5
Nuclear Power
Nuclear power could make a substantial contribution to the base-load electrical system of the United States in the intermediate term. Advanced converters or fission breeders could enlarge this contribution, and extend it many decades or thousands of years. Nevertheless, the expansion and further development of nuclear power face uncertainties and controversies.
-
The demand for electricity is difficult to predict.
-
The amount of uranium that will be available to fuel the present generation of reactors at economical prices is uncertain.
-
The safety of nuclear reactors is a controversial topic.
-
Policies for disposal of radioactive waste have not been developed, and delay in their development has heightened concern about the efficacy of proposed methods.
-
The possibility that terrorists or other groups might divert nuclear materials is a matter of concern. The degree of protection that can be achieved against diversion has been discussed and argued without resolution.
-
The contribution nuclear power might make to increasing or decreasing the risks of nuclear weapons proliferation and nuclear war is controversial, and the obvious importance of this issue makes it a matter of urgent concern.*
|
* |
See statement 5–1, by E.J.Gornowski, Appendix A. |
These and related issues are addressed in this chapter. We first present a summary statement and principal conclusions. The balance of the chapter takes up these items in detail.†
SUMMARY
Nuclear power contributes to diversity in the sources of energy on which the United States can draw. In 1978, 66 light water reactors (LWR’s) supplied close to 13 percent of the electricity generated in the United States. In some regions of the country, the share of electricity generated by nuclear power exceeded 40 percent. The distribution of nuclear plants is illustrated in Figure 5–1. The generating capacity of nuclear power plants totals 52 gigawatts (electric) (GWe).1
Of the energy sources that can be used to generate large amounts of electricity, only coal and nuclear power offer reasonably assured ability to support significant expansion in electrical generating capacity over the next few decades. The costs of electricity produced from coal and nuclear power are roughly comparable and depend on plant location and financing conditions. Nevertheless, new orders for nuclear power plants were offset by cancellations of previous orders the past 3 years, and this will create a pause in the expansion of nuclear capacity after 1985 unless the licensing of nuclear plants is accelerated and their construction time reduced. The nuclear industry in the United States can produce at least 500 GWe of nuclear generating capacity for installation by the year 2000, and more than 750 GWe by 2010. The actual rate at which this capability will be called upon depends on several factors.
First, there is the question what the demand for electricity will be. The Supply and Delivery Panel evaluated a number of projections and concluded that an annual average growth rate of 4 percent to the year 2010 represents a reasonable figure for planning the growth of electrical capacity.2 This would lead to a total demand for just under 2000 GWe of capacity in 2010, if the total system’s capacity factor is unchanged. In the scenario of highest energy consumption considered by CONAES (assuming constant real prices and 3 percent annual average rate of growth in gross national product (GNP)), the required electrical capacity falls below 1500 GWe in 2010. Assuming a higher rate of electrification, the required capacity might be about 1750 GWe in 2010. (See chapter 11.) Although these projected rates fall below the historical rate of growth, they may still
|
† |
See statement 5–2, by E.J.Gornowski, Appendix A. |
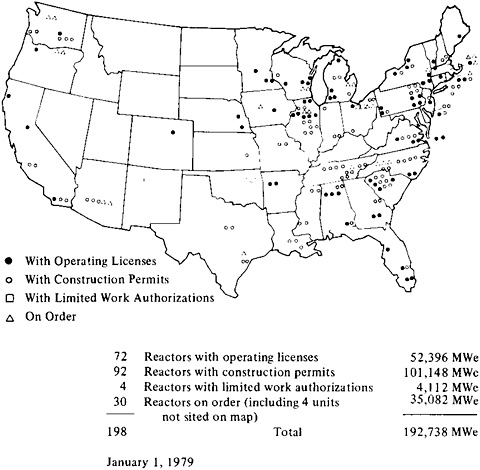
FIGURE 5–1 Nuclear power plants in the United States as of January 1, 1979. Source: Atomic Industrial Forum, Electricity from Nuclear Power (Washington, D.C.: Atomic Industrial Forum, 1979).
be unrealistically high. Models constructed for the CONAES study project lower and declining rates of growth in GNP than the rates experienced in the past.3 The CONAES models have also explored the effects of higher and increasing prices for energy, or equivalent policies. These assumptions lead to scenarios in which the demand for electricity ranges from below present values to just under 3 times present values (2.8 times) by 2010.4
Correspondingly, utility capacity would be between about 400 and 1450 GWe of installed central station power (1978 capacity was 560 GWe). These estimates assume that the fraction of total energy demand satisfied
by electrical end-use remains constant.* In the past, electricity has tended to displace the direct use of fuels at the point of consumption, and the fraction of total energy demand met by electricity has increased.
How much of this demand for electricity will be met by nuclear power is also uncertain. Nuclear power has a slight economic advantage over coal. This advantage has good prospects for enhancement, but also has some chance of reversal. Prudent utility planners are likely to plan mixed systems of nuclear power and coal, given these contingencies, but the proportion of each can only be guessed. In addition to cost, planners must also consider the reliability of supply, the stability of regulatory requirements, and prospective public policy. Some considerations will favor nuclear power, others, coal.
A major reservation against too great a reliance on nuclear power may arise from uncertain availability of natural uranium, the primary resource for nuclear fuel. The Uranium Resource Group of this study5 concluded in 1977 that not more than 1.8 million tons of minable domestic uranium oxide (U3O8) reserves and probable resources should be considered as a basis for prudent planning. CONAES has revised its own figure to 2.4 million tons, reflecting higher estimates recently published by the U.S. Department of Energy. (Table 5–1, under the section “Availability of Uranium,” sets out the pertinent estimates.) Translating these figures into nuclear power capacity, 2.4 million tons of U3O8 would meet the lifetime fueling requirements of about 400 GWe of installed capacity, assuming the continued use of light water reactors on once-through fuel cycles. The total nuclear capacity in operation, under construction, or planned in the United States in 1979 amounts to 193 GWe.6 According to the Supply and Delivery Panel, the uranium production rates required to reach installed nuclear capacities much above 200 GWe by 2010 would demand a national commitment to uranium resource exploration and extraction.7
Further expansion and continuation of nuclear power could be accommodated if fuel reprocessing were permitted. The industrial position is that expansion much beyond current commitments would not be undertaken unless the durability of nuclear power were confirmed by commitment to a breeder reactor (or to equivalent fuel production systems, such as accelerator breeders, or fusion-fission devices).† Without firm plans for reactor designs to follow light water reactors, or for fuel reprocessing and recycle, nuclear capacity would have to be gradually phased out as reactors were retired, beginning early in the twenty-first century. However, if (as some resource economists believe) considerably more uranium is found as the price rises, then nuclear capacity could be
|
* |
See statement 5–3, by L.F.Lischer, Appendix A. |
|
† |
Statement 5–4, by E.J.Gornowski: It is unlikely that there is unanimous opinion that no new LWR’s would be built if the breeder were forever excluded. |
expanded even if the introduction of new reactors and fuel cycles were to be postponed.
Some expansion of light water reactor capacity (with a once-through fuel cycle) could also be achieved by reconfiguring the light water reactor to minimize U3O8 consumption, and also by lowering enrichment tails to 0.1 percent or less (see “Uranium Enrichment”). This might raise the allowable capacity in the year 2000 for the same resource base by nearly 25 percent, to 500 GWe.
Another possibility for a more durable industry is to switch from the present generation of light water reactors on the once-through cycle (no reprocessing or other reuse of spent fuel) to reactors and nuclear fuel cycles that make more efficient use of uranium. Under present conditions, only about 0.6 percent of the fission energy potentially available is used. The fission of uranium-235 (235U) contributes 0.4 percent, and the fission of plutonium-239 (239Pu) created in the reactor contributes 0.2 percent. If the spent fuel removed from the reactor were reprocessed, and the 235U and 239Pu recycled in fuel, the use of uranium could be raised to 0.9 or 1 percent. Such reactor types as the Canadian CANDU or advanced high-temperature gas-cooled reactors (HTGR’s) could be designed and operated to use up to 2.0 percent of the energy embodied in uranium on a once-through cycle. Combining lower enrichment tails and the possible stripping of existing accumulated tails with the use of the enriched CANDU once-through cycle might further increase the capacity that could be safely committed by 2000, perhaps to more than 525 GWe.
By loading uranium and plutonium into breeder reactors, and recycling the load many times through similar reactors after reprocessing, it is possible to recover perhaps 70 percent of the energy in the original uranium ore—an improvement in energy recovery by about a factor of 100 over light water reactors. This possibility not only multiplies the energy from existing resources (including existing enrichment plant tails), but permits economic recovery of energy from much less concentrated and more widely distributed uranium ores, essentially making uranium a potential source of energy for hundreds of thousands of years.
In addition to recovering a large fraction of the energy in 238U, it is possible to recover the energy in another element, thorium, that is probably 4 times more abundant in the earth’s crust than uranium. The single isotope of thorium, thorium-232 (232Th), can be converted to another fissile isotope of uranium, 233U, in nuclear reactors. Various combinations of thorium-uranium and uranium-plutonium fuel cycles can greatly multiply energy resources.
Making more efficient use of nuclear fuel resources depends on using new designs for reactors and operating these reactors in combination with fuel reprocessing.8 These reactor designs may be divided into two classes:
advanced converters designed for the use of thermal neutrons and generally operating on the thorium cycle, and fast breeders designed for the use of fast neutrons that can generate more plutonium from 238U than they consume in generating power. Breeders can also generate 233U from thorium. Advanced converters using thorium and 233U can be designed to function as thermal breeders. With sufficiently careful design and frequent fuel reprocessing, they can operate without additional fissile isotopes from nature. However, these conditions are not likely to yield economical power generation.9
The breeder design closest to commercial status in the United States and elsewhere is the liquid-metal fast breeder reactor (LMFBR). In the most resource-efficient version, this reactor would be fueled with plutonium separated from the spent fuel of light water reactors and with depleted uranium left behind in the enrichment process for today’s light water reactor fuel. The energy available from uranium already mined and stored as depleted tails from domestic enrichment plants, if used in LMFBR’s, could provide one third to one half of the energy recoverable from domestic coal reserves and resources.
Advanced converters can also extend resources, but unless they are fueled with plutonium from the spent fuel of light water reactors, their operation will require some additional uranium feed. The amount of this required feed can be minimized by frequent reprocessing and by features in the converter designed to hold down the loss of neutrons to fission products, control rods, and structural materials. The advanced converter most widely used in the world is the natural-uranium, heavy water CANDU, developed in Canada. The advanced converters closest to commercial status in the United States are the high-temperature gas-cooled reactor and the light water breeder reactor (LWBR). They both use the thorium-uranium cycle with enriched 235U feed. Both require more uranium for their initial inventories of fuel than light water reactors.10 This uranium requirement can be reduced somewhat by mixing in plutonium from reprocessed light water reactor fuel. Advanced converters require far less uranium ore over their operating lives than light water reactors.
The thorium-233U fuel cycle can be used to greatest advantage in thermal advanced converters, and the uranium-plutonium fuel cycle can be used to greatest advantage in fast breeders. This suggests the possibility of using various integrated fuel cycles: combinations of fast breeders, advanced converters, and light water reactors.
These technical possibilities are unlikely to be realized unless nuclear power is publicly acceptable. Public opinion may show swings and trends in the future, as it has in the past. Public concern about nuclear power has centered on four issues: the safety of routine operation of the nuclear fuel
cycle and of reactors; the possibility and effects of major nuclear accidents; the handling of radioactive wastes; and the production of nuclear bombs by nations or subnational groups using fissile materials obtained from nuclear-powered facilities.
At all stages of the nuclear fuel cycle, some radioactivity is released to the environment. The largest burden from these releases has come from the underground mining of uranium and from the milling process by which uranium is concentrated from its ores. The hazards of uranium mining have been estimated as resulting in about 15 deaths per year per 10,000 miners. The radioactivity in the mine increases the hazard of cancer, although the risk of accidental fatality in mining accidents is higher than the increased cancer risk.11 Per miner-year, the hazards of uranium mining are comparable to those of coal mining, but because the same energy is recoverable from only about 1 percent as much material, the mortality of uranium mining is, per unit of power, far less serious than that of coal mining. (See chapter 9.)
Additional radioactive emissions come from the mill tailings—the residues from the uranium concentration process—which contain over 80 percent of the ore’s original radioactivity. Past practices have been careless, resulting in exposure of the tailings to weathering, which releases some of the radioactivity to the environment, and in their incorporation into concrete and landfill for homes and schools, in extreme cases. Although the total morbidity from such handling has been quite small, these consequences have cast doubt on the seriousness with which the industry and the responsible federal agencies approach the job of protecting the public.*
Other routine sources of emission are the releases permitted from nuclear power plants (within set limits) of materials that have become radioactive, and potential releases of radioactive gases (such as krypton-85 (85Kr), tritium, and carbon-14 dioxide) from reprocessing plants.
All these “normal” or routine releases of radioactivity are estimated to increase environmental radiation by a small fraction of the existing background, and on this basis, their effects per unit of power generated are small compared to the mining risks, or to the risks of other energy sources.†
More controversial is the possibility of reactor accidents. Much of the controversy has focused on the validity of risk assessments made in the Reactor Safety Study for the Nuclear Regulatory Commission (also known as the Rasmussen Report or WASH-1400). This report attempted to
|
* |
See statement 5–5, by E.J.Gornowski, Appendix A. |
|
† |
Statement 5–6, by J.P.Holdren: The statement is too sweeping. NAS estimates prepared for CONAES imply 0.5–2.0 excess cancer deaths per GWe-year from routine exposures and emissions, excluding tailings. |
estimate the probability (per reactor-year of operation) that accidents of varying severity would occur.12 Its stated findings are that the actuarial risks (sums of the probabilities of consequences multiplied by the severity of consequences) are very small, and that the chances of severe accidents that would cause large numbers of casualties are extremely small—so small as to be within the range of risks we hardly deign to consider. Nevertheless, these findings have been challenged on several grounds: that the statistical treatment is in some respects incorrect and in others misleadingly presented; that casualty figures for the most severe types of accidents are underestimated; and that accident frequencies may have been overestimated (industry analysts typically arguing the latter, and nuclear critics, the former).13
The Risk and Impact Panel of this study examined the controversy, but could not reach more than qualitative conclusions. These conclusions are, briefly, that the statistical inferences of the report should be corrected upward, owing to the report’s use of medians rather than means of certain probability distributions where the correct procedure would have been to use the mean values, and that in addition to this upward correction in the “best estimate” of the accident risk, the counterclaims of optimism and pessimism for accident frequencies and consequences ought at least to be interpreted as indicating that the uncertainties accompanying both probabilities and consequences are greater than the uncertainty factors stated in WASH-1400.
We would estimate higher average risks than WASH-1400—not so high as to be alarming, but with sufficient uncertainty that there remain legitimate grounds for controversy whether the risk of reactor accidents ought to be an important consideration in decisions about nuclear power. Thus on safety grounds alone, the expansion of nuclear power would be acceptable,* provided the rate of expansion were consistent with the rate of improvement of knowledge about accident risks, especially reductions in uncertainty.
The reactor accident at Three Mile Island occurred after most of CONAES’s deliberations had been completed. That fact and the fact that several investigations of the accident are still in progress make it inappropriate for CONAES to discuss its implications at length, and impossible to do so with authority. The information so far released about the accident (and interpreted by nuclear specialists on the committee) seems consistent with CONAES’s cautious, positive findings on reactor safety.
Another element of public concern is apprehension about the ability of
institutions and industry to manage or dispose of radioactive wastes. The most acute concern is the fate of high-level wastes generated in reprocessing plants or contained in spent fuel, but the management or disposal of the much larger bulk of intermediate- and low-level waste generated throughout the nuclear industry also raises public apprehension. Most experts are of the opinion that no technological obstacles stand in the way of safe management of any of these wastes,14 but governmental inaction, changes of program and emphasis, and the lack of approved facilities are not reassuring.
In the reprocessing and refabrication of fuel essential to making effective use of resources in advanced converters or breeders on either the thorium or the uranium fuel cycle, fissile material (either 233U or 239Pu) is separated from the spent fuel elements and is thus more readily subject to theft or illicit diversion than if it remained in the spent fuel elements. The appearance of pure plutonium or 233U in some stages of the fuel cycle presents the troubling possibility that weapons-usable material could be stolen by terrorists. Proposals have been advanced for reprocessing methods that avoid separation of plutonium in pure form. These schemes are given the generic name “coprocessing” when the plutonium is chemically mixed with its parent uranium throughout the cycle, and “Civex” when it is given the additional protection of retaining some highly radioactive fission products. Such processes are not now available and would require development.
A graver possibility than illicit diversion is that countries installing reprocessing plants would thereby have the means to build up arsenals of nuclear weapons in short order. This concern is particularly acute for breeder reactors, which have little or no value without reprocessing, and it was this consideration that persuaded the Carter administration to defer both commercial reprocessing and commitment to the fast breeder.
A possible advantage of the thorium-233U fuel cycle for fast breeders or advanced converters (it can be used in either) is that the 233U or 235U used to feed these reactors can be diluted with 238U in a 4:1 ratio (for 235U) or a 7:1 ratio (for 233U), making either undesirable as weapons material without physical isotope separation as well as chemical reprocessing. This is the “denatured” thorium cycle. The efficacy of denaturing is now the subject of extensive debate. It is being studied in the United States and will be studied further in the ongoing program of the International Nuclear Fuel Cycle Evaluation (INFCE).
In spite of the unsettled state of the reactor-safety issue following the Three Mile Island incident (which occurred late in the committee’s deliberations), the committee continued to regard proliferation and diversion as the most important—perhaps the overriding—issue in nuclear power. The degree to which the risks of national proliferation of nuclear
armaments or subnational diversion of material for nuclear weapons could be controlled was discussed at length. The problem was acute: Subjective estimates of the magnitude of these risks were balanced against equally subjective estimates of the benefits that nuclear power might provide in easing the world’s problems of energy supply.
There was general agreement that the greatest threat of nuclear technology lies in existing stockpiles of nuclear weapons and weapons material throughout the world. There was further agreement that to the extent that high enrichment of 235U and isolation of 233U and plutonium are needed for a civilian nuclear power industry, these steps of the fuel cycle should be conducted in secured plants, preferably under international control. However, some members of the committee believe that the economic importance of nuclear energy is not great enough to warrant accepting significantly increased risk of international proliferation or subnational use of nuclear weapons, and that such increased risk will attend the spread and growth of nuclear power if these should occur more rapidly than improvements can be made in existing safeguards and deterrents. Other members of the committee believe that the world’s energy problems already pose a greater long-term threat than does proliferation, and that the benefits of the rapid spread of nuclear power in alleviating these problems outweigh any plausible increase in the risks of proliferation and diversion.* Divergent opinions on what steps to take follow from these beliefs.
Some argue that international solutions such as the Non-Proliferation Treaty, safeguards (monitoring by the International Atomic Energy Agency), and strengthened controls on fuel cycles can only be effected if the United States is an active participant, a reliable supplier of nuclear materials and know-how. These are arguments for carrying forward, and very probably exploiting, the development of reprocessing and breeder reactors, since both increase our ability to provide nuclear fuel.
Others argue that the current policy of the United States—staying the commercialization of reprocessing for the time being and limiting the development of breeders to technology-level studies—is essential as an example to others.† They maintain that this forbearance is necessary to avoid a situation in which countries that have legitimate domestic needs for major nuclear power enterprises are tempted to manufacture nuclear weapons. The argument is that the moral position of the United States is strengthened in international negotiations by what may be some self-sacrifice.
|
* |
See statement 5–8, by E.J.Gornowski, Appendix A. |
|
† |
Statement 5–9, by E.J.Gornowski: The United States has lost this argument. Reprocessing is going ahead in other countries regardless of the U.S. position. |
The issues of diversion and proliferation make the future of reprocessing and the breeder reactor uncertain. As a consequence, the future of nuclear power beyond the point of resource scarcity is also uncertain. The undecided future of reprocessing adds to uncertainty about the form of waste that must ultimately be banished from the environment. The committee cannot resolve these uncertainties, but in the recommendations that follow, suggests ways they might be reduced by improving the reliability of information, by narrowing and clarifying areas of dispute, and by instituting interim programs that preserve flexibility of response in anticipation of better information.
CONCLUSIONS
The committee draws the following conclusions about technical factors that should be considered in formulating nuclear policy.
-
The rate of growth in the use of electricity is a primary factor affecting the strategy of nuclear power development. Low rates of growth allow the electric utilities sufficient flexibility to regard coal and nuclear capacity as interchangeable to a considerable degree. This becomes increasingly difficult for higher electricity growth rates; rapid expansion of both coal and nuclear capacity would be required. The highest growth rates in electricity use examined by the committee call for technically achievable rates of expansion of both new coal and nuclear capacity that many members of the committee regard as incompatible with environmental and political restrictions.
-
The growth of conventional nuclear power (today’s light water reactors) will be limited by the producibility of domestic uranium resources, probably before the year 2000. With today’s once-through fuel cycle and no change in the prevailing policy against reprocessing, a maximum nuclear capacity of about 400 GWe could be reached by 2000, diminishing thereafter. This contribution could be extended to about 600 GWe with reprocessing and recycle of fuel in light water reactors. A more complete assessment is needed of domestic and world uranium resources, and of the rate at which they can be produced at various costs.
-
A greater, or more sustained, contribution of nuclear power beyond 400 GWe and past the year 2000 could only be supported by the installation of advanced reactor systems, particularly those using recycle of nuclear fuel. Even if very extensive new uranium resources are identified before 1990, advanced converters would still be attractive because they could extend the uranium energy base appreciably. Nevertheless, only the
-
breeder can provide insurance of satisfying very high demand, or of abating a shortage of uranium.
-
Several different breeder reactors could serve in principle as candidates for an indefinitely sustainable source of energy. Only the liquid-metal fast breeder reactor could be built and operated by the year 2000.
With regard to the major domestic issues that surround nuclear power, the committee draws the following conclusions.
-
The short-term health risks from routine operation of the LWR nuclear fuel cycle appear to be far below the risks from the coal fuel cycle. This remains the case if reactor accidents are included, using the risk estimates of the Reactor Safety Study (WASH-1400). The accuracy of the WASH-1400 results and the validity of this type of comparison are disputed both inside and outside CONAES. Long-term risks are even more difficult to compare. The maximum estimates of nuclear power risks are within the range of risks for the coal cycle. An analysis of reactor safety such as WASH-1400 cannot be carried out for advanced reactors until specific commercial designs are available.
-
No insurmountable technical obstacles are foreseen to preclude safe disposal of nuclear wastes in geological formations. All necessary process steps for immobilizing high- and low-level wastes have been developed, and there are no technical barriers to their implementation. Geological emplacement can be carried out with standard mining techniques. There is still some controversy about the assured integrity of the backfill.
-
The main problems with geological waste disposal are site-specific: characterizing sites that exhibit a high degree of stability, transmit water only by pore flow, and offer no ready access to groundwater. Storage of waste at such sites would engender much smaller risk to the public than that of routine emissions from the rest of the fuel cycle, Routine emissions from the nuclear fuel cycle are generally recognized to present very small risks to health.
-
Radiation has been released from stored nuclear waste, notably from the wastes of military production operations, but also from some wastes of civilian operations. These incidents have not so far resulted in public hazards. They do, however, illustrate the inadequacies of existing surveillance and regulatory practices, and they emphasize the need for permanent disposal facilities.
-
Nuclear waste disposal has suffered in the past from decision making by the federal government that has been both dilatory and capricious. The time for decisions is upon us, but we have not yet arrived at a decision-making process that is both legitimate and authoritative.
Finally, with regard to international issues, we note the following conclusions.
-
The United States, with relatively large reserves of both coal and uranium, is in a very favorable position compared to many countries of the world that have little or no indigenous fuels. In the absence of practical alternatives, these countries may well find nuclear power, especially breeder reactors, attractive as an energy source that greatly reduces reliance on fuel imports.
-
The problem of diversion of nuclear materials by terrorist or criminal groups, and the related question of the vulnerability of nuclear facilities to sabotage, are serious matters. Domestic security measures, such as those practiced in laboratories and facilities handling enriched materials, can be effective. However, if our society moves in the direction of turbulence and polarization, questions might be raised about our ability to carry out domestic security measures properly.
-
The proliferation threat must be viewed from the perspective that the overriding security problem is to avoid war; failing this, it is to avoid war between or among superpowers, and failing that, to avoid devastating nuclear exchanges among them. Nuclear power can reduce this threat by reducing the competition for scarce resources, one of the causes of war. Nuclear power can also increase this threat by facilitating the acquisition of nuclear weapons, particularly by countries whose possession or use of them might catalyze superpower war.
-
Nuclear power is not the most likely route countries with the will to acquire nuclear armaments might follow, but it is not an impossible one. The most likely scenario by which nuclear power could contribute to nuclear armament is the appropriation of plutonium or 233U from nuclear fuel cycles by a country that might not, in the absence of this opportunity, have made the decision to acquire nuclear weapons.
RECOMMENDATIONS
The committee’s principal recommendations are listed below. More detailed recommendations appear in subsequent sections of this chapter.
-
National policy should support the continued use of nuclear power for the next few decades.15 The rationale for such support rests on the availability of nuclear power as a domestic energy resource whose risks are
-
at worst comparable* to those of other energy sources, its competitive economics, and the undesirability of relying too heavily on coal or nuclear power, to the exclusion of the other, until the risks of each are better understood.
-
Advanced reactor types should be developed to the point that they can be evaluated for possible introduction, if needed. Three advanced reactor types can be considered: the liquid-metal fast breeder reactor, the high-temperature gas-cooled reactor, and advanced versions of heavy water reactors. Of these, the LMFBR would be recommended if industrial economic factors were the only consideration. The LMFBR has the best chance of providing insurance that a nuclear industry could in the long term meet any electrical demands that might eventuate.† A major consideration is, of course, the question whether the fuel reprocessing necessary to operate LMFBR’s (and eventually, other advanced converters) is compatible with national antiproliferation objectives. We recommend that development of proliferation-resistant reprocessing for LMFBR’s proceed, pending a decision whether such methods as coprocessing, Civex, or radioactive spiking are sufficient or necessary to counter this policy objection to the LMFBR.
-
High-temperature gas-cooled reactors or heavy water reactors (HWR’s) (or both) are appropriate advanced reactors if (as in most CONAES scenarios) electrical demand grows at a moderate rate, or if appreciable uranium supplies can be produced at a price in the range of $100–$200/lb of U3O8. Uranium supplies in this price range are not believed to be abundant in the United States. These reactors could probably be installed with fewer siting restrictions than LMFBR’s, and they would be compatible with LMFBR’s in a mixed system, with the breeders operating as fuel factories. They might, for a considerable period, produce power more economically than breeders. Their development at some level of effort is therefore recommended, regardless of the decision on the LMFBR.
-
Exploration for uranium resources and their specification must continue at a vigorous pace. This information is basic to the timing of expanding the existing light water reactor industry, and to the pace of commercializing advanced reactors.
-
Technological designs and licensing requirements should be available “off the shelf” for the reprocessing plants that would handle fuel from the various reactor types to reduce the lead time needed if reprocessing is approved. This means that both research and development activities
|
* |
Statement 5–10, by J.P.Holdren: I disagree. Implied here is a kind of apples-plus-oranges comparison that cannot be done. The public might well decide some nuclear risks are intolerable. |
|
† |
See statement 5–11, by L.F.Lischer, Appendix A. |
-
through the pilot-plant stage must be undertaken, as well as licensing of these pilot plants. In view of the uncertain future for commercial reprocessing as a consequence of national policy, there is no alternative to government funding of this activity.
-
Within the national nuclear energy program specifically, and as an integral part of the national technological research program, a much higher level of support is needed for fundamental engineering and materials sciences. These include basic studies in heat transfer, fluid flow, the mechanics of materials under dynamic stresses, corrosion, solid-state diffusion in technical materials, and many other similar fields. Such studies are necessary to resolve questions of reactor safety by means other than complicated and expensive overdesign, and should be the core of any nuclear safety research program.
-
Ore tailings and low-level radioactive wastes from the nuclear industry need a sound program of environmental protection to ensure that they do not present significant health risks to the public. Since even the worst-case risks from these sources are quite low, the steps required should be based on the most probable values of costs and risks, rather than on the most conservative possible assumptions.*
-
High-level and transuranic wastes from the nuclear industry should be disposed of by emplacement in a geological repository. The responsibility for site selection must ultimately rest with the federal government, as the benefits from a well-chosen repository accrue to the nation as a whole, and the risks to local populations are not high.† The target for placing a first repository in operation should be the mid-1990s. In the interim, spent-fuel storage should be practiced without assuming or precluding reprocessing.
-
Radioactive wastes from weapons-material production facilities, military propulsion reactors, weapons fabrication facilities, and other military activities must be dealt with independently. These wastes exist in quantity and in less-than-ideal form for geological disposal. They should, at minimum, be immobilized and collected at a few isolated sites. After these steps, they can be prepared for geological disposal (probably at the same sites). Within the category of defense wastes, the transuranic waste and (possibly) the high-level waste from propulsion-reactor reprocessing are the only types that can be considered for disposal at the Waste Isolation Pilot Plant near Carlsbad, New Mexico.
|
* |
Statement 5–12, by J.P.Holdren: I disagree. Conservatism is particularly appropriate when the potential victims are in future generations, as is the case with tailings and low-level wastes. |
|
† |
See statement 5–13, by J.P.Holdren, Appendix A. |
Finally, what can we say about the problem of nuclear weapons proliferation that is not a homily or a statement of alternatives? It should be continued national policy to search for ways to avoid nuclear war, but it is by no means clear that this country is prepared to give up the option of nuclear weapons for use in extreme circumstances. In this quandary, any technical or industrial policy steps are, at best, supportive of larger national policy. All the antiproliferation measures we can conceive have an experimental character, including such relatively recent measures as the deferral of domestic plans for reprocessing and breeder reactors. If they promote a more peaceful and prosperous world (and thus, necessarily, a more peaceful and prosperous United States), such policies warrant continuation and can be used as the foundations for far-reaching policies. If they do not promote these ends, they should be firmly and quietly scrapped. In the committee’s view, therefore, the country should recognize that any antiproliferation measures (Civex, no reprocessing, or whatever) are not ends in themselves, but only practical measures in the service of policy decisions.
NUCLEAR GROWTH RATES AND THE EFFECT OF REACTOR TYPES ON DEMAND FOR URANIUM
Nuclear power, as visualized now or for the intermediate period of this study, can contribute to our energy supply as a source of electricity. Thus, the contribution we can expect from nuclear power is tied to projections of the demand for electricity. A number of sources compete to satisfy this demand—coal, nuclear, hydroelectric, geothermal, and advanced systems. Nuclear power will only be a part of a mix. If a large part is desirable for nuclear power, it could be constrained by the availability of uranium, or by the availability of reactor types and fuel cycles that would make more efficient use of the available uranium.
This section takes up these questions within the context of a neutral policy. Policy decisions are rarely neutral. Even domestic decisions to select one reactor or fuel cycle over another cannot be simplified and isolated from their context, a context determined by the mutual interaction of technology, the environment, human values, and world affairs. The relative importance of various measures to achieve resistance to diversion and proliferation, the measure of risk and benefit the public assigns to nuclear power, the desirability of competing technologies, and the interaction between the United States and the rest of the world will figure as prominently in domestic decisions about nuclear power as considerations of demand and supply.
NUCLEAR GROWTH SCENARIOS, URANIUM REQUIREMENTS, AND REACTOR TYPES
The question examined here is, “What are the implications for uranium requirements of filling nuclear demands by various reactor and fuel cycle strategies?” The number of variables that affect the answer is large. Several studies have been conducted on the subject, using slightly different assumptions, The results, however, exhibit similar characteristics. The results presented here have their own set of detailed assumptions, but we consider them to be typical. The conclusions to be drawn are qualitative rather than quantitative. (The examples are taken from Zebroski and Sehgal.)16
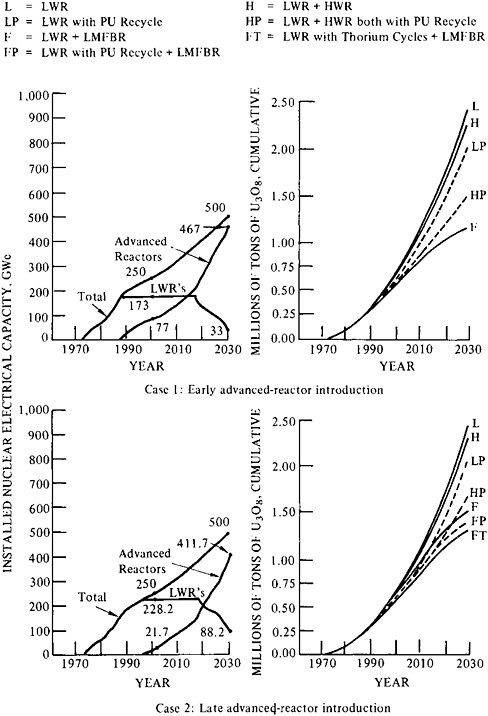
Figure 5–2 shows introduction and installation schedules with corresponding requirements for uranium of two cases, both of which correspond to fairly low growth of demand for nuclear electricity. Although 200 GWe of installed electrical generating capacity from nuclear power plants in 1990 is slightly higher than the installed capacity now scheduled or expected by that date, the approximately 50 GWe assumed in the figure to be installed from 1990 to 2000, and the approximately 60 GWe to be installed between, 2000 and 2010, are well below the expected figures. Case 1 assumes early introduction of advanced reactors (1987) and a rate of installation that results in about 80 GWe by 2000, or a third of total nuclear generating capacity. Case 2 assumes that advanced reactors are introduced in 1995* and installed at a rate resulting in 20 GWe by 2000 (about 8 percent of total nuclear generating capacity), and in 310 GWe by 2030 (62 percent of total nuclear generating capacity). In both cases, the total of the uranium consumption and forward commitments for fueling any of the combinations of reactor types that might be selected to achieve the postulated demand for nuclear generating capacity falls below 2 million tons, even by 2030. Indeed, with only light water reactors on a once-through cycle (the most resource-intensive system), cumulative ore requirements by 2010 would amount to just 1.2 million tons of U3O8. The use of more separative work in the enrichment process, leaving tails at an assay of 0.1 or 0.15 percent, would hold the resource requirement below 2 million tons until 2030, but not beyond.
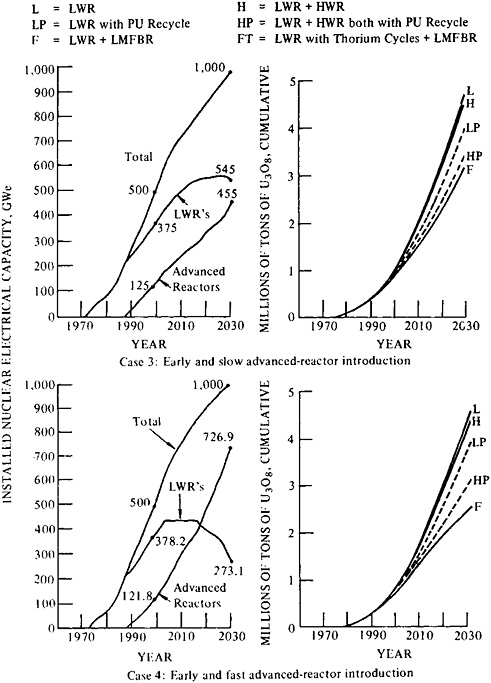
Figure 5–3 illustrates three schedules of nuclear installation to meet moderately high growth of demand for nuclear-generated electricity. The starting point in 1990 is about 250 GWe rather than 200, but this difference does not affect the conclusions that can be drawn. The cases are (1) early introduction of advanced reactors and gradual installation, (2)
|
* |
See statement 5–14, by L.F.Lischer, Appendix A. |
early introduction and rapid installation, and (3) late introduction and rapid installation. A resource base of 2 million tons of U3O8 is sufficient to meet any of these three schedules to 2010, but only those cases that assume the introduction of fast breeders or high utilization of 235U from natural uranium (low tails assay) and fissile-isotope recycle in advanced converters (or both) manage to avoid outstripping 4 million tons of U3O8 in consumption and forward commitments by 2030.
Figure 5–4 depicts a very high rate of growth in nuclear generating capacity. In fact, Figure 5–4 is based on an unrealistic initial rate of installation—400 GWe by 1990—but as with Figure 5–3, the qualitative conclusions are not affected. In all cases, 2 million tons of uranium resources are exhausted soon after the year 2000, and 4 million tons are exhausted soon after 2010. Only those schedules that show early introduction and rapid installation of fast breeder reactors keep consumption and forward commitments of uranium oxide under 6 million tons by 2030. The simultaneous introduction and installation of advanced converters helps relieve some of the pressure on resources, but in all the cases illustrated, the demands on uranium, and possibly on thorium, exceed the rate of production the committee considers possible with present methods, as detailed in the section on uranium production. This scenario is therefore unrealistic unless a “heroic” program of breeder deployment is begun immediately.
The projections illustrated in Figures 5–2, 5–3, and 5–4 correspond roughly to the CONAES study scenarios, assuming that half or more of the electrical generating capacity required is supplied by nuclear power plants. Figure 5–2 represents projections compatible with the low- to medium-consumption scenarios I3, II2, and III2; Figure 5–3, with the medium-high to high scenarios III3, IV2, and IV3; and Figure 5–4, with the specific high-electrification variants of these latter scenarios. (See chapter 11 for details of the study scenarios.) One difference between the examples presented here and the CONAES study scenarios is that the former show demand for electricity continuing to rise at a signficant rate, while the latter show demand for electricity tending to saturate by 2010.
AVAILABILITY OF URANIUM17
In the previous section, the demands of various schedules for installation of nuclear electrical capacity, and of various strategies for developing and installing reactor systems, were expressed in terms of the amounts of uranium they might require. The availability of uranium could limit some of these strategies or schedules.
The federal government, for many years the only customer for uranium,
FIGURE 5–2 Low growth of installed nuclear capacity (350 GWe by 2010): comparison of reactor types and cumulative ore requirements from 1972 forward In case 1, LWR’s without recycle would approach an estimated 2.4 million tons of uranium supplies by 2030. Advanced converters (introduced in 1987 and reaching 80 GWe installed capacity by 2000) with reprocessing, along with reprocessing in LWR’s, could significantly extend the life of nuclear power within the uranium supply estimate of the Uranium Resource Group (1.8 million tons) through 2030. In case 2, late introduction of advanced converters offers less extension time than case 1, but still more than 10 years. Advanced converters, introduced in 1995, reach an installed capacity of 20 GWe by 2000. Source: Adapted from E.L.Zebroski and B.Sehgal, “Advanced Reactor Development Goals and Near-Term and Mid-Term Opportunities for Development” (Paper presented to the American Nuclear Society, Washington, D.C., November 18, 1976).
publishes a systematic annual estimate of potential resources and reserves. The figures for reserves represent ore deposits that have been measured by drilling, and assayed within a 20 percent degree of accuracy, and material available under existing practices as by-products from mining or treating other materials. Reserves can be characterized as the supply of ore the mining industry is confident it can produce. Potential resources represent ore deposits inferred to exist from tacit knowledge and field judgments: the amount of ore that can reasonably be expected to occur in producing strata, or in nonproducing areas that display the characteristics of producing areas.
Figure 5–5 shows the annual estimates made in recent years. The estimates are set out by incremental or forward costs: the additional cost to recover uranium oxide over expenditures already incurred in exploring, filing claims, buying or leasing mineral rights, and determining the extent of deposits. “Forward costs” make no allowance for profit; they represent neither total costs to recover ores nor market prices. The reserves of uranium set out in various categories of forward cost should not be interpreted as representing the ores that can be economically recovered today. They do represent relative cost; for example, reserves in the $30/lb category will be about twice as expensive to recover as those in the $15/lb category. The estimates of potential resources are set out in three categories: probable, possible, and speculative.
The Uranium Resource Group of this study reviewed the 1976 estimate and compared it to information from geologists, experts advising the uranium industry, and others. The group concluded that the possible and speculative categories were not well enough established for resource planning. It judged that the estimate for probable resources, 1.06 million tons, was the closest approximation to a quantitative estimate for all potential resources18 and estimated the domestic uranium producible by
conventional mining at 1.7 million tons. The federal government has reported higher estimates since, partly as the result of better information on uranium in the $30–$50/lb forward-cost category. This uranium is not producible under prevailing market conditions, but its production will become economic as higher-grade deposits are depleted.
Table 5–1 shows the increase in estimates of reserves and probable potential resources since 1976. CONAES has adjusted its resource estimate, reflecting this increase, to 2.4 million tons of U3O8 (excluding by-product recovery).
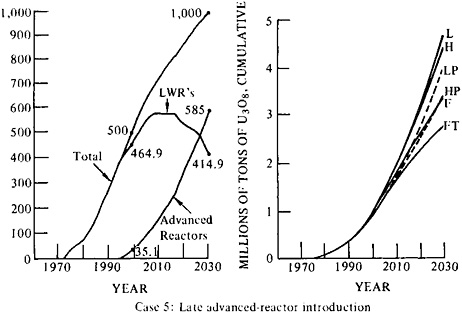
FIGURE 5–3 Moderately high growth of installed nuclear capacity (700 GWe by 2010): comparison of reactor types and cumulative ore requirements from 1972 forward, In all three cases, 2.4 million tons of uranium can sustain moderate rates of growth in installed nuclear capacity to 2010, with recycle of fissile isotopes in the selected mix of reactors. In case 5, however, the late introducton of advanced converters would realize significant savings in resource consumption only if the uranium resource base is more than 3 million tons. At the lower estimate, the late-arriving breeder would be necessary to sustain growth in installed nuclear capacity beyond 2020, In all three cases, the introduction of advanced converters or breeders, or both, is necessary to hold the consumption of uranium and forward commitments below 3 million tons to 2030. Source: Adapted from E.L. Zebroski and B. Sehgal, “Advanced Reactor Development Goals and Near-Term and Mid-Term Opportunities for Development” (Paper presented to the American Nuclear Society, Washington, D.C., November 18, 1976).
Experience with other minerals indicates that projections of potential resources usually fall far below the resources subsequently discovered and produced.19 This is because increasing quantities of lower-grade ores are exploitable as the price rises. But uranium deposits that have been identified in the United States are either rather high grade (>700 ppm uranium by weight) or quite low grade (<100 ppm, or 0.01 percent) with no large intermediate range. Many geologists believe that this is an intrinsic and unique feature of uranium mineralization. Moreover, uranium deposits tend to be discrete and sharply bounded; the edges of the deposits, contrary to experience with other minerals, are barren. Finally, low-grade deposits (less than 0.01 percent uranium oxide), such as those in black shale, may not be producible in quantity because of the massive rock volumes to be moved, uncertain milling requirements, and vast quantities of mill tailings to be managed.
Uranium exploration is a chancy venture. Discovery of the Grants mineral belt in New Mexico (1956–1957), and of the Wyoming Basins deposit (1969–1970) added 60,000 tons/yr to uranium reserves, but
FIGURE 5–4 High growth of installed nuclear capacity (1300 GWe by 2010): comparison of reactor types and cumulative ore requirements from 1972 forward, Conditions can be met only by introducing fast breeder reactors and assuming abundant supplies of uranium—5 million tons for early introduction and rapid rates of installation. Source: Adapted from E.L. Zebroski and B.Sehgal, “Advanced Reactor Development Goals and Near-Term and Mid-Term Opportunities for Development” (Paper presented to the American Nuclear Society, Washington, D.C., November 18, 1976).
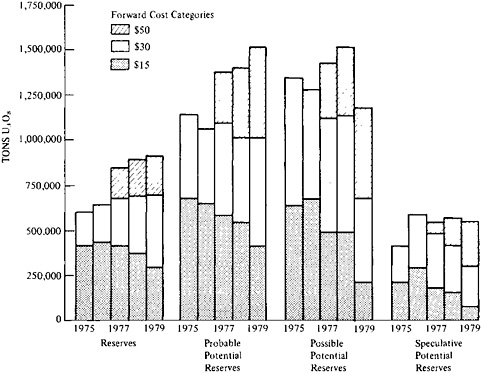
FIGURE 5–5 Changes in domestic uranium estimates, as of January 1 in each of the years 1975–1979. Source: Leon T.Silver, “Discussion of U.S. Uranium Supplies,” testimony before the Subcommittee on Energy Research and Production, Committee on Science and Technology, U.S. House of Representatives, May 31, 1979.
between these two discovery periods, annual additions totaled only 10,000–30,000 tons/yr. Exploration for new uranium deposits concentrates on areas similar to those with known deposits, Only one new district has been discovered in more than 15 years. The odds that uranium exploration will lead to discovery can be improved by better understanding of the factors that govern deposition.
The Uranium Resource Group has estimated that under prevailing
TABLE 5–1 Estimates of Reserves and Probable Potential Resources of Uranium in the United States (tons of U3O8)
|
Year |
Reserves |
Probable Potential |
Total |
|
1976 |
640,000 |
1,060,000 |
1,700,000 |
|
1977 |
840,000 |
1,370,000 |
2,210,000 |
|
1978 |
890,000 |
1,395,000 |
2,285,000 |
|
1979 |
920,000 |
1,505,000 |
2,425,000 |
|
Source: U.S. Department of Energy, Statistical Data of the Uranium Industry (Grand Junction, Colo.: Department of Energy (GJO-100 [79]), 1979), pp. 21, 30–31. |
|||
conditions, uranium discovery will increase to a rate of 50,000 tons/yr in 1985 and decline thereafter. Under conditions favoring discovery, the rate would improve (for a resource base of 1.8 million tons) to 62,000 tons/yr by 1991, and under conditions favoring all-out discovery efforts, rates of 80,000 tons/yr might be reached by 1989.
To maintain adequate reserves—8–12 years’ worth of production at prevailing rates—new ore deposits must be discovered and measured continuously. Production of these reserves normally lags behind discovery about 5 years. Under conditions of uncertainty in future demand similar to those prevailing today, this lag would lengthen to about 10 years. The 5-yr lag could be maintained if there were reasonable assurance of demand.20
Expansion of exploration and mining must be accompanied by expansion of milling21 capacity. Discovery of new ore deposits takes 1–5 years after exploratory effort begins; evaluation by drilling and assay takes an additional 1.5–2 years; mine development, 1–3 years, and construction of a mill, 2–3 years. Some of these steps must be completed before others can be undertaken. The deposit must prove sufficient to justify a new mill, for example. Others may be undertaken simultaneously. Nevertheless, 10 years usually elapses between the decision to expand uranium exploration and the production of uranium oxide.
The nuclear power industry must plan for relatively distant futures, A light water reactor requires at least 10 years to license and build, and the utility must be assured fuel at reasonably predictable prices for at least 10 full-power years of its 30-yr life. In planning for future advanced converters or breeders, the nuclear power industry will be particularly concerned that fuel is both available and producible at rates that correspond to the planned rate of buildup of the industry. Here the
uncertainties that plague uranium resource availability interact with other uncertainties to complicate the planning of both suppliers and consumers,
Under conditions that constitute business as usual for uranium producers, and under prevailing reactor fueling practices, the rate of uranium production in 2000 could fuel only 228 GWe from light water reactors. Accelerating the rates of discovery and increasing production could provide fuel for 310 GWe22 around the year 2000. If the producible uranium oxide is 2.25 million tons, a 310 GWe capacity might then be sustained for 5–10 years before being restricted by a declining production rate as reserves decline.
URANIUM ENRICHMENT
Several enrichment methods were tested in pilot plants during World War II, including gaseous diffusion, thermal diffusion, and modified mass spectrography. Gaseous diffusion proved most effective and economical, In this process, the lighter isotope 235U, in the form of gaseous uranium hexafluoride (UF6), passes more readily than 238U through porous barriers. By repeating the process in successive stages of a cascade, any degree of enrichment can be achieved. In the United States, three facilities (in Oak Ridge, Tennessee, Paducah, Kentucky, and Portsmouth, Ohio) are operated as a single enrichment complex. Enrichment plants using gaseous diffusion also exist in Great Britain, France, the U.S.S.R., and China.
Gaseous diffusion is itself energy intensive. On the average, a little over 5 percent of the electrical energy generated by a light water reactor is needed for the enrichment process.
The increasing cost of energy has made the alternative method of separation by gas centrifuge economically competitive. In this process, the heavier 238U (again, as uranium hexafluoride) is spun to the outside of a centrifuge and the lighter 235U withdrawn from the center. The process requires one twentieth or less of the electricity needed in gaseous diffusion. The savings in electricity justify a large capital cost, and the Department of Energy is now planning to add at least one gas centrifuge to its enrichment facilities, at a cost of $4.2–$4.5 billion.23 Abroad, a tripartite consortium (URENCO), sponsored by Great Britain, the Federal Republic of Germany, and the Netherlands, is building one plant in England and planning others on the European continent.
Among other processes in the United States, laser separation of isotopes has been demonstrated in the laboratory. A chemical exchange method is being investigated in France, and separation by jet nozzles, a process developed in Germany, is being tried on a commercial scale in Brazil and (by a different process) in South Africa. Developers of the chemical exchange method claim it is practical for slightly enriched uranium, but
not for the higher enrichments that would be needed for weapons. If this is confirmed, it would be preferred as a proliferation-resistant technology. The laser process has some promise as a method for almost entirely removing 235U from the rejected material (tails) of existing enrichment plants, in which case it could increase effective uranium reserves by perhaps 20–30 percent (this is the fraction of 235U in natural uranium that is now discarded). The nozzle separation method does not appear practical for the United States, as it is too energy intensive.
Both the laser separation method (which is not yet proved) and the gas-centrifuge method have the potential to produce weapons-grade 235U in significant quantity at relatively small-scale installations.* Both processes employ sophisticated technology that few countries can use. However, technology always spreads. Over time, these processes might provide the easiest access to nuclear weapons: Because of the low level of radioactivity involved, enrichment plants could be built secretly with greater ease than reactors or reprocessing plants.
The capacity of enrichment plants is measured in separative work units (SWU’s) per year, which have the dimensions of mass flow rate (e.g., kilograms of uranium per year). The amount of uranium and separative work required to deliver a given amount of reactor fuel at a given enrichment can be varied within the limits of enriching plants to operate at different tails assays. For the Department of Energy’s existing enriching complex, the tails assay can be varied from 0.2 percent to 0.3 percent 235U without loss of separative capacity. Table 5–2 illustrates the variation possible in feed and separative work per kilogram of light water reactor fuel within this range.
To a limited extent, separative work substitutes for natural-uranium feed. As illustrated in Table 5–2, light water reactor fuel enriched to 3 percent 235U can be produced at 0.2 percent tails assay with about 20 percent less natural-uranium feed, but with 26 percent more separative work, than at 0.3 percent tails assay.
The three-plant complex operating in the United States has a capacity of 18 million SWU/yr, and as of 1977 was intended to reach 28 million SWU/yr in 1981.24 (Requirements have since decreased, and expansion has been delayed accordingly.) The expanded plants are expected to reach full-capacity production by 1985.25 This expanded capacity has been committed to domestic and foreign obligations (323 GWe of light water reactors—two thirds in the United States and one third in foreign countries). The new centrifuge plant is expected to operate at its full capacity of 8.8 million SWU/yr in 1988.26
TABLE 5–2 Ratios of Uranium Feed and Separative Work Units to a Kilogram of Enriched Reactor Fuel, 3 Percent 235U, for Selected Tails Assays
|
|
Tails Assay (percent 235U) |
||
|
0.20 |
0.25 |
0.30 |
|
|
Natural uranium, kg |
5.479 |
5.965 |
6.569 |
|
Separative work units |
4.306 |
3.811 |
3.425 |
|
Source: A.de la Garza, “An Overview of U.S. Enriching Resources,” report to the Supply and Delivery Panel, Committee on Nuclear and Alternative Energy Systems, National Research Council, Washington, D.C., 1976. |
|||
Figure 5–6 illustrates production expected from enrichment plants over the next decade and contract commitments for separative work. The apparent gap between commitments and production from 1981 to 1988 could be closed if operation at high tails assay—about 0.36 percent—were possible. But this inordinately high tails assay would require more feed at a time when uranium supplies may become scarce. Conversely, since enrichment plants are being added in Europe, and since the Soviet Union apparently has spare enrichment capacity, some relief might be available from these sources.
A recent report points out that utilities holding long-term fixed-commitment contracts are required to provide uranium feed to the enrichment complex in amounts that may not agree with their fuel requirements, and it suggests that most of the apparent gap between production capacity and commitments could be eliminated through case-by-case adjustments.27 The substitution of fuel “enriched” by the addition of plutonium from reprocessed old fuel could also help prevent an “enrichment gap.”28
Whether additional enrichment capacity will be needed beyond 1990, and if so when, depends on the number and type of reactors built and their particular fuel needs. Existing and planned enrichment capacity, for example, can supply the fuel for 215 GWe generated by today’s light water reactors using a once-through cycle. Domestic capacity might approach this figure in the early 1990s, and in addition, it would be proper for the United States to supply its share of the enrichment needs of countries that must buy this service. The introduction of new reactors in the form of advanced converters would also affect projected demands for enrichment. Heavy water reactors using natural uranium, or uranium enriched to 1.2 percent 235U, have no enrichment requirements in the first case, and
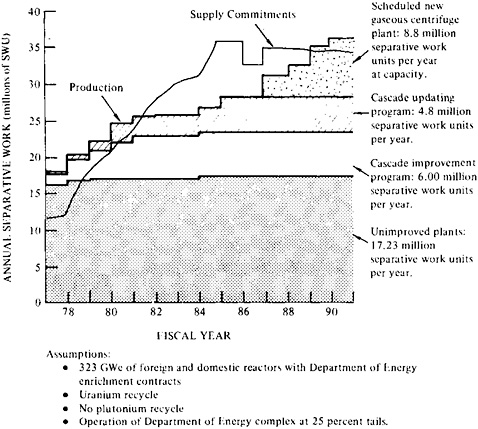
FIGURE 5–6 Separative capacity schedule for the Department of Energy complex of uranium enrichment plants (in Oak Ridge, Tennessee, Portsmouth, Ohio, and Paducah, Kentucky) and contract supply commitments to domestic and foreign utilities.
modest requirements in the second. Advanced converters on the thorium-uranium cycle (such as the HTGR or LWBR) with fuel recycle have heavy requirements for highly enriched uranium for their initial critical loadings, but after the first loading, they use less separative work than LWR’s.29 Breeder reactors started on plutonium have no separative work requirements and could indeed provide some fissile fuel (above and beyond what is needed to fuel new breeders) to converters.
However, in spite of the uncertainties in future demand for separative work, enrichment is not a bottleneck in nuclear expansion. An enrichment plant takes only as much lead time as a reactor to plan and build, so enrichment capacity can be scheduled to match reactor commitments.*
|
* |
See statement 5–16, by E.J.Gornowski, Appendix A. |
NUCLEAR REACTORS AND FUEL CYCLES
Nuclear power is generated from the fission of heavy elements. The heat of fission is liberated in nuclear reactors. This heat is then transported by the reactor coolant to external equipment, where it is converted into electricity. The different types of reactors that have been developed for this purpose employ the major approaches that have been considered attractive. Table 5–3 lists these reactors and the status of their development.
Nuclear fuel is made of isotopes that are easily fissioned by slow neutrons. These are known as fissile isotopes. The only fissile isotope that exists in nature in usable abundance is 235U. This makes up about 0.7 percent of natural uranium, and natural uranium is therefore the basic resource for nuclear power. There are two other isotopes from which fissile isotopes can be made in a reactor. These are 238U, the more common isotope and the major constituent of natural uranium, and 232Th, naturally occurring thorium. Fissile 239Pu, the commonest form of plutonium, is made from 238U, and fissile 233U is made from thorium (238U and 232Th are called fertile isotopes).
As the fissile isotopes originally loaded in a reactor are destroyed, new fissile isotopes are formed from fertile isotopes. The relative rate of replacement is the conversion ratio. When the conversion ratio is greater than 1, new fissile isotopes are formed faster than the original fissile isotopes undergo fission, and the reactor is called a breeder. A reactor that operates at conversion ratios below 1 is known as a converter.
The fuel cycle for a reactor describes the way the fissile and fertile isotopes are used. If natural or slightly enriched uranium is the fuel, plutonium is formed and partly fissioned during reactor operation. Spent fuel still contains some 235U and 239Pu. It could be recycled, or the spent fuel could be considered waste material. Recycle reduces the amount of fresh 235U that has to be supplied, and thus reduces the commitment of natural uranium needed to fuel the reactor.
More highly enriched uranium can also be mixed with thorium in reactor fuel, creating a Th-U fuel cycle. This is not generally economic unless the 235U and 233U left in spent fuel are recycled; however, some once-through cycles in some reactors are only slightly more expensive than their uranium counterparts. A version of the Th-U cycle, in which some 238U is added to keep concentrations of fissile isotopes in uranium below weapons-usable amounts, is the denatured U-Th cycle. This cycle is discussed later in this chapter (under “Reprocessing Alternatives”). Similarly, plutonium could be extracted from spent reactor fuel and combined with 238U to form new fuel. This would be done in recycling natural or slightly enriched uranium fuel, using plutonium at low concentrations. This is the fuel cycle for fast breeder reactors—at higher
TABLE 5–3 Nuclear Reactors and Fuel Cycles: Development Status
|
Reactor Type |
Fuel Cycles |
Development Status |
Possible Commercial Introduction in the United Statesa |
|
Light water reactor (LWR) |
Slightly enriched U (~3 percent 235U) |
Commercial in United States |
1960 |
|
Spectral-shift-control reactor (SSCR) |
Th-Ub |
Conceptual designs, small experiment run; borrows LWR technology |
1990; fuel cycle, 1995 or laterc |
|
Light water breeder reactor (LWBR) |
Th-Ub |
Experiment running; borrows LWR technology; fuel cycle not developed |
1990; fuel cycle, 1995 or laterc |
|
Heavy water reactor (CANDU or HWR) |
Natural uranium |
Commercial in Canada, some U.S. experience |
1990 |
|
|
Slightly enriched U (~1.2 percent 235U) |
Modification of existing designs |
1995 |
|
|
Th-Ub |
Modification of designs; fuel cycle not developed |
1995 |
|
High-temperature gas-cooled reactor (HTGR) |
Th-Ub |
Demonstration running; related development in Germany; fuel cycle partly developed |
1985; fuel cycle, 1995 or laterc |
|
Molten-salt (breeder) reactor (MSR or MSBR) |
Th-Ub |
Small experiment run; much more development needed |
2005 |
|
Liquid-metal fast breeder reactor (LMFBR) |
U-Pub |
Many demonstrations in the United States and abroad |
1995 |
|
|
Th-Ub |
Fuel cycle not developed |
1995 |
|
Gas-cooled fast breeder reactor (GCFBR) |
U-Pub Th-Ub |
Concepts only; borrows LMFBR and HTGR technology |
2000 |
|
aBased on the assumption of firm decisions in 1978 to proceed with commercialization. No institutional delays have been considered except those associated with adapting foreign technology. On the basis of light water reactor experience, it can be estimated that it would take about an additional 15 years after introduction to have significant capacity in place. bIndicated fuel cycles demand reprocessing. cThorium-uranium fuel reprocessing is less developed than uranium-plutonium reprocessing. Indicated reactors could operate for several years before accumulating enough recyclable material for reprocessing. |
|||
concentrations of plutonium—although a Th-U cycle could also be used. The cycle with plutonium and uranium is designated the U-Pu cycle; as with the Th-U cycle, recycle is always assumed.
Table 5–4 presents fuel cycle characteristics of the reactors in Table 5–3. Some caution is needed in interpreting Table 5–4. There are continuous possibilities for varying enrichments, fuel concentrations, lattice spacings, and other reactor fueling parameters. How designers choose to operate a fuel cycle depends on finding a minimum fueling cost within broad technical limits, and only those parameters that seem interesting now are presented. Table 5–4 is schematic and highly simplified. In some cases, particularly those involving future developments, relatively crude estimates have been used.
Virtually all the nuclear power in the United States today, and all planned expansion of nuclear power, is in the form of light water reactors. As can be seen in Table 5–4, these reactors make large demands on supplies of natural uranium at economical prices. Such supplies (as pointed out under “Availability of Uranium”) are limited; therefore, this type of reactor has a limited term of service. How long this term might be depends, of course, on the demand for nuclear power as well as on the supply of uranium. A very long-lasting nuclear industry could only persist by the use of some form of breeder reactor, whose ultimate source of fuel, the fertile isotopes, could probably be supplied at economical prices for hundreds of millennia.
All U-Pu recycle schemes and all Th-U schemes that do not use denatured uranium present the problem that pure fissile material could be chemically isolated during the recycle steps. In particular, breeder reactors are intrinsically fuel-recycle systems. As such, they present the possibility that nuclear materials usable in weapons could be diverted, or that the fuel cycle could be used in national proliferation of nuclear arsenals. On these grounds, the United States has deferred civilian nuclear fuel reprocessing and is attempting to persuade other countries to do the same. On the same grounds, the Administration has opposed proceeding with the latest demonstration breeder reactor project in the United States, the Clinch River breeder reactor (CRBR), a subject discussed later in this chapter.
Nevertheless, a vigorous and durable nuclear power industry could be a very important part of our future energy supply system. Therefore, in what follows, we explore the various reactor systems that might be used, even though many interesting systems rely on recycle of nuclear fuel.
ADVANCED REACTORS30
It has already been noted (Table 5–4) that the current generation of power reactors in the United States, consisting of light water reactors, is not very
TABLE 5–4 Fueling Characteristics of Various Reactors (each 1 GWe capacity)
|
Reactor Type |
Fuel Cycle |
Conversion Ratio |
Natural U3O8 Committed to System Inventory (tons)a |
Natural U3O8 Consumed over System Life (tons)b |
Thorium Requirements (initial inventory plus tons per year) |
Referencec |
|
Light water reactor |
~3 percent 235U, once-through ~3 percent 235U, U recycle ~3 percent 235U, U and Pu recycle Th-235U, once-through Th-235U, U recycle |
0.5–0.65 0.5–0.65 0.45–0.55 0.6 0.65 |
700 ~1000 ~1000 900 ~1200 |
5300 4200 3200 6200 2800 |
— — . 120+30 120+30 |
|
|
Spectral-shift-control reactor |
Th-235U, U recycle |
0.75 |
~1300 |
2300 |
120+10 |
|
|
Light water breeder reactor |
Th-235U, seed recycle Th-233U, recycle |
~0.75 ~1 |
700 ~1000d |
5000 0 |
120+40 4e |
|
|
Heavy water reactor (CANDU) |
Natural U, once-through Natural U, Pu recycle 1.2 percent 235U, once-through Th-235U, once-through Th-235U, U recycle |
0.7 0.6 0.7 0.75 0.9 |
160 ~350 310 700 ~1200 |
4300 1800 3300 2800 1200 |
— — — 160+140 160+140 |
|
|
High-temperature gas-cooled reactor (pebble bed) |
~8 percent 235U, once-through Th-235U, once-through |
0.58 0.58 |
280 300 |
4400 4100 |
— not available |
|
High-temperature gas-cooled reactor (prismatic) |
Th-235U, once-through Th-235U, U recycle |
0.6 0.7–0.9 |
280 ~600 |
4200 2300 |
150+10 150+10 |
|
|
Molten-salt breeder reactor |
Th-233U |
0.9–1.07 |
~400d |
0 |
Inventory not available; 3 |
|
|
Liquid-metal fast breeder reactor |
U-Pu Th-233U |
>1.15 > 1.1 |
1000–2000d 1000–2000d |
Breeder Breeder |
— Not available |
|
|
Gas-cooled fast breeder reactor |
U-Pu Th-233U |
>1.2 >1.15 |
1000–2000d 1000–2000d |
Breeder Breeder |
— Not available |
|
|
a Estimate of reactor inventory plus out-of-reactor inventory for recycle systems. bDoes not include inventory, which is assumed to be recoverable at end of reactor life. cSources: As indicated on the table, the following sources were consulted for descriptions of reactor fueling characteristics. The conversion ratios and tonnages listed in the table (intended for comparative purposes) are the responsibility of CONAES. 1. P.R.Kasten et al., Assessment of the Thorium Fuel Cycle in Power Reactors (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL/TM-5565), 1977), Appendix B. 2. Resources Planning Associates, The Economics of Utilization of Thorium in Nuclear Reactors—Textual Annexes 1 and 2 (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL/TM-6332), n.d.). Table 2.13, p. 158. 3. Battelle Columbus Laboratories, Study of Advanced Fission Power Reactor Development for the United States, vol. 3 (Columbus, Ohio: Battelle Columbus Laboratories (BCL-NSF-C946–2), 1976), pp. C-80–C-120. 4. J.S.Foster and E.Critoph, “The Status of the Canadian Nuclear Power Program and Possible Future Strategies,” Annals of Nuclear Energy 2 (1975):689–703. 5. E.Teuchert et al., “Once-Through Cycles in the Pebble Bed HTR,” ANS Transactions 27 (1977):460. 6. Battelle Columbus Laboratories, Study of Advanced Fission Power Reactor Development for the United States, vols. 2 and 3 (Columbus, Ohio: Battelle Columbus Laboratories (BCL-NSF-C946–2), 1976), vol. 2, pp, IV-II and vol. 3, pp. C-80–C-120. 7. National Research Council, U.S. Energy Supply Prospects to 2010. Committee on Nuclear and Alternative Energy Systems, Supply and Delivery Panel (Washington, D.C.: National Academy of Sciences, 1979). dEstimates based on requirements for equivalent highly enriched 235U to initiate fuel cycles. ePublished estimates of LWBR thorium requirements apparently assume refabrication. |
||||||
efficient in its use of uranium. This is particularly significant if fuel is not recycled, as the fissile isotopes in spent fuel could replace some of the 235U in freshly mined uranium. It is possible to use uranium more efficiently. The key is to use reactors with higher conversion ratios and long fuel lifetimes. Higher conversion ratios can substitute for recycling to the extent that the fissile atoms formed undergo fission in place. With recycle, the higher conversion ratio permits more fissile atoms to be substituted for natural 235U.
Although light water reactors do not now have high conversion ratios, a great deal of the plutonium created in their operation undergoes fission in place. About one third of all the energy in LWR’s is obtained from plutonium fission, and at the end of fuel life, more than 60 percent of the fissions occur in 239Pu. LWR’s could, in principle, be designed for higher conversion ratios and better use of natural uranium, a fact that should be remembered in comparing them to other reactors. Such designs would have lower enrichments and burnups than existing LWR cycles and could only achieve better use of natural uranium through plutonium recycle.31 The required rate of reprocessing might be twice as high, per unit of electrical energy generated, as that for the standard LWR recycle mode estimated in Table 5–4, but lifetime uranium consumption would be less than 3000 tons. For all reactors, the conversion ratio varies with the composition of the fuel loaded and with fuel management. Differences among reactors often correspond to differences in the conversion ratio that can be readily achieved for fuel loading and management practices permitting economical power generation.
The reactors proposed to achieve greater efficiency in the use of fissile resources fall into two classes: advanced converters and breeders. Advanced converters can be designed to achieve conversion ratios ranging from 0.7 to slightly more than 1. (Light water reactors operate at conversion ratios of 0.6 or less.) Breeder reactors can be designed to achieve conversion significantly greater than 1, although they could obviously be designed and operated at lower conversion ratios. For some breeders, such as the molten-salt breeder reactor (MSBR), the reduction in fissile inventory could be sufficient for greater economy (i.e., the savings in charges against inventory could be greater than the loss of income from product sale and the extra cost of feed material).
Prototypes and designs for various types of advanced converters and breeder reactors have been developed in the United States and other countries. The functional and practical points that must be considered to evaluate the relative merits of these reactors and fuel cycles cannot all be assessed equally for the designs and prototypes. Some reactor designs are only conceptual, others have been tested through small pilot plants, and others are close to commercial status. A complete safety assessment, for
example, requires a detailed commercial design. The probability of various accidents depends more on the details of design than on the reactor’s generic characteristics. The only design that has been completely assessed for safety by the standards of the United States is the light water reactor.
ADVANCED CONVERTER DESIGNS
Light water reactors can be designed for conversion of thorium to 233U. Although a light water reactor operated on a Th-U fuel cycle with reprocessing could achieve conversion ratios of about 0.7, the initial fissile inventory would require highly enriched uranium. (This highly enriched fuel may be ruled out by regulations to safeguard the fuel cycle.) The lifetime fuel requirements of a light water reactor on this fuel cycle could be 50–60 percent lower than those of an LWR on the once-through cycle, but the reactor would have to operate some time before enough 233U accumulated for reprocessing. Preliminary studies suggest that Th-U fueling of light water reactors would be uneconomical;32 however, the relatively modest changes required represent the most immediate opportunity to begin learning the engineering of Th-U fuel cycles. Spectral-shift-control reactors (SSCR’s) are essentially similar to pressurized-water reactors (PWR’s), one of the two light water reactors in use today. The coolant/moderator is changed during operation from heavy water to ordinary light water as the fissile content of the fuel in the core decreases. The development of this reactor consists mostly of conceptual studies, although a small pilot plant has been operated in Belgium.33
The light water breeder reactor, in spite of its name, is actually an advanced converter or break-even thermal breeder. Its design goal is to convert enough fertile material to fissile material to completely reload the core after accounting for fuel cycle losses. A demonstration LWBR achieved criticality in 1977, and experimenters anticipate that the reactor will be fully demonstrated by 1985.
The CANDU, an advanced converter designed in Canada to operate efficiently on natural (unenriched) uranium, supplies about 10 percent of the kilowatt-hours (kWh) generated by nuclear power in North America. This reactor employs heavy water as the reactor’s moderator and coolant, and allows on-line refueling. The introduction and installation of heavy water reactors offers a relatively near-term opportunity in the United States to improve uranium efficiency on the once-through fuel cycle. The use of slightly enriched uranium oxide, perhaps 1.0–1.2 percent 235U, would reduce the fuel requirements of a heavy water reactor 40 percent below the fuel requirements of a comparable light water reactor on a once-through fuel cycle. A CANDU-type reactor could also be designed to
incorporate thorium fuel elements. This reactor could be operated either on a once-through fuel cycle or with reprocessing to recover the 233U and thorium for recycle. A once-through cycle would require, however, considerably more uranium mining and enrichment (See Table 5–4 for comparative uranium consumption.)
The high-temperature gas-cooled reactor loads fuel elements into graphite blocks that serve as the reactor’s moderator. The coolant is high-temperature helium. A 330-megawatt (electric) (MWe) commercial plant is operating near Fort Saint Vrain, Colorado. HTGR’s appear capable of operating at both higher thermal efficiency and higher conversion ratios than light water reactors, but their expanded use depends on successful development of economical reprocessing for graphite-based fuel. If the high-temperature gas used to cool the graphite core could be used to drive a gas turbine directly, the thermal efficiency of this reactor could be further improved and the reactor’s operation would be freed of requirements for water. Moreover, HTGR’s, or a version of the related German pebble-bed reactor (whose fuel is contained inside balls of graphite), could be used to supply process heat as well as electricity.
In the molten-salt reactors, solid fuel assemblies are replaced by uranium fluoride and thorium fluoride dissolved in a molten fluoride-salt mixture. The salt is circulated to a heat exchanger external to the core, The molten-salt breeder reactor adds chemical kidneys in the fuel’s external circulation system for continuous reprocessing of the fuel/coolant. This feature reduces the total inventory of fissile material committed to the power plant and its fuel cycle, as compared to other breeder reactors. Volatile fission products are also continuously removed, a step that permits true breeding in the Th-U fuel cycle with a thermal-neutron spectrum. A small pilot molten-salt reactor (10 megawatt (thermal) (MWt)) was operated at Oak Ridge National Laboratories. The development of this concept is far behind that of other advanced converters and breeders. Success cannot be guaranteed because of formidable materials problems, but the advantages that might be realized in this type of system are considerable.
EVALUATION OF ADVANCED CONVERTERS
Several factors must be considered in evaluating advanced converters: ease of development, economic prospects, and compatibility with other policy objectives of the nuclear program. On this latter point, since policy objectives change, the important criterion is that a reactor type perform well under different, or variable, policy limitations.
Two advanced converter systems stand out as being clearly favored by these criteria: the prismatic HTGR (named for the shape of its fuel
elements, which are hexagonal blocks) and the CANDU. A third, the spectral-shift-control reactor, is also worth mention. The prismatic HTGR has the lead in the United States by virtue of the licensed prototype unit (Fort Saint Vrain). Further development of the reactor requires only that this unit be scaled up to fully commercial size. Most generic problems have been identified. The CANDU coded by pressurized heavy water is fully commercial in Canada, and Canadian affiliates of companies in the United States are CANDU designers and vendors. Nevertheless, these companies are skeptical of the ability of the CANDU to meet domestic licensing requirements in its present design. Possible points of difficulty (under the industry’s understanding of licensing philosophy) are the use of on-line refueling, thin-walled fuel cladding, and the design of the emergency core-cooling system.34 If these design features must be changed, the CANDU would evolve in the direction of the British steam-generating heavy water reactor (SGHWR), a system that has been essentially abandoned in Great Britain as uneconomical. The SSCR is claimed to be a straightforward extension of LWR engineering, with its main point of development being an auxiliary unit: an in plant heavy water reconcentration unit that requires separate commercial development, but whose development may be considered independent of other reactor problems.
Two other systems seem to present more formidable development problems, but exhibit some development advantages. The organic-cooled CANDU has been developed in Canada to a point just short of prototype construction. One evaluation35 suggests that its economic prospects are more favorable than those of the CANDU cooled by pressurized heavy water, but not by a margin sufficient to justify the cost of commercialization. However, if the United States were to undertake development of a CANDU for domestic use, there might be keen Canadian interest in joint development of the organic-cooled version.
The pebble-bed reactor is a version of the HTGR that has had successful prototype operation in Germany, and the vendor of the domestic HTGR (General Atomic) has access to its technology.
ECONOMIC PROSPECTS
Capital Costs Under present economic circumstances, none of the advanced converters appears to be competitive with LWR’s. All types seem to involve appreciably higher capital costs. These capital costs must be counterbalanced by savings in fuel cycle costs.
The size of the capital-cost disadvantage for various systems is highly controversial; it is only natural that proponents of a concept present optimistic data, and it is extremely difficult to find economic evaluations for systems not yet built that are free of subjective judgments. With the
TABLE 5–5 Estimates of Nuclear Reactor Capital Costs (dollarsa per kilowatt-electric)
|
Reactor Type |
Cost |
|
Light water reactor |
625 |
|
Spectral-shift-control reactor |
690b |
|
High-temperature gas-cooled reactor |
715 |
|
CANDU |
915c |
|
Fast breeder reactor |
800 |
|
a1977 dollars; includes interest during construction. bIncludes cost of heavy water. cFueled by natural uranium; includes cost of heavy water. Source: Resources Planning Associates, The Economics of Utilization of Thorium in Nuclear Reactors—Textual Annexes 1 and 2 (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL/TM-6332), n.d.), Table D.2, p. 15. |
|
warning that the numbers are not firm, we offer Table 5–5 as a set of estimates for reactor capital costs.
As a rule of thumb, a capital cost of about $30/kWe can be translated, under today’s charge rates for private capital, into an electrical cost of 1 mill/kWh. On this basis, the CANDU reactor would be ruled out. However, it should be noted that the cost cited for the CANDU is for the natural-uranium version, a larger machine that uses more heavy water than a CANDU designed for a slightly enriched uranium or thorium fuel cycle. Capital-cost estimates for these versions of CANDU are not available.
Once-Through Cycles The comparative cost of once-through cycles may be estimated as the differential cost of uranium enrichment and ore purchases. The arbitrary assumption is made here that the costs of fuel fabrication and storage (lesser factors in fuel cycle costs in any case) do not vary much per kilogram of fuel. To the extent that they do vary, small adjustments in comparative evaluations would have to be made. For this evaluation, we assign a separative work cost of $100/swu, expressed as kilograms.
On a once-through uranium cycle, the spectral-shift-control reactor requires very little less uranium feed (about 10 tons/yr less of U3O8 per GWe) than its light water counterpart (a PWR)36; thus, this cycle is not listed in Table 5–4.
The natural-uranium CANDU requires no separative work and uses about 30 tons/yr less natural uranium per GWe than an LWR. Equivalent
fuel cycle cost savings are about 2 mills/kWh on enrichment. At today’s spot prices of $40/lb of U3O8, or $100/kg of uranium, the purchase of ore contributes about 4 mills/kWh to the cost of electricity. The savings in ore purchases are just under 1 mill/kWh. A natural-uranium CANDU could save 5 mills/kWh in fuel cycle costs (compared to an LWR), but only if uranium cost about $120/lb of U3O8. (This is, in fact, an underestimate of the break-even uranium price. As the cost of uranium rises relative to the cost of enrichment, the system shifts toward lower tails enrichment, and the fuel cycle cost rises less rapidly than this simple treatment indicates.)
The CANDU fueled with uranium enriched to 1.2 percent 235U would require only about a fourth the separative work that an LWR fueled with uranium enriched to 3 percent 235U would require for production of a given amount of electricity, and only about two thirds as much U3O8. In comparison to an LWR of the same size (1 GWe), operated at the same capacity factor (70 percent), a CANDU would use about 70 tons less U3O8 per year. At today’s prices for ore and enrichment, the savings in the cost of ore purchases would be 1.5 mills/kWh, and in the cost of enrichment, 1.6 mills/kWh. The price of U3O8 would have to rise to $90/lb for these savings to balance the disadvantage of capital charges for the CANDU of 5 mills/kWh.
Finally, an HTGR on a once-through fuel cycle (using fuel enriched to 8 percent 235U) would require essentially the same amount of separative work per kWh of electricity sold, and would save about 20 percent of the cost of ore purchases incurred by an LWR. The price of uranium would have to rise by a factor of about 6—to $240/lb of U3O8—before these savings on ore purchases counterbalanced a 5 mill/kWh capital-charge disadvantage. Fueling costs for prismatic and pebble-bed HTGR’s on Th-U once-through cycles would be similar.
Table 5–6 lists these results for quick inspection.
Recycle Systems Recycle costs have been estimated, but not experienced. The estimated costs of fuel reprocessing and refabrication show little uniformity. A recent report37 suggests that the cost (in constant dollars) to reprocess LWR fuel (recycling uranium) would be $200–$300/kg of heavy metal, whereas the cost of reprocessing HTGR fuel (Th-U) would range from $500–$900/kg, and the cost of reprocessing fuel from fast breeder reactors (U-Pu), from $300–$500/kg. These numbers are midrange among various estimates. Refabrication costs show an even larger reactor-dependent range of values. The fuel cycle cost savings that might be achieved by recycle in various reactors are difficult to estimate. Benchmark cases have been computed for LWR’s using today’s price schedules.38 The results indicate that a slight saving could be achieved with recycle. The uncertainties in these calculations are large enough that slightly negative
TABLE 5–6 Cost of U3O8 for the Fuel Cost of Various Reactors on Once-Through Fuel Cycles to Fall 5 mills/kWh Below That of a Nominal Light Water Reactor (1978 dollars per pound)
|
Reactor |
U3O8 Cost |
|
CANDU (1.2 percent enriched uranium) |
90 |
|
CANDU (natural uranium) |
120 |
|
High-temperature gas-cooled reactor |
240 |
|
Spectral-shift-control reactor |
very high |
results are also possible. The only definite conclusion that can be drawn is that economics is a minor factor (today) in the decision to reprocess. As uranium prices rise, however, recycle will become progressively more attractive—offering fuel cycle savings, for example, or the means to stay further increases in the prices of coal and uranium.
FLEXIBILITY
Both the CANDU and HTGR have the flexibility to operate on once-through uranium cycles and (with recycled uranium) on the Th-U cycle. In both cases, significant savings can be realized in the amount of uranium required (relative to the corresponding LWR case). The savings from heavy water reactors are generally greater than those from HTGR’s (see Table 5–4). The SSCR, on the other hand, is only attractive as a recycle option: On a once-through cycle, it shows little better fuel economy than a standard LWR.
Again, both the CANDU and HTGR may be used in conjunction with a denatured Th-U cycle, in which fissile uranium is diluted below weapons grade with 238U (see “Reprocessing Alternatives”). In both cases, the quantities of plutonium produced are far below those produced in a once-through uranium cycle. The SSCR would, on a denatured cycle, probably produce at least one third as much plutonium as the LWR it replaces.
The information just presented leads to the conclusion, drawn by other studies as well,39 that the CANDU and HTGR are the best choices if an advanced converter is to be selected for the United States at this time.
In favor of the CANDU is that it consistently shows the smallest resource requirements (of natural uranium) for any given mode of fuel cycle operation (compare HWR and HTGR in Table 5–4). It has considerable flexibility of design, and a given reactor could accommodate a large range
of fuel loadings, from natural uranium to denatured Th-U with recycle. (The reactor would have to be derated if a new fuel loading were significantly different from that for which the reactor was designed.) Besides making efficient use of resources, HWR’s make efficient use of reactor fuel, as measured by fissions per initial fissile atom (FIFA). High FIFA translates into low frequency of reprocessing of fissile atoms, per unit of energy generated, and correspondingly small out-of-reactor inventories and process losses. Finally, HWR’s can operate on a denatured Th-U fuel cycle with relatively small concomitant production of plutonium—about one tenth that of an equivalent LWR.
Among the disadvantages of heavy water reactors are that their capital costs appear high to evaluators in the United States, and that the plutonium produced in once-through operation is less contaminated with 238Pu and 240Pu, both isotopes that detract from the desirability of plutonium as a weapons material.
The HTGR displays significant advantages, irrespective of resource considerations. It permits operation at high temperature, giving a high efficiency for the conversion from thermal to electric power with less waste heat; it might achieve even higher thermal efficiencies with a gas-turbine topping cycle; its decay heat might be more easily dissipated in a loss-of-coolant accident, thereby reducing the probability of a major radioactive release; and evaluators rate its capital costs below those of HWR’s. The HTGR also has considerable flexibility of operation and could be run on the denatured cycle without producing large amounts of plutonium. The very high burnups considered achievable in HTGR’s make their plutonium product, even from a once-through cycle, relatively undesirable for weapons.40
Of the advanced reactors, the HTGR is considered the easiest to develop for application in the United States, but facilities for its fuel cycles require a longer development and commercialization period than those for a heterogeneous reactor such as the LWR or HWR. The fuel cycle operations are rated as correspondingly high in cost.
A balance of all of these considerations seems to favor the HTGR as the advanced converter whose development offers the best prospects of fuel economy, flexibility of operation, and economy in power generation. Nevertheless, it would be appropriate to undertake a careful cost comparison between an HTGR and an HWR optimized for domestic economic conditions and safety regulations before making a final choice between these two types.
Fast Breeder Reactors These reactors take their name from the fast neutrons (energy greater than 50 keV) that produce most fissions in their
operation. The neutron energy spectrum in fast breeder reactors has several advantages.
-
The number of neutrons produced in fission is slightly higher.
-
The ratio of capture reactions to fissions is smaller.
-
The fast neutron spectrum enables some 238U to undergo fission, generating additional neutrons and raising the breeding gain beyond that possible with plutonium alone.
-
Neutron absorption in structural materials, relative to fissions, is lower than in thermal reactors.
Fast breeder reactors obtain the fast-neutron spectrum by eliminating moderators such as graphite, water, or heavy water that slow down the neutrons emitted in fission to thermal energies before they produce additional fissions in the chain reaction.
The fuel for fast breeder reactors is considerably more concentrated than the fuel for thermal reactors. Fissile atoms may be 10–20 percent, or more, of the heavy-metal atoms in the reactor core (the region where most of the fissions occur and most of the power is generated).
Surrounding the core is a blanket of pure fertile material. In systems of reasonable size, a significant fraction of the neutrons produced escape from the core, and these must be caught in the blanket in order to achieve breeding.
The design of the liquid-metal fast breeder reactor seeks to conserve these gains by employing a coolant with good heat-transfer properties and low neutron absorption (liquid sodium).
The development of the LMFBR is significantly more advanced than other breeder concepts both in this country and abroad. In the United States, four reactors (EBR-I, EBR-II, Enrico Fermi-I, and SEFOR) have been operated, a test reactor (the Fast Test Reactor, or FTR) is under construction,41 and a pilot commercial plant (the Clinch River breeder reactor, or CRBR) is in the design stage. Several large pieces of equipment have been fabricated, but further procurement is in abeyance as a result of the Administration’s decision to terminate the pilot plant Various test reactors and demonstration plants have already been operated abroad, notably pilot-size commercial plants in France, Great Britain, and the U.S.S.R. Other pilot-size plants are under construction in Germany and Japan, Commercial-size plants are under construction in France and the U.S.S.R. Preliminary results indicate that losses of fissile isotopes in reprocessing can be held to 1 percent—low enough to preserve the breeding gain in LMFBR’s42—but full-scale reprocessing experiments with high-burnup fuel are just beginning.
The gas-cooled fast breeder reactor (GCFBR) is essentially similar to an
LMFBR. The principal design differences stem from the use of pressurized helium rather than sodium as the coolant. No test or pilot-plant GCFBR has been built, but much of the needed technology can be borrowed from HTGR’s. Calculations on reference designs for GCFBR’s indicate a higher breeding gain than that of the LMFBR, but the GCFBR would require a higher fissile inventory. The principal safety question is whether the core temperature can be held well below its melting point should the helium cooling system suddenly lose pressure.
Since this reactor has not been under intensive development, its program would lag at least 5 years behind that of the LMFBR in the United States.
Core and Blanket Cycles Fast breeders such as the LMFBR and GCFBR can be fueled with 239Pu or 233U, and can breed the same fissile materials by neutron capture in 238U or 232Th, respectively. In practice, the U-Pu cycle (238U+239Pu) is preferred, as it yields a much better breeding ratio in the core. The use of thorium in LMFBR’s leads to marginal breeding gain; the gain in GCFBR’s could be significant
The amount of plutonium produced in the core relative to the amount of whatever fissile product is bred in the blanket can be adjusted. Cores of smaller critical mass and with a higher ratio of plutonium to uranium will produce less plutonium in the core and leak more neutrons for breeding in the blanket. The breeding ratio of the system may actually increase with this adjustment. Either the LMFBR or the GCFBR could serve as a combination breeder-converter, with break-even (or less) plutonium production in the core, and with large production of 233U in a thorium blanket, which could be used to fuel advanced converters. Up to now, this possibility has not received much attention, perhaps because the necessary design changes would result in fuel that could not remain so long in the reactor, and because more frequent reprocessing would be required. The possibility may be particularly suitable for the secured fuel cycle parks that have been proposed to safeguard the fuel cycle. In a fuel cycle park, the reprocessing, fuel fabrication, and some power generation would be colocated, eliminating the problems of transporting large volumes of fissile material outside a secure area.43
DEVELOPMENT STATUS OF VARIOUS CONCEPTS
The development status of the various reactor concepts was noted briefly in the reactor descriptions. The purpose of this section is to provide some insight into the effort that would be required to bring various reactor concepts to a state of readiness for introduction over the next few decades. The key considerations from the point of view of uranium-ore require-
ments are the date by which such new systems could be introduced, and the rate at which they might be installed in place of additional LWR’s.
Experience with LWR’s provides ample evidence for the time-consuming nature of developing and marketing large, technically sophisticated facilities. Generally, laboratory-scale experiments and component development are followed by a small-scale reactor experiment to test the technical feasibility of the concept. This is followed by one or more intermediate-size pilot or demonstration plants operated within utility grids. The purpose of these plants is to evaluate different design approaches for the concept (e.g., “loop” versus “pool” LMFBR), to begin developing the capacity to design and manufacture the special larger-scale components needed for a given concept, and to work out practical operating procedures in the context of producing power on a utility grid. Assuming that the demonstration is successful and efforts toward commercialization are warranted, the next step is to undertake construction and operation of one or more prototype plants—the first-of-a-kind, full-scale plants of the new concept. This step provides the kind of engineering data from which relatively firm cost estimates can be made. Bringing new reactor systems to this stage of development and commercial suitability may take 20–30 years after the decision has been made to proceed with development. The lengthy period includes time to budget, license, construct, and briefly operate the various plants, typically about 15 years. It also allows for some overlapping of steps; for example, proceeding with detailed designs of a prototype reactor while pilot or demonstration plants are in final construction phases. Some steps might be telescoped—pilot plant and intermediate-size demonstration plant—or bypassed altogether to save time and resources. Different levels of component testing conducted prior to building a plant can modify the technical and economic risks of that plant. Obviously, judgment must be exercised to prevent corner cutting that would increase the risk of failure.
Some concepts described above—such as LWR’s with improved fuel utilization, spectral-shift-control reactors, and LWBR’s—represent extensions or modifications of light water reactor technology. The development of these concepts could rely heavily on existing industrial capacity and experience to reduce developmental requirements; similarly, the GCFBR could make use of LMFBR and HTGR technologies, if these continue to evolve at a sufficiently rapid rate.
Significant development programs abroad have resulted in foreign capacity to construct nuclear power plants. Requirements for efforts in the United States could be reduced to the extent that the experience and capacity developed abroad can be transferred to domestic industry and regulatory organizations. The difficulty of useful transfer based on the trade secrets of industrial organizations, and differing regulatory criteria in
various countries, should not be underestimated. In particular, differences in the treatment of proprietary information make government-to-government transfers to the United States impractical. Transfer can only be accomplished by the licensing of firms in the United States that agree to protect the information. The necessity of government involvement, however, makes it difficult to estimate how much the United States could benefit from CANDU technology in Canada or from LMFBR technology in France.
Developing the capacity to build and operate a large number of plants for a new concept is so costly (measured in billions of dollars) that no single private-sector entity is likely to make a conscious decision to proceed with the process on its own. Even with licenses from foreign sources, the domestic version of any new system would probably require a large pilot-size plant (about 300 MWe) to confirm satisfaction of regulatory requirements. There is a major risk that considerable redesign would be required for domestic application.
A cooperative program between government and the private sector would be required to bring these concepts to readiness, selecting those most attractive to vendors and buyers, and encouraging the development and commercialization of reactor types that have potential long-term economic benefits.
Our estimates for the earliest possible dates of commercial introduction for the principal breeder and advanced-converter designs, based on brisk efforts by government and industry, indicate that of the advanced converters, only the HTGR could have a commercial-size prototype operating before 1990. Of the breeders, the LMFBR could be readied for operation by the mid-1990s, and the GCFBR, 10 years later. Estimated schedules are included in Table 5–3. The schedule takes only technical problems into account; limitations implied by institutional or policy matters have not been (and cannot be) estimated. Any advanced-converter or breeder reactor system introduced by a date listed in Table 5–3 will need an additional 10–20 years to gain a significant share of the nuclear power market, and as noted in the table, any institutional delays (which must be expected if today’s pattern of political, legal, regulatory, and financial uncertainties persists) would further lengthen the schedule.
One other aspect of the development problem has come to our attention. This is the matter of industry morale. In addition to the impetus from federal direction and funding, the LMFBR program has received considerable support from industry. This support includes industrial funding for the Clinch River breeder reactor program (about $250 million), as well as the assignment of vital personnel. The commitment was made under very strong federal pressure to do so from the Congress and the former Atomic Energy Commission, and industry personnel feel betrayed, both financially
and psychologically, by the cancellation of the CRBR. Nevertheless, the LMFBR still commands industrial support because of this commitment
Reprocessing followed the same course of strong federal encouragement, industrial commitment, and federal foreclosure. The industry is not likely to commit itself again to similar projects. Future reactor development and commercialization will probably require much more federal capital, with the industry assuming the role of developers, component vendors, and operators under contract. It is difficult to quantify the difference this might make, but it could tilt development schedules in favor of the LMFBR, relative to other reactors, by 2–5 years. This would contribute to the head start the LMFBR already has by virtue of a large array of developmental facilities: among others, the Fast Flux Test Facility at Richland, Washington, the Large Components Test Facility at Santa Susana, California, and the EBR-II and other major experimental reactors operated by Argonne National Laboratory at the National Reactor Test Station, Idaho. Exchange of noncommercial information between the United States and foreign countries operating breeders in the 300-MWe class (Great Britain and France) could also help accelerate the LMFBR’s schedule.
BREEDERS VERSUS ADVANCED CONVERTERS
The most important point of comparison among reactor types and fuel cycles is likely to be contingent on their appropriateness under the conditions prevailing when a market appears for a new system, The conditions that dominate will be the accrued and projected growth of demand for electricity, the availability of uranium resources, the competitive economics of electrical generation, and the measures adopted to discourage or forestall diversion and proliferation.
The following conditions (not all of equal weight) would favor the use of fast breeder reactors over advanced converters in the United States.
-
The demand for electricity in the United States grows steadily after the year 2000.
-
Total domestic uranium resources are found to be at the low end of recent estimates.
-
Very little intermediate-grade uranium ore is found that can be produced at costs in the range of $100–$200/lb.
-
The world growth of nuclear capacity in conventional light water reactors exerts pressure on the United States to export some of its uranium or enriched fuel (or both) to offset the balance-of-payments deficit from oil imports, to discourage recycle of fissile isotopes or installation of breeder reactors elsewhere, or to meet other needs.
-
Enrichment technologies reducing the cost of low enrichment tails do not become available early.
The following conditions would generally favor the use of advanced converters for nuclear-generated electricity.
-
The demand for electricity in the United States grows slowly, especially after 2000.
-
Sufficient uranium resources are found to fuel advanced converters at their projected rate of introduction and installation, particularly intermediate-grade ores producible at costs around $100–$200/lb.
-
Capital costs of advanced converters turn out to be significantly less than those of breeders.
-
The operation of advanced converters and their fuel cycles offers advantages for safeguarding against proliferation or diversion.
-
New enrichment technologies that permit economic operation at low tails assays become available early.
Both lists of conditions require some qualification. As noted, economics and the measures adopted by the world to slow proliferation of nuclear weapons could dominate the choice. Both are highly uncertain factors. We can only estimate future costs qualitatively, and we can expect surprises in international decision making.
The conditions most favorable to a large role for advanced converters—low growth in demand for nuclear-generated electricity, and abundant uranium supplies—may also act against their development. Under these conditions, LWR’s would also have a future. A slowly growing industry could not be expected to sponsor new and expensive development. More likely, it would continue to market a proved product—the LWR—with perhaps incremental improvements.
There are long-term reasons for developing breeders and advanced converters simultaneously. The availability of both could permit optimal mixes of the two types that would be superior to either type alone. For example, the fuel produced by breeders would compete economically with natural uranium purchases, holding down the fuel cycle costs of converters; advanced converters would provide customers for breeder operators. Breeders might fit well, along with reprocessing and fuel fabrication plants, into a system of secure fuel cycle parks, while advanced converters could be located near existing load centers. In short, these “symbiotic” systems might offer the best combination of economical power generation and security. But the strongest reason for parallel development is simply that one or both of the two types may be needed to
permit substitution of nuclear power for coal if necessary, or to provide a desirable diversity of power sources.
CONCLUSIONS AND RECOMMENDATIONS: ADVANCED CONVERTERS AND BREEDERS
The relative roles advanced converters and breeders might play in the energy supply sector cannot easily be predicted. Conditions that have a reasonable chance of eventuating would be favorable to the installation of both types of reactors (steady growth in electrical demand; economic attractiveness of nuclear power relative to other sources of electricity; and satisfactory resolution of the political and social issues discussed later in this chapter).
Breeders are more flexible in their ability to respond to quite rapid growth in demand as well as to rather moderate growth. Thus, although the two types of reactors both serve, in a sense, as insurance that increased supplies of electricity could be provided if needed, breeders provide broader coverage. The probability that such coverage will be needed by, say, 2010 may not be very high. However, the risk of inadequate supply could be high, and insurance is of greatest value against high-risk, low-probability events.
Advanced converters offer insurance against moderate growth in demand for electricity, compared to past experience, and limited supplies of uranium, so long as they are not expensive. Advanced converters would also be a useful adjunct to breeders in a breeder economy. Thus, conditions favorable to their development are also flexible.*
CONAES concludes that these considerations lead to the recommendation that both types of reactors should be developed. If only one type can be developed, breeders should receive priority, as covering more contingencies. If, for whatever reasons, development of the breeder is so long deferred as to preclude the option of commercialization in the early twenty-first century, the commitment should be made to expeditious development of the advanced converter.
The committee recommends the following course of action.
-
Development of the LMFBR should continue, but without immediate commitment to construction of prototype reactors. CONAES was divided on the issue of whether to recommend construction of the Clinch River breeder reactor as part of this development program.
-
A majority of the committee considered the Clinch River breeder
|
* |
See statement 5–17, by L.F.Lischer, Appendix A. |
-
reactor undesirable or unnecessary for reasons that varied within the majority, including: inappropriateness of its design as a developmental facility,† its incompatibility with President Carter’s antiproliferation policies, and its possible contribution toward committing the United States to commercialization of the LMFBR. A minority considered it necessary, as a technological step that is well short of commitment to commercialization, but necessary if early commercialization turned out to be desirable.
-
A reference design should be produced for a commercial-scale LMFBR to identify the problems that require solution in the research program.
-
Commercial-scale experiments should be conducted to ensure that a workable fuel cycle for the LMFBR can be operated.* Proliferation-resistant schemes, such as the proposed Civex cycle or the denatured Th-U cycle, should receive particular attention.
-
Development of the HTGR to full commercial scale should be encouraged (either the prismatic or pebble-bed version).
-
A pioneer-scale reprocessing plant (a few hundred tons per year) for Th-U fuels should be built and operated. Further work on recycle of HTGR fuels in such a plant should be supported, and increased attention should be given to off-gas problems and to the special requirements of coated-particle composites.
-
A joint program should be undertaken with Canada to explore and, if attractive, develop toward commercialization an advanced heavy water reactor design that can be adapted to the regulatory and economic climates of both countries. Such a design should be considered as the next major improvement of the heavy water line.
As is well known, many decisions about the domestic nuclear power program have been deferred pending the outcome of the International Nuclear Fuel Cycle Evaluation. The following actions should follow completion of that program, based on international agreements.
-
The United States should act expeditiously to provide fuel cycle facilities of the types recommended. The best alternative breeder to the plutonium-fueled LMFBR should also be developed through a joint program with other supplier countries. This program of development should be carried out regardless of an INFCE recommendation for the LMFBR and U-Pu fuel cycle. We anticipate that world demand for nuclear fuel will lead to a breeder, rather than a break-even converter, as the most suitable alternative.
-
After completion of INFCE and associated programs in the United
|
† |
See statement 5–18, by L.F.Lischer, Appendix A. |
|
* |
Statement 5–19, by J.P.Holdren: The concept of a “commercial-scale experiment” is vague. If it means building a commercial-size plant for fast reactor fuel, I oppose it. |
-
States, the schedule for breeder and advanced converter development or installation (or both) should be reevaluated and any required programs initiated.
DOMESTIC ISSUES IN THE FUTURE OF NUCLEAR POWER
PUBLIC APPRAISAL OF NUCLEAR POWER
Public opinion polls have repeatedly shown that the majority of people in the United States view nuclear power favorably.44 Referenda introduced in seven states in 1976 that would have halted, postponed, or forestalled the expansion of nuclear power were all defeated. On April 7, 1979, just a week after the accident at the nuclear power plant near Harrisburg, Pennsylvania, citizens of Austin, Texas, voted to retain their 16 percent interest in a nuclear power plant under construction, and they extended the city council additional borrowing authority to cover anticipated and unanticipated costs.
Nevertheless, nuclear power is controversial, and is likely to remain so. The same polls cited indicate that there is a significant core of very strong opposition to nuclear power—opponents who will continue efforts to persuade the public to abandon this source of energy.
A factor that increases the effectiveness of the opponents of nuclear power is their development of a comprehensive information network. The bulk of the information circulated is, as might be expected, highly partisan, but it contains enough factual statements that the nuclear opposition is much better informed about nuclear issues than the general public.*
While it is no doubt important to understand the rational arguments and irrational appeals that may sway individual voters, the nuclear controversy can ultimately be explained only as a contest among groups in the society. The leadership of the antinuclear movement today appears to be in the hands of environmental organizations. The pronuclear forces are led by industries and professional associations within the nuclear power field. These groups are vying with one another to win public support.
For the foreseeable future, the scientific community will occupy a strategic position in this debate for at least two reasons. First, scientists themselves are found on both sides of the nuclear controversy. Second, other parties to the controversy are eager to claim scientific support for their views. This helps to account for the recent “proliferation of petitions, polls, and statements purporting to reveal what the nation’s scientists and
engineers … really think about the controversial technology.”45 Moreover, scientists enjoy a great deal of public confidence—much more than any of the other main parties to the controversy and any of the other main sources of information, according to the Harris surveys. Indeed, Harris notes that many believe the pivotal factor in the California initiative was the widely shared impression that scientists support nuclear power.46 But the public perception that scientists are not of one mind is itself an obstacle to acceptance of nuclear energy.
Public appraisal of nuclear power is difficult to analyze. Technical, political, and social issues flow together, change, and diverge. Public attitudes are influenced by technical information and opinion, and nuclear technology, in its continuing development, responds to political and social influences. For example, the publication in 1957 of the Atomic Energy Commission’s report “Theoretical Possibilities and Consequences of Major Accidents in Large Nuclear Power Plants” (also known as WASH-740 or the Brookhaven Report) was an important factor in the emergence of public apprehension about reactor safety. The resulting demands for greater assurance of safety have profoundly influenced modern regulatory practice, leading to designs that are both higher in cost and protected against accidents that the industry would consider inconceivable.
For policy guidance, some principal concerns with the future and expansion of nuclear power are separated here into those that are primarily technical, institutional, or social.
Some public concerns about nuclear energy center on technical issues that have not been resolved to the satisfaction of a substantial number of scientific critics.
-
The effectiveness of technical means to prevent or hinder diversion of weapons-usable material from the fuel cycle.
-
The safety of nuclear reactors, including protection against sabotage.
-
The long-term management of nuclear waste.
-
Release of long-lived radioactive effluents from the nuclear fuel cycle.
Other concerns arise from public distrust of institutions responsible for the management of nuclear energy programs in the past. Primarily institutional issues include the following.
-
Whether human institutions can be relied on to provide long-term management of radioactive waste, reactor safety, and secured weapons-usable material.
-
Whether international institutions can be created and maintained to work effectively against the proliferation of nuclear weapons.
Other aspects of the public appraisal of nuclear power are principally social. They reflect differing perceptions of desirable future conditions.
-
Nuclear power has become the most visible symbol of large-scale centralized technology for which many citizens feel they have surrendered control to experts who cannot be held accountable.
-
A significant number of citizens dislike nuclear power, particularly the breeder, because if offers the continuation of a high-growth materialistic society that, in their view, will eventually prove disastrous to the physical and social environment of mankind.
-
Some see nuclear power as competing for capital with other energy systems that are more nearly autonomous and under local control, and therefore, both in itself and as a symbol, as excluding social organizational patterns that are based on such autonomy.47
-
On the other hand, some see nuclear power as essential if people are to have sufficient energy to live with dignity, achieve their aspirations, and improve their own lives and those of their children.48
-
Many people feel that institutions, including utilities, government, and regulatory bodies, exist to provide services to citizens; that they can and should be economical (whether large or small); and that technologies, including nuclear power, can be controlled to serve man in a safe, environmentally acceptable way.
How should government and other institutions respond to these concerns? Even in controversies whose main content is technical, judgments are influenced by social and institutional preferences. On questions of fact or of likelihood, we can use existing institutions or create new ones. Ultimately, such questions are resolved in retrospect: by drawing inferences from experience.
On the social and institutional questions that have been raised, we can make no recommendation. They should be worked out through the political process. Each of us has opinions, but we agree that the only ethical way to act on them is through action outside the scope of this study.
It is obvious that a high level of confidence in nuclear power depends on consensus that the nuclear industry and the government have workable institutions to manage properly the whole enterprise, including the complete nuclear fuel cycle.
COSTS OF NUCLEAR POWER
Nuclear power plants began to be installed in quantity in the late 1960s in response to (1) the promise of more economical generation of electricity,
(2) features demanded of coal-fired generation, and (3) anticipated insecurity of oil supply. Nuclear power plants, in large sizes, appeared to be somewhat higher in capital costs than coal-fired plants, but the fuel cycle costs of nuclear plants were competitive with those of fossil fuels and had the added advantage that the price of uranium was considered much more stable than the price of fossil fuels.
Since that time, the costs of both nuclear and coal-fired power plants have escalated rapidly—nuclear somewhat more, both at a higher rate than overall inflation. The escalation was due partly, but not completely, to changes and additions to plant design required by regulation for improved safety or environmental protection. The costs of labor and construction materials have risen more rapidly than the general rate of inflation. The competition for new electrical generating plants is now between coal and nuclear power, with the two sources exhibiting the following characteristics.
-
Coal plants take less time to plan, license, and build than nuclear plants (6–10 years versus 9–12 years).
-
Coal plants have slightly lower capital costs than nuclear plants (0–25 percent cost differential).
-
Nuclear plants have lower operating costs.
-
Nuclear plants have lower fueling costs.
-
Nuclear fuel is easily stored and does not have to be delivered frequently, making it less vulnerable to interruptions in mining and transportation.
One consequence of weighing these considerations has been that, for most private utilities in the United States, nuclear power is rated slightly to considerably less expensive than coal-fired electricity. However, there are uncertainties in future costs, both because there has been a history of downtime in nuclear plants arising from changes in safety regulations, and because escalations are intrinsically unpredictable (for both coal and nuclear plants). Therefore, utilities will usually order some mix of both types. This gives them flexibility: If one or the other type shows superior operational performance or lower cost, that type can be exploited more heavily.
The balance is more strongly in favor of nuclear power for publicly financed utilities, both in the United States and abroad. These utilities have lower carrying charges on capital, and the cost disadvantage of the initial nuclear investment is a less important consideration.
Although cost is not the only criterion for either utility or public decision making, it is an important one, Confusion is easily found in this area. For example, a period of rapid inflation, such as we are now
experiencing, guarantees that future costs (in dollars of the future) will be greater than current costs (in today’s dollars). There are also features of tax laws, of utility accounting practices, and of the regulatory requirements governing investment capital that compensate (or fail to compensate) in various ways for the effects of inflation and market fluctuations. Utility capitalization is based on the actual purchase costs, rather than on the replacement values of plants, and in times of rapid inflation, the plants’ income-producing value diminishes correspondingly. To compensate, a very high capital charge rate is adopted. The result is that new investments with high capital costs are discouraged, including many that would be clearly economical in a noninflationary period. This result is paradoxical, since high capital-cost equipment is normally considered a prudent hedge against inflation if it reduces recurrent costs that are subject to inflationary pressures.
Because utility rates are subject to public regulation, they are affected by a variety of social issues. Should utilities be heavily taxed to support other regional services, or taxed only according to the services they use? Do they demand services that are not obvious, whose provision amounts to subsidy? What is a fair distribution of costs and economies between the utility owner (public or private) and the consumer? How much of future capital costs should be borne by the current consumer in anticipation of future benefits, and how much should be paid by the future consumer? Different answers to these questions, arising from the larger social debate, affect rates, the way those rates are evaluated, and thus the relative economies of nuclear power compared to other sources.
Capital Costs
A number of studies have estimated the capital costs of nuclear plants. In particular, the costs of nuclear power plants have been compared to the costs of competitive electrical plants; in the short and intermediate term, this means specifically coal plants.
The following expectations are typical.
-
Bechtel Corporation49 projected typical costs in New England for fossil and nuclear plants. The most economical fossil fuel plant for this region consisted of three coal units burning high-Btu eastern coal and equipped with scrubbers. The plants were each of 700-MWe capacity. The cost in 1985 was projected as $850/kWe. A 5 percent inflation rate was implicit, and this cost becomes $600/kWe in 1978 dollars.
A comparable nuclear plant, consisting of two 1100-MWe units, was estimated to cost $1030/kWe in 1985, and with the same adjustment for inflation, $730/kWe in 1978 dollars. The larger total capacity of the
-
nuclear plant is required because of adjustment for the reserve capacities of larger units.
-
Commonwealth Edison50 cited comparable figures leading to a typical cost of about $530/kWe (1976 dollars) for coal units with scrubbers and to a virtually identical cost for nuclear units. Inflating to 1978 at a yearly rate of 6 percent, this cost would be about $595/kWe. The utility indicated an uncertainty band of 10 percent in the numbers. In 1978 dollars, their “high” estimates were $620/kWe and $630/kWe for coal and nuclear power, respectively. Part of the difference between the estimates of Bechtel and Commonwealth Edison is attributable to lower costs of construction in the Midwest.
-
Numbers cited by those who oppose nuclear power are often higher, but after correction for assumed inflation rates, are not very different. For example, Komanoff51 indicates an expectation of $1200/kWe for nuclear plants and $950/kWe for coal plants in 1985. Deflated to 1978 dollars at 6 percent, these become $800/kWe and $630/kWe, respectively.
There seems to be general agreement, therefore, that the capital costs of nuclear power plants are between $600/kWe and $800/kWe in 1978 dollars, and those of coal plants are about the same to 20 percent less.
Cost Escalation
Estimates of future costs are colored by the estimators’ expectations of cost escalations, over and above general inflation. Escalation of this kind makes future plants more expensive than present ones, even in terms of constant-value dollars. Brush, in testimony presented to a New England state utility commission,52 introduced curves suggesting that escalation has added half again as much as general inflation to the costs of nuclear power plants over the last 10 years. This is an annual rate of roughly 3 percent. Brush’s data imply that for fossil fuel plants, the annual rate has averaged about 2 percent. Bupp53 agrees with the 2–3 percent figure for extra cost escalation of nuclear power up to 1975, but points out that there has been a much faster escalation of estimates since that date.
Arguments about future escalation center on the perceived causes. Industry representatives emphasize that past escalations have resulted either from general shifts in the costs of construction (i.e., field-labor rates, productivity decreases) or from changes in the scope of the jobs (thicker containment, more rigid quality assurance, extra engineered safety features for nuclear plants, scrubbers and other pollution-abatement systems for coal plants). The general cost shifts are felt in all large construction projects. As to changes in scope, the utilities argue that most of the regulatory tightening that increased the magnitude of nuclear projects has already occurred, whereas the standards for emissions from coal plants are
still changing. This would lead to an expectation that the costs of coal plants will escalate faster than those of nuclear plants.
Another source of escalation is simply delay. Again, it is argued that nuclear plant schedules are now as protracted as can reasonably be expected, and further escalation from this cause is unlikely.
While it remains a matter of opinion, we find these arguments, based on attempts to identify the causes of escalation, more convincing than the arguments based on simple trends. Escalation is likely to make the future cost of electricity higher, but is not likely to make nuclear plants less competitive with coal.
Capacity Factors
Charges against capital continue no matter whether a plant is being used, whereas revenues are proportional to the product sold. Therefore, that part of the cost of the product that arises from capital charges is minimized if the plant works at maximum capacity. The measure of performance is the capacity factor, the ratio of electricity actually sent out to that which would have been sent out if the plant were in round-the-clock operation at maximum dependable capacity.
Capacity factors fall below 100 percent for three reasons: scheduled maintenance, unscheduled outage (for repair of malfunction or for other technical reasons), and lack of demand for the product electricity (economic downtime). Nuclear plants are designed for scheduled refueling and maintenance periods that vary between 7 percent and 15 percent of the time. Thus, their theoretical capacity factors for an average year are between 85 percent and 93 percent. Those of coal-fired plants are comparable. During refueling outages, turbine inspection and deferred repair work can be carried out.
Balancing this disadvantage of nuclear plants is an advantage in economic downtime. When demand is low, utilities operate at base load only, keeping in operation those plants whose incremental operating costs are minimal. For large plants, these incremental costs are almost exclusively fuel costs, and nuclear fuel is the cheapest source for thermal plants. This is offset to some extent by the difficulty of starting up and shutting down various plants, but the incentive remains to rule on nuclear plants more completely than on fossil plants for base loads. This effect suggested to early nuclear planners that capacity factors as high as 80 percent would be reasonable. More conservative estimators—realizing that the unscheduled outages commonly experienced in fossil fuel units would also occur in nuclear plants—tended to expect about a 70 percent capacity factor as a goal.
Experience has been disappointing. Although their physical condition is
normally superior to that of fossil plants by virtue of the strict regulation imposed on nuclear plants, this strict regulation has also on occasion required shutdown for inspection or for repairs that in a fossil fuel plant would have been deferred to a scheduled maintenance period. Reserve margins have been high because of sharp reductions in demand growth in response to the events of recent years, and even some base-load plants have not experienced full-capacity demand. This latter fact has made the concept of availability—the fraction of time during which a plant could operate at full power if called upon—a measure of technical performance, while capacity factor remains the measure of economic performance.
Table 5–7 presents the histories of capacity factors and availability factors for large coal and nuclear units from 1970 to 1975. The chief feature of this table is year-to-year fluctuation. Komanoff54 has interpreted the data as indicating a trend toward decreasing capacity and availability factors for nuclear plants, and has attributed this trend primarily to decreased availability of the larger sizes of nuclear units that entered service progressively during the period. Perl55 has criticized both Komanoff’s statistical inferences from his limited data base and some of Komanoff’s data adjustments. A separate analysis by Commonwealth Edison56 generally agrees with Perl, but is even more sanguine about the likelihood of high capacity factors for large units.
On this controversy, we find that the data base for nuclear units is too small for these analyses to be significant. For example, it may be noted that if entries in Table 5–7 had been dated from 1971 rather than 1970, no general trend in capacity factors would be apparent. Few large nuclear plants were in service in 1970. There may be a trend to lower availability for large nuclear units, but it is too early to tell. In any case, it seems that capacity factors for large coal and nuclear units will be similar, as they have been in the past, hovering between 55 and 60 percent. Capacity factors for both types of plants will increase if reserve margins decrease and peak loads are leveled. New pricing policies, such as off-peak cost reductions, would promote this outcome. Higher capacity factors for both coal-fired and nuclear plants would improve the relative economies of nuclear power by decreasing the fraction of power cost represented by capital charges.
Nuclear Fuel Cycle Costs
Throughout the discussion of costs, the point was repeatedly made that nuclear fueling is less expensive than fueling with coal. We now examine the data on this point.
Table 5–8 was prepared by CONAES to illustrate the main features of nuclear fuel cycle costs. The major variables are the price of uranium and
TABLE 5–7 1970–1977 Average Annual Capacity and Availability Factors for Coal Units over 400-MWe Capacity and Nuclear Units (percent)
|
Year |
Capacity Factor |
Equivalent Availability |
||
|
Coal |
Nuclear |
Coal |
Nuclear |
|
|
1970 |
59 |
53 |
66 |
67 |
|
1971 |
61 |
58 |
68 |
72 |
|
1972 |
61 |
54 |
66 |
68 |
|
1973 |
63 |
57 |
69 |
71 |
|
1974 |
56 |
55 |
64 |
68 |
|
1975 |
58 |
59 |
65 |
72 |
|
1976 |
59 |
57 |
66 |
69 |
|
1977 |
58 |
63 |
61 |
75 |
|
Source: For coal, Edison Electric Institute, Report on Equipment Availability for the Ten-year Period—1968–1977 (Washington, D.C.: Edison Electric Institute, 1979), and for nuclear, Division of Nuclear Power Development, Operating History of U.S. Central Station Nuclear Power Plants (Washington, D.C.: U.S. Department of Energy, April 1979). |
||||
the cost of working capital. The other factors were selected as typical by the following line of reasoning.
-
Enrichment cost of $100/swu is slightly above the present value. Including associated chemical conversion, present cost is a little over $90/swu. In the past 3 years, enrichment charges have been tracking general inflation (after a threefold increase in 1975 to account for changes in government accounting of both capital charges and power costs). The present charges are very close to estimates of cost if the plants were privately owned and operated. (European enrichment plants are charging slightly higher prices for future services.) The new gas-centrifuge facility planned for the 1980s is projected to have similar costs and charges. Future price increases could arise from increases in the cost of electricity to run enrichment plants, but these electricity prices are not expected to rise faster than the rate of general inflation, The minimum combined cost of enrichment and uranium purchases is quite insensitive to the exact tails assay chosen. A conventional and representative value is 0.25 percent 235U tails assay.
-
Fabrication charges of $100/kg of uranium dioxide (UO2) have been typical for more than 10 years. The cost in constant dollars has therefore
TABLE 5–8 Contributions to Electricity Cost of Nuclear Fuel Cycle Operations (mills per kilowatt-hour)a
|
Capital Charge Rate |
Fuel Cycle Costs, Without Uranium Purchase |
Uranium Purchase Cost, with UO2 at: |
Total Fuel Cycle Cost with UO2 at: |
|||||||
|
Enrichment |
Fabrication |
Storage |
Total |
$50/kg |
$100/kg |
$200/kg |
$50/kg |
$100/kg |
$200/kg |
|
|
6 |
1.61 |
0.49 |
0.43 |
2.53 |
1.54 |
3.07 |
6.14 |
4.07 |
5.60 |
8.67 |
|
10 |
1.84 |
0.54 |
0.38 |
2.76 |
1.78 |
3.56 |
7.11 |
4.57 |
6.32 |
9.87 |
|
15 |
2.14 |
0.62 |
0.33 |
3.09 |
2.12 |
4.25 |
8.50 |
5.21b |
7.34b |
11.59 |
|
20 |
2.48 |
0.72 |
0.29 |
3.49 |
2.52 |
5.04 |
10.07 |
6.01b |
8.53b |
13.56 |
|
aAssumptions: 1. Once-through (throwaway or stowaway) cycle. 2. Enrichment to 3 percent at 0.25 percent 235U tails assay. 3. Burnup at 3 MWd (heat) per kilogram of UO2. 4. Conversion efficiency at 31 percent, heat to electricity. 5. Unit costs and payment schedules: Uranium—variable cost, mean payment 4 years before mean receipt of revenue; includes costs of mining, milling, exploration. Enrichment—$100 per separative work unit (uranium), paid 3 1/2 years before receipt of revenue; includes chemical conversion costs. Fabrication—$100/kg of UO2, paid 3 years before mean receipt of revenue; includes chemical conversion costs. Storage fee—$125/kg of UO2, paid 3 years after mean receipt of revenue; includes transportation costs. bThese values give the approximate range of present market conditions. |
||||||||||
-
been declining. At worst, these charges can be expected to increase at the general inflation rate.
-
Storage fee charges of $125/kg of UO2 in spent fuel are a guess. Numbers between $25 and $150 have been mentioned. The fuel cycle cost is not very sensitive to this charge, because the cost is incurred after the revenue has been produced and is therefore discounted.
-
The schedule of payments is a crude approximation to conditions that would exist if well-developed markets for all the cost components existed. In fact, except for fabrication charges, actual payment schedules are a complicated collection of advance payments, interest credits, and (in the case of uranium purchasing) investment sharing and crediting. To that extent, the payment schedules are both approximate and arbitrary.
The capital charge rates used in Table 5–8 roughly correspond to the following economic circumstances: 6 percent, no inflation; 10 percent, mild (3–4 percent per year) inflation; 15 percent, mild inflation plus use of equity rather than borrowed capital; and 20 percent, strong inflation, equity capital. The rate is now between 15 and 20 percent for most utilities.
The price of uranium is generally quoted commercially in units of dollars per pound of uranium oxide. The uranium costs listed in Table 5–8 ($50, $100, and $200/kg of UO2) correspond, respectively, to prices of $22, $44, and $88/lb of U3O8. Prices average about $18/lb, but include deliveries made under existing purchase contracts, negotiated when uranium prices were very low. The present “spot” price—the price of immediate delivery of a new order—is about $40/lb, having declined slightly from $45/lb in 1977. The high value of $88/lb is representative of a price that might be reached if low-grade deposits have to be mined.
Under present market conditions, then, the nuclear fuel cycle contributes between 5.2 and 8.6 mills/kWh to the cost of electricity (see footnote b in Table 5–8).
For comparison, Commonwealth Edison57 lists nuclear fuel cycle costs in 1976 dollars of 6 mills/kWh (equal to 6.72 mills/kWh in 1978 dollars), and Perl58 lists costs of 6.89 mills/kWh in 1985, presumably deflated to 1978 dollars.
Commonwealth Edison59 suggested 10 mills/kWh as the fuel cost from high-sulfur coal (in 1976 dollars—closer to 11 mills/kWh in 1978 dollars) and 16 mills/kWh as the cost from low-sulfur coal. These are on the same basis as the nuclear fuel cycle costs: estimated costs of new fuel supplies.
Depending on the type of coal used, the cost advantage of the nuclear fuel cycle is about 4 mills/kWh (compared to high-sulfur coal, for a midwestern utility).
Add-On Costs
One contention of opponents to nuclear power is that the cost of nuclear power is not complete. They argue as follows that subsidies, either from the government or future electric ratepayers, will be required.
-
Past research and development by the government has not been incorporated into the bill.
-
The cost of current research and development by the government ought to be borne by the industry.
-
Government services are provided at a loss to the taxpayer (for example, enrichment services, licensing charges, insurance, and waste disposal, when available).
-
Costs of decommissioning nuclear power plants ought to be added into the bill.
While CONAES has not investigated these items in great detail, we are of the opinion that none should, or can, be a source of large increase in the cost of nuclear power, for the following reasons.
-
It has not been the practice of the government to recover sunk research and development costs from industries that profit from the work. The rationale has been that the economic stimulus from new products yields a return to the government in general taxes. There is a certain amount of ideology involved in any contention of this nature. However, the existing practice serves the clear economic benefit of minimizing marginal costs for the benefits provided.
-
The industry is supporting most of the research and development that it considers necessary for its own continued profit. A great deal of the ongoing government research and development (such as safety research) consists of projects intended to support the general welfare. Other areas receiving large government support are justified by future general economic benefits, which are not recoverable by the industries involved. Both the LMFBR and solar power benefit from this policy.
-
We have not yet found a government “subsidy” by the accounting standards in force, Both licensing and Price-Anderson “insurance”60 seem to be charging fair fees for the services offered.* Enrichment services have been continuously scrutinized and found to be without subsidy. The price has gone up because the capital costs of plants have been allocated to the users. The government was originally the main customer for separative
-
work, but the main customer now is the nuclear industry, and the industry pays base costs rather than marginal costs.
-
Decommissioning costs are far in the future and can in no way be considered comparable to construction costs. The Atomic Industrial Forum, the only organization that has conducted a detailed study of these costs, concludes that they might, in constant-dollar terms, be as much as 10 percent of original costs.61 Discounting this estimate at 5 percent over 50 years yields a present worth for this item of less than 1 percent of the original plant cost.
In summary, we consider that the costs of nuclear power, as computed now or projected into the future, represent a fair statement, and that no significant additions to these costs have been identified.*
Risk Costs
Nuclear power, as an industry subject to accidents and government regulation, may incur costs from uninsured risks. These arise from the excess costs of replacement power when plants are shut down following an accident or regulatory action. A related set of costs may result from delays in licensing that add to the capital costs of plants under construction.
These risks are subsumed under the capacity-factor projections and the contingencies included in construction schedules that are now part of the industry’s standard accounting. The accident at the Three Mile Island nuclear power plant in 1979 raises the question whether the accounting is adequate. Are the capacity-factor projections and construction schedules that seemed reasonable before this incident still reasonable?
These questions cannot yet be answered. The rate of regulatory shutdown does not appear much greater than the rate prevailing before the accident at Three Mile Island. A licensing hold that has been in effect since then has delayed the schedules of several new reactors, but it may be lifted in the future.
The prolonged shutdown of the Three Mile Island plant represents a financial blow to its operating utility. The loss could be mitigated by an assessment against other nuclear units that would add less than 1 percent to nuclear generating costs. Institutions and arrangements to spread the risks in this or similar ways do not yet exist, but they are being explored. If there were many accidents, the costs would become significant, but in that case, nuclear power would no longer be considered a major energy option.
LMFBR Costs
Unlike those of existing coal-fired or light water reactor power plants, the costs of a commercial breeder reactor have only been estimated, not experienced However, it is generally acknowledged that the capital costs of LMFBR’s will be higher than those of today’s light water reactors. This is explained partly by the need for an extra intermediate heat-transfer loop, partly by the extra complexity involved in using sodium as coolant, and partly by the more complex refueling and auxiliary systems. These additional costs are partly offset by the inherent advantages of eliminating the highly pressurized primary system of the LWR’s, and by economies in the turbine-generator and condenser systems resulting from the higher thermal efficiency of the LMFBR power cycle.
For a light water reactor, the nuclear steam-supply system accounts for 10–20 percent of the total capital costs of the plant. The other 80–90 percent of the cost is for the so-called balance-of-plant, mostly conventional structures (piping, turbine, generator, condenser), cable, installation labor, engineering, and indirect costs. There seems to be no intrinsic reason why these costs should be higher for a developed LMFBR than for an LWR. On the other hand, the nuclear steam-supply system is expected to cost 2–3 times as much as the system for an LWR. Adding and subtracting these items, the capital cost of a developed LMFBR power plant is expected to be 10–40 percent higher than the cost of an equivalent LWR power plant.
An analysis conducted of cost estimates for the Clinch River breeder reactor, of the actual costs of the French LMFBR (Phenix), and of the economic improvements expected for a commercial plant suggests that capital costs of commercial LMFBR’s should be about 40 percent greater than those of LWR’s.62
The Clinch River breeder reactor is a first-of-a-kind demonstration plant, with costs much higher than those expected for commercial plants. Its construction cost is estimated as 3–5 times that of an LWR of equal capacity. Cost reductions of a magnitude sufficient to bring LMFBR costs down from this starting point to a target value within 40 percent of LWR costs are not uncommon in industrial development, but they are large enough that achievement of the target is uncertain.
Balancing the higher capital costs expected of LMFBR’s is the expectation that their fueling costs will be lower than those of LWR’s. There is no need for continuous fissile feed, for example, and the excess fissile material has by-product value. High fuel burnup is not limited by large reactivity losses. The reduction in fuel costs expected from these factors could compensate for the high fuel reprocessing and fabrication costs assumed for LMFBR’s.
TABLE 5–9 Typical Nuclear Fuel Cycle Costs in the 1990s (1976 dollars)
|
|
Light Water Reactor |
Liquid-Metal Fast Breeder Reactor |
|
Assumptions |
|
|
|
U3O8 ($/lb) |
60 |
N.A. |
|
Enrichment ($/swu) |
100 |
N.A. |
|
Fabrication ($/kg) |
100 |
800 |
|
Reprocessing ($/kg) |
200 |
350 |
|
Capacity factor (percent) |
70 |
70 |
|
Fuel burnup (percent heavy metal atoms) |
3 |
6 |
|
Fissile plutonium value ($/g) |
24 |
24 |
|
Waste managementa |
— |
— |
|
Contributions to generating costs (mills/kWh) |
|
|
|
U3O8, net |
4.33 |
0 |
|
Enrichment |
2.36 |
0 |
|
Fabrication |
0.55 |
1.92 |
|
Reprocessing |
0.74 |
0.63 |
|
Plutonium sale |
(0.43) |
(0.43) |
|
Plutonium inventory |
N.A. |
1.03 |
|
TOTAL |
7.6 |
3.2 |
|
aNot included, but assumed identical. |
||
Table 5–9 compares projected fuel cycle costs for an LMFBR and LWR (with uranium and plutonium recycle), at a U3O8 price of $60/lb in 1977 dollars. At this uranium price, and with the warning that the unit costs for such items as fabrication and reprocessing are estimates rather than firm values, the LMFBR fuel cycle cost would be 4.4 mills/kWh less than the fuel cycle costs of an LWR.
Anticipating a result obtained later in this section (that capital charges now contribute about 20 mills/kWh to the price of electricity), an LMFBR that was 40 percent more expensive would contribute 8 additional mills to that cost. From Table 5–9, we may infer that the LMFBR will save 8 mills in fueling costs when the price of U3O8 reaches $110/lb. Uranium concentrates might cost $60/lb (constant value) in the late 1980s, but are unlikely to cost $110/lb until well past 2000 (unless the demand for nuclear power and the parallel demand for uranium accelerate in the intervening period). On the other hand, if the LMFBR cost targets are met, these results also
indicate that the breeder could place a cost ceiling on electric power at a level no higher than 30 percent above present prices.
To the extent that breeders replace LWR’s, they enable more economic operation of the remaining LWR’s by slowing the demand for uranium and the escalation of its cost. They would also exert downward pressure on the costs of coal. A system of breeders and LWR’s would be economically more attractive than a system of either by itself. A system of breeders and advanced converters would show even greater mutual cost benefits, With reprocessing and recycling, various types of breeders and converters could compete for the market.
Summary of Costs
Table 5–10 represents an appraisal of the costs of LWR and coal power. These are planning figures from a midwestern utility, based on replacement-cost accounting as of 1976. They are therefore useful for comparing expected costs. Table 5–10 gives nuclear power plants an expected 18 percent cost advantage over coal plants with scrubbers.
Another recent comparison is presented in Figure 5–7. According to these data, if both coal and nuclear plants are run at 70 percent capacity factor, and particularly if the best available emission control technology is required for coal plants, nuclear power is cheaper in most regions of the United States. However, if nuclear plants are run at capacity factors around 55 percent and coal-fired plants at around 70 percent, with no new emission control technology required, coal-generated electricity would be cheaper. (As all new coal-burning plants are now required to install scrubbers, this latter comparison may be of little interest.)
There is a strong incentive to minimize investment risk in the utility sector. This will lead to decisions for a mix of coal and nuclear plants, since future costs for both sources of power have large uncertainties and each is a hedge against the other. If at some time the costs become more reliably predictable, the mix can be adjusted.
We have not found any costs within the nuclear estimates that can be identified as sources of differential cost escalation (relative to the costs of coal-generated electricity), nor are there any new charges against nuclear power that would increase its relative cost. There are potential requirements for further emission control devices on coal-burning plants that could significantly increase the cost of coal power. Therefore, if we were forced to make a prediction, we would guess that nuclear power would dominate the electrical generation market if cost were the only consideration. Fortunately, this guess is unnecessary, as the decision will be made on an investment-by-investment basis, and cost is not the only consideration.
TABLE 5–10 Comparison of Estimated Total Busbar Generating Costs (1976 dollars) for a Midwestern Utility (mills per kilowatt-hour)a
|
Costs |
Nuclear |
Low-Sulfur Coal Without Scrubbers |
High-Sulfur Coal With Scrubbers |
Oil |
|
Carrying charges |
20 |
14 |
20 |
14b |
|
Fuel (replacement costs) |
6 |
16 |
10c |
23d |
|
Other |
2 |
2 |
4e |
1 |
|
TOTAL |
28 |
32 |
34 |
38 |
|
Nuclear advantage |
|
4 (13%) |
6 (18%) |
10 (26%) |
|
aNuclear Fuel Assumptions: 1. $35/lb of U3O8. 2. $75 per separative work units for enrichment—0.20 percent tails assay. 3. $100/kg for fabrication. 4. Burnup in megawatt-days per metric ton: 33,000 for pressurized-water reactors and 29,000 for boiling-water reactors. 5. Net salvage cost (cost of reprocessing and waste disposal, less salvage recoveries) equivalent to about 0.5 mill/kWh. Fossil Fuel Assumptions (delivered cost to Chicago area): 1. High-sulfur coal, $1.00 per million Btu. 2. Low-sulfur coal, $1.60 per million Btu. 3. No. 6 oil, $2.30 per million Btu. bRoughly the same as coal without flue-gas scrubbers. cIncludes 0.5 mill/kWh for fuel required to generate power for scrubber operation. dBased on $2.30 per million Btu oil—roughly equivalent to $15 per barrel for no. 6 oil and 10,000 Btu/kWh. eIncludes 2 mills/kWh for flue-gas scrubber operation and maintenance expense other than fuel. (Actual expenses have been costlier so far.) Source: Gordon R.Corey, testimony before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part I),” 95th Cong., 1st Sess., Sept. 12–19, 1977, p. 883. |
||||
The following points should be noted for breeders and advanced converters.
-
It is very unlikely that more than a few pioneer commercial-scale units of either type can be put on line this century.
-
The economics of a single unit or a few units will not have a significant effect. The economies of new types of reactors will only be realized after they are fully commercial.
-
Advanced reactors, and particularly breeders, offer the prospect of a durable cost ceiling on the price of electricity. Projections of the cost of
-
electricity from commercial breeders range from 10 percent to 30 percent above today’s cost; similar costs can be expected for advanced converters.
-
The economics of a mixed system of breeders and converters is likely to be more favorable than the economics of either reactor type by itself.
-
The decision for or against the ultimate possibility of a breeder economy will have a profound effect on decisions about other reactors—LWR’s in particular—but advanced converters as well, since breeders would help hold down future uranium demand and cost.
REGULATION OF NUCLEAR POWER PLANTS
The regulatory process affects the industry by lengthening the time between planning a new generating facility and placing it in operation, by retroactive changes in plant design arising from the unique surveillance responsibility of the Nuclear Regulatory Commission, and by providing a special forum for public opposition to nuclear plants.
Fossil fuel plants typically require 8–10 years from the start of planning to the completion of construction, while nuclear plants require 10–12 years or more. A large part of this extra time is claimed by the extensive reviews required for nuclear plants prior to construction. The extra time costs money and adds to the uncertainty that a project’s cost targets will be met. The nuclear industry has a large stake in shortening the period taken up with regulatory processes.
Many rules and regulations engender large capital and operating costs, not all of which (in the industry’s view) can be easily justified by cost-benefit analysis or improved public safety. Contentions of this type between regulators and regulated are fairly standard in our political system. A more vexatious matter is the changes in regulations imposed during the plant’s construction or operation. Such changes are more expensive to implement than changes during the design stage—often an order of magnitude greater.63 Justification for these retroactive changes is always at issue, particularly since each change in regulations increases the opportunities for further legal and administrative interventions.
Interventions have been used to build up opposition to nuclear power, and in some cases, have forced postponement or cancellation of nuclear power plants, either by generating resistance in the region affected, or by delays that bring the economics of the plant into question. The regulatory process, reinforced by judicial interpretations, serves the clear function of guaranteeing that all proper points at issue are raised and judged. However, very little is accomplished if the same points are debated again and again.
Although frequently frustrating to the industry, the close attention paid to nuclear power through the regulatory process has contributed to the
safety record of this energy technology. Moreover, a competent and independent regulatory agency is necessary for public acceptance of nuclear power. The involvement of the Nuclear Regulatory Commission in events following the accident at Three Mile Island is widely credited as decisive in maintaining confidence that a nuclear accident need not lead to public catastrophe. In streamlining regulation of the nuclear power industry, therefore, close attention to the objective requirements of protecting the public must be maintained, as well as attention to the requirements of public confidence: legitimate participation in technical decisions and observance of due process. Three complicated aspects of the regulatory process could be simplified without affecting its integrity.
-
Responsibility is shared but not clearly partitioned among federal and state agencies for safeguarding fuels, overseeing the storage of nuclear wastes, monitoring the health and safety of plant employees, regulating financing and insurance, and protecting the environment. This responsibility is in a state of flux. A division of responsibility should be worked out that is clear, reasonable, and stable.
-
Issues in nuclear power should be settled as much as possible on generic bases. Given generic findings based on comprehensive hearings, regulatory agencies can conduct hearings on individual systems, taking up specific items of contention that have not been previously heard and judged. The records of hearings on generic issues provide a sound basis for determining whether new data can affect the findings, and if so, whether such data should be sought and heard.
-
The regulatory process too often degenerates into an adversary process when, in fact, all parties share an interest in safety, economy, and service. The regulatory process should be scrutinized and corrected so as not to discourage conciliation and mediation.
SAFETY OF NUCLEAR REACTORS AND THEIR FUEL CYCLES
This section examines technological aspects of nuclear power safety. Chapter 9 discusses the health effects of the nuclear energy cycle and compares them with those of fossil fuel use. A recent report of the National Academy of Sciences presents an analysis of these and other safety issues, drawing on a review of the literature to lay an ample base and set legitimate bounds for dicussion.64
Radioactivity is an inherent feature of nuclear energy. Nuclear fuels and fertile materials are radioactive. The chain reaction produces neutrons and gamma rays from fission, as well as new radioactive materials. These latter are the products of fission and of nonfissioning neutron capture. It has long been known that radiation causes damage to living tissues. The safety of
nuclear power depends on isolating these sources of radiation from the biosphere.
Isolation cannot, and need not, be absolute. Radiation is a natural part of our lives. By comparing the radioactivity added by nuclear power to natural radiation backgrounds, or to the variation in background among locations generally considered to be healthy living environments, exposure standards can be set at some fraction of “normal” background. (See chapter 9.) At some level of addition, small increases of radioactivity may be judged to be inconsequential, as a practical matter, simply because the consequences are very much less than those from risks already accepted in exchange for similar benefits. This is a judgment that must be made by society (“how safe is safe enough?”). In making this judgment, the most appropriate standards (in the case of nuclear power) will be based on comparisons with the risks of alternative sources, or with the risks of not having this source of power.
At least two characteristics of radioactive emissions must be supplied to inform this judgment: their frequency (probability per unit time of emissions of given magnitude), and their consequences. Of these characteristics, the frequency is the more difficult number to ascertain. The health effects of radiation have been studied more completely than the effects of any other type of emission. There is still some controversy (as detailed in chapter 9), but models of radiation effects that most authorities believe to be conservative (i.e., that predict more severe consequences than are considered likely to result) are used by regulatory agencies to set exposure limits and to estimate the consequences of very low levels of irradiation.
Such studies, however, do not address the probability of radioactive emissions—the frequency variable. They serve to dramatize the principle that releases of large magnitudes must not be allowed. This is then a design criterion for nuclear systems. The success of design must be judged against this criterion by a numerical estimate of the frequency of a release. If the frequency is sufficiently small, a case can be made for the practical impossibility of the event. However, at smaller frequencies the background of experience disappears. Estimates must be made on the basis of event models, and the uncertainties of the estimates become very large.
“Risk” is the measure of the expected average value of consequences from some unit value of operation of a system. For example, “fatalities per passenger-mile of commercial aircraft,” “person-days lost through accidents per person-year of employment in industry X,” or “corrosion damage costs (in dollars) per unit of release of chemical Y” are all measures of specific risks. We have adopted the GWe-year of reactor operation as the unit of system operation, since this gives some insight into the risks associated with a single large nuclear power plant. Actuarial risk, of course, says nothing about the distribution of risk (for example, among
different models of commercial aircraft), or about whether the risk arises from many small accidents or a few large ones.
Safety of Normal Operations Although greater public concern surrounds reactor accidents, controversy surrounds the safety of the normal operations of nuclear power systems. Three separate areas of operation have figured in these controversies.
-
The operation of reactors involves some discharge of radioactivity to the environment. Attempts have been made to implicate these discharges as significant causes of public morbidity.
-
During the course of reactor operations, and specifically during maintenance periods, workers at nuclear power plants are exposed to radiation from contaminated equipment. It has been charged that this represents undue and uncompensated risk to the workers.
-
At various stages in the nuclear fuel cycle, radioactive effluents are discharged. This is another potential source of public morbidity.
These points are discussed at greater length in chapter 9 of this report. The last point will be treated in the subsequent discussion of nuclear waste management and disposal. It is worth noting here that continued review has indicated that normal reactor discharges, within existing regulatory limits, are not significant causes of public morbidity. The evidence on which the first charge is based has not stood the test of scientific scrutiny. With regard to occupational exposures of workers in nuclear installations, on the other hand, it follows from the linear dose-response hypothesis that existing limits on occupational exposure to radiation present a marginal risk to workers of slightly increased risk of cancer. (This hypothesis is still in dispute, as discussed in detail in chapter 9.)
Reactor Accidents More than 98 percent of the radioactive atoms made in a reactor, and an even greater fraction of those that remain radioactive after a few seconds, are generated in the fuel. The two types of new radioactive atoms are fission products and actinides. Fission products are a congeries of nuclides of medium atomic mass number (66–172 amu, with the largest quantities found near mass numbers 95 and 140). Actinides are the elements beyond actinium, and include both natural and manufactured isotopes of thorium, protactinium, uranium, neptunium, plutonium, and heavier elements. The half-lives of fission products vary from fractions of a second to very large values, and different fission products become important at different times after they are formed. The artificial actinides of consequence have longer half-lives than the more important fission products, and correspondingly less radioactivity (for a given number of
atoms, radioactivity is inversely proportional to half-life). The radioactivity of the actinides in nuclear fuel just after reactor operation is inconsequential compared to that of fission products, and it is not until after several centuries of fission-product decay that the two sources of radioactivity become about equal.
Reactors are designed to contain the radiation they produce. Thick biological shields of concrete, or deep pools of water, are used to attenuate neutrons and gamma rays. In addition, their fuel elements (with the exception of the molten-salt reactor) are designed to contain the fission products and actinides produced. If individual fuel elements fail, the primary reactor system can contain the radioactivity they release, and as a backup, the whole system is placed inside a stout, pressure-type containment building.
The efficacy of these multiple containments is the subject of reactor accident analysis. Accident conditions are postulated, and the consequences of the accident are modeled analytically. From this analysis, further engineered safeguards may then be indicated, which either reduce the probability (frequency) of the accident or mitigate its consequences (chiefly, exposure of people to radiation).
No reactor considered for nuclear power generation can explode like an atomic bomb; nevertheless, certain of the postulated types of accidents might have severe consequences.
The efficacy of containment is also tested by reactor incidents and accidents. In the accident at Three Mile Island, for example, the contaminated water in the reactor building was not contained there.* This is now recognized as a flaw in the plant’s design.
In the case of light water reactors, there is general agreement that the worst type of accident would be one that led to melting of a large part of the reactor core. Such an accident might conceivably be caused by a large power increase, beyond the capability of the coolant to remove the energy generated, or by an interruption of coolant flow. Of these potential mechanisms, the power increase can be ruled out because it would immediately result in a decrease in water density, which would act to decrease the power again. In this respect, light water reactors are inherently self-regulating. An interruption of coolant flow could, however, be brought about by a break in the coolant-flow line—a loss-of-coolant accident (LOCA).
Since in a LOCA the loss of water would quickly quench the fission reaction, only the decay power of the radioactive products would remain. This is about 6 percent of reactor thermal power immediately after
shutdown, and it decreases with time.65 One hour after shutdown, a 1200-MWe (3800-MWt) LWR would still be generating about 50 MWt of heat from radioactive decay. (This is about the time after shutdown that the most serious event in the Three Mile Island accident occurred. We infer from the information published so far that the decay heat from the reactor core had raised the fuel temperature to the point that its cladding began to react chemically with the reactor’s water.)
To remove this heat under the assumed conditions that the normal water circulation would have been lost in the “blowdown” following the LOCA, an emergency core-cooling system (ECCS) is an engineered safeguard feature of LWR’s. This is a system of several subsystems, each capable of removing the heat from a reactor after a LOCA and designed to function independently.
If the ECCS were to fail to cool the reactor fuel, the fuel could melt, forming a large glob. Further cooling would be extremely difficult, and the molten fuel could melt through the reactor vessel. Throughout this period, it would be releasing fission products, whose radioactive decay would heat the containment building, as well as the structures and the air within it. The steam escaping from the reactor through the ruptured pipe could further heat the building. In the absence of a cooling system for the building, or controlled venting, the containment might conceivably rupture from the internal pressure. Alternatively, the containment might be faulty from some other cause, or the molten fuel might itself melt through the floor of the building and be released to the earth below.*
While engineering safety features, such as the containment building, the ECCS, containment cooling devices, and filtered vent lines, can reduce the likelihood and consequences of serious accidents in light water reactors, the probability of the core’s melting followed by release of a large amount of radioactivity cannot be reduced to zero.
Using an analytical technique called fault-tree analysis, the Reactor Safety Study (also referred to as the Rasmussen Report or WASH-1400)66 has estimated the expected median frequency and consequences of various accidents in light water reactors of contemporary design.67 The method consists of analyzing failure rates of various components in the operating reactor system (including operator failure where appropriate), determining what further events must occur to lead to significant accidents, determining what failure rates are appropriate to these further events, and so on. The result is statistical assignment of a very large number of events into a frequency distribution relating magnitude of release (of radioactivity) to frequency of release.
|
* |
See statement 5–24, by L.F.Lischer, Appendix A. |
There are so many components and potential pathways for release that the process can never claim to have evaluated all possible accidents exhaustively; however, the authors of the study maintain that they have analyzed enough cases (which, in truth, comprise a very large number) that their statistical treatment is likely to be of a valid sample. Moreover, they have corrected their frequencies upward, as is proper, to account for unsampled accident chains. A major effort of the study was to conceive of events that have the potential for very large releases. Therefore, the authors believe (and we consider it reasonable) that the frequency of large releases is not likely to be low by virtue of “missed” accident sequences.*
Any fault-tree analysis depends on its input data. Many criticisms of WASH-1400 from the nuclear reactor industry are based on the contention that the fault frequencies of individual components were consistently overestimated. This is a consequence of two separate factors. First, failure data from eclectic sources were used in the absence of statistically valid samples from the nuclear industry itself (for example, with regard to pipe breaks, losses of motive or signal power, and so on). Second, the translation of these data into failure analysis in nuclear systems requires engineering judgment. The engineering protocol of basing such judgment on “conservative” or worst-case analysis is almost automatic, and it is claimed that much of the translation was on this basis.
A degree of conservatism that has been documented since the time most critiques were filed can be found in the assumptions made about the rate of heating of uncooled reactor fuel68 and about the release of fission products from melted fuel.69 It is now believed that a “best value” assumption of the heat input would imply delayed onset of meltdown and a lengthier period over which meltdown might occur, significantly improving the likelihood of corrective action. A “best value” assumption of fission-product release would significantly reduce the estimate of fission-product escape from a core melt.†
Criticisms have been directed against the treatment of “common-mode” failures in WASH-1400. A common-mode failure is an accident in which a single initiating event dislocates protective sequences designed to deal with the consequences of that event. For example, protective circuits are usually arranged so that a failure of one is backed up by the operation of another; if the event that caused one protective circuit to fail also caused the others to fail, that would be a common-mode failure. Data on common-mode failures are very difficult to validate; nevertheless, some WASH-1400 assumptions on this point are questionable.70 The Risk and Impact Panel, after investigating this point, concluded that the criticisms were valid, but
|
* |
Statement 5–25, by J.P.Holdren: The history of attempts to identify a priori the ways that complex systems could fail warrants more skepticism than is expressed here. |
|
† |
Statement 5–26, by J.P.Holdren: See my dissenting view, statement 5–27, Appendix A. |
could not specify what the effects of the WASH-1400 assumptions might be. Other critics contend that the assumptions are optimistic (i.e., low) in their effect on the predicted frequency of large release.
It has also been noted that the statistical presentation of risk in WASH-1400 is not appropriate for actuarial purposes. The report presents median values for frequencies of accidents of varying severity, whereas for actuarial purposes, mean values are more appropriate. Means are “expectation values.” For the sort of frequency distribution assumed (log normal), and in the case of a large spread (standard deviation) of such a distribution—as in the WASH-1400 results—the mean may be many times greater than the median.
A number of organizations, including the Atomic Energy Commission, the American Physical Society, the Environmental Protection Agency, and the Union of Concerned Scientists,71 identified a number of omissions, errors, and additional sources of uncertainty in the report. The Nuclear Regulatory Commission responded to these criticisms by commissioning the Risk Assessment Review Group. The group’s recently issued report72 states essentially the same conclusions presented here. It is notable that there was little consultation between members of CONAES and members of the review group. The conclusions—both positive and cautionary—may represent a growing consensus on the status of reactor safety.
At the time of our review, detailed evaluations of the recent incident at the Three Mile Island nuclear power plant near Harrisburg, Pennsylvania, were not available. It is possible that it may be an example of the class of accidents—potentially catastrophic, but in the event, controllable—from which improved safety practices would follow. If improved practices do result, then the outlook for nuclear safety might be favorable in a technical sense, regardless of the justifiably negative effect of the accident on public appraisal of nuclear power.
The Risk and Impact Panel also considered WASH-1400 and concluded that there did not seem to be any consistent biases in the study, but concurred in the qualitative judgment that the uncertainties in accident frequencies and consequences should be larger than reported.73
An important critique of WASH-1400 was presented by the Nuclear Energy Policy Study Group. Their conclusion was that considering all the uncertainties (those highlighted above, and others), LWR’s are unlikely to have an actuarial risk more than 500 times greater than that inferred from the median value points presented in the report. The group notes that at this upper limit, the risk from an LWR is not higher than the upper limit of risk from coal power, and therefore concludes that reactor safety against
accidents ought not to be a factor inhibiting nuclear expansion, at least as compared with coal.74*
WASH-1400 is a monumental piece of work, one that can be used to define those safety problems and system designs for which further work would be most significant, but it cannot prove either that reactors are safe or that they are dangerous.
With all the qualifications just presented, it is nevertheless useful to examine the Reactor Safety Study’s conclusions (set out in Table 5–11). It should be noted that risk is the product of frequency (chance per reactor-year) and consequences. Table 5–12 presents the data in this form. The frequency (probability per year) of loss of coolant is estimated as 1 in 2000 per reactor-year. The probability that emergency systems designed to prevent meltdowns in loss-of-coolant accidents will fail is estimated as 1 per 10 accidents, leading to a meltdown frequency of 1 in 20,000 per reactor-year. Further, WASH-1400 has estimated that only 1 meltdown in 100 would release large enough amounts of radioactivity to cause 10 or more deaths among members of the general public, The product of this 1-in-1000 figure and the estimated probability for meltdown of 1 in 20,000 per reactor-year gives the much-quoted WASH-1400 estimate of the probability of severe accidents in light water reactors: 1 in 2 million per reactor per year.
The Reactor Safety Study can be used to draw certain inferences, in spite of the large uncertainties that must be attached to the frequencies at which accidents of various consequences might occur.
The shape of the risk curve is a good deal less sensitive to uncertainty than is the actual magnitude of risk. This is because the larger releases tend to be consequent to the same initiating events. The entire risk curve will go up or down as the frequencies of the initiating events are changed, but the relative frequencies of the larger-consequence events will not change, From this, we may draw other inferences.
For example, accidents with lesser consequences will be far more prevalent than those with greater consequences. What this implies is that a vigorous program to diagnose and correct flaws in reactor safety systems as they become apparent through small accidents will not fail to decrease the expected frequency of severe accidents.
Another inference that is relatively firm concerns the nature of the dominant risk. This is the type of accident from which (over the long term) the greatest damages are expected to accrue. Table 5–12 exhibits maximum values among the entries for a particular column in the region of dominant risk. It can be noted that this is roughly the “one-in-a-million-reactor-
|
* |
See statement 5–27, by J.P.Holdren, Appendix A. |
TABLE 5–11 Consequences of Reactor Accidents as a Function of Median Frequency of Their Occurrence
|
Frequency (chance per reactor-year)a |
Early Fatalities |
Cases of Early Illness |
Total Property Damage (billions of dollars) |
Decontamination Area (square miles) |
Relocation Area (square miles) |
Latent Cancersb |
Thyroid Nodulesc |
Genetic Effectsd |
|
One in 20,000e |
— |
1 |
— |
— |
— |
— |
— |
— |
|
One in one million |
— |
300 |
0.9 |
2,000 |
130 |
170 |
1,400 |
25 |
|
One in 10 million |
110 |
3,000 |
3 |
3,200 |
250 |
460 |
3,500 |
60 |
|
One in 100 million |
900 |
14,000 |
8 |
8,000f |
290 |
860 |
6,000 |
110 |
|
One in one billion |
3,300 |
45,000 |
14 |
14,000f |
300 |
1,500 |
8,000 |
170 |
|
Uncertaintyg |
0.25–4 |
0.25–4 |
0.20–2 |
0.20–2 |
0.20–2 |
0.17–3 |
0.33–3 |
0.33–6 |
|
aThese are median probabilities, with uncertainties of a factor of 5 in either direction. bFatal cancer cases per year over an assumed 30-year latency period, resulting from the postulated release. cNodules per year in an assumed 11- to 40-year period following the postulated release. The thyroid nodules counted here are benign or successfully treatable. Thyroid cancers resulting in death are included in the latent cancers. dInduced effects per year over the span of one human generation following the postulated release. Later generations would have fewer cases from that release. eOne in 20,000 years is the estimated median frequency of meltdown, per reactor. fThere is no risk measure for these quantities; they are ways of characterizing consequences. gFactors within which there is 95 percent confidence that consequences have been accurately predicted. Source: U.S. Nuclear Regulatory Commission, Reactor Safety Study: An Assessment of Accident Risks in U.S. Commercial Nuclear Power Plants (Washington, D.C.: U.S. Nuclear Regulatory Commission (WASH-1400), 1975). |
||||||||
TABLE 5–12 Risks of Reactor Accidents as a Function of Median Frequency of Their Occurrence (per reactor-year)a
|
Frequency (chance per reactor-year) |
Early Fatalities |
Cases of Early Illness |
Total Property Damage (thousands of dollars) |
Decontamination Area (square miles)b |
Relocation Area (square miles)b |
Latent Cancersc |
Thyroid Nodulesd |
Genetic Effectse |
|
One in 20,000f |
— |
0.00005 |
— |
— |
— |
— |
— |
— |
|
One in one million |
— |
0.0003 |
0.9 |
— |
— |
0.0051 |
0.042 |
0.0025 |
|
One in 10 million |
0.000011 |
0.0003 |
0.3 |
— |
— |
0.0014 |
0.011 |
0.0006 |
|
One in 100 million |
0.000009 |
0.00014 |
0.08 |
— |
— |
0.00026 |
0.0018 |
0.00011 |
|
One in one billion |
0.0000033 |
0.000045 |
0.014 |
— |
— |
0.000045 |
0.00024 |
0.000017 |
|
aThese are median probabilities, with uncertainties of a factor of 5 in either direction. bThere is no risk measure for these quantities; they are ways of characterizing consequences. cFatal cancer cases per reactor-year. dNodules per reactor-year. The thyroid nodules counted here are benign or successfully treatable. Thyroid cancers resulting in death are included in the latent cancers. eGenetic effects per reactor-year if the total number of cases is 100 times the annual rate exhibited in the first generation. fOne in 20,000 years is the estimated median frequency of meltdown, per reactor. Source: U.S. Nuclear Regulatory Commission, Reactor Safety Study: An Assessment of Accident Risks in U.S. Commercial Nuclear Power Plants (Washington, D.C.: U.S. Nuclear Regulatory Commission (WASH-1400), 1975). |
||||||||
year” category, characterized by few immediate casualties of any type, by tens to hundreds of delayed health effects, and by hundreds of millions of dollars in property damage. Therefore, such cases should be considered as characteristic of “catastrophic” nuclear accidents. We infer that a similar type of reasoning may have guided the Nuclear Energy Policy Study Group in concluding that “the consequences of an extremely serious accident are not out of line with other peacetime catastrophes that our society has been able to handle….”75 Damages in the same range as those from dominant nuclear accidents have, after all, been experienced in other industries: refinery and chemical plant fires and explosions, airplane crashes, shipwrecks, and toxic chemical and metal releases.
The assumption implicit (if not explicit) in the reactor safety studies conducted so far is that the equipment and the people operating it and regulating its use behave approximately according to the conditions specified. Nevertheless, there may be shortcomings in the people and equipment. Mistakes, laxity, and incompetence can overcome technological barriers. In the nuclear power industry, as in any industry in which mistakes can have expensive consequences, human errors and inadequacies constitute a significant source of risk that is difficult to quantify. It would seem that the uncertainties in estimations of risk have themselves been underestimated by failing to take these factors into account.
On the other hand, human ingenuity eventually brought the two most serious nuclear power accidents (Brown’s Ferry and Three Mile Island) under control, and this quality has evidenced itself in the prevention and mitigation of many other incidents.
Thus, we find reason to assign an uncertainty to the possibilities calculated for nuclear power accidents, ranging higher or lower than those published, and note as a consequence the great value of maintaining as well trained a work force as possible for the design, construction, operation, maintenance, inspection, and supervision of nuclear power plants.
Safety of Other Reactors Fault-tree, event-tree analysis can also be applied to compare the risks of various reactor systems against one another. The analyses require information that has not yet been assembled for advanced converters or fast breeders: specific designs, recognized design criteria, and results of accident analyses.
The only document produced in the United States on the safety of LMFBR’s and available for study is the draft environmental statement for the Clinch River breeder reactor. Events that might lead to large-scale release of radioactivity to the public are the class-8 and class-9 accidents listed in Table 5–13.
Accidents that form the basis for the plant’s design are grouped in class
TABLE 5–13 Postulated Liquid-Metal Fast Breeder Reactor Accidents That Could Result in Radioactive Release to Public, with Postulated Light Water Reactor Accidents Shown for Comparison
|
Accident Classification |
Description |
Light Water Reactor |
Liquid-Metal Fast Breeder Reactor |
|
8 |
Accident-initiating events considered in design-basis safety evaluation |
Transients in reactivity; rupture in primary piping system; steam-line breaks |
Leaks in steam generator; steam-line breaks, failures in primary sodium storage tank; leaks cold trap; large rupture in primary piping systema; events leading to core disruptionb |
|
9 |
Hypothetical sequences leading to accidents more severe than those in class 8 |
Successive failures of multiple barriers provided and maintained to prevent the escape of large amounts of radioactive material |
Successive failures of multiple barriers provided and maintained to prevent the escape of large amounts of radioactive materialb |
|
aThe Clinch River breeder reactor design has a closed-cycle secondary heat-transport system that separates the primary coolant from the power-conversion system. Class-4 failures (events that release radioactivity into the primary cooling system) and coincident heat-exchanger leaks would not result in release of significant amounts of radioactive material to the environment. bWhile the Nuclear Regulatory Commission does not consider these events as design-basis accidents for the plant, the commission has asked that mitigating features be provided. Source: National Research Council, Risks and Impacts of Alternative Energy Systems, Committee on Nuclear and Alternative Energy Systems, Risk and Impact Panel (Washington, D.C.: National Academy of Sciences, in preparation). |
|||
8. The plant must be designed to withstand such accidents without failure of containment. These postulated accidents begin with the failure of major components or piping and threaten the release of significant amounts of radioactive material from the reactor’s primary system. The design must incorporate special features to mitigate the consequences of class-8 accidents: sealed equipment cells, a double-level containment, and two independent, diverse systems to shut the reactor down. Siting regulations require the offsite doses calculated for the full range of class-8 accidents to fall within guideline values.
In the LMFBR, the sodium coolant circulates at atmospheric pressure and at temperatures well below the boiling point. Thus, loss of the coolant by sudden evaporation (blowdown) is impossible. This represents a significant safety advantage over light water reactors.
Potentially severe accidents that are physically possible, but so extremely improbable that it is not considered reasonable to counter them by expensive engineered safeguards or consider them in siting decisions, fall into class 9. Class-9 accidents require that major failures in the reactor system be accompanied by independent and concurrent failure of safety systems and barriers to the escape of radioactive material. The consequences of these accidents could in some cases exceed the consequences calculated for the worst accidents considered in the safety report on light water reactors. It seems intuitively reasonable to assume that the likelihood of such accidents would be substantially lower than even the very low values predicted by the Reactor Safety Study for high-consequence LWR accidents, but this intuition must be confirmed by a probabilistic fault-tree analysis.
The compact core of a liquid-metal fast breeder reactor displays both high power density and high plutonium content. This core is not designed for maximum reactivity. The principal concern over a possible disruption of the core is that it might take on a more reactive shape, or that shifting pieces of the core might create areas of high reactivity. The energy released in these cases could exert enough pressure to disassemble the core, terminating the chain reaction. But core disruption or subsequent recriticality might conceivably release sufficient energy to generate mechanical forces that threaten the containment. The Department of Energy has sponsored considerable research on this topic because of its crucial importance, and it is now believed that a containment-threatening, core-disruptive accident is precluded by proper design.76
The Nuclear Regulatory Commission takes the position that designs for prototype liquid-metal fast breeder reactors must attempt to reduce the probability of class-9 accidents leading to large-scale core melting to 10−6 per year. In addition, the Nuclear Regulatory Commission recommends that features be provided in the design to protect against the effects of
mechanical forces that might be generated by core melting, and to contain the bulk of fission products in a class-9 accident. This would be a stricter criterion than those applied to light water reactors.
A number of commentators point out that reducing the probability of core melting 10–50 times below the probability of core melting in LWR’s can be set as an objective for LMFBR designs, but that it is unrealistic to expect conclusive demonstration that the objective has been met.77
The unanswered questions for LMFBR safety, then, are: whether inherent or engineered safety features eliminate or greatly reduce the probability of core melting; whether, if this probability cannot be reduced to desirable unlikelihood, engineered features can contain the consequences; and by what mechanisms reasonable consensus can be reached that these objectives have or have not been met.
A preliminary analysis of HTGR safety has been conducted by the vendor, and there has been some assessment of its features in design reviews of the Fort Saint Vrain reactor and other proposed HTGR installations. Salient features of this analysis are discussed in the report of the Risk and Impact Panel. The Working Group on HTGR Safety of that panel concluded tentatively that the HTGR may be less susceptible to large radioactive releases than the LWR. The HTGR has demonstrably better tolerance for storing decay heat without releasing fission products and may be less subject to a large LOCA, but it has the extra mechanism of graphite oxidation for potential release of fission products and heat. In case of loss of the helium coolant, for example, air could not be used for emergency cooling because it would burn up the graphite.78
Sabotage of Nuclear Facilities As already noted, deliberate sabotage has not been included in the discussion of nuclear accidents, as it is not usually included in accident analysis of other systems. Nevertheless, the question has been raised whether the existence of nuclear facilities presents an “attractive nuisance” to would-be terrorists, who might use the threat of sabotage to extract concessions from society, or to people bent on destruction.
This discussion is limited to a general review. Details can be found in the recently issued report of the National Academy of Sciences on the safety of nuclear power.79 That report (like this discussion) is necessarily limited to information in unclassified literature.
Three points must be considered: the degree of vulnerability of nuclear installations, consequences that might credibly ensue, and comparison of vulnerability and consequences with those of other energy sources.
Nuclear systems are probably less accessible, and harder to sabotage, than many competing energy systems. Access is more carefully controlled than to other thermal power plants, and all thermal plants are inherently
easier to protect (because of their relative compactness) than more dispersed sources, such as dams or solar or wind installations. Thus, with regard to vulnerability against loss of generating capacity, nuclear plants must be rated as highly secure.
With regard to vulnerability against attacks or threats aimed at endangering the public, nuclear plants have considerable intrinsic protection. The main reason is that the plants themselves are complex. A limited number of individuals have knowledge sufficient to initiate a severe accident, and the steps that must be taken to ensure that an accident leads to large release are numerous. The multiplicity of systems available to the defense-in-depth strategy of nuclear plants would have to be disabled, and the degree of planning required would seem to demand the collusion of a great many “insiders.” Preparations would be necessarily time consuming, and no threat could be voiced until they were complete, since shutting a reactor down would quickly decrease the severity of consequences from delayed releases of radioactivity.
Nevertheless, the consequences of sabotage against nuclear plants must be rated as potentially severe. In a hierarchy of risk, nuclear plant sabotage could lead to consequences of the same order of severity as those following the breach of a major dam or sabotage of a natural gas storage facility. Oil refineries would present a medial level of risk, and coal plants and solar and wind facilities would be at the bottom.
The range of consequences that can be produced by sabotage of a reactor is probably rather similar to that for reactor accidents. The aim of a saboteur, presumably, would be maximum release of radioactivity at a time when the weather conditions would be most conducive to directing the radioactivity to populated areas nearby. This aim would complicate the saboteur’s task by introducing a further element of timing. Not only would the sabotage have to be prepared and set, but also perpetrated at the most damaging time.
We might also consider the additional threat to the public that might ensue from bombing a nuclear plant (in the course of war, for example). For anything but direct hits on the reactor building with very heavy conventional bombs or penetrating missiles, the reactor containment is an effective barrier. Although a direct hit on a spent-fuel storage pool would disperse the radioactivity contained in it, experience with reactor destructive tests, such as BORAX,80 and accidents, such as that of the SL-1, indicate that little of the radioactivity is transferred a significant distance, and that the overwhelming bulk of the contamination would be confined to the plant site. However, a direct hit with a nuclear bomb would very much enhance (by orders of magnitude) the subsequent damage due to fallout
Three points can be made about the sabotage potential of nuclear systems.
-
It is extremely difficult to make quantitative estimates of the expected frequency of effective attempts to sabatoge nuclear plants.
-
Nuclear systems are easy to sabotage into a state of inoperability, if penetrated by saboteurs, but they are very difficult to sabotage into a public hazard because of the redundancy of safety features.
-
Many other systems in our society offer more dangerous combinations of vulnerability to sabotage and likelihood of causing major damage: large aircraft, tanks of liquefied natural gas, major dams, and chemical plants.*
As with many other large industrial installations, it would appear that the greatest degree of defense against sabotage should be concentrated at sites near large population centers. The Nuclear Regulatory Commission is responsible for plant protection standards and appears to have given the matter of sabotage adequate emphasis.
Conclusions and Recommendations
It is important to recognize three quite separate issues in reactor safety that many published discussions of the subject fail to distinguish clearly.
-
The set of “best estimates” of the probabilities and consequences of various kinds of accidents, and the ranges of uncertainty that bracket these values.
-
Interpretation of these values and uncertainties to yield some understanding of expected values of consequences.
-
What “mean” values of probabilities and consequences of accidents in nuclear power plants, and what degrees of uncertainty about these values, are acceptable in exchange for the benefits of nuclear power.
The first two issues are essentially technical; the third is essentially social and political.
The probabilities of very low frequency accidents are difficult to estimate with precision. One expert suggests that the probabilities and consequences of catastrophic nuclear accidents can never be estimated more accurately than within an order of magnitude.81 Thus, the safety of nuclear reactors will continue to be a matter of judgment. Perhaps the most valuable feature of fault-tree, event-tree analysis is that it points out where design improvements could be most effective. Those improvements that make a
significant contribution to further reducing the likelihood of accidents can be identified and should be incorporated in new plants. Similarly, “safety” systems that do not make such a contribution might be dropped from licensing requirements, particularly if significant cost savings result.
There are three legitimate ways by which judgments of risk may be better specified for policy purposes. Improved precision could result in part from more reactor-years of experience and from improved methods of analysis. It might also be possible to improve the data base of the models used in reactor safety studies by conducting better tests and analyses of nuclear-system components. The third way would be to improve the design of nuclear power plants and to make stringent inspections at critical stages of construction.
These considerations permit CONAES to reach a conclusion on the question of reactor safety. It is consonant with that of many other review groups, but is both more optimistic and more cautionary. We believe that the expansion of nuclear power can proceed without untoward public risk from reactor accidents, but only under certain conditions.
-
Institutions to review experience and enforce improvement of safety design must be vigorous and independent. In the case of the United States, this institution is the Nuclear Regulatory Commission. For international concerns, such bodies as the International Atomic Energy Agency (IAEA), which has safety consultation and review authority, should be strengthened for maximum effect of its recommendations.
-
Both the nuclear industry and regulatory authorities must be more receptive to design modifications that would enhance safety. The design and licensing process is now so lengthy that there are strong economic disincentives to consideration of any design changes. It must be recognized that improvements in safety by design are difficult to prove; under these circumstances, there is a natural tendency to continue with existing practices. Nevertheless, it is only by change that improvements can be made. Evaluations of proposed improvements (with regard to their effect on the results of the Reactor Safety Study’s estimates of accident frequencies and consequences) should be a continuous process, and the proposed improvements that receive favorable evaluation should be instituted.
-
It goes without saying that research on the safety of nuclear power should receive continued support. There is need to reconsider the sort of work to be emphasized. Significant advances in our understanding of reactor safety come from improved knowledge in the engineering sciences, Such topics as two-phase flow, mechanics of materials, metal-water chemical reaction processes, steam explosion theory, fission-product decay heat, fission-product chemistry and volatility, and others are basic to
-
understanding the phenomena that might occur during accident conditions, and to quantitative specifications of the consequences. These deserve strong research support, preferably as independent studies, but if necessary, under efforts to improve safety. (Their level of support as research topics has been grossly inadequate for more than a decade.) Conversely, integral experiments to “verify” conclusions should be used sparingly; too often, they are demonstrations of the already known. For example, measurements of the physics or heat-transfer behavior of systems that have already been simulated by critical experiments or electrically heated loops should only be performed if there is some question about the feedbacks among various types of phenomena in the reactor.
-
The Reactor Safety Study gives valuable guidance for decisions the public must make about expansion of nuclear power.* The committee makes the following recommendations.
-
For existing reactor types, such as LWR’s now operating in the United States, studies should be updated every 10 years. The purpose of the update should be to quantify the rate of improvement in knowledge pertinent to safety and safety records.
-
The safety of new reactor types, such as LMFBR’s or advanced converters, should be compared with the safety of existing reactors. This should be done before commissioning a large number of new reactors. An appropriate time to conduct such studies might be after about the first 10 such reactors have been granted construction permits. Before this point, there is likely to be too much design variation to permit generic comparision. If the study is delayed too long, the same sort of conflicts may develop that trouble the country today about LWR safety.
MANAGEMENT OF RADIOACTIVE WASTE
Radioactive wastes consist of a variety of natural uranium and thorium decay products, fission products, products of neutron activation, and transuranic isotopes or actinides with intermediate to very long half-lives. These wastes must be sequestered from the biosphere for as long as their radiation represents a hazard. Burial or “geological isolation” is the reference method for doing this.
The principal technical consideration in assessing modes of geological isolation is the transport of radionuclides by groundwater. For longer-lived wastes, this requires selection of geological formations that themselves would be proof against the failure of containers after one or two hundred
years, in the sense that migration of the waste nuclides must be slow enough, or accompanied by so much dilution, that the radioactivity of the water when it reaches the biosphere is a small fraction of natural radioactivity.
Public concern with the management of radioactive waste centers on society’s ability to achieve and maintain the necessary isolation. Both technical and social aspects of this topic have received lively attention. The technical aspects will be taken up first; the social considerations, while not wholly separable, will be discussed briefly near the end of this section.
What Is the Required Isolation?
The degree of isolation required of radioactive materials varies from one form to another. Some materials are easily mobilized and transported (e.g., by groundwater), some are not. Some materials are concentrated by living tissue, some are not. Radioactive materials decay, losing their radioactivity, at different rates.
Over and above these considerations there is the question of what level of concentration or release is dangerous, Standards of comparison can be natural radioactivity (background) or projected health effects. Some types of radioactivity released during the nuclear cycle can occur naturally: uranium, thorium, and the decay daughters of uranium and thorium, which are the nuclides at issue in mining and milling wastes, and 14C and tritium, which can be formed during reactor irradiation and released either at that time or during the course of reprocessing. For such materials, comparisons of the contribution from the nuclear cycle with their natural abundance can provide insight into the safety factors needed to render them effectively harmless. (See chapter 9.) Other materials—fission products, higher actinides, and activation products—have no natural source of any consequence. For these, projected health-effect calculations are used to determine the standards for release. Complicating these simple criteria is the fact that they are not necessarily consistent with one another. Projected health-effect calculations (that attempt to err always on the side of predicting higher mortality and morbidity than a best estimate) can predict effects from natural radiation that are well beyond observed values; in the other direction, predictions of health effects have led to hypotheses that some natural sources of radiation are significant causes of morbidity.
The differences due to concentration effects are also important. Low concentrations of radioactivity have a statistically low probability of harming any given individual. As with toxic heavy metals, society tends to accept “low enough” concentrations in the environment. The rationale seems to be that at some level, the risk posed can be ignored in comparison to risks that are orders of magnitude larger. Yet, it is (properly) considered
poor practice to manage most wastes by dilution to an acceptable concentration level, even when that is feasible. We have (again, properly) confidence in our ability to sequester or destroy wastes if we concentrate and package them to delay or prevent their return to the biosphere. Yet, paradoxically, the existence of concentrated wastes, specifically radioactive wastes, is viewed with more apprehension than the existence of radioactivity in very dilute form already mobilized within the biosphere (such as radioactivity from fallout of weapons tests). It therefore appears that the problem of disposing of radioactive wastes has two parts. The first is to package and isolate the wastes as well as possible, and the second is to arrange for sufficient delay and dilution, in case the isolation fails, to ensure that the concentration possibly returning to the biosphere is not a major source of risk by prevailing standards.
The most radioactive materials (e.g., most fission products and most products of neutron activation) have relatively short half-lives; indeed, specific radioactivity is inversely proportional to half-life for a given number of atoms. It is reasonable to demand a high degree of assurance that the isolation of these products will continue until natural decay has reduced their radioactivity to low levels. As half-life increases (and radioactivity decreases), such assurance becomes both more difficult to provide and less necessary. The difficulty is, of course, a direct result of the long time required for decay, but the radioactivity is subsequently less intense and the consequences of release would be less severe.
The result of these considerations is a waste management philosophy that incorporates and combines two separate types of waste isolation: physical and chemical isolation, and disposal in a geological setting expected to delay and hinder the return of radioactivity to the biosphere, or at worst to dilute it considerably during the course of such return. The physical and chemical isolation is more of a backup precaution, forming an additional barrier to the geological isolation that constitutes the main safety factor for long-lived waste. For the short-lived waste, physical and chemical isolation is designed to be an effective barrier in its own right.
Types of Radioactive Waste
Uranium Ore Tailings About 80 percent of the original radioactivity in uranium ore remains in uranium ore tailings. Processing (milling) removes only the uranium, leaving behind about 7 percent of the uranium (as processing loss), the daughters 234Th, 231Th, and 230Th, their decay products, and any natural 232Th in the ore. Among these radioactive materials, the most troublesome are 230Th (half-life: 77,000 years), radium-226 (226Ra) (half-life: 1600 years), and their daughters. These radioactive isotopes are widely dispersed in nature through the natural weathering of
uranium-containing rock. It is therefore the surface concentration, rather than the quantity of these nuclides in the ore tailings, that must be dealt with. The total quantity of tailings accumulated since 1948 totals 123 million tons, or approximately 70 million yd3. The quantity is large because virtually all the uranium ore mined appears in the tailings: Typical uranium concentrations are well under 1 percent.
A standard light water reactor requires the mining of about 150 tons/yr of U3O8, and at the typical concentration of 0.1 percent in the ore, this amounts to about 150,000 tons of rock, or about 40,000 yd3 (30,000 m3). The contained radioactivity is about 500 times greater than that of ordinary soil. The parent nuclide of most of the radioactivity is 230Th, and all the lighter radioactive nuclides in the uranium radioactive series are “fed” by it. At any given time, there are about 10 other radioactive disintegrations for each disintegration of 230Th, the whole chain decaying with the thorium half-life.
The most important members of the disintegration series from the point of view of radiation hazard are 226Ra and radon-222 (222Rn). Radium is chemically a member of the alkaline earth family (along with calcium, strontium, and barium) and shares with other members a relatively high leachability and mobility in the presence of groundwater. Radon is a noble gas that, unless trapped in a crystalline medium or in sub-surface pores, diffuses into the air. Radon is the daughter of radium, so that if the radium has migrated, radon is released at the point of radium disintegration.
Abandoned mines and active piles of ore tailings are the sources of this radiation. Protective measures should meet the criterion of reducing this source to the same order of magnitude as ordinary soil. As noted, this means reduction by a factor of about 500. Achieving such reduction is not a technically difficult matter, although it represents a small incremental cost to the mine operator. Filling in mines, burying tailings, and avoiding massive invasion of the tailing piles by water are recommended procedures. A few feet of earth above the pile is an effective seal against the escapes of radon, because the gas has only a 3.85-day half-life and diffuses slowly. Asphalt seals further delay the release of radon, but serve the more important function of preventing or slowing seepage of surface water through the pile. Soil fillers or conditioners could also be used to inhibit seepage.
Over the course of time, wind or water erosion could conceivably reexpose the pile. However, filling and covering are equally likely results of both wind and water action.* In the United States, uranium is mined mostly in arid regions where surface water is not common, but care must
be taken not to disturb subsurface aquifers. Further exposure is a possibility, but the level of exposure would not be significantly above background and would not result in serious consequences to any human generation. If our descendants are sophisticated and “radiation conscious,” they would find detection of the source to be very simple, and correction of the condition equally simple. If they are not, it is likely to be because their technology has so regressed that they are subject to much more pressing dangers.
Wastes of Reprocessing Four major types of waste are produced in fuel reprocessing: the aqueous raffinates from solvent extraction, the metal hulls sheared from the fuel rods, the scrubbing solutions or solids generated by reaction with the gases containing radioactive iodine-129 (129I), 131I, tritium, and 14C, and the gases released during dissolution of the fuel.
The aqueous raffinates are by far the most radioactive of these wastes. They contain almost all the fission products generated, together with a small fraction of the actinide elements uranium, plutonium, neptunium, and americium. Regarding the fission products, strontium-90 (90Sr) and cesium-137 (137Cs) are the most notorious components. Each has a half-life of about 30 years, and the reduction of their activities by a factor of 1000 in 300 years, 1 million in 600 years, and 1 billion in 900 years establishes the period 500–1000 years for social concern with their custody. The ratio of atoms of actinides to fission products is less than 1 percent. Their radioactivity is at first negligible compared to that of fresh fission products, but their radioactivity lasts longer. In conventional reprocessing, the raffinates appear as concentrated nitric acid solutions, amounting to about 56 gal/ton of spent fuel. These raffinates are commonly considered to be the crux of the radioactive waste management program.
Other radioactive wastes from reprocessing—insoluble residues, raffinates from product purification steps, hulls and off-gases—are treated separately. In particular, off-gases are of short half-life or low radioactivity. The most troublesome is 85Kr, which is to be collected and stored for about a century when the amounts become significant.
Tables 5–14 and 5–15 set out, respectively, concentrations of chemical elements in spent light water reactor fuel and the radioactivity of important nuclides.
Table 5–14 is primarily of interest in considering source concentrations of elements that would be subject to both total and isotopic dilution during migration. Table 5–15 presents the radioactivities of spent fuel up to 10 years after reactor discharge. Of interest is the very high radioactivity after 10 years of cooling, and the increasing contribution of the actinides. Thirty
days after discharge, less than 2 percent of the activity is from actinides, but after 10 years of cooling, the actinides produce almost 20 percent of the activity.
Alpha-Active Wastes82 Throughout the nuclear industry, uranium is converted from one chemical form to another: from uranium oxide to uranium hexafluoride for enrichment, from uranium hexafluoride to uranium dioxide for fuel-material preparation, and so on. There are radioactive wastes from these processes. They are not as radioactive as mill tailings, and ought probably to be mingled with them for disposal,* but institutional arrangements have not been made for this step.
Laboratory operations involving plutonium, 233U, and other actinides also produce wastes that contain long-lived alpha activity. In the past, the concentration of alpha activity governed the method of disposal: Low concentrations were considered low-level wastes, to be buried or dispersed. However, the increased concentrations and quantities of this material being disposed of, and public fear of the consequences of its dispersal, stimulated a change in policy, and this material is now considered in the same disposal category as high-level waste.
Looking to the future, we can expect to see a large increase in the generation of alpha-active waste if 233U and plutonium are recycled. A considerable amount of waste is generated during nuclear fuel fabrication: dusts from grinding operations, contaminated fabrics from filters, contaminated crucibles and tools, contaminated metal pieces from rejected fuel elements, and so on. (For a given number of atoms, radioactivity is inversely proportional to half-life, and the half-lives of possible contaminants are 700 million years for 235U, 160,000 years for 233U, 23,000 years for 239Pu, and 6500 years for 240Pu.)
A further consideration in recycled fuel is the contamination introduced by other actinide nuclides. These materials (232U, 238Pu, 241Pu, americium-241 (241Am), and others) have even shorter half-lives and higher activities than those just mentioned, and for that reason, they have been suggested as radioactive “spikes” to safeguard nuclear fuel, discussed in this chapter under “Safeguarding the Domestic Fuel Cycle.”
Reprocessing Wastes from Military Production Although not within the responsibility of a civilian industry, military production wastes are the major focus of current concerns about waste management. The important wastes are the aqueous raffinates from reprocessing of fuel to recover plutonium for weapons. Three different processes have been used in the
TABLE 5–14 Element Concentrations in Spent Light Water Reactor Fuel (grams per metric ton of heavy metal)a
|
Element |
Concentrations |
|||||
|
After 30 days |
After 90 days |
After 150 days |
After 1 year |
After 3 years |
After 10 years |
|
|
3H |
0.075 |
0.074 |
0.074 |
0.071 |
0.064 |
0.043 |
|
Kr |
383 |
383 |
382 |
381 |
378 |
369 |
|
Xe |
5,580 |
5,590 |
5,590 |
5,590 |
5,590 |
5,590 |
|
Rb |
341 |
341 |
341 |
342 |
346 |
355 |
|
Cs |
2,830 |
2,810 |
2,800 |
2,750 |
2,630 |
2,380 |
|
Sr |
932 |
921 |
914 |
903 |
877 |
794 |
|
Ba |
1,410 |
1,420 |
1,440 |
1,490 |
1,610 |
1,850 |
|
Y |
486 |
482 |
480 |
477 |
477 |
477 |
|
La |
1,300 |
1,300 |
1,300 |
1,300 |
1,300 |
1,300 |
|
Ce |
2,890 |
2,830 |
2,790 |
2,690 |
2,570 |
2,550 |
|
Pb |
1,210 |
1,220 |
1,230 |
1,230 |
1,230 |
1,230 |
|
Nd |
3,910 |
3,950 |
3,990 |
4,090 |
4,200 |
4,230 |
|
Pm |
113 |
109 |
104 |
88.8 |
52.3 |
8.2 |
|
Sm |
824 |
829 |
834 |
849 |
885 |
926 |
|
Eu |
194 |
192 |
191 |
189 |
184 |
172 |
|
Gd |
111 |
113 |
113 |
116 |
122 |
136 |
|
Te |
1.9 |
1.9 |
1.9 |
1.9 |
1.9 |
1.9 |
|
Dy |
1.1 |
1.1 |
1.1 |
1.2 |
1.2 |
1.2 |
TABLE 5–15 Radioactivity of Selected Nuclides in Spent Light Water Reactor Fuel (curies per metric ton of heavy metal)a
|
Nuclide |
Half-life |
Radioactivity |
|||||
|
After 30 days |
After 90 days |
After 150 days |
After 1 year |
After 3 years |
After 10 years |
||
|
Fission Products |
|
|
|
|
|
|
|
|
3H |
12.3 years |
727 |
720 |
713 |
690 |
616 |
415 |
|
85Kr |
10.8 years |
11,400 |
11,300 |
11,200 |
10,800 |
9,490 |
6,060 |
|
131Xeb |
12.0 days |
2,600 |
104 |
3.2 |
— |
— |
— |
|
133Xe |
5.3 days |
37,300 |
14 |
— |
— |
— |
— |
|
134Cs |
2.1 years |
250,000 |
237,000 |
224,000 |
184,000 |
93,300 |
8,750 |
|
136Cs |
13.0 days |
12,800 |
522 |
21 |
— |
— |
— |
|
137Cs |
30.0 years |
111,000 |
110,000 |
110,000 |
108,000 |
103,000 |
87,900 |
|
137Bab |
2.6 min |
103,000 |
103,000 |
103,000 |
101,000 |
96,600 |
82,200 |
|
89Sr |
52.1 days |
464,000 |
209,000 |
93,800 |
5,340 |
0.3 |
— |
|
90Sr |
28.1 years |
78,900 |
78,600 |
78,300 |
77,200 |
73,500 |
61,800 |
|
140Ba |
12.8 days |
277,000 |
10,800 |
417 |
— |
— |
— |
|
90Y |
64.0 hours |
78,900 |
78,700 |
78,300 |
77,200 |
73,500 |
61,800 |
|
91Y |
59.0 days |
642,000 |
316,000 |
156,000 |
12,400 |
2.2 |
— |
|
140La |
40.2 hours |
319,000 |
12,400 |
480 |
— |
— |
— |
|
141Ce |
32.3 days |
716,000 |
198,000 |
55,000 |
553 |
— |
— |
|
144Ce |
284 days |
1,020,000 |
880,000 |
760,000 |
450,000 |
75,500 |
150 |
|
144Prb |
17.3 min |
1,020,000 |
880,000 |
760,000 |
450,000 |
75,500 |
150 |
|
143Pr |
13.7 days |
287,000 |
13,800 |
663 |
— |
— |
— |
|
147Nd |
11.1 days |
87,900 |
2.070 |
49 |
— |
— |
— |
|
147Pm |
2.6 years |
104,000 |
101,000 |
96,400 |
82,500 |
48,600 |
7,630 |
|
93Zr |
1.5×106 years |
1.9 |
1.9 |
1.9 |
1.9 |
1.9 |
1.9 |
|
93Nbb |
13.6 years |
0.2 |
0.2 |
0.2 |
0.2 |
0.4 |
0.9 |
|
95Zr |
65.2 days |
973,000 |
513,000 |
271,000 |
27,300 |
11 |
— |
|
95Nbb |
90.0 hours |
20,700 |
10,900 |
5,750 |
580 |
0.2 |
— |
|
95Nb |
35.0 days |
1,250,000 |
852,000 |
508,000 |
58,100 |
24 |
— |
|
99Tc |
2.1×105 years |
15 |
15 |
15 |
15 |
15 |
15 |
|
103Ru |
39.5 days |
710,000 |
249,000 |
86,900 |
2,020 |
— |
— |
|
103Rhb |
57 min |
711,000 |
249,000 |
86,900 |
2,020 |
— |
— |
|
106Rub |
1.0 year |
524,000 |
468,000 |
418,000 |
278,000 |
70,000 |
560 |
|
106Rh |
30.0 s |
524,000 |
468,000 |
418,000 |
278,000 |
70,000 |
560 |
|
129I |
1.7×107 years |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
|
131I |
8.0 days |
65,600 |
375 |
2 |
— |
— |
— |
|
Actinides |
|
|
|
|
|
|
|
|
234U |
2.5×105 years |
0.7 |
0.7 |
0.7 |
0.7 |
0.8 |
0.8 |
|
236U |
2.4×107 years |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
237U |
6.7 days |
39,500 |
86 |
2.7 |
2.5 |
2.3 |
1.6 |
|
238U |
4.5×109 years |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
236Pu |
2.8 years |
0.4 |
0.4 |
0.3 |
0.3 |
0.2 |
— |
|
238Pu |
88.9 years |
2,970 |
3,010 |
3,030 |
3,070 |
3,060 |
2,900 |
|
239Pu |
24,400 years |
323 |
323 |
323 |
323 |
323 |
323 |
|
240Pu |
6,760 years |
485 |
485 |
485 |
485 |
486 |
487 |
|
241Pu |
14.6 years |
108,000 |
107,000 |
106,000 |
103,000 |
94,000 |
67,400 |
|
242Pu |
3.8×105 years |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
1.5 |
|
241Am |
433 years |
105 |
134 |
162 |
260 |
575 |
1,460 |
|
243Am |
7,650 years |
20.2 |
20.2 |
20.2 |
20.2 |
20.2 |
20.2 |
|
243Cm |
163 days |
32,000 |
24,800 |
19,200 |
7,710 |
352 |
7.7 |
|
244Cm |
18.1 years |
2,820 |
2,810 |
2,790 |
2,730 |
2,520 |
1,930 |
|
Sum, Fission Products |
|
1.06×107 |
6.14×106 |
4.38×106 |
2.24×106 |
806,000 |
325,000 |
|
Sum, Actinides |
|
1.89×105 |
1.39×105 |
1.32×105 |
1.18×105 |
101,000 |
74,600 |
|
TOTAL |
|
1.08×107 |
6.28×106 |
4.51×106 |
2.36×106 |
907,000 |
400,000 |
|
aAssumptions: 3.3 percent enriched uranium fuel; burnup, 34,000 MWd/metric ton of heavy metal; specific power, 29.5 MWe/metric ton of U3O8. bNuclides in metastable states that can decay into more stable form by emission of a gamma ray. Source: H.O.Haug, Calculations and Complications of Composition, Radioactivity, Thermal Power, Gamma and Neutron Release Rates of Fission Products, and Actinides of Spent Power Reactors’ Fuels (Karlsruhe, Federal Republic of Germany: Reactor Research Institute, 1974). |
|||||||
past (bismuth coprecipitation, Redox, and Purex), and they differ in the quantities and types of process chemicals that accompany the waste to liquid storage. However, the main features of these wastes are similar, and they are different from those of civilian Purex wastes.
-
The actinide content is much lower. Almost no higher actinides are formed during irradiation to make weapons-grade plutonium, and process yields of plutonium are more nearly total, leaving only a small fraction in the waste.
-
The waste solution is neutralized. This produces wastes, most of which have precipitated readily from solution (as hydroxides or carbonates), loading the solution with sodium salts. After prolonged liquid storage, the storage tanks contain both solid “salt cake” and a salt solution. Unfortunately, almost all these wastes from the weapons programs were stored in carbon-steel tanks, an expediency adopted during World War II and regrettably continued until recent times. The salt cake and salt solution have corroded some of these tanks and are expected to corrode more. The wastes cannot be redissolved and pumped out without also dissolving the tanks. Thus, these weapons wastes present a unique one-time problem not to be experienced in any civilian program, where the use of acid solutions in stainless steel tanks eliminates the difficulty.
The carbon-steel tanks at the Hanford reservation have leaked many times, and severely at least once. The leaks have been without hazard to the public83 because of the low rate of ion migration of radioisotopes through the soil at Hanford, and the low concentration of long-lived actinides indicates that they will never be a danger. The radioactivity will have decayed to innocuous levels by the time the material reappears in the biosphere.
However, the peculiar nature of this waste has made it very difficult to find a disposal method that is more appropriate than letting the salt cake evaporate to dryness and entombing the tank in concrete.
Chemically similar wastes are stored in carbon-steel tanks of better design at the Savannah River plant in South Carolina, and in relatively small amounts at West Valley, New York. Leaks at Savannah River have been minute, and zero at West Valley, but the local hydrology is by no means so favorable in those two places as in Hanford.
Spent Fuel as Waste President Carter announced in 1977 that the United States would defer reprocessing of spent reactor fuel indefinitely, to avoid potential diversion of reprocessed plutonium for weapons. Many have interpreted this policy as tantamount to declaring spent fuel to be waste. CONAES has not accepted this interpretation, and prefers to consider the announcement to be one of reserving judgment. Even those members who
oppose nuclear power consider it prudent for now to preserve spent fuel as a potential resource. For the short term, the stowaway fuel cycle (guarded storage of spent fuel) protects against subnational diversion because the spent fuel is literally too hot (radioactive) to handle.
In the future, a decision could be made to treat spent fuel as waste. The fuel is in a ceramic form and is not expected to be easily leachable. However, it is at least as concentrated a heat source as solidified reprocessing wastes, and contains 50–100 times more actinide radioactivity per GWe-plant-year than reprocessed waste.
Treatment of Radioactive Waste for Disposal
The recommended treatment of mill tailings has already been discussed. The same considerations ought to govern the treatment of small quantities of alpha-active waste, and indeed we consider it reasonable to define the term “small” to be that quantity that could be blended with mill tailings without noticeably increasing their radioactivity. In fact, a considerable amount of low-level wastes might actually be disposed of with mill tailings.
The more concentrated wastes require separate treatment. Cladding hulls, reprocessing-plant solids, and medium-level solid wastes from reactor operations are all representative of materials that can be (and have been) treated by encapsulating them in drums and filling the drums with asphalt, cement, concrete, or thermosetting plastics. The key to the success of this technique is the low heat generated by these wastes, which permits them to be handled without concern for cooling or ventilation.
Cladding hulls, which are voluminous, are generally compressed and stored in water-filled drums. To reduce their volume further and with an eye to eventual recovery of zirconium, which is a valuable metal, various forms of chemical reprocessing of the zirconium are under development. The aim today is simply to get more zirconium into a drum.
It is usually assumed that these miscellaneous materials, in their drums, will be buried underground. The Federal Republic of Germany has already committed a salt formation (Asse) to this use. The same facilities used for alpha-active waste disposal should also serve here. In the case of both alpha-active and medium- to high-level activation products, disposal in a high-level waste repository appears to be a straightforward matter and should not increase the problems of handling the high-level waste.
Radioactive off-gases from reprocessing get variable treatment. At present, only the radioiodines are permanently fixed, as silver iodides. There are few problems with this technique, since most of the radioactivity is in the form of 8-day 131I, which decays into stable xenon, and since 129I, which has a very long half-life (16 million years), is formed at such low yield that it can be allowed to build up indefinitely in the filter bed, until the plant itself is decommissioned.
The gas 85Kr is not trapped. It contributes a small amount to our radioactive background—of the order of 0.01 person-rem/yr. However, if large-scale reprocessing for a large nuclear industry (>1000 GWe) is instituted, it will be desirable to isolate this gas. A thorough analysis of the problem has been conducted at Karlsruhe, in Germany.84 The preferred handling method is isolation by cryogenic distillation, and storage in pressurized steel spheres. If small spheres are used and stored in an underground cavern, even an occasional leak or rupture would not lead to significant radiological hazard.
Tritium and 14C in off-gases represent small additions to natural radioactivity and are rapidly dispersed, mostly in the atmosphere and the oceans, which provide enormous dilution. In consequence, their level of hazard is small, though spread worldwide. They also emit relatively low-energy beta radiation, which reduces their biological consequences, and they have relatively short biological half-lives (mean residence time in vivo), which somewhat mitigates the severity of local exposures.
The environmental inventory of 14C comes from nuclear reactions initiated by cosmic radiation in the atmosphere. It is estimated to be 280 megacuries (MCi)85 and to deliver a whole-body dose of 0.7 person-rem/yr to the average individual. To this, an LWR reprocessing plant of 1500 tons/yr uranium throughput (serving about 50 GWe of reactors) would, if all the 14C in the fuel were released, add 0.000006 person-rem/yr for each year of operation.86 After 500 years of operating such a plant, the dose from this source would be 0.003 person-rem/yr, and if 40 such plants were operating worldwide for that period (a 2000-GWe world industry), the dose from 14C from that source would be 0.12 person-rem/yr. The maturing of a large HTGR industry could, however, increase this dose appreciably. In HTGR’s, 14C is formed from the following reactions (the latter from residual nitrogen in graphite):
Similarly, if all the tritium produced in LWR fuel from a 2000-GWe world industry were released, the dose to each individual would reach a constant value of about 0.03 person-rem/yr after about 20 years; further additions to the environmental inventory would be balanced by its decay. The widespread use of heavy water could significantly increase the quantity of tritium formed, due to the following reaction.
Since the nuclear industry is well below the 2000-GWe level used for the examples above, and since the incremental dose is small compared to
background 14C (0.7 person-rem/yr) and the total background (100 person-rem/yr), reprocessing that is now being practiced releases both 14C and tritium (as well as 85Kr, from which the dose is about the same as from tritium). Research is in progress on methods for trapping even this small amount of radioactivity,87 and since the chemistries of hydrogen and carbon are very well known, processes for trapping at least 90 percent of these effluents can almost certainly be developed. It is expected that these processes will be used ultimately, in accordance with the philosophy of ALARA (as low as reasonably achievable) used by regulatory commissions for effluents from the nuclear industry.
The technology for handling wastes from the reprocessing of spent LWR fuel is relatively well developed in both the United States and Europe. The typical waste is a nitric acid solution of fission products and actinides partially evaporated immediately after discharge from the reprocessing plant to reduce the volume to be stored and to recover nitric acid for recycle. After evaporation, the concentrated wastes—amounting to about 56 gal/ton of spent fuel—are routed to large double-walled underground storage tanks cooled by water and made of stainless steel, where they are held for up to 5 years from the time of reactor discharge.
When the waste has aged to the point that its radioactivity no longer requires strong cooling (less than 5 years after reprocessing), it can be solidified. For example, the Waste Calcination Facility at the Idaho Reprocessing Plant (which reprocesses highly enriched fuels from propulsion and research reactors) reduces the nitric acid solution to a frit (the partly fused state necessary for glass making, or for introduction into ceramics) of oxide granules by fluid-bed calcination. The French reprocessing plants at Marcoule and La Hague carry the process a step further by continuously incorporating the calcined solids into glass cylinders encased in steel cans. The radioactive solids are an integral part of the glass, which has the appearance of an opaque, smoothly glazed ceramic. It is expected that all reprocessing plants will ultimately use either glass or a metal or ceramic matrix as the vehicle for incorporating solidified high-level wastes.
If spent fuel is not reprocessed, it must be stored. To relieve utilities of the responsibility for storing increasing amounts of spent fuel in their temporary cooling ponds, the government proposed in 1977 to accept title and transfer of spent reactor fuel on payment of a one-time storage fee.88 At least for the time being, the stowaway fuel cycle will prevail, and the high-level waste process will involve early storage of discharaged spent fuel in water-filled canals at the reactor site (to provide gamma-ray shielding and a medium for heat dissipation), later encapsulation of the unprocessed assemblies in sealed containers, and delivery of canned assemblies to the government for storage. The waste forms and storage facilities will have to be designed for safe isolation over two or three decades, and for economical recovery of spent fuel.
Status of Technology for Ultimate Disposal in Underground Repositories Several methods have been considered for the ultimate disposal of encapsulated waste, including ejection into extraterrestrial orbit by rockets, disposal on or under the seabed, nuclear transmutation of long-lived actinides, and isolation at depth in suitable continental geological formations. Only the last of these options, using conventional underground mining technology, is believed to be practical in the near future.
Desirable geological properties for an underground repository include absence of groundwater, low permeability, high plasticity, freedom from joints and faults, good ion-exchange capability, and location in an area of low seismic activity. Rock types that exhibit some or all of these desired qualities include bedded evaporites such as salt and potash, marine shales, unjointed and unfaulted crystalline rocks (igneous and metamorphic), and limestone in arid regions.
Since percolation of groundwater is the only significant mechanism for releasing waste forms from their matrix, evaluation of the suitability of an area depends substantially on properly modeling the transport of radioactive atoms once dissolved. The highly active fission-product waste is hardly at issue here. The matrix in which it is incorporated is expected to be at least mostly resistant to leaching over the period (300–1000 years) during which the waste—principally 90Sr and 137Cs—decays away. (But see below for the U.S. Geological Survey’s reservations on this point) The actinides of medium half-life, such as 239Pu (24,000 years), 240Pu (6500 years), and 241Am (450 years), are the critical nuclides. This underscores the significance of dealing properly with alpha-active waste. There is a good chance that if groundwater were to intrude upon the repository, the matrix could be leached out before these nuclides decayed.
Most actinide transport studies that have been conducted indicate that migration in groundwater will be very slow, being governed by absorption-desorption equilibria with solids in the aquifer, rather than solution transport mechanisms.89 This could be the controlling process in return to the biosphere, and indicates that the ion-exchange behavior of the disposal environment for actinide ions is the most important selection criterion. The resistance to leaching of the glass and the absence of groundwater serve only as “insurance” factors.
This question has recently been reviewed by the American Physical Society (APS) and the U.S. Geological Survey (USGS).90 Although the tones of the reports are different, the APS being generally optimistic and the USGS emphasizing reservations, our study of these documents indicates that their findings are similar. In both cases, there is confidence, primarily based on past experience with radium as a natural tracer of mineral migration in groundwater, that a site chosen with reasonable care will provide the necessary holdup of waste radioactive nuclides. Both reports
emphasize the importance of improved characterization of the geochemistry and chemical hydrology of the chosen site. The USGS suggests that the geochemical reactions that might occur in the presence of the heat sources from high-level waste might rapidly alter the form of that waste. By inference, the authors are more optimistic about the chances that properly encapsulated alpha-active waste (which produces far less heat than high-level waste) would remain intact. Both sources, finally, believe that underground emplacement in mined salt cavities is not necessarily the best method of geological isolation, and they particularly recommend that emplacement in deep drill holes, and in such conventional rocks as granites and basalts, be reevaluated. Interestingly, a very thorough study by the Swedish commission appointed to study the question91 has selected temporary storage (for decay of the heat production), corrosion-resistant jacketing (to hinder geochemical reactions), and ultimate emplacement in granite as the preferred method.
The drilling and mining operations required for waste emplacement are considered to be conventional technologies.
Three other problems, which we believe to be quite minor, have also received attention. One of these concerns the absolute dedication of disposal sites to that purpose, foreclosing further exploitation of subsurface minerals, geothermal energy, and so forth. We consider that any reasonable characterization of a site would confirm the presence or absence of mineral deposits of high value, that the foreclosure of mineral exploration at an unpromising site would be readily accepted, and that the monitoring of a site to confirm adherence to regulations against drilling would be easy.
Another problem concerns the protection of the underground emplacement from surface-water seepage, and the parallel problem of protecting the surface against escape of radioactivity through the bore holes. As we understand these problems, they are within the capabilities of sound practice in mining engineering, and the sort of leakage that could be expected would be minor.
A third problem is that of designing the underground repository in such a way that it does not disrupt its geological milieu. The problem seems similar to that involved in drilling deep tunnels, a technology in which, again, standards of good practice are well established. In some settings, however, this problem may cause deep drilling to be a preferred technique over excavation of a mined cavity; for example, when the integrity of the rock above the cavity is uncertain.
The Nature of the Waste Disposal Hazard An informed public response to the hazard presented by stored radioactive waste must begin with a qualitative understanding of its nature. Because hazards from high-level
reprocessing wastes are potentially much larger than those from other parts of the nuclear industry, we use them for illustration.
A basis for conceptualization can be found in an estimate of the consequences of simply abandoning the accumulated high-level wastes at the Savannah River plant.92 This estimate was prepared to quantify the base case against which various waste treatment and disposal techniques could be evaluated for incremental costs and benefits. Such abandonment, with no precautions taken against tank corrosion and seepage of the waste, is estimated to result in the eventual delivery of 620,000 person-rem to a surrounding area population of 70,000 people. Criticisms of this report have shown that the risk is underestimated, particularly with regard to persistent radiation in the water and soil. It is nevertheless true that such cavalier treatment of the waste should be a wild upper limit to the hazards. This particular limit is not much greater than the lifetime dose to the same population from natural background, and the expected consequences (most importantly, 100 extra cases of cancer, compared to a normal incidence of about 10,000 cases) in the population affected would be tragic but not catastrophic.
The Savannah River plant has turned out a quantity of high-level radioactive waste over its lifetime that is large in absolute terms, but small compared to the product of a civilian reprocessing plant. The plant size usually considered to be of full commercial scale would handle 1500 tons of spent fuel per year, the annual throughput from about 50 GWe of LWR’s. The fission products handled would be many times greater than at Savannah River, and there would be orders of magnitude more plutonium and higher actinides in the waste. However, the waste would be relatively quickly converted to a solid, rather than stored indefinitely as a liquid, and we are concerned here with its hazards after solids have been emplaced underground. These extra steps offer orders-of-magnitude reductions in public exposure that counterbalance the increases in scale, so that the results for Savannah River remain an easily improvable upper limit.
The APS study93 considered a large number of ways in which this extra level of protection might be negated. The natural forces of tectonic activity (earthquakes), volcanism, erosion, and meteoritic impact can be ruled out in any well-chosen site, at least to the extent that such events might bring wastes back to the biosphere by physical movement. Similarly, the effects of random anthropogenic activity, such as drilling, surface blasting, and so on, would not be a credible threat to the integrity of emplacement, and sabotage after emplacement would be virtually impossible. The only event that needs to be considered seriously is transport of radioactive materials by groundwater. Groundwater could exist in the repository as a result of poor choice, or could enter the repository as a secondary consequence of other catastrophes. The chain of events would be this: intrusion of the
water; contact of water with waste bodies; dissolution of radioactive material; transport by the water through the host rock, and finally delivery (still by water) to the surface in springs or seepage outlets. The modeling of the various steps is an imperfect art. There is a very wide spread in estimates of transport times and amounts and concentrations of the radioactivity delivered.
Nevertheless, some limiting considerations can be applied. First, the process of leaching and transport does not produce highly concentrated solutions of radioactive material; rather, the conditions that favor rapid water transport also favor dilution of the material. These conditions include high permeabilities of the rock, and they are associated with rapid flow rates. Thus, any rapid delivery of radioactive waste to the biosphere would be at low concentration, and the consequences would be expected to be measurable as small increases in background radiation over a large area.
The conditions just outlined are quite rare. More common is a condition of naturally low flow rates of water through small pores. The water percolates, rather than flows. This condition favors an ionic absorption-desorption mechanism as the detailed transport phenomenon. A limiting case would be the chromographic process, in which such mechanisms permit certain ionic species to migrate in a concentrated “band” at a lower rate than the water. These processes are many times slower than ordinary convection—slow enough that they are only significant for longer-lived radioactivities (if applicable to shorter-lived species, the transport time becomes many half-lives, and the radioactivity decays en route). Thus, only the longer-lived radioactivities, primarily actinides and a limited number of fission products such as technetium-99 (99Tc) or 129I, present any possibility of reappearing in the biosphere in concentrated form, and such reappearance can be expected to occur in the very far future (millennia to eons).
Thus, the nature of the public hazard lies between two extremes: a relatively widespread, highly diluted reappearance of fission-product activities, and a much delayed, relatively more concentrated reappearance of actinides and a few fission products of long half-life. The consequences of this reappearance would in both cases mean an increase of environmental radioactivity, either in the form of an increase in background radiation over a large area, or in the form of pockets of radioactive materials. In the former case, the results of this increased radioactivity might be seen as similar to those from the fallout of distant nuclear weapons tests. In the latter case, they would be similar to those arising from natural concentrations of radiaoctive ores. A widespread increase in background radiation is not necessarily negligible in its public health implications, but is nevertheless a very small risk to any individual. An addition to surface concentrations of long-lived radioactivity would not affect a considerable
number of people in any generation. It is only by adding these “occasional” effects over many generations that a large total effect could be inferred, and this would persist only as long as the radioactivity remains unidentified and therefore unabated.
To estimate the effects of this latter possibility, one study94 considered “abandoned” wastes from the Idaho Chemical Processing Plant. The wastes are stored as solids in steel bins contained in concrete bunkers just below ground level. The worst exposures to individuals from a variety of pathways, including radon emanations from the bins, intruders digging superficially at the site, or settlements and farms appearing on the site, amounted to less than 5 rads per individual, or a doubling of the lifetime dose exposure to natural background radiation. The frequencies of exposure to such doses were estimated to be of the order of a few individuals per century, or less.
Whatever risk exists from release of radioactive waste stored in repositories can therefore be characterized qualitatively as one that is very small to any individual, but in the worst cases, as one that may be quite persistent or broadly diffused. It is definitely not a catastrophic risk in the usual meaning of that term.
Some Social and Institutional Considerations Understanding the problems of nuclear waste management requires discussion of more than the technology. The decisions to be made are principally of a social nature, such as how safe is safe enough? How should questions of equity be settled when the benefits are widely distributed but the wastes are disposed of in one (or a few) places?
The whole matter has received a great deal of public attention since about 1971, but little before. Present difficulties have three main causes: the generally negligent and uncommunicative attitude of the Atomic Energy Commission (AEC), even up to its dissolution; continuing federal indecision about the acceptability of nuclear power; and an unfortunate mistaking of goals for working policy. The management of radioactive waste has been a political issue, which itself has several features.
Regarding the historical role of the AEC, we have already discussed briefly how the handling of nuclear waste in the weapons program led to severe difficulties which are, however, of a nonrecurring type. The unfortunate technical choices were at the same time accompanied by the practice of holding information about the weapons waste program to a minimum, sometimes justified on grounds of national security, sometimes not. The AEC was at the same time declaring the nuclear waste problem to be tractable and straightforward, and was also deluding itself that an abandoned salt mine in Lyons, Kansas, originally selected only for nonradioactive experiments, was ideally suited as a permanent repository,
despite the presence of solution mining in the same salt bed less than 2 miles away, and despite the many unrecorded drill holes throughout the strata. When all these matters came to light, the AEC and its successor agencies lost credibility that they have not yet regained.
Regarding the second cause, federal indecision about the long-term acceptability of nuclear power seems to be amplified in continuing indecision and changes in goals for handling nuclear wastes. First, the distinction between the problems posed by weapons wastes and those of commercial wastes have never been adequately emphasized publicly, nor has an adequate working policy been laid out for either. On the contrary, goals have been stated (e.g., a demonstrated repository by 1985), as if they themselves were the policy being carried out, and the distinction has been ignored.
Regarding the third point, the issue is naturally—almost ideally— political. Storing the wastes anywhere involves questions of equity, of local political jurisdictions, and of local acceptance. The difficulty is acute, considering that hazards with much larger social costs are routinely accepted merely because they are distributed. Many other much larger social costs are inequitably borne (e.g., coal-mining hazards) mainly because the problems are less recent and fashionable.
The quality of the information circulated to the public about the nuclear waste problem can only be described as abysmal. Indeed, misinformation is rife. At one extreme, many nuclear proponents have claimed that the waste is less radioactive than the ore after 500 years, a statement that is simply not true. At the other extreme, opponents have raised spectres of 250,000-yr hazards without a hint of their minute magnitude, or of the minute consequences of such hazards.*
Discussion
Before presenting our conclusions and recommendations, a few further points of discussion are necessary.
Until about 2 years ago, all plans for waste disposal anticipated that the fuel from LWR’s would be reprocessed and that the resulting high-level wastes from the reprocessing plants would be solidified and encapsulated in glass or ceramic matrices for geological storage, most likely in salt formations. For many years, waste disposal was treated by government and industry as a problem that could safely be postponed, since it appeared to have a number of reasonable technical solutions. In the meantime, spent fuel from existing nuclear plants was temporarily stored in water basins
|
* |
See statement 5–32, by L.F.Lischer, Appendix A. |
adjacent to reactors, pending reprocessing. The amount of waste to be handled was modest and did not present a problem that was viewed as urgent by the responsible technicians. Public distrust of this approach, however, was exacerbated by difficulties with the weapons wastes, already described, and was further increased when official statements continued to change in response to criticism.
Regardless, it is important to emphasize that waste management, unlike reactor safety, diversion, and proliferation, does not present hazards of large consequence. The risk is of slow leakage due to unforeseen contact with water and consequent exposure of people to low levels of radiation.
Finally, we must point out that the most general sorts of geological and geochemical knowledge, on which predictions of geological repository performance are based, cannot be expected to improve in the foreseeable future. To expect confirmation by experiment of expectations for integrity beyond 1000 years is simply impossible.
Our own conclusions and recommendations are essentially identical with those reached by the American Physical Society’s study group on nuclear fuel cycles and waste management, with regard to the feasibility of radioactive waste isolation.95 Among other points, the study group notes that waste isolation is feasible in salt and other media; that detailed technology for waste solidification, encapsulation, transport, and emplacement in mixed salt caverns is within the scope of existing knowledge; that confidence in geological isolation arises primarily from limitations on the rate of ion migration in underground formations; that continued investigation of geological and geochemical transport modeling is the most important current research topic; and that unreprocessed spent fuel should not be considered as waste, at least at this time.
Our own comments follow up these conclusions. While it appears that adequate technical solutions to radioactive waste disposal exist (e.g., geological disposal), the implementation of a program will require overcoming several political and institutional barriers. The foremost of these barriers is misunderstanding by the public of the nature of the problem. As evidenced by local hostility in many places to investigation of sites, it appears that the public is under the misapprehension that waste management poses local, high-intensity risks, rather than (at worst) widespread, low-intensity risks.
A second barrier is the failure of the government to agree which agency is responsible for setting standards—the Department of Energy or the Nuclear Regulatory Commission. Thus, standards (which can be set) are still pending.
A third barrier is the widespread opinion (at least in government) that a demonstration facility is necessary before a working repository is engineered. This seems to have led the public to conclude that there remains
some significant doubt that waste disposal can be engineered on the basis of existing knowledge and site-specific investigations. This leads to the paradox of requiring proved demonstration in advance of establishing the facility. Arguments over what is meant by “demonstration” could be used to delay action for many years. Finally, there is an unnecessary emphasis on waste retrievability, which leads (again) to an impression of uncertainty and also raises costs.
Even if civilian nuclear power were to disappear, there would be a substantial radioactive waste inventory from military programs. Efforts should be instituted as soon as possible to develop and act on a workable program to manage military wastes. At Hanford alone there are about 200,000 m3 (47 million gal) of high-level wastes, containing well over 200,000 tons (mostly sodium salts) of solids and over 200 Mci of radioactivity, primarily longer-lived fission products.96
Solving the political and institutional problems connected with management of this waste would be an important step forward. The chief problem seems to be devising a method of balancing federal and state interests in such a way that legitimate concerns are adequately addressed. The current policy, which gives localities and states arbitrary veto power, seems to be unworkable because local opinion has proved particularly vulnerable to scare tactics.
The potential for the future development of improved methods of dealing with radioactive wastes should not be ignored. Further research and development could be very valuable, while not impugning the validity of whatever short-term decisions are made. (An analogy may be made with enrichment. While the gas centrifuge process appears to be superseding the gaseous diffusion process, this does not mean that the latter is not a good process.)
Conclusions and Recommendations
Our recommendations follow from these observations and address primarily the institutional problems.
-
The nature of the risks from geological disposal of nuclear waste must be clearly spelled out and vigorously publicized. The risks are those of chronic, dispersed, low-level radiation and are not comparable, for example, with risks from catastrophic reactor accidents.
-
The federal government should immediately proceed to set criteria for geological waste disposal. These should be performance criteria (leach rates, heat rates) for waste forms in categories that recognize the risks from different types of wastes, and site criteria (groundwater standards,
-
seismic stability, resource and mining restrictions). The Nuclear Regulatory Commission has this responsibility, which is appropriate.
-
The problem of disposal must be separated from the problem of spent fuel storage.
-
The weapons waste problem must be settled, and the issue separated from that of commercial wastes. It may well be that long-term entombment is appropriate. If so, it should be effected. We note again that this waste is mostly fission products, and that its period of high risk is therefore relatively short.
-
The federal government should accept full responsibility for any waste in existence, leaving the question of joint state-federal responsibility to be resolved for wastes generated in the future.
-
Standards must be set and enforced for the treatment of abandoned mines and of tailings from mines and mills. These standards should permit disposal of low-level alpha-active wastes (i.e., alpha-active wastes that, if blended with the tailings, would not significantly increase the risk from tailings) in tailings piles.* This will require collaborative effort between the federal government and the uranium-mining states.
-
While retrievability of waste forms after emplacement is a desirable feature of a test facility, and such a facility would be useful for a research program, retrievability ought not to be a consideration in designing a repository for actual waste disposal.
SAFEGUARDING THE DOMESTIC FUEL CYCLE
Atomic bombs are made from fissile material, and fissile material is the fuel of nuclear power. The term “safeguards” is the rubric under which we collect all the measures by which the manufacture of bombs from nuclear fuel materials can be prevented.
An intrinsic safeguard is isotopic dilution. Natural or slightly enriched uranium contains fissile 235U in low concentration, with most of the uranium being 238U. Enrichment is necessary for bomb material: A bomb requires a high concentration of 235U. Since enrichment is a laborious, high-technology operation, now carried out in national or international facilities, stealing low-enrichment uranium would not be of much use for making bombs.
Another intrinsic safeguard is radioactivity. Spent reactor fuel is so radioactive that it must be handled under water or behind thick shielding. Most such fuel contains plutonium or 233U, both of which are also bomb
|
* |
Statement 5–33, by J.P.Holdren: See my statement 5–31 and my longer dissenting view, statement 1–36, Appendix A. |
materials, but to separate these fissile isotopes, a high-technology reprocessing operation must be carried out. While technically simpler, this process is even harder to carry out secretly than enrichment. Reprocessing releases radioactive gases that are readily detected at a considerable distance.
Spent nuclear fuel is a material of potential value. Recovery of its fertile material—thorium or low-enrichment uranium—permits substitution of recycled chemical elements for material that would otherwise have to be mined. Of greater value is the fissile material it contains: 233U when thorium is the fertile material, 239Pu when uranium is the fertile material. The recycle of this material in breeders or advanced converters could markedly reduce the need for 235U from nature. However, both 233U and 239Pu can be made into bombs. Therefore, if they are to be recycled, they must be safeguarded from the time they are recovered from the spent fuel to the time they are inserted as recycled fuels into a reactor.
REPROCESSING
Reprocessing is the key operation that triggers concern about safeguards. The reference process for uranium-rich fuels is known as Purex, and for thorium-rich fuels, Thorex. They have similar characteristics. In both cases, fuel pieces from power reactors are chopped up or chemically declad, to expose the fuel material, and the fuel material is dissolved in acid. Uranium and plutonium (or thorium and uranium, in Thorex) are extracted by a solution of tributyl phosphate (TBP) in dodecane (a light paraffin oil). The TBP forms a complex chemical compound with the heavy elements and remains dissolved in dodecane, which is immiscible with the original acid solution. The fission products remain behind in the acid, which now is high-level waste. After separating the solution of dodecane and TBP, the heavy elements are washed (stripped).
The result of reprocessing can be either separated uranium and plutonium (or uranium and thorium) or mixed fuel materials.
The plants in which reprocessing takes place are very heavily shielded, and until the purified product is isolated, the physical barriers of the shielding and the intense radioactivity of the material make diversion essentially impossible. The type of construction needed also makes it convenient to provide very secure vaults for storage of the product material. Therefore, concern about safeguarding the reprocessing operation centers on prevention of “inside jobs” that might lead to diversion of the final product. The methods used for control of this product include materials accounting (i.e., checking that fissile material brought into the plant is either in process or has been shipped to a legitimate user) and personnel screening. However, the chief safeguard is security: limitation
and control of access (as with vaults for precious materials); multiple checking of shipment authorizations and deliveries to shipping; and, since the product fissile material is still quite radioactive, multiple radiometric monitoring of access points and the personnel using them.
Radioactivity of Reprocessed Fuel
Recovered uranium from slightly enriched reactor fuel has more 236U than natural uranium, but after purification from fission products and plutonium, it can still be handled essentially as virgin material. Recovered thorium is highly contaminated with 228Th and its radioactive daughters. It must be stored for 10–20 years before it can be reused. By that time, the activity from 228Th will have decayed to its low natural level.
The plutonium produced from 238U and the uranium produced from 232Th are much more radioactive. Typical plutonium recovered from spent LWR fuel contains 3 percent 238Pu, 57 percent 239Pu, 23 percent 240Pu, 12 percent 241Pu, and 5 percent 242Pu. After several recycles, concentrations might be 5 percent 238Pu, 31 percent 239Pu, 27 percent 240Pu, 17 percent 241Pu, and 20 percent 242Pu.97 Representative numbers from thorium cycles are less firm, since there is far less experience with these fuels. The main radioactive constituent of 233U, however, is 232U, and it has been calculated that 233U from HTGR irradiation could have 300–1000 ppm (i.e., 0.03–0.1 percent) of 232U.98
Each kilogram of the mixture of plutonium isotopes coming from spent reactor fuel has typically over 600 curies (Ci) of alpha activity and more than 10,000 Ci of beta activity. (One curie is 3.7×1010 disintegrations per second, the radioactivity of 1 g of purified radium.) While these radiations are readily shielded by relatively thin containers, they are accompanied by penetrating gamma rays and neutrons. A kilogram of reactor plutonium emits 3×108 gamma rays per second with energy greater than 300 keV, considered “hard” gammas, and 3×105 fast neutrons per second from spontaneous fission of 240Pu. These radiations deliver a dose of about 140 rads/hr, on contact (as in a pocket), that is dangerous and easily detectable, even with shielding. Only a minute quantity could escape detection unless lead or other heavy shielding is used in its removal, and these materials are readily sensed by other means.
Freshly reprocessed 233U has less radiation; its alpha activity, mostly from 232U, is only about 30 Ci/kg, and the penetrating gamma radiation that accompanies it is one tenth that of plutonium. This is probably detectable in large quantities. However, it may be desirable to “age” 233U in order to increase its detectability, as illustrated below.
If the fissile material is not used after reprocessing, its short-lived isotopes (238Pu, 241Pu, and 232U) begin to decay. From plutonium, the
principal new radioactive constituent is 241Am, the product of 241Pu beta decay. After 3 months, gamma radiation from americium starts to contribute to the gamma-ray field surrounding plutonium, and after about a year, it almost doubles the emission of the most penetrating gamma rays (over 600 keV). Thus, aged plutonium is slightly easier to detect than freshly reprocessed material.
The situation with 233U is more dramatic. Decay of the accompanying 232U produces, in turn, 228Th (1.9-yr half-life) and all the short-lived daughter nuclides, of this isotope, which quickly come into radioactive equilibrium. These daughters include some very hard gamma-ray emitters, such as thallium-208 (208Tl), lead-122 (122Pb), and lead-212 (212Pb). After about 1 year, the alpha activity from these decay products is several hundred curies per kilogram of 233U, and the penetrating gamma radiation is several hundred to several thousand times greater than that from plutonium. Aged 233U is radiologically self-protected against diversion.
REPROCESSING ALTERNATIVES: DENATURING URANIUM-233, CIVEX FOR PLUTONIUM, AND OTHER DEVICES
Since reprocessing is the fuel cycle step in which potentially weapons-usable material is isolated, ways of achieving reprocessing while supplying extra technological safeguards would be very desirable. The principal approach for 233U is “denaturing”: blending in enough 238U to make isotopic separation necessary to recover weapons-usable material. The principal approach for plutonium is to increase its radioactivity so that further, highly shielded reprocessing would be necessary to convert it to weapons-usable material.
Denaturing of 233U is readily performed by mixing a quantity of depleted uranium solution in the fuel-dissolution stage of thorium fuel-element processing. The isotopes of uranium behave identically in the chemical process. After separation of the uranium from the thorium and isotopic analysis of the separated uranium, further blending of the product would finally yield 10–12 percent concentration of 233U. This is a concentration at which a nuclear weapon would be so large and awkward as to be essentially impractical.
The chief drawback with denatured 233U is the effect of depleted uranium (238U) on nuclear fuel cycles. The 238U substitutes for thorium, so that denatured fuel is, chemically, largely uranium. For example, the nuclear equivalent of 3 percent 235U in slightly enriched uranium, such as that used in LWR’s, is about 6 percent 233U in thorium. If 233U is used in denatured (say, 12 percent) concentration, almost 40 percent of fuel material would be uranium, and only 60 percent would be thorium. Almost 30 percent of the new fissile material produced would be
plutonium, which is a less desirable fissile material in thermal reactors. A heavy water reactor would be markedly superior in this regard, since it would require less fissile material in its fuel, and a feasible loading might be of the order of 15 percent uranium at 12 percent 233U concentration, and 85 percent thorium. Only 10 percent, or less, of the new fissile material would be plutonium.
A second effect is that the concentration of 233U in spent fuel is less than 12 percent of the uranium. In order to recycle the fissile material, either more highly enriched uranium must be added, or the reactor must be reloaded even more heavily with uranium in subsequent cycles. The preferred method would be to “re-enrich” with 20 percent 235U in 238U, which is also undesirable for weapons (the critical concentrations of 235U are higher than those of 233U in assemblies of the same dimensions). Ultimately, however, unless the reactor is virtually a breeder, this will also lead to a “mostly uranium” fuel.
Denatured fuel cycles require further study before definitive conclusions can be drawn. The heavy water reactors, HTGR’s, and LWBR’s appear the most likely candidates for denatured fuel application.99 The denatured Th-U cycle does not appear attractive for conventional LWR’s.
For plutonium, a recently proposed alternative is the Civex cycle.100 In this cycle, the uranium and plutonium are extracted together, and enough fission products are intentionally carried along with them that the product is still highly radioactive. Only a small sidestream of separated uranium is permitted, to bring the plutonium concentration of the product to reactor-usable levels. Coextracting some of the fission products along with the fuel materials actually simplifies the flow sheet of the Purex process. In principle, it can be accomplished using less careful purification of the tributyl phosphate. TBP is partially decomposed by the intense radiation of the reprocessing environment, and its decomposition products extract trivalent elements such as zirconium, lanthanides (rare earths), and higher actinides.
These elements include several fission products with sufficiently high activities to make the product material “hot” enough to be disabling, yet with half-lives long enough to persist. However, the lanthanides include a number of nuclides that strongly absorb low-energy neutrons. As a result, the Civex cycle has only been proposed for recycle of plutonium in fast reactors. Other flow sheets, rejecting lanthanides but retaining zirconium, other transition elements, and (preferably) strontium and cesium, would be needed for “activity-protected” thermal fuel recycle.
A related idea is to denature plutonium with 238Pu. Because of its high rate of radioactive decay (half-life: 88 years) through alpha particle emission, this nuclide produces a great deal of heat (for which reason it is used as a heat source for remote power systems and, in very small
amounts, for implanted cardiac pacemakers). If the concentration of 238Pu (in plutonium) is above about 5 percent, any large pieces of weapons material would have to be cooled, or they would at least deform, and (if metal) possibly melt. Such material would be very unattractive, even for use in a national nuclear weapons arsenal, and would be virtually forbidding as weapons material for a subnational diverter. It could, however, be used as reactor fuel.
The 238Pu would be manufactured by mixing a substantial quantity of neptunium-237 (237Np) with the first recycle of plutonium fuel from LWR’S and subsequently coprocessing neptunium. The nuclear reaction is the following.
(In the discussion that follows we subsume this concept under Civex for brevity, although its originators are different from the originators of the Civex concept.)
Many in the nuclear industry question Civex on the grounds that it would require remote fuel fabrication. Today’s fuel elements are manufao tured by processes that include a variety of direct manipulations, but this would not be possible with highly radioactive fuel material. However, we consider that the development of remote fabrication processes is inevitable. Recycled plutonium already demands some shielding, and aged 233U requires either reprocessing (to remove 228Th) or remote handling. Development of remote, automated inspection equipment is already quite advanced, and its use would obviate the direct inspection steps that figure among the main reasons for human intervention in the fabrication process.
Moreover, remote fabrication would, in our opinion, eliminate one more diversion route; the fewer the hands in the pie, the fewer the possible number of sticky fingers. Finally, the Civex cycle requires colocation of reprocessing and fabrication facilities to minimize shipment of radioactive material, and this is a highly desirable antidiversion practice in itself.
However, it must also be noted that wastes from the fabrication of Civex fuel (in contrast to the alpha-active wastes from fabrication of ordinary nuclear fuels) would be both alpha active and high-level. Minimizing population exposure from such wastes would require meticulous process design and operation.*
Finally, to complete the picture of technical measures that have been proposed to discourage diversion, we might mention the suggestion (from Manson Benedict) that conventionally recycled fuel elements be irradiated
in a research-type reactor before shipment. A relatively short irradiation period, of the order of 1 week, would be required. This would build up, in situ, a fission-product inventory with sufficient radioactivity of a persistent nature to obviate diversion of fresh fuel in shipment
All of these measures require further research and development: for denatured 233U, mostly analytical studies to follow the process in specific reactors, but also further investigation of Thorex reprocessing with heavy uranium loadings; and for Civex, technological development of both the reprocessing and fabrication steps. Even pre-irradiation would require some special reactor development, albeit of a straightforward nature. Moreover, standard security measures may be sufficient to guard adequately against diversion in the United States; if so, the added security of such technical safeguards as denaturing and Civex might be very costly and of small incremental value.
HIJACKING AND THEFT IN STORAGE AND SHIPMENT
If separated plutonium or fissile uranium is the end product of reprocessing, transport of this material between reprocessing and fabricating plants presents a major period of vulnerability to theft. For security, these two plants could be colocated in a guarded fuel cycle park. Colocation would be convenient, since rejected material from the fabrication plant must sometimes be returned to the processing plant for recovery of fissile isotopes.
Shipment of fabricated reactor fuel to the reactor plant and management of the fuel before loading into the reactor pose the greatest diversion hazards. The fuel must be loaded onto a vehicle for shipment; the vehicle could be hijacked, misplaced in railroad systems, or waylaid.
Some maintain that shipments of nuclear fuel must be accompanied by unusual security measures. These measures include lead and follow vehicles with guards, air surveillance of the shipment, and super safes to contain the material. Safes might be tagged with radioactivity for easy location by air surveillance. Aerial radiometric surveillance has been used to locate lost gamma-ray sources. Proponents of reprocessing point out that similar security measures have been successful in the far more dangerous transportation of nuclear weapons, and that the attendant military presence has been unobtrusive. The expense of these measures would contribute little to the cost of nuclear power.
Fortunately, reactor fuel comes in large pieces that cannot be moved without the cooperative interaction of several people to operate cranes, hoists, and fueling machines. In view of this, sabotage is generally considered a greater threat to nuclear power plants than diversion.
Security measures are generally acknowledged to be effective deterrents
to theft under two conditions. First, the protective force available must be sufficient in numbers and equipment so as to resist effectively the maximum attacking force that could be expected in the case of overt theft. There is some disagreement as to how large such an attacking force might be, but a report of the Nuclear Regulatory Commission suggests that an on-duty force of about a dozen guards, properly equipped and with reinforcements available, would be sufficient to protect a nuclear facility such as a reprocessing plant.101 This is judged to be a competent group to defeat a small attacking force or to delay a large one until reinforcements could be brought in.
The other condition is that the security force and key operating personnel (particularly, managers and professional employees) be loyal to security. Indeed, preventing “inside jobs,” particularly those of a covert nature, appears to be a major concern of much of our existing policy. For example, there is considerable emphasis on materials accounting as a safeguards measure. This is a tool specifically to detect covert theft Security against dissident personnel depends on personnel clearance, separation of function control over detailed responsibilities, and management integrity to enforce controls.
Some argue that these measures might or might not work. There has been insufficient critical discussion of their efficacy. Even in the domestic weapons program, there have been incidents of lax security. Security measures tend to deteriorate with time. In a turbulent society, the rate of attempts might be quite high—higher, for example, than the rate of attempts on payroll trucks—in view of the enormous possibilities for blackmail presented by nuclear weapons. Concern has been expressed that the measures necessary for dealing with such a threat might erode civil liberties. Others find these expressed concerns to be contrived and exaggerated.
CONCLUSIONS
The subject of nuclear power security is evolving, and it is difficult to reach a firmly based conclusion on the adequacy of measures proposed or the threat they might pose to civil liberties.102
Levels of security now used or proposed are not unusual in our society. Some degree of personnel clearance for employment is normal for operations involving access to precious or dangerous materials, and voluntary citizen cooperation with police activities is high enough on matters of great public risk that civil liberties are rarely compromised. The point at issue is therefore whether measures required in the nuclear power area, particularly when fissile materials are recycled, will reach a
magnitude so great as to convert acceptable security into a choice between insufficient security and unacceptable intrusion.*
It therefore seems premature to us to commit the United States to expensive, additional technical safeguards, such as denatured fuel cycles, Civex, pre-irradiation, and so forth. They deserve further development, because such development might reduce their expense or demonstrate further benefits that would counterbalance their cost. Among the benefits of such development is that of having such safeguards available in the case that other security measurements are either insufficient or socially unacceptable.
Colocation of fuel cycle facilities is an example of a measure likely to lead to both improved economy and increased security. Improved methods of materials accounting also seem to be in this desirable category.
Finally, our conclusions pertain only to the United States. Other countries have different national and social institutions, including different standards of citizen rights against government intrusion. We must therefore expect that different countries will evolve different internal safeguards, and we should avoid judging these safeguards by too detailed comparisons with our own.
NUCLEAR POWER AND PROLIFERATION OF NUCLEAR WEAPONS
The view is increasingly expressed that the most serious liability of commercial nuclear power is the link between this technology and the international proliferation of nuclear weapons. This was the conclusion, for example, of the report issued in 1976 by the United Kingdom’s Royal Commission on Environmental Pollution103 and of a study sponsored by the Australian government to assess the effects of uranium mining in that country.104 In the United States, the concern permeating recent reports on nuclear power was bluntly summed up as follows in a 1977 report sponsored by the Ford Foundation.105
In our view, the most serious risk associated with nuclear power is the attendant increase in the number of countries that have access to technology, materials, and facilities leading to a nuclear weapons capability…. If widespread proliferation actually occurs, it will prove an extremely serious danger to U.S. security and to world peace and stability in general.
The broad spectrum of views held by informed analysts results from the complexity of the issue. Proliferation is not one problem, but the intersection of many. The motivations of nations to acquire both nuclear reactors and nuclear weapons are as much an issue as the technical means, and those motivations arise from concerns for the security and independence of energy supplies, trustworthiness of military alliances, regional antagonisms, disparities in arsenals of conventional armaments, aspirations to greater status in the community of nations, and perceptions of rich-poor, big-small, and north-south inequities. It is this complexity and diversity on the motivational side that led one analyst to insist, “There are no simple solutions that are feasible, no feasible solutions that are simple, and no solutions at all that are applicable across the board.”106
How should concern for proliferation influence the use of nuclear technology in the United States and shape the action this country takes to help or hinder the use of nuclear power technologies abroad? Differences of opinion on this issue can be usefully classified by the question that first elicits disagreement in the following hierarchy.
-
Is proliferation very important?
-
If so, is the link between nuclear power and proliferation very important?
-
If so, is the influence of the position taken by the United States on nuclear power very important?
-
If so, what should the United States do?
In what follows we try to state concisely the principal positions held on each of these questions by various parties to the proliferation debate, in the hope that this will illuminate policy alternatives.
IS PROLIFERATION VERY IMPORTANT?
The “yes” position holds that proliferation is important because the more nations that possess nuclear weapons, the greater the likelihood of nuclear war. Countries that do not have nuclear arms cannot use them in whatever conflicts they enter. For a given number of conflicts, the greater the fraction in which one or more parties have nuclear arms, the greater the chance that these weapons will be used.
The “no” position* holds that proliferation may as easily be a stabilizing as a destabilizing force. It would diminish one form of inequality, where inequality is correlated with instability. In a world where many countries
have nuclear weapons, all countries will tread more carefully and there will be fewer conflicts altogether.
The antiproliferation position replies that this view relies too heavily on the universality of rational decision making. What of revolutions in which national stockpiles of nuclear weapons fall into the hands of fanatic groups or unstable dictators? What of miscalculations in the face of perceived threats to national survival? The pro-proliferation or neutralist position responds that there is only one historical example of the use of nuclear weapons in hostilities, and in that instance, only one of the adversaries had them. The U.S.-Soviet nuclear “balance” has been stable for almost 30 years.
An intermediate position of sorts has been stated by Greenwood107: a world of many nuclear weapons states may not be intrinsically less stable than today’s world of a few, but there is danger in the possibility that the rate of spread of weapons will be too fast for political systems and international institutions to make the appropriate adjustments.
There are irreducible uncertainties in speculating about the future behavior of nations. This study takes proliferation to be important and dangerous, not necessarily by virtue of a general relation between the number of weapons states and the probability that nuclear weapons will be used, but in considering that proliferation may occur in particularly fragile regions or at rates too high for institutions to accommodate.
IS THE LINK BETWEEN NUCLEAR POWER AND PROLIFERATION VERY IMPORTANT?
The spectrum of opinion on this question is encompassed by three positions: first, that the spread of nuclear power has little to do with the real prospects for proliferation; second, that the spread of nuclear power is likely to alleviate pressures for proliferation; and third, that the spread of nuclear power is likely to aggravate the proliferation problem.
There is general agreement that the spread of nuclear weapons among nations has been limited up until now by various combinations of the following four factors: (1) good intentions, as manifested by the Non-Proliferation Treaty (NPT) signed by more than a hundred nations; (2) lack of the technical skills required to design and fabricate a reliable nuclear weapon; (3) reluctance to commit the necessary technical and financial resources to this particular task; and (4) lack of the means to acquire the necessary fissile materials, which until recently only a few nations have had the technical wherewithal to obtain. The category “good intentions” includes the case of national policy that open acquisition of nuclear weapons is not in the country’s interest, and cases in which fear of
detection by the International Atomic Energy Agency deters a clandestine nuclear weapons program.
In this context, the link between nuclear power and proliferation rests on the fact that commercial nuclear power programs can spread both bomb-relevant technical skills and bomb-quality fissile materials into countries that formerly possessed neither, undermining two of the four obstacles listed. The spread of pertinent knowledge has already proceeded to the point that access to fissile material almost certainly poses the greater threat.
The various types of nuclear power technologies differ in the ease with which they can be made to yield bomb-quality fissile material. The most sensitive technologies in this respect are enrichment and reprocessing. As indicated in the section on enrichment (in “Nuclear Reactors and Fuel Cycles”), low concentrations of 235U can be “cascaded” to produce the much higher enrichment needed for nuclear weapons. In the reprocessing of spent fuel, fissile plutonium (or 233U) is separated from the highly radioactive fission products in a form that requires no further isotopic separation for use in nuclear bombs.
One argument against a strong link between nuclear power and proliferation holds that nuclear weapons can be manufactured by a number of methods that are far less costly and troublesome than trying to use nuclear fuel cycles. For example, almost any country that sets out to acquire nuclear weapons can expend a few years of effort and a few tens of millions of dollars to build a natural-uranium, graphite-moderated, air-cooled reactor capable of producing a few bombs’ worth of plutonium each year. More ambitious countries could build bigger production reactors with much higher outputs of plutonium, at a cost below that of commercial power reactors with comparable plutonium production. Fuel for reactors dedicated to the production of plutonium is easier to reprocess. Moreover, the isotopic quality of plutonium from reactors dedicated to the production of weapons material can be used more readily to make efficient bombs than can the plutonium from most kinds of commercial reactors dedicated to the economic production of power. Moreover, several different enrichment processes now at various stages of development—centrifuges, nozzles, laser schemes—might become widely accessible in the future, even to countries with no commercial nuclear power programs.
Some counterarguments can be advanced. All enrichment schemes known today are, as far as can be deduced from the open literature, high-technology enterprises. If not spread through nuclear power programs, they may remain out of the reach of many developing countries for years—perhaps long enough to slow proliferation by enrichment. The use of dedicated production or research reactors and modest indigenous repro-
cessing capacity is probably a more troublesome possibility, considering the case of India, but at least two arguments are put forward by those contending that this possibility does little to alleviate legitimate concern about proliferation through commercial nuclear power.
First, production reactors and reprocessing plants of the size readily constructed by most of the countries in question dribble out plutonium, whereas commercial-size reactors pour it out, on the order of 20 Nagasaki bombs per year for each large light water reactor. With a commercial nuclear power program operating, particularly if it includes reprocessing, a country can go from no bombs to many bombs in short order.
Without a nuclear power program, a nation must commit itself to the construction of a reactor and reprocessing plant, or set up uranium enrichment for the sole purpose of making nuclear weapons. Reluctance to make such a commitment or to risk being caught in a clandestine operation has served as a barrier to proliferation. Commercial nuclear power, or more explicitly, national reprocessing or enrichment facilities, lowers the barrier. It supplies the option without the commitment, and by shortening the time between decision and bombs if a country’s intentions change, it enhances the option. The time between commitment and possession of nuclear weapons, the argument goes, is likely the period when a country’s decision makers feel most vulnerable to censure or intervention from their own people, other nations, or international organizations. The shorter the period, the less the risk. In a world of well over a hundred nations there must be some that would not initiate a weapons program from scratch but might succumb to the temptation of having most of the needed ingredients at hand in the form of commercial nuclear facilities and materials.
The principal argument for the view that nuclear power is of less importance than international diplomatic measures rests on the relative success to date of measures to enforce international agreements to limit the spread of nuclear weapons. Since the period immediately after World War II, only three nations have actually been added to the “weapons club.” At the same time, nuclear technology has become common knowledge throughout the advanced and developing world. The spread of nuclear technology has been accompanied by general acceptance—with some outstanding exceptions—of IAEA safeguards and the Non-Proliferation Treaty. The terms of the treaty and safeguards explicitly offer favorable status for nuclear energy development as a reward for nonproliferation, an apparently effective bargain.
The most optimistic view, of course, is that the spread of commercial nuclear power will exercise an influence against the proliferation of weapons greater than the temptation it represents. The argument underlying this position is that poverty in general and shortage of energy
in particular are major causes of tension among nations. By providing a source of energy needed for economic development, the spread of nuclear power reduces this tension. Poor and developing nations cannot be expected to compete with the United States and other industrial nations in bidding for Middle Eastern oil. Widespread intensive use of nuclear power reduces the possibility of a war erupting over access to Middle Eastern oil.
A counterargument on this point is that nuclear power is rather badly matched in scale, energy quality, and other characteristics to the needs of many developing countries. Thus, the spread of nuclear power may narrow the rich-poor gap only slightly—and, if it contributes mostly to the well-being of urban areas, may even widen that gap within some countries—all at the cost of making nuclear weapons more accessible. On the other hand, it can make a major contribution if the availability of power expedites such uses as water pumping to increase productivity of small agricultural holdings, or if power is used to improve rural life, as exemplified by the Rural Electric Association in this country.
As in other questions where perceptions, motivations, and future actions of sovereign states play a role, the effect of the spread of nuclear power on the spread of nuclear weapons defies completely persuasive argumentation for any position. On one point, however, the members of CONAES agree: Stopping the spread of nuclear power, or limiting its evolution to forms considered proliferation resistant, cannot stop proliferation. Countries with sufficient determination will get bombs by other routes. The issue is more a quantitative than a qualitative one: whether the spread or evolution of nuclear power would speed proliferation, either in the number of nations that decide to take the plunge or in the number of bombs they have at any given time. Accordingly, the argument that there is a potentially malign link between nuclear power and nuclear weapons proliferation (which, by implication, should be manipulated if possible to reduce the threat) is most plausibly an argument for buying time, for slowing a process that almost all analysts agree can no longer be completely stopped. And, naturally, buying time will avail nothing if statesmen do not use it to fashion institutions capable of deterring the use of nuclear weapons by however many nations possess them.
IS THE INFLUENCE OF THE POSITION OF THE UNITED STATES ON NUCLEAR POWER VERY IMPORTANT?
Again the spectrum of opinion can be illuminated by considering three answers to this question: yes; yes, but only if the position of the United States is one of continued active participation in the world nuclear power market; and no, regardless.
The “no” viewpoint is perhaps the easiest to state simply. It is basically
that “the genie is out of the bottle.” Nuclear power is going to spread whether the United States likes it or not, since so many nations besides the United States can supply it, and since so many others have valid and legitimate reasons for wanting it. And since the main driving force behind proliferation of weapons is the motivation of countries to possess them, not technology itself, posturing about technology by the United States can only distract attention from the more important issues and thus diminish this country’s influence over the course of events.
The “yes, but…” position argues that only by remaining an active participant in the international nuclear market can the U.S. hope to retain any influence over the kinds of nuclear technologies that are supplied to other countries, and over the kinds of safeguards against proliferation that are exercised. If this country backs out of the export market, no one will listen to advice from the United States about how that enterprise should be managed, a situation that could well worsen if the United States begins to phase out nuclear power at home.
An important component of the “yes, but…” position pertains to the Non-Proliferation Treaty. The NPT is, in the view of many, the most important single instrument available for discouraging proliferation. Accordingly, any position taken by the United States on proliferation must be at pains not to undermine the treaty. Since the treaty requires that weapons states cooperate with non-weapons states in making available the technology for peaceful applications of fission, the United States could not withdraw from the international nuclear market, and perhaps cannot even limit selectively the export of particularly proliferation-prone technologies without weakening support for the treaty among non-weapons states.
The unequivocal “yes” viewpoint—that the United States can exercise influence against proliferation through its position on exports of nuclear technology, on the one hand, and through the character of its own nuclear energy supply, on the other—rests on three propositions. The first is that the substantial share of the world nuclear export market now controlled by the United States is in itself sufficient to give this country considerable leverage in governing what is available for other countries to buy. Other nations could even totally fill orders the United States refuses, but the time new suppliers require is time gained against proliferation. Second, the United States has some influence over other nations supplying nuclear power, in the form of persuasion, in the form of the direct dependence of their nuclear programs on our parts and enrichment services, and in the form of other political and economic incentives and disincentives this country might be prepared to wield in pursuit of nonproliferation. Third, the nuclear power policies of the United States might serve as an example. The United States is still a model that influences the behavior and aspirations of many other countries. Even where the direct force of the
example on other governments is minimal, a strong position taken by the United States against proliferation may undermine the public support in other countries for proliferation-prone policies pursued by their governments, or strengthen the hand of antinuclear movements within those countries.
It cannot be doubted in any case, the argument continues, that if the United States does nothing against proliferation, other countries are likely to do nothing as well. Business as usual in the country with the biggest nuclear industry in the world can only be taken as a clear signal for business as usual everywhere. The more the United States pushes nuclear energy at home, particularly plutonium recycle and plutonium breeders (which are claimed by some to be more subject to proliferation than other technologies), the more convinced other nations will become that they cannot do without these technologies. The counterargument is that heavy-handed use of persuasion and political pressure by the United States to dissuade other countries from deploying certain nuclear technologies may backfire by hardening their resolve and even accelerating their programs.*
The “yes” viewpoint—that positions taken by the United States restricting the export or use of nuclear technologies can slow proliferation—must confront the liability of potential damage to the NPT. Supporters of this view often do so by suggesting that the treaty is not doing as much good as is often supposed, thus the possibility of undermining it should not stand in the way of measures likely to be much more effective. Many nations have not signed it, including some thought to be particularly interested in proliferation, or having signed, have not ratified it. Those that have ratified it can withdraw on 3 months’ notice. The IAEA safeguards provided by the treaty can at best detect diversion, not prevent it, and some have asserted that the IAEA inspectorate is too overtaxed to provide even reasonable assurance of detection. Moreover, it is argued, the sanctions likely to be exercised if a country happens to be caught in violation are too feeble to be effective. Finally, the weapons-states parties to the treaty have themselves already undermined it by not meeting their own obligations, either in making significant progress toward nuclear disarmament, or in giving preferential treatment to other parties in transfers of nuclear power technology.
WHAT SHOULD THE UNITED STATES DO?
The policy options open to the United States in pursuit of nonproliferation can be organized for purposes of discussion in terms of the following hierarchy.
-
Approaches seeking to reduce motivations toward proliferation, versus approaches dealing with the potentially proliferation-related characteristics of nuclear power itself.
-
Among those approaches dealing with nuclear power, seeking increased resistance to proliferation in the characteristics of reactors and fuel cycles, versus developing management techniques and institutional arrangements for nuclear power that act against proliferation.
-
Among the measures in the previous category, elements that are part of purely domestic policies on nuclear power, versus policies on U.S. exports of nuclear technologies, versus other kinds of policies intended to influence the behavior of other nations.
Naturally, the elements in this hierarchy are not mutually exclusive. Many could be pursued at once. In the following subsections, we give brief attention to approaches seeking to reduce motivations, followed by more detailed treatment of proliferation-resistant management.
Motivations
Some of the reasonably obvious ways to try to reduce the motivations driving nations toward acquisition of nuclear weapons are the following.
-
Maintaining, strengthening, or extending security guarantees provided by the United States to certain non-weapons states.
-
Working to resolve or stabilize regional disputes.
-
Reducing the prestige and symbolic importance of nuclear weapons in world politics, including working vigorously for reduction of the nuclear arsenals of the superpowers.
-
Seeking to satisfy some of the economic and political ambitions of certain potential weapons states.
While these approaches seem attractive in the perspective of nonproliferation, many of them have significant economic, military, and political costs. Investigation of these matters was well beyond the scope of this study.
Proliferation-Resistant Management and Institutions It should be apparent from the preceding sections of this chapter that there is no technical
antidote to the possible use of nuclear power for the proliferation of nuclear weapons. We list here the major kinds of management measures and institutional changes that have received serious attention in the proliferation debate, emphasizing the role the United States could play. The following list moves from moderate to drastic measures.
-
Ways to strengthen the NPT.
-
Bilateral (U.S.-recipient country) agreements stronger than the NPT.
-
International control of sensitive parts of the fuel cycle.
-
Barriers to the spread of fission technology or to its evolution in particularly proliferation-prone directions.
For each category, we consider the role of domestic policy, policy on nuclear exports, and other policies.
In principle, the Non-Proliferation Treaty could be strengthened by the following: tightening the safeguards incorporated into agreements concluded under the treaty between the IAEA and non-weapons-state parties; funding more inspectors to enforce the safeguards; adding sanctions to be imposed on violators; bringing the behavior of weapons-state parties into line with the letter and spirit of the treaty. Some of these measures could be accomplished without rewriting the treaty, a tedious and risk-laden procedure.
The United States could contribute expertise and money to achieve other measures, refuse expertise and materials to non-party nations, stop withholding assistance and materials from party nations, use its influence to encourage other supplier nations to do the same, and upgrade efforts to make real progress toward nuclear disarmament. A particularly dramatic—some think dangerous—measure in this last category would be to take unilateral steps to reduce the domestic stockpile of nuclear weapons. Modifying the treaty to add sanctions would probably be very difficult, and the likelihood that the United States would find the benefits worth the required political investment seems low.
The United States could choose, in its own relations with recipient nations as a supplier, to reach safeguards agreements more stringent than those enforced under the treaty. One such possibility is to lease nuclear fuel to non-weapons states rather than selling it, requiring return of the fuel, when spent, for reprocessing or storage without reprocessing in this country. A recipient country could break such an agreement and reprocess a batch of fuel to extract the plutonium, but detection would be assured and it would only work once—perhaps a small consolation. Some domestic opposition to taking back spent fuel from other countries might be expected, on grounds that this would burden us with environmental liabilities from the energy use of other countries. Furthermore, as a
practical matter, the United States (despite positive statements by the Administration and members of Congress) has not acted on any plan to provide adequate spent-fuel storage—even for anticipated domestic needs.
A politically much more difficult, but also more promising, approach to proliferation control is to place the most sensitive parts of the fuel cycle under international control, including enrichment plants, reprocessing plants, plants for the fabricating of plutonium fuel, and shipping links wherein plutonium flows unprotected by accompanying fission products. Breeder reactors using undenatured fuel would perhaps also come under international control. Colocation of many of these facilities in international fuel cycle centers would reduce the problem of surveillance. Dispersed reactors supplied with denatured fuel from these centers and returning their spent fuel to the centers could be under national control. The political and organizational problems connected with this degree of international cooperation are generally considered to be formidable obstacles. Nevertheless, it is possible that a really vigorous effort by the United States to muster support for the approach, also underway in the IAEA, could lead to several pilot examples.
The most drastic category of measures the U.S. might entertain consists of erecting and maintaining barriers to the spread of fission technology and to its evolution in particularly proliferation-prone directions. For any or all of the sensitive technologies—enrichment plants, reprocessing plants, breeder reactors—the United States could refuse export, restrict their domestic use or development, and influence other supplier nations to do the same. Carrying this approach to its limit would require refusing to export reactors or fuel cycle facilities of any kind, and sharply limiting or phasing out domestic nuclear power in an attempt to force the world away from the nuclear option.
Any approach in this category has the liability of undermining to some degree the Non-Proliferation Treaty, as discussed above. These approaches also open the United States to the accusation that this country is insensitive to the needs of countries poorer and less well endowed with energy alternatives than itself, and they run the risk of aggravating non-weapons states into developing unsafeguarded nuclear facilities. Proponents of the “barrier” approaches believe that the risk of aggravating others is one the United States must take, and that necessary accompaniments to the barriers are that the United States must supply a plausible substitute for what has been denied and also begin to exercise some real leadership in nuclear disarmament.
The Administration’s proposal is to guarantee supply of enrichment services for converter reactors as a compensation for the denial of enrichment and reprocessing plants. Countries capable of developing plutonium recycle and marketing plutonium breeders are urged to defer
these steps. The United States would defer as an example. These proposals are seen by some nations as self-serving, inasmuch as they promote what the United States has to offer (enrichment services) while disparaging alternatives (reprocessing and breeders) being promoted by competitors in the world market. The offer to provide enrichment services is questioned on grounds of this country’s record on nuclear policy, and on grounds that the United States is doing little to stretch its own uranium supply.
Certainly it is a liability of these proposals that non-weapons states are not likely to be much more inclined to rely on the United States for enriched uranium than they are to rely on OPEC for oil. This problem may diminish with the development of a strong international market in enrichment services, characterized by a number of independent suppliers. But critics who think the proposals too mild assert that continuing to export reactors themselves will encourage the owners to complete a measure of energy independence by seeking, as quickly as possible, domestic enrichment or reprocessing capacity, or both, and critics who think the proposals too severe believe that resentment of moralizing from the United States on these matters will diminish any influence we might have had in securing better international safeguards for the full range of nuclear facilities sure to be demanded almost everywhere.
If the United States were to go further than the Administration’s proposals by trying to erect barriers against the spread of all nuclear technologies, it seems clear that, for consistency, domestic policy would have to phase out nuclear power, and foreign policy would have to assist other countries with a variety of alternative energy supplies. This assistance might well emphasize “income” energy sources available in particular abundance in some of the poorest regions. But the sorts of trades that would have to be considered in this situation would likely include diverting Alaskan oil and gas to Japan and exporting a good deal of coal mined in the United States. Many Americans might think this too high a price to pay.
CONCLUSIONS
The nature of the proliferation problem is such that even stopping nuclear power completely could not stop proliferation completely. Countries can acquire nuclear weapons by means independent of commercial nuclear power. It is reasonable to suppose if a country is strongly motivated to acquire nuclear weapons, it will have them by 2010, or soon thereafter, no matter how nuclear power is managed in the meantime. Unilateral and international diplomatic measures to reduce the motivations that lead to proliferation should be high on the foreign policy agenda of the United States. Nevertheless, the potential links between nuclear power and
proliferation of nuclear weapons must be taken seriously, both because the rate of proliferation may be increased by the availability of commercial nuclear power facilities, and because the “attractive nuisance” that indigenous stocks of weapons-usable material constitute may nudge some nations, not sufficiently motivated to develop a separate weapons program from scratch, to topple into weapons status.
A minimum antiproliferation prescription for the management of nuclear power is to try to raise the political barriers against proliferation through misuse of nuclear power by strengthening the Non-Proliferation Treaty, and to seek to raise the technological barriers by placing fuel cycle operations involving weapons-usable material under international control Any such measures should be considered tactics to slow the spread of nuclear weapons and thus earn time for the exercise of statesmanship. It is essential that statesmen use this earned time to find ways to end proliferation, to begin to shrink the weapons stockpiles, and to reduce the probability that nuclear weapons will ever again be used.
The question remains whether measures more comprehensive and more disruptive of the nuclear enterprise are warranted by antiproliferation goals. Weighing the often counteracting political and technological considerations outlined in the body of this section, different members of the committee reach different answers.
So many of the political factors, particularly, contain unpredictable elements, that no completely convincing analysis of the likely outcomes of given measures is possible. It is hardly surprising, therefore, that individuals with different perceptions of the likely future behavior of governments, of the incremental dangers of risk reduction associated with given technological changes, and of the likelihood and jeopardy of energy shortages, do not agree whether the United States should try to accelerate or decelerate the use and spread of nuclear power in general and breeder reactors in particular.
We do agree that any proposed policy should recognize the possibility that it is based on wrong judgments, and accordingly, should incorporate escape routes—ways to pull back from a policy decision if evidence accumulates that the consequences run counter to its aims.
NOTES
|
|
|
|
|
3. National Research Council, Alternative Energy Demand Futures to 2010, Committee on Nuclear and Alternative Energy Systems, Demand and Conservation Panel (Washington, D.C.: National Academy of Sciences, 1979); and National Research Council, Supporting Paper 2: Energy Modeling for an Uncertain Future, Committee on Nuclear and Alternative Energy Systems, Synthesis Panel, Modeling Resource Group (Washington, D.C.: National Academy of Sciences, 1978). |
|
|
4. See chapter 11 for display and discussion of study scenarios. |
|
|
5. National Research Council, Supporting Paper 1: Problems of U.S. Uranium Resources and Supply to the Year 2010, Committee on Nuclear and Alternative Energy Systems, Supply and Delivery Panel, Uranium Resource Group (Washington, D.C.: National Academy of Sciences, 1978). |
|
|
6. Atomic Industrial Forum, op. cit., p. 1. This figure includes some reactors not scheduled to operate until as late as 1995. |
|
|
7. Supply and Delivery Panel, U.S. Energy Supply Prospects to 2010, op. cit., chap. 5. |
|
|
8. Assuming that the available resource base is sufficient to provide initial fuel requirements. See, for example P.R. Kasten et al., Assessment of the Thorium Fuel Cycle in Power Reactors (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL/TM-5565), January 1977), p. xi. |
|
|
9. Ibid.; and Alfred M.Perry, “Thermal Breeders in Today’s Context” (Paper presented at the International Scientific Forum on an Acceptable Nuclear Energy Future of the World, Fort Lauderdale, Fla., November 7–11, 1977). |
|
|
10. Kasten, op. cit.; and Perry, op. cit. |
|
|
11. U.S. Atomic Energy Commission, Comparative Risk-Cost-Benefit Study of Alternative Sources of Electrical Energy (Washington, D.C.: U.S. Atomic Energy Commission, (WASH-1224), 1974); L.A.Sagan, “Human Costs of Nuclear Power,” Science 177 (August 1972): 487–493; E.E.Pochin, Estimated Population Exposure from Nuclear Power Production and Other Radiation Sources, Organization for Economic Cooperation and Development (Paris, France: Nuclear Energy Agency, 1976); U.S. Nuclear Regulatory Commission, Final Generic Environmental Statement on the Use of Recycle Plutonium in Mixed Oxide Fuel in Light Water Cooled Reactors, vol. 4 (Washington, D.C.: U.S. Nuclear Regulatory Commission (NUREG-0002, or GESMO), 1976); Nuclear Energy Policy Study Group, Spurgeon M. Keeny, Jr., Chairman, Nuclear Power: Issues and Choices (Cambridge, Mass.: Ballinger Publishing Co., 1977), pp. 173–174. |
|
|
12. U.S. Nuclear Regulatory Commission, Reactor Safety Study: An Assessment of Accident Risks in U.S. Commercial Nuclear Power Plants (Washington, D.C.: U.S. Nuclear Regulatory Commission (WASH-1400), 1975). |
|
|
13. For a complete review of the literature on reactor safety, see National Academy of Sciences, Risks Associated with Nuclear Power: A Critical Review of the Literature, Committee on Science and Public Policy, Committee on Literature Survey of Risks Associated with Nuclear Power (Washington, D.C.: National Academy of Sciences, 1979). |
|
|
14. See, for example, “Report to the American Physical Society by the Study Group on Nuclear Fuel Cycles and Waste Management,” Reviews of Modern Physics 50 (January 1978): S-1–S-185; and National Research Council, Radioactive Wastes at the Hanford Reservation: A Technical Review, Commission on Natural Resources, Committee on Radioactive Waste Management (Washington, D.C.: National Academy of Sciences, 1978). |
|
|
15. It must be recognized that this statement reflects the least common denominator of a wide range of views in CONAES, and is accordingly ambiguous. Some members of CONAES, for example, believe that nuclear energy is the lowest-cost and least environmentally risky technology available for the generation of electricity and should be encouraged to expand as rapidly as warranted by the electricity demand projections of the industry. Others believe that nuclear power is the technology of last resort and its expansion should be restricted to the |
|
|
|
|
|
49. Harvey Brush, testimony before the Connecticut Public Utilities Control Authority, January 21, 1976. |
|
|
50. Gordon R.Corey, testimony before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part I),” 95th Cong., 1st Sess., September 12–19, 1977. |
|
|
51. Charles Komanoff, testimony before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part II),” 95th Cong., 1st Sess., September 20–22, 1977, p. 1186. |
|
|
52. Harvey Brush, testimony before the Connecticut Public Utilities Control Authority, January 21, 1976. |
|
|
53. Irvin Bupp, testimony before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part II),” 95th Cong., 1st Sess., September 20–22, 1977, p. 1401. |
|
|
54. Komanoff, op. cit., p. 1187. |
|
|
55. Lewis J. Perl, testimony before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part I),” 95th Cong., 1st Sess., September 12–19, 1977, p. 661 et seq. |
|
|
56. A.David Rossin, paper prepared for the Commonwealth Edison Company, submitted with testimony of Gordon R.Corey before the Environment, Energy, and Natural Resources Subcommittee, Committee on Government Operations, U.S. House of Representatives, “Nuclear Power Costs (Part I),” 95th Cong., 1st Sess., September 12–19, 1977, pp. 843–884. |
|
|
57. Ibid. |
|
|
58. Perl, op. cit., p. 694. |
|
|
59. Rossin, op. cit., p. 881. |
|
|
60. The Price-Anderson Act (Public Law 85–256, as amended) provides for insurance and partial indemnification of the civilian suppliers and users of nuclear power equipment, |
|
|
61. Atomic Industrial Forum, Engineering Evaluation of Nuclear Power Reactor Decommissioning Alternatives (Washington, D.C.: Atomic Industrial Forum (NESP), 1976). |
|
|
62. M.Levenson and C.P.L.Zaleski, “Economic Perspective of the LMFBR,” unpublished monograph, 1976. |
|
|
63. The Future Development and Acceptance of Light- Water Reactors in the U.S., report prepared by the Energy Lab in collaboration with the Department of Nuclear Engineering, Massachusetts Institute of Technology (Cambridge, Mass.: MIT (MIT-EL-78–035), 1978). |
|
|
64. Committee on Science and Public Policy, Committee on Literature Survey of Risks Associated with Nuclear Power, op. cit., summary and synthesis chapter. |
|
|
65. Decay Heat Power in Light Water Reactors, ANS-5.1 Proposed Standard, American Nuclear Society, June 1978. |
|
|
66. U.S. Nuclear Regulatory Commission, Reactor Safety Study, op. cit. |
|
|
67. WASH-1400 specifically excludes reactor sabotage as a cause of release of radioactivity. This omission has been criticized. We nevertheless concur that the exclusion of sabotage and its consequences is proper. Other energy sources, to which nuclear power must be compared, are not normally evaluated on the basis of sabotage risks. |
|
|
68. The ANS-5.1 Proposed Standard (see note 65) is 20 percent below the previous standard. |
|
|
69. Fission Product Behavior in LWR’s, quarterly report (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL/NUREG/TM-186), 1978). |
|
|
70. National Research Council, Risks and Impacts of Alternative Energy Systems, Committee on Nuclear and Alternative Energy Systems, Risk and Impact Panel (Washington, D.C.: National Academy of Sciences, in preparation), chap. 4. |
|
|
71. Critiques, WASH-1400. |