2
Ammonia
John T. James, Ph.D. NASA-Johnson Space Center Habitability and Environmental Factors Office Houston, Texas
TABLE 2-1 Physical and Chemical Properties of Ammoniaa
OCCURRENCE AND USE
Ammonia (see Table 2-1) is not routinely measured in air samples from space vehicles; however, ground-based data from simulated space habitats show that under nominal conditions, concentrations of ammonia remain less than 0.2 milligrams per cubic meter (mg/m3); however, unexpected sources can boost the concentration to 0.9 mg/m3 or more (James et al. 2002). During the 90-day (d) phase of the Lunar Mars Life Support Test (LMLST), the venting of headspace gases from the bioreactor and wastewater containers caused an increase in the airborne ammonia. The lower concentration (0.2 mg/m3) represents a steady-state condition between the release of ammonia, primarily from human metabolism, and the removal of ammonia by charcoal filters and humidity conden-
sate. The typical concentrations of ammonia found in humidity condensate and processed water from space habitats are shown in Table 2-2.
The U.S. Laboratory Module of the International Space Station (ISS) uses anhydrous ammonia in the external loop of the heat exchanger, and there is a remote chance that some of this ammonia could enter the internal heat-exchange loop containing water and then reach the inhabited portion of the module. Because there are many liters of anhydrous ammonia in the external loop, such a series of leaks could result in a catastrophe. If such a leak were suspected, the water-recovery systems would be stopped until the atmospheric ammonia concentration returned to nominal levels. In addition, some payload proponents want to use ammonia in the cooling of their hardware.
TOXICOKINETICS
Ingested ammonia, like endogenously produced ammonia, is readily absorbed from the gastrointestinal (GI) tract, metabolized to urea primarily in the liver, and then excreted (as urea) mostly by the kidney. A healthy person has a high capacity for metabolizing ingested ammonia.
Absorption
Bacteria in the digestive tract produce ammonium ions (NH4+) from the metabolism of nitrogen-containing compounds that come from in-
TABLE 2-2 Ammonia in Water Samplesa
gested food. The total amount of ammonia produced is about 4,000 mg/d, and about 99% of that is absorbed from the colon (Summerskill and Wolpert 1978). Using a method free of significant interference from glutamine-containing compounds, Brown et al. (1957) found that the range of plasma concentrations of ammonia as nitrogen in 10 healthy young males was 0.30-0.55 mg per liter (L). Thus, the body normally has a high capacity to deal with ammonia and allows very little of it to remain unchanged in the general circulation.
An ingested dose of ammonia appears to be readily absorbed and metabolized to urea in the liver (Conn 1972). In 20 healthy human subjects given ammonium chloride (NH4Cl) tablets, as done in ammonia tolerance tests (dose of 20.0 mg per pound [mg/lb] of body weight, with a maximum of 3 g administered), the arterial blood concentration of ammonia showed a small transient increase. The peak, which was small, occurred in most subjects at 15 minutes (min), but in a few subjects, the peak occurred after 30 min. However, in a group of cirrhotic patients, the increase in the concentration of ammonia in the blood was much greater and was slower to return to baseline levels. These findings suggest that in healthy subjects, ammonia is readily absorbed from the GI tract and that the liver removes it from the blood (Conn 1972).
In a study of four human subjects who had received surgical colon bypass for chronic hepatic encephalopathy, solutions of different pH and NH4Cl concentrations were infused. Approximately half as much ammonia was absorbed under acidic conditions (pH = 5), where there is little ammonia, unlike under basic conditions (pH = 9), where a much higher portion of ammonia compared with ammonium ions is present (Castell and Moore 1971).
In people with normal renal function, an increase in dietary protein causes a decrease in the retention of ammonium hydroxide (15N) from NH4Cl (Richards et al. 1975). Subjects were dosed with 15N-labeled NH4Cl in five equal doses, each given at 4-hour (h) intervals, with total doses between 9 and 17 mg per kilograms (mg/kg) body weight. About 30% of the isotope was retained in the first 7 d in people on a normal diet; however, those restricted to a low-protein diet for 3 weeks (wk) before the dosing, retained about 70% of the isotope in 6 d (Richards et al. 1975).
Distribution
Ammonium ions that are absorbed from the gut into the portal circulation are converted to urea in the liver and excreted in the urine. Un-
ionized ammonia diffuses readily into cells, whereas ammonium ions hardly reach the intracellular compartment (Stabenau et al. 1958). Because of the chemical equilibrium between dissolved ammonia and ammonium ions, the latter can be absorbed indirectly. Ammonium compounds that reach the circulatory system can penetrate into cells in the rest of the body as ammonia, where it can be incorporated into proteins.
Metabolism
Ammonia and ammonium ions are metabolized to urea and glutamine in the process shown in Figure 2-1. At physiologic pH (7.25), only 1% of the ammonia is in the form of ammonia (pKa = 9.25). Bacteria in the gut decompose nitrogenous compounds into ammonia, which is absorbed into the portal circulation and then converted into urea in the liver. Conversely, urea passed from the liver to the GI tract is converted to ammonia by bacterial urease (Powers-Lee and Meister 1988). In the liver, ammonia is also converted to glutamine, which is the storage and transport form of ammonia. Many other enzymatic reactions in the liver produce significant amounts of ammonia (Powers-Lee and Meister 1988); however, a treatise on those is beyond the scope of this document.
Elimination
Ingested ammonia is metabolized to urea and primarily excreted in the urine; however, lesser amounts are excreted in the feces, sweat, and exhaled breath (Richards et al. 1975; Utell et al. 1989). As one would expect, this pattern is similar to that for elimination of endogenously produced ammonia.
TOXICITY SUMMARY
A small number of animal toxicity studies conducted mostly on rodents, suggest that ingested ammonia causes little, if any, systemic toxicity. Exposures at 40 mg/kg/d and above seem necessary to cause any adverse effects, and this dosage should be considered a lowest-observed-adverse-effect level (LOAEL). Exposures of about 20 mg/kg/d appear to be a no-observed-adverse-effect level (NOAEL) (ATSDR 2002). Some
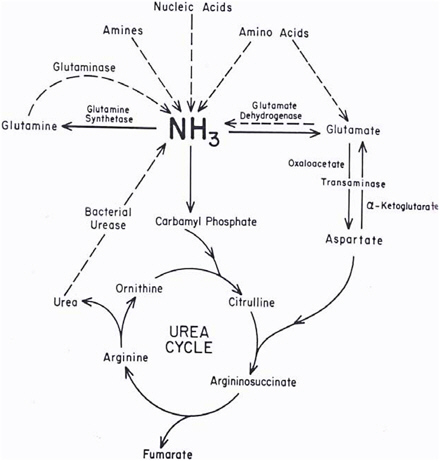
FIGURE 2-1 Metabolism of endogenous ammonia. Source: Powers-Lee and Meister 1988. Reprinted with permission; copyright 1988, Raven Press, New York.
studies are targeted at understanding the neurologic effects of high concentrations of ammonia in the blood as seen in liver failure; however, massive oral doses are necessary to increase and sustain these concentrations in healthy test animals. Effects on neuroreceptor binding and bone formation have been reported. Because only a few studies involve ammonia ingestion, they will not be grouped in the traditional exposure-time intervals. The toxicity database is insufficient to support a discussion of the comparative toxicity of different forms of ingested ammonia;
furthermore, the form present in potable water is not known a priori. For the ISS, it is likely to be NH4Cl, but that could change in future systems.
Esophageal lesions and edema were reported in five persons after single ingestions of household ammonia (ammonium hydroxide [15NH]) (Christesen 1995). Ammonia solutions in excess of 4% are generally considered caustic. In adults, only those with symptoms after ingesting strong alkali compounds and other caustic material are at risk for complications, the most common of which is esophageal injury. Thus, systemic injury from ingested ammonia is unlikely for one-time, large aqueous doses.
In a study designed to assess the effect of NH4Cl on the hypercalcemic effect of parathyroid hormone, one group of male Sprague-Dawley rats was given only NH4Cl. The dose was a 1.5% (15,000 mg/L) concentration of NH4Cl in their drinking water for 5-6 d. At this time, the average serum calcium in rats ingesting NH4Cl was 0.6 mg/dL higher than in rats drinking regular distilled water (Barzel 1975). In an earlier study with the same protocol except that the rats drank the spiked water for 330 d, the investigators found that, compared with the bones of controls, the exposed animals had reduced calcium and less fat-free solid (Barzel and Jowsey 1969). NH4Cl-induced osteoporosis in growing dogs was reported as part of a study assessing the effects of this model on serum phosphatase (Bodansky et al. 1932).
Effects on neuroreceptor function have been studied in models of hyperammonemia. In one study, Wistar rats were exposed through the dam to ammonia administered as ammonium acetate (20% concentration by weight) from day 1 of pregnancy through weaning (21 d postnatal) (Minana et al. 1995). They demonstrated a long-lasting impairment of N-methyl-D-aspartate (NMDA) receptor function in cerebellar neurons placed in culture and tested for binding to [3H]MK-801, which labels open NMDA channels. Body-weight increases in rats held for 140 d and exposed to ammonia either early in life (gestational day 1 to 21 d after birth) or during their entire life were lower than in the controls (Minana et al. 1995). Using another hyperammonemia model in which Wistar rats were given ammonium acetate (20% concentrate by weight in their diet) and 5 millimolar (mM) ammonium acetate in their drinking water for 3, 7, or 15 d, Boyano-Adanez et al. (1996) found a decrease in the equilibrium measures of somatostatin binding to synaptosomes from the fronto-parietal cortex and hippocampus. The effect was statistically significant only in the groups exposed for 7 or 15 d.
Renal effects, which are probably adaptive rather than adverse, can be induced by sustained oral doses of NH4Cl, which result in chronic
acidosis (Benyajati and Goldstein 1975). Repeated administration of NH4Cl (5 millimoles per kg [mmol/kg]) twice daily for 3 d to rats about 10 d old resulted in increased ammonia excretion and increased phosphate-dependent glutaminase activity in kidney homogenates. Following cessation of the treatments, the ammonia excretion and enzyme activity fell rapidly to normal levels. The authors considered these reversible changes to be adaptive.
A 90-d administration of ammonium sulfamate to groups of 20 albino rats of various ages at doses of 0, 100, 250, and 500 mg/kg resulted in a NOAEL (Gupta et al. 1979), although the highest-dose group of adult females failed to gain as much weight as the other groups. Clinical pathology results, gross necropsy findings, and histopathology results were not changed by the dose of ammonium sulfamate to the animals.
The most important biologic property of ammonia for our purposes is its odor. The odor threshold for ammonia in air is 5.2 ± 2.0 parts per million (ppm), and it is 1.5 ppm when dissolved in water (Amoore and Hautala 1983). These concentrations are well below any that might induce toxic effects even with prolonged exposure; however, the ingestion of water having an obvious odor would not be tolerated by astronauts for very long.
Cancer
Ammonia alone does not seem to cause cancer in rodents; however, when it is given in conjunction with known carcinogens, it can increase the tumorigenic response. Toth (1972) gave 0.1%, 0.2%, and 0.3% concentrations of 15NH to Swiss mice for their entire lifetimes after they were 5-6 wk old. Similarly, inbred C3H mice were given the lowest dose for their lifetimes. In the Swiss mice, there was no apparent increase in tumor incidence with increasing doses or in comparison to control mice, and the dose given to the C3H mice did not increase the incidence of spontaneous breast tumors.
In a much more limited study, Uzvolgyi and Bojan (1980, 1985) found that neither ammonia alone nor diethyl pyrocarbonate alone caused an increase in lung tumors when given to female Kid: CFLP mice in gavage doses twice per wk for 4 wk, but when the two were given together, more than half the mice showed lung tumors. The authors suggest that the tumors could have resulted from the formation of urethane as a reaction between the two compounds.
Ammonia may promote tumors induced by N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) in male Sprague-Dawley rats given 83 mg/L/d of the carcinogen for 24 wk in their drinking water (Tsujii et al. 1995). After the administration of the MNNG, rats were given a 0.01% solution of ammonia or tap water for an additional 24 wk. The number of gastric tumors was approximately double in the MNNG-treated animals receiving the ammonia water compared with those receiving tap water.
Genotoxicity
The database on genotoxicity is scanty at best, and many of the standard assays have never been done. There is one report that 22 workers exposed to ammonia gas at a fertilizer factory had an increase in chromosomal aberrations and sister chromatid exchanges (Yadav and Kaushik 1997). Other studies show mixed results of the potential for ammonia to cause genotoxicity; however, they were carried out before the development of standard assays and consensus-control procedures for such assays (summarized in ATSDR 2002).
Reproductive Toxicity
No studies of the reproductive effects of ingested ammonia were found.
Developmental Toxicity
Except for the study by Minana et al. (1995) described above, no reports of the developmental effects of ingested ammonia were found. Minana et al. reported that exposure to ammonia during gestation and lactation results in offspring with long-lasting NMDA-receptor impairment and decreased body-weight gains. The dose was given in feed as ammonium acetate at 20% of the food by mass. This study did not follow standard protocol for the detection of developmental toxicants and involved a large dose of ammonium acetate, so its relevance to the risk assessment of ammonia in drinking water is uncertain.
Spaceflight Effects
Bone resorption during spaceflight constitutes one of the more serious, progressive effects of being in microgravity for long periods (Schneider et al. 1994). Even with exercise countermeasures, some bonemineral-density losses were noted in specific bones. Massive doses of NH4Cl (about 1.5% in drinking water) that cause metabolic acidosis result in bone resorption and bone deformities in rabbits and dogs (Seegal 1927; Bodansky et al. 1932; Barzel and Jowsey 1969). Given the doses of NH4Cl necessary to affect bone metabolism, it is extremely unlikely that astronauts could ingest enough ammonia to increase their propensity for bone loss in space.
Synergistic Effects
Synergistic effects of ammonium salts with other chemicals in the body have not been reported except as noted in the cancer section above.
LIMITS SET BY OTHER ORGANIZATIONS
The U.S. Environmental Protection Agency (EPA) has not set a maximum contaminant level (MCL) for ammonia in drinking water, nor is there a reference dose (RfD) for chronic oral exposure. The lifetime health advisory level (HAL) set by EPA (2000) for ammonia is 30 mg/L as nitrogen. A HAL is an estimate of the concentration of a chemical in drinking water that is not expected to cause any adverse noncancer effects for a lifetime of exposure. The World Health Organization (WHO) noted the water odor threshold of 1.5 mg/L and the taste threshold at 35 mg/L and declined to propose a health-based water standard for ammonia (WHO 2002 as cited in the ATSDR 2002). No other drinking water standards were found in a literature search.
The most recent Agency for Toxic Substances and Disease Registry (ATSDR 2004) toxicologic profile for ammonia does not list an oral minimal risk level (MRL).
The Food and Drug Administration (FDA 1973) concluded that ammonia and ammonium salts in food are not a health concern; however, limits were placed on a number of products. Most relevant for the purposes here is the limit set on nonalcoholic beverages of 0.003%, or 30 ppm, for dibasic ammonium phosphate.
RATIONALE FOR SWEGS
In setting a spacecraft water exposure guideline (SWEG) for ammonia, it is essential that potable water remain free of any factors that might discourage crew water consumption. Although crew members are expected to consume water at a rate of 2,000 mL/d, the amount typically consumed is from 2,000 to less than 1,000 mL/d (Lane and Smith 1999). Urine production is correspondingly reduced from 2,800 mL/d to less than 500 mL/d. Thirst sensation appears to be reduced during spaceflight. The NOAEL in studies of various lengths is approximately 20 mg/kg/d, which for a 70 kg person drinking 2.8 L of water per day leads to a water concentration of 500 mg/L. This is an ingestion of 1,400 mg/d, or about one-third of the amount produced in the adult GI tract (Summerskill and Wolpert 1978). From a strictly toxicologic perspective, that amount would seem to be safe; however, the average odor threshold for ammonia in water is only 1.5 mg/L (Amoore and Hautla 1983). This threshold is based on only two reports with quite different thresholds reported (standard error = 2). The SWEGs were set to ensure that, at least for long periods of time, the crew drinks ample water.
1-d SWEG = 5 mg/L
10-d SWEG = 1 mg/L
100-d SWEG = 1 mg/L
1,000-d SWEG = 1 mg/L
The rationale for the 1-d SWEG is that the crew could drink water with a slight odor for 1 d by mixing something with it that masks the smell. For the longer-term SWEGs (10-1,000 d), there should be little chance that the crew would ever detect an odor from their drinking water and thus drink less. All of these limits are well below any that might be expected to cause adverse health effects. If an ammonia-like odor was coming from the water, the crew would also be expected to adapt and become less sensitive to it.
Finally, these limits are consistent with those accepted for alkylamines (see Chapter 4). For monoalkylamines, the limits for 1, 10, 100, and 1,000 d are 4, 2, 2, and 2 mg/L, respectively. Limits for di- and trialkylamines are somewhat lower than those for monoalkylamines (see Chapter 4). These SWEGs were based on the odor thresholds for these compounds.
REFERENCES
Amoore, J.E., and E. Hautala. 1983. Odor threshold as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities from 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3:272-290.
ATSDR (Agency for Toxic Substances and Disease Registry). 2002. Toxicological Profile for Ammonia, Draft for Public Comment (Update). U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, GA.
Barzel, U.S. 1975. The effect of chronic ammonium chloride ingestion on parathyroid hormone function. Nephron 14:339-346.
Barzel, U.S., and J. Jowsey. 1969. The effects of chronic acid and alkali administration on bone turnover in adult rats. Clin. Sci. 36:517-524.
Benyajati, S., and L. Goldstein. 1975. Renal glutaminase adaptation and ammonia excretion in infant rats. J. Physiol. 228:693-698.
Bodansky, A., H.L. Jaffe, and J.P. Chandler. 1932. Serum phosphatase changes in calcium deficiency and in ammonium chloride osteoporosis. Proc. Soc. Exp. Biol. Med. 29:871-873.
Boyano-Adanez, M.C., G. Bodega, V. Barrios, and E. Arilla. 1996. Response of rat cerebral somatostatinergic system to a high ammonia diet. Neurochem. Int. 29:469-476.
Brown, R.H., G.D. Duda, S. Korkes, and P. Handler. 1957. A Colorimetric micromethod for determination of ammonia; the ammonia content of rat tissue and human plasma. Arch. Biochem. and Biophys. 66:301-309.
Castell, D.O., and E.W. Moore. 1971. Ammonia absorption from the human colon. Gastroenterology 60:33-42.
Christesen, H.B.T. 1995. Prediction of complications following caustic ingestion in adults. Clin. Otolaryngol. 20:272-278.
Conn, H.O. 1972. Studies of the source and significance of blood ammonia IV. Early ammonia peaks after ingestion of ammonium salts. Yale J. Biol. Med. 45:543-549.
EPA (U.S. Environmental Protection Agency). 2000. Drinking Water Standards and Health Advisories. EPA 822-B-00-001. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
FDA (U.S. Food and Drug Administration). 1973. Generally recognized as safe food ingredients-ammonium ion. FDA PB-221-235. U.S. Food and Drug Administration, Washington, DC.
Gupta, B.N., R.N. Khanna, and K.K. Datta. 1979. Toxicological studies of ammonium sulfamate in rat after repeated oral administration. Toxicology 13:45-49.
James, J.T., T.F. Limero, S.W. Beck, M. Martin, P.A. Covington, L. Yang, D. Lind, and J.F. Boyd. 2002. Environmental monitoring: Air quality. Chapter 4.1 in Isolation: NASA Experiments in Closed-Environment Living,
H.W. Lane, R.L. Sauer, and D.L. Feeback, eds. San Diego: American Astronautical Society.
Lane H.W., and S.M. Smith. 1999. Nutrition in Space. Pp 783-788 in Modern Nutrition in Health and Disease. Baltimore: Lippincott Williams & Wilkins.
Merck Index. 1989. An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th Ed. S. Budavari, M.J. O’Neil, and A. Smith, eds. Whitehouse Station, NJ: Merck & Co.
Minana, M.D., G. Marcaida, S. Grisolia, and V. Felipo. 1995. Prenatal exposure of rats to ammonia impairs NMDA receptor function and affords delayed protection against ammonia toxicity and glutamate neurotoxicity. J. Neuropathol. Exp. Neurol. 54:644-650.
Pierre, L.M., J.R. Schultz, S.M. Johnson, R.L. Sauer, Y.E. Sinyak, V.M. Skuratov, and N.N. Protasov. 1996. Collection and chemical analysis of reclaimed water and condensate from the Mir space station. SAE-ICES Paper 961569. Warrendale, PA: Society of Automotive Engineers.
Pierre, L.M., J.R. Schultz, S.E. Carr, and R.L. Sauer. 2002. Water chemistry monitoring. Chapter 4.2 in Isolation: NASA Experiments in Closed-Environment Living, H.W. Lane, R.L. Sauer, D.L. Feeback, eds. San Diego: American Astronautical Society.
Plumlee, D.K., P.D. Mudgett, and J.R. Schultz. 2003. ISS potable water sampling and chemical analysis: Expeditions 4 & 5. SAE International paper 2003-01-2401. Presented at the 33rd International Conference on Environmental Systems (ICES), July 7-10, 2003, Vancouver, Canada.
Powers-Lee, S.F., and A. Meister. 1988. Urea synthesis and ammonia metabolism. Ch. 17 in The Liver: Biology and Pathobiology, 2nd Ed., I.M. Arias, W.B. Jacoby, H. Popper, D. Schachter, and D.A. Shafritz, eds. New York: Raven Press.
Richards, P., C.L. Brown, B.J. Houghton, and O.M. Wong. 1975. The incorporation of ammonia nitrogen into albumin in man: The effects of diet, uremia and growth hormone. Clin. Nephrol. 3:172-179.
Schneider, V.S., A.D. Le Blanc, and L.C. Taggart. 1994. Bone and mineral metabolism. Ch. 17 in Space Medicine and Physiology, 3rd Ed., A. Nicogossian, C. Huntoon, and S. Pool, eds. Philadelphia: Lea & Febiger.
Seegal, B.C. 1927. Chronic acidosis in rabbits and in dogs. Arch. Intern. Med. 39:550-563.
Stabenau, J.R., K.S. Warren, and D.P. Rall. 1958. The role of pH gradient in the distribution of ammonia between blood and cerebral spinal fluid, brain, and muscle. J. Clin. Invest. 38:373-383.
Summerskill, D.M., and E. Wolpert. 1970. Ammonia metabolism in the gut. Am. J. Clin. Nutr. 23:633-639.
Toth, B. 1972. Hydrazine, methylhydrazine and methylhydrazine sulfate carcinogenesis in Swiss mice: Failure of ammonium hydroxide to interfere in the development of tumors. Int. J. Cancer 9:109-118.
Tsujii, M., S. Kawano, S. Tsuji, Y. Takei, K. Tamura, H. Fusamoto, and T. Kamada. 1995. Mechanism for ammonia-induced promotion of gastric carcinogenesis in rats. Carcinogenesis 16:563-566.
Utel, M.J., J.A. Martiglio, and P.E. Morrow. 1989. Effects of inhaled acid aerosol on respiratory function: The role of endogenous ammonia. J. Aerosol Med. 2:141-147.
Uzvolgyi, E., and F. Bojan. 1980. Possible in vivo formation of a carcinogenic substance from diethyl pyrocarbonate and ammonia. J. Cancer Res. Clin. Oncol. 97:205-207.
Uzvolgyi, E., and F. Bojan. 1985. In vivo formation of a carcinogenic substance from diethyl pyrocarbonate in the presence of ammonia. Arch. Toxicol. Suppl. 8:490-493.
Wyle Laboratories. Report dated January 9, 2002. Subject: Final chemical analysis results for the STS-104/7A ISS water samples. Wyle Laboratories, Houston, TX.
Yadav, J.S., and V.K. Kaushik. 1997. Genotoxic effect of ammonia exposure on workers in a fertilizer factory. Indian J. Exp. Biol. 35:487-492.













