7
Formaldehyde
J. Torin McCoy NASA-Johnson Space Center Habitability and Environmental Factors Office Houston, Texas
OCCURRENCE AND USE
Formaldehyde (HCHO) is an organic substance that is widely used and can occur as a result of both natural and anthropogenic processes (see Table 7-1). At room temperature, formaldehyde exists as a colorless gas with a distinct and pungent odor. Formaldehyde has been widely used since the early 1900s in commercial and industrial applications. Much of its use centers around the manufacturing of plastics and resins (such as urea-formaldehyde resins) or applications in the production of chemical intermediates. Other diverse uses include application as a preservative for biologic samples, an ingredient in shampoos and household cleaning agents, use as an agricultural fumigant, and use in various products as an antimicrobial agent. In addition to these numerous commercial
TABLE 7-1 Physical and Chemical Properties
|
Formula |
HCHO |
|
|
Synonym |
methanal, formic aldehyde, methyl aldehyde |
|
|
CAS registry no. |
50-00-0 |
|
|
Molecular weight |
30.0 |
|
|
Boiling point |
−19.5°C |
|
|
Melting point |
−92°C |
|
|
Water solubility |
55 g/100 mL |
|
|
Vapor pressure |
10 mm Hg at 88°C |
|
|
Vapor density |
1.08 |
|
|
Specific gravity |
0.81 at 20°C |
|
|
Log Kow |
0.35 |
|
|
Source: Data from ATDSR 1999. |
||
applications, formaldehyde is a common combustion byproduct and can be measured at low concentrations in almost all ambient air samples. Although it is often thought of as strictly an environmental pollutant, formaldehyde is a normal metabolic product formed endogenously from the breakdown of serine (Restani and Galli 1991) and serves as an intermediary cellular metabolite in the biosynthesis of purines, thymidine, and several amino acids (ATSDR 1999). Formaldehyde is also a normal byproduct from the metabolism of many N-methyl substituted drugs and other xenobiotics (Dahl and Hadley 1983). Endogenous concentrations of formaldehyde in blood are estimated at 2.6 micrograms per gram (µg/g) for humans (Heck et al. 1985). Dietary exposure is also relevant because formaldehyde is found naturally (and even as a food additive) in some animal products, fruits, vegetables, cheeses, seafood, and other commodities (Restani and Galli 1991).
Formaldehyde is a common contaminant that may be discharged to the spacecraft environment from both direct and indirect sources. Its occurrence in water is also closely tied to releases to air, thus providing a good example of the interdependency of these media onboard the space shuttle and International Space Station (ISS). Formaldehyde is occasionally detected, although at relatively low concentrations (1-3 µg per liter [L]), in drinking water provided to the ISS from ground-based sources (Schultz 2004). However, much higher concentrations of formaldehyde (up to 9,000 µg/L) have been measured in the humidity condensate onboard the ISS (Schultz 2004).
The relatively high concentrations of formaldehyde in this condensate are largely attributable to the number of sources that may release formaldehyde, as well as to its chemical and physical properties. Formaldehyde is one of the most common indoor air pollutants. Formaldehyde can be off-gassed from textiles, foam insulation, resins, epoxies, and a myriad of other substances commonly encountered in the indoor environment (both ground based and in orbit). Furthermore, formaldehyde can be formed through secondary reactions of other indoor air pollutants (for example, methane, pinene), especially in the presence of higher temperatures and/or chemical oxidizers. Studies by the National Aeronautics and Space Administration (NASA) have frequently observed formaldehyde releases from Delrin and other commonly used industrial materials (James 2004). Once present in the air, the high water solubility of formaldehyde relative to its vapor pressure (a relationship expressed by its Henry’s law constant) results in a significant removal of formaldehyde from the air by condensing moisture. Given this interdependency, exposure to formaldehyde through drinking water ingestion is a relevant exposure pathway on-orbit, despite ground-based experiences that suggest
that this pathway might be insignificant in comparison to inhalation exposures.
TOXICOKINETICS
For the purposes of this document, the toxicokinetics of ingested formaldehyde will be highlighted. As with any chemical, a thorough understanding of its absorption, metabolism, distribution, and elimination is crucial to gaining a perspective on its toxicity and to the setting of appropriate spacecraft water exposure guidelines (SWEGs).
Absorption
Formaldehyde is readily absorbed into gastrointestinal (GI) mucosal cells following oral exposures (IARC 1995). However, it is difficult to distinguish the fraction of formaldehyde that is ultimately absorbed across the GI tract (ATSDR 1999). As evidenced by rapid increases in formic acid concentrations in the blood (within minutes per observations of Eells et al. 1981), absorbed formaldehyde is either metabolized to formate in the GI tract (which is then quickly absorbed), or formaldehyde is quickly absorbed and metabolized to formate in the blood (Galli et al. 1983; Burkhart et al. 1990). No studies were found that were able to distinguish the oral absorption kinetics of formaldehyde apart from the kinetics of its metabolism to formic acid.
Metabolism
Although metabolism rates are thought to vary somewhat across species, the basic sequence of metabolism is the same in all mammalian systems (Pandey et al. 2000). Figure 7-1 describes the basic metabolic process by which formaldehyde is converted to formate/formic acid and ultimately to carbon dioxide and water. The conversion of formaldehyde to formic acid is mainly catalyzed by the formaldehyde dehydrogenase (FDH)/alcohol dehydrogenase 3 complex (with glutathione [GSH] and nicotinamide adenine dinucleotide [NAD+] acting as cofactors), because of its specific affinity for formaldehyde, although other enzyme systems also have the capability to oxidize aldehydes (Teng et al. 2001). FDH (which is also defined as alcohol dehydrogenase [ADH3] according to
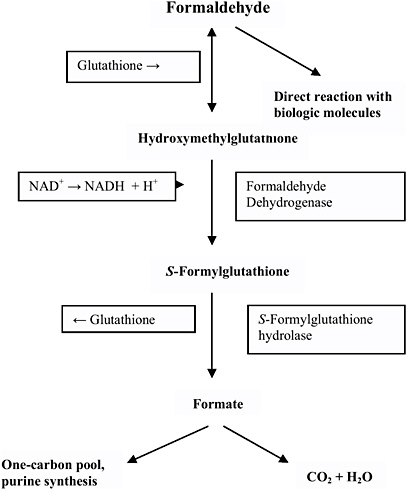
FIGURE 7-1 Metabolism and biologic fate of formaldehyde. Source: Modified figure printed with permission; copyright 1995, World Health Organization.
current nomenclature) is present in almost all tissues but is especially prevalent in the liver and erythrocytes and is very efficient in acting upon endogenous or exogenous formaldehyde. Researchers have observed polymorphisms in the ADH3 gene that encodes FDH/ADH3 among several population groups, and this variability suggests that the capacity to metabolize formaldehyde will likely exhibit some interindividual variation (Hedberg et al. 2001). Upon metabolism from formaldehyde, formic acid can then be metabolized to carbon dioxide and water, although much more slowly than the FDH/ADH3-dependent metabolism of formaldehyde (55 minute [min] plasma half-life as compared to 1 min with
formaldehyde) (Stratemann et al. 1968). This metabolic step relies upon tetrahydrofolate-dependent enzymatic action (Pandey et al. 2000). Alternately, formic acid can be excreted as a salt in the urine or can enter the one-carbon metabolic pool (Restani and Galli 1991) where it can be incorporated as a methyl group into nucleic acids and proteins (Thrasher and Kilburn 2001).
Distribution and Elimination
In rodents, Buss et al. (1964) observed that about 40% of radiolabeled formaldehyde that was administered orally (7 milligrams per kilogram [mg/kg] of body weight dose) was eliminated as carbon dioxide within 12 hours (h), while another estimated 10% was eliminated in urine and 1% in feces. Incorporation into macromolecules was postulated by the authors to account for much of the remaining radiolabeled carbon. Formaldehyde is not generally expected to be absorbed into the blood-stream and carried as an unmetabolized molecule to other organ systems. Thus, distribution and excretion are not thought to be significant considerations with formaldehyde exposure and toxicity. Instead, its rapid metabolism and reactivity suggest that it will either be metabolized or it will exert its toxic effects locally at the point of exposure. It is postulated that the toxicity of formaldehyde is evidenced when exposure is of sufficient magnitude that this detoxification mechanism for formaldehyde is saturated and the reactive formaldehyde molecule is allowed to exert its effects on local tissues and macromolecules (for example, proteins and DNA) (Heck and Casanova 1990).
TOXICITY SUMMARY
Given its commercial importance and potential for human exposure, formaldehyde has been the subject of a relatively large amount of toxicologic research. Much of this work has been focused on investigations of inhalation exposures to formaldehyde, and the literature on its oral toxicity is not quite as robust. However, there are a number of studies (both human and animal) focusing on a variety of toxicologic end points and exposure durations that provide a basis for setting SWEGs for formaldehyde. The following discussion is not meant to represent a comprehensive review of all available data on the toxicity of formaldehyde. Instead, the focus is on those studies that appear to be the most applica-
ble to SWEG development (that is, exposure to formaldehyde through drinking water).
The discussion of the oral toxicity of formaldehyde is organized into four different categories based on the duration of the exposures: acute (1-5 days [d]), short-term (6-30 d), subchronic (30-180 d), and chronic (180 d to lifetime). Data for these individual exposure durations generally correspond to timeframes relevant to the development of 1-, 10-, 100-, and 1,000-d SWEGs, respectively. Complete descriptions of studies cited in the discussion below are provided in Table 7-2. It is important to note that some of the data discussed below came from studies involving gavage exposures to formaldehyde. These results should be viewed with some caution because gavage exposures may have a greater potential to overwhelm formaldehyde metabolic capabilities, resulting in greater distribution and toxicity of formaldehyde than might be experienced with episodic drinking water exposures.
Acute Toxicity (1-5 d)
Several human cases of accidental, homicidal, or suicidal ingestion of formaldehyde have been reported in the literature (Eells et al. 1981; Kochhar et al. 1986; Burkhart et al. 1990). Eells et al. (1981) reported on the death of a 41-year [y]-old woman following the intentional ingestion of formaldehyde (620 mg/kg). The woman was cyanotic and severely hypotensive when admitted to the emergency room and died within 28 h of admission. Observed toxicologic effects prior to death included renal failure, abdominal pains, and symptoms of metabolic acidosis. Similar effects were reported by Burkhart et al. (1990) after the ingestion of formaldehyde at 520 mg/kg in a suicide attempt. The victim complained of difficulty breathing and severe abdominal pains before experiencing a significant drop in blood pressure and slipping into a coma. Upon autopsy, the stomach of the victim was reported to be hard, white, and leathery. In a nonfatal case, Kochhar et al. (1986) described a 26-y-old woman who accidentally ingested formaldehyde at 230 mg/kg. The patient experienced ulceration and sloughing of the soft palate and posterior pharyngeal wall and epiglottis, as well as ulceration of the stomach and upper GI tract. Mild tachycardia was also observed following the ingestion of formaldehyde. In summary, acute oral human exposures to formaldehyde have resulted in adverse respiratory, cardiac, GI, metabolic, renal, and neurologic effects.
TABLE 7-2 Toxicity Summary
|
Dose/Route |
Exposure Duration |
Species |
Effects |
Reference |
|
Acute Exposures |
||||
|
520 mg/kg/dm, oral |
One event |
Human, male (n = 1) |
Death; decreased blood pressure; GI irritation; cardiac arrest; acidosis |
Burkhart et al. 1990 |
|
620 mg/kg/d, oral |
One event |
Human, female (n = 1) |
Death; decreased blood pressure; GI irritation; acidosis; loss of consciousness |
Eells et al. 1981 |
|
230 mg/kg/d, oral |
One event |
Human, female (n = 1) |
Ulceration of esophagus mucosa; GI irritation; tachycardia |
Kochhar et al. 1986 |
|
Short-Term Exposures |
||||
|
185 mg/kg/d (LOAEL), gavage |
Gestation days 6-15 |
CD-1 mice, female |
Increased mortality (22 of 34 mice); no observed teratogenic effects |
Marks et al. 1980 |
|
40 ppm (NOAEL), formaldehyde vapor |
Gestation days 6- 20, 6 h/d |
Sprague-Dawley rats, female (n = 25) |
No reproductive or developmental effects observed at concentrations of 0, 5, 10, 20, and 40 ppm; maternal toxicity in the 40-ppm group was observed |
Saillenfait et al. 1989 |
|
10 ppm (NOAEL), formaldehyde vapor |
Gestation days 6- 15, 6 h/d |
Sprague-Dawley rats, female (n = 25) |
No reproductive or developmental effects observed with exposures of 0, 2, 5, or 10 ppm |
Martin 1990 |
|
10,000 mg/L (as HMT) (NOAEL), drinking water |
2 wk (exposures to dams continued through gestation and lactation) |
Wistar rats, male and female |
No malformations noted in 124 pups born to females exposed to 0 or 1% hexamethylenetetramine (HMT) |
Della Port et al. 1970 |
|
80 mg/kg/d (LOAEL), gavage |
4 wk |
Wistar rats, male |
Reduced weight gain |
Vargova et al. 1993 |
|
20 mg/kg/d (LOAEL), gavage |
4 wk |
Wistar rats, male |
Dose-dependent reduced antibody response |
Vargova et al. 1993 |
|
125 mg/kg/d (LOAEL), 25 mg/kg/d (NOAEL), drinking water |
4 wk |
Wistar rats, male and female |
Significant hyperkeratosis of the stomach; focal gastritis; bodyweight reductions; decreases in blood protein and albumin concentrations; increased kidney weight |
Til et al. 1988 |
|
30 mg/L (0.003 %) (LOAEL), auxiliary spray test |
2 wk |
Humans |
No ACD other than very mild dermatitis observed (2/13 subjects) in formaldehyde- sensitized individuals at this concentration |
Jordan et al. 1979 |
|
300 mg/kg/d (LOAEL), drinking water |
24 mo, but effects observed after several wk |
Wistar rats, male and female |
Mortality as early as 9 d, reduced weight gain and food/water intake |
Tobe et al. 1989 |
|
82 mg/kg/d (LOAEL), drinking water |
24 mo, but effects observed after 1 wk |
Wistar rats, male |
Decreased bodyweight and liquid consumption |
Til et al. 1989 |
|
Subchronic Exposures |
||||
|
150 mg/kg/d (rats) (LOAEL), drinking water. 100 mg/kg/d (dogs) (LOAEL), 50 mg/kg/d (NOAEL), oral in feed |
90 d |
Sprague-Dawley rats, male and female; beagles, male and female |
Reduced weight gain; reduction in water/feed consumption |
Johannsen et al. 1986 |
|
9.4 mg/kg/d (NOAEL), oral in feed |
52 d, gestation days 4-56 |
Beagles, female |
No effect on pregnancy rate, weight gain, length of gestation, or malformation |
Hurni and Ohder 1973 |
|
Dose/Route |
Exposure Duration |
Species |
Effects |
Reference |
|
Chronic Exposures |
||||
|
258 mg/kg/d (LOAEL), drinking water |
32 wk |
Wistar rats, male |
Reduced weight gain; gastric ulceration; papillomas; regenerative mucosa |
Takahashi et al. 1986 |
|
82 mg/kg/d (LOAEL), 15 mg/kg/d (NOAEL), drinking water |
24 mo |
Wistar rats, male |
Reduced body weight; thickened limiting ridge; gastric ulceration; mucosal thickening |
Til et al. 1989 |
|
300 mg/kg/d (severe effects), 50 mg/kg/d (LOAEL), 10 mg/kg/d, (NOAEL), drinking water |
24 mo |
Wistar rats, male and female |
Significant mortality; non-neoplastic gastric lesions; hyperplasia of fundic mucosa; reduced weight gain; lower food and water intake |
Tobe et al. 1989 |
|
(1) 188 mg/kg/d (2) 125 mg/kg/d (3) 63 mg/kg/d (4) 13 mg/kg/d (5) 6 mg/kg/d (6) 1 mg/kg/d |
104 wk |
Sprague-Dawley rats, male and female (n = 50 each) |
Leukemia incidence (male and female) by dose group (1) 22,14% (2) 12,14% (3) 16,8% (4) 10,8% (5) 10,8% (6) 2,4% |
Soffritti et al. 1989 |
|
Drinking water |
|
|
4,3% (control) 10,6% (methanol) |
|
|
(1) 188 mg/kg/d (2) 125 mg/kg/d (3) 63 mg/kg/d (4) 13 mg/kg/d (5) 6 mg/kg/d (6) 1 mg/kg/d |
104 wk |
Sprague-Dawley rats, male and female |
GI tract malignant neoplasias (male and female) (1) 6,0% (2) 2,0% (3) 0,0% (4) 0,0% (5) 0,4% (6) 4,0% |
Soffritti et al. 1989 |
|
Drinking water |
|
|
0% (control) 0% (methanol) |
|
|
313 mg/kg/d |
104 wk |
Sprague-Dawley breeders (n = 36) |
Malignant GI neoplasias (male and female): 2.8% in breeders |
Soffritti et al. 1989 |
|
Drinking water |
|
and their offspring (n = 73) |
15.1% in offspring 0% in controls Leukemias (male and female) 11% in breeders 5.5% in offspring 2.5% in control 5.5% methanol |
|
|
Dose/Route |
Exposure Duration |
Species |
Effects |
Reference |
|
(1) 188 mg/kg/d (2) 125 mg/kg/d (3) 63 mg/kg/d (4) 13 mg/kg/d (5) 6 mg/kg/d (6) 1 mg/kg/d |
104 wk |
Sprague-Dawley rats, male and female (n = 50 each) |
Leukemias (M,F) (1) 22,18% (2) 18,24% (3) 6,20% (4) 16,12% (5) 8,12% (6) 4,6% |
Soffritti et al. 2002 |
|
Drinking water |
|
|
14,6% (control) 11,10% (methanol) |
|
|
(1) 188 mg/kg/d (incidences primarily limited to highest dose group), drinking water |
104 wk |
Sprague-Dawley rats, male and female (n = 50 each) |
Glandular stomach: 12% adenomatous polyp (male only), 0% in control Intestine: 10% malignant tumors (male only), 0% in control |
Soffritti et al. 2002 |
|
(1) 188 mg/kg/d (2) 125 mg/kg/d (3) 63 mg/kg/d (4) 13 mg/kg/d (5) 6 mg/kg/d (6) 1 mg/kg/d |
104 wk |
Sprague-Dawley rats, male (n = 50) |
Testes: interstitial-cell adenoma (1) 18% (2) 24% (3) 20% (4) 12% (5) 12% (6) 6% |
Soffritti et al. 2002 |
|
Drinking water |
|
|
10% (control) 6% (methanol) |
|
One consistent symptom following acute human ingestion to formaldehyde is GI irritation and abdominal pains, with varying degrees of severity. It is thought that the irritation produced by formaldehyde may be related to the precipitation of proteins or the formation of protonated hydroxymethyl amino acid derivatives that occurs in conjunction with high concentrations of formaldehyde exposure (Loomis 1979). Another common observation following acute exposures is that metabolic acidosis occurs as a result of the metabolism of formaldehyde and the subsequent build up of formic acid (Eells et al. 1981).
Short-Term Toxicity (6-30 d)
Marks et al. (1980) found that formaldehyde (in a solution with 12-15% methanol) administered by gavage on days 6-15 of gestation at doses of 185 mg/kg/d resulted in the death of 22 of 34 treated pregnant mice within 18 d. No mortality was observed at a dose of 75 mg/kg/d. However, the authors noted that the methanol fraction may have contributed to the observed mortality. Tobe et al. (1989) observed mortality within the first 9 d of drinking water administration of formaldehyde at 300 mg/kg/d in Wistar rats, but not at 10 or 50 mg/kg/d. Other drinking water (Johannsen et al. 1986; Takahashi et al. 1986) and gavage (Vargova et al. 1993) studies failed to observe mortality in rats exposed to formaldehyde orally at doses as high as 258 mg/kg/d.
Tobe et al. (1989) reported significantly reduced bodyweight gain and water and food intake in Wistar rats evaluated weekly after being exposed to formaldehyde at 300 mg/kg/d (5,000 mg/L) in drinking water. Values for similar end points evaluated for the 10 and 50 mg/kg/d groups (200 and 1,000 mg/L, respectively) were not statistically different than the control group values. Johannsen et al. (1986) reported that rats administered formaldehyde at 150 and 225 mg/kg/d by gavage in a 2-week (wk) pilot study displayed mean body weight reduction proportional to dose, along with reduced food and water consumption. Vargova et al. (1993) found significantly reduced mean body weights relative to controls for rats exposed to 80 mg/kg/d, but not for the 20 and 40 mg/kg/d exposure groups. Body weight was also not reduced in rats exposed for 4 wk to formaldehyde in drinking water at doses as high as 125 mg/kg/d in a study conducted by Til et al. (1988).
Til et al. (1988) did report other effects following subacute oral exposures to formaldehyde. The authors exposed Wistar rats to formaldehyde at 0, 5, 25, and 125 mg/kg/d in drinking water for 4 wk. In the
highest-dose group, the authors observed decreased protein and albumin concentrations in blood plasma and slight increases in the weight of the kidneys relative to controls (female only). However, the most marked observation was significant hyperkeratosis and thickening of the limiting ridge in the forestomach and focal gastritis in the glandular stomach, all observed in the 125 mg/kg/d group. The authors did not observe these effects in the 25 mg/kg/d group and suggested that dose as a no-observed-adverse-effect level (NOAEL) for formaldehyde. This distinct portal-of-entry irritation is consistent with results seen in both shorter-and longer-duration studies of formaldehyde across a variety of species including humans (ATSDR 1999).
Vargova et al. (1993) investigated the potential for formaldehyde to affect the immune system during short-term subacute exposures. Rats were administered formaldehyde by gavage at doses of 0, 20, 40, and 80 mg/kg/d for 28 d. Hematologic and clinical chemistry data revealed some significant differences relative to controls (for example, increased hematocrit, hemoglobin, and erythrocytes, and decreased monocyte and lymphocyte percentages), primarily in the highest-dose group. Increased absolute and relative lymph node weights were observed in the 40- and 80-mg/kg/d groups. Perhaps the most notable finding was dose-dependent reductions in antibody responses (immunoglobulin G [IgG] and M [IgM]) in a hemagglutination assay across all dose groups. Results from several other immunologic assays that were utilized in this study failed to show significant adverse effects.
Subchronic Toxicity (30-180 d)
A small number of studies addressing subchronic oral exposures to formaldehyde were available in the scientific literature. Johannsen et al. (1986) administered formaldehyde to rats and dogs for 90 d via their drinking water and diet, respectively. Sprague-Dawley rats were dosed with 0, 50, 100, and 150 mg/kg/d, and dogs were exposed to formaldehyde through their feed at doses of 0, 50, 75, and 100 mg/kg/d. Compared to controls, treated rats exhibited a dose-dependent decrease in the volume of water consumed (up to 31% in the males from the 150 mg/kg/d group). Significant reductions in body weight gain were also observed for rats of both sexes at 150 mg/kg/d and in male rats at 100 mg/kg/d. For the dogs, reduced food consumption compared to controls was statistically significant above 50 mg/kg/d, although reduced weight gain was only observed at the 100 mg/kg/d dose. For both rats and dogs,
hematologic and clinical chemistry analysis did not demonstrate any significant exposure-related changes from nonexposed controls. Additionally, no differences in the weights of critical organs including kidney, liver, heart, spleen, and thyroid were observed. Another important observation was that gross and histopathologic evaluations did not reveal inflammation of the GI mucosa or lesions in either species, even in the highest-exposure groups. These negative findings were consistent with observations of Vargova et al. (1993), but were in contrast to the Til et al. (1988) findings, which observed signs of GI irritation at similar doses.
Chronic Toxicity (180 d to lifetime)
Systemic Effects
Several chronic-duration studies in which the systemic oral toxicity of formaldehyde was assessed are available in the scientific literature. Some of these studies included end points with shorter-term observations by the authors (for example, increased mortality, reduced weight gain, lower water consumption) that have already been presented and discussed in the acute, short-term, or subchronic sections of this document.
Tobe et al. (1989) conducted a 2-y study in which formaldehyde at 0, 10, 50, and 300 mg/kg/d was administered to male and female Wistar rats in drinking water (0, 200, 1,000, and 5,000 mg/L). The general condition of the rats in the highest-dose group was poor, with reduced weight gain and reduced water and food intake noted by the authors. No significant differences were noted with the lower-dose groups. Mortality, which was observed as early as 9 d into the study, was 100% by the end of the study for both males and females in the highest-dose groups.
A significant observation in the Tobe et al. (1989) study was marked ulceration and tissue changes within the forestomach and glandular stomach of rats of either sex following chronic formaldehyde exposure at the 300 mg/kg/d and 50 mg/kg/d doses. The highest-dose group, necropsied after 12 months (mo) of exposure, showed the highest frequency of effects with all of the rats exhibiting squamous cell hyperplasia (with or without hyperkeratosis) and 10/12 exhibiting basal cell hyperplasia of the forestomach. Effects were also observed in the glandular stomach at this dose, with erosion and ulceration and glandular hyperplasia along the limiting ridge. No effects were observed in the lower-dose groups at the 12-mo necropsy. At the 18-mo and 2-y necropsies, forestomach hyperkeratosis (2/14 rats) was reported for the 50-mg/kg group,
but no effects on the glandular stomach were observed. No lesions of the forestomach or glandular stomach were observed in the 10 mg/kg/d exposure group, and this concentration was viewed by the authors as a NOAEL for formaldehyde with respect to these effects.
Til et al. (1989) conducted a similar 2-y study in which Wistar rats (70 male and 70 female in each dose group) were dosed with 0, 1.2, 15, and 82 mg/kg/d (males) and 0, 1.8, 21, and 109 mg/kg/d (females) via drinking water . Unlike the aforementioned Tobe et al. (1989) study, the general condition of the animals was not significantly affected, even for the highest-dose groups. No differences in mortality were noted relative to controls. However, the high-dose group did exhibit statistically significant decreases in water and food consumption and reduced mean body weights. Absolute decreases in kidney, testes, heart, and liver weights were noted, although they were attributed to the overall reductions in weight gain for the rats.
As with the Tobe et al. (1989) study, one of the notable findings of the Til et al. (1989) study was the observed GI effects following drinking water exposures to formaldehyde. Necropsies performed at weeks 53, 79, and 105 noted pronounced inflammatory changes in the forestomach and glandular stomach of rats in the high-dose group. These effects were noted in both male and female rats. Within the forestomach, histopathologic examination revealed papillary epithelial hyperplasia along with hyperkeratosis along the limiting ridge. In many rats in this dose group, this inflammation was advanced enough to be considered ulceration. In the glandular stomach, almost all rats exhibited chronic atrophic gastritis of varying severity. The affected mucosa was often reduced in width and had extensive lesions and ulceration. Rats in the 15 mg/kg/d (male) and 21 mg/kg/d groups (female) did not exhibit statistically significant gastric effects relative to controls, and these formaldehyde concentrations were considered to be NOAELs for these effects.
Carcinogenic Potential
Human Epidemiology Summary
Because of its commercial importance and because of widespread occupational exposures to formaldehyde, there is a wealth of human epidemiologic studies (over 40 case-control or cohort studies and several meta-analyses) that have assessed chronic inhalation exposures to formaldehyde and the potential for these exposures to result in the devel-
opment of cancer (see ATSDR 1999 and WHO 2002 for a comprehensive review of available studies). No human epidemiologic studies specific to oral cancers associated with formaldehyde exposures were found.
Given the reactivity and water solubility of formaldehyde and the available evidence from laboratory animal inhalation exposures, many epidemiologic studies have focused on upper respiratory tract cancer incidences. With respect to sino-nasal and nasopharyngeal cancers, some recent studies and at least two meta-analyses reported an exposure-response relationship (Blair et al. 1990; Partanen 1993; Hauptmann et al. 2004), although other studies and reviews failed to find such a relationship (Collins et al. 1997; Marsh et al. 2002; Coggon et al. 2003; Pinkerton et al. 2004), and the evidence is considered by some to be equivocal (ATSDR 1999; WHO 2002). Hauptmann et al. (2004) reported an association between increasing risk of nasopharyngeal cancers and highest peak and cumulative exposures (but not for average exposures or duration of exposure). The significant trend was based on a small number (nine) of cancer cases, two of which were from the control group. In contrast, Collins et al. (1997) conducted a meta-analysis of sino-nasal and nasopharyngeal cancers reported in 47 occupational epidemiologic studies involving formaldehyde inhalation. They found that although a few of the studies reported increased incidences of these cancers, the findings from the majority of the studies were negative. After correcting for underreporting of negative study results, a relative risk estimate of 1.8 (95% confidence interval [CI] = 1.4-2.3) for case-control studies and 0.3 (95% CI = 0.1-0.9) for cohort studies was found. The authors concluded that these observations do not support the contention that there is a significant association between formaldehyde inhalation and sino-nasal or nasopharyngeal cancers. Similarly, Coggon et al. (2003) conducted a follow-up evaluation of a cohort of over 14,000 chemical workers who were exposed to formaldehyde by inhalation. The authors did not find an association between formaldehyde exposures and nasopharyngeal cancers and concluded that “overall, the epidemiologic evidence now available indicates that if formaldehyde does cause nasopharyngeal cancers, then the increased risk is small.” However, the International Agency for Research on Cancer (IARC 2004) recently upgraded its classification of formaldehyde from Group 2A to Group 1 (sufficient evidence from experimental animals and humans to conclude it is carcinogenic to humans), based on their opinion that there is sufficient epidemiologic evidence that formaldehyde causes nasopharyngeal cancers in humans. The National Toxicology Program (NTP) classifies formaldehyde as “reasonably anticipated to be a human carcinogen” (NTP 2005).
Although scanty, there is some epidemiologic evidence that formaldehyde could result in lymphohematopoietic cancers and lung cancers. In regard to lung cancer, Coggon et al. (2003) reported increased incidences for their cohort (standardized mortality rate [SMR] of 1.28 for the high-exposure group), but there was no association with duration of exposure, and the authors cautioned that these results need to be further investigated (for example, closer evaluation of confounding factors). Collins et al. (1997) found no increased lung cancer incidences for industrial workers in the available cohorts or in the case-control studies and concluded that the available epidemiologic evidence did not support an association between formaldehyde exposure and lung cancers. Similarly, Hauptmann et al. (2004) conducted a follow-up study of a cohort of over 25,000 industrial workers from 10 U.S. plants and did not find a positive association between formaldehyde exposure and lung cancer risks.
With respect to lymphohematopoietic cancers in this same cohort, Hauptmann et al. (2003) reported relative risks for leukemia of 1.15 (95% CI = 0.4-3.2) and 2.49 (95% CI = 1-6) when average formaldehyde exposures were 0.5-0.9 parts per million (ppm) and > 1 ppm, respectively (larger relative risks were reported when grouping by peak formaldehyde exposure concentrations). However, the leukemia relative risks were not positively associated with cumulative formaldehyde exposure or duration of exposure, and all myeloid tumor types (for example, acute and chronic) were lumped together despite their different etiologies. The authors stated that these results should be viewed with caution because the overall body of evidence for an association between formaldehyde and leukemia is mixed. Pinkerton et al. (2004) also reported an increased risk of myeloid leukemia in association with duration of formaldehyde exposure in their review of a cohort of over 11,000 U.S. garment workers. In contrast, Coggon et al. (2003) did not find increased mortality from leukemia in their cohort, even in evaluating the subset of workers with highest formaldehyde exposure. The need for caution in interpreting non-respiratory tract cancers with formaldehyde is echoed by the World Health Organization (WHO 2002) who states, “Available evidence for these tumors at sites other than the respiratory tract does not, therefore, fulfill traditional criteria of causality (for example, consistency, biologic plausibility) for associations observed in epidemiological studies.” IARC (2004) concluded that there was “strong, but not sufficient evidence for a causal association between leukemia and occupational exposure to formaldehyde,” citing existing uncertainties and inconsistencies among different cohorts.
Discussion of Animal Data
There is a wealth of animal data on formaldehyde carcinogenesis associated with inhalation exposure, and these findings are important to consider in evaluating cancer risks from oral exposures, especially with respect to the assumed mode of action and the types of tissues most likely to be affected. Inhalation of formaldehyde has been shown to induce nasal tumors (squamous cell carcinomas) in rats in several animal bioassays (Swenberg et al. 1980; Albert et al. 1982; Kerns et al. 1983; Monticello et al. 1996). Largely based on this inhalation evidence, formaldehyde is classified by the U.S. Environmental Protection Agency (EPA) as a probable (B1) human carcinogen (IRIS 2004). As mentioned above, IARC recently elevated its rating of formaldehyde and now lists it as a Group 1 carcinogen (IARC 2004). The carcinogenic potential of formaldehyde is supported by its demonstrated cytotoxicity and genotoxicity (Yager et al. 1986; Casanova et al. 1989; Ballarin et al. 1992), the reactivity of the molecule, and its ability to be incorporated into other critical macromolecules (Heck and Casanova 1990). There has been a significant focus in the scientific literature on the mechanism of nasal tumor induction with formaldehyde (CIIT 1999; WHO 2002; Conolly et al. 2003) and the relevancy of rodent tumor data to human health risk assessment (particularly in extrapolating from high to low levels of exposure). Nasal tumors are believed to be largely dependent on cytotoxicity and cell proliferation at the portal-of-entry (that is, nasal epithelium), although mutagenicity mediated through DNA-protein cross-links (DPX) may also be a factor (Schlosser et al. 2003). Inhalation studies have generally observed that exposure concentrations of formaldehyde that were high enough to produce nasal tumors were also high enough to produce nasal lesions and necrosis of the epithelium and that exposure concentrations that were not sufficient to result in these necrotic effects also did not cause nasal tumors (Heck and Casanova 1990; Morgan 1997). Tumors at sites distant from the portal of entry have generally not been demonstrated in inhalation studies with formaldehyde (ATSDR 1999).
Fewer studies have attempted to evaluate the carcinogenicity of formaldehyde following oral ingestion. However, five animal studies are available that provide data pertinent to the setting of guidelines for acceptable concentrations (ACs) of formaldehyde in drinking water.
As described previously, both Tobe et al. (1989) and Til et al. (1989) conducted 2-y exposure studies in which Wistar rats were administered formaldehyde through drinking water. Both of these studies observed inflammation and necrosis in the forestomachs and glandular
stomachs of the rats. However, in both studies, evaluation of multiple organ systems did not find any increased tumor incidence in the formaldehyde-treatment groups relative to controls.
Takahashi et al. (1986) assessed the potential for oral exposures of formaldehyde (single dose group at 258 mg/kg/d) to promote gastric tumors in rats. Forestomach papillomas were observed in 8 of 10 formaldehyde-treated rats, which was statistically significant relative to controls. Til et al. (1989) have questioned whether these benign papillomas, which were not found to be statistically significant during their own study, might actually be characterized as papillary epithelial hyperplasia. It is unclear, however, whether this difference, species variations, or other factors might explain apparent inconsistencies between these animal studies.
There are two studies that reported positive associations between cancer and the ingestion of formaldehyde by rats (Soffritti et al. 1989, 2002). The earlier study involved two separate experiments. The first involved the administration of 0, 1, 6, 13, 63, 125, and 188 mg/kg/d to male and female Sprague-Dawley rats through their drinking water (0, 10, 50, 500, 1,000, 1,500, and 2,500 mg/L, respectively) for a total of 104 wk. In addition to the normal controls, another control group of rats were exposed to drinking water containing 15 mg/L of methanol. In the second experiment from the same Soffritti et al. (1989) study, two groups of male and female breeders were exposed to formaldehyde in drinking water at doses of 0 and 313 mg/kg/d from 25 wk of age for 104 wk. Offspring from these breeders were then exposed to the same concentrations of formaldehyde for 104 wk.
The findings from the Soffritti et al. (1989) study were very different from other similarly designed long-term studies of oral exposure to formaldehyde with respect to the assessment of its carcinogenic potential. Increased incidences of GI tract tumors (both benign and malignant) were observed in the highest-does group (188 mg/kg/d) for male and female rats exposed from 6 wk of age and in both female breeders and offspring when dosed with 313 mg/kg/d. Although the observed incidences were low, the authors noted that no similar tumors were reported in the control groups and that these cancers are very rare for untreated rats in their breeding colony. There were some inconsistencies in GI tumor incidence between breeders and offspring (for example, female breeders dosed with 313 mg/kg/d had no malignant GI tumors, whereas 21% of female offspring were positive at this exposure concentration) observed in the second experiment from this study.
Cancers at other sites were also reported in the Soffritti et al. (1989) study. An increased incidence of leukemias (mostly lymphoblastic leukemias and lymphosarcomas) was observed in both male and female rats exposed to formaldehyde in the first experiment of the study. This effect was most observable at the highest exposure concentration, where leukemias were found in 22% of the male rats and 14% of the female rats compared to only 3-4% of controls. Leukemia incidence for both male and female rats and their offspring were approximately twice as high as controls in the second experiment (Soffritti et al. 1989). Soffritti et al. (2002) followed up with a similar 2-y study of oral formaldehyde exposure to Sprague-Dawley rats through drinking water (0, 10, 50, 500, 1,000, 1,500, and 2,500 mg/L). Estimated dose concentrations in this study were 0, methanol control, and formaldehyde at 1, 6, 13, 63, 125, and 188 mg/kg/d (ATSDR 1999). Fifty male and 50 female rats per group were dosed from 6 wk of age. This study found similar results to the earlier Soffritti et al. study (1989), with some malignant intestinal cancers (adenocarcinomas and leiomyosarcomas) largely limited to the 188 mg/kg/d group (3/50 in male rats). This study also noted adenomatous polyps (6/50 for male rats) and adenocarcinomas (2/50 for female rats) in the glandular stomach in the 188 mg/kg/d group (not reported in the earlier study).
Cancers within hemolymphoreticular tissue (lymphomas or leukemias) were observed in 46% of the male rats and 20% of the female rats at the highest-dose concentration, compared with only 7-8% of the rats in the control group. Other tumors of significance were interstitial cell adenomas within the testis (significant relative to controls at the 63, 125, and 188 mg/kg/d groups) and mammary gland adenocarcinomas (significant relative to controls at the top two doses and in the 13 mg/kg/d group).
Organoleptic Considerations
Although not strictly a health consideration, organoleptic characteristics (for example, taste, odor) can affect the palatability of drinking water and can indirectly contribute to or exacerbate crew dehydration if noticeable enough to discourage crew water consumption. Formaldehyde has a characteristic odor that is frequently described as strong, pungent, and irritating (ACGIH 1994). In water, this odor threshold is approximately 50 mg/L (ATSDR 1999; HSDB 1999). As cited in these same sources, formaldehyde in drinking water can also impart an undesirable taste at this concentration.
Genotoxicity
Formaldehyde is a very reactive molecule that can readily interact with proteins, DNA, RNA, and other critical macromolecules (formaldehyde has an electrophilic carbonyl group that has an affinity for nucleophilic sites on these molecules) (Feron et al. 1991). The genotoxicity of formaldehyde has been observed (although not strictly by the oral-ingestion route) through a number of in vivo (Lam et al. 1985; Chebotarev et al. 1986; Yager et al. 1986; Casanova et al. 1991; Ballarin et al. 1992) and in vitro (Glass et al. 1986; Schmid et al. 1986; Grafstrom et al. 1993; Merk and Speit 1998) studies. In vivo and in vitro studies in which genotoxicity was not observed are also available (DeFlora 1981; Dallas et al. 1992; Vasudeva and Anand 1996). These and other studies have been the focus of several thorough genotoxicity reviews for formaldehyde (Ma and Harris 1988; Heck and Casanova 1990; IARC 1995; ATSDR 1999; WHO 2002). General genotoxicity observations are summarized briefly in the following sections.
In Vitro Assays
The genotoxicity of formaldehyde has been observed in many, but not all, bacterial and mammalian in vitro assays (IARC1995; WHO 2002). Positive mutagenicity with Salmonella typhimurium and Escherichia coli has been reported for formaldehyde (Donovan et al. 1983; Connor et al. 1985; Glass et al. 1986) in both the presence and absence of metabolic activation. With cultured human cell lines (for example, bronchial fibroblast, lymphocytes, and tracheal epithelial cells) genotoxic effects ranging from chromosomal aberrations, sister chromatid exchange, and DNA damage have been observed (Kreiger and Garry 1983; Schmid et al. 1986; Dresp and Bauchinger 1988). Similar results have been reported in cell lines from other mammalian test species (Miller and Costa 1989; Grafstrom et al. 1993).
In Vivo Observations
Although no genotoxicity studies involving oral exposures to formaldehyde are available, there are a number of in vivo inhalation exposure studies (in both humans and rodents) that suggest formaldehyde is genotoxic. Other negative studies are also available (Dallas 1992;
Vasudeva and Anand 1996). With humans, positive genotoxic effects include lymphocyte chromosomal aberrations (Chebotarev et al. 1986) and sister chromatid exchange (Yager et al. 1986), along with increases in micronuclei formation in cells of the nasal passage. As discussed previously, genotoxicity at sites distant from the portal of entry is unlikely. This conclusion is generally supported by available genotoxicity studies (Klingerman et al. 1984; Dallas 1992; ATSDR 1999; WHO 2002).
There has been a particular research focus placed on the significance of DNA-protein crosslinks. These complexes between DNA bases and proteins bound by crosslinks are formed in response to formaldehyde exposure (Swenberg et al. 1980). It is postulated that these crosslinks could result in mutations and/or chromosomal aberrations if not repaired prior to cell replication (Morgan 1997; Klaassen 2001) or that repair and DNA regeneration could promote cell proliferation (Heck and Casanova 1990). This potential is heightened by the observation that these crosslinks are relatively stable and can be formed following formaldehyde exposures that are not cytotoxic (Merk and Speit 1998). However, there is still some debate in the literature as to whether genotoxic expression through DNA-protein crosslinks is the exact mechanism of formaldehyde carcinogenesis.
Reproductive and Developmental Toxicity
The reactivity of formaldehyde and the ability of the body to metabolize it significantly limit the potential for formaldehyde exposure to result in adverse reproductive or developmental effects (Collins et al. 2001). Several publications have reviewed the human epidemiologic data on reproductive and developmental effects of formaldehyde (for example, rates of spontaneous abortion, birth defects, worker infertility, etc.) and concluded that these types of effects are unlikely with occupational exposures to formaldehyde (ATSDR 1999; Collins et al. 2001; WHO 2002).
There are a number of published animal studies that have assessed the reproductive and/or developmental toxicity of formaldehyde through a variety of exposure routes. Collins et al. (2001) conducted a thorough review of these animal studies and concluded that they generally did not report positive reproductive or developmental effects in association with formaldehyde exposure. In briefly describing those studies here, a focus was placed on summarizing those exposures most relevant to SWEG development. Hurni and Ohder (1973) evaluated the reproductive toxicity
of formaldehyde in female beagles exposed to formaldehyde at 9.4 mg/kg/d in their food on days 4-56 after mating. When compared to controls, no effects on pregnancy rates, length of gestation, litter size, or other reproductive end points were noted. Also, no internal or skeletal teratogenic anomalies were observed in the pups born to exposed females. In another study, Marks et al. (1980) exposed pregnant mice to formaldehyde orally (gavage) on days 6-15 of gestation. Formaldehyde doses were as high as 185 mg/kg/d. Despite the gavage exposures and significant observed mortality (23/34) to the dams in the highest-dose group, necropsy revealed no statistically significant teratogenic effects in the fetuses when compared to controls. Similarly, Della Porta et al. (1970) observed no malformations in 124 pups from female rats exposed to formaldehyde through drinking water at 0 or 1% hexamethylenetetramine over 2 wk of exposure.
One inhalation study that did observe reproductive effects with formaldehyde was a Russian study (Guseva 1972) in which groups of male rats were exposed to formaldehyde by inhalation for 4 h/d, 5 d/wk for 6 mo. The formaldehyde concentrations were 0, 0.1, and 0.2 ppm. After mating with unexposed females, reproductive effects were assessed. The males in the highest-exposure group exhibited a significant decrease is testicular DNA, although there were no observed effects on the fetus (abnormalities in fetal weight, litter size, incidence of birth defects, etc.). There are also several Russian studies that have suggested that there is a relationship between formaldehyde exposure and various developmental effects in rats (Gofmekler 1968; Gofmekler et al. 1968; Pushkina et al. 1968). In these studies, rats were exposed to formaldehyde 24 h/d by inhalation for 10-15 d, at concentrations of 0, 0.01, and 0.83 ppm. These effects included increases in fetal body weight, a reduced number of fetuses per litter, reduced nucleic acid levels in fetuses, and fetal histopathologic changes. Specific study details have been found to be lacking or inconsistent, and these results have not been duplicated in other studies (Collins et al. 2001). They are also inconsistent with the findings of Saillenfait et al. (1989) who evaluated Sprague-Dawley rats exposed to up to 40 ppm on gestations days 6-20 and observed no changes in a number of reproductive variables (for example, number of resorptions, implantation), and they are inconsistent with the work of Martin (1990) who evaluated a wide variety of reproductive and developmental end points and observed no effects in Sprague-Dawley rats exposed to formaldehyde at concentrations as high as 10 ppm (6 h/d on gestation days 6-15).
Allergic Contact Dermatitis
Sufficient exposure to formaldehyde can elicit allergic contact dermatitis (ACD), and patch test results suggest that approximately 1-4% of individuals may have ACD from exposure (Marks et al. 1995). These reactions may be exhibited as simple drying and reddening of the skin to perifolicular dermatitis and edema (Loomis 1979). There are a tremendous number of studies that have demonstrated that closed-patch testing can result in ACD following formaldehyde exposure (see ATSDR 1999). Many of these studies are designed to evaluate relatively high concentrations of formaldehyde (that is, 1-2%), concentrations that have been shown to result in irritation in the general population (Trattner et al. 1998; WHO 2002). These studies do not provide useful information on water concentrations likely to produce a response in individuals with ACD.
One study (Jordan et al. 1979) provided particularly useful information on the potential for low-level exposure to formaldehyde in water to elicit ACD. In this human study, nine individuals with ACD consented to continuous closed-patch testing with formaldehyde at concentrations of 1, 30, 60, and 100 ppm (0.003-0.01%). The testing was continued for a total of 168 h, with midstudy evaluation at 72 and 120 h. The same testing regime was followed with four non-ACD individuals, and these subjects failed to exhibit any ACD response, even to the highest concentration tested. For the individuals with ACD, a concentration-dependent response to formaldehyde was observed, with 6/9 subjects responding at 100 ppm, 5/9 responding at 60 ppm, and 4/9 responding at 30 ppm by the end of the study. The authors noted that the reaction times and responses seen with the 100 ppm exposure group clearly indicated that 100 ppm was sufficient to produce ACD. However, with the 30 ppm exposure group, the intensity of the response lessened with time and there was some question as to the significance of the patch test findings, especially given that closed-patch testing is extremely rigorous. To address this issue, the authors also performed repeated axillary spray tests on 13 subjects with ACD. Solutions of formaldehyde at 30 ppm were sprayed onto the axilla twice a day for 2 wk. This type of experimental design is likely to be more applicable than the closed-patch testing to on-orbit exposures to formaldehyde in water. With the exception of two individuals, no response was observed in this testing. The two individuals who might be characterized as responders only displayed very mild perifolicular dermatitis after 2 wk of testing. The subjects reacting to formaldehyde at 30 ppm in the closed-patch testing did not respond to this same concentra-
tion of formaldehyde in the axillary spray testing. The authors concluded that contact with formaldehyde below 30 ppm can be tolerated without notable response (even by individuals with ACD) for extended-exposure durations (Jordan et al. 1979).
Spaceflight Effects
As discussed previously, human oral exposure to formaldehyde has been shown to cause adverse cardiac effects ranging from sinus tachycardia, reduced blood pressure, and even cardiac arrest. However, these effects have only been observed at acute doses (200-600 mg/kg/d) during accidental or suicidal poisoning incidences described previously. These are not expected to be critical effects of formaldehyde that are relevant to exposures which could foreseeably occur in the spacecraft environment. With respect to hematologic effects, only one of the reviewed acute poisoning cases observed any effect (intravascular coagulopathy), and numerous reviewed animal studies of various durations generally did not report any statistically significant hematologic effects.
LIMITS SET BY OTHER ORGANIZATIONS
In order to provide context for the development of SWEGs for formaldehyde, an attempt was made to summarize health-based exposure limits for formaldehyde in drinking water that have been adopted or published by various organizations external to NASA (Table 7-3). These organizations include federal agencies such as EPA and the Agency for Toxic Substances and Disease Registry (ATSDR), as well as state environmental agencies and other organizations that may have their own drinking water quality standards. Certain exposure assumptions (for example, drinking water intake rates), assumed receptors (for example, child, adult), and policy decisions (for example, target cancer risk levels) are inherent to some of the drinking water standards provided in Table 7-3, in accordance with their intended application. Because these exposure assumptions differ, any differences in allowable concentrations among the various groups may be attributable to factors other than just the toxicity factor that is utilized. In cases where a toxicity value (for example, EPA’s reference dose [RfD]) was available, standard drinking water risk assessment assumptions for the general population (70 kg body weight, 2 L/d ingestion) were used to derive a water equivalent concentration.
TABLE 7-3 Standards and Guidelines for Formaldehyde Set by Other Organizations
EPA References
No maximum contaminant level (MCL) has been established by EPA for formaldehyde in drinking water. However, nonenforceable
health-effects guideline values have been calculated for formaldehyde by the EPA Office of Water. These guidelines are based on the oral RfD and are calculated for different exposure durations (1 d, 10 d, lifetime). Although the guidelines are one of the few sets of available guidelines that include consideration for shorter-duration exposures, they are not completely applicable to the SWEG process, because they are not specifically based on short-term toxicity studies and are intended to address sensitive childhood exposures.
The EPA has established an oral RfD for formaldehyde for use in evaluating its chronic noncancer health risks. An RfD is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of adverse effects over a lifetime (IRIS 2004). The oral RfD for formaldehyde is 0.2 mg/kg/d and was last revised on EPA’s Integrated Risk Information System (IRIS) in 1990. The basis for the RfD is the Til et al. (1989) 2-y study that observed adverse noncancer effects following oral exposure to formaldehyde in drinking water. Administered formaldehyde doses were 0, 1.2, 15, and 82 mg/kg/d for males and 0, 1.8, 21, and 109 mg/kg/d for females. At the highest dose level in both sexes, significant changes in the histopathology of the forestomach and glandular stomach were noted. These effects were evidenced as papillary epithelial hyperplasia accompanied by erosions/ulceration in the forestomach and chronic gastritis in the glandular stomach. These effects were not observed in the lower-exposure groups, and a NOAEL of 15 mg/kg/d was used as the basis for the RfD. A total uncertainty factor (UF) of 100 was applied to account for interspecies (10) and intraspecies differences (10).
ATSDR MRLs
In its 1999 Toxicologic Profile for Formaldehyde, ATSDR established minimal risk levels (MRLs) for formaldehyde. An oral MRL is a daily exposure dose for the general population (including sensitive receptors) that is expected to be without appreciable risk of adverse noncancer health effects. ATSDR has provided intermediate duration (15-364 d) and chronic duration (365 d to lifetime) MRLs for formaldehyde. An acute-duration MRL was not derived because of an overall lack of suitable dose-response data for these types of exposures to formaldehyde (ATSDR 1999).
The intermediate-duration MRL is based on the 4-wk Wistar rat
study conducted by Til et al. (1988). Male and female rats were administered formaldehyde at doses of 0, 5, 25, and 125 mg/kg/d in drinking water. In the highest-dose group, exposed rats exhibited decreased food and water intake, along with slight changes is blood chemistry. However, the most marked effects were seen in the GI tract, with significant thickening of the limiting ridge in the forestomach along with hyperkeratosis and mucosal lesions. Focal atrophic gastritis was also observed in the glandular stomach. ATSDR utilized the NOAEL from this study (25 mg/kg/d) and applied a UF of 10 for extrapolation from animals to humans and another factor of 10 for human variability (final intermediate duration MRL of 0.3 mg/kg/d).
Consistent with the EPA oral RfD, the chronic-duration ATSDR MRL for formaldehyde is based on the results from Til et al. (1989). This 2-y drinking water study, which observed significant histopathologic changes in the rat forestomach and glandular stomach, has been described previously in this document. In deriving the MRL, ATSDR used the NOAEL of 82 mg/kg/d and applied a combined UF of 100 (10 for human variability and 10 for extrapolation from animals to humans). This resulted in a final chronic-duration MRL of 0.2 mg/kg/d.
Applying drinking water risk assessment assumptions for the general population (70 kg body weight, 2 L/d ingestion), these MRLs for intermediate- and chronic-duration exposure to formaldehyde would correspond to drinking water equivalent concentrations of 10 mg/L and 7 mg/L, respectively.
State Approaches
Most states do not derive their own toxicity factors. Instead, they typically have a hierarchy of recognized sources of toxicity information (for example, EPA RfD, ATSDR MRL) that are utilized. These toxicity factors are applied in risk calculations that also incorporate exposure assumptions and policy decisions (for example, evaluating a child versus an adult receptor) in the derivation of drinking water standards, action levels, or health-based guidelines. As can be seen from those examples provided in Table 7-3, state drinking water guidelines for formaldehyde can vary significantly (0.03-6 mg/L). Some of this variation can be explained solely by the addition of relative source contribution terms (often a factor of 5), which lower the allowable concentration of formaldehyde in water to account for non-drinking water exposures. California, which has an action level of 0.1 mg/L for formaldehyde in drinking water, used
the Til et al. (1989) NOAEL but applied an additional UF (as compared to EPA or ATSDR) of 10 to account for inadequacies in the toxicity database. The action level is also reflective of a relative source contribution of 20% in drinking water (that is, lowering the action level by a factor of 5). California raised this action level in 2000, having previously used an action level for formaldehyde of 0.03 mg/L. These observations may explain the low drinking water guidelines listed for New Jersey (0.1 mg/L) and Maine (0.03 mg/L), because it is not uncommon for other states to replicate California’s approaches in developing their own guidelines. It should be noted that all state and federal agencies appeared to use the systemic (noncancer) toxicity of formaldehyde as the basis for decision making.
RATIONALE FOR SWEGS
In the next section of this SWEG document, the justification for the establishment of formaldehyde SWEGs for 1-, 10-, 100-, and 1,000-d durations (as shown in Table 7-4) is provided. ACs for each end point are summarized in Table 7-5. To a significant degree, critical toxicity studies that formed the basis for many of the state and federal drinking water guidelines discussed will also play a role in SWEG development. However, because the target population is not the same for SWEGs and state and federal drinking water standards, it should not be surprising that the guidelines will differ as well.
Several studies involving gavage exposures to formaldehyde were not considered in SWEG development. Although data from gavage studies can often be utilized for toxicologic purposes, with formaldehyde it is especially critical that the route of administration match up with conditions that are most relevant to drinking water exposures (that is, sustained, relatively low-level exposures). Gavage exposures may increase
TABLE 7-4 Spacecraft Water Exposure Guidelines for Formaldehyde
|
Duration |
Concentration (mg/L) |
Target Toxicity |
|
1 d |
20 |
Gastric irritation; allergic contact dermatitis |
|
10 d |
20 |
Gastric irritation; allergic contact dermatitis |
|
100 d |
12 |
Gastric irritation; allergic contact dermatitis |
|
1,000 d |
12 |
Gastric irritation; allergic contact dermatitis |
TABLE 7-5 Acceptable Concentrations (ACs)
|
End Point, Exposure Data, Reference |
Species |
Uncertainty Factor |
ACs (mg/L) |
|||||||
|
NOAEL |
Exposure Time |
Species |
Space-flight |
Inter-Individual |
1 d |
10 d |
100 d |
1,000 d |
||
|
Gastric Irritation |
||||||||||
|
25 mg/kg/d (NOAEL) 4- wk drinking water study (Til et al. 1988) |
Wistar rats |
1 |
1 |
10 |
1 |
3 |
20 |
20 |
—a |
— |
|
15 mg/kg/d (NOAEL) 2-y drinking water study (Til et al. 1989) |
Wistar rats |
1 |
1 |
10 |
1 |
3 |
— |
— |
12 |
12 |
|
Allergic Contact Dermatitis |
||||||||||
|
30 mg/L (LOAEL)b 2-wk axillary spray test (Jordan et al. 1979) |
Human |
1 |
1 |
1 |
1 |
1 |
30 |
30 |
30 |
30 |
|
Organoleptic Concerns |
||||||||||
|
50 mg/L taste and odor threshold (HSDB 1999) |
Human |
1 |
1 |
1 |
1 |
1 |
— |
50 |
50 |
50 |
|
SWEG |
20 |
20 |
12 |
12 |
||||||
|
a—, not applicable bIn this study, a very mild response was noted in 2 of 13 susceptible individuals. The authors concluded that concentrations below 30 mg/L should not cause notable ACD effects, even for susceptible individuals. A much higher LOAEL/NOAEL would be more appropriate for the 96-99% of the population that does not have ACD. |
||||||||||
the potential for formaldehyde to remain unmetabolized and circulate through the bloodstream in a manner that would not occur following administration of equivalent daily doses in drinking water.
Irritation
Studies from both humans and laboratory animals have shown that irritation and other adverse GI effects can occur with sufficient oral exposure to formaldehyde. Although no designed studies were found that dealt specifically with very short-term exposures (1-2 d) to formaldehyde, observations from longer-duration studies suggest that this is a credible adverse effect to be considered in AC development.
The AC for this irritation is based on the NOAEL (25 mg/kg/d) for the Til et al. (1988) 4-wk administration of formaldehyde to rats in drinking water. At the next highest dose level (125 mg/kg/d), significant thickening of the limiting ridge and hyperkeratosis in the forestomach, along with gastritis in the glandular stomach were observed. A UF of 10 was applied to account for necessary species extrapolation, and a standard drinking water rate (2.8 L/d) and body weight (70 kg) for astronauts was assumed in calculating the AC. It is not possible to ascertain exactly how long an exposure was necessary to result in the observed gastric effects. However, this and other studies (Til et al. 1989; Tobe et al. 1989), have reported reduced food and drinking water intake (proceeding observed GI effects) in formaldehyde-exposed rats as early as the first week of exposure. Although a single day of exposure may not be sufficient to produce the marked GI changes observed in these animal studies, the establishment of an AC for this end point is supported by the potential for milder gastric irritation.
With inhalation exposures, some variability in human response to the irritant effects of formaldehyde has been shown. Individual sensitivity may be partially related to observed genetic polymorphisms which influence the activity of FDH/ADH3, the key enzyme involved in the rapid metabolism and detoxification of formaldehyde (Hedberg 2001). Given that a range of irritant responses is likely with drinking water exposures to formaldehyde, and considering that this type of sensitivity is not evaluated in astronaut health screening, an additional UF of 3 was applied in calculating the AC to account for variability in individual response.
In regard to the 100- and 1,000-d ACs, Til et al. (1989) conducted a 2-y drinking water study where formaldehyde was administered to male and female rats at various doses. At the highest doses (82 mg/kg/d in males, 109 mg/kg/d in females), significant and marked GI effects were observed relative to controls. Effects were observed during the 53-, 79-, and 105-wk autopsy evaluations. These effects consisted of ulceration and hyperkeratosis in the forestomach (especially along the limiting ridge) and focal gastritis and significant mucosal inflammation in the glandular stomach. A NOAEL of 15 mg/kg/d was noted for these effects. These results were consistent with the findings of Til et al. (1988), who conducted a similar study over a 4-wk exposure period. A UF of 10 was applied to account for species extrapolation, along with an additional factor of 3 to address variations in individual response to formaldehyde. A standard drinking water rate (2.8 L/d) and body weight (70 kg) for astronauts was assumed in calculating the AC.
The rationale for applying the results of a chronic study to the 100-d SWEG is that there is sufficient evidence that the gastric effects observed in the chronic study could occur over a shorter timeframe. Til et al. (1988) observed the same gastric changes in their 4-wk study, at very similar doses (125 mg/kg/d as compared to 82 and 109 mg/kg/d in the chronic study). Also, Til et al. (1989) observed significantly reduced weight gain, water intake, and food intake throughout much of their study (especially with the male rats), which is suggestive that there were treatment-related effects relevant to the 100-d exposure timeframe.
Allergic Contact Dermatitis
Jordan et al. (1979) evaluated low-level exposure to formaldehyde with respect to the potential for elicitation of ACD. Among other tests, the authors performed repeated axillary spray tests on 13 human volunteers with ACD. Solutions of 30 ppm (0.003%) formaldehyde were sprayed onto the axilla twice a day for 2 wk. This type of experimental design is likely to be more applicable than closed patch testing to on-orbit exposures to formaldehyde in water. With the exception of two in-
dividuals, no response was observed in this testing. The two individuals who might be characterized as responders only displayed very mild perifolicular dermatitis after 2 wk of testing. The authors concluded that formaldehyde concentrations below 30 ppm can be tolerated without notable response (even by individuals with ACD) for extended exposure durations (Jordan et al. 1979).
In developing ACs for ACD, the 30 mg/L threshold was taken as the starting concentration that will be protective of responsive individuals. Adjustment to estimate a NOAEL was not accomplished. With ACD, it can be difficult, if not impossible, to establish an AC that will protect all individuals (Loomis 1979). For example, one highly responsive individual was reported to elicit an ACD response to formaldehyde at concentrations in water as low as 0.2 ppm (Horsfall 1934). However, contact with water containing less than 30 mg/L of formaldehyde is unlikely to result in any notable ACD response, even for individuals who already have ACD (WHO 2002). Since the Jordan et al. results are based on a human study of individuals with ACD, no adjustment was necessary for species extrapolation or to ensure protection of a susceptible population. Also, accounting for the small number of volunteers is not considered necessary given the populations studied and the mildness of the potential response to this low concentration of formaldehyde.
There is no evidence in the literature to suggest that low-level exposures to formaldehyde in water will cause ACD in crew members who do not already have ACD. Also, given the ubiquitous ground-based use of formaldehyde (for example, use in consumer products, laboratories), it is likely that crew members would have experienced more-significant exposures to formaldehyde prior to flight. With respect to the 100-d and 1,000-d ACs for ACD, it is probable that any ACD response would have been experienced within the timeframe (2-wk study) observed by Jordan et al., and no adjustment was made in setting the 100- or 1,000-d ACs. A significantly higher AC for this end point would be acceptable for the vast majority (96-99%) of the population (Marks et al. 1995) that does not already have contact dermatitis from exposure.
All timeframe ACs = 30 mg/L.
Carcinogenicity
Interpretation of the Soffritti et al.’s (1989, 2002) cancer findings for formaldehyde in terms of their applicability to SWEG derivation is complex. First, two chronic duration animal studies (Til et al. 1989; Tobe
et al. 1989) did not report increased tumor incidence in the GI tract or other evaluated organ systems. As discussed previously, Soffritti et al. (1989, 2002) reported both GI tumors and cancers at other sites distant from the portal of entry. With respect to observed GI tumors, it is biologically plausible that sufficient formaldehyde exposure could result in cancers at the portal of entry (through incorporation of formaldehyde into critical macromolecules and tissue damage and cell proliferation at the point of contact). However, given the rapid metabolism of formaldehyde, it is likely that a threshold would exist below which formaldehyde ingestion would not pose a credible cancer risk (CIIT 1999). Additionally, both Soffritti et al. studies showed a relatively low incidence of treatment-related GI tumors, and these tumors were generally limited to the high-exposure groups (188 and 313 mg/kg/d). Consistent with the understood mechanism of action of formaldehyde in the development of tumors in association with inhalation exposures, oral portal-of-entry tumors would likely be dependent on cytotoxicity, hyperplasia, and regenerative cell proliferation (CIIT 1999; WHO 2002). Even when considering the possibility of a mutagenic mechanism of action (expressed through DNA-protein crosslink formation), cancer risks associated with low levels of formaldehyde exposure would be much lower than those based on extrapolations from doses sufficient to evidence cytotoxicity and cell proliferation (Schlosser et al. 2003). Overall, these studies suggest that formaldehyde exposures that do not result in GI irritation or damage are also unlikely to represent a concern for GI cancers. ACs were not established for GI cancers based on this assessment. This approach is consistent with the WHO (2002) conclusion that the “lack of evidence for the potential carcinogenicity of ingested formaldehyde precludes an analysis of exposure–response for this effect.”
With respect to the Soffritti et al. observations of tumors at sites distant from the portal of entry (that is, leukemias, mammary gland adenocarcinomas, testicular adenomas), there is uncertainty as to their applicability to SWEG development. The Soffritti et al. findings contradict other drinking water studies (Til et al. 1989; Tobe et al. 1989) in which these types of tumors were not observed, and are inconsistent with the lack of increased concentrations of formaldehyde in the blood (Heck et al. 1985) and the general absence of tumors at sites other than the portal of entry with inhalation exposures to formaldehyde. The findings from the Soffritti et al. (1989) study were reviewed by WHO (2002) and IRIS (2004) but were not utilized in development of oral-cancer risk estimates by these organizations. Similarly, ACs for these cancer end points were not developed in this document.
REFERENCES
ACGIH (American Conference of Governmental Industrials Hygienists). 1994. Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th Ed., Vol. I. Cincinatti, OH: ACGIH.
Albert, R.E., A.R. Sellakumar, S. Laskin, M. Kuschner, N. Nelson, and C.A. Snyder. 1982. Gaseous formaldehyde and hydrogen chloride induction of nasal cancer in the rat. J. Natl. Cancer Inst. 68:597-603.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Formaldehyde. Prepared by Syracuse Research Corporation, July 1999. Agency for Toxic Substances and Disease Registry, Atlanta, GA.
Ballarin, C., F. Sarto, L. Giacomelli, G.B. Bartolucci, and E. Clonfero. 1992. Micronucleated cells in nasal mucosa of formaldehyde-exposed workers. Mutat. Res. 280:1-7.
Blair, A., R. Saracci, P.A. Stewart, R.B. Hayes, and C. Shy. 1990. Epidemiologic evidence on the relationship between formaldehyde and cancer. Scand. J. Work Environ. Health 16:381-393.
Bolt, H.M. 1987. Experimental toxicology of formaldehyde. J. Cancer Res. Clin. Oncol. 113:305-309.
Burkhart, K.K., K.W. Kulig, and K.E. McMartin. 1990. Formate levels following formalin ingestion. Vet. Hum. Toxicol. 32(2):135-137.
Buss, J., K. Kuschinsky, H. Kewitz, and W. Koransky. 1964. Enteric resorption of formaldehyde. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 247:380-381 (as cited in IARC 1995).
Casanova, M., D.F. Deyo, and H.D. Heck. 1989. Covalent binding of inhaled formaldehyde to DNA in the nasal mucosa of Fischer 344 rats: Analysis of formaldehyde and DNA by high-performance liquid chromatography and provisional pharmacokinetic interpretation. Fundam. Appl. Toxicol. 12:319-417.
Casanova, M., K.T. Morgan, W.H. Steinhagen, J.I. Everitt, J.A. Popp, H.D. Heck. 1991. Covalent binding of inhaled formaldehyde to DNA in the respiratory tract of Rhesus monkeys: Pharmacokinetics, rat-to-monkey interspecies scaling and extrapolation to man. Fundam. Appl. Toxicol. 17:409-428.
CDHS (California Department of Health Services). 2003. Drinking Water Notification Levels: An Overview [online]. Available: www.dhs.ca.gov/ps/ddwem/chemicals/al/notificationoverview.pdf [accessed March 23, 2005].
Chebotarev, A.N., N.V. Titenko, T.G. Selezneva, V.N. Fomenko, and L.M. Katosova. 1986. Comparison of the chromosome aberrations, sister chromatid exchanges and unscheduled DNA synthesis in the evaluation of the mutagenicity of environmental factors [in Russian]. Tsitol. Genet. 20:21-26.
CIIT (Chemical Industry Institute of Toxicology). 1999. Formaldehyde: hazard characterization and dose-response assessment for carcinogenicity by the
route of inhalation. Chemical Industry Institute of Toxicology, Research Triangle Park, NC.
Coggon, D., E.C. Harris, J. Poole, and K.T. Palmer. 2003. Extended follow-up of a cohort of British chemical workers exposed to formaldehyde. J. Natl. Cancer Inst. 95(21):1608-1615.
Collins, J.J., J.F. Acquavella, and N.A. Esmen. 1997. An updated meta-analysis of formaldehyde exposure and upper respiratory tract cancers. J. Occup. Environ. Med. 39(7):639-651.
Collins, J.J., R. Ness, R.W. Tyl, N. Krivanet, and N.A. Esmen. 2001. A review of adverse pregnancy outcomes and formaldehyde exposure in human and animal studies. Regul. Toxicol. Pharmacol. 34(10):17-34.
Connor, T.H., J.B. Ward, and M.S. Legator. 1985. Absence of mutagenicity in the urine of autopsy service workers exposed to formaldehyde: factors influencing mutagenicity testing in urine. Int. Arch. Occup. Environ. Health 56:225-237.
Conolly, R. J. Kimbell, D. Janszen, P.M. Schlosser, D. Kalisak, J. Preston, and F.J. Miller. 2003. Biologically motivated computational modeling of formaldehyde carcinogenicity in the F344 rat. Toxicol. Sci. 75:432-447.
Dahl, A.R., and W.M. Hadley. 1983. Formaldehyde production promoted by rat nasal cytochrome P-450-dependent monooxygenases with nasal decongestants, essences, solvents, air pollutants, nicotine, and cocaine as substrates. Toxicol. Appl. Pharmacol. 67:200-205
Dallas, C.E., M. Scott, and J. Ward. 1992. Cytogenetic analysis of pulmonary lavage and bone marrow cells of rats after repeated formaldehyde inhalation. J. Appl. Toxicol. 12:199-203.
DeFlora, S. 1981. Study of 106 organic and inorganic compounds in the Salmonella/microsome test. Carcinogenesis 2:283-298.
Della Porta, G., J.R. Cabral. and G. Parmiani. 1970. Transplacental toxicity and carcinogenesis studies in rats with contragestational agents. Contraception 13:571-582.
Donovan, S.M., D.F. Krahn, J.A. Stewart, and A.M. Sarrif. 1983. Mutagenic activities of formaldehyde (HCHO) and hexamethylphosphoramide (HMPA) in reverse and forward Salmonella typhimurium mutation assays. Environ. Mutagen. 5:476.
Dresp, J., and M. Bauchinger. 1988. Direct analysis of the clastogenic effect of formaldehyde in unstimulated human lymphocytes by means of the premature chromosome condensation technique. Mutat. Res. 204:349-352.
Eells, J.T., K.E. McMartin, K. Black, V. Virayotha, R.H. Tisdell, and T.R Tephly. 1981. Formaldehyde poisoning: Rapid metabolism to formic acid. JAMA 246:1237-1238.
EPA (U.S. Environmental Protection Agency). 2002. 2002 Edition of the Drinking Water Standards and Health Advisories. EPA 822-R-02-035. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
Feron, V.J., H.P. Til, and F. de Vrijer. 1991. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 259:363-385.
FSTRAC (Federal-State Toxicology and Risk Analysis Committee). 1999. Summary of State and Federal Drinking Water Standards and Guidelines 1998-1999. Federal-State Toxicology and Risk Analysis Committee, U.S. Environmental Protection Agency, Washington, DC.
Galli, C.L., C. Ragusa, and P. Resmini. 1983. Toxicological evaluation in rats and mice of the ingestion of a cheese made from milk with added formaldehyde. Food Chem. Toxicol. 21:313-317.
Glass, L.R., T.H. Connor, J.C. Theiss, C.E. Dallas, and T.S. Matney. 1986. Genotoxic evaluation of the offgassing products of particle board. Toxicol. Lett. 31:75-83.
Gofmekler, V.A. 1968. The embryotrophic action of benzene and formaldehyde in experimental administration by inhalation. Gig. Sanit. 33(3):12-16.
Gofmekler, V.A., N.N. Pushkina, and G.N. Klevtsova. 1968. Various biochemical shifts during a study of the embryotrophic effect of benzene and formaldehyde, based on data on morphological studies. Gig. Sanit. 33(7):96-98.
Grafstrom, R.C., I.C. Hsu, and C.C. Harris. 1993. Mutagenicity of formaldehyde in Chinese hamster lung fibroblasts: synergy with ionizing radiation and N-nitroso-N-methylurea. Chem. Biol. Interact 86:41-49.
Guseva, V. 1972. Gonadotrophic effect of formaldehyde on male rats during its simultaneous introduction with air and water. Gig. Sanit. 37:102-103.
Hauptmann, M., J. Lubin, P. Stewart, R.B. Hayes, and A. Blair. 2003. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J. Natl. Cancer Inst. 95(21):1615-1623.
Hauptmann, M, J. Lubin, P. Stewart, R.B. Hayes, and A. Blair. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am. J. Epidemiol. 159(12):1117-1130.
Heck, H.D., and M. Cassanova. 1990. Formaldehyde toxicity—new understandings. Crit. Rev. Toxicol. 21(6):397-426.
Heck, H.D., M. Casanova-Schmitz, and P.B. Dodd. 1985. Formaldehyde (CH20) concentrations in the blood of humans and Fischer-344 rats exposed under controlled conditions. Am. Ind. Hyg. Assoc. J. 46:1-3.
Hedberg, J.J., M. Backlund, P. Stromberg, S. Lonn, M.L. Dahl, M. Ingelman-Sundberg, and J.O. Hoog. 2001. Functional polymorphism in the alcohol dehydrogenase 3 (ADH3) promoter. Pharmacogenetics 11(9):815-824.
Horsfall, F. 1934. Formaldehyde hypersensitiveness: An experimental study. J. Immunol. 27:571-581.
HSDB (Hazardous Substances Data Bank). 1999. National Library of Medicine, National Toxicology Information Program, Bethesda, MD.
Hurni, H., and H. Ohder. 1973. Reproduction study with formaldehyde and hexamethylenetetramine in beagle dogs. Food Cosmet. Toxicol. 11:459-462.
IARC (International Agency for Research on Cancer). 1995. IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans. Vol. 62: Wood dusts and formaldehyde. International Agency for Research on Cancer, World Health Organization, Lyon, France.
IARC (International Agency for Research on Cancer). 2004. Formaldehyde (Group 1). IARC Monographs Programme on the Evaluation of Carcinogenic Risks to Humans, Vol. 88. International Agency for Research on Cancer, World Health Organization, Lyon, France.
IRIS (Integrated Risk Information System). 2004. Formaldehyde. Integrated Risk Information System, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/iris/subst/0419.htm [accessed March 23, 2005].
Johannsen, F.R., G.J. Levinskas, and A.S. Tegeris. 1986. Effects of formaldehyde in the rat and dog following oral exposure. Toxicol. Lett. 30:1-6.
Jordan, W., W. Sherman, and S. King. 1979. Threshold responses in formaldehyde-sensitive subjects. J. Am. Acad. Dermatol. 1:44-58.
Kerns, W.D., K.L. Pavkov, D.J. Donofrio, E.J. Gralla, and J.A. Swenberg. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43:4382-4391.
Klaassen, C.D., ed. 2001. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 6th ed. New York: McGraw-Hill.
Klingerman, A.D., M.C. Phelps, and G.L. Erexsan. 1984. Cytogenetic analysis of lymphocytes from rats following formaldehyde inhalation. Toxicol. Lett. 21:241-246.
Kochhar, R., V. Nanda, B. Nagi, and S.K. Mehta. 1986. Formaldehyde-induced corrosive gastric cicatrisation: case report. Hum. Toxicol. 5:381-382.
Kreiger, R.A., and V.F. Garry. 1983. Formaldehyde-induced cytotoxicity and sister-chromatid exchanges in human lymphocyte cultures. Mutat. Res. 120(1):51-55.
Lam, C-W, M. Casanova, and H.D. Heck. 1985. Depletion of nasal mucosal glutathione by acrolein and enhancement of formaldehyde-induced DNA-protein cross-linking by simultaneous exposure to acrolein. Arch. Toxicol. 58:67-71.
Loomis, T.A. 1979. Formaldehyde Toxicity. Arch. Pathol. Lab. Med. 103:321-324.
Ma, T-H, and M.M. Harris. 1988. Review of the genotoxicity of formaldehyde. Mutat. Res. 196:37-59.
Marks, J.G., D.V. Belsito, V.A. DeLeo, J.F. Fowler, A.F. Fransway, H.I. Maibach, C.G.T. Mathias, J.R. Nethercot, R.I. Rietschel, E.F. Sherertz, F.J. Storrs, and J.S. Taylor. 1995. North American contact dermatitis group standard tray patch test results (1992-1994). Am. J. Contact Dermat. 6:160-165.
Marks, T.A., W.C. Worthy, and R.E. Staples. 1980. Influence of formaldehyde and Sonacide (potentiated acid glutaraldehyde) on embryo and fetal development in mice. Teratology 22:51-58.
Marsh, G.M, A.O. Youk, L.D. Buchanich, L.D. Cassidy, L.J. Lucas, N.A. Esman, and I.M. Gathuru. 2002. Pharyngeal cancer mortality among chemical plant workers exposed to formaldehyde. Toxicol. Ind. Health 18(6):257-268.
Martin, W.J. 1990. A teratology study of inhaled formaldehyde in the rat. Reprod. Toxicol. 4:237–239.
Merk, O., and G. Speit. 1998. Significance of formaldehyde-induced DNA protein crosslinks for mutagenesis. Environ. Mol. Mutagen. 32(3):260-8.
Miller, C.A., and M. Costa. 1989. Analysis of proteins cross-linked to DNA after treatment of cells with formaldehyde, chromate, and cisdiamminechloroplatinum. Mol. Toxicol. 2:11-26.
Monticello, T.M., J.A. Swenberg, E.A. Gross, J.R. Leininger, J.S. Kimbell, S. Seilkop, T.B. Starr, J.E. Gibson, and K.T. Morgan. 1996. Correlation of regional and nonlinear formaldehyde-induced nasal cancer with proliferating populations of cells. Cancer Res. 56:1012-1022.
Morgan, K.T. 1997. A brief review of formaldehyde carcinogenicity in relation to rat nasal passages and human health risk assessment. Toxicol. Pathol. 25(3):291-307.
NTP (National Toxicology Program). 2005. 11th report on carcinogenesis. National Toxicology Program, U.S. Department of Health and Human Services, Research Triangle Park, NC.
Pandey, C.K., A. Agarwal, and A. Baronia. 2000. Toxicity of ingested formalin and its management. Hum. Exp. Toxicol. 19(6):360-6.
Partanen, T. 1993. Formaldehyde exposure and respiratory cancer- a metaanalysis of the epidemiologic evidence. Scan. J. Work Environ. Health 19:8-15.
Pinkerton, L.E, M.J. Hein, and L.T. Stayner. 2004. Mortality among a cohort of garment workers exposed to formaldehyde: An update. Occup. Environ. Med. 61:193-200.
Pushkina, N.N., V.A. Gofmekler, and G.N. Kievtsova. 1968. Changes in content of ascorbic acid and nucleic acids produced by benzene and formaldehyde. Bull. Exp. Biol. Med. 66:868-870.
Restani, P., and C.L. Galli. 1991. Oral toxicity of formaldehyde and its derivatives. Crit. Rev. Toxicol. 21(5):315-78.
Saillenfait, A.M., P. Bonnet, and J. de Ceaurriz. 1989. The effects of maternally inhaled formaldehyde on embryonal and foetal development in rats. Food Chem. Toxicol. 8:545–548.
Schlosser, P.M., P.D. Lilly, R.B. Conolly, D.B. Janszen, and J.S. Kimbell. 2003. Benchmark dose risk assessment for formaldehyde using airflow modeling and a single-compartment, DNA-protein cross-link dosimetry model to estimate human equivalent doses. Risk Anal. 23(3):473-487.
Schmid, E., W. Googelmann, and M. Bauchinger. 1986. Formaldehyde-induced cytotoxic, genotoxic, and mutagenic response in human lymphocytes and Salmonella typhimurium. Mutagenesis 1:427-431.
Soffritti, M., C. Maltoni, F. Maffei, and R. Biagi. 1989. Formaldehyde: an experimental multipotential carcinogen. Toxicol. Ind. Health 5:699-730.
Soffritti, M., F. Belpoggi, L. Lambertin, M. Lauriola, M. Padovani, and C. Maltoni. 2002. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann. NY Acad. Sci. 982: 87-105.
Stratemann, K., W. Bredt, W. Herken, and N. Rietbrock. 1968. The folate content as limiting factor for formate detoxification and methanol metabolism. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 260:208.
Swenberg, J.A., W.D. Kerns, R.I. Mitchell, E.J. Gralla, and K.L. Pavkov. 1980. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposures to formaldehyde vapor. Cancer Res. 40:3398-3402.
Takahashi, M., R. Hasegawa, F. Furukawa, K. Toyoda, H. Sato, and Y. Hayashi. 1986. Effects of ethanol, potassium metabisulfite, formaldehyde and hydrogen peroxide on gastric carcinogenesis in rats after initiation with N-methyl-N’-nitro-N-nitrosoguanidine. Jpn. J. Cancer Res. 77:118-124.
TCEQ (Texas Commission on Environmental Quality). 2003. Chapter 350 in Texas Risk Reduction Program Protective Concentration Limit Table. Updated October 2003. Texas Commission on Environmental Quality, Austin, TX.
Teng, S., K. Beard, J. Pourahmad, M. Moridani, E. Easson, and R. Poon. 2001. The formaldehyde metabolic detoxification enzyme system and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 130-132(1-3):285-286.
Thrasher, J.D., and H. Kiliburn. 2001. Embryo toxicity and tetraogenicity of formaldehyde. Arch. Environ. Health 56(4):300-311.
Til, H.P., R.A. Woutersen, V.J. Feron, and J.J. Clary. 1988. Evaluation of the oral toxicity of acetaldehyde and formaldehyde in a 4-week drinking-water study in rats. Food Chem. Toxicol. 26:447-452.
Til, H.P., R.A. Woutersen, V.J. Feron, V.H. Hollanders, H.E. Falke, and J.J. Clary. 1989. Two-year drinking-water study of formaldehyde in rats. Food Chem. Toxicol. 27:77-87.
Tobe, M., K. Natio, and Y. Kurokawa. 1989. Chronic toxicity study on formaldehyde administered orally to rats. Toxicology 56:79-86.
Trattner, A., J. Johansen, and T. Menne. 1998. Formaldehyde concentrations in diagnostic patch testing: comparison of 1% with 2%. Contact Derm. 38:9-13.
Vargova, M., J. Wagnerova, A. Liskova, J. Jakubovsky, M. Gajdova, E. Stolcova, J. Kubova, J. Tulinska, and R. Stenclova. 1993. Subacute immunotoxicity study of formaldehyde in male rats. Drug Chem. Toxicol. 16:255-275.
Vasudeva, N., and C. Anand. 1996. Cytogenic evaluation of medical students exposed to formaldehyde vapor in the gross anatomy dissection level. J. Am. Coll. Health 44:177-179.
WHO (World Health Organization). 2002. Concise International Chemical Assessment Document 40. Formaldehyde. World Health Organization, Geneva, Switzerland.
Yager, J.W., K.L. Cohn, R.C. Spear, J.M. Fisher, and L. Morse. 1986. Sisterchromatid exchange in lymphocytes of anatomy students exposed to formaldehyde-embalming solution. Mutat. Res. 174:135-139.










































