2
Chlorine Trifluoride1
Acute Exposure Guideline Levels
SUMMARY
Chlorine trifluoride (ClF3) is a greenish-yellow liquid at temperatures <11.7°C and a colorless gas with a sweet, suffocating odor at higher temperatures. While it is not flammable, ClF3 is an extremely reactive and corrosive oxidizing agent that is used in nuclear reactor fuel processing; as a fluorinating agent; as an incendiary, igniter and propellant for rockets; and as a pyrolysis inhibitor for fluorocarbon polymers. It is unstable in air and rapidly hydrolyzes to hydrogen fluoride (HF) and a number of chlorine-containing compounds including chlorine dioxide
(ClO2). The toxic effects of ClF3 are due, at least in part, to the actions of HF and ClO2.
Chlorine trifluoride is a potent, rapidly-acting mucous membrane irritant. Skin and eye contact with ClF3 produces burns and inhalation causes acute pulmonary irritation and edema. Inhalation studies with the monkey, dog, rat, and mouse for several end points and exposure durations have been performed. Data on irritant effects were available for the dog and rat; data on sublethal and lethal concentrations were available for the monkey, rat, and mouse. One report of a very brief (1-2 min) human exposure was located, but no data on exposure concentrations were available. The data were considered adequate for derivation of the three AEGL classifications for five exposure periods. Regression analyses of the reported concentration-exposure durations for lethality for the animal species determined that the relationship between concentration and time is C1.3 × t = k.
The AEGL-1 was based on slight irritation as evidenced by rhinorrhea (nasal discharge) observed in two of two dogs during the first 3 h of a 6-h exposure to an average concentration of 1.17 ppm (Horn and Weir 1956). Nasal discharge in response to an irritant gas in the sensitive nose of dogs was considered a NOAEL for the AEGL-1. No signs were observed in 20 rats exposed to this concentration for 6 h. Exposure of the dogs for longer than 3 h resulted in obvious lacrimation. Repeated, daily exposures of rats and dogs to 1.17 ppm resulted in severe signs of irritation. The rhinorrhea in dogs exposed for 3 h was considered an appropriate end point for development of the AEGL-1. Exposure to 1.17 ppm for 3 h was extrapolated using a combined interspecies and intraspecies uncertainty factor of 10 (3 for interspecies differences [the dog was more sensitive than the rat] and 3 for intraspecies differences in sensitivity [slight irritation should occur at a similar level among the general population]). Time-scaling was not applied to the AEGL-1 as adaptation to slight sensory irritation occurs. Therefore, the calculated value of 0.12 ppm was adopted for all AEGL-1 time points. The 0.12 ppm value is similar to the ClO2 AEGL-1 of 0.15 ppm and is one-eighth of the HF AEGL-1 value of 1.0 ppm. Application of an intraspecies factor of 3 is sufficient, since application of a larger factor would result in AEGL-1 values that are not consistent with those of ClO2 and HF, two of the major decomposition products of ClF3 (breakdown of one mole of ClF3 potentially forms three moles of HF and one mole of ClO2).
The AEGL-2 was based on signs of irritation (salivation, lacrima-
tion, rhinorrhea, and blinking of the eyes) in two of two dogs exposed to a concentration of 5.15 ppm for 6 h (Horn and Weir 1955). These effects were reversible by the end of the first exposure day (i.e. dogs “did not appear markedly affected”), and therefore, were not considered an impairment to the ability to escape. Twenty rats exposed to this concentration for 6 h appeared unaffected. However, repeated daily exposures of rats and dogs to this concentration resulted in increasingly severe signs of irritation. The 6-h concentration of 5.15 ppm was divided by a combined interspecies and intraspecies uncertainty factor of 10 (3 for interspecies differences as the dog was more sensitive than the rat and 3 for intraspecies differences). The resulting value of 0.52 ppm was scaled across time using Cn × t = k, where n = 1.3; this concentration-exposure duration relationship was determined from several lethality studies (Appendix A). Because time-scaling data were available over the exposure duration of 13.5 to 222 min, the 10-min AEGL-2 was not set equal to the 30-min value as is usually done when the exposure duration of the key study is greater than 4 h. An intraspecies uncertainty factor of 3 is sufficient as these AEGL-2 values are considerably lower than those of HF (10- and 30-min and 1-, 4-, and 8-h values of 95, 34, 24, 12, and 12 ppm, respectively) and similar to the longer-term AEGL-2 values for ClO2. The 10- and 30 min AEGL-2 values for ClF3 (8.1 and 3.5 ppm) are higher than those of ClO2 (both 1.4 ppm) because information was available for time-scaling ClF3 values, whereas, in the absence of time-scaling information, the conservative value of n = 3 was used for scaling to the shorter time periods for ClO2.
Lethality data (1 h LC50 values) were available for the monkey, rat, and mouse. Based on similar respiratory rates, gross respiratory tract anatomy, amount and distribution of types of respiratory epithelium, and airflow patterns, the monkey was considered the most appropriate model for deposition of ClF3 and its decomposition products in the human respiratory tract. The AEGL-3 values were based on the highest 1 h concentration that resulted in no deaths in monkeys (MacEwen and Vernot 1970). This concentration, 127 ppm, was divided by interspecies and intraspecies uncertainty factors of 2 and 3, respectively (for a total of 6), and scaled across time using the C1.3 × t = k relationship. This time-scaling relationship was determined from several lethality studies (Appendix A). The interspecies uncertainty factor of 2 was considered appropriate as LC50 values were similar in three species and the monkey is an appropriate model for humans. A smaller interspecies uncertainty fac-
tor would result in values that are inconsistent with the HF values. The intraspecies uncertainty factor of 3 was considered appropriate because chlorine trifluoride is a direct-acting irritant and differences among individuals should not differ greatly. In cases where animals died, death was due to massive lung hemorrhaging. Applying the same procedures to the calculated 1 h LC01 from the mouse data (135 ppm) results in similar values. The 8-h AEGL-3 value was set equal to the 4-h value because the time-scaled 8 h value of 4.3 ppm is inconsistent with the experimental data. Dogs exposed to 21 ppm for two days did not die during the following month of observation, and dogs and rats tolerated repeated 6 h exposures to 5.15 ppm for several weeks before the first death was recorded (Horn and Weir 1955).
The values appear in Table 2-1.
INTRODUCTION
Chlorine trifluoride (ClF3) is a colorless, corrosive gas at ambient temperature and pressure. It is one of the most reactive of the halogen
TABLE 2-1 Summary of AEGL Values for Chlorine Trifluoride
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End point (Reference) |
|
AEGL–1 (Nondisabling) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
Slight irritation - dog (Horn and Weir 1956) |
|
AEGL–2 (Disabling) |
8.1 ppm (31 mg/m2) |
3.5 ppm (13 mg/m3) |
2.0 ppm (7.6 mg/m3) |
0.70 ppm (2.7 mg/m3) |
0.41 ppm (1.6 mg/m3) |
Threshold, impaired ability to escape -dog (Horn and Weir 1955) |
|
AEGL–3 (Lethal) |
84 ppm (320 mg/m3) |
36 ppm (140 mg/m3) |
21 ppm (80 mg/m3) |
7.3 ppm (28 mg/m3) |
7.3 ppm (28 mg/m3) |
Threshold for lethality - monkey (MacEwen and Vernot 1970) |
fluorides; it is a powerful oxidizing agent that reacts violently with water and may explode on contact with organic materials (Matheson 1980; O’Neil et al. 2001). Chemical and physical properties are listed in Table 2-2. In the vapor phase, ClF3 is unstable and decomposes by hydrolysis to a variety of substances including ClOF (the initial product), ClF, ClO2F, ClO3F, ClO2, Cl2, and HF; the proportion of products depends on the availability of water (Bougon et al. 1967; Cooper et al. 1972; Dost et al. 1974). Increased humidity increases the rate of decomposition (MacEwen and Vernot 1970).
Chlorine trifluoride has been used in nuclear reactor fuel processing (to convert uranium to gaseous uranium hexafluoride), as a fluorinating agent, as an incendiary, igniter and propellant for rockets, and as a pyrolysis inhibitor for fluorocarbon polymers (O’Neil et al. 2001). It is
TABLE 2-2 Chemical and Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
Chlorine fluoride chlorotrifluoride |
HSDB 2005 |
|
Molecular formula |
ClF3 |
O’Neil et al. 2001 |
|
Molecular weight |
92.45 |
O’Neil et al. 2001 |
|
CAS Registry Number |
7790-91-2 |
HSDB 2005 |
|
Physical description |
Colorless (gas) greenish-yellow (liquid) white (solid) |
O’Neil et al. 2001 |
|
Solubility in water |
Violent hydrolysis with water |
O’Neil et al. 2001 |
|
Vapor pressure |
1064 mm Hg at 20°C |
Matheson 1980 |
|
Vapor density (air = 1) |
3.21 at 20°C |
Matheson 1980 |
|
Liquid density (water = 1) |
1.9 kg/L at 0°C |
Matheson 1980 |
|
Melting point |
−76.34°C |
O’Neil et al. 2001 |
|
Boiling point |
11.75°C |
O’Neil et al. 2001 |
|
Flammability |
Not flammable, but may cause fire in contact with some materials |
U.S. DOT 1985 |
|
Conversion factors |
1 ppm = 3.85 mg/m3 |
AIHA 2004 |
|
|
1 mg/m3 = 0.26 ppm |
|
used as a chlorine/fluorine source for plasma etching in the semiconductor industry and for in-situ cleaning of chemical vapor deposition reactors (BOC Group 1997). It is produced commercially by the continuous gas-phase reaction of chlorine and fluorine in a nickel reactor at 290°C. In 1993, U.S. production was estimated at several metric tons per year with most of the product used in nuclear fuel processing. It is shipped as a liquified compressed gas in steel cylinders in quantities of 82 kg/cylinder or less (Bailey and Woytek 1994). The BOC Group (1997) ships cylinders containing either 13 or 26 pounds.
Chlorine trifluoride is a potent, rapidly-acting mucous membrane irritant. Contact with the skin and eyes produces burns and inhalation causes pulmonary irritation and edema (Teitelbaum 2001). No data on human exposures to measured concentrations were found. Inhalation studies for several exposure durations with the monkey, dog, rat, and mouse were located. Because of the reactive nature of ClF3, difficulty in generating and monitoring the compound was encountered in some of the studies (Horn and Weir 1955; Dost et al. 1974).
2.
HUMAN DATA
2.1.
Acute Lethality
Deichmann and Gerarde (1969) concluded that exposure to 50 ppm may be fatal within 30 min to 2 h. No further details were given, and neither the source nor the basis of that conclusion was cited.
2.2.
Nonlethal Toxicity
Although an odor threshold was not located, Teitelbaum (2001) states that the pungent odor and irritation associated with ClF3 are detectable at such a low concentration that exposed individuals would escape before experiencing severe effects. The odor has been described as sweet and suffocating (O’Neil et al. 2001). Signs experienced by a worker exposed to an unknown concentration for 1-2 min included headache, abdominal pain, and dyspnea that lasted about 2 h (Longley et al. 1965). No systemic or local effects were found. Except for fatigue (duration not given), there were no apparent sequelae.
2.3.
Developmental/Reproductive Effects
No data concerning potential developmental or reproductive toxicity of ClF3 in humans were identified.
2.4.
Genotoxicity
No data concerning the genotoxicity of ClF3 in humans were identified.
2.5.
Carcinogenicity
No data concerning the potential carcinogenicity of ClF3 in humans were identified.
2.6.
Summary
No studies of developmental or reproductive toxicity, genotoxicity, or carcinogenicity of ClF3 in humans were located. Exposure to sufficiently high ClF3 concentrations may cause skin and mucous membrane irritation (Teitelbaum 2001) as well as headache, abdominal pain, and dyspnea (Longley et al. 1965). Deichmann and Gerarde (1969) report that 50 ppm may be fatal within 30 min to 2 h, but neither the source nor basis of that conclusion was cited.
3.
ANIMAL TOXICITY DATA
3.1.
Acute Toxicity
Acute toxicity data, available for the monkey, dog, rat, and mouse, are summarized in Table 2-3 and discussed below. Longer-term studies using dogs and rats described irritant effects during the first day of exposure. It should be noted that in the 2-day and longer-term studies reported by Horn and Weir (1955, 1956), groups of 2 dogs and 20 rats were exposed at the same time, presumably in the same 500-liter exposure chamber.
TABLE 2-3 Acute Inhalation Toxicity in Laboratory Animals
|
Species |
Concentration (ppm) |
Exposure Time |
Effecta |
Reference |
|
Monkey |
230 |
1 h |
LC50 |
MacEwen and Vernot 1970 |
|
|
127 |
1 h |
No deaths; signs of sneezing, coughing, and gagging |
|
|
Dog |
21 |
6 hb |
Extreme eye and mucous membrane irritation, singed hair; recovery by next morning except inflamed eyes irritation, salivation, sneezing, lacrimation, coughing nasal discharge within 45 min, lacrimation after 3 h |
Horn and Weir 1955; 1956 |
|
|
5.15 |
6 hb |
||
|
|
1.17 |
6 hb |
||
|
Rat |
800 |
13-14 min |
Approximate LC50 |
Dost et al. 1974 |
|
|
|
10 min |
No deaths; severe inflammation of mucosal surfaces, preening, skin burns, lacrimation, brittle hair, corneal ulceration, shallow respiration |
|
|
|
400 |
28 min |
Approximately LC50 |
|
|
|
400 |
25 min |
Same signs as 10-min exposure to 800 ppm |
|
|
Rat |
480 |
70 min |
100% mortality |
Horn and Weir 1955 |
|
|
|
40 min |
ET50c |
|
|
Rat |
299 |
1 h |
LC50 |
MacEwen and Vernot 1970; Vernot et al. 1977 |
|
|
200 |
1 h |
No deaths; signs of lacrimation, salivation, labored breathing, and rhinorrhea; bloody discharge from eyes and nares |
|
|
Rat |
96 |
4.5 h |
80 %mortality |
Horn and Weir 1955 |
|
|
|
3.7 h |
ET50c |
|
|
Rat |
21 |
6 hb |
Rhinorrhea, lacrimation, singed hair; recovery by morning |
Horn and Weir 1955 |
|
|
5.15 |
6 hb |
Little observed effect |
|
|
|
1.17 |
6 hb |
No effects |
|
3.1.1.
Nonhuman Primates
Groups of four Rhesus monkeys of both sexes inhaled 0, 127, 150, 200, 300, or 400 ppm for 1 h (MacEwen and Vernot 1970). Concentrations were measured based on the reaction of ClF3 or its decomposition products with dimethylamine. Measurements showed that concentrations of ClF3 were stable in the test chamber. Observations were made during exposure and continued 14 days post-exposure. Signs observed in exposed animals included sneezing, coughing, and gagging. Animals exposed to lethal concentrations (150 ppm) demonstrated general paresis, labored breathing, and cyanosis prior to coma and death. Massive alveolar and interstitial hemorrhages involving the entire lungs were present in all animals that died. Most of the deaths occurred 2 to 3 h after the cessation of exposure. Animals were cyanotic, but no methemoglobin was formed during these exposures. Mortality ratios were 0/4, 2/4, 1/4, 2/4, and 4/4 animals at the 127, 150, 200, 300, and 400 ppm concentrations, respectively. The 1-h LC50 was 230 ppm. Pulmonary congestion, edema, hemorrhage, and emphysema were observed in surviving monkeys at 14 days post-exposure. No differences in clinical chemistry parameters were found between exposed and control animals.
3.1.2.
Dogs
Two dogs were exposed to 21 ppm for 6 h/day for two days (Horn and Weir 1955). Shortly after exposure was initiated, lacrimation, cough, rhinorrhea, rapid respiration and salivation were observed. Rhinorrhea and lacrimation were observed approximately 10 min after the exposure was initiated. The dogs became nauseated, coughed up a small quantity of mucoid material, and had rapid respiration and salivation. The eyes were extremely irritated by the end of the exposure and the hair had a “singed feel.” The morning after the first exposure the animals appeared essentially normal with the exception of inflamed eyes. During the second day of exposure, the study was terminated due to equipment failure which resulted in a concentration considerably higher than 21 ppm. Corneal ulcers and a burn in the vicinity of the nose developed following the exposures. No deaths were reported during the following month of observation
Two male dogs were exposed to 5.15 ppm for 6 h/day, 5 days/week
for 6 weeks (Horn and Weir 1955). Signs of exposure occurred during the first day and included salivation, lacrimation, and rhinorrhea; coughing and sneezing were also noted. However, by the end of the first day of exposure, the dogs “did not appear markedly affected.” Respiratory distress was evident at the midpoint of the study, and the dogs died on days 17 and 26. The authors experienced difficulty in maintaining constant concentrations in the exposure chambers, and during the exposures, concentrations of one-half to two times the average value were recorded.
Two dogs were exposed to an average concentration of 1.17 ppm for 6 h/day, 5 days/week for 6 months (Horn and Weir 1956). During the early part of the study, signs of irritation included rhinorrhea, usually within 45 min of exposure, and obvious lacrimation after 3 hs of exposure. All animals developed a "singed feel" of the hair following the first exposure. The animals appeared normal by the following morning. By the 28th day, the dogs were coughing up bloody mucoid material and showed signs of blinking of the eyes and a change in respiratory pattern at the beginning of each exposure. After more than 60 days (42 exposures) the dogs began showing signs of pneumonia. Penicillin was administered, but one dog died on the 115th day of the study (during the 82nd exposure). The other dog was sacrificed at the termination of the experiment. Examination of the lungs revealed purulent bronchitis and pulmonary abscesses in the dog that died and alveolar hemorrhage, interstitial edema, and irritation in the surviving dog.
3.1.3.
Rats
Groups of 4-10 male Sprague-Dawley rats inhaled 400 ppm for 20, 25, 30, 35, or 40 min or 800 ppm for 10, 13, 15, 20, 25, or 30 min (Dost et al. 1974). Gas flow rates were measured with mass flow meters; exposure chamber ClF3 concentrations were verified by infrared spectral analysis. Rats began preening at initiation of ClF3 exposure, and exposures produced severe inflammation of all exposed mucosal surfaces. Time-respective mortalities for the 400 ppm exposure were: 0/8, 0/4, 4/6, 7/8, and 8/8. Time-respective mortalities for the 800 ppm concentration were: 0/10, 1/8, 10/10, 8/8, 6/6, and 4/4. LC50 values were not calculated by the authors.
The authors found that prolonged exposures or high ClF3 concentrations caused burning of the exposed skin, and the hair became brittle
and yellowed. Corneal ulceration was a frequent occurrence. Respiration became shallow without an appreciable increase in rate. Exposures to 400 ppm for 30 min or 800 ppm for 15 min were lethal. Deaths occurred within 3 h of exposure. Although the post-exposure observation period was not given in terms of days, the authors stated that "rats that survived 4 h after exposure to ClF3 in air were able to survive the primary inhalation injury indefinitely, with minimal after care."
Groups of 20 rats (sex and strain not stated) were exposed to 96 or 480 ppm under conditions of continuous exposure until all animals were dead (Horn and Weir 1955). Chlorine trifluoride was measured by drawing a known quantity of the chamber atmosphere through sodium hydroxide solution and then analyzing for chloride. Deaths were concentration-related as mortalities were 50 and 80% after 3.7 and 4.5 h, respectively, at a concentration of 96 ppm. Exposure to 480 ppm killed 50 and 100% after 40 and 70 min, respectively (Table 2-3). Excessive preening, deep gasping respiration, rhinorrhea, salivation, and lacrimation occurred soon after the exposures began. Marked eye irritation, corneal damage, and singed hair were observed. The authors noted the corrosive effect of the gas on the equipment; deviations from the measured concentrations of one-half to two times the average value occurred.
Groups of 8 male Wistar rats inhaled 200 or 400 ppm for 1 h (MacEwen and Vernot 1970; Vernot et al. 1977). Although both publications described the same study, MacEwen and Vernot (1970) report the strain of rats as Wistar, whereas Vernot et al. (1977) report the strain as Sprague-Dawley. Observations were made during the exposures and for 14 days post-exposure. Signs observed in all exposed animals included lacrimation, salivation, labored breathing, and rhinorrhea. No deaths occurred at 200 ppm, but 6 of 8 rats died at 400 ppm. The LC50 was 299 ppm. Massive alveolar and interstitial hemorrhage involving the entire lung was present in all animals that died. Deaths occurred within 2 to 3 h after the exposure. Survivors developed bloody discharge from the eyes and nares that lasted for several days. Near lethal concentrations induced congestion, edema, hemorrhage, and emphysema in localized or discrete areas of the lungs.
Twenty rats (sex and strain not stated) were exposed to 21 ppm for 6 h/day for two days (Horn and Weir 1955). Shortly after exposure was initiated, the rats began preening and showed signs of rhinorrhea and lacrimation. At the end of the first day, rhinorrhea and lacrimation were observed and the fur had a “singed feel.” The animals appeared normal the
following morning. During the second day of exposure, the study was terminated due to equipment failure resulting in a considerably higher concentration. No deaths were reported.
Horn and Weir (1955) exposed 20 rats (sex and strain not stated) to an average concentration of 5.15 ppm, 6 h/day, 5 days/week for 6 weeks (31 exposures). At the end of the first day of exposure the authors noted that “the rats did not appear to be affected.” On the second day the rats appeared restless, and there was a moderate amount of preening, salivation, and rhinorrhea. Signs and symptoms of irritation increased with increasing exposure time; one death occurred at the end of the 36th day of the experiment. No further deaths occurred before termination of the experiment on the 43rd day. Animals sacrificed at the termination of the experiment had severe lung pathology, including varying degrees of hyperemia, hemorrhage, and edema. During the exposures, concentrations of one-half to two times the average value were recorded.
Twenty rats (sex and strain not stated) were exposed to an average concentration of 1.17 ppm for 6 h/day, 5 days/week for 6 months (Horn and Weir 1956). During the early part of the study, the rats appeared unaffected by the exposures. By the 9th day the rats began preening immediately after exposure began. After 10 min, preening was followed by reduced physical activity that lasted throughout the exposure period. After several weeks, blood tinged exudate around the nares and eyes occurred on occasion. Five deaths (days 56-178) occurred in the treated group versus two in the concurrent control. Examination of the lungs revealed pulmonary edema and bronchopneumonia in the rats that died and pulmonary irritation in the survivors.
3.1.4.Mice
Groups of 15 mice were exposed to concentrations of 125, 150, 175, 200, or 400 ppm for 1 h (MacEwen and Vernot 1970). Signs observed during exposure included lacrimation, salivation, labored breathing, and rhinorrhea. Most fatalities occurred 2 to 3 h after exposure; all deaths occurred within 36 h post-exposure. Deaths occurred at concentrations 150 ppm; the LC50 was 178 ppm (95% confidence limits, 169-187 ppm). Mortality ratios were 0/15, 2/15, 4/15, 14/15, and 15/15 at concentrations of 125, 150, 175, 200, and 400 ppm, respectively. Examination of surviving mice at 14 days post-exposure revealed localized areas of congestion, edema, hemorrhage, and emphysema in the lungs.
3.2.
Developmental/Reproductive Toxicity
No information on potential developmental or reproductive toxicity associated with ClF3 exposure was located in the available literature.
3.3.
Genotoxicity
No information on genotoxicity was located in the available literature.
3.4.
Chronic Toxicity/Carcinogenicity
No information on the chronic toxicity or carcinogenic potential of ClF3 in animals was located in the available literature.
3.5.
Summary
Data were available on lethality in three species (monkey, rat, and mouse) and the consequences of ClF3-induced irritation in two species (dog and rat). The 1-h LC50 values for the monkey, rat, and mouse were 230, 299, and 178 ppm, and the 1-h exposures resulting in no deaths for the respective species were 127, 200, and 125 ppm. Concentration-dependent ocular, mucous membrane, and pulmonary tract irritation was noted in dogs at 1.17, 5.15, and 21 ppm for an exposure duration of 6 h. The dog was more sensitive than the rat to the irritant effects of airborne ClF3 as no signs of irritation were observed in a group of 20 rats during the first day of exposure to 1.17 or 5.15 ppm; rhinorrhea, lacrimation, and singed hair were observed in both species during the first day of exposure to 21 ppm. No information on potential developmental/reproductive toxicity, genotoxicity, or carcinogenicity was located.
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
Tissue distribution of fluoride ion was analyzed at 0, 2, 6, and 24-h
after male Sprague-Dawley rats inhaled 400 ppm ClF3 for 15 min (Dost et al. 1970). Distribution was uniform among soft tissues. There was no evidence of increased fluoride burden in the lung at any time after exposure. Nonsignificant, transient increases in fluoride were observed in the spleen immediately after exposure and at 2 h post-exposure. Bone fluoride increased from 118 μg F/g tissue at 0 h to 172 μg F/g tissue at 24 h post-exposure.
4.2.
Mechanism of Toxicity
ClF3 is corrosive to all tissues. In rats that inhaled 400 ppm and had previously been injected with 14C-labeled NaHCO3, 14CO2 production was reduced sharply during the first 30 min and remained so for up to 6 h (Dost et al. 1974). A decrease in CO2 expiration indicates damage to the lungs.
Analysis of ClF3 in ambient air (at 65% relative humidity) by an infrared technique found approximately 85% of the ClF3 had degraded within 6 sec (MacEwen and Vernot 1970). Dilutions of ClF3 with air and 50% humidity at nominal concentrations of 1000, 2000, and 5000 ppm showed ClF3 when analyzed within 30 sec. Reaction products had little or no infrared absorption (hydrogen fluoride [HF] and chlorine [Cl2] have little or no infrared absorption).
In the moist respiratory tract, ClF3 is predicted to hydrolyze to ClOF which further degrades to ClO2F and ClF (Dost et al. 1974). ClO2F rapidly hydrolyzes to ClO2, HF, and ClOx anions; the first two products predominate and are thought to be responsible for ClF3 toxicity as the ClOx anions are relatively nontoxic.
4.3.
Structure-Activity Relationships
The chemical reactivity of the halogenated fluorine compounds in order of decreasing reactivity is: chlorine pentafluoride (ClF5) > ClF3 bromine pentafluoride (BrF5) > iodine heptafluoride (IF7) > chlorine monofluoride (ClF) > bromine trifluoride (BrF3) > bromine monofluoride (BrF) (Bailey and Woytek 1994).
Signs produced by ClF3 exposure are similar to those of other respiratory irritants including ClF5 and HF. MacEwen and Vernot (1971) and
Darmer et al. (1972) compared the toxicity of ClF3 with that of oxygen difluoride (OF2, a potent oxidizer), HF, and ClF5. Table 2-4 lists 1 h LC50 values for these chemicals in order of decreasing toxicity. In the monkey, ClF3 is slightly less potent than ClF5 but 7 times more toxic than HF (in all three species for which data are available, ClF5 is almost exactly ten times more toxic than HF). In the rat and mouse, ClF3 is approximately 4 times more toxic than HF.
MacEwen and Vernot (1970) found ClF3 exposure induced toxicity similar to that of its principal hydrolysis product, HF; thus, the relative toxicities of HF and ClF3 on an equivalent molar basis (3 moles of HF formed per mole of ClF3) would be 690, 897, and 534 ppm (3 times the ClF3 1-h LC50 values of 230, 299, and 178 ppm), respectively. The response to ClF3 in monkeys was not due entirely to HF, but HF was a major factor in the response of rats and mice. Additional effects were thought due to one or more chlorine-containing degradation products. The hydrolysis of ClF5 is very exothermic which may contribute to its enhanced toxicity compared with HF (Syage 1994).
Inhalation studies with rats also indicated that the toxicity of ClF3 was comparable to that of ClO2 based on the number of chlorine equivalents (Dost et al. 1974). Two of five rats that inhaled 500 ppm ClO2 for 15 min died, and all 8 rats exposed to 1,000 ppm ClO2 for 30 min died. Exposure to 2,000 ppm ClO3F for 25 min or to 1,000 ppm ClO3F for 60 min was not lethal. The authors stated that the toxicity of ClF3 was comparable to that of ClO2 on a chlorine equivalent basis and that the toxicity of inhaled ClF3 was comparable to that of HF on a fluorine equivalent basis.
4.4.
Other Relevant Information
4.4.1.
Species Differences
There is little variation in species sensitivity to lethal concentrations
TABLE 2-4 Comparative 1 h LC50 Values for ClF3 and Related Compounds
|
Species |
OF2 |
ClF5 |
ClF3 |
HF |
|
Monkey |
16.0 |
173 |
230 |
1774 |
|
Dog |
26.0 |
122 |
— |
— |
|
Rat |
2.6 |
122 |
299 |
1276 |
|
Mouse |
1.5 |
57 |
178 |
501 |
|
Source: Darmer et al. 1972. |
||||
of ClF3. The 1-h LC50 values for the monkey, rat, and mouse were 230, 299, and 178 ppm, respectively (MacEwen and Vernot 1970). No monkeys died at 127, and no mice died at 125 ppm. Data from the MacEwen and Vernot (1970) study allowed calculation of LC01 values for the mouse and rat (Table 2-5); however, only two data points were available for the rat. The data set for the monkey did not allow calculation of a reliable LC01.
Although 1 h LC50 values were not reported in studies of Horn and Weir (1955) and Dost et al. (1974), these values can be calculated (see section 4.4.3 for time scaling). In the study by Horn and Weir (1955), the 1-h ET50 values would be 262 and 351 ppm based on the 96 and 480 ppm exposures, respectively. It should be noted that these values underestimate the LC50 as the protocol did not allow for post-exposure observation. In the Dost et al. (1974) study, the 1-h LC50 concentration would be 222 ppm. If these values are considered, then all 1 h LC50 values across species are within a factor of two (range, 178-351 ppm).
The nasal passages vary considerably in size and shape among species. The nasal passages of rodents and primates differ in gross anatomy, the amount and distribution of types of respiratory epithelium, and airflow patterns. The noses of primates (humans and monkeys) show great similarity in these three factors (Schreider 1986), and the monkey is a more appropriate model for extrapolation of inhalation toxicity data for irritants to humans than is the rodent.
The respiratory rate of primates is lower than that of rodents. Therefore, the delivered dose to the respiratory tract in primates is lower than that of rodents exposed to the same concentration. Furthermore, based on relative body size, the respiratory rate of humans is lower that of monkeys, with a resulting lesser dose to the target tissues in the respiratory tract.
TABLE 2-5 Comparative ClF3 1-H Lethal Values for Three Species
|
Species |
LC50 (95% confidence limits) |
No deaths (ppm) |
LC01 (ppm) |
|
Monkey |
230 |
127 |
— |
|
Rat |
299 |
200 |
156 |
|
Mouse |
178 (169-187) |
125 |
135 |
|
Source: MacEwen and Vernot 1970. |
|||
4.4.2.
Susceptible Populations
Asthmatics may respond to exposure to primary irritants with increased bronchial responsiveness. No information on the relative susceptibility of asthmatic and otherwise healthy people to inhaled ClF3 was located. The elderly and those with compromised lung function could potentially experience increased susceptibility to airborne ClF3, but there are neither animal data nor clinical experience to suggest that such is the case.
People engaged in emergency situations and those engaged in physical activity will experience greater ClF3 deposition and pulmonary irritation than sedentary individuals. Furthermore, individuals who breathe through their mouth would receive a larger pulmonary delivered dose since scrubbing and deposition in the nasal passages would be bypassed.
4.4.3.
Concentration-Exposure Duration Relationship
Lethality data were available for three species and for exposure durations of 13.5 to 222 min. These data are graphed in Appendix A. The inverse of the slope of the line (n) is 1.3. This value of n was used in the time-scaling relationship, Cn t = k, for both the AEGL-2 and AEGL-3 because tissue damage from a direct-contact irritant is a matter of degree between the AEGL-2 and AEGL-3. Comparing the LC01 value for the mouse, 135 ppm, with the lower confidence limit on the LC50 value, 169 ppm, shows that the concentration-response curve is very steep. In the Dost et al. (1974) study, a 3-min time difference separated highest nonlethal and LC50 values for exposures to both 400 and 800 ppm.
5.
DATA ANALYSIS FOR AEGL-1
5.1.
Human Data Relevant to AEGL-1
No human data relevant to the calculation of an AEGL-1 were located.
5.2.
Animal Data Relevant to AEGL-1
The only animal study using a concentration relevant to AEGL-1 was the subchronic study in dogs and rats exposed to 1.17 ppm, 6 h/day, 5 days/week, for 6 months (Horn and Weir 1956). While the rats remained unaffected during the early exposures, dogs displayed rhinorrhea within 45 min of the first exposure; lacrimation developed after 3 h. Signs of irritation became increasingly severe with continued exposures.
5.3.
Derivation of AEGL-1
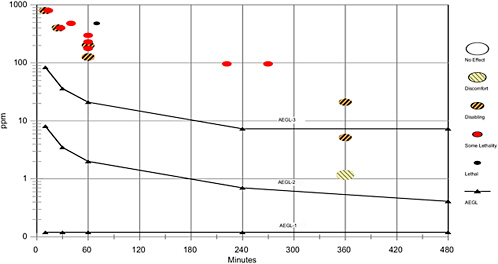
Although the Horn and Weir (1956) account did not clearly state on which day the signs of irritation to ClF3 began in dogs, nasal discharge indicates slight sensory irritation as the end point (NOAEL) for the AEGL-1. Obvious lacrimation was not observed until after 3 h of exposure. The AEGL-1 was based on nasal discharge observed in dogs during the first 3 h of exposure to 1.17 ppm. The 1.17 ppm concentration for an exposure duration of 3 h was divided by a combined interspecies and intraspecies uncertainty factor of 10 (3 for interspecies differences [the dog was more sensitive than the rat] and 3 for intraspecies differences in sensitivity [slight irritation should occur at a similar level among the general population]). Time-scaling was not applied because adaptation to slight irritation occurs. Therefore, the calculated value of 0.12 ppm was adopted for all AEGL-1 timepoints. The 0.12 ppm AEGL-1 value is similar to the ClO2 AEGL-1 of 0.15 ppm (also based on slight sensory irritation; EPA 2005), and the AEGL-1 is one-eighth of the HF AEGL-1 value of 1.0 ppm (NRC 2004). Application of an intraspecies factor of 3 is sufficient, since application of a larger factor would result in AEGL-1 values that are not consistent with those of ClO2 and HF, two of the major decomposition products of ClF3 (breakdown of one mole of ClF3 potentially forms three moles of HF and one mole of ClO2). The AEGL-1 values are listed in Table 2-6. Figure 2-1 category plot contains ClF3 data including the AEGL-1 end point and the AEGL-1 value.
TABLE 2-6 AEGL-1 Value for Chlorine Trifluoride
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m2) |
6.
DATA ANALYSIS FOR AEGL-2
6.1.
Human Data Relevant to AEGL-2
No human data relevant to derivation of an AEGL-2 were located.
6.2.
Animal Data Relevant to AEGL-2
There were no single exposure studies designed to evaluate nonlethal effects. Two dogs exposed to 5.15 ppm, 6 h/day, 5 days/week, for 6 weeks showed salivation, lacrimation, rhinorrhea, blinking of the eyes, severe coughing, and sneezing. The duration of ClF3 exposure required to induce these signs was not clearly stated, but “signs of mucous membrane irritation” observed in dogs during the first day appeared to be reversible by the end of the first day as the dogs at that time “did not appear markedly affected.” A group of twenty rats exposed to the same concentration did not appear to be affected either during the exposure or at the end of the first day (Horn and Weir 1955).
6.3.
Derivation of AEGL-2
The signs in dogs exposed to 5.15 ppm for 6 h - irritation, salivation, lacrimation, rhinorrhea, coughing, and sneezing - may be extremely uncomfortable but are reversible. Thus, these signs are considered consistent with a NOAEL for disabling effects or an impaired ability to escape. Twenty rats exposed to this concentration for 6 h appeared unaffected. However, repeated daily exposures of rats and dogs to this concentration resulted in increasingly severe signs of irritation. The AEGL-2 was calculated based on the 6-h exposure of dogs to 5.15 ppm. A combined uncertainty factor of 10 was applied: 3 for interspecies differences in sensitivity (the value is based on the dog which was considerably more sensitive than the rat) and 3 for intraspecies differences in sensitivity (irritation should occur at a similar concentration among the general population). Scaling across time was based on the time-scaling relationship derived in Section 4.4.3. Although the end point for time scaling was lethality in several species, the same relationship can be utilized for the AEGL-2 because the difference between severe irritation (AEGL-2) and lethality from tissue destruction (AEGL-3) is one of degree. The 10-min value was time-scaled from the 6-h point of departure because time-scaling data were available for 13.5 to 222 min. An intraspecies uncer-
tainty factor of 3 is sufficient as these AEGL-2 values are considerably lower than those of HF (10- and 30-min and 1-, 4-, and 8 h values of 95, 34, 24, 12, and 12 ppm, respectively) and similar to the longer-term AEGL-2 values for ClO2. The 10- and 30 min AEGL-2 values for ClF3 (8.1 and 3.5 ppm) are higher than those of ClO2 (both 1.4 ppm) because information was available for time-scaling ClF3 values, whereas, in the absence of time-scaling information, the conservative value of n = 3 was used for scaling to the shorter time periods for ClO2. Calculations appear in Appendix B and values are listed in Table 2-7. The AEGL-2 end point and AEGL-2 values are plotted in Figure 2-1.
7.
DATA ANALYSIS FOR AEGL-3
7.1.
Human Data Relevant to AEGL-3
Deichmann and Gerarde (1969) state that 50 ppm may be fatal in 30 min to 2 h. No further details were given. The source of that conclusion was not cited and cannot be treated as reliable.
7.2.
Animal Data Relevant to AEGL-3
Exposure of 20 rats or 2 dogs for 6 h to 21 ppm resulted in lacrimation, rhinorrhea, cough, rapid respiration, and salivation shortly after initiation of exposure, but no deaths were reported, even when the animals were exposed a second day (Horn and Weir 1955). Thus, this concentration-exposure duration may be below the threshold for lethality as defined by the AEGL-3.
The 1-h LC50 values for the monkey, rat, and mouse were 230, 299, and 178 ppm, respectively, and the 1-h values resulting in no deaths for the same species were 127, 200, and 125 ppm, respectively (MacEwen and Vernot 1970). Data were sufficient to derive LC01 values for mouse and rat. The data set for the monkey did not allow calculation of a reliable LC01.
TABLE 2-7 AEGL-2 Values for Chlorine Trifluoride
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
8.1 ppm (31 mg/m3) |
3.5 ppm (13 mg/m3) |
2.0 ppm (7.6 mg/m3) |
0.70 ppm (2.7 mg/m3) |
0.41 ppm (1.6 mg/m3) |
7.3.
Derivation of AEGL-3
Of the three species tested, the mouse is the most sensitive species as determined by the 1-h LC50 of 178 ppm. However, based on similar respiratory rates, gross respiratory tract anatomy, amount and distribution of types of respiratory epithelium, and nasal airflow patterns, the monkey is a more appropriate model for chemical deposition in the human respiratory tract. The AEGL-3 values were based on the highest 1 h concentration that resulted in no deaths in monkeys (MacEwen and Vernot 1970). This concentration, 127 ppm, was divided by interspecies and intraspecies uncertainty factors of 2 and 3, respectively (for a total of 6), and scaled across time using the C1.3 × t = k relationship. The interspecies uncertainty factor of 2 was considered adequate as the monkey is an appropriate model for extrapolation to humans. An intraspecies uncertainty factor of 3 was considered appropriate as differences among individuals in sensitivity to contact irritants (the concentration at which lung tissue damage occurs) should not differ greatly. The 8-h AEGL-3 value was set equal to the 4-h value because the time-scaled 8 h value of 4.3 ppm is inconsistent with the experimental data. Dogs exposed to 21 ppm for two days did not die during the following month of observation, and dogs and rats tolerated repeated 6 h exposures to 5.15 ppm for several weeks before the first death was recorded (Horn and Weir 1955). The AEGL-3 end point and AEGL-3 values are plotted in Figure 2-1. The AEGL-3 values are listed in Table 2-8.
Probit analyses of the mouse data resulted in an LC01 value of 135 ppm. Using the same uncertainty factors and time scaling relationship as for the monkey, the AEGL-3 values based on the mouse data are very similar, 89, 38, 23, 7.7, and 7.7 ppm for the 10- and 30 min and 1-, 4-, and 8 h time periods, respectively.
Chlorine trifluoride decomposes within seconds to HF among other products (MacEwen and Vernot 1970). Compared with the 1-h LC50 value for HF in monkeys (1774 ppm), the response to inhaled ClF3 in monkeys cannot be attributed entirely to HF (3 moles of HF formed), but HF may be a major factor (MacEwen and Vernot 1970). The difference in expected lethality may be due to the high water solubility and consequent scrubbing in the upper respiratory tract of HF compared with ClF3.
TABLE 2-8 AEGL-3 Values for Chlorine Trifluoride
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
84 ppm (320 mg/m3) |
36 ppm (140 mg/m3) |
21 ppm (80 mg/m3) |
7.3 ppm (28 mg/m3) |
7.3 ppm (28 mg/m3) |
Nevertheless, the AEGL-3 values for ClF3 are appropriate to those of HF.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
The AEGL-1 was based on slight irritation (rhinorrhea in the sensitive nose of the dog) during the first 3 h of a 6-h exposure to ClF3 at 1.17 ppm; notable discomfort (lacrimation) was observed after 3 h. A combined uncertainty factor of 10: 3 for interspecies differences in sensitivity (the dog was considerably more sensitive to ClF3 than the rat) and 3 for intraspecies differences in sensitivity was applied. No time scaling was applied as tolerance develops to the slight irritation that defines the AEGL-1.
The AEGL-2 was based on obvious irritation observed in dogs exposed to an average concentration of 5.15 ppm for 6 h. Although these signs resolved by the end of the day, they were considered a threshold for impaired ability to escape. Rats exposed to 5.15 ppm for 6 h appeared unaffected. The 6-h concentration of 5.15 ppm was divided by a combined interspecies and intraspecies uncertainty factor of 10 and scaled across time using the C1.3 × t = k relationship.
The AEGL-3 was based on the highest non-lethal 1 h value for the Rhesus monkey (127 ppm). The 127 ppm concentration was divided by a combined interspecies and intraspecies uncertainty factor of 6 and scaled across time using the same reasons and relationships as for the AEGL-1 above. Were the AEGL-3 values to be based on the LC01 for the mouse (135 ppm), essentially the same values would be derived.
The AEGL values for three levels and five exposure periods are summarized in Table 2-9.
8.2.
Comparisons with Other Standards
Standards and guidance levels for community and occupational exposures are listed in Table 2-10. The 1-h AEGL-1 value is similar to the 1-h ERPG-1 value, and the 1-h AEGL-2 and -3 values are higher than the respective ERPG values. The AEGL-1 and -2 values were based on studies with ClF3, whereas the ERPG-1 and -2 values for ClF3 are based on
analogies with chlorine and HF. The AEGL-3 and ERPG-3 values were based on the same lethality study (MacEwen and Vernot 1970), but used different species. The ERPG-3 was calculated by dividing the 1-h LC50
TABLE 2-9 Summary of AEGL Values
|
Classification |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 (Nondisabling) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
0.12 ppm (0.46 mg/m3) |
|
AEGL-2 (Disabling) |
8.1 ppm (31 mg/m3) |
3.5 ppm (13 mg/m3) |
2.0 ppm (7.6 mg/m3) |
0.70 ppm (2.7 mg/m3) |
0.41 ppm (1.6 mg/m3) |
|
AEGL-3 (Lethal) |
84 ppm (320 mg/m3) |
36 ppm (140 mg/m3) |
21 ppm (80 mg/m3) |
7.3 ppm (28 mg/m3) |
7.3 ppm (28 mg/m3) |
TABLE 2-10 Extant Standards and Guidelines for Chlorine Trifluoride (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
0.12 |
0.12 |
0.12 |
0.12 |
0.12 |
|
AEGL-2 |
8.1 |
3.5 |
2.0 |
0.70 |
0.41 |
|
AEGL-3 |
84 |
36 |
21 |
7.3 |
7.3 |
|
ERPG-1 (AIHA)a |
|
|
0.1 (0.3 as HF) |
|
|
|
ERPG-2 (AIHA) |
|
|
1 (3 as HF) |
|
|
|
ERPG-3 (AIHA) |
|
|
10 (30 as HF) |
|
|
|
EEGL (NRC)b |
7 |
3 |
1 |
|
|
|
IDLH (NIOSH)c |
|
20 |
|
|
|
|
Ceiling (OSHA)d |
|
|
|
|
0.1 |
|
Ceiling (NIOSH)e |
|
|
|
|
0.1 |
|
TLV-Ceiling (ACGIH)f |
|
|
|
|
0.1 |
|
MAC-Ceiling (Netherlands)g |
|
|
|
|
0.1 |
|
aERPG (Emergency Response Planning Guidelines, American Industrial Hygiene Association (AIHA 2004). The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one hour without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one hour without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to one hour without experiencing or developing life-threatening health effects. bEEGL (Emergency Exposure Guidance Levels, National Research Council (NRC 1984) The EEGL is the concentration of contaminants that can cause discomfort or other evidence of irritation or intoxication in or around the workplace, but avoids death, other severe acute effects and long-term or chronic injury. cIDLH (Immediately Dangerous to Life and Health, National Institute of Occupational Safety and Health) (Ludwig et al. 1994; NIOSH 2004) represents the maximum concentration from which one could escape within 30 minutes without any escape-impairing symptoms, or any irreversible health effects. dOSHA PEL-Ceiling (Permissible Exposure Limits - Ceiling Term Exposure Limit) (NIOSH 2004) must not be exceeded during any part of the workday. eNIOSH Ceiling (National Institute of Occupational Safety and Health, Recommended Exposure Limits - Ceiling) (NIOSH 2004) must not be exceeded during any part of a 10-hour workday. fACGIH TLV-Ceiling (American Conference of Governmental Industrial Hygienists, Threshold Limit Value - Ceiling) (ACGIH 2004) is the concentration that should not be exceeded during any part of the workshift. gMAC (Maximaal Aanvaarde Concentratie [Maximal Accepted Concentration]) (SDU Uitgevers [under the auspices of the Ministry of Social Affairs and Employment], The Hague, The Netherlands 2000) is defined analogous to the ACGIH-TLV-TWA. |
|||||
for the mouse (178 ppm) by approximately 20, whereas the AEGL-3 was derived by adjusting the highest non-lethal value in the monkey by a total uncertainty factor of 6.
The AEGL-1 value for ClF3 is less than the corresponding value for HF (1 ppm for all time periods; NRC 2004). The HF data base is exten-
sive and the AEGL-1 value for HF is based on markers of exposure in human subjects exposed to 0.2 to 6.3 ppm for 1 h. A total uncertainty factor of 3 was applied in development of the HF values.
The 10-min EEGL of 7 ppm is 58 times the 10-min AEGL-1, but it is similar to the 10-min AEGL-2. Exposure at the EEGL may involve discomfort, and the EEGL is considered by AIHA as an acceptable level for healthy adults, whereas the AEGL values are designed to account for the entire population. The 30-min and 1 h EEGL values are approximately 25 and eight times higher than the corresponding AEGL-1 values, but are similar to the corresponding AEGL-2 values. The basis for the 1984 EEGL values was not explained.
The 30-min NIOSH IDLH of 20 ppm (Ludwig et al. 1994) lies between the 30-min AEGL-2 and AEGL-3. The IDLH is based on 6 h exposures of dogs and rats to 21 ppm (Horn and Weir 1955). The 20 ppm IDLH was considered conservative by the NIOSH authors in light of the estimates of human fatal exposure concentrations by Deichmann and Gerarde (1969).
Workplace exposure limits, as 8 h ceiling values, are all 0.1 ppm and are intended to control and prevent the noxious ocular, mucous membrane and pulmonary irritation associated with exposure to ClF3. The workplace 0.1 ppm concentration limits are similar to the AEGL-1 value of 0.12 ppm. A German maximum workplace concentration (MAK) has not been established for this material.
8.3.
Data Adequacy and Research Needs
Human data were lacking. Lethal and sublethal data were available for four animal species and several exposure durations. For each species, relatively consistent values for lethality were observed among studies. However, monitoring data and analytical techniques demonstrated problems in maintaining consistent exposure concentrations. The most recently published study was in 1974; techniques for the analysis of ClF3 in air have improved since that time.
Values similar to the derived AEGL-3 values can be derived from the rat data. There were no deaths following 1 h exposures of rats to 200 ppm (MacEwen and Vernot 1970) or during 25- or 10-min exposures to 400 or 800 ppm, respectively (Dost et al. 1974). Application of a total uncertainty factor of 10 (an interspecies uncertainty factor of 3 would be
applied to the non-primate data) to each of these concentrations and time scaling with an n value of 1.3 results in 10 min time-scaled values of 79-81 ppm. Application of a total uncertainty factor of 10 to the 1-h mouse non-lethal (125 ppm) or LC01 (135 ppm) values results in lower 10 min values, 50 and 54 ppm, respectively. The rat data support selection of the monkey data and use of an interspecies uncertainty factor less than 3.
Few data are available to support the AEGL-1 and AEGL-2 values. However, both key studies were 6 h in duration (eliminating the uncertainty of extrapolating from short-term to long exposure durations) and used two species. The values were based on the dog, the more sensitive species as indicated by signs of irritation.
MacEwen and Vernot (1970) suggested that the toxicity of ClF3 may be associated with the formation of HF. Three moles of HF would be formed from the spontaneous decomposition of one mole of ClF3 in moist air. The AEGL-2 values for HF (12-95 ppm; NRC 2004) are approximately a factor of 10 times the respective AEGL-2 values for ClF3 (0.41-8.1 ppm), showing that the AEGL-2 values for ClF3 may be conservative (the data base for HF is extensive). The AEGL-3 values for HF and ClF3 more closely follow the molar relationship.
9.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2004. Documentation of the Threshold Limit Values and Biological Exposure Indices: Chlorine trifluoride. Sixth ed.,. Cincinnati, OH: ACGIH.
AIHA (American Industrial Hygiene Association). 2004. The AIHA Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guidelines Handbook. Fairfax VA: AIHA.
Bailey, W.I. and A.J. Woytek. 1994. Fluorine compounds, inorganic (halogens). In Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 11, 4th ed. New York: John Wiley & Sons, p. 349.
BOC Group. 1997. Chlorine trifluoride data sheet. The British Oxygen Company, Chertsey Road, Windlesham, Surrey GU20 6HJ, United Kingdom.
Bougon, R., M. Charles and J. Aubert. 1967. Reaction du trifluorure de
chlore avec l'eau. C.R. Acad. Sci. Ser. D 265:179-182. (Cited in Dost et al., 1974).
Cooper, T.D., F.N. Dost and C.H. Wang. 1972. Evidence for ClOF as a primary product of the reaction of ClF3 with H2O. J. Inorg. Nucl. Chem. 34:3564-3567.
Darmer, K.I., C.C. Haun, and J.D. MacEwen. 1972. The acute inhalation toxicity of chlorine pentafluoride. Am. Ind. Hygiene Assoc. J. 33:661-668.
Deichmann, W.B. and H.W. Gerarde. 1969. Chlorine trifluoride. In: Toxicology of Drugs and Chemicals. New York: Academic Press.
Dost, F.N., D.J. Reed. T.D. Cooper and C.H. Wang. 1970. Fluorine distribution in rats following acute intoxication with nitrogen and halogen fluorides and with sodium fluoride. Toxicol. Appl. Pharmacol. 17:573-584.
Dost, F.N., D.J. Reed. V.N. Smith and C.H. Wang. 1974. Toxic properties of chlorine trifluoride. Toxicol. Appl. Pharmacol. 27:527-536.
Horn, H.J. and R.J. Weir. 1955. Inhalation toxicology of chlorine trifluoride. I. Acute and subacute toxicity. A.M.A. Archives Indust. Health 12:515-521.
Horn, H.J. and R.J. Weir. 1956. Inhalation toxicology of chlorine trifluoride. II. Chronic toxicity. A.M.A. Archives Indust. Health 13:340-345.
HSDB (Hazardous Substances Data Bank). 2005. Chlorine trifluoride. MEDLARS Online Information Retrieval System, National Library of Medicine, National Institute of Health, Department of Health and Human Services, retrieved 4/26/05.
Longley, M.Y., J.F. Pierce and E.C. Griesemer. 1965. A toxic hazard study of selected missile propellants (1965). AMRL-TR-65-99, Wright Patterson Air Force Base, OH. (Cited in NRC, 1984).
Ludwig, H.R., S.G. Cairelli, and J.J. Whalen. 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHS). National Institute for Occupational Safety and Health (NIOSH), Cincinnati, OH; PB94195047, National Technical Information Service, Springfield, VA.
MacEwen, J.D. and E.H. Vernot. 1970. Toxic Hazards Research Unit Annual Technical Report: 1970. AMRL-TR-70-77, Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, OH; National Technical Information Service, Springfield, VA.
MacEwen, J.D. and E.H. Vernot. 1971. Toxic Hazards Research Unit
Annual Technical Report: 1971. AD-734 543; AMRL-TR-71-83; National Technical Information Service, Springfield, VA.
Matheson. 1980. Matheson Gas Data Book, 6th ed., Division Searle Medical Products USA, Inc., Lyndhurst, NJ.
Ministry of Social Affairs and Employment (SDU Uitgevers). 2000. Nationale MAC (Maximum Allowable Concentration) List, 2000. The Hague, The Netherlands.
NIOSH (National Institute for Occupational Safety and Health). 2004. NIOSH Pocket Guide to Chemical Hazards. Publication 94-116, U.S. Department of Health and Human Services; U.S. Government Printing Office, Washington, DC. See also http://www.cdc.gov/niosh/idlh/74873.html.
NRC (National Research Council). 1984. Emergency and Continuous Exposure Limits for Selected Airborne Contaminants, Volume 2. Committee on Toxicology, National Research Council, National Academy Press, Washington, DC.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2004. Acute Exposure Guideline Levels for Selected Airborne Chemicals, Volume 4. Washington, DC: National Academy Press.
O'Neil, M.J., A. Smith, and P.E. Heckelman (Eds.). 2001. The Merck Index, 13th ed. Whitehouse Station, NJ: Merck & Co., Inc.
Schreider, J.P. 1986. Chapter 1: Comparative anatomy and function of the nasal passages. In: C.S. Barrow, ed., Toxicology of the Nasal Passages. New York: Hemisphere Publishing Corp.
Syage, J.A. 1994. Launch Safety, Toxicity, and Environmental Effects of the High Performance Oxidizer ClF5. ADA286095; Available from National Technical Information Service, Springfield, VA.
Teitelbaum, D.T. 2001. The halogens. Pp. 731-825 in: Patty's Toxicology, Vol. 3, E. Bingham et al., eds. New York: John Wiley & Sons, Inc.
U.S. DOT (U.S. Department of Transportation, U.S. Coast Guard). 1985. Chemical Hazard Response Information System: chlorine trifluoride.
U.S. EPA (U.S. Environmental Protection Agency). 2005. Chlorine Dioxide: Interim Acute Exposure Guideline Levels. Washington, DC.
Vernot, E.H., J.D. MacEwen, C.C. Haun and E.R. Kinkead. 1977. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol. 42:417-423.
APPENDIX A
TIME: CONCENTRATION RELATIONSHIP FOR LETHALITY
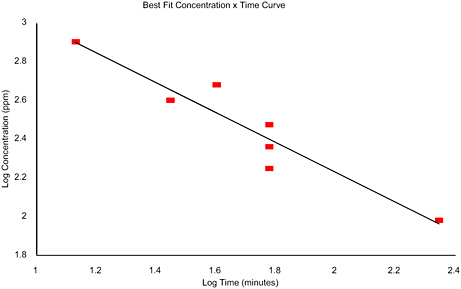
FIGURE A-1 Chlorine trifluoride: LC50 values for three species—monkey, rat, and mouse (Horn and Weir 1955; MacEwen and Vernot 1970; Dost et al. 1974).
|
Time |
Conc. |
Log Time |
Log Conc. |
|
13.5 |
800 |
1.1303 |
2.9031 |
|
28 |
400 |
1.4472 |
2.6021 |
|
40 |
480 |
1.6021 |
2.6812 |
|
60 |
178 |
1.7782 |
2.2504 |
|
60 |
230 |
1.7782 |
2.3617 |
|
60 |
299 |
1.7782 |
2.4757 |
|
222 |
96 |
2.3464 |
1.9823 |
|
n = |
1.3 |
|
|
|
k = |
79325.99 |
|
|
|
Regression Output: |
|
|
Intercept |
3.7684 |
|
Slope |
−0.7692 |
|
R Squared |
0.9014 |
|
Correlation |
−0.9494 |
|
Degrees of Freedom |
5 |
|
Observations |
7 |
APPENDIX B
DERIVATION OF AEGL VALUES
DERIVATION OF AEGL-1 FOR CHLORINE TRIFLUORIDE
|
Key study: |
Horn and Weir 1956 |
|
Toxicity end point: |
Mucous membrane irritation as evidenced by nasal discharge, the only sign of irritation during the first 3 h of a 6-h exposure of dogs to 1.17 ppm. |
|
Uncertainty factors: |
3 for interspecies 3 for intraspecies combined uncertainty factor of 10a |
|
Scaling: |
No time scaling was utilized. Adaptation occurs to the slight irritation that defines the AEGL-1. |
|
Calculation: |
(Concentration/uncertainty factors) = AEGL-1 (1.17 ppm/10) = 0.12 ppm |
|
|
Because tolerance develops to the slight irritation that defines the AEGL-1, the 0.12 ppm value was used for all AEGL-1 exposure durations. |
|
aEach uncertainty factor of 3 is actually the geometric mean of 10 which is 3.16; 3.16 × 3.16 = 10. |
|
Derivation of AEGL-2 for Chlorine Trifluoride
|
Key study: |
Horn and Weir 1955 |
|
Toxicity end point: |
Strong irritation in dogs exposed to a concentration of 5.15 ppm for 6 h. |
|
Uncertainty Factors: |
3 for interspecies 3 for intraspecies combined uncertainty of 10 |
|
Scaling: |
C1.3 × t = k (this document; based on LC50 concentration and exposure duration relationships in Horn and Weir [1955], MacEwen and Vernot [1970], and Dost et al. [1974]). |
|
Calculations: |
|
|
|
(C1.3/uncertainty factors) × t = k ([5.15 ppm1.3] /10) × 360 min = 151.93 ppm1.3 · min |
|
|
10 min AEGL-2: 151.93 ppm1.3 · min/10 min = 8.1 ppm |
|
|
30 min AEGL-2: 151.93 ppm1.3 · min/30 min = 3.5 ppm |
|
|
1 h AEGL-2: 151.93 ppm1.3 · min/60 min = 2.0 ppm |
|
|
4 h AEGL-2: 151.93 ppm1.3 · min/240 min = 0.70 ppm |
|
|
8 h AEGL-2: 151.93 ppm1.3 · min/480 min = 0.41 ppm |
Derivation of AEGL-3 for Chlorine Trifluoride
|
Key study: |
MacEwen and Vernot 1970 |
|
Toxicity end point: |
Highest 1 h non-lethal value in monkeys (127 ppm) |
|
Uncertainty Factors: |
2 for interspecies 3 for intraspecies combined uncertainty factor of 6 |
|
Scaling: |
C1.3 × t = k (this document; based on LC50 concentration and exposure duration relationships in Horn and Weir [1955], MacEwen and Vernot [1970], and Dost et al. [1974]). |
|
Calculations: |
(C1.3/uncertainty factors) × t = k (127 ppm1.3/6) × 60 min = 3173.2 ppm1.3 · min |
|
|
k = 3173.2 ppm1.3· min |
|
10 min AEGL-3: |
3173.2 ppm1.3 · min/10 min = 84 ppm |
|
30 min AEGL-3: |
3173.2 ppm1.3 · min/30 min = 36 ppm |
|
1 h AEGL-3: |
3173.2 ppm1.3 · min/60 min = 21 ppm |
|
4 h AEGL-3: |
3173.2 ppm1.3 · min/240 min = 7.3 ppm |
|
8 h AEGL-3: |
3173.2 ppm1.3 · min/480 min = 4.3 ppmb |
|
bBecause the time-scaled 8 h AEGL-3 value of 4.3 ppm is inconsistent with the experimental data, the 8-h value was set equal to the 4-h value of 7.3 ppm. |
|
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLORINE TRIFLUORIDE (CAS REG. NO. 7790-91-2)
DERIVATION SUMMARY
|
AEGL-1 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
0.12 ppm |
0.12 ppm |
0.12 ppm |
0.12 ppm |
0.12 ppm |
|
Key Reference: Horn, H.J. and R.J. Weir. 1956. Inhalation toxicology of chlorine trifluoride. II. Chronic toxicity. A.M.A. Arch. Indust. Health 13:340-345. |
||||
|
Test Species/Strain/Number: Two dogs and 20 rats, breed and strain not stated. |
||||
|
Exposure Route/Concentration/Duration: Inhalation: 1.17 ppm, 6 h/day, 5 days/week for 6 months. |
||||
|
Effects during first day: Dogs: 1.17 ppm for 6 h - nasal discharge (began within 0 to 45 min) obvious lacrimation (after 3 h). Rats: 1.17 ppm for 6 h - no observed effects. |
||||
|
End point/Concentration/Rationale: A concentration of 1.17 ppm for 3 h resulted in no signs of irritation in dogs other than nasal discharge. Nasal discharge is considered to be within the definition of the AEGL-1 (mild sensory irritation). Lacrimation after 3 h of exposure was considered the threshold for notable discomfort. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—The dog is a sensitive species for nasal irritation and provides a good model for humans. Dogs exposed to 1.17 ppm showed obvious lacrimation after 3 h yet rats showed no effects at the same concentration for 6 h. Intraspecies: 3—The concentration at which slight irritation is induced in the general population should not differ greatly. |
||||
|
Modifying Factor: Not applicable. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: Not applied; adaptation occurs to the slight sensory irritation that defines the AEGL-1. |
||||
|
Data Adequacy: Although only two dogs were tested in the key study, the concomitant exposure of 20 rats contributes to confidence in the data. The value was based on the dog, which appeared to be more sensitive to respiratory irritants than the rat. Although no histopathological examinations were performed until the termination of the experiment or death, exposure continued for 56 days (39 exposures) before a death occurred in the treated rats. The hydrolysis of ClF3 potentially produces three moles of hydrogen fluoride (HF). Confidence in the AEGL-1 values is boosted by the fact that the values for ClF3 are one-eighth of the AEGL-1 values for HF. The database for HF is extensive. |
|
AEGL-2 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
8.1 ppm |
3.5 ppm |
2.0 ppm |
0.70 ppm |
0.41 ppm |
|
Key Reference: Horn, H.J. and R.J. Weir. 1955. Inhalation toxicology of chlorine trifluoride. I. Acute and subchronic toxicity. A.M.A. Arch. Indust. Health 12:515-521. |
||||
|
Test Species/Strain/Sex/Number: Two dogs and 20 rats, breed and strain not stated. |
||||
|
Exposure Route/Concentration/Duration: Inhalation: 5.15 ppm for 6 h/day, 5 days/week for 6 months. |
||||
|
Effects (observed during the first day) for exposures to 5.15 ppm for 6 h: Dogs: strong irritation (salivation, lacrimation, rhinorrhea, coughing, sneezing) apparent recovery at end of day. Rats: no observed effects. |
||||
|
End point/Concentration/Rationale 5.15 ppm for 6 h resulted in strong signs of irritation (salivation, lacrimation, rhinorrhea, coughing, sneezing) in the dog. These signs and symptoms are consistent with the definition of the AEGL-2 (threshold for irreversible or other serious, long-lasting effects or impaired ability to escape). Following 2 days of exposure to 21 ppm, corneal ulcers were observed. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—The dog is a sensitive species for nasal irritation and provides a good model for the human. Dogs exposed to 5.15 ppm |
||||
|
showed signs of strong irritation (salivation, lacrimation, rhinorrhea, coughing, sneezing) during a 6-h exposure period yet rats showed no effects at the same concentration for 6 h. Intraspecies: 3—The concentration that induces irritation among the general population should not vary greatly. |
||||
|
Modifying Factor: Not applicable. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: Cn × t = k where n = 1.3; based on the time-concentration relationship for LC50 values in monkeys, rats, and mice for exposure durations of 13.5-222 min (Horn and Weir 1955; MacEwen and Vernot 1970; Dost et al. 1974). |
||||
|
Data Adequacy: Although only two dogs were tested in the key study, the concomitant exposure of 20 rats contributes to confidence in the data. The value was based on the dog which appeared to be more sensitive to respiratory irritants than the rat. No histopathological examinations were performed until termination of the experiment or death; exposures continued for 26 days before a death occurred in the treated dogs. |
|
AEGL-3 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
84 ppm |
36 ppm |
21 ppm |
7.3 ppm |
7.3 ppm |
|
Key Reference: MacEwen, J.D. and E.H. Vernot. 1970. Toxic Hazards Research Unit Annual Technical Report: 1970, AMRL-TR-70-77, Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, OH. |
||||
|
Test Species/Strain/Sex/Number: Male and female rhesus monkeys, 4/exposure group. |
||||
|
Exposure Route/Concentration/Duration: Inhalation: 127, 150, 200, 300, or 400 ppm for 1 h. |
||||
|
Effects from 1 h exposure: |
||||
|
Concentration |
Mortality |
|
|
|
|
127 ppm: |
0/4 |
|
|
|
|
150 ppm: |
2/4 |
|
|
|
|
200 ppm: |
1/4 |
|
|
|
|
300 ppm: |
2/4 |
|
|
|
|
400 ppm: |
4/4 |
|
|
|
|
1 h LC50 is 230 ppm (provided in reference) 1 h LC01 could not be calculated |
||||
|
End point/Concentration/Rationale: 127 ppm for 1 h, the highest non-lethal value in the monkey, was considered the threshold for lethality, the defined end point for the AEGL-3. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 6 Interspecies: 2—Based on the similarity in respiratory parameters among primates. In addition, effects were similar among species and LC50 values varied by less than a factor of two for the monkey, rat, and mouse (indicating similar species sensitivity). Intraspecies: 3—The concentration at which extreme irritation and pulmonary damage may lead to lethality should not differ by more than a factor of 3 among the general population. |
||||
|
Modifying Factor: Not applicable. |
||||
|
Animal to Human Dosimetric Adjustment: Insufficient data. |
||||
|
Time Scaling: Cn × t = k where n = 1.3; based on the time-concentration relationship for LC50 values in monkeys, rats, and mice for exposure durations of 13.5-222 min (Horn and Weir 1955; MacEwen and Vernot 1970; Dost et al. 1974). |
||||
|
Data Adequacy: The key study was well conducted and documented. LC50 values from several additional studies were within a factor of two for all tested species. Similar values can be derived using the rat data (MacEwen and Vernot 1970; Dost et al. 1974) and a total uncertainty factor of 10. |
||||