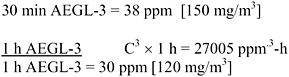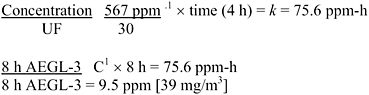3
Cyclohexylamine1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/ AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed
for each of five exposure periods (10 and 30 min, 1 h, 4 h, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure levels that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, non-sensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to unique or idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
EXECUTIVE SUMMARY
Cyclohexylamine is a respiratory, eye, and skin irritant, as well as a strong base (pKa = 10.7) with a fishy, amine odor. It is used primarily for boiler water treatment (corrosion inhibition) as well as organic synthesis of rubber and agricultural chemicals. Occupational exposure to cyclohexylamine has been reported to cause headache, nausea, dizziness, vomiting, eye, nose and throat irritation, and rapid and irregular heart-
beat. Acute exposure of animals resulted in extreme mucous membrane irritation, gasping, tremors, clonic muscular spasms, lung hemorrhage, opaque corneas, vascular lesions, and hemolysis.
The level of distinct odor awareness (LOA) for cyclohexylamine is 2.0 ppm (see Appendix B for LOA derivation). The LOA represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, about 10% of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception.
AEGL-1, AEGL-2, and AEGL-3 values were derived from a study in which Sprague-Dawley rats (5/sex/dose) were exposed for 4 h to 54.2 ppm (Group III) or 567 ppm (Group II) cyclohexylamine vapor, or to a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol (Group I) (Bio/dynamics, Inc. 1990). This well-conducted study was the most comprehensive of the available acute exposure studies. At 54.2 ppm, rats developed labored breathing, partially closed eyes, and red nasal discharge. Rats exposed to the two higher concentrations also exhibited rales, gasping, dried red facial material, tremors, body weight loss, and ocular lesions (corneal opacity, ulceration). Corneal opacity and ulceration were irreversible in Groups I and II (i.e., still present 3 weeks after exposure), but most other effects were reversible in both Groups I and II or only in Group II. Two Group I rats died and developed alopecia and lesions in the nasal turbinates, lungs, and/or urinary bladder.
AEGL-1 values were obtained by dividing 54.2 ppm by a modifying factor of 3, because the effects seen in rats at 54.2 ppm were more severe than prescribed by the AEGL-1 definition. Because mild sensory irritation is not expected to vary greatly over time, the same AEGL value was adopted for exposures of 10 min to 8 h. Uncertainty factors of 3 were applied for interspecies and intraspecies variability, respectively. Mild sensory irritation is not likely to vary greatly among humans or animals, and both human and additional animal data indicate that a greater UF was not warranted. The AEGL-1 is consistent with a study in which chemical workers exposed to 4-10 ppm for an undefined duration (<8 h) reported “no symptoms of any kind” (Watrous and Schulz 1950), but which was inappropriate for AEGL-1 derivation because effects were below AEGL-1 severity criteria. The AEGL-1 values are also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989), from which it is predicted that 2.7 or 5.1 ppm
should result in some sensory irritation in humans, whereas 0.27 or 0.51 ppm should cause no sensory irritation (Alarie 1981).
AEGL-2 values were based on the Bio/dynamics, Inc. (1990) inhalation exposure of rats to cyclohexylamine for 4 h to 54.2 ppm. The rats had moderate respiratory effects and ocular irritation but no irreversible ocular lesions, consistent with an earlier study in which rats, a rabbit, and guinea pigs exposed to 150 ppm 7 h/day for up to 2 weeks had no eye lesions, but those exposed to 800 ppm had corneal opacity (Watrous and Schulz 1950). Data were not available to determine the concentration-time relationship for cyclohexylamine toxicity. The concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain protective AEGL-2 values, scaling across time was performed using n = 3 to extrapolate to exposure times <4 h (exposure duration in the key study), except for the 10-min values, and n = 1 to extrapolate to exposure times >4 h. The 30-min values were adopted as 10-min values due to unacceptably large uncertainty in extrapolating from ≥4 h to 10 min and to be protective of human health (NRC 2001). A total uncertainty factor of 10 was applied (3 for interspecies variability and 3 for intraspecies variability) because effects seen at 54.2 ppm were clearly reversible, and a larger uncertainty factor yields values at or below the AEGL-1.
The AEGL-3 values were based on irreversible ocular lesions and an estimated lethality threshold in rats, from exposure for 4 h to 567 ppm. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the ten Berge et al. (1986) equation Cn × t = k and n = 1 or n = 3, as for the AEGL-2 (NRC 2001). A total uncertainty factor of 30 was used: 10 for interspecies variability because, although tissue destruction caused by a severely corrosive agent is not expected to vary greatly among animals, the dose spacing in the key study failed to delineate the LOAEL for ocular lesions or the threshold for lethality in rats, and the set of animal studies was limited. An intraspecies uncertainty factor of 3 was applied because tissue destruction caused by a severely corrosive agent is not expected to vary greatly among humans; a greater uncertainty factor is not warranted because it yields concentrations comparable to AEGL-2 values in Table 3-1.
TABLE 3-1 Summary of AEGL Values for Cyclohexylamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End point (Reference) |
|
AEGL-1a (Non-disabling) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
Mild sensory and/or ocular irritation in rats (Bio/dynamics, Inc. 1990). |
|
AEGL-2 (Disabling) |
11 ppm (45 mg/m3) |
11 ppm (45 mg/m3) |
8.6 ppm (35 mg/m3) |
5.4 ppm (22 mg/m3) |
2.7 ppm (11 mg/m3) |
Moderate respiratory effects and ocular irritation; NOAEL for irreversible ocular lesions (Bio/dynamics, Inc. 1990). |
|
AEGL-3 (Lethal) |
38 ppm (150 mg/m3) |
38 ppm (150 mg/m3) |
30 ppm (120 mg/m3) |
19 ppm (77 mg/m3) |
9.5 ppm (39 mg/m3) |
Lethality threshold and irreversible ocular lesions (Bio/dynamics, Inc. 1990). |
|
aReported odor thresholds vary from 2.6 to 110 ppm. |
||||||
1.
INTRODUCTION
Cyclohexylamine is a strong base (pKa = 10.6; HSDB 2002) and a flammable liquid. Cyclohexylamine occurs naturally, in the wood of the plant Toddalia asiatica (Tsai et al. 1998). Cyclohexylamine is produced by the catalytic hydrogenation of aniline at elevated temperatures and pressures, by the ammonolysis of cyclohexanol, or by the reduction of nitrocyclohexane (Sandridge and Staley 1978). Its uses include boiler water treatment (corrosion inhibition), synthesis of rubber chemicals, agricultural chemicals, plasticizers, and emulsifying agents (HSDB 2002). In the 1960s, a major use of cyclohexylamine was the production of cyclamate sweeteners for beverages and food products; this practice was banned by the U.S. Food and Drug Administration in 1970 (IARC 1980). U.S. demand for cyclohexylamine was estimated as 25 million lbs for 2002, which included exports up to 5 million pounds per year (HSDB 2002). As of 1994, the U.S. International Trade Commission listed only two U.S. producers, but the amounts produced or sold were not disclosed (USITC 1995).
Cyclohexylamine is a respiratory, eye, and skin irritant. The marked corrosive, irritant properties and foul odor generally limit human exposure to airborne cyclohexylamine. Watrous and Schulz (1950) pointed out that “the strong, disagreeable smell of cyclohexylamine, and its intensely bitter taste provide good warning properties.” Reported odor thresholds vary widely, ranging from 2.6 ppm (Amoore and Hautala 1983) to 26-110 ppm (Ruth 1986). Occupational exposure to cyclohexylamine has caused symptoms including headache, nausea, dizziness, vomiting, eye, nose, and throat irritation, and rapid and irregular heartbeat. Acute exposure of animals resulted in extreme mucous membrane irritation, gasping, lung hemorrhage, opaque corneas, tremors, restlessness and clonic spasm of the trunk and paw muscles, hemolysis, and vascular lesions.
The primary fate of cyclohexylamine in the atmosphere is reaction with hydroxyl radicals, with a half-life of approximately 1.82 days (EPA 1987). Chemical and physical properties of cyclohexylamine are listed in Table 3-2.
TABLE 3-2 Chemical and Physical Data
|
Parameter |
Value |
Reference |
|
Synonyms |
Cyclohexanamine, aminocyclohexane, aminohexahydrobenzene, cyclohexylamine, hexahydroaniline, hexahydrobenzenamine |
IARC 1980 |
|
Chemical formula |
C6H13N |
Budavari et al. 1996 |
|
Molecular weight |
99.18 |
Budavari et al. 1996 |
|
CAS Registry Number |
108-91-8 |
IARC 1980 |
|
Physical state |
Liquid |
IARC 1980 |
|
Color |
Colorless or yellow |
HSDB 2002 |
|
Solubility in water |
Completely miscible |
Budavari et al. 1996 |
|
Acid ionization constant, pKa |
10.6 |
HSDB 2002 |
|
Vapor pressure |
8.4 mm Hg at 20°C |
Eastman Kodak 1984 |
|
Vapor density (air = 1) |
3.42 |
Verschueren 1996 |
|
Liquid density (water = 1) |
0.8647 at 25/25°C |
Budavari et al. 1996 |
|
Melting point |
−17.7°C |
Budavari et al. 1996 |
|
Boiling point |
134.5°C at 760 mm |
Budavari et al. 1996 |
|
Flammability/explosive limits |
1.5-9.4% |
NIOSH 2005 |
|
Conversion factors |
1 mg/m3 = 0.247ppm; 1ppm = 4.06 mg/m3 |
Verschueren 1996 |
2.
HUMAN TOXICITY DATA
2.1.
Acute Lethality
No reports of human lethality resulting from acute cyclohexylamine exposure were located.
2.2.
Nonlethal Toxicity
2.2.1.
Odor Threshold/Odor Awareness
The odor detection threshold for cyclohexylamine was reported to be 2.6 ppm by Amoore and Hautala (1983). The value of 2.6 ppm was stated to be the geometric mean of all available literature data (not given), omitting extreme points and duplicate values, although the methods used to obtaining the individual odor thresholds were not described. Another secondary source listed the low and high reported odor detection thresholds for cyclohexylamine of 26 and 110 ppm, respectively (Ruth, 1986). It is possible that the low threshold in the latter source was a typographic error and should have been 2.6 ppm.
2.2.2.
Occupational Exposure
Watrous and Schulz (1950) described three cases of industrial cyclohexylamine exposure. A 42-year-old chemical operator, exposed for about an hour, noticed a strong, fishy smell, felt lightheaded and became very anxious. Later the same day, he reported loss of appetite, anxiety that prevented his falling asleep, burning in his throat, and a rapid heartbeat. Medical examination the following day revealed no abnormalities although the man still complained of anxiety. Air cyclohexylamine concentrations were not obtained at the time but were measured at a later time (not specified) and found to be 4-10 ppm. The location and number of operators involved were not given, though “this exposure caused no symptoms of any kind in the operators.”
In the second case, a 27-year-old operator was splattered on the face with liquid cyclohexylamine dissolved in a caustic solution (not identified). The skin on his face became red and developed many small white spots characteristic of coagulative necrosis. The man became nau-
seated and vomited an hour after the accident and twice more later. He had a normal pulse and blood pressure but became drowsy, had slurred speech and widely dilated pupils that responded poorly to light. The next day he apparently recovered except for some facial crusting.
The third clinical case described by Watrous and Schulz (1950) was of a supervisor of the process that used cyclohexylamine. He was exposed to cyclohexylamine vapor on several occasions and said that he became nauseated, although he did not vomit (no further details were provided).
NIOSH conducted a Health Hazard Evaluation of the Cincinnati Electronics Corp. (Hills and Lushniak 1989; Hills et al. 1990). Employees reported symptoms including headache, rapid and irregular heartbeat, nausea, dizziness, vomiting, and eye, nose and throat irritation that persisted for several hours to several days. They worked in an area humidified by a water boiler to which was added four times the normal amount of a corrosion inhibitor containing cyclohexylamine (and diethylaminoethanol). The odor was described as musty-acrid, ammonia-like, musty radiator, or pungent. Air samples were not collected during the incident. Samples collected by NIOSH several days later (2 h, 0.2 liters/min [LPM]) had cyclohexylamine concentrations below detectable limits (~0.08 ppm), likely due to the six boiler steam purgings and daily addition of clean replacement water to the boiler after the incident prior to the NIOSH investigation.
2.3.
Neurotoxicity
No descriptions of neurologic involvement other than the case descriptions by Watrous and Schulz (1950) were located.
2.4.
Developmental/Reproductive Toxicity
There are no confirmed reports of cyclohexylamine-induced reproductive or developmental toxicity in humans. Microgastria, a rare congenital anomaly arising from a defect in embryological development and resulting in effects including asplenia, upper limb hypoplasia, and intestinal malrotation, was reported in a 2.75-year old boy conceived as the result of insemination by donor following Clomid stimulation (Hanson et al. 1990). While this individual was in utero, his mother was ex-
posed to cyclohexylamine on pregnancy day 34 or 35. The boy was born a normal size (7.5 lbs) and had a normal karyotype. No details of the cyclohexylamine exposure were given. No causal relationship between the microgastria and cyclohexylamine exposure was asserted by the study authors.
2.5.
Genotoxicity
Cyclohexylamine did not induce cytogenetic changes (rings, dicentrics, chromatid breaks or aberrations) in human lymphocytes in vitro (Brewen et al. 1971). Cells were exposed at the G0, G1, S, or G2 stages of the cell cycle with up to 500 μg/mL cyclohexylamine.
2.6.
Carcinogenicity
No epidemiologic studies or case reports of carcinogenicity occurring from cyclohexylamine exposure by any route were located. IARC (1987) and the ACGIH (2004) concluded that there is inadequate evidence in humans and in experimental animals to establish the carcinogenicity of cyclohexylamine. IARC placed cyclohexylamine (with cyclamates) in carcinogenicity Group 3 and the ACGIH placed it in Group A4. The Environmental Protection Agency (EPA) has not provided a carcinogenicity weight-of-evidence classification for cyclohexylamine (EPA 2005).
2.7.
Summary
Several occupational studies described effects from acute inhalation exposure to unknown air concentrations of cyclohexylamine. Workers reported headache, rapid and irregular heartbeat, nausea, dizziness, vomiting, and eye, nose and throat irritation. Watrous and Schulz (1950) found that exposure during an unspecified fraction of a workday to 4-10 ppm cyclohexylamine “caused no symptoms of any kind,” but there was no indication whether the workers were able to detect the characteristic odor. Widely varying odor detection thresholds were reported for cyclohexylamine in two secondary sources (2.6 ppm and 26-110 ppm). There
was no evidence in humans of cyclohexylamine inhalation causing carcinogenicity, reproductive or developmental effects, or genotoxicity.
3.
ANIMAL TOXICITY DATA
Only a small number of animal studies were located; these used rats, mice, guinea pigs, and rabbits. Unfortunately, most had incomplete reporting of the methods and/or results. The cyclohexylamine inhalation studies are summarized in Table 3-3.
3.1.
Acute Lethality
3.1.1.
Rats
In a 4-h acute inhalation toxicity study conducted by Bio/dynamics, Inc. (1990), Sprague-Dawley rats (5/sex/dose) were administered nominal concentrations of 8.8 (Group I), 6.4 (Group II), or 0.57 mg/L (Group III) cyclohexylamine (determined using the test substance weight and delivered air volume). Exposure was in a 100-L Plexiglas chamber. The analytical concentrations of cyclohexylamine vapor were measured hourly, and for Groups I, II, and III were, respectively, 2.2, 2.3, and 0.22 mg/L (542 ppm, 567 ppm, and 54.2 ppm). The Group I atmosphere also contained cyclohexylamine aerosol (mean of 612 mg/m3 range of 5.5-1,500 mg/m3; mean mass median diameter [MMMD] of 11 μM, measured hourly). The aerosol appeared to have formed by reaction of the vapor with moisture from the animals, since aerosol was not seen during empty chamber trials at the same target concentration. Additional desiccation of the chamber air during exposure of Group II and III animals eliminated all but small amounts of aerosol (Group II: 0.00011-0.59 mg/m3, 12 μM MMMD; Group III: 0.88-54 mg/m3, 2.2 μM MMMD). Rats were observed for 2 weeks (Group III) or 3 weeks (Groups I, II) post exposure and were weighed on days 1 (preceding exposure), 2, 3, 5, 8, 15, and for Groups I and II, also on day 22. Necropsies were performed on all animals (no histopathology was performed).
Two Group I rats died on day 2. They had alopecia and nasal, lung, and urinary bladder lesions. Groups I and II rats had dyspnea, gasping, tremors, partly or completely shut eyes, profuse lacrimation, corneal opacity and ulceration, red nasal discharge, dried red or brown stains on
TABLE 3-3 Cyclohexylamine Inhalation Exposure Animal Studies
|
Single-Exposure Studies |
|||||
|
Species |
Exposure Time |
Conc. (ppm) |
Time of Death |
Mortality |
Effects, Comments (Reference) |
|
Rat |
6 h |
1,000 |
(none) |
0/3 |
No death or reported effects |
|
|
6 h |
12,000 |
48 h |
2/3 |
Death; no other details (Eastman Kodak,1984) |
|
Rat |
4 h |
4,000 |
(none) |
0/6 |
No death or reported effects |
|
|
4 h |
8,000 |
? |
6/6 |
Death; no other reported effects |
|
|
≤2 h |
~15,000 |
(none) |
0/6 |
No death, reported effects (Smyth, et al. 1969) |
|
Rat |
4 h |
54.2 |
(none) |
0/10 |
Lacrimation, red nasal discharge (see Table 3) Corneal lesions, severe respiratory effects |
|
|
4 h |
567 |
(none) |
0/10 |
|
|
|
4 h |
>542a |
Day 2 |
2/10 |
As above; 2/10 died (Bio/dynamics, Inc. 1990) |
|
Rat |
Not given |
443 |
(none) |
LC0 |
Effects reported but not ascribed to a specific dose or species include irritation of mucous membranes, restlessness, muscle spasm, hemolysis, vascular lesions, organ weight changes. Mice given 2.5 ppm had changes in subthreshold impulse summation. (Lomonova 1965) |
|
|
(<8 h ?) |
1,059 |
Mainly on days |
LCLo |
|
|
|
|
1,847 |
7-14 |
LC50 |
|
|
|
|
2,833 |
|
LC100 |
|
|
Mouse |
not given |
2.5, 12.3 |
(none) |
LC0 |
|
|
|
(<8 h ?) |
24.6 |
Mainly on days |
LCLo |
|
|
|
|
264 |
1-5 |
LC50 |
|
|
|
|
1,059 |
|
LC100 |
|
|
Mouse |
15 min |
26-84 |
(none) |
0/6 |
RD50 = 51 ppm (Gagnaire et al. 1989; 1993) |
|
|
120 min |
79-220 |
(none) |
0/6 |
Tracheally cannulated RD50 = 51 ppm |
|
Mouse |
30 min |
4-355 |
(none) |
0/4 |
RD50 = 27 ppm; 75% lower respiration rate at 355 ppm (Nielsen and Yamagiwa 1989) |
|
|
|
32-345 |
(none) |
0/4 |
Tracheally cannulated RD50 = 78 ppm |
|
Multiple-Exposure Studies |
|||||
|
Rat |
2 h/d x 2 mo |
172 |
2 mo |
3/6 |
↓ BW gain, vascular changes, organ lesions |
|
|
4 h/d x 5 mo |
24.6 |
4 mo |
1/20 |
Leukocytosis at 3-5 mo. (Lomonova 1965) |
|
Rat |
7 h/d x 10 d |
150 |
≤10 day |
1/5 |
1 death; no other reported effects |
|
|
|
800 |
24 h |
0/5b |
Corneal opacity; death possibly (unclear) |
|
|
|
1,200 |
7 h |
4/5b |
Extreme irritation, lung hemorrhage, opaque corneas, death(Watrous and Schulz 1950) |
|
Guinea pig |
7 h/d x 10 d |
150 |
≤10 day |
0/2?b |
No reported effects |
|
|
|
800 |
14 h |
2/?b |
Corneal opacity; 2 deaths |
|
|
|
1,200 |
7 h |
All |
Extreme irritation, lung hemorrhage, opaque corneas, death (Watrous and Schulz 1950) |
|
Rabbit |
7 h/d x 10 d |
150 |
7 h |
1/?b |
1 death; no other reported effects |
|
|
|
800 |
14 h |
1/?b |
Corneal opacity; 1 death |
|
|
|
1,200 |
7 h |
All |
Extreme irritation, lung hemorrhage, opaque corneas, death (Watrous and Schulz 1950) |
|
?, Unknown; not reported. aHighest conc. was 542 ppm vapor + ~612 mg/m3 aerosol. bTotal number of animals tested and the results for the entire 70-h exposure period were not reported. |
|||||
face, moist and dry rales, yellow ano-genital stains, alopecia, and marked body weight loss. Many of these effects persisted during the 3-week post-exposure observation period in Group I. Necropsy revealed numerous eye lesions (ulcerations, opacity, corneal scars, tissue damage, and discoloration) in Groups I and II but no other significant findings. Group III rats had a much milder response, consisting of reversible respiratory effects and sensory irritation. The Bio/dynamics, Inc. (1990) study is summarized in Table 3-4.
White rats were exposed to 150, 800, or 1,200 ppm cyclohexylamine for up to 70 h (7 h/day, 5 days/week) by Watrous and Schulz (1950). A titration method was used to measure air cyclohexylamine concentrations. It was not explicitly stated how many rats were treated at each concentration (although it appeared to be 5) or how many days the animals were exposed to each concentration (appeared to be 2 weeks). All but one rat died after the first 7 h exposure to 1,200 ppm; the rats showed signs of extreme mucous membrane irritation and had lung hemorrhage. Of the rats exposed to 800 ppm, “five survived 24 h of exposure;” it was not stated how many animals died after 24 h of exposure. Of the rats exposed to 150 ppm cyclohexylamine, 4/5 survived 70 h of exposure. The higher concentrations (presumably 800 and 1,200 ppm) caused the corneas of all the animals to become opaque. No convulsant effects were seen.
Several published rat inhalation studies were poorly reported but help provide an overall picture of cyclohexylamine acute toxicity. Smyth et al. (1969) showed that the maximum period for which albino rats survived exposure to air saturated with cyclohexylamine vapor was 2 h (cyclohexylamine air saturation occurs at ~15,000 ppm; Amoore and Hautala 1983). Exposure of rats to 4,000 ppm cyclohexylamine for 4 h resulted in 0/6 deaths, whereas exposure to 8,000 ppm for 4 h resulted in 6/6 deaths after 14 days (Smyth et al., 1969). Exposure of rats to 1000 ppm cyclohexylamine for 6 h resulted in 0/3 deaths, whereas 2/3 rats died after exposure for 6 h to 12,000 ppm (Eastman Kodak 1984). Lomonova (1965) exposed rats to 443-2833 ppm for an single unspecified duration and obtained a 14-day LC50 of 1847 ppm; rats had visible mucous membrane irritation, as well as clonic muscle spasms, decreased body weight, body temperature, respiration rate, and urine output, 8-16% methemoglobinemia, evidence of hemolysis, organ weight changes, and vascular lesions (not clearly defined). Lomonova (1965) exposed other groups of rats to172 ppm cyclohexylamine 2 h/day for 2 months or to 24.6 ppm 4 h/day for 5 months. Both treatment groups had premature
TABLE 3-4 Observations in Rats Inhaling Cyclohexylamine Vapor for 4 h
|
Observation |
Number of Affected Animals (n = 10) during Exposurea |
Number of Affected Animals (n = 8-10) Post-Exposureb |
||||||||
|
1/4 |
1/2 |
3/4 |
1 |
2 |
4 |
2 h |
7 d |
14 d |
22 d |
|
|
GROUP I (2.2 mg/L + 612 mg/m3aerosol) |
|
|
|
Observations could not be made due to clouding or residual build-up inside the chamber |
n=10 |
n=8 |
n=8 |
n=8 |
||
|
Excessive lacrimation |
Few |
All |
All |
2 |
1 |
0 |
1 |
|||
|
Mucoid nasal discharge |
— |
Few |
Most |
0 |
0 |
0 |
0 |
|||
|
Red nasal discharge (wet or dried) |
— |
— |
— |
3 |
1 |
0 |
0 |
|||
|
Dried brown material on face |
— |
— |
— |
1 |
4 |
0 |
0 |
|||
|
Labored breathing |
Few |
Most |
Most |
10 |
2 |
1 |
1 |
|||
|
Gasping |
— |
— |
— |
7 |
0 |
0 |
1 |
|||
|
Rales: moist or dry |
— |
— |
— |
6 |
4 |
5 |
5 |
|||
|
Eyes closed |
All |
All |
All |
0 |
0 |
0 |
0 |
|||
|
Coarse tremors |
— |
— |
— |
6 |
0 |
0 |
0 |
|||
|
Corneal opacity |
— |
— |
— |
2 |
7 |
8 |
7 |
|||
|
Corneal irregularity or ulceration |
— |
— |
— |
9 |
1 |
0 |
8 |
|||
|
Yellow ano-genital stains |
— |
— |
— |
2 |
2 |
2 |
0 |
|||
|
Alopecia |
— |
— |
— |
0 |
0 |
1 |
2 |
|||
|
Decreased activity |
— |
— |
— |
10 |
0 |
0 |
0 |
|||
|
GROUP II (567 ppm) |
|
|
|
|
Observations could not be made due to residual build-up inside the chamber |
n=10 |
n=10 |
n=10 |
n=10 |
|
|
Excessive lacrimation |
— |
Few |
Few |
Few |
5 |
0 |
0 |
0 |
||
|
Chromodacryorrhea |
— |
— |
— |
— |
0 |
0 |
0 |
0 |
||
|
Mucoid nasal discharge |
— |
Few |
Few |
Few |
0 |
0 |
0 |
0 |
||
|
Observation |
Number of Affected Animals (n = 10) during Exposurea |
Number of Affected Animals (n=8-10) Post-Exposureb |
||||||||
|
1/4 |
1/2 |
3/4 |
1 |
2 |
4 |
2 h |
7 d |
14 d |
22 d |
|
|
GROUP II (567 ppm) |
|
|
|
|
Observations could not be made due to residual build-up inside the chamber |
n=10 |
n=10 |
n=10 |
n=10 |
|
|
Red nasal discharge (wet or dried) |
— |
— |
— |
— |
6 |
2 |
0 |
0 |
||
|
Dried brown material on face |
— |
— |
— |
— |
8 |
8 |
1 |
0 |
||
|
Labored breathing |
Few |
Some |
Some |
Some |
10 |
0 |
5 |
0 |
||
|
Gasping |
— |
Few |
Few |
Few |
0 |
0 |
0 |
0 |
||
|
Rales: moist or dry |
— |
— |
— |
— |
10 |
10 |
10 |
7 |
||
|
Eyes partially closed |
All |
All |
All |
All |
|
|
0 |
0 |
0 |
0 |
|
Coarse tremors |
— |
— |
— |
— |
|
|
2 |
0 |
0 |
0 |
|
Corneal opacity |
— |
— |
— |
— |
|
|
0 |
9 |
10 |
7 |
|
Corneal irregularity or ulceration |
— |
— |
— |
— |
|
|
10 |
1 |
10 |
10 |
|
Yellow ano-genital stains |
— |
— |
— |
— |
|
|
8 |
6 |
0 |
0 |
|
Alopecia |
— |
— |
— |
— |
|
|
0 |
0 |
5 |
2 |
|
GROUP III (54.2 ppm) |
|
|
|
|
|
|
n=10 |
n=10 |
n=10 |
All sacrificed on day 15 |
|
Excessive lacrimation |
— |
— |
— |
— |
— |
— |
1 |
0 |
0 |
|
|
Chromodacryorrhea |
— |
— |
— |
— |
— |
— |
0 |
0 |
0 |
|
|
Mucoid nasal discharge |
— |
— |
— |
— |
— |
— |
0 |
0 |
0 |
|
|
Red nasal discharge (wet or dried) |
— |
— |
— |
Few |
Few |
Few |
0 |
1 |
0 |
|
|
Dried brown/red material on face |
— |
— |
— |
— |
— |
— |
9 |
1 |
0 |
|
|
Labored breathing |
Few |
Some |
Most |
Most |
All |
All |
0 |
0 |
0 |
|
|
Eyes partially closed |
— |
— |
— |
Most |
All |
All |
0 |
0 |
0 |
|
|
aThe animals (5/sex) were observed as a group, and the number of affected animals was presented as few = 10-30%, some = 40-60%, most = 70-90%, all = 100%. bObservations were made 30 min, 1 h and 2 h, after exposure and thereafter daily until sacrifice. The daily incidence at the stated time is presented. One male and one female Group I rat died on post-treatment day 2. Source: Bio/dynamics, Inc. 1990. |
||||||||||
decedents, decreased body temperature and respiratory rate, and the 172-ppm rats had decreased body weight gain, evidence of hemolysis, vascular changes in most internal organs, and lesions in the heart, kidneys, trachea, lungs, and adrenal cortex.
3.1.2.
Mice
Mice received a single exposure of 12.3-1,059 ppm cyclohexylamine for an unspecified duration; most died within 1-5 days of exposure (Lomonova 1965; summarized in Table 3-3). Mice appeared more sensitive than rats to acute cyclohexylamine exposure. However, the doselethality pattern for mice was different than for rats. The “absolute lethal,” “median lethal,” and “minimum lethal” concentrations were about 2.7-fold, 7-fold, and 43-fold lower, respectively, for mice than for rats.
3.1.3.
Rabbits
Rabbits were exposed to 150, 800, or 1,200 ppm cyclohexylamine for 7 h/day, 5 days/week by Watrous and Schulz (1950). Air cyclohexylamine concentrations were measured by a titration method. It was not specified how many animals were treated at each concentration, but only one rabbit was mentioned at any given concentration. The total number of exposures which the animals survived was not stated; 10 days was the maximum exposure duration. At 1,200 ppm, all animals showed signs of extreme mucous membrane irritation, developed pulmonary hemorrhages, and died after the first exposure. Animals exposed to the “higher concentrations” (presumably 800 and 1,200 ppm) developed opaque corneas. The mortality patterns of the rabbits were difficult to distinguish due to the limited number of rabbits studied. At 800 ppm, one rabbit died after the second 7 h exposure; at 150 ppm one rabbit died after 7 h.
3.1.4.
Guinea Pigs
Guinea pigs were exposed to 150, 800, or 1,200 ppm cyclohexylamine for 7 h/day, 5 days/week (Watrous and Schulz 1950). Air cyclohexylamine concentrations were measured by a titration method. It was not specified how many animals were included at each concentration, but
only two guinea pigs were mentioned at any given concentration. The total number of exposures which the animals survived was not stated; 10 days was the maximum exposure duration. At 1,200 ppm, all animals showed extreme mucous membrane irritation, had lung hemorrhage, and died after the first 7 h exposure. At 800 ppm, two guinea pigs died after the second 7 h exposure and at 150 ppm two guinea pigs survived 70 h exposure.” Animals exposed to “higher concentrations” (presumably 800 and 1,200 ppm) developed opaque corneas.
3.2.
Nonlethal Toxicity
3.2.1.
Rats
In a GLP study conducted by Bio/dynamics, Inc. (1990), Sprague-Dawley rats were exposed for 4 h to 542 ppm cyclohexylamine vapor + 612 mg/m3 aerosol (Group I), 567 ppm vapor (Group II), or 54.2 ppm vapor (Group III). As shown in Table 3-4 and described in Section 3.1.1, 2/10 Group I rats died and Group I and II rats had severe respiratory, neurological, and ocular effects. These alterations persisted to the end of the 3-week observation period in Group I and in some cases (corneal lesions and rales) in Group II. Rats inhaling 54.2 ppm displayed milder effects, which during exposure consisted of labored breathing, partially closed eyes and red nasal discharge. Over the 2-h following exposure, rats had lacrimation, chromodacryorrhea, mucoid or red nasal discharge, and dried red or brown nasal discharge or facial material. Red nasal discharge was seen in several animals during the 2-week observation but was no longer present by day 14. The rats did not gain weight until post-exposure day 4.
Several other studies described in Section 3.1.1 and summarized in Table 3-4 included non-lethal exposures. Lomonova (1965) found mucous membrane irritation, CNS excitability, decreased body weight, increased blood methemoglobin, hemolysis (not at 24.6 ppm), vascular lesions, and/or organ weight changes, but no mortality in groups of albino rats exposed once to 443 ppm (duration unknown), for <2 months (2 h/day) to 172 ppm, or <4 months (4 h/day) to 24.6 ppm. No mortality occurred among 3 rats exposed for 6 h to 1,000 ppm (Eastman Kodak 1984) or among 6 rats exposed for 4 h to 4,000 ppm (Smyth et al. 1969).
3.2.2.
Mice
The concentration of cyclohexylamine causing a 50% decrease in the breathing rate of male OF1 Swiss mice (RD50) was determined to be 51 ppm in an oronasal exposure study conducted by Gagnaire et al. (1989). Mice were exposed for 15 min to 26-84 ppm cyclohexylamine vapor (6/concentration) by enclosing the head in a 200-liter stainless steel exposure chamber into which cyclohexylamine vapor was delivered by bubbling air through the liquid amine. Respiratory rates were measured using plethysmographic techniques (American Standard Method E981-84). The effect on breathing rate was maximal 10-15 min into the exposure, and recovery after the 15-min exposure occurred within 1 min. The decreased breathing rate is due to stimulation of the trigeminal nerves in the nasal mucosa, and was monitored with a pressure transducer. (Gagnaire et al. [1993] reports the same RD50 results; however, the stated exposure was 60 min; it is unclear whether the 1993 reference was for a new experiment.)
Pulmonary irritation was similarly assessed in anesthetized, tracheally cannulated (TC) mice by determining the RD50TC (i.e., concentration causing 50% respiratory inhibition). RD50TC values for upper airway irritants are typically higher than RD50 values (for nasally exposed mice) due to the bypass of the trigeminal nerves in the nasal mucosa of the TC animals. The TC mice were exposed to 79-220 ppm cyclohexylamine for 120 min (Gagnaire et al. 1989; results also reported in Gagnaire et al. 1993), and an RD50TC of 184 ppm was determined. The maximal decrease in the breathing rate was seen after 120 min of exposure; recovery of breathing rate to pre-exposure levels was incomplete (animals were observed 20-30 min after exposure based on Gagnaire et al. 1993). The lower value of the RD50 compared to the RD50TC indicates that the respiratory toxicity of cyclohexylamine is primarily related to its upper airway irritant effects. The decrease in breathing rate is due to a reflex reaction induced by stimulation of the irritant and mechanosensitive alveolar receptors.
Nielsen and Yamagiwa (1989) also determined RD50 values for cyclohexylamine. Respiratory rates and tidal volumes were measured using plethysmographic techniques (American Standard Method E981-84). Male Ssc:CF-1 mice (4/concentration) were exposed for 30 min. Exposure was head-only in a 3.3-L chamber into which evaporated cyclohexylamine was delivered in the air (52-97 L/min). Chamber concentrations were monitored continuously by infrared spectroscopy. The
RD50 for cyclohexylamine was determined to be 27 ppm by exposure of the mice to 4-355 ppm cyclohexylamine. A slight (~20%) decrease in the respiratory rate occurred at 4 ppm and the maximal inhibition (about 75%) was seen at 355 ppm, but no animals died. For tracheally cannulated mice exposed to 32-345 ppm cyclohexylamine, the 50% respiratory inhibition (“tRD50”, analogous to RD50TC), was 78 ppm. Maximal pulmonary irritation occurred at ~100 ppm, but no animals died at that concentration or at a 3.5-fold higher concentration (345 ppm), indicating that the decrease in respiratory rate is not directly correlated to the mortality rate. The concentration-response curve for cyclohexylamine was linear for non-cannulated mice, suggesting that the decreased respiratory rate at all tested concentrations were due to sensory irritation. Tidal volume was slightly altered (~20% increase) at only the highest dose tested in non-cannulated mice, and sensory and pulmonary irritation reached a plateau within 10 min of exposure.
Lomonova (1965) reported that the lowest single exposure concentration that altered the CNS capacity for summation of subthreshold impulses in mice was 2.5 ppm and that 12.3 ppm was the “maximum tolerated” dose (see Section 3.1.1. and Table 3-3).
3.3.
Neurotoxicity
No studies were located assessing the neurotoxicity of cyclohexylamine in animals.
3.4.
Developmental/Reproductive Toxicity
No animal studies were located that addressed the developmental or reproductive effects of cyclohexylamine following inhalation exposure.
Oral studies have generally associated cyclohexylamine with developmental and/or reproductive toxicity but not teratogenicity in mammals (reviewed in IARC 1980; Beard and Noe 1981). Subchronic oral treatment (63-90 days) of rats and dogs with 200-400 mg/kg-day cyclohexylamine or lifetime administration of 50-300 mg/kg-day cyclohexylamine caused effects including testicular tubular atrophy and calcium deposition, decreased testicular weights, impaired spermatogenesis, and/or lowered sperm count and motility (Gaunt et al. 1974; Oser et al.
1976; Mason and Thompson 1977; James et al. 1981; Roberts et al. 1989). Conversely, mice fed 3000 ppm (~390 mg/kg) cyclohexylamine hydrochloride for up to 80 weeks failed to develop testicular atrophy or degeneration (Hardy et al. 1976). Reproductive performance was not significantly affected in the Gaunt et al. (1976) chronic rat study or in the Gaunt et al. (1974) 13-week study, but was impaired slightly in the Oser et al. (1976) multigeneration study (reduction in litter size and weaning weight). Embryotoxicity was observed in a six-generation mouse study by Kroes et al. (1977), in which mice given 0.5% dietary cyclohexylamine sulfate had a significant reductions in implantations, live-born fetuses, and the offspring showed evidence of growth retardation. Parental toxicity was not evaluated, although it was noted that treated mice had significantly lower body weight but a better survival rate than controls. Oser et al. (1976) and Gaunt et al. (1974; 13-week study) found no treatment-related malformations in offspring of treated parents. No evidence of teratogenesis was seen in ICR mice or Rhesus monkeys treated orally with 75 or 100 mg/kg-day or in Wistar rats given 1.8-36 mg/kg-day during organogenesis (Takano and Suzuki 1971; Wilson 1972; Tanaka et al. 1973).
3.5.
Genotoxicity
Cyclohexylamine (33-10,000 μg/plate) was not mutagenic in Salmonella typhimurium tester strains TA98, TA100, TA1535, or TA1537 when tested using a modified preincubation assay, with or without rat or hamster liver S9 mix (Mortelmans et al. 1986). In vitro, cyclohexylamine enhanced the virus-mediated transformation of Syrian hamster embryo cells and induced chromosome aberrations in kangaroo rat cells (IARC 1987). It did not, however, induce somatic or sex-linked recessive lethal mutations, aneuploidy, or heritable translocations in Drosophila (Knaap et al. 1973). Negative results were obtained in most of the reported dominant-lethal rodent assays; questionable or positive findings in two assays were difficult to interpret because either postimplantation loss or the experimental design were not adequately reported (EPA 1987). Weakly positive results were obtained in the mouse spot test (IARC 1987). Cyclohexylamine induced chromosomal aberrations in vivo in hamster lymphocytes and in rat spermatogonia but not in rat leukocytes, hamster, lamb, or rat bone marrow, or hamster and mouse spermatogonia (IARC 1987).
Cultured (48-68 h) peripheral blood lymphocytes from living sheep fetuses that had been infused in utero with 50-250 mg/kg cyclohexylamine for 5 or 18 h had increased levels of chromosome aberrations. The number of aberrations at 18 h as 4-5 times greater than at 5 h (Turner and Hutchinson 1974).
Cyclohexylamine was negative in the mammalian CHO/HGPRT forward mutation assay (172-1720 μg/mL tested) and in the unscheduled DNA synthesis assay in rat hepatocytes (4.3-860 μg/mL) (Brusick et al. 1989). The upper concentrations tested in each of the assay systems were cytotoxic.
3.6.
Carcinogenicity
No inhalation carcinogenicity bioassays were located in the literature. Several oral exposure studies were conducted that showed no definitive evidence of cyclohexylamine carcinogenicity. ASH-CS1 mice (48-50/sex) that received up to 3,000 ppm (~390 mg/kg BW) dietary cyclohexylamine hydrochloride for 80 weeks had tumor incidences and types within historical control ranges (Hardy et al. 1976). Sprague-Dawley rats (52/sex) fed cyclohexylamine at 200 mg/kg BW for 30 months, FDRL rats (30/sex) fed at 15-150 mg/kg BW for 2 years, and Wistar rats (48/sex) fed at 24-440 mg/kg BW for 2 years had tumor incidences similar to controls (Schmahl 1973; Oser et al. 1976; Gaunt et al. 1976). A rare invasive bladder carcinoma was reported in 1/25 male Sprague-Dawley rats (0/25 females) given 15 mg/kg BW dietary cyclohexylamine sulfate for 2 years (0.15 and 1.5 mg/kg-day were also tested) (Price et al. 1970). This appeared to be a spontaneous tumor unrelated to treatment, since bioassays conducted by other investigators who used greater numbers of animals did not find treatment-related increases in tumor incidence or any bladder tumors (EPA 1987).
Both IARC (1987) and the ACGIH (2004) concluded that there was inadequate evidence in humans and in experimental animals to establish the carcinogenicity of cyclohexylamine. EPA has not provided a carcinogenicity weight-of-evidence classification for cyclohexylamine (EPA 2005).
3.7.
Summary
Cyclohexylamine acute lethality inhalation studies were located for rats, mice, guinea pigs, and rabbits. The most comprehensive study
was that conducted by Bio/dynamics, Inc. (1990), in which Sprague-Dawley rats were exposed for 4 h to ≥54.2 ppm cyclohexylamine vapor. Many of the other available studies had incomplete reporting of the methods and/or results. Several multiple-exposure inhalation studies were also conducted using rats and mice. Rats appeared less sensitive to cyclohexylamine than mice, guinea pigs, or rabbits with respect to lethality in two studies. The data, however, were inadequate to define the most sensitive species. No studies were suitable for determination of cyclohexylamine concentration-time relationships.
RD50 values of 51 ppm (Gagnaire et al. 1989) and 27 pm (Nielsen and Yamagiwa 1989) were obtained for cyclohexylamine in male OF1 Swiss mice. RD50 values for tracheally cannulated mice were higher in both experiments (184 ppm and 78 ppm, respectively), indicating that the response was primarily related to upper airway irritation.
No animal inhalation studies were located that addressed the developmental or reproductive effects of cyclohexylamine. Several subchronic and chronic oral studies with rats and dogs indicated that cyclohexylamine had developmental and reproductive but not teratogenic potential. However, these effects were not seen by other investigators using mice and monkeys. Genotoxicity assays yielded mixed results. Carcinogenicity studies did not show conclusive evidence of cyclohexylamine-induced neoplasms. Both IARC (1987) and ACGIH (2004) concluded that there was inadequate evidence in humans or in experimental animals to determine the carcinogenic potential of cyclohexylamine (IARC Group 3; ACGIH Group A4).
4.
SPECIAL CONSIDERATIONS
4.1.
Metabolism and Disposition
No human or animal studies were located that described the metabolism or disposition of cyclohexylamine following inhalation exposure. However, oral studies showed that cyclohexylamine was absorbed rapidly and nearly completely from the gastrointestinal tract by humans, rats, rabbits, and guinea pigs (Renwick and Williams 1972). Excretion was primarily by urine, with 61-90% of radiolabel from 14C-cyclohexylamine-HCl recovered within 24 h of administration. All tested animals as well as man excreted primarily the parent compound, with 4-5% of the administered dose metabolized in 24 h in the rat and guinea
pig, 1-2% in man and 30% in rabbits. In rats, metabolism of cyclohexylamine was mainly through hydroxylation of the cyclohexane ring, in guinea pigs and rabbits by ring hydroxylation and deamination, and in humans by deamination. The extent of hydroxylation was not related to the development of testicular toxicity in rats (Roberts et al. 1989). Mice absorbed and eliminated orally administered cyclohexylamine more rapidly than rats; the steady-state plasma clearance rates of 33 and 66 mL/min/kg were estimated for rats and mice, respectively (Roberts and Renwick 1986; Roberts et al. 1989).
4.2.
Mechanism of Toxicity
Cyclohexylamine is a strong base (pKa = 10.7) and a severe eye, skin and respiratory irritant. Its alkalinity is likely responsible for the corneal lesions (opacity, irregularities, ulceration) found in rats, guinea pigs, and rabbits exposed to ≥542 ppm cyclohexylamine (Watrous and Schulz 1950; Bio/dynamics, Inc. 1990). The marked irritancy of cyclohexylamine has been held responsible for pulmonary edema and hemorrhage, along with extreme mucous membrane irritation and gasping seen in rodents. Occupationally exposed workers reported similar effects, including eye, nose and throat irritation, headache, rapid and irregular heartbeat, nausea, dizziness, and vomiting. Other effects have also been observed in animals treated by inhalation, such as tremors, clonic muscular spasms, vascular lesions, and hemolysis. The mechanism by which these systemic effects occur is less clear.
Lee and Dixon (1972) have reported that cyclohexylamine (150-1250 mg/kg) injected into Swiss Webster mice caused sniffling, vicious fighting, panting, hyperexcitability, hyperpyrexia, and excessive salivation. High ambient temperatures and crowding increased the cyclohexylamine-induced mortality. Death by systemic cyclohexylamine intoxication was prevented by the administration of the anti-adrenergic drugs reserpine, chlorpromazine, tolazoline, and phenoxybenzamine (Lee and Dixon 1972). These signs and the response to high ambient temperatures, crowding, and sympatholytic drugs are characteristic of other sympathomimetic amines such as amphetamine. However, it is unclear whether a direct parallel can be drawn between exposure to inhaled, very basic cyclohexylamine (100% pure) and the injection of cyclohexylamine hydrochloride that is dissolved in water and neutralized to pH 7.4 with sodium hydroxide (purity not stated). For example, in the
Bio/dynamics, Inc. (1990) study, 10/10 rats in the high-dose group (542 ppm aerosol + 612 mg/m3 vapor) exhibited “decreased activity” up to 2 h after the 4-h exposure, and 1/10 rats felt “cold to the touch” two days after exposure, whereas hyperthermia and hyperactivity were two hallmark features of rats injected with cyclohexylamine hydrochloride. Additionally, Lee and Dixon (1972) showed that pre-treatment with monoamine oxidase (MAO) inhibitors (pargyline or JB-516) had no effect on cyclohexylamine lethality, whereas Brittain et al. (1964) found that pretreatment with MAO inhibitors (phenelzine and tranylcypromine) increased amphetamine toxicity 15- to 20-fold.
4.3.
Structure-Activity Relationships
In addition to investigations with cyclohexylamine, Lomonova (1965) also examined the inhalation toxicity of dicyclohexylamine (DCHA). Compared to cyclohexylamine, the LC100 of DCHA for mice was 17-fold lower, although the minimum lethal concentrations (LCLo) were comparable; the animals died within 1-2 h. The mice were initially restless; increased motor activity was followed by tonic-clonic spasms. Repeated exposure to a sublethal concentration of DCHA (2 h/day for 30 days) did not cause lesions in mice but caused liver and kidney degeneration in rats.
N,N-dimethylcyclohexylamine, a compound structurally similar to cyclohexylamine, was somewhat less irritating than cyclohexylamine. It produced CNS effects such as weakness, tremor, salivation, gasping, and convulsions (Beard and Noe 1981).
Gagnaire et al. (1993) obtained RD50 values for n-hexylamine of 42 ppm for nasally exposed mice and 93 ppm for tracheally cannulated mice. These RD50 values are comparable to the RD50 values obtained for cyclohexylamine in the same study (51 and 184 ppm for nasally exposed and cannulated mice, respectively) and by Nielsen and Yamagiwa (1989; RD50 of 27 and 78 ppm for nasally exposed and cannulated mice, respectively).
4.4.
Other Relevant Information
4.4.1
Species Variability
Because cyclohexylamine is a surface-contact alkaline irritant that
is water and lipid soluble, it is reasonable to expect that at low concentrations, i.e. those that result primarily in sensory irritation, there is little variability in the degree of irritation among animal species. At higher concentrations, where deep lung damage and/or systemic toxicity may result, the sensitivity of various species to cyclohexylamine vapor could not be established from the available studies.
The Lomonova (1965) experiments with rats and mice could be taken to indicate that mice were more sensitive than rats, since the mouse LC50 was 7-fold lower than the rat LC50. This study protocol appeared flawed because exposure durations were not given and outcomes were not given at specific concentrations nor separately for each species. Watrous and Schulz (1950) suggested that rats were less sensitive to inhaled cyclohexylamine than guinea pigs or rabbits, but some of the results were inconsistent and the study lacked sufficient methodological details to draw a definitive conclusion regarding species sensitivities.
4.4.2.Susceptible Populations
No studies were located identifying specific populations susceptible to cyclohexylamine exposure.
4.4.3.
Concentration-Exposure Duration Relationship
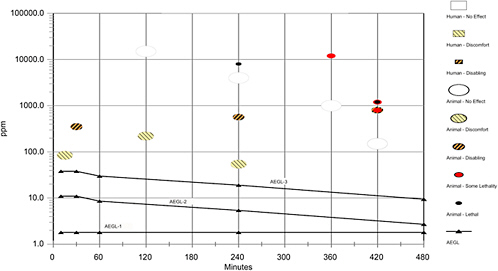
No data were available from which to determine the concentration-time relationship for cyclohexylamine inhalation toxicity. Ten Berge et al. (1986) determined that the concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5, and n ranged from 1 to 3 for 90% of the chemicals examined. To obtain protective AEGL-2 and AEGL-3 values for 30, 60, and 480 min, scaling across time was performed using n = 3 to extrapolate to shorter exposure times and n = 1 to extrapolate to longer exposure times, to provide AEGL values that would be protective of human health (NRC 2001) (the AEGL-1 was not scaled). The 10-min value was not extrapolated from 4 h (exposure duration in key study) because extrapolating from ≥4 h to 10 min is associated with unacceptably large inherent uncertainty. Therefore, the 30-min value was adopted for 10 min to be protective of human health.
5.
RATIONALE FOR AEGL-1
5.1.
Summary of Human Data Relevant to AEGL-1
Watrous and Schulz (1950) reported that exposure of chemical operators for an undetermined time period to 4-10 ppm cyclohexylamine “caused no symptoms of any kind,” although it was not noted whether the operators could detect an odor. In the same chemical plant, workers previously exposed to higher (unknown) concentrations of cyclohexylamine for a short period (≤1 1/2 h) reported headache, rapid and irregular heartbeat, nausea, dizziness, vomiting, and eye, nose and throat irritation. This study is not appropriate for derivation of AEGL-1 values because in one case, effects were below AEGL-1 severity criteria, and in the other case, the exposure concentration was unknown.
5.2.
Summary of Animal Data Relevant to AEGL-1
The 4-h inhalation rat study by Bio/dynamics, Inc. (1990) can be used for AEGL-1 derivation. “At the two higher test concentrations (567 ppm and the 542 ppm vapor/aerosol mixture), rats also had rales, gasping, dried red facial material, tremors, weight loss, and irreversible ocular lesions. Two of 10 rats exposed to the aerosol/vapor mixture died, and necropsy revealed nasal, lung, and urogenital lesions.”
5.3.
Derivation of AEGL-1
AEGL-1 values were derived from the Bio/dynamics, Inc. (1990) rat study, in which 54.2 ppm caused notable respiratory and ocular effects (labored breathing, red nasal discharge, partially closed eyes). Because the effects seen at 54.2 ppm are more severe than prescribed by the AEGL-1 definition, 54.2 ppm was divided by a modifying factor of 3 (per Section 2.6.2., NRC 2001). Mild sensory irritation is not expected to vary greatly over time, so the same AEGL value was adopted for 10 min to 8 h. An uncertainty factor of 3 was applied for interspecies variability, and 3 was applied for sensitive humans (yielding 1.8 ppm for 10 min to 8 h), because mild sensory irritation from an alkaline irritant gas is not likely to vary greatly among humans or animals, and both human and additional animal data indicate that a greater UF is not needed. The
AEGL-1 is consistent with a study in which chemical workers exposed to 4-10 ppm for an undefined duration (<8 h) reported “no symptoms of any kind” (Watrous and Schulz 1950), but which was inappropriate for AEGL-1 derivation because effects were below AEGL-1 severity criteria. The AEGL-1 values are also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989), from which it is predicted that 2.7 or 5.1 ppm should result in some sensory irritation in humans, whereas 0.27 or 0.51 ppm should cause no sensory irritation (Alarie 1981). The latter assertion is based on two mouse RD50 studies, in which exposure to 4 ppm for 30 min caused a 20% decrease in respiratory rate in mice, and RD50 values of 27 and 51 ppm were obtained (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989). According to Alarie (1981), exposure to 0.1 of the RD50 (i.e., 2.7 or 5.1 ppm) for several hours-days should result in some sensory irritation in humans, whereas 0.01 × RD50 (0.27 or 0.51 ppm) should cause no sensory irritation. The AEGL-1 values are shown in Table 3-5 and calculations are detailed in Appendix A.
6.
RATIONALE FOR AEGL-2
6.1.
Summary of Human Data Relevant to AEGL-2
No human data was located that was appropriate for derivation of AEGL-2 values.
6.2.
Summary of Animal Data Relevant to AEGL-2
The Bio/dynamics, Inc. (1990) study was considered appropriate for AEGL-2 derivation. In this study, rats were exposed for 4 h to 54.2 or 567 ppm cyclohexylamine vapor, or to a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol. At 54.2 ppm, rats displayed dyspnea and had partially closed eyes and red nasal discharge.
TABLE 3-5 AEGL-1 Values for Cyclohexylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.8 (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
At ≥542 ppm, rats also had rales, gasping, dried red facial material, tremors, weight loss, irreversible ocular lesions (corneal opacity, ulceration), and two rats exposed to the aerosol-containing atmosphere died.
Another study that potentially has end points within the scope of AEGL-2 is that of Watrous and Schulz (1950), in which rats, rabbits and guinea pigs were exposed to 150, 800, or 1,200 ppm cyclohexylamine 7 h/day, 5 days/week for up to 10 days. Animals exposed to 800 or 1200 ppm developed opaque corneas and died before 10 days; at 150 ppm, no animals had opaque corneas, four of five rats and two guinea pigs (not stated out of how many) survived 70 h of exposure, and one rabbit died after one 7-h exposure (no dose-response). Although this study was not considered useful for AEGL-2 derivation because of the inconsistency in response and incomplete reporting of results, the study provides a higher NOAEL for corneal opacity (i.e., 150 ppm), and supports the Bio/dynamics (1990) study for this end point.
6.3.
Derivation of AEGL-2
AEGL-2 values were derived from the Bio/dynamics, Inc. (1990) study, based on exposure for 4 h to 54.2 ppm, at which concentration the rats had moderate respiratory effects and ocular irritation, and which was a NOAEL for irreversible ocular lesions. This NOAEL is consistent with an earlier study in which rats, a rabbit, and guinea pigs exposed to 150 ppm 7 h/day for up to 2 weeks had no eye effects but those exposed to 800 ppm had corneal opacity (Watrous and Schulz 1950). Data were not available to determine the concentration-time relationship for cyclohexylamine toxicity. The concentration-time relationship for many irritant and systemically acting vapors and gases may be described by Cn × t = k, where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain protective AEGL-2 values, scaling across time was performed using n = 3 to extrapolate to exposure times <4 h (exposure duration in the key study), except for the 10-min values, and n = 1 to extrapolate to exposure times >4 h. The 30-min values were adopted as 10 min values due to unacceptably large uncertainty in extrapolating from ≥4 h to 10 min (NRC 2001). An uncertainty factor of 10 was applied (3 for interspecies variability and 3 for intraspecies variability) because the effects seen at 54.2 ppm were clearly reversible, and a larger uncertainty factor yields values at or below the AEGL-1. The resulting AEGL-2 values are shown in Table 3-6 and calculations are detailed in Appendix A.
TABLE 3-6 AEGL-2 Values for Cyclohexylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
11 ppm (45 mg/m3) |
11 ppm (45 mg/m3) |
8.6 ppm (35 mg/m3) |
5.4 ppm (22 mg/m3) |
2.7 ppm (11 mg/m3) |
7.
RATIONALE FOR AEGL-3
7.1.
Summary of Human Data Relevant to AEGL-3
No quantitative information on lethal cyclohexylamine exposure in humans was located.
7.2.
Summary of Animal Data Relevant to AEGL-3
Studies considered appropriate for AEGL-3 derivation, for which sufficient details of the experimental methods and study results were given include: (1) the Bio/dynamics, Inc. (1990) study, in which rats were exposed for 4 h to 54.2 ppm had labored breathing, partially closed eyes, and red nasal discharge, and rats exposed to 567 ppm or to a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol additionally had rales, gasping, dried red facial material, tremors, weight loss, irreversible ocular lesions, and two rats exposed to the aerosol-containing atmosphere died; (2) the sensory irritation study by Nielsen and Yamagiwa (1989) in which mice exposed for 30 min to 355 ppm cyclohexylamine did not die but had a 75% decrease in breathing rate; and (3) the multiple-exposure study by Watrous and Schulz (1950) in which rats exposed to 150 ppm cyclohexylamine 7 h/day for up to 10 days had fractional mortality (use one 7 h exposure for derivation).
7.3.
Derivation of AEGL-3
The study chosen for AEGL-3 derivation was the GLP study in which Sprague-Dawley rats (5/sex/dose) exposed to 567 ppm cyclohexylamine vapor for 4 h had reversible dyspnea, tremors, weight loss, and irreversible ocular lesions, although none died within the 3-week observation period (Bio/dynamics, Inc. 1990). The AEGL-3 end points were irreversible ocular lesions and an estimated lethality threshold in rats (at the next higher exposure concentration, i.e., 542 ppm + 612
mg/m3 aerosol), lethality occurred in 1/5 males and 1/5 females, and animals that died had nasal, lung, and urogenital tissue damage. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the ten Berge et al. (1986) equation Cn × t = k where n = 1 or n = 3, as was done for the AEGL-2. A total uncertainty factor of 30 was used: 10 for interspecies variability because, although tissue destruction caused by a severely corrosive agent is not expected to vary greatly among animals, the dose spacing in the key study did not precisely delineate the LOAEL for ocular lesions or the threshold for lethality in rats, and the set of animal studies was limited. An intraspecies uncertainty factor of 3 was applied because tissue destruction caused by a severely corrosive agent is not expected to vary greatly among humans; a greater uncertainty factor is not warranted because it yields concentrations comparable to AEGL-2 values. The resulting AEGL-3 values are shown in Table 3-7; calculations are detailed in Appendix A.
8.
SUMMARY OF AEGLs
8.1.
AEGL Values and Toxicity End Points
A summary of the AEGL values for cyclohexylamine and their relationship to one another are shown in Table 3-8. AEGL-1, AEGL-2, and AEGL-3 values were derived from a study in which Sprague-Dawley rats (5/sex/dose) were exposed for 4 h to 54.2 or 567 ppm cyclohexylamine vapor, or to a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol (Bio/dynamics, Inc. 1990). At 54.2 ppm, rats had labored breathing, partially closed eyes, and red nasal discharge; rats exposed to 567 ppm or to the vapor/aerosol combination also had rales, gasping, dried red facial material, tremors, weight loss, and irreversible ocular lesions. Two of the rats exposed to the aerosol-containing atmosphere died, and had alopecia, red areas in the lining of the nasal turbinates, GI tract (male), and bladder (male), and the female had lung hemorrhage and edema and thin red fluid in the urinary bladder.
AEGL-1 values were derived from the Bio/dynamics, Inc. (1990) rat study, in which 54.2 ppm caused notable respiratory and ocular effects (labored breathing, red nasal discharge, partially closed eyes). Because the effects seen at 54.2 ppm are more severe than prescribed by the AEGL-1 definition, 54.2 ppm was divided by a modifying factor of 3.
TABLE 3-7 AEGL-3 Values for Cyclohexylamine
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
38 ppm (150 mg/m3) |
38 ppm (150 mg/m3) |
30 ppm (120 mg/m3) |
19 ppm (77 mg/m3) |
9.5 ppm (39 mg/m3) |
TABLE 3-8 Summary of AEGL Values for Cyclohexylamine
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (Nondisabling) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
1.8 ppm (7.3 mg/m3) |
|
AEGL-2 (Disabling) |
11 ppm (45 mg/m3) |
11 ppm (45 mg/m3) |
8.6 ppm (35 mg/m3) |
5.4 ppm (22 mg/m3) |
2.7 ppm (11 mg/m3) |
|
AEGL-3 (Lethal) |
38 ppm (150 mg/m3) |
38 ppm (150 mg/m3) |
30 ppm (120 mg/m3) |
19 ppm (77 mg/m3) |
9.5 ppm (39 mg/m3) |
Mild sensory irritation is not expected to vary greatly over time, so the same AEGL value was adopted for 10 min to 8 h. An uncertainty factor of 3 was applied for interspecies variability and 3 for sensitive humans, yielding AEGL values of 1.8 ppm for 10 min to 8 h. Both human and additional animal data indicate that a greater UF was not needed. The AEGL-1 is consistent with a study in which chemical workers exposed to 4-10 ppm for an undefined duration (<8 h) reported “no symptoms of any kind” (Watrous and Schulz 1950), but which was inappropriate for AEGL-1 derivation because effects were below AEGL-1 severity criteria. The AEGL-1 values are also consistent with two mouse respiratory irritation studies (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989), from which it is predicted that 2.7 or 5.1 ppm should result in some sensory irritation in humans, whereas 0.27 or 0.51 ppm should cause no sensory irritation (Alarie 1981).
AEGL-2 values were based on exposure for 4 h to 54.2 ppm, at which concentration the rats had moderate respiratory effects and ocular irritation, and which was a NOAEL for irreversible ocular lesions. This NOAEL is consistent with an earlier study in which rats, a rabbit, and guinea pigs exposed to 150 ppm 7 h/day for up to 2 weeks had no eye effects, but those exposed to 800 ppm had corneal opacity (Watrous and Schulz 1950). Data were not available to determine the concentration-time relationship for cyclohexylamine toxicity and scaling across time was performed using the equation Cn × t = k, using the exponent n = 3 to extrapolate to exposure times <4 h (exposure duration in the key study),
except for the 10-min values, and n = 1 to extrapolate to exposure times >4 h, as described in Section 4.4.3. The 30-min values were adopted as 10 min values due to unacceptably large uncertainty in extrapolating from ≥4 h to 10 min and to be protective of human health. A total uncertainty factor of 10 was applied (3 for interspecies variability and 3 for intraspecies variability) because the effects seen at 54.2 ppm were clearly reversible, and a larger uncertainty factor yields values at or below the AEGL-1.
The AEGL-3 values were based on irreversible ocular lesions and an estimated lethality threshold in rats, which resulted from exposure for 4 h to 567 ppm. Data were not available to determine the concentration-time relationship, and scaling across time was performed using the equation Cn × t = k with n = 3 or n = 1, as was done for the AEGL-2. A total uncertainty factor of 30 was used: 10 for interspecies variability because, although tissue destruction caused by a severely corrosive agent is not expected to vary greatly among animals, the dose spacing in the key study failed to delineate the LOAEL for ocular lesions or the threshold for lethality in rats, and the set of animal studies was limited. An intraspecies uncertainty factor of 3 was applied because tissue destruction caused by a severely corrosive agent is not expected to vary greatly among humans; a greater uncertainty factor is not warranted because it yields concentrations comparable to AEGL-2 values in Table 3-8.
8.2.
Comparison with Other Standards and Guidelines
The existing standards and guidelines for cyclohexylamine are shown in Table 3-9.
The 8-h TWA of 10 ppm was adopted by ACGIH in 1974, and the OSHA PEL of 10 ppm was established to be consistent with the ACGIH value. OSHA encourages employers to follow the 10 ppm limit, although
TABLE 3-9 Extant Standards and Guidelines for Cyclohexylamine (ppm)
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-1 |
1.8 |
1.8 |
1.8 |
1.8 |
1.8 |
|
AEGL-2 |
11 |
11 |
8.6 |
5.4 |
2.7 |
|
Guideline |
Exposure Duration |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
|
AEGL-3 |
38 |
38 |
30 |
19 |
9.5 |
|
REL-TWA (NIOSH)a |
|
|
|
|
10 |
|
TLV-TWA (ACGIH)b |
|
|
|
|
10 |
|
MAK (Germany)d |
|
|
|
|
(10)c |
|
MAK Peak Limitation (Germany)e |
10 (15 min) |
|
|
|
|
|
MAC (Netherlands)g |
|
|
|
|
(5)f |
|
LLV (Sweden)h |
|
|
|
|
5 |
|
STV (Sweden)i |
10 (15 min) |
|
|
|
|
|
aNIOSH REL-TWA (National Institute of Occupational Safety and Health, Recommended Exposure Limits - Time Weighted Average) (NIOSH 2005) is defined analogous to the ACGIH-TLV-TWA. bACGIH TLV-TWA (American Conference of Governmental Industrial Hygienists, Threshold Limit Value - Time Weighted Average) (adopted 1974; ACGIH 1996) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect. cThis 8 h value is superceded by the assignment of 10 ppm as also the “peak limit.” dMAK (Maximale Arbeitsplatzkonzentration [Maximum Workplace Concentration]) (DFG [Deutsche Forschungs-gemeinschaft or German Research Association] 2002) is defined analogous to the ACGIH-TLV-TWA. eMAK Peak Limitation (Category I, excursion factor = 1) (DFG 2002) is the maximum “momentary value” concentration which should not be exceeded at any time. The peak values are determined using a sampling period of 15 min. The peak limitation value is intended to avoid short exposure peaks that could lead to significant irritating effects, which could occur because the MAK value is close to the irritation threshold determined in the RD50 study of Gagnaire et al. (1993) (Greim, 2002). fA value of 1.2 ppm was proposed upon a recent reassessment of the Health Council of the Netherlands (HCN 2000) and is likely the current Dutch exposure limit, although post-2000 Dutch values were not available to verify this. gMAC (Maximaal Aanvaarde Concentratie [Maximal Accepted Concentration]) (SDU Uitgevers [under the auspices of the Ministry of Social Affairs and Employment], The Hague, The Netherlands 2000) is defined analogous to the ACGIH-TLV-TWA. A skin notation was present, indicating a danger of percutaneous absorption.) |
|||||
its PEL of 10 ppm was deleted in 1992 by the 11th Circuit Court of Appeals. The ACGIH (1996) documentation of TLV and BEI indicates that the TWA was based on the Watrous and Schulz (1950) study, in which “4-10 ppm caused no symptoms of any kind in workmen exposed, apparently, under acute conditions.” No accounting is made for inurement of the workers to the smell, and no intraspecific uncertainty factors were applied in generating this TLV-TWA. The German MAK of 10 ppm was based on the RD50 of 51 ppm obtained by Gagnaire et al. (1989; 1993), although the German documentation for the value, as well as a footnote for cyclohexylamine in the 2002 “List of MAK and BAT Values,” states that the 10 ppm should be regarded as a momentary value that should not be exceeded (Greim 2002). Therefore the 8-h TLV/MAK of 10 ppm loses its meaning as an average value because no excursions above the "moment value" are permitted. The lower RD50 of 27 ppm obtained by Nielsen and Yamagiwa (1989) was not cited in the rationale for the German TLV/MAK.
A recent health-based reassessment of administrative occupational exposure limits for the Netherlands (HCN 2000) recommends a health-based occupational exposure limit (HBROEL) of 1.2 ppm as an 8-h time-weighed average for systemic effects. This value was derived by route-to-route extrapolation from a chronic, multigenerational study in rats with a NOAEL was 15 mg/kg BW (at higher doses found decreased weight gain and food intake, decreased kidney weight, and microscopic changes in the testes, kidneys, and bladder). The Dutch rationale also states that because cyclohexylamine is an irritant, and adequate data on irritation from inhalation exposure are lacking, it is unclear whether the limit of 1.2 ppm will protect workers from irritation.
Nielsen and Yamagiwa (1989) estimated a theoretical occupational TLV for cyclohexylamine using mouse RD50 study data. They multiplied the RD50 (27 ppm) by 0.03 (Alarie 1981) or the RD0 (2 ppm) by 0.2
(Nielsen et al. 1985), obtaining TLVs of 0.8 and 0.4 ppm, respectively. They also obtained a TLV of 0.8 ppm by multiplying the tRD50 (78 ppm) by 0.01, using the approach of Ferguson et al. (1986). The latter approach is less well-established because the relationship between pulmonary irritation and the TLVs is not as well defined as the relationship between sensory irritation and the TLV. Nielsen and Yamagiwa (1989) concluded that the current 10 ppm NIOSH and ACGIH occupational TLVs for cyclohexylamine may “need reconsideration.”
8.3.
Data Quality and Research Needs
The data set used to derive cyclohexylamine AEGL values was limited and would be improved by the availability of (1) additional studies with end points within the scope of AEGL-2, since in the key study there was a 10-fold difference in the lowest test concentration, which was used for AEGL-2 derivation, and the next higher test concentration, which was used for AEGL-3 derivation, (2) lethality studies with species other than rats to determine the species variability, (3) a study that could be used to determine the concentration-time relationship (n in Cnt = k), and (4) human data to define the odor and irritation thresholds and intraspecies variability.
Despite these limitations, there is a reasonable degree of confidence in the derived AEGL-1, AEGL-2, and AEGL-3 values because they are based on a reliable and robust GLP rat study (Bio/dynamics, Inc. 1990) that was consistent with the other available data, and uncertainty factors (total of 10 for AEGL-2 and 30 for AEGL-3) were applied to account for the limited data set.
9.
REFERENCES
ACGIH. 1996. American Conference of Government Industrial Hygienists: cyclohexylamine. In: Documentation of the Threshold Limit Values and Biological Exposure Indices, Suppl. to 6th ed., pp. 364-366, ACGIH, Cincinnati, OH.
ACGIH. 2004. American Conference of Government Industrial Hygienists: cyclohexylamine. In: Threshold Limit Values and Biological Exposure Indices for chemical substances and physical agents. ACGIH, Cincinnati, OH.
Alarie, Y. 1981. Dose-response analysis in animal studies: Prediction of human responses. Environ. Health. Perspect. 42:9-13.
Amoore, J.E. and E. Hautala. 1983. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272-290.
Beard, R.R. and J.T. Noe. 1981. Aliphatic and alicyclic amines. In: Patty’s Industrial Hygiene and Toxicology, 3rd ed, Vol. 2B, Clayton and Clayton, Eds., pp. 3135-3173.
Bio/dynamics, Inc. 1990. An acute inhalation toxicity study of C-1388 in the rat. Final Report. Project no. 89-8214. December 4, 1990.
Brewen J.G., F.G. Pearson, K.P. Jones, and H.E. Luippold. 1971. Cytogenetic effects of cyclohexylamine and N-OH-cyclohexylamine on human leukocytes and Chinese hamster bone marrow. Nature New Biol. 230:15-16.
Brittain, R.T., D. Jack and P.S.J. Spencer. 1964. Mechanism of monoamine oxidase inhibitors in enhancing amphetamine toxicity. J. Pharm. Pharmacol. 16: 565-567.
Brusick, D., M. Cifone, R. Young and S. Benson. 1989. Assessment of the genotoxicity of calcium cyclamate and cyclohexylamine. Environ. Molec. Mutagen. 14: 188-199.
Budavari S., M.J. O'Neil, A. Smith, P.E. Heckelman, J.F. Kinneary (Eds.). 1996. The Merck Index, 12th ed. Merck & Co., Inc., Whitehouse Station, NJ., p. 439.
DFG (Deutsche Forschungsgemeinschaft). 2002. List of MAK and BAT Values, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area, Report No. 38. Weinheim, Federal Republic of Germany: Wiley VCH.
Eastman Kodak. 1984. Eastman Kodak Company Material Safety Data Sheet - Cyclohexylamine. Prepared 2/13/84.
Ferguson, J.S., M. Schaper, M.F. Stock, D.A. Weyel and Y. Alarie. 1986. Sensory and pulmonary irritation with exposure to methyl isocyanate. Toxicol. Appl. Pharmacol. 82: 329-335.
Gagnaire, F., S. Azim, P. Bonnet, P. Simon, J.P. Guenier and J. De Ceaurriz. 1989. Nasal irritation and pulmonary toxicity of aliphatic amines in mice. J. Appl. Toxicol. 9:301-304.
Gagnaire, F., S. Azim, P. Simon, B. Cossec, P. Bonnet and J. De Ceaurriz. 1993. Sensory and pulmonary irritation of aliphatic amines in mice: a structure-activity relationship study. J. Appl. Toxicol. 13:129-135.
Gaunt, I.F., M. Sharratt, P. Grasso, A.B.G. Lansdown and S.D. Gangolli. 1974. Short-term toxicity of cyclohexylamine hydrochloride in the rat. Food Cosmet. Toxicol. 12: 609-624.
Gaunt, I.F., J. Hardy, P. Grasso, S.D. Gangolli and K.R. Butterworth. 1976. Long-term toxicity of cyclohexylamine hydrochloride in the rat. Food Cosmet. Toxicol. 14: 255-267.
Greim, H., Ed. 2002. Gesundheitsschaedliche Arbeitsstoffe, Toxikologisch-arbeitsmedizinische Begruendungen von MAK-Werten, German edition, Weinheim, 34th. (Translation by F. Kalberlah, FoBiG GmbH, Freiburg, Germany as personal communication).
Hanson, J.W. M.T. Greally and A.H. Olney. 1990. Microgastria and limb reduction defects: a possible developmental field defect. Am. J. Hum. Genet. 47 (Suppl 3): A60.
Hardy, J., I.F. Gaunt, J. Hooson, R.J. Hendy and K.R. Butterworth. 1976. Long-term toxicity of cyclohexylamine chloride in mice. Food Cosmet. Toxicol. 14: 269-276.
HCN (Health Council of the Netherlands). 2000. Cyclohexylamine. In: Health-based reassessment of administrative occupational exposure limits. Health Council of the Netherlands (Gezondheidsraad) Vol 15OSH/021, 34 p.
Hills, B. and B. Lushniak. 1989. Health Hazard Evaluation Report HETA 89-057-2003, Cincinnati Electronics Corp., Cincinnati, Ohio. NIOSH/00194415.
Hills, B., B. Lushniak and T. Sinks. 1990. Workplace exposures to the corrosion-inhibiting chemicals from a steam humidification system. Appl. Occup. Environ. Hyg. 5: 672-673.
HSDB. 2002. Hazardous Substances Data Bank. Cyclohexylamine. MEDLARS Online Information Retrieval System, National Library of Medicine (http://toxnet.nlm.nih.gov; retrieved 7/2005).
IARC. 1980. International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risk of chemicals to humans. Vol. 22: Some non-nutritive sweetening agents. Cyclohexylamine, pp. 59-109.
IARC. 1987. International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Supplement 7: Overall Evaluation of Carcinogenicity: An updating of IARC Monographs Volumes 1 to 42. Cyclohexylamine, pp. 178-182.
James, R.W., R. Heywood and D. Crook. 1981. Testicular responses of rats and dogs to cyclohexylamine overdosage. Food Cosmet. Toxicol. 19: 291-296.
Knaap, A.G., P.G. Kramers and F.H. Sobels. 1973. Lack of mutagenicity of the cyclamate metabolites in Drosophila. Mutat. Res. 21: 341-344.
Kroes, R., P.W.J. Peters, J.M. Berkvens, H.G. Verschuuren, Th. De Vries and G.J. van Esch. 1977. Long-term toxicity and reproduction study (including a teratogenicity study) with cyclamate, saccharin and cyclohexylamine. Toxicology 8: 285-300.
Lee, I.P. and R.L. Dixon. 1972. Various factors affecting the lethality of cyclohexylamine. Toxicol. Appl. Pharmacol. 22: 465-473.
Lomonova, G.V. 1965. Toxicity of cyclohexylamine and dicyclohexylamine. Fed. Proc. Transl. Suppl. 24(pt. 2): T96-T98. [Translation of original 1963 article in Gigiena Truda I Professional’nye Zabolevaniya, 7 (11) 51-57.]
Mason, P.L. and G.R. Thompson. 1977. Testicular effects of cyclohexylamine hydrochloride in the rat. Toxicol. 8: 143-156.
Mortelmans, K., S. Haworth, T. Lawlor, W. Speck, B. Tainer and E. Zeiger. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutagen. 8 (Suppl.7): 1-119.
NRC (National Resource Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. National Academy Press, Washington, DC.
Nielsen, G.D., J. Olsen, J.C. Bakbo and E. Holst. 1985. Propyl ether. I. Interaction with the sensory irritant receptor. Acta Pharmacol. Toxicol. 56: 158-164.
Nielsen, G.D. and M. Yamagiwa. 1989. Structure-activity relationships of airway irritating aliphatic amines. Receptor activation mechanisms and predicted industrial exposure limits. Chem.-Biol. Interact. 71: 223-244.
NIOSH. 2005. National Institute for Occupational Safety and Health. Cyclohexylamine. In: Pocket Guide to Chemical Hazards. U.S. Department of Health and Human Services, Public Health Service, Cincinnati, OH. Online at http://www.cdc.gov/niosh/npg/npgd0168.html; retrieved 5/2005].
Oser, B.L., S. Carson, G.E. Cox, E.E. Vogin and S.S. Sternberg. 1976. Long-term and multigeneration studies with cyclohexylamine hydrochloride. Toxicology 6: 47-65.
Price, J.M., C.G. Biava, B.L. Oser, E.E. Vogin, J. Steinfeld and H.L. Ley. 1970. Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science 167: 1131-1132.
Renwick, A.G. and R.T. Williams. 1972. The metabolites of cyclohexylamine in man and certain animals. Biochem. J. 129: 857-867.
Roberts, A. and A.G. Renwick. 1986. The pharmacokinetics and tissue distribution of cyclohexylamine in the rat and mouse. In: Proc. 4th Int. Cong. Toxicol, Tokyo, Japan, July 21-25. Toxicol Lett. 31: 1986.
Roberts, A., A.G. Renwick, G. Ford, D.M.. Creasy and I. Gaunt. 1989. The metabolism and testicular toxicity of cyclohexylamine in rats and mice during chronic dietary administration. Toxicol. Appl. Pharmacol. 98: 216-229.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: a review. Am. Ind. Hyg. Assoc. 47:142-151.
Sandridge, R.L. and H.B. Staley. 1978. Amines by reduction. In: Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., Volume II, M. Grayson and D. Eckroth, Ed. John Wiley and Sons, Inc., N.Y., p. 360.
Schmahl, D. 1973. Lack of carcinogenic effects of cyclamate, cyclohexylamine and saccharin in rats. Arzneim. Forsch. 23: 1466-1470.
SDU Uitgevers (Ministry of Social Affairs and Employment). 2000. National MAC (Maximum Allowable Concentration) List, 2000. The Hague, The Netherlands.
Smyth, H.F., C.P. Carpenter, C.S. Weil, U.C. Pozzani, J.A. Striegel and J.S. Nycum. 1969. Range-finding toxicity data: List VII. Am. Ind. Hyg. Assoc. J. 30: 470-476.
Swedish National Board of Occupational Safety and Health. 2000. Swedish Occupational Exposure Limits: LLV (Level Limit Values), CLV (Ceiling Limit Values), and STV (Short-Term Values), Adopted 28th July, 2000 by Ordinance of the Swedish National Board of Occupational Safety and Health.
Takano, K. and M. Suzuki. 1971. Cyclohexylamine, a chromosome-aberration inducing substance: No teratogenicity in mice (Jpn.). Congenital Anomalies 11: 51-57.
Tanaka, S., S. Nakaura, K. Kawashima, S. Nagao, T. Kuwamura and Y. Omori. 1973. Studies on the teratogenicity of food additives. 2.
Effects of cyclohexylamine and cyclohexylamine sulfate on the fetal development in rats. J. Food Hyg. Soc. 14: 542-548.
ten Berge, W.F., A. Zwart and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Materials. 13:302-309.
Tsai, I.L., M.F. Wun, C.M. Teng, T. Ishikawa and I.S. Chen. 1998. Anti-platelet aggregation constituents from Formosan Toddalia asiatica. Phytochemistry 48: 1377-1382.
Turner, J.H. and D.L. Hutchinson. 1974. Cyclohexylamine mutagenicity: An in vivo evaluation utilizing fetal lambs. Mutation Res. 26: 407-412.
USITC. 1995. United States International Trade Commission, publication 2810. Synthetic organic chemicals - United States production and sales, p. 3–42.
U.S. EPA (U.S. Environmental Protection Agency). 1987. Health and Environmental Effects Document for Cyclohexylamine. U.S. EPA. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, U.S. EPS, Cincinnati, OH.
U.S. EPA (U.S. Environmental Protection Agency). 2005. Integrated Risk Information System (IRIS): Cyclohexylamine. Cincinnati, OH, Office of Health and Environmental Assessment. Online at http://www.epa.gov/iris/subst/0319.htm; retrieved 7/2005.
Van Doorn, R., M. Ruijten and T. Van Harreveld. 2002. Guidance for the application of odor in 22 chemical emergency response. Version 2.1, 29.08.2002.
Verschueren, K. (Ed.) 1996. Cyclohexylamine. In: Handbook of Environmental Data on Organic Chemicals, Third Edition. Van Nostrand Reinhold Co., New York, p. 574.
Watrous, R.M. and H.N. Schulz. 1950. Cyclohexylamine, p-chloronitrobenzene, 2-aminopyridine: Toxic effects in industrial use. Ind. Med. Surg. 19: 317-320.
Wilson, J.G. 1972. “Use of primates in teratological investigations.” In: Medical Primatology, E.I. Goldsmith and J. Moor-Jankowski, Eds., Karger, Basel, 1972, pp. 286-295.
APPENDIX A
Derivation of AEGL Values
Derivation of AEGL-1
|
Key study: |
Bio/dynamics, Inc. 1990. Rats exposed for 4 h to 54.2 ppm cyclohexylamine had labored breathing, partially closed eyes, and red nasal discharge; 54.2 ppm was divided by a modifying factor of 3 because the effects seen at 54.2 ppm are more severe than prescribed by the AEGL-1 definition, yielding a concentration (18 ppm) expected to cause mild sensory irritation. At the two higher doses, rats also displayed rales, gasping, dried red material on the facial area, tremors, weight loss, ocular irritation, and irreversible ocular lesions; 2/10 rats exposed to the aerosol/vapor mix died (had nasal, lung, and urogenital lesions). |
|
Toxicity end point: |
Mild respiratory and/or ocular irritation from exposure to 18 ppm for 4 h |
|
Scaling: |
None; using the same value across time was considered appropriate since mild irritant effects do not vary greatly over time |
|
Uncertainty factors: |
Total Uncertainty Factor: 10 |
|
Interspecies: |
3—Mild sensory irritation from an alkaline irritant gas is not likely to vary greatly among animals; supported by two mouse respiratory irritation studies (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989). |
|
Intraspecies: |
3—Mild sensory irritation from an alkaline irritant gas is not likely to vary greatly among humans; supported by occupational exposure data (Watrous and Schulz 1950). |
|
Modifying Factor: |
3—Effects seen at 54.2 ppm are more severe than prescribed by the AEGL-1 definition. |
Calculations:
10 min AEGL-1 = 54.2 ppm/30 = 1.8 ppm [7.3 mg/m3]
30 min AEGL-1 = 54.2 ppm/30 = 1.8 ppm [7.3 mg/m3]
1 h AEGL-1 = 54.2 ppm/30 = 1.8 ppm [7.3 mg/m3]
4 h AEGL-1 = 54.2 ppm/30 = 1.8 ppm [7.3 mg/m3]
8 h AEGL-1 = 54.2 ppm/30 = 1.8 ppm [7.3 mg/m3]
Derivation of AEGL-2
|
Key study: |
Bio/dynamics, Inc. 1990. Rats were exposed for 4 h to 54.2 ppm, 567 ppm or a vapor/aerosol combination (542 ppm vapor + ~612 mg/m3 aerosol). At 54.2 ppm, rats had labored breathing, red nasal discharge, ocular irritation. At the two higher doses, rats also displayed rales, gasping, dried red material on the facial area, tremors, weight loss, ocular irritation, and irreversible ocular lesions; 2/10 rats exposed to the aerosol/vapor mix died (had nasal, lung, and urogenital lesions). |
|
Toxicity end point: |
Moderate respiratory effects and ocular irritation, NOAEL for irreversible ocular lesions (54.2 ppm) |
|
Scaling: |
Cn × t = k (ten Berge et al. 1986); no data were available to derive n, so used n = 3 to extrapolate to <4 h and n = 1 to extrapolate to >4 h, except the 30-min values were adopted as 10 min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
|
Uncertainty factors: |
Total Uncertainty Factor: 10 |
|
Interspecies: |
3—Effects seen at 54.2 ppm were clearly reversible and a larger uncertainty factor yields values at or below the AEGL-1. |
|
Intraspecies: |
3—Effects seen at 54.2 ppm were clearly reversible and a larger uncertainty factor yields values at or below the AEGL-1. |
Calculations for 10 and 30 min, and 1 h:
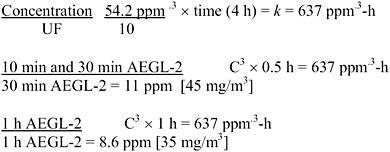
Calculations for 4 h:
Calculations for 8 h:
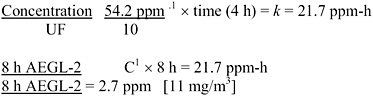
Derivation of AEGL-3
|
Key study: |
Bio/dynamics, Inc. 1990. Rats were exposed for 4 h to 54.2 ppm, 567 ppm or a vapor/aerosol combination (542 ppm vapor + ~612 mg/m3 |
|
|
aerosol). At 54.2 ppm, rats had labored breathing, red nasal discharge, ocular irritation. At the two higher doses, rats also displayed rales, gasping, dried red material on the facial area, tremors, weight loss, ocular irritation, and irreversible ocular lesions; 2/10 rats exposed to the aerosol/vapor mix died (had nasal, lung, and urogenital lesions). |
|
Toxicity end point: |
567 ppm was considered the threshold for lethality and caused irreversible ocular lesions |
|
Scaling: |
Cn × t = k (ten Berge et al. 1986); no data were available to derive n, so used n = 3 to extrapolate to <4 h and n = 1 to extrapolate to >4 h, except the 30-min values were adopted as 10 min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
|
Uncertainty factors: |
Total Uncertainty Factors: 30 |
|
Interspecies: |
10—Although tissue destruction caused by a severely corrosive agent is not expected to vary greatly among animals, the dose spacing in the key study did not precisely delineate the LOAEL for ocular lesions or the threshold for lethality in rats, and the set of animal studies was limited. |
|
Intraspecies: |
3—Tissue destruction caused by a severely corrosive agent is not expected to vary greatly among humans; a greater uncertainty factor is not warranted because it yields concentrations comparable to AEGL-2 values. |
Calculations for 10 and 30 min, and 1 h:
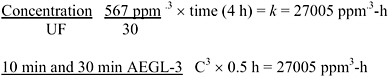
APPENDIX B
Derivation of the Level of Distinct Odor Awareness
The level of distinct odor awareness (LOA) represents the concentration above which it is predicted that more than half of the exposed population will experience at least a distinct odor intensity, about 10 % of the population will experience a strong odor intensity. The LOA should help chemical emergency responders in assessing the public awareness of the exposure due to odor perception. The LOA derivation follows the guidance given by van Doorn et al. (2002).
An odor detection threshold (OT; stated to be the geometric mean of all available literature data, omitting extreme points and duplicate values; method of obtaining values not stated) of 2.6 ppm was obtained for cyclohexylamine from Amoore and Hautala (1983). The same citation listed an odor detection threshold of 0.83 for n-butanol, as compared to the reference value of 0.04 ppm as the odor threshold provided by van Doorn et al (2002). Based on the differences in n-butanol values from the two sources, an “inter-laboratory” correction factor is applied to 0.83 ppm as follows:
The concentration C leading to an odor intensity (I) of distinct odor detection (I=3) is derived using the Fechner function:
For the Fechner coefficient, the default of kw = 2.33 will be used due to the lack of chemical-specific data:
The resulting concentration is multiplied by an empirical field correction factor. It takes into account that in every day life, factors such as sex, age, sleep, smoking, upper airway infections and allergies, as well as distraction, increase the odor detection threshold by a factor of 4. In addi-
tion, it takes into account that odor perception is very fast (about 5 sec) which leads to the perception of concentration peaks. Based on the current knowledge, a factor of 1/3 is applied to adjust for peak exposure. Adjustment for distraction and peak exposure lead to a correction factor of 4/3 = 1.33.
The LOA for Cyclohexylamine is 2.0 ppm.
APPENDIX C
ACUTE EXPOSURE GUIDELINES FOR CYCLOHEXYLAMINE (108-91-8)
DERIVATION SUMMARY
|
AEGL-1 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
1.8 ppm |
1.8 ppm |
1.8 ppm |
1.8 ppm |
1.8 ppm |
|
Key Reference: Bio/dynamics, Inc. 1990. An acute inhalation toxicity study of C-1388 in the rat. Final Report. Project no. 89-8214. December 4, 1990. |
||||
|
Test Species/Strain/Sex/Number: Sprague-Dawley rats; 5/sex/dose. |
||||
|
Exposure Route/Concentrations/Durations: Inhalation for 4 h of 54.2 ppm, 567 ppm, or a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol. |
||||
|
Effects: At 54.2 ppm, rats had labored breathing, red nasal discharge, and partly closed eyes primarily during the 4-h exposure. Rats exposed to 567 ppm or the vapor/aerosol combination had severe respiratory effects and irreversible ocular lesions; 2/10 rats exposed to the aerosol-containing atmosphere died. |
||||
|
End point/Concentration/Rationale: Mild respiratory and/or ocular irritation is expected to result from exposure to 18.1 ppm (obtained by dividing 54.2 ppm by the modifying factor of 3). |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—Mild sensory irritation from an alkaline irritant gas is not likely to vary greatly among animals. Supported by other animal (RD50) data. Intraspecies: 3—Mild sensory irritation from an alkaline irritant gas is not likely to vary greatly among humans. Supported by human (occupational) data. |
||||
|
Modifying Factor: 3—Effects seen at 54.2 ppm are more severe than prescribed by the AEGL-1 definition. |
||||
|
Animal to Human Dosimetric Adjustment: Not performed. |
||||
|
Time Scaling: None; using the same value for 10 min to 8 h was considered appropriate because mild irritant effects do not vary greatly over time. |
||||
|
Data Adequacy: The data set was small, but the key study was well-conducted (GLP). The derived AEGL-1 level (and appropriateness of the total UF) is supported by a human study (chemical workers exposed to 4-10 ppm for <8 h reported “no symptoms of any kind”; Watrous and Schulz 1950) and by two mouse respiratory depression (RD50) studies (Gagnaire et al. 1989; Nielsen and Yamagiwa 1989), from which it is predicted that 2.7 or 5.1 ppm should result in some sensory irritation in humans, whereas 0.27 or 0.51 ppm should cause no sensory irritation (Alarie 1981). |
|
AEGL-2 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
11 ppm |
11 ppm |
8.6 ppm |
5.4 ppm |
2.7 ppm |
|
Key Reference: Bio/dynamics, Inc. 1990. An acute inhalation toxicity study of C-1388 in the rat. Final Report. Project no. 89-8214. December 4, 1990. |
||||
|
Test Species/Strain/Sex/Number: Sprague-Dawley rats; 5/sex/dose. |
||||
|
Exposure Route/Concentrations/Durations: Inhalation for 4 h of 54.2 ppm, 567 ppm, or a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol. |
||||
|
Effects: At 54.2 ppm, rats had labored breathing, red nasal discharge, and partly closed eyes primarily during the 4-h exposure. Rats exposed to 567 ppm or the vapor/aerosol combination had severe respiratory effects and irreversible ocular lesions; 2/10 rats exposed to the aerosol-containing atmosphere died. |
||||
|
End point/Concentration/Rationale: Exposure to 54.2 ppm for 4 h caused moderate respiratory effects and ocular irritation; is NOAEL for irreversible ocular lesions. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 10 Interspecies: 3—Effects seen at 54.2 ppm were clearly reversible and a larger uncertainty factor yields values at or below the AEGL-1. |
||||
|
Intraspecies: 3—Effects seen at 54.2 ppm were clearly reversible and a larger uncertainty factor yields values at or below the AEGL-1. |
||||
|
Modifying Factor: none. |
||||
|
Animal to Human Dosimetric Adjustment: Not performed. |
||||
|
Time Scaling: Cn × t = k (ten Berge et al. 1986); no data were available to derive n, so used n = 3 to extrapolate to <4 h and n = 1 to extrapolate to >4 h, except the 30-min values were adopted as 10-min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
||||
|
Data Adequacy: The data set was small but the key study was well-conducted (GLP guidelines used). There were no data to determine the value of n for time scaling. The NOAEL for corneal opacity was supported by another study with several species. |
|
AEGL-3 VALUES |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
38 ppm |
38 ppm |
30 ppm |
19 ppm |
9.5 ppm |
|
Key Reference: Bio/dynamics, Inc. 1990. An acute inhalation toxicity study of C-1388 in the rat. Final Report. Project no. 89-8214. December 4, 1990. |
||||
|
Test Species/Strain/Sex/Number: Sprague-Dawley rats; 5/sex/dose |
||||
|
Exposure Route/Concentrations/Durations: Inhalation; 4 h; 54.2 ppm, 567 ppm, or a vapor/aerosol combination containing 542 ppm vapor and ~612 mg/m3 aerosol. |
||||
|
Effects: At 54.2 ppm, rats had labored breathing, red nasal discharge, and partly closed eyes primarily during the 4-h exposure. Rats exposed to 567 ppm or the vapor/aerosol combination had labored breathing, rales, gasping, dried red material on the facial area, tremors, weight loss, irreversible ocular lesions, and 1/5 males and 1/5 females exposed to the aerosol-containing atmosphere died and had nasal, lung, and urogenital tissue damage. |
||||
|
End point/Concentration/Rationale: Exposure to 567 ppm for 4 h caused irreversible ocular lesions and was the lethality threshold. |
||||
|
Uncertainty Factors/Rationale: Total uncertainty factor: 30 Interspecies: 10—Although tissue destruction caused by a severely corrosive agent is not expected to vary greatly among animals, the dose spacing in the key study did not precisely delineate the LOAEL for ocular lesions or the threshold for lethality in rats, and the set of animal studies was limited. |
||||
|
Intraspecies: 3—Tissue destruction caused by a severely corrosive agent is not expected to vary greatly among humans; a greater uncertainty factor is not warranted because it yields concentrations comparable to AEGL-2 values. |
||||
|
Modifying Factor: None. |
||||
|
Animal to Human Dosimetric Adjustment: No data. |
||||
|
Time Scaling: Cn × t = k (ten Berge et al. 1986); no data were available to derive n, so used n = 3 to extrapolate to <4 h and n = 1 to extrapolate to >4 h, except the 30-min values were adopted as 10-min values to be protective of human health (NRC 2001; see Section 4.4.3.). |
||||
|
Data Adequacy: The data set was small but the key study was well-conducted (GLP guidelines used). There were no data to determine the value of n for time scaling. Supporting studies with lethality as an end point were limited. |