Dietary Allowance (RDA). The use of nonlinear regression and two-phase linear cross-over regression analysis could be helpful in defining the variability.
Using piglets as subjects, a feeding study could be helpful in identifying a surrogate measure that would indicate that a particular amino acid is potentially toxic (e.g., that it can no longer be metabolized if intake exceeds a certain amount). In a study that fed higher and higher levels of phenylalanine, the data curve showed a point at which the data went straight up—that is, it identified the phenylalanine intake at which phenylalanine hydroxylase is overwhelmed. The break point could serve as a surrogate measure that the particular amino acid could be toxic.
Methods Related to the EAR for Protein
Dr. Pencharz discussed the following views regarding methods related to protein requirements:
-
Nitrogen balance is a cumbersome tool and is expensive and time consuming.
-
Failure to include all available data in past analyses may have resulted in an underestimation of protein requirements.
-
Longer-term feeding studies are needed (e.g., Garza et al. [1977] provided evidence that short-term nitrogen balance studies probably underestimate the protein requirement).
-
New methods need to be applied. Preliminary data obtained by measuring breath 13CO2 following the oxidation of orally administered L-[1-13C] phenylalanine over various protein intakes suggest that the break point for the protein requirement occurs at about 0.9 g/kg of body weight, which is consistent with a reanalysis of nitrogen balance data.
Methods for determining amino acid requirements include growth, which is useful only during rapid growth; nitrogen balance; direct oxida-
tion; indicator amino acid oxidation (IAAO); 24-hour direct amino acid balance; and 24-hour indicator amino acid balance (IAAB). Either the indicator of oxidation or the 24-hour balance is currently considered the state of the art for determining amino acid requirements.
Genetic variability merits consideration as related to research on protein requirements. Within-subject variability is smaller than between-subject variability. Farm animals with selective breeding have much less variability than humans, but ongoing studies in dogs of different breeds are showing a degree of variability that is similar to that in humans.
The IAAO could be used as a direct reflection of protein synthesis for two purposes: (1) comparing a commercially available parenteral amino acid solution to a new parenteral solution, and (2) identifying limiting amino acids in both parenteral solutions. A corollary with food could be to determine how processing alters the bioavailability of an amino acid. Within one day, this method can identify the limiting amino acids.
Concluding Remarks
In conclusion, there is a need to relate the determination of DRIs to long-term health. Determine whether short-term balance studies relate to long-term health. Conduct studies with graded levels of intake to define an appropriate intake level, and then study health effects of that intake over a long term, as was done by Garza and colleagues (1977). Consider additional ways to examine function—possibly the Harvard Step Test as a measure of iron status. Look at findings in new ways, for example, How do studies that show a suppression of parathyroid concentration with increased 25-hydroxyvitamin D relate to bone health and hence to vitamin D requirements? Would the answer require long-term studies or would medium- or short-term studies suffice?
“-OMICS” AND SYSTEMS BIOLOGY
Presenter: Jose M. Ordovas
The focus of this presentation was how the science of genetics can be used to reduce the risk of chronic disease, with examples mainly related
to reducing the risk of coronary heart disease. In response to research recommendation 8-A,2 genetics can be very helpful in determining the individual risk for chronic or age-related diseases. Genetics may help predict, as early as possible, those people who will be more susceptible to common disorders, so that specific attention can be directed to them. Earlier detection of diabetes mellitus, for example, could have an enormous impact: using current methods, persons with diabetes already have lost about 50 percent of their beta-cell function by the time the diabetes is diagnosed.
What is involved in this earlier detection? It requires capturing and interpreting information at different levels and using a variety of novel techniques. Thus, genomics involves studying the genomes, which contain the information (an indication of what can happen). Transcriptomics is used to analyze gene expression (what appears to be happening). Proteomics is used to investigate proteins (compounds that make things happen). Metabolomics is used measure metabolites (substances that indicate what has happened and is happening).
Genetic information provided by the Framingham Study (Lahoz et al., 2001) shows a differential cardiovascular disease (CVD) risk associated with the presence of one or another allele at candidate genes (i.e., APOE) and a gender difference as well. Genetics may help provide more specific information related to the need for different recommendations for men and women, and provide such information early. In one example, genetics could identify 12 or 15 percent of the population who would not be identified by low-density lipoprotein (LDL) cholesterol, the classical biomarker for CVD risk. Their LDL cholesterol could be low despite a genetic predisposition to have as much cardiovascular disease risk as do those with high LDL cholesterol.
Because the technology has moved forward, an approach called unbiased knowledge acquisition is examining the genome and obtaining data on hundreds of thousands of SNPs. A National Institutes of Health initiative is working to put all this information in the public domain. In the near future, the entire human genome variation will be covered, allowing investigation of genes of interest in terms of whatever pathway is being studied.
|
2 |
See Appendix C to find the wording that corresponds to the recommendation numbers given in this chapter. The number refers to the ID number for the recommendation in the DRI Research Synthesis Database, and the letter refers to the specific DRI report. |
Possible Revisions of Selected Research Recommendations
Dr. Ordovas suggested a revision of research recommendation 282-E related to genetic variants that contribute to the intraindividual variation in LDL. In particular, he added high-density lipoprotein cholesterol (HDL-C) and triglyceride concentrations as biomarkers of interest and fatty acids as a dietary component.
In addition to the traditional CVD risk biomarkers (usually measured in the fasting state), increasing evidence supports the notion that biomarkers measured in the nonfasting (postprandial) state may have a key role in identifying CVD risk. Many, many genes are involved in the complex postprandial process of handling dietary fat and cholesterol. Thus, genes may affect the concentration of atherogenic triglyceride-rich lipoproteins, and CVD risk varies with the concentration of these lipoproteins during the postprandial period. For example, research at one of the candidate genes examined (APOA5) shows that for the people who do not have a specific polymorphism—approximately 80 to 85 percent of the U.S. population—polyunsaturated fatty acid intake has little effect on this risk factor. However, for those who are carriers of the polymorphism, circulating triglyceride-rich lipoprotein concentrations tend to be higher; and this condition is associated with greater CVD risk. This negative aspect of this gene variant is expressed only in the case of higher n-6 polyunsaturated fatty acid intake.
Dr. Ordovas also suggested revising research recommendation 256-E relating to dose–response studies by adding n-6 and n-3 polyunsaturated fatty acids to the examples of essential macronutrients. The negative effect associated with the higher intake of polyunsaturated fatty acids (discussed in the previous paragraph) is exclusive to n-6 fatty acids.
With regard to research recommendation 266-E, genetics might be used, for example, to predict who is going to benefit from the classical approaches to successful or lasting weight loss. Figure 12-4 shows a 12-month follow-up of obese subjects who were placed on hypocaloric diets (Corella et al., 2005). The carriers of the allele of the perilipin gene did not lose weight during the year, whereas the group without the polymorphism did. (The perilipin gene is expressed in the adipocytes and protects the adipocytes from lipolysis.) Thus, this is an example of how to distinguish the people who may be able to succeed with a traditional weight-reduction approach from those who need a different intervention.
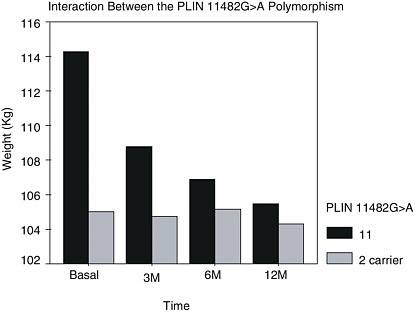
FIGURE 12-4 Weight reduction over 1 year (obese subjects on a low-calorie diet), by PLIN (11482G > A) polymorphism status.
NOTE : M = month.
SOURCE: From Corella et al. (2005). Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J Clin Endocrinol Metab. 90(9):5121–5126. Copyright 2005, The Endocrine Society.
Making Use of Metabolomics
Metabolomics can be used to distinguish people who have severe atherosclerosis from those who have normal coronary arteries (see Figure 12-5).The partial least squares for discriminant analysis (PLS-DA) model (Brindle et al., 2002) is a noninvasive, multivariate statistical approach to predicting coronary artery status that makes catheterization (a very invasive process) unnecessary. A yet unpublished example of a metabolomic technique combines ultraperformance linkage chromatography with mass spectrometry. The technique produces PLS-DA score plots for three different diets, which allows identification of the dietary phase (saturated fat, polyunsaturated fat, or monounsaturated fat) for each subject. In the future, this kind of approach might be used to assess compliance or the intake of certain nutrients.
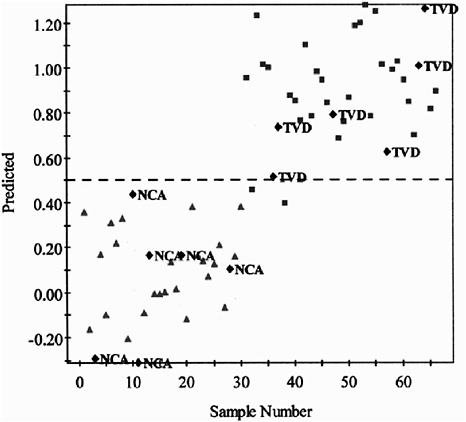
FIGURE 12-5 Prediction of coronary artery status using the PLS-DA model. Comparison of patients with severe atherosclerosis (TVD) and patients with normal coronary arteries (NCA)
SOURCE: Brindle et al. (2002). Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. 8:1439–1445. Copyright 2002.
Because of the huge amount of information that is being generated by these new techniques, it is important to use bioinformatics to process the information and make it readily usable. By using this approach and the different connections between genes and gene products, it will be possible to define, for example, the mechanisms by which arachidonic acid and docahexaenoic acid (DHA) inhibit the synthesis and production of cholesterol. By knowing the key players along pathways, it will be
possible to increase the points at which specific nutrients can be targeted for disease prevention and even treatment.
Concluding Remarks
Dr. Ordovas concluded that the ultimate goal of nutrigenomics is determining optimal nutrition for everyone—not just for 95 percent—with any genetic constitution, in any environment, at any life stage. He predicts that what is called nutrigenomics today will be called nutrition within the next 10 years.
DISCUSSION
Dr. Zeisel commented that a parallel use of metabolomics can be developed to analyze food composition. The concept is to look at the 3,000 components in a food and characterize them simultaneously. Thus, the investment in metabolomics could help both the clinical studies and the food composition studies.
In response to Dr. Pencharz’s comments on variability, Dr. Appel noted that the DRI reports tended not to deal with measurement error issues related to intraindividual variability, and he asked for a research objective to document the variation and to catalogue it in future reports.
Dr. Rosenberg commented that despite all the power of genomics, he does not expect to be able to explain individual variation only in those terms. For example, differences in absorption and even metabolic turnovers will not be completely explained by measuring these genotypes. Dr. Ordovas agreed, stating that genetics can explain up to 50 percent.














