9
Risks of Energy Systems
Worldwide concern for the protection of public health and the environment has shown remarkable growth in the past 15 years, evidenced in the United States by the passage of landmark legislation, the creation of the Environmental Protection Agency, and the proliferation of regulations to mitigate, for example, the health and environmental risks of energy systems.
This concern is one of many that must be balanced in formulating energy policy. Furthermore, many aspects of this concern are new: Our knowledge of several important risks, as well as our knowledge of how to control them, is recent and incomplete.
Energy policy must be formulated with the knowledge available. Even were such knowledge greater than it is today, difficult decisions would still have to be made. The risks of various energy systems are of different types that cannot all be reduced to common measures. Judgment will continue to dominate these decisions.
The purpose of this chapter is to review the known risks, to indicate the difficulties of ascertaining some of the most important suspected risks, and to recommend both practical courses of action in the face of uncertainty, and steps to improve judgment with better information. Among the major categories of risk considered are those relating to industrial operations, to atmospheric pollution, to shortage of water supply, and to change in climate. For each of these, we have considered the risks posed by energy systems based on fossil fuels, nuclear fuels, and solar energy.
The chapter takes up the nature of risk and the government’s actions to control the risks of energy systems. The risks posed by the major energy
systems to health, agriculture, climate and water supply, and ecosystems are described, and wherever possible, compared. After some discussion, the major findings are summed up in nine conclusions. The reader may wish to read these conclusions first.
RISK
In ordinary language, as well as in this report, the word risk is used in two ways: to convey the possibility (probability) of loss or to denote a dangerous element or factor. This chapter examines the risks associated with the three principal groups of energy systems—fossil fuel, nuclear, and solar—particularly in the generation of electricity, as this provides a convenient base for comparison.
Risks have been grouped by origin in the various steps of each energy cycle, including extraction and processing of the energy resource; its transportation and storage; its use in the production of another fuel (liquid fuels from coal, for example), electricity, or power; the disposal of waste, and finally end-use. (In engineering literature, fuel cycle is usually synonymous with energy cycle, but in official regulatory practice,1 fuel cycle excludes mining, operation of waste disposal sites, and transportation.)
The complete evaluation of risk depends on the nature and amount of the dangerous element or factor (termed “insult” by some environmentalists) and an understanding of how it stresses or interacts with its targets, of how the targets are affected (termed “insult” in medical literature), and of how they react in turn. Such target reactions can then affect other objects or systems.2
The comparison of risks is often simplified by consistent comparisons—similar kinds of risks that arise when different energy systems are employed for the same specific purpose, such as the production of a stated amount of electricity. The matching of risks may be difficult. Consider, for example, the number of deaths associated with the production of 1 quadrillion Btu (quad) of electricity in 1 year from oil or uranium. Practically all cancer deaths due to the use of oil for 1 year would occur within the 30 years following, but those from uranium might be projected to occur over thousands of years. Would 30 deaths in 30 years be better, worse, or equal to 30 deaths in 1000 years? The decision calls for judgment, or for specific sociological information that shows how the bunching of deaths leads to more or less damage than spreading them over the years.
ASSESSMENT OF TOTAL RISK
Owing to the many quite different types of risks incurred in the operation of an energy system, we considered it undesirable to force them into the terms of some arbitrary measure to obtain a sum. Instead (where possible), comparisons were made on the basis of individual, similar risks. Since the facts are frequently insufficient for clear-cut quantitative analysis, estimates of cost and benefit may become highly speculative. This is especially true if sociological data are in question, but as explained later in this chapter, it is also a frustrating problem even in dealing with the chemical and toxicological aspects of atmospheric pollution. The evaluation of total damage, based on all contributing factors, must therefore be a matter of judgment.
Additional judgment must be brought to bear on decisions about “acceptable levels of risk.” In this case, the assessment of cost may have to be considered and should include the consequences of being wrong. Society may (or may not) prefer a larger risk that can be estimated with confidence to one estimated to be smaller, but with great uncertainty.
REGULATION
The federal government attempts to protect public health and the quality of the environment through the Environmental Protection Agency (EPA), the Nuclear Regulatory Commission (NRC), the Occupational Safety and Health Administration (OSHA), and other agencies, based on such acts of Congress and subsequent amendments as the Clean Air Act of 1970 and the Clean Water Act of 1972 (as amended), the Nuclear Regulatory Commission Act of 1975, the Federal Mines Safety and Health Act of 1977, the Surface Mining Control and Regulation Act of 1977, the Energy Supply and Environmental Coordination Act of 1974, and the Resource Conservation and Energy Act of 1976. Some 20 congressional committees deal with this field and compete with one another in producing legislation.3 At a conservative estimate, close to 90 units of the federal government, most of which function independently of one another, set or enforce standards.4
While there is no doubt about the need for regulation, its explosive growth and resulting complexity is bewildering and, on occasion, too costly or perhaps even self-defeating. An example of the complexity may be drawn from the electric utilities industry, where the regulation of risk, though not the only consideration of national policy, is a major factor in determining the 8- to 10-yr lead times for construction of fossil-fueled plants, and the 10- to 12-yr lead times for nuclear power plants.5 It has
been estimated that some 90 permits are now required to open and operate a surface coal mine.6*
Administrative and Legal Aspects
Most of the standards governing the risks of concern in this chapter have been established by administrative action under federal legislation. The administrator of the Environmental Protection Agency, for example, lists environmental pollutants and sets standards for their allowable concentrations in, say, drinking water or the atmosphere by a process that usually includes a hearing. After proposed regulations are reviewed by the public and departmental consultants, and a final version adopted, the final action is the promulgation of a regulation with the force of law, which is published in the Federal Register.
The enforcement of the regulation is by administrative and court action, by court action in civil proceedings for an injunction and civil penalties, or by court action in criminal proceedings. Where the risks arise from a utility plant or other facility licensed under a quasi-judicial process (such as a nuclear plant licensed by the Nuclear Regulatory Commission), consideration of the elements of risk as applied to that facility is an important aspect of the licensing process.
The attainment and maintenance of ambient air quality at the levels set by the Environmental Protection Agency are principally the responsibility of the states,7 a responsibility they exercise under state implementation plans that have been approved by the EPA. The plans vary greatly in detail from state to state. Local agencies may also comprise parts of the regulatory process.
The complexity and repercussions of such implementation plans are worthy of more than passing notice. For example, as will be considered under “Emissions and Wastes,” the vast majority of urban areas are not in compliance with the ozone standard, and the agency expects that even 10 years from now, some will find compliance very difficult despite envisioned steps to curb automobile emissions and to limit traffic patterns.
Practical Aspects
Should an unattainable standard be relaxed? Under the Clean Air Act, standards are to be reviewed every 5 years and may be revised by the administrator of the Environmental Protection Agency on the basis of that review. The act requires, however, that the standards be based squarely on
|
* |
See statement 9–1, by H.Brooks, Appendix A. |
scientific criteria for adequate health protection: Neither the cost of achieving such standards, nor even the availability of the requisite technology to achieve them, is germane to such a determination.8
Whether governmental regulation extends in such cases beyond practicable or just limits may be questioned. As a case in point, the ozone standard was recently reviewed and relaxed, a decision that aroused criticisms of “not enough” and “too much.” The several problems of how to regulate need to be studied, and the ozone case will provide a profitable subject for one such analysis.
Since the enactment of the Clean Air Act of 1970, several major policy steps have been taken that will tend to keep permissible ambient air levels of pollutants low or impede their rise. In the case of the new ozone standard, the administrator has set it with consideration for groups of “particularly sensitive citizens such as bronchial asthmatics and emphysematics who in the normal course of daily activity are exposed to the ambient environment.”9 This requirement will be of particular importance for the determination and interpretation of epidemiological exposure-effect curves (see “Dose-Effect Curves”) and poses major problems in determining how much weight should be given to small sensitive subgroups of the population.
Two striking and important changes have been made in the regulation of emissions. Areas of “no significant deterioration” have been designated where only a 10 percent increment over preexisting ambient air levels of pollutants would be permitted.10 Enforcement here will be complicated by the inability to control extraterritorial emissions that are atmospherically transported to the restricted region over distances that are sometimes as great as many hundreds of miles.
Second, it is now required of new utility plants (among other stationary sources) that the best available control technology (BACT) be employed regardless of ambient air quality levels, emission standards, or the quality of fuel used.
While this may provide benefits, one economic effect will be to lessen the advantage of using low-sulfur coal or oil and to enhance the market for high-sulfur eastern coal. The cost of power plant construction is increased by about 25 percent. (See chapter 4.)
Among the major arguments to support this policy is that of caution. It is not certain that adequate protection is provided by present primary and secondary ambient air quality standards. “Best available control technology” represents a philosophy of playing it safe. Its principal disadvantage is cost. On the other hand, if present standards prove materially inadequate, retrofitting would cost much more, or it could even be impractical.
Although dissatisfaction with various aspects of the government’s handling of the regulatory process is often expressed (e.g., of nuclear
waste, as described in chapter 5 under “Management of Radioactive Waste”), the tremendous importance of regulation cannot be ignored. Considering the legislative expansion of environmental and public health protection in the last 15 years,11 it is too much to expect that any society could master the art of such regulation so rapidly. We are still in the period of learning by sequential experience the extent to which our regulatory objectives can be achieved, and how.
There are limitations to the control of risk. As the reduction in risk becomes more refined, the incremental benefit eventually diminishes and the cost rises disproportionately. No amount of regulation can ensure a risk-free society, nor should it be assumed that such a goal is desirable.*
HEALTH
The risks associated with several energy systems are compared here on the basis of the production of electricity. Electricity now accounts for 11 percent of final energy demand and 29 percent of primary energy use, and its use is increasing relative to that of other forms of energy. Comparisons are given for each step in the cycle; thus, some of the results (e.g., mining, transportation) are applicable to other end-uses.
The fossil fuel and nuclear systems are of primary interest. Estimates for the risks of solar, fusion, and geothermal energy cycles are still speculative, although it does appear that many uses and forms of solar energy would be no more hazardous than energy systems now in use and, in some forms, less so.12
The Risk and Impact Panel of this study reported health effects relative to the operation of an electric generating plant of 1-GWe (109 watts=1 gigawatt (electric) (GWe)) capacity, at 33 percent efficiency and 75 percent capacity factor for 1 year. Such a “GWe-plant-year” corresponds to a fuel input of about 0.0673 quads, and an electrical output of 0.0225 quads, approximately 1/300 of current national electricity production. The fuel required to operate such a plant for 1 year would be 3 million tons of coal, 12 million barrels of oil, 67 billion ft3 of natural gas, or 150 tons of uranium oxide (U3O8) obtained from 75,000 tons of mined ore (0.2 percent) for a light water reactor operated on today’s once-through fuel cycle. (With reprocessing and recycle of uranium, this latter requirement could be reduced to 120 tons of uranium oxide.) It should be noted that a GWe-plant-year of electricity is equal to 75 percent of the GWe-year of electricity used in regulatory procedures. A 1-GWe power station will
|
* |
See statement 9–2, by H.Brooks, Appendix A. |
serve a regional population of about 1 million, including urban, suburban, industrial, and rural components.
ROUTINE INDUSTRIAL RISKS
The routine risks engendered by industrial activities to supply energy involve fatal and nonfatal outcomes.
Fatal Accidents
The lowest accidental death rates in the generation of electricity are for light water reactors and natural gas systems (0.2 deaths per GWe-plant-year). The rate of accidental deaths for electricity from oil is somewhat higher (0.35) and that for coal is very much the highest (2.6 for surface mining, 4.0 for deep mining).* These rates of accidental death are set out in Table 9–1. To place them in perspective, recall that a GWe-plant serves a population of about 1 million. In 1974, the accidental death rate of the general population was 500 per million, of which 220 deaths were due to motor vehicle accidents, 80 to falls, 30 to burns, and 10 to accidents with firearms.
The higher rate of accidental deaths for electricity from coal is largely due to deep mining and to transportation. Both could be improved. In general, small mines suffer twice as many accidental deaths as large mines, per ton of coal mined. If the standards observed in all coal mines were brought up to the standards of the safest, the accidental death rate could be reduced to perhaps a quarter of its present value. However, future trends are difficult to estimate. Until 5 years ago, the accident rate was declining about 4 percent/yr. Since then, it has risen slightly (per ton).13 In the future, automation and other technical improvements in deep mining should enhance safety, but the rapid expansion of production, with a less-experienced and younger work force, and a possible shortage of mining engineers may raise accident rates.
The high mortality rate due to coal transportation is an estimate that is not directly based on coal-train-miles. It reflects conditions that are likely to change. For future planning, this estimate could easily be high by a factor of 2 or 3, in our opinion. The matter needs more precise study.
TABLE 9–1 Accidental Deaths During Routine Operation, by Energy Source (per gigawatt-plant-year)
|
Energy Source and Quantity Required |
Extraction |
Processing |
Transport |
Power Station |
Totala |
|
Coal (3×106 tons) |
|
0.02 |
2.3b |
0.01 |
|
|
Deep |
1.7 |
|
|
|
4.0 |
|
Surface |
0.3 |
|
|
|
2.6 |
|
Oil, onshore and offshore (12×106 barrels) |
0.2 |
0.08 |
0.05 |
0.01 |
0.4 |
|
Natural gas (67×109 ft3) |
0.16 |
0.01 |
0.02 |
0.01 |
0.2 |
|
Uranium oxidec (150 tons from 75,000 tons of ore) |
0.2 |
0.001 |
0.01 |
0.01 |
0.2 |
|
aTotals do not add due to rounding. bThe estimates are not based on coal trains per se, but on the overall rate of train accidents. Furthermore, many accidents with trains are not the fault of cargo nor of the carrier, and the responsibility for them may be incorrectly charged, For meaningful statistics, the matter needs further study. A forthcoming review cites figures based on the exclusive use of unit trains that scale to 0.5 deaths per gigawatt-plant-year. less than one-fourth the entry in the table. Carl W.Gehrs, David S.Shriner, Steven E.Herbes, Harry Perry, and Eli Salmon, “Environmental. Health, and Safety Implications of Increased Coal Utilization,” in Chemistry of Coal Utilization, tech. ed. M.A.Elliott. chap. II, suppl. vol. 2 (New York: Wiley Interscience, in press). cWith reprocessing, the uranium oxide requirement could be reduced to 1.4 tons. Presumably, the mean extraction risk would be reduced proportionately, and the processing risk increased. The net result could be lower total risk. Source: Data for coal are from MITRE Corporation, Metrek Division, Accidents and Unscheduled Events Associated with Non-Nuclear Energy Resources and Technology (Washington, D.C.: MITRE Corporation, (M76–68), December 1976), p. 51, except power-station entry. For oil, natural gas, and power stations, U.S. Council on Environmental Quality, Energy and the Environment—Electric Power (Washington, D.C.: U.S. Government Printing Office, 1973). For uranium oxide extraction, U.S. Atomic Energy Commission, Comparative Risk-Cost-Benefit Study of Alternative Sources of Electrical Energy (Washington, D.C.: U.S. Atomic Energy Commission (WASH-1224), December 1974). For uranium oxide processing, Nuclear Energy Policy Study Group, Spurgeon M. Keeny, Jr., Chairman. Nuclear Power: Issues and Choices (Cambridge, Mass.: Ballinger Publishing Co., 1977), p. 175. For uranium oxide transport, U.S. Atomic Energy Commission, Directorate of Regulatory Standards. Environmental Survey of Transportation of Radioactive. Materials to and from Nuclear Power Plants (Washington, D.C.: U.S. Atomic Energy Commission (WASH-1238), 1972); and U.S. Nuclear Regulatory Commission, Final Generic Statement on the Use of Recycle Plutonium in Mixed Oxide Fuel in Light Water Cooled Reactors (Washington, D.C.: U.S. Nuclear Regulatory Commission (NUREG-0002, or GESMO), 1976). |
|||||
Nonfatal Accidents and Occupational Disease
Underground coal mining is again the most hazardous energy-cycle activity, as shown in Table 9–2 (15,000 days lost per GWe-plant-year), followed by oil and surface mining (about 3500 days lost), fission and natural gas (1500–2000 days lost). These estimates are less certain than those for low side. The production and use of synthetic fuels from coal (with which we have little recent experience) may be considered to have the same risk as coal from extraction through transport and perhaps about the same as oil in processing.
Underground coal mining is more hazardous than underground mining for other materials: The frequency of injuries is about 50 percent higher and their severity is about 25 percent greater. In the case of oil, extraction accidents account for 60 percent of work days lost. With natural gas, accidents related to drilling were at least 10 times greater than accidents in other steps of the energy cycle (per quad).14
Routine accidents in hydroelectric plants in 1972 were about one half as frequent and one tenth as severe as the average for all electric generating plants.
From a medical point of view, underground coal mining adds considerably to the risk of the coal fuel cycle, but it has never been completely and satisfactorily analyzed, owing to the many factors
TABLE 9–2 Accidental Injuries and Workdays Lost During Routine Operations, by Energy Source (per gigawatt-plant-year)a
|
Energy Source |
Accidents |
Workdays Lost |
|
Coal miningb |
|
|
|
Mined underground |
112 |
15,000 |
|
Surface mined |
41 |
3,000 |
|
Oil |
32 |
3,600 |
|
Gas |
18 |
2,000 |
|
Nuclear |
15 |
1,500 |
|
aA permanently disabling accident was credited with 6000 workdays lost, and a temporary disability with 100 workdays lost. The figures are for 1977. bSynthetic liquid fuel from coal might be estimated to have a rate equal to that for coal plus an allowance for the conversion process. Source: National Research Council, Risks and Impacts of Alternative Energy Systems, Committee on Nuclear and Alternative Energy Systems, Risk and Impact Panel (Washington, D.C.: National Academy of Sciences, in preparation), chap. 2. |
||
involved—sociological as well as occupational.15–18 The principal physical factors are dust, noise, and gases (including engine emissions). The present respirable dust standard is set at 2 mg/m3 of air. “Respirable dust” signifies particles that are small enough to reach the depths of the lung (bronchioles and alveoli). Larger particles may be eliminated in the larger air passages (nose, trachea, bronchi). Respirable dust is defined in practice as the material trapped by a particular “respirable mass sampler,” whose deposition characteristics are assumed to represent the depths of the lung.
The problem may first be seen as coal worker’s pneumoconiosis, a reaction of the lung to coal dust, diagnosed by X-ray, that is unimportant in itself, but that may develop into progressive massive pulmonary fibrosis, which seriously impairs pulmonary function. In 1969–1971, the prevalence of pneumoconiosis among miners was 30 percent and of massive pulmonary fibrosis, 2.5 percent. On the basis of meticulous studies abroad, the present dust standards—if enforced—should lower the incidence of simple pneumoconiosis to less than 3 percent, and of massive fibrosis more or less proportionately to 0.25 percent. Although the standard applies to respirable dust, it should be emphasized that dust composed of larger particles can have irritating effects on the larger air passages, and perhaps the gastrointestinal tract, that may be quite important.
“Black lung” is not a medically defined disease but a legally established state of eligibility for benefits, loosely defined by Congress in 1972. The term includes coal worker’s pneumoconiosis and progressive massive fibrosis, as well as other diseases affecting the heart and respiratory system that may not necessarily result from coal mining. Measures that reduce the threat of pneumoconiosis-fibrosis types of illness may therefore have a much smaller effect on the whole spectrum of black lung conditions, for which disability payments now total more than $1 billion/yr (a total certain to rise). The larger dusts, noise, and gases (including engine emissions) presumably give rise to significant types of illness outside the pneumoconiosis-fibrosis category. The prevalence of such illnesses should be investigated.
It is essential that the entire problem be studied, with attention to both the medical and sociological factors (and particular attention to such complicating factors as smoking habits). The spectrum of conditions that can be attributed to work in the mines must be clarified to deal with them medically and to establish fair standards for disability compensation that are in line with national policy for all workers.
With respect to cancer,19 a study of deaths in coal miners only (the working population), excluding disabled and retired men, did not detect an excess risk. In contrast, a study of 533 miners employed in 1937 and followed for 28 years did show an excess of deaths from cancer, particularly for digestive-system cancer. The problem should be reviewed
again and prospective studies initiated, especially since recent legislation has led to major improvements in mining conditions. It should not be automatically assumed that the exposures experienced in mining will increase the cancer incidence rate. Significant evidence from the United Kingdom shows that lung cancer is diminished for unknown reasons in underground coal miners.20
In the case of uranium miners, a lung cancer mortality rate of about 0.2 per GWe-plant-year can be estimated from the doses and cancer-induction factors given later in this chapter (1400 person-rem×(2×10−4) cancers per person-rem).*
PUBLIC HEALTH RISKS
Epidemiological Methods
In dealing with the quantitative assessment of risk due to emissions and wastes, the dose-effect curve is used whenever feasible. For the discussions that follow, it is important to consider the advantages and limitations of the method, particularly in the examples detailed below.
Two kinds of toxic agents are generated in energy cycles, artificially radioactive elements (fission products or elements activated by neutron adsorption) whose half-lives may be short or very long, and chemicals. Much more is known (for present purposes) about the mode of action of the ionizing radiations than that of the chemical agents at the low levels of dosage that are of primary concern. It appears that radiation primarily induces late effects (e.g., cancer), whereas the chemicals produce more immediate effects, although they might produce late effects as well.
Ionizing Radiations The systematic study of the Japanese atomic bomb survivors has provided an outstanding example of the knowledge that can be gained from a large-scale epidemiological study, and the time and effort required to achieve it.
Results for leukemia are illustrated by the dose-effect curves in Figure 9–1, for Hiroshima and Nagasaki. The death rate (mean annual rate for the period 1950–1974) is plotted against radiation dose (per person) received in 1945.21 For the entire study period, there was a crude excess of 67 cases of leukemia in the total population of about 83,000 persons. The curves illustrate the great sensitivity of the epidemiological method at its best, when a specific class of rare disease due to a specific cause is fully
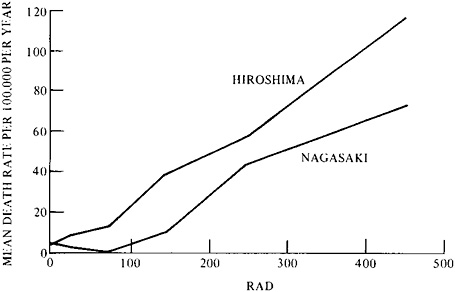
FIGURE 9–1 Mean annual leukemia death rate at Hiroshima and Nagasaki per 100,000 persons at risk, by dose and city from 1950 to 1974. The initial numbers at risk were as follows: for Hiroshima—controls, 43,700; 10–49 rads, 10,800; 50–400 rads or more, 6,000; and for Nagasaki—controls, 15,200; 10–49 rads, 3,800; 50–400 rads or more, 4,100. The number of leukemia cases were as follows: for Hiroshima, 42 control and 68 exposed; and for Nagasaki, 12 control and 22 exposed. Based on the observed control rate for both cities combined, the expected number of leukemias in the exposed population was 23, and the excess, 67. Source: G.W.Beebe, H.Kato, and C.E.Land, Mortality Experience of Atomic Bomb Survivors, 1950–1974 (Hiroshima, Japan: Radiation Effects Research Foundation (RERF TR 1–77), 1977), p. 24.
investigated over a period of 25 years.22 If, however, leukemia had not been specified, the 67 extra deaths would have been lost in the variations of the overall gross mortality rate.
In addition to illustrating the importance of a suitable endpoint, the Japanese studies demonstrate the importance of dosimetry. From individual estimates, made years before death, each decedent could be placed in one of five broad dose classes. Dose (rads, rems, or grays) relates to the energy absorbed by the target organs.23 But even dose may require further specification. The Nagasaki curve lies below that for Hiroshima in Figure 9–1 because the Nagasaki doses involved less than a 2 percent contribution from neutrons, and those at Hiroshima a 19–27 percent contribution. There is reason to believe that the shape of dose-effect curves for neutrons differs from that for gamma rays (and X-rays), as suggested by Figure 9–1, and there is a possibility that neutrons and gamma rays differentially
induce different types of leukemia.24,25 The neutron-weighted curve (Hiroshima) resembles a linear one, whereas the other curve does not.
Sensitive though the Japanese study may be, is it sensitive enough to estimate the risk in the regulatory range of particular interest today, below 5 rads, and especially below 1 rad? This region of dose effect is poorly defined statistically by the two curves in Figure 9–1 owing to the small numbers of leukemia cases below 50 rads. In fact, the two curves are not significantly different statistically. They illustrate the uncertainty of extrapolating from higher, relatively well-established regions of the dose-effect curve to the lowest regions, which are of greatest interest. The problem of allowing for natural background radiation in the very low dose region, where effects even in very large populations have been undetectable, is discussed later in this section of chapter 9 under “Fission.”
Finally, the nature of the population at risk must be examined. Is it of uniform sensitivity or does it contain subpopulations whose response in fact determines the overall results? In the case of the Japanese data, age at exposure determined how rapidly leukemia first occurred (Figure 9–2).26 As a result, early reports concluded that children under 15 were the most sensitive, but after 10 years, disease began to occur in those who had been exposed at 45 years of age or older, and this group can now be seen to be the most affected. The occurrence of other forms of cancer only began some 15 years after exposure (in all age groups) and is still increasing after 30 years.
Chemical Agents The problem is much more complex for chemical agents, since the dose to the target tissue is known only under exceptional circumstances. Even the exposure level (concentration in air or water) may not be known quantitatively, and it is rarely specified for individuals on the basis of where they spend their time, e.g., indoors or outdoors. The parameter of exposure in air pollution studies is almost invariably a measurement taken at some distance from those at risk, for example, from a single monitoring station in a metropolitan area.
Additional confusion may result from the imprecise use of the term “dose.” Dose might refer to the ambient exposure, or as in medicine, it might specify the amount of agent entering the body (by mouth or lung) but not necessarily reaching the target organ. (This could be true of radioactive substances that emit alpha and beta particles.) Thus, the agent may be inactivated in the digestive tract or excreted; if absorbed, it may be inactivated by the blood or liver, or it may be excreted by the kidneys. All these mechanisms tend to reduce the effect per unit quantity of agent or even to establish a threshold of exposure below which there is no damage.27
However, some innocuous substances are activated in the body. In the
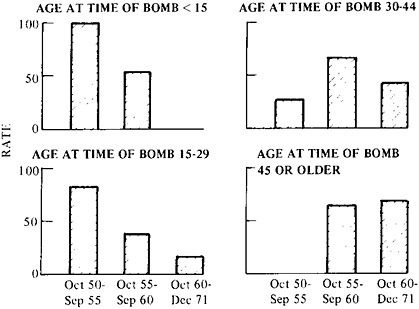
FIGURE 9–2 Leukemia death rate per 100,000 persons during three successive time intervals as dependent on the individual’s age at exposure to atomic bomb radiation. The exposure occurred in 1945. The rate is adjusted for sex and city, and for doses of 100 rads or more. Note the much longer latent period in the case of the older cohort. Source: M.Ichimaru, I.Ishimaru, and J.L.Belsky, Incidence of Leukemia in Atomic Bomb Survivors, by Dose, Years After Exposure, Age, and Type of Leukemia, 1950–1971, Hiroshima and Nagasaki (Hiroshima, Japan: Radiation Effects Research Foundation (RERF TR 10–76), 1976), p. 14.
case of the respiratory and digestive tracts, and also the skin, direct contact between chemical and epithelial tissue does occur and may enhance the tissue’s vulnerability.
Two examples illustrate the complications.28 First, the accumulation of lead in the human lung appears to be a threshold phenomenon. No increment in tissue level occurs until the atmospheric level rises above 1.35 µg/m3. Second, the levels of cadmium in liver, lung, and kidney are largely independent of the atmospheric level but dependent on smoking. To investigate the toxicity of atmospheric cadmium at the usual levels would likely be impossible in a population of unidentified smokers.
Other variables that are known to have major effects on the dose-effect curve in epidemiological studies are past medical history, socioeconomic status, and the weather.
It should be noted that the laboratory demonstration of a toxic agent’s entering the cells does not guarantee a toxic epidemiological outcome. First, it has been argued on probabilistic grounds that unless the
concentration within the cell exceeds a certain level, the toxic agent is unlikely to reach its intracellular target.29 Second, although the target is hit, recovery is likely to occur unless the toxic dose is high enough to saturate the cell’s recovery system.30 Third, if a cancer cell is produced, that cell must override the resisting host to produce a tumor. And fourth, the toxic substance may not always be toxic. Selenium, for example, becomes progressively more toxic and eventually lethal at levels of 5–10 ppm (and above) in the diet, but it is an essential element for the good health of many domestic animals at a minimum level of 0.1 ppm.31 Such factors will affect the shape of the dose-effect curve (the toxic effect of selenium has a threshold) and may complicate its interpretation, especially in the low-dose region.
In the case of man, the epidemiological study of smoking provides the major chemical example of the knowledge that can be gained when large numbers of exposed and cooperative individuals are available and when the level of exposure is relatively high.32 As with all exposures continuing over a period of years, assessment at one particular time (e.g., at the time of the study inquiry, as is often the case in pollution studies) may have little to do with health at that time or in the future. Thus, in the case of smoking, even after termination of the habit, significant though diminished increments in death rate occur 10 or more years afterward from chronic bronchitis and emphysema, and from pulmonary heart disease.33
A cancer dose-effect equation for cigarette smoking has been obtained using data drawn from a 20-yr prospective study of some 34,000 British physicians.34 The equation was based on the age-standardized incidence of bronchial carcinoma in those physicians who began smoking at 16–25 years of age and who had each reported the number of cigarettes smoked per day at a relatively constant rate (but not more than 40/day) from that age onward. No cancers were observed prior to 40 years of age. For the age range 40–79 years, the fitted equation for annual risk of bronchial cancer (per person) was the following.
Cancer therefore was a nonlinear function of exposure, and its incidence rate depended on intensity of exposure and years of exposure.
Other types of dose-effect curves induced by irritating particulates are seen in asbestosis, byssinosis, and silicosis.35
Estimating Health Risks
Adequate dose-effect curves are rarely available for the very low ranges of exposure that are now at issue in the regulatory process, especially when
applied to the whole population. Nonetheless, risk must be estimated in many such cases. Some approaches that have been employed to make these estimates are listed below.
-
When the mode of action of the toxic agent is adequately known, as well as the dose-effect curve, and the population at risk is sufficiently well defined, the extrapolation or interpolation of the curve poses no great difficulty (e.g., carbon monoxide).36 This is rarely the case.
-
Experiments with animals might be used to set upper bounds of permissible doses, provided that additional margins of safety in dosage or exposure have been incorporated in the process.37,38
-
Further large-scale epidemiological studies could be undertaken to define the dose-response curve in the low-dose region. The sensitivity of such investigations, however, may not be sufficient to supply the desired information.39–41 The logistics of such studies must be carefully prepared in advance. Especially important is the accuracy of the dosimetry (compare approach 1, above). It is also important to know if a threshold of response is likely and if there are particularly sensitive groups of individuals whose reactions differ significantly from the mean.
-
High-dose results have been extrapolated on the assumption that effect will continue to follow dose on a curve of the same shape in the unexplored lower-dose region. In the case of radiation protection, this has been done by linear extrapolation.42 That is to say, if one cancer death results from a dose of 100 rem to each of 100 persons (104 person-rem), one death is assumed to follow a dose of 10 rem to each of 1000 persons (104 person-rem), or even 0.1 rem to 100,000 persons (104 person-rem). In view of the discussion above regarding thresholds (for chemicals, the population dose or exposure would be in person-mg), such a step most likely overestimates the risk (at least in the case of toxic substances). For this reason, decisions based on this assumption are often considered to be conservative.
Although such a conservative decision may appear to be the prudent one, is it in fact? Reduction of exposure to extremely low levels of pollutants may be both costly and troublesome. Would the cost and trouble be warranted, considering that the risk is hypothetical and that the expenditure of equal cost and effort elsewhere would yield tangible benefits (including the reduction of other health risks)? A recent example of the complexity of such choices was the resetting of the ozone standard (discussed in previous and subsequent sections).
Fission
Natural Background and Federal Regulations The natural radiation background to which all of us are exposed—from earth, rocks, outer space, building materials, and food—might be a source of cancer or mutation (Table 9–3).43 In the United States, the mean annual dose per individual is about 80 millirem (mrem) to the soft tissues, 120 to bone surfaces, and 180 to the lungs. The total-body mean is about 85 mrem. In Denver, the city of highest exposure owing to its elevation, the annual dose averaged over the entire body is about 125 mrem.
Total background includes man-made sources as well as natural background. Medical X-ray exposure is the chief anthropogenic component and averages about 70 mrem/yr in the United States. Fallout adds 3 mrem. Nuclear power now adds less than 0.01 mrem, and for a 300-reactor program, the contribution would not exceed 0.1–0.2 mrem/yr.
The damage from background has been estimated two ways: by the use of factors based on a combination of experience, experiments, and
TABLE 9–3 Average Dose-Equivalent Rates in the United States from Various Sources of Natural Background Radiation (millirem per year)
|
Source |
Site |
||||
|
Gonads |
Lung |
Bone Surfaces |
Bone Marrow |
Gastrointestinal Tract |
|
|
Cosmic radiationa |
28 |
28 |
28 |
28 |
28 |
|
Cosmogenic radionuclides |
0.7 |
0.7 |
0,8 |
0.7 |
0.7 |
|
External terrestrialb |
26 |
26 |
26 |
26 |
26 |
|
Inhaled radionuclidesc |
— |
100d |
— |
— |
— |
|
Radionuclides in the bodye |
27 |
24 |
60 |
24 |
24f |
|
TOTALg |
80 |
180 |
120 |
80 |
80 |
|
aIncludes a 10 percent reduction to account for structural shielding. bIncludes a 20 percent reduction for shielding by housing and a 20 percent reduction for shielding by the body. cDoses to organs other than lung included in “Radionuclides in the Body.” dLocal dose-equivalent rate to segmental bronchioles is 450 mrem/yr. eExcluding the cosmogenic contribution shown separately. fThis does not include any contribution from radionuclides in the gut contents. gTotals do not add due to rounding. The mean annual whole-body dose for the United States is approximately 85 mrem. Source: Adapted from National Council on Radiation Protection and Measurements, Natural Background Radiation in the United States (Washington, D.C.: National Council on Radiation Protection and Measurements (NCRP Rep. 45), 1975). |
|||||
judgment, and by field studies. If the cancer factor of the Risk and Impact Panel (2×10−4 cancer deaths per person-rem) applies at such low dosage, it might be projected that natural background contributes some 4000 cancer deaths to the annual total of 350,000 deaths each year from cancer in the United States.44 Since the factor is based on a linear extrapolation from a much higher dose range, it may very likely be an overestimate.
Another approach has been to compare the cancer death rates of geographic regions whose natural backgrounds differ appreciably. Large background differences in India and in Brazil are known, but other uncontrolled variables preclude a sufficiently sensitive analysis.45 In the United States, cancer mortality by state has been studied against natural background.46 The analysis indicated that the states with the highest background have the lowest cancer mortality rates. It is interesting to conjecture how much more publicity the study would have received had it shown an increase rather than a decrease in cancer. The investigators attempted to remove the possibly confounding effects of a variety of socioeconomic factors but failed to change the result. The study (as its reporters no doubt realized) fails to meet the rigorous criteria (discussed in “Dose-Effect Curves” of this chapter) that are considered essential when small differences are at stake: The dosimetry was not individualized but was based on a state-wide mean of exposure. The vital statistics were based on death certificates, whose diagnostic bias was not controlled, and the socioeconomic variables were not related to individuals. As it stands, therefore, the study performs the function of again raising an interesting question that would require great effort and sophistication to resolve, assuming that such resolution is feasible.
Federal regulations to limit radiation exposure had their origin in the recommendations proposed by radiologists and medical physicists through their International Commission on Radiological Protection (ICRP) and National Council on Radiation Protection and Measurements (NCRP), long before nuclear power plants were built. These permissible doses have been progressively lowered as knowledge of radiation biology has grown and as the technical ability to limit exposure has improved. The maximum permissible doses (prospective guidance limits) recommended by the NCRP47 are based on annual cumulative dose.
|
Occupational exposure: |
5 rem to the individual |
|
Population exposure: |
0.5 rem for any one individual 0.17 rem, average for population |
Medical exposure and natural background are not counted. The guidance limits for strictly partial-body dosage are higher.48 Since proper use of the guidance limits involves the ALARA principle (exposure to be as low as reasonably achievable), almost no one receives the maximum under ordinary circumstances, and the average for the exposed population is far below it. However, as standards are driven lower and lower by regulation, protection tends to become more costly, and cost-benefit considerations become important. It may well be that unnecessarily costly environmental restrictions would lead suppliers to use alternative technologies that have greater risks. Any fresh evidence of effects—or lack of effects—in the low-dosage range (relative to the standards) would therefore be of the greatest value.
A recent highly publicized study based on death certificates49 claimed that cancer mortality associated with employment at an atomic plant was significantly increased in that segment of the working population that had received cumulative doses of less than 10 rem. The doses to the entire population ranged from below 1 rem to above 25 rem (with 75 percent below 5 rem), accumulated over periods up to 20 years and more.
The same statistical data have been reinvestigated by others,50–52 who employed better methods of analysis, and the whole issue has been reviewed.53 No increase in leukemia was observed, including myelogenous leukemia, the hallmark of radiation-induced cancer. No increase in cancer from doses below 10 rem was noted. For doses above 10 rem, small excesses of cancer of the pancreas and of multiple myeloma were noted, but they could not be shown to have been caused by occupational exposure to radiation. Further and more refined study is to be undertaken.
In the case of fission products from the present generation of light water reactors, the principal sources of public exposure are radioactive gases and emissions into the atmosphere—radon-222 (222Rn) in uranium mines and mills, and krypton-85 (85Kr), tritium, iodine-129 (129I), and carbon-14 (14C) in other parts of the fuel cycle. Inhalation makes the lung a primary target. The target for iodine is the thyroid gland. Other agents include bone seekers.
In the routine operation of light water reactors, the general population is exposed to radiation by emissions into the atmosphere and by the cooling water discharged into local water (Figure 9–3). Spent fuel is handled separately, for eventual reprocessing or long-term storage. For regulatory purposes, the dose to an individual member of the population is maximized by estimation for a hypothetical individual who spends all his or her time at the plant boundary and obtains all his or her food and water from the immediate area. Such an individual is subjected to airborne radioactive gases, receives external exposure from radioactive particulates deposited
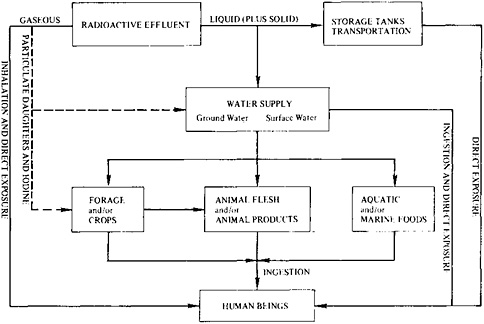
FIGURE 9–3 Generalized exposure pathways to man from the operation of a light water reactor. Source: U.S. Nuclear Regulatory Commission, Final Generic Environmental Statement on the Use of Recycle Plutonium in Mixed-Oxide Fuel in Light-Water Cooled Reactors (Washington, D.C.: U.S. Nuclear Regulatory Commission (NUREG-0002, or GESMO), 1976).
on the ground, inhales airborne radionuclides, and drinks water and eats foodstuffs, including fish, that contain radioactivity, as indicated by Figure 9–3.
The radioactivity of these emissions declines with radioactive decay and with dilution or transport by natural factors. Obviously, human exposure will be very sensitive to the action of these factors as well as to lodgement in the food chain and the rate at which radioactive materials are excreted.
For regulatory purposes, 85Kr, tritium, 129I, and 14C are especially important nuclides. (Radon gas is important for mining and milling.) The emissions data in Table 9–4 are associated with the routine operation of a light water reactor for 1 GWe-plant-year.
The federal regulations that control exposure are promulgated by the EPA and the NRC, and the regulations of both agencies are enforced by the NRC. The NRC has decreed that reactor emissions should be as low as reasonably achievable. Current design guides for a light water reactor limit the annual air doses to any member of the public from that plant to 10 millirads (mrad) for gamma radiation; 15 mrem for iodine and particu-
TABLE 9–4 Major Radionuclides in the Emissions of a Light Water Reactor
|
Radionuclide |
Half-life (days) |
Radioactivity (curies per giga-watt-year)a |
|
Effluent Gases |
|
|
|
Carbon-14 |
2.1×106 |
9.5 |
|
Iodine-131 |
8.1 |
0.3 |
|
Iodine-133 |
0.9 |
1.1 |
|
Krypton-85 |
3,900 |
290 |
|
Tritium |
4,480 |
47 |
|
Xenon-133 |
5.3 |
3,200 |
|
Xenon-135 |
0.38 |
1,100 |
|
Effluent Liquids |
|
|
|
Cesium-134 |
750 |
0.01 |
|
Cesium-137 |
11,000 |
0.02 |
|
Iodine-131 |
8.1 |
0.26 |
|
Tritium |
4,480 |
43 |
|
aFor conversion to curies per gigawatt-plant-year, multiply by 0.75. Source: Extracted from U.S. Nuclear Regulatory Commission, Final Generic Environmental Statement on the Use of Recycle Plutonium in Mixed Oxide Fuel in Light Water Cooled Reactors (Washington, D.C.: U.S. Nuclear Regulatory Commission (NUREG-0002, or GESMO), 1976). |
||
lates; and for effluents, 3 mrem (whole body) or 10 mrem to any one organ.54
The Environmental Protection Agency has declared that by 1983, emissions entering the general environment from the uranium fuel cycle (per GWe-year produced by the fuel cycle) are to be less than 50,000 Ci55 of 85Kr, 5 mCi (millicuries) of 129I, and 0.5 mCi (combined) of 239Pu and other transuranic radionuclides with half-lives greater than 1 year. (It should be noted that the EPA “uranium fuel cycle” includes neither mining nor waste disposal and that a “GWe-year” is 1.33 times as much electricity as produced in a “GWe-plant-year,” which is corrected for load factors.)
The EPA has also directed that beginning in 1980, the dose received by any member of the public from the uranium fuel cycle shall not be more than 25 mrem whole-body, 75 mrem thyroid, and 25 mrem other organs (radon and its daughters excepted).56
The EPA takes the position that permissible levels should be as low as regulation can drive them at some practical cost. The specification of what
cost is practical, however, is a matter of opinion. One definition by the Nuclear Regulatory Commission for reactor design is that as an interim measure, $1000 per reduction of 1 person-rem is a favorable cost-benefit ratio.57 Taking the risk of cancer death as 2×10−4 per person-rem, the commission’s policy entails a cost of $5 million per avoided cancer death.
On the other hand, while equally concerned for health, and emphasizing that industrial and other practices should always involve the ALARA principle (as low as reasonably achievable), the National Council on Radiation Protection and Measurements is not convinced that its current permissible doses should be radically changed.58 These doses are also recommended by the International Commission on Radiological Protection59 and are generally used today throughout the world. The National Council considers the risk of cancer to be overestimated in the low-dosage range for gamma and beta rays by the process of linear extrapolation from high-dosage and high dose rate experience. It cautions government policymaking agencies against taking such extrapolations as accurate, and as a result, adding heavy margins of safety in setting permissible doses that could become unduly restrictive. To examine the whole matter as fully as possible, the council has set four committees to work. Their reports should begin to appear late in 1979. As noted above, the council was the original proponent of protection standards, and its views cannot be dismissed as those of a biased party.
There is merit in the intent of both positions. The problem is not a matter of choosing between them but of using both to make the best decision possible. A basic difficulty, of course, is that data for the exposure range of interest are not available. Present decisions must rely on conjecture. Government agencies may tend to take a more protective position, in part because they prefer to err on the side of caution. Furthermore, as recently evidenced in the case of ozone, such a position can turn out to be impractical, and it may be reversed. This committee observes that the EPA 25-mrem annual standard for individual members of the general population is equivalent to one quarter of the average natural-background dose for the United States, and is within the range of its regional variation. The annual background dose in Denver, for example, is about 50 mrem higher than that in the Mississippi Valley. The utility of promulgating such a standard is not clear.
Routine Reactor Operations The individual steps of the nuclear energy cycle—from mining to waste disposal—are outlined in Table 9–5, with their individual contributions to occupational and population radiation dosage.60 Several facts stand out First, the larger risks to employees are from mining, milling, and reactor operation; to the general population, they are from mining, milling, and reprocessing. For the employees, the
TABLE 9–5 Estimated Radiation Dose Commitments Delivered by Routine Operation of Various Parts of the Uranium Energy Cycle to Existing Populations
|
Operation |
Contributions to Lifetime Dose Commitment (person-rems to whole body, or, in parentheses, to key organs with significantly greater exposure, per gigawatt-year of energy produced)a |
||
|
Employees |
Domestic Population |
Foreign Populations |
|
|
250 (lung 1370) |
600 (bone 1960, kidney 2285) |
— |
|
|
Millingc |
80 (lung 660, bone 320) |
120 (bone 390, kidney 450) |
— |
|
Conversion |
1 (lung 8, bone 320) |
10 (bone 24, kidney 3) |
— |
|
Enrichment |
0.7 (lung 14, bone 6) |
0.02 (gastrointestinal tract 1.6, kidney 0.7, if fuel is recycled) |
— |
|
Fuel fabrication |
12 (lung 462) |
0.6 (bone 10, kidney 1.6) |
— |
|
Reactor operationd |
1240 |
76 (bone 272, thyroid 195) |
52 (bone 250) |
|
Reprocessinge |
25 |
360 (bone 890, skin 2200) |
240 (bone 750, skin 7900) |
|
Transportation, irradiated fuel storage, and waste management |
4 |
0.2 |
— |
|
TOTALf |
|
|
|
|
Without reprocessing |
1600 (lung 3800) |
800 (kidney 2800, bone 2700) |
50 (bone 250) |
|
With reprocessing |
1600 (lung 3300) |
1000 (kidney 2600, bone 3100) |
270 (bone 1000, skin 8300) |
|
aThe dose commitment delivered to any person by a given release of radioactivity to the environment is the integrated dose he or she will receive for the rest of his or her life from the decay of the released radioactivity, assuming the person remains in the neighborhood and continues to be exposed to radiation from ground, water, and locally produced food. Values given in person-rems equal the number of persons at risk multiplied by the mean dose per person (in rems). (For conversion to curies per gigawatt-(electric)-plant-year, multiply by 0.75.) bThe figures overestimate the risk from mining by a factor of 2 (Conyers Herring, personal communication to H.I.Kohn, August 1979). cFigures for mining and milling assume proper management of mill tailings and abandoned mines and allow for the escape of radon gas from open mines and mill tailings before being covered. dOccupational doses per unit of energy produced have varied severalfold, depending on such factors as the age of the reactor and the sophistication of the protective measures employed. Population doses from noble gas effluents of boiling-water reactors have until recently been several times larger than the value shown. Reactors with a thorium-uranium fuel cycle (not yet developed commercially) would produce an additional occupational hazard via gamma radiation from 232U. (See chapter 5.) eFigures assume no retention of the nuclides 3H, 14C, and 85Kr in the body. Population doses would be greatly reduced if any or all of these nuclides were retained. Skin dose is mainly due to 85Kr. fTotals do not add due to rounding. Source: Taken from National Research Council, Risks Associated with Nuclear Power: A Critical Review of the Literature, Summary and Synthesis Chapter, Committee on Science and Public Policy, Committee on Literature Survey of Risks Associated with Nuclear Power (Washington, D.C.: National Academy of Sciences, 1979), p. 40. |
total risk is practically the same with or without reprocessing (about 1200 person-rem/GWe-plant-year). In the general population, the total risk is about 750 person-rem with reprocessing and 600 person-rem without. Using a cancer-rate factor of 2×10−4 per person-rem, the total cancer risk would be 0.4 per GWe-plant-year, an estimate adopted by the Risk and Impact Panel and about equal to that of the United Nations.61
Of the principal emissions from a nuclear plant under routine conditions (see Table 9–4), 14C eventually achieves universal distribution into all living things through the food chain (as illustrated in Figure 9–3). This and its long half-life (5570 years) indicate that its long-term risk should be calculated. Estimating that 7.5 Ci of 14C will be generated per GWe-plant-year, and assuming that the world population will be constant at 4 billion, the worldwide population dose would be 1700 person-rem/GWe-plant-year, equivalent by application of the linear hypothesis to 0.3 cancers per GWe-plant-year.62
The number of serious genetic defects per GWe-plant-year has been estimated to be about 0.5 by the Risk and Impact Panel.63 That panel reported an estimate of 100 cases of all kinds of severe genetic diseases per million person-rem, due to gene mutation and chromosomal abnormalities, and expressed in the first generation after irradiation, with diminishing frequency for the next half-dozen generations. In addition, there is an important class of diseases for which mutation is a partial cause but for which the magnitude of the mutational component is unknown. This includes a variety of congenital abnormalities and constitutional diseases, such as diabetes, cancer, heart disease, and mental retardation. The uncertainty in estimating the radiation-induced incidence of diseases in this group is very great, but as a crude estimate, was taken to be equal to the first. “Severe genetic defects,” then, implies conditions or diseases that substantially reduce life expectancy, seriously impair normal physical or mental activity, or require prolonged medical attention.
The panel estimates a risk of 2×10−4 per person-rem for severe genetic defects, expressed mostly within the first 5 to 10 generations. The estimates are very uncertain, depending heavily on mouse data and on speculations about the role of mutation in human disease. The estimates could easily err by a factor of 5 in either direction.
For a large domestic nuclear power program of 300 reactors (each of 1-GWe capacity), the projected annual increment in risk (on the basis of the linear hypothesis, and recalling the dangers of extrapolating genetic effects from mice to people) would ultimately be about 100 cancer deaths (added to an annual rate of 340,000) and about the same number of serious genetic defects (added to an annual rate of about 30,000). Foreign populations (in toto) would suffer about 5–15 percent of these estimates.
Disposal of Radioactive Waste This subject is discussed at length in chapter 5, and the literature about its risks has recently been reviewed.64 The principal point is that under routine conditions, radioactive waste contributes only a small fraction of the total risk (dose) of the nuclear energy cycle. Nevertheless, the government’s failure to institute a waste disposal program has led to a loss of public confidence: The public now sees waste disposal as a difficult and dangerous undertaking.
Although the numbers of curies to be handled and sequestered is impressively large (as indicated in Table 9–6), many experts agree that their disposal—intelligently managed—will do no more than elevate background in some areas, or give rise to small pockets of higher exposure levels that can be effectively isolated. Table 9–5 estimates less than 4 person-rem/GWe-plant-year as the lifetime dose commitment.
It is important to emphasize that none of the events leading to possible catastrophe in nuclear power reactors can occur in a properly designed radioactive waste repository.65 The principal dangers are that some of the waste might be carried to the discharge areas of an aquifer, or that the waste-storage area might be accidentally tapped in mining operations. The health hazard in the first case would arise from prolonged low-level exposure to radionuclides that enter drinking water or the food chain. The maximum credible risk in either case is comparable to that of improperly treated tailings from uranium mills.
The greatest health hazard associated with high-level waste disposal is likely to arise in connection with the transportation of the waste to the permanent repository or to a reprocessing plant. For this reason, locating the repository near its satellite reactors or reprocessing plants would be desirable (though not essential).
Under routine operations, the waste originates as follows. Approximately one fourth the charge of nuclear fuel in a light water reactor is replaced each year. The spent fuel elements are now stored in pools near the reactor. At some time in the future, these elements will have to be stored in separate facilities (away from reactor, or AFR pools). For long-term retrievable storage, most would be placed in separate canisters, as damaged fuel elements are now stored.
Between 90 and 99 percent of the actinide toxicity of the fuel would be removed in reprocessing, but the spent fuel from fuel elements containing recycled actinides may have up to 10 times the actinide toxicity of spent fuel removed from reactors loaded with fresh, slightly enriched uranium fuel. Most of this increased toxicity is due to plutonium isotopes plutonium-238 (238Pu) and 241Pu, with half-lives of 88.9 and 14.6 years, respectively) that are not important as long-term disposal risks (i.e., risks that persist for millennia). Table 9–6 sets out the principal fission products in spent fuel and their activities over a lengthy period.
After several hundred years of relatively rapid decline (as illustrated in Figure 9–4), the radioactivity is within a factor of 10 of that in the original ore, and from that point forward, the radioactivity is dominated by the slowly decaying actinides. The shielding requirements for this latter phase are much less demanding than the initial requirements.
The technical problems of waste disposal are not considered major. (These are discussed in detail in chapter 5.) Among the schemes that have been proposed, deep-mined repositories in geologically sound locations seem to offer storage at reasonable cost and acceptable risk.*
The government urgently needs to initiate a program of radioactive waste management, including that of mines and mills. While improved schemes may be developed in the future, waiting for their emergence and demonstration does not seem sensible in view of the practical measures that can be taken now. Rather than searching for a once-and-for-all solution, research should be undertaken to assure that each increment of waste is disposed of by the best technology available. The committee’s recommendations on the management of radioactive waste can be found in chapter 5.
Combustion
To control pollution of the atmosphere from combustion, the Environmental Protection Agency establishes and enforces two sets of standards: one set that limits emissions and another that stipulates the ambient air quality to be maintained (or bettered, if possible). Standards for emissions from power plants are detailed in chapter 4. In this chapter, five principal pollutants are considered. For each, one or more national ambient air quality standards have been set that may not be exceeded anywhere. These standards are defined as the amount of pollutant in a cubic meter of air, or as an allowable fraction of the total atmosphere.
There are similarities and differences in the nature of the standards applied to chemical and radioactive pollutants. The emission standards for combustion devices (chapter 4) are analogous to those for radionuclides emitted from reactors. On the other hand, the ambient air quality standards for chemicals relate to possible exposure, whereas the standards for radiation are stated in terms of the absorbed dose in the tissue of interest. In this respect, the practice of radiation protection is more sophisticated. The difference is not academic, since the major difficulty in estimating the hazards of the chemical pollutants stems from lack of knowledge of the dosage.
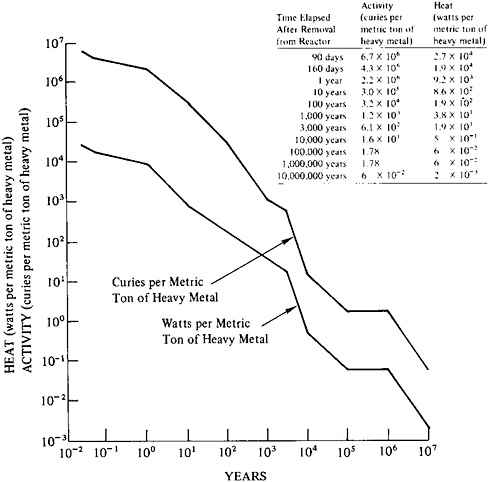
FIGURE 9–4 Decay of radioactivity in spent fuel from a light water reactor: time dependence of activity (curies per metric ton of heavy metal) and of heat production (watts per metric ton of heavy metal). Fuel characteristics: burnup, 25,000 MWd per metric ton of heavy metal; power at shutdown, 35 MWe per metric ton of heavy metal. Source: For activity and power projections up to 3000 years, U.S. Energy Research and Development Administration, Alternatives for Managing Wastes from Reactors and Post-Fission Operations in the LWR Fuel Cycle (Washington, D.C.: Energy Research and Development Administration (ERDA-76–43), 1976), Table 2–17; for projections over 3000 years, B.L.Cohen, “The Management of Radioactive Waste: Waste Partitioning as an Alternative” (Paper prepared for the U.S. Nuclear Regulatory Commission, Washington, D.C., 1976).
Regulations and Current Levels of Pollution The standards represent the considered judgment of experts, based on available data from experience with man, plants, and animals, and supplemented by the results of laboratory experimentation. (See “Research on the Health Effects of Air Pollutants,” below.) Whether the standards now in force were set at the
TABLE 9–6 Some Fission Products of Biological Interest Associated with a Typical Batch of Spent Fuel Removed from a 1-GWe Boiling-Water Reactora
|
Radionuclide |
Half-life |
Radioactivity (curies) |
|
Cesium-134 |
2.19 years |
5.3×106 |
|
Cesium-137 |
30 years |
2.8×106 |
|
Carbon-14 |
5,770 years |
0 |
|
Iodine-129 |
17,000,000 years |
1 |
|
Iodine-131 |
8.1 days |
1.7×107 |
|
Krypton-85 |
10.4 years |
2.8×105 |
|
Strontium-90 |
28 years |
1.9×106 |
|
Tritium |
12 years |
1.8×104 |
|
Xenon-133 |
5.3 days |
3.1×107 |
|
Total of all activity, including actinides |
|
2.7×109 |
|
aThese data are for a typical reload of 172 bundles, one-quarter of the total assembly. The bundles contain about 32 metric tons of uranium (enrichment about 2.6 percent). The total inventory of the reactor core is therefore 4 times that indicated in the table, plus an additional allowance for all the actinides, See chapter 5, Table 5–15, for a detailed listing. Source: Extracted from U.S. Nuclear Regulatory Commission, Final Generic Environmental Statement of the Use of Recycle Plutonium in Mixed Oxide Fuel in Light Water Cooled Reactors (Washington, D.C.: U.S. Nuclear Regulatory Commission, (NUREG-0002, or GESMO), 1976), p. IV-C-12. |
||
most efficient levels for all circumstances cannot be decided, since more information is needed in the lower ranges of exposure. Table 9–7 displays data for certain of these pollutants as of 1977. As more experience and knowledge are gained, the standards will be reevaluated as required by law, and possibly improved.
Accepting the standards as they are, the practical comparison of risks from various fuels amounts to a comparison of how readily their emissions can be controlled to comply with the standards. For stationary sources, the use of any fossil fuel will lead to the production of nitrogen dioxide from the nitrogen of the atmosphere and, in the case of oil and coal, an increment from their own nitrogen content. Oil and coal contain sulfur, from which sulfur dioxide will be produced. Finally, the combustion of oil and especially coal releases a variety of organic compounds and trace metals, and the combustion of coal releases a small amount of radioactivity. The combustion of coal will therefore be the most costly to control, that of natural gas the least.66 It should also be noted that the hydrocarbon and nitrogen oxide emissions of the transportation sector are both
significant and costly to control. The principal emissions from combustion are set out by source in Table 9–8.
With the trend in recent years toward more stringent control, some diminution in emissions is to be expected, although it may be partially masked by increased consumption of fossil fuel. The total emissions for 1970, 1974, and 1977 (Table 9–8, bottom three lines) show a steady decline in particulates that totals 45 percent, declines of about 5 percent in sulfur oxides and volatile organic compounds (approximately the equivalent of nonmethane hydrocarbons), and increases of 3 percent in carbon monoxide and 10 percent in nitrogen dioxide. The increases in coal and oil consumption during this period were 19 and 20 percent, respectively.
Considering the United States as a whole, a rough idea of how well some of the standards were being met in 1977 can be obtained from an EPA report67 compiled from the records of state and local monitoring agencies in the National Aerometric Data Bank. Some figures from that report are given below.
-
Total suspended particulates (annual geometric mean): Based on 2699 stations, 40 percent exceeded the secondary standard (a guide only) and 17 percent exceeded the primary standard.
-
Sulfur dioxide: Of 1355 stations, 2 percent exceeded the primary annual mean; of 2635 stations, 2 percent exceeded the primary 24-hour mean.
-
Nitrogen dioxide (annual mean): Of 933 stations, 2 percent exceeded the primary standard.
-
Carbon monoxide (8-hour mean): Of 456 stations, 46 percent exceeded the primary standard.
-
Ozone (1-hour mean): Of 524 stations, 86 percent exceeded the primary standard.
Clearly, the pollutants can be divided into one class that is fairly well controlled (sulfur and nitrogen oxides), and one that is not (particulates, carbon monoxide, and ozone). The “uncontrolled class” is largely a product of the automobile, reinforced by industrial chemical processes, petroleum refining, oil and gas production, and the use of organic solvents (as indicated in Table 9–8).
The stationary and transportation sources also interact. Both produce emissions (nitrogen dioxide, hydrocarbons) that under the influence of sunlight lead to the formation of ozone and the photochemical oxidants (Table 9–8).
In addition, the mix of emissions from the two sectors can interact at the cellular level to increase or diminish individual toxic reactions, at least in plants (see “Agriculture and Plant Life”).
TABLE 9–7 National Ambient Air Quality Standardsa
|
Pollutantb |
Standardc |
Period |
Maximum Permissible Concentrationd |
|
|
Micrograms per Cubic Meter |
Parts per Million by Volume |
|||
|
Particulates (total suspended particulates) |
primary |
annual 24 hours |
75 260 |
|
|
secondary |
annual 24 hours |
60 150 |
|
|
|
Sulfur dioxide (SO2) |
primary |
annual 24 hours |
80 365 |
0.03 0.14 |
|
|
secondary |
3 hours |
1,300 |
0.50 |
|
Carbon monoxide (CO) |
primary |
8 hours 1 hour |
10,000 40,000 |
9.0 35 |
|
Ozone (O3)e |
primary and secondary |
1 hour (between 9 a.m. and 9 p.m.) |
240 |
0.12 |
|
Nitrogen dioxide (NO2) |
primary |
annual |
100 |
0.05 |
|
aThe standards are maximum allowable rather than desirable levels. Annual standards are mean levels that are not to be exceeded. Short-term standards are means that may be exceeded not more than once a year. The objective of regulation is to promote keeping ambient air levels below the standards (which must be reviewed by the Environmental Protection Agency every 5 years, and revised if necessary). This table is accurate as of February 8, 1979. Announcements on reviews will be made in the summer of 1979 for nitrogen dioxide and carbon monoxide and in May 1980 for sulfur dioxide and the particulates. The standards were set in 1970 (excepting ozone in 1978), so their 5-year reviews are overdue. A standard was promulgated for lead in 1978 (1.5 µg/m3, averaged on a quarterly basis). When originally promulgated, the ambient air quality standards applied throughout the United States regardless of pollution levels. The law has since been broadened to permit EPA to apply standards differentially on the basis of three classes: (1) where air quality should be preserved as it is, well below the standard, (2) where moderate deterioration would accompany controlled growth, and (3) where air quality could be allowed to deteriorate to national standards from present levels. bFederal Reference Methods are specified for the determination of pollutants. Sulfur oxides U.S. are calculated as sulfur dioxide (SO2), and nitrogen oxides (NOx) as nitrogen dioxide (NO2). Ozone is no longer an indicator for the class of photochemical oxidants (including peroxyacetylnitrates and aldehydes), but stands only for itself. There is no standard for a class of photochemical oxidants. In addition, hydrocarbons (nonmethane) averaged over a 3-hour period, have a maximum of 160 µg/m3 (0.24 ppm), for use as a guide in devising implementation plans to achieve oxidant standards. cThe primary standard is for the protection of human health. The secondary standard is to protect “welfare,” which in the words of the act “includes, but is not limited to, effects on soils, water, crops, vegetation, man-made materials, animals, wildlife, weather, visibility, and climate, damage to and deterioration of property, and hazards to transportation, as well as effects on economic values and on personal comfort and well being.” |
||||
TABLE 9–8 Nationwide Emissions from Combustion in 1977, with Totals for 1970 and 1974 for Comparison, by Source (millions of metric tons per year)a
|
Source |
Particulates |
Sulfur Oxides (SOx) |
Nitrogen Oxides (NOx) |
Volatile Organic Compounds (voc) |
Carbon Monoxide (CO) |
|
Transportation |
1.1 |
0.8 |
9.2 |
11.5 |
85.7 |
|
Stationary fuel combustion |
4.8 |
22.4 |
13.0 |
1.5 |
1.2 |
|
Electric utility |
(3.4) |
(17.6) |
(7.1) |
(0.1) |
(0.3) |
|
Industrial processesb |
5.4 |
4.2 |
0.7 |
10.1 |
8.3 |
|
Miscellaneous |
1.1 |
0.1 |
0.2 |
5.2 |
7.5 |
|
1977 TOTAL |
12.4 |
27.4 |
23.1 |
28.0 |
102.7 |
|
Comparison totals |
|
|
|
|
|
|
1974 |
17.0 |
28.4 |
21.7 |
28.6 |
99.7 |
|
1970 |
22.2 |
29.8 |
19.6 |
29.5 |
102.2 |
|
aThe emission estimates for particulates, sulfur oxides, and nitrogen oxides embrace a broader range of substances than are measured by routine ambient air quality monitoring equipment (see footnote b, Table 9–7). voc are not quite equivalent to nonmethane hydrocarbons, the usual category. bIndustrial processes include emissions from chemicals, petroleum refining, metals, mineral products, oil and gas production and marketing, organic solvent use, and other processes. Source: U.S. Environmental Protection Agency, National Air Quality, Monitoring, and Emissions Trends Report, 1977 (Washington, D.C.: Environmental Protection Agency (EPA-450/2–78–052), 1978). |
|||||
Finally, combustion of organic fuels from whatever source produces carbon dioxide, whose direct effect on health is observed only under extreme conditions68 but whose potential effect on climate is a major concern (discussed under “Global Climate”). Per Btu of energy released,
carbon dioxide production stands in the ratio of 1.0 to 0.8 to 0.6 for coal, oil, and natural gas; for coal-derived liquids it is 1.4.69
The Clean Air Act as amended requires that the Environmental Protection Agency review the ambient air quality standards every 5 years and change them if the criteria indicate such action. The first such review was completed early in 1979.70 Ozone, which had been regarded as a parameter of the photochemical oxidants, was redefined as a pollutant, and its permissible level was raised from 0.08 to 0.12 ppm.71 The agency argued that no significant disadvantage to health or welfare would result from such a change and that the level of smog would be unaffected, As a formal result, it is expected that the 86 percent violation rate recorded in 1977 (and noted above) will be decreased in 1979.
Court actions are being instituted challenging the agency’s position, some arguing that the standard should not have been relaxed, others that it was not relaxed enough. A critical survey of the decision-making process for the new ozone standard would enable standard-setters of the future to learn from this experience. The scientific and sociological factors that led to relaxing the ozone standard should be compared to those that led to greatly increased stringency in the radiation standard discussed previously.
Projections The National Energy Plan (NEP) of 1977 called for the use of 13.5 additional quads of coal by utilities and 4.5 quads by industry in 1990. A joint analysis by six national laboratories72 concluded that, of various factors, the degradation of air quality would be the major constraint in reaching this goal and that its prevention depends critically on siting. The analysis, which concentrated on particulates and sulfur oxides, was based on county-by-county (not within-county) data and assumed that the increase in use would tend to be directly proportional to current use (August 1977). Because such a distribution places increased use in or near many nonattainment or limited areas, approximately 50 percent of the projected industrial use of coal and 25 percent of that projected for utilities was constrained.
These estimates are inflated by the use of county-level analysis that ignores the adjustments possible within counties. Overall, the estimated atmospheric levels of pollutants were about the same on regional and national maps as today’s levels, owing to the emission controls required of new plants.
We judge that these results do not rule out doubling the use of coal. They demonstrate the necessity for integrating the several problems of siting (on both a within-county and a regional basis) with other aspects of energy planning.
As indicated in the following section, the epidemiological evidence concerning the health impact of air pollution from coal combustion
products is inconclusive. The widely quoted studies indicating severe impact of sulfates resulting from the atmospheric transformation of sulfur dioxide emissions are seriously flawed. On the other hand, there is also no evidence to suggest conclusively that present standards are too tight, or even that there are no health risks at present ambient levels. The present regulatory strategy must be considered as conservative in the light of existing evidence, but probably justified in view of the prospective rapid growth in the use of coal, especially for electric power generation, and the much higher cost of retrofit compared with tight standards on new plants. The case for conservatism is reinforced by the prospect that the largest growth in electric generation is likely to occur during the next 15 years, with a slowdown thereafter, as suggested by the various CONAES scenarios. Moreover, the possibility of a slowdown or even a moratorium on nuclear growth owing to public opposition also argues for holding coal emissions as low as practical in case coal expansion has to take place at an even greater rate than projected in the CONAES estimates.
The one emission whose effect is independent of siting is carbon dioxide. Increasing fossil fuel combustion will increase carbon dioxide emissions and tend to raise the level of carbon dioxide in the atmosphere. This problem is discussed under “Global Climate.”
Research on the Health Effects of Air Pollutants The setting of national air quality standards was a landmark in the history of public health protection in this country. The original standards were selected in 1970 by reviewing the available epidemiological and clinical evidence, deciding on a level at which a minimal effect was observed in man, and setting the standard at a level below it.73–77 For the fossil fuels, the major designated pollutants that emanate from stationary sources are the particulates,78 sulfur oxides,79–81 and nitrogen oxides.82–84 For the mobile sources85 they are ozone,86 carbon monoxide,87 nonmethane hydrocarbons,88 nitrogen oxides, and as a secondary product, photochemical oxidants.89 Toxicity was judged by excess morbidity, primarily of the respiratory system, by excess mortality occurring during major fog pollution episodes, or on a daily or other short-term basis associated with fluctuations in the sulfur dioxide and particulate levels over the course of a year or more.
In addition, it had been recognized that coal tar is a classical source of chemical carcinogens. An excess of cancer had been demonstrated as an occupational hazard in certain industrial operations where the workers were heavily exposed to fumes from coal processes90 (present-day practices would reduce such exposure). These carcinogens may include mutagens. Control of particulates would presumably diminish exposure of the public to the chemical carcinogens and mutagens released by combustion. It is
anticipated that the use of synthetic liquids or high-Btu gaseous fuels from coal will be cleaner than burning coal directly.91*
The following discussion reviews some trends in health research that bear on the primary standards. The analysis establishing sulfate (at prevailing levels) as an important determinant of mortality has been rejected. There is expanding interest in the many roles played by the particulates. Appreciation has increased for the great complexity of the epidemiology; for example, the difficulty of assessing the risk is magnified (perhaps disproportionately) as the level of pollution diminishes. Quantitatively significant health effects have not been established below the standards; if they exist (and they might), more sophisticated investigation must be used to find them.
Past studies on sulfur dioxide and sulfates were most valuable in showing the complexity of the epidemiological problems. With increased experience, investigators and reviewers have become much more critical of investigational design.92–97 In fact, two investigators who supplied the classical findings that related fluctuating daily mortality rates to changing ambient levels of sulfur dioxide have independently concluded that their original analyses (considered sophisticated at the time) were not sophisticated enough, and they no longer accept their own conclusions.98,99 One investigator100 could show the same associated fluctuations several years later, even though the general level of sulfur dioxide had fallen by a factor of 10. He considers that both mortality and sulfur dioxide levels are fluctuating in response to some other factor. The other investigator101 has undertaken a study of how the many variables at work, including daily fluctuations in temperature, may interact or otherwise confound the analysis, and of what the statistical and methodological requirements of such investigations might be.
Another study102 that attempted to establish the mortality risk factor for sulfate, based on intercity comparisons, concluded that per 100,000 persons at risk, 3.25 deaths occurred per microgram of sulfate in a cubic meter of air. The study has been widely quoted, and the coefficient has been used to quantify the hazards of increasing the use of coal.
The study was a retrospective one, based on vital and other public statistics. The investigators had no control over the chemical methods used to estimate ambient air levels of sulfate. The parameter of exposure for any given year was usually obtained thus: Each urban area had one or perhaps two sampling stations; one analysis was performed biweekly; of the 26 analyses per annum available in about two thirds of the urban areas studied, the lowest value of each set was selected as the best parameter of annual exposure for that area.
The study and its mortality-rate factor for sulfate have been criticized103,104 and rejected.105–107* The critical comments point out defects, for example, that result from multicolinearity of the sulfate measure with a number of urban characteristics. When one critic redid the analysis, correcting for defects, the original positive effect for sulfate disappeared.108,109 A report issued by the National Academy of Sciences110 concludes that there is insufficient evidence to establish an ambient air quality standard for sulfate in addition to that for sulfur dioxide.
For particulates, one review111 that synthesized the published statistics of 17 major pollution episodes found an excess of 1 percent in the concurrent mortality rate for each increment of 100 µg/m3 of total suspended particulates (TSP). The levels in 16 of the incidents, occurring from 1930 to 1975, were measured or estimated to fall between 500 and 5000 µg/m3, well above the present primary standard (Table 9–7).
In time-series studies in single cities (mean annual levels, 130–215 µg/m3), the mean annual mortality increment was 0.6 percent per 100 µg/m3. For intercity comparisons (cross-sectional studies) based on mean annual rates, an almost significant excess of 6 percent mortality per 100 µg/m3 was found.
The positive association between particulates and mortality leaves open the question of “cause of death”—the toxic agent and the pathological process.112 Particulates could be the surrogate for a large number of combustion products, including sulfates. Population mortality rates serve as indicators of many different factors, social and biological, and thus depend not only on immediate circumstances, but also (and generally much more) on the population’s past history. It will be of great importance to determine if such results can be found in the range of exposures at or below the levels achieved under current standards113 (the data were drawn chiefly from 1950–1972).
The same report114 also found a positive association for manganese in a few specific locations and concluded that these areas should be restudied when the 1980 census data become available.
The linear relationship between mortality and the level of total suspended particulates in the range above the standard is of major interest and indicates the need for further intensive research (some is already under way). Presumably, a more sensitive and specific endpoint than mortality would be desirable.115 Improved dosimetry, especially at or below the levels of current standards, would be essential in sorting out the many interrelated problems and factors that apply to atmospheric pollutants in general, as illustrated by the following considerations.116–118
-
Probably less than 1 percent of total suspended particulates from an uncontrolled source can reach the lungs, owing to filtration by the upper respiratory tract (the respirable particulates (RP) are less than 2 µm in diameter). The trapped particulates are mostly excreted through the digestive tract
-
As fly-ash emissions from stationary sources continue to be reduced, the larger particles are removed more and more efficiently, but the smaller respirable particles (less than 1 µm) continue to be emitted. If (as generally assumed) the respirable fraction is the toxic fraction (because it reaches the lungs), the proposed standard of 99 percent reduction in particulate emissions from stationary sources would not yield proportional benefits to health, although tangible gains (cleanliness, visibility) will be realized.
-
The fine particulates include complex organic compounds (including mutagens and carcinogens) and various toxic metals such as arsenic, lead, mercury, and zinc (from trace amounts in coal). During transit, the emitted gases (sulfur dioxide, nitrogen dioxide) form aerosols through condensation and coagulation and may react with other fine particulates,119 which can lead to increases in particulate size and may change their eventual distribution in the body.
-
The particulates may travel hundreds of miles from their point of origin. The emitted gases that accompany them may be oxidized to sulfates and nitrates (catalyzed in part by transportation pollutants), and other chemical changes may occur, influenced by varying conditions of temperature, humidity, and solar irradiation.120 Precipitation may bring them down, or their nature may be influenced by substances originating in the territory over which they travel. The qualitative nature of the particulates, therefore, changes in transit and also with place and season. Both increases and decreases in toxicity may be significant.121
-
A number of sources, perhaps widely distributed, are responsible for the pollutants observed at any particular place, since pollutants travel far. These independent contributions vary diurnally and seasonally, and are differentially affected by meteorological conditions. The task of estimating the pollution profile for large regions (or metropolitan areas) on the basis of projected energy plans is complicated by the multiplicity and uncertainty of all these factors. The analysis and modeling necessary to the task have been initiated122–125 and a projection for the National Energy Plan of 1977 has been completed.126 Such profiles, moreover, must ultimately be related to individual dosage to determine epidemiological effects.
Besides dosimetry, the factor of time must be emphasized in further epidemiological research. Experience has shown that important aspects of the epidemiological studies necessary in this area cannot be hurried to meet the demands of policy,127 despite the significance of this consider-
ation. Chronic and late effects take time to develop. We know of one study well under way that tends to meet the requirements discussed here.128 It is a prospective study of comparable populations in six small cities. One such study, however, cannot answer the massive array of questions that confront the policy maker who needs critical examination of the validity of present standards. Much more work will have to be done, and over an extended period of time. We favor conducting much of this work outside government laboratories to ensure its independence, and to provide flexibility to draw upon the country’s scientific manpower.
CATASTROPHES
Nuclear power plants, liquefied natural gas, and large dams pose a danger of catastrophic accidents. The greatest potential risk is that of catastrophic accidents in nuclear power plants.*
Gas129 The principal potential hazard of natural gas is associated with leaks in the pipeline distribution system. In the case of liquefied natural gas (LNG), its cryogenic properties create additional hazards. Contact, for example, will damage human tissue. On vaporization and exposure to oxygen and a source of ignition, the gas will burn and possibly explode. The worst LNG accident on record occurred at a storage facility in Cleveland in 1944: The accident killed 130 people and caused $10 million worth of property damage. It is highly unlikely that an accident of this type could occur today, since the development and use of materials that resist brittle failure at cryogenic temperatures have largely eliminated the cause. Nevertheless, if liquefied natural gas is released in an accident, it may form a vapor cloud that travels several miles before igniting. In 1972, 40 workers were killed by inhalation of the vapors in an emptied tank on Staten Island. Liquefied natural gas is now being shipped from foreign countries in tankships for receipt in ports with large storage facilities. The degree of hazard in shipping and storing LNG is controversial.130
Hydroelectric Power131 In the 40-yr period from 1918 to 1958, 1680 deaths occurred from the failure of five dams: a statistical average of 40 deaths per year. The total number of dam failures was 33. Assuming that the average number of dams in the United States over this period was 1000, a failure rate of about 8×10−4 per dam-year can be estimated, and a major disaster rate of 1.3×10−4 per dam-year.
|
* |
See statement 9–8, by H.Brooks, Appendix A. |
From 1959 to 1965, nine major dams of the world failed (of an estimated 7800). The worldwide failure rate was about 2×10−4 per dam-year.
The probability of failure of a particular dam is difficult to estimate, but can be strongly dependent on geological setting, surface faulting and seismicity, as well as on the type of construction, size, and other factors. Damage will depend on such factors as topography, storage volume, the cause and mode of failure, the size of the population at risk (some thousands to more than 100,000), and on mitigating factors, such as evacuation.
Nuclear Power Reactor accidents are discussed in chapter 5 from a physical and engineering point of view. A major conclusion of that discussion is that the more serious the accident, the less probable its occurrence. Two important implications follow. First, the relatively frequent minor accidents provide opportunities to improve design and lessen overall risk. Second, there is a “dominant risk,” defined by maximum cumulative damage, that may be used to characterize and compare particular reactors. The dominant risk is chosen on the basis of a maximum value for the expression: (probability of incident)×(damage per incident).
It should be noted that even large accidents in light water reactors are not analogous to the explosion of an atomic bomb. There is no explosion or release of neutrons; buildings outside the reactor plant are not damaged physically, nor are fires ignited. Property, ground, and water damage are due to radioactive contamination. The danger to human beings is the inhalation or ingestion of radioactive substances or gases, or irradiation from such substances released to the atmosphere or deposited on the ground. (The occupational hazard within the plant could at some time involve exposure to neutrons.)
Three Mile Island In the case of light water reactors, accidents with dire consequences have not occurred in some 400 reactor-years of operation, but an accident that severely damaged the reactor core did occur at the Three Mile Island plant in Pennsylvania on March 28, 1979. It involved a partial failure of the cooling system. The analysis of what happened is being conducted by a presidential commission, whose report is due in October 1979, and other groups.
Of particular interest is the report of the Ad Hoc Population Dose Assessment Group, staffed by technical experts from three government agencies.132 During the period March 28 to April 7, it was necessary to vent quantities of radioactive gas. These gases constituted an atmospheric risk to approximately 2 million people who reside within a 50-mile radius of the plant. The principal emissions were xenon-133 (133Xe) and, to a
lesser degree, 131I, whose half-lives are 5.3 and 8.1 days, respectively. The initial estimate of the total population dose was 3500 person-rem. The mean dose was about 2 mrem (0.002 rem) per person. The highest dose anyone received was below 100 mrem. These estimates are likely to be too high, since they do not allow for the protective effects of evacuation, nor for protective shielding by buildings in the case of persons staying indoors. The amount of radioactivity in milk due to the incident was trivial.
Using the linear dose-response hypothesis, it can be estimated that less than one cancer and less than one serious genetic effect were induced in the entire population at risk (the existing cancer mortality rate for that population is 3400 per year).
At the time of the committee’s final deliberations, it appeared that the health risks of this accident were negligible (a single automobile accident during the evacuation would have done more damage), but the doubts and fears raised by the incident could have far-reaching consequences.
WASH-1400 In 1975 the Nuclear Regulatory Commission published the results of a study undertaken to estimate the probability of the great variety of accidents that might occur in the operation of nuclear reactors. The Reactor Safety Study (known as WASH-1400 or the Rasmussen Report)133,134 employed decision-tree and fault-tree analysis, based on judgment of risk at each step of a specific sequence of events leading to an accident, and tempered by the operating experience that had been accumulated.
The NRC has recently withdrawn its approval of the absolute probabilities of risk of reactor accidents calculated in the report,135 without commenting whether individually or as a group they are too high or too low. Nonetheless, as the external review group of the NRC has pointed out,136 WASH-1400 is still the most comprehensive attack on the problem of reactor safety, and its approach, collected materials, discussions, and experience are of great importance in providing a base from which the examination of this complex problem can advance.
Reactor accidents that involve “breach of containment” release radioactive materials into the atmosphere and thus may affect the public. The accident at Three Mile Island did not breach containment; its radioactive emissions were released by the operator in the course of controlling the rising pressure within the reactor during its shutdown (necessitated by insufficient cooling).*
WASH-1400 estimated the frequency of accidents of this severity as 1 in 300 to 1 in 30,000 reactor-years of operation. The range has proved to be
correct, since the accident is the first in about 400 reactor-years of experience.†
Table 9–9 lists three types of progressively more serious accidents and their diminishing probabilities. WASH-1400 is interesting in its attempt to deal with human dosage, which is especially sensitive to meteorological conditions at the time of the accident and the distribution of population around the reactor site.
Taking the 68 reactor sites then in operation or under construction, six typical weather-type sites were selected, each located in one of six geographical regions (eastern river valley, eastern seacoast, southeastern, midwest lakeside, midwest plains, west coast). For each site, a specific surrounding population was constructed over a 50-mile radius (on the basis of the actual populations associated with reactors in that geographical region). Although for each composite population the area distribution is less variable than that for the group of existing populations that it represents, care was taken not to average out the extremes within it. Weather data obtained from six typical sites were employed to predict conditions at the six composite sites. However, the calculations do not allow for variations in wind direction as the plume travels downwind from the reactor, during which it might be dispersed over hundreds of miles. Dosage calculations were made separately for external exposure, inhalation, and ingestion. A correction was made for evacuation of the population from an area within 25 miles downwind of the reactor, which typically would reduce the early health effects by a factor of 3.137
Table 9–9 gives the WASH-1400 estimates of damage for (1) core meltdown, (2) core meltdown followed by aboveground breach of containment, and (3) an accident of the type described in (2) followed by adverse conditions of wind, weather, and population density in the path of the radioactive cloud that is released. Although the nominal probabilities given in Table 9–9 are now in question, it is useful to consider them with respect to order of magnitude, and especially relative to one another: 1 in 20,000, 1 in 1 million (dominant risk; see chapter 5, “Reactor Accidents”), and 1 in 1 billion reactor-years, respectively.138 The reactors are assumed to be 1-GWe plants. Power plant experience in the United States totaled about 400 reactor-years by January 1979, and we are accumulating about 70 reactor-years of experience every year.
Table 9–9 provides estimates of property damage and health effects appearing within 1 year of the accident, and those appearing over a period of years—thyroid nodules (for which there is effective treatment), cancer deaths, and genetic defects. The table illustrates the range of morbidity
and mortality that might result from different types of accidents and also indicates the tremendous complexity of making such estimates.
The table reveals that the consequences of a single accident may be very large but that such accidents are improbable, and the consequences are small per year when averaged over a period of years. Thus, if the chance of an accident is 1 in 1 billion years, a total of 3000 deaths averages only 1 death per 333,000 years. Such averaging, however, does not consider that the social disruption of a catastrophe can be much greater than that associated with a distributed set of small accidents producing the same number of deaths.
It is of interest to compare the hypothetical catastrophic risks of Table 9–9 with those of routine power plant operation. Core meltdown with aboveground breach of containment (the dominant risk) carries a probability of once in a million reactor-years and leads (in round numbers) to less than 10,000 deaths from all causes. Over the same hypothetical period of 1 million reactor-years, the routine operation of the nuclear plant would be associated with 200,000 deaths, that of an oil-fired plant also with 200,000, and that for coal with 2 million (Table 9–1).* If we suppose that the nuclear catastrophe is underrated tenfold, the risks of routine operation and those of catastrophes become approximately equal.
WASH-1400 has been criticized for (inter alia) underestimating some hazards owing to the use of median rather than mean estimates,139–141 and for underestimating the uncertainty of its results. The recent report by the Risk Assessment Review Group to the Nuclear Regulatory Commission142 and the Ford/Mitre Report143 both make the latter point. The review group of the NRC points out both overly conservative and inadequately conservative assumptions in the probability estimates, and the group concludes that the uncertainty is seriously understated. Both groups note that reactor experience provided an upper bound at the times of their reports.
AGRICULTURE AND PLANT LIFE
The effects of pollutants on plant life are judged in terms that are quite different from those applied to judge the effects of pollutants on human health. Cancer, for example, is not a problem in the case of crop plants, nor are the late effects of exposure that take years to develop. While genetic effects might be of interest in special cases, the rare occurrence of a mutant in a wild or cultivated population is not important. Major interest
|
* |
Statement 9–11, by J.P.Holdren: Table 9–1 includes only the deaths caused by industrial accidents, mostly to workers except in the case of coal trains. The comparison is meaningless. |
TABLE 9–9 WASH-1400 Estimates of Probabilities of and Damage to the Public from Three Types of Major Accidents to Light Water Reactors, with Statistical Rates of U.S. Population for Comparisona
|
Accident |
Public Property Damage Excluding Reactor (millions of dollars) |
Health Effects Within One Year |
First Year’s Health Effects Plus Delayed Health Effects |
||||||
|
Type |
Probability Per Reactor Yearb |
Deaths |
Illnesses |
Population at Risk Within 25 mic |
Cancer Deathsd |
Thyroid Modulesd |
Genetic Defectse |
Population at Risk Within 500 mic |
|
|
Core meltdown |
5×10−5 |
1 |
negl. |
negl. |
negl. |
3 (0.1) |
3 (0.1) |
75 (0.5) |
1×104 |
|
Plus above-ground breach of containment (dominant risk) |
1×10−6 |
1,000 |
1 |
300 |
4,000 (within 5 mi; density 50 per square mile) |
5,100 (170) |
42,000 (1,400) |
3,750 (25) |
2×106 (density 77 per square mile) |
|
Plus adverse wind, weather, and population density |
1×10−9 |
14,000 |
3,000 |
45,000 |
1×106 (within 25 mi; density 500 per square mile) |
45,000 (1,500) |
240,000 (8,000) |
26,000 (170) |
10×106 (density, includes maximum)f |
|
Statistical rates for U.S. populationg |
— |
— |
9,000 |
— |
1×106 |
1,700 |
800 |
800 |
1×106 |
|
aAmong the major factors allowed for in projecting damage are the following: absolute probability of core melt, relative probability of various radioactive release categories after core melt; probability of various types of weather conditions; probability that a particular population density distribution will be exposed. See text. Specific details are given in WASH-1400 (especially Tables 5–4 and 5–5, and in Appendix VI, Section 9). A survey and some additional discussion are given in U.S. Nuclear Regulatory Commission, Overview of the Reactor Safety Study Consequence Model (Washington, D.C.: U.S. Nuclear Regulatory Commission (NUREG-0340), 1977). The ranges of uncertainty in these estimates are under review, and most likely will be increased. The present ranges are as follows: for accident probabilities, one-fifth to five times the stated value; for cancer deaths, one-sixth to six times; for thyroid nodules, one-third to three times; for prompt health effects, one-fourth to four times; and for genetic effects, one-sixth to six times. Effective evacuation is assumed for 30 percent of the population, partially effective evacuation for 40 percent, and ineffective evacuation for 30 percent. Evacuation for up to 25 miles downwind of the reactor is allowed for. bThe Nuclear Regulatory Commission no longer accepts the absolute probabilities given here. They are still of interest, especially relative to one another. See text. cHealth effects within one year are seen only in those persons with doses of more than 50 rem. Their number may be quite small, although large numbers may receive very small doses and be liable to delayed effects. Population figures were supplied by Norman Rasmussen. dAssumed to occur from the tenth to the fortieth year after the accident. The statistical mean annual rate during this period is given in parentheses; the actual annual rates would vary considerably. The estimates are based on a sum of an organ by organ survey. The probability of cancer death (per personrem) depends on three dose-factor components: that for internally deposited radionuclides (5×10−4 cancer deaths per person-rem), that for external exposure (1.2×10−4), and that for a “dose-rate and dose factor.” (Below 10 rem/day or a total dose of 25 rem, the absorbed dose is progressively less effective; the maximum reduction of 80 percent is at less than 1 rem/day or at a total dose of 10 rem or less.) By and large, this comes to a rule of thumb of 1×10−4 cancer deaths per rem (whole body). eTotal for all generations. The mean annual rate is given in parentheses. All types of detect are included: 20 percent of the dominant effects and 10 percent of the recessive effects are assumed lost per generation. fMean population density in the United States is 77 per square mile. In this case, urban areas of great density are included. gThe health rates given are for the United States, per million of population per annum. Source: Compiled from U.S. Nuclear Regulatory Commission, Reactor Safety Study: An Assessment of Accident Risks in U.S. Commercial Nuclear Power Plants (Washington, D.C.: U.S. Nuclear Regulatory Commission (WASH-1400), 1975). |
centers on the immediate effects of pollutants on plant growth,144–147 the associated effects in ecosystems, or both.
It was estimated in 1973148 that about $100 million of crop loss was due to photochemical oxidants and that another $13 million loss was due to sulfur dioxide. Such estimates do not account for all effects on plant life, but do establish that a general problem exists. In heavily polluted areas such as the Los Angeles Basin, it has become necessary to abandon many citrus groves, and elsewhere truck crops, particularly of leafy plants, are increasingly hard to cultivate. Such effects extend to natural ecosystems. In some forested areas under observation, lower dosages of pollutants tend to eliminate trees, then, as duration of exposure lengthens, affect tall shrubs, and later, other plants.
The photochemical oxidants149 (primarily the products of vehicles) are considered the most important agents, followed by sulfur dioxide.150,151 Sulfate, which has been considered in epidemiological studies, is not directly toxic to plants (see “Water and Climate”).
For sulfur dioxide, there may be a practical threshold of toxicity, owing to the presence of a detoxification system.152 When the system is saturated, toxicity will occur. The absorption of sulfur dioxide by the plant can be beneficial to growth in soils that are deficient in sulfur.153 Nitrogen dioxide alone is relatively innocuous but indirectly important through interaction with the photochemical-oxidant system.154
Biological interactions between sulfur dioxide and the photochemical oxidants (e.g., ozone) depend on plant species and apparently vary a great deal: They can lead to additive, reduced, or more than additive responses. Much more research is needed to clarify the nature and importance of their combined effects on vegetation.155
One of the most striking aspects of the general problem is that plants vary so greatly in their sensitivity.156 Among trees, the white pine is especially sensitive.157 Examples among vegetables are pinto beans, potatoes, and lettuce. Most sensitive of all appear to be the mosses and lichens that may show signs of injury after exposure to sulfur dioxide at levels one third those of the human annual ambient air quality standard.158 The first European Congress on the Influence of Air Pollution on Plants and Animals (1968) recommended that mosses and lichens be employed as biological indicators of pollution because they are easily handled and exhibit a range of sensitivity that greatly exceeds that of most higher plants.
The variation in sensitivity among species indicates clearly that genetic factors are important, and within species, clones of more or less resistant plants have been produced experimentally.159,160 In Connecticut, the selection, breeding, and production of tobaccos resistant to ozone saved
the shade-tobacco industry. On the other hand, the production of spinach declined sharply, in part because the plants available were so susceptible.
Aside from the importance of such work in its own right, the investigations with plants may provide information about the subtle biochemical interactions of pollutants that would be much more difficult to discover initially with animals, but once known or suspected, could be effectively sought.
The interaction between sulfur dioxide and the photochemical oxidants supports the view that under various circumstances, interactions within the biological system negate analysis based on a model of “one agent, one effect.” Furthermore, the two (or more) agents are likely in this case to come from two different sources—sulfur dioxide from an electric utilities plant, photochemical oxidants from vehicular traffic. The apportionment of responsibility, therefore, may likewise involve interactions.
WATER AND CLIMATE
This section is concerned with the effects of energy systems on precipitation, climate, and water supply. As dealt with here, these tend to be very large-scale problems, affecting regions of the nation or even the entire world.
ACID PRECIPITATION
The formation of acids in the atmosphere from combustion-generated sulfur dioxide (SO2) and nitrogen dioxide (NO2) acidifies rain and snow.161–163 It was estimated that about 60 percent of the effect 10 years ago was due to acid-sulfate aerosols, and 40 percent to acid-nitrate aerosols. During the past 10 years, however, the nitric acid moiety has become relatively more important, presumably because of the increased use of low-sulfur fuels. Best available control technology (cf. chapter 4) will continue for some years to reduce sulfur oxide emissions relative to those of nitrogen oxides.
The distribution pattern of acid rain, determined by meteorological conditions, may extend for many hundreds of miles from the source, as first dramatically demonstrated in Sweden and Norway, where the effects were attributed to emissions from central Europe and the United Kingdom. In the United States, the Northeast is the focal area, and the pH of precipitation now averages annually about 4–4.3, with values as low as 2–3 observed at particular locations during storms. To the west and northwest, the pH gradient rises (to fall eventually in certain restricted areas), and in the desert regions, the pH averages about 7 (neutrality). The
FIGURE 9–5 Acidity of precipitation falling in the United States during June 1966. Source: Gene E.Likens, “Acid Precipitation,” Chemical and Engineering News, November 22, 1976, 54 (48):30.
maps in Figures 9–5 and 9–6 illustrate the increasing spread of the low-pH region over a period of some 15 years (1955–1972). These trends were confirmed in 1978.164
The ecological effects are greatest in waters that contain the least dissolved matter—waters that are poorly buffered. Thousands of lakes in southern Norway and Sweden have shown a decline in fish populations, associated with increased acidity of the water, in turn associated with acid precipitation. A similar trend has been reported for the Adirondack Mountain region. Effects on terrestrial systems have been more difficult to isolate unambiguously, perhaps because changes register less quickly. A recent report165 points out that as a result of acid precipitation, the forest-floor leaching mechanism in a New England coniferous ecosystem has changed from a carbonic-organic acid type to a mineral acid type, which may accelerate leaching and increase the concentrations of dissolved trace metals of potential toxicity. Damage to forests and sport fishing has been estimated at $100 million annually.166
Comparing various fuel cycles, it appears that the use of all fossil fuels involves some risk owing to the production of NO2, and the use of those containing sulfur (coal, oil) present an additional risk.
Energy systems can influence climate on a large scale by the production of heat and by the production of carbon dioxide, both discharged into the atmosphere.172 Other less important factors include the discharge of particulate matter and water vapor, and the resulting changes in albedo. From a global point of view, heat production is trivial: It amounts to just 0.01 percent of the sun’s input of 150 W/m2, when averaged over the earth’s surface. On a regional or local basis, however, the concentration of industrial and other energy systems may produce sufficient heat to change patterns of wind, precipitation, humidity and cloudiness, and to elevate temperature. While of significant local interest, these effects—which can develop and disappear rapidly since they are reversible—are much less important than those of carbon dioxide. Carbon dioxide production could become the chief factor limiting the use of fossil fuels.
Although quantitatively a minor constituent of the atmosphere (330 parts per million by volume (ppmv) in 1979), carbon dioxide takes part in the control of temperature through the so-called “greenhouse effect.” Virtually transparent to visible light, carbon dioxide strongly absorbs certain infrared wavelengths (heat) radiated from the earth’s surface, to which other atmospheric gases are transparent. An increase in carbon dioxide in the troposphere, therefore, alters the path of the radiation of heat from earth into space, and thereby elevates the temperature of the lower troposphere—that portion of the atmosphere 5 miles from the earth’s surface and below—in which variations in weather are largely determined.
The concentration of carbon dioxide in the atmosphere has been growing in parallel with increasing fossil fuel consumption throughout the world.173 The future rate of increase will depend on the continuing availability of oil and natural gas and especially on the increasing use of coal. To the extent that coal is substituted for the other two fuels, the problem will be worsened: Coal produces some 200 lb of carbon dioxide per million Btu (89 metric tons per trillion joules), oil produces 80 percent as much, and natural gas about 57 percent. Synthetic liquids from coal produce 140 percent as much.174 Nuclear power makes no contribution to this problem, nor do solar technologies.*
It is estimated that the atmospheric concentration of carbon dioxide has increased some 10 percent since the beginning of continuous measurements in 1958. At present rates of growth in the consumption of energy (according to one estimate175), it could rise from the present level of 330
|
* |
See statement 9–12, by H.Brooks, Appendix A. |
FIGURE 9–7 The record of carbon dioxide concentration from 1860 to 1975, measured at several locations, and some estimates of possible future trends. Source: W.W.Kellogg, Effects of Human Activities on Global Climate (Geneva, Switzerland: World Meteorological Organization (Tech. Note 156), 1977).
ppmv to 360–400 by the year 2000, and might double by 2040 (Figure 9–7). Uncertainty about the quantitative role of the biosphere in the overall carbon dioxide cycle adds uncertainty to these estimates,176 but the Risk and Impact Panel177 and other experts consider the projected trend correct.
Figure 9–8 illustrates one set of gross estimates for additional increments of atmospheric carbon dioxide and resulting rise in temperature. The estimates indicate the following.
-
For a doubling of atmospheric carbon dioxide concentration there is a 2°C–3°C rise in the average temperature of the lower atmosphere at middle latitudes, and a 7 percent increase in average precipitation.
-
The temperature rise is threefold to fourfold greater in the polar regions in this model.
-
For each 1°C rise in average temperature for the middle latitudes, there might be a 10-day average increase in the growing season. In the higher latitudes (40°C–50°C), the average growing season could be
FIGURE 9–8 The mean surface temperature record for the Northern Hemisphere from 1850 to the present (solid line), and one estimate of the course that it might have taken without the addition of anthropogenic carbon dioxide (dashed line). Source: W.W.Kellogg, Effects of Human Activities on Global Climate (Geneva, Switzerland: World Meteorological Organization (Tech. Note 156), 1977).
-
lengthened by 2 or 3 times this amount. (There could be wide local variations from the average.)
-
Extensive and complex changes in precipitation patterns might occur as a result of the diminution in the polar-equator temperature difference, and general enhancement of the hydrological cycle.
A doubling of the carbon dioxide level presumably would have no significant direct effect on human health.178
The implications of the changes indicated above are potentially great. Increased temperature in the polar regions would lead to changes in precipitation patterns. Not only would the duration of the seasons be affected in other latitudes, but also humidity, cloudiness, and rainfall, which in turn would affect the extent and location of agricultural lands. Shifts in grazing, agricultural, and forest belts might occur, as well as increases or decreases in their extent. Such regional modulations might be much more important than the rise in temperature alone. Increased
temperatures in the polar regions might lead to a “slow” melting of polar ice, in turn leading to a slowly rising water level and changing coastline.
It is important to emphasize, however, that while the trend of such a global picture may be generally correct, it has no degree of certainty today for particular times or places. Present models cannot project when and what will happen at Fargo, North Dakota, or Paris, France. Obviously, the regional effects might be good or bad. But even if the ultimate effects are good, the transitional period will presumably involve significant dislocations and inconvenience at the very least, if not problems that are far worse. Furthermore, the realization that major changes are anticipated is bound to cause distress and political tension.
The problem is a global one that should be analyzed and planned for at an international level. Further study is required to predict when major climatic changes might occur in relation to anticipated carbon dioxide levels, and to determine the influence of changes in carbon dioxide output now, or 15 years from now, on the course of climatic events. The graphs in Figures 9–7 and 9–8 project the possibility of perceptible changes by the year 2000, and the possibility of significant changes within 50 years. Study is required to predict with greater certainty and precision if and where the changes will occur, to outline their varied distribution, and to indicate more precisely what their consequences will be. Areas of uncertainty as well as certainty in these predictions should be defined as precisely as possible. On the basis of such estimates, society will have to consider building sufficient flexibility into its economic and international organizations to have some chance of adjusting to the changes gradually—in advance of their occurrence, if possible. Such adjustments might well include reduction in dependence on fossil fuels. This is one important reason to increase the diversification in our energy supply system, and to have nonfossil energy sources available for rapid substitution in the future.
WATER SUPPLY
A detailed discussion of the risk that increased energy consumption will induce a shortage of water, or that the limitations in water supply may curtail the use of energy, is given in chapter 4, largely based on the report of the Risk and Impact Panel.179 Here we note briefly that the water problem may become a major limiting factor of energy availability. The difficulties pertain to mining, to the increased production of electricity, and to the proposed production of synthetic fuels from coal.
Some water is consumed in the routine processes of coal mining. The mining of coal or oil shale can disrupt aquifers and contaminate local drainage systems with acidic wastes.
More dramatic, however, is the issue of land reclamation in the arid
western regions containing large deposits of surface coal (the upper Colorado and Missouri hydrological regions). By law, the mined land must be reclaimed, but the report of the Risk and Impact Panel warns that the success of reclamation in the high arid plains of these regions will depend critically on the ability of the soil to redevelop its capacity for aeration, water retention, and biological nutrient regeneration. The amount of water required per year to assist this process, for how many years, and with what promise of success remains an open question. Adequate water supply depends on the particular conditions at specific sites, but in general, the outlook for satisfying additional demands for water from indigenous supplies in these regions is very poor.
On the other hand, large amounts of coal in the eastern production regions lie within the basins of the upper Mississippi, Ohio, and Tennessee Rivers. Even though increased mining and reclamation may indeed be feasible in these and certain western areas, the use of that coal in the production of synthetic fuels or for the production of additional electricity could again raise the question whether local supplies of water are adequate. The generation of electricity consumes 15 times more water than mining the coal it burns.
Suppose the nation required an additional 18 quads of coal per year for the generation of electricity (11.5 quads) and the production of synthetic fuels (6.5 quads) for the next 20 years (a significant amount in national plans). Based on Samuels’s criterion, the analysis presented in chapter 4 indicates that it would be desirable (perhaps necessary) to shift a major part of the burden to hydrological regions outside the western states. The adequacy of Samuels’s criterion may be questioned: By attempting to guarantee that water flow in the area of interest will not fall below the weekly minimum observed the past 10 years, it may be too restrictive. It may, however, serve as a goal for ecologically sound practices.
An equivalent problem has been studied in greater detail by the six national laboratories that analyzed the water requirements of the President’s National Energy Plan of 1977.180 That plan called for an additional 18 quads of coal—13.5 for electricity and 4.5 for industrial use—and the findings were considered to apply by and large to the plans under the subsequent National Energy Act of 1978. Using a less demanding water shortage criterion (critical surface supply) than that employed by the Risk and Impact Panel, the laboratories’ report concludes that such an increase is feasible, provided that particular attention is paid to the many siting problems that will occur.* The problem will not be in the mining of the coal, but in its use.
It is clear that regional and interregional as well as local hydrological analysis must become an integral part of national energy planning, both to prevent water-supply failure and especially to obtain optimal use of our hydrological resources. We recommend that the relatively unstudied hydrological regions be examined. Water resources are largely under the control of the states. Two different approaches in law have been used to control them (the Riparian Doctrine and the Appropriation Doctrine). Their utilization in national planning will not be a simple matter. The energy-water problem is, in fact, a part of a much broader one of water as a limiting factor in the activities of society.
ECOSYSTEMS181
The following review offers brief summaries of the effects of the principal energy systems on ecosystems, based largely on the report of the Ecosystems Resource Group of the Risk and Impact Panel.182 The field of study is still young. Its magnitude and extreme diversity are added burdens to investigators who recognize the value of expressing their findings in simple, generally applicable, quantitative terms. The resource group set the following criteria for adverse effects: loss of arable land and of water resources; loss of open space in or near urban areas; intrusion into wilderness areas and loss of beauty; and loss of habitat and loss of wild populations, particularly when leading to extinction of species. It is recognized that much more work should be done to translate qualitative descriptions or observations into quantitative assessments that can be related to energy system activity per unit of time. However, the National Environmental Policy Act explicitly states that unquantified environmental amenities and values must be given appropriate consideration in decision making, as well as economic and technical information.183
Decisions on the expanded production and use of energy taken in the face of predicted effects on ecosystems may involve a difficult balancing of values that cannot be made comparable.184 Threats to ecosystems are generally speculative and subject to a high degree of uncertainty. The causal connections leading from the source to the ultimate (and long-term) consequences are long and complicated, and the consequences themselves are judged differently. To one group, the extinction of a few endangered species may seem a small price to pay for expanded consumer choice in material consumption, but to another group, the benefit of a few more material goods may seem frivolous—hardly worth the destruction of an endangered species.
HYDROELECTRIC POWER185
The ecological effects of hydroelectric projects are difficult to quantify and are extremely variable from case to case. If hydroelectric capacity expands in the future, the total ecological damage is likely to increase more than proportionately as the more suitable sites are used. This could be temporarily offset by a major development such as the Canadian James Bay project, but few suitable sites remain in the United States.
Among the adverse ecological consequences of new dam construction are the loss of habitat in the immediate area of the reservoir, subtle effects on the biological productivity of the river below the dam, damage to scenic areas along the wild stretches of the river, damage to the ecological balance of estuaries due to alteration of freshwater flow patterns, accelerated siltation and eutrophication186 in the artificial lakes behind dams, adverse effects on fish species (such as salmon) that swim up rivers to spawn, and excess evaporation of water from artificial lakes and the resulting increased salinity, particularly in arid regions.
Some of these effects can be reduced or mitigated by proper design measures, but in the opinion of the Ecosystems Resource Group of the Risk and Impact Panel,187 the ecological damage per unit of energy produced is probably greater for hydroelectricity than for any other energy source.* The social value assigned to these ecological losses varies. There is no question, however, that free-flowing rivers constitute a nonrenewable and rapidly disappearing feature of the American landscape. Their complete destruction would be too high a price for a small contribution to solving the energy problem.
GEOTHERMAL ENERGY188
In considering the ecological effects of this source, it is necessary to distinguish existing technologies for sources of steam and hot water from future technologies for hot dry rock or geopressured brines. The existing technologies are limited in their capacity to supply a significant fraction of total energy needs. Their ecological effects are specific to location: Some sources could destroy habitats and release toxic emissions that would affect local flora and fauna. Hydrogen sulfide is a particular problem for some sites.
Hot dry rock may be cleaner, but its exploitation as a source of energy will require methods of fracturing the rock at depth to provide access for water over a sufficiently large volume. In some cases, the procedures used
|
* |
See statement 9–14, by H.Brooks, Appendix A. |
(such as high explosives) could contaminate groundwater or trigger seismic disturbances. Seismic effects could present significant hazards if large-scale exploitation of the normal thermal gradient in the earth’s crust ever becomes feasible.
In the case of geopressured sources, the volume of brine that must be handled is enormous, and its removal could create ecological problems unless a satisfactory technique for reinjection is available: Land subsidence, for example, could be significant.
Too little is known about feasible methods of exploiting geopressured brines or hot dry rock to judge the ecological effects or techniques to avoid them.
SOLAR POWER189
The diversity of solar power systems is so great (photothermal, photoelectric, photochemical) that generalizations about the effects of these systems are difficult. The evaluation is complicated in the case of most solar power systems by a complete lack of practical experience. They appear to be no worse as a group than other energy systems, and in many forms, far superior. The caution must be voiced here (as for other alternative sources of energy) that the ecological effects depend on specific processes and siting plans.
In one respect, all solar systems may be expected to be benign. They do not directly contaminate the atmosphere, nor do they dig deep into the earth or strip its surface, particularly in areas that are difficult to reclaim. The centralized stations required for large-scale power production would affect land areas and even the ocean. Thermal stations would require about 6 acres/MWe; placed in the desert for optimal operation, their effect would be amplified by the ecological fragility of the location. On a perGWe basis, solar central stations would require about 6000 acres, or 15 times the area of a nuclear plant and 10 times the area of a coal plant of comparable capacity (not counting the land required to mine the coal over its life).190*
Decentralized rooftop solar collectors represent a minor environmental risk compared to centralized solar collectors. They offer the possibility of generating electricity and space heat, thus using a greater fraction of incident solar energy. The main problems will result from the necessity of removing shade trees. With clustered buildings, this problem can be reduced by sharing the energy among the buildings; Each building does not have to be unshaded throughout the day.
|
* |
See statement 9–15, by H.Brooks, Appendix A. |
Wind power would require 120,000 acres/GWe (about 20 times the land required for a solar thermal plant), but the land between towers could be used for other purposes, such as agriculture. Access roads would be required for maintenance, as well as transmission lines interconnecting the numerous towers. The environmental effects of these requirements would depend on competing demands for use of the land. Wind power would be most valuable in areas where it could be integrated into a grid supplied by hydroelectric power, which could be used to compensate for the fluctuations in wind. Besides presenting aesthetic problems in many areas, wind-turbine installations may endanger migratory birds and interfere with TV and other communications.
The development of ocean thermal power (OTEC) is almost certain to affect marine ecosystems, particularly if this source of energy is eventually relied upon for a significant fraction of total energy. The mixing of surface and deep waters will bring nutrients to the surface and may also release carbon dioxide to the atmosphere, although in lesser amounts than would be released by producing an equivalent amount of energy through the combustion of fossil fuel. Large-scale deployment of ocean thermal power might ultimately modify ocean currents and temperature distributions, with significant effects on regional or global ecosystems. Very little attention has been given to any of these environmental questions. Most of the effects mentioned, however, would only be of concern with very large-scale deployment and cannot be regarded as major deterrents to the development of OTEC.
Large-scale bioconversion or “biomass” is a popular option advocated by proponents of solar energy. To the extent that biomass is derived from organic wastes or from materials grown on special areas of the ocean or unused lands, the ecological effects would be minimal. This source of energy could supply perhaps 5 quads of total demand by 2010. Bioconversion is estimated to become progressively less desirable above this level as more land is dedicated to the cultivation of energy crops. To obtain an economic harvest and avoid soil depletion, chemical fertilizers and large amounts of water would have to be used. The land requirements would be enormous. At 1 percent average photosynthetic efficiency, 1.5 percent of the land area of the United States—an area about the size of Arkansas—would be required to generate about 10 quads of primary energy. The development of especially hardy species to increase the yields and improve the overall economics of energy from biomass would run some risk of spreading these species where they would be undesirable.
COAL
One of the most disruptive energy sources is coal. Underground mining can affect underground water systems and their associated drainage patterns and lead to subsidence. The underground waters that drain through the mine carry off toxic substances, and the mine itself disturbs the pattern of underground drainage. Strip mining without suitable reclamation is ruinous to surface land, and in some areas the possibility of lasting reclamation must be doubted.
Reclamation does not imply restitution (return to original conditions), but its water requirements may be high in areas where water supply is low. Areas that are relatively flat and possess an abundant water supply with high humidity are the most suitable for reclamation. Much strippable coal exists in areas where it may be effectively impossible to return the land to its prestripped state, even with the abundant use of water transported from elsewhere. If the consumption of coal increases rapidly, pressures will mount to initiate production in areas where reclamation will be relatively ineffective.
Deep mining is less destructive of the surface environment but can lead to subsidence and toxic effects in associated aquatic systems. These latter problems, however, appear susceptible to proper management, especially with improved mining technology.
As discussed in chapter 4, large resources of coal can be neither mined nor stripped but could be recovered by underground gasification. Little is known about the environmental effects of this practice. It could have adverse effects on important groundwater resources and thus indirectly affect ecosystems. The potential problems need to be kept in mind as the technology is developed.
In combustion, coal emits air pollutants that affect plant growth and lead to acid precipitation, which affects freshwater aquatic systems and forests (as explained earlier). The accumulation of carbon dioxide released in all fossil fuel combustion could ultimately result in drastic alteration of both natural and agricultural ecosystems.
The conversion of coal to synthetic fuels (liquids or gas) produces wastes, including contaminated liquids (phenol is a major constituent) that are unsuitable for immediate discharge. Present regulations and experience with coking operations offer assurance that adequate control can be achieved, but this can only be established in practice. The combustion of these fuels should be a relatively clean process (less polluting than combustion of oil or coal), since many impurities are removed in the process of conversion.
OIL
Drilling for oil can lead to accidental discharges that are harmful to local ecosystems. Likewise, spills of oil in transit or from routine operations are a serious hazard that can have devastating effects on marine and freshwater systems. The delayed as well as the prompt effects vary. The refining of oil and the release of pollutants in its combustion may have widely dispersed effects. Pipelines may promote erosion and, when overland, may hinder species migration. That suitable planning can prevent such untoward effects is now being tested by the operation of the Alaska pipeline. The overall ecosystem effects of oil are less serious than those of coal for corresponding energy production levels. Automobile emissions affect not only human health and comfort but also agricultural and plant life systems. (See “Agriculture and Plant Life.”)
NATURAL GAS
The extraction and delivery of natural gas can threaten natural habitats. Pipeline leaks, by blanketing with natural gas and thus excluding oxygen, may leave an area barren for several months after the leak has been stopped. Fires and leaks from receiving facilities in marine areas are particularly hard on estuary life. The combustion of natural gas is indirectly damaging to ecosystems through the accompanying oxidation of atmospheric nitrogen. The nitrogen oxides thus formed are an important factor in acid precipitation (discussed under “Water and Climate”). In association with the photochemical-oxidant system or sulfur dioxide, they can be toxic to plant life (as previously noted). In total, however, the effects are much less severe than those of coal.
SHALE OIL AND COAL-DERIVED SYNTHETIC FUELS
The ecological effects of coal-derived fuels are much the same as those for coal itself. On the other hand, the production of oil from shale carries with it the threat of considerably more ecological damage than conventional production of oil. The attractive oil shale resources in the United States are located in limited areas of the western states that are ecologically fragile and that are short of water, of which large quantities would be needed. Solid-waste disposal is a problem. Experimentation has been initiated on conversion of oil shale in situ, a technique that might or might not be less damaging ecologically than retorting in aboveground plants but that may involve serious aquifer disruption.
NUCLEAR POWER
The principal ecological effects of nuclear power result from uranium mining—effects simliar to coal mining, but far less severe (only 3–4 percent as much ore is required today). Recycling uranium and plutonium in light water reactors and lowering enrichment tails could reduce the uranium ore required per gigawatt (electric) by as much as 40 percent and thereby reduce the ecological consequences of uranium mining and also of waste disposal. Light water reactors use about 50 percent more water than fossil-fueled generating plants. If breeders were widely adopted, the remaining ecological effects would be those from thermal pollution, similar to that accompanying the generation of electricity from other sources. The same would tend to be true for advanced converters. If the breeder option is foreclosed but dependence on nuclear power continues, the demand for uranium will lead to the mining of ever lower grade ores, gradually increasing the adverse ecological effects per gigawatt (electric) capacity. Use of the very lowest-grade sources, such as shales, would create environmental disruption comparable to that of strip-mined coal.
DISCUSSION
Before stating the conclusions drawn in this chapter, it may be useful to review some of the problems encountered in formulating them, as well as the concept of risk assessment itself.
LIMITATIONS IN RISK ASSESSMENT
In assessing the risks of energy systems to health, biological systems, and the environment, we have been constrained by the limited data in certain important areas. The major factor here is the early age of this field. While it is true that public health considerations have been in the public view for many years, they have centered on infectious diseases. Our concerns today center on diseases induced by chemicals or radiation. They are more difficult to detect because they are not uniquely associated with their causative agents, and are more difficult to cure because in general they are not so precisely defined and understood.
Two other factors materially increase the difficulty of risk assessment—the no-threshold dose-effect curve and the “late” effect. The latter, exemplified by cancer, may not be observed earlier than 20 years after exposure. The assumption of a no-threshold dose-effect curve is equivalent to the claim that any dose, no matter how small, has a finite chance of damaging someone in the exposed population. Together, both factors
create a state of uncertainty in the public mind that leads to the setting of standards at progressively lower levels of dose and smaller probabilities of effect. Epidemiological investigation may not always be able to keep up with the pace of such standard setting, and the validation of a new standard—by scientific study and cost-benefit analysis—may be impossible.
In some cases that deal with very low levels of exposure, it may be asked if such validation could ever be achieved, Nonetheless, epidemiological and other related studies should be conducted, if only to establish the liminal value for risk that can be determined by research, No society can be free of risk, nor is the goal of a risk-free society necessarily worth striving for.
Somewhat analogously, the estimate of risks from rare nuclear accidents may never be precise enough to satisfy those taking a position against nuclear power. Two factors enter here. First, there is the analysis for mechanical failure, such as that reported in great detail in WASH-1400. Second, there is another risk that was recognized in that report but that could not be dealt with so extensively without a great deal more industrial experience—the risk of human error, that management may not be adequate under the stress of unforeseen and previously unexperienced circumstances. It is our impression that this second risk contributed to the damage in the Three Mile Island reactor accident.191
Finally, we note the inherent difficulty of comparing one energy system with another, since some of their important risks may be different and thus not strictly comparable. Nor can such differences be reduced to the terms of one common measure. Value judgments must therefore be made in the final determination of the risks society may prefer, from which sources, and at what cost.
For perspective, it may be of interest to note some of the other risks that are current in our society. In 1974, the following annual accident death rates per 100,000 persons at risk applied to the United States: motor vehicle, 22.0; falls, 7.7; drowning, 3.1; burning, 2.9; and firearms, 1.2. A British investigator192 has summarized United Kingdom experience thus: A one-in-a-million risk of death has been attributed to 400 miles by air, 60 miles by car, three fourths of a cigarette, 1.5 min of rock climbing, 1.5 weeks of typical factory work, and 20 min of being a man 60 years old.
PERCEPTION OF RISK
Society’s tolerance of risks that have been lived with is greater than its tolerance of risks associated with new technologies. The personal evaluation of risks tends to be formed when a technology first becomes visible. At the time older technologies were introduced, risks were more
readily accepted than they are today. An important question is how subjective evaluations should be taken into account along with the more objective measures, such as fatalities per GWe-plant-year of energy production. If the subjective values are ignored, energy policy may stalemate.
The recent incident at the Three Mile Island reactor bears on this point From the dosimetry (see “Nuclear Power”), it is clear that damage to public health was negligible, but the public’s perception of the dangers of nuclear power (rightly or wrongly) has been greatly heightened. The uncertainty of management and the tenor of the information released in the early days after the accident no doubt played a role in this. It will be of interest to see how public perception is affected by the report of the presidential commission.
A point of difference between the objective quantification of risk and its sociopolitical assessment is the role of “attitude toward risk” in the latter. An energy system may be viewed as a hazard by a particular group of people (organized or unorganized) for reasons that may not reflect biological or ecological assessments of the risks. Nuclear power, for example, may be opposed as a symbol of big government, impersonal corporate business, or unrestrained economic growth. Gasoline shortages are viewed by many as tricks on the part of the big oil corporations rather than as a consequence of conditions of supply and demand on the world market.
One conclusion that may be drawn is that adequate information must continually be made easily available to the public, to inform the decision-making process, and to prevent the spread of false conclusions and impressions. We emphasize the continuous nature of the task, since energy policy in general and knowledge of its associated risks in particular will be developing for many years to come.
REGULATION
From the point of view of practical governance, the foregoing considerations are encompassed by the regulatory process, a function that has been evolving rapidly and in a somewhat uncoordinated way (in the areas dealt with here). It should not occasion surprise that this is so. As we have noted, many congressional committees and many units of the executive branch are involved.
Steps should be taken to simplify the regulatory process, but the extent to which such improvements can actually be effected is a matter of conjecture. It would be unrealistic to suppose that the work of a very large number of departments and agencies could be rapidly coordinated and made efficient during a period of rapid expansion in governmental
responsibility. The government today is still in the process of learning through its own experience how to regulate and control the risks of energy systems in ways that satisfy many diverse groups.
With respect to the evaluation of risk, it should be recognized as a matter of policy that energy plans require time for their implementation. The assessment of risk therefore can be advantageously made a sequential process. In moving forward, the goal might be set to control overall total risk: As one energy system expands, the risk per unit of product should decline. Ideally, the total risk would not rise and might even fall. Progress toward this goal will also occur whenever the expansion of one system replaces another that is riskier.
SOCIAL AND POLITICAL RISKS193
The concerned citizen and policy maker will have to go beyond the scientific and technical comparisons we have made and consider sociological and political risk comparisons as well. An extreme example would be to compare the loss of unique natural beauty with the advantages of local business development. There are many less extreme but important examples. To change the activity of a fuel system, for example, in response to its health risks might be weighed against the risk of reduced employment or of forcing a change in established cultural patterns. Other major comparisons lie completely within the sociopolitical domain, such as the risk of an accelerating oil shortage compared to the economic inflation engendered by increasing the price of oil.
An important aspect of such sociopolitical considerations is the geographical dissociation of risks and benefits. For example, western states might bear more than their share of the adverse consequences of rapidly developed mining (e.g., the boomtown), but the economic benefits would not return to the affected communities in the same proportion. Appalachia has already demonstrated such effects over the years. Likewise, air and water pollution generated at any stage of the fuel cycle are not necessarily borne by the users of the energy in proportion to use.
Traditional cost-benefit analysis, as applied to energy decisions, does not usually include these distributional effects.194 Extension of the analysis can in principle identify costs and benefits to particular groups or geographical regions, but the balancing of costs to one group against benefits to another, or to the general welfare, is inherently a political judgment. There is a need for some kind of compensation to redress the imbalance in the distributed effects of energy systems.
Finally, we wish to mention four sociopolitical risks that may figure prominently in the deliberation of energy policy and that serve to place other sociopolitical risks in perspective.
-
In the minds of some, the greatest risk associated with the production of nuclear energy is that the associated technology and materials can assist in the proliferation of nuclear weapons. In comparison to nuclear war, all other risks are small. But the closeness of the connection between development and the threat of nuclear hostilities or war is uncertain. As detailed in chapter 5, CONAES is divided on this issue,
-
A related risk is that the protection of nuclear fuel cycles against sabotage and theft will lead to unacceptable security measures, incompatible with our standards for civil liberties.* Some argue that such measures will be necessary in a world of increasing violence and terrorism. Other energy sources, in particular, dams and storage facilities for liquefied natural gas, are subject to sabotage, as are such nonenergy facilities as supplies of drinking water. Nuclear facilities, in principle, may be easier to guard without intrusion into the rest of society by virtue of the small area involved and the limited number of personnel.
-
The third risk is associated with the further large-scale development of national energy systems, whose operation and control become increasingly centralized and increasingly out of the reach of the ordinary citizen. It is argued that numerous self-sufficient energy systems at the household and neighborhood level would be more flexible and responsive to local needs. The development of such systems for the future is certainly desirable, if only to provide for greater diversity of energy alternatives.† Nevertheless, large-scale electrical and gas-distribution systems are working well today. Decentralized systems, to serve their intended purposes, would have to be mass produced, widely distributed, and maintained on a large scale. Without more experience, it is impossible to say how sturdy such decentralized systems could be.
-
The fourth risk is not having enough energy. CONAES has not considered the risks that could be faced by a society in which energy supplies fall short of the citizens’ legitimate needs.‡
CONCLUSIONS
1.
LIMITS OF RISK CONTROL
The increasing interest in protecting public health and natural resources is of major historical significance, and no doubt will continue to increase in
|
* |
See statement 9–16, by B.I.Spinrad and H.Brooks, Appendix A. |
|
† |
See statement 9–17, by B.I.Spinrad and H.Brooks, Appendix A. |
|
‡ |
Statement 9–18, by J.P.Holdren: The Demand and Conservation Panel found very low growth in energy use compatible with high prosperity. Only sudden shortfalls were not considered. |
extent and influence. Regulations will always engender controversy. This is especially true today, owing to the tremendous expansion of regulatory activity and to the fact that we are still learning how to manage it. In dealing with these problems, we must recognize that there are practical limits to the refined ascertainment of risk and its control. Energy systems (or other systems) cannot be made risk-free, nor can all improvements be made as a matter of course without significant economic penalty or other adjustment. The public should be made aware of these limitations, and of the possibility that the absolute control of risk may not only be impossible, but undesirable.
2.
CONSERVATION
For the most part, conservation is the least risky strategy from the standpoint of direct effects on the environment and public health. (One potential problem is the possibility of indoor air pollution buildup in connection with certain conservation measures in buildings.) The main reason that conservation cannot be the only strategy is that at some level of application, it would give rise to indirect socioeconomic and political effects, mostly through economic adversity, that would predominate over its direct benefits. We cannot be sure where that point is, but all the CONAES technical analyses suggest that it is far from where we are now, possibly at an energy/GNP ratio of about half its present value, given several decades for adjustment. The maximum conservation achievable without adverse socioeconomic effects will likely have health and environmental benefits and therefore should have highest priority in policies to reduce the risks of energy systems.
3.
FOSSIL FUELS
Among fossil fuels, natural gas presents the smallest health and environmental risks in both production and consumption, although there is the possibility of serious accidents in the transportation and storage of liquefied natural gas. Oil is next, and coal is much higher in risk. This ranking is likely to persist, although the gap may narrow with improvements in technology. Research is most urgently needed on the health effects of coal combustion by utilities and industry, and on the possible occupational and public health hazards of producing and using synthetic fuels.
We must be prepared for the possibility that adverse health effects, global CO2 increase and associated climatic change, freshwater supply problems, and ecological considerations will eventually severely restrict
continuing expansion of coal use. These problems are likely, though not certain, to become critical at about 3 times current coal output, or less.
4.
CARBON DIOXIDE
The accelerating increase in the level of atmospheric carbon dioxide, paralleling the increase in combustion of fossil fuels worldwide, may affect global climate by elevating the mean global temperature. The substitution of coal for oil (or synthetic liquids for oil) and oil for gas will tend to hasten this process, but the combustion of any fossil fuel contributes CO2 to the atmosphere. Assuming continued growth in the use of fossil fuels, a perceptible change has been projected by one model for the year 2000, and significant change by the year 2030. A serious concern is that climatic changes due to CO2 would be practically irreversible by the time they were detected. It should be noted that the ultimate effects may be good as well as bad (or mixed), but the transitional period could be disruptive in its effects on agriculture and industry in some regions. Vigorous international efforts should be undertaken to predict the course of carbon dioxide buildup and to determine its climatic and consequent ecological effects. There is a parallel need for planning studies to mitigate possible economic and social disruption, including plans to curtail the use of fossil fuels.
5.
NUCLEAR POWER
The routine risks of nuclear power include the induction of cancer and genetic effects by ionizing radiation released throughout the nuclear energy cycle. These risks are very small in comparison to the overall incidence of cancer and genetic effects in the general population, and they could be significantly smaller yet if the most important source of radiation in the nuclear energy cycle—uranium mill tailings—were generally better protected.* There are also risks of severe accidents, whose probabilities have been estimated with a great deal of uncertainty, but whose severities could be comparable to those of large dam failures and liquefied natural gas storage system fires.† There are also risks from the disposal of radioactive waste; these are less than those of the other parts of the nuclear energy cycle, but only if appropriate action is taken to find suitable long-term disposal sites and methods.
It should be clear from the earlier general discussion of risk comparisons that any ranking of the risks of technologies as disparate as coal-fired and nuclear electricity generation is subject to very broad, and in some cases
|
* |
Statement 9–19, by H.I.Kohn: This may contradict Table 9–5. Presumably, it assumes that the improper and now illegal practices of the past will be continued. |
|
† |
See statement 9–20, by H.I.Kohn, Appendix A. |
irreducible, uncertainties. However, if one takes all health effects into account (including mining and transportation accidents and the estimated expectations from nuclear accidents), the health effects of coal production and use appear to be a good deal greater than those of the nuclear energy cycle. If in this comparison, though, one takes the most optimistic view of the health effects of coal-derived air pollution and the most pessimistic view of the risk of nuclear accidents, coal might have a small advantage in such a comparison.†
Nuclear power is associated also with risks of nuclear weapons proliferation and terrorism, but the magnitude of these risks (and even whether nuclear power increases or decreases the risks) cannot be assessed in terms of probabilities and consequences.
6.
WATER SUPPLY
The supply of water may constrain the continued growth of electrical power, the mining and the conversion of coal to synthetic fuels, the production of oil from shale, and the use of coal by industry. Water scarcity is greater in the West than the East, but affects particular localities in both parts of the country. The projection reported in this study shows that the constraint could indeed be significant when, for example, the amount of additional electricity and synthetic fuels that could be produced from 40 quads of coal is produced nationally. (See chapter 4.) The projection assumes present technology (which could be improved) and does not allow for large-scale use of brackish water or seawater. We urge that regional and interregional hydrological analyses become an integral part of energy production planning, to prevent actual water shortage, and especially to distribute production to make optimal use of our hydrological resources. Such planning will probably be difficult. We note that the water-energy problem is a single manifestation of the broader problem of water as a limiting factor in the growth of society.
7.
SOLAR ENERGY
Several solar energy technologies appear very promising from the standpoint of health and environmental risk. Hydroelectric power (classed by convention with solar energy), however, while benign with regard to air pollution, is quite destructive of ecosystems per unit of output. Terrestrial energy farms are also likely to be ecologically destructive if deployed on a scale large enough to provide more than a few percent of total energy
|
† |
See statement 9–21, by J.P.Holdren, Appendix A. |
needs. (Biomass production at sea could avoid this problem.) For most solar technologies, the main risks are those associated with extracting and processing the requisite large amounts of construction materials.
8.
AIR QUALITY STANDARDS AND RESEARCH
The difficult tasks of setting standards for ambient air quality and emissions have been greatly complicated by a lack of precise knowledge of the levels at which epidemiological effects first appear and of the diversity of such effects. Pragmatic decisions must therefore be made in the face of uncertainty—uncertainty magnified by the periodic (and appropriate) review of these standards. Industrialists may claim that the standards are set too low, in order to make them safe regardless of cost and convenience; this inhibits industrial planning and has been a deterrent in the further use of coal. The situation calls for a major research effort into the effects of pollutants, including emissions from mobile sources (nitrogen oxides, ozone, nonmethane hydrocarbons), as well as from the stationary sources (nitrogen oxides, sulfur oxides, particulates) that this chapter considers at length.
The committee recommends that investigation center on the dose-effect curve (or exposure-effect curve) in the region near and below the present ambient air quality standards.
-
The quantitative assessment of exposure, and if possible, of dose per individual, is essential to advance knowledge in this field. One or two centrally located stations are insufficient to monitor an urban area for epidemiological research (they may be sufficient for other purposes). Measurements of indoor and outdoor, residential and occupational exposures are necessary.
-
Mortality is too gross an endpoint to be used alone. Others must be selected for specific types of morbidity and for physiological and biochemical response.
-
While immediate responses are important, late effects in specific individuals may be even more important.
-
The effects of emissions on plants and ecosystems should receive major attention.
-
The magnitude of the several problems to be investigated necessitates undertaking and maintaining long-term studies. Some will take decades to complete.
-
Much of the work cannot be planned from first to last detail, and none of it should be subject to political control. Coupled with the need for an effort adequate in scale to the problems under investigation, there is a
-
need for flexibility and independence in pursuing the studies that suggests scientists outside the government should conduct much of the work.
-
We should realize that answers will come slowly and decisions will have to be made on an uncertain basis for some time in the future.
9.
PUBLIC APPRAISAL OF ENERGY SYSTEMS
There is a need for research that will contribute to better understanding of the factors that determine public perceptions of the health and environmental risks of energy systems, and their acceptance by different subgroups within the public. No strategy for risk reduction in energy systems can be fully acceptable if it does not take into account these public perceptions and judgments, even when they are seen as unfounded by experts.* It is unlikely that the appraisal of risk will ever be able to avoid difficult relative value judgments between different kinds of risks, as well as between risks and economic or other benefits of energy technologies. This is not to say that present methods of risk assessment cannot be improved. Nevertheless, the judgmental factor will continue to predominate in decisions among energy alternatives, and is unlikely ever to be superseded by formal analysis of risks and benefits. This underscores the importance of an informed and open public debate.
NOTES
|
|
1. 40 Code of Federal Regulations 190,02 (a), (b), 1978, “Environmental Radiation Protection Standards for Nuclear Power Operations,” |
|
|
2. In addition to the risks associated with the operation of an energy system itself, those associated with construction of power plants and the occupational risks of manufacturing its parts might also be considered (as done in chapter 6). It was recently claimed (H.Inhaber, Risk of Energy Production (Ottawa, Ontario: Atomic Energy Control Board (AECB 1119), March 1978); and H.Inhaber, “Risks from Conventional and Unconventional Sources,” Science 203 (1979):718–723) that inclusion of these risks brings solar power to a level of risk approximately equal to that of power from coal or oil. The calculations supporting these widely publicized conclusions have been rejected. (See, for example, J.P.Holdren, K.R. Smith, and G.Morris, “Energy: Calculating the Risks (II),” Science 204 (1979):564–568; R. Caputo, “Energy: Calculating the Risks,” Science 204 (1979):454; R.Lemberg, “Energy: Calculating the Risks,” Science 204 (1979):454, and John P.Holdren et al., Risk of Renewable Energy Sources: A Critique of the Inhaber Report, Energy and Resources Group (Berkeley, Calif.: University of California, June 1979).) The inclusion of these risks is worth consideration, but the ramifications might be endless, and ultimately the definition of the risks under investigation would blur. For further discussion of risk and its estimation, see National Research Council, Risks and Impacts of Alternative Energy Systems, Committee on |
|
* |
See statement 9–22, by H.Brooks, D.J.Rose, and B.I.Spinrad, Appendix A. |
|
|
Nuclear and Alternative Energy Systems, Risk and Impact Panel (Washington, D.C.: National Academy of Sciences, in preparation), chaps. 1, 2, and 3. |
|
|
3. J.Clarence Davies III and Barbara S. Davies, The Politics of Pollution, 2nd ed., Studies in Contemporary American Politics, general ed. Richard E.Morgan (Indianapolis, Ind.: Pegasus, 1975). |
|
|
4. Estimated from inspection of the list of agencies publishing in the Federal Register (1979). |
|
|
5. T.J.Nagel, “Operating a Major Electric Utility Today,” Science 201 (1978):985–993. |
|
|
6. John A.Phinney, consultant to Continental Oil Co., personal communication to H.I.Kohn, September 15, 1978. |
|
|
7. Public Law 95–95 (August 7, 1977), Clean Air Act Amendments of 1977, Sect. 110. |
|
|
8. Although the standard is set without reference to technological considerations, penalties or other sanctions resulting from legal proceedings may be withheld if the required technology is unavailable. |
|
|
9. Federal Register 44 (1979):8202–8233. The preamble to this regulation provides a useful summary of several points raised in this chapter about the Clean Air Act and the problems of regulation in general. |
|
|
10. Public Law 95–95 (August 7, 1977), Clean Air Act Amendments of 1977, Sects. 160–169, “Prevention of Significant Deterioration of Air Quality.” |
|
|
11. Davies and Davies, op. cit. |
|
|
12. Risk and Impact Panel, op. cit. |
|
|
13. T.Falkie, “Perspectives on Coal Mining, Preparation, and Transportation,” in Actions to Increase the Use of Coal: Today to 1990 (McLean, Va.: Mitre Corp., 1978). |
|
|
14. Risk and Impact Panel, op. cit., chap. 4. |
|
|
15. Robert B.Cameron, An Estimation of the Tangible Costs of Black Lung Disease Related Disability to the Bituminous Coal Mine Operations of Appalachia, Appalachian Resources Project no. 47 (Knoxville, Tenn.: University of Tennessee, 1976), p. 123. |
|
|
16. National Research Council, Mineral Resources and the Environment, Supplementary Report: Coal Workers’ Pneumoconiosis, Commission on Natural Resources, Committee on Mineral Resources and the Environment (Washington, D.C.: National Academy of Sciences, 1975). |
|
|
17. Office of Technology Assessment, The Direct Use of Coal: Prospects and Problems of Production and Combustion (Washington, D.C.: Government Printing Office (052–003–000664–2), 1979). |
|
|
18. Mitre Corp., Accidents and Unscheduled Events Associated with Non-Nuclear Energy Resources and Technology, Metrek Division (McLean, Va.: Mitre Corp. (M76–68), December 1976). |
|
|
19. Risk and Impact Panel, op. cit., chaps. 4 and 5. |
|
|
20. F.E.Speizer, “Questionnaire Approaches and Analysis of Epidemiological Data in Organic Dust Lung Diseases,” Annals of the New York Academy of Sciences 221 (1974):50–58. |
|
|
21. G.W.Beebe, H.Kato, and C.E.Land, Mortality Experience of Atomic Bomb Survivors, 1950–1974 (Hiroshima, Japan: Radiation Effects Research Foundation (RERF TR 1–77), 1977). |
|
|
22. The analysis can be further refined by considering acute leukemia and chronic granulocytic leukemia separately. T.Ishimaru, M.Otake, and M.Ichimaru, “Dose-Response Relationship of Neutrons and Gamma Rays to Leukemia Incidence Among Atomic Bomb Survivors in Hiroshima and Nagasaki by Type of Leukemia, 1950–1971,” Radiation Research 77 (1979):377–394. |
|
|
23. 1 rad equals 100 ergs of absorbed radiation per gram of tissue. Since different radiations (gamma rays, alpha particles, neutrons) are more or less biologically effective (per rad), the |
|
|
doses are normalized for comparison to rem, the biologically equivalent rad dose of gamma rays. |
|
|
24. For gamma rays, X-rays, and beta rays (low LET radiations) the following dose-effect relation has frequently proved useful: effect=aD+bD2, where a and b are constants. The initial part of the curve (e.g., up to 50 rads) is practically linear; thereafter, the slope increases constantly. The Nagasaki curve might be of this type. (See Ishimaru, Otake, and Ichimaru, op. cit.) A definitive review of the subject is being prepared by the National Council on Radiation Protection (in press, 1979). |
|
|
25. See note 22. |
|
|
26. M.Ichimaru, T.Ishimaru, J.L.Belsky, et al., Incidence of Leukemia in Atomic Bomb Survivors, Hiroshima and Nagasaki, 1959–1971 (Hiroshima, Japan: Radiation Effects Research Foundation (RERF 10–76), 1976). |
|
|
27. B.D.Dinman, “Non-Concept of ‘No-Threshold’: Chemicals in the Environment,” Science 175 (1972):495–497. |
|
|
28. D.M.Bernstein, “The Influence of Trace Metals in Disperse Aerosols on the Human Body Burden of Trace Metals” (Thesis submitted to the Department of Environmental Health Sciences, New York University, Graduate School of Arts and Sciences, 1977). |
|
|
29. Dinman, op. cit. |
|
|
30. R.H.Haynes, “The Influence of Repair Processes on Radiobiological Survival Curves,” in Cell Survival After Low Doses of Radiation, ed. T.Alper (New York: John Wiley and Sons, 1975). See also, R.F.Kimball, “The Relation of Repair Phenomena to Mutation Induction in Bacteria,” Mutation Res. 55 (1978):85–120; and H.I.Kohn, “X-Ray Mutagenesis: Results with the H-Test Compared with Others, and the Importance of Selection and/or Repair,” Genetics 92 (1979):S63–S66 (supplement). |
|
|
31. E.J.Underwood, Trace Elements in Human and Animal Nutrition, 3rd ed. (New York: Academic Press, 1971). |
|
|
32. National Cancer Institute, Epidemiological Study of Cancer and Other Chronic Diseases, ed. W.Haenszel (Washington, D.C.: Government Printing Office, 1966). |
|
|
33. R.Doll and R.Peto, “Mortality in Relation to Smoking: 20 Years’ Observations on Male British Doctors,” British Medical Journal 11 (1976):1525–1536, |
|
|
34. R.Doll and R.Peto, “Cigarette Smoking and Bronchial Carcinoma: Dose and Time Relationships Among Regular Smokers and Lifelong Non-Smokers,” Journal of Epidemiology and Community Health 32 (1978):303–313. |
|
|
35. For asbestosis see P.E.Enterline, “Pitfalls in Epidemiological Research,” Journal of Occupational Medicine 18 (1976):150–156; for cotton dust, J.A.Merchant, et al., “Dose Response Studies in Cotton Textile Workers,” Journal of Occupational Medicine 15 (1973):222–230; for silicosis, T.H.Hatch, “Criteria for Hazardous Exposure Limits,” Archives of Environmental Health 27(4) (1973):231–235, |
|
|
36. National Research Council, Carbon Monoxide, Assembly of Life Sciences, Committee on Medical and Biological Effects of Environmental Pollutants (Washington, D.C.: National Academy of Sciences, 1977). |
|
|
37. N.Mantel and M.A.Schneiderman, “Estimating ‘Safe’ Levels, A Hazardous Undertaking,” Cancer Research 35 (1975):1379–1386. |
|
|
38. N.Mantel, et al., “An Improved Mantel-Bryan Procedure for ‘Safety’ Testing of Carcinogens,” Cancer Research 35 (1975):865–872. |
|
|
39. Mantel and Schneiderman, op. cit. |
|
|
40. Frederick W.Lipfert, “The Association of Human Mortality with Air Pollution: Statistical Analyses by Region, by Age, and by Cause of Death” (Dissertation submitted to Union Graduate School, Yellow Springs, Ohio, 1978); Frederick W.Lipfert, “Differential Mortality and the Environment,” in Energy Systems and Policy (in press, 1979); and Frederick W.Lipfert, “Statistical Studies of Mortality and Air Pollution: 1. Multiple |
|
|
|
|
|
163. G.E.Likens, “Acid Precipitation,” Chemical and Engineering News 54 (1976):26–49; and personal communication to H.I.Kohn, October 1978. |
|
|
164. Ibid. |
|
|
165. Christopher S.Cronan and William A.Reiners, “Forest Floor Leaching: Contributions from Mineral, Organic, and Carbonic Acids in New Hampshire Subalpine Forest,” Science 200 (1978):309–311. |
|
|
166. Commission on Natural Resources, Nitrates: An Environmental Assessment, op. cit. |
|
|
167. National Research Council, Energy and Climate, Assembly of Mathematical and Physical Sciences (Washington, D.C.: National Academy of Sciences, 1977). |
|
|
168. William W.Kellogg, “Review of Mankind’s Impact on Global Climate” (Typescript prepared for the Workshop on Multidisciplinary Research Related to the Atmospheric Sciences, National Center for Atmospheric Research, Boulder, Colo., June 21, 1977). |
|
|
169. C.F.Base, H.E.Goeller, J.S.Olson, and R.M.Rotty, The Global Carbon Dioxide Problem (Oak Ridge, Tenn.: Oak Ridge National Laboratory (ORNL-5194), 1976). |
|
|
170. Risk and Impact Panel, op. cit., chap. 7. |
|
|
171. J.Williams, ed., Carbon Dioxide, Climate, and Society (New York: Pergamon Press, 1978). |
|
|
172. In addition, dust and nitrogen oxides have the potential to make a quantitative contribution. |
|
|
173. And possibly also widespread deforestation, although the role of the biosphere is not clear. Reforestation has been recommended: See, for example, G.M.Woodwell, G.J. MacDonald, R.Revelle, and C.D.Keeling, The Carbon Dioxide Problem: Implications for Policy in the Management of Energy and Other Resources, report to the Council on Environmental Quality (Washington, D.C.: National Academy of Sciences, July 1979). |
|
|
174. MacDonald and Carter, op. cit. |
|
|
175. Kellogg, op. cit. |
|
|
176. Williams, op. cit. |
|
|
177. Risk and Impact Panel, op. cit., chap. 7. |
|
|
178. Schaefer, op. cit. |
|
|
179. J.Harte and M.El-Gasseir, “Energy and Water,” Science 199 (1978):623–633; and Risk and Impact Panel, op. cit., chap. 6. |
|
|
180. U.S. Department of Energy, op. cit. |
|
|
181. National Research Council, Energy and the Fate of Ecosystems, Committee on Nuclear and Alternative Energy Systems, Risk and Impact Panel, Ecosystems Resource Group (Washington, D.C.: National Academy of Sciences, in preparation). |
|
|
182. Ibid. |
|
|
183. Public Law 91–190 (January 1, 1970), National Environmental Policy Act, Sec. 102(B). See also S.F.Singer, “A Quantified Environment,” Science 203 (1979):400, |
|
|
184. H.Brooks, “Environmental Decision Making: Analysis and Values,” in When Values Conflict, eds. L.H.Tribe, C.Schelling, and J.Voss. (Cambridge, Mass.: Ballinger Publishing Co., 1976), pp. 115–135. |
|
|
185. Risk and Impact Panel, Risks and Impacts of Alternative Energy Systems, op. cit., chap. 6. |
|
|
186. Eutrophication refers to the enhancement of the basic nutritional level of lakes and other bodies of water that eventually disrupt the natural ecological relationships between species, permitting “undesirable” ones to outgrow and inhibit the rest, and leading to profound changes in the entire habitat, |
|
|
187. Risk and Impact Panel, Risks and Impacts of Alternative Energy Systems, op. cit., chap. 6. |
|
|
188. See chapter 8, “Geothermal Energy,” and National Research Council, Supporting Paper 4: Geothermal Resources and Technology in the United States, Committee on Nuclear and |
|
|
Alternative Energy Systems, Supply and Delivery Panel, Geothermal Resource Group (Washington, D.C.: National Academy of Sciences, 1979). |
|
|
189. See “Solar Energy,” Risk and Impact Panel, Risks and Impacts of Alternative Energy Systems, op. cit., chap. 6; and National Research Council, Supporting Paper 6: Domestic Potential of Solar and Other Renewable Energy Sources, Committee on Nuclear and Alternative Energy Systems, Supply and Delivery Panel, Solar Resource Group (Washington, .D.C: National Academy of Sciences, 1979). |
|
|
190. Ibid. |
|
|
191. The President’s Commission on the Accident at Three Mile Island, The Need for Change: The Legacy of TMI (Washington, D.C.: U.S. Government Printing Office, 1979). |
|
|
192. Sir Edward E.Pochin, Why Be Quantitative About Radiation Risk Estimates?, Lecture no. 2, Lauriston S.Taylor Lecture Series in Radiation Protection and Measurements (Washington, D.C.: National Council on Radiation Protection and Measurements, 1978). |
|
|
193. Risk and Impact Panel, Risks and Impacts of Alternative Energy Systems, op. cit., chap. 8. |
|
|
194. National Research Council, Implications of Environmental Regulations for Energy Production and Consumption, Commission on Natural Resources, Committee on Energy and the Environment (Washington, D.C.: National Academy of Sciences, 1977). |